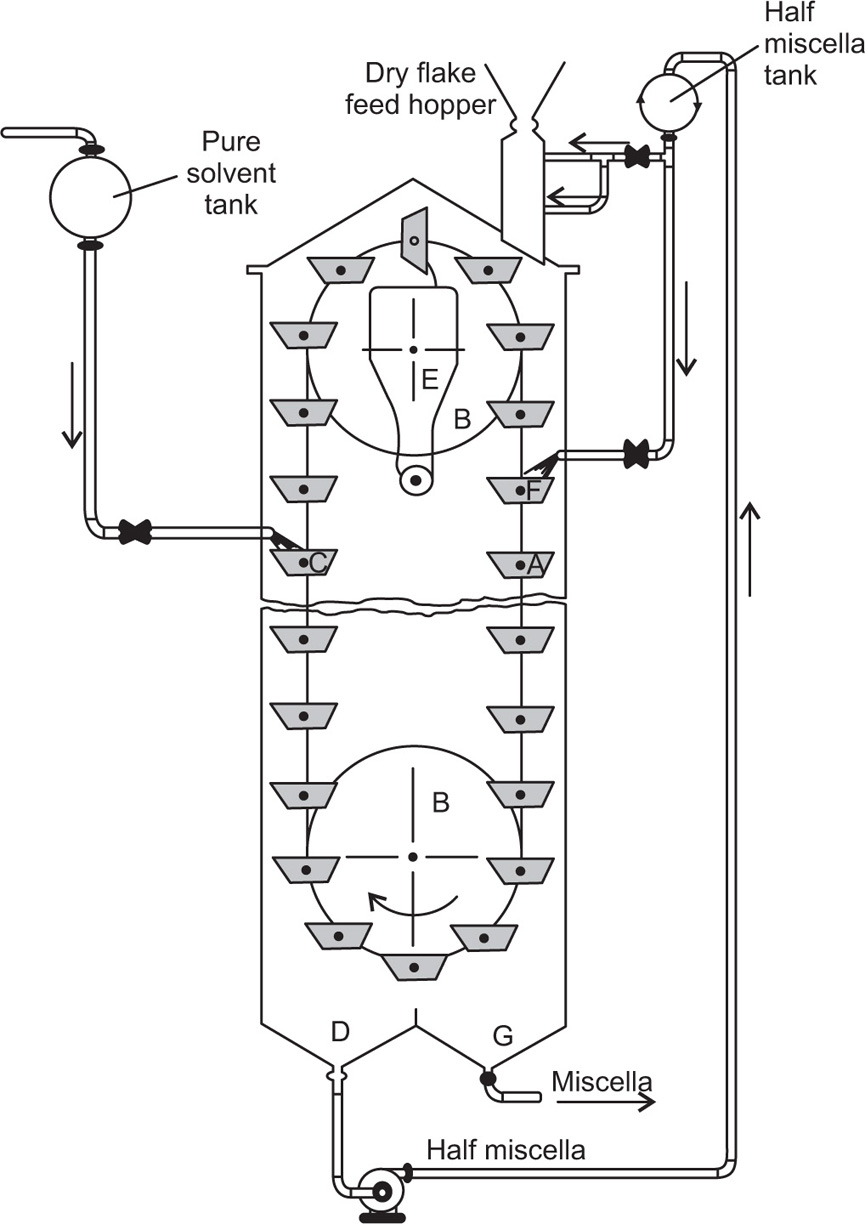
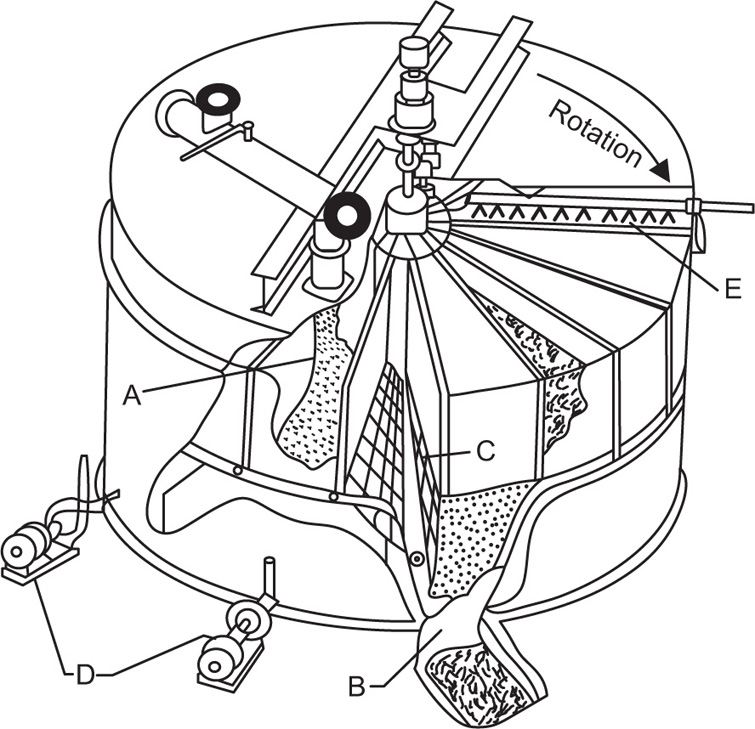
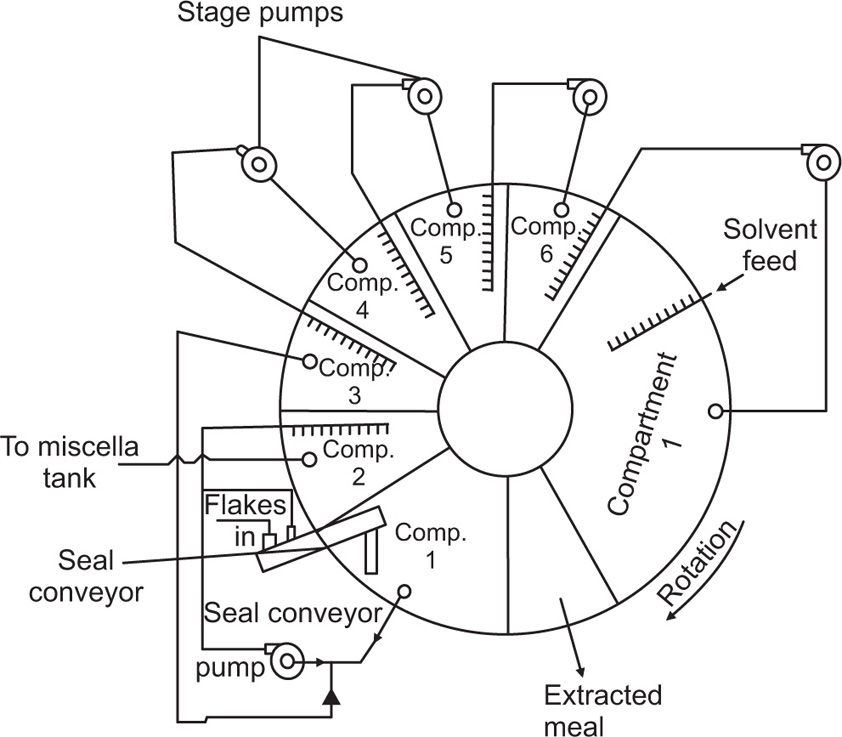
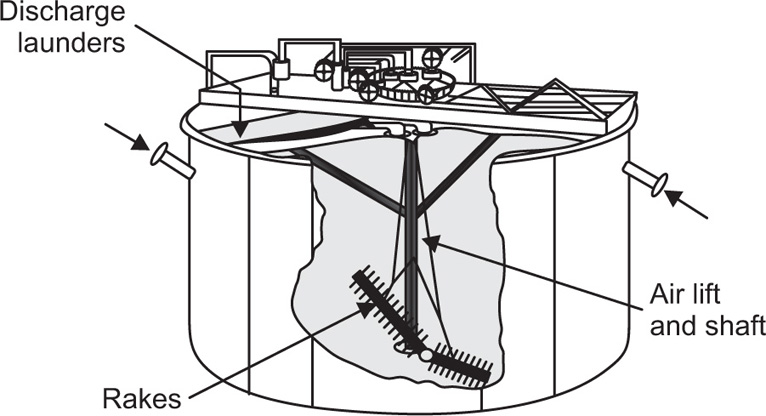
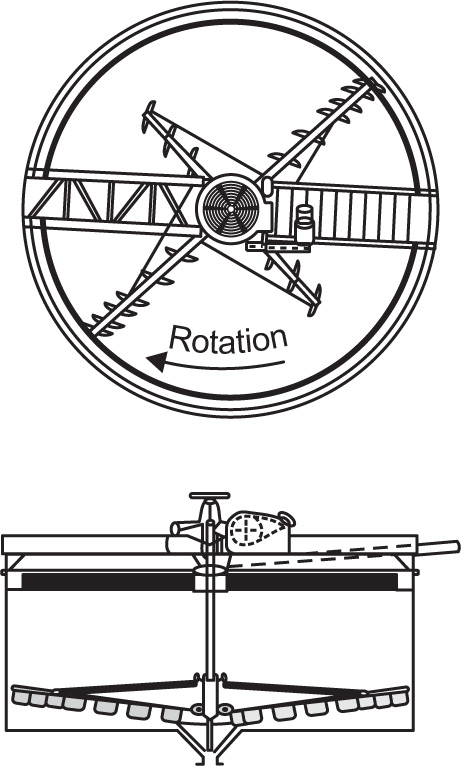
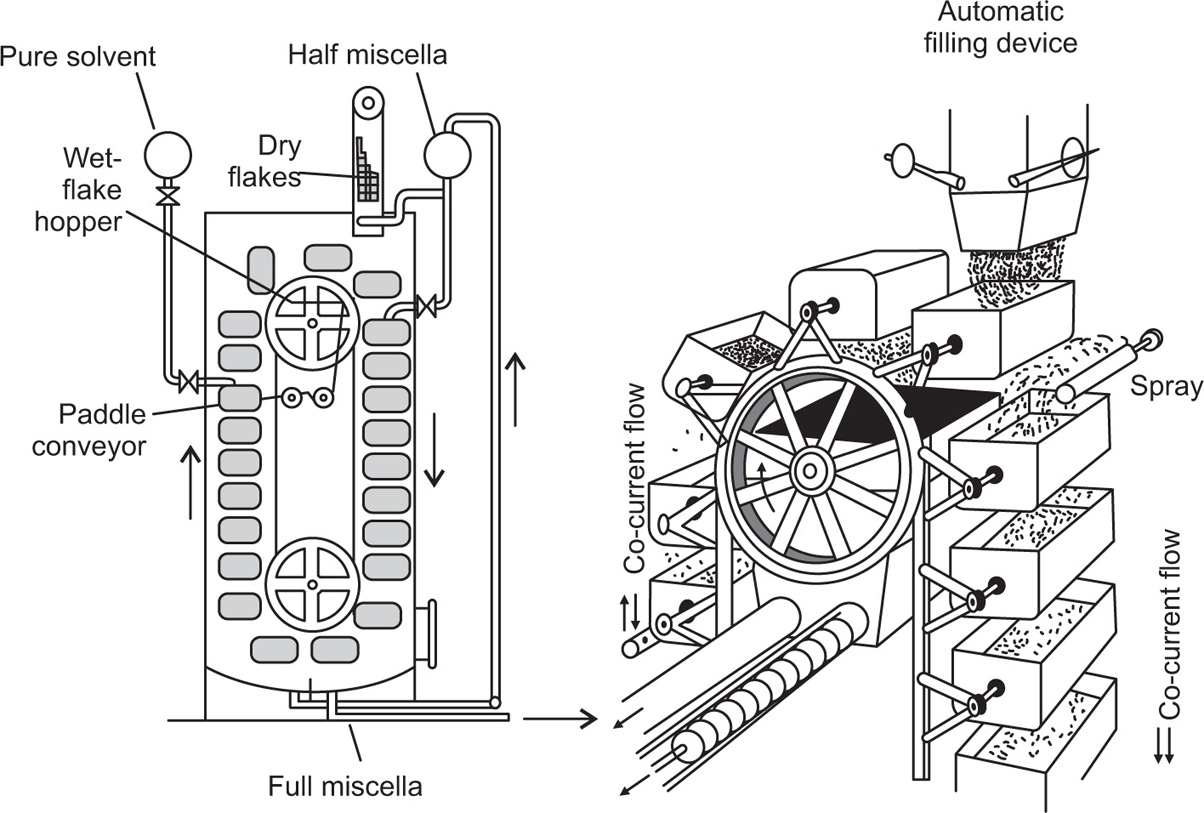
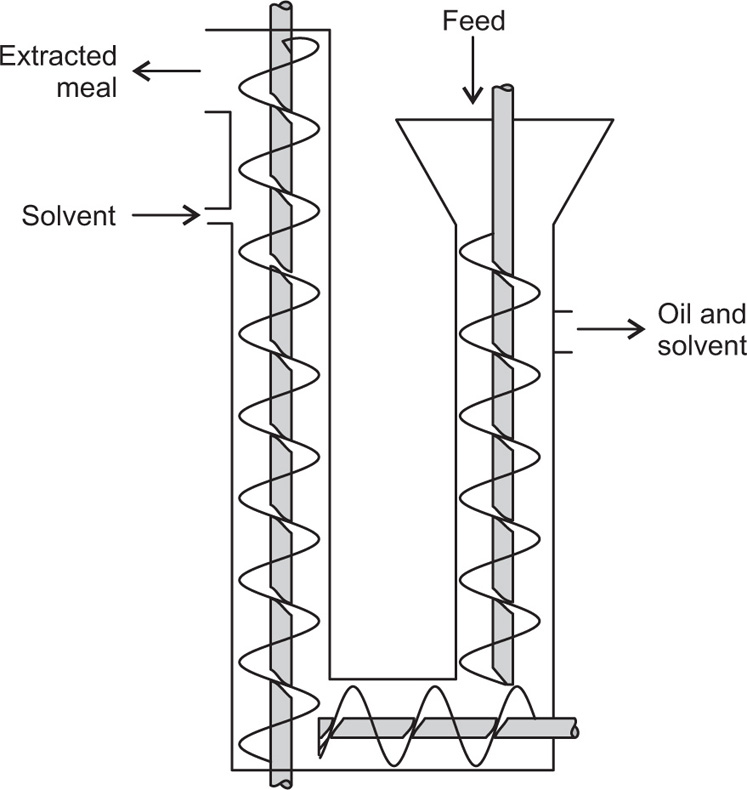
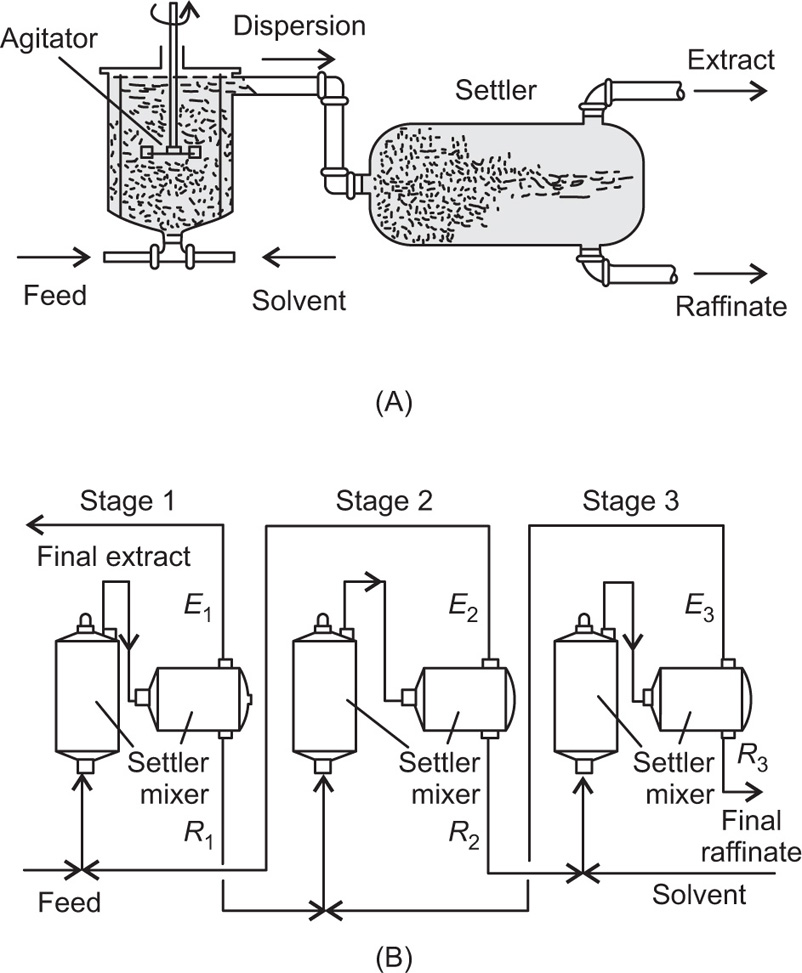
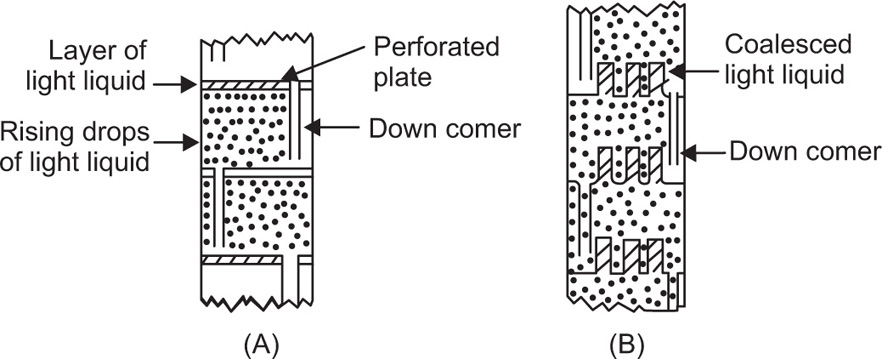
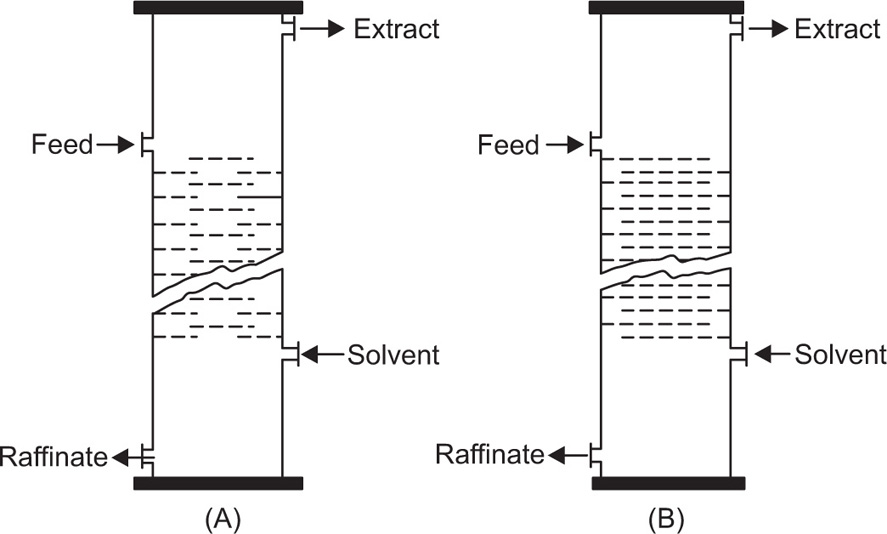
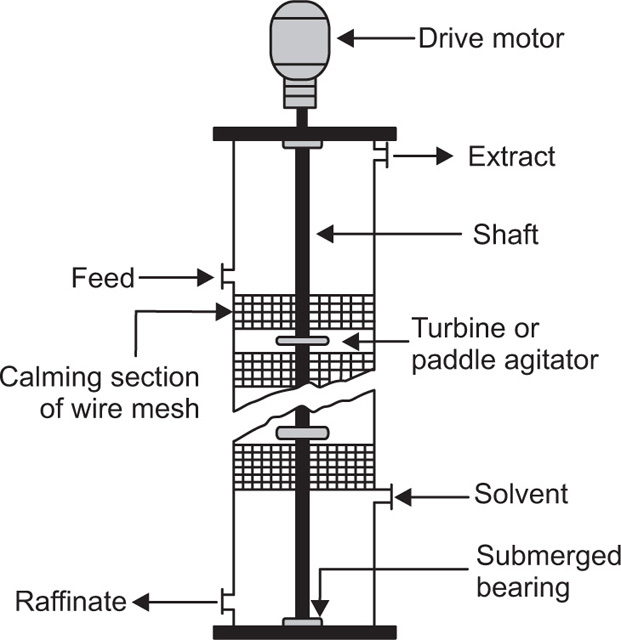
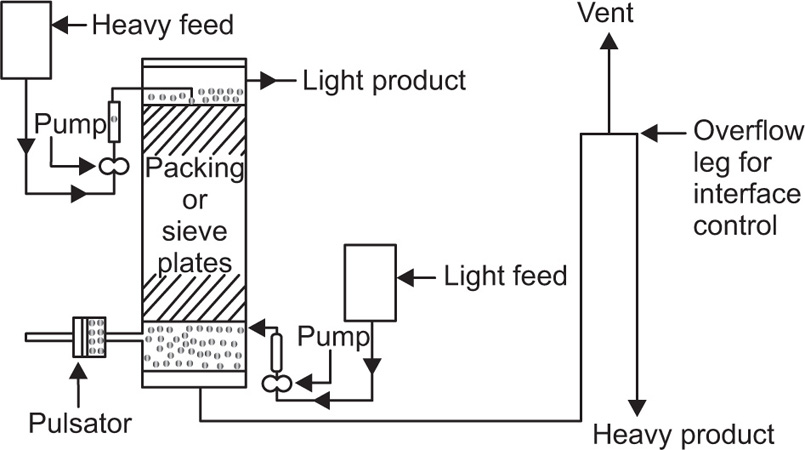
Pretreatment of The Raw material and the Mechanism of Leaching
Some preliminary treatment of the raw material is nearly always necessary in order to facilitate extraction of the active principles. Where these