Pain following Spinal Cord Injury
Introduction
Although loss of mobility is often considered the most serious consequence of spinal cord injury (SCI), people with SCI consistently rate pain as one of the most difficult problems associated with their injury (Widerström-Noga et al 1999). It not only is a cause of suffering but also has a direct bearing on the ability of spinally injured persons to participate in rehabilitation and regain their optimal level of activity (Anke et al 1995, Widerström-Noga et al 1999). In one survey, when given the option, one-third of people with SCI stated they would trade relief of pain for loss of bladder, bowel, or sexual function (Nepomuceno et al 1979).
Prevalence of Pain
A large number of studies have examined the prevalence of pain following SCI (Levi et al 1995, Störmer et al 1997, Rintala et al 1998, Widerström-Noga et al 1999, Ravenscroft et al 2000, Siddall et al 2003). Although the prevalence is variable, depending on the methodology used, most studies indicate that around two-thirds of people with SCI experience pain and in one-third of them the pain is severe. When specific types of SCI pain are examined, differences are found in the prevalence of these different types of pain. A long-term follow-up study found that at 5 years following injury, musculoskeletal pain was the most common and was present in 58% of people, “at-level” neuropathic pain (i.e., located in the region close to the level of injury) was present in 42%, and “below-level” neuropathic pain (i.e., below the level of spinal injury) in 34% (Siddall et al 2003). Below-level neuropathic pain was the most likely to be described as severe or excruciating and was found to develop months and even years following injury. From this study, those who experience neuropathic pain in the first 3–6 months following injury are likely to continue to experience ongoing pain at 3–5 years (Siddall et al 2003).
Factors Related to Pain
An ability to predict the development of pain and provide guidance on prognosis would enable earlier treatment and better follow-up. However, research identifying factors linked to the development and severity of pain has been inconclusive.
A significant relationship between the level of injury and the presence of pain has been suggested but is difficult to confirm. Several clinical observational studies have proposed that neuropathic pain is more common in people with incomplete lesions (Davidoff et al 1987b, Beric et al 1988), a proposition that is supported by findings at autopsy (Kakulas et al 1990). This contrasts with other studies that have failed to find any relationship between the extent of injury and the presence of pain (Richards et al 1980, Summers et al 1991).
The role of the spinothalamic tracts in the development of neuropathic pain following SCI remains an area of intense interest. Although a spinothalamic lesion (marked by loss of cutaneous temperature and evoked pain sensations in the area of pain) is considered necessary for the development of neuropathic pain below the level of injury, it has traditionally been thought to be insufficient to explain the presence of neuropathic pain (Finnerup et al 2003).
Finally, a number of studies have found that psychosocial factors (e.g., disturbed mood and acceptance of disability) are more closely correlated with the presence and severity of pain following SCI than physical factors are (Richards et al 1980, Summers et al 1991, Störmer et al 1997). It is difficult to make definitive conclusions on the relationship between pain and psychological factors and to attribute causality from these studies. However, it is clear that pain has a major impact on the person’s ability to participate in daily activities (Ravenscroft et al 2000, Widerström-Noga et al 2001) and may have a stronger influence on quality of life than the extent of SCI does (Rintala et al 1998).
Types of Pain after Spinal Cord Injury
Many classification systems have been used to categorize the types of pain that have been observed to occur following SCI. In 2002, a taxonomy was developed by the SCI Pain Task Force of the International Association for the Study of Pain (IASP) and has been used widely (Table 68-1) (Siddall et al 2002). Since then, a working party of people from various professional organizations has been attempting to achieve more widespread consensus on SCI pain taxonomy (Widerstrom-Noga et al 2008). The final report from this group has not yet been published. As a result, this chapter will use the IASP taxonomy as a framework for discussing the various types of pain associated with SCI. This taxonomy proposes a three-tiered classification, with the first tier being nociceptive and neuropathic and the second tier being musculoskeletal, visceral, and above-level, at-level, or below-level neuropathic pain.
Table 68-1
Proposed International Association for the Study of Pain Taxonomy of Pain following Spinal Cord Injury
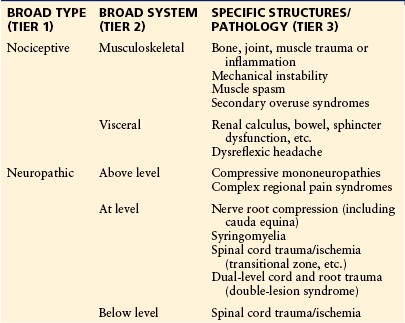
From Siddall PJ, Yezierski RP, Loeser JD 2002 Taxonomy and epidemiology of spinal cord injury pain. In: Yezierski RP, Burchiel KJ (eds) Spinal cord injury pain: assessment, mechanisms, management. Progress in pain research and management, vol 23. IASP Press, Seattle, pp 9–24.
Musculoskeletal Pain
Musculoskeletal pain typically occurs in normally innervated regions rostral to the level of the SCI. Most people who sustain an injury to the spinal cord also sustain trauma to the vertebral column and its supporting structures, including ligaments, muscles, intervertebral discs, and facet joints. This inevitably results in acute nociceptive pain that can be made worse by ongoing spinal column instability. Typically, musculoskeletal pain is related to activity and position. Pain may be referred to the limbs or trunk and can be difficult to distinguish from radicular (nerve root) pain. Flexion and extension plain radiography, computed tomography, and magnetic resonance imaging may help identify spinal instability.
Chronic musculoskeletal pain can occur with overuse or “abnormal” use of the extremities (e.g., with manual wheelchair use and transfers) and is very common in people with paraplegia (Dalyan et al 1999). Musculoskeletal pain can also occur when there is limited functional use of the extremities, such as persons with tetraplegia, in whom shoulder pain may be due to muscle atrophy and recurrent dislocation (Irwin et al 2007). Muscle spasm pain is also common following SCI and may contribute to musculoskeletal pain, particularly in those with incomplete injuries.
Heterotopic ossification (the formation of ectopic bone in soft tissue surrounding peripheral joints) may occur below the level of injury. Acute symptoms may include fever, swelling of the joint, reduced range of motion, and pain. In this setting, other causes, including infection, fracture, venous thrombosis, and pressure ulceration, need consideration. Findings on imaging (including bone scans) and blood tests such as the erythrocyte sedimentation rate and serum alkaline phosphatase level may also be altered.
Visceral Pain
Pathology or altered function in visceral structures located in the chest, abdomen, and pelvis, such as urinary tract infection, renal calculi, and constipation, is a common source of pain in people with SCI (Finnerup et al 2008). However, the level of the injury will affect the quality of the pain. Therefore, paraplegics may experience visceral pain that is identical to the pain in those without SCI, whereas tetraplegics may experience more vague generalized symptoms of unpleasantness that are difficult to interpret. Visceral pain can be identified by location (e.g., abdomen or chest) and by characteristics of the pain (dull, poorly localized, bloating, and cramping), which may be intermittent or spasmodic.
The diagnosis of visceral pain is often difficult to make when sensory input from visceral structures is disturbed. If investigations fail to find evidence of visceral pathology and treatments directed at visceral pathology do not relieve the pain, it is reasonable to consider whether the pain is neuropathic rather than visceral.
Neuropathic Pain
A redefinition of neuropathic pain has recently been proposed (Treede et al 2008). This redefinition emphasizes the need to demonstrate pathology within the nervous system that can plausibly explain the pain. Although the presence of central nervous system damage is inherent following spinal cord injury, patients do not always report pain. A diagnosis of neuropathic pain therefore remains reliant on the clinical features (history and examination) of the person reporting pain. Even though the history and bedside examination remain fundamental to the diagnosis of neuropathic pain, screening questionnaires (Jensen 2006) and confirmatory tests (e.g., quantitative sensory testing) may also be helpful in assessing pain and response to treatment (Cruccu et al 2010).
Though not diagnostic, neuropathic pain is suspected when certain pain descriptors are used (shooting, electric, burning, tingling, pricking, itching, cold) and the location of the pain is in a region of sensory disturbance. Although the IASP classification referred to earlier identifies three types of neuropathic pain, in this chapter we focus on the two main types of neuropathic pain that are specific to SCI: at-level and below-level neuropathic pain.
At-Level Neuropathic Pain
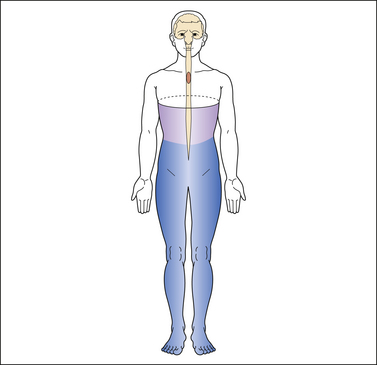
At-level neuropathic pain refers to pain that occurs in a segmental or dermatomal pattern within the dermatome at the level of neurological injury and three dermatomes below this level (Widerstrom-Noga et al 2008) (Fig. 68-1). This type of pain is also referred to as segmental, transitional zone, border zone, end zone, and girdle zone pain, names that reflect its characteristic location in the dermatomes close to the level of injury. It is often associated with allodynia or hyperesthesia of the affected dermatomes.
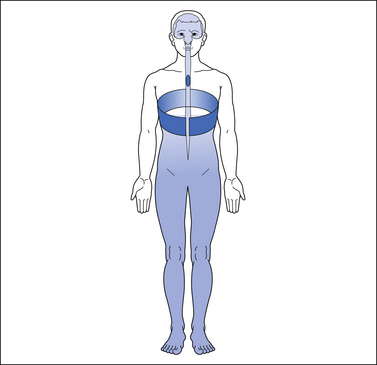
Figure 68-1 Typical pattern of at-level neuropathic pain following spinal cord injury (T4 neurological level).
The dark shading represents the distribution of pain. The lighter shading represents the distribution of sensory disturbance below the spinal cord lesion.
