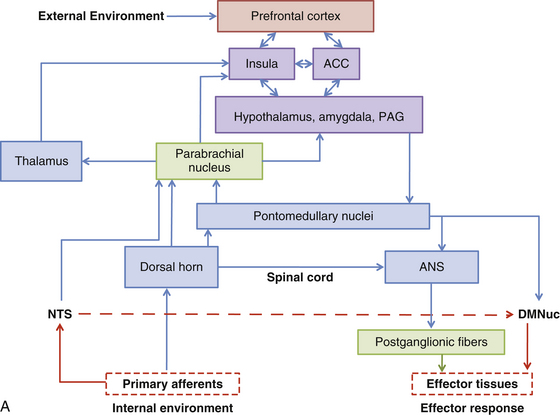
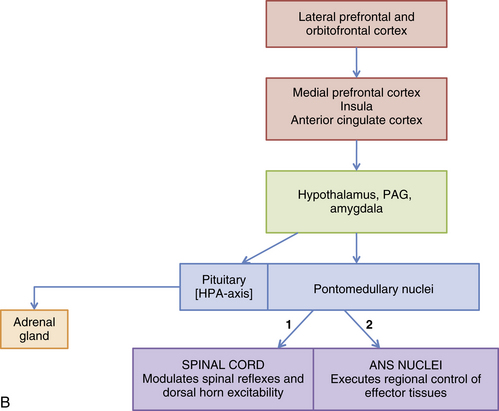
Spinal Afferent Fibers
As mentioned, input conveyed through spinal afferent fibers is concerned with visceral sensation, especially visceral nociception. The sensations that reach consciousness are poorly localized and, in the case of pain, may be referred and may produce hyperalgesia (see Appendix II for definitions of terms related to pain), as well. The cell bodies of these fibers are located in the dorsal root ganglia of the thoracic and upper lumbar dorsal roots. These visceral afferent fibers travel from the peripheral receptors in the cardiac, pulmonary, and splanchnic nerves; continue through the sympathetic trunk, white rami communicantes, and dorsal roots; and finally terminate in the spinal cord.
Central Projections and the Referral of Pain
As mentioned, pain of visceral origin differs from somatic pain, both in the neurophysiologic characteristics associated with the production of the pain and more obviously in the clinical presentation to the painful stimuli. Numerous conditions help to define visceral pain. Overdistension of the hollow muscular walled organs, ischemia and the resulting release of chemicals such as bradykinin, and inflammation of the viscera are stimuli that induce pain. However, the severity of the condition resulting in pain is not always a reflection of the severity of the pain experienced. For example, mild pain may be experienced with early appendicitis, whereas severe pain may be present with the fairly normal presence of gas in the GI tract. The least sensitive of the viscera tend to be the solid organs, whereas the serosae of the hollow organs appear to be the most sensitive. Also, a mildly painful or even nonpainful stimulus can result in severe pain (Al-Chaer & Traub, 2002) if an organ is already inflamed or altered pathologically (e.g., irritable bowel syndrome [IBS]). Visceral pain often is described as being either true or referred. True visceral pain is diffuse pain that is perceived as originating from deep, midline structures in the thorax or abdomen. It is often accompanied by the sense of nausea and ill-being. An example of this is during the early stage of appendicitis when the pain is initially felt in the midline. Referred pain also is diffuse but is localized to a distant cutaneous site or to muscles. The following is a list of important clinical characteristics associated with visceral pain:
1. Not all viscera (e.g., liver, kidney) are sensitive to painful stimuli. This is because of functional variances of the peripheral receptors and the phenomenon that some receptors of visceral afferents, when stimulated, do not evoke a conscious perception (Cervero & Laird, 1999).
2. Visceral pain is not always linked to an injurious event. For example, distension of the bladder, although painful, does not damage the tissue, whereas cutting the gut wall is not painful.
3. Visceral pain is referred to distant locations based on the convergence of visceral afferent fibers and somatic afferent fibers on viscerosomatic neurons in the spinal cord.
4. Visceral pain is poorly localized and diffuse. This is a result of such factors as the following:
a. The viscera are less densely innervated compared with somatic structures.
b. In the gut there are few (7%) visceral afferent fiber cell bodies in the dorsal root ganglia (DRG) compared to the number of somatic afferent cell bodies in DRG (Beyak et al., 2006).
c. In the gut the sensory innervation of an organ arises from a range of DRG although there are peak distributions. This results in a generalized, overlapping, and viscerotropic distribution of visceral afferent fibers (Knowles & Aziz, 2009).
d. The distribution of visceral afferent fibers terminating in cord segments is broader than that of somatic fibers, spanning as many as five segments (Chandler, Zhang, & Foreman, 1996; Jänig, 1996).
e. A specific visceral sensory pathway is lacking.
f. Viscerovisceral convergence occurs in the cord (Al-Chaer & Traub, 2002).
5. Visceral pain is accompanied by autonomic reflex and motor responses such as vomiting, changes in heart rate, or hypertonicity in skeletal muscles.
Neurotransmission of Visceral Pain
Peripheral receptors are located in the blood vessels, walls of hollow organs, parenchyma of visceral organs, and serosae (outer covering of certain organs). The receptors conveying nociception are sensitive to chemical, mechanical, and thermal stimuli and are classified physiologically as being either high- or low-threshold receptors. The high-threshold receptors respond to noxious mechanical stimuli and are the lone receptors found in organs in which pain is the only conscious sensation (e.g., ureter). There is a paucity of these in organs that respond to both innocuous and noxious sensations. Low-threshold receptors respond primarily to innocuous mechanical stimuli but some encode a wide range of stimuli that extends into the noxious range. These are located, for example, in the gut and bladder (Cervero & Laird, 1999; Al-Chaer & Traub, 2002; Knowles & Aziz, 2009). The distribution of these receptors varies among the visceral organs. A third type of receptor, called the silent nociceptor, also is located in the viscera. The silent receptors are minimally responsive or unresponsive to normally occurring stimuli, but become activated by inflammation and various chemical insults.
