The Difficult Airway
Risk, Assessment, Prophylaxis, and Management
Robin Russell MBBS, MD, FRCA, Mansukh Popat MBBS, FRCA
Chapter Outline
Maternal Morbidity and Mortality
Physiologic and Anatomic Changes of Pregnancy
Atlanto-occipital Joint Extension
Fasting and Antacid Prophylaxis
Denitrogenation (Preoxygenation)
Rapid-Sequence Induction and Cricoid Pressure
Awake Intubation before General Anesthesia
Indirect Optical/Video Laryngoscopy
Awake Tracheostomy or Surgery Standby
Local Anesthesia for Cesarean Delivery
THE UNANTICIPATED DIFFICULT AIRWAY
Features of the Obstetric Patient
Cannot Intubate but Can Ventilate
Cannot Intubate and Cannot Ventilate
Laryngeal Tube and Esophageal-Tracheal Combitube
Cannula and Surgical Cricothyrotomy
EXTUBATION OF THE PATIENT WITH A DIFFICULT AIRWAY
Risk
Definitions
A difficult airway can be defined in several ways. A practitioner may be said to encounter a difficult airway when he or she experiences difficulty providing adequate maintenance or protection of the airway that leads to hypoxemia or soiling of the tracheobronchial tree.1 This definition includes difficulty in providing ventilation via a facemask or supraglottic airway (e.g., laryngeal mask airway [LMA]) or tracheal intubation. The American Society of Anesthesiologists (ASA) Task Force on Management of a Difficult Airway defines a difficult airway as the clinical situation in which a conventionally trained anesthesiologist experiences difficulty with facemask ventilation of the upper airway, difficulty with tracheal intubation, or both.2
The prevalence of difficult facemask ventilation is dependent on the definition. In one study,3 5% of 1502 nonpregnant patients experienced difficulty in facemask ventilation, which was defined as an oxyhemoglobin saturation value less than 92%.3 A multivariate analysis identified five independent risk factors for difficult facemask ventilation: (1) age older than 55 years, (2) body mass index (BMI) greater than 26 kg/m2, (3) presence of a beard, (4) lack of teeth, and (5) a history of snoring. Impossible mask ventilation, defined as an inability to exchange air during bag-mask ventilation despite multiple providers, airway adjuncts, and neuromuscular blockade, was reported in 77 of 50,000 (0.15%) nonobstetric anesthetic procedures.4 Independent predictors of impossible mask ventilation were previous neck irradiation, male gender, diagnosis of sleep apnea, and a Mallampati class III or IV (see later discussion).4 Difficult laryngeal mask ventilation may be defined as the inability within three attempts of device insertion to produce expired tidal volumes more than 7 mL/kg (leak pressure > 15 to 20 cm H2O).1 In a study of 11,910 nonobstetric patients,5 the incidence of difficult laryngeal mask ventilation was 0.19%.
Although failed tracheal intubation is a tangible endpoint, defining difficult intubation is more complex. Difficulty may be encountered because of failure to visualize the glottis (difficult laryngoscopy) or due to an anatomic laryngeal or tracheal abnormality. Difficulty has been variously defined by (1) the time taken to intubate, (2) the number of attempts, (3) the view at laryngoscopy, and (4) the requirement for special equipment.
Although a dramatic decrease in the number of anesthesia-related deaths has been reported in the Confidential Enquiries into Maternal Deaths in the United Kingdom over the past 40 years, complications from general anesthesia, primarily complications of airway management, continue to be the leading cause of anesthesia-related maternal mortality.6 Similarly, data from the United States have demonstrated a higher case-fatality rate with general anesthesia compared with neuraxial anesthesia.7 Although the development of national guidelines2,8,9 has resulted in a more systematic approach to the management of the difficult airway, deaths directly resulting from anesthesia still occur owing to failures in ventilation, tracheal intubation, or airway management following extubation. Despite widespread use of neuraxial anesthesia for operative delivery, general anesthesia may still be required in emergency situations, if neuraxial anesthesia is contraindicated or patients refuse it, or if neuraxial anesthesia has failed to provide adequate anesthesia.
Incidence and Epidemiology
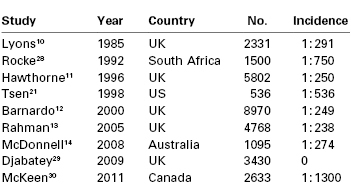
The incidence of failed intubation in obstetrics has long been considered to be approximately 1 in 250 to 300,10-14 which is approximately eight times greater than that in the general population (Table 30-1).15 A number of reasons have been proposed to explain the increased difficulty with obstetric airway management. There are significant physiologic and anatomic changes of pregnancy (see later discussion) affecting the airway, oxygenation, and metabolism. The majority of obstetric general anesthetics are administered for emergency deliveries, often during off hours16; these anesthetic procedures may be conducted by inexperienced anesthesia providers with less proficiency in difficult airway management. Indeed, in one U.K. study,12 the relative risk of failed intubation at emergency compared with elective cesarean delivery was 1.79 (95% confidence interval [CI], 0.61 to 5.26). Excessive cricoid pressure applied by a poorly trained assistant can worsen the glottic view at laryngoscopy,17 as can positioning the parturient with left lateral tilt. Marfin et al.18 proposed that the introduction of disposable airway equipment may adversely affect airway management; single-use, disposable gum elastic bougies are less reliable than reusable devices.
An increase in the incidence of airway-related complications in obstetric patients has been predicted.19,20 The change in maternal demographics, most notably an increase in the prevalence of maternal obesity, may increase the risk for complications from general anesthesia, especially when performed for emergency procedures. With a decrease in the number of cesarean deliveries performed under general anesthesia, trainees have fewer opportunities to become familiar with challenges of the obstetric difficult airway.21-23 Changes in anesthesia training, notably the reduction in trainee working hours and the advent of supraglottic airway (SGA) devices mean that, overall, laryngoscopy and intubation are now less commonly performed than previously. Therefore, the skills required to manage a challenging tracheal intubation are less likely to have been gained before working on the labor and delivery unit without direct supervision. In an editorial, Russell19 suggested that failure to intubate during emergency cesarean delivery may be a self-fulfilling prophecy as practitioners draw on less experience with intubating the trachea during general anesthesia for an emergency cesarean delivery.
The increasing prevalence of maternal obesity is of significant concern. Obese women are at increased risk for obstetric interventions requiring anesthesia24 and are more likely to have unsuccessful neuraxial anesthesia, necessitating the use of general anesthesia for emergency delivery (see Chapter 50). Difficulty with intubation has been reported to occur in 15.5% of the nonobstetric obese population.25 A large Danish cohort study of more than 90,000 nonobstetric patients found that BMI of greater than 35 kg/m2 was a significant risk factor for difficult intubation (odds ratio, 1.34)26; BMI was a more accurate predictor of difficult intubation than weight alone. Data collected from one U.K. region from 1993 to 1998 identified 26 parturients with failed intubation at cesarean delivery; the mean BMI was 33.1 kg/m2.12 Poor head and neck positioning at induction of anesthesia, inappropriate cricoid pressure, and operator anxiety may be responsible for a higher incidence of difficult airway management in obese patients.27
In contrast to some experts, others have questioned whether the rate of difficult and failed intubation is increasing in obstetric anesthesia practice.28-30 A more liberal attitude toward the use of general anesthesia has been suggested to lead to greater familiarity with maternal airway management and subsequent reduced rates of difficulty.29 The ethics of using a technique potentially associated with greater risk for individual harm to reduce the overall incidence of anesthesia-related mortality associated with the technique presents an interesting dilemma. Certainly the presence of experienced anesthesia staff during induction of general anesthesia is recommended and should reduce the morbidity and mortality, and perhaps the frequency, of difficulty with airway management.16 It is hoped that the introduction and widespread acceptance of simulation training in obstetrics31 will lead to improvement in staff performance during critical events such as difficult airway management.
Maternal Morbidity and Mortality
For many years the U.K. Confidential Enquiries into Maternal Deaths have reported thromboembolism, hypertensive disease, and hemorrhage as the leading causes of maternal mortality. In the most recent report, covering the 2006 to 2008 triennium, pregnancy-related mortality from anesthetic causes was the 11th most common cause, accounting for 2.7% of maternal deaths.6 In the United States between 1991 and 2002, 1.6% of maternal deaths were related to complications of anesthesia care, representing a 59% reduction in anesthesia-related mortality compared with data from 1979 to 1990.7,32 Experience from both countries demonstrates dramatic improvements in anesthesia-related maternal mortality in the past three decades. This improvement likely reflects the tremendous efforts that been made by national anesthesia organizations in defining standards of care that lead to improved maternal safety.
Compared with neuraxial anesthesia, general anesthesia is associated with a greater risk for maternal mortality (Table 30-2, see Chapter 40).7 Using data from the Centers for Disease Control and Prevention (CDC), the estimated case-fatality risk ratio for general compared with neuraxial anesthesia was 16.7 between the years 1985 and 1990.32 However, the estimated risk ratio for the period between 1997 and 2002 was only 1.7 (95% CI, 0.6 to 4.6, P = .2).32 Improvements in monitoring and the publication of algorithms for difficult airway management have been suggested to account for the reduction in mortality from general anesthesia. The case-fatality risk for general anesthesia from the earlier period may have overstated the relative risk because the accuracy of data was questionable and it is likely that general anesthesia was used for more complex cases for which mortality was expected to be greater.33
TABLE 30-2
Case-Fatality Rates and Risk Ratios of Anesthesia-Related Mortality during Cesarean Delivery in the United States: 1979 to 2002
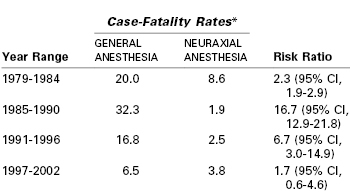
* Deaths per million anesthetics.
CI, confidence interval.
From Hawkins JL, Chang J, Palmer SK, et al. Anesthesia-related maternal mortality in the United States: 1979-2002. Obstet Gynecol 2011; 117:69-74.
Unfortunately, maternal deaths directly attributable to general anesthesia are still reported.6 Although protocols for the management of a difficult airway are now ubiquitous, they are not always followed.12,34
Hypoventilation and airway obstruction after extubation are now increasingly recognized as causes of maternal mortality.6,35 In Michigan between 1985 and 2003, eight maternal deaths were believed related to anesthesia care; all deaths occurred during emergence from general anesthesia or the recovery period, and six of the eight patients were obese. System errors in which the care of the patient did not meet recognized standards were identified in five of the eight cases.35 These errors included inadequate supervision by an anesthesiologist and lapses in postoperative monitoring.
Physiologic and Anatomic Changes in Pregnancy
Of the multitude of anatomic and physiologic changes that occur in pregnancy (see Chapter 2), some have a significant effect on the degree of difficulty of laryngoscopy and tracheal intubation (Box 30-1).
Airway Edema
Fluid retention makes the tissues of the head and neck less compliant and may lead to narrowing of the upper airway, especially in the supine position. Consequently, nasal congestion, snoring, and voice changes all occur more frequently in advanced pregnancy.36 A 34% increase in Mallampati class IV scores15,37 from the first to the third trimester of pregnancy has been observed (see later discussion).38 Difficulty with intubation has been shown to be more than 11 times more common in women with Mallampati class IV than class I scores.28
Although changes in the airway develop gradually during pregnancy, more acute changes may be observed during labor. Mallampati class scores deteriorate during labor.39-41 Decreases in upper airway volume during labor have been demonstrated by acoustic reflectometry.39 The volume of both the oral component of the airway (from the incisors to the oropharyngeal junction) and the pharyngeal component (from the oropharyngeal junction to the glottis) are decreased, presumably as a result of increasing soft tissue edema. Airway narrowing may be more significant in women with preeclampsia. The airway edema that has been observed during labor may be exacerbated by expulsive efforts during the second stage of labor42 or after extubation after cesarean delivery.28 It is therefore prudent to reevaluate the airway before induction of general anesthesia rather than rely solely on a prelabor assessment.39
Nasal capillary engorgement during pregnancy increases the risk for epistaxis after nasal instrumentation and has led many practitioners to believe that nasal intubation is relatively contraindicated in pregnancy. In a 2011 review, Arendt et al.43 challenged this opinion, suggesting that nasal fiberoptic intubation is acceptable after careful and appropriate preparation of the nasal mucosa with topical vasoconstrictors. However, the effects of topical agents on both the prevention of epistaxis and maternal hemodynamic parameters and uteroplacental perfusion must be evaluated and the relative risk of this procedure should be assessed on an individual basis.
Respiratory and Metabolic Changes
As pregnancy progresses, the gravid uterus increasingly encroaches on the diaphragm and lung volumes are reduced. By term, expiratory reserve volume decreases by 25% and residual volume decreases by 15%, resulting in a 20% reduction in functional residual capacity (FRC). This decrease is more marked in the supine than upright position, and in the obese than lean patient. Closing volume is unchanged in pregnancy, but the decrease in FRC results in airway closure in 50% of women if they are supine.44 Metabolic requirements for oxygen increase by nearly 60% during pregnancy, predominantly because of fetal demands. Oxygen requirement is further increased during labor (see Chapter 2). These changes make pregnant women more likely to become hypoxemic during periods of apnea such as during the induction of general anesthesia.45 Therefore, adequate denitrogenation (so-called preoxygenation—replacing nitrogen in the FRC with oxygen) is vital to delay the onset of hypoxemia during periods of apnea (see later discussion).
Preoxygenation and the rate of hemoglobin desaturation have been investigated by computer modeling.46-48 In these models, labor, morbid obesity, and sepsis all hasten preoxygenation; however, desaturation also occurs more rapidly in the moderately ill and the obese (Figure 30-1). Significantly, the time to life-threatening hypoxemia is significantly shorter than that for recovery from paralysis from succinylcholine.46 Therefore, should ventilation be impossible, it cannot be assumed that the patient will recommence breathing before dangerously low levels of oxygen saturation have been reached.
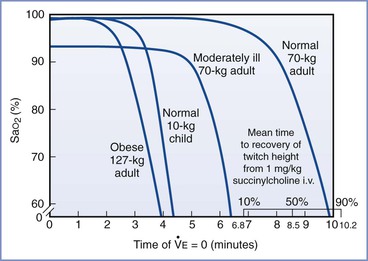
FIGURE 30-1 Time to hemoglobin desaturation (initial SaO2 = 0.87). SaO2 versus time of apnea for various types of patients. (From Benumof JL, Dagg R, Benumof R. Critical hemoglobin desaturation will occur before return to an unparalyzed state following 1 mg/kg intravenous succinylcholine. Anesthesiology 1997; 87:979-82.)
Weight Gain
During pregnancy, most women gain 10 to 15 kg (22 to 33 lb) or more. This weight gain is composed of increases in fat deposition, blood and interstitial fluid volume, and uterine and fetal mass. High BMI is associated with difficulty in mask ventilation and tracheal intubation4,27 and with a greater risk for requiring emergency cesarean delivery.24 BMI is directly associated with more rapid oxygen desaturation during apnea during the induction of general anesthesia.
Breast Enlargement
Breast enlargement during pregnancy may impede intubation by interfering with correct placement of the laryngoscope blade and laryngoscopic manipulation to improve visualization of the larynx. Various strategies can minimize this problem, the most important of which is optimizing the patient's position. With both arms abducted, breast tissue falls away from the chest. Ensuring that the patient is in the ideal intubating position (discussed later) further helps with laryngoscope blade insertion; a short-handled laryngoscope is recommended. The handle can be directed toward the shoulder on insertion of the blade and then redirected once the blade is in the oropharynx.
Full Dentition
Full dentition is typically present in young pregnant women and can interfere with direct laryngoscopy, particularly if the maxillary incisors are protruding or the thyromental distance is small.28
Gastroesophageal Changes
Pregnancy-induced changes in the gastroesophageal system do not per se make laryngoscopy and intubation more difficult. However, owing to the increased risk for regurgitation and aspiration from the second trimester onward (see Chapters 2 and 29), rapid-sequence induction of anesthesia is advocated for almost all parturients, thus potentially increasing the risk for difficult airway management. Antacid prophylaxis is therefore mandatory when surgical intervention is required.
Airway Assessment
Preanesthetic assessment of the airway is necessary before both general or neuraxial anesthesia, so that plans for airway management can be made in advance. A variety of bedside tests have been used, either singularly or in combination, to predict the airway difficulty. The validity of many tests has been questioned, and it is useful to consider how these assessments have been investigated.49 First, airway difficulty, the outcome, must be defined. A number of definitions have been used (see earlier discussion), including difficulty or failure with ventilation (with or without a supraglottic airway) or intubation. Second, various predictive factors that are associated with difficult airway management have been tested on different sample populations of patients.
For an assessment to be useful it must be both sensitive (i.e., correctly identify those whose tracheas are difficult to intubate) and specific (i.e., correctly identify those whose tracheas are easy to intubate). Despite having both reasonably high sensitivity and specificity, many predictive tests have limited use in the clinical environment because failed intubation is rare; the number of false-positive tests (those predicted to be difficult that are not) will always be significantly higher than the number of true-positive tests (those predicted to be difficult that are).49 The positive predictive value (ratio of true-positive tests to the total number of positive tests) for individual difficult airway tests is typically less than 50%, that is, fewer than half of the procedures predicted to be difficult will actually be difficult.49 However, combining difficult airway tests can raise the index of suspicion for difficulty with airway management. Despite these shortcomings in difficult airway prediction, airway assessment is a vital part of anesthetic management. Preanesthetic assessment allows the consideration of potential airway problems and the creation of a stepwise plan for dealing with difficulties should they arise. Common methods of airway assessment used in clinical practice are discussed next.
