Therapist’s Management of Tendon Transfers
▪ A team approach is critical to effectively address preoperative issues and enhance postoperative success.
▪ Preoperative factors considered by the team include (1) psychosocial issues, (2) status of neural recovery, (3) musculoskeletal readiness, (4) degree of sensibility, (5) motor learning aptitude, and (6) functional requirements.
▪ Preoperative therapy may be required to maintain or increase the strength of the muscle(s) proposed for transfer, to maximize preoperative passive range of motion and correct joint contractures, and to provide education regarding anticipated postoperative restrictions and requirements.
▪ Therapy after surgery has three phases: (1) immobilization, (2) activation of the transfer, and (3) strengthening/return to function.
▪ The use of custom-fabricated orthoses is an important part of the therapy program after tendon transfers.
1. The specifics of the surgical procedure as well as the patient’s clinical presentation will dictate the exact positioning requirement of the orthosis.
2. In general, the orthosis is fabricated with the joints affected by the transfer positioned to protect the resting tension of the transfer.
3. Familiarity with the specific surgical procedure, healing characteristics, and positioning requirements are essential in the fabrication and ongoing monitoring and modification of an upper extremity orthosis. An experienced certified hand therapist is uniquely qualified to fabricate the orthoses required.
Once neural recovery from peripheral nerve injury has plateaued, tendon transfers offer a means to enhance prehension and related function. Yet, the decision to perform tendon transfers requires a great deal of consideration preoperatively to ensure a successful outcome.
This chapter (1) outlines preoperative considerations, (2) reviews evaluations used to establish a baseline and monitor progress, and (3) considers postoperative rehabilitation methods used to foster prehensile skill after tendon transfer(s).
Tendon transfers done immediately after a traumatic injury as a salvage procedure require quick decision making by the surgeon, patient, and family. However, if tendon transfers are to be done after rehabilitation from peripheral nerve injury is complete, then a preoperative team assessment is often used to direct preoperative intervention strategies in preparation for the transfer procedure and to aid decision making regarding surgical procedures to be performed.
A team approach can effectively address preoperative issues and enhance postoperative success. The ideal team would consist of a hand surgeon, physician assistant or nurse practitioner, hand therapist (occupational or physical therapist), electrodiagnostician, social worker, the client, and his or her family. The client’s primary therapist is also an important team member because he or she is usually the most familiar with the client’s condition. Ideally, details on current function and preoperative goals will be exchanged among team members.
Preoperative factors considered by the team include (1) psychosocial issues, (2) status of neural recovery, (3) musculoskeletal readiness, (4) degree of sensibility, and (5) motor learning aptitude.
Success with postoperative rehabilitation depends on issues such as cognition, past and current abilities/interests, client roles, motivation, and compliance. Pertinent information can be obtained from the primary therapist, family/client interview, and observations of nonverbal behavior.1 Cognitive screening should be done with young children and individuals with impairments that may affect tendon transfer training. The use of the Disability of the Arm, Shoulder, and Hand Questionnaire2 or a graphic display of the client’s roles and responsibilities may supply useful information. A record of participation in therapy and carryover with home programs can help gauge future motivation and compliance.
Poor compliance can diminish the success of tendon transfer surgery and may stem from
1. Denial. Many individuals believe that the situation will resolve itself or determine that because the other limb is working well, they really “don’t need to improve.”
2. Frustration. The client and his or her family may have made many sacrifices to attend therapy and perform exercises without sufficient gratification of nerve recovery.
3. Lack of trust in the therapy program. The client may not believe in the rehabilitation process or have confidence in the team members because of outcome or other issues.
4. Finances. Therapy visits can be very costly when time, insurance copays, and travel expenses are also an issue.
5. Time. Other roles as a family member, job holder, and maintainer of the household may occupy the client’s day and limit the availability to participate or attend therapy sessions.
If the underlying reasons for poor compliance can be resolved, the chance for success may improve.
Before tendon transfers, the team should ensure that the opportunity for reinnervation has passed and that recovery has plateaued. For example, repeated assessment of muscle strength, sensibility, and dexterity should reveal gradual slowing in progress or lack of progress over the past few months. To verify current ability, the contribution of stabilization techniques used to augment movement and function (e.g., orthotic stabilization or neuromuscular electrical stimulation [NMES]) can be compared with the client’s individual effort (see Chapter 45) (Fig. 59-1). Findings from electrodiagnostic tests such as electromyography or nerve conduction studies can further verify that recovery has plateaued, and provide support that the client is ready for surgical intervention.
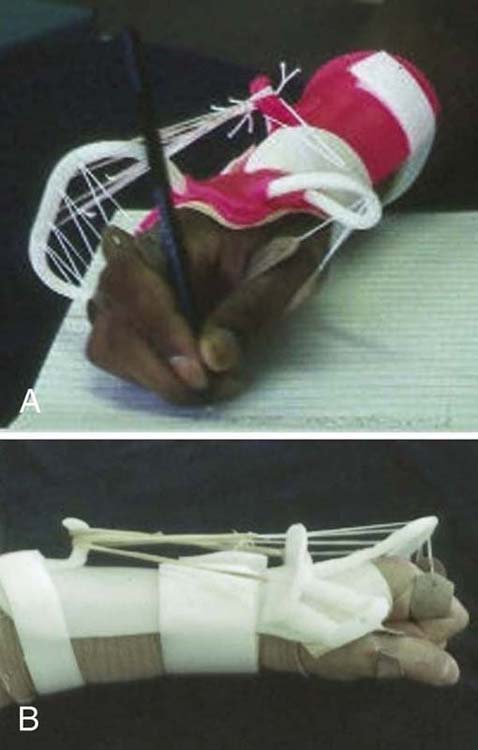
Figure 59-1 Two versions of a modified splint for a radial nerve injury without and with dynamic components. Both support the wrist and MCP joints to allow the client to write or grasp effectively.
Range of Motion. Ideally, full passive and active range of motion (ROM) should be present in the involved extremity before surgery, particularly at the associated joint(s). The involved side should be compared with the noninvolved side with a goniometer. Active ROM should also be assessed during functional tasks because the quality of the motion may differ from what is displayed during goniometry.
