Neurologic Complications of Pregnancy and Neuraxial Anesthesia
Felicity Reynolds MD, MBBS, FRCA, FRCOG ad eundem
Chapter Outline
THE INCIDENCE OF NEUROLOGIC SEQUELAE
Compression of the Lumbosacral Trunk
Compression as a Risk Factor for Peripheral Neuropathy
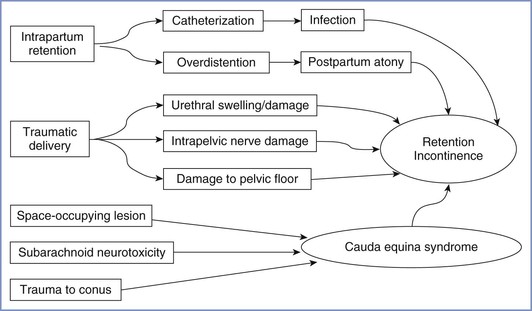
POSTPARTUM BLADDER DYSFUNCTION
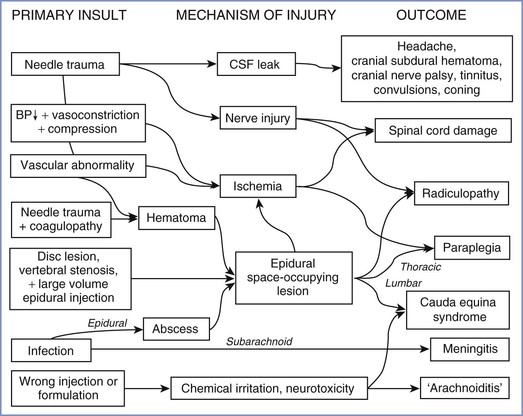
CENTRAL NERVOUS SYSTEM LESIONS
Neurologic Sequelae of Dural Puncture
Trauma to Nerve Roots and the Spinal Cord
Space-Occupying Lesions of the Vertebral Canal
Diagnosis of Possible Neurologic Injury
Neurologic complications of childbirth may be associated with neuraxial analgesia and anesthesia or may result from childbirth itself. Complications of neuraxial anesthesia may be immediate, such as motor blockade, unexpectedly high or prolonged blockade, and seizures after unintentional intravenous injection of local anesthetic, or they may be prolonged or delayed (sequelae). Immediate complications of neuraxial anesthesia are described in Chapter 23; here the discussion is focused on neurologic sequelae.
Although neurologic disorders after childbirth are more likely to have obstetric than anesthetic causes, neuraxial anesthesia is all too often blamed. For example, Tubridy and Redmond1 described seven women referred with neurologic symptoms after childbirth, all of which had been attributed to epidural analgesia. The women suffered from brachial neuritis, peroneal neuropathy, femoral neuropathy, neck strain, and leg symptoms for which there was no obvious physical cause. In such circumstances, a careful history and neurologic examination, together with diagnostic aids such as electromyography, nerve conduction studies, and imaging techniques, can localize the lesion and differentiate obstetric from anesthetic causes. For example, it should be possible to distinguish by simple clinical means between a mononeuropathy, which is likely to have an obstetric cause, and a radiculopathy resulting from neuraxial blockade. Accurate and prompt diagnosis is essential.
The Incidence of Neurologic Sequelae
Patients frequently ask obstetricians and anesthesia providers about the incidence of complications of neuraxial anesthesia, but even if accurate data were available, the question has no true answer. The incidence of neurologic complications varies widely according to local practice and the skill and training of the practitioners. Some old surveys are based on accurate local records, but the data relate to a time when obstetric and anesthetic practices, equipment, and drugs were less safe than they are today. The incidence of serious complications is now too low to be estimated accurately on a local basis. Nonetheless, anesthesia providers have a duty to inform patients of the complications associated with a proposed procedure and are expected to give some figure for the level of risk, however meaningless such an estimate may be.
Obstetric Surveys
In early surveys, the reported incidence of neurologic deficits in obstetric patients ranged from 1 in 2100 to 1 in 6400.2,3 During this period, long labor and difficult rotational forceps delivery were commonplace and neuraxial anesthesia was relatively unusual. Many surveys have subsequently attempted to assess the incidence of neurologic complications of neuraxial anesthesia, overlooking obstetric problems and other sources of error (Box 32-1).
Some of the more relevant surveys are listed in Table 32-1, those of the 20th century having been ably reviewed by Loo et al.4 in 2000. Ong et al.5 reviewed the medical records of all women who delivered in Winnipeg over a 9-year period and interviewed all those who received anesthesia care. All neurologic deficits in this series were transient, with none lasting more than 72 hours. The incidence of neurologic symptoms was similar after epidural and general anesthesia, but symptoms were more likely to be reported by women who received anesthesia than by those who received none. This is not surprising because only women who received anesthesia were interviewed. Indeed, the survey identified 45 cases of neurologic deficit, but only 10 had been noted in the hospital record, suggesting that many deficits may have been missed in the patients who were not interviewed. Moreover, modern statistical methods such as logistic regression analysis were not used to tease out the influence of prolonged labor and traumatic delivery, which the investigators noted as possible causative factors.
TABLE 32-1
Surveys of Neurologic Complications of Childbirth and of Neuraxial Blocks in Obstetrics
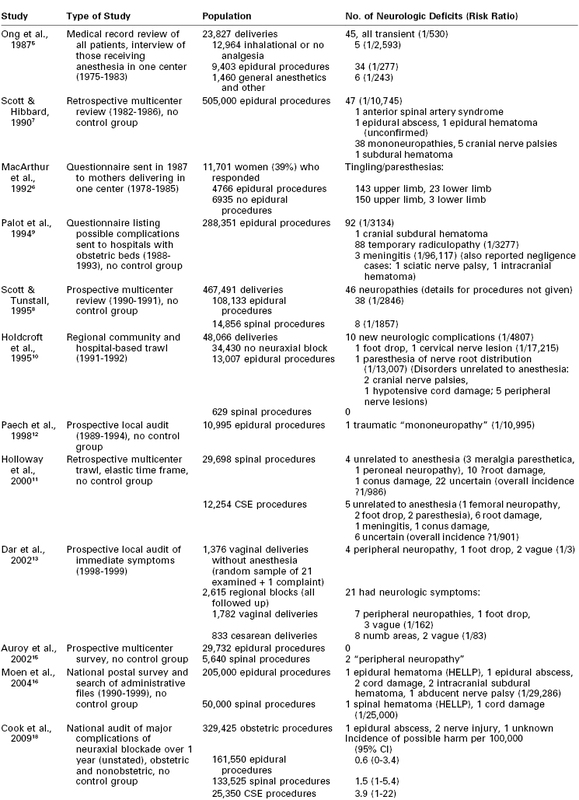
CSE, combined spinal-epidural.
In a large retrospective survey of long-term symptoms, women who delivered in one hospital in Birmingham, United Kingdom, were asked to recall events 2 to 9 years after delivery.6 The response rate was only 39%, and all those (and only those) who received epidural analgesia had been interviewed intensively about their symptoms immediately postpartum, thereby enhancing subsequent recall in this subset of the population. Logistic regression analysis demonstrated a link between epidural analgesia and many symptoms, including tingling and numbness in the arms, calling into question any causative link with neuraxial anesthesia. No major neurologic sequelae were detected.
Scott and Hibbard7 conducted a retrospective British survey of more than 500,000 epidural procedures administered between 1982 and 1986, which detected a number of serious sequelae, including one epidural abscess, one hematoma, and one anterior spinal artery syndrome, but many of the diagnoses were presumptive.7 This survey probably failed to detect many minor lesions, but it was followed by a smaller prospective survey by Scott and Tunstall,8 which included some spinal anesthetics and involved a self-selected group of respondents. The investigators found no major neurologic disorders but a more believable number of what were termed mononeuropathies.
A French survey based on a questionnaire covering the years 1988 to 1993, and sent to all hospitals with maternity units, recorded one cranial subdural hematoma but no cases of epidural abscess or hematoma in 288,351 obstetric epidural procedures.9 Seven deaths were reported. Complications of spinal anesthesia were not included in this survey.
Both the Scott surveys7,8 and the French inquiry9 overlooked the need for a control group of laboring patients who did not receive neuraxial analgesia/anesthesia. The study by Holdcroft et al.10 avoided this pitfall; the denominator consisted of all 48,066 women who delivered in one region over a year, and every effort was made to detect genuine neurologic symptoms in the community. Because the women themselves were not sent questionnaires, however, the response rate could not be estimated. The investigators judged that only one case of paresthesia, without physical signs, could be attributed to epidural analgesia and none to spinal anesthesia. Peripheral nerve damage was more common. The most serious was one case of foot drop in a woman who had a spontaneous delivery of a large baby with inhalation analgesia only.
With the increase in popularity of spinal anesthesia, concern about the growing numbers of reports of paresthesias and possible root trauma led Holloway et al.11 to conduct a retrospective U.K. national survey of spinal and combined spinal-epidural (CSE) anesthesia in the 1990s. No difference in frequency of neuropathy was detected between Whitacre and Sprotte needles or between single-shot spinal and CSE techniques. Imprecise diagnoses made it difficult to differentiate anesthetic from coincidental causes, but after eliminating obvious obstetric or peripheral nerve palsies while otherwise erring on the pessimistic side, the investigators estimated that the incidence of neurologic sequelae was approximately 1 in 1000, including two cases of conus damage, one of meningitis, and the rest minor root palsies.
Two thorough local audit reports of immediate postpartum symptoms provided somewhat contrasting findings. One from Perth, Australia,12 involved prospective recording of complications in 10,995 parturients who received epidural analgesia, but it regrettably did not include a control group. The investigators detected only a single lasting neurologic problem. Although they termed the injury a traumatic mononeuropathy, it was apparently a radiculopathy, because it was attributed to a traumatic epidural procedure.
The second report, from Leeds, United Kingdom, involved 3991 women who delivered in one center in 1 year.13 Twenty-one women presenting with symptoms after neuraxial blockade were matched with 21 asymptomatic control patients who had also received neuraxial blockade and 21 who had not. Only 1 woman who had not had a neuraxial block presented with symptoms, and she was found to have foot drop after a vacuum extraction. Typical peripheral neuropathies occurred among those who delivered vaginally; sacral numbness was most commonly detected after cesarean delivery. All changes were transient, and none could be attributed to neuraxial anesthesia. Similar neurologic deficits were detected among the randomly selected, asymptomatic 21 control patients who had had no anesthetic intervention. In contrast, negligible deficits could be detected among the 21 asymptomatic control women who had had an anesthetic intervention. These results demonstrate that minor neurologic deficits are to be found postpartum quite frequently if sought, but only those who have had anesthetic intervention are likely to complain.
A prospective survey among 6057 women who delivered in 1 year in Chicago14 does not feature in Table 32-1 because the patients were not grouped by type of analgesia, but the findings of this survey corroborate those of the Leeds study.13 The incidence of lower limb nerve injuries was approximately 1% (24 lateral femoral cutaneous nerve, 22 femoral nerve, 3 peroneal nerve, 3 lumbosacral plexus, 2 sciatic nerve, 3 obturator nerve, and 5 radicular injuries).14 Significant risk factors identified by logistic regression analysis included nulliparity and a prolonged second stage of labor but not neuraxial anesthesia.
In a nationwide 10-month prospective French survey, only two so-called peripheral neuropathies and no major sequelae were detected among 5,640 spinal and 29,732 epidural procedures in obstetric patients.15 In contrast, in a retrospective Swedish national survey covering the years 1990 through 1999, Moen et al.16 reported nine serious sequelae among an estimated 200,000 epidural and 50,000 spinal procedures in parturients.
Ruppen et al.17 attempted to conflate the findings of various surveys to derive a consensus incidence of neurologic injury after obstetric epidural block. Unfortunately, this study took no account of the now widespread use of spinal and CSE techniques, denominators were calculated inaccurately, and the findings of surveys were not always interpreted correctly. For example, cranial subdural hematoma was counted as a spinal hematoma. Therefore, reliable information cannot be derived from this publication.
A U.K. national audit of neuraxial blocks, without controls, published in 2009, found that the risk for major complications was 6- to 14-fold higher for perioperative than for obstetric procedures. Among the obstetric patients, the risk was highest for CSE, intermediate for spinal, and lowest for epidural procedures.18
Several conclusions can be drawn from these surveys. Despite an increased cesarean delivery rate, obstetric palsies (albeit now more short-lived) still occur, and the reported frequency of neurologic sequelae depends on how hard one seeks them. The risk for transient mild deficits after childbirth may be quite high.13,14 A true figure for anesthetic complications cannot be calculated even from thorough surveys because (1) the diagnosis is rarely accurate; (2) definitions, severity, and duration are often ill defined; and (3) anesthesia provider skills vary. Table 32-1 demonstrates a variation in the incidence of neurologic sequelae from 1 in 3 for mild symptoms with no neuraxial block13 to 1 in 30,000 for epidural analgesia.16 Moreover, bias is created when more attention is paid postpartum to patients who have received neuraxial blockade than to those who have not.
Other Surveys
Modern surveys of neurologic complications of spinal and epidural anesthesia among nonobstetric populations may yield more reliable results but still lack sensitivity to detect all potential problems and are commonly conducted in relatively elderly and sick populations. Moreover, the occurrence of case clusters gives the lie to the existence of a “true” incidence of complications.16 Auroy et al.,15 Moen et al.,16 and Cook et al.18 surveyed mixed populations and found a lower incidence of serious sequelae in obstetric than in other patients. It is therefore invalid to extrapolate findings from one population to the other. The reported risk for neurologic problems varies greatly with the patient population, local practice and skill, completeness of detection, and inclusion criteria. Hence, it is meaningless to attempt to put any firm figure on the risk for neurologic complications after neuraxial anesthesia.