are contained in cells near the epidermis, drastic comminution is unnecessary, and bruising or slicing thinly is adequate before maceration. Further treatment could, indeed, lead to an inferior product due to loss of volatile constituents. Occasionally, the raw material needs to be freshly prepared; thus cardamom seed must be freshly removed from the fruit and used immediately after being bruised or coarsely powdered.
Tissues which yield their active principles less readily must be reduced to a powder before extraction and it is very important that this should produce material of the right
particle size. The smaller this is, the greater will be the surface area exposed to the solvent, and the shorter will be the distance through which this must diffuse to reach the solute and extract it. Increasing the degree of comminution will therefore lead to a faster rate of extraction. The most suitable particle size, however, is not necessarily the smallest, and it will depend on the physical nature of the raw material and of the solvent employed. Hard, woody, materials that are leached by a solvent which causes little swel1ing of the tissues, can be reduced to a fine powder, e.g. ipecacuanha, which is extracted with 80 per cent alcohol. Tissues that swell in the solvent, however, would produce a bed of low permeability if similarly treated. Also, although the cell walls of organized drugs retard the leaching of active constituents, they perform a useful function in preventing the extraction of unwanted high molecular weight constituents. Rupture of a considerable proportion of the cells by reduction to a fine powder may therefore yield an excessive amount of inert material which could hinder subsequent purification of the active principle or render difficult the clarification of a galenical (
Bull, 1935;
1936). Fine dry grinding may also affect the chemical properties of plant constituents and thereby initiate chemical reactions in which the active principle forms complexes with other cell constituents from which the pure compound becomes difficult to separate (
Pirie, 1956).
