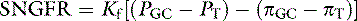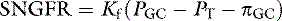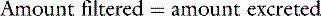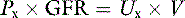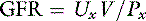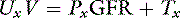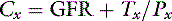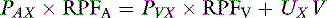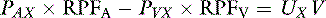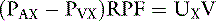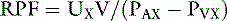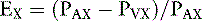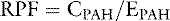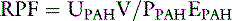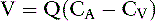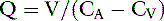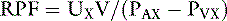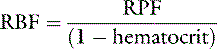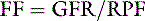Chapter 2 Applied Renal Physiology
“Superficially it might be said that the function of the kidneys is to make urine; but in a more considered view, one can say that the kidneys make the stuff of philosophy itself.”
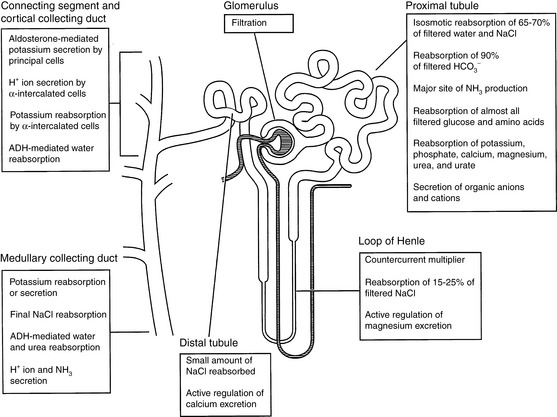
Each day the glomeruli of the kidneys filter an enormous volume of plasma water, and the tubules must reabsorb most of this water along with vital solutes so that only a small volume of water and unneeded solutes are excreted as urine. For example, a normal 10-kg dog may have a glomerular filtration rate (GFR) of 4 mL/min/kg. In the course of one day, this dog would filter 57.6 L of plasma water in its kidneys. If 60% of body weight is water, this volume represents almost 10 times the dog’s total body water. The same dog may have a urine output of 33 mL/kg/day. Thus, more than 99% of plasma water filtered by the glomeruli is reabsorbed by the tubules. The proximal tubules and loops of Henle reabsorb approximately 85% of the filtered water and solutes, whereas the collecting ducts adjust the final composition of urine to compensate for fluctuations in intake and prevent changes in the volume and composition of body fluids. The major functions of the various segments of the nephron are depicted in Figure 2-1.
Concept of renal clearance
An appreciation of the concept of clearance is crucial to understanding how renal function is evaluated clinically. The renal clearance of a substance is the volume of plasma that contains the amount of the substance excreted in the urine in 1 minute. It is the volume of plasma that must be filtered each minute to account for the amount of the substance appearing in the urine each minute under steady-state conditions. If the concentration of the substance in urine is Ux and the urine flow rate is V, the amount of the substance excreted in the urine per minute is UxV. If the concentration of the substance in plasma is Px, the volume of plasma that contains the same quantity of that substance or the volume of plasma that must be filtered per minute to account for that amount in the urine is UxV/Px, the standard clearance formula. The clearance of any substance may be calculated, but the clearance of certain substances (e.g., inulin, p-aminohippuric acid [PAH], and creatinine) provides important information about renal function (see later discussion of measurement of glomerular filtration rate and measurement of renal blood flow and renal plasma flow).
Glomerular filtration
Glomerular morphology
The glomerular capillary wall or filtration barrier consists of three components: the capillary endothelium, basement membrane, and visceral epithelium (Fig. 2-2). The glomerulus is a unique vascular structure consisting of a capillary bed interposed between two arterioles: the afferent and efferent arterioles. The glomerular capillary divides into several branches, each of which forms a lobule of the glomerulus. The capillary endothelium of the glomerulus is fenestrated by openings 50 to 100 nm in diameter. These openings exclude cells from the ultrafiltrate, but macromolecules are not restricted based on size. The luminal surface of the endothelium is covered by negatively charged sialoglycoproteins that contribute to the charge selectivity of the filtration barrier.
