Nerve Mobilization and Nerve Gliding
BIOMECHANICAL AND PHYSIOLOGIC CONCEPTS
NEUROPATHOLOGY AND ITS MANIFESTATIONS
▪ Neuropathic pain is often a major component of neural tension dysfunction.
▪ The nervous system is designed to accommodate movement via excursion and strain, and excess strain can lead to compromise of the nervous system’s function.
▪ Neuropathology manifests as alterations in neurophysiology (symptoms) and mechanics (limitation of motion).
▪ The application of neurodynamic testing and treatment via neural mobilization requires a profound respect for the sensitivity of the nervous system to excess movement, especially in the diseased state.
▪ Treatment requires continuous and sound clinical reasoning when applying these techniques.
The use of neural provocation testing (NPT) and specifically the upper limb neural tension test (ULNTT) and nerve mobilization (NM) as an examination and treatment approach requires a clinician’s understanding of neural biomechanics and the consequences of neuropathology, pain, and movement dysfunction. Knowledge of treatment principles, guidelines, progression, precautions, and contraindications is essential for executing a safe and effective examination and treatment strategy. This is best summed up by the concept of neurodynamics developed by Michael Shacklock.1 It combines mechanical and physiologic properties of the peripheral nervous system that are dynamically interdependent and correlates the effects of tension and excursion on the peripheral nervous system. Attempting to separate these features clinically is nearly impossible. Alterations in neurodynamics will manifest as a pathomechanical (excursion, tension) and/or a pathophysiologic (pain sensory changes) problem. Shacklock2 now refers to this as neural tension dysfunction (NTD) that incorporates both of these concepts. Changes in neural physiology and mobility may result in the development of the patient’s symptoms and the limitation of motion confirmed via neurodynamic evaluation. This concept of neurodynamics also applies to treatment to restore normal neural physiology and mobility and alleviate pain originating in the nervous system.
Using NPT and NM mandates that the clinician understand that the vast majority of patients with NTD present with neuropathic pain as a primary feature. The International Association for the Study of Pain defined peripheral neuropathic pain as “pain initiated or caused by a primary lesion or dysfunction in the peripheral nervous system.”3 The mechanisms of neuropathic pain are not fully understood as evidenced by the list of reported mechanisms compiled by Allen in Box 118-1.4 In the normal pain state, afferent (nociceptor) and central nervous system (spinal cord, thalamic, cortical) hyperexcitability occurs. Neuropathic pain may be a dysfunction of this process.5 For successful use of these examination and treatment techniques, the therapist must appreciate the interaction between the patient’s pain and accompanying limitations in motion.
Box 118-1 Potential Neuropathic Pain Mechanisms
Primary afferent nociceptor neuropeptide changes
Neuronal sprouting: peripheral and central
Possible theoretical neuropathic pain mechanisms compiled by Allen.4
It appears that the concept of abnormal impulse-generating sites (AIGSs) plays a key role in neuropathic pain.6 These sites are sources of ectopic stimulus generation or discharges related to an alteration in ion channel function and number. These ion channel changes usually occur at the site of demyelination, which can occur when a nerve is damaged by trauma or disease. This damage may result in the formation of microneuromas in continuity.7 The pain may arise from impulses in demyelinated, damaged, or regenerating afferent fibers. A number of different stimuli, such as temperature, cytokines, catecholamines, and metabolic and mechanical factors, can stimulate an AIGS.6 AIGSs will eventually cause changes in normal function of the dorsal root ganglion and neurons in the central nervous system (dorsal horn, thalamus, and cortical neurons), known as central sensitization. Pain from AIGSs has also been described as dysesthetic pain.8
Neuropathic pain can also be attributed to nerve trunk pain,8 which may result from increased activity in nociceptive endings in the connective tissue of the peripheral nerves called nervi nervorum.8-11 Asbury and Fields8 theorize that nociceptive activity causing nerve trunk pain is created by chemically mediated increased mechanosensitivity of the neural tissue. This theory is also supported by Sauer and associates.12 These researchers reported that the nervi nervorum are responsible for the release of calcitonin gene–related peptide (CGRP), leading to an inflammatory cascade within the nerve.12 Because of this increased sensitivity, the application of an adequate stimulus, tension, and compression will result in a noxious response.
One final theory to consider is the direct effect of chemical sensitization of the peripheral nervous system. The chemical may be of non-neurogenic origin as a result of injury to connective tissue. Endogenous chemicals such as bradykinin, serotonin, histamine, prostaglandins, and leukotrienes are released and have been shown to affect nociceptive afferents.13 These endogenous chemicals may also be of neurogenic origin and are called neuropeptides and include substance P, CGRP, vasoactive intestinal peptide, and enkephalins. These chemicals are released by primary afferent neurons as a result of chemical or physical damage to the peripheral nociceptive afferent.13 These chemicals will result in the formation of neurogenic inflammation and potentially produce neuropathic pain.14,15 The result of this neurogenic inflammation is increased mechanosensitivity of the nervous system.16,18 As discussed later in the chapter, this increase in mechanosensitivity plays a role in the neural hyperalgesia experienced by patients in the form of antalgic posturing, mechanical tension, or compression via palpation.
Neuropathic pain presents with a variety of symptoms that are summarized in Box 118-2.3 Patients with neuropathic pain often present with continuous and spontaneous pain that may not be related to any particular stimulus and may linger after the removal of an evoking stimulus (hyperpathia). The existence of spontaneous discharge has been confirmed in animal studies by Eliav and colleagues18 and Bove and colleagues16 in the presence of induced neurogenic inflammation. This neurogenic inflammation and increased mechanosensitivity may also be responsible for the distant projection of pain or its sometimes widespread nature.17,18 Neuropathic pain may be perceived as deep (cramping, aching, throbbing) or superficial (burning, pinching, stabbing). There often is a delayed response to mechanical stimuli, which may occur after repeated stimulation.19 Paroxysmal pain may also occur and is described as electric shock–like or shooting. Spontaneous ectopic discharge and lowered threshold mechanoreceptor function may be responsible for some of the bizarre symptoms reported by patients with neuropathic pain.5 Pain may radiate from a focal point along a continuous track or may be referred to other areas described as clusters or clumps of pain.5,6,17,18 The specific evaluation of neuropathic pain has been postulated by Galer and Jensen20 using the Neuropathic Pain Scale (NPS).20 The scale is designed to measure the distinct qualities of neuropathic pain. Preliminary testing shows the NPS to be discriminant and have predictive validity. Further testing of this scale is needed to confirm its usefulness in evaluating neuropathic pain. The reader is referred to Chapter 113 for a more detailed discussion of pain.
Box 118-2 Neuropathic Pain Terminology
Allodynia: Nonpainful stimulus provokes pain
Hyperalgesia: Increased response to painful stimulus
Hyperpathia: Increased response to painful stimulus continues after it is withdrawn
Dysesthesia: Unpleasant abnormal sensation (spontaneous or evoked)
The nervous system is a continuum.21,22 The peripheral, central, and autonomic nervous systems all combine to form one system that interacts as a unit of input and output. This continuum is achieved mechanically, electrically, and chemically. Figure 118-1 is an anatomic prosection demonstrating this concept visually prepared by Dr. Rufus Weaver and displayed in 1893 at the Columbian Exposition in Chicago. This anatomic preparation demonstrates how placing tension or strain on either the peripheral or central nervous system could have a potential effect on the nervous system in another location. For example, the ULNTT alters neuraxial or meningeal tension and provides the clinician with screening maneuvers to examine the irritability of the patient’s nervous system and its accompanying interfacing tissues.6,23,24

Figure 118-1 Photograph of the dissected nervous system by Rufus Weaver, MD, entitled “Harriet,” demonstrating the concept of the nervous system continuum. (Courtesy of Drexel University Department of Anatomy, Philadelphia, Pennsylvania.)
The nervous system as a continuum requires mechanisms for elongation, tension, and glide. Sunderland and Bradley25,26 examined the mechanical properties of the peripheral nerve and nerve root to investigate strain failure rate. They reported that the elastic limit in the peripheral nerve varied 7% to 20% and failure strain varied 7% to 30%. For the nerve root, the maximal elastic limit was less than 15%, and failure strain occurred at 25%, indicating that the nerve root failed at lower loads than the peripheral nerve.25,26 Haftek27 investigated the effect of slow and quick stretch on albino rat tibial nerves. He reported that the initial process of elongation did not affect the nerve fiber but was physiologic in nature, described as unfolding. Progressive strain to failure demonstrated that histologic rupture of the epineurium occurred first. Before epineural rupture, damage in the form of neurapraxia or axonotmesis occurs.27 These strain levels are much higher than the clinician would want to impart when performing NPT or NM.
The strain and/or stress that occur along the course of the nerve as a result of upper extremity joint motion must also be considered. Strain is the change in length that occurs in a nerve as a result of unfolding in response to extremity movement. Millesi and colleagues,28 Zoech and colleagues,29 and most recently Wright and colleagues30-32 and Kleinrensink and colleagues33-35 demonstrated in cadavers that motion of the upper extremity results in stress being imparted along the entire course of the nervous system as measured by strain. Kleinrensink and colleagues,33-35 studying the effect of the ULNTT of strain on the peripheral nerves, determined that motion at the wrist increased the strain at the cord level of the brachial plexus. In addition, contralateral lateral flexion of the cervical spine increased strain in the cords of the brachial plexus and the three major nerves in the arm. Finally, the ULNTT of the median nerve was the most sensitive for the three major nerves and the most specific for the median nerve compared to the other ULNTT for the ulnar and radial nerves. Research performed in animal, cadaver, and limited human models36 verifies that the nervous system has multiple mechanisms to attenuate strain, tension, and elongation and that upper extremity and spinal motion can affect tension throughout the nervous system.
Tension within the nerve can also affect intraneural blood flow and nerve function.27,37-40 Lundborg and Rydevik38 determined that lower limits of strain (5%–10%) demonstrated the first signs of changes in blood flow in the epineural and perineural vessels. The upper stretch limit was 11% to 18%, causing complete occlusion that resolved after relaxation of the nerve. Using rabbit sciatic nerve, Ogata and Naito39 found that strain limits greater than 15.7% resulted in complete ablation of blood flow to the nerve. Complete ablation of blood flow also occurred when external compression was greater than 50 to 70 mm Hg. Studying the effect of strain on rat tibial nerve function, Kwan et al.37 reported strains of 6% or greater resulted in a 60% decrease in compound nerve action potential (CNAP) after 20 minutes. They concluded that longstanding low stress could affect the functional properties of the nerve.