Because spinal visceral afferents are similar to somatic afferents in their basic morphology and in that their first synapse is in the dorsal horn, it is likely that the mechanism of visceral pain transmission is similar to the mechanism of somatic pain transmission. In the context of this chapter, the discussion of visceral pain transmission will be based on studies of pain mechanisms relative to the GI system. (See Chapter 11 and Appendix II for a discussion of terms related to pain and pain referral and a discussion of peripheral and central hyperalgesia [sensitization] of nociception from somatic tissues related to the spine.) Both high and low threshold afferent fibers are activated in brief acute visceral pain events. In this case a noxious stimulus initiates the opening of voltage-gated sodium channels, which produces an action potential. The activation of the sodium channels can occur directly or secondarily by the activation of transducer channels that respond to noxious mechanical, chemical, and thermal stimuli (Fig. 10-26, A). Examples of these channels, transient receptor potential (TRP), purinoceptors (P2X) and acid-sensing ion channels (ASICs), have been identified on neurons in the gut. If the stimulation is prolonged or repetitive (e.g., in hypoxia or inflammation), a change in the chemical environment allows the nociceptors (including the now activated silent nociceptors) to fire at lower thresholds than they would have in an acute noxious event. This causes decreased pain thresholds at the location of the injury, resulting in the phenomenon called primary hyperalgesia. This form of stimulus-evoked nociceptor plasticity is referred to as peripheral sensitization. The peripheral sensitization of these nociceptors is caused by the release of chemical mediators from the injured or inflamed tissue cells, which subsequently lower the firing threshold of the nociceptors. These events result in nociceptors being sensitive to and activated by normal innocuous stimuli. There is evidence suggesting that some GI disorders such as gastroesophageal reflux disease demonstrate peripheral sensitization. Using GI disorders as an example, it has been suggested (Knowles & Aziz, 2009) that the following three membrane events result in lowering of the threshold (Fig. 10-26, B): (1) G protein–coupled receptors are activated by sensitizers such as kinins, biogenic amines (histamine and 5-HT), prostanoids (prostaglandin E2 [PGE2] and growth factors), proteases, chemokines, and cytokines; decreased pH; and increased levels of ATP. The binding to the receptors activates intracellular signaling pathways, which in turn reduce the transduction thresholds in cation channels. (2) Neuroimmune interactions occur with neighboring epithelial cells and inflammatory cells such as mast cells and lymphocytes. This interaction causes the release of bioactive amines, which stimulate the release of neuropeptides such as substance P and CGRP (neurogenic inflammation). These in turn stimulate nearby target cells, which release mediators including nerve growth factor (NGF). (3) NGF activates a phenotypic switch through retrograde signaling in the dorsal root ganglion cell bodies and up-regulates neuropeptide expression, especially cations. These proteins can be transported centrally and peripherally, causing an extended peripheral and central sensitized phenotype (Knowles & Aziz, 2009).
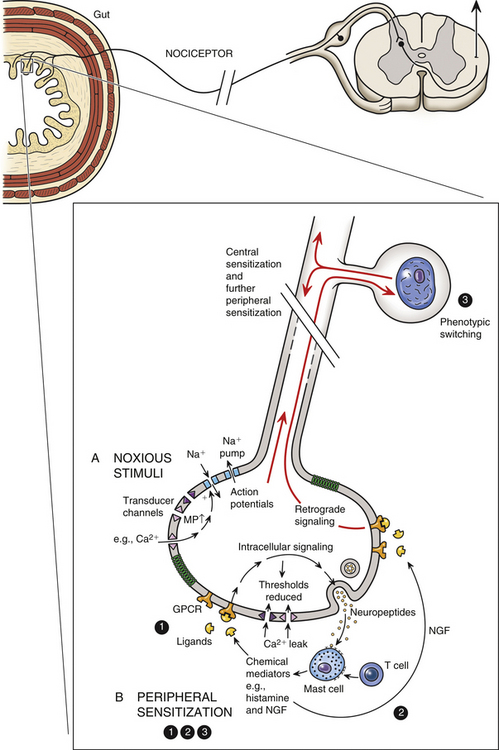
FIG. 10-26 Visceral nociception. A, Noxious stimuli (mechanical, chemical, thermal) activate Na+ channels directly or indirectly through the utilization of transducer channels, resulting in an action potential. B, Peripheral sensitization. The following three events may cause the threshold for nociceptor firing to be lowered: (1) Sensitizing mediators such as histamine and kinins bind to G protein–coupled receptors, which initiate intracellular signaling pathways. This results in lowering the thresholds for activating transducer channels and ultimately decreasing pain thresholds. (2) Biogenic amines released by nearby immune cells (neurogenic inflammation) stimulate the release of neuropeptides such as substance P, which in turn activates the release of inflammatory mediators such as nerve growth factor (NGF). (3) Through retrograde signaling, NGF acts on the nociceptor cell body, promoting a switch in the phenotype and leading to the up-regulation of neuropeptide and cation channels. These proteins can be transported to peripheral or to central terminal sites on the nociceptor, allowing for continued sensitization peripherally and sensitization centrally in the dorsal horn (Knowles & Aziz, 2009).
The repetitive firing of these afferent fibers produces a barrage of sensory input to dorsal horn viscerosomatic neurons, which results in hyperexcitability and hyperactivity of these neurons. The continuous firing of the afferent fibers and their release of neurotransmitter causes a progressive increase in the excitability of the dorsal horn neurons referred to as “wind-up.” All of this activity leads to visceral pain, which may be referred, and often is accompanied by hyperalgesia and allodynia. Hyperalgesia is an increase in sensitivity, resulting in enhanced pain perception. Allodynia is the perception of pain from a stimulus that is normally innocuous. The term hyperalgesia is used in this section to refer to both hyperalgesia and allodynia. These dorsal horn neuronal changes are highly organized and specific, corresponding only to those neurons receiving input directly from the injured tissue (Cervero, 2000b). The dorsal horn neurons are described as forming an “irritable focus,” and their collective alterations in excitability are called central sensitization (Cervero & Laird, 1999; Basbaum & Jessell, 2000; Cervero, 2000a,b; Al-Chaer & Traub, 2002). Afferent nociceptive fibers release glutamate (a neurotransmitter common to somatic afferent fibers) as well as neuropeptides (substance P, serotonin) and trophic factors (brain-derived neurotrophic factor), which act as neurotransmitters and modulators. Experimental studies show that receptors for these mediators are located on the postsynaptic membranes of dorsal horn neurons (Knowles & Aziz, 2009). It is speculated that central sensitization may be mediated by the phosphorylation of the N-methyl-d-aspartate (NMDA) glutamate receptors (through intracellular signaling) with a change in their receptor kinetics resulting in an increased responsiveness to glutamate. It is also possible that neurokinin-1 substance P receptors are involved (Basbaum & Jessell, 2000; Cervero, 2000b; Al-Chaer & Traub, 2002; Knowles & Aziz, 2009). In addition, the neuronal hyperexcitability may be facilitated by positive feedback loops established between the dorsal horn neurons and higher centers. The presence of these feedback loops may explain the motor and autonomic reflex responses (e.g., nausea and hypertonicity in abdominal muscles) that accompany visceral pain experiences (Cervero & Laird, 1999). As long as peripheral sensitization of the nociceptors continues during the inflammatory process, normal physiologic (innocuous) stimuli activate afferent fibers and perpetuate the increased volley of sensory input to the “irritable focus” that was initiated by the acute injury. The increased excitability of these neurons in turn facilitates and sustains the effects of the sensory input from the viscera, resulting in an increase in pain intensity and duration. In addition, the normal function of the affected viscera may be altered because of the injury and inflammation, resulting in further activation of the sensitized nociceptors and even the recruitment of more distant nociceptors. Thus the presence of the central sensitization phenomenon in conjunction with normal physiologic visceral afferent activity results in the persistence of visceral pain. Central sensitization may be the mechanism that produces hyperalgesia and allodynia both in sites to which pain is referred (referred hyperalgesia) (see section Central Projections and the Referral of Pain) and in the same or nearby viscera (visceral hyperalgesia). The processing of nociceptive input in the dorsal horn can be modulated by descending pathways from supraspinal regions. The PAG receives input from the anterior cingulate cortex and is able to modulate nociceptive input by activating neurons in the rostral ventromedial medulla and dorsolateral pons. These in turn project to the dorsal horn. Also, PAG activation of brain stem regions such as the locus ceruleus (noradrenaline region), raphe nuclei, and the rostral ventrolateral medulla results in the initiation of a gating effect on nociceptive input in the dorsal horn (Knowles & Aziz, 2009).
