Neuroanatomy of the Spinal Cord
The vertebral column and its adjacent musculature are discussed in detail in the previous chapters. Because of the intimate anatomic and functional relationship between the vertebral column and spinal cord (which is protected by the vertebral column), knowledge of both is equally important. Chapter 3 describes the meninges, external surface, and vasculature of the spinal cord. In addition, it provides a cursory description of the cord’s internal organization. The purpose of this chapter is to elaborate on this internal organization by discussing the neurons, which form the circuitry of the spinal cord, and the ascending and descending tracts, which provide a connection among the spinal cord, the peripheral nerves, and the higher centers of the central nervous system. This information forms the basis of important neuroanatomic concepts that are necessary for an understanding of clinical neuroscience. The knowledge of these concepts is imperative for diagnosing pathologic conditions of the cord, some of which may be caused by vertebral column dysfunction. Examples demonstrating the application of these neuroanatomic principles to pathologic conditions are presented at the end of the chapter.
Peripheral Nervous System
Peripheral receptors are sensory endings of peripheral nerves; they are scattered throughout the body. They are found in great numbers along the vertebral column and within the ligaments, muscles, and skin that surround the spine. These receptors and the sensory systems that transmit their input provide information concerning one’s environment. Each receptor is sensitive to a particular form of physical energy, or stimulus, and transduces the stimulus into electrochemical energy or an action potential, which is the “language” the central nervous system (CNS) can understand.
Receptors may be divided into two types, rapidly adapting and slowly adapting. A slowly adapting receptor, such as a Merkel’s disc, responds continuously to a sustained stimulus, whereas a rapidly adapting receptor does not. A rapidly adapting receptor, such as a pacinian corpuscle, responds to any dynamic change in the receptor. For example, if the pressure onto a pacinian corpuscle is continuously increased, the corpuscle will continue to produce action potentials until the stimulus strength becomes constant. A stimulus furnishes a receptor with four basic characteristics: modality (type of stimulus), intensity (strength of stimulus), duration (perceived time that the stimulus is present), and location (where on the body the stimulus is being perceived). When a receptor is adequately stimulated, a generator potential occurs across its membrane and may lead to an action potential. The action potential propagates along the sensory neuron into the CNS. The CNS then is able to combine the four characteristics of the stimulus into a perceived sensation.
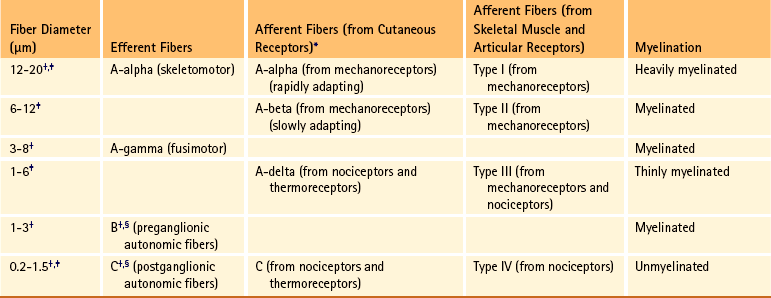
The sensory neurons of the peripheral nervous system (PNS) are pseudounipolar neurons. Their cell bodies are located in the dorsal root ganglia. The peripheral process, which may be myelinated or unmyelinated, is the part of the fiber continuous with the receptor. It is the sensory component of a peripheral nerve. The other part of the fiber, the central process, enters the CNS. A bundle of central processes forms a dorsal root. The peripheral processes are classified according to their conduction velocity, and conduction velocity is related to the axon diameter. Fibers with large diameters conduct the fastest. Based on the relationship between velocity and diameter, cutaneous fibers are classified alphabetically as Aβ (beta), Aδ (delta), and C fibers. Similarly, afferents from muscle tissue usually are classified numerically from heavily myelinated to unmyelinated as I, II, III, and IV. Type I also has subgroups of Ia and Ib. Afferents from visceral interoceptors often are classified as Aδand C fibers. Motor (efferent) fibers also are classified according to the alphabetic listing. Large somatic motor neurons correspond to the Aα(alpha) and Aγ(gamma) group, and autonomic efferent fibers correspond to the B and C groups. Table 9-1 summarizes the classifications of the afferent and efferent fibers.
Table 9-1
Summary of the Classification of Peripheral Fibers
∗Afferent fibers from visceral receptors are classified as A-delta and C fibers.
†From Kiernan JA. (2009). The human nervous system (7th ed.). Philadelphia: JB Lippincott.
‡From Gardner EP, Martin JH, & Jessell TM. (2000). The bodily senses. In ER Kandel, JH Schwartz, & TM Jessell (Eds.). Principles of neural science (4th ed.). New York: McGraw-Hill.
§From Standring et al. (2008). Gray’s anatomy (38th ed.). Edinburgh: Churchill Livingstone.
Peripheral receptors can be classified by their morphology, location, and the type of stimulus to which they respond. Morphologically, receptors may be encapsulated by connective tissue and nonneural cells, or they may simply be nonencapsulated, bare arborizing endings. Receptors classified by their location of distribution are called exteroceptors, proprioceptors, or interoceptors. Exteroceptors are superficial and located in the skin. Modalities such as nociception (pain), temperature, and touch (and the submodalities of pressure and vibration) are conveyed by these receptors. Proprioceptors are located in the muscles, tendons, and joints of the body and provide information concerning limb position, while the limbs are either stationary (static) or moving (dynamic or kinesthetic) (Pearson & Gordon, 2000a; Nolte, 2002; Standring et al. 2008; Kiernan, 2009). Interoceptors are located in the viscera, glands, and vessels and convey poorly localized information from such systems as the digestive and urinary systems. Examples of the types of information conveyed by interoceptors include distention or fullness and ischemic pain.
Receptors classified by the type of stimulus to which they respond are called mechanoreceptors, thermoreceptors, chemoreceptors, or nociceptors. Mechanoreceptors respond to deformation or displacement of self or adjacent cells. Thermoreceptors respond to changes in temperature. Chemoreceptors respond to chemical stimuli and are important in the special senses of taste and olfaction. Nociceptors respond to stimuli that damage tissue cells, and their stimulation results in the sensation of pain. The classification of receptors by location overlaps with the classification by stimulus type, such that nociceptors also can be exteroceptors, and mechanoreceptors also can be proprioceptors.
Cutaneous Receptors
Cutaneous receptors (exteroceptors) include mechanoreceptors, thermoreceptors, and nociceptors and subserve such modalities as touch, pressure, vibration, temperature, and nociception (pain) (Fig. 9-1). Mechanoreceptors include the nonencapsulated Merkel’s discs, nonencapsulated endings surrounding hair follicles (peritrichial), and encapsulated endings such as Ruffini endings, pacinian corpuscles, and Meissner’s corpuscles. The fibers supplying these receptors are primarily A-beta. Thermoreceptors are nonencapsulated, free nerve endings that occupy areas approximately 1 mm in diameter. Cold thermoreceptors respond in the range of 5° C (41° F) to 40° C (104° F) relative to the normal skin temperature of 34° C (93.2° F) and fire most frequently at 25° C (77° F). Warm thermoreceptors are stimulated in the temperature range 29° C (84.2° F) to 45° C (113° F) and are most active at 45° C (113° F) (Gardner, Martin, & Jessell, 2000). Cold receptors are supplied by A-delta or C fibers, but warm receptors are supplied by C fibers alone.
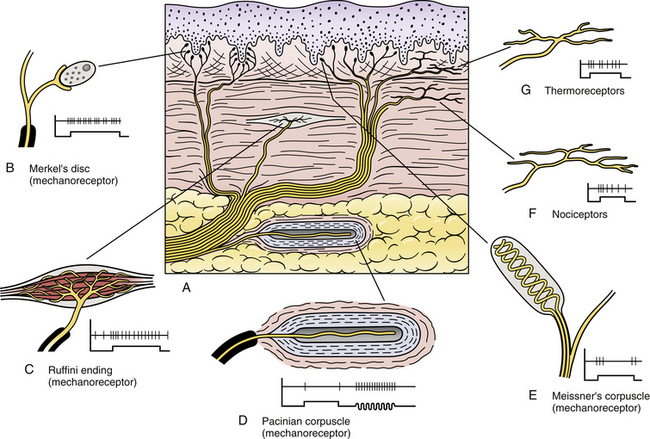
FIG. 9-1 Types of cutaneous receptors. Receptors C, D, and E are encapsulated. Receptors D and E are rapidly adapting and receptors B, C, F, and G are slowly adapting. The tracings illustrate that the slowly adapting receptors fire continuously throughout the stimulus and the rapidly adapting receptors fire at the onset and offset of the stimulus.
An understanding of nociceptors is helpful in the comprehension of pain of spinal origin. Nociceptors are free nerve endings, and they respond to stimuli that may threaten or actually damage adjacent tissue cells. The damage to cells causes the release of chemical mediators that either may sensitize (e.g., prostaglandins, leukotrienes, substance P) previously unresponsive free nerve endings by lowering their threshold for activation or may activate (e.g., histamine, bradykinin, potassium, serotonin) the free nerve endings. In some instances, activated free nerve endings release substance P and calcitonin gene–related peptide into the surrounding area, causing vasodilation, extravasation of fluid, and release of histamine from tissue cells. These changes in turn lead to lowering of the threshold for activation of more nociceptors. The inflammatory process that results from the activity of these nociceptors is termed neurogenic inflammation (Basbaum & Jessell, 2000). Three types of nociceptors appear to exist: (a) mechanical, which are stimulated by mechanical damage such as by a sharp object; (b) thermal, which are stimulated by temperatures higher than 45° C (113° F) or lower than 20° C (68° F); and (c) polymodal, which respond to damaging mechanical, thermal, or chemical stimuli. Mechanical and thermal nociceptors send their information via A-delta fibers, whereas polymodal receptors use C fibers. The three types of nociceptors often are activated simultaneously and thus work together. For example, if a person stubs a toe, initially there is sharp (fast) pain resulting from stimulating mechanical nociceptors followed by an achy prolonged pain resulting from stimulating polymodal nociceptors that use slower C fibers.
The cutaneous fibers of these receptors form overlapping horizontal plexuses in the dermis and subcutaneous layers of the skin. The density and variety of receptors vary in different regions. For example, in hairy skin the peritrichial endings are most common, but Merkel’s discs and free nerve endings are also present. In glabrous (hairless) skin, free nerve endings are present, as are Merkel’s discs and Meissner’s corpuscles. The latter two receptors have small receptive fields and help to discriminate the spatial relationship of stimuli. This ability to discriminate is well developed on the fingertips. In fact, Meissner’s corpuscles have been located only in primate animals (Kiernan, 2009). The subcutaneous tissues of both types of skin are provided with pacinian corpuscles and Ruffini endings, both of which have large receptive fields and therefore are less discriminatory (Gardner, Martin, & Jessell, 2000).
Cutaneous modalities that may be easily tested during a neurologic examination include vibration, temperature, pain (nociception), and tactile sensation. Tactile sensation can be described as simple touch (which includes light touch, touch pressure, and crude localization) and tactile discrimination (which includes deeper pressure and spatial localization), which is sometimes called two-point discriminatory touch. On the fingertips, for example, tactile discrimination is precise enough to localize two points of stimulation applied simultaneously 2 mm apart. Such tactile discrimination is necessary for further analysis of objects concerning their size, shape, texture, and movement pattern. This analysis is completed in sensory integrative areas of the cerebral cortex. Identifying common objects held in the hand and recognizing letters drawn on the back of the hand without visual cues are called stereognosis and graphesthesia, respectively. These are further examples that demonstrate tactile discrimination. The clinical relevance of these cutaneous modalities is discussed at the end of this chapter.
Muscle, Tendon, and Joint Receptors
The majority of the receptors located in muscles, tendons, and joints are involved with the sense of proprioception. The receptors are classified as proprioceptors based on their location. They are classified as mechanoreceptors based on the type of stimulus to which they respond. These receptors convey proprioception, the term used to describe the sensory information that contributes to the sense of movement and position of one’s own limbs and body without visual input. The mechanoreceptors involved with providing proprioception (excluding the vestibular system of the inner ear) are the joint receptors, neuromuscular spindles, and Golgi tendon organs (neurotendinous spindles). They function in the coordination and control of movements and the maintenance of upright posture by monitoring both the stationary position and the movement (kinesthesia) of body parts, and then relaying that information into the CNS. This information, often called joint position sense, may be perceived consciously. In addition, cutaneous mechanoreceptors also may function in the overall assessment of proprioception. For example, they may aid other proprioceptors in the task of discriminating different thicknesses of objects held between the thumb and finger and in the manipulation of objects of different shapes and sizes (McCloskey, 1994; Gardner, Martin, & Jessell, 2000).
Joint receptors are located in the superficial and deep layers of the joint capsules and in the ligaments. Four types of receptors exist, classified as I, II, III, and IV. The first three types are encapsulated mechanoreceptors that have a proprioceptive function. The fourth type consists of unmyelinated free nerve endings. The receptors classified as I, II, or III provide information about such activities as the direction, velocity, and initiation of joint movements. They do this by responding to tension applied to the connective tissue surrounding them. The group IV free nerve endings, which mediate nociception and normally are silent, respond to potentially injurious mechanical or inflammatory processes (Wyke, 1985).
Neuromuscular spindles are complex encapsulated proprioceptors that monitor muscle fiber length (see Fig. 9-24). They are located within a skeletal muscle close to the tendon and are surrounded by muscle fibers. They provide the sensory arc of the stretch, or myotatic, reflex. Each spindle consists of a connective tissue capsule that is fixed at each end to adjacent muscle fibers. The capsule encloses specialized muscle fibers called intrafusal fibers, which are either nuclear bag fibers or nuclear chain fibers. The total and individual numbers of nuclear bag and chain fibers vary among spindles. The innervation of the nuclear bag and chain fibers of the spindle is provided by group Ia and II sensory fibers. In addition, the spindle is a unique peripheral receptor in that it also has a motor innervation furnished by gamma motor neurons. The motor innervation of the intrafusal fibers allows the spindle to remain sensitive to muscle fiber length during muscle contraction. The section Spinal Motor Neurons and Motor Coordination describes the function of the muscle spindle and stretch reflex.
Golgi tendon organs (GTOs), or neurotendinous spindles, respond to tension that is applied to a tendon. They consist of tendon collagen fibers surrounded by connective tissue, with group Ib afferent fibers twisted among the collagen fibers in such a way that they may become “squeezed” under the appropriate amount of tension. On stimulation, the group Ib afferent fiber stimulates a Ib interneuron. This interneuron then inhibits the alpha motor neuron that supplies the skeletal muscle associated with that stimulated GTO. This action is opposite that of a stimulated neuromuscular spindle, which produces excitation of the alpha motor neuron supplying the skeletal muscle associated with that spindle. The GTOs and spindles not only function at the spinal level, but also send input to higher centers (see Ascending Tracts). The section Spinal Motor Neurons and Motor Coordination describes the functional relationship between muscle spindles and GTOs.
Visceral Receptors
These receptors also may be classified as interoceptors because of their location. They include mechanoreceptors that respond to movement or distention of the viscera. These are found in locations such as the mesentery, connective tissue enclosing the organs, and along blood vessels. Nociceptors are found in the viscera, as well. These are capable of responding to noxious mechanical, thermal, and chemical stimuli (Willis & Coggeshall, 1991). Chapter 10 discusses the relationship of these receptors and their afferent fibers to the somatic and autonomic nervous systems.
Peripheral Nerves
The spinal cord receives impulses from receptors and sends output to effectors via the PNS. Because the PNS transmits this essential information, its components are considered in this section.
Thirty-one pairs of spinal nerves exist, and each is formed by the convergence of a dorsal root and a ventral root usually within the intervertebral foramen (IVF). Just distal to this union, each spinal nerve divides into a dorsal ramus (posterior primary division, PPD) and a ventral ramus (anterior primary division, APD) (see Fig. 3-3). The dorsal rami of spinal nerves innervate the zygapophysial joints, skin over the back, and deepest muscles of the neck and back. The ventral rami innervate the extremities and the ventrolateral aspect of the wall of the trunk. Successive thoracic ventral rami retain a clear segmental distribution along the thoracic region. However, the back of the head, the anterior and lateral neck, the shoulders, and upper and lower extremities are innervated by plexuses. Each plexus is formed by a regrouping of adjacent ventral rami. The plexuses are called the cervical, brachial, and lumbosacral plexuses, and each is briefly described here.
The cervical plexus is formed by ventral rami of the C1 through C4 cervical nerves. It supplies cutaneous innervation to the dorsolateral part of the head, neck, and shoulder. Motor fibers in this plexus course to the deep cervical muscles, hyoid muscles, diaphragm, and sternocleidomastoid and trapezius muscles (see Chapter 5).
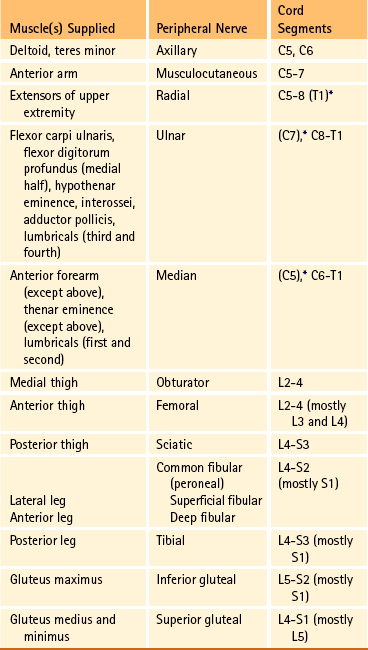
The brachial plexus is formed by ventral rami of the C5 through T1 spinal nerves (often with a contribution from C4 and T2). This plexus supplies the upper extremity. (See Chapter 5 for a full description of the brachial plexus and its proximal branches.) Subsequent to the mixing of the ventral rami in the plexus, numerous branches are formed, including five large terminal branches: the axillary, musculocutaneous, radial, ulnar, and median nerves. The axillary nerve (C5 to C6) supplies cutaneous branches to the deltoid region and muscular branches to the deltoid and teres minor muscles. The musculocutaneous nerve (C5 to C7) provides sensory innervation to the anterolateral and posterolateral aspect of the forearm. It supplies motor innervation to the flexors of the arm, which includes the biceps brachii. The radial nerve (C5 to C8, possibly T1) has an extensive area of distribution in both the arm and the forearm. Its cutaneous branches innervate the posterior aspect of the arm and forearm. The superficial radial nerve supplies the skin of the lateral half of the dorsum of the hand and the first three and a half digits, excluding the nails. The radial nerve also innervates the extensor muscles of the upper extremity. The ulnar nerve (C8 and T1, sometimes C7) courses through the arm to supply structures in the forearm and hand. Its motor distribution includes one and a half forearm flexors (ulnar side) and intrinsic hand muscles, including the hypothenar and all interossei muscles and some thenar muscles. Its cutaneous distribution is present only in the hand and encompasses the ulnar half of the hand, including the fifth and medial half of the fourth digits. The median nerve (C6 to C8 and T1, sometimes C5) supplies motor fibers to the forearm flexors (excluding those with ulnar innervation) and some intrinsic hand muscles, including most of the thenar muscles. As with the ulnar nerve, its sensory area of distribution is only in the hand and includes the lateral palmar surface and the first three and a half digits, including the nails (Standring et al., 2008) (Figs. 9-2 and 9-3 and Table 9-2).
