Therapist’s Management of Peripheral Nerve Injury
▪ After a peripheral nerve lesion, muscle denervation and sensory loss contribute to upper extremity prehensile limitations, leading to incoordination and maladaptive strategies.
▪ The key to successful rehabilitation is to adhere to precautions initially to protect the nerve after repair, then provide carefully designed therapeutic activity and exercise as the individual progresses through the phases of recovery.
▪ Greater emphasis placed on fostering neural reorganization and recovery of sensorimotor control may enhance achievement of flexible and efficient prehensile skill following nerve injury and repair.
Prehensile skill is often taken for granted until it is lost or diminished, as in the case of peripheral nerve injury (PNI). After a peripheral nerve lesion, muscle denervation and sensory loss contribute to prehensile limitations, leading to incoordination and maladaptive strategies. To effectively foster neural recovery and prehension following injury, clinicians should address not only impairments and function but motor control through the provision of specific sensory and motor experiences.
This chapter (1) considers adaptations to injury; (2) reviews the clinical presentation following injury; (3) discusses evaluations to establish a baseline and monitor neural recovery; and (4) reviews rehabilitation tactics used to prevent deformity and enhance recovery and prehensile skill.
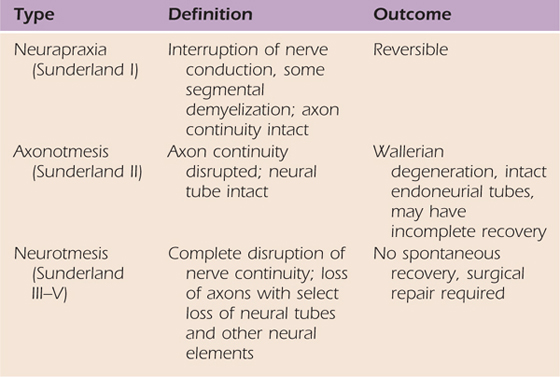
Immediately after PNI the central (CNS) and peripheral nervous systems (PNS) adapt to a reduction in sensory input.1-3 Neural adaptation to changes in input, or plasticity,4 ranges from axonal degeneration to topographic reorganization.1,5 The residual effects of PNI depend on the degree of axon and connective tissue involvement (see Chapters 42 and 43). Table 45-1 (online) shows the relationship between injury classification schemes by Seddon6 and Sunderland.1,7 An appreciation of postinjury adaptations may aid in the design of effective interventions.
Table 45-1 Classification of Injury
Postinjury peripheral changes include the onset of Wallerian degeneration and initiation of neural recovery. Wallerian degeneration involves axonal disintegration and demyelination distal to the lesion site,8 and phagocytic macrophages interact with Schwann cells to remove debris.9 With long-standing nerve injury, the endoneurial tubes shrink and collapse due to a reduction in cross-sectional area and the end-organs often degenerate.
Following a PNI, nerve function diminishes in the largest diameter fibers first and later the smallest. Large-diameter fibers (group A and B) are myelinated with a fast conduction rate, and small fibers (C or IV) are unmyelinated with a slower conduction rate.8 After a nerve lesion, functional loss may proceed in the following sequence: motor, proprioception/vibration, touch, pain, and sudomotor function. Large-diameter fibers carrying motor, proprioceptive, and vibration information may be lost first, yet may be the last to return. Because pain information is primarily carried by the smallest diameter fibers, it is usually one of the last sensations to be lost and the first to return.
Recovery from PNI involves remyelination, collateral sprouting of axons, and axonal regeneration.9 Regeneration following neurapraxia or axonotmesis begins immediately, whereas a neurotmesis usually requires nerve repair. After a 2- to 3-week latency period, a repaired nerve begins to regenerate. For the nerve to regenerate, the central axon must survive, the environment must support axonal growth, the axon must make timely contact with receptors, and the CNS must integrate signals from the PNS.9 Axonal regeneration is guided by Schwann cells. In a favorable environment, the axon sprouts a “finger-like” growth cone initiating the process, and the Schwann cells provide a source of neurotrophic factors, which diffuse across the distal to proximal stump.10 The regrowth rate in adults is 1 to 3 mm a day or more for a nerve laceration and repair11,12 and about 3 to 4 mm a day after a crush.12 The timing for axon regrowth after relief from nerve compression is more variable.
Factors that influence nerve recovery include (1) the lesion type (e.g., crush, stretch, laceration); (2) distance from the cell body; (3) age; (4) time since injury; (5) health; and (6) genetic factors.13 Genetic factors include muscle fiber type, density, motor unit number, height, and weight. Constraints that influence recovery include the ability of the axon to cross the repair site, a slow rate of axon regrowth, and mismatching of sensory and motor fibers. Research examining methods to overcome constraints has included the use of neurotrophic factors, electrical stimulation, and exercise.10,14-16 These methods are reviewed later in the chapter.
Lost or diminished sensory input induces CNS changes, including remodeling of cortical and subcortical structures and alterations at the spinal cord level. Neural reorganization may stem from any one of the following mechanisms:
1. Removal or unmasking of inhibitory controls in the affected region.17,18 For example, changes in excitatory and inhibitory mechanisms in the spinal cord may alter tonic inhibition, causing tactile hypersensitivity.19
2. Migration of cells that serve other body parts into deafferented cortical regions3,20
3. Strengthening of existing connections and subthreshold excitatory inputs based on postinjury experience21-23
4. Reorganization of subcortical regions that project to the cortex such as the basal ganglia, brainstem, or thalamus17,24
5. Neurogenesis and sprouting of new pathways25,26
Somatosensory feedback and anticipatory control are essential components of grasp and manipulation.27-30 Current visual information and memories from past sensorimotor experiences are used to preshape the hand (Fig. 45-1) in advance of contact to accommodate object size and shape,30 aiding the acquisition of stable grasp points.31 Visual and somatosensory information are also used to anticipate the frictional conditions at the digit–object interface and estimate object weight to adequately grade fingertip forces (grip and load) used to grasp and lift objects.32,33 If anticipatory control is sufficient, the peak rate of increase in grip (normal or squeeze) and load (tangential or lift) force will be higher for heavier or more slippery objects.29
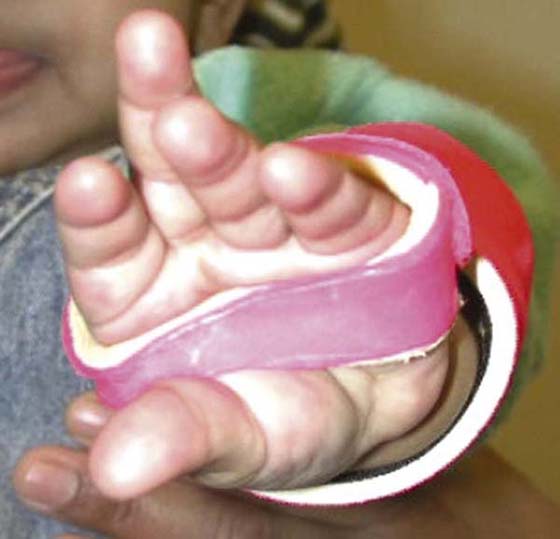
Figure 45-1 Anticipatory grip formation during reach-to-grasp facilitated with support from a dorsal wrist orthosis.
Performance during reach-to-grasp tasks, including the movement path, velocity, and finger width (aperture), and exhibited during grip formation can be examined using kinematic (spatial–temporal) analysis. Kinetic (force) information on fingertip force scaling can be obtained from multiaxial force transducers shown in Figure 45-2A and documented as shown in Figure 45-2B. Kinematic and kinetic analyses allow for close examination of alterations in the spatiotemporal features of the movement and force control as occurs when sensory input is distorted or absent, as in cases of denervation,27,34 focal hand dystonia,35 and large-fiber neuropathy.36

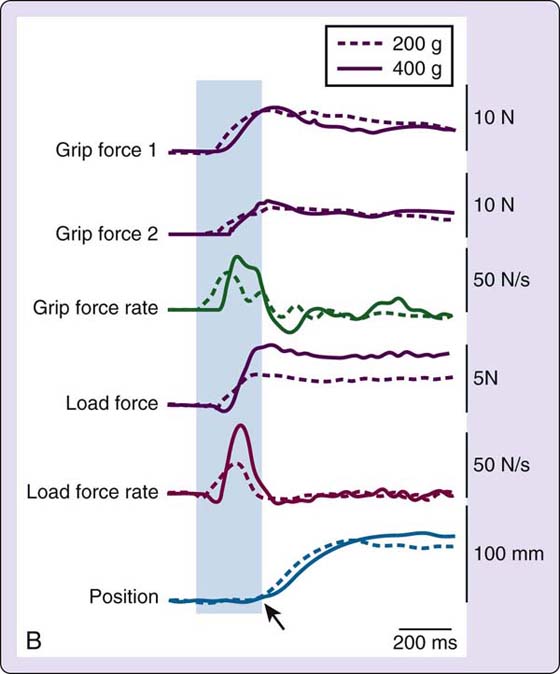
Figure 45-2 A, Grip device equipped with two parallel multiaxial force transducers and a magnetic position sensor (in box); B, The type of data obtained with this device includes grip force for each digit, grip force rate (first derivative of grip force), load force, load force rate (first derivative of load force), position (object lift-off table), and lift acceleration (second derivative of position—not shown). The arrow on “Position” depicts when the object is lifted off the table. The area shown in grey occurs before the weight of the object is known. Because the grip and load force rates (rate of onset of force) are higher for the heavier object, this subject displayed anticipatory control or advanced planning of fingertip forces.
