CHAPTER 5 Pattern recognition and interpretation
The clinical application of electronic fetal heart rate (FHR) monitoring consists of three distinct, interdependent elements: definition, interpretation, and management.
This chapter addresses the standardized terminology used to define FHR patterns and the standardized, evidence-based interpretation of FHR patterns with respect to underlying physiology. The principles developed in this chapter are applied to a standard approach to fetal heart rate management that will be discussed in Chapter 6.
The evolution of standardized fetal heart rate terminology
Electronic FHR monitoring was introduced into clinical practice before consensus was achieved regarding standardized definitions of FHR patterns. This resulted in wide variations in the description and interpretation of common FHR observations. Lack of standardization was a major impediment to effective communication. In 1995 and 1996, the National Institute of Child Health and Human Development (NICHD) convened a workshop to develop “standardized and unambiguous definitions for fetal heart rate tracings.”11 The resulting recommendations were published in 1997 in the American Journal of Obstetrics and Gynecology and in the Journal of Obstetric, Gynecologic and Neonatal Nurses. However, the NICHD recommendations were not incorporated rapidly into clinical practice and wide variations persisted. In May 2005, the American College of Obstetricians and Gynecologists (ACOG) endorsed the 1997 NICHD recommendations in Practice Bulletin # 62, entitled “Intrapartum Fetal Heart Rate Monitoring” (subsequently updated in December 2005, Practice Bulletin # 70).2 Shortly after ACOG published Practice Bulletin # 62, the Association of Women’s Health, Obstetric and Neonatal Nurses (AWHONN) endorsed the NICHD terminology stating, “AWHONN’s decision is based on recognition of the importance of ensuring the use of standardized terminology among health care providers to communicate fetal heart monitoring and promote patient safety.”6 In December 2006, the American College of Nurse Midwives (ACNM) issued a position statement encouraging the use of NICHD nomenclature as the standard when using electronic fetal monitoring.1
The 2008 NICHD consensus report
In 2008, a second NICHD consensus panel was convened to review and update the standardized definitions published in 1997 and to reach consensus regarding basic principles of FHR interpretation. 26 The standardized NICHD FHR definitions published in 2008 are summarized in Table 5-1. In addition to clarifying and reiterating the standardized FHR definitions proposed by the 1997 NICHD consensus statement, the 2008 report recommended a simplified system for classifying FHR tracings and introduced into the literature for the first time consensus in the form of two key statements that together represent a fundamental principle of FHR interpretation:26
TABLE 5-1 Standardized Fetal Heart Rate Definitions
Adapted from: Macones GA, et al. Obstet Gynecol. 2008 Sep;112(3):661-666.
The proposed classification system groups every FHR tracing into one of three categories (Table 5-2). Category I includes tracings with a normal baseline rate (110-160), moderate variability and no variable, late or prolonged decelerations. Category III includes tracings with at least one of the following: absent variability with recurrent late decelerations, absent variability with recurrent variable decelerations, absent variability with bradycardia for at least 10 minutes or a sinusoidal pattern for at least 20 minutes. Category II includes all tracings that do not meet criteria for classification as Category I or Category III.
TABLE 5-2 Three-tier FHR Classification System
| Category I | |
| Normal | |
| Category II | |
| Indeterminate | Includes all FHR tracings not assigned to Categories I or III |
| Category III | |
| Abnormal | |
Adapted from: Macones GA, et al. Obstet Gynecol. 2008 Sep;112(3):661-666.
The proposed FHR categories represent a shorthand method of defining FHR tracings. Because Category II includes an extremely wide range of FHR tracings, the categories alone do not provide sufficient information for FHR interpretation or management. In other words, categorizing a FHR tracing does not replace a complete description of baseline rate, variability, accelerations, decelerations, and changes or trends over time.
The recommendations of the 2008 NICHD consensus report are summarized in ACOG Practice Bulletins 106 and 116.3,4 The 2008 NICHD consensus report also addressed the confusion surrounding the description of uterine activity. Normal uterine contraction frequency was defined as 5 or fewer contractions in a 10-minute window averaged over 30 minutes. Contraction frequency of more than 5 in 10 minutes averaged over 30 minutes is defined as tachysystole. The terms hyperstimulation and hypercontractility are poorly defined, therefore the consensus report recommended that they be avoided.26
The fetal physiology underlying common FHR patterns has been the subject of scientific investigation for at least four decades. However, a surprising number of FHR theories have not been substantiated by appropriate analytic research. In an area as critical as intrapartum FHR monitoring, it is imperative that care providers recognize the difference between concepts that are based on sound scientific evidence and those that are based on unsubstantiated theories.
This chapter will review the relationship between fetal physiology and FHR patterns with particular emphasis on the underlying scientific evidence. Principles of FHR interpretation will be stratified by supporting evidence according to the method outlined by the U.S. Preventive Services Task Force as summarized in Box 5-1.38 Level I evidence is considered to be the most robust and level III the least. Specifically, Level I and II evidence is considered analytic and is capable of establishing statistically significant relationships. Level III evidence is descriptive and serves to generate theories and hypotheses. It does not test theories or hypotheses and therefore is not capable of proving them.
BOX 5-1 Stratification of Scientific Evidence*
| Level I | Evidence obtained from at least one properly designed randomized controlled trial. |
| Level II-1 | Evidence obtained from well-designed controlled trials without randomization. |
| Level II-2 | Evidence obtained from well-designed cohort or case-control analytic studies, preferably from more than one center or research group. |
| Level II-3 | Evidence obtained from multiple time series with or without the intervention. Dramatic results in uncontrolled experiments also could be regarded as this type of evidence. |
| Level III | Opinions of respected authorities, based on clinical experience, descriptive studies, or reports of expert committees. |
*According to method outlined by U.S. Preventive Services Task Force.38
NICHD definitions general considerations
The standardized definitions proposed by the NICHD in 1997 and reiterated in 2008 apply to the interpretation of FHR patterns produced by a direct fetal electrode detecting the fetal ECG or by an external Doppler device detecting fetal cardiac motion using the autocorrelation technique. Autocorrelation is a computerized method of minimizing the artifact associated with Doppler ultrasound calculation of the FHR. This technology is incorporated in all modern FHR monitors. Other general considerations are as follows:
 Deceleration onset is defined as abrupt if the onset to nadir (lowest point) is less than 30 seconds and gradual if the onset to nadir is 30 seconds or greater.
Deceleration onset is defined as abrupt if the onset to nadir (lowest point) is less than 30 seconds and gradual if the onset to nadir is 30 seconds or greater. Although terms such as beat-to-beat variability, short-term variability, and long-term variability are used frequently in clinical practice, the NICHD panel recommended that no distinction be made between short-term (or beat-to-beat) and long-term variability because in actual practice they are visually determined as a unit.