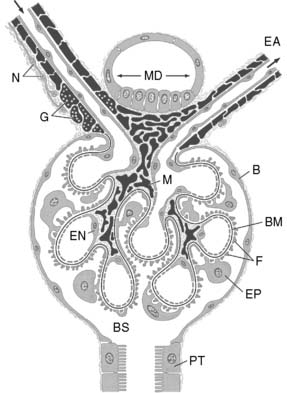
Figure 2-2 Schematic representation of the glomerulus demonstrating the afferent and efferent arterioles, juxtaglomerular apparatus, and glomerular capillary loops. At the vascular pole, an afferent arteriole (AA) enters and an efferent arteriole (EA) leaves the glomerulus. At the urinary pole, the Bowman space (BS) becomes the tubular lumen of the proximal tubule (PT). The epithelial cells composing the Bowman capsule (B) enclose the Bowman space. Smooth muscle cells proper of the arterioles and all cells derived from smooth muscle are shown in black, including the granular cells (G).The afferent arteriole is innervated by sympathetic nerve terminals (N). The extraglomerular mesangial cells are located at the angle between AA and EA and continue into the mesangial cells (M) of the glomerular tuft. The glomerular capillaries are outlined by fenestrated endothelial cells (EN) and covered from the outside by the epithelial cells (EP) with foot processes (F). The glomerular basement membrane (BM) is continuous throughout the glomerulus. At the vascular pole, the thick ascending limb touches the macula densa (MD), the extraglomerular mesangium.31
The glomerular basement membrane is composed of the lamina rara interna on the endothelial side, the central lamina densa, and the lamina rara externa on the epithelial side. The lamina rara interna and lamina rara externa contain polar noncollagenous proteins that contribute to the negative charge of the filtration barrier. The lamina densa contains nonpolar collagenous proteins that contribute primarily to the size selectivity of the filtration barrier. The filtration barrier is permeable to molecules with effective molecular radii less than 2 nm and impermeable to those with radii greater than 4 nm.
The visceral epithelial cells or podocytes constitute the outermost portion of the filtration barrier. They cover the glomerular basement membrane and glomerular capillaries on the urinary side of the barrier with their primary and interdigitating secondary foot processes. Filtration slits, 10 to 30 nm in width, are located between the secondary foot processes. The podocytes are phagocytic and may engulf macromolecules trapped by the filtration slits. They are invested with a negatively charged sialoglycoprotein coat that contributes to the charge selectivity of the filtration barrier. It is believed that the visceral epithelial cells synthesize the glomerular basement membrane.
The mesangium is not a part of the filtration barrier but a stabilizing core of tissue, forming an anchor for the glomerulus at the vascular pole and along the axes of the capillary lobules. The mesangial cells are in contact with the basement membrane in areas where there is no capillary endothelium. The extraglomerular mesangium fills the space between the macula densa and the glomerular arterioles and constitutes part of the juxtaglomerular apparatus (JGA). The mesangial cells contain microfilaments and can contract in response to specific hormones (e.g., angiotensin II), thus altering the surface area available for filtration. They also synthesize prostaglandins that contribute to renal vasodilatation. The mesangium also contains macrophages that can clear filtration residues from the mesangial space by phagocytosis.
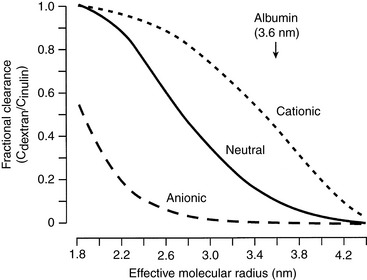
The glomerular capillary wall is a size-selective and a charge-selective barrier to filtration. Its size selectivity resides primarily in the lamina densa of the glomerular basement membrane. The glomerulus generally excludes molecules with radii greater than 4 nm. Inulin, with a molecular mass of 5200 daltons and radius of 1.4 nm, permeates freely, whereas serum albumin, with a molecular mass of 69,000 daltons and radius of 3.6 nm, permeates minimally.
