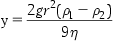Suspensions
 The pharmaceutical uses of suspensions
The pharmaceutical uses of suspensions
 The properties of an ideal suspension
The properties of an ideal suspension
Introduction
Suspensions contain one or more insoluble medicaments in a vehicle, with other additives such as preservatives, flavours, colours, buffers and stabilizers. Most pharmaceutical suspensions are aqueous, but an oily vehicle is sometimes used. Suspensions may be used for oral administration, inhalation, topical application, as ophthalmic preparations, for parenteral administration and as aerosols.
A pharmaceutical suspension may be defined as a disperse system in which one substance (the disperse phase) is distributed in particulate form throughout another (the continuous phase). Most are classified as a coarse suspension, which is a dispersion of particles with a mean diameter>1 μm. A colloidal suspension is a dispersion of particles with a mean diameter<1 μm. Suspended solids slowly separate on standing, but redispersion may be difficult if they form a compacted sediment.
Pharmaceutical applications of suspensions
Suspensions may be used pharmaceutically for a number of reasons. Some are given below:
 Drugs with low solubility in the continuous phase can be formulated as suspensions
Drugs with low solubility in the continuous phase can be formulated as suspensions
 Patient acceptability – a liquid form rather than a solid dosage form
Patient acceptability – a liquid form rather than a solid dosage form
 Drugs that have an unpleasant taste in their soluble form can be made into insoluble derivatives, and formulated as a suspension, which will be more palatable, e.g. chloramphenicol (soluble) and chloramphenicol palmitate (insoluble)
Drugs that have an unpleasant taste in their soluble form can be made into insoluble derivatives, and formulated as a suspension, which will be more palatable, e.g. chloramphenicol (soluble) and chloramphenicol palmitate (insoluble)
 In oral suspensions, the drug is delivered in finely divided form, therefore optimal dissolution occurs in the gastrointestinal (GI) fluids and hence the rate of absorption is increased
In oral suspensions, the drug is delivered in finely divided form, therefore optimal dissolution occurs in the gastrointestinal (GI) fluids and hence the rate of absorption is increased
 Insoluble forms of drugs may prolong the action of a drug by preventing rapid degradation in the continuous phase
Insoluble forms of drugs may prolong the action of a drug by preventing rapid degradation in the continuous phase
 If the drug is unstable when in contact with the vehicle, suspensions should be prepared immediately prior to handing out to the patient in order to reduce the amount of time that the drug particles are in contact with the dispersion medium. For example, in ampicillin suspension, water is added to powder or granules prior to giving out to the patient. A 14-day expiry date is given, if the product is to be kept in the fridge
If the drug is unstable when in contact with the vehicle, suspensions should be prepared immediately prior to handing out to the patient in order to reduce the amount of time that the drug particles are in contact with the dispersion medium. For example, in ampicillin suspension, water is added to powder or granules prior to giving out to the patient. A 14-day expiry date is given, if the product is to be kept in the fridge
 Drugs which degrade in aqueous solution may be suspended in a non-aqueous phase, e.g. tetracycline hydrochloride has been suspended in a fractionated coconut oil for ophthalmic use
Drugs which degrade in aqueous solution may be suspended in a non-aqueous phase, e.g. tetracycline hydrochloride has been suspended in a fractionated coconut oil for ophthalmic use
 Bulky, insoluble powders can be formulated as a suspension so that they are easier to take, e.g. kaolin, chalk and magnesium trisilicate (see Examples 34.1 and 34.2)
Bulky, insoluble powders can be formulated as a suspension so that they are easier to take, e.g. kaolin, chalk and magnesium trisilicate (see Examples 34.1 and 34.2)
 Intramuscular, intra-articular or subcutaneous injections are often formulated as suspensions to prolong the release of the drug
Intramuscular, intra-articular or subcutaneous injections are often formulated as suspensions to prolong the release of the drug
 Lotions containing insoluble solids are formulated to leave a thin coating of medicament on the skin. As the vehicle evaporates, it gives a cooling effect and leaves the solid behind. Examples are Calamine Lotion BP (see Example 34.3) and Sulphur Lotion Compound BPC (see Ch. 36).
