Radiopharmacy
 Types of radionuclides and the principles of their medical use
Types of radionuclides and the principles of their medical use
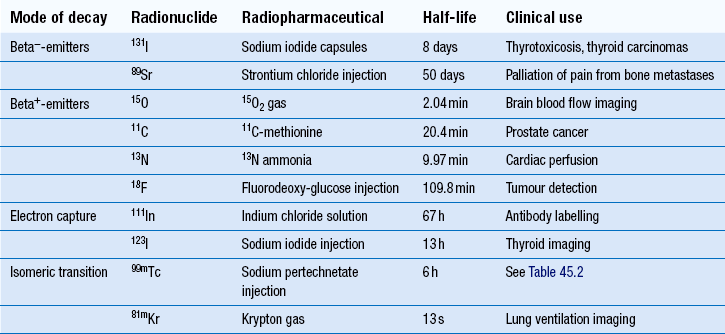
 Examples of alpha-emitters, beta−- and beta+-emitters, electron capture and isomeric transitions
Examples of alpha-emitters, beta−- and beta+-emitters, electron capture and isomeric transitions
 Radionuclide production of beta+-emitters
Radionuclide production of beta+-emitters
 Principles of using a molybdenum-technetium generator
Principles of using a molybdenum-technetium generator
Introduction
Elements that emit radiation are known as radionuclides and have a number of applications in medicine. Radiopharmacy in hospital practice is concerned with the manufacture or preparation of radioactive medicines known as radiopharmaceuticals. These have two main applications in medicine:
 As an aid to the diagnosis of disease (diagnostic radiopharmaceuticals)
As an aid to the diagnosis of disease (diagnostic radiopharmaceuticals)
 In the treatment of disease (therapeutic radiopharmaceuticals).
In the treatment of disease (therapeutic radiopharmaceuticals).
Diagnostic radiopharmaceuticals may be classified into two types:
 Radiopharmaceuticals used in tracer techniques for measuring physiological parameters (e.g. 51Cr-EDTA for measuring glomerular filtration rate)
Radiopharmaceuticals used in tracer techniques for measuring physiological parameters (e.g. 51Cr-EDTA for measuring glomerular filtration rate)
 Radiopharmaceuticals for diagnostic imaging (e.g. 99mTc-methylene diphosphonate (MDP) used in bone scanning).
Radiopharmaceuticals for diagnostic imaging (e.g. 99mTc-methylene diphosphonate (MDP) used in bone scanning).
In diagnostic imaging, gamma-emitting radionuclides are used, since their interaction with tissue is much less than that of particulate emitters and will cause significantly less damage to tissue. Radiopharmaceuticals are administered to the patient, usually by the intravenous (IV) route, and distribute into a particular organ. The radiation is then detected externally using a special scintillation detector known as a gamma-camera. These are used by nuclear medicine departments to image the distribution of the radiopharmaceutical within the patient’s body. Using the gamma-camera in conjunction with a computer system it is not only possible to produce static images of an organ, but also to examine how the radiopharmaceutical moves through an organ. These dynamic images describe how the organ is functioning. It is also possible to create images in three dimensions, a process known as single photon emission computerized tomography (SPECT) when used in combination with gamma-emitting radionuclides such as 99mTc and positron emission tomography (PET) when used in combination with positron-emitting radionuclides such as 18F.
It is important to note that for the safe production of radiopharmaceuticals, the radiopharmacy must be designed to comply with, and procedures must follow, good manufacturing practice and good radiation protection practice. Radiopharmacists working in this field are part of a multidisciplinary team which includes physicians, physicists, radiochemists and technicians from the field of pharmacy as well as nuclear medicine. As part of this team, they not only ensure that the radiopharmaceuticals will give high-quality clinical information, but also that they are safe for both patient and user alike.
Radionuclides used in nuclear medicine
Alpha-emitters
Alpha-decay is the process whereby a nucleus emits a helium nucleus, or alpha-particle. This commonly occurs with heavy nuclei (e.g. 226Ra:22688Ra→22286Rn+alpha).
