Studies of Pain in Human Subjects
Methods of Experimental Pain Stimulation
Studies of pain in normal humans have one feature in common: an external stimulus must be applied to create the experience of pain. Once produced, this experience can be evaluated by a number of verbal, behavioral, and physiological measures. Choice from the large number of combinations of stimulus and response methods is based on the properties of each method and on the goals of the experiment. Increasingly, this choice is not restricted to a single modality and stimulation site to provide a profile of pain responsivity.
The multiple properties of stimulation methods can be organized around a consideration of desirable traits. Beecher (1959) described 10 properties: an ideal pain stimulus should (1) be applied to body parts exhibiting minimal neurohistological variation between individuals, (2) provoke minimal tissue damage, (3) show a relationship between stimulus and pain intensity, (4) provide information about discrimination between stimuli, (5) result in repeatable stimulation without temporal interaction, (6) be easily applied and produce a distinct pain sensation, (7) allow a quantifiable determination of pain quality, (8) be sensitive, (9) show an analgesic–dose relationship, and (10) be applicable to both humans and animals. Additional requirements emerged as the scope of pain research broadened from the demonstration of experimental analgesia. These requirements include (11) rapid, controlled onset for studies in which the stimulus event must be timed precisely, such as studies using averaged measures of cortical or muscle activity; (12) rapid termination for stimuli administered at fast rates, such as one every 1 to 3 seconds; (13) natural stimulation that is experienced in everyday life or could be experienced by an animal in the wild; (14) suppression of specific afferent activity; (15) ability to sensitize neurons and/or activate processes involved in persistent pain states; (16) demonstration of similar sensitivities in different individuals; and (17) ability to excite a restricted group of primary afferents.
Heat
Heat is one of the most commonly used methods of evoking experimental pain sensations. Its temporal and spatial properties are easily varied and the stimulation excites a known group of nociceptors. Heat pain is commonly applied by contact or by radiant sources. Objects heated by water baths or by contact thermodes can be used to apply contact heat. Many modern contact thermodes use the Peltier principle, in which a direct current through a semiconductor substrate results in an increase in temperature on one side and a decrease in temperature on the other. The magnitude and direction of change in the stimulus are proportional to the magnitude and polarity of the stimulating current (Kenshalo and Bergen 1975). Other contact stimulators use circulating fluid or electrical heaters, which may be cooled by circulating fluid (Chen et al 2001, Petzke et al 2003a). The rate of change is relatively slow with the Peltier units and fast with electrically heated, fluid-cooled units. Contact heat can also be achieved by simple immersion in hot water or by infusion of hot water into muscle (Graven-Nielsen et al 2002).
Radiant heat is a classic stimulation method. An infrared light source is focused on a skin site, usually blackened to improve absorption of energy. Stimulus intensity is determined by lamp voltage and stimulus duration by a mechanical shutter. Modern adaptations have used similar methodology (Sternberg et al 2001) but generally involve a laser stimulus source than can vary in wavelength and hence stimulation properties, depending on the source (e.g., CO2, argon, infrared diode, thulium:yttrium-aluminum-garnet [YAG], neodymium:YAG) (Satero et al 2000, Lefaucheur et al 2001, Romaniello et al 2002, Iannetti et al 2006, Tran et al 2008).
Cold
Cold stimuli are administered by the contact stimulators described earlier, by the administration of coolant sprays, or by immersion in fluid. These methods can be divided into those delivering discrete stimuli and those producing continuous stimulation. A common example of the latter is the cold pressor method, in which pain is produced by immersion of a limb in very cold (0–4°C) water (Sternberg et al 2001, Polianskis et al 2002, Lowery et al 2003, Mechlin et al 2005, Dowman et al 2008, Dawson and List 2009, Neziri et al 2011). It produces a severe pain that increases quickly and can be tolerated for only a few minutes by most people.
Ischemia
Arresting blood flow in an arm with a tourniquet while simultaneously exercising the hand produces ischemic pain by isometric or isotonic contractions (Byas-Smith et al 1999, Edwards et al 2001, Straneva et al 2002, Graven-Nielsen et al 2003, Mechlin et al 2005, Tuveson et al 2006, Campbell et al 2008a). This method produces a severe, continuous, and increasing pain that can generally be tolerated for 20 minutes. It is similar to the cold pressor method and is used both as a pain stimulus and as an experimental stressor.
Mechanical Pressure
Mechanical pressure is a classic method in which pain sensations are evoked by deformation of the skin via von Frey hairs and needles, by the application of gross pressure, by pinching, by high-velocity impact via probes or projectiles, and by balloon or fluid distention of viscera. Phasic or tonic stimulation with sharp or punctuate mechanical probes is useful in studies of nociceptor function and phenomena such as temporal summation (Andrew and Greenspan 1999). Increased sensitivity to painful blunt pressure is associated with myofascial pain syndromes and with fibromyalgia and is found in visceral conditions such as irritable bowel syndrome (Andrew and Greenspan 1999, Naliboff et al 2003, Petzke et al 2003a). Thus, methods that deliver painful pressure provide a relevant, adequate stimulus for mechanistic studies of these pain disorders. Mechanical methods produce a wide range of pain intensities and durations. The results are influenced by physical factors such as tissue elasticity, stimulation area, and rate and degree of compression, as well as by gender and age (Magerl et al 2010) and psychological factors such as distress (Petzke et al 2003b).
Electrical
Electrical stimulation is applied to the skin (Sang et al 2003, Kunz et al 2009), teeth (Fujii-Abe et al 2010), muscle (Kosek and Hansson 2002), and stomach or intestine (Rossel et al 2001) and is applied directly to peripheral (Weidner et al 2002) and central (Lenz et al 1998b, Davis et al 2000, Patel et al 2006) neurons. Stimulus current is often used as the independent variable, and current ranges for pulsed stimuli are usually 0 to 30 mA for skin (depending on pulse density) and 0 to 100 μA for teeth. The precise timing of onset and termination and capabilities for short-duration stimuli are useful for studies of evoked reflexes or studies of cerebral potentials or magnetic fields that require precise timing, as well as for studies that require brief stimulation at specific times, such as during different phases of the cardiac cycle (Edwards et al 2008).
Chemical
Chemical stimulation has been applied to intact, punctured, or blistered skin; to esophageal, gastric, intestinal, or nasal mucosa; to teeth; and to the eye; it can also be injected intramuscularly. Chemical stimuli activate unique pain processes not evoked by other methods. The degree of stimulus control is generally less, although several more recent methods provide increased control comparable to other stimulus modalities, including delivery of CO2 to the nasal mucosa (Anton et al 1992); manipulation of tissue pH (Steen et al 1995); iontophoresis of adenosine triphosphate, protons, or potassium (Humphries et al 1996); microdialysis of inflammatory mediators and agents mediating itch or pain (Lischetzki et al 2001, Drewes et al 2003); and intramuscular infusion of hypertonic saline (Arendt-Nielsen et al 2008).
The use of topical or intradermal capsaicin, the pungent ingredient in chili pepper, is a special case in which the primary pain of application is of less interest than the phenomena of primary heat hyperalgesia and secondary mechanical allodynia and hyperalgesia (Sang et al 1996, Byas-Smith et al 1999, Khalili et al 2001, Sumikura et al 2006, Frymoyer et al 2007, Wang et al 2008). These methods, the use of other agents such as mustard oil, bee venom, glutamate, and nerve growth factor (Sumikura et al 2006, Rukwied et al 2010, Wang et al 2010), and other methods such as continuous electrical stimulation, experimental burns, or freezing of the skin have been used widely to evoke a condition of central sensitization usually found only in clinical conditions of persistent pain. Capsaicin also desensitizes nociceptors and is used both clinically and experimentally to block nociceptor activation.
Properties of Stimulation Methods
The relationship between research goals and types of experimental pain stimuli is shown in Table 20-1. It is apparent that specific pain production methods satisfy some but not all criteria of an ideal pain stimulus. For example, electrical tooth pulp stimulation provides a controllable, repeatable sensation with minimal temporal effects, excites a relatively restricted group of primary afferent fibers, and exhibits a precise onset and termination. Thus, it is an ideal stimulus for many investigations. However, it is an inappropriate stimulus for studies that compare sensitivities between groups because the range of intensities required to elicit pain sensations varies widely between individuals, probably as a consequence of individual tooth geometry. Electrical tooth pulp stimulation also bypasses receptor mechanisms to produce a synchronous barrage of afferent activity and resultant unnatural sensation. Electrical stimulation of the skin also produces unnatural sensations, but sensitivities are similar between individuals, thus permitting between-group comparisons. However, sensations evoked by electrical skin stimulation can contain a powerful, Aβ-mediated pressure–vibration component. The evoked sensation can be felt as an aversive intense stab or vibration without actually being painful. In studies of Aβ-mediated mechanical allodynia or tactile hypersensitivity, electrical stimuli can selectively activate Aβ afferents at detection-level stimulus intensities (Sang et al 2003). In studies of nociceptive afferents, the contribution of Aδ stimulation may be reduced by stimulus preparation or minimized by stimulating teeth. Although Aβ fibers have been identified in tooth pulp, the majority of the afferent fibers are nociceptive and conduct in the Aδ- and C-fiber range (Dong et al 1985). The sensation evoked by electrical tooth pulp stimulation contains a measurable pre-pain component (Chatrian et al 1982, McGrath et al 1983) at near-threshold levels. However, suprathreshold stimulation results in a distinct pain sensation without the significant non-pain qualities found with electrical skin stimulation. Radiant heat stimulation produces similar sensations in different individuals, thus allowing comparison of pain sensitivity across groups. It excites a restricted group of primary afferents and its onset is rapid. Termination is slow, however, which renders these methods less appropriate for studies in which stimulation must be repeated quickly. Contact heat stimulation has a fast termination and can be used for such studies. It excites a restricted group of primary afferent fibers but also activates slowly adapting mechanoreceptors. Laser stimulation contains all the advantages of a radiant source. Return to baseline temperature is faster because of the small area stimulated. However, this small area may not be adequate for studies of summation or warmth, which require variable or large surface stimulation. Sharp or nearly sharp (punctate) pressure activates predominately Aδ nociceptors, whereas blunt pressure is characterized by a predominately C-fiber response (Treede et al 2002).
Table 20-1
Properties of Experimental Pain Stimulation Methods
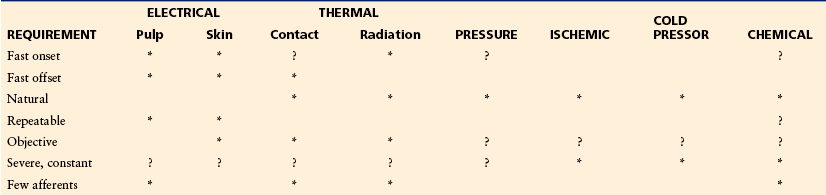
Stimulation requirements are shown for electrical tooth pulp and electrical skin stimulation, thermal stimulation by contact or radiant heat, pressure stimulation, ischemic pain produced by exercising a limb in which circulation has been occluded by a tourniquet, cold pressor stimulation achieved by immersion of a limb in cold water, and chemical stimulation of the skin, teeth, or mucosa. Asterisks indicate that the method satisfies the requirement; question marks indicate that the method may satisfy the requirement under specific conditions.
Chemical methods range from very controllable (CO2 applied to nasal mucosa) to moderately (pH buffers) and minimally controllable (application of capsaicin or mustard oil). Stimulation is natural and, in the case of substances such as capsaicin or mustard oil, is capable of mimicking many of the significant features of a clinical syndrome. Prolonged pain evoked by the infusion of hypertonic saline or other chemicals into muscle provides a deep, diffuse pain sensation that may more closely resemble clinical pain. This stimulus has been shown to be useful for a variety of investigations, including evaluation of jaw muscle reflexes (Svensson et al 1999), visceral nociception (Drewes et al 2003), and brain opioid binding and genetic influence on such binding (Berthele et al 2005). Iontophoresis can provide steady-state levels of stimulation over a period of many minutes.
Subjective Measures: Pain Psychophysics
Single-Point Measures Such as Threshold and Tolerance
The “pain threshold” is often used incorrectly to refer to general pain sensitivity and variability of this sensitivity between different individuals. One person may have a “high pain threshold” whereas another has a “low pain threshold.” These differences in pain threshold imply differences in the nervous system such that a person with a high threshold needs extra input to feel pain and greater input to feel the same level of pain as a person with a normal or low threshold. To complicate matters, the pain threshold may also reflect the labels chosen to describe sensations processed by similarly sensitive nervous systems. Reports of minimal pain can represent either an insensitive nervous system or a stoical reporting style in which the label “non-painful” is used to describe a painful sensation. One elusive goal in pain measurement is assessment of pain sensitivity independent of pain labeling behavior, that is, assessment of subjective pain without the biases that influence verbal report. Of course, this goal assumes that these biases represent arbitrary choices and not the known effects of the multiple physiological, psychological, and social factors that modulate pain. Increasing evidence of physiological changes in response to factors such as empathy and expectation blurs the distinction between pain sensitivity and labeling behavior.
The pain threshold is defined as the minimum amount of stimulation that reliably evokes a report of pain. Pain tolerance is similarly defined as the time that a continuous stimulus is endured or the maximally tolerated stimulus intensity. Threshold and tolerance measures are attractive because of their simplicity for both the administrator and the subject. In addition, the response is expressed in physical units of stimulus intensity or time, thereby avoiding the subjectivity of a psychological scale of pain. These methods are commonly used and have been found to be useful for many measurement situations, especially for the evaluation of sensory function in the clinic. However, both are poor psychophysical measures. Both are single measures that are usually confounded with time or increasing intensity. A subject can easily be biased to respond sooner or later or to a lower or higher intensity. Unlike determination of sensory thresholds in which a subject must choose between the presence or absence of sensation, in most cases the pain threshold is a judgment about the quality of a sensation that is always present. Pain thresholds are thus more subjective, and the judgment can be made on the basis of irrelevant stimulus features. Tolerance measures share the same problem. In addition, tolerance of a painful stimulus has been shown repeatedly to be related to a separate endurance factor that is not associated with pain intensity (Cleeland et al 1996). Another problem with these methods is that they assess only the extremes of the perceptual pain range. They provide little information about levels of pain that are observed clinically and that can be produced by experimental methods. In addition, because of the vulnerability to scaling bias, these measures can be contaminated by factors such as psychological distress that do not affect the suprathreshold measures described next (Petzke et al 2003b).
A number of psychophysical methods can be used to assess the range of pain sensation from threshold to tolerance. Some consist of an ascending series and are vulnerable to the same biases that can affect ascending measures of threshold or tolerance. More sophisticated methods control many of these biases. The domain of suprathreshold pain measures can be divided into three classes depending on the target of the measurement: (1) methods that treat pain as a single dimension and assess the range from pain threshold to intense pain levels; (2) separation of the single dimension of pain into two dimensions of sensory intensity and unpleasantness; and (3) multidimensional assessment of the many attributes of pain sensation, including its intensive, qualitative, and aversive aspects.
Pain as a Single Dimension
Most human research studies assess “pain” by treating the experience as a single dimension varying in magnitude, much like varying the sound level by turning the volume knob on a radio. Both classic threshold and suprathreshold measures treat pain as a single dimension. The following sections describe the application of these psychophysical methods to pain assessment.
Pain Threshold
The pain threshold can be determined by the classic method of limits, which administers ascending and descending trials; the method of adjustment, in which the subject adjusts the stimulus intensity; and the method of constant stimuli, in which a set of fixed-intensity stimuli are presented several times in a random sequence (Chen et al 1996). The result of each method is a specific magnitude of stimulus intensity that is inversely related to pain sensitivity; the lower the threshold, the greater the sensitivity. As noted earlier, the pain threshold is not a discrete event but rather a probability function, and the subjective criteria used to attach the label of “pain” to a specific sensation vary between and within individuals.
There have been simple and sophisticated applications of threshold methodology to pain assessment. The simplest methods use a modification of the method of limits. For example, in the Marstock method (Fruhstorfer et al 1976), a thermal stimulus slowly increases or decreases from a neutral baseline. Subjects indicate either the warm or cool detection threshold or the heat or cold pain threshold by pressing a button that returns the stimulus to baseline or initiates a stimulus excursion in the opposite direction. Although this method lacks rudimentary psychophysical controls, it is adequate for the large changes in threshold observed in many clinical conditions, and, when appropriate, it efficiently describes altered thermal sensibility.
In striking contrast to the detection of large changes by the simple Marstock method, other procedures use sophisticated judgment models to evaluate pain scaling behavior. The powerful methods of sensory decision theory (SDT) have been applied both to the analysis of pain thresholds and to category responses of suprathreshold pain sensations. This method yields not one but two parameters. The beta, or response criterion, parameter is a direct measure of the subjective criteria used to attach the label of pain. For example, the criteria may be stoical, with only clearly painful (or greater) sensations labeled as pain. The second SDT parameter (classically called d′) is a measure of discrimination, or the ability to distinguish between two stimuli. At first glance it seems as though application of this method could achieve the elusive goal of separating pain sensitivity from pain-reporting behavior. A number of studies have investigated this goal. This research identified a number of issues and focused interest on pain measurement (Chapman 1977, Rollman 1977). One issue is the role of extraneous components of discrimination. For example, Figure 20-1 shows that measures such as d′ are also influenced by variability in sensation and variability in choosing labels to describe sensations (Coppola and Gracely 1983). Changes in discrimination do not necessarily indicate analgesia, although unchanged discrimination is strong evidence that pain sensitivity has not changed (Clark and Clark 1980). Another issue is interpretation of changes in the response criterion. These can represent changes in labeling behavior, or they can represent changes in other aspects of the sensation, such as unpleasantness or painfulness, that do not alter discrimination. In these situations, a change in this response parameter could represent an analgesic effect that reduces the unpleasantness of the pain sensation.
