Substance Abuse
Lisa R. Leffert MD
Chapter Outline
Abuse of licit and illicit substances by pregnant women can pose a significant risk to maternal and fetal health. Estimates of the prevalence of substance abuse in pregnant women vary depending on the particular licit or illicit substance, the maternal age group, and the data source.1-3 The rate of illicit drug use during pregnancy as reported in the National Survey on Drug Use and Health from 2009 to 2010 was 16.2% among pregnant women aged 15 to 17 years, 7.4% among pregnant women aged 18 to 25 years, and 1.9% among pregnant women aged 26 to 44 years. During the same time period, among pregnant women aged 15 to 44 years, 4.4% were current illicit drug users. This 4.4% rate was lower than the rate among nonpregnant women (10.9%).1
Depending on the particular substance ingested, pregnant women may experience little to no acute and chronic adverse effects, or alternatively, manifest one or more of the following: (1) cardiovascular, pulmonary, and neurologic complications; (2) obstetric complications (e.g., decreased intrauterine fetal growth, preterm labor, placental abruption, fetal death); and (3) heightened sensitivity to pain.4-12 Anesthesia providers often care for these patients during the provision of analgesia for labor and anesthesia for cesarean delivery as well as during other obstetric procedures before and after delivery. Patients with substance abuse may have altered pain sensitivity, and anesthesia providers are often consulted for help with management of acute postoperative pain.
Drug Detection
Optimal care requires developing a therapeutic bond with these patients and identifying what substances have been ingested.13 The anesthesia provider should ask questions in a respectful and nonjudgmental manner. It is vital to respect patient confidentiality, and it may be necessary to speak to the patient without family or friends present. Self-reporting typically underrepresents the true incidence of substance abuse.14-16 Therefore, health care providers should be familiar with the characteristic signs and symptoms associated with acute and chronic intoxication. The myriad of methods available to test pregnant patients and their infants for the presence of illicit drugs include analysis of urine, blood, saliva, hair, meconium, and umbilical cord tissue. Amniotic fluid and neonatal gastric aspirate have also been analyzed (Tables 54-1 and 54-2).14-19 It is vital to understand which compounds a particular drug test identifies before interpreting the results. Caregivers should be aware that the immunoassays most commonly used in drug testing can have false-positive or false-negative results in the presence of structurally related drugs or additives. Gas chromatography with mass spectrometry ideally should be used to provide confirmation of positive results.16
TABLE 54-1
Drug Detection: Overview
| Specimen | Advantages | Limitations |
| Urine | Detection of diverse group of illicit substances (except volatile alcohols) Specimen and test readily available Short turnaround time (30 minutes at point of care; 2 hours for laboratory specimens) More sensitive test (compared with meconium and hair) for cannabis | Underrepresents most illicit drug use Significant false-positive rate for phencyclidine (PCP) Narrow detection window compared with that for meconium and hair Specimen can more easily be adulterated |
| Blood | Most commonly used for volatile alcohols (can detect other illicit substances) Specimen and test readily available | Invasive Narrow detection window compared with that for urine, meconium, and hair |
| Meconium | Highly sensitive (compared with urine testing) for cocaine and opioids Wide detection window No false-positive results for cocaine | Report may be delayed (days) Low sensitivity and specificity for detecting cannabinoids and amphetamines via immunoassay |
| Hair | Highly sensitive test for detecting cocaine (three times that of urine) and opioids Wide detection window (reflects chronic cumulative use) Samples can be stored at room temperature Samples can be analyzed remote from collection | Multiple hairs required; harvested close to scalp Environmental contamination may cause false-positive result Low sensitivity for detecting tetrahydrocannabinol |
| Umbilical cord blood | Comparable to meconium with more rapid results May reflect a wide window of detection | Specimen not available before delivery |
| Oral fluid | Highly sensitive for methamphetamine and other basic drugs Easy, noninvasive Primarily detects parent compound | If mouth is dry, salivary stimulation may be associated with a decreased drug concentration in oral fluid |
Data from references 3, 14, 15, 17, and 165.
TABLE 54-2
Drug Detection Window in Urine*
| Drug† | Analyte | Detection Window |
| Tobacco | Cotinine Nicotine | 19 h (urine T1/2) 2 h (urine T1/2) |
| Cocaine | Cocaine Benzoylecgonine | 3-6 h IV use: 1-2 days Intranasal use: 2-3 days |
| Amphetamines | Amphetamine Methamphetamine | 1-3 days Smoked: 60 h |
| Methylenedioxymethamphetamine (MDMA, ecstasy) | MDMA | 1-3 days |
| Marijuana (cannabis) | Tetrahydrocannabinol (THC) THCCOOH | Smoked: 10 h Up to 25 days |
| Lysergic acid diethylamide (LSD) | LSD 2-Oxo-3-OH-LSD | 24 h 96 h |
| Heroin | 6-Acetyl morphine Morphine | IV use: 2-4.5 h 19-54 h |
| Benzodiazepines | Flunitrazepam: 7-aminoflunitrazepam | < 72 h Chronic use: 4-6 wk |
| γ-Hydroxybutyric acid (GHB) | Rapidly metabolized to CO2 and H2O | < 12 h |
* Average values based on recent use; precise values may vary according to method of ingestion, assay employed, and duration of use.
† Detection of methadone, buprenorphine, oxycodone, and oxymorphone typically requires an additional screening test.
IV, intravenously; T1/2, half-life.
Data from references 5, 19, 74, 120, and 166.
Licit Drugs
Alcohol
Epidemiology
Alcohol (ethanol) abuse represents a significant problem for pregnant women and their developing fetus. Since 1981, official advisories have warned against the use of alcohol by pregnant women or women considering pregnancy.20 Yet the 2010 National Survey on Drug Use and Health noted that 10.8% of pregnant women 15 to 44 years old reported current alcohol use, 3.7% reported binge drinking, and 1.0% reported heavy drinking. These rates are significantly lower than those of nonpregnant women in the same age brackets (54.7%, 24.6%, and 5.4%, respectively). Survey data indicate that among heavy drinkers, approximately 1.8% also use illicit drugs.1
Pharmacology
Alcohol, usually ingested orally, is absorbed through the gastrointestinal tract, primarily within the small intestine. Ingested alcohol is metabolized by alcohol and acetaldehyde dehydrogenases. This process leads to the production of acetaldehyde and the reduction of nicotinamide adenine dinucleotide (NAD) to its reduced form NADH. The excess reduced form relative to NAD results in metabolic derangements. A small residual amount (2% to 8%) of alcohol is excreted via the lungs, urine, and sweat.21-23
Systemic Effects
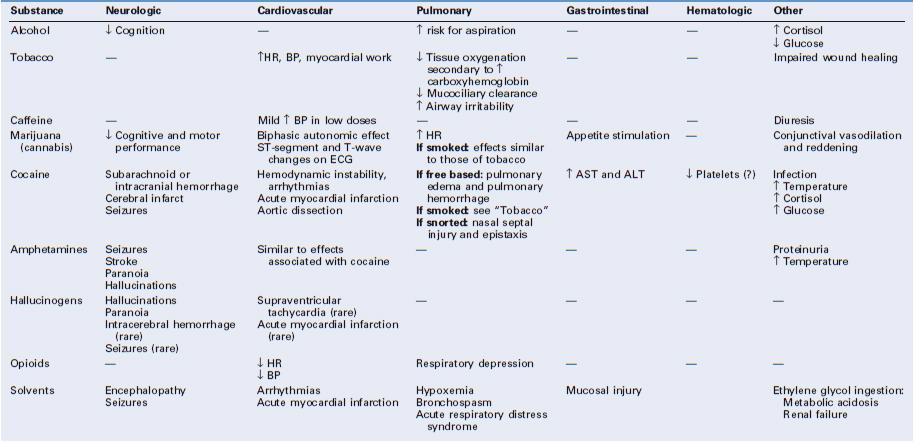
According to legal statutes, the definition of “intoxication” requires a blood alcohol level of at least 80 to 100 mg/dL, although behavioral, cognitive, and psychomotor changes can occur at levels of 20 to 30 mg/dL (e.g., after one to two drinks) (Table 54-3).21-23
TABLE 54-3
Acute Intoxication and Organ Dysfunction
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BP, blood pressure; ECG, electrocardiogram; HR, heart rate; ↑, increase in; ↓, decrease in; ?, questionable.
Alcohol has complex effects on the central nervous system (CNS); it acts as both a depressant and a stimulant through a variety of neurotransmitter pathways.23 When alcohol is consumed in conjunction with barbiturates or benzodiazepines, these effects can be compounded. Endogenous opioids interact with alcohol to “reinforce” further alcohol use; this effect is blunted by opioid antagonists.23
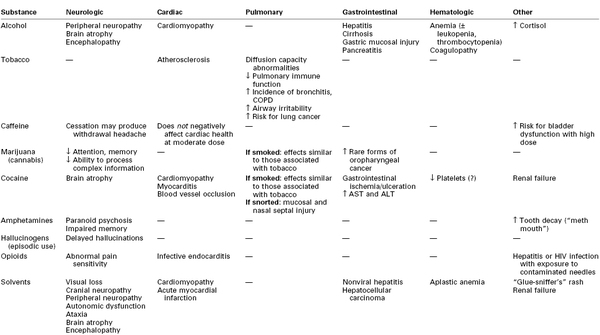
Alcohol and its metabolites (e.g., acetaldehyde) can be directly toxic to brain tissue.21,22 Chronic alcoholism is associated with brain atrophy that results in impairment of memory, abstract problem-solving, verbal learning, and visual-spatial processing.21,22 Additional adverse neurologic effects result from vitamin (e.g., thiamine, vitamin B12) deficiencies.21,22
Heavy alcohol consumption also damages other organs. Over time, hepatic cirrhosis can develop, which, in turn, can lead to encephalopathy, coagulopathy, and esophageal varices (Table 54-4). Gastrointestinal mucosa injury, pancreatitis, and cardiomyopathy may also occur.9,10,22,24
TABLE 54-4
Effects of Chronic Substance Abuse
ALT, alanine aminotransferase; AST, aspartate aminotransferase; COPD, chronic obstructive pulmonary disease; HIV, human immunodeficiency virus; ↑, increase in; ↓, decrease in; ?, questionable.
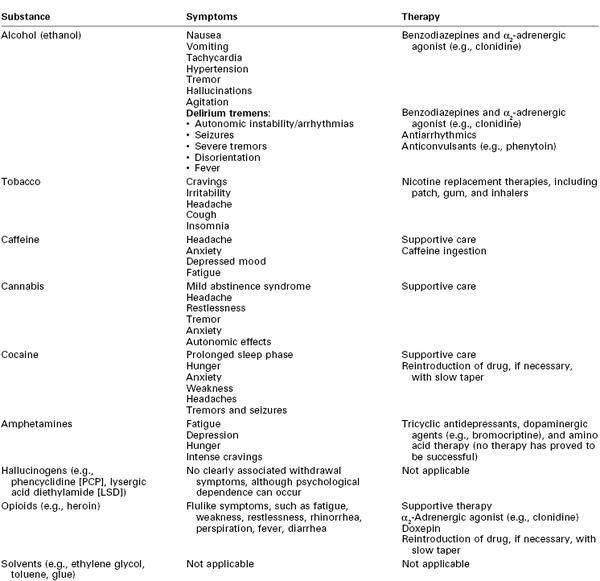
Women who abuse alcohol are at increased risk for depression, suicide, and accidents.7 Symptoms of acute alcohol withdrawal (e.g., nausea, vomiting, tachycardia, hypertension, arrhythmias, tremor, hallucinations, agitation, seizures) usually occur within 6 to 48 hours after cessation of alcohol consumption (Table 54-5).9,10,25 Pharmacologic therapy to minimize the signs and symptoms of alcohol withdrawal includes the use of benzodiazepines and α2-adrenergic agonists (e.g., clonidine).9 The most severe form of withdrawal symptoms, delirium tremens, manifests as agitation, disorientation, hallucinations, and fever combined with autonomic instability. Delirium tremens occurs rarely in pregnant women, but it can lead to maternal and fetal death if untreated.6, 26
Effects on Pregnancy and the Fetus
Intrauterine alcohol exposure is the leading cause of preventable birth defects in the United States.27,28 No safe level of alcohol consumption by pregnant women has been identified.26,27 In contrast to other investigations of high-risk populations, a 2010 population-based cohort study found no association between low or moderate prenatal alcohol exposure before or during pregnancy and the occurrence of birth defects.29 However, the authors recognized that the incidence of birth defects is low and the sample size may not be sufficient to draw definitive conclusions. Fetal alcohol syndrome is defined as the presence of particular neonatal facial features (e.g., small palpebral fissures, flat midface with a short upturned nose, thin upper lip) and significant impairment in neurodevelopment and physical growth.9,10,27,30 Fetal alcohol spectrum disorders refer to the wide range of possible adverse effects of fetal exposure to alcohol.27 The extent to which an individual fetus is affected is related to the characteristics of the exposure, genetic variables, and the intrauterine environment.31
Alcohol exposure is also associated with pregnancy loss. Harlap and Shiono32 reported an increased risk for second-trimester fetal loss in pregnant women who consumed alcohol early in pregnancy (relative risk, 1.98 for those consuming one to two drinks daily and 3.53 for those consuming more than three drinks daily, compared with nondrinkers). Identification of mothers at risk can facilitate treatment and perhaps improve pregnancy outcomes.27
Anesthetic Management
Alcohol-intoxicated parturients are at increased risk for behavioral problems, electrolyte abnormalities, greater gastric acid secretion, and co-intoxication with other substances.9,10,26 Determining whether the patient can protect her airway is of paramount importance because acute intoxication increases the risk for pulmonary aspiration of gastric contents. In addition, these patients may have intravascular volume depletion secondary to vomiting, inadequate oral intake, diuresis, and hypoalbuminemia. Significant alcohol ingestion in the setting of poor oral intake may also manifest as severe hypoglycemia.22,24,33
Neuraxial analgesia or anesthesia can be safely administered for labor or cesarean delivery provided that (1) the patient is cooperative, (2) there is no evidence of coagulopathy (as a result of liver disease), (3) the patient is volume replete, and (4) baseline neurologic deficits (e.g., peripheral neuropathy, cognitive deficits) are assessed and documented.9
If emergency delivery is required and the patient is either uncooperative or too sedated to protect her airway, general anesthesia will be necessary. The patient should receive pharmacologic aspiration prophylaxis (e.g., nonparticulate antacid, histamine-2 (H2)-receptor antagonist, metoclopramide) and should undergo a rapid-sequence induction of general anesthesia.34
Evidence from published reports is inconclusive about predictable differences in anesthetic requirements in patients with acute and chronic alcohol use.35 Acute alcohol intoxication is believed to decrease a patient's anesthetic requirements, in part because of the additive effect of alcohol and other CNS depressants. The notion that chronic alcoholics require more anesthesia than their non–alcohol-using counterparts is based primarily on data from an abstract published by Han in 1969,36 who demonstrated that the mean minimum alveolar concentration (MAC) for halothane in six chronic alcoholic patients who had been heavy drinkers for more than 10 years was significantly greater than that for six healthy adults. Subsequently, Swerdlow et al.37 assessed the response to thiopental in 11 nonpregnant, chronic alcohol users. After eliminating potential confounders such as acute intoxication, withdrawal, polysubstance abuse, and end-organ dysfunction, they found that chronic alcohol intake did not alter thiopental dose requirements, pharmacokinetics, or pharmacodynamics. No large population studies have assessed dose requirements for volatile anesthetic agents or hypnotic agents in patients who chronically abuse alcohol.