At-level neuropathic pain may be due to damage to either nerve roots or the spinal cord itself. Pain arising from nerve root damage is typically unilateral and suggested by characteristics such as increased pain in relation to spinal movement. The pain may be due to direct damage to the nerve root during the initial injury or be secondary to spinal column instability and impingement by facet or disc material. Electromyographic or somatosensory evoked potential abnormalities may be present. Diagnosis is assisted by radiographic evidence of compression of the nerve root in the foramen that correlates with the location of the pain.
In the past, pain that occurs at the level of the lesion and that has features of nerve root pain has often been classified as radicular even in the absence of definitive evidence of nerve root damage. However, segmental neuropathic pain may occur in the absence of nerve root damage and may be due to spinal cord rather than nerve root pathology. Animal models of SCI that have damage confined to the spinal cord without root involvement exhibit pain behavior that is similar to that seen in people with at-level neuropathic pain (Hao et al 1992, Yezierski et al 1998). Although this type of pain may be difficult to distinguish from nerve root pain on the basis of descriptors, such distinction is important because the underlying mechanisms and therefore treatment may be different.
Syringomyelia must always be considered in patients with delayed onset of segmental pain, especially when it is associated with a rising level of sensory loss. Loss of pain and temperature sensation is typical, but all sensory and motor functions can be affected. Nashold (1991) reported that 65% of a group of paraplegics who had a delayed onset of pain exhibited a syrinx, with the average onset being 6 years following the initial spinal injury. Patients describe a constant, burning pain that may be associated with allodynia or hyperalgesia. The diagnosis is established by magnetic resonance imaging.
An important variant of at-level neuropathic pain is seen after injury to the cauda equina. Even though pain caused by damage to the cauda equina may occur in a diffuse distribution in the lower limbs, it is classified as at-level neuropathic pain because it is due to nerve root damage. Cauda equina pain is reported in the lower lumbar and sacral dermatomes and is usually described as burning, stabbing, and hot. It is constant but may fluctuate with activity or autonomic activation.
Below-Level Neuropathic Pain
Below-level neuropathic pain is also referred to as central dysesthesia syndrome, central pain, phantom pain, or deafferentation pain. It is spontaneous and/or evoked pain that is often diffusely caudal to the level of SCI. It is defined as neuropathic pain and occurs in the region more than three dermatomes below the neurological level of injury (Widerstrom-Noga et al 2008) (Fig. 68-2). Sudden noises or jarring movements may trigger this type of pain. Differences in the nature of below-level neuropathic pain may be apparent in those with complete and incomplete lesions. Both complete and partial injuries may be associated with the diffuse, burning pain that appears to be related to spinothalamic tract damage. However, incomplete injuries are more likely to have an allodynic component because of sparing of tracts conveying touch sensation.
Psychological Aspects of Pain
As would be expected, SCI results in significant psychological disruption. Persistent pain following SCI is associated with more depressive symptoms and perceived stress than in those without pain (Rintala et al 1998). There is also a strong relationship between pain, spasticity, “abnormal non-painful sensations,” and sadness (Widerström-Noga et al 1999). However, the ongoing prevalence and severity of disruption are not as high as may be expected since many psychological symptoms return to normal limits within the first year following injury.
Pain itself may have an impact on a person’s psychological status and quality of life (Lundqvist et al 1991, Rintala et al 1998, Westgren and Levi 1998), and there is no doubt that psychological responses have tremendous importance in the experience and expression of pain. Pain-related psychological factors (e.g., catastrophizing and self-efficacy) play an important role in determining disability, even in the early stages following injury (Nicholson Perry et al 2009).
Some authors have included psychological or psychogenic as a type of pain that occurs following SCI. Such a diagnosis is rare and is extremely difficult to validate even by specialist mental health professionals. In daily practice, it is more beneficial to consider how psychological factors contribute to a person’s pain, distress, and disability than to consider whether the pain is “psychogenic.”
Mechanisms
The mechanisms of SCI pain can be considered within the framework of the taxonomy described earlier (Fig. 68-3). First, acute SCI is often associated with extensive damage to deep somatic structures such as bones, joints, discs, ligaments, and muscles. Input generated from any of these structures may give rise to nociceptive pain. In the long term, overuse, spasm, and postural problems may also cause nociceptive pain. Similarly, nociceptive pain may arise from stimulation of visceral nociceptors. The mechanisms underlying both musculoskeletal and visceral pain are similar to those in people without SCI and are well described in other chapters in this book.
Neuropathic Pain
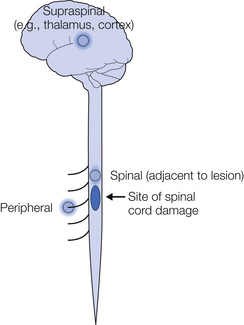
Although neuropathic pain is not unique to SCI, certain condition-specific mechanisms are worthy of further discussion and will therefore be addressed in more detail here. Neuropathic SCI pain may occur as a result of pathophysiology at three broad levels: peripheral, spinal, and supraspinal.
Peripheral Mechanisms
Damage to bony spinal structures may result in impingement of nerve roots entering the spinal cord. This may lead to the generation of impulses within primary afferents and the production of radicular at-level neuropathic pain in a similar manner to other peripheral neuropathic pain conditions involving nerve root trauma. The inflammatory process associated with SCI leads to the release of multiple agents, such as nerve growth factor, that can activate and sensitize nociceptive sensory fibers projecting to areas of damage.
Spinal Mechanisms
At least some cases of at-level neuropathic pain appear to be dependent on the presence of a “spinal generator” rather than just nerve root trauma. This is supported by animal research suggesting that at-level neuropathic pain is dependent on preservation of the superficial dorsal horn (Yezierski et al 2004) and human studies demonstrating that specific ablation of structures in the superficial dorsal horn can provide pain relief (Falci et al 2002). Several case reports of intrathecally administered local anesthetic in people with SCI pain also describe complete (though temporary) abolition of pain when the sensory blockade is above the level of injury (Loubser and Clearman 1993).
This ability of spinal local anesthetic to relieve neuropathic pain following SCI led to the proposition of an “irritated focus,” “neural pain generator,” or “spinal generator” located at the distal end of the spinal cord proximal to the injury. This proposition has been supported by a case report in which electrophysiological recordings demonstrated abnormal spontaneous neuronal activity in cells just above the level of injury in a man with upper lumbar SCI (Loeser et al 1968). Studies using quantitative sensory testing also demonstrate an association between at-level neuronal hyperexcitability and at-level neuropathic pain, although it is difficult to determine the precise location of the hyperexcitable neurons with this approach (Finnerup et al 2007).
These clinical observations have also been supported, to some extent, by subsequent investigations using animal models of SCI pain. In an early animal study, Loeser and Ward (1967) found abnormal spontaneous activity in the spinal cord close to the level of injury. Several animal models of neuropathic SCI pain have been developed over the years, including complete or partial cord transection (Christensen et al 1996, Vierck and Light 1999), irradiation (Hao et al 1991), excitotoxic intraspinal injections of quisqualate (Yezierski and Park 1993), and contusion (Siddall et al 1995, Hubscher and Johnson 1999, Hulsebosch et al 2000, Lindsey et al 2000). Though not identical, these models all result in varying but similar behavioral features suggestive of neuropathic pain, including increased sensitivity to mechanical and thermal stimulation and over-grooming. These behavioral changes suggestive of pain and hyperesthesia occur predominantly, though not exclusively, in the dermatomes close to the level of SCI. Importantly, animals in which these models are used display behavioral features of at-level neuropathic pain even when histological examination reveals no evidence of nerve root damage (Hao et al 1991, Yezierski and Park 1993). Thus, with at-level neuropathic pain, both spinal and peripheral pain mechanisms should be considered.
The physiological and biochemical changes underlying such behavior have also been investigated in several animal models. Electrophysiological recordings from spinal cord neurons demonstrate increased responsiveness to peripheral stimuli, an increase in the level of background neuronal activity, and an increased duration of afterdischarge responses following cessation of a stimulus (Hao et al 1992, Yezierski and Park 1993, Christensen et al 1996, Drew et al 2001, Hoheisel et al 2003). It has been suggested that the changes in neuronal responsiveness described may be due to damage to the normal inhibitory processes in the spinal cord involving opioids, monoamines, γ-aminobutyric acid (GABA), and glycine.
Trauma to the spinal cord results in an injury cascade that includes excitotoxic, neurochemical, inflammatory, and anatomical components that may contribute to the development of pain (Yezierski 2009). This injury cascade may affect inhibitory neurons and thus the production and release of inhibitory neurotransmitters. Much of the focus in research has been on interference in GABAergic inhibition, and several studies have provided evidence that neuropathic pain is linked to GABAergic dysfunction within the spinal cord close to the site of injury (Drew et al 2004, Gwak et al 2006, Cramer et al 2008, Meisner et al 2010). These changes may underlie a reduction in GABAergic inhibitory tone and lead to amplification of ascending input.
It has also been proposed that pain may be due to an increase in glutamatergic excitatory activity at N-methyl-D-aspartate (NMDA), non-NMDA, and metabotropic glutamate receptors. Ultimately, excitatory factors are thought to activate intracellular signaling cascades that are associated with neuronal sensitization (Yu and Yezierski 2005, Crown et al 2006). Up-regulation of the tetrodotoxin-sensitive sodium channel Nav1.3 in second-order dorsal horn sensory neurons may also contribute to neuronal hyperexcitability and pain (Hains et al 2003).
Besides pharmacological and functional changes, spinal cord trauma may also result in anatomical or morphological changes that produce pain. Following SCI, several changes take place that appear to reflect attempts at anatomical reorganization in the spinal cord, including structural remodeling of the terminals of primary afferents within the dorsal horn (Christensen and Hulsebosch 1997, Kalous et al 2007). SCI also results in activation of microglia, which has been linked to the development of below-level neuropathic pain (see Chapter 4) (Hains and Waxman 2006, Zhao et al 2007a).
