Respiratory Disease
Karen S. Lindeman MD
Chapter Outline
Asthma
Definition
Asthma is defined by the presence of the following three characteristic findings: (1) reversible airway obstruction, (2) airway inflammation, and (3) airway hyperresponsiveness. Airway obstruction produces the clinical manifestations of wheezing, cough, and dyspnea. Airway inflammation modulates the course of asthma by independently producing airway obstruction and enhancing airway hyperresponsiveness. Airway hyperresponsiveness is marked by exaggerated responses to a wide variety of bronchoconstrictor stimuli, including histamine, methacholine, prostaglandin F2α, hypo-osmotic solutions, and cold air.
Epidemiology
Asthma is an increasingly common problem among young, otherwise healthy women of childbearing age. Morbidity and mortality rates from this disease increased during the 1980s and 1990s. From 2001 to 2010, the prevalence of asthma in the United States increased from 7.3% to 8.4%.1
The prevalence of asthma in women of childbearing age also continues to rise. The rate was approximately 3% in the 1990s and has increased to approximately 8.8% in the early 2000s.2
Pathophysiology
Asthma is believed to occur under a variety of environmental influences in the presence of genetic susceptibility.3 The underlying defect that produces the clinical syndrome of asthma is unknown. The most important potential mechanisms are (1) an enhancement of contractility or an impairment of relaxation of airway smooth muscle, (2) a neural imbalance, (3) airway inflammation, and (4) changes in the function of the airway epithelium.
Airway Smooth Muscle
Contraction of airway smooth muscle is believed to be the most important factor in producing acute airway obstruction. For many years, an enhancement of airway smooth muscle responsiveness to contractile agonists was assumed to be a major mechanism of asthma. To test this hypothesis, investigators attempted to correlate airway responsiveness in vivo and in vitro in humans4-8 and in the basenji-greyhound dog model of asthma.9 These studies did not demonstrate a significant correlation between the airway response to histamine or cholinergic agonists in vivo and airway smooth muscle contraction in vitro. Some studies actually demonstrated a negative correlation between the in vivo and in vitro responses,8,9 suggesting that diminished responsiveness may represent a chronic adaptive response of airway smooth muscle.
Instead of an enhancement in responsiveness to contractile stimuli, a reduction in responsiveness to relaxant stimuli may contribute to airway obstruction. One study demonstrated impaired relaxant responses to isoproterenol in airway smooth muscle from human asthmatic subjects in comparison with the responsiveness of airway smooth muscle from controls.10 Other evidence substantiates the presence of impaired airway relaxation in asthmatic subjects in vivo.11 Although the mechanism for this effect is poorly understood, a reduction in airway sensitivity to beta-adrenergic agonists could contribute to airway hyperresponsiveness by altering the balance between constricting and dilating influences.
Neural Components
A balance between constricting and dilating influences also exists with respect to the autonomic nervous system. A shift in this balance, with an increase in constricting influences, may be a mechanism of asthma.
The parasympathetic nervous system provides the dominant constrictor input to the airways (Figure 53-1). Efferent cholinergic fibers travel in the vagus nerve to synapse in ganglia within the airway wall.12 Postganglionic fibers release acetylcholine to activate muscarinic receptors and stimulate airway smooth muscle contraction. A negative feedback system limits release of acetylcholine from nerve terminals. Muscarinic autoreceptors, or receptors on the nerve ending,13 also are activated by acetylcholine and inhibit further release of acetylcholine from the nerve terminal.
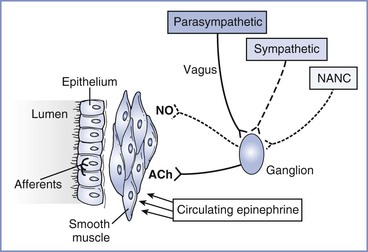
FIGURE 53-1 Neural control of the airway. Parasympathetic, sympathetic, and nonadrenergic noncholinergic (NANC) efferents innervate ganglia within the airway wall. Postganglionic cholinergic efferents release acetylcholine (ACh) to constrict airway smooth muscle. Postganglionic NANC efferents release nitric oxide (NO) to relax airway smooth muscle. Circulating epinephrine relaxes the airway. Afferents from the airway originate in the epithelium and are activated by airway irritation, as occurs with tracheal intubation.
The importance of exaggerated cholinergic efferent activity in the pathogenesis of airway hyperreactivity has been debated extensively. The relatively limited efficacy of anticholinergic agents in relieving clinical bronchospasm, as well as growing evidence supporting other mechanisms, suggests that this pathway has a limited role in the pathophysiology of asthma. However, this mechanism appears to be very important in the perioperative management of asthmatic subjects. Reflex stimulation of airway smooth muscle by placement of a tracheal tube represents one of the most important causes of bronchospasm in the perioperative period.
An alternative mechanism by which the parasympathetic nervous system may contribute to airway hyperresponsiveness is through dysfunction of the muscarinic autoreceptors. Dysfunction of these receptors allows increased postganglionic release of acetylcholine after reflex stimulation.14 This mechanism is well established in a guinea pig model of viral infection15 and may explain the airway hyperresponsiveness that occurs for several weeks after an upper respiratory tract infection, although additional autoreceptor-independent mechanisms may also be present.16 The role of this mechanism in the pathophysiology of clinical asthma is unclear.
The sympathetic nervous system primarily acts to decrease airway tone. In contrast to the parasympathetic nervous system, sympathetic innervation of airway smooth muscle in human subjects is either sparse or absent.17 Circulating catecholamines activate beta-adrenergic receptors in airway smooth muscle and provide the primary sympathetic efferent input to human airways. Because airways of normal human subjects do not become hyperresponsive after beta-adrenergic blockade,18 it is unlikely that impaired catecholamine secretion contributes significantly to the pathogenesis of asthma.
The alpha-adrenergic system is thought to play a relatively minor role in determining the state of airway responsiveness. Although alpha-adrenergic receptors are present in human airways,19 the protective effects of alpha-adrenergic antagonists have been disappointing and can be attributed to other properties, such as antihistamine activity.
In addition to cholinergic and adrenergic input, a third neural system, the nonadrenergic noncholinergic (NANC) system, provides efferent nerves to the airways. Both constricting and dilating pathways have been identified.20 Nitric oxide serves as the inhibitory NANC neurotransmitter in human airways.21 Potentially, a relative increase in constricting influences or a decrease in dilating influences in the NANC system could contribute to asthma. However, asthmatic subjects demonstrated no deficit in NANC inhibitory pathways,22 and inhibition of NANC excitatory neurotransmission did not improve airway hyperresponsiveness.23 Thus, current evidence does not support imbalance of the NANC system as a major mechanism of asthma.
Airway Inflammation
Airway inflammation appears to serve primarily as a modulating influence in asthma. Inflammation is certainly present in some but not all asthmatic subjects.24 The process of inflammation involves the occurrence of airway wall edema and infiltration of the mucosa by a variety of inflammatory cells, including neutrophils, mast cells, helper T lymphocytes, macrophages, and eosinophils.25 These cells produce and release mediators of inflammation, such as histamine, leukotrienes, platelet-activating factor, prostaglandins, thromboxanes, cytokines, serotonin, and nitric oxide.25 Mediators can modulate airway responsiveness by stimulating airway smooth muscle contraction,26 directing migration of inflammatory cells,27 modifying neural control of the airways,28 increasing mucosal permeability,29 or disrupting airway epithelium.30 In addition, airway inflammation can reduce airway diameter. Airway hyperresponsiveness is correlated with increased baseline airway tone.31 The overall importance of inflammation in asthma has been debated. Although inflammation appears to modulate the course of asthma, other factors certainly contribute to the pathogenesis.
Airway Epithelium
The epithelium provides a barrier to protect the subepithelial layers against stimuli that could provoke bronchospasm. Airways of asthmatic subjects demonstrate areas of epithelial destruction,32 and the clinical significance of this finding has been demonstrated.33
The epithelium not only serves as a barrier but also plays an active role in the maintenance of airway tone. The epithelium produces constricting and dilating factors.34,35 An alteration in the balance between these factors could alter airway responsiveness. The relative importance of alterations in epithelial function in the pathogenesis of asthma is unknown.
Diagnosis
Medical History
The classic symptoms of asthma include wheezing, cough, dyspnea, and chest tightness. A patient's medical history also should include information about the pattern and severity of the symptoms, precipitating and aggravating factors, and the duration and course of these symptoms.
Physical Examination
Physical examination is directed to the respiratory tract. Auscultation of the chest may reveal wheezing and a prolonged phase of expiration.
Laboratory Studies
Laboratory studies that aid in the diagnosis of asthma depend on findings from the medical history and physical examination. In general, pulmonary function tests are useful to document the severity and establish the reversibility of obstruction (Box 53-1). In the absence of additional findings, other tests are not as useful in establishing the diagnosis of asthma. Bronchoprovocation tests (with agents such as methacholine or histamine) are used when the history and physical examination strongly suggest the presence of asthma but spirometry does not show airway obstruction.
Interaction with Pregnancy
Effects of Pregnancy on Asthma
The overall course of asthma has been reported to improve, worsen, or remain the same during pregnancy.36 Earlier evidence suggested that patients with more severe asthma are more likely to experience deterioration during pregnancy,36 but a recent study demonstrated that asthma severity during pregnancy is similar to severity during the year before pregnancy, provided that patients continue to use their prescribed medication during pregnancy. The investigators concluded that even mild asthma is likely to become significantly more severe if women discontinue their prescribed medication during pregnancy.37 A likely reason for the variation in the results of published studies is the difference in methods of assessing the severity of asthma. Most studies have used either clinical symptoms or requirements for pharmacologic therapy as indicators of the course of the disease. These measures do not correlate with objective measures of airway obstruction.38 Juniper et al.39 measured methacholine sensitivity before, during, and after pregnancy. Measurements of sensitivity to methacholine made during the second and third trimesters were lower than preconception or postpartum measurements (Figure 53-2). Although these findings suggest a reduction in airway hyperresponsiveness during pregnancy, the limited study population (16 subjects) makes extrapolation of the data to the general population unclear. Exacerbations of asthma during labor and delivery occur in as many as 20% of subjects37 and occur more frequently after cesarean delivery than after vaginal delivery (41% and 4%, respectively).40
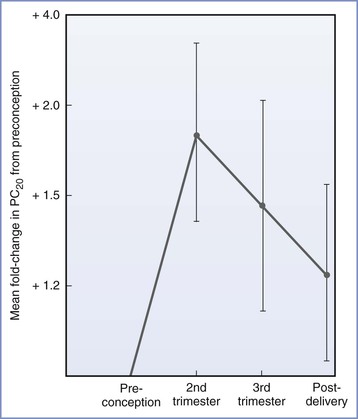
FIGURE 53-2 Airway responsiveness before, during, and after pregnancy expressed as fold change in PC20—dose of methacholine needed to reduce FEV1 (forced expiratory volume in 1 second) by 20%—compared with values before conception (n = 16; P = .033 for the effect of pregnancy on airway responsiveness). (From Juniper EF, Daniel EE, Roberts RS, et al. Improvement in airway responsiveness and asthma severity during pregnancy. Am Rev Respir Dis 1989; 140:924-31.)