Although terms such as beat-to-beat variability, short-term variability, and long-term variability are used frequently in clinical practice, the NICHD panel recommended that no distinction be made between short-term (or beat-to-beat) and long-term variability because in actual practice they are visually determined as a unit. A number of FHR characteristics are dependent upon gestational age, so gestational age must be considered in the full evaluation of the pattern. In addition, the FHR tracing should be evaluated in the context of maternal medical condition, prior results of fetal assessment, medications, and other factors.
A number of FHR characteristics are dependent upon gestational age, so gestational age must be considered in the full evaluation of the pattern. In addition, the FHR tracing should be evaluated in the context of maternal medical condition, prior results of fetal assessment, medications, and other factors.It is essential to recognize that FHR patterns do not occur alone and generally evolve over time. Therefore, a full description of a FHR tracing requires a qualitative and quantitative description of each of the five essential FHR components
Definition and physiology of FHR components
Baseline rate
Definition
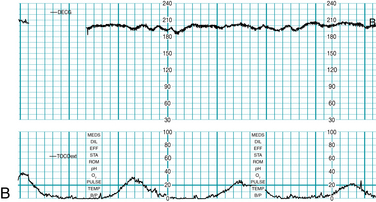
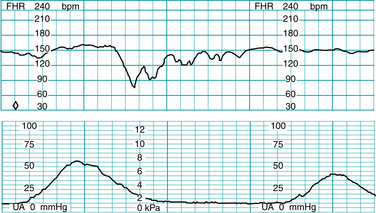
Baseline FHR is defined as the approximate mean FHR rounded to increments of 5 bpm (beats per minute) during a 10-minute segment, excluding accelerations, decelerations, and periods of marked variability (Figures 5-1 through 5-4). Baseline rate is defined as a single number (for example 145 bpm), not as a range (for example “140-150 bpm” or “140s”) because the definitions of other FHR components, including accelerations and decelerations, are based on the degree of deviation from the baseline rate. In any 10-minute window the minimum baseline duration must be at least 2 minutes (not necessarily contiguous) or the baseline for that period is deemed indeterminate. If the baseline during any 10-minute segment is deemed indeterminate, it may be necessary to refer to previous 10-minute segment(s) for determination of the baseline.
FIGURE 5-1 FHR baseline. Note that the 2-minute minimum for identifiable baseline does not require 2 contiguous or continuous minutes; rather, it is the total identifiable baseline in the 10-minute window that must add up to 2 minutes. Also, baseline may occur (and be interpreted) during contractions, as seen here. Baseline (highlighted) is identified over the entire 10-minute window, exceeding the 2-minute minimum.
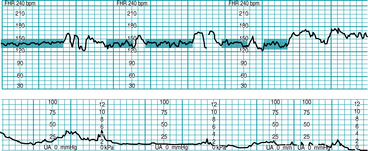
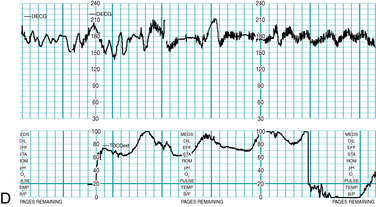
FIGURE 5-2 FHR baseline. Note that the 2-minute minimum for identifiable baseline does not require 2 contiguous or continuous minutes; rather, it is the total identifiable baseline in the 10-minute window that must add up to 2 minutes. Also, baseline may occur (and be interpreted) during contractions, as seen in Figure 5-1. Baseline (highlighted) is identified between accelerations or between segments differing by 25 bpm or more; identifiable baseline is approximately 5 minutes total (note the 2-minute minimum is met).
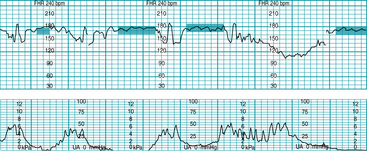
FIGURE 5-3 FHR baseline. Note that the 2-minute minimum for identifiable baseline does not require 2 contiguous or continuous minutes; rather, it is the total identifiable baseline in the 10-minute window that must add up to 2 minutes. Also, baseline may occur (and be interpreted) during contractions, as seen in Figure 5-1. Baseline (highlighted) is identified between decelerations or between segments differing by 25 bpm or more; identifiable baseline is approximately 5 minutes total (note the 2-minute minimum is met).
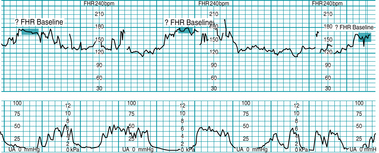
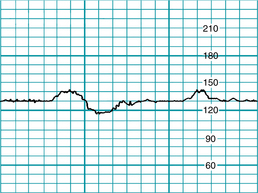
FIGURE 5-4 Indeterminate baseline. Note that there are less than 2 minutes of identifiable baseline during this 10-minute window. The baseline would be labeled “indeterminate” for this portion of the tracing, and the clinician would need to refer to previous portions of the strip to determine baseline. Prior baseline was 155 bpm.
Summary of baseline rate characteristics
Physiology
Baseline FHR is regulated by intrinsic cardiac pacemakers (SA node, AV node) and conduction pathways, autonomic innervation (sympathetic, parasympathetic), humoral factors (catecholamines), extrinsic factors (medications) and local factors (calcium, potassium). Sympathetic innervation and plasma catecholamines increase baseline FHR while parasympathetic innervation reduces the baseline rate. Autonomic input regulates the FHR in response to fluctuations in PO2, PCO2 and blood pressure detected by chemoreceptors and baroreceptors located in the aortic arch and carotid arteries. A normal FHR baseline of 110 to 160 bpm is consistent with normal neurologic regulation of the FHR.
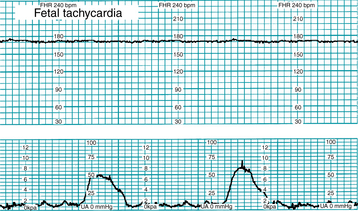
Fetal tachycardia is defined as a baseline rate above 160 bpm for at least 10 minutes (Figure 5-5). Conditions potentially associated with fetal tachycardia are summarized below. Most supporting evidence is level II or III.
Conditions potentially associated with fetal tachycardia:
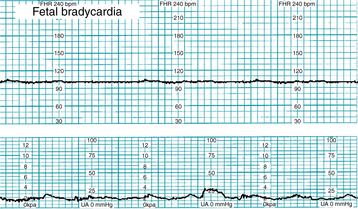
Fetal bradycardia is defined as a baseline rate below 110 bpm for at least 10 minutes (Figure 5-6). Conditions associated with fetal bradycardia are summarized below. Most supporting evidence is level II or III.