In 1992, Wall and associates,41 using rabbit tibial nerves and measuring nerve conduction, also determined that strain rates of 6% or greater resulted in a 70% decrease in CNAP after 20 minutes. Recovery occurred to within 10% of prestretch values when the load was removed. At 12% strain, there was a rapid reduction in CNAP with complete conduction block after 50 minutes. There was only a 40% recovery once the load was removed. It was their opinion that mechanical deformation contributed to decreased nerve conduction and ischemia. Wall and colleagues concluded that the response to stretch might not be immediate; however, prolonged stretch may cause irreversible damage. Porter and Wharton40 examined the effect of nerve function by occluding the nerve’s blood supply and measuring the irritability of a muscle innervated by that nerve. They discovered that irritability in muscle increased within 2 to 11 minutes after the blood flow was occluded. The role of repeated versus continuous strain on nerve function was studied by Watanabe and colleagues.42 The authors applied a continuous traction at 1, 2, 5, and 0 N of force and a repetitive traction at 60 and 120 cycles per hour to the brachial plexus of a rat. They determined that there was no change in blood–nerve barrier permeability, functional grip strength, and electrophysiologic function, as measured by CNAP. In contrast, repetitive traction resulted in significant changes in all three measures with the higher repetitions (120 cycles per hour) causing the greatest change. The practical application of this information is that it supports that maximal strain rates using the ULNTT and NM should be less than 4% to 6%. Repetitive applications or oscillations of NM may result in damage to the nerve. The clinician must rely on the patients’ response (pain/paresthesia) to determine the amount of strain because it cannot be measured clinically.
The nervous system’s ability to accommodate tension is a product of an intraneural and extraneural anatomic design. Internally, the nerve is designed with undulations creating a tortuous nature.43 The nerves’ connective tissue layers form these undulations and were described by Clarke and Bearn44 when investigating the presence of the spiral bands of Fontana. These bands are present in the relaxed state of the nerve and disappear as tension is applied. A second mechanism that the nerve uses to tolerate elongation is intraneural gliding.28,43,45 The unique framework of the nerve’s connective tissue allows intraneural excursion between individual nerve fibers and their surrounding endoneurium and the endoneurium surrounding each nerve fiber. The epineurium allows excursion to occur between it and the perineurium of each fascicle. Finally, the nerve’s internal ability to tolerate tension and permit elongation results from an intrafunicular plexus formation described by Sunderland.46
Extraneural gliding provides for attenuation of tension via a gliding surface between the paraneurium and the epineurium.28 Extraneural excursion or gliding has been demonstrated in the central nervous system47,48 and the peripheral nervous system.11,30,32,43,46
In the central nervous system, Reid48 demonstrated that 1.8 cm of excursion occurred in the spinal cord when performing movements of cervical and lumbar spine flexion and extension. He reported that 11.3% strain occurred in the cervical spine with the greatest amount of strain between the C2 and C5 levels. This increased to 17.6% when C2–T1 levels were combined. Movement of the nerve root that occurred was transmitted via the dural sheath and dentate ligaments and not directly to the rootlets. O’Connell22 demonstrated that cervical flexion caused excursion of the cord in the cephalad direction with increased nerve root tension. Cervical extension caused movement in the opposite direction and decreased nerve root tension. Cervical spine flexion caused 5 mm of excursion in the cervical region, 4 mm in the thoracic region, and 1 mm in the lumbar region. Straight leg raising and prone knee bend created caudal excursion of the spinal cord and increased nerve root tension at the lumbar level. Finally, Lew and colleagues,21 examining baboon cadavers, also reported that cervical flexion caused cephalad movement of the spinal cord.
Neural excursion has also been demonstrated to occur in the peripheral nervous system. McLellan and Swash49 demonstrated that the median nerve underwent an excursion of 7.4 mm distally and 4.3 mm proximally with wrist and finger motion and elbow flexion/extension. In 1986, Wilgis and Murphy50 measured excursion of the brachial plexus, median, ulnar, and radial nerves in 15 cadaver arms. The greatest excursions occurred at the brachial plexus level (15.3 mm) with movement of the shoulder, the median nerve wrist level (proximal 14.5 mm and distal 6.8 mm), the ulnar nerve wrist level (13.8 mm), and 6.8 mm distal excursion of the ulnar nerve with elbow extension to flexion. Using fresh frozen cadaver specimens, Wright and colleagues30-32 examined excursion of the median, ulnar, and radial nerves. A summary of their findings is presented in Tables 118-1 through 118-3.
Table 118-1 Median Nerve Excursion
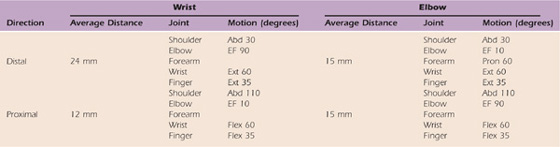
Excursion of the median nerve measured at the wrist and elbow adapted from the work of Wright et al.31
Abd, abduction; EF, elbow flexion; Ext, extension; Flex, flexion; Pron, pronation.
Table 118-2 Ulnar Nerve Excursion
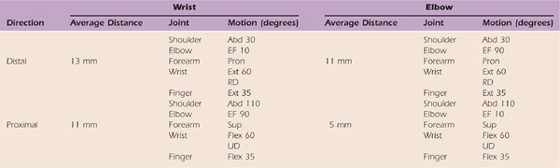
Excursion of the ulnar nerve measured at the wrist and elbow adapted from the work of Wright et al.30
Abd, abduction; EF, elbow flexion; Ext, extension; Pron, pronation; RD, radial deviation; Sup, supination; UD, ulnar deviation.
Table 118-3 Radial Nerve Excursion
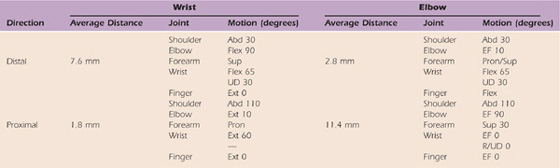
Excursion of the ulnar nerve measured at the wrist and elbow adapted from the work of Wright et al.32
Abd, abduction; EF, elbow flexion; Ext, extension; Flex, flexion; Pron, pronation; R/UD, radial/ulnar deviation; Sup, supination; UD, ulnar deviation.
Wright and associates31 demonstrated that the average median nerve distal excursion measured at the wrist was 24 mm and occurred when the upper extremity was positioned in shoulder abduction of 30 degrees, elbow flexion of 90 degrees, and wrist/finger extension. Average proximal median nerve excursion measured at the wrist was 12 mm with the shoulder abducted 110 degrees, elbow extension of 10 degrees, and wrist/finger flexion. Excursion of the median nerve measured at the elbow revealed that an average distance of 15 mm occurred distally with shoulder abduction of 30 degrees, elbow extension of 10 degrees, and wrist/finger extension. Proximal excursion averaged 15 mm with 110 degrees of shoulder abduction, elbow flexion of 90 degrees, and combined wrist and finger flexion.
The average excursion of the ulnar nerve measured at the wrist was 13 mm distally with shoulder abduction of 30 degrees, elbow extension, pronation, and wrist/finger extension. The average proximal excursion was 11 mm with shoulder abduction of 110 degrees, elbow flexion of 90 degrees, supination, and wrist/finger flexion. Measured at the elbow, the average distal excursion was 11 mm with shoulder abduction of 30 degrees, elbow flexion of 90 degrees, pronation, and wrist/finger extension. Proximal excursion of 5 mm occurred with shoulder abduction of 110 degrees, elbow extension of 10 degrees, supination, and wrist/finger flexion. The total distance necessary to accommodate upper extremity motion was 24 mm at the wrist and 17 mm at the elbow. The total motion required to accommodate upper extremity motion was 36 mm at the wrist and 30 mm at the elbow.30
The radial nerve demonstrated less excursion than the median or ulnar nerve.32 A total of 11.4 mm of proximal excursion occurred at the elbow in the position of shoulder abduction of 110 degrees, elbow flexion of 90 degrees, and distal joints at 0 degrees. Distal excursion measured at the elbow was 2.8 mm with shoulder abduction of 30 degrees, elbow extension, and wrist/finger flexion. Excursion measured at the wrist was 1.8 mm in the proximal direction with the shoulder in abduction of 110 degrees, elbow extension, and wrist/finger extension. The greatest distal excursion (7.6 mm) occurred with the shoulder in 30 degrees of abduction, elbow flexion of 90 degrees, with the wrist flexed and fingers extended.32
In vivo studies using ultrasonography confirmed the presence of nerve excursion.36,51,52 Dilley and colleagues51 reported total median nerve excursion of 10.4 mm in the upper arm and 4.2 mm in the forearm with elbow extension as the moving component. They also confirmed the concept of the nervous system continuum by demonstrating that the distal motion affects the nerve proximally. Passive wrist and index finger extension also result in similar median nerve excursions of up to 4.5 mm. Finally, the excursions that occur in vivo are less than those that occur in the cadaver studies previously mentioned. From these data, it can be seen that changing the position of the shoulder, elbow, wrist, and fingers can affect nerve excursion distally or proximally. These central and peripheral nervous system studies support the continuum theory, which explains the nerve tensioning and gliding that is imparted with the ULNTT and NM.
An integral component in understanding peripheral nerve excursion is appreciating that the interfacing tissues surrounding the nerve along its entire course are also required to adapt in length in relationship to joint motion. Millesi and colleagues11,53 reported that the median nerve bed must adapt by as much as 20% in length at the elbow and wrist to accommodate motion. Zoech and colleagues29 specifically investigated the difference in the length of the median nerve bed in positions of maximal flexion and extension of the upper extremity. They demonstrated that maximal extension required a 4.3% change in length, and flexion resulted in as much as a 14.9% decrease in overall length. Therefore, not only must the peripheral nerve be able to adapt to elongation and tension, but the interfacing tissues that form the nerve bed must also adapt independently to changes in length resulting from joint motion. This same adaptation of the nerve bed occurs within the central nervous system.21,22,47,48,54
In addition to the peripheral nervous system’s ability to accommodate movement, its physiology and function are also dependent on its vascularity and the maintenance of a pressure gradient system. This system allows for the maintenance of vascular perfusion and physiology and is best depicted by Sunderland’s46 model as shown by the formula PA > PC > PF > PV > PT. This model requires arterial pressure (PA) to be greater than capillary pressure (PC), which is greater than fascicular pressure (PF), which exceeds venous return pressure (PV) and ultimately is greater than tunnel pressure (PT). Alterations of any one of these five pressures have the potential to affect circulation46 and axoplasmic flow55,56 throughout the nerve. Alterations in tunnel pressure often result in the more common peripheral nerve entrapments in the upper extremity such as cubital tunnel syndrome, ulnar tunnel syndrome, and carpal tunnel syndrome (CTS).