A number of mechanisms may be responsible for the changes in the clinical course of asthma during pregnancy (Box 53-2). An increase in the progesterone level is thought to be one mechanism that improves asthma during pregnancy. Progesterone relaxes uterine and gastrointestinal smooth muscle and may or may not have similar effects on airway smooth muscle. However, Juniper et al.39 did not demonstrate a strong association between methacholine responsiveness and progesterone levels during pregnancy, suggesting that progesterone does not play a central role in attenuating airway hyperresponsiveness. In contrast, progesterone may actually worsen asthma by enhancing inflammation.41 Thus, effects of pregnancy on asthma appear to involve a number of factors other than direct effects of hormones on airway smooth muscle.
Effects of Asthma on the Parturient and Fetus
Many investigators have questioned whether maternal asthma adversely affects perinatal outcome. Differences in study design (e.g., retrospective, prospective) and differences in severity and treatment of asthma may account for different study results. Some studies have reported an increased incidence of preeclampsia,42,43 cesarean delivery,44-46 low-birth-weight (LBW) infants,47 preterm labor,45,48 antepartum and postpartum hemorrhage,49 and perinatal mortality.50 Diabetes mellitus appears to be more common among asthmatic patients treated with corticosteroids.51 Severe or poorly controlled asthma is a predictor of adverse outcome.40 Although asthma in pregnancy is associated with an increased risk for adverse perinatal outcomes, a meta-analysis of cohort studies suggested that active asthma management, which is intended to reduce the exacerbation rate, may reduce the risk for perinatal complications, particularly preterm delivery.52 No controlled studies have documented better perinatal outcome with aggressive asthma treatment. Potential mechanisms of increased perinatal morbidity and mortality in patients with uncontrolled asthma include hypoxemia and hypocapnia, inflammation, and altered placental function from asthma-associated mediator release.53 Siddiqui et al.54 have documented an association between preeclampsia and airway hyperresponsiveness and have proposed that the mechanism involves an interaction between mast cells and smooth muscle. A large prospective study is needed to confirm this association.
Medical Management
Pharmacologic therapy for asthma during pregnancy is directed toward avoiding acute exacerbations and episodes of status asthmaticus. Ideally, management should begin before conception. Although general principles typically dictate that unnecessary medication should be avoided during pregnancy, studies investigating the effects of asthma on perinatal outcome suggest that the risks for uncontrolled asthma are significantly higher than medication-associated risks.55 Medications that are currently used to treat asthma fall into two general categories: bronchodilators and anti-inflammatory agents. These agents generally are safe for the fetus. The prophylactic use of antibiotics is unnecessary.
Bronchodilators
Beta-adrenergic agonists exert beneficial effects in asthmatic patients by activation of β2-adrenergic receptors, which mediate a number of processes (Box 53-3). Short-acting beta-adrenergic agonists represent the most effective therapy for acute exacerbations of asthma.56 Daily use of long-acting beta-adrenergic agonists is controversial. Long-acting beta-adrenergic agonist therapy is associated with a significant increase in the risk for death,57 but controlled studies have not confirmed a cause-and-effect relationship.58 Certain genetic polymorphisms affect responses to short-acting but not long-acting beta-adrenergic agonists,59 leading to hopes that a personalized approach to therapy would improve clinical efficacy. Although regular use of beta-adrenergic agonists in asthma may be beneficial in conjunction with other forms of therapy, these agents do not appear to provide optimal control when used alone. Conversely, no compelling evidence requires that beta-adrenergic agonists be discontinued after conception or that their use be reserved for treatment of an acute exacerbation.
These agents may be administered as aerosols, orally, or parenterally. The aerosol route is generally preferred during pregnancy because high concentrations of the medication can be delivered directly to the site of activity in the airways, with relatively less drug delivered to the uteroplacental circulation.
The limited number of human studies investigating the fetal safety of long-term administration of a beta-adrenergic agonist have not shown significant adverse neonatal outcomes.60,61 In addition, the long history of use of these agents without reports of teratogenicity suggests that their use should not be restricted because of fetal concerns. Optimal control of maternal symptoms of asthma appears to be more important for the fetus than potential detrimental effects of beta-adrenergic agonists.
On the basis of the potential risks of long-term single-agent therapy with a beta-adrenergic agonist, a paradoxical approach to the treatment of asthma may involve long-term administration of a beta-adrenergic antagonist.62 This approach is analogous to the paradigm based in the cardiovascular system, in which long-term administration of a beta-adrenergic antagonist is beneficial in patients with congestive heart failure. Studies in asthmatic patients are ongoing.
Methylxanthines (e.g., theophylline, aminophylline) were used for many years in the long-term treatment of asthma. Although their mechanism of action is controversial, relaxation of airway smooth muscle is the most prominent effect. The ability of the agents to inhibit intracellular phosphodiesterase and increase concentrations of cyclic adenosine monophosphate (cAMP) is not the mechanism of bronchodilation, because these effects do not occur at clinically relevant concentrations in vivo.63 Furthermore, in the patient taking anti-inflammatory agents and beta-adrenergic agonists, methylxanthines add little to optimal asthmatic control.64 Although their use is now limited to patients whose asthma responds poorly to other forms of therapy, methylxanthines do not appear to cause significant adverse fetal outcomes.61 Serum concentrations of theophylline should be monitored carefully, especially in the third trimester, when theophylline clearance decreases.65
Bronchodilation with anticholinergic agents occurs through the blockade of muscarinic receptors on airway smooth muscle. Overall, anticholinergic agents alone are not as effective as beta-adrenergic agonists, but some patients show better response to anticholinergic agents.66 The effects of adding anticholinergic agents to beta-adrenergic agonists for acute67 and chronic68 asthma were evaluated in meta-analyses of randomized trials. Anticholinergic agents improved lung function in acute asthma67 but had little benefit in chronic asthma.68 The quaternary anticholinergic agent ipratropium bromide can be delivered as an aerosol, allowing higher concentrations in the lung with reduced systemic absorption and potential effects on the fetus. Human data on the safety of anticholinergic agents and on potential teratogenicity are lacking, but ipratropium bromide is not associated with teratogenicity in animal studies.69
Magnesium sulfate relaxes airway smooth muscle, most likely via its antagonism of calcium entry into airway smooth muscle cells.70 Its use is limited primarily to acute bronchospasm.71
Anti-Inflammatory Agents
Proposed mechanisms of action for corticosteroids are (1) decreases in cellular infiltration and mediator release, (2) reductions in airway permeability, and (3) up-regulation of the beta-adrenergic system.72 Unlike bronchodilators, corticosteroids not only reduce airway sensitivity to a constrictor stimulus73 but also decrease the maximal extent of airway narrowing, a feature that may predict severity of an acute asthmatic episode.74
The use of inhaled corticosteroids has gained popularity. This route of administration is effective and may limit fetal side effects. Studies have assessed the effects of systemic and inhaled corticosteroids on the fetus. Neither systemic nor inhaled corticosteroids have been proven to increase the risk for congenital malformations in humans. Inhaled corticosteroids do not affect glucocorticoid-regulated pathways in the fetus and therefore are unlikely to cause adverse effects on fetal growth and development.75 Although oral corticosteroid use is associated with an increased incidence of LBW infants,76 inhaled corticosteroids do not appear to increase perinatal risk.77 Further, a meta-analysis did not show an association between inhaled corticosteroid use and any adverse perinatal outcome.78 Of interest, use of inhaled corticosteroids during pregnancy can be guided by measurements of exhaled nitric oxide, which, in a randomized trial, were shown to significantly reduce exacerbations when compared with use of a clinical algorithm based on symptoms alone.79
Corticosteroids may increase perinatal morbidity by exacerbating maternal glucose intolerance, especially in women who also receive treatment with a beta-adrenergic agonist. Thus, careful monitoring of maternal glucose concentration is indicated in asthmatic women who require treatment with a corticosteroid during pregnancy. However, because of the efficacy of corticosteroids in controlling severe asthma during pregnancy, these agents should not be withheld from the medical regimen.
Some authorities have recommended that corticosteroid-dependent asthmatic women receive large doses of parenteral corticosteroids during labor to prevent complications related to adrenal suppression.55,80,81 The scientific basis for this recommendation is questionable. Although physiologic glucocorticoid replacement reduced hemodynamic instability and mortality in adrenalectomized primates that underwent surgery, supraphysiologic doses provided no additional benefit.82 Furthermore, inhaled corticosteroids in moderate doses do not produce adrenocortical suppression.83 There is little information about the benefit of corticosteroid replacement therapy during labor. The potential for adrenal insufficiency in infants of asthmatic mothers taking inhaled or oral corticosteroids appears to be very low,81 most likely owing to the widespread use of either prednisone or prednisolone. In the mother, prednisone is converted rapidly to prednisolone, which crosses the placental barrier to a very limited extent.
Cromolyn sodium and nedocromil sodium belong to a class of drugs that are thought to reduce inflammation and mediator release primarily by stabilizing mast cells and perhaps other inflammatory cells. Nedocromil also inhibits cellular chloride ion flux, a feature that may explain its ability to affect a range of airway cells, including nerve cells.84
Cromolyn and nedocromil are administered as aerosols. Limited studies suggest that cromolyn is safe during pregnancy,85 and clinical experience is greater with cromolyn than with nedocromil. Thus, use of cromolyn is preferred.