Certain abdominal and pelvic disorders are characterized by persistent abnormal visceral sensations (including allodynia and hyperalgesia). These sensations are commonly organized under the term of visceral hypersensitivity (Vergnolle, 2010). As mentioned previously, the initial inflammatory process or infection results in drastic changes in neuronal functions and membrane receptor expression that appear to be caused by the release of inflammatory mediators. These chemicals cause a hyperexcitabiltiy in the membranes of afferent fibers innervating the diseased organ. Once the acute inflammatory or infectious state is resolved, instead of returning to normal function, visceral afferents remain hypersensitized, producing chronic pain symptoms. In this postinflammatory stage, it is thought that the chemical mediators may not be directly responsible for the hyperexcitability of the visceral afferent fibers. Instead, the mediators may influence molecular structures on the membrane. Studies of the P2X ligand-gated ionotropic receptors have shown that they may be important for normal mechanosensitivity in the colon and that they may also play a role in postinflammatory hypersensitivity (Vernolle, 2010). In addition, members of the transient receptor potential vanilloid (TRPV) family of ion channels (specifically TRPV1 and TRPV4) and TRPA1 (an ion channel similar and functionally related to TRPV1) have been identified as being involved in nociception and in the development of visceral afferent sensitization and colonic hypersensitivity such as IBS. In fact, it has been suggested that the TRPV4 receptor may function as a central mediator. In this role, the release of chemical mediators involved in the pathophysiology of IBS, such as histamine, serotonin, and protease-activated receptor 2 (PAR2), activate TRPV4, which then initiates colonic hypersensitivity (Christianson et al., 2009; Vernolle, 2010). Knowing the functional importance of these receptors, treatment regimens may be designed to target them rather than target the inflammatory mediators, which have not yet been specifically identified in the postinflammatory tissues.
It has been suggested that conditions of the GI tract that present as chronic unexplained symptoms, including abdominal pain in the absence of any clear pathology, can be divided into two overlapping groups. One group includes gastrointestinal neuromuscular diseases (GINMDs) and the other includes the functional gastrointestinal disorders (FGIDs) (Knowles & Aziz, 2009). The underlying pathology of GINMD appears to be abnormalities in the components involved with normal GI sensory and motor functions, which include the interstitial cells of Cajal, smooth muscle, and the enteric nervous system and its extrinsic neurons. Symptoms of impaired motor activity manifested as slow or blocked transit are common in this group of diseases. Most GINMDs appear later in life and include enteric dysmotility, intestinal pseudoobstruction, and slow-transit constipation but some may appear as congenital defects such as Hirschsprung’s disease. In these disorders the mechanisms underlying the pain may be neuropathic. In FGIDs the predominant and defining symptom is chronic abdominal pain. This group of conditions includes irritable bowel syndrome, functional dyspepsia, functional heartburn, and functional abdominal pain syndrome. The distinguishing pathophysiologic characteristic of FGID is visceral hypersensitivity. The main mechanisms that are believed to underlie visceral hypersensitivity are “peripheral sensitization, central sensitization, altered descending excitatory or inhibitory influences (neural and humoral), and misinterpretation of non-noxious sensation as noxious due to cognitive and emotional biasing” (Knowles & Aziz, 2009). As mentioned earlier, treating these conditions may include using agents that act on voltage-gated ion channels, cation channels, and G protein–coupled receptors in the periphery (i.e., the serotonin receptors) and dorsal horn (i.e., NMDA receptors), thus reducing peripheral and central sensitization.
Visceral afferent fibers entering the spinal cord may initiate reflex responses or synapse on tract neurons. Although they comprise a minority of the total number of afferent fibers entering the spinal cord, they synapse in numerous laminae, including I, II, V, VII, and X (Beyak et al., 2006; Wood, 2007; Knowles & Aziz, 2009; Mayer, 2011).
These predominantly A-delta and C fibers, including those transmitting visceral nociception, enter the dorsolateral tract of Lissauer. They immediately enter the dorsal horn, as well as send collateral branches up and down as many as five segments within Lissauer’s tract before they enter the dorsal horn and synapse. The axons of the second-order neurons project to the brain through numerous ascending pathways including the spinothalamic, dorsal column (see below), spinoreticular, spinomesencephalic/parabrachial, spinohypothalamic, and spinosolitary tracts. The spinothalamic tract projects to the thalamus and from here to the somatosensory cortex (SI and SII) where the localization and intensity of the stimulus are provided. The other tracts project to brain stem nuclei (e.g., the reticular formation) and higher centers such as the amygdala, anterior cingulate cortex, and insula of Reil, which are involved with the unconscious and automatic responses that alter one’s emotional state and behavior. The activation of the cortex by all of these tracts allows a conscious perception of the nociception as pain and also allows the individual to respond to the pain. The spinothalamic tract helps to localize pain, although pain from the viscera is localized much less accurately than pain of somatic origin. Activating the reticular formation permits some localization and a conscious attentiveness to the pain. The widespread distribution to cortical areas beyond the somatosensory cortex and the interconnections subsequently established may account for the often accompanying discomfort and unpleasantness that can produce a particular affective mental state and subsequent behavioral patterns. The anterior cingulate cortex is an integrative center that may be responsible for linking pain and emotional states, and through connections with other neural structures it is an important source of descending pain modulation. The insula appears to have an important function in the integration of visceral, emotional, and cognitive functions. Through its connections with other areas such as the prefrontal cortex it is involved with the higher control of autonomic visceromotor and behavioral responses (Beyak et al., 2006; Wood, 2007; Knowles & Aziz, 2009; Mayer, 2011).
