CHAPTER 2 Physiologic basis for electronic fetal heart rate monitoring
The objective of intrapartum fetal heart rate (FHR) monitoring is to prevent fetal injury that might result from interruption of normal fetal oxygenation during labor. The underlying assumption is that interruption of fetal oxygenation leads to characteristic physiologic changes that can be detected by changes in the FHR. Understanding the physiologic basis for electronic FHR monitoring requires a realistic appraisal of this basic assumption. The role of intrapartum FHR monitoring in assessing the fetal physiologic changes caused by interrupted oxygenation can be summarized as follows:
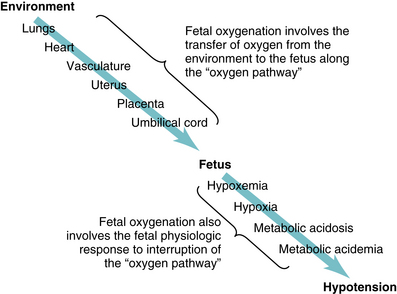
This chapter reviews the physiology underlying fetal oxygenation, including transfer of oxygen from the environment to the fetus and the fetal response to interruption of oxygen transfer (Figure 2-1). Chapters 5 and 6 explore the relationship between fetal oxygenation and electronic FHR patterns.
Transfer of oxygen from the environment to the fetus
Oxygen is carried from the environment to the fetus by maternal and fetal blood along a pathway that includes the maternal lungs, heart, vasculature, uterus, placenta, and umbilical cord. The oxygen pathway from the environment to the fetus, illustrated in Figure 2-1, is a central concept in FHR monitoring. Interruption of oxygen transfer can occur at any or all of the points along the oxygen pathway. Therefore, it is essential to understand the physiology and pathophysiology involved in each step.
External environment
Oxygen comprises approximately 21% of inspired air. Therefore, in inspired air, the partial pressure exerted by oxygen gas (PO2) is approximately 21% of total atmospheric pressure (760 mmHg) minus the pressure exerted by water vapor (47 mmHg). At sea level, this translates to approximately 150 mmHg. As oxygen is transferred from the environment to the fetus, the partial pressure declines. By the time oxygen reaches fetal umbilical venous blood, the partial pressure is as low as 30 mmHg. After oxygen is delivered to fetal tissues, the PO2 of deoxygenated blood in the umbilical arteries returning to the placenta is approximately 15-25 mmHg.4,6-9 The sequential transfer of oxygen from the environment to the fetus and potential causes of interruption at each step are described below.
Maternal lungs
Inspiration carries oxygenated air from the external environment to the distal air sacs of the lung, the alveoli. On the way to the alveoli, inspired air mixes with less-oxygenated air leaving the lungs. As a result, the PO2 of air within the alveoli is lower than that in inspired air. At sea level, alveolar PO2 is approximately 105 mmHg. From the alveoli, oxygen diffuses across a thin blood-gas barrier into the pulmonary capillary blood. The pulmonary blood-gas barrier consists of three layers: a single-cell layer of alveolar epithelium, a layer of extracellular collagen matrix (interstitium) and a single-cell layer of pulmonary capillary endothelium.
Interruption of oxygen transfer from the environment to the alveoli can result from airway obstruction or depression of central respiratory control caused by narcotics, magnesium or convulsions. Interruption of oxygen transfer from the alveoli to the pulmonary capillary blood can be caused by a number of factors including ventilation-perfusion mismatch and diffusion defects due to conditions such as pulmonary embolus, pneumonia, asthma, atelectasis, or adult respiratory distress syndrome.
Maternal blood
After diffusing from the pulmonary alveoli into maternal blood, more than 98% of oxygen combines with hemoglobin in maternal red blood cells. Approximately 1%-2% remains dissolved in the blood and is measured by the partial pressure of oxygen in arterial blood (PaO2). The amount of oxygen bound to hemoglobin depends directly upon the PaO2. Hemoglobin saturations at various PaO2 levels are illustrated by the oxyhemoglobin dissociation curve (Figure 2-2). A normal adult PaO2 value of 95-100 mmHg results in hemoglobin saturation of approximately 95%-98%, indicating that hemoglobin is carrying 95%-98% of the total amount of oxygen it is capable of carrying. A number of factors affect the affinity of hemoglobin for oxygen and can shift the oxyhemoglobin dissociation curve to the left or right. In general, the tendency for hemoglobin to release oxygen is increased by factors that signal an increased requirement for oxygen. Specifically, oxygen release is enhanced by factors that indicate active cellular metabolism. These factors shift the oxyhemoglobin saturation curve to the right and include by-products of anaerobic metabolism (reflected by increased 2,3-DPG concentration), production of lactic acid (reflected by decreased pH), and heat.
