Pain of Spinal Origin
Anterior Primary Divisions (Ventral Rami)
Posterior Primary Divisions (Dorsal Rami)
Nerves Associated with the Sympathetic Nervous System
Pain Generators Unique to the Cervical Region
Central Transmission of Nociception
Important Terms and General Concepts Related to Pain
Differentiation Between Pain of Somatic Origin and Radicular Pain
The purpose of this chapter is to apply much of the information from previous chapters to the clinical setting. This is accomplished by discussing the case of a typical patient with low back pain. In addition, structural features of other regions of the spine particularly susceptible to injury or pathologic conditions also are mentioned briefly. The aspects of pain discussed in this chapter are meant to include the most common causes of discomfort. Exhaustive lists are beyond its scope. Fortunately, this subject has been reported thoroughly elsewhere (Kirkaldy-Willis, 1988a; Haldeman, 2005; Cavanaugh, 1995; Greenspan, 1995; Bogduk, 1992; 2005 Siddal & Cousins, 1997). This chapter discusses a challenging problem that faces clinicians continuously: their patients’ pain.∗
Patient Background
On a bright Monday afternoon in January, Mr. S., a 40-year-old man, enters the waiting room. The receptionist recognizes Mr. S. as a previous patient. As she hands him a form inquiring about the details of his chief complaint, she notices his extremely slow gait and guarded stance. The receptionist retrieves his previous records while Mr. S. diligently fills out the form. At the earliest opportunity the receptionist alerts the clinician to the electronic file previously compiled on Mr. S. and mentions that he appears to have “a nasty case of low back pain.” As the practitioner (you) reviews the records, he or she immediately recalls Mr. S. as a patient from 3 years earlier. He had strained the muscles of his lower back while unloading bags of dry cement mix that he was planning to use for a home improvement project. The clinician suspects his complaint today may be related to this prior incident, and begins to analyze the perception of pain.†
Perception of Pain
The general approach to patients complaining of pain is that it is real. It has both physical and psychological components, one of which may predominate, and the pain always alters the personality of the individual (Kirkaldy-Willis, 1988b; Burton et al., 1995; Gillette, 1996; Kummel, 1996; Hildebrandt et al., 1997). This alteration of personality usually returns to the prepain state when the physical cause of the discomfort has sufficiently healed. In addition, pain always has a subjective component and is perceived by patients in relation to previous experiences with pain, usually from their early years (Weinstein, 1988).
Pain has been defined by the International Association for the Study of Pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage” (Loeser et al., 2011). This group’s committee on taxonomy goes on to state, “If a patient regards their experience as pain and if they report it in the same ways as pain caused by tissue damage, it should be accepted as pain” (Loeser et al., 2011). Therefore not all pain is the result of a nociceptive stimulus received and transmitted by a sensory receptor of a peripheral nerve (Weinstein, 1988).
Many other factors may influence the patient’s perception of pain, including the following: the individual’s general health, the nervous system’s overall status, the pain’s chronicity, and even the environment in which the patient lives (Colloca et al., 2005). In addition, a person’s work environment, work activities, and work satisfaction have all been found to affect the occurrence and outcome of back pain (Macfarlane et al., 1997; Pappageotgiou et al., 1997). Finally, the dorsal root ganglia, spinal cord, and higher centers are all capable of adjusting and regulating (modulating) painful stimuli. Therefore to continue with the example of Mr. S., the clinician may not fully appreciate and understand the severity of Mr. S.’s pain until he or she has had an opportunity to observe him on several different occasions (Kirkaldy-Willis, 1988b).
The characteristics and quality of pain, such as that experienced by Mr. S., can be important. For example, diffuse burning pain, which may or may not radiate into the lower extremity, can be of sympathetic origin. The afferent fibers of the recurrent meningeal nerve travel with sympathetic fibers. The peripheral (sensory) receptors of the nerves may be stimulated by arachnoiditis and postoperative fibrosis and could be a source of diffuse burning pain of sympathetic origin (Kirkaldy-Willis, 1988b). However, aching usually is the result of muscle tightness or soreness and frequently is relieved by stretching and short periods of rest. Other generalized lower extremity pain, excluding aching pain, often is associated with a vascular or neurogenic cause (Weinstein, 1988).
Pain of Somatic Origin
The clinician now begins to consider the possible causes of Mr. S.’s current discomfort. Low back pain (LBP) can be defined as pain in the lumbar or sacral region that is “not more than a handbreadth” away from either side of the patient’s vertebral column (Bogduk, 1992). Even though LBP is one of the most common complaints seen by physicians, it is one of the most difficult to understand (Weinstein, 1988). Recall that an anatomic structure must be supplied by nociceptive nerve endings (nerve endings sensitive to tissue damage; see Chapter 9) to be a cause of LBP, and Mr. S.’s perception of his LBP greatly depends on the factors described previously. Noncutaneous nociceptors are found in muscles, tendons, joint capsules, periosteum, perivenous tissues (vasa nervorum), and several visceral tissues (Greenspan, 1997). Therefore these are all potential sources of Mr. S.’s discomfort. Also recall that nociceptors may be stimulated by mechanical, thermal, or chemical means. Because the structures that receive nociceptive innervation are able to “generate pain,” they are sometimes called pain generators. (Notice that the presence of the nociceptive endings in the damaged tissue is what actually allows the structure to function as a pain generator.) The nociceptive nerve endings respond to tissue-damaging or potentially tissue-damaging stimuli; that is, nociceptive nerve endings can fire before tissue damage actually occurs, thereby possibly preventing injury (Greenspan, 1997).
Once a nociceptor has depolarized, intrinsic and extrinsic physiologic changes can occur in its membrane properties that frequently allow it to become more sensitive to subsequent noxious stimuli. This increased sensitivity is known as primary hyperalgesia. The central nervous system (CNS) has several mechanisms by which it, too, may create hyperalgesia (secondary hyperalgesia) from an area of injury (see Chapter 9). Therefore after tissue is damaged, it is usually more sensitive to further nociception until healing has occurred. After pathologic conditions or injury, hyperalgesia also may be present in the healthy tissues surrounding the site of the lesion.
Frequently nociception of spinal origin is the result of damage to several structures, and the effects of hyperalgesia allow for nociception to be felt from tissues that, if injured to the same degree independently, might have gone unnoticed (Haldeman, 1992). (Hyperalgesia and other related concepts are discussed in further detail in the section Important Terms and General Concepts Related to Pain and in Appendix II.)
Most pain has a physical cause, even though not all the structures supplied by nociceptors, and therefore capable of producing pain, are known (Haldeman, 1992). Those tissues that are supplied by nociceptive nerve endings usually can undergo a number of different pathologic processes that can lead to direct stimulation or sensitization of nociceptors (Haldeman, 1992).
One of the best ways to organize Mr. S.’s possible pain generators is to list them according to the four main sources of neural innervation to spinal structures: the anterior primary division (APD, ventral ramus), posterior primary division (PPD, dorsal ramus), recurrent meningeal nerve, and sensory fibers that course with the sympathetic nervous system (Fig. 11-1). All these afferent nerves have their cell bodies in the dorsal root ganglia (DRGs), which, with the exception of C1 and C2 (see Chapter 5), are located within the intervertebral foramina (IVFs) of the vertebral column. Note that the sensory fibers that are associated with the recurrent meningeal nerve and sympathetic nervous system provide a route for the transmission of nociception from somatic structures of the vertebral column’s anterior aspect. Fibers arising from these sources pass through the APD for a short distance before reaching the spinal nerve. They then enter the dorsal root. Even though these nerves briefly pass through the ventral ramus, they are best considered separately because they are important to nociception of spinal origin.
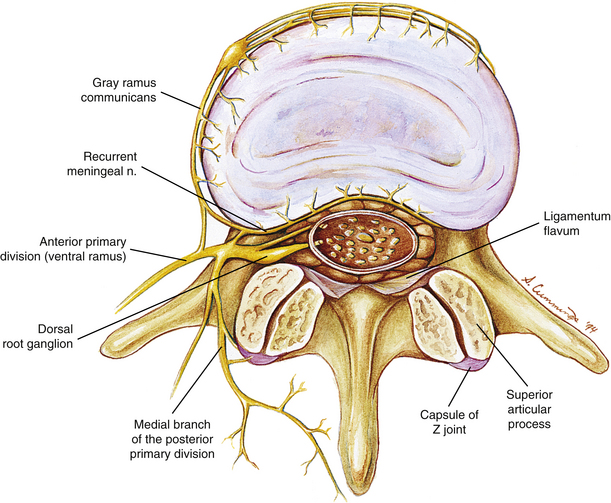
FIG. 11-1 Horizontal view of a lumbar vertebra, the intervertebral foramina, the vertebral foramen, and the nerves associated with this region. Notice the innervation to the zygapophysial joint by the medial branch of the posterior primary division. Also notice the recurrent meningeal nerve innervating the posterior aspect of the intervertebral disc. The recurrent meningeal nerve also innervates the posterior longitudinal ligament and the anterior aspect of the spinal dura mater.
Anterior Primary Divisions (Ventral Rami)
To approach the cause of the discomfort experienced by Mr. S., let’s first consider those structures innervated by the lumbar APDs (Box 11-1). The APDs of the lumbar region innervate much of the gluteal and inguinal regions, as well as the entire lower extremity. Although these regions may refer to the low back, they usually are accompanied by more localized pain from the structure that is either injured or affected by some form of pathologic process. More likely causes of back pain originating from structures innervated by APDs (ventral rami) are several muscles, including the psoas major, quadratus lumborum, and lateral intertransversarii. Strain or possibly increased tightness (what some would call “spasm”) of these muscles can be a source of back pain. Abscess within the psoas muscle also is a possible source of pain. The transverse processes (TPs) are innervated by the APD too, and a fracture of a TP or bruise to its periosteum may result in pain (Bogduk, 1983).
Posterior Primary Divisions (Dorsal Rami)
Discomfort such as that experienced by Mr. S. also may arise from structures innervated by the PPDs (dorsal rami) listed in Box 11-2. This list contains some of the most frequent causes of LBP, including the deep back muscles, which receive nociceptive innervation by means of nerves accompanying the vessels that supply these muscles; spinal ligaments (nociceptors are most numerous in the posterior longitudinal ligament, innervated by the recurrent meningeal nerve, and fewest in the interspinous ligament and ligamentum flavum innervated by the PPDs; see Box 11-2 for other ligaments innervated by the PPDs); the zygapophysial joints (Z joints) and superficial and deep fascia. These are all high on the list of possible causes of pain similar to that experienced by Mr. S. (Cavanaugh, 1995). Each of these groups of structures may be affected by a number of pathologic conditions or injuries. The muscles may be strained or affected by areas of myofascial tenderness (“trigger points”) (Bogduk, 1983; Hubbard & Berkoff, 1993). The ligaments may be sprained. Pain from the Z joints may be difficult to localize because each Z joint receives innervation from the PPD of the same level and also from the PPD of the level above (Bogduk, 1976) and below (Jeffries, 1988). The Z joints may be fractured (fracture of an articular process) or inflamed as a result of arthritic changes. Discomfort also can arise from a Z joint articular capsule or synovial fold (Giles, 1987; Schwarzer et al., 1994) that has become entrapped within the Z joint or pinched between the articular surfaces (see Chapter 7). Degeneration of articular cartilage may produce inflammatory agents that may stimulate nociceptors of the Z joint articular capsules. Inactivity of the spinal joints, even if this inactivity is imposed on these joints by muscle guarding, may promote degeneration (Cramer et al., 2004) (Fig. 11-2) and pain (Kirkaldy-Willis, 1988b). In addition, the spinous processes may be fractured or may repeatedly collide with one another (Baastrup’s syndrome). Finally, Sihvonen and colleagues (1995) found that the medial branch of the posterior primary division can become irritated or entrapped along its course, causing weakness of transversospinalis muscles (i.e., semispinalis, multifidus, and rotatores muscles) and low back pain. The LBP in this instance presumably results both from irritation of the medial branch of the posterior primary division and from abnormal stresses and loads being placed on pain generators of the spine as a result of the biomechanical changes caused by the muscle weakness.
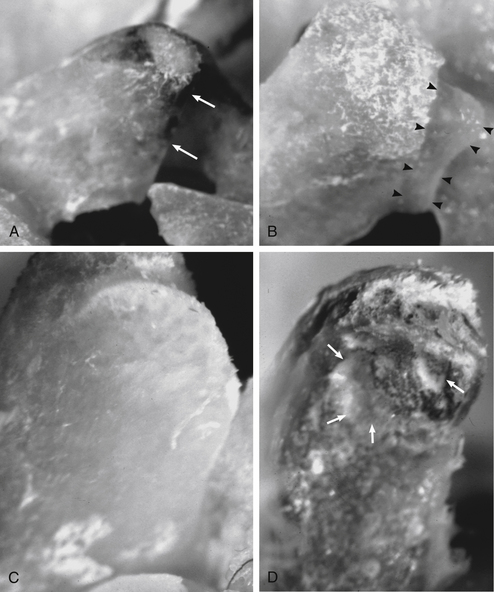
FIG. 11-2 Zygapophysial joint osteophyte formation and facet surface degeneration following induced hypomobility in an animal model (rat). The external surfaces of two L5 superior articular processes are shown in A (8-week control animal) and B (8-week fixation/hypomobility animal). A, Notice that the cephalad edge of the articular process is smooth in the control animal (small white arrows) with no signs of osteophytes, whereas in B a prominent osteophyte (arrowheads) is seen on the cephalad portion of the articular process of the 8-week fixation/hypomobility animal. The internal surfaces of two L5 superior articular facets are shown in C (1-week control animal) and D (4-week fixation/hypomobility animal). C, Notice that the articular facet of the control animal is smooth, whereas in D that of the 4-week fixation/hypomobility animal has marked roughening, pitting, and remodeling. The deep pitting is identified with white arrows. D, The remodeling is so marked that the ventral portion of the articular process (bottom) is out of the plane of maximum focus. (From Cramer GC et al. [2004]. Degenerative changes following spinal fixation in a small animal model. J Manipulative Physiol Ther, 27, 141-154.)
Recurrent Meningeal Nerve
Structures innervated by the recurrent meningeal (sinuvertebral) nerve also may be a source of back pain (Raoul et al., 2002). The list in Box 11-3 identifies the structures supplied by these nerves. The periosteum of a vertebral body may be affected by fracture or neoplasm within the vertebral body. The basivertebral veins may be affected by intraosseous hypertension, crush fractures, or neoplasms of the vertebral body (Bogduk, 1983). The epidural veins may be affected by venous engorgement. The posterior aspect of the intervertebral disc (IVD) is also a pain generator (Schwarzer et al., 1994; Cavanaugh, 1995) that can be affected by internal disc disruption, protrusion of the nucleus pulposus through the outer layers of the anulus fibrosus, or tearing (sprain) of the outer layers of the anulus fibrosus (AF). Of related importance is that sensory innervation of degenerated IVDs extends into the deeper layers of the anulus fibrosus. That is, the process of IVD degeneration seems to stimulate the nerves innervating the posterior, lateral, and anterior aspects of the IVD to grow deeper into the IVD, probably making them more capable of providing nociceptive sensation (Yoshizawa, O’Brien, & Thomas-Smith, 1980). Also innervated by the recurrent meningeal nerves is the posterior longitudinal ligament, which can be torn (sprained) during severe hyperflexion injuries or may be pierced by an IVD protrusion. In addition, the anterior aspect of the dura mater may be compressed by an IVD protrusion or irritated by the release of chemical mediators associated with internal disc disruption (see Chapter 7).
Nerves Associated with the Sympathetic Nervous System
Finally, recall that several structures are innervated by nerves that arise directly from the sympathetic trunk and gray communicating rami (Box 11-4). The sensory fibers of these nerves follow the gray rami to the APD, where they enter the spinal nerve. They then reach the spinal cord by coursing through the dorsal roots. Pathologic conditions of the periosteum of the anterior and lateral aspects of the vertebral body, which are innervated by sensory fibers traveling with gray rami, may result in pain. Some of the most common causes of this type of pathologic condition include fracture, neoplasm, and osteomyelitis (Bogduk, 1983). Sprain of the anterior longitudinal ligament or outer layers of the anterior or lateral part of the anulus fibrosis also may result in nociception conducted by fibers that course with the gray communicating rami.