Table 9-2
Muscles Supplied by Terminal Branches of the Brachial Plexus and the Lumbosacral Plexus
∗This level represents clinically significant contributions in a small portion of the population.
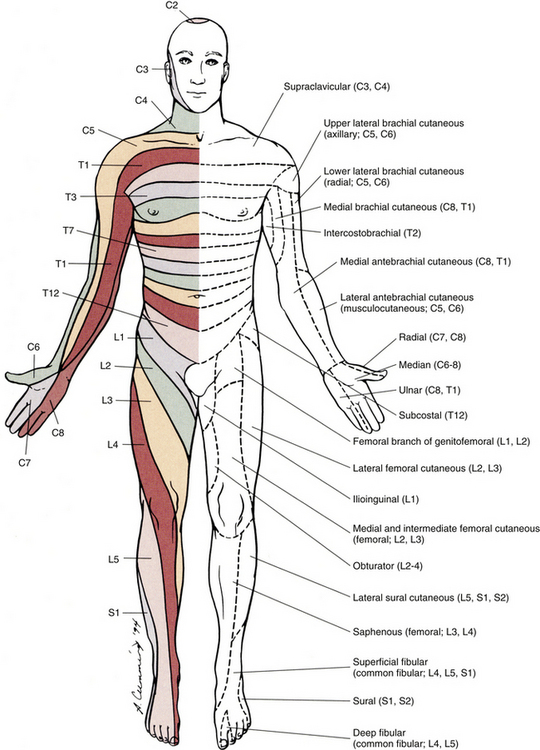
FIG. 9-2 Anterior view of the body showing its cutaneous innervation. Left, Dermatomal pattern, which may vary according to different authors. This dermatomal mapping is based on studies by JG Keegan and FV Garrett (1948). Right, Areas of cutaneous peripheral nerve distributions. Note the similarity of cord segment origins between the two sides.
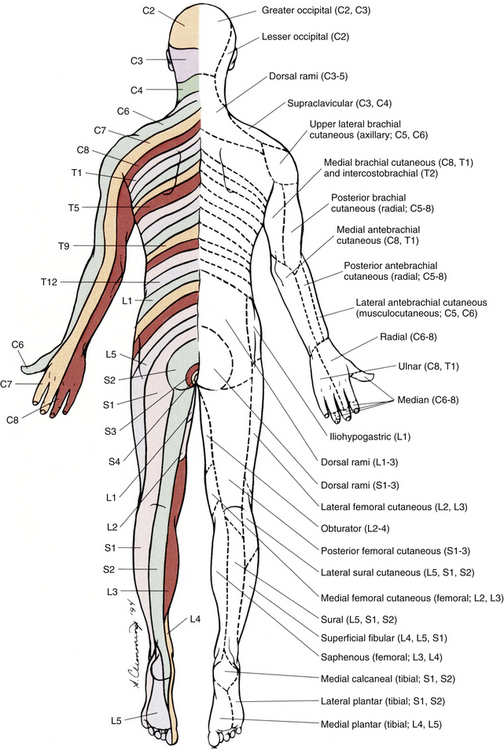
FIG. 9-3 Posterior view of the body showing its cutaneous innervation. Left, Dermatomal pattern, which may vary according to different authors. This dermatomal mapping is based on studies by JG Keegan and FV Garrett (1948). Right, Areas of cutaneous peripheral nerve distributions. Note the similarity of cord segment origins between the two sides.
The lumbosacral plexus (sometimes described as a lumbar plexus and a sacral plexus) is the third plexus, which is composed of ventral rami L2 through S2 (with contributions from L1 and S3). The major branches of this network are the femoral, obturator, gluteal, sciatic, common fibular (peroneal) (and its branches), and tibial nerves. Cutaneous nerves with large areas of distribution include, but are not limited to, the lateral femoral cutaneous, saphenous, posterior femoral cutaneous, and sural nerves. (Chapters 7 and 8 describe the lumbar plexus and its smaller branches [iliohypogastric and ilioinguinal] and the sacral plexus and its branches.)
The obturator nerve (L2 to L4) supplies motor branches to the adductor muscles of the thigh and gracilis muscle. Also it provides cutaneous branches to the inner thigh. The femoral nerve (L2 to L4) sends motor branches to the anterior thigh muscles (e.g., quadriceps), which extend the leg. Cutaneous innervation by the femoral nerve supplies the anterior and anteromedial thigh and, via the saphenous nerve (L3 and L4), the medial leg and foot. The thigh’s lateral side is innervated by cutaneous branches of the lateral femoral cutaneous nerve (L2 and L3). The sciatic nerve (L4 to S3) is the body’s largest nerve. This nerve actually is composed of two parts (tibial and common fibular) but usually is ensheathed to form one nerve in the posterior thigh. Motor branches in the posterior thigh innervate the hamstring muscles (biceps femoris, semitendinosus, semimembranosus), which flex the leg. The cutaneous innervation of the posterior thigh is furnished by the posterior femoral cutaneous nerve (S1 to S3). The sciatic nerve divides into the common fibular (peroneal) and tibial nerves at varying levels proximal to the knee.
The common fibular (peroneal) nerve (L4, L5, S1, and S2) courses laterally around the neck of the fibula and divides into two major branches: superficial fibular (peroneal) and deep fibular (peroneal). The superficial fibular (peroneal) nerve supplies muscular branches to the fibularis (peronei) muscles, which are responsible for eversion of the foot, and cutaneous branches to the distal and anterolateral third of the leg and dorsum of the foot (excluding the first digital interspace). The deep fibular (peroneal) nerve sends motor fibers to the anterior leg muscles, which provide dorsiflexion of the foot and extension of the toes. Cutaneous branches supply the skin between the first two toes. The other branch of the sciatic nerve is the tibial nerve (L4, L5, S1, S2, and S3). This nerve provides motor innervation to posterior leg muscles (including the gastrocnemius muscle), which are responsible for plantar flexion of the foot. A branch of the tibial nerve and contributing fibers from the common fibular (peroneal) nerve form the sural nerve. This supplies sensory innervation to the posterior and lateral surfaces of the leg. The tibial nerve divides into medial and lateral plantar nerves at the region of the medial malleolus. These nerves supply motor and sensory innervation to the plantar aspect of the foot (see Figs. 9-2 and 9-3 and Table 9-2).
The inferior and superior gluteal nerves innervate muscles moving the hip joint. The inferior gluteal nerve (L5, S1, and S2) is responsible for the motor innervation of the strongest hip extensor, the gluteus maximus. The superior gluteal nerve (L4, L5, and S1) innervates the gluteus medius and minimus muscles and the tensor fascia latae muscle, which are responsible for hip abduction (see Table 9-2).
This has been a cursory description of the innervation of major individual muscles and muscle groups and the cutaneous distribution of the major nerves. Because of developmental events, one muscle is innervated by multiple cord segments, and one cord segment may be involved with the innervation of more than one muscle. The intermingling of dorsal root fibers in the plexuses produces a peripheral nerve with an area of distribution that is different from a dermatomal pattern—that is, a pattern indicating the cutaneous area supplied by one dorsal root and its ganglion. However, the origin (cord segments) of a peripheral nerve innervating a particular cutaneous region includes the same cord segments as those supplying the dermatomes of that same area. For example, the lateral femoral cutaneous nerve is formed from cord segments L2 and L3, and its peripheral nerve pattern includes parts of the L2 and L3 dermatomal regions. There is much variation in the dermatome maps that are found in textbooks. Although most maps stem from two major references, Foerster (1933) and/or Keegan and Garrett (1948), other studies have also resulted in mapped dermatomes as well. The variability in dermatomes may be due to two or more dorsal roots innervating an area of the skin. Another possibility may be that the intrathecal intersegmental anastomoses that can occur between dorsal rootlets allow a sensory fiber from one dorsal root ganglion to enter the cord at a different level. A detailed description of these studies as well as a new and unique evidence-based dermatome map has been presented by Lee and colleagues (2008).
Knowing a cutaneous nerve’s distribution and its related dermatomal pattern (variable as it may be) as well as both the segmental and the peripheral innervation of major skeletal muscles is very important (see Figs. 9-2 and 9-3). Knowledge of the innervation of muscles and skin at both the PNS and CNS levels is imperative, because a neurologic examination includes the assessment of a patient’s motor functions (reflexes and muscle strength; Table 9-3) and sensory functions. The information gained from this assessment is useful for distinguishing if the lesion is in the CNS or PNS and subsequently for determining the specific location of the lesion along one of these two systems.
Table 9-3
Muscle Testing and Deep Tendon (Muscle Stretch) Reflexes
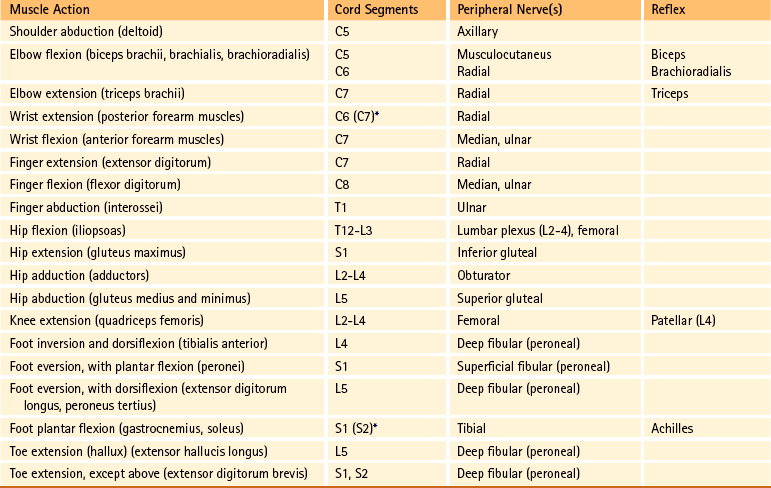
∗This level represents clinically significant contributions in a small portion of the population.
Internal Organization of the Spinal Cord
The gray matter of the spinal cord appears in cross section as an H- or butterfly-shaped region. Each of the two symmetric halves consists of a dorsal horn, which includes a head, neck, and base; an intermediate region; and a ventral horn. An additional small lateral horn located between the dorsal and ventral horns is present in cord segments T1 through L2 or L3. In general, the dorsal horn is a receptacle for sensory afferent input, and the ventral horn is involved in motor functions, including housing the cell bodies of motor neurons. Microscopically the gray matter is a dense region of neuron cell bodies, cell processes and their synapses, neuroglia cells, and capillaries.
The multipolar neurons (see Chapter 14, Neural Tissue) that compose the gray matter are subdivided into four groups: motor neurons, the axons of which leave the spinal cord and innervate the effector tissues (skeletal, smooth, and cardiac muscles; glands); tract neurons, the axons of which ascend in the white matter to higher centers; interneurons, which have short processes; and propriospinal neurons, the axons of which provide communication between cord segments. Propriospinal neurons are classified as long, intermediate, or short. Long propriospinal neurons extend the length of the cord in the ventral funiculus and ventral part of the lateral funiculus (see White Matter). Descending propriospinal fibers (axons) course bilaterally and the ascending axons project mostly contralaterally. Short propriospinal neurons extend up to six to eight segments in the ipsilateral lateral funiculus, and intermediate propriospinal neurons course primarily ipsilaterally more than eight segments but less than the cord’s entire length. Most of the propriospinal fibers are located immediately adjacent to the gray matter in the fasciculus proprius, but some travel more laterally in the funiculi. The axons of medial propriospinal neurons are long and include extensive branches. For example, some extend the length of the cord to coordinate the movements of neck and pelvic axial musculature for postural corrections. Laterally placed propriospinal fibers influence neurons innervating more distal muscles, communicate with a smaller number of segments, and branch less extensively. Because the distal musculature is less connected to other muscle groups, these muscles function more independently. This independence permits more diverse somatic motor activities (Ghez & Krakauer, 2000; Standring et al., 2008). Through their communication with neurons in other cord segments, propriospinal neurons are involved not only with the coordination of somatic motor activity, but also with the autonomic innervation of sweat glands, smooth muscle of the vasculature, and viscera such as the bladder and bowel (Standring et al., 2008).
In the early 1950s, Rexed (1952) studied feline spinal cords and proposed that the organization of the gray matter formed 10 layers, or laminae. He described lamina I as being located at the tip of the dorsal horn, followed sequentially into the ventral horn by laminae II through IX. Lamina X formed the connecting crossbar of the gray matter, that is, the gray commissure. This organization has been accepted for the human spinal cord as well (Fig. 9-4). Each lamina includes at least one of the four general types of neurons: motor, tract, interneuron, or propriospinal. Each lamina also may be the site of the termination of primary afferents, descending tracts, propriospinal neurons, and interneurons of neighboring laminae. The laminae may vary in size throughout regions of the spinal cord and even may be absent in some regions. Also within each lamina, neurons may be organized into smaller groups, called nuclei or cell columns, based on commonalities such as cell morphology and function. The following is a brief description of each of these laminae.
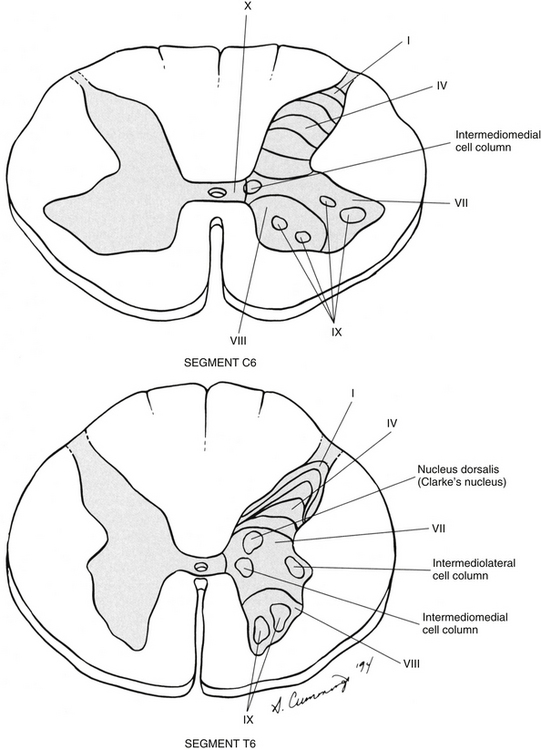
FIG. 9-4 Cross sections of the C6 and T6 spinal cord segments showing the lamination of the gray matter. Lamina VI is often diminished or absent in thoracic cord levels. Examples of nuclei located within the laminae are shown.
Laminae I through VI (Dorsal Horn)
The dorsal horn consists of laminae I through VI. Laminae I through IV, which form the head of the dorsal horn, are the main termination sites of cutaneous afferent fibers and their collateral branches. Lamina V forms the neck, and receives thinly myelinated fibers from the skin, muscle, and viscera. Lamina VI forms the base of the dorsal horn and receives proprioceptive as well as some cutaneous fibers (Standring et al., 2008). Laminae I and II are collectively known as the superficial dorsal horn and are heavily involved with the processing of nociception. The majority of A-delta and C fibers terminate here. Lamina I is also known as the marginal zone of Waldeyer. It is a very thin layer at the dorsolateral tip of the dorsal horn and includes neuron cell bodies that intermingle with thin and thick fibers, a characteristic reflected by its reticular appearance. Most of the primary afferent input into lamina I originates from cutaneous nociceptors and thermoreceptors via A-delta fibers. Additional input is conveyed by C fibers (from nociceptors [peptidergic fibers], thermoreceptors, and histamine-sensitive receptors conveying the sensation of “itch” [Schmelz et al., 1997; Andrew & Craig, 2001; Craig, Zhang, & Blomqvist, 2002]) and by a small group of thinly myelinated muscle, joint, and visceral afferent fibers (Willis & Coggeshall, 1991; Standring et al., 2008). Many neurons within lamina I are considered to be nociceptive specific, whereas others are classified as wide-dynamic-range neurons that respond to both noxious and innocuous stimuli. Thermoreceptive-specific neurons also terminate in lamina I (Han, Zhang, & Craig, 1998) along with numerous interneurons. Tract (projection) neurons originate in lamina I as well, and provide the major output for the superficial dorsal horn. They are the targets of both interneurons and primary afferent neurons. The majority of the axons from these neurons cross the midline although some project bilaterally. Tract neurons are a heterogeneous population and attempts have been made to classify them. In rat studies, data show that 80% of the tract neurons express the main receptor to which substance P binds (neurokinin 1 receptor [NK1R]). Neurons that express NK1R are both large and small, and among those that lack the NK1R is a population that has very large multipolar cell bodies. The dendrites of tract neurons remain in lamina I and based on their morphology these tract neurons may be described as fusiform, pyramidal, or multipolar. Studies using anterograde tracing methods suggest that tract neuron axons terminate in numerous locations within brain stem nuclei (caudal ventrolateral medulla, nucleus of the solitary tract, lateral parabrachial area, and periaqueductal gray) and thalamic nuclei (ventral posterior lateral and posterior). There is widespread collateral branching and some axons may project to at least three locations although evidence suggests that certain neurons preferentially terminate in specific targets. Based on these termination sites, it appears that these tract neurons are involved with numerous functions pertaining to the autonomic nervous system and aspects of cortical nociception processing, including motivation and affect and the sensory-discriminatory component of pain perception (Todd, 2010).
Lamina II is known as the substantia gelatinosa of Rolando. The many processes, the presence of small neurons, and the absence of myelinated axons give this layer a gelatinous appearance on close inspection. It is divided into an outer (IIo) and an inner (IIi) layer, the latter showing a lower density of neurons. The primary afferent input into lamina IIo enters by C afferent fibers from thermoreceptors and peptidergic nociceptive C fibers from various tissues and deep regions of the skin. A few A-delta fibers also terminate here. Nonpeptidergic nociceptive C fibers convey input from the epidermis of the skin and terminate in a narrow band within the central part of lamina II. Afferent input from hair follicles (both A-beta and A-delta fibers) and from tactile afferents (A-beta fibers) terminates in the more ventral lamina IIi (Todd, 2010). The neurons of lamina II are almost entirely interneurons (both excitatory and inhibitory), the dendrites of which arborize within the lamina and also project into other laminae. Some interneurons respond to noxious stimuli, whereas others respond to both noxious and innocuous stimuli (Basbaum & Jessell, 2000).