The charge selectivity of the glomerulus resides in the negatively charged sialoglycoproteins (e.g., laminin and fibronectin) and peptidoglycans (e.g., heparan sulfate) of the capillary endothelium, lamina rara interna, lamina rara externa, and visceral epithelium. At any given effective molecular radius, negatively charged macromolecules experience greater restriction to filtration than neutral ones. Positively charged macromolecules experience less restriction to filtration than neutral ones of the same size (Fig. 2-3).
Determinants of glomerular filtration
The term glomerular filtration rate refers to the total filtration rate of both kidneys and represents the sum of the single-nephron glomerular filtration rates (SNGFRs) of all nephrons. The number of nephrons per kidney reflects the size of the animal. The feline kidney has approximately 200,000 nephrons, the canine kidney approximately 400,000, and the human kidney approximately 1,200,000 nephrons. SNGFR may differ among some groups of nephrons under normal conditions, and additional changes may occur in response to such factors as water deprivation, increased water intake, increased salt intake, or increased protein intake. Superficial cortical nephrons have short loops of Henle with little or no penetration into the renal medulla. These nephrons tend to excrete relatively more solute and water. Juxtamedullary nephrons have long loops of Henle that penetrate the inner medulla, and these nephrons tend to conserve solute and water. All of the nephrons in the canine and feline kidneys are thought to have long loops of Henle.
The glomerular ultrafiltrate is a protein-free ultrafiltrate of plasma containing water and all of the crystalloids of plasma in concentrations similar to those in plasma. The concentrations are not exactly the same because of the Gibbs-Donnan effect. The same Starling forces that govern the movement of fluid across other capillaries in the body determine SNGFR, but there are some important differences in the glomerulus that account for the relatively high rate of filtration:
where PGC is the hydrostatic pressure in the glomerular capillary, which falls slightly along the length of the glomerular capillary, averaging 55 mm Hg; PT is the hydrostatic pressure in the Bowman space, which is higher than systemic interstitial pressure, averaging 20 mm Hg; πGC is the oncotic pressure in the glomerular capillary, which increases along the length of the capillary because of loss of protein-free ultrafiltrate into the Bowman space, averaging 20 mm Hg; and πT is the oncotic pressure in the Bowman space and is negligible because the ultrafiltrate is nearly protein free. If πT is neglected, the formula for SNGFR simplifies to:
These relationships are depicted in Figure 2-4, in which average pressure values are those reported for dogs36 and cats.7 If the average pressures just described are considered alone, it can be seen that the net filtration pressure in the glomerulus is approximately 15 mm Hg, which is similar to values obtained for systemic capillaries. The fact that GFR is so much higher than the movement of fluid across systemic capillaries is explained by different values for Kf.
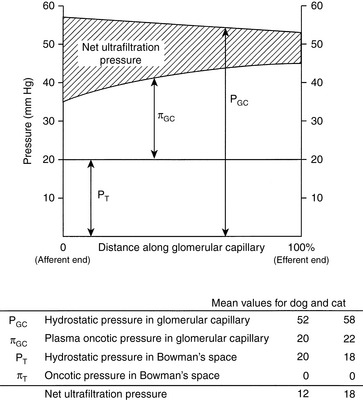
Figure 2-4 Graphic representation of the generation of net filtration pressure in the glomerulus as governed by Starling forces.
(Drawing by Tim Vojt.)
The ultrafiltration constant, Kf, is dependent on the surface area available for filtration and the permeability per unit area of capillary to crystalloids and water. The morphology of the glomerulus is such that the surface area available for filtration is much greater than that found in the capillary beds of skeletal muscle, and the unit permeability of the glomerular endothelium is more than 100 times that of skeletal muscle capillaries. This much higher value for Kf in glomerular capillaries than in systemic capillaries accounts for the much higher rate of filtration. The ultrafiltration coefficient, Kf, is not constant and can change as a result of disease and in response to hormones that cause mesangial cells to contract (e.g., angiotensin II).
