Neuroanatomy of the Autonomic Nervous System
Sympathetic, Parasympathetic, and Enteric Divisions
Innervation of Autonomic Effectors
Innervation of Peripheral Effectors
Innervation of the Heart and Lungs
Efferent Response and the Cholinergic Anti-inflammatory Pathway
Neurotransmission Occurring at the Autonomic Ganglia and Neuroeffector Junctions
The autonomic nervous system (ANS) functions to maintain homeostasis by providing the optimal internal environment for the cellular components of the organism during normal and stressful periods. The ANS accomplishes this task through its control of visceral function, and generally it is considered to be a motor system consisting of fibers that innervate the smooth muscle, cardiac muscle, and glands of the body. However, sophisticated neuroanatomic techniques, such as immunocytochemistry and axonal tracing methods, have produced data indicating that visceral control involves much more than the innervation of effectors by the efferents of the sympathetic and parasympathetic divisions of the ANS. Information suggests that other structures and regions are intimately associated with these efferents. These include visceral afferent fibers and the reflexes they may initiate, the widespread influence and variety of chemical mediators, and the central autonomic circuitry, which is involved with the integration and dissemination of visceral input. Detailed description of both the anatomic and physiologic relationships of the ANS with all of the structures involved with homeostatic mechanisms is beyond the scope of this chapter. Therefore each of these topics generally is described along with the origin and course of the sympathetic and parasympathetic efferent fibers to present a composite picture of the ANS. Certain topics that seemingly are not typically discussed in standard textbooks are elaborated on in this chapter. The chapter concludes with examples of pathologies that affect the ANS.
Sympathetic, Parasympathetic, and Enteric Divisions
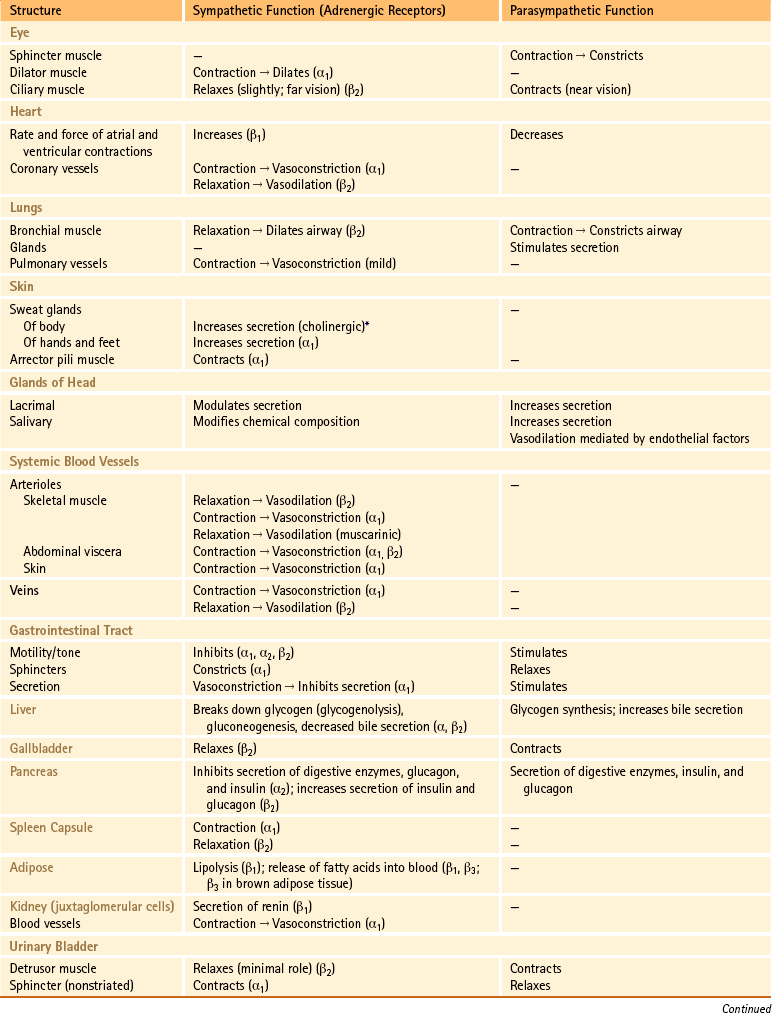
The ANS is composed of a sympathetic and a parasympathetic division. These two divisions are discussed here, followed by a description of a third division of the ANS. This third division is the enteric nervous system, which is a complex network of neurons located within the wall of the gut. Typically the anatomy and functions of the parasympathetic and sympathetic divisions are described separately. This method of introducing the basic concepts and components of the divisions may lead to the misconception that they function antagonistically and separately. This is certainly not the case. In fact, the fibers of both divisions are tonically active, and in most cases do not function as either “on” or “off.” Although some tissues are predominantly or exclusively innervated by one division (e.g., sympathetic peripheral effector tissues), many tissues have a dual innervation. A balance of activity exists between the division fibers in these cases, such that when one division increases its activity, the other division decreases its activity. The central nervous system (CNS) has the ability to alter the balance of activity between these divisions. In addition, the sympathetic and parasympathetic divisions interact with the somatic nervous system and different hormonal systems to stabilize the internal environment and maintain homeostasis during normal conditions or emergency situations.
The ANS can be described best after certain characteristics common to both the sympathetic and parasympathetic divisions have been reviewed. The parasympathetic and sympathetic innervation of autonomic effectors (i.e., organs, vessels, glands) is organized differently than the innervation of skeletal muscle (Fig. 10-1). Although the axons of alpha and gamma motor neurons course directly to skeletal muscles, the innervation of ANS effectors requires a chain of two neurons (see Fig. 10-1), called the preganglionic and postganglionic neurons. The cell body of the preganglionic neuron always is located in the CNS—either in the spinal cord or in the brain stem (Fig. 10-2). The axon is thinly myelinated and immediately leaves the CNS within a specific ventral root of the spinal cord or within certain cranial nerves exiting the brain stem. The cell body of the postganglionic neuron is located in an autonomic ganglion that may be found in numerous places outside the CNS (see Fig. 10-2). The preganglionic neuron synapses with the postganglionic neuron within this ganglion. The axon of the postganglionic neuron is unmyelinated and innervates the effector. Both the preganglionic and postganglionic neurons frequently travel in components of the peripheral nervous system (PNS) (i.e., spinal nerves, cranial nerves) and are intermingled with afferents and somatic motor neurons of peripheral nerves. As stated, most effectors are innervated by both sympathetic and parasympathetic fibers (Table 10-1; see also Fig. 10-2). These fibers produce antagonistic but coordinated responses in the effectors. Descending input from higher integrative centers such as the hypothalamus and areas of the brain stem reaches the cell bodies of preganglionic fibers to regulate and adjust their activity. This descending input is a part of several specific visceral reflex pathways and also is used by higher centers to institute widespread bodily changes.
Table 10-1
Functions of the Sympathetic and Parasympathetic Divisions
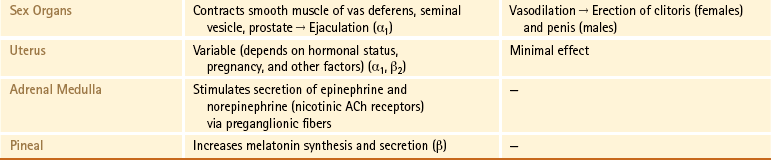
∗Sweat glands are an exception; their sympathetic fibers release ACh, which binds to cholinergic receptors.
From Benarroch EE et al. (1999). Medical neurosciences (4th ed.). New York: Lippincott Williams & Wilkins; Bray JJ et al. (1994). Lecture notes on human physiology (3rd ed.). Cambridge, UK: Blackwell Science; FitzGerald MJT & Folan-Curran J. (2002). Clinical neuroanatomy and related neuroscience (4th ed.). Philadelphia: WB Saunders; Tortora GJ & Derrickson B. (2009). Principles of anatomy and physiology (12th ed.). Hoboken, NJ: John Wiley & Sons; Waxman SG. (2003). Clinical neuroanatomy (25th ed.). Chicago: McGraw-Hill.
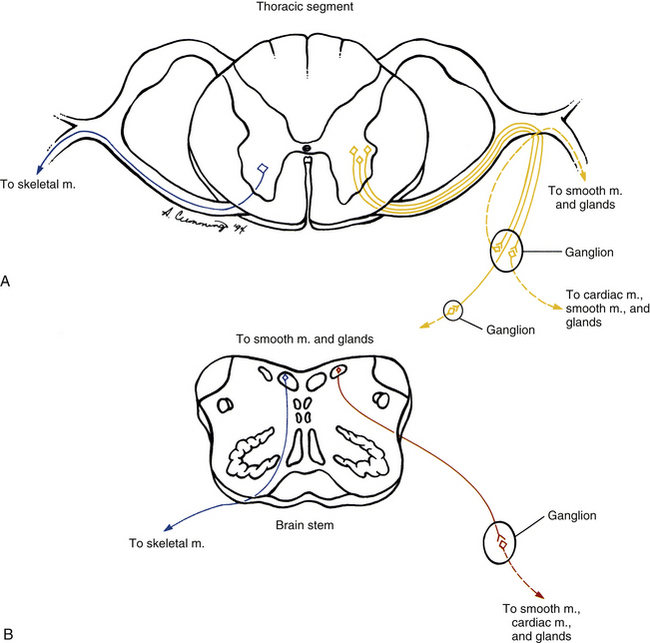
FIG. 10-1 General organization of autonomic preganglionic (solid line) and postganglionic (dashed line) neurons (right) compared with somatic efferent neurons (left). A, Sympathetic output and somatic output from the spinal cord. B, Parasympathetic output and somatic output from the brain stem.
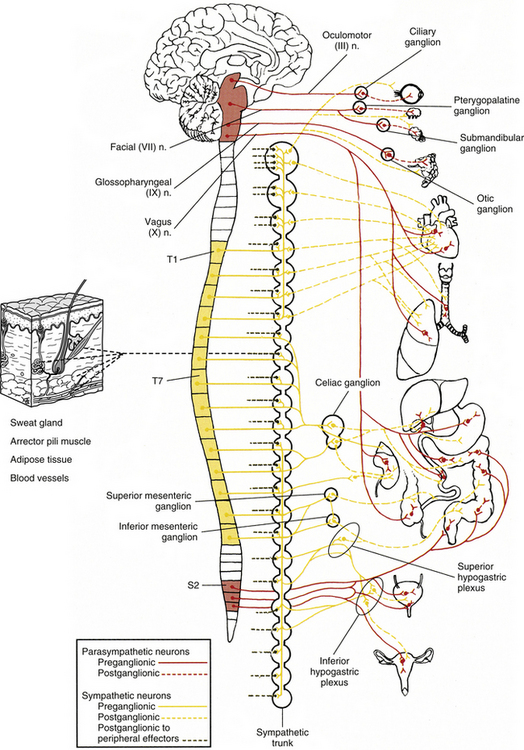
FIG. 10-2 Overview of the parasympathetic and sympathetic divisions of the autonomic nervous system. Preganglionic neuron cell bodies are located in the brain stem and sacral cord segments (parasympathetic or “cranio-sacral” division) and thoracic and upper lumbar cord segments (sympathetic or “thoraco-lumbar” division). The axons of these neurons synapse with postganglionic neurons, which course to the smooth muscle, cardiac muscle, and glands of the body. The postganglionic neuron cell bodies may be located in distinct autonomic ganglia, or in the wall or very near the wall of the innervated visceral organ. Note that sympathetic fibers provide the only innervation to peripheral effectors (sweat glands, arrector pili muscles, adipose tissue, and blood vessels).
Sympathetic Division
The general function of the sympathetic nervous system (SNS) is to help the body cope with stressful situations. The response usually is the rapid release, and subsequent use, of energy. This is best exemplified by the reaction of the body to a dangerous situation. In this instance, sympathetic involuntary responses occur, including increased heart and respiratory rates, cold and clammy hands, wide-eyed stare, and dilated pupils. Blood is redistributed by means of vasoconstriction and vasodilation from such areas as the abdominal and pelvic organs and skin, to more important tissues such as the brain, heart, and skeletal muscles. The level of blood glucose increases, as does the blood pressure. Activity of the gastrointestinal (GI) and urinary systems is less important during this stressful situation; therefore the nonvascular smooth muscle of these organs is inhibited. The sympathetic division often is called the fight-or-flight division of the ANS because of this overall response.
Preganglionic Sympathetic Neurons
The cell bodies of the preganglionic sympathetic neurons are located in the spinal cord in all thoracic segments and in the upper two or three lumbar segments (see Fig. 10-2). Because of the distribution of these preganglionic cell bodies, the sympathetic division of the ANS often is called the thoracolumbar division. These preganglionic neurons comprise a heterogeneous population within the spinal cord. The dendritic arrangement of these neurons ranges from simple to complex arborizations. The cell bodies are of different shapes, and their size falls in a range between the size of smaller dorsal horn neurons and that of larger somatic motor neurons. Of the total membranous surface area of these neurons, the cell body of each composes a maximum of 15%, which likely indicates the importance of the dendritic surface area of that neuron (Cabot, 1990).
The cell bodies of the preganglionic neurons are found in four nuclei within the intermediate gray matter of the spinal cord (Fig. 10-3) (Cabot, 1990). The largest group of these cell bodies is the intermediolateral (IML) cell column that forms the lateral horn. Throughout this column are clusters of 20 to 100 neurons that are separated by distances ranging from 200 to 500 µm in the thoracic region and from 100 to 300 µm in the lumbar region. The cell bodies are approximately 12 to 13 µm in diameter and histologically are similar to motor neurons (Harati, 1993). The diameters of the axons range from 2 to 5 µm, and their speed of conduction is approximately 3 to 15 m/sec. These fibers often are classified in the B group (see Chapter 9). At the T6 and T7 levels, the mean number of these cells is approximately 5000, but it has been shown that the number decreases with age at the rate of approximately 8% per decade (Harati, 1993).
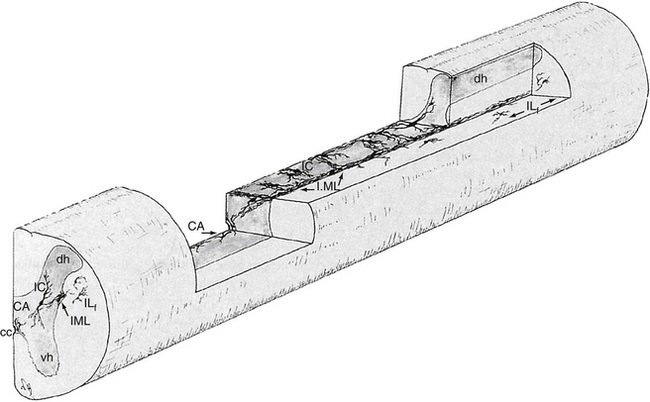
FIG. 10-3 Location of the four groups of sympathetic preganglionic neurons within the spinal cord gray matter. In the middle of the spinal cord, the horizontal plane shows the “ladderlike” arrangement of these neurons. CA, Central autonomic nucleus; cc, central canal; dh, dorsal horn; IC, intercalated nucleus; ILf, lateral funicular nucleus; IML, intermediolateral nucleus; vh, ventral horn. (From Cabot JB. [1990]. Sympathetic preganglionic neurons: cytoarchitecture, ultrastructure, and biophysical properties. In AD Loewy & KM Spyer [Eds.]. Central regulation of autonomic functions. New York: Oxford University Press.)
The other three nuclear groups of preganglionic neurons have been described by Cabot (1990) and are the lateral funicular area (located lateral and dorsal to the intermediolateral group), the intercalated cell group (located medial to the IML column and possibly the same cluster of neurons typically called the intermediomedial group), and the central autonomic nucleus (located lateral and dorsal to the central canal). The combination of these groups forms a ladderlike structure in longitudinal sections in which the paired IML cell columns form the sides of the ladder and the interconnected central autonomic nucleus and intercalated cell group form the rungs (Fig. 10-3). The IML cell column is the origin of the vast majority of preganglionic fibers, but the other three nuclei also give rise to some preganglionic fibers. The four nuclei are the recipients of extensive input from higher centers such as the hypothalamus and brain stem nuclei. These sources release various neurotransmitters that have been identified as monoamines, neuropeptides, and amino acids. Although the anatomic characteristics of these four nuclei have been described, the exact functions of each specific nucleus still remain unclear.
Postganglionic Sympathetic Neurons
According to the general rule of organization of the ANS, two neurons are necessary for the impulse to reach the effector. One is the preganglionic neuron, just discussed. The second neuron in the pathway to an autonomic effector is the postganglionic neuron. This neuron’s axon is classified as a group C fiber (see Chapter 9). Generally it is described as unmyelinated, with a diameter ranging from 0.3 to 1.3 µm and a slow conduction speed ranging from 0.7 to 2.3 m/sec (Carpenter & Sutin, 1983). The cell body is located outside the CNS in an autonomic ganglion. Unlike a sensory ganglion of cranial nerves and a dorsal root ganglion of spinal nerves, in which no synapses occur, an autonomic ganglion is the location of the synapse between the preganglionic and postganglionic neurons. Preganglionic fibers disseminate their information by diverging and synapsing on numerous postganglionic fibers. This principle of divergence is based on studies of the superior cervical ganglion of mammals. Results of different studies show preganglionic to postganglionic ratios of 1:4 (Loewy, 1990a), 1:15 to 1:20, and 1:196 in a human superior cervical ganglion (Ebbesson, 1968; Standring et al., 2008). (The parasympathetic division also has been found to exhibit divergence, but to a lesser degree.) This divergence may allow the effects of sympathetic stimulation to be more widespread throughout the body and to be of greater magnitude.
The autonomic ganglion in which the synapse occurs may be one of a chain of ganglia (called the sympathetic chain, sympathetic trunk, or paravertebral ganglia) located near the vertebral bodies of the spinal column, or it may be a prevertebral ganglion, such as the celiac ganglion (see Fig. 10-2), found within one of the autonomic nerve plexuses.
These plexuses surround the large arteries in the abdominal and pelvic cavities. The ganglion, regardless of location, is encapsulated by connective tissue. The connective tissue capsule is continuous with the epineurium of the bundle of entering preganglionic neurons and the bundle of exiting postganglionic neurons. Within the capsule are predominantly multipolar, spheroidal-shaped postganglionic neurons (Fig. 10-4). These neurons consist of cell bodies that have diameters ranging from 25 to 50 µm and dendrites that branch in a complex pattern. These dendrites are the location where preganglionic neuron axons commonly synapse. Satellite cells (neuroglial cells) that are similar to those found in the dorsal root ganglia surround the cell bodies and dendrites. These cells provide support and help maintain the chemical environment. Interneurons are also located in the ganglion. One type, called small (cell bodies ranging in diameter from 15 to 20 µm) intensely fluorescent cells (SIFs), is present singly or in clusters. These cells contain the neurotransmitters epinephrine, serotonin, and dopamine (Hamill, 1996), which, when released, bind to and modulate the activity of postganglionic neurons. Another type of interneuron found in the ganglion is the small chromaffin cell, which also contains catecholamines. The exact difference between the chromaffin cell and SIF is unclear (Carpenter & Sutin, 1983; Harati, 1993; Standring et al., 2008).
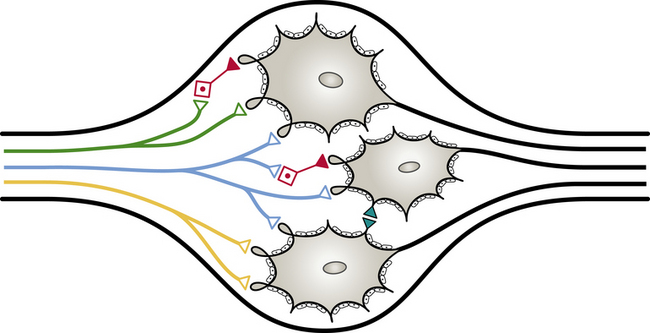
FIG. 10-4 An autonomic ganglion showing the synaptic connections between preganglionic (green, blue, and yellow) and postganglionic neurons. Note the interneurons (red), the dendro-dendritic synapse (teal), and the satellite cells surrounding the postganglionic cell bodies. ∆, Excitatory; ▲, inhibitory.
Sympathetic Trunk
Two sympathetic trunks are located in the body, each of which lies on the anterolateral side of the vertebral column (Fig. 10-5). They both extend from the base of the skull to the coccyx. The ganglia of the sympathetic trunks are also called the paravertebral ganglia because they lie next to the vertebral column. Inferiorly the two trunks join in the midline and terminate on the anterior surface of the coccyx as the ganglion impar.
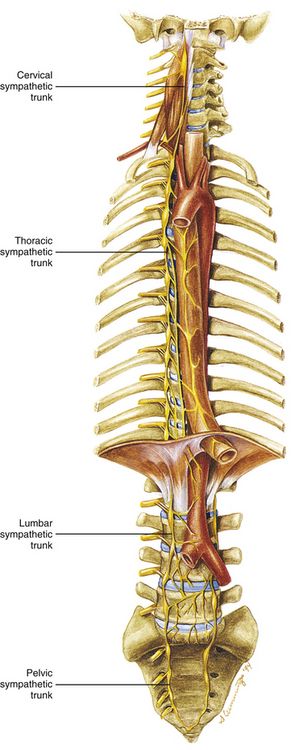
FIG. 10-5 Sympathetic chain ganglia (trunk) and its anatomic location in the cervical region and the thoracic, abdominal, and pelvic cavities. (The left sympathetic trunk in the cervical and thoracic regions has been omitted for the sake of clarity.)
Each sympathetic trunk shares important anatomic relationships with surrounding structures. In the neck it lies between the carotid sheath and prevertebral muscles, which cover the transverse processes (TPs) of the cervical vertebrae. It is found anterior to the heads of the ribs in the thorax, anterolateral to the bodies of the lumbar vertebrae in the abdomen, and medial to the anterior sacral foramina in the pelvis (Standring et al., 2008). As the name sympathetic chain ganglia implies, this structure consists of approximately 22 ganglia that are linked together by connective tissue surrounding ascending and descending fibers. The total number of ganglia does not correspond exactly to the number of spinal nerves because some of the ganglia have fused with one another. This fusion is most evident in the cervical region, where there are only three cervical ganglia (see Cervical Sympathetic Trunk later in this chapter and Cervical Sympathetics in Chapter 5). The thoracic portion of the sympathetic trunk includes 10 to 12 ganglia (70% of the time there are 11), the lumbar region exhibits 4 ganglia (although this number may vary), and 4 or 5 ganglia appear in the sacral region of the trunk. The union of the two sympathetic trunks forms the one coccygeal ganglion.
The preganglionic fibers exit the spinal cord in the ventral roots of cord segments T1 to L2 or L3 to reach the postganglionic neurons. Therefore at these particular levels, the ventral roots include both preganglionic sympathetic fibers and fibers to skeletal muscle (i.e., alpha and gamma motor neurons). The preganglionic fibers continue into the spinal nerve, and at the division of the spinal nerve into its dorsal and ventral rami (posterior and anterior primary divisions, respectively), the myelinated preganglionic fibers exit, forming the white (myelin is a white substance) ramus communicans, and then continue into the sympathetic trunk. (There are only 14 or 15 white rami on each side because there are only 14 or 15 spinal cord segments [T1 to L2-3] that provide preganglionic sympathetic fibers.)
The sympathetic system innervates autonomic effectors throughout the entire body. In general, cord segments T1 through T6 are involved with sympathetic innervation of autonomic effectors in the head, neck, upper extremities, and thorax. The cord segments from approximately T7 through L2 or L3 innervate the effectors in the lower extremities, abdominal cavity, and pelvic cavity. Recall that the sympathetic trunk is where synapses occur between preganglionic and postganglionic sympathetic fibers. Because the sympathetic trunk extends rostrally, adjacent to cervical vertebrae to reach the base of the skull, and caudally, adjacent to the sacrum to reach the coccyx, this trunk provides the means by which preganglionic fibers may ascend or descend to reach spinal nerves formed above or below the levels of T1 through L2 or L3. The preganglionic fibers may proceed in different directions once they pass through the white rami communicantes and enter the sympathetic trunk.
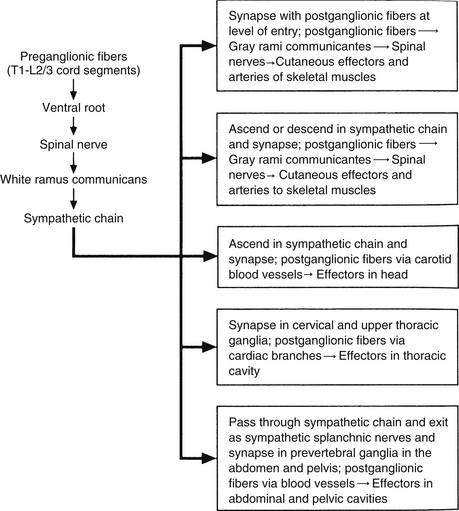
Autonomic fibers innervating peripheral blood vessels (including those in the skeletal muscles and skin), sweat glands, and arrector pili muscles of hair follicles travel in spinal nerves and subsequently peripheral nerves to innervate the appropriate effectors. These effectors are located in the area of distribution of each of the peripheral nerves. After entering the sympathetic trunk, preganglionic fibers associated with these effectors do one of three things (Fig. 10-6): ascend to synapse with postganglionic neurons in ganglia above T1 (for cervical nerves); synapse with postganglionic neurons at the level of entry into the trunk (i.e., T1 to L2 or L3 for those corresponding nerves); or descend to synapse with postganglionic neurons in ganglia below L2-3 (for lumbar and sacral nerves). From the sympathetic trunk the postganglionic fibers course through gray (these are unmyelinated fibers) rami communicantes (usually located proximal to the white rami), enter the spinal nerve at the location of its division into dorsal and ventral rami, and continue to the ANS effectors. Therefore the dorsal and ventral rami and subsequently formed peripheral nerves include sensory afferent fibers, motor neurons to skeletal muscle, and postganglionic sympathetic fibers. The ventral roots of T1 to L2-3 cord segments are unique in that they contain motor neurons to skeletal muscle and also preganglionic sympathetic fibers.
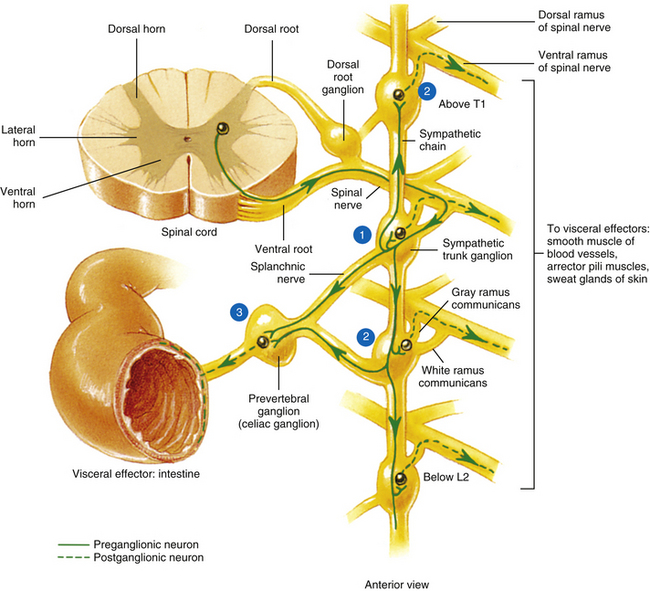
FIG. 10-6 Summary of synapses of sympathetic preganglionic and postganglionic neurons. 1, Preganglionic fibers enter the chain via the white ramus communicans and may synapse at that level, ascend to a more superior ganglion and synapse, descend to a more inferior ganglion and synapse, or pass through the chain. 2, Postganglionic fibers, destined to innervate peripheral effectors, exit the sympathetic chain via the gray ramus communicans (at least one for every spinal nerve) and enter into a ventral (anterior) ramus (they may enter a dorsal [posterior] ramus). 3, Preganglionic fibers, destined to innervate viscera in the abdominal and pelvic cavities, exit the sympathetic chain without synapsing and travel to a prevertebral ganglion and synapse on postganglionic neurons. (From Tortora GJ & Grabowski SR. [2003]. Principles of anatomy and physiology [10th ed.]. New York: John Wiley & Sons, Inc.]