Muscle Strength. A strength assessment helps determine which muscles are strong enough to transfer. Although muscle physiologic cross-sectional area is proportional to maximum muscle strength, it is not easily measured.3 Therefore, strength is often documented with manual muscle testing or hand-held dynamometry (e.g., M500 Myometer; Jtech Medical Industries, Salt Lake City, UT) (Fig. 59-2). Traditionally, a muscle must be graded as 4/5 on a standard 0 to 5 manual muscle testing scale4 and have sufficient excursion in the new position to be considered for transfer. Proximal muscle weakness and incoordination can reduce muscle power, resulting in substitution patterns that the therapist should be cognizant of (see Table 45-2). Grip and pinch dynamometers help determine prehensile strength.5 However, there are no established recommendations associated with dynamometry and tendon transfers.
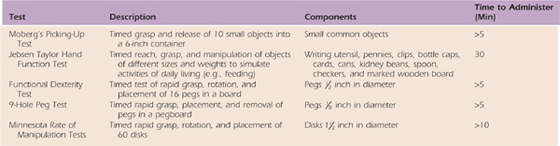
Assessments of Dexterity/Function. Measurement of coordination, skill, and function using standardized tests can validate the client’s need for tendon transfers and document preoperative performance. Table 59-1 (online) provides a list and descriptions of useful dexterity and functional tests. (See Chapter 12 for a detailed description of functional tests.)
Table 59-1 Dexterity and Functional Tests
Because it plays an important role in object manipulation,6,7 an accurate assessment of passive and active sensibility should be made. Touch pressure and discrimination are two forms of passive sensibility. The touch-pressure threshold is commonly tested with Semmes–Weinstein monofilaments8,9 or the Weinstein Enhanced Sensibility Test, as shown in Figure 59-3.10 Discrimination can be assessed with static/moving two-point discrimination using the Disk-Criminator.11,12 An alternative is the Pressure-Specifying Sensory Device,13 which was designed to determine the amount of pressure required to perceive one or two metal prongs. Active sensibility or stereognosis can be tested with the Moberg’s Picking-Up Test14,15 or the Byl-Cheney-Boczai Sensory Discriminator Test.16 The clinician is advised to follow the nerve distribution and assess both limbs for comparison during sensibility testing.
Activation of the muscle–tendon unit after transfer requires learning new control strategies. Preoperative motor learning ability can be measured behaviorally at four levels: acquisition, retention (consistency), transfer (flexibility), and efficiency.17,18 Initial practice or performance of a new task involves acquisition of a skill or a control aspect of a skill. Retention or consistency is evident when a skill or some aspect of a skill can be demonstrated after a short or long time delay. Transfer or flexibility involves the ability to execute a similar skill or slight variation (e.g., altered force or timing) after a time delay. Finally, efficiency refers to the ability to minimize energy expenditure during task performance such as during the execution of fine motor activities. The ideal test would measure all four levels.
Motor learning aptitude should be assessed with the noninvolved versus the involved limb. Acquisition can be assessed by observing performance of a novel task. For example, a client could be asked to quickly place 10 small objects in a set sequence into a container (acquisition). At the next therapy session, retention could be measured by having the client repeat placement of the objects in the same order without coaching. As a transfer task, the client could be asked to place the objects in reverse order without previous review. Finally, efficiency could be measured by timing performance.
When a client does not yet meet the surgical criteria, he or she may be referred back to therapy to further improve ROM and strength. For instance, muscle wasting or contractures may have developed during the primary healing phase. It is possible that the therapeutic regimen was insufficient or compliance with the recommended protocol was poor, detracting from the outcome. These issues should be resolved before proceeding with tendon transfer surgery.
Joint contractures often interfere with successful outcome from tendon transfers. Thus, it is imperative that maximum passive ROM be achieved in the involved joints preoperatively, which can be done with static progressive orthoses or serial casting.
Serial casting can be quite effective at resolving distal contractures. It provides quick improvement in ROM by incorporating a low-load sustained stretch to shortened tissue(s).19,20 Its use ensures client compliance because the cast cannot be easily removed. Unfortunately, prolonged cast wear may interfere with client roles, alter skin integrity, and temporarily weaken muscles needed for transfer. In those situations, a static progressive orthosis would be best (Fig. 59-4).
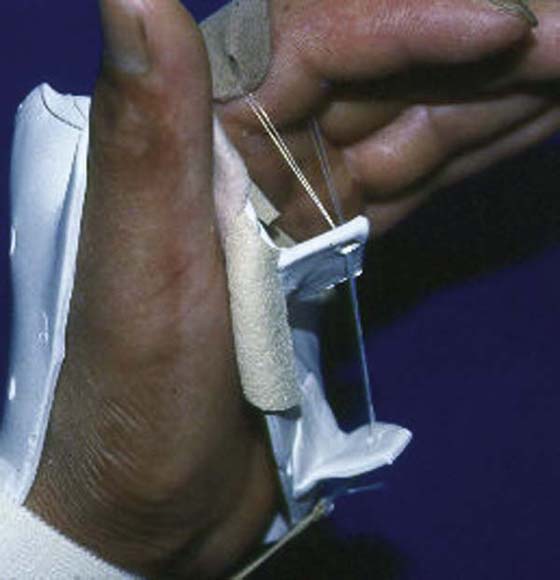
Figure 59-4 Static progressive orthosis to provide a low-load sustained stretch to the metacarpophalangeal joint.
Structures susceptible to contracture vary depending on the nerve injured. After a radial nerve injury, due to the loss of finger extension, extrinsic finger flexor muscle–tendon unit shortening may occur and this will limit combined wrist and finger extension. In this case, a forearm to finger tip serial cast or orthosis positioned at the end range of finger and wrist extension may be warranted. The orthosis can be serially adjusted to result in gradual lengthening of the shortened finger flexors and allow increased combined extension of the wrist and fingers. After a median nerve injury, the webspace between the thumb and index finger is vulnerable to an adduction contracture. A thumb webspacer orthosis or serial cast with stabilization of the metacarpophalangeal (MCP) joints of the thumb and index may help to maximize thumb abduction or extension. After an ulnar nerve injury, chronic intrinsic minus or claw posturing can lead to MCP joint hyperextension and interphalangeal (IP) joint flexion contractures. In this instance, orthoses or serial casts for MCP flexion and IP joint extension are an effective way to increase passive ROM at both joints (Fig. 59-5).