Laminae III and IV are similar and are described together. These laminae (and sometimes the upper part of lamina V) often are called the nucleus proprius. The majority of the primary afferent input arrives via A-beta fibers, which transmit innocuous input from mechanoreceptors such as pacinian corpuscles, peritrichial endings surrounding hair follicles, and Meissner’s corpuscles. Although direct afferent input synapses on the interneurons within laminae III and IV, the dendrites of these interneurons also project dorsally into lamina II. Some lamina II neurons also project axons ventrally into these laminae and thus influence laminae III and IV neurons and their sensory input. Thus considerable interlaminar communication occurs. The types of neurons present in these laminae include interneurons and some tract neurons.
Lamina V forms the neck of the dorsal horn. It is a thick layer that is interlaced by bundles of fibers. Primary afferent input comes via A-delta fibers from cutaneous mechanical nociceptors and group III and IV muscle and joint afferents, and nociceptive visceral afferents (Willis & Coggeshall, 1991; Basbaum & Jessell, 2000). Additional input to this lamina (such as input from C fibers) is most likely received via the dendritic projections located within more dorsal laminae. Many neurons within this lamina are wide-dynamic-range tract neurons that are the site of viscerosomatic convergence for visceral referred pain (Benarroch et al., 1999), whereas others are interneurons and propriospinal neurons. Cortical and subcortical fibers terminate in lamina V as well, indicating its involvement in motor function.
Lamina VI is the base of the dorsal horn and is anatomically close to motor regions in the ventral horn, suggesting it is involved with regulation of movement. This lamina is diminished and sometimes absent in segments other than those forming the cervical and lumbosacral enlargements in which it is prominent. Proprioceptive information enters via large-diameter afferent fibers such as the group Ia and A-beta fibers. These fibers form the primary afferent input to this lamina. In addition, many cortical and subcortical descending tracts terminate in this area. Numerous interneurons and propriospinal neurons are present in this lamina also.
In summary, the first six laminae that comprise the dorsal horn are the major receiving areas for sensory information. Pain and temperature input appears to terminate primarily in superficial layers; mechanical types of stimuli terminate in the middle region; and proprioceptive input ends in the base of the dorsal horn near the motor regions. Each primary afferent fiber has many collateral branches that synapse in the circuitry of more than one lamina and feed into more than one ascending pathway. For example, one Ia afferent fiber from a neuromuscular spindle may have 500 or more branches terminating within the gray matter of the cord (Nolte, 2002). Many of the laminae communicate with each other via profuse dendritic branching and the axonal projections of their interneurons. This provides a mechanism by which incoming sensory signals may be processed and modified (modulated) before ascending to higher centers. This modulation occurs at the level of the synapse between presynaptic and postsynaptic neurons and involves not only the release of neurotransmitters and neuromodulators but also the presence of the different receptors with which these chemicals bind. This is exemplified by studies of the nociceptive interneurons in lamina II that show that numerous chemicals can modulate synaptic transmission.
Dorsal horn interneurons: Interneurons in the dorsal horn play a key role in modifying sensory information and ongoing movements, as well as controlling all reflexive movements. They form the neuronal circuitry that links sensory fibers with the tract neurons that provide the major output of the dorsal horn. Numerous studies have described the interneurons located in the superficial dorsal horn (Todd, 2010). There are two classes of interneurons: excitatory, which utilize the neurotransmitter glutamine; and inhibitory, which use gamma-aminobutyric acid (GABA) and/or glycine as their neurotransmitter. Since most primary afferent terminals exhibit axoaxonic synapses, it has been suggested that one function of GABAergic interneurons is to provide presynaptic inhibitory input to afferent fibers. Interneurons in lamina II have been classified as either islet, central, vertical, or radial on the basis of their dendritic morphology. Studies using immunocytochemistry methods indicate that the islet cells are GABAergic, radial cells are glutaminergic, vertical cells are mostly GABAergic but can be glutaminergic and central cells which can be either type. An additional 30% have yet to be classified. Lamina I interneurons have been described as pyramidal, fusiform, or multipolar. However, projection neurons are present in this lamina and may have been inadvertently classified as part of the population because they have not been distinguished from the interneurons. The extensive branching of the afferent inputs to the spinal cord results in simultaneous activation of many types of interneurons. The activity of these interneurons and their subsequent synapses on cells in the ventral horn (motor systems), ascending tracts (sensory perception of stimuli), descending tracts (sensitivity of motor and sensory pools), and other interneurons in the spinal cord controls the overall sensitivity and general responsiveness of all neurons in the spinal cord. The balance between the firing of excitatory and inhibitory interneurons is critical in the maintenance of normal sensory function. Disruptions in the transmission from interneuronal circuits can result in allodynia (see Appendix II) or the development and maintenance of inflammation and neuropathic pain (Todd, 2010). In addition to the input from afferent fibers, the spinal interneurons receive input from other sources including descending fibers from higher centers. The superficial dorsal horn receives input from serotonergic fibers, noradrenergic fibers, and GABAergic fibers, all of which originate in different brain stem nuclei (Todd, 2010). Additional sources that influence spinal interneurons include ventral horn alpha motor neurons, local (at the level) interneurons, short propriospinal neurons (from one or two levels above or below), and long propriospinal neurons (ascending and descending between all levels of the cord). This vast array of interconnections among and between the various interneurons also contributes to the general responsiveness of the spinal cord to both afferent (sensory or reflexive) and efferent (motor or voluntary) stimuli. The interconnections between the interneurons are mutually inhibitory, meaning that only one set of pathways can be active at any time. When one particular response pathway and its corresponding interneurons are activated, all other interneuronal pathways typically are inhibited. This allows the spinal cord to respond selectively to any input, either afferent or efferent, without inappropriately activating other antagonistic pathways that might hinder the appropriate response. In addition, interneurons can modulate or alter the normal output of higher-order neurons. One example of this is related to the gate control theory of pain (see Fig. 11-6), in which nonnociceptive afferents reduce the output of second-order pain fibers via inhibitory interneurons in the dorsal horn. Therefore interneurons are the key building blocks of all spinal reflexes, and can control or modulate most afferent and efferent information at the level of the spinal cord (see the Spinal Motor Neurons and Motor Coordination section later in this chapter).
Relationship between the dorsal horn and trigeminal nerve: The dorsal horn of the upper three or four cervical cord segments has an interesting relationship with the trigeminal nerve. The trigeminal nerve (cranial nerve V) provides sensory innervation to the skin of the face and other structures, including the paranasal sinuses, cornea, temporomandibular joint, and oral and nasal mucosae. The trigeminal nerve also supplies motor fibers to the muscles of mastication and several other small muscles of the head. The afferent fibers of the trigeminal nerve enter the brain stem at the level of the pons and synapse in a nuclear column that extends from the midbrain through the pons and medulla and into the upper cervical cord segments. The portion of the nuclear column located in the medulla and upper cervical cord segments is known as the spinal trigeminal nucleus (Fig. 9-5). Afferent fibers conveying pain and temperature (and some touch) that enter the brain stem within the trigeminal nerve (and also in the facial and glossopharyngeal nerves) descend as the spinal trigeminal tract and synapse in this nucleus. The most inferior portion of the spinal trigeminal nucleus is the nucleus caudalis. This nuclear region continues from the caudal medulla into the upper three or four cervical cord segments and blends with laminae of the dorsal horn of those segments (Carpenter, 1991; Standring et al., 2008). This area of gray matter that is the site of convergence of trigeminal and cervical afferents is also called the trigeminocervical (Bogduk, 1992; Biondi, 2001) or trigeminospinal nucleus. The descending spinal trigeminal tract fibers also continue into the dorsolateral tract of Lissauer in the upper cervical segments. Afferent fibers conveying similar information and traveling in dorsal roots of the upper three or four cervical nerves also synapse in these same laminae. In fact, some of these cervical dorsal root afferents may ascend into the rostral medulla and synapse in the spinal trigeminal nucleus (Abrahams, 1989).
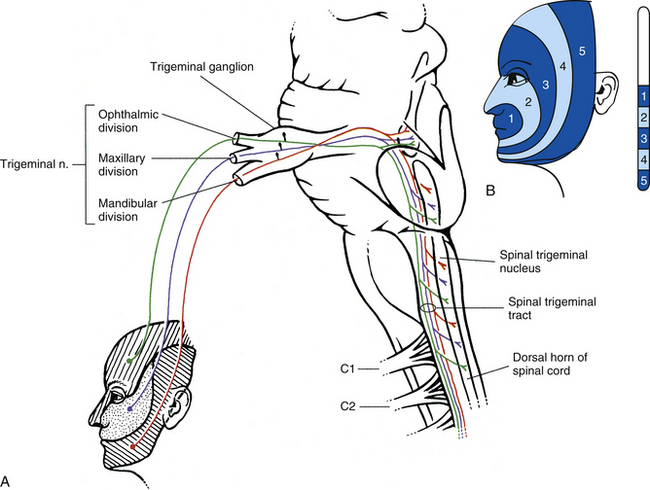
FIG. 9-5 Afferent portion of the trigeminal system. A, The trigeminal nerve fibers conducting pain and temperature enter the pons of the brain stem and descend in the medulla (as the spinal trigeminal tract) and into the upper three or four cervical cord segments. They synapse in the adjacent spinal trigeminal nucleus of the medulla and dorsal horn of the upper cervical cord segments. This provides the anatomic substrate for pain referral (see text). Note that the descending fibers are arranged within the medulla such that the ophthalmic fibers are ventral, the mandibular fibers are dorsal, and the maxillary fibers are in between. B, Representation of trigeminal afferents in the nucleus caudalis of the spinal trigeminal nucleus. Note the “onion skin” pattern created by the five zones and that the most posterior zone (5) is represented in the most caudal part of the nucleus (approximately at the C3 cord level). The rostral portion of the nucleus (white area) receives afferents from the oral cavity and teeth.
The relationship between the dorsal horn and trigeminal system is clinically significant (Pollman, Keidel, & Pfaffenrath, 1997; Biondi, 2000, 2001; Bogduk, 2001; Bogduk & Govind, 2009). The upper three cervical spinal nerves innervate the muscles (including the trapezius and sternocleidomastoid muscles), ligaments, and joints such as the zygapophysial joints in the region of the upper three cervical vertebral segments, C2-3 intervertebral discs, vertebral and internal carotid arteries, and dura mater of the upper spinal cord and posterior cranial fossa of the skull. Second-order neurons in the dorsal horn, which project to the brain, receive nociceptive afferents traveling in the C1 to C3 spinal nerves from these structures and from afferents coursing in adjacent cervical spinal nerves. Afferents from lower cervical spinal nerves do not appear to have a direct anatomic relationship with these second-order neurons (Bogduk & Govind, 2009). Because of the convergence of cervical afferents from the occiput with cervical spinal afferents in the dorsal horn, a cervical-cervical pain referral pattern may occur. In this case nociceptive input from upper cervical pain generators is perceived in the region of the head that is innervated by cervical nerves (e.g., external occiput or the posterior cranial fossa [Bogduk, 2004]). In addition, second-order neurons receive input from pain generators innervated by the trigeminal nerve primary afferents. When the brain receives this nociceptive information from these spinal pain generators, it can misinterpret the input as coming from the more familiar source, resulting in a cervical-trigeminal pain referral pattern (i.e., the perception that the origin is in the territory of the trigeminal nerve). Thus this region of convergence becomes the anatomic basis for pain referral from the neck to regions innervated by the trigeminal nerve (typically the forehead, parietal region, or orbital region), and vice versa (sometimes called the trigeminocervical reflex [Lance, 1989; Pollmann, Keidel, & Pfaffenrath, 1997; Browne et al., 1998; Sessle, 1998; Milanov & Bogdanova, 2003]). Head pain or headache that is the result of stimulation of pain generators located in the cervical spine is called cervicogenic headache.
Laminae VII through X
Lamina VII composes most of the intermediate region of the gray matter and also extends into the ventral horn (see Fig. 9-4). The shape of lamina VII varies in different regions. For example, in the T1 to L2 or L3 segments, lamina VII includes the lateral horn. Most primary afferent input into this lamina (excluding the lateral horn) and the remaining ventral horn concerns proprioception, although some neurons receive noxious stimuli via polysynaptic connections (Basbaum & Jessell, 2000). The lateral part of lamina VII (excluding the lateral horn) is involved with the regulation of posture and movement. These neurons, including tract neurons, have numerous ascending (tract neurons) and descending connections with the cerebellum and midbrain. The medial part of lamina VII includes interneurons and propriospinal neurons that connect adjacent laminae and cord segments for reflexes concerned with movement and autonomic activities. In the ventral part of the lamina, inhibitory interneurons, such as Renshaw cells and Ia inhibitory interneurons, have been identified (Standring et al., 2008). Clearly defined nuclei and cell columns also are within lamina VII. One of these is the nucleus dorsalis of Clarke (nucleus thoracicus). This oval nucleus consists of tract neurons and is located in the medial part of lamina VII in segments C8 through L3. It is best defined in the T10 to L2 segments (Carpenter & Sutin, 1983). The axons of these neurons ascend ipsilaterally in the spinal cord white matter to the cerebellum as the dorsal spinocerebellar tract (see Ascending Tracts). A cluster of four nuclei that is associated with the innervation of autonomic effectors also is located in lamina VII (see Chapter 10). The largest of the four is the intermediolateral cell column, which is located in the lateral horn in cord segments T1 to L2 or L3 and consists of autonomic motor neuron cell bodies. The axons of these motor neurons are preganglionic sympathetic fibers that exit in the ventral root and synapse in autonomic sympathetic ganglia. They are involved with the innervation of smooth and cardiac muscles and glands. The sacral autonomic nucleus is found in cord segments S2 to S4, and although there is no lateral horn at that level, this nucleus is located in a similar position to that of the intermediolateral cell column. The sacral autonomic nucleus contains cell bodies, the axons of which form preganglionic parasympathetic fibers. These fibers exit via the S2 to S4 ventral roots, synapse in autonomic ganglia, and innervate the smooth muscle and glands of the pelvic and lower abdominal regions. An additional group of neurons forms the intermediomedial nucleus of lamina VII. This is found in the medial aspect of this lamina and is considered by some authors to be the termination site of visceral afferent fibers (Carpenter & Sutin, 1983). From this description, it is apparent that lamina VII is composed of all four neuron types: interneurons, tract, propriospinal, and motor neurons.
Lamina VIII is also located in the ventral horn. In spinal cord segments of the cervical and lumbar enlargements, it is found in the medial aspect of the ventral horn. In thoracic segments, lamina VIII is located in the base of the ventral horn. Input to the interneurons and propriospinal neurons of this lamina originates from descending tracts and some proprioceptive afferents. Interneurons from adjacent laminae and the contralateral lamina VIII project here as well. The axons of lamina VIII interneurons excite gamma motor neurons, which in turn influence alpha motor neurons bilaterally via muscle spindle activation. Interneurons may also synapse on alpha motor neurons directly.
Lamina IX is found in the ventral horn and consists of well-defined medial and lateral longitudinal nuclear columns and a central nuclear group, all of which are composed of motor neurons (anterior horn cells) (see Fig. 9-4) (Standring et al., 2008). The position and presence of the groups vary at different spinal regions. The medial group is found throughout the cord although it is sometimes absent in the L5 and S1 segments. In the T1 to L4 cord segments it is divided into dorsomedial and ventromedial groups. In the C1 segment it consists of a dorsomedial group, and in the L5 to C0 segments only the ventromedial component is present. It provides the axons that innervate the epaxial muscle groups (“true” or “deep” back muscles) and hypaxial muscle groups (prevertebral muscles, intercostal and anterior abdominal wall muscles) (see Chapter 12). The lateral group (subdivided into dorsal, ventral, and retrodorsal groups) of the cervical and lumbar enlargements is responsible for innervating the muscles of the upper and lower extremities, respectively. These clusters of neurons are somatotopically organized within the enlargement: neurons innervating proximal limb muscles are found more superiorly than the neurons innervating distal muscles; motor neurons innervating distal (hand or foot) muscles are located dorsally in the ventral horn and just ventral to these are the neurons supplying the intrinsic limb muscles; and neurons innervating the muscles of the shoulder and pelvic girdles are located in the ventrolateral region. In addition, one specific group of ventrolateral neurons located in the S1 and S2 segments forms Onuf’s nucleus. The axons from this nucleus innervate the perineal musculature (Standring et al., 2008). The addition of these lateral motor neuronal groups in cord segments supplying the extremity muscles creates a distinctive lateral enlargement in the ventral horn.
The third nuclear group of lamina IX is the central group. This group includes three nuclei located in specific cord segments. One of these, the lumbosacral nucleus, is located in the L2-S1 cord segments. The projections from this nucleus are unknown. Another nuclear column forms the phrenic nucleus, which is found in the C3 to C5 and even as far caudal as the C7 segments (Standring et al., 2008). The axons of these segments, and in particular C4, form the phrenic nerve, which provides innervation to the diaphragm. Based on cadaveric studies, Routal and Pal (1999) suggest that the phrenic nucleus is not part of a central column but instead actually is a subdivision of the medial column. The diaphragm develops as an axial muscle and therefore the phrenic nucleus is located near the other motor neurons that innervate the axial musculature (i.e., the medial motor column). The location of the nucleus in the cervical cord segments and the location of the nucleus’ efferent fibers also may have clinical significance. As exemplified by two case studies, severe cases of cervical spondylotic myelopathy could traumatize the phrenic nucleus or its efferent fibers, resulting in phrenic paresis (Parke & Whalen, 2001). The other nucleus is the accessory nucleus, which is located at the ventral border of the ventral horn (Standring et al., 2008) and extends from the lower medulla into the C5 cord segment (Routal & Pal, 1999). Axons from this nucleus form the spinal root of the spinal accessory nerve (CN XI). They ascend in the vertebral canal dorsal to the denticulate ligament and travel through the foramen magnum to enter the cranial cavity and briefly join the cranial root of CN XI. Subsequently this nerve exits the cranial cavity, and the spinal root fibers branch away to innervate the sternocleidomastoid and trapezius muscles. This nucleus may be somatotopically organized in a craniocaudal direction and subdivided into two parts, with the upper part of the nucleus projecting to the sternocleidomastoid muscle, and the lower part projecting to the trapezius muscle (Routal & Pal, 1999).