Short-term consumption of alcohol inhibits the metabolism of drugs by the liver (through competition for cytochrome P450), which results in higher plasma concentrations of drugs metabolized by the liver. Long-term consumption of alcohol increases the levels of cytochrome P450, resulting in decreased levels of medications such as diazepam and labetalol, and increased levels of toxic metabolites that occur from hepatic degradation of illicit drugs such as cocaine.22 Both pregnancy and liver disease can lead to decreased plasma concentrations of pseudocholinesterase; however, this decrease does not seem to have a clinically significant effect on the degradation of succinylcholine and ester local anesthetics.35
Parturients who regularly consume large amounts of alcohol and undergo general anesthesia for cesarean delivery may be at high risk for awareness under anesthesia.38 The high doses of volatile anesthetics often recommended in nonpregnant, chronic alcohol-using patients can lead to significant uterine atony and potential increased blood loss. Therefore, a balanced anesthetic technique that combines induction with generous doses of the hypnotic agent with succinylcholine (if not otherwise contraindicated), followed by maintenance with a volatile anesthetic agent (limited to 0.5 to 0.8 MAC after delivery to prevent uterine atony), nitrous oxide (up to 67%), and an opioid and a benzodiazepine (for analgesia and amnesia), should be employed. Withholding additional muscle relaxation after induction and adding a brain function monitor if time and circumstance permits39 may help to identify patients who could benefit from additional anesthesia.
Tobacco
Epidemiology
Cigarette smoking is the most common form of substance abuse during pregnancy. As public awareness has grown regarding the hazards of smoking during pregnancy, the prevalence of cigarette smoking during pregnancy has declined over the past 40 years. An estimated 18% of pregnant women reported smoking (in the past month) in 2003,40 compared with 16% in 2010.1 Although the overall smoking rate in pregnant women is lower than in nonpregnant women, the rate among pregnant teens aged 15 to 17 years is actually higher than that among nonpregnant teens (22.7% versus 13.4%).1 Among the approximately 40% of patients who stop smoking when they discover that they are pregnant, 60% to 80% return to smoking by 6 months postpartum.41
Pharmacology
More than 4000 chemicals are found in tobacco, including nicotine, carbon monoxide, and cyanides.23,42 Tobacco is most often smoked, but it can also be chewed or sniffed. Nicotine, the principal drug of abuse in tobacco, acts at peripheral and central nicotinic (acetylcholine) receptors throughout the body to affect the release of catecholamines. Nicotine's effects begin immediately on exposure; it is then rapidly metabolized in the liver and the lungs and excreted by the kidneys. The half-life is typically a few hours. The duration of the acute effects of nicotine is shorter in heavy smokers than in light smokers.23
Carbon monoxide, another constituent of cigarette smoke, interferes with oxygen delivery to the cells by competitively binding to hemoglobin, thereby decreasing the oxygen-binding capacity and shifting the oxyhemoglobin dissociation curve to the left.43 Depending on the extent of smoke inhalation, carbon monoxide may occupy 3% to 15% (or more) of the oxygen-carrying capacity of the blood.44
Systemic Effects
Smoking alters maternal physiology through both the acute pharmacologic actions of tobacco's chemical constituents and their contribution to comorbid disease. Peripherally, nicotine increases sympathetic tone, thereby increasing maternal heart rate, blood pressure, and cardiac work (see Table 54-3).45 Nicotine affects neurotransmitter release in different areas of the brain, producing feelings of alertness, euphoria, and, ultimately, dependence.23,45
Increased production of carboxyhemoglobin is thought to be a major factor in the impaired wound healing observed in smokers.43 Smoking also promotes atherosclerosis. The effects of tobacco smoking on the lungs include changes in the volume and composition of mucus, impaired mucociliary clearance, and an increased incidence of bronchitis and chronic obstructive pulmonary disease (see Table 54-4).45
Tobacco is addictive, and cessation of its use produces withdrawal symptoms, including cravings, irritability, headache, cough, and insomnia (see Table 54-5). Smoking cessation interventions include counseling and therapy, hypnosis, acupuncture, and pharmacologic therapy. The use of nicotine replacement therapy (e.g., nicotine gum, patch, inhaler) has not undergone sufficient evaluation of safety during pregnancy; thus, the American College of Obstetricians and Gynecologists (ACOG) has recommended that nicotine replacement therapy be used only when nonpharmacologic interventions have failed.40 The physiologic benefits of smoking cessation are progressive. Even brief smoke-free intervals can result in a reduction in the carboxyhemoglobin concentration, improved ciliary function, and decreased small airway obstruction.46
Effects on Pregnancy and the Fetus
Nicotine has a low molecular weight and readily crosses the placenta.4 Smoking may result in decreased fetal oxygenation as a result of increased concentrations of carboxyhemoglobin and reduced uteroplacental perfusion. It also leads to decreased uptake of nourishing amino acids by the placenta.42 Smoking is associated with a higher incidence of ectopic pregnancy, spontaneous fetal loss, placental abruption, and sudden infant death syndrome (SIDS).32,40,47 Smoking may protect against the occurrence of preeclampsia (see Chapter 36).48
Smoking adversely affects fetal growth.42,49,50 Low birth weight (LBW) is associated with increased neonatal and infant mortality.42,50 Salihu et al.50 documented that infant mortality was 40% higher in the offspring of women who smoked than in the offspring of nonsmoking women; this risk increased in a dose-dependent fashion for the infants who were small for gestational age (SGA). Smoking cessation before the third trimester ameliorates the smoking-associated reduction in birth weight.40
Intrauterine exposure to smoking (tobacco and/or marijuana) may also have long-term effects. Fried et al.51 examined the cognitive performance in 145 adolescents (13 to 16 years of age) from a low-risk, middle-class population. After controlling data for socioeconomic status and polysubstance abuse, they found that measures of overall intelligence and auditory memory were negatively related to prenatal maternal smoking in a dose-related fashion.
Anesthetic Management
Smoking is a risk factor for several perioperative complications, including respiratory sequelae and impaired wound healing.43,45 Smoking results in increased airway secretions, decreased ciliary motility, and impaired gas exchange.45 Smoking is also associated with an increase in nonspecific airway reactivity, and tracheal intubation may provoke bronchospasm. Wilkes et al.52 observed that coughing after exposure to desflurane was more pronounced in smokers. By contrast, Kim and Bishop53 observed that although most patients coughed as they awakened from general anesthesia maintained with isoflurane, smokers were not more likely to cough than nonsmokers.
Six months of abstinence may be required before the function of alveolar macrophages and pulmonary cytokines during and after general anesthesia in former smokers is similar to that of nonsmokers.46 Administration of neuraxial anesthesia avoids airway manipulation and is typically preferred in parturients who smoke.
Caffeine
Epidemiology
On a daily basis, 80% to 98% of women drink caffeine-containing beverages.9,54 The prevalence of consumption of caffeine-containing beverages during pregnancy is unknown.
Pharmacology
Caffeine (1,3,7-trimethylxanthine) is a naturally occurring alkaloid found in coffee, tea, cocoa, and some soft drinks and medicines.9,54,55 The primary sources of caffeine in the adult diet are coffee (56 to 100 mg/100 mL if brewed) and tea (20 to 73 mg/100 mL). Caffeine is readily absorbed through the gastrointestinal tract, and maximum blood concentrations of caffeine are attained 1 to 1.5 hours after ingestion. Caffeine undergoes hepatic metabolism and is then excreted in the urine.54,55 The elimination half-life of caffeine is 3 to 7 hours. In pregnancy, the half-life increases from 4 hours in the first trimester to 18 hours by the third trimester. Caffeine crosses the placenta and can also be found in breast milk.54,56 Habitual use of caffeine at levels greater than 500 to 600 mg/day is defined as abuse.54,56
The half-life of caffeine in the neonate is prolonged in comparison with that in children and nonpregnant women.54
Systemic Effects
Caffeine acts as an antagonist at the adenosine receptor. In the absence of the inhibitory effects of adenosine, the neurotransmitters norepinephrine, dopamine, and serotonin are released in increased concentrations.54,56 Systemic effects of caffeine include CNS stimulation, changes in blood pressure and metabolic rate, and diuresis (see Table 54-3).55 The side effects commonly attributed to caffeine vary among individuals, in part related to the doses ingested and the degree of chronic use. Studies of the effects of caffeine on alertness, vigilance, mood, and memory have produced inconsistent results.54
Moderate caffeine intake (≤ 400 mg/day or ≤ 4 cups of coffee/day) does not seem to negatively affect cardiovascular health in most people. Although some people who ingest caffeine report tachycardia and palpitations, doses lower than 450 mg/day do not appear to increase significant cardiac arrhythmias in either healthy patients or in those with ischemia or ventricular ectopy. Caffeine doses as low as 250 mg have been reported to have a hypertensive effect after acute ingestion (an increase in systolic blood pressure of 5 to 15 mm Hg and an increase in diastolic blood pressure of 5 to 10 mm Hg), particularly in caffeine-naive individuals; however, epidemiologic studies have produced inconsistent results. In general, people who ingest caffeine on a long-term and frequent basis are less likely than occasional users to have difficulty sleeping.54
Caffeine appears to affect bladder function in women. Moderate caffeine intake may exacerbate preexisting bladder symptoms, and excessive intake (> 400 mg/day) increases the risk for bladder dysfunction.54
Evidence suggests that caffeine is not a human carcinogen.55 The lethal dose of caffeine in humans has been estimated to be 10 g; however, only a few such cases have been reported.54
Caffeine withdrawal is associated with headache, anxiety, depressed mood, and fatigue (see Table 54-5). Typically, symptoms begin 12 to 24 hours after cessation of use, peak at 20 to 48 hours, and last up to 7 days. The severity and likelihood of symptoms are not predictable.54
Effects on Pregnancy and the Fetus
Caffeine readily crosses the placenta. Whereas animal studies have shown that very high doses can have a teratogenic effect, moderate doses do not appear to result in teratogenesis in humans.54,57,58 There is some evidence that caffeine at doses greater than 300 mg/day may result in fetal growth restriction (also known as intrauterine growth restriction) and decreased birth weight, particularly in women who also smoke or drink significant amounts of alcohol.54 Although previous studies56 have not shown an association between low or moderate caffeine intake (< 300 mg/day) and greater risk for spontaneous abortion or preterm delivery, Weng et al.59 found that caffeine consumption greater than 200 mg/day was associated with an increased risk for miscarriage, particularly among pregnant women who did not have a previous history of miscarriage. Moderate intake of caffeine in lactating women does not adversely affect postnatal development.54,55
Two studies have suggested that moderate caffeine intake during pregnancy may actually have beneficial effects on the mother and the fetus. Adeney et al.60 found that women who consumed caffeinated coffee before conception had a significantly lower risk for development of gestational diabetes mellitus than women who did not consume coffee. No reduction in risk was associated with ingestion of tea or soda. Back et al.61 showed that neonatal mice exposed to caffeine and subsequently subjected to hypoxia seemed to have less neurologic injury (i.e., less ventriculomegaly, less disruption in myelination) than non–caffeine-exposed mice.
Anesthetic Management
Caffeine may enhance the side effects of beta-adrenergic receptor agonists such as epinephrine and albuterol. Caffeine may also increase the risk for a hypertensive crisis in patients taking monoamine oxidase (MAO) inhibitors. Caffeine slows the elimination of theophylline and acetaminophen, resulting in higher drug concentrations in the blood. In contrast, serum concentrations of lithium may be decreased secondary to caffeine-enhanced elimination.55
Perhaps of greatest significance to the anesthesia provider is the potential for caffeine withdrawal headache in parturients who abruptly decrease their usual caffeine intake during labor and delivery. Caffeine withdrawal should be considered in a postpartum patient who has a nonspecific, nonpositional headache without associated lateralizing neurologic findings (see Chapter 31). Evidence for the efficacy of caffeine in the treatment of post–dural puncture headache is scant (see Chapter 31).62 Resumption of caffeine ingestion should be considered for treatment of caffeine withdrawal headache.
