CHAPTER 6 Intrapartum management of the fetal heart rate tracing
Chapters 2 and 4 provided the physiologic basis for fetal heart rate (FHR) monitoring and evaluation of uterine activity. Chapter 5 reviewed the standardized National Institute of Child Health and Human Development (NICHD) definitions and introduced an evidence-based approach to the interpretation of fetal heart rate patterns. This chapter incorporates those previously developed concepts and presents a systematic, comprehensive, and multidisciplinary approach to management of intrapartum fetal heart rate tracings.
Fundamental principles
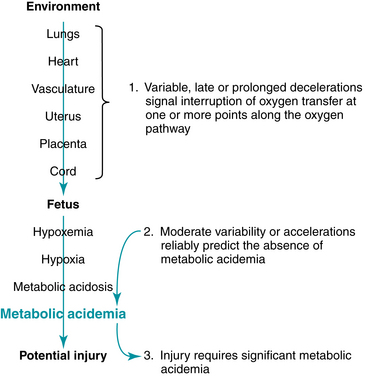
As introduced at the close of Chapter 5, three central principles of evidence-based fetal heart rate interpretation provide the foundation for a systematic approach to fetal heart rate management. Figure 6-1 illustrates the relationship of these three principles.
Standardized management decision model
Advances in standardized fetal monitoring definitions and interpretation make it possible to construct a standardized approach to intrapartum fetal heart rate management that is evidence-based and reflects consensus in the literature. A common misconception is that standardized FHR management is a “one-size-fits-all” approach that removes individual clinical judgment and dictates the timing and method of delivery. On the contrary, standardized intrapartum FHR management is intended to encourage the timely application of individual clinical judgment and to serve as a systematic reminder of potential sources of preventable error in the effort to optimize outcomes and minimize risk. The model described in this chapter uses the standardized FHR definitions and categories proposed by the NICHD in 2008.30 It does not include adjunctive tests of fetal status such as fetal scalp blood sampling, fetal pulse oximetry, and fetal ST-segment analysis that are currently unavailable for general clinical use in the United States. These techniques are reviewed at the end of the chapter.
Standard of care
The standard of care mandates that practitioners provide patient care that is reasonable and prudent. Reasonableness, in turn, is determined by factual accuracy and the ability to articulate a clear and understandable plan. Standard definitions and interpretation help to ensure factual accuracy. A standardized approach to management provides a framework for organized, evidence-based planning that can minimize variation, reduce the potential for preventable error, and be articulated clearly and understandably.
Confirm FHR and uterine activity
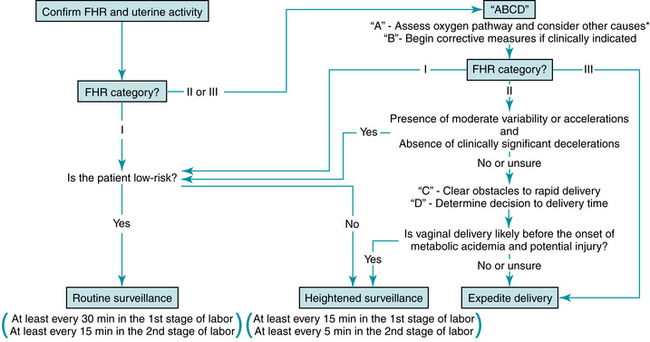
Reliable information is vital to the success of intrapartum FHR monitoring. Therefore, the first step is to confirm that the monitor is recording the FHR and uterine activity accurately (Figure 6-2). If external monitoring is not adequate for definition and interpretation, a fetal scalp electrode and/or intrauterine pressure catheter might be helpful. Under certain circumstances, the FHR monitor can inadvertently record the maternal heart rate. For example, if the fetus is not alive, an internal fetal scalp electrode will record the maternal heart rate. An external Doppler device can record the maternal heart rate even if the fetus is alive. Particularly in the setting of maternal tachycardia, the maternal heart rate can appear deceptively similar to a normal fetal heart rate. At times the monitor can alternately record the fetus and the mother. When switching from one to the other, the tracing does not necessarily demonstrate discontinuity. Therefore continuity of the tracing alone should not be relied upon to exclude this phenomenon. Unless the monitor is recording the fetal heart rate, it can provide no information regarding the condition of the fetus. Therefore, it is essential to distinguish between maternal and fetal heart rates. If there is any question, consider other methods such as ultrasound, palpation of the maternal pulse, fetal scalp electrode, or maternal pulse oximetry.
Evaluate five FHR components
Thorough, systematic evaluation of a FHR tracing includes assessment of uterine contractions along with the five FHR components defined by the NICHD: baseline rate, variability, accelerations, decelerations, and changes or trends in the tracing over time. The 2008 NICHD consensus report defined three categories of FHR tracings as summarized in Table 6-1.30 If all FHR components are normal (Category I), the FHR tracing reliably predicts the absence of fetal metabolic acidemia and on going hypoxic neurologic injury. In low-risk patients, ACOG Practice Bulletin 106 and ACOG-AAP Guidelines for Perinatal Care recommend that the FHR tracing should be reviewed at least every 30 minutes during the active phase of the first stage of labor and at least every 15 minutes during the second stage.1,3 In high-risk patients, the FHR tracing should be reviewed at least every 15 minutes during the active phase of the first stage of labor and at least every 5 minutes during the second stage (Figure 6-2). Nursing documentation should comply with hospital policies and procedures. Physician and midwife documentation should be performed periodically.3 Documentation and risk management issues are discussed in detail in Chapter 10.