Lotions containing insoluble solids are formulated to leave a thin coating of medicament on the skin. As the vehicle evaporates, it gives a cooling effect and leaves the solid behind. Examples are Calamine Lotion BP (see Example 34.3) and Sulphur Lotion Compound BPC (see Ch. 36).
Properties of a good pharmaceutical suspension
In preparing a pharmaceutically elegant product, several desirable properties are sought:
Formulation of suspensions
The three steps that can be taken to ensure formulation of an elegant pharmaceutical suspension are:
1. Control particle size. On a small scale, this can be done using a mortar and pestle, to grind down ingredients to a fine powder
2. Use a thickening agent to increase viscosity of the vehicle, by using suspending or viscosity-increasing agents
Some of the theoretical and practical aspects of these will be considered in the context of extemporaneous dispensing.
The insoluble medicament may be a diffusible solid or an indiffusible solid:
Diffusible solids (dispersible solids). These are insoluble solids that are light and easily wetted by water. They mix readily with water, and stay dispersed long enough for an adequate dose to be measured. After settling, they redisperse easily. Examples include magnesium trisilicate; light magnesium carbonate; bismuth carbonate and light kaolin (see Example 34.1).
Indiffusible solids. Most insoluble solids are not easily wetted, and may form large porous clumps in the liquid. These solids will not remain evenly distributed in the vehicle long enough for an adequate dose to be measured. They may not redisperse easily. Examples for internal use include phenobarbital and chalk (see Example 34.2), and for external use calamine, hydrocortisone, sulphur and zinc oxide.
Problems encountered when formulating insoluble solids into a suspension
Various factors need to be considered when formulating insoluble solids into a suspension.
Sedimentation
The factors affecting the rate of sedimentation of a particle are described in Stokes’ equation:
where y=velocity of a spherical particle of radius r, and density ρ1, in a liquid of density ρ2, and viscosity η, and where g is the acceleration due to gravity.
The basic consequences of this equation are that the rate of fall of a suspended particle in a vehicle of a given density is greater for larger particles than it is for smaller particles. Also, the greater the difference in density between the particles and vehicle, the greater will be the rate of descent. Increasing the viscosity of the dispersion medium, within limits, so that the suspension is still pourable, will reduce the rate of sedimentation of a solid drug. Thus, a decrease in settling rate in a suspension may be achieved by reducing the size of the particles and by increasing the density and the viscosity of the continuous phase.
Flocculation
The natural tendency of particles towards aggregation will determine the properties of a suspension. In a deflocculated suspension, the dispersed solid particles remain separate and settle slowly. However, the sediment that eventually forms is hard to redisperse and is described as a ‘cake’ or ‘clay’. In a flocculated suspension, individual particles aggregate into clumps or floccules in suspension. Because these flocs are larger than individual particles, sedimentation is more rapid, but the sediment is loose and easily redispersible. Excess flocculation may prevent ‘pourability’ due to its effect on rheological properties.
The ideal is to use either a deflocculated system with a sufficiently high viscosity to prevent sedimentation, or controlled flocculation with a suitable combination of rate of sedimentation, type of sediment and pourability.
Wetting
Air may be trapped in the particles of poorly wetted solids, which causes them to float to the surface of the preparation and prevents them from being readily dispersed throughout the vehicle. Wetting of the particles can be encouraged by reducing the interfacial tension between the solid and the vehicle, so that adsorbed air is displaced from solid surfaces by liquid. Suitable wetting agents have this effect, but decrease interparticular forces, thereby affecting flocculation.
