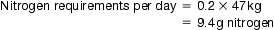Parenteral nutrition and dialysis
 Provision of nutritional support for patients
Provision of nutritional support for patients
 Indications for total parenteral nutrition (TPN)
Indications for total parenteral nutrition (TPN)
 Components and compounding of a TPN/home parenteral nutrition (HPN) formulation
Components and compounding of a TPN/home parenteral nutrition (HPN) formulation
 Addition of medicines to a TPN or HPN bag
Addition of medicines to a TPN or HPN bag
 HPN training and potential problems
HPN training and potential problems
 Administration of a TPN/HPN formulation
Administration of a TPN/HPN formulation
 British Parenteral Nutrition Group and British Association of Parenteral and Enteral Nutrition
British Parenteral Nutrition Group and British Association of Parenteral and Enteral Nutrition
 Introduction to home-care for patients on dialysis
Introduction to home-care for patients on dialysis
 Haemodialysis (HD), peritoneal dialysis (PD), including continuous ambulatory peritoneal dialysis (CAPD), intermittent peritoneal dialysis (IPD) and automated peritoneal dialysis (APD)
Haemodialysis (HD), peritoneal dialysis (PD), including continuous ambulatory peritoneal dialysis (CAPD), intermittent peritoneal dialysis (IPD) and automated peritoneal dialysis (APD)
 Provision of services from a hospital renal unit, including home dialysis
Provision of services from a hospital renal unit, including home dialysis
Introduction
Today, an increasing number of patients are requesting and being provided with healthcare services at home. Such services include the provision of home parenteral nutrition and home dialysis. This chapter will explore the provision of parenteral nutrition and dialysis for patients in hospital and will explain how these services can be transferred to the home-care setting.
Provision of nutritional support
Studies have shown that up to 50% of medical and surgical patients can suffer from nutritional deficiencies. If nutritional support is indicated, enteral feeding should be considered as the first option. Patients can receive nutrients orally or via a tube feed, e.g. by nasogastric feeding. This is only possible if the gastrointestinal tract is functional. If this is not the case, parenteral nutrition may be considered. Short-term (e.g. postoperative) intravenous (IV) administration of fluids, such as 5% dextrose or saline may be sufficient. This could provide the patient with around 500 calories per day but does not provide any protein, vitamins, minerals or trace elements.
Patients who need longer-term nutrition support may require total parenteral nutrition (TPN) which is a method of administering adequate nutrients via the parenteral route. The components of a TPN formulation are added to a sterile infusion bag and administered to the patient via a catheter. Administration can be via a peripheral Venflon, a peripherally inserted central catheter (PICC) or a central line. However, TPN fluids are normally highly concentrated mixtures, which on a long-term basis could cause damage to peripheral veins. For this reason, peripheral veins are only used for TPN administration lasting up to 4 weeks.
If parenteral nutrition is supplied to patients at home, it is known as home parenteral nutrition (HPN) and is more likely to be provided by a commercial company than a hospital TPN unit. Patients on HPN administer their nutrition via a central line into a central vein.
Parenteral nutrition formulations are prepared under strict aseptic conditions (see Ch. 40) following guidelines published by the MHRA in Rules and Guidance for Pharmaceutical Manufacturers(2002) and by the DH in Aseptic Dispensing for NHS Patients (Farwell 1995).
HPN is becoming increasingly prevalent. Guidelines have been published by the British Association of Parenteral and Enteral Nutrition (BAPEN) and the National Institute for Health and Care Excellence (NICE) to ensure that adequate provision is made for patients receiving HPN. Patients who are suitable candidates for HPN will be, initially, stabilized on TPN bags while in hospital. They can then undergo appropriate training to enable them to administer their TPN bags at home. If the patient is unable to care for their line, then a carer or nurse would be trained to administer the TPN at home. However, HPN patients will still need to return to the hospital for regular check-ups. This means that pharmacists involved in the care of HPN patients will require a working knowledge of the procedures adopted to provide care for patients in hospital and at home. They may also have to liaise with the patient’s GP and the community nurse.
This chapter concentrates on the provision of adult TPN, although neonatal TPN is available.
Indications for TPN
TPN can be required for finite periods of time or for life. Some of the main indications for TPN are:
 Gastrointestinal disease, including Crohn’s disease, ulcerative colitis, pancreatitis and malabsorption syndrome
Gastrointestinal disease, including Crohn’s disease, ulcerative colitis, pancreatitis and malabsorption syndrome
 Major trauma including severe burns, severe septicaemia
Major trauma including severe burns, severe septicaemia
 Major abdominal surgery; severely malnourished patients may benefit from early peri and postoperative parenteral nutrition if surgery has resulted in a non-functioning gastrointestinal tract
Major abdominal surgery; severely malnourished patients may benefit from early peri and postoperative parenteral nutrition if surgery has resulted in a non-functioning gastrointestinal tract
 Radiation enteritis, when TPN is considered if enteritis is severe after treatment of a primary malignancy
Radiation enteritis, when TPN is considered if enteritis is severe after treatment of a primary malignancy
 High-dose chemotherapy, radiotherapy and bone marrow transplantation. Patients are often ill for a limited time (3–6 weeks) and are unable to eat. TPN can be administered during this period to ensure that the patient’s nutritional requirements are adequately met.
High-dose chemotherapy, radiotherapy and bone marrow transplantation. Patients are often ill for a limited time (3–6 weeks) and are unable to eat. TPN can be administered during this period to ensure that the patient’s nutritional requirements are adequately met.
Several other conditions may require the nutritional support of TPN, e.g. patients in a prolonged coma or AIDS patients.