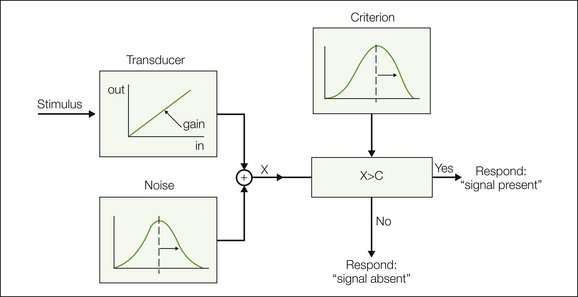
Figure 20-1 Model describing sensory discrimination and factors that influence discrimination sensitivity.
A noisy transducer results in a perceptual signal “X” that is compared with a variable criterion “C.” The mean location of the varying criterion determines the response bias parameter, whereas discrimination sensitivity is determined by transducer gain and also by noise in the transducer and noise in the criterion (choice mechanism). (From Coppola R, Gracely RH 1983 Where is the noise in SDT pain assessment? Pain 17:257–266. Copyright 1983 Elsevier Ltd.)
The method of two alternative forced choice (2-AFC) is related to SDT and provides a measure of discrimination that is not influenced by the subject’s response criterion. A stimulus is presented at one of two locations or in one of two temporal intervals during each trial, and the subject indicates the correct location or interval. The proportion of correct responses above the 50% chance level corresponds to the SDT discrimination parameter. The 2-AFC method yields a bias-free measure of discrimination sensitivity but does not indicate the magnitude or direction of the bias. Figure 20-2 shows an example of this method in which different painfully hot or cold stimulus temperatures were delivered to the tongue by the combination of a contact thermode and an insulating strip that provided a 0.5°C temperature difference between the left and right sides of the tongue (Albin et al 2008). Subjects indicated which side of the tongue was more painful and rated the intensity of the pain evoked on each side, with both methods providing similar levels of statistically significant measures of sensitivity at this temperature difference.
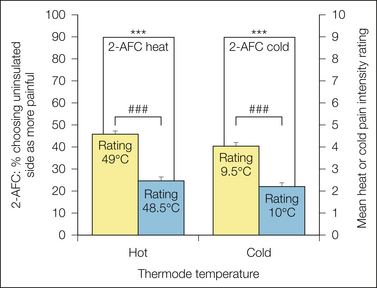
Figure 20-2 Results of two alternative forced choice (2-AFC) measures compared with pain ratings.
Painfully hot or cold stimulus temperatures were delivered to the tongue by the combination of a contact thermode and an insulating strip that provided a 0.5°C temperature difference between the left and right sides. Subjects both indicated which side of the tongue was more painful and rated the intensity of the pain evoked on each side. Indication of the more painful side provides an example of the method of 2-AFC, which provides a bias-free measure of discrimination sensitivity. In this example, both methods provided similar levels of statistically significant measures of sensitivity at this temperature difference. (From Albin KC, Carstens MI, Carstens E 2008 Modulation of oral heat and cold pain by irritant chemicals. Chemical Senses 33:3–15.)
The precision of SDT or 2-AFC is gained at the expense of extended time and increased number of stimuli. In studies of pain, these increased requirements may be excessive because of the nature of the stimulus (very painful, prolonged) or the subject (chronic pain patient). Newer methods developed specifically for assessment of pain reduce the amount of stimulation required. A subset of these use interactive methodology based on older methods that were initially applied to the analysis of visual thresholds. These “stimulus-dependent” methods are described next.
Scaling Suprathreshold Pain Sensation: Response-Dependent Methods
Tolerance measures and the threshold procedures described earlier can be considered to be “stimulus-dependent” methods because the dependent variable is an amount of stimulus intensity (or time) corresponding to a fixed response of the pain threshold. In contrast, many of the suprathreshold scaling procedures can be classified as “response-dependent” methods. These methods deliver a series of discrete stimuli of varying intensity in random sequence. The dependent variable is some measure of subjective response.
These response-dependent measures are more complex than methods that assess threshold or tolerance by an ascending series. However, these methods minimize the numerous biases associated with the ascending methods discussed earlier. Presentation of stimulus sequences in which stimulus intensity is varied randomly avoids confounders associated with time or order. The difference in stimulus intensities should be small enough to create confusion between adjacent stimulus intensities and thus force choices based on judgment of sensation and not on identification of specific stimulus intensities (e.g., this is the second stimulus from the bottom, which I call “4”). As a further advantage, these methods deliver sensations over the entire perceptual range and do not focus only on the bottom, the threshold level, or on the top, the tolerance region.
These methods all assume that subjects can meaningfully quantify the evoked sensation on a psychological scale of pain magnitude but vary in both the type of response and the analysis of these responses. Figure 20-3 shows common responses, including both discrete numerical (0–10) and verbal (mild, moderate, severe) categorical scales, a combined numerical and verbal scale, and a bounded continuous response dimension, in this case the common visual analog scale (VAS). Theoretically unbounded measures, such as numbers or time duration, are used with the psychophysical scaling techniques of magnitude estimation and cross-modality matching.
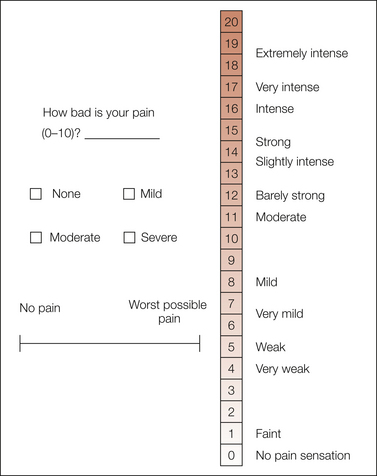
Figure 20-3 Common pain measures.
Three scales are shown on the left. Pain is often rated on a simple number scale of 0–10, and 4-point category scales are a classic standard in clinical pharmacology. The most widely used scale is the visual analog scale, which is commonly displayed as a horizontal 10-cm line labeled at the extremes, although it can be presented in several possible orientations and label formats. A combined verbal–numerical category scale of pain intensity is shown on the right. Previously quantified verbal descriptors are spaced logarithmically along a spatial 0–20 numerical category scale. Subjects are instructed to use the semantic space on the right to form a response and then report the appropriate number on the left. This type of scale is especially useful for situations such as telephone surveys or neuroimaging studies in which a manual response is to be avoided or is impossible.
Simple category scales such as the four-point “none, mild, moderate, and severe” or the common 0 to 10 numerical scale can be scored in several ways. The simplest, the “method of equal-appearing intervals,” assigns successive integers to verbal categories or uses numerical categories directly. The more complex, the “method of successive categories,” determines specific category values depending on the proportion of responses made to each stimulus intensity. An additional approach determines specific numerical values for each category in a separate session. Subjects use several types of scaling methods to quantify the magnitude implied by each response category.
Category scales have been the standard in clinical trials and in many pain studies, and their reliability and validity have been demonstrated repeatedly with limited 4-point scales of pain or pain relief. Issues include the resolution provided by a limited number of categories and a number of biases associated with the limits of the available categories, described later in the discussion on bounded scales. In addition, the response is easily remembered, which confounds measures of repeat reliability or studies of pain memory.
As shown in Figure 20-3, the VAS scale usually consists of a 10-cm line labeled at the anchor points with “no pain” and “most intense pain imaginable” or similar descriptions. Subjects indicate their pain magnitude by marking the line at the appropriate point. The ease of administration and scoring has contributed to the widespread use of this method. The lack of a distinct response category avoids the confounding factor of remembering discrete responses. The concerns about reliability that apply to many types of suprathreshold procedures may not be specific to the VAS scale (Yarnitsky et al 1996).
Both VAS and category scales are “bounded”; that is, they provide a limited range of measurement confined by fixed end points. When using these scales to describe a range of painful stimuli, subjects typically spread their responses out to cover the entire range of possible responses. In the extreme case, this tendency results in the same scale for any stimulus set. In most cases it makes VAS, category, and other bounded scales very sensitive to stimulus range, spacing, and frequency. This effect would tend to reduce the sensitivity of a scale to a pain control intervention because subjects would use the same responses before and after the manipulation. This tendency would be most problematic in situations that deliver multiple painful stimuli to normal individuals and, theoretically, less of a problem in clinical assessment. Despite these theoretical problems, VAS scales have been used successfully for assessment of the sensory intensity and unpleasantness of experimental pain sensations and for evaluation of the mechanisms and efficacy of both pharmacological and non-pharmacological interventions. Use of longer VAS scales (Price and McHaffie 1988) and specific instructions appears to avoid many of the problems of bounded scales.
Many modern psychophysical scaling methods also avoid the problem of bounded scales by using scales with an unbounded response range. The most widely used example is the method of “magnitude estimation” (Beydoun et al 1996), in which subjects describe the magnitude of the sensation evoked by the first stimulus with a number and then assign numbers to subsequent stimuli in proportion to this judgment. If the second sensation is judged to be twice as great as the first, the number given is twice that made for the first sensation. The first stimulus may be either arbitrary or fixed (the standard), and the first response value may be either arbitrary or fixed (the modulus). These methods theoretically produce ratio scales with a true zero point that allows multiplicative statements such as “the pain is one-third of what it was before the analgesic.” Price and McHaffie (1988) provided evidence that VAS scales also provide ratio-level measurement. Although the ratio properties of these various methods have been debated in both the psychophysical and pain literature (Gracely and Dubner 1981), these methods provide more information and are less sensitive to the biases associated with the bounded response range of VAS and category scales. Ratio scaling methods have been used to assess pain magnitude, including variations in which the response is another adjustable stimulus modality (Gracely et al 1978a), the response is made to both painful and non-painful stimulus modalities (Duncan et al 1988), or various responses are used to quantify the magnitude implied by the labels in a pain category scale. These values are used in analysis of the scale when it is applied to pain measurement (Gracely et al 1979). One important, but rarely used feature of quantified verbal categories is the presentation of response choices in random order, which requires a unique cognitive task that avoids a common problem with other methods. Rather than choose from an actual response space, be it either a VAS scale or a category scale (which can be treated as a VAS scale by ignoring the meaning of the words), a randomized response scale forces responses based on the meaning of the descriptor rather than its spatial location in a list. Though possibly difficult for the subject, this method avoids the rating biases (e.g., spreading responses over the scale) found with all bounded spatial scales. Forcing choices based on meaning may facilitate discrimination of different pain dimensions, as discussed later.
In addition to randomization, quantified category values permit the use of hybrid scales that combine verbal and graphic ratings by placing descriptors in appropriate locations on an analog or category continuum as shown in Figure 20-3 (Naliboff et al 1997, Sternberg et al 2001). Unlike the VAS, these scales allow measurement without marking a line, a feature useful for telephone evaluations and for studies such as brain imaging in which a motor response is difficult or undesirable (Gracely et al 2002).
A variety of VAS scales have been incorporated into automated systems that can provide continuous measures of pain sensitivity over time. Such measures can indicate pathological states, such as abnormally prolonged sensations, that are not evaluated by ordinary scaling methods (Gracely 1991, Graven-Nielsen et al 1997). Automated systems have been developed recently that collect sequential discrete responses over longer periods by using handheld PDA (personal digital assistant) devices. Though designed to measure clinical pain, these methods use VAS or analog descriptor response scales and have been validated in studies involving normal, pain-free subjects (Jamison et al 2002). A comparison between ratings of pain made with either electronic or paper VAS scales is shown in Figure 20-4.
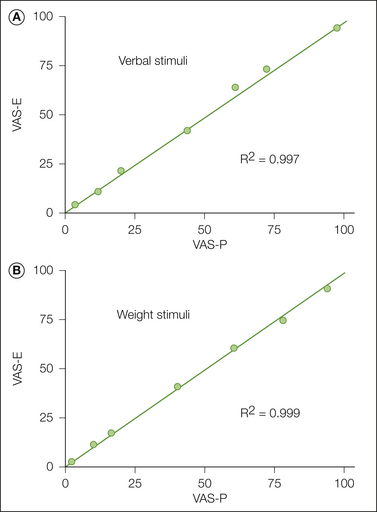
Figure 20-4 Comparison of pain ratings on a 10-cm paper and 5-cm electronic visual analog scale (VAS-P and VAS-E).
Subjects used either scale to measure an actual sensation, the heaviness of weights, or sensations implied by verbal descriptors ranging from “faint” to “extremely intense.” There is very close agreement between the scaling methods for both common sensory and cognitive stimuli. (From Jamison RN, Gracely RH, Raymond SA, et al 2002 Comparative study of electronic vs. paper VAS ratings: a randomized, crossover trial using healthy volunteers. Pain 99:341–347.)
Scaling methods that require greater cognitive demands have been applied to pain assessment. Two similar methods, functional measurement and conjoint measurement, require a single response to not one stimulus but rather to an integrated impression of two or more stimuli. These stimuli can both be painful, or subjects can respond to a combination of pain evoked by somatosensory stimulation and pain implied by either a verbal descriptor (Gracely and Wolskee 1983) or the discomfort of an aversive tone (Algom et al 1986). These stimulus integration methods provide more information than that available from single-stimulus, single-response designs. They simultaneously evaluate subjective magnitude and, in addition, can evaluate each subject’s ability to perform the scaling task. The method may also be used to assess physiological interaction or additivity (Lautenbacher et al 2007).
Scaling Suprathreshold Pain Sensation: Stimulus-Dependent Methods
Similar to measures of pain threshold and tolerance, these procedures use a physical measure of stimulus intensity as the dependent measure. These “staircase” or “adaptive” methods, which are commonly used to measure pain threshold, have been adapted to assess suprathreshold pain sensation. In these methods, an interactive computer program continuously adjusts the intensity of stimuli so that some fall within specific response categories. Figure 20-5 shows an example in which staircases are titrated between “no pain” and “mild,” “mild” and “moderate,” or “moderate” and “intense.” The algorithm for this adjustment can be based on either staircase rules or probability estimates (Gracely et al 1988). In each case the magnitude of responses to specific stimulus intensities are used to adjust future stimulus intensities to maintain response magnitudes at specific levels. These stimulus-dependent scaling procedures are useful in clinical populations because they automatically equalize the psychological range of stimulus-evoked sensations, thereby ensuring that subjects with widely varying pain sensitivity receive similar sensory experiences. Because the stimuli are adjusted continuously to present the same perceptual experience, this method minimizes the extraneous cues (e.g., reduced stimulus range) that an analgesic has been delivered. In addition, the response is expressed in units of stimulus intensity, which allows comparison of effects across different experiments.
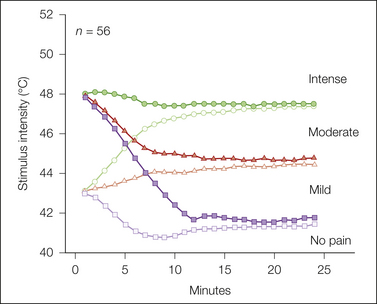
Figure 20-5 Multiple random staircase evaluation of pain intensity evoked by a 1-cm2 contact thermode.
Fifty-six subjects received 5-second heat stimuli applied to the volar side of the forearm. On each trial, one of six independent staircases is chosen at random and a stimulus temperature associated with that staircase is delivered. A response from the 4-point response scale shown at the right, a criterion between a pair of categories, and the response history determine the next stimulus to be delivered by that staircase the next time that it is randomly chosen. In this example a pair of staircases is associated with each of the three intervals between the response categories. To demonstrate the ability of the algorithm to converge to a common stimulus temperature, one staircase from each pair is initiated at 43°C, and the other is initiated at 48°C.
Scaling Suprathreshold Pain Sensations: Minimizing the Influence of Factors Such as Distress in Clinical Evaluations of Pain Sensitivity
There is considerable evidence that pain ratings may be influenced by a number of psychological factors. Whether this modulation is in the experience of pain or just an effect on the pain rating method (i.e., rating bias), it is an important research question specific to each type of modulating factor and experimental pain modality. In clinical assessment, a prime example of this phenomenon is determination of tenderness in patients with fibromyalgia. In addition to widespread ongoing pain, the American College of Rheumatology criteria (1990) for fibromyalgia required that 4 kg of manual pressure elicit pain in at least 11 of 18 defined tender points. Because patients were increasingly aware of the location of these tender points and the required response to label the sensation as painful, this method was easily biased. Use of a mechanical dolorimeter provided a more objective measure of the pain threshold; however, it was still biased because the procedure was based on a predictable ascending series. The influence of psychological factors in the determination of clinical tenderness is supported by the results of population studies that have found a correlation between the number of tender points and various measures of psychological distress (Wolfe 1997). Perhaps in part because of these issues, the tender point count has been dropped from the recently revised diagnostic criteria. Whether this modulation represents an effect of bias or an effect on perceived pain was addressed in a study by Petzke and colleagues (2003b) that compared the influence of distress on the number of tender points, dolorimeter measures of tenderness at these points and at the thumb, and suprathreshold scaling of randomized discrete pressure stimuli applied to the thumb. These measures were collected from a sample of 47 subjects that included a mixture of healthy controls and pain patients to produce a normally distributed range of tenderness when a dolorimeter was applied to the tender points. Regression analysis showed a significant influence of distress on the tender point measure and a lesser, but significant effect on the dolorimeter measures. In contrast, the suprathreshold scaling methods showed a non-significant, minimal association with distress. This result was validated by a subsequent study that used factor analysis in 97 patients and showed that a commonly used clinical measure of tenderness is contaminated by psychological distress and also that the nature of this influence is on the rating process and is not reflected in a physiological effect of increased pain (Giesecke et al 2003).
Pain Psychophysics: Role of Gender, Age, Race, and Ethnic Identity
A growing literature demonstrates gender differences in pain evoked by heat, cold, pressure, chemical, and electrical stimulation (Fillingim et al 2009), and the general topic of gender differences is addressed in Chapter 15 by Greenspan and Traub.
