Neuroanatomical Substrates of Spinal Nociception
Introduction
The dorsal horn of the spinal cord is the major receiving zone for primary afferent axons that transmit information from sensory receptors in the skin, viscera, joints, and muscles of the trunk and limbs to the central nervous system. Nociceptive primary afferent axons (i.e., those that respond to tissue-damaging stimuli) terminate almost exclusively in the dorsal horn, which is therefore the site of the first synapse in ascending pathways conveying the sensory information that underlies conscious perception of pain. In addition, it contains neuronal circuits involved in generating local reflexes.
In the Gate Control Theory of pain, Melzack and Wall (1965) proposed that inhibitory interneurons in the superficial part of the dorsal horn play a crucial role in controlling incoming sensory information before it is transmitted to the brain. This theory aroused a great deal of interest in organization of the dorsal horn. However, despite intensive study since then, our knowledge of the neuronal circuitry of the region remains limited. The dorsal horn contains four neuronal components: (1) central terminals of primary afferent axons, which arborize in different areas, depending on their diameter and the type of sensory stimulus that they respond to; (2) interneurons, with axons that remain in the spinal cord, either terminating locally or extending into other spinal segments; (3) projection neurons, with axons that pass rostrally in white matter to reach various parts of the brain; and (4) descending axons that pass caudally from several brain regions and play an important role in modulating the transmission of nociceptive information. In this chapter we review the anatomical organization of the mammalian dorsal horn, with particular emphasis on primary afferents and interneurons. Certain features of projection neurons are covered here, but they are described in more detail in Chapter 12. Descending modulatory systems are dealt with in Chapter 8, but here we discuss possible targets of the monoamine neurotransmitters released by axons projecting from the brain stem. Because many of the anatomical studies of the dorsal horn have been carried out on cats or rodents, our account is based on these species.
The Laminae of Rexed
Rexed (1952) divided the dorsal horn of the cat spinal cord into six parallel laminae based on differences in the size and packing density of neurons (cytoarchitectonics). This scheme has since been extended to other species, including human, monkey, and rat (Fig. 5-1), and serves as a useful basis for describing its anatomical organization. Lamina II is often subdivided into two parts: inner (IIi) and outer (IIo). Laminae I and II, which are referred to as the superficial dorsal horn, constitute the main target for nociceptive primary afferents (see later). We concentrate our account on this region, partly because of its obvious importance in pain mechanisms and partly because more is known about its neuronal organization. However, the deeper laminae (III–VI) also have an important role in pain: some nociceptive primary afferents terminate in this region, and many neurons in these laminae (including some projection cells) are activated by noxious stimulation. In addition, low-threshold afferents that terminate in laminae IIi–V are at least partially responsible for the tactile allodynia (pain felt in response to touch) that occurs in certain pathological pain states (Campbell et al 1988).
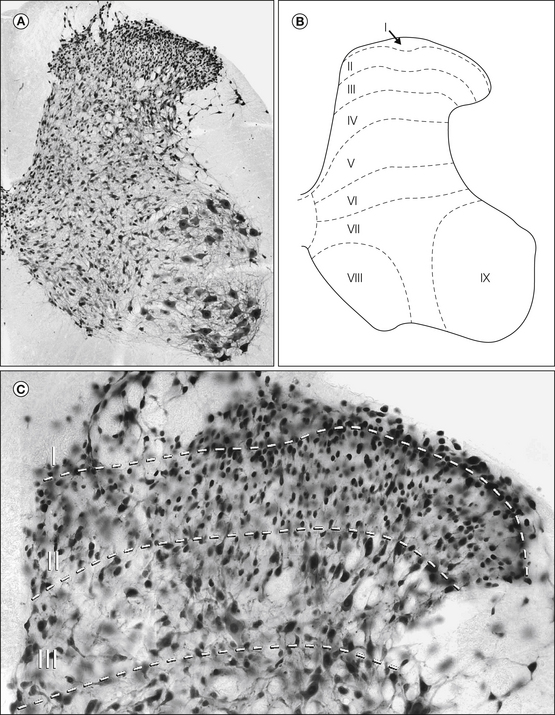
Figure 5-1 Rexed’s laminae applied to the rat spinal cord.
A, Transverse section of the rat lumbar spinal cord (L4 segment) stained with antibody to NeuN, a neuronal nuclear protein. This results in immunostaining of all neurons in the spinal cord. B, The positions of Rexed’s laminae as applied to the rat lumbar spinal cord. Laminae I–VI constitute the dorsal horn. C, Higher-magnification view of laminae I–III stained with the NeuN antibody. Approximate positions of the laminar boundaries are shown with dashed lines. Laminae I and II contain numerous densely packed small neurons, whereas those in lamina III are generally slightly larger.
Lamina I, also known as the marginal layer, forms a thin sheet covering the dorsal aspect of the dorsal horn and contains both projection neurons and interneurons. Although this lamina contains the highest density of projection neurons in the dorsal horn, they are thought to make up only ≈5% of its neuronal population, with the remainder being interneurons (Spike et al 2003). Most of the cells have dendrites that remain within the lamina. Lamina I neurons vary considerably in size and shape, with projection cells being larger than interneurons (Al Ghamdi et al 2009). A few particularly large projection neurons, known as marginal cells of Waldeyer, can be recognized. Lamina II is also known as the substantia gelatinosa because the lack of myelinated fibers gives it a translucent appearance. Virtually all the neurons in this lamina are interneurons, and they are densely packed in its outer part. Lamina III also contains a high density of neurons. Most are interneurons and are generally somewhat larger than those of lamina II, but scattered large projection neurons are also present. Although Rexed’s scheme was based on cytoarchitectonic criteria, the border between laminae II and III can be identified more easily by the absence of myelinated axons in lamina IIi and their presence in lamina III. This can be seen with myelin stains or dark-field microscopy of unstained sections. It should be noted that the correlation between the substantia gelatinosa and Rexed’s lamina II, originally determined in cats, may differ in rodents (Woodbury et al 2000) because lamina IIi receives abundant input from some large myelinated low-threshold mechanoreceptive afferent fibers in rodents but not in cats (Brown 1982, Woolf 1987, Woodbury et al 2001). Laminae IV–VI are more heterogeneous, with neurons of various size, some of which are projection cells. The borders between these laminae are difficult to determine with certainty.
Primary Afferent Fibers
Primary sensory neurons provide constant feedback on the external environment, as well as the ongoing state of the body. The somata of those that innervate the limbs and trunk are located in sensory ganglia associated with spinal nerves (dorsal root ganglia). Their axons bifurcate within the ganglion and give rise to a peripheral branch that innervates various tissues and a central branch that travels through a dorsal root to enter the spinal cord, where it forms synapses with second-order neurons. The peripheral targets of these fibers provide a convenient means for classification. Fibers innervating skin are described as cutaneous sensory neurons. Likewise, those innervating abdominal or pelvic viscera are termed visceral afferents. Within these populations, fibers can respond to various sensory modalities, including mechanical, thermal, and chemical stimuli. Modality-specific groups are further divided according to the intensity of their adequate peripheral stimuli. Those that respond to gentle mechanical force or innocuous thermal stimuli are low-threshold mechanoreceptors or innocuous cooling or warming afferents. Fibers responding only to stimulus intensities considered tissue threatening or potentially tissue damaging are termed nociceptors.
As a group, primary sensory neurons exhibit a rich diversity in morphological and functional properties, including somatic membrane properties, laminar location of central projections, neurochemical content, and response properties of the central networks that they activate (Koerber and Mendell 1992, Djouhri et al 1998). The most common means of classifying primary sensory neurons is based on the conduction velocity of their peripheral axons, which is directly related to axon diameter and whether the axon is myelinated. From the distribution of these peripheral conduction velocities, primary sensory neurons are routinely divided into different groups: Aα/β, Aδ, and C.
The Aα/β group consists of large myelinated axons with the fastest peripheral conduction velocity, the Aδ group contains smaller fibers that are thinly myelinated and conduct at an intermediate velocity, and the C group consists of the smallest, unmyelinated, and most slowly conducting fibers. Within each group there is a wide range of functional types of primary afferents, as defined by sensory modality. Most sensory neurons with fibers conducting in the Aα/β range respond to innocuous mechanical stimuli, do not encode noxious stimulus intensities, and are classified as low-threshold mechanoreceptors. Some of these fibers, however, respond to relatively innocuous mechanical stimuli but also encode stimulus intensities in the noxious range and in some cases respond to noxious heating of the skin. This trend reverses with decreasing conduction velocity, with a majority of Aδ fibers and most C fibers being classified as nociceptors. The relative number of functional types in specific conduction velocity groups varies between species and the areas of the body that the fibers innervate. However, it is important to point out that both nociceptors and non-nociceptors exist in all three conduction velocity groups (Fig. 5-2).
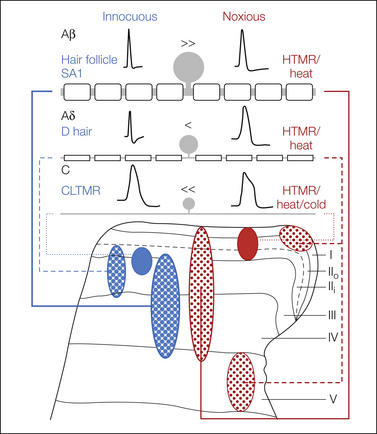
Figure 5-2 Schematic representation of the general properties of cutaneous afferent fibers.
Inequality signs denote the relative numbers of afferent fibers within a conduction velocity that respond to innocuous (blue) or noxious (red) stimuli. The density of the terminals of each type of fiber is shown as varying stippling within each projection zone. Note that not all C fibers are nociceptors and not all Aβ fibers are low-threshold mechanoreceptors. HTMR, high-threshold mechanoreceptor.
Vesicular Glutamate Transporters
All primary afferents are thought to use glutamate as their principal neurotransmitter since the excitatory post-synaptic currents produced by these afferents can be blocked with antagonists of the α-amino-3-hydroxy-5-methyl-4-isoxazoleproprionate (AMPA)-type glutamate receptor (Yoshimura and Jessell 1990), glutamate is enriched in their central terminals (Broman et al 1993), and these are associated with post-synaptic AMPA receptors (Nagy et al 2004a). Until recently it was difficult to identify glutamatergic axons, but this changed with the discovery of the vesicular glutamate transporters (VGLUTs). Two of these transporters, VGLUT1 and VGLUT2, are widely distributed in the spinal gray matter (Varoqui et al 2002, Todd et al 2003), whereas the third (VGLUT3) is present at much lower levels (Seal et al 2009). Within the dorsal horn, VGLUT1 is largely restricted to the deeper part (laminae IIi–VI) and is apparently present in the central terminals of all low-threshold mechanoreceptive myelinated primary afferents, as well as in some descending glutamatergic axons (Todd et al 2003). VGLUT2-immunoreactive axons are found throughout the gray matter, and most belong to local interneurons (see later). The central terminals of many myelinated nociceptors in lamina I also show strong VGLUT2 immunoreactivity. However, although most C fibers appear to express VGLUT2 (Brumovsky et al 2007), it is present at very low or undetectable levels in their central terminals (Todd et al 2003). For example, dorsal rhizotomy does not lead to detectable loss of VGLUT2 in the dorsal horn, but there is a substantial reduction in VGLUT1 (Alvarez et al 2004, Brumovsky et al 2007). Seal and colleagues (2009) recently identified a population of low-threshold mechanoreceptive unmyelinated primary afferents that express VGLUT3 and project mainly to the innermost part of lamina II and the dorsal part of lamina III.
Physiological Properties
Early studies of primary sensory neurons revealed heterogeneity in the shapes of somatic action potentials (Yoshida et al 1978, Gorke and Pierau 1980), and subsequent studies have shown strong correlation between these shapes and receptor function (e.g., Belmonte and Gallego 1983, Koerber and Mendell 1988). Myelinated fibers that respond to innocuous mechanical stimulation of the skin have narrow somatic action potentials without breaks in the rising or falling phase. Unmyelinated and myelinated nociceptive fibers have broad somatic action potentials that most often have a distinct inflection on the falling phase. This correlation is very consistent for myelinated fibers. However, all unmyelinated fibers have broad inflected somatic action potentials regardless of their peripheral response properties.
Spinal Projections of Primary Sensory Neurons
The central projections of primary sensory neurons have been visualized with various labeling methods, including the Golgi technique, detection of degenerating axon terminals, bulk-labeling techniques in which tracer substances are administered to a nerve or peripheral tissue and transported by many primary afferents, and intracellular staining of individual identified fibers. In his landmark studies, Cajal (1909) used the Golgi technique and suggested that fine primary afferent fibers project to the superficial part of the dorsal horn. As newer bulk-labeling techniques were introduced, the organization of central projections of different fiber types was further refined (e.g., Light and Perl 1977, Mesulam and Brushart 1979). However, the uncertainties inherent in bulk-labeling techniques left many questions unanswered. The use of intracellular labeling has clarified the specific central targets of different afferent fiber types (e.g., Light and Perl 1979, Brown 1982). We will therefore focus on the findings of studies using these intracellular staining techniques.
Cutaneous Sensory Neurons
Myelinated Low-Threshold Mechanoreceptors
Low-threshold mechanoreceptive fibers enter the spinal cord and bifurcate into main ascending and descending branches that travel in the dorsal columns and migrate medially as they move away from their point of entry. Collateral fibers arise from these main branches, turn ventrally, and pass through the dorsal horn before terminating in dense arborizations that lie within a region extending from lamina IIi to lamina V. The morphological characteristics of these projections vary with the specific fiber type and the target tissue innervated, their relative position within the dorsal horn, and the distance from the point of entry into the spinal cord (Brown 1982, Woolf 1987, Millecchia et al 1991, Koerber et al 1995). In all cases the most superficial and dense central projections lie near the point of entry. Away from the entry zone they become more diffuse and occupy more ventral and medial positions (Koerber et al 1995). Another consistent feature is that those innervating hair follicles terminate more superficially than do those innervating slowly adapting receptors. Among fibers innervating hair follicles, those conducting in the Aδ range (D-hair afferents) occupy the most superficial position of all low-threshold fibers and project extensively into lamina IIi (Light et al 1979, Woodbury and Koerber 2003, Woodbury et al 2008).
Myelinated Nociceptive Afferent Fibers
Myelinated nociceptors, which are thought to signal fast pricking or sharp pain, were first identified in the pioneering studies of Burgess and Perl (1967) and have since been studied extensively by other groups (e.g., Campbell et al 1979, Light and Perl 1979, Reeh et al 1987). They span a very large range of conduction velocities, from the slowest in the Aδ spectrum to well into the Aβ range (Campbell et al 1979, Koerber and Mendell 1988). They respond to different stimulus modalities (e.g., mechanical and thermal) and have threshold stimulus intensities ranging from innocuous to noxious (e.g., Burgess and Perl 1967, Fitzgerald and Lynn 1977). Recent studies also show that they exhibit different central morphologies and have a range of neurochemical phenotypes (Light and Perl 1979, Lawson et al 1997, Lawson et al 2002, Woodbury and Koerber 2003, McIlwrath et al 2007, Lawson et al 2008).
Most early studies examining individual myelinated nociceptors used extracellular recording techniques, either from peripheral nerves using microelectrodes (Burgess and Perl 1967) or from nerve strands draped over metal electrodes (Reeh et al 1987). With these techniques investigators are limited to using peripheral response properties, such as mechanical threshold, to identify myelinated nociceptors. Although it has long been known that some putative myelinated nociceptors could be activated by non-noxious moderate pressure (Burgess and Perl 1967), threshold criteria were commonly used to avoid ambiguity, and thus neurons that code for both non-noxious and noxious mechanical stimuli have largely been overlooked.