Peripheral Nerve Palsies
Postpartum nerve injury is often assumed to be due to neuraxial anesthesia, but peripheral nerve palsies, which generally have obstetric causes, are much more common, with a reported incidence between 0.6 and 92 per 10,000.19 They may arise from compression in the pelvis by the fetal head, or from more distal compression, the signs of which may be overlooked in the presence of neuraxial anesthesia. In contemporary obstetric practice, cesarean delivery is usually preferred to prolonged labor and difficult or forceps delivery. The incidence of pelvic nerve trauma and compression should therefore be lower than in years past. Surveys have shown that although obstetric palsies still occur,11,13,14 most are short-lived and less disabling than hitherto.2,3 Foot drop, however, is still reported,10,13,14,20 primarily in cases in which the effort to avoid cesarean delivery leads to vaginal delivery of a disproportionately large baby.10,13 Abnormal presentation, persistent occiput posterior position, fetal macrosomia, breakthrough pain during epidural labor analgesia, a prolonged second stage of labor, difficult instrumental delivery, and prolonged use of the lithotomy position may presage postpartum neuropathy.
Reference to the distribution of spinal dermatomes and peripheral nerve sensory innervation clearly demonstrates the distinction between peripheral and central lesions (Figure 32-1). Spinal nerve root lesions are also manifested by weakness that involves several lower extremity joints and movements (Figure 32-2).

FIGURE 32-1 Segmental (right leg) and peripheral (left leg) sensory nerve distributions useful in distinguishing central from peripheral nerve lesions. (From Redick LF. Maternal perinatal nerve palsies. Postgrad Obstet Gynecol 1992; 12:1-6.)
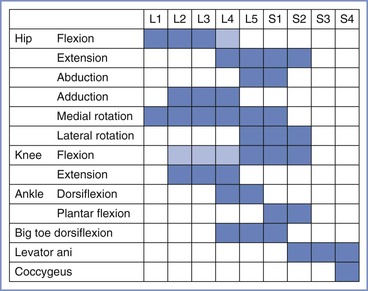
FIGURE 32-2 The spinal segments involved in movements of joints in the leg. Lighter shading denotes a minor contribution. (Data from Russell R. Assessment of motor blockade during epidural analgesia in labour. Int J Obstet Anesth 1992; 4:230-4.)
Compression of the Lumbosacral Trunk
Compression of the lumbosacral trunk by the fetal head at the pelvic brim (Figure 32-3) preferentially affects the more medial fibers that make up the peroneal rather than the tibial nerve.19 In addition to weakness that predominantly affects ankle dorsiflexion (foot drop), compression of the lumbosacral trunk produces sensory disturbance mainly involving the L5 dermatome (see Figure 32-1). This palsy most often results from some cephalopelvic disproportion and is therefore typically seen after prolonged labor and difficult vaginal delivery.2,3,11-14
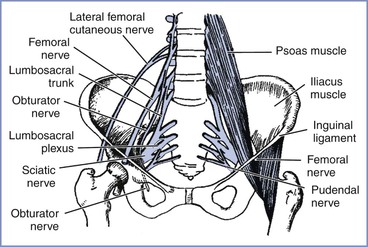
FIGURE 32-3 The principal nerves in the pelvis. The lumbosacral trunk (L4 to L5) and obturator nerve (L2 to L4) are vulnerable to pressure as they cross the pelvic brim, particularly in cases of cephalopelvic disproportion. The femoral (L2 to L4) and lateral femoral cutaneous (L2 to L3) nerves are particularly vulnerable in the lithotomy position, where they pass beneath the inguinal ligament. (Adapted from Cole JT. Maternal obstetric paralysis. Am J Obstet Gynecol 1946; 52:374.)
Obturator Nerve Palsy
The obturator nerve is susceptible to compressive injury as it crosses the brim of the pelvis or within the obturator canal (see Figure 32-3). The mother may complain of pain when the damage occurs, followed by weakness of hip adduction and internal rotation, with sensory disturbance over the upper inner thigh (see Figure 32-1). Cases are reported after both labor and cesarean delivery20-22; three were detected in a prospective study by Wong et al.14 Because the nerve would appear to be in a vulnerable position, it may be that injury occurs more often than is reported, but is misdiagnosed.
Femoral Nerve Palsy
Approximately one third of the postpartum palsies detected by Wong et al.14 were femoral nerve palsies. Dar et al.13 detected five cases in their small population, although the symptoms were transient. The femoral nerve does not enter the pelvis and is therefore not vulnerable to compression by the fetal head but is vulnerable to stretch injury as it passes beneath the inguinal ligament. Damage may result from prolonged flexion, abduction, and external rotation of the hips during the second stage of labor and also during procedures conducted in an excessive lithotomy position.23 The hips should therefore never remain continuously flexed during the second stage of labor. In femoral neuropathy, the nerve supply to the iliopsoas muscle is spared, so that some hip flexion is still possible. The patient with a femoral neuropathy may walk satisfactorily on a level surface but may be unable to climb stairs; the patellar reflex is diminished or absent.
Meralgia Paresthetica
Meralgia paresthetica is a neuropathy of the lateral femoral cutaneous nerve, a purely sensory nerve also known as the lateral cutaneous nerve of the thigh. First described more than 100 years ago, meralgia paresthetica is commonly encountered in pregnancy and childbirth.13,14 It may arise both during pregnancy, typically at about 30 weeks' gestation, and intrapartum,14,24 in association with increasing intra-abdominal pressure. It may recur during successive pregnancies. The most likely cause is entrapment of the nerve as it passes around the anterior superior iliac spine beneath or through the inguinal ligament, where its vulnerability is increased by a large intra-abdominal mass or by retractors used during pelvic surgery. The compressive effect of edema may also contribute. Meralgia paresthetica manifests as numbness, tingling, burning, or other paresthesias affecting the anterolateral aspect of the thigh. The distribution is quite unlike that of a nerve root lesion (see Figure 32-1), yet the disturbance is commonly attributed to neuraxial blockade by those ignorant of neuroanatomy. The condition can be expected to resolve after childbirth; transient pain may be relieved by local infiltration analgesia.
Sciatic Nerve Palsy
Sciatic nerve palsy arises from compression of the nerve, usually in the buttock. It is not commonly mentioned in surveys or generally recognized as a complication of childbirth, possibly because it is mistaken for a lesion of the lumbosacral trunk. It gives rise to loss of sensation below the knee with sparing of the medial side and loss of movement below the knee. Posterior cutaneous nerve and gluteal function are preserved, implying damage distal to the lumbosacral plexus, where the gluteal nerves branch off the sciatic nerve (Figure 32-4). It has occurred during childbirth under neuraxial blockade, either from sitting in one position too long25 or from a hip wedge misplaced during cesarean delivery.25-27 Hypotension may be contributory. Three cases were detected by Wong et al.14 Despite the peripheral location of the lesions, neuraxial anesthesia cannot be exonerated, because the symptoms of nerve compression, which otherwise would have prompted a change of position, may be overlooked or wrongly attributed to local anesthetic-induced sensory blockade.
Peroneal Nerve Palsy
The common peroneal nerve is vulnerable to compression as it passes around the head of the fibula below the knee. It is also susceptible to damage while it still forms part of the sciatic nerve as it leaves the pelvis. Peroneal nerve palsy may be caused by prolonged squatting,28 sometimes popular in “natural childbirth,” by excessive knee flexion for any reason, by compression of the lateral side of the knee against any hard object, even the parturient's hand,29 and by prolonged use of the lithotomy position. These risks are compounded by the presence of neuraxial blockade. When the peroneal nerve is damaged at the knee, there is sensory impairment on the anterolateral calf and the dorsum of the foot; foot drop may be profound, with steppage gait and weak ankle eversion, but plantar flexion and inversion at the ankle are preserved.
Compression as a Risk Factor for Peripheral Neuropathy
During pregnancy, nerve compression due to edema may be a factor in the genesis of several peripheral neuropathies, such as carpal tunnel syndrome, Bell's palsy, and meralgia paresthetica.24,30,31 Anesthesia providers cannot be wholly absolved from responsibility for peripheral neuropathies, the signs of which may be overlooked or attributed to neuraxial anesthesia.26,29 Adverse factors can be minimized by attention to simple rules (Box 32-2). One group of patients, those with hereditary neuropathy with liability to pressure palsy, requires particular attention. In these women even relatively brief periods of immobility or pressure on any one site must be avoided.32,33
Postpartum Bladder Dysfunction
There are several mechanisms by which bladder function may be disturbed postpartum (Figure 32-5). In theory, neuraxial blockade (1) may provoke the need for bladder catheterization with increased risk for infection, (2) may allow bladder distention to go undetected, and (3) on very rare occasions, may be associated with cauda equina syndrome (see later discussion). However, several postpartum studies of bladder function have found no association with neuraxial analgesia34,35 or only a weak correlation between epidural analgesia and an increased residual volume immediately postpartum.36 In contrast, a prolonged second stage of labor, instrumental delivery, and perineal damage have been identified as significant factors for postpartum bladder dysfunction.34-36 In the previously described large survey of long-term symptoms after childbirth conducted in Birmingham, United Kingdom, no association was found between epidural analgesia and stress incontinence or urinary frequency.8,37 By far the most common cause of bladder dysfunction appears to be non-neurologic. Nevertheless, it must be part of the anesthesia provider's responsibility to ensure that the bladder does not become overdistended either intrapartum or postpartum.
Central Nervous System Lesions
Lesions of the central nervous system (CNS) after childbirth have complex causes (Figure 32-6), which may be classified as traumatic (to nervous tissue, meninges, or blood vessels), infective, ischemic, or chemical (to nervous tissue or meninges). Anesthesia providers should bear in mind that even central lesions may have causes other than neuraxial block, the most obvious being a prolapsed intervertebral disc. Apart from sequelae of dural puncture, serious iatrogenic complications are remarkably rare.
Neurologic Sequelae of Dural Puncture
The subject of post–dural puncture headache is discussed in detail in Chapter 31. There are several other causes of severe postpartum headache, some of which have serious neurologic implications. Postpartum headache requires diagnosis first and foremost, followed by treatment that is curative rather than palliative.
Postpartum cortical vein and venous sinus thrombosis are more common than expected because of the hypercoagulable state of the blood.38-40 Cortical vein thrombosis has been associated with dural puncture and post–dural puncture headache. Headaches caused by meningitis, venous sinus thrombosis, preeclampsia, hypertensive encephalopathy, subdural hematoma, internal carotid artery dissection, and posterior reversible encephalopathy syndrome may cause difficulty in diagnosis, particularly if they occur after epidural bolus injection, unintentional dural puncture, or administration of an epidural blood patch. Seizures may occur in patients with eclampsia, hypertensive encephalopathy, meningitis, or pneumocephalus but may also follow dural puncture or performance of an epidural blood patch.38-49
It is commonly assumed that headache after dural puncture will resolve spontaneously over time, but unfortunately a dural leak can persist and may occasionally have more serious consequences, including cranial nerve palsy and subdural hematoma. Neglected cerebrospinal fluid (CSF) leak has also been known to induce medullary and tentorial coning.50 Although serious problems are more likely to occur from unintentional dural puncture with a large epidural needle and a neglected headache, they may occasionally follow deliberate dural puncture with a small-gauge spinal needle.50
Cranial Nerve Palsy
Major loss of CSF, usually following unintentional dural puncture with a large needle, may cause a number of cranial nerve palsies; those affecting cranial nerves VI, VII and VIII are the most frequently reported.51-57 Because of its long course within the cranium, the abducens nerve (VI) is the most vulnerable. All cranial nerve palsies require prompt epidural blood patch, but even after the blood patch, recovery may be delayed. In the case of cranial nerve VIII dysfunction, tinnitus may become permanent.56,57 Trigeminal nerve dysfunction is usually a transient effect of high spinal blockade.
Cranial Subdural Hematoma
More seriously, reduced CSF pressure may cause rupture of bridge meningeal veins and result in cranial subdural hematoma, a potentially fatal condition that has been reported sporadically over many years.50 Palot et al.9 found one case in 288,351 obstetric epidural procedures; in 2000, Loo et al.4 identified eight obstetric cases, and more have been reported since.58-62 Although commonly believed to result only from neglect of a dural puncture with a large needle or a cutting spinal needle, subdural hematoma requiring craniotomy has been reported after puncture with a small-gauge, pencil-point spinal needle60 and after an unintentional dural puncture that had been appropriately treated with an epidural blood patch.58 Whenever headache persists after treatment with an epidural blood patch (particularly if the headache is accompanied by altered consciousness, seizures, or other focal neurologic findings), magnetic resonance imaging (MRI) is warranted to exclude subdural hematoma, which may be fatal without urgent surgery.
One case of cranial epidural hematoma arose after spinal anesthesia for removal of the placenta; the hematoma occurred after a seizure.63 Nothing was as it seemed, however; the seizure was not eclamptic, but rather epileptic, and the hematoma was not a result of spinal anesthesia.
Trauma to Nerve Roots and the Spinal Cord
Insertion of a spinal needle or epidural catheter is not infrequently accompanied by paresthesia that is sometimes painful. Although a flexible catheter is unlikely to do lasting damage to a nerve root in the epidural space, nerve roots in the subarachnoid space are more vulnerable.