Under certain circumstances, the sympathetic nervous system can play a complex role with regard to pain (Jorgensen & Fossgreen, 1990; Budgell, Hotta, & Sato, 1995; Siddall & Cousins, 1997). Changes in vasculature, changes in perspiration (sudomotor), and changes in nail, hair, and bone structure can accompany chronic pain associated with the sympathetic nervous system (Siddall & Cousins, 1997). This pain often is burning in nature and is associated with increased stimulation of sensory receptors. This is accompanied by an increased perception of pain in areas not associated with tissue damage (hyperalgesia), and also by an increased sensitivity to mechanical stimulation, including mechanical stimulation that previously would not be considered capable of producing pain (allodynia). The mechanism may be related to the finding that nociception from damaged or regrowing nerves and even nearby axons sometimes can be activated by norepinephrine from sympathetic stimulation. This activation is thought to result from alpha-adrenergic (norepinephrine) receptors developing on C (nociceptive) fibers after partial nerve injury (Greenspan, 1997). Once known as reflex sympathetic dystrophy, the term complex regional pain syndrome is now preferred (Plancarte & Calvillo, 1997). The pain of some patients with complex regional pain syndrome is related to the sympathetic nervous system (sympathetically maintained pain), although others’ pain is not (sympathetically independent pain) (Greenspan, 1997). (See Sympathetically Maintained Pain in Appendix II and Complex Regional Pain Syndrome in Chapter 10).
Pain Generators Unique to the Cervical Region
If Mr. S. is also presenting with pain in the cervical region, other structures should be included on the list of possible pain generators. These include irritation of the nerves surrounding the vertebral artery and nociception arising from uncovertebral “joints.”
Nociception arising from almost any structure innervated by the upper four cervical nerves may refer to the head, resulting in head pains and headaches (Campbell & Parsons, 1944; Edmeads, 1978; Bogduk, Lambert, & Duckworth, 1981; Bogduk, 1984; Aprill, Dwyer, & Bogduk, 1990; Dwyer, Aprill, & Bogduk, 1990; Darby & Cramer, 1994; Cramer, 1998). Pain originating from the region of the basiocciput and occipital condyles frequently refers to the orbital and frontal regions (Campbell & Parsons, 1944). A discussion of neck pain and its relationship to headache and head pain has been fully covered elsewhere (Vernon, 2001), and is beyond the scope of this chapter.
Autonomic reactions such as sweating, pallor, nausea, alterations of pulse rate, and other autonomic disturbances frequently have been observed in association with disturbances of the suboccipital and upper cervical spine. The intensity of these autonomic reactions seems to be proportional to the stimulus and proximity of the stimulus to the suboccipital region. The autonomic response ranges from mild subjective discomforts to measurable objective signs (Campbell & Parsons, 1944).
Pain Generators Unique to the Thoracic Region
If Mr. S. should present with discomfort of the thoracic region, the costocorporeal and costotransverse articulations, as well as fracture of the proximal aspect of a rib, should be added to the list of possible pain generators (see Chapter 6). Also a compression fracture of one or more of the thoracic vertebral bodies could be a realistic source of acute pain arising from the thoracic region.
Dorsal Root Ganglia
The neurons located in the dorsal root ganglia (DRGs) serve as modulators of spinal nociception. They contain many neuropeptides (see Chapter 9) associated with the transmission of nociception (e.g., substance P, calcitonin gene–related peptide [CGRP], vasoactive intestinal peptide) (Weinstein, Claverie, & Gibson, 1988). These neuropeptides and other neuromodulators are secreted from the peripheral terminals of sensory nerves that transmit nociception. These substances are manufactured in the cell bodies of the DRG and reach the peripheral terminals (sensory endings) and the synaptic boutons found in the dorsal horn of the spinal cord by axonal transport mechanisms. The presence of neuropeptides and neuromodulators around the receptors may sensitize the receptors, making them more susceptible to depolarization (Weinstein, Claverie, & Gibson, 1988). (See the sections Important Terms and General Concepts Related to Pain, and Modulation of Nociception for more information on the modulation of nociception at the level of the peripheral nerve endings.)
Somatic Referred Pain
Nociception arising from any of the somatic structures previously listed may be perceived by Mr. S. or a similar patient as being a considerable distance from the pain generator, even in an area innervated by different nerves than those innervating the pain generator. This is known as pain referral. The term somatic referred pain has been used to describe this type of back pain (Bogduk, 1992, 1997).
Several possible mechanisms of pain referral exist. Perhaps one of the most important mechanisms is the result of the internal organization of the spinal cord. The nociceptive information being transmitted by a pain generator is dispersed by either ascending or descending fibers that comprise the dorsolateral tract (zone) of Lissauer (see Chapter 9). These fibers may ascend or descend several cord segments before synapsing. Thus nociceptive information, entering from several different spinal cord segments, converges on the same interneuronal pool; therefore this interneuronal pool receives primary sensory information from different somatic regions (Fig. 11-3). More specifically, the dorsal horn neurons in the extreme lateral aspect of the dorsal horn have been found to receive input from a wide variety of superficial and deep tissues. In fact, spinal tissues have been found to produce more convergence in the spinal cord than other tissues, a phenomenon termed hyperconvergence (Gillette, Kramis, & Roberts, 1993). Gillette and colleagues (1993) found that neurons within the skin, Z joint, spinal ligaments, and paraspinal muscles all caused firing of the same dorsal horn neurons. This dispersal of incoming afferents onto different-tract neurons, in combination with the convergence of several different afferents onto single tract neurons, most likely decreases the ability of the CNS to localize nociception (Song & Ruppert, 2005; Darby & Cramer, 1994). In addition, excitatory and inhibitory interneurons found in laminae I and II in the spinal cord can be activated by non-nociceptive afferents, as well as by descending pathways, and also can modulate the output of second-order neurons, thus further altering the ability of the CNS to localize nociception. This type of dispersal and convergence also may be found at the second synapse along the nociceptive pathway. That synapse occurs in the ventral posterior lateral nucleus of the thalamus (see later discussion on pain pathway).
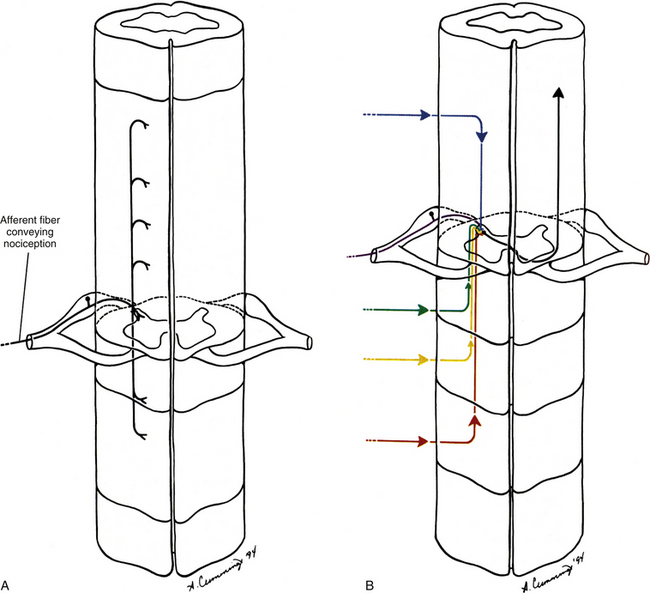
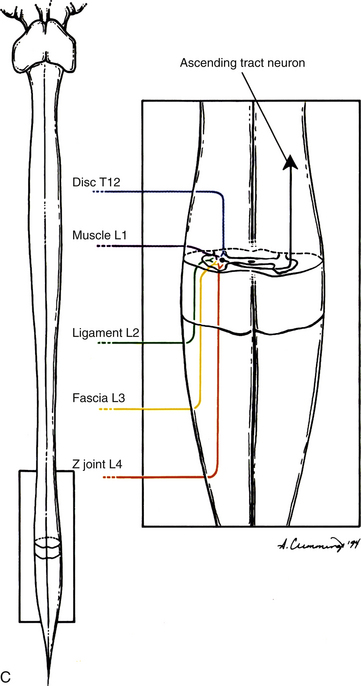
FIG. 11-3 A, Dispersion of afferents conducting nociception as they enter the spinal cord. B, Convergence of afferents conducting nociception onto a tract neuron. C, Nociception from a variety of sources may influence the same pool of tract neurons.
The ventral posterior lateral thalamic nucleus projects to the postcentral gyrus of the cerebral cortex. The region of the back is represented on a small area of the postcentral gyrus (sensory homunculus) of the cerebral cortex (see Fig. 9-15). The small size of the sensory cortex devoted to the back also may contribute to the poor localization of nociception of spinal origin (Song & Ruppert, 2005). In addition, the tract neurons for ascending pain pathways most frequently carry nociceptive information from cutaneous areas. Therefore when the tract neurons are stimulated to fire, the cerebral cortex (where conscious awareness of nociception occurs) may interpret the impulse as originating from a cutaneous or other recently injured region. Either of these regions may be distant to the structure that is currently damaged or inflamed. This phenomenon is called pain memory (Carpenter & Sutin, 1983; Wyke, 1987; Nolte, 1988).
The existence of pain referral between somatic structures has been documented for some time (Kellgren, 1938; Inman & Saunders, 1944; Hockaday & Whitty, 1967; McCall, Park, & O’Brien, 1979). The term somatic referred pain is used currently when discussing pain of somatic origin that is felt distant to the structure generating the nociception (Box 11-5). This type of pain is characterized as dull and aching, difficult to localize, and fairly constant in nature (Bogduk, 2005). For future reference, these characteristics of somatic referred pain are highlighted in Box 11-6.
Increased tenderness to deep palpation of the back muscles and hyperalgesia of innervated tissues may occur in areas of referred pain (Weinstein, 1988). An example of somatic referred pain is the pain arising from an inflamed lumbar Z joint, which may refer to the groin, buttock, greater trochanter of the femur, and posterior aspect of the thigh, extending to the knee and occasionally extending inferiorly to the leg’s posterior and lateral calf (Weinstein, 1988; Yukawa et al., 1997).
Takebayashi and colleagues (2001) identified an additional explanation for pain referring to regions innervated by nerves originating at higher spinal levels than the nerves that typically supply a particular pain generator. These investigators found that, in rats, some of the nerve fibers innervating spinal tissues originate from dorsal root ganglia several segments above those at the same segmental levels as the damaged tissues. In other words, some of the nerves innervating Mr. S.’s L4-5 IVD may originate from his L1 or L2 DRG and refer pain into his inguinal region (innervated by L1 and L2). In rats the fibers that eventually reach the higher dorsal root ganglia course from the pain generator of origin to gray rami communicantes at the level of the pain generator, ascend in the sympathetic chain, and then course through another gray communicating ramus that connects to an anterior primary division at the higher segmental level. From here the fibers pass through the APD, spinal nerve, DRG, and dorsal root at the higher level before synapsing in the more superior spinal cord segment. This work has been supported by the animal research (also rats) of others (Ohtori et al., 1999, 2001). Therefore the anulus fibrosus of the IVD is supplied by nerves originating from several segments and from both the left and right sides (Nakamura et al., 1996). Although more work is needed to corroborate these animal studies in humans, the results are consistent with the findings of clinical research showing that approximately 4.1% of patients with protrusion of the IVD at L4-5 or L5-S1 experience groin pain (Yukawa et al., 1997).
The threshold of nociceptors typically is lower than that of pain perception. This may result from the polysynaptic nature of the connections associated with most nociceptors. Many A-delta and most C fibers synapse on one or more interneurons before reaching tract neurons of the ascending pathway that will transmit their information to the cortex, and each of these interneuronal connections is a site for possible modulation (inhibition). Another possible explanation of the difference in thresholds may result from central modulation of nociception via stimulation of non-nociceptive afferents (Greenspan, 1997). Consequently, afferent input from activity of the muscles and the Z joints, as well as spinal manipulation of the Z joints, tends to decrease pain via a “gate control” type of mechanism (Melzack & Wall, 1965; Kirkaldy-Willis, 1988b) or via neuroplastic changes that alter the nociceptive circuits (Ianuzzi & Khalsa, 2005). The work of Indahl and colleagues (1997) illustrates this point. In an elegant experiment using 29 adolescent pigs, these investigators stimulated the L3-4 IVD while recording muscle activity (by means of needle electromyography) from the multifidus and longissimus muscles. The stimulation of the IVD created an increased number of action potentials recorded from the spinal muscles. The researchers then injected physiologic saline into the Z joint innervated by the same segmental level. The physiologic saline stretched the Z joint capsule. This resulted in significant decreased muscle activity. The authors concluded that stretching the Z joint capsule decreased multifidus muscle tightness (spasm) that was caused by pain arising from the IVDs (Indahl et al., 1997). Therefore if the pain was of somatic origin, Mr. S. might benefit most from treatment designed to promote activity and movement (Kirkaldy-Willis, 1988b). (See the section Modulation in the Spinal Cord for a more detailed explanation of the gate control theory.) Of related interest is that patients with LBP have been found to have decreased proprioception, as measured by difficulty with standing and then four-point kneeling 10 times in 30 seconds (Gill & Callaghan, 1998). Consequently, increased activity, joint movement, and rehabilitative and proprioceptive training exercises also may help to improve Mr. S.’s joint position sense. However, recall that under certain circumstances mechanoreceptors can be sensitized to cause pain once tissue damage is well established, probably by means of central sensitization at the dorsal horn of the spinal cord (mechanical hyperalgesia) (Greenspan, 1997). Therefore precautions also should be taken to avoid further compromising any damaged tissue.
All the discussed information was quickly recalled even before the clinician entered the examination room to see Mr. S. Just as the clinician steps into the room, he or she quickly remembers the pathways for the transmission of pain.
Central Transmission of Nociception
Pain is the perception that results from the cerebral cortex’s interpretation of nociceptive input by a variety of CNS structures (Basbaum & Jessell, 2000). Some of the CNS structures that have been implicated in contributing to this process include the dorsal horn of the spinal cord; excitatory and inhibitory interneurons in the spinal cord; ascending pathways; reticular formation of the brain stem; thalamus; and several areas of the cerebral cortex, including the primary sensory cortex, cingulate gyrus, and insular cortex (Craig & Bushnell, 1994; Craig et al., 1996). The interconnections of these areas and subsequent integration of the information result in the components associated with the sensation of pain. These components include discriminatory qualities, emotions, attentiveness to the painful area, and reflex responses involving both the autonomic and endocrine systems (Haldeman, 1992).
The afferent fibers that convey nociception are group A-delta and C fibers. These fibers enter the dorsolateral tract (zone) of Lissauer, located at the tip of the cord’s dorsal horn. Some fibers continue directly into the gray matter of the dorsal horn, whereas their collateral branches ascend or descend numerous cord segments before entering the dorsal horn (see Fig. 11-3, A). The A-delta fibers convey nociception quickly and rapidly and terminate in lamina I and laminae V to VII. The group C fibers convey what is interpreted as a dull sensation of pain at a slow rate and terminate directly in lamina II and may indirectly (via interneurons) terminate in laminae V and VII. In addition, many of the neurons synapsing in lamina VII originate from both sides of the body, and may further contribute to the diffuse nature of many pain conditions (Basbaum & Jessell, 2000). The neurons that transmit the information to higher centers are located in various laminae of the gray matter (see Chapter 9). Surgical cordotomy procedures that relieve pain have shown that the most important fibers transmitting nociception to higher centers decussate in the ventral white commissure and then ascend in the anterolateral quadrant of the cord’s white matter (Hoffert, 1989). Alternative pathways also may be involved, although their course and function in humans remain unclear (Besson, 1988; Hoffert, 1989) (see Chapter 9).
Spinothalamic Tract
The response of the brain to painful stimuli is intricate and complex, and there are several pathways associated with Mr. S.’s LBP. Nociceptive information is conveyed to higher centers by tracts in the anterolateral quadrant of the spinal cord. Two major tracts conduct this information in the cord’s anterolateral quadrant—the spinothalamic tract (also called the neospinothalamic tract) and the spinoreticular tract. The spinothalamic tract conveys nociceptive information, which is perceived as sharp, pinprick-like pain, via fast conducting A-delta fibers. The integrity of this tract commonly is tested during a neurologic examination by asking the patient to differentiate the sensations of sharp versus dull. The cell bodies of the tract neurons are both nociceptive-specific and wide–dynamic-range neurons (sensitive to many types of stimuli) that are located in the dorsal horn primarily in laminae I and V to VII (Basbaum & Jessell, 2000). These axons decussate in the ventral white commissure within one or two segments of entry and ascend in the anterolateral white matter of the cord and through the brain stem. They synapse in the ventral posterior lateral nucleus and posterior nucleus, which comprise the lateral nuclear group of the thalamus (Fig. 11-4, A). From the thalamus, axons of the next order neurons course to the somesthetic region of the cortex, which is the postcentral gyrus and the posterior part of the paracentral lobule of the parietal lobe. As the axons of the spinothalamic tract ascend, body parts are represented in specific regions of the tract. This specific pattern is retained in the cerebral cortex, such that a specific area of cortex corresponds to the region of the body from which the sensory fibers originated. This cortical representation is called the sensory homunculus. The size of the body part represented on the homunculus reflects the amount of sensory innervation devoted to that body area. As mentioned, this unequal neuronal representation may be one reason that localization of sensations, such as pain, is more difficult in one region (e.g., back) than another (e.g., fingertips or lips). The information conveyed in the spinothalamic tract is processed in the region of the sensory homunculus. Here the nociception is perceived as pain that is acute, localized, and discriminatory.