Cormack and Lehane Grade
Cormack and Lehane50 devised a glottic view grading system in 1984. The purpose of the system was to grade the glottic view obtained with direct laryngoscopy as a means of training for general anesthesia in the obstetric patient. Therefore, the Cormack and Lehane grade is not a preoperative assessment tool but rather a classification method to describe the relative difficulty with subsequent tracheal intubation. The original description includes four grades of laryngoscopy (Figure 30-2):
• Grade 1: Full view of glottis
• Grade 2: Partial view of glottis or arytenoids
Subsequent modifications have been proposed. Grade 2 may be divided into 2A (part of vocal cords visible) and 2B (only arytenoids or very posterior origin of vocal cords visible).51,52 Further, Grade 3 may be divided into those in which the epiglottis is visible and lifted, such as with a gum elastic bougie (Grade 3A), and those in which the epiglottis is visible but not able to be lifted (Grade 3B).52,53 Increasing difficulty with intubation is to be expected with each progressive grade of the Cormack and Lehane classification.
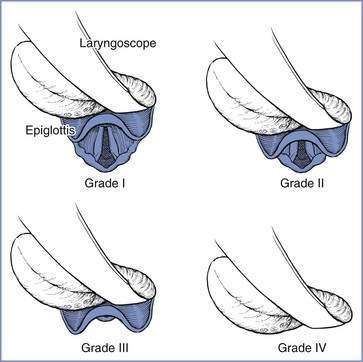
FIGURE 30-2 Cormack and Lehane laryngoscopic view grades. Grade I is visualization of the entire laryngeal aperture. Grade II is visualization of only the posterior portion of the laryngeal aperture. Grade III is visualization of only the epiglottis. Grade IV is visualization of only the soft palate. (From Cormack RS, Lehane J. Difficult tracheal intubation in obstetrics. Anaesthesia 1984; 39:1105-11.)
Because of the widespread acceptance of the Cormack and Lehane grading system, some useful information can be gained by reviewing the anesthetic records of patients who have a previous history of direct laryngoscopy; the Cormack and Lehane glottic view grade is often documented. However, prior reports should be treated with caution because grades given in the nonpregnant state will likely differ from those determined during pregnancy, and the potential for inter-observer and intra-observer variability exists.
Mallampati Class
In 1985 Mallampati et al.37 described a three-point scale of the oropharyngeal view of the open mouth based on concealment of the faucal pillars, soft palate, and uvula by the base of the tongue; the more the view was obscured, the greater the difficulty with laryngoscopy and intubation. Samsoon and Young15 later modified the scoring system into a four-point scale (Figure 30-3):
Class I: visualization of soft palate, uvula, and tonsillar pillars
Class II: visualization of soft palate and base of uvula
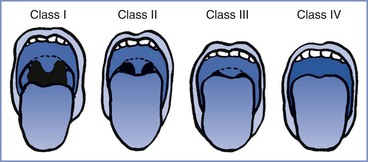
FIGURE 30-3 Modified Mallampati classification of the oropharynx. Classification of the upper airway in terms of the size of the tongue and the pharyngeal structures that are visible with the mouth open. In class I, the soft palate, uvula, and anterior and posterior tonsillar pillars can be seen. In class II, the soft palate and uvula can be seen; the tonsillar pillars are hidden by the tongue. In class III, the soft palate and the base of the uvula can be seen. In class IV, only the hard palate can be seen. (From Mallampati SR, Gatt SP, Gugino LD, et al. A clinical sign to predict difficult tracheal intubation: a prospective study. Can Anaesth Soc J 1985; 32:429-34.)
The test should be performed with the patient sitting upright with her head in the neutral position. The patient is instructed to open her mouth as wide as possible and protrude her tongue as far as possible without phonation. Increasing difficulty with laryngoscopy and tracheal intubation has been demonstrated with greater Mallampati scores in both obstetric28 and nonobstetric populations.37
Mallampati scores are frequently used as part of an assessment to predict difficult intubation. It is important to remember that scores change during pregnancy38 and during labor.39-41 When used as the sole predictor of difficult airway, the incidence of both significant false-positive and false-negative results is high.54 This poor predictive value may be explained by the use of phonation, poor patient positioning, involuntary arching of the tongue, and interobserver variability in interpretation. A meta-analysis of the Mallampati score concluded that the test had limited accuracy for predicting a difficult airway and was not a useful screening test.55 Consequently, the Mallampati score is best used in combination with other tests.
Thyromental Distance
During laryngoscopy, the tongue is normally pushed into the mandibular space. The thyromental distance, the distance from the tip of the chin to the notch of the thyroid cartilage, can be used to estimate the size of this space and therefore whether the tongue can easily be displaced to facilitate laryngoscopy.56 In the absence of other abnormalities, if the thyromental distance is more than 6.5 cm and the horizontal mandibular length more the 9 cm, intubation should proceed without difficulty. A thyromental distance of less than 6 cm suggests an increased risk for difficulty.54 However, lack of detail in various studies regarding precisely how the thyromental distance was measured (whether it was performed from the inner or outer border of the mandible) make interpretation of this test difficult.
Anatomically, if the mandibular space is small and unable to accommodate the tissues displaced by the laryngoscope blade, few alterations will improve the line of vision during direct laryngoscopy (Figure 30-4).56 When the mandibular space is small, the larynx lies relatively anterior and the tongue must be pulled forward maximally and compressed to expose the larynx.
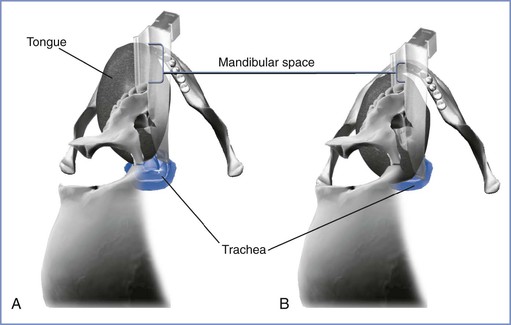
FIGURE 30-4 The mandibular space viewed by the laryngoscopist inserting a curved laryngoscope blade into the airway in a supine patient. The mandibular space is the area bounded by the plane of the line of vision and the part of the mandibular arch in front of this plane (lower and upper extent of brackets, respectively). A, Normal-size mandibular space with room for the tongue. The laryngoscopist has an unimpeded view of the glottis. B, Small mandibular space—the tongue impedes the view of the glottis. (Drawing by Naveen Nathan, MD, Northwestern University Feinberg School of Medicine, Chicago, IL.)
Atlanto-occipital Joint Extension
Extension of the atlanto-occipital joint is necessary for the patient to be in the ideal intubating position in which the oral, pharyngeal, and laryngeal axes are aligned (see later discussion). Movement can be assessed with the patient seated with the head and neck in the neutral position facing forward and then with the joint maximally extended (Figure 30-5). Normal extension should be 35 degrees or more; difficulty with intubation can be expected when joint movement is decreased.56 The accuracy of this assessment is subject to inter-observer variability, making its role in routine airway assessment questionable.
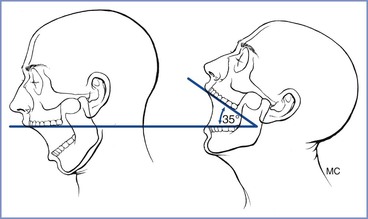
FIGURE 30-5 Clinical method for quantifying atlanto-occipital joint extension. When the head is held erect and faces forward, the plane of the occlusal surface of the upper teeth is horizontal and parallel to the floor. When the atlanto-occipital joint is extended, the occlusal surface of the upper teeth form an angle with the plane parallel to the floor. The angle between the erect and the extended planes of the occlusal surface of the upper teeth quantifies the atlanto-occipital joint extension. A normal person can produce 35 degrees of atlanto-occipital joint extension. (From Bellhouse CP, Dore C. Criteria for estimating likelihood of difficulty of endotracheal intubation with Macintosh laryngoscope. Anaesth Intensive Care 1988; 16:329-37.)
Mandibular Protrusion
The patient's ability to extend the mandibular teeth anteriorly beyond the line of the maxillary teeth may predict adequate visualization of the larynx during direct laryngoscopy. In the mandibular protrusion test, patients are asked to protrude their mandible as far as possible (Figure 30-6); one of three classes is assigned57,58:
• Class A: The lower incisors can protrude anterior to the upper incisors.
• Class B: The lower incisors can be brought edge to edge with the upper incisors.
• Class C: The lower incisors cannot be brought edge to edge with the upper incisors.
Class A is a good predictor of a good glottic view with direct laryngoscopy whereas class C is associated with poor glottic view.57
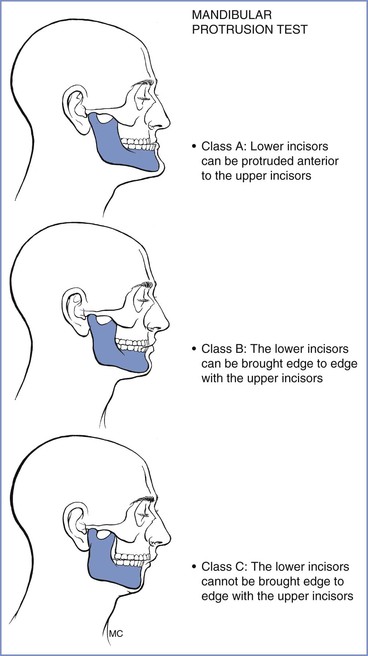
FIGURE 30-6 Mandibular protrusion test. Three classifications are based on the test, which is also referred to as the upper lip bite test. (Redrawn from Munnur U, de Boisblanc B, Suresh MS. Airway problems in pregnancy. Crit Care Med 2005; 33:S259-68.)
The upper lip bite test (ULBT) is similar to mandibular protrusion. In class 1 the lower incisors can bite the upper lip above the vermillion border (i.e., the normally sharp demarcation between the lip and the adjacent normal skin); in class 2, the lower incisors can bite the upper lip below the vermillion border; and in class 3, the lower incisors cannot bite the upper lip.59 The ULBT has been shown to be a better predictor than a Mallampati score for predicting ease with laryngoscopy and intubation.59 The ULBT cannot be assessed in edentulous patients.
Other Assessments
Sternomental distance has been suggested to predict difficult laryngoscopy. This distance is measured between the chin and sternum with the head fully extended on the neck and the mouth closed. Unfortunately, the assessment has extremely weak predictive power, and consequently it has largely been abandoned.
Limited mouth opening impedes the introduction of laryngoscope blade as well as other airway devices; an interincisor distance of less than 5 cm may predict difficult intubation. Mouth opening of less than two fingerbreadths has been shown to reduce the prevalence of easy intubation from 95% to 62%.60 Mouth opening also is influenced by cervical spine movement; if movement is limited, mouth opening may also be restricted.61 Protruding maxillary incisors, a single maxillary incisor, and missing maxillary incisors have been shown to be predictive of difficult intubation in obstetric patients.28
Comorbidities, including those not related to pregnancy, may influence airway management and should be considered before anticipated airway management. Most notably, maternal obesity is associated with an increased incidence of airway problems (see earlier discussion).24,25,27 Similarly, difficulties in airway management should be anticipated in patients with severe preeclampsia.
Multivariable Assessments
Individual tests are poorly predictive of airway difficulty; therefore, a number of investigators have combined assessments in an effort to improve specificity. Wilson et al.62 assessed five risk factors (weight, head and neck movement, jaw movement, presence or absence of a receding mandible, prominent teeth). Each variable was scored from 0 to 2, giving a Wilson risk sum. Although 75% of cases of difficult laryngoscopy could be predicted, 12% were falsely predicted to be difficult.62 Subsequent work using the Wilson risk sum found a positive predictive value of only 9%, and consequently it is now rarely used in clinical practice.63
Frerk64 demonstrated that a combination of the Mallampati score and thyromental distance was more predictive than either test alone; the combined assessment had a sensitivity of 81% and a specificity of 98% in predicting a difficult airway. However, owing to the rarity of difficult intubation, the positive predictive value was only 64%.64 Realizing that there was an absence of a clear description and agreement as to the method of performing individual tests, Lewis et al.65 assessed different methods of grading the oropharyngeal view and the mandibular space as predictors of difficult laryngoscopy. Twenty-four different oropharyngeal assessments were considered using two body positions, three head positions, and two tongue positions, each with and without phonation. Similarly, the mandibular space was measured in 24 ways using two body positions, three head positions, and two distal and two proximal endpoints. The results were subject to logistic regression analysis. Although most difficult intubations could be predicted, half of those that were anticipated to be difficult were ultimately found to be easy, even with the most predictive combination of tests.65
Tse et al.66 combined the angle at full head extension (in an upright position, the angle made by a line joining the ear tragus [apex] and the corner of the mouth to a line parallel to the floor [horizontal]), thyromental distance, and Mallampati classification in an attempt to predict difficult intubation in a general surgery population. Although these tests were likely to identify easy intubations, they had low sensitivity for predicting those in whom intubation was difficult.66
In a study of 400 pregnant women scheduled for elective cesarean delivery, Honarmand and Safavi67 evaluated Mallampati class score, ratio of height to thyromental distance, and the ULBT, both in isolation and combination. A total of 8.75% patients had a Cormack and Lehane grade 3 or 4 laryngoscopic view; the ratio of height to thyromental distance was the best predictor of this outcome.67
Recommendations
The thoroughness of the airway assessment often depends on the urgency with which surgery needs to be performed. For emergency procedures, relatively little time is available; and so it is prudent to assess all women in the labor and delivery suite soon after their arrival, focusing on those with the greatest risk for intervention.68 However, changes in assessment during the course of labor must be anticipated, and reevaluation before inducing anesthesia is vital to the safe care of these patients.
The assessment should attempt to identify the patients who will be difficult to ventilate and whose tracheas will be difficult to intubate. It should start with a history to detect factors that may indicate the presence of a difficult airway, as well as the potential risk for pulmonary aspiration. Examination of previous anesthetic records, if available, may indicate problems with ventilation or intubation. The presence of comorbidities such as obesity and preeclampsia should be considered. The ASA Practice Guidelines for Management of the Difficult Airway2 list 11 components that can be assessed (Table 30-3), acknowledging the absence of a single test that can reliably predict who is likely to present difficulty with airway management. Consequently, a combination of assessments is generally considered preferable.
TABLE 30-3
Components of Preoperative Airway Examination
| Airway Examination Component | Nonreassuring Findings |
| 1. Length of upper incisors | Relatively long |
| 2. Relation of maxillary and mandibular incisors during normal jaw closure | Prominent overbite (maxillary incisors anterior to mandibular incisors) |
| 3. Relation of maxillary and mandibular incisors during voluntary protrusion | Patient cannot bring mandibular incisors anterior to maxillary incisors |
| 4. Interincisor distance | Less than 3 cm |
| 5. Visibility of uvula | Not visible when tongue protruded with patient sitting (e.g., Mallampati class > II) |
| 6. Shape of palate | Highly arched or very narrow |
| 7. Compliance of mandibular space | Stiff, indurated, occupied by mass or nonresilient |
| 8. Thyromental distance | Less than three ordinary fingerbreadths |
| 9. Length of neck | Short |
| 10. Thickness of neck | Thick |
| 11. Range of motion of head and neck | Patient cannot touch tip of chin to chest or cannot extend neck |
Modified from American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Practice guidelines for management of the difficult airway. Anesthesiology 2013; 118:251-70.
Performing and documenting mouth opening, the Mallampati class, atlanto-occipital mobility, thyromental distance, and mandibular protrusion may be performed relatively quickly and should identify most patients who will present difficulties with airway management. The preanesthesia evaluation should seek to identify risk factors for difficulty with mask ventilation, laryngoscopy, airway device insertion (including intubation), and performance of a surgical airway. When risk factors are identified, appropriate plans for airway management, such as the ready availability of additional equipment and personnel (e.g., individuals experienced with airway management and the creation of a surgical airway) should be made. The proposed plan should consider that the administration of a neuraxial anesthetic technique may be the safest option for both mother and infant, even in the presence of nonreassuring fetal status. The plan must also include alternatives for situations in which the initial plan is not possible. The risks and benefits of various alternatives should be discussed with the patient and the obstetric, neonatal, and nursing teams and documented in the patient's medical record.
Prophylaxis
Neuraxial Labor Analgesia
The widespread acceptance and use of neuraxial analgesic and anesthetic techniques for obstetric patients has significantly reduced the need for general anesthesia and airway manipulation. In obstetric patients in whom difficulty in airway management or neuraxial technique administration is anticipated or when risk factors for an urgent or emergent cesarean delivery are present, early or prophylactic placement of an epidural catheter should be encouraged. A prophylactic epidural catheter is one that is placed and tested with a small dose of local anesthetic; analgesia is not established until active labor begins, the patient requests analgesia, and/or an operative delivery is required. Such a catheter provides a readily available conduit for providing neuraxial analgesia or anesthesia, especially if rapid onset (e.g., an emergency cesarean delivery) is desirable. Early epidural catheter placement also allows the procedure to take place in a controlled setting and allows time for catheter manipulation and replacement, if necessary, before further pathophysiologic changes (e.g., decreased platelet count, worsening airway edema) occur. The correct placement of the epidural catheter in the epidural space should be tested with the injection of a local anesthetic test dose and careful bilateral sensory testing to confirm the presence of bilateral neuroblockade.