Evidence gained from experimental studies and midline myelotomies (intentionally placed midline lesions of the spinal cord) performed to treat pain from pelvic cancer indicates that visceral information also ascends in the dorsal column of the cord (Cervero & Laird, 1999; Willis et al., 1999; Joshi & Gebhart, 2000; Nauta et al., 2000; Ness, 2000; Westlund, 2000; Al-Chaer & Traub, 2002; Palecek, Paleckova, & Willis, 2002). Postsynaptic dorsal column cells (PSDCs) are located around the central canal of the spinal cord. These cells are responsive to noxious and innocuous cutaneous stimuli, as well as to mechanical and chemical visceral noxious stimuli. Axons from these cells in the sacral cord segments conveying pelvic visceral nociception ascend ipsilaterally in the midline of the dorsal column in an area distinct from mechanoreceptive ascending fibers and synapse in the gracile nucleus. Fibers from thoracic PSDCs carrying thoracic visceral nociception ascend at the lateral edge of the fasciculus gracilis and synapse in the gracile and cuneate nuclei. From these nuclei, axons ascend in the medial lemniscus and terminate in the ventral posterior thalamic nucleus. In addition to the fibers coursing in the medial lemniscus, some fibers synapse in the medullary reticular formation, periaqueductal gray, hypothalamus, amygdala, and medial areas of the thalamus. These dorsal column fibers transmitting visceral nociception to higher centers have been suggested as being as important as or more important than the spinothalamic tract (Beyak et al., 2006; Wood, 2007).
The data that have demonstrated the termination of visceral afferent fibers in the spinal cord gray matter also lend credence to the convergence-projection theory of referred pain (Ruch, 1946). This theory maintains that referred pain occurs because of the high degree of convergence of visceral and somatic afferent fibers on the same pool of viscerosomatic neurons in the cord (see Fig. 10-25). Because somatic pain is more common than visceral pain, the higher centers misread the visceral input as originating from somatic afferent fibers. Therefore pain is referred to the area of skin and deep structures (e.g., muscle, bone) supplied by the somatic afferent fibers that have entered the same cord segments as the visceral afferent fibers. The viscerosomatic neurons, located in laminae I, V, and X (Jänig, 1996) of the cord, are either tract neurons or local interneurons. The site of referral may be to the skin, muscles (or other deep somatic tissues), or both. In addition, referred pain often is accompanied by hyperalgesia. The hyperalgesia, which is an increased sensitivity to pain produced by noxious and innocuous (allodynia—see previous discussion) stimuli, is the result of peripheral sensitization of nociceptors and, more importantly, central sensitization. Although hyperalgesia is evident at the onset of the painful experience, it often persists longer than the pain associated with the initial visceral insult. This may occur for several reasons: (a) the persistence of the previously described plastic changes in the dorsal horn neurons make this area of the spinal cord function independently of peripheral nociceptive activation; (b) changes in the viscera persist longer than the initial focal lesion and send afferent input to the dorsal horn, maintaining the hyperactivity of the neurons; (c) viscerosomatic reflexes are activated back toward the peripheral sites, causing increased sensitivity of nociceptors (including muscle nociceptors) and therefore hyperalgesia; and (d) trophic changes occur in hyperalgesic somatic tissue. Studies in rat models of hyperalgesic muscle have shown morphofunctional changes (essentially characteristics associated with muscle atrophy) indicative of muscles undergoing sustained contractions, which often occur as a consequence of visceral referred pain.
Examples of visceral pain referral to muscle are the referral to abdominal oblique and quadratus lumborum muscles seen in patients experiencing pain from renal calculus, referred pain to the rectus abdominis muscle as a result of biliary calculus, and referred pain to the inferior aspect of the rectus abdominis and pelvic muscles as a result of dysmenorrhea (Vecchiet, Vecchiet, & Giamberardino, 1999; Giamberardino, 2003).
A common example of pain referral to cutaneous sites occurs after a myocardial infarction or angina pectoris episode. The relationship between visceral afferent fibers and the spinothalamic tract for pain originating from the heart was determined from data obtained from experiments on primates. These investigations were designed to demonstrate that cardiac ischemia stimulates cardiac afferent fibers, which in turn synapse on spinothalamic tract cells. Bradykinin, a peptide released from ischemic cells, was injected into cardiac tissue and resulted in stimulation of afferent fibers. By measuring tract neuron discharge rates, it was shown that 15 seconds after bradykinin injection (the time needed for receptor activation), 75% of the spinothalamic tract cells increased their firing rate (Cervero & Foreman, 1990). These data support the theory that visceral afferent fibers converge on the same tract cells on which somatic afferent fibers terminate. The peripheral distribution of these same somatic afferent fibers becomes the general location of the pain referral. The afferent fibers subserving nociception from the heart course primarily in the middle and inferior cardiac nerves and left thoracic cardiac branches (Kiernan, 2009) and eventually enter the first five thoracic cord segments. Pain is most frequently referred superficially to the left side of the chest and left inner arm; however, pain often is referred to the neck and jaw also. It is speculated that the mechanism for this referral pattern is the convergence of cardiac afferent fibers with the upper cervical cord segments. Cardiac afferent fibers travel in sympathetic nerves and the vagus nerve. The vagal fibers enter the medulla and synapse in the nucleus of the tractus solitarius (NTS) and also send branches inferiorly into the C1-3 cord segments (see Relationship between the Dorsal Horn and Trigeminal Nerve in Chapter 9). Fibers from the NTS also may send relay neurons to the C1-2 segments (Chandler, Zhang, & Foreman, 1996). These segments are the site of convergence of trigeminal afferent fibers serving the head, and somatic afferent fibers conveying sensory information from the area served by the C1-3 cord segments. This region of the dorsal horn provides the anatomic substrate for convergence of the trigeminal, somatic, and vagal afferent fibers on tract neurons (Chandler, Zhang, & Foreman, 1996; Chandler et al., 1999; Foreman, 1999) and subsequent misinterpretation of the origin by higher centers. Another theory explaining this referral pattern is that afferent information synapsing in thoracic spinal cord segments may ascend to upper cervical segments via propriospinal fibers. Also, visceral afferent fibers that enter the zone of Lissauer may ascend or descend as many as five cord segments before synapsing in the dorsal horn. These connections may provide a route for cardiac afferent fibers to directly terminate in the upper cervical segments (Chandler, Zhang, & Foreman, 1996), thus referring pain to the neck and even the head (via the connections to the spinal nucleus of the trigeminal nerve).