Illicit Drugs
Marijuana (Cannabis)
Use of marijuana for medical and recreational purposes can be traced back thousands of years.63 In 2010, approximately 2.4 million people in the United States used marijuana for the first time.1 Marijuana was the illicit drug with the largest number of new users aged 12 or older. Estimates of marijuana use in the pregnant population vary from 6% to 7%.17,64
Pharmacology
Marijuana contains more than 400 compounds, including 60 cannabinoids. Most of the psychotropic effects are caused by 9-tetrahydrocannabinol (THC).63,65 Noncannabinoid constituents of marijuana are similar to those in tobacco without the nicotine. Inhaled THC is absorbed through the lungs and reaches the brain within minutes. Oral ingestion of marijuana results in blood THC concentrations that are 25% to 30% of those obtained by smoking, but with a delayed onset of up to 2 hours. Cannabinoids are highly lipid-soluble compounds that are sequestered in fatty tissues and are gradually released into other tissues. Thus, a single ingestion can have an elimination half-life of up to 7 days; complete elimination of the inactive metabolite 11-nor-9-carboxy-delta-9-tetrahydrocannabinol (THCCOOH) takes as long as 25 to 30 days. Metabolism occurs in the liver, with excretion in the urine. Measured concentrations of THC and other cannabinoid metabolites in the blood and urine correlate poorly with the degree of intoxication.65
Systemic Effects
Cannabis interacts with specific cannabinoid receptors in the brain and peripheral nerves. Psychoactive effects include anxiolysis, analgesia, appetite stimulation, euphoria, and, sometimes, dysphoria (see Table 54-3).65 Marijuana intoxication impairs cognitive and psychomotor function. Impairment of memory, attention, and the ability to process complex information can occur with long-time heavy use (see Table 54-4). It is unclear whether these effects are reversible.66
Acute intoxication with marijuana appears to have a biphasic effect on the autonomic nervous system; low doses cause tachycardia and higher cardiac output due to increased sympathetic and decreased parasympathetic tone. In contrast, high doses produce sympathetic inhibition and parasympathetic stimulation, resulting in bradycardia and hypotension.5,63 Although ventricular ectopy can occur, life-threatening arrhythmias in patients without preexisting cardiac disease are rare and autonomic disturbances are generally well tolerated. Reversible ST-segment and T-wave abnormalities may occur, perhaps as a result of the higher heart rate associated with marijuana use.5
As with tobacco, the respiratory consequences of smoking marijuana include mucociliary dysfunction, increased susceptibility to bronchitis, and chronic obstructive pulmonary disease (see Table 54-4). Acute intoxication may cause conjunctival vasodilation and visible reddening of the eyes. Although high doses of marijuana can cause hallucinations and psychosis, fatal overdose has not been documented.6,65
Withdrawal from long-time marijuana use may produce a mild abstinence syndrome that includes headache, restlessness, tremor, anxiety, and autonomic effects, similar to those of withdrawal from benzodiazepines and hypnotic drugs (see Table 54-5).5,66
Effects on Pregnancy and the Fetus
It is difficult to ascertain specific effects of marijuana on pregnancy, because women who use marijuana often engage in polysubstance abuse. In a prospective study of more than 3800 pregnancies, Hatch and Bracken66 showed that regular marijuana use by white women was associated with an increased risk for delivering an LBW and/or SGA infant, but occasional use was not. The risk for these adverse outcomes in nonwhite marijuana-using women was not elevated above their baseline level of increased risk. In a prospective multicenter study of more than 7000 multiethnic pregnant women, marijuana use was not associated with increased risk for LBW, preterm delivery, or placental abruption.67
Intrauterine exposure to marijuana has not been proven to be teratogenic, but some research suggests that it is associated with subtle postnatal neurologic derangements. Fried et al.51 did not find an association between maternal marijuana use and overall intelligence quotient (IQ) and verbal memory in exposed offspring; however, certain aspects of cognition, such as memory at 4 years of age, attention at 6 years, attention and visually related cognition at 9 to 12 years, and stability of attention at 13 to 16 years, were negatively associated with prenatal marijuana exposure.
Anesthetic Management
Neuraxial analgesia is typically preferred during labor and delivery and can be safely performed in patients who use marijuana, in the absence of other contraindications. Drug-related changes in heart rate and blood pressure are usually well tolerated in otherwise healthy patients who are acutely intoxicated. However, the administration of atropine, pancuronium, and ketamine can exacerbate existing tachycardia.63
Long-time marijuana smokers are at risk for many of the same respiratory complications during and after general anesthesia as are tobacco cigarette smokers; these patients may have increased airway secretions, impaired mucociliary clearance, and potentially increased airway reactivity.45,65 Persistent postextubation laryngospasm in a patient with a history of heavy marijuana smoking has been reported.68
Acute intoxication with marijuana can have additive effects with those of sedative agents and volatile anesthetic agents, including myocardial depression24,63; careful titration to clinical effect is recommended.
The effect of marijuana on pain perception has been explored. Wallace et al.69 presented a model of human experimental pain in which smoked marijuana had a differential effect on pain scores; a low dose had no effect, a medium dose reduced pain, and a high dose significantly increased pain. The clinical implications of these findings are unclear.
Cocaine
The use of cocaine, extracted from the leaf of the South American Erythroxylum coca bush, can be dated as far back as 600 ad.70 Cocaine was introduced into clinical practice as a local anesthetic in the 1880s. During this period, Sigmund Freud also experimented with cocaine's ability to combat hunger and fatigue and opiate addiction.70,71 Cocaine has both vasoconstrictive and local anesthetic properties as a result of its ability to block sodium channels during depolarization.
Epidemiology
The prevalence of cocaine use during pregnancy is difficult to estimate. Studies using a variety of detection techniques have reported rates that range from 1.8% to greater than 10% depending on the method and the population studied.70 Survey data demonstrate that cocaine abuse in pregnancy is distributed broadly across ethnic and socioeconomic groups. In addition, an estimated 60% to 90% of pregnant cocaine users engage in polysubstance abuse, including the use of tobacco.72,73 As with other illicit substances, the self-reporting of cocaine use results in identification of fewer drug users than urine testing and use of other biologic markers.3 There is an association between cocaine use and an increased risk for sexually transmitted diseases, preterm labor, and failure to obtain prenatal care.3
Pharmacology
Cocaine (benzoylmethylecgonine) is an ester of benzoic acid and the base ecgonine, which is the parent compound of atropine and scopolamine.71,74 Cocaine is consumed in a variety of forms. When dissolved in hydrochloric acid to form a water-soluble powder (cocaine hydrochloride), it can be chewed, administered intravenously (“mainlined”), or taken intranasally (“snorted”). Intrarectal and intravaginal use has also been reported.75 When cocaine is processed with either sodium bicarbonate (“crack”) or ammonia and ether (“free base”), the resulting cocaine alkaloid can be smoked.71,74,76 The amount (dose) and duration of exposure are more important determinants of the effects of cocaine than is the chemical formulation. Smoked cocaine (“crack” or “free base”) is rapidly absorbed through the lungs and reaches the brain in 6 to 8 seconds, intravenous cocaine reaches the brain in 12 to 16 seconds, and snorted cocaine reaches the brain in 3 to 5 minutes.77 The typical half-life of cocaine is 30 to 90 minutes, although its effects can last as long as 6 hours.71
Cocaine is metabolized to ecgonine esters and benzoylecgonine (biologically inactive) by plasma and hepatic cholinesterases and to norcocaine (biologically active) by nonenzymatic hydrolysis. Only small amounts of cocaine are excreted unchanged in the urine. In the presence of alcohol, cocaine is transesterified to cocaethylene, which has a longer half-life and greater physiologic effects than cocaine.76,77
Systemic Effects
Cocaine has complex actions on the central and peripheral nervous systems and on nerve conduction. The powerful sympathomimetic effects of cocaine are due to the drug's inhibition of the reuptake of norepinephrine, dopamine, and serotonin, which allows these neurotransmitters to accumulate at the synaptic clefts and produce sustained stimulation.70,76-78 This process can result in as much as a fivefold increase in circulating concentrations of catecholamines,5 which, in turn, may lead to feelings of euphoria, increased energy, and decreased fear.70,77 Repetitive use of cocaine eventually leads to depletion of neurotransmitter stores, up-regulation of receptors, and a higher dose requirement to achieve the desired euphoric effects.71
The peripheral nervous system effects of cocaine and its derivatives result from binding to tissue receptors involved in monoamine reuptake, which results in hypertension and/or labile blood pressure and tachycardia. Cocaine produces widespread small and large vessel occlusion through vasospasm, thrombosis, and endothelial injury, which may result in significant end-organ damage.71
Cocaine has profound effects on the cardiovascular system, and pregnancy appears to enhance them. Acute administration of cocaine increases peripheral vascular resistance, cardiac contractility, and myocardial oxygen demand (see Table 54-3).71,79 Coronary vasoconstriction also occurs; a greater effect occurs in diseased vessel segments than in nondiseased segments.76 Studies in vitro have indicated that cocaine can have a procoagulant effect in small and large vessels.5 This may lead to thrombus formation and coronary plaque rupture in the setting of cocaine-induced hypertension.71,80
Cocaine-induced chest pain is a common complaint among young people presenting to the emergency department.81 Mittleman et al.80 found that cocaine use was associated with a significant, abrupt, and transient increase in the risk for acute myocardial infarction in patients who were otherwise at low risk.82 The occurrence of cocaine-induced electrocardiographic changes is fairly common and is not necessarily associated with true ischemia.76,83 If true ischemia is suspected, treatment with supplemental oxygen, aspirin, vasodilators, with or without reperfusion therapy,81 as well as measurement of troponin I levels,82 is indicated. Although cocaine abusers who suffer a myocardial infarction have fewer postinfarction sequelae than the general population, the incidence of major cardiovascular complications is not trivial; 5% to 7% have congestive heart failure, 4% to 17% have ventricular arrhythmias, and up to 2% die.76
Prompt recognition of acute cocaine-induced cardiovascular toxicity facilitates management. However, not all cocaine-induced hypertension in pregnant women requires intervention. If pharmacotherapy is used, it is important to understand the potential undesired consequences. Beta-adrenergic receptor blockade may result in unopposed alpha-adrenergic receptor–mediated vasoconstriction that can lead to coronary artery vasoconstriction and myocardial failure. Labetalol, which is both an alpha- and a beta-adrenergic receptor antagonist, may be preferred, although it does not ameliorate cocaine-induced coronary artery vasoconstriction. Direct vasodilators (e.g., nitrates, hydralazine) can be used but may cause further tachycardia.5,76 Hydralazine treated the hypertension in cocaine-intoxicated pregnant ewes but did not restore uterine blood flow.84 Calcium entry–blocking agents can potentiate the toxic effects of cocaine.5 Sedatives (e.g., benzodiazepines) or magnesium sulfate may ameliorate cocaine's cardiovascular effects.5,76
Other acute cardiovascular effects of cocaine include QT prolongation, bradycardia, and arrhythmias, including supraventricular or ventricular tachycardia and ventricular fibrillation.71 Severe bradycardia can be treated with atropine or electrical pacing. If supraventricular tachycardia (SVT) occurs and is well tolerated, close observation, vagal maneuvers, and/or the use of adenosine are warranted. In the unstable patient with SVT, direct current (DC) cardioversion may be required.85,86
The use of lidocaine as an antiarrhythmic agent appears to be acceptable in patients who have used cocaine. In a retrospective multicenter study, Shih et al.87 found that the use of lidocaine in nonpregnant patients with cocaine-induced myocardial infarction was not associated with significant cardiovascular or CNS toxicity. Although amiodarone therapy for maternal and fetal arrhythmias has been described as having only minor adverse effects in some patients, there are also reports of associated fetal hypothyroidism and fetal growth restriction.85,88,89 Thus, the use of amiodarone in pregnant women is reserved for malignant arrhythmias that are refractory to other therapies.
Long-time cocaine abuse can cause left ventricular hypertrophy and dilated cardiomyopathy with accompanying systolic dysfunction (see Table 54-4).71,76 Aortic dissection has also been reported.76 Intravenous use of cocaine and other injectable drugs increases the risk for development of infective endocarditis. Noncardiogenic pulmonary edema, pulmonary hypertension, and right-sided heart failure can also occur in the setting of cocaine abuse.5,71
The neurologic complications of cocaine may be transient or permanent. Morbidity and mortality may result from subarachnoid hemorrhage, intracerebral hemorrhage, cerebral vasculitis, and/or transient ischemic attacks.8,90,91 Many cocaine-abusing patients in whom cerebral infarct(s) and hemorrhage developed had additional risk factors for stroke, including hypertension, alcohol abuse, and smoking. Cocaine-induced seizures, if self-limited, are typically treated with supportive care and benzodiazepines.90
Respiratory complications occur in 25% of cocaine users. As with tobacco, smoking cocaine can have profound respiratory effects, which include bronchospasm, chronic cough, and diffusion capacity abnormalities.75,77 Cocaine-abusing parturients are at increased risk for peripartum wheezing.8 Inhaled cocaine vapor can produce thermal airway burns. “Snorting” cocaine can lead to epistaxis, oral ulcers, and nasal septal injury. The intense pulmonary and bronchial arterial vasoconstriction produced by cocaine can cause interstitial and alveolar hemorrhage. Pneumothorax, pneumomediastinum, and pneumopericardium have also been reported.71
Cocaine ingestion can result in serious gastrointestinal complications, such as ischemia, ulceration, and perforation.77 In addition, cocaine's anticholinergic effects include delayed gastric emptying and an increased risk for aspiration.71 Although some cocaine users have abnormal liver enzyme levels, cocaine is not clearly hepatotoxic.77
Hematologic consequences of cocaine exposure during pregnancy may include thrombocytopenia.63,92,93 Cocaine-induced thrombocytopenia has a clinical course similar to that of idiopathic thrombocytopenic purpura, with platelet counts normalizing after termination of drug exposure, therapy with corticosteroids, and, in one published case, splenectomy.93 Kain et al.92 examined the prevalence of cocaine-associated thrombocytopenia in an inner-city obstetric hospital; the rate of thrombocytopenia was higher in the cocaine group than in the drug-free group (6.7% versus 1.5%, respectively), even when human immunodeficiency virus (HIV)–positive patients were excluded. However, Gershon et al.94 compared platelet counts in a group of more than 7000 pregnant patients, 671 of whom tested positive for cocaine; only 2.5% of the cocaine-positive women had a platelet count lower than 140,000/mm3, compared with 4.7% in the cocaine-negative group.
Renal failure can result from cocaine abuse on the basis of rhabdomyolysis, renal infarction, and impaired immunologic functions.71 Cocaine-abusing patients have a higher prevalence of syphilis, HIV infection, and other infectious diseases compared with non–cocaine-using patients, even after controlling for intravenous drug abuse.77,95 Studies of the endocrine system in gravid ewes have shown that cocaine exposure results in increases in maternal adrenocorticotropic hormone and cortisol as well as maternal and fetal plasma glucose and lactate.96,97
Cocaine also impairs cutaneous vasodilation and sweating. The lethal effects of cocaine are related, in part, to the drug's tendency to produce hyperthermia, particularly in hot weather.98
Cocaine use has been associated with sudden death from a variety of the above mentioned factors, including cardiac arrhythmias, respiratory arrest, status epilepticus, and impaired thermoregulation.