Fetal heart rate variability
Definition
Variability is defined as fluctuations in the baseline FHR that are irregular in amplitude and frequency. Variability is quantitated in beats per minute and is measured from the peak to the trough in beats per minute. No distinction is made between “short-term” (“beat-to-beat”) variability and “long-term” variability because in actual practice they are visually determined as a unit. In addition, there is no consensus that “beat-to-beat” differences in rate can be quantitated accurately with the unaided eye. Major organizations representing providers of obstetric care in the United States have endorsed the 1997 and 2008 NICHD recommendations discouraging the use of the terms “short-term” variability, “beat-to-beat” variability, and “long-term” variability. In the contemporary practice of obstetrics, continued use of these terms in the medical record (a legal document) raises a red flag, indicating that the person using the terms is not aware of the recommendations of prominent professional societies or is aware of the recommendations but chooses to ignore them. In either event, it promotes inconsistent communication, jeopardizes the credibility of the healthcare team and should be abandoned in favor of standard definitions.
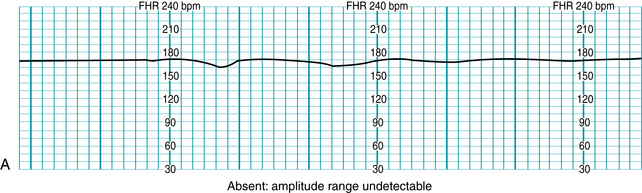
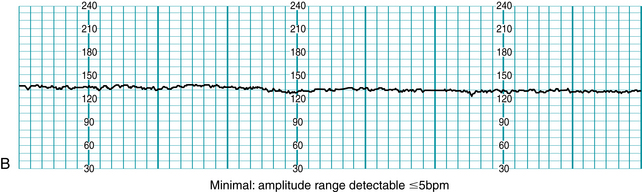
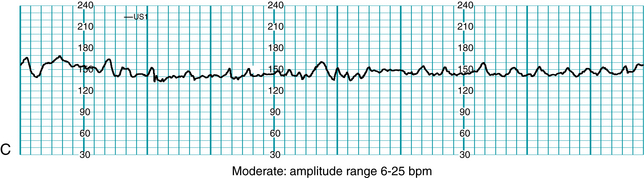
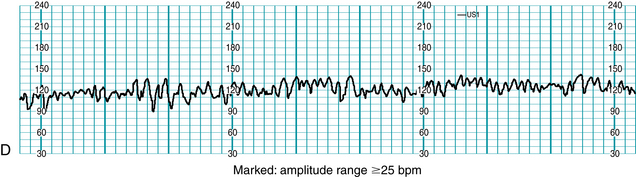
Standardized NICHD nomenclature classifies variability as absent, minimal, moderate or marked (Figure 5-7). Variability is defined as absent when the amplitude range of the fetal heart rate fluctuations is undetectable to the unaided eye. Variability is defined as minimal when the amplitude range is detectable but less than or equal to 5 beats per minute. When the amplitude range of the fetal heart rate fluctuations is 6-25 beats per minute, variability is defined as moderate. Variability is defined as marked when the amplitude range of the fetal heart rate fluctuations is greater than 25 beats per minute.
Physiology
Many factors interact to regulate FHR variability, including cardiac pacemakers (SA node, AV node) and the cardiac conduction system, autonomic innervation (sympathetic, parasympathetic), humoral factors (catecholamines), extrinsic factors (medications), and local factors (calcium, potassium). Fluctuations in PO2, PCO2, and blood pressure are detected by chemoreceptors and baroreceptors located in the aortic arch and carotid arteries. Signals from these receptors are processed in the medullary vasomotor center, possibly with regulatory input from higher centers in the hypothalamus and cerebral cortex. Sympathetic and parasympathetic signals from the medullary vasomotor center modulate the FHR in response to moment-to-moment changes in fetal PO2, PCO2, and blood pressure. With every heart beat, slight corrections in the heart rate help to optimize fetal cardiac output and maximize the distribution of oxygenated blood to the fetal tissues. This variation is referred to as FHR variability and is displayed visually on the FHR graph as an irregular horizontal line.
As discussed in Chapter 2, the 2008 NICHD consensus report stated unequivocally that moderate variability reliably predicts the absence of fetal metabolic acidemia at the time it is observed.26 However, the converse is not true. Minimal or absent variability cannot confirm the presence of fetal metabolic acidemia. Other conditions potentially associated with minimal or absent variability include fetal sleep, fetal tachycardia, medications, prematurity, congenital anomalies, fetal anemia, cardiac arrhythmias, infection, and preexisting neurologic injury. Most supporting evidence is Level II-3 and III.
Conditions potentially associated with minimal-absent FHR variability and the absence of accelerations:
It is important to note that most of the literature regarding “decreased” variability does not differentiate between absent variability (amplitude range undetectable) and minimal variability (amplitude range detectable but ≤5 bpm). Therefore, it is not possible to draw valid conclusions regarding the relative clinical significance of these two categories. The significance of marked variability is not known. Possible explanations include a normal variant or an exaggerated autonomic response to interruption of fetal oxygenation. Scientific evidence regarding marked variability is limited to Level III.
Sinusoidal pattern
Definition
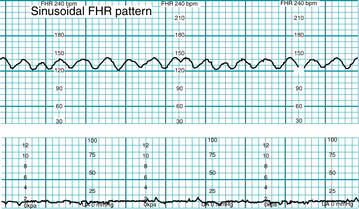
The sinusoidal pattern, illustrated in Figure 5-8, is a smooth, sine wave-like undulating pattern in FHR baseline with a cycle frequency of 3-5/min that persists for at least 20 minutes. It is specifically excluded from the definition of variability. The sinusoidal pattern can be distinguished from variability because it is characterized by fluctuations in the baseline that are regular in amplitude in frequency.
Physiology
Although the pathophysiologic mechanism is not known, this pattern classically is associated with severe fetal anemia. Variations of the pattern have been described in association with chorioamnionitis, fetal sepsis, or administration of narcotic analgesics.16 Scientific evidence regarding associated factors is Level II-2 to Level III. Evidence regarding pathophysiology is Level III.
Acceleration
Definition
An acceleration is an abrupt (onset to peak <30 seconds) increase in FHR above the baseline. The peak is at least 15 bpm above the baseline and the acceleration lasts at least 15 seconds from the onset to return to baseline, as illustrated in Figure 5-9. Before 32 weeks of gestation, an acceleration is defined as having a peak at least 10 bpm above the baseline and a duration of at least 10 seconds. An acceleration lasting at least 2 minutes but less than 10 minutes is defined as a prolonged acceleration. An acceleration lasting 10 minutes or longer is defined as a baseline change.