Previous kinematic and kinetic analyses indicate there is a strong relationship between impaired visual skills or somatosensation and anticipatory control.27-29,31 Visual or somatosensory deficits may lead to insufficient finger opening, object grasp at incorrect contact points, slips at object contact, temporal delays, and the use of excessive fingertip forces during fine-motor tasks. PNI can impair the sebaceous glands in the fingertips, causing objects to seem more slippery. If somatosensory input is diminished, grip force and movement time may increase to prevent slips and ensure success.29 To examine the effect of injury on fingertip force scaling, Cole and colleagues34 induced median nerve compression and then generated sensory nerve action potentials via electric stimulation proximal to the carpal canal. It was not until the compression caused a 50% reduction in sensory nerve action potentials that sensibility began to diminish and subjects began to use greater than 50% increase in grip force to secure objects.
Secondary to diminished sensation and muscle denervation experienced after PNI, the number of motor units37 and joint motion available is reduced. Thus, altered sensory and motor function reduces the degrees of freedom or the number of ways the involved hand and arm can move,38 necessitating the use of alternative prehensile patterns. For example, after a radial nerve injury there is little active wrist and finger–thumb extension. Thus, it may be difficult to preshape the hand in preparation for object contact. After median nerve injury, thenar muscle denervation may require a shift from precision to power grips or the use of bilateral versus unilateral grip patterns. With a low ulnar nerve injury, a visible Froment’s sign (excess thumb interphalangeal [IP] joint flexion during lateral pinch) suggests that the adductor pollicis is affected, resulting in overpowering from the flexor pollicis longus to pinch. Fingertip force generation is significantly affected by median and ulnar nerve injuries, influencing dexterity and handedness. Although activation of intact muscles aids function, coordination is compromised, increasing the effort used, and, therefore, the cost of engaging in prehensile tasks.39
Injury to the radial nerve can stem from humeral shaft fractures (middle and distal third), elbow dislocations, fractures, and compression between the head of the radius and the supinator (radial tunnel syndrome).13 Nerve compression in the axilla from the incorrect use of crutches or awkward sleeping postures (Saturday night palsy) can also lead to a radial nerve injury. The effect of injury on function depends on the level of the injury (Fig. 45-3). A “wrist drop” is the classic deformity associated with a radial nerve injury characterized by forearm pronation, wrist flexion, thumb flexion and abduction, slight metacarpophalangeal (MCP) joint flexion, and IP joint extension (Fig. 45-4). The individual is unable to extend the wrist and fingers simultaneously or abduct the extended thumb. Depending on the level of the injury, there may be atrophy of the dorsal forearm due to involvement of the finger and thumb extensors and the extensor carpi ulnaris. With higher level injuries there may be atrophy of the extensor muscle mass near the lateral epicondyle due to involvement of the brachioradialis, extensor carpi radialis longus and brevis, or the triceps.
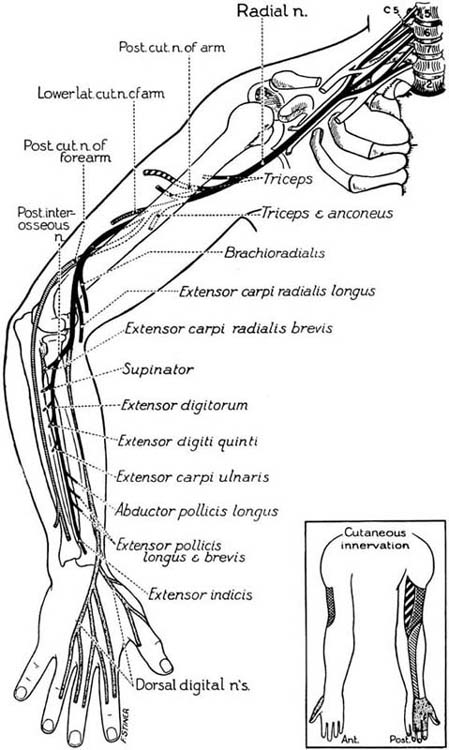
Figure 45-3 The course and distribution of the radial nerve. (From Haymaker W, Woodhall B. Peripheral Nerve Injuries. Philadelphia: W.B. Saunders, 1953).
The sensory branch of the radial nerve can be injured at the wrist as it resurfaces in the anatomic snuff box. Injury mechanisms may include a radioulnar joint dislocation or nerve compression (wrist-band injury). A lesion at this level only affects sensibility on the dorsal thumb, index, middle, and radial side of the ring finger to the PIP joint (excluding the nail beds), with some variation.
Forearm-level injury can result in full or partial denervation of the following muscles: extensor carpi ulnaris (ECU), extensor digitorum communis (EDC), extensor digiti minimi (EDM), abductor pollicis longus (APL), extensor pollicis longus (EPL), extensor pollicis brevis (EPB), and extensor indicis proprius (EIP). Forearm-level injuries can result in lost or diminished ulnar wrist extension, MCP joint extension of all digits, and thumb abduction or extension. As described for low-level injuries, sensibility through the dorsoradial aspect of the hand is affected.
Elbow-level lesions result in diminished sensibility to the dorsoradial portion of the hand and full or partial denervation of the muscles described earlier with the addition of the supinator, extensor carpi radialis longus (ECRL), and extensor carpi radialis brevis (ECRB). With injury at this level, radial wrist extension is lost or weakened, and supination is weakened. Lesions proximal to the elbow also involve the brachioradialis and triceps, resulting in the addition of slightly weakened elbow flexion and lost or weakened elbow extension.
Lost or diminished sensibility on the dorsoradial aspect of the hand from a wrist-level injury (or higher) has little effect on hand function because the volar surface continues to obtain the sensory input needed for prehension. However, the injured individual should remain vigilant during daily tasks to prevent superficial injury or scalds and burns to the dorsum of the hand. Forearm- or elbow-level lesions limit the ability to turn the hand over and extend the wrist to receive objects. Inadequate wrist stabilization may prevent the formation of stable prehension patterns, such as a spherical grip or radial digital grasp used to open jars or bottles and to perform manipulative tasks such as buttoning. Limited finger and thumb extension will hinder grip formation and object release and may lead to hand asymmetries as found in other populations.40 To compensate for poor grip formation, the wrist may flex to passively extend the fingers or objects may be transferred to the involved hand by the noninvolved hand. Object release may be accomplished by wrist flexion, shaking objects free, or through use of the noninvolved hand to retrieve them. Reach-to-grasp activities and tasks such as writing or typing are difficult to complete with the wrist in flexion and the fingers and thumb in extension, limiting the use of adequate grip force.
Injuries to the median nerve can result from lacerations, humeral fractures, elbow dislocations, distal radius fractures, and lunate dislocations into the carpal tunnel.13 Nerve compression can occur between the two heads of the pronator teres (pronator syndrome), when it branches off as the anterior interosseous nerve and in the carpal tunnel. Functional changes associated with median nerve injury depend on the level of injury (Fig. 45-5). The classic deformity associated with a median nerve injury is the sign of “benediction” with the thumb resting in adduction and the index and long fingers in extension and adduction. With higher-level lesions there may be atrophy of the pronator teres and flexor carpi radialis resulting in loss of volar muscle mass near the medial epicondyles. Thenar muscle atrophy is often visible with long-standing injury (Fig. 45-6).
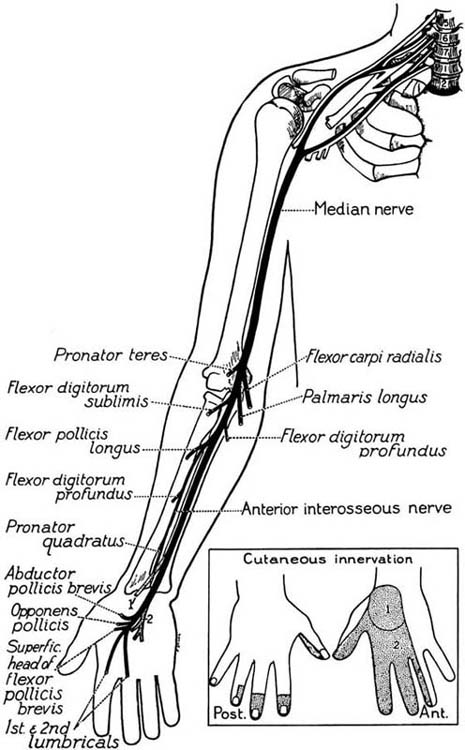
Figure 45-5 The course and distribution of the medial nerve. (From Haymaker W, Woodhall B. Peripheral Nerve Injuries. Philadelphia: W.B. Saunders, 1953).
Wrist-level injuries result in full or partial denervation of the opponens pollicis (OP), abductor pollicis brevis (APB), flexor pollicis brevis-superficial head (FPB), and the first and second lumbricals. Thumb flexion, palmar abduction, and opposition are lost or weakened with wrist-level lesions. Sensibility may be impaired through the volar thumb, index, long, and the radial half of the ring finger and the nail beds. If the injury occurs in the carpal tunnel, sensibility to the thenar eminence is spared because the cutaneous branch travels outside of the carpal tunnel.
Forearm- or elbow-level lesions result in full or partial denervation of the muscles previously mentioned with the addition of the pronator teres (PT), flexor carpi radialis (FCR), flexor digitorum superficialis (FDS), palmaris longus (PL), flexor pollicis longus (FPL), flexor digitorum profundus (FDP) to the index and long fingers, and the pronator quadratus (PQ). Motions lost or weakened include pronation, wrist flexion and radial deviation, thumb IP joint flexion, and flexion of the index and middle fingers at the PIP and DIP joints. MCP joint hyperextension may be observed due to the overpowering activation of the EDC.
