Phantom Limb
Introduction
The phenomenon of phantom limbs has probably been known since antiquity, but the first medical descriptions were not published until the 16th century. Authors such as Ambroise Paré, René Descartes, Aaron Lemos, and Charles Bell were similar in their descriptions of the clinical characteristics of phantom limbs but differed when it came to explaining the phenomenon. Historically, Silas Weir Mitchell (1829–1914) is credited with coining the term “phantom limb.” More than anyone else, Mitchell brought phantom limbs to the attention of the medical community. In his “Injuries of Nerves and Their Consequences” (1872), he presented the results from clinical studies of amputees and approached phantom limbs physiologically, experimentally, and therapeutically (for historical review see Finger and Hustwit 2003).
In modern times, World War II and the Vietnamese, Israeli, Iraqi (Ebrahimzadeh et al 2006), Yugoslavian, and Afghani wars have been responsible for many sad cases of traumatic amputations in otherwise healthy people. Land mine explosions in Cambodia still result in many amputations (Husum et al 2002), and during the civil war in Sierra Leone, the opposing factions performed limb amputations to terrorize the enemy (Lacoux et al 2002). In Western countries, the main reasons for amputation are diabetes and peripheral vascular disease in elderly people and, less often, tumors.
Amputation is followed by phantom sensations, painful or not, in almost all patients. In some patients the missing limb becomes the site of severe pain, and although much research has been done on the subject, no major treatment advances in phantom pain have taken place. Phantom phenomena may occur following amputation of body parts other than limbs, but the present chapter focuses on the clinical characteristics, mechanisms, treatment, and possible preventive measures for phantom pain after limb amputation.
Clinical Characteristics
Prevalence
The prevalence of phantom pain shows great discrepancy in the literature. Early studies report figures in the range of 2–4% (Ewalt et al 1947, Henderson and Smyth 1948), but most recent studies agree that 60–80% of patients experience phantom pain following amputation (Table 64-1 for details). This variation may be ascribed to differences in study populations, research design, and cutoff levels for phantom pain. Studies based on medical records of pain and analgesic requirements are likely to underestimate the prevalence (Sherman and Sherman 1983, Campbell et al 2000).
The prevalence of phantom pain is probably not influenced by age in adults, gender, side or level of amputation, and cause (civilian versus traumatic) of the amputation (Jensen et al 1983, Sherman and Sherman 1985, Houghton et al 1994, Montoya et al 1997). However, a recent prospective study of 85 amputees showed that female gender and upper limb amputation were associated with a higher risk for phantom pain (Bosmans et al 2010). Phantom pain is less frequent in very young children and congenital amputees (Melzack et al 1997, Wilkins et al 1998, Kooijman et al 2000), but phantom pain develops in older children and adolescents almost to the same extent as in adults (Krane and Heller 1995, Wilkins et al 1998).
Onset and Duration
Prospective studies in patients undergoing amputation mainly because of peripheral vascular disease have shown that the onset of phantom pain usually occurs within the first week after amputation (Jensen et al 1983, Nikolajsen et al 1997a, Richardson et al 2006, Hanley et al 2007). The appearance of phantom pain may, however, be delayed for months or even years (Schley et al 2008). Rajbhandari and colleagues (1999) described a 58-year-old man who had undergone left below-knee amputation at the age of 13. Eight months before the diagnosis of diabetes, he began to complain of a typical diabetic neuropathic pain in the phantom leg, which was followed by a similar complaint in the intact limb. In a retrospective study of individuals who either were born limb deficient or underwent amputation before the age of 6 years, Melzack and co-workers (1997) found that the mean time for the onset of phantom pain was 9 years in the group of congenital amputees and 2.3 years in the group of individuals with early amputations.
It is not possible to give exact descriptions of the time course of phantom pain because no prospective studies with long-term (many years) follow-up exist. Prospective studies show that the prevalence of phantom pain decreases only slightly during a maximum follow-up period of 3.5 years (Jensen et al 1985, Nikolajsen et al 1997a, Richardson et al 2006, Hanley et al 2007, Bosmans et al 2010). However, the severity and frequency of phantom pain attacks show a gradual decrease with time in most patients. In a retrospective survey of 526 veterans, phantom pain had disappeared in 16%, decreased markedly in 37%, remained similar in 44%, and increased in 3% of the respondents reporting phantom pain (Wartan et al 1997).
Intensity, Frequency, Localization, and Character
Although phantom pain is seen in 60–80% of amputees, the number of patients with severe pain is substantially smaller, in the range of 5–10%. In a prospective study of lower limb amputees, the mean intensity of pain 6 months after amputation was 22 (range, 3–82) on a visual analog scale (VAS, 0–100) 6 months after amputation (Nikolajsen et al 1997a). Similar results were reported in another prospective study (Hanley et al 2007). Houghton and co-workers retrospectively asked 176 amputees to recall on a VAS (0–10) how much phantom pain they had postoperatively at 6 months and at 1, 2, and 5 years after amputation; mean scores were 4, 3, 3, 2, and 1, respectively (Houghton et al 1994).
Phantom pain is usually intermittent and only a few patients are in constant pain. Episodes of pain attacks are most often reported to occur daily or at daily or weekly intervals (Ehde et al 2000, Kooijman et al 2000, Whyte and Niven 2001, Richardson et al 2006, Schley et al 2008, Desmond and Maclachlan 2010). In a survey of 141 upper limb amputees, Desmond and Maclachlan (2010) found that the duration of pain attacks was seconds or a few minutes in 43% of amputees, several minutes to hours in 20%, and of longer duration in the rest of the amputees.
Phantom pain is primarily localized to the distal parts of the missing limb. In upper limb amputees, pain is normally felt in the fingers and palm of the hand, and in lower limb amputees, pain is generally experienced in the toes, foot, or ankle (Jensen et al 1985, Katz and Melzack 1990, Nikolajsen et al 1997a). The reason for this clear, but the vivid phantom experience of distal limb parts is not clear. Perhaps the larger cortical representation of the hand and foot as opposed to the lesser representation of the more proximal parts of the limb may play a role.
The character of phantom pain is often described as shooting, pricking, and burning. Other terms used are stabbing, pricking, pins and needles, tingling, throbbing, cramping, and crushing. Some patients have vivid descriptions such as “a hammer is slammed at my calf” and “ants are crawling around inside my foot” (Montoya et al 1997, Nikolajsen et al 1997a, Wartan et al 1997, Wilkins et al 1998, Ehde et al 2000).
The following case is illustrative of a person with severe phantom limb pain:
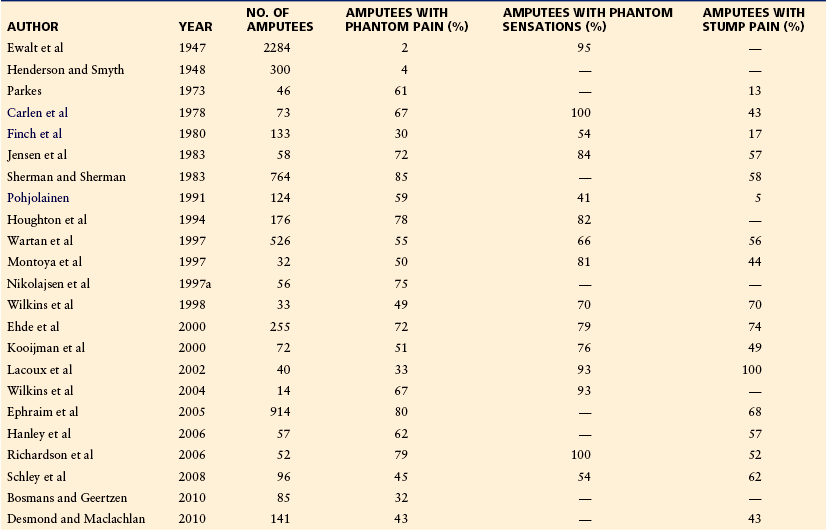
A 55-year-old man lost his arm at the age of 32 years because of an explosion accident at work. After the amputation, he had severe constant pain localized in the phantom hand and fingers. The phantom arm was extended in front of the thorax, occasionally with the perception of voluntary and involuntary painful movements of the hand (Fig. 64-1). The pain waxed and waned, and during instances of severe pain the phantom moved involuntarily to the dorsum. The amputee described his pain as follows: “The pain is always there. I have a constant burning sensation in my hand and a feeling that my fingers are being crushed. It feels as if somebody is ripping off my fingernails and like sand is running through my veins. A frequent nightmare is that a wolf is eating my arm. I only sleep 1 or 2 successive hours.” Tricyclic antidepressants, anticonvulsants, including gabapentin and pregabalin, and opioids had been tried, but the patient experienced either a lack of analgesic effect or intolerable side effects. Physical examination revealed amputation of the right arm and sensory abnormalities in the amputated area. The patient had several trigger zones in the neck and the amputation stump from where referred phantom pain could be elicited. Findings on neurological examination were otherwise normal.