On the basis of the observation that leukotrienes are released into the airways by immune cells and contribute to the inflammatory process, other forms of anti-inflammatory therapy are leukotriene receptor antagonists and leukotriene synthesis inhibitors. Safety data for the use of these agents in pregnancy are scarce. Bracken et al.47 did not observe adverse neonatal outcomes in nine women exposed to these agents. A later prospective study of 96 women showed that use of leukotriene receptor antagonists was not associated with a specific pattern of congenital abnormalities, but the investigators cautioned that extrapolation of the data to a large population would require additional studies because of the limited sample size of the study.85
Obstetric Management
The following aspects of obstetric management of the asthmatic parturient may differ from that of the nonasthmatic patient: (1) induction of labor, (2) management of postpartum hemorrhage, and (3) treatment of hypertension.
For induction of labor, prostaglandins should be administered cautiously in women with asthma. Prostaglandin F2α constricts airways in vivo86 and in vitro.87 Airways of asthmatic subjects demonstrate greater sensitivity to prostaglandin F2α, and its use to induce labor is associated with bronchospasm.88 Prostaglandin E2 can have either dilating or constricting effects on the airways, perhaps because of its ability to activate a variety of different types of prostaglandin receptors.89 Because of the known risk for bronchospasm after exposure to prostaglandin F2α and the possible risk after exposure to prostaglandin E2, alternative methods of induction of labor are preferred in asthmatic women.
Likewise, asthma represents a relative contraindication to the administration of 15-methyl prostaglandin F2α (carboprost, Hemabate) for the treatment of postpartum hemorrhage. The use of ergot alkaloids to treat postpartum hemorrhage in asthmatic women has also been questioned. Although controlled studies have not been performed, ergot alkaloids have been associated with episodes of acute bronchospasm,90,91 on the basis of either their tryptaminergic actions or their ability to activate α1-adrenergic receptors on airway smooth muscle cells. Oxytocin, which does not significantly affect airway tone, is the preferred ecbolic agent in asthmatic patients.
Beta-adrenergic receptor antagonists are used to treat hypertension in some pregnant women. In asthmatic women, these agents may provoke bronchospasm when used acutely.92 Other antihypertensive agents, such as hydralazine and sodium nitroprusside, do not seem to enhance airway responsiveness.
Anesthetic Management
Preoperative Assessment
During the preoperative evaluation, the anesthesia provider should assess the severity of the disease and whether an acute asthmatic episode is present. The medical history should include information about symptoms of wheezing, dyspnea, and cough. Further information should be sought about the frequency and severity of symptoms, the course of these symptoms during pregnancy, and the date of the most recent exacerbation. Patients who have frequent, severe attacks are at increased risk for morbidity in the peripartum period.
Physical examination should focus on the pulmonary system. Chest auscultation may demonstrate wheezing with or without a prolonged expiratory phase. However, wheezing may not be audible if air movement is markedly reduced. Additional signs of an acute exacerbation of asthma include tachypnea, an exaggerated (> 20 mm Hg) pulsus paradoxus, and the use of accessory respiratory muscles.
In a pregnant woman with stable asthma, laboratory tests add little to anesthetic management. However, if an acute exacerbation is suspected, chest radiographic examination, arterial blood gas measurements, and pulmonary function tests may assist with diagnosis and therapy. Chest radiographic examination helps diagnose precipitating or complicating conditions such as pneumonia, pneumothorax, and heart failure. During an episode of acute asthma, arterial blood gas measurements often show hypoxemia and respiratory alkalosis. After a prolonged, severe episode, arterial carbon dioxide tension increases as a result of fatigue. Spirometry measures the volume of gas exhaled over time (see Box 53-1). The most convenient indirect measurement for assessing airway obstruction during labor is the peak expiratory flow rate, which can be measured at the bedside with a Wright peak flowmeter.93
Management during Labor and Vaginal Delivery
The goals of analgesia for labor and delivery in asthmatic women include (1) provision of pain relief, (2) reduction in the stimulus to hyperpnea, and (3) prevention or relief of maternal stress. The goal of adequate pain relief does not differ for asthmatic women. It is important to prevent hyperpnea and stress in women who describe asthmatic episodes triggered by exercise or stress. These goals should be accomplished with minimal sedation, minimal paralysis of the muscles of respiration, and minimal depression of the fetus. Possible analgesic regimens include systemic opioids, paracervical block, pudendal nerve block, lumbar sympathetic block, and epidural or spinal analgesia using local anesthetic agents, opioids, or both.
Systemic opioids may provide reasonable pain relief and reduce the stimulus to hyperpnea, especially during the early part of the first stage of labor. In theory, opioids reduce the risk for bronchospasm in asthmatic subjects. Opiate receptors are believed to be present in the respiratory tract94 and to inhibit release of excitatory neuropeptides. The clinical relevance of these findings is unknown, because moderate doses of inhaled morphine do not significantly alter airway tone.95 Conversely, high doses of some opioids (e.g., morphine) may increase the risk for bronchospasm by releasing histamine. The risk of using moderate doses of morphine does not seem excessive, because airway tone does not change in subjects with moderate to severe asthma after inhalation of morphine.95 An opioid that does not release histamine (e.g., fentanyl, remifentanil) may be a better choice for the asthmatic parturient. High doses of opioids are not desirable in subjects with active wheezing because of the risks for maternal and neonatal respiratory depression (see Chapter 22).
Paracervical block and pudendal nerve block performed by an obstetrician are acceptable choices for analgesia during the first and second stages of labor, respectively. These techniques provide analgesia without sedation or paralysis of the respiratory muscles. The problems with these techniques in asthmatic women are similar to those in nonasthmatic parturients (see Chapter 24).
Lumbar sympathetic block also provides pain relief without sedation or motor block during the first stage of labor. This technique has the same limitations as for women without asthma (see Chapter 24).
Intrathecal and epidural opioid techniques are useful during the first stage of labor and do not produce motor block (see Chapter 23). The advantage of the absence of motor block should be weighed against the risk for respiratory depression in asthmatic subjects.
Advantages of the use of local anesthetic agents for lumbar epidural analgesia in asthmatic patients include continuous pain relief and a reduction in the stimulus to hyperventilation. These goals typically are achieved without maternal sedation or neonatal depression. Unlike other analgesic techniques, continuous lumbar epidural analgesia adds a margin of safety by providing the opportunity to extend the sensory block for cesarean delivery. The possibility of extension allows the anesthesia provider to avoid some of the risks of general anesthesia. The most significant disadvantage of epidural local anesthetics in an asthmatic subject is the risk for a high thoracic motor block and respiratory insufficiency. Use of an appropriate epidural catheter test dose and maintenance of a sensory level at the 10th thoracic dermatome minimize this risk. In addition, the use of a dilute concentration of local anesthetic combined with a modest dose of an opioid produces satisfactory analgesia with less motor block than local anesthetic alone.96
Management during Cesarean Delivery
The choice between neuraxial anesthesia and general anesthesia for cesarean delivery depends on obstetric considerations and the respiratory status of the parturient. In general, avoidance of airway instrumentation is desirable, because tracheal intubation markedly increases airway tone in asthmatic subjects.97
The most significant advantage of neuraxial anesthesia in the asthmatic patient is that this technique obviates the necessity for tracheal intubation. Neuraxial anesthesia is associated with a lower incidence of bronchospasm than general anesthesia in asthmatic subjects.98 Stable asthmatic patients can undergo either spinal anesthesia or epidural anesthesia. In unstable asthmatic patients who require the use of accessory muscles of respiration, neuraxial anesthesia may be hazardous because of impaired ventilatory capacity in the presence of a high thoracic motor block. Intrathecal administration of either ropivacaine or levobupivacaine, which may produce less motor block than bupivacaine, does not confer any advantage with respect to pulmonary function in women receiving spinal anesthesia for cesarean delivery.99
The adrenal medulla receives innervation from preganglionic sympathetic fibers arising from the sixth thoracic to the second lumbar spinal segment.100 Some authors have postulated that neuraxial anesthesia and the ensuing sympathectomy could precipitate or potentiate bronchospasm during cesarean delivery in asthmatic subjects by reducing adrenal output of epinephrine. This possibility seems remote. First, although epinephrine infusion can reduce airway reactivity in asthmatic subjects,101 epinephrine concentrations do not decrease during nonobstetric surgery performed with neuraxial anesthesia that achieves high thoracic sensory levels.102,103 Second, the idea that neuraxial anesthesia may prevent increases in circulating epinephrine that are required to compensate for stress-induced bronchospasm does not appear to be valid. Bronchoconstriction does not stimulate epinephrine secretion in human asthmatic subjects.104 Thus, neuraxial anesthesia is appropriate for cesarean delivery in stable asthmatic subjects.
General anesthesia for asthmatic women undergoing cesarean delivery requires a balance between the competing considerations of pulmonary aspiration and intraoperative bronchospasm. Although airway instrumentation provides a great stimulus for bronchospasm, the high risk for aspiration mandates tracheal intubation during administration of general anesthesia in parturients.
Most commonly, options for tracheal intubation include awake intubation and rapid-sequence induction, although mask induction of general anesthesia with sevoflurane has been described in a parturient with status asthmaticus.105 Indications for awake intubation in asthmatic subjects are similar to those for nonasthmatic patients, and pretreatment with a local anesthetic and a beta-adrenergic agonist can attenuate reflex-induced bronchoconstriction after awake intubation.97 The benefits of topical local anesthetics and airway nerve blocks for awake intubation should be weighed against a possible increase in the risk for aspiration from the loss of protective airway reflexes. Rapid-sequence induction for cesarean delivery in asthmatic patients is most often accomplished using either propofol or ketamine. A sympathomimetic agent, ketamine has long been considered the intravenous induction agent of choice for asthmatic subjects. Ketamine relaxes airway smooth muscle and inhibits neural reflexes.106 Propofol provides better protection than thiopental against bronchospasm associated with tracheal intubation in asthmatic patients,107 but it has not been compared directly with ketamine in humans. Beneficial airway effects of propofol, like those of ketamine, also appear to occur via inhibition of airway reflexes.106 Intravenous lidocaine, which also inhibits airway reflexes, attenuates irritant-induced bronchoconstriction,108 including tracheal intubation, and produces an additional protective effect above that of beta-adrenergic agonist pretreatment alone.109
In patients without asthma, maintenance of general anesthesia typically includes administration of a low concentration of a volatile halogenated anesthetic agent, with or without nitrous oxide, before delivery of the infant. After delivery, maintenance of anesthesia typically consists of nitrous oxide and an intravenous opioid, with or without a low concentration of a volatile halogenated agent. In asthmatic parturients, the volatile halogenated anesthetic agents are considered the agents of choice for the maintenance of anesthesia. These agents attenuate airway responsiveness through direct effects on airway smooth muscle,110-112 inhibition of airway reflexes,113 and effects on the epithelium.114
A high concentration of a volatile halogenated anesthetic agent has salutary effects on the airways but also increases the risk for hemorrhage during cesarean delivery by causing dose-dependent uterine relaxation.115 Alternatively, nitrous oxide, an intravenous opioid, and a low concentration of a volatile halogenated agent may be given. Although halothane and isoflurane are approximately equipotent bronchodilators at high concentrations, halothane produces greater bronchodilation at lower concentrations116 and therefore may be preferable for anesthesia for cesarean delivery. Sevoflurane acts as a bronchodilator in large and small airways117 and reverses airway constriction associated with tracheal intubation.118 Effects of desflurane are controversial. Desflurane protects against a direct stimulus to the airways119 but may be less effective against reflex stimuli, such as tracheal intubation.120
A bronchodilator can be added if bronchospasm occurs. The potential disadvantage of this technique is that the most effective bronchodilators (i.e., the beta-adrenergic agonists) also relax uterine smooth muscle. The administration of a beta-adrenergic agonist by aerosol delivers a relatively greater dose of drug to the airways and minimizes uterine relaxation.