Prehensile skill is compromised after median nerve injury. Lost or diminished finger flexion, thumb flexion, palmar abduction, and opposition impedes grip formation during reach-to-grasp tasks. The ability to stabilize opposing forces between the radial fingers to grasp, and to use the intrinsic muscles for in-hand manipulation, is lost or diminished.41-43 Insufficient activation of lumbricals I and II prevents finger-to-thumb-pad approximation used for precision grip, resulting in a raking pattern or a weak lateral pinch. Inadequate thumb mobility prevents in-hand manipulation of small objects, thus, compensatory patterns are often used such as two-handed manipulation or stabilizing items against the body. In-hand manipulation used to rotate (turn), translate (move palm to fingertips or fingertips to palm), and shift (move along fingertips) coins, pegs, or a key in one hand is affected. A complete median nerve injury may also result in a 60% loss of lateral pinch strength and 32% loss of power grasp strength.42
Lost or diminished sensation in the volar–radial side of the hand impairs somatosensory feedback needed for object recognition, coordination, and grading of movement. If feedback is impaired, excess grip force is often employed to prevent object slippage and drops.29 Premature fatigue from the use of excess grip force may limit endurance during writing and related tasks as shown in other groups.44 Reach-to-grasp and pointing movements are less accurate with diminished tactile feedback.27 Furthermore, insufficient tactile feedback makes it difficult to determine the start and end position in tasks such as typing, text messaging, and calculator use.45 Median nerve injury significantly limits the ability to complete activities of daily living (ADL), often requiring individuals to change hand dominance if the dominant hand is injured.
Causes of ulnar nerve injury include direct lacerations and fracture of the medial epicondyle of the humerus or olecranon of the ulna.13,46 Compression may occur at the cubital or Guyon’s canal.47 The effect of injury on function depends on the individual’s age and level of the lesion (Fig. 45-7). High-level lesions in young individuals recover fairly well after repair, whereas adults rarely regain motor function.48 The classic deformity associated with an ulnar nerve injury is a “partial intrinsic minus” deformity (Fig. 45-8) due to loss of the interossei and lumbricals III and IV with less posturing in the index and long fingers (lumbricals intact). Yet, as reinnervation occurs, the posturing often increases. This deformity is noted for MCP joint hyperextension with PIP joint flexion at the ring and small fingers. With long-standing injury the hypothenar eminence and intrinsics between the IV and V metacarpals may be atrophied. After high-level injuries, there may be medial forearm atrophy.
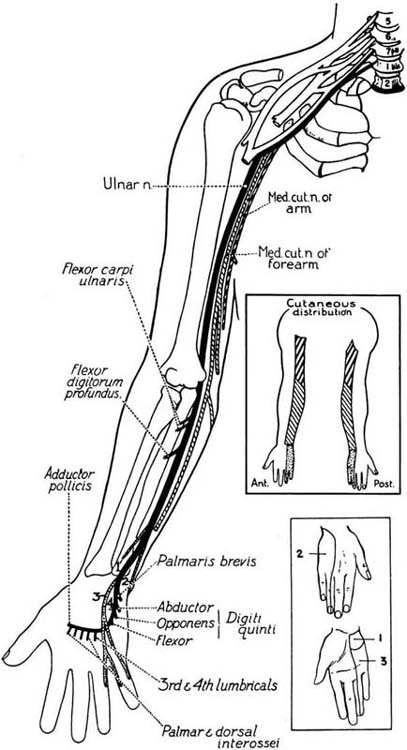
Figure 45-7 The course and distribution of the ulnar nerve. (From Haymaker W, Woodhall B. Peripheral Nerve Injuries. Philadelphia: W.B. Saunders, 1953).
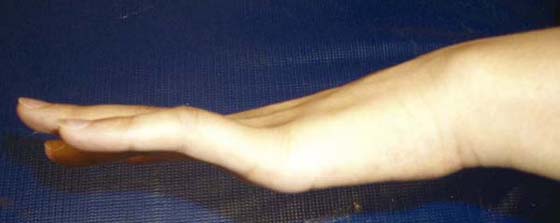
Figure 45-8 Intrinsic minus posturing with hyperextension at the ulnar metacarpophalangeal joints due to ulnar nerve injury.
Low-level lesions result in full or partial denervation of: abductor digiti minimi (ADM), flexor digiti minimi (FDM), opponens digiti minimi (ODM), III and IV lumbricals, dorsal interossei (DI), palmar interossei (PI), flexor pollicis brevis (deep head) (FPB-deep), and adductor pollicis (AP). Due to partial or full denervation of the intrinsics, finger abduction and adduction, thumb adduction, and opposition of the small finger are lost or weakened. Also, MCP joint flexion at the small and ring finger with simultaneous extension of the IP joints is lost or weakened. Because FDS and FDP function remain intact and the EDC can contract, ulnar-sided “intrinsic minus,” or claw, posturing is more pronounced than in individuals with high ulnar nerve lesions.49 With a wrist-level injury, sensibility is lost or diminished through the volar–ulnar aspect of the palm distally and the small and ulnar half of the ring finger.
Lesions at the elbow and above result in full or partial denervation of the muscles listed earlier plus the flexor carpi ulnaris (FCU) and FDP to the ring and small fingers, resulting in lost or weakened ulnar deviation and distal interphalangeal (DIP) joint flexion. Without the FDP and FDS, the “intrinsic minus” position through the ring and small fingers is less severe but noticeable due to contraction of the EDC. Sensibility is now lost or diminished through the dorsal and volar surface of the small and ulnar half of the ring fingers and the ulnar aspect of the proximal palm.
During many daily tasks the ulnar border of the hand interfaces with a contact surface area. Therefore, lost or diminished sensation through the ulnar side of the hand increases the risk for burns, lacerations, and skin breakdown. To prevent injury, it is vital that the injured individual remains vigilant. Individuals should be advised to wear gloves when exposed to extreme temperature, sharp or rough surfaces, or friction during repetitive physical labor tasks.
The ulnar side of the hand acts as a point of stability for powerful grip and pinch as well as manipulation. Weakness after ulnar nerve injury affects gross grasp needed for opening jars and carrying heavy objects. Lost or weakened intrinsic muscle function restricts active grasp and release and reduces strength.42 Following an ulnar nerve block, Kozin and colleagues42 found a mean decrease of 38% gross grasp strength and 77% key pinch strength. Limited key pinch strength makes it difficult to secure fasteners and open doors. Froment’s sign is an inefficient substitution for weak key pinch. Posturing into intrinsic minus prevents ulnar digit extension to accommodate large objects during grasping or attempts to catch a football or basketball. Quarterbacks and pitchers with ulnar nerve injuries may have difficulty controlling ball release during throws. Lost or weakened FDP function prevents DIP joint flexion used to secure items against the palm during the translation component (palm-to-fingers or fingers-to-palm movement) of in-hand manipulation.
Clinicians have an opportunity to influence prehensile recovery following PNI by conducting sensitive evaluations and implementing creative treatment strategies. Individual participation in carefully designed, therapeutic activity after injury may contribute to neural regeneration and reorganization following injury and repair.
As with other hand injuries it is important to consult with the referring physician to discuss how much tension was placed on the nerve repair, the ideal orthotic position and the duration of limb immobilization. Nerve injuries are typically immobilized for a minimum of 3 weeks.
After obtaining relevant information from the physician, pertinent medical history and demographics can be obtained from a questionnaire or interview, including the cause and timing of the injury, date of injury, age, type of treatment to date, social/work information, and hand dominance. The nature of current problems, comorbidities, and an assessment of psychosocial factors, ADL status, and vocational or school requirements should also be acquired. A pain assessment using an instrument such as the McGill Pain Questionnaire50 or the visual analog scale (or pain faces scale for children)51 should be conducted. The presence of an advancing Tinel’s sign, induced by tapping on the nerve (to elicit a distal tingling sensation), may be used to gauge current and ongoing progression in regeneration. The physical assessment needs to be customized to the phase of recovery and compared with the noninvolved limb.
Sympathetic function is assessed via observation and palpation and includes an assessment of (1) vasomotor function (e.g., skin color, skin temperature, edema); (2) sudomotor function (sweat); (3) pilomotor function (gooseflesh); and (4) trophic changes (nutrition to skin and nails). Figure 45-9 shows the effect of loss of function in the sebaceous glands of the palm innervated by sympathetic nerve fibers. A quick test of sympathetic function is the wrinkle test, found to have 97% sensitivity and 95% specificity.52 (See Chapter 11 for more detail.) Absence of sympathetic function is highly suggestive of absent sensibility because of the high resilience those fibers have to mechanical trauma.53 Some sympathetic changes are seen immediately, but others are not noticeable until 3 to 6 weeks after nerve injury.
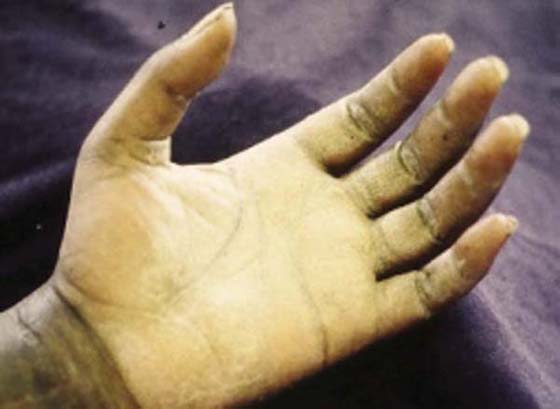
Figure 45-9 Evidence of trophic changes, which alter skin texture due to sympathetic denervation of the sebaceous glands.