Unfortunately, labor analgesia cannot always be successfully converted to surgical anesthesia for an operative delivery; reported failure rates are as high as 8%.69 A 2012 meta-analysis has demonstrated the need for conversion to general anesthesia in 5% of women who receive epidural analgesia in labor70; higher failure rates are observed among women requiring more physician interventions for inadequate epidural labor analgesia, in settings of need for urgent delivery, and when an anesthesiologist without specialty training or experience in obstetric anesthesia is providing care. Consequently, women receiving labor epidural analgesia must be evaluated at regular intervals; if analgesia is inadequate, re-siting the epidural catheter must be considered. A meta-analysis of studies comparing different local anesthetics for conversion of epidural analgesia to anesthesia found that lidocaine with epinephrine has a faster onset than bupivacaine, levobupivacaine, or ropivacaine.71 The addition of bicarbonate to chloroprocaine or lidocaine with epinephrine further hastens the onset of local anesthetic action (see Chapter 26).
In situations in which conversion of epidural analgesia to anesthesia is not possible, general anesthesia may still be avoided if time permits the initiation of spinal or combined spinal-epidural anesthesia. However, care should be taken when performing a spinal anesthetic after a failed epidural top-up dose of local anesthetic, because cases of high and total spinal anesthesia have been reported in this setting (see Chapter 26). An airway management plan must always be in place, even if the primary plan is for the administration of neuraxial anesthesia.
Fasting and Antacid Prophylaxis
All obstetric patients requiring surgical anesthesia are at risk for pulmonary aspiration of gastric contents, particularly if airway difficulties are encountered (see Chapter 29). Because conversion from neuraxial to general anesthesia may be required either before or during surgery, strategies must be adopted to minimize this risk. The ASA and the American College of Obstetricians and Gynecologists (ACOG) recommendations allow modest amounts of clear liquid with uncomplicated labor, but they recommend the avoidance of solid foods in laboring women.72,73 Clear liquids and solids are allowed up to 2 hours and 6 to 8 hours, respectively, before an elective operative procedure.72,73 However, more liberal policies on oral intake in labor have become increasingly widespread as maternal death from aspiration becomes less common. The U.K. National Institute for Health and Clinical Excellence suggests that women be allowed to drink isotonic fluids in labor and eat a light diet unless they develop risk factors that make general anesthesia more likely.74 Consequently, all laboring women should be assessed and oral intake restricted if surgical intervention appears likely.
Once surgical intervention is required, antacids such as intravenous histamine-2 (H2)-receptor antagonists or proton-pump inhibitors should be administered in case general anesthesia is necessary. However, these drugs take up to 30 minutes to become effective. If emergency general anesthesia is required, oral administration of a nonparticulate antacid such as sodium citrate is used to increase the pH of gastric contents. A dose of sodium citrate (0.3 molar) 30 mL is effective for approximately 30 minutes and should be administered shortly before the induction of general anesthesia.75
Metoclopramide promotes gastric emptying and increases lower esophageal sphincter tone, although its efficacy is decreased by concurrent use of opioids. It may be given orally in labor or intravenously before general anesthesia.
A number of deaths from presumed aspiration after tracheal extubation were reported in the most recent Confidential Enquiries into Maternal Deaths in the United Kingdom report.6 The report's authors recommended that when general anesthesia is administered to woman with a potentially full stomach, consideration should be given to passing an “in and out” orogastric tube before extubation.
Patient Positioning
The optimal view during laryngoscopy, which yields the best chance for a successful intubation, requires appropriate patient positioning. The sniffing position, with 35 degrees of neck flexion and 15 degrees of head extension, has been considered the ideal position for facilitating a view of the glottis by aligning the oral, pharyngeal, and laryngeal axes (Figure 30-7).76 Although use of the sniffing position has recently been questioned, most studies find it superior to other positions. The correct use of the sniffing position requires that the external auditory meatus and sternum are in horizontal alignment. Some video laryngoscopes (see later discussion) do not require the patient's head and neck to be in the sniffing position for successful device use.
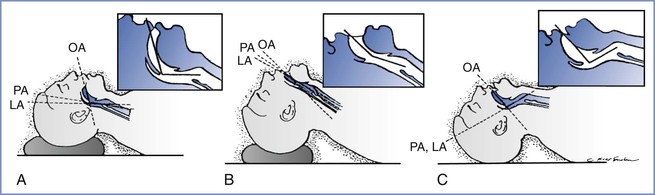
FIGURE 30-7 Head and neck position during laryngoscopy. As the head position changes from neutral, the alignment of the oral axis (OA), pharyngeal axis (PA), and laryngeal axis (LA) changes within the upper airway. A, The head is resting on a large pad that flexes the neck on the chest and aligns the LA with the PA (the neutral position). B, The head is resting on a pad (which flexes the neck on the chest) and concomitant extension of the head on the neck can be seen, which brings all three axes into alignment (the sniffing position). C, Extension of the head on the neck without concomitant elevation of the head on a pad, which results in nonalignment of the PA and LA with the OA. (From Benumof JL. Conventional [laryngoscopic] orotracheal and nasotracheal intubation [single-lumen type]. In Benumof JL, editor. Clinical Procedures in Anesthesia and Intensive Care. Philadelphia, JB Lippincott, 1991:115-48.)
For obese patients, a ramped position is preferable (Figure 30-8). The anteroposterior chest diameter is increased in obese patients, making 35 degrees of neck flexion unachievable in the supine position. Consequently, the shoulders and upper torso need to be raised; this position can be achieved with the use of blankets or pillows or one of the many commercially available, wedge-shaped positioning cushions. Optimal elevation is verified by checking that the external auditory meatus and sternoclavicular joint are in horizontal alignment. Elevating the back of the operating table by 25 degrees may make laryngoscopy easier and also aids preoxygenation (discussed later). The operating room table should be elevated to a height at which the laryngoscopist is most comfortable, with space at the head of the bed to accommodate access for the anesthesia team and necessary equipment.
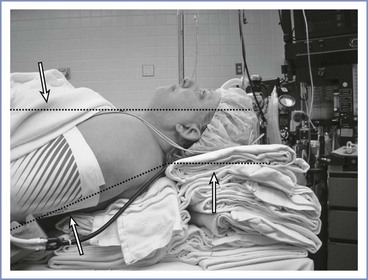
FIGURE 30-8 A morbidly obese patient is in an optimal position for direct laryngoscopy when an imaginary horizontal line can be drawn from the sternal notch through (or slightly anterior to) the external auditory meatus. To achieve this, the upper back and shoulders should be significantly elevated with pads or blankets (or commercial elevation wedge/pillow) to allow the head to be extended at the atlanto-occipital joint. Additional blankets should be used to support the head in this position.
Left uterine displacement to minimize aortocaval compression should be maintained during preparation for, and induction of, general anesthesia. This may be achieved by tilting the operating table or by placing a wedge under the right hip.
Denitrogenation (Preoxygenation)
In pregnancy, the decrease in FRC and increase in oxygen requirement result in rapid oxygen desaturation during periods of apnea (e.g., during induction of general anesthesia). The FRC is the primary reservoir for oxygen during apnea. Therefore, effective denitrogenation, or preoxygenation, of the FRC is vital to delay the onset of hypoxemia.
The standard technique for preoxygenation has been to breathe 100% oxygen through a tight-fitting facemask at normal tidal volumes for 3 to 5 minutes. Given the urgent nature of obstetric general anesthesia, attention has focused on whether several maximal deep breaths over a shorter period can be as effective. Chiron et al.77 compared a traditional 3-minute technique with either eight deep breaths over 1 minute (8 DB/1 min) or four deep breaths over 30 seconds (4 DB/30 sec). By monitoring with end-tidal fractional oxygen concentration (FETO2), which is probably the best marker of lung denitrogenation, the authors found that 3 minutes of tidal volumes or the 8 DB/1 min technique was more effective than the 4 DB/30 sec technique. They suggested using the 8 DB/1 min technique in the setting of emergency obstetric anesthesia.
The use of maximal deep breaths to achieve denitrogenation may cause maternal hypocarbia and, therefore, should be limited. However, preoxygenation with the 4 DB/30 sec technique leads to more rapid desaturation than standard normal tidal volume breathing or the 8 DB/1 min technique, indicating that a longer period of time is required to maximize oxygen storage in tissue and vascular body compartments. Indeed, during apnea, the time to desaturation depends on (1) the amount of oxygen stored in the lungs, tissue, and blood; (2) the mixed venous oxyhemoglobin saturation; and (3) the presence of intrapulmonary shunting.
A tight-fitting facemask is necessary to prevent air entrainment, which reduces the efficiency of preoxygenation. With normal tidal volume breathing, preoxygenation is best achieved with oxygen flow rates in excess of 10 L/min for 3 minutes,78 although this may still be inadequate due to air entrainment; some authors suggest FETO2 should be greater than 0.8 before anesthesia is induced.79 A 20 degree to 30 degree head-up tilt increases the FRC and delays the time to desaturation, especially in obese patients.80,81
Rapid-Sequence Induction and Cricoid Pressure
In an attempt to minimize the risk for aspiration, the rapid-sequence induction has become the standard technique for induction of obstetric general anesthesia. It usually consists of preoxygenation, rapid intravenous injection of a predetermined dose of induction agent followed immediately by succinylcholine administration, application of cricoid pressure, and avoidance of positive-pressure ventilation before tracheal intubation with a cuffed endotracheal tube. The relative urgency of intubation, in a patient in whom ventilation is avoided, may increase the likelihood of failure.
Although in widespread use, the conduct of rapid-sequence induction is not uniform. Induction agents should provide rapid loss of consciousness with minimal hemodynamic instability while improving the quality of intubating conditions.82 Thiopental has traditionally been the induction agent of choice for obstetric general anesthesia.83 When used with succinylcholine, it provides intubating conditions that are as good or better than other agents. An additional advantage is that it can be reconstituted and stored for future use. Furthermore, some evidence suggests that, compared with propofol, thiopental leads to less maternal hypotension and fewer detrimental effects on the neonate.83 Propofol, however, appears to offer better intubating conditions when combined with rocuronium; compared with thiopental in nonobstetric patients, visualization of the vocal cords was significantly more likely with propofol.84 Its superiority to thiopental is most likely related to its greater ability to suppress pharyngeal and laryngeal reflexes. However, when succinylcholine is used, the choice of induction agent has no significant effect on intubating conditions.82
Opioids have traditionally not been part of rapid-sequence induction because of concerns of respiratory depression should intubation fail. Moreover, in the obstetric population, the potential for neonatal depression is greater if opioids are used because of rapid placental transfer of these drugs. In nonobstetric patients, the addition of opioids produces better intubating conditions when combined with rocuronium; this improvement has not been demonstrated with succinylcholine.82
Succinylcholine is associated with a number of undesirable side effects, most notably a prolonged duration of action in patients with cholinesterase deficiency and a trigger for malignant hyperthermia and anaphylaxis. However, because of its rapid onset, succinylcholine has traditionally been the muscle relaxant of choice for rapid-sequence induction of anesthesia in obstetric patients. Despite reduced levels of plasma pseudocholinesterase in pregnancy, the duration of action of succinylcholine remains clinically unchanged in the obstetric patient.85 The ideal dose of succinylcholine, traditionally 1 mg/kg, remains controversial86; when combined with opioids in nonobstetric patients, succinylcholine 1 mg/kg fails to produce good intubating conditions at 1 minute in up to 8% of cases.87 Naguib et al.87 found that succinylcholine doses as high as 2 mg/kg still do not guarantee excellent intubating condition in all patients; however, the authors found little extra benefit from using doses above 1.5 mg/kg.
The potential disadvantage of increasing the succinylcholine dose is delayed return of spontaneous respiration; return of spontaneous ventilation is vital should intubation not be achieved. Reducing the succinylcholine dose to 0.5 to 0.6 mg/kg does not appear to compromise intubating conditions, at least when administered in the nonobstetric population with propofol and fentanyl; the lower dose slightly shortens the recovery time.88 In pregnancy, however, the reduced maternal FRC and greater oxygen demands make significant desaturation likely before the return of spontaneous respiration, no matter the succinylcholine dose. Because thiopental remains a widely used induction agent, and opioids are not commonly administered, continued use of succinylcholine 1 to 1.5 mg/kg is recommended.
The side effect profile of succinylcholine has resulted in consideration of alternative muscle relaxants for the rapid-sequence technique. Rocuronium is often used when succinylcholine is contraindicated; however, its prolonged duration of action is a significant concern when failure to ventilate or intubate occurs. Sugammadex, with its ability to rapidly reverse the effects of rocuronium, may help resolve this controversial issue; however, the drug is not available in the United States and there have been no clinical trials in pregnancy. When used with propofol for rapid-sequence induction, a 2008 meta-analysis indicated that succinylcholine (1 mg/kg or greater) produced superior intubating conditions compared with rocuronium (0.6 to 0.7 mg/kg) (relative risk [RR], 0.88; 95% CI, 0.80 to 0.97).89 No significant difference was observed between the two agents when a larger dose of rocuronium (1.2 mg/kg) was used.89 Therefore, succinylcholine remains the preferred muscle relaxant for use in rapid-sequence induction.
Cricoid pressure as a method to decrease pulmonary aspiration during induction of anesthesia was first described in 1961.90 Interest in the technique was promoted by the reports of deaths due to aspiration under general anesthesia.91 Although the effectiveness of cricoid pressure in preventing pulmonary aspiration of gastric contents has recently been challenged,92,93 it is frequently used during the induction of obstetric general anesthesia (see Chapters 26 and 29). However, the use of cricoid pressure can adversely affect the ease of ventilation, laryngoscopy, and intubation. In a comparison of cricoid pressure with 20 N, 30 N, and 44 N of force, increasing pressure was more likely to lead to cricoid deformity and esophageal occlusion, particularly in women.94 Difficulty with ventilation is less likely when 30 N is applied (currently accepted practice) than with 44 N (the previously suggested optimum value).95 When correctly applied, with an increase in force from 10 N to 30 N with the induction of general anesthesia, there is little evidence of harm. However, when difficulty with intubation or ventilation arises, pressure may need to be reduced or released (see later discussion).
Although the cricoid pressure technique originally described by Sellick90 was a one-handed technique, the placement of a second hand behind the patient's neck to prevent excessive neck flexion has been observed to provide a superior laryngoscopic view.96 However, it should also be remembered that the two-handed cricoid pressure technique does not allow the anesthetic assistant to assist with other procedures, such as holding additional equipment necessary for difficult airway management.
Management
Planning
The approach to the difficult airway in the obstetric patient depends on the situation as well as the skill set of the anesthesia provider. In Figure 30-9 a suggested approach is outlined for management of obstetric patients with an anticipated difficult airway. After an initial assessment of the patient, an airway management plan should be created and shared with the patient and other members of the multidisciplinary team. In extreme cases, the anesthesia considerations may influence the mode and timing of delivery. Despite a thorough airway assessment and management plan, unanticipated or unrecognized airway issues and complications may arise; alternative algorithms and equipment should be readily available to ensure oxygenation and ventilation. Emergence and extubation should also be planned in advance. Lack of forethought and planning can lead to poor decision-making in crisis situations.
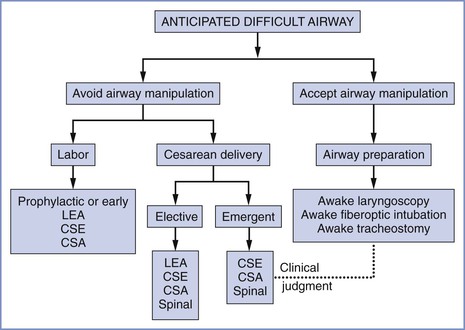
FIGURE 30-9 Algorithm for anticipated difficult airway. This algorithm is not intended to provide comprehensive guidance that addresses every contingency. Rather, it should help anesthesia providers consider the various options that are available. Management should be individualized, and the anesthesia provider's clinical skills and judgment should guide decision-making. For additional information, the reader is referred to the American Society of Anesthesiologists practice guidelines.2 CSA, continuous spinal anesthesia technique; CSE, combined spinal-epidural technique; LEA, lumbar epidural analgesia or anesthesia technique; spinal, spinal anesthesia technique.
Neuraxial Anesthesia
The value of establishing and confirming a functional epidural catheter during labor in patients with an anticipated difficult airway has been described (see earlier discussion). A neuraxial anesthetic technique may also be preferable in patients with an anticipated difficult airway undergoing urgent or elective cesarean delivery. The choice of anesthetic technique (e.g., single-shot spinal, combined spinal-epidural, epidural, continuous spinal techniques) depends on the circumstances and preferences of the anesthesia provider. Neuraxial techniques do not obviate the necessity of planning airway management. High spinal anesthesia necessitating urgent airway intervention is a complication of all neuraxial techniques. Epidural anesthesia may be complicated by unintentional intravascular or intrathecal injection. Despite optimal planning and execution, a neuraxial anesthetic technique may fail to provide a surgical blockade of adequate density or duration.33,58,97 Therefore, plans for securing the airway must always be preformulated, and standard and alternative airway equipment should be readily available.