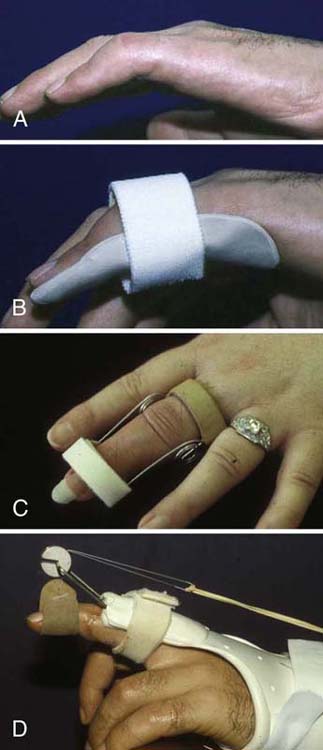
Figure 59-5 A, Proximal interphalangeal (PIP) joint contracture. B, Static three-point extension orthosis to increase extension. C, Spring wire PIP extension orthosis. D, Dynamic PIP extension orthosis.
Before surgery, methods to maintain or increase strength of the muscle(s) proposed for transfer should be encouraged. Biofeedback is effective in cueing individuals with various disabling conditions to activate muscles with more strength and vigor.21 NMES has also been used to augment muscle contraction in potential transfer muscles, as shown in Figure 59-6.22 Aquatic therapy or whirlpool provides a gravity-reduced environment useful for strengthening muscle without stress and strain. Once muscle activation is sufficient, resistance can be provided in the form of free weights, cuff weights, Thera-Band (The Hygienic Corporation, Akron, OH), theraputty, exercise machines, work simulators, and computer games (e.g., Biometrics device; Biometrics Ltd., Ladysmith, VA). If prehension patterns are limited, cuff weights can provide resistance without requiring grasp of a free weight.
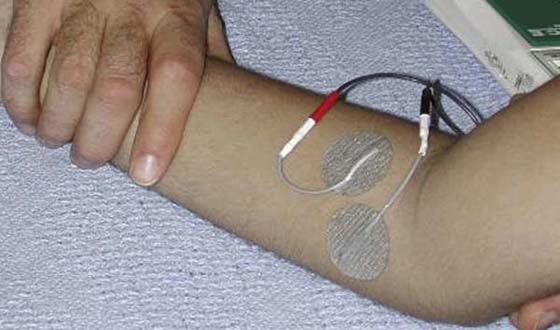
Figure 59-6 Neuromuscular electrical nerve stimulation to condition the pronator teres muscle before tendon transfer in its new role as a wrist extensor.
Postoperative guidelines should be reviewed before and after surgery to clarify expectations. Guidelines may include information regarding orthoses, therapy frequency, precautions, and activity limitations. The commitment required to achieve success from surgery should be emphasized, including the therapy timeline and the value of compliance with home exercise programs. These discussions may prompt the client to plan life events such as sports participation around the surgery schedule. Postoperative success and motivation can be enhanced by establishing realistic functional outcomes. A social worker can aid in planning for medical costs or travel to ensure postoperative therapy participation. Referral to another client who has undergone tendon transfers may enhance understanding of the experience.
Treatment after surgery consists of three phases: (1) immobilization, (2) activation of the transfer, and (3) strengthening/return to previous function (see Table 59-2 for goals at each phase).
Table 59-2 General Goal and Treatment Methods for Each Phase
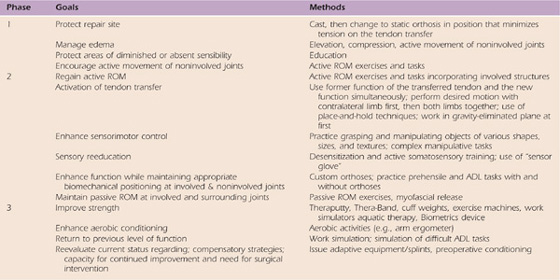
ADL, activities of daily living; ROM, range of motion.
After tendon transfer the limb is usually immobilized in a bulky dressing with a plaster shell at first and then a cast or custom-fabricated thermoplastic orthosis fabricated by a hand therapist for 3 to 5 weeks. Limited evidence has been published exploring the application of early motion after tendon transfers. In a clinical study23 of 20 patients after transfer of the extensor indicis proprius to the extensor pollicis longus to restore thumb extension, patients were treated with either immobilization or early dynamic motion (dynamic thumb extension with active, limited range flexion). The authors found that the dynamic motion group had better results after 4 weeks, but hand function was similar in both groups after 6 and 8 weeks. Patients treated with early dynamic motion recovered their hand function more rapidly than the immobilized patients, shortening total rehabilitation time and making dynamic motion treatment cost-effective. In a similar study, Rath24 reported that immediate postoperative active mobilization reduced rehabilitation time by an average of 19 days after opposition tendon transfer compared with immobilization. An earlier return to activities of daily living was a further benefit to patients.
A more recent study by Giessler et al.25 compared early free active motion with early dynamic motion with extension orthoses. Both regimens (dynamic vs. early active) achieved comparable clinical results.
Nevertheless, a period of immobilization is the more typical practice after tendon transfers and is particularly recommended for the younger or less compliant patient.
Orthotic Fabrication. After cast removal, the client is usually referred to therapy for fabrication of an orthosis to continue to protect the integrity of the transfer. The specifics of the surgical procedure as well as the patient’s clinical presentation will dictate the exact positioning requirements. In general, the orthosis is fabricated with the joints affected by the transfer positioned to protect the resting tension of the transfer. For example, after a transfer of the flexor digitorum superficialis (FDS) tendon of the ring finger to the thumb to restore opposition, a wrist-based thumb spica orthosis may be used to position the wrist in 10 to 20 degrees of flexion with thumb opposition. If a swan-neck deformity with PIP joint hyperextension develops in the ring finger, a static three-point hyperextension block orthosis may be warranted to limit PIP joint hyperextension. A protective orthosis should be removed only for exercise and bathing.
