Emulsions
 The uses of pharmaceutical emulsions
The uses of pharmaceutical emulsions
 The different types of emulsion and their identification
The different types of emulsion and their identification
Introduction
An emulsion consists of two immiscible liquids, one of which is uniformly dispersed throughout the other as fine droplets normally of diameter 0.1–100 μm. To prepare a stable emulsion, a third ingredient, an emulsifying agent, is required. Oral emulsions are stabilized oil-in-water dispersions that may contain one or more active ingredients. They are a useful way of presenting oils and fats in a palatable form. Emulsions for external use are known as lotions, applications or liniments if liquid, or creams if semi-solid in nature. Some parenteral products may also be formulated as emulsions. Most important of these is total parenteral nutrition (see Ch. 44). Pharmaceutically, the term ‘emulsion’, when no other qualification is used, is taken to mean an oil-in-water preparation for internal use.
Pharmaceutical applications of emulsions
Emulsions have a wide range of uses, including:
 Oral, rectal and topical administration of oils and oil-soluble drugs
Oral, rectal and topical administration of oils and oil-soluble drugs
 Formulation of oil- and water-soluble drugs together
Formulation of oil- and water-soluble drugs together
 To enhance palatability of oils when given orally by disguising both taste and oiliness
To enhance palatability of oils when given orally by disguising both taste and oiliness
 Increasing absorption of oils and oil-soluble drugs through intestinal walls
Increasing absorption of oils and oil-soluble drugs through intestinal walls
 Intramuscular injections of some water-soluble vaccines: these provide slow release and therefore a greater antibody response and longer-lasting immunity
Intramuscular injections of some water-soluble vaccines: these provide slow release and therefore a greater antibody response and longer-lasting immunity
 Total parenteral nutrition (see Ch. 44).
Total parenteral nutrition (see Ch. 44).
Examples of traditionally used emulsions for oral use are cod liver oil emulsion (see Example 35.1), Liquid Paraffin Oral Emulsion BP (see Example 35.2). An example of emulsion for external use is Oily Calamine Lotion BP (see Example 35.3).
Emulsion types
Emulsions may be oil-in-water (o/w) emulsions, where oil is the disperse phase in a continuous phase of water, or water-in-oil (w/o) emulsions, where water is the disperse phase in a continuous phase of oil. It is also possible to form a multiple emulsion, e.g. a water droplet enclosed in an oil droplet, which is itself dispersed in water–a w/o/w emulsion. Multiple emulsions are increasingly used in manufactured pharmaceutical products and are used for delayed action drug delivery systems.
If the emulsion is for oral or intravenous administration it will always be oil-in-water. Intramuscular injections may be water-in-oil for depot therapy. When selecting emulsion type for preparations for external use, the therapeutic use, texture and patient acceptability will be taken into account. Oil-in-water emulsions are less greasy, easily washed off the skin and more cosmetically acceptable than water-in-oil emulsions. They have an occlusive effect, which hydrates the upper layers of the skin (called an emollient, see Ch. 36). Water-in-oil emulsions rub in more easily.
Identification of emulsion type
There is a range of tests available to identify the emulsion type. Some of the tests that can be used are outlined below.
Miscibility test. An emulsion will only mix with a liquid that is miscible with its continuous phase. Therefore an o/w emulsion is miscible with water, a w/o emulsion with an oil.
Conductivity measurement. Systems with an aqueous continuous phase will conduct electricity, while systems with an oily continuous phase will not.
Staining test. A dry filter paper impregnated with cobalt chloride turns from blue to pink on exposure to stable o/w emulsions.
Dye test. If an oil-soluble dye is used, o/w emulsions are paler in colour than w/o emulsions. If examined microscopically, an o/w emulsion will appear as coloured globules on a colourless background while a w/o emulsion will appear as colourless globules against a coloured background.
Formulation of emulsions
An ideal emulsion has globules of disperse phase that retain their initial character, that is the mean globule size does not change and the globules remain evenly distributed. The formulation of emulsions involves the prevention of coalescence of the disperse phase (often called ‘cracking’) and reducing the rate of creaming.