Sympathetic Preganglionic and Postganglionic Fibers
Sympathetic preganglionic fibers sending nerve impulses to effectors in the head enter the sympathetic trunk, ascend to the superior cervical ganglion, and synapse with postganglionic neurons. The postganglionic fibers course with large blood vessels to reach effectors located in the head region (Fig. 10-7, A). Such effectors include glands, the smooth muscle of blood vessels, and the smooth muscle of the eye. Some preganglionic fibers sending impulses to smooth muscle, cardiac muscle, and glands of the thorax also ascend on entering the trunk and synapse at rostral levels, whereas others synapse with postganglionic fibers at the level of entry. These postganglionic fibers leave the chain as branches that merge with other nerve fibers, including parasympathetic vagal fibers, to form plexuses innervating the heart and lungs. Abdominal and pelvic effectors are innervated in a different manner than the effectors of the head, thorax, and cutaneous regions. Preganglionic fibers enter the sympathetic trunk via white rami communicantes but do not synapse in the chain ganglia. Instead they pass through the chain ganglia and emerge as a collection of fibers coursing in splanchnic (referring to the viscera) nerves. These fibers within the splanchnic nerves course inferiorly in an anteromedial direction, pass through the diaphragm, and terminate in various prevertebral ganglia. Here they synapse on postganglionic neurons that then continue to the effectors of the abdominal and pelvic cavities (Fig. 10-7, B). The sympathetic prevertebral ganglia are enmeshed in plexuses of sympathetic and parasympathetic fibers and are located near large arteries found in the abdominal cavity. Examples are the celiac, superior mesenteric, aorticorenal, and inferior mesenteric ganglia.
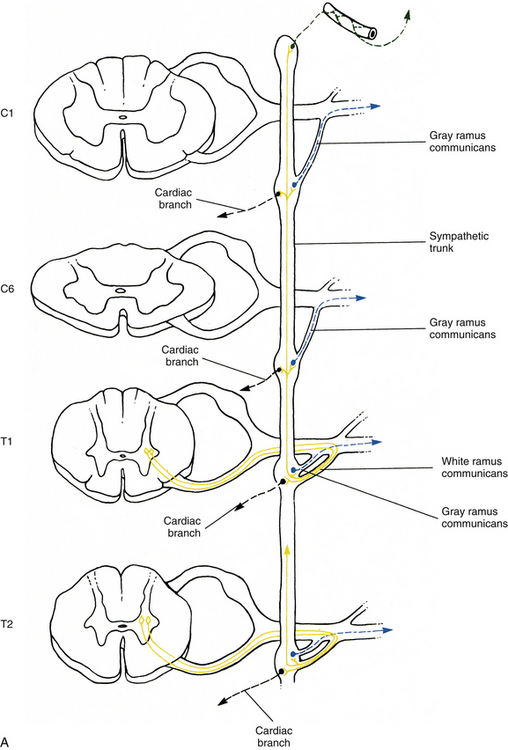
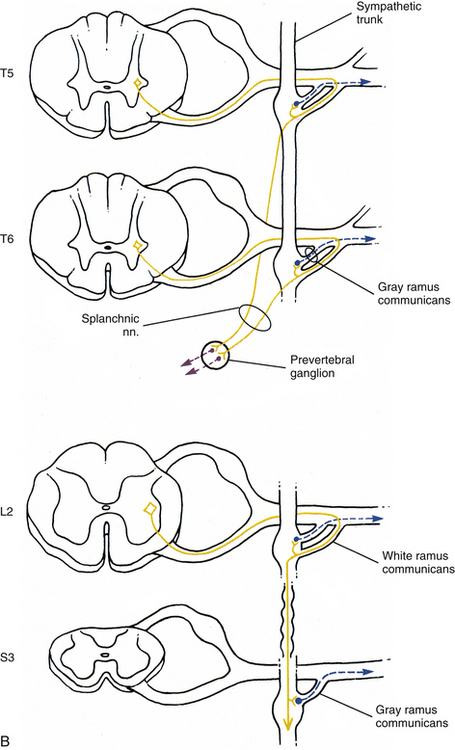
FIG. 10-7 Diagrammatic scheme showing the options of sympathetic neurons. A, This shows that once preganglionic fibers (yellow) have entered the chain, they may ascend to higher levels and synapse with postganglionic fibers that may enter gray rami (blue) or travel on blood vessels (green); synapse in numerous ganglia with postganglionic neurons that leave the chain as cardiac branches (black); or synapse at the level of entry with postganglionic neurons that enter gray rami. B, This shows that once preganglionic fibers (yellow) have entered the chain they may synapse at the level of entry with postganglionic neurons that enter gray rami (blue); descend to lower ganglia and synapse on postganglionic neurons that enter gray rami; or pass through the chain without synapsing and travel to prevertebral ganglia, where they synapse with postganglionic neurons (purple), the axons of which course to effectors in the abdominal and pelvic cavities.
On entering the sympathetic trunk, a typical preganglionic neuron (other than those forming splanchnic nerves) may either ascend or descend and, in each case, subsequently synapse in more than one ganglion. A preganglionic neuron also may synapse at the entry level and send collateral branches up or down to other ganglia. However, less than 2% of the neurons send a branch both up and down (Cabot, 1990). In all cases described thus far, a preganglionic neuron has synapsed with a postganglionic neuron. However, a notable exception is the innervation of the medulla of the adrenal gland which is directly innervated by preganglionic sympathetic neurons. The adrenal medulla develops from the same embryonic neural crest as postganglionic neurons. Although the medullary chromaffin cells do not resemble postganglionic neurons in appearance, they do function in a similar manner. Preganglionic neurons innervate the medulla directly, which in turn releases epinephrine and some norepinephrine into the bloodstream. These neurotransmitters circulate throughout the body, stimulating effectors and assisting in the overall sympathetic response.
A summary of the various sympathetic nerve pathways is provided in Figures 10-6, 10-7, and 10-8.
Specific Regions of the Sympathetic Trunk
Cervical sympathetic trunk: This part of the sympathetic trunk is located anterior to the TPs of the cervical vertebrae, behind and medial to the carotid sheath, which houses the vagus nerve, internal jugular vein, and internal carotid artery. It lies anteriorly on the prevertebral fascia covering the longus capitus and colli muscles. At the level of the C6 vertebra the diameter of the trunk has been measured to be between 1.0 and 4.7 mm with a mean of 2.8 mm (Saylam et al., 2009). A common surgical approach to the cervical spine is from an anterior direction and involves retracting the carotid sheath and/or the longus colli muscle (the longest and most medial of the prevertebral muscles) to expose the lateral structures of the cervical vertebral bodies. Understanding the anatomy of the sympathetic trunk and its ganglia is necessary in order to reduce the risk of injury during these surgical procedures. The fusion of the eight cervical ganglia results in three distinct ganglia in the region of the neck (Figs. 10-9, A, and 10-10; see also Fig. 10-5). These are known as the superior, middle, and cervicothoracic (stellate) ganglia. (Approximately 20% of the time the T1 ganglion is separate, in which case the cervicothoracic ganglion is called the inferior cervical ganglion [Pather et al., 2006].) The superior ganglion (Fig. 10-11, A; see also Figs. 10-9, A, and 10-10) is the largest of the three and lies high in the neck on the TPs of vertebrae C2 and C3, anterior to the longus capitis muscle and posterior to the cervical part of the internal carotid artery. It is also in the vicinity of the internal jugular vein and the glossopharyngeal, vagus, spinal accessory, and hypoglossal cranial nerves (Standring et al., 2008). The proximity of the ganglion to these nerves may account for the autonomic effects seen when these nerves are lesioned in this location (Cross, 1993b). The ganglion is formed by the fusion of the first four cervical ganglia. Its mean length is 3.3 cm and its mean width is 0.8 cm (Saylam et al., 2009), and it includes more than 1 million neurons (Carpenter & Sutin, 1983; Harati, 1993). Postganglionic fibers leaving this ganglion course to various regions. Some ascend as perivascular plexuses on the internal and external carotid arteries. A large branch (internal carotid nerve) from the superior cervical ganglion ascends with the internal carotid artery and divides into branches that form the internal carotid plexus (see Fig. 10-9, A) (Standring et al., 2008). This plexus, which surrounds the artery and innervates its wall, continues to travel with that artery, and within the cranial cavity the fibers innervate the autonomic effectors. Examples of these effectors are the dilator pupillae muscle of the eye, the superior tarsal muscle (Müller’s muscle) of the eyelid, and sweat glands in the medial part of the forehead (Watson & Vijayan, 1995; Salvesen, 2001). In addition, some are sympathetic vasoconstrictor fibers and innervate cerebral branches of the internal carotid artery. Other postganglionic fibers leave the ganglion as medial, lateral, and anterior branches and course directly to effectors. The lateral branches include slender filaments that communicate with the inferior vagal ganglion, hypoglossal nerve, the superior jugular bulb, and jugular glomus of the internal jugular vein, and the meninges of the posterior cranial fossa. One branch, the jugular nerve, branches at the cranial base to join the inferior glossopharyngeal ganglion and the superior vagal ganglion (Standring et al., 2008). Other lateral branches are gray rami that join the first four cervical spinal nerves. The latter travel with those spinal nerves to effectors in the areas of distribution of the nerves. The medial branches include the laryngopharyngeal branches, which supply the carotid body and help form the pharyngeal plexus and cardiac branches. The cardiac branches contain efferent fibers and no nociceptive cardiac afferent fibers. The anterior branches travel with the common and external carotid arteries. The fibers surround the arteries as delicate plexuses within which small ganglia are sometimes found. An external carotid plexus continues with branches of the external carotid artery to innervate such structures as the arrector pili muscles, cutaneous blood vessels, and facial sweat glands not innervated by the internal carotid plexus. These fibers travel with terminal branches of the trigeminal nerve (cranial nerve [CN] V) (Watson & Vijayan, 1995; Salvesen, 2001; Standring, 2008).
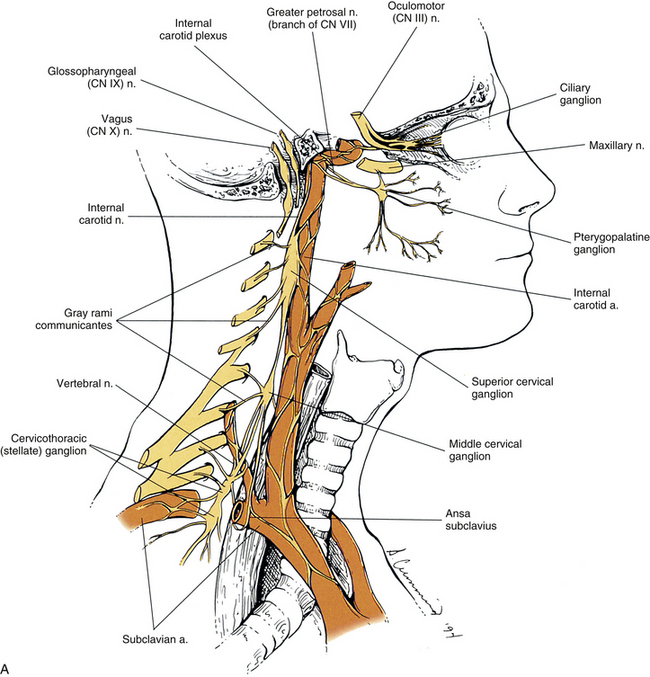
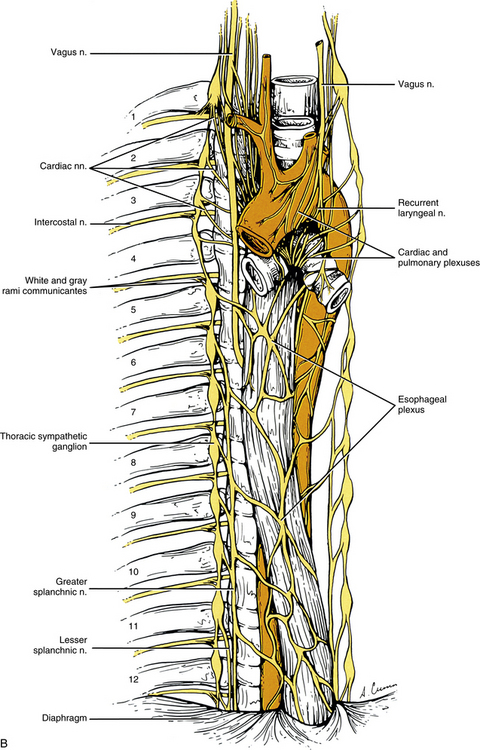
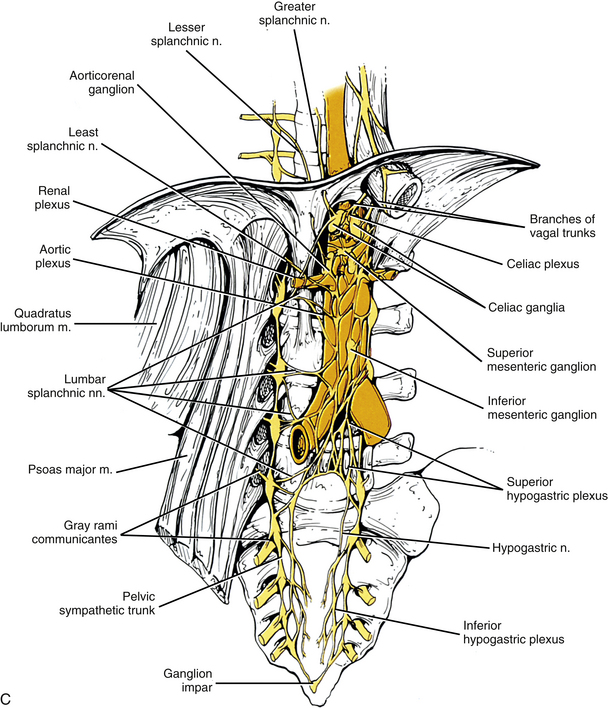
FIG. 10-9 A, Cervical sympathetic trunk and the continuation of autonomic fibers to effectors in the head. Note the relationship of the superior cervical ganglion to the vagus and glossopharyngeal cranial nerves and the internal carotid artery. Gray (not white) rami communicantes course from the cervical chain to the cervical spinal nerves. Leaving the cervicothoracic ganglion are the vertebral nerve and plexus that travel with the vertebral artery. Fibers of some postganglionic neuron cell bodies located in the superior cervical ganglion initially form the internal carotid nerve, which travels with the internal carotid artery, and subsequently branches to form the internal carotid plexus. Fibers of other postganglionic neuron cell bodies located in the superior cervical ganglion course with branches of the external carotid artery to sweat glands (not shown). Note that postganglionic fibers leave the blood vessels and travel with branches of cranial nerves. On the way to their destination, the sympathetic fibers may pass through, but do not synapse in, parasympathetic ganglia (e.g., ciliary and pterygopalatine). B, The thoracic sympathetic trunk shows that gray (medial) and white (lateral) rami communicantes are present. Thoracic autonomic plexuses (e.g., cardiac, pulmonary, and esophageal), which are formed by postganglionic sympathetic fibers and vagal preganglionic fibers, are shown. Cardiac nerves and greater and lesser splanchnic nerves are also illustrated.
C, Lumbar and pelvic sympathetic trunks and autonomic plexuses. The psoas major muscle has been reflected laterally to show the lumbar chain more clearly. The left and right pelvic sympathetic trunks can be seen uniting on the anterior surface of the coccyx to form the ganglion impar. Gray rami communicantes connecting the sympathetic trunk with spinal nerves are present at all levels. Also notice the major autonomic plexuses found in the abdominal and pelvic cavities. Sympathetic prevertebral ganglia located in the abdominal cavity (such as the celiac, superior mesenteric, and inferior mesenteric) also are shown. In the pelvic cavity the superior hypogastric plexus continues as the left and right hypogastric nerves that, with parasympathetic fibers, form the left and right inferior hypogastric (pelvic) plexuses.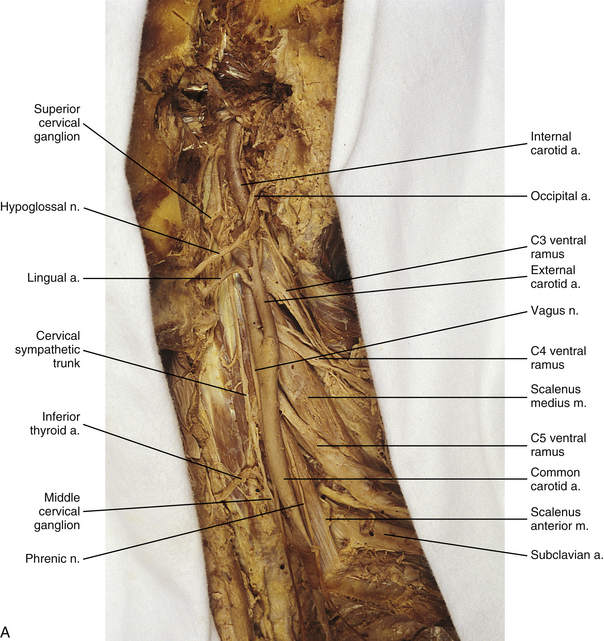
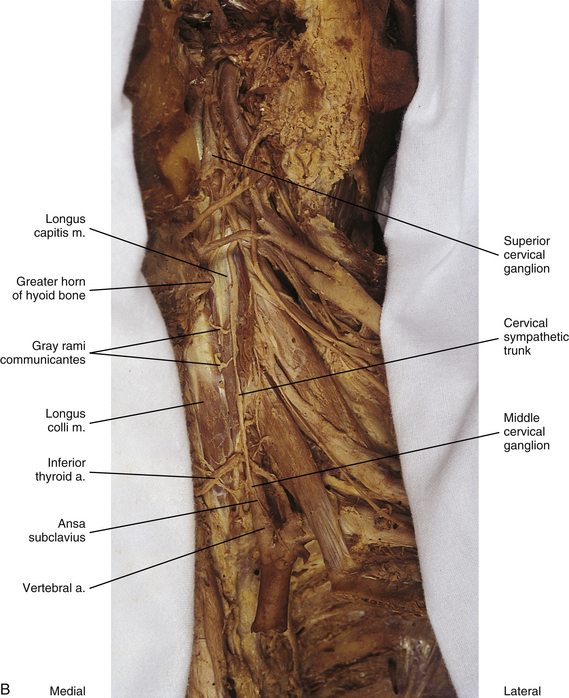
FIG. 10-10 A, Left side of the neck showing the cervical sympathetic trunk. The veins and superior portion of the external carotid artery have been resected. B, The common carotid artery has been reflected laterally to expose the vertebral artery. Notice the relationship of the sympathetic trunk to the longus colli and capitis muscles and note the gray rami communicantes coursing between the two muscles.
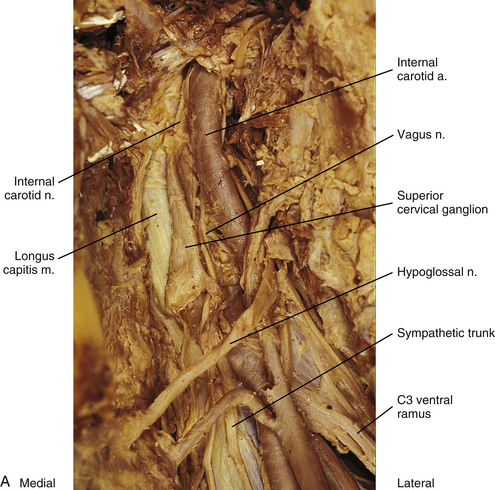
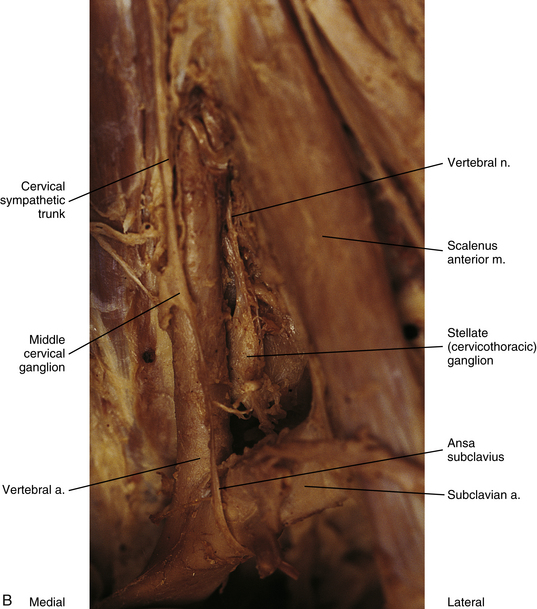
FIG. 10-11 A, Lateral view of the superior aspect of the deep region of the neck near the base of the skull showing the superior cervical ganglion. The internal carotid nerve (postganglionic fibers) is coursing with the internal carotid artery into the carotid canal. B, Stellate ganglion, middle cervical ganglion, and ansa subclavius. The inferior thyroid artery has been resected. The vertebral nerve, which travels with the vertebral artery, courses from the superior aspect of the stellate ganglion.
The middle cervical ganglion (see Figs. 10-9, A, and 10-10) is formed by the fusion of the C5 and C6 ganglia. It is the smallest (≈0.89 cm long and 0.5 cm wide), and often is absent. It lies anterior to the transverse process of the C6 vertebra and near the inferior thyroid artery, which is a branch of the thyrocervical trunk. Measurements show that the ganglion is located 5.95 cm (mean distance) inferior to the superior ganglion and 1.28 cm (mean distance) superior to the cervicothoracic ganglion. In one study, instead of one ganglion, upper and lower ganglia were present 10% of the time (Saylam et al., 2009). Postganglionic fibers include gray rami that enter the C5 and C6 spinal nerves (sometimes the fourth and seventh), thyroid branches (to the thyroid and parathyroid glands), and the largest sympathetic cardiac branch. The cardiac branch often arises from the trunk itself superior or inferior to the ganglion. In addition, slender branches from the ganglion course to the trachea and esophagus (Standring et al., 2008). The ganglion is continuous with the cervicothoracic ganglion by anterior and posterior branches. Although there is variation to this connection, typically the posterior branch splits around the vertebral artery as it descends to the cervicothoracic ganglion; the anterior component descends and loops around the first part of the subclavian artery before connecting with the cervicothoracic ganglion. This loop is called the ansa subclavia (see Fig. 10-11, B).
The cervicothoracic (stellate) ganglion (CTG) (see Figs. 10-9, A, and 10-11, B) is formed by the fusion of the seventh, eighth, and first thoracic ganglia (and sometimes even the second, third, and fourth thoracic ganglia). Most studies indicate the CTG is present 75% to 88% of the time. In a study of 48 cadavers (Pather et al., 2006) in which the cervicothoracic ganglion was present 84% of the time, it was observed that the ganglion was in the shape of either a spindle (28%), a dumbbell (27%), or an inverted “L” (45%). The mean length and width were 16.51 and 6.65 mm, respectively. Another study (Zhang et al., 2009) found the CTG was present 80% of the time and its measurements were about 19.3 mm long and 6.5 mm wide. It is located between the base of the TP of C7 and the neck of the first rib and adjacent to the first intercostal space. It lies on or just lateral to the longus colli muscle and typically behind the vertebral vessels (Saylam et al., 2009) and is in close proximity to the C8 and T1 roots of the brachial plexus (Pather et al., 2006). With the neck fully extended, the ganglion lies two finger’s breadths above the sternoclavicular joint at the level of the transverse process of C7 (Standring et al., 2008). A small ganglion, called the vertebral ganglion, may be present on the sympathetic trunk. It lies anterior or anteromedial to the origin of the vertebral artery and directly above the subclavian artery. The ganglion is thought to be a detached portion of the middle cervical ganglion or stellate/inferior ganglion. Fibers from the ganglion may form or join the ansa subclavia. Other fibers surround the vertebral artery and connect to the stellate ganglion supplying gray rami to the fourth and fifth or the fifth and sixth cervical spinal nerves along the way (Kiray et al., 2005; Standring et al., 2008). The presence of the vertebral ganglion is variable. Saylam and colleagues (2009) found it in only 8% of cadaveric sympathetic trunks whereas Kiray and colleagues (2005) found it in 33% of cadaveric specimens. The ganglion was observed with (12.5%) or without (20.8%) the presence of a middle cervical ganglion (Kiray et al., 2005).
Some postganglionic fibers of the cervicothoracic ganglion travel in gray rami communicantes to enter the C6, C7, C8, and T1 spinal nerves. Song and colleagues (2010) analyzed the morphologic characteristics of these gray rami found in 33 cadavers. The results showed that the gray rami were divided into lateral and medial groups. The lateral group included rami from the CTG to the C8 and T1 nerves. Usually 2 rami coursed to the T1 nerve and only 1 to the C8 nerve although there was some variation, 1-4 and 0-3, respectively. The medial group of rami were associated with the C6, C7, and C8 spinal nerves. The gray ramus joining the C6 nerve root was present on only six sides. The ramus ascended on the posteromedial side of the vertebral artery. Before joining its C6 root, two of the six sides were observed to give off a branch that ascended along the artery to join the C5 nerve root. Medial gray rami connected the CTG with the C8 and C7 nerve roots in 66 and 63 sides, respectively, and in most cases they were shown to give a branch to the adjacent superior nerve root before joining the C8 or C7 root. The gray ramus destined for the C8 nerve root gave off a branch to the C7 nerve root that usually ascended through the C7 transverse foramen. The gray ramus coursing to the C7 root gave off a major branch that then diverged into other small branches. The majority of these ascended through the transverse foramen posteromedially to the vertebral artery to the C6 root and gave off branches to the artery and local joint capsules.