Edema Control. Immediately postoperatively, limb elevation prevents distal accumulation of fluid. Exercise proximal to the surgical site also prevents edema formation and may reduce the incidence of secondary joint stiffness. Compressive garments should be used as needed.
Soft-Tissue Mobility. Postoperative treatment includes interventions to prevent adhesions, increase tendon excursion, and enhance mobility at the surgical and donor sites. This can be accomplished in a few ways. One is through massage during activation of the transfer or separately, performed at least twice daily. If needed, vibration can be used to reduce pain before massage.26 Another method to improve soft tissue mobility and minimize hypertrophic scarring is with the use of a silicone gel sheet, which acts through a process of skin hydration.27 Use of an elasticized tubular bandage sleeve (Tubigrip) over the gel insert can keep it in place and increase the compression on the scar while contributing to edema control.
Home Program. During the immobilization phase, the primary goal is to protect the transfer. This is accomplished with casting and orthoses. Other goals such as edema control, active ROM of noninvolved joints, and soft tissue mobility can be reinforced at home.
Mobilization of the transfer requires learning to activate the muscle–tendon unit in a new role. To recruit the transfer, the client is encouraged to move into the former and new role simultaneously. For example, if the pronator teres is transferred to a new role as a wrist extensor, the transfer can be recruited by pronating the forearm and extending the wrist at the same time (Fig. 59-7A). It may be easier to initiate isolated motion such as wrist extension in a gravity eliminated plane (Fig. 59-7B). If he or she is unable to recruit the transfer in a gravity-eliminated plane, it may be easier to first try to place the limb in the new position passively and then ask the client to hold it (place-and-hold technique). Another strategy is to perform the desired motion with the noninvolved limb first and then attempt to move the involved limb actively or move both limbs simultaneously.
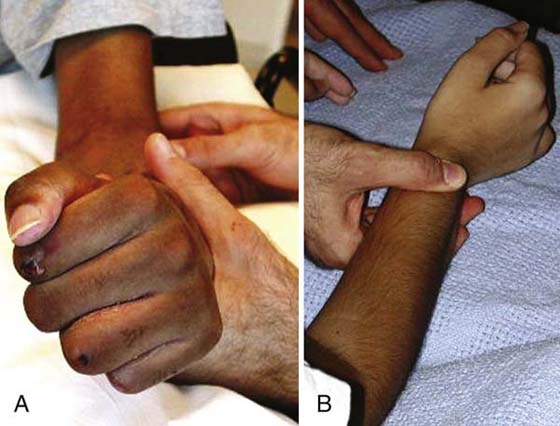
Figure 59-7 Initiation of active motion after pronator teres transfer for wrist extension. A, Attempted wrist extension in combination with forearm pronation. B, Isolated wrist extension in gravity-eliminated plane.
The client may feel that it is necessary to move vigorously and force movement of the transfer; however, he or she must be educated to move slowly and perform short sessions of exercise to prevent fatigue of the transferred muscle. A recommended strategy is for the client to activate the transfer through 10 repetitions and then rest while attending to scar massage, edema control, and passive ROM. This sequence could be repeated three times in the session, and the orthosis reapplied at the end of the session.
Below are examples of common transfers used for the radial, median, and ulnar nerve palsies (Table 59-3) along with methods to activate select transfers.
Table 59-3 Common Tendon Transfers
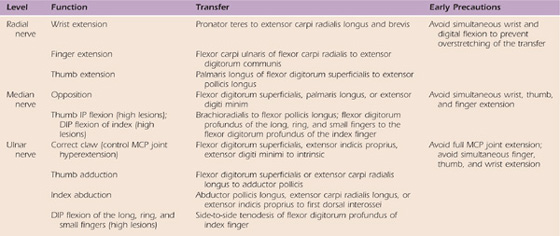
DIP, distal interphalangeal; IP, interphalangeal; MCP, metacarpophalangeal.
Radial Nerve Tendon Transfers. These transfers are often performed to augment wrist or finger/thumb extension after an injury at the forearm/elbow level. The pronator teres is often used to improve wrist extension. If the client has difficulty activating the transfer, the limb may be positioned with the forearm in approximately 20 degrees pronation, while the therapist provides counterpressure and the client attempts further active pronation. Resisted pronation should stimulate the transferred muscle to activate wrist extension (see Fig. 59-7A). To augment digit extension, the flexor carpi ulnaris may be transferred to the extensor digitorum communis (EDC). During therapy, the client may be encouraged to flex the wrist to neutral (from an extended position) while attempting to extend the MCP joints. Forceful wrist flexion past neutral is not encouraged because this may promote more tenodesis compared with active movement of the transfer. The client would then follow the same rest and exercise sequence as mentioned earlier.
Median Nerve Tendon Transfers. The most common median nerve transfer done after a wrist-level injury is an opponensplasty. To achieve this, the FDS tendon of the ring finger may be transferred to a thenar muscle such as the opponens pollicis or abductor pollicis brevis to enhance thumb opposition. During therapy, activation of the transferred FDS tendon may be encouraged if ring finger MCP flexion is blocked during attempted opposition. This may be further facilitated by active assistance of the thumb into opposition manually or through the assistance of an orthosis to position the thumb in opposition for light activity (Fig. 59-8).
Another transfer for higher level injuries is transfer of the brachioradialis to the flexor pollicis longus. Initially, activation of the transfer may take place in elbow flexion/forearm neutral to activate the brachioradialis in its former role as an elbow flexor. Once the client is able to activate the transfer, repetitive practice in the new role ensues (Fig. 59-9).