Emulsifying agents
Emulsifying agents help the production of a stable emulsion by reducing interfacial tension and then maintaining the separation of the droplets by forming a barrier at the interface. Most emulsifying agents are surface-active agents. Emulsion type is determined mainly by the solubility of the emulsifying agent. If the emulsifying agent is more soluble in water (i.e. hydrophilic), then water will be the continuous phase and an o/w emulsion will be formed. If the emulsifying agent is more soluble in oil (i.e. lipophilic), oil will be the continuous phase and a w/o emulsion will be formed. If a substance is added which alters the solubility of the emulsifying agent, this balance may be altered and the emulsion may change type. The process is called phase inversion. The ideal emulsifying agent is colourless, odourless, tasteless, non-toxic, non-irritant and able to produce stable emulsions at low concentrations.
Emulsifying agents can be classed into three groups: naturally occurring, synthetic surfactants and finely divided solids.
Naturally occurring emulsifying agents
These agents come from vegetable or animal sources. Therefore, the quality may vary from batch to batch and they are susceptible to microbial contamination and degradation.
Polysaccharides. Acacia is the best emulsifying agent for extemporaneously prepared oral emulsions as it forms a thick film at the oil–water interface to act as a barrier to coalescence. It is too sticky for external use. Tragacanth is used to increase the viscosity of an emulsion and prevent creaming. Other polysaccharides, such as starch, pectin and carrageenan, are used to stabilize an emulsion.
Semi-synthetic polysaccharides. Low-viscosity grades of methylcellulose (see Example 35.2) and carboxymethylcellulose will form o/w emulsions.
Sterol-containing substances. These agents act as water-in-oil emulsifying agents. Examples include beeswax, wool fat and wool alcohols (see Ch. 36).
Synthetic surfactants
These agents are classified according to their ionic characteristics as anionic, cationic, non-ionic and ampholytic. The latter are used in detergents and soaps but are not widely used in pharmacy.
Anionic surfactants. These are organic salts which, in water, have a surface-active anion. They are incompatible with some organic and inorganic cations and with large organic cations such as cetrimide. They are widely used in external preparations as o/w emulsifying agents. They must be in their ionized form to be effective and emulsions made with anionic surfactants are generally stable at more alkaline pH.
Some pharmaceutical examples of anionic surfactants include:
 Alkali metal and ammonium soaps such as sodium stearate (o/w)
Alkali metal and ammonium soaps such as sodium stearate (o/w)
 Soaps of divalent and trivalent metals such as calcium oleate (w/o) (see Example 35.3)
Soaps of divalent and trivalent metals such as calcium oleate (w/o) (see Example 35.3)
Cationic surfactants. These are usually quaternary ammonium compounds which have a surface-active cation and so are sensitive to anionic surfactants and drugs. They are used in the preparation of o/w emulsions for external use and must be in their ionized form to be effective. Emulsions formed by a cationic surfactant are generally stable at acidic pH. The cationic surfactants also have antimicrobial activity. Examples include cetrimide and benzalkonium chloride.
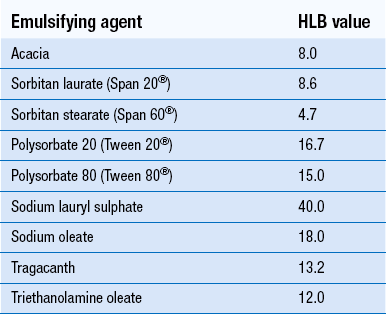
Non-ionic surfactants. These are synthetic materials and make up the largest group of surfactants. They are used to produce either o/w or w/o emulsions for both external and internal use. The non-ionic surfactants are compatible with both anionic and cationic substances and are highly resistant to pH change. The type of emulsion formed depends on the balance between hydrophilic and lipophilic groups which is expressed as the HLB (hydrophilic–lipophilic balance) number (see below). Examples of the main types include glycol and glycerol esters, macrogol ethers and esters, sorbitan esters and polysorbates.