Other postganglionic fibers (83.7%) exit and form a cardiac branch. Most of the time (97.9%) two branches form (an inferior cervical ganglion ramus and a first thoracic ganglion ramus) and contribute to the cardiac plexus located behind the aortic arch (Pather et al., 2006). Some other fibers form plexuses that course on the subclavian artery and its branches, such as the internal thoracic artery, the first part (typically) of the axillary artery, and the vertebral artery. The ascending branch coursing on the vertebral artery is large (see Figs. 10-9, A, and 10-11, B), and frequently called the vertebral nerve (see Chapter 5 and Fig. 5-32). Saylam and colleagues (2009) showed the nerve arising singly from the inferior cervical/cervicothoracic ganglion 85% of the time and arising as two branches, one from that ganglion as well as the vertebral ganglion 15% of the time. In the majority of the specimens (70%) the vertebral nerve was located posterior to the vertebral artery but it also was found traveling anterior, posteromedial, and posterolateral to the artery. Some authors suggest the nerve is essentially a long deep gray ramus from the CTG coursing to the lower cervical spinal nerves within the transverse foramen. Others propose that it looks like a nerve because fibers of cervical gray rami interconnect with each other and with ventral rami of cervical nerves within and between the transverse foramina (Tubbs et al., 2007; Standring et al., 2008; Song et al., 2010). Articular branches, branches to the disc, and branches to the dura come off of the ramus/nerve (Tubbs et al., 2007; Standring et al., 2008). Also, other slender branches intermingle with gray rami communicantes to form the vertebral plexus, which is associated with the vertebral artery (see Cervical Sympathetics in Chapter 5 for further details on the vertebral artery sympathetic nerve plexus). Deep rami communicantes branch from the vertebral plexus and travel with ventral rami of the first five or six cervical spinal nerves. In addition to sympathetic (efferent) fibers, the plexus also contains cervical somatic afferent fibers that innervate the adventitia of the vertebral arterial wall. The plexus travels into the cranial cavity on the vertebral artery and continues on the basilar artery (and its branches) as far as the posterior cerebral artery, where it continues anteriorly to join the internal carotid artery plexus (Standring et al., 2008). The vertebral plexus may be the major continuation of the sympathetic system into the cranium.
Because of its location in the sympathetic chain and the fact that its fibers course to upper extremity and thoracic cavity effectors, lesions of the cervicothoracic ganglion can affect the sympathetic innervation of the head, neck, upper extremity, and thoracic viscera. Having knowledge of the anatomy of this ganglion as well as the anatomy of its gray rami is important (see Kiray et al., 2005; Saylam et al., 2009; Song et al., 2010; for specific morphometric dimensions). Thoracoscopic sympathectomy procedures are commonly used for sympathetically mediated syndromes affecting the upper extremities such as complex regional pain syndrome (CRPS) and hyperhidrosis and also for refractory anginal pain. In some cases the cervicothoracic ganglion needs to be preserved and in other situations the ganglion is selectively blocked. Care must be taken when performing procedures in this area; if the ganglion is injured during thoracic sympathectomy, Horner’s syndrome may result (Pather et al., 2006) (see Clinical Applications section).
Although the cervical sympathetic chain has no white rami communicantes associated with it, numerous gray rami are associated with each spinal nerve. Also, cervical gray rami may pierce the longus capitis and scalenus anterior muscles as they course to the cervical spinal nerves (Standring et al., 2008). Pathological conditions in cervical vertebrae and cervical soft tissue may affect gray rami associated with cervical spinal nerves and the brachial plexus (Song et al., 2010).
Thoracic sympathetic trunk: Eleven small ganglia usually (70% of the time) are found in the thoracic sympathetic chain (Fig. 10-12; see also Fig. 10-9, B, Figs. 6-11 to 6-13). (Note that approximately 80% of the time the T1 ganglion is fused with the inferior cervical ganglion, in which case the succeeding ganglion is still named the second [T2].) Each ganglion includes 90,000 to 100,000 neurons (Harati, 1993). The thoracic chain lies adjacent to the heads of the ribs and posterior to the costal pleura. In this region of the chain, white rami communicantes, as well as the gray rami communicantes, are clearly evident (see Fig. 10-12, B). The white rami lie more distal (lateral) than the gray rami, and two or more rami may be connected to one spinal nerve. A mixed ramus formed by the fusion of the gray and white rami sometimes may be present. The anatomy of the upper sympathetic thoracic trunk and any variations are clinically important because the trunk is often the site for surgical procedures (sympathectomy, sympathicotomy, clipping of the sympathetic chain, and ramicotomy) as treatments for pathologies such as essential palmar hyperhidrosis, complex regional pain syndrome (CRPS), and Raynaud’s phenomenon. Because of the importance of this region, Cho and colleagues (2005) and Zhang and colleagues (2009) performed cadaveric studies on the anatomy of the upper thoracic trunk. Zhang and colleagues (2009) found that rather than being located anterior to the proximal aspect of the ribs the T2, T3, and T4 ganglia were located in the intercostal spaces 92%, 68%, and 50% of the time, respectively, and that as the trunk descended the ganglia were more likely to be found either at the upper border or on the surface of the lower rib. Additional rami communicantes were also noted and described as follows: intercostal rami that connected adjacent intercostal nerves or ventral rami; ascending rami that connected the sympathetic ganglion with the intercostal nerve or ventral rami at the level above; descending rami that connected the ganglion to the intercostal nerve at the level below. The first intercostal ramus ascending from the second ganglion (nerve of Kuntz) was present 40% of the time but only bilaterally 16% of the time. The only other ramus found was the second intercostal ramus, which was seen in only 6% of the specimens. Ascending rami coursing from the T2, T3, and T4 ganglia were present 40%, 16%, and 6% (Zhang et al., 2009) and 54%, 6%, and 5% (Cho et al., 2005) of the time, respectively, and descending rami coursing from these ganglia were present 30%, 10%, and 8% (Zhang et al., 2009) and 46%, 25%, and 8% (Cho et al., 2005) of the time, respectively. It was infrequent that a ganglion would have both ascending and descending branches. The additional rami communicantes and their relationship with intercostal nerves may act as a conduit for sympathetic fibers to bypass the chain and may be an explanation as to why surgeries on the sympathetic trunk or rami sometimes fail and symptoms reoccur. Another study looked at the relationship of the sympathetic trunk and the intercostal veins at the level of the third and fourth intercostal spaces. The size of the vein and whether the vein crossed anteriorly or posteriorly to the trunk were analyzed. The results showed that large veins (defined as being more than half the width of the intercostal space), which are susceptible to bleeding, and veins crossing anteriorly to the trunk were found more often on the right side than the left. Surgical procedures performed in these intercostal spaces on the right side would present a greater risk for bleeding to occur (Haam et al., 2010). Surgeons should be aware of these variations when upper limb sympathectomies are performed. Postganglionic fibers originating from all thoracic ganglia course in gray rami and enter the thoracic spinal nerves and travel with them to effectors. Some postganglionic fibers from the T1 to T5 ganglia form direct branches to the thoracic aortic, cardiac, and pulmonary plexuses of the thorax. Other large branches of the T5 to T12 ganglia supply the aorta and are associated with the three splanchnic nerves involved with the sympathetic innervation of the abdominal and pelvic viscera. These splanchnic nerves are mixed nerves in that they consist of preganglionic fibers that synapse in prevertebral ganglia located in the abdominal cavity and visceral afferent fibers (see section on Visceral Afferents).
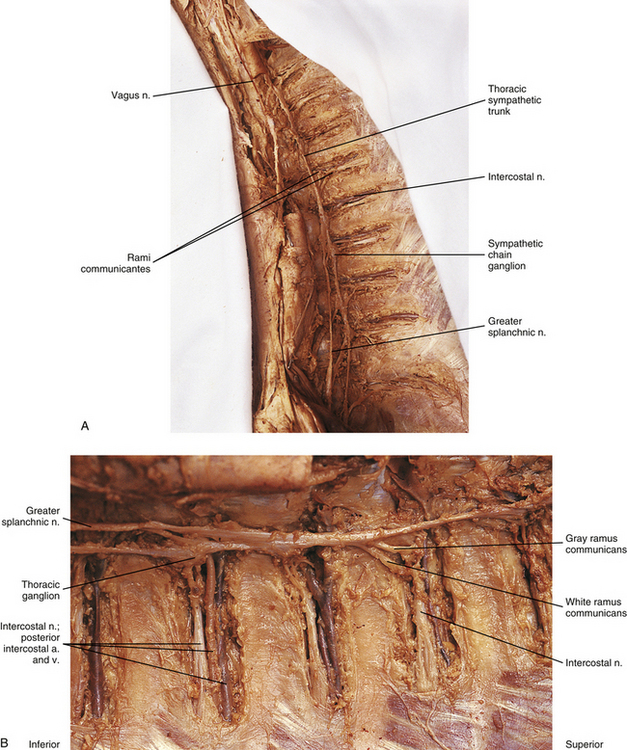
FIG. 10-12 A, Thoracic sympathetic trunk. B, Closer view of the left thoracic sympathetic trunk. Both white and gray rami communicantes are shown in relationship to the intercostal nerve, artery, and vein. The greater splanchnic nerve (preganglionic fibers) is coursing inferiorly and medially from the sympathetic trunk into the abdominal cavity.
The greater splanchnic nerve (see Figs. 10-9, B and 10-12, A) contains preganglionic fibers exiting medially from the T5 to T9 or T10 ganglia, although there is some variation (see below). As it descends obliquely on the vertebral bodies, it sends branches to the descending thoracic aorta and then pierces the crus of the diaphragm. The main trunk enters the celiac ganglion whereas other fibers course to the medulla of the adrenal gland and sometimes the aorticorenal ganglion. In the ganglia, the preganglionic fibers of the greater splanchnic nerve synapse on postganglionic neurons and interneurons (Standring et al., 2008). The lesser splanchnic nerve consists of preganglionic fibers from the T9 and T10 or T10 and T11 ganglia (see below) and is present 94% of the time. It traverses the diaphragm and enters the abdominal cavity to synapse in the aorticorenal ganglion (the detached lower part of the celiac ganglion) and may supply the lateral side of the celiac ganglion. The third splanchnic nerve is the lowest or least splanchnic nerve and is present 56% of the time. Sometimes this nerve is called the renal nerve. It emerges medially from the T12 (or lowest) ganglion although this is variable (see below) and travels with the sympathetic chain under the medial arcuate ligament of the diaphragm and inferiorly to enter the renal plexus. The majority of the fibers enter the aorticorenal ganglion and may also supply the lateral side of the celiac ganglion. Sometimes it may appear as a part of the lesser splanchnic nerve connecting the aorticorenal ganglion to the renal plexus (Harati, 1993; Standring et al., 2008). From these prevertebral ganglia, postganglionic fibers participate in the formation of the various perivascular plexuses as they travel to abdominal effectors. Because of the clinical relevancy of the splanchnic nerves (see below), Gest and Hildebrandt (2009) conducted a cadaveric study on the splanchnic nerves and their course through the diaphragm. All 3 splanchnic nerves were found 57% (25 of 44 sides) of the time; the least splanchnic was missing in 43% of the sympathetic chains whereas the greater and lesser nerves were always present. Of the 6 different patterns by which the nerves passed through the crura of the diaphragm, the 2 most common were all 3 passing through a hiatus together (16 of 44) and the greater and lesser nerves passing through together (18 of 44). In 3 specimens the greater nerve traveled through alone and the lesser and least nerves ran together, and in 4 of 44 specimens the greater and lesser nerves coursed together and the least nerve passed through alone. In only 3 of 43 specimens did the nerves traverse the diaphragm separately; 65% of the time the pattern was symmetric between the right and left sides. In only two specimens did the least nerve pass through the diaphragm with the sympathetic trunk on both sides. It was also noted in 17 of 35 specimens that the angle of the subdiaphragmatic portion of the greater splanchnic nerve coursing to the celiac ganglion was 90 degrees or greater relative to the intrathoracic portion of the nerve.
Thoracic splanchnic nerves (and the celiac ganglion) are very important from a clinical standpoint because visceral afferent fibers conveying nociception from upper abdominal organs course within these nerves. Splanchnicectomy is a surgical procedure that has been used to treat a number of pathologies such as chronic spastic constipation, chronic intestinal pseudoobstruction, and hypertension. The procedure is also performed to manage intractable abdominal pain related to pancreatic cancer and chronic pancreatitis. Patients with chronic pancreatitis complain of severe intermittent or long-lasting and persistent pain caused likely by damage to intrapancreatic nerves. Patients with pancreatic cancer, although presenting initially with weight loss and jaundice, experience pain thought to be caused by the infiltration of cancer cells into the perineurium of local nerves and extrapancreatic nerves such as those related to the celiac and/or mesenteric plexuses. It appears therefore that pancreatic neuropathy may be the stimulus for the intense pain experienced by patients with both of these conditions. The splanchnicectomy may be performed at the intrathoracic level or at the subdiaphragmatic, retroperitoneal level. Variations of the procedure include dissection of the nerves or transection of the main trunks of the nerve, thermocoagulation, division or transection of the nerve branches, and excision of small sections of the nerve (Loukas et al., 2010). There have been numerous anatomic studies (reviewed by Loukas and colleagues [2010]) indicating (1) variations in the levels and ganglia contributing to the formation of each of the thoracic splanchnic nerves, (2) the absence of a contributing root from a ganglion to the nerve, (3) variations in the frequency of the lesser and least nerves being present, (4) a lack of symmetry between right and left sides in an individual, (5) alternate neural pathways (e.g., thin fibers that interconnect the greater and lesser nerves or the greater and least nerves, and the presence of a fourth accessory splanchnic nerve), and (6) anatomic variations in the distal subdiaphragmatic segments of the nerves (Gest & Hildebrandt, 2009). It is clear that an understanding of the detailed anatomy of these structures and their variations between right and left sides and among patients is essential to have successful outcomes from surgical procedures.
Lumbar sympathetic trunk: The thoracic sympathetic trunk passes posterior to the medial arcuate ligament (or sometimes through the crura of the diaphragm) to become continuous with the lumbar sympathetic trunk found within the abdominal cavity. The trunk has been described as consisting of 4 interconnected lumbar ganglia (each of which contains 60,000 to 85,000 neurons) (Harati, 1993; Standring et al., 2008). However, other data indicate that the number of ganglia varies (Mitchell, 1953; Rocco, Palombi, & Raeke, 1995). Murata and colleagues (2003) studied cadaveric specimens and looked at the anatomic variations of the ganglia and associated rami. They found that the number of ganglia on one side ranged from 2 to 6 (mean, 3.9) and that the majority of ganglia were located on the L2 and L3 vertebrae. Typically no ganglia were found on the L1 and L4 vertebrae. Approximately 40% of the cadavers showed the same number of ganglia on both sides, and those were asymmetrically located. In addition, Murata and colleagues (2003) found 5 to 12 rami communicantes per side (mean of 7.2); in addition, they noted that more than 1 ramus communicans could connect to a lumbar ventral ramus and that a lumbar ventral ramus often received rami communicantes from more than 1 ganglion. In fact, one third of the ganglia associated with the L2 and L3 vertebrae included rami that traveled to three spinal nerves. Rami associated with the L1-5 spinal nerves were measured, and it was found that the ramus of the L4 spinal nerve was significantly similar in length to the L2 and L3 rami. Although this is contrary to what would be expected because the sympathetic chain lies closer to the intervertebral foramina (IVFs) at the L4-5 vertebral levels, it may be due to the fact that there are fewer ganglia at the L4 level and the rami have farther to travel to reach a more superiorly located ganglion. The L1 rami also were significantly longer than the other lumbar rami, most likely for the same reason. The rami of L5 were significantly shorter than those of L1-4. The presence of anatomic variations in the rami and ganglia in this region may be of importance relative to the nociceptive pathway for lower lumbar structures. Based on studies in rats that may apply to humans as well, it has been suggested that nociceptive input from low back structures is carried in two different pathways. Some nociceptive fibers from low back pain generators travel in a segmental fashion, directly within spinal nerves, and terminate in local cord segments. Other nociceptive fibers terminate centrally in a nonsegmental fashion. These course in lower lumbar spinal nerves, enter the sympathetic chain and ascend, and then exit the chain through more superiorly located rami connected to the L2 spinal nerve. These fibers terminate in the lower region of the thoracolumbar cord segments associated with the sympathetic division (Murata et al., 2003). If this pathway is present in humans it may contribute to the wide referral pattern seen in lower lumbar intervertebral discs, lower lumbar zygapophysial (Z) joints, and sacroiliac joint pain conditions (Murata et al., 2000) (see Chapters 7 and 11 for further information).
The lumbar trunk lies adjacent to the anterolateral aspect of the upper lumbar vertebrae and becomes more posterior relative to lower lumbar vertebrae (Murata et al., 2003). It also lies adjacent to the medial margin of the psoas major muscle (Fig. 10-13; see also Fig. 10-9, C). The inferior vena cava, right ureter, and lumbar lymph nodes lie anterior to the right sympathetic trunk. The left sympathetic trunk lies posterior to the aortic lymph nodes and lateral to the aorta. These relationships are important surgically because lumbar ganglia may have to be removed (lumbar sympathectomy; see Innervation of Peripheral Effectors) to treat certain arterial diseases of the lower extremities (Snell, 2008; Standring et al., 2008).
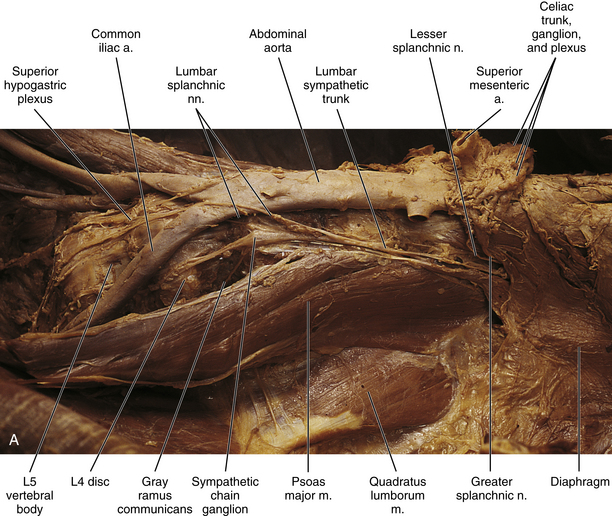
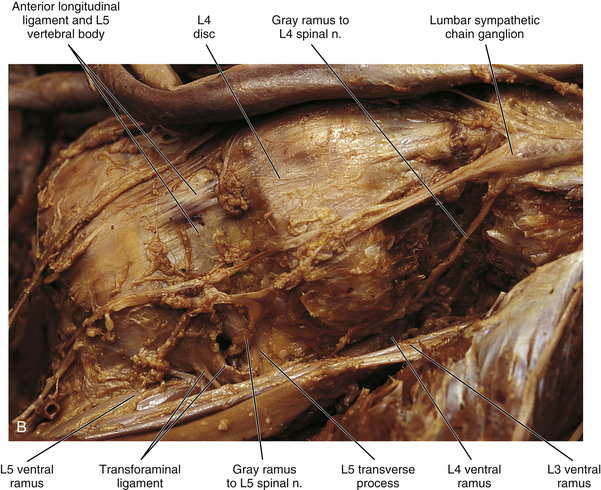
FIG. 10-13 Left lumbar sympathetic trunk. A, Notice the relationship of the sympathetic trunk with the psoas major muscle and vertebral bodies and discs. Lumbar splanchnic nerves are coursing from the sympathetic trunk to the superior hypogastric plexus. The greater and lesser splanchnic nerves pass through the diaphragm to synapse in the celiac ganglion and aorticorenal ganglion. The celiac trunk, superior and inferior mesenteric, and renal arteries have been resected. The inferior vena cava also has been resected. B, Lumbar sympathetic trunk at the level of the L4 and L5 vertebrae. The left common iliac artery has been reflected. The psoas major muscle also has been reflected. Notice the long gray rami communicantes. A transforaminal ligament spanning the intervertebral foramen is present in this specimen. Note the relationship of the L5 ventral ramus and gray ramus communicans to this ligament.
White rami communicantes are associated with the upper two or three ganglia. Some preganglionic fibers descend in the chain to synapse in any lumbar ganglia below the L2 or L3 ganglia and in pelvic ganglia (see below). The gray rami are long (see previous section) and they course with lumbar arteries along the sides of the vertebral bodies to join each lumbar spinal nerve (see Fig. 10-13). The majority of these postganglionic fibers are thought to use the femoral nerve, obturator nerve, and their muscular and cutaneous branches to supply vasoconstrictor fibers to the adjoining blood vessels and fibers to cutaneous effectors. In a manner similar to the lower thoracic ganglia, some preganglionic fibers pass through the lumbar ganglia to form lumbar splanchnic nerves, which will also contain visceral afferent fibers. In general, each lumbar splanchnic nerve corresponds to its ganglion of the same number, although the second lumbar splanchnic nerve receives additional fibers from the third ganglion and the third lumbar splanchnic nerve also receives a contribution from the fourth ganglion. The four splanchnic nerves course into the abdomen and become part of the abdominal plexuses: the first splanchnic nerve courses within the celiac, abdominal aortic (intermesenteric), inferior mesenteric, and renal plexuses; the second splanchnic nerve contributes to the inferior part of the abdominal aortic (intermesenteric) plexus or inferior mesenteric plexus; the third splanchnic nerve passes anterior to the common iliac vessels and travels within the superior hypogastric plexus (see Figs. 10-9, C, and 10-13); the fourth splanchnic nerve also courses anterior to the common iliac vessels and contributes to the lowest portion of the superior hypogastric plexus (or hypogastric nerve). All lumbar ganglia give off vascular branches that enter the abdominal aortic plexus. Lower lumbar splanchnic nerves give off branches that run on the common iliac arteries and continue as a network that extends onto the internal and external iliac arteries (Standring et al., 2008). The lumbar portion of the sympathetic trunk passes inferiorly, posterior to the common iliac vessels, and becomes continuous with the pelvic portion of the trunk.
Pelvic sympathetic trunk: The pelvic chain consists of four or five ganglia that lie in extraperitoneal tissue on the anterior aspect of the sacrum beneath presacral fascia. Within the chain are descending preganglionic fibers from the lower thoracic and the upper two or three lumbar cord segments that will synapse in pelvic ganglia. Each side unites to form the ganglion impar on the anterior aspect of the coccyx (Fig. 10-14; see also Fig. 10-9, C). Postganglionic fibers leave the chain in gray rami to enter the sacral spinal nerves and coccygeal nerve. Fibers destined for blood vessels in the leg and foot course primarily with the tibial nerve to connect subsequently with (and supply) the popliteal artery and its branches in the leg and foot. Other fibers travel with the pudendal and gluteal nerves to the internal pudendal artery and gluteal arteries and their branches. In addition, some medial fibers from the first two sacral ganglia referred to as the sacral splanchnic nerves travel within the inferior hypogastric plexus (or hypogastric nerve) to the pelvic viscera as a delicate network of pelvic nerves (the pelvic plexus) (Standring et al., 2008).
Plexuses of the Autonomic Nervous System
The autonomic plexuses that have been mentioned are a network of autonomic fibers (both sympathetic and parasympathetic) and ganglia found in the thoracic, abdominal, and pelvic cavities. They surround, and usually are named after, the large blood vessels with which they travel. In addition to the named ganglia associated with the plexuses, there are often small ganglia scattered within the plexuses. A considerable amount of intermingling of fibers of the plexuses and of the ganglia occurs, especially in the major plexuses near the abdominal aorta. The plexuses supply the autonomic effectors within the thorax, abdomen, and pelvis. The effectors and their specific innervation are discussed later in this chapter.
The cardiac, pulmonary, celiac, and hypogastric plexuses are the major plexuses (Standring et al., 2008), although secondary plexuses may emanate from each one. The cardiac plexus, which is divided into deep and superficial parts, consists of cardiac branches from cervical and upper thoracic ganglia mixed with cardiac branches of the vagus nerve (see Fig. 10-9, B). A continuation of the cardiac plexus forms secondary coronary and atrial plexuses. The pulmonary plexus is an extension of fibers of the cardiac plexus that course with the pulmonary arteries to the lungs. Therefore the cardiac and pulmonary plexuses consist of the same sympathetic and vagal branches.
The celiac plexus is the largest autonomic plexus (see Fig. 10-9, C). It is located at the level of the T12 and L1 vertebrae and surrounds the celiac artery and the base of the superior mesenteric artery. It is posterior to the stomach and lesser omentum and anterior to the crura of the diaphragm and the beginning of the abdominal aorta. The adrenal glands lie on either side of the plexus. This plexus is a dense fibrous network that interconnects the paired celiac ganglia. Mingling with the celiac plexus and ganglia are the greater and lesser splanchnic nerves and also branches of the vagus and phrenic nerves (Standring et al., 2008). Numerous subsidiary ganglia and fibers extend from the celiac plexus and course along abdominal blood vessels to autonomic effectors. These fibers and ganglia form (in some cases with the help of the lesser and least splanchnic nerves) plexuses that include the phrenic, hepatic, gastric, splenic, testicular, ovarian, superior mesenteric (to small and large intestines), renal, and suprarenal. As one can see, these ganglia and plexuses are responsible for the innervation of the abdominal viscera. The celiac ganglia are irregular masses located on each side of the celiac trunk medial to the adrenal glands and anterior to the crura of the diaphragm. Both are related to other blood vessels in the region (i.e., the left lies posterior to the origin of the splenic artery and the right lies posterior to the inferior vena cava). As observed in other structures related to the sympathetic system, there is much variation in the location of the ganglia (usually at the level of T12 or L1) and in their morphology. Because a celiac plexus block is a surgical procedure used to manage pancreatic pain in conjunction with or as an alternative to splanchnicectomy, understanding the normal anatomy and any variations in this region is essential in order to have positive surgical outcomes (Loukas et al., 2010). The greater splanchnic nerve enters the upper part of each ganglion and the lesser splanchnic nerve ends in the lower part. A portion of the lower part of each ganglion forms a distinct region called the aorticorenal ganglion, which also receives fibers from the lesser splanchnic nerve (Loukas et al., 2010). Fibers from this ganglion form the majority of the renal plexus, which lies anterior to the beginning of the renal artery. A superior extension of the celiac ganglion develops into the phrenic plexus, with the left usually larger than the right. The left innervates the left adrenal gland and stomach (cardiac orifice); the right travels with the right phrenic nerve, creating a small phrenic ganglion, and subsequently innervates the right adrenal gland and inferior vena cava, and contributes to the hepatic plexus. The hepatic plexus supplies innervation to the liver, gallbladder, bile ducts, stomach, duodenum, and pancreas. It contains afferent branches and efferent sympathetic and parasympathetic fibers. An inferior extension of the celiac plexus is the superior mesenteric plexus. These fibers, which also include posterior vagal fibers, course with the superior mesenteric artery; secondary plexuses course with its branches. The plexus and its ganglion are near the origin of the superior mesenteric artery, with the ganglion located superior to the artery’s origin. Associated with the aorta and lying between the origins of the superior and inferior mesenteric arteries is the abdominal aortic plexus (intermesenteric plexus). This plexus consists of 4 to 12 interconnecting nerves and is created by parasympathetic and sympathetic fibers traveling from the celiac plexus and branches of the first and second lumbar sympathetic nerves. It is continuous with the superior mesenteric plexus above and with the superior hypogastric plexus below and it sends fibers to the gonadal, inferior mesenteric, superior hypogastric, and iliac plexuses. The inferior mesenteric plexus consists of fibers primarily from the aortic plexus and the first and second lumbar splanchnic nerves and also from the superior hypogastric plexus. It is located near the origin of the inferior mesenteric artery and its fibers course with the artery and its branches (Standring et al., 2008).