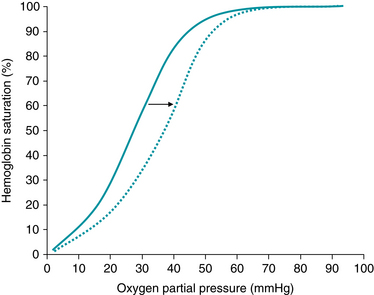
FIGURE 2-2 Fetal oxygen dissociation curve. The tendency for hemoglobin to release oxygen is increased by factors that signal an increased requirement for oxygen. Specifically, oxygen release is enhanced by factors that indicate active cellular metabolism. These factors shift the oxyhemoglobin saturation curve to the right and include anaerobic glycolysis (reflected by increased 2, 3-DPG concentration), production of hydrogen ions (reflected by decreased pH), and heat. (Courtesy David A. Miller, MD.)
Interruption of oxygen transfer from the environment to the fetus due to abnormal maternal oxygen-carrying capacity can result from severe anemia or from hereditary or acquired abnormalities affecting oxygen binding, such as hemoglobinopathies or methemoglobinemia. In an obstetric population, reduced maternal oxygen-carrying capacity rarely interferes with fetal oxygenation. Maternal hemoglobin saturation can be estimated non-invasively by transmission pulse oximetry (SpO2). In recent years, investigators studying the efficacy of fetal oxygen saturation (FSpO2) monitoring have provided valuable insights into fetal physiology (Chapter 6).
Maternal heart
From the lungs, pulmonary veins carry oxygenated blood to the maternal heart. Blood enters the left atrium with a PaO2 of approximately 95 mmHg. Oxygenated blood passes from the left atrium, through the mitral valve into the left ventricle and out the aorta for systemic distribution. Normal transfer of oxygen from the environment to the fetus is dependent upon normal cardiac function, reflected by cardiac output. Cardiac output is the product of heart rate and stroke volume.
Heart rate is determined by intrinsic cardiac pacemakers (SA node, AV node), the cardiac conduction system, autonomic regulation (sympathetic, parasympathetic), humoral factors (catecholamines), extrinsic factors (medications), and local factors (calcium, potassium). Stroke volume is determined by preload, contractility, and afterload. Preload is the amount of stretch on myocardial fibers at the end of diastole when the ventricles are full of blood. It is determined by the volume of venous blood returning to the heart. Contractility is the force and speed with which myocardial fibers shorten during systole to expel blood from the heart. Afterload is the pressure that opposes the shortening of myocardial fibers during systole and is estimated by the systemic vascular resistance or systemic blood pressure.
Interruption of oxygen transfer from the environment to the fetus at the level of the maternal heart can be caused by any condition that reduces cardiac output, including:
 impaired contractility (ischemic heart disease, diabetes, cardiomyopathy, congestive heart failure),
impaired contractility (ischemic heart disease, diabetes, cardiomyopathy, congestive heart failure), structural abnormalities of the heart and/or great vessels that impede the ability to pump blood (valvular stenosis, valvular insufficiency, pulmonary hypertension, coarctation of the aorta).
structural abnormalities of the heart and/or great vessels that impede the ability to pump blood (valvular stenosis, valvular insufficiency, pulmonary hypertension, coarctation of the aorta).In a healthy obstetric patient, the most common cause of reduced cardiac output is reduced preload resulting from hypovolemia or compression of the inferior vena cava by the gravid uterus.
Maternal vasculature
Oxygenated blood leaving the heart is carried by the systemic vasculature to the uterus. The path includes the aorta, common iliac artery, internal iliac (hypogastric) artery, anterior division of the internal iliac artery, and the uterine artery. From the uterine artery, oxygenated blood travels through the arcuate arteries, the radial arteries, and finally the spiral arteries before exiting the maternal vasculature and entering the intervillous space of the placenta.