Pechan and Julis,57 examining ulnar nerve pressures at the elbow, demonstrated a twofold increase in cubital tunnel pressure with cervical spine and shoulder motion and a sixfold increase with ulnar nerve provocative testing (elbow flexion, wrist extension, and the arm above the head). This study further supports the concept that remote motions can have an influence on not only neural motion and strain but also neural pressures and vascular flow. Studying the rabbit vagus nerve, Dahlin and McLean58 verified a conduction block with 30 mm and 50 mm of external compression sustained for 2 hours was reversible within 24 hours. At 200 mm of external compression for 2 hours, reversal took as long as 3 days, and at 400 mm, reversal took 7 days. Nemoto and colleagues59 clamped a dog sciatic nerve at one of two locations proximal and distal to the experimental compression site. Their work established that two low-grade compressions along the nerve trunk created greater damage than a single compression and the damage from dual compression exceeded the expected damage caused by an isolated compression. This supports the theory of double crush described by Upton and McComas.56 In addition to affecting vascular flow, axoplasmic flow is also altered with less external pressure than occurs with minor CTS.55
Taken collectively, these general principles emphasize the key point—that the nervous system is designed for movement. As with any other soft tissue structure, when the nervous system is in a diseased and hyperirritable state, mechanical stresses such as compression and tension may provoke pain syndromes and movement dysfunction associated with the nervous system and its interfacing tissues. The clinician should maintain awareness that not only is direct and local tissue affected, but other tissues innervated by the involved nerves may also be the source of the patient’s pain and movement dysfunction.
Although it is not the intent of this chapter to discuss neural pathology in detail, the neuropathologic consequences on the mechanical properties, vascular flow, and axoplasmic flow of the nerve must be considered when using the ULNTT and NM (gliding, sliding, or tensioning) techniques for treatment. These three factors contribute to the cause of hyperirritability of the peripheral nervous system and its interfacing tissues. The vascular system may be compromised by external compression or adverse tension. NTD may be the result of adaptive shortening of the peripheral nerve from positioning or scarring of the nerve (intra- or extraneural). Compromise of the nerve’s vascularity can lead to a state of inflammation in or around the connective tissues of the nerve, increasing its level of irritability.6,55,60 Vascular changes may also lead to alterations in neurovascular dynamics and intraneural fibrosis.28,46,61 External compression can lead to compromise in axoplasmic flow. This compromise reduces the transport of neural filaments, microtubules, and neurotransmitters along the axon to its terminal ending and the return of metabolic by-products, potentially altering the nerve’s physiology and function. As a result of this chemically mediated inflammatory process and/or the loss of intra- or extraneural gliding capabilities, mechanical irritability of the nerve will occur, resulting in repetitive forces being placed across the fixed (adherent) nerve segment. This loss in neural motion tolerance in one segment requires force attenuation to be achieved over a shorter segment of the nerve, exposing it to further damage or injury.
This neuropathologic process increases the nerve’s vulnerability throughout the upper extremity such as in the confined space of the cubital or carpal tunnel. The interfacing tissues that surround the nerve along its course also create these spaces or tunnels. Two examples of these are the median nerve passing through the pronator muscle or the radial nerve passing through the supinator muscle. Nerves are also more vulnerable at relatively fixed points where motion introduced at these levels minimizes the nerve’s capability of tolerating elongation forces. This frequently occurs where the nerve branches or is relatively fixed such as passing through fascia. Finally, the nerve is vulnerable to external compression whenever it rests across a hard surface such as the radial sensory nerve as it passes across the radius.
The final consequence of peripheral neuropathology is fibrosis.46,60,62,63 Occurring in two ways, intra- and extraneural fibrosis removes the nerve’s inherent ability to elongate or potentiate tension within the nerve fascicles and the gliding that occurs between the connective tissue layers of the nerve and its interfacing tissues. Intraneural fibrosis causes the loss of the tortuous course of the nerve or its undulations,43 resulting in the loss of internal glide and the unfolding capability of the nerve. Extraneural fibrosis limits the nerve’s ability to move within its nerve bed or between the interfacing tissues, creating mechanical interference. In either scenario, the lack of nerve mobility results in increased stress or strain delivered to a shorter nerve segment as joint motion occurs.29 This fibrosis may ultimately lead to the onset of pain and adaptive shortening of the nervous system, altering joint movement and extremity function.
Elvey,64,65 Butler,6,23,24 and Gifford and Butler66 have all described the clinical manifestations of this pathologic process. Simplified, these manifestations are pathophysiologic: symptoms reported by the patient such as paresthesia and pain and pathomechanical alterations in neural mechanics, which are limiting isolated or multisegmented motion of the upper extremity. Elvey65 also described the clinical response observed through a series of clinical examination techniques. These techniques result in provocation of the patient’s symptoms (pathophysiologic) and identifying motion dysfunction or limitations (pathomechanical) of the involved extremity, spinal segment, or both. Elvey65 and Butler23,24 describe a resistance encountered with passive extremity motion resulting from altered neural mechanics. Butler refers to these as “tissue barriers” encountered while performing the ULNTT.23,24 Elvey65 and Balster and Jull67 theorize that the resistance encountered results from hyperirritability of nervous tissue, causing a protective reflexive muscle contraction.
It is often assumed that the ULNTT and NM are neural tissue specific. These techniques are multisegmental and often involve mechanical deformation of other structures such as arteries, fasciae, and nerve roots and accompanying meningeal tissues. To confirm that there is a relationship with neural tissue, Edgar and colleagues68 studied the relationship of upper trapezius length and extensibility of neural tissues in normals. Using the ULNTT, they concluded that there was a relationship between upper trapezius and neural tissue extensibility. Subjects with less neural extensibility also have less upper trapezius length. In a cadaver study by Wilson and colleagues,69 strain in the lateral cord of the brachial plexus and segments of the subclavian artery was measured. They determined that applying the ULNTT resulted in strain in the lateral cord and the subclavian artery. Because of common sympathetic innervations, they theorized, the subclavian artery could be a potential source of symptom provocation leading to a false-positive result. Moses and Carman70 examined three cadaver specimens to determine whether the ULNTT was specific to neuromeningeal tissue. They concluded that the nerve roots of the lower cervical spine attach to innervated structures and that these tissues may also be stressed with the ULNTT, possibly causing a false-positive result.
Additional cadaver studies have been conducted to examine neural excursion and its relationship to component movements of the ULNTT.71-73 Bay and colleagues71 demonstrated that wrist position (extension) plays a significant role in excursion with movement of the fingers (extension–flexion). Coppieters and Alshami73 confirmed that greater excursion occurred with a sliding technique, a two-component motion or moving two joints simultaneously, than with a one-component movement (one joint), and the least amount of excursion occurred with full tension technique (ULNTT).
There is anatomic evidence supporting that the ULNTT and NM also produce changes in strain in the brachial plexus and peripheral nerves.33-35,74 Lewis and colleagues74 studied five fresh cadavers to measure strain in the median nerve distal to the axilla with the application of the ULNTT. They reported a significant increase in tension with elbow and wrist extension, contralateral cervical lateral flexion, and ipsilateral straight leg raise. The importance of these findings is that remote motions increase tension in the nerves at locations distant to the motion. This is further evidence of the continuum theory of the nervous system.
Kleinrensink and colleagues33 reported additional evidence of strain within the brachial plexus and peripheral nerves. In three cadavers they investigated the strain of the brachial plexus and the three major nerves of the upper extremity while applying the ULNTT and a modification with cervical contralateral lateral flexion. Significant increases in strain in the nerves and brachial plexus occurred in all three tests. A specific ULNTT for each nerve also caused strain in the brachial plexus and the other peripheral nerves. These results question the specificity of each ULNTT and confirm the sensitivity. They concluded that the median nerve ULNTT was the most sensitive. These findings support earlier work that demonstrated that strain in the median nerve measured in the axilla and the proximal and distal forearm was increased even with remote joint positioning.34,35 These two studies support the work of Lewis and colleagues74 that remote joint motion will cause strain along the entire course of the nerve. Therefore, the clinical ability of the ULNTT or NM to isolate a particular segment of the nerve is difficult.
Additional cadaver studies confirmed increases in strain within the median and ulnar nerve.72,73 Byl and colleagues72 determined that maximum nerve strain occurred with the full median ULNTT: 8.2% ulnar nerve and 6.7% median nerve. Coppieters and Alshami73 also demonstrated increases in median nerve strain using six different components of the median nerve ULNTT. Maximum tension occurred with the full median nerve ULNTT measured at the wrist (4.7%) and above the elbow (4.2%). These results contradict one another in terms of the “safe zone” of strain in the nerve (4%–6%) to avoid compromise of blood flow and conduction. Finally, Coppieters and Butler75 determined that strain on the median nerve was significantly less when performing sliding movements compared with tension movements. All three tensioning techniques resulted in strains greater than 4%.
A number of authors have investigated the response of the ULNTT in normal subjects. Kenneally and colleagues76,77 reported on the response of the ULNTT applied to 100 normal subjects. In 80% to 90% of the subjects, the most common response was pain/stretch and/or paresthesia over the radial aspect of the forearm, thumb, and radial three fingers, occasionally the ulnar side of the hand, and a deep stretch sensation in the cubital fossa and palm. In 1991, Yaxley and Jull78 investigated the responses of 50 normal subjects to a modification of the ULNTT for the radial nerve. The most frequent response was a strong painful stretch over the radial aspect of the proximal forearm and upper arm, followed by stretch over the biceps. An increase in arm symptoms occurred in 90% of the extremities when cervical contralateral lateral flexion was added. The modified test of wrist/finger extension revealed a different response of pain over the medial elbow, anterior forearm, palm, and the middle three fingers or the entire hand excluding the thumb.
Other authors have investigated the neural and kinetic response to the ULNTT. Balster and Jull67 studied 20 asymptomatic subjects using surface electromyography, elbow goniometry, and pain perception in two groups of more or less extensible neural tissues. In the less extensible group, there was greater electromyographic activity and less elbow extension. Pain perception was not significantly different between the two groups. The result of this study is evidence of a reflexive muscle contraction tissue barrier in response to the application of the ULNTT and a possible correlation between the patient’s perceived pain and limitation of motion measured at the elbow. Coppieters and colleagues79 attempted to quantify this increased upper trapezius tone and limitation of motion by measuring the scapula elevation force and combined wrist/elbow motion using electrogoniometry in a pilot experiment with five asymptomatic (normal) subjects. They determined that there was a greater scapula elevation force in the ULNTT than in the nontension position. In a further study of 35 normal subjects, the ULNTT was applied in five test variations. As each variant was applied, there was an increase in the patient’s subjective response and a decrease in elbow range of motion. Coppieters and colleagues80 concluded that although neurodynamic testing assessed non-neural and neural structures, the high level of paresthesia supports that some of the response is neurogenic in nature.
In all these studies, the authors reported a high level of intrarater reliability for the application of the ULNTT. Because all these studies on normal subjects reported subjective responses, it makes confirmation of neurogenic involvement in the patient population more difficult and complex. Several of these studies also attempted to quantify the ULNTT. These results provide early evidence of the potential for objective measurement of neurodynamic testing and confirm the hypothesis of altered extremity mobility as a component of a positive neurodynamic test.