Emergence from general anesthesia, as with induction, requires a balance between reducing the risk for aspiration and lowering the risk for bronchospasm. Extubation of the trachea when the patient is awake minimizes the risk for aspiration, but the tracheal tube may stimulate reflexes and precipitate bronchospasm as the depth of anesthesia is reduced. If bronchospasm occurs during emergence, bronchodilators can be administered. For refractory bronchospasm, continued mechanical ventilation in an intensive care unit may be required.
Cigarette Smoking
Epidemiology
Cigarette smoking is a significant, preventable cause of maternal morbidity and perinatal morbidity and mortality.121 The prevalence of smoking among pregnant women in the United States declined from 18.1% in 1991 to 16% in 2010.122 Approximately 46% of women who smoke quit smoking during pregnancy.123
Pathophysiology
Cigarette smoke contains a large number of separate components that have a variety of biologic effects. Nonrespiratory effects of cigarette smoking are described in Chapter 54.
The primary respiratory effects of cigarette smoking include alterations in small airway function, increased mucus secretion, and impairment of ciliary transport.124 The precise mechanisms for these effects are unknown. Smoking also is associated with an increase in nonspecific airway reactivity, possibly through epithelial damage, altered airway geometry due to increased mucus secretion, or up-regulation of endothelin receptors.125 These changes lead to a marked increase in the incidence of postoperative pulmonary complications.124
Interaction with Pregnancy
Few studies have documented the respiratory effects of cigarette smoking during pregnancy. In one study, reductions in forced expiratory flow rates suggested that pregnant women who smoke cigarettes have greater small airway resistance than those who do not smoke.126 These and other abnormalities were similar to the changes in airway function observed in nonpregnant smokers. Although further studies are warranted, other respiratory effects of cigarette smoking in pregnant women are likely to be similar to those effects in nonpregnant women.
Cigarette smoking adversely affects pregnancy in a number of ways. The association between smoking and LBW has a genetic influence, such that the specific variation of the nicotinic acetylcholine receptor gene cluster is associated with lower newborn birth weight in smokers but not in nonsmokers.127 Although observational, this study suggests a causal relationship between smoking during pregnancy and lower offspring birth weight. Further details regarding adverse maternal and fetal effects of smoking are described in Chapter 54.
Medical Management
Cessation of smoking is the preferred form of medical management. Smoking cessation programs are effective in pregnant women.128 Nonpharmacologic methods are preferred to pharmacologic methods (e.g., nicotine patches) because of insufficient safety information for the latter.123 Das et al.129 demonstrated that smoking cessation before or early in pregnancy results in prompt improvement in maternal airway function. Smoking cessation reduces perioperative complications in the nonpregnant patient undergoing surgery,130 but no controlled studies have evaluated effects of smoking cessation on peripartum outcome.
Anesthetic Management
Tracheal intubation is associated with bronchospasm in smokers.131 For vaginal delivery, any of the analgesic techniques described earlier for asthmatic parturients are acceptable. For cesarean delivery, neuraxial anesthesia achieves the goal of avoiding airway instrumentation and is therefore preferable to general anesthesia, although no controlled studies have documented differences in peripartum morbidity. If general anesthesia is required, the methods for reducing the risk for intraoperative bronchospasm described previously may be considered. During induction of general anesthesia in smokers, the formulation of propofol containing sulfite results in greater respiratory resistance after tracheal intubation than the formulation containing ethylenediaminetetraacetic acid (EDTA).132 The clinical significance of this finding is unknown. One study noted that respiratory resistance did not decrease after tracheal intubation in smokers anesthetized with desflurane,120 suggesting that other volatile halogenated anesthetic agents might be preferable.
Cystic Fibrosis
Epidemiology
Cystic fibrosis, a lethal genetic disorder that is transmitted as an autosomal recessive trait, affects approximately 1 in 3200 white neonates in the United States.133 Because of improvements in diagnosis and therapy, a growing number of women with cystic fibrosis survive to reproductive age. The number of pregnancies reported to a national cystic fibrosis registry increased from fewer than 100 in the 1980s134 to approximately 240 in 2010.135
Pathophysiology
Clinical features of cystic fibrosis result from abnormalities of epithelial tissues, especially in the respiratory, digestive, and reproductive tracts. The underlying mechanism is a defect in cAMP-mediated activation of chloride (Cl−) conductance in the epithelium.136,137 Normal epithelial cells secrete Cl− in response to an increase in intracellular cAMP. In cystic fibrosis, a genetic mutation makes epithelial cells unable to alter Cl− permeability in response to changes in cAMP. The gene responsible for cystic fibrosis is located on chromosome 7 and encodes a protein known as the cystic fibrosis transmembrane regulator (CFTR).138,139 The CFTR acts as a Cl− channel but also has a number of other actions.140 Cystic fibrosis is characterized by obstruction of exocrine glands with mucus, but the precise molecular mechanism is not well understood. In sweat glands, however, patients with cystic fibrosis show abnormalities that can be readily explained by impairment of the CFTR, which limits reabsorption of Cl− and therefore of salt. In the airways, two opposing hypotheses, one in which the airway epithelium behaves similarly to the sweat duct epithelium and one in which the airway epithelium “behaves in a fashion essentially opposite to that of the sweat duct,” have been proposed to explain alterations in fluid and electrolyte composition of airway secretions in patients with cystic fibrosis.140
In the lungs, abnormalities of electrolyte transport alter the composition of airway secretions. Inflammation, with infiltration of polymorphonuclear leukocytes, also contributes to changes in airway secretions.141 Large numbers of disintegrating neutrophils release DNA in quantities sufficient to overwhelm the ability of deoxyribonuclease I (DNAse I), an endogenously released enzyme, to digest extracellular DNA. Undigested DNA increases the viscosity of airway secretions, which causes obstruction of small airways and reduced lung volumes. The ensuing ventilation-perfusion inequalities produce arterial hypoxemia. Some patients have hyperreactive airways. Spontaneous pneumothorax often occurs. Chronic airway obstruction and impaired mucus clearance increase the frequency of pulmonary infection. Most patients become colonized or infected with Pseudomonas aeruginosa. Eventually, tissue damage leads to bronchiectasis and pulmonary insufficiency. Chronic hypoxemia and lung destruction may produce pulmonary hypertension and cor pulmonale. Nonrespiratory manifestations of cystic fibrosis include pancreatic exocrine insufficiency, intestinal obstruction, and infertility.
Diagnosis
Clinical criteria for the diagnosis of cystic fibrosis include (1) the presence of chronic obstructive lung disease and colonization with Pseudomonas aeruginosa before age 20 years, (2) exocrine pancreatic insufficiency, and (3) a family history of cystic fibrosis. Laboratory findings include (1) sweat Cl− concentrations greater than 60 mEq/L, (2) CFTR genotype with two known cystic fibrosis mutations, and (3) detection of CFTR dysfunction by nasal potential difference test.142 Chest radiographic examination often demonstrates hyperinflation, and arterial blood gas measurements may show hypoxemia. Pulmonary function tests, which can reveal obstructive or restrictive lung patterns, are useful to assess the severity of the disease. With serial measurements, clinicians should look for evidence of an increased residual volume and a reduced FEV1 (forced expiratory volume in 1 second).143
Interaction with Pregnancy
Effect of Pregnancy on Cystic Fibrosis
The following factors may contribute to the deterioration of pulmonary function during pregnancy: (1) increased airway responsiveness and obstruction (as can occur in patients with asthma), (2) increased work of breathing, and (3) cardiovascular changes such as congestive heart failure and pulmonary hypertension associated with the increased blood volume of pregnancy.
In spite of potential negative effects of pregnancy on the course of cystic fibrosis, long-term survival does not appear to be affected.144 However, enthusiasm for these results should be tempered by the knowledge that pregnant women with cystic fibrosis require more intensive medical care than healthy pregnant women.145,146
Effect of Cystic Fibrosis on Pregnancy
Cystic fibrosis has been associated with an increased risk for LBW infants and preterm delivery,146 but recent evidence suggests that improved care of these patients can mitigate these problems.147 Potential mechanisms for these complications include chronic hypoxemia and poor maternal nutrition. Low prepregnancy FEV1 and BMI are associated with an increased risk for adverse fetal outcome.148
Medical Management
Respiratory management of cystic fibrosis is primarily symptomatic. Patients with large volumes of mucus production undergo mechanical airway clearance. Some patients inhale recombinant human deoxyribonuclease I to reduce viscosity of lung secretions caused by accumulating DNA.133 Hypertonic saline inhalation aids clearance of airway mucus.149 Bronchodilators may help those patients who manifest a reversible component of airway obstruction. Continuous oxygen therapy may benefit patients with hypoxemia and cor pulmonale.
Long-term antibiotic therapy with inhaled tobramycin reduces both the incidence of recurrent pulmonary infection and the frequency of exacerbations in patients with cystic fibrosis.150 Long-term administration of oral azithromycin also decreases exacerbations from cystic fibrosis151 through either its antibiotic or its anti-inflammatory properties. Effects of long-term antibiotic therapy on the fetus are unknown.