Preamputation Pain and Phantom Pain
Some retrospective studies, but not all, have pointed to preamputation pain as a risk factor for phantom pain (Wall et al 1985, Houghton et al 1994, Krane and Heller 1995). The hypothesis is that preoperative pain may sensitize the nervous system, which explains why some individuals may be more susceptible to the development of chronic pain. For example, Houghton and colleagues found a significant relationship between preamputation pain and phantom pain in the first 2 years after amputation in vascular amputees, but in traumatic amputees, phantom pain was related to preamputation pain only immediately after the amputation (Houghton et al 1994). The relationship between preamputation pain and phantom pain has been confirmed in prospective studies (Jensen et al 1985, Nikolajsen et al 1997a, Hanley et al 2007). In the study by Nikolajsen’s group, a relationship between preoperative pain and phantom pain was found 1 week and 3 months after the amputation, but not later in the course. However, phantom pain never developed in some patients with severe preoperative pain, whereas it did develop in others with only modest preoperative pain (Nikolajsen et al 1997a).
The complexity of the relationship between preamputation pain and phantom pain is supported by the notion that phantom pain develops in patients with traumatic amputations, some of whom never experienced pain before the amputation, to the same extent as in patients with long-standing preamputation pain who undergo amputation for medical reasons. In addition, Lacoux and associates examined 40 upper limb amputees who had lost their limbs following injury by a machete, axe, or gunshot during the civil war in Sierra Leone. About half the amputees (56%) lost their limbs at the time of injury (primary), whereas the remainder had an injury and subsequent amputation at the hospital on average 10 days after the injury (secondary). It is reasonable to assume that the latter group suffered from severe pain between the two events. However, there was no correlation between the development of phantom pain and whether the amputation was primary or secondary (Lacoux et al 2002).
Another issue concerns the extent to which pain experienced before the amputation may survive as phantom pain. Striking case reports show that phantom pain may mimic preamputation pain in both character and localization (Katz and Melzack 1990, Hill et al 1996, Nikolajsen et al 1997a). In a retrospective study by Katz and Melzack (1990), 68 amputees were questioned about preamputation pain and phantom pain from 20 days to 46 years after amputation. Fifty-seven percent of those who had experienced preamputation pain claimed that their phantom pain resembled the pain that they had before the amputation. The number of patients with similar descriptions of preamputation pain and phantom pain was much lower, however, in two prospective studies (Jensen et al 1985, Nikolajsen et al 1997a).
In the study by Nikolajsen’s group, the character and localization of pain were recorded before and at specific intervals after the amputation. Although 42% of patients claimed that their phantom pain was similar to the pain that they experienced before the amputation, the actual similarity when comparing pre- and postamputation descriptions of pain was not higher in patients who claimed similarity than in those who found no similarity between phantom pain and preamputation pain (Nikolajsen et al 1997a). Thus, retrospective memories about pain should be judged carefully. It is likely that pain experienced preoperatively may survive as phantom pain in some patients, but this is not the case in the vast majority of patients.
Psychological Factors
Amputation of a limb is a traumatic experience in most patients, and many amputees exhibit a range of psychological symptoms such as depression, anxiety, self-pity, and isolation. In a survey of 914 amputees, depressive symptoms were shown to be a significant predictor of the intensity of phantom pain (Ephraim et al 2005). As with other chronic pain conditions, coping strategies are important for the experience of pain (Hill et al 1995, Jensen et al 2002). Passive coping strategies, especially catastrophizing, are associated with phantom limb pain (Richardson et al 2007, Vase et al 2011). Other psychosocial factors, as, for example, social support, also play an important role in the adjustment to phantom pain (Jensen et al 2002, Hanley et al 2004).
Others have looked at pain-related disability and rehabilitation (Sinha and van den Heuvel 2011). The impact on working life is especially relevant for amputees who become handicapped at a young age. Schoppen and colleagues (2002) examined the occupational situation of people with lower limb amputations in The Netherlands and found that amputees who experienced a long delay between the amputation and return to work had difficulty finding suitable jobs and had fewer opportunities for promotion.
Other Modulating Factors
Phantom pain may be modulated by several other internal and external factors, such as attention, distress, coughing, urination, and manipulation of the stump. It is unclear whether the use of a functionally active prosthesis as opposed to a cosmetic prosthesis reduces phantom pain (Lotze et al 1999, Weiss et al 1999, Kooijmann et al 2000, Hunter et al 2008).
Both experimental and clinical studies have shown a significant genetic contribution to the development of chronic pain, including neuropathic pain after nerve injury (Seltzer et al 2001, Nissenbaum et al 2010, Reimann 2010). However, an inherited component is not always a feature of phantom pain. Schott (1986) described a case in which five members of a family sustained traumatic amputation of their limbs. The development of phantom pain was unpredictable despite the individuals being first-degree relatives.
It has been claimed that phantom pain may be provoked by spinal anesthesia in lower limb amputees (Mackenzie 1983). However, Tessler and Kleiman (1994) prospectively investigated 23 spinal anesthetics in 17 patients, and phantom pain developed in only 1 patient but resolved in 10 minutes.
Phantom Sensations
Phantom sensations are more frequent than phantom pain and are experienced by nearly all amputees (see Table 64-1 for details). Phantom sensations rarely pose any major clinical problem. The two phenomena are strongly correlated. In a study by Kooijmann’s group, phantom pain was present in 36 of 37 upper limb amputees with phantom sensations but in only 1 of 17 without phantom sensations (Kooijman et al 2000).
As with phantom pain, non-painful sensations usually appear within the first days after amputation (Schley et al 2008). The amputee frequently wakes up from anesthesia with a feeling that the amputated limb is still there. Immediately after amputation, the phantom limb often resembles the preamputation limb in shape, length, and volume. Over time the phantom fades, but sensation in the distal parts of the limb remains. For example, upper limb amputees may feel hand and fingers, and lower limb amputees may feel foot and toes.
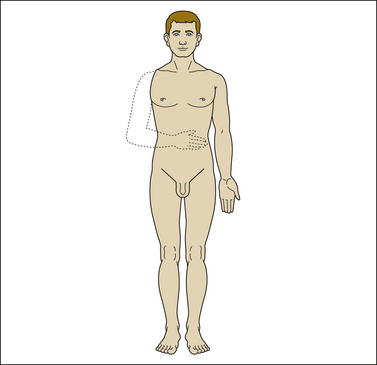
A common position of the phantom in upper limb amputees is that the fingers are clenched in a fist, whereas the phantom limb of lower limb amputees is commonly described as toes flexed (Wilkins et al 1998). In some cases, phantom sensations are very vivid and include feelings of movement and posture; in other cases, only suggestions of the phantom are felt. Telescoping (shrinkage of the phantom) is reported to occur in about one-third of patients. The phantom gradually approaches the amputation stump and eventually becomes attached to it. Sometimes it may even be experienced within the residual limb (Fig. 64-2). It has been postulated that phantom pain prevents or retards shrinkage of the phantom, but Montoya and co-workers failed to find such a relationship: 12 of 16 patients with phantom pain and 5 of 10 patients without pain reported telescoping (Montoya et al 1997).
Stump Pain
Stump pain is common in the early postamputation period (Parkes 1973, Jensen et al 1983). In a prospective study including lower limb amputees, 54 patients had some stump pain in the first week after amputation, with a median intensity of 15.5 (range, 0–61) on a VAS (0–100) (Nikolajsen et al 1997a). Stump pain can, however, persist beyond the stage of post-surgical healing. The prevalence of chronic stump pain varies a lot in the literature, but severe pain is probably seen in only 5–10% of cases (see Table 64-1 for details). In a survey of 78 traumatic amputees, Pezzin and associates (2000) found that 14.1% suffered from severe and constant pain in the stump. In another survey of 914 amputees, the prevalence of stump pain was 67.7%, but the pain was mild in most cases (Ephraim et al 2005). The incidence of chronic stump pain is likely to be higher in war zones (Husum et al 2002, Lacoux et al 2002).