If the cell walls remain intact, diffusion through them may be the rate determining step and this will be in accordance with Fick’s first law of diffusion (see
Chapter 9). Extraction is not, however, always governed by molecular diffusion since dialysis and slow dissolution may be controlling factors (
Karnofsky, 1949a). Other factors may also be important; for example,
Othmer and coworkers (1955,
1959) demonstrated that, when soybean flakes are shaken with solvent, the oil extraction rate is controlled by capillary action within the broken cells. They showed the dependence of this upon the surface tension, density and viscosity of the solvent. Hydrostatic pressure, particle cohesion and convection currents are also important.
Dean et al. (1953) observe, however, that the rate of leaching finally depends upon the permeability of the cell wall. They suggest that Belladonna and Stramonium Tinctures may be rapidly prepared by passage of the drug suspended in the solvent through a colloid mill followed by clarification in a centrifuge. Another method which, on a small scale, might increase the efficiency of extraction of plant tissue is to subject the macerating material to ultrasonic vibration.
Thompson and Sutherland (1955) postulated that this decreased interfacial resistance to mass transfer, increased the interfacial area by reducing particle size, and increased the rate of dispersion of active principle away from the interface into the bulk of the solvent. As has been said above, however, such methods may lead to the extraction of unwanted material.
Since the raw material must form a bed of adequate permeability the particle size distribution should be sufficiently narrow to achieve this. A wide distribution would lead to a low porosity, because the smaller particles would tend to fill the voids between the larger particles. For this reason the monographs of the official preparations indicate a suitable degree of comminution and reference should be made to the official classification of powders.
The Nature of the Solvent
The ideal solvent should be selective in dissolving only the wanted constituents, but is rarely if ever met. The commonest solvent, water, is almost nonselective and alcohol is often insufficiently so. In practice dilute alcohols are used for many extractions but in some cases stronger a1cohol may be necessary to avoid solution of unwanted substances of high molecular weight, such as gums.
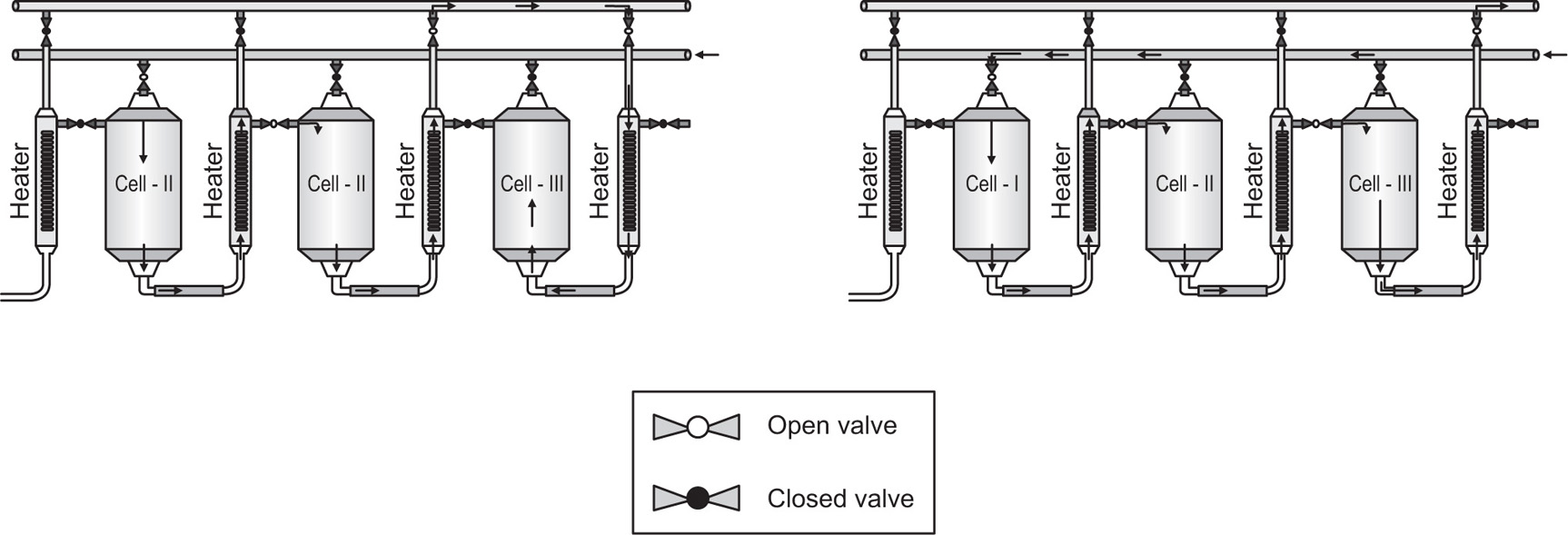
Additional processing is occasionally needed in order to remove undesirable constituents. Thus, before extracting ergot alkaloids with acid alcohol, the powdered ergot must first be defatted with low-boiling-point petroleum. In other cases the extract may be defatted. Thus on a small scale, in making Nux Vomica Liquid Extract, a liquid–liquid extraction may be used; the percolate, after concentration, is heated and shaken with hard paraffin, which retains the fat on cooling. Nux Vomica can also be extracted by a ‘cover and run down’ process using boiling water. Several of these extractions, each lasting about a day, may be needed to extract an economical amount of alkaloid. The aqueous extract can then be separated from the oil, concentrated by evaporation and re-extracted with strong alcohol to remove unwanted colloidal material. Further evaporation gives a soft extract which, after assay, can be suitably diluted with alcohol to make the tincture.