Other forms of therapy include gene therapy and lung transplantation. Gene therapy uses viral (recombinant adeno-associated) or nonviral (liposome) vectors to transfer the normal CFTR to airway epithelium of patients with cystic fibrosis. A meta-analysis of randomized trials provided no evidence that topical CFTR gene replacement therapy improved clinical outcome, but further studies are needed owing to a limited number of study subjects.152 Significant pulmonary deterioration sometimes leads to double-lung transplantation, although it is unclear whether transplantation alters survival.153
Obstetric Management
Because of the influence of pregravid maternal health on pregnancy outcome, the primary obstetric issue centers on the advisability of pregnancy in patients with cystic fibrosis. Criteria for the termination of pregnancy are not clearly defined. Genetic counseling regarding the risks of cystic fibrosis in the offspring is another important component of obstetric management.
Anesthetic Management
Considerations for anesthetic management focus primarily on the pulmonary system. Because of the high incidence of hypoxemia in patients with cystic fibrosis, continuous monitoring of oxygen saturation and appropriate oxygen therapy are advisable.
The goals of pain relief during labor are to provide adequate analgesia and to prevent maternal hyperventilation while avoiding high thoracic motor block and respiratory depression. High thoracic motor block may impair the parturient's ability to cough and eliminate thick secretions. Hyperventilation increases the work of breathing and may cause decompensation in patients with severe pulmonary dysfunction. For pain relief during labor, parenteral opioid analgesia may worsen pulmonary function by depressing respiratory drive and inhibiting cough. Intrathecal opioids have been used successfully,154 but patients should be monitored carefully for respiratory depression. An ideal way to deliver a short-acting intrathecal opioid might be through a microcatheter, which would provide a margin of safety by allowing repeated administration of small doses of the opioid.155 Alternatively, continuous lumbar epidural analgesia, with a sensory nerve block maintained at the level of the 10th thoracic dermatome, can provide excellent pain relief and reduce the stimulus for hyperventilation, with minimal motor block of the thorax. A dilute solution of bupivacaine, with or without an opioid, provides sensory analgesia with minimal motor block and is therefore nearly ideal in this setting.156 In healthy parturients, this technique actually improves respiratory function slightly.157
Cesarean delivery necessitates the choice between general anesthesia and neuraxial anesthesia. Among patients with cystic fibrosis, no studies have documented differences in outcome between general anesthesia and neuraxial anesthesia. Neuraxial anesthesia offers the advantage of avoiding tracheal intubation, which may be associated with bronchospasm or obstruction of the tracheal tube with secretions. Neuraxial anesthesia also avoids positive-pressure ventilation, which may enlarge a preexisting pneumothorax. The primary consideration for neuraxial anesthesia during cesarean delivery is to avoid a high thoracic motor block, which may impair ventilation and the ability to cough. Effective spinal anesthesia for cesarean delivery slightly decreases vital capacity.158 Methods for reducing the risk for excessively high motor block include the use of a continuous catheter technique, which allows titration of the local anesthetic agent to achieve the desired sensory level, and the use of the lowest concentration of local anesthetic (with or without an opioid) that provides surgical anesthesia. Both epidural anesthesia159 and combined spinal-epidural anesthesia160,161 have been used in parturients with cystic fibrosis.
For general anesthesia, techniques to reduce the risk for bronchospasm, as described for patients with asthma (see earlier discussion), may be warranted. Additional considerations include (1) humidification of gases to prevent inspissation of mucus, (2) frequent suctioning to remove excess secretions, and (3) use of ventilator settings that allow an appropriately long expiratory phase to prevent air trapping and pneumothorax. It may also be prudent to avoid nitrous oxide in the parturient with cystic fibrosis, because of the risk for pneumothorax. Patients with cystic fibrosis should be allowed to awaken fully before extubation of the trachea. Chest physiotherapy may be required in the immediate postoperative period.
Respiratory Failure
Epidemiology
The prevalence of respiratory failure during pregnancy is unknown. A significant subset of patients with respiratory failure suffers from acute respiratory distress syndrome (ARDS). The prevalence of ARDS in pregnancy has been estimated at approximately 1 in 6000 to 7000 deliveries.162,163 Mortality from respiratory failure during pregnancy is high.164
Pathophysiology
The pathophysiology of respiratory failure depends on the underlying disorder. ARDS results from a group of predisposing conditions, but a common final pathway leads to similar manifestations.165 Damage to the alveolar and capillary membranes initiates a cascade of events leading to fluid transudation that often is accompanied by pulmonary venoconstriction. Direct injury to the alveolar and capillary membranes can result from pulmonary aspiration of gastric contents and perhaps oxygen toxicity. Indirect toxicity can result from humoral and cellular mechanisms caused by triggers such as sepsis and amniotic fluid embolism. Transudation of fluid leads to atelectasis, airway obstruction, reduced lung compliance, and altered ventilation-perfusion relationships. Both physiologic dead space and shunt fractions are increased.
Diagnosis
A variety of disorders can cause acute respiratory failure during pregnancy (Box 53-4). Specific diagnostic criteria depend on the disorder.
The diagnosis of ARDS requires the exclusion of other disorders. Prominent characteristics of ARDS include arterial hypoxemia, radiographic evidence of pulmonary infiltrates, and reduced lung compliance in the setting of a recognized predisposing condition.166
Interaction with Pregnancy
Pregnancy is not known to alter the overall course of respiratory failure. However, differences in outcome between pregnant and nonpregnant patients have been observed in subsets of patients with respiratory failure. In a series of patients with severe acute respiratory syndrome (SARS), pregnant patients had greater morbidity.167 Mortality rates are similar in pregnant and nonpregnant subjects.164
The most significant effect of respiratory failure on pregnancy is a reduction in oxygen delivery to the fetus. This reduction results most commonly from maternal arterial hypoxemia or maternal hypotension, which often accompanies respiratory failure. Hypotension may result from associated underlying conditions or from elevated mean airway pressures during mechanical ventilation. High rates of prenatal complications with or without preterm delivery have been reported.162,163
Medical Management
Therapeutic strategies for managing respiratory failure during pregnancy do not differ qualitatively from those in nonpregnant patients. The primary goals of medical management are to (1) eliminate predisposing conditions, (2) limit fluid transudation, and (3) maintain maternal oxygen delivery. Fluid restriction and diuretics help limit fluid transudation, although this therapy must be used cautiously when the underlying cause of respiratory failure is associated with intravascular fluid depletion. The goals for maintenance of oxygen delivery may differ quantitatively during pregnancy. Oxygen delivery to the fetus worsens significantly when PaO2 decreases below 70 mm Hg or oxygen saturation (SaO2) falls below 95%.162,168 Standard methods of maintaining oxygen delivery include (1) administration of a higher inspired concentration of oxygen, (2) administration of bronchodilators in the presence of reversible airway obstruction, (3) administration of pharmacologic agents to support the circulation as needed, and (4) mechanical ventilation. A higher inspired oxygen concentration, delivered by face mask, is safe during pregnancy and may obviate the necessity of tracheal intubation and its risks for aspiration and difficult airway management. Bronchodilator therapy can also be used for respiratory failure, as described earlier for asthma. Pharmacologic agents for circulatory support include agents with both alpha- and beta-adrenergic receptor activity.
Indications for tracheal intubation and mechanical ventilation are similar for pregnant and nonpregnant patients with respiratory failure.167,169 Maternal and fetal effects of current approaches to mechanical ventilation, including use of low tidal volumes and permissive hypercapnia, have not been studied in pregnant patients. Positive end-expiratory pressure may be used if cardiac output is maintained to allow sufficient blood flow to the uterus.
Some pregnant patients with respiratory failure do not show adequate response to conventional methods of treatment. For these patients, treatment options include extracorporeal membrane oxygenation (ECMO),170 high-frequency oscillatory ventilation,171 and inhaled nitric oxide.172,173 Nitric oxide relaxes vascular smooth muscle. Rapid inactivation of nitric oxide by binding to hemoglobin in the circulation allows inhaled nitric oxide to produce pulmonary vasodilation without systemic vascular effect. Selective pulmonary vasodilation in well-ventilated areas of the lung presumably would improve oxygen delivery. The safety of these alternative forms of treatment in pregnancy is unknown because reports of their use are anecdotal.
Obstetric Management
Because the beneficial effects of delivery on the course of respiratory failure have not been proved, indications for induction of labor or cesarean delivery in this setting are not well defined. Small observational studies have not clearly shown an association between delivery and improved respiratory status in pregnant women with respiratory failure.163,174,175 Further, data to support decisions regarding mode of delivery are limited. Vaginal delivery is possible during mechanical ventilation176,177 and may avoid complications of major intra-abdominal surgery in a critically ill woman.
Anesthetic Management
The anesthetic management of patients with respiratory failure requires appropriate medical management. During labor, analgesia for mechanically ventilated patients can be achieved with intravenous opioids, which are often used for sedation during mechanical ventilation. Lumbar epidural analgesia provides pain relief without the neonatal respiratory depression associated with high doses of opioids. Labor epidural analgesia also reduces oxygen consumption,178 which may be beneficial in hypoxemic patients. The use of labor epidural analgesia in patients with respiratory failure depends on underlying conditions and ongoing therapy. Close attention should be paid to intravascular volume, adequacy of coagulation, and presence or absence of infection.
In mechanically ventilated patients, general endotracheal anesthesia is often the most convenient choice for cesarean delivery. Aside from the issues of medical management (as discussed earlier), the techniques and pharmacologic agents do not differ substantially from those used in patients without respiratory failure.
References
1. Akinbami LJ, Moorman JE, Bailey C, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001-2010. NCHS Data Brief. 2012;1–8.
2. Kwon HL, Triche EW, Belanger K, Bracken MB. The epidemiology of asthma during pregnancy: prevalence, diagnosis, and symptoms. Immunol Allergy Clin North Am. 2006;26:29–62.
3. Melen E, Pershagen G. Pathophysiology of asthma: lessons from genetic research with particular focus on severe asthma. J Intern Med. 2012;272:108–120.
4. Vincenc KS, Black JL, Yan K, et al. Comparison of in vivo and in vitro responses to histamine in human airways. Am Rev Respir Dis. 1983;128:875–879.
5. Armour CL, Lazar NM, Schellenberg RR, et al. A comparison of in vivo and in vitro human airway reactivity to histamine. Am Rev Respir Dis. 1984;129:907–910.