Trauma Associated with Attempted Epidural Catheter Insertion
An epidural catheter may injure nerve roots either because it is inappropriately rigid64 or because an undue length is threaded and ensnares a root.65 A catheter seemingly threaded into the epidural space may lodge in an intervertebral foramen or even pass into the paravertebral space. In rare instances the epidural catheter and the artery of Adamkiewicz share the same foramen. If the epidural catheter is stiff enough to compress the artery within the unyielding foramen, the blood supply to the spinal cord may be impaired. This is a possible cause of anterior spinal artery syndrome. Clinical reports indicate that the condition resolves rapidly and completely if the catheter is withdrawn before permanent damage has occurred.66,67
Injury to the spinal cord may result from attempted identification of the epidural space in the presence of undetected spina bifida occulta or a tethered cord68 or as a result of an unsteady grip or uncontrolled advancement of the epidural needle. Insertion of an epidural catheter in an anesthetized patient greatly increases the risk for spinal cord damage, and catastrophic injury may occur with injection of fluid into the substance of the spinal cord.69
Trauma Associated with Spinal Anesthesia
Insertion of a spinal needle below the level of the spinal cord sometimes causes brief radiating pain or paresthesia, which may be associated with persistent paresthesia in the same dermatomal distribution. Prolonged symptoms involving more than one spinal segment suggest damage to the spinal cord itself. Damage to the terminal portion of the cord (the conus medullaris) without intracord injection has also been reported in healthy conscious parturients receiving spinal or CSE anesthesia using a pencil-point needle.11,16,70,71 Typically, the patient complains of pain on needle insertion before any fluid is injected, often followed by the normal appearance of CSF from the needle hub, easy injection of the local anesthetic agent, and a normal onset of neural blockade. On recovery, there is unilateral numbness, which is succeeded by pain and paresthesia in the L5 to S1 distribution and foot drop, and in some cases urinary symptoms; sensory symptoms may last for months or years. The MRI appearance is one of a small syrinx or hematoma within the conus at the level of the body of T12 on the same side as the pain on insertion and subsequent leg symptoms (Figure 32-7).71 In the majority of cases, the anesthesia provider believed the interspace selected was L2-L3. In one patient who subsequently died of other causes, hematomyelia was confirmed at autopsy.72 Since a spate of cases of conus damage in the 1990s, the practice of spinal needle insertion may have been modified, but an abnormally long cord may still be damaged with the best of techniques.73
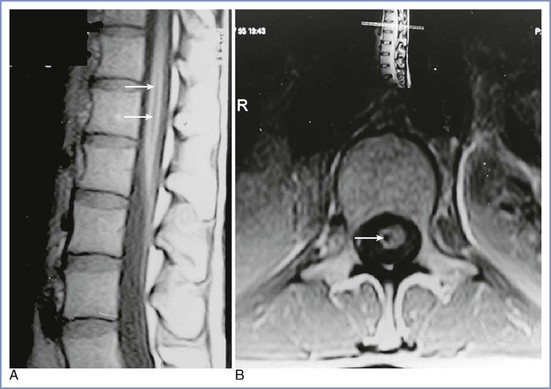
FIGURE 32-7 A and B, Magnetic resonance images of a conus medullaris lesion (arrows). (From Reynolds F. Damage to the conus medullaris following spinal anaesthesia. Anaesthesia 2001; 56:238-47.)
These injuries may have occurred for the following reasons:
• Anesthesia providers, accustomed to siting epidural needles and catheters, had forgotten the precautions necessary to avoid contact with the spinal cord during dural puncture. Moreover, the option to use an upper lumbar interspace was sanctioned by some reputable textbooks.74
• Identification of lumbar interspaces was far from accurate. Studies showed that it was common to select a space that is higher than assumed by one, two, or even more segments (Figure 32-8).75,76 Anesthesia providers had little opportunity for feedback to improve their skill in interspace identification.
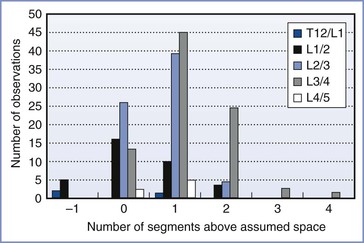
FIGURE 32-8 Identification of lumbar interspaces by Oxford anesthetists. The horizontal axis shows the position of the actual interspace identified on magnetic resonance imaging, relative to the assumed space, in 200 observations. (Data from Broadbent CR, Maxwell WB, Ferrie R, et al. Ability of anaesthetists to identify a marked lumbar interspace. Anaesthesia 2000; 55:1122-6.)
• Although the spinal cord typically ends level with the lower body of L1 or the L1-L2 interspace, the length varies (Figure 32-9).77 From the L1-L2 interspace, the needle tip can easily reach the conus in 27% of men and 43% of women.78
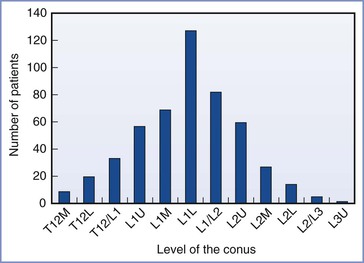
FIGURE 32-9 Variation in the level of the tip of the conus medullaris assessed by magnetic resonance imaging of the lumbar spine among 504 consecutive adults. L, lower third of vertebral body listed; M, middle third of vertebral body listed; T12/L1, interspace between T12 and L1; U, upper third of vertebral body listed. (Data derived from Saifuddin A, Burnett SJ, White J. The variation of position of the conus medullaris in an adult population: a magnetic resonance imaging study. Spine 1998; 23:1452-6.)
• The standard method of identifying lumbar interspaces involves the use of Tuffier's line, the imaginary line joining the two iliac crests. This method can be inaccurate, however, particularly in obese or pregnant women (Figure 32-10). Moreover, even when accurately assessed, Tuffier's line is an inconstant landmark.79 Although typically at the level of the L4 spinous process, it may lie anywhere between the L3-L4 and L5-S1 interspaces. Other means of identifying the interspace, such as counting down from C7 or finding the vertebra that is attached to the 12th rib, are tedious and of little help in obese patients.
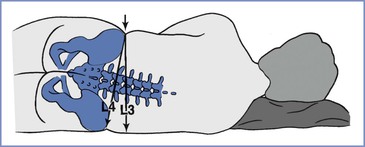
FIGURE 32-10 Error that may arise if Tuffier's line is judged in a pregnant patient in the lateral position, when a line is drawn perpendicularly from the upper iliac crest rather than through both iliac crests. In pregnant patients at term, the hips may have a greater width than the shoulders. The resulting cephalad pelvic tilt may lead to an error in the cephalad direction.
• Pencil-point spinal needles must be advanced further than cutting needles before the orifice is within the subarachnoid space, at which point the tip may impinge on the spinal cord.
Medical students and residents are usually instructed to select the L4-L5 interspace for diagnostic lumbar puncture, but anesthesia providers have been more liberal in their approach. Given the inaccuracy of identification of lumbar interspaces and the variability of the position of the conus, it is both logical and prudent to insert a spinal needle below the spinous process of L3, or at least into a lower lumbar interspace, especially in women. Box 32-3 summarizes the problems and precautions in identifying lumbar interspaces and avoiding damage to the conus medullaris.
Space-Occupying Lesions of the Vertebral Canal
Space-occupying lesions of the vertebral canal include intraspinal hematomas (epidural or subdural), epidural abscess, and intraspinal tumors, any of which, within the rigid confines of the bony spinal canal, can cause dangerous compression of nervous tissue and its blood supply. Urgent laminectomy is required to avoid permanent neurologic damage. Delayed recognition and treatment (> 6 to 12 hours after onset of symptoms) may have a catastrophic outcome and grave medicolegal consequences.
Epidural analgesia in labor does not normally behave like a space-occupying lesion and produces no lasting deformation of the thecal sac on MRI.80 Nevertheless, in the presence of vertebral stenosis or lumbar disc protrusion, a large volume injected into the epidural space may tip the balance and produce signs of nervous tissue compression that normally resolve in a few hours.81,82
The neurologic deficit that may arise from a compressive lesion depends on the vertebral level; lower thoracic lesions are associated with leg weakness or paraplegia, and lumbar lesions with cauda equina syndrome, including urinary retention and incontinence. Back pain (often radiating to the legs) is a common feature.
Spinal Hematoma
Spinal hematomas may be classified as epidural, subdural, or subarachnoid.83 Keppel et al.83 found 613 cases of spinal hematoma published in the medical literature between 1826 and 1996, 461 of which were epidural, 25 subdural, and 96 subarachnoid. Spinal hematomas were described at all vertebral levels. Twice as many patients were male as female. Many patients were elderly and only five were pregnant (details unknown). The majority of cases (44%) were spontaneous or followed minor trauma, and 22.5% were related to coagulopathy or anticoagulant treatment; only 63 cases followed lumbar puncture or neuraxial anesthesia. Of these 63 patients, 26 were without coagulopathy.
Similarly, during pregnancy and the puerperium, spontaneous epidural hematoma is reported more frequently than hematoma associated with neuraxial blockade. Loo et al.4 found three cases (that may have been included in the earlier review83) and more have been reported in the 21st century.84-88 Epidural hematoma in pregnancy associated with coagulopathy but without neuraxial anesthesia is also reported.89,90 It has been suggested that pregnancy-induced structural changes in vessel walls, together with hemodynamic changes, may predispose to spinal hematoma.86
Epidural Hematoma after Neuraxial Blockade.
Incidence.
Epidural hematoma after neuraxial blockade typically causes neurologic deficit in elderly patients with arterial disease; it is very rare in obstetric patients, despite the engorgement and possible fragility of epidural veins. Nine surveys, covering 1,331,171 obstetric epidural procedures,* found two cases (see Table 32-1), one without confirmatory details7 and the other in a patient with HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome.16 This gives an incidence of 0.150 per 100,000 epidural procedures (95% confidence interval [CI], 0.018 to 0.543). Any estimate of the incidence of epidural hematoma in the surgical population is equally meaningless because, as with obstetric cases, it depends on how assiduously neuraxial blockade is avoided in the presence of coagulopathy and also on the incidence of vessel puncture, which in turn is affected by the skill of the anesthesia provider.
Causation.
Risk factors identified from comprehensive reviews of case reports include (1) difficult or traumatic epidural needle/catheter placement, (2) coagulopathy or therapeutic anticoagulation, (3) spinal deformity, and (4) spinal tumor.4,91,92 Antiplatelet therapy with nonsteroidal anti-inflammatory drugs (NSAIDs) is believed not to increase the incidence of neurologic dysfunction after neuraxial anesthesia,93 although NSAIDs were involved in three cases in one survey of a mixed population.91
Of the five cases of epidural hematoma in obstetric patients found by Loo et al.,4 four were without details and the other associated with coagulopathy. In the most recent analysis from the American Society of Anesthesiologists' (ASA) Closed-Claims Project database,94 there were four cases of epidural hematoma, of which only one had coagulopathy; again, details were not provided. Two further case reports are of interest. An eclamptic patient with thrombocytopenia suffered a persistent lower limb deficit after a traumatic epidural catheter insertion using the loss-of-resistance-to-air technique.95 Laminectomy revealed multiple bubbles and a 4-mL blood clot, the exact site of which was not stated. Such a small volume could have caused neurologic deficit only if it was subdural rather than epidural. An epidural hematoma was reported presenting nine days after removal of an epidural catheter that had been sited and used uneventfully for labor analgesia.96 Apparently the only risk factors were a traumatic insertion and self-medication with ibuprofen postpartum. The coagulation assessment was normal, but the hematoma was extensive and required decompressive surgery. It is of course possible that this was a spontaneous hematoma and neuraxial analgesia was coincidental.
Both vessel damage and coagulopathy (whether inherited, acquired, or due to anticoagulation) are usually necessary to produce a hematoma large enough to cause a neurologic deficit in the parturient. Safe epidural catheter insertion in coagulopathic parturients has often been recorded, but the frequency of vessel trauma is rarely mentioned.97-100 Vessel trauma can arise not only during insertion but also on removal of the epidural catheter.
Protective Factors.
It may be that, in obstetric patients, many epidural hematomas arise but are too small to cause neurologic deficit. One factor may be the hypercoagulable status of blood peripartum. Another is the ease with which a large volume of anticoagulated blood may flow out of the unrestricting intervertebral foramina in young patients. Injected blood is known to disappear from the epidural space rapidly in the parturient.80,101 During performance of an epidural blood patch, 20 mL of blood is commonly injected with impunity. Although compressive symptoms may be experienced with a volume larger than 20 mL, they do not normally presage any neurologic deficit in obstetric patients.
Subdural and Subarachnoid Hematoma.
Spinal subdural hematoma has been reported in obstetric patients, one in association with an ependymoma,4 one after spinal anesthesia and an epidural blood patch,102 and another in a woman with preeclampsia, known vessel puncture during epidural catheter insertion, and mild coagulopathy.103 A subarachnoid hematoma after spinal anesthesia was reported in a patient with HELLP syndrome.104 More worryingly, one woman suffered a major subarachnoid hematoma after apparently straightforward CSE anesthesia.105 All four patients developed cauda equina syndrome and laminectomy was required in three.
Dural puncture (with or without arachnoid puncture) is a prerequisite for subdural and subarachnoid hematoma. On the other hand, coagulopathy may not be a prerequisite, because the extravasated blood is confined in a small space and may compress adjacent nerve roots more readily than in the capacious epidural space.
Prevention, Diagnosis, and Management.
It is clearly important to check coagulation status in an at-risk parturient not only when inserting but also when removing an epidural catheter. If neuraxial blockade is found to have been conducted in the presence of risk factors for spinal hematoma, it is an essential responsibility of the anesthesia provider to examine the lower extremities after delivery, to confirm and document the return of normal motor and sensory function, and to request subsequent checks by the nursing staff. Severe back pain and a significant delay in normal recovery or deterioration of lower extremity or bladder function signal the need for emergency imaging of the spine. If intraspinal compression is confirmed by MRI, a neurosurgical opinion must be urgently sought.
The dangers of neuraxial anesthesia in the presence of coagulopathy and anticoagulant treatment are discussed in Chapters 39 and 44.
Infection
Neuraxial infection (epidural abscess and meningitis) was identified as the most common cause of neuraxial injury in obstetric cases in the ASA Closed-Claims Project database between 1980 and 1999.106 Infections that have been reported include epidural abscess, paraspinal and other epidural-related infection, and meningitis.
Epidural Abscess
Frequency.
Epidural abscess may occur spontaneously in pregnancy and the puerperium as at other times.4,107 An analysis of 915 reports of spinal epidural abscess published between 1954 and 1997 found that epidural blockade had been performed in only 5.5% of cases.108 After neuraxial blockade, epidural abscess, like spinal hematoma, appears to be rare in obstetric patients. Three cases were found among 1,331,171 epidural procedures listed in nine surveys summarized in Table 32-1, a frequency of 0.225 per 100,000 (95% CI, 0.047 to 0.659). The incidence among surgical patients has been reported as 10-fold16 to 100-fold109 higher, with most cases arising in elderly and immunocompromised patients. A careful 4-year Australian study of 9482 obstetric patients who underwent childbirth in a center where correct sterile procedures were used found 49 epidural catheter–related infections (0.52%): 45 superficial, 2 epidural, and 2 paraspinal, giving an epidural infection rate of 21 per 100,000,110 which was 100-fold higher than the calculated frequency from larger, but apparently less sensitive, surveys.