During phase one (immobilization) the motor assessment includes an examination of active (AROM) and passive range of motion (PROM) of noninvolved joints. After immobilization (phase two), AROM and PROM of the involved joints can be tested as well as prehension patterns through general observation or with tests of hand function such as the Sollerman’s Test of Hand Grip,54 which is a standardized hand function test based on common hand grips and consisting of 20 ADL items (see Chapter 12). To guide testing of muscle function the therapist can follow the predictable sequential pattern of muscle reinnervation and the ability to (1) produce an observable and palpable contraction without joint motion; (2) hold a position, yet not produce the same position; (3) move the involved joint actively; and (4) tolerate resistance.55
After the patient has been cleared by the surgeon for strength testing and resistive exercise, there are a few ways to measure strength. The manual muscle test (MMT) is the most widely used and involves a standard 0 to 5 grading scale (absent to normal grades). The action of the muscle being tested is resisted at the distal end of the moving bone while the proximal joint is stabilized. Resistance can be provided isometrically by asking the individual to hold the position or isotonically by resisting throughout the range. Another frequently used tool is the hand-held dynamometer. This device measures the magnitude of force generated versus the amount of resistance tolerated. Although calibrated grip-and-pinch dynamometers measure gross hand strength, hand-held dynamometry tests the strength of individual muscles.43 During muscle testing it is important to be aware of substitution patterns.13 Classic substitution patterns for each nerve injury are listed in Table 45-2.
Table 45-2 Potential Substitution Patterns for Each Nerve Injury
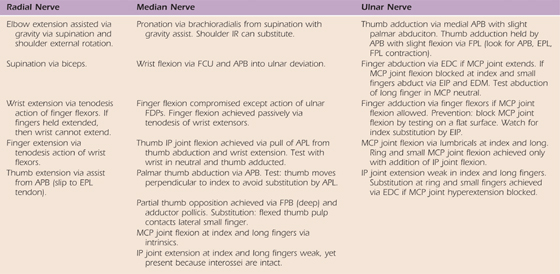
APB, abductor pollicis brevis; APL, abductor pollicis longus; EDC, extensor digitorum communis; EDM, extensor digiti minimi; EIP, extensor indicis proprius; FCU, flexor carpi ulnaris; FDP, flexor digitorum profundis; FPB, flexor pollicus brevis; FPL, flexor pollicus longus; IP, interphalangeal; IR, internal rotation; MCP, metacarpophalangeal.
The sequence of return in sensibility is from (1) deep pressure and pinprick, to (2) moving touch, to (3) static light touch, and then to (4) discriminative touch.13 Initially, localization is poor, and the sensation elicited radiates proximally or distally. Accurate touch localization is one of the last functions to return. Passive sensibility can be tested using the Semmes–Weinstein monofilaments or the Weinstein Enhanced Sensory Test (WEST) for touch–pressure; and the Discriminator for static and moving two-point discrimination. Active sensibility can be tested using Moberg’s Picking-Up Test or general tests of tactile gnosis or stereognosis (see Chapter 11 for more details).
Patients with PNIs often require alternative strategies and prehension patterns to perform everyday tasks, increasing the effort while decreasing the movement efficiency.39 Timed dexterity tests can be used to document baseline performance and improvements in fine-motor efficiency. Common dexterity tests (see Chapter 12 for details) include the Nine Hole Peg Test, the Purdue Pegboard Test, and the Jebsen Taylor Hand Function Test.56 These and other tests can be used to test components of in-hand manipulation. Although normative data are available on most tests, it is worthwhile to also compare performance against the noninvolved hand.
Impairments and function are important issues to address following PNI. Yet, methods aimed at motor learning and control can facilitate the transition from poor to smooth coordination, enhancing function. Initially, therapeutic strategies that encourage the use of noninvolved structures or provide supplemental feedback may aid this process. Substituting one form of feedback for another while awaiting peripheral nerve regeneration may preserve central neural function.57,58 Once regeneration ensues engagement in meaningful but carefully planned tasks as used with focal hand dystonia59 or central lesions60 may aid reorganization and lead to better functional recovery.
Postinjury management can be organized into three phases of recovery: phase one refers to early healing when immobilization is required; phase two is the period of reinnervation when remobilization, sensory reeducation, and active motor control is emphasized; and phase three stresses strengthening and functional performance. Rehabilitation methods often include orthotic positioning, AROM, biofeedback, neuromuscular electrical stimulation (NMES), and functional tasks.13 Adjunctive methods include massage, edema management, and sensory reeducation. The goals and common treatment methods for each phase are listed in Table 45-3. Orthotic positioning is reviewed in greater detail in the next section.
Table 45-3 General Goals and Treatment Methods for Each Phase
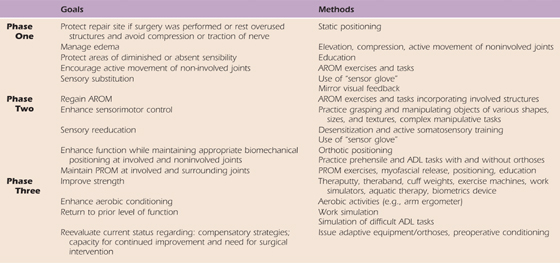
ADL, activities of daily living; AROM, active range of motion; PROM, passive range of motion.
Phase One. Following nerve repair, a custom-made immobilization orthosis or cast is used to protect the repair by limiting tension on the repair site for a minimum of 3 weeks. The specific orthosis and position depend on the nerve repaired. Static orthoses provide appropriate biomechanical positioning to prevent overstretching of denervated muscles and deformity of associated structures. Management of pain, edema, and inflammation can be addressed with anti-inflammatory medications and modalities such as ultrasound, phonophoresis, and cryotherapy. Education is often provided to (1) aid in edema reduction via elevation and active movement of noninvolved joints; (2) protect areas of diminished or absent sensation; and (3) review home programs. During this period of immobilization, limitations in performance of ADL are addressed using adapted methods and assistive devices.
Phase Two. After immobilization, place-and-hold techniques and active motion foster muscle activation and movement. Place-and-hold techniques require muscle activation at various lengths and are performed by having the clinician or the individual place the limb in the desired position where it is briefly held. Active motion is initially encouraged through a protected range with gravity eliminated then progressed to full motion against gravity. Aquatic therapy can be beneficial, as the buoyancy of the water can provide assistive AROM. Orthotic positioning is often used to facilitate new movement and function as reinnervation occurs.
To enhance function and neural control in phases two and three, therapeutic activities should focus on motor relearning and adaptive strategies. Traditionally, biofeedback and NMES assist with motor learning and control. Biofeedback provides visual or auditory feedback (or both) when the targeted muscle contracts, aiding relearning of muscle activation or relaxation. NMES augments muscle activation when there is evidence of innervation, but also provides proprioceptive and tactile input to enhance muscle activation and awareness (Fig. 45-10). However, the use of electrical stimulation in full or partially denervated muscle remains controversial.
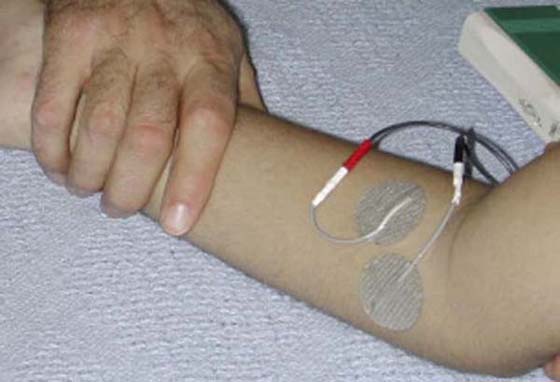
Figure 45-10 Electrode placement for neuromuscular electrical stimulation (NMES) used to facilitate muscle activation in the pronator muscle group.
Multisensory approaches can help maintain cortical representation and allow for faster integration of sensory input on reinnervation. The “sensory glove” introduced by Rosén and Lundborg57 provides auditory feedback to substitute for tactile input during fine-motor tasks after PNI. The type of auditory stimuli varies depending on the texture of the object being grasped. Mirror visual feedback (MVF),20,58 which uses a mirror image to provide an illusion of movement in the involved hand, may also be another useful strategy. During MVF the individual is instructed to observe a movement being completed by the noninvolved hand in a mirror image located at midline (involved hand is behind the mirror). Theoretically, the visual system interprets the mirror image to be movement of the involved hand, thus stimulating mirror neurons in the brain. The goal is to restore volitional control in the affected hand. During the phase of immobilization, the patient simply observes the reflected image and during the phase of reinnervation, the patient attempts to perform the motion observed in the mirror. Based on the reported success relieving phantom limb pain and complex regional pain syndrome,20 MVF may be successful after PNI58 and warrants further investigation.
During the design of therapeutic activities it is important to remember that visual information influences anticipatory control during reach to grasp and object manipulation.32 Engagement with familiar objects and tasks may initially aid motor control. However, as improvement is noted, the introduction of novel objects and tasks may challenge the nervous system to better refine sensorimotor control.41
Phase Three. Once full available AROM is obtained with gravity eliminated and against gravity, strengthening can begin. Resistance can be in the form of theraputty, theraband, free weights, cuff weights, exercise machines, work simulators, and computer games (e.g., Biometrics Device). Aquatic therapy can also be useful since buoyancy of the water can also provide a form of resistance. As in other phases, correct technique should be emphasized to prevent injury.