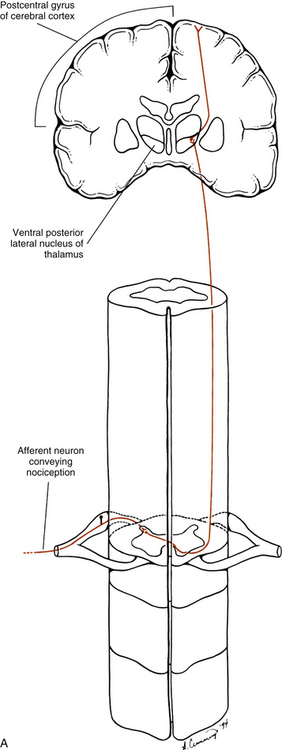
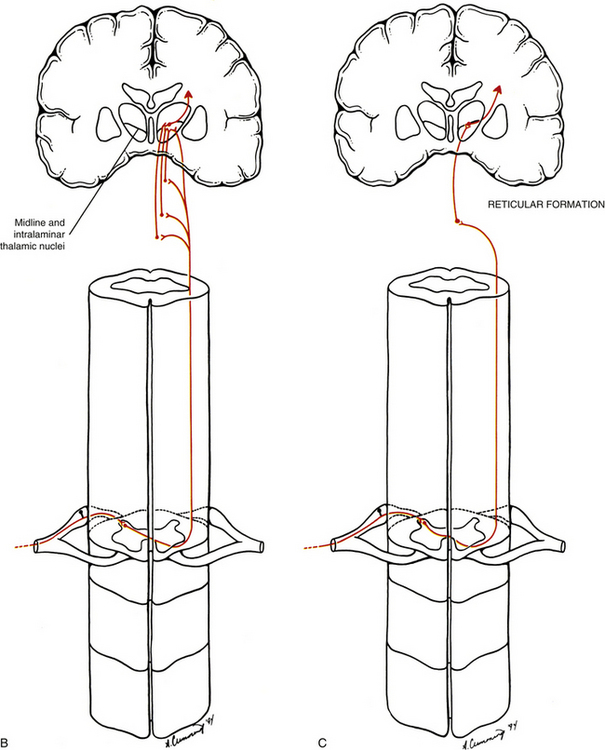
FIG. 11-4 Ascending spinal cord tracts associated with nociception. A, The neospinothalamic tract is associated with localization of nociceptive stimulation. This pathway also is associated with the evaluation of the intensity of nociceptive stimulation. B, The paleospinothalamic tract, which sends collateral branches into the brain stem reticular formation, and, C, the spinoreticular tract are most likely associated with the evaluation of nociceptive input as being unpleasant (painful). They both project to widespread areas of the cerebral cortex and are associated with the body’s autonomic response to nociceptive stimuli (e.g., increased sympathetic stimulation).
Spinoreticular Tract
The other tract that ascends in the anterolateral quadrant is the spinoreticular tract (Fig. 11-4, B and C). The cell bodies of the spinoreticular tract are located in laminae VII and VIII. The nociceptive input to these neurons is polysynaptic; therefore these neurons are likely involved with more complex response properties of pain. The axons of these neurons ascend to the reticular formation of the brain stem and to the thalamus. The reticular formation is a complex network of neurons located throughout the core of the brain stem. It has numerous functions and is a major component of the ascending reticular activating system (ARAS), along with the thalamus and cerebral cortex. The ARAS provides the circuitry through which arousal and attentiveness are maintained. The tract neurons synapsing in the reticular formation form complex connections within this region and subsequently project to brain stem nuclei, the hypothalamus, and the midline and intralaminar nuclei of the thalamus. These latter nuclei are part of the medial nuclear group of the thalamus. Subsequent thalamic projections course to widespread, nonspecific areas of the cerebral cortex. In addition, the paleospinothalamic tract (also known as the spinoreticulothalamic tract) contributes collateral branches to the reticular formation as it ascends through the brain stem to the midline and intralaminar thalamic nuclei. The spinoreticular tract and spinoreticulothalamic fibers are not somatotopically organized. The nociceptive information conveyed in these fibers also may be involved with the generation of chronic pain and the qualities associated with that sensation. The focusing of the individual’s attention on the painful area is most likely a function of the ARAS.
Dimensions of Pain
Pain appears to be of two dimensions. One is a sensory portion that is involved with identifying the location, intensity, quality, and other characteristics of the pain for an appropriate motor response. The other is the dimension of affect and motivation. This is the subjective awareness of the unpleasantness of the pain: the desire (motivation) to terminate, reduce, or escape the stimulus. It includes the emotions involved with present, short-term, or long-term implications of the pain (i.e., interference with one’s life) and the cognitive processes of how to cope with the pain. Pain is a complex sensation, and multiple pathways that ascend both in series and in parallel terminate in numerous brain stem and cortical regions to participate in its processing. The pathway that serves the dimension of pain sensation is the (neo)spinothalamic pathway, sometimes called the lateral and more direct pathway. As discussed, this pathway projects to the somatosensory cortex, which in turn projects to other cortical areas such as the posterior parietal lobe and insular cortex. From these two areas, projections continue to other areas (e.g., the amygdala and anterior cingulate cortex) that have reciprocal connections with the prefrontal cortex. This pathway may be involved not only with the discrimination of the rudimentary aspects of pain such as intensity, quality, and location, but also with the integration of numerous somatosensory inputs. The integration of these inputs provides an overall feeling of the seriousness or threat of the pain stimulus to the body and self (Price, 2000). The aspect of affect and motivation to pain sensation is served by numerous pathways sometimes collectively called the medial pathway (Cross, 1994; Hudson, 2000; Sewards & Sewards, 2002). The spinoreticular and spinoreticulothalamic tracts (see previous section) and spinomesencephalic and spinohypothalamic tracts (see Chapter 9) terminate in brain stem nuclei, the thalamus, and hypothalamus (Price, 2000). Processing at these levels may produce elementary aspects of pain behavior by involving autonomic activation, escape responses, arousal, and fear. These attributes occur early in pain-processing stages when fear, defensive behavior, and visceral responses occur. The thalamic nuclei of this pathway (midline and intralaminar) project to the limbic system, which allows an individual to perceive a sensation as being uncomfortable, aching, or hurting (Song & Rupert, 2005). The component of the limbic system receiving this input is the anterior cingulate cortex (see Fig. 9-14). This region is considered to be a major cortical area for processing the emotional (motivational) component of pain (Hudson, 2000; Price, 2000; Raineville, 2002; Sewards & Sewards, 2002). The anterior cingulate cortex is indirectly and directly connected with many other areas including the posterior cingulate cortex, amygdala (which functions in the memory aspects of painful experiences), insular cortex (which functions in the autonomic component of the entire painful episode), parietal cortex, and prefrontal cortex. Because of these interactions, the anterior cingulate cortex may be a region that integrates pain information received from sensory cortices concerning recognition and threat of the painful experience with information received from the prefrontal cortex. This integration is considered important for individuals like Mr. S. to plan how to respond and cope with the painful experience.
Important Terms and General Concepts Related to Pain
The sensations that are generally thought of as painful are all perceptions by the cerebral cortex, and therefore subjective. These perceptions are based on previous experience and understanding that something injurious has occurred to the body. Most painful conditions experienced or described by patients have a physiologic basis, and understanding the mechanisms associated with the stimulation of nociceptors and the subsequent perception of pain is important to properly diagnose and treat any injury. Appendix II (Terms Related to Pain) contains a list of many of the common terms used in further describing and differentiating diagnoses related to pain. Many of the terms used to describe, define, or classify pain for diagnostic purposes were developed specifically for use in clinical practice, hence the need here for additional physiologic descriptions to provide information related to the mechanisms associated with a particular term. Each term in Appendix II is followed immediately (in quotes) by the current definition as described by the International Association for the Study of Pain (Loeser et al., 2011), a physiologic or anatomic description for that term, and, finally, a brief description, where appropriate, relating to Mr. S. The important concepts of primary and secondary hyperalgesia not only are defined and described in Appendix II but also are illustrated in Figure 11-5.
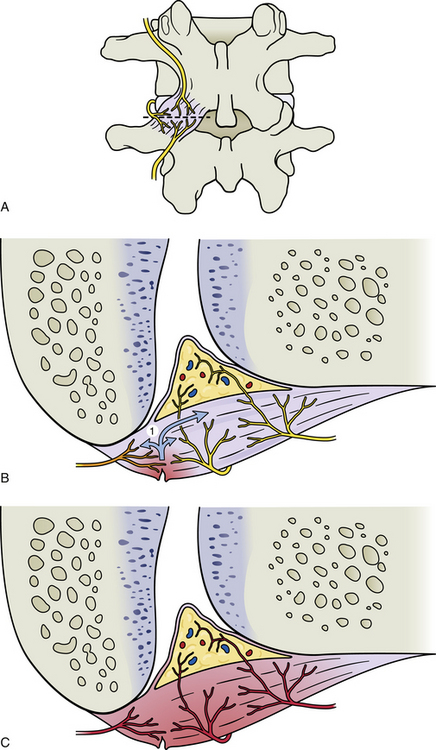
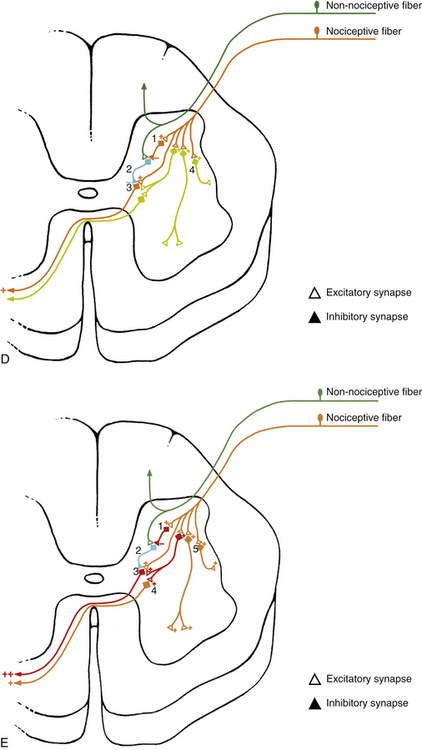
FIG. 11-5 A-C, Primary and, D and E, secondary hyperalgesia, also known as, A-C, peripheral and, D and E, central sensitization. A, Posterior view of a zygapophysial (Z) joint showing the innervation of the Z joint capsule from the posterior primary division (dorsal ramus) of the same level, the level above, and the level below. For example, if the vertebrae depicted are L3 and L4, the nerve that is the furthest lateral, left, in this figure would be the posterior primary division of the L3 spinal nerve, and the nerves coming from the level above and below would be the posterior primary divisions of the L2 and L4 spinal nerves, respectively. The dashed line through the joint shows the plane of section shown in B and C. B, Horizontal section of the same Z joint showing a torn area of the posterior aspect of the capsule. The orange color of the nerve to the left indicates that it is firing as a result of the chemokines, 1, that have been released in the area of tissue damage. C, Primary hyperalgesia (peripheral sensitization). The red color of the nerves has been used to indicate that they are firing at an increased rate. This means the nerves are responding to the same stimuli they previously experienced (e.g., movement) with a greater number of action potentials. In addition, the neurons are also firing spontaneously because of the chemokines (1 in B) first released from the damaged tissue and subsequently also released from the nerve endings themselves. D, Cross section of a corresponding spinal cord segment showing examples of afferent neurons shown in B. Short-term stimulation of nociceptive fibers results in the activation of many dorsal horn interneurons. Activation of inhibitory interneurons, 1, results in a decrease in output of gate control interneurons, 2, which leads to decreased inhibition of second-order pain fibers, 3. In addition, direct activation of second-order fibers results in an increased output of these fibers and an increase in the perception of pain. The orange color indicates the second-order neuron is currently firing. Short stimulation of nociceptive fibers may not be sufficient to bring other excitatory interneurons, 4, to threshold. E, Secondary hyperalgesia (central sensitization). The spinal cord segment now corresponds to the scenario depicted in C. Long-term stimulation of nociceptive fibers results in increased excitability of dorsal horn interneurons and secondary hyperalgesia. Activation of inhibitory neurons, 1, results in profound decreases in gate control neurons, 2, and little or no inhibition of second-order pathways, 3. Second-order neurons may also undergo changes in excitability, leading to spontaneous and/or increased output to nociceptive input, resulting in increased output of these fibers, 4. Long-term nociceptive activity may also lower the threshold of excitatory interneurons in the dorsal horn, leading to the recruitment of other second-order pain pathways, 4, and compensatory effects, 5, such as activation of intermediolateral (autonomic) and ventral horn neurons. Red indicates neurons that are firing at a higher rate than normal. Orange indicates neurons that are currently firing, but normally would not be firing if the peripheral stimulus was of relatively short duration.
General Concepts Related to Pain
Physiologic and Pathophysiologic Pain
One method of categorizing pain is to divide it into two basic types—physiologic and pathophysiologic. The latter also is known as clinical pain. Physiologic pain is related to a stimulus (tissue damage) and the response to that stimulus by the nervous system (e.g., nociceptive pain). The response involves the spinal cord, as well as higher centers of the CNS. This type of pain is associated with the “hard-wiring” of the nervous system when responding to tissue damage. Pathophysiologic pain is the result of tissue (including nerve) inflammation and nerve injury inside or outside the CNS. The concept of pathophysiologic pain has developed as more knowledge has been acquired about the changes (some are long-term changes) that occur in the nervous system after tissue damage, and how these changes affect the response of the nervous system to nociceptive input (e.g., peripheral and central sensitization). The concept of pathophysiologic pain is particularly important when considering subacute and chronic pain syndromes (Siddall & Cousins, 1997).
Modulation of Nociception
Modulation at the Level of the Peripheral Nerves and Spinal Tissues (Pain Generators)
Damage to or section of a peripheral nerve results in biochemical and neurophysiologic changes in the nerve and surrounding tissues that lower the threshold of the affected nerve and the nerve endings of other nerves within the affected surrounding tissues (Fig. 11-5, A through C). These changes are mediated by the nerve endings themselves or by the DRG, and also can cause the nociceptive fibers to fire spontaneously (ectopically). In addition, a decrease in the blood supply anywhere along the course of myelinated nerves can cause a reduction of the myelination that also can lead to ectopic firing of the nerves. Ectopic firing of the afferent fibers is interpreted by the CNS as if a real stimulus onto a specific receptor had produced the event, and these impulses can be perceived as stabbing (sharp), radiating, or burning pain (Siddall & Cousins, 1997). Of related importance is that a much higher incidence of blocked lumbar segmental arteries and the middle sacral artery exists in patients with LBP compared with age-matched controls (Kauppila & Tallroth, 1993). Recall that these arteries supply the dorsal and ventral roots, spinal nerve, proximal portion of the anterior primary division, and a considerable portion of the posterior primary division and its medial and lateral branches. Therefore ischemia to any of these nerve fibers (with the exception of the ventral roots) can lead to an increase in the amount of perceived pain through ectopic firing, even though no true peripheral nociceptive stimulus may be present.