Each of these three major groups of lamina IX includes alpha motor neurons, which project to extrafusal skeletal muscle fibers and are subdivided into tonic (to slow muscle fibers) and phasic (to clusters of fast muscle fibers) neurons; beta motor neurons, which course to extrafusal and intrafusal fibers; and gamma motor neurons, which innervate the contractile portion of the neuromuscular spindles located within skeletal muscles and are subdivided into static and dynamic types (FitzGerald & Folan-Curran, 2002; Standring et al., 2008). The alpha and gamma motor neurons are tightly packed into pools responsible for the innervation of a particular skeletal muscle. Both types of motor neurons receive excitatory and inhibitory input from neighboring interneurons that have formed local reflex circuits, propriospinal neurons, and some descending tract fibers. These connections modulate the activity of the motor neurons. Alpha motor neurons also receive the primary afferent fibers from neuromuscular spindles that form the sensory arc of the stretch (myotatic) reflex (see Spinal Motor Neurons and Motor Coordination). The cell bodies of the alpha motor neurons, which are large and range from 25 to 70 µm in diameter, receive a tremendous amount of synaptic input. An estimated 10,000 excitatory boutons of descending fibers from the brain and propriospinal neurons may synapse on the extensive dendritic tree of a typical alpha motor neuron, and about 5,000 inhibitory propriospinal boutons synapse on the cell bodies of alpha motor neurons (FitzGerald & Folan-Curran, 2002). These connections indicate the extensive level of neuronal integration occurring at this cell. Gamma motor neurons are smaller (15 to 25 µm in diameter) and have a lower threshold to stimuli than alpha motor neurons (Davidoff & Hackman, 1991; Standring et al., 2008). In addition to the motor neurons, lamina IX includes some interneurons and propriospinal neurons (Carpenter & Sutin, 1983; Standring et al., 2008; Kiernan, 2009).
The last lamina to be mentioned is lamina X. This region forms the commissural area between the two halves of the gray matter and surrounds the central canal. It consists of interneurons and some decussating axons. Lamina X receives input from A-delta fibers transmitting information from mechanical nociceptors and group C visceral afferents (Willis & Coggeshall, 1991).
In summary, the gray matter is divided into 10 laminae, each of which may vary in size and shape within the different cord segments (Fig. 9-6). This variation results in a notable difference in the overall amount and shape of gray matter present in the various regions of the spinal cord (see Chapter 3, Regional Characteristics). Also, there is a difference in the appearance and amount of white matter throughout the cord because of the presence or absence of certain tracts at different levels. For example, the amount of white matter is greater in the cervical cord than in other areas because all ascending and descending tracts to and from the brain traverse this region (Fig. 9-6). Table 9-4 summarizes the types of neuron cell bodies found in each lamina, the primary afferent fiber types terminating in each lamina, and the laminae in which descending (motor) fibers from higher centers synapse.
Table 9-4
General Summary of Input to and Neurons in the Laminae of Rexed

∗All parentheses indicate minor contribution.
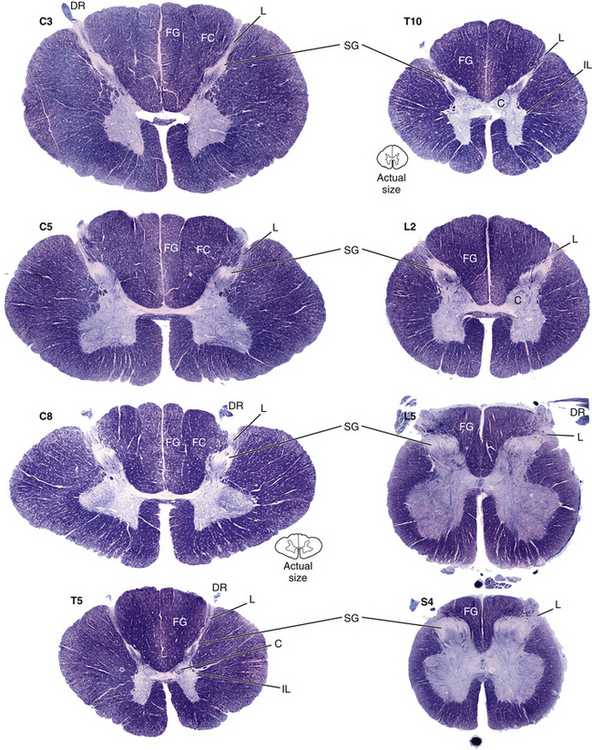
FIG. 9-6 Cross sections of the spinal cord. Note the variation in the overall shapes and the different amounts and appearances of gray matter and white matter present in the various regions of the spinal cord. C, Clarke’s nucleus; DR, dorsal root; FC, fasciculus cuneatus; FG, fasciculus gracilis; IL, intermediolateral cell column; L, Lissauer’s tract; SG, substantia gelatinosa. (From Nolte J. [2002]. The human brain [5th ed.]. St Louis: Mosby.)
Dorsal Root Entry Zone
Peripheral receptors and the spinal gray matter are linked together through sensory afferent fibers. Stimulated receptors transmit their information to the CNS via peripheral processes. These processes may be classified according to their velocity of conduction as a group A or group C cutaneous or visceral fiber or as a group I, II, III, or IV fiber from muscle or joint receptors. Peripheral processes course with efferent motor fibers in peripheral nerves, dorsal and ventral rami, and spinal nerves. They are processes of pseudounipolar neurons, the cell bodies of which form a dorsal root ganglion. This ganglion is located in the IVF. The pseudounipolar neuron also has a central process, which, with many other central processes, forms a dorsal root. Because each peripheral and central process is a component of one neuron, this sensory or afferent neuron does not synapse until it reaches the CNS. As the dorsal root approaches the spinal cord within the vertebral canal, it consists of medial and lateral fascicles, each of which branches into numerous rootlets (Standring et al., 2008). Each rootlet becomes a myriad of fibers conveying various types of sensory information.
As the rootlet fibers enter the dorsal root entry zone, they become arranged into lateral and medial divisions (Fig. 9-7). The lateral division contains thinly myelinated and unmyelinated fibers, which include the nociceptive (pain) and temperature, or A-delta and C, fibers. These fibers first enter an area of white matter located at the tip of the dorsal horn called the dorsolateral tract of Lissauer and then continue into the gray matter of the cord. The dorsolateral tract of Lissauer is found in all cord segments, but it is most developed in the upper cervical segments. Collateral branches of the entering fibers are given off within the dorsolateral tract of Lissauer, some of which ascend or descend a few segments before also synapsing in the gray matter. It has been suggested that visceral afferent fibers may give off collateral branches that span as many as five segments (Chandler, Zhang, & Foreman, 1996; Jänig, 1996). In addition to the ascending and descending branches, the dorsolateral tract of Lissauer also contains fibers from the substantia gelatinosa (lamina II) that interconnect with laminae II of other levels. Other propriospinal fibers also have been identified in the dorsolateral tract (Standring et al., 2008). At all levels, the lateral division primary afferent fibers may synapse on tract neurons in the dorsal horn and intermediate gray, interneurons in the dorsal horn, and interneurons involved with somatic and visceral reflex responses.
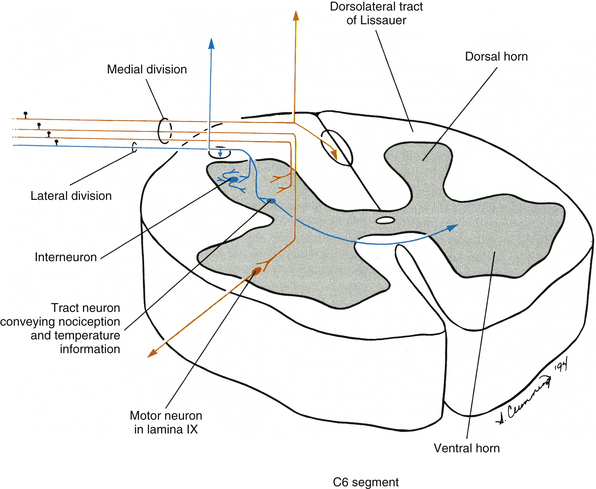
FIG. 9-7 Dorsal root entry zone illustrating fibers in one rootlet. The lateral division fibers use the dorsolateral tract of Lissauer to enter the laminae associated with nociception (pain) and temperature. Collateral branches of these fibers ascend and descend in Lissauer’s tract before they enter the dorsal horn of nearby segments. The medial division fibers, which include large-diameter fibers, enter the cord medial to Lissauer’s tract, where many ascend and descend. Others enter the medial aspect of the gray matter to synapse in various laminae, such as lamina IX of the ventral horn, the location of alpha motor neurons.
The medial division of the dorsal root entry zone contains large- and intermediate-diameter fibers from such receptors as proprioceptors (e.g., neuromuscular spindles) and mechanoreceptors (e.g., Meissner’s and pacinian corpuscles). These fibers enter medial to Lissauer’s tract. Branches of these fibers may ascend directly in the dorsal white column of the spinal cord to the medulla of the brain stem, or give off collateral branches to the dorsal horn and then ascend. In addition, branches may synapse on tract neurons in the intermediate gray (e.g., Clarke’s nucleus in lamina VII) or on interneurons and motor neurons involved with segmental reflexes (e.g., lamina IX and the stretch reflex), or synapse on dorsal horn interneurons involved with pain modulation.
As stated, after entering the cord each of these primary afferent fibers is the origin of many branches that synapse with numerous neurons in various laminae and in cord segments at multiple levels. Consequently, one primary afferent fiber may be involved with many local neuronal circuits and pathways and thus have an impact on a variety of neuronal activities.
When the primary afferent fibers enter the dorsal horn, they synapse in the gray matter and release neurotransmitters. These neurotransmitters include excitatory amino acids and neuropeptides. Many primary afferent fibers appear to release glutamate (or aspartate, or both) as their primary neurotransmitter. Glutamate has been localized in peripheral nerves, dorsal root ganglia, dorsal roots, and the dorsal horn. Excitatory neuropeptides found in the presynaptic terminals of small-diameter primary afferent fibers include substance P and calcitonin gene–related peptide (CGRP). Substance P is synthesized in the cell bodies found in the dorsal root ganglia and is transported (via fast axonal transport) to the terminals of small-diameter nociceptive fibers in the dorsal horn. The fibers’ terminals are prevalent in laminae I and II. CGRP has been identified in the cell bodies found in the dorsal root ganglia (often colocalized along with substance P), in group A-delta and C dorsal root fibers, in Lissauer’s tract, and in the presynaptic terminals of afferent fibers that synapse in dorsal horn laminae. Additional excitatory neuropeptides released by small-diameter afferent fibers, which are less well-known, include somatostatin, cholecystokinin, thyrotropin-releasing hormone, and vasoactive intestinal polypeptide (Willis & Coggeshall, 1991).
In summary, the pathway for sensory information generally can be described as beginning in a peripheral receptor and continuing through peripheral nerves, dorsal or ventral rami, spinal nerves, dorsal roots, and dorsal rootlets. Within each rootlet a fiber travels in either the medial or the lateral division. Once in the spinal cord, the fiber’s future course depends on the type of information it is conveying. Most fibers terminate in various laminae of the gray matter. The next section describes the white matter of the spinal cord and the continuation of sensory information to higher centers.
White Matter, Ascending Tracts, and Descending Tracts
The white matter of the spinal cord is seen in cross section to be a distinct region located peripheral to the gray matter. It contains myelinated and unmyelinated axons ranging in diameter from less than 1 to 10 µm, glial cells, and capillaries. The white matter consists of three regions: a dorsal funiculus (column) between the dorsal horns, a lateral funiculus (column) between each dorsal horn and ventral horn, and a ventral funiculus (column) between the ventral horns. Tracts, or fasciculi, are present within each of these regions (Fig. 9-8). These may be ascending tracts, which convey sensory information to higher centers, or descending tracts, which originate in higher centers and send descending signals to the cord. These descending signals are involved primarily with some type of motor information. Other axons are confined to the spinal cord and interconnect cord segments at various levels. These form the fasciculus proprius, which is an area of white matter located immediately adjacent to the gray matter. It consists of descending and ascending axons of propriospinal neurons. Concerning the ascending and descending tracts associated with higher centers, each tract contains axons that have a common origin, destination, and function. During a neurologic examination, the integrity of certain tracts is tested. Therefore familiarity of the location of these tracts aids the clinician in localizing lesions within the CNS.
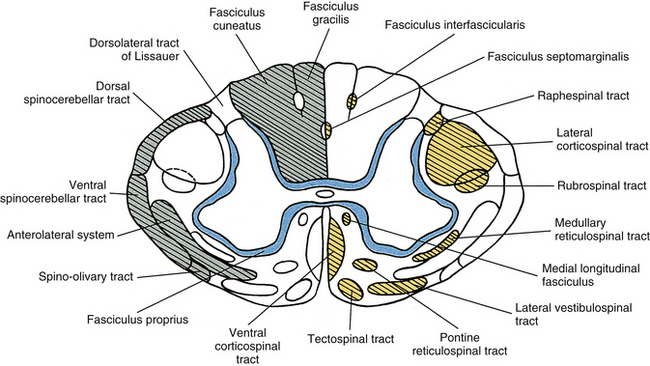
FIG. 9-8 Cross section of the spinal cord illustrating the organization of white matter into fasciculi and tracts. Boundaries usually overlap but are well-defined here for illustrative purposes. Ascending tracts are indicated on the left side (green). Descending tracts are indicated on the right side (yellow); the fasciculus proprius (blue) consists of axons of propriospinal neurons.
Ascending Tracts
In general, an ascending tract can be classified into one of three groups based on the three different functions that are provided by the somatosensory system. One group contains tracts that transmit information concerning the type, location, and intensity of a stimulus. These are considered to be direct or discriminatory tracts. A second group consists of tracts that transmit information about the initiation of reflexes that may be concerned with arousal, affective, and motivational responses to a stimulus. The third group of tracts includes those that transmit information concerning the unconscious monitoring and control of motor activity, such as posture and movement (Benarroch et al., 1999). Ascending tracts convey information that has originated from a stimulated peripheral receptor located in the skin, muscles, tendons, joints, or viscera. When a receptor is stimulated, it transmits an action potential via the peripheral and central processes of sensory (afferent) neurons to the CNS. The sensory fibers that convey the action potential from the peripheral receptors are sometimes called first-order neurons, because they are the first neuron in a chain of neurons that proceeds to a higher center such as the cerebral cortex for conscious awareness of the stimulus, or the cerebellar cortex for regulation of motor patterns (Fig. 9-9). On entering the cord, numerous first-order neurons synapse on neurons in the gray matter of the spinal cord, whereas others, after contributing collateral branches to the gray matter, ascend and synapse in nuclear gray matter in the caudal medulla of the brain stem. The next neuron of the chain that leaves the gray matter of the cord or medulla to ascend to higher centers is known as the second-order neuron. Along with many others, this neuron makes up a specific tract. If sensory information is perceived consciously, the second-order neuron decussates and subsequently synapses with a third-order neuron in the thalamus, which is located in the diencephalon of the brain. The thalamus is an oval-shaped area of gray matter that consists of numerous nuclear subgroups (Fig. 9-10). All sensory information traveling to the cerebral cortex (except olfaction) synapses in one of these thalamic nuclei. From here, the third-order thalamic neuron fibers course in a thick bundle of axons called the internal capsule (which also includes efferent fibers from the cerebral cortex) and terminate in the cortex, thus completing the chain. The thalamus is not simply a relay station through which sensory information is routed to reach its cortical destination. In fact, there is a crude awareness of pain and temperature sensations at this level. In reality, the thalamus functions more like a complex processing center, or gatekeeper. Through the processing within a thalamic nucleus, and in conjunction with excitatory and inhibitory input from other centers (e.g., brain stem nuclei, reticular formation, and cerebral cortex), the thalamus is able to modulate its sensory output to the cerebral cortex based on the immediate needs of the individual (Amaral, 2000).
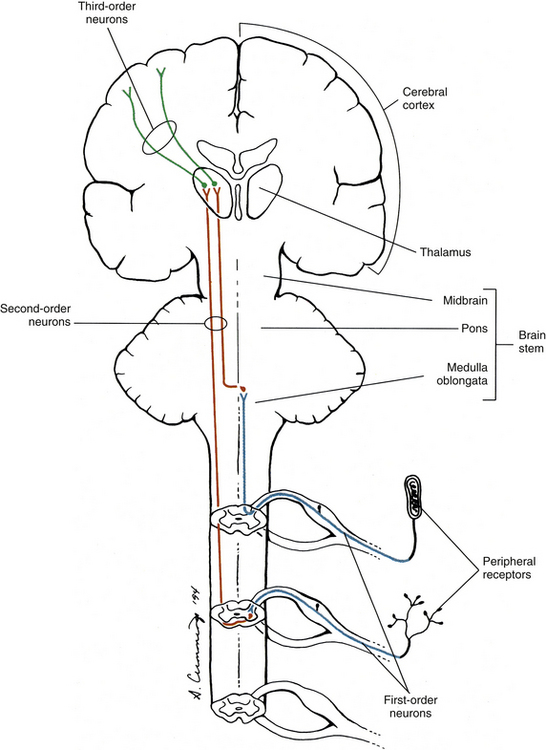
FIG. 9-9 Neuronal organization of ascending information to higher centers such as the cerebral cortex. Note that there are three neurons involved. The first-order neuron (blue) cell body is in the dorsal root ganglion. The second-order neuron cell body is located in either the gray matter of the spinal cord or the medulla of the brain stem. Its axon (red) ascends contralaterally and terminates in the thalamus. The third-order neuron (green) courses to the cerebral cortex.
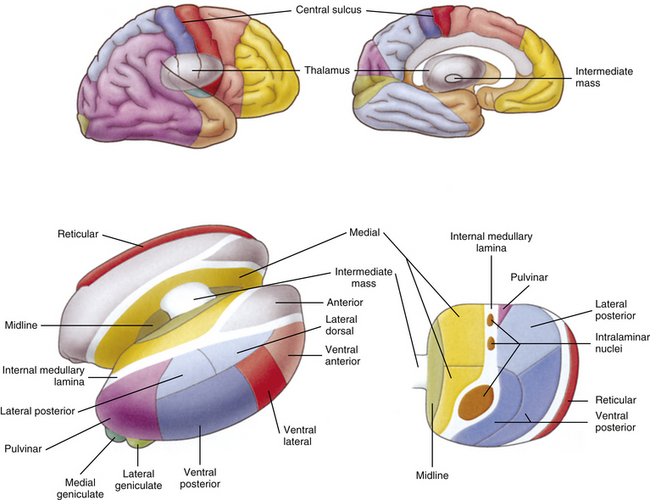
FIG. 9-10 Thalamus. This area of gray matter is located approximately in the center of the brain and is divided into nuclear subgroups. Note the ventral posterior nucleus and intralaminar nuclei, which are the synaptic locations for tract axons conveying general sensory information from the body. Above, areas of the cerebral cortex that receive thalamic projections are color coded to correspond to the thalamic nuclei from which their thalamic projections arise. (From Tortora GJ & Grabowski SR. [2003]. Principles of anatomy and physiology [10th ed.]. New York: John Wiley & Sons.)