Assessment of the patient in hospital
TPN aims to provide patients with all their nutritional requirements in one formulation which can then be infused directly into the body via the veins, either central or peripheral. In order to determine exactly what the patient’s nutritional requirements are, clinical and biochemical assessments must take place. The Malnutrition Universal Screening Tool (MUST) is used to identify patients who may benefit from TPN. The patient’s body weight, height and body mass index (BMI) can be recorded and comparison made with their ideal body weight which would be available from standard charts. In most hospitals a dietitian would review the patient and calculate their nutritional requirements.
Factors investigated will include urea and electrolytes, full blood counts, liver function tests, triglycerides, blood glucose and fluid balance. Trace elements are only monitored if the patient receives TPN for longer than 28 days. The NICE guidelines for nutrition support contain a section on the monitoring required for TPN patients.
Each hospital has its own particular way of designing a TPN regimen. Most hospitals use a range of standard formulations which are routinely used to treat TPN patients. Standard bags can be altered if the need arises. In general, additions to the finished TPN bags outside of the pharmacy aseptic unit are not recommended in order to minimize microbial contamination.
More recently, pharmaceutical companies have introduced a range of three-in-one ready-to-use multi-chambered TPN bags. These bags have three chambers, which contain amino acid, dextrose and lipid. When a bag of TPN is required, the seal separating the chambers can easily be broken and the three solutions are mixed together in one chamber. Before mixing, these bags have a long expiry date of around 2 years and do not need to be stored in the fridge. Many hospitals have swapped to using these bags as they are cost-effective and reduce the time for manufacture. Trace elements and vitamins need to be added to these bags before use.
Some hospitals tailor regimens to individual patients and carry out a number of calculations to determine baseline requirements for each component. In this way they can build up a formulation by matching up the patient’s requirements to commercially available solutions which contain the required components in the correct proportions. Individualized bags tend to be used in patients on long-term TPN. Patients on HPN will always have bags tailored exactly to their nutritional needs.
The nutrition team
In most hospitals where TPN is supplied, there will be a nutrition team to coordinate the delivery of the parenteral nutrition service. This team can include the following:
The role of these individuals in provision of patient care can vary from one hospital to another. In general, the consultant is responsible for prescribing the TPN formulation and liaising with the patient’s GP to provide care for HPN patients, although with the introduction of non-medical prescribing, this role is increasingly taken over by nurses and pharmacists.
The pharmacist can provide information on aseptic techniques for handling and setting up TPN bags, formulation requirements, potential complications or stability problems, and storage conditions required. In some hospitals, the pharmacist’s role can be extended to include the following:
 Training nursing staff in the techniques required for IV administration of TPN fluids
Training nursing staff in the techniques required for IV administration of TPN fluids
 Helping with patient training for HPN
Helping with patient training for HPN
 Monitoring of patients in HPN clinics
Monitoring of patients in HPN clinics
 Liaising with the staff from the home-care company
Liaising with the staff from the home-care company
The nutrition nurse and dietitian will together give advice on a day-to-day basis regarding the nutritional status of the patients and will advise on necessary dietary requirements.
Commercial companies supplying home-care services have a nutrition nurse who provides medical care, support and advice (on a 24-hour basis if required), a patient coordinator who deals with the ordering of HPN bags and ancillaries, and a designated delivery person who will supply the necessary equipment and HPN bags to the patient’s home.
In the rare circumstances that the HPN is supplied by the hospital pharmacy, patients can be provided with the support from the hospital and their GP.
Components of a TPN formulation
TPN formulations can contain the following components:
Baseline water requirements
Water accounts for over 50% of body weight. To prevent patients becoming dehydrated, daily water losses and gains must be carefully considered. Water can be lost through urine and faeces and through ‘insensible losses’, i.e. through the skin and lungs. Patients with burns and gastrointestinal losses will require increased volume. Patients with renal and cardiac failure should be given reduced volumes.
Several methods are available for estimating daily fluid requirements, but most take into consideration body weight and measured urine output, and an allowance is made for insensible losses. The average adult requires between 1500 and 4000 mL of fluid per day. A TPN regimen will need to provide this volume of fluid on a daily basis.
Protein source
Protein requirements vary from one patient to another and are highly dependent on the metabolic status of the patient. Undernourished patients requiring parenteral nutrition are, generally, said to have a negative nitrogen balance. This means that the amount of nitrogen excreted in urine and faeces is greater than the amount of nitrogen administered.
Lack of nitrogen in the body can result in poor wound healing and interference with the body’s defence mechanisms. To overcome this problem, a utilizable source of nitrogen must be administered to the patient. This is achieved by administering amino acid solutions in a TPN formulation. Nitrogen requirements can be estimated from a 24-hour urine collection. This is done by analysing the total amount of urea excreted and by considering the individual patient’s body weight and clinical ‘type’.
Energy sources
Carbohydrates and fats are chosen to provide optimal energy sources for TPN patients. The relative proportions of each will be dependent on the clinical requirements of the patient and formulation considerations. The carbohydrate of choice is normally dextrose and is available in solution with concentrations ranging from 5% to 70% weight in volume (w/v). Like amino acid solutions, dextrose solutions are hypertonic and have a low pH (3–5). If high concentrations of dextrose are added to the TPN bags, they must only be given centrally.
The fat component in a TPN formulation is administered in the form of an oil-in-water emulsion. Fat emulsions are isotonic with plasma, have neutral pH and provide a high calorie source in a low volume. As a result, they are often used in combination with dextrose to provide the necessary calorie content, thereby avoiding the potential problems encountered with excessive dextrose administration.