Nerve fibers in the region become more sensitive to stimulation once tissue damage occurs, and motions or pressures that normally would not cause pain may then be perceived as quite painful (allodynia). The phenomenon of increased sensitivity of nociceptors after tissue damage is known as peripheral sensitization or primary hyperalgesia. Primary hyperalgesia (sensitization) has been verified experimentally in spinal tissues (Yamashita et al., 1990). This type of sensitization is caused by a combination of pain-mediating chemicals (e.g., substance P, bradykinin, histamine) released from tissue cells in the area of tissue damage, including inflammatory tissue cells (e.g., lymphocytes, mast cells), as well as neurotransmitters and related substances released from the nerve endings innervating the injured tissues. These events can lead to a prolonged sensation of pain to nociceptive stimuli. In addition, certain nociceptors, sometimes called “silent nociceptors,” which normally have an extremely high threshold and do not fire even with mild to moderate tissue damage, begin to fire frequently once this “chemical soup” of pain mediators is found in the damaged tissues. Silent nociceptors have been well documented in muscle tissue and joint capsules and are thought to be present in the IVD. They are not found in skin (Cavanaugh, 1995; Greenspan, 1997). These silent nociceptors are also a component of peripheral sensitization. Conversely, endogenous opioids in the peripheral tissue may be released and activate opioid receptors found on the afferent fibers, thus modulating the membrane potential of the afferent fibers and resulting in a decrease of nociceptive input and ultimately in pain perception (Siddall & Cousins, 1997). Therefore the response of the peripheral nervous system to tissue damage is a complex one, and is the result of the competitive effects of pain mediators and endogenous opioids in the region surrounding the sensory nerve endings or a damaged nerve.
Modulation in the Spinal Cord
The neurotransmission of nociception can be modulated at the segmental level by the action of interneurons in the dorsal horn (Fig. 11-6). This mechanism has been known to exist since Melzack and Wall (1965) proposed the gate control theory. Since then the dorsal horn, and especially the superficial dorsal horn, has been extensively investigated. The data from this research have led to numerous questions concerning the details of the gate control theory, and subsequently the theory was revised. However, the general concept of the gate control theory appears to be accepted (McMahon, 1990; Willis & Coggeshall, 1991). Briefly this theory states that increased activity of large-diameter, low-threshold (non-nociceptive) afferents competitively inhibits the transmission of small-diameter (nociceptive) afferent fibers, thus decreasing nociceptive input by tract neurons to higher centers, resulting in a decrease in the perception of pain. This concept has led to effective therapies for relief of pain, such as transcutaneous nerve stimulation and dorsal column stimulation (McMahon, 1990).
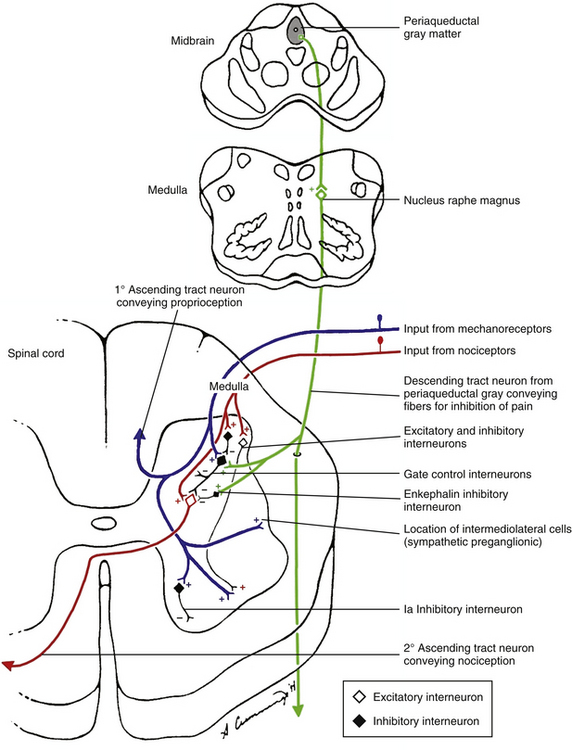
FIG. 11-6 Modulation of nociception in the spinal cord. Local afferents conducting impulses from mechanoreceptors and descending fibers from the nucleus raphe magnus are capable, via inhibitory interneurons, of inhibiting the second-order tract neurons conducting nociception. Afferents conducting impulses from nociceptors also synapse onto interneurons in the dorsal horn. In addition, excitatory and inhibitory interneurons in the dorsal horn influence the gate control interneurons affecting nociception, as well as other spinal circuits to the ventral horn and sympathetic preganglionic cells in the intermediolateral cell column. Interneurons therefore play a key role in the modulation of nociception.
The inhibition of the tract neurons conducting nociception most likely comes from a population of interneurons located in lamina II (substantia gelatinosa). Many of these interneurons use enkephalins as neurotransmitters. These interneurons receive excitatory input (possibly indirectly) from large-diameter, low-threshold mechanoreceptive afferents. The interneurons, in turn, inhibit the neurons conducting nociception (Basbaum, 1984). Consequently, a balance between the non-nociceptive inhibitory input and the excitatory nociceptive input from small-diameter fibers is mediated by inhibitory interneurons, and is likely to be necessary for the normal processing of nociception (McMahon, 1990). This balance is probably the result of modulation originating from both the complex circuitry of the superficial dorsal horn and the descending supraspinal pathways, and may explain, in part, the wide variability associated with tolerance to pain. In addition, stimulation of the non-nociceptive mechanoreceptors immediately after the activation of C fiber nociceptive afferents can reduce the output of the nociceptive neurons and possibly reduce the amount of central sensitization associated with activation of pain pathways. Further research is necessary to clarify this circuitry and its relationship to the descending input from regions in the brain stem (see following discussion).
Supraspinal Modulation
Evidence from studies in which electrical stimulation of regions of the brain stem produced analgesia (Basbaum & Fields, 1978) indicates that descending pathways can modulate nociceptive signals. One of the components of this endogenous pain control system is the periaqueductal gray (PAG) matter of the midbrain. This region has a major projection to the nucleus raphe magnus, which is located in the midline of the rostroventral medulla (see Fig. 11-6). This nucleus is rich in the neurotransmitter serotonin. Serotonergic fibers course into the dorsolateral funiculus of the spinal cord (raphe-spinal tract) from this region, and many fibers synapse on neurons in the superficial dorsal horn (laminae I and II). A second pathway stimulated by the PAG originates in the lateral tegmental nucleus of the brain stem and sends adrenergic neurons to stimulate interneurons that use enkephalin as a neurotransmitter. These interneurons also are found in the superficial dorsal horn of the spinal cord (Basbaum & Fields, 1984; Hendry, Hsiao, & Bushnell, 1999). The superficial dorsal horn receives input from afferent fibers conveying nociception. In addition, this is the location of the origin of the spinothalamic tracts (Basbaum & Fields, 1978; Jessell & Kelly, 1991) and the area involved with the segmental modulation of nociception (see previous section). The descending fibers synapse on several types of neurons. These include the inhibitory interneurons containing enkephalins (an opioid peptide) just discussed, and also the nociceptive projection (tract) neurons. The opioid-containing (enkephalin) inhibitory interneurons are close to both primary nociceptive afferents and the tract neurons. In fact, both the axonal afferent nociceptive endings and the dendrites of the tract neurons contain opioid receptors (Jessell & Kelly, 1991). Pharmacologic studies have shown that the release of opioid peptides from the inhibitory interneurons blocks transmission of nociception by two mechanisms (see Fig. 11-6). One proposed mechanism is by binding to receptors on the presynaptic terminals of the primary afferent fibers and inhibiting their release of neurotransmitters, such as substance P. The enkephalins may bind to µ-opioid receptors by diffusing from their site of release to the presynaptic membrane of the primary afferent fibers (Basbaum, 1987; Besson, 1988; Jessell & Kelly, 1991; Basbaum & Jessell, 2000). The effect of these enkephalins on the presynaptic membrane is to hyperpolarize the membrane or shorten the duration of the incoming action potential (or both), which leads to a decrease in the amount of neurotransmitter released, and therefore a reduction of nociceptive information being transmitted to the CNS.
The second mechanism by which inhibitory interneurons can mediate spinal neurotransmission of nociception is by directly synapsing with the postsynaptic membrane of the tract neuron (see Fig. 11-6). This occurrence has been well documented (Basbaum, 1987; Besson, 1988; Jessell & Kelly, 1991). Through these connections, the tract neuron is made less excitable (by increasing K+ conductance and thus hyperpolarizing the cell) and nociceptive transmission can be reduced substantially. Recall that the descending fibers from the PAG stimulated the enkephalin interneurons. Therefore analgesia can be produced by neural stimulation of the PAG. Analgesia also can be produced by the administration of opiates, such as morphine, into the CNS. The areas activated by the opiates are similar to those that produce analgesia when the PAG, rostroventral medulla, and enkephalin interneurons in the superficial dorsal horn are stimulated by normal neural transmission. This lends credence to the theory that endogenous opioid peptides, which have been found in the brain, can activate the descending control system to modulate pain perception (Jessell & Kelly, 1991).
In addition to the serotonergic descending pathway, other fibers descend from the pons (Basbaum, 1987; Hoffert, 1989) and appear to be involved with modulation of the nociceptive system. These descending fibers contain norepinephrine and also appear to inhibit nociception at the dorsal horn level. However, at the same time, collateral branches of these fibers synapse on the serotonergic neurons of the raphe nuclei. The subsequent release of norepinephrine at this level results in tonic inhibition of the raphe-spinal neurons (Basbaum, 1987), which tends to increase nociceptive transmission in the dorsal horn. Thus both systems provide a descending component to the mechanism for controlling pain. Feeding into these two systems is the nociceptive information transmitted through the ascending pathways (Basbaum & Fields, 1978). These ascending pathways possibly include the spinomesencephalic tract and input from the reticular formation (see Chapter 9). Also, possibly feeding into the two descending systems is stress-induced input channeled through the limbic system and hypothalamus (Jessell & Kelly, 1991). Therefore supraspinal modulation of pain is a complicated process that can be modulated at many levels by numerous different ascending and descending systems. This allows significant variability in the overall perception of pain based on the degree of tissue injury, physical activity of the individual, stimulation of other sensory systems, mental activity, psychological factors (including stress), and other influences. Consequently, once the physical cause of Mr. S.’s problem is determined, many factors must be considered when developing a comprehensive treatment plan.
After a brief pause to review the nature of mechanical back pain, the clinician enters the room to greet Mr. S., and is now mentally prepared to consider the pain that is bothering Mr. S.
Differentiation Between Pain of Somatic Origin and Radicular Pain
On meeting Mr. S., the clinician notices that he is not seated in the consultation room but instead is standing and partially supporting himself on the edge of the desk located near the center of the room. Mr. S. appears to be in great pain. As the clinician approaches, Mr. S. lets go of the desk and slowly reaches to shake the clinician’s hand. The clinician notices that in doing so, Mr. S. leans dramatically to the right and has his left hand placed along his left buttock. The clinician has read Mr. S.’s account of his chief complaint and has noted that he has been experiencing rather mild LBP intermittently over the past 2 years. However, this morning while unloading his truck (Mr. S. drives a truck for a prominent soft drink manufacturer, and his job requires him to deliver the soda to grocery and convenience stores), he heard a “pop,” and shortly thereafter felt extreme pain in his back that shot “like a lightning bolt” down his left leg (Fig. 11-7). During questioning, Mr. S. states the pain is a dull ache in his low back region (he moves his hand around a rather large area of his lower lumbar area and into his left buttock). He goes on to say that the lightning bolt pain is “on and off” and extends (he points) into his left posterior thigh and leg and the lateral aspect of the sole of his left foot. The clinician carefully questions Mr. S. about somatic and visceral symptoms of the head and neck, thorax, abdomen, and pelvis, and other possible injury to his lower extremity. The inquiries reveal that Mr. S. has no significant difficulties or symptoms arising from these regions.
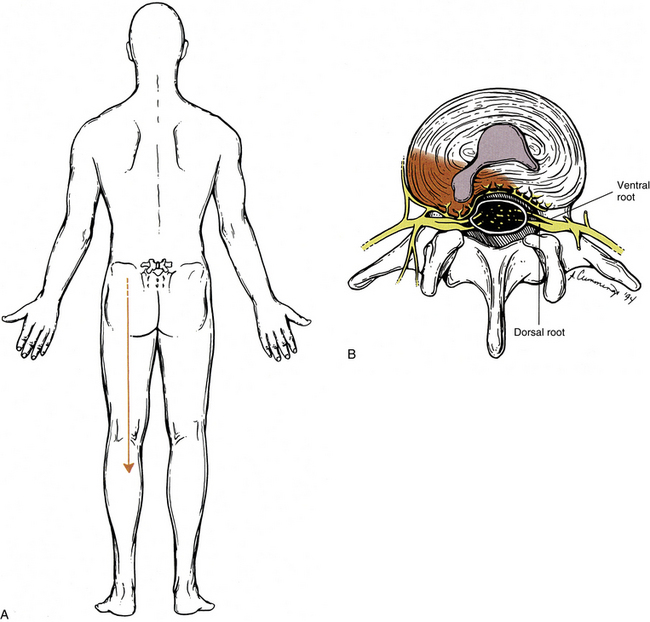
FIG. 11-7 A, Pain radiating into the lower extremity as a result of, B, an intervertebral disc protrusion. The disc protrusion is shown compressing the dorsal (and ventral) roots. This is one mechanism by which the sensation described as pain (and called radicular pain) may be felt radiating into the lower extremity. The sensation of pain also may be felt in the lower extremity after injury to or pathologic conditions of somatic structures (e.g., zygapophysial joints, ligaments, deep back muscles). The term somatic referred pain has been used to describe radiating discomfort produced by this latter mechanism (which is not demonstrated in this figure).
The physical examination reveals Mr. S. to be an individual who, his present state excluded, is physically fit. His vital signs are normal. Chest and abdomen are normal to palpation, percussion, and auscultation, and he has no palpable inguinal hernia. Rectal examination is normal. Results from examination of his head, anterior neck, and cervical and upper thoracic regions are normal. Cranial nerves and upper extremity sensation, reflexes, and muscle strength are all normal.
Mr. S. has a great deal of muscle guarding of his lumbar region during the examination. The clinician notes marked tightness of his erector spinae muscles (possibly associated with hyperalgesia), and that he is particularly sensitive to percussion over the L5 spinous process. Reflexes, sensory findings (e.g., pinprick, ability to identify touch from a cotton swab, vibration sense), and motor strength of his right lower extremity are all normal. His left extremity reveals slight weakness of plantar flexion, slightly diminished Achilles reflex, and diminished sensation to pinprick and a wisp of cotton along the posterior leg and lateral aspect of the sole of the left foot. Nerve tension signs (straight leg raising and well leg raising) produce pain at 40 degrees on the left and 60 degrees on the right, reproducing the lightning bolt pain that extends down the left lower extremity into the sole of the left foot.
Because of his antalgic posture, description of a sharp stabbing pain, positive nerve tension signs, decreased sensation, and diminished Achilles reflex, the clinician strongly suspects that Mr. S. has a disc bulge or protrusion of the L5-S1 IVD. The clinician believes that the disc is compressing the S1 dorsal and ventral nerve roots (Figs. 11-7 through 11-9). Compression of this kind results in a type of pain frequently encountered in clinical practice, known as radicular pain. Radicular pain is caused by activation of sensory fibers at the level of the dorsal root or DRG. It is experienced as a thin band of sharp, shooting pain along the distribution of the nerve or nerves supplied by the affected dorsal root (Box 11-7).
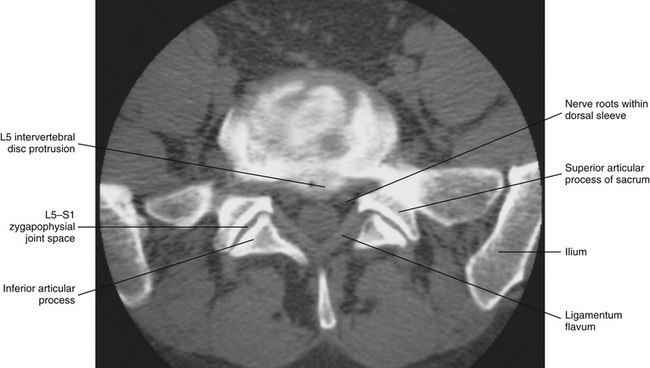
FIG. 11-8 Computed tomography discogram (radiopaque dye injected into the nucleus pulposus) of the L5-S1 region. Notice that a protrusion of the intervertebral disc can be seen on this horizontal image. The extension of the dye throughout the intervertebral disc (irregular radiopacity throughout intervertebral disc) is an indication of internal disc disruption. The circular structure contained within the “V” formed by the ligamenta flava is the cauda equina within the lumbar cistern and is surrounded by arachnoid and dura (thecal sac). (Computed tomography discogram courtesy Dr. Dennis Skogsbergh.)