Summary of acceleration characteristics
 At less than 32 weeks of gestation, an acceleration peaks ≥ 10 bpm above the baseline and lasts ≥ 10 seconds but < 2 min.
At less than 32 weeks of gestation, an acceleration peaks ≥ 10 bpm above the baseline and lasts ≥ 10 seconds but < 2 min.Physiology
Accelerations in FHR frequently occur in association with fetal movement, possibly as a result of stimulation of peripheral proprioceptors, increased catecholamine release, and autonomic stimulation of the heart. Another suspected mechanism of FHR acceleration is transient compression of the umbilical vein, resulting in decreased fetal venous return and a reflex rise in heart rate.
The 2008 NICHD consensus report stated that FHR accelerations reliably predict the absence of fetal metabolic acidemia at the time they are observed.26 However, the converse is not true. The absence of accelerations does not confirm the presence of fetal metabolic acidemia. Conditions potentially associated with the absence of accelerations are similar to conditions potentially associated with minimal-absent variability and include: fetal sleep, fetal tachycardia, medications (narcotics, barbiturates, phenothiazines, tranquilizers, general anesthetics, atropine, phenothiazines), prematurity, congenital anomalies, fetal anemia, cardiac arrhythmias, infection, preexisting neurologic injury, and fetal metabolic acidemia.13,15,22,40 Supporting evidence is Level II-2, II-3, and III. In the absence of spontaneous accelerations, fetal scalp stimulation or vibroacoustic stimulation can provoke fetal movement and FHR accelerations.
Decelerations
Decelerations in the FHR are categorized as early, late, variable or prolonged and are quantitated by depth in bpm below the baseline and duration in minutes and seconds. An abrupt deceleration reaches its nadir (lowest point) in less than 30 seconds. A gradual deceleration reaches its nadir in ≥30 seconds. Decelerations that occur with at least 50% of uterine contractions in a 20-minute period are defined as recurrent. Decelerations occurring with fewer than 50% of contractions in a 20-minute period are defined as intermittent. Classification of decelerations as “mild,” “moderate,” or “severe” has not been shown to correlate with metabolic acidemia or newborn outcome independent of other FHR characteristics such as moderate variability and accelerations. Therefore, such classification is not included in standardized NICHD terminology.26
Early deceleration
Definition
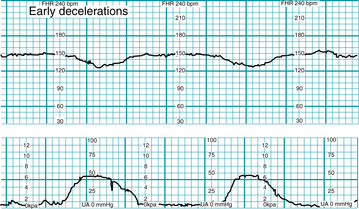
Early deceleration is defined as a gradual (onset to nadir ≥30 seconds) decrease in FHR from the baseline and subsequent return to baseline associated with a uterine contraction. In most cases the onset, nadir, and recovery of the deceleration occur at the same time as the beginning, peak, and end of the contraction, respectively (Figure 5-10).
Physiology
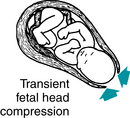
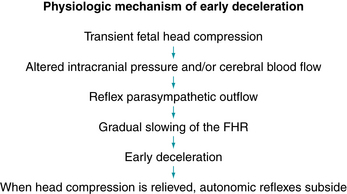
Although the precise physiologic mechanism is not known, early decelerations are considered to represent a fetal autonomic response to changes in intracranial pressure and/or cerebral blood flow caused by intrapartum compression of the fetal head during uterine contractions, as illustrated in Figure 5-11. Early decelerations are not correlated with adverse outcome and are considered clinically benign. Evidence is Level II-3 and Level III. Analytic evidence in the literature does not support the notion that mechanical compression of the fetal head by uterine contractions can cause local cerebral ischemia and neurologic injury in the absence of metabolic acidemia and neonatal encephalopathy.
Late deceleration
Definition
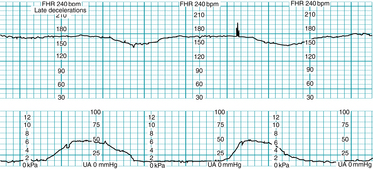
Late deceleration of the FHR is defined as a gradual (onset to nadir ≥30 seconds) decrease of the FHR from the baseline and subsequent return to the baseline associated with a uterine contraction, as illustrated in Figure 5-12. In most cases the onset, nadir, and recovery of the deceleration occur after the beginning, peak, and ending of the contraction, respectively.
Physiology
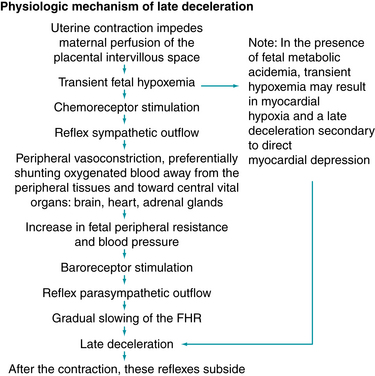
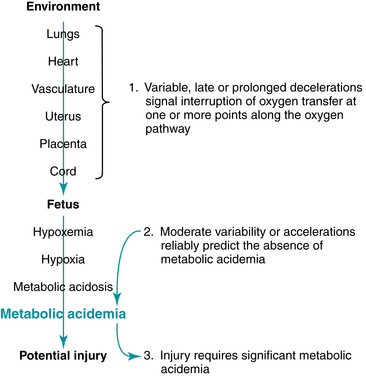
A late deceleration is a reflex fetal response to transient hypoxemia during a uterine contraction. Myometrial contractions can compress maternal blood vessels traversing the uterine wall and reduce maternal perfusion of the intervillous space of the placenta. Reduced delivery of oxygenated blood to the intervillous space can reduce the diffusion of oxygen into the fetal capillary blood in the chorionic villi, leading to a decline in fetal PO2. If the fetal PO2 falls below the normal range (approximately 15 – 25 mm Hg in the umbilical artery), chemoreceptors detect the change and signal the medullary vasomotor center in the brainstem to initiate a protective autonomic reflex response. Initially, sympathetic outflow causes peripheral vasoconstriction, shunting oxygenated blood flow away from non-vital vascular beds and toward vital organs such as the brain, heart, and adrenal glands. The resulting increase in fetal blood pressure is detected by baroreceptors, which trigger a parasympathetic reflex slowing of the heart rate to reduce cardiac output and return the blood pressure to normal. After the contraction, fetal oxygenation is restored, autonomic reflexes subside, and the FHR gradually returns to baseline. This combined sympathetic-parasympathetic reflex response to transient interruption of fetal oxygenation, summarized in Figure 5-13, has been confirmed in animal studies.7,8,10,12,18,21,27,29-31 Rarely, fetal oxygenation can be interrupted sufficiently to result in metabolic acidemia. In that event, a late deceleration may result from direct hypoxic myocardial depression.27 Since the latter mechanism requires metabolic acidemia, it can be excluded by the observation of moderate variability or accelerations. Late decelerations classically have been attributed to “uteroplacental insufficiency.” However, this term can be misleading because it implicates only the uterus and placenta as potential sources of interrupted fetal oxygenation. In reality, interruption of the oxygen pathway at any point can contribute to a late deceleration. For example, conditions such as maternal hypoxemia, poor cardiac output, or hypotension can result in late decelerations even with mild uterine contractions and a normally functioning placenta. Regardless of the underlying mechanism, all late decelerations reflect interruption of oxygen transfer from the environment to the fetus at one or more points along the oxygen pathway. Supporting evidence is Level II-1 and II-2.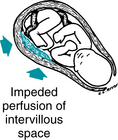
Variable deceleration
Definition
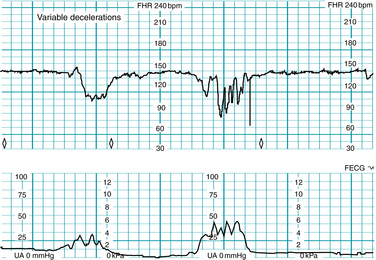
Variable deceleration of the FHR is defined as an abrupt (onset to nadir <30 seconds) decrease in FHR below the baseline (Figure 5-14). The decrease is at least 15 bpm below the baseline and the deceleration lasts at least 15 seconds and <2 minutes from onset to return to baseline. Variable decelerations can occur with or without uterine contractions.