Fat emulsions provide the patient with essential fatty acids and also act as a vehicle for fat-soluble vitamins which are required in the TPN formulation. Fat is not required in every TPN formulation, but fat deficiency can occur in patients who do not receive fat components for periods longer than 1 month.
Commercially available preparations are based on soya bean oils and are composed of varying combinations of long and medium chain triglycerides. Newer fat solutions have been developed, incorporating olive oil and fish oils, which are claimed to protect patients on long-term parenteral nutrition from complications. Larger and longer trials are required to prove these claims. The energy content of commercially available solutions for both carbohydrates and fats is expressed in kcal/L, e.g. Intralipid 10% provides 550 kcal/500 mL; dextrose 5% provides 210 kcal/500 mL.
Electrolytes
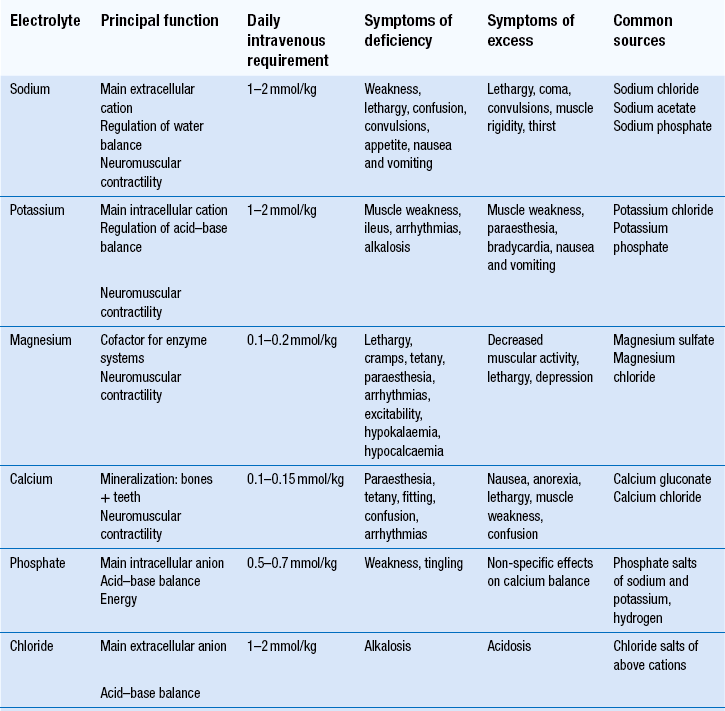
The main electrolytes of clinical significance in a TPN formulation include sodium, potassium, magnesium, calcium, phosphate and chloride. The requirement for electrolytes can be met in the form of injectable solutions of varying percentage content. Electrolyte content of each is expressed in terms of mmol/L. The individual role of each electrolyte in a TPN formulation is given in Table 44.1.
Trace elements
Trace elements act as metabolic cofactors and are said to be essential for the proper functioning of several enzyme systems in the body. Despite being termed essential, they are only required in very small quantities, expressed in micromoles. The main trace elements required in a TPN formulation are zinc, copper, manganese and chromium.
Vitamins and minerals
Vitamin requirements fall into two categories: fat soluble and water soluble. Four fat-soluble vitamins (vitamins A, D, E and K) and nine water-soluble vitamins (vitamins B1, B2, B3, B5, B6, B12, C, folic acid and biotin) are said to be essential.
Vitamins and minerals are normally included in foods taken in orally and must therefore be included in TPN formulations for patients on long-term parenteral nutrition. The NICE guidelines published in 2006 recommend that patients must receive vitamins and trace elements daily in their TPN bags.
Compounding of TPN and HPN formulations
Compounding can take place within a hospital pharmacy using aseptic dispensing facilities within a clean room or within a designated compounding unit in a commercial pharmaceutical company.
Preparation and training
For patients in hospital, a suitable TPN regimen will be prescribed. On receipt of the prescription, the pharmacist checks the suitability and compatibility of the formulation, the required volume of each component is calculated and details are transferred to a worksheet. Patient details are entered into a computer and labels generated for the worksheet and the final product. In the preparation area, items required for the compounding process are collected together in an appropriate tray ready for transfer to the clean room facility. Batch numbers and expiry dates for each product used are recorded on the worksheet. The pharmacist checks all details, including calculations, before the compounding procedure begins.
Compounding of a TPN formulation is carried out under strict aseptic conditions (in a Grade A environment) using a laminar airflow (LAF) cabinet within a clean room facility. Chapter 40 gives details regarding clean room facilities, gowning-up procedures for entry to clean rooms and working procedures for using LAF cabinets. SOPs should be available for all staff carrying out aseptic dispensing procedures.
TPN/HPN bags
The components of a TPN formulation are sterile and are prepared under sterile conditions as the formulation is eventually infused directly into the bloodstream of the patient. It is therefore essential that the bags used to hold the TPN formulation are also sterile. In the past, only polyvinyl chloride (PVC) bags were used for TPN formulations. However, because of the problems of leaching of plasticisers from PVC bags containing a fat component, ethylvinyl acetate (EVA) bags (which contain no plasticizers) are now recommended. However, EVA bags have been shown to be permeable to oxygen; hence multilayer EVA bags are now available for formulations requiring prolonged storage.