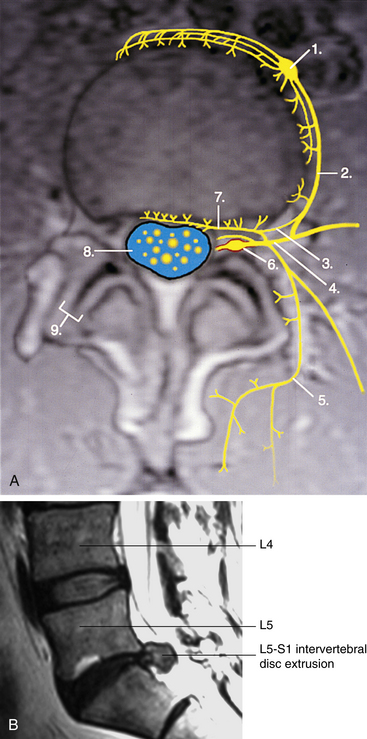
FIG. 11-9 Innervation of the intervertebral disc in horizontal section. A, Neural elements have been drawn onto a horizontal magnetic resonance imaging (MRI) scan. The top of the illustration is anterior and the bottom is posterior. Numbers indicate the following: 1, sympathetic ganglion; 2, gray ramus communicans; 3, branch of the gray ramus coursing toward the intervertebral foramen to contribute to the recurrent meningeal (sinuvertebral) nerve; 4, anterior primary division (ventral ramus); 5, medial branch of posterior primary division (the lateral branch is seen coursing to the reader’s right of the medial branch); 6, dorsal root (spinal) ganglion and dural root sleeve (red) within the intervertebral foramen; 7, recurrent meningeal (sinuvertebral) nerve; 8, cauda equina (yellow) within the cerebrospinal fluid (blue) of the lumbar cistern of the subarachnoid space; 9, zygapophysial joint. Notice that the intervertebral disc is receiving innervation from branches of the sympathetic ganglion (anteriorly), gray communicating ramus (laterally and posterolaterally), and recurrent meningeal nerve (posteriorly). Also notice that the zygapophysial joint is receiving innervation from the medial branch of the posterior primary division. B, Magnetic resonance imaging scan performed in a sagittal plane, showing extrusion of the L5-S1 intervertebral disc. (A, Photograph courtesy Ron Mensching and illustration courtesy Dino Juarez, The National University of Health Sciences;(A, Photograph courtesy Ron Mensching and illustration courtesy Dino Juarez, The National University of Health Sciences; B, magnetic resonance imaging courtesy Dr. Dennis Skogsbergh.)
Some of the causes of radicular pain include IVD protrusion, spinal (vertebral) canal stenosis (see Chapter 7), and other space-occupying lesions. The list in Box 11-8 shows several additional causes of radicular pain. Boxes 11-9 and 11-10 list the most likely mechanisms of radicular pain.
Roles of the Dorsal Root Ganglia and Nerve Roots in Pain of Radicular Origin
The results of compression on the DRGs and nerve roots are myriad. Notice in Box 11-9 that mechanical deformation affects not only the nerve fibers within the dorsal root, but also the blood vessels and connective tissue elements associated with the root (Dahlin et al., 1992). The spinal cord, nerve roots, and especially the dorsal root ganglia are more susceptible to compression than peripheral nerves, and compression or inflammation anywhere along the cauda equina or DRGs can be associated with radiculopathy (Garfin et al., 1995). The distal side of the DRG is particularly sensitive to compression (Parke, 2005). Weinstein (1988) has stated, “The intensity of the pain and its radicular nature are dependent on the strength of the stimulus” (i.e., amount of compression). As noted, the DRG is particularly sensitive to compression (Hanai, Matsui, & Hongo, 1996). Notice also in Box 11-9 that pressure on the DRG results in DRG edema. This edema is primarily from venous congestion, which can occur with little pressure on the DRG (Parke, 2005). The edema eventually results in decreased blood flow to sensory nerve cell bodies because oxygenated blood from the externally located DRG capillaries can no longer easily reach the neuronal cell bodies. Lack of oxygenated blood to the DRG results in neural ischemia, and the neural ischemia is perceived as radicular pain (Rydevik, Myers, & Powell, 1989). Short-term neural ischemia has also been related to a decrease in the speed impulses are transmitted along nerves (i.e., decreased nerve conduction velocity); studies of the relationship between long-term neural ischemia and nerve conduction velocities are still needed (Takamori et al., 2011). Compression of nerve roots has also been found to decrease axoplasmic flow within the neurons, affecting neuron (including neurotransmitter) metabolism; these changes may be another reason for decreased nerve function following nerve root compression (Kobayashi et al., 2005a). In addition, histamine-like chemicals and membrane-bound substances (e.g., phospholipase A2) within the nucleus pulposus (not in the anulus fibrosus) of a prolapsed or extruded IVD cause inflammation of the DRG, which can result in radicular pain, demyelination of nerve roots, and decreased nerve conduction velocities in the nerves affected by these chemical mediators (Rydevik, Brown, & Lundborg, 1984; Garfin, Rydevik, & Brown, 1991; Chen et al., 1997; Otani et al., 1997; Kayama et al., 1998; Özaktay, Kallakuri, & Cavanaugh, 1998). In addition, nucleus pulposus in contact with nerve roots has been found to result in increased endoneurial fluid pressure, causing what has been called a “compartment syndrome of DRG.” This compartment syndrome decreases blood flow in the DRG and increases venous congestion, augmenting radicular pain (Yabuki et al., 1998; Yabuki, Igarashi, & Kikuchi, 2000; Parke, 2005). Ectopic impulses (depolarization of neurons without extrinsic stimulation) are thought to occur from these changes and as a result to be one cause of radicular pain. These impulses normally occur during slight mechanical pressure on the DRG, and may continue for up to 25 minutes after the removal of the compression. These continued ectopic impulses are known as “after-discharges.” Such ectopic discharges may be related to the demyelination and inflammation, augmented by persisting changes in blood flow (e.g., venous congestion) mentioned earlier in this paragraph (Parke, 2005). In addition, substance P (a neuromodulator) has been found in the DRGs of experimentally chronically compressed sensory roots in pigs (Cornefjord et al., 1995), and voltage-dependent ion channels are altered in DRGs associated with radiculopathy (Abe et al., 2002). These findings also may contribute to the ectopic discharges. Finally, the receptive fields of the neurons supplied by a compressed DRG are sensitized and expanded after the compression is removed (Cavanaugh, 1995; Hanai, Matsui, & Hongo, 1996). If compression of a dorsal root occurs at two sites (e.g., in the lateral recess and in the intervertebral foramen proper) or rapidly, the events described in this section may be significantly augmented (Parke, 2005).
Exposure of the DRG and nerve roots to both normal and degenerated nucleus pulposus (without compression of the roots) also causes a decrease in the nerve conduction velocities of the axons passing through them (Otani et al., 1997; Iwabuchi et al., 2001; Ozawa, Atsuta, & Kato, 2001) that can take up to 8 weeks to resolve after exposure to nucleus pulposus tissue (Otani et al., 1997). Added to this are the findings that nucleus pulposus placed on nerve roots results in the induction of hyperalgesia and mechanical allodynia in rats (Anzai et al., 2002; Park et al., 2011). In experiments using rats, Anzai and colleagues (2002) found that nucleus pulposus placed on nerve roots caused wide–dynamic-range neurons of the dorsal horn of the spinal cord to have “enhanced responses to noxious stimuli for hours.” One potential mechanism appears to be related to the expression of the cytokine fractalkine in the spinal cord, which affects both neurons and glia (Park et al., 2011).
The dorsal and ventral roots receive sensory innervation themselves via nervi nervorum. The nervi nervorum provide another mechanism by which pressure on the nerve roots can result in pain. The nervi nervorum are most sensitive to stretch, which may help to explain the effectiveness of orthopedic tests designed to stretch the spinal nerve and nerve roots (e.g., the straight leg raise test) (Cavanaugh, 1995). The dorsal and ventral roots (ventral roots only through stimulation of nervi nervorum) originally were verified as a source of back pain by Smyth and Wright (1958), who tied nylon ligatures around nerve roots of patients during lumbar disc surgery and then pulled on the ligatures during the postsurgical recovery period. They found that nerve roots associated with IVD prolapse were much more sensitive to pulling (stretching) of the ligatures than adjacent nerve roots.
The nerve roots in the IVF may respond to pressure differently than those comprising the cauda equina within the spinal canal. The cauda equina may be more sensitive to compression than the distal aspects of the dorsal roots (Dahlin et al., 1992), and even a small amount of pressure (compression) may produce venous congestion of the intraneural microcirculation of nerve roots in the cauda equina (Olmarker et al., 1989). As mentioned when discussing DRGs, the presence of nucleus pulposus (NP) cells (not extracellular matrix) on porcine cauda equina also results in decreased nerve conduction velocities. The decreased conduction velocities result from Schwann cell damage (Olmarker, Rydevik, & Nordborg, 1993; Olmarker et al., 1996, 1997). Kirkaldy-Willis (1988b) stated:
Compromise of the cauda equina as a result of spinal stenosis may result in unusual sensations which may be “bizarre” in nature and may affect one or both limbs…. He may say that the legs feel as though they do not belong to him…or are made of rubber.
Other sensory and even motor modalities are also influenced when a dorsal nerve root is affected (Box 11-11). Therefore radicular pain usually is accompanied by paresthesia, hypesthesia, and diminished reflexes (because the sensory limb of the deep tendon reflex is affected). For the reason that the dorsal root and ventral nerve root are adjacent to each other, compression of the dorsal root usually is accompanied by compression of the ventral nerve root as well. In addition to stimulation of the nervi nervorum of the root, which results in pain, compression of the ventral (motor) root results in motor weakness. Therefore radicular pain often may be accompanied by motor weakness (see Box 11-11). Because of the decreased motor function found with compression of the ventral roots, needle electromyography of the paraspinal muscles (innervated by the dorsal rami, or posterior primary divisions, to be distinguished from the dorsal roots) can be useful in verifying the diagnosis of radiculopathy in difficult cases (Haig, LeBreck, & Powley, 1995).
Chronic compression of nerve roots and DRGs results in other unique physiologic responses. For example, serotonin, a neurotransmitter previously discussed as being involved in pain modulation, may be released by aggregating platelets and result in vasoconstriction in the blood vessels supplying nerve roots that are chronically compressed but causes vasodilation of vessels of healthy (uncompressed) nerve roots (Sekiguchi et al., 2002). However, the chronically compressed nerve roots also seem to adapt to some extent. Kikuchi and colleagues (1996) found that chronically compressed nerve roots are less sensitive to acute compression than noncompressed nerve roots.
What may seem to some as an unrelated activity can cause changes in the DRG in certain individuals. Whole body vibration has been established as one cause of LBP and has been linked to IVD protrusion (especially when combined with repetitive, sudden, intense [“shock”] loading of the IVD) (Yates & McGill, 2011). In addition, morphologic changes in the DRGs (i.e., increase in number of mitochondria, lysosomes, and nuclear membrane clefts) have been found with whole body vibration in rabbits. These changes are consistent with the formation of neuropeptides. This suggests the potential for similar changes in humans with occupations attended by whole body vibration. Such occupations include truck driving, certain highway maintenance, and some construction occupations (McLain & Weinstein, 1991). Because Mr. S. has this type of occupation (driving a truck), his sensory neurons may be sensitized to additional stimulation.
Combined Somatic Referred and Radicular Pain
In addition to the radicular signs and symptoms just discussed, Mr. S. also has diffuse LBP. This leads the clinician to suspect that he may be experiencing two different types of pain: somatic referred (nociceptive) and radicular (neuropathic) pain.
Recall that pain of somatic origin displays certain characteristics. Some of the generalized discomfort experienced by Mr. S. may be emanating from a lesion of a somatic structure. A review of possible somatic pain generators is useful, because only those structures innervated by nociceptive nerve endings are capable of producing pain (see Boxes 11-1 through 11-4).
Mr. S. also appears to be experiencing pain and some loss of function resulting from irritation of the left S1 nerve roots (dorsal and ventral). Therefore Mr. S. is simultaneously experiencing both radicular pain and pain arising from somatic structures. This is not unusual. Pain of spinal origin frequently arises from more than one pain generator. In addition, the referral zones of somatic referred pain and radicular pain frequently overlap. A patient with the symptoms and signs described for Mr. S. could be experiencing somatic referred pain originating from the posterior aspect of the anulus fibrosus, posterior longitudinal ligament, and anterior aspect of the dural root sleeve. The nociceptive input from these structures is carried by the recurrent meningeal nerve to the dorsal horn of the spinal cord and then follows the described pathways to higher centers.
The radicular pain and functional deficits (i.e., decreased Achilles reflex and loss of plantar flexion) of the left lower extremity described in this particular case are caused by compression of the S1 nerve roots between the protruding L5 IVD and the left superior articular process of S1. The mechanisms of nociception arising from compression of the dorsal root, as well as the mechanism of the loss of motor function from compression of the ventral root, were described earlier in this section.
Unique Role of the Intervertebral Discs in Low Back Pain
Like Mr. S., many patients experience low back and leg pain resulting from pathology of the IVD and from pressure on nerve roots or a DRG from a protruding IVD. A great deal of research related to the biology of the IVD and its role in back pain has been completed in recent years. This section summarizes some of the key findings of that research.
Common Pathologies of the Intervertebral Disc
Two of the most common problems associated with the IVD are IVD degeneration and IVD protrusion (herniation). The following sections discuss each of these pathologies separately; however, the two processes are not mutually exclusive. For example, extruded nucleus pulposus spontaneously produces increased amounts of matrix metalloproteinases, nitric oxide, interleukin-6, prostaglandin E2, and other chemical mediators. These products may be intimately involved in both the biochemistry of disc degeneration and the pathophysiology of radiculopathy (Herzog, 1996; Kang et al., 1996; Kobayashi et al., 2005b).
Intervertebral disc degeneration: Intervertebral disc degeneration is sometimes difficult to separate from the normal aging process of the IVD. For these reasons, some authors discuss disc degeneration and normal aging of the disc interchangeably, whereas others (Adams, 2005) insist that the disc degeneration is distinctly different from IVD aging. Generally, disc degeneration has many features that are similar to the most severe aspects of the IVD aging process. The IVD seems to age differently than other tissues of the body (probably as a result of its avascular nature), and many authors now conclude that the disc is unique in that it begins the degenerative process quite early in life (approximately the second decade). However, there is a wide variation in the aging and degenerative process of the disc, and some septuagenarians have the IVDs of 30-year-olds (and vice versa).
The hallmarks of disc degeneration are loss of fluid and fluid pressure, disruption or breakdown of collagen (including tearing of the anulus fibrosus) and proteoglycans, and sclerosis of the cartilaginous end plate (CEP) and adjacent subchondral bone.
There are several conditions that have been found to promote or even possibly initiate disc degeneration. Some of these conditions may not appear to have an obvious relationship. For example, advanced aortic atherosclerosis, presenting as calcium deposits in the posterior wall of the aorta, increases a person’s risk of developing disc degeneration and is associated with the occurrence of back pain (Kauppila et al., 1997). Because of the histologic and biochemical nature of IVD degeneration, and the relationship of this condition to the aging process of the IVD, a more thorough discussion of IVD aging and the pathophysiology of IVD degeneration is presented in Chapter 14.