Supraspinal Mechanisms
In addition to a contribution from changes at a spinal level, it has long been suspected that supraspinal changes may contribute to the development of pain. Most notably, Melzack and Loeser (1978) described a group of people with neuropathic SCI pain that continued despite peripheral sympathetic spinal blockade and even surgical removal of cord segments above the level of injury. These findings suggested the possibility that in some patients neuropathic pain may be due to a pain generator in supraspinal structures.
One brain region with strong evidence of a role in pain is the thalamus. Studies using animal models have demonstrated that lesions of the spinothalamic tract result in an increase in spontaneous and evoked thalamic neuronal responses linked with changes in thalamic NMDA receptor function (Koyama et al 1993, Weng et al 2000), sodium channel expression (Hains et al 2005), and microglial activation (Zhao et al 2007b). Electrophysiological studies in humans have demonstrated that deafferented thalamic neurons in SCI patients with pain have abnormal patterns of activity, including high rates of spontaneous bursting (Lenz et al 1989, Jeanmonod et al 1993), although abnormal bursting alone is not sufficient to explain the presence of pain (Radhakrishnan et al 1999). Further evidence obtained with magnetoencephalography (Llinas et al 1999) and electroencephalography (Boord et al 2008, Wydenkeller et al 2009) in people with neuropathic SCI pain, as well as electrophysiological studies in animals (Gerke et al 2003), has demonstrated changes in the rhythmicity of thalamic neurons that may underlie pain. Chemical changes have also been demonstrated with magnetic resonance spectroscopy, and correlations between pain intensity and concentrations of N-acetylaspartate and myoinositol in the thalamus suggest neuronal loss or dysfunction (Pattany et al 2002).
In addition to changes in the thalamus, there is now evidence that pain following SCI is associated with structural and functional changes at a cortical level. In people with complete thoracic injuries and below-level neuropathic pain, reorganization of the somatosensory cortex has been demonstrated to occur and correlates with pain intensity (Wrigley et al 2009).
The extent to which these supraspinal changes and pain are dependent on preserved ascending fiber tracts remains unclear. Ascending fiber tracts include surviving tracts that traverse the injury site. Clinical electrophysiological studies suggest that approximately 50% of people with clinically complete injuries still have preserved transmission in spinal pathways (Finnerup et al 2004). Therefore, preserved spinal cord pathways have been proposed as an important factor underlying the development of below-level neuropathic pain following SCI. Based on quantitative sensory testing studies enhanced by chemical sensitization of primary afferents, this would seem to be the case, at least in a proportion of people (Wasner et al 2008). These pathways may transmit information generated by spinal neurons that are sensitized as a result of changes induced by inflammatory mediators, microglial activation, and second-messenger activation below the level of injury (Detloff et al 2008).
Summary
Thus, there is accumulating evidence of pathophysiological changes occurring at the peripheral, spinal, and supraspinal levels. The relative contribution of these changes to the development of neuropathic SCI pain varies among individuals, depending on the injury and their response to the injury.
Patient Evaluation
Evaluation of a patient with pain is described in detail elsewhere in this text (e.g., Chapters 20 to 24). A few specific considerations regarding the history, physical examination, and investigations are worth mentioning in people with pain following SCI.
Attempts to determine the type of pain will assist in formulating a treatment plan. A thorough history will explore the biological, psychological, and social contributors to the pain and its associated disability. The physical examination must precisely describe the patient’s neurological status (neurological level and completeness of injury). If it is changing, the relationship to the pain complaint must also be determined to identify the presence of progressive spinal conditions such as a syrinx. Determination of the stability of the traumatized spine may also require the use of imaging studies. Electrodiagnostic studies (e.g., quantitative sensory testing, evoked potential studies) for examining central and peripheral nerve function may be useful in determining the extent of injury to the nervous system.
Some measure of physical and emotional function needs to accompany pain measurement when assessing a person with persistent pain. Measurement may entail the use of questionnaires (self-report) or involve standardized observations. They may be generic or be adapted specifically to SCI. Although many measures exist, a consensus statement regarding clinical pain trial outcome measures has been published (Dworkin et al 2005). An SCI-specific core pain data set has also been proposed (Widerstrom-Noga et al 2008).
Management
As described previously, pain following SCI is dependent on a wide range of pathophysiological mechanisms. In addition, the disability and distress associated with pain are highly influenced by individual psychological factors. Ideally, therefore, management should be aimed at the various contributing biopsychosocial factors identified in an individual person. Even though some treatments may reduce the severity of pain, complete relief is uncommon. Strategies aimed at managing the impact of pain on a person’s life are consequently of central importance to any treatment plan.
A great deal of discussion has occurred over the years regarding the clinical applicability of a mechanism-based approach to persistent pain, including SCI pain (Finnerup and Jensen 2006, Widerstrom-Noga et al 2009). Although the principles of such an approach are intuitively reasonable, the clinical tools required to enable this approach on an individual level have yet to be developed. Until such tools are available, a management plan based on pain type provides a pragmatic starting point (Siddall and Middleton 2006).
The number of well-designed trials examining the effectiveness of treatments aimed at relief of pain following SCI continues to slowly increase. Over the years, the quality of clinical trials has been affected by low numbers of subjects and failure to specifically target the type of pain. A summary of treatments used for SCI pain is provided here with an emphasis on randomized controlled trials (RCTs).
Nociceptive Pain (Musculoskeletal and Visceral Pain)
Many of the treatments used for this type of pain are similar to those used for other types of nociceptive pain and therefore will not be covered in detail. Treatment is initially directed toward identifying and addressing any pathology thought to be responsible for the pain while at the same time offering strategies aimed at reducing the impact of ongoing pain (e.g., pain self-management) and palliative symptom control (both pharmacological and non-pharmacological).
Of all the potential sources of musculoskeletal pain, the one pain that occurs almost universally independent of the injury level is shoulder pain. A generic functional restoration approach can be used for overuse shoulder pain since treatments aimed at specific anatomical contributors lack evidence of definitive superior benefit. Because of the extended periods of extreme disability following surgery to the shoulder, operative treatment is usually considered an absolute last resort in people with upper extremity pain (Irwin et al 2007). Management of heterotopic ossification involves a physical therapy program to maintain range of motion and can include the use of bisphosphonates. Surgery may be considered in established extreme cases (Irwin et al 2007).
As mentioned previously, the diagnosis of visceral pain in a person with SCI is complicated by changes in sensory processing. In view of the challenges inherent in assessment, as well as the possibility that abdominal pain may be neuropathic, any visceral pathology identified should be treated appropriately as in other populations. The onset of headache in a person with upper thoracic or cervical SCI should alert clinicians to the possibility of a visceral disturbance such as bowel impaction or bladder distention producing autonomic dysreflexia. This can be a life-threatening situation associated with malignant hypertension and cerebrovascular hemorrhage and needs to be recognized and addressed expeditiously. A treatment algorithm for nociceptive SCI pain is presented in Figure 68-4.
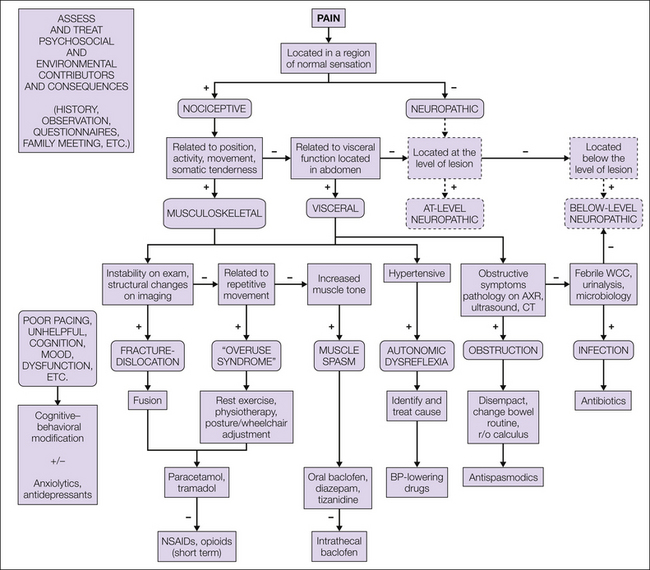
Figure 68-4 Assessment and treatment algorithm for the management of nociceptive pain following spinal cord injury.
BP, blood pressure; CT, computed tomography; NSAIDs, non-steroidal anti-inflammatory drugs; r/o, removal of; AXR, abdominal x-ray; WCC, white cell count. (Modified from Siddall PJ, Middleton JW 2006 A proposed algorithm for the management of pain following spinal cord injury. Spinal Cord 44:67–77.)
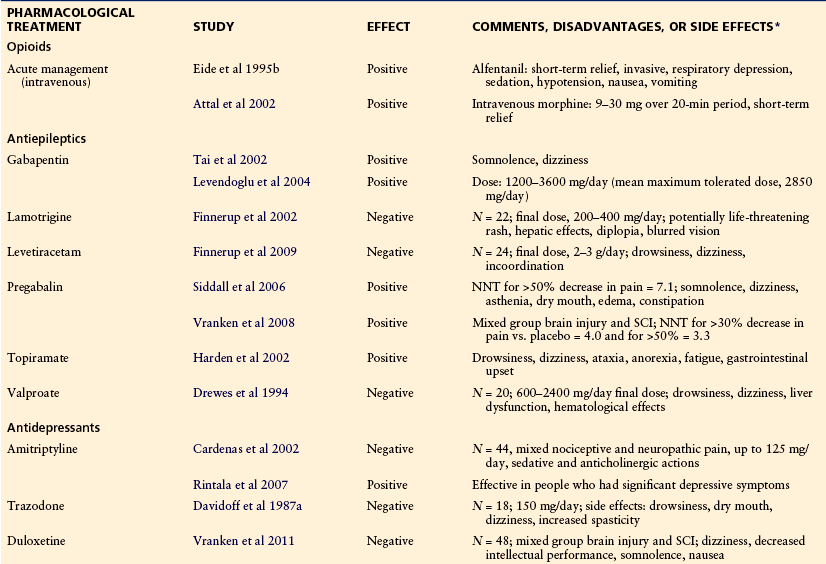
Pharmacological Treatment (Table 68-2)
Simple analgesics and non-steroidal anti-inflammatory drugs are helpful for musculoskeletal pain but, because of their mode of action, are largely ineffective in the management of neuropathic SCI pain. Some patients with neuropathic SCI pain do respond to parenteral opioids (Eide et al 1995a, Attal et al 2002). However, sustained relief with oral opioids is often difficult to obtain and long-term benefit is commonly reduced by constipation, drowsiness, tolerance, and dependence. An RCT examining the effectiveness of tramadol found significant improvement in pain in comparison to placebo but was associated with a high rate of adverse events, including tiredness, dry mouth, and dizziness (Norrbrink and Lundeberg 2009). Although spinal opioid administration has been shown to be effective in some patients, the intrathecal route is also associated with long-term side effects and tolerance, which has an impact on benefit (Fenollosa et al 1993).