Sixty years ago an anthropologist described ethnic differences in pain expression, with Jewish and Italian Mediterranean cultures being more expressive than American and Irish cultures (Zborowski 1952). This report further differentiated these groups: Italians were supposedly more present centered, whereas Jews were concerned about the future. Irish were influenced by negative social connotations of pain expression, whereas Americans were thought to genuinely be stoical. Within 2 decades these differences were partly confirmed in the experimental pain laboratory. Turksy and Sternbach, using electrical stimulation of the skin, compared the pain sensitivity of housewives in these ethnic groups who had immigrated to the United States. Both psychophysical and psychophysiological measures provided experimental confirmation of Zborowski’s observations (Sternbach and Tursky 1965, Tursky and Sternbach 1967).
Ethnic differences have been demonstrated repeatedly by using a variety of experimental pain measures. In the United States, the majority of these studies have compared Caucasians and African Americans. Beginning with the original study of Chapmen and Jones (1944), which actually preceded Zborowski’s anthropological studies, experiments have found similar or increased sensitivity in African American subjects (Edwards et al 2001; Campbell et al 2005; Mechlin et al 2005; Rahim-Williams et al 2007; Campbell et al 2008a, 2008b). In comparison to Caucasian subjects, these studies have generally found similar pain thresholds but less tolerance and increased pain ratings in response to cold pain, heat pain, and ischemic pain in African Americans. This pattern of reduced tolerance and increased sensitivity to suprathreshold stimulation has been interpreted in terms ranging from psychological mechanisms such as hypervigilance (Campbell et al 2005) to physiological mechanisms of impaired endogenous pain regulatory systems (Mechlin et al 2005).
Increased pain sensitivity has also been observed in minority groups, such as Asian Indian Singaporeans in comparison to Chinese and Malays (Tan et al 2008), South Indians in comparison to Danish Caucasians (Gazerani and Arendt-Nielsen 2005), Middle Eastern subjects in comparison to Swedes (Dawson and List 2009), and Chinese in comparison to European Canadians (Hsieh et al 2010). The study of Singaporeans was interesting because it used a natural, acute painful stimulus, cesarean section, instead of laboratory stimulation, and the dependent measures included both pain ratings and morphine consumption. A study of Libyans in Libya noted that sensitivity was decreased in the majority ethnic group (Tashani et al 2010) but still found increased sensitivity when compared with the results of a reference group of “Western” subjects from London who participated in a separate experiment (Keogh et al 2005).
In addition to the demonstrated effects of gender and ethnic or racial identity, the influence of age on pain perception has been evaluated, with common findings of increased sensitivity in clinical conditions and varying results in experimental settings (Gibson and Helme 2001, Lautenbacher et al 2005). One source of this variability is probably methodological and results from the use of different single-stimulus modalities and methods across experiments. As with studies of analgesics, the use of multiple modalities may provide a more consistent profile of aging effects. Lautenbacher and colleagues used such an approach and observed decreased sensitivity to non-noxious stimuli and contrary effects with painful heat and pressure; heat thresholds were not affected by age, whereas heat temporal summation was increased with age (Lautenbacher et al 2005). In contrast, pressure pain thresholds decreased with age but pressure temporal summation was not influenced by age. Because pressure pain is assumed to be more strongly influenced by descending inhibition, this result may be consistent with more recent evidence of decreased descending regulatory systems with aging (Gibson and Farrell 2004, Farrell and Gibson 2007, Cole et al 2010, Riley et al 2010). Further studies using multiple modalities and loci are needed to support this concept.
This brief description of unidimensional pain measurement indicates how conventional measures such as magnitude estimation or procedures such as randomized verbal descriptors, magnitude-matching methods, or stimulus-dependent scaling methods are adapted to the measurement of suprathreshold pain magnitude. These methods may control for specific biases such as those associated with spreading responses to cover the range of a scale. However, they condense the experience of pain into a single dimension of pain magnitude. They do not adequately assess the relevant dimensions of the experience.
Dual Dimensions of Sensory Intensity and Unpleasantness
The dual nature of pain has been recognized throughout philosophical and scientific history. Pain is both a somatic sensation and a powerful feeling state that evokes behavior to minimize bodily harm and promote healing (Wall 1979). Single measures of pain magnitude blur this distinction and create confusion because the underlying meaning of an expressed pain magnitude is not known.
This confusion may be minimized by scales that essentially ask, “how intense is your sensation, and how much does it bother you?” There is a precedent for such scales because the sensation of pain is not uniquely endowed with motivational characteristics. A sensory intensity and a feeling state also characterize hunger and thirst, the chemical senses (taste, olfaction), and the thermal senses (warm, cool). Studies involving these modalities have demonstrated different psychophysical functions for scales of sensory intensity and “hedonic” scales of pleasantness–unpleasantness. In addition, manipulation of the internal state (core temperature, hunger) has been shown to shift the hedonic responses without altering judgments of sensory intensity (Gracely et al 1978b).
The intensity and hedonic components (unpleasantness) of pain have been assessed by a number of scaling methods. In some cases, different types of scales were used to measure the two dimensions. The results of such studies must be interpreted with caution. Because these studies confound the different dimensions with the type of scale, the results could be due to method variance and not to a differential effect of pain dimension (Gracely et al 1978b).
Verbal category scales with words descriptive of each dimension have distinguished between pain intensity and unpleasantness in a number of situations (Gracely et al 1978b, 1979; Gracely and Kwilosz 1988). The use of language specific to a dimension is assumed to facilitate discrimination of these dimensions. Commonly used VAS and other similar scales have also distinguished between pain intensity and unpleasantness. Although verbal methods have been found to be more discriminative than VAS scales (Gracely et al 1978b, 1979; Duncan et al 1989; Gendreau et al 2003), the combination of extensive instructions to the subject and the labels on a VAS scale (“the most intense pain sensation imaginable,” “the most unpleasant feeling imaginable”) probably promotes the discrimination of intensity and unpleasantness (Price 1988). The ability of subjects to describe these dimensions with each method and the role of instructions and training are obvious topics for future research.
The non-sensory aspects of the pain experience have been referred to as the reaction, emotional, affective, or evaluative component, as well as other terms such as discomfort, distress, and suffering. The number and structure of these components have not been firmly established, although recent proposals include both an immediate unpleasantness component, similar to the feelings associated with other senses, and a secondary affective component that includes emotions and feelings of distress mediated through cognitive appraisal. These types of studies and those described in the next section should continue to clarify the feeling and emotional components of pain sensation.
Multiple Pain Dimensions
Multidimensional scales emphasize the differences between pain sensations, or the distinguishing features that separate various pain syndromes. Sensory intensity and unpleasantness scales are “a priori” scales in the sense that they assume two significant dimensions of pain. In contrast, multidimensional methods empirically determine the number and character of the relevant dimensions. They do not make a priori assumptions about the structure of the pain experience.
Our own experience verifies the variety of pain qualities. Pain can be deep or superficial, pricking, burning, throbbing, aching, or shooting. This breadth of the pain experience is evaluated in normal individuals by three types of studies: (1) multidimensional scaling of experimentally evoked pain sensation to determine scale dimensions, (2) multidimensional scaling of verbal descriptor items to construct a scale or verify the structure of an existing scale, and (3) use of existing scales to assess experimentally evoked pain sensations.
Multidimensional scaling of sensations evoked by electrical or thermal stimulation provides examples of the first type. In these studies, similarity judgments of stimulus pairs resulted in a primary dimension of sensory intensity and secondary dimensions of either painfulness or frequency when the frequency of the stimulus was varied (Janal et al 1993).
Examples of the second type of multidimensional investigations include several studies that have examined the structure of the McGill Pain Questionnaire (MPQ), which is probably the most widely used multidimensional instrument (see Chapter 21). The questionnaire was developed from a study by Melzack and Torgerson (1971) in which a large number of pain descriptors were ultimately classified into 20 categories describing sensory qualities, affective qualities, and an evaluative dimension. A total of 78 descriptors appear in the present instrument, with 2–6 descriptors per category. A short form presents a subset of 15 words, and subjects rate the magnitude of the sensation or feeling on a scale of none, mild, moderate, and severe (Melzack 1987). Subsequent studies have replicated this method or have derived a structure with the use of multidimensional scaling methods (Gracely and Naliboff 1996). The results of these experiments confirm the two main dimensions of sensory intensity and affect/unpleasantness, but they have resulted in different category assignments and variations in the overall organizational scheme of hierarchical categories. An extensive study by Torgerson and colleagues (1998) developed the ideal type model, which rates each descriptor on an intensity continuum and, in addition, quantifies “quality” in terms of a number of primary ideal qualities or types. The number of primary qualities and the degree to which each of them contributes to a specific descriptor are specified, much like the primary components of a color mixture. The MPQ, in contrast, assigns only one quality to each descriptor. A review of all these descriptor structures reveals many commonalties. Pain sensation is described by thermal qualities; by temporal patterning; by location or changing location (superficial or deep, spreading, moving); and by a series of mechanical qualities such as punctate, traction, and compression pressure. Subsequent analyses have made finer distinctions. For example, whereas the MPQ places “pricking,” “stabbing,” “drilling,” and “boring” in the same class, the ideal type model places “pricking” and “stabbing” in a class separate from “drilling” and “boring,” as distinguished by the rotational character of the latter class. The most variability appears in the affective components of pain with dimensions that describe unpleasantness, suffering, fear, autonomic reactions, and fatigue.
The third class of multidimensional study uses multidimensional scales to assess the magnitude and quality of pain sensations produced by experimental stimulation. Few such studies have been performed because these scales are used predominately in clinical evaluations. An early study compared the MPQ responses of both patients and normal subjects receiving painful electrical skin stimulation (Crockett et al 1977). Factor analysis identified five common factors, thus emphasizing the utility of assessing common dimensions of experimental and clinical pain. Another experiment by Klepac and co-workers (1981) assessed high or low levels of either cold pressor pain or electrical tooth pulp pain in a 2 × 2 factorial design. Overall intensity scores differentiated the two types of stimulation, which also resulted in qualitatively different responses.
In summary, validated methods have been developed to assess one, two, and more dimensions of the pain experience. What should an investigator do? The answer again depends on the experimental question. Naliboff (Gracely & Naliboff 1996) identified four criteria for increasing the number of dimensions:
A multidimensional system may increase utility if it: (1) leads to an increase in accuracy of pain reports. If for example a rating of intensity and affect misses or blurs critical aspects of a pain sensation then a patient or subject’s pain may change due to treatment or experimental manipulation and this change could be missed. This is essentially an issue of reliability. (2) Increases greater diagnostic sensitivity. If, for example, the amount of prickliness of a pain is a clear marker of certain types of tissue pathology then assessment of only sensory and affective intensity (painfulness) may yield poorer diagnostic discrimination. Similarly, pain ratings with very unusual patterns of multidimensional ratings might indicate malingering or confusion. (3) Increases communication about pain, and therefore empathy with patients suffering, and (4) improves the correspondence between neurophysiological and psychological data. With the dramatic increase in sensitivity in brain imaging we might expect to see more specificity in terms of which brain areas correspond to which pain dimensions.
Further choices between double and multiple dimensions must be made in the context of the measurement situation. As noted earlier, multidimensional methods do not make assumptions about the number or type of significant dimensions. The goal of multidimensional methods is to discover the salient dimensions, although the results of such discoveries support the concept of dual dimensions (Gracely and Naliboff 1996).
Non-Verbal Measures
Concerns about the reliability and validity of verbal judgments have motivated the development of physiological and behavioral “objective measures” of pain magnitude that would be relatively insensitive to biasing factors and the psychological demands associated with requests for introspective reports. There are also instances in which such measures are necessary, such as assessment of pain in animals and infants or in adults with poorly developed language skills.
Although arguments have been made for the exclusive use of non-verbal methods, these procedures can also be influenced by extraneous factors. In addition, non-verbal methods lack the face validity of verbal report. They use similarity to verbal report to establish concurrent validity, thus suggesting that verbal measures are preferable if available. Generally, arguments for the superiority of one method over another often reflect the tendency of research laboratories to specialize in a single method. The resulting differences have sparked lively debate, identified important measurement flaws, and generally improved the technology of pain assessment. There is a growing consensus that in most situations, effective pain assessment may ultimately result from an approach that integrates information from these separate, complementary sources of information. However, as discussed further, the considerable plasticity in pain processing and the many examples of disassociation between non-verbal measures and subjective reports indicate that ultimately, pain can be defined only by a verbal description of the perceptual experience.
Behavioral Measures
It is well known that pain elicits stereotypical behavior in both humans and animals. Grimacing, vocalization, licking, limping, and rubbing are often elicited by a painful stimulus. Both these naturally occurring reactions and trained operant behavior (such as manipulating a bar to escape a painful stimulus) have been used to assess the magnitude of stimulus-evoked pain sensation. Many have been used more extensively for the assessment of clinical pain syndromes (Keefe and Dolan 1986, McDaniel et al 1986). Exceptions include studies of facial expression evoked by experimental stimulation (Patrick et al 1986) or analysis of pain expressions from photographs (LeResche 1982).
The behavioral measure of reaction time latency to painful heat has been shown to be monotonically related to stimulus intensity, a finding that permits the use of reaction time, in controlled conditions, as a measure of pain magnitude (Kenshalo et al 1989, Sternberg et al 1998).
Physiological Measures
Studies have long focused on an objective measure of pain processing, and this field remains a very active area of research. Early studies focused on autonomic measures such as heart rate and skin conductance but found that these responses habituated quickly and were non-specific because they were evoked by painful, non-painful, or novel stimulation. Autonomic measures continue to be assessed, but the bulk of such studies examine physiological measures related to neural processing. Progressing from the periphery to the brain, these methods examine microneurographical recordings of primary afferent activity, spinal reflexes, evoked and ongoing cortical activity, recording and stimulation of the thalamus and brain during neurosurgical procedures, and functional brain imaging.
Microneurography
Neurophysiological recording of afferent activity in animals has provided a wealth of information about the function of nociceptors. Human microneurography uses recording methods tested in animal research to investigate peripheral mechanisms in unanesthetized normal volunteers. The pioneering experiments (Vallbo and Hagbarth 1968) recorded from myelinated A fibers and, subsequently, from unmyelinated C-fiber afferents (Torebjork and Hallin 1970). Human microneurography is a powerful tool that can compare intervening primary afferent activity in response to both the evoking stimulus and the resulting sensation. This method has characterized several functional classes of C fibers, including polymodal nociceptors that are sensitive to heat, mechanical, and chemical stimulation. A marking method has been used to identify another class of mechano-insensitive C fibers that probably mediate blunt pressure pain and, when inflamed, respond to mechanical punctate stimulation (Namer and Handwerker 2009, Schmelz and Schmidt 2010). A third class of C fibers responds to cool temperatures (Campero et al 2009). The mechanically insensitive C fibers have been shown to be involved in aspects of central sensitization, for example, in mediating spontaneous pain following injections of capsaicin and the resultant mechanical hyperalgesia (Schmelz et al 2000). Microneurography has identified neural signatures for itch and compared flare responses in human, pig, monkey, and rat subjects and activity in the terminal arborization of C-fiber nociceptors (Weidner et al 2003, Namer and Handwerker 2009, Schmelz and Schmidt 2010). This method remains a valuable conduit to the functioning of pain primary afferents in both health and disease.
Spinal Reflexes
Most studies of pain-related reflexes assess the spinally mediated electromyogram (EMG) response to a brief intense stimulus. Several measures of reflex activity, such as the H-reflex, the nociceptive (RIII) reflex, jaw inhibitory reflexes, and the blink reflex, have been investigated in human subjects. These reflexes are commonly elicited by a train of electrical pulses (typically five 1-msec pulses at 200–300 Hz) and, rarely, by laser stimulation (Andersen et al 2006) and mechanical stimulation, such as ballistic impacts (Beise et al 1999). Basic studies have assessed nociceptive specificity (Kaube et al 2000, de Tommaso et al 2001, Romaniello et al 2002), optimized parameters (Katsarava et al 2002), and determined the spatial organization of reflexes as a function of intensity and locus of stimulation (Andersen et al 2001). These studies have provided information about the neural organization of the nociceptive system, including the convergence of occipital and trigeminal cutaneous afferents. These measures also respond appropriately to many pain interventions, including attenuation by both transcutaneous electrical nerve stimulation (TENS) and opiates and demonstration of the stress-produced changes antagonized by naloxone. In addition, the amplitude of evoked reflexes has been shown to correlate with other physiological parameters such as cerebral evoked potentials or the concentration of administered analgesics and anesthetics or circulating opioids (Skljarevski and Ramadan 2002).
Nociceptive reflexes share the temporal resolution that is a feature of the following methods in this section. This resolution has been used to investigate mechanisms mediating pain and pain modulation. Edwards and colleagues (2002) took advantage of the precise timing characteristics of cutaneous electrical stimulation to deliver stimuli to the sural nerve during different phases of the cardiac cycle. Using either the amplitude of the nociceptive reflex produced by specific stimulus intensities or the amount of current needed to evoke the reflex, these authors found reduced sensitivity during the systolic pressure pulse consistent with an arterial baroreceptor mechanism of hypertensive hypoalgesia. Several studies have also demonstrated modulation of lower limb or jaw muscle reflexes by deep muscle pain evoked by the infusion of hypertonic saline (Andersen et al 2000), by intramuscular electrical stimulation (Andersen et al 2006), by brief heat (Andersen et al 1998), and by simple limb movement (Don et al 2008). These studies indicate that inhibition of reflexes is not a unitary, homogeneous effect but varies depending on at least the type and location of the conditioning tonic stimulation.