Herniated disc: Although IVD herniation can occur suddenly, more often a protruded or extruded nucleus pulposus (or other components of the IVD, see below) is the end stage of a rather long process (Adams & Hutton, 1983). This process includes tearing of the inner layers of the AF with associated localized (focal, <25% of the IVD circumference) bulging outward of the remaining AF, followed by complete extrusion of part of the NP through the remaining layers of the AF (see Fig. 14-17, E and F). The terminology associated with this topic is confusing. When discussing IVD pathology with another clinician, agreeing upon a common set of terms is important. The following terminology is a synopsis of that originally developed by a consensus process supported by the International Society for the Study of the Lumbar Spine (Andersson & Weinstein, 1996) and further developed by Combined Task Forces of the North American Spine Society (NASS), the American Society of Spine Radiology (ASSR), and the American Society of Neuroradiology (ASNR) (Fardon, 2001) (Fig. 11-10):
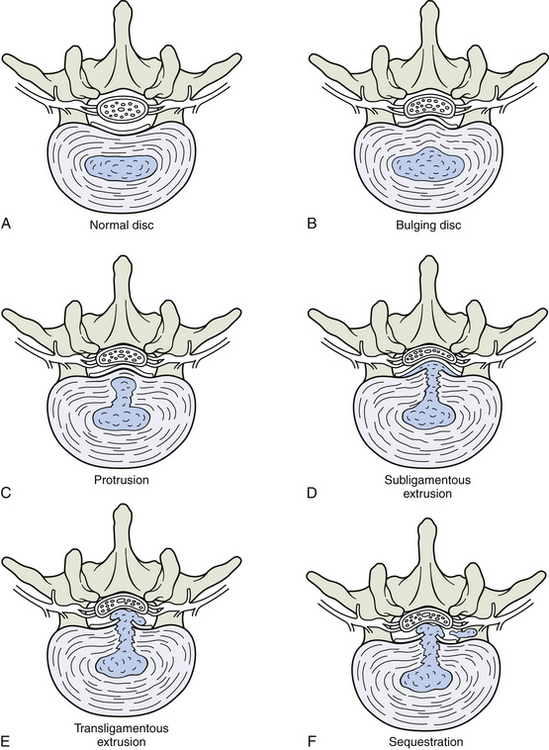
FIG. 11-10 Demonstration of terms related to intervertebral disc (IVD) bulging. A, Normal disc. B, IVD bulge. C, IVD protrusion. Notice the nucleus pulposus has herniated through several lamellae of the anulus fibrosus but has not passed through the most external lamella. D and E, IVD extrusion. The nucleus pulposus has passed through the most external lamella of the anulus fibrosus but remains attached to the host IVD. Extrusions can be further subdivided into, D, subligamentous or, E, transligamentous extrusions, depending upon whether or not the nucleus pulposus remains anterior to the posterior longitudinal ligament (subligamentous) or has pierced the ligament (transligamentous). F, Sequestration. Same as D and E except that a piece of the herniated fragment is no longer in contact with the remainder of the host nucleus pulposus. Note the sequestered fragment in F is to the reader’s right (patient’s left) of the transligamentous extrusion.
• Disc bulge. Expansion of disc material beyond its normal border (e.g., a normal disc during significant compression; or a degenerated, or aging, disc with decreased disc height). The AF bulges in both cases.
• IVD herniation. Discal material has pushed through several layers of anulus fibrosus. The discal material is usually NP in individuals less than 30 years of age, but is more often AF in individuals older than age 30. Cartilage from the CEP can also be in herniated discal material. The presence of NP and/or cartilage in the herniated material has been related to increased pain, and a higher percentage of cartilage in the herniated material has been related to decreased deep tendon reflexes. Finally, sensory impairment appears to increase when AF and cartilage predominate in the herniation. The decreased motor and sensory findings in patients with higher concentrations of cartilage (and to a lesser extent AF) in the herniation are probably due to the “harder” nature of the herniation, which increases the compressive force of the herniation on the ventral (motor) and dorsal (sensory) roots (Willburger et al., 2004). IVD herniation has several subcategories, listed immediately below.
• Protrusion. Discrete localized bulge in the AF; the disc material is displaced (i.e., the NP or other fragments of the IVD have protruded through the inner layers of the AF). Therefore a true herniation (through at least the inner fibers of the AF) is present; protrusion is a type of IVD herniation.
• Extrusion. A pedunculated protrusion—that is, a protrusion in which the diameter of the peripheral herniated disc material has a greater diameter than the attachment site (base) to the host disc. This frequently indicates that the NP (or other fragments of the IVD) has protruded (herniated) through all layers of the AF while remaining attached to the disc of origin. Some authors subcategorize extrusion into subligamentous extrusion, which does not penetrate the posterior longitudinal ligament, and transligamentous extrusion, which does penetrate the posterior longitudinal ligament (Herzog, 1996).
• Sequestration. A free disc fragment with no attachment to the host IVD is located in the epidural space. This free fragment can migrate superiorly, inferiorly, medially, or laterally. Sequestrations also can be subcategorized into subligamentous sequestration (does not penetrate the posterior longitudinal ligament) and transligamentous sequestration (does penetrate the posterior longitudinal ligament). On the rare occasions when the dura mater is adherent to the posterior longitudinal ligament, a transligamentous sequestration may penetrate the dura mater (Herzog, 1996).
The reader is encouraged to refer to the original sources (Andersson & Weinstein, 1996; Fardon, 2001) for more comprehensive definitions of these listed terms and for additional definitions related to the IVDs.
Jönsson and Stromqvist (1996) found that a patient (such as Mr. S.) usually, but not always, exhibits more serious signs and symptoms with an increase in the severity of disc pathology from bulge, to prolapse and extrusion, to sequestration.
Although a significant number of people in their twenties suffer from painful, bulging IVDs (Salminen et al., 1995), protrusion and extrusion of the IVD occurs most commonly during the fourth and fifth decades of life. At this age the NP retains its relatively high fluid content and high hydrostatic pressure, but the AF may have acquired tears and fissures. In addition, during the fourth and fifth decades there is a relatively high level of activity, with exposure to external forces in jobs and sports. These factors combine to increase the likelihood of IVD protrusion and extrusion during this age period (Kraemer, 1995). Kraemer (1995) has called the increased incidence of IVD protrusion and extrusion during the fourth and fifth decades of life, “the midlife crisis of the disc.”
Contrariwise, in the later decades, even though the AF has more fissures and ruptures, the NP is relatively dehydrated and does not move toward the periphery. In addition, external forces during sports and lifting on the job usually are reduced. The vertebral column has reduced motion (becomes “stiff”), but frequently becomes relatively pain free (Kraemer, 1995). This has been termed “the comfortable rigidity of the aging spine” (Kraemer, 1995). However, bony narrowing and stenosis of the spinal canal occurs more frequently in the elderly than earlier in life.
Once a disc fragment has extruded through the AF a complex series of events occurs (Fig. 11-11). First, the NP begins to absorb more fluid (i.e., swells) once it is extruded from the AF. This can result in increased pressure on the nearby DRG or dorsal and ventral nerve roots during the first several days after extrusion of the NP (Kraemer, 1995). In addition, an extruded, and even just a protruded, IVD initiates an inflammatory response. This inflammation has been found to be mediated by substances originating from the NP, as well as the response of the surrounding tissue to the NP-related substances. Such chemical mediators include nitric oxide, prostaglandin E2, phospholipase A2, interleukin-6, interleukin-8, substance P, and mononuclear cells (Kang et al., 1996, 1997; O’Donnell & O’Donnell, 1996; Chen et al., 1997; Hashizume et al., 1997; Kawakami et al., 1997; Nygaard, Mellgren, & Østerud, 1997; Piperno et al., 1997; Ahn et al., 2002). These substances, and the inflammation associated with them, are probably significant in the production of the radicular pain associated with protruded, extruded, and sequestered (sequestrated) nuclei pulposi (protruded nucleus pulposus [PNP]). The pressure of a PNP on the DRG or dorsal root (which has recently been quantified in the lumbar region to be >53 mm Hg) is also a cause of the radicular pain (Takahashi, Shima, & Porter, 1999). Experimentally, the combination of inflammatory substances and pressure on the DRG (or dorsal root) has been found to produce the most pronounced decrease in nerve conduction velocities. These changes in nerve conduction velocities may not be fully expressed until after 1 week of compression and exposure to the by-products of inflammation (Takahashi et al., 2003). Therefore treatment often is directed at decreasing the inflammation, as well as decreasing the intradiscal pressure of the host disc (to “pull” the herniated NP back into the confines of the AF). Various nutritional and pharmaceutical approaches are used to treat the inflammation, and many manipulative procedures (such as flexion-distraction and other manipulative, or adjusting, techniques) and surgical procedures are used to treat the pressure on the neural elements caused by the PNP. In fact, Onel and colleagues (1989) found in a study using computed tomography (CT) to evaluate PNPs in 30 subjects that traction resulted in “retraction” of extruded nuclear material in significant numbers of extruded IVDs. Decreasing the pressure on the neural elements also is thought to decrease inflammation as well.

FIG. 11-11 Healing of an intervertebral disc (IVD) protrusion. A, A left posterolateral protrusion of the nucleus pulposus (NP, blue) through the majority of the lamellae of the anulus fibrosus (AF) has resulted in an inflammatory response (red). B, A close-up of the protruding IVD and the surrounding tissues shows that the inflammation has affected the nerves innervating the outer lamellae of the IVD. Both the IVD and nerves are shown releasing chemokines, 1, including fibroblast growth factor, angiogenin, transforming growth factor-beta, platelet-derived endothelial cell growth factor, and vascular endothelial growth factor. C, An isolated view of the protruding IVD shows that in response to the chemokines just listed, new blood vessels grow (neovascularization) into the inflamed AF and NP. D, A close-up of the protruded NP and surrounding AF shows that macrophages (the cells in the NP) have made their way from the new blood vessels into the protruded NP. The macrophages in turn release matrix metalloproteinases (mm). The matrix metalloproteinases break down the extracellular matrix (primarily collagen and proteoglycans) within the protruded NP, and the absorption of the extracellular matrix into the vascular system results in shrinkage of the protrusion. E, Granulation (scar) tissue (gray) is laid down in the region of the AF surrounding the originally protruded and now retracting NP. The scar tissue eventually constricts, resulting in resolution of the IVD bulge. The greatest reduction in size of a protrusion (or extrusion or sequestration) usually occurs between 6 months and 1 year after the initial protrusion occurs (Herzog, 1996).
Much research related to IVD protrusion in the cervical and lumbar regions (PNP in the thoracic region has not been studied as extensively) has found that PNP is a dynamic process, and that after approximately 1 to 3 weeks PNPs will usually begin a 2-month to 1-year process of resolution, resulting in significant resorption, and from a patient’s standpoint (i.e., pain) often complete remission of signs and symptoms (Kraemer, 1995; Bush et al., 1997; Haro et al., 1997; Otani et al., 1997). In fact, Carreon and colleagues (1996) provided histologic evidence of resorption of sequestered NPs, and shrinkage of PNP has been seen on both CT and magnetic resonance imaging (MRI) (Koike et al., 2003). The greatest reduction in size of a PNP (with or without extrusion) usually occurs between 6 months and 1 year after the initial protrusion occurs (Herzog, 1996). However, approximately 0.25% of LBP patients require surgery for PNP. Cauda equina syndrome, severe acute motor weakness, and intolerable pain requiring permanent high-dose analgesics are among the strong indications for surgery (Kraemer, 1995; Andersson & Weinstein, 1996).
The process of PNP remission and resorption seems to begin with the ingrowth of new blood vessels onto the region of prolapse or into the extruded disc fragment (Fig. 11-11, C and D). Although the healthy adult IVD is avascular, the surrounding ligaments are supplied by blood vessels. In addition, the epidural space contains the relatively dense internal vertebral venous plexus. These vessels can allow a rather extensive system of branches to grow into the IVD after IVD protrusion, extrusion, and sequestration (Virri et al., 1996; Koike et al., 2003). This “neovascularization” probably results from the inflammatory process that was briefly described earlier, and the expression of chemokines, or cytokines, by the PNP. Some of the chemokines associated with the repair response also may be released from the nerve endings innervating the IVD (Ashton & Eisenstein, 1996; Palmgren et al., 1996) (Fig. 11-11, B). The release of cytokines by the nerve endings probably is related to the primary role of these nociceptive fibers to respond to noxious, including chemical, stimuli. These nerve endings are probably sensitive to the internal environment of the deeper layers of the AF as the chemicals seep toward the AF from the NP (McCarthy, 1993). The chemokines specifically associated with neovascularization include fibroblast growth factor, angiogenin, transforming growth factor-beta, platelet-derived endothelial cell growth factor, and vascular endothelial growth factor (Koike et al., 2003). Neovascularization seems to be closely associated with the resorption and scar formation (granulation tissue) associated with the healing process of PNP. In addition, chemokines are also expressed in the scar tissue of PNP, and inflammatory mediators are released from mononuclear cells found along the edge of the granulation tissue associated with PNP. These cytokines and inflammatory mediators are associated with the granulation tissue function to promote and continue the neovascularization and resorption process begun with the initial occurrence of the PNP (Doita et al., 1996; Haro et al., 1997; Minamide et al., 1999). In addition, chondrocytes within the IVD have been found to be chemotactic and they recruit monocytes (macrophages) that participate in IVD resorption (Kikuchi et al., 1998). The new vessels formed by the neovascularization process carry the macrophages into the PNP. The macrophages in turn secrete matrix metalloproteinases. These metalloproteinases further break down the extracellular matrix within the PNP, and the absorption of the extracellular matrix results in shrinkage of the PNP (Koike et al., 2003) (see Fig. 11-11, E).
As mentioned previously, portions of NP, AF, and CEP are found in varying concentrations in the extruded or sequestered IVD fragment. In such cases, NP undergoes resorption most rapidly, followed by AF, and then CEP (Kraemer, 1995; Carreon et al., 1996, 1997). In fact, a recent study showed that CEP inhibits the neovascularization associated with the resorption of the extruded disc fragment. Therefore higher concentrations of CEP in a given extruded fragment seem to slow the resorption process. This may help to explain the wide variation of healing rates seen clinically. In addition, cervical herniations often contain more CEP (Kokubun, Sakurai, & Tanaka, 1996). This also may help to explain why cervical disc problems can be challenging clinically. However, sometimes a patient may, after several weeks, experience a decrease in pain even though the PNP remains the same size. This is thought to result from a change in the hydration of the extruded fragment and a complete or partial resolution of the inflammation and edema surrounding the affected nerve roots or DRG (Kraemer, 1995).
Clinicians often are amazed at the wide variation in pain patterns associated with disc problems. Recall that the AF of the IVD receives sensory innervation (Figs. 11-1 and 11-9, A). Consequently, damage to the AF can be a primary source of pain. In addition, degenerated and injured IVDs, as well as PNPs themselves, have been found to be more heavily innervated with sensory nerve endings (both nociceptive and mechanoreceptive) than “healthy discs,” and these more numerous nerve endings are accompanied with a higher concentration of the peptide neuromodulators substance P (Coppes et al., 1997) and calcitonin gene-related peptide (CGRP), which are related to nociception secondary to inflammation (Ozawa et al., 2006). This ingrowth of nerves after IVD injury and the accompanying higher concentration of substance P in the region of the nerve endings make such an IVD more likely to be pain producing (a pain generator) if it is not allowed to heal properly, or if it is reinjured. In addition, the IVD also may contain silent nociceptors (see section Modulation at the Level of the Peripheral Nerves and Spinal Tissues [Pain Generators]), and the chemical mediators within the NP that were discussed earlier in this section may stimulate these silent nociceptors when the disc is injured, again resulting in pain (Cavanaugh, 1995). Recall that the specific nerves that supply the IVD are the recurrent meningeal nerve (to the posterior IVD) and other fibers that course with the autonomic nervous system (to the anterior and lateral IVDs). These nerves have a variable distribution pattern. Therefore discogenic pain (not including radicular pain) can present in a wide variety of patterns, including radiation into the groin or lower extremity. Because of the strong contribution of afferents associated with the sympathetics, discogenic pain may also have characteristics similar to those of visceral pain (Edgar, 2007). In addition, compression or inflammation of the DRG or the dorsal roots from a PNP or the inflammation associated with it, as described, results in the sharp, stabbing pain known as radicular pain, which radiates along the distribution of the nerves that are affected (see discussion of radicular pain in previous sections). Recall that radicular pain usually has a more distal radiation pattern than discogenic pain.
Animal studies have shown that an incision in the AF results in decreased nerve conduction velocities and morphologic changes in the dorsal roots. The cut AF allows “leakage of NP material,” and the NP material (not a bulge or extrusion) close to the nerve roots is thought to be responsible for causing the morphologic and physiologic changes in the nerve root (Kayama et al., 1996). Other studies also have shown that NP tissue simply placed near dorsal roots or DRGs results in decreased nerve conduction velocities (Otani et al., 1997), and phospholipase A2, which is associated with PNP, has been found to cause demyelination of nerve roots (Chen et al., 1997). These findings help to explain the LBP (because of tearing of the AF) and radicular pain (from the DRG or dorsal roots) radiating into the lower extremity in patients with no evidence of PNP on MRI or even during surgery (Kayama et al., 1996). These findings are further supported by those from human studies showing aching pain along with radicular pain in patients with disc disruption but without compromise of the outer anular fibers (Ohnmeiss, Vanharanta, & Ekholm, 1997). Therefore even if Mr. S. has no evidence of PNP on MRI, he may still have an IVD injury. This makes a thorough history and physical examination important in all cases of LBP, and additional laboratory and neurophysiologic tests are important in many cases of serious acute or chronic LBP.