Changes in the resistance of the afferent (preglomerular) and efferent (postglomerular) arterioles may have a marked effect on GFR. Alterations in resistance in the afferent arterioles lead to parallel changes in GFR and renal blood flow (RBF), but changes in resistance in the efferent arterioles lead to divergent changes in GFR and RBF (Fig. 2-5). The interplay of the effects of neural and hormonal factors on vascular tone in the kidneys is complex, but the main purpose of these effects is to minimize even slight changes in GFR, which could have drastic adverse effects on the volume and composition of the extracellular fluid.
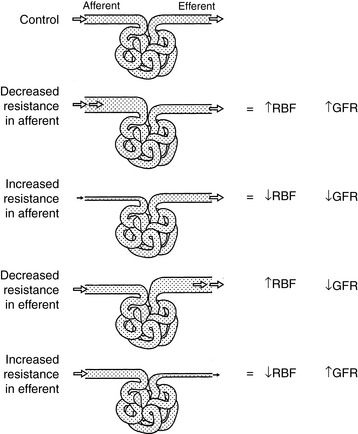
Figure 2-5 Effects of alterations in afferent and efferent arteriolar tone on renal blood flow and glomerular filtration rate.
(Drawing by Tim Vojt.)
The resistance of these arterioles is regulated by the autonomic nervous system and by numerous vasoactive mediators (Table 2-1). Stimulation of the sympathetic nervous system results in release of norepinephrine from nerves terminating on the afferent and efferent arterioles. Norepinephrine can cause afferent and efferent vasoconstriction, but efferent arteriolar constriction usually predominates. As a result, RBF decreases with minimal changes in GFR (i.e., filtration fraction [FF] increases). Angiotensin II also causes efferent more than afferent vasoconstriction and has similar effects on RBF and GFR. Stimulation of dopaminergic receptors causes afferent and efferent vasodilatation and increased RBF with little change in GFR at low concentrations of dopamine. Norepinephrine, angiotensin II, and antidiuretic hormone (ADH, vasopressin) cause vasoconstriction, at the same time promoting the production of prostaglandins that cause vasodilatation. These prostaglandins (PGE2 and PGI2) play an important role in maintaining RBF in hypovolemic states when angiotensin II and norepinephrine concentrations are increased. The effects of these prostaglandins are limited to the kidneys because they are rapidly metabolized in the pulmonary circulation. Nonsteroidal anti-inflammatory drugs that inhibit generation of prostaglandins by the cyclooxygenase pathway may cause renal ischemia and acute renal insufficiency in hypovolemic patients.10,12 Locally produced kinins also cause vasodilatation and favor redistribution of RBF to inner cortical nephrons. Mediators produced locally by the vascular endothelium also contribute to afferent and efferent vasoconstriction (e.g., endothelin and thromboxane) and vasodilatation (e.g., nitric oxide and prostacyclin).
Table 2-1 Effects of Selected Vasoactive Mediators on Glomerular Hemodynamics
| Substance | Afferent Arteriole | Efferent Arteriole |
|---|---|---|
| Vasodilators | ||
| Acetylcholine | Relax | Relax |
| Nitric oxide | Relax | Relax |
| Dopamine | Relax | Relax |
| Bradykinin | Relax | Relax |
| Prostacyclin | Relax | Relax |
| Prostaglandin E2 | Relax | No effect |
| Prostaglandin I2 | Relax | Relax |
| Vasoconstrictors | ||
| Norepinephrine | Constrict | Constrict |
| Angiotensin II | Constrict | Constrict |
| Endothelin | Constrict | Constrict |
| Thromboxane | Constrict | Constrict |
| Vasopressin | No effect | Constrict |
From Valtin H, Schafer JA. Renal function. Boston: Little, Brown, 1995: 107.