TABLE 6-1 Three-tier FHR Classification System
| Category I | |
| Normal | |
| Category II | |
| Indeterminate | Includes all FHR tracings not assigned to Categories I or III |
| Category III | |
| Abnormal | |
Adapted from Macones GA, et al. Obstet Gynecol 112(3): 661-666, 2008.
A standardized “ABCD” approach to FHR management
If assessment of all five FHR components indicates that the tracing is anything other than Category I, further evaluation is warranted. A practical, systematic “ABCD” approach to management is as follows:
A – Assess the oxygen pathway (and consider other causes of FHR changes)
B – Begin conservative corrective measures as needed
C – Clear obstacles to rapid delivery
D – Determine decision to delivery interval
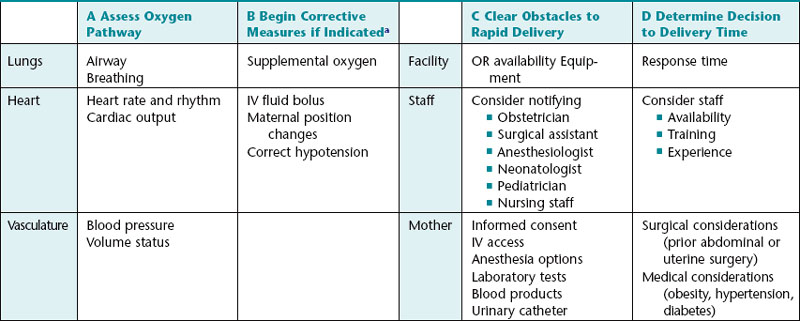
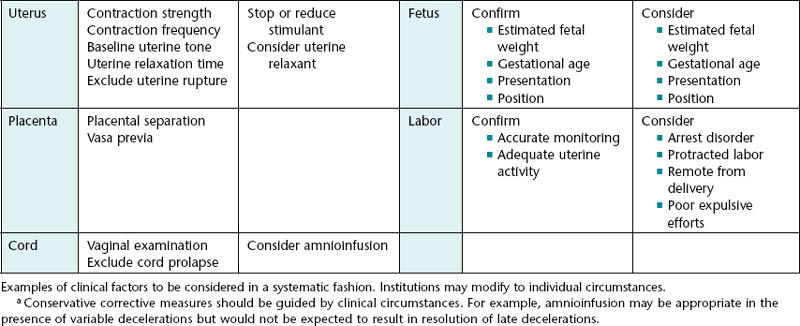
This approach is summarized in Table 6-2.
A: assess the oxygen pathway and consider other causes of FHR changes
Rapid, systematic assessment of the pathway of oxygen transfer from the environment to the fetus can identify potential sources of interrupted oxygenation. Assessment of the maternal lungs can be as simple as checking the respiratory rate. The heart and the vasculature usually can be assessed by checking the maternal pulse and blood pressure. Uterine activity can be assessed by palpation or by review of the information obtained from a tocodynamometer or intrauterine pressure catheter. The possibility of placental separation can be assessed by checking for vaginal bleeding. Finally, the possibility of umbilical cord prolapse can be assessed by visual examination or by a digital vaginal examination. If rapid evaluation of these steps suggests that further investigation is warranted, it should be undertaken as deemed necessary by individual clinical judgment.
Chapter 5 identified a number of maternal and fetal factors that can influence the appearance of the FHR tracing by mechanisms other than interruption of fetal oxygenation (Box 6-1). If the FHR changes are thought to be due to any condition not directly related to fetal oxygenation, individualized management must be directed at the specific cause. Correction of fetal oxygenation cannot reasonably be expected to resolve FHR abnormalities that are not related to oxygenation. For example, supplemental oxygen, maternal position changes, and intravenous fluid boluses are unlikely to correct fetal tachycardia that is secondary to fetal supraventricular tachycardia or severe fetal anemia caused by parvovirus infection or Rh isoimmunization. Corrective interventions for fetal arrhythmias or severe fetal anemia can be extremely complex and usually require subspecialty consultation. Management of conditions such as these is beyond the scope of this chapter.
B: begin corrective measures as indicated
At each point along the oxygen pathway, conservative corrective measures are initiated, if indicated, to optimize fetal oxygenation (Table 6-2). Scientific evidence supporting the efficacy of each of these measures is detailed in an excellent review by Simpson and James.41
These conservative corrective measures are summarized below:
Supplemental oxygen
Fetal oxygenation is dependent upon the oxygen content of maternal blood perfusing the intervillous space of the placenta, as discussed in Chapter 2. Administration of supplemental oxygen by nasal cannula or face mask can increase the Po2 of inspired air, increasing both the partial pressure of oxygen dissolved in maternal blood and the amount of oxygen bound to hemoglobin. This can increase the oxygen concentration gradient across the placental blood—blood barrier and lead to increased fetal Po2 and oxygen content. Several studies have reported resolution of fetal heart rate decelerations after administration of supplemental oxygen to the mother, providing indirect evidence of improved fetal oxygenation.41 Direct evidence is provided by fetal pulse oximetry studies demonstrating increased fetal hemoglobin saturation following maternal administration of oxygen. Although the optimal method and duration of oxygen administration is not known, available data support the use of a non-rebreather face mask to administer oxygen at a rate of 10 L/min for approximately 15 to 30 minutes.41
Maternal position changes
There are sound physiologic reasons to avoid the supine position during labor. Supine positioning increases the likelihood that pressure on the inferior vena cava will impair venous return, cardiac output, and perfusion of the intervillous space. It also increases the likelihood that pressure on the descending aorta and/or iliac vessels will impede the delivery of oxygenated blood to the intervillous space. Prospective fetal pulse oximetry data confirm that left or right lateral positioning results in higher fetal hemoglobin saturation levels than does supine positioning.41 In the setting of suspected umbilical cord compression, maternal position changes may result in fetal position changes and relief of pressure on the umbilical cord.