Cocaine withdrawal can be difficult to recognize because its signs and symptoms are nonspecific; they consist of a prolonged sleep phase followed by hunger, anxiety, weakness, headaches, tremors, and seizures (see Table 54-5). Recommended therapy involves supportive care and reintroduction of the drug, if necessary, followed by a slow taper of the dose over days to weeks.24
Effects on Pregnancy and the Fetus
Pregnant women metabolize cocaine to norcocaine to a greater extent than their nonpregnant counterparts, exposing both mother and fetus to this more potent metabolite.70,74 Cocaine has a low molecular weight and high lipophilicity, and it is mostly un-ionized at physiologic pH; thus, it readily crosses the placenta.8,70
Woods et al.99 demonstrated that cocaine increases both heart rate and myocardial oxygen consumption and decreases cardiac output to a greater extent in gravid ewes than in nonpregnant ewes. Cocaine also increases maternal blood pressure and decreases uterine blood flow in gravid ewes.100
In a 2011 meta-analysis, Gouin et al.101 found that cocaine use during pregnancy was associated with significantly higher risk of preterm birth (odds ratio [OR], 3.38), SGA infants (OR, 3.23), and LBW infants (OR, 3.66). Obstetric complications associated with maternal cocaine use include a higher incidence of placental abruption and preterm labor; the latter occurs in 17% to 29% of women who use cocaine.102 Acute cocaine toxicity can mimic preeclampsia or eclampsia when pregnant women present with hypertension, headache, blurred vision, and/or seizures. In one case series, cocaine-induced changes were distinguished through a positive urine test result for cocaine, the presence of normal laboratory measurements, and rapid resolution of symptoms without delivery.103
The impact of maternal use of cocaine (particularly “crack”) on the fetus has been the subject of intense legal, political, and scientific debate since the 1980s.104 Initial animal data and retrospective human studies have suggested an increased risk for major congenital anomalies such as genitourinary and abdominal wall defects in fetuses exposed to cocaine. However, these reports are confounded by concurrent use of other drugs and low statistical power.105 Subsequent studies found no significant difference in type or number of congenital anomalies between infants who had and those who had not been exposed to cocaine in utero, after accounting for confounding variables.106,107
Frank et al.108 reviewed studies published between 1984 and 2000 to assess the possible relationships between maternal cocaine use during pregnancy and childhood outcome. After controlling for possible confounding factors, they found no consistent negative association between intrauterine cocaine exposure and physical growth, developmental test scores within the first 6 years of life, or the presence of expressive or receptive language skills. They observed less optimal motor performance up to 7 months of age, but after this age, these effects did not appear to persist. They also indicated that there were insufficient data to comment on cocaine's effects on developmental scores in infants born preterm.
Subsequently, Mayes et al.109 examined the trajectories of motor and mental development between the ages of 3 and 36 months in an impoverished high-risk population. These investigators found (1) a general decline (compared with age-adjusted equivalents) in motor performance for these high-risk children and a trend toward a greater decrease in performance in cocaine-exposed children; (2) a decline in performance on mental tasks until 24 months, with a similar trajectory of decline for the cocaine-exposed and non–cocaine-exposed cohorts; and (3) evidence of lower mental performance in cocaine-exposed children compared with non–cocaine-exposed children at all assessment ages.
Bauer et al.106 found that infectious complications, such as hepatitis, syphilis, and, to a lesser extent, HIV infection, were more common in infants of cocaine-abusing mothers. Bae and Zhang110 used a rat model to test prenatal cocaine exposure. Their results showed abnormal apoptosis and myocyte hypertrophy in the postnatal heart, which they speculated might result in greater susceptibility to myocardial ischemia and reperfusion injury.
Anesthetic Management
Cocaine-abusing patients are at risk for acute and chronic multiorgan system dysfunction95 and the need for urgent cesarean delivery. During labor, early administration of neuraxial anesthesia should be encouraged provided that the patient is cooperative and has a platelet count above the threshold of concern for the anesthesia provider.73 The use of neuraxial anesthesia can reduce levels of circulating catecholamines and thereby may mitigate the systemic effects of cocaine.8 Existing epidural analgesia facilitates the extension to anesthesia for emergency cesarean delivery.
In one study, cocaine users had a greater incidence of hypotension during administration of epidural anesthesia for cesarean delivery; however, there was no difference between cocaine users and nonusers in the incidence of hypotension during spinal anesthesia.8 When it occurs, treatment of hypotension should include volume resuscitation, and, if needed, careful titration of vasopressors. Depending on the level of circulating catecholamines, cocaine-intoxicated patients can be either more or less responsive to ephedrine; thus, phenylephrine may be a better choice for treatment of hypotension.11,63,73
When an ester local anesthetic or succinylcholine is administered to a patient who has ingested cocaine, the medication might compete with cocaine for available plasma cholinesterase.8,74 Kain et al.8 described a prolonged response to succinylcholine in a parturient who had abused cocaine chronically and had a normal dibucaine number but a low level of pseudocholinesterase.
Changes in µ- and κ-opioid receptors and altered baseline endorphin levels may result in an increased perception of pain in cocaine-abusing patients despite the presence of an apparently satisfactory level of neuraxial anesthesia.63 Ross et al.111 observed a reduction in duration of intrathecal sufentanil analgesia during labor in cocaine-abusing women, although the quality of analgesia was not diminished.
Cocaine-abusing patients who receive general anesthesia are at greater risk for hypertension and tachycardia during and after laryngoscopy and tracheal intubation.8,9 If general anesthesia is required, premedication with a benzodiazepine or an opioid may help attenuate the acute physiologic effects of cocaine.
The anticholinergic effects of cocaine can delay gastric emptying and may also increase the risk for aspiration; pharmacologic prophylaxis and a rapid-sequence induction of general anesthesia are indicated. Ketamine and etomidate should be avoided because ketamine may stimulate the CNS and etomidate may precipitate myoclonus or hyperreflexia.63 Induction of general anesthesia with propofol is commonly used in clinical practice. Dexmedetomidine, an α2-adrenoreceptor agonist, has been shown in animal studies to delay the onset of cocaine-induced seizures and may be useful in selected patients.112 Despite the theoretical risk for altered metabolism of succinylcholine, a standard intubating dose should be administered.73 Early studies in dogs demonstrated that acute cocaine intoxication was associated with a dose-dependent increase in halothane dose requirements.113 Whether this extends to other volatile agents has not been studied.
Cocaine can cause hyperthermia in both laboratory animals and humans71,98; core temperature should be monitored in cocaine-abusing patients. However, given the propensity for nasal septal defects in such patients, temperature probes (and other tubes and monitors) should not be inserted intranasally. Measures that provide active warming should be used only if needed.
Amphetamines and “Club” Drugs
Because of their high potential for abuse, amphetamines have been categorized by the U.S. Drug Enforcement Agency (DEA) as Schedule II stimulants since 1971.64 Amphetamines have historically been prescribed as components of nasal decongestants, bronchodilators, weight loss drugs, and therapies for narcolepsy and attention deficit hyperactivity disorder.114
Methamphetamine (“ice”) has become the most common illicit substance requiring medical treatment during pregnancy.115 The availability of this compound is facilitated by its production in low-cost, home laboratories within and outside the United States.114 It and MDMA (3,4-methylenedioxymethamphetamine) (“ecstasy”) are thought to be the most widely abused amphetamines.64
Epidemiology
The number of new users of methamphetamine among persons aged 12 years or older was 105,000 in 2010, similar to the 2009 estimate (154,000) but lower than the 2002 to 2007 estimates (ranging from 157,000 to 318,000).1 In a 2010 sample population between 12 and 49 years of age, the average age of new methamphetamine users was 18.8 years, which was not significantly different from corresponding 2002 to 2009 estimates.1
Arria et al.64 observed that 5.2% of high-risk pregnant women had used methamphetamine at some time during pregnancy. Polydrug use in women who use methamphetamine and MDMA appears to be common.64,116 According to analysis of national administrative data, the hospitalization ratio for amphetamine abuse has doubled, whereas that for cocaine abuse has decreased 44%.117
Pharmacology
Amphetamines (and related compounds) are amines that exist as either salts of various acids or free bases. Used illicitly, they can be ingested orally, inhaled, or, less commonly, injected, resulting in significant CNS penetration.23 The plasma half-life ranges from 5 to 30 hours. Metabolism is variable; up to 30% of the parent compound can be found in the urine. Detection of these compounds and their metabolites in the urine is possible up to several days after ingestion.5
Methamphetamine (“speed” or “crystal meth”) is a congener of amphetamine that contains a methyl radical.64 This white, odorless, bitter-tasting powder can be smoked, snorted, ingested orally, or administered rectally.116 Methamphetamine is more potent than amphetamine and has a longer half-life; 50% of the drug is cleared in 12 hours. When it is smoked or injected intravenously, the “flash” from this drug is intense and of short duration. Snorting produces euphoria within 5 minutes, and oral ingestion does so within 20 minutes.114
The amphetamine analogue, MDMA, or ecstasy, has been steadily growing in popularity, particularly in young women.118 The methylenedioxy group that is attached to the aromatic ring of the amphetamine molecule confers some of the pharmacologic hallucinogenic effects.119 The effects of MDMA typically begin approximately 20 minutes after ingestion and last approximately 6 hours; large doses have effects for up to 2 days. MDMA is metabolized by the liver and excreted by the kidneys.5
The “club” drug, γ-hydroxybutyric acid (GHB), derived from gamma-aminobutyric acid (GABA), readily enters the brain when ingested, producing anxiolytic, euphoric, and sedative effects.120 Severe cardiorespiratory depression, coma, and seizures can occur at high doses. Overdose is common, owing in part to the variable individual responses to the drug.120,121 Chronic use may be associated with a down-regulation of GABA receptors, and withdrawal manifests as insomnia, tachycardia, hypertension, and nausea/vomiting.120
The newest designer drug of the phenethylamine class is 3,4-methylenedioxypyrovalerone (MDPV), also known as “bath salts.” Typically sold in crystal or powder form that can be snorted, smoked, or injected, these substances act as norepinephrine and dopamine reuptake inhibitors.122 Until recently, “bath salts” could be purchased legally over the Internet, at some convenience stores, or in “head shops,” but its active ingredients were banned by the DEA after several deaths were reported.123
Systemic Effects
Acute amphetamine ingestion leads to indirect sympathetic activation through the release of norepinephrine, dopamine, and serotonin from adrenergic nerve terminals (see Table 54-3).5,124 The physiologic effects of amphetamines are similar to those of cocaine and other stimulants, with two important differences: (1) amphetamines and their derivatives lack local anesthetic properties, and (2) amphetamine can inhibit monoamine oxidase activity, leading to decreased degradation of catecholamines.5 Amphetamine-induced seizures can masquerade as eclampsia in parturients who have ingested the drug in the peripartum period.125
Long-term use of high doses of amphetamines can have a number of adverse maternal effects, including damage to the cardiovascular and neurologic systems, and behavioral changes such as hostility, violence, hallucinations, and paranoid psychosis (see Table 54-4).116
The cardiovascular effects of amphetamines and their derivatives are similar to those of cocaine.5 Methamphetamine has a much longer duration of action than cocaine, because a smaller fraction of the former drug is metabolized.114 Drug-induced effects include vasoconstriction, tachycardia, and labile blood pressure.24 Patients are typically hypertensive, although catecholamine depletion over time can result in hypotension. Arrhythmias, myocardial ischemia, endothelial damage, and acceleration of atherosclerosis can also occur. Recommendations for the management of cardiovascular complications are similar to those for cocaine toxicity.5
The pleasurable effects of methamphetamine and the deleterious neurologic sequelae are believed to be the result of high levels of dopamine in the brain. In addition to positive feelings, patients who have taken methamphetamine may experience anxiety, mood disturbances, paranoia, and hallucinations. Severe intracranial hypertension6 and hemorrhagic stroke91 has been reported in the setting of acute use. Long-time use has been associated with impairment of motor function and verbal learning as well as with significant changes in the areas of the brain associated with memory and emotion (see Table 54-4).114 Volkow et al.126 observed that prolonged abstinence (12 to 17 months) resulted in significant recovery of brain dopamine transporters, although performance on neuropsychological tests did not improve to the same extent. In addition, psychotic features of long-time amphetamine use may be precipitated by stress in former users after months or even years of abstinence.114 Ecstasy users are prone to drink excessive amounts of water, predisposing them to hyponatremia and the resulting CNS effects.63
Psychostimulant withdrawal causes fatigue, depression, hunger, and intense cravings (see Table 54-5). Pharmacologic therapy for stimulant withdrawal (e.g., tricyclic antidepressants, dopaminergic agents [e.g., bromocriptine], amino acid replacement therapy) has not been particularly successful.127 In the setting of methamphetamine overdose, the ensuing seizures, severe hypertension, and hyperthermia can be fatal. Treatment goals include provision of a calm environment (with or without a benzodiazepine) and airway protection. Active cooling, antihypertensives, and anticonvulsants should be used as needed.119,127
Effects on Pregnancy and the Fetus
Ingestion of amphetamines results in high levels of circulating catecholamines, which may lead to vasoconstriction and decreased uteroplacental blood flow. Animal studies have suggested that intrauterine exposure to methamphetamine is associated with an increased incidence of retinal defects, cleft palate, and rib malformations and a decreased overall rate of growth and motor development.116,128 Results from a meta-analysis revealed that exposure to amphetamines in pregnancy had a negative impact on birth outcomes, specifically, increasing the risk for preterm birth, LBW, and SGA infants.129 Cernerud et al.130 conducted long-term follow-up of 65 Swedish children with intrauterine exposure to amphetamines and found that a higher percentage of these children were in a grade lower than their chronologic age and that their performance in mathematics, language, and sports was significantly below that of their peers.
Specific pregnancy and fetal effects of ecstasy and bath salts are not known.