Interruption of oxygen transfer from the environment to the fetus at the level of the maternal vasculature commonly results from hypotension caused by regional anesthesia, hypovolemia, impaired venous return, impaired cardiac output, or medications. Alternatively, it may result from vasoconstriction of distal arterioles in response to endogenous vasoconstrictors or medications. Conditions associated with chronic vasculopathy, such as chronic hypertension, long-standing diabetes, collagen vascular disease, thyroid disease, and renal disease may result in chronic suboptimal transfer of oxygen and nutrients to the fetus at the level of the maternal vasculature. Preeclampsia is associated with abnormal vascular remodeling at the level of the spiral arteries and can impede perfusion of the intervillous space. Acute vascular injury (trauma, aortic dissection) is rare.
In a healthy obstetric patient, the most common cause of interrupted oxygen transfer at the level of the maternal vasculature is transient hypotension. Chronic vascular conditions can exacerbate this interruption and should be considered in the course of thorough evaluation.
Uterus
Between the maternal uterine arteries and the intervillous space of the placenta, the arcuate, radial, and spiral arteries traverse the muscular wall of the uterus. Interruption of oxygen transfer from the environment to the fetus at the level of the uterus commonly results from uterine contractions that compress intramural blood vessels and impede the flow of blood. Uterine contractions and uterine injury (rupture, trauma) are the most common causes of interruption of fetal oxygenation at this level. Uterine activity is discussed in Chapter 4.
Placenta
The placenta facilitates the exchange of gases, nutrients, wastes, and other molecules (for example: antibodies, hormones, medications) between maternal blood in the intervillous space and fetal blood in the villus capillaries. On the maternal side of the placenta, oxygenated blood exits the spiral arteries and enters the intervillous space to surround and bathe the chorionic villi. On the fetal side of the placenta, paired umbilical arteries carry blood from the fetus through the umbilical cord to the placenta (Figure 2-3). At term, the umbilical arteries receive 40% of fetal cardiac output. Upon reaching the placental cord insertion site, the umbilical arteries divide into multiple branches and fan out across the surface of the placenta. At each cotyledon, placental arteries dive beneath the surface en route to the chorionic villi (Figure 2-4). The chorionic villi are microscopic branches of trophoblast that protrude into the intervillous space. Each villus is perfused by a fetal capillary bed that represents the terminal distribution of an umbilical artery. At term, fetal villus capillary blood is separated from maternal blood in the intervillous space by a thin blood-blood barrier similar to the blood-gas barrier in the maternal lung. The placental blood-blood barrier is comprised of a layer of placental trophoblast and a layer of fetal capillary endothelium with intervening basement membranes and villous stroma. Substances are exchanged between maternal and fetal blood by a number of mechanisms, including simple diffusion, facilitated diffusion, active transport, bulk flow, pinocytosis, and leakage. These mechanisms are summarized in Table 2-1. Oxygen is transferred from the intervillous space to the fetal blood by a complex process that depends upon the PaO2 of maternal blood perfusing the intervillous space, maternal blood flow within the intervillous space, chorionic villus surface area and diffusion across the placental blood-blood barrier.
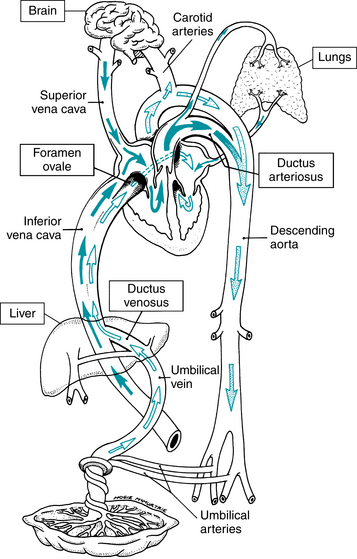
FIGURE 2-3 Fetal circulation. Oxygenated and nutrient-rich blood is carried to the fetus by the umbilical vein to the fetal heart. Oxygen-poor and waste product-rich blood circulates back to the placenta via the umbilical arteries. Three anatomic shunts (the ductus venosus, the foramen ovale, and the ductus arteriosus) permit fetal blood to bypass the liver and the lungs. (From Bloom RS: Delivery room resuscitation of the newborn. In Martin RJ, Fanaroff AA, Walsh MC, editors: Fanaroff and Martin’s neonatal-perinatal medicine: Diseases of the fetus and infant, ed 8, Philadelphia, 2006, Mosby.)