Because they are heavy and positively charged, alpha-particles travel only short distances in air (~5 mm) and only micrometre distances in tissues. Their ionizing nature would result in a highly localized radiation dose if taken internally and hence they tend not to be used as diagnostic radiopharmaceuticals but may have a place as therapeutic agents.
Some alpha-emitters (e.g. 137Cs) when encapsulated are used as sealed sources, emitting X-rays or gamma-rays for radiotherapy applications. Here the body is exposed to radiation externally in an attempt to treat malignant tumours.
Beta-emitters
Beta-decay occurs in two ways, one that involves the emission of a negatively charged beta−-particle, or electron, and the other that involves the emission of a positively charged beta+-particle, or positron.
Beta−-emitters
Radionuclides which decay by beta−-decay tend to have nuclei that are neutron rich. They attempt to reach a more stable state by the transformation of a neutron into a proton with the emission of a beta− -particle (e.g. 32P: 3215P → 3216S + beta−). Despite beta−-particles having a range in air of up to several metres, their range in tissues is only a few millimetres. Because of this and their highly ionizing nature, beta−-emitters tend to be used in therapeutic radiopharmaceuticals (Table 45.1).
The principle of therapeutic treatment with radionuclides is to target the radionuclide to a specific tissue within the body in an attempt to selectively damage or destroy that tissue. Ideally, therapeutic beta−-emitting radionuclides should have energies of 0.5–1.5 MeV and a half-life of several days to provide a prolonged radiobiological effect.
The most widely used example of this is 131I- sodium iodide, which is used in the treatment of hyperactive thyroid disease and in certain thyroid tumours. Here the physiological property of thyroid tissue is exploited to target the radionuclide to the site of action. Since thyroid tissue avidly takes up iodine in the normal synthesis of the hormone levothyroxine, radioactive iodine is also taken up and held in the thyroid tissue. Hence, the radiation damage is targeted to the thyroid tissue specifically and the normal excretion of any excess iodine results in no significant damage to other organs and tissues.
Beta+-emitters (positrons)
Radionuclides that emit positrons are becoming more widely used in nuclear medicine. In this transformation, a proton-rich nuclide attempts to achieve stability by converting a proton to a neutron with the emission of a positron (e.g. 11C:116C → 115S + beta+ + gamma). The positron is very short-lived, since it interacts with an electron resulting in an annihilation reaction and the conversion of both particles into electromagnetic (EM) radiation. This EM radiation is in the form of two gamma-rays, each having energy of 0.511 MeV, which are emitted at an angle of 180° to each other.
When used in conjunction with a specialized gamma-camera with detectors placed 180° apart, it is possible to create images in all three dimensions with the position of the radiopharmaceutical being very precisely known. This type of imaging technique is known as positron emission tomography (PET). There are a number of positron emitting radionuclides which are becoming important tools in diagnostic imaging. Currently 18F-labelled glucose, known as 18F-fluoro deoxy-glucose (18F-FDG), is the most commonly used PET radiopharmaceutical in hospital practice and as a result the production processes for it will be described in simplified form and used as an example (see below). However, it should be noted there are four main positron emitters used to prepare radiopharmaceuticals (see Table 45.1). PET imaging with 18F-FDG, in combination with X-ray computerized tomography (CT) is rapidly becoming an important imaging technique in the diagnosis of cancer.
Electron capture
Nuclei that are proton rich may, as an alternative to positron emission, capture electrons from the atom’s electron orbital. This process results in the transformation of a proton to a neutron within the nucleus. The subsequent rearrangement of the electrons orbiting the nucleus results in a characteristic emission of X-rays or gamma-rays (e.g. 123I:12353I + electron→12353Te+gamma).
Radionuclides which decay by electron capture are useful in diagnostic imaging since they emit gamma-rays; examples are given in Table 45.1.