The recent development of procedures that allow intracellular recordings from the cell soma combined with labeling of the central projections of the recorded fiber has increased the number of criteria that can be used to identify nociceptive sensory neurons (Koerber and Woodbury 2002, Woodbury and Koerber 2003). For example, myelinated nociceptors have broad inflected somatic action potentials that can easily be distinguished from those of low-threshold mechanoreceptors (Koerber and Mendell 1988, Djouhri et al 1998). Although relatively little information is available on the neurochemical properties of myelinated nociceptors, it is known that some contain neuropeptides, such as substance P and calcitonin gene–related peptide (CGRP), and express the high-affinity neurotrophin receptor TrkA, thus being responsive to nerve growth factor (NGF) (Lawson et al 1997, 2002). Others contain TrkC and are sensitive to neurotrophic factor 3 (NT3) (McIlwrath et al 2007). In addition, these fibers often possess acid-sensing ion channel 3 (ASIC3) and the transient receptor potential vanilloid type 2 (TRPV2) receptor (McIlwrath et al 2007, Lawson et al 2008).
The central projections of myelinated nociceptors were first described by Light and Perl (1979), who focused on fibers conducting in the Aδ range in cats. They found that on entry into the spinal cord, the main branches were located laterally in the dorsal column, often in or near Lissauer’s tract. Terminal arbors from these afferents were centered primarily on laminae I and IIo, with some passing ventrally to terminate in lamina V. However, some of the faster-conducting fibers had collateral branches that penetrated deeply into the dorsal horn and then recurved dorsally and projected into the ventral part of lamina IV. The findings of this landmark study led to the widespread belief that myelinated nociceptors project only to laminae I and V.
More recently, Woodbury and Koerber used an ex vivo preparation consisting of isolated spinal cord and attached innervated skin to examine the projections of these and other afferent types in neonatal and adult mice (Woodbury et al 2003, 2008). Two distinct morphological types of myelinated nociceptor were observed. The first closely resembled the thinly myelinated nociceptors described in the cat by Light and Perl (1979). On entry into the spinal cord, their axons bifurcated to give rise to ascending and descending branches that extended over several segments. Some of these afferents gave rise to axons that ascended in the dorsal columns. However, most maintained a lateral position in the vicinity of Lissauer’s tract. The second type had main branches that ascended and descended in the dorsal columns and gave rise to numerous collaterals that penetrated ventrally through the depth of the dorsal horn before recurving dorsally, as seen with many low-threshold myelinated mechanoreceptive afferents. However, in marked contrast to the latter, the arbors of this group extended through the full depth of the dorsal horn, including laminae IIo and I (Fig. 5-3). Once in lamina I, they typically turned to run along the rostrocaudal axis while continuing to arborize. In general, the arbors of these fibers were somewhat more diffuse than those of low-threshold mechanoreceptors with similar peripheral conduction velocities. Interestingly, those with central projections focused in laminae I and IIo had higher mechanical thresholds than did those with projections spanning the dorsal horn. Some fibers of each type also responded to noxious heating of the skin (Woodbury and Koerber 2003, Woodbury et al 2008).
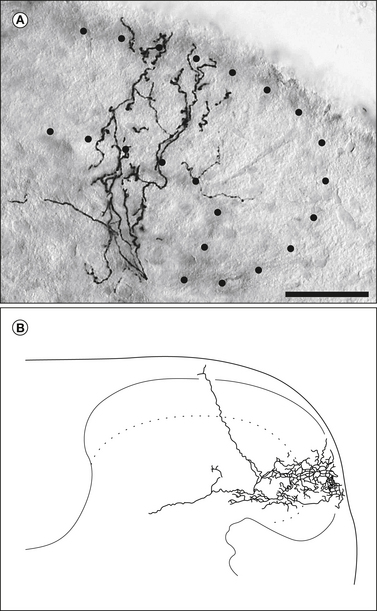
Figure 5-3 Spinal projections of a myelinated cutaneous nociceptive fiber from a 3-week-old mouse showing novel morphology.
A, Photomicrograph of a section of spinal cord dorsal horn showing the laminar distribution of a single recurving collateral. The dotted lines indicate the boundaries of laminae I–II. B, Camera lucida drawing of a different collateral from the same afferent fiber. Scale bar, 50 μm. (Modified from Woodbury CJ, Koerber HR 2003 Widespread projections from myelinated nociceptors throughout the substantia gelatinosa provide novel insights into neonatal hypersensitivity. Journal of Neuroscience 23:601–610. Copyright 2003 by the Society for Neuroscience.)
Unmyelinated Afferent Fibers
Cutaneous primary afferent neurons with unmyelinated peripheral axons are diverse in terms of response properties and neurochemical phenotypes. As a group, they exhibit a wide range in the stimulus intensity necessary for their activation. Some unmyelinated fibers respond to gentle brushing of the skin and/or innocuous cooling, whereas others require more intense mechanical or thermal stimulation in the noxious range (Bessou and Perl 1969). Individual neurons can respond to different modalities of noxious stimuli, including mechanical, thermal, and chemical. Although some respond only to a single type of stimulus, others are equally responsive to two or more and are referred to as polymodal nociceptors (Bessou and Perl 1969). Still other afferent fibers are normally insensitive to peripheral stimulation and become sensitized only after prolonged noxious stimulation or in response to injury (e.g., Meyer and Campbell 1988).
In terms of phenotypic diversity, unmyelinated cutaneous afferents can express a large number of neuroactive compounds and receptors. Frequently, C fibers are divided into two major groups based on the combination of neurochemical phenotype and sensitivity for different neurotrophins (Snider and McMahon 1998). One group consists of axons that are sensitive to NGF, express TrkA, and usually contain neuropeptides such as CGRP, substance P, and galanin (Averill et al 1995, Molliver et al 1995, Bennett et al 1996, Zhang et al 1993). Neurons in the second group are responsive to members of the glial cell line–derived neurotrophic factors (GDNFs) and neurturin, express the receptor tyrosine kinase (RET), have binding sites for the lectin isolectin B4 (IB4, from Bandeiraea simplicifolia), usually possess the purinergic receptors P2X3 and P2Y1 (Molliver et al 1997, Bennett et al 1998, Bradbury et al 1998, Vulchanova et al 1998, Guo et al 1999, Moriyama et al 2003, Gerevich et al 2005), and lack CGRP and substance P (Averill et al 1995). However, despite the obvious differences between these two populations, it is not clear whether they correspond to different functional types. For example, the capsaicin receptor (TRPV1), which responds to heat and protons (Caterina et al 1997) and is believed to transduce noxious heat stimuli, is expressed in a variety of sensory neurons in the rat, including both TrkA/peptidergic and IB4-binding populations (Guo et al 1999, Michael and Priestley 1999). However, there is still debate over the location of the receptor in the peripheral and central projections of these fibers (Guo et al 1999). The distribution of TRPV1 is different in mice because it does not appear to be expressed by the IB4-binding population (Zwick et al 2002). Interestingly, TRPV1-knockout mice exhibit relatively modest changes in their behavioral response to noxious heat (Caterina et al 2000). In addition, it has been shown that in these knockout mice, C fibers responding to both mechanical and heat stimuli have normal heat responses (Woodbury et al 2004). Taken together, these results suggest that both populations of C fibers respond to heat stimuli and that TRPV1 is not the only receptor capable of transducing such stimuli. Recently, it has been shown that in naïve mice, cutaneous C fibers expressing TRPV1 are mechanically insensitive but respond robustly to heat stimuli (C heat, CH) whereas mice lacking TRPV1 also lack CH fibers, thus suggesting that TRPV1 is required for heat sensitivity in this fiber type (Lawson et al 2008).
The central projections of these two different groups of unmyelinated primary afferents differ, with the IB4-positive (“non-peptidergic”) fibers projecting to the central part of lamina II (dorsal part of lamina IIi) and peptidergic fibers arborizing mainly in laminae I and IIo, but with scattered terminals in deeper laminae (IIi–V) (Silverman and Kruger 1988, Plenderlieth et al 1990, Averill et al 1995; but also see Riberio-da-Silva et al 1986, Woodbury et al 2000).
The central axons of individual unmyelinated fibers have been visualized with intracellular labeling techniques, most notably by Sugiura and colleagues (Sugiura et al 1986, 1989). They stained functionally identified C fibers from the guinea pig and reconstructed their central projections. Although relatively few fibers were examined, examples of low- and high-threshold mechanoreceptors, as well as polymodal nociceptors, were recovered. These fibers entered the spinal cord and ran rostrally and/or caudally along the surface of the dorsal funiculus near or in Lissauer’s tract while sending off collateral branches that penetrated ventrally into laminae I and II and ended in dense terminal fields. Although individual fibers had projections that were focused more or less in different parts of the superficial dorsal horn, most were found in both laminae I and II, with the primary focus usually being in lamina II (Sugiura et al 1989). Recent studies in mice have largely confirmed these original reports by demonstrating that most nociceptive C fibers project extensively to lamina IIo and, to a more limited extent, to lamina I (Woodbury et al 2004, Albers et al 2006).
Advances in transgenic technology have recently been used to identify specific biomarkers for additional subsets of sensory fibers (e.g., Zylka et al 2005, Seal et al 2009). The inclusion of fluorescent reporter constructs combined with intracellular recording and staining has allowed functional identification of some of these fiber types. For example, the Mas-related G protein–coupled receptor D (Mrgprd) is selectively expressed in unmyelinated cutaneous fibers that lack peptides and bind IB4 (Zylka et al 2005). Intracellular recordings demonstrated that all these fibers were responsive to mechanical stimulation and that the vast majority were also sensitive to heat and occasionally cold stimuli (Rau et al 2009). These predominantly polymodal fibers have central projections that precisely overlap the IB4-positive labeling within lamina II.
Afferent Fibers Innervating Muscle and Viscera
Afferent fibers innervating deep structures such as muscles, tendons, or viscera share many characteristics with those innervating skin. They have a large range of peripheral conduction velocities and respond to various stimulus modalities over a wide spectrum of intensities (Hoheisel et al 1989, Habler et al 1993, Sengupta and Gebhart 1994). Myelinated fibers innervating muscles and tendons generally fall into two categories. One group innervates specific receptors (e.g., muscle spindles or Golgi tendon organs) and these afferents are generally referred to as proprioceptors. They have very low mechanical thresholds and their central projections are usually confined to the deeper parts of the dorsal horn (laminae IV–VI) and the ventral horn. Another group of myelinated mechanoreceptive fibers innervating muscle, tendon, or fascia is not associated with specialized endings. These fibers respond over a range of stimulus intensities and can be divided into low- and high-threshold groups (Hoheisel et al 1989). High-threshold myelinated mechanoreceptors exhibit two different patterns of central termination. One type has projections confined exclusively to lamina I, whereas a second type projects to lamina I and also to laminae IV–V. Low-threshold mechanoreceptors have a different central morphology and generally cover a greater rostrocaudal extent in the dorsal horn. They project predominantly to lamina II, as well as to laminae IV and VI (Hoheisel et al 1989) (Fig. 5-4).
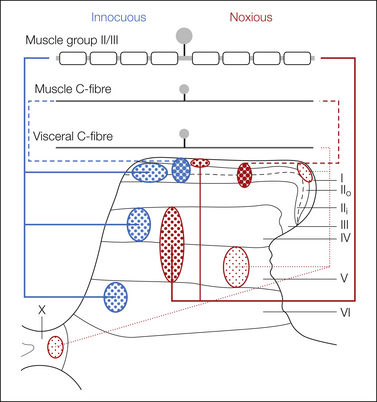
Figure 5-4 Schematic representation of the spinal projections of afferent fibers innervating muscle and viscera.
Myelinated muscle afferents conveying information about innocuous muscle stimuli are myelinated and have widespread projections. Myelinated fibers that respond to noxious stimuli project to the deeper dorsal horn, whereas C fibers responding to painful stimuli project to the superficial (muscle) or superficial and deep (visceral) dorsal horn laminae. The relative density of fiber terminals among these fibers and with respect to cutaneous fiber projections (see Fig. 5-2) is depicted by the varying stippling.
Similarly, myelinated fibers innervating abdominal and pelvic viscera can be divided into low- and high-threshold mechanoreceptive groups, although many of the low-threshold group also encode into the noxious range (see Chapter 51). However, little is known about the morphology of their central projections. Results from bulk-labeling studies suggest that they are most likely to project to lamina I and/or laminae V–VI (de Groat et al 1981, Morgan et al 1981).
Unmyelinated fibers innervating muscle and viscera respond to a variety of noxious stimuli, including mechanical and chemical, and thus can be considered polymodal nociceptors (Kumazawa 1996). These neurons contain many of the same neuroactive compounds and receptor types seen in their cutaneous counterparts (Molander et al 1987, O’Brien et al 1989, Perry and Lawson 1998). However, one notable difference is that they rarely bind IB4, thus suggesting some fundamental differences between the populations (Bennett et al 1996, Perry and Lawson 1998). Early studies using bulk-labeling methods suggested that unmyelinated fibers innervating viscera (de Groat et al 1981, Morgan et al 1981) or muscle (Craig and Mense 1983, but see Brushart et al 1981) project to lamina I and deeper parts of the dorsal horn, but not to lamina II, as is the case with cutaneous C fibers. However, intracellular staining of individual unmyelinated fibers innervating these structures (Sugiura et al 1989, Ling et al 2003) has shown conclusively that these fibers do project to lamina II, as well as to the other parts of the dorsal horn.
Ling and colleagues (2003) examined the central projections of six unidentified unmyelinated afferents contained in the nerve to the lateral gastrocnemius muscle. These fibers were shown to enter the spinal cord and run rostrally and caudally in the superficial part of the dorsal funiculus. They gave rise to two different types of projections. One type had small focused projections in lamina I, the overlying white matter, and lamina IIi. They were less dense than those of cutaneous C fibers. The second type had projections throughout lamina II that were relatively sparse in comparison to cutaneous fibers. Both types also gave rise to sporadic projections into the dorsal part of lamina III. Additional studies are needed to determine whether these two morphological groups represent different functional types.
Individual unmyelinated fibers innervating abdominal viscera have also been examined (Sugiura et al 1989). These fibers were identified by electrical stimulation of the celiac ganglion but were otherwise uncharacterized. On entering the spinal cord, they bifurcated and ran rostrally and caudally in the dorsal funiculus or Lissauer’s tract for several segments and gave rise to many collaterals that ramified in several different locations in the spinal cord, including laminae I–II, V, and X. The terminal ramifications formed relatively diffuse narrow sheets. Overall, comparison of unmyelinated fibers innervating different peripheral tissues shows that cutaneous fibers have the most focused and dense projections, visceral afferents have the most wide-ranging and diffuse projections, and those innervating muscle have projections that lie between these two extremes. These differences in central projection patterns could contribute to the difficulty in localizing muscle and visceral pain.
Summary of Spinal Projections
It is clear that both myelinated and unmyelinated afferent fibers that respond to noxious stimulation in the periphery project predominantly to the superficial dorsal horn. However, it is also clear that myelinated and unmyelinated fibers that signal the presence of innocuous mechanical and thermal stimuli also project to these same laminae. Therefore, the anatomical substrate for processing pain information cannot easily be distinguished from those involved in other functions such as homeostasis. Interestingly, although there is significant overlap in the projections of fibers signaling different stimulus intensities, there appears to be at least some degree of functional segregation at the post-synaptic level in the superficial laminae (e.g., Light and Willcockson 1999, Andrew and Craig 2001, Wilson et al 2002).