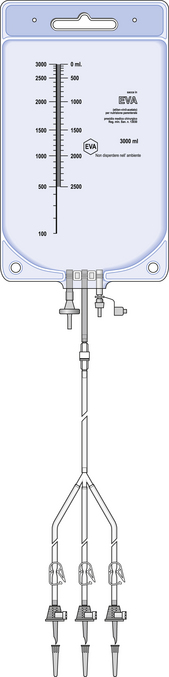
Bags are usually supplied with a pre-mounted sterile filling set attached. The filling set consists of a number of hollow plastic tubes (up to six) with a plastic spike attached to the end of each. The spikes are used to pierce the rubber septum of the bottles and bags of amino acids, glucose and fat emulsion to enable filling of the components into the TPN bag. Clamps fitted with air vents are attached to each filling tube to clamp off the source bottles and bags when they are empty. Filling sets are used for compounding purposes only and are disconnected and replaced with a sterile hub before being sent out to the patient. Every HPN bag is supplied with a sterile giving set which allows the bag to be infused into the patient.
TPN bags vary in size, ranging from small 250 mL bags used for neonatal TPN up to 4 L bags for adult TPN. Figure 44.1 shows a TPN bag with filling set attached.
Addition of components to a TPN bag
Components are added into the TPN bag in a strictly defined procedure. Small-volume additives can be added directly into large-volume fluids (but not directly into the fat component) or directly into the additive port on the bag (depending on manufacturers’ recommendations). Amino acid solutions and glucose are added into the bag first, followed by any fat emulsion if required. To prevent precipitation of vitamins, they are, generally, only added immediately before administration.
Filling of the TPN bags can be achieved under gravity. The bag is placed on the floor of the LAF cabinet and the solution components suspended from a retort stand, enabling the solution to flow freely into the bag. If several bags require to be compounded in a limited time period, the bag can be placed in a vacuum chamber to speed up the filling process. Electronic devices, known as compounders, are also available. They are usually under microprocessor control and can be pre-programmed to fill TPN bags with set volumes of individual components.
When all the components are added, the bag can be clamped off and the filling set removed. The bag is gently shaken to ensure adequate mixing of all components. The TPN bag and compounding materials are transferred back to the preparation area. A visual inspection of the bag is made, including checking of the additive port, for integrity. All necessary documentation is completed and the TPN bag is labelled. Details to be included on the label are shown in Box 44.1.
The TPN bag is then sealed into a dark-coloured outer plastic bag (to protect the formulation from light) and an outer label that is identical to the label on the bag itself is attached.
To maintain stability of the formulated product, it is refrigerated until required. All TPN and HPN formulations must be stored in a designated pharmaceutical grade refrigerator. Coolboxes packed with ice packs can be used for transportation of formulations to the ward or the patient’s home.
Compounding of HPN formulations by commercial companies
A designated compounding unit is used for preparing HPN formulations. Conditions used will be the same as those used in the hospital sector and the same government regulations apply. The compounding unit must hold a manufacturing licence prior to supplying TPN bags (see Ch. 4).
The commercial company providing the home-care service must be in receipt of a prescription for the HPN formulation prior to compounding.
However, when the health care is transferred to the home-care setting in Scotland, the patient’s GP will take on the responsibility for supplying the HPN prescription. In England and Wales, the primary care commissioner is responsible for providing the HPN prescription, although with the current changes to the NHS in England and Wales the responsibilities may change. Subsequent prescriptions will then be forwarded to the commercial company in advance of the patient’s requirements. The hospital nutrition team will issue the prescriptions for the patients at home. The patient coordinator will deal with orders for sundries and ancillaries such as pumps, dressings, needles, etc.
Potential complications arising during compounding and administration of TPN formulations
The components of a TPN formulation will individually and collectively contribute to the overall stability of the resulting formulation. However, with several hospitals now using standard TPN formulations, many of these problems can be overcome. For hospital pharmacies which have a manufacturing licence, standard bags can be made up in advance of requirements and stored in a refrigerator for periods of 30 days or more. The shelf-life given to individual formulations must be based on validated stability studies previously carried out on the formulation. The stability of any regimen will be confirmed before manufacture.
Individual components of the formulation such as vitamins, electrolytes and fat can cause formulation complications. Vitamin stability is very poor, particularly in the presence of light and with extended storage time. Stability is also affected by solution pH, hence the need for careful consideration of the overall formulation.
The requirement for administration of calcium and phosphate in a formulation can lead to precipitation of calcium phosphate. This reaction is said to be affected by factors such as the relative amounts of each component present, solution pH, concentration of amino acid solutions present and the mixing process used. To overcome this type of problem, manufacturers of parenteral nutrition fluids can supply tables which give details of the amount of each component that can be safely combined to ensure stability of the formulation is maintained. These tables are specific to an individual formulation and details cannot be interchanged between formulations.
The presence of fat in a TPN formulation can cause stability problems. As storage time increases, the fat component of the formulation becomes less stable, resulting in a process of ‘cracking’ where the oil and water phases of the emulsion separate out. If the formulation is administered to the patient in this unstable condition, this can lead to potentially dangerous fat deposits arising in the lungs and other body tissues.
The factors a pharmacist must consider when formulating a TPN bag with a fat component are:
Addition of medicines to a TPN or HPN bag
Stability studies have been carried out on a number of medicines to determine their compatibility and stability in a TPN bag. So far, studies have confirmed the suitability of only a limited range of medicines which includes: heparin, insulin, aminophylline, cimetidine, famotidine, ranitidine and certain antibiotics. Reference to manufacturers’ literature and compatibility studies will provide current recommendations. Although stability is available on the drugs listed above, the addition of drugs to TPN is not recommended. This is because the drugs may affect the long-term stability of the TPN bag and it will affect the pharmacokinetics of the drugs added to the TPN. Most hospitals in the UK allow no additions to the TPN bags.