Isomeric transition
Some radionuclides exist for measurable periods in excited, or isomeric, states prior to reaching ground state. This form of decay involves the emission of a gamma-ray and is known as isomeric transition. When radionuclides exist in this transitional state, they are known as metastable, which is denoted by the letter ‘m’ and written thus: 99mTc.
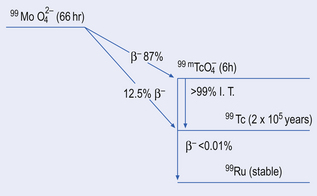
A simplified decay scheme for 99mTc-technetium is shown in Figure 45.1 where 99mTc’s parent radionuclide, molybdenum (99Mo), decays by beta−-emission to the ground state 99Tc either directly or indirectly.
The indirect route, which is the most common, involves the isomer 99mTc, which in turn decays from its metastable state to 99Tc by isomeric transition.
Radionuclides which decay by this process are used in diagnostic imaging since they emit gamma-rays (see Table 45.1). It should be noted that 99mTc is the most widely used radionuclide in hospital radiopharmacy today, making up the radionuclide component of around 90% of the radiopharmaceuticals produced. For these reasons the production processes for 99mTc-radiopharmaceuticals will be especially emphasized (see below).
Principles of 99mTc-radiopharmaceutical production
The physical and chemical properties of 99mTc make it nearly ideal for imaging purposes as outlined below:
 It has a 6-h half-life (T1/2); long enough to allow imaging to take place in the working day, while also being short enough that patients are not radioactive for long periods (in 24 h, or 4 half-lives, the radioactivity will have decayed by 94%)
It has a 6-h half-life (T1/2); long enough to allow imaging to take place in the working day, while also being short enough that patients are not radioactive for long periods (in 24 h, or 4 half-lives, the radioactivity will have decayed by 94%)
 99mTc emits gamma-rays of 140 keV energy: ideal for use with the modern gamma-camera
99mTc emits gamma-rays of 140 keV energy: ideal for use with the modern gamma-camera
 There are no particulate emissions that, if present, would add to the patient’s radiation dose
There are no particulate emissions that, if present, would add to the patient’s radiation dose
 By purchasing a device known as a 99Mo/99mTc -generator, 99mTc can be made readily available to the hospital site in a sterile and pyrogen-free form
By purchasing a device known as a 99Mo/99mTc -generator, 99mTc can be made readily available to the hospital site in a sterile and pyrogen-free form
 99mTc has versatile coordination chemistry and will allow a large number of ligands to complex with it. By using different ligands in the radiopharmaceutical’s formulation, a wide range of radiopharmaceuticals can be prepared in the radiopharmacy, providing for the many different investigations carried out in nuclear medicine departments (Table 45.2).
99mTc has versatile coordination chemistry and will allow a large number of ligands to complex with it. By using different ligands in the radiopharmaceutical’s formulation, a wide range of radiopharmaceuticals can be prepared in the radiopharmacy, providing for the many different investigations carried out in nuclear medicine departments (Table 45.2).