Receptors Associated with Primary Afferent Neurons
Primary afferent fibers possess a rich diversity of ligand-gated ionotropic, metabotropic, and tyrosine kinase receptors. Although a complete description of these receptors is beyond the scope of this chapter, several are present on the central terminals of primary afferent fibers, and since their activation appears to regulate the release of neurotransmitters, they merit consideration. These include both the AMPA and N-methyl-D-aspartate (NMDA) classes of ionotropic glutamate receptors (Tachibana et al 1994) and metabotropic glutamate receptors (Ohishi et al 1995). Both GABAA and GABAB receptors are expressed by sensory neurons; although GABAB receptors have been localized to presynaptic terminals (Poorkhalkali et al 2000), little is yet known about the arrangement of GABAA receptor subunits on primary afferent terminals. The three main opioid receptors (μ, δ, and κ) are also found in primary sensory neurons, and μ- and δ-opioid receptors have been identified on fine-diameter primary afferent terminals (Wang et al 2010). In addition, both nicotinic and muscarinic cholinergic receptors are present on afferent fibers (Flores et al 1996, Haberberger et al 1999). α2-Adrenergic receptors are also found in sensory neurons and are thought to be localized at the central terminals of peptidergic fibers (Stone et al 1998).
Ultrastructure of Primary Afferent Terminals
Although most primary afferent boutons have relatively simple synaptic arrangements in the dorsal horn, some form complex structures involving several synapses and are known as synaptic glomeruli (Fig. 5-5). Since the central axons of all synaptic glomeruli are of primary afferent origin (Ribeiro-da-Silva 2003), this provides a convenient way of identifying primary afferent terminals with electron microscopy. Synaptic glomeruli consist of a central primary afferent bouton surrounded by several other profiles with which the central axon forms synapses. The peripheral profiles are either GABAergic axons, which are presynaptic to the primary afferent bouton at axo-axonic synapses, or dendrites. In most cases the dendrites are post-synaptic to the primary afferent bouton. However, some contain synaptic vesicles and form dendro-axonic synapses onto the central axon. These vesicle-containing dendrites, which are also GABAergic (Todd 1996), may be involved in reciprocal synapses with the primary afferent bouton (Fig. 5-5A). Another type of arrangement that occurs in glomeruli is the synaptic triad, in which the central axon forms synapses with two peripheral profiles that are themselves linked by a synapse. It is clear from this description that synaptic glomeruli provide the basis for complex modulation of incoming sensory information, including GABAergic presynaptic inhibition of the primary afferent terminal by axo-axonic and dendro-axonic synapses.
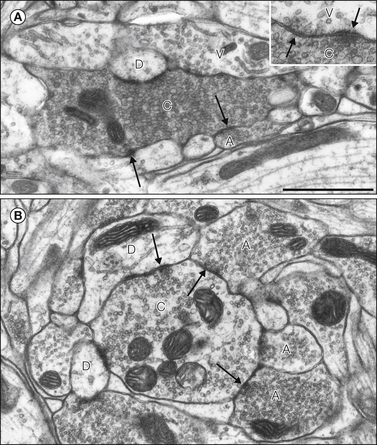
Figure 5-5 Synaptic glomeruli in the superficial dorsal horn of the rat spinal cord.
A, Type I glomerulus. The central axon (C) is indented and filled with vesicles of various sizes. It is surrounded by several profiles, including an axon (A) and dendrites, one of which is labeled (D). A vesicle-containing dendrite (V) is also present, and on an adjacent section this formed a reciprocal axodendritic/dendro-axonic synapse with the central axon (inset). Some of the synapses are indicated by arrows. B, The central axon (C) of this type II glomerulus contains several mitochondrial profiles and synaptic vesicles that are clustered at synapses. The central axon receives axo-axonic synapses from three other axons (A) and is presynaptic to two dendrites (D). Some of the synapses are shown with arrows. Scale bar for both parts, 1 μm. (Modified from Todd AJ 1996 GABA and glycine in synaptic glomeruli of the rat spinal dorsal horn. European Journal of Neuroscience 8:2492–2498, with permission from Blackwell Publishing Ltd.)
Ribeiro-da-Silva and Coimbra (1982) recognized two types of synaptic glomeruli in the rat spinal cord. Central axons of type I glomeruli typically have indented contours, are packed with synaptic vesicles of varying diameter, have few mitochondria, and receive a single axo-axonic synapse from a peripheral axon (Ribeiro-da-Silva 2003) (Fig. 5-5A). They occupy a narrow band in the middle of lamina II, and their central axons usually belong to C fibers that lack neuropeptides. Type II glomeruli are located slightly more ventrally, on either side of the lamina II/III border. Their central axons are generally larger than those of type I glomeruli and are derived from Aδ D-hair afferents (Rèthelyi et al 1982). Type II central axons contain clustered synaptic vesicles and several mitochondrial profiles, and they often receive several axo-axonic synapses (Fig. 5-5B), thus suggesting that they are under powerful presynaptic inhibitory control.
In the rat, most peptide-containing primary afferent terminals in the superficial dorsal horn form simple synaptic arrangements (Ribeiro-da-Silva et al 1989), whereas in the monkey they may be involved in synaptic glomeruli (Knyihar-Csillik et al 1982). The central terminals of peptidergic primary afferents also differ from those of non-peptidergic C fibers in that they receive very few axo-axonic synapses.
Central terminals of Aδ mechanical nociceptors have been identified in the cat and monkey (Rèthelyi et al 1982, Alvarez et al 1992). In lamina I, some of these nociceptors formed simple axodendritic synapses, but many had glomerular arrangements in which they were presynaptic to several dendrites and post-synaptic to GABAergic axons. Terminals of these afferents in lamina V had simpler synaptic arrangements.
The ultrastructure of several different types of Aβ low-threshold mechanoreceptive afferents has been studied in the cat (Maxwell and Rèthelyi 1987). Most of them have a “non-glomerular” synaptic organization in which they are presynaptic to one or more dendrites and frequently receive axo-axonic GABAergic synapses.
Projection Neurons, Substance P, and the Neurokinin 1 Receptor
Neurons with axons that project to the brain are concentrated in lamina I and scattered through the deep dorsal horn (laminae III–VI) and the ventral horn. Those in lamina I, together with some of the projection cells in deeper laminae, have axons that cross the midline and ascend to several supraspinal targets, including the thalamus, midbrain periaqueductal gray (PAG), lateral parabrachial area (LPb) of the pons, and various parts of the medulla (Craig 1995, Villanueva and Bernard 1999, Willis and Coggeshall 2004) (see Chapter 12). Quantitative studies on the rat spinal cord indicate that there are around 400 projection neurons on each side in lamina I in the L4 segment and that ≈95% of these neurons send axons to the LPb, around a third to the PAG, a quarter to the nucleus of the solitary tract, but <5% to the thalamus (Al-Khater et al 2008, Polgár et al 2010a, Todd 2010). However, spinothalamic lamina I neurons are far more numerous in the cervical region of the rat and in both lumbar and cervical enlargements in the cat and monkey (Zhang et al 1996, Zhang and Craig 1997). Many projection neurons send their axons to more than one supraspinal target.
In addition to their supraspinal targets, projection neurons also generate local axon collaterals and thus contribute to processing of information in the dorsal horn, as well as to segmental reflex pathways. For example, Szücs and colleagues (2010) have recently shown that the majority of lamina I projection neurons in the rat give rise to axon collaterals in the dorsal and/or ventral horn.
Many nociceptive primary afferents contain substance P (Lawson et al 1997), and there is evidence that this peptide and the neurokinin 1 (NK1) receptor, on which it acts, have a significant role in spinal pain mechanisms. The NK1 receptor is present in the dorsal horn, with its highest concentration in lamina I (e.g., Nakaya et al 1994) (Fig. 5-6). The receptor is expressed by some neurons in each lamina, including ≈80% of lamina I projection neurons (Fig. 5-7B). There is also a population of large NK1 receptor–immunoreactive projection neurons with cell bodies in laminae III–IV and dendrites that pass dorsally into lamina I (Todd 2010) (Fig. 5-7E). These neurons are far less numerous than the lamina I projection neurons, with only ≈25 per side in the L4 segment. Immunocytochemical studies have shown that many NK1 receptor–immunoreactive neurons in laminae I, III, and IV internalize the receptor following acute noxious stimulation, presumably as a result of its activation by substance P released from nociceptive primary afferents (Mantyh et al 1995).
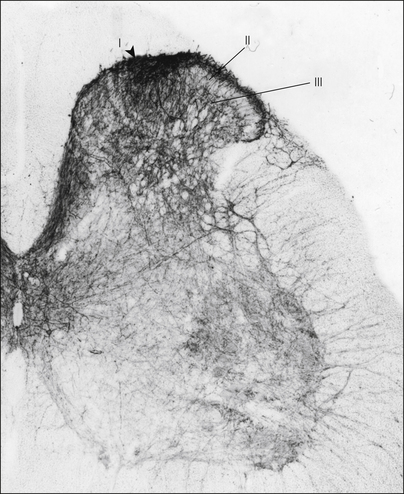
Figure 5-6 Neurokinin 1 (NK1) receptor immunostaining in a section from the lumbar spinal cord of a rat.
The receptor is found throughout the spinal cord but is present at the highest concentration in lamina I. (Modified from Nakaya Y, Kaneko T, Shigemoto R, et al 1994 Immunohistochemical localization of substance P receptor in the central nervous system of the adult rat. Journal of Comparative Neurology 347:249–274. Reprinted with permission of Wiley-Liss, Inc., a subsidiary of John Wiley and Sons, Inc.)
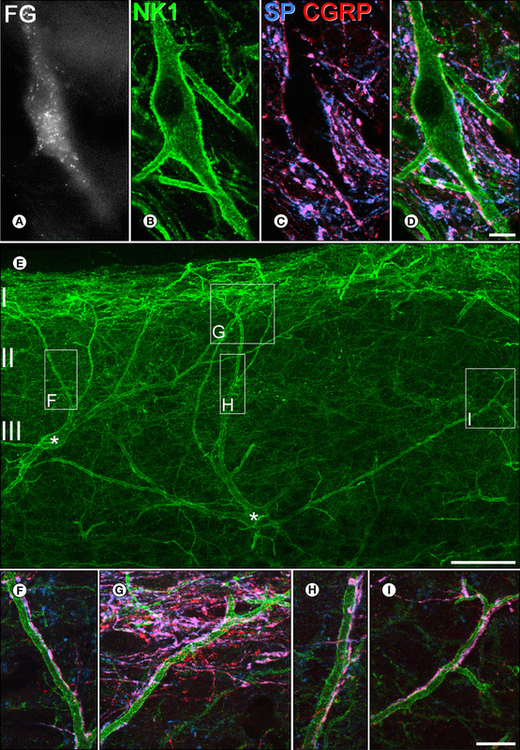
Figure 5-7 Contacts formed by substance P (SP)-containing primary afferents and projection neurons that express the neurokinin 1 (NK1) receptor in the rat lumbar spinal cord.
A-D show a lamina I neuron in a horizontal section. A, The cell was retrogradely labeled with the tracer Fluorogold (FG), which had been injected into the medullary reticular formation. B, It expresses the NK1 receptor (green), which outlines its cell body and dendrites. C and D show immunostaining with antibodies against SP (blue) and calcitonin gene–related peptide (CGRP, red). Axonal boutons that contain both peptides appear pink, and several of them are adjacent to the labeled cell. The presence of both these peptides identifies an axon as being an SP-containing (nociceptive) primary afferent. E, A sagittal section through the dorsal horn reveals two large NK1 receptor–immunoreactive neurons (asterisks) with cell bodies in lamina III and dendrites that extend up to lamina I. All cells of this type are projection neurons. Boxes indicate areas shown at higher magnification in F–I. In these images, SP (blue) and CGRP (red) are also shown, and it can be seen that dendrites of the two cells receive numerous contacts from SP-containing primary afferents. Scale bars for A–D, 10 μm; E, 50 μm; F–I, 10 μm. (A–D Reproduced with permission from Todd AJ, Puskár Z, Spike RC, et al 2002 Projection neurons in lamina I of rat spinal cord with the neurokinin 1 receptor are selectively innervated by substance P–containing afferents and respond to noxious stimulation. Journal of Neuroscience 22:4103–4113. Copyright 2002 by the Society for Neuroscience.)
Both the lamina I and the lamina III–IV projection neurons that express the NK1 receptor are densely innervated by substance P–containing primary afferents (Fig. 5-8; also see Fig. 5-7), which form numerous synapses on their dendrites and cell bodies (Naim et al 1997, Todd et al 2002). These afferents provide around half the glutamatergic synaptic input to the NK1 receptor–expressing lamina I projection neurons (Polgár et al 2010b). For the lamina III–IV cells, most of the synapses from substance P–containing afferents are located on dendrites in the superficial dorsal horn, but they also receive significant input from these afferents on their deeper dendrites and cell bodies. Since they have large dendritic trees that can extend from lamina I to lamina V, these cells could potentially be innervated by various types of primary afferent. However, they receive only limited input from myelinated low-threshold mechanoreceptive afferents in laminae III and IV and have very few contacts from unmyelinated primary afferents that lack neuropeptides (Todd 2010). This indicates that the primary afferent input to projection neurons is arranged in a highly selective manner. Substance P is released from primary afferents at extrasynaptic sites and acts on NK1 receptors through “volume transmission,” whereas the asymmetrical synapses that these projection cells receive from substance P–containing afferents underlie glutamatergic transmission. These findings demonstrate a strong disynaptic connection from nociceptive primary afferents that contain substance P to various sites in the brain, including the thalamus and LPb.
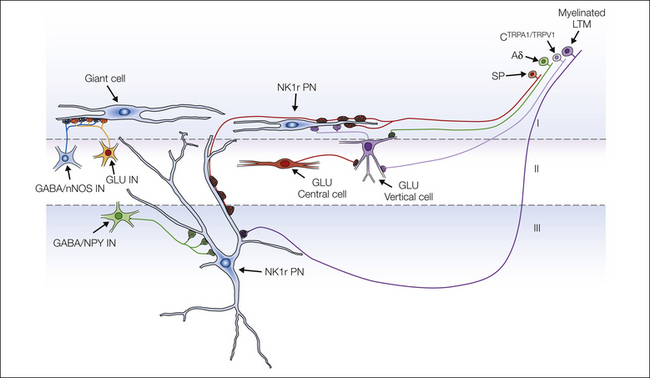
Figure 5-8 Diagram showing some of the synaptic circuits identified in laminae I–III.
Three types of projection neuron (PN) are shown: a neurokinin 1 receptor (NK1r)-expressing cell in lamina I (NK1r PN), an NK1r-expressing cell in lamina III, and a giant lamina I neuron. Both types of NK1r-expressing projection neuron are densely innervated by substance P–containing primary afferents (SP), and the lamina III neurons also have input from myelinated low-threshold mechanoreceptive (LTM) afferents. The lamina III NK1r cells receive substantial input from GABAergic interneurons that contain neuropeptide Y (GABA/NPY IN), whereas inhibitory interneurons that contain neuronal nitric oxide synthase (GABA/nNOS IN) innervate the giant lamina I cells. The giant cells receive a high density of synapses from vesicular glutamate transporter 2–containing boutons derived from unknown populations of glutamatergic interneuron (GLU IN). NK1r-expressing lamina I projection neurons also receive input from glutamatergic vertical cells (GLU Vertical cell), which are innervated by glutamatergic central cells (GLU Central cell). The primary afferents that synapse onto vertical cells include Aδ fibers, as well as C fibers that express both transient receptor potential A1 (TRPA1) and transient receptor potential vanilloid 1 (TRPV1). (Modified from Todd AJ 2008 Neuronal circuits and receptors involved in spinal pain processing. In: Castro-Lopes J (ed) Current topics in pain: 12th World Congress on Pain, Glasgow, UK. Seattle, Wash., IASP Press, p 25–51, Fig. 3.)