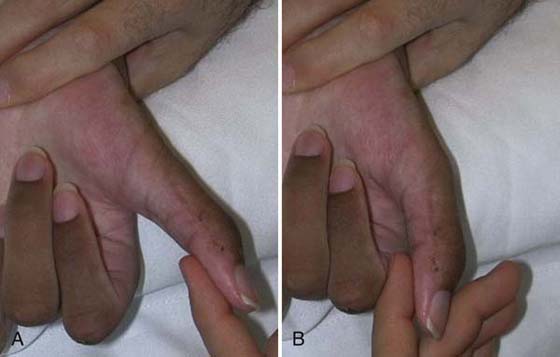
Figure 59-9 Training thumb flexion after transfer of the brachioradialis to the flexor pollicis longus.
Ulnar Nerve Tendon Transfers. Transfers after a wrist or forearm/elbow-level ulnar nerve injury are typically done to reduce posturing in the intrinsic minus or claw position. A common procedure in this case is the Zancolli lasso procedure in which the FDS to the ring and small fingers is detached from its insertion and wrapped around the second annular ligament near the volar aspect of the MCP joint(s), which acts as a pulley. The effect of the transfer is to limit the action of the EDC at the MCP joint by preventing MCP joint hyperextension. The action of the EDC is then transmitted distally, allowing PIP and distal IP (DIP) joint extension, eliminating the claw deformity. To activate this transfer, the client is encouraged to flex and extend the MCP joints in very small ranges (between 20 and 30 degrees from full MCP flexion resting posture), with special attention to avoid full MCP extension, which would stretch out and defeat the transfer. Initially, a wrist-based orthosis positioning the MCP joints in flexion and the IP joints in extension may be used. As treatment progresses, the patient may progress to a hand-based orthosis, as shown in Figure 59-10.
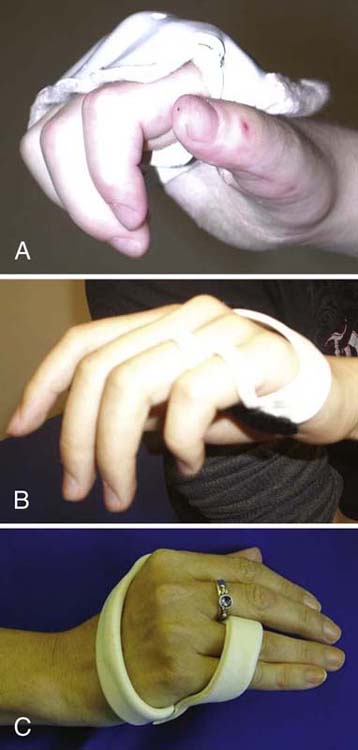
Figure 59-10 Hand-based orthoses to block hyperextension of the metacarpophalangeal joints digits 2 through 5 (A) and digits 4 and 5 (B, C).
Augmenting Muscle Activation. There are various options available to enhance activation of the transferred muscle including mirror visual feedback, therapeutic whirlpool, and vibration.28,29 Mirror visual feedback requires movement of the noninvolved limb in front of a mirror, which provides visual and proprioceptive feedback while the involved limb rests behind the back of the mirror. This process has been shown to engage mirror neurons in the contralateral hemisphere, which may aid in the initiation of movement in the involved limb.30 Another option is to place both limbs in a low agitation whirlpool or therapeutic pool, which provides buoyancy and assists active motion. Another useful method is to apply vibration to the muscle–tendon unit to foster muscle activation.28
Biofeedback and NMES. Activation of the transfer may be difficult to obtain initially because there may not be any observeable joint motion at first. Therapeutic devices that provide visual and/or auditory biofeedback when activation of the transfer is attempted may aid this process. If biofeedback is not successful at enhancing activation, NMES may be required. Units as simple as a hand-held device such as the M500 Myotrac can be used.
If the transferred muscle cannot be held isometrically during place-and-hold techniques or if the client cannot be encouraged to move into the new position, NMES may help activate the muscle. NMES parameters are customized to the client’s age, need, and tolerance. After full ROM with gravity eliminated can be achieved, gravity-resisted motion may be used and then progressed to resisted motion.
Home Program. During the activation phase, an orthosis is often worn during the day but is removed three to five times daily for exercise and practice of specific tasks. Soft tissue mobility techniques and edema control should also be reinforced at home.
By the eighth week postoperatively, if the client can activate the transfer without assistance, he or she is ready for strengthening and functional task practice. During this phase, nighttime wear of an orthosis may continue for positioning only, or it may be discontinued.
Strengthening. Resistive grip, pinch, and upper limb exercises may be initiated. One form of resistive training that is useful is the Biometrics Exercise Kit (Biometrics Ltd., Ladysmith, VA). This device allows the client to activate or strengthen the transferred muscle through progressive resistance using varied prehension patterns while being visually engaged in a game on a computer screen (Fig. 59-11).

Figure 59-11 Strengthening through engagement in computer-based devices such as the Biometrics Exercise Kit.
Task Practice. Participation in functional activities should be encouraged in this phase in preparation for return to full activities of daily living and work/school/leisure activities. For example, after a brachioradialis to flexor pollicis longus transfer, thumb flexion/adduction can be used to hold a pen during handwriting (Fig. 59-12). Two components associated with successful intervention are the type of practice used in treatment and the form of feedback provided by the clinician. Part of a task or the whole task can be practiced. Practice may also be organized in a blocked, random, or serial (combination) fashion. Research in typical adults supports the use of blocked practice during acquisition; however, random or serial practice seems to better support retention and skill transfer.31 Other studies report that blocked practice may lead to a better outcome in clinical populations.32-34

Figure 59-12 Practicing handwriting after transfer of the brachioradialis to the flexor pollicis longus to enhance thumb flexion/adduction.
Feedback. How frequently and what type of feedback should be provided? Intermittent feedback has been shown to be more helpful for skill acquisition than continuous feedback.35 Thus, if knowledge of results on errors is provided in a bandwidth or when the error exceeds a tolerance limit, motor learning is facilitated.36 Informational feedback is more conducive to learning than just positive reinforcement such as “that was good” or “nice job.”18 For example, you may state “your wrist muscles seem stronger than last week, you were able to hold your wrist up for 10 seconds.” Furthermore, intermittent feedback is more conducive to learning than concurrent or constant feedback.37,35
Home Program. During this phase, activities to strengthen the transfer should be done. The key to success is practice; therefore, a specific practice schedule should be recommended.