In order for clinicians to be able to describe the location of a patient’s pain, an abdominal viscus, or a mass in the abdominal cavity, the anterior abdominal surface has been divided into nine arbitrary regions. From superior to inferior, these are as follows: right side—right hypochondrium, right lumbar, right iliac fossa; middle—epigastric, central/umbilical, suprapubic/hypogastrium; left side—left hypochondrium, left lumbar, left iliac fossa. The specfic surface of the body to which visceral pain is referred is related to the embryologic origin of the organ, which is segmentally linked to the somatic innervation. Relative to the abdominal viscera, pain in general is referred to the three middle abdominal regions; that is, organs derived from the foregut (e.g., stomach [although the gastroesophageal junction refers to the subxiphoid and retrosternal areas], gallbladder, pancreas) will refer visceral pain to the central epigastrium region; organs derived from the midgut (e.g., appendix, jejunum, ileum) will refer pain to the umbilical region; and organs derived from the hindgut (e.g., descending colon, anal canal) will refer pain to the suprapubic region. Knowledge of the innervation of the peritoneum is also clinically important. The visceral peritoneum (as well as the pleura), supplied by branches of the afferent fibers (predominantly vagal) innervating the underlying viscera, is sensitive to stretch. When this occurs it causes a poorly localized sensation of discomfort and often initiates cardiovascular reflex responses likely mediated through connections among brain stem nuclei. The parietal peritoneum (and pleura) as well as the overlying skin and muscles is innervated by branches of somatic afferent and efferent nerve fibers. [This difference in innervation is important to note relative to certain surgical procedures involving incisions in the parietal peritoneum. Spinal anesthesia will anesthetize local regions of the parietal peritoneum. However, because the vagus nerve bypasses the cord and synapses in the brain stem, stretching of the visceral peritoneum that it supplies may have serious effects and initiate reflexes that may result in acute vascular instability in the underlying viscera causing ischemia and abdominal pain that is poorly localized (Standring et al., 2008).] The parietal peritoneum, pleura, or pericardium near the diseased organ includes nociceptors that may be activated by the spreading of the disease process that has caused the visceral pain referral. (The capsules of some viscera such as the liver and spleen are also innervated by branches of nerves that innervate the parietal peritoneum.) The somatic afferent fibers of these nociceptors are segmentally related to muscles and skin in the body wall overlying the viscus. The pain pattern, different than the visceral referred pain pattern, is well-localized, lateralized, and often limited to one or two dermatomes for each somatic afferent fiber’s area of distribution. (The involvement of the parietal serous membranes lining the inside wall of the abdominal and thoracic cavities has been described as viscerosomatic pain [FitzGerald & Folan-Curran, 2002].) In addition, these somatic afferent fibers terminate in corresponding segments that innervate muscles. The activation of these afferent fibers may cause reflex muscle contraction and, in the case of the abdominal wall musculature, hypercontractility (guarding) or rigidity. A good example of the change in the nature and location of pain patterns is a diseased appendix. Initially, visceral afferent fibers that course in the splanchnic nerves are stimulated and terminate in the T10 to T11 cord segments on viscerosomatic neurons. The result is poorly localized aching pain and cramping that is referred to the periumbilical region in the T10 to T11 skin surface area of the abdominal wall. The parietal peritoneum adjacent to the appendix is innervated by somatic afferent fibers that terminate approximately in the T12 to L1 cord segments on the right side. Therefore when the parietal peritoneum also becomes inflamed the pain now becomes sharp and localized to the right lower abdominal quadrant (Standring et al., 2008).
Another example of pain referral is that which occurs as a result of biliary calculus (gallstones). Sensory afferents from the gallbladder and bile ducts course in the right greater splanchnic nerve and into the T7 and T8 cord segments. The referral site is to the central epigastrium and, because of the involvement of the overlying somatic peritoneum, to the right upper quadrant of the abdomen and right infrascapular region, corresponding to the T7 and T8 dermatomal patterns. As the disease progresses, inflammation of the peritoneum associated with the diaphragm occurs. The chemical mediators involved with the inflammatory process activate the peritoneal sensory fibers that travel in the phrenic nerve. These afferent fibers terminate in the C3-5 cord segments and thus can refer pain to the top of the shoulder.
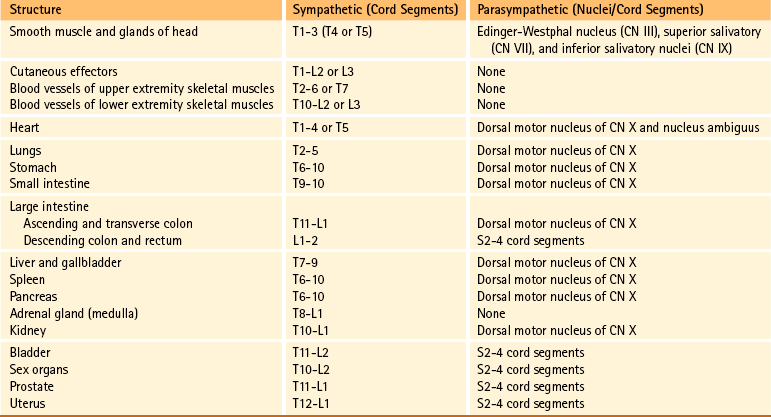
Because pain is the most important clinical visceral sensation, knowledge of visceral pain referral patterns and the spinal cord segments to which visceral afferent fibers project (which is the same location as the sympathetic preganglionic cell bodies) is extremely important. This knowledge allows a clinician to more effectively diagnose pathologic conditions occurring in the viscera (Fig. 10-27 and Table 10-2).
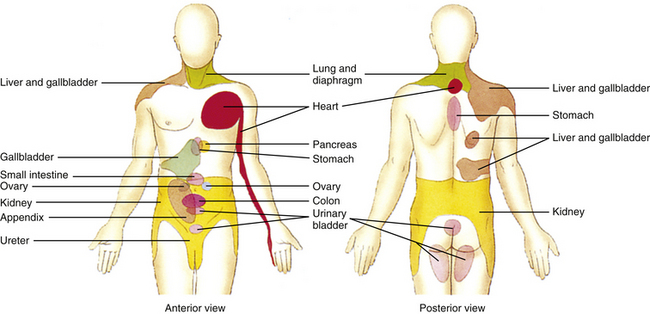
FIG. 10-27 Referral patterns. The colored regions show cutaneous areas to which visceral pain is referred (see text for discussion). (From Tortora GJ & Grabowski SR. [2003]. Principles of anatomy and physiology [10th ed.]. New York: John Wiley & Sons.)
There is evidence suggesting there is viscerovisceral referral and sensitization (called cross-organ sensitization) among different organs as well (Al-Chaer & Traub, 2002; Giamberardino, 2003; Brumovsky & Gebhart, 2010). Based on data from experiments on animals, both central and peripheral mechanisms have been proposed to explain this phenomenon. In the spinal cord there is viscerovisceral convergence on neurons similar to the segmental neuronal arrangement seen in viscerosomatic convergence. Afferent fibers from two different organs converge on the same second-order spinal neurons such that when one organ becomes diseased, the afferent fibers from that organ sensitize the spinal neurons. This results in an increased excitability to afferent fibers transmitting normal stimuli from the other organ producing the signs and symptoms as if the “normal” organ was diseased. For example, a pathologic condition of the esophagus can produce the same symptomology and referral pattern commonly seen in patients with myocardial ischemia. In addition to hyperexcitability of second-order spinal neurons, interneurons in the dorsal horn may also be involved. Afferent fibers from a diseased organ may synapse on interneurons that excite afferent fibers from another organ, resulting in an antidromic axon response leading to neurogenic inflammation in the healthy organ. There are also some data that suggest that there may be viscerovisceral convergence at the level of the brain stem and possibly the thalamus (Brumovsky & Gebhart, 2010).