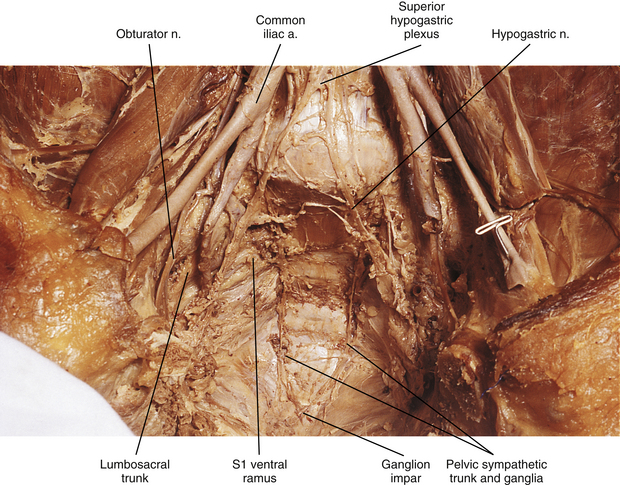
The superior hypogastric plexus (Fig. 10-13, A, and see Fig. 10-9, C) lies in extraperitoneal connective tissue and is formed by the third and fourth lumbar splanchnic nerves and fibers of the aortic plexus. The size and extent of the interconnections of the branches vary. It may contain some pelvic afferent (nociceptive) fibers (Correia et al., 2010) and some parasympathetic fibers of the pelvic splanchnic nerves that have ascended from the inferior hypogastric plexuses within the two hypogastric ‘nerves’. The plexus lies anterior to the bifurcation of the aorta at the level of the L5 vertebral body usually to the left of the midline. It lies between the common iliac arteries and anterior to the left common iliac vein and medial sacral blood vessels. It is anatomically closely related to the adjacent viscera in that anterior and to the left of the inferior part of the plexus is the attachment of the sigmoid mesocolon and the superior part of the mesorectum within which superior rectal vessels travel. Only a thin layer of connective tissue separates the plexus and these visceral structures. This plexus sends branches to the testicular, ureteric, ovarian, and common iliac plexuses and to plexuses around the superior rectal arteries. As the superior hypogastric plexus descends into the pelvic cavity, it divides into left and right hypogastric nerves on each side of the rectum that continue caudally to form the left and right inferior hypogastric plexuses (see Fig. 10-9, C). The superior hypogastric plexus, in general, contains the sympathetic fibers that innervate the rectum, oviducts, and uterus and the fibers that provide vesical tone. It also contains the afferent fibers supplying pelvic viscera. Specifically, it contains sympathetic fibers that control the ejaculatory response and the closing of the sphincter of the bladder neck, which prevents reflux of the ejaculate into the bladder. Muscle tone of the internal urethral sphincter is also controlled by these fibers. In females, some of the sympathetic fibers are vasoconstrictive to the blood vessels supplying the uterus, vulva, and vagina. The superior hypogastric plexus, like other autonomic plexuses, is not immune to anatomic variations. Correia and colleagues (2010) studied male and female adult cadavers and fetuses and found variations in the height and width of the plexus, the distance from the plexus to the point where the common iliac artery bifurcates, the angle at which the hypogastric nerves emerge from the plexus, and the morphology of the plexus. Awareness of variations is essential to prevent functional losses, such as ejaculatory dysfunction, during medical procedures performed in the pelvic region. These procedures may be necessary to remedy pathologies related to the medical fields of proctology, urology, gynecology, angiology, and orthopedics and for procedures such as anesthetic blockades of the plexus, video-laparoscopic surgeries, and retroperitoneal lymphadenectomy surgeries. Knowledge of these variations is also relevant so that medical images may be accurately read and evaluated (Corrreia et al., 2010).
The inferior hypogastric plexus lies in thin extraperitoneal connective tissue on the side of the pelvic wall and is located on the internal iliac vessels. It lies medial to the attachments of the levator ani, coccygeus, and obturator internus muscles. The plexus lies on each side of and posterolateral to the posterior part of the bladder, seminal vesicles, and prostate gland in males. It is lateral to the posterior part of the bladder, cervix of the uterus, and fornix of the vagina in females and may extend into the broad ligament of the uterus. It is formed from pelvic splanchnic parasympathetic fibers (originating from the S2-4 cord segments) and sympathetic sacral splanchnic fibers from sacral ganglia. A few sympathetic fibers from lower lumbar ganglia also contribute to the plexus. Some sympathetic fibers are found within lumbar splanchnics that have traveled via the inferior hypogastric nerves (see below) from the superior hypogastric plexus. The inferior hypogastric plexus forms small branches, the ‘pelvic plexus’, that course directly to or run with arteries to supply the vas deferens, penis, and reproductive glands in the male; the ovary, oviducts, uterus, and vagina in the female; and the bladder and distal ureter in both males and females. Also coursing within the inferior hypogastric plexus are branches of the parasympathetic pelvic splanchnic nerves. The inferior hypogastric ‘nerves’ that connect the inferior and superior hypogastric plexuses consist primarily of sympathetic and some parasympathetic fibers. Most of these are ascending (coursing up and out of the pelvis) from the inferior hypogastric plexus. The ‘nerves’, which may be double or form a plexus, are located in loose connective tissue posterolateral to the origin of the mesorectum and travel medial to the internal iliac vessels over the pelvic brim (Standring et al., 2008). Extensions of the inferior hypogastric plexus, which include the middle rectal, prostatic, uterovaginal, and vesical plexuses, continue along the branches of the internal iliac artery to innervate autonomic effectors of the pelvis. The ANS innervation of the most clinically important effectors of the pelvis is discussed later in this chapter.
Parasympathetic Division
The parasympathetic division generally is concerned with conserving and restoring energy. It is coordinated with the sympathetic division in the dual and antagonistic innervation of autonomic effectors (see Table 10-1). However, there is no parasympathetic innervation of autonomic effectors located in the extremities and body wall (i.e., sweat glands, arrector pili muscles, peripheral blood vessels). Because the sympathetic division has been nicknamed the fight-or-flight division, the parasympathetic division could appropriately be named the rest-and-digest division, because in general, parasympathetic activation results in decreased heart rate and increased GI glandular secretion and peristalsis. In contrast to the widespread control by the sympathetic system, the parasympathetic division controls effectors at a more local level. This relates to the overall pattern of organization of the parasympathetic division of the ANS. Compared with the sympathetic division, each parasympathetic preganglionic neuron synapses with fewer postganglionic neurons, and the location of the parasympathetic ganglia is near, or frequently within, the wall of the effector organ. The ganglia are the site of synapses between preganglionic and postganglionic neurons; however, afferent fibers, postganglionic sympathetic fibers, and even branchial arch efferent fibers can course through them.
The parasympathetic division also is called the craniosacral division. As with the thoracolumbar (sympathetic) division, this name refers to the location of the cell bodies of preganglionic neurons (see Fig. 10-2). These cell bodies are located in autonomic nuclei of the brain stem (cranio) and in the second, third, and fourth sacral cord segments (sacral). The parasympathetic efferents of the sacral cord course within the ventral roots and within pelvic splanchnic nerves. These nerves do not use the sympathetic trunk. Axons of the cell bodies located in the brain stem leave the brain stem traveling within the oculomotor, facial, glossopharyngeal, and vagus cranial nerves. Although numerous branches of various cranial nerves include these parasympathetic efferents, only the major branches are described. The somatic functions of the four cranial nerves are not discussed because this chapter is devoted to the innervation of autonomic effectors.
Cranial Portion of the Parasympathetic Division
Oculomotor nerve: The oculomotor nerve (CN III) emerges from the ventral surface of the midbrain of the brain stem (see Chapter 9, Fig. 9-20). The origin of the autonomic efferents is in the Edinger-Westphal nucleus, which is located in the midbrain ventral to the cerebral aqueduct of Sylvius. These preganglionic fibers course within the oculomotor nerve to the ciliary ganglion, where they synapse with postganglionic neurons (Fig. 10-15). This ganglion is less than 2 mm long and contains 3000 multipolar neurons (Harati, 1993). It is located in the orbit just anterior to the superior orbital fissure. Postganglionic fibers course in the short ciliary nerves to the eye and travel between the choroid and sclera of the eye wall. Here the fibers innervate the smooth muscle of the iris (sphincter pupillae) and ciliary body (ciliary muscle). The sphincter muscle of the iris functions to constrict the pupil during the pupillary light reflex and during the accommodation-convergence reflex. Contraction of the ciliary muscle occurs during the accommodation-convergence reflex. The result of this contraction is a thickening of the lens, which improves near vision.
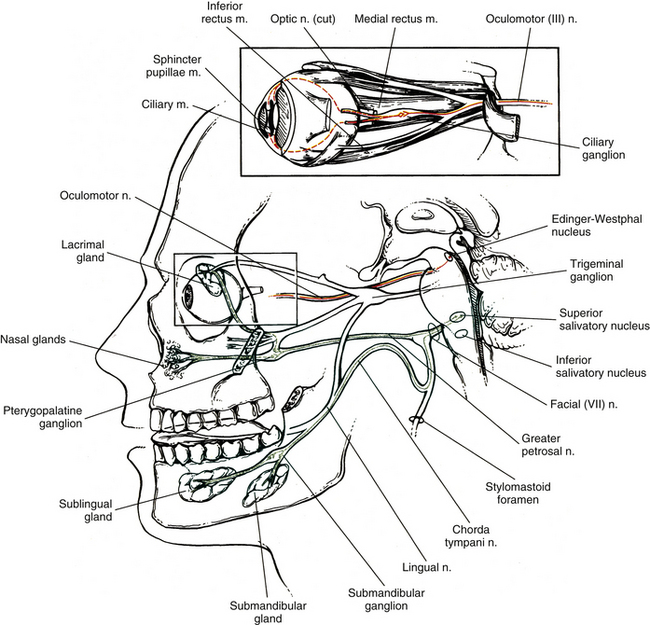
FIG. 10-15 Course of parasympathetic fibers in the oculomotor (red) (cranial nerve III) and facial nerves (green) (cranial nerve VII). Oculomotor preganglionic neuron cell bodies are found in the midbrain in the Edinger-Westphal nucleus. Their axons (red solid line) synapse with postganglionic neurons in the ciliary ganglion located in the orbit. Postganglionic axons (red dashed line) travel to the smooth muscles of the eye (sphincter pupillae and ciliary). Facial nerve preganglionic neuron cell bodies are found in the caudal pons in the superior salivatory nucleus. Their axons (green solid line) synapse with postganglionic neurons in the pterygopalatine ganglion and submandibular ganglion. From these ganglia, postganglionic axons (green dashed line) travel to the lacrimal gland, nasal mucosal, and sublingual and submandibular salivary glands.
Facial nerve: The facial nerve (CN VII) also contains preganglionic fibers. The cell bodies of these fibers are located in the superior salivatory nucleus. This nucleus is located in the caudal part of the pons near the facial motor nucleus. The fibers emerge from the pontomedullary junction in the nervus intermedius portion of CN VII (see Chapter 9, Fig. 9-20). Some of the fibers travel in the chorda tympani nerve, which in turn joins the lingual branch of the mandibular division of the trigeminal nerve (CN V). These preganglionic fibers continue to the submandibular (sublingual) ganglion, where they synapse with postganglionic neurons (see Fig. 10-15). The postganglionic fibers are secretomotor and course to minor salivary glands, as well as to the larger submandibular and sublingual salivary glands. (It has been reported also that stimulation of the chorda tympani nerve results in vasodilation in the salivary glands [Standring et al., 2008].) In addition to the preganglionic fibers en route to the submandibular ganglion, other secretomotor preganglionic fibers from the lacrimal portion of the superior salivatory nucleus course in the greater petrosal nerve to the pterygopalatine ganglion (see Fig. 10-15). This ganglion is approximately 3 mm long and contains 56,500 compactly arranged neurons (Harati, 1993). It is located in the pterygopalatine fossa behind and below the orbit. Postganglionic fibers exit from here and travel in the zygomatic nerve (a branch of the maxillary division of the trigeminal nerve) and terminate in the lacrimal gland. Other secretomotor branches of the pterygopalatine ganglion course to the glands and mucous membranes of the palate and nasal mucosa.
Glossopharyngeal nerve: The glossopharyngeal nerve is CN IX. Preganglionic neurons that course in this nerve originate in the inferior salivatory nucleus, which is located caudal to the superior salivatory nucleus. CN IX emerges as three to five rootlets from the dorso-olivary sulcus on the lateral side of the medulla of the brain stem (see Chapter 9, Fig. 9-20). The preganglionic fibers travel in the lesser petrosal nerve to the otic ganglion, where they synapse with postganglionic neurons (Fig. 10-16, A). The postganglionic fibers are secretomotor, and the axons of these neurons travel in the auriculotemporal nerve (a branch of the mandibular division of the trigeminal nerve) to reach the parotid gland that they innervate. Evidence shows that stimulation of the lesser petrosal nerve results in vasodilation in the parotid gland, as well as serous secretion (Standring et al., 2008).
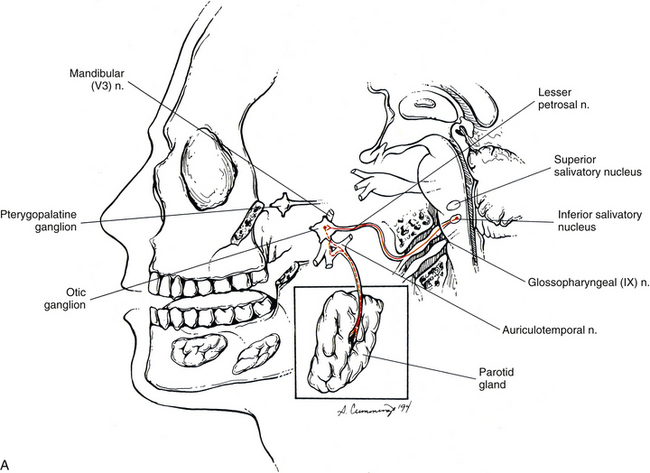
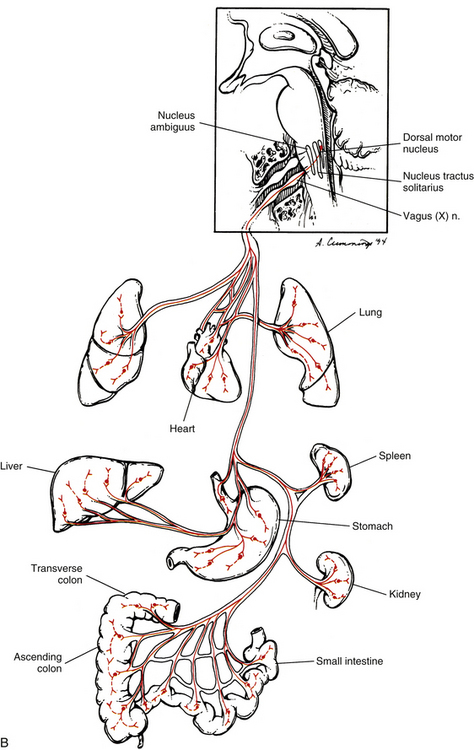
FIG. 10-16 A, Course of parasympathetic fibers within the glossopharyngeal nerve (cranial nerve IX) to the parotid gland. Preganglionic neuron cell bodies are found in the inferior salivatory nucleus in the rostral medulla. Their axons (red solid line) synapse with postganglionic neurons (red dashed line) in the otic ganglion. B, Course of parasympathetic fibers within the vagus nerve (cranial nerve X) to smooth muscle, cardiac muscle, and glands. Preganglionic neuron cell bodies are found primarily in the dorsal motor nucleus located in the medulla. Some cell bodies are located in the nucleus ambiguus (see text). Their axons (solid line) synapse with postganglionic neurons (dashed line) that are in or very near the wall of innervated thoracic and abdominal visceral organs. Some axons (solid line) synapse directly on enteric neurons (dashed line) in the wall of GI organs (see Enteric Nervous System).
Regarding these three cranial nerves and their ganglia, it is of interest to note that sympathetic postganglionic fibers coursing to their effectors may pass through (but not synapse in) the parasympathetic ganglia. They also may travel along with branches of various cranial nerves. Parasympathetic fibers also may “hitch a ride” with peripheral branches of cranial nerves other than III, VII, and IX.
Vagus nerve: The vagus nerve (CN X) also conveys parasympathetic fibers. Although 75% of all parasympathetic efferent fibers are carried by the vagus nerve, only about 10% of vagal fibers are efferent. This nerve is closely related to the glossopharyngeal nerve both anatomically and functionally. Just caudal to the glossopharyngeal nerve the vagus nerve emerges as 8 to 10 rootlets from the dorso-olivary sulcus of the medulla (see Chapter 9, Fig. 9-20). Most preganglionic fibers (some of which are extremely long) arise from the dorsal motor nucleus, which is a column of cell bodies located in the medulla of the brain stem. Some (possibly the majority) of the preganglionic fibers destined for cardiac muscle originate in or near the nucleus ambiguus (Loewy & Spyer, 1990; Noback, Strominger, & Demarest, 1991; Wang, Holst, & Powley, 1995; Iversen, Iversen, & Saper, 2000; Nolte, 2002; Standring et al., 2008; Kiernan, 2009), which is located in the medulla ventral to the dorsal motor nucleus. However, the nucleus ambiguus is involved primarily with supplying skeletal muscles via the glossopharyngeal, vagus, and accessory cranial nerves. All the preganglionic fibers travel in the vagus nerve and its numerous branches (see Fig. 10-16, B). Some mingle with sympathetic fibers to form the extensive autonomic plexuses of the thoracic and abdominal cavities. The long preganglionic fibers are destined to synapse in small ganglia located within plexuses near the effector organ or ganglia within the wall of the organ itself. Some of the specific branches that conduct preganglionic parasympathetic fibers are the following: cardiac, pulmonary, and esophageal branches that join the plexuses of the same name within the thorax; and gastric and intestinal branches within the abdomen that join in the celiac plexus (and its subsidiary plexuses) en route to the stomach, small intestine, ascending colon and most of the transverse colon, accessory glands, and kidneys.
The vagus nerve has an extensive area of distribution as can be seen in Figure 10-16, B. However, note that the vagus nerve does not supply autonomic effectors of the head. These are innervated by CNs III, VII, and IX. Although vagal efferents are important, the vast majority of the vagal fibers are afferent fibers. The sensory distribution of the vagus nerve is so widespread that this peripheral nerve may be one of the most important nerves in the body. The vagus nerve is also discussed in the following sections: Enteric Nervous System, Visceral Afferents, and ANS and the Immune System.
Sacral Portion of the Parasympathetic Division
Most effectors innervated by parasympathetic fibers are served by cranial nerves as can be noted from the previous discussion. The remaining effectors (e.g., the smooth muscle and glands of the pelvis) not innervated by the vagus nerve are innervated by the sacral portion of the craniosacral parasympathetic division. The origin of these preganglionic fibers is in the sacral autonomic nucleus of lamina VII of sacral cord segments two, three, and four (Fig. 10-17). The preganglionic fibers exit the cord in the ventral roots of these cord segments and leave the ventral rami within pelvic splanchnic nerves. [It is important to remember that the cell bodies are located in the conus medullaris and that the axons are within the cauda equina. Syndromes related to these two structures include signs and symptoms resulting from disruptions of these neurons.] Most of these fibers course through the inferior hypogastric plexus and enter the pelvic plexus along with sympathetic fibers to the pelvic viscera. They synapse in ganglia within those plexuses or in ganglia within the wall of the effector organ. Some enter the inferior hypogastric nerve and ascend out of the pelvis or enter the superior hypogastric plexus. A few course in a superior and lateral direction over the iliac vessels into the retroperitoneum and into the posterior mesentery of the sigmoid and descending colon (Standring et al., 2008). Generally, these fibers supply motor innervation to part of the transverse colon, descending colon, sigmoid colon, rectum, anus, and reproductive organs. They innervate and are excitatory to the detrusor muscle of the bladder wall but are inhibitory to the vesical sphincter. Also, some are vasodilatory to the penis and clitoris (erectile tissue), testes, ovaries, uterine tubes, and uterus. Coursing with these fibers are visceral afferent fibers that convey important sensory information (Standring et al., 2008; Kiernan, 2009) that provides reflex control of normal bladder, colon, and reproductive organ function.
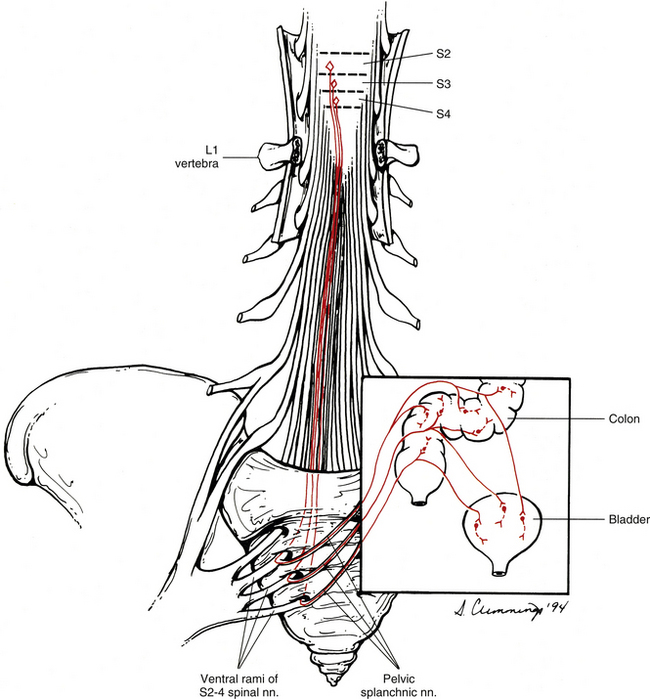
FIG. 10-17 Parasympathetic fibers originating from the spinal cord. The lumbar vertebral bodies have been removed to expose the sacral cord segments and cauda equina. The preganglionic neurons originate in the S2-4 cord segments, course within ventral roots of the cauda equina (red solid line), and exit through their corresponding intervertebral foramina. They branch from ventral rami of S2-4 spinal nerves and travel within pelvic splanchnic nerves to the walls of the pelvic viscera. Here they synapse on enteric neurons (red dashed line) in the colon or postganglionic neurons (red dashed line) in the other pelvic viscera.
Enteric Nervous System
The third division of the ANS is the enteric nervous system (ENS). Experiments performed in the late nineteenth and early twentieth centuries led to the theory that motility of the GI system was under autonomous control by an intrinsic nervous system, when well-coordinated and purposeful motility still occurred independently after severing nerves to the GI system (Bayliss & Starling, 1899; Langley, 1903; Langley & Magnus, 1905). Since that time the concept that an intrinsic group of neurons exists in the wall of the gut, pancreas, and gallbladder has been fully accepted. This extensive integrated network of neurons is located in the esophagus, stomach, small and large intestines, gallbladder, and pancreas. Groups of these neurons acting as microcircuits process sensory information such as the current contractile state of muscle, mechanical stimulation of the mucosa, or changes in the chemical contents of the lumen. They respond by generating output to coordinate muscle contraction, to influence secretory activity, and to adjust blood flow to accommodate the metabolic needs of the gut. In addition, extrinsic vagal and spinal afferents signal information coming from the gut to the CNS, and sympathetic fibers from prevertebral ganglia (splanchnic nerves) and parasympathetic fibers via the vagus and pelvic splanchnic nerves synapse on the enteric neurons. This input can adjust, regulate, and (in some emergency situations) override this intrinsic system (Fig. 10-18).
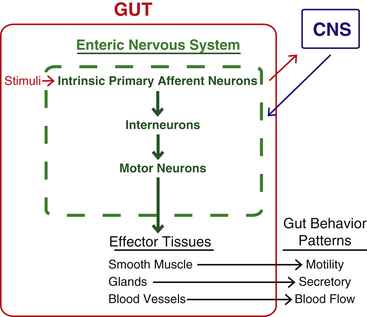
FIG. 10-18 Overview of the function of the enteric nervous system. Enteric neurons are interconnected to form microcircuitry. When stimulated, synaptic transmission flows through this circuitry to the effector tissues, allowing for the organization and coordination of motility, glandular secretion, and blood flow within the gut. Extrinsic afferent fibers send information to the CNS. The CNS may modulate the enteric circuitry through the sympathetic and parasympathetic divisions.
The enteric nervous system (ENS) is found within the 4 layers of the wall of the GI tract and is considered to contain as many neurons as the spinal cord itself—approximately 100 million (Noback, Strominger, & Demarest, 1991; Camilleri, 1993; Kiernan, 2009) or, according to some studies on humans, as many as 200 to 600 million (Wood, 2009). The enteric system consists of two major interconnected plexuses of various functional classes of neuron cell bodies (ganglia) and their processes (Fig. 10-19) as well as glial cells that outnumber the neurons. One of these is the myenteric plexus of Auerbach, which is located between the inner circular and outer longitudinal smooth muscle layers of the muscularis externa and extends from the esophagus to the internal anal sphincter. The second plexus is the submucosal plexus of Meissner, which is found in the submucosa of the GI tract. The submucosal plexus is most prominent in the small and large intestine and is absent or sparse in the esophagus and stomach. In large mammals it consists of an inner plexus (Meissner’s plexus) located at the deep side of the muscularis mucosa and an outer plexus (Schabadasch’s plexus) located next to the luminal side of the circular layer of muscle. In humans, an additional intermediate plexus lies between the two within the intestines. Motor neurons of the submucosal plexus innervate the secretory glands of the intestines (e.g., Brunner’s glands and the crypts of Lieberkühn). Interconnections between the submucosal and myenteric plexuses allow for the integration and processing of information received from each plexus (Brookes & Costa, 2006; Wood, 2009). Nonganglionated nerve plexuses also are located in and supply various layers of the wall of the gut (Standring et al., 2008).
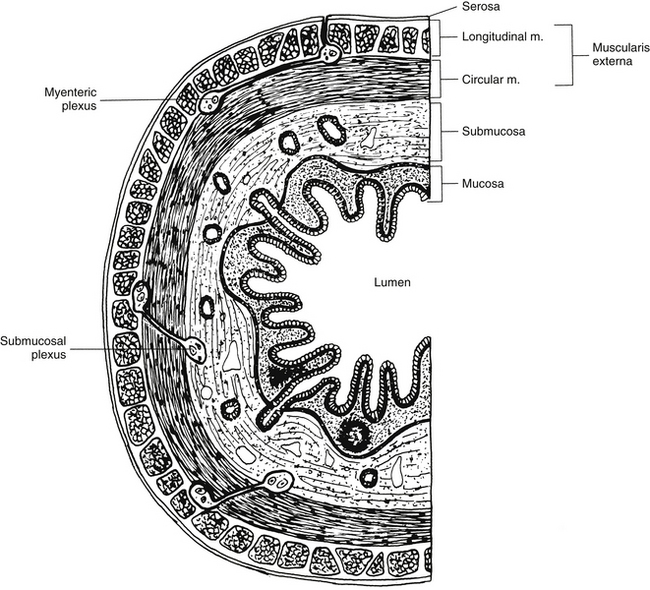
FIG. 10-19 Cross section through the wall of the gut showing the myenteric plexus (located between the two layers of smooth muscle) and the submucosal plexus (located within the submucosa layer) of the enteric nervous system. Each plexus consists of small ganglia that are interconnected.