M.C. is a lively 7-year-old boy who sustained a combined high median, radial, and ulnar nerve injury of his right dominant extremity. He received therapy in our outpatient facility for 1 year to monitor nerve recovery, provide sensory reeducation, and improve active ROM and strength. His family had a history of poor compliance with therapeutic regimens and therapy visits; however, they remained quite invested in his anticipated recovery. M.C. revisited the surgeon for a yearly follow-up examination, who recommended tendon transfers to augment wrist and thumb function. M.C. was referred back to therapy for preoperative evaluation of passive ROM, strength, sensibility, and dexterity (Jebsen Taylor Hand Function Test and the 9-Hole Peg Test). One week later, M.C. went to the operating room and had the pronator teres tendon dissected from the radial shaft and transferred to the extensor carpi radialis brevis. The right index extensor indicis tendon was transferred to the abductor pollicis brevis to aid thumb opposition.
M.C. was immobilized in a long arm cast for 5 weeks as follows: 90 degrees of elbow flexion with forearm neutral, 30 degrees of wrist extension, and 30 degrees of thumb palmar abduction. At 2 weeks postoperatively, he was recasted in the same position. At 4 weeks postoperatively, the cast was removed and M.C. was referred back to therapy for fabrication of a resting orthosis, which placed him in 30 degrees of wrist extension, 30 degrees of thumb palmar abduction, 30 degrees of MCP flexion, and 5 degrees of PIP and DIP flexion. The night orthosis was designed to protect the transfers and the muscle donor sites and preserve the arches of the hand because he lacked intrinsic muscle control before surgery. He was also issued a thumb spica orthosis for day wear (Fig. 59-13). M.C. had a history of poor compliance with orthosis wear. Therefore, his family was educated in detail regarding the importance of orthosis wear and home exercise compliance to maximize the results of the surgery.
The goals of the first session were as follows: (1) enhance soft tissue mobility, (2) increase passive ROM, (3) reduce pain, and (4) activate the transfers (Table 59-4, online). Treatment began with forearm and thumb scar massage to his forearm and thumb scars and passive ROM. M.C. was able to passively stretch into 50 degrees of wrist extension and 60 degrees of thumb abduction. He had a pain level of 6/10 on the Wong-Baker FACES Pain Rating Scale with passive ROM. To address pain during treatment, frequent breaks were taken and snacks provided. To activate the transfer, M.C.’s arm was positioned on the therapy table in 90 degrees of elbow flexion and forearm neutral. With guidance, he was instructed to pronate the forearm to elicit wrist extension. M.C. was then instructed to straighten his index finger while trying to move the thumb away from the palm into abduction. With these strategies combined, M.C. was able to exhibit 25 degrees of active wrist extension and 10 degrees of palmar abduction. Interestingly, overflow from activation of simultaneous movements from his uninvolved extremity seemed to enhance wrist extension and thumb abduction. The sequence of scar massage and passive and active ROM exercises were repeated three times in the session.
Table 59-4 Case Study M.C.: Therapy Goals
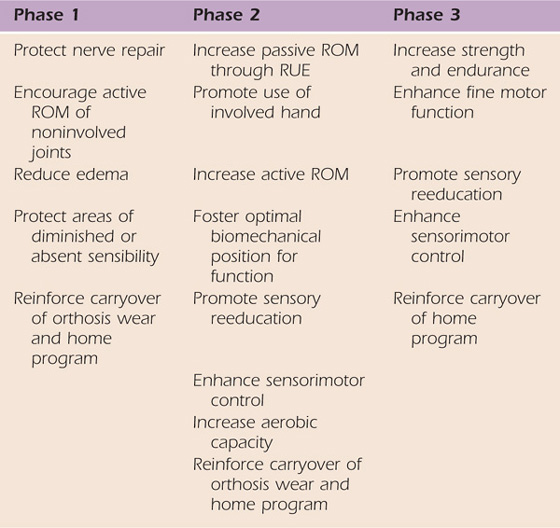
ROM, range of motion; RUE, right upper extremity.
Because of M.C.’s family’s past issues with compliance, it was even more important for them to have specific guidelines to follow. M.C. and his family were thoroughly instructed to perform the same sequence of activities at home five times per day and issued a detailed written home program with orthosis wear details, exercises, and scar massage. M.C. was seen 5 days after the initial session, and the orthosis was visibly dirty with an intense odor. This was a good sign that M.C. was wearing the orthosis; however, according to the family, M.C. did not take off the orthosis once over the 5-day period, despite all the education provided on the first visit. Therefore, the family was again reeducated on the importance of following the whole regimen at home.
For the next week, M.C. practiced eliciting wrist extension and palmar abduction in a gravity-eliminated plane. During therapy, table hockey games were played using light objects such as cotton balls, foam blocks, and peg dowels. The objects were to be moved across the table using only the dorsum of his hand with gravity-eliminated wrist extension. Biofeedback was also used to improve strength of the transfer, especially into opposition because this motion was more difficult for M.C. to visualize. In addition, M.C. only had 60 degrees of available MCP joint flexion at the time, so the thumb was not able to approximate the PIP joint as efficiently. M.C. quickly progressed to gravity-resisted wrist extension 1-½ weeks after cast removal. Active wrist extension against gravity was 10 degrees and palmar abduction was approximately 2 degrees.
The family reported that alternating between wear of the the night and day orthoses was challenging, so the night orthosis was discontinued. Thus, M.C. was only required to wear the thumb spica orthosis for day and night wear. Therapy activities were shifted to include placement and removal of objects from vertical incline surfaces such as loose fitting pegboards, water-cling foam blocks, and a vertical coin slot. M.C. was also instructed to grasp and release ping pong balls using a tenodesis grasp from a surface at shoulder height to engage the entire arm. M.C. used the Biometrics unit with a ROM arc of 0 to 25 degrees of nonresistive active ROM to exercise the wrist into extension. After 2 weeks, M.C. was only required to wear his orthosis for rough play activities and at night.