Hydrophilic colloids such as acacia and tragacanth can act as wetting agents. However, care should be taken when using these agents, as they can promote deflocculation. Intermediate HLB (hydrophilic–lipophilic balance) surfactants (see Ch. 35) such as polysorbates and sorbitan esters are used for internal preparations. Solvents such as ethanol, glycerol and the glycols also facilitate wetting. Sodium lauryl sulphate and quillaia tincture are used in external preparations.
Suspending agents
Suspending agents increase the viscosity of the vehicle, thereby slowing down sedimentation. Most agents can form thixotropic gels which are semi-solid on standing, but flow readily after shaking. Care must be taken when selecting a suspending agent for oral preparations, as the acid environment of the stomach may alter the physical characteristics of the suspension, and therefore the rate of release of the drug from suspension. Some suspending agents may also bind to certain medicaments, making them less bioavailable.
Suspending agents can be divided into five broad categories: natural polysaccharides, semi-synthetic polysaccharides, clays, synthetic thickeners and miscellaneous compounds.
Natural polysaccharides
The main problem with these agents is their natural variability between batches and microbial contamination. Tragacanth is a widely used suspending agent and is less viscous at pH4-7.5. As a rule of thumb, 0.2 g Tragacanth Powder is added per 100 mL suspension or 2 g Compound Tragacanth Powder per 100 mL suspension. Tragacanth Powder requires to be dispersed with the insoluble powders before water is added to prevent clumping (see Example 34.2). Compound Tragacanth Powder BP 1980 contains tragacanth, acacia, starch and sucrose and so is easier to use. Other examples include acacia gum, starch, agar, guar gum, carrageenan and sodium alginate. These materials should not be used externally as they leave a sticky feel on the skin.
Semi-synthetic polysaccharides
These are derived from the naturally occurring polysaccharide cellulose. Examples include methylcellulose (Cologel®, Celacol®), hydroxyethylcellulose (Natrosol 250®), sodium carboxymethylcellulose (Carmellose sodium) and microcrystalline cellulose (Avicel®).
Clays
These are naturally occurring inorganic materials, which are mainly hydrated silicates. Examples include bentonite and magnesium aluminium silicate (Veegum®).
Preservation of suspensions
Water is the most common source of microbial contamination. All pharmaceutical preparations that contain water are therefore susceptible to microbial growth. Also the naturally occurring additives such as acacia and tragacanth may be sources of microbes and spores. Preservative action may be diminished because of adsorption of the preservative onto solid particles of drug, or interaction with suspending agents. Useful preservatives in extemporaneous preparations include chloroform water, benzoic acid and hydroxybenzoates.
The dispensing of suspensions
The method of dispensing suspensions is the same for most, with some differences for specific ingredients.
 Crystalline and granular solids are finely powdered in the mortar. The suspending agent should then be added and mixed thoroughly in the mortar. Do not apply too much pressure, otherwise gumming or caking of the suspending agent will occur and heat of friction will make it sticky.
Crystalline and granular solids are finely powdered in the mortar. The suspending agent should then be added and mixed thoroughly in the mortar. Do not apply too much pressure, otherwise gumming or caking of the suspending agent will occur and heat of friction will make it sticky.
 Add a little of the liquid vehicle to make a paste and mix well until smooth and free of lumps. Continue with gradual additions until the mixture can be poured into a tared bottle. Further liquid is used to rinse all the powder into the bottle, where it is made up to volume.
Add a little of the liquid vehicle to make a paste and mix well until smooth and free of lumps. Continue with gradual additions until the mixture can be poured into a tared bottle. Further liquid is used to rinse all the powder into the bottle, where it is made up to volume.
Variations
 If wetting agents are included in the formulation, add them before forming the paste
If wetting agents are included in the formulation, add them before forming the paste
 If syrup and/or glycerol are in the formulation, use this rather than water to form the initial paste
If syrup and/or glycerol are in the formulation, use this rather than water to form the initial paste
 If soluble solids are being used, dissolve them in the vehicle before or after making the paste
If soluble solids are being used, dissolve them in the vehicle before or after making the paste
 Leave addition of volatile components, colourings or concentrated flavouring tinctures such as chloroform spirit, liquid liquorice extract and compound tartrazine solution until near the end.