In the past it was thought that the ganglia of these plexuses functioned as parasympathetic relay-distribution centers similar to other autonomic ganglia in the body. In other words, preganglionic parasympathetic fibers synapsed on postganglionic neuron cell bodies within a ganglion and then the fibers of these synapsed on smooth muscle and glands. This concept has been discarded because a closer look at the plexuses reveals that they are an independent integrative neuronal network that also receives input from sympathetic postganglionic fibers as well as preganglionic parasympathetic fibers (Fig. 10-20). In fact, only 10% of vagal efferents supply the gut and other viscera (the other 90% are afferents), indicating that parasympathetic influence on gut function must be to activate large neuronal circuits instead of individual effectors (Schemann & Mazzuoli, 2010). The networks or microcircuits are positioned near effector organs (smooth muscle and glands and blood vessels) and are programmed to control individual effectors or to coordinate the actions of multiple groups of effectors. In addition, the plexuses consist of cell populations different from and more complex than those found in other autonomic ganglia. Each plexus contains clusters of neurons that are interconnected, and each cluster consists of a heterogeneous population of neurons (Loewy, 1990a; Camilleri, 1993; Kiernan, 2009). These neurons can be classified numerous ways including by the type of receptor expressed, morphologic characteristics, electrophysiologic properties, and chemical coding (Furness, 2000; Hansen, 2003a; Brookes & Costa, 2006; Standring et al., 2008; Wood, 2009). In fact, it has been suggested that peptidergic and monoaminergic neurons are the predominant neurons in the ENS (Standring et al., 2008). Based on the number of ENS neurons, the complexity of their interactions and formation of microcircuits, and the similarity in neurotransmission and signaling molecules to the brain, the ENS has been referred to as a ‘mini-brain’ (Wood, 2009; Mayer, 2011). In the late 1970s and early 1980s studies on this vast number of intermingled neurons showed there were different chemical markers associated with these neurons, which led to the chemical coding hypothesis. Simply stated, it suggested that functional classes of neurons could be distinguished from each other on the basis of chemical attributes that constituted a chemical code. This code included neuropeptides, which are useful because they are targets in immunohistochemical studies. Extensive investigations of the ENS of the guinea pig, primarily, but also humans, mice, rats, pigs, and sheep, led to the chemical codes of enteric neurons. It appears that codes related to essential functions of neurons are highly conserved between species—that is, all neurons regardless of species or gut region that have similar roles will contain the same primary neurotransmitter, although there is considerable variation in the other codes between species. There may also be subtle differences in the codes within a particular enteric population. Data from the investigations of chemical coding led to the conclusion that although the same neuron releases several transmitters, one neurotransmitter, called the primary transmitter, has a dominant role. For example, in the guinea pig there are eight transmitters in the chemical code for cholinergic nonvasodilator secretomotor neurons. This type of transmission where several neurotransmitters from one neuron are utilized is called plurichemical transmission (Furness, 2009). Evidence indicates that more than 30 neurotransmitters are involved in the synaptic neurotransmission. These are small molecules, peptides, or gases that modulate the release or action of the primary neurotransmitter or provide a trophic influence. Combining characteristic features from morphologic, chemical, electrophysiologic, and receptor classifications, three classes of neurons have been identified based broadly on functional properties. These are motor neurons, interneurons, and sensory (intrinsic primary afferent) neurons (see Fig. 10-20). These neurons ultimately control motility, secretory activity, and blood flow patterns of the gut by activating each effector system in a coordinated manner such that the organ functions as an integrated system.
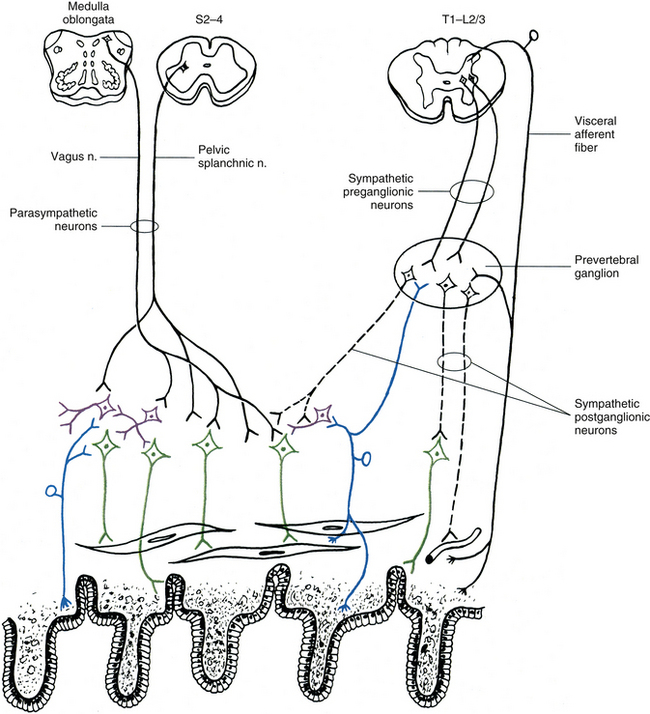
FIG. 10-20 Simplified illustration showing the intrinsic primary afferent neurons (sensory) (blue), motor neurons (green), and interneurons (purple) that compose a plexus of the enteric nervous system. Input into this group of neurons is from parasympathetic neurons and sympathetic postganglionic neurons, both of which may modulate the function of the enteric neurons. Notice that the prevertebral sympathetic ganglion integrates input from collateral branches of primary visceral afferent fibers destined for the spinal cord, and sensory enteric neurons, thus establishing a reflex arc with sympathetic postganglionic neurons.
Motor neurons are classified as those that innervate smooth muscle and those that innervate glands and blood vessels. Muscle motor neurons only innervate smooth muscle of the GI system and are excitatory or inhibitory. Most of the cell bodies are located in the myenteric plexus in small mammals but in humans there is a substantial population located in the outer submucosal plexus. The single axon of the motor neuron extends within the plexus before terminating as branches within the muscle. Motor neurons to intestinal circular muscle are excitatory or inhibitory. Excitatory neurons course in an oral direction or project locally and inhibitory neurons project aborally. Thus motor contraction will occur above the focal point of excitation with relaxation below this point (the law of the intestine suggested by Bayliss and Starling in 1899). Although less numerous than excitatory neurons, the firing of inhibitory neurons dominates when the two are activated simultaneously. Motor neurons that innervate longitudinal muscle are located within the myenteric plexus and their axons course within the muscle and parallel to the fibers. These neurons are primarily excitatory and project to local muscle fibers, allowing for graded motor control of the muscle in the immediate area. Long ascending or descending fibers of interneurons may activate these neurons if muscle contraction needs to be coordinated over long distances. The motor neurons to longitudinal muscle are a separate population from those that innervate circular muscle and although both can act independent of each other, they may also be activated simultaneously during muscle stretching (Brookes & Costa, 2006). In excitatory neurons the primary neurotransmitter is acetylcholine (ACh), which acts on muscarinic receptors (usually the subtypes M2 and M3), but there are also tachykinins, such as substance P, that function as cotransmitters. The inhibitory neurons use the co-transmitters nitric oxide (NO), vasoactive intestinal polypeptides (VIPs), and adenosine triphosphate (ATP), although the primary transmitter is generally NO (Furness, 2009).
The activity of inhibitory motor neurons, which is controlled by interneurons, determines if motor contraction occurs, and when it does occur it controls the length of the contracting segment and the direction of the ensuing propagation. Since some inhibitory neurons innervating circular muscle fire continuously, contraction of circular muscle in response to the interstitial cells of Cajal (see below) will only occur when interneurons inactivate the inhibitory neurons. (The control of sphincter musculature by inhibitory neurons is the opposite in that the neurons are normally quiet and are activated only at the appropriate time.) The control of inhibitory neurons is such that inactivation of inhibitory neurons results in specific gut segments contracting while inhibitory neurons firing to adjacent segments results in relaxation. Controlling the firing of inhibitory neurons such that they are progressively inactivated in an aboral direction allows contraction to occur, resulting in the normal propagation of luminal contents in the aboral direction. Reversal of this pattern would be required for the process of emesis to occur (Wood, 2007). Much of the effect of the motor neurons is thought to be mediated by the interstitial cells of Cajal (ICCs). These cells are specialized smooth muscle cells that act as pacemaker cells for the gastric musculature and intestinal circular musculature. They generate slow waves of depolarization and form an electrical syncytium such that action potentials and the pacemaker potentials propagate from muscle cell to muscle cell through gap junctions. Circular musculature is able to contract in response to these electrical slow waves when their inhibitory neurons are inactivated. Based on morphology and location within the gut wall, it has been shown that there are different types of ICCs (Komuro, 2006; Ward & Sanders, 2006; Mostafa, Moustafa, & Hamdy, 2010). They are in contact with each other and with smooth muscle cells, and are located in the vicinity of enteric motor neurons and vagal afferent fibers. Because of these interconnections, the ICCs may be involved with the mechanism of neurotransmission for intrinsic gut motility and for sensory signaling. It has been suggested that changes in the number, structure, or density of ICCs may be related to GI disorders such as achalasia, gastroparesis, chronic intestinal pseudoobstruction, and chronic constipation. Abnormal pacemaker activity may occur if there are few or no ICCs, resulting in decreased smooth muscle contractility and diminished intestinal transit (Mostafa, Moustafa, & Hamdy, 2010). Inhibitory motor neurons may be destroyed or inactive as a result of degenerative noninflammatory or inflammatory ENS neuropathies. This leads to no control of ICC activity and no organization of the muscle contractions. The smooth muscle will become hyperactive and contract continuously, behaving as an obstruction. This decrease in inhibitory neurons is the pathophysiologic beginning of disinhibitory motor disease, which includes several forms of chronic intestinal pseudoobstruction and sphincteric achalasia. Initially the symptoms appear similar to those presented in irritable bowel syndrome (IBS) cases but then as more neurons degenerate the symptoms worsen and finally manifest as chronic intestinal pseudoobstruction (Wood, 2007).
The movement of luminal contents through the intestines is the result of various patterns of propulsive contractions created by the polysynaptic peristaltic reflex circuitry. Wood (2009) referred to this as the intestinal analog of spinal motor reflexes. Like the spinal reflex, the peristaltic reflex is hardwired to appropriately synchronize the activation of excitatory and inhibitory motor neurons. Distension of the intestinal wall and mechanical stimulation of the mucosa result in a stereotypic motor response—relaxation of the circumferential muscle group and contraction of the longitudinal muscle group below the site of stimulation and contraction of the circumferential group above the point of stimulation. The reflex circuit must activate the excitatory and inhibitory motor neurons controlling longitudinal muscle groups and circular muscle groups appropriately in the receiving segment for the forward-moving intraluminal contents and coordinate the firing of the excitatory and inhibitory motor neurons to the two muscle groups in the propulsive segment. It is thought that the reflex circuits are arranged in units that are interconnected in series within the intestinal wall. When one of the circuits is activated, it is able to recruit other circuits, providing the mechanism for propulsion to occur over various distances of the intestine. The hardwired circuitry involved in somatic spinal reflexes may be modified according to the motor program that is to be used (i.e., reflexes, automatic movements, and purposeful movements). ENS circuits are also modified by sensory feedback and other input to compensate for local changes or for changes that affect the entire intestine in order to adjust the strength of contraction and the rate of repeating a particular motor pattern. The reflex circuitry is also under control by other ENS circuits during various digestive states and during emesis, when the distance over which propulsion occurs and the direction in which it occurs vary. Similar to spinal networks involved in the programming of somatic motor neurons for repetitive, rhythmic types of movement such as walking, it is thought that central pattern generators also exist within the ENS circuitry in the intestine. Wood (2009) suggests that each circuit is multifunctional, consisting of a ‘library’ of programs that can be reconfigured by modulatory input, resulting in various motor patterns. Some of the programs are designed to control motility and secretion occurring during various digestive states such as the postprandial state and the interdigestive state, and also during the emetic state. In addition, there is a protective program resulting in power propulsion that is activated in response to potentially dangerous agents within the lumen such as food allergens or toxins. The neuromodulatory signals altering the neurotransmission within the programmed neuronal circuits may be chemical mediators released from enteroendocrine cells and immune or inflammatory cells within the gut wall, such as the histamine released from enteric mast cells, or they may be signals from neurons (Wood, 2009) (see section Enteric Nervous System and the Immune System).
Secretomotor and vasomotor (dilator) neurons of the enteric nervous system regulate secretions and blood flow in response to chemical and mechanical stimuli. Vasomotor fibers control the diameter of submucosal and mucosal arterioles to adjust blood flow relative to the level of gut secretory activity. These cholinergic fibers cause nitric oxide (NO) to be released from the vascular endothelium, resulting in dilation and increased blood flow. Secretomotor fibers are the major pathways that regulate secretion from the intestinal crypts of Lieberkühn, Brunner’s glands, and goblet cells in the intestine and activate ion fluxes and water movement across the epithelium. Many of these submucosal neurons project to both targets, thus coordinating blood flow with secretory demand. When activation occurs, water, NaCl, bicarbonate, and mucus from glands are secreted into the lumen and blood flow is increased (Wood, 2007). Prominent among the submucosal neurons are neurons (noncholinergic) that use the VIP family peptides as the primary transmitter. These neurons receive both excitatory and inhibitory synaptic input from a variety of sources including intrinsic primary afferent neurons (IPANs), interneurons, and sympathetic postganglionic neurons These VIP family neurons appear to play the major role in physiologic responses by adjusting and regulating most of the local reflex responses. Other secretomotor/vasodilator neurons use ACh as their primary transmitter. One population of vasodilator neurons uses ACh as its primary transmitter along with seven different neuropeptides. Nonneural cells located in the mucosa and submucosa, such as enterochromaffin cells, mast cells, and other immune/inflammatory cells, release chemicals that activate the VIP family neurons (Brookes & Costa, 2006; Wood, 2007; Furness, 2009).
Secretomotor neurons may be inhibited by intrinsic enteric neurons and by postganglionic sympathetic neurons. Norepinephrine (NE) released from the sympathetic fibers binds to α2 receptors, inhibiting the firing of the secretomotor neurons with subsequent reduced secretory activity and a decrease in the concentrations of water and electrolytes. This mechanism of sympathetic activation can occur for normal homeostatic maintenance where blood needs to be shunted from the viscera to the systemic circulation and also in pathologic conditions resulting in a constipated state. Secretomotor neurons also express receptors that promote excitation and therefore an increase in secretory activity by the intestinal epithelium. In a pathologic state this may lead to secretory diarrhea. VIP, ACh, and substance P are able to activate these receptors on the neurons. An excessive amount of serotonin released from enterochromaffin cells stimulates secretomotor neurons and may be the mechanism by which the diarrhea state exists in the irritable bowel syndrome (IBS) diarrhea-predominant condition in patients. Inflammation of the intestinal mucosa or submucosa causes histamine and other mediators to be released, which can result in secretory diarrhea. These chemicals excite secretomotor neurons and also act presynaptically on postganglionic sympathetic fibers to inhibit their release of NE. The latter results in the removal of sympathetic inhibition on the secretomotor neurons and allows for an increase in their activity and more secretion to occur (Wood, 2007).
Interneurons aid in forming the circuitry necessary for processing sensory input and for determining the proper motility pattern. The interneuronal circuits are established for organizing reflex responses and also for housing the integrative programs for the diverse motor functions of the gut such as migrating myoelectric complexes (cyclical waves of intestinal contractions occurring between meals), mixing patterns during digestion, and powerful propulsive contractions during peristalsis. Interneurons receive input from intrinsic primary afferent neurons (IPANs), from other interneurons, and from extrinsic neurons and they project to all classes of enteric neurons. The interneurons are arranged into functional chains, which allows for reflex circuits to extend farther superiorly and inferiorly within the gut wall. It has been suggested that this organization can allow a segment of the chain that controls a specific set of enteric neurons to act as a switch, controlled by sympathetic or vagal fibers, between motility patterns utilized during feeding and fasting periods (Brookes & Costa, 2006). Two classes of interneurons are found in the ENS. One class consists of three populations of interneurons that have axons that are descending (aborally directed). Two populations are cholinergic and project to both plexuses. However, one of these two populations is also somatostatin immunoreactive and synapses on interneurons and inhibitory motor neurons whereas the other is also serotonin (5-hydroxytryptamine, 5-HT) immunoreactive and does not likely synapse on inhibitory motor neurons. The third population of descending interneurons is comprised of a heterogeneous neuronal group of which many are cholinergic and all are similar morphologically. The other class of interneurons comprises about 5% of myenteric neurons. This interneuron is predominantly cholinergic and has a short ascending (orally) directed axon. It synapses with other ascending interneurons and with excitatory motor neurons, propagating excitation up the gut wall and coordinating motor contractility over long distances (Brookes & Costa, 2006).
The sensory neurons of the enteric nervous system are called intrinsic primary afferent neurons (IPANs) (see later discussion and Fig. 10-21). They are found in both the submucosal and myenteric plexuses of the small and large intestines but are absent from the esophagus and rare in the stomach (Furness, 2008). Their processes, which greatly outnumber those of vagal or spinal afferent fibers, terminate in the lamina propria of the mucosa. They are located throughout the wall of the gut and are subjected to gut wall distortion, toxins, nutrients, hormones, inflammatory mediators, and gut metabolic changes and therefore influence reflex circuitry for multiple reasons. They are able to act as mechanoreceptors and chemoreceptors and may be activated by mechanical stimuli of the mucosa, by distension of the gut wall or distortion of their processes, or by luminal chemical stimuli such as fatty acids, acids, alkaline solutions, and glucose. Evidence shows that the activation of neurons by distortion and chemical stimuli is caused by the release of serotonin from enteroendocrine cells in response to these stimuli (Gershon, 2005) (see later discussion). Based on responses to stimuli, it appears that there are three classes of IPANs: submucosal IPANs that respond to mechanical stimuli of the mucosa; myenteric IPANs, the majority of which respond to chemical stimulation of the mucosa; and other myenteric IPANs that respond to stretching of the gut wall (Brookes & Costa, 2006). IPANs have processes with extensive connections onto most other enteric neurons including other IPANs, interneurons, and motor neurons, and so they also play a major role in activating the enteric neuronal circuitry for the physiologic reflex control of motility, secretion, and blood flow. The peristalsis reflex is an example of the interaction of these neurons. Distension or the presence of chemical stimuli activates the IPANs that project to interneurons. The interneurons in turn synapse with orally directed excitatory motor neurons and anally directed inhibitory motor neurons (Standring et al., 2008), thus initiating peristalsis. Also, IPANs that are activated in response to luminal nutrients produce a reflex response by stimulating secretomotor fibers that results in secretion. This response not only is local but also can be widespread throughout the GI tract by means of the integrated circuitry of the plexuses that activates secretomotor neurons at different levels. IPANs may also initiate reflex responses to potentially harmful conditions. They are able to detect mucosal irritants such as bacterial by-products or parasitic infestations and initiate a protective response of heightened secretion and motility manifested as diarrhea (Furness, 2006). In the small intestine of the guinea pig, all types of neurons have been identified. The IPANs in these animals and likely in humans have been shown to use ACh and tachykinins as the primary neurotransmitters although secondary neurotransmitters also are involved in the secretory process (Furness, 2009).
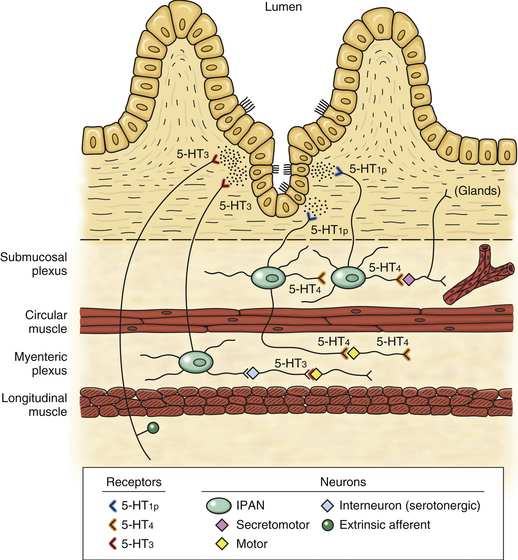
FIG. 10-21 The effect of serotonin (5-HT), which is released from enterochromaffin cells on numerous enteric neurons and extrinsic afferent fibers through various 5-HT receptors (see text for discussion).
The enteric neurons and effectors of the gut wall are modulated and innervated, respectively, by fibers from extrinsic sources. These are vagal, pelvic, and spinal afferent fibers and parasympathetic and sympathetic efferent fibers. All of these fibers terminate as varicose fibers within the enteric ganglia. The extrinsic afferent neurons are other sensory neurons located in the GI system. These neurons send input to the CNS about the GI environment with regard to normal physiologic processes and the feelings of discomfort and pain. They provide the input for spinal and brain stem reflex mechanisms and the input to the central autonomic network that regulates behaviors associated with the GI system. These afferents course in the vagus nerve, pelvic splanchnic nerves (referred to as pelvic afferents), and thoracolumbar splanchnic nerves (referred to as spinal afferents) along with efferent fibers and encode activity within physiologic levels. However, the spinal afferents are also activated by pathophysiologic stimuli and mediate the sensation of pain (see Spinal Afferent Fibers under section Visceral Afferents). The cell bodies of both spinal afferents and pelvic afferents are located in dorsal root ganglia. The cell bodies of the spinal afferents exhibit a generalized overlapping viscerotopic distribution in that a dorsal root ganglion will have a preponderance of fibers associated with one particular area although it innervates many areas. Spinal afferent fibers constitute about 10% to 20% of the splanchnic fibers; pelvic afferents constitute about 30% to 50 % of the pelvic splanchnic fibers (Beyak et al., 2006). Spinal afferents terminate in muscle layers near ganglionic cell bodies and smooth muscle cells, in the mucosa, serosa, and mesentery. They are predominantly high-threshold mechanonociceptors. A small minority of spinal afferents that terminate in the muscle layers are muscle afferents that are sensitive to mechanical stimuli. Another small population of endings are mucosal mechanoreceptors that respond to light mechanical stimuli (not stretch) (Zagorodnyuk, Brookes, & Spencer, 2010). There is little direct evidence that suggests they are involved with sensing luminal contents (Beyak et al., 2006). Other spinal afferents are mechanosensitive and terminate on intramural blood vessels in the submucosa. These have been shown to be activated by large-amplitude circumferential stretch of the submucosa. Nonmechanosensitive collaterals of the afferent fibers branch in the myenteric and submucosal ganglia and terminate on blood vessels. Blood vessels in the mesentery are also innervated by mechanosensitive afferents. These afferents fire in response to blood vessel compression, perfusion changes, and large-amplitude stretch. Vascular afferents comprise a distinct and significant type of afferent fiber and may be a contributing component in the neural transmission of nociception. In general, spinal afferent fibers respond to mechanical stimuli and to potentially noxious chemical or mechanical stimuli resulting from ischemia, tissue damage, or inflammation. These afferent fibers express numerous receptors such as the 5-HT3 receptor and receptors for bradykinin, ATP, prostaglandins, histamine, and mast cell proteases (Wood, 2007). When these afferent fibers are activated, they send input regarding noxious stimuli into the dorsal horn where they initiate reflex responses or synapse on tract neurons. These tract neurons convey information to higher centers for further processing. In some instances these afferent fibers and dorsal horn neurons can undergo the process of sensitization, resulting in functional gastrointestinal disorders such as IBS (see Neurotransmission of Visceral Pain under section Visceral Afferents).
Pelvic afferents supply the distal colon and rectum, terminating as intraganglionic laminar endings (IGLEs), which are similar to vagal intraganglionic laminar endings. These fibers transmit information from low-threshold stretch-sensitive mechanoreceptors of predominantly the rectum where the density of the IGLEs is greater than that in the distal colon (Zagorodnyuk, Brookes, & Spencer, 2010). Pelvic afferents also terminate in the mucosa on mechanoreceptors. Some of these receptors respond to light stroking whereas others respond to light stroking and distension. Like vagal afferents (see below) the pelvic afferents signal information to the CNS concerning normal physiologic functioning of the gut and they initiate centrally mediated reflexes (Brookes & Costa, 2006). Spinal afferent neurons are immunoreactive for calcitonin gene–related peptide (CGRP) and tachykinins (e.g., substance P). These neurotransmitters are known to be chemical mediators involved in the neurogenic inflammatory process and GI inflammation. Pelvic afferents are nonpeptidergic and are immunoreactive for vesicular glutamate transporters (VGluT1 and VGluT2) (Zagorodnyuk, Brookes, & Spencer, 2010).
Vagal afferent fibers signal the physiologic states of the gut to the CNS and are part of the inflammatory reflex (see section ANS and the Immune System). Afferent fibers coursing in the vagus nerve outnumber efferents by a 9:1 ratio (Beyak et al., 2006). Three types of afferent endings have been described. One type is associated with branching varicose fibers that are interspersed among the circular and longitudinal muscle cell layers. These are called intramuscular arrays and they are sparse in the intestine but fairly dense in the lower esophagus, upper stomach, and pylorus. It has been speculated that they may be a type of mechanoreceptor sensitive to length (Beyak et al., 2006; Brookes & Costa, 2006; Zagorodnyuk, Brookes, & Spencer, 2010). The second type, called intraganglionic laminar endings (IGLEs), consists of endings that surround the myenteric ganglia. These vagal IGLEs are present in the esophagus and stomach, decrease in number throughout the intestines, and are absent in the rectum (Beyak et al., 2006; Brookes & Costa, 2006). These monitor the stress and strains produced by muscle stretch or contraction. A third type of afferent fiber terminates as mucosal endings. These have been described in the gut wall from the esophagus to the distal colon and extend throughout the lamina propria of villi near the basal lamina of epithelial cells and are often close to enteroendocrine cells. They are sensitive to light mechanical stimuli such as gentle stroking or compression but not to stretch or distension and thus may be able to detect luminal contents moving across the mucosal surface (Zagorodnyuk, Brookes, & Spencer, 2010). They also are activated indirectly by a variety of chemicals including nutrients involved with the digestion process and by changes in osmolarity and acidity in the contents of the lumen. An extensive discussion of GI physiology relevant to the functions of all chemical mediators is beyond the scope of this chapter. However it is important to mention some of the mediators and their relationships with extrinsic afferent fibers and IPANs.