Mantyh and associates (1997) showed that NK1 receptor–expressing neurons in the dorsal horn can be selectively destroyed by the intrathecal injection of substance P conjugated to the cytotoxin saporin. Rats treated in this way show normal responses to acute noxious stimuli but dramatically reduced signs of hyperalgesia following an inflammatory stimulus. In contrast, mice lacking either the NK1 receptor or the preprotachykinin gene (from which substance P is derived) show little reduction in signs of hyperalgesia in inflammatory models (Cao et al 1998, De Felipe et al 1998), and NK1 receptor antagonists have proved disappointing as analgesics (Hill 2000). This suggests that NK1 receptor–expressing cells are essential for the development of hyperalgesia but that this effect is not mediated through the NK1 receptor. It is likely that glutamatergic transmission from substance P–containing nociceptive primary afferents to NK1 receptor–expressing projection neurons plays a central role in the development of hyperalgesia.
There is a population of large lamina I projection cells that lack the NK1 receptor and can be recognized by the very high density of excitatory and inhibitory synapses on their cell bodies and dendritic trees. These “giant” cells, which correspond in part to Waldeyer cells, were initially identified by the presence of numerous synapses containing the glycine receptor–associated protein gephyrin (Puskár et al 2001). Though very distinctive, they are infrequent and make up only 2–3% of the projection neurons in lamina I. Unlike the NK1 receptor–expressing projection neurons, these cells appear to receive little direct synaptic input from primary afferents (Polgár et al 2008a).
Less is known about the role of other lamina I projection neurons that do not express the NK1 receptor. Although some of these cells respond to noxious stimulation (Todd et al 2002), it is likely that this population also includes neurons selectively activated by innocuous thermal stimuli (e.g., Dostrovsky and Craig, 1996).
Recent studies have investigated the expression of AMPA receptor subunits by dorsal horn projection neurons. AMPA receptors are tetramers consisting of four subunits (GluR1–4). Subunit composition affects the properties of the receptor; for example, those lacking GluR2 are Ca2+ permeable, whereas either GluR1 or GluR4 is required for activity-dependent insertion during long-term potentiation. Conventional immunocytochemical techniques do not reveal synaptic receptors because of the cross-linking of proteins at glutamatergic synapses during fixation (Nagy et al 2004a). Polgár and colleagues (2010b) used an antigen retrieval technique to reveal synaptic AMPA receptors and reported that NK1 receptor–expressing lamina I projection neurons could be divided into two distinct classes: the smaller ones possess GluR1-containing (but not GluR4-containing) receptors, whereas the large ones have AMPA receptors that contain GluR4 but not GluR1. Previous studies had shown that the giant lamina I cells (Polgár et al 2008a) and the NK1 receptor–expressing lamina III projection neurons (Todd et al 2009) also have GluR4-containing AMPA receptors. Since interneurons in the superficial laminae are thought to express GluR1-containing receptors (see later), it appears that the GluR4 subunit is restricted to projection neurons in the superficial laminae.
Spinal Interneurons
Interneurons make up the great majority of the neuronal population throughout the dorsal horn. The packing density of neurons is particularly high in laminae I–III (see Fig. 5-1), and therefore this region contains a very large number of interneurons. Szentágothai (1964) proposed that laminae I–III represent a “closed system,” with the axons of intrinsic neurons either terminating locally or else extending a few segments rostrally or caudally in Lissauer’s tract before re-entering the superficial dorsal horn. This suggestion provided one of the first important insights into the function of the superficial dorsal horn, although it has had to be modified with the discovery that some interneurons in these laminae send their axons to other parts of the spinal cord (see later).
Axonal Projections of Interneurons
Results from anatomical studies using the Golgi technique or from electrophysiological studies in which individual cells have been labeled have shown that interneurons in laminae I–III give rise to axons that arborize in the same segment and generally close to the cell body (e.g., Beal and Cooper 1978, Gobel 1978, Light et al 1979, Bennett et al 1980, Rèthelyi et al 1989, Schneider 1992, Grudt and Perl 2002, Yasaka et al 2010). Bice and Beal (1997) reported that following injection of a retrograde tracer into the mid-thoracic spinal cord, 7% of lamina II neurons in the L1 segment were labeled retrogradely, which suggests that a significant proportion of cells in this lamina also have long propriospinal projections. Less is known about the intersegmental connections of interneurons in other laminae, although Schneider (1992) identified two populations of small neurons (presumed interneurons) in lamina III–V: cells with short axons that arborize close to the cell body (local axon cells) and those with axons that pass ventral to the soma and bifurcate into long branches that extend beyond the segment of origin and have few local axon collaterals (deep axon cells).
The studies just referred to have shown that many dorsal horn interneurons have axons that remain in the same lamina as the cell body, but it is also common for cells to give rise to axons that extend into other laminae. For example, some lamina II interneurons have axons that enter lamina I or else pass ventrally into laminae III–V (Gobel 1978, Light and Kavookjian 1988, Grudt and Perl 2002, Yasaka et al 2010).
Classification of Interneurons
Inhibitory and Excitatory Interneurons
Interneurons in the dorsal horn can be divided into two main functional types: inhibitory cells, which release GABA and/or glycine, and excitatory glutamatergic cells. The cell bodies of inhibitory interneurons can be identified by immunocytochemistry with antibodies against GABA or glycine (Todd and Sullivan 1990, Polgár et al 2003). Although glycine has other functions apart from acting as an inhibitory neurotransmitter, there is good evidence that it is present at much higher concentration in glycinergic neurons than in other cell types. At the electron microscopic level these antibodies can be used to detect GABAergic and glycinergic axon terminals (e.g., Todd 1996). GABAergic axons can also be identified with antibodies against the GABA-synthesizing enzyme glutamic acid decarboxylase (GAD) (McLaughlin et al 1975), and glycinergic axons can be identified by the presence of the glycine transporter GLYT2 (Spike et al 1997). There are two isoforms of GAD, known as GAD65 and GAD67 on the basis of their molecular weights, and both are present in GABAergic axons in the dorsal horn (Mackie et al 2003). Axons that use GABA and/or glycine can also be detected with antibodies against the vesicular GABA transporter (VGAT), which transports both transmitters into synaptic vesicles.
Immunocytochemical studies have shown that GABAergic neurons and axon terminals are present in large numbers throughout the dorsal horn. Glycinergic neurons and terminals, however, are much more common in the deeper laminae (III–VI) (Todd and Sullivan 1990). All projection neurons in the dorsal horn are thought to be glutamatergic, and therefore all the GABAergic and glycinergic neurons in the dorsal horn are presumably interneurons. GABAergic cells make up 25–30% of the neurons in laminae I–II and around 40% of those in lamina III (Todd and Sullivan 1990, Polgár et al 2003). Immunocytochemical studies have suggested that virtually all the glycinergic neurons in laminae I–III are also GABAergic, although in deeper laminae there are neurons that are glycinergic but not GABAergic (Todd and Sullivan 1990). GABA and glycine are also co-localized in many axon terminals in the dorsal horn (Todd et al 1996). However, even though there is physiological evidence that the two transmitters can be co-released, it has been suggested that the distribution of GABAA and glycine receptors means that at many synapses in the dorsal horn, only one transmitter is functional even when both are released (Chéry and De Koninck 1999).
Although the great majority of the GABAergic and glycinergic axons in the dorsal horn are probably derived from local inhibitory interneurons, some are descending fibers with cell bodies in the brain. For example, Antal and colleagues (1996) identified a system of spinally projecting GABAergic axons originating from cells in the region of the medullary raphe nuclei.
Most GABAergic and glycinergic axons form axodendritic or axosomatic synapses onto dorsal horn neurons, and these generate post-synaptic inhibition. However, some form axo-axonic synapses onto primary afferent terminals and are responsible for GABA-mediated presynaptic inhibition.
An important insight into the roles of GABA and glycine in spinal cord sensory processing came from the studies of Yaksh (1989), who showed that intrathecal administration of GABAA or glycine receptor antagonists to rats led to an aversive response to brushing of hairs, which was thought to correspond to tactile allodynia. Sivilotti and Woolf (1994) subsequently reported that spinal administration of these antagonists also lowered the mechanical threshold for eliciting a flexion withdrawal reflex in decerebrate rats. These findings suggest that GABAergic and glycinergic inhibition in the dorsal horn reduces the ability of low-threshold mechanoreceptive afferents to activate pathways that convey nociceptive information to the brain and to circuits involved in withdrawal reflexes. Loss of this inhibition, which is presumably mediated mainly by local interneurons, would lead to allodynia, as proposed in the Gate Theory of Pain (Melzack and Wall 1965). Sandkühler (2009) has recently identified several functions of inhibitory synapses in the dorsal horn: attenuation of responses of nociceptive neurons, silencing of these neurons in the absence of noxious stimuli, prevention of crosstalk between sensory modalities, and restriction of the spread of activity to somatotopically appropriate areas. These functions are likely to be brought about by neuronal circuits involving specific populations of inhibitory interneurons. There is also evidence that a distinct population of inhibitory interneurons in the superficial laminae has a role in the suppression of itch (Ross et al 2010).
Until recently it has proved difficult to identify glutamatergic interneurons or their axons. It has been assumed that most (if not all) of the cells that did not use GABA or glycine as a transmitter were glutamatergic, and this has been confirmed directly by demonstrating the presence of VGLUT2 in their axons (Yasaka et al 2010). Most of the plexus of VGLUT2-containing axons in the dorsal horn is likely to originate from excitatory interneurons since VGLUT2 immunoreactivity has been found in the axons of many interneurons in the superficial laminae (Maxwell et al 2007, Yasaka et al 2010). We know little about the roles of these cells, mainly because of the difficulty in distinguishing between the action of glutamate released from excitatory interneurons and that released from primary afferents. However, it is now possible to investigate the synaptic connections formed by glutamatergic interneurons and thus their involvement in neuronal circuits within the dorsal horn.
Morphological Classification
Since the morphology of neurons is often closely related to function in other parts of the central nervous system, numerous attempts have been made to classify interneurons in the dorsal horn based on the shapes of their cell bodies and dendritic trees. These attempts, which have particularly concentrated on lamina II, have been partially successful in that certain morphological types of neurons have been consistently identified. However, they have failed to produce a comprehensive classification scheme. The most widely accepted scheme for lamina II interneurons is that of Grudt and Perl (2002), who identified four main morphological classes (Fig. 5-9A). Islet and central cells both have rostrocaudally elongated dendritic trees, with those of islet cells being much more extensive. Radial cells have short radiating dendrites, which give rise to a compact dendritic tree. All these types are found throughout the depth of lamina II. Vertical cells are typically located in lamina IIo and have dendrites that pass ventrally and often extend into lamina III. Many of the vertical cells have numerous dendritic spines, and these cells had previously been recognized as a distinct class known as stalked cells (Gobel 1978).
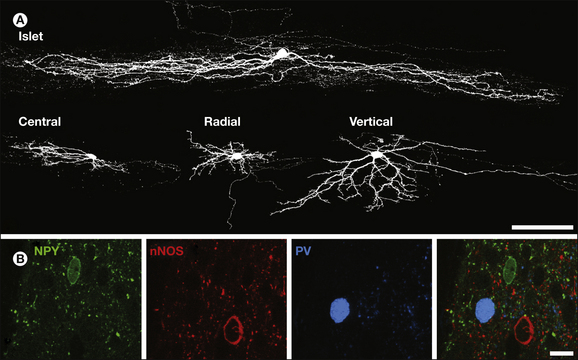
Figure 5-9 Examples of anatomical methods that have been used to classify interneurons.
A, Confocal images of four lamina II neurons labeled with Neurobiotin following whole-cell patch-clamp recording. The cells are seen in sagittal sections and correspond to each of the main classes identified by Grudt and Perl. Islet cells have dendritic trees that are elongated in the rostrocaudal axis with little dorsoventral or mediolateral spread. Central cells are similar but have much shorter dendritic trees. Radial cells have compact dendritic trees with primary dendrites radiating in several directions. Vertical cells typically have a dorsally placed soma and dendrites that fan out ventrally, often occupying a conical shape. Scale bar, 100 µm. B, This image shows a confocal optical slice through part of lamina II in a section immunostained to reveal neuropeptide Y (NPY, green), the neuronal form of nitric oxide synthase (nNOS, red), and parvalbumin (PV, blue), compounds that are present in the dorsal horn and found in non-overlapping groups of inhibitory interneurons. A single cell body containing each compound is visible. Scale bar, 10 µm. (A from Yasaka T, Tiong SYX, Hughes DI, et al 2010 Populations of inhibitory and excitatory interneurons in lamina II of the adult rat spinal dorsal horn revealed by a combined electrophysiological and anatomical approach. Pain 151:475–488; B from Todd A J 2010 Neuronal circuitry for pain processing in the dorsal horn. Nature Reviews of Neuroscience 11:823–836.)
Gobel had suggested that islet cells were inhibitory whereas stalked cells were excitatory interneurons that conveyed input from primary afferents terminating in lamina II to projection neurons in lamina I. Recent electrophysiological studies that have combined whole-cell patch-clamp recording with morphological analysis and post hoc identification of neurotransmitter phenotype have provided further insight into the relationship between structure and function (Maxwell et al 2007, Yasaka et al 2010). The results of these and previous studies (Todd and Spike 1993) indicate that although morphology is related to transmitter content for lamina II neurons, the relationship is not straightforward. Islet cells are invariably GABAergic, whereas radial cells and most vertical cells are glutamatergic. However, some small vertical cells are GABAergic, and central cells can be of either type. A further limitation of this approach is that a substantial proportion of lamina II interneurons (≈30% in most studies) cannot be assigned to any of the recognized morphological classes. Yasaka and co-workers (2010) found a clear relationship between neurotransmitter content and the firing pattern of cells in response to injection of depolarizing current. The gap, delayed and reluctant firing patterns (which have been attributed to the presence of A-type potassium, IA, channels) were associated with most excitatory but with few inhibitory interneurons.
Less is known about the morphological classes of interneurons in other laminae, although it has been reported that many of those in lamina III with rostrocaudally orientated dendrites are GABAergic whereas cells with dorsally directed dendrites are not (Todd and Spike 1993). Schneider and Walker (2007) reported that glutamatergic interneurons in laminae III and IV of the hamster spinal cord were morphologically diverse and included both local and deep axon varieties. Lamina I neurons have been allocated to three main morphological types—fusiform, pyramidal, and multipolar—although a complication here is that this classification has been applied to both projection neurons and interneurons. Prescott and De Koninck (2002) reported a clear correlation between morphology and firing pattern for a sample of small neurons (presumably interneurons) in lamina I. However, we know little about the relationship between either of these parameters and neurotransmitter phenotype for lamina I interneurons.
Neurochemical Classification
An alternative approach to classification is based on the use of immunocytochemistry to detect neurochemical markers such as neuropeptides, calcium-binding proteins, enzymes, and receptors that are expressed by discrete populations of neurons in laminae I–III (Todd and Spike 1993, Todd 2010) (Table 5-1). Several neuropeptides can be detected in dorsal horn neurons, particularly in laminae I and II. Neuropeptide concentrations are often low (and may be undetectable) in cell bodies, and for some neuropeptides, antibodies against precursor proteins have therefore been used to reveal the cells that express them.