Intravenous fluid administration
Optimal uterine perfusion depends upon optimal cardiac output and intravascular volume. Normal blood pressure does not necessarily reflect optimal intravascular volume, venous return, preload, or cardiac output. An intravascular bolus of isotonic fluid can improve cardiac output not only by increasing circulating volume but also by increasing venous return, left ventricular end diastolic pressure, ventricular preload, and ultimately, stroke volume in accordance with the Frank-Starling mechanism. In this way, a relatively small increase in intravascular volume can have a significant impact on cardiac output and uterine perfusion.
An intravenous fluid bolus of 500 – 1,000 cc can result in improved fetal oxygenation even in an apparently euvolemic patient.41 Excessive fluid administration can have serious consequences, and caution must be exercised in patients at risk for volume overload, pulmonary edema, or both. The optimal rate of intravenous fluid administration during labor is not known. Potential maternal and fetal complications argue against administering large-volume intravenous boluses of glucose-containing fluids.
Correct maternal blood pressure
A number of factors predispose laboring women to transient episodes of hypotension. These include inadequate hydration, insensible fluid losses, supine position resulting in compression of the inferior vena cava, decreased venous return and reduced cardiac output, and peripheral vasodilation due to sympathetic blockade during regional anesthesia. Maternal hypotension can reduce uterine perfusion and fetal oxygenation. Hydration and lateral or Trendelenburg positioning usually correct the blood pressure. However, medication may be necessary if these measures do not achieve the desired result. Ephedrine is a sympathomimetic amine with weak α- and β-agonist activity. The primary mechanism of action is the release of norepinephrine from presynaptic vesicles, resulting in stimulation of postsynaptic adrenergic receptors. Ephedrine has no known adverse impact on fetal outcome.
Reduce uterine activity
As discussed in detail in previous chapters, excessive uterine activity is a common cause of interrupted fetal oxygenation. It is also a common source of medical-legal liability. A number of terms have been used by clinicians to describe excessive uterine activity. Examples include hyperstimulation, hypercontractility, tachysystole, hypertonus, and tetanic contraction. As discussed previously, these terms are defined inconsistently in the literature and are used inconsistently by clinicians. The 2008 NICHD consensus statement recommended using the term tachysystole to describe uterine contraction frequency in excess of 5 contractions in 10 minutes averaged over 30 minutes.26 Normal contraction frequency is defined as 5 or fewer contractions in 10 minutes averaged over 30 minutes. The report specifically noted that other features of uterine activity are clinically important as well, including contraction duration, intensity, resting tone, and time between contractions.
For the purposes of fetal heart rate management, if an abnormal fetal heart rate pattern is thought to be related to excessive uterine activity, options include position changes, intravenous hydration, reduction in dose or discontinuation of uterine stimulants, and/or administering uterine relaxants. The evaluation and management of uterine activity is discussed in detail in Chapter 4.
Alter second-stage pushing technique
During the second stage of labor, maternal expulsive efforts can be associated with FHR decelerations. Suggested corrective approaches include open-glottis rather than Valsalva-style pushing, fewer pushing efforts per contraction, shorter individual pushing efforts, pushing with every other or every third contraction, and, in patients with regional anesthesia, pushing only with perceived urge.41
Amnioinfusion
Intrapartum amnioinfusion involves infusion of isotonic fluid through an intrauterine catheter into the amniotic cavity in order to restore the amniotic fluid volume to normal or near-normal levels. The procedure is intended to relieve intermittent umbilical cord compression, variable fetal heart rate decelerations, and transient fetal hypoxemia, and to dilute thick meconium in an attempt to prevent meconium aspiration syndrome. Amnioinfusion performed for the indication of oligohydramnios and umbilical cord compression can reduce the occurrence of variable decelerations and lower the rate of cesarean delivery. It has no known impact on late decelerations. Routine amnioinfusion for meconium-stained amniotic fluid without variable decelerations is not recommended by ACOG.2 A procedure for amnioinfusion is described in Appendix A.
A systematic approach to FHR management does not require the use of all these measures in every situation. It simply helps to ensure that important considerations are not overlooked and that decisions are made in a timely manner. In addition, it provides a framework to help clinicians articulate a thoughtful, organized plan of management, a key element of reasonableness and the standard of care.
Reevaluate the FHR tracing
After assessing the oxygen pathway and beginning corrective measures that are deemed appropriate, the tracing is reevaluated. The time frame for reevaluation is based on clinical judgment but usually ranges from 5-30 minutes, in accordance with ACOG-AAP guidelines.1,3
 If the FHR tracing returns to Category I, continued surveillance is appropriate. The decision to perform routine or heightened surveillance is based on clinical judgment, taking into account the entire clinical situation.