The use of high-frequency ultrasound images has demonstrated the effectiveness in assessing median nerve excursion in vivo.51 Dilley and associates81 examined ulnar nerve excursion during four different upper limb movements similar to components of the ulnar nerve ULNTT. They demonstrated that ulnar nerve excursion occurred in the forearm and to a lesser extent in the upper arm. Additionally, the amount of excursion was dependent on the wrist and shoulder position. Echigo and colleagues82 also used ultrasonography to examine median nerve excursion during nerve gliding exercises. Their study examined the component motions of passive wrist/finger extension and active finger flexion measuring median nerve excursion in the forearm. Passive wrist and digital extension resulted in distal nerve gliding of 1.9 mm in elbow extension and pronation and 3.0 mm in elbow flexion and supination. Proximal excursion of 0.8 mm occurred for active hook fist and 1.3 mm for active grasp. Coppieters and colleagues83 confirmed the earlier cadaver findings of Coppieters and Alshami.73 Using ultrasonography, they confirmed that more excursion occurred using the sliding technique than the tension technique and the difference was significant.83
Cadaver studies have examined the sensitivity and specificity of the ULNTT.33,74 Recently, the specificity of neurodynamic testing has been examined using various sensory perception84,85 and experimentally induced pain models86,87 using a thermal pain sensitivity model in a quasi-experimental design in healthy subjects. Beneciuk and colleagues84 determined that the group treated with NM had a decrease in temporal summation, improved range of motion, and a decrease in sensory descriptors rating. The ULNTT alters the sensory perception threshold, and there is a weak but significant effect of age, with perception leaning toward hypoesthesia.85 In two separate studies using an experimentally induced pain model,86,87 the straight leg raise and slump test87 and the median nerve ULNTT86 did not affect the experimentally induced pain, indicating that the neurodynamic test is specific to the nervous system. Further support for the specificity of neurodynamic testing was provided by Boyd and colleagues88 when they confirmed the mechanosensitivity of the lower extremity nervous system using the straight leg neurodynamic test. Collectively, these experiments support the specificity of neurodynamic testing.
Clinical research has been limited to reliability,89 validity,1,90,92 normal responses to the ULNTT,93 and responses of select pathologic groups.94-97 Viikari-Juntura89 investigated the interexaminer reliability of various cervical examination techniques including the ULNTT. A group of 52 patients were examined with four special tests including the ULNTT by two examiners before undergoing cervical myelography. The authors reported poor interrater reliability; however, the test was only performed on patients 17 through 52 and not as typically described. Additionally, only the patients’ response was measured with no consideration for any limitation of motion. Sandmark and Nisell90 investigated the validity of five common manual neck pain–provoking tests including the ULNTT. They determined that the ULNTT had a specificity of 94%, a sensitivity of 77%, a positive predictive value of 85%, and negative predictive value of 91% in 22 of 75 subjects randomly selected with reported neck pain. In a single case study, Shacklock1 examined the validity of the ULNTT in a surgically proven neuropathy of the ulnar nerve at the elbow. He reported a positive ULNTT result based on loss of motion in shoulder abduction of 10% compared with the contralateral side and an abnormal response based on Kenneally’s76 work.
Multiple authors91,94,96,97 have also investigated the presence of positive ULNTT results in patient populations. In a study of 60 patients with repetitive strain injury, Elvey and associates93 found that the ULNTT was positive in 59 of the patients for reproduction of their symptoms. In a study of 40 patients reporting pain and paresthesia associated with a neck injury, the ULNTT was performed and compared with 20 normal subjects. A positive test result was based on symptom provocation. Yeung and colleagues97 reported a sensitivity value of 0.9 and a specificity value of 1.0; however, the exact statistical method was not elucidated. Quintner,98 in a retrospective study, reported on 22 patients, all with positive findings on the ULNTT with a stretch-induced cervical–arm pain syndrome. Byng99 examined three different groups—patients with upper limb overuse syndrome (ULOS), asymptomatic keyboard users, and asymptomatic nonkeyboard users—and found that the ULNTT used produced positive results in 100% of the patients with ULOS and the asymptomatic keyboard users. Perhaps this indicates that the ULNTT may be useful in identifying those people that may have subclinical pathology. The ULNTT was also found to be useful in identifying and having diagnostic accuracy for those patients with cervical radiculopathy.100 Patient symptom provocation and reduced tissue extensibility (limitation of motion) were the criteria for a positive test result.
Assessment of variables other than symptom response to the ULNTT has also been measured. Yaxley and Jull,96 in a series of 20 patients with tennis elbow, used the radial nerve ULNTT with positive criteria of symptom response and the limitation of motion at the glenohumeral joint as determined by tissue resistance. They determined that glenohumeral motion (abduction) was significantly different on the symptomatic side based on wrist position. Selvaratnam and colleagues91 assessed range of motion for the ULNTT. In one of the few control group studies, they compared three groups of subjects: a group with a known brachial plexopathy, a group with sports injury, and an asymptomatic group of 25 subjects. A goniometric apparatus was used to measure upper extremity joint position and cervical motion while performing the ULNTT. The upper extremity joints were sequentially moved until the motion elicited pain or end range was achieved based on pain onset and tolerance. The authors found a significant difference in range of motion to sensory change between the brachial plexus group and the sports group with less motion and earlier onset of pain in the range of motion in the brachial plexus group. Similarly, there was a significant group interaction with a greater reduction of motion in the brachial plexus group than the asymptomatic group.
Other authors have also examined range-of-motion limitations. Pullos94 compared the limitation of elbow motion with the ULNTT between asymptomatic and neck–arm pain patients. No difference was found between left and right arms in 100 normal subjects. The normal motion deficit was 16.5 to 53.2 degrees with an 8-degree difference between left and right considered normal. In 25 neck–arm pain patients, 68% had positive ULNTT results with asymmetrical elbow motion and symptom provocation. Finally, in a whiplash injury population, Yeung and colleagues97 examined limitations of knee motion with the slump test (another neurodynamic test) and symptom onset. The addition of knee extension in the slump test increased cervical pain and produced a greater limitation of knee extension.
From the available clinical research, it appears that a positive response is the provocation of the patient’s pain, not to be confused with the normal response in asymptomatic subjects (normal subjects). Additionally, a limitation of motion was present in those studies that compared patients with normal subjects. Interrater reliability is still questionable; therefore, caution should be exercised when comparing the results of two clinicians, even in the same patient population. Although intrarater reliability was reported to be strong, the criteria used was not clearly defined. The specificity and sensitivity have been reported to be high, and recent studies appear to support this concept. The clinician is also wise to continually consider that non-neural structures are being mechanically deformed, which may lead to potential errors in specificity and sensitivity.
The purpose of using NM is to effect a change in the compliance (excursion and strain) of the neural tissue and to decrease its increased state of mechanosensitivity. As discussed earlier in great detail, the mechanosensitivity is increased in peripheral nerves in patients with neuropathic pain for a number of reasons. Wilgis and Murphy50 theorized that neural excursion is compromised in compression syndromes and certain diseased states. There is a growing body of literature that supports this theory of altered neural compliance. Valls-Sole and colleagues,101 measuring latencies of sensory nerve action potentials, theorized that longitudinal sliding of the nerve was compromised in individuals with CTS. Using ultrasonography, Nakamichi and Tachibana102 demonstrated a significant decrease in sliding of the median nerve with wrist extension–flexion motion in patients with CTS compared with normal subjects. The finding of significantly reduced median nerve excursion in patients with CTS was supported by Hough and colleagues.103 In contrast, Erel and colleagues104 reported no significant change in median nerve excursion in patients with CTS when examining metacarpal phalangeal motion. Greening and colleagues,105 in two separate reports, demonstrated decreased median nerve excursion in patients with nonspecific arm pain and while measuring median nerve excursion in the forearm with inspiration in patients with whiplash and nonspecific arm pain.106 These findings may partly play a role in the underlying pathophysiology of these conditions.
NM as a form of treatment was reported as early as 1883 by Marshall107 when he described the use of “nerve stretching” to relieve pain. The author describes NM and its effects on the peripheral and central nervous systems. Among the many points that Marshall107 discusses are the importance of the rate of application and that less force should be applied in the case of a diseased nerve. Today, the clinical technique for the application of NM as a treatment approach is based on an eclectic compilation of theoretical concepts based on scientific evidence and empirical experience. There are reported clinical studies using NM for the treatment of neuropathic pain. Sweeney and Harms108 treated 29 patients with unilateral mechanical allodynia of the hand using a neural self-mobilization program. Using components of the ULNTT, the patient applied tension to the end point of perceived discomfort for a 10-second period with a 10-second interval of rest, repeated for 10 cycles once daily. The patients were reevaluated 2 weeks after starting the program. There was improvement in the ULNTT range of motion, with 89% achieving full elbow extension and 66% reporting improvement in their symptoms. The range of onset of the symptoms was large, which could have skewed the data. In a case report of a patient with shoulder pain and disability, Haddick109 used NM as one of several manual treatments. He reported an improved Shoulder Pain and Disability Index (SPADI) score and increased shoulder range of motion.
NM has also been used in the conservative treatment of CTS. Reporting on 197 patients in a retrospective study, Rozmaryn and colleagues110 divided the patients into two groups, each receiving standard conservative measures and one group receiving nerve and tendon gliding exercises. Using distal components of the ULNTT, stretch was applied and maintained for 7 seconds and repeated five times three to five times per day. In the control group, 71% of patients required surgery, and in the experimental group, 43% required surgery. In the experimental group, 89% of the patients responded to follow-up interviews, with 70% reporting good to excellent results and 19% remaining symptomatic. Seradge and colleagues111 performed a prospective study of 286 production workers who performed carpal tunnel decompression exercises, which were composed of components of the ULNTT. The exercises were performed for 3 minutes and 10 seconds before the start of production tasks. The incidence of cumulative trauma disorder was reduced by 37% and CTS was reduced by 45%. In the study by Pinar and colleagues,112 two groups of patients received a volar resting wrist orthosis and one group performed nerve gliding exercises described by Totten and Hunter.113 Each of the six exercises was performed for 10 repetitions five times per day for 10 weeks. Both groups reported significant improvement, and the experimental group also reported a more rapid pain reduction and greater functional movement.113 In a similar randomized study using orthotic positioning and nerve and tendon gliding as described by Totten and Hunter, Akalin and colleagues114 reported significant improvement in both groups: 72% in the orthosis-only group versus 93% in the orthosis plus exercises group. However, the difference was not significant. In contrast, Brininger and colleagues115 found no difference between the group receiving tendon and nerve gliding exercises and the control group of patients with CTS. A similar finding was reported by Heebner and Roddey116 comparing two groups, one receiving standard care and the other standard care plus nerve and tendon gliding. The nerve gliding exercise was performed for 10 repetitions with a 5-second hold three to five times per day. The nerve gliding exercise as described was a nerve-tensioning exercise and not a sliding exercise. Bialosky and colleagues117 found no significant difference between the sham treatment and an actual neurodynamic technique treatment in subjects with CTS. However, they did report that there was a reduction in temporal summation in the neurodynamic technique group. Beneciuk and colleagues84 applied neural tensioning using 10 cycles of passive elbow extension and flexion at a rate of 6 seconds per cycle when measuring the effectiveness in improving neuromechanics in asymptomatic individuals. Tal-Akabi and Rushton118 evaluated the effectiveness of NM compared with carpal mobilization in patients with CTS and a control group. Patient scores significantly improved on the pain relief scale in both treatment groups; however, there was no significant difference between them. Of the three groups, the NM group went on to fewer surgeries. In a case report, Coppieters and colleagues reported improvement in symptoms in a patient with cubital tunnel syndrome. The NM technique of sliding was used in conjunction with joint mobilization and therapeutic exercise.119 From these studies it appears that there is no clear indication for the proper duration, dose, frequency, or type of exercise to be used.