Table 45.2
Examples of 99mTc-radiopharmaceuticals
| Radiopharmaceutical | Organ or tissue of distribution | Main clinical application |
| 99mTc-sodium pertechnetate | Thyroid | Imaging the thyroid gland and ectopic tissue |
| Salivary gland | Dynamic images of accumulation and drainage to show gland function | |
| Gastric mucosa | Presence of Meckel’s diverticulum containing gastric mucosa | |
| 99mTc-methylene diphosphonate (MDP) | Skeleton | Bone metastases from carcinoma of lung, breast and prostate |
| 99mTc-macro-aggregates of albumin (MAA) | Lung blood flow | Lung perfusion studies most commonly for the diagnosis of pulmonary embolism |
| 99mTc-exametazime (HMPAO) | Brain blood flow | Regional cerebral imaging in stroke and tumours |
| Diagnosis of Alzheimer’s dementia | ||
| 99mTc-exametazime (HMPAO) labelled leucocytes | Infection or inflammation | Identification of abscesses associated with pyrexia of unknown origin. Extent of inflammatory bowel disease |
| 99mTc-tetrofosmin | Heart | Cardiac perfusion imaging |
| 99mTc-sestamibi (MIBI) | Heart | Cardiac perfusion imaging |
| 99mTc-tin colloid | Liver | Location of hepatic tumours, abscesses and cysts. Detection of cirrhosis |
| 99mTc-mercapto triglycine (MAG 3) | Kidney | Dynamic studies to study kidney function |
| 99mTc-dimercapto-succinic acid (DMSA) | Kidney | Static imaging showing the kidney structure |
The production of 99mTc – the molybdenum/technetium generator
Radionuclides with long half-lives (e.g. 131I, T1/2=8 days) can be easily transported from production site to the user hospital. With shorter half-life radionuclides, e.g. 99mTc, this supply system would be extremely difficult. As a result, a device known as the radionuclide generator is used to provide 99mTc to the hospital site.
Radionuclide generators work on the principle that they contain a relatively long-lived ‘parent’ radionuclide that decays to produce a ‘daughter’ radionuclide. The chemical nature of parent and daughter are different, allowing separation of the daughter from the parent.
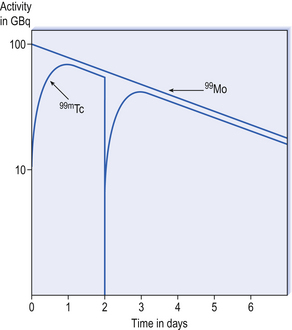
The molybdenum/technetium generator consists of 99Mo (long-lived ‘parent’) absorbed onto an alumina-filled column, the 99Mo being present in the form of molybdate (99MoO42−). 99Mo decays to its ‘daughter’ radionuclide 99mTc, as pertechnetate, 99mTcO4− (see Fig. 45.1). The amount of 99mTcO4− grows as a result of the decay of 99Mo, until a transient equilibrium is reached. At this point the amount of 99mTc in the column appears to decay with the half-life of 99Mo (Fig. 45.2).
By drawing a solution of sodium chloride 0.9% weight in volume (w/v) through the column, 99mTc is removed from the column in the form of sodium pertechnetate, Na99mTcO4. This process is known as eluting the generator and the resulting solution as the eluate. This process results in the production of a sterile solution of sodium pertechnetate that may now be used to make 99mTc -radiopharmaceuticals.
99Mo remains on the column where it decays to produce further 99mTc, the equilibrium being re-established about 23 h after elution. Elution of the generator is repeated daily to provide the radiopharmacy with a supply of 99mTc for 7–14 days, beyond which the yield of 99mTc becomes too small to be useful. Hospital radiopharmacies tend to buy generators on a weekly basis to provide a continuous supply of 99mTc.
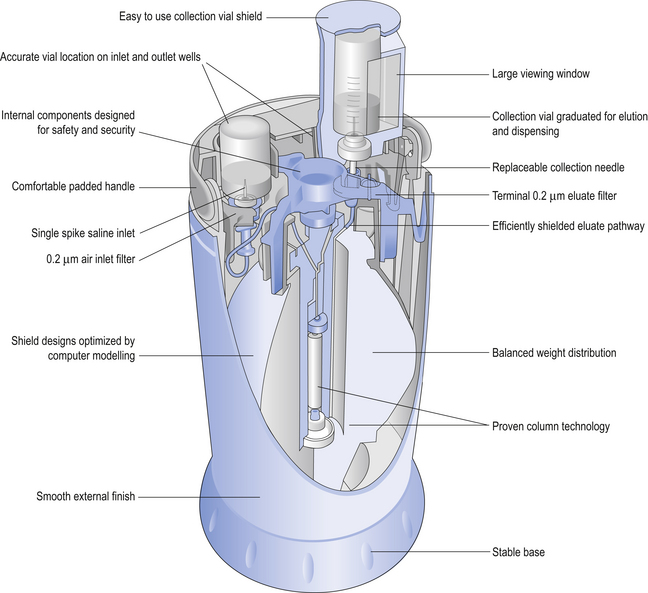
Design of a 99mTc-generator
The design of a typical generator will be described by reference to the GE Healthcare generator, Drytec® (Fig. 45.3). The main components of this generator are:
 A needle connected to the top end of the alumina column by tubing (it is this needle upon which a vial of IV Sodium Chloride Intravenous Infusion BP 0.9% w/v will be placed)
A needle connected to the top end of the alumina column by tubing (it is this needle upon which a vial of IV Sodium Chloride Intravenous Infusion BP 0.9% w/v will be placed)
 A sterile alumina column to which is bound 99Mo
A sterile alumina column to which is bound 99Mo
 An elution needle which is connected to the bottom end of the alumina column
An elution needle which is connected to the bottom end of the alumina column
These components are housed within a compact plastic casing. The alumina column is encased in lead to give protection from the radiation.