If the FHR tracing returns to Category I, continued surveillance is appropriate. The decision to perform routine or heightened surveillance is based on clinical judgment, taking into account the entire clinical situation. If the FHR tracing progresses to Category III despite appropriate conservative corrective measures, delivery usually is expedited.
If the FHR tracing progresses to Category III despite appropriate conservative corrective measures, delivery usually is expedited. Tracings that remain in Category II warrant additional evaluation. Category II is extremely broad. It includes FHR tracings for which continued surveillance is appropriate. However, it also includes tracings that require preparations for rapid delivery. If a Category II FHR tracing reveals clinically insignificant interruption of fetal oxygenation (absent or infrequent decelerations) and excludes fetal metabolic acidemia (moderate variability and/or accelerations), continued surveillance is reasonable (Figure 6-2). Category II tracings that do not meet these criteria require further measures. If there is any question regarding the clinical significance of any decelerations, the presence of moderate variability or the presence of accelerations, the safest and easiest approach is to take the next step in the ABCD management model.
Tracings that remain in Category II warrant additional evaluation. Category II is extremely broad. It includes FHR tracings for which continued surveillance is appropriate. However, it also includes tracings that require preparations for rapid delivery. If a Category II FHR tracing reveals clinically insignificant interruption of fetal oxygenation (absent or infrequent decelerations) and excludes fetal metabolic acidemia (moderate variability and/or accelerations), continued surveillance is reasonable (Figure 6-2). Category II tracings that do not meet these criteria require further measures. If there is any question regarding the clinical significance of any decelerations, the presence of moderate variability or the presence of accelerations, the safest and easiest approach is to take the next step in the ABCD management model.C: clear obstacles to rapid delivery
If conservative corrective measures do not result in moderate variability (and/or accelerations) and resolution of clinically significant decelerations, it is prudent to plan ahead for the possible need for rapid delivery. Planning ahead does not constitute a commitment to a particular time or method of delivery. Instead, it serves as a systematic reminder of common sources of unnecessary delay so that important factors are not overlooked and decisions are made in a timely manner. This can be accomplished by systematically gathering necessary information and communicating proactively with other members of the team. This step involves many common sense considerations that do not necessarily require medical expertise. As a result, this step does not always receive the serious, systematic attention it warrants and is often left to the vagaries of random recall. Failure to take simple precautions to avoid unnecessary delay can jeopardize patient safety and can be a source of intense criticism in the event of an undesired outcome. Fortunately, there is an alternative to random recall. Potential sources of unnecessary delay can be grouped into 5 major categories. Organized in non-random order, from largest to smallest, these five categories include the facility, staff, mother, fetus, and labor. Table 6-2 identifies some examples of potential sources of unnecessary delay at each level. Standardized intrapartum FHR management does not mandate that each of these measures is carried out. It simply provides a practical checklist of factors to consider. The checklist approach promotes optimal team communication, encourages timely decision-making, and minimizes preventable errors.
D: decision to delivery time
After appropriate conservative measures have been implemented, it is sensible to take a moment to estimate the time needed to accomplish delivery in the event of a sudden deterioration of the FHR tracing. The anticipated decision to delivery time must be taken into consideration when weighing the risks and benefits of continued expectant management versus expeditious delivery. This step can be facilitated by systematically considering individual characteristics of the facility, staff, mother, fetus, and labor (Table 6-2).
Management steps A, B, C, and D are largely uncontroversial. They are readily amenable to standardization and represent the overwhelming majority of decisions that must be made during labor. However, once they are exhausted, further management decisions rely on the individual clinical judgment of the care provider who ultimately is responsible for the safety of the mother and the fetus.
Expectant management versus delivery
If conservative measures are unsuccessful, the clinician must decide whether to await spontaneous vaginal delivery or to expedite delivery by other means. This decision demands individual clinical judgment, weighing the estimated time until vaginal delivery against the estimated time until the onset of metabolic acidemia and potential injury. Prediction of the time until vaginal delivery is inexact at best. It is based on many clinical factors, including but not limited to parity, estimated fetal weight, adequacy of uterine activity, previous progress in labor, adequacy of the maternal pelvis, and effectiveness of maternal expulsive efforts. Adding to the complexity of the prediction, available information in the literature regarding the rate of progression of metabolic acidemia is extremely limited. Retrospective data suggest that, in the setting of minimal-absent variability and recurrent decelerations, metabolic acidemia usually does not appear suddenly. Instead, it can evolve over a period of approximately 60 minutes, assuming that the previous tracing was normal and there are no acute events.35
It is imperative to recognize that this process can occur much more rapidly (for example, over the course of minutes), more slowly (for example, over the course of hours), or not at all, depending on many factors, including but not limited to the frequency and duration of decelerations. Despite the paucity of data, a clinical decision must be made using the best information available at the time. The ultimate decision may differ from case to case. However, a standardized, systematic approach can help ensure that management decisions are organized, timely, and based, to the extent possible, on scientific evidence and consensus in the literature.