6. Roberts JA, Raeburn D, Rodger IW, Thomson NC. Comparison of in vivo airway responsiveness and in vitro smooth muscle sensitivity to methacholine in man. Thorax. 1984;39:837–843.
7. Roberts JA, Rodger IW, Thomson NC. Airway responsiveness to histamine in man: effect of atropine on in vivo and in vitro comparison. Thorax. 1985;40:261–267.
8. Goldie RG, Spina D, Henry PJ, et al. In vitro responsiveness of human asthmatic bronchus to carbachol, histamine, beta-adrenoceptor agonists and theophylline. Br J Clin Pharmacol. 1986;22:669–676.
9. Downes H, Austin DR, Parks CM, Hirshman CA. Comparison of drug responses in vivo and in vitro in airways of dogs with and without airway hyperresponsiveness. J Pharmacol Exp Ther. 1986;237:214–219.
10. Cerrina J, Le Roy Ladurie M, Labat C, et al. Comparison of human bronchial muscle responses to histamine in vivo with histamine and isoproterenol agonists in vitro. Am Rev Respir Dis. 1986;134:57–61.
11. Skloot G, Permutt S, Togias A. Airway hyperresponsiveness in asthma: a problem of limited smooth muscle relaxation with inspiration. J Clin Invest. 1995;96:2393–2403.
12. Richardson JB. Nerve supply to the lungs. Am Rev Respir Dis. 1979;119:785–802.
13. Starke K, Gothert M, Kilbinger H. Modulation of neurotransmitter release by presynaptic autoreceptors. Physiol Rev. 1989;69:864–989.
14. Barnes PJ. Muscarinic autoreceptors in airways: their possible role in airway disease. Chest. 1989;96:1220–1221.
15. Fryer AD, Jacoby DB. Parainfluenza virus infection damages inhibitory M2 muscarinic receptors on pulmonary parasympathetic nerves in the guinea-pig. Br J Pharmacol. 1991;102:267–271.
16. Sorkness R, Clough JJ, Castleman WL, Lemanske RF Jr. Virus-induced airway obstruction and parasympathetic hyperresponsiveness in adult rats. Am J Respir Crit Care Med. 1994;150:28–34.
17. Nadel JA, Barnes PJ. Autonomic regulation of the airways. Annu Rev Med. 1984;35:451–467.
18. Tattersfield AE, Leaver DG, Pride NB. Effects of beta-adrenergic blockade and stimulation on normal human airways. J Appl Physiol. 1973;35:613–619.
19. Spina D, Rigby PJ, Paterson JW, Goldie RG. Alpha 1-adrenoceptor function and autoradiographic distribution in human asthmatic lung. Br J Pharmacol. 1989;97:701–708.
20. Krishnakumar S, Holmes EP, Moore RM, et al. Non-adrenergic non-cholinergic excitatory innervation in the airways: role of neurokinin-2 receptors. Auton Autacoid Pharmacol. 2002;22:215–224.
21. Belvisi MG, Stretton CD, Yacoub M, Barnes PJ. Nitric oxide is the endogenous neurotransmitter of bronchodilator nerves in humans. Eur J Pharmacol. 1992;210:221–222.
22. Lammers JW, Minette P, McCusker MT, et al. Capsaicin-induced bronchodilation in mild asthmatic subjects: possible role of nonadrenergic inhibitory system. J Appl Physiol. 1989;67:856–861.
23. Kraan J, Vink-Klooster H, Postma DS. The NK-2 receptor antagonist SR 48968C does not improve adenosine hyperresponsiveness and airway obstruction in allergic asthma. Clin Exp Allergy. 2001;31:274–278.
24. Hogg JC, James AL, Pare PD. Evidence for inflammation in asthma. Am Rev Respir Dis. 1991;143:S39–S42.
25. Tattersfield AE, Knox AJ, Britton JR, Hall IP. Asthma. Lancet. 2002;360:1313–1322.
26. Veerappan A, Reid AC, Estephan R, et al. Mast cell renin and a local renin-angiotensin system in the airway: role in bronchoconstriction. Proc Natl Acad Sci U S A. 2008;105:1315–1320.
27. Luster AD, Tager AM. T-cell trafficking in asthma: lipid mediators grease the way. Nat Rev Immunol. 2004;4:711–724.
28. Black JL. Control of human airway smooth muscle. Am Rev Respir Dis. 1991;143:S11–S12.
29. Schwartz LB. Cellular inflammation in asthma: neutral proteases of mast cells. Am Rev Respir Dis. 1992;145:S18–S21.
30. Nadel JA. Biologic effects of mast cell enzymes. Am Rev Respir Dis. 1992;145:S37–S41.
31. Bergner A, Kellner J, Kemp da Silva A, et al. Bronchial hyperreactivity is correlated with increased baseline airway tone. Eur J Med Res. 2006;11:77–84.
32. Hogg JC. Pathology of asthma. J Allergy Clin Immunol. 1993;92:1–5.
33. Knight DA, Holgate ST. The airway epithelium: structural and functional properties in health and disease. Respirology. 2003;8:432–446.
34. Zietkowski Z, Skiepko R, Tomasiak MM, Bodzenta-Lukaszyk A. Endothelin-1 in exhaled breath condensate of allergic asthma patients with exercise-induced bronchoconstriction. Respir Res. 2007;8:76.
35. Qin XQ, Xiang Y, Liu C, et al. The role of bronchial epithelial cells in airway hyperresponsiveness. Sheng Li Xue Bao. 2007;59:454–464.
36. Schatz M, Dombrowski MP, Wise R, et al. Asthma morbidity during pregnancy can be predicted by severity classification. J Allergy Clin Immunol. 2003;112:283–288.
37. Belanger K, Hellenbrand ME, Holford TR, Bracken M. Effect of pregnancy on maternal asthma symptoms and medication use. Obstet Gynecol. 2010;115:559–567.
38. Teeter JG, Bleecker ER. Relationship between airway obstruction and respiratory symptoms in adult asthmatics. Chest. 1998;113:272–277.
39. Juniper EF, Daniel EE, Roberts RS, et al. Improvement in airway responsiveness and asthma severity during pregnancy: a prospective study. Am Rev Respir Dis. 1989;140:924–931.
40. Mabie WC, Barton JR, Wasserstrum N, Sibai BM. Clinical Observations on Asthma in Pregnancy. J Matern Fetal Med. 1992;1:45–50.
41. Hellings PW, Vandekerckhove P, Claeys R, et al. Progesterone increases airway eosinophilia and hyper-responsiveness in a murine model of allergic asthma. Clin Exp Allergy. 2003;33:1457–1463.
42. Martel MJ, Rey E, Beauchesne MF, et al. Use of inhaled corticosteroids during pregnancy and risk of pregnancy induced hypertension: nested case-control study. BMJ. 2005;330:230.
43. Enriquez R, Griffin MR, Carroll KN, et al. Effect of maternal asthma and asthma control on pregnancy and perinatal outcomes. J Allergy Clin Immunol. 2007;120:625–630.
44. Dombrowski MP, Schatz M, Wise R, et al. Asthma during pregnancy. Obstet Gynecol. 2004;103:5–12.
45. Sorensen TK, Dempsey JC, Xiao R, et al. Maternal asthma and risk of preterm delivery. Ann Epidemiol. 2003;13:267–272.
46. Sheiner E, Mazor M, Levy A, et al. Pregnancy outcome of asthmatic patients: a population-based study. J Matern Fetal Neonatal Med. 2005;18:237–240.
47. Bracken MB, Triche EW, Belanger K, et al. Asthma symptoms, severity, and drug therapy: a prospective study of effects on 2205 pregnancies. Obstet Gynecol. 2003;102:739–752.
48. Getahun D, Ananth CV, Oyelese Y, et al. Acute and chronic respiratory diseases in pregnancy: associations with spontaneous premature rupture of membranes. J Matern Fetal Neonatal Med. 2007;20:669–675.
49. Tata LJ, Lewis SA, McKeever TM, et al. A comprehensive analysis of adverse obstetric and pediatric complications in women with asthma. Am J Respir Crit Care Med. 2007;175:991–997.
50. Sobande AA, Archibong EI, Akinola SE. Pregnancy outcome in asthmatic patients from high altitudes. Int J Gynaecol Obstet. 2002;77:117–121.
51. Kallen B, Otterblad Olausson P. Use of anti-asthmatic drugs during pregnancy. 2. Infant characteristics excluding congenital malformations. Eur J Clin Pharmacol. 2007;63:375–381.
52. Murphy VE, Namazy JA, Powell H, et al. A meta-analysis of adverse perinatal outcomes in women with asthma. BJOG. 2011;118:1314–1323.
53. Murphy VE, Gibson PG, Smith R, Clifton VL. Asthma during pregnancy: mechanisms and treatment implications. Eur Respir J. 2005;25:731–750.
54. Siddiqui S, Goodman N, McKenna S, et al. Pre-eclampsia is associated with airway hyperresponsiveness. BJOG. 2008;115:520–522.
55. Rey E, Boulet LP. Asthma in pregnancy. BMJ. 2007;334:582–585.
56. Najafizadeh K, Sohrab Pour H, Ghadyanee M, et al. A randomised, double-blind, placebo-controlled study to evaluate the role of formoterol in the management of acute asthma. Emerg Med J. 2007;24:317–321.
57. Nelson HS, Weiss ST, Bleecker ER, et al. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest. 2006;129:15–26.
58. Oppenheimer J, Nelson HS. Safety of long-acting beta-agonists in asthma: a review. Curr Opin Pulm Med. 2008;14:64–69.
59. Szefler SJ, Chinchilli VM, Israel E, et al. Key observations from the NHLBI Asthma Clinical Research Network. Thorax. 2012;67:450–455.
60. Osur SL. The management of asthma and rhinitis during pregnancy. J Womens Health (Larchmt). 2005;14:263–276.
61. Gluck JC, Gluck PA. Asthma controller therapy during pregnancy. Am J Obstet Gynecol. 2005;192:369–380.
62. Bond RA, Spina D, Parra S, Page CP. Getting to the heart of asthma: can “beta blockers” be useful to treat asthma? Pharmacol Ther. 2007;115:360–374.
63. Bukowskyj M, Nakatsu K, Munt PW. Theophylline reassessed. Ann Intern Med. 1984;101:63–73.
64. Lam A, Newhouse MT. Management of asthma and chronic airflow limitation. Are methylxanthines obsolete? Chest. 1990;98:44–52.