Anesthetic Management
Amphetamines cause (indirect) sympathetic activation. Intoxicated patients are at risk for dangerous cardiovascular events, including hemodynamic instability and cardiac arrest. Recommendations for anesthetic management are similar to those for patients with cocaine toxicity (as discussed earlier).5
A cooperative patient may be a candidate for neuraxial analgesia/anesthesia, although refractory hypotension has been reported in a case of a long-time amphetamine user undergoing neuraxial anesthesia with intravenous propofol sedation.131 The authors attributed the response to down-regulation of beta-adrenergic receptors and catecholamine depletion. As with cocaine, phenylephrine may be a better choice than ephedrine for the treatment of hypotension in amphetamine-intoxicated patients.
Parturients who are amphetamine abusers may be at increased risk for urgent cesarean delivery requiring general anesthesia. Evidence from animal studies suggests that acute ingestion of amphetamines increases the MAC for volatile halogenated anesthetic agents, whereas chronic ingestion of amphetamines decreases the MAC of volatile agents.132,133 There is a well-recognized association between methamphetamine abuse and severe tooth decay (i.e., “meth mouth”) which can present a significant hazard during laryngoscopy. The airway assessment should include attention to the presence of fragile or loose teeth that might be dislodged during laryngoscopy as well as to the possible presence of burns throughout the airway. High doses of MDMA can cause rhabdomyolysis, and dantrolene has been used successfully in these cases.119
Hallucinogens
LSD (lysergic acid diethylamide), PCP (phencyclidine), psilocybin, and mescaline are drugs of abuse that are hallucinogens.127 3,4-Methylenedioxymethamphetamine (MDMA) also has hallucinogenic effects.
Epidemiology
Hallucinogen use is usually episodic.9,134 In 2006, 1.1 million Americans 12 years or older used hallucinogens for the first time; more than 75% of these encounters involved the hallucinogen MDMA.135 The prevalence of hallucinogen use during pregnancy is unknown.
Pharmacology
The hallucinogens are a diverse group of drugs notable for their complex mechanism of actions, which include agonist, partial agonist, and antagonist effects at serotonergic, dopaminergic, and adrenergic receptors. Overall, the adrenergic effects of these drugs are mild compared with those of cocaine and amphetamine.5
Both psilocybin, the hallucinogen in some wild mushrooms, and LSD, manufactured synthetically,127 are indole derivatives that chemically resemble serotonin.5 When ingested, they evoke auditory, visual, and tactile hallucinations. Clinical effects usually develop over 15 to 60 minutes and last for 6 to 12 hours.9,127 LSD is 100 times more potent than psilocybin and can be detected in urine or plasma for up to 3 days. LSD is metabolized by the liver and has a plasma half-life of 100 minutes.5 The potency of individual samples of psilocybin varies, and the physiologic effects typically occur at doses of 20 to 60 mg. Animal studies indicate that 65% of psilocybin is excreted in urine and 15% to 20% is excreted in bile or feces. Most of the drug is excreted in the first 8 hours, although small amounts are excreted for up to a week.127
Phencyclidine, initially developed as a general anesthetic agent, was removed from the legal market in 1978. Ketamine, a related compound, is a clinically used anesthetic agent that is also a drug of abuse. In powder form these drugs can be ingested orally, intranasally, intravenously, or rectally and can be smoked. The psychological effects of PCP typically last 12 to 48 hours. Ketamine has a shorter duration of action.127
The phenethylamines include MDMA, GHB (see earlier discussion), and mescaline. Mescaline, the active ingredient in peyote cactus buttons, is typically eaten or drunk as a tea. The effects of mescaline, which last approximately 12 hours, include visual hallucinations, nausea, and vomiting.127
Systemic Effects
Ingestion of hallucinogens causes activation of the sympathetic nervous system, which results in hypertension, tachycardia, dilated pupils, and increased core body temperature (see Table 54-3).9 The cardiovascular effects of these drugs are rarely serious, although some instances of supraventricular tachycardia and acute myocardial infarction have been reported. Myocardial infarction may result from vasospasm and increased platelet aggregation.5
Carotid artery occlusion has been reported after the use of LSD. PCP use has been associated with seizures, delayed hypertensive crisis, and intracerebral hemorrhage.6 Overdose with PCP can be associated with confusion and combativeness, which may progress to seizures and catatonia.
Some users of LSD experience a “bad trip,” which is likely to be a manifestation of an acute anxiety reaction. Other users report “flashbacks” or systemic effects of these drugs that occur months or even years after ingestion. Some of these episodes are likely due to delayed release of small amounts of drug from fatty tissues. The use of LSD may unmask an underlying psychiatric disorder in vulnerable patients.127
The psychological effects of psilocybin (“magic mushrooms”) include giddiness, visual hallucinations, and gastrointestinal dysfunction. Morbidity is associated primarily with inadvertent ingestion of toxic species of mushrooms.127 Although psychological dependence on these drugs has been observed, no clearly associated withdrawal symptoms occur with abstinence (see Table 54-5).9
A direct causal relationship between abuse of these drugs and death has not been documented; however, hallucinogens can cause feelings of paranoia and panic that can lead to accidents or fatalities.6,9
Effects on Pregnancy and the Fetus
There is conflicting evidence as to whether intrauterine PCP exposure has deleterious fetal effects. Mvula et al.136 found that PCP-positive women had smaller and more preterm infants compared with non–PCP-positive women. The PCP-positive women were also more likely to have syphilis and diabetes and to use tobacco, alcohol, and marijuana. Early reports of chromosomal damage secondary to LSD were not confirmed by later studies.6
Anesthetic Management
Management of a hallucinogen-intoxicated patient is primarily supportive and noninterventional.5 Stressful situations can provoke panic attacks, which in turn can intensify the physiologic effects of these drugs. Specific recommendations include provision of a quiet, supportive environment and administration of a benzodiazepine if needed. Neuroleptic medications are relatively contraindicated in such a patient, because they can intensify toxic reactions.5, 9
Hemodynamic perturbations are usually relatively mild and well tolerated.63 Occasionally, patients experience supraventricular tachycardia, hypertension, and myocardial ischemia.5 The acutely intoxicated patient may have an exaggerated response to ephedrine9; phenylephrine may be preferable and should be titrated to effect. LSD may have some intrinsic anticholinesterase activity, but the clinical significance is unclear. Hallucinogens may also prolong opioid-induced analgesia and respiratory depression.63
Either neuraxial or general anesthesia can be administered for vaginal or cesarean delivery as the clinical situation warrants.
Opioids
Opioids refer to the class of naturally occurring and synthetic drugs that are structurally and functionally related to morphine. The term opiate specifically describes any of the narcotic alkaloids found in the juice of poppy plants, including morphine and codeine.23
Epidemiology
The estimated prevalence of opioid use during pregnancy varies from 1.6% to 7.2%, depending on the methodology of the survey and the studied population.137 The National Survey on Drug Use and Health estimated that more than 359,000 Americans 12 years of age and older used heroin in 2010, including approximately 140,000 who used it for the first time that year.1 Among youths and young adults, nonmedical use of prescription drugs, particularly pain relievers, was the second most common form of drug abuse.1
Pharmacology
The naturally occurring opioids are metabolized first to morphine, which has a plasma half-life of 2 to 3 hours. Morphine then undergoes rapid metabolism in the liver and is excreted in the urine, where both active and inactive metabolites can be detected for up to 2 days in occasional users, and longer in chronic users.5,23
Heroin (diacetylmorphine or diamorphine) is a commonly abused and highly addictive semisynthetic analogue of morphine. It can be smoked, snorted, or injected intravenously or intramuscularly. Most fatal overdoses occur in users of intravenous heroin. The speed of onset varies from less than 1 minute to 15 minutes, depending on the delivery method; the elimination half-life is typically 1 to 2 hours. Heroin is metabolized by the liver and excreted in the urine.5,23,24,138 The formulation of street heroin has become increasingly pure. In 1990, a typical 100-mg bag of powder had up to 8 mg of heroin mixed with inert, often toxic additives; subsequently the drug has become available with a purity as high as 45% to 90%. Heroin is more lipid soluble than other opioids; thus, it rapidly crosses the blood-brain barrier and has significant and toxic CNS effects.138
Rapidly acting semisynthetic opioids such as oxycodone and hydrocodone are prescribed legitimately and distributed illicitly. The oral bioavailability of oxycodone is higher than that of morphine, and its potency with oral ingestion is greater.23
Methadone is a synthetic compound that is structurally unrelated to the other opioids but has similar effects.23,139 It is formulated as a racemic mixture of two enantiomers: R-methadone, which is a potent µ- and δ-opioid receptor agonist, and S-methadone, which is a noncompetitive N-methyl-d-aspartate (NMDA) antagonist that prevents the reuptake of 5-hydroxytryptamine and norepinephrine.140 Typically available in powder form, methadone can be reconstituted for oral, rectal, or intravenous use.141 Oral bioavailability of methadone is typically three times that of morphine.140,141 There is significant inter-individual variability in its clinical effect and duration of action. The peak effect occurs approximately 3 hours after oral ingestion, and its half-life varies from 15 to 40 hours.23,24,140-142 Life-threatening complications may result from the cumulative effects of successive doses.140
Unlike morphine, methadone undergoes biotransformation (rather than conjugation) in the liver and is excreted primarily by the fecal route. Methadone has been used since the 1960s for stabilization and maintenance therapy for patients suffering from opioid-addictive disorders and since the 1970s for the same reason in pregnant patients. It is also used for analgesia in patients with chronic pain.140,142 Pregnancy is associated with greater methadone metabolism and reduced methadone bioavailability because of greater maternal blood volume and changes in hepatic enzymatic activity and glomerular filtration rate.23,142,143
Buprenorphine is a semisynthetic opioid that acts as a partial µ-receptor agonist (with low intrinsic opioid activity) and k-receptor antagonist and therefore can also be used as part of maintenance therapy for patients suffering from opioid-addictive disorders.61,144,145 The two sublingual formulations of this drug (Subutex and Suboxone) contain naloxone, which becomes active only if the user attempts to snort, inject, or cook the drug.144 Buprenorphine binds up to 1000 times more tightly to the µ- and k-receptors than morphine, and it has a slow dissociation half-life of approximately 166 minutes.144 Primary metabolism of buprenorphine occurs in the liver with excretion in the bile.146 Buprenorphine has a ceiling effect at 24 to 32 mg, which makes respiratory depression less likely and contributes to the opioid tolerance of treated patients.144,147
Two additional formulations for intravenous and transdermal administration of buprenorphine are used for analgesia and are not approved for opiate addiction therapy.147
When used as part of a maintenance therapy for patients with addiction to opioids, methadone must be prescribed and dispensed on a daily basis by a registered substance abuse treatment program; buprenorphine, however, can only be prescribed by physicians who have been specially trained and credentialed for distribution in an office-based setting.148 All dose adjustments needed in the course of pregnancy or the postpartum period must be done in close consultation with the addiction treatment program (for methadone) or the providers responsible for prescribing and managing the patient's buprenorphine therapy.148
Systemic Effects
Opioids mimic the activity of endogenous peptides and exert their effects through binding to µ-, δ-, and κ-opioid receptors. Morphine and heroin exert their euphoric, analgesic, and reinforcing effects primarily through stimulation of the µ-opioid receptors. Long-time opioid use causes neuroadaptations in the brain that may explain the manifestations of withdrawal.23,138
Opioids act in the CNS to reduce sympathetic activity and increase parasympathetic activity; they also promote histamine release from mast cells. The resulting cardiovascular effects include bradycardia, hypotension, and, in some cases, potentially lethal tachyarrhythmias and bradyarrhythmias (see Table 54-3). Noncardiogenic pulmonary edema has been observed in some cases of overdose and is believed to be caused by hypoxia, intense pulmonary vasoconstriction, and, perhaps, the use of reversal agents (e.g., naloxone).5
Opioid-induced respiratory depression occurs through a direct effect on the brainstem that reduces the ventilatory response to hypercarbia.138 Opioid overdose can progress from miosis and respiratory depression to obtundation and death.6,10 Treatment of overdose includes maintenance of a patent airway, provision of hemodynamic support, and, if necessary, administration of an opioid antagonist (e.g., naloxone).5
Women who abuse opioids or other drugs intravenously are at risk for infective endocarditis (usually affecting right-sided valves), HIV infection, viral hepatitis, septic emboli, and pulmonary abscess formation.5,24 In addition, hilar adenopathy may develop as a result of ingestion of additives such as quinine and starch. Opioid-dependent patients may have additional end-organ damage and may exhibit opioid tolerance, physical dependence, and withdrawal.139
Animal studies have found an association between opioid administration and abnormal pain sensitivity, including hyperalgesia and allodynia (see Table 54-4).149 Opioid tolerance develops with chronic use and is related to amount and duration of drug exposure; tolerance results from changes in drug distribution and metabolism (pharmacokinetics) and in receptor density and activity (pharmacodynamics).139 Patients receiving methadone maintenance therapy often have diminished pain tolerance.150
Acute opioid withdrawal results from sympathetic hyperactivity, resulting in flulike signs and symptoms such as fatigue, weakness, restlessness, rhinorrhea, perspiration, fever, and diarrhea (see Table 54-5).6,24 These can persist for several days if not treated. The onset and duration of withdrawal symptoms vary according to the opioid ingested; morphine or heroin withdrawal symptoms typically begin within 6 to 18 hours after the last dose, reach their peak intensity by 3 days, and last for 7 to 10 days. Methadone withdrawal symptoms are delayed—with an onset within 24 to 48 hours, a peak intensity within 3 to 21 days, and a duration of up to 6 to 7 weeks.139 Clonidine, an α2-adrenergic agonist, can modulate these effects, although postural hypotension may result.24 Doxepin, a tricyclic antidepressant that inhibits the reuptake of serotonin and norepinephrine, has also been administered in this setting. Acute withdrawal can also be treated by administration of a short-acting opioid and initiation of a gradual dose taper.9
Substitution pharmacology (e.g., methadone maintenance or buprenorphine therapy) has been recommended for the treatment of opioid dependency if abstinence is not attainable and withdrawal is not feasible. Typically, long-acting µ-agonists (e.g., methadone) or partial agonists (e.g., buprenorphine) are substituted for the opioid of abuse in combination with behavioral modification therapy. Drug maintenance therapy decreases withdrawal symptoms and attenuates both craving and the positive rewards associated with subsequent opioid use (see later discussion on effects on the neonate).142
The U.S. Food and Drug Administration (FDA) guidelines for methadone administration in pregnancy were developed in 1970.4,142 Several studies have shown that methadone maintenance therapy in heroin-addicted pregnant women is beneficial for both the mother and the infant. Specific benefits include better medical and prenatal care,151 a lower incidence of unplanned pregnancy,152 decreased neonatal abstinence symptoms and days of hospitalization, and less maternal illicit drug use at delivery.141,143
Effects on Pregnancy and the Fetus
In general, published reports have not shown an increased risk for birth defects after prenatal exposure to oxycodone, meperidine or propoxyphene, but they have shown an association between congenital heart defects and early use of codeine.148 A retrospective population-based study (data from 1997 to 2005) identified an association between maternal therapeutic use of opioid medications early in pregnancy and atrial and ventricular septal cardiac defects, hypoplastic left heart syndrome, spina bifida, and gastroschisis.153 Data were obtained retrospectively via maternal interviews and did not control for dose, therapeutic indication (including if the drug was administered within the context of a surgical procedure), duration, or frequency of opioid exposure.