Table 5-1
Localization of Various Neuropeptides and Proteins in Different Classes of Interneurons in Laminae I–III of the Dorsal Horn
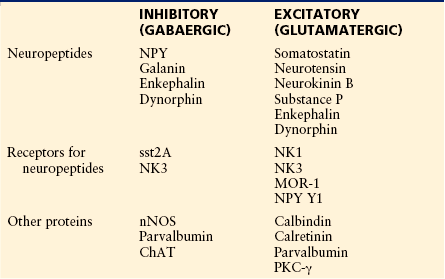
For further details and references, see text.
ChAT, choline acetyltransferase; MOR-1, μ-opioid receptor 1; NK1, neurokinin 1; nNOS, neuronal form of nitric oxide synthase; NPY, neuropeptide Y; PKC-γ, protein kinase C-γ.
It has been shown that all the neurons in the superficial dorsal horn that contain neuropeptide Y (NPY) or galanin are GABAergic whereas somatostatin, neurotensin, and neurokinin B (NKB) are restricted to glutamatergic cells (Todd and Spike 1993, Todd et al 2003, Todd 2010). Although immunocytochemical studies have failed to detect substance P–containing cell bodies in the dorsal horn, axons of local neurons (which can be detected by the presence of substance P and lack of CGRP) are invariably VGLUT2 immunoreactive (Todd et al 2003). This suggests that substance P is restricted to glutamatergic neurons, and these probably include both interneurons and projection cells (Blomqvist and Mackerlova 1995). Many neurons in laminae I and II contain the enkephalin peptides, and these include both glutamatergic and GABAergic cells. Similarly, dynorphin is expressed by both glutamatergic and GABAergic neurons (Marvizón et al 2009, Sardella et al 2011a). Interestingly, NPY and galanin are restricted to GABAergic cells that are not also glycinergic, and most or all of the galanin-containing cells also express dynorphin (Sardella et al 2011a).
Because of the large number of neuropeptides in the superficial laminae of the dorsal horn, it is not surprising that several neuropeptide receptors are expressed by neurons in this region. These include the NK1 and NK3 tachykinin receptors, μ-opioid receptor 1 (MOR-1), the somatostatin receptor sst2A, and the NPY receptor Y1. Although the NK1 receptor is present on many projection neurons in laminae I, III, and IV, the majority of cells with this receptor are interneurons and can be distinguished by their smaller size (Al Ghamdi et al 2009). The other peptide receptors listed are found mainly on interneurons in the superficial dorsal horn. MOR-1 and the NK1 receptor are virtually restricted to glutamatergic cells but are present on different populations of neurons (Spike et al 2002). The Y1 receptor is found on several populations, including excitatory interneurons and projection cells (Brumovsky et al 2006). In contrast, the sst2A receptor is present only on GABA-immunoreactive neurons (Todd et al 1998, Yasaka et al 2010). The NK3 receptor is expressed by interneurons that contain the neuronal form of nitric oxide synthase (nNOS), which are presumed to be inhibitory, and also by MOR-1–immunoreactive (excitatory) interneurons (Seybold et al 1997, Ding et al 2002). μ-Opioid receptors in the spinal cord have attracted attention because of their role in opiate analgesia. Antibodies against MOR-1 produce strong staining in laminae I and II, part of which is on primary afferents and part on intrinsic spinal neurons. Although electrophysiological studies generally report that a high proportion of neurons in the superficial dorsal horn are hyperpolarized by μ-opioid agonists (e.g., Yoshimura and North 1983), Spike and colleagues (2002) estimated that only ≈10% of lamina II neurons are MOR-1–immunoreactive. Part of this discrepancy may be caused by the presence of other splice variants of MOR-1.
Various other proteins are present in restricted populations of neurons in laminae I–III, and some of these are so widely distributed throughout the cytoplasm or on the plasma membrane that immunocytochemistry can be used to reveal the morphology of the neurons, thus making them useful markers for anatomical studies. The calcium-binding proteins calbindin D28k and parvalbumin are present in largely non-overlapping neuronal populations. Calbindin-containing cells are nearly all excitatory, whereas the majority of cells with parvalbumin are inhibitory interneurons that use GABA and glycine (Antal et al 1991, Laing et al 1994). Another calcium-binding protein, calretinin, is found in many neurons in laminae I–II (Ren and Ruda 1992), and these neurons are thought to be mainly glutamatergic (Albuquerque et al 1999).
nNOS is present in numerous GABAergic neurons in laminae I–III, many of which are also glycinergic (Spike et al 1993, Sardella et al 2011b). Cholinergic neurons are scattered throughout the deeper laminae (III–VI) (Barber et al 1984). All those in lamina III contain both GABA and nNOS but are not glycinergic (Spike et al 1993). Each of the neuronal isoforms of protein kinase C (PKC) has been found in neurons in the superficial laminae, and PKC-γ has attracted particular attention since the demonstration that mice deficient in this enzyme fail to develop neuropathic pain after peripheral nerve injury (Malmberg et al 1997). Neurons with high levels of PKC-γ are concentrated in the ventral half of lamina II but are also fairly numerous in lamina III. Most of these cells are not GABAergic, and many contain neurotensin, somatostatin, or NKB (Polgár et al 1999a). In contrast, PKCβII is expressed by many GABAergic interneurons in lamina II (Heinke et al 2004).
The large number of neurochemical markers in laminae I–III indicates the need for caution when using the expression of individual neuropeptides or proteins to define populations. Some of these compounds are present in a relatively high proportion of neurons or are expressed by both excitatory and inhibitory interneurons, thus suggesting that they do not define discrete populations. On the other hand, certain markers show a restricted distribution and occur in non-overlapping groups, and these may represent functional populations. For example, we have found that among the inhibitory interneurons in laminae I–III, NPY, galanin, nNOS, and parvalbumin are present in separate populations (Laing et al 1994, Tiong et al 2011) (Fig. 5-9B). The NPY-, galanin-, and nNOS-containing cells constitute around two-thirds of the inhibitory interneurons in lamina I and nearly half of those in lamina II (Sardella et al 2011b).
This account of interneurons in the dorsal horn demonstrates that there is a high degree of complexity since several different populations can be identified by using either morphological or neurochemical criteria. In future studies it will be important to integrate the results of these two different approaches with physiological data obtained from single-cell recording in an attempt to develop a satisfactory functional classification scheme for dorsal horn interneurons.
Synaptic Circuits Involving Interneurons
At present, our knowledge of the synaptic circuits involving interneurons is still limited, although recent studies have begun to shed light on their primary afferent input, their interconnections, and their output to projection cells and primary afferents. Some of the neuronal circuits that have been identified are shown in Figure 5-8.
The primary afferent input to different classes of lamina II interneurons has been examined in combined electrophysiological and morphological studies (Grudt and Perl 2002, Lu and Perl 2003, 2005, Yasaka et al 2007, Zheng et al 2010). These studies have shown that islet and central cells receive their main primary afferent input from unmyelinated fibers whereas radial and vertical cells are innervated by both Aδ and C afferents. However, this approach does not differentiate between different types of C fibers or between nociceptive and hair follicle Aδ afferents. Uta and co-authors (2010) reported that C fibers expressing both transient receptor potential A1 (TRPA1) and TRPV1 are presynaptic to most vertical and radial cells, but not to islet or central cells. PKC-γ–expressing excitatory interneurons in laminae IIi and III receive at least part of their primary afferent input from low-threshold mechanoreceptive myelinated fibers (Neumann et al 2008).
Electrophysiological studies involving paired recordings have begun to reveal the organization of synaptic circuits linking different types of interneurons in lamina II. These studies have identified inhibitory input from islet to central cells and excitatory input from central to vertical cells (Lu and Perl, 2003, 2005). A recent study by Zheng and colleagues (2010) examined a population of lamina II inhibitory central cells and showed that these cells could be linked through GABAergic synapses to islet cells (in some cases with reciprocal synapses) and were also presynaptic to vertical cells.
Two populations of inhibitory interneurons that innervate spinal projection neurons have been identified. GABAergic axons that contain NPY and are presumably derived from interneurons in laminae I–III form numerous synapses with NK1 receptor–immunoreactive projection neurons in laminae III and IV, but not with another type of projection cell in this region, post-synaptic dorsal column cells (Polgár et al 1999b) (Fig. 5-10). In contrast, the giant lamina I projection cells are often densely innervated by axons containing nNOS and GABA (again, presumably originating from local interneurons), which provide around a quarter of their GABAergic input. Less is known about the excitatory interneurons that are presynaptic to projection cells, although Lu and Perl (2005) have identified synaptic input from glutamatergic vertical cells in lamina II that were innervated by Aδ primary afferents to lamina I projection neurons with the NK1 receptor. The giant lamina I projection neurons are densely innervated by VGLUT2-containing boutons that probably originate from local excitatory interneurons (Polgár et al 2008a), although the types of interneurons and their laminar location are not yet known.
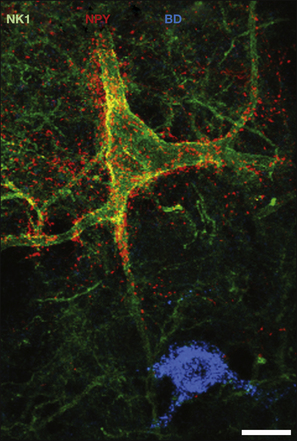
Figure 5-10 Confocal images showing the association between neuropeptide Y (NPY)-containing axons (red) and the cell body and the proximal dendrites of a neurokinin 1 (NK1) receptor–immunoreactive (green) projection neuron in lamina III.
This field, from a parasagittal section of a rat spinal cord, also contains the cell body of a post-synaptic dorsal column (PSDC) neuron that was retrogradely labeled with biotin dextran (BD, blue) injected into the gracile nucleus. The NK1 receptor–expressing cell receives numerous contacts from NPY-containing axons, which are thought to be derived from local GABAergic interneurons. In contrast, the PSDC cell receives very few contacts. This indicates the selective input from interneurons to projection cells in the dorsal horn. Scale bar, 20 µm. (From Polgár E, Shehab SAS, Watt C, et al 1999b GABAergic neurons that contain neuropeptide Y selectively target cells with the neurokinin 1 receptor in laminae III and IV of the rat spinal cord. Journal of Neuroscience 19:2637–2646. Copyright 1999 by the Society for Neuroscience.)
Presynaptic inhibition of primary afferents is mediated by axo-axonic synapses, and the axons presynaptic to the central boutons of type I and type II synaptic glomeruli differ since those in type I glomeruli are only GABA immunoreactive whereas those in type II glomeruli often contain GABA and glycine (Todd 1996). This suggests that different populations of GABAergic interneuron are responsible for presynaptic inhibition of non-peptidergic C-fiber nociceptors and D-hair afferents. Cholinergic cells make up a very small proportion of dorsal horn interneurons but apparently contribute a significant component of the peripheral axons in type II glomeruli (Ribeiro-da-Silva and Cuello 1990), which suggests that their axons selectively target D-hair afferent terminals.
These preliminary findings indicate that circuits involving dorsal horn interneurons can be arranged in a selective manner, with each type of interneuron preferentially innervating particular target neurons.
Receptors for the Amino Acid Neurotransmitters on Spinal Neurons
Most studies of receptors for glutamate, GABA, and glycine have not distinguished between projection neurons and interneurons in the dorsal horn, and we will therefore briefly describe the distribution of these receptors in this section.
Glutamate Receptors
All three types of ionotropic glutamate receptor (AMPA, NMDA, and kainate) are present in the dorsal horn, and antigen retrieval techniques have been used to map the distribution of AMPA and NMDA receptor subunits at synapses in this region (Nagy et al 2004a, 2004b, Polgár et al 2008b). AMPA receptors containing GluR2, GluR3, and GluR4 are widely distributed in laminae IV–VI and the ventral horn. In contrast, at most glutamatergic synapses in laminae I–II and at many of those in lamina III, GluR1 replaces GluR4, and it is likely that most (if not all) interneurons in the superficial dorsal horn express GluR1. Virtually all glutamatergic synapses throughout the spinal cord contain GluR2 subunits, and therefore at synapses that possess Ca2+-permeable (GluR2-lacking) AMPA receptors, they will be intermingled with GluR2-containing (Ca2+-impermeable) receptors. Of the NMDA receptor subunits that have been identified at synapses in the dorsal horn, the obligatory NR1 subunit is widespread, while NR2B is found at high concentration in laminae I–II and NR2A in the deeper laminae.
Although less is known about kainate receptors, mRNA for the GluR5, GluR7, KA1, and KA2 subunits has been found in scattered neurons in the superficial laminae.
mRNA for several of the metabotropic glutamate receptors has been identified in dorsal horn neurons, and of these receptors, mGluR5 appears to be the most abundant in the superficial laminae and is expressed by many small neurons in laminae I–III (Jia et al 1999, Alvarez et al 2000).
GABA and Glycine Receptors
GABAA and glycine receptors are widely distributed in the spinal cord and are probably expressed by all dorsal horn neurons. For the GABAA receptor, the β3 and γ2 subunits are present throughout the spinal cord, whereas the α subunits are distributed differentially. The α2 and α3 subunits are found in all dorsal horn laminae, whereas the α1 and α5 subunits are expressed in laminae III–VI but excluded from laminae I–II (Bohlhalter et al 1996). This is consistent with the finding that analgesia can be obtained by activating GABAA receptors containing the α2 and/or α3 subunits (Knabl et al 2008).
Although there are monoclonal antibodies against the main ligand-binding subunit of the glycine receptor (GlyR α1), these antibodies do not work well on fixed tissue, and most immunocytochemical studies designed to investigate glycinergic transmission have been carried out with antibodies against the glycine receptor–associated protein gephyrin. Gephyrin shows a high degree of co-localization with the GlyR α1 subunit in the spinal cord (Todd et al 1996), although it is also expressed at low levels at GABAergic synapses that lack glycine receptors. Synapses with strong gephyrin (or GlyR α1) immunoreactivity are present throughout the dorsal horn but are far more numerous in the deeper laminae (III–VI), thus matching the distribution of glycinergic axon terminals. The GlyR α3 subunit (a target for prostaglandin E2, which suppresses inhibitory post-synaptic currents) has also been identified at inhibitory synapses in lamina II, where it shows some co-localization with GlyR α1 (Harvey et al 2004).
GABAB receptors can be demonstrated in the dorsal horn by radioligand binding, and high levels of the receptor are seen in the superficial laminae. Results from both immunocytochemical and in situ hybridization studies indicate that as well as being present on primary afferent terminals, GABAB receptors are also expressed by dorsal horn neurons (Towers et al 2000).
Descending Monoaminergic Axons
Among the various populations of axons that project from the brain to the spinal cord, the serotoninergic and norepinephrinergic systems have attracted particular attention because of their probable involvement in the modulation of spinal pain mechanisms, including stimulation-induced analgesia (see Chapter 8). Spinal serotoninergic axons originate in the medullary raphe nuclei, whereas those that contain norepinephrine are derived from the locus coeruleus and adjacent areas of the pons. Serotonin-containing axons are widely distributed throughout the dorsal horn but are most numerous in laminae I and IIo (Ruda et al 1982). Norepinephrine-containing axons are found throughout the dorsal horn, but at relatively high density in laminae I and II (Westlund and Coulter 1980).
Since monoamines are likely to act through volume transmission, it is more important to know about the cellular distribution of their receptors than about contacts or synapses formed by the monoaminergic axons themselves. Although various types of monoamine receptors have been identified with radioligand binding and in situ hybridization (Coggeshall and Carlton 1997), we have little information about the cell types that express these receptors. The 5-HT3 receptor is found on numerous axon terminals in laminae I and II, and it is likely that at least some of these axon terminals belong to excitatory interneurons (Maxwell et al 2003), while others are of primary afferent origin. Both α2A- and α2C-adrenergic receptors are present on axons in the superficial laminae (Stone et al 1998). Axons with α2A are primary afferents, whereas those with α2C appear to be derived from glutamatergic interneurons (Olave and Maxwell 2003a). Interestingly, the axons of these interneurons form synapses with NK1 receptor–immunoreactive projection neurons in lamina I (Olave and Maxwell 2003b), which suggests that part of the antinociceptive action of norepinephrine may result from suppression of transmission between excitatory interneurons and projection cells.