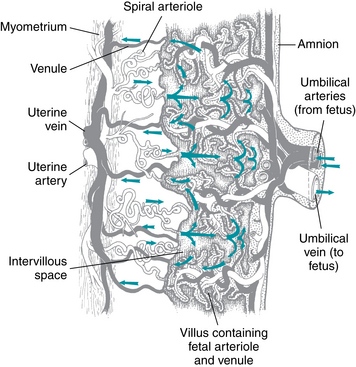
FIGURE 2-4 Schema of placenta. As maternal blood enters the intervillous space, it spurts from the uterine spiral arterioles and spreads laterally through the space. White vessels carry oxygenated blood. Gray vessels carry oxygen-poor blood.
TABLE 2-1 Mechanisms of Exchange Between Fetal and Maternal Blood
| Mechanism | Description | Substances |
| Simple diffusion | Passage of substances from a region of higher concentration to one of lower concentration along a concentration gradient that is passive and does not require energy | |
| Facilitated diffusion | Passage of substances along a concentration gradient with the assistance of a carrier molecule involved | |
| Active transport | Passage of substances against a concentration gradient; carrier molecules and energy are required | |
| Bulk flow | Transfer of substances by a hydrostatic or osmotic gradient | |
| Pinocytosis | Transfer of minute, engulfed particles across a cell membrane | |
| Breaks and leakage | Small breaks in the placental membrane allowing passage of plasma and substances | Maternal or fetal blood cells (potentially resulting in isoimmunization) |
Intervillous space PaO2
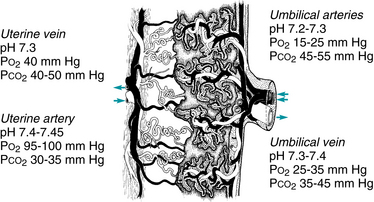
As described previously, oxygenated maternal blood leaves the maternal heart with a PaO2 of approximately 95 mmHg. There are no capillary beds between the maternal heart and the spiral arteries. Therefore, the oxygenated maternal blood exiting the spiral arteries and entering the intervillous space has a PaO2 of approximately 95 mmHg. Oxygen is released from maternal hemoglobin and diffuses across the placental blood-blood barrier into fetal blood where it combines with fetal hemoglobin. As a result, maternal blood in the intervillous space becomes relatively oxygen-depleted and exits the intervillous space via uterine veins with a PaO2 of approximately 40 mmHg (Figure 2-5). Therefore, the average PaO2 of maternal blood in the intervillous space is between the PaO2 of blood entering the intervillous space (95 mmHg) and the PaO2 of blood exiting the intervillous space (40 mmHg). The average intervillous space PaO2 is approximately 45 mmHg. Interruption of fetal oxygenation can result from conditions that reduce the PaO2 of maternal blood entering the intervillous space. These conditions have been discussed previously.
Intervillous space blood flow
At term, uterine perfusion accounts for 10%-15% of maternal cardiac output, or approximately 700 to 800 cc per minute. Most of this blood is located in the intervillous space of the placenta surrounding the chorionic villi. Conditions that can reduce the volume of the intervillous space include collapse or destruction of the intervillous space due to placental abruption, infarction, thrombosis or infection.
Chorionic villus surface area
Optimal oxygen exchange requires normal chorionic villous surface area. Normal transfer of oxygen from the environment to the fetus at the level of the placenta can be interrupted by conditions that limit or reduce the chorionic villous surface area available for gas exchange. These conditions can be acute or chronic and include primary abnormalities in the development of the villous vascular tree or secondary destruction of normal chorionic villi by infarction, thrombosis, hemorrhage, inflammation, or infection.
Diffusion across the blood-blood barrier
Diffusion of a substance across the placental blood-blood barrier is dependent upon concentration gradient, molecular weight, lipid solubility, protein-binding, and ionization. In addition, diffusion rate is inversely proportional to diffusion distance. At term, the placental blood-blood barrier is very thin and the diffusion distance is short. Under normal circumstances, oxygen and carbon dioxide diffuse readily across this thin barrier. However, normal diffusion can be impeded by conditions that increase the distance between maternal and fetal blood. These conditions can be acute, subacute, or chronic, and include villous hemorrhage, inflammation, thrombosis, infarction, edema, fibrosis, and excessive cellular proliferation (syncytial knots).2,3,8
Interruption of placental blood vessels
Fetal blood loss caused by injury to blood vessels at the level of the placenta warrants brief consideration. Damaged chorionic vessels can allow fetal blood to leak into the intervillous space, leading to fetal-maternal hemorrhage. This may be a consequence of abdominal trauma, but can occur in association with placental abruption or invasive procedures. A specific cause is not always identified. Ruptured vasa previa is a rare cause of fetal hemorrhage. Vasa previa is a placental vessel traversing the chorioamniotic membrane in close proximity to the cervical os. Such a vessel may be damaged by normal cervical change during labor or injured inadvertently during membrane rupture or digital exam.