One of the most common preventable errors at this point in FHR management is to postpone a difficult decision in the hope that the situation will resolve on its own. It is highly advisable during this step of FHR management to resist the urge to delay an indicated decision. Instead, the clinician should use discipline and individual clinical judgment to make and document a plan based on the best information available. If the clinician decides to expedite delivery, the rationale should be documented and the plan should be implemented. If the clinician decides to continue to wait for vaginal delivery, the rationale and plan should be documented and the decision should be revisited after a reasonable period of time. It is critical to recognize that, both medically and legally, “deciding to wait” is distinctly different from “waiting to decide.” The former reflects the application of clinical judgment, whereas the latter can be construed as procrastination. As long as reasonable judgment is exercised, “deciding to wait” is likely to be defensible, regardless of the eventual outcome. “Waiting to decide,” however, puts the clinician in the difficult position of trying to explain why he or she neglected to make a medically necessary decision in a timely fashion.
The standardized management algorithm detailed in this chapter expands the recommendations of ACOG Practice Bulletin 116 to provide an organized, systematic framework that can help clinicians at all levels of training and experience formulate a care plan that is factually accurate and articulate.4
Adjuncts to electronic fetal monitoring
One of the major shortcomings of electronic fetal monitoring is a high rate of false-positive results. Even the most abnormal patterns are poorly predictive of neonatal morbidity. This has led to exploration of alternative methods of evaluating fetal status, including fetal scalp pH determination, scalp stimulation or vibroacoustic stimulation, computer analysis of fetal heart rate, fetal pulse oximetry, and ST segment analysis. In assessing the immediate condition of the newborn, umbilical cord acid—base determination is an adjunct to the Apgar score.
Intrapartum fetal scalp pH and lactate determination
Intermittent sampling of scalp blood for pH determination was described in the 1960s and studied extensively in the 1970s. However, its use has been limited by many factors, including the requirements for cervical dilation and membrane rupture, technical difficulty of the procedure, the need for serial pH determinations, and uncertainty regarding interpretation and application of results. It is used infrequently in the U.S. but remains a common practice in many countries outside the U.S. A recent meta-analysis revealed that fetal scalp lactate determination was accomplished successfully more frequently than scalp pH determination. However, there were no differences in maternal, fetal, neonatal or infant outcome.19
Fetal scalp stimulation and vibroacoustic stimulation
A number of studies in the 1980s reported that fetal heart rate acceleration in response to fetal scalp stimulation or vibroacoustic stimulation was highly predictive of normal scalp blood pH. 10,11,20,21,25,37,43,44 A literature review and meta-analysis by Skupski and colleagues confirmed the utility of various methods of intrapartum fetal stimulation, including scalp puncture, atraumatic stimulation with an Allis clamp, vibroacoustic stimulation, and digital stimulation.42
Procedure
Stimulation methods include the following:
It is crucial for clinicians to recognize that fetal scalp stimulation and vibroacoustic stimulation are diagnostic tools used to provoke fetal heart rate accelerations in order to exclude the presence of fetal metabolic acidemia. As noted previously, fetal stimulation procedures should be performed at times when the fetal heart rate is at baseline. Neither fetal scalp stimulation nor vibroacoustic stimulation is appropriate during fetal heart rate decelerations or bradycardia.
Computer analysis of fetal heart rate
Subjective interpretation of fetal heart rate tracings by visual analysis has been hampered by inconsistency and imprecision. In an attempt to overcome this limitation, Dawes and others derived a system of numeric analysis of fetal heart rate.12 Computer analysis of intrapartum fetal heart rate records has been reported to be more precise than visual assessment.13,36
However, intrapartum computer analysis has not been shown to improve prediction of neonatal outcome. Keith and colleagues reported the results of a multicenter trial of an intelligent computer system using clinical data in addition to fetal heart rate data.26 In 50 cases analyzed, the system’s performance was indistinguishable from that of 17 expert clinicians. The authors reported that the system was highly consistent, recommended no unnecessary intervention, and performed better than all but two of the experts.
Fetal pulse oximetry
Intrapartum reflectance fetal pulse oximetry is a modification of transmission pulse oximetry that indirectly measures the oxygen saturation of hemoglobin in fetal blood. An intrauterine sensor placed in contact with fetal skin uses the differential absorption of red and infrared light by oxygenated and deoxygenated fetal hemoglobin to provide continuous estimation of fetal oxygen saturation. A number of studies have examined the utility of intrapartum fetal pulse oximetry.8,14-18,22,27,28,32,34,40 Although the technology appears to reduce the incidence of cesarean delivery for fetal indications, no consistent impact on overall cesarean rates or newborn outcomes has been demonstrated. The results of a number of randomized trials led the manufacturer to announce that it would no longer distribute the sensors, effectively withdrawing the product from the market.
ST segment analysis
Study of the fetal electrocardiogram has produced some promising results.7 In sheep, fetal heart rate decelerations that accompanied hypoxemia were associated with characteristic changes in the fetal P-R interval. In 2000, Strachan compared standard electronic fetal monitoring with electronic fetal monitoring plus P-R interval analysis in 1,038 women.45 The groups demonstrated statistically similar rates of operative intervention for presumed fetal distress and no differences in newborn outcomes.
The ST segment of the fetal electrocardiogram represents myocardial repolarization. Myocardial hypoxia can lead to elevation of the ST segment and T wave secondary to catecholamine release, β-adrenoceptor activation, glycogenolysis, and tissue metabolic acidosis.24,39,49 These observations have led to the development of technology to analyze the fetal electrocardiogram plus the ST waveform (STAN; Neoventa Medical, Göteborg, Sweden).6,29
One randomized trial in 2,434 patients demonstrated a 46% reduction in operative intervention for fetal distress when ST segment analysis was added to standard electronic fetal monitoring.48 Operative interventions for dystocia and other indications were not increased. Fewer cases of metabolic acidemia and low 5-minute Apgar scores were observed in the group with electronic fetal monitoring plus ST segment analysis; however, these differences did not reach statistical significance.