Measurement of glomerular filtration rate
Consider a substance that is filtered by the glomeruli but neither reabsorbed nor secreted by the tubules. Under steady-state conditions, the following mass balance equation may be written:
where Px is the plasma concentration of x (milligrams per milliliter), Ux is the urine concentration of x (milligrams per milliliter), V is the urine flow rate (milliliters per minute), and GFR is the glomerular filtration rate (milliliters per minute). Dividing both sides of the equation by Px:
Note that this equation is the same as the formula for clearance presented before. Thus, the renal clearance of a substance that is neither reabsorbed nor secreted is equal to GFR. Inulin is a polymer of fructose with a molecular mass of 5200 da. It is not bound to plasma proteins and is freely filtered by the glomeruli. It is neither reabsorbed nor secreted by the tubules. It is not metabolized by the kidneys or any other organ. It is uncharged and not subject to the Gibbs-Donnan effect. In summary, inulin is an ideal substance for the measurement of GFR, and inulin clearance is the laboratory standard for GFR determination. Normal values for GFR as measured by inulin clearance are 3 to 5 mL/min/kg in the dog16,21 and 2.5 to 3.5 mL/min/kg in the cat.16,45
Inulin clearance is not used clinically because it requires intravenous infusion of inulin and an assay that is not routinely available in most clinical pathology laboratories. Creatinine is produced endogenously in the body and excreted primarily by glomerular filtration, so its clearance can be used to estimate GFR in the steady state. The only requirements for determination of endogenous creatinine clearance are an accurately timed urine sample (usually 24 hours), determination of the patient’s body weight, and measurement of serum and urine creatinine concentrations.
In the dog and cat, creatinine is filtered by the glomeruli and is neither reabsorbed nor secreted by the tubules.18-20,22 In most clinical pathology laboratories, creatinine is measured by the alkaline picrate reaction. This reaction is not entirely specific for creatinine and measures another group of substances collectively known as noncreatinine chromogens. These substances are found in plasma, where they may constitute up to 50% of the measured creatinine at normal serum creatinine concentrations, but only small amounts appear in urine.21,22 When the creatinine concentration is determined using the alkaline picrate reaction, the presence of noncreatinine chromogens causes endogenous creatinine clearance to underestimate GFR. This problem may be avoided by using more accurate methods (e.g., peroxidase-antiperoxidase) to measure the creatinine concentration.22 Values for endogenous creatinine clearance in the dog and cat are approximately 2 to 5 mL/min/kg.5,17,22
To circumvent the problem of noncreatinine chromogens and to improve accuracy, some investigators have advocated determination of exogenous creatinine clearance. In this test, which is somewhat more cumbersome, creatinine is administered subcutaneously to the animal to increase the serum creatinine concentration and reduce the relative effect of the noncreatinine chromogens. For example, a normal dog may have a serum creatinine concentration of 1.0 mg/dL, of which 0.5 mg/dL represents noncreatinine chromogens. This measurement represents a 50% error. If, however, the dog’s serum creatinine concentration is increased to 10 mg/dL by subcutaneous administration of creatinine, the noncreatinine chromogens still represent only 0.5 mg/dL, and the error is reduced to 5%. Exogenous creatinine clearance exceeds endogenous creatinine clearance and more closely approximates inulin clearance in the dog.20
The amount of any substance excreted by the kidneys is the algebraic sum of the amount filtered and the amount handled by the tubules:
where Tx is the amount handled by tubules (milligrams per minute).
The term Tx is a positive number if the substance experiences net secretion and a negative number if it experiences net reabsorption. Dividing both sides of the equation by Px yields the familiar clearance formula:
Thus, the clearance of a substance experiencing net reabsorption is less than GFR (Tx is negative), and the clearance of a substance experiencing net secretion is greater than GFR (Tx is positive). The ratio of the clearance of a substance to inulin clearance gives an indication of the net handling of that substance by the kidneys. If the ratio is less than 1.0, the substance experiences net reabsorption; if it is greater than 1.0, it experiences net secretion.
Renal blood flow and renal plasma flow
The kidneys receive 25% or more of cardiac output. The major sites of resistance within the kidneys are the afferent and efferent arterioles, with an approximately 80% to 90% decrease in perfusion pressure across this region of the renal vasculature (Fig. 2-6). Blood flow is not uniform throughout the kidneys. In dogs, more than 90% of RBF is normally directed to the renal cortex, less than 10% to the outer medulla, and only 2% to 3% to the inner medulla.51 The actual rate of flow to the renal cortex is approximately 100 times that of resting muscle and is required for glomerular filtration. Blood flow to the medulla is similar to that of resting muscle, and this reduced flow is necessary for normal function of the urinary concentrating mechanism.