Operating the generator is fairly straightforward. A vial of Sodium Chloride Intravenous Infusion BP 0.9% w/v, supplied with the generator, is first placed on the left hand needle (see Fig. 45.3). A sterile evacuated vial, also supplied with the generator, is placed in a lead pot designed for the elution process. Then, by placing this on the elution needle, the vacuum draws sterile Sodium Chloride Intravenous Infusion BP 0.9% w/v from the vial through the column and into the evacuated vial. When eluate has been collected, air enters the elution vial after first passing through the column. This dries the column as well as removing excess vacuum in the elution vial. The elution process is now complete and the vial may be removed from the generator.
The sterility of the eluates is maintained throughout the useful life of the generator by the following means:
 The eluting solution is terminally sterilized Sodium Chloride Intravenous Infusion BP 0.9% w/v
The eluting solution is terminally sterilized Sodium Chloride Intravenous Infusion BP 0.9% w/v
 Air entering the system passes through a 0.22 μm filter
Air entering the system passes through a 0.22 μm filter
 A terminal eluate 0.22 μm filter is placed between the column and the elution needle
A terminal eluate 0.22 μm filter is placed between the column and the elution needle
 Between elutions, the needle is protected by a single-use, disposable, sterile needle guard.
Between elutions, the needle is protected by a single-use, disposable, sterile needle guard.
The elution of the generator should be carried out in a Grade A environment (see Ch. 40).
Preparation of 99mTc-radiopharmaceuticals
The daily supply of 99mTc is provided by the elution of the generator, resulting in a sterile solution of sodium pertechnetate that is subdivided to provide the radioactive component of the radiopharmaceutical. Some nuclear medicine investigations use sodium pertechnetate alone as the radiopharmaceutical (see Table 45.2). In this case, preparation of sodium pertechnetate injection requires only the subdivision from the generator eluate with perhaps some further dilution with Sodium Chloride Intravenous Infusion BP 0.9% w/v.
Other investigations, and these are in the majority, use radiopharmaceuticals that involve the chemical transformation of the sodium pertechnetate into another radiochemical form.
In order to make the preparation of 99mTc-radiopharmaceuticals as simple as possible, commercially available ‘kits’ are used to manufacture these radiopharmaceuticals. These kits allow the radiopharmacist, in the hospital environment, to transform the pertechnetate, via complex chemical reactions performed within the vial, into the desired radiopharmaceutical. This is achieved by the simple addition of pertechnetate into the vial followed by shaking to dissolve the contents.