Awake Intubation before General Anesthesia
Performing an awake intubation may be the safest option for the patient with an anticipated difficult airway, particularly if very difficult or impossible mask ventilation is anticipated or if neuraxial anesthesia is contraindicated or fails. Even patients with an advanced upper airway pathologic process have the ability to breathe when awake. However, the induction of general anesthesia with paralysis can distort the airway anatomy by allowing soft tissue relaxation and movement of the larynx in an anterior direction; this distortion can make attempts at direct laryngoscopy more difficult. Therefore, an appropriate sequence of events includes securing the airway of these patients while they are awake and spontaneously breathing, before the induction of general anesthesia.98 There is a perceived notion, particularly among practitioners with limited experience with the technique, that an awake intubation is time consuming, results in patient discomfort and anxiety, and is often difficult. In skilled hands, the technique can be accomplished quickly and comfortably with a high success rate.99
Awake intubation can be performed with a number of airway management devices, but the flexible fiberoptic bronchoscope offers unique advantages (Box 30-2). Proper planning and execution, with attention to detail, are keys to patient cooperation and a high success rate. Appropriate equipment must be readily available and experienced assistance is desirable. It is useful to have two anesthesia providers: one to perform the endoscopy and another to monitor the patient.100 Pulse oximetry, capnography, continuous electrocardiography, and blood pressure monitoring are mandatory. The level of conscious sedation must be constantly monitored to obtain the desired level for the procedure (see later discussion). Supplemental oxygen should be administered.
An unhurried, thorough explanation of the technique to the patient helps to allay anxiety. Pharmacologic premedication should include prophylaxis for pulmonary aspiration and an antisialagogue such as intravenous glycopyrrolate 0.2 mg. A dry mouth improves topical oral anesthesia by ensuring better contact between the local anesthetic and the mucosa.101 Secretions may also cause internal reflection from the light source and distort the fiberoptic view. Performing the procedure with the patient in the upright, rather than supine, position minimizes airway obstruction and aortocaval occlusion, enhances drainage of secretions, and allows better acceptance of topical anesthesia by the patient.
Conscious Sedation
The term awake intubation is a misnomer because in practice most patients receive some form of sedation to relieve anxiety, produce amnesia, and reduce pain and discomfort during the procedure. Moderate sedation/analgesia, also termed conscious sedation, is a drug-induced depression of consciousness during which the patient can respond purposefully to verbal or tactile stimulation. No interventions are required to maintain a patent airway, and spontaneous ventilation is adequate.102 An overdose of the sedative/hypnotic or analgesic drugs can result in airway obstruction, hypoxemia, and cardiorespiratory depression; maintenance of continuous verbal contact is the optimal method for avoiding oversedation.103
The choice of drugs to produce conscious sedation depends on the preference and experience of the anesthesia provider. Small boluses of intravenous midazolam (0.5 to 1 mg) and fentanyl (25 to 50 µg) are usually adequate104; the use of a propofol infusion has also been described.105 Remifentanil may confer some advantages over fentanyl in providing rapid onset, more precise titration with the ability to use an infusion, and rapid metabolism and dissipation of effects; decreased respiratory rate or apnea may be quickly reversed by stopping the infusion. Remifentanil infusion rates between 0.05 and 0.175 µg/kg/min have been used for awake fiberoptic intubation in nonobstetric patients106; target-controlled infusions of remifentanil, with or without propofol, can also provide ideal conditions.107 Neonatal effects of the drugs used for sedation are usually minimal; however, the neonatologist should be informed of the drugs administered to the mother before delivery.
Topical Anesthesia
Providing adequate topical anesthesia of the upper respiratory tract is one of the most critical elements of successful awake fiberoptic intubation. Local anesthetic agents can be used in two basic ways to provide topical upper airway anesthesia: direct application to the mucosa or the injection for laryngeal nerve blocks. Topical application of local anesthetic is the most commonly used technique, owing to its ease and effectiveness. There are a number of techniques. For example, the patient can be asked to gargle and slowly swallow viscous lidocaine (2% or 4%), or lidocaine (2%, 4%, or 10%) can be aerosolized and sprayed onto the tongue and oropharynx.
A number of commercially available devices, which are produced in a variety of shapes and sizes, can aerosolize and spray local anesthetic solutions in a jetlike stream. The Mackenzie technique uses an intravenous cannula with an injection port (e.g., 20- or 18-gauge) connected to oxygen tubing via a three-way connector (Figure 30-10). Administration of local anesthetic from a syringe through the connector, with the oxygen flowing at 2 L/min, creates a jetlike spray.108 An additional method uses a nebulizer mask or mouth piece, with 4% lidocaine (4 to 6 mL) placed in the nebulizer bowl and connected to an oxygen source at a flow rate of 8 L/min. This method is easy to administer, noninvasive, and comfortable for the patient, with minimal or absent coughing. Each of these techniques may be insufficient as a single entity and may be combined with other methods, including instillation of local anesthetic through the working channel of the fiberoptic bronchoscope channel.
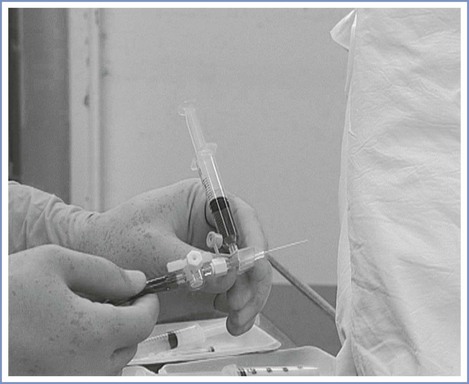
FIGURE 30-10 Topical airway anesthesia. The MacKenzie technique108 uses a 20-gauge intravenous cannula with an injection port connected to oxygen tubing via a three-way tap to produce a jetlike spray of local anesthetic administered from a syringe connected to the cannula with the oxygen flowing at 2 L/min.
The “spray as you go” (SAYGO) technique uses the working channel of the fiberoptic bronchoscope to instill local anesthetic onto the mucous membranes of the airway. The working channel of an intubating fiberoptic bronchoscope, such as the Olympus LF-2 (Olympus America Inc. Centre Valley, PA, USA), is 600 mm long and 1.5 mm in diameter. If a small syringe is directly attached to the working channel port and the solution is merely injected, the local anesthetic is likely to stay in the channel rather than be sprayed onto the mucosa. This problem can be overcome by placing an epidural catheter through the working channel; using a Luer-Lok connector for the epidural catheter allows a direct and tight connection with the local anesthetic syringe and avoids leakage. The local anesthetic agent is drawn up in a 2-mL syringe and “dripped” on the mucus membranes; this instillation can be better targeted if the distal tip of the epidural catheter is allowed to protrude approximately 1 cm from the tip of the fiberoptic bronchoscope.
Nerve Blocks
The nerve supply to the upper airway is derived from branches of cranial nerves V, VII, IX and X. The lingual branch of the glossopharyngeal nerve (IX), which innervates the submucosal pressure receptors at the base of the tongue, can be blocked with the bilateral administration of 1% lidocaine (2 mL) just under the mucosa at the base of the anterior tonsillar pillars. The value or necessity of this block during performance of awake intubation in obstetric patients is controversial.109 Laryngeal and tracheal sensation can be minimized with blockade of the internal branch of the superior laryngeal nerve and transtracheal administration of lidocaine, respectively. Blockade of the superior laryngeal nerve may be performed by locating the greater cornu of the hyoid bone, advancing a small-bore needle until the bone is contacted, walking the needle off the edge of the bone into the thyrohyoid membrane, and injecting 1% lidocaine, approximately 3 mL. The injection is then repeated on the other side of the neck.
Historically, nerve blocks were an essential part of preparing the upper airway; today, meticulous topical application is easier to perform, less invasive, and provides effective intubating conditions. The preferred airway anesthesia technique used at our institution for topical anesthesia for oral awake fiberoptic intubation is described in Box 30-3.
Airway Anesthesia and Risk for Aspiration
Some anesthesia providers are concerned that local anesthesia of the larynx might obtund the reflexes for protecting the airway. An early study found that an unprotected glottis might result from translaryngeal block.110 There is evidence that local anesthetic solutions spread to the superior aspect of the vocal cords after a translaryngeal block.111 However, in a series of 129 patients, both the translaryngeal injection and SAYGO techniques were effective and safe, with no evidence of regurgitation or aspiration in any patient.112 It has been suggested that topical anesthesia of the larynx does not impair voluntary motor function of the vocal cords, such as coughing on request,113 thus allowing the patient to protect her airway. The SAYGO technique may be preferred because the interval between topical anesthesia and endoscopy is minimal and if gastric reflux occurs during endoscopy it can be visualized and the gastric juice aspirated through the fiberoptic bronchoscope. The key to minimizing the risk for aspiration, however, is avoidance of oversedation. Nonetheless, administration of aspiration prophylaxis is advised and the patient should be monitored for possible reflux or emesis.
Fiberoptic Intubation
Fiberoptic laryngoscopy can be performed orally or nasally, but the oral route is more common because of the engorgement of the nasal mucosa and the potential for epistaxis. However, in very specific situations (i.e., when the oral aperture is insufficient to allow fiberoptic bronchoscope passage), the nasal route can be successfully used in the obstetric patient with careful topical preparation of the nasal mucosa with agents that provide anesthesia and vasoconstriction.43
A common impediment to successful fiberoptic laryngoscopy is being able to easily advance the endotracheal tube (ETT) into the correct position. Guiding the ETT over the fiberoptic bronchoscope is a “blind” procedure. The ETT most commonly arrests at the right arytenoid cartilage.114 In a review of the causes, incidence, and solutions to this issue, Asai and Shingu115 noted that impingement can be minimized by selecting the appropriate size and type of ETT and by using proper advancement technique. Smaller-diameter tracheal tubes (6 to 7 mm internal diameter [ID]) that fit more snugly onto the bronchoscope generally advance more easily. The design and flexibility of the tube and tip may also determine success; for example, the intubating LMA endotracheal tube with its Huber tip is easier to advance than a flexometallic ETT during nasal fiberoptic intubation, probably owing to the acute angle of the Huber tip (Figure 30-11).116
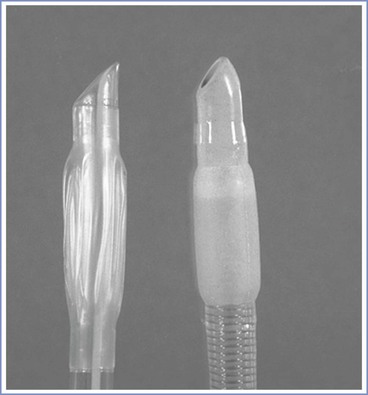
FIGURE 30-11 Endotracheal tube tips. Left, The tip of a conventional endotracheal tube. Right, The tip of an endotracheal tube used with an intubating laryngeal mask airway (LMA). The Huber tip of the intubating LMA endotracheal tube is less likely to impinge on other structures, thus increasing the success of advancement and correct placement in the trachea.
The lubricated ETT is loaded over the fiberoptic bronchoscope in its normal position (curve facing anterior, leading edge [tip] on the right, bevel facing left) and the fiberoptic bronchoscope is advanced into the airway. After the tip of the fiberoptic bronchoscope is positioned above the carina, the ETT is advanced over the fiberoptic bronchoscope into the airway. If impingement occurs, the ETT is withdrawn approximately 1 cm and rotated 90 degrees counterclockwise to bring the tip of the ETT anteriorly and the ETT is reinserted. If this does not work, the ETT is rotated a further 90 degrees counterclockwise and advancement is reattempted. Alternatively, the tube can be loaded on the fiberoptic bronchoscope with the tip facing anteriorly or other maneuvers may be employed, such as keeping the airway patent with jaw thrust and application of pressure on the neck to shift the vocal cords posteriorly.
Awake direct laryngoscopy results in more noxious stimulation than fiberoptic laryngoscopy, but a well-prepared and highly motivated patient may tolerate the procedure surprisingly well. Awake intubation also has been described using indirect or video laryngoscopes (see later discussion), but their role in awake intubation in the obstetric setting has yet to be determined. Other techniques for awake intubation, such as blind nasal intubation and retrograde intubation, are performed infrequently in obstetric patients.
Indirect Optical/Video Laryngoscopy
Since the introduction of the Bullard laryngoscope in the late 1980s, a number of rigid indirect-optical laryngoscopes have been developed. Frequently referred to as video laryngoscopes because of a video camera eye positioned near the tip of the blade, these devices can capture an image of the glottis in real time and transmit it to a video screen.117 Video laryngoscopes offer several advantages over conventional direct laryngoscopy. To obtain a good view of the glottis with a direct laryngoscope, a line of sight from the oral opening to the glottis must be obtained by neck flexion and head extension (see earlier discussion). With video laryngoscopes, a direct line of sight to the glottis is unnecessary. In patients with an anteriorly positioned larynx, an assistant is frequently required to apply pressure on the thyroid cartilage to move the larynx posteriorly and improve the view; with the use of video laryngoscopes, the assistant may watch the screen image to more directly witness the effect of the pressure. Randomized controlled studies have found higher success rates for intubation with several types of video laryngoscopes compared with the Macintosh laryngoscope blade in adult nonobstetric patients with predicted difficult airways.118-122 Video laryngoscopes are perceived to cause less trauma and stress to patients owing to less need to reposition the head and neck, less pressure on the neck, and less frequent use of ETT introducers118,123; however, data are currently insufficient to confirm this perception.117 Data are conflicting as to whether the elapsed time required to intubate using video laryngoscopy is longer or shorter than that required using conventional laryngoscopy.117
The indirect-optical and video laryngoscopes may be classified into three categories117:
1. Macintosh type (e.g., C–MAC, Karl Storz Endoscopy, Tuttlingen, Germany). These devices have a Macintosh-type blade and an insertion method similar to that used with conventional laryngoscopy. The glottis is visualized either directly or on the video screen. In the setting of anticipated difficult airway, the success rate is generally higher with these devices than with direct laryngoscopy, but external pressure and an ETT introducer are more frequently required.118
2. Anatomically shaped without a tube guide (e.g., GlideScope video laryngoscope, Verathon Inc., Bothell, WA, USA; McGrath video laryngoscope, LMA North America, San Diego, CA, USA). The curved shape of the blade allows a view of the glottis without flexing or extending the head and neck; however, directing the ETT toward the glottis maybe difficult, resulting in trauma. Several reports have described pharyngeal and palatal injury with use of the GlideScope.124-128
3. Anatomically shaped blade with tube guide (e.g. Airtraq, King Systems Corporation, Noblesville, IN, USA; The Airway Scope AWS-S100, Hoya-Pentax, Tokyo, Japan). The tip of the tube is captured on the video screen even before the device is inserted, and hence its location can be continuously confirmed during the entire course of intubation.
There are few studies that compare the use of different video laryngoscopes in patients with an anticipated difficult airway.129,130 Furthermore, it not known whether the preoperative assessments used to predict difficult direct laryngoscopy are valid predictors of difficult video laryngoscopy. Additional investigation is necessary to compare various devices and to ascertain whether specific devices or device types are better for specific airway variations.
Dhonneur et al.131 reported the successful use of a difficult airway algorithm in which the Airtraq device was used in parturients as a rescue device if tracheal intubation failed after 2 minutes of direct laryngoscopy. During a 6-month period, 69 parturients underwent emergency cesarean delivery under general anesthesia; 2 morbidly obese parturients required the Airtraq device for successful tracheal intubation. The investigators suggested that the device might be an acceptable primary airway management tool in cases of emergency cesarean delivery in parturients with an anticipated difficult airway. Aziz et al.132 retrospectively analyzed 180 tracheal intubations over a 3-year period in their obstetric unit. Traditional direct laryngoscopy resulted in 157 of 163 successful intubations on first attempt, with one failed intubation (95% CI, 92% to 99%). Video laryngoscopy with a GlideScope resulted in 18 of 18 successful intubations on the first attempt (95% CI, 81% to 100%) and a successful intubation in the patient with the failed direct laryngoscopy. Of note, the patients whose tracheas were intubated with the video laryngoscope were more likely to require urgent or emergency surgery and/or have predictors of difficult direct laryngoscopy than the patients whose tracheas were intubated using direct laryngoscopy.
The GlideScope has also been used for awake intubation or to assist fiberoptic laryngoscopy intubation.133,134
Awake Tracheostomy or Surgery Standby
It is possible to perform an awake tracheostomy with local anesthesia, a technique that may be required in some situations in which airway management is anticipated to be extremely difficult and dangerous.103,135,136 In some cases, particularly if there is a known airway pathologic process, it is prudent to request that a surgical team proficient in emergency surgical airway management be immediately available before the induction of anesthesia for cesarean delivery.