Aqueous extracts are subject to degradation by enzyme action. This can be inhibited by including alcohol to give a concentration of 25 per cent or more, but the enzyme is not destroyed and care must be taken to avoid subsequent conditions in which the enzyme activity can be restored. Water also supports the life of microorganisms which may degrade the drug constituents and it is therefore usually necessary to include preservative to prevent this. For most purposes alcohol is used, as it inhibits microbial activity at about the same concentration as is required to inhibit the isolated enzymes. Chloroform Water is used for the cold extraction of liquorice on a small scale, but it is lost when the extract is concentrated by evaporation, and preservation is maintained by addition of alcohol.
It has already been pointed out that some tinctures may need to mature for some time before being finally clarified. Undue deposition of inactive constituents may sometimes be prevented by enhancing the solvent power of the final product, e.g. glycerol is included in Compound Rhubarb Tincture as a solvent for tannins. Alternatively, precipitation of the unwanted material may be ensured by adjustment of the solvent; thus ammonia added to Senega Liquid Extract produces a slight alkalinity which precipitates inert matter and a bright product is obtained after filtration. With liquorice, however, a bright extract of nearly neutral pH can be obtained without the addition of ammonia and so a check for its absence is included in the official monograph. An increase in pH above neutrality considerably enhances the colour intensity and a variable product would be obtained if unspecified amounts of ammonia were present (
Oakley & Stuckey, 1949). The colour intensity of Liquorice Liquid Extract is also influenced by its trace metal content (
Collett, 1950;
1953). The equipment used for making it must therefore be carefully chosen.
If the solvent is to remain in the final product, it must be nontoxic. A number of liquids which are good solvents for the principles to be extracted may therefore be unsuitable for use. If, however, the solvent is to be removed by evaporation, freedom from toxicity is less important, provided that there is no hazard in its use. It must be completely volatilized during the evaporation process, but its boiling point should not be so low as to cause difficulties during the leaching process, which might necessitate the use of specially constructed equipment operating under pressure. The solvent should also be plentiful and cheap and have a low specific heat in order to reduce the cost of its removal. Preferably it should also be noninflammable. It should also have a low viscosity, for this facilitates the leaching of solute from the tissues, the percolation of solvent through the bed of the material and the pressing of the marc and subsequent handling of the solution. Concentration of the solution by evaporation is also influenced by the viscosity, since a high viscosity reduces the rate of heat transfer and increases the risk of overheating thermolabile solutes. A high solution viscosity also restricts the design of suitable evaporators.
For the solvent extraction of oils, such solvents as benzene, light petroleum, or trichloroethylene can be used, while ether is used for the extraction of Male Fern. Where traces of water might interfere with the extraction process, a water-miscible solvent, such as acetone, may be preferred. Acetone is used for the extraction of spices and Ginger Oleoresin. For Capsicum Oleoresin, however, alcohol may be preferred on an industrial scale, since a liquid–liquid extraction may be used later to get rid of water-soluble material. This is an alternative to percolation with acetone and removal of this, followed by cold extraction of the residue with alcohol.
Besides alcohol, hydrocarbons are also used to extract alkaloids. The raw material must, however, first be moistened with water or an alkaline solution to liberate the free alkaloidal base. This technique can be used to obtain reserpine, the moistened rauwolfia powder being extracted with hot benzene in a Soxhlet apparatus (
Boehringer et al., 1957). Purification of an alkaloid may be facilitated by such a procedure. The extract as first obtained will consist of a mixture of compounds, often chemically related but of differing pharmacological activity. One or more of the alkaloids will need to be further extracted from this mixture, perhaps by a change of solvent. An alkaloid may be partitioned from solution in a hydrocarbon into acidified water. By careful adjustment of the pH of the aqueous extract, separation of closely related compounds can be achieved by further liquid–liquid extraction. Final purification of alkaloids may also be assisted by careful choice of the acid used to make a crystalline salt (
Svoboda & Shahovsky, 1953).
Careful selection of solvent may be necessary, not only to achieve a maximum extraction of active principle, but also to prevent undue destruction during the extraction process (
Campo & Gramling, 1953). Racemization may also be an undesirable result of poor choice of solvent (
Carkhuff & Gramling, 1952).
Various workers have investigated the effect of adding surfactants to the solvent (
Helman, 1969). Nonionic compounds in alcoholic solvents may improve the extraction of alkaloids and, by enhancing the imbibition of solvent by the vegetable tissue, lead to a more rapid extraction with increased selectivity for alkaloid (
Butler & Wiese, 1953;
Srivastava & Chadha, 1963). In comparing the different classes of surfactants however,
Brochmann–Hanssen (1954) concluded that nonionic surfactants had little or no effect on the yield of alkaloid. He demonstrated that salts of cationic surfactants gave the best extraction, whereas anionic compounds were unsuitable due to solubilization and an increased permeability of the cell wall, although an ion exchange mechanism was postulated for cationic surfactants, many alkaloids being combined with cellular constituents such as acids, proteins and cell wall components (
Witt et al., 1953). Such adjuvants can only be used where the alkaloids are to be further purified, otherwise they would be present in the final product.
Temperature
The use of elevated temperatures is often precluded by the thermolability or volatility of the active principle, or by an increased extraction of unwanted constituents. For the isolation of a pure thermostable compound, however, raising the temperature of the solvent has the effect of hastening the leaching process, owing to the increased rate of diffusion, stronger convection currents and better solubility of the active principles. An enhanced solvent action is also assisted by loss of the integrity of cell walls and membranes.
Obsolete processes involving the use of hot solvents include the preparation of fresh infusions and decoctions. Fresh infusions were prepared by macerating the drug in a covered vessel for periods ranging from 15
minutes to 2 hours, followed by straining. Generally the water was boiling when first added, but occasionally cold water was used either to prevent too much leaching or to avoid gelatinization of starch. Fresh infusions were not adjusted to volume. Decoctions were prepared by boiling the drug with water, the process being restricted to drugs whose active principles were not volatile in steam. After straining, the volume was adjusted by passing water over the contents of the strainer.