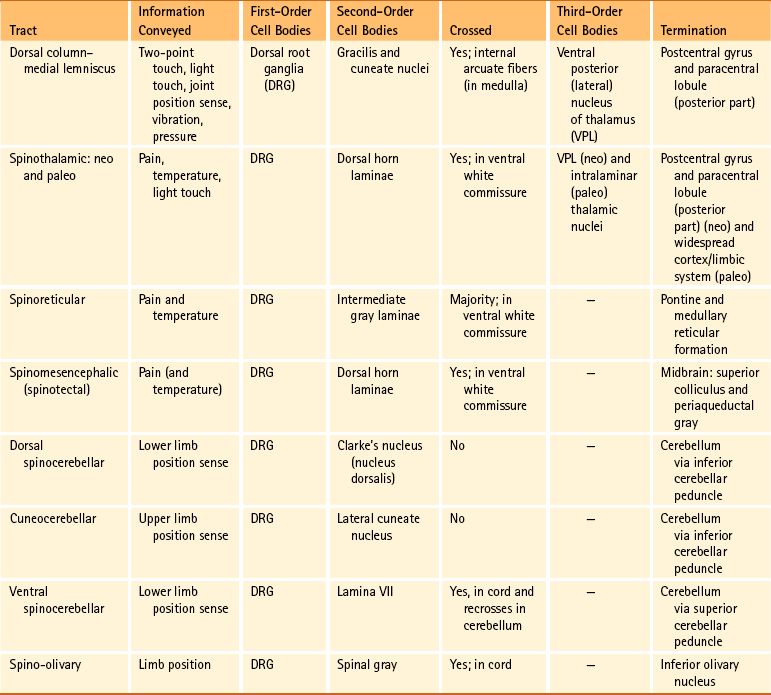
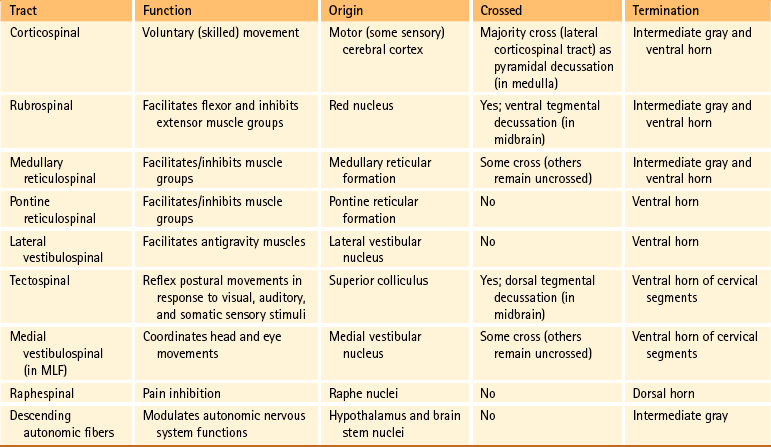
Certain important characteristics should be identified when considering the tracts of the CNS. These include the direction of the tract (i.e., ascending or descending, which usually is indicated by the name), the specific type of information the tract is conveying, and the location where the tract crosses, if applicable. This information is summarized in Tables 9-5 and 9-6. The ascending tracts are discussed first, beginning with the most clinically and anatomically relevant. These major tracts are well-defined, and much information has been gathered about them. Secondary tracts are then discussed, about which limited information is available.
Dorsal column-medial lemniscal system: The first system of ascending fibers to be discussed is the dorsal column–medial lemniscus (DC-ML). Dorsal column refers to the first-order fibers located ipsilaterally (in reference to the side of fiber entry) in the dorsal white column of the spinal cord. Medial lemniscus refers to the second-order fibers located contralaterally in the brain stem. The DC-ML system conveys discriminatory (two-point) touch, some light (crude) touch, pressure, vibration, and joint position sense (conscious proprioception). This input provides temporal and spatial discriminatory qualities that allow for the conscious awareness of body part position at rest and during movements, and the conscious appreciation of the intensity and localization of touch, pressure, and vibration.
The peripheral receptors, which are mechanoreceptors (e.g., neuromuscular spindles, joint receptors, GTOs), have been discussed. The afferent fibers of these mechanoreceptors are large diameter; therefore they are located in the medial division of the dorsal root entry zone and subsequently enter the cord just medial to the dorsolateral tract of Lissauer. As these first-order neurons enter, they bifurcate into long ascending and short descending branches. The descending branches descend as the fasciculus interfascicularis in the upper half of the cord and as the fasciculus septomarginalis in the lower cord segments (see Fig. 9-8). These synapse in spinal gray matter and are involved in mediating reflex responses. The longer fibers contribute collateral branches to the spinal gray matter to participate in intersegmental reflexes and ascend ipsilaterally in the dorsal (white) column of the cord and continue into the medulla of the brain stem (Fig. 9-11). The first synapse occurs here in the nuclei gracilis and cuneatus, which are deep to the tubercles of the same name (Fig. 9-12). As the first-order neurons enter the dorsal white column, each neuron comes to lie more laterally to the fibers that entered more inferiorly. For example, information entering via a lumbar nerve ascends in the dorsal column in axons located lateral to the axons conveying information entering via a sacral nerve. In a cross section of the C3 spinal cord, first-order neurons conveying information (specific for the DC-ML system) from areas of the body innervated by sacral nerves are found most medial, followed in a lateral sequence by axons conveying information from areas innervated by lumbar, thoracic, and cervical nerves (see Fig. 9-11).
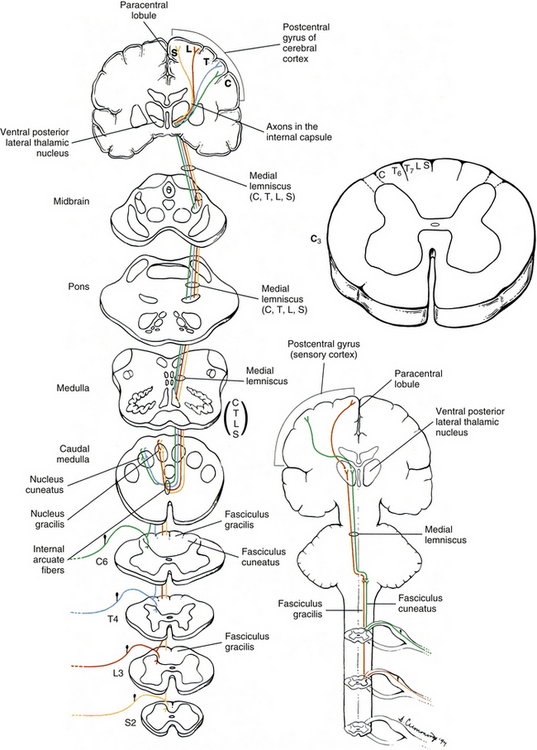
FIG. 9-11 Dorsal column–medial lemniscus system. The cross sections are through various regions of the central nervous system and show the locations of the ascending fibers. The ascending fibers are color coded (yellow, sacral; red, lumbar; blue, thoracic; green, cervical) to correspond to their cord level of entry. Note the somatotopic organization of these fibers in the spinal cord and brain stem as they ascend to the cerebral cortex.
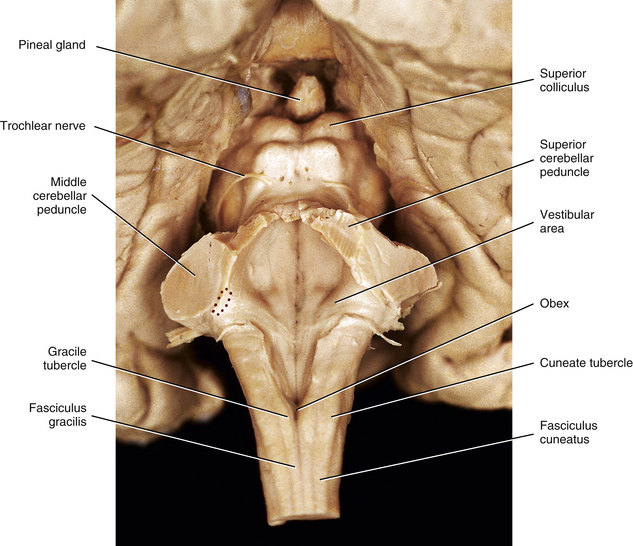
FIG. 9-12 Dorsal view of the brain stem. The cerebellum has been removed to expose the floor of the fourth ventricle. Red stippled area indicates the left inferior cerebellar peduncle.
At the midthoracic level of the cord and above, the dorsal column is divided by the dorsal intermediate sulcus into a medial fasciculus gracilis and a lateral fasciculus cuneatus. The dorsal intermediate sulcus extends ventrally from the cord’s periphery to approximately one half of the way into the dorsal column. This sulcus acts as a mechanical barrier preventing medial migration of the cuneate fibers (Smith & Deacon, 1984). Thus the fasciculus gracilis, which is found in all cord segments, includes axons of middle and lower thoracic, lumbar, and sacral nerves and therefore generally conveys information from the ipsilateral lower extremity. Smith and Deacon (1984) investigated the dorsal columns of human spinal cords and found that the orientation of the fasciculus gracilis fibers varied. In the most caudal part of the fasciculus, the fibers were oriented parallel to the medial side of the dorsal horn, whereas the upper lumbar and lower thoracic fibers were parallel to the dorsal median septum. However, the fibers in the fasciculus gracilis located in the remaining cord segments were oriented obliquely in a ventromedial-to-dorsolateral fashion. The authors also found that some overlapping of fibers occurred within the fasciculus gracilis but little if any occurred between the fasciculus gracilis and fasciculus cuneatus.
The fasciculus cuneatus includes axons of upper and middle thoracic and cervical nerves and, in general, conveys information from the ipsilateral upper extremity (see Fig. 9-11).
In addition to the mediolateral arrangement of fibers in the dorsal column, fibers are also organized by the type of modality they convey such that input from hair receptors courses in superficial fibers, whereas tactile and vibratory information travels in deeper fibers (Standring et al., 2008). Smith and Deacon (1984) demonstrated that the cross-sectional shape of each fasciculus (both gracilis and cuneatus) is different in each of the upper thoracic and cervical segments, and thus characteristic of that particular segment. The authors also believe that the fasciculi gracilis and cuneatus should be regarded as separate anatomic entities.
The first-order neurons of the fasciculi gracilis and cuneatus synapse with second-order neurons in the nuclei of the same name in the caudal medulla. The axons of the second-order neurons decussate (cross) in the caudal medulla (in a region called the sensory or lemniscal decussation); as they do so, they form a bundle of fibers known as the internal arcuate fibers (see Fig. 9-11). These second-order neurons then ascend through the brain stem as a fiber bundle known as the medial lemniscus. The lemniscal fibers are organized in the medulla such that information originating from the lower extremity and transmitted to the spinal cord in lumbar and sacral nerves is conveyed by fibers that are ventral to fibers conveying information originating from the upper extremity and transmitted to the cord in (primarily) cervical nerves. In the pons the fibers shift so that the lower extremity information is conveyed by fibers located lateral to the fibers conveying information from the upper extremity. In the midbrain of the brain stem, the lower extremity fibers become located dorsolateral to the upper extremity fibers. Clinically, it is imperative to recognize that the decussation of fibers occurs in the medulla. A unilateral lesion (e.g., trauma, vascular insufficiency, tumor) in the medial lemniscus of the brain stem produces contralateral deficits (e.g., loss of vibration, loss of joint position sense), whereas a lesion in the dorsal column of the spinal cord produces ipsilateral deficits.
The medial lemniscus ascends to the ventral posterior (lateral part) nucleus of the thalamus and synapses on third-order neurons (see Figs. 9-10 and 9-11). The axons of the third-order neurons travel in the internal capsule (a mass of axons going to and coming from the cerebral cortex) by way of the corona radiata to the primary sensory area of the cerebral cortex, which is located in the postcentral gyrus and paracentral lobule (posterior part) of the parietal lobe (Figs. 9-13 and 9-14; see also Fig. 9-11). From here, neurons project to adjacent cortical areas for further higher-level processing. This includes the integration of the information that is useful for the execution of movements and for the appreciation of the significance of the sensory input. For example, through the sense of touch and without visual input, one is able to recognize an object placed in the hand (stereognosis) or identify numbers or letters traced on the skin (graphesthesia). The DC-ML system maintains the spatial relationships of all parts of the body throughout its course in the CNS and allows the surface and underlying body structures to be mapped onto the primary sensory area of the cerebral cortex. This arrangement is called somatotopic organization. This organization is illustrated by a mapping of the entire body surface on the somatosensory cortex that depicts the location and amount of cortex dedicated to the processing of sensory information from a particular part of the body. The size of the relative body part represented in the map, called the homunculus, is related to the number of receptors projecting to that area of cortex (Fig. 9-15).
FIG. 9-13 Lateral view of the brain showing the primary motor (precentral gyrus), premotor, and primary sensory (postcentral gyrus) areas of the cerebral cortex. Prefrontal cortex is also indicated. The colored line lies within the central sulcus.
FIG. 9-14 Medial view of the brain. The paracentral lobule, which is the continuation of the primary motor area (precentral gyrus) and primary sensory area (postcentral gyrus) of the cerebral cortex, is indicated. Purple stippled area outlines the periaqueductal gray. Solid red area indicates the location of the red nucleus. Green stippled area indicates the reticular formation.
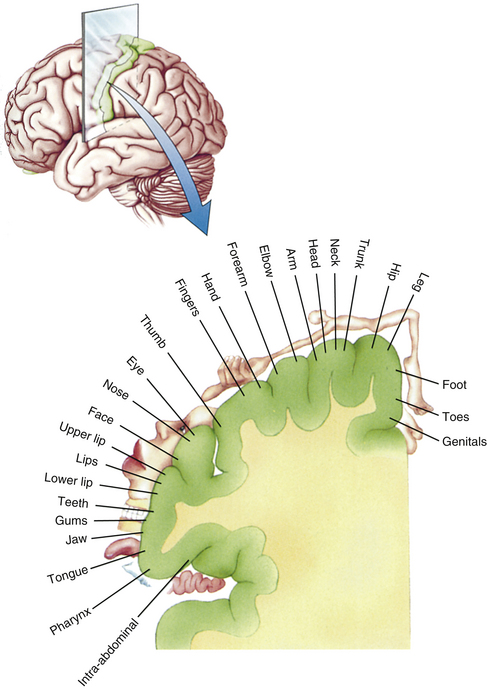
FIG. 9-15 The homunculus. The sensory homunculus is a somatotopic mapping of the body surface on the cortex (postcentral gyrus and posterior part of the paracentral lobule) indicating the location and amount of cortex assigned to the processing of sensory information from a particular part of the body. (From Bear MF, Connors BW, & Paradiso MA. [2001]. Neuroscience: exploring the brain [2nd ed.]. Baltimore: Lippincott Williams & Wilkins.)
Anterolateral system: The remaining tracts discussed in this chapter follow a basic plan in which first-order neurons terminate in the spinal cord gray matter. One group of tracts conveys nociception (pain) and innocuous stimuli such as temperature and some light touch. The tracts of this group ascend in the anterolateral quadrant of the spinal cord white matter and are collectively called the anterolateral system (ALS). This system consists of the spinothalamic, spinoreticular, and spinomesencephalic tracts (Young, 1986; Willis & Coggeshall, 1991; Basbaum & Jessell, 2000; Nolte, 2002). Nociception is conveyed by fast-conducting A-delta fibers (6 to 30 m/s) and slow-conducting C fibers (0.5 to 2 m/s) and when processed it is divided into two main types of pain. The A-delta fibers convey nociceptive information that is precisely localized and is perceived as being sharp, acute, or pinprick-like pain. It is perceived almost immediately (less than 0.1 second) after the stimulus has occurred and acts to alert the individual to potential tissue damage. It is typically confined to the skin. Slow-conducting C fibers convey nociceptive information that is described as burning, aching, or throbbing pain and is felt 1 second or later after the stimulus has occurred. This pain is appreciated secondarily to the fast pain, lasts much longer, and is poorly localized. It can occur from damage in any tissue (Snell, 2001). Temperature sense is also conveyed by A-delta and C fibers.
The first-order neurons of all of the ALS tracts enter the cord via the lateral division of the dorsal root entry zone and pass into the dorsolateral tract of Lissauer. Here, collateral branches ascend and descend, as well as enter and then synapse, in spinal cord laminae (Fig. 9-16). The spinothalamic tract originates primarily in laminae I, IV to VI, and even from laminae VII and VIII (Young, 1986; Hodge & Apkarian, 1990; Noback, Strominger, & Demarest, 1991; Willis & Coggeshall, 1991; Willis & Westlund, 1997; Standring et al., 2008). The second-order fibers decussate in the cord’s ventral white commissure within one or two segments of entry, although some studies suggest that the fibers cross transversely rather than diagonally (Nathan, Smith, & Deacon, 2001). The fibers then ascend in the anterolateral white matter of the cord and brain stem to terminate in the thalamus. As they travel through the brain stem, collateral branches are given off to the reticular formation. Fibers from the thalamus course through the internal capsule to terminate in the cerebral cortex.