Stump pain may be described as pressing, throbbing, burning, squeezing, or stabbing (Jensen et al 1985). Some patients have spontaneous movements of the stump ranging from slight, hardly visible jerks to severe contractions. Careful sensory examination of amputation stumps may reveal areas with sensory abnormalities such as hypoesthesia, hyperalgesia, or allodynia (Nikolajsen et al 1998). In a prospective study of 35 amputees, low mechanical thresholds (pressure algometry) at the stump were associated with stump and phantom pain 1 week after amputation (Nikolajsen et al 2000b). However, other studies have shown that there is no simple correlation between phantom pain and sensory function of the stump (Hunter et al 2005, 2008).
Stump pain and phantom pain are strongly correlated. In a survey of 648 amputees, Sherman and Sherman (1983) found that stump pain was present in 61% of amputees with phantom pain but in only 39% of those without phantom pain. Similar results have been reported in more recent studies (e.g., Richardson et al 2006, Schley et al 2008, Desmond and Maclahan 2010). The association between stump and phantom pain is consistent with experimental studies in amputees. Nystrøm and Hagbarth (1981) observed abnormal activity in the peroneal and median nerve fibers of two amputees with ongoing pain in their phantom foot and hand, respectively. Percussion of neuromas in these two patients produced increased nerve fiber discharges and augmentation of their phantom pain.
Mechanisms of Phantom Pain
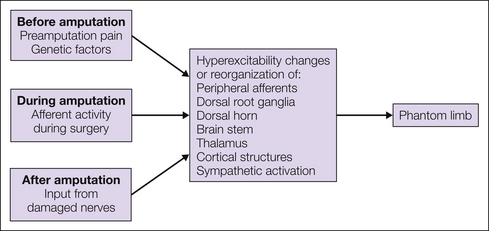
The mechanisms underlying phantom limb pain are not fully known despite research in the area. Results from research on animal models and other neuropathic pain conditions have, however, contributed significantly to the understanding of phantom limb pain. It is now clear that nerve injury is followed by a number of morphological, physiological, and chemical changes in both the peripheral and central nervous system and that all these changes are likely to play a role in the induction and maintenance of phantom limb pain (Flor et al 2006). The first events are likely to occur in the periphery, which subsequently generates a cascade of events that sweep more centrally until cortical brain structures finally become recruited. The involvement of cortical structures may be responsible for the complex and vivid characteristics of certain phantom phenomena (Silva et al 2010). In the following text a brief overview of peripheral, spinal, and supraspinal mechanisms is presented. An understanding of the mechanisms underlying phantom pain is likely to lead to new and rationally founded types of treatment. Figure 64-3 shows a proposed model of the development of phantom pain.
Peripheral Factors
Both experimental and clinical studies confirm that mechanisms in the periphery (i.e., in the stump or in central parts of the sectioned afferents) play a role in the phantom limb concept. Following a nerve cut, formation of neuromas is seen universally. Such neuromas show spontaneous and abnormal evoked activity following mechanical or chemical stimulation (for details see Chapters 1 and 61). The ectopic and increased spontaneous and evoked activity from the periphery is assumed to be the result of an increased and also novel expression of sodium channels (Novakovic et al 1998, Black et al 2008).
Percussion of the stump or of identified stump neuromas induces stump and phantom pain. In a classic microneurographic study involving two amputees, Nyström and Hagbarth (1981) showed that tapping of neuromas was associated with increased activity in afferent C fibers and increased phantom pain sensation. Consistent with these findings, a more recent study has shown that the intensity of phantom pain is inversely correlated with the pressure pain threshold of the stump early after amputation (Nikolajsen et al 2000b). Local anesthesia of the stump may reduce or abolish phantom pain temporarily. Chabal and co-workers showed that modulating peripheral output by locally anesthetizing stump neuromas with lidocaine reduces tap-evoked stump pain. In contrast, perineuromal injection of a potassium channel blocker, gallamine, produced clear exacerbation of the pain (Chabal et al 1989). These findings are consistent with the notion of abnormal input from peripheral nociceptors as an important pain generator.
It has been claimed that surgical neuroma removal abolishes phantom pain. For example, Sehirlioglu and colleagues (2009) retrospectively studied 75 lower limb amputees who underwent neuroma removal and reported that all patients were free of all pain symptoms after a mean follow-up period of 2.8 years. However, in a prospective study of six patients, pain was relieved in only two following surgical neuroma removal (Nikolajsen et al 2010).
In dorsal root ganglion (DRG) cells, changes occur following a complete nerve cut. Cell bodies in the DRG show similar abnormal spontaneous activity and increased sensitivity to mechanical and neurochemical stimulation (Kajander et al 1992). In the study by Nyström and Hagbarth (1981), local anesthesia of neuromas abolished tap-induced afferent discharges and tap-induced accentuation of phantom pain, but spontaneous pain and recorded spontaneous activity were unchanged. This is consistent with the generation of activity in DRG cells. DRG cells exhibit major changes in the expression of sodium channels and an altered expression pattern of different channels (Waxman 1999). Coward and colleagues have confirmed these findings in various human pain states (Coward et al 2000).
The sympathetic nervous system may also play an important role in generating and in particular in maintaining phantom pain. From animal studies it is well known that the application of noradrenaline or activation of post-ganglionic sympathetic fibers excites and sensitizes damaged, but not normal nerve fibers (Koltzenburg and McMahon 1991). Sympatholytic blocks can abolish neuropathic pain, and in patients whose pain is relieved after sympatholytic blockade, it can be rekindled by a minute’s injection of noradrenaline into the skin (Torebjork et al 1995). Long after limb amputation, injection of epinephrine around a stump neuroma is reported to be intensely painful (Chabal et al 1992). Lin and co-workers (2006) showed in 20 patients that perineuronal administration of norepinephrine resulted in a dose-dependent increase in pain. There was partial reversal of the pain by pretreatment with phentolamine. The catecholamine sensitivity may also be manifested in the skin, with a cooler extremity on the amputated side, and it has been suggested that phantom pain intensity is inversely related to the skin temperature of the stump (Sherman and Glenda 1987). Sympathetic–sensory coupling at the level of the DRG may also contribute to the increased pain response following a change in sympathetic activity.
Spinal Factors
Clinical observations show that spinal factors must be involved in the generation of phantom limb pain. For example, phantom limb pain may appear or disappear following spinal cord neoplasia. Aydin and colleagues described a woman who experienced phantom limb pain following lower limb amputation at the age of 5 years. At the age of 65 years, the pain gradually disappeared, in parallel with the evolution of cauda equina compression as a result of an intraspinal tumor. The phantom limb pain gradually reappeared after surgical removal of the tumor (Aydin et al 2005).
A very large number of experimental studies support the importance of spinal factors. After nerve injury there is an increase in this general excitability of spinal cord neurons, with C fibers and Aδ afferents gaining access to secondary pain-signaling neurons. Sensitization of dorsal horn neurons is mediated by release of glutamate and neurokinins. This sensitization may be manifested in several ways, including lowered threshold, increased persistent neuronal discharges with prolonged pain after stimulation, and expansion of peripheral receptive fields (for details see Chapter 6).
Another type of anatomical reorganization may also be present and contribute to central sensitization. Substance P is normally expressed in small afferent fibers, but following nerve injury, substance P may be expressed in large Aβ fibers; this phenotypic switch of large Aβ fibers into nociceptive-like nerve fibers may be one of the reasons why non-noxious stimuli can be perceived as painful (Hökfelt et al 1997).
Some amputees have abnormal sensitivity to pressure and to repetitive stimulation of the stump with a von Frey filament, which can often provoke attacks of phantom pain. The pharmacology of spinal sensitization involves increased activity in N-methyl-D-aspartate (NMDA) receptor–operated systems, and many aspects of central sensitization can be reduced by NMDA receptor antagonists. In human amputees, the stump or phantom pain evoked by repetitively stimulating the stump can be reduced with the NMDA antagonist ketamine (Nikolajsen et al 1996).
Supraspinal Factors
Amputation produces a cascade of events in the periphery and in the spinal cord. It is reasonable to assume that these changes will eventually sweep more centrally and alter neuronal activity in cortical and subcortical structures. Also, the phantom limb concept with its complex perceptual qualities and its modification by various internal stimuli (e.g., attention, distraction, stress) shows the phantom image to be a product of the brain.