The HLB (hydrophilic–lipophilic balance) system. An HLB number, usually between 1 and 20, is allocated to an emulsifying agent and represents the relative proportions of the lipophilic and hydrophilic parts of the molecule. The lower the number, the more oil soluble the emulsifying agent. Higher numbers (8–18) indicate a hydrophilic molecule which produces an o/w emulsion. Low numbers (3–6) indicate a lipophilic molecule which produces a w/o emulsion. Oils and waxy materials have a ‘required HLB number’ which helps in the selection of appropriate emulsifying agents when formulating emulsions. Liquid paraffin, for example, has a required HLB value of 4 to obtain a w/o emulsion and 12 for an o/w emulsion. Two or more surfactants can be combined to achieve a suitable HLB value and often give better results than one surfactant alone. HLB values of some commonly used emulsifying agents are given in Table 35.1.
Finely divided solids
Finely divided solids can be adsorbed at the oil–water interface to form a coherent film that prevents coalescence of the dispersed globules. If the solid particles are preferentially wetted by oil, a w/o emulsion is formed. Conversely, if the particles are preferentially wetted by water, an o/w emulsion is formed. They form emulsions with good stability which are less prone to microbial contamination than those formed with other naturally derived agents. Examples are bentonite, aluminium magnesium silicate and colloidal silicon dioxide. Colloidal aluminium and magnesium hydroxides are used for internal preparations. For example liquid paraffin and magnesium hydroxide oral emulsion is stabilized by the magnesium hydroxide.
Choosing an emulsifying agent
The active ingredients that are to be emulsified and the intended use of the product will determine the choice of emulsifying agent. The natural polysaccharides (acacia) and non-ionic emulsifying agents are useful for internal emulsions because they are non-toxic and non-irritant. Quillaia can be used in low concentrations, but soap emulsions irritate the gastrointestinal tract and have a laxative effect. The taste should be bland and palatable, for example, natural polysaccharides. Polysorbates have a disagreeable taste, therefore flavouring ingredients are necessary. Only certain non-ionic emulsifying agents are suitable for parenteral use including lecithin, polysorbate 80, methylcellulose, gelatin and serum albumin. A wider range of emulsifying agents can be used externally, although the polysaccharides are normally considered too sticky.
Antioxidants
Some oils are liable to degradation by oxidation and therefore antioxidants may be added to the formulation. They should be preferentially soluble in the oily phase.
Antimicrobial preservatives
Emulsions contain water, which will support microbial growth. Microbes produce unpleasant odours, colour changes and gases. In addition they may affect the emulsifying agent, possibly causing the breakdown of the emulsion. Other ingredients in emulsions can provide a growth medium for microbes. Examples include arachis oil which supports Aspergillus species and liquid paraffin which supports Penicillium species. Contamination may be introduced from a variety of sources including:
Antimicrobial preservative agents should be free from toxic effects, odour, taste (for internal use) and colour. They should be bactericidal rather than bacteriostatic, have a rapid action and wide antibacterial spectrum over a range of temperatures and pH. Additionally emulsion ingredients should not affect their activity and they should be resistant to attack by microorganisms. The effect of the partition coefficient is also important. Microbial growth normally occurs in the aqueous phase of an emulsion; therefore it is important that a sufficient concentration of preservative is present in the aqueous phase. A preservative with a low oil/water partition coefficient will have a higher concentration in the aqueous phase. A combination of preservatives may give the best preservative cover for an emulsion system. The ratio of the disperse phase volume to the total volume is known as the phase volume or phase volume ratio. If, for example, a preservative is soluble in the oil and if the proportion of oil is increased, the concentration of preservative in the aqueous phase decreases. This could reduce the concentration in the aqueous phase below an effective concentration.