If an individual plateaus, therapy may shift to consideration of long-term solutions to address residual deficits. Solutions may include the use of devices or orthoses to substitute for absent movement, stability, or strength. If surgical intervention is a consideration, the individual should receive preoperative education and therapy.
Because of full or partial denervation after injury, individuals are at risk for secondary impairments, such as muscle atrophy, overstretching of the involved muscle–tendon unit, and joint contracture, if care is not properly taken to prevent them.61 Orthoses can immobilize or mobilize involved structures via static or dynamic components. Orthotic design and wear schedule vary depending on the type, extent, and location of the injury along with the phase of neural recovery. Multiple client factors (e.g., cognition, age) and the desired outcome also influence design. Regardless of recovery phase, patient education is vital to maximize compliance with orthosis care and wear.
Orthosis design after PNI requires attention to detail to ensure that there is sufficient support, reasonable fit, allowance for optimum sensation, ease in donning and doffing, a proper line of pull, and appealing aesthetics.61 In phase one, orthotic positioning (or casting) aims to (1) protect the nerve repair (or rest the involved nerve), (2) maintain anatomic integrity, and (3) prevent deformity. The method and time period of immobilization depend on the severity of the injury and extent of initial medical care. If surgical repair was performed, the individual is immobilized for a minimum of 3 weeks. PNIs not requiring surgical care should be positioned based on the type and level of injury. Positioning goals in phase two are to (1) maintain passive ROM and (2) enhance function during reinnervation.
Phase One. After this injury the fingers and wrist need to be supported to prevent shortening of the flexors and overstretching of the extensors. A volar resting orthosis with the wrist in neutral to partial extension and the MCP joints in extension should be worn nightly. During the day a dorsal wrist cock-up supporting the MCP joints in extension with elastic slings allows for digit movement and leaves the volar surface free for sensory input.
As with other compression injuries, rest is needed to reduce the pain and inflammation that occurs with radial tunnel syndrome. According to Gelberman and colleagues46 the optimal position for radial nerve decompression is 90 degrees of elbow flexion, forearm midposition or supination, and slight wrist extension. This position of rest can be provided in a long-arm orthosis (Fig. 45-11).
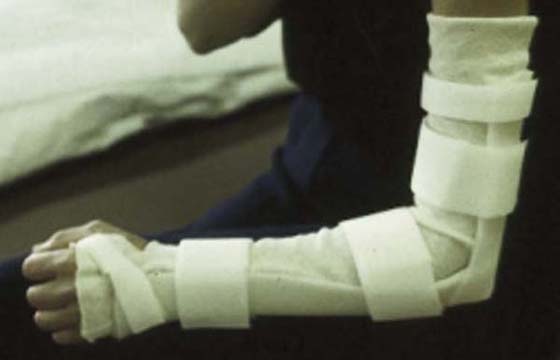
Figure 45-11 Elbow orthosis for use with cubital tunnel syndrome or radial nerve decompression contoured to enhance stability.
Phase Two. At this phase wrist and digit extension often remain absent or weak. A dorsal wrist cock-up orthosis with MCP joint support stabilizes the wrist and MCP joints, allowing for the execution of basic fine-motor skills. Another option is to reestablish a tenodesis pattern to improve hand function during reinnervation (Fig. 45-12A). The tenodesis orthosis uses the intact finger flexors to control wrist extension.62 Static nylon cord attached to finger slings suspends the proximal phalanges from an outrigger attached to a dorsal forearm base (Fig. 45-12B). Within the orthosis, active finger flexion passively places the wrist in neutral for a power grip, whereas active wrist flexion passively extends the fingers, allowing for object release. The radial nerve orthosis can be varied to include the thumb, expanding the available prehension (Fig. 45-12C, D). Both orthoses foster a power grip and allow use of the impaired hand in ADL. As strength and control improve, other options such as a dynamic Lycra glove or kinesiotaping can be used.
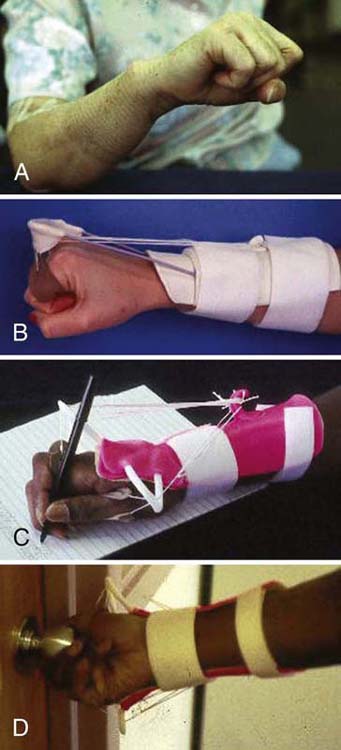
Figure 45-12 A, Use of tenodesis after radial nerve injury to extend the wrist by flexing the fingers. B, Tenodesis orthosis: Finger flexion stabilizes wrist in extension (Colditz JC. Splinting for radial nerve palsy. J Hand Ther 1987;1(1):18-23). C, D, Demonstration of functional use of a modified radial nerve orthosis, which extends beyond the wrist and includes the thumb.
Phase Three A functional assessment determines if positioning is necessary at this time. If contractures are present, static progressive orthotic positioning may help increase PROM. If an individual has no return, or if recovery of function is insufficient, a referral to a hand surgeon may be warranted to explore surgical options.
Phase One. To prevent a thumb adduction or webspace contracture, individuals with wrist-level lesions need to wear a night orthosis to maintain passive range in the first webspace. With a high-level lesion, an immobilization orthosis fit in a functional position or a wrist-based thumb spica (Fig. 45-13A) should be worn nightly to maintain length of the intrinsics to the index and middle fingers while supporting the thumb in opposition with the webspace maintained. A short opponens orthosis (Fig. 45-13B) can be worn during the day to provide support to the thumb and allow for use of the digits.
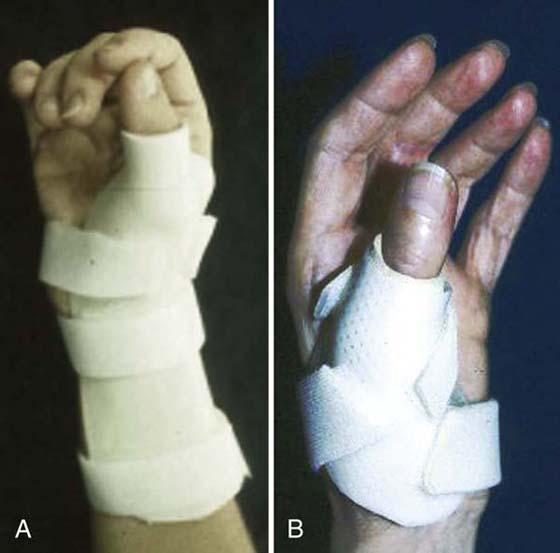
Figure 45-13 A, Wrist-based thumb spica supports wrist and thumb in functional position. B, In-hand thumb spica orthosis supports the thumb and allows use of the digits.
Phase Two. Absent or limited active thumb mobility is the primary impediment to function at this time. A short opponens orthosis that positions the thumb in palmar abduction and opposition (Fig. 45-14) allows thumb-to-fingerpad contact for a radial–digital or pincer grasp. If thenar atrophy is present, the short opponens orthosis may be too uncomfortable. An option is to form a soft orthosis using a “putty-like” material; securing it with an elastic band (Fig. 45-15) or covering it with a thin plastic orthosis. As strength and motor control improve, other options, such as a Benik Hand Splint or neoprene thumb strap, can provide limited support during motion.
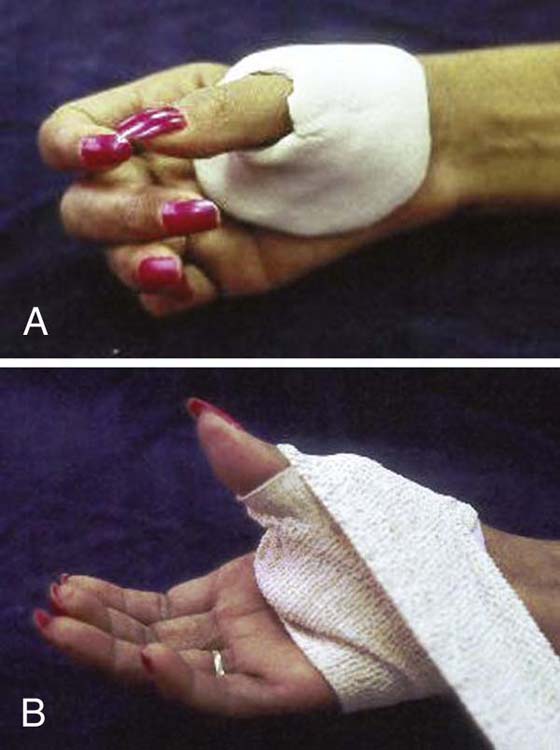
Figure 45-15 A, Alternative opponens orthosis using a “putty-like” material to form a soft orthosis, which can be secured with an elastic band (B).
Phase Three. Evaluation of function will determine how to proceed at this time and may include the continued use of orthoses. If the individual has no return, or functional recovery is insufficient, surgical options may need to be explored.