α-Adrenergic Agonists
Clonidine administered spinally either alone or in combination with morphine may be effective in controlling neuropathic SCI pain. In one study, clonidine administered by the epidural route was found to be more effective than morphine for pain relief in patients with SCI pain (Glynn et al 1986). Combinations of clonidine with other agents may also be effective. A randomized placebo-controlled study demonstrated that a combination of intrathecal morphine and clonidine produced better relief of neuropathic SCI pain than did saline placebo, although the proportion obtaining substantial relief was low and evidence of long-term efficacy is not available (Siddall et al 2000).
Antidepressants
Despite the widespread use of antidepressants for treating neuropathic SCI pain, the results from RCTs are limited and mixed. Trazodone at a dose of 150 mg/day produced no significant difference in relief of pain when compared with placebo (Davidoff et al 1987a). One study examining the effectiveness of amitriptyline found no significant difference in pain relief or other outcome measures when compared with the active placebo benztropine (Cardenas et al 2002). A subsequent study found a positive effect of amitriptyline on neuropathic pain in those who had significant depressive symptoms (Rintala et al 2007).
There has been recent interest in the use of selective noradrenergic reuptake inhibitors for the treatment of neuropathic pain. Despite some evidence in people with neuropathic SCI pain, the results to date have not been promising. Though not ruling out its usefulness, an RCT found no significant difference in pain intensity when duloxetine was compared with control (Vranken et al 2011).
Several uncontrolled studies have examined the effectiveness of antidepressants in combination with other drugs, including combinations of amitriptyline with melitracen (another tricyclic antidepressant; Heilporn 1977), flupentixol (a thioxanthene antipsychotic), carbamazepine (anticonvulsant; Sandford et al 1992, Erzurumlu et al 1996), and lamotrigine (anticonvulsant; Canavero and Bonicalzi 1996). Although the evidence from these studies is weak, it does suggest that a combination of a tricyclic antidepressant with other agents may result in better pain relief.
Anticonvulsants
Though used widely, only anecdotal evidence supports the efficacy of older anticonvulsants such as carbamazepine (Farkash and Portenoy 1986), and a controlled trial investigating the effectiveness of valproate found no significant analgesic effect in comparison to placebo (Drewes et al 1994). Newer anticonvulsants such as gabapentin, pregabalin, lamotrigine, topiramate, and levetiracetam have been studied in people with neuropathic SCI pain with mixed results. Although the number of subjects in some trials is small, positive results have been obtained with both gabapentin (Tai et al 2002, Levendoglu et al 2004) and pregabalin (Siddall et al 2006, Vranken et al 2008), as well as with topiramate (Harden et al 2002). However, negative results were obtained in RCTs with both lamotrigine (Finnerup et al 2002) and levetiracetam (Finnerup et al 2009).
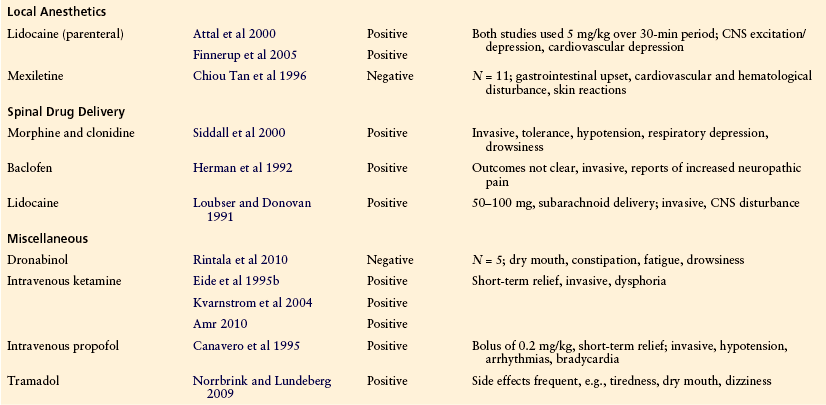
Local Anesthetics
In several RCTs, intravenous administration of lidocaine (lignocaine) produced a significant improvement in pain levels when compared with placebo (Backonja and Gombar 1992, Attal et al 2000, Finnerup et al 2005). Unfortunately, results with the use of mexiletine (an oral congener of lidocaine) have been less encouraging (Chiou Tan et al 1996). In one of the previously mentioned studies (N = 12 subjects; Attal et al 2000), treatment with oral mexiletine following response to lidocaine was associated with troublesome adverse effects, and few (25%) gained pain relief. Lidocaine has also been administered intrathecally and in a placebo-controlled study was found to be effective in relieving constant (most often burning) or stabbing, paroxysmal pain at or below the level of the lesion (Loubser and Donovan 1991). Continuation of such therapy would of course be problematic.
NMDA Receptor Antagonists
Several RCTs have evaluated the efficacy of intravenous ketamine infusion for the management of neuropathic SCI pain (Eide et al 1995b, Kvarnstrom et al 2004, Amr 2010). These studies reported significant improvement in pain intensity, but the relief is short-term and use of ketamine can result in unpleasant side effects.
GABA Receptor Agonists
The effect of the GABA receptor agonist baclofen on SCI pain has been assessed in one controlled trial and some uncontrolled trials. There is good evidence that both oral and intrathecal baclofen is effective in the relief of muscle spasm pain (Loubser and Akman 1996). However, clinical studies of people with neuropathic pain have not been so positive. One controlled trial assessing the effect of acute intrathecal baclofen on chronic, “dysesthetic,” and muscle spasm pain in patients with spinal spasticity found that baclofen significantly suppressed neuropathic and muscle spasm pain but did not influence pinch-induced and musculoskeletal (low back) pain (Herman et al 1992). In another uncontrolled study, although 5 of 6 patients had significant relief of their musculoskeletal pain, 7 of 12 patients with neuropathic pain exhibited no significant change in pain severity (Loubser and Akman 1996). Intravenous administration of propofol, a GABAA receptor agonist, has been reported to be more effective than placebo in relieving neuropathic SCI pain but is not suitable for long-term administration (Canavero et al 1995).
Cannabinoids
Although many people with neuropathic pain following SCI report that the use of marijuana provides them with pain relief, trials with synthetic cannabinoids have not been promising. A small RCT of dronabinol in people with below-level neuropathic pain found no significant improvement in pain levels over active placebo (Rintala et al 2010).
Anesthetic Blockade
Anesthetic blockade at several levels, including sympathetic, epidural, or spinal blockade, may be useful in reducing pain following SCI. As mentioned earlier, there is anecdotal evidence of spinal blockade resulting in disappearance of below-level neuropathic pain (Davis 1954, Loubser and Donovan 1991, Loubser and Clearman 1993). However, the pain relief is only temporary and there is no evidence that even multiple local anesthetic blocks result in long-term relief of pain. The results with sympathetic blockade are also disappointing. Therefore, though possibly useful as a diagnostic procedure for the assessment of visceral pain, sympathetic blockade has a limited role in the management of neuropathic SCI pain. Injection of botulinum toxin is another treatment that has attracted interest and may be helpful in the treatment of pain associated with muscle spasm (O’Brien 2002).
Stimulation Techniques
Transcutaneous Electrical Nerve Stimulation
Evidence of the effectiveness of transcutaneous electrical nerve stimulation (TENS) in treating SCI pain is limited and the results have been contradictory. Some evidence suggests that people with neuropathic pain at the level of their injury are more likely to obtain relief with TENS (Davis and Lentini 1975, Eriksson et al 1979). However, it is not clear how many of those with pain at the level of injury had musculoskeletal pain. On the other hand, another study reported lack of success in treating at-level neuropathic pain, which in this case was presumed to be due to nerve root involvement (Heilporn 1977). Side effects of TENS have been noted in people with SCI. Long-term use of TENS may be associated with postural detrusor sphincter dyssynergia in some tetraplegics.
Acupuncture
It has been suggested from case studies that acupuncture may be beneficial in treating neuropathic SCI pain (Nayak et al 2001, Rapson et al 2003). In addition, two controlled trials comparing acupuncture with either a manual therapy intervention (Dyson-Hudson et al 2001) or sham acupuncture (Dyson-Hudson et al 2007) found significant reductions in pain in both groups in each trial (Dyson-Hudson et al 2001). This would suggest that the non-specific components of the treatments (placebo) significantly contributed to the pain relief achieved.
Spinal Cord Stimulation
Spinal cord stimulation (SCS) may be effective in some people with neuropathic SCI pain, although it is dependent on the type of injury and type of pain. To obtain success, at least partial preservation of sensation in the area of pain is normally required. In many cases of neuropathic SCI pain it is difficult to achieve perceived paresthesia in the area of pain. There are reports that SCS may be more useful if the spinal cord lesion is incomplete (Cioni et al 1995). Efficacy may also be dependent on the type of pain. SCS appears to be more effective for at-level than for below-level neuropathic SCI pain (Cioni et al 1995); however, long-term data on the efficacy of SCS in patients with SCI pain are limited. Evidence from case series suggests that SCS may be effective initially in 20–75% of selected patients but that long-term efficacy declines to 10–40% (Richardson et al 1980). Of course, it is difficult for subjects to be blinded to treatment with these stimulators.