There are also a number of possibilities that may explain a peripheral mechanism for cross-organ sensitization. One is that there are some dichotomizing primary afferent neurons that innervate viscera—that is, two different organs are innervated by the same primary afferent neuron, thus contributing to cross-tissue/organ sensitization. Another theory is that there may be chemical and electrical coupling between neuron cell bodies in close proximity to each other in the dorsal root ganglion or between lightly myelinated primary afferent fibers found in the same nerve fascicle. Through this coupling between the cell body or fiber from a diseased viscus with the cell body or fiber from a healthy organ, cross-organ sensitization could occur. Another possibility is through the connection visceral afferent fibers (including intestinofugal afferent fibers) have with the autonomic ganglia. Collateral branches of an afferent fiber from a diseased organ may excite sensory or motor neurons in an autonomic ganglion that innervates a healthy organ and could result in cross-organ sensitization and neurogenic inflammation in that nondiseased viscus. Although peripheral and central mechanisms have been studied as processes that work independently, it has also been suggested (Brumovsky & Gebhart, 2010) that the initiation and persistence of cross-organ sensitization may be the result of peripheral and central mechanisms working simultaneously. In this scenario, a disease process such as inflammation causes peripheral excitation/sensitization to occur and subsequently intensify through positive feedback loops. This results in an increased amount of convergence on spinal neurons, causing amplified transmission of input to higher centers. Consideration has also been given to glial (e.g., microglia) interactions with the neurons that receive converging input at the spinal cord level and higher as a mechanism that may affect neuronal excitability and contribute to cross-organ sensitization.
Based on experimental and clinical research it appears that cross-organ sensitization occurs between organs found within certain organ groups. One group includes organs in the thoraco–upper abdominal region, which are the esophagus, heart, lower airways, gallbladder, stomach, and duodenum. Clinical data and data from animal studies indicate that cross-organ sensitization may occur between the heart and esophagus, heart and stomach or gallbladder, and duodenum and esophagus. The other group includes the colon, rectum, ureter, bladder, pelvic urethra, prostate, and uterus, which are located in the pelvic–lower abdominal region. In this group, the most common cross-organ sensitization occurs between the colon and bladder, which have a close functional relationship under normal physiologic conditions. Clinical studies have shown that patients with IBS may present with signs and symptoms of bladder hypersensitivity (e.g., back pain, frequency and urgency of micturition, and nocturia), and data from animal studies also indicate there is sensitization between these two organs. Other studies show that cross-organ sensitization occurs between the bladder and uterus, uterus and pelvic urethra, pelvic urethra and female reproductive organs, uterus and vagina, and prostate and pelvic viscera such as the lower urinary tract. Clinical studies show that cross-organ sensitization between lower abdominal organs and pelvic urinary or reproductive organs is a common occurrence. Because of this phenomenon, diagnosing and initiating proper treatment for the pathologic organ becomes problematic (Brumovsky & Gebhart, 2010).
Autonomic Reflexes
Reflexes are common events mediated by the nervous system. A reflex can be described simply as an involuntary action that occurs fairly quickly, regulates some effector function, and has no direct involvement with the cerebral cortex. The components of a reflex arc include a peripheral receptor and its afferent fiber, which form the sensory limb; an efferent fiber that forms the motor limb; and an effector. Depending on whether the reflex arc is monosynaptic or polysynaptic, the presence of interneurons connecting the afferent and efferent fibers is variable. Both types of afferents (somatic and visceral) and efferents (somatic and visceral) may be involved, thus creating four major kinds of reflex arcs. These are somatosomatic, viscerosomatic, viscerovisceral, and somatovisceral.
Somatosomatic Reflexes
Somatosomatic reflexes consist of somatic afferent fibers that influence somatic effectors, that is, skeletal muscle. Chapter 9 discusses examples of this type of reflex, which included the muscle stretch reflex and superficial reflexes (cremasteric and abdominal). The flexor (withdrawal) reflex and the crossed extensor reflex also are examples of somatosomatic reflexes.
Viscerosomatic Reflexes
The existence of polysynaptic viscerovisceral and viscerosomatic reflexes implies that visceral afferent fibers are involved not only with the mediation of visceral functions but also with the functions of somatic effectors (i.e., skeletal muscles). Physiologic activities that exemplify viscerosomatic reflex responses concern respiratory function and GI activity. Regulation of respiratory rhythmicity is under the control of respiratory centers located in the medulla. These neurons receive input from lung receptors that inhibit inspiration and facilitate expiration. The Hering-Breuer reflex is activated to prevent overinflation of the lung in the hyperinflated state and when the tidal volume increases to greater than 1.5 L. Stretch receptors that lie in the bronchi and bronchioles of the lungs increase their firing rate as the lungs inflate. This information is conveyed via visceral afferent fibers in the vagus nerve to the nucleus of the tractus solitarius of the brain stem medulla. From this nucleus, neurons project into the respiratory center that inhibits inspiration. From here, descending fibers inhibit the motor neurons that innervate the skeletal muscles of respiration and subsequently terminate the inspiration phase. Other visceral afferent fibers that reflexively influence respiratory skeletal muscles course in the glossopharyngeal and vagus nerves from chemoreceptors located in the carotid and aortic bodies. A change in the carbon dioxide concentration causes a reflex change in the rate and depth of respiration. Abnormal stimuli such as visceral nociception also can produce skeletal muscle contractions. An example of this type of viscerosomatic reflex is the contraction of the abdominal skeletal musculature after excessive distension of a viscus or the inflammation of peritonitis (see Central Projections and the Referral of Pain).
Experiments on rabbits have shown that stimulation of organs such as the renal pelvis and small intestine causes reflex paravertebral muscle contractions. In addition, some pathologic conditions (e.g., coronary artery disease) cause stimulation of afferent fibers that produce not only skeletal muscle contractions but also concurrent activation of autonomic effectors in somatic tissue that results in cutaneous vasomotor and sudomotor changes (Beal, 1985).
Viscerovisceral Reflexes
Visceral afferent fibers also mediate visceral reflex responses. Viscerovisceral reflex responses are common occurrences and are best exemplified in the functioning of the cardiovascular and GI systems. Changes in blood pressure are monitored by baroreceptors of the carotid sinus and aortic arch. For example, an increase in blood pressure stimulates the baroreceptors. The visceral afferent fibers from these course in the glossopharyngeal and vagus nerves to the brain stem, causing a reflex slowing of the heart rate via visceral efferent fibers in the vagus nerve and peripheral vasodilation via inhibition of sympathetic efferent fibers. Visceral afferent fibers from the GI tract and bladder convey information allowing for the normal functioning of digestion, elimination, and voiding. Sensory input such as distension produces reflex responses, including contraction of smooth muscle (in the wall and sphincters) and mucosal secretion.
The enteric nervous system is intimately involved with viscerovisceral reflex responses. For example, a toxic microbial organism may stimulate the intrinsic sensory neurons of the submucosal plexus that innervate the epithelium of the gut. Although the circuitry is not completely understood, these neurons cause reflex secretion of water and ions, a decrease in absorption, and, by means of the myenteric plexus, an increase in motility of the gut (Loewy, 1990a) (see section Enteric Nervous System).