Summary of placental causes of interrupted oxygenation
Many conditions can interfere with the transfer of oxygen across the placenta. Those involving the microvasculature frequently are diagnosed by histopathologic examination after delivery. Clinically detectable causes, such as placental abruption, bleeding placenta previa or vasa previa should be considered but may not be amenable to conservative corrective measures.
Fetal blood
After oxygen has diffused from the intervillous space across the placental blood-blood barrier and into fetal blood, the PaO2 is in the range of 30 mmHg and fetal hemoglobin saturation is between 50% and 70%. Although fetal PaO2 and hemoglobin saturation are low in comparison to adult values, adequate delivery of oxygen to the fetal tissues is maintained by a number of compensatory mechanisms. For example, fetal cardiac output per unit weight is greater than that of the adult. Hemoglobin concentration and affinity for oxygen are greater in the fetus as well, resulting in increased oxygen-carrying capacity. Finally, oxygenated blood is directed preferentially toward vital organs by way of anatomic shunts at the level of the ductus venosus, foramen ovale, and ductus arteriosus.
Conditions that can interrupt the transfer of oxygen from the environment to the fetus at the level of the fetal blood are rare, but may include fetal anemia (alloimmunization, infections, fetomaternal hemorrhage, vasa previa) and conditions that reduce oxygen-carrying capacity (Bart’s hemoglobinopathy, methemoglobinemia).
Umbilical cord
After oxygen combines with fetal hemoglobin in the villous capillaries, oxygenated blood returns to the fetus by way of villous veins that coalesce to form placental veins on the surface of the placenta. Placental surface veins unite to form a single umbilical vein within the umbilical cord.
Interruption of the transfer of oxygen from the environment to the fetus at the level of the umbilical cord can result from simple mechanical compression. Other uncommon causes may include vasospasm, thrombosis, atherosis, hypertrophy, hemorrhage, inflammation, or a “true knot.”
From the environment to the fetus, maternal and fetal blood carry oxygen along the oxygen pathway illustrated in Figure 2-1. Common causes of interrupted oxygen transfer at each step along the pathway are summarized in Table 2-2. In the interest of simplicity, the above discussion was limited to one gas, oxygen. It is critical to note that gas exchange also involves the transfer of carbon dioxide in the opposite direction—from the fetus to the environment. Any condition that interrupts the transfer of oxygen from the environment to the fetus has the potential to interrupt the transfer of carbon dioxide from the fetus to the environment. However, carbon dioxide diffuses across the placental blood-blood barrier more rapidly than does oxygen. Therefore, any interruption of the pathway is likely to impact oxygen transfer to a greater extent than carbon dioxide transfer.
TABLE 2-2 Some Causes of Interrupted Transfer of Oxygen from the Environment to the Fetus
| Oxygen Pathway | Causes of Interrupted Oxygen Transfer |
| Lungs | |
| Heart | |
| Vasculature | |
| Uterus | |
| Placenta | |
| Umbilical cord |
ARDS, adult respiratory distress syndrome; SLE, systemic lupus erythematosus
As summarized previously, oxygen transfer from the environment to the fetus represents the first basic component of fetal oxygenation. The second basic component of fetal oxygenation involves the fetal physiologic response to interrupted oxygen transfer.
Fetal response to interrupted oxygen transfer
Depending upon frequency and duration, interruption of oxygen transfer at any point along the oxygen pathway may result in progressive deterioration of fetal oxygenation. The cascade begins with hypoxemia, defined as decreased oxygen content in the blood. At term, hypoxemia is characterized by an umbilical artery PaO2 below the normal range of 15-25 mmHg. Recurrent or sustained hypoxemia can lead to decreased delivery of oxygen to the tissues and reduced tissue oxygen content, termed hypoxia. Normal homeostasis requires an adequate supply of oxygen and fuel in order to generate the energy required by basic cellular activities. When oxygen is readily available, aerobic metabolism efficiently generates energy in the form of ATP. By-products of aerobic metabolism include carbon dioxide and water. When oxygen is in short supply, tissues may be forced to convert from aerobic to anaerobic metabolism, generating energy less efficiently and resulting in the production of lactic acid. Accumulation of lactic acid in the tissues results in metabolic acidosis. Lactic acid accumulation can lead to utilization of buffer bases (primarily bicarbonate) to help stabilize tissue pH. If the buffering capacity is exceeded, the blood pH may begin to fall, leading to metabolic acidemia. Eventually, recurrent or sustained tissue hypoxia and acidosis can lead to loss of peripheral vascular smooth muscle contraction, reduced peripheral vascular resistance, and hypotension leading to potential hypoxic-ischemic injury to many tissues including the brain and heart.