Administration of TPN/HPN formulations
For patients requiring TPN for longer than 4 weeks, central venous access is required. During their stay in hospital, patients have a catheter inserted into the subclavian vein under anaesthesia. It has an exit site on the lower chest wall, allowing patients easy access for care of the catheter site.
Catheters can be made of materials such as polyvinyl chloride or silicone. For long-term feeding, a permanent catheter (a Hickman catheter or a Portacath) is used. It is held in place by a Dacron cuff (an internal woven plastic used to connect arteries and veins under the skin). Good aseptic techniques are essential to ensure that the catheter site does not become contaminated. Infection around the catheter site can be difficult to treat successfully and may eventually result in removal of the catheter and replacement at another site.
Catheter sites should only be used for administration of TPN fluids and not for blood sampling or administration of other medicines. In some instances, a triple lumen catheter can be used with one line being kept for administration of the TPN bag only. To infuse the TPN formulation into the patient, the catheter is connected via an extension set to a volumetric infusion pump. These devices use positive pressure as the driving force to allow accurate infusion at pre-set rates (see Ch. 41).
The infusion period varies from 24 h in hospital to around 12–14 h for home patients (as HPN can often be administered overnight). Most pumps now have the ability to be programmed to give an infusion rate which ‘steps up’ at the beginning and ‘steps down’ at the end of the infusion period, avoiding potential problems with high concentrations of dextrose in the formulation. They are also fitted with an alarm which will alert the patient if a technical fault arises.
Potential problems for HPN patients
Mechanical problems
Problems of pneumothorax, or air embolism, are more likely to occur in the hospital environment in the early stages of catheter placement and are dealt with before the patient commences on HPN. However, daily connection and disconnection of the catheter hub may result in cracking and possible leakage of the HPN fluid. Repair kits are available, and if used promptly when the problem first arises, catheter replacement may not be necessary.
Internal line blockage of the catheter can arise. Patients are taught to flush out the catheter port with heparinized saline to prevent thrombus formation. Blockage of the line arising during administration of the HPN fluid can cause changes in flow rate which are recognized by the pump, and the alarm is activated.
Metabolic problems
Metabolic complications include:
 Problems with electrolyte levels leading to conditions such as hypernatraemia or hyponatraemia
Problems with electrolyte levels leading to conditions such as hypernatraemia or hyponatraemia
 Problems with glucose levels leading to hyperglycaemia or hypoglycaemia
Problems with glucose levels leading to hyperglycaemia or hypoglycaemia
 Balancing of fluid intake (to ensure adequate hydration is achieved)
Balancing of fluid intake (to ensure adequate hydration is achieved)
 Altered liver enzymes which can be resolved by amending the TPN prescription
Altered liver enzymes which can be resolved by amending the TPN prescription
The majority of the metabolic complications which can affect HPN patients can be overcome by careful monitoring of the patient initially in hospital and with regular check-ups and home visits by the nutrition nurse.
Catheter-related complications
Catheter-related infections can arise as a result of poor management of the catheter exit site. Infection is distinguished by pain, redness and tenderness around the site and rigors when feeding through the line. To minimize such infections, staff are trained to use strict aseptic procedures when changing TPN bags and use of the catheter port is restricted to administration of the TPN bag only. HPN patients are taught the same aseptic techniques and are required to carry out these procedures at all times when changing bags at home. Home-care patients are also taught to be aware of their own physical condition and to be alert to any deterioration in their medical condition at the earliest possible time. Patients are asked to contact their nutrition nurse if they experience any signs or symptoms of infection around the catheter site.
Psychological and social problems
Patients receiving TPN in hospital or at home must learn to adapt to the changes occurring in their lifestyle. Some patients have, over a prolonged period of time, suffered from a general deterioration in their health and as a result adapt well to the initiation of parenteral nutrition as it improves their quality of life. Other patients require TPN as a result of major trauma and these patients find the dramatic changes in their lifestyle very difficult to cope with.
While in the hospital receiving treatment, patients have the constant support of medical and nursing staff to help them to cope with any practical difficulties encountered. The clinical psychologist will review many patients before discharge and coping strategies will be discussed. The ability of patients to adapt to HPN is highly dependent on a number of factors:
 Patient’s underlying medical condition
Patient’s underlying medical condition
 Physical ability and capability of the patient
Physical ability and capability of the patient
 Training and counselling prior to leaving hospital
Training and counselling prior to leaving hospital
 Home circumstances, particularly support from family members and the patient’s GP
Home circumstances, particularly support from family members and the patient’s GP
 Ability to deal with physical and emotional changes in lifestyle, e.g. dependence on others.
Ability to deal with physical and emotional changes in lifestyle, e.g. dependence on others.
 Disruption to normal sleeping pattern during administration of the HPN bag overnight and loss of ‘social’ eating can be difficult for many patients, particularly in the initial stages of HPN.
Disruption to normal sleeping pattern during administration of the HPN bag overnight and loss of ‘social’ eating can be difficult for many patients, particularly in the initial stages of HPN.
To enable a smooth transition from hospital to home to be achieved, patients require the services of the nutrition nurse and other healthcare workers to teach them the necessary skills required for handling, setting up their HPN bags and disconnecting them once the procedure is complete.
Training for HPN patients
Health care which can be provided at home has a number of advantages. Patients have a better quality of life and can become more independent as their confidence in providing self-care increases. However, motivation and confidence to carry out the required manipulations at home are essential. Thus, training in the hospital environment is required to build up the necessary skills and techniques.