A kit consists of a pre-packed set of sterile ingredients designed for the preparation of a specific radiopharmaceutical. Most commonly, the ingredients are freeze dried, enclosed within a rubber-capped nitrogen-filled vial. Normally, the kit contains sufficient materials to prepare a number of patient doses. In a typical formulation, the following may be found:
 The compound to be complexed to the 99mTc. These are known as ligands (e.g. methylene diphosphonate)
The compound to be complexed to the 99mTc. These are known as ligands (e.g. methylene diphosphonate)
 Stannous ions (e.g. stannous chloride or fluoride) which are present as a reducing agent. The reduction of 99mTcO4− to a lower valance state is required to allow the ligands to form a complex with the 99mTc
Stannous ions (e.g. stannous chloride or fluoride) which are present as a reducing agent. The reduction of 99mTcO4− to a lower valance state is required to allow the ligands to form a complex with the 99mTc
 Other compounds that act as stabilizers, buffers or antioxidants.
Other compounds that act as stabilizers, buffers or antioxidants.
Given below is an example of how 99mTc-radiopharmaceutical production may be performed. The compounding procedures must be carried out within the facilities described in Chapter 40 using aseptic technique and carried out as ‘closed’ procedures (GMP).
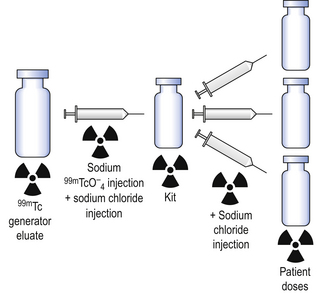
The production method (Fig. 45.4) involves two simple steps:
Step 1. The freeze-dried kit is reconstituted by aseptically transferring the necessary activity of sodium pertechnetate using a sterile syringe and needle. This step may also include a further dilution of the eluate with a suitable diluent. The amount of activity withdrawn for the reconstitution of the kit vial depends on two factors:
 The number of patient doses to be manufactured
The number of patient doses to be manufactured
 The amount of activity required at injection time for each of the patient doses. The calculation would take into account the decay of 99mTc.
The amount of activity required at injection time for each of the patient doses. The calculation would take into account the decay of 99mTc.
Manufacturers normally specify a maximum activity that may be added to the vial.
Step 2. The reconstituted kit is aseptically subdivided to provide each patient dose with sufficient activity to allow proper imaging after administration. As in Step 1, a diluent may be added to the final dose to give the desired radioactive concentration.
99mTc-radiopharmaceuticals must be administered on the day of production, for the following reasons:
 Sterility. Aseptically prepared pharmaceuticals should ideally be administered within a few hours of production, in accordance with GMP
Sterility. Aseptically prepared pharmaceuticals should ideally be administered within a few hours of production, in accordance with GMP
Radiochemical stability. 99mTc-complexes are generally stable for a period between 4 and 8 h after production.
Principles of 18F-fluoro deoxy-glucose (18F-FDG) production
In the manufacture of 18F-FDG there are two main processes. First there is the production of the radionuclide itself, which is produced in a cyclotron facility. This is followed by the radio-synthesis of the 18F-FDG, which is carried out in an automated apparatus, known as a synthesis module. The resulting solution of 18F-FDG has then to be sterilized and may have to be sub-dispensed in some way so as to provide the injection in a ready to administer form. Since the 18F-FDG has been synthesized, it will require analysis and other quality control checks prior to administration to the patient.
Radionuclide production
A cyclotron is a device used to produce radionuclides. It accelerates atomic or subatomic particles in a circular orbit, increasing the energy of the particles until a high-energy beam of particles is created. Once the particles have reached their maximum energy they are extracted using high voltage and allowed to bombard target materials. The composition of the target material and the nuclear reactions that take place determine the radionuclides that are produced.
For 18F production, the most common nuclear reaction used is the 18O (p,n) 18F reaction, where protons are produced from hydrogen gas and are accelerated until, at a required energy, a ‘beam’ of the protons are allowed to bombard a target of 18O-enriched water (H218O). 18O is a naturally occurring isotope of oxygen, but much less abundant than the normal 16O. By forcing a proton into the 18O nucleus the now unstable nucleus ejects a neutron and the result is the production of 18F – a positron-emitting radionuclide.