Leave addition of volatile components, colourings or concentrated flavouring tinctures such as chloroform spirit, liquid liquorice extract and compound tartrazine solution until near the end.
Most ‘official’ suspensions will be prepared from the constituent ingredients. There may be some occasions where an oral solid dosage form, such as a tablet or capsule, will have to be reformulated by the pharmacist into an oral suspension, e.g. where the medicine is for a child (see Example 34.4). It is important to obtain as much information (physical, chemical and microbiological) as possible about the manufactured drug and its excipients. Typically, the tablet will be crushed or capsule contents emptied into the mortar and a suspending agent added. A paste is formed with the vehicle and then diluted to a suitable volume, with the addition of any other desired ingredients such as preservative or flavour. A short expiry of no more than 7 days should be given owing to the lack of knowledge about the stability of the formulation.
Containers for suspensions
Suspensions should be packed in amber bottles. There should be adequate air space above the liquid to allow shaking and ease of pouring.
Special labels and advice for suspensions
The most important additional label for suspensions is ‘Shake well before use’, as some sedimentation of medicament would normally be expected. Shaking the bottle will redisperse the medicament and ensure that the patient can measure an accurate dose.
‘Store in a cool place’. Stability of suspensions may be adversely affected by both extremes and variations of temperature. Some suspensions, such as those made by reconstituting dry powders, may need to be stored in a refrigerator.
Extemporaneously prepared and reconstituted suspensions will have a relatively short shelf-life. They are usually required to be recently or freshly prepared, with a 1–4-week expiry date. Some official formulae state an expiry date, but many do not. The pharmacist may have to make judgements about the expiry date for a particular preparation, based on its constituents and likely storage conditions. The manufacturer’s literature for reconstituted products will give recommended storage conditions.
Inhalations
Suspensions are useful formulations for inhalations. The volatile components are adsorbed onto the surface of a diffusible solid to ensure uniform dispersion throughout the liquid. When hot water is added, the oils vaporize. Where quantities are not stated, 1 g of light magnesium carbonate is used for each 2 mL of oil (such as eucalyptus oil) or 2 g of volatile solid (such as menthol). An example of a traditional inhalation is menthol and eucalyptus inhalation.
Traditionally used, but no longer recommended, for the treatment of acute diarrhoea.
Formulation notes. Light kaolin is a diffusible solid; therefore no suspending agent is required.
Method of preparation. Weigh the light kaolin and place in the mortar. Dissolve the sodium bicarbonate in about 100 mL of water. Gradually add this to the light kaolin in the mortar with mixing to disperse the solid. Add the chloroform and morphine tincture. Wash the mixture into a tared, amber medicine bottle, and make up to volume with water.
Traditionally used, but no longer recommended, as an antidiarrhoeal mixture for children.
Formulation notes. Chalk is practically insoluble in water and is an indiffusible solid, which requires a suspending agent. Tragacanth Powder is used in this formulation. The concentrated cinnamon water is a flavouring agent and the syrup increases the viscosity as well as acting as a sweetener. Chloroform water is the preservative.
Method of preparation. The chalk and tragacanth should be weighed and lightly mixed in a mortar and pestle. Add the syrup and mix to make a paste. The double strength chloroform water should be gradually added, with mixing, followed by the concentrated cinnamon water. The mixture should be rinsed into a previously tared 100 mL amber medicine bottle and made up to volume with water. Seal the bottle and shake the suspension well.
Action and uses. As a cooling lotion for sunburn or skin irritation and pruritus.