A discharge plan is required for each patient working towards home-care. The British Association for Parenteral and Enteral Nutrition (BAPEN), a registered charity formed in 1992, has laid down guidelines for the provision of nutritional care at home. Individual hospitals will develop their own guidelines based on the advice given by BAPEN. The scope of BAPEN includes guidelines on the following matters:
 Details which should be included in a patient discharge plan
Details which should be included in a patient discharge plan
 Knowledge and practical skills which must be achieved by patients prior to discharge
Knowledge and practical skills which must be achieved by patients prior to discharge
 Guidelines for GPs on the provision of HPN
Guidelines for GPs on the provision of HPN
 Advice on how to liaise with the patient’s GP to ensure that everyone is aware of their responsibilities
Advice on how to liaise with the patient’s GP to ensure that everyone is aware of their responsibilities
 Information regarding the supplier of the HPN bags and equipment and how this service will be provided
Information regarding the supplier of the HPN bags and equipment and how this service will be provided
 Details of appropriate people who patients can contact for advice and help with any problems they have.
Details of appropriate people who patients can contact for advice and help with any problems they have.
The length of time required for training can vary depending on the patient’s underlying medical condition and personal approach to training. Patients must be taught aseptic techniques and the importance of ensuring that they are carried out correctly. They must demonstrate their skills and competence on several occasions prior to leaving the hospital. Areas covered during the training period include:
 Aseptic techniques for setting up and disconnecting the HPN bag
Aseptic techniques for setting up and disconnecting the HPN bag
 How to deal with problems of the catheter blocking
How to deal with problems of the catheter blocking
Information booklets on HPN and educational videos can be used with patients to reinforce the training received in hospital.
Services provided by home-care companies
Patients receiving home-care will require certain practical arrangements to be put in place before HPN can be initiated. Home-care companies who provide services to HPN patients normally provide the following items for patient use: a refrigerator for storing HPN bags; a trolley for patients to set up their HPN bags aseptically; a drip stand and an infusion pump. Patients are required to have adequate storage space to keep any extra components which may be required for HPN administration and easy access to hand washing facilities for use prior to setting up their HPN bag. A home assessment will be completed by the home-care company and a nutrition nurse from the hospital before discharge to ensure the patient’s home circumstances are suitable for HPN.
Support services provided for HPN patients
Patients will be metabolically stable prior to transfer to the home-care setting, hence frequency of monitoring will be reduced to a minimum. Patients can have monthly check-ups at the hospital initially, reducing to 3-monthly as they adapt to life on HPN. During visits, patients may be seen by the multidisciplinary nutrition team and reviewed by each member of the team. The pharmacist on the team will arrange any changes in the patient’s TPN prescription. Routine monitoring can be carried out during these visits, including the following:
 Checking the patient’s underlying medical condition
Checking the patient’s underlying medical condition
 Reviewing the patient’s nutritional status, particularly in relation to their weight
Reviewing the patient’s nutritional status, particularly in relation to their weight
 Routine haematological and biochemical tests
Routine haematological and biochemical tests
The nutrition nurse will make home visits if required to check on aseptic techniques and any practical difficulties being encountered by patients and/or their partner or carer.
Patients on HPN can benefit from the support of others undergoing nutrition therapy at home. This is made possible by an organization called ‘PINNT’ (Patients on Intravenous and Nasogastric Nutrition Therapy). This charitable organization aims to support and bring together people who have similar medical conditions and could benefit from the moral support of others who understand the problems they face. PINNT provides practical help in areas such as provision of portable equipment for people on HPN who wish to go on holiday, help with holiday arrangements, including appropriate travel insurance and general advice on benefits available to HPN patients. A newsletter is produced on a regular basis and close links are kept between PINNT and BAPEN to ensure that patient needs are adequately met.
The British Parenteral Nutrition Group
Pharmacists in the UK can keep up-to-date with the working of organizations like PINNT and BAPEN by joining the British Parenteral Nutrition Group (BPNG). Currently, BPNG has a large membership, most of whom are hospital pharmacists working in the NHS. However, membership also includes dietitians, nutrition nurses, research workers and members of commercial companies who work in the field of TPN and HPN. The BPNG exists to further the practice of TPN through a number of activities including research, contributing to the work of BAPEN and arranging symposia on practical and scientific developments in the field. This group is also one of five constituent groups which make up BAPEN.
Introduction to kidney disease and dialysis therapy
In December 2010, there were over 50 000 adult patients receiving renal replacement therapy in the UK. Patients requiring renal replacement therapy (RRT) have end-stage renal disease/failure (ESRF) which may have occurred acutely or may be the result of chronic kidney disease. Transplantation is the most common treatment modality (48%) with haemodialysis accounting for 44% and peritoneal dialysis 8% of RRT. Home therapies make up 9% of RRT. Home therapy was used by 17.6% of prevalent dialysis patients in 2010, with 2.9% on home haemodialysis and the remaining patients having either continuous ambulatory peritoneal dialysis or automated peritoneal dialysis. While still small in number, more patients are now receiving home haemodialysis, with numbers increasing by 23% since 2009, from 636 patients to 780 patients.
Chronic kidney disease
Chronic kidney disease (CKD) is relatively common, affecting approximately 1 in 10 people in the general population. The most common cause is diabetes. Glomerulonephritis and hypertension are also responsible and less common causes include pyelonephritis. CKD may also be inherited, e.g. polycystic kidney disease. Some common drug therapies may also lead to kidney disease.
ESRF is the result of progressive kidney disease, which leads to an irreversible and life-threatening loss of function. Patients with ESRF may be suitable for RRT or may choose conservative treatment. There are a number of types of RRT, e.g. kidney transplantation, haemodialysis or peritoneal dialysis. Unfortunately, over 30% of patients are unsuitable for transplantation and for a number of patients, a suitable donor may not be found. For these patients, transplantation may not therefore be an option and chronic RRT is required. For most patients with ESRF who wish to have RRT, there are two choices, either long-term haemodialysis (HD) or peritoneal dialysis (PD) therapy, although patients may not be suitable for both modalities and may change from one to the other at various times according to need.