Animal studies have demonstrated functional plasticity of the primary somatosensory cortex after amputation. After dorsal rhizotomy, a lowered threshold to evoked activity in the thalamus and cortex can be demonstrated, and adult monkeys display cortical reorganization in which the mouth and chin invade cortices corresponding to representation of the arm and digits that have lost their normal afferent input (Pons et al 1991, Florence and Kaas 1995).
Studies in humans have also documented cortical reorganization after amputation with different cerebral imaging techniques (Grüsser et al 2001). In a series of studies, Flor’s group showed a correlation between phantom pain and the amount of reorganization in the somatosensory cortex (Flor et al 1995, 1998). Birbaumer and colleagues (1997) studied the effect of regional anesthesia on cortical reorganization in upper limb amputees and found that brachial plexus blockade abolished pain and reorganization in three of six amputees. Huse and co-workers (2001) showed in a small group of amputees that cortical reorganization and pain were reduced during treatment with morphine.
Changes have also been observed at more subcortical levels. Using neuronal recording and stimulation techniques, Davis and associates (1998) found that thalamic neurons that do not normally respond to stimulation begin to respond in amputees and show enlarged somatotopic maps. In addition to functional plasticity, structural alterations also follow amputation. Draganski and colleagues recently demonstrated a decrease in the gray matter of the thalamus in 28 amputees. The decrease was correlated with the time span after amputation and was explained as a structural correlate of the loss of afferent input (Draganski et al 2006, Dostrovsky 1999).
Treatment
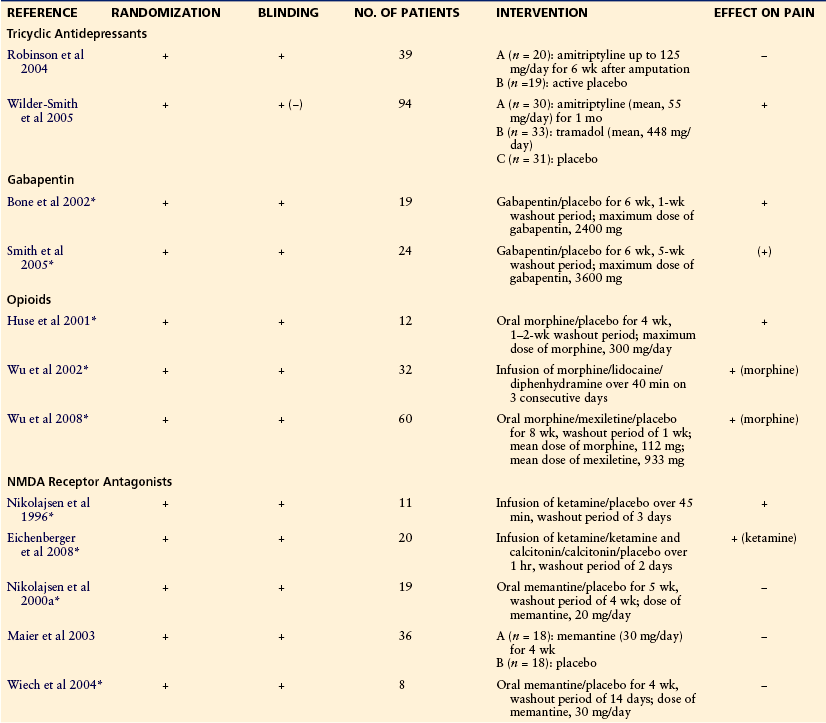
Treatment of chronic pain after amputation represents a major challenge to the clinician, in particular, treatment of phantom pain. There is not much evidence from randomized trials to guide clinicians in treatment, and most studies dealing with phantom pain suffer from major methodological errors: samples are small, randomization and blinding are either absent or inappropriate, controls are often lacking, and follow-up periods are short. Halbert and colleagues (2002) performed a systematic literature search (Medline 1966–1999) to determine the optimal management of phantom pain. The authors identified 186 articles, but after exclusion of letters, reviews, descriptive trials without intervention, case reports, and trials with major methodological errors, only 12 articles were left for review. Since then, some well-designed studies have been published. Until more clinical data become available, however, guidelines analogous to the treatment regimens used for other neuropathic pain conditions are probably the best approximation, especially for the treatment of stump pain (Finnerup et al 2010). A combination of medical and non-medical treatment may be advantageous. In general, treatment should be non-invasive. Surgery on the peripheral or central nervous system always involves further deafferentation and therefore increased risk for persistent pain. Suggestions for the treatment of postamputation pain are presented in Box 64-1.
Medical Treatment
At least two studies have examined the effect of tricyclic antidepressants on phantom pain. In one study, 39 patients were randomized to receive either amitriptyline or active placebo during a 6-week trial period. The dosage of amitriptyline was increased until the patient reached the maximum tolerated dose of 125 mg/day. Unfortunately, the study showed no effect of amitriptyline on pain intensity or secondary outcome measures such as satisfaction with life (Robinson et al 2004). In the other study, 49 post-traumatic amputees were randomized to receive amitriptyline (mean dose, 55 mg), tramadol (mean dose, 448 mg), or placebo for 1 month. The administration of tramadol and placebo was blinded; amitriptyline was given non-blinded as an open comparison. Non-responders (less than 10-mm pain relief on a VAS from baseline to day 3) were switched to the alternative active treatment (e.g., tramadol to amitriptyline treatment and vice versa). Placebo non-responders were switched to tramadol or amitriptyline. Both tramadol and amitriptyline had almost abolished the stump and phantom pain at the end of the treatment period (Wilder-Smith et al 2005).
Gabapentin
The effect of gabapentin on established phantom limb pain has been examined in two studies. Bone’s group examined the effect of gabapentin in a double-blind crossover study that included 19 patients with phantom pain. The dose of gabapentin was titrated in increments of 300 mg to the maximum dosage of 2400 mg/day. After 6 weeks of treatment, gabapentin was better than placebo in reducing phantom pain (Bone et al 2002). Smith and colleagues administered gabapentin or placebo for 6 weeks to 24 amputees in a double-blind, crossover fashion with a maximum dose of 3600 mg. Gabapentin did not decrease the intensity of pain significantly, but the participants rated the decrease in pain as more meaningful during the treatment period with gabapentin (Smith et al 2005). Thus far, the effect of pregabalin on phantom pain has not been examined in controlled trials.
Opioids
Failure to provide efficient pain relief should not be accepted until after a trial of opioids. In a placebo-controlled, crossover study that included 12 patients, a significant reduction in phantom pain was found during a 4-week treatment phase with oral morphine (Huse et al 2001). In another randomized, double-blind, crossover study with active placebo, 31 amputees received a 40-minute infusion of lidocaine, morphine, or diphenhydramine. When compared with placebo, morphine reduced both stump and phantom pain, whereas lidocaine reduced only stump pain (Wu et al 2002). The same group examined the effect of oral treatment with morphine, mexiletine, or placebo in 60 amputees during an 8-week treatment period. Postamputation pain was significantly reduced only during treatment with morphine (Wu et al 2008).
NMDA Receptor Antagonists
The effect of NMDA receptor antagonists has been examined in different studies. In a double-blind, placebo-controlled, crossover trial, intravenous ketamine reduced pain, hyperalgesia, and wind-up–like pain in 11 amputees with stump and phantom pain (Nikolajsen et al 1996). Eichenberger and colleagues studied the effect of a 1-hour infusion of ketamine alone, a combination of ketamine and calcitonin, calcitonin alone, and placebo in 20 amputees with phantom pain. Ketamine alone significantly reduced phantom pain. The combination of ketamine and calcitonin provided no additional effect, and calcitonin alone had no effect on pain (Eichenberger et al 2008). Three other trials have examined the effect of memantine, an NMDA receptor antagonist available for oral use. In all studies, memantine was administered in a blinded, placebo-controlled, crossover fashion to patients with established stump and phantom pain. Memantine at doses of 20 or 30 mg/day failed to have any effect on spontaneous pain, allodynia, and hyperalgesia (Nikolajsen et al 2000a, Maier et al 2003, Wiech et al 2004).