Both maceration and percolation processes are amenable to the use of hot solvent. Maceration is accomplished by heating the drug and solvent in a closed vessel, this modification being known as digestion. Hot solvent is sprayed over the bed of raw material in some industrial processes.
Hot water is used for extracting liquorice and cascara, as a precursor to the official processes, but apart from thermolability or volatility of active principle, many drugs are not amenable to such treatment since inert extractive may tend to precipitate for months afterwards. An additional advantage with cascara, however, is that the elevated temperature reduces enzymatic degradation (
Fairbairn & Simic, 1970). Hot extraction is also used for nonofficial or nonmedicinal products: thus quassia is extracted with hot water and buchu with hot alcohol.
Occasionally it is necessary to subject the raw material to the action of hot solvent for an extended period of time, particularly when the solute is not readily soluble, or penetration of cellular tissue is slow. Such cases include the extraction of fixed oils from seeds and of alkaloids by means of such solvents as hydrocarbons, chloroform or methanol, and of ginger oleoresin with acetone.
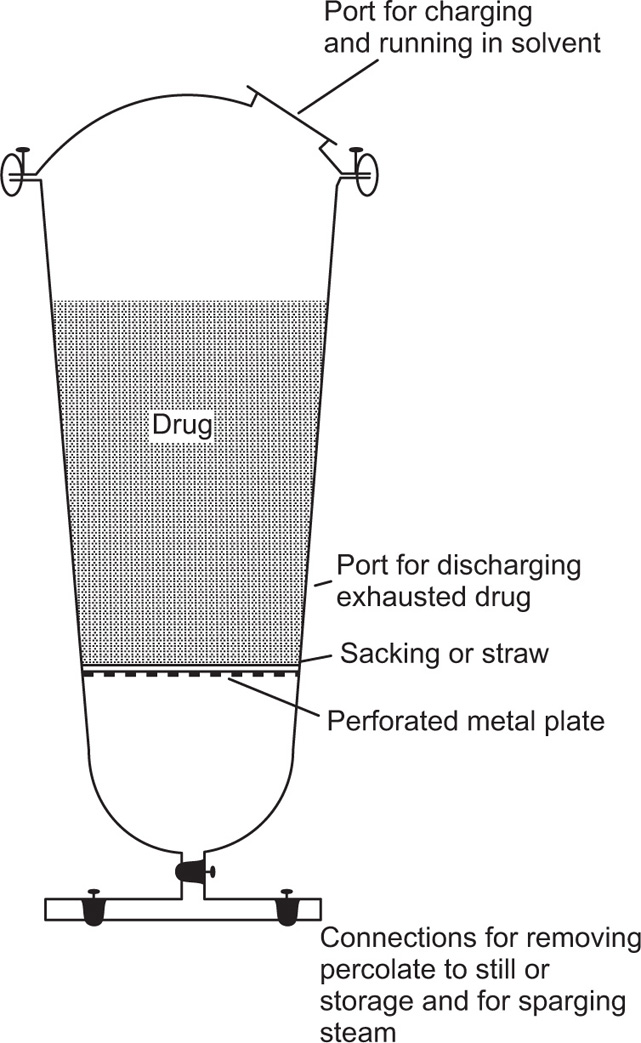
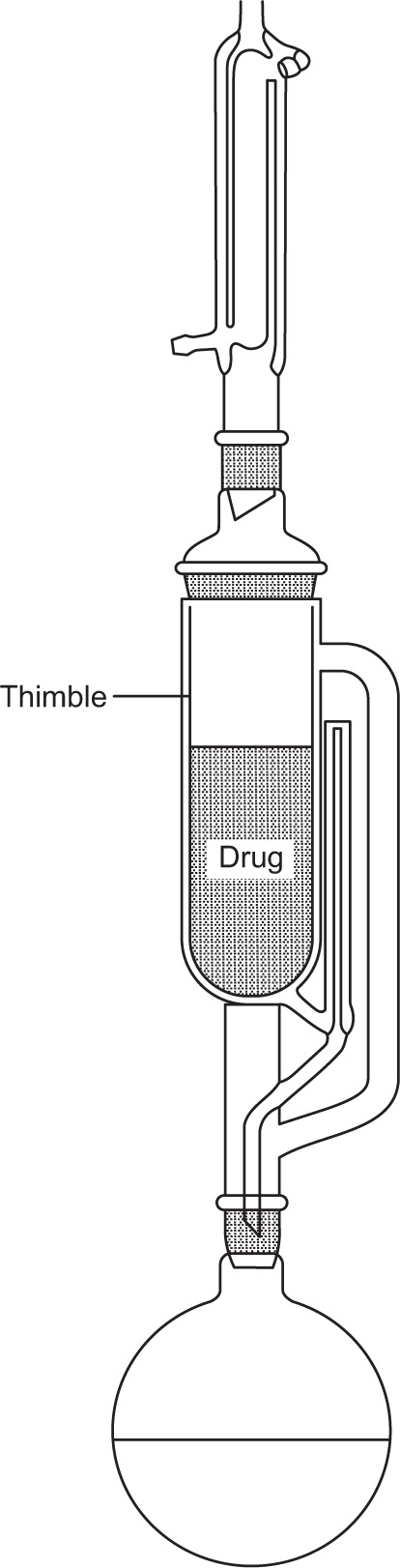
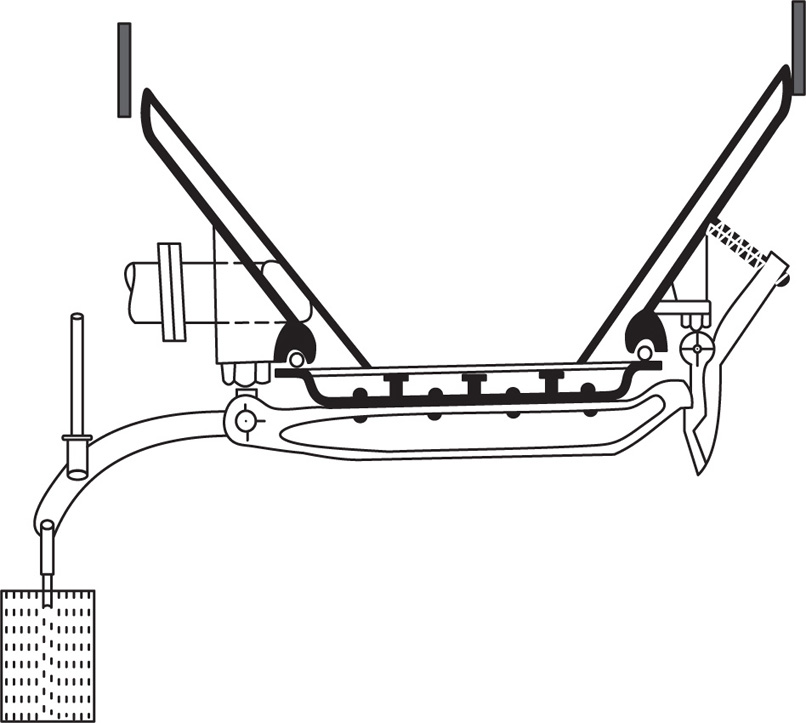
A small scale extraction apparatus is shown in
Fig. 13.2. It consists of a flask, a Soxhlet extractor and a reflux condenser. The raw material is usually placed in a ‘thimble’ made of filter paper and inserted into the wide central tube of the extractor. Solvent is placed in the flask and boiled, its vapour passing up the large right hand tube into the central space above the drug and thence to the condenser. The condensate then drops back on to the drug, through which it percolates, leaching solutes in the