Conclusion
Despite substantial recent advances in our understanding of the neuroanatomy of the spinal dorsal horn, many important issues remain to be addressed. Our knowledge of the circuitry within the dorsal horn is still fragmentary. For example, little is known about the post-synaptic targets of the different functional classes of nociceptive primary afferent. We also have limited information about the involvement of interneurons in the neuronal circuitry and thus about their roles in sensory processing. Although pharmacological studies have provided some insight into the part played by inhibitory interneurons, we know very little about the functions of excitatory interneurons, even though they make up the majority of the neuronal population in the superficial laminae. The excitatory interneurons may be involved in distributing information from particular types of primary afferent to different segments or to other laminae, but it is likely that they also have other functions in sensory processing. The recent finding that these cells show IA currents (Yasaka et al 2010) suggests that they may have an important role in dorsal horn plasticity in inflammatory pain states because the Kv4.2 channel, which is thought to underlie these currents (Hu et al 2006), is a downstream target for phosphorylation by extracellular signal–regulated kinases, which are activated in lamina II neurons following noxious stimulation (Ji et al 1999).
Most neuroanatomical studies of the dorsal horn have concentrated on the superficial laminae, mainly because this region is the principal termination zone for nociceptive primary afferents, but also because the neurochemistry of the region provides useful intrinsic markers for different neuronal populations. However, many neurons in the deeper laminae of the dorsal horn respond to noxious stimuli, and this area includes a significant number of projection neurons. The deep laminae clearly play an important role in pain mechanisms, and a great deal more work is therefore needed to unravel the neuronal and synaptic organization of this region. Unfortunately, the neurochemical approaches that have proved useful in studies of the superficial laminae may be less helpful here.
The superficial dorsal horn contains a wide range of neuropeptides, which are present in the axons of both primary afferents and interneurons, and many of the corresponding receptors have been identified on specific populations of neurons in this region. Interestingly, many of these peptides and some of their receptors can be either up- or down-regulated in pathological conditions. Although substance P and the NK1 receptor have been implicated in nociception, there is still controversy about their precise role. Even less is known about the functions of the other peptides or their receptors.
NMDA receptors are thought to play a major role in both induction and maintenance of chronic pain. Although it is known that NMDA receptor subunits are expressed by many (if not all) spinal neurons, we know little about the types of synapses at which they are present. This issue clearly needs to be addressed if we are to understand the mechanisms that underlie chronic pain. It has been suggested that changes in ionotropic glutamate receptor function at synapses within the dorsal horn may be important in pain states. The demonstration of rapid phosphorylation of synaptic GluR1 subunits in response to noxious stimulation (Nagy et al 2004a) opens up the possibility of investigating plasticity at the level of individual glutamatergic synapses.
Acknowledgment
Work in the authors’ laboratories is supported by grants from the Wellcome Trust (AJT) and the National Institute of Neurological Disorders and Stroke of the National Institutes of Health (HRK), which are gratefully acknowledged.
The references for this chapter can be found at www.expertconsult.com.
References
Albers K.M., Woodbury C.J., Ritter A.M., et al. Glial cell-line–derived neurotrophic factor expression in skin alters the mechanical sensitivity of cutaneous nociceptors. Journal of Neuroscience. 2006;26:2981–2990.
Albuquerque C., Lee C.J., Jackson A.C., et al. Subpopulations of GABAergic and non-GABAergic rat dorsal horn neurons express Ca2+-permeable AMPA receptors. European Journal of Neuroscience. 1999;11:2758–2766.
Al Ghamdi K.S., Polgár E., Todd A.J. Soma size distinguishes projection neurons from NK1 receptor–expressing interneurons in lamina I of the rat lumbar spinal dorsal horn. Neuroscience. 2009;164:1794–1804.
Al-Khater K.M., Kerr R., Todd A.J. A quantitative study of spinothalamic neurons in laminae I, III, and IV in lumbar and cervical segments of the rat spinal cord. Journal of Comparative Neurology. 2008;511:1–18.
Alvarez F.J., Kavookjian A.M., Light A.R. Synaptic interactions between GABA-immunoreactive profiles and the terminals of functionally defined myelinated nociceptors in the monkey and cat spinal cord. Journal of Neuroscience. 1992;12:2901–2917.
Alvarez F.J., Villalba R.M., Carr P.A., et al. Differential distribution of metabotropic glutamate receptors 1a, 1b, and 5 in the rat spinal cord. Journal of Comparative Neurology. 2000;422:464–487.
Alvarez F.J., Villalba R.M., Zerda R., et al. Vesicular glutamate transporters in the spinal cord, with special reference to sensory primary afferent synapses. Journal of Comparative Neurology. 2004;472:257–280.
Andrew D., Craig A.D. Spinothalamic lamina I neurons selectively sensitive to histamine: a central neural pathway for itch. Nature Neuroscience. 2001;4:72–77.
Antal M., Petko M., Polgár E., et al. Direct evidence of an extensive GABAergic innervation of the spinal dorsal horn by fibres descending from the rostral ventromedial medulla. Neuroscience. 1996;73:509–518.
Antal M., Polgár E., Chalmers E., et al. Different populations of parvalbumin- and calbindin-D28k-immunoreactive neurons contain GABA and accumulate 3H-d-aspartate in the dorsal horn of the rat spinal cord. Journal of Comparative Neurology. 1991;314:114–124.
Averill S., McMahon S.B., Clary D.O., et al. Immunocytochemical localization of trkA receptors in chemically identified subgroups of adult rat sensory neurons. European Journal of Neuroscience. 1995;7:1484–1494.
Barber R.P., Phelps P.E., Houser C.R., et al. The morphology and distribution of neurons containing choline acetyltransferase in the adult rat spinal cord: an immunohistochemical study. Journal of Comparative Neurology. 1984;229:329–346.
Beal J.A., Cooper M.H. The neurons of the gelatinosal complex (laminae II and III) of the monkey (Macaca mulatta): a Golgi study. Journal of Comparative Neurology. 1978;179:89–122.
Belmonte C., Gallego R. Membrane properties of cat sensory neurones with chemoreceptor and baroreceptor endings. Journal of Physiology. 1983;342:603–614.
Bennett D.L., Dmietrieva N., Priestley J.V., et al. trkA, CGRP and IB4 expression in retrogradely labelled cutaneous and visceral primary sensory neurones in the rat. Neuroscience Letters. 1996;206:33–36.
Bennett D.L., Michael G.J., Ramachandran N., et al. A distinct subgroup of small DRG cells express GDNF receptor components and GDNF is protective for these neurons after nerve injury. Journal of Neuroscience. 1998;18:3059–3072.
Bennett G.J., Abdelmoumene M., Hayashi H., et al. Physiology and morphology of substantia gelatinosa neurons intracellularly stained with horseradish peroxidase. Journal of Comparative Neurology. 1980;194:809–827.
Bessou P., Perl E.R. Response of cutaneous sensory units with unmyelinated fibers to noxious stimuli. Journal of Neurophysiology. 1969;32:1025–1043.
Bice T.N., Beal J.A. Quantitative and neurogenic analysis of the total population and subpopulations of neurons defined by axon projection in the superficial dorsal horn of the rat lumbar spinal cord. Journal of Comparative Neurology. 1997;388:550–564.
Blomqvist A., Mackerlova L. Spinal projections to the parabrachial nucleus are substance P–immunoreactive. Neuroreport. 1995;6:605–608.
Bohlhalter S., Weinmann O., Mohler H., et al. Laminar compartmentalization of GABAA-receptor subtypes in the spinal cord: an immunohistochemical study. Journal of Neuroscience. 1996;16:283–297.
Bradbury E.J., Burnstock G., McMahon S.B. The expression of P2X3 purinoceptors in sensory neurons: effects of axotomy and glial-derived neurotrophic factor. Molecular and Cellular Neuroscience. 1998;12:256–268.
Broman J., Anderson S., Ottersen O.P. Enrichment of glutamate-like immunoreactivity in primary afferent terminals throughout the spinal cord dorsal horn. European Journal of Neuroscience. 1993;5:1050–1061.
Brown A.G. The dorsal horn of the spinal cord. Quarterly Journal of Experimental Physiology. 1982;67:193–212.
Brumovsky P., Hofstetter C., Olson L., et al. The neuropeptide tyrosine Y1R is expressed in interneurons and projection neurons in the dorsal horn and area X of the rat spinal cord. Neuroscience. 2006;138:1361–1376.
Brumovksy P., Watanabe M., Hökfelt T. Expression of the vesicular glutamate transporters-1 and -2 in adult mouse dorsal root ganglia and spinal cord and their regulation by nerve injury. Neuroscience. 2007;147:467–490.
Brushart T.M., Henry E.W., Mesulam M.M. Reorganization of muscle afferent projections accompanies peripheral nerve regeneration. Neuroscience. 1981;6:2053–2061.
Burgess P.R., Perl E.R. Myelinated afferent fibres responding specifically to noxious stimulation of the skin. Journal of Physiology. 1967;190:541–562.
Cajal S.R. Histologie du système nerveux de l`homme et des vertébrés. Paris: Maloine; 1909.
Campbell J.N., Meyer R.A., LaMotte R.H. Sensitization of myelinated nociceptive afferents that innervate monkey hand. Journal of Neurophysiology. 1979;42:1669–1679.
Campbell J.N., Raja S.N., Meyer R.A., et al. Myelinated afferents signal the hyperalgesia associated with nerve injury. Pain. 1988;32:89–94.
Cao Y.Q., Mantyh P.W., Carlson E.J., et al. Primary afferent tachykinins are required to experience moderate to intense pain. Nature. 1998;392:390–394.
Caterina M.J., Leffler A., Malmberg A.B., et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313.
Caterina M.J., Schumacher M.A., Tominaga M., et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824.
Chéry N., De Koninck Y. Junctional versus extrajunctional glycine and GABA(A) receptor–mediated IPSCs in identified lamina I neurons of the adult rat spinal cord. Journal of Neuroscience. 1999;19:7342–7355.
Coggeshall R.E., Carlton S.M. Receptor localization in the mammalian dorsal horn and primary afferent neurons. Brain Research Reviews. 1997;24:28–66.
Craig A.D. Distribution of brainstem projections from spinal lamina I neurons in the cat and monkey. Journal of Comparative Neurology. 1995;361:225–248.
Craig A.D., Mense S. The distribution of afferent fibers from the gastrocnemius-soleus muscle in the dorsal horn of the cat, as revealed by the transport of horseradish peroxidase. Neuroscience Letters. 1983;41:233–238.
De Felipe C., Herrero J.F., O’Brien J.A., et al. Altered nociception, analgesia and aggression in mice lacking the substance P receptor. Nature. 1998;392:394–397.
de Groat W.C., Nadelhaft I., Milne R.J., et al. Organization of the sacral parasympathetic reflex pathways to the urinary bladder and large intestine. Journal of the Autonomic Nervous System. 1981;3:135–160.
Ding Y.-Q., Lu C.R., Wang H., et al. Two major distinct subpopulations of neurokinin-3 receptor–expressing neurons in the superficial dorsal horn of the rat spinal cord. European Journal of Neuroscience. 2002;16:551–556.
Djouhri L., Bleazard L., Lawson S.N. Association of somatic action potential shape with sensory receptive properties in guinea-pig dorsal root ganglion neurones. Journal of Physiology. 1998;513:857–872.
Dostrovsky J.O., Craig A.D. Cooling-specific spinothalamic neurons in the monkey. J Neurophysiol. 1996;76:3656–3665.
Fitzgerald M., Lynn B. The sensitization of high threshold mechanoreceptors with myelinated axons by repeated heating. Journal of Physiology. 1977;265:549–563.
Flores C.M., DeCamp R.M., Kilo S., et al. Neuronal nicotinic receptor expression in sensory neurons of the rat trigeminal ganglion: demonstration of alpha3beta4, a novel subtype in the mammalian nervous system. Journal of Neuroscience. 1996;16:7892–7901.
Gerevich Z., Muller C., Illes P. Metabotropic P2Y1 receptors inhibit P2X3 receptor-channels in rat dorsal root ganglion neurons. European Journal of Pharmacology. 2005;521:34–38.
Gobel S. Golgi studies of the neurons in layer II of the dorsal horn of the medulla (trigeminal nucleus caudalis). Journal of Comparative Neurology. 1978;180:395–414.
Gorke K., Pierau F.K. Spike potentials and membrane properties of dorsal root ganglion cells in pigeons. Pflugers Archiv: European Journal of Physiology. 1980;386:21–28.
Grudt T.J., Perl E.R. Correlations between neuronal morphology and electrophysiological features in the rodent superficial dorsal horn. Journal of Physiology. 2002;540:189–207.
Guo A., Vulchanova L., Wang J., et al. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. European Journal of Neuroscience. 1999;11:946–958.
Haberberger R., Henrich M., Couraud J.Y., et al. Muscarinic M2-receptors in rat thoracic dorsal root ganglia. Neuroscience Letters. 1999;266:177–180.
Habler H.J., Janig W., Koltzenburg M. Myelinated primary afferents of the sacral spinal cord responding to slow filling and distension of the cat urinary bladder. Journal of Physiology. 1993;463:449–460.
Harvey R.J., Depner U.B., Wässle H., et al. GlyR α3: an essential target for spinal PGE2-mediated inflammatory pain sensitization. Science. 2004;304:884–887.
Heinke B., Ruscheweyh R., Forsthuber L., et al. Physiological, neurochemical and morphological properties of a subgroup of GABAergic spinal lamina II neurones identified by expression of green fluorescent protein in mice. Journal of Physiology. 2004;560:249–266.
Hill R.G. NK1 (substance P) receptor antagonists—why are they not analgesic in humans? Trends in Pharmacological Science. 2000;21:244–246.
Hoheisel U., Lehmann-Willenbrock E., Mense S. Termination patterns of identified group II and III afferent fibres from deep tissues in the spinal cord of the cat. Neuroscience. 1989;28:495–507.
Hu H.J., Carrasquillo Y., Karim F., et al. The Kv4.2 potassium channel subunit is required for pain plasticity. Neuron. 2006;50:89–100.
Ji R.R., Baba H.H., Brenner G.J., et al. Nociceptive-specific activation of ERK in spinal neurons contributes to pain hypersensitivity. Nature Neuroscience. 1999;2:1114–1119.
Jia H., Rustioni A., Valtschanoff J.G. Metabotropic glutamate receptors in superficial laminae of the rat dorsal horn. Journal of Comparative Neurology. 1999;410:627–642.
Knabl J., Witschi R., Hösl K., et al. Reversal of pathological pain through specific spinal GABAA receptor subtypes. Nature. 2008;451:330–334.
Knyihar-Csillik E., Csillik B., Rakic P. Periterminal synaptology of dorsal root glomerular terminals in the substantia gelatinosa of the spinal cord in the rhesus monkey. Journal of Comparative Neurology. 1982;210:376–399.
Koerber H.R., Mendell L.M. Functional specialization of central projections from identified primary afferent fibers. Journal of Neurophysiology. 1988;60:1597–1614.
Koerber H.R., Mendell L.M. Functional heterogeneity of dorsal root ganglion cells. In: Scott S.A., ed. Sensory neurons: diversity development and plasticity. New York: Oxford University Press; 1992:77–96.