Somatovisceral Reflexes
The existence of somatovisceral reflexes indicates that visceral afferent fibers are not the sole initiators of visceral responses; somatic afferent fibers also can reflexively stimulate autonomic efferent fibers. This usually occurs when changes of skin temperature result in cutaneous vasomotor and sudomotor responses. Although evidence exists that stimulating the receptors of somatic afferent fibers produces changes in visceral activity, the exact neural circuitry for somatovisceral reflexes is not clearly understood.
Sato and colleagues (Sato, Sato, & Schmidt, 1984; Sato & Swenson, 1984; Sato, 1992a,b) have provided much evidence supporting the presence of somatovisceral reflexes. Using anesthetized rats, they stimulated the receptors of somatic afferent fibers from the skin, muscle, and knee joint and measured the reflex changes in heart rate, gut motility, bladder contractility, adrenal medullary nerve activity, and secretion of the adrenal medulla. Reflex responses to cutaneous stimuli produced the following varied responses depending on the type of stimuli and organ involved:
1. Noxious and innocuous mechanical stimuli and thermal stimuli produced an increase in heart rate.
2. Noxious pinching of the abdominal skin resulted in inhibited gastric motility, although motility sometimes was facilitated when the hindpaw was pinched.
3. Stimulation of the perianal area caused increased efferent firing to and reflex contractions in a quiescent (slightly expanded) bladder, but this caused the inhibition of bladder contractions in an expanded bladder.
4. Noxious pinching of the skin and noxious thermal stimuli resulted in an increase in the secretory activity of and neural activity to (via the greater splanchnic nerve) the medulla of the adrenal gland, whereas innocuous stimuli had the opposite effect.
Type III and IV muscle afferent fibers, stimulated by intraarterial injections of potassium chloride (KCl) and bradykinin (both of which are analgesic agents), produced the following effects on heart rate and smooth muscle of the bladder:
1. “Injection of KCl regularly accelerates heart rate. With bradykinin, both accelerations and decelerations can be observed” (Sato, 1992a).
2. Both substances had effects on the bladder similar to those initiated by cutaneous stimuli (i.e., excitation to the quiescent bladder and inhibition to the contractions of an expanded bladder).
Joint receptors from both a normal and an inflamed knee joint were stimulated by movements both within and beyond the joint’s normal range of motion. Results showed that heart rate and secretory and nerve activity of the adrenal medulla increased when the normal knee joint was moved beyond its normal range and when the inflamed knee joint was moved within and beyond its normal range, with a greater increase occurring during the latter. These data indicated the variability that can occur in different effectors in response to various stimuli of somatic afferent fibers. These experiments showed that effectors could be mediated through both sympathetic and parasympathetic efferent fibers and that the response could be excitatory or inhibitory. Further, reflex responses may be integrated at the segmental level (spinal cord) or at the supraspinal level, and the data indicated that both paths were used. For example, segmental integration occurred for the cutaneovesical reflex of the quiescent bladder, cutaneoadrenal reflex, and cutaneogastric reflex, and supraspinal integration was necessary for the cutaneocardiac reflex and cutaneovesical reflex of the expanded bladder.
In other experiments exploring somatovisceral reflexes, different forces were applied to the lateral aspect of two regions of immobilized spines of anesthetized rats (Fig. 10-28) to study the effect on heart rate, blood pressure, and activity in the adrenal nerve to the adrenal medulla and the renal nerve to the kidney (Sato & Swenson, 1984; Sato, 1992a). Lateral flexion resulting from applied mechanical force stimulated afferent fibers supplying the vertebral column and produced the following results:
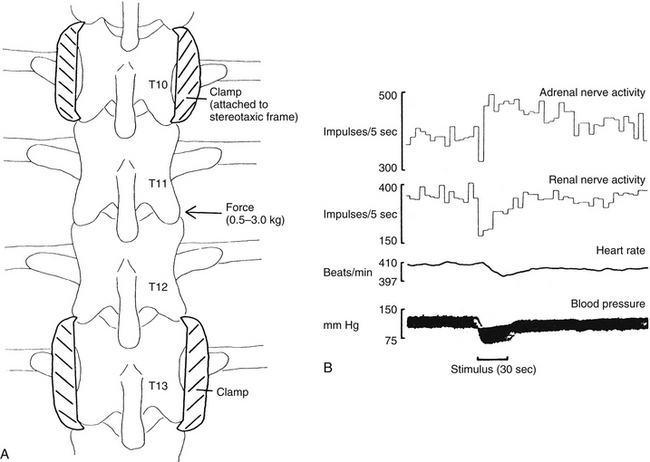
FIG. 10-28 A, Illustration of the stimulation procedure (thoracic spine shown). Segments isolated from skin and muscle, upper and lower segments fixed in a spinal stereotaxic device, and force exerted (0.5 to 3 kg) on mobile segments. B, Sample record from a central nervous system–intact animal with thoracic spine stimulation. Force (3 kg) delivered during the period marked by the dark bar below the blood pressure trace. (From Sato A & Swenson RS. [1984]. Sympathetic nervous system response to mechanical stress of the spinal column in rats. J Manipulative Physiol Ther, 7(3), 141-147.)
1. A consistently large decrease in blood pressure that returned to normal after the stimulus was removed
2. An inconsistently small decrease in heart rate
3. A decrease in blood flow to the gastrocnemius and biceps femoris muscles, with a concomitant decrease in systemic arterial blood pressure
4. An initial decrease in activity in the renal nerve and subsequent recovery, both during the period of stimulation
5. An initial decrease in activity in the adrenal nerve with a gradual return to baseline activity, which was followed by an additional increase in activity (likely caused by a baroreceptor-mediated reflex response)
A study in 2006 (Bolton, Budgell, & Kimpton, 2006) investigated the effect of innocuous mechanical stimuli of cervical vertebrae on the activity in the sympathetic nerve to the adrenal gland. Rats were used to see if localized cervical intervertebral displacement resulted in a change in sympathetic efferent activity. To prevent activation of the vestibular system and any resulting vestibulosympathetic reflex responses, the heads of the rats were fixed in place. Sympathetic nerve responsiveness was first tested by innocuous mechanical cutaneous stimuli (brushing of the skin of the neck) and noxious stimuli (pinching the forepaw). The results showed an increase in activity to noxious stimuli and showed no response to innocuous stimuli, which were consistent with data of other studies. Low amplitude, low velocity displacement of the C2 vertebra at various ranges within the normal physiologic range was performed on the rats. In general, the results from the study suggest that sympathetic nerve activity to the adrenal gland was not reflexively altered by innocuous mechanical stimulation induced by low amplitude and low velocity movements of the C2 vertebra. However, it was noted in a few rats that C2 vertebral displacements at greater than 20-degree rotation (beyond the normal physiologic range) did result in modulation of adrenal nerve activity and a change in blood pressure. These data, which are typical of a sympathetic response to an injurious event, may indicate that a noxious event occurred as a result of the mechanical displacement of C2 at those higher degrees of rotation that occur beyond the normal limits of rotation.