Local Anesthesia for Cesarean Delivery
Rarely, the infiltration of local anesthesia may be used as a primary anesthetic technique for emergency cesarean delivery in the patient with an anticipated difficult airway. This technique, which has been well described, is most often used in developing countries, where contemporary anesthetic techniques may not be readily available.137-139 Few obstetricians today are familiar or proficient with this technique, but some resident training programs still provide instruction on its use.58 A large volume (i.e., 75 to 100 mL) of a dilute local anesthetic solution, such as 0.5% lidocaine, is often required. Administration of such a large volume entails a risk for systemic local anesthetic toxicity.
Mei et al.140 described four cases in which cesarean delivery was performed with bilateral transversus abdominis plane (TAP) block and ilioinguinal-iliohypogastric (IIIH) nerve blocks using 0.5% ropivacaine 40 mL.
In some cases it is possible to perform the entire surgical procedure with local infiltration, provided the obstetrician makes a midline abdominal incision, makes minimal use of retractors, and does not exteriorize the uterus. Alternatively, the obstetrician might begin surgery and deliver the infant with the aid of local infiltration. Temporary hemostasis may be achieved until the airway is secured and then surgery completed after the induction of general anesthesia.
Cesarean delivery performed with local infiltration, if successful, has the advantages of preserving maternal hemodynamic stability and a patent airway while allowing emergency delivery of the infant. However, the technique requires a skilled and patient obstetrician. Maternal anesthesia is typically incomplete and often inadequate, a fact that subsequently presents significant management issues, given that the abdomen has been opened, positioning options are limited, and the consequences of the surgical procedure such as hemorrhage may require immediate attention.
The Unanticipated Difficult Airway
Features of the Obstetric Patient
Despite attempts to adequately assess parturients preoperatively, cases of unanticipated difficulty with airway management do occur (Figure 30-12).141 Therefore, the anesthesia provider and the entire operating team should have a plan to manage unanticipated difficulties in airway management before administering general anesthesia to obstetric patients. Although national guidelines exist for this scenario in the nonobstetric patient,2,8 none exist specifically for the obstetric patient. The lack of guidelines likely reflects the unique and often difficult conflict posed by the failure to intubate the mother's trachea: the optimal and safe management of the mother may threaten the life of the fetus and vice versa. A classification schema for the degree of urgency of cesarean delivery142 may help clarify the risks to the mother and fetus, thereby improving communication among providers, particularly between anesthesia providers and team members whose primary focus may be directed toward the fetus (obstetricians, midwives, neonatologists). Communication between anesthesia providers and other members of the multidisciplinary team is vital,73 especially if the anesthesia provider(s) believes it is in the best interest of the mother to delay delivery of the fetus to manage the difficult airway. The entire operating room team should be familiar with local protocols to manage this infrequent but life-threatening situation. Examples of failed intubation guidelines are available on the website of the Obstetric Anaesthetists' Association (OAA).143
FIGURE 30-12 Algorithm for unanticipated difficult airway. This algorithm is not intended to provide comprehensive guidance that addresses every contingency. Rather, it should help anesthesia providers consider the various options that are available. Management should be individualized, and the anesthesia provider's clinical skills and judgment should guide decision-making. For additional information, the reader is referred to the American Society of Anesthesiologists practice guidelines.2 SGA, supraglottic airway; LMA, laryngeal mask airway.
The safest method to prevent difficulty in airway management is by making the first attempt at laryngoscopy the best attempt. This is achieved by using optimal head and neck position, applying cricoid pressure correctly, and waiting for an adequate dose of muscle relaxant to act (see earlier discussion). If an adequate view of the larynx is not achieved, a second attempt should be made using a gum elastic bougie and/or a different laryngoscopy blade or device. In experienced hands, laryngoscopy attempts should be limited to no more than three, and the second or third attempt should be performed only in those cases in which a portion of the laryngeal anatomy is visible (Cormack and Lehane grade 3A or better). If a grade 3B or 4 laryngoscopic view is identified with the initial laryngoscopy attempt, the anesthesia provider should immediately focus attention on ensuring adequate oxygenation and ventilation of the mother.144
The fact that the trachea cannot be intubated should be conveyed loudly to the entire operating room team and help should be summoned immediately. Maintenance of maternal and fetal oxygenation becomes the anesthesia provider's first priority. Facemask ventilation should be attempted with maintenance of cricoid pressure. Placement of an oropharyngeal airway may be helpful. If difficulty is encountered, a two-person mask technique should be employed, with one person firmly holding the mask in place with two hands while providing a jaw lift as the second person squeezes the reservoir bag.58,141 Once facemask ventilation is attempted, several scenarios may arise, which are discussed next.
Cannot Intubate but Can Ventilate
In the “cannot intubate but can ventilate” scenario, maternal and fetal status should be rapidly assessed in consultation with the obstetrician. If the mother and fetus are not in immediate jeopardy, then the safest course is to awaken the mother. Once this is accomplished, other anesthetic options, such as an awake intubation or a neuraxial anesthetic technique, should be considered. The risk for proceeding with surgery in this scenario represents an elective commitment to the possibility of mask airway failure, more airway manipulation, aspiration, and progression to a “cannot ventilate” scenario.
If the situation is immediately life threatening to the mother secondary to hemorrhage (e.g., uterine rupture, placental abruption), it may be necessary to proceed with cesarean delivery to optimize outcome for both the mother and infant. Significant angst and controversy often accompany decision-making in the management of a stable mother with evidence of life-threatening fetal compromise, such as fetal bradycardia as a result of a prolapsed umbilical cord. In such cases, if mask ventilation is easy and adequate, the risk-benefit ratio of proceeding with an unsecured airway and an increased risk for aspiration should be weighed against the benefits of prompt delivery of the infant. In cases in which the maternal risk for aspiration is considered low and mask ventilation is easy, it may be reasonable to continue mask ventilation and avoid further intubation attempts. It is unclear as to whether continued mask ventilation or repeated intubation attempts represent the greater risk to the mother; even insertion of an LMA may further traumatize the airway or precipitate regurgitation.
The anesthesia provider should carefully consider the maternal risks of proceeding with cesarean delivery in a mother with an unsecured and unprotected airway, especially if no urgency exists and/or mask ventilation is difficult. Some obstetric anesthesiologists argue that even a nonreassuring (but non–life-threatening) fetal heart rate tracing does not always justify proceeding with cesarean delivery under general anesthesia in a patient with an unsecured airway. Alternatively, in some of these cases, proceeding with cesarean delivery via mask ventilation or with an SGA may be a better option than awakening the patient, especially in those in whom neuraxial techniques are contraindicated. In these cases, the importance of communication between the obstetric and anesthesia teams cannot be overemphasized (see Chapter 11).
Cannot Intubate and Cannot Ventilate
In the “cannot intubate and cannot ventilate” scenario, the first objective remains the maintenance of maternal and fetal oxygenation. This scenario indicates that facemask ventilation has failed despite optimizing head and neck position, inserting an oropharyngeal airway, and using the two-person mask technique. If partial ventilation exists and succinylcholine has been given, it may be possible to allow the neuromuscular blockade to resolve and spontaneous ventilation to return. If ventilation is not possible, insertion of an SGA (e.g., LMA, laryngeal tube) should be considered (see next section). Initial SGA insertion should be attempted with the application of cricoid pressure144; however, pressure may need to be reduced or removed to facilitate insertion. If SGA insertion or ventilation fails, the management team should secure an airway using an anterior neck technique (i.e., jet ventilation via a cannula inserted through the cricothyroid membrane, or surgical cricothyrotomy or tracheostomy). If these methods prove successful, the risks and benefits of proceeding with surgery should be discussed among team members; both maternal and fetal health should be considered. A 14- or 16-gauge cannula placed through the cricothyroid membrane is inherently unstable; thus, ideally, a more definitive airway should be established before surgery is started.58
Laryngeal Mask Airway
The LMA is arguably the SGA with which anesthesia providers are most familiar. The introduction of the LMA into anesthetic practice was a significant advance in airway management that resulted in major alterations to the difficult airway algorithms of the ASA and other societies.2,8 The insertion of an LMA in an obstetric patient who can easily be ventilated by facemask is controversial, because little additional ventilation benefit is obtained and the placement of an LMA can induce vomiting and aspiration in this setting. However, in any situation in which conventional facemask ventilation is difficult or impossible, an SGA is the rescue device of choice.
The LMA has many advantages, most notably its ease of use and a very high initial success rate.145 Moreover, the LMA need not be perfectly positioned over the larynx to allow adequate ventilation. When assessed by flexible fiberoptic endoscopy, radiography, and magnetic resonance imaging, the placement of the LMA around the larynx is variable145; however, 94% to 99% of patients with an LMA have little or no difficulty with ventilation.
In a prospective study, an LMA was inserted by experienced users in 1067 healthy parturients undergoing elective cesarean delivery under general anesthesia.146 The investigators demonstrated that a clinically effective and acceptable airway was obtained on the first attempt in 98% of the patients and on the second or third attempt in an additional 1%. Fewer than 1% of patients required intubation for failure to obtain satisfactory LMA placement within 90 seconds, or for an SpO2 less than 94%, or an end-tidal CO2 greater than 45 mm Hg. Moreover, the airway management (which was accomplished with the LMA, maintenance of cricoid pressure until delivery, and mechanical tidal-volume ventilation of 8 to 12 mL/kg) was associated with no episodes of hypoxemia (SpO2 < 90%), regurgitation, aspiration, laryngospasm, bronchospasm, or gastric insufflation. The investigators concluded that, in experienced hands, an LMA is effective and “probably safe” for ventilation and the administration of a volatile anesthetic agent for general anesthesia in selected healthy patients undergoing elective cesarean delivery.
A number of reports have described the use of an LMA as a rescue device for obstetric patients in whom conventional methods of securing the airway have failed.147-152 In a national case control study performed in the United Kingdom from 2007 to 2009, 39 of 57 patients with a failed intubation were managed with a classic LMA.153
An LMA may also act as a conduit for intubation; however, passage of an ETT without visualization has a low success rate.154 By contrast, fiberoptic-guided intubation through the LMA has a success rate that has approached 100% in some studies.145
Despite these benefits, the LMA has been associated with the following disadvantages: (1) placement can induce vomiting; (2) aspiration of gastric contents is not prevented; (3) improper positioning can lead to gastric insufflation; (4) multiple insertion attempts may be required for correct placement, which may result in airway trauma; and (5) use of positive-pressure ventilation may be limited. In 0.4% to 0.6% of patients with normal airway findings, the placement of an LMA leads to inadequate ventilation145; reasons for this outcome have been reported to include (1) backfolding of the distal cuff, (2) occlusion of the glottis by the distal cuff, (3) complete downfolding of the epiglottis, and (4) 90- to 180-degree rotation of the mask around its long axis.
Most of the data just cited pertain to the use of the original classic LMA; several variations in LMA design have since become available. Updated designs that have been found to be useful in difficult airway management of parturients are the ProSeal LMA (LMA North America San Diego, CA, USA) and the Fastrach LMA (LMA North America, San Diego, CA, USA). The ProSeal LMA has a specialized high-volume/low-pressure cuff that allows the device to achieve a better fit over the glottis than a classic LMA (Figure 30-13).155 This design allows the use of higher ventilation pressures (up to 30 to 40 cm H2O) with less air leakage around the cuff and a lower risk for air entry into the stomach. The ProSeal LMA also contains a specialized drainage conduit that bypasses the bowl of the LMA to minimize the entry of gastric fluid into the glottis. The drainage conduit has been shown to be effective in venting both passive and active regurgitation156,157 and can accommodate the passage of a gastric tube, which can assist in decompressing or emptying the stomach.
FIGURE 30-13 The ProSeal LMA (laryngeal mask airway). This LMA device has a specialized high-volume/low-pressure cuff, which allows glottis coverage that enables the use of higher ventilation pressures (up to 30-40 cm H2O) with less air leakage around the cuff and a lower risk for entry of air into the stomach. The ProSeal LMA also contains a specialized drainage tube that bypasses the bowl of the LMA and prevents gastric fluid from entering the glottic area. A gastric tube can be passed down this drainage lumen to assist in emptying the stomach contents.
Cook et al.158 randomly assigned 180 nonparalyzed, nonpregnant anesthetized patients to airway management with a ProSeal or classic LMA. The ProSeal more reliably allowed positive-pressure ventilation than the classic LMA. Halaseh et al.159 described the use of the ProSeal LMA in 3000 patients undergoing cesarean delivery who had fasted for more than 4 hours and were not thought to have a difficult airway. They established an “effective” airway on the first attempt in 2992 (99.7%) women, with only 8 patients (0.3%) requiring a change to a different LMA size. None of the patients required tracheal intubation, and only one patient experienced regurgitation of gastric contents into the mouth.159 Minor side effects such as sore throat occurred in 21 patients (0.7%). A number of case reports have described the successful use of the ProSeal LMA after failed intubation in obstetric patients.160-162
A disadvantage of use of the ProSeal LMA in an emergency is that it requires practice and experience to use correctly. Further, because the gastric drainage conduit traverses the LMA bowl, the passage of an ETT may be more difficult.163 A disposable, single-use version of the ProSeal LMA—the LMA Supreme—is now available.
Although designed specifically to facilitate blind tracheal intubation,164 the Fastrach or intubating LMA can also be combined with fiberoptic bronchoscopy (Figure 30-14).165,166 Both reusable and disposable versions are available. When properly placed, the intubating LMA allows ventilation similar to that with the original LMA; however, a more rigid J-shaped design improves the alignment of the mask over the glottic opening and better accommodates a special soft-tipped tracheal tube for blind intubation (see Figure 30-11). This special silicone ETT minimizes the risk for airway trauma and is available in diameter sizes 6.0 to 8.0 mm. In addition, the intubating LMA has an epiglottis elevator bar at its distal end that acts to lift the epiglottis anteriorly as the ETT exits the intubating LMA into the glottis.164 When a fiberoptic bronchoscope is used with an intubating LMA, the ETT should be advanced far enough to partially elevate the epiglottis elevator bar to assist the passage of the fiberoptic bronchoscope into the trachea. A variation of the intubating LMA, the Air-Q LMA (Mercury Medical, Clearwater, FL, USA), has a different mask tip, a keyhole-shaped airway outlet, and a removable circuit connector that accommodates an 8.5-mm ETT (Figure 30-15). The successful use of the intubating LMA during a failed intubation at emergency cesarean delivery has been reported.167
FIGURE 30-14 Intubating laryngeal mask airway (LMA). This device features a more rigid J-shaped design than the conventional LMA to facilitate the alignment of the mask over the glottic opening and better accommodate a special soft-tipped tracheal tube for blind intubation (see Figure 30-11).
FIGURE 30-15 The Air-Q laryngeal mask airway (LMA). This intubating LMA device features a unique mask tip, a keyhole-shaped airway outlet that elevates the epiglottis, and a removable circuit connector that accommodates an 8.5-mm endotracheal tube (ETT). The LMA depicted has an ETT within its lumen.
Laryngeal Mask Airway and Cricoid Pressure
With the possible exception of the ProSeal, an LMA does not protect against pulmonary aspiration of gastric contents, and its placement may precipitate regurgitation in a lightly anesthetized patient.168 Therefore, it is generally recommended that continuous cricoid pressure be maintained after the placement of an LMA in obstetric patients. Although a correctly placed LMA does not appear to compromise the effectiveness of cricoid pressure,169 the use of cricoid pressure can inhibit proper insertion of the LMA and, in some cases, may make correct insertion impossible (Figure 30-16).170-175 The application of cricoid pressure can prevent the tip of the LMA from fully occupying the hypopharynx behind the arytenoid and cricoid cartilages. Therefore, if difficulty with insertion of an LMA is encountered during an obstetric airway emergency, consideration should be given to releasing cricoid pressure temporarily while a second attempt is made at insertion.172 The risk for hypoxemia after failed LMA placement is most likely greater than the small risk for aspiration due to the temporary release of cricoid pressure. Several authors have reported that this maneuver increases the chances of a successful LMA placement.
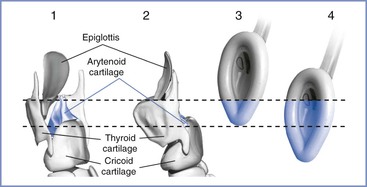
FIGURE 30-16 The position of the laryngeal mask airway (LMA) with and without cricoid pressure. The blue-shaded area indicates the distal part of the LMA that occupies the hypopharynx. The dashed lines indicate anatomic correlation. 1, Posterior view of the larynx. 2, Lateral view of the larynx. 3, Position of the tip of the LMA when cricoid pressure is applied. When cricoid pressure is applied before placement, the LMA, in theory, might be wedged in the hypopharynx but is more likely to occupy the space behind the arytenoid cartilages. The LMA is positioned at least 2 cm more proximal than usual. 4, Position of the LMA when no cricoid pressure is applied. When the LMA is placed correctly, the distal tip is at the distal end of C5 (fifth cervical vertebra), and the distal part of the LMA should fully occupy the hypopharynx and the pharyngeal space behind both the arytenoid and cricoid cartilages. A, arytenoid cartilages; C, cricoid cartilage; E, epiglottis. (Drawing by Naveen Nathan, MD, Northwestern University Feinberg School of Medicine, Chicago, IL.)