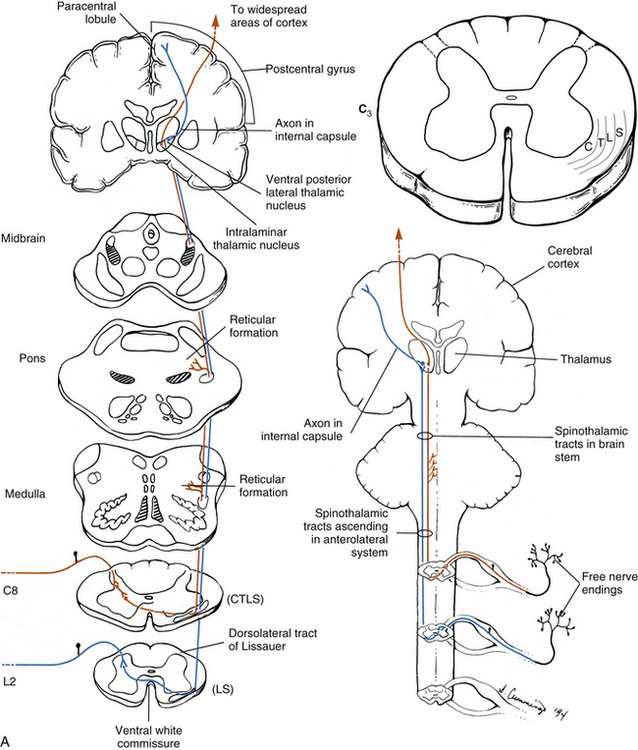
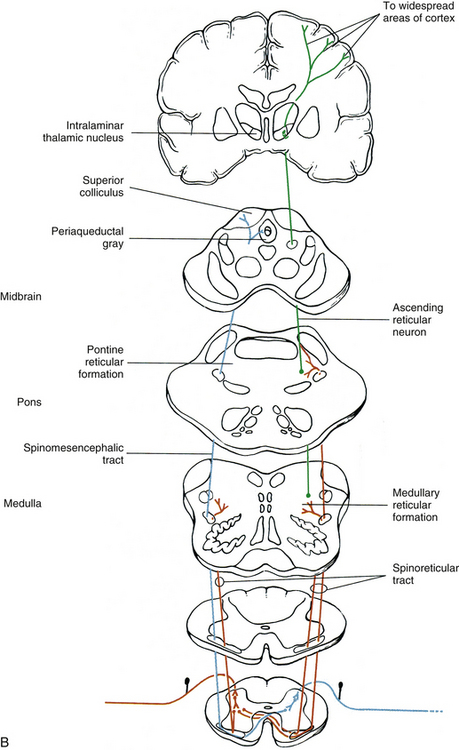
FIG. 9-16 Anterolateral system. A, Neospinothalamic (blue) and paleospinothalamic (red) tracts. Note that the second-order neurons cross in the ventral white commissure of the spinal cord. The somatotopic organization of the neospinothalamic tract is illustrated in the C3 cross section. The medial lemniscus is shaded to show its anatomic relationship to the spinothalamic tracts within the brain stem. B, Spinoreticular tract (red) terminates in both the pontine and the medullary reticular formations. Information from the reticular formation (represented by the neuron [green]) continues to the thalamus and then to the cortex. Spinomesencephalic tract is also shown (blue).
The spinothalamic and spinoreticular tracts are sometimes differentiated based on the order of their appearance during vertebrate evolution. The spinoreticular tract appeared first (i.e., is present in lower vertebrate animals), terminating in the reticular formation. Third-order neurons then project to the medial thalamic nuclear group. When mammals evolved, a group of fibers developed that coursed directly to the medial thalamic nuclear group but included collateral branches to the reticular formation. These fibers are called the paleospinothalamic tract or spinoreticulothalamic tract. This tract is similar to the spinoreticular tract. Lastly, a tract evolved that coursed directly to the lateral thalamic nuclear group and became the most developed in primates. This is called the neospinothalamic tract (Basbaum & Jessell, 2000; FitzGerald & Folan-Curran, 2002). The neospinothalamic tract originates from tract neurons primarily in laminae I and V to VII (Basbaum & Jessell, 2000) and conveys sharp or A-delta fiber nociception (pain), temperature, light (crude) touch, and pressure.
In addition, many of the neospinothalamic tract neurons in thoracic cord segments also receive convergent input from visceral afferent fibers and thus are called viscerosomatic neurons. This intersection of visceral input and somatic input on the same tract neuron may explain the phenomenon of visceral referred pain (Basbaum & Jessell, 2000; Strandring et al., 2008) (see Chapter 10). The fibers of the neospinothalamic tract then ascend contralaterally through the brain stem to the ventral posterior (lateral part) nucleus (see Fig. 9-10) and possibly the posterior nucleus of the thalamus, where they synapse on third-order neurons (see Fig. 9-16, A). The third-order neurons ascend to the sensory part of the cerebral cortex, which is the postcentral gyrus and paracentral lobule (posterior part) of the parietal lobe (see Figs. 9-13 and 9-14). As mentioned earlier, thermal sense from the skin travels in fibers within this tract and after being relayed in the thalamus is processed in the primary sensory cortex. This pathway is responsible for the perception and discriminatory qualities of cutaneous temperature. Nakamura and Morrison (2008) performed a study using rats in which they stimulated thermoreceptors by innocuous skin cooling. The data from the study showed that thermoreceptive-specific dorsal horn neurons, which are glutamatergic, directly terminated on neurons in the lateral parabrachial nucleus of the brain stem without relaying in the thalamus. From the lateral parabrachial nucleus, glutamatergic neurons projected directly to the preoptic area. The preoptic area is located in the hypothalamus and is considered to be the location of the neural circuitry responsible for thermoregulatory homeostatic responses such as shivering. Based on their results, Nakamura and Morrison (2008) suggested that this pathway from the dorsal horn to the preoptic area be regarded as a “thermoregulatory afferent” pathway that conveys information concerning environmental cutaneous thermal changes and that is distinct from the spinothalamic tract for conscious awareness of temperature.
Nociceptive input (consciously perceived as pain) also travels in the neospinothalamic tract. The type of pain information ascending in this tract, exemplified by a pinprick, is well localized and discriminatory. The fibers ascend in the spinal cord in a dorsolateral-to-ventromedial somatotopic pattern, with axons conveying information from sacral nerves found dorsolaterally (more superficial) and axons conveying information from cervical nerves located mostly ventromedially (closer to the ventral horn) (see Fig. 9-16, A). There is also evidence that fibers are segregated within the tract such that fibers conveying thermal sense are located dorsolaterally in the anterolateral white followed in a ventromedial direction by fibers conveying nociception, touch, and pressure in that order (Friehs, Schrottner, & Pendi, 1995; Snell, 2001; Standring et al., 2008). A cutaneous sensation that is associated with pain is itch, also known as pruritus. This sensation is defined as “an unpleasant cutaneous sensation that serves as a physiological self-protective mechanism to prevent the body from being hurt by harmful external agents” (Sun & Chen, 2007). Itch and pain share common characteristics: pruritic agents activate nociceptors producing the pain and itch sensations simultaneously; lesioning the anterolateral white of the spinal cord relieves chronic pain and blocks itch. Pain and itch are also different from each other: the stimuli of each produce different behavioral responses and itch is diminished by counter-stimuli (scratching) that activate nociceptors (Davidson & Giesler, 2010). Acute pruritus, which is abolished by scratching, occurs daily but a chronic condition can be the result of serious skin disorders, renal diseases and liver diseases. The primary afferents conveying the itch sensation include a histamine pathway and a histamine-independent pathway that utilize mechanically insensitive C fibers and polymodal C fibers, respectively (Davidson & Giesler, 2010). Their cell bodies are a subset of dorsal root ganglion (DRG) neurons that express gastrin-releasing peptide (GRP) colocalized with CGRP and substance P. Data from studies indicate that the central processes of these afferent fibers synapse on the neurons in lamina I of the dorsal horn that express the GRP receptor (GRPR). Tract neurons conveying pruritic stimuli synapse in the posterior and ventral posterior areas of the thalamus. It appears that these neurons are not necessary for the transmission of nociceptive information although it is not definitive as to whether or not they respond to nociceptive stimuli. Evidence suggests that a specific GRPR-mediated molecular signaling pathway for pruritus exists in the dorsal horn and may be the driving force for scratching behavior. Although it is unclear as to whether the GRPR+ neurons are interneurons or spinothalamic tract neurons, it is possible that they could be a central therapeutic target for drug therapy in patients with chronic pruritus (Sun & Chen, 2007; Sun et al., 2009; Davidson & Giesler, 2010). Itch can be blocked by counter-stimuli such as scratch, electrical stimulation, heat and cold, pinprick, and capsaicin injection. These stimuli excite mechanically sensitive polymodal C and A-delta fibers, which likely inhibit itch at the central level. A number of explanations have been proposed regarding the mechanism of counter-stimuli on pruritus. Counter-stimuli may block itch by activating dorsal horn neurons which release chemicals that modulate the central terminals of the pruriceptive afferents (retrograde signaling). In addition, it has been shown that itch can be reduced through the activation of the periaqueductal gray (PAG) of the midbrain. When simultaneously activated by both noxious and pruritic stimuli the PAG provides descending input to the dorsal horn in a manner similar to its inhibitory action on nociceptive circuitry in the dorsal horn. Finally, activated pruriceptive afferent fibers synapse on dorsal horn spinothalamic tract neurons (directly or indirectly) and inhibitory interneurons. These interneurons also receive input from nociceptive afferents. When both pruriceptive and nociceptive afferent fibers are activated simultaneously (the latter by counter-stimuli) the summation of the excitatory effect on the inhibitory interneurons produces a strong inhibitory effect on spinothalamic tract neurons that block the itch response (Davidson & Giesler, 2010).
The paleospinothalamic tract conveys dull, achy, or slow C-fiber nociception (pain) and temperature. The cell bodies of second-order neurons are located in laminae VII and VIII, and their axons decussate in the ventral white commissure to ascend in the brain stem (see Fig. 9-16, A). Their dendrites extend into laminae I through IV, and numerous interneurons are involved in the neurotransmission between first- and second-order neurons. The tract sends some collateral branches to the reticular formation of the brain stem on its course to the thalamus (Snell, 2001; Kiernan, 2009) (see Fig. 9-16, A). When reaching the thalamus, the second-order neurons of the paleospinothalamic tract synapse in the intralaminar nucleus (see Fig. 9-10). From this nucleus, third-order neurons travel to the same cortical areas as the spinoreticular tract (see the following), where the information relative to the affective and motivational aspects of pain is processed. Sometimes, perhaps unnecessarily (Snell, 2001; Nolte, 2002; Standring et al., 2008; Kiernan, 2009), the spinothalamic tract is divided into a lateral spinothalamic tract, which is thought to convey nociception and temperature, and an anterior (ventral) spinothalamic tract, which conveys light (crude) touch and pressure. However, physiologic studies indicate that all of these fibers are extensively intermingled and they should be considered as one entity (Standring et al., 2008).
The spinoreticular tract originates from second-order neurons located primarily in laminae VII and VIII (Young, 1986; Willis & Westlund, 1997; Basbaum & Jessell, 2000) but also in the dorsal horn, especially lamina V (Standring et al., 2008). The second-order fibers ascend to synapse in various nuclei in the medullary and pontine reticular formation. The majority of these axons ascend contralaterally, although a substantial number from cervical segments ascend ipsilaterally (Willis & Westlund, 1997; Basbaum & Jessell, 2000; Standring et al., 2008) (see Fig. 9-16, B). The reticular formation is a network of neurons located in the core of the brain stem (see Fig. 9-14), which is similar to the intermediate gray matter of the spinal cord. It consists of highly organized and differentiated neuronal populations that receive input from fibers of sensory systems ascending through the cord and brain stem. The reticular formation has numerous functions, including mediating cranial nerve reflexes, modulating somatic motor activity, influencing autonomic functions and the endogenous pain system (see Chapters 10 and 11), and regulating the level of consciousness. From the reticular formation, neurons project to the intralaminar and midline thalamic nuclei (see Fig. 9-10) and then, after synapsing, these neurons project to widespread areas of the cerebrum via the anterior cingulate (see Fig. 9-14) and insular cortices. These areas are important components of the limbic system, which consists of areas of the brain that are involved with the emotions and behaviors necessary for survival. The spinoreticular tract is thought to convey cutaneous information associated with alertness and consciousness and also dull, achy pain. Unlike the spinothalamic tract, which transmits information pertaining to the discriminatory qualities of painful stimuli (i.e., location and intensity), the spinoreticular tract is involved with transmitting information that is part of the affective and motivational aspects of pain (i.e., the unpleasantness of the pain and the desire to eliminate or reduce it). These latter aspects of pain result in autonomic, behavioral, and emotional responses to the painful stimuli. The brain stem reticular formation also is part of a group of structures comprising the ascending reticular activating system (ARAS). This system functions in arousal of the cortex to maintain alertness and attentiveness and, relative to the spinoreticular tract, the nature of the painful stimuli and subsequent responses.
The third tract found in the anterolateral quadrant consists of a group of fibers, called the spinomesencephalic tract, which terminates in the midbrain (see Fig. 9-16, B) (Young, 1986; Willis & Westlund, 1997; Basbaum & Jessell, 2000; Nolte, 2002; Standring et al., 2008). The spinomesencephalic tract also is likely to be involved with the motivational-affective component of pain. In addition, it is associated with the activation of a descending analgesic system. This tract originates primarily in laminae I and V but also in laminae IV and VI through VIII. It is predominantly crossed, although some fibers from the upper cervical levels remain ipsilateral. The tract conveys nociceptive information to midbrain nuclei, such as the superior colliculus (the fibers to this nucleus are called the spinotectal tract [Snell, 2001; FitzGerald & Folan-Curran, 2002; Standring et al., 2008]), the mesencephalic reticular formation, the pretectal nuclei, the parabrachial nucleus, and the periaqueductal gray (PAG) of the midbrain (see Figs. 9-12, 9-14, and 9-16, B). The superior colliculus is thought to be concerned with spinovisual reflexes; for example, turning the head and eyes toward a stimulus. The PAG has been implicated as being part of an endogenous pain control system. The PAG is capable of modulating pain circuitry in the dorsal horn of the spinal cord via the descending raphe-spinal tract (see Other Descending Fibers).
Remember that most conscious pain and temperature information ascends contralaterally in the anterolateral region and that the decussation of the second-order fibers occurs within the ventral white commissure within one or two segments of entry. Therefore a lesion in the spinal cord or brain stem produces a contralateral loss of pain and temperature below the level of the lesion (injury).
Spinocervicothalamic tract: The spinocervicothalamic tract is involved with conveying tactile, thermal, and nociceptive information (Willis & Westlund, 1997; Basbaum & Jessell, 2000; Nolte, 2002; Standring et al., 2008). First-order neurons terminate in the gray matter of the dorsal horn. Axons of tract neurons in laminae III to V ascend ipsilaterally in the dorsolateral funiculus as the spinocervical tract to synapse in the lateral cervical nucleus. This nucleus is located in the lateral white matter ventrolateral to the dorsal horn in the first two cervical cord segments. Axons of this nucleus decussate and ascend with the medial lemniscus to the thalamus, where they synapse. Others synapse in the midbrain. Axons from the thalamic nuclei subsequently project to the cerebral cortex. Although the lateral cervical nucleus is prominent in many mammals, especially carnivores, and is likely part of an important somatosensory pathway, its presence and importance in humans is unclear.
Spinohypothalamic tract: This tract originates in various laminae of the spinal cord gray matter (e.g., laminae VII and VIII [Sewards & Sewards, 2002]; laminae I, VII, and X [Willis & Westlund, 1997]; and laminae I, V, and VIII [Basbaum & Jessell, 2000]) and ascends bilaterally to the hypothalamus (see Fig. 9-14). The tract may be involved with the motivational aspect of pain and the resulting emotional and autonomic responses.
Postsynaptic dorsal column pathway: This pathway originates from neurons located around the central canal in lamina X. It ascends in the dorsal column and synapses in the dorsal column nuclei of the caudal medulla. From here fibers travel to visceroceptive neurons in the ventral posterior lateral (VPL) nucleus of the thalamus. This tract is a visceral nociceptive pathway and is viscerotopically organized such that fibers conveying information from pelvic viscera travel in the midline of the dorsal column and fibers from upper abdominal organs ascend more laterally in the dorsal column (Nauta et al., 1997; Willis & Westlund, 1997, 2001).
The previous description of ascending tracts has emphasized the fact that these tracts terminate in specific CNS targets. Neuroanatomic and neurophysiologic evidence collected through the use of more advanced techniques in tract-tracing methods and low threshold point antidromic mapping methods indicates that there are spinal dorsal horn neurons that project to double or multiple targets (Lu & Willis, 1999). This is not unexpected, because axons are known to display extensive branching and collateralization. Identified systems that appear to have double or multiple projecting systems include subpopulations that are related to the spinothalamic tract system, dorsal column postsynaptic tract system, and spinohypothalamic tract system. When compared with the anatomic description of the more classically defined multisynaptic and direct projecting systems, these pathways appear to be a phylogenetically intermediate group of tracts. Also, these pathways may function to transmit afferent information that has converged and been processed in the dorsal horn to multiple sites. These multiple sites may influence autonomic, affective, and motivational responses, as well as modulate descending control systems.
Spinocerebellar tracts: The next group of ascending tracts terminates in the cerebellum and conveys unconscious proprioception. Numerous spinocerebellar tracts have been implicated, although all their origins are not well-known (Ekerot, Larson, & Oscarsson, 1979; Grant & Xu, 1988; Xu & Grant, 1988). The best known of these are the dorsal spinocerebellar tract, cuneocerebellar tract, and ventral spinocerebellar tract.
Dorsal spinocerebellar tract: The dorsal spinocerebellar tract (DSCT) is located on the periphery of the lateral funiculus ventral to the dorsolateral tract of Lissauer and lateral to the lateral corticospinal tract (see Fig. 9-8). It begins in the L2 or L3 segments and ascends (Fig. 9-17). The cell bodies of these tract fibers are located in the nucleus dorsalis (thoracicus), also known as Clarke’s nucleus. This nucleus is located in lamina VII in cord segments C8 or T1 through L2 or L3 and is best developed in the upper lumbar and lower thoracic segments (Nolte, 2002; Kiernan, 2009). It consists of distinct subclasses of neurons that may allow for different mechanisms for processing proprioceptive input (Hantman & Jessell, 2010). Evidence suggests that axons of neurons located within the intermediate and dorsal laminae of adjacent segments also contribute to the DSCT (Bosco & Poppele, 2001). The DSCT is believed to carry proprioceptive and cutaneous touch and pressure information from the trunk and lower extremities. The vast majority of first-order neurons, along with collaterals of dorsal column primary afferents (Nolte, 2002; Standring et al., 2008), enter at the levels of C8 or T1 to L3 and synapse in Clarke’s nucleus. However, first-order neurons entering in dorsal roots L4 and inferiorly first ascend in the fasciculus gracilis to reach Clarke’s nucleus in the lower thoracic and upper lumbar segments, where they then synapse. Because the second-order neurons originating from Clarke’s nucleus and adjacent laminae ascend in the lateral white column as the DSCT, the tract itself is only present at the levels in which Clarke’s nucleus is found and superiorly (i.e., L3 and above). The DSCT ascends into the medulla of the brain stem and then exits the medulla via the inferior cerebellar peduncle (see Fig. 9-12) to terminate in the vermal and paravermal regions (spinocerebellum) of the cerebellum (Figs. 9-17 and 9-18). Before exiting the medulla, some of the DSCT axons that are conveying conscious proprioception from the lower extremity synapse in the nucleus Z of Brodal and Pompeiano, which is found immediately rostral to the nucleus gracilis. Internal arcuate fibers from the nucleus Z decussate and join the medial lemniscus (Standring et al., 2008; Kiernan, 2009).