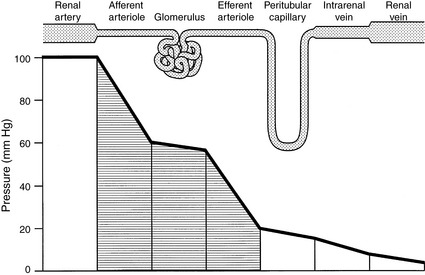
Figure 2-6 Pattern of hydrostatic pressure and vascular resistance in the renal circulation.
(Drawing by Tim Vojt.)
Autoregulation
Autoregulation refers to the intrinsic ability of an organ to maintain blood flow at a nearly constant rate despite changes in arterial perfusion pressure. In the kidneys, between perfusion pressures of 80 and 180 mm Hg, GFR and RBF vary less than 10% (Fig. 2-7). Flow (Q) is equal to pressure (P) divided by resistance (R). As pressure increases, flow can remain constant only if resistance increases proportionately. The site of this resistance change in the kidneys is the afferent arteriole. Autoregulation is intrinsic to the kidneys and occurs in the isolated, denervated kidney and in the adrenalectomized animal. However, it is impaired by anesthesia in proportion to the depth of anesthesia. The afferent arterioles are maximally dilated at mean arterial pressures of 70 to 80 mm Hg, and at lower pressures, GFR declines linearly with RBF (i.e., autoregulation is lost). It is likely that autoregulation of RBF is a consequence of the need to regulate GFR closely and thus maintain tight control over water and salt balance.Two physiologic mechanisms contribute to autoregulation. The myogenic mechanism is based on the principle that smooth muscle tends to contract when stretched and relax when shortened. As the afferent arteriole is stretched by increased perfusion pressure, it constricts, thus limiting transmission of this increased pressure to the glomerulus and minimizing any change in glomerular capillary hydrostatic pressure and SNGFR. The myogenic mechanism represents a coarse control that operates with a delay of 1 to 2 seconds.
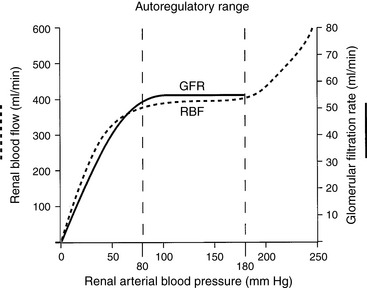
Figure 2-7 Autoregulation of renal blood flow and glomerular filtration rate.
(Drawing by Tim Vojt.)
Tubuloglomerular feedback represents a local intrarenal negative feedback mechanism for individual nephrons. The morphologic basis for this physiologic mechanism is the JGA. Increased sodium chloride concentration or transport in the distal tubule is sensed by the extraglomerular mesangial cells of the JGA as they monitor sodium chloride transport across the tubular cells of the macula densa. Transport of NaCl by the tubular cells of the macula densa requires functional NKCC2 (the Na+, K+, 2Cl- cotransporter) and ROMK (a potassium channel) in the luminal membranes and functional Na+, K+-ATPase in the basolateral membranes.46 Transcellular transport of NaCl causes generation of adenosine, which together with angiotensin II causes afferent arteriolar constriction in the parent glomerulus. The afferent arteriolar constriction causes SNGFR to decrease, thus decreasing filtration and minimizing NaCl loss in that nephron. This effect occurs locally in the region of the juxtaglomerular interstitium. Tubuloglomerular feedback represents a fine control that operates with a 10- to 12-second delay.
Measurement of renal blood flow and renal plasma flow
Consider the following mass balance equation52:
Amount entering the kidneys = amount leaving the kidney
where PAX is the renal arterial plasma concentration of x, RPFA is the arterial renal plasma flow (RPF), PVX is the renal venous plasma concentration of x, RPFV is the venous RPF, UX is the urine concentration of x, and V is the urine flow.
If we ignore the slight difference between renal arterial and venous plasma flow (with probably less than 1% error), the equation is simplified