process. When sufficient of the solution has collected to raise its level to that of the top of the siphon tube, shown on the right hand side, the whole of the collected percolate siphons over into the flask. The suction effect of the siphoning assists permeation of solvent through the drug. A limited amount of hot solvent is thus made to percolate repeatedly through the raw material, the solutes from which are transferred to the flask.
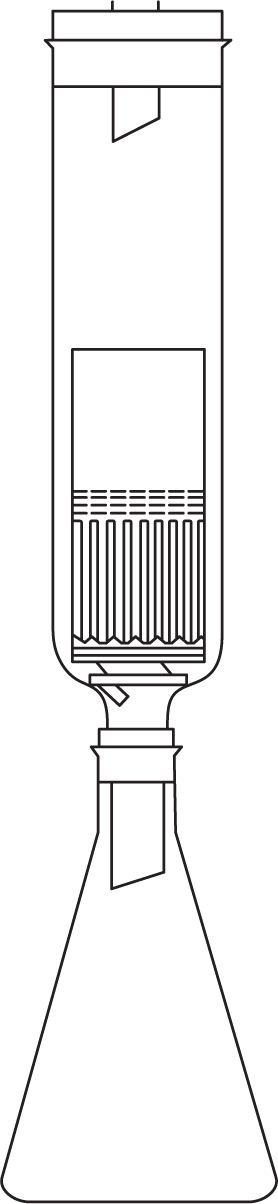
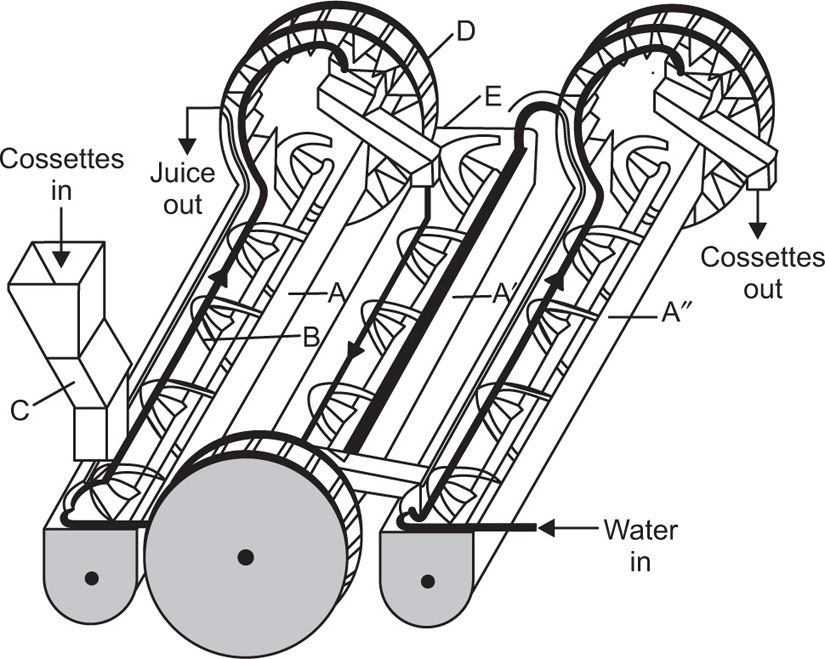
This principle of continuous hot extraction is sometimes used to extract a drug for the purposes of assay. A simple form of apparatus is described in the British Pharmacopoeia. It is shown in
Fig. 13.3 and has the advantage that the hot, rising vapours encircle the material to be extracted, though the short period of maceration undergone in the Soxhlet apparatus is sacrificed.
A large-scale plant has also been designed based on this principle.
Enzyme Activity
Enzyme action may increase solvent penetration of tissues, for example autolysis of lung tissue facilitates the extraction of heparin. Sometimes, fermentation may alter the composition of the soluble constituents, but occasionally the actual yield of an
active principle is increased. The sapogenin content of Dioscorea, for example, is increased if the tubers are homogenized with water and allowed to ferment before extraction.
Generally, however, enzyme activity needs to be minimized. The use of strong alcohol and elevated temperatures has been mentioned previously. Protein precipitation may also be used. In the Stoll process for the extraction of Digitalis glycosides, for example, hydrolytic enzymes are inhibited by precipitation with ammonium sulphate added directly to the homogenized tissues. The glycosides can then be extracted by maceration with 70 per cent alcohol with minimum loss.