Other Drugs
Calcitonin significantly reduced phantom pain when used intravenously in the early postoperative phase in one study (Jaeger and Maier 1992). However, a more recent study found no effect of such treatment (Eichenberger et al 2008). A large number of other treatments, for example, dextromethorphan, topical application of capsaicin, intrathecal opioids, various anesthetic blocks, injection of botulinum toxin, and topiramate, have been claimed to be effective for phantom pain, but none of them has proved to be effective in well-controlled trials with a sufficient number of patients. Table 64-2 presents an overview of studies on the medical treatment of phantom pain.
Non-medical Treatment
A recent survey of treatments used for phantom pain revealed that after pharmacological treatment, physical therapy was the treatment modality most often used (Hanley et al 2006). Physical therapy involving massage, manipulation, and passive movements may prevent trophic changes and vascular congestion in the stump. Other treatments, such as transcutaneous electrical nerve stimulation, acupuncture, biofeedback, and hypnosis, may in some cases have a beneficial effect on stump and phantom pain. One study showed an effect of Farabloc, a metal-threaded sock to be worn over the stump (Conine et al 1993). It has been suggested that mirror therapy can reduce phantom pain (Chan et al 2007, Diers et al 2010). In a larger clinical trial of 80 amputees, however, Brodie and colleagues (2007) failed to find any significant effect of mirror treatment. Flor’s group demonstrated that sensory discrimination training achieved by applying stimuli at the stump reduced pain in five upper limb amputees (Flor et al 2001). The advantage of most of the aforementioned methods is the absence of side effects and complications and the fact that the treatment can easily be repeated. However, most studies are uncontrolled observations.
Surgical and Other Invasive Treatment
Surgery on amputation neuromas and more extensive amputation previously played important roles in the treatment of stump and phantom pain. Today, stump revision is probably performed only in cases of obvious stump pathology, and in properly healed stumps there is almost never any indication for proximal extension of the amputation because of pain. In a recent prospective study of patients with neuropathic pain, including phantom pain, pain was relieved in only two of six patients following surgical neuroma removal (Nikolajsen et al 2010). The results of other invasive techniques such as, for example, dorsal root entry zone lesions, sympathectomy, and cordotomy have generally been unfavorable, and most of them have been abandoned. Surgery may produce short-term pain relief, but the pain often reappears. Spinal cord stimulation and deep brain stimulation may be used for the treatment of phantom limb pain (Bittar et al 2005, Viswanathan et al 2010). Because these methods are invasive and associated with considerable cost, they should be used only in carefully selected patients.
Prevention
The idea of using perioperative analgesic interventions to prevent the development of phantom limb pain was prompted by the following observations:
These observations led to the theory that preamputation pain created an imprint in memorizing structures of the central nervous system and that such imprint could be responsible for persistent pain after amputation.
Epidurals
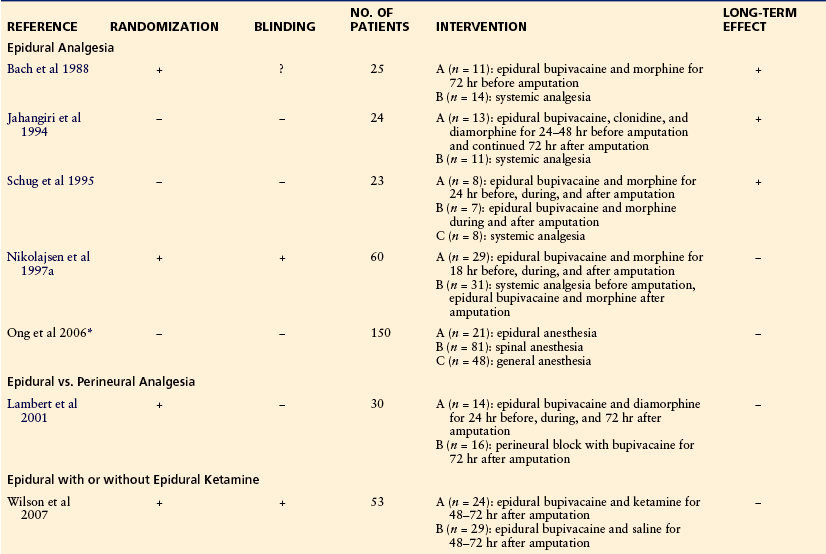
Inspired by this concept, Bach and colleagues carried out the first study on prevention of phantom pain: 25 patients were randomized by birth year to either epidural pain treatment 72 hours before the amputation (11 patients) or conventional analgesics (14 patients). All patients had spinal or epidural analgesia for the amputation, and both groups received conventional analgesics to treat postoperative pain. Blinding was not described. After 6 months the incidence of phantom pain was lower in patients who had received the preoperative epidural blockade (Bach et al 1988).
Jahangiri and co-workers examined the effect of perioperative epidural infusion of diamorphine, bupivacaine, and clonidine on postamputation stump and phantom pain. Thirteen patients received the epidural treatment 5–48 hours preoperatively and for at least 3 days postoperatively. A control group of 11 patients received opioid analgesia on demand. All patients underwent general anesthesia for the amputation. The incidence of severe phantom pain was lower in the epidural group 7 days, 6 months, and 1 year after amputation. The study was not randomized or blinded (Jahangiri et al 1994).
Schug and associates presented in a letter the results of a study in which 23 patients had either epidural analgesia before, during, and after the amputation (n = 8), intra- and postoperative epidural analgesia (n = 7), or general anesthesia plus systemic analgesia (n = 8). After 1 year the incidence of phantom pain was significantly lower in patients who received pre-, intra-, and postoperative epidural analgesia than in patients who received general anesthesia plus systemic analgesia (Schug et al 1995). Several abstracts with similar study designs have claimed a preventive effect of perioperative epidurals, but the results have never been published in articles.
Nikolajsen’s group carried out a randomized, double-blind, placebo-controlled study in which 60 patients scheduled for lower limb amputation were randomly assigned to one of two groups: a blockade group that received epidural bupivacaine and morphine before the amputation and during the operation (29 patients) and a control group that received epidural saline and oral or intramuscular morphine (31 patients). Both groups underwent general anesthesia for the amputation, and all patients received epidural analgesics for postoperative pain management. Patients were interviewed about preamputation pain on the day before the amputation and about stump and phantom pain after 1 week and 3, 6, and 12 months. The median duration of the preoperative epidural blockade (blockade group) was 18 hours. After 1 week the percentage of patients with phantom pain was 51.9 in the blockade group and 55.6 in the control group. Subsequently, the figures were (blockade/control) 82.4/50 at 3 months, 81.3/55 at 6 months, and 75/68.8 at 12 months. The intensity of stump and phantom pain and consumption of opioids were also similar in the two groups at all four postoperative interviews (Nikolajsen et al 1997b). In a retrospective review of 150 amputees, no difference was found in the incidence of phantom pain 24 months after the amputation in those who had received epidural, spinal, or general anesthesia for the amputation (Ong et al 2006).
Other Nerve Blocks
Others have examined the effect of peri- or intraneural blockade on phantom pain. Fischer and Meller (1991) introduced a catheter into the transected nerve sheath at the time of amputation and infused bupivacaine for 72 hours in 11 patients. Phantom pain did not develop in any patient during a 12-month follow-up. Two retrospective studies have found negative and positive effects, respectively, of a similar treatment (Elizaga et al 1994, Grant and Wood 2008). Pinzur et al (1996) prospectively randomized 21 patients to continuous postoperative infusion of either bupivacaine or saline but failed to find any difference between the two groups with regard to the incidence of phantom pain after 3 and 6 months (Pinzur et al 1996). Lambert and colleagues compared two techniques of regional analgesia: 30 patients were randomized to epidural bupivacaine and diamorphine started 24 hours before the amputation and continued for 3 days postoperatively or to an intraoperative perineural catheter for intra- and postoperative administration of bupivacaine. All patients underwent general anesthesia for the amputation. The pre-, peri-, and postoperative epidural pain treatment was not superior to the intra- and postoperative perineural pain treatment in preventing phantom pain since the incidence of phantom pain was similar in the two groups after 3 days and 6 and 12 months (Lambert et al 2001).
In a very recent study, interesting results have been reported following a prolonged infusion of local anesthetic via a perineural catheter. Seventy-one patients received perineural infusion of 0.5% ropivacaine for a median period of 30 days (range, 4–83 days) after the amputation. The infusion of ropivacaine was discontinued at regular intervals but restarted if the intensity of phantom pain exceeded 1 on a 5-point verbal scale. The incidence of severe to intolerable phantom pain was only 3% after 12 months (Borghi et al 2010).