Neuron-orthopedic examination of the patient requires that the therapist perform a complete upper quarter screening examination (see Chapter 10). In addition, a differential tissue assessment must be completed to rule out any potential tissue that may be an attribute of the patient’s signs and symptoms. Only after these two components are addressed should the therapist proceed with the neural component of the examination.
Use of NM and gliding techniques requires the careful assessment of the patient to establish the presence of a neurogenic component of the patient’s symptoms. The five components of the neuron-orthopedic examination are summarized in Box 118-3.65 The examination requires the presence of active motion dysfunction. The purpose of examining for active dysfunction is to determine the patient’s willingness to move and for the therapist to gain insight into the patient’s limitation of motion and pain. Before initiating the examination, the therapist should observe the presenting posture of the patient for evidence of antitension posture; an example is shown in Figure 118-2. In this patient with a diagnosis of right cubital tunnel syndrome, after submuscular anterior transposition, note how the patient is attempting to minimize tension in her nervous system by posturing in cervical rotation and lateral flexion to the ipsilateral side. There is increased tone in the upper trapezius, as noted by scapular elevation, and the right extremity is positioned in shoulder adduction and internal rotation, elbow flexion, pronation, and wrist flexion.
Box 118-3 Summary of Neuron-orthopedic Examination
1. Active Abduction/Forward Flexion
Observe cervical spine and extremity joint positioning, patient’s willingness to move
Specific motions: observe joint position changes in upper quarter
Coronal plane shoulder abduction with elbow extended
Coronal plane shoulder abduction with proximal (cervical spine) component
Ipsilateral lateral flexion or side bending
Coronal plane shoulder abduction with distal component
Variation with elbow/forearm position
Upper limb neural tension tests
Local peripheral nerves palpation sites
Distal: flexor carpi ulnaris origin
Radial sensory: brachioradialis insertion
Local tissue tender points in tissues innervated by the peripheral nerve or cervical segment involved
Cervical spine segmental stiffness for the segmental levels composing the brachial plexus and/or the peripheral nerves involved
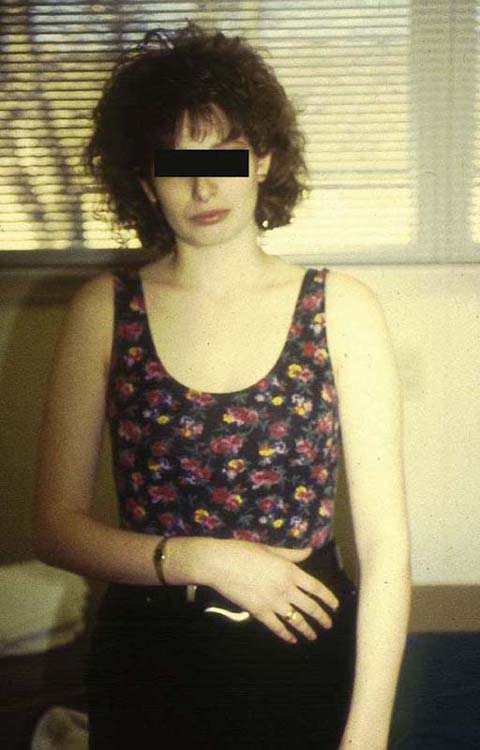
Figure 118-2 Example of antitension posture (neural tension dysfunction). Note how the patient is attempting to minimize the tension in the right upper extremity.
An example of the active dysfunction examination is depicted in the series of photographs in Figure 118-3 (online) of a patient status post repair of the left median nerve at the wrist. In Figure 118-3A (online), the initial test of the patient’s willingness to move is examined by asking the patient to comfortably place his hands over his head. In the following photographs, the patient is specifically requested to perform shoulder abduction in the coronal plane within his comfort range while various components are altered to determine the effect on motion and symptoms. The therapist should note how the position of the head and joints of the upper extremity changes with each movement. The active dysfunction examination is also summarized in Box 118-3.
Figure 118-3 Active motion dysfunction of a patient status post median nerve repair at the wrist. A, Active shoulder elevation, coronal plane abduction of the noninvolved side (B) and involved side (C). Coronal plane abduction with wrist extension, noninvolved side (D) and involved side (E). (Note the change in shoulder abduction when comparing sides.) Coronal plane abduction with cervical rotation to the contralateral side (F), cervical rotation to the ipsilateral side (G), and cervical lateral flexion to the contralateral side (H). (Note again the change in shoulder abduction when tension is altered using a proximal component.)
After active motion assessment, passive motion is examined using the ULNTT. The base component motions of the ULNTT for the three major nerves in the upper extremity are outlined in Box 118-4. As each successive sequence of motion is applied, the previous positions are maintained to successively apply tension to the nervous system. The therapist must pay close attention for the presence of encountered resistance (reflexive muscle contraction)6,65,67 and the level of irritability (patient response)6,23,65 to avoid progressing beyond the endpoint of protective resistance and symptoms that may exacerbate the patient’s symptoms. The encountered resistance was investigated by van der Heide and colleagues120 by examining 20 asymptomatic subjects by measuring the electromyographic activity. They found that the onset of pain and the increase in electromyographic activity were highly reliable and compared favorably with the onset of muscle activity. An example of the sequence of this motion for the median nerve ULNTT is demonstrated in Figure 118-4. The therapist can vary the order in which each component motion is applied; however, it is recommended that the test for each nerve first be applied in a standardized manner. The final position of each joint and the patient response are recorded for baseline purposes to monitor progress.
Box 118-4 Upper Extremity Neural Tension Testing Components
Cervical contralateral lateral flexion
Upper limb nerve tension test components for the three major nerves in the upper extremity. Note that the sensitizing components are added after the primary component motion if necessary.
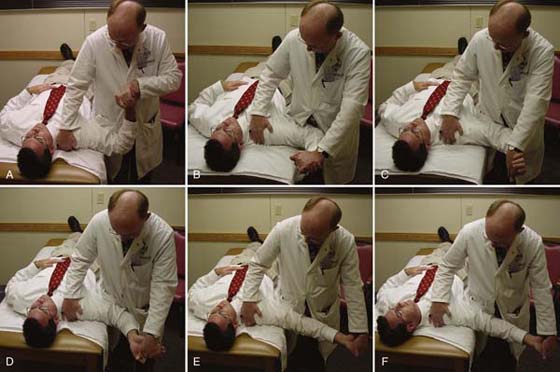
Figure 118-4 Successive application of the median nerve (base) upper limb neural tension test. Shoulder abduction (A), shoulder external rotation (B), forearm supination (C), wrist/hand extension (D), elbow extension (E), additional sensitizing motions of contralateral cervical flexion and scapular depression (F).
It is recommended that the sensitizing components of scapular depression and cervical contralateral lateral flexion be applied only after the base components have been tested. The addition of the sensitizing motions needs only to be applied to confirm the involvement of the nervous system if it is still suspect as a possible mediator of the patients’ symptoms and there is no response to the primary components. The therapist should keep in mind that the test affects multiple aspects including neurophysiology, blood flow, inflammation, neural mechanical sensitivity, and muscle response.2 Each ULNTT should be applied slowly to avoid going beyond the encountered resistance. Although there are no clear data, it is the author’s recommendation that each component be applied at a rate of 10 to 15 seconds. Video application of each of the three main neurodynamic tests for the upper extremity can be found on the companion Web site.
In addition to active and passive motion dysfunction, the therapist should be able to demonstrate palpable neural hyperalgesia along the course of the hypothesized nerves involved.19 As with the passive test, neural palpation is subject not only to the magnitude but also the rate of the load.19 Repeated palpation may also preload the neural tissue, causing increased mechanosensitivity, which may lead to a false-positive result.19 Box 118-3 includes a summary of the anatomic locations where each of the peripheral nerves can be palpated, keeping in mind that many of these locations will be an indirect palpation and the nerve itself will not be palpable. Elvey65 described the presence of local tender points in tissues innervated by the hypothesized peripheral nerve or nerve root segment. The therapist should anticipate that these structures will be sensitive. Often, muscle tenderness may be confused with myofascial trigger points. Finally, there will be the presence of local dysfunction within the cervical segment(s) contributing to the peripheral nerve involved.65 This local cervical dysfunction may present as segmental hypomobility or tenderness to palpation of the segments contributing to the involved peripheral nerve.
Normal responses to the ULNTT have been reported by Kenneally76,77 and others.67,78,79 The interpretation of a positive ULNTT result is reproduction of the patient’s symptoms and motion limitations determined by patient tolerance or encountered resistance. During the initial testing, the endpoint at which the therapist ceases continuing the motion or adding components should be the onset of patient symptoms or resistance encountered. Altering a distal or proximal component (joint) motion should result in a change in the baseline results of the ULNTT. For example, placing the wrist in a neutral position may increase elbow extension or cervical contralateral lateral flexion may decrease elbow extension when performing a particular ULNTT.33,91 These changes in motion or response are then documented for comparison with the baseline test performed. A positive test may also be confirmed by identifying a difference in response or motion limitation by comparing the involved to the noninvolved side. It is beyond the scope of this chapter to explain in complete detail the principles of ULNTT examination and its findings. Readers are referred to the additional sources in the references and urged to pursue furthering their knowledge for a more in-depth understanding.
Once the therapist has determined the presence of a neural component of the patient’s problem, the development of a logical treatment approach using neural tensioning and gliding (sliding)73 techniques can be guided by three basic principles.121 First is education of the patient and the therapist. It is imperative the patient understand the role that the nervous system plays in his or her pathology, the concept of neural mechanics, and how these interact to alter movement and function. A sound comprehension of the concepts of neural tension and gliding is crucial to preventing exacerbation and assists with treatment progression. Sound clinical reasoning that formulates a working hypothesis is required. These hypotheses must include (1) the source of the symptoms or dysfunction, (2) contributing factors, (3) precautions and contraindications to examination or treatment, (4) management, and (5) prognosis.122
The second basic principle is exercise application and prescription. In most cases, the therapist is presented with a patient whose pathologic process has occurred over an extended period of time, resulting in pain and movement dysfunction. These adaptive changes may take months to alter with treatment. Although NM techniques are used in the clinic, the restoration of movement and elimination of pain require the diligent use of a home program that is periodically adjusted based on reassessment and response to treatment. Finally, the therapist plays a major role in empowering the patient in the performance and follow-through of the exercise program. This is achieved through assisting the patient in observing and appreciating the changes in his or her symptomatology, improvements in impairments such as specific joint or multisegment motion limitations, and ability to use his or her upper extremity for functional activities.