Physiology
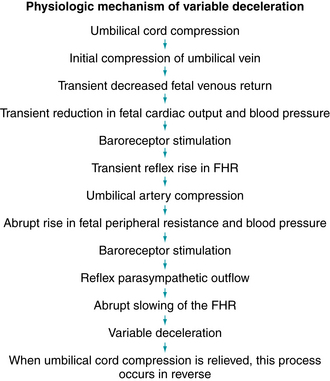
A variable deceleration represents a fetal autonomic reflex response to transient mechanical compression of the umbilical cord.17-20,24,25,28,35,37,41 Initially, compression of the umbilical cord occludes the thin-walled, compliant umbilical vein, decreasing fetal venous return and triggering a baroreceptor-mediated reflex rise in FHR (sometimes described as a “shoulder”). Further compression occludes the umbilical arteries, causing an abrupt increase in fetal peripheral resistance and blood pressure. Baroreceptors detect the abrupt rise in blood pressure and signal the medullary vasomotor center in the brainstem, which in turn triggers an increase in parasympathetic outflow and an abrupt decrease in heart rate. As the cord is decompressed, this sequence of events occurs in reverse (Figure 5-15). It is important to remember that umbilical cord compression can coincide with interruption at any or all of the other points along the oxygen pathway. The observation of a variable deceleration does not preclude interruption of fetal oxygenation at the level of the maternal lungs, heart, vasculature, uterus or placenta. Therefore, for the purposes of standardized FHR interpretation, a variable deceleration reflects interruption of oxygen transfer from the environment to the fetus at one or more points along the oxygen pathway. Supporting evidence is Level II-1, II-2, II-3, and III.
Prolonged deceleration
Definition
Prolonged deceleration of the FHR is defined as a decrease (either gradual or abrupt) in FHR at least 15 bpm below the baseline lasting at least 2 minutes from onset to return to baseline (Figure 5-16). According to NICHD terminology, a prolonged deceleration lasting 10 minutes or longer is considered a baseline change.
Physiology
If the physiologic mechanisms responsible for late or variable decelerations persist, a deceleration can last long enough to be defined as a prolonged deceleration. A prolonged deceleration reflects interruption of oxygen transfer from the environment to the fetus at one or more points along the oxygen pathway. For example, at the level of the maternal lungs, a prolonged deceleration can result from maternal apnea during a convulsion. At the level of the maternal heart, a cardiac arrhythmia can compromise cardiac output and cause a prolonged FHR deceleration. At the level of the vasculature, the oxygen pathway can be interrupted by maternal hypotension associated with regional anesthesia or compression of the vena cava by the gravid uterus. Examples of interruption of the oxygen pathway at the level of the uterus include excessive uterine activity or uterine rupture. Examples at the level of the placenta include abruption or bleeding placenta previa. Finally, at the level of the umbilical cord, examples include cord compression or prolapse.
Standardized FHR interpretation
Intrapartum FHR interpretation can be summarized in three central principles that are evidence-based and reflect consensus in the medical literature. These three principles can be applied to any FHR tracing to produce a factually accurate, logical, and consistent interpretation that can help guide management. The first two principles have been reviewed in detail in this chapter. The third principle was introduced in Chapter 2.
Several maternal and fetal factors and conditions can influence the appearance of a FHR tracing by mechanisms that are not directly related to oxygenation. Examples are summarized in Table 5-3.