Once the LMA is in place, cricoid pressure can be reapplied and should not prohibit adequate ventilation.169 However, in some patients, reapplication of cricoid pressure may decrease tidal volumes during positive-pressure ventilation through an LMA.176 Difficult ventilation after placement of an LMA should raise suspicion of overzealous administration of cricoid pressure; in such cases, a reduction in cricoid pressure typically allows adequate ventilation.
Laryngeal Mask Airway as a Conduit for Intubation
Intubation through an LMA can be achieved blindly, especially with an intubating LMA, or with the assistance of a fiberoptic bronchoscope. Before starting this maneuver, the risks and benefits of an intubation attempt must be weighed.177 Intubation attempts should never supersede or compromise active ventilation; however, in certain situations, such as a patient at significant risk for aspiration or if ventilation is marginal, securing the airway with an ETT may take precedence. The use of a fiberoptic bronchoscope through an LMA has been reported to be nearly 100% successful in achieving intubation.177 A 6-mm ID cuffed ETT may be passed over the fiberoptic bronchoscope and through the shaft of a size 3 or 4 LMA; of note, a nasal RAE tube (Nellcor, Boulder, CO, USA) is a suitable match for this purpose, owing to its adequate length and widespread availability.8 A 7-mm ID cuffed ETT may be passed in a similar manner through the shaft of a size 5 LMA.
When a fiberoptic bronchoscope is passed through an LMA, the bronchoscope should be introduced through the self-sealing diaphragm of an elbow adapter attached to the ETT and the airway circuit to allow continuous ventilation (Figure 30-17). The space available for ventilation around a 4-mm outer diameter (OD) fiberoptic bronchoscope placed within a 6-mm ID ETT is equivalent to that available with a 4.5-mm ID tube. Alternatively, a two-stage method can be employed, in which a fiberoptic bronchoscope is placed through the lumen of an Aintree Intubation Catheter (Cook Critical Care, Bloomington, IN, USA) and the stem of a size 3 or 4 LMA. With the LMA in the oropharynx, the fiberoptic bronchoscope and Aintree Catheter are advanced through the vocal cords, the fiberoptic bronchoscope withdrawn, and then an ETT is guided over the Aintree Catheter.178 There are no studies assessing the success rate of these advanced techniques that use the LMA as a conduit for tracheal intubation; however, these techniques take time and skill and should be performed by anesthesia providers experienced in their use.
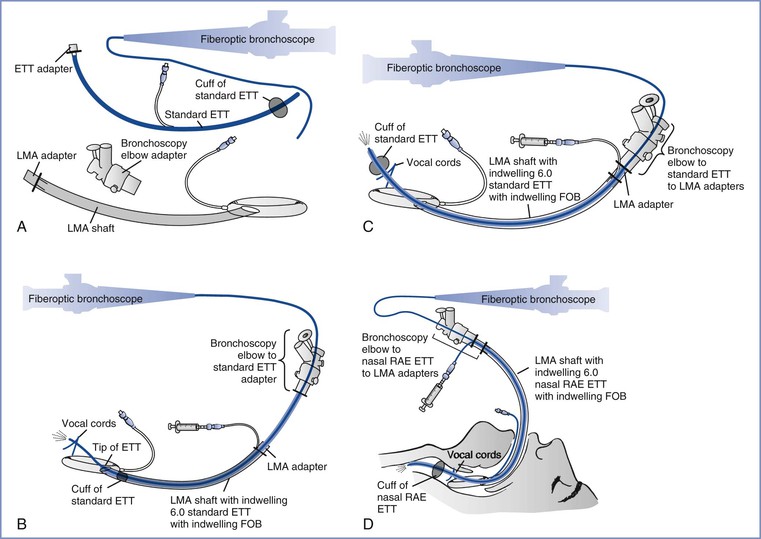
FIGURE 30-17 Schematic diagram of set-up for continuous ventilation during fiberoptic bronchoscopy–guided tracheal intubation using a laryngeal mask airway (LMA) as a conduit for both the endotracheal tube (ETT) and fiberoptic bronchoscope (FOB). In A and B, a standard 28-cm-long ETT is shown. (A 6-mm inner diameter nasal RAE tube [Nellcor, Boulder, CO] may also be used.) A, Components of the continuous ventilation system. B, With passage of the tip of a cuffed 6-mm internal diameter tracheal tube to the level of the LMA grille and a 4-mm outer diameter FOB through the self-sealing diaphragm of a bronchoscope elbow adapter, ventilation can occur around the FOB but within the ETT lumen; the deflated cuff inside the shaft of the LMA makes an airtight seal and permits positive-pressure ventilation. C, After the fiberoptic bronchoscope is passed into the trachea, the ETT is advanced over the FOB into the trachea until the ETT adapter is near that of the LMA (if using a nasal RAE ETT) or is flush up against the adapter of the LMA (if using a standard ETT). The preformed curvature of the nasal RAE tube presents no problem with insertion if the outside of the tube is adequately lubricated. D, Schematic of C, with superimposed upper airway anatomy.
Laryngeal Tube and Esophageal-Tracheal Combitube
The laryngeal tube (VBM Medizintechnik GmbH, Sulz, Germany) is another SGA device.179,180 Laryngeal tubes are manufactured from either silicone or polyvinylchloride (PVC) and have ventilation apertures between a proximal oropharyngeal cuff and a distal esophageal cuff. The laryngeal tube is inserted into the oropharynx until resistance is met, which should result in positioning of the ventilation apertures directly above the glottic opening. These devices are reported to provide seal pressures similar to those with the ProSeal LMA (40 cm H2O) and insertion times and success rates comparable to those of the LMA.181 The Laryngeal Tube-S (LTS) contains a second lumen that can be used for drainage of the stomach.182 The LTS has been used successfully after a failed intubation and ventilation in a patient undergoing emergency cesarean delivery.183
The esophageal-tracheal Combitube (ETC) (Sheridan Catheter Corporation, Argyle, NY, USA) is a twin-lumen plastic tube with an outer diameter of 13 mm. One lumen has an open distal end and thus resembles a tracheal tube (i.e. the tracheal lumen), and the other (esophageal) lumen has a closed distal end. The ETC has a 100-mL proximal pharyngeal balloon; when the ETC is correctly positioned, the pharyngeal balloon fills the space between the tongue base and soft palate. When inflated, the proximal balloon seals the oral and nasal cavities. Distal to the pharyngeal balloon, but proximal to the level of the larynx, are eight perforations in the esophageal lumen. A smaller 15-mL distal cuff, similar to that on an ETT, seals either the esophagus or trachea when inflated. The ETC is inserted, with or without the aid of a laryngoscope, but its insertion does not require visualization of the larynx. Indeed, in the usual clinical context, the larynx cannot be visualized. The ETC enters the esophagus 96% of the time, allowing ventilation through the esophageal lumen perforations.184 If the ETC enters the trachea, the patient's lungs can be ventilated directly through the tracheal lumen. Therefore, regardless of whether the distal end of the ETC enters the trachea or esophagus, the anesthesia provider can ventilate the lungs, assuming correct identification of which lumen should be used for ventilation.
The ETC allows adequate ventilation while preventing aspiration of gastric contents. If the distal end of the ETC enters the esophagus, the ETC can assist in removing gastric fluids through suction applied to the “tracheal” lumen. When long-term ventilation is anticipated or required, the ETC should be exchanged for an endotracheal tube.
Use of the ETC in the out-of-hospital setting has been associated with a notable incidence of serious complications, including upper airway bleeding, esophageal laceration and perforation, and mediastinitis.185 Although a lower incidence of serious complications would be expected in the more controlled operating room environment with an anesthesiologist using a laryngoscope to facilitate placement, the stiffness and the anterior curvature of the ETC, as well as the potential for balloon overinflation, still represent potential sources of airway and esophageal injury.
Cannula and Surgical Cricothyrotomy
The anesthesia provider must diagnose the failure of facemask and alternative devices to oxygenate and ventilate the patient and decide that direct tracheal access is necessary. A delay in performing cricothyrotomy results in greater morbidity and mortality than complications resulting from the attempt.35,186 Three techniques for cricothyrotomy are available187:
1. Narrow-bore cricothyrotomy using a purpose-designed cannula less than 2 mm ID (e.g., Ravussin; VBM Medizintechnik GmbH, Sulz, Germany)
2. Wide-bore cricothyrotomy using a purpose-designed cannula greater than 4 mm ID (e.g., QuickTrach; VBM Medizintechnik GmbH, Sulz, Germany)
3. Surgical cricothyrotomy using a scalpel to enable placement of a small (< 6 mm ID) standard tracheal or tracheostomy tube
Narrow-bore cannula cricothyrotomy requires the use of a high-pressure ventilation source (e.g., Manujet) for transtracheal jet ventilation (TTJV). The other two techniques use standard ventilation circuits. Most TTJV systems have an in-line regulator that allows the delivery pressure to be controlled to between 0 and 50 psi. A minimum pressure of 20 to 30 psi is required in the majority of patients to inflate the chest and provide appropriate tidal volumes and minute ventilation.188 During TTJV, it is critical that the operator allow time for exhalation of inspired gas to avoid hyperinflation of the lungs, potential air trapping, and barotrauma.189-191 Exhalation can be facilitated by keeping the airway patent by some means (e.g., nasal/oral airway, jaw thrust, LMA). There are numerous reports of the use of TTJV as a means to prevent hypoxemia during life-threatening airway emergencies192-194 and as a temporizing measure before a more definitive surgical airway can be established.
Although cannula cricothyrotomy is faster and carries significantly fewer risks, its success rate is much lower than that of surgical cricothyrotomy.187 Skill fade associated with increased length of time since training is likely to have a more significant impact on outcome than choice of device.187 The choice of technique can therefore be determined by local protocols. The anesthesia provider should evaluate and practice various airway techniques to maximize success during an emergency.
Extubation of the Patient with a Difficult Airway
General Principles
Tracheal extubation is a critical step during emergence from general anesthesia. Airway condition at time of tracheal extubation may be less favorable than at induction of anesthesia. The patient's disease process (e.g., preeclampsia) may contribute to worsening airway edema, as do the fluid and blood products that have been administered during the procedure. Comorbidities, such as obesity and obstructive sleep apnea, may contribute to an increased risk for airway compromise after extubation of the trachea. Although the majority of extubations occur without incident, a number of serious adverse events, including hypoxic brain injury, occur during emergence from general anesthesia and tracheal extubation or in the postoperative period.35,195-198
After publication of the ASA guidelines for management of the difficult airway,2 there was a statistically significant reduction in airway claims arising from injury at induction of anesthesia.199 However, the number of claims arising from intraoperative events and those at extubation and during recovery has not changed. Death and brain injury occur more commonly after extubation and during recovery than during induction of anesthesia.199 Not surprisingly, extubation problems are more common in obese patients and in those with obstructive sleep apnea.199 In the fourth National Audit Project (NAP4) of the Royal College of Anaesthetists and the Difficult Airway Society (DAS), major airway complications in patients receiving anesthesia occurred during emergence or recovery in approximately one third of the reported cases.100
Extubation is an elective procedure that should always have a management strategy.200-202 The goals of extubation management are to ensure uninterrupted oxygen delivery, avoid airway stimulation, and allow ventilation and possibly reintubation with minimum difficulty and delay should extubation fail. The U.K. Difficult Airway Society Extubation Guidelines9 describe a four-step approach to the safe management of tracheal extubation:
The planning and preparation steps allow the anesthesia provider to stratify risk and categorize the patient as either at low risk or at risk for extubation complications. A fasted patient with an uncomplicated airway is at low risk, whereas a patient at risk for aspiration in whom the ability to oxygenate and reintubate is potentially difficult is at risk.9 By definition, all obstetric patients fall into the “at-risk” group owing to the risk for pulmonary aspiration.9 A crucial decision is to determine whether it is safe to extubate the trachea or to postpone the extubation. Unfortunately, studies attempting to identify risk factors that can reliably predict difficulty with extubation, performed almost entirely in the critical care population, have been inconclusive.203 The leak test, in which the ETT cuff is deflated, the proximal end of the tube is occluded, and the patient is evaluated to determine if she can spontaneously ventilate around the tube, has not been uniformly demonstrated to predict success with extubation.204
If the surgical procedure was long, or massive bleeding and significant fluid replacement has occurred, consideration should be given to transferring the patient to the intensive care unit and delaying tracheal extubation. The decision to remove the ETT should follow a full assessment of the patient (i.e., ability to follow commands, appropriate level of consciousness, recovery from neuromuscular blockade). The head of the bed can be elevated or the patient positioned on her left side. Patients for whom oxygenation and/or reintubation is predicted to be difficult may benefit from the insertion of an airway exchange catheter before tracheal extubation (see later discussion).
Airway Exchange Catheters
In situations in which the patient appears ready for extubation but concerns exist regarding potential difficulty with—and/or need for—re-intubation (e.g., difficult intubation), establishing continuous airway access may be of benefit; such access can be achieved with an airway-exchange catheter (AEC) inserted through the ETT before extubation.205,206 There are many commercially available AECs: the Cook AEC (Cook Critical Care, Bloomington, IN, USA) is a long, thin, hollow tube made from semi-rigid thermostable polyurethane. It has a blunt end with distal terminal and side holes; it is radiopaque and has length markings on its outer surface. The catheter is supplied with a removable 15-mm connector that is compatible with anesthetic circuits for oxygenation and Luer-Lok connectors for use with high-pressure source (jet) ventilation. AECs are available in various sizes; the most appropriate sizes for extubation are of sufficient length (e.g., 83 cm) and diameter (e.g., 11- to 14-gauge) to remain between the vocal cords while removing and/or reinserting an ETT. These catheters are compatible with tracheal tubes of internal diameters greater than 4 and 5 mm, respectively. Successful reintubation can be enhanced by the use of a smaller tracheal tube and rotation of the ETT 90 degrees during insertion to facilitate passage of the bevel through the vocal cords.115
Mort207 evaluated 354 patients with potentially difficult airways who were extubated over an AEC; 51 required reintubation. The reintubation success rate was greater than 90%. Complications during airway management, including low oxygen saturation, bradycardia, hypotension, esophageal intubation, and the need for accessory airway adjuncts, were less common when an AEC was used to assist re-intubation. Successful outcomes have been reported in other similar studies.205,208 Visualization of the larynx, either by direct or video laryngoscopy, during AEC use can also increase the success of reintubation and reduce the incidence of complications.209
Morbidity associated with AEC use is predominantly a result of inappropriate positioning and failure of oxygenation. The distal tip of the AEC is ideally positioned in the mid trachea; oxygen insufflation and jet ventilation through an AEC should only be undertaken with extreme caution, because barotrauma and death have been reported.206,210,211
References
1. Pearce A. Evaluation of the airway and preparation for difficulty. Best Pract Res Clin Anaesthesiol. 2005;19:559–579.
2. American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Practice guidelines for management of the difficult airway. Anesthesiology. 2013;118:251–270.
3. Langeron O, Masso E, Huraux C, et al. Prediction of difficult mask ventilation. Anesthesiology. 2000;92:1229–1236.
4. Kheterpal S, Martin L, Shanks AM, Tremper KK. Prediction and outcomes of impossible mask ventilation: a review of 50,000 anesthetics. Anesthesiology. 2009;110:891–897.
5. Verghese C, Brimacombe JR. Survey of laryngeal mask airway usage in 11,910 patients: safety and efficacy for conventional and nonconventional usage. Anesth Analg. 1996;82:129–133.
6. Cantwell R, Clutton-Brock T, Cooper G, et al. Saving Mothers’ Lives: Reviewing maternal deaths to make motherhood safer: 2006-2008. The Eighth Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. BJOG. 2011;118(Suppl 1):1–203.
7. Hawkins JL, Chang J, Palmer SK, et al. Anesthesia-related maternal mortality in the United States: 1979-2002. Obstet Gynecol. 2011;117:69–74.
8. Henderson JJ, Popat MT, Latto IP, et al. Difficult Airway Society guidelines for management of the unanticipated difficult intubation. Anaesthesia. 2004;59:675–694.
9. Difficult Airway Society Extubation Guidelines Group. Difficult Airway Society guidelines for the management of tracheal extubation. Anaesthesia. 2012;67:318–340.
10. Lyons G. Failed intubation: six years’ experience in a teaching maternity unit. Anaesthesia. 1985;40:759–762.
11. Hawthorne L, Wilson R, Lyons G, Dresner M. Failed intubation revisited: 17-yr experience in a teaching maternity unit. Br J Anaesth. 1996;76:680–684.
12. Barnardo PD, Jenkins JG. Failed tracheal intubation in obstetrics: a 6-year review in a UK region. Anaesthesia. 2000;55:690–694.
13. Rahman K, Jenkins JG. Failed tracheal intubation in obstetrics: no more frequent but still managed badly. Anaesthesia. 2005;60:168–171.
14. McDonnell NJ, Paech MJ, Clavisi OM, et al. Difficult and failed intubation in obstetric anaesthesia: an observational study of airway management and complications associated with general anaesthesia for caesarean section. Int J Obstet Anesth. 2008;17:292–297.
15. Samsoon GL, Young JR. Difficult tracheal intubation: a retrospective study. Anaesthesia. 1987;42:487–490.
16. Palanisamy A, Mitani AA, Tsen LC. General anesthesia for cesarean delivery at a tertiary care hospital from 2000 to 2005: a retrospective analysis and 10-year update. Int J Obstet Anesth. 2011;20:10–16.