Acidemia is defined as increased hydrogen ion content (decreased pH) in the blood. With respect to fetal physiology, it is critical to distinguish between respiratory acidemia, caused by accumulation of CO2, and metabolic acidemia, caused by accumulation of fixed (lactic) acid. These distinct categories of acidemia have entirely different clinical implications and will be discussed later in this chapter.
Mechanisms of injury
If interrupted oxygen transfer progresses to the stage of metabolic acidemia and hypotension, as described above, multiple organs and systems (including the brain and heart) can suffer hypoperfusion, reduced oxygenation, lowered pH, and reduced delivery of fuel for metabolism. These changes can trigger a cascade of cellular events including altered enzyme function, protease activation, ion shifts, altered water regulation, disrupted neurotransmitter metabolism, free radical production, and phospholipid degradation. Disruption of normal cellular metabolism can to lead to cellular dysfunction, tissue dysfunction, and even death.
Injury threshold
The relationship between fetal oxygen deprivation and neurologic injury is complex. Electronic FHR monitoring was introduced with the expectation that it would reduce the incidence of neurologic injury (specifically cerebral palsy) caused by intrapartum interruption of fetal oxygenation. In recent years, it has become apparent that most cases of cerebral palsy are unrelated to intrapartum events and therefore cannot be prevented by intrapartum FHR monitoring. Nevertheless, some cases of cerebral palsy may be related to intrapartum events and continue to generate controversy.
In 1999, the International Cerebral Palsy Task Force published a consensus statement identifying specific criteria that must be met in order to establish intrapartum interruption of fetal oxygenation as a possible cause of cerebral palsy.5 In January 2003, ACOG and the American Academy of Pediatrics (AAP) Cerebral Palsy Task Force published a monograph entitled “Neonatal Encephalopathy and Cerebral Palsy: Defining the Pathogenesis and Pathophysiology” summarizing the world literature regarding the relationship between intrapartum events and neurologic injury.1 Agencies and professional organizations that reviewed and endorsed the ACOG-AAP Cerebral Palsy Task Force report include the Centers for Disease Control and Prevention, the Child Neurology Society, the March of Dimes Birth Defects Foundation, the National Institute of Child Health and Human Development, the Royal Australian and New Zealand College of Obstetricians and Gynecologists, the Society for Maternal-Fetal Medicine, and the Society of Obstetricians and Gynaecologists of Canada. The consensus report established four essential criteria defining an acute intrapartum event sufficient to cause cerebral palsy (Box 2-1).
BOX 2-1 Essential Criteria that Define an Acute Intrapartum Event Sufficient to Cause Cerebral Palsy*
The first criterion provides crucial information regarding the threshold of fetal injury in the setting of intrapartum interruption of fetal oxygenation. Specifically, it indicates that intrapartum interruption of fetal oxygenation does not result in neurologic injury in the form of cerebral palsy unless the fetal physiologic response progresses at least to the stage of significant metabolic acidemia (umbilical artery pH <7 and base deficit ≥12 mmol/L). It is important to note that fetal injury is uncommon even when metabolic acidemia is present. It is also important to understand that respiratory acidemia is not a recognized risk factor for fetal injury. This information has significant implications for the interpretation and management of intrapartum FHR patterns and will be reviewed in Chapter 6.
The second criterion highlights an equally important point. Specifically, intrapartum interruption of fetal oxygenation does not result in cerebral palsy unless it first causes moderate-severe neonatal encephalopathy. The report further clarified that neonatal encephalopathy has many possible causes. Hypoxic-ischemic encephalopathy resulting from intrapartum interruption of fetal oxygenation represents only a small subset of the larger category of neonatal encephalopathy.