Koerber H.R., Mirnics K., Mendell L.M. Properties of regenerated primary afferents and their functional connections. Journal of Neurophysiology. 1995;73:693–702.
Koerber H.R., Woodbury C.J. Comprehensive phenotyping of sensory neurons using an ex vivo somatosensory system. Physiology and Behavior. 2002;77:589–594.
Kumazawa T. The polymodal receptor: bio-warning and defense system. Progress in Brain Research. 1996;113:3–18.
Laing I., Todd A.J., Heizmann C.W., et al. Subpopulations of GABAergic neurons in laminae I–III of rat spinal dorsal horn defined by coexistence with classical transmitters, peptides, nitric oxide synthase or parvalbumin. Neuroscience. 1994;61:123–132.
Lawson J.J., McIlwrath S.L., Woodbury C.J., et al. TRPV1 unlike TRPV2 is restricted to a subset of mechanically insensitive cutaneous nociceptors responding to heat. Journal of Pain. 2008;9:298–308.
Lawson S.N., Crepps B.A., Perl E.R. Relationship of substance P to afferent characteristics of dorsal root ganglion neurones in guinea-pig. Journal of Physiology. 1997;505:177–191.
Lawson S.N., Crepps B., Perl E.R. Calcitonin gene–related peptide immunoreactivity and afferent receptive properties of dorsal root ganglion neurones in guinea-pigs. Journal of Physiology. 2002;540:989–1002.
Light A.R., Kavookjian A.M. Morphology and ultrastructure of physiologically identified substantia gelatinosa (lamina II) neurons with axons that terminate in deeper dorsal horn laminae (III–V). Journal of Comparative Neurology. 1988;267:172–189.
Light A.R., Perl E.R. Differential termination of large-diameter and small-diameter primary afferent fibers in the spinal dorsal gray matter as indicated by labeling with horseradish peroxidase. Neuroscience Letters. 1977;6:59–63.
Light A.R., Perl E.R. Spinal termination of functionally identified primary afferent neurons with slowly conducting myelinated fibers. Journal of Comparative Neurology. 1979;186:133–150.
Light A.R., Trevino D.L., Perl E.R. Morphological features of functionally defined neurons in the marginal zone and substantia gelatinosa of the spinal dorsal horn. Journal of Comparative Neurology. 1979;186:151–171.
Light A.R., Wilcockson H.H. Spinal laminae I–II neurons in rat recorded in vivo in whole cell, tight seal configuration: properties and opioid responses. Journal of Neurophysiology. 1999;82:3316–3336.
Ling L.J., Honda T., Shimada Y., et al. Central projection of unmyelinated (C) primary afferent fibers from gastrocnemius muscle in the guinea pig. Journal of Comparative Neurology. 2003;461:140–150.
Lu Y., Perl E.R. A specific inhibitory pathway between substantia gelatinosa neurons receiving direct C-fiber input. Journal of Neuroscience. 2003;23:8752–8758.
Lu Y., Perl E.R. Modular organization of excitatory circuits between neurons of the spinal superficial dorsal horn (laminae I and II). Journal of Neuroscience. 2005;25:3900–3907.
Mackie M., Hughes D.I., Maxwell D.J., et al. Distribution and colocalisation of glutamate decarboxylase isoforms in the rat spinal cord. Neuroscience. 2003;119:461–472.
Malmberg A.B., Chen C., Tonegawa S., et al. Preserved acute pain and reduced neuropathic pain in mice lacking PKCgamma. Science. 1997;278:279–283.
Mantyh P.W., DeMaster E., Malhotra A., et al. Receptor endocytosis and dendrite reshaping in spinal neurons after somatosensory stimulation. Science. 1995;268:1629–1632.
Mantyh P.W., Rogers S.D., Honore P., et al. Ablation of lamina I spinal neurons expressing the substance P receptor profoundly inhibits hyperalgesia. Science. 1997;278:275–279.
Marvizón J.C.G., Chen W., Murphy N. Enkephalins, dynorphins, and β-endorphin in the rat dorsal horn: an immunofluorescence colocalization study. Journal of Comparative Neurology. 2009;517:51–68.
Maxwell D.J., Belle M.D., Cheunsuang O., et al. Morphology of inhibitory and excitatory interneurons in superficial laminae of the rat dorsal horn. Journal of Physiology. 2007;584:521–533.
Maxwell D.J., Kerr R., Rashid S., et al. Characterisation of axon terminals in the rat dorsal horn that are immunoreactive for serotonin 5-HT3A receptor subunits. Experimental Brain Research. 2003;149:114–124.
Maxwell D.J., Rèthelyi M. Ultrastructure and synaptic connections of cutaneous afferent fibres in the spinal cord. Trends in Neurosciences. 1987;10:117–123.
McIlwrath S.L., Lawson J.J., Anderson C.E., et al. Overexpression of neurotrophin-3 enhances the mechanical response properties of slowly adapting type 1 afferents and myelinated nociceptors. European Journal of Neuroscience. 2007;26:1801–1812.
McLaughlin B.J., Barber R.P., Saito K., et al. Immunocytochemical localization of glutamate decarboxylase in rat spinal cord. Journal of Comparative Neurology. 1975;164:305–322.
Melzack R., Wall P.D. Pain mechanisms: a new theory. Science. 1965;150:971–979.
Mesulam M.M., Brushart T.M. Transganglionic and anterograde transport of horseradish peroxidase across dorsal root ganglia: a tetramethylbenzidine method for tracing central sensory connections of muscles and peripheral nerves. Neuroscience. 1979;4:1107–1117.
Meyer R.A., Campbell J.N. A novel electrophysiological technique for locating cutaneous nociceptive and chemospecific receptors. Brain Research. 1988;441:81–86.
Michael G.J., Priestley J.V. Differential expression of the mRNA for the vanilloid receptor subtype 1 in cells of the adult rat dorsal root and nodose ganglia and its downregulation by axotomy. Journal of Neuroscience. 1999;19:1844–1854.
Millecchia R.J., Pubols L.M., Sonty R.V., et al. Influence of map scale on primary afferent terminal field geometry in cat dorsal horn. Journal of Neurophysiology. 1991;66:696–704.
Molander C., Ygge J., Dalsgaard C.J. Substance P-, somatostatin- and calcitonin gene–related peptide-like immunoreactivity and fluoride resistant acid phosphatase-activity in relation to retrogradely labeled cutaneous, muscular and visceral primary sensory neurons in the rat. Neuroscience Letters. 1987;74:37–42.
Molliver D.C., Radeke M.J., Feinstein S.C., et al. Presence or absence of TrkA protein distinguishes subsets of small sensory neurons with unique cytochemical characteristics and dorsal horn projections. Journal of Comparative Neurology. 1995;361:404–416.
Molliver D.C., Wright D.E., Leitner M.L., et al. IB4-binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron. 1997;19:849–861.
Morgan C., Nadelhaft I., de Groat W.C. The distribution of visceral primary afferents from the pelvic nerve to Lissauer’s tract and the spinal gray matter and its relationship to the sacral parasympathetic nucleus. Journal of Comparative Neurology. 1981;201:415–440.
Moriyama T., Iida T., Kobayashi K., et al. Possible involvement of P2Y2 metabotropic receptors in ATP-induced transient receptor potential vanilloid receptor 1–mediated thermal hypersensitivity. Journal of Neuroscience. 2003;23:6058–6062.
Nagy G.G., Al-Ayyan M., Andrew D., et al. Widespread expression of the AMPA receptor GluR2 subunit at glutamatergic synapses in the rat spinal cord and phosphorylation of GluR1 in response to noxious stimulation revealed with an antigen unmasking method. Journal of Neuroscience. 2004;24:5766–5777.
Nagy G.G., Watanabe M., Fukaya M., et al. Synaptic distribution of the NR1, NR2A and NR2B subunits of the NMDA receptor in the rat lumbar spinal cord revealed with an antigen unmasking technique. European Journal of Neuroscience. 2004;20:3301–3312.
Naim M., Spike R.C., Watt C., et al. Cells in laminae III and IV of the rat spinal cord which possess the neurokinin-1 receptor and have dorsally-directed dendrites receive a major synaptic input from tachykinin-containing primary afferents. Journal of Neuroscience. 1997;17:5536–5548.
Nakaya Y., Kaneko T., Shigemoto R., et al. Immunohistochemical localization of substance P receptor in the central nervous system of the adult rat. Journal of Comparative Neurology. 1994;347:249–274.
Neumann S., Braz J.M., Skinner K., et al. Innocuous, not noxious, input activates PKCgamma interneurons of the spinal dorsal horn via myelinated afferent fibers. Journal of Neuroscience. 2008;28:7936–7944.
O’Brien C., Woolf C.J., Fitzgerald M., et al. Differences in the chemical expression of rat primary afferent neurons which innervate skin, muscle or joint. Neuroscience. 1989;32:493–502.
Ohishi H., Nomura S., Ding Y.Q., et al. Presynaptic localization of a metabotropic glutamate receptor, mGluR7, in the primary afferent neurons: an immunohistochemical study in the rat. Neuroscience Letters. 1995;202:85–88.
Olave M.J., Maxwell D.J. An investigation of neurones that possess the α2C-adrenergic receptor in the rat dorsal horn. Neuroscience. 2003;115:31–40.
Olave M.J., Maxwell D.J. Neurokinin-1 projection cells in the rat dorsal horn receive synaptic contacts from axons that possess α2C-adrenergic receptors. Journal of Neuroscience. 2003;23:6837–6846.
Perry M.J., Lawson S.N. Differences in expression of oligosaccharides, neuropeptides, carbonic anhydrase and neurofilament in rat primary afferent neurons retrogradely labelled via skin, muscle or visceral nerves. Neuroscience. 1998;85:293–310.
Plenderleith M.B., Haller C.J., Snow P.J. Peptide coexistence in axon terminals within the superficial dorsal horn of the rat spinal cord. Synapse. 1990;6:344–350.
Polgár E., Al Ghamdi K.S., Todd A.J. Two populations of neurokinin 1 receptor–expressing projection neurons in lamina I of the rat spinal cord that differ in AMPA receptor subunit composition and density of excitatory synaptic input. Neuroscience. 2010;167:1192–1204.
Polgár E., Al-Khater K.M., Shehab S., et al. Large projection neurons in lamina I of the rat spinal cord that lack the neurokinin 1 receptor are densely innervated by VGLUT2-containing axons and possess GluR4-containing AMPA receptors. Journal of Neuroscience. 2008;28:13150–13160.
Polgár E., Fowler J.H., McGill M.M., et al. The types of neuron which contain protein kinase C gamma in rat spinal cord. Brain Research. 1999;833:71–80.
Polgár E., Hughes D.I., Riddell J.S., et al. Selective loss of spinal GABAergic or glycinergic neurons is not necessary for development of thermal hyperalgesia in the chronic constriction injury model of neuropathic pain. Pain. 2003;104:229–239.
Polgár E., Shehab S.A.S., Watt C., et al. GABAergic neurons that contain neuropeptide Y selectively target cells with the neurokinin 1 receptor in laminae III and IV of the rat spinal cord. Journal of Neuroscience. 1999;19:2637–2646.
Polgár E., Watanabe M., Hartmann B., et al. Expression of AMPA receptor subunits at synapses in laminae I–III of the rodent spinal dorsal horn. Molecular Pain. 2008;4:5.
Polgár E., Wright L.L., Todd A.J. A quantitative study of brainstem projections from lamina I neurons in the cervical and lumbar enlargement of the rat. Brain Research. 2010;1308:58–67.
Poorkhalkali N., Juneblad K., Jonsson A.C., et al. Immunocytochemical distribution of the GABA(B) receptor splice variants GABA(B) R1a and R1b in the rat CNS and dorsal root ganglia. Anatomy and Embryology (Berlin). 2000;201:1–13.
Prescott S.A., De Koninck Y. Four cell types with distinctive membrane properties and morphologies in lamina I of the spinal dorsal horn of the adult rat. Journal of Physiology. 2002;593:817–836.
Puskár Z., Polgár E., Todd A.J. A population of large lamina I projection neurons with selective inhibitory input in rat spinal cord. Neuroscience. 2001;102:167–176.
Rau K.K., McIlwrath S.L., Wang H., et al. Mrgprd enhances excitability in specific populations of cutaneous murine polymodal nociceptors. Journal of Neuroscience. 2009;29:8612–8619.
Reeh P.W., Bayer J., Kocher L., et al. Sensitization of nociceptive cutaneous nerve fibers from the rat’s tail by noxious mechanical stimulation. Experimental Brain Research. 1987;65:505–512.
Ren K., Ruda M.A. A comparative study of the calcium-binding proteins calbindin-D28K, calretinin, calmodulin and parvalbumin in the rat spinal cord. Brain Research Reviews. 1994;19:163–179.
Rèthelyi M., Light A.R., Perl E.R. Synaptic complexes formed by functionally defined primary sensory afferent units with fine myelinated fibers. Journal of Comparative Neurology. 1982;207:381–393.
Rèthelyi M., Light A.R., Perl E.R. Synaptic ultrastructure of functionally and morphologically characterized neurons of the superficial dorsal horn of cat. Journal of Neuroscience. 1989;9:1846–1863.
Rexed B. The cytoarchitectonic organization of the spinal cord in the cat. Journal of Comparative Neurology. 1952;96:415–495.
Ribeiro-da-Silva A. Substantia gelatinosa of the spinal cord. In: Paxinos G., ed. The rat nervous system. Sydney: Academic Press, pp 129–148, 2003.
Ribeiro-da-Silva A., Castro-Lopes J.M., Coimbra A. Distribution of glomeruli with fluoride-resistant acid phosphatase (FRAP)-containing terminals in the substantia gelatinosa of the rat. Brain Research. 1986;377:323–329.
Ribeiro-da-Silva A., Coimbra A. Two types of synaptic glomeruli and their distribution in laminae I–III of the rat spinal cord. Journal of Comparative Neurology. 1982;209:176–186.
Ribeiro-da-Silva A., Cuello A.C. Choline acetyltransferase–immunoreactive profiles are presynaptic to primary sensory fibers in the rat superficial dorsal horn. Journal of Comparative Neurology. 1990;295:370–384.
Ribeiro-da-Silva A., Tagari P., Cuello A.C. Morphological characterization of substance P–like immunoreactive glomeruli in the superficial dorsal horn of the rat spinal cord and trigeminal subnucleus caudalis: a quantitative study. Journal of Comparative Neurology. 281, 1989. 497–515, 1989
Ross S.E., Mardinly A.R., McCord A.E., et al. Loss of inhibitory interneurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron. 2010;65:886–898.
Ruda M., Coffield J., Steinbusch H.W.M. Immunocytochemical analysis of serotonergic axons in laminae I and II of the lumbar spinal cord of the cat. Journal of Neuroscience. 1982;2:1660–1671.
Sandkühler J. Models and mechanisms of hyperalgesia and allodynia. Physiological Reviews. 2009;89:707–758.
Sardella T.C.P., Polgár E., Garzillo F., et al. Dynorphin is expressed primarily by GABAergic neurons that contain galanin in the rat dorsal horn. Molecular Pain. 2011;7:76.
Sardella T.C.P., Polgár E., Watanabe M., et al. A quantitative study of neuronal nitric oxide synthase expression in laminae I–III of the rat spinal dorsal horn. Neuroscience. 2011;192:708–720.
Schneider S.P. Functional properties and axon terminations of interneurons in laminae III–V of the mammalian spinal dorsal horn in vitro. Journal of Neurophysiology. 1992;68:1746–1759.
Schneider S.P., Walker T.M. Morphology and electrophysiological properties of hamster spinal dorsal horn neurons that express VGLUT2 and enkephalin. Journal of Comparative Neurology. 2007;501:790–809.