Interaction between the Zygapophysial Joints and Intervertebral Discs
Even though the Z joints probably are not the primary source of back pain in the case of Mr. S., the structures of the posterior vertebral arch, and particularly the Z joints, are related to 15% to 63% of spine-related pain, depending on the region of the spine (Manchukonda et al., 2007). The Z joints also may contribute to radicular pain of discal origin, because Z joint facet arthrosis may further decrease the space available for the exiting nerve roots (Kirkaldy-Willis, 1988a) (Fig. 11-12). In addition, the inflammatory cytokines associated with Z joint inflammation (Igarashi et al., 2004) could quite conceivably affect the exiting nerve roots in the same way cytokines from PNP can cause radicular pain; however, more research is needed to confirm the effects of Z joint inflammation on the exiting nerve roots and spinal nerve. Chapter 7 discusses the role that may be played by facet arthrosis in the development of spinal (vertebral) canal stenosis and of IVF stenosis.
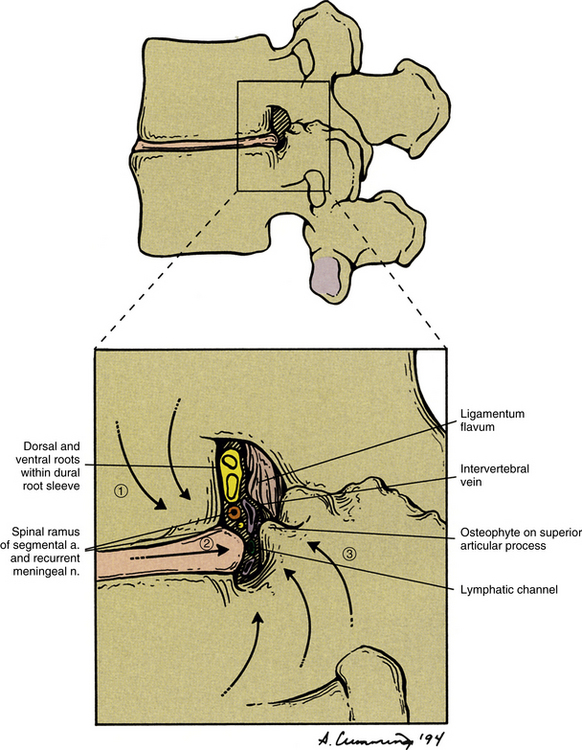
FIG. 11-12 Foraminal encroachment from a variety of sources (arrows), including intervertebral disc narrowing (1), intervertebral disc bulge (2), and zygapophysial joint arthrosis (3). Notice that as the two vertebrae approximate each other, the more superior body also shifts slightly posteriorly because of the disc narrowing.
The reverse also is true. Disc degeneration can alter Z joint motion both at the level of degeneration and at adjacent levels (Li et al., 2011). This altered motion along with the narrowing of the IVD may lead to increased stress on the Z joints. This can result in somatic pain, not originating from the articular cartilage but from pressure on the subchondral bone underlying the articular cartilage. The added pressure on the Z joints secondary to disc degeneration also may result in a small piece of soft tissue (articular capsule or Z joint synovial fold) being pinched between the facets (Hutton, 1990), which can also cause pain.
Recall that lumbar radiculopathy may be caused by chemical irritation. This also has been reproduced by selective nerve sheath injection with hypertonic saline (Rauschning, 1987). In addition, escape of radiopaque contrast medium into the nerve root canals (IVFs) has been repeatedly observed during facet joint arthrography. Because extraarticular synovial fluid is known to have strong tissue-irritating properties, Rauschning (1987) believes that “leakage of synovial fluid from ruptured intraspinal synovial cysts or weakened facet joint capsules may cause pain and possibly transient nerve root dysfunction.” Therefore simultaneous radicular pain and somatic pain arising from the Z joints are a real possibility (see Fig. 11-12).
Other Considerations
Focus often is placed on the dermatomal pattern of radicular pain. Yet there is significant variation in dermatomal patterns from individual to individual, and the dermatomes themselves overlap. So the dermatomes are only a crude means to determine the origin of radicular pain. In addition, the peripheral portions of the nerves affected by compression or inflammation of a dorsal root or DRG also supply sensory innervation to structures of myotomal and sclerotomal origin (e.g., muscles, periosteum of bones, ligaments of the back and lower extremities). Therefore pain resulting from compression of a dorsal root or DRG sometimes is felt deeper than a dermatomal distribution, corresponding to the myotomal and sclerotomal distribution of the peripheral extension of these nerves (Weinstein, 1988). This deep pain can be felt superiorly, inferiorly, medially, or laterally (or any combination of these) from the corresponding dermatome.
Pain of Somatic Origin
Somatic referral of pain also is related to the embryologic origin of somatic pain generators. The paraxial mesoderm, which surrounds the embryonic neural tube, condenses to form paired somites (see Chapter 12). Each somite subdivides into a dermomyotome (to form dermis and muscle) and sclerotome (to form the vertebrae, Z joints, the ligaments between the vertebrae, and the AF of the IVDs). Some embryologic myotomes migrate and fuse with one another. Therefore the dull aching somatic referred pain associated with myotomal patterns of pain referral, initiated by tissue damage of a somatic pain generator, may be large. Such myotomal patterns can occur with strain of the large erector spinae muscles. The deeper muscles (e.g., the transversospinalis group, interspinales, and intertransversarii muscles) remain more segmental in distribution and innervation. However, even the smaller muscles and the small joints of the spine frequently receive innervation from more than one spinal nerve. This overlap of segmental innervation also contributes to the broad referral patterns of somatic pain arising from the deep back structures (see the section Somatic Referred Pain for more details).
The clinician turns to Mr. S. and notices that Mr. S.’s eyes are closed. The clinician’s heart rate quickens, imagining that Mr. S. may have expired from old age during the time that the clinician has been considering the various ramifications of pain of spinal origin. However, the clinician is relieved as respiratory movements by Mr. S. are noticed. Mr. S. is roused from his deep slumber so the examination can be finished. The clinician focuses the care of Mr. S. on his disc protrusion with radiculopathy, and during the next few weeks, Mr. S. responds well to the treatment. The investigation and treatment of Mr. S’s pain have reignited the clinician’s interest in the mechanism of back pain. Consequently, Mr. S. has provided a service to the clinician of almost equal value to the help that the clinician has given Mr. S.
The work of Bogduk (2005) provides a clear, well-organized, and well-referenced account of structures of the spine receiving nociceptive innervation. It also describes the mechanisms, as well as they are understood, of somatic referred and radicular pain. The books of Kirkaldy-Willis (1988a,b) and Haldeman (2005) and the papers by Cavanaugh (1995), Greenspan (1995), and Siddall and Cousins (1997) provide excellent correlations between the basic science considerations of pain of spinal origin and clinical practice.
References
Abe, M., et al. Changes in expression of voltage-dependent ion channel subunits in dorsal root ganglia of rats with radicular injury and pain. Spine. 2002;27:1517–1524.
Adams, M. Pathophysiology of disc degeneration. In: Haldeman S., ed. Principles and practice of chiropractic. ed 3. New York: McGraw-Hill Companies, Inc; 2005:383–400.
Adams, M.A., Hutton, W.C. The effect of fatigue on the lumbar intervertebral disc. J Bone Joint Surg Br. 1983;65:199–203.
Ahn, S.H., et al. mRNA expression of cytokines and chemokines in herniated lumbar intervertebral discs. Spine. 2002;27:911–917.
Andersson, G.B.J., Weinstein, J.N. Disc herniation. Spine. 1996;21:1S.
Anzai, H., et al. Epidural application of nucleus pulposus enhances nociresponses of rat dorsal horn neurons. Spine. 2002;27:E50–E55.
Aprill, C., Dwyer, A., Bogduk, N. Cervical zygapophyseal joint pain patterns II: a clinical evaluation. Spine. 1990;15:458–461.
Ashton, I.K., Eisenstein, S.M. The effect of substance P on the proliferation and proteoglycan deposition of cells derived from rabbit intervertebral disc. Spine. 1996;21:421–426.
Basbaum, A.I. Anatomical substrates of pain and pain modulation and their relationship to analgesic drug action. In: Kuhar M., Pasternak G., eds. Analgesics: neurochemical, behavioral, and clinical perspectives. New York: Raven Press, 1984.
Basbaum, A.I. Cytochemical studies of the neural circuitry under-lying pain and pain control. Acta Neurochir Suppl (Wien). 1987;38:5–15.
Basbaum, A.I., Fields, H.L. Brain stem control of spinal pain-transmission neurons. Annu Rev Physiol. 1978;40:217–248.
Basbaum, A.I., Fields, H.L. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309–338.
Basbaum, A.I., Jessell, T.M. The perception of pain. In Kandel E.R., Schwartz J.H., Jessell T.M., eds.: Principles of neural science, 4th ed., New York: McGraw-Hill, 2000.
Benarroch, E.E., et al. Leptin receptor immunoreactivity in sympathetic prevertebral ganglion neurons of mouse and rat. Neurosci Lett. 1999;265(2):75–78.
Besson, J.M. The physiological basis of pain pathways and the segmental controls of pain. Acta Anaesthesiol Belg. 1988;39(3 Suppl 2):47–51.
Bogduk, N. The anatomy of the lumbar intervertebral disc syndrome. Med J Aust. 1976;1:878–881.
Bogduk, N. The innervation of the lumbar spine. Spine. 1983;8:286–293.
Bogduk, N. The rationale for patterns of neck and back pain. Patient Management. 1984;13:17–28.
Bogduk, N. The causes of low back pain. Med J Aust. 1992;156:151–153.
Bogduk, N. Clinical anatomy of the lumbar spine, 4th ed. London: Churchill Livingstone; 2005.
Bogduk, N., Lambert, G., Duckworth, J. The anatomy and physiology of the vertebral nerve in relation to cervical migraine. Cephalalgia. 1981;1:11–24.
Budgell, B., Hotta, H., Sato, A. Spinovisceral reflexes evoked by noxious and innocuous stimulation of the lumbar spine. J Neuromusculoskel Syst. 1995;3:122–131.
Burton, A.K., et al. Psychosocial predictors of outcome in acute and subchronic low back trouble. Spine. 1995;20:722–728.
Bush, K., et al. The pathomorphologic changes that accompany the resolution of cervical radiculopathy: a prospective study with repeat magnetic resonance imaging. Spine. 1997;22:183–186.
Campbell, D., Parsons, C. Referred head pain and its concomitants. J Nerv Ment Dis. 1944;99:544–551.
Carpenter, M.B., Sutin, J. Human neuroanatomy, 8th ed. Baltimore: Williams & Wilkins; 1983.
Carreon, L.Y., et al. Histologic changes in the disc after cervical spine trauma: evidence of disc absorption. J Spinal Disord. 1996;9:313–316.
Carreon, L.Y., et al. Neovascularization induced by anulus and its inhibition by cartilage end plate. Its role in disc absorption. Spine. 1997;22(13):1429–1434.
Cavanaugh, J.M. Neural mechanisms of lumbar pain. Spine. 1995;20:1804–1809.
Chen, C., et al. Effects of phospholipase A2 on lumbar nerve root structure and function. Spine. 1997;22:1057–1064.
Colloca, C.J., et al. The use of measurement instruments in chiropractic practice. In Haldeman S., ed.: Principles and practices of chiropractic, 3rd ed, New York: McGraw Hill Companies Inc, 2005.
Coppes, M.H., et al. Innervation of “painful” lumbar discs. Spine. 1997;22(20):2342–2349.
Cornefjord, M., et al. Neuropeptide changes in compressed spinal nerve roots. Spine. 1995;20:670–673.
Cramer, G.C., et al. Degenerative changes following spinal fixation in a small animal model. J Manipulative Physiol Ther. 2004;27:141–154.
Cramer, L.E. Preventing low back pain in industry. JAMA. 1998;280(23):1993–1994.
Craig, A.D., Bushnell, M.C. The thermal grill illusion: unmasking the burn of cold pain. Science. 1994;265(5169):252–255.
Craig, A.D., et al. Functional imaging of an illusion of pain. Nature. 1996;384(6606):258–260.
Cross, S.A. Pathophysiology of pain. Mayo Clin Proc. 1994;69(4):375–383.
Dahlin, L.B., et al. Physiology of nerve compression. In Haldeman S., ed.: Principles and practice of chiropractic, 2nd ed., East Norwalk, Conn: Appleton & Lange, 1992.
Darby, S., Cramer, G. Pain generators and pain pathways of the head and neck. In: Curl D., ed. Chiropractic approach to head pain. Baltimore: Williams & Wilkins, 1994.
Doita, M., et al. Immunohistologic study of the ruptured intervertebral disc of the lumbar spine. Spine. 1996;21(2):235–241.
Dwyer, A., Aprill, C., Bogduk, N. Cervical zygapophyseal joint pain patterns. I: A study in normal volunteers. Spine (Phila Pa 1976). 1990;15:453–457.
Edgar, M.A. The nerve supply of the lumbar intervertebral disc. J Bone Joint Surg Br. 2007;89:1135–1139.
Edmeads, J. Headaches and head pains associated with diseases of the cervical spine. Med Clin North Am. 1978;62:533–544.
Fagni, L., Chavis, P., Ango, F., et al. Complex interactions between mGluRs, intracellular Ca2+ stores and ion channels in neurons. Trends Neurosci. 2000;23:80–88.
Fardon, D.F. Nomenclature and classification of lumbar disc pathology. Spine (Phila Pa 1976). 2001;26:461–462.
Fields, H.L. Pain. New York: McGraw-Hill; 1987.
Garfin, S.R., Rydevik, B.L., Brown, R.A. Compression neuropathy of spinal nerve roots. Spine. 1991;16:162–166.
Garfin, S.R., et al. Spinal nerve root compression. Spine. 1995;20:1810–1820.
Giles, L.G.F. Lumbo-sacral zygapophysial joint tropism and its effect on hyaline cartilage. Clin Biomechan. 1987;2:2–6.
Gill, K.P., Callaghan, M.J. The measurement of lumbar proprioception in individuals with and without low back pain. Spine. 1998;23:371–377.
Gillette, R.D. Behavioral factors in the management of back pain. Am Fam Physician. 1996;53:1313–1318.
Gillette, R.G., Kramis, R.C., Roberts, W.J. Characterization of spinal somatosensory neurons having receptive fields in lumbar tissues of cats. Pain. 1993;54:85–98.
Greenspan, A. Bone island (enostosis): current concept. A review. Skeletal Radiol. 1995;24(2):111–115.
Greenspan, J.D. Nociceptors and the peripheral nervous system's role in pain. J Hand Ther. 1997:78–85. [April-June].
Haig, A.J., LeBreck, D.B., Powley, S.G. Paraspinal mapping: quantified needle electromyography of the paraspinal muscles in persons without low back pain. Spine. 1995;20:715–721.
Haldeman, S. The neurophysiology of spinal pain. In Haldeman S., ed.: Principles and practice of chiropractic, 2nd ed., East Norwalk, Conn: Appleton & Lange, 1992.
Haldeman S., ed. Principles and practice of chiropractic, 3rd ed., New York: McGraw Hill Companies Inc, 2005.
Hanai, F., Matsui, N., Hongo, N. Changes in responses of wide dynamic range neurons in the spinal dorsal horn after dorsal root or dorsal root ganglion compression. Spine. 1996;21:1408–1415.
Haro, H., et al. Sequential dynamics of monocyte chemotactic protein-1 expression in herniated nucleus pulposus resorption. J Orthop Res. 1997;15(5):734–741.
Hashizume, H., et al. Histochemical demonstration of nitric oxide in herniated lumbar discs: a clinical and animal model study. Spine. 1997;22:1080–1084.
Hendry, S.H.C., Hsiao, S.S., Bushnell, M.C. Somatic sensation. In: Zigmond M.J., et al, eds. Fundamental neuroscience. San Diego: Academic Press, 1999.
Herzog, R.J. The radiologic assessment for a lumbar disc herniation. Spine. 1996;21:19S–38S.
Hildebrandt, J., et al. Prediction of success from a multidisciplinary treatment program for chronic low back pain. Spine. 1997;22:990–1001.
Hockaday, J.M., Whitty, C.W. Patterns of referred pain in the normal subject. Brain. 1967;90(3):481–496.
Hoffert, M.J. The neurophysiology of pain. Neurol Clin. 1989;7(2):183–203.
Hubbard, D.R., Berkoff, G.M. Myofascial trigger points show spontaneous needle EMG activity. Spine. 1993;18(13):1803–1807.
Hudson, A.J. Pain perception and response: central nervous system mechanisms. Can J Neurol Sci. 2000;27(1):2–16.
Hutton, W.C. The forces acting on a lumbar intervertebral joint. J Manual Med. 1990;5:66–67.