The nociceptive reflex is also modulated by psychological variables such as emotion, anticipation, and expectation (Rhudy et al 2006; Goffaux et al 2007, 2009) and has been observed to be suppressed in clinical pain conditions (Langemark et al 1993) and to vary between ethnic populations (Campbell et al 2008b) and during the menstrual cycle (Tassorelli et al 2002). It provides a useful marker of central summation (Biurrun Manresa et al 2010) and attenuation of temporal summation (Guirimand et al 2000, Bajaj et al 2005). A study combining the nociceptive reflex and functional magnetic resonance imaging (fMRI) found at least two separate effects in subjects, with sustained activation by a tonic pain stimulus in the orbital frontal cortex predicting subjective analgesia and sustained activation in the primary somatosensory cortex and the periaqueductal gray predicting suppression of the nociceptive reflex (Piche et al 2009). Nociceptive reflexes provide considerable information, especially in concert with supraspinal and other physiological measures and subjective pain reports. Recent studies that provide normative values (Neziri et al 2010) and more sensitive analytical methods (Neziri et al 2009) have further enhanced this utility.
Supraspinal Processing
There is increasing growth both in the methods used to assess supraspinal processing and in the knowledge gained from these methods. As an example, the third edition of this textbook, published in 1994, described two studies using positron emission tomography (PET); the method of fMRI was in its developmental stages and had not yet been applied to pain. By the time of publication of the next edition in 1999, PET had become a mature technology with dozens of applications to pain, and fMRI pain studies were emerging. The field has expanded dramatically in the past decade, and the large body of functional brain imaging evidence is presented in a separate chapter (see Chapter 7). The remainder of this section highlights the active field of supraspinal processing using the evoked and spontaneous electroencephalogram (EEG), magnetoencephalogram (MEG), and intercellular recording of physiological responses directly from the brain.
Cortical Evoked Potentials
Application of a temporally controlled stimulus evokes a small, synchronized response in the EEG embedded in non-synchronized (noise) EEG activity. Averaging multiple trials reduces the influence of random, non-synchronized activity and reveals a waveform of about 1 second in duration that can be characterized by the amplitude and latency of positive and negative peaks. Early, short-latency components of the waveform are associated with sensory components, whereas later components have been associated with perceptual processing. These measures are described by several names such as cortical evoked potentials (CEPs), somatosensory evoked potentials (SEPs), or event-related potentials (ERPs). These measures have been studied extensively and under certain conditions correlate with both stimulus intensity and verbal report (Kanda et al 2002). Potentials evoked by electrical, laser, contact heat, and mechanical stimulation have been used to assess a number of research goals. Many of these have examined the waveform and topography of evoked responses to stimuli applied to skin, muscle, and viscera (Arendt-Nielsen and Yarnitsky 2009).
The principal advantage of these evoked methods is high resolution in the time domain. Figure 20-6 shows an example of this resolution in which laser evoked potentials (LEPs) are used to assess whether the baroreceptor modulation of pain observed by using measures of blood pressure within and between can be observed dynamically within individuals during different phases of the cardiac cycle. LEPs from 10 subjects were attenuated during systole in comparison to diastole (Edwards et al 2008), thus providing evidence consistent with the observation of dynamic baroreceptor modulation using the nociceptive reflex (Edwards et al 2002). In another example, both LEPs and contact heat evoked potentials were used to evaluate putative differences in nociceptor activation in hairy and glabrous skin. The inability to evoke first pain sensation by contact heat in glabrous skin is attributed to a lack of type II AMH (A-fiber mechano-heat–sensitive) nociceptors in hairy skin. The response to contact heat was delayed and attenuated in comparison to responses evoked by laser stimulation in glabrous skin, and the response to contact heat in glabrous skin was delayed in comparison to the response to contact heat in hairy skin (Iannetti et al 2006). Potentials in hairy and glabrous skin were similar when evoked by laser stimulation. These results suggest that the current concept that glabrous skin is devoid of type II AMH nociceptors is an artifact of the evaluation method. A third type of study involves stimulation at the level of the spinal cord and evaluation of the timing and topography of evoked cerebral responses. The results of two studies revealed multiple spinal pathways with faster conduction velocities to the sensory cortex (Tsuji et al 2006, Valeriani et al 2007).
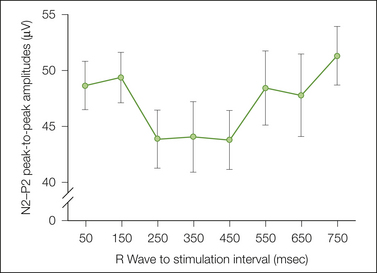
Figure 20-6 Hypertension in normal individuals is associated with reduced experimental pain ratings, an effect assumed to reflect baroreceptor modulation of pain. This figure shows the results of a study that assessed whether the baroreceptor modulation of pain observed by using measures of blood pressure within and between individuals can be observed dynamically within individuals during different phases of the cardiac cycle. Laser evoked potentials (LEPs) were evaluated during high- and low-pressure components of the cardiac cycle. LEPs from 10 subjects were attenuated during systole in comparison to diastole. (From Edwards L, Inui K, Ring C, et al 2008 Pain-related evoked potentials are modulated across the cardiac cycle. Pain 137:488–494.)
The temporal resolution of evoked potentials should allow analysis of effects closely coupled in time, but often only one response is perceived to either two stimuli delivered at short intervals or to a single stimulus that activates multiple nociceptor afferents with different conduction velocities (Lee et al 2009). In the latter case, a laser stimulus should activate a pricking pain sensation mediated by Aδ nociceptors followed by a diffuse burning sensation mediated by the slower C fibers, yet only the pricking sensation is felt and the C-fiber–mediated LEP is not observed. If Aδ activation is avoided, the C-fiber–evoked activity is observed. This result cannot be explained by relative refractory periods (Mouraux et al 2004) and may represent an inhibitory interaction of either fiber type on the other (Tran et al 2008).
In addition to speed, evoked methods can localize the origin of the signals. Source analysis provides information about the cerebral region evoked by electrical, laser, and contact heat stimulation (Drewes et al 2004, Chen et al 2006, Hobson et al 2010, van den Broeke et al 2010). Source analysis locates areas of activation that are also found in functional imaging studies (Brown et al 2008a, Nir et al 2008). Precise localization of these regions has been improved by methods that use MRI scans of the same subjects (Mobascher et al 2009, Piche et al 2009) to transform the results into standard coordinate space. The results of several studies have localized peaks of the potentials to generators in multiple regions of the pain “matrix,” a general term describing consistently activated regions such as the anterior/posterior cingulate cortex, posterior parietal cortex, anterior insular cortex, and regions corresponding to the bilateral secondary somatosensory cortex. Regardless of whether supplemented by fMRI, the EEG evoked potentials and MEG evoked magnetic activity discussed later provide high resolution in the time domain that complements the spatial resolution provided by methods such as fMRI. For example, although fMRI studies show activation in the primary and secondary somatosensory cortices, studies using evoked EEG activity show a consistent initial sequence in which the contralateral primary and secondary somatosensory cortices are activated first and the ipsilateral secondary somatosensory cortex is activated 13 to 18 msec later (Inui et al 2002, Frot and Mauguiere 2003). Analysis of the laser-evoked P2 component and fMRI confirms subsequent activation in the anterior cingulate cortex and also in the amygdala and thalamus (Iannetti et al 2005, Mobascher et al 2009). The source analysis method of “low-resolution brain electromagnetic tomography” (sLORETA) has been applied to contact or laser heat evoked potentials, with initial activation being associated with subjective pain ratings localized in the primary somatosensory cortex that are most closely associated with subjective pain ratings and activation in an attentional network that included the dorsolateral prefrontal, posterior cingulate, and inferior parietal cortices and was associated with uncertainty about the intensity of a stimulus (Brown et al 2008a, Nir et al 2008). Certainty was associated with activity in structures outside the pain matrix that involved semantic and prospective memory (left inferior frontal cortex, inferior temporal cortex, right anterior prefrontal cortex) (Brown et al 2008a).
In addition to the temporal dynamics of cerebral responses to painful stimulation, these methods address common experimental questions, including the effects of spatial and temporal summation (Chen et al 2002); effects of cutaneous, muscle, and visceral stimulation (Arendt-Nielsen and Yarnitsky 2009, Brock et al 2010, Hobson et al 2010); and determination of conduction velocity (Tsuji et al 2006, Valeriani et al 2007). A number of studies have assessed the effects of modulation of CEPs by cognitive factors such as attention (Ohara et al 2006; Dowman 2007a, 2007b), point localization tasks (Kanda et al 1999, Valeriani et al 2000), and cognitive strategies during hypnosis (De Pascalis et al 2001). Similar to related psychophysical and reflex experiments, evoked potentials are also modulated by painful stimulation (Valeriani et al 2006, Brock et al 2010). Laser stimulation attenuates both subjective pain ratings and the amplitudes of tooth-related evoked potentials, consistent with activation of diffuse noxious inhibitory controls (DNICs) (Fujii-Abe et al 2010).
Evoked potentials are modulated by non-pharmacological interventions such as theta-burst stimulation (Poreisz et al 2008) and by a wide variety of psychological interventions such as empathy (Bufalari et al 2007, Valeriani et al 2008), trait anxiety, and depression (Vossen et al 2006). However, modulation by known or putative analgesic agents is providing an increasingly important tool for basic pharmacological studies and for the early-phase clinical trials elaborated at the end of this section. As an example, Renner and associates (2007) demonstrated the sensitivity of a method using evoked potentials in response to CO2 applied to the nasal mucosa. This method showed analgesic effects of the minor analgesic acetaminophen and enhancement by caffeine. This method has also demonstrated effects on other minor non-opioid analgesics such as aspirin and ibuprofen (Staahl et al 2009b) without changes in subjective ratings, thus suggesting improved sensitivity with evoked potentials. However, the finding of greater sensitivity of subjective ratings to the effects of imipramine (Staahl et al 2009b) indicates that these differences should be interpreted in terms of selective sensitivity rather than overall superiority of a single method.
One universal advantage of methods such as CEPs and motor reflex responses is assessment in individuals with poorly developed or compromised language skills and in children or infants (Norman et al 2008). In an interesting variant of this approach, Opsommer and Plaghki (2001) compared CEPs evoked by laser stimulation of the hand in children (mean of 10 years) and adults (mean of 24 years). They found a decrease in late CEP amplitude and decreased reaction times with age that may reflect maturation of the heat pain system.
Spontaneous Electroencephalogram
In contrast to the time-coupled EEG response to stimulation, a number of methods use ongoing EEG activity as a dependent measure in human pain studies. The results can be analyzed by the standard method of determining the power in different EEG frequencies by simple Fourier transformation or by advanced methods. In an example of the latter, independent component analyses demonstrate both phase-locked and non–phase-locked responses to brief electrical or laser stimulation that are localized to the thalamus and to the somatosensory, cingulate, and insular cortices (Stancak et al 2010). Thus, these methods can quantify brain responses to temporally precise, brief stimulation in a manner similar to the evoked methods described earlier and provide both temporal and spatial information. In addition, ongoing EEG methods possess an additional unique advantage of evaluating the effects of prolonged stimuli, stimuli that are poorly controlled, or stimuli without a precise onset. Recent studies have assessed the effects of tonic heat (Huber et al 2006; Nir et al 2008, 2010) and cold (Dowman et al 2008) and the effects of procedural pain in newborn infants (Norman et al 2008). Figure 20-7 shows the effects that cold produced by immersing the left hand in a bucket of ice water. These methods have also been used to assess the effects of confidence and anticipation of pain on EEG responses (Babiloni et al 2007, 2008; Brown et al 2008b) and have used EEG responses during anticipation to predict subjective pain intensity (Babiloni et al 2006). In addition to this experimental advantage, these methods may have considerable clinical utility. For example, a recent study found differences in the spontaneous EEG parameters of absolute and relative spectral power and of coherence in patients with fibromyalgia with respect to a normative database, with specific differences being associated with pain severity (Hargrove et al 2010).
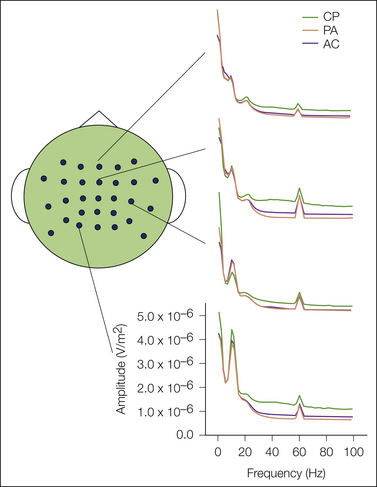
Figure 20-7 Effect on ongoing electroencephalography (EEG) if tonic pain evoked the cold pressor method.
Fifteen subjects participated in three conditions, pain anticipation (AP), arithmetic (AC), and lowering a hand into a bucket of 4.3–4.5°C ice water (CP) during ongoing recording of EEG via 29 scalp electrodes. Tonic cold resulted in reduced amplitudes of alpha activity in the contralateral temporal scalp, shown in the third tracing from the top at the peak over 10 Hz. Tonic cold also increased alpha activity in posterior cortex, shown in the bottom tracing in the peak over 10 Hz. (From Dowman R, Rissacher D, Schuckers S 2008 EEG indices of tonic pain-related activity in the somatosensory cortices. Clinical Neurophysiology 119:1201–1212.)
Spontaneous EEG methods have been used to assess modulation of pain as a result of interventions ranging from empathy (Mu et al 2008) and acupuncture (Hori et al 2010) to administration of benzodiazepines (Barbanoj et al 2007) and opioids (Quante et al 2004, Noh et al 2006). The wide clinical use of EEG, the ability to combine spontaneous EEG with evoked EEG responses, and the low cost in comparison to brain imaging procedures suggest that these methods will continue to provide information about brain processing that includes individual differences and response to treatment interventions.
Magnetic Methods
The electrical currents measured by the EEG can also be assessed by measuring the minute magnetic fields generated by variation in these currents. The magnetocephalogram (MEG) is measured by sensitive, super-cooled detectors (superconductivity quantum induction device [SQUID]) placed near the head. An important feature is that similar to CEPs, source analysis of the magnetic signals can localize the regions responsible for evoked activity (however, the analyses of evoked potentials and MEG differ significantly). Similar to studies of CEPs, recent MEG studies have demonstrated pain-related magnetic fields evoked by electrical stimulation (Inui et al 2002), CO2 laser (Kanda et al 2000, Kakigi et al 2003), mechanical distention of the esophagus (Loose et al 1999), and painful mechanical ballistic impacts (Arendt-Nielsen et al 1999, Druschky et al 2000). Similar to evoked potentials, MEG methods have superior temporal resolution. For example, Figure 20-8 shows the results of a study (Nakata et al 2008) that applied laser stimuli to the thigh instead of to the hand to provide greater cortical separation between activations in the posterior parietal cortex and primary somatosensory cortex (SI). The results show an initial response in the contralateral SI at 152 msec, followed by responses in the contralateral secondary somatosensory cortex (SII) 18 msec later, followed by nearly simultaneous responses in the ipsilateral SII (11 msec later) and contralateral inferior parietal lobule (13 msec later). These regions are activated in conventional brain imaging studies of evoked pain responses, but the second resolution of these methods cannot reveal the sequence of activations provided by the millisecond resolution of evoked potentials or MEG. Second-generation studies are now examining modulation of the MEG response. Previous studies found that distraction has been shown to have no effect on early components associated with the primary and secondary somatosensory cortices and the insula and to attenuate later responses from other brain regions. A subsequent study found effects of distraction on brain regions that included effects on the primary and secondary somatosensory cortices and the insula, with more statistically significant effects being observed in the insular and cingulate cortex (Qiu et al 2004).
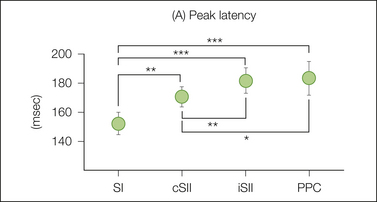
Figure 20-8 Latency of magnetocephalogram signals localized to the sensory cortex and parietal cortex following painful laser stimulation of the thigh.
An initial response in the contralateral primary somatosensory cortex (SI) is observed at 152 msec, followed by responses in the contraeral secondary somatosensory cortex (cSII) 18 msec later, followed by nearly simultaneous responses in the ipsilateral SII (11 msec later) and contralateral inferior parietal lobule (13 msec later). PPC, posterior parietal cortex. (From Nakata H, Tamura Y, Sakamoto K, et al. 2008 Evoked magnetic fields following noxious laser stimulation of the thigh in humans. NeuroImage 42:858–868.)
Additional studies have examined reduced effects by non-pharmacological and pharmacological interventions. In an interesting example of non-pharmacological effects, a combined MEG and fMRI study found attenuation of laser-evoked pain brain responses during meditation by a Yoga master (Kakigi et al 2005). In a more conventional pharmacological manipulation, intravenous clonidine attenuated both subjective pain ratings of electrical intracutaneous stimulation and activity in the contralateral secondary somatosensory cortex, although the lack of correlation between these effects across subjects does not suggest a simple correspondence between SII activity and subjective pain magnitude (Hauck et al 2006). This lack of correspondence extends to similar methods. Hoshiyama and Kakigi (2000) found that TENS attenuated measures of CEPs but had no effect on either pain ratings or evoked magnetic cortical fields, thus challenging the concept that measures of MEG and cortical potentials provide different measures of the same underlying neurophysiological mechanisms.