Medical Interventions
A few studies have examined the effect of medical interventions applied in the peri- and postoperative period. In an open study with historical controls, Dertwinkel and colleagues (2002) suggested that ketamine infused intraoperatively and for 72 hours after amputation could reduce phantom pain. A randomized, double-blind trial that included 45 patients found no effect of a similar treatment (Hayes et al 2004). In another double-blind study, 19 patients with acute traumatic amputation of the upper extremity were randomized to memantine, 20–30 mg daily, or placebo for 4 weeks after amputation. All patients received postoperative analgesia by continuous brachial plexus analgesia. Memantine treatment reduced phantom pain after 4 weeks and 6 months but not after 12 months (Schley et al 2006). Nikolajsen and colleagues randomized 46 lower limb amputees to either gabapentin or placebo for the first 30 days after amputation. The first dose of 300 mg gabapentin/placebo was given on the first postoperative day, and the dosage was gradually increased until the maximum of 2400 mg was reached. The intensity, frequency, and duration of phantom pain attacks were recorded daily in the first 30 days and after 3 and 6 months. The intensity of stump pain was also recorded and sensory testing of the stump was performed. The two treatment groups were similar in almost all outcome parameters. Thus, early and prolonged treatment with gabapentin did not seem to reduce the incidence of phantom pain (Nikolajsen et al 2006).
In conclusion, perioperative interventions, such as epidurals, other nerve blocks, and medical treatments, are effective in the treatment of immediate postoperative stump pain. However, it is evident that more well-designed controlled trials are necessary to further evaluate the potential effectiveness of different perioperative treatment regimens in reducing chronic phantom pain. Table 64-3 presents an overview of studies on the prevention of phantom pain (for review see also Ypsilantis and Tang 2010).
Table 64-3
Summary of Studies on Prevention of Phantom Pain

A, B, and C refer to the different treatment arms in each study.
The references for this chapter can be found at www.expertconsult.com.
References
Aydin M.D., Cesur M., Aydin N., et al. Disappearance of phantom limb pain during cauda equina compression by spinal meningioma and gradual reactivation after decompression. Anesthesia and Analgesia. 2005;101:1123–1126.
Bach S., Noreng M.F., Tjéllden N.U. Phantom limb pain in amputees during the first 12 months following limb amputation after preoperative lumbar epidural blockade. Pain. 1988;33:297–301.
Birbaumer N., Lutzenberger W., Montoya P., et al. Effects of regional anesthesia on phantom limb are mirrored in changes in cortical reorganization in upper limb amputees. Journal of Neuroscience. 1997;17:5503–5508.
Bittar R.G., Otero S., Carter H., et al. Deep brain stimulation for phantom limb pain. Journal of Clinical Neuroscience. 2005;12:399–404.
Black J.A., Nikolajsen L., Kroner K., et al. Multiple sodium channels isoforms and mitogen-activated protein kinases are present in painful human neuromas. Annals of Neurology. 2008;64:644–653.
Bone M., Critchley P., Buggy D.J. Gabapentin in postamputation phantom limb pain: a randomized, double-blind, placebo-controlled, cross-over study. Regional Anesthesia and Pain Medicine. 2002;27:481–486.
Borghi B., D’Addabbo M., White P.F., et al. The use of prolonged peripheral neural blockade after lower extremity amputation: the effect on symptoms associated with phantom limb syndrome. Anesthesia and Analgesia. 2010;111:1308–1315.
Bosmans J.C., Geertzen J.H.B., Post W.J., et al. Factors associated with phantom limb pain: a 31⁄2-year prospective study. Clinical Rehabilitation. 2010;24:444–453.
Brodie E.E., Whyte A., Niven C.A. Analgesia through the looking-glass? A randomized controlled trial investigating the effect of viewing a “virtual” limb upon phantom limb pain, sensation and movement. European Journal of Pain. 2007;11:428–436.
Campbell W.B., Marriott S., Eve R., et al. Anaesthesia and analgesia for major lower limb amputation. Cardiovascular Surgery. 2000;8:572–575.
Carlen P.L., Wall P.D., Nadvorna H., et al. Phantom limbs and related phenomena in recent traumatic amputations. Neurology. 1978;28:211–217.
Chabal C., Jacobson L., Russell L.C., et al. Pain responses to perineuromal injection of normal saline, gallamine, and lidocaine in humans. Pain. 1989;36:321–325.
Chabal C., Jacobson L., Russell L.C., et al. Pain response to perineuronal injection of normal saline, epinephrine, and lidocaine in humans. Pain. 1992;49:9–12.
Chan B.L., Witt R., Charrow A.P., et al. Mirror therapy for phantom limb pain. New England Journal of Medicine. 2007;357:2206–2207.
Conine T.A., Herschler C., Alexander S.T., et al. The efficacy of Farabloc in the treatment of phantom limb pain. Canadian Journal of Rehabilitation. 1993;6:155–161.
Coward K., Plumpton C., Facer P., et al. Immunolocalization of SNS/PN3 and NaN/SNS2 sodium channels in human pain states. Pain. 2000;85:41–50.
Davis K.D., Kiss Z.H., Luo L. Phantom sensations generated by thalamic microstimulation. Nature. 1998;391:385–387.
Dertwinkel R., Heinrichs C., Senne I., et al. Prevention of severe phantom limb pain by perioperative administration of ketamine—an observational study. Acute Pain. 2002;4:9–13.
Desmond D.M., Maclachlan M. Prevalence and characteristics of phantom limb pain and residual limb pain in the long term after upper limb amputation. International Journal of Rehabilitation Research. 2010;33:279–282.
Diers M., Christmann C., Koeppe C., et al. Mirrored, imagined and executed movements differentially activate sensorimotor cortex in amputees with and without phantom limb pain. Pain. 2010;149:296–304.
Dostrovsky J.O. Immediate and long-term plasticity in human somatosensory thalamus and its involvement in phantom limbs. Pain. 1999;6(Suppl):37–43.
Draganski B., Moser T., Lummel N., et al. Decrease of thalamic gray matter following limb amputation. NeuroImage. 2006;31:951–957.
Ebrahimzadeh M.H., Fattahi A.S., Nejad A.B. Long-term follow-up of Iranian veteran upper extremity amputees from the Iran-Iraq war (1980-1988). Journal of Trauma. 2006;61:886–888.
Ehde D.M., Czerniecki J.M., Smith D.G., et al. Chronic phantom sensations, phantom pain, residual limb pain, and other regional pain after lower limb amputation. Archives of Physical Medicine and Rehabilitation. 2000;81:1039–1044.
Eichenberger U., Neff F., Sveticic G., et al. Chronic phantom limb pain: the effects of calcitonin, ketamine, and their combination on pain and sensory thresholds. Anesthesia and Analgesia. 2008;106:1265–1273.
Elizaga A.M., Smith D.G., Sharar S.R., et al. Continuous regional analgesia by intraneural block: effect on postoperative opioid requirements and phantom limb pain following amputation. Rehabilitation Research and Development. 1994;31:179–187.
Ephraim P.L., Wegener S.T., MacKenzie E.J., et al. Phantom pain, residual limb pain, and back pain in amputees: results of a national survey. Archives of Physical Medicine and Rehabilitation. 2005;86:1910–1919.
Ewalt J.R., Randall G.C., Morris H. The phantom limb. Psychosomatic Medicine. 1947;9:118–123.
Finch D.R.A., MacDougal M., Tibbs D.J., et al. Amputation for vascular disease: the experience of a peripheral vascular unit. British Journal of Surgery. 1980;67:233–237.
Finger S., Hustwit M.P. Five early accounts of phantom limb in context: Paré, Descartes, Lemos, Bell, and Mitchell. Neurosurgery. 2003;52:675–686.
Finnerup N.B., Sindrup S.H., Jensen T.S. The evidence for pharmacological treatment of neuropathic pain. Pain. 2010;150:573–581.
Fischer A., Meller Y. Continuous postoperative regional analgesia by nerve sheath block for amputation surgery—a pilot study. Anesthesia and Analgesia. 1991;72:300–303.
Flor H., Denke C., Schaefer M., et al. Effect of sensory discrimination training on cortical reorganisation and phantom limb pain. Lancet. 2001;357:1763–1764.
Flor H., Elbert T., Knecht S. Phantom limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature. 1995;375:482–484.