More studies have confirmed the fact that noxious and innocuous stimuli affect cardiovascular and other autonomic responses. Kurosawa and colleagues (2006) used anesthetized rats and studied the effects of innocuous mechanical (brushing) stimuli on the blood flow to the dorsal spinal cord. Blood flow rate was measured when the forepaw, forelimb, upper and lower back, hindlimb, and hindpaw were stimulated. The data indicated that an ipsilateral increase in blood flow occurred with no change in blood pressure and that it was segmentally organized by neuronal excitation in the cord. Kurosawa and colleagues suggested that sympathetic nerves and α-adrenoceptors may be involved in this somato-autonomic reflex response but that metabolic effects (e.g., release of local vasodilators) induced by neuronal excitation may contribute as well. Toda et al. (2008) studied anesthetized rats and the effects of noxious mechanical (pinching) cutaneous stimuli on blood flow of the dorsal spinal cord. In this study, spinal cord blood flow (SCBF), which was measured in the spinal cord region associated with the hindlimb, was increased when all four paws were stimulated although blood flow increased the greatest to the ipsilateral hindpaw stimulation. Mean arterial pressure (MAP) also increased when stimuli were applied to the paws. To gain greater insight into the mechanism of the increase in SCBF, experiments were performed in which baroreceptors were denervated, phenoxybenzamine (α-adrenoceptor antagonist) and phenylephrine (α-adrenoceptor agonist) were administered intravenously, and rats were spinalized. Based on the data, the authors suggest that somato-autonomic (sympathetic) reflexes and autoregulatory responses (both possibly through the activation of α-adrenoceptors) as well as systemic arterial pressure provide mechanisms that generally regulate SCBF. However, because an increase in SCBF in spinalized rats and in rats treated with phenoxybenzamine and phenylephrine occurred when the ipsilateral hindpaw was stimulated, it was proposed that a mechanism also existed that was specific for that local region. Organized ipsilaterally and segmentally, sensory input would result in neuronal activation inducing metabolic effects on spinal vasomotor tone that may already have been altered in response to increased systemic arterial pressure and/or noxious mechanical cutaneous stimulation. Anatomic and experimental evidence has shown that numerous mechanoreceptors are found in structures of the neck (e.g., skin, muscles, tendons, ligaments, periosteum, intervertebral discs, zygapophysial joints) that work in conjunction with the vestibular system to provide reflex responses to postural changes (Bolton, 1998). Reflexes associated with postural adjustments include the cervicocollic reflex, tonic neck reflex, and cervico-ocular reflex. In addition, there is evidence that activation of neck receptors elicits reflexes that produce autonomic responses. Experimental studies on cats (Bolton et al., 1998) suggested that stimulation of afferents of cervical neck muscles produced cervicosympathetic and cervicorespiratory reflex responses. This was evident by the changes seen in nerve activity in the greater splanchnic and abdominal nerves (respiratory motoneurons to the abdominal wall musculature), as well as the hypoglossal nerve. It has been established that input from neck afferents is relayed through brain stem vestibular nuclei to sympathetic and respiratory neurons. In addition, brain stem transections through the caudal medulla performed to separate the vestibular nuclei connections from the cord have shown that afferent fibers from the neck also stimulate sympathetic and respiratory nerves without relaying through the vestibular nuclei. However, transections through the middle of the medulla, which possibly interrupt descending fibers from the rostral ventrolateral (VL) medulla (part of the central autonomic control network), have been found to alter the activity of the sympathetic and respiratory neurons. Therefore based on the data from these experiments, a complex mechanism appears to exist to produce the physiologically normal cervicosympathetic and cervicorespiratory reflexes. This complex mechanism involves caudal brain stem and spinal cord structures that must be intact for these reflexes to take place. In addition, numerous studies on anesthetized animals also indicate that autonomic reflex responses are seen in visceral organs in response to somatic stimuli. Some of the effector organs studied that demonstrated changes are the gastrointestinal tissues, heart, urinary bladder, vasculature of the peripheral nerves, vasculature of the cerebrum, medulla of the adrenal gland, and spleen (see overview article by Sato [1997]).
Other animal studies also confirm that stimulation of somatic structures has an effect on autonomic responses. In one study, the effects of somatic stimuli were observed on the motility of the quiescent bladder. The results showed that there was an increase in bladder muscle tone in response to noxious chemical stimuli of the interspinous tissue but little change in bladder pressure in response to innocuous somatic stimuli (Budgell, Hotta, & Sato, 1998). Another animal study examined the effects of noxious chemical stimulation of interspinous tissues on gastric motility. The results showed that motility was strongly inhibited and that the reflex arc responsible for this action was segmentally located in the middle to lower thoracic region. Although both vagus and sympathetic nerve supply to the stomach was found to be involved in the reflex, the sympathetic innervation appeared to be more important (Budgell & Suzuki, 2000).
It also has been demonstrated that cardiac function can be altered by the innocuous stimulation of mechanoreceptors through spinal manipulation. These reflexes are sometimes referred to as spinovisceral reflexes. Budgell and Igarashi (2001) reported one case study of a young man with bradycardia and an arrhythmia. While being monitored continuously by electrocardiography, one cervical (C2) spinal manipulation was performed on the patient, which resulted in the coincidental disappearance of the arrhythmia. Although the result was clearly apparent, no distinct mechanism has yet to be proposed to explain the event. Another study investigated the effects of spinal manipulation (C1 and C2 levels) of healthy young adults on cardiac function. Results from this study, which used sham and authentic manipulations (non-noxious stimuli within normal physiologic ranges of motion), demonstrated significant changes in heart rate and heart rate variability (HRV) when only the authentic manipulations were performed (Budgell & Hirano, 2001).
In summary, many experiments show that somatic afferent stimulation, especially noxious stimulation, can modulate autonomic output, resulting in changes in heart rate, blood pressure, and activity in sympathetic efferents to the kidney and medulla of the adrenal gland. This includes studies that show that various stimuli applied to spinal structures can initiate somatovisceral reflex arcs. For example, studies show that noxious chemical stimulation results in modulation of sympathetic output related to cardiovascular and adrenal medullary activity (Budgell, Hotta, & Sato, 1995; Budgell, Sato, & Suzuki, 1997) and that mechanical stimulation causes varied responses depending on the location and type of stimulus applied (Sato & Swenson, 1984; Bolton, Budgell, & Kimpton, 2006). Pathologic processes affecting the spine also may result in reflex changes in visceral activity (Sato, 1992a). Based on this evidence, the neural components for this type of somatovisceral reflex do exist. In addition, spinal manipulation performed within the normal physiologic range of motion may stimulate somatic afferent fibers to create somatovisceral reflex responses.