Sixteen case reports of well-authenticated epidural abscess after epidural analgesia in obstetric patients111-126 have been tabulated elsewhere.127 All cases occurred after epidural catheterization, with three as part of CSE anesthesia. None followed spinal anesthesia alone. One case was reported as meningitis but was actually an epidural abscess.122
One additional case report concerned a mother who developed back pain 12 months after uneventful epidural analgesia for labor.128 After 2 further months she developed leg weakness; diagnostic lumbar puncture provoked further acute neurologic deterioration, and she was found to have an epidural abscess from the cervical to the lumbar region.
Possible risk factors identified from these cases are outlined in Table 32-2. An epidural abscess typically follows prolonged epidural catheterization, usually between 1 and 4 days in obstetric cases. Other possible etiologic factors are traumatic or difficult insertion of the catheter,112,114,117,120 epidural administration of opioid without local anesthetic,110,112,118,119 and diabetes or immunosuppression from any cause.109,112,115,121 Inflammation at the epidural catheter entry point may presage epidural space infection.110,114,119 In light of these reports it may be prudent to avoid prolonged epidural catheterization in the patient with other risk factors for infection.
Some practitioners have suggested that administration of an epidural blood patch necessitates prior blood culture. However, a Medline search carried out in April 2012 for “epidural abscess AND epidural blood patch” did not yield any cases, and the only recorded instance of an infected blood patch is one that appears to have entered the subcutaneous fat (see later discussion).
Clinical Presentation.
Symptoms of epidural abscess typically start between 4 and 10 days after removal of the epidural catheter. The interval of 14 months cited earlier is extreme and poses the question whether the epidural catheter was causative. Severe backache (with local tenderness) and fever, with or without radiating or root pain, are the presenting features. The catheter entry point may be inflamed with some fluid leak, and a hematology screen typically reveals leukocytosis and increased C-reactive protein. Fever, neck stiffness, headache, and signs of inflammation serve to differentiate epidural abscess from hematoma. These signs and symptoms should prompt MRI, which may allow early diagnosis before the onset of neurologic changes (Figure 32-11).129 If untreated, symptoms may progress to leg weakness, paresthesias, bladder dysfunction, and other evidence of cauda equina syndrome. Blood culture may identify the organism before or without surgical drainage. Diagnostic lumbar puncture is contraindicated.128
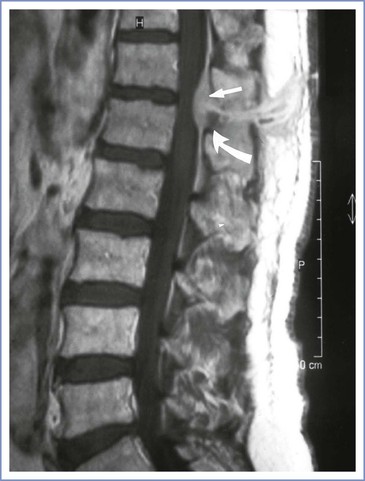
FIGURE 32-11 Epidural abscess. Midsagittal T1-weighted magnetic resonance image of the lumbar and lower thoracic region, after intravenous gadolinium DTPA. Note the dorsal epidural mass located at T12-L1 (arrows), convex anteriorly but not compressing the conus. Normal epidural fat is flat anteriorly. (From Royakers AANM, Willigers H, van der Ven AJ, et al. Catheter-related epidural abscesses—don't wait for neurological deficits. Acta Anaesthesiol Scand 2002; 46:611-5.)
Etiology.
Staphylococcus aureus is the most common causative organism in cases of epidural abscess, with the occasional infection with Streptococcus and Pseudomonas species. The skin appears to be the most likely source of infection.4
The skin is commonly colonized by Staphylococcus epidermidis and other weakly pathogenic bacteria and occasionally by S. aureus. The highest concentration of colonies is found in the hair follicles,130 where organisms may be protected from briefly applied disinfectants. Infectious organisms from the skin can reach deeper tissue planes via the needle track or an implanted epidural catheter to create a localized abscess in the paraspinal or epidural space. Despite all aseptic precautions, some level of detectable bacterial colonization of the epidural catheter is very common, but robust host defenses normally prevent infection. When defenses are weak and infection containment breaks down, epidural abscess formation begins.
Management.
As with spinal hematoma, once neurologic signs are present, early diagnosis with prompt laminectomy is essential to recovery. In the presence of mild symptoms without neurologic changes, successful conservative treatment with antibiotics116 and successful percutaneous needle drainage131 of epidural abscesses have also been reported, although only laminectomy can ensure that all loculations are drained under direct vision. Prompt identification of the infectious organism(s) and directed antibiotic therapy are mandatory. Antibiotic treatment should be continued for 2 to 4 weeks.129
Epidural-Related Infection
Paraspinal abscess and osteomyelitis after epidural analgesia132-137 and discitis after spinal blockade138 have been reported in obstetric patients. Catheter-site inflammation is relatively common with prolonged postoperative epidural analgesia.110,139 One report described both a subdural abscess after CSE anesthesia and infection in the subcutaneous tissues after an apparently misplaced epidural blood patch.140
A variety of organisms have been associated with epidural-related infections.127 All such conditions are associated with back pain and signs of inflammation and pose a threat of spread to the epidural space. Moreover, paraspinal abscess may itself cause neurologic deficit.132,133
Meningitis
Although not consistently included in surveys, post–spinal meningitis has become a cause for concern141 and is an important serious neurologic complication of neuraxial labor analgesia. It was suspected in two cases in the prospective survey of 108,133 epidural procedures and 14,865 spinal anesthetic procedures by Scott and Tunstall,8 although the specific type of anesthesia was not stated. Palot et al.9 reported three cases of meningitis among 288,351 obstetric epidural procedures but did not state whether they followed dural puncture. One case was identified in a survey of spinal and CSE anesthesia (1/42,000 procedures).11 A recent review found an incidence derived from surveys of spinal and CSE anesthesia in obstetrics of 1 in 39,000.127 Table 32-3 summarizes 41 published reports of post–spinal meningitis in obstetric patients.45,141-165
TABLE 32-3
Case Reports of Post–Dural Puncture Meningitis Among Obstetric Patients
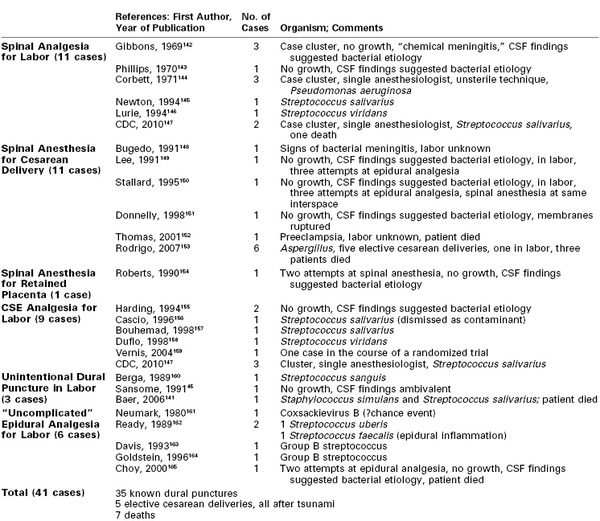
CSE, combined spinal-epidural anesthesia; CSF, cerebrospinal fluid.
Causative Organisms.
Community-acquired meningitis may occur in pregnancy as at other times. It is commonly caused by Neisseria meningitidis, Streptococcus pneumoniae, or Haemophilus influenzae, while occasional cases due to tuberculosis, several β-hemolytic streptococci, and viruses are also reported in pregnancy. Post–spinal meningitis, by contrast, is most commonly caused by streptococci of the viridans type (α-hemolytic streptococci such as S. salivarius, S. sanguis, and S. uberis) (see Table 32-3). These organisms are found in the upper airway and the vagina. Pseudomonas meningitis has also been reported.144 Neither Pseudomonas nor α-hemolytic streptococci are normally virulent; they do not, for example, cause wound infection, but they thrive in a watery medium and flourish if introduced into CSF. In several early cases (before the use of the polymerase chain reaction), no organisms were grown on culture and chemical meningitis was diagnosed. In most cases, however, there were features of bacterial meningitis, including low CSF glucose concentration. Of note, streptococci of the viridans type do not grow readily in conventional culture media and may well have been present but not detected.
Risk Factors.
Dural puncture is probably a prerequisite for iatrogenic meningitis. A retrospective review of surgical patients in one hospital in Brazil found three cases among 38,128 patients receiving spinal anesthesia (1/12,709) and none among 12,822 patients receiving other types of anesthesia.166 Among 73 women with β-hemolytic streptococcal infections in the puerperium identified in a survey from Iowa,167 the only woman who suffered meningitis had received spinal anesthesia. In normal circumstances, the blood-brain barrier (the endothelial lining of the capillaries, which are continuous with tight junctions and no pinocytotic vesicles) protects the CNS against weakly pathogenic bacteria. The dura mater should not be confused with the blood-brain barrier, but dural puncture is commonly associated with vascular trauma,168 which allows blood to enter the CSF.
Of the 41 published cases of puerperal post–spinal meningitis for which details are available (see Table 32-3), 35 occurred after known dural puncture. Among the six cases that followed apparently uncomplicated epidural analgesia, one was viral and may have been a chance event,161 one was probably an epidural abscess,162 and two were blood borne from vaginal infection due to group B streptococcus.163,164 One case, sadly fatal, followed multiple attempts at epidural catheter insertion.165 Uncomplicated epidural catheterization itself is unlikely to increase the risk for puerperal meningitis. Although it is used more commonly than spinal analgesia during labor, case reports of meningitis after spinal analgesia far outnumber those after epidural analgesia. A causative relationship between epidural catheterization and meningitis after vaginal delivery may be attributed to unrecognized dural puncture, which may occur during multiple attempts at epidural catheter insertion or even with apparently uncomplicated catheter insertion.
Labor may also be a risk factor for meningitis. The great majority of parturients with nosocomial meningitis had labored (see Table 32-3). In the latest survey from Sweden, where spinal and CSE anesthesia are rarely used during labor, meningitis was found only among surgical patients.16 Meningitis appears surprisingly rare after elective cesarean delivery, despite the extensive use of spinal anesthesia in this context. The five exceptions were among six unusual cases in Sri Lanka, which resulted from Aspergillus contamination of syringes that had been donated after the 2005 tsunami and stored in an unsuitable warehouse at 41° C and 75% humidity.153
The possible reasons why meningitis is reported more commonly in laboring women than among those undergoing elective cesarean delivery are as follows:
1. The vagina may be colonized by streptococci, and vaginal delivery is commonly followed by mild bacteremia. Thus labor, with its potential for vaginal trauma, is clearly an important risk factor. Unlike vaginal delivery, elective cesarean delivery is not normally associated with streptococcal bacteremia.
2. For elective cesarean delivery, spinal anesthesia is administered in the operating room, which is a cleaner environment than the labor and delivery room.
3. The anesthesia provider is more likely to wear a mask in the operating room.
4. The nonlaboring patient is not thrashing about in a (possibly) contaminated bed.
5. An antibiotic is usually administered immediately before or after cesarean delivery.
Infection at a remote site may also be a risk factor for meningitis. Bacteremia has been detected in approximately 8% of women with chorioamnionitis,169 although two small studies found no evidence of spinal infection among 12 women with bacteremia who received epidural blockade without antibiotic treatment.170,171 Although such negative findings are reassuring, they are not conclusive and do not apply to spinal anesthesia. Human immunodeficiency virus (HIV) infection and acquired immunodeficiency syndrome (AIDS) should not be regarded as contraindications to neuraxial analgesia, in view of the early presence of the virus within the CNS (see Chapter 45).172 Performing an epidural blood patch in the presence of bacteremia is also a theoretical risk for both meningitis and abscess, but neither has been reported in this context. Neuraxial analgesia in the presence of maternal fever is discussed in detail in Chapter 37.
Other risk factors for meningitis include faulty technique, in particular failure to wear a mask (see later discussion). Manual removal of the placenta is a postulated risk factor for meningitis, and one such case has been reported,154 although given the popularity of spinal anesthesia for this indication, one would perhaps expect a higher frequency. It may be postulated that use of the CSE technique, with the presence of a foreign body next to a dural hole, may increase the risk for meningitis.
Clinical Presentation and Management.
Fever, headache, photophobia, nausea, vomiting, and neck stiffness are typical symptoms of meningitis; when they are accompanied by confusion, drowsiness, and Kernig's sign (inability to straighten the knee when the hip is flexed), meningitis should be strongly suspected. The onset of nosocomial meningitis may be 12 hours to a few days after delivery. Diagnostic lumbar puncture (best avoided in the presence of raised intracranial pressure or suspicion of epidural abscess) shows increased CSF pressure, increases in protein level and white blood cell count (mainly polymorphonuclear leukocytes in patients with bacterial meningitis), and a CSF glucose concentration that is lower than that in the blood. Because of the nature of the S. viridans group, culture on plates rather than in broth may have negative results, particularly if antibiotics have been given, or the growth may be assumed to be a contaminant.141 Treatment with an appropriate antibiotic should not await the microbiology results and should result in full recovery.4 Vancomycin and third-generation cephalosporins have been recommended as first-line treatment.166 The treatment regimen should be adjusted according to results of culture and sensitivity testing.
Prevention of Intraspinal Infection after Neuraxial Anesthesia
Measures to prevent intraspinal infection are described in Chapter 12 and summarized in Box 32-4. Means of preventing meningitis and epidural abscess are not identical, because abscess usually follows epidural catheterization and is commonly caused by S. aureus, which enters via the skin, whereas meningitis classically follows dural puncture, is caused by vaginal or nasal organisms, may be bloodborne, and is usually caused by streptococcus and never by S. aureus.
Because adverse outcomes are rare, the use of sterile precautions can rarely be supported by evidence from randomized trials. The components of good sterile technique should be guided by common sense and the best available indirect evidence. It is notable that in many case reports of neuraxial infection, sterile precautions used in initiating neuraxial blockade receive no mention.