Another trial using newer technology included 4,966 women randomized to electronic fetal monitoring alone versus electronic fetal monitoring plus ST segment analysis.6 When analyzed according to intention to treat, the incidence of umbilical artery acidemia was 53% lower in the electronic fetal monitoring plus ST segment analysis group. In the electronic fetal monitoring plus ST segment analysis group, the incidence of cesarean section for fetal distress was 8%, compared to 9% in the group monitored with electronic fetal monitoring alone (p = 0.047). After excluding patients with inadequate fetal heart rate recordings and fetal malformations, these differences were slightly more pronounced.
A meta-analysis of four studies, including 9,829 women, concluded that adjunctive ST segment analysis was associated with significantly fewer cases of severe metabolic acidemia at birth, fewer cases of neonatal encephalopathy, and fewer operative vaginal deliveries.31 There were no significant differences in cesarean delivery rates, low 5-minute Apgar scores, or neonatal intensive care unit admissions. A recent, large multicenter trial randomized 5,681 women to intrapartum electronic FHR monitoring alone versus electronic monitoring plus ST segment analysis.47 No significant difference was observed in the primary outcome of metabolic acidosis, defined as an umbilical artery pH <7.05 with a base deficit of more than 12 mmol/L in the extracellular fluid. In the group with electronic monitoring plus ST analysis, there were statistically fewer cases of fetal blood sampling during labor (10.6% versus 20.4%, relative risk 0.52, 95% confidence interval 0.46-0.59), umbilical artery pH <7.05 and base deficit >12 mmol/L (1.6% versus 2.6%, relative risk 0.63, 95% confidence interval 0.42-0.97) and fewer cases of umbilical artery pH <7.05 (1.9% versus 2.7%, relative risk 0.67, 95% confidence interval 0.46-0.97). Total operative deliveries, cesarean deliveries, and instrumented vaginal deliveries occurred with statistically similar frequency in both groups. There were no differences in operative deliveries for fetal distress. There were no other statistically significant differences in newborn outcome.
The Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal Fetal Medicine Units Network is at this writing recruiting patients in the United States for a Phase III trial of the STAN monitor as an adjunct to electronic fetal monitoring (Identifier NCT01131260). The estimated study completion date is June 2012.
Umbilical cord acid—base determination
Umbilical cord blood gas and pH assessment is a useful adjunct to the Apgar score in assessing the immediate condition of the newborn. There are no contraindications to obtaining cord gases.
ACOG5 suggests obtaining cord gases in the following clinical situations:
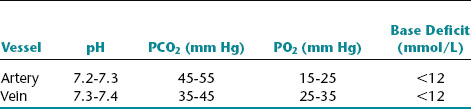
Umbilical arterial values reflect fetal condition, whereas umbilical venous values reflect placental function. Normal findings preclude the presence of acidemia at, or immediately before, delivery. Approximate normal values for cord blood are summarized in the following chart.23,33,38,46
Approximate normal values for cord blood
The base deficit reflects utilization of buffer bases to help stabilize pH, usually in the setting of peripheral tissue hypoxia, anaerobic metabolism, and accumulation of lactic acid. An umbilical artery pH less than 7.20 usually is considered to define acidemia. Note that a much lower pH (7.0) is defined as the threshold of potential injury.
Acidemia is categorized as respiratory, metabolic, or mixed. Isolated respiratory acidemia is diagnosed when the umbilical artery pH is less than 7.20, the Pco2 is elevated, and the base deficit is <12 mmol/L. This reflects disrupted exchange of blood gases, usually as a transient phenomenon related to umbilical cord compression. Isolated respiratory acidemia is not associated with fetal neurologic injury. Isolated metabolic acidemia is diagnosed when the pH is less than 7.20, the Pco2 is normal, and the base deficit is at least 12 mmol/L. Metabolic acidemia can result from recurrent or prolonged interruption of fetal oxygenation that has progressed to the stage of peripheral tissue hypoxia, anaerobic metabolism, and lactic acid production in excess of buffering capacity. Although most cases of fetal metabolic acidemia do not result in injury, the risk is increased in the setting of significant metabolic acidemia (umbilical artery pH <7.0 and base deficit ≥12 mmol/L). Mixed (respiratory and metabolic) acidemia is diagnosed when the pH is below 7.20, the Pco2 is elevated, and the base deficit is 12 mmol/L or greater. The clinical significance of mixed acidemia is similar to that of isolated metabolic acidemia.
The types of acidemia (respiratory, metabolic, or mixed) are summarized in the following chart.
Types of acidemia
The procedure for obtaining umbilical cord blood consists of double-clamping a 10- to 20-cm segment of the umbilical cord immediately after delivery. A specimen should be drawn with a 1-ml plastic syringe that has been flushed with heparin solution (1000 U/ml). Using separate syringes, draw blood from an umbilical artery first, then from the umbilical vein.