Phase One. Ulnar nerve injuries not requiring surgical repair should be positioned with a dorsal MCP joint blocking orthosis with the MCP joints in flexion to prevent a fixed claw deformity of the ring and small fingers in the intrinsic minus position (Fig. 45-16). An MCP joint blocking orthosis helps redistribute force from the EDC to the IP joints to promote full IP joint extension. If IP joint contracture is present, the immediate goal is to regain motion with serial casting or positioning. Mild contractures are correctable with a static progressive or dynamic PIP joint extension orthosis.
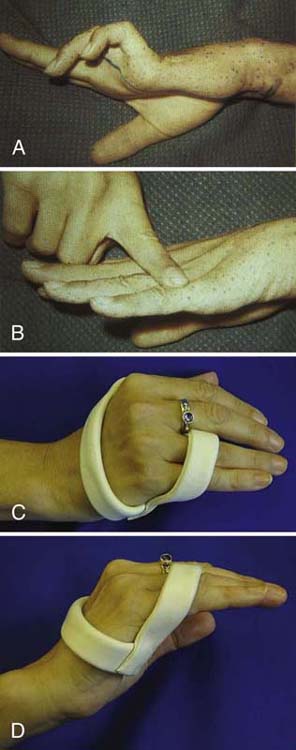
Figure 45-16 Dorsal metacarpophalangeal (MCP) joint block orthosis to prevent intrinsic minus or claw posturing. A, Ulnar nerve claw deformity. B, By blocking MCP joint hyperextension, redistribution of the action of the extensor digitorum communis to the interphalangeal (IP) joints allows IP joint extension. C, Ulnar claw orthosis blocks MCP joint hyperextension. D, Active IP joint extension is possible when MCP joint hyperextension is blocked by the orthosis.
Phase Two. Once strength and motor control improve, less restrictive orthoses can be used. For example, finger loops or cuffs placed over the ring and small fingers can be attached to a palmar bar or wristband with a static line to minimize intrinsic minus posturing, while allowing greater digit flexion and sensibility.
Phase Three. Assessment of function at this time determines how to proceed and may include continued orthosis wear in cases of long-standing injury (Fig. 45-17). If the individual has no return, or recovery is insufficient, surgical options such as tendon transfers may be explored.
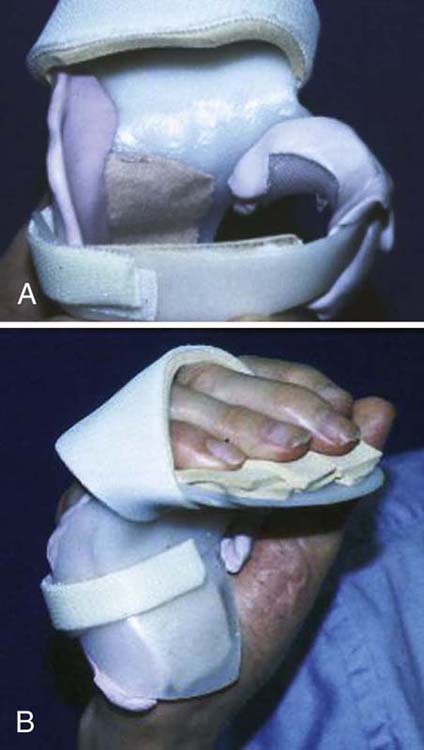
Figure 45-17 A, Orthosis fabricated for long-standing ulnar nerve injury with joint contractures, padded for comfort due to atrophy and excessive bony prominences. B, Orthosis in place with MCP joints in flexion and IP joints in extension.
Methods used at the time of nerve repair or afterward to enhance neural recovery include the application of neurotrophic factors, direct electrical stimulation to the repaired nerve, exercise or activity, and sensory reeducation.
Neurotrophic factors are important for regeneration of neurons.63 Unfortunately, after PNI there is a loss in retrograde transport of neurotrophic factors back to the cell body.64 This can lead to cell death and halt regeneration. Research suggests that the application of multiple neurotrophic factors (e.g., brain-derived [BDNF] and glial-derived [GDNF]) acting on two different populations of cells may increase survival of damaged neurons and enhance regeneration.65 Alternatively, the use of human bone marrow stromal cells (MSCs) may enhance peripheral nerve regeneration.66 MSCs are easily harvested through bone marrow aspiration and differentiate into a wide variety of cells, such as stem cells. Early findings suggest that MSCs grow into thick, robust Schwann cells useful in the production of neurotrophic factors. Although neurotrophic factors have been shown to have a positive effect on nerve regeneration, there currently is no clinical application because of the concerns regarding correct dosage and method of application.67 Studies explored in animal models may alleviate these issues in the future.
Brushart and colleagues14,15 used a rat model to determine that intraoperative low-frequency electrical stimulation provided to the nerve 1 hour after repair accelerates the axons’ ability to cross the repair site and helps direct them to the appropriate sensory or motor fiber. Electrical stimulation was also found to correlate with an up-regulation of neurotrophic factors in the neurons.14 In the future it may be feasible and safe to provide electrical stimulation directly to human nerves intraoperatively to enhance success of nerve repair.68
Neural regeneration and reorganization are activity-dependent processes21 induced more favorably in enriched environments.69 Exercise has proven beneficial for axon regeneration in animals,16 yet for humans participation in meaningful, and challenging, goal-directed activity that engages attention and motivation may best enhance neural recovery.21,59 If used prudently, biofeedback and NMES can augment feedback and promote task success.
Even if motor return is good, functional outcome can be poor if hand sensibility is not restored.70 Sensory retraining is one method of fostering neural reorganization. According to Lundborg,71 sensory reeducation aims to (1) aid interpretation and promote normalization of distorted hand maps, (2) refine cortical receptive fields to a higher resolution of sensory information, and (3) improve sensory processing. The two phases of sensory retraining are (1) desensitization to address neuropathic pain and (2) sensory reeducation to enhance functional prehension. Please see Chapter 46 for further details on sensory reeducation.
MC is a 5-year-old right-hand-dominant male who fell through a glass door and sustained a deep laceration to his right axilla, resulting in a complete transaction of the brachial artery and vein, the ulnar and median nerves, and the medial brachial cutaneous nerve. The structures were all repaired within 4 hours of injury. The biceps muscle was partially lacerated but not repaired. Immediately after surgery, MC was placed in a right shoulder immobilizer for 4 weeks.
At 4 weeks after surgery, the hand surgeon determined that MC had complete motor and sensory deficits through the median and ulnar nerve distributions. He was referred to therapy for fabrication of orthoses, exercises, and education. At the initial visit, his hand was postured in an intrinsic minus position in digits 2 through 5, yet PROM was within normal limits (WNL). Two orthoses were fabricated and issued: (1) a nighttime resting hand orthosis in slight wrist extension, MCP joint flexion, and IP joint extension and (2) a static daytime orthosis to prevent posturing in the intrinsic minus position. The orthoses were fabricated and issued, but due to insurance issues MC was unable to attend therapy at our facility and was referred out to another center. Unfortunately, MC and his family were not compliant with follow-up therapy and orthotic wear. He also missed several postoperative physician visits. Five months after the initial referral (6 months after surgery), MC returned for weekly therapy at our facility with a new insurance policy.
Six months postoperatively, medical and social history were reviewed and an assessment was made of (1) resting posture and available prehension patterns, (2) sensibility, (3) AROM and PROM, (4) strength (Myometer and Jamar dynamometer), (4) dexterity (9-hole peg test, Jebsen Taylor Hand Function Test), (5) ADL skills, and (6) individual and family preferred learning styles.
At rest MC postured his wrist in flexion, MCP joints in slight flexion, and IP joints in flexion. Because of weakness and joint contracture, MC could only exhibit the following patterns: a raking grasp, weak lateral pinch, and a weak scissors grasp between his fingers. During bimanual tasks, MC could not stabilize objects with his right hand, and he relied on both hands to grasp and lift objects. Findings for the initial and final objective assessments are listed in Tables 45-4 through 45-8 (online).
Table 45-4 Case Study: Sensibility (Median and Ulnar Nerve Distributions)
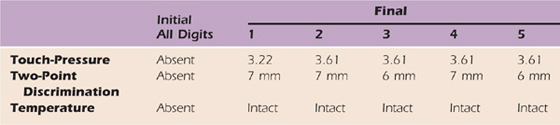
Table 45-5 Case Study: Right Upper Extremity AROM
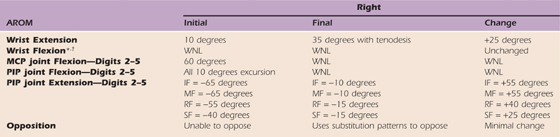
AROM, active range of motion; IF, index finger; LUE, left upper extremity; MCP, metacarpophalangeal; MF, middle finger; PIP, proximal interphalangeal; PROM, passive range of motion; RF, ring finger; SF, small finger; WNL, within normal limits.
LUE AROM and PROM WNL.
*Full passive wrist extension achieved with full finger flexion with c/o 2/10 pain and end-range tightness. Composite extension with wrist past neutral induced c/o 7/10 pain and end-range tightness.
†Full passive digit extension only with wrist neutral.