Seal R.P., Wang X., Guan Y., et al. Injury-induced mechanical hypersensitivity requires C-low threshold mechanoreceptors. Nature. 2009;462:651–655.
Sengupta J.N., Gebhart G.F. Characterization of mechanosensitive pelvic nerve afferent fibers innervating the colon of the rat. Journal of Neurophysiology. 1994;71:2046–2060.
Seybold V.S., Grkovic I., Portbury A.L., et al. Relationship of NK3 receptor–immunoreactivity to subpopulations of neurons in rat spinal cord. Journal of Comparative Neurology. 1997;381:439–448.
Silverman J.D., Kruger L. Lectin and neuropeptide labeling of separate populations of dorsal root ganglion neurons and associated “nociceptor” thin axons in rat testis and cornea whole-mount preparations. Somatosensory Research. 1988;5:259–267.
Sivilotti L., Woolf C.J. The contribution of GABAA and glycine receptors to central sensitization: disinhibition and touch-evoked allodynia in the spinal cord. Journal of Neurophysiology. 1994;72:169–179.
Snider W.D., McMahon S.B. Tackling pain at the source: new ideas about nociceptors. Neuron. 1998;20:629–632.
Spike R.C., Puskár Z., Andrew D., et al. A quantitative and morphological study of projection neurons in lamina I of the rat lumbar spinal cord. European Journal of Neuroscience. 2003;18:2433–2448.
Spike R.C., Puskár Z., Sakamoto H., et al. MOR-1–immunoreactive neurons in the dorsal horn of the rat spinal cord: evidence for non-synaptic innervation by substance P–containing primary afferents and for selective activation by noxious thermal stimuli. European Journal of Neuroscience. 2002;15:1306–1316.
Spike R.C., Todd A.J., Johnston H.M. The coexistence of NADPH diaphorase with GABA, glycine and acetylcholine in rat spinal cord. Journal of Comparative Neurology. 1993;335:320–333.
Spike R.C., Watt C., Zafra F., et al. An ultrastructural study of the glycine transporter GLYT2 and its association with glycine in the superficial laminae of the rat spinal dorsal horn. Neuroscience. 1997;77:543–551.
Stone L.S., Broberger C., Vulchanova L., et al. Differential distribution of α2A and α2C adrenergic receptor immunoreactivity in the rat spinal cord. Journal of Neuroscience. 1998;18:5928–5937.
Sugiura Y., Lee C.L., Perl E.R. Central projections of identified, unmyelinated (C) afferent fibers innervating mammalian skin. Science. 1986;234:358–361.
Sugiura Y., Terui N., Hosoya Y. Difference in distribution of central terminals between visceral and somatic unmyelinated (C) primary afferent fibers. Journal of Neurophysiology. 1989;62:834–840.
Szentágothai J. Neuronal and synaptic arrangement in the substantia gelatinosa Rolandi. Journal of Comparative Neurology. 1964;122:219–239.
Szücs P., Luz L.L., Lima D., et al. Local axon collaterals of lamina I projection neurons in the spinal cord of young rats. Journal of Comparative Neurology. 2010;518:2645–2665.
Tachibana M., Wenthold R.J., Morioka H., et al. Light and electron microscopic immunocytochemical localization of AMPA-selective glutamate receptors in the rat spinal cord. Journal of Comparative Neurology. 1994;344:431–454.
Tiong S.Y.X., Polgár E., van Kranlingen J.C., et al. Galanin-immunoreactivity identifies a distinct population of inhibitory interneurons in laminae I–III of the rat spinal cord. Molecular Pain. 2011;7:36.
Todd A.J. GABA and glycine in synaptic glomeruli of the rat spinal dorsal horn. European Journal of Neuroscience. 1996;8:2492–2498.
Todd A.J. Neuronal circuitry for pain processing in the dorsal horn. Nature Reviews of Neuroscience. 2010;11:823–836.
Todd A.J., Hughes D.I., Polgár E., et al. The expression of vesicular glutamate transporters VGLUT1 and VGLUT2 in neurochemically-defined axonal populations in the rat spinal cord with emphasis on the dorsal horn. European Journal of Neuroscience. 2003;17:13–27.
Todd A.J., Polgár E., Watt C., et al. Neurokinin 1 receptor–expressing projection neurons in laminae III and IV of the rat spinal cord have synaptic AMPA receptors that contain GluR2, GluR3 and GluR4 subunits. European Journal of Neuroscience. 2009;29:718–726.
Todd A.J., Puskár Z., Spike R.C., et al. Projection neurons in lamina I of rat spinal cord with the neurokinin 1 receptor are selectively innervated by substance P–containing afferents and respond to noxious stimulation. Journal of Neuroscience. 2002;22:4103–4113.
Todd A.J., Spike R.C. The localization of classical transmitters and neuropeptides within neurons in laminae I–III of the mammalian spinal dorsal horn. Progress in Neurobiology. 1993;41:609–646.
Todd A.J., Spike R.C., Polgár E. A quantitative study of neurons which express neurokinin 1 or somatostatin sst2A receptor in rat spinal dorsal horn. Neuroscience. 1998;85:459–473.
Todd A.J., Sullivan A.C. A light microscope study of the coexistence of GABA-like and glycine-like immunoreactivities in the spinal cord of the rat. Journal of Comparative Neurology. 1990;296:496–505.
Todd A.J., Watt C., Spike R.C., et al. Colocalization of GABA, glycine, and their receptors at synapses in the rat spinal cord. Journal of Neuroscience. 1996;16:974–982.
Towers S., Princivalle A., Billinton A., et al. GABAB receptor protein and mRNA distribution in rat spinal cord and dorsal root ganglia. European Journal of Neuroscience. 2000;12:3201–3210.
Uta D., Furue H., Pickering A.E., et al. TRPA1-expressing primary afferents synapse with a morphologically identified subclass of substantia gelatinosa neurons in the adult rat spinal cord. European Journal of Neuroscience. 2010;31:1960–1973.
Varoqui H., Schäfer M.K.-H., Zhu H., et al. Identification of the differentiation-associated Na+/PI transporter as a novel vesicular glutamate transporter expressed in a distinct set of glutamatergic synapses. Journal of Neuroscience. 2002;22:142–155.
Villanueva L., Bernard J.-F. The multiplicity of ascending pain pathways. In: Lydic R., Baghdoyan H.A., eds. Handbook of behavioral state control: cellular and molecular mechanisms. Boca Raton, FL: CRC Press; 1999:569–585.
Vulchanova L., Riedl M.S., Shuster S.J., et al. P2X3 is expressed by DRG neurons that terminate in inner lamina II. European Journal of Neuroscience. 1998;10:3470–3478.
Wang H.-B., Zhao B., Zhong Y.Q., et al. Coexpression of δ- and μ-opioid receptors in nociceptive sensory neurons. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:13117–13122.
Westlund K.N., Coulter J.D. Descending projections of the locus coeruleus and subcoeruleus/medial parabrachial nucleus in monkey: axonal transport studies and dopamine–β-hydroxylase immunohistochemistry. Brain Research Reviews. 1980;2:235–264.
Willis W.D., Coggeshall R.E. Sensory mechanisms of the spinal cord, ed 3. New York: Kluwer Academic; 2004.
Wilson L.B., Andrew D., Craig A.D. Activation of spinobulbar lamina I neurons by static muscle contraction. Journal of Neurophysiology. 2002;87:1641–1645.
Woodbury C.J., Koerber H.R. Widespread projections from myelinated nociceptors throughout the substantia gelatinosa provide novel insights into neonatal hypersensitivity. Journal of Neuroscience. 2003;23:601–610.
Woodbury C.J., Kullmann F.A., McIlwrath S.L., et al. Identity of myelinated cutaneous sensory neurons projecting to nocireceptive laminae following nerve injury in adult mice. Journal of Comparative Neurology. 2008;508:500–509.
Woodbury C.J., Ritter A.M., Koerber H.R. On the problem of lamination in the superficial dorsal horn of mammals: a reappraisal of the substantia gelatinosa in postnatal life. Journal of Comparative Neurology. 2000;417:88–102.
Woodbury C.J., Ritter A.M., Koerber H.R. Central anatomy of individual rapidly adapting low-threshold mechanoreceptors innervating the “hairy” skin of newborn mice: early maturation of hair follicle afferents. Journal of Comparative Neurology. 2001;436:304–323.
Woodbury C.J., Zwick M., Wang S., et al. Nociceptors lacking TRPV1 and TRPV2 have normal heat responses. Journal of Neuroscience. 2004;24:6410–6415.
Woolf C.J. Central terminations of cutaneous mechanoreceptive afferents in the rat lumbar spinal cord. Journal of Comparative Neurology. 1987;261:105–119.
Yaksh T.L. Behavioral and autonomic correlates of the tactile evoked allodynia produced by spinal glycine inhibition: effects of modulatory receptor systems and excitatory amino acid antagonists. Pain. 1989;37:111–123.
Yasaka T., Kato G., Furue H., et al. Cell-type–specific excitatory and inhibitory circuits involving primary afferents in the substantia gelatinosa of the rat spinal dorsal horn in vitro. Journal of Physiology. 2007;581:603–618.
Yasaka T., Tiong S.Y.X., Hughes D.I., et al. Populations of inhibitory and excitatory interneurons in lamina II of the adult rat spinal dorsal horn revealed by a combined electrophysiological and anatomical approach. Pain. 2010;151:475–488.
Yoshida S., Matsuda Y., Samejima A. Tetrodotoxin-resistant sodium and calcium components of action potentials in dorsal root ganglion cells of the adult mouse. Journal of Neurophysiology. 1978;41:1096–1106.
Yoshimura M., Jessell T. Amino acid–mediated EPSPs at primary afferent synapses with substantia gelatinosa neurones in the rat spinal cord. Journal of Physiology. 1990;430:315–335.
Yoshimura M., North R.A. Substantia gelatinosa neurones hyperpolarised in vitro by enkephalin. Nature. 1983;305:529–530.
Zhang E.T., Craig A.D. Morphology and distribution of spinothalamic lamina I neurons in the monkey. Journal of Neuroscience. 1997;17:3274–3284.
Zhang E.T., Han Z.S., Craig A.D. Morphological classes of spinothalamic lamina I neurons in the cat. Journal of Comparative Neurology. 1996;367:537–549.
Zhang X., Nicholas A.P., Hökfelt T. Ultrastructural studies on peptides in the dorsal horn of the spinal cord. I. Co-existence of galanin with other peptides in primary afferents in normal rats. Neuroscience. 1993;57:365–384.
Zheng J., Lu Y., Perl E.R. Inhibitory neurones of the spinal substantia gelatinosa mediate interaction of signals from primary afferents. Journal of Physiology. 2010;588:2065–2075.
Zwick M., Davis B.M., Woodbury C.J., et al. Glial cell line–derived neurotrophic factor is a survival factor for isolectin B4–positive, but not vanilloid receptor 1–positive, neurons in the mouse. Journal of Neuroscience. 2002;22:4057–4065.
Zylka M.J., Rice F.L., Anderson D.J. Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron. 2005;45:17–25.
Burgess P.R., Perl E.R. Myelinated afferent fibres responding specifically to noxious stimulation of the skin. Journal of Physiology. 1967;190:541–562.
Djouhri L., Bleazard L., Lawson S.N. Association of somatic action potential shape with sensory receptive properties in guinea-pig dorsal root ganglion neurones. Journal of Physiology. 1998;513:857–872.
Grudt T.J., Perl E.R. Correlations between neuronal morphology and electrophysiological features in the rodent superficial dorsal horn. Journal of Physiology. 2002;540:189–207.
Hoheisel U., Lehmann-Willenbrock E., Mense S. Termination patterns of identified group II and III afferent fibres from deep tissues in the spinal cord of the cat. Neuroscience. 1989;28:495–507.
Hu H.J., Carrasquillo Y., Karim F., et al. The Kv4.2 potassium channel subunit is required for pain plasticity. Neuron. 2006;50:89–100.
Ji R.R., Baba H.H., Brenner G.J., et al. Nociceptive-specific activation of ERK in spinal neurons contributes to pain hypersensitivity. Nature Neuroscience. 1999;2:1114–1119.
Lawson J.J., McIlwrath S.L., Woodbury C.J., et al. TRPV1 unlike TRPV2 is restricted to a subset of mechanically insensitive cutaneous nociceptors responding to heat. Journal of Pain. 2008;9:298–308.
Lawson S.N., Crepps B.A., Perl E.R. Relationship of substance P to afferent characteristics of dorsal root ganglion neurones in guinea-pig. Journal of Physiology. 1997;505:177–191.
Light A.R., Perl E.R. Spinal termination of functionally identified primary afferent neurons with slowly conducting myelinated fibers. Journal of Comparative Neurology. 1979;186:133–150.
Ling L.J., Honda T., Shimada Y., et al. Central projection of unmyelinated (C) primary afferent fibers from gastrocnemius muscle in the guinea pig. Journal of Comparative Neurology. 2003;461:140–150.
Mantyh P.W., Rogers S.D., Honore P., et al. Ablation of lamina I spinal neurons expressing the substance P receptor profoundly inhibits hyperalgesia. Science. 1997;278:275–279.
Melzack R., Wall P.D. Pain mechanisms: a new theory. Science. 1965;150:971–979.
Ribeiro-da-Silva A. Substantia gelatinosa of the spinal cord. In: Paxinos G., ed. The rat nervous system. Sydney: Academic Press, 2003.
Sandkühler J. Models and mechanisms of hyperalgesia and allodynia. Physiological Reviews. 2009;89:707–758.
Seal R.P., Wang X., Guan Y., et al. Injury-induced mechanical hypersensitivity requires C-low threshold mechanoreceptors. Nature. 2009;462:651–655.
Sugiura Y., Lee C.L., Perl E.R. Central projections of identified, unmyelinated (C) afferent fibers innervating mammalian skin. Science. 1986;234:358–361.
Todd A.J. Neuronal circuitry for pain processing in the dorsal horn. Nature Reviews of Neuroscience. 2010;11:823–836.
Todd A.J., Puskár Z., Spike R.C., et al. Projection neurons in lamina I of rat spinal cord with the neurokinin 1 receptor are selectively innervated by substance P–containing afferents and respond to noxious stimulation. Journal of Neuroscience. 2002;22:4103–4113.
Willis W.D., Coggeshall R.E. Sensory mechanisms of the spinal cord, ed 3. New York: Kluwer Academic; 2004.
Woodbury C.J., Koerber H.R. Widespread projections from myelinated nociceptors throughout the substantia gelatinosa provide novel insights into neonatal hypersensitivity. Journal of Neuroscience. 2003;23:601–610.
Woodbury C.J., Ritter A.M., Koerber H.R. On the problem of lamination in the superficial dorsal horn of mammals: a reappraisal of the substantia gelatinosa in postnatal life. Journal of Comparative Neurology. 2000;417:88–102.
Woodbury C.J., Zwick M., Wang S., et al. Nociceptors lacking TRPV1 and TRPV2 have normal heat responses. Journal of Neuroscience. 2004;24:6410–6415.
Yaksh T.L. Behavioral and autonomic correlates of the tactile evoked allodynia produced by spinal glycine inhibition: effects of modulatory receptor systems and excitatory amino acid antagonists. Pain. 1989;37:111–123.
Yasaka T., Tiong S.Y.X., Hughes D.I., et al. Populations of inhibitory and excitatory interneurons in lamina II of the adult rat spinal dorsal horn revealed by a combined electrophysiological and anatomical approach. Pain. 2010;151:475–488.
Zylka M.J., Rice F.L., Anderson D.J. Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron. 2005;45:17–25.