RRT with dialysis replaces only some of the functions of the kidneys and is an artificial method of filtering toxins and breakdown products from the blood. It does not replicate normal renal function and does not provide any of the metabolic functions of the kidney such as insulin metabolism or the hormonal functions such as erythropoietin production. RRT with HD or PD uses a combination of dialysis therapy to remove unwanted solutes by the process of diffusion and haemofiltration and ultrafiltration to remove water.
Epidemiology
In 2010, the incidence rate in the UK was 107 per million population. The median age of all incident patients was 64.9 years and for non-whites 57.1 years. Diabetic renal disease is identified as the single most common cause in 24% of patients. There is no identified relationship between social deprivation and presentation pattern according to the renal registry data.
Dialysis
Dialysis is commenced to treat, or to prevent, life-threatening hyperkalaemia, acidosis or hypervolaemic pulmonary oedema or to treat complications of CKD, e.g. pericarditis, uraemic neuropathy or seizures.
Haemodialysis
HD is a process where blood is filtered to remove waste products. The patient is connected to a dialysis machine where blood is removed from the patient’s body and filtered by passing it over an artificial semi-permeable membrane into dialysis fluid. The waste products are retained within the dialysis fluid and the blood returned into the body.
To facilitate HD, access to the patient’s bloodstream must be established, either using a surgically created arteriovenous fistula, where an artery is joined to a vein during a minor surgical operation, a graft, and the join between the artery and vein is made using a synthetic tube, or by inserting a permanent or temporary central vascular catheter into a large vein, such as the subclavian, jugular or femoral vein.
HD usually takes 3–4 hours each time and will be required, on average, three times a week for most patients. The blood is removed and passed over a membrane with a large surface area to allow solutes to be exchanged between the blood and dialysis fluid. Dialysis membranes are sterile, disposable membranes made of cellulose or polycarbonate materials. Pressure is applied to the blood in the machine to induce an ultrafiltration process and allow removal of excess water in addition to the removal of toxins.
Dialysis fluid is composed of similar constituents to plasma:
To promote potassium removal from the blood, the dialysate potassium concentration is variable and is usually lower than that in the plasma. To prevent the blood clotting in the dialysis circuit, unfractionated heparin, low molecular weight heparin or prostacyclin may be used.
During the HD process, there are a number of potential complications such as low blood pressure, air embolus and blood loss.
HD may be carried out in a variety of settings providing the appropriate equipment and water supply is available. Locations include specialist hospital units or in the patient’s own home. The dialysis process follows the same principles in all settings.
Hospital-based haemodialysis
Patients occasionally have direct responsibility for their treatment; however, it is more common for the dialysis to be managed by a team of doctors, nurses and other healthcare professionals. Patients travel to the unit for HD according to an arranged schedule.
In a satellite unit, patients sometimes play a more active role in their treatment. They are supervised by trained staff but may prepare the dialysis machine or carry out the dialysis process themselves.
Home haemodialysis
Home HD may be suitable for a limited number of patients. NICE (TAC 48) recommends that all suitable patients should be offered home HD.
There are a number of advantages to home HD:
 Greater independence for the patient
Greater independence for the patient
 Improved blood pressure control
Improved blood pressure control
 Lower hospital admission rates
Lower hospital admission rates
There are a number of factors that determine a patient’s suitability for home HD. For example, patients must be able and motivated to learn and perform dialysis at home and be capable of maintaining and monitoring their own treatment observations. They must be medically stable and be free of complications that make dialysis difficult. Patients also require good functioning vascular access, support from family or carers and suitable space and facilities must be available. Any patients considered suitable for home HD will be assessed, including their home circumstances. They will undergo a comprehensive training programme to develop skills and techniques in addition to developing confidence and self-reliance.
Peritoneal dialysis
In PD, the dialysis fluid is passed directly into the patient’s body and, in contrast to HD, no blood removal occurs. The peritoneal membrane which lines the abdominal cavity has a large surface area and a good capillary blood supply. It is this semi-permeable membrane that is used to perform PD and allows excess water and waste products to be removed from the blood.
Dialysis fluid is instilled into the peritoneal cavity through a surgically inserted indwelling catheter, which goes through the abdominal wall. The distal end of the catheter has tiny holes in it to allow the dialysis fluid to flow freely into the peritoneal cavity. Fluid is removed from the blood by ultrafiltration down an osmotic pressure gradient. Solutes and toxins cross the peritoneal membrane through diffusion and solvent drag with water.
There are two main methods of PD: continuous ambulatory peritoneal dialysis (CAPD) and automated peritoneal dialysis (APD).
In CAPD, patients generally carry out three or four PD exchanges every 24 h and this is the most common form of home dialysis. In APD, patients are connected to a machine for 8–12 h, often overnight. The machine utilizes a pump delivery system which warms the dialysis fluid prior to administration and delivers a carefully programmed volume of dialysis fluid which exchanges throughout the infusion period. The home patient or a carer will set the machine every night by connecting it to the catheter. This method of dialysis has advantages for the patient as it allows freedom from dialysis during the day.
A variety of dialysis fluids are available and each patient will be prescribed a specific tailored regimen of dialysis fluids. The volume will be determined in part by the available abdominal space. For adults, the range is 1–7 L.
The composition of the dialysate consists of sodium, calcium, glucose or dextran to increase or decrease osmolality.