Ianuzzi, A., Khalsa, P.S. Comparison of human lumbar facet joint capsule strains during simulated high-velocity, low-amplitude spinal manipulation versus physiological motions. Spine J. 2005;5:277–290.
Igarashi, A., Kikuchi, S., Konno, S., et al. Inflammatory cytokines released from the facet joint tissue in degenerative lumbar spinal disorders. Spine (Phila Pa 1976). 2004;29:2091–2095.
Indahl, A., et al. Interaction between the porcine lumbar intervertebral disc, zygapophysial joints, and paraspinal muscles. Spine. 1997;22:2834–2840.
Inman, V.T., Saunders, J.B. Referred pain from skeletal structures. J Nerv Ment Dis. 1944;996:660–667.
Iwabuchi, M., et al. Effects of anulus fibrosus and experimentally degenerated nucleus pulpous on nerve root conduction velocity. Spine. 2001;26:1651–1655.
Jeffries, B. Facet joint injections. Spine State Art Rev. 1988;2:409–417.
Jessell, T.M., Kelly, D.D. Pain and analgesia. In Kandel E.R., Schwartz J.H., Jessell T.M., eds.: Principles of neural science, 3rd ed., East Norwalk, Conn: Appleton & Lange, 1991.
Jönsson, B., Stromqvist, B. Neurologic signs in lumbar disc herniation. Preoperative affliction and postoperative recovery in 150 cases. Acta Orthop Scand. 1996;67(5):466–469.
Jorgensen, L.S., Fossgreen, J. Back pain and spinal pathology in patients with functional upper abdominal pain. Scand J Gastroenterol. 1990;25(12):1235–1241.
Kang, J.D., et al. Herniated lumbar intervertebral discs spontaneously produce matrix metalloproteinases, nitric oxide, interleukin-6, and prostaglandin E2. Spine. 1996;21:271–277.
Kang, J.D., et al. Toward a biochemical understanding of human intervertebral disc degeneration and herniation: contributions of nitric oxide, interleukins, prostaglandin E2, and matrix metalloproteinases. Spine. 1997;22:1065–1073.
Kawakami, M., et al. The role of phospholipase A2 and nitric oxide in pain-related behavior produced by an allograft of intervertebral disc material to the sciatic nerve of the rat. Spine. 1997;22:1074–1079.
Kawasaki, Y., et al. Ionotropic and metabotropic receptors, protein kinase A, protein kinase C, and Src contribute to C-fiber-induced ERK activation and cAMP response element-binding protein phosphorylation in dorsal horn neurons, leading to central sensitization. J Neurosci. 2004;24:8310–8321.
Kauppila, L.I., et al. Disc degeneration/back pain and calcification of the abdominal aorta: a 25-year follow-up study in Framingham. Spine. 1997;22:1642–1649.
Kauppila, L.I., Tallroth, K. Postmortem angiographic findings for arteries supplying the lumbar spine: their relationship to low-back symptoms. J Spinal Disord. 1993;6:124–129.
Kayama, S., et al. Incision of the anulus fibrosus induces nerve root morphologic, vascular, and functional changes: an experimental study. Spine. 1996;21:2539–2543.
Kayama, S., et al. Cultured autologous nucleus pulposus cells induce functional changes in spinal nerve roots. Spine. 1998;23:2155–2158.
Kellgren, J.H. Observations on referred pain arising from muscle. Clin Sci. 1938;3:175–190.
Kikuchi, S., et al. Increased resistance to acute compression injury in chronically compressed spinal nerve roots. Spine. 1996;21:2544–2550.
Kikuchi, T., et al. Monocyte chemoattractant protein-1 in the intervertebral disc. A histologic experimental model. Spine. 1998;23(10):1091–1099.
Kirkaldy-Willis, W.H. The pathology and pathogenesis of LBP. In Kirkaldy-Willis W., ed.: Managing low back pain, 2nd ed., New York: Churchill Livingstone, 1988.
Kirkaldy-Willis, W.H. The mediation of pain. In Kirkaldy-Willis W., ed.: Managing low back pain, 2nd ed., New York: Churchill Livingstone, 1988.
Kobayashi, S., Baba, H., Uchida, K., et al. Effect of mechanical compression on the lumbar nerve root: localization and changes of intraradicular inflammatory cytokines, nitric oxide, and cyclooxygenase. Spine (Phila Pa 1976). 2005;30:1699–1705.
Kobayashi, S., Kokubo, Y., Uchida, K., et al. Effect of lumbar nerve root compression on primary sensory neurons and their central branches: changes in the nociceptive neuropeptides substance P and somatostatin. Spine (Phila Pa 1976). 2005;30:276–282.
Koike, Y., et al. Angiogenesis and inflammatory cell infiltration in lumbar disc herniation. Spine. 2003;28(17):1928–1933.
Kokubun, S., Sakurai, M., Tanaka, Y. Cartilaginous end plate in cervical disc herniation. Spine. 1996;21(2):190–195.
Kraemer, J. Natural course and prognosis of intervertebral disc diseases. International Society for the Study of the Lumbar Spine, Seattle, Washington, June 1994. Spine. 1995;20(6):635–639.
Kummel, B.M. Nonorganic signs of significance in low back pain. Spine. 1996;21:1077–1081.
Latremoliere, A., Woolf, C.J. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926.
Li, W., et al. Lumbar facet joint motion in patients with degenerative disc disease at affected and adjacent levels: an in vivo biomechanical study. Spine (Phila Pa 1976). 2011;36:E629–E637.
Loeser, et al, IASP Committee on Taxonomy Pain terms: a list with definitions and notes on usage. Accessed at, 2011. www.iasp-pain.org/AM/Template.cfm?Section=Pain_Definitions
Macfarlane, G.J., et al. Employment and physical work activites as predictors of future low back pain. Spine. 1997;22:1143–1149.
Manchukonda, R., et al. Facet joint pain in chronic spinal pain: an evaluation of prevalence and false-positive rate of diagnostic blocks. J Spinal Disord Tech. 2007;20:539–545.
McCall, I.W., Park, W.M., O'Brien, J.P. Induced pain referral from posterior lumbar elements in normal subjects. Spine. 1979;4(5):441–446.
McCarthy, P.W. Sparse substance P-like immunoreactivity in intervertebral discs. Nerve fibers and endings in the rat. Acta Orthop Scand. 1993;64(6):664–668.
McLain, R.F., Weinstein, J.N. Ultrastructural changes in the dorsal root ganglion associated with whole body vibration. J Spinal Disord. 1991;4:142–148.
McMahon, S. The spinal modulation of pain. In: Paterson J.K., Burn L., eds. Back pain: an international review. Dordrecht, Netherlands: Kluwer, 1990.
Melzack, R., Wall, P.D. Pain mechanisms: a new theory. Science. 1965;150(699):971–979.
Minamide, A., et al. Effects of basic fibroblast growth factor on spontaneous resorption of herniated intervertebral discs: an experimental study in the rabbit. Spine. 1999;24:940.
Nakamura, S., et al. Origin of nerves supplying the posterior portion of lumbar intervertebral discs in rats. Spine. 1996;21:917–924.
Nolte, D. Bronchial hyperreactivity: pingpong between cells, nerves and mediators. Med Klin (Munich). 1988;83(4):149–150.
Nygaard, Ø.P., Mellgren, S.I., Østerud, B. The inflammatory properties of contained and noncontained lumbar disc herniation. Spine. 1997;22:2484–2488.
O'Donnell, J.L., O'Donnell, A.L. Prostaglandin E2 content in herniated lumbar disc disease. Spine. 1996;21:1653–1656.
Ohnmeiss, D.D., Vanharanta, H., Ekholm, J. Degree of disc disruption and lower extremity pain. Spine. 1997;22:1600–1605.
Ohtori, S., et al. Sensory innervation of the dorsal portion of the lumbar intervertebral disc in rats. Spine. 1999;24:2295.
Ohtori, S., et al. Neurones in the dorsal root ganglia of T13, L1 and L2 innervate the dorsal portion of lower lumbar discs in rats. A study using diI, an anterograde neurotracer. J Bone Joint Surg Br. 2001;83(8):1191–1194.
Olmarker, K., et al. Effects of experimental graded compression on blood flow in spinal nerve roots. A vital microscopic study on the porcine cauda equina. J Orthop Res. 1989;7(6):817–823.
Olmarker, K., et al. Ultrastructural changes in spinal nerve roots induced by autologous nucleus pulposus. Spine. 1996;21:411–414.
Olmarker, K., et al. The effects of normal, frozen, and hyaluronidase-digested nucleus pulposus on nerve root structure and function. Spine. 1997;22:471–476.
Olmarker, K., Rydevik, B., Nordborg, C. Autologous nucleus pulposus induces neurophysiologic and histologic changes in porcine cauda equina nerve roots. Spine. 1993;18:1425–1432.
Onel, D., et al. Computed tomographic investigation of the effect of traction on lumbar disc herniations. Spine. 1989;14(1):82–90.
Otani, K., et al. Experimental disc herniation. Spine. 1997;22:2894–2899.
Özaktay, A.C., Kallakuri, S., Cavanaugh, J.M. Phospholipase A2 sensitivity of the dorsal root ganglion. Spine. 1998;23:1297–1306.
Ozawa, K., Atsuta, Y., Kato, T. Chronic effects of the nucleus pulposus applied to nerve roots on ectopic firing and conduction velocity. Spine. 2001;26:2661–2665.
Ozawa, T., et al. The degenerated lumbar intervertebral disc is innervated primarily by peptide-containing sensory nerve fibers in humans. Spine (Phila Pa 1976). 2006;31:2418–2422.
Palmgren, T., et al. Immunohistochemical demonstration of sensory and autonomic nerve terminals in herniated lumbar disc tissue. Spine. 1996;21(11):1301–1306.
Pappageotgiou, A.C., et al. Psychosocial factors in the workplace: do they predict new episodes of low back pain? Spine. 1997;22:1137–1142.
Park, H.W., et al. Changes in spinal cord expression of fractalkine and its receptor in a rat model of disc herniation by autologous nucleus pulposus. Spine (Phila Pa 1976). 2011;36:E753–E760.
Parke, W. Role of epidural and radicular veins in chronic back pain and radiulopathy. In: Kambin P., ed. Arthoscopic and endoscopic spinal surgery text and atlas second edition. 2nd ed. Totowa, NJ: Humana Press; 2005:151–165.
Pezet, S., Marchand, F., D'Mello, R., et al. Phosphatidylinositol 3-kinase is a key mediator of central sensitization in painful inflammatory conditions. J Neurosci. 2008;28:4261–4270.
Piperno, M., et al. Phospholipase A2 activity in herniated lumbar discs: clinical correlations and inhibition by piroxicam. Spine. 1997;22:2061–2065.
Plancarte, R., Calvillo, O. Complex regional pain syndrome type 2 (causalgia) after automated laser discectomy. Spine. 1997;22:459–462.
Portenoy, R. Neuropathic pain. In: Portenoy R., Kanner R., eds. Pain management: theory and practice. Philadelphia: FA Davis, 1996.
Price, D.D. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–1772.
Raineville, P. Brain mechanisms of pain affect and pain modulation. Curr Opin Neurobiol. 2002;12:195–204.
Raoul, S., et al. Role of the sinu-vertebral nerve in low back pain and anatomical basis of therapeutic implications. Surg Radiol Anat. 2002;24:366–371.
Rauschning, W. Normal and pathologic anatomy of the lumbar root canals. Spine. 1987;12:1008–1019.
Rydevik, B., Brown, M., Lundborg, G. Pathoanatomy and pathophysiology of nerve root compression. Spine. 1984;9:7–15.
Rydevik, B.L., Myers, R.R., Powell, H.C. Pressure increase in the dorsal root ganglion following mechanical compression. Spine. 1989;14(6):574–576.
Salminen, J.J., et al. Low back pain in the young. Spine. 1995;20:2101–2108.
Schwarzer, A.C., et al. The relative contributions of the disc and zygapophyseal joint in chronic low back pain. Spine. 1994;19:801–806.
Sekiguchi, M., et al. Nerve vasculature changes induced by serotonin under chronic cauda equina compression. Spine. 2002;27:1634–1639.
Sewards, T.V., Sewards, M.A. The medial pain system: neural representations of the motivational aspect of pain. Brain Res Bull. 2002;59(3):163–180.
Siddal, P.J., Cousins, M.J. Spine update: spinal pain mechanisms. Spine. 1997;22:98–104.
Sihvonen, T., et al. Dorsal ramus irritation associated with recurrent low back pain and its relief with local anesthetic or training therapy. J Spinal Disord. 1995;8:8–14.
Smyth, M.J., Wright, V. Sciatica and the intervertebral disc: an experimental study. J Bone Joint Surg Am. 1958;40-A(6):1401–1418.
Song, X., Rupert, R.L. Central projections of spinal receptors. In Haldeman S., ed.: Principles and practices of chiropractic, 3rd ed, New York: McGraw Hill Companies Inc, 2005.
Takahashi, I., Kikuchi, S., Sato, K., et al. Effects of the mechanical load on forward bending motion of the trunk: comparison between patients with motion-induced intermittent low back pain and healthy subjects. Spine (Phila Pa 1976). 2007;32:E73–E78.
Takahashi, K., Shima, I., Porter, R.W. Nerve root pressure in lumbar disc herniation. Spine. 1999;24:2003.
Takahashi, N., et al. Pathomechanisms of nerve root injury caused by disc herniation: an experimental study of mechanical compression and chemical irritation. Spine. 2003;28:435–441.
Takamori, Y., et al. Combined measurement of nerve root blood flow and electrophysiological values: intraoperative straight-leg-raising test for lumbar disc herniation. Spine (Phila Pa 1976). 2011;36:57–62.
Takebayashi, T., et al. Effect of nucleus pulposus on the neural activity of the dorsal root ganglion. Spine. 2001;26:940–945.
Vernon, H. The cranio-cervical syndrome. Oxford, UK: Butterworth Heinemann, 2001.
Virri, J., et al. Prevalence, morphology, and topography of blood vessels in herniated disc tissue. A comparative immunocytochemical study. Spine. 1996;21(16):1856–1863.
Wei, F., Vadakkan, K.I., Toyoda, H., et al. Calcium calmodulin-stimulated adenylyl cyclases contribute to activation of extracellular signal-regulated kinase in spinal dorsal horn neurons in adult rats and mice. J Neurosci. 2006;26:851–861.
Weinstein, J., Claverie, W., Gibson, S. The pain of discography. Spine. 1988;13:1344–1348.
Weinstein, W.H. The perception of pain. In Kirkaldy-Willis W., ed.: Managing low back pain, 2nd ed., New York: Churchill Livingstone, 1988.
Willburger, R.E., Ehiosun, U.K., Kuhnen, C., et al. Clinical symptoms in lumbar disc herniations and their correlation to the histological composition of the extruded disc material. Spine (Phila Pa 1976). 2004;29:1655–1661.
Willis, W.D., Jr., Coggeshall, R.E. Sensory mechanisms of the spinal cord, 2nd ed. New York: Plenum Press; 1991.
Wyke, B. The neurology of back pain. In Jayson M.I.V., ed.: The lumbar spine and back pain, 3rd ed., New York: Churchill Livingstone, 1987.
Yabuki, S., et al. Acute effects of nucleus pulposus on blood flow and endoneurial fluid pressure in rat dorsal root ganglia. Spine. 1998;23:2517–2523.
Yabuki, S., Igarashi, T., Kikuchi, S. Application of nucleus pulposus to the nerve root simultaneously reduces blood flow in dorsal root ganglion corresponding hindpaw in the rat. Spine. 2000;25:1471–1476.
Yamashita, T., et al. Mechanosensitive afferent units in the lumbar facet joint. J Bone Joint Surg. 1990;72-A:865–870.
Yashpal, K., et al. Differential effects of NMDA and group I mGluR antagonists on both nociception and spinal cord protein kinase C translocation in the formalin test and a model of neuropathic pain in rats. Pain. 2001;94:17–29.
Yates, J.P., McGill, S.M. The effect of vibration and posture on the progression of intervertebral disc herniation. Spine (Phila Pa 1976). 2011;36:386–392.
Yoshizawa, H., O'Brien, J., Thomas-Smith, W. The neuropathology of the intervertebral discs removed for low back pain. J Pathol. 1980;132:95.
Yukawa, Y., et al. Groin pain associated with lower lumbar disc herniation. Spine. 1997;22:1736–1740.
∗Because of the clinical nature of the material in this chapter, no red rules to highlight information have been placed in the margins.
†Refer to Appendix II: Terms Related to Pain.