As discussed earlier in the literature review, no clear-cut protocol for the development and implementation of treatment exists. A thorough working knowledge of the nervous system and the ULNTT components is required. The ULNTT components are transformed into mobilization techniques and exercises for treatment and home exercises. NTD is a dynamic process, changing daily, affecting diagnosis and treatment hypotheses. Therefore, examination, evaluation, and ongoing re-evaluation will continuously guide treatment progression and implementation as the hypothesis(es) regarding the specific tissues at fault and the location changes or becomes refined. The concepts of nerve tension (stress and strain) and glide (excursion) play a major role in treatment formulation. Tension creates strain within the nerve by pulling on both ends of the nerve simultaneously, causing the nerve to unfold. This will have a profound effect on the nerve’s neurophysiology due to alterations in vascular and axoplasmic flow. In contrast, glide refers to placing tension on the nerve at one point while simultaneously releasing it at another. Coppieters and Butler75 refer to this as a sliding technique, which they feel is different from gliding exercises, which is a general exercise principle. The overall resting tension or strain is significantly minimized with sliding.73,75 Gliding can also occur within the nerve itself28 or between the nerve and its interfacing tissue.30-33,49,50 Figure 118-5 is an illustration of these two concepts. A clinical example of these two concepts is presented in Figure 118-6.

Figure 118-6 Examples of home exercises used for nerve mobilization. A and B, Gliding. Note that as tension is placed in one location, it is relieved in another location. C and D, Tension. Note that tension is applied using one joint only while maintaining one component in the slack position and maintaining the tension position of the other joints of the upper extremity. E, Progression to terminal tension by adding cervical contralateral flexion.
The presenting level of patient symptom irritability guides the use of tension and/or glide. In patients with highly irritable symptoms, sliding techniques may be the most appropriate approach. When using sliding techniques, the therapist needs to be aware of the tissue barriers (reflexive muscle contraction) encountered. Treatment and home exercises are performed in the pain- and tension-free range of motion below the stimulus threshold. It may be necessary to begin at remote sites from the hypothesized location, progressing toward the specific site of involvement as tolerated. Patients whose symptoms are of low irritability may be able to tolerate single-joint progressing to multijoint tension when symptoms are mild or absent at rest and recovery from exacerbation is rapid. It may be appropriate to tension the involved nerve to the point of the tissue barrier directly or at a more remote site and progress toward the hypothesized location. Perhaps restoring mobility or improving tolerance to tension at a remote location may assist the nerve in attenuating tension throughout its course, thereby decreasing symptoms. As irritability decreases, the therapist can begin to work through the tissue barriers to restore movement to the extremity and increase function. At this level, there may be a transient response of paresthesia or discomfort that should quickly resolve. At no time should there be a sustained stretch.2 Figure 118-7 is an algorithm to depict treatment progression.
The tissues hypothesized to be at fault, neural or non-neural, will also guide treatment. The treatment of non-neural tissues may initially require the nervous system be placed in a position of antitension or slack. This would allow for direct treatment of the non-neural tissues while minimizing mechanical deformation of the sensitized nerve and avoiding further risk of exacerbation. For example, if the clinician is attempting to improve glenohumeral motion, the combination of ipsilateral cervical flexion and scapular elevation may minimize the additional neural tension created by glenohumeral motion. In contrast to the treatment of non-neural tissue, it may be desirable to place the nerve under tension to benefit both systems in the patient with less neural irritability. The clinician may want to mobilize the glenohumeral joint to recover abduction with the elbow extended or the cervical spine laterally flexed to the contralateral side. Care should always be taken not to exceed the clinical limit of the nervous system.
Finally, the therapist should have a working hypothesis as to the existence of any intraneural or extraneural fibrosis.23,28 If intraneural fibrosis is suspected, it may be more appropriate to attempt to increase neural compliance at a location that is remote from the hypothesized site of the lesion. It is my opinion that these therapeutic techniques are unlikely to improve the internal structure of a fibrotic nerve based on our knowledge of connective tissue. However, if it is hypothesized that the nerve’s ability to glide (slide) within its tissue bed is compromised (extraneural fibrosis), treatment of the interfacing tissues at the site(s) hypothesized may be indicated. This may be performed in conjunction with glide or tension of the nerve in question, depending on the level of irritability. Box 118-5 summarizes the guidelines that the treating practitioner should consider when developing a treatment strategy.
Box 118-5 Treatment Guidelines
WORKING KNOWLEDGE/UNDERSTANDING OF THE UPPER LIMB NERVE TENSION TEST COMPONENTS
Response to treatment-changing hypothesis
HYPOTHESIS OF LOCATION AND TISSUES AT FAULT
Tension Versus Glide (Excursion)
Tension: Lengthens the nerve, stresses vascular supply
Glide: Tension in one location and release in another
Irritable: Gliding (tissue barriers), selected component distal to site (lower extremity or trunk), pain-free range tension free
Nonirritable: Tension, (+) symptoms (mild) rapid recovery, select component/nerve directly involved
Neural Versus Non-neural Components
Treat non-neural tissues directly with tension eliminated (brachial plexus slack position)
Treat non-neural tissues under tension (nonirritable)
Intraneural Versus Extraneural Fibrosis
Intraneural: Attempt to increase mobility away from site
Extraneural: Treat interfacing tissue in conjunction with glide/tension
Using other treatment techniques can augment NM exercises or techniques. Superficial and deep heating modalities may be used to precondition the tissues, keeping in mind this may alter the stimulus threshold for provoking symptoms, which could result in a delayed exacerbation response. Massage, joint mobilization, or other manual techniques can be applied to assist in restoring mobility and decreasing interfacing tissue adherence with the nerve under tension or in a slackened (antitension) position. Postural education and awareness and the use of physiologic range-of-motion exercises to improve soft tissue mobility and strength can be used in conjunction with NM techniques. The list of combining and augmenting treatments is exhaustive as long as the clinical state of the nerve and its interfacing tissues is respected.
The progression of NM techniques is depicted in Figure 118-7 and is dictated by the patient’s response. Progression requires continual clinical reasoning to avoid further injury or exacerbation. In the clinic, the use of Maitland’s123,124 gradation for joint mobilization has been advocated.23,24 This concept divides the total range of available motion into four parts for any given joint. These are then applied clinically using motion in the first half of available range for symptom control and motion in the last half of available motion for restoring mobility. An alternative way to look at this is that the first half of the available range is the pain free or “through range”6,123 within a given total available range; the last half of the range includes the tissue barriers or end ranges23,24 that must eventually be overcome to restore limited motion. This is an oversimplification of the concept, but the most important aspect is its consideration for the level of irritability of the tissues involved. These concepts can be applied actively by the patient, passively by the therapist, via active physiologic joint motion using individual or multiple ULNTT components in small- or large-amplitude movements. It is this author’s recommendation that all treatments start with sliding techniques.
At present, no clear guidelines or research supports the most effective amplitude, dose, duration, or frequency necessary to achieve the desired result. Therefore, sound clinical reasoning regarding these parameters is necessary to progress NM techniques and exercises. As a general rule, Elvey and colleagues93 recommend that end-range grades should not be used, and the duration should be less than that used for joint mobilization. Shacklock2 also recommends avoiding sustained end-range hold. In contrast, Butler6,23 recommends that for an irritable disorder, pain-free midrange (through-range) mobilizations and end-range mobilizations may be used for an initial treatment, with each movement lasting 20 to 30 seconds. For the present, the progression can only be based on the patient’s clinical response to the treatment.
The use of additional component joint motions of the ULNTT will result in increased tension within the nervous system. As irritability decreases and mobility improves, additional component motions can be combined as appropriate and necessary. Working from a remote site and progressing toward the hypothesized involved site or nerve is another way to progress NM. For example, if the ulnar nerve was involved at the level of the elbow, it may be more appropriate because of irritability to initiate nerve gliding at the shoulder, cervical spine, or wrist before using the elbow joint for mobilizing the nerve. The adaptive changes of nerve length, structure, chemistry, mechanics, and electrical properties that occur over time will require time to reverse. Therefore, these principles are best applied in conjunction with a home program with periodic visits to adjust the exercise prescription.
The contraindications and precautions for the use of ULNTT and NM are listed in Box 118-6. The application of the ULNTT or NM techniques can easily result in significant exacerbation and prolonged recovery of the patient’s symptoms when applied incorrectly. Cord and nerve root signs, especially in the presence of hard neurologic signs (reflex changes, motor weakness, and sensory changes), may indicate the presence of adverse neural tension within the central nervous system, necessitating that these patients be approached with care. Unremitting night pain not associated with a mechanical cause or undiagnosed may indicate pain of visceral origin. For example, cardiac, hepatic, diaphragmatic, and upper gastrointestinal structures can refer pain to the upper quadrant. Recent sensory changes may indicate a systemic neurologic or inflammatory disease such as multiple sclerosis and rheumatoid arthritis or may be indicative of a recent central nervous system pathology such as nerve root compression and a peripheral lesion. Complex regional pain syndrome may be an underlying component of the patient’s pain, and aggressive handling may contribute to further exacerbation of the patient’s symptoms. Pain originating from cervical spine structures may also be referring pain to the upper extremity. In all these cases, the clinician must exercise caution in the application of the ULNTT and NM techniques. Mechanical mobilization of the nervous system is contraindicated in the case of a recently repaired nerve, malignancy (primary or metastatic) involving a nerve or its surrounding tissues, and active neurologic or inflammatory diseases.
Box 118-6 Precautions and Contraindications
Severe unremitting night pain, lacking a diagnosis
Recent paresthesia/anesthesia/complex regional pain syndrome types I and II
Mechanical spine pain with peripheralization
Recently repaired peripheral nerve
The judicious use of the ULNTT and NM requires the therapist to have a sound knowledge of the nervous system’s histology, vascularity, gross anatomy, biomechanics, and pathology. Only through the application of this knowledge is it possible to effectively examine and treat the peripheral nervous system. Clinically recognizing how nervous system pathology may manifest itself is required before proceeding with the ULNTT and NM. Research on this topic has centered on normal responses and selected patient populations. Basic science research with regard to neural biomechanics and the ULNTT theoretically supports the validity of ULNTT sensitivity and specificity. Clinical and basic science studies have supported the validity of the ULNTT, but with the one exception that they lack the rigors of random control. High intrarater reliability has been reported; however, interrater reliability has not been confirmed, which can lead to multiple problems clinically. Several studies have examined the use of NM as a treatment. Although the results are promising, further controlled studies are required to identify the appropriate patient population. There are no studies to support dose, duration, or frequency in the application of NM techniques, which leaves it up to the clinician to determine these parameters.