Some preservatives in use are listed below:
 Benzoic acid: effective at a concentration of 0.1% at a pH below 5
Benzoic acid: effective at a concentration of 0.1% at a pH below 5
 Esters of parahydroxybenzoic acid such as methyl paraben (0.01–0.3%)
Esters of parahydroxybenzoic acid such as methyl paraben (0.01–0.3%)
 Chloroform, as chloroform water (0.25% v/v)
Chloroform, as chloroform water (0.25% v/v)
 Quaternary ammonium compounds, e.g. cetrimide, which can be used as a primary emulsifying agent but can also be used as a preservative
Quaternary ammonium compounds, e.g. cetrimide, which can be used as a primary emulsifying agent but can also be used as a preservative
 Organic mercurial compounds such as phenyl mercuric nitrate and acetate (0.001–0.002%).
Organic mercurial compounds such as phenyl mercuric nitrate and acetate (0.001–0.002%).
Colours and flavourings
Colour is rarely needed in an emulsion, as most have a white colour and thick texture. Emulsions for oral use will usually contain some flavouring agent.
Stability of emulsions
Phase inversion
This is the process in which an emulsion changes from one type to another, say o/w to w/o. The most stable range of disperse phase concentration is 30–60%. As the amount of disperse phase approaches or exceeds a theoretical maximum of 74% of the total volume, so the tendency for phase inversion to occur increases. Addition of substances which alter the solubility of an emulsifying agent may also cause phase inversion. The process is irreversible.
Creaming
The term ‘creaming’ is used to describe the aggregation of globules of the disperse phase at the top or bottom of the emulsion, similar to cream on milk. The process is reversible and gentle shaking redistributes the droplets throughout the continuous phase. Creaming is undesirable because it is inelegant, and inaccurate dosing is possible if shaking is not thorough. Additionally, creaming increases the likelihood of coalescence of globules and therefore the breakdown of the emulsion due to cracking.
Cracking
Cracking is the coalescence of dispersed globules and separation of the disperse phase as a separate layer. It is an irreversible process and redispersion cannot be achieved by shaking.
Causes and prevention of cracking or creaming
 Globule size. Stable emulsions require a maximal number of small sized (1–3 μm) globules and as few as possible larger (>15 μm) diameter globules. A homogenizer will efficiently reduce droplet size and may additionally increase the viscosity if more than 30% of disperse phase is present. Homogenizers force the emulsion through a small aperture to reduce the size of the globules
Globule size. Stable emulsions require a maximal number of small sized (1–3 μm) globules and as few as possible larger (>15 μm) diameter globules. A homogenizer will efficiently reduce droplet size and may additionally increase the viscosity if more than 30% of disperse phase is present. Homogenizers force the emulsion through a small aperture to reduce the size of the globules
 Storage temperature. Extremes of temperature can lead to an emulsion cracking. When water freezes it expands, so undue pressure is exerted on dispersed globules and especially the emulsifying agent film, which may lead to cracking. Conversely, an increased temperature decreases the viscosity of the continuous phase and disrupts the integrity of the interfacial film. An increasing number of collisions between droplets will also occur, leading to increased creaming and cracking
Storage temperature. Extremes of temperature can lead to an emulsion cracking. When water freezes it expands, so undue pressure is exerted on dispersed globules and especially the emulsifying agent film, which may lead to cracking. Conversely, an increased temperature decreases the viscosity of the continuous phase and disrupts the integrity of the interfacial film. An increasing number of collisions between droplets will also occur, leading to increased creaming and cracking
 Potential for globule coalescence. Increasing the viscosity of the continuous phase will reduce the potential for globule coalescence as this reduces the movement of globules. Emulsion stabilizers, which increase the viscosity of the continuous phase, may be used in o/w emulsions, e.g. tragacanth, sodium alginate and methylcellulose
Potential for globule coalescence. Increasing the viscosity of the continuous phase will reduce the potential for globule coalescence as this reduces the movement of globules. Emulsion stabilizers, which increase the viscosity of the continuous phase, may be used in o/w emulsions, e.g. tragacanth, sodium alginate and methylcellulose
 Changes which affect the interfacial film. These may be chemical, physical or biological effects: microbiological contamination may destroy the emulsifying agent, especially if a polysaccharide emulsifying agent is being used, addition of a common solvent, addition of an emulsifying agent of opposite charge, for instance cationic to anionic
Changes which affect the interfacial film. These may be chemical, physical or biological effects: microbiological contamination may destroy the emulsifying agent, especially if a polysaccharide emulsifying agent is being used, addition of a common solvent, addition of an emulsifying agent of opposite charge, for instance cationic to anionic
Dispensing emulsions
Emulsions can be extemporaneously prepared on a small scale using a mortar and pestle. Electric mixers can also be used, although incorporation of excess air may be a problem. All equipment used must be thoroughly clean and dry. All oil-soluble and water-soluble components of the emulsion are separately dissolved in the appropriate phase. A suitable emulsifying agent must then be used.