TABLE 5-3 Factors not Specifically Related to Fetal Oxygenation that May Influence Fetal Heart Rate
| Factor | Reported FHR Associations (Most Evidence Level II-3 and Level III) |
| Fever/infection | Increased baseline rate, decreased variability |
| Medications | Effects depend upon specific medication and may include changes in baseline rate, frequency and amplitude of accelerations, variability, and sinusoidal pattern |
| Hyperthyroidism | Tachycardia, decreased variability |
| Prematurity | Increased baseline rate, decreased variability, reduced frequency and amplitude of accelerations |
| Fetal anemia | Sinusoidal pattern, tachycardia |
| Fetal heart block | Bradycardia, decreased variability |
| Fetal tachyarrhythmia | Variable degrees of tachycardia, decreased variability |
| Congenital anomaly | Decreased variability, decelerations |
| Preexisting neurologic abnormality | Decreased variability, absent accelerations |
| Sleep cycle | Decreased variability, reduced frequency and amplitude of accelerations |
Fetal cardiac arrythmias
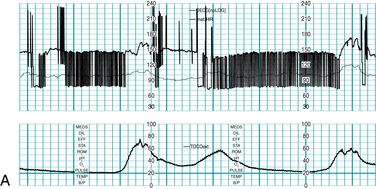
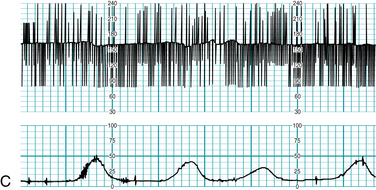
Precise characterization of fetal cardiac arrhythmias can challenge the clinical skills of the most experienced specialist, even with the benefit of direct, magnified visualization of the fetal heart using state-of-the-art sonographic equipment with color, pulse-wave, and M-mode Doppler capability. Therefore, any attempt to classify fetal cardiac arrhythmias using electronic fetal heart rate monitoring alone is destined to result in a tentative diagnosis at best. This imprecision is compounded by the fact that rates above 240 bpm may be halved or not printed at all by the monitor. This severely limits the ability of the FHR monitor to distinguish between fetal conditions such as supraventricular tachycardia, atrial fibrillation, and atrial flutter, all of which can result in fetal heart rates above 240 bpm (the upper limit of the FHR graph on standard paper). Electronic FHR monitoring cannot determine whether a fetal heart beat is initiated by an electrical impulse originating in the atrium or in the ventricle. In other words, an electronic monitor cannot reliably distinguish an atrial arrhythmia from a ventricular arrhythmia. Nevertheless, electronic fetal heart rate monitoring can offer some clues to the presence of an abnormal fetal heart rhythm. For example, dropped beats might appear on the FHR monitor as sharp downward spikes that nadir at approximately half of the baseline rate. A premature beat with a compensatory pause might appear as a sharp upward spike followed immediately by a downward spike. Bradycardia due to heart block can appear persistently or intermittently as a baseline rate that is half of the normal rate. Sinus bradycardia should be suspected if the baseline rate is lower than 110 bpm but higher than half of the normal rate. Any FHR baseline of less than 110 bpm requires thorough evaluation before it can be attributed to a benign condition. If the fetal heart is not generating electrical activity, as in the case of fetal demise, a fetal scalp electrode may detect the electrical impulses from the maternal heart and record the maternal heart rate. If there is any question about the clinical significance of any unusual fetal heart rhythm that is seen on the fetal monitor or detected audibly, further evaluation with other modalities is necessary to establish an accurate diagnosis (Figure 5-17 A-E).
Patterns not defined by the nichd
Several terms and concepts that are not defined by the NICHD are common in clinical practice. The 2008 NICHD consensus report specifically stated that FHR characteristics such as “overshoot,” “shoulders,” “variability within a deceleration,” “variable deceleration with a late component,” and “biphasic” decelerations require further research investigation to determine clinical significance.26 These and other undefined terms are discussed below.
“Wandering baseline”
An FHR baseline that is within the normal range (110-160 bpm) but that is not stable at a single rate for long enough to define a mean has been described as a “wandering baseline” (Figure 5-18). Absent variability and absent accelerations are prominent features. Decelerations can be present or absent. This combination of FHR findings has been suggested to indicate preexisting neurologic injury and impending fetal death. The physiologic mechanism is not known and published data are limited (Level III). If this pattern is observed, it should be interpreted in the context of other FHR observations and clinical factors.
“Lambda pattern”
The “lambda” FHR pattern is characterized by a brief acceleration followed by a small deceleration, as illustrated in (Figure 5-19).9 Common during early labor, this pattern has no known clinical significance. The underlying physiologic mechanism is not known (Level III).
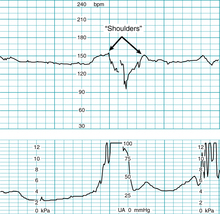
“Shoulder(s)”
As discussed above, variable decelerations result from transient mechanical compression of umbilical blood vessels within the umbilical cord. Initial compression of the umbilical vein reduces fetal venous return and triggers a baroreceptor-mediated reflex rise in FHR that commonly is described as a “shoulder,” as illustrated in Figure 5-20.20,25 As the cord is decompressed, a second “shoulder” frequently follows the deceleration and likely reflects the same underlying mechanism. The precise mechanism has not been confirmed. There is no known association with adverse newborn outcome. On the other hand, there is no firm evidence that the observation reflects normal fetal oxygenation. The observation requires further research investigation to determine clinical significance.26 Supporting evidence is Level III.
“Checkmark pattern”
The “checkmark pattern” is an unusual FHR pattern that has been described in association with neurologic injury, neonatal convulsions, and possible in utero fetal seizure activity. It is depicted in Figure 5-21. Unlike most FHR patterns described in association with neurologic injury, the “checkmark” pattern is not necessarily accompanied by absent baseline variability. All evidence related to the visual appearance of the pattern and the putative clinical significance is Level III.
“End-stage bradycardia” and “terminal bradycardia”
Standardized NICHD FHR terminology clearly indicates that the term bradycardia applies to the baseline FHR. The term specifically does not apply to a prolonged deceleration that interrupts the baseline. The terms “end-stage bradycardia” and “terminal bradycardia” have been used to describe a prolonged deceleration observed at the end of the second stage of labor (Figure 5-22). Such decelerations are common in the course of normal vaginal delivery and usually are of little clinical significance. The precise cause is unknown. However, suggested mechanisms include umbilical cord compression, umbilical cord stretching, fetal head compression, and transient fetal hypoxemia due to excessive uterine activity and/or maternal expulsive efforts. The impact on immediate newborn outcome is variable and depends upon a number of interacting factors, including, but not limited to, the physiologic cause of the deceleration, prior condition of the fetus, and duration of the deceleration. Consistent with NICHD terminology, the terms “end-stage bradycardia” and “terminal bradycardia” should be discarded in favor of the more precise term “prolonged deceleration.” Evidence is Level III.
“Uniform accelerations”
Various terms have been used to describe FHR accelerations. Examples include “uniform sporadic accelerations,” “variable sporadic accelerations,” “uniform periodic accelerations,” “sporadic periodic accelerations,” and “crown accelerations.” These terms are not included in standardized NICHD definitions and there is no confirmed physiologic basis for such sub-classification.
“Atypical” variable decelerations
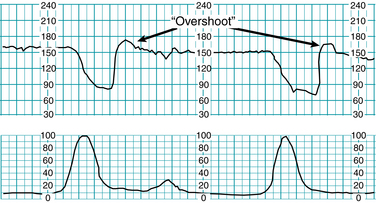
“Overshoot”
The term “overshoot” has been used to describe a FHR pattern characterized by persistently absent variability, absent accelerations, and a variable deceleration followed by a smooth, prolonged rise in the FHR above the previous baseline with gradual return.14,32-34,39 It is depicted in Figure 5-23. As with the “wandering baseline,” essential elements of this uncommon pattern include the persistent absence of variability and the absence of accelerations. The “overshoot” pattern has been attributed to a range of conditions, including “mild fetal hypoxia above the deceleration threshold,” “chronic fetal distress,” and “repetitive transient central nervous system ischemia.” However, all these associations are speculative and none has been substantiated by appropriate analytic scientific evidence. The physiologic mechanisms responsible for the “overshoot” pattern are not known. However, the pattern has been described in association with abnormal neurologic outcome with or without metabolic acidemia, suggesting that it might indicate preexisting neurologic injury. Because of the wide variation in reported associations and the lack of agreement regarding the definition, visual appearance or clinical significance of “overshoot,” it is best to avoid the use of this term in favor of specific terminology. All information regarding the “overshoot” pattern in humans is descriptive Level III evidence that is not capable of establishing statistically significant relationships.