Flor H., Elbert T., Mühlnickel W. Cortical reorganization and phantom phenomena in congenital and traumatic upper-extremity amputees. Experimental Brain Research. 1998;119:205–212.
Flor H., Nikolajsen L., Jensen T.S. Phantom limb pain: a case of maladaptive CNS plasticity? Nature Reviews. Neuroscience. 2006;7:873–881.
Florence S.L., Kaas J.H. Large-scale reorganization at multiple levels of the somatosensory pathway follows therapeutic amputation of the hand in monkeys. Journal of Neuroscience. 1995;15:8083–8095.
Grant A.J., Wood C. The effect of intra-neural local anaesthetic infusion on pain following major lower limb amputation. Scottish Medical Journal. 2008;53:4–6.
Grüsser S.M., Winter C., Mühlnickel W., et al. The relationship of perceptual phenomena and cortical reorganization in upper extremity amputees. Neuroscience. 2001;102:263–272.
Halbert J., Crotty M., Cameron I.D. Evidence for the optimal management of acute and chronic phantom pain: a systematic review. Clinical Journal of Pain. 2002;18:84–92.
Hanley M.A., Jensen M.P., Ehde D.M., et al. Psychosocial predictors of long-term adjustment to lower-limb amputation and phantom limb pain. Disability and Rehabilitation. 2004;26:882–893.
Hanley M.A., Ehde D.M., Campbell K.M., et al. Self-reported treatments used for lower-limb phantom pain: descriptive findings. Archives of Physical Medicine and Rehabilitation. 2006;87:270–277.
Hanley M.A., Jensen M.P., Smith D.G., et al. Preamputation pain and acute pain predict chronic pain after lower extremity amputation. Journal of Pain. 2007;8:102–109.
Hayes C., Armstrong-Brown A., Burstal R. Perioperative intravenous ketamine infusion for the prevention of persistent post-amputation pain: a randomized, controlled trial. Anaesthesia and Intensive Care. 2004;32:330–338.
Henderson W.R., Smyth G.E. Phantom limbs. Journal of Neurology, Neurosurgery, and Psychiatry. 1948;11:88–112.
Hill A., Niven C.A., Knussen C. The role of coping in adjustment to phantom limb pain. Pain. 1995;62:79–86.
Hill A., Niven C.A., Knussen C. Pain memories in phantom limbs: a case story. Pain. 1996;66:381–384.
Hökfelt T., Zhang X., Xu Z.-Q., et al, Phenotypic regulation in dorsal root ganglion neurons after nerve injury: focus on peptides and their receptors. Borsook D., ed. Molecular neurobiology of pain. Progress in pain research and management, vol 9. Seattle: IASP Press; 1997:115–143.
Houghton A.D., Nicholls G., Houghton A.L., et al. Phantom pain: natural history and association with rehabilitation. Annals of the Royal College of Surgeons of England. 1994;76:22–25.
Hunter J.P., Katz J., Davis K.D. Dissociation of phantom limb phenomena from stump tactile spatial acuity and sensory thresholds. Brain. 2005;128:308–320.
Hunter J.P., Katz J., Davis K.D. Stability of phantom limb phenomena after upper limb amputation: a longitudinal study. Neuroscience. 2008;156:939–949.
Huse E., Larbig W., Flor H., et al. The effect of opioids on phantom limb pain and cortical reorganization. Pain. 2001;90:47–55.
Husum H., Resell K., Vorren G., et al. Chronic pain in land mine accident survivors in Cambodia and Kurdistan. Social Science & Medicine. 2002;55:1813–1816.
Jaeger H., Maier C. Calcitonin in phantom limb pain: a double-blind study. Pain. 1992;48:21–27.
Jahangiri M., Jayatunga A.P., Bradley J.W.P., et al. Prevention of phantom pain after major lower limb amputation by epidural infusion of diamorphine, clonidine and bupivacaine. Annals of the Royal College of Surgeons of England. 1994;76:324–326.
Jensen M.P., Ehde D.M., Hoffman A.J., et al. Cognitions, coping and social environment predict adjustment to phantom pain. Pain. 2002;95:133–142.
Jensen T.S., Krebs B., Nielsen J., et al. Phantom limb, phantom pain and stump pain in amputees during the first 6 months following limb amputation. Pain. 1983;17:243–256.
Jensen T.S., Krebs B., Nielsen J., et al. Immediate and long-term phantom limb pain in amputees: incidence, clinical characteristics and relationship to preamputation limb pain. Pain. 1985;21:267–278.
Kajander K.C., Wakisaka S., Bennett G.J. Spontaneous discharge originates in the dorsal root ganglion at the onset of a painful peripheral neuropathy in the rat. Neuroscience Letters. 1992;138:225–228.
Katz J., Melzack R. Pain “memories” in phantom limbs: review and clinical observations. Pain. 1990;43:319–336.
Koltzenburg M., McMahon S.B. The enigmatic role for the sympathetic nervous system in chronic pain. Trends in Pharmacological Sciences. 1991;12:399–402.
Kooijman C.M., Dijkstra P.U., Geertzen J.H.B., et al. Phantom pain and phantom sensations in upper limb amputees: an epidemiological study. Pain. 2000;87:33–41.
Krane E.J., Heller L.B. The prevalence of phantom sensation and pain in pediatric amputees. Journal of Pain and Symptom Management. 1995;10:21–29.
Lacoux P.A., Crombie I.K., Macrae W.A. Pain in traumatic upper limp amputees in Sierra Leone. Pain. 2002;99:309–312.
Lambert A.W., Dashfield A.K., Cosgrove C., et al. Randomised prospective study comparing preoperative epidural and intraoperative perineural analgesia for the prevention of postoperative stump and phantom pain following major amputation. Regional Anesthesia and Pain Medicine. 2001;26:316–321.
Lin E.E., Horasek S., Agarwal S., et al. Local administration of norepinephrine in the stump evokes dose-dependent pain in amputees. Clinical Journal of Pain. 2006;22:482–486.
Lotze M., Grodd W., Birbaumer N., et al. Does use of a myoelectric prosthesis prevent cortical reorganization and phantom limb pain? Nature Neuroscience. 1999;2:501–502.
Mackenzie N. Phantom limb pain during spinal anaesthesia. Anaesthesia. 1983;38:886–887.
Maier C., Dertwinkel R., Mansourian N., et al. Efficacy of the NMDA-receptor antagonist memantine in patients with chronic phantom limb pain—results of a randomized double-blinded, placebo-controlled trial. Pain. 2003;103:277–283.
Melzack R., Israel R., Lacroix R., et al. Phantom limbs in people with congenital limb deficiency or amputation in early childhood. Brain. 1997;120:1603–1620.
Montoya P., Larbig W., Grulke N. Relationship of phantom limb pain to other phantom limb phenomena in upper extremity amputees. Pain. 1997;72:87–93.
Nikolajsen L., Black J., Krøner K., et al. Neuroma removal for neuropathic pain: efficacy and predictive value of lidocaine infusion. Clinical Journal of Pain. 2010;26:788–793.
Nikolajsen L., Finnerup N.B., Kramp S., et al. A randomized study of the effects of gabapentin on postamputation pain. Anesthesiology. 2006;105:1008–1015.
Nikolajsen L., Gottrup H., Kristensen A.G.D., et al. Memantine (a N-methyl D-aspartate receptor antagonist) in the treatment of neuropathic pain following amputation or surgery: a randomised, double-blind, cross-over study. Anesthesia and Analgesia. 2000;91:960–966.
Nikolajsen L., Hansen C.L., Nielsen J., et al. The effect of ketamine on phantom pain: a central neuropathic disorder maintained by peripheral input. Pain. 1996;67:69–77.
Nikolajsen L., Ilkjaer S., Jensen T.S. Effect of preoperative epidural bupivacaine and morphine on stump sensations in lower limb amputees. British Journal of Anaesthesia. 1998;81:348–354.
Nikolajsen L., Ilkjaer S., Jensen T.S. Relationship between mechanical sensitivity and postamputation pain: a prospective study. European Journal of Pain. 2000;4:327–334.
Nikolajsen L., Ilkjaer S., Krøner K., et al. The influence of preamputation pain on postamputation stump and phantom pain. Pain. 1997;72:393–405.
Nikolajsen L., Ilkjaer S., Krøner K., et al. Randomised trial of epidural bupivacaine and morphine in prevention of stump and phantom pain in lower-limb amputation. Lancet. 1997;350:1353–1357.