Treatment begins with the clinician’s awareness of his or her knowledge, clinical limitations, and the contraindications and/or precautions for NM. Application of NM is guided by the principles of education, exercise, and encouragement. The clinician is reminded that NM as a treatment rarely stands alone and is augmented by other treatment techniques used each day for his or her patients. Finally, progression is based on continuous reassessment of the patient’s response to the treatment strategies used. NM is an additional treatment tool available to the clinician and his or her patients presenting with difficult and complicated diagnoses of the upper extremity.
1. Shacklock M. Positve upper limb tension test in a case of surgically proven neuropathy: analysis and validity. Manual Ther. 1996;1:154–161.
2. Shacklock M. Improving application of neurodynamic (neural tension) testing and treatments: a message to researchers and clinicians. Manual Ther. 2005;10:175–179.
3. Merskey H, Bogduk N. Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms. 2nd ed Seattle: International Association for the Study of Pain; 1994.
4. Allen R. Neuropathic pain in the cancer patient. Neurol Clin. 1998;16(4):869–887.
5. Bennett G. Neuropathic pain. In: Wall P, Melzack R, eds. Textbook of Pain. 3rd ed New York: Churchill Livingstone; 1994.
6. Butler D. The Sensitive Nervous System. Adelaide, Australia: Noigroup Publications; 2000.
7. Devor M. The pathophysiology of damaged peripheral nerves. In: Melzak R, Wall P, eds. Textbook of Pain. Edinburgh: Churchill Livingstone; 1994:79–100.
8. Asbury A, Fields H. Pain due to peripheral nerve damage: an hypothesis. Neurology. 1984;34:1587–1590.
9. Allan C. Functional results of primary nerve repair. Hand Clin. 2000;16(1):67–72.
10. Hormada J. On the nerve supply of the connective tissue of some peripheral nervous system components. Acta Anat. 1963;55:343–351.
11. Millesi H, Zoch G, Rath T. The gliding apparatus of peripheral nerve and its clinical significance. Ann Hand Upper Limb Surg. 1990;9:87–96.
12. Sauer SK, Bove GM, Averbeck B, Reeh PW. Rat peripheral nerve components release calcitonin gene-related peptide and prostaglandin E2 in response to noxious stimuli: evidence that nervi nervorum are nociceptors. Neuroscience. 1999;92(1):319–325.
13. Weinstein J. Neurogenic and non-neurogenic pain and inflammatory mediators. Orthop Clin North Am. 1991;22:235–246.
14. Carter GT, Galer BS. Advances in the management of neuropathic pain. Phys Med Rehabil Clin North Am. 2001;12(2):447–459.
15. Eliav E, Herzberg U, Ruda MA, Bennett GJ. Neuropathic pain from an experimental neuritis of the rat sciatic nerve. Pain. 1999;83:169–182.
16. Bove GM, Ransil BJ, Lin HC, Leem JG. Inflammation induces ectopic mechanical sensitivity in axons of nociceptors innervating deep tissues. J Neurophysiol. 2003;90:1949–1955.
17. Dilley A, Lynn B, Pang SJ. Pressure and stretch mechanosensitivity of peripheral nerve fibres following local inflammation of the nerve trunk. Pain. 2005;117:462–472.
18. Eliav E, Benoliel R, Tal M. Inflammation with no axonal damage of the rat saphenous nerve trunk induces ectopic discharge and mechanosensitivity in myelinated axons. Neurosci Lett. 2001;311:49–52.
19. Clarke EC, McNulty PA, Macefield VG, Bilston LE. Mechanically evoked sensory and motor responses to dynamic compression of the ulnar nerve. Muscle Nerve. 2007;35(March):303–311.
20. Galer BS, Jensen MP. Development and preliminary validation of a pain measure specific to neuropathic pain: the neuropathic pain scale. Neurology. 1997;48:332–338.
21. Lew PC, Morrow CJ, Lew AM. The effect of neck and leg flexion and their sequence on the lumbar spinal cord. Spine. 1994;19:2421–2425.
22. O’Connell JE. The clinical signs of meningeal irritation. Brain. 1946;69:9–21.
23. Butler D. Adverse mechanical tension in the nervous system: a model for assessment and treatment. Austral J Physiother. 1989;35:227–238.
24. Butler D. Mobilisation of the Nervous System. London: Churchill Livingstone; 1991.
25. Sunderland S, Bradley K. Stress-strain phenomena in denervated peripheral nerve trunks. Brain. 1961;64:125–127.
26. Sunderland S, Bradley K. Stress-strain phenomena in human spinal nerve roots. Brain. 1961;64:102–124.
27. Haftek J. Stretch injury of peripheral nerve: acute effects of stretching on rabbit nerve. J Bone Joint Surg. 1970;52B:354–365.
28. Millesi H, Zoch G, Reihsner R. Mechanical properties of peripheral nerves. Clin Orthop Related Res. 1995;314:76–83.
29. Zoech G, Reihsner R, Beer R, Millesi H. Stress strain in peripheral nerves. Neuron-orthopedics. 1991;10:73–82.
30. Wright T, Glowczewskie F Jr, Cowin D, et al. Ulnar nerve excursion and strain at the elbow and wrist associated with upper extremity motion. J Hand Surg. 2001;26A:655–662.
31. Wright T, Glowczewskie F Jr, Wheeler D, et al. Excursion and strain of the median nerve. J Bone Joint Surg. 1996;78-A:1897–1903.
32. Wright TW, Cowin D, Wheeler DL. Radial nerve excursion and strain at the elbow and wrist associated with upper-extremity motion. J Hand Surg. 2005;30A(5):990–996.
33. Kleinrensink GJ, Stoeckart R, Mulder PG, et al. Upper limb tension tests as tools in the diagnosis of nerve and plexus lesions—anatomical and biomechanical aspects. Clin Biomech. 2000;15:9–14.
34. Kleinrensink GJ, Stoeckart R, Vleeming A, et al. Mechanical tension in the median nerve: the effects of joint positions. Clin Biomech. 1995;10:240–244.
35. Kleinrensink GJ, Stoeckart R, Vleeming A, et al. Peripheral nerve tension due to joint motion: a comparison between embalmed and unembalmed human bodies. Clin Biomech. 1995;10:235–239.
36. Dilley A, Lynn B, Greening J, DeLeon N. Quantitative in vivo studies of median nerve sliding in response to wrist, elbow, shoulder and neck movements. Clin Biomech. 2003;18:899–907.
37. Kwan M, Wall EJ, Massie J, Garfin SR, Strain. stress, and stretch of peripheral nerve: rabbit experiments in vitro and in vivo. Acta Orthop Scand. 1992;63:267–272.
38. Lundborg G, Rydevik B. Effects of stretching the tibial nerve of the rabbit: a preliminary study of the intraneural circulation and the barrier function of the perineurium. J Bone Joint Surg. 1973;55B:390–401.
39. Ogata K, Naito M. Blood flow of peripheral nerve effects of dissection, stretching and compression. J Hand Surg. 1986;11B:10–14.
40. Porter EL, Wharton PS. Irritability of mammalian nerve following ischemia. J Neurophysiol. 1949;12:109–116.
41. Wall E, Massie JB, Kwan MK, et al. Experimental strectch neuropathy: changes in nerve conduction under tension. J Bone Joint Surg. 1992;74B:126–129.
42. Watanabe M, Yamaga M, Kato T, et al. The implication of repeated versus continuous strain on nerve function in a rat forelimb model. J Hand Surg. 2001;26A:663–669.
43. Sunderland S. The connective tissues of peripheral nerves. Brain. 1965;88:841–854.
44. Clarke E, Bearn JG. The spiral bands of Fontana. Brain. 1972;95:1–20.
45. Lundborg G., et al. Peripheral nerve: the physiology of injury and repair. In: Woo S, Buckwalter J, eds. American Academy Orthopedic Surgeon Symposium Injury and Repair of the Musculoskeletal Soft Tissues. Park Ridge: Illinois; 1998.
46. Sunderland S. Nerve and Nerve Injuries. London: Churchill Livingstone; 1978.
47. Breig A. Biomechanical considerations in the straight-leg-raising test: cadaveric and clincal studies of the effects of medial hip rotation. Spine. 1979;4(3):242–250.
48. Reid JD. Effects of flexion-extension movements of the head and spine upon the spinal cord and nerve roots. J Neurol Neurosurg Psychiatry. 1960;23:214–221.
49. McLellan DL, Swash M. Longitudinal sliding of the median nerve during movements of the upper limb. J Neurol Neurosurg Psychiatry. 1976;39:566–570.
50. Wilgis ES, Murphy R. The significance of longitudinal excursion in peripheral nerves. Hand Clin. 1986;2:761–766.
51. Dilley A, Greening J, Lynn B, et al. The use of cross-correlation analysis between high-frequency ultrasound images to measure longitudinal median nerve movement. Ultrasound Med Biol. 2001;27(9):1211–1218.
52. Nakamichi K, Tachibana S. Transverse sliding of the median nerve beneath the flexor retinaculum. J Hand Surg. 1992;17B:213–216.
53. Millesi H. The nerve gap therapy in clinical practice. Hand Clin. 1986.(4):651–653.
54. Breig A. Adverse Mechanical Tension in the Central Nervous System. Stockholm Sweden: Almqvist & Wiksell International; 1978.
55. Lundborg G. Intraneural microcirculation. Orthop Clin North Am. 1988;19:1–12.
56. Upton A, McComas A. The double crush in nerve entrapment syndrome. Lancet. 1973;2:359–362.
57. Pechan J, Julis I. The pressure measurement in the ulnar nerve. a contribution to the pathophysiology of the cubital tunnel syndrome. J Biomech. 1975;8:75–79.
58. Dahlin L, McLean G. Effects of graded experimental compression on slow and fast axonal transport in rabbit vagus nerve. J Neurosci. 1986.(72):19–30.
59. Nemoto K, Matsumoto N, Tazaki K, et al. An experimental study of the “double crush” hypothesis. J Hand Surg. 1987;12A:552–559.
60. Mackinnon S, Dellon AL. Experimental study of chronic nerve compression clinical implicatons. Hand Clin. 1986;2:639–650.
61. Lundborg G., et al. Injury and repair of the musculoskeletal soft tissues. In: Symposium on Injury and Repair of the Musculoskeletal Soft Tissues. Savannah, GA: American Academy of Orthopaedic Surgeons; 1988.
62. Lundborg G, Dahlin L. The pathophysiology of nerve compression. Hand Clin. 1992;8:215–227.
63. MacKinnon S. Double and multiple “crush” syndromes: double and multiple entrapment neuropathies. Hand Clin. 1992;8:369–390.
64. Elvey R. Brachial plexus tension test and the pathoanatomical origin of arm pain. In: Glasgow E, Tavomey L, eds. Aspects of Manipulative Therapy. Melbourne, Australia: Lincoln Institute of Health Sciences; 1979:105–109.