MEG has also been used to assess interventions that augment pain, such as mechanical hyperalgesia evoked by electrical stimulation of mechano-insensitive C-fiber nociceptors (Maihofner et al 2010). An interesting combined EEG and MEG study found that a preparatory cue increased pain intensity (Hauck et al 2007). Increasing the delay from cue to pain stimulation increased the magnitude of pain ratings and EEG-derived activity in the mid-cingulate cortex, whereas MEG-derived activity in the secondary somatosensory cortex was not altered by changes in the cue interval. Similar to the results with clonidine discussed earlier, these results suggest further studies of the role of the secondary somatosensory cortex in pain perception.
Physiological Recording and Stimulation of Brain Structures in Humans
Studies performed during neurosurgery for pain or movement disorders provide an opportunity to assess the function of central structures by several methods. Paralleling electrophysiological studies in animals, investigators can record from single cells while applying peripheral stimulation. These methods have identified cells in the principal sensory nucleus of the human thalamus that respond with greater frequency as the magnitude of innocuous mechanical or thermal stimuli is increased into the noxious range (Lee et al 1999). These methods can also be used to examine the temporal properties of neuronal firing in the absence of peripheral stimulation, for example, comparing patterns from central neurons projecting from the painful region in patients to those projecting from the unaffected region in the same patients or those from similar regions in pain-free patients (Lenz et al 1998a). Evoked potentials can also be recorded from electrodes implanted on the surface or within the brain. The results confirm the sequences of activation observed with MEG and evoked potentials.
Relevance of Physiological Methods: Informative without Being Necessarily Specific
It is possible to stimulate and record neural activity at both the periphery and brain, as well as in between, to assess spinal and supraspinal activity by EMG reflexes and by ongoing and evoked EEG, evoked MEG, and a number of brain imaging methods of ongoing and evoked activity. These approaches can be applied to multiple species and thus bridge the gap between animal and human studies. The results have clearly increased the knowledge of pain mechanisms and promise to aid in diagnosis and the choice of treatment. As a group, these methods can provide a profile of responses from an individual or a profile of group responses to an analgesic intervention. The latter concept is of interest to pharmaceutical companies because the predictive accuracy of current preclinical evaluations in animals has largely been disappointing (Chizh et al 2008, 2009; Leiser et al 2011). A collection of physiological measures may serve as surrogate biomarkers for analgesic development targeted toward specific pain syndromes. These biomarkers may be mechanistically based, such as the use of blood cholesterol to predict coronary artery disease, or empirical, to provide prediction without a clear understanding of the mechanisms mediating the association. Recent evidence and reviews suggest that much of the evidence from these physiological methods may in fact be empirical. The effects of painful stimulation are often similar to the effects of non-painful somatosensory stimulation or even non-somatosensory auditory or visual stimulation. These effects may represent a non-specific measure of stimulus salience rather than pain-specific processing (Iannetti and Mouraux 2010, Mouraux et al 2011). This does not invalidate their utility as biomarkers in preclinical development or as clinical aids to diagnosis and treatment of an individual patient but, instead, the description of these applications as empirical rather than mechanistic or theory based. In other words, these methods may be very informative without being necessarily specific to a mediating mechanism.
Interestingly, these issues probably also apply to verbal measures of evoked pain. As elaborated later, the field has evolved from specialization of a specific stimulus and location (such as the author’s recent use of pressure applied to the thumb [Gracely et al 2003]) to a profile of multiple modalities applied to different locations and types of tissue. Danish investigators have formalized this approach in the concept of “multimodal and tissue-differentiated experimental pain assessment” in which multiple modalities of heat, pressure, and electrical stimulation are applied to skin, muscle, and viscera (Staahl et al 2006, Arendt-Nielsen et al 2009). Similarly, other investigators have provided evidence of the need for multiple measures to establish a pain phenotype (Bhalang et al 2005), and German scientists have formalized a clinical evaluation using an array of non-painful and painful stimuli (Magerl et al 2010, Maier et al 2010). Similar to the goals of physiological measures, a professed goal of these verbal measures is to provide an individual profile of a patient, a profile of functional subgroups, or a profile predicting analgesic efficacy. Subgroups have been found by comparing different experimental pain methods (Hastie et al 2005) or a combination of pain and psychological methods (Giesecke et al 2003). Despite the general consensus that experimental pain “profiles” hold great promise, the reality of this potential has not been fully achieved because of issues such as confounders by method variance (Angst and Clark 2007) and the likely possibility that specific profiles may not be specific to a single condition but rather represent a group of conditions (Serra 2010).
Comparison of Physiological and Verbal Experimental Pain Methods
One implicit if not explicit goal of physiological and verbal measures of pain processing is to provide an “objective” measure of pain in contrast to “subjective” verbal pain reports. These objective methods are validated by studies that show a close association with verbal reports (Dowman 1993, Skljarevski and Ramadan 2002). However, instances in which verbal reports and physiological measures differ have also been used to support the validity of physiological measures that are not contaminated by the biases that are known to influence subjective measures of pain (Walsh et al 2000, Terkelsen et al 2001). The reader can appreciate that these two scenarios approach a closed belief system. Investigators cannot have it both ways, using both association and non-association to support validity. The primary examples of disassociation have been observed in response to non-pharmacological manipulations (e.g., attention, hypnosis, segmental or heterotopic noxious stimulation, and gastric or rectal stimulation) and to pharmacological agents (e.g., opioids, non-steroidal anti-inflammatory drugs [NSAIDs], anesthetics, and antidepressants) (Gracely 1999, Guirimand et al 2000, Skljarevski and Ramadan 2002). In addition to challenging the validity of the physiological measures, these disassociations can be informative. For example, verbal reports and also CEPs and MEG can be considered to be the ultimate final product of pain processing, whereas spinal reflexes represent effects observed at the spinal level. Similar effects of an intervention on these two types of measures suggest an effect observed at the spinal levels mediated by local spinal mechanisms with a possible contribution of ascending and descending modulation. Effects only on verbal report or evoked potentials and MEG suggest a supraspinal effect only, and a variety of such effects are difficult to interpret. For example, Terkelsen and colleagues (2001) found that heterotopic and segmental conditioning reduced subjective ratings with variable effects on the nociceptive withdrawal reflex and poor correlation between the reflex and subjective effects (see Fig. 20-8). These functional relationships become more complicated when comparing supraspinal measures such as evoked potentials and MEG with verbal report. What does disassociation mean? In studies of interventions, effects only on the physiological measures could represent an action independent of analgesia, such as the action of a barbiturate that may alter physiological responses. Effects could also conceivably be due to reporting bias. France and co-workers (2002) have shown that catastrophizing, assessed by the Coping Strategies Questionnaire, was associated with subjective pain ratings but not with reflex thresholds to electrical sural nerve stimulation.
A more empirical approach extends the concept of response patterns or profiles to include all measures of pain, whether verbal, physiological, or behavioral. Such a profile can be very discriminating if the various measures are relatively independent. It is likely that in the future, current studies using either psychophysical or physiological approaches may further integrate these measures into a combined battery of multiple modalities and methods.
Because pain is private and conceptual, verbal report will remain the ultimate standard of measurement. Yet physiological and behavioral correlates are useful in a number of scenarios, such as in children or infants (Andrews and Fitzgerald 1999, Norman et al 2008), adults with poorly developed language skills, or anyone unable to perform the cognitive task of describing perceived pain. In research, physiological measures may be the only way to assess the effects of stimulation at rapid intervals.
Relevance of Experimental Methods
The Classic Goal of Assessment of Analgesic Efficacy
Evaluation of analgesic efficacy has been a classic goal of studies using experimental stimulation (Beecher 1959, Gracely 1991). The ideal method would avoid the uncontrolled and highly variable nature of the pain “stimulus” associated with clinical syndromes. The pioneering studies enjoyed initial success, followed by criticism and repeated failure. Methodological improvements resulted in renewed success with the demonstration of opioid analgesia, which is routinely observed in contemporary experiments (Staahl et al 2009a).
Many of the important features of present methods evolved from these early studies. Initially, successful methods used the pain threshold to thermal stimuli as the dependent measure in uncontrolled studies. Positive effects vanished with the introduction of double-blind placebo controls but reappeared with the use of severe, long-lasting pain sensations produced by the continuous, increasing pain of the tourniquet ischemia technique (Beecher 1959, Smith et al 1966) or by the use of discrete stimuli to stimulate an increasing continuous pain sensation (Parry et al 1972). The demonstration of opioid analgesia with these stimulus modalities was attributed to their severity, which was deemed sufficient to evoke a sufficient “reaction component,” an affective component associated with clinically significant pain but not usually found with brief discrete stimuli. Present evidence suggests that this success was not due to the presence of a reaction component but rather to the use of suprathreshold stimulation. A wide range of discrete suprathreshold stimuli and several response methods have repeatedly demonstrated significant effects of pharmacological pain control interventions (Staahl et al 2009a, 2009b). Nonetheless, as elaborated in the section on the dual dimensions of pain, the reaction component has remained an influential concept in pain measurement and treatment.
Presently, experimental demonstrations of opiate analgesia are commonplace. Opioids routinely produced significant analgesia in comparison to placebo and have shown significant dose–response relationships (Oertel et al 2008). The renewed interest in central sensitization has prompted a number of studies that target the putative central mechanisms responsible for secondary hyperalgesia and mechanical allodynia. Studies of experimental sensitization have demonstrated opioid attenuation of secondary hyperalgesia and the extent of mechanical allodynia following the administration of multiple opioids, such as morphine, fentanyl, alfentanil, and remifentanil, and multiple methods of inducing peripheral and central sensitization, such as intradermal injection of capsaicin, heat and topical capsaicin, heat and inflammation, ultraviolet B radiation, continuous electrical stimulation, burn injury, and freeze lesion (Sethna et al 1998, Staahl et al 2009a). This battery of measures and additional procedures has also been used to assess the activity of non-opioid analgesics such as local anesthetics, N-methyl-D-aspartate (NMDA) antagonists, NSAIDs, anticonvulsants, and antidepressants (Brennum et al 1992, Staahl et al 2009b). The results indicate that multiple analgesic actions are more effective with specific models. Opioids produce analgesia in response to most pain stimulus modalities, although the effect is greater with longer-lasting stimulation and may be greater for muscle versus cutaneous stimulation. In contrast to opioids, lidocaine appears to be more effective for brief, localized stimulation by laser, electrical, or mechanical stimuli and also attenuates secondary hyperalgesia (Brennum et al 1992, 1993).
Studies focusing on central sensitization often use more severe, less controlled stimulation such as experimental burns, intradermal injection of capsaicin, or topical application of capsaicin or other substances such as mustard oil. The use of continuous electrical stimulation (Koppert et al 2001) or a combination of topical capsaicin and heat (Petersen and Rowbotham 1999) provides more stimulus control. Administration of NMDA antagonists such as ketamine and dextromethorphan has reduced signs of experimental central sensitization (Ilkjaer et al 1997, Sang et al 1998, Staahl et al 2009b) without robust effects on brief painful stimuli. Similar studies of adrenergic mechanisms have also found specific effects. Drummond (1995) administered noradrenaline and observed increased primary heat hyperalgesia, thus suggesting α-adrenergic involvement in the mechanisms mediating mechanical allodynia but not mechanical punctate secondary hyperalgesia. Administration of the α-adrenergic antagonist phentolamine decreased ongoing pain and the extent of capsaicin-induced mechanical allodynia but had no effect on the extent of pinprick secondary hyperalgesia (Liu et al 1996, Kinnman et al 1997). These results indicate a differential action of adrenergic mechanisms on peripheral and central hyperalgesia and provide further evidence that altered sensitivity to Aβ and nociceptor input is mediated by independent mechanisms.
The results of studies of weaker analgesics are less consistent. Gender may play a role. A recent study of the potent NSAID ketorolac on cold pressor pain found no overall effect. However, after separate analyses for each gender, the researchers found a significant effect in women, but not in men (Compton et al 2003). In contrast, a study of the effects of ibuprofen found a significant reduction in pain evoked by electrical stimulation of the earlobe in men but not in women (Walker and Carmody 1998). Topical application of agents such as acetylsalicylic acid has been shown to suppress the pain evoked by tissue acidosis (Steen et al 1995) and attenuate the spontaneous pain, the area of flare, secondary hyperalgesia, and the mechanical allodynia produced by topical capsaicin (Schmelz and Kress 1996). Oral administration of NSAIDs such as aspirin and ibuprofen has demonstrated analgesia in a large number of cutaneous models, including laser, single and repetitive electrical stimulation, CO2 applied to the nasal mucosa, mechanical stimulation of the finger web, and acid (Staahl et al 2009b). These agents have also been shown to reduce the hyperalgesia associated with freeze, burn, or mechanical injuries, presumably by peripheral attenuation of the nociceptive input maintaining central sensitization (Bickel et al 1998, Sycha et al 2003) and possibly by more direct central effects (Burian et al 2003).
Methods using experimental pain stimulation in normal individuals have also been used to assess the effects of other drugs, including the analgesic effects observed with nitrous oxide, tramadol, imipramine, and intradermal lidocaine in combination with morphine (Gracely 1999). The results of recent studies with the tricyclic antidepressant imipramine provide an example of the specificity—and complexity—of analgesic mechanisms. Imipramine reduces experimental esophageal pain and demonstrates analgesia in models of pressure pain tolerance, electrical stimulation tolerance, and electrical summation threshold (Peghini et al 1998, Enggaard et al 2001). However, imipramine shows no analgesic activity in the cold pressor test (whereas the relatively weak analgesic codeine does) or in models of brief pain such as the electrical pain threshold, laser-evoked pain thresholds, or evoked cerebral potentials (Poulsen et al 1995, Sindrup et al 1998, Enggaard et al 2001).
The expanding literature on experimental pain pharmacology is supplemented by a growing literature on non-pharmacological modulation of experimental pain perception. Many of these studies assess methods used for clinical treatment. Recent studies of TENS have demonstrated the influence of stimulating parameters and experimental pain modality. Using the same methods in separate studies of experimental pain evoked by blunt pressure and by the cold pressor method, Chen and Johnson (2010a, 2010b) found essentially opposite results. In 35 pain-free subjects, TENS delivered at 3 Hz, in comparison to TENS delivered at 80 Hz, produced significant analgesia in response to pain produced by the cold pressor method in which a hand was immersed in 1°C water (Chen and Johnson 2010a). In contrast, 80-Hz TENS was superior to 3-Hz TENS if the experimental pain stimulus was either blunt pressure pain or tourniquet ischemia (Chen and Johnson 2010a, 2010b). These studies showed an effect of TENS treatment at both frequencies, reported as significant in the cold pressor study (Chen and Johnson 2010a). However, these studies did not use a placebo control but instead compared the effects of the two frequencies with each other. Non-painful TENS has been assumed to reduce pain by a peripheral mechanism of “gate control” in which touch fiber input inhibits pain mediated by smaller nociceptor input (Melzack and Wall 1965). Consistent with this view, a number of studies have provided evidence that TENS analgesia is not mediated by a central opioid mechanism (Leonard et al 2010). However, this lack of an effect may be due to methodological issues. A recent study has demonstrated antagonism of TENS analgesia by using a high dose but not low doses of the narcotic antagonist naloxone (Leonard et al 2010). This result, the low efficacy of low-frequency TENS in opioid-tolerant animals and patients (Léonard et al 2011), and the recent findings of tolerance to daily TENS (Liebano et al 2011) suggest that TENS analgesia is mediated by central opioid mechanisms.
A variation of TENS delivers electrical current sequentially through a rectangular array of needle electrodes positioned at the dermal–epidermal junction. Termed cutaneous field stimulation, this method has been shown to evoke prolonged attenuation of Aδ or C fiber–mediated laser-evoked pain and pinch-evoked pain, effects that may be similar to those of long-term depression found in animal studies (Nilsson et al 2003).
Even though acupuncture is a different technique than TENS with a long history of clinical application, experimental studies commonly use electroacupuncture. Thus the main differences are delivery of current beneath or above the skin and the use of acupuncture at specified locations or “points.” Recent studies of the influence of acupuncture on pressure, heat, and repeated electrical stimulation suggest that electroacupuncture shows greater analgesia than manual acupuncture does and that higher-intensity electrical acupuncture is more efficacious (Kong et al 2005, Barlas et al 2006, Zheng et al 2010). PET ligand-binding studies suggest that both short- and long-term effects involve opioid systems (Harris et al 2009). Imaging studies also suggest that the effects of actual and sham acupuncture, as well as modulation by expectation, are mediated by distinctly different mechanisms (Kong et al 2009, Zyloney et al 2010).
Studies that assess analgesic efficacy by experimental methods have been criticized for not being relevant to the clinical situation. Critics rightfully point out that laboratory administration of experimentally painful stimuli cannot duplicate the physiological features of an acute or chronic pain condition or produce the accompanying psychological features such as anxiety, uncertainty, suffering, and foreboding. However, this inability to exactly duplicate clinical pain syndromes imposes only modest limits on the inferential utility of these methods. The consistent results with opiates suggest that the antinociceptive efficacy of opioid agonists or antagonists can be evaluated by using laboratory procedures. The important issues may relate to whether the dose and potency relationships that have been established experimentally predict clinical findings and whether the models are developed sufficiently to accurately predict poor clinical analgesic action. In addition, it is increasingly becoming clear that the pain afferent system is not a simple transmission line but a complex series of processing stages that change and increase in number as acute pain is prolonged. Specific experimental pain paradigms are able to activate specific components, such as Aδ-fiber activation, C-fiber activation, Aβ temporal summation, C-fiber “wind-up,” central sensitization, and progressive tactile hypersensitivity (Ma and Woolf 1996, Eliav and Gracely 1998, Gracely 1999). The results of recent studies strongly suggest that the complexity of pain processing may best be assessed—and in some cases only be assessed—through the administration of a battery of experimental pain methods that target specific components of this system. In this context, the usefulness of experimental models naturally extends beyond the measurement of whether an analgesic works to why it works and to identification of its mechanisms of analgesic action.