Radio-synthesis
2-[18F] fluoro-2-deoxy-D glucose (18F-FDG) may be prepared by various chemical pathways, but whatever radio-synthetic route is used it must be rapid to minimize the radiation risk and be of high yield. An automated apparatus, the synthesis module, is used to synthesize the 18F-FDG to assure production efficiency and keep radiation exposure to a minimum. There are now commercially available synthesis modules such as the TRACERlabFx Synthesizer® as shown in Figure 45.5, which are supplied with good manufacturing practice (GMP) standard raw materials and reagents for use in the module. Using this equipment, the following synthetic pathway is performed:

Figure 45.5 Synthesis module for 18F-FDG production (TRACERlabFx Synthesizer®). (Courtesy of GE Healthcare plc.) Module located in lead-shielded (5.5 cm) specialized fume cupboard known as ‘hot cell’.
1. The 18F-fluoride is adsorbed by an anion-exchange resin.
2. The retained 18F-fluoride is eluted using an aqueous potassium carbonate solution.
3. A phase transfer catalyst (Kriptofix 222) dissolved in acetonitrile is then added, to bind the potassium ion and to enhance the nucleophilic reactivity of the 18F-fluoride.
4. After evaporation of the solvents, the 18F-fluoride is allowed to react with the reagent mannose triflate at elevated temperature (1,3,4,6-O-Acetyl-2-O-trifluoromethanesulfonyl-beta-D-mannopyranose). The structure of mannose triflate is similar to that of glucose with a leaving group (triflate) and four acetyl protecting groups to ensure the mannose ring undergoes nucleophilic substitution at the second carbon atom.
5. Hydrolysis under acidic or alkaline conditions also at elevated temperature yields 2-[18F] fluoro-2-deoxy-D glucose (18F-FDG).
Sterilization and sub-dispensing
Sterilization of the 18F-FDG is achieved either by filtration through a 0.2 μm filter, although there are automated systems that use high temperature short sterilization cycles (e.g. 10 min cycle consisting of 4 min heat up; 135°C for 3.5 min; 2.5 min cool down). Sub-dispensing or fractionation of the bulk solution into patient doses is then carried out using robotic dispensing systems that perform the aseptic transfers since the radiation hazard is too great for regular manual aseptic transfer. This aseptic manipulation is carried out within a specialized isolator specifically designed for handling PET radiopharmaceuticals.
Quality control
Since the 18F-FDG has been synthesized, it must undergo stringent testing as outlined in the monograph given in the European Pharmacopoeia. A detailed description is beyond the scope of this chapter, but in summary the tests will include:
 Determination of bacterial endotoxins
Determination of bacterial endotoxins
 Determination of chemical purity
Determination of chemical purity
 Determination of radionuclide purity
Determination of radionuclide purity
Facilities required for the production of radiopharmaceuticals
The majority of radiopharmaceuticals are intended for IV administration; therefore it is of paramount importance that these preparations are sterile. They also contain radionuclides with short half-lives that require their preparation and administration on the same day. Because of the thermal lability of some of these products, it is not possible to use terminal sterilization by autoclaving and hence these injections must be prepared using aseptic techniques. Here, highly skilled operators work with sterile ingredients within clean room facilities containing either laminar flow safety cabinets or isolators. The facilities for carrying out such manipulations are more fully described in Chapter 40, but there is specialized equipment as well as design criteria specifically for handling radiopharmaceuticals. More information can be found in Further Reading (see Appendix 4).