65. Elvey R. Physical evaluation of the peripheral nervous system in disorders of pain and dysfunction. J Hand Ther. 1997;10:122–129.
66. Gifford L, Butler D. The intergration of pain sciences into clinical practice. J Hand Ther. 1997;10:86–95.
67. Balster SM, Jull GA. Upper trapezius muscle activity during the brachial plexus tension test in asymptomatic subjects. Manual Ther. 1997;2:144–149.
68. Edgar D, Jull G, Sutton S. The relationship between upper trapezius muscle length and upper quadrant neural tissue extensibility. Austral Physiother. 1994;40:99–103.
69. Wilson S, Selvaratnam P, Briggs C. Strain at the subclavian artery during the upper limb tension test. Austral Physiother. 1994;40:243–248.
70. Moses A, Carman J. Anatomy of the cervical spine: implications for the upper limb tension test. Austral Physiother. 1996;42:31–35.
71. Bay BK, Sharkey NA, Szabo RM. Displacement and strain of the median nerve at the wrist. J Hand Surg. 1997;22A(4):621–627.
72. Byl C, Puttlitz C, Byl N, et al. Strain in the median and ulnar nerves during upper-extremity positioning. J Hand Surg. 2002;27A(6):1032–1040.
73. Coppieters MW, Alshami AM. Longitudinal excursion and strain in the median nerve during novel nerve gliding exercises for carpal tunnel syndrome. J Orthop Res. 2007(July).972–980.
74. Lewis J, Ramot R, Green A. Changes in mechanical tension in the median nerve: possible implications for the upper limb tension test. Physiotherapy. 1998;84:254–261.
75. Coppieters MW, Butler DS. Do ‘sliders’ slide and ‘tensioners’ tension? An analysis of neurodynamic techniques and considerations regarding their application. Manual Ther. 2008;13:213–221.
76. Kenneally M. The upper limb tension test. In: Proceedings of the Fourth Biennial Conference of the Manipulative Therapists Association of Australia. Australia: Melbourne; 1983.
77. Kenneally M, Rubenach H, Elvey R. The upper limb tension test: the slr test of the arm: physical therapy of the cervical and thoracic spine. In: Grant R, ed. Physical Therapy of the Cervical and Thoracic Spine. Clinics in Physical Therapy 17. New York: Churchill Livingstone; 1988:167–194.
78. Yaxley G, Jull G. A modified upper limb tension test: an investigation of responses in normal subjects. Austral Physiother. 1991;37:143–152.
79. Coppieters MW, Stappaerts KH, Everaert DG, Staes FF. A qualitative assessment of shoulder girdle elevation during upper limb tension test 1. Manual Ther. 1999;4:33–38.
80. Coppieters MW, Stappaerts KH, Staes FF, Everaert DG. Addition of test components during neurodynamic testing: effect on range of motion and sensory responses. J Orthop Sports Phys Ther. 2001;5:226–237.
81. Dilley A, Summerhayes C, Lynn B. An in vivo investigation of ulnar nerve sliding during upper limb movements. Clin Biomech. 2007;22:774–779.
82. Echigo A, Aoki M, Ishiai S, et al. The excursion of the median nerve during nerve gliding exercise: an observation with high-resolution ultrasonography. J Hand Ther. 2008;21:221–228.
83. Coppieters MW, Hough AD, Dilley A. Different nerve-gliding exercises induce different magnitudes of median nerve longitudinal excursion: an in vivo study using dynamic ultrasound imaging. J Orthop Sports Phys Ther. 2009;39(3):164–171.
84. Beneciuk JM, Bishop MD, George SZ. Effects of upper extremity neural mobilization on thermal pain sensitivity: a sham-controlled study in asymptomatic participants. J Orthop Sports Phys Ther. 2009;30(6):428–438.
85. Costantini M, Tunks K, Wyatt C, et al. Age and upper limb tension testing affects current perception thresholds. J Hand Ther. 2006;19:307–317.
86. Coppieters MW, Alshami AM, Hodges PW. An experimental pain model to investigate the specificity of the neurodynamic test for the median nerve in the differential diagnosis of hand symptoms. Arch Phys Med Rehabil. 2006;87:1412–1417.
87. Coppieters MW, Mortensen T, Richards N, et al. The impact of neurodynamic testing on the perception of experimentally induced muscle pain. Manual Ther. 2005;10:52–60.
88. Boyd B, Wanek L, Gray AT, Topp KS. Mechanosensitivity of the lower extremity nervous system during straight-leg raise neurodynamic testing in healthy individuals. J Orthop Sports Phys Ther. 2009;39(11):780–790.
89. Viikari-Juntura E. Interexaminer reliability of observations in physical examinations of the neck. Phys Ther. 1987;67:1526–1532.
90. Sandmark H, Nisell R. Validity of five common manual neck pain provoking tests. Scand J Rehabil Med. 1995;27:131–136.
91. Selvaratnam PJ, Matyas TA, Glasgow EF. Noninvasive discrimination of brachial plexus involvement in upper limb pain. Spine. 1994;19(1):26–33.
92. Viikari-Juntura E, Porras M, Laasonen EM. Validity of clinical tests in the diagnosis of root compression in cervical disc disease. Spine. 1989;14:253–257.
93. Elvey R, Quinter J, Thomas A. A clinical study of RSI. Austral Family Physician. 1986;15:1314–1322.
94. Pullos J. The upper limb tension test. Austral J Physiother. 1986;32:258–259.
95. Quintner JL. A study of upper limb pain and paraesthesiae following neck injury in motor vehicle accidents: assessment of the brachial plexus tension test of Elvey. Br J Rheumatol. 1989;28(6):528–533.
96. Yaxley GA, Jull GA. Adverse tension in the neural system: a preliminary study of tennis elbow. Austral Physiother. 1993;39(1):15–22.
97. Yeung E, Jones M, Hall B. The response to the slump test in a group of female whiplash patients. Austral Physiother. 1997;43(4):245–252.
98. Quintner J. Stretch induced cervicobrachial pain syndrome. Austral Physiother. 1990;36:99–103.
99. Byng J. Overuse syndromes of the upper limb and the upper limb tension test: a comparison between patients, asymptomatic keyboard workers and asymptomatic non-keyboard workers. Manual Ther. 1977;2(3):157–164.
100. Wainner R, Fritz JM, Irrgang JJ, et al. Reliability and diagnostic accuracy of the clinical examination and patient self-report measures for cervical radiculopathy. Spine. 2003;28(1):52–62.
101. Valls-Sole J, Alvarez R, Nunez M. Limited longitudinal sliding of the median nerve in patients with carpal tunnel syndrome. Muscle Nerve. 1995;18:761–767.
102. Nakamichi K, Tachibana S. Restricted motion of the median nerve in carpal tunnel syndrome. J Hand Surg. 1995;20B(4):460–464.
103. Hough AD, Moore AP, Jones MP. Reduced longitudinal excursion of the median nerve in carpal tunnel syndrome. Arch Phys Med Rehabil. 2007;88:569–576.
104. Erel E, Dilley A, Greening J, et al. Longitudinal sliding of the median nerve in patients with carpal tunnel syndrome. J Hand Surg. 2003;28B(5):439–443.
105. Greening J, Lynn B, Leary R, et al. The use of ultrasound imaging to demonstrate reduced movement of the median nerve during wrist flexion in patients with non-specific arm pain. J Hand Surg. 2001;26B:401–406.
106. Greening J, Dilley A, Lynn B. In vivo study of nerve movement and mechanosensitivity of the median nerve in whiplash and non-specific arm pain patients. Pain. 2005;115:248–253.
107. Marshall J. Nerve-stretching for the relief or cure of pain. Lancet. 1883.1029–1036.
108. Sweeney J, Harms A. Persistent mechanical allodynia following injury of the hand: treatment through mobilization of the nervous system. J Hand Ther. 1996;9:328–338.
109. Haddick E. Management of a patient with shoulder pain and disability: a manual physical therapy approach addressing impairments of the cervical spine and upper limb neural tissue. J Orthop Sports Phys Ther. 2007;37(6):342–350.
110. Rozmaryn L, Dovelle S, Rothman ER, et al. Nerve and tendon gliding exercises and the conservative management of carpal tunnel syndrome. J Hand Ther. 1998;11:171–179.
111. Seradge H, Bear C, Bithell D. Preventing carpal tunnel syndrome and cumulative trauma disorder: effect of carpal tunnel decompression excercises: an Oklahoma experience. J Oklahoma State Med Assoc. 2000;93(4):150–153.
112. Pinar L, Enhos A, Ada S, Güngör N. Can we use nerve gliding exercises in women with carpal tunnel syndrome? Adv Ther. 2005;22(5):467–475.
113. Totten P, Hunter J. Therapeutic techniques to enhance nerve gliding in thoracic outlet syndrome and carpal tunnel syndrome. Hand Clin. 1991;7:505–520.
114. Akalin E, El O, Peker O, et al. Treatment of carpal tunnel syndrome with nerve and tendon gliding exercises. Am J Phys Med Rehabil. 2002;81(2):108–113.
115. Brininger MTL, Rogers JC, Holm MB, et al. Efficacy of a fabricated customized splint and tendon and nerve gliding exercises for the treatment of carpal tunnel syndrome: a randomized controlled trial. Arch Phys Med Rehabil. 2007;99:1429–1435.
116. Heebner ML, Roddey TS. The effects of neural mobilization in addition to standard care in persons with carpal tunnel syndrome from a community hospital. J Hand Ther. 2008;21:229–241.
117. Bialosky J, Bishop MD, Price DD, et al. A randomized sham-controlled trial of a neurodynamic technique in the treatment of carpal tunnel syndrome. J Orthop Sports Phys Ther. 2009;39:709–723.
118. Tal-Akabi A, Rushton A. An investigation to compare the effectiveness of carpal bone mobilisation and neurodynamic mobilisation as methods of treatment for carpal tunnel syndrome. Manual Ther. 2000;5(4):214–222.
119. Coppieters MW, Batholomeeusen KE, Stappaerts KH. Incorporating nerve-gliding techniques in the conservative treatment of cubital tunnel syndrome. J Manipulative Physiol Ther. 2004;27(9):560–568.
120. van der Heide B, Allison G, Zusman M. Pain and muscular responses to a neural tissue provocation test in the upper limb. Manual Ther. 2001;6(3):154–162.
121. Walsh M. Upper limb neural tension testing and mobilization: fact, fiction, and practical approach. J Hand Ther. 2005;18(2):241–258.
122. Jones M. Clinical reasoning in manual therapy. Phys Ther. 1992;72:875–884.
123. Maitland G. Peripheral Manipulation. 2nd ed London: Butterworths; 1970.
124. Maitland GD. Treatment of the glenohumeral joint by passive movement. Physiotherapy. 1983;60(1):3–7.