Additional Goals of Experimental Pain Methods
The experimental goal of evaluating analgesic efficacy was the second of several goals listed at the beginning of this chapter. The first goal of measurement development and validation is intrinsic to the methods and has been alluded to in descriptions of the methods. An additional goal describes a major utility of experimental methods: evaluation of the mechanisms of pain and pain control. Examples of these applications are provided throughout this volume. When reviewing these, it may be helpful to divide these studies into anatomic divisions of peripheral, spinal, and supraspinal mechanisms. Both psychophysical and physiological measures can assess the function of nociceptive afferents, and the recent focus on central sensitization has emphasized the importance of also evaluating the function of fibers that normally mediate non-painful, tactile sensation. Once entering the dorsal horn, primary afferent information can be modulated by a number of mechanisms. Indeed, attenuation of this input either by other peripheral input or by central endogenous opioid and non-opioid mechanisms has marked major milestones in pain research. In contrast to these mechanisms of attenuation, current studies have focused on the mechanisms that exacerbate symptoms in conditions of persistent pain. Though predominantly spinal, these mechanisms can be investigated successfully by all the methods described in this chapter. Models of spinal sensitization are produced by fast trains of noxious stimuli (wind-up), by the application of chemicals or burns (central sensitization), and in cases of allodynia or peripheral inflammation, by the use of tactile stimuli. Innocuous cold stimuli are also useful. For example, Chen and colleagues (1996) used both an adaptive, stimulus-dependent method and the classic method of constant stimuli to assess cold detection and pain thresholds and different adapting temperatures. These authors found evidence of separate afferent systems mediating the sensation of cool and of cold pain. Campero and co-workers (1996) assessed C-polymodal nociceptors responsive to heat and mechanical stimulation and found that about 40% of these fibers were also activated by cold stimulation. These fibers may represent the afferent nociceptive channel of Chen and colleagues (1996) and may mediate the symptom of cold hyperalgesia found in neuropathic pain syndromes (Campero et al 1996). Additional psychophysical studies and functional brain imaging studies of supraspinal processing have further identified interactions between cold and warm fiber symptoms that are responsible for the classic thermal grill illusion and probably contribute to the symptom of cold hyperalgesia (Craig et al 1996).
A further goal involves evaluation of the psychological factors involved in the experience of pain and the influence of these factors on pain measurement. The controlled environment of experimental studies has demonstrated the influence of cognitive factors such as attention, expectation, memory, and suggestion, as well as the influence of the mood states of anxiety and depression (Gracely 1999). One important issue is the relevance of these findings for clinically significant acute and chronic pain. Like experimental measures of analgesic efficacy, these types of experiments must ultimately be cross-validated in the clinic. Many of the studies cited used groups of patients and volunteers or delivered experimental stimuli to pain patients. These types of studies are represented by another important goal, which is the use of experimental methods in the clinical situation. Methods such as experimental pain matching can be used to improve pain assessment. The growth of clinical studies using quantitative sensory testing is an excellent example of the successful merging of experimental procedures and clinical evaluation. New studies are now extending this concept further and exploring how clinical conditions may modulate measures of spinal and supraspinal processing. These experiments will probably provide important parts of the puzzle of the multiple mechanisms of pain perception. They will also probably continue to approach one of the most elusive goals, a physiological signature associated with what is otherwise an unobservable and private event.
Acknowledgment
The author thanks Kirsten Ambrose for her technical assistance.
The references for this chapter can be found at www.expertconsult.com.
References
Albin K.C., Carstens M.I., Carstens E. Modulation of oral heat and cold pain by irritant chemicals. Chemical Senses. 2008;33:3–15.
Algom D., Raphaeli N., Cohen-Raz L. Integration of noxious stimulation across separate somatosensory communications systems: a functional theory of pain. Journal of Experimental Psychology. Human Perception and Performance. 1986;12:92–102.
Andersen O.K., Graven-Nielsen T., Matre D., et al. Interaction between cutaneous and muscle afferent activity in polysynaptic reflex pathways: a human experimental study. Pain. 2000;84:29–36.
Andersen O.K., Morch C.D., Arendt-Nielsen L. Modulation of heat evoked nociceptive withdrawal reflexes by painful intramuscular conditioning stimulation. Experimental Brain Research. 2006;174:775–780.
Andersen O.K., Sonnenborg F.A., Arendt-Nielsen L. Reflex receptive fields for human withdrawal reflexes elicited by non-painful and painful electrical stimulation of the foot sole. Clinical Neurophysiology. 2001;112:641–649.
Andersen O.K., Svensson P., Ellrich J., et al. Conditioning of the masseter inhibitory reflex by homotopically applied painful heat in humans. Electroencephalography and Clinical Neurophysiology. 1998;109:508–514.
Andrew D., Greenspan J.D. Peripheral coding of tonic mechanical cutaneous pain: comparison of nociceptor activity in rat and human psychophysics. Journal of Neurophysiology. 1999;82:2641–2648.
Andrews K., Fitzgerald M. Cutaneous flexion reflex in human neonates: a quantitative study of threshold and stimulus-response characteristics after single and repeated stimuli. Developmental Medicine and Child Neurology. 1999;41:696–703.
Angst M.S., Clark J.D., Comment on Koltzenburg, et al, Differential sensitivity of three experimental pain models in detecting the analgesic effects of transdermal fentanyl and buprenorphine. Pain 2006;126:165–174. Pain 128:292–294, 2007.
Anton F., Euchner I., Handwerker H.O. Psychophysical examination of pain induced by defined CO2 pulses applied to the nasal mucosa. Pain. 1992;49:53–60.
Arendt-Nielsen L., Olesen A.E., Staahl C., et al. Analgesic efficacy of peripheral kappa-opioid receptor agonist CR665 compared to oxycodone in a multi-modal, multi-tissue experimental human pain model: selective effect on visceral pain. Anesthesiology. 2009;111:616–624.
Arendt-Nielsen L., Sluka K.A., Nie H.L. Experimental muscle pain impairs descending inhibition. Pain. 2008;140:465–471.
Arendt-Nielsen L., Yamasaki H., Nielsen J., et al. Magnetoencephalographic responses to painful impact stimulation. Brain Research. 1999;839:203–208.
Arendt-Nielsen L., Yarnitsky D. Experimental and clinical applications of quantitative sensory testing applied to skin, muscles and viscera. Journal of Pain. 2009;10:556–572.
Babiloni C., Brancucci A., Capotosto P., et al. Different modalities of painful somatosensory stimulations affect anticipatory cortical processes: a high-resolution EEG study. Brain Research Bulletin. 2007;71:475–484.
Babiloni C., Brancucci A., Del Percio C., et al. Anticipatory electroencephalography alpha rhythm predicts subjective perception of pain intensity. Journal of Pain. 2006;7:709–717.
Babiloni C., Del Percio C., Brancucci A., et al. Pre-stimulus alpha power affects vertex N2-P2 potentials evoked by noxious stimuli. Brain Research Bulletin. 2008;75:581–590.
Bajaj P., Arendt-Nielsen L., Andersen O.K. Facilitation and inhibition of withdrawal reflexes following repetitive stimulation: electro- and psychophysiological evidence for activation of noxious inhibitory controls in humans. European Journal of Pain. 2005;9:25–31.
Barbanoj M.J., Urbano G., Antonijoan R., et al. Different acute tolerance development to EEG, psychomotor performance and subjective assessment effects after two intermittent oral doses of alprazolam in healthy volunteers. Neuropsychobiology. 2007;55:203–212.
Barlas P., Ting S.L., Chesterton L.S., et al. Effects of intensity of electroacupuncture upon experimental pain in healthy human volunteers: a randomized, double-blind, placebo-controlled study. Pain. 2006;122:81–89.
Beecher H.K. Measurement of subjective responses. New York: Oxford University Press; 1959.
Beise R.D., Kohlloffel L.U., Claus D. Blink reflex induced by controlled, ballistic mechanical impacts. Muscle & Nerve. 1999;22:443–448.
Berthele A., Platzer S., Jochim B., et al. COMT Val108/158Met genotype affects the mu-opioid receptor system in the human brain: evidence from ligand-binding, G-protein activation and preproenkephalin mRNA expression. NeuroImage. 2005;28:185–193.
Beydoun A., Dyke D.B., Morrow T.J., et al. Topical capsaicin selectively attenuates heat pain and A delta fiber–mediated laser-evoked potentials. Pain. 1996;65:189–196.
Bhalang K., Sigurdsson A., Slade G.D., et al. Associations among four modalities of experimental pain in women. Journal of Pain. 2005;6:604–611.
Bickel A., Dorfs S., Schmelz M., et al. Effects of antihyperalgesic drugs on experimentally induced hyperalgesia in man. Pain. 1998;76:317–325.
Biurrun Manresa J.A., Morch C.D., Andersen O.K. Long-term facilitation of nociceptive withdrawal reflexes following low-frequency conditioning electrical stimulation: a new model for central sensitization in humans. European Journal of Pain. 2010;14:822–831.
Brennum J., Arendt-Nielsen L., Horn A., et al. Quantitative sensory examination during epidural anaesthesia and analgesia in man: effects of morphine. Pain. 1993;52:75–83.
Brennum J., Arendt-Nielsen L., Secher N.H., et al. Quantitative sensory examination in human epidural anaesthesia and analgesia: effects of lidocaine. Pain. 1992;51:27–34.
Brock C., Andresen T., Frokjaer J.B., et al. Central pain mechanisms following combined acid and capsaicin perfusion of the human oesophagus. European Journal of Pain. 2010;14:273–281.
Brown C.A., Seymour B., Boyle Y., et al. Modulation of pain ratings by expectation and uncertainty: behavioral characteristics and anticipatory neural correlates. Pain. 2008;135:240–250.
Brown C.A., Seymour B., El-Deredy W., et al. Confidence in beliefs about pain predicts expectancy effects on pain perception and anticipatory processing in right anterior insula. Pain. 2008;139:324–332.
Bufalari I., Aprile T., Avenanti A., et al. Empathy for pain and touch in the human somatosensory cortex. Cerebral Cortex. 2007;17:2553–2561.
Burian M., Tegeder I., Seegel M., et al. Peripheral and central antihyperalgesic effects of diclofenac in a model of human inflammatory pain. Clinical Pharmacology and Therapeutics. 2003;74:113–120.
Byas-Smith M.G., Bennett G.J., Gracely R.H., et al. Tourniquet constriction exacerbates hyperalgesia-related pain induced by intradermal capsaicin injection. Anesthesiology. 1999;91:617–625.
Campbell C.M., Edwards R.R., Fillingim R.B. Ethnic differences in responses to multiple experimental pain stimuli. Pain. 2005;113:20–26.
Campbell C.M., France C.R., Robinson M.E., et al. Ethnic differences in diffuse noxious inhibitory controls. Journal of Pain. 2008;9:759–766.
Campbell C.M., France C.R., Robinson M.E., et al. Ethnic differences in the nociceptive flexion reflex (NFR). Pain. 2008;134:91–96.
Campero M., Baumann T.K., Bostock H., et al. Human cutaneous C fibres activated by cooling, heating and menthol. Journal of Physiology. 2009;587:5633–5652.
Campero M., Serra J., Ochoa J.L. C-polymodal nociceptors activated by noxious low temperature in human skin. Journal of Physiology. 1996;497:565–572.
Chapman C.R. Sensory decision theory methods in pain research: a reply to Rollman. Pain. 1977;3:295–305.
Chapman C.R., Casey K.L., Dubner R., et al. Pain measurement: an overview. Pain. 1985;22:1–31.
Chapman C.R., Loeser J.D. Advances in pain research and therapy: issues in pain measurement, ed 12. New York: Raven Press; 1989.
Chatrian G.E., Fernandes de Lima V.M., Lettich E., et al. Electrical stimulation of tooth pulp in humans. II. Qualities of sensations. Pain. 1982;14:233–246.
Chen A.C., Niddam D.M., Arendt-Nielsen L. Contact heat evoked potentials as a valid means to study nociceptive pathways in human subjects. Neuroscience Letters. 2001;316:79–82.
Chen A.C., Niddam D.M., Crawford H.J., et al. Spatial summation of pain processing in the human brain as assessed by cerebral event related potentials. Neuroscience Letters. 2002;328:190–194.
Chen C.C., Johnson M.I. A comparison of transcutaneous electrical nerve stimulation (TENS) at 3 and 80 pulses per second on cold-pressor pain in healthy human participants. Clinical Physiology and Functional Imaging. 2010;30:260–268.
Chen C.C., Johnson M.I. An investigation into the hypoalgesic effects of high- and low-frequency transcutaneous electrical nerve stimulation (TENS) on experimentally-induced blunt pressure pain in healthy human participants. Journal of Pain. 2010;11:53–61.
Chen C.C., Rainville P., Bushnell M.C. Noxious and innocuous cold discrimination in humans: evidence for separate afferent channels. Pain. 1996;68:33–43.
Chen S., Xia W., Li L., et al. Gray matter density reduction in the insula in fire survivors with posttraumatic stress disorder: a voxel-based morphometric study. Psychiatry Research. 2006;146:65–72.
Chizh B.A., Greenspan J.D., Casey K.L., et al. Identifying biological markers of activity in human nociceptive pathways to facilitate analgesic drug development. Pain. 2008;140:249–253.
Chizh B.A., Priestley T., Rowbotham M., et al. Predicting therapeutic efficacy—experimental pain in human subjects. Brain Research Reviews. 2009;60:243–254.
Clark W.C., Clark S.B. Pain responses in Nepalese porters. Science. 1980;209:410–412.
Cleeland C.S., Nakamura Y., Howland E.W., et al. Effects of oral morphine on cold pressor tolerance time and neuropsychological performance. Neuropsychopharmacology. 1996;15:252–262.
Cole L.J., Farrell M.J., Gibson S.J., et al. Age-related differences in pain sensitivity and regional brain activity evoked by noxious pressure. Neurobiology of Aging. 2010;31:494–503.
Compton P., Charuvastra V.C., Ling W. Effect of oral ketorolac and gender on human cold pressor pain tolerance. Clinical and Experimental Pharmacology & Physiology. 2003;30:759–763.
Coppola R., Gracely R.H. Where is the noise in SDT pain assessment? Pain. 1983;17:257–266.
Craig A.D., Reiman E.M., Evans A., et al. Functional imaging of an illusion of pain. Nature. 1996;384:258–260.
Crockett D.J., Prkachin K.M., Craig K.D. Factors of the language of pain in patient and volunteer groups. Pain. 1977;4:175–182.
Davis K.D., Taub E., Duffner F., et al. Activation of the anterior cingulate cortex by thalamic stimulation in patients with chronic pain: a positron emission tomography study. Journal of Neurosurgery. 2000;92:64–69.
Dawson A., List T. Comparison of pain thresholds and pain tolerance levels between Middle Easterners and Swedes and between genders. Journal of Oral Rehabilitation. 2009;36:271–278.
De Pascalis V., Magurano M.R., Bellusci A., et al. Somatosensory event-related potential and autonomic activity to varying pain reduction cognitive strategies in hypnosis. Clinical Neurophysiology. 2001;112:1475–1485.
de Tommaso M., Libro G., Guido M., et al. The blink reflex and the corneal reflex are followed by cortical activity resembling the nociceptive potentials induced by trigeminal laser stimulation in man. Neuroscience Letters. 2001;310:37–40.
Don R., Pierelli F., Ranavolo A., et al. Modulation of spinal inhibitory reflex responses to cutaneous nociceptive stimuli during upper limb movement. European Journal of Neuroscience. 2008;28:559–568.
Dong W.K., Chudler E.H., Martin R.F. Physiological properties of intradental mechanoreceptors. Brain Research. 1985;334:389–395.
Dowman R. A noninvasive strategy for identifying and quantifying innocuous and nociceptive peripheral afferent activity evoked by nerve stimulation. Physiology & Behavior. 1993;53:1163–1169.
Dowman R. Neural mechanisms of detecting and orienting attention toward unattended threatening somatosensory targets. I. Intermodal effects. Psychophysiology. 2007;44:407–419.
Dowman R. Neural mechanisms of detecting and orienting attention toward unattended threatening somatosensory target stimuli. II. Intensity effects. Psychophysiology. 2007;44:420–430.
Dowman R., Rissacher D., Schuckers S. EEG indices of tonic pain-related activity in the somatosensory cortices. Clinical Neurophysiology. 2008;119:1201–1212.
Drewes A.M., Babenko L., Birket-Smith L., et al. Induction of non-painful and painful intestinal sensations by hypertonic saline: a new human experimental model. European Journal of Pain. 2003;7:81–91.
Drewes A.M., Rossel P., Le Pera D., et al. Dipolar source modelling of brain potentials evoked by painful electrical stimulation of the human sigmoid colon. Neuroscience Letters. 2004;358:45–48.
Drummond P.D. Noradrenaline increases hyperalgesia to heat in skin sensitized by capsaicin. Pain. 1995;60:311–315.
Druschky K., Lang E., Hummel C., et al. Pain-related somatosensory evoked magnetic fields induced by controlled ballistic mechanical impacts. Journal of Clinical Neurophysiology. 2000;17:613–622.
Duncan G.H., Bushnell M.C., Lavigne G.J. Comparison of verbal and visual analogue scales for measuring the intensity and unpleasantness of experimental pain. Pain. 1989;37:295–303.
Duncan G.H., Feine J.S., Bushnell M.C., et al. Use of magnitude matching for measuring group differences in pain perception. In: Dubner R., Gebhart G.R., Bond M.R., eds. Proceedings of the Vth World Congress on Pain. Amsterdam: Elsevier, 1988.
Edwards C.L., Fillingim R.B., Keefe F. Race, ethnicity and pain. Pain. 2001;94:133–137.
Edwards L., Inui K., Ring C., et al. Pain-related evoked potentials are modulated across the cardiac cycle. Pain. 2008;137:488–494.