Radiation protection in the radiopharmacy
There are three basic principles to radiation protection:
 Shielding. By placing shielding around the radioactive source, the radiation dose rate may be reduced. Materials used as shielding must be appropriate to the type of radiation being emitted by the radionuclide. Plastic, Perspex and metals of low molecular weight such as aluminium are appropriate materials for shielding beta -emitters. For gamma-emitters, high molecular weight metals such as lead and tungsten should be used. The thickness of shielding material necessary for gamma-emitters is dependent on the gamma-ray energy – the greater the energy, the thicker the shield required
Shielding. By placing shielding around the radioactive source, the radiation dose rate may be reduced. Materials used as shielding must be appropriate to the type of radiation being emitted by the radionuclide. Plastic, Perspex and metals of low molecular weight such as aluminium are appropriate materials for shielding beta -emitters. For gamma-emitters, high molecular weight metals such as lead and tungsten should be used. The thickness of shielding material necessary for gamma-emitters is dependent on the gamma-ray energy – the greater the energy, the thicker the shield required
 Distance. The radiation dose from a radioactive source is inversely proportional to the square of the distance (i.e. by doubling the distance the radiation dose is quartered)
Distance. The radiation dose from a radioactive source is inversely proportional to the square of the distance (i.e. by doubling the distance the radiation dose is quartered)
 Time. Minimizing the time spent handling a radioactive source will reduce the radiation dose. It is important for new operators to practice the handling operation prior to working with radioactive materials.
Time. Minimizing the time spent handling a radioactive source will reduce the radiation dose. It is important for new operators to practice the handling operation prior to working with radioactive materials.
In working practice, all three of these principles may be used in isolation or together, to reduce the radiation dose to the operator. For example, in the dispensing operation outlined in Figure 45.4, all vials containing radioactive material would be contained in a 3 mm lead pot. This will attenuate 99mTc’s gamma-rays by a factor of approximately 1000. The syringes used to carry out the transfers would be only half full (i.e. 1 mL of radioactive solution would be transferred with a 2 mL syringe) in order to maximize the distance between the operator’s fingers and the source, without compromising the accuracy of the dispensing operation.
The syringes, during the operation, should also be contained within a syringe shield. These are made of materials such as lead, tungsten, lead glass or lead acrylic, the latter two being transparent. Lead and tungsten syringe shields have lead glass/acrylic windows incorporated to allow the operator to see the graduations on the syringe. Alternatively, the whole syringe shield may be made of lead glass/acrylic which would have the advantage of giving greater visibility.
Handling the vials outside their lead pots should be carried out using long forceps and not with the fingers. The dispensing process should be carried out over a ‘drip tray’ that allows easy containment of any accidental spillage. It also should be carried out within a laminar flow safety cabinet or negative pressure isolator that provides operator protection as well as product protection (see Ch. 40).
The staff working in the radiopharmacy will be constantly monitored to assess their radiation exposure and to ensure compliance with safety legislation. Whole-body dose may be monitored with film badges and the radiation dose to the finger pulp with thermo luminescent dosimeters.
Key Points
 Radiopharmaceuticals may be used in therapy or diagnosis, the latter either as tracers or in imaging
Radiopharmaceuticals may be used in therapy or diagnosis, the latter either as tracers or in imaging
 PET-CT imaging with 18F-FDG is becoming an important imaging technique in the diagnosis of cancer
PET-CT imaging with 18F-FDG is becoming an important imaging technique in the diagnosis of cancer
 18F-FDG may be prepared by first producing 18F in a cyclotron, followed by the radio-synthesis of the 18F-FDG, which is carried out in an automated apparatus, known as a synthesis module
18F-FDG may be prepared by first producing 18F in a cyclotron, followed by the radio-synthesis of the 18F-FDG, which is carried out in an automated apparatus, known as a synthesis module
 99mTc is the most widely used radionuclide
99mTc is the most widely used radionuclide
 A molybdenum/technetium generator will provide a daily supply of 99mTc for 7–14 days, as sodium pertechnetate
A molybdenum/technetium generator will provide a daily supply of 99mTc for 7–14 days, as sodium pertechnetate
 Reacting with a suitable ligand can chemically modify sodium pertechnetate. Different ligands give different bio-distributions, resulting in the wide range of scans that may be performed with 99mTc
Reacting with a suitable ligand can chemically modify sodium pertechnetate. Different ligands give different bio-distributions, resulting in the wide range of scans that may be performed with 99mTc
 Radiation protection should be provided for operators using a combination of shielding, distance and time
Radiation protection should be provided for operators using a combination of shielding, distance and time