Formulation notes. Calamine is a coloured zinc carbonate and is practically insoluble in water, as is zinc oxide. Both are indiffusible solids. Sodium citrate is added to control the flocculation of calamine. Bentonite is a thickening agent and glycerol will thicken the product and help powder adherence to the skin. Liquefied phenol acts as a preservative and antiseptic.
Method of preparation. The dry powders should be weighed and mixed in a mortar so that the bentonite is well distributed. Add the glycerol to the powders and mix. The sodium citrate is dissolved in about 140 mL of water, and gradually added to the mixture in the mortar, so that a smooth paste is produced. Add the liquefied phenol, taking care not to splash, as it is caustic. Transfer the mixture to a tared, amber ribbed glass bottle, adding washings from the mortar, and make up to volume. Seal with a child-resistant closure.
Shelf-life and storage. Store in a cool, dry place. It is recently prepared; therefore, a shelf-life of 2–3 weeks is applicable.
Advice and labelling. ‘For external use only’, ‘Shake well before use’ and ‘Do not apply to broken skin’. The lotion should be applied to the affected areas when required and allowed to dry.
Action and uses. A potassium-sparing diuretic used in oedema of heart failure and nephrotic syndrome.
Formulation notes. Spironolactone is practically insoluble in water. Cologel® (methylcellulose) acts as the suspending agent. Compound orange spirit is a flavouring agent.
Method of preparation. Tablets may be used, and sufficient crushed in a mortar and pestle to give 300 mg spironolactone (e.g. 6 × 50 mg tablets). Alternatively, weigh the powder and transfer to a mortar and pestle. Add the Cologel® and mix to a paste. Gradually add some of the water. Add the compound orange spirit. Rinse the suspension into a tared, amber medicine bottle and make up to volume with water. Shake the bottle well and seal with a child-resistant closure. Polish and label the bottle and give a 5 mL medicine spoon with the medicine.
Shelf-life and storage. It is recently prepared with a shelf-life of 4 weeks when stored in a refrigerator. Spironolactone should be protected from light.
Advice and labelling. ‘Shake well before use’ and ‘Give one 5 mL spoonful three times a day’. BNF Label 21 should be used. Reinforce the storage conditions.
Key Points
 Suspensions can be used to administer an insoluble solid by the oral route
Suspensions can be used to administer an insoluble solid by the oral route
 Suspensions may be used to replace tablets, to improve dissolution rate, to prolong action and to mask a bad taste
Suspensions may be used to replace tablets, to improve dissolution rate, to prolong action and to mask a bad taste
 Solids may be diffusible or indiffusible and require different dispensing techniques
Solids may be diffusible or indiffusible and require different dispensing techniques
 Stokes’ equation can be applied when formulating a suspension to help ensure accurate dosage of the drug
Stokes’ equation can be applied when formulating a suspension to help ensure accurate dosage of the drug
 Flocculated particles settle quickly and redisperse easily, while deflocculated particles settle slowly but tend to cake
Flocculated particles settle quickly and redisperse easily, while deflocculated particles settle slowly but tend to cake
 Hydrophobic solids may require wetting agents
Hydrophobic solids may require wetting agents
 Suspending agents are added to slow down the rate of settling of the solid
Suspending agents are added to slow down the rate of settling of the solid
 Suspending agents may be natural polysaccharides, semi-synthetic polysaccharides, clays or synthetic polymers
Suspending agents may be natural polysaccharides, semi-synthetic polysaccharides, clays or synthetic polymers
 Some suspensions are made by adding water to reconstitute manufactured powders when stability is a problem
Some suspensions are made by adding water to reconstitute manufactured powders when stability is a problem
 ‘Shake well before use’ and ‘Store in a cool place’ should be part of the labels on a suspension
‘Shake well before use’ and ‘Store in a cool place’ should be part of the labels on a suspension
 Inhalations are suspensions of a volatile material adsorbed onto a diffusible solid
Inhalations are suspensions of a volatile material adsorbed onto a diffusible solid