Edwards L., McIntyre D., Carroll D., et al. The human nociceptive flexion reflex threshold is higher during systole than diastole. Psychophysiology. 2002;39:678–681.
Eliav E., Gracely R.H. Sensory changes in the territory of the lingual and inferior alveolar nerves following lower third molar extraction. Pain. 1998;77:191–199.
Enggaard T.P., Poulsen L., Arendt-Nielsen L., et al. The analgesic effect of codeine as compared to imipramine in different human experimental pain models. Pain. 2001;92:277–282.
Farrell M., Gibson S. Age interacts with stimulus frequency in the temporal summation of pain. Pain Medicine. 2007;8:514–520.
Fillingim R.B., King C.D., Ribeiro-Dasilva M.C., et al. Sex, gender, and pain: a review of recent clinical and experimental findings. Journal of Pain. 2009;10:447–485.
France C.R., Froese S.A., Stewart J.C. Altered central nervous system processing of noxious stimuli contributes to decreased nociceptive responding in individuals at risk for hypertension. Pain. 2002;98:101–108.
Frot M., Magnin M., Mauguiere F., et al. Human SII and posterior insula differently encode thermal laser stimuli. Cerebral Cortex. 2007;17:610–620.
Frot M., Mauguiere F. Dual representation of pain in the operculo-insular cortex in humans. Brain. 2003;126:438–450.
Fruhstorfer H., Lindblom U., Schmidt W.C. Method for quantitative estimation of thermal thresholds in patients. Journal of Neurology, Neurosurgery, and Psychiatry. 1976;39:1071–1075.
Frymoyer A.R., Rowbotham M.C., Petersen K.L. Placebo-controlled comparison of a morphine/dextromethorphan combination with morphine on experimental pain and hyperalgesia in healthy volunteers. Journal of Pain. 2007;8:19–25.
Fujii-Abe K., Oono Y., Motohashi K., et al. Heterotopic CO2 laser stimulation inhibits tooth-related somatosensory evoked potentials. Pain Medicine. 2010;11:825–833.
Gazerani P., Arendt-Nielsen L. The impact of ethnic differences in response to capsaicin-induced trigeminal sensitization. Pain. 2005;117:223–229.
Gendreau M., Hufford M.R., Stone A.A. Measuring clinical pain in chronic widespread pain: selected methodological issues. Best Practice & Research. Clinical Rheumatology. 2003;17:575–592.
Gibson S.J., Farrell M. A review of age differences in the neurophysiology of nociception and the perceptual experience of pain. Clinical Journal of Pain. 2004;20:227–239.
Gibson S.J., Helme R.D. Age-related differences in pain perception and report. Clinics in Geriatric Medicine. 2001;17:433–456. v–vi
Giesecke T., Williams D.A., Harris R.E., et al. Subgrouping of fibromyalgia patients on the basis of pressure-pain thresholds and psychological factors. Arthritis and Rheumatism. 2003;48:2916–2922.
Goffaux P., de Souza J.B., Potvin S., et al. Pain relief through expectation supersedes descending inhibitory deficits in fibromyalgia patients. Pain. 2009;145:18–23.
Goffaux P., Redmond W.J., Rainville P., et al. Descending analgesia—when the spine echoes what the brain expects. Pain. 2007;130:137–143.
Gracely R.H. Psychophysical assessment of human pain. In: Bonica J.J., ed. Advances in pain research and therapy. New York: Raven Press, 1979.
Gracely R.H. Theoretical and practical issues in pain assessment in central pain syndromes. In: Casely K.L., ed. Pain and central nervous system disease. New York: Raven Press, 1991.
Gracely R.H. Pain measurement. Acta Anaesthesiologica Scandinavica. 1999;43:897–908.
Gracely R.H., Dubner R. Pain assessment in humans—a reply to Hall. Pain. 1981;11:109–120.
Gracely R.H., Dubner R., McGrath P.A. Narcotic analgesia: fentanyl reduces the intensity but not the unpleasantness of painful tooth pulp sensations. Science. 1979;203:1261–1263.
Gracely R.H., Grant M.A., Giesecke T. Evoked pain measures in fibromyalgia. Best Practice & Research. Clinical Rheumatology. 2003;17:593–609.
Gracely R.H., Kwilosz D.M. The Descriptor Differential Scale: applying psychophysical principles to clinical pain assessment. Pain. 1988;35:279–288.
Gracely R.H., Lota L., Walter D.J., et al. A multiple random staircase method of psychophysical pain assessment. Pain. 1988;32:55–63.
Gracely R.H., McGrath F., Dubner R. Ratio scales of sensory and affective verbal pain descriptors. Pain. 1978;5:5–18.
Gracely R.H., McGrath P., Dubner R. Validity and sensitivity of ratio scales of sensory and affective verbal pain descriptors: manipulation of affect by diazepam. Pain. 1978;5:19–29.
Gracely R.H., Naliboff B. Measurement of pain sensation. In: Kruger L., ed. Handbook of perception and cognition: somatosensory systems. New York: Raven Press; 1996:243–313.
Gracely R.H., Petzke F., Wolf J.M., et al. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis and Rheumatism. 2002;46:1333–1343.
Gracely R.H., Wolskee P.J. Semantic functional measurement of pain: integrating perception and language. Pain. 1983;15:389–398.
Graven-Nielsen T., Arendt-Nielsen L., Mense S. Thermosensitivity of muscle: high-intensity thermal stimulation of muscle tissue induces muscle pain in humans. Journal of Physiology. 2002;540:647–656.
Graven-Nielsen T., Jansson Y., Segerdahl M., et al. Experimental pain by ischaemic contractions compared with pain by intramuscular infusions of adenosine and hypertonic saline. European Journal of Pain. 2003;7:93–102.
Graven-Nielsen T., McArdle A., Phoenix J., et al. In vivo model of muscle pain: quantification of intramuscular chemical, electrical, and pressure changes associated with saline-induced muscle pain in humans. Pain. 1997;69:137–143.
Guirimand F., Dupont X., Brasseur L., et al. The effects of ketamine on the temporal summation (wind-up) of the R(III) nociceptive flexion reflex and pain in humans. Anesthesia and Analgesia. 2000;90:408–414.
Hargrove J.B., Bennett R.M., Simons D.G., et al. Quantitative electroencephalographic abnormalities in fibromyalgia patients. Clinical EEG and Neuroscience. 2010;41:132–139.
Harris R.E., Zubieta J.K., Scott D.J., et al. Traditional Chinese acupuncture and placebo (sham) acupuncture are differentiated by their effects on mu-opioid receptors (MORs). NeuroImage. 2009;47:1077–1085.
Hastie B.A., Riley J.L., 3rd., Robinson M.E., et al. Cluster analysis of multiple experimental pain modalities. Pain. 2005;116:227–237.
Hauck M., Bischoff P., Schmidt G., et al. Clonidine effects on pain evoked SII activity in humans. European Journal of Pain. 2006;10:757–765.
Hauck M., Lorenz J., Zimmermann R., et al. Duration of the cue-to-pain delay increases pain intensity: a combined EEG and MEG study. Experimental Brain Research. 2007;180:205–215.
Hobson A.R., Chizh B., Hicks K., et al. Neurophysiological evaluation of convergent afferents innervating the human esophagus and area of referred pain on the anterior chest wall. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2010;298:G31–G36.
Hori E., Takamoto K., Urakawa S., et al. Effects of acupuncture on the brain hemodynamics. Autonomic Neuroscience: Basic & Clinical. 2010;157:74–80.
Hoshiyama M., Kakigi R. After-effect of transcutaneous electrical nerve stimulation (TENS) on pain-related evoked potentials and magnetic fields in normal subjects. Clinical Neurophysiology. 2000;111:717–724.
Hsieh A.Y., Tripp D.A., Ji L.J., et al. Comparisons of catastrophizing, pain attitudes, and cold-pressor pain experience between Chinese and European Canadian young adults. Journal of Pain. 2010;11:1187–1194.
Huber M.T., Bartling J., Pachur D., et al. EEG responses to tonic heat pain. Experimental Brain Research. 2006;173:14–24.
Humphries S.A., Johnson M.H., Long N.R. An investigation of the gate control theory of pain using the experimental pain stimulus of potassium iontophoresis. Perception & Psychophysics. 1996;58:693–703.
Iannetti G.D., Mouraux A. From the neuromatrix to the pain matrix (and back). Experimental Brain Research. 2010;205:1–12.
Iannetti G.D., Zambreanu L., Cruccu G., et al. Operculoinsular cortex encodes pain intensity at the earliest stages of cortical processing as indicated by amplitude of laser-evoked potentials in humans. Neuroscience. 2005;131:199–208.
Iannetti G.D., Zambreanu L., Tracey I. Similar nociceptive afferents mediate psychophysical and electrophysiological responses to heat stimulation of glabrous and hairy skin in humans. Journal of Physiology. 2006;577:235–248.
Ilkjaer S., Dirks J., Brennum J., et al. Effect of systemic N-methyl-D-aspartate receptor antagonist (dextromethorphan) on primary and secondary hyperalgesia in humans. British Journal of Anaesthesia. 1997;79:600–605.
Inui K., Tran T.D., Qiu Y., et al. Pain-related magnetic fields evoked by intra-epidermal electrical stimulation in humans. Clinical Neurophysiology. 2002;113:298–304.
Jamison R.N., Gracely R.H., Raymond S.A., et al. Comparative study of electronic vs. paper VAS ratings: a randomized, crossover trial using healthy volunteers. Pain. 2002;99:341–347.
Kakigi R., Nakata H., Inui K., et al. Intracerebral pain processing in a Yoga master who claims not to feel pain during meditation. European Journal of Pain. 2005;9:581–589.
Kakigi R., Tran T.D., Qiu Y., et al. Cerebral responses following stimulation of unmyelinated C-fibers in humans: electro- and magneto-encephalographic study. Neuroscience Research. 2003;45:255–275.
Kanda M., Matsuhashi M., Sawamoto N., et al. Cortical potentials related to assessment of pain intensity with visual analogue scale (VAS). Clinical Neurophysiology. 2002;113:1013–1024.
Kanda M., Nagamine T., Ikeda A., et al. Primary somatosensory cortex is actively involved in pain processing in human. Brain Research. 2000;853:282–289.
Kanda M., Shindo K., Xu X., et al. Cortical mechanisms underlying point localization of pain spot as studied by event-related potentials following CO2 laser stimulation in man. Experimental Brain Research. 1999;127:131–140.
Katsarava Z., Ellrich J., Diener H.C., et al. Optimized stimulation and recording parameters of human “nociception specific” blink reflex recordings. Clinical Neurophysiology. 2002;113:1932–1936.
Kaube H., Katsarava Z., Kaufer T., et al. A new method to increase nociception specificity of the human blink reflex. Clinical Neurophysiology. 2000;111:413–416.
Keefe F.J., Dolan E. Pain behavior and pain coping strategies in low back pain and myofascial pain dysfunction syndrome patients. Pain. 1986;24:49–56.
Kenshalo D.R., Bergen D.C. A device to measure cutaneous temperature sensitivity in humans and subhuman species. Journal of Applied Physiology. 1975;39:1038–1040.
Kenshalo D.R., Jr., Anton F., Dubner R. The detection and perceived intensity of noxious thermal stimuli in monkey and in human. Journal of Neurophysiology. 1989;62:429–436.
Keogh E., Bond F.W., Hanmer R., et al. Comparing acceptance- and control-based coping instructions on the cold-pressor pain experiences of healthy men and women. European Journal of Pain. 2005;9:591–598.
Khalili N., Wendelschafer-Crabb G., Kennedy W.R., et al. Influence of thermode size for detecting heat pain dysfunction in a capsaicin model of epidermal nerve fiber loss. Pain. 2001;91:241–250.
Kinnman E., Nygards E.B., Hansson P. Peripheral alpha-adrenoreceptors are involved in the development of capsaicin induced ongoing and stimulus evoked pain in humans. Pain. 1997;69:79–85.
Klepac R.K., Dowling J., Hauge G. Sensitivity of the McGill Pain Questionnaire to intensity and quality of laboratory pain. Pain. 1981;10:199–207.
Kong J., Fufa D.T., Gerber A.J., et al. Psychophysical outcomes from a randomized pilot study of manual, electro, and sham acupuncture treatment on experimentally induced thermal pain. Journal of Pain. 2005;6:55–64.
Kong J., Kaptchuk T.J., Polich G., et al. An fMRI study on the interaction and dissociation between expectation of pain relief and acupuncture treatment. NeuroImage. 2009;47:1066–1076.
Koppert W., Dern S.K., Sittl R., et al. A new model of electrically evoked pain and hyperalgesia in human skin: the effects of intravenous alfentanil, S(+)-ketamine, and lidocaine. Anesthesiology. 2001;95:395–402.
Kosek E., Hansson P. The influence of experimental pain intensity in the local and referred pain area on somatosensory perception in the area of referred pain. European Journal of Pain. 2002;6:413–425.
Kunz M., Mylius V., Scharmann S., et al. Influence of dementia on multiple components of pain. European Journal of Pain. 2009;13:317–325.
Langemark M., Bach F.W., Jensen T.S., et al. Decreased nociceptive flexion reflex threshold in chronic tension-type headache. Archives of Neurology. 1993;50:1061–1064.
Lautenbacher S., Kunz M., Strate P., et al. Age effects on pain thresholds, temporal summation and spatial summation of heat and pressure pain. Pain. 2005;115:410–418.
Lautenbacher S., Prager M., Rollman G.B. Pain additivity, diffuse noxious inhibitory controls, and attention: a functional measurement analysis. Somatosensory & Motor Research. 2007;24:189–201.
Lee J., Dougherty P.M., Antezana D., et al. Responses of neurons in the region of human thalamic principal somatic sensory nucleus to mechanical and thermal stimuli graded into the painful range. Journal of Comparative Neurology. 1999;410:541–555.
Lee M.C., Mouraux A., Iannetti G.D. Characterizing the cortical activity through which pain emerges from nociception. Journal of Neuroscience. 2009;29:7909–7916.
Lefaucheur J.P., Debray S., Jarry G. Laser evoked potentials using the Nd:YAG laser. Muscle & Nerve. 2001;24:496–501.
Leiser S.C., Dunlop J., Bowlby M.R., et al. Aligning strategies for using EEG as a surrogate biomarker: a review of preclinical and clinical research. Biochemical Pharmacology. 2011;81:1408–1421.
Lenz F.A., Garonzik I.M., Zirh T.A., et al. Neuronal activity in the region of the thalamic principal sensory nucleus (ventralis caudalis) in patients with pain following amputations. Neuroscience. 1998;86:1065–1081.
Lenz F.A., Gracely R.H., Baker F.H., et al. Reorganization of sensory modalities evoked by microstimulation in region of the thalamic principal sensory nucleus in patients with pain due to nervous system injury. Journal of Comparative Neurology. 1998;399:125–138.
Léonard G., Cloutier C., Marchand S. Reduced analgesic effect of acupuncture-like TENS but not conventional TENS in opioid-treated patients. Journal of Pain. 2011;12:213–221.
LeResche L. Facial expression in pain: a study of candid photographs. Journal of Nonverbal Behavior. 1982;7:56–76.
Liebano R.E., Rakel B., Vance C.G., et al. An investigation of the development of analgesic tolerance to TENS in humans. Pain. 2011;152:335–342.
Lischetzki G., Rukwied R., Handwerker H.O., et al. Nociceptor activation and protein extravasation induced by inflammatory mediators in human skin. European Journal of Pain. 2001;5:49–57.
Liu M., Max M.B., Parada S., et al. The sympathetic nervous system contributes to capsaicin-evoked mechanical allodynia but not pinprick hyperalgesia in humans. Journal of Neuroscience. 1996;16:7331–7335.
Loose R., Schnitzler A., Sarkar S., et al. Cortical activation during oesophageal stimulation: a neuromagnetic study. Neurogastroenterology and Motility. 1999;11:163–171.
Lowery D., Fillingim R.B., Wright R.A. Sex differences and incentive effects on perceptual and cardiovascular responses to cold pressor pain. Psychosomatic Medicine. 2003;65:284–291.
Ma Q.P., Woolf C.J. Progressive tactile hypersensitivity: an inflammation-induced incremental increase in the excitability of the spinal cord. Pain. 1996;67:97–106.
Magerl W., Krumova E.K., Baron R., et al. Reference data for quantitative sensory testing (QST): refined stratification for age and a novel method for statistical comparison of group data. Pain. 2010;151:598–605.
Maier C., Baron R., Tolle T.R., et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. Pain. 2010;150:439–450.
Maihofner C., Jesberger F., Seifert F., et al. Cortical processing of mechanical hyperalgesia: a MEG study. European Journal of Pain. 2010;14:64–70.
McDaniel L.K., Anderson K.O., Bradley L.A., et al. Development of an observation method for assessing pain behavior in rheumatoid arthritis patients. Pain. 1986;24:165–184.
McGrath P.A., Gracely R.H., Dubner R., et al. Non-pain and pain sensations evoked by tooth pulp stimulation. Pain. 1983;15:377–388.
Mechlin M.B., Maixner W., Light K.C., et al. African Americans show alterations in endogenous pain regulatory mechanisms and reduced pain tolerance to experimental pain procedures. Psychosomatic Medicine. 2005;67:948–956.
Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975;1:277–299.
Melzack R. Pain measurement and assessment. New York: Raven Press; 1983.
Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191–197.
Melzack R., Torgerson W.S. On the language of pain. Anesthesiology. 1971;34:50–59.
Melzack R., Wall P.D. Pain mechanisms: a new theory. Science. 1965;150:971–979.