Summary
Recent progress toward consensus in FHR monitoring makes it possible to construct a practical, standardized approach to FHR interpretation and management. The intrapartum FHR management model described in this chapter is not intended to dictate actions that must be taken in response to specific fetal heart rate patterns. Instead, it is intended to serve as a reminder of common sources of preventable error and an indicator of actions that should be considered to ensure that management decisions are made in a timely fashion. Fetal heart rate definition, interpretation, and management should be guided by a few basic principles:
1. American Academy of Pediatrics, American College of Obstetricians and Gynecologists. Guidelines for perinatal care, ed 6. Washington DC: AAP, ACOG; 2007.
2. American College of Obstetricians and Gynecologists. Amnioinfusion does not prevent meconium aspiration syndrome. ACOG Committee Opinion no. 346. Washington DC: ACOG; 2006.
3. American College of Obstetricians and Gynecologists: Intrapartum fetal heart rate monitoring: nomenclature, interpretation, and general management principles. ACOG Practice Bulletin no. 106. Obstet Gynecol. 2009;114:192-202
4. American College of Obstetricians and Gynecologists. Management of intrapartum fetal heart rate tracings. ACOG Practice Bulletin no. 116. Obstet Gynecol. 2010;116:1232-1240.c.
5. American College of Obstetricians and Gynecologists. Umbilical cord blood gas and acid-base analysis. ACOG Committee Opinion. Obstet Gynecol. 2006;108(5):1319-1322.
6. Amer-Wåhlin I, Hellsten C, Norén H, et al: Cardiotocography only versus cardiotocography plus ST analysis of fetal electrocardiogram for intrapartum fetal monitoring: a Swedish randomised controlled trial. Lancet. 2001;358(9281)534-538
7. Arulkumaran S, Lilja H, Lindecrantz K, et al. Fetal ECG waveform analysis should improve fetal surveillance in labour. J Perinatal Med. 1990;18(1):13-22.
8. Bloom SL, Spong CY, Thom E, et al: National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network: fetal pulse oximetry and cesarean delivery. N Engl J Med. 2006;355(21)2195-2202
9. Bolnick JM, Garcia G, Fletcher BG, et al. Cross-over trial of fetal heart rate response to halogen light and vibroacoustic stimulation. J Matern Fetal Neonatal Med. 2006;19:215-219.
10. Clark SL, Gimovsky ML, Miller FC. Fetal heart rate response to scalp blood sampling. Am J Obstet Gynecol. 1982;144(6):706-708.
11. Clark SL, Gimovsky ML, Miller FC: The scalp stimulation test: a clinical alternative to fetal scalp blood sampling. Am J Obstet Gynecol. 1984;148(3)274-277
12. Dawes GS. Computerised analysis of the fetal heart rate. Eur J Obstet Gynecol Reprod Biol. 1991;42:S5-S8. Suppl
13. Dawes GS, Moulden M, Sheil O, et al. Approximate entropy, a statistic of regularity, applied to fetal heart rate data before and during labor. Obstet Gynecol. 1992;80(5):763-768.
14. Dildy DA, Clark SL, Loucks CA: Intrapartum fetal pulse oximetry: past, present, and future. Am J Obstet Gynecol. 1996;175(1)1-9
15. Dildy GA, Clark SL, Loucks CA. Preliminary experience with intrapartum fetal pulse oximetry in humans. Obstet Gynecol. 1993;81(4):630-635.
16. Dildy GA, Thorp JA, Yeast JD, et al: The relationship between oxygen saturation and pH in umbilical blood: implications for intrapartum fetal oxygen saturation monitoring. Am J Obstet Gynecol. 1996;175(3 Pt 1)682-687
17. Dildy GA, van den Berg PP, Katz M, et al: Intrapartum fetal pulse oximetry: fetal oxygen saturation trends during labor and relation to delivery outcome. Am J Obstet Gynecol. 1994;171(3)679-684
18. East CE, Brennecke SP, King JF, et al: The effect of intrapartum fetal pulse oximetry, in the presence of a nonreassuring fetal heart rate pattern, on operative delivery rates: a multicenter, randomized, controlled trial (the FOREMOST trial). Am J Obstet Gynecol. 2006;194(3)606.e1-606.e16
19. East CE, Leader LR, Sheehan P, et al: Intrapartum fetal scalp lactate sampling for fetal assessment in the presence of a non-reassuring fetal heart rate trace. Cochrane Database Syst Rev 3: CD006174. DOI: 10.1002/14651858.
20. Edersheim TG, JM Hutson JM, Druzin ML, et al. Fetal heart rate response to vibratory acoustic stimulation predicts fetal pH in labor. Am J Obstet Gynecol. 1987;157(6):1557-1560.
21. Elimian A, Figueroa R, Tejani N: Intrapartum assessment of fetal well-being: a comparison of scalp stimulation with scalp blood pH sampling. Obstet Gynecol. 1997;89(3)373-376
22. Garite TJ, Dildy GA, McNamara H, et al. A multicenter controlled trial of fetal pulse oximetry in the intrapartum management of nonreassuring fetal heart rate patterns. Am J Obstet Gynecol. 2000;183(5):1049-1058.
23. Helwig JT, Parer JT, Kilpatrick SJ, et al: Umbilical cord blood acid-base state: what is normal. Am J Obstet Gynecol. 1996;174(6)1807-1812
24. Hökegård KH, Eriksson BO, Kjellemer I, et al. Myocardial metabolism in relation to electrocardiographic changes and cardiac function during graded hypoxia in the fetal lamb. Acta Physiol Scand. 1981;113(1)1-7
25. Ingemarsson I, Arulkumaran S. Reactive fetal heart rate response to VAS in fetuses with low scalp blood pH. Br J Obstet Gynaecol. 1989;96(5):562-565.