“Variable deceleration with a late component”
“Variable deceleration with a late component” describes a deceleration with an abrupt onset and a gradual return to baseline, as illustrated in Figure 5-24. The abrupt onset suggests that the deceleration begins as a reflex autonomic response to an abrupt rise in blood pressure caused by umbilical cord compression (the “variable” component of the pattern). The gradual return to baseline suggests a gradual reduction of autonomic outflow upon resolution of transient hypoxemia, as occurs in a late deceleration (the “late” component of the pattern). A plausible explanation of the pattern would be initial umbilical cord compression causing a reflex fall in FHR and a transient decline in fetal PO2. The PO2 probably drops below the threshold that triggers the reflex sympathetic outflow and peripheral vasoconstriction characteristic of a late deceleration. Decompression of the umbilical cord brings about rapid resolution of the variable deceleration; however, the physiologic mechanism responsible for late deceleration resolves more slowly, causing the FHR to return slowly to the previous baseline. The specific physiologic mechanism has not been studied systematically. Scientific evidence regarding the underlying physiologic mechanism is limited to Level III. Second stage variable decelerations with slow recovery have been reported to increase the likelihood of operative delivery; however, no consistent impact on newborn outcome has been described.36 In the absence of a standard definition of this pattern, its use is best avoided in favor of standard terminology. For example: “variable deceleration with gradual return to baseline.”
“Mild,” “moderate,” and “severe” variable decelerations
The depth and duration of variable decelerations have been suggested as predictors of newborn outcome. Kubli and colleagues proposed three categories of variable decelerations based upon these characteristics.23 According to this classification system, a mild variable deceleration was defined by a duration <30 seconds regardless of depth, a depth no lower than 80 bpm, or a depth of 70-80 bpm lasting <60 seconds. A moderate variable deceleration was defined by a depth <70 bpm lasting 30-60 seconds or a depth of 70-80 bpm lasting more than 60 seconds. A severe deceleration was defined as a deceleration below 70 bpm lasting more than 60 seconds. There is no analytic evidence in the literature that the depth of any type of deceleration (early, variable, late, or prolonged) is predictive of fetal metabolic acidemia or newborn outcome independent of other important FHR characteristics such as baseline rate, variability, accelerations, and frequency of decelerations. Therefore, “mild,” “moderate,” and “severe” categories are not included in standard NICHD definitions of FHR decelerations. The 2008 NICHD report specifically states that such classification requires further research investigation to determine clinical significance.26 Consistent with NICHD terminology, all decelerations may be further quantitated by depth (to nadir) in beats per minute and duration in minutes and seconds from onset to offset.
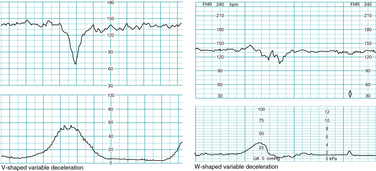
“V-shaped” variables and “w-shaped” variables
The visual appearance of a variable deceleration has been suggested to predict the underlying cause. For example, a “V-shaped” variable deceleration has been suggested to indicate umbilical cord compression due to oligohydramnios, while a “W-shaped” variable deceleration has been suggested to reflect umbilical cord compression due to a nuchal cord. Examples are depicted in Figure 5-25. Although such claims likely have little impact on patient care, there is no supporting analytic evidence in the literature. These terms are not included in standardized NICHD terminology.
“Good variability within the deceleration”
At the nadir of a variable or late deceleration, the FHR frequently appears irregular, similar to the appearance of moderate variability. The visual similarity has led some to suggest that “variability” during a deceleration has the same clinical significance as baseline variability. While the concept may sound plausible, it has never been studied. In addition, it is inconsistent with standard terminology. Variability is a characteristic of the FHR baseline. The term “variability” is not used to qualify periodic or episodic decelerations that interrupt the baseline. In the absence of evidence, the safest approach is to avoid assigning undue significance to this observation.
Summary
The three basic elements of FHR monitoring are (1) definition, (2) interpretation, and (3) management. A standardized, evidence-based approach to each element facilitates effective communication, promotes patient safety, and helps ensure optimal outcomes.
Standardized definitions, including the newly introduced FHR Categories, have been endorsed by all major professional organizations in the United States representing providers of obstetric care. Standardized FHR interpretation requires a critical assessment of the scientific evidence underlying the relationships between FHR patterns and fetal physiology.
As described in Chapter 2, the physiologic basis of FHR monitoring can be summarized in a few simple principles.
This chapter has reviewed in detail the relationships between specific FHR patterns and fetal physiology with particular emphasis on evidence-based interpretation. Regarding the relationship between FHR patterns and fetal oxygenation, evidence-based FHR interpretation can be summarized in three central principles that are illustrated in Figure 5-26.
The concepts developed in this chapter form the basis of systematic management of FHR patterns discussed in Chapter 6.
1. American College of Nurse-Midwives. Standard nomenclature for electronic fetal monitoring. Position Statement 2006. Silver Spring, MD: ACNM; 2006.
2. American College of Obstetricians and Gynecologists. Intrapartum fetal heart rate monitoring. ACOG Practice Bulletin no. 70. Obstet Gynecol. 2005;106:1453-1461.
3. American College of Obstetricians and Gynecologists: Intrapartum fetal heart rate monitoring: nomenclature, interpretation, and general management principles. ACOG Practice Bulletin no. 106. Obstet Gynecol. 2009;114:192-202
4. American College of Obstetricians and Gynecologists. Management of intrapartum fetal heart rate tracings. ACOG Practice Bulletin no. 116. Obstet Gynecol. 2010;116:1232-1240.
5. American College of Obstetricians and Gynecologists’ Task Force on Neonatal Encephalopathy and Cerebral Palsy, American College of Obstetricians and Gynecologists, American Academy of Pediatrics: Neonatal encephalopathy and cerebral palsy. defining the pathogenesis and pathophysiology. Washington DC;ACOG, AAP: 2003
6. Association of Women’s Health, Obstetric and Neonatal Nurses. Changes to the FHMPP program. Available at www.awhonn.org /?pg=873-2180-17530, August 22, 2005. Accessed
7. Ball RH, Espinoza MI, Parer JT. Regional blood flow in asphyxiated fetuses with seizures. Am J Obstet Gynecol. 1994;170:156-161.