The mucosa of the GI tract is lined predominantly by simple columnar epithelial cells and in the intestine these absorptive cells are called enterocytes. Located among the enterocytes is a population of enteroendocrine (EE) cells and within this group is a small subpopulation (1% to 3% of all human epithelial cells [Bertrand & Bertrand, 2010]) of cells called enterochromaffin (EC) cells. These EE and EC cells are likely the predominant sensory cell population functioning as mechanical and nutrient sensors (a process referred to as “nutrient tasting” [Raybould, 1999; Grundy, 2002]). In other words, it appears that most stimuli of the gut wall do not directly interact with sensory nerve terminals but instead activate these sensory cells (Xue et al., 2007; Bertrand & Bertrand, 2010). These cells thus provide the site for sensory transduction to occur, resulting in the release of mediators such as ATP and the hormones secretin, cholecystokinin (CCK), and serotonin (5-HT). Intestinal glucose is one nutrient that is detected in the lumen by the enteroendocrine and enterochromaffin cells. Studies indicate that the membrane transport protein at least on EC cells may be a glucose-specific sensor that when activated results in depolarization and release of 5-HT (Raybould, 2007; Raybould, 2010) instead of a glucose transporter. Several G protein–coupled receptors are also expressed on EE cells that are activated by luminal chemicals such as glucose, calcium, umami, protein hydrolysates (strong stimulants resulting in EE cell secretion), and both long- and short-chain fatty acids. Evidence indicates that fatty acids and protein hydrolysates induce the release of CCK and of GLP-1 (glucagon-like peptide 1) and CCK, respectively (Raybould, 2010). Interestingly, some gut epithelial cells and EE cells also express the same G protein–coupled receptors (T2R) and G proteins that are part of the components that mediate bitter taste in the oral cavity. When activated, the cells release CCK. Research also shows that EE cells express toll-like receptors, which recognize bacterial breakdown products such as lipopolysaccharide. Depending on the bacterial ligand, studies show that CCK, defensin, and chemokines may be released, which activate pro-inflammatory pathways. Because it appears that EE cells respond to bacterial breakdown products, it may be that this response is a method to defend the integrity of the mucosa (Raybould, 2010). As one can see, enteroendocrine cells play a very important role in the sensory transduction caused by chemical and mechanical stimuli within the lumen of the gut by releasing chemical mediators. Enterochromaffin (EC) cells in the gut are also very important. These cells synthesize, store, and release serotonin (5-HT). Serotonin is a very important mediator that has a pivotal role with the mediation of a variety of GI reflexes through its interactions with enteric neurons. The GI tract has the largest accumulation (approximately 90%) of 5-HT in the body. In addition to the EC cells, 5-HT is also found in enteric neurons (it is expressed in 2% to 20% of all enteric neurons) (McLean, Borman, & Lee, 2007). Other molecules stored in the EC cell include gamma-aminobutyric acid (GABA), ATP, uroguanylin, and melatonin (Bertrand & Bertrand, 2010). Along with mediators from EE cells such as CCK and ATP, serotonin initiates and regulates motor and secretory behaviors of the gut in response to various stimuli of the lumen at any given time and in any region. 5-HT acts in a paracrine manner and activates the 5-HT receptors on intrinsic and extrinsic sensory nerve terminals. Based on in vitro studies, it appears that these receptors are important for the initiation and propagation of peristalsis and other gut reflexes including secretory activity (Bertrand & Bertrand, 2010). Studies indicate that 5-HT is released in response to the presence of luminal nutrients and chemicals and as a result of mechanical stimuli such as compression of the mucosal epithelium and circular and longitudinal muscle contractions (Bertrand & Bertrand, 2010). Because there are no serotonergic neurons in the mucosa, enterocytes function to terminate 5-HT signaling, thus preventing excessive stimulation of the receptors and possibly desensitization. These cells express a membrane serotonin reuptake transporter (SERT), which mediates the transmembrane transport of 5-HT into the cell where it is degraded. The effects of 5-HT on the enteric neurons are through its activation of several different receptor types such as the 5-HT3 and 5-HT4 receptors and the putative 5-HT1P receptors (Gershon, 2005) (Fig. 10-21). 5-HT1P receptors are expressed on submucosal IPANs. These neurons initiate peristaltic and secretory reflexes in response to mucosal stimuli. 5-HT4 receptors are presynaptic and are found on the terminal endings of submucosal IPANs, at synapses with myenteric neurons, and at the neuromuscular junction. It is postulated that by binding to the 5-HT4 receptors on submucosal IPANs, 5-HT increases the release of neurotransmitters (ACh and CGRP), thus strengthening neurotransmission and enhancing the peristaltic and secretory responses. 5-HT3 receptors are postsynaptic and are expressed on the terminals of extrinsic afferent neurons where they may respond to noxious stimuli. In addition, they are found on myenteric IPANs and on neurons in the myenteric plexus.
These receptors have been targeted by modulating drugs (agonists and antagonists) in the treatment of functional GI disorders such as IBS (Gershon, 2005; McLean, Borman, & Lee, 2007). Because the subtypes of 5-HT receptors are on distinct populations of neurons (i.e., the submucosal IPANs, which initiate peristaltic and secretory reflexes, versus the extrinsic afferent neurons, which are activated by noxious stimuli), drugs can be used to treat symptoms resulting from the activation of one group without affecting the other (Gershon, 2005). 5-HT has a very important role in mediating physiologic responses such as modulation of smooth muscle activity and of neuronal function and intestinal secretion within normal GI function parameters. Although it appears that decreased levels of 5-HT do not disrupt normal GI function, there is evidence to suggest that very high levels of 5-HT release can contribute to the diarrhea associated with cholera, the nausea associated with chemotherapy or radiation therapy, and similar symptoms manifested in chronic intestinal diseases such as chronic constipation, enterocolitis, inflammatory bowel disease, and irritable bowel syndrome. Also, studies show that a mechanism may exist during inflammation that can alter the number of EC cells and amount of 5-HT (Bertrand & Bertrand, 2010). In general, understanding the cellular and molecular mechanisms of 5-HT release, the activation of its numerous receptors, and the serotonin reuptake transporter may allow for a better understanding of the pathologic processes occurring in chronic GI disorders and treatment protocols.
Receptors for these mediators are expressed on enteric neurons and on extrinsic (primarily vagal) sensory afferents. Vagal afferents express receptors for many regulatory molecules such as CCK, glucagon-like peptides, peptide YY, leptin, orexin, ghrelin, and serotonin. Activation of these afferents by mediators attributable to nutrient or chemical stimuli can influence food intake and initiate a vago-vagal reflex regulating GI functions such as intestinal fluid secretion, gastric emptying, and pancreatic exocrine secretion. At times, the reflex may be of a protective nature (propulsion and elimination) in response to the sensing by EE cells of bacterial breakdown products or to bitter substances (Raybould, 2007; Raybould, 2010).
As mentioned earlier, IPANs are the intrinsic sensory neurons that respond to luminal contents and distension and initiate reflexes adjusting motility, blood flow, and secretion. There are other afferent enteric neurons that appear to have a broader function that involves the sympathetic system. These intestinofugal or viscerofugal (i.e., impulses traveling away from the intestine/viscera) neurons are in-parallel stretch receptors that monitor the degree of stretch of the circular muscle. They function as slowly adapting mechanoreceptors that detect changes in volume (Szurszewski & Miller, 2006). They are likely to be active most of the time, providing peripheral input to prevertebral ganglia and initiating ongoing reflexes mediated by postganglionic sympathetic fibers that control normal functions. The fibers synapse only with postganglionic sympathetic neurons that regulate secretion and motility. Intestinofugal neuron cell bodies are located primarily in the myenteric ganglia. Animal studies indicate that there is a proximodistal gradient in the number of cell bodies, that the greatest number is at the end of the ascending colon, and that 90% of cell bodies of intestinofugal fibers terminating in the celiac, superior, and inferior mesenteric ganglia are found in the colon (Szurszewski & Miller, 2006). The axons of the intestinofugal neuron cell bodies project to sympathetic prevertebral ganglia (see Fig. 10-20). The axons that use ACh as the primary neurotransmitter do not appear to form collateral branches to the gut wall (Brookes & Costa, 2006). Because of the preponderance of cell bodies in the distal areas of the gut, they are likely involved with coordinating the activity of proximal regions of the gut by activating sympathetic neurons in prevertebral ganglia that are evenly distributed within the gut wall. Prevertebral ganglia (PVGs) neurons do not form circuits that control stereotypic patterns of motor activity. It appears that the axons of these neurons modify the programs established in enteric circuitry by inhibiting the firing of excitatory myenteric motor neurons and preventing the release of inhibitory neurotransmitters from secretomotor neurons. Based on the termination of their projection fibers, PVGs are arranged organotropically such that the cranial PVGs innervate the more proximal region of the gut and distal regions of the gut are innervated by the more caudal PVGs. The celiac ganglion innervates mainly the stomach and small intestine; the superior mesenteric ganglion, the small intestine; the inferior mesenteric ganglion, the colon; and the pelvic ganglion, the distal colon and rectum (Szurszewski & Miller, 2006). Animal studies of the prevertebral celiac ganglion show that three populations of noradrenergic neurons innervate the gut. One population, which is also immunoreactive for neuropeptide Y, functions as a vasoconstrictor and projects to the spleen, mesenteric blood vessels, and arterioles in the gut wall. A second population, which is immunoreactive for somatostatin, inhibits secretomotor neurons. This population terminates in the submucosal ganglia of the small intestine, cecum, and proximal colon. The third population inhibits motility and projects to the myenteric ganglia. Intestinofugal sensory neurons project to the two populations of postganglionic neurons that inhibit secretion and inhibit motility but not to the vasomotor neurons (Furness, 2003). Activation of postganglionic sympathetic fibers that course back to the myenteric plexus results in reflex sympathetic inhibition on regions of the GI wall. Studies of the colon indicate that this enteroenteric reflex is initiated by the activation of intestinofugal neurons by distension of the intestine. It appears that these neurons can be stimulated directly by the distension and by local neurons that synapse with them. Acidic chyme in the small intestine also activates an enteroenteric reflex, resulting in inhibition of gastric motility. Based on numerous experimental data, the reflex response produced by the sympathetic efferent fibers is thought to inhibit the motility of a more proximal (orally directed) region of the GI tract relative to the location of the initial stimulus. Therefore it appears that the enteroenteric reflex, via prevertebral ganglia, regulates the movement of luminal contents from a proximal to a distal direction (Furness, 2003).
The functional relationship between the intestinofugal fibers and the sympathetic system becomes somewhat more complex when it is noted that all prevertebral ganglia neurons receive additional input. This input is from the CNS via preganglionic sympathetic fibers within splanchnic nerves, from collateral branches of spinal afferent fibers that are conveying information to the spinal cord (see Fig. 10-20), and, as seen from studies in rat celiac ganglia, from the dorsal motor nucleus of the vagus (Szurszewski & Miller, 2006). Therefore the prevertebral ganglia may serve a variety of functions related to GI activity. Unlike the paravertebral (sympathetic chain) ganglia, which receive input only from intermediolateral cell column preganglionic fibers, the prevertebral sympathetic ganglia receive input from the IML cell column as well as convergent input from enteric neurons and collateral branches of spinal afferent fibers and, in the case of some neurons, fibers from the dorsal motor nucleus of the vagus. The input from preganglionic fibers to the postganglionic neurons in the prevertebral ganglia is divergent, which allows for spatial and synaptic amplification of the incoming signals. Although there is variation among different ganglia and among species, in general the ratio is 1:200. Sympathetic fibers controlling the vasculature may use these ganglia simply as a relay station in which preganglionic fibers cause the firing of postganglionic vasomotor fibers. However, and more importantly, the ganglia also may serve as an integrative center for collecting CNS input from preganglionic fibers, as well as peripheral input from sensory neurons in the GI wall. Neither of these inputs alone is capable of causing the postganglionic neurons to reach their firing threshold; therefore the continual activity of sensory neurons from the gut, in essence, determines the firing threshold of the postganglionic neurons. As long as this activity is at a high enough level, the spatial summation of these afferent fibers, together with the input from preganglionic sympathetic fibers, allows CNS information to reach the effectors. The necessity of summation to allow for proper GI function demonstrates the importance of sensory input to the prevertebral sympathetic ganglia. This input provides a means by which the prevertebral ganglia can regulate and adjust (modulate) incoming information from the CNS that is destined for the GI tract (Jänig, 1988).
Perhaps of equal importance is that the prevertebral ganglia are thought to be involved in the circuitry that protects the GI tract from potential or real injury. Spinal afferents, the cell bodies of which are located in dorsal root ganglia, have been shown to transmit information from mechanoreceptors and receptors sensitive to molecules such as bradykinin and hydrogen chloride. As they course to the spinal cord, these afferent fibers send collateral branches into the prevertebral ganglia. This input to the prevertebral ganglia, which is only approximately 10% of all of the synapses at this location (Furness, 2003), and to the spinal cord provides the sensory limb for viscerovisceral, viscerosympathetic, and viscerosomatic reflexes (discussed later in this chapter), as well as for visceral sensation. The collateral branches synapsing in the ganglia are thought to cause a lowering of the firing threshold of the postganglionic fibers. This would allow spatial summation of the sensory afferent fibers and preganglionic fibers to occur more readily, thereby facilitating, for example, the motor limb of the intestino-intestinal reflex (Jänig, 1988). PVGs consist of populations of neurons that are different from each other based on characteristics such as their morphology, chemical coding, and type of neurotransmission. They function as coordinating and distributing centers. Because PVG visceromotor neurons exhibit the properties of plurichemical transmission, convergence, modulation, weak synaptic input, and possession of a variety of receptors and numerous ionic channels, they are able to function as integrating centers (Szurszewski & Miller, 2006). PVGs act as an interface for the ENS and CNS and help regulate and control GI functions.
In the somatic motor system, the final neuronal pathway to skeletal muscle is provided by alpha and gamma motor neurons. In the ANS, the final neuronal pathway that modulates the activity of ENS neurons is provided by parasympathetic and sympathetic fibers. Parasympathetic fibers are preganglionic fibers and are predominantly cholinergic. They arise from the dorsal motor nucleus of the vagus and the sacral spinal cord segments S2-4. A few have been shown to innervate EE cells but the majority terminate primarily in the myenteric ganglia. The terminal endings have varicosities and course throughout the ganglia, giving off collateral branches that contact many enteric neurons. The proximal and distal parts of the GI tract receive the vast majority of the parasympathetic fibers. Parasympathetic fibers coursing in the vagus are excitatory to enteric neurons, and activation of those to the stomach results in both excitatory (increased secretion and motility in the distal stomach) and inhibitory effects (relaxation of the upper stomach), the latter via inhibitory neurons. The rectum and distal colon are strongly activated by parasympathetic fibers coursing in pelvic nerves and their branches. In general, parasympathetic efferent fibers are excitatory and modulate the ongoing activity of neuronal circuits involved with local reflex responses and increase their effectiveness. Because they comprise a small number of vagal fibers, they do not affect individual motor neurons but instead they allow the CNS to control the activation of blocks of integrated microcircuits in the gut wall. In addition, they provide a powerful input to the motility patterns resulting in defecation and emesis (Brookes & Costa, 2006). Sympathetic postganglionic fibers provide the major extrinsic input into the gut wall. In general, they have an inhibitory influence on enteric networks controlling motility, they play a minor role in the relaxation of non–sphincter musculature, and they cause contraction of the sphincters through direct innervation. Sympathetic activity is involved in maintaining homeostasis by its control of the cardiovascular system and the body’s water and electrolyte balance. Fibers directly innervate blood vessels and activation of the fibers causes vasoconstriction of vessels, thereby shunting blood from the viscera to deprived vascular beds in tissues. Sympathetic fibers also reduce the secretion of water and electrolytes by inhibiting secretomotor neurons (Brookes & Costa, 2006; Furness, 2008). They arise from prevertebral ganglia and terminate as varicose endings densely covering all neuron cell bodies in both the myenteric and submucosal ganglia. Based on their chemical coding characteristics, it appears that different effectors (i.e., myenteric and submucosal plexus neurons and blood vessels) are innervated by different populations of sympathetic fibers, implying that these effectors and thus the functions of motility, blood flow, and secretion can be regulated separately. The primary neurotransmitter released in the enteric ganglia is norepinephrine (NE, noradrenaline). Binding of NE to α2 receptors expressed on enteric neurons causes presynaptic inhibition of neurotransmitter release. This may be a mechanism for suppressing reflex pathways and for quieting spontaneous activity created within enteric neuronal networks and from parasympathetic input. NE binding to the α2 receptor expressed on submucosal secretomotor neurons containing VIP results in direct, postsynaptic inhibition. NE may have an excitatory effect on enteric neurons by binding to α1 receptors (Brookes & Costa, 2006).
In summary, the enteric nervous system consists of neurons that form integrative circuits within the gut wall that are organized to function independently of the CNS—somewhat like a “local minibrain” or “brain-in-the-gut.” These circuits are arranged into microcircuits that appear to be programs that can control specific functions for the muscular wall, the glands, and the blood vessels to act independently of one another for local homeostatic adjustments. These microcircuits can also coordinate and control these effectors so that the entire organ functions as an integrated unit (Wood, 2004; Wood, 2009). The output of the enteric circuits is based upon the sensory information it receives. In general, the input concerns the current status of the effector such as the tone of the muscular wall, the contents of the lumen, or the presence of changes in the environment of the lumen (such as osmolarity or pH). The sensory fibers include the IPANs (initiate tone-dependent peristaltic contractions), interneurons (initiate tone-independent stretch-activated reflex contractions), vagal afferents (allow the gut to respond to tension), viscerofugal/intestinofugal neurons (allow the gut to fill), and spinal afferents (signal nociceptive information) (Szurszewski & Miller, 2006). Although the ENS functions autonomously, the CNS is not totally “blind” to the activity of the gut and receives sensory information through vagal afferents (primarily) and spinal afferents. It provides input to the ENS for integrative functions via the sympathetic preganglionic/postganglionic fibers and parasympathetic efferent fibers. These fibers modify programmed circuits and coordinate ENS activity in different regions of the gut, resulting in the activation of effectors controlling motility patterns, secretion, and blood flow. In general, parasympathetic input to the ENS results in the stimulation of gut motility and secretion and also the relaxation of the GI sphincters. Sympathetic input in general results in the inhibition of GI motility and secretion and also the constriction of the sphincters. To maintain gut homeostasis, local neural circuits and CNS structures establish a hierarchy of gut reflexes and loops (Mayer, 2011) (Fig. 10-22). Many local responses to stretch or chemical stimuli that cause peristalsis and secretomotor activity may utilize enteric circuits confined to the gut wall. Vago-vagal reflexes (e.g., gastric reflexes) use the nucleus tractus solitarius (NTS) and dorsal motor nucleus and reflexes involving coordinating distinct regions of the gut (intestino-intestinal) use prevertebral ganglia. Responses to nociceptive input involve spinal and supraspinal reflex loops, which may elicit strong autonomic and emotional behaviors such as motivation and affect. Integration of visceral and somatic input occurs in the brain stem and is further integrated with external environmental stimuli and behavioral control regions in cortical areas. In cortical regions such as the insula and cingulate cortex, the input is processed, leading to complex gut-related homeostatic states with affective and motivational aspects such as nausea, pain, hunger, and the feeling of ‘well-being’. Through top-down modulation the supraspinal areas allow for optimal homeostatic regulation of GI function by activating autonomic efferent fibers, the neuroendocrine system, and pain modulatory pathways (see section Control of Autonomic Afferents: Central Autonomic Network). Research has already produced much data that describe the enteric neuronal population and its interactions with other neuronal populations. However, future studies will further elucidate details on this extensive population of neurons and its complex interactions with prevertebral ganglia and CNS neurons (i.e., spinal cord, medulla of the brain stem, and higher centers) in normal physiologic states and in the realm of GI pathologies.
Innervation of Autonomic Effectors
Innervation of Peripheral Effectors
Cutaneous Effectors
Cutaneous autonomic effectors include blood vessels, sweat glands, adipose tissue, and arrector pili muscles. Similar to tissues of the immune system, but unlike most autonomic effectors, these cutaneous effectors are innervated by only the sympathetic division. Preganglionic fibers originate from cell bodies located in the intermediolateral cell column of the T1 to L2 or L3 cord segments, leave via ventral roots, and, after traversing the white rami communicantes, synapse in the sympathetic chain ganglia. Postganglionic fibers include vasomotor fibers, sudomotor fibers (to sweat glands), pilomotor fibers, and fibers to adipose tissue. Vasomotor fibers provide tone to the blood vessel wall, and when their firing is increased they vasoconstrict the vessel. Sudomotor fibers cause sweat to be secreted. Pilomotor fibers innervate the arrector pili muscles of the hair follicle. When the fibers are activated the hair is moved into a more vertical position and its surrounding epidermis forms a small elevation (‘goose bump’). In lower animals piloerection allows a layer of air to be trapped next to the skin for insulation purposes. The innervation of fat cells in adipose tissue is necessary for the mobilization of lipid, which is especially important during exercise and fasting and for thermoregulation. The fibers to all of these peripheral effectors travel in gray rami to join the spinal nerve on its course to its dermatomal area of supply. (However, the area of skin innervated by sympathetic fibers has been found to be wider than the dermatomal distribution of the somatic fibers [Ogawa & Low, 1993].) More specifically, superior and middle cervical ganglia send postganglionic fibers to the head and neck; the stellate (cervicothoracic) ganglion (in conjunction with a small contribution from the middle ganglion and possibly upper thoracic ganglia) supplies the upper extremities; thoracic ganglia supply the trunk; and lower lumbar and upper sacral ganglia furnish postganglionic fibers for the skin of the lower extremities (Jänig, 1990; Standring et al., 2008). Because sympathetic efferents innervate cutaneous effectors covering the entire body, nearly all spinal nerves are likely to contain postganglionic sympathetic fibers. The sympathetic innervation of these cutaneous effectors is controlled primarily by the hypothalamus, and stimulation of these effectors is important for thermoregulation.
Blood Vessels Supplying Skeletal Muscles
During muscle activity, vasodilation (and subsequent increased blood flow) is primarily a result of local muscle tissue effects; for example, decreased oxygen concentration in the contracting muscle causes the release of vasodilator substances. However, sympathetic fibers also innervate these blood vessels, providing continuous vasomotor tone, and when further activated they cause vasoconstriction (sympathetic vasodilator fibers also exist in some lower animals) (Guyton, 1991). The adrenal medulla (innervated by preganglionic sympathetic fibers) is also involved in the regulation of blood flow to skeletal muscles by causing vasoconstriction (via the neurotransmitter norepinephrine, which binds to vasoconstrictor alpha receptors) and some vasodilation (via the neurotransmitter epinephrine, which binds to vasodilator beta receptors) (Guyton, 1991).
The origin of preganglionic neurons for the upper extremity blood vessels is thought to be in the intermediolateral cell column of the T2 or T3 to T6 or T7 cord segments. The axons enter the sympathetic chain ganglia through white rami communicantes and synapse in the stellate (cervicothoracic) ganglion and upper thoracic ganglia. In general, the postganglionic fibers leave the ganglion within gray rami to join spinal nerves and, subsequently, ventral rami destined to form the brachial plexus. The C8 and T1 ventral rami receive the greatest contribution of fibers; therefore the lower trunk of the brachial plexus conveys most of the peripheral sympathetic efferents. The lower trunk provides fibers to numerous terminal branches of the brachial plexus, including the median, ulnar, and radial nerves. As the postganglionic neurons travel with these nerves, they supply branches to the accompanying brachial, ulnar, and radial arteries, respectively. The lower extremity muscular arteries are supplied by sympathetic fibers originating in the T10 to L2 or L3 cord segments. These preganglionic fibers enter the sympathetic chain and synapse in the lumbar and sacral ganglia. Postganglionic neurons traverse the gray rami, join spinal nerves, and then enter ventral rami of the lumbar and sacral plexuses. Postganglionic efferents course with the femoral nerve and its branches to supply vasoconstrictor branches to their adjacent blood vessels in the thigh and with the obturator nerve to supply the obturator artery. Others travel with the tibial nerve to innervate the popliteal artery and the tibial vascular tree and with the pudendal and gluteal nerves to innervate their respective arteries. Some postganglionic fibers to vessels in the upper thigh travel with the iliac arteries (Standring et al., 2008).
Sympathectomy
Surgical procedures such as endoscopic thoracic sympathectomy, clipping of the sympathetic chain, or ramicotomy have been performed as a method of treating conditions such as palmar hyperhidrosis, Raynaud’s disease, and CRPS (see section on CRPS and sympathectomy). These procedures performed at the appropriate levels interrupt fibers innervating the peripheral vasculature and sweat glands. As noted earlier (see Thoracic Sympathetic Trunk), there are many anatomic variations of the upper thoracic trunk relative to intercostal veins and intercostal nerves and communicating ascending and descending rami that provide alternate sympathetic pathways. Having an understanding of these variations and the anatomy of the cervicothoracic ganglion is necessary for a surgeon to perform a sympathectomy successfully and minimize any side effects. Interrupting fibers to the vasculature provides a treatment for relief of vasomotor spasms that occur in such disorders as Raynaud’s disease and intermittent claudication. Sympathectomy also has been performed to influence vasomotor tone in hypertensive patients. The most common indicator for thoracic sympathectomy is palmar hyperhidrosis, and because of its high success rate it has become the preferred treatment (Haam et al., 2010). Some data suggest that the sweat glands of the hand are innervated by fibers from T2 to T3 and possibly T4 ganglia (Cho et al., 2005). Based on anatomic studies, it appears that a fourth thoracic ganglionectomy is most effective in treating this condition and reducing side effects such as compensatory sweating and Horner’s syndrome (Cho et al., 2005; Singh & Ramsaroop, 2007). Sympathectomy may also be performed on the lumbar sympathetic trunk to treat plantar hyperhidrosis and arterial disease and symptoms related to causalgia. The portion of the chain removed is the segment from and including the L2 ganglion through the L3 ganglion, sparing the L1 ganglion, which is involved with ejaculatory function (Snell, 2008; Standring et al., 2008).
Innervation of the Heart and Lungs
As with most autonomic effectors, the heart and lungs are innervated by both the sympathetic and the parasympathetic divisions of the ANS. Sympathetic preganglionic neurons to both the heart and lungs originate in the T1 to T4 or T5 cord segments, enter the sympathetic chain, and synapse. The preganglionic sympathetic fibers associated with the innervation of the heart synapse in the thoracic ganglia that correspond to the spinal cord segments of origin. Many also ascend to synapse in all three cervical ganglia. Postganglionic fibers leave the ganglia as cardiac branches (nerves), which form part of the cardiac plexus (see Fig. 10-9, B). Sympathetic innervation of the heart results in cardiac acceleration and increased force of ventricular contraction. Coronary blood flow is primarily controlled by autoregulation of the coronary arteries in response to increased and decreased cardiac activity and subsequent metabolic needs of the muscle tissue. However, the coronary arteries are well innervated by sympathetic fibers and, although of minor importance functionally, these fibers on stimulation cause either vasoconstriction or vasodilation, depending on which receptor (alpha or beta, respectively) is activated (Guyton, 1991). Afferent information from the heart travels in all cardiac branches except the branch associated with the superior cervical ganglion (Standring et al., 2008).
Postganglionic sympathetic fibers to the lungs originate from the T2 to T5 sympathetic chain ganglia and pass through the cardiac plexus. However, they continue and course along the pulmonary arteries to form the pulmonary plexus. These postganglionic efferents provide bronchodilation and vasoconstriction to the lungs.
Parasympathetic Innervation
Parasympathetic innervation to the heart and lungs is provided by the vagus nerve (CN X). Cardiac preganglionic fibers originate in the brain stem medulla. Although most parasympathetic visceral efferents originate in the dorsal motor nucleus, some, possibly the majority, of the efferents to the heart originate in or near the nucleus ambiguus (Loewy & Spyer, 1990; Noback, Strominger, & Demarest 1991; Nolte, 2002; Standring et al., 2008; Kiernan, 2009). Regardless of origin, the preganglionic fibers descend within the vagus nerve into the thoracic cavity as cardiac branches and become part of the cardiac plexus (see Fig. 10-9, B). They synapse in small cardiac ganglia located in the cardiac plexus and in the walls of the atria. Postganglionic parasympathetic fibers cause a decrease in ventricular contraction and cardiac deceleration. Although autoregulation of coronary arteries based on local metabolic needs is the primary mechanism of controlling coronary blood flow, a few parasympathetic fibers innervate these arteries and, on stimulation, result in a slight vasodilation (Guyton, 1991).