Heroin use during pregnancy is associated with first-trimester spontaneous abortion, preterm delivery, and fetal growth restriction, in part as a result of poor maternal nutrition.4,141,151,154 Maternal and neonatal infection and neonatal abstinence syndrome have also been described. Neonatal abstinence syndrome (NAS), which occurs in neonates repeatedly exposed to opioids in utero, is characterized by irritability, poor feeding, abnormal sleep patterns, diarrhea, fever, and seizures. Affected neonates may also have autonomic symptoms such as yawning and mottling. If prolonged and untreated, NAS can result in death.4,154 Children born to women using heroin have been found to have normal development at the time of entry into school.4 However, in utero heroin exposure appears to be associated with a high rate of attention deficit hyperactivity disorder.155
In 2010, Jones et al.145 performed a multicenter, randomized controlled trial comparing the neonatal effects of methadone and buprenorphine therapy on NAS when used as part of an opioid dependence program during pregnancy. They found the length of hospitalization and amount of morphine therapy needed for NAS was significantly less in the infants of buprenorphine-treated mothers, although the percentage of neonates requiring treatment for NAS did not differ between groups.
When obstetric complications were compared in methadone- and buprenorphine-treated pregnant women, maternal weight gain, cesarean delivery rates, birth weights, and Apgar scores were similar.147 However, medical complications and overdoses were less frequent in buprenorphine-treated women.147 Based on these data the ACOG issued a 2012 committee statement suggesting that buprenorphine be considered “a potential first-line medication for pregnant opioid-dependent women who are new to treatment.”148
Anesthetic Management
Preanesthesia Assessment and Communication.
Establishing trust and effective communication is critical in the care of opioid-tolerant, methadone- or buprenorphine-maintained or opioid-addicted patients and may improve the ability to elicit a more complete drug history. Ideally, before the patient presents for delivery, a thorough anesthesia consultation and development of a mutually agreeable strategy for pain management with appropriate goals for pain intensity scores should be conducted.
Providers, family, and patients may express concern that exposing a previously addicted patient to opioids or other sedatives will prompt cravings or a frank relapse. However, withholding appropriate analgesic and anxiolytic medications from such a patient cannot be justified. Patient monitoring (e.g., drug screens, pill counts) may be necessary.139
Opioid Maintenance Requirements.
Opioids used on a regular basis for chronic pain or methadone or buprenorphine maintenance therapy should be continued during the hospitalization,108,139-141 with additional therapies added for acute pain management. The precise dose of methadone must be confirmed with the dispenser, because improper dosing can result in inadequate analgesia, withdrawal phenomena, or life-threatening overdose.140 Any methadone or buprenorphine dose adjustments needed in the course of pregnancy or the postpartum period must be done in close consultation with the prescribing physicians and addiction treatment program.148 Although some providers advocate trying to substitute a pure opioid agonist for buprenorphine in advance of elective induction of labor or elective cesarean delivery to maximize the effect of peripartum opioid analgesics, there are several potential pitfalls to this approach. First, most admissions for delivery are unplanned. Second, it can be extremely challenging to effectively manage predelivery transition from buprenorphine to opioid-agonist therapy in the outpatient setting. Third, reintroduction of buprenorphine therapy in the postpartum period may induce acute withdrawal symptoms.156
An alternative approach to switching to pure opioid agonist therapy is to continue the buprenorphine through the peripartum period. Kronfeld and Manfredi156 described five nonpregnant patients who continued their buprenorphine through the operative period; postoperative pain was successfully controlled using full doses of agonist opioids. A third approach is to continue the buprenorphine through the operative period but to divide the usual dose into three or four partial doses that maximize the analgesic properties of the medication.144,148 Importantly, the patient must receive the correct total methadone or buprenorphine dose; therefore, dividing the dose should only be attempted if administration of the correct total dose can be ensured.
Alterations of Drug Levels.
Sedative medications can increase the depressant effects of chronically ingested opioids. Methadone has a long half-life and is metabolized by cytochrome P450; thus, women receiving methadone are particularly vulnerable to changes in metabolism that result from administration of other medications. Anticonvulsants (e.g., phenobarbital, phenytoin) increase methadone clearance, and selective serotonin reuptake inhibitors decrease it. Methadone dose requirements are typically established by titrating to effect; thus, the discontinuation of these other medications can have unintended consequences.139
Anesthetic Technique and Dose Requirements.
Neuraxial analgesia or anesthesia is the technique of choice for chronic opioid-abusing parturients or those receiving methadone or buprenorphine maintenance therapy undergoing vaginal or cesarean delivery, assuming that the patient is cooperative and has no evidence of coagulopathy or other contraindication to a neuraxial technique.148
Providers should not assume that the cause of inadequate neuraxial labor analgesia is solely a result of the patient's addiction. Opioid-addicted patients can have objectively inadequate (failed) neuraxial analgesia and anesthesia just like their nonaddicted counterparts.
In a review of publications on opioid-dependent parturients, Cassidy and Cyna157 catalogued the needs of these patients across a broad range of anesthetic techniques and modes of delivery. The proportion of opioid-dependent women who required general anesthesia for cesarean delivery, or the number who requested epidural labor analgesia, did not differ compared with the proportions in non–opioid-dependent women. However, a significant incidence of pain management challenges were observed, particularly after cesarean delivery; 26% of the patients required consultation for inpatient pain management in addition to usual labor analgesia, and 74% of the patients had difficulties with postcesarean analgesia. These patients also had significant vascular access problems and generated more emergency calls for fainting and collapse. Meyer et al.152 noted that pregnant women on methadone maintenance therapy had similar pain scores and analgesia requirements during labor compared with matched control women, but the methadone-maintained patients required 70% more opioid analgesia after cesarean delivery.152
Although mixed opioid agonists/antagonists (e.g., nalbuphine) are commonly employed for labor analgesia or treatment of neuraxial opioid-induced pruritus in parturients, these agents should be avoided in patients with a history of opioid abuse because they can precipitate withdrawal.
If neuraxial techniques are contraindicated for labor, intravenous patient-controlled analgesia can be used. Patients who use either physician-prescribed or illicit opioids over the long term demonstrate a relative insensitivity to opioids. Their opioid dose requirements for intraoperative and postoperative analgesia may be 30% to 100% higher than those of opioid-naive patients.139,158
During administration of general anesthesia for cesarean delivery, the anesthesia provider should consider the potential for decreased MAC in a patient with acute opioid intoxication as well as the potential for cross-tolerance with CNS depressants in a patient with a history of long-time opioid use.9
Postpartum Analgesia.
Postoperative pain management can be challenging in patients with a history of chronic opioid use. In one study, methadone-maintained women had higher pain scores but did not receive greater doses of opioids after vaginal delivery. However, methadone-maintained women had higher pain scores and received greater doses of opioids after cesarean delivery.152 Multimodal therapy, including nonsteroidal anti-inflammatory drugs (NSAIDs) and acetaminophen, should be used after vaginal delivery.139 For cesarean delivery, multimodal analgesia with neuraxial morphine and systemic NSAID therapy is recommended. Epidural analgesia, transversus abdominis plane (TAP) block, and intravenous patient-controlled opioid analgesia can also be considered, similar to that in women who are not chronic opioid users (see Chapters 27 and 28).
Solvents
Solvents are a chemically diverse group of readily available substances (e.g., toluene, ethylene glycol) that can be sniffed, aerosolized, or ingested to produce feelings of euphoria, excitement, and invulnerability.5,159,160
Epidemiology
In 2010, more than 700,000 individuals in the United States used an inhalant for the first time.1 Of the 3 million persons aged 12 years or older who used illicit drugs for the first time within that year, 9% reported that their first drugs were inhalants. The prevalence of solvent abuse in pregnant patients is not known.
Pharmacology
Inhalants are quickly absorbed through the lungs and typically are rapidly metabolized in the liver. The precise mechanism of action of inhalants is variable and is not completely understood.
Detection, although difficult, can be suspected by the presence of the “glue sniffer's” facial rash24 or the odor of the abused inhalant.160 Laboratory assays can detect many solvents in the blood within 10 hours of exposure. Urine assays are available only for certain volatile substances (e.g., toluene, chlorinated solvents) but may provide a longer window of detection.5
Ethylene glycol, a bittersweet-tasting component of antifreeze, brake fluids, and industrial solvents, is sometimes ingested by substance-abusing adults as an inexpensive substitute for alcohol. Readily absorbed via the gastrointestinal tract with ingestion, this solvent reaches maximal blood concentrations within 1 to 4 hours. It is metabolized by the hepatic enzyme alcohol dehydrogenase to four toxic organic acids. The primary screening test findings for ethylene glycol intoxication include serum anion-gap metabolic acidosis, high urine osmolality with an osmol gap, and the presence of calcium oxalate crystals in the urine.161,162
Toluene, a primary component of household paint and cleaning agents, is also sniffed or ingested orally for its transient euphoric effects.63
Systemic Effects
Typically, the user of inhalants feels an initial sense of euphoria followed by a brief period of disinhibition and impulsivity. Intoxication can be prolonged through repeated inhalation of the solvent. As the dose increases, dizziness, diplopia, and disorientation manifest, followed by headache, nausea, vomiting, drowsiness, and sleep.160
Mucous membrane irritation can result in rhinorrhea, epistaxis, excessive salivation, and conjunctival redness. Nausea, vomiting, and diarrhea can also occur.160 Ingestion of volatile substances can lead to potentially lethal tachyarrhythmias, likely secondary to sympathetic stimulation, or bradyarrhythmias from decreased sinoatrial node automaticity or direct vagal stimulation and myocardial depression (see Table 54-3).5 Hypoxemia may occur as a result of formation of carboxyhemoglobinemia, methemoglobinemia, or suffocation.5,160,163
Chronic inhalant abuse can cause multiorgan system disease, with cardiac (e.g., cardiomyopathy, arrhythmias, myocardial infarction), pulmonary (e.g., wheezing, acute respiratory distress syndrome, pulmonary hypertension), central and peripheral nervous system (e.g., cognitive impairment, ataxia, muscle weakness, peripheral neuropathy), and autonomic dysfunction (see Table 54-4).5,6 Renal toxicity, aplastic anemia, and hepatocellular carcinoma have also been reported.127,160 Imaging studies have demonstrated loss of brain mass, white matter degeneration, and subcortical abnormalities in long-term users. Significant methemoglobinemia with attendant cyanosis can also occur from exposure to some compounds.5,160,163
Cessation of chronic use can lead to reversal of many of the pathophysiologic changes, although the CNS effects may persist. Death from inhalant use typically occurs from suffocation, aspiration, or accidental injury. Sudden sniffing death syndrome, likely secondary to a fatal arrhythmia in the setting of a sensitized myocardium, electrolyte abnormalities, and hypoxemia, has been reported in both new and long-term users.160
Ingestion of ethylene glycol has inebriating effects similar to those of alcohol with more devastating consequences. Accumulation of the toxic metabolite glycolic acid occurs, resulting in CNS depression and seizure activity. Cardiopulmonary manifestations are often delayed (12 to 24 hours after ingestion) and may be fatal. If the patient survives, she will likely experience renal failure 24 to 72 hours after ingestion. The estimated lethal dose of undiluted ethylene glycol is 1.4 mL/kg. Treatment of ethylene glycol ingestion may require the induction of emesis with ipecac in the alert and awake patient or the use of gastric lavage. The conversion of ethylene glycol to its toxic metabolites can be prevented with the use of the antidote fomepizole or ethanol. Hemodialysis may be warranted.162
Ingestion of toluene can similarly cause cardiac arrhythmias, acute respiratory distress syndrome, hepatic failure, cerebral edema, and death.63
Effects on Pregnancy and the Fetus
Solvent abuse has been associated with fetal growth restriction, preterm delivery, and perinatal mortality.9 Infants born of inhalant-abusing women exhibit NAS symptoms, including excessive and high-pitched crying, sleep disorders, CNS irritability, and poor feeding. Phenobarbital can be somewhat effective in ameliorating these manifestations.164
Ethylene glycol intoxication has been misdiagnosed as eclampsia in a patient who presented at 26 weeks' gestation with hypertension and seizures followed by coma. Her postoperative course was complicated by hemodynamic instability, severe metabolic acidosis, and acute renal failure, which prompted suspicion of substance intoxication. Toxicology investigation confirmed ethylene glycol poisoning. Subsequently, the patient was successfully treated with hemodialysis and intravenous ethanol; her neonate was treated with diuresis and replacement transfusion.161
Anesthetic Management
Identification of solvent abuse in the parturient is critical to safe anesthetic care.163 The parturient's skin and clothing should be decontaminated if inhalation abuse is suspected.160 A careful physical examination, including documentation of preexisting sensory and motor deficits, should be undertaken, and electrolyte abnormalities should be identified and corrected.9
If the patient is cooperative and able to protect her airway, neuraxial labor analgesia can be administered. It may be prudent to avoid administration of an epinephrine-containing local anesthetic solution, given the significant risk for arrhythmias in these patients. The treatment of hypotension should include the use of intravenous fluids. Phenylephrine may be preferable to ephedrine because the latter may predispose to arrhythmias. Atropine should be readily available but used with caution. In a stable patient with sustained tachyarrhythmias, beta-adrenergic receptor antagonists should be the first line of therapy.5
In intoxicated or otherwise uncooperative patients, rapid-sequence induction of general anesthesia for cesarean delivery may be indicated.9
References
1. Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. U.S. Department of Health and Human Services. [Available at] http://www.samhsa.gov/data/NSDUH/2k10ResultsRev/NSDUHresultsRev2010.htm#2.6 [Accessed March 2013] .