The dialysis exchange requires strict aseptic technique and a number of different systems may be employed. The most popular is a disconnect system. Dialysis fluid is warmed to body temperature and both this and a drainage bag are attached to the abdominal catheter. Fluid is drained out from the abdominal catheter into the empty bag and new dialysate is instilled from the warmed bag. The bags are then disconnected and the fluid left in place for 4–8 h. The dialysate in the abdominal cavity drains in and out under gravity and by capillary blood flow.
Community dialysis teams
Community dialysis teams provide support to patients undertaking dialysis at home – both HD and PD. Most teams are multidisciplinary with highly trained medical and nursing staff making decisions regarding the treatment and providing the care and support through regular home visits to monitor patients. The team will usually have strong links with the wider multidisciplinary team, which includes dietitians, pharmacists, renal technicians and social workers.
Each member of the renal team will have specific responsibilities:
 The medical and nursing team will be involved with prescribing of dialysis programmes and clinical monitoring
The medical and nursing team will be involved with prescribing of dialysis programmes and clinical monitoring
 The dietitian advises on nutritional intake and any dietary restrictions required
The dietitian advises on nutritional intake and any dietary restrictions required
 The pharmacist provides medicines advice and may have a role in the ordering and supply of dialysis fluids and ancillary products
The pharmacist provides medicines advice and may have a role in the ordering and supply of dialysis fluids and ancillary products
 The social worker provides advice and practical help for patients
The social worker provides advice and practical help for patients
 The renal technician is responsible for the programming, servicing and functioning of dialysis machines.
The renal technician is responsible for the programming, servicing and functioning of dialysis machines.
UK Renal Pharmacy Group
The UK Renal Pharmacy Group (UKRPG) is affiliated to the British Renal Society and is a specialist interest group for pharmacists and pharmacy technicians working in the field of renal medicine or with an interest in renal pharmacy. The UKRPG uses its clinical pharmacy experience to compile The Renal Drug Handbook and Introduction to Renal Therapeutics; both publications are excellent reference sources for further reading.
Key Points
 Up to half of medical and surgical patients may have nutritional deficiencies
Up to half of medical and surgical patients may have nutritional deficiencies
 TPN/HPN formulations are prepared under strict aseptic conditions
TPN/HPN formulations are prepared under strict aseptic conditions
 Before starting TPN, a full assessment of the patient’s nutritional needs must be made
Before starting TPN, a full assessment of the patient’s nutritional needs must be made
 The nutrition team contribute their expertise to provide good patient care by meeting regularly to monitor patient needs
The nutrition team contribute their expertise to provide good patient care by meeting regularly to monitor patient needs
 A TPN formulation may contain water, protein, carbohydrate, fat, electrolytes, trace elements, vitamins and minerals
A TPN formulation may contain water, protein, carbohydrate, fat, electrolytes, trace elements, vitamins and minerals
 Most TPN patients have a negative nitrogen balance and so require amino acids
Most TPN patients have a negative nitrogen balance and so require amino acids
 Care must be taken when administering dextrose in a TPN/HPN formulation to prevent problems of hyper or hypoglycaemia
Care must be taken when administering dextrose in a TPN/HPN formulation to prevent problems of hyper or hypoglycaemia
 Strictly defined procedures are followed when adding ingredients to TPN bags during preparation
Strictly defined procedures are followed when adding ingredients to TPN bags during preparation
 Stability of TPN formulations is one of the major issues which must be carefully considered
Stability of TPN formulations is one of the major issues which must be carefully considered
 Controlling quantities can minimize incompatibilities such as that between calcium and phosphate
Controlling quantities can minimize incompatibilities such as that between calcium and phosphate
 TPN bags containing a fat component become less stable on prolonged storage and could result in fat deposits arising in lungs and capillaries if administered in this unstable condition
TPN bags containing a fat component become less stable on prolonged storage and could result in fat deposits arising in lungs and capillaries if administered in this unstable condition
 For TPN lasting longer than 4 weeks, a central vein should be used
For TPN lasting longer than 4 weeks, a central vein should be used
 A number of problems can arise during TPN/HPN administration. For HPN patients, adequate training to deal with problems arising at home is essential
A number of problems can arise during TPN/HPN administration. For HPN patients, adequate training to deal with problems arising at home is essential
 HPN patients are required to make psychological and social adjustments, but can also have an improvement in quality of life
HPN patients are required to make psychological and social adjustments, but can also have an improvement in quality of life
 BAPEN has laid down standards for home nutritional care which are used as the basis for patient training prior to discharge
BAPEN has laid down standards for home nutritional care which are used as the basis for patient training prior to discharge
 Dialysis is used to remove toxic metabolites, correct acid–base balance and avoid fluid overload
Dialysis is used to remove toxic metabolites, correct acid–base balance and avoid fluid overload
 In haemodialysis, the patient’s blood is passed over a semi-permeable membrane to allow exchange of small solutes with dialysis fluid
In haemodialysis, the patient’s blood is passed over a semi-permeable membrane to allow exchange of small solutes with dialysis fluid
 Peritoneal dialysis uses the peritoneal membrane as the semi-permeable membrane, the dialysis fluid staying in the peritoneal cavity during the exchange
Peritoneal dialysis uses the peritoneal membrane as the semi-permeable membrane, the dialysis fluid staying in the peritoneal cavity during the exchange
 CAPD has a number of advantages and disadvantages for patients
CAPD has a number of advantages and disadvantages for patients
 HD solutions do not need to be sterile, but PD solutions must be sterile and aseptic technique used in handling
HD solutions do not need to be sterile, but PD solutions must be sterile and aseptic technique used in handling