Brain Stimulation
Electrical stimulation of the various brain regions, including the thalamus, periventricular gray matter, internal capsule, and motor cortex, has been used for the treatment of neuropathic SCI pain and may be beneficial in a limited number of intractable cases (Hosobuchi 1986, Nguyen et al 1999). However, information is available only from limited case reports, and although some report early relief, the procedure is very invasive and associated with potential serious adverse effects and questionable long-term efficacy (Hosobuchi 1986, Nguyen et al 1999).
Other Stimulation Techniques
Several other stimulation techniques have been used, with two RCTs being available, one investigating the effectiveness of direct current stimulation applied transcranially over the motor cortex (Fregni et al 2006) and the other through a clip attached to the ear (Tan et al 2006). The first study demonstrated short-term but significant reduction in pain following a 5-day treatment trial (Fregni et al 2006), but longer-term improvement was achieved when the treatment was combined with a visual illusion technique (Soler et al 2010). The second study demonstrated a significant difference in pain reduction from baseline between the two groups, although the change in the active group was small and would not normally be considered clinically significant (<1-point reduction on an 11-point visual analog scale) (Tan et al 2006).
Surgical Procedures
Orthopedic and neurosurgical procedures that stabilize the spine immediately after trauma and decompress impinged nerve roots can be very effective in eliminating pain caused by instability or nerve root compression. Decompressive surgery with lysis of adhesions at the site of SCI is also effective in the treatment of syringomyelia, although the pain associated with this condition may continue even though the syrinx is collapsed. Various ablative spinal cord surgical procedures have been performed in an attempt to provide relief to patients with neuropathic SCI pain, including cordotomy, cordectomy, and midline (commissural) myelotomy. However, it is now recognized that the success of these various procedures is often disappointing and does vary according the nature of the pain (Tasker et al 1992). For below-level neuropathic SCI pain, ablative surgery and intrathecal administration of agents such as phenol and alcohol offer a low chance of success.
Dorsal Root Entry Zone Lesions
Dorsal root entry zone (DREZ) lesions that involve two to three spinal segments have been proposed as being effective in the management of SCI pain (Nashold and Bullitt 1981, Edgar et al 1993, Sindou et al 2001). The effectiveness is thought to be due to abolition of abnormal activity in dorsal horn neurons close to the level of injury, interruption of ascending pain pathways, or rebalancing of inhibitory and excitatory input within a damaged sensory network (Nashold and Bullitt 1981). The reported efficacy of DREZ lesions varies. Most studies indicate that 50–85% will obtain good (>50%) relief of their pain (Nashold and Bullitt 1981, Sindou et al 2001), although superior results are claimed with computer-assisted microcoagulation (Edgar et al 1993). As with SCS, efficacy may be dependent on the type of pain. Those who have unilateral, radicular cauda equina pain or intermittent, at-level neuropathic pain are more likely to have a favorable outcome, whereas those who have sacral, continuous, below-level neuropathic pain or a syrinx are less likely to do well (Nashold and Bullitt 1981, Edgar et al 1993, Sindou et al 2001). Despite positive effects in some people, DREZ lesions can be associated with a number of serious adverse effects, and even if initially successful, the pain may return.
Cordotomy
Cordotomy has been used historically as treatment of neuropathic SCI pain, with reports of positive results from several case series (Davis and Martin 1947, White and Sweet 1969). Once again, associated severe adverse effects (including induction of a higher level of sensory loss) and pain recurrence have limited the ongoing use of this approach.
Cordectomy
The use of cordectomy as a pain-relieving measure is controversial. Some studies have suggested good relief of at-level neuropathic pain with removal of the cord at least two to three segments above the level of injury (Nashold 1991). This success is presumably due to removal of the focus of aberrant neuronal activity in the spinal cord. Despite these reports of pain relief, many spinally injured patients are understandably reluctant to consider removal of sections of their spinal cord. Their reluctance is supported by a number of reports suggesting that even with complete anatomical section of the spinal cord, there is either poor or no relief of below-level neuropathic pain (Davis and Martin 1947, Davis 1954, Tasker et al 1992).
Other Surgical Procedures
Other surgical procedures that have been used for the management of SCI pain include anterior spinal cord decompression, commissurotomy, and surgical sympathectomy and cordomyelotomy (Pagni and Canavero 1995). Once again, evidence is limited, the results are mixed, and the possible benefits need to be weighed against the likelihood of adverse effects.
Physical Treatment
Physical treatment is important in the management of SCI pain, particularly in those with musculoskeletal pain. Changes in posture, exercise (Hicks et al 2003), adjustments to wheelchairs, and other forms of physical treatment modalities such as massage and heat (Norrbrink Budh and Lundeberg 2004) may be helpful in treating pain that is arising from a mechanical source. In particular, exercises may be helpful in addressing the shoulder pain that can occur in long-term wheelchair users (Curtis et al 1999). Pain syndromes associated with overuse or pressure can often be managed with physical measures alone. Prosthetic devices, orthotics, attention to seating, and exercise routines may assist in the management of these problems.
Psychological Treatment
Patients with SCI undergo a huge adjustment in relationships, lifestyle, vocation, and self-image that needs to be addressed. Patients with SCI usually have significant psychological distress, especially in the early stages following injury (Rintala et al 1998). The superimposition of chronic pain is a major factor that prevents the expected rehabilitation and return to employment and function in domestic life.
Psychological assessment should be part of the evaluation of every SCI patient with persistent pain of any type. Psychological factors may both contribute to and be a consequence of persistent pain. Reports of pain may be an expression of difficulty in adjustment, and therefore psychological approaches that attempt to deal with these issues may be helpful in reducing the experience of pain. Use of pain management strategies based on a cognitive–behavioral therapy approach can reduce the distress associated with pain, improve mood (particularly in those with high levels of depression), and help in long-term adjustment and return to maximal functional status (Umlauf 1992, Craig et al 1997). Joining a community SCI association may also be a positive step for some people.
Treatment Summary
Evidence to support many interventions for the management of SCI pain is limited, which makes generalized definitive recommendations difficult to formulate. However, guidelines are available that provide a general approach that may be tailored to the individual (Siddall and Middleton 2006), and a algorithm suggested for both nociceptive and neuropathic SCI pain is included in this chapter (Fig. 68-5; also see Fig. 68-4).
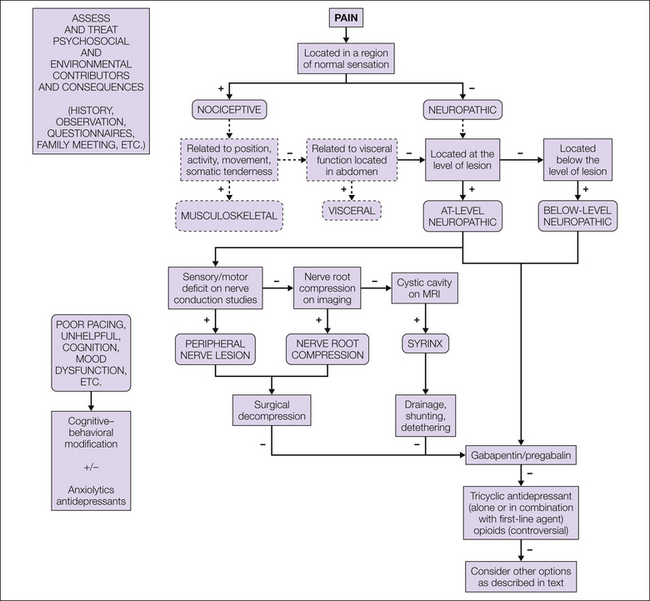
Figure 68-5 Assessment and treatment algorithm for the management of neuropathic pain following spinal cord injury.
MRI, magnetic resonance imaging. (Modified from Siddall PJ, Middleton JW 2006 A proposed algorithm for the management of pain following spinal cord injury. Spinal Cord 44:67–77.)
This algorithm provides a three-level approach to the management of neuropathic SCI pain. First-line treatments are those that have been validated for SCI pain, second-line treatments have been validated for other neuropathic pain states, and third-line treatments are limited by their side effect profile, invasiveness, or lack of efficacy data. In the acute setting, parenteral lidocaine is suggested as a first-line agent and a gabapentinoid in the subacute or chronic setting. As second-line treatment, use of a tricyclic antidepressant such as amitriptyline or nortriptyline or a low-dose long-acting opioid is suggested. The use of opioids long-term is controversial, and consideration must be given to the particular issues surrounding long-term opioid use. The combination of an anticonvulsant with either a tricyclic antidepressant or an opioid may produce additional relief.
Third-line treatments include many of the other treatments described earlier. If the response to first- and second-line treatments is inadequate, third-line treatments may be considered. However, the likelihood of benefit must be weighed against possible adverse side effects of the treatment. In parallel with these treatments, which focus largely on the biological aspects of the pain, it is crucial to implement treatments aimed at addressing psychosocial issues such as mood, cognition, and environmental factors that routinely contribute to both pain and disability.
The references for this chapter can be found at www.expertconsult.com.
References
Amr Y.M. Multi-day low dose ketamine infusion as adjuvant to oral gabapentin in spinal cord injury related chronic pain: a prospective, randomized, double blind trial. Pain Physician. 2010;13:245–249.
Anke A.G.W., Stenehjem A.E., Stanghelle J.K. Pain and life quality within 2 years of spinal cord injury. Paraplegia. 1995;33:555–559.
Attal N., Gaud V., Brasseur L., et al. Intravenous lidocaine in central pain: a double-blind, placebo-controlled, psychophysical study. Neurology. 2000;54:564–574.