2. Ebrahim SH, Gfroerer J. Pregnancy-related substance use in the United States during 1996-1998. Obstet Gynecol. 2003;101:374–379.
3. Kline J, Ng SK, Schittini M, et al. Cocaine use during pregnancy: sensitive detection by hair assay. Am J Public Health. 1997;87:352–358.
4. Huestis MA, Choo RE. Drug abuse's smallest victims: in utero drug exposure. Forensic Sci Int. 2002;128:20–30.
5. Ghuran A, Nolan J. Recreational drug misuse: issues for the cardiologist. Heart. 2000;83:627–633.
6. Brust JC. Neurologic complications of substance abuse. J Acquir Immune Defic Syndr. 2002;31(Suppl 2):S29–S34.
7. Curet LB, Hsi AC. Drug abuse during pregnancy. Clin Obstet Gynecol. 2002;45:73–88.
8. Kain ZN, Mayes LC, Ferris CA, et al. Cocaine-abusing parturients undergoing cesarean section: a cohort study. Anesthesiology. 1996;85:1028–1035.
9. Kuczkowski KM. Anesthetic implications of drug abuse in pregnancy. J Clin Anesth. 2003;15:382–394.
10. Khan H, Nagda J. Substance abuse. Pian-Smith MCM, Leffert L. Obstetric Anesthesia. Cambridge University Press: New York; 2007.
11. Bloomstone JA. The drug-abusing parturient. Int Anesthesiol Clin. 2002;40:137–150.
12. Birnbach DJ. Anesthetic management of the drug-abusing parturient: are you ready? J Clin Anesth. 2003;15:325–327.
13. Keegan J, Parva M, Finnegan M, et al. Addiction in pregnancy. J Addict Dis. 2010;29:175–191.
14. Ostrea EM Jr, Knapp DK, Tannenbaum L, et al. Estimates of illicit drug use during pregnancy by maternal interview, hair analysis, and meconium analysis. J Pediatr. 2001;138:344–348.
15. Montgomery D, Plate C, Alder SC, et al. Testing for fetal exposure to illicit drugs using umbilical cord tissue vs meconium. J Perinatol. 2006;26:11–14.
16. Lester BM, Andreozzi L, Appiah L. Substance use during pregnancy: time for policy to catch up with research. Harm Reduct J. 2004;1:5.
17. Lester BM, ElSohly M, Wright LL, et al. The Maternal Lifestyle Study: drug use by meconium toxicology and maternal self-report. Pediatrics. 2001;107:309–317.
18. Musshoff F, Madea B. Review of biologic matrices (urine, blood, hair) as indicators of recent or ongoing cannabis use. Ther Drug Monit. 2006;28:155–163.
19. Verstraete AG. Detection times of drugs of abuse in blood, urine, and oral fluid. Ther Drug Monit. 2004;26:200–205.
20. Alcohol consumption among pregnant and childbearing-aged women—United States, 1991 and 1995. MMWR Morb Mortal Wkly Rep. 1997;46:346–350.
21. Spampinato MV, Castillo M, Rojas R, et al. Magnetic resonance imaging findings in substance abuse: alcohol and alcoholism and syndromes associated with alcohol abuse. Top Magn Reson Imaging. 2005;16:223–230.
22. Lieber CS. Medical disorders of alcoholism. N Engl J Med. 1995;333:1058–1065.
23. Reynolds EW, Bada HS. Pharmacology of drugs of abuse. Obstet Gynecol Clin North Am. 2003;30:501–522.
24. Wood PR, Soni N. Anaesthesia and substance abuse. Anaesthesia. 1989;44:672–680.
25. Kuczkowski KM. Labor analgesia for the drug abusing parturient: is there cause for concern? Obstet Gynecol Surv. 2003;58:599–608.
26. Kuczkowski KM. Labor analgesia for the tobacco and ethanol abusing pregnant patient: a routine management? Arch Gynecol Obstet. 2005;271:6–10.
27. Floyd RL, O’Connor MJ, Sokol RJ, et al. Recognition and prevention of fetal alcohol syndrome. Obstet Gynecol. 2005;106:1059–1064.
28. Wright A, Walker J. Management of women who use drugs during pregnancy. Semin Fetal Neonatal Med. 2007;12:114–118.
29. O’Leary CM, Nassar N, Kurinczuk JJ, et al. Prenatal alcohol exposure and risk of birth defects. Pediatrics. 2010;126:e843–e850.
30. Kuczkowski KM. Drug misuse in pregnancy. Br J Psychiatry. 2004;184:182.
31. Mayes LC. Genetics of childhood disorders: LV. Prenatal drug exposure. J Am Acad Child Adolesc Psychiatry. 2003;42:1258–1261.
32. Harlap S, Shiono PH. Alcohol, smoking, and incidence of spontaneous abortions in the first and second trimester. Lancet. 1980;2:173–176.
33. Ackland GL, Smith M, McGlennan AP. Acute, severe hypoglycemia occurring during general anesthesia in a nondiabetic adult. Anesth Analg. 2007;105:553–554.
34. American Society of Anesthesiologists Task Force on Obstetric Anesthesia. Practice guidelines for obstetric anesthesia: an updated report. Anesthesiology. 2007;106:843–863.
35. Bruce DL. Alcoholism and anesthesia. Anesth Analg. 1983;62:84–96.
36. Han YH. Why do chronic alcoholics require more anesthesia. Anesthesiology. 1969;30:2.
37. Swerdlow BN, Holley FO, Maitre PO, Stanski DR. Chronic alcohol intake does not change thiopental anesthetic requirement, pharmacokinetics, or pharmacodynamics. Anesthesiology. 1990;72:455–461.
38. Ghoneim MM. Awareness during anesthesia. Anesthesiology. 2000;92:597–602.
39. Robins K, Lyons G. Intraoperative awareness during general anesthesia for cesarean delivery. Anesth Analg. 2009;109:886–890.
40. American College of Obstetricians and Gynecologists. Smoking cessation during pregnancy. [ACOG Committee Opinion No. 316. Washington, DC] October 2005.
41. Lerman C, Patterson F, Berrettini W. Treating tobacco dependence: state of the science and new directions. J Clin Oncol. 2005;23:311–323.
42. Sastry BV. Placental toxicology: tobacco smoke, abused drugs, multiple chemical interactions, and placental function. Reprod Fertil Dev. 1991;3:355–372.
43. Hlastala MP, McKenna HP, Franada RL, Detter JC. Influence of carbon monoxide on hemoglobin-oxygen binding. J Appl Physiol. 1976;41:893–899.
44. Klausen K, Andersen C, Nandrup S. Acute effects of cigarette smoking and inhalation of carbon monoxide during maximal exercise. Eur J Appl Physiol Occup Physiol. 1983;51:371–379.
45. Warner DO. Perioperative abstinence from cigarettes: physiologic and clinical consequences. Anesthesiology. 2006;104:356–367.
46. Kotani N, Kushikata T, Hashimoto H, et al. Recovery of intraoperative microbicidal and inflammatory functions of alveolar immune cells after a tobacco smoke-free period. Anesthesiology. 2001;94:999–1006.
47. Kistin N, Handler A, Davis F, Ferre C. Cocaine and cigarettes: a comparison of risks. Paediatr Perinat Epidemiol. 1996;10:269–278.
48. Klonoff-Cohen H, Edelstein S, Savitz D. Cigarette smoking and preeclampsia. Obstet Gynecol. 1993;81:541–544.
49. Bada HS, Das A, Bauer CR, et al. Low birth weight and preterm births: etiologic fraction attributable to prenatal drug exposure. J Perinatol. 2005;25:631–637.
50. Salihu HM, Aliyu MH, Pierre-Louis BJ, Alexander GR. Levels of excess infant deaths attributable to maternal smoking during pregnancy in the United States. Matern Child Health J. 2003;7:219–227.
51. Fried PA, Watkinson B, Gray R. Differential effects on cognitive functioning in 13- to 16-year-olds prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol. 2003;25:427–436.
52. Wilkes AR, Hall JE, Wright E, Grundler S. The effect of humidification and smoking habit on the incidence of adverse airway events during deepening of anaesthesia with desflurane. Anaesthesia. 2000;55:685–689.
53. Kim ES, Bishop MJ. Cough during emergence from isoflurane anesthesia. Anesth Analg. 1998;87:1170–1174.
54. Nawrot P, Jordan S, Eastwood J, et al. Effects of caffeine on human health. Food Addit Contam. 2003;20:1–30.
55. Higdon JV, Frei B. Coffee and health: a review of recent human research. Crit Rev Food Sci Nutr. 2006;46:101–123.
56. Bech BH, Obel C, Henriksen TB, Olsen J. Effect of reducing caffeine intake on birth weight and length of gestation: randomised controlled trial. BMJ. 2007;334:409.
57. Browne ML. Maternal exposure to caffeine and risk of congenital anomalies: a systematic review. Epidemiology. 2006;17:324–331.
58. Browne ML, Bell EM, Druschel CM, et al. Maternal caffeine consumption and risk of cardiovascular malformations. Birth Defects Res A Clin Mol Teratol. 2007;79:533–543.
59. Weng X, Odouli R, Li DK. Maternal caffeine consumption during pregnancy and the risk of miscarriage: a prospective cohort study. Am J Obstet Gynecol. 2008;198:279 e1–8.
60. Adeney KL, Williams MA, Schiff MA, et al. Coffee consumption and the risk of gestational diabetes mellitus. Acta Obstet Gynecol Scand. 2007;86:161–166.
61. Back SA, Craig A, Luo NL, et al. Protective effects of caffeine on chronic hypoxia-induced perinatal white matter injury. Ann Neurol. 2006;60:696–705.
62. Halker RB, Demaerschalk BM, Wellik KE, et al. Caffeine for the prevention and treatment of postdural puncture headache: debunking the myth. Neurologist. 2007;13:323–327.
63. Hernandez M, Birnbach DJ, Van Zundert AA. Anesthetic management of the illicit-substance-using patient. Curr Opin Anaesthesiol. 2005;18:315–324.
64. Arria AM, Derauf C, Lagasse LL, et al. Methamphetamine and other substance use during pregnancy: preliminary estimates from the Infant Development, Environment, and Lifestyle (IDEAL) study. Matern Child Health J. 2006;10:293–302.
65. Ashton CH. Pharmacology and effects of cannabis: a brief review. Br J Psychiatry. 2001;178:101–106.
66. Hatch EE, Bracken MB. Effect of marijuana use in pregnancy on fetal growth. Am J Epidemiol. 1986;124:986–993.
67. Shiono PH, Klebanoff MA, Nugent RP, et al. The impact of cocaine and marijuana use on low birth weight and preterm birth: a multicenter study. Am J Obstet Gynecol. 1995;172:19–27.
68. White SM. Cannabis abuse and laryngospasm. Anaesthesia. 2002;57:622–623.
69. Wallace M, Schulteis G, Atkinson J, et al. Dose-dependent effects of smoked cannabis on capsaicin-induced pain and hyperalgesia in healthy volunteers. Anesthesiology. 2007;107:12.
70. Fajemirokun-Odudeyi O, Lindow SW. Obstetric implications of cocaine use in pregnancy: a literature review. Eur J Obstet Gynecol Reprod Biol. 2004;112:2–8.
71. Shanti CM, Lucas CE. Cocaine and the critical care challenge. Crit Care Med. 2003;31:1851–1859.
72. Bauer CR, Langer JC, Shankaran S, et al. Acute neonatal effects of cocaine exposure during pregnancy. Arch Pediatr Adolesc Med. 2005;159:824–834.
73. Kuczkowski KM. Peripartum care of the cocaine-abusing parturient: are we ready? Acta Obstet Gynecol Scand. 2005;84:108–116.
74. Fleming JA, Byck R, Barash PG. Pharmacology and therapeutic applications of cocaine. Anesthesiology. 1990;73:518–531.
75. Ettinger NA, Albin RJ. A review of the respiratory effects of smoking cocaine. Am J Med. 1989;87:664–668.
76. Lange RA, Hillis LD. Cardiovascular complications of cocaine use. N Engl J Med. 2001;345:351–358.
77. Warner EA. Cocaine abuse. Ann Intern Med. 1993;119:226–235.
78. Kuczkowski KM. Cardiovascular complications of recreational cocaine use in pregnancy: myth or reality? Acta Obstet Gynecol Scand. 2005;84:100–101.
79. Feng Q. Postnatal consequences of prenatal cocaine exposure and myocardial apoptosis: does cocaine in utero imperil the adult heart? Br J Pharmacol. 2005;144:887–888.
80. Mittleman MA, Mintzer D, Maclure M, et al. Triggering of myocardial infarction by cocaine. Circulation. 1999;99:2737–2741.
81. Hollander JE, Henry TD. Evaluation and management of the patient who has cocaine-associated chest pain. Cardiol Clin. 2006;24:103–114.
82. Livingston JC, Mabie BC, Ramanathan J. Crack cocaine, myocardial infarction, and troponin I levels at the time of cesarean delivery. Anesth Analg. 2000;91:913–915.
83. Kuczkowski KM. More on the idiosyncratic effects of cocaine on the human heart. Emerg Med J. 2007;24:147.
84. Vertommen JD, Hughes SC, Rosen MA, et al. Hydralazine does not restore uterine blood flow during cocaine-induced hypertension in the pregnant ewe. Anesthesiology. 1992;76:580–587.
85. Gowda RM, Khan IA, Mehta NJ, et al. Cardiac arrhythmias in pregnancy: clinical and therapeutic considerations. Int J Cardiol. 2003;88:129–133.
86. Mason BA, Ricci-Goodman J, Koos BJ. Adenosine in the treatment of maternal paroxysmal supraventricular tachycardia. Obstet Gynecol. 1992;80:478–480.