Emulsions for oral use
Acacia gum is usually used when making extemporaneous o/w emulsions for oral use, unless otherwise specified. A primary emulsion should be prepared first. This is a thick, stable emulsion prepared using optimal proportions of the ingredients. These vary with the nature of the oil.
Calculating quantities for primary emulsions
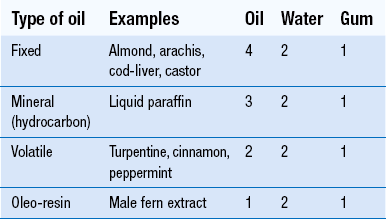
Proportions or ‘parts’ for preparation of primary emulsions are given in Table 35.2. These refer to parts by volume for the different types of oil and water and weight for the acacia gum. If more than one oil is to be incorporated, the quantity of acacia for each is calculated separately and the sum of the quantities used.
Variations to primary emulsion calculations
If the proportion of oil is too small, modifications must be made. Acacia emulsions containing less than 20% oil tend to cream readily. A bland, inert oil, such as sesame, cottonseed or maize oil, should be added to increase the amount of oil.
Methods of preparation of extemporaneous emulsions
There are two possible methods, the dry gum method being the more popular.
Dry gum method of preparation
 Measure the oil accurately in a dry measure. It is important that the measure is dry
Measure the oil accurately in a dry measure. It is important that the measure is dry
 Allow measure to drain into a dry mortar with a large, flat bottom
Allow measure to drain into a dry mortar with a large, flat bottom
 Measure the water for the primary emulsion in a clean measure
Measure the water for the primary emulsion in a clean measure
 Add acacia to the oil and mix lightly to disperse lumps. Do not over-mix, and keep the suspension in the bottom of the mortar
Add acacia to the oil and mix lightly to disperse lumps. Do not over-mix, and keep the suspension in the bottom of the mortar
 Immediately add all of the water (aim to do this within 10–15 s of adding the acacia to the oil) and stir continuously and vigorously until the mixture thickens and the primary emulsion is formed. The mixture thickening, becoming white and producing a ‘clicking’ sound, characterizes this
Immediately add all of the water (aim to do this within 10–15 s of adding the acacia to the oil) and stir continuously and vigorously until the mixture thickens and the primary emulsion is formed. The mixture thickening, becoming white and producing a ‘clicking’ sound, characterizes this
 Continue mixing for a further 2–3 min to produce the white stable emulsion. The whiter the product, the smaller the globules
Continue mixing for a further 2–3 min to produce the white stable emulsion. The whiter the product, the smaller the globules
 Gradually dilute the primary emulsion with small volumes of the vehicle, ensuring complete mixing between additions
Gradually dilute the primary emulsion with small volumes of the vehicle, ensuring complete mixing between additions
 Gradually add any other ingredients, transfer to a measure and make up to final volume with the vehicle.
Gradually add any other ingredients, transfer to a measure and make up to final volume with the vehicle.
Wet gum method of preparation
Water is added to the acacia gum and quickly triturated until the gum has dissolved to make a mucilage. Oil is added to this mucilage in small portions, triturating the mixture thoroughly after each addition until a thick primary emulsion is obtained. The primary emulsion should be stabilized by mixing for several minutes and then completed in the same way as for the dry gum method.