A practice advisory published in 2010 by the ASA Task Force on Infectious Complications Associated with Neuraxial Techniques173 concerns prevention, diagnosis, and management of infectious complications. Certain key aspects are discussed here.
Mask.
Several surveys indicate widespread disregard of surgical masks for infection control during neuraxial block administration.166,174,175 Among case reports of nosocomial meningitis, a mask was not mentioned143,154,160-162 or was not worn (“as it is of doubtful value”149 or because it “contributes little to prevent infection during spinal or epidural anesthesia,”146 or is not considered part of “full aseptic technique”163).
Confusion has arisen because randomized trials have demonstrated that omission of masks in the operating room does not increase the occurrence of wound infection.176 This is not surprising, however, because organisms from the upper airway do not cause wound infection, but they certainly do cause nosocomial meningitis. The effect of wearing a mask in the prevention of such rare complications cannot readily be ascertained by a randomized controlled trial. Nevertheless, the obvious value of masks in reducing the dispersion of bacteria from the mouth and nose has been well demonstrated.177,178 A mask is an essential part of aseptic precautions that should be taken for neuraxial needle and catheter insertion.141,173 Masks must be of good quality, preferably fiberglass and not simply woven linen or paper, not allowed to become wet, and changed for each patient.167
Sterile Gown.
Although undeniably part of “full aseptic precautions” employed by surgeons, a sterile gown is rarely worn for spinal needle placement. For insertion of an epidural catheter, a gown is commonly worn in the United Kingdom, although this is not the typical practice in the United States or France. The value of wearing a gown is not supported by good evidence, but it can only be safer than not doing so. During epidural catheter insertion by a novice who is not wearing a gown, the catheter may inadvertently come into contact with the skin of the provider's upper arm or unsterile clothing. This occurrence may facilitate entry, via the catheter, of skin organisms that may cause epidural abscess.
Sterilizing the Skin.
Evidence from laboratory and clinical studies shows that chlorhexidine in 70% alcohol consistently outperforms povidone-iodine for skin disinfection.130,139,179 The concentration of chlorhexidine used varies from 0.5% to 2%. It is superior in speed of onset and duration of action, it is less likely to provoke a skin reaction, and unlike povidone-iodine it is effective in the presence of blood or pus, it stays sterile in the container, and bacterial resistance to it is unlikely.127 Alcohol provides the rapid onset, and chlorhexidine provides the longer duration of action.
The one question over its use concerns that of neurotoxicity. Neither chlorhexidine nor iodine is licensed for skin sterilization before neuraxial block administration. The risk for infectious complications, however, far outweighs that for neurotoxicity, and the superiority of chlorhexidine as an antiseptic should be paramount. It is appropriate, nevertheless, to take precautions, such as using the lowest effective concentration and not allowing it to come into contact with solutions or equipment that will enter the patient's neuraxis. Use of a spray can overcome this concern; otherwise the chlorhexidine container must be removed from the cart before equipment for neuraxial insertion is deployed. The ASA practice advisory states that the aseptic technique for neuraxial procedures should include use of chlorhexidine in alcohol with adequate drying time.173
Maintaining Sterility of the Epidural Catheter, Its Contents, and the Entry Point.
The entry point of the epidural catheter clearly needs to be protected from contamination by a suitable dressing.180 For prolonged analgesia, racemic bupivacaine may be safer than an opioid alone or the pure L-isomers of local anesthetics, which may permit bacterial growth in the solution.127 Although it would seem logical, there is no evidence to support or discourage the use of a bacterial filter during a short-term (1 to 2 days) epidural infusion.180 Prolonged catheterization is best avoided after dural puncture, whether unintentional or deliberate, and when sepsis or immunocompromise is present or suspected.
Vascular Disorders
Ischemic Injury to the Spinal Cord
Ischemic injury to the spinal cord is typically seen in elderly patients after epidural or spinal anesthesia, often with an epinephrine-containing solution, and indeed after general anesthesia with accompanying hypotension. It is rare in the obstetric population, in whom arterial disease is unusual and hypotension is treated aggressively.
The blood supply to the spinal cord depends on a single anterior spinal artery and bilateral posterior spinal arteries. The arteries arise from the circle of Willis and receive reinforcements during their descent in the spinal canal. The posterior spinal arteries receive regular contributions from radicular arteries, but the single anterior spinal artery, which supplies the anterior two thirds of the spinal cord, receives only sporadic reinforcement. Anterior spinal artery syndrome, which may result from arterial compression or hypotension, is characterized by a predominantly motor deficit, with or without loss of pain and temperature sensation, but with sparing of vibration and joint sensations, which are transmitted in the posterior columns. The condition has been reported among obstetric patients with particular risk factors (see later discussion).4,7,181 One report described a series of accidents.182 A parous woman received epidural analgesia with lidocaine, then bupivacaine with epinephrine followed by 2-chloroprocaine, when she required urgent cesarean delivery. Hypotension due to blood loss from a placenta previa and a ruptured uterus was followed by typical irreversible anterior spinal artery syndrome. Hypotension due to blood loss is likely to cause a greater degree of ischemia than that due to vasodilation, and the use of epinephrine may have contributed to the adverse outcome in this case.
Chemical Injury
The Epidural Space
The epidural space is remarkably tolerant of foreign and potentially neurotoxic substances because of two protective factors. First, vascular uptake and outward flow via the intervertebral foramina remove a large proportion of solutions deposited in the epidural space. Second, nerve roots within the epidural space are protected by a cuff of dura and arachnoid as well as pia mater. Severe neuraxial damage occurs only when these defenses are overwhelmed either by gross overdose or if there is unintentional contamination of the subarachnoid space. There are many case reports of unintentional epidural injection of the wrong substance, including the following:
1. Vasopressors (ephedrine and metaraminol). Epidural administration resulted in severe hypertension.183
2. Potassium chloride. At least four well-documented cases have been reported.184,185 All the patients had profound motor and sensory block with pain or depolarizing spasms. Only one, who received the largest epidural dose (15 mL of 11.25% KCl), remained permanently paraplegic.185
3. Other potentially noxious substances. Administration of an unknown substance, possibly paraldehyde, given in error as an epidural bolus injection during labor, resulted in permanent painful quadriplegia and the largest monetary award for damages in the United Kingdom at that time.186 Unintentional misconnections of intravenous and epidural infusion systems have led to large-volume epidural infusions of potentially harmful substances, including total parenteral nutrition solutions with a high osmolality187 and ranitidine in a phenol-containing solution.188 Fortunately, in most cases of this type of drug error, neurologic sequelae have not been reported.
In summary, with a few exceptions, the epidural space appears to be merely an exotic means of systemic administration of analgesia/anesthesia. Nevertheless, the possibility of occult dural puncture means that unintentional administration of a potentially neurotoxic substance (e.g., traces of alcohol, antioxidant, or preservative) may migrate into the subarachnoid space. Vigilance and systems to avoid these errors are mandatory.
The Subarachnoid Space
The subarachnoid space, with its poorly protected nerve roots and direct communication with intracranial structures, presents a greater risk than the epidural space for adverse outcome after unintentional injection of toxic substances. Intrathecal potassium does not merely maim, it can kill.189 Irritant solutions may cause neurotoxicity and arachnoiditis (see later discussion). Transient neurologic syndrome (see later discussion) may be a minor and transient form of neurotoxicity. Neurotoxicity may manifest as cauda equina syndrome or, if more extensive, as paraplegia or quadriplegia.
Nerve roots within the subarachnoid space are highly vulnerable to chemical damage, particularly the sacral roots, which are poorly myelinated. Therefore, neurotoxicity associated with a small-volume intrathecal injection classically produces cauda equina syndrome. For example, in 1937, 14 cases of severe cauda equina syndrome were reported after spinal anesthesia using a solution called “heavy duracaine,” a mixture (in 15% ethanol) of procaine, glycerin, and gliadin or gum acacia, which presumably was added in an attempt to prolong the action of procaine.190 In the 1940s and 1950s in the United Kingdom, spinal injection of 10 mL of hypo-osmolar dibucaine was associated with paraplegia, but whether the paraplegia resulted from disturbance of the intrathecal milieu or contamination with phenol is argued.
More recently, there were numerous reports of cauda equina syndrome after intrathecal injection of lidocaine (all types of administration, both intended and unintended, most commonly hyperbaric 5%)191-193 and occasionally after intrathecal administration of other local anesthetics.194,195 None of these cases involved obstetric patients. In all cases, other causes of neurologic deficit (trauma, ischemia, infection, compression, contamination, and adverse positioning) were excluded. An upper safe dose limit for intrathecal lidocaine of 60 mg192,196 has been recommended. Hyperbaric lidocaine is not available in either Australia or the United Kingdom. The various risk factors for neurotoxic damage are summarized in Box 32-5.
Conus damage and cauda equina syndrome may appear similar. Although conus damage may involve upper motor neuron signs, these are not always present, and both conditions may have unilateral or bilateral features.71 However, the causation is different. Whereas conus damage may result from ischemia or trauma, cauda equina syndrome typically results from either compression or chemical damage within the lumbar spinal canal.
Transient Neurologic Syndrome
Transient neurologic syndrome (also called transient radicular irritation) is not associated with any detectable neurologic deficit, but the distribution of pain in the back, buttocks, and thighs mirrors the distribution of nerve damage in cauda equina syndrome sufficiently to support the theory that the nerves are indeed irritated by a noxious intrathecal injection. Like cauda equina syndrome, it follows spinal anesthesia, most commonly with lidocaine. Moreover, other risk factors for cauda equina syndrome and transient neurologic syndrome are similar (see Box 32-5), although transient neurologic syndrome may be less dependent on lidocaine dose or the presence of a vasoconstrictor.197 Transient neurologic syndrome occurs more than four times more frequently with spinal lidocaine than with other local anesthetics.198 It is, however, much more common in surgical than obstetric patients, with a median frequency, according to one review, of 22%.197 Parturients are not exempt from transient neurologic symptoms, with reported frequencies of 0%,199 4.2%,200 5.3%,201 and 8.9%.202
Arachnoiditis
Arachnoiditis is a disastrous condition, usually with a delayed onset of permanent quadriplegia. It is extremely rare, and it has not been detected in any surveys of neurologic sequelae of obstetric neuraxial blockade. Among parturients, chronic adhesive arachnoiditis of chemical origin has arisen after unintentional intrathecal injection of a large dose of 2-chloroprocaine with antioxidant and preservative intended for the epidural space,203 while seven cases occurred in Miami after epidural analgesia for childbirth with 2% lidocaine, probably with preservative.204 Six cases were reported among Italian surgical patients after apparently standard epidural anesthesia with bupivacaine and/or mepivacaine, usually with epinephrine.205 The local anesthetic agents, however, were obtained from multidose vials containing parabens as preservative, and the glass syringes used for loss-of-resistance identification of the epidural space had been washed in detergent. With earlier publications it is not always possible to distinguish the cause of arachnoiditis, but it seemed to appear in clusters, suggesting that there may have been shortcomings in anesthetic technique. In a more recent single case in the United Kingdom, a woman suffered severe arachnoiditis after spinal anesthesia for elective cesarean delivery.206 Her skin had been cleaned with iodine and then chlorhexidine in alcohol and allowed to dry. It was unclear whether the tray containing antiseptic solutions was removed before the rest of the procedure. Because of pain during attempted insertion of the spinal needle, the skin was infiltrated at least three times with lidocaine before intrathecal bupivacaine was given. Shortly thereafter she became disturbed and experienced a severe headache, so she was given general anesthesia for the operation. Postpartum she developed obstructive hydrocephalus and extensive adhesive arachnoiditis. The judge determined that, on balance of probabilities, there must have been contact in some way between the chlorhexidine and the local anesthetic solution.
Vulnerable Patients
Various conditions may render some women more vulnerable than normal to neurologic injury precipitated by neuraxial anesthesia. The following discussion of conditions is not exhaustive.
Vertebral Abnormality
Skeletal abnormalities involving the spine, including congenital anomaly, trauma, and back surgery, can make epidural or spinal needle insertion difficult. Patients with spina bifida are at risk for accidental dural puncture and nerve root damage unless the needle is inserted above the defect. Those with tethered cord syndrome are at risk for cord damage if spinal or epidural needle insertion is attempted at a vertebral level that would normally be expected to be below the conus.68 Occasionally, a low-lying conus may be present without any premonitory signs.73 Pressure from spinal stenosis or prolapsed intervertebral disc, coupled with a large-volume epidural injection, may result in spinal cord compression and neurologic deficit.82
Vascular Abnormalities
Vascular disease and malformation are risk factors for spinal cord ischemia, hematoma, and compression. The major supply to the lumbar enlargement of the spinal cord is the artery of Adamkiewicz, a unilateral structure that typically arises from the lower thoracic or upper lumbar portion of the aorta between T9 and L2. Compression of this single vessel may therefore jeopardize the blood supply to the lower cord in susceptible individuals. In 15% of individuals, a secondary blood supply to the spinal cord that ascends from the internal iliac arteries207 assumes a major role. These ascending arteries lie close to the lumbosacral trunk and are, in theory, vulnerable to pressure from the fetal head or damage by obstetric instrumentation, thus causing conus ischemia.
An arteriovenous malformation is an obvious cause for concern for the obstetric anesthesia provider.208 Small arterial feeders from a segmental intercostal artery supply dilated serpiginous epidural veins that may extend over many segments of the spinal canal.209 The resulting hemangioma raises the pressure on epidural veins and reduces spinal cord blood flow. Oxygen delivery to local tissues is reduced, and the risks of spinal hematoma or ischemic damage and compression are increased. Pregnancy and epidural analgesia have been known to precipitate paraplegia in previously asymptomatic patients210,211; aortocaval compression, a large blood volume, and a large epidural injection (which may cause severe pain) all increase epidural pressure.
The preanesthesia examination should include inspection of the back for cutaneous angiomas or macular areas of skin discoloration, which may suggest the presence of an underlying spinal angioma at the same segmental level. Because spinal cord capillary flow is compromised in the drainage area of an arteriovenous malformation, systemic arterial pressure should be kept close to normal throughout the peripartum period, regardless of anesthetic technique.