Table 45-6 Case Study: Right Upper Extremity PROM
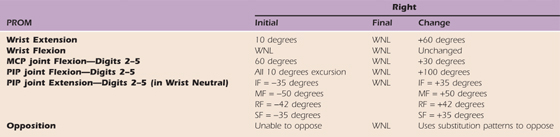
IF, index finger; MCP, metacarpophalangeal; MF, middle finger, PIP, proximal interphalangeal; PROM, passive range of motion; RF, ring finger; SF, small finger; WNL, within normal limits
Table 45-7 Case Study: Strength

Table 45-8 Case Study: Right Hand
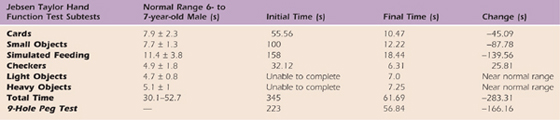
MC’s primary concerns at this time were his inability to use his right hand to hold toys, assist with dressing, or wipe himself after toileting. He also had difficulty with bimanual tasks such as holding the handlebars on his bike, securing fasteners, and stabilizing a plate and food for cutting. MC had to change his dominance from his right to his left hand due to his limitations.
It was important to consider neural recovery factors when establishing treatment goals with MC and his family and speculating on outcomes. MC’s age, health, and timeliness of repair were supportive of good recovery. He was now a 6-year-old male in good general health and had received his nerve repair within 4 hours of injury. He was active and energetic and enjoyed participating in therapeutic activities and had specific functional goals that he wished to address. Constraints to achieving a favorable outcome included the level of his lesion, the involvement of multiple peripheral nerves, and compliance. MC’s laceration was at the axilla level, the median and ulnar nerve injuries were severe, and he and his family had a history of noncompliance. His initial delay in acquiring rehabilitation services contributed to joint contracture and suboptimal resting hand posture and may have delayed sensory and motor recovery.
For MC it was essential to prioritize goals and treatment and continually reevaluate his progress due to his significant involvement from multiple PNIs. At this phase of treatment MC exhibited significant AROM impairments in his right upper extremity (RUE). The late initiation of his rehabilitation program and his age required modification of the typical treatment goals and strategies listed earlier. His goals for treatment and the strategies used to attain them are shown in Table 45-9 (online). Individual education included a review of therapy goals, precautions, timeline/expectations in recovery, and explanation of functional deficits and the cause.
Table 45-9 Case Study: Therapy Goals
AROM, active range of motion RUE, right upper extremity; PROM, passive range of motion.
Due to his age, MC required multimodal individual education, including demonstration, hand-over-hand assistance, verbal, and written or pictorial instructions. To initiate and retrain grasp and release we used MVF. Strategies used to enhance AROM included aquatic tasks using a “water table” for wrist flexion–extension exercises, bilateral dowel exercises, volleyball and baseball with the arm in an “Aircast” to strike the ball, prone scooter board play, play in a tire swing, table hockey using elbow flexion–extension or wrist flexion–extension, and an arm ergometer. To isolate muscle activation we used biofeedback and place-and-hold exercises, which were both successful. We progressed to strengthening via progressive resistive exercises using cuff weights, theraband, theraputty, a biometrics device, and a medicine ball for bilateral games. To enhance sensorimotor control we used the biometrics device to encourage grading of fingertip forces while playing video games. We also encouraged construction of wooden projects (toy boat, car), and participation in manipulative games such as Jenga, Connect Four, Yahtzee, Legos, and Connex. We used virtual reality games and cuff weights to reinforce use of the RUE and to enhance endurance. Sensory reeducation tasks incorporated textured discrimination tiles, stereognosis games, and touch localization with and without vision. Participation in preferred bimanual tasks such as meal preparation and dressing gave MC the opportunity to improve function.
Orthosis type and wear schedule were adapted as progress was made. The long-arm opponens orthosis issued in phase one to provide wrist stability and position the thumb for grasp was used until wrist extension returned. We then issued a short opponens orthosis to position the thumb for grasp combined with kinesiotaping to facilitate active wrist extension. At this time we also issued a figure-of-8 orthosis to prevent intrinsic minus posturing.
Unfortunately, MC’s poor sensibility and neuropathic pain (which increased on reinnervation) reduced his willingness to use his RUE for ADL tasks. Sensory reeducation strategies progressed as follows: (1) desensitization, (2) identification of static versus moving touch, (3) touch localization, (4) tactile gnosis, and (5) tactile discrimination.
Goals for this phase are listed in Table 45-9 (online). MC primarily focused on strengthening his RUE and improving his prehensile control. After 1 year of therapy, MC displayed maximum therapeutic benefit and was referred to a hand surgeon for consultation regarding surgical options. He continued to display intrinsic minus posturing and had evidence of muscle wasting (Fig. 45-18A). He also was unable to oppose his thumb or small finger (Fig. 45-18B).
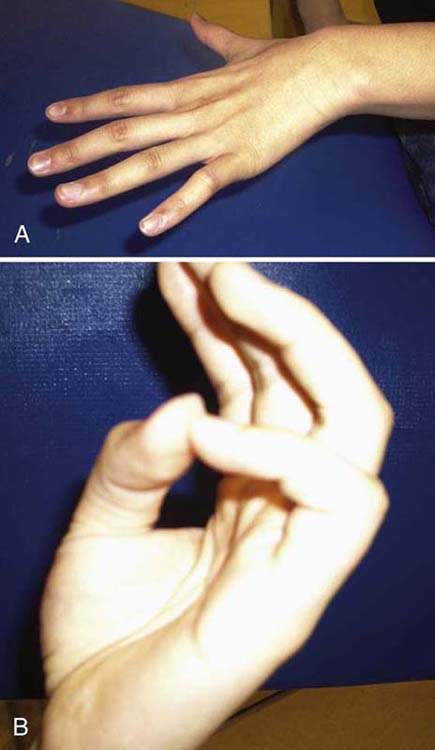
Figure 45-18 Case study: Median and ulnar nerve injury. A, Intrinsic minus posturing and muscle atrophy between metacarpals 2 through 5. B, Inability to oppose with thumb or small finger after 6 months.
At discharge his reevaluation showed progress in most areas as shown in Tables 45-4 through 45-8 (online) including dexterity. Based on his current function and surgical consultation it was determined that he would receive tendon transfers. See Chapter 59 for tendon transfer results.
Because of the tremendous potential for peripheral nerve regeneration, rehabilitation after compression or traction injuries or after lacerations and repair can be quite rewarding. The key to success is to adhere to precautions initially to rest and protect the nerve, then provide an enriched therapeutic environment as the individual progresses through the phases of recovery. This strategy enhances not only peripheral recovery but central reorganization. Furthermore, if greater emphasis is placed on fostering reorganization and recovery of sensorimotor control, the patient may be able to regain flexible and efficient prehensile skill after nerve injury.
Video 45-1 Grip Formation: note wide aperture (width between thumb and fingers) during reach-to-grasp of blocks.
Video 45-2 Demonstration of two of three components of in-hand manipulation; translation (palm to fingers) and shift (movement along fingertips).
Video 45-3 Use of an orthosis with ring and small finger loops attached to a palmar bar to minimize “intrinsic minus” or claw posturing after ulnar nerve injury.
Video 45-4 CASE: subtest form the Jebsen Test of Hand Function, turning cards.
1. Sunderland S. Nerves and Nerve Injuries. 2nd ed London: Churchill Livingstone; 1978.
2. Xu J, Wall JT. Rapid changes in brainstem maps of adult primates after peripheral injury. Brain Res. 1997;774(1-2):211–215.
3. Kolarik RC, Rasey SK, Wall JT. The consistency, extent, and locations of early-onset changes in cortical nerve dominance aggregates following injury of nerves in primate hands. J Neurosci. 1994;14:4269–4288.
4. Leeber J. How much brain does a mind need? Scientific, clinical, and educational implications of ecological plasticity. Dev Med Child Neurol. 1998;40(5):352–357.
5. Wall JT, Xu J, Wang X. Human brain plasticity: an emerging view of the multiple substrates and mechanisms that cause cortical changes and related sensory dysfunctions after injuries of sensory inputs from the body. Brain Res Brain Res Rev. 2002;39(2-3):181–215.
6. Seddon HJ. Three types of nerve injury. Brain. 1943;66:237.
7. Terzis JK, Smith KL. The Peripheral Nerve: Structure, Function, and Reconstruction. New York: Raven Press; 1990.
8. Keirnan JA. The Human Nervous System: An Anatomical Viewpoint. Peripheral nervous system 7th ed Philadelphia: Lippincott-Raven Press; 1998.
9. Liuzzi FJ, Tedeschi B. Peripheral nerve regeneration. Neurosurg Clin N Am. 1991;2(1):31–42.
10. Gerenghi G. Peripheral nerve regeneration and neurotrophic factors. J Anat. 1999;194:1–14.
11. Chan H, Smith RS, Snyder RE. Junction between parent and daughter axons in regenerating myelinated nerve: properties of structure and rapid axonal transport. J Comp Neurol. 1989;28:391–404.
12. Tapia M, Inestrosa NC, Alvarez J. Early axonal regeneration: repression by Schwann cells and a protease? Exp Neurol. 1995;131:124–132.
13. Skirven TM. Nerve injuries. In: Stanley BG, Stribuzi SM, eds. Concepts in Hand Rehabilitation. Philadelphia: FA Davis; 1992:323–352.
14. Al-Majed AA, Neumann CM, Brushart TM, Gordon T. Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. J Neurosci. 2000;20:2602–2608.
15. Brushart TM, Jari R, Verge V, et al. Electrical stimulation restores the specificity of sensory axon regeneration. Exp Neurol. 2005;194:221–229.
16. Sabatier MJ, Redmon N, Schwartz G, English AW. Treadmill training promotes axon regeneration in injured peripheral nerves. Exp Neurol. 2008 June;211(2):489–493.