Problems when producing the primary emulsion
The primary emulsion may not form and a thin oily liquid is formed instead. Possible causes are:
 Incorrect quantities of oil or water were used
Incorrect quantities of oil or water were used
 There was cross-contamination of water and oil
There was cross-contamination of water and oil
 The mortar was too small and curved, or the pestle head was too round, giving insufficient shear
The mortar was too small and curved, or the pestle head was too round, giving insufficient shear
 Excessive mixing of oil and gum before adding water (dry gum method)
Excessive mixing of oil and gum before adding water (dry gum method)
 Diluting the primary emulsion too soon
Diluting the primary emulsion too soon
Emulsions for external use
Liquid or semi-liquid emulsions may be used as applications, liniments and lotions (see Ch. 36). The extemporaneous preparation of emulsions for external use does not require the preparation of a primary emulsion. Soaps are commonly used as the emulsifying agent and some are prepared ‘in situ’ by mixing the oily phase containing a fatty acid and the aqueous phase containing the alkali. Alternatively the emulsifying agent can be dissolved in the oily or aqueous phase and the disperse phase added to the continuous phase, either gradually or in one portion.
Creams are semi-solid emulsions which may be o/w (e.g. aqueous cream) or w/o (e.g. oily cream). (These are considered in more detail in Ch. 36.)
Shelf-life and storage
Emulsions should be stored at room temperature and will either be recently or freshly prepared. Some official preparations will have specific expiry dates. They should not be frozen.
Special labelling and advice for emulsions
 ‘Store in a cool place’. This is to protect the emulsion against extremes of temperature which will adversely affect its stability
‘Store in a cool place’. This is to protect the emulsion against extremes of temperature which will adversely affect its stability
Worked Examples
Formulation notes. Cod-liver oil is a fixed oil that requires the addition of acacia gum as an emulsifying agent. The proportions are: 4 oil; 2 water; 1 gum. Therefore 60 mL cod-liver oil, 30 mL of cinnamon water and 15 g of acacia gum will be used to prepare the primary emulsion. Cinnamon water acts as a flavouring agent and vehicle. It may need to be prepared from concentrated cinnamon water, at a dilution of 1 part to 39 parts of water. Since 60 mL of the emulsion is the cod-liver oil, it is not necessary to prepare 200 mL of cinnamon water, so 160 mL is adequate. Therefore, 4 mL of concentrated cinnamon water will be diluted to 160 mL with water. Chloroform is dense and only slowly soluble and acts as a preservative.
Method of preparation. Use the dry gum method. Weigh 15 g of acacia, measure 60 mL of cod-liver oil and 30 mL of cinnamon water, which will be used to create the primary emulsion. Place the cod-liver oil in a dry, flat-bottomed mortar. Add the acacia and mix very lightly and briefly. Immediately add the cinnamon water, mixing vigorously until a clicking sound is heard and a white primary emulsion is formed. Continue mixing for a few minutes to stabilize the primary emulsion. Scrape the mortar and pestle with a spatula to ensure that all the oil is incorporated. Add the chloroform by pipette and mix thoroughly. Gradually add most of the remainder of the cinnamon water to the emulsion in the mortar, stirring well between additions. Transfer the emulsion to a 200 mL measure, rinsing the mortar with cinnamon water, adding these washings to the measure. Make up to volume with cinnamon water and pack in an amber medicine bottle with a child-resistant closure.
Storage. Store in a cool, dry place.
Formulation notes. Methylcellulose 20 at a concentration of 2% acts as an emulsifying agent for the mineral oil, liquid paraffin. A primary emulsion is not required. Benzoic acid and chloroform act as preservatives and vanillin and saccharin sodium act as flavouring and sweetening agent respectively. The amount of saccharin sodium is not weighable on a dispensing balance and will be obtained by trituration using water as the diluent (since this is the vehicle for the emulsion).