17. Haslam N, Parker L, Duggan JE. Effect of cricoid pressure on the view at laryngoscopy. Anaesthesia. 2005;60:41–47.
18. Marfin AG, Pandit JJ, Hames KC, et al. Use of the bougie in simulated difficult intubation. 2. Comparison of single-use bougie with multiple-use bougie. Anaesthesia. 2003;58:852–855.
19. Russell R. Failed intubation in obstetrics: a self-fulfilling prophecy? Int J Obstet Anesth. 2007;16:1–3.
20. D’Angelo R. Anesthesia-related maternal mortality: a pat on the back or a call to arms? Anesthesiology. 2007;106:1082–1084.
21. Tsen LC, Pitner R, Camann WR. General anesthesia for cesarean section at a tertiary care hospital 1990-1995: indications and implications. Int J Obstet Anesth. 1998;7:147–152.
22. Searle RD, Lyons G. Vanishing experience in training for obstetric general anaesthesia: an observational study. Int J Obstet Anesth. 2008;17:233–237.
23. Panni MK, Camann WR, Tsen LC. Resident training in obstetric anesthesia in the United States. Int J Obstet Anesth. 2006;15:284–289.
24. Dresner M, Brocklesby J, Bamber J. Audit of the influence of body mass index on the performance of epidural analgesia in labour and the subsequent mode of delivery. BJOG. 2006;113:1178–1181.
25. Juvin P, Lavaut E, Dupont H, et al. Difficult tracheal intubation is more common in obese than in lean patients. Anesth Analg. 2003;97:595–600.
26. Lundstrom LH, Moller AM, Rosenstock C, et al. High body mass index is a weak predictor for difficult and failed tracheal intubation: a cohort study of 91,332 consecutive patients scheduled for direct laryngoscopy registered in the Danish Anesthesia Database. Anesthesiology. 2009;110:266–274.
27. Saravanakumar K, Rao SG, Cooper GM. Obesity and obstetric anaesthesia. Anaesthesia. 2006;61:36–48.
28. Rocke DA, Murray WB, Rout CC, Gouws E. Relative risk analysis of factors associated with difficult intubation in obstetric anesthesia. Anesthesiology. 1992;77:67–73.
29. Djabatey EA, Barclay PM. Difficult and failed intubation in 3430 obstetric general anaesthetics. Anaesthesia. 2009;64:1168–1171.
30. McKeen DM, George RB, O’Connell CM, et al. Difficult and failed intubation: incident rates and maternal, obstetrical, and anesthetic predictors. Can J Anaesth. 2011;58:514–524.
31. Pratt SD. Focused review: simulation in obstetric anesthesia. Anesth Analg. 2012;114:186–190.
32. Hawkins JL, Koonin LM, Palmer SK, Gibbs CP. Anesthesia-related deaths during obstetric delivery in the United States, 1979-1990. Anesthesiology. 1997;86:277–284.
33. Chestnut DH. Anesthesia and maternal mortality. Anesthesiology. 1997;86:273–276.
34. Cook TM, McCrirrick A. A survey of airway management during induction of general anaesthesia in obstetrics: are the recommendations in the confidential enquiries into maternal deaths being implemented? Int J Obstet Anesth. 1994;3:143–145.
35. Mhyre JM, Riesner MN, Polley LS, Naughton NN. A series of anesthesia-related maternal deaths in Michigan, 1985-2003. Anesthesiology. 2007;106:1096–1104.
36. Izci B, Riha RL, Martin SE, et al. The upper airway in pregnancy and pre-eclampsia. Am J Respir Crit Care Med. 2003;167:137–140.
37. Mallampati SR, Gatt SP, Gugino LD, et al. A clinical sign to predict difficult tracheal intubation: a prospective study. Can Anaesth Soc J. 1985;32:429–434.
38. Pilkington S, Carli F, Dakin MJ, et al. Increase in Mallampati score during pregnancy. Br J Anaesth. 1995;74:638–642.
39. Kodali BS, Chandrasekhar S, Bulich LN, et al. Airway changes during labor and delivery. Anesthesiology. 2008;108:357–362.
40. Boutonnet M, Faitot V, Katz A, et al. Mallampati class changes during pregnancy, labour, and after delivery: can these be predicted? Br J Anaesth. 2010;104:67–70.
41. Farcon EL, Kim MH, Marx GF. Changing Mallampati score during labour. Can J Anaesth. 1994;41:50–51.
42. Jouppila R, Jouppila P, Hollmen A. Laryngeal oedema as an obstetric anaesthesia complication: case reports. Acta Anaesthesiol Scand. 1980;24:97–98.
43. Arendt KW, Khan K, Curry TB, Tsen LC. Topical vasoconstrictor use for nasal intubation during pregnancy complicated by cardiomyopathy and preeclampsia. Int J Obstet Anesth. 2011;20:246–249.
44. Russell IF, Chambers WA. Closing volume in normal pregnancy. Br J Anaesth. 1981;53:1043–1047.
45. Archer GW Jr, Marx GF. Arterial oxygen tension during apnoea in parturient women. Br J Anaesth. 1974;46:358–360.
46. Benumof JL, Dagg R, Benumof R. Critical hemoglobin desaturation will occur before return to an unparalyzed state following 1 mg/kg intravenous succinylcholine. Anesthesiology. 1997;87:979–982.
47. McClelland SH, Bogod DG, Hardman JG. Apnoea in pregnancy: an investigation using physiological modelling. Anaesthesia. 2008;63:264–269.
48. McClelland SH, Bogod DG, Hardman JG. Pre-oxygenation and apnoea in pregnancy: changes during labour and with obstetric morbidity in a computational simulation. Anaesthesia. 2009;64:371–377.
49. Yentis SM. Predicting difficult intubation—worthwhile exercise or pointless ritual? Anaesthesia. 2002;57:105–109.
50. Cormack RS, Lehane J. Difficult tracheal intubation in obstetrics. Anaesthesia. 1984;39:1105–1111.
51. Yentis SM, Lee DJ. Evaluation of an improved scoring system for the grading of direct laryngoscopy. Anaesthesia. 1998;53:1041–1044.
52. Rutter JM, Murphy PG. Cormack and Lehane revisited. Anaesthesia. 1997;52:927.
53. Cook TM, Nolan JP, Gabbott DA. Cricoid pressure—are two hands better than one? Anaesthesia. 1997;52:179–180.
54. Shiga T, Wajima Z, Inoue T, Sakamoto A. Predicting difficult intubation in apparently normal patients: a meta-analysis of bedside screening test performance. Anesthesiology. 2005;103:429–437.
55. Lee A, Fan LT, Gin T, et al. A systematic review (meta-analysis) of the accuracy of the Mallampati tests to predict the difficult airway. Anesth Analg. 2006;102:1867–1878.
56. Bellhouse CP, Dore C. Criteria for estimating likelihood of difficulty of endotracheal intubation with the Macintosh laryngoscope. Anaesth Intensive Care. 1988;16:329–337.
57. Calder I, Calder J, Crockard HA. Difficult direct laryngoscopy in patients with cervical spine disease. Anaesthesia. 1995;50:756–763.
58. Munnur U, de Boisblanc B, Suresh MS. Airway problems in pregnancy. Crit Care Med. 2005;33:S259–S268.
59. Khan ZH, Mohammadi M, Rasouli MR, et al. The diagnostic value of the upper lip bite test combined with sternomental distance, thyromental distance, and interincisor distance for prediction of easy laryngoscopy and intubation: a prospective study. Anesth Analg. 2009;109:822–824.
60. Rose DK, Cohen MM. The airway: problems and predictions in 18,500 patients. Can J Anaesth. 1994;41:372–383.
61. Calder I, Picard J, Chapman M, et al. Mouth opening: a new angle. Anesthesiology. 2003;99:799–801.
62. Wilson ME, Spiegelhalter D, Robertson JA, Lesser P. Predicting difficult intubation. Br J Anaesth. 1988;61:211–216.
63. Oates JD, Macleod AD, Oates PD, et al. Comparison of two methods for predicting difficult intubation. Br J Anaesth. 1991;66:305–309.
64. Frerk CM. Predicting difficult intubation. Anaesthesia. 1991;46:1005–1008.
65. Lewis M, Keramati S, Benumof JL, Berry CC. What is the best way to determine oropharyngeal classification and mandibular space length to predict difficult laryngoscopy? Anesthesiology. 1994;81:69–75.
66. Tse JC, Rimm EB, Hussain A. Predicting difficult endotracheal intubation in surgical patients scheduled for general anesthesia: a prospective blind study. Anesth Analg. 1995;81:254–258.
67. Honarmand A, Safavi MR. Prediction of difficult laryngoscopy in obstetric patients scheduled for Caesarean delivery. Eur J Anaesthesiol. 2008;25:714–720.
68. Morgan BM, Magni V, Goroszenuik T. Anaesthesia for emergency caesarean section. Br J Obstet Gynaecol. 1990;97:420–424.
69. Riley ET, Papasin J. Epidural catheter function during labor predicts anesthetic efficacy for subsequent cesarean delivery. Int J Obstet Anesth. 2002;11:81–84.
70. Bauer ME, Kountanis JA, Tsen LC, et al. Risk factors for failed conversion of labor epidural analgesia to cesarean delivery anesthesia: a systematic review and meta-analysis of observational trials. Int J Obstet Anesth. 2012;21:294–309.
71. Hillyard SG, Bate TE, Corcoran TB, et al. Extending epidural analgesia for emergency Caesarean section: a meta-analysis. Br J Anaesth. 2011;107:668–678.
72. American College of Obstetricians and Gynecologists. Oral intake during labor. ACOG Committee Opinion No. 441. Washington, DC (reaffirmed 2011). (Obstet Gynecol 2009; 114:714).
73. American Society of Anesthesiologists. Practice guidelines for obstetric anesthesia. Anesthesiology. 2007;106:843–863.
74. National Institute for Health and Clinical Excellence. Intrapartum Care: Care of Healthy Women and Their Babies during Childbirth. RCOG Press: London; 2007.
75. O'Sullivan GM, Bullingham RE. The assessment of gastric acidity and antacid effect in pregnant women by a non-invasive radiotelemetry technique. Br J Obstet Gynaecol. 1984;91:973–978.
76. El-Orbany M, Woehlck H, Salem MR. Head and neck position for direct laryngoscopy. Anesth Analg. 2011;113:103–109.
77. Chiron B, Laffon M, Ferrandiere M, et al. Standard preoxygenation technique versus two rapid techniques in pregnant patients. Int J Obstet Anesth. 2004;13:11–14.
78. Russell EC, Wrench I, Feast M, Mohammed F. Pre-oxygenation in pregnancy: the effect of fresh gas flow rates within a circle breathing system. Anaesthesia. 2008;63:833–836.
79. Porter R, Wrench IJ, Freeman R. Preoxygenation for general anaesthesia in pregnancy: is it adequate? Int J Obstet Anesth. 2011;20:363–365.
80. Hignett R, Fernando R, McGlennan A, et al. A randomized crossover study to determine the effect of a 30 degrees head-up versus a supine position on the functional residual capacity of term parturients. Anesth Analg. 2011;113:1098–1102.
81. Dixon BJ, Dixon JB, Carden JR, et al. Preoxygenation is more effective in the 25 degrees head-up position than in the supine position in severely obese patients: a randomized controlled study. Anesthesiology. 2005;102:1110–1115.
82. El-Orbany M, Connolly LA. Rapid sequence induction and intubation: current controversy. Anesth Analg. 2010;110:1318–1325.
83. Russell R. Propofol should be the agent of choice for caesarean section under general anaesthesia. Int J Obstet Anesth. 2003;12:276–279.
84. Dobson AP, McCluskey A, Meakin G, Baker RD. Effective time to satisfactory intubation conditions after administration of rocuronium in adults. Comparison of propofol and thiopentone for rapid sequence induction of anaesthesia. Anaesthesia. 1999;54:172–176.
85. Blitt CD, Petty WC, Alberternst EE, Wright BJ. Correlation of plasma cholinesterase activity and duration of action of succinylcholine during pregnancy. Anesth Analg. 1977;56:78–83.
86. Donati F. The right dose of succinylcholine. Anesthesiology. 2003;99:1037–1038.
87. Naguib M, Samarkandi AH, El-Din ME, et al. The dose of succinylcholine required for excellent endotracheal intubating conditions. Anesth Analg. 2006;102:151–155.
88. Naguib M, Samarkandi A, Riad W, Alharby SW. Optimal dose of succinylcholine revisited. Anesthesiology. 2003;99:1045–1049.
89. Perry JJ, Lee JS, Sillberg VA, Wells GA. Rocuronium versus succinylcholine for rapid sequence induction intubation. Cochrane Database Syst Rev. 2008;(2).
90. Sellick BA. Cricoid pressure to control regurgitation of stomach contents during induction of anaesthesia. Lancet. 1961;2:404–406.
91. Vanner R. Cricoid pressure. Int J Obstet Anesth. 2009;18:103–105.
92. Fenton PM, Reynolds F. Life-saving or ineffective? An observational study of the use of cricoid pressure and maternal outcome in an African setting. Int J Obstet Anesth. 2009;18:106–110.
93. Paech MJ. “Pregnant women having caesarean delivery under general anaesthesia should have a rapid sequence induction with cricoid pressure and be intubated”. Can this ‘holy cow’ be sent packing? Anaesth Intensive Care. 2010;38:989–991.
94. Palmer JHMG, Ball DR. The effect of cricoid pressure on the cricoid cartilage and vocal cords: an endoscopic study in anaesthetised patients. Anaesthesia. 2000;55:263–268.
95. Hartsilver EL, Vanner RG. Airway obstruction with cricoid pressure. Anaesthesia. 2000;55:208–211.
96. Yentis SM. The effects of single-handed and bimanual cricoid pressure on the view at laryngoscopy. Anaesthesia. 1997;52:332–335.
97. Ezri T, Szmuk P, Evron S, et al. Difficult airway in obstetric anesthesia: a review. Obstet Gynecol Surv. 2001;56:631–641.
98. Popat MT, Rai M. Awake fibreoptic intubation. Difficult Airway Management. Oxford University Press: Oxford, New York; 2009:65–76.
99. Ovassapian A. Fiberoptic tracheal intubation in adults. Ovassapian A. Fiberoptic Endoscopy and the Difficult Airway. Lippincott-Raven: Philadelphia; 1996:72–103.
100. Cook TM, Woodall N, Frerk C. 4th National audit project: Major complications of airway management in the UK. [Available at] http://www.rcoa.ac.uk/nap4/ [Accessed June 2013] .
101. Watanabe H, Lindgren L, Rosenberg P, Randell T. Glycopyrronium prolongs topical anaesthesia of oral mucosa and enhances absorption of lignocaine. Br J Anaesth. 1993;70:94–95.
102. American Society of Anesthesiologists. Continuum of depth of sedation: definition of general anesthesia and levels of sedation/analgesia. [Available at] http://www.asahq.org/For-Members/Standards-Guidelines-and-Statements.aspx [Accessed June 2013] .
103. Benumof JL. Management of the difficult adult airway: with special emphasis on awake tracheal intubation. Anesthesiology. 1991;75:1087–1110.
104. Popat MT, Chippa JH, Russell R. Awake fibreoptic intubation following failed regional anaesthesia for caesarean section in a parturient with Still's disease. Eur J Anaesthesiol. 2000;17:211–214.
105. Popat M, Russell R. Awake fibreoptic intubation following previous failed intubation. Int J Obstet Anesth. 2001;10:332–333.
106. Puchner W, Egger P, Puhringer F, et al. Evaluation of remifentanil as single drug for awake fiberoptic intubation. Acta Anaesthesiol Scand. 2002;46:350–354.
107. Rai MR, Parry TM, Dombrovskis A, Warner OJ. Remifentanil target-controlled infusion vs propofol target-controlled infusion for conscious sedation for awake fibreoptic intubation: a double-blinded randomized controlled trial. Br J Anaesth. 2008;100:125–130.
108. Mackenzie I. A new method of drug application to the nasal passage. Anaesthesia. 1998;53:309–310.
109. Sitzman BT, Rich GF, Rockwell JJ, et al. Local anesthetic administration for awake direct laryngoscopy. Are glossopharyngeal nerve blocks superior? Anesthesiology. 1997;86:34–40.
110. Claeys DW, Lockhart CH, Hinkle JE. The effects of translaryngeal block and Innovar on glottic competence. Anesthesiology. 1973;38:485–486.
111. Walts LF, Kassity KJ. Spread of local anesthesia after upper airway block. Arch Otolaryngol. 1965;81:77–79.
112. Ovassapian A, Krejcie TC, Yelich SJ, Dykes MH. Awake fibreoptic intubation in the patient at high risk of aspiration. Br J Anaesth. 1989;62:13–16.
113. Mahajan RP, Murty GE, Singh P, Aitkenhead AR. Effect of topical anaesthesia on the motor performance of vocal cords as assessed by tussometry. Anaesthesia. 1994;49:1028–1030.
114. Marfin AG, Iqbal R, Mihm F, et al. Determination of the site of tracheal tube impingement during nasotracheal fibreoptic intubation. Anaesthesia. 2006;61:646–650.