Spinal Tumor
Epidural blockade has been reported to precipitate neurologic symptoms in the presence of previously undiagnosed spinal tumors. Spinal tumors also predispose to spinal hematoma after neuraxial blockade,4 and epidural analgesia may precipitate extreme pain.212
Coagulopathy or Anticoagulation
The risk for neuraxial procedures in patients with a preexisting coagulopathy or anticoagulation therapy is discussed earlier in this chapter and also in Chapters 39 and 44.
Immunocompromise
The majority of cases of epidural infection that have been reported in surveys involve elderly patients with immunocompromise.16,109 This topic is covered in an excellent review.213 It is advisable to avoid prolonged epidural catheterization in immunocompromised patients.
Preexisting Neurologic Disorder
Relapse rates in patients with multiple sclerosis are increased after delivery, and the fear is that neuraxial blockade will be blamed.214 It has been postulated that spinal anesthesia may worsen demyelinating conditions, although surveys are inconclusive.215 If affected nerve roots are indeed at higher risk for neurotoxicity, epidural anesthesia may be safer than spinal anesthesia. Indeed, some anesthesia providers prefer epidural or even general anesthesia over spinal anesthesia in women with multiple sclerosis. It is clearly important to document neurologic status and to discuss relapse rates with the mother before and after any anesthetic intervention.
Patients with hereditary neuropathy with liability to pressure palsy are particularly sensitive to compression neuropathy during the course of labor and delivery.32,33,216
It is postulated that patients with preexisting peripheral neuropathies may be more susceptible to nerve injury when exposed to a second insult, the so-called double crush phenomenon.215 To explore whether neuraxial anesthesia represented such an insult, Hebl et al.215 reviewed the charts of 567 patients with peripheral neuropathies who had spinal or epidural blockade, including 12 obstetric patients. There were two instances of worsening neurologic status, both in elderly diabetic patients. There were no control patients, but clearly, if there is a risk that neuraxial anesthesia will exacerbate a neuropathy, it is very small.
Diabetes
Diabetic patients are vulnerable to neurologic injury for three reasons. They are susceptible to infection, they may have vascular disease, and they may have a peripheral neuropathy. Diabetic patients are at increased risk for epidural abscess, either catheter-associated or spontaneous.16,109 Anterior spinal artery syndrome has also been described in diabetic parturients,181 and worsening neuropathy has been observed in diabetic surgical patients.215
Risk Management and Follow-Up
High-risk women should be referred to the obstetric anesthesia clinic during pregnancy, so that an anesthesia provider can obtain a thorough history, examine the patient, organize consultations or imaging studies as needed, and plan the anesthetic strategy. The recommended points to explore during the history and physical examination are summarized in Boxes 32-6 and 32-7.
In addition to minimizing risk by adopting good and safe practices, vigilance must be extended postpartum to detect, diagnose, and treat any disorders that may arise. Early hospital discharge presents a problem in the detection of post–dural puncture headache and space-occupying lesions, both of which require urgent assessment and treatment. It is important that those caring for women in the puerperium are taught to look for signs of neurologic pathology and that the women themselves, once home, are taught to recognize postanesthesia symptoms and to contact an anesthesia provider if they experience them.
Diagnosis of Possible Neurologic Injury
Through simple clinical examination coupled with knowledge of basic neuroanatomy outlined in this chapter, it should be possible to distinguish peripheral from central lesions. Preanesthesia history and physical examination may reveal some helpful clues, and any preexisting signs or deficits documented in the record will narrow the search or even provide an immediate answer. The history and physical examination should seek to establish whether the complaint is genuine and to determine its site and cause.
The basic physical examination outlined in Box 32-7 should be repeated, and the results should be compared with the preanesthesia findings. Fever and leukocytosis indicate an infective cause. Severe back pain and localized tenderness suggest epidural abscess, and pain radiating to the legs or buttocks is a late and urgent sign of spinal cord or cauda equina compression. Headache, fever, and neck stiffness suggest meningitis. Viral or other community-acquired types of meningitis may coincide with pregnancy, but the responsible organisms differ from those causing nosocomial infection.
MRI has revolutionized the speed and precision with which intraspinal lesions can be identified, and gadolinium enhancement further improves its sensitivity. MRI cannot distinguish clearly between blood and other fluid, although the distinction can usually be made on other grounds. In the presence of meningitis, MRI with gadolinium enhancement shows swelling of the cord and punctate areas of increased density, reflecting inflammatory cell infiltrates. Arteriovenous malformations of the cord or dura may be visible, and the enlarged veins draining them may be seen as serpiginous signal voids.
References
1. Turbridy N, Redmond JMT. Neurological symptoms attributed to epidural analgesia in labour: an observational study of seven cases. Br J Obstet Gynaecol. 1996;103:832–833.
2. Tillman AJB. Traumatic neuritis in the puerperium. Am J Obstet Gynecol. 1935;29:660–666.
3. Chalmers JA. Traumatic neuritis of the puerperium. J Obstet Gynaecol Br Emp. 1949;56:205–216.
4. Loo CC, Dahlgren G, Irestedt L. Neurological complications in obstetric regional anaesthesia. Int J Obstet Anesth. 2000;9:99–124.
5. Ong BY, Cohen MM, Esmail A, et al. Paresthesias and motor dysfunction after labor and delivery. Anesth Analg. 1987;66:18–22.
6. MacArthur C, Lewis M, Knox EG. Investigation of long term problems after obstetric epidural anaesthesia. BMJ. 1992;304:1279–1282.
7. Scott DB, Hibbard BM. Serious nonfatal complications associated with extradural block in obstetric practice. Br J Anaesth. 1990;64:537–541.
8. Scott DB, Tunstall ME. Serious complications associated with epidural/spinal blockade. Int J Obstet Anesth. 1995;4:133–139.
9. Palot M, Visseaux H, Botmans C, Pire JC. Epidémiologie des complications de l’analgésie péridurale obstétricale. Cah Anesthesiol. 1994;42:229–233.
10. Holdcroft A, Gibberd FB, Hargrove RL, et al. Neurological problems associated with pregnancy. Br J Anaesth. 1995;75:522–526.
11. Holloway J, Seed PT, O'Sullivan G, Reynolds F. Paraesthesiae and nerve damage following combined spinal epidural and spinal anaesthesia. Int J Obstet Anesth. 2000;9:151–155.
12. Paech MJ, Godkin R, Webster S. Complications of obstetric epidural analgesia and anaesthesia: a prospective analysis of 10995 cases. Int J Obstet Anesth. 1998;7:5–11.
13. Dar AQ, Robinson APC, Lyons G. postpartum neurological symptoms following regional blockade: a prospective study with case controls. Int J Obstet Anesth. 2002;11:85–90.
14. Wong CA, Scavone BM, Dugan S, et al. Incidence of postpartum lumbosacral spine and lower extremity nerve injuries. Obstet Gynecol. 2003;101:279–288.
15. Auroy Y, Benhamou D, Bargues L, et al. Major complications of regional anesthesia in France: the SOS regional anesthesia hotline service. Anesthesiology. 2002;97:1274–1280.
16. Moen V, Dahlgren N, Irestedt L. Severe neurological complications after central neuraxial blockades in Sweden 1990-1999. Anesthesiology. 2004;101:950–959.
17. Ruppen W, Derry S, McQuay H, Moore RA. Incidence of epidural hematoma, infection and neurologic injury in obstetric patients with epidural analgesia/anesthesia. Anesthesiology. 2006;105:394–399.
18. Cook TM, Counsell D, Wildsmith JA, Royal College of Anaesthetists Third National Audit Project. Major complications of central neuraxial block: report on the Third National Audit Project of the Royal College of Anaesthetists. Br J Anaesth. 2009;102:179–190.
19. Wong CA. Nerve injuries after neuraxial anaesthesia and their medicolegal implications. Best Pract Res Clin Obstet Gynaecol. 2010;24:367–381.
20. Haas DM, Meadows RS, Cottrell R, Stone WJ. Postpartum obturator neurapraxia: a case report. J Reprod Med.. 2003;48:469–470.
21. Hong BY, Ko YJ, Kim HW, et al. Intrapartum obturator neuropathy diagnosed after cesarean delivery. Arch Gynecol Obstet. 2010;282:349–350.
22. Hakoiwa S, Hoshi T, Tanaka M, Mishima H. Case of bilateral obturator neuropathy after caesarean section. Masui. 2011;60:721–723.
23. Peirce C, O’Brien C, O’Herlihy C. Postpartum femoral neuropathy following spontaneous vaginal delivery. J Obstet Gynaecol. 2010;30:203–204.
24. Van Diver T, Camann W. Meralgia paresthetica in the parturient. Int J Obstet Anesth. 1995;4:109–112.
25. Silva M, Mallinson C, Reynolds F. Sciatic nerve palsy following childbirth. Anaesthesia. 1996;51:1144–1148.
26. Roy S, Levine AB, Herbison GJ, Jacobs SR. Intraoperative positioning during cesarean as a cause of sciatic neuropathy. Obstet Gynecol. 2002;99:652–653.
27. Postaci A, Karabeyoglu I, Erdogan G, et al. A case of sciatic neuropathy after caesarean section under spinal anaesthesia. Int J Obstet Anesth. 2006;15:317–319.
28. Babayev M, Bodack MP, Creatura C. Common peroneal neuropathy secondary to squatting during childbirth. Obstet Gynecol. 1998;91:830–832.
29. Radawski MM, Strakowski JA, Johnson EW. Acute common peroneal neuropathy due to hand positioning in normal labor and delivery. Obstet Gynecol. 2011;118:421–423.
30. LeBlanc KE, Cestia W. Carpal tunnel syndrome. Am Fam Physician. 2011;83:952–958.
31. Farrar D, Raoof N. Bell's palsy, childbirth and epidural analgesia. Int J Obstet Anesth. 2001;10:68–70.
32. Peters G, Hinds NP. Inherited neuropathy can cause postpartum foot drop. Anesth Analg. 2005;100:547–548.
33. Lepski GR, Alderson JD. Epidural analgesia in labour for a patient with hereditary neuropathy with liability to pressure palsy. Int J Obstet Anesth. 2001;10:198–201.
34. Weissman A, Grisaru D, Shenhav M, et al. Postpartum surveillance of urinary retention by ultrasonography: the effect of epidural analgesia. Ultrasound Obstet Gynecol. 1995;6:130–134.
35. Chien PFW, Khan KS, Agustsson P, et al. The determinants of residual bladder volume following spontaneous vaginal delivery. J Obstet Gynecol. 1996;16:146–150.
36. Liang C-C, Wong S-Y, Tsay P-T, et al. The effect of epidural analgesia on postpartum urinary retention in women who deliver vaginally. Int J Obstet Anesth. 2002;11:164–169.
37. MacArthur C, Lewis M, Knox EG. Health after childbirth. HMSO: London; 1991.
38. Borum SE, Naul LG, McLeskey CH. Postpartum dural venous sinus thrombosis after postdural puncture headache and epidural blood patch. Anesthesiology. 1997;86:487–490.
39. Ravindran RS, Zandstra GC, Viegas OJ. Postpartum headache following regional analgesia: a symptom of cerebral venous thrombosis. Can J Anaesth. 1989;36:705–707.
40. Ariola SE, Russo TO, Marx GF. Postdural puncture headache or incipient eclampsia? Int J Obstet Anesth. 1991;1:33–34.
41. Diemunsch P, Balabaud VP, Petiau C, et al. Bilateral subdural hematoma following epidural anesthesia. Can J Anaesth. 1998;45:328–331.
42. Shah JL. Severe headache following an epidural top-up. Int J Obstet Anesth. 1991;1:29–31.
43. Waidelich JM, Bullough AS, Mhyre JM. Internal carotid artery dissection: an unusual cause of postpartum headache. Int J Obstet Anesth. 2008;17:61–65.
44. Torillo TM, Bronster DJ, Beilin Y. Delayed diagnosis of posterior reversible encephalopathy syndrome (PRES) in a parturient with preeclampsia after inadvertent dural puncture. Int J Obstet Anesth. 2007;16:171–174.
45. Sansome AJT, Barnes GR, Barrett RF. An unusual presentation of meningitis as a consequence of inadvertent dural puncture. Int J Obstet Anesth. 1991;1:35–37.
46. Shearer VE, Jhaveri HS, Cunningham FG. Puerperal seizures after post dural puncture headache. Obstet Gynecol. 1995;85:225–260.
47. Rodrigo P, Garcia JM, Ailagas J. General convulsive crisis related to pneumocephalus after inadvertant dural puncture in an obstetric patient. Rev Esp Anestesiol Reanim. 1997;44:247–249.
48. Oliver CD, White SA. Unexplained fitting in three parturients suffering from postdural puncture headache. Br J Anaesth. 2002;89:782–785.
49. Marfurt D, Lyrer P, Rüttimann U, et al. Recurrent post-partum seizures after epidural blood patch. Br J Anaesth. 2003;90:247–250.
50. Reynolds F. Dural puncture and headache: avoid the first but treat the second. BMJ. 1993;306:874–876.
51. Heyman HJ, Salem MR, Klimov I. Persistent sixth cranial nerve paresis following blood patch for postdural puncture headache. Anesth Analg. 1982;61:948–949.
52. Dunbar SA, Katz NP. Failure of delayed epidural blood patching to correct persistent cranial nerve palsies. Anesth Analg. 1994;79:806–807.
53. Chohan U, Khan M, Saeed-uz-zafar. Abducent nerve palsy in a parturient with a 25-gauge Sprotte needle. Int J Obstet Anesth. 2003;12:235–236.
54. Perez M, Olmos M, Garrido FJ. Facial nerve paralysis after epidural blood patch. Reg Anesth. 1993;18:196–198.
55. Carrero EJ, Agusti M, Fabregas N, et al. Unilateral trigeminal and facial nerve palsies associated with epidural analgesia in labour. Can J Anaesth. 1998;45:893–897.