65. Carter BL, Driscoll CE, Smith GD. Theophylline clearance during pregnancy. Obstet Gynecol. 1986;68:555–559.
66. Chhabra SK, Pandey KK. Comparison of acute bronchodilator effects of inhaled ipratropium bromide and salbutamol in bronchial asthma. J Asthma. 2002;39:375–381.
67. Lanes SF, Garrett JE, Wentworth CE 3rd, et al. The effect of adding ipratropium bromide to salbutamol in the treatment of acute asthma: a pooled analysis of three trials. Chest. 1998;114:365–372.
68. Westby M, Benson M, Gibson P. Anticholinergic agents for chronic asthma in adults. Cochrane Database Syst Rev. 2004;(3).
69. Barsky HE. Asthma and pregnancy: a challenge for everyone concerned. Postgrad Med. 1991;89:125–130.
70. Gourgoulianis KI, Chatziparasidis G, Chatziefthimiou A, Molyvdas PA. Magnesium as a relaxing factor of airway smooth muscles. J Aerosol Med. 2001;14:301–307.
71. Rowe BH, Camargo CA Jr. The role of magnesium sulfate in the acute and chronic management of asthma. Curr Opin Pulm Med. 2008;14:70–76.
72. Barnes PJ. Molecular mechanisms and cellular effects of glucocorticosteroids. Immunol Allergy Clin North Am. 2005;25:451–468.
73. Berry M, Morgan A, Shaw DE, et al. Pathological features and inhaled corticosteroid response of eosinophilic and non-eosinophilic asthma. Thorax. 2007;62:1043–1049.
74. Bel EH, Timmers MC, Zwinderman AH, et al. The effect of inhaled corticosteroids on the maximal degree of airway narrowing to methacholine in asthmatic subjects. Am Rev Respir Dis. 1991;143:109–113.
75. Hodyl NA, Stark MJ, Osei-Kumah A, et al. Fetal glucocorticoid-regulated pathways are not affected by inhaled corticosteroid use for asthma during pregnancy. Am J Respir Crit Care Med. 2011;183:716–722.
76. Namazy JA, Murphy VE, Powell H, et al. Effects of asthma severity, exacerbations and oral corticosteroids on perinatal outcomes. Eur Respir J. 2013;41:1082–1090.
77. Schatz M, Dombrowski MP, Wise R, et al. The relationship of asthma medication use to perinatal outcomes. J Allergy Clin Immunol. 2004;113:1040–1045.
78. Rahimi R, Nikfar S, Abdollahi M. Meta-analysis finds use of inhaled corticosteroids during pregnancy safe: a systematic meta-analysis review. Hum Exp Toxicol. 2006;25:447–452.
79. Powell H, Murphy VE, Taylor DR, et al. Management of asthma in pregnancy guided by measurement of fraction of exhaled nitric oxide: a double-blind, randomised controlled trial. Lancet. 2011;378:983–990.
80. Greenberger PA. Asthma during pregnancy. J Asthma. 1990;27:341–347.
81. Chung KF, Barnes PJ. Treatment of asthma. Br Med J (Clin Res Ed). 1987;294:103–105.
82. Udelsman R, Ramp J, Gallucci WT, et al. Adaptation during surgical stress: a reevaluation of the role of glucocorticoids. J Clin Invest. 1986;77:1377–1381.
83. Barnes NC. The properties of inhaled corticosteroids: similarities and differences. Prim Care Respir J. 2007;16:149–154.
84. Alton EW, Norris AA. Chloride transport and the actions of nedocromil sodium and cromolyn sodium in asthma. J Allergy Clin Immunol. 1996;98:S102–S105.
85. Bakhireva LN, Jones KL, Schatz M, et al. Safety of leukotriene receptor antagonists in pregnancy. J Allergy Clin Immunol. 2007;119:618–625.
86. Mathe AA, Hedqvist P. Effect of prostaglandins F2 alpha and E2 on airway conductance in healthy subjects and asthmatic patients. Am Rev Respir Dis. 1975;111:313–320.
87. Ishimura M, Kataoka S, Suda M, et al. Effects of KP-496, a novel dual antagonist for leukotriene D4 and thromboxane A2 receptors, on contractions induced by various agonists in the guinea pig trachea. Allergol Int. 2006;55:403–410.
88. Kreisman H, Van de Weil W, Mitchell CA. Respiratory function during prostaglandin-induced labor. Am Rev Respir Dis. 1975;111:564–566.
89. Tilley SL, Hartney JM, Erikson CJ, et al. Receptors and pathways mediating the effects of prostaglandin E2 on airway tone. Am J Physiol Lung Cell Mol Physiol. 2003;284:L599–L606.
90. Sellers WF, Long DR. Bronchospasm following ergometrine. Anaesthesia. 1979;34:909.
91. Crawford JS. Bronchospasm following ergometrine. Anaesthesia. 1980;35:397–398.
92. Leff AR. Endogenous regulation of bronchomotor tone. Am Rev Respir Dis. 1988;137:1198–1216.
93. Wright BM, McKerrow CB. Maximum forced expiratory flow rate as a measure of ventilatory capacity: with a description of a new portable instrument for measuring it. Br Med J. 1959;2:1041–1046.
94. Belvisi MG, Rogers DF, Barnes PJ. Neurogenic plasma extravasation: inhibition by morphine in guinea pig airways in vivo. J Appl Physiol. 1989;66:268–272.
95. Otulana B, Okikawa J, Linn L, et al. Safety and pharmacokinetics of inhaled morphine delivered using the AERx system in patients with moderate-to-severe asthma. Int J Clin Pharmacol Ther. 2004;42:456–462.
96. Chestnut DH, Owen CL, Bates JN, et al. Continuous infusion epidural analgesia during labor: a randomized, double-blind comparison of 0.0625% bupivacaine/0.0002% fentanyl versus 0.125% bupivacaine. Anesthesiology. 1988;68:754–759.
97. Groeben H, Schlicht M, Stieglitz S, et al. Both local anesthetics and salbutamol pretreatment affect reflex bronchoconstriction in volunteers with asthma undergoing awake fiberoptic intubation. Anesthesiology. 2002;97:1445–1450.
98. Shnider SM, Papper EM. Anesthesia for the asthmatic patient. Anesthesiology. 1961;22:886–892.
99. Lirk P, Kleber N, Mitterschiffthaler G, et al. Pulmonary effects of bupivacaine, ropivacaine, and levobupivacaine in parturients undergoing spinal anaesthesia for elective caesarean delivery: a randomised controlled study. Int J Obstet Anesth. 2010;19:287–292.
100. Bonica JJ. Autonomic innervation of the viscera in relation to nerve block. Anesthesiology. 1968;29:793–813.
101. Knox AJ, Campos-Gongora H, Wisniewski A, et al. Modification of bronchial reactivity by physiological concentrations of plasma epinephrine. J Appl Physiol. 1992;73:1004–1007.
102. Pflug AE, Halter JB. Effect of spinal anesthesia on adrenergic tone and the neuroendocrine responses to surgical stress in humans. Anesthesiology. 1981;55:120–126.
103. Shimosato S, Etsten BE. The role of the venous system in cardiocirculatory dynamics during spinal and epidural anesthesia in man. Anesthesiology. 1969;30:619–628.
104. Emerman CL, Cydulka RK. Changes in serum catecholamine levels during acute bronchospasm. Ann Emerg Med. 1993;22:1836–1841.
105. Que JC, Lusaya VO. Sevoflurane induction for emergency cesarean section in a parturient in status asthmaticus. Anesthesiology. 1999;90:1475–1476.
106. Brown RH, Wagner EM. Mechanisms of bronchoprotection by anesthetic induction agents: propofol versus ketamine. Anesthesiology. 1999;90:822–828.
107. Pizov R, Brown RH, Weiss YS, et al. Wheezing during induction of general anesthesia in patients with and without asthma: a randomized, blinded trial. Anesthesiology. 1995;82:1111–1116.
108. Groeben H, Schwalen A, Irsfeld S, et al. Intravenous lidocaine and bupivacaine dose-dependently attenuate bronchial hyperreactivity in awake volunteers. Anesthesiology. 1996;84:533–539.
109. Groeben H, Silvanus MT, Beste M, Peters J. Combined intravenous lidocaine and inhaled salbutamol protect against bronchial hyperreactivity more effectively than lidocaine or salbutamol alone. Anesthesiology. 1998;89:862–868.
110. Mercier FJ, Naline E, Bardou M, et al. Relaxation of proximal and distal isolated human bronchi by halothane, isoflurane and desflurane. Eur Respir J. 2002;20:286–292.
111. Jones KA, Housmans PR, Warner DO, et al. Halothane alters cytosolic calcium transient in tracheal smooth muscle. Am J Physiol. 1993;265:L80–L86.
112. Duracher C, Blanc FX, Gueugniaud PY, et al. The effects of isoflurane on airway smooth muscle crossbridge kinetics in Fisher and Lewis rats. Anesth Analg. 2005;101:136–142.
113. Warner DO, Vettermann J, Brichant JF, Rehder K. Direct and neurally mediated effects of halothane on pulmonary resistance in vivo. Anesthesiology. 1990;72:1057–1063.
114. Park KW, Dai HB, Lowenstein E, Sellke FW. Epithelial dependence of the bronchodilatory effect of sevoflurane and desflurane in rat distal bronchi. Anesth Analg. 1998;86:646–651.
115. Gultekin H, Yildiz K, Sezer Z, Dogru K. Comparing the relaxing effects of desflurane and sevoflurane on oxytocin-induced contractions of isolated myometrium in both pregnant and nonpregnant rats. Adv Ther. 2006;23:39–46.
116. Brown RH, Zerhouni EA, Hirshman CA. Comparison of low concentrations of halothane and isoflurane as bronchodilators. Anesthesiology. 1993;78:1097–1101.
117. Burburan SM, Xisto DG, Ferreira HC, et al. Lung mechanics and histology during sevoflurane anesthesia in a model of chronic allergic asthma. Anesth Analg. 2007;104:631–637.
118. Rooke GA, Choi JH, Bishop MJ. The effect of isoflurane, halothane, sevoflurane, and thiopental/nitrous oxide on respiratory system resistance after tracheal intubation. Anesthesiology. 1997;86:1294–1299.
119. Lele E, Petak F, Fontao F, et al. Protective effects of volatile agents against acetylcholine-induced bronchoconstriction in isolated perfused rat lungs. Acta Anaesthesiol Scand. 2006;50:1145–1151.