FIG. 9-17 Spinocerebellar tracts as they ascend through the spinal cord, medulla, and rostral pons: the dorsal spinocerebellar tract (and its first-order neuron) (blue), ventral spinocerebellar tract (and its first-order neuron) (red), and cuneocerebellar tract (and its first-order neuron) (green). The second crossing of the ventral spinocerebellar tract is within the white matter of the cerebellum. Note that the side of the cerebellum that receives the input is ipsilateral to the side of the body where the input originated.
Cuneocerebellar tract: The upper limb equivalent to the DSCT is the cuneocerebellar tract. Its first-order fibers enter the spinal cord and ascend in the fasciculus cuneatus into the caudal medulla (see Fig. 9-17). Here they synapse in the lateral or accessory cuneate nucleus, which is lateral to the nucleus cuneatus of the DC-ML system. Axons from the lateral cuneate nucleus, called the posterior external arcuate fibers, form the cuneocerebellar tract and course with the DSCT, leaving the brain stem via the inferior cerebellar peduncle and terminating in the vermal and paravermal regions of the cerebellum (Standring et al., 2008).
Ventral spinocerebellar tract: A third tract, which like the DSCT is involved with lower extremity unconscious proprioception, is the ventral spinocerebellar tract (VSCT) (see Fig. 9-17). This tract does not originate in Clarke’s nucleus but instead originates from spinal border cells located in the periphery of the ventral horn (Grant & Xu, 1988; Xu & Grant, 1988) and from other neurons located in laminae V through VII in cord segments L1 and below (Carpenter & Sutin, 1983; Noback, Strominger, & Demarest, 1991). The majority of the tract fibers decussate in the ventral white commissure and are first observed in the lower lumbar cord segments (Carpenter & Sutin, 1983). They ascend in the lateral white column just ventral to the DSCT. The VSCT ascends through the medulla and into the rostral pons and then exits the brain stem via the superior cerebellar peduncle (see Fig. 9-12). Before terminating in the vermal and paravermal regions of the cerebellum (see Fig. 9-18), the majority of the tract fibers decussate again within the white matter of the cerebellum and terminate in the cerebellar hemisphere ipsilateral to the side of the body where the primary afferent fibers originated. The upper extremity equivalent to the VSCT, called the rostral spinocerebellar tract, rarely is seen in humans and is not described here
The tracts just discussed are components of the mossy fiber system of the cerebellum. The tract fibers are arranged such that information entering into lower spinal segments will travel in fibers that are most superficial. Their termination in the cerebellum is highly organized and is somatotopically arranged. The DSCT and VSCT terminate in the region of the cerebellar cortex for the lower limbs, and the cuneocerebellar tract ends in the upper limb area of the cerebellum. These tracts are involved with muscle coordination during movements and maintenance of posture (Carpenter & Sutin, 1983). The DSCT and cuneocerebellar tract neurons receive input monosynaptically from neuromuscular spindles and GTOs, joint receptors, and also from cutaneous (touch and pressure) receptors. Data from a murine study by Hantman and Jessell (2010) suggest that nearly all sensory input to DSCT neurons is from proprioceptors and that cutaneous input to the tract neurons is by way of interneurons. The proprioceptive input may arise from an individual limb muscle or from synergistic muscles acting at a common joint (Ekerot, Larson, & Oscarsson, 1979; Carpenter & Sutin, 1983; Standring et al., 2008). The classical view of the function of the DSCT was that it transmits information that is used for the precise coordination of individual limb muscles during posture and limb movements. However, several investigators have proposed that the DSCT neurons are more than a simple relay nucleus and serve an integrative role and process sensory input about global limb parameters, such as the position of the limb endpoint and its direction of movement. Although there are well-defined ipsilateral monosynaptic connections with DSCT neurons, there is also a bilateral polysynaptic afferent input via interneurons to DSCT neurons. This allows the DSCT to convey information about the status of the coordination of both lower limbs (Bosco & Poppele, 2001; Poppele, Rankin, & Eian, 2003). Studies of the step cycle in decerebrate cats with cut dorsal roots indicate that the sensory feedback to the cerebellum via the DSCT occurs only during ongoing movements (Ghez & Thach, 2000). There is also evidence to suggest that there is a strong excitatory cerebral cortical influence on a subset of DSCT neurons. These corticospinal fibers appear to be able to evoke pre- and postsynaptic inhibition of proprioceptive input to the tract neurons. DSCT neurons become the site where converging descending fibers and proprioceptive afferent fibers assemble interneuronal circuits “…with the potential to predict and modulate proprioceptive feedback signals” (Hantman & Jessell, 2010). The other major spinal input to the cerebellum is the ventral spinocerebellar tract. The VSCT neurons receive a complex array of input from type I afferents (mainly GTOs) and cutaneous afferents and also from segmental motor centers that may be excitatory or inhibitory (Ekerot, Larson, & Oscarsson, 1979). The motor centers are complex interneuronal pools that receive input from primary afferents and descending tracts and then synapse on local motor neurons. Some of the centers are pattern generator areas for automatic movements such as stepping. Studies show that the VSCT functions to transmit information concerning the activity of this central locomotor rhythmic area and interneuronal pools, as well as the activity of primary afferent fibers (Ghez & Thach, 2000). It is strongly modulated by the descending tracts, especially during locomotion; in response, it may relay information back to the cerebellum. In doing so, the VSCT may aid in monitoring the activity of the descending pathways (Noback, Strominger, & Demarest, 1991).
Spino-olivary tract: In addition to the spinocerebellar and cuneocerebellar tracts, which project to the cerebellum with few synapses, the less direct spino-olivary tract also conveys proprioceptive and exteroceptive information to the cerebellum by way of brain stem nuclei. Second-order neurons from deep laminae of the spinal gray matter decussate in the cord and ascend to the inferior olivary nucleus, which is located deep to the olive of the medulla (see Fig. 9-20). From the inferior olivary nucleus, the axons project through the inferior cerebellar peduncle to the contralateral side of the cerebellum. This tract may transmit information about motor learning occurring at the level of the cerebellum, as well as input to alter olivocerebellar connections concerning the correction of a moving body part meeting an obstacle (FitzGerald & Folan-Curran, 2002).
In summary, unconscious proprioception to the cerebellum is conveyed in the DSCT, cuneocerebellar tract, and VSCT, which use the fewest synapses and are thus the fastest, and also in the spino-olivary tract. Pain and temperature sensations are conveyed in the anterolateral quadrant in the spinothalamic, spinoreticular, and spinomesencephalic tracts, which cross in the spinal cord. Discriminative qualities of sensation (e.g., two-point touch and conscious proprioception) ascend in the DC-ML system, which decussates in the lower medulla. Light (crude) touch ascends in both the spinothalamic tract and the DC-ML system. Descending pathways from the cerebral cortex and brain stem nuclei also play an important role in the ascending systems. These descending pathways modulate (inhibit or facilitate) the transmission of the ascending tract neurons of the spinal cord.
Clinically, the most important ascending tracts are the DC-ML and spinothalamic. These tracts convey information that can be tested during a neurologic examination, such as vibration, joint position sense, stereognosis, light touch, pain, and temperature. Because lesions may disrupt these tracts, it is crucial that their functions, locations within the CNS, and points of decussation be remembered in order to localize the lesion site.
Descending Tracts
As discussed in the previous section, ascending tracts convey sensory information to higher centers. Some of this processed information is integrated to enable the human brain to form a conscious perception of the environment. Sensory input also is used by autonomic centers to help maintain homeostasis and by motor centers to allow for efficient control of somatic movement. Continuous sensory input such as visual, auditory, cutaneous, and proprioceptive input keeps higher centers informed about such facts as an object’s location in space relative to body position, and body position (stationary or moving) in space. This information is integrated and assessed, and is used for programming and adjusting movements (Ghez & Krakauer, 2000).
Three major motor areas receive this input and are involved with controlling movements. They are arranged in a hierarchy; the first is the spinal cord. Neurons in the spinal cord involved with motor activity either supply muscles directly (via alpha and gamma motor neurons) or form local circuits that mediate reflexes and automatic movements such as locomotion. Although some reflexes are monosynaptic, most reflex circuitry is complex and includes polysynaptic interneuronal pools. Descending inputs from higher centers terminate on motor neurons and interneurons, thus coordinating motor activity by influencing, through excitation or inhibition, the output of the interneuronal pools on the motor neurons. The second motor area is the brain stem, which includes nuclear regions that receive input from ascending tracts and also information from the eyes, inner ear, and even higher centers. The brain stem in turn sends information back to spinal cord neurons to modulate circuitry and thus influence the alpha and gamma motor neurons involved with postural adjustments, purposeful movements, and the control of coordinated head and eye movements. The third motor area is the cerebral cortex. The frontal lobe of the cerebral cortex includes three specific motor areas: (a) primary (located in the precentral gyrus and anterior part of the paracentral lobule); (b) premotor (located in the region anterior to the precentral gyrus); and (c) supplementary (located in the medial frontal gyrus anterior to the paracentral lobule) (see Figs. 9-13 and 9-14). These areas project directly to the spinal cord and brain stem nuclei, which in turn project to the spinal cord. The premotor and supplementary areas receive input from the sensory cortex and prefrontal cortex, project to the adjacent primary motor area, and, in general, are involved with coordinating and planning complex motor activities (Ghez & Krakauer, 2000). Two additional higher centers, the basal ganglia and cerebellum, through their projections to the cortex and brain stem nuclei, are also involved with planning, coordinating, and correcting motor activities.
All these motor areas provide control over such activities as maintaining balance and posture and performing purposeful, skilled movements. In general, three classes of movements can be described. These classes, which may function separately or be combined, are reflex movements, which, although coordinated and elicited by somatosensory input, are the simplest and are involuntary; automatic rhythmic movements (e.g., chewing, swallowing, and locomotion), which typically are initiated by somatosensory input to motor circuits located in the cord and brain stem and are voluntary at their initiation and cessation; and voluntary movements, which are performed for a purpose and may be learned and subsequently improved. Voluntary movements vary in complexity from turning a doorknob to playing the piano (Ghez & Krakauer, 2000).
All three types of movements are influenced by the brain stem and cerebral cortex. These areas produce two sets of parallel descending pathways, through which they can control somatic motor activity by indirectly (via interneurons) and directly influencing the alpha and gamma motor neurons that innervate the muscles that produce movements. Although most descending tracts are involved with somatic motor control, some influence primary sensory afferents and autonomic functions. The descending pathways (tracts) are described in the subsections that follow, beginning with the pathway that descends from the cerebral cortex, and followed by those that descend from the brain stem. The locations of the tracts within the spinal cord are described in general terms because their boundaries often overlap.
Corticospinal tract: The largest descending tract is the corticospinal tract (CST), which is often called the pyramidal tract (Fig. 9-19). This tract transmits information concerning voluntary (especially skillful) motor activity. The majority (approximately two thirds) of the fibers originate in the motor cortex (precentral or primary motor and premotor cortices) of the frontal lobe (Davidoff, 1990; Schoenen, 1991; Nolte, 2002; Standring et al., 2008), whereas other cell bodies are located in the adjacent sensory cortex of the parietal lobe. The CST courses through the posterior limb of the internal capsule and continues to descend within the ventral (basal) portion of the brain stem (see Fig. 9-19). The fibers in the medulla become very compact and form two elevations called the medullary pyramids (Fig. 9-20). At the caudal level of the medulla, 80% to 90% of the fibers cross forming the pyramidal decussation. The crossed fibers become the lateral CST and descend in the lateral white column (funiculus) of the spinal cord between the fasciculus proprius and the DSCT (see Fig. 9-8). When the DSCT is not present (below the L2 or L3 spinal cord segments), the lateral CST may extend to the periphery of the cord.
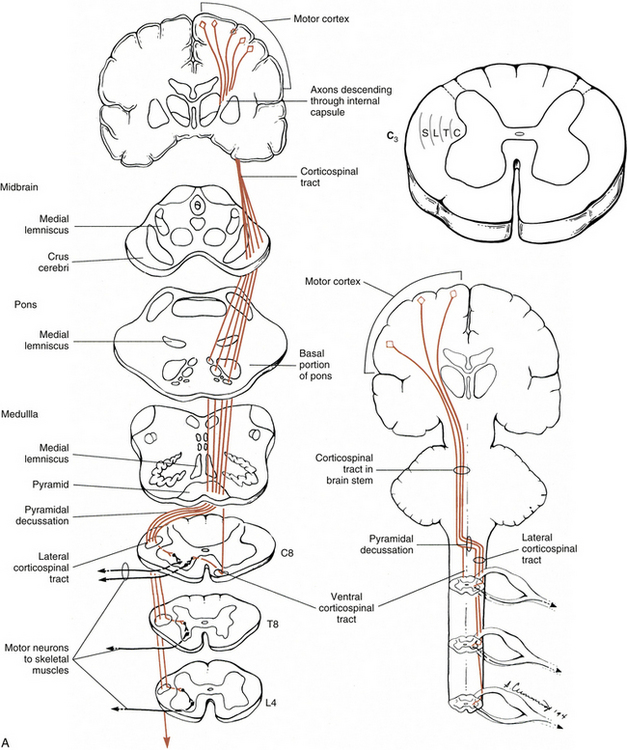
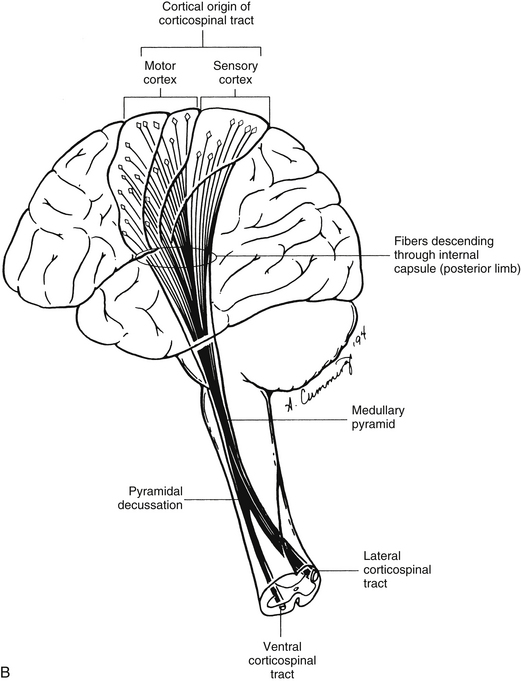
FIG. 9-19 Corticospinal tract (CST). A, CST descends within the basal (ventral) region of the brain stem. Most fibers cross in the caudal medulla and descend in the lateral funiculus of the cord as the lateral CST. Note the somatotopic organization of the lateral CST at the level of C3. The uncrossed fibers descend in the ventral funiculus as the ventral CST. These fibers cross before terminating in the gray matter. B, Origin of the CST, its course through the internal capsule, and its continuation into the brain stem and spinal cord.
FIG. 9-20 View of the ventral surface of the brain stem. Notice the olive immediately lateral to the pyramid on the medulla and cranial nerves III (oculomotor), VII (facial), IX (glossopharyngeal), and X (vagus). (From Lundy-Ekman L. [2002]. Neuroscience: fundamentals for rehabilitation [2nd ed.]. Philadelphia: Saunders.)
The CST is relatively new phylogenetically and found only in mammals. It is best developed in humans and becomes fully myelinated by the end of the second year of life. In each human medullary pyramid, there are approximately 1 million axons, and the vast majority are myelinated (DeMyer, 1959; Standring et al., 2008). (However, a study using a more discriminating staining technique suggests that there actually are less than 100,000 axons in the human medullary pyramid [Wada et al., 2001].) Axons with a diameter of 1 to 4 µm comprise approximately 90% of the tract fibers, 9% have a diameter of 5 to 10 µm, and less than 2% of the axons range from 11 to 22 µm in diameter (Carpenter & Sutin, 1983; Strandring et al., 2008). As the tract descends in the white matter, the size of the tract becomes progressively smaller. In the cervical cord segments, 55% of the fibers leave the tract and synapse on neurons in the gray matter. (It is understandable that such a large percentage of fibers terminate in these segments, because this is the location of the neurons that supply the muscles of the upper extremity, including the hand, which can perform highly skilled movements.) The gray matter in the thoracic cord segments receives 20% of the descending fibers, and the gray matter of the lumbar and sacral segments receives the remaining 25% of the tract axons. Like the somatosensory cortex, the motor cortex also is associated with a mapping of the body (motor homunculus) relative to the density of cortical neurons associated with a specific motor neuron pool (see Fig. 9-21). In addition, the lateral CST, as with the spinothalamic and DC-ML tracts, is somatotopically organized. The fibers that synapse in the cervical segments are located most medial, followed laterally by the fibers that synapse in the thoracic, lumbar, and sacral segments, respectively (see Fig. 9-19, A). The lateral CST fibers terminate in laminae IV to VIII. Some also synapse directly on motor neurons in lamina IX.
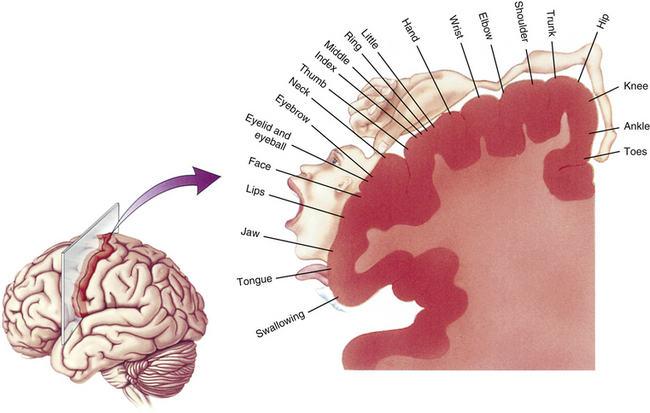
FIG. 9-21 The motor homunculus is a somatotopic mapping indicating the location and amount of cortex (precentral gyrus and anterior part of the paracentral lobule) associated with a specific motor neuron pool, which controls muscles of a particular part of the body. (From Bear MF, Connors BW, & Paradiso MA. [2001]. Neuroscience: exploring the brain [2nd ed.]. Baltimore: Lippincott Williams & Wilkins.)