Attal N., Guirimand F., Brasseur L., et al. Effects of IV morphine in central pain—a randomized placebo-controlled study. Neurology. 2002;58:554–563.
Backonja M., Gombar K.A. Response of central pain syndromes to intravenous lidocaine. Journal of Pain and Symptom Management. 1992;7:172–178.
Beric A., Dimitrijevic M.R., Lindblom U. Central dysesthesia syndrome in spinal cord injury patients. Pain. 1988;34:109–116.
Boord P., Siddall P.J., Tran Y., et al. Electroencephalographic slowing and reduced reactivity in neuropathic pain following spinal cord injury. Spinal Cord. 2008;46:118–123.
Canavero S., Bonicalzi V. Lamotrigine control of central pain. Pain. 1996;68:179–181.
Canavero S., Bonicalzi V., Pagni C.A., et al. Propofol analgesia in central pain—preliminary clinical observations. Journal of Neurology. 1995;242:561–567.
Cardenas D.A., Turner J.A., Warms C.A., et al. Classification of chronic pain associated with spinal cord injuries. Archives of Physical Medicine and Rehabilitation. 2002;83:1708–1714.
Chiou Tan F.Y., Tuel S.M., Johnson J.C., et al. Effect of mexiletine on spinal cord injury dysesthetic pain. American Journal of Physical Medicine & Rehabilitation. 1996;75:84–87.
Christensen M.D., Everhart A.W., Pickelman J.T., et al. Mechanical and thermal allodynia in chronic central pain following spinal cord injury. Pain. 1996;68:97–107.
Christensen M.D., Hulsebosch C.E. Spinal cord injury and anti-NGF treatment results in changes in CGRP density and distribution in the dorsal horn of the rat. Experimental Neurology. 2007;147:463–475.
Cioni B., Meglio M., Pentimalli L., et al. Spinal cord stimulation in the treatment of paraplegic pain. Journal of Neurosurgery. 1995;82:35–39.
Craig A.R., Hancock K., Dickson H., et al. Long-term psychological outcomes in spinal cord injured persons: results of a controlled trial using cognitive behavior therapy. Archives of Physical Medicine and Rehabilitation. 1997;78:33–38.
Cramer S.W., Baggott C., Cain J., et al. The role of cation-dependent chloride transporters in neuropathic pain following spinal cord injury. Molecular Pain. 2008;4:36.
Crown E.D., Ye Z., Johnson K.M., et al. Increases in the activated forms of ERK 1/2, p38 MAPK, and CREB are correlated with the expression of at-level mechanical allodynia following spinal cord injury. Experimental Neurology. 2006;199:397–407.
Cruccu G., Sommer C., Anand P., et al. EFNS guidelines on neuropathic pain assessment: revised 2009. European Journal of Neurology. 2010;17:1010–1018.
Curtis K.A., Tyner T.M., Zachary L., et al. Effect of a standard exercise protocol on shoulder pain in long-term wheelchair users. Spinal Cord. 1999;37:421–429.
Dalyan M., Cardenas D.D., Gerard B. Upper extremity pain after spinal cord injury. Spinal Cord. 1999;37:191–195.
Davidoff G., Guarracini M., Roth E., et al. Trazodone hydrochloride in the treatment of dysesthetic pain in traumatic myelopathy: a randomized, double-blind, placebo-controlled study. Pain. 1987;29:151–161.
Davidoff G., Roth E., Guarracini M., et al. Function-limiting dysesthetic pain syndrome among traumatic spinal cord injury patients: a cross-sectional study. Pain. 1987;29:39–48.
Davis L. Treatment of spinal cord injuries. AMA Archives of Surgery. 1954;69:488–495.
Davis L., Martin J. Studies upon spinal cord injuries. Journal of Neurosurgery. 1947;4:483–491.
Davis R., Lentini R. Transcutaneous nerve stimulation for treatment of pain in patients with spinal cord injury. Surgical Neurology. 1975;4:100–101.
Detloff M.R., Fisher L.C., McGaughy V., et al. Remote activation of microglia and pro-inflammatory cytokines predict the onset and severity of below-level neuropathic pain after spinal cord injury in rats. Experimental Neurology. 2008;212:337–347.
Drew G.M., Siddall P.J., Duggan A.W. Responses of spinal neurones to cutaneous and dorsal root stimuli in rats with mechanical allodynia after contusive spinal cord injury. Brain Research. 2001;893:59–69.
Drew G.M., Siddall P.J., Duggan A.W. Mechanical allodynia following contusion injury of the rat spinal cord is associated with loss of GABAergic inhibition in the dorsal horn. Pain. 2004;109:379–388.
Drewes A.M., Andreasen A., Poulsen L.H. Valproate for treatment of chronic central pain after spinal cord injury. A double-blind cross-over study. Paraplegia. 1994;32:565–569.
Dworkin R.H., Turk D.C., Farrar J.T., et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113:9–19.
Dyson-Hudson T.A., Kadar P., LaFountaine M., et al. Acupuncture for chronic shoulder pain in persons with spinal cord injury: a small-scale clinical trial. Archives of Physical Medicine and Rehabilitation. 2007;88:1276–1283.
Dyson-Hudson T.A., Shiflett S.C., Kirshblum S.C., et al. Acupuncture and Trager Psychophysical Integration in the treatment of wheelchair user’s shoulder pain in individuals with spinal cord injury. Archives of Physical Medicine and Rehabilitation. 2001;82:1038–1046.
Edgar R.E., Best L.G., Quail P.A., et al. Computer-assisted DREZ microcoagulation: posttraumatic spinal deafferentation pain. Journal of Spinal Disorders. 1993;6:48–56.
Eide K., Stubhaug A., Oye I., et al. Continuous subcutaneous administration of the N-methyl-D-aspartic acid (NMDA) receptor antagonist ketamine in the treatment of post-herpetic neuralgia. Pain. 1995;61:221–228.
Eide P.K., Stubhaug A., Stenehjem A.E. Central dysesthesia pain after traumatic spinal cord injury is dependent on N-methyl-D-aspartate receptor activation. Neurosurgery. 1995;37:1080–1087.
Eriksson M.B., Sjolund B.H., Nielzen S. Long term results of peripheral conditioning stimulation as an analgesic measure in chronic pain. Pain. 1979;6:335–347.
Erzurumlu A., Dursun H., Gunduz S. The management of chronic pain in spinal cord injured patients. The comparison of effectiveness of amitriptyline and carbamazepine combination and electroacupuncture application. Journal of Rheumatology and Medical Rehabilitation. 1996;7:176–180.
Falci S., Best L., Bayles R., et al. Dorsal root entry zone microcoagulation for spinal cord injury–related central pain: operative intramedullary electrophysiological guidance and clinical outcome. Journal of Neurosurgery. 2002;97:193–200.
Farkash A.E., Portenoy R.K. The pharmacological management of chronic pain in the paraplegic patient. Journal of the American Paraplegia Society. 1986;9:41–50.
Fenollosa P., Pallares J., Cervera J., et al. Chronic pain in the spinal cord injured: statistical approach and pharmacological treatment. Paraplegia. 1993;31:722–729.
Finnerup N.B., Biering-Sorensen F., Johannesen I.L., et al. Intravenous lidocaine relieves spinal cord injury pain: a randomized controlled trial. Anesthesiology. 2005;102:1023–1030.
Finnerup N.B., Faaborg P., Krogh K., et al. Abdominal pain in long-term spinal cord injury. Spinal Cord. 2008;46:198–203.
Finnerup N.B., Grydehoj J., Bing J., et al. Levetiracetam in spinal cord injury pain: a randomized controlled trial. Spinal Cord. 2009;47:861–867.
Finnerup N.B., Gyldensted C., Fuglsang-Frederiksen A., et al. Sensory perception in complete spinal cord injury. Acta Neurologica Scandinavica. 2004;109:194–199.
Finnerup N.B., Jensen T.S. Mechanisms of disease: mechanism-based classification of neuropathic pain—a critical analysis. Nature Clinical Practice. Neurology. 2006;2:107–115.
Finnerup N.B., Johannesen I.L., Fuglsang-Frederiksen A., et al. Sensory function in spinal cord injury patients with and without central pain. Brain. 2003;126:57–70.
Finnerup N.B., Pedersen L.H., Terkelsen A.J., et al. Reaction to topical capsaicin in spinal cord injury patients with and without central pain. Experimental Neurology. 2007;205:190–200.
Finnerup N.B., Sindrup S.H., Flemming W.B., et al. Lamotrigine in spinal cord injury pain: a randomized controlled trial. Pain. 2002;96:375–383.
Fregni F., Boggio P.S., Lima M.C., et al. A sham-controlled, phase II trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injury. Pain. 2006;122:197–209.
Gerke M.B., Duggan A.W., Xu L., et al. Thalamic neuronal activity in rats with mechanical allodynia following contusive spinal cord injury. Neuroscience. 2003;117:715–722.
Glynn C.J., Jamous M.A., Teddy P.J., et al. Role of spinal noradrenergic system in transmission of pain in patients with spinal cord injury. Lancet. 1986;2:1249–1250.
Gwak Y.S., Tan H.Y., Nam T.S., et al. Activation of spinal GABA receptors attenuates chronic central neuropathic pain after spinal cord injury. Journal of Neurotrauma. 2006;23:1111–1124.
Hains B.C., Klein J.P., Saab C.Y., et al. and functional involvement in neuronal hyperexcitability associated with central neuropathic pain after spinal cord injury. Journal of Neuroscience 23. Upregulation of sodium channel Na. 2003;v. 1:8881–8892.
Hains B.C., Saab C.Y., Waxman S.G. Changes in electrophysiological properties and sodium channel Na(v)1.3 expression in thalamic neurons after spinal cord injury. Brain. 2005;128:2359–2371.
Hains B.C., Waxman S.G. Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. Journal of Neuroscience. 2006;26:4308–4317.