120. Goff MJ, Arain SR, Ficke DJ, et al. Absence of bronchodilation during desflurane anesthesia: a comparison to sevoflurane and thiopental. Anesthesiology. 2000;93:404–408.
121. Cnattingius S. The epidemiology of smoking during pregnancy: smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine Tob Res. 2004;6(Suppl 2):S125–S140.
122. U.S. Department of Health and Human Services. Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. [Available at] http://www.samhsa.gov/data/NSDUH/2k10ResultsRev/NSDUHresultsRev2010.htm#2.6 [Accessed date, October 2013] .
123. The American College of Obstetricians and Gynecologists Committees on Healthcare for Underserved Women and Obstetric Practice. Committee Opinion No. 471. Smoking cessation during pregnancy. [Washington, DC] Obstet Gynecol. 2010;116:1241–1244.
124. Pearce AC, Jones RM. Smoking and anesthesia: preoperative abstinence and perioperative morbidity. Anesthesiology. 1984;61:576–584.
125. Zhang Y, Edvinsson L, Xu CB. Up-regulation of endothelin receptors induced by cigarette smoke—involvement of MAPK in vascular and airway hyper-reactivity. Scientific World Journal. 2010;10:2157–2166.
126. Das TK, Moutquin JM, Parent JG. Effect of cigarette smoking on maternal airway function during pregnancy. Am J Obstet Gynecol. 1991;165:675–679.
127. Tyrrell J, Huikari V, Christie JT, et al. Genetic variation in the 15q25 nicotinic acetylcholine receptor gene cluster (CHRNA5-CHRNA3-CHRNB4) interacts with maternal self-reported smoking status during pregnancy to influence birth weight. Hum Mol Genet. 2012;21:5344–5358.
128. Pollak KI, Oncken CA, Lipkus IM, et al. Nicotine replacement and behavioral therapy for smoking cessation in pregnancy. Am J Prev Med. 2007;33:297–305.
129. Das TK, Moutquin JM, Lindsay C, et al. Effects of smoking cessation on maternal airway function and birth weight. Obstet Gynecol. 1998;92:201–205.
130. Warner DO. Perioperative abstinence from cigarettes: physiologic and clinical consequences. Anesthesiology. 2006;104:356–367.
131. Kim ES, Bishop MJ. Endotracheal intubation, but not laryngeal mask airway insertion, produces reversible bronchoconstriction. Anesthesiology. 1999;90:391–394.
132. Rieschke P, LaFleur BJ, Janicki PK. Effects of EDTA- and sulfite-containing formulations of propofol on respiratory system resistance after tracheal intubation in smokers. Anesthesiology. 2003;98:323–328.
133. Saeed Z, Wojewodka G, Marion D, et al. Novel pharmaceutical approaches for treating patients with cystic fibrosis. Curr Pharm Des. 2007;13:3252–3263.
134. Kotloff RM, FitzSimmons SC, Fiel SB. Fertility and pregnancy in patients with cystic fibrosis. Clin Chest Med. 1992;13:623–635.
135. Cystic fibrosis foundation. Patient registry: annual data report 2010. [Bethesda, Maryland. Available at] http://www.cff.org/UploadedFiles/LivingWithCF/CareCenterNetwork/PatientRegistry/2010-Patient-Registry-Report.pdf.
136. Hwang TC, Lu L, Zeitlin PL, et al. Cl− channels in CF: lack of activation by protein kinase C and cAMP-dependent protein kinase. Science. 1989;244:1351–1353.
137. Li M, McCann JD, Liedtke CM, et al. Cyclic AMP-dependent protein kinase opens chloride channels in normal but not cystic fibrosis airway epithelium. Nature. 1988;331:358–360.
138. Riordan JR, Rommens JM, Kerem B, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073.
139. Rommens JM, Iannuzzi MC, Kerem B, et al. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 1989;245:1059–1065.
140. Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352:1992–2001.
141. Konstan MW, Hilliard KA, Norvell TM, Berger M. Bronchoalveolar lavage findings in cystic fibrosis patients with stable, clinically mild lung disease suggest ongoing infection and inflammation. Am J Respir Crit Care Med. 1994;150:448–454.
142. Boyle MP. Adult cystic fibrosis. JAMA. 2007;298:1787–1793.
143. Kerem E, Reisman J, Corey M, et al. Prediction of mortality in patients with cystic fibrosis. N Engl J Med. 1992;326:1187–1191.
144. Goss CH, Rubenfeld GD, Otto K, Aitken ML. The effect of pregnancy on survival in women with cystic fibrosis. Chest. 2003;124:1460–1468.
145. Cheng EY, Goss CH, McKone EF, et al. Aggressive prenatal care results in successful fetal outcomes in CF women. J Cyst Fibros. 2006;5:85–91.
146. Whitty JE. Cystic fibrosis in pregnancy. Clin Obstet Gynecol. 2010;53:369–376.
147. Burden C, Ion R, Chung Y, et al. Current pregnancy outcomes in women with cystic fibrosis. Eur J Obstet Gynecol Reprod Biol. 2012;164:142–145.
148. Lau EM, Barnes DJ, Moriarty C, et al. Pregnancy outcomes in the current era of cystic fibrosis care: a 15-year experience. Aust N Z J Obstet Gynaecol. 2011;51:220–224.
149. Reeves EP, Molloy K, Pohl K, McElvaney NG. Hypertonic saline in treatment of pulmonary disease in cystic fibrosis. Scientific World Journal. 2012;2012:465230.
150. Ramsey BW, Pepe MS, Quan JM, et al. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. Cystic Fibrosis Inhaled Tobramycin Study Group. N Engl J Med. 1999;340:23–30.
151. McArdle JR, Talwalkar JS. Macrolides in cystic fibrosis. Clin Chest Med. 2007;28:347–360.
152. Lee TW, Southern KW. Topical cystic fibrosis transmembrane conductance regulator gene replacement for cystic fibrosis-related lung disease. Cochrane Database Syst Rev. 2012;(10).
153. Liou TG, Adler FR, Cox DR, Cahill BC. Lung transplantation and survival in children with cystic fibrosis. N Engl J Med. 2007;357:2143–2152.
154. Hyde NH, Harrison DM. Intrathecal morphine in a parturient with cystic fibrosis. Anesth Analg. 1986;65:1357–1358.
155. Arkoosh VA, Palmer CM, Yun EM, et al. A randomized, double-masked, multicenter comparison of the safety of continuous intrathecal labor analgesia using a 28-gauge catheter versus continuous epidural labor analgesia. Anesthesiology. 2008;108:286–298.
156. Deshpande S. Epidural analgesia for vaginal delivery in a patient with cystic fibrosis following double lung transplantation. Int J Obstet Anesth. 1998;7:42–45.
157. von Ungern-Sternberg BS, Regli A, Bucher E, et al. The effect of epidural analgesia in labour on maternal respiratory function. Anaesthesia. 2004;59:350–353.
158. von Ungern-Sternberg BS, Regli A, Bucher E, et al. Impact of spinal anaesthesia and obesity on maternal respiratory function during elective Caesarean section. Anaesthesia. 2004;59:743–749.
159. Bose D, Yentis SM, Fauvel NJ. Caesarean section in a parturient with respiratory failure caused by cystic fibrosis. Anaesthesia. 1997;52:578–582.
160. Cameron AJ, Skinner TA. Management of a parturient with respiratory failure secondary to cystic fibrosis. Anaesthesia. 2005;60:77–80.
161. Muammar M, Marshall P, Wyatt H, Skelton V. Caesarean section in a patient with cystic fibrosis. Int J Obstet Anesth. 2005;14:70–73.
162. Catanzarite V, Willms D, Wong D, et al. Acute respiratory distress syndrome in pregnancy and the puerperium: causes, courses, and outcomes. Obstet Gynecol. 2001;97:760–764.
163. Mabie WC, Barton JR, Sibai BM. Adult respiratory distress syndrome in pregnancy. Am J Obstet Gynecol. 1992;167:950–957.
164. Vasquez DN, Estenssoro E, Canales HS, et al. Clinical characteristics and outcomes of obstetric patients requiring ICU admission. Chest. 2007;131:718–724.
165. Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349.
166. Nunn JF. Applied Respiratory Physiology. Butterworth-Heinemann: London; 1987.
167. Lam CM, Wong SF, Leung TN, et al. A case-controlled study comparing clinical course and outcomes of pregnant and non-pregnant women with severe acute respiratory syndrome. BJOG. 2004;111:771–774.
168. Cole DE, Taylor TL, McCullough DM, et al. Acute respiratory distress syndrome in pregnancy. Crit Care Med. 2005;33:S269–S278.
169. Deblieux PM, Summer WR. Acute respiratory failure in pregnancy. Clin Obstet Gynecol. 1996;39:143–152.
170. Cunningham JA, Devine PC, Jelic S. Extracorporeal membrane oxygenation in pregnancy. Obstet Gynecol. 2006;108:792–795.
171. Raphael JH, Bexton MD. Combined high frequency ventilation in the management of respiratory failure in late pregnancy. Anaesthesia. 1993;48:596–598.
172. Huang S, DeSantis ER. Treatment of pulmonary arterial hypertension in pregnancy. Am J Health Syst Pharm. 2007;64:1922–1926.
173. Bugge JF, Tanbo T. Nitric oxide in the treatment of fulminant pulmonary failure in a young pregnant woman with varicella pneumonia. Eur J Anaesthesiol. 2000;17:269–272.
174. Collop NA, Sahn SA. Critical illness in pregnancy: an analysis of 20 patients admitted to a medical intensive care unit. Chest. 1993;103:1548–1552.
175. Tomlinson MW, Caruthers TJ, Whitty JE, Gonik B. Does delivery improve maternal condition in the respiratory-compromised gravida? Obstet Gynecol. 1998;91:108–111.
176. Jenkins TM, Troiano NH, Graves CR, et al. Mechanical ventilation in an obstetric population: characteristics and delivery rates. Am J Obstet Gynecol. 2003;188:549–552.
177. Pacheco LD, Gei AF, VanHook JW, et al. Burns in pregnancy. Obstet Gynecol. 2005;106:1210–1212.
178. Ackerman WE 3rd, Molnar JM, Juneja MM. Beneficial effect of epidural anesthesia on oxygen consumption in a parturient with adult respiratory distress syndrome. South Med J. 1993;86:361–364.