56. Martin-Hirsch DP, Martin-Hirsch PL. Vestibulocochlear dysfunction following epidural anaesthesia in labour. Br J Clin Pract. 1994;48:340–341.
57. Viale M, Narchi P, Veyrac P, Benhamou D. Chronic tinnitus and hearing loss caused by cerebrospinal fluid leak treated with success with peridural blood patch: apropos of 2 cases. Ann Otolaryngol Chir Cervicofac. 1996;113:175–177.
58. Davies JM, Murphy A, Smith M, O'Sullivan G. Subdural haematoma after dural puncture headache treated by epidural blood patch. Br J Anaesth. 2001;86:720–723.
59. Vaughan DJ, Stirrup CA, Robinson PN. Cranial subdural haematoma associated with dural puncture in labour. Br J Anaesth. 2000;84:518–520.
60. Cantais E, Benhamou D, Petit D, Palmier B. Acute subdural hematoma following spinal anesthesia with a very small spinal needle. Anesthesiology. 2000;93:1354–1355.
61. Kardash K, Morrow F, Béïque F. Seizures after epidural blood patch with undiagnosed subdural hematoma. Reg Anesth Pain Med. 2002;27:433–436.
62. Kayacan N, Atrici G, Karsli B, Erman M. Acute subdural haematoma after accidental dural puncture during epidural anaesthesia. Int J Obstet Anesth. 2004;13:47–49.
63. Ayorinde BT, Mushambi M. Extradural haematoma in a patient following manual removal of the placenta under spinal anaesthesia: was the spinal to blame? Int J Obstet Anesth. 2002;11:216–218.
64. Yoshii WY, Rottman RL, Rosenblatt RM, et al. Epidural catheter induced traumatic radiculopathy in obstetrics: one center's experience. Reg Anesth. 1994;19:132–135.
65. Loo CC, Cheong KF. Monoplegia following obstetric epidural anaesthesia. Ann Acad Med Singapore. 1997;26:232–234.
66. Richardson J, Bedder M. Transient anterior spinal cord syndrome with continuous postoperative epidural analgesia. Anesthesiology. 1990;72:764–766.
67. Ben-David B, Vaida S, Collins G, et al. Transient paraplegia secondary to an epidural catheter. Anesth Analg. 1994;79:598–600.
68. Reynolds F. Litigation in obstetric regional anaesthesia. Van Zurdert A. Highlights in Pain Therapy and Regional Anaesthesia V. Publicidad Permanyer: Barcelona; 1996:39–43.
69. Bromage PR, Benumof JL. Paraplegia following intracord injection during attempted epidural anesthesia under general anesthesia. Reg Anesth. 1998;23:104–107.
70. Rajakulendran Y, Rahman S, Venkat N. Long-term neurological complication following traumatic damage to the spinal cord with a 25 gauge Whitacre spinal needle. Int J Obstet Anesth. 1999;8:62–66.
71. Reynolds F. Damage to the conus medullaris following spinal anaesthesia. Anaesthesia. 2001;56:238–247.
72. Greaves JD. Serious spinal cord injury due to haematomyelia caused by spinal anaesthesia in a patient treated with low-dose heparin. Anaesthesia. 1997;52:150–154.
73. Ahmad FU, Pandey P, Sharma BS, Garg A. Foot drop after spinal anesthesia in a patient with a low-lying cord. Int J Obstet Anesth. 2006;15:233–236.
74. Reynolds F. Logic in the safe practice of spinal anaesthesia (editorial). Anaesthesia. 2000;55:1045–1046.
75. Van Gessel EF, Forster A, Gamulin Z. Continuous spinal anesthesia: where do spinal catheters go? Anesth Analg. 1993;76:1004–1007.
76. Broadbent CR, Maxwell WB, Ferrie R, et al. Ability of anaesthetists to identify a marked lumbar interspace. Anaesthesia. 2000;55:1122–1126.
77. Saifuddin A, Burnett SJ, White J. The variation of position of the conus medullaris in an adult population: a magnetic resonance imaging study. Spine. 1998;23:1452–1456.
78. Thomson A. Fifth annual report of the committee of collective investigation of the Anatomical Society of Great Britain and Ireland for the year 1893-94. J Anat Physiol. 1894;29:35–60.
79. Render CA. The reproducibility of the iliac crest as a marker of lumbar spine level. Anaesthesia. 1996;51:1070–1071.
80. Davidson EM, Sklar E, Bhatia R, et al. Magnetic resonance imaging findings after uneventful continuous infusion neuraxial analgesia: a prospective study to determine whether epidural infusion produces pathologic magnetic resonance imaging findings. Anesth Analg. 2010;110:233–237.
81. Horlocker TT. Neuraxial blockade in patients with spinal stenosis: between a rock and a hard place. Anesth Analg. 2010;110:13–15.
82. Forster MR, Nimmo GR, Brown AG. Case report: prolapsed intervertebral disc after epidural analgesia in labour. Anaesthesia. 1996;51:773–775.
83. Kreppel D, Antoniadis G, Seeling W. Spinal hematoma: a literature survey with meta-analysis of 613 patients. Neurosurg Rev. 2003;26:1–22.
84. Case AS, Ramsey PS. Spontaneous epidural hematoma of the spine in pregnancy. Am J Obstet Gynecol. 2005;193:875–877.
85. Bose S, Ali Z, Rath GP, Prabhakar H. Spontaneous spinal epidural haematoma: a rare cause of quadriplegia in the post-partum period. Br J Anaesth. 2007;99:855–857.
86. Tada S, Yasue A, Nishizawa H, et al. Spontaneous spinal epidural hematoma during pregnancy: three case reports. J Obstet Gynaecol Res. 2011;37:1734–1738.
87. Matsubara S, Inoue H, Takamura K, et al. Spontaneous spinal epidural hematoma at the 16th week of a twin pregnancy. J Obstet Gynaecol Res. 2011;37:1466–1469.
88. Jo YY, Lee D, Chang YJ, Kwak HJ. Anesthetic management of a spontaneous spinal-epidural hematoma during pregnancy. Int J Obstet Anesth. 2012;21:185–188.
89. Forsnes E, Occhino A, Acosta R. Spontaneous spinal epidural hematoma in pregnancy associated with using low molecular weight heparin. Obstet Gynecol. 2009;113:532–533.
90. Doblar DD, Schumacher SD. Spontaneous acute thoracic epidural hematoma causing paraplegia in a patient with severe preeclampsia in early labor. Int J Obstet Anesth. 2005;14:256–260.
91. Wulf H. Epidural anaesthesia and spinal haematoma. Can J Anaesth. 1996;43:1260–1271.
92. Esler MD, Durbridge J, Kirby S. Epidural haematoma after dural puncture in a parturient with neurofibromatosis. Br J Anaesth. 2001;87:932–934.
93. Horlocker TT, Wedel DJ, Schroder DR, et al. Preoperative antiplatelet therapy does not increase the risk of spinal hematoma associated with regional anesthesia. Anesth Analg. 1995;80:303–309.
94. Davies JM, Posner KL, Lee LA, et al. Liability associated with obstetric anesthesia: a closed claims analysis. Anesthesiology. 2009;110:131–139.
95. Yuen TST, Kua JSW, Tan IKS. Spinal haematoma following epidural anaesthesia in a patient with eclampsia. Anaesthesia. 1999;54:350–371.
96. Guffey PJ, McKay WR, McKay RE. Epidural hematoma nine days after removal of a labor epidural catheter. Anesth Analg. 2010;111:992–995.
97. Rasmus KT, Rottman RL, Kotelko DM, et al. Unrecognized thrombocytopenia and regional anesthesia in parturients: a retrospective review. Obstet Gynecol. 1989;73:943–946.
98. Hew-Wing P, Rolbin SH, Hew E, Amato D. Epidural anaesthesia and thrombocytopenia. Anaesthesia. 1989;44:775–777.
99. Beilin Y, Zahn J, Comerford M. Safe epidural analgesia in thirty parturients with platelet counts between 69,000 and 98,000 mm3. Anesth Analg. 1997;85:385–388.
100. Chi C, Lee CA, England A, et al. Obstetric analgesia and anaesthesia in women with inherited bleeding disorders. Thromb Haemost. 2009;101:1104–1111.
101. Beards SC, Jackson A, Griffiths AG, Horsman EL. Magnetic resonance imaging of epidural blood patches: appearances from 30 minutes to 18 hours. Br J Anaesth. 1993;71:182–189.
102. Verduzco LA, Atlas SW, Riley ET. Subdural hematoma after an epidural blood patch. Int J Obstet Anesth. 2012;21:189–192.
103. Lao TT, Halpern SH, MacDonald D, Huh C. Spinal subdural haematoma in a parturient after attempted epidural anaesthesia. Can J Anaesth. 1993;40:340–345.
104. Koyama S, Tomimatsu T, Kanagawa T, et al. Spinal subarachnoid hematoma following spinal anesthesia in a patient with HELLP syndrome. Int J Obstet Anesth. 2010;19:87–91.
105. Walters MA, Van de Velde M, Wilms G. Acute intrathecal haematoma following neuraxial anaesthesia: diagnostic delay after apparently normal radiological imaging. Int J Obstet Anesth. 2012;21:181–185.
106. Lee LA, Posner KL, Domino KB, et al. Injuries associated with regional anesthesia in the 1980s and 1990s. Anesthesiology. 2004;101:143–152.
107. Anderson BL, Nau GJ, Simhan HN. Idiopathic vertebral abscess in pregnancy: case report and literature review. Am J Perinatol. 2007;24:377–379.
108. Reihsaus E, Waldbaur H, Seeling W. Spinal epidural abscess: a meta-analysis of 915 patients. Neurosurg Rev. 2000;23:175–204.
109. Wang LP, Hauerberg J, Schmidt JF. Incidence of spinal epidural abscess after epidural analgesia: a national 1-year survey. Anesthesiology. 1999;91:1928–1936.
110. Green LK, Paech MJ. Obstetric epidural catheter-related infections at a major teaching hospital: a retrospective case series. Int J Obstet Anesth. 2010;19:38–43.
111. Crawford JS. Pathology in the extradural space. Br J Anaesth. 1975;47:412–415.
112. Ngan Kee WD, Jones MR, Thomas P, Worth RJ. Extradural abscess complicating extradural anaesthesia for caesarean section. Br J Anaesth. 1992;69:647–652.
113. Borum SE, McLeskey CH, Williamson JB, et al. Epidural abscess after obstetric epidural analgesia. Anesthesiology. 1995;82:1523–1526.
114. Kindler C, Seeberger M, Siegmund M, Schneider M. Extradural abscess complicating lumbar extradural anaesthesia and analgesia in an obstetric patient. Acta Anaesthesiol Scand. 1996;40:858–861.
115. Jenkin G, Woolley IJ, Brown GV, Richards MJ. Post partum epidural abscess due to group B streptococcus. Clin Infect Dis. 1997;25:1249.
116. Dysart RH, Balakrishnan V. Conservative management of extradural abscess complicating spinal-extradural anaesthesia for caesarean section. Br J Anaesth. 1997;78:591–593.
117. Dhillon AR, Russell IF. Epidural abscess in association with obstetric analgesia. Int J Obstet Anesth. 1998;6:118–121.
118. Collier CB, Gatt SP. Epidural abscess in an obstetric patient. Anaesth Intensive Care. 1999;27:662–666.
119. Rathmell JP, Garahan MB, Alsofrom GF. Epidural abscess following epidural analgesia. Reg Anesth Pain Med. 2000;25:79–82.
120. Unseld H, Eisinger I. Epidural abscess following repeated epidural catheter placement for delivery. Anaesthetist. 2000;49:960–963.
121. Rohrbach M, Plotz J. Epidural abscess following delivery with peridural analgesia: the question of prevention. Anaesthetist. 2001;50:411–415.
122. Trautmann M, Lepper PM, Schmitz FJ. Three cases of bacterial meningitis after spinal and epidural anesthesia. Eur J Clin Microb Infect Dis. 2002;21:43–45.
123. Evans PR, Misra U. Poor outcome following epidural abscess complicating epidural analgesia for labour. Eur J Obstet Gynecol Reprod Biol. 2003;109:102–105.
124. Veiga Sanchez AR. Vertebral osteomyelitis and epidural abscess after epidural anesthesia for a cesarean section. Rev Esp Anestesiol Rean. 2004;51:44–46.
125. Schroeder TH, Krueger WA, Neeser E, et al. Spinal epidural abscess—a rare complication after epidural analgesia for labour and delivery. Br J Anaesth. 2004;92:896–898.
126. Chiang HL, Chia YY, Chen YS, et al. Epidural abscess in an obstetric patient with patient-controlled epidural analgesia—a case report. Int J Obstet Anesth. 2005;14:242–245.
127. Reynolds F. Neurological infections after neuraxial anesthesia. Anesthesiol Clin. 2008;26:23–52.
128. Doonan J, Murphy P, Timothy J, Marks P. Acute neurologic deterioration following lumbar puncture in an epidural abscess occurring 14 months after epidural catheter placement. J Neurosurg Anesthesiol. 2000;12:364–365.
129. Royakkers AA, Willigers H, van der Ven AJ, et al. Catheter-related epidural abscesses—don't wait for neurological deficits. Acta Anaesthesiol Scand. 2002;46:611–615.
130. Sato S, Sakuragi T, Dan K. Human skin flora as a potential source of epidural abscess. Anesthesiology. 1996;85:1276–1282.
131. Tabo E, Ohkuma Y, Kimura S, et al. Successful percutaneous drainage of epidural abscess with epidural needle and catheter. Anesthesiology. 1994;80:1393–1395.
132. Kinahan AM, Douglas MJ. Piriformis pyomyositis mimicking epidural abscess in a parturient. Can J Anaesth. 1995;42:240–245.
133. Raj V, Foy J. Paraspinal abscess associated with epidural in labour. Anaesth Intensive Care. 1998;26:424–426.
134. Hill JS, Hughes EW, Robertson PA. A Staphylococcus aureus paraspinal abscess associated with epidural analgesia in labour. Anaesthesia. 2001;56:871–878.