Mobascher A., Brinkmeyer J., Warbrick T., et al. Laser-evoked potential P2 single-trial amplitudes covary with the fMRI BOLD response in the medial pain system and interconnected subcortical structures. NeuroImage. 2009;45:917–926.
Mouraux A., Diukova A., Lee M.C., et al. A multisensory investigation of the functional significance of the “pain matrix,”. NeuroImage. 2011;54:2237–2249.
Mouraux A., Guerit J.M., Plaghki L. Refractoriness cannot explain why C-fiber laser-evoked brain potentials are recorded only if concomitant Adelta-fiber activation is avoided. Pain. 2004;112:16–26.
Mu Y., Fan Y., Mao L., et al. Event-related theta and alpha oscillations mediate empathy for pain. Brain Research. 2008;1234:128–136.
Nakata H., Tamura Y., Sakamoto K., et al. Evoked magnetic fields following noxious laser stimulation of the thigh in humans. NeuroImage. 2008;42:858–868.
Naliboff B.D., Berman S., Chang L., et al. Sex-related differences in IBS patients: central processing of visceral stimuli. Gastroenterology. 2003;124:1738–1747.
Naliboff B.D., Munakata J., Fullerton S., et al. Evidence for two distinct perceptual alterations in irritable bowel syndrome. Gut. 1997;41:505–512.
Namer B., Handwerker H.O. Translational nociceptor research as guide to human pain perceptions and pathophysiology. Experimental Brain Research. 2009;196:163–172.
Neziri A.Y., Andersen O.K., Petersen-Felix S., et al. The nociceptive withdrawal reflex: normative values of thresholds and reflex receptive fields. European Journal of Pain. 2010;14:134–141.
Neziri A.Y., Curatolo M., Bergadano A., et al. New method for quantification and statistical analysis of nociceptive reflex receptive fields in humans. Journal of Neuroscience Methods. 2009;178:24–30.
Neziri A.Y., Scaramozzino P., Andersen O.K., et al. Reference values of mechanical and thermal pain tests in a pain-free population. European Journal of Pain. 2011;15:376–383.
Nilsson H.J., Psouni E., Schouenborg J. Long term depression of human nociceptive skin senses induced by thin fibre stimulation. European Journal of Pain. 2003;7:225–233.
Nir R.R., Lev R., Moont R., et al. Neurophysiology of the cortical pain network: revisiting the role of S1 in subjective pain perception via standardized low-resolution brain electromagnetic tomography (sLORETA). Journal of Pain. 2008;9:1058–1069.
Nir R.R., Sinai A., Raz E., et al. Pain assessment by continuous EEG: association between subjective perception of tonic pain and peak frequency of alpha oscillations during stimulation and at rest. Brain Research. 2010;1344:77–86.
Noh G.J., Kim K.M., Jeong Y.B., et al. Electroencephalographic approximate entropy changes in healthy volunteers during remifentanil infusion. Anesthesiology. 2006;104:921–932.
Norman E., Rosen I., Vanhatalo S., et al. Electroencephalographic response to procedural pain in healthy term newborn infants. Pediatric Research. 2008;64:429–434.
Oertel B.G., Preibisch C., Wallenhorst T., et al. Differential opioid action on sensory and affective cerebral pain processing. Clinical Pharmacology and Therapeutics. 2008;83:577–588.
Ohara S., Anderson W.S., Lawson H.C., et al. Endogenous and exogenous modulators of potentials evoked by a painful cutaneous laser (LEPs). Acta Neurochirurgica Supplement. 2006;99:77–79.
Opsommer E., Plaghki L. Maturational changes in the thermoalgesic system in humans from childhood to adulthood revealed by CO(2) laser evoked brain potentials following cutaneous heat stimuli. Neuroscience Letters. 2001;316:137–140.
Parry W.L., Smith G.M., Denton J.E. An electric-shock method of inducing pain responsive to morphine in man. Anesthesia and Analgesia. 1972;51:573–578.
Patel S., Ohara S., Dougherty P.M., et al. Psychophysical elements of place and modality specificity in the thalamic somatic sensory nucleus (ventral caudal, vc) of awake humans. Journal of Neurophysiology. 2006;95:646–659.
Patrick C.J., Craig K.D., Prkachin K.M. Observer judgments of acute pain: facial action determinants. Journal of Personality and Social Psychology. 1986;50:1291–1298.
Peghini P.L., Katz P.O., Castell D.O. Imipramine decreases oesophageal pain perception in human male volunteers. Gut. 1998;42:807–813.
Petersen K.L., Rowbotham M.C. A new human experimental pain model: the heat/capsaicin sensitization model. Neuroreport. 1999;10:1511–1516.
Petzke F., Clauw D.J., Ambrose K., et al. Increased pain sensitivity in fibromyalgia: effects of stimulus type and mode of presentation. Pain. 2003;105:403–413.
Petzke F., Gracely R.H., Park K.M., et al. What do tender points measure? Influence of distress on 4 measures of tenderness. Journal of Rheumatology. 2003;30:567–574.
Piche M., Arsenault M., Rainville P. Cerebral and cerebrospinal processes underlying counterirritation analgesia. Journal of Neuroscience. 2009;29:14236–14246.
Polianskis R., Graven-Nielsen T., Arendt-Nielsen L. Modality-specific facilitation and adaptation to painful tonic stimulation in humans. European Journal of Pain. 2002;6:475–484.
Poreisz C., Antal A., Boros K., et al. Attenuation of N2 amplitude of laser-evoked potentials by theta burst stimulation of primary somatosensory cortex. Experimental Brain Research. 2008;185:611–621.
Poulsen L., Arendt-Nielsen L., Brosen K., et al. The hypoalgesic effect of imipramine in different human experimental pain models. Pain. 1995;60:287–293.
Price D.D. Psychological and neural mechanisms of pain. New York: Raven Press; 1988.
Price D.D., McHaffie J.G. Effects of heterotopic conditioning stimuli on first and second pain: a psychophysical evaluation in humans. Pain. 1988;34:245–252.
Qiu Y., Inui K., Wang X., et al. Effects of distraction on magnetoencephalographic responses ascending through C-fibers in humans. Clinical Neurophysiology. 2004;115:636–646.
Quante M., Scharein E., Zimmermann R., et al. Dissociation of morphine analgesia and sedation evaluated by EEG measures in healthy volunteers. Arzneimittelforschung. 2004;54:143–151.
Rahim-Williams F.B., Riley J.L., 3rd., et al. Ethnic identity predicts experimental pain sensitivity in African Americans and Hispanics. Pain. 2007;129:177–184.
Renner B., Clarke G., Grattan T., et al. Caffeine accelerates absorption and enhances the analgesic effect of acetaminophen. Journal of Clinical Pharmacology. 2007;47:715–726.
Rhudy J.L., Williams A.E., McCabe K.M., et al. Emotional modulation of spinal nociception and pain: the impact of predictable noxious stimulation. Pain. 2006;126:221–233.
Riley J.L., 3rd., King C.D., Wong F., et al. Lack of endogenous modulation and reduced decay of prolonged heat pain in older adults. Pain. 2010;150:153–160.
Rollman G.B. Signal detection theory measurement of pain: a review and critique. Pain. 1977;3:187–211.
Romaniello A., Arendt-Nielsen L., Cruccu G., et al. Modulation of trigeminal laser evoked potentials and laser silent periods by homotopical experimental pain. Pain. 2002;98:217–228.
Rossel P., Pedersen P., Niddam D., et al. Cerebral response to electric stimulation of the colon and abdominal skin in healthy subjects and patients with irritable bowel syndrome. Scandinavian Journal of Gastroenterology. 2001;36:1259–1266.
Rukwied R., Mayer A., Kluschina O., et al. NGF induces non-inflammatory localized and lasting mechanical and thermal hypersensitivity in human skin. Pain. 2010;148:407–413.
Sang C.N., Gracely R.H., Max M.B., et al. Capsaicin-evoked mechanical allodynia and hyperalgesia cross nerve territories. Evidence for a central mechanism. Anesthesiology. 1996;85:491–496.
Sang C.N., Hostetter M.P., Gracely R.H., et al. AMPA/kainate antagonist LY293558 reduces capsaicin-evoked hyperalgesia but not pain in normal skin in humans. Anesthesiology. 1998;89:1060–1067.
Sang C.N., Max M.B., Gracely R.H. Stability and reliability of detection thresholds for human A-beta and A-delta sensory afferents determined by cutaneous electrical stimulation. Journal of Pain and Symptom Management. 2003;25:64–73.
Satero P., Klingenstierna U., Karlsson T., et al. Pain threshold measurements with cutaneous argon laser, comparing a forced choice and a method of limits. Progress in Neuro-psychopharmacology & Biological Psychiatry. 2000;24:397–407.
Schmelz M., Kress M. Topical acetylsalicylate attenuates capsaicin induced pain, flare and allodynia but not thermal hyperalgesia. Neuroscience Letters. 1996;214:72–74.
Schmelz M., Schmidt R. Microneurographic single-unit recordings to assess receptive properties of afferent human C-fibers. Neuroscience Letters. 2010;470:158–161.
Schmelz M., Schmid R., Handwerker H.O., et al. Encoding of burning pain from capsaicin-treated human skin in two categories of unmyelinated nerve fibres. Brain. 2000;123:560–571.
Serra J. Sensory profiles: the cliché and the challenge. Pain. 2010;150:384–385.
Sethna N.F., Liu M., Gracely R., et al. Analgesic and cognitive effects of intravenous ketamine-alfentanil combinations versus either drug alone after intradermal capsaicin in normal subjects. Anesthesia and Analgesia. 1998;86:1250–1256.
Sindrup S.H., Nielsen J.C., Bjerring P., et al. Imipramine does not affect argon-laser–induced pin-prick pain thresholds and laser-evoked cerebral potentials. European Journal of Pain. 1998;2:127–132.
Skljarevski V., Ramadan N.M. The nociceptive flexion reflex in humans—review article. Pain. 2002;96:3–8.
Smith G.M., Egbert L.D., Markowitz R.A., et al. An experimental pain method sensitive to morphine in man: the submaximum effort tourniquet technique. Journal of Pharmacology and Experimental Therapeutics. 1966;154:324–332.
Staahl C., Olesen A.E., Andresen T., et al. Assessing analgesic actions of opioids by experimental pain models in healthy volunteers—an updated review. British Journal of Clinical Pharmacology. 2009;68:149–168.
Staahl C., Olesen A.E., Andresen T., et al. Assessing efficacy of non-opioid analgesics in experimental pain models in healthy volunteers: an updated review. British Journal of Clinical Pharmacology. 2009;68:322–341.
Staahl C., Reddy H., Andersen S.D., et al. Multi-modal and tissue-differentiated experimental pain assessment: reproducibility of a new concept for assessment of analgesics. Basic & Clinical Pharmacology & Toxicology. 2006;98:201–211.
Stancak A., Polacek H., Bukovsky S. Bursts of 15-30 Hz oscillations following noxious laser stimulus originate in posterior cingulate cortex. Brain Research. 2010;1317:69–79.
Steen K.H., Reeh P.W., Kreysel H.W. Topical acetylsalicylic, salicylic acid and indomethacin suppress pain from experimental tissue acidosis in human skin. Pain. 1995;62:339–347.
Sternbach R.A., Tursky B. Ethnic differences among housewives in psychophysical and skin potential responses to electric shock. Psychophysiology. 1965;1:241–246.
Sternberg W.F., Bailin D., Grant M., et al. Competition alters the perception of noxious stimuli in male and female athletes. Pain. 1998;76:231–238.
Sternberg W.F., Bokat C., Kass L., et al. Sex-dependent components of the analgesia produced by athletic competition. Journal of Pain. 2001;2:65–74.
Straneva P.A., Maixner W., Light K.C., et al. Menstrual cycle, beta-endorphins, and pain sensitivity in premenstrual dysphoric disorder. Health Psychology. 2002;21:358–367.
Sumikura H., Andersen O.K., Drewes A.M., et al. Secondary heat hyperalgesia induced by melittin in humans. European Journal of Pain. 2006;10:121–125.
Svensson P., McMillan A.S., Graven-Nielsen T., et al. Modulation of an inhibitory reflex in single motor units in human masseter by tonic painful stimulation. Pain. 1999;83:441–446.
Sycha T., Gustorff B., Lehr S., et al. A simple pain model for the evaluation of analgesic effects of NSAIDs in healthy subjects. British Journal of Clinical Pharmacology. 2003;56:165–172.
Tan E.C., Lim Y., Teo Y.Y., et al. Ethnic differences in pain perception and patient-controlled analgesia usage for postoperative pain. Journal of Pain. 2008;9:849–855.
Tashani O.A., Alabas O.A., Johnson M.I. Cold pressor pain responses in healthy Libyans: effect of sex/gender, anxiety, and body size. Gender Medicine. 2010;7:309–319.
Tassorelli C., Sandrini G., Cecchini A.P., et al. Changes in nociceptive flexion reflex threshold across the menstrual cycle in healthy women. Psychosomatic Medicine. 2002;64:621–626.
Terkelsen A.J., Andersen O.K., Hansen P.O., et al. Effects of heterotopic- and segmental counter-stimulation on the nociceptive withdrawal reflex in humans. Acta Physiologica Scandinavica. 2001;172:211–217.
Torebjork H.E., Hallin R.G. C-fibre units recorded from human sensory nerve fascicles in situ. A preliminary report. Acta Societatis Medicorum Upsaliensis. 1970;75:81–84.
Tran T.D., Matre D., Casey K.L. An inhibitory interaction of human cortical responses to stimuli preferentially exciting Adelta or C fibers. Neuroscience. 2008;152:798–808.
Treede R.D., Rolke R., Andrews K., et al. Pain elicited by blunt pressure: neurobiological basis and clinical relevance. Pain. 2002;98:235–240.
Tsuji T., Inui K., Kojima S., et al. Multiple pathways for noxious information in the human spinal cord. Pain. 2006;123:322–331.
Tursky B., Sternbach R.A. Further physiological correlates of ethnic differences in response to shock. Psychophysiology. 1967;4:67–74.
Tuveson B., Leffler A.S., Hansson P. Time dependent differences in pain sensitivity during unilateral ischemic pain provocation in healthy volunteers. European Journal of Pain. 2006;10:225–232.
Valeriani M., Betti V., Le Pera D., et al. Seeing the pain of others while being in pain: a laser-evoked potentials study. NeuroImage. 2008;40:1419–1428.
Valeriani M., Le Pera D., Restuccia D., et al. Parallel spinal pathways generate the middle-latency N1 and the late P2 components of the laser evoked potentials. Clinical Neurophysiology. 2007;118:1097–1104.
Valeriani M., Restuccia D., Barba C., et al. Sources of cortical responses to painful CO(2) laser skin stimulation of the hand and foot in the human brain. Clinical Neurophysiology. 2000;111:1103–1112.
Valeriani M., Tonali P., Le Pera D., et al. Modulation of laser-evoked potentials by experimental cutaneous tonic pain. Neuroscience. 2006;140:1301–1310.
Vallbo A.B., Hagbarth K.E. Activity from skin mechanoreceptors recorded percutaneously in awake human subjects. Experimental Neurology. 1968;21:270–289.
van den Broeke E.N., van Rijn C.M., Biurrun Manresa J.A., et al. Neurophysiological correlates of nociceptive heterosynaptic long-term potentiation in humans. Journal of Neurophysiology. 2010;103:2107–2113.
Vossen H.G., van Os J., Hermens H., et al. Evidence that trait-anxiety and trait-depression differentially moderate cortical processing of pain. Clinical Journal of Pain. 2006;22:725–729.
Walker J.S., Carmody J.J. Experimental pain in healthy human subjects: gender differences in nociception and in response to ibuprofen. Anesthesia and Analgesia. 1998;86:1257–1262.
Wall P.D. On the relation of injury to pain. The John J. Bonica lecture. Pain. 1979;6:253–264.
Walsh D.M., Noble G., Baxter G.D., et al. Study of the effects of various transcutaneous electrical nerve stimulation (TENS) parameters upon the RIII nociceptive and H-reflexes in humans. Clinical Physiology. 2000;20:191–199.
Wang H., Bolognese J., Calder N., et al. Effect of morphine and pregabalin compared with diphenhydramine hydrochloride and placebo on hyperalgesia and allodynia induced by intradermal capsaicin in healthy male subjects. Journal of Pain. 2008;9:1088–1095.
Wang K., Svensson P., Sessle B.J., et al. Interactions of glutamate and capsaicin-evoked muscle pain on jaw motor functions of men. Clinical Neurophysiology. 2010;121:950–956.
Weidner C., Schmelz M., Schmidt R., et al. Neural signal processing: the underestimated contribution of peripheral human C-fibers. Journal of Neuroscience. 2002;22:6704–6712.
Weidner C., Schmidt R., Schmelz M., et al. Action potential conduction in the terminal arborisation of nociceptive C-fibre afferents. Journal of Physiology. 2003;547:931–940.
Wolfe F., Smythe H.A., Yunus M.B., et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheumatism. 1990;33:160–172.
Wolfe F. The relation between tender points and fibromyalgia symptom variables: evidence that fibromyalgia is not a discrete disorder in the clinic. Annals of the Rheumatic Diseases. 1997;56:268–271.
Yarnitsky D., Sprecher E., Zaslansky R., et al. Multiple session experimental pain measurement. Pain. 1996;67:327–333.
Zborowski M. Cultural components in response to pain. Journal of Social Issues. 1952;8:16–30.
Zheng Z., Feng S.J., Costa C., et al. Acupuncture analgesia for temporal summation of experimental pain: a randomised controlled study. European Journal of Pain. 2010;14:725–731.
Zyloney C.E., Jensen K., Polich G., et al. Imaging the functional connectivity of the periaqueductal gray during genuine and sham electroacupuncture treatment. Molecular Pain. 2010;6:80.