A small number (approximately 2%) of the fibers that do not decussate descend ipsilaterally just ventral to the lateral CST. These thin fibers synapse in lamina VII and in the base of the dorsal horn (Carpenter, 1991). The larger remaining group of uncrossed fibers forms the ventral CST (see Fig. 9-19). This tract descends in the ventral white funiculus and usually has disappeared by the midthoracic region. It is best seen in cervical segments. Before terminating in the intermediate gray and ventral horn (primarily lamina VII), most fibers cross in the ventral white commissure; therefore approximately 98% of all corticospinal fibers terminate contralaterally.
CST fibers terminate on various neurons in the spinal cord, including Renshaw cells (see Spinal Motor Neurons and Motor Coordination), excitatory and Ia inhibitory interneurons, and primary afferent fibers (FitzGerald & Folan-Curran, 2002). Studies suggest that the axon terminals release the excitatory neurotransmitters glutamate or aspartate (Carpenter, 1991; Standring et al., 2008). In addition, fibers terminate on and coactivate both the alpha and the gamma motor neurons that innervate the same muscle, facilitating flexors and inhibiting extensors. Various laminae are the recipients of human CST fibers. Fibers from the motor cortices terminate mostly on interneurons in lamina VI (lateral part) but also in laminae V, VII, VIII, and IX (dorsolateral group and the lateral aspects of the central and ventrolateral groups of nuclei [Standring et al., 2008]). Although it is accepted that CST fibers terminate almost exclusively via polysynaptic connections in lower mammals, there is evidence to support the theory that CST fibers project directly (monosynaptically) on limb motor neuronal pools in humans and primates (Pierrot-Deseilligny, 1996; Maertens de Noordhout et al., 1999; Nicolas et al., 2001; Standring et al., 2008). Studies also show that the descending excitatory cortical fibers can influence upper limb motor neuronal pools indirectly via propriospinal premotoneurons. These are neurons located at the superior (C3-4) edge of the cervical enlargement that receive direct input from CST fibers, input from low threshold upper limb peripheral afferents, and input from both “feed forward” inhibitory interneurons and feedback inhibitory interneurons (which both serve to increase the gain of the incoming sensory signal by reducing peripheral inputs via inhibition from interneurons stimulated by the input [feed forward] or output [feedback] connections in the spinal cord). The projections of the propriospinal premotoneurons to motor neurons are excitatory. This intervening pool of premotoneurons neurons may integrate cortical commands for movement with sensory feedback from the moving upper limb, and help regulate and adjust the activation of the selected motor neuronal pool (Pierrot-Deseilligny, 1996; Nicolas et al., 2001).
Studies by Nathan and colleagues (1990) have resulted in more information concerning adult human spinal cord CSTs. They found that the extent of the area occupied by the lateral CST varied as a result of the size of the dorsal and ventral horns, the width of the fasciculus proprius that surrounds the gray matter, and the shape of the cord. In some cases the CST extended throughout a wide area of the dorsolateral white column, reaching the cord’s periphery, and even extending ventral to a coronal plane through the central canal. In cervical regions the lateral CST varied from segment to segment, whereas in thoracic segments it became more constant.
This variability is important clinically because surgical procedures, such as percutaneous cordotomy and dorsal root entry zone coagulation, may damage these fibers because of the tract’s proximity (although the denticulate ligament provides a landmark) to the anterolateral system and entering dorsal roots, respectively. The ventral CST also was described by Nathan and colleagues (1990) as being located in the ventral white funiculus adjacent to the ventral median fissure. Although some authors believe it is found primarily in cervical and upper to midthoracic levels (Davidoff, 1990; Carpenter, 1991; Snell, 2001; FitzGerald & Folan-Curran, 2002; Nolte, 2002; Standring et al., 2008), Nathan and colleagues (1990) found that it was a distinct tract, and in some cases it extended into sacral segments. Curiously, but of related interest, they also found that cord asymmetry was a common characteristic. In the 50 normal spinal cords studied, 74% were asymmetric, and in spinal cords of 22 patients with amyotrophic lateral sclerosis, 73% were unequal. Of the total asymmetric cords, 75% were found to be larger on the right side. This asymmetry appears to be caused by the size of the CSTs. In cases in which the right side was larger than the left, it was observed that a larger number of CST fibers were located in the right side. This was because more CST fibers crossed from the left pyramid into the cord’s right side than CST fibers crossing from the right pyramid into the left side. Also, more ventral CST fibers were found on the right side of the cord; whereas usually a reciprocal relationship exists between the number of ventral CST fibers and the number of contralateral lateral CST fibers. Therefore the larger (right) half included the larger lateral CST and also the larger ventral CST. A disproportionately large number of uncrossed fibers in some spinal cords (not the ventral CST fibers) that cross in the spinal cord before terminating may explain why lesions in the internal capsule may produce ipsilateral hemiplegia. Although this asymmetry is quite interesting, it does not appear to be related to handedness, and handedness does not appear to be related to the fact that fibers decussating from left to right do so at a higher level than those that decussate from right to left (Nathan, Smith, & Deacon, 1990).
The CST functions to augment the brain stem’s control of motor activity and also to provide voluntary, skilled (purposeful) movements. The CST allows movements of manual dexterity and manipulative movements to be performed, primarily by control of the distal musculature of the extremities, through the independent use of individual muscles of the hand and fingers. This type of fine motor activity is called fractionation, and the integrity of the motor cortex is essential for it to occur. Buttoning a shirt and tying a shoelace are examples of this type of motor activity. In addition, the CST adds precision and speed to fractionation and other basic voluntary movements (Wise & Evarts, 1981). However, the CST is not necessary for the production of voluntary movements. This is demonstrated by lesions specific to the CST, which result in loss of independent finger and hand movements and not paralysis. Although the basic features of voluntary movements are generated by other descending tracts (see the following), a functioning CST is necessary for providing the skill, precision, fractionation, speed, and agility used during voluntary motor activity. As stated, the CST takes its origin in part from the sensory cortex (parietal lobe). These CST fibers descend and provide supraspinal modulation to sensory relay areas such as the dorsal horn (lateral part of primarily laminae IV and V but also VI and VII [Standring et al., 2008]) and gracile and cuneate nuclei (of the DC-ML system). The corticocuneate fibers have been implicated in the primate to function as a means of regulating and adjusting (modulating) spatial and temporal input to this nucleus before and during hand movements (Bentivoglio & Rustioni, 1986). The descending fibers also are likely to modulate sensory input to motor areas. The variability of the CST in mammalian species may indicate its functional importance in various species. In the rat the CST synapses in the dorsal horn and intermediate zone, indicating that it may be part of that animal’s sensory system (Miyabayashi & Shirai, 1988). In species in which manual dexterity is nonexistent, such as the pig, the CST is not evident in the spinal cord (Palmieri et al., 1986). It is best developed in humans, providing feedback to sensory systems and also controlling voluntary skilled movements.
Other descending tracts: general considerations: The remaining descending tracts that influence motor activity originate in the brain stem and can be divided into two groups based on their location in the cord’s white matter, their termination in the gray matter, and their general functions. The ventromedial group fibers course in the ventral funiculus and ventrolateral aspect of the white matter and include the vestibulospinal tracts, the reticulospinal tracts, and the tectospinal tract (see Fig. 9-8). The fibers of the lateral group travel in the lateral funiculus and include the rubrospinal tract. Although the tracts are assigned to one of the two groups, the groups are not completely isolated from each other. There is communication between the two groups at the supraspinal level, and the termination of the two groups of tracts is on shared interneuronal pools. Also, there is a functional relationship between the two groups such that postural muscles frequently are caused to contract by one group as a stabilizing force during the simultaneous activation of distal musculature for purposeful movements by another group (Kuypers, 1981; Canedo, 1997; Standring et al., 2008).
The tracts of the ventromedial group, in addition to their location, also have the following functional characteristics in common: They give off many collateral fibers (collateralization), which become involved with maintaining posture; they integrate axial and limb movements and provide input associated with movements of an entire limb; and they govern head and body position in response to visual and proprioceptive stimuli. Also, they terminate in the ventromedial gray (e.g., laminae VII and VIII) on long and intermediate propriospinal neurons (which in turn project bilaterally in the fasciculus proprius) and on interneurons associated with the motor neurons supplying the axial muscles and proximal muscles of the extremities.
The fibers found in the lateral group are located near the lateral corticospinal tract. They have little collateralization and enhance the functions of the ventral group by providing independent flexion-based movements of the extremities, especially through their influence on the neurons that innervate the distal muscles of the upper extremity. These tracts terminate in laminae V, VI, and VII (dorsal part) on short propriospinal neurons (which in turn project ipsilaterally in the fasciculus proprius), numerous interneurons, and some motor neurons (Kuypers, 1981; Carpenter & Sutin, 1983; Schoenen, 1991; Strandring et al., 2008). Each of the tracts in these two groups is discussed in detail in the following paragraphs.
Reticulospinal tracts: These tracts originate from the reticular formation (RF), which, as stated, is a phylogenetically old group of neurons and processes that has a network appearance in cross section. The RF consists of various nuclei located within the core of the brain stem (see Fig. 9-14). Through extensive neuronal processes, the nuclei directly and indirectly connect with all areas of the CNS and are involved in numerous activities, including mediating the sleep-arousal cycle, regulating visceral responses such as cardiovascular and respiratory functions, modulating pain perception, and controlling somatic motor function. This chapter discusses the fibers involved with somatic muscle control. The reticulospinal tracts (ReSTs) consist of the medullary and pontine tracts (Fig. 9-22, A). These tracts originate in the medullary and pontine reticular formation, respectively, and show little if any somatotopic organization. The medullary ReST arises from neurons in the medial two thirds of the medullary reticular formation and descends bilaterally in the ventral and ventrolateral white columns of the cord to terminate in laminae VI, VII, and VIII and directly on motor neurons. The pontine ReST originates in the reticular formation of the medial pontine tegmentum (core of the pons) and descends ipsilaterally in the ventral white column to terminate in laminae VII (central and medial parts) and VIII. These tracts are difficult to evaluate in humans, and most data have been compiled from feline studies. However, Nathan and colleagues (1996) studied human spinal cords and looked at characteristics of reticulospinal fibers. The fibers, which did not form well-defined tracts, were located in the ventral, ventrolateral, and lateral white columns, and descended primarily ipsilaterally, although some were bilateral. They were intermingled with other ascending and descending tracts in the white matter. Many fibers shifted their position from the ventral white into the lateral white matter as they descended in the cord. In general, during movements these tracts adjust and regulate reflex actions. Either facilitation or inhibition can be produced depending on the area of stimulation in the reticular formation.
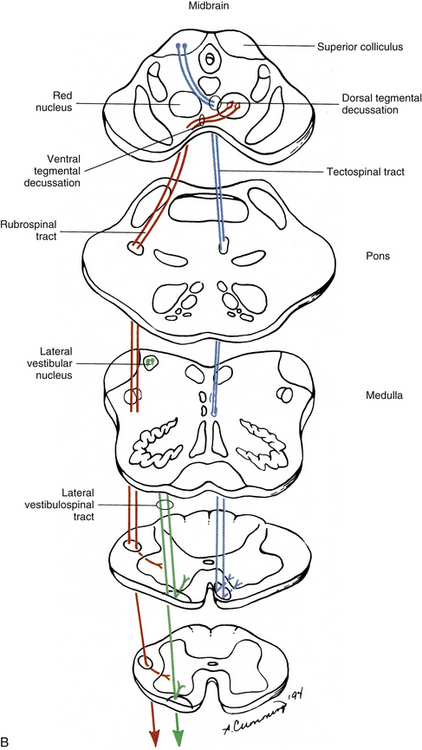
FIG. 9-22 Descending tracts that originate in the brain stem. A, Reticulospinal tracts: medullary (green) and pontine (red). Descending input from the cerebral cortex is represented by arrows and the neuron (blue). B, Lateral vestibulospinal tract (green), rubrospinal tract (red), and tectospinal tract (blue).
The pontine ReST facilitates motor neurons supplying axial muscles and limb extensors. The medullary ReST may inhibit or excite motor neurons innervating cervical muscles and excites motor neurons supplying back muscles in the thoracic and lumbosacral regions. Both alpha and gamma motor neurons are influenced by these tracts via monosynaptic and polysynaptic connections (Standring et al., 2008). The reticular formation integrates large amounts of information from the motor areas of the cerebral cortex (corticoreticular fibers) and other systems involved with motor control (e.g., cerebellum and brain stem nuclei). It functions to control and coordinate automatic movements such as locomotion (rhythmic actions such as walking and running) and posture (see Spinal Motor Neurons and Motor Coordination). Locomotion is regulated by networks of spinal interneurons known as pattern generators that activate flexor and extensor motor neurons in a rhythmic and reciprocal manner. However, the initiation of locomotion, as well as the control of speed, is a function of the mesencephalic locomotor region of the brain stem. Neurons from the mesencephalic locomotor region project to the medullary RF, which in turn sends fibers in the ventrolateral white region of the cord to the locomotor cord pattern generators, thus modulating their activity. To fine-tune locomotor activity (e.g., controlling balance, refining and coordinating movements for actions such as avoiding an obstacle), afferent input about the terrain from the skin, muscles, tendons, visual system, and inner ear (vestibular apparatus), which projects to segmental neurons, cerebellum, and the motor cortex, is also necessary (Pearson & Gordon, 2000a). Posture, simply defined either as the position of body parts held between movements (standing or sitting) or as the stabilization of proximal limb joints so voluntary movement of distal limbs can occur, is also influenced by the RF through ReSTs. For example, when a standing cat lifts its front paw off the ground (corticospinal input), its body weight shifts to other paws to ensure balance while the movement is occurring. However, if the medullary reticular formation is inoperative, the postural correction does not occur although the limb movement is attempted (Ghez, 1991). In general, the ReSTs influence stereotypical movements (i.e., nonskillful movements) concerned with posture, crude stereotypical limb movements, and steering head and trunk movements in response to external stimuli (Standring et al., 2008).
Vestibulospinal tracts: Two tracts originate from the vestibular nuclei of the brain stem. These nuclei (lateral, medial, inferior, and superior) are located in the vestibular area in the floor of the brain stem’s fourth ventricle (see Fig. 9-12). The nuclei receive proprioceptive input from the inner ear and cerebellum. They project to brain stem nuclei for coordinating conjugate eye movements and to gray matter in the spinal cord. One of these tracts is the lateral vestibulospinal tract, which originates in the lateral vestibular (Deiter’s) nucleus. The tract fibers descend the length of the cord ipsilaterally. Initially they are in the peripheral part of the ventrolateral white column of the cord, but move medially into the ventral white column at lower cord levels. The fibers synapse in the medial area of the ventral horn in lamina VIII and the medial part of lamina VII (Fig. 9-22, B). The tract affects antigravity muscles by facilitating (polysynaptically and monosynaptically) the motor neurons supplying the extensor muscles of the neck, back, and limbs, and by inhibiting the motor neurons (via Ia inhibitory interneurons) supplying the limb flexor muscles (Standring et al., 2008). Its function is to maintain balance and upright posture by compensating for unanticipated movements made by the body while standing or during locomotion.
The other vestibulospinal tract is the medial vestibulospinal tract, which originates primarily from the medial vestibular nucleus. These fibers descend bilaterally near the midline in the ventral white column of the cord within the medial longitudinal fasciculus (MLF), and synapse in laminae VII and VIII in the cervical and upper thoracic cord segments. Animal studies indicate that motor neurons innervating axial muscles of the neck and upper back are monosynaptically inhibited by these fibers. The tract is responsible for stabilizing head position and accurately maintaining head position in relation to eye movements in response to vestibular stimuli.
Tectospinal tract and medial longitudinal fasciculus: The tract fibers that have just been discussed are located throughout the spinal cord and most likely influence somatic motor activity in the trunk and all four extremities. The tectospinal tract and MLF are two additional descending tracts located in the ventral funiculus that also influence skeletal muscle activity, but they are not found in all cord segments.
The tectospinal tract originates in the superior colliculus of the midbrain (see Figs. 9-12 and 9-14). The fibers cross in the midbrain as the dorsal tegmental decussation and descend through the brain stem and into the medial part of the ventral white column of the cord (see Fig. 9-22, B). The majority of fibers terminate in the upper four cervical cord segments in laminae VI through VIII (Carpenter, 1991; Standring et al., 2008), although in lower mammals (e.g., rat, cat, monkey) fibers also terminate in the segments of the cervical enlargement (Coulter et al., 1979). This tract is involved with orienting an animal toward stimuli in the environment (e.g., visual, auditory, cutaneous) by reflex turning of the head (and eyes via tectal fibers to cranial nerve nuclei) toward the stimulus. It is also involved with the head and eye movements used in tracking a moving visual stimulus (e.g., watching a tennis match from seats at midcourt).
The MLF (descending component of the MLF) is located in the cord’s ventral white column and terminates in the cervical cord segments. (Ascending fibers of the MLF are involved with eye movements and course to brain stem motor nuclei of the oculomotor, trochlear, and abducens nerves.) This pathway includes (houses) the medial vestibulospinal tract and possibly the tectospinal, ventral corticospinal, and cervical portions of reticulospinal tracts (FitzGerald & Folan-Curran, 2002; Nolte, 2002; Standring et al., 2008; Kiernan, 2009). The tract is responsible for accurately maintaining head position relative to eye movements in response to vestibular stimuli.
Rubrospinal tract: The rubrospinal tract is part of (with the lateral corticospinal tract) the lateral group of tracts. The rubrospinal tract originates in the red nucleus of the midbrain (see Fig. 9-14). The tract crosses in the midbrain as the ventral tegmental decussation and descends somatotopically in the lateral white column just ventral to the lateral CST (see Fig. 9-22, B). The fibers synapse in the dorsal part of lamina VII and in laminae V and VI. Although the rubrospinal tract is well established in the cat and monkey and extends the length of the spinal cord, its localization, termination, and function in the human cord are still unclear. Nathan and Smith (1982) studied the human rubrospinal tract from the brains of nine patients and found that the tract was small, and when it could be traced into the cord, it extended only into the upper cervical segments. The red nucleus receives input from the cerebellum and motor cortex and may be an indirect route to the motor neurons of the spinal cord. Stimulation of the red nucleus produces facilitation of the contralateral motor neurons supplying flexor muscles and inhibition of the contralateral motor neurons supplying extensors. Although it is well developed in lower mammals, its importance probably diminished phylogenetically as the CST became more developed.