Hao J.X., Xu X.J., Aldskogius H., et al. Allodynia-like effects in rat after ischaemic spinal cord injury photochemically induced by laser irradiation. Pain. 1991;45:175–185.
Hao J.X., Xu X.J., Yu Y.X., et al. Transient spinal cord ischaemia induces temporary hypersensitivity of dorsal horn wide dynamic range neurons to myelinated, but not unmyelinated, fiber input. Journal of Neurophysiology. 1992;68:384–391.
Harden R.N., Brenman E., Saltz S., et al, Topiramate in the management of spinal cord injury pain: a double-blind, randomized, placebo-controlled pilot study. Yezierski R.P., Burchiel K.J., eds. Spinal cord injury pain: assessment, mechanisms, management. Progress in pain research and management, vol 23. Seattle: IASP Press; 2002:393–407.
Heilporn A. Two therapeutic experiments on stubborn pain in spinal cord lesions: coupling melitracen-flupenthixol and the transcutaneous nerve stimulation. Paraplegia. 1977;15:368–372.
Herman R.M., D’Luzansky S.C., Ippolito R. Intrathecal baclofen suppresses central pain in patients with spinal lesions: a pilot study. Clinical Journal of Pain. 1992;8:338–345.
Hicks A.L., Martin K.A., Ditor D.S., et al. Long-term exercise training in persons with spinal cord injury: effects on strength, arm ergometry performance and psychological well-being. Spinal Cord. 2003;41:34–43.
Hoheisel U., Scheifer C., Trudrung P., et al. Pathophysiological activity in rat dorsal horn neurones in segments rostral to a chronic spinal cord injury. Brain Research. 2003;974:134–145.
Hosobuchi Y. Subcortical electrical stimulation for control of intractable pain in humans: report of 122 cases. Journal of Neurosurgery. 1986;64:543–553.
Hubscher C.H., Johnson R.D. Changes in neuronal receptive field characteristics in caudal brain stem following chronic spinal cord injury. Journal of Neurotrauma. 1999;16:533–541.
Hulsebosch C.E., Xu G.Y., Perez-Polo J.R., et al. Rodent model of chronic central pain after spinal cord contusion injury and effects of gabapentin. Journal of Neurotrauma. 2000;17:1205–1217.
Irwin R.W., Restrepo J.A., Sherman A. Musculoskeletal pain in persons with spinal cord injury. Topics in Spinal Cord Injury and Rehabilitation. 2007;13:43–57.
Jeanmonod D., Magnin M., Morel A. Thalamus and neurogenic pain: physiological, anatomical and clinical data. Neuroreport. 1993;4:475–478.
Jensen M.P. Review of measures of neuropathic pain. Current Pain and Headache Reports. 2006;10:159–166.
Kakulas B.A., Smith E., Gaekwad U., et al, The neuropathology of pain and abnormal sensations in human spinal cord injury derived from the clinicopathological data base at the Royal Perth Hospital: recent achievements in restorative neurology. Altered sensation and pain, vol 3. Basel: Karger; 1990:****. pp 37–41
Kalous A., Osborne P.B., Keast J.R. Acute and chronic changes in dorsal horn innervation by primary afferents and descending supraspinal pathways after spinal cord injury. Journal of Comparative Neurology. 2007;504:238–253.
Koyama S., Katayama Y., Maejima S., et al. Thalamic neuronal hyperactivity following transection of the spinothalamic tract in the cat: involvement of N-methyl-D-aspartate receptor. Brain Research. 1993;612:345–350.
Kvarnstrom A., Karlsten R., Quiding H., et al. The analgesic effect of intravenous ketamine and lidocaine on pain after spinal cord injury. Acta Anaesthesiologica Scandinavica. 2004;48:498–506.
Lenz F.A., Kwan H.C., Dostrovsky J.O., et al. Characteristics of the bursting pattern of action potentials that occurs in the thalamus of patients with central pain. Brain Research. 1989;496:357–360.
Levendoglu F., Ogun C.O., Ozerbil O., et al. Gabapentin is a first line drug for the treatment of neuropathic pain in spinal cord injury. Spine. 2004;29:743–751.
Levi R., Hultling C., Nash M.S., et al. The Stockholm Spinal Cord Injury Study: 1. Medical problems in a regional SCI population. Paraplegia. 1995;33:308–315.
Lindsey A.E., LoVerso R.L., Tovar C.A., et al. An analysis of changes in sensory thresholds to mild tactile and cold stimuli after experimental spinal cord injury in the rat. Neurorehabilitation and Neural Repair. 2000;14:287–300.
Llinas R.R., Ribary U., Jeanmonod D., et al. Thalamocortical dysrhythmia: a neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:15222–15227.
Loeser J.D., Ward A.A., Jr. Some effects of deafferentation on neurons of the cat spinal cord. Archives of Neurology. 1967;17:629–636.
Loeser J.D., Ward A.A., White L.E. Chronic deafferentation of human spinal cord neurons. Journal of Neurosurgery. 1968;29:48–50.
Loubser P.G., Akman N.M. Effects of intrathecal baclofen on chronic spinal cord injury pain. Journal of Pain and Symptom Management. 1996;12:241–247.
Loubser P.G., Clearman R.R. Evaluation of central spinal cord injury pain with diagnostic spinal anesthesia. Anesthesiology. 1993;79:376–378.
Loubser P.G., Donovan W.H. Diagnostic spinal anaesthesia in chronic spinal cord injury pain. Paraplegia. 1991;29:25–36.
Lundqvist C., Siosteen A., Blomstrand C., et al. Spinal cord injuries: clinical, functional, and emotional status. Spine. 1991;16:78–83.
Meisner J., Marsh A., Marsh D. Loss of GABAergic interneurons in laminae I-III of the spinal cord dorsal horn contributes to reduced GABAergic tone and neuropathic pain after spinal cord injury. Journal of Neurotrauma. 2010;27:729.
Melzack R., Loeser J.D. Phantom body pain in paraplegics: evidence for a central “pattern generating mechanism” for pain. Pain. 1978;4:195–210.
Nashold B.S. Paraplegia and pain. In: Nashold B.S., Ovelmen-Levitt J., eds. Deafferentation pain syndromes: pathophysiology and treatment. New York: Raven Press; 1991:301–319.
Nashold B.S., Jr., Bullitt E. Dorsal root entry zone lesions to control central pain in paraplegics. Journal of Neurosurgery. 1981;55:414–419.
Nayak S., Shiflett S.C., Schoenberger N.E., et al. Is acupuncture effective in treating chronic pain after spinal cord injury? Archives of Physical Medicine and Rehabilitation. 2001;82:1578–1586.
Nepomuceno C., Fine P.R., Richards J.S., et al. Pain in patients with spinal cord injury. Archives of Physical Medicine and Rehabilitation. 1979;60:605–609.
Nguyen J.P., Lefaucheur J.P., Decq P., et al. Chronic motor cortex stimulation in the treatment of central and neuropathic pain. Correlations between clinical, electrophysiological and anatomical data. Pain. 1999;82:245–251.
Nicholson Perry K., Nicholas M.K., Middleton J. Spinal cord injury–related pain in rehabilitation: a cross-sectional study of relationships with cognitions, mood and physical function. European Journal of Pain. 2009;13:511–517.
Norrbrink C., Lundeberg T. Tramadol in neuropathic pain after spinal cord injury: a randomized, double-blind, placebo-controlled trial. Clinical Journal of Pain. 2009;25:177–184.
Norrbrink Budh C., Lundeberg T. Non-pharmacological pain-relieving therapies in individuals with spinal cord injury: a patient perspective. Complementary Therapies in Medicine. 2004;12:189–197.
O’Brien C.F. Treatment of spasticity with botulinum toxin. Clinical Journal of Pain. 2002;18:S182–S190.
Pagni C.A., Canavero S. Cordomyelotomy in the treatment of paraplegia pain—experience in two cases with long-term results. Acta Neurologica Belgica. 1995;95:33–36.
Pattany P.M., Yezierski R.P., Widerström-Noga E.G., et al. Proton magnetic resonance spectroscopy of the thalamus in patients with chronic neuropathic pain after spinal cord injury. American Journal of Neuroradiology. 2002;23:901–905.
Radhakrishnan V., Tsoukatos J., Davis K.D., et al. A comparison of the burst activity of lateral thalamic neurons in chronic pain and non-pain patients. Pain. 1999;80:567–575.
Rapson L.M., Wells N., Pepper J., et al. Acupuncture as a promising treatment for below-level central neuropathic pain: a retrospective study. Journal of Spinal Cord Medicine. 2003;26:21–26.
Ravenscroft A., Ahmed Y.S., Burnside I.G. Chronic pain after SCI. A patient survey. Spinal Cord. 2000;38:611–614.
Richards J.S., Meredith R.L., Nepomuceno C., et al. Psycho-social aspects of chronic pain in spinal cord injury. Pain. 1980;8:355–366.
Richardson R.R., Meyer P.R., Cerullo L.J. Neurostimulation in the modulation of intractable paraplegic and traumatic neuroma pains. Pain. 1980;8:75–84.
Rintala D., Loubser P.G., Castro J., et al. Chronic pain in a community-based sample of men with spinal cord injury: prevalence, severity, and relationship with impairment, disability, handicap, and subjective well-being. Archives of Physical Medicine and Rehabilitation. 1998;79:604–614.
Rintala D.H., Holmes S.A., Courtade D., et al. Comparison of the effectiveness of amitriptyline and gabapentin on chronic neuropathic pain in persons with spinal cord injury. Archives of Physical Medicine and Rehabilitation. 2007;88:1547–1560.
Rintala D.H.P., Fiess R.N., Tan G.P., et al. Effect of dronabinol on central neuropathic pain after spinal cord injury: a pilot study. American Journal of Physical Medicine & Rehabilitation. 2010;89:840–848.
Sandford P.R., Lindblom L.B., Haddox J.D. Amitriptyline and carbamazepine in the treatment of dysesthetic pain in spinal cord injury. Archives of Physical Medicine and Rehabilitation. 1992;73:300–301.