8. Ball RH, Parer JT, Caldwell LE, et al. Regional blood flow and metabolism in ovine fetuses during severe cord occlusion. Am J Obstet Gynecol. 1994;171:1549-1555.
9. Brubaker K, Garite TJ: The lambda fetal heart rate pattern: an assessment of its significance in the intrapartum period. Obstet Gynecol. 1988;72:881-885
10. Cohn HE, Sacks EJ, Heymann MA, et al. Cardiovascular response to hypoxemia and acidemia in fetal lambs. Am J Obstet Gynecol. 1974;120:817-824.
11. Electronic fetal heart rate monitoring. research guidelines for interpretation. National Institute of Child Health and Human Development Research Planning Workshop. Am J Obstet Gynecol. 1997;177:1385-1390.
12. Field DR, Parer JT, Auslander RA, et al. Cerebral oxygen consumption during asphyxia in fetal sheep. J Dev Physiol. 1990;14:131-137.
13. Giannina G, Guzman ER, Lai YL, et al. Comparison of the effects of meperidine and nalbuphine on intrapartum fetal heart rate tracings. Obstet Gynecol. 1995;86:441-445.
14. Goodlin RC, Lowe EW: A functional umbilical cord occlusion heart rate pattern: the significance of overshoot. Obstet Gynecol. 1974;43:22-30
15. Hallak M, Martinez-Poyer J, Kruger ML, et al: The effect of magnesium sulfate on fetal heart rate parameters: a randomized, placebo-controlled trial. Am J Obstet Gynecol. 1999;181:1122-1127
16. Hatjis CG, Meis PJ. Sinusoidal fetal heart rate pattern associated with butorphanol administration. Obstet Gynecol. 1986;67:377-380.
17. Itskovitz J, LaGamma EF, Rudolph AM. Heart rate and blood pressure responses to umbilical cord compression in fetal lambs with special reference to the mechanism of variable deceleration. Am J Obstet Gynecol. 1983;147:451-457.
18. Itskovitz J, LaGamma EF, Rudolph AM. The effect of reducing umbilical blood flow on fetal oxygenation. Am J Obstet Gynecol. 1983;145:813-818.
19. Itskovitz J, LaGamma EF, Rudolph AM. Effect of cord compression on fetal blood flow distribution and O2 delivery. Am J Physiol. 1987;252:H100-H109.
20. James LS, Yeh MN, Morishima HO, et al: Umbilical vein occlusion and transient acceleration of the fetal heart rate: experimental observations in subhuman primates. Am J Obstet Gynecol. 1976;126:276-283
21. Jensen A, Roman C, Rudolph AM. Effects of reducing uterine blood flow on fetal blood flow distribution and oxygen delivery. J Dev Physiol. 1991;15:233-309.
22. Kopecky EA, Ryan ML, Barrett JF, et al. Fetal response to maternally administered morphine. Am J Obstet Gynecol. 2000;183:424-430.
23. Kubli FW, Hon EH, Khazin AF, et al. Observations on heart rate and pH in the human fetus during labor. Am J Obstet Gynecol. 1969;104:1190-1206.
24. Lee CY, Di Loreto PC, O’Lane JM. A study of fetal heart rate acceleration patterns. Obstet Gynecol. 1975;45:142-146.
25. Lee ST, Hon EH. Fetal hemodynamic response to umbilical cord compression. Obstet Gynecol. 1963;22:553-562.
26. Macones GA, Hankins GD, Spong CY, et al: The 2008. National Institute of Child Health and Human Development workshop report on electronic fetal monitoring: update on definitions, interpretation, and research guidelines. Obstet Gynecol. 2008;112(3)661-666
27. Martin CBJr, de Haan J, van der Wildt B, et al: Mechanisms of late decelerations in the fetal heart rate: a study with autonomic blocking agents in fetal lambs. Eur J Obstet Gynecol Reprod Biol. 1979;9(6)361-373
28. Mueller-Heubach E, Battelli AF. Variable heart rate decelerations and transcutaneous PO2 during umbilical cord occlusion in fetal monkeys. Am J Obstet Gynecol. 1982;144:796-802.
29. Peeters LL, Sheldon RD, Jones MD, et al. Blood flow to fetal organs as a function of arterial oxygen content. Am J Obstet Gynecol. 1979;135:466-637.
30. Reid DL, Parer JT, Williams K, et al. Effects of severe reduction in maternal placental blood flow on blood flow distribution in the sheep fetus. J Dev Physiol. 1991;15:183-188.
31. Richardson BS, Rurak D, Patrick JE, et al. Cerebral oxidative metabolism during prolonged hypoxemia. J Dev Physiol. 1989;11:37-43.
32. Saito J, Okamura K, Akagi K, et al. Alteration of FHR pattern associated with progressively advanced fetal acidemia caused by cord compression. Nippon Sanka Fujinka Gakkai Zasshi. 1988;40:775-780.
33. Schifrin BS, Hamilton-Rubinstein T, Shield JR. Fetal heart rate patterns and the timing of fetal injury. J Perinatol. 1994;14:174-181.
34. Shields JR, Schifrin BS. Perinatal antecedents of cerebral palsy. Obstet Gynecol. 1988;71:899.
35. Siassi B, Wu PY, Blanco C, et al. Baroreceptor and chemoreceptor responses to umbilical cord occlusion in fetal lambs. Biol Neonate. 1979;35:66-73.
36. Spong CY, Rascul C, Collea JY, et al. Characterization and prognostic significance of variable decelerations in the second stage of labor. Am J Perinatol. 1998;15:369-374.
37. Towell MD, Salvador HS. Compression of the umbilical cord. In: Crasignoni P, Pardi G, editors. An experimental model in the fetal goat, fetal evaluation during pregnancy and labor. New York: Academic Press; 1971:143-156.
38. United States Preventive Services Task Force. Guide to clinical preventive services. Report of the U.S. Preventive Services Task Force, ed 2. Williams & Wilkins; 1996.
39. Westgate JA, Bennet L, de Haan HH, et al. Fetal heart rate overshoot during repeated umbilical cord occlusion in sheep. Obstet Gynecol. 2001;97:454-459.
40. Wright JW, Ridgway LE, Wright BD, et al. Effect of MgSO4 on heart rate monitoring in the preterm fetus. J Reprod Med. 1996;41:605-608.
41. Yeh MN, Morishima HO, Niemann WE, et al: Myocardial conduction defects in association with compression of the umbilical cord: experimental observations on fetal baboons. Am J Obstet Gynecol. 1975;121:795-951