17. Rasmusson DD, Webster HH, Dykes RW. Neuronal response properties within subregions of racoon somatosensory cortex 1 week after digit amputation. Somatosens Mot Res. 1992;9(4):279–289.
18. Wellman CL, Arnold LL, Garman EE, Garraghty PE. Acute reductions in GABA-A receptor binding in layer IV of adult primate somatosensory cortex after peripheral nerve injury. Brain Res. 2002;954(1):68–72.
19. Stendel R, Jahnke U, Straschill M. Changes of medium-latency SEP-components following peripheral nerve lesion. J Brachial Plex Peripher Nerve Inj. 2006;1:4–10.
20. Ramachandran VS, Rogers-Ramachandran D, Stewart M. Perceptual correlates of massive cortical reorganization. Science. 1992;258(5085):1159–1160.
21. Beggs S, Torsney C, Drew LJ, Fitzgerald M. The postnatal reorganization of primary afferent input and dorsal horn cell receptive fields in the rat spinal cord is an activity dependent process. Eur J Neurosci. 2002;16(7):1249–1258.
22. Churchill JD, Tharp JA, Wellman CL, et al. Morphological correlates of injury-induced reorganization in primate somatosensory cortex. BMC Neurosci. 2004;5:13.
23. Merzenich MM, Sameshima K. Cortical plasticity and memory. Curr Biol. 1993;3(2):187–196.
24. Garraghty PE, Kaas JH. Large-scale functional reorganization in the adult monkey cortex after peripheral nerve injury. Proc Natl Acad Sci USA. 1991;88(16):6976–6980.
25. Jones EG. Cortical and subcortical contributions to activity-dependent plasticity in primate somatosensory cortex. Ann Rev Neurosci. 2000;23:1–37.
26. Wainer BH, Kwon J, Eves EM, et al. Neural plasticity as studied in neuronal cell lines. Adv Neurol. 1997;72:133–142.
27. Rao AK, Gordon AM. Contribution of tactile information to accuracy in pointing movements. Exp Brain Res. 2001;138:438–445.
28. Gentilucci M, Toni I, Chieffi S, Pavesi G. The role of proprioception in the control of prehension movements: a kinematic study in a peripherally deafferented individual and in normal subjects. Exp Brain Res. 1994;99:483–500.
29. Westling G, Johansson RS. Factors influencing the force control during precision grip. Exp Brain Res. 1984;53:277–284.
30. Jeannerod M. The timing of natural prehension. J Mot Behav. 1984;16(3):235–254.
31. Goodale MA, Meenan JP, Bülthoff HH, et al. Separate visual pathways for perception and action. Trends Neurosci. 1994;15:20–25.
32. Gordon AM, Forssberg H, Johansson RS, Westling G. Integration of sensory information during the programming of precision grip: comments on the contributions of size cues. Exp Brain Res. 1991;85:226–229.
33. Johansson RS, Cole KJ. Sensory-motor coordination during grasping and manipulative actions. Curr Opin Neurobiol. 1992;2(6):815–823.
34. Cole KJ, Steyers CM, Graybill EK. The effects of graded compression of the median nerve in the carpal canal on grip force. Exp Brain Res. 2003;148:250–257.
35. Byl NN, Melnick M. The neural consequences of repetition: clinical implications of a learning hypothesis. J Hand Ther. 1997;10(2):160–174.
36. Sainburg RL, Poizner H, Ghez C. Loss of proprioception produces deficits in interjoint coordination. J Neurophysiol. 1993;70(5):2136–2147.
37. Rafuse VF, Gordon T, Orozco R. Proportional enlargement of motor units after partial denervation of cat triceps surae muscles. J Neurophys. 1992;68(4):1261–1276.
38. Bernstein N. The Coordination and Regulation of Movements. Oxford, UK: Pergamon; 1967.
39. Cruse H, Wischmeyer M, Bruwer P, et al. On the cost functions for the control of the human arm movement. Biol Cybern. 1990;62(6):519–528.
40. Gordon AM, Lewis SR, Eliasson AC, Duff SV. Object release under varying task constraints in children with hemiplegic cerebral palsy. Dev Med Child Neurol. 2003;45:440–448.
41. Duff SV. Impact of peripheral nerve injury on sensorimotor control. J Hand Ther. 2005;18:277–291.
42. Kozin SH, Porter S, Clark P, Thoder JJ. The contribution of the intrinsic muscles to grip and pinch strength. J Hand Surg. 1999;24(1):64–72.
43. Schreuders TAR, Roebroeck M, van Der Kar TJ, et al. Strength of the intrinsic muscles of the hand measured with a hand-held dynamometer: reliability in patients with ulnar and median nerve paralysis. J Hand Surg [Br]. 2000;25:560–565.
44. Gordon AM, Duff SV. Relationship between clinical measures and fine manipulative control in children with hemiplegic cerebral palsy. Dev Med Child Neurol. 1999;41:986–991.
45. Bock O, Eckmiller R. Goal-directed arm movements in humans after damage to somatic sensory cortex. Exp Brain Res. 1986;109:92–100.
46. Gelberman RH. Operative Nerve Repair and Reconstruction. Vol. 1. Philadelphia: J.B. Lippincott; 1991. 711-844
47. Leffert RD. Anterior submuscular transposition of the ulnar nerves by the Learmonth technique. J Hand Surg [Am]. 1982;7:247–255.
48. Gaul JS. Intrinsic motor recovery—a long-term study of ulnar nerve repair. J Hand Surg. 1982;7:502–508.
49. Pfaeffle HJ, Waitayawinyu T, Trumble TE. Ulnar nerve laceration and repair. Hand Clin. 2007;23:391–399.
50. Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975;1:377–399.
51. Wong D, Baker C. Pain in children: comparison of assessment scales. Ped Nurs. 1988;14(1):9–17.
52. Vasudevan TM, Van Rij AM, Nukada H, Taylor PK. Skin wrinkling for the assessment of sympathetic function in the limbs. ANZ J Surg. 2000;70(1):57–59.
53. Tindall A, Dawood R, Povlsen B. Case of the month: the skin wrinkle test: a nerve injury test for paediatric and uncooperative patients. Emerg Med J. 2006;23:883–886.
54. Sollerman C. Assessment of Grip Function: Evaluation of a New Method. 1980.
55. Haymaker W, Woodhall B. Peripheral Nerve Injuries: Principles of Diagnosis. 2nd ed Philadelphia: WB Saunders; 1967.
56. Jebsen RH, Taylor N, Trieschmann RB, et al. An objective and standard test of hand function. Arch Phys Med Rehabil. 1969;50(6):311–319.
57. Rosén B, Lundborg G. Early use of artificial sensibility to improve sensory recovery after repair of the median and ulnar nerve. Scand J Plast Reconstr Surg Hand Surg. 2003;37(1):54–57.
58. Rosén B, Lundborg G. Training with a mirror in rehabilitation of the hand. Scan J Plast Reconstr Surg. 2005;39:204–208.
59. Byl NN, Nagajaran S, McKenzie AL. Effect of sensory discrimination training on structure and function in individuals with focal hand dystonia. Arch Phys Med Rehabil. 2003;84(10):1505–1514.
60. Park SW, Butler AJ, Cavalheiro V, et al. Changes in serial optical topography and TMS during task performance after constraint-induced movement therapy in stroke: a case study. Neurorehabil Neural Repair. 2004;18:95–105.
61. Colditz JC. Splinting the hand with a peripheral nerve injury. In: Osterman AL, Skirven TM, Fedorcyzk J, Sneider L, eds. Rehabilitation of the Hand and Upper Extremity, 5 ed, 622-633.
62. Colditz JC. Splinting for radial nerve palsy. J Hand Ther. 1987;1:18–23.
63. Ernfors H, Henschen A, Olson L, Persson H. Expression of nerve growth factor receptor mRNA is developmentally regulated and increased after axotomy on rat spinal cord motoneurons. Neuron. 1989;2:1605–1613.
64. Terenghi G. Peripheral nerve regeneration and neurotrophic factors. J Anat. 1999;194:1–14.
65. Gravvanis AI, Lykoudis EG, Tagaris GA, et al. Microsurgical repair of nerve lesions with nerve grafts: the effect of nerve growth factor 7S. Euro J Plast Surg. 2002;25:187–192.
66. Shimizu S, Kitada M, Ishikawa H, et al. Peripheral nerve regeneration by the in vitro differentiated-human bone marrow stromal cells with Schwann cell property. Biochem Biophys Res Comm. 2007;399:915–920.
67. Boyd JC, Gordon T. A dose-dependent facilitation and inhibition of peripheral nerve regeneration by brain-derived neurotrophic factor. Euro J Neuroscienc. 2002;15:613–626.
68. Gordon T, Brushart TM, Amirjani N, Chan KM. The potential of electrical stimulation to promote functional recovery after peripheral nerve injury—comparisons between rats and humans. Acta Neurochir Suppl. 2007;100:3–11.
69. Schmidhammer R, Hausner T, Hopf R, et al. In peripheral nerve regeneration environment enriched with activity stimulating factors improves functional recovery. Acta Neurochir Suppl. 2007;100:161–167.
70. Shieh SJ, Chiu HY, Lee JW, Hsu HY. Evaluation of the effectiveness of sensory reeducation following digital replantation and revascularization. Microsurg. 1995;6:578–582.
71. Lundborg G, Richard P. Bunge memorial lecture. Nerve injury and repair—a challenge to the plastic brain. J Peripher Nerv Syst. 2003;8:209–226.