Siddall P.J., Cousins M.J., Otte A., et al. Pregabalin in central neuropathic pain associated with spinal cord injury: a placebo-controlled trial. Neurology. 2006;67:1792–1800.
Siddall P.J., McClelland J.M., Rutkowski S.B., et al. A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain. 2003;103:249–257.
Siddall P.J., Middleton J.W. A proposed algorithm for the management of pain following spinal cord injury. Spinal Cord. 2006;44:67–77.
Siddall P.J., Molloy A.R., Walker S., et al. The efficacy of intrathecal morphine and clonidine in the treatment of pain after spinal cord injury. Anesthesia and Analgesia. 2000;91:1493–1498.
Siddall P.J., Xu C.L., Cousins M.J. Allodynia following traumatic spinal cord injury in the rat. Neuroreport. 1995;6:1241–1244.
Siddall P.J., Yezierski R.P., Loeser J.D., Taxonomy and epidemiology of spinal cord injury pain. Yezierski R.P., Burchiel K.J., eds. Spinal cord injury pain: assessment, mechanisms, management. Progress in pain research and management, vol 23. Seattle: IASP Press; 2002:9–24.
Sindou M., Mertens P., Wael M. Microsurgical DREZotomy for pain due to spinal cord and/or cauda equina injuries: long-term results in a series of 44 patients. Pain. 2001;92:159–171.
Soler M.D., Kumru H., Pelayo R., et al. Effectiveness of transcranial direct current stimulation and visual illusion on neuropathic pain in spinal cord injury. Brain. 2010;133:2565–2567.
Störmer S., Gerner H.J., Grüninger W., et al. Chronic pain/dysaesthesiae in spinal cord injury patients: results of a multicentre study. Spinal Cord. 1997;35:446–455.
Summers J.D., Rapoff M.A., Varghese G., et al. Psychosocial factors in chronic spinal cord injury pain. Pain. 1991;47:183–189.
Tai Q., Kirshblum S., Chen B., et al. Gabapentin in the treatment of neuropathic pain after spinal cord injury: a prospective, randomized, double-blind, crossover trial. Journal of Spinal Cord Medicine. 2002;25:100–105.
Tan G., Rintala D.H., Thornby J.I., et al. Using cranial electrotherapy stimulation to treat pain associated with spinal cord injury. Journal of Rehabilitation Research and Development. 2006;43:461–474.
Tasker R.R., DeCarvalho G.T.C., Dolan E.J. Intractable pain of spinal cord origin: clinical features and implications for surgery. Journal of Neurosurgery. 1992;77:373–378.
Treede R.D., Jensen T.S., Campbell J.N., et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70:1630–1635.
Umlauf R.L. Psychological interventions for chronic pain following spinal cord injury. Clinical Journal of Pain. 1992;8:111–118.
Vierck C.J., Light A.R. Effects of combined hemotoxic and anterolateral spinal lesions on nociceptive sensitivity. Pain. 1999;83:447–457.
Vranken J.H., Dijkgraaf M.G.W., Kruis M.R., et al. Pregabalin in patients with central neuropathic pain: a randomized, double-blind, placebo-controlled trial of a flexible-dose regimen. Pain. 2008;136:150–157.
Vranken J.H., Hollmann M.W., von der Vegt M.H., et al. Duloxetine in patients with central neuropathic pain caused by spinal cord injury or stroke: a randomized, double-blind, placebo-controlled trial. Pain. 2011;152:267–273.
Wasner G., Lee B.B., Engel S., et al. Residual spinothalamic tract pathways predict development of central pain after spinal cord injury. Brain. 2008;131:2387–2400.
Weng H.R., Lee J.I., Lenz F.A., et al. Functional plasticity in primate somatosensory thalamus following chronic lesion of the ventral lateral spinal cord. Neuroscience. 2000;101:393–401.
Westgren N., Levi R. Quality of life and traumatic spinal cord injury. Archives of Physical Medicine and Rehabilitation. 1998;79:1433–1439.
White J.C., Sweet W.H. Pain and the neurosurgeon: a forty-year experience. Springfield, IL: Charles C Thomas; 1969.
Widerström-Noga E., Biering-Sorensen F., Bryce T., et al. The international spinal cord injury pain basic data set. Spinal Cord. 2008;46:818–823.
Widerström-Noga E.G., Felipe-Cuervo E., Broton J.G., et al. Perceived difficulty in dealing with consequences of spinal cord injury. Archives of Physical Medicine and Rehabilitation. 1999;80:580–586.
Widerström-Noga E.G., Felipe-Cuervo E., Yezierski R.P. Chronic pain after spinal injury: interference with sleep and daily activities. Archives of Physical Medicine and Rehabilitation. 2001;82:1571–1577.
Widerström-Noga E.G., Finnerup N.B., Siddall P.J. Biopsychosocial perspective on a mechanisms-based approach to assessment and treatment of pain following spinal cord injury. Journal of Rehabilitation Research and Development. 2009;46:1–12.
Wrigley P.J., Press S.R., Gustin S.M., et al. Neuropathic pain and primary somatosensory cortex reorganization following spinal cord injury. Pain. 2009;141:52–59.
Wydenkeller S., Maurizio S., Dietz V., et al. Neuropathic pain in spinal cord injury: significance of clinical and electrophysiological measures. European Journal of Neuroscience. 2009;30:91–99.
Yezierski R.P. Spinal cord injury pain: spinal and supraspinal mechanisms. Journal of Rehabilitation Research and Development. 2009;46:95.
Yezierski R.P., Liu S., Ruenes G.L., et al. Excitotoxic spinal cord injury: behavioural and morphological characteristics of a central pain model. Pain. 1998;75:141–155.
Yezierski R.P., Park S.H. The mechanosensitivity of spinal sensory neurons following intraspinal injections of quisqualic acid in the rat. Neuroscience Letters. 1993;157:115–119.
Yezierski R.P., Yu C.G., Mantyh P.W., et al. Spinal neurons involved in the generation of at-level pain following spinal injury in the rat. Neuroscience Letters. 2004;361:232–236.
Yu C.G., Yezierski R.P. Activation of the ERK1/2 signaling cascade by excitotoxic spinal cord injury. Molecular Brain Research. 2005;138:244–255.
Zhao P., Waxman S.G., Hains B.C. Extracellular signal–regulated kinase–regulated microglia-neuron signaling by prostaglandin E2 contributes to pain after spinal cord injury. Journal of Neuroscience. 2007;27:2357–2368.
Zhao P., Waxman S.G., Hains B.C. Modulation of thalamic nociceptive processing after spinal cord injury through remote activation of thalamic microglia by cysteine cysteine chemokine ligand 21. Journal of Neuroscience. 2007;27:8893–8902.
Christensen M.D., Everhart A.W., Pickelman J.T., et al. Mechanical and thermal allodynia in chronic central pain following spinal cord injury. Pain. 1996;68:97–107.
Finnerup N.B., Gyldensted C., Fuglsang-Frederiksen A., et al. Sensory perception in complete spinal cord injury. Acta Neurologica Scandinavica. 2004;109:194–199.
Finnerup N.B., Johannesen I.L., Fuglsang-Frederiksen A., et al. Sensory function in spinal cord injury patients with and without central pain. Brain. 2003;126:57–70.
Hains B.C., Klein J.P., Saab C.Y., et al. Upregulation of sodium channel Na v. 1.3 and functional involvement in neuronal hyperexcitability associated with central neuropathic pain after spinal cord injury. Journal of Neuroscience. 2003;23:8881–8892.
Hains B.C., Waxman S.G. Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. Journal of Neuroscience. 2006;26:4308–4317.
Hao J.X., Xu X.J., Aldskogius H., et al. Allodynia-like effects in rat after ischaemic spinal cord injury photochemically induced by laser irradiation. Pain. 1991;45:175–185.
Lenz F.A., Kwan H.C., Dostrovsky J.O., et al. Characteristics of the bursting pattern of action potentials that occurs in the thalamus of patients with central pain. Brain Research. 1989;496:357–360.
Melzack R., Loeser J.D. Phantom body pain in paraplegics: evidence for a central “pattern generating mechanism” for pain. Pain. 1978;4:195–210.
Siddall P.J., McClelland J.M., Rutkowski S.B., et al. A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain. 2003;103:249–257.
Siddall P.J., Middleton J.W. A proposed algorithm for the management of pain following spinal cord injury. Spinal Cord. 2006;44:67–77.
Siddall P.J., Yezierski R.P., Loeser J.D., Taxonomy and epidemiology of spinal cord injury pain. Yezierski R.P., Burchiel K.J., eds. Spinal cord injury pain: assessment, mechanisms, management. Progress in pain research and management, vol 23. Seattle: IASP Press; 2002:9–24.
Wasner G., Lee B.B., Engel S., et al. Residual spinothalamic tract pathways predict development of central pain after spinal cord injury. Brain. 2008;131:2387–2400.
Widerström-Noga E., Biering-Sorensen F., Bryce T., et al. The international spinal cord injury pain basic data set. Spinal Cord. 2008;46:818–823.
Widerström-Noga E.G., Felipe-Cuervo E., Broton J.G., et al. Perceived difficulty in dealing with consequences of spinal cord injury. Archives of Physical Medicine and Rehabilitation. 1999;80:580–586.
Wrigley P.J., Press S.R., Gustin S.M., et al. Neuropathic pain and primary somatosensory cortex reorganization following spinal cord injury. Pain. 2009;141:52–59.
Yezierski R.P., Liu S., Ruenes G.L., et al. Excitotoxic spinal cord injury: behavioural and morphological characteristics of a central pain model. Pain. 1998;75:141–155.
Yezierski R.P., Park S.H. The mechanosensitivity of spinal sensory neurons following intraspinal injections of quisqualic acid in the rat. Neuroscience Letters. 1993;157:115–119.