115. Asai T, Shingu K. Difficulty in advancing a tracheal tube over a fibreoptic bronchoscope: incidence, causes and solutions. Br J Anaesth. 2004;92:870–881.
116. Rai MR, Scott SH, Marfin AG, et al. A comparison of a flexometallic tracheal tube with the intubating laryngeal mask tracheal tube for nasotracheal fibreoptic intubation using the two-scope technique. Anaesthesia. 2009;64:1303–1306.
117. Asai T. Videolaryngoscopes: do they truly have roles in difficult airways? Anesthesiology. 2012;116:515–517.
118. Aziz MF, Dillman D, Fu R, Brambrink AM. Comparative effectiveness of the C-MAC video laryngoscope versus direct laryngoscopy in the setting of the predicted difficult airway. Anesthesiology. 2012;116:629–636.
119. Asai T, Liu EH, Matsumoto S, et al. Use of the Pentax-AWS in 293 patients with difficult airways. Anesthesiology. 2009;110:898–904.
120. Jungbauer A, Schumann M, Brunkhorst V, et al. Expected difficult tracheal intubation: a prospective comparison of direct laryngoscopy and video laryngoscopy in 200 patients. Br J Anaesth. 2009;102:546–550.
121. Malik MA, Subramaniam R, Maharaj CH, et al. Randomized controlled trial of the Pentax AWS, Glidescope, and Macintosh laryngoscopes in predicted difficult intubation. Br J Anaesth. 2009;103:761–768.
122. Enomoto Y, Asai T, Arai T, et al. Pentax-AWS, a new videolaryngoscope, is more effective than the Macintosh laryngoscope for tracheal intubation in patients with restricted neck movements: a randomized comparative study. Br J Anaesth. 2008;100:544–548.
123. Hirabayashi Y, Fujita A, Seo N, Sugimoto H. Cervical spine movement during laryngoscopy using the Airway Scope compared with the Macintosh laryngoscope. Anaesthesia. 2007;62:1050–1055.
124. Cross P, Cytryn J, Cheng KK. Perforation of the soft palate using the GlideScope videolaryngoscope. Can J Anaesth. 2007;54:588–589.
125. Hirabayashi Y. Pharyngeal injury related to GlideScope videolaryngoscope. Otolaryngol Head Neck Surg. 2007;137:175–176.
126. Chin KJ, Arango MF, Paez AF, Turkstra TP. Palatal injury associated with the GlideScope. Anaesth Intensive Care. 2007;35:449–450.
127. Malik AM, Frogel JK. Anterior tonsillar pillar perforation during GlideScope video laryngoscopy. Anesth Analg. 2007;104:1610–1611.
128. Hsu WT, Hsu SC, Lee YL, et al. Penetrating injury of the soft palate during GlideScope intubation. Anesth Analg. 2007;104:1609–1610.
129. Liu EH, Goy RW, Tan BH, Asai T. Tracheal intubation with videolaryngoscopes in patients with cervical spine immobilization: a randomized trial of the Airway Scope and the GlideScope. Br J Anaesth. 2009;103:446–451.
130. Malik MA, Maharaj CH, Harte BH, Laffey JG. Comparison of Macintosh, Truview EVO2, Glidescope, and Airwayscope laryngoscope use in patients with cervical spine immobilization. Br J Anaesth. 2008;101:723–730.
131. Dhonneur G, Ndoko S, Amathieu R, et al. Tracheal intubation using the Airtraq in morbid obese patients undergoing emergency cesarean delivery. Anesthesiology. 2007;106:629–630.
132. Aziz MF, Kim D, Mako J, et al. A retrospective study of the performance of video laryngoscopy in an obstetric unit. Anesth Analg. 2012;115:904–906.
133. Jones PM, Harle CC. Avoiding awake intubation by performing awake GlideScope laryngoscopy in the preoperative holding area. Can J Anaesth. 2006;53:1264–1265.
134. Moore MS, Wong AB. GlideScope intubation assisted by fiberoptic scope. Anesthesiology. 2007;106:885.
135. Benumof JL. Management of the difficult airway. Ann Acad Med Singapore. 1994;23:589–591.
136. Burtner DD, Goodman M. Anesthetic and operative management of potential upper airway obstruction. Arch Otolaryngol. 1978;104:657–661.
137. Ranney B, Stanage WF. Advantages of local anesthesia for cesarean section. Obstet Gynecol. 1975;45:163–167.
138. Busby T. Local anesthesia for cesarean section. Am J Obstet Gynecol. 1963;87:399–404.
139. Cooper MG, Feeney EM, Joseph M, McGuinness JJ. Local anaesthetic infiltration for caesarean section. Anaesth Intensive Care. 1989;17:198–201.
140. Mei W, Jin C, Feng L, et al. Bilateral ultrasound-guided transversus abdominis plane block combined with ilioinguinal-iliohypogastric nerve block for cesarean delivery anesthesia. Anesth Analg. 2011;113:134–137.
141. Suresh M. Difficult airway in the parturient. Probl Anesth. 2001;13:88–99.
142. Lucas DN, Yentis SM, Kinsella SM, et al. Urgency of caesarean section: a new classification. J R Soc Med. 2000;93:346–350.
143. Obstetric Anaesthetists’ Association. Clinical guidelines: Failed intubation. [Available at] http://www.oaa-anaes.ac.uk/content.asp?ContentID=303 [Accessed June 2013] .
144. Benumof JL. The unanticipated difficult airway. Can J Anaesth. 1999;46:510–511.
145. Benumof JL. Laryngeal mask airway and the ASA difficult airway algorithm. Anesthesiology. 1996;84:686–699.
146. Han TH, Brimacombe J, Lee EJ, Yang HS. The laryngeal mask airway is effective (and probably safe) in selected healthy parturients for elective Cesarean section: a prospective study of 1067 cases. Can J Anaesth. 2001;48:1117–1121.
147. Chadwick IS, Vohra A. Anaesthesia for emergency caesarean section using the brain laryngeal airway. Anaesthesia. 1989;44:261–262.
148. McClune S, Regan M, Moore J. Laryngeal mask airway for caesarean section. Anaesthesia. 1990;45:227–228.
149. McCrirrick A. The laryngeal mask airway for failed intubation at caesarean section. Anaesth Intensive Care. 1991;19:135.
150. Priscu V, Priscu L, Soroker D. Laryngeal mask for failed intubation in emergency caesarean section. Can J Anaesth. 1992;39:893.
151. Storey J. The laryngeal mask for failed intubation at caesarean section. Anaesth Intensive Care. 1992;20:118–119.
152. Bailey SG, Kitching AJ. The laryngeal mask airway in failed obstetric tracheal intubation. Int J Obstet Anesth. 2005;14:270–271.
153. Quinn AC, Milne D, Columb M, et al. Failed tracheal intubation in obstetric anaesthesia: 2 yr national case-control study in the UK. Br J Anaesth. 2013;110:74–80.
154. Heath ML, Allagain J. Intubation through the laryngeal mask: a technique for unexpected difficult intubation. Anaesthesia. 1991;46:545–548.
155. Brimacombe J, Keller C. The ProSeal laryngeal mask airway: a randomized, crossover study with the standard laryngeal mask airway in paralyzed, anesthetized patients. Anesthesiology. 2000;93:104–109.
156. Evans NR, Gardner SV, James MF. ProSeal laryngeal mask protects against aspiration of fluid in the pharynx. Br J Anaesth. 2002;88:584–587.
157. Keller C, Brimacombe J, Kleinsasser A, Loeckinger A. Does the ProSeal laryngeal mask airway prevent aspiration of regurgitated fluid? Anesth Analg. 2000;91:1017–1020.
158. Cook TM, Nolan JP, Verghese C, et al. Randomized crossover comparison of the proseal with the classic laryngeal mask airway in unparalysed anaesthetized patients. Br J Anaesth. 2002;88:527–533.
159. Halaseh BK, Sukkar ZF, Hassan LH, et al. The use of ProSeal laryngeal mask airway in caesarean section—experience in 3000 cases. Anaesth Intensive Care. 2010;38:1023–1028.
160. Awan R, Nolan JP, Cook TM. Use of a ProSeal laryngeal mask airway for airway maintenance during emergency Caesarean section after failed tracheal intubation. Br J Anaesth. 2004;92:144–146.
161. Keller C, Brimacombe J, Lirk P, Puhringer F. Failed obstetric tracheal intubation and postoperative respiratory support with the ProSeal laryngeal mask airway. Anesth Analg. 2004;98:1467–1470.
162. Sharma B, Sahai C, Sood J, Kumra VP. The ProSeal laryngeal mask airway in two failed obstetric tracheal intubation scenarios. Int J Obstet Anesth. 2006;15:338–339.
163. Matioc A, Arndt GA. Intubation using the ProSeal laryngeal mask airway and a Cook airway exchange catheter set. Can J Anaesth. 2001;48:932.
164. LMA-Fastrach Instruction Manual. The Laryngeal Mask Company, Ltd. [Available at] http://www.lmana.com/files/fastrach_ifu.pdf [Accessed June 2013] .
165. Joo HS, Rose DK. The intubating laryngeal mask airway with and without fiberoptic guidance. Anesth Analg. 1999;88:662–666.
166. Kamiya I, Satomoto M, Tokunaga M, et al. Intubation in a patient with lingual tonsil hypertrophy using an intubating laryngeal mask airway in combination with fiberoptic intubation. Masui. 2002;51:523–525.
167. Minville V, N’Guyen L, Coustet B, et al. Difficult airway in obstetric using ILMA-Fastrach. Anesth Analg. 2004;99:1873.
168. Asai T, Appadurai I. LMA for failed intubation. Can J Anaesth. 1993;40:802.
169. Strang TI. Does the laryngeal mask airway compromise cricoid pressure? Anaesthesia. 1992;47:829–831.
170. Asai T, Barclay K, Power I, Vaughan RS. Cricoid pressure impedes placement of the laryngeal mask airway. Br J Anaesth. 1995;74:521–525.
171. Aoyama K, Takenaka I, Sata T, Shigematsu A. Cricoid pressure impedes positioning and ventilation through the laryngeal mask airway. Can J Anaesth. 1996;43:1035–1040.
172. Brimacombe J, White A, Berry A. Effect of cricoid pressure on ease of insertion of the laryngeal mask airway. Br J Anaesth. 1993;71:800–802.
173. Brimacombe J, Berry A. LMA for failed intubation. Can J Anaesth. 1993;40:802–803.
174. Ansermino JM, Blogg CE. Cricoid pressure may prevent insertion of the laryngeal mask airway. Br J Anaesth. 1992;69:465–467.
175. Harry RM, Nolan JP. The use of cricoid pressure with the intubating laryngeal mask. Anaesthesia. 1999;54:656–659.
176. Asai T, Barclay K, McBeth C, Vaughan RS. Cricoid pressure applied after placement of the laryngeal mask prevents gastric insufflation but inhibits ventilation. Br J Anaesth. 1996;76:772–776.
177. Benumof JL. Laryngeal mask airway: indications and contraindications. Anesthesiology. 1992;77:843–846.
178. Atherton DP, O'Sullivan E, Lowe D, Charters P. A ventilation-exchange bougie for fibreoptic intubations with the laryngeal mask airway. Anaesthesia. 1996;51:1123–1126.
179. Asai T, Shingu K. The laryngeal tube. Br J Anaesth. 2005;95:729–736.
180. Agro F, Cataldo R, Alfano A, Galli B. A new prototype for airway management in an emergency: the Laryngeal Tube. Resuscitation. 1999;41:284–286.
181. Figueredo E, Martinez M, Pintanel T. A comparison of the ProSeal laryngeal mask and the laryngeal tube in spontaneously breathing anesthetized patients. Anesth Analg. 2003;96:600–605.
182. Genzwurker H, Finteis T, Hinkelbein J, Ellinger K. First clinical experiences with the new LTS: a laryngeal tube with an oesophageal drain. Anaesthesist. 2003;52:697–702.
183. Zand F, Amini A. Use of the laryngeal tube-S for airway management and prevention of aspiration after a failed tracheal intubation in a parturient. Anesthesiology. 2005;102:481–483.
184. Lefrancois DP, Dufour DG. Use of the esophageal tracheal combitube by basic emergency medical technicians. Resuscitation. 2002;52:77–83.
185. Vezina MC, Trepanier CA, Nicole PC, Lessard MR. Complications associated with the Esophageal-Tracheal Combitube in the pre-hospital setting. Can J Anaesth. 2007;54:124–128.
186. Frerk C, Frampton C. Cricothyroidotomy; time for change. Anaesthesia. 2006;61:921–923.
187. Frerk C, Cook TM. Management of the “can't intubate can't ventilate” situation and the emergency surgical airway. Report on the findings of the 4th National Audit Project of the Royal College of Anaesthetists. [Available at] http://www.rcoa.ac.uk/nap4/; 2011 [Accessed June 2013] .
188. Benumof JL, Scheller MS. The importance of transtracheal jet ventilation in the management of the difficult airway. Anesthesiology. 1989;71:769–778.
189. Bellemain A, Ghimouz A, Goater P, et al. Bilateral tension pneumothorax after retrieval of transtracheal jet ventilation catheter. Ann Fr Anesth Reanim. 2006;25:401–403.
190. Cook TM, Bigwood B, Cranshaw J. A complication of transtracheal jet ventilation and use of the Aintree intubation catheter during airway resuscitation. Anaesthesia. 2006;61:692–697.
191. Craft TM, Chambers PH, Ward ME, Goat VA. Two cases of barotrauma associated with transtracheal jet ventilation. Br J Anaesth. 1990;64:524–527.
192. McLellan I, Gordon P, Khawaja S, Thomas A. Percutaneous transtracheal high frequency jet ventilation as an aid to difficult intubation. Can J Anaesth. 1988;35:404–405.
193. Chandradeva K, Palin C, Ghosh SM, Pinches SC. Percutaneous transtracheal jet ventilation as a guide to tracheal intubation in severe upper airway obstruction from supraglottic oedema. Br J Anaesth. 2005;94:683–686.
194. McHugh R, Kumar M, Sprung J, Bourke D. Transtracheal jet ventilation in management of the difficult airway. Anaesth Intensive Care. 2007;35:406–408.
195. Peskett MJ. Clinical indicators and other complications in the recovery room or postanaesthetic care unit. Anaesthesia. 1999;54:1143–1149.
196. Rose DK, Cohen MM, Wigglesworth DF, DeBoer DP. Critical respiratory events in the postanesthesia care unit: patient, surgical, and anesthetic factors. Anesthesiology. 1994;81:410–418.
197. Auroy Y, Benhamou D, Pequignot F, et al. Mortality related to anaesthesia in France: analysis of deaths related to airway complications. Anaesthesia. 2009;64:366–370.
198. Lewis G. Saving Mothers’ Lives: Reviewing Maternal Deaths to make Motherhood Safer—2003–2005. The Seventh Report on Confidential Enquiries into Maternal Deaths in the United Kingdom. CEMACH: London; 2007.
199. Peterson GN, Domino KB, Caplan RA, et al. Management of the difficult airway: a closed claims analysis. Anesthesiology. 2005;103:33–39.
200. Cooper RM. Extubation and changing endotracheal tubes. Hagberg CA. Benumof's Airway Management: Principles and Practice. 2nd edition. Mosby: Philadelphia; 2007:1164–1180.
201. Calder I, Pearce A. Basic principles of airway management. Core Topics in Airway Management. Cambridge University Press: Cambridge; 2011.
202. Dravid R, Lee G. Extubation and re-intubation strategy. Popat M. Difficult Airway Management. Oxford University Press: Oxford; 2009:131–144.
203. Caroleo S, Agnello F, Abdallah K, et al. Weaning from mechanical ventilation: an open issue. Minerva Anestesiol. 2007;73:417–427.
204. Kriner EJ, Shafazand S, Colice GL. The endotracheal tube cuff-leak test as a predictor for postextubation stridor. Respir Care. 2005;50:1632–1638.
205. Dosemeci L, Yilmaz M, Yegin A, et al. The routine use of pediatric airway exchange catheter after extubation of adult patients who have undergone maxillofacial or major neck surgery: a clinical observational study. Crit Care. 2004;8:R385–R390.
206. Benumof JL. Airway exchange catheters: simple concept, potentially great danger. Anesthesiology. 1999;91:342–344.
207. Mort TC. Continuous airway access for the difficult extubation: the efficacy of the airway exchange catheter. Anesth Analg. 2007;105:1357–1362.
208. Loudermilk EP, Hartmannsgruber M, Stoltzfus DP, Langevin PB. A prospective study of the safety of tracheal extubation using a pediatric airway exchange catheter for patients with a known difficult airway. Chest. 1997;111:1660–1665.
209. Mort TC. Tracheal tube exchange: feasibility of continuous glottic viewing with advanced laryngoscopy assistance. Anesth Analg. 2009;108:1228–1231.
210. Baraka AS. Tension pneumothorax complicating jet ventilation via a cook airway exchange catheter. Anesthesiology. 1999;91:557–558.
211. Duggan LV, Law JA, Murphy MF. Brief review: Supplementing oxygen through an airway exchange catheter: efficacy, complications, and recommendations. Can J Anaesth. 2011;58:560–568.