26. Keith RDF, Beckly S, Garibaldi JM, et al. A multicentre comparative study of 17 experts and an intelligent computer system for managing labour using the cardiotocogram. Br J Obstet Gynaecol. 1995;102(9):688-700.
27. Klauser CK, Christensen EE, Chauhan SP, et al: Use of fetal pulse oximetry among high-risk women in labor: a randomized clinical trial. Am J Obstet Gynecol. 2005;192(16)1810-1819
28. Kuhnert M, Seelbach-Goebel G, Butterwegge M: Predictive agreement between the fetal arterial oxygen saturation and fetal scalp pH: results of the German multicenter study. Am J Obstet Gynecol. 1998;178(2)330-335
29. Lilja H, Karlsson K, Lindecrantz K, et al. Microprocessor based waveform analysis of the fetal electrocardiogram during labor. Int J Gynecol Obstet. 1989;30:109-116.
30. Macones GA, Hankins GD, Spong CY, et al: The 2008 National Institute of Child Health and Human Development workshop report on electronic fetal monitoring: update on definitions, interpretation, and research guidelines. Obstet Gynecol. 2008;112(3)661-666
31. Neilson JP. Fetal electrocardiogram (ECG) for fetal monitoring during labour. Cochrane Database Syst Rev. 3, 2006. CD000116
32. Nijland R, Jongsma HW, Nijhuis JG, et al. Arterial oxygen saturation in relation to metabolic acidosis in fetal lambs. Am J Obstet Gynecol. 1995;172(3):810-819.
33. Nodwell A, Carmichael L, Ross M, et al. Placental compared with umbilical cord blood to assess fetal blood gas and acid-base status. Obstet Gynecol. 2005;105(1):129-138.
34. Oeseburg B, Ringnalda BEM, Crevels J, et al: Fetal oxygenation in chronic maternal hypoxia: what’s critical. Adv Exp Med Biol. 1992;317:499-502
35. Parer JT, King T, Flanders S, et al: Fetal acidemia and electronic fetal heart rate patterns: is there evidence of an association. J Matern Fetal Neonatal Med. 2006;19(5)289-294
36. Pello LC, Rosevear BM, Dawes GS, et al. Computerized fetal heart rate analysis in labor. Obstet Gynecol. 1991;78(4):602-610.
37. Polzin GB, Blakemore KJ, Petrie RH, et al: Fetal vibro-acoustic stimulation: magnitude and duration of fetal heart rate accelerations as a marker of fetal health. Obstet Gynecol. 1988;72(4)621-626
38. Richardson B, Nodwell A, Webster K, et al. Fetal oxygen saturation and fractional extraction at birth and the relationship to measures of acidosis. Am J Obstet Gynecol. 1988;178(3):572-579.
39. Rosén KG, Dagbjartsson A, Henriksson BA, et al. The relationship between circulating catecholamine and ST waveform in the fetal lamb electrocardiogram during hypoxia. Am J Obstet Gynecol. 1984;149(2):190-195.
40. Seelbach-Gobel B, Butterwegge M, Kuhnert M, et al: Fetal reflectance pulse oximetry sub partu: experiences, prognostic significance, and consequences and goals. Zeitschr Geburtshilfe Perinatol. 1994;198:67-71
41. Simpson KR, James DC. Efficacy of intrauterine resuscitation techniques in improving fetal oxygen status during labor. Obstet Gynecol. 2005;105(6):1362-1368.
42. Skupski DW, Rosenberg CR, Eglington GS: Intrapartum fetal stimulation tests: a meta-analysis. Obstet Gynecol. 2002;99(1)129-134
43. Smith CV, Nguyen HN, Phelan JP, et al: Intrapartum assessment of fetal well-being: a comparison of fetal acoustic stimulation with acid-base determinations. Am J Obstet Gynecol. 1986;155(4)726-728
44. Spencer JA. Predictive value of a fetal heart rate acceleration at the time of fetal blood sampling in labour. J Perinat Med. 1991;19(3):207-215.
45. Strachan BK, van Wijngaarden WJ, Sahota D, et al: Cardiotocography only versus cardiotocography plus PR-interval analysis in intrapartum surveillance: a randomized, multicentre trial. Lancet. 2000;355(9202)456-459
46. Victory R, Penava D, Da Silva O, et al. Umbilical cord pH and base excess values in relation to adverse outcome events for infants delivering at term. Am J Obstet Gynecol. 2004;191(6):2021-2028.
47. Westerhuis MEMH, Visser GHA, Moons KGM, et al: Cardiotography plus ST analysis of fetal electrocardiogram compared with cardiotocography only for intrapartum monitoring: a randomized trial. Obstet Gynecol. 2010;115:1173-1180
48. Westgate J, Harris M, Curnow JS, et al. Plymouth randomized trial of cardiotocogram only versus ST waveform plus cardiotogram for intrapartum monitoring in 2400 cases. Am J Obstet Gynecol. 1993;169(5):1151-1160.
49. Widmark C, Jansson T, Lindecrantz K, et al. ECG waveform, short term heart rate variability and plasma catecholamine concentrations in response to hypoxia in intrauterine growth retarded guinea pig fetuses. J Dev Physiol. 1991;15(3):161-168.