By week 8, M.C. began to verbalize the excitement of holding a bike handle, tying shoes, and grasping a mini football because these were activities that he had enjoyed previously, but was unable to perform for more than a year after the initial injury. M.C.’s index finger exhibited 80 degrees of MCP flexion, so he was able to elicit an inferior pincer grasp. However, efficiency of the pinch was inhibited by DIP hyperextension. Kinesiotaping was used to position the DIP joint into 5 degrees of flexion to block DIP hyperextension and provide stability for thumb to index finger opposition. Strengthening activities at this phase included the use of a Thera-Band, theraputty, the Theraband Power Web Exerciser, and graded clothes pins for resisted pinch. Other modalities included the Biometrics unit (for resisted gross grasp, wrist extension, and key pinch tasks), a nut-and-bolt assembly board, and practice securing fasteners such as shirt buttons and shoe laces. These prehensile tasks had frustrated M.C. in the period just after the nerve injury. Sensory reeducation activities were added including texture wands and use of the Dynagel unit (an aqueous gel medium tank that has general thermal and cold properties for resistive exercise and proprioceptive feedback). Coordination activities were introduced such as shifting playing cards, in-hand coin manipulation, and timed grasp and release of various sized pegs and dowels.
By 3 months postoperatively, M.C. was practicing more proximal strengthening activities such as wall push ups, Thera-Band chest presses, and graded ball tosses onto a rebounder. We also added conditioning activities such as the arm ergometer (Fig. 59-14). At 3 months postoperatively, he was progressing nicely in terms of dexterity and function. At 6 months postoperatively, M.C. had improved functional strength for wrist extension, thumb opposition, and key pinch (Fig. 59-15). The family also reported that he was back to performing some of his preinjury activities. At 6 months postoperatively, he had continued to make gains in dexterity and function. Tables 59-5 through 59-9, online, depict clinical findings at 3 and 6 months postoperatively. After a successful postoperative treatment program, he was seen less frequently and finally discharged with a home program and followed only in the hand clinic.
Table 59-5 Case Study M.C.: Sensibility (Right Hand Median/Ulnar Nerve Distributions)
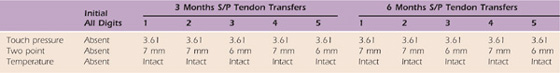
S/P, status/post.
Table 59-6 Case Study M.C.: Right Upper Extremity Active Range of Motion
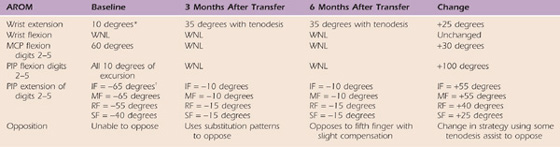
Left upper extremity active and passive range of motion was within normal limits.
*Full passive wrist extension achieved with full finger flexion with c/o 2/10 pain and end-range tightness. Composite extension with wrist past neutral induced complaints of 7/10 pain and end range tightness.
†Full passive digit extension only with wrist neutral.
IF, index finger; MCP, metacarpophalangeal; MF, middle finger; PIP, proximal interphalangeal; RF, ring finger; SF, small finger; WNL, within normal limits.
Table 59-7 Case Study M.C.: Right Upper Extremity Passive Range of Motion
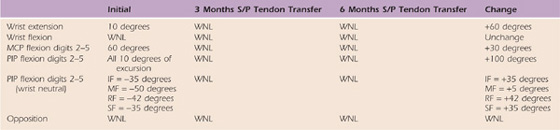
MCP, metacarpophalangeal; MF, middle finger; PIP, proximal interphalangeal; RF, ring finger; SF, small finger; WNL, within normal limits.
Table 59-8 Case Study M.C.: Strength via Dynamometry
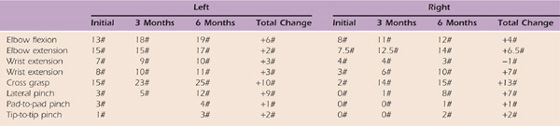
#, pounds.
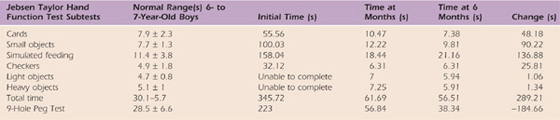
Table 59-9 Case Study M.C.: Dexterity Right Hand
If tendon transfers are an option after a client has plateaued after a peripheral nerve injury, the potential outcome must be fully explored by the hand team and the family because the preparation, surgery, and rehabilitation are lengthy and demanding. The preoperative phase requires a thorough examination with baseline objective tests and maintenance or achievement of maximum passive ROM at associated joints and maximum strength of the muscle(s) to be transferred. The postoperative and rehabilitation phase must include carefully positioned orthoses to protect the transfer, intense muscle reeducation procedures, and effective practice schedules to improve active ROM, strength, and prehension patterns in the involved limb. With diligence and creative programming, the functional benefit of tendon transfers may be achieved, enhancing function in everyday activities.
Video 59-1: Three months post-op Jebsen—small items
Video 59-2: Three months post-op Jebsen—cards
Video 59-3: Three months post-op 9-hole peg test
Video 59-4: Six months post-op Jebsen—small items
Video 59-5: Six months post-op Jebsen—cards
Video 59-6: Six months post-op 9-hole peg test
1. Starck-Schier J, Chan J. Changes in life roles after hand injury. J Hand Ther. 2007;20:57–59.
2. Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: the DASH (Disabilities of the Arm, Shoulder, and Hand). [corrected]. The Upper Extremity Collaborative Group (UECG). Am J Ind Med. 1996;29(6):602–608.
3. An KN, Linscheid RL, Brand PW. Correlation of physiological cross-sectional areas of muscle and tendon. J Hand Surg. 1991;16(1):66–67.
4. Hislop H, Montgomery J. Daniels and Worthingham’s Muscle Testing: Techniques of Manual Examination. 8th ed Philadelphia: WB Saunders; 2007.