87. Shih RD, Hollander JE, Burstein JL, et al. Clinical safety of lidocaine in patients with cocaine-associated myocardial infarction. Ann Emerg Med. 1995;26:702–706.
88. Schleich JM, Bernard Du Haut Cilly F, Laurent MC, Almange C. Early prenatal management of a fetal ventricular tachycardia treated in utero by amiodarone with long term follow-up. Prenat Diagn. 2000;20:449–452.
89. Widerhorn J, Bhandari AK, Bughi S, et al. Fetal and neonatal adverse effects profile of amiodarone treatment during pregnancy. Am Heart J. 1991;122:1162–1166.
90. Spivey WH, Euerle B. Neurologic complications of cocaine abuse. Ann Emerg Med. 1990;19:1422–1428.
91. Westover AN, McBride S, Haley RW. Stroke in young adults who abuse amphetamines or cocaine: a population-based study of hospitalized patients. Arch Gen Psychiatry. 2007;64:495–502.
92. Kain ZN, Mayes LC, Pakes J, et al. Thrombocytopenia in pregnant women who use cocaine. Am J Obstet Gynecol. 1995;173:885–890.
93. Orser B. Thrombocytopenia and cocaine abuse. Anesthesiology. 1991;74:195–196.
94. Gershon RY, Fisher AJ, Graves WL. The cocaine-abusing parturient is not at an increased risk for thrombocytopenia. Anesth Analg. 1996;82:865–866.
95. Kain ZN, Rimar S, Barash PG. Cocaine abuse in the parturient and effects on the fetus and neonate. Anesth Analg. 1993;77:835–845.
96. Owiny JR, Jones MT, Sadowsky D, et al. Cocaine in pregnancy: the effect of maternal administration of cocaine on the maternal and fetal pituitary-adrenal axes. Am J Obstet Gynecol. 1991;164:658–663.
97. Owiny JR, Sadowsky D, Jones MT, et al. Effect of maternal cocaine administration on maternal and fetal glucose, lactate, and insulin in sheep. Obstet Gynecol. 1991;77:901–904.
98. Crandall CG, Vongpatanasin W, Victor RG. Mechanism of cocaine-induced hyperthermia in humans. Ann Intern Med. 2002;136:785–791.
99. Woods JR Jr, Scott KJ, Plessinger MA. Pregnancy enhances cocaine's actions on the heart and within the peripheral circulation. Am J Obstet Gynecol. 1994;170:1027–1033.
100. Woods JR Jr, Plessinger MA, Clark KE. Effect of cocaine on uterine blood flow and fetal oxygenation. JAMA. 1987;257:957–961.
101. Gouin K, Murphy K, Shah PS. Effects of cocaine use during pregnancy on low birthweight and preterm birth: systematic review and metaanalyses. Am J Obstet Gynecol. 2011;204:340 e1–12.
102. Little BB, Snell LM, Trimmer KJ, et al. Peripartum cocaine use and adverse pregnancy outcome. Am J Hum Biol. 1999;11:598–602.
103. Towers CV, Pircon RA, Nageotte MP, et al. Cocaine intoxication presenting as preeclampsia and eclampsia. Obstet Gynecol. 1993;81:545–547.
104. Shankaran S, Lester BM, Das A, et al. Impact of maternal substance use during pregnancy on childhood outcome. Semin Fetal Neonatal Med. 2007;12:143–150.
105. Richardson GA, Hamel SC, Goldschmidt L, Day NL. Growth of infants prenatally exposed to cocaine/crack: comparison of a prenatal care and a no prenatal care sample. Pediatrics. 1999;104:e18.
106. Bauer CR, Shankaran S, Bada HS, et al. The Maternal Lifestyle Study: drug exposure during pregnancy and short-term maternal outcomes. Am J Obstet Gynecol. 2002;186:487–495.
107. Behnke M, Eyler FD, Garvan CW, Wobie K. The search for congenital malformations in newborns with fetal cocaine exposure. Pediatrics. 2001;107:E74.
108. Frank DA, Augustyn M, Knight WG, et al. Growth, development, and behavior in early childhood following prenatal cocaine exposure: a systematic review. JAMA. 2001;285:1613–1625.
109. Mayes LC, Cicchetti D, Acharyya S, Zhang H. Developmental trajectories of cocaine-and-other-drug-exposed and non-cocaine-exposed children. J Dev Behav Pediatr. 2003;24:323–335.
110. Bae S, Zhang L. Prenatal cocaine exposure increases apoptosis of neonatal rat heart and heart susceptibility to ischemia-reperfusion injury in 1-month-old rat. Br J Pharmacol. 2005;144:900–907.
111. Ross VH, Moore CH, Pan PH, et al. Reduced duration of intrathecal sufentanil analgesia in laboring cocaine users. Anesth Analg. 2003;97:1504–1508.
112. Whittington RA, Virag L, Vulliemoz Y, et al. Dexmedetomidine increases the cocaine seizure threshold in rats. Anesthesiology. 2002;97:693–700.
113. Stoelting RK, Creasser CW, Martz RC. Effect of cocaine administration on halothane MAC in dogs. Anesth Analg. 1975;54:422–424.
114. American College of Obstetricians and Gynecologists. Methamphetamine abuse in women of reproductive age. [ACOG Committee Opinion No. 479. Washington, DC] March 2011.
115. Terplan M, Smith EJ, Kozloski MJ, Pollack HA. Methamphetamine use among pregnant women. Obstet Gynecol. 2009;113:1285–1291.
116. Wouldes T, LaGasse L, Sheridan J, Lester B. Maternal methamphetamine use during pregnancy and child outcome: what do we know? N Z Med J. 2004;117:U1180.
117. Cox S, Posner SF, Kourtis AP, Jamieson DJ. Hospitalizations with amphetamine abuse among pregnant women. Obstet Gynecol. 2008;111:341–347.
118. Ho E, Karimi-Tabesh L, Koren G. Characteristics of pregnant women who use ecstasy (3, 4-methylenedioxymethamphetamine). Neurotoxicol Teratol. 2001;23:561–567.
119. Kalant H. The pharmacology and toxicology of “ecstasy” (MDMA) and related drugs. CMAJ. 2001;165:917–928.
120. Rodgers J, Ashton CH, Gilvarry E, Young AH. Liquid ecstasy: a new kid on the dance floor. Br J Psychiatry. 2004;184:104–106.
121. Ludlow J, Christmas T, Paech MJ, Orr B. Drug abuse and dependency during pregnancy: anaesthetic issues. Anaesth Intensive Care. 2007;35:881–893.
122. Smith C, Cardile AP, Miller M. Bath salts as a “legal high.”. Am J Med. 2011;124:e7–e8.
123. Falgiani M, Desai B, Ryan M. “Bath salts” intoxication: a case report. Case Rep Emerg Med. 2012;2012:976314.
124. Samuels SI, Maze A, Albright G. Cardiac arrest during cesarean section in a chronic amphetamine abuser. Anesth Analg. 1979;58:528–530.
125. Kuczkowski KM. Postpartum convulsions and acute hemodynamic instability in the parturient with recent amphetamine intake. Arch Gynecol Obstet. 2009;280:1059–1061.
126. Volkow ND, Chang L, Wang GJ, et al. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci. 2001;21:9414–9418.
127. Sajo E. Pharmacology of substance abuse. Lippincotts Prim Care Pract. 2000;4:319–335.
128. Oro AS, Dixon SD. Perinatal cocaine and methamphetamine exposure: maternal and neonatal correlates. J Pediatr. 1987;111:571–578.
129. Ladhani NN, Shah PS, Murphy KE. Prenatal amphetamine exposure and birth outcomes: a systematic review and metaanalysis. Am J Obstet Gynecol. 2011;205:219 e1–7.
130. Cernerud L, Eriksson M, Jonsson B, et al. Amphetamine addiction during pregnancy: 14-year follow-up of growth and school performance. Acta Paediatr. 1996;85:204–208.
131. Hanzawa S, Nemoto M, Etoh S, et al. A case of amphetamine-induced down-regulation of beta-adrenoceptor. Masui. 2001;50:1242–1245.
132. Johnston RR, Way WL, Miller RD. Alteration of anesthetic requirement by amphetamine. Anesthesiology. 1972;36:357–363.
133. Michel R, Adams AP. Acute amphetamine abuse: problems during general anaesthesia for neurosurgery. Anaesthesia. 1979;34:1016–1019.
134. The NSDUH Report; Patterns of Hallucinogen Use and Initiation: 2004 and 2005. U.S. Department of Health and Human Services. Substance Abuse and Mental Health Services Administration, Office of Applied Studies. [Available at] http://www.samhsa.gov/data/2k7/hallucinogen/hallucinogen.pdf [Accessed March 2013] .
135. Methamphetamine Abuse and Addiction. National Institute on Drug Abuse Research Report Series. [NIH Publication Number 06-4210. Washington, DC, U.S. Health and Human Services, National Institutes of Health, National Institute on Drug Abuse, 1998, revised 2006. Available at] http://www.drugabuse.gov/publications/research-reports/methamphetamine-abuse-addiction [Accessed March 2013] .
136. Mvula MM, Miller JM Jr, Ragan FA. Relationship of phencyclidine and pregnancy outcome. J Reprod Med. 1999;44:1021–1024.
137. Crome IB, Kumar MT. Epidemiology of drug and alcohol use in young women. Semin Fetal Neonatal Med. 2007;12:98–105.
138. Sporer KA. Acute heroin overdose. Ann Intern Med. 1999;130:584–590.
139. Mitra S, Sinatra RS. Perioperative management of acute pain in the opioid-dependent patient. Anesthesiology. 2004;101:212–227.
140. Peng PW, Tumber PS, Gourlay D. Review article: perioperative pain management of patients on methadone therapy. Can J Anaesth. 2005;52:513–523.
141. Fajemirokun-Odudeyi O, Sinha C, Tutty S, et al. Pregnancy outcome in women who use opiates. Eur J Obstet Gynecol Reprod Biol. 2006;126:170–175.
142. Kandall SR, Doberczak TM, Jantunen M, Stein J. The methadone-maintained pregnancy. Clin Perinatol. 1999;26:173–183.
143. McCarthy JJ, Leamon MH, Parr MS, Anania B. High-dose methadone maintenance in pregnancy: maternal and neonatal outcomes. Am J Obstet Gynecol. 2005;193:606–610.
144. Bryson EO, Lipson S, Gevirtz C. Anesthesia for patients on buprenorphine. Anesthesiol Clin. 2010;28:611–617.
145. Jones HE, Kaltenbach K, Heil SH, et al. Neonatal abstinence syndrome after methadone or buprenorphine exposure. N Engl J Med. 2010;363:2320–2331.
146. Gevirtz C, Frost EA, Bryson EO. Perioperative implications of buprenorphine maintenance treatment for opioid addiction. Int Anesthesiol Clin. 2011;49:147–155.
147. Alto WA, O’Connor AB. Management of women treated with buprenorphine during pregnancy. Am J Obstet Gynecol. 2011;205:302–308.
148. American College of Obstetricians and Gynecologists. Opioid abuse, dependence, and addiction in pregnancy. ACOG Committee Opinion No. 524. Washington, DC. (Obstet Gynecol 2012; 119:1070-6.).
149. Mao J. Opioid-induced abnormal pain sensitivity: implications in clinical opioid therapy. Pain. 2002;100:213–217.
150. Compton P, Charuvastra VC, Ling W. Pain intolerance in opioid-maintained former opiate addicts: effect of long-acting maintenance agent. Drug Alcohol Depend. 2001;63:139–146.
151. Fischer G. Treatment of opioid dependence in pregnant women. Addiction. 2000;95:1141–1144.
152. Meyer M, Wagner K, Benvenuto A, et al. Intrapartum and postpartum analgesia for women maintained on methadone during pregnancy. Obstet Gynecol. 2007;110:261–266.
153. Broussard CS, Rasmussen SA, Reefhuis J, et al. Maternal treatment with opioid analgesics and risk for birth defects. Am J Obstet Gynecol. 2011;204:314 e1–11.
154. Sinha C, Ohadike P, Carrick P, et al. Neonatal outcome following maternal opiate use in late pregnancy. Int J Gynaecol Obstet. 2001;74:241–246.
155. Ornoy A, Ergaz Z. Alcohol abuse in pregnant women: effects on the fetus and newborn, mode of action, and maternal treatment. Int J Environ Res Public Health. 2010;364–369.
156. Kornfeld H, Manfredi L. Effectiveness of full agonist opioids in patients stabilized on buprenorphine undergoing major surgery: a case series. Am J Ther. 2010;17:523–528.
157. Cassidy B, Cyna AM. Challenges that opioid-dependent women present to the obstetric anaesthetist. Anaesth Intensive Care. 2004;32:494–501.
158. Rapp SE, Ready LB, Nessly ML. Acute pain management in patients with prior opioid consumption: a case-controlled retrospective review. Pain. 1995;61:195–201.
159. Kuczkowski KM. Ethylene glycol and other solvent use in pregnancy: a new phenomenon and a new diagnostic dilemma. Acta Anaesthesiol Scand. 2006;50:1037.
160. Williams JF, Storck M. Inhalant abuse. Pediatrics. 2007;119:1009–1017.
161. Kralova I, Stepanek Z, Dusek J. Ethylene glycol intoxication misdiagnosed as eclampsia. Acta Anaesthesiol Scand. 2006;50:385–387.
162. Leth PM, Gregersen M. Ethylene glycol poisoning. Forensic Sci Int. 2005;155:179–184.
163. Kuczkowski KM. Solvents in pregnancy: an emerging problem in obstetrics and obstetric anaesthesia. Anaesthesia. 2003;58:1036–1037.
164. Tenenbein M, Casiro OG, Seshia MM, Debooy VD. Neonatal withdrawal from maternal volatile substance abuse. Arch Dis Child Fetal Neonatal Ed. 1996;74:F204–F207.
165. Gray TR, LaGasse LL, Smith LM, et al. Identification of prenatal amphetamines exposure by maternal interview and meconium toxicology in the Infant Development, Environment and Lifestyle (IDEAL) study. Ther Drug Monit. 2009;31:769–775.
166. Haufroid V, Lison D. Urinary cotinine as a tobacco-smoke exposure index: a minireview. Int Arch Occup Environ Health. 1998;71:162–168.