Nissenbaum J., Devor M., Seltzer Z., et al. Susceptibility to chronic pain following nerve injury is genetically affected by CACNG2. Genome Research. 2010;20:1180–1190.
Novakovic S.D., Tzoumaka E., McGivern J.G., et al. Distribution of the tetrodotoxin-resistant sodium channel PN3 in rat sensory neurons in normal and neuropathic pain conditions. Journal of Neuroscience. 1998;18:2174–2187.
Nyström B., Hagbarth K.E. Microelectrode recordings from transected nerves in amputees with phantom limb pain. Neuroscience Letters. 1981;27:211–216.
Ong B.Y., Arneja A., Ong E.W. Effects of anesthesia on pain after lower-limb amputation. Journal of Clinical Anesthesia. 2006;18:600–604.
Parkes C.M. Factors determining the persistence of phantom pain in the amputee. Journal of Psychosomatic Research. 1973;17:97–108.
Pezzin L.E., Dillingham T.R., Mackenzie E.J. Rehabilitation and the long-term outcomes of persons with trauma-related amputations. Archives of Physical Medicine and Rehabilitation. 2000;81:292–300.
Pinzur M.S., Garla P.G.N., Pluth T., et al. Continuous postoperative infusion of a regional anaesthetic after an amputation of the lower extremity, Journal of Bone and Joint Surgery. American Volume. 1996;78:1501–1505.
Pohjolainen T. A clinical evaluation of stumps in lower limb amputees. Prosthetics and Orthotics International. 1991;15:178–184.
Pons T.P., Garraghty P.E., Ommaya A.K., et al. Massive cortical reorganization after sensory deafferentation in adult macaques. Science. 1991;252:1857–1860.
Rajbhandari S.M., Jarett J.A., Griffiths P.D., et al. Diabetic neuropathic pain in a leg amputated 44 years previously. Pain. 1999;83:627–629.
Reimann F., Cox J.J., Belfer I., et al. Pain perception is altered by a nucleotide polymorphism in SCN9A. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:5148–5153.
Richardson C., Glenn S., Nurmikko T., et al. Incidence of phantom phenomena including phantom limb pain 6 months after major lower limb amputation in patients with peripheral vascular disease. Clinical Journal of Pain. 2006;22:353–358.
Richardson C., Glenn S., Horgan M., et al. A prospective study of factors associated with the presence of phantom limb pain six months after major lower limb amputation in patients with peripheral vascular disease. Journal of Pain. 2007;8:793–801.
Robinson L.R., Czerniecki J.M., Ehde D.M., et al. Trial of amitriptyline for relief of pain in amputees: results of a randomized controlled study. Archives of Physical Medicine and Rehabilitation. 2004;85:1–6.
Schley M., Topfner S., Wiech K., et al. Continuous brachial plexus blockade in combination with the NMDA receptor antagonist memantine prevents phantom pain in acute traumatic upper limb amputees. European Journal of Pain. 2006;11:299–308.
Schley M.T., Wilms P., Toepfner S., et al. Painful and nonpainful phantom and stump sensations in acute traumatic amputees. Journal of Trauma. 2008;65:858–864.
Schoppen T., Boonstra A., Groothoff J.W., et al. Employment status, job characteristics, and work-related health experience of people with a lower limb amputation in the Netherlands. Archives of Physical Medicine and Rehabilitation. 2002;82:239–245.
Schott G.D. Pain and its absence in an unfortunate family of amputees. Pain. 1986;25:229–231.
Schug S.A., Burell R., Payne J., et al. Preemptive epidural anaesthesia may prevent phantom limb pain. Regional Anesthesia. 1995;20:256.
Sehirlioglu A., Ozturk C., Yazicioglu K., et al. Painful neuroma requiring surgical excision after lower limb amputation caused by landmine explosions. International Journal of Ortopedics. 2009;33:533–536.
Seltzer Z., Wu T., Max M.B., et al. Mapping a gene for neuropathic pain–related behavior following peripheral neurectomy in the mouse. Pain. 2001;93:101–106.
Sherman R., Sherman C. Prevalence and characteristics of chronic phantom limb pain among American veterans: results of a trial survey. American Journal of Physical Medicine. 1983;62:227–238.
Sherman R.A., Glenda G.M. Concurrent variation of burning phantom limb and stump pain with near surface blood flow in the stump. Orthopedics. 1987;10:1395–1402.
Sherman R.A., Sherman C.J. A comparison of phantom sensations among amputees whose amputations were of civilian and military origins. Pain. 1985;21:91–97.
Silva S., Bataille B., Jucla M., et al. Temporal analysis of regional anaesthesia–induced sensorimotor dysfunction: a model for understanding phantom limb. British Journal of Anaesthesia. 2010;105:208–213.
Sinha R., Van den Heuvel W.J.A. A systematic literature review of quality of life in lower limb amputees. Disability and Rehabilitation. 2011;33:883–899.
Smith D.G., Ehde D.M., Hanley M.A., et al. Efficacy of gabapentin in treating chronic phantom limb and residual limb pain. Journal of Rehabilitation Research and Development. 2005;42:645–654.
Tessler M.J., Kleiman S.J. Spinal anaesthesia for patients with previous lower limb amputations. Anaesthesia. 1994;49:439–441.
Torebjork E., Wahren L., Wallin G., et al. Noradrenaline-evoked pain in neuralgia. Pain. 1995;63:11–20.
Vase L., Nikolajsen L., Christensen B., et al. Cognitive-emotional sensitization contributes to wind-up-like pain in phantom limb pain patients. Pain. 2011;152:157–162.
Viswanathan A., Phan P.C., Burton A.W. Use of spinal cord stimulation in the treatment of phantom limb pain: case series and review of the literature. Pain Practice. 2010;10:479–484.
Wall R., Novotny-Joseph P., Macnamara T.E. Does preamputation pain influence phantom limb pain in cancer patients? Southern Medical Journal. 1985;78:34–36.
Wartan S.W., Hamann W., Wedley J.R., et al. Phantom pain and sensation among British veteran amputees. British Journal of Anaesthesia. 1997;78:652–659.
Waxman S.G. The molecular pathophysiology of pain: abnormal expression of sodium channel genes and its contributions to hyperexcitability of primary sensory neurons. Pain. 1999;6(Suppl):133–140.
Weiss T., Miltner W.H., Adler T., et al. Decrease in phantom limb pain associated with prosthesis-induced increased use of an amputation stump in humans. Neuroscience Letters. 1999;272:131–134.
Whyte A.S., Niven C.A. Variation in phantom limb pain: result of a diary study. Journal of Pain and Symptom Management. 2001;22:947–953.
Wiech K., Kiefer R.T., Töpfner S., et al. A placebo-controlled randomized crossover trial of the N-methyl-D-aspartic acid receptor antagonist, memantine, in patients with chronic phantom limb pain. Anesthesia and Analgesia. 2004;98:408–413.
Wilder-Smith C.H., Hill L.T., Laurent S. Postamputation pain and sensory changes in treatment-naive patients: characteristics and responses to treatment with tramadol, amitriptyline, and placebo. Anesthesiology. 2005;103:619–628.
Wilkins K.L., McGrath P.J., Finley G.A., et al. Phantom limb sensations and phantom limb pain in child and adolescent amputees. Pain. 1998;78:7–12.
Wilkins K.L., McGrath P.J., Finley G.A., et al. Prospective diary study of nonpainful and painful phantom sensations in a preselected sample of child and adolescent amputees reporting phantom limbs. Clinical Journal of Pain. 2004;20:293–301.
Wilson J.A., Nimmo A.F., Fleetwood-Walker S.M., et al. A randomised double blind trial of the effect of pre-emptive epidural ketamine on persistent pain after lower limb amputation. Pain. 2008;135:108–118.
Wu C.L., Agarwal S., Tella P.K., et al. Morphine versus mexiletine for treatment of postamputation pain: a randomized, placebo-controlled, crossover trial. Anesthesiology. 2008;109:289–296.
Wu C.L., Tella P., Staats P.S., et al. Analgesic effects of intravenous lidocaine and morphine on postamputation pain. Anesthesiology. 2002;96:841–848.
Ypsilantis E., Tang T.Y. Pre-emptive analgesia for chronic limb pain after amputation for peripheral vascular disease: a systematic review. Annals of Vascular Surgery. 2010;24:1139–1146.