5. Mathiowetz V. Comparison of Rolyan and Jamar dynamometers for measuring grip strength. Occup Ther Int. 2002;9(3):201–209.
6. Gordon AM, Duff SV. Relation between clinical measures and fine manipulative control in children with hemiplegic cerebral palsy. Dev Med Child Neurol. 1999;41(9):586–591.
7. Nowak DA, Hermsdörfer J. Selective deficits of grip force control during object manipulation in patients with reduced sensibility of the grasping digits. Neurosci Res. 2003;47(1):65–72.
8. Bell-Krotoski J, Buford WL. The force/time relationship of clinically used sensory testing instruments. J Hand Ther. 1997;10(4):297–309.
9. Bell-Krotoski JA, Fess EE, Figarola JH, Hiltz D. Threshold detection and Semmes-Weinstein monofilaments. J Hand Ther. 1995;8(2):155–162.
10. Weinstein S. Fifty years of somatosensory research: from the Semmes-Weinstein monofilaments to the Weinstein Enhanced Sensory Test. J Hand Ther. 1993;6(1):11–22.
11. Crosby PM, Dellon AL. Comparison of two-point discrimination testing devices. Microsurgery. 1989;10(2):134–137.
12. Moberg E. Two-point discrimination test: a valuable part of hand surgical rehabilitation, e.g., in tetraplegia. Scand J Rehabil Med. 1990;22:127–134.
13. Dellon ES, Mourey R, Dellon AL. Human pressure perception values for constant and moving one- and two-point discrimination. Plast Reconstr Surg. 1992;90(1):112–117.
14. Amirjani N, Ashworth NL, Gordon T, et al. Normative values and the effects of age, gender, and handedness on the Moberg Pick-Up Test. Muscle Nerve. 2007;35(6):788–792.
15. Moberg E. Objective methods of determining functional value of sensibility in the hand. J Bone Joint Surg. 1958;40B:454–466.
16. Byl N, Leano J, Cheney LK. The Byl-Cheney-Boczai Sensory Discriminator: reliability, validity, and responsiveness for testing stereognosis. J Hand Ther. 2002;15(4):315–330.
17. Duff SV, Quinn L. Motor learning and motor control. In: Cech D, Martin S, eds. Functional Movement Development Across the Lifespan. 2nd ed Philadelphia: WB Saunders; 2002:86–117.
18. Magill RA. Motor Learning and Control: Concepts and Applications. 7th ed New York: McGraw-Hill; 2004.
19. Kottke FJ, Pauley DL, Ptak RA. The rationale for prolonged stretching for the correction of shortening of connective tissue. Arch Phys Med Rehabil. 1966;47:345–352.
20. MacKay-Lyons M. Low-load, prolonged stretch in treatment of elbow flexion contractures secondary to head trauma: a case report. Phys Ther. 1989;69:292–296.
21. Byl NN, McKenzie A. Treatment effectiveness for patients with a history of repetitive hand use and focal hand dystonia: a planned, prospective follow-up study. J Hand Ther. 2000;13(4):239–301.
22. Stackhouse SK, Binder-Macleod SA, Stackhouse CA, et al. Neuromuscular electrical stimulation versus volitional isometric strength training in children with spastic diplegic cerebral palsy: a preliminary study. Neurorehabil Neural Repair. 2007;21(6):475–485.
23. German G, Wagner H, Blome-Eberwein S, et al. Early dynamic motion versus postoperative immobilization in patients with extensor indicis proprius transfer to restore thumb extension: a prospective randomized study. J Hand Surg. 2001;26A:1111–1115.
24. Rath S. Immediate active mobilization versus immobilization for opposition tendon transfer in the hand. J Hand Surg. 2006;31A:754–759.
25. Giessler GA, Przybilski M, Germann G, et al. Early free active versus dynamic extension splinting after extensor indicis proprius tendon transfer to restore thumb extension: a prospective randomized study. J Hand Surg. 2008;33A:864–868.
26. Taylor AG, Galper DI, Taylor P, et al. Effects of adjunctive Swedish massage and vibration therapy on short-term postoperative outcomes: a randomized, controlled trial. J Altern Complement Med. 2003;9(1):77–89.
27. Tandara AA, Mustoe TA. The role of the epidermis in the control of scarring: evidence for mechanism of action for silicone get. J Plast Reconstr Aesthet Surg. 2008;61(10):1219–1225.
28. Luo J, McNamara BP, Moran K. A portable vibrator for muscle performance enhancement by means of direct muscle tendon stimulation. Med Eng Phys. 2005;27(6):513–522.
29. Ramachandran VS, Rogers-Ramachandran D, Stewart M. Perceptual correlates of massive cortical reorganization. Science. 1992;258(5085):1159–1160.
30. Ramachandran VS. Plasticity and functional recovery in neurology. Clin Med. 2005;5(4):368–373.
31. Shea JB, Morgan RL. Contextual interference effects on the acquisition, retention, and transfer of a motor skill. J Exp Psychol: Human Learn Memory. 1979;5:183.
32. Bouwsema H, van der Sluis CK, Bongers RM. The role of order of practice in learning to handle an upper-limb prosthesis. Arch Phys Med Rehabil. 2008;89(9):1759–1764.
33. Duff SV, Gordon AM. Learning of grasp control. Dev Med Child Neurol. 2003;45(11):746–757.
34. Lin CH, Sullivan KJ, Wu AD, et al. Effect of task practice order on motor skill learning in adults with Parkinson’s disease: a pilot study. Phys Ther. 2007;87(9):1120–1131.
35. Winstein CJ, Pohl PS, Cardinale C, et al. Learning a partial-weight bearing skill: effectiveness of two forms of feedback. Phys Ther. 1997;76(9):985–993.
36. Lee TD, Carhahan H. Bandwidth knowledge of results and motor learning: More than just a relative frequency effect. Quart J Exp Psych. 1990;42(4):777–789.
37. Ranganathan R, Newell KM. Influence of augmented feedback on coordination strategies. J Mot Behav. 2009;41(4):317–330.