Trituration for saccharin sodium:
| Saccharin sodium | 100 mg |
| Water | to 100 mL |
| 5 mL of water will contain | 5 mg of saccharin sodium. |
Method of preparation. First, prepare a mucilage by mixing the methylcellulose 20 with about six times its weight of boiling water and allow to stand for 30 min to hydrate. Add an equal weight (about 15 g) of ice and stir mechanically until the mucilage is homogeneous. Dissolve the vanillin in the benzoic acid solution and chloroform. Add this mixture to the mucilage and stir for 5 min. Make up the saccharin sodium trituration and stir in the appropriate volume of solution to the mucilage. Make the volume of the mucilage up to 50 mL, taking care to ensure that there is no entrapped air in the mucilage. Make the emulsion by mixing together 50 mL of liquid paraffin and 50 mL of prepared mucilage with constant stirring. The emulsion is more stable if passed through a hand homogenizer. Pack in an amber medicine bottle with a child-resistant closure. Shake well to ensure that the emulsion is thoroughly mixed. Polish and label the bottle and give a 5 mL medicine spoon with the medicine.
Storage. Store in a cool, dry place.
Formulation notes. The emulsifying agent for the arachis oil is the soap calcium oleate produced from the calcium hydroxide and oleic acid when they are shaken together. Wool fat is included as an emulsion stabilizer. This is a w/o emulsion.
Method of preparation. The wool fat, oleic acid and arachis oil should be warmed gently together in an evaporating basin (using a water bath or heating block) until melted. Mix them thoroughly. The calamine should be sieved and weighed and placed on a warm ointment tile. Add a little of the oily mixture and rub in with a large spatula until smooth. Gradually add more of the oily mixture until it is fluid. Transfer back to the evaporating basin and stir to evenly distribute the calamine powder. Pour into a previously tared, amber ribbed bottle and add the calcium hydroxide solution to the bottle in small amounts, shaking well between additions. Make up to volume and seal with a child-resistant closure. Polish and label the bottle.
Key Points
 Emulsions may be oil-in-water (o/w) or water-in-oil (w/o)
Emulsions may be oil-in-water (o/w) or water-in-oil (w/o)
 Emulsions may be used orally, externally or by intramuscular and intravenous injection
Emulsions may be used orally, externally or by intramuscular and intravenous injection
 The type of emulsion may be determined by miscibility, conductivity, staining and dye tests
The type of emulsion may be determined by miscibility, conductivity, staining and dye tests
 Emulsifying agents are required to reduce the interfacial tension and act as a barrier between the oil and water phases
Emulsifying agents are required to reduce the interfacial tension and act as a barrier between the oil and water phases
 Naturally occurring emulsifying agents include polysaccharides (acacia), semi-synthetic polysaccharides (methylcellulose) and sterols (wool fat)
Naturally occurring emulsifying agents include polysaccharides (acacia), semi-synthetic polysaccharides (methylcellulose) and sterols (wool fat)
 Synthetic surfactants can be used and are selected using the HLB number
Synthetic surfactants can be used and are selected using the HLB number
 Care is required to avoid anion–cation incompatibilities
Care is required to avoid anion–cation incompatibilities
 Some finely divided solids will stabilize emulsions
Some finely divided solids will stabilize emulsions
 Emulsions require antimicrobial preservation
Emulsions require antimicrobial preservation
 Phase inversion, creaming and cracking are instabilities of emulsions which must be avoided
Phase inversion, creaming and cracking are instabilities of emulsions which must be avoided
 A primary emulsion is prepared when making an emulsion using acacia as the emulsifying agent using either the ‘dry gum’ or ‘wet gum’ method
A primary emulsion is prepared when making an emulsion using acacia as the emulsifying agent using either the ‘dry gum’ or ‘wet gum’ method
 The ratio of oil:water:acacia used for the primary emulsion will vary with the type of oil in the formulation
The ratio of oil:water:acacia used for the primary emulsion will vary with the type of oil in the formulation
 Liquid emulsions should have ‘Shake well before use’ and ‘Store in a cool place’ labels and should not be frozen
Liquid emulsions should have ‘Shake well before use’ and ‘Store in a cool place’ labels and should not be frozen