Vagal preganglionic fibers that innervate the lung aid the sympathetics in forming the pulmonary plexus. The preganglionic parasympathetic fibers synapse in small ganglia adjacent to the lung hilum. Postganglionic fibers continue into the lung to stimulate bronchoconstriction, vasodilation, and glandular secretion (Standring et al., 2008). These actions help to maintain the integrity of the epithelial lining of the bronchial tree.
Innervation to the Head
In general, preganglionic sympathetic cell bodies for the head (and neck) are located in the T1 (primarily) to T3 (and some in T4 and T5) spinal cord segments. These fibers enter the sympathetic chain and ascend to the superior cervical ganglion, where they synapse. Postganglionic fibers leave the ganglion and travel with branches of the carotid arteries to gain access to autonomic effectors in the head (see Fig. 10-9, A). One large branch (the internal carotid nerve) leaves the ganglion and branches to form the internal carotid plexus, which travels on the internal carotid artery into the cranial cavity. Some fibers (vasoconstrictors) of the plexus continue to the cerebral arteries, where they meet additional sympathetic fibers of the vertebral plexus. Other fibers leave the artery and travel to their destination by “hitching a ride” on cranial nerves in the region.
The perivascular plexus surrounding the external carotid artery and its branches contains fibers that are vasomotor, pilomotor, and secretomotor to all sweat glands of the face except the sweat glands in the medial part of the forehead. These are supplied by branches of the internal carotid plexus (Watson & Vijayan, 1995; Salvesen, 2001).
Although sympathetic efferents employ arterial transportation, parasympathetic fibers to the head emerge from the brain stem as a part of the oculomotor, facial, and glossopharyngeal nerves. These nerves innervate glandular tissue and smooth muscle.
There is dual innervation to the lacrimal, mucosal, and salivary glands. In general, parasympathetic fibers are secretomotor and sympathetic fibers are vasoconstrictive although they modulate lacrimal glandular secretion (Standring et al., 2008) and salivary gland function (see below). Parasympathetic fibers that innervate the lacrimal gland, nasal and palate mucosal glands, and major salivary glands (sublingual and submandibular) travel with the facial nerve. Preganglionic neurons originate in the superior salivatory nucleus located in the brain stem. Those en route to the lacrimal and mucosal glands synapse in the pterygopalatine ganglion, and those destined for the salivary glands synapse in the submandibular ganglion. Parasympathetic efferents innervating the parotid salivary gland course in the glossopharyngeal cranial nerve. These preganglionic fibers originate in the inferior salivatory nucleus of the brain stem and course to the otic ganglion. After synapsing, the postganglionic secretomotor fibers travel to the parotid gland (see Figs. 10-15 and 10-16, A). Secretion by salivary glands (parotid, submandibular, and sublingual) is a reflex mediated by autonomic fibers. There is always a resting flow of saliva that serves to protect the oral mucosal surface. During short periods of intense reflex stimulation of taste receptors, olfactory receptors, mechanoreceptors, and nociceptors attributable typically to tasting food and chewing, increased amounts of saliva are produced. Not only will the volume of saliva increase but also the composition of saliva can vary as a result of the mixing of secretions from the three different glands. In addition, the composition of saliva from one gland can change based upon different afferent stimuli. Afferent input to the brain stem leads to activation of salivary nuclei, which appear to have connections with other nuclei. There is evidence of projections to the lateral hypothalamus where feeding, drinking, and body temperature are integrated. Although the exact circuitry is unclear, it appears that there is central modulation of salivary secretion because excitatory and inhibitory modulation of the salivary nuclei may occur (Proctor & Carpenter, 2007). Because there is no peripheral inhibition, interconnections with higher centers and the ability to centrally inhibit salivary nuclei may be the mechanism by which anxiousness produces dry mouth. The parasympathetic and sympathetic innervation of the salivary glands is typical in the sense that parasympathetic fibers are cholinergic and utilize muscarinic receptors and sympathetic fibers are adrenergic and employ the α1 and β1 adrenoceptors. However, they do not function in the typical antagonistic fashion but instead interact synergistically. Sympathetic preganglionic neurons originate in the upper thoracic segments, and postganglionic neurons are located in the superior cervical ganglion. Although sympathetic fibers do control glandular blood flow (i.e., vasoconstriction), it does not occur as part of the salivary reflex response. The increase in blood flow during the reflex response occurs in response to parasympathetic stimulation mediated by the release of endothelial factors such as nitric oxide, resulting in vasodilation. Parasympathetic secretomotor activity is predominant and stimulates most of the salivary fluid secretion, most of the mucin secretion, and a variable amount of cellular exocytosis (protein secretion). Sympathetic activity does not result in the inhibition of salivary secretion, but instead it has a synergistic effect on the same cells innervated by parasympathetic fibers. It modifies the molecular composition of saliva by increasing cellular exocytosis, and like the parasympathetic fibers it also induces the contraction of myoepithelial cells (Proctor & Carpenter, 2007).
The ANS is also intimately involved with the innervation of effectors located in the region of the orbit. These include the smooth muscle (Müller’s muscle or superior tarsal muscle) of the eyelids, blood vessels of the eye, and the smooth muscle of the iris and ciliary body. This innervation is responsible for some of the functions that can be assessed during a neurologic examination. Regulation of blood flow to the eye is extremely important in maintaining an adequate nutrient supply to the retina. Retinal arterioles are autoregulated, but choroidal arterioles are autonomically innervated. Sympathetic activation causes vasoconstriction, whereas parasympathetic stimulation, via the facial nerve, is vasodilatory (Loewy, 1990b).
The upper eyelid contains skeletal muscle (levator palpebrae superioris), which is innervated by somatic efferents of the oculomotor nerve. It (and the lower eyelid to a lesser extent) also contains smooth muscle fibers (Müller’s muscle or superior tarsal muscle). The smooth muscle is innervated by sympathetic fibers. Because ptosis (drooping) of the upper eyelid is an important indicator of damage to the sympathetic nervous system, knowledge of the two innervations is necessary for differentiating a lesion involving the oculomotor nerve from a lesion of the sympathetic system.
Other smooth muscle fibers in this region are the intrinsic muscles of the eye (i.e., the sphincter and dilator pupillae muscles of the iris and the ciliary muscle of the ciliary body). The iris acts as a diaphragm and regulates the amount of light entering the eye. The dilator muscle is innervated by sympathetic postganglionic efferents that have left the internal carotid plexus to travel with the ophthalmic fibers of the trigeminal nerve. The parasympathetic innervation supplies the sphincter muscle fibers and is the motor arc of the pupillary light reflex. The origin of the parasympathetic preganglionic fibers is the Edinger-Westphal nucleus of the midbrain. The fibers emerge from the brain stem, lie superficially in the dorsomedial aspect of the oculomotor nerve, and travel to the ciliary ganglion located in the orbit (see Fig. 10-15). After synapsing, postganglionic fibers innervate the sphincter muscle fibers of the iris. When these fibers are activated to cause pupillary constriction, there is an accompanying inhibition of the innervation to the dilator muscle (Loewy, 1990b). During alert but resting periods, a constant sympathetic tone is sustained through hypothalamic input; parasympathetic drive to the sphincter pupillae muscle is decreased at the same time (Cross, 1993b; Standring et al., 2008).
An additional smooth muscle, the ciliary muscle, also is involved in the normal function of the eye. This muscle controls the tension of the suspensory ligaments attached to the lens. Parasympathetic and some sympathetic fibers (the function of the latter is unclear) innervate the ciliary muscle. Controlling the regulation of the curvature and thus the refractive power of the lens via this muscle is essentially a parasympathetic function. This innervation allows focusing to occur when an object is close to the eye (accommodation). Of the total number of preganglionic fibers leaving the Edinger-Westphal nucleus, 94% travel to the ciliary muscle, whereas the remaining fibers supply the iris (Cross, 1993b). The accommodation-convergence reflex is necessary during near vision to correct for an unfocused image on the fovea (region of the retina associated with the most acute vision). The reflex response (mediated by CN III) results in an increase in lens curvature, pupillary constriction, and convergence of the eyes.
Knowledge of the innervation of the eye is important because pathologic conditions affecting the autonomic innervation can occur and because these eye functions can be tested in a neurologic examination. Pathologic conditions caused by disruption of parasympathetic fibers include the Argyll Robertson pupil (associated with neurosyphilis), internal ophthalmoplegia, light-near dissociation, and Adie’s pupil (Cross, 1993a). Horner’s syndrome is an example of a condition attributed to a lesion in the sympathetic system (see Clinical Applications).
Innervation of the Lower Urinary Tract
The lower urinary tract consists of the bladder, the neck of the bladder (essentially the internal urethral orifice, which is histologically distinct from the smooth muscle of the bladder wall [Standring et al., 2008]), the urethra, and the urethral sphincter. The lower urinary tract is involved with the process of micturition, which is organized into two phases of bladder function—storage and elimination (voiding) of urine. The neural control of the lower urinary tract is somewhat different than the mechanism by which other viscera are autonomously regulated in that it is dependent on CNS control. The process consists of storage and emptying phases controlled by ‘on’ and ‘off’ neural circuits rather than continual tonic control. It also has a volitional component that becomes operational as the nervous system matures (Fowler et al., 2008). Control of lower urinary tract function occurs by a complex integration and coordination of afferent fibers, sympathetic and parasympathetic efferent fibers, somatic efferent fibers (Fig. 10-23), pontine micturition centers, periaqueductal gray, hypothalamic nuclei, and cortical areas (Carpenter & Sutin, 1983; de Groat & Steers, 1990; Abdel-Azim, Sullivan, & Yalla, 1991; Bradley, 1993; Blok & Holstege, 1998; Shefchyk, 2001, 2002; Fowler et al., 2008). Sympathetic preganglionic neurons originate in cord segments T11 or T12 through L2 and synapse in corresponding ganglia and small ganglia in the superior and inferior hypogastric plexuses. Postganglionic adrenergic fibers course in the vesical plexus (anterior fibers of the inferior hypogastric plexus) to the bladder. Some of these fibers inhibit the smooth muscle (detrusor) of the bladder wall through activation of β3-adrenergic receptors, but many, and possibly the majority, are also vasomotor, suggesting that sympathetic efferents may have no essential role in micturition. Other sympathetic fibers richly supply (especially in the male) and stimulate the smooth muscle in the neck of the bladder (sphincter vesicae) and urethra by activating α1-adrenergic receptors. In males, the smooth muscle of the neck of the bladder includes an additional distinct bundle of muscle fibers forming a sphincter (preprostatic sphincter). This is not a urinary sphincter and has a minor, if any, role in urinary continence. During the processes of emission and ejaculation, which are under the control of sympathetic fibers, there is a concomitant stimulation of sympathetic fibers to the bladder neck to contract the sphincter and prevent reflux of ejaculate into the bladder. In females the neck of the bladder is profusely supplied by cholinergic fibers and very few adrenergic fibers. Because the bladder neck does not include an anatomic sphincter, the integrity of the bladder neck in females is more likely to be dependent upon the ligaments surrounding it (Snell, 2001; Fowler et al., 2008; Standring et al., 2008).
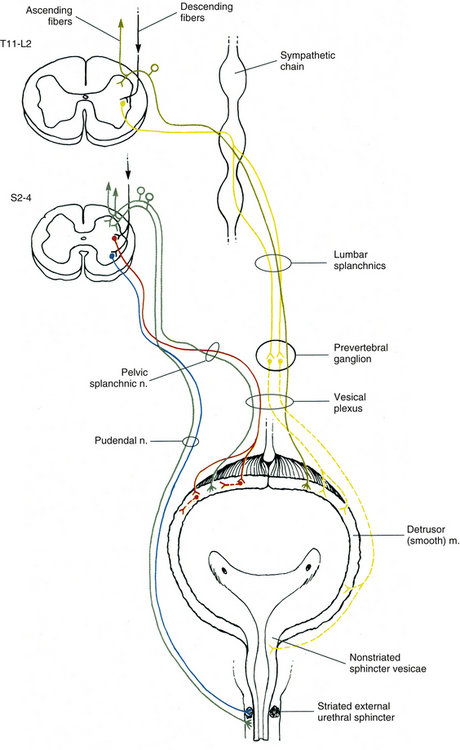
FIG. 10-23 General description of the innervation of the bladder. Parasympathetic fibers (red) innervate the detrusor (smooth) muscle. Sympathetic fibers (yellow) are primarily vasomotor to the bladder wall but also supply the sphincter vesicae (smooth) muscle in the neck of the bladder, which functions to close the lumen of the neck during ejaculation. Somatic efferents (blue) coursing in the pudendal nerve also provide an important innervation to the external urethral sphincter (striated muscle). Afferent fibers (green) entering the cord may transmit information to higher centers, as well as provide the afferent arc for initiating the voiding response. Descending information from higher centers also may modulate the spinal cord neurons. See text for discussion on the supraspinal control of bladder function.
The parasympathetic innervation is another important autonomic efferent supply to the detrusor muscle of the bladder. The parasympathetic fibers originate in the S2 to S4 spinal cord segments. The neuron cell bodies are located in the sacral autonomic nucleus and are divided into tonic and phasic types. Dendrites course into lamina I and the dorsal gray commissure. The commissure includes interneurons that are thought to be active during micturition and likely influence the parasympathetic neuron cell bodies (Birder et al., 2010). The parasympathetic fibers pass into the cauda equina, emerge from the S2 to S4 pelvic (ventral) sacral foramina, and travel in the pelvic splanchnic nerves (see Figs. 10-17 and 10-23). These nerves travel within the inferior hypogastric plexus and continue distally into the vesical plexus. These preganglionic fibers synapse with postganglionic neurons located in ganglia within the vesical plexus or within the bladder wall. The postganglionic cholinergic fibers provide excitatory innervation to the detrusor muscle. (A few fibers supply and inhibit the sphincter vesicae, which is mediated by the neural release of nitric oxide.) The acetylcholine (ACh) that is released activates the M3 muscarinic receptors and causes contraction of the detrusor muscle. The parasympathetic presynaptic nerve terminals at the neuromuscular junction express M1 and M4 receptors. Depending on the intensity of neural firing, neurotransmission at this site can be modulated such that activation of these M1 and M4 receptors can enhance or suppress, respectively, transmitter release. Some fibers also co-release ATP and cause detrusor muscle contraction through ATP’s action on P2X purinergic receptors (Fowler et al., 2008).
Autonomic postganglionic neuron cell bodies that innervate the lower urinary tract (as well as the reproductive tract and lower bowel) are located in pelvic ganglia. Most of the postganglionic cell bodies are found in the ganglia located at the vesico-ureteric junction but other ganglia are located in the bladder wall, the region of the bladder neck, the trigone, the prostate, and the proximal urethra. Excitatory noradrenergic (NA) and cholinergic fibers, and inhibitory noncholinergic and nonadrenergic fibers, synapse in the ganglia. These neurons, found in groups of up to 20 neurons, show an abundance of acetylcholinesterase. Within the ganglia numerous neurotransmitters and neuromodulators have been identified. Also, data indicate that postganglionic sympathetic fibers terminate within the ganglia as well (Standring et al., 2008; Birder et al., 2010).
Another important muscle involved with normal bladder function is the striated (voluntary) urethral sphincter muscle. This muscle does not contain neuromuscular spindles and consists of slow-twitch fibers. This urethral sphincter is important in maintaining urinary continence because the muscle fibers maintain a contracted state for long periods of time and in doing so contribute to the tone that closes the urethra. Somatic motor neurons in Onuf’s nucleus (S1-S2 cord segments) and possibly other neurons in the ventral horn of the S2 to S4 cord segments innervate this muscle.These neurons travel in the perineal branches of the pudendal nerve to reach the skeletal muscle fibers of the sphincter (Standring et al., 2008).
In addition to the sympathetic, parasympathetic, and somatic efferent innervation to the bladder, there are also afferent fibers that course from the bladder, neck of the bladder, and urethra to the spinal cord. Afferent fibers from the neck of the bladder and urethra travel in the pudendal and hypogastric nerves. Input regarding bladder fullness travels in afferent fibers running in the pelvic splanchnic and hypogastric (lumbar splanchnic) nerves. In animal studies, research shows there are four types of mechanosensitive afferents, which are low-threshold A-delta fibers. These are distributed to the serosa, to the urothelial (transitional epithelial) layer, and throughout the muscle layers of the bladder. Lumbar splanchnic nerves (hypogastric) include afferent fibers primarily from the serosa and muscle layers. Pelvic splanchnic nerves include afferent fibers of all four classes that synapse on interneurons in cord segments S2 to S4. The majority of these are from muscle layers signaling passive distension and active contraction and likely are involved in mediating the micturition reflex via the spinobulbospinal pathway. Other afferents include a small population of unmyelinated C fibers that are distributed in the lamina propria and urothelium (transitional epithelium). These fibers contain peptides such as substance P and CGRP and course in the hypogastric nerve. They are high-threshold fibers that respond to distension and are usually insensitive to muscle contraction. Other unmyelinated C fibers are concerned with nociception and temperature sense. These C fibers are silent during normal bladder filling but will respond primarily to noxious stimuli such as cooling or chemical irritation. These afferent fibers course in pelvic nerves. Afferent fibers enter Lissauer’s tract and terminate on spinal neurons, some of which will mediate segmental reflexes. For example, lamina I neurons receive afferent fibers and project to the IML cell column and to brain stem integration nuclei (Drake et al., 2010). Other entering afferent fibers terminate on interneurons in laminae V to VII and X. Some fibers terminate in the vicinity of the sacral autonomic nucleus and in the dorsal gray commissure. The termination of afferent fibers from the bladder and afferent fibers from the urethra and urethral sphincter overlaps in the lateral and basal part of the dorsal horn and dorsal gray commissure. This may be an integration area that coordinates the functions of the bladder and sphincter (Fowler et al., 2008). Axons from some spinal neurons convey information from the bladder to higher centers and course in the dorsal and anterolateral white columns. In disease states such as malignancies, stones, or inflammation, distension and nociception are brought to one’s consciousness. Because nociceptive information ascends in the anterolateral white, bilateral anterolateral cordotomy results in pain relief. Fibers conveying mechanosensitive information regarding distension ascend in the dorsal column and thus the awareness of bladder fullness and need to void is preserved (Standring et al., 2008).
In addition to the afferent fibers coursing from the bladder to the cord, an intrinsic nerve plexus has been shown to exist immediately below the urothelium in the lamina propria. Fibers of the plexus project into the urothelium. The plexus is especially dense in the region of the bladder neck but is sparse in the bladder fundus and inferolateral walls. It is considered to be an essential component for sensory processing by the urothelium. Also, a population of nonneuronal cells has been identified in animal models that has also been implicated in sensory mechanisms. These are cells that comprise the urothelium and are able to respond to chemical and mechanical stimuli. The cells have unique sensory and signaling properties that allow for communication with underlying nerve fibers, myofibroblasts, smooth muscle cells, and inflammatory cells. One of these properties is the expression of numerous receptors and ion channels that are typically found on nociceptive and mechanoreceptive fibers. Examples of these receptors are purinergic, adrenergic, nicotinic, and muscarinic receptors that are known to mediate responses to stimuli such as stretching and to various signaling molecules. Other unique properties are their close proximity to the afferent and efferent fibers and the ability of these cells to release chemicals that can act on neighboring nerve fibers affecting bladder contraction and vascular flow. These attributes allow the cells to have a bidirectional chemical communication with adjacent nerves in the wall of the bladder. Defects in these mechanisms of sensory transduction may contribute to the pathophysiology of bladder diseases (Fowler et al., 2008; Birder et al., 2010).
In addition to the areas in the spinal cord gray matter involved with lower urinary tract function, there are many higher centers also involved in controlling the bladder, urethra, and urethral sphincter. These include the raphe nuclei in the medulla, the locus ceruleus, and the noradrenergic (NA) A5 cell group in the brain stem, which project to the cord and act as nonspecific ‘level-setting’ mechanisms. Other areas involved with the micturition response include the pontine micturition center (PMC; also known as Barrington’s nucleus), periaqueductal gray (PAG), hypothalamic nuclei, amygdala, and regions of the cerebral cortex such as the anterior cingulate gyrus, insula, and prefrontal cortex. These supraspinal areas are interconnected and regulate the bladder and lower urinary tract function by sending projections to the lumbosacral spinal cord via multiple pathways (Fowler et al., 2008).
As mentioned previously, micturition consists of two phases involving the bladder: filling/storage and voiding. The neural circuits involved with this system are organized as ‘on-off’ switching circuits interrelating activity in the bladder with the outlet structures (i.e., neck of the bladder, urethra, and urethral sphincter). In general the spinal cord circuitry is involved with the filling of the bladder whereas the circuitry mediating the voiding process is organized in the higher centers. Afferent information is processed in the spinal cord, establishing a reflex response and activating tract neurons. The tracts include the fasciculus gracilis, spinoreticular, spinothalamic, and spinohypothalamic. The axons ascend in the dorsal column, and the dorsolateral funiculus and lateral funiculus, and give off collateral branches as they terminate in higher centers. One of these areas is the periaqueductal gray (PAG) matter located in the midbrain, which controls the primary input to the pontine micturition center (PMC). The PMC neurons subsequently transmit signals back to the sacral spinal cord. The PMC, which also receives ascending projections from the spinal cord directly, has two components: the medial or ‘M’ center and the lateral or ‘L’ center. The ‘L’ center (pontine storage center) is thought to project to Onuf’s nucleus in the sacral cord and to facilitate the contraction of the external (striated) urethral sphincter. The ‘M’ center projects to the sacral autonomic nucleus to activate parasympathetic neurons and possibly inhibit Onuf’s nucleus. This spinobulbospinal voiding-reflex pathway functions as a switch. It is in the ‘off’ position for filling (storage) and is in the ‘on’ position for voiding. During bladder filling, afferents from stretch receptors project into the cord where interneuronal circuits function as a gating mechanism and reflexively inhibit parasympathetic innervation to the detrusor muscle; also, some sympathetic fibers facilitate bladder filling by causing inhibition of the detrusor muscle whereas many fibers cause contraction of the sphincter vesicae. In addition, through input from the ‘L’ center, somatic motor neurons fire and maintain the external urethral sphincter muscle in a tonically active state. The sum of all of these urethral reflex circuits that promote continence is known as the ‘guarding reflex’. During a critical level of bladder distension, bladder afferent activity reaches a specific threshold, sensory fibers activate ascending pathways to higher centers including the brain stem, and the pathway is switched on. Descending fibers from the ‘M’ pontine micturition center course through the lateral funiculus of the cord and, via interneurons, cause inhibition of somatic motor neurons, resulting in relaxation of the external urethral sphincter followed a few seconds later by excitation of sacral preganglionic parasympathetic neurons, resulting in detrusor muscle contraction (Blok & Holstege, 1998; Shefchyk, 2001, 2002; Fowler et al., 2008). Although the pontine center coordinates the micturition process, it in turn is strictly modulated by the cerebral cortex, allowing for voluntary control. The decision to void is based on numerous factors that include one’s emotional state, input from the bladder, and one’s environment. The PAG has a dominant role in this process, acting as a hub by interconnecting with cortical regions and providing the major input to the PMC. Afferent signals from the bladder terminate in the PAG and from here axons project to the cortex where conscious awareness of the bladder sensation occurs. Additional input may ascend in the fasciculus gracilis and spinothalamic tract. The prefrontal cortex, especially the medial and dorsolateral areas and often more on the right side (Drake et al., 2010), projects back to the PAG to influence its activation of the PMC: during filling the PAG firing is suppressed and voiding is prevented; if voiding is desired, excitation by the PAG is allowed. Other areas of cortex involved with bladder function have also been identified in functional magnetic resonance imaging (fMRI) studies. During bladder filling the PAG not only is activated but also appears to project, possibly via the thalamus, to the insula (especially the right), which is shown to be very active during bladder filling. Visceral sensations including the desire to void are thought to be mapped in the insular cortex. The PAG also interconnects with the anterior cingulate cortex, which likely determines one’s attentiveness to bladder sensation and deciding whether to initiate or delay voiding, and the prefrontal cortex. The prefrontal cortex in turn has connections with the anterior cingulate cortex, the hypothalamus, and other autonomic control centers. It functions as the CEO of the brain, orchestrating complex cognitive behaviors, including those that are socially acceptable, and providing the mechanisms that allow one to be aware of a situation and respond appropriately. It is possible that through its connection with the PAG, the prefrontal cortex has a tonic suppressive effect on voiding that is reduced when voiding is desired (de Groat & Steers, 1990; Bradley, 1993; Blok & Holstege, 1998; Fowler et al., 2008). This descending cortical input not only allows voluntary control of voiding but also allows one to start and stop micturition on demand. The connections between the spinal cord and brain stem forming the spinobulbospinal reflex are instrumental for sustaining detrusor muscle contraction and relaxing the striated sphincter during micturition. In an excellent review, Drake and colleagues (2010) proposed a working model for the control of bladder function by higher centers. Sphincter relaxation and detrusor contraction are under the control of the PMC, which in turn receives input from the hypothalamus and, seemingly more important, the PAG. The PAG acts as an integrative center and modulates the activity of the PMC based on the input it has received from bladder afferents and higher centers. The higher centers that project to the PAG and are most understood include the insula (which maps and processes visceral sensations), the anterior cingulate gyrus (which monitors stress and conflict and initiates the correct autonomic response), and the medial prefrontal cortex (which evaluates information from all of the sensory cortices and makes a decision taking into account the current emotional and social state of the individual). Other areas of the brain that project to the PAG are the basal ganglia and cerebellum.
The functional and clinical relevance of the connections of the prefrontal cortex with the brain stem is exemplified in pathologic conditions that include this region (both cortex and underlying white matter) such as in stroke and normal pressure hydrocephalus (NPH). In NPH, which is often misdiagnosed as Parkinson’s or Alzheimer’s disease, three distinguishing characteristics are recognized: abnormal gait, dementia, and urinary incontinence. Imaging scans show enlarged anterior horns of the lateral ventricles, which are located in the frontal lobe. The frontal lobe is also the site of the descending fibers of the prefrontal cortex (as well as the connections between the basal ganglia and the frontal lobe) (Victor & Ropper, 2001). Studies of other frontal lobe pathologies resulting in urinary dysfunction (Drake et al., 2010) have shown the lesion to be localized anterior to the tips of the ventricles and the genu of the corpus callosum. Patients with acute hemispheric strokes that present with urinary dysfunction commonly show infarcts in the frontal lobe. In these cases, the cognitive abilities of the individual are normal and the act of voiding is normal. However, when the patient is sleeping the result of the lesion is incontinence. When the patient is awake, the loss of higher control causes frequency and extreme urgency of the voiding response. Because of the lesion, the individual is unaware of the sensation of normal gradual bladder filling and the sensation that voiding is about to occur. This often results in embarrassing and humiliating situations.