135. Huang YY, Zuo Z, Yuan HB, et al. A paraspinal abscess following spinal anaesthesia for caesarean section and patient-controlled epidural analgesia for postoperative pain. Int J Obstet Anesth. 2005;14:252–255.
136. Lee BB, Ngan Kee WD, Griffith JF. Vertebral osteomyelitis and psoas abscess occurring after obstetric epidural anesthesia. Reg Anesth Pain Med. 2002;27:220–224.
137. Yang YW, Chen WT, Chen JY, et al. Bacterial infection in deep paraspinal muscles in a parturient following epidural analgesia. Acta Anaesthesiol Taiwan. 2011;49:75–78.
138. Bajwa ZH, Ho C, Grush A, et al. Discitis associated with pregnancy and spinal anesthesia. Anesth Analg. 2002;94:415–416.
139. Cameron CM, Scott DA, McDonald WM, Davies MJ. A review of neuraxial epidural morbidity. Anesthesiology. 2007;106:997–1002.
140. Collis RE, Harris SE. A subdural abscess and infected blood patch complicating labour analgesia. Int J Obstet Anesth. 2005;14:246–251.
141. Baer ET. Post-dural puncture bacterial meningitis. Anesthesiology. 2006;105:381–393.
142. Gibbons RB. Chemical meningitis following spinal anesthesia. JAMA. 1969;210:900–902.
143. Phillips OC. Aseptic meningitis following spinal anesthesia. Anesth Analg. 1970;49:866–871.
144. Corbett JJ, Rosenstein BJ. Pseudomonas meningitis related to spinal anesthesia: report of three cases with a common source of infection. Neurology. 1971;21:946–950.
145. Newton JA, Lesnik IK, Kennedy CA. Streptococcus salivarius meningitis following spinal anesthesia. Clin Infect Dis. 1994;18:840–841.
146. Lurie S, Feinstein M, Heifetz C, Mamet Y. Iatrogenic bacterial meningitis after spinal anesthesia for pain relief in labor. J Clin Anesth. 1999;11:438–439.
147. Center for Disease Control and Prevention. Bacterial meningitis after intrapartum spinal anesthesia—New York and Ohio, 2008-2009. MMWR Morb Mortal Wkly Rep. 2010;59:65–69.
148. Bugedo G, Valenzuela J, Munoz H. Aseptic meningitis following spinal anesthesia: report of a case. Rev Med Chile. 1991;119:440–442.
149. Lee JJ, Parry H. Bacterial meningitis following spinal anaesthesia for caesarean section. Br J Anaesth. 1991;66:383–386.
150. Stallard N, Barry P. Another complication of the combined extradural-subarachnoid technique. Br J Anaesth. 1995;75:370–371.
151. Donnelly T, Koper M, Mallaiah S. Meningitis following spinal anaesthesia: a coincidental infection? Int J Obstet Anesth. 1998;7:170–172.
152. Thomas T, Cooper G. In Why mothers die 1997-1999. Confidential Enquiries into Maternal Deaths in the United Kingdom. RCOG Press: London; 2001.
153. Rodrigo N, Perera KNT, Ranwala R, et al. Aspergillus meningitis following spinal anaesthesia for caesarean section in Colombo, Sri Lanka. Int J Obstet Anesth. 2007;16:256–260.
154. Roberts SP, Petts HV. Meningitis after obstetric anaesthesia. Anaesthesia. 1990;45:376–377.
155. Harding SA, Collis RE, Morgan BM. Meningitis after combined spinal-extradural anaesthesia in obstetrics. Br J Anaesth. 1994;73:545–547.
156. Cascio M, Heath G. Meningitis following a combined spinal-epidural technique in a labouring term parturient. Can J Anaesth. 1996;43:399–402.
157. Bouhemad B, Dounas M, Mercier FJ, Benhamou D. Bacterial meningitis following combined spinal-epidural analgesia for labour. Anaesthesia. 1998;53:292–295.
158. Duflo F, Allaouchiche B, Mathon L, Chassard D. Bacterial meningitis following combined obstetric spinal and peridural anesthesia. Ann Fr Anesth Reanim. 1998;17:1286.
159. Vernis L, Duale C, Storme B, et al. Perispinal analgesia for labour followed by patient-controlled infusion with bupivacaine and sufentanil: combined spinal-epidural vs. epidural analgesia alone. Eur J Anaesthesiol. 2004;21:186–192.
160. Berga S, Trierweiler MW. Bacterial meningitis following epidural anesthesia for vaginal delivery: a case report. Obstet Gynecol. 1989;74:437–439.
161. Neumark J, Feichtinger W, Gassner A. Epidural block in obstetrics followed by aseptic meningitis. Anesthesiology. 1980;52:518–519.
162. Ready LB, Helfer D. Bacterial meningitis in parturients after epidural anesthesia. Anesthesiology. 1989;71:988–990.
163. Davis L, Hargreaves C, Robinson PN. Postpartum meningitis. Anaesthesia. 1993;48:788–789.
164. Goldstein MJ, Parker RL, Dewan DM. Status epilepticus amauroticus secondary to meningitis as a cause of postpartum cortical blindness. Reg Anesth. 1996;21:595–598.
165. Choy JC. Mortality from peripartum meningitis. Anaesth Intensive Care. 2000;28:328–330.
166. Videira RLR, Ruiz-Neto PP, Neto MB. Post spinal meningitis and sepsis. Acta Anaesthesiol Scand. 2002;46:639–646.
167. White CA, Koontz FP. β-Hemolytic streptococcus infections in postpartum patients. Obstet Gynecol. 1973;41:27–32.
168. Knowles PR, Randall NP, Lockhart AS. Vascular trauma associated with routine spinal anaesthesia. Anaesthesia. 1999;54:647–650.
169. Gibbs RS, Castillo MS, Rodgers PJ. Management of acute chorioamnionitis. Am J Obstet Gynecol. 1980;136:709–713.
170. Bader AM, Gilbertson L, Kirz L, Datta S. Regional anesthesia in women with chorioamnionitis. Reg Anesth. 1992;17:84–86.
171. Goodman EJ, DeHorta E, Taguiam JM. Safety of spinal and epidural anesthesia in parturients with chorioamnionitis. Reg Anesth. 1996;21:436–441.
172. Gershon RY, Manning-Williams D. Anesthesia and the HIV-infected parturient: a retrospective study. Int J Obstet Anesth. 1997;6:76–81.
173. American Society of Anesthesiologists Task Force on Infectious Complications Associated with Neuraxial Techniques. Practice advisory for the prevention, diagnosis, and management of infectious complications associated with neuraxial techniques. Anesthesiology. 2010;112:530–545.
174. Panikkar KK, Yentis SM. Wearing of masks for obstetrical regional anaesthesia: a postal survey. Anaesthesia. 1996;51:398–400.
175. Sellors JE, Cyna AM, Simmons SW. Aseptic precautions for inserting an epidural catheter: a survey of obstetric anaesthetists. Anaesthesia. 2002;57:593–606.
176. Tunevall TG. Postoperative wound infections and surgical face masks: a controlled study. World J Surg. 1992;16:147–148.
177. Phillips BJ, Fergusson S, Armstrong P, Wildsmith JAW. Surgical facemasks are effective in reducing bacterial contamination caused by dispersal from the upper airway. Br J Anaesth. 1992;69:407–408.
178. McLure HA, Talboys CA, Yentis SM, Azadian BS. Surgical face masks and downward dispersal of bacteria. Anaesthesia. 1998;53:624–626.
179. Sakuragi T, Yanagisawa K, Dan K. Bactericidal activity of skin disinfectants on methicillin-resistant Staphylococcus aureus. Anesth Analg. 1995;81:555–558.
180. Hebl JR. The importance and implications of aseptic techniques during regional anesthesia. Reg Anesth Pain Med. 2006;31:311–323.
181. Eastwood DW. Anterior spinal artery syndrome after epidural anesthesia in a pregnant diabetic patient with scleredema. Anesth Analg. 1991;73:90–91.
182. Ackerman WE, Juneja MM, Knapp RK. Maternal paraparesis after epidural anesthesia and cesarean section. South Med J. 1990;83:695–697.
183. Savage R, Beattie C. Inadvertent epidural administration of metaraminol. Anaesthesia. 2004;59:624–625.
184. Shanker KB, Palkar NV, Nishkala R. Paraplegia following epidural potassium chloride. Anaesthesia. 1985;40:45–47.
185. Lin D, Becker K, Shapiro HM. Neurologic changes following epidural injection of potassium chloride and diazepam: a case report with laboratory correlations. Anesthesiology. 1986;65:210–212.
186. Brahams D. Record award of personal injuries sustained as a result of negligent administration of epidural anaesthesia. Lancet. 1982;1:159.
187. Patel PC, Sharif AMY, Farnando PUE. Accidental infusion of total parenteral nutrition solution through an epidural catheter. Anaesthesia. 1984;39:383–384.
188. McGuinness JP, Cantees KK. Epidural injection of a phenol-containing ranitidine preparation. Anesthesiology. 1990;73:553–555.
189. Meel B. Inadvertent intrathecal administration of potassium chloride during routine spinal anesthesia: case report. Am J Forensic Med Path. 1998;19:255–257.
190. Ferguson FR, Watkins KH. Paralysis of the bladder and associated neurological sequelae of spinal anaesthesia (cauda equina syndrome). Br J Surg. 1937;25:735–752.
191. Rigler ML, Drasner K, Krejchie TC, et al. Cauda equina syndrome after continuous spinal anesthesia. Anesth Analg. 1991;72:275–281.
192. Loo CC, Irestedt L. Cauda equina syndrome after spinal anaesthesia with hyperbaric 5% lidocaine: a review of six cases of cauda equina syndrome reported to the Swedish Pharmaceutical Insurance 1993-1997. Acta Anaesthesiol Scand. 1999;43:371–379.
193. Cheng ACK. Intended epidural anesthesia as possible cause of cauda equina syndrome. Anesth Analg. 1994;78:157–159.
194. Yamauchi Y, Nomoto Y. Irreversible damage to the cauda equina following repeated intrathecal injection of hyperbaric dibucaine. J Anesth. 2002;16:176–178.
195. Chabbouh T, Lentschener C, Zuber M, et al. Persistent cauda equina syndrome with no identifiable facilitating condition after an uneventful single spinal administration of 0.5% hyperbaric bupivacaine. Anesth Analg. 2006;101:1847–1848.
196. Drasner K. Local anesthetic neurotoxicity: Clinical injury and strategies that may minimize risk. Reg Anesth Pain Med. 2002;27:576–580.
197. Pollock JE. Transient neurologic symptoms: etiology, risk factors and management. Reg Anesth Pain Med. 2002;27:581–586.
198. Zaric D, Christiansen C, Pace NL, Punjasawadwong Y. Transient neurologic symptoms after spinal anesthesia with lidocaine versus other local anesthetics: a systematic review of randomized, controlled trials. Anesth Analg. 2005;100:1811–1816.
199. Wong CA, Slavenas P. The incidence of transient radicular irritation after spinal anesthesia in obstetric patients. Reg Anesth Pain Med. 1999;24:55–58.
200. Viitanen H, Porthan L, Viitanen M, et al. Postpartum neurologic symptoms following single-shot spinal block for labour analgesia. Acta Anaesthesiol Scand. 2005;49:1015–1022.
201. Philip J, Sharma SK, Vijaya NR, et al. Transient neurologic symptoms after spinal anesthesia with lidocaine in obstetric patients. Anesth Analg. 2001;92:405–409.
202. Rorarius M, Suominen P, Haanpaa M, et al. Neurologic sequelae after caesarean section. Acta Anaesth Scand. 2001;45:34–41.
203. Reisner LS, Hochman BN, Plumer MH. Persistent neurologic deficit and adhesive arachnoiditis following intrathecal 2-chloroprocaine injection. Anesth Analg. 1980;59:452–454.
204. Sklar EM, Quencer RM, Green BA, et al. Complications of epidural anesthesia: MR appearance of abnormalities. Radiology. 1991;181:549–554.
205. Sgchirlanzoni A, Marazzi R, Pareyson D, et al. Epidural anaesthesia and spinal arachnoiditis. Anaesthesia. 1989;44:317–321.
206. Bogod D. The truth, the whole truth? Anaesth News. 2010;issue 271:7–8.
207. Lazorthes G, Poulhes J, Bastide G, et al. La vascularization de la moelle épinière (étude anatomique et physiologique). Rev Neurol. 1962;106:535–537.
208. Ong BY, Littleford J, Segstro R, et al. Spinal anaesthesia for Caesarean section in a patient with a cervical arteriovenous malformation. Can J Anaesth. 1996;43:1052–1058.
209. Doppman JL, Wirth FP, Di Chiro G, Ommaya AK. Value of cutaneous angiomas in the arteriographic localization of spinal cord arteriovenous malformations. N Engl J Med. 1969;281:1440–1444.
210. Hirsch NP, Child CS, Wijetilleka SA. Paraplegia caused by spinal angioma: possible association with epidural analgesia. Anesth Analg. 1985;64:937–940.
211. Liu C-L, Yang D-J. Paraplegia due to vertebral hemangioma during pregnancy: a case report. Spine. 1988;13:107–108.
212. Martin HB, Gibbons JJ, Bucholz RD. An unusual presentation of spinal cord tumor after epidural anesthesia. Anesth Analg. 1992;75:844–846.
213. Horlocker TT, Wedel DJ. Regional anesthesia in the immunocompromised patient. Reg Anesth Pain Med. 2006;1:334–345.
214. Drake E, Drake M, Bird J, Russell R. Obstetric regional blocks for women with multiple sclerosis: a survey of UK experience. Int J Obstet Anesth. 2006;15:115–123.
215. Hebl JR, Kopp SL, Schroeder DR, Horlocker TT. Neurologic complications after neuraxial anesthesia or analgesia in patients with preexisting peripheral sensorimotor neuropathy or diabetic polyneuropathy. Anesth Analg. 2006;103:1294–1299.
216. Chilvers RJ, Salman MM. Hereditary neuropathy with a liability to pressure palsies presenting as a case of sensory neuropathy following spinal anaesthesia for caesarean delivery. Int J Obstet Anesth. 2011;20:95–96.