The third criterion emphasizes that different subtypes of cerebral palsy have different clinical origins. Spastic quadriplegia is associated with injury to the parasagittal cerebral cortex and involves abnormal motor control of all four extremities. The dyskinetic subtype of cerebral palsy is associated with injury to the basal ganglia and involves disorganized, choreoathetoid movements. The report concluded that these are the only two subtypes of cerebral palsy that are associated with term hypoxic-ischemic injury. Specifically, spastic diplegia, hemiplegia, ataxia, and hemiparetic cerebral palsy are “unlikely to result from acute intrapartum hypoxia.” The report further concluded that other conditions, including epilepsy, mental retardation, and attention deficit hyperactivity disorder do not result from birth asphyxia in the absence of cerebral palsy.
The fourth criterion underscores the fact that intrapartum hypoxic-ischemic injury is a potential factor in only a small subset of all cases of cerebral palsy. Other identifiable etiologies include trauma, coagulation disorders, infectious conditions, or genetic disorders.
The Task Force authors identified five additional criteria that can help establish the timing of injury, emphasizing that these criteria are “nonspecific to asphyxial insults” (Box 2-2).
BOX 2-2 Criteria that Collectively Suggest Event Occurred Within 48 Hours of Birth
Summary
The physiology of fetal oxygenation involves the sequential transfer of oxygen from the environment to the fetus and the subsequent fetal response to interruption of this pathway (Figure 2-1). Although interruption of oxygen transfer can occur at any point along the oxygen pathway, examples of causes that might be encountered in a typical obstetric population are summarized in Table 2-2. Recurrent or sustained interruption of oxygen transfer can lead to progressive deterioration of fetal oxygenation and eventually to potential fetal injury. However, the International Cerebral Palsy Task Force and the ACOG-AAP Cerebral Task Force each published consensus reports identifying significant metabolic acidemia (umbilical artery pH <7.0 and base deficit ≥12 mmol/L) as an essential prerequisite to intrapartum hypoxic neurologic injury in the form of cerebral palsy.1,5 With respect to the relationship between fetal oxygenation and potential injury, there is now consensus in the literature that interrupted oxygenation does not result in cerebral palsy unless the fetal response progresses at least to the stage of significant metabolic acidemia. As depicted in Box 2-3, the physiologic basis of FHR monitoring can be summarized in a few key concepts. Later chapters will expand on these concepts and apply them to standardized interpretation and management of FHR patterns.
BOX 2-3 Key Concepts of the Physiologic Basis of Intrapartum FHR Monitoring
1. American College of Obstetricians and Gynecologists Task Force on Neonatal Encephalopathy and Cerebral Palsy, American College of Obstetricians and Gynecologists, American Academy of Pediatrics. Neonatal encephalopathy and cerebral palsy:defining the pathogenesis and pathophysiology. Washington, DC: ACOG, AAP; 2003.
2. Arabin B, Jimenez E, Vogel M, et al. Relationship of utero- and fetoplacental blood flow velocity wave forms with pathomorphological placental findings. Fetal Diagn Ther. 1992;7(3–4):173-179.
3. Giles WB, Trudinger BJ, Baird PJ: Fetal umbilical artery flow velocity waveforms and placental resistance: pathological correlation. Br J Obstet Gynaecol. 1985;92(1)31-38
4. Helwig JT, Parer JT, Kilpatrick SJ, et al: Umbilical cord blood acid-base state: what is normal. Am J Obstet Gynecol. 1996;174(6)1807-1812
5. MacLennan A: A template for defining a causal relation between acute intrapartum events and cerebral palsy: international consensus statement. BMJ. 1999;319(7216)1054-1059
6. Nodwell A, Carmichael L, Ross M, et al. Placental compared with umbilical cord blood to assess fetal blood gas and acid-base status. Obstet Gynecol. 2005;105(1):129-138.
7. Richardson B, Nodwell A, Webster K, et al. Fetal oxygen saturation and fractional extraction at birth and the relationship to measures of acidosis. Am J Obstet Gynecol. 1998;178(3):572-579.
8. . Trudinger B . : Fetus and neonate. physiology and clinical applications: the circulation. Cambridge;Cambridge University Press: 1993:323-338
9. Victory R, Penava D, Da Silva O, et al. Umbilical cord pH and base excess values in relation to adverse outcome events for infants delivering at term. Am J Obstet Gynecol. 2004;191(6):2021-2028.