There is also a synergistic physiologic relationship between the lower urinary tract and other pelvic organs. Studies on animals show that the sensory input from a distended rectum inhibits the efferent arc of the micturition reflex (i.e., voiding response). Data indicate that many neurons in the pontine micturition center have a dual connection with neurons controlling colon and bladder activity whereas only a few center neurons have separate connections. Also, in spinal cord injury patients, low level stimulation of S3 roots by implanted electrodes results in the contraction of the external urethral and anal sphincters and pelvic floor muscles. Stimulation of the S3 roots at high levels results in detrusor muscle contraction and subsequent bladder emptying when the sphincter muscle is relaxed (Birder et al., 2010). Other studies have shown that a small percentage of lumbosacral afferent neurons dually innervate both the colon and the bladder. It is thought that this bidirectional communication may be involved in the enhancement of reflex activity in one when the other is chemically irritated (de Groat, 2006). This mechanism is referred to as cross-organ hypersensitivity (see section on Visceral Afferents).
Innervation of Reproductive Organs
The innervation of the reproductive organs, which is similar to that of the bladder, consists of sympathetic, parasympathetic, and somatic fibers (de Groat & Steers, 1990; Seftel, Oates, & Krane, 1991; Stewart, 1993). This section is primarily concerned with the innervation of male reproductive organs, although innervation to homologous female organs is somewhat similar.
The sympathetic preganglionic fibers originate from approximately the T10 to L2 cord segments (Fig. 10-24). The route of these fibers varies. Some preganglionic fibers synapse with postganglionic neurons in the sympathetic chain. The axons of these postganglionic neurons enter into the hypogastric nerves and continue into the inferior hypogastric (pelvic) plexus. Other preganglionic fibers travel in the superior hypogastric plexus and synapse in ganglia scattered in the inferior hypogastric plexus (Seftel, Oates, & Krane, 1991). In both cases the postganglionic fibers coursing within the inferior hypogastric plexus continue distally into the prostatic plexus. These fibers then leave the plexus as the cavernous nerve and innervate erectile tissue of the penis and smooth muscle in the seminal vesicles, prostate gland, vas deferens, and the nonstriated sphincter in the bladder neck. Parasympathetic preganglionic fibers originate from S2 to S4 and course in the pelvic splanchnic nerves (see Fig. 10-24). The pelvic splanchnic nerves synapse in ganglia in the inferior hypogastric (pelvic) plexus. Postganglionic fibers, in conjunction with postganglionic sympathetic fibers, continue to (and are the primary innervation of) erectile tissue, as well as glandular tissue in the seminal vesicles, prostate gland, and urethra.
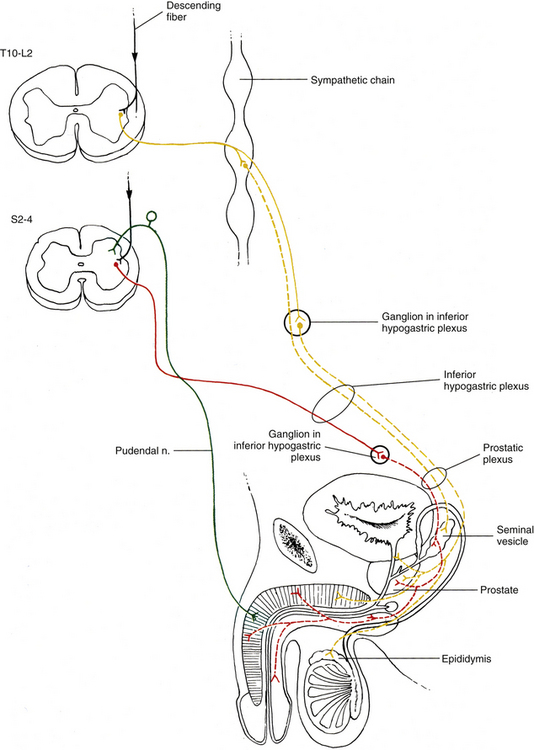
FIG. 10-24 Innervation of the male reproductive organs. Sympathetic fibers (yellow) are vasoconstrictive to the erectile tissue and also supply the glandular smooth muscle tissue. Parasympathetic fibers (red) are the major supply of the penile erectile tissue but also supply the glandular tissue. The pudendal nerve contains somatic efferent fibers (not pictured here) to the bulbocavernosus and ischiocavernosus muscles and afferent fibers (green) conveying sensory information from the penis. These afferent fibers form the sensory arc for reflexogenic erections. Descending fibers from the hypothalamus and limbic system structures send input to the spinal cord neurons, which initiate psychogenic erections.
The somatic nervous system is also involved with sexual function. The pudendal nerve contains sensory fibers that course from the penis to sacral cord segments. It also contains motor fibers that travel from the spinal cord to the bulbocavernosus and ischiocavernosus skeletal muscles. These motor neurons originate in the ventral horn of the S2 to S4 cord segments.
The erection phase of sexual function can be initiated by stimuli such as visual, auditory, imaginative, and tactile. Evidence from spinal cord–injured patients indicates that there are two types of erection reflexes: psychogenic and reflexogenic. In healthy individuals, both types of reflexes probably act synergistically. Reflexogenic erections are sacral spinal reflexes consisting of afferent fibers in the pudendal nerve activating sacral parasympathetic efferent fibers (a small number of afferent fibers ascend in the dorsal columns to higher centers). Psychogenic erections begin in supraspinal centers, including the limbic system and the hypothalamus. The hypothalamus has been implicated as the integration center for the erection response (de Groat & Steers, 1990; Stewart, 1993).
Fibers from these supraspinal centers descend through the brain stem and the lateral white column (funiculus) of the spinal cord to synapse on lower thoracic and lumbar preganglionic sympathetic neurons and the sacral preganglionic parasympathetic neurons. The parasympathetic fibers initiate the erectile response by causing dilation of the arteries within the erectile tissue. However, the sympathetic fibers contribute to this reflex because they also can initiate a psychogenic erection (possibly through the use of different neurotransmitters) when the parasympathetic preganglionic neurons are lesioned (de Groat & Steers, 1990; Seftel, Oates, & Krane, 1991).
Emission and ejaculation are also a part of sexual function. Cortical modulation occurs, but the mechanism is complex and unclear. Emission is sympathetically controlled by neurons originating in the T10 to L2 or L3 cord segments (see previous discussion for the route of these sympathetic preganglionic and postganglionic fibers). This event includes the smooth muscle contraction of the vas deferens, seminal vesicles, prostate gland, and nonstriated sphincter vesicae (to prevent reflux of semen into the bladder during ejaculation), resulting in the deposition of semen into the prostatic urethra. The process of ejaculation consists of propelling the semen from the prostatic urethra through the membranous and penile parts of the urethra and out the urethral orifice. The bulbocavernosus and ischiocavernosus skeletal muscles, which are innervated by the pudendal nerve, contract during this event. The coordination of emission and ejaculation probably occurs by the integration of sensory afferent fibers, descending supraspinal input, and motor efferents in an ejaculation center located in the T12 to L2 cord segments (Seftel, Oates, & Krane, 1991).
Integrative Applications
Having explained the innervation of individual systems by the ANS, it is important to remember that systems work together to maintain homeostasis. Exercise and the stress response are two examples that will be discussed in general to demonstrate the concept of integration among physiologic systems.
Exercise
Other than the muscular system, the major systems involved in exercise are the cardiovascular and respiratory systems. During exercise, the active skeletal muscles have a tremendous increase in their demand for oxygen, which arrives by way of the bloodstream. During rest, metabolic demand is low and therefore some capillaries in muscle tissue have little blood flow in them. When exercise begins, all capillaries are opened and the demand for blood flow increases. A decrease in available oxygen in the muscle tissue causes local vasodilation in the arterioles attributable to increased flaccidity in the arteriolar wall and the release of vasodilator substances. Chemical vasodilators released locally from cells to allow increased perfusion through the muscle include bradykinin, adenosine, potassium, carbon dioxide, lactate, and ATP. In addition, nitric oxide released from endothelial cells in response to the increased shear stress resulting from the increased flow also contributes to the vasodilation. While the higher centers of the CNS initiate contraction of the skeletal muscles, they also activate the vasomotor center in the brain stem. This area initiates a mass sympathetic discharge (and inhibits parasympathetic innervation to the heart) that affects other parts of the body. This leads to an escalation in heart rate and contractile force via cardiac nerves; vasoconstriction of peripheral arterioles located in the noncontracting muscles and nonparticipating tissues (coronary arteries and cerebral arteries are exempt), thus redirecting blood to the active muscles; and vasoconstriction of the veins, which increases venous return to the heart. Vasoconstriction occurs both directly through sympathetic innervation to the blood vessels and indirectly through sympathetic innervation of the medulla of the adrenal gland by the greater splanchnic nerve. The adrenal medulla releases norepinephrine, which causes vasoconstriction, and epinephrine, which increases cardiac output. The extensive increase in sympathetic stimulation to the heart and blood vessels raises cardiac output, supports arterial blood pressure, and provides significantly increased blood flow to the working muscles. In addition to these adaptive changes to provide increased oxygen and nutrient supply to the working muscles, there must also be increased blood flow directed to peripheral subcutaneous regions as part of the thermoregulatory response to shed the additional heat being generated by the working muscles. Temperature-regulating centers in the hypothalamus are activated to inhibit sympathetic fibers directed to peripheral subcutaneous vessels to cause vasodilation and allow increased blood flow to these regions for the purpose of heat dissipation. In addition, the thermoregulatory response will stimulate sympathetic cholinergic fibers that innervate sweat glands to activate heat dissipation by evaporation of the sweat from the skin surface.
The respiratory system functions to deliver and remove the oxygen and the carbon dioxide, respectively, to the bloodstream that flows through the lungs. As higher centers signal the start of muscle contraction and activate the vasomotor center, the brain stem respiratory center also receives excitatory input, which results in the increase in ventilation experienced during exercise. In fact, it appears that a large percentage of the total ventilatory increase occurs immediately at the initiation of exercise, indicating that the brain is able to anticipate the subsequent increase in oxygen consumption and carbon dioxide generation. Respiratory center neurons inhibit parasympathetic neurons and activate sympathetic neurons. The effects of sympathetic fibers that directly innervate bronchiolar muscle tissue are minimal. It is the innervation to the adrenal medulla and subsequent release of epinephrine and norepinephrine into the blood that is more effective. The result is dilation of the bronchial tree primarily through the effect of epinephrine, which has a greater stimulatory effect through its binding to bronchial β-adrenergic receptors.
Stress Response
In order to survive and maintain the well-being of the individual, physiologic responses are required to maintain homeostasis while the body is challenged by various stressors. In general, stress can be defined as “an actual or anticipated disruption of homeostasis or an anticipated threat to well-being” (Ulrich-Lai & Herman, 2009). Combating stress requires activation of the neuroendocrine system and the ANS. Some forms of stress (called eustress) are helpful in preparing the body to meet challenges whereas other forms of stress (called distress) can be damaging. An increase in body temperature is an example of a stressor, and through the control of just a limited number of effector tissues (i.e., sweat glands and smooth muscle in the cutaneous blood vessels), sympathetic nerves can restore the normal body temperature. In cases of distress where the challenge is chronic or extreme, resulting from severe and uncontrollable situations of physical and emotional stress, normal homeostatic mechanisms are insufficient. These situations can result in a sequence of changes to maintain the physiologic integrity of the body. This is sometimes referred to as the adaptive stress response or the general adaptation syndrome (Tortora & Derrickson, 2009). The ANS provides the most immediate response to stress. It is initiated by inputs into the brain indicating homeostatic disturbances. In some cases such as blood loss, infection, or pain, the immediate response is reflexive through connections in the rostral ventrolateral medulla of the brain stem and the intermediolateral cell column of the spinal cord. In other cases, the stressor may be psychogenic or nonphysical. In this case, the input is processed in areas of the limbic forebrain, which includes the amygdala, hippocampus, and medial prefrontal cortex. These areas have received input from other regions of the brain that are involved in higher-order sensory processing, attention and arousal, and memory. From here, most connections are made with various hypothalamic nuclei for integration and necessary modulation based on the current physiologic status of the individual. Output is relayed to the paraventricular nucleus (PVN) of the hypothalamus, which has been suggested as being the principal integrator of stress signals (Ulrich-Lai & Herman, 2009). This nucleus sends signals to various nuclei in the brain stem and to the spinal cord. The involvement of the sympathetic neurons results in a simultaneous mass discharge of sympathetic output to widespread areas of the body by utilizing both postganglionic peripheral fibers and preganglionic fibers that innervate the medulla of the adrenal glands (the sympathoadrenomedullary connection), causing the release of epinephrine and norepinephrine into the blood. The immediate goal of the activation of the sympathetic system is to ensure that there are increased amounts of glucose and oxygen transported to essential tissues for the necessary increased rates of cellular metabolism for immediate action (increase in muscle strength and increased mental activity). To achieve this, the innervation of numerous tissues and organs is modulated and their functions coordinated. This response demonstrates the widespread connections of the sympathetic nervous system throughout the body. Some of the more obvious organs and tissues that are involved include the heart (increase in heart rate and force of contraction), peripheral blood vessels in nonessential tissues (redistribution in blood flow), lungs (vasodilation and bronchial dilation), liver (glycogenolysis), and kidneys (reduced renal blood flow resulting in the release of renin). At the same time that sympathetic neurons are activated, parasympathetic neuronal activation is modulated. CNS areas such as the PVN and the prelimbic cortex influence the parasympathetic neurons in the nucleus ambiguus and dorsal motor nucleus of the vagus directly or possibly through connections with the nucleus tractus solitarius (NTS). This connection alters vagal tone to the heart and lungs and helps to limit the duration of the response (Ulrich-Lai & Herman, 2009). In addition to the activation of the ANS for an immediate response, the neuroendocrine system is employed through the hypothalamic-pituitary-adrenal (HPA) axis. The brain stem and circumventricular areas also provide input into the PVN as well as the limbic forebrain structures mentioned previously. The PVN secretes releasing hormones such as corticotropin-releasing hormone (CRH), which acts on the anterior pituitary gland. It also synthesizes arginine vasopressin, which is released in the posterior pituitary. These hormones stimulate the release of adrenocorticotropic hormone (ACTH), which acts on the adrenal cortex to initiate the synthesis and release of glucocorticoids (e.g., cortisol) into the blood. Glucocorticoids quickly mobilize stored amino acids and fats so they are available for synthesizing other necessary molecules such as glucose, and in general protect against acute hypoglycemia. If these neuroendocrine responses are insufficient to maintain homeostasis, the body’s organ systems may incur pathologic changes. Research indicates that the brain’s continual effort of attempting to combat chronic stress can result in plastic changes in areas such as the amygdala, hippocampus, medial prefrontal cortex, and PVN. These include changes in dendritic morphology, changes in glucocorticoid receptor expression, increased corticotropin-releasing hormone expression and release, and increased stress excitability. Studies suggest that the excitability of the HPA axis and the sympathoadrenomedullary pathway seems to be enhanced by chronic stress (McEwen, 2007; Ulrich-Lai & Herman, 2009). In addition, it appears that the activation of neural pathways from the brain attributable to psychological stress and negative life experiences may cause degranulation of mast cells in the gut and exacerbate symptoms in patients with gut disorders such as IBS and colitis. Mast cell degranulation results in the activation of the same enteric defense program of the gut that normally is activated when there is a threatening circumstance within the intestinal lumen, such as the presence of dietary antigens, bacteria, toxins, parasites, and viruses. The defense program (i.e., coordinated mucosal secretion and power propulsion) is manifested clinically by the symptoms of abdominal pain, fecal urgency, and diarrhea, which are present in GI disorders such as IBS (Wood, 2007).
Visceral Afferents
Although the ANS is considered by some to consist primarily of an efferent limb, visceral afferent fibers have an important relationship with the efferent fibers. Visceral afferent fibers, which since 1894 have been known to accompany autonomic efferents (Cervero & Foreman, 1990), provide information about changes in the body’s internal environment. This input becomes integrated in the CNS and may participate in reflexes via autonomic and somatic efferents. These reflexes, such as the regulation of blood pressure and the chemical composition of the blood, aid the ANS in the control of homeostasis. However, visceral afferent fibers also mediate some conscious feelings, such as the visceral sensations of hunger, nausea, and distension. Although receptors of visceral afferent fibers do not respond to stimuli such as cutting or burning (as cutaneous receptors do), a pathologic condition or excessive distension produces visceral nociception. The continual barrage of impulses via visceral afferent fibers on the CNS is a probable cause of an individual’s feeling of well-being or of discomfort.
Visceral afferent fibers convey information from peripheral receptors called interoceptors. These endings, which may be encapsulated or free nerve endings, are found in the walls of the viscera, glands, adventitia of blood vessels, epithelium, mesentery, and serosae. Some are polymodal nociceptors, which are predominantly free nerve endings of unmyelinated fibers. They are located throughout the GI and respiratory tracts and respond to various stimuli such as noxious chemicals or damaging mechanical stimuli (Standring et al., 2008). Others are described as mechanoreceptors and include numerous pacinian corpuscles. These are located in the abdominal mesenteries. Additional mechanoreceptors are found in the serosal covering of the viscera and also in the blood vessels, and they may be stimulated by movement or distension. Still others, found in smooth muscle layers such as that of the bladder and gut, monitor both contraction and distension. In addition, chemoreceptors and baroreceptors are special interoceptors that are located specifically in the aortic arch and the bifurcation of the left and right common carotid arteries.
Receptors of somatic afferent fibers show a clear distinction between those detecting noxious stimuli and those that detect innocuous stimuli. This distinction is not as clear regarding visceral afferent receptors. For example, GI and pelvic spinal afferents, which are mechanosensitive, express receptors that function in visceral nociception. Also, unlike somatic receptors, those expressed on visceral sensory neurons may respond to more than one stimulus—for example, GI mucosal afferents respond to both light brushing and chemical irritants and muscle afferents in the wall of the gut respond to distension but may also fire in response to chemical stimuli (Christianson et al., 2009). The visceral afferent fibers (excluding the enteric nervous system afferent fibers that have already been discussed) travel with the autonomic efferents of both the sympathetic and parasympathetic divisions. (To be clear, the terms sympathetic and parasympathetic refer only to efferent fibers.) The vast majority are unmyelinated, with the exception of those from the pacinian corpuscles located in the mesentery (see previous discussion). There are numerous receptors expressed on visceral afferent fibers that are involved with nociception, including the adenosine and 5-HT3 receptors. Another is the P2X receptor to which ATP binds. ATP is a mediator released from epithelial cells in response to cell injury and plays a role in nociception and inflammation. The P2X receptors have been identified on vagal C afferent fibers innervating the lung and esophagus and spinal afferent fibers in the bladder and colon. The transient receptor potential (TRP) family of transmembrane cation-permeable channels has also been identified on visceral afferents. Members of this group of receptors have been implicated in being involved in visceral nociception and inflammation and may also play a role in the development of hypersensitivity (Christianson et al., 2009). Although many of these afferent fibers are purely sensory, some fibers also have an efferent function. These fibers, sometimes called sensory-motor nerves, have the ability to release neurotransmitters such as substance P, CGRP, and ATP from their peripheral endings during the axon reflex. The release of these chemicals results in neurogenic inflammation, which includes vasodilation, increased vascular permeability, altered smooth muscle contractility, and functional changes in cells typically involved with the inflammatory response, such as degranulation of mast cells. This event appears to be a widespread phenomenon that occurs in autonomic tissues within the cardiovascular, respiratory, and GI systems (Standring et al., 2008). The cell bodies of visceral afferent fibers that travel within the glossopharyngeal (CN IX) and vagus (CN X) nerves are located in the inferior (petrosal) ganglion of CN IX and the superior (jugular) (Christianson et al., 2009) and inferior (nodose) ganglia of CN X. The dorsal root ganglia of the second, third, and fourth sacral roots house visceral afferent cell bodies of fibers that travel with pelvic parasympathetic efferents in pelvic splanchnic nerves. These are referred to as pelvic afferent fibers. Cell bodies of afferent fibers associated with sympathetic fibers are located in the dorsal root ganglia of the thoracic and upper lumbar dorsal roots. These fibers course from the periphery along with sympathetic efferent fibers in the splanchnic nerves, pass through the prevertebral ganglia without synapsing, and enter the sympathetic trunk (Fig. 10-25). Then they pass through the white rami communicantes into the dorsal root and terminate in the cord segment from which the accompanying preganglionic efferent fibers originate. These afferent fibers are referred to as spinal afferent fibers. Some general characteristics of somatic afferent fibers apply to visceral afferent fibers. Both course with efferent fibers, the cell bodies are located in sensory ganglia, and there is only one sensory fiber that extends from the receptor into the CNS. One unique feature relative to the sensory innervation of the viscera is that for each organ the origin of the sensory input is from two well-defined sources and each has a different role in sensory processing. The thoracic and abdominal viscera are supplied both by fibers of cell bodies located in the dorsal root ganglia (spinal afferents), which appear to be involved with acute nociceptive function, and by fibers of cell bodies located in the ganglia of the vagus (predominantly) and glossopharyngeal nerves. The fibers within these cranial nerves function more often in the realm of normal physiologic states and in the related sensations of well-being. The pelvic viscera are supplied by fibers of cell bodies located in the dorsal root ganglia of sacral cord segments (pelvic afferents) and thoracolumbar cord segments. These appear to function in sensitizing processes such as the postinflammatory state but not in the initial process of nociception (Christianson et al., 2009).
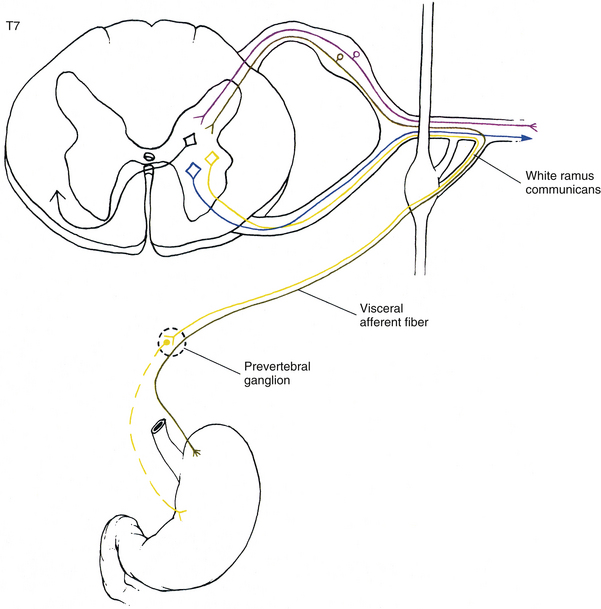
FIG. 10-25 The pathway of visceral afferent information into the spinal cord is shown using the stomach as an example. (Afferent fibers that travel in the vagus nerve and convey visceral information are not shown.) Note how the afferent fiber (green) travels with the sympathetic efferent fiber (yellow) but does not synapse in the prevertebral ganglion. Instead the afferent fiber synapses in the dorsal horn and has the capability of influencing numerous neurons, including preganglionic efferents, somatic efferents (blue), or tract neurons (black). In the case of visceral pain, viscerosomatic neurons also are receiving input from cutaneous sources (purple), thus providing a mechanism for pain referral (see text for discussion).
Electron microscopic and retrograde tracing methods show that a low density of fibers innervates the viscera in comparison to the innervation of the skin. Feline studies demonstrate that approximately 16,000 spinal afferent fibers exist, and 6000 to 7000 of these are found in the greater splanchnic nerves (Cervero & Foreman, 1990). However, the total number of afferent fibers represents less than 20% of all fibers in these nerves and approximately 2% of the fibers located in dorsal roots of thoracic and lumber spinal nerves. An obvious disparity is noticed when the vagal and pelvic afferent fibers are enumerated. Feline visceral afferent fibers in the vagus nerve number approximately 40,000 and pelvic afferent fibers approximately 7500. Of the total number of fibers in the vagus, 80% are afferent. In the pelvic nerves 50% of the fibers are afferent (Cervero & Foreman, 1990). In general, concerning the two groups of mixed nerves (i.e., the vagus and splanchnic nerves), the vagus nerve consists predominantly of afferent fibers and the splanchnic nerves consist predominantly of efferent fibers. The specific functional differences between these two major groups of afferent fibers are not clear. The vagal, primarily, and pelvic afferent fibers generally are thought to transmit input concerning physiologic activity of the viscera, such as GI motility and secretion. By monitoring the activity, these afferent fibers are able to mediate reflexes necessary for the proper regulation of visceral function. The spinal afferent fibers appear to be concerned with sensations arising from the viscera, especially the sensation of pain (Cervero & Foreman, 1990). However, Kollarik and colleagues (2010) have introduced the concept that there is a subset of vagal afferent fibers referred to as “putative vagal nociceptors” that convey nociception. These have characteristics similar to nociceptors in somatic tissues. Although it is possible that these vagal afferent fibers are yet to be identified in other viscera, two regions that do show evidence of these are the lungs and the esophagus. In the lungs, putative vagal nociceptors are bronchopulmonary C fibers. These comprise the majority (75%) of the lung afferent fibers and innervate all components of the lungs. They are inactive in healthy lungs and are activated by exogenous noxious factors such as cigarette smoke, environmental pollutants, ozone, and particulate matter, and by endogenous inflammatory mediators. When activated, reflexes such as coughing, tachypnea, and mucus secretion are induced in order to remove or limit the effects of the noxious stimulus. Both peripheral (induced by inflammatory mediators such as prostaglandin E2) and central sensitization have been described. Although tension mechanosensors comprise a large number of receptors in the esophagus, there exists another population of distension-sensitive afferent fibers that can be characterized as putative vagal nociceptor fibers. These fibers have a different response pattern to distension and express membrane receptors that respond directly to noxious chemicals, are peripherally sensitized by inflammatory mediators, and are likely to be sensitized at the central level. The vast majority have a conduction rate that corresponds to C fiber conduction speeds.
Vagus, Glossopharyngeal, and Pelvic Afferent Fibers
Afferent fibers coursing in the vagus nerve relay input from many sources (Standring et al., 2008). A partial list includes the following:
• Mucosa and smooth muscle of the bronchial tree of the lung
• Connective tissue adjacent to the alveoli of the lung
The glossopharyngeal nerve conveys visceral afferent information from the posterior region of the tongue, upper pharynx, tonsils, and the carotid sinus and carotid body.
The cell bodies of afferent fibers for both the vagus and glossopharyngeal nerves are located in their respective sensory ganglia. These ganglia are located adjacent to the jugular foramen of the skull. The afferent fibers of both CNs IX and X synapse in the nucleus of the tractus solitarius, which is located in the medulla oblongata of the brain stem. From here, axons project to numerous areas in the central autonomic network of the brain stem and diencephalon (see Control of Autonomic Efferents: Central Autonomic Network and Figs. 10-33 and 10-34) and the cerebral cortex. The projections to the cerebral cortex travel by way of the thalamus. These cerebral projections allow for conscious awareness of sensations, such as hunger. When appropriate, reflexes also may be elicited. Examples include the swallowing, cough, cardiovascular, and respiratory reflexes.
Visceral afferent fibers from the pelvic viscera and distal colon enter the spinal cord via pelvic splanchnics. Based on their structure and function at the peripheral level, the endings of these fibers act primarily as mechanosensors monitoring stretch in the hollow viscera. The extent of their participation in mediating nociception originating in the bladder and colon is unclear (Carpenter & Sutin, 1983; Cervero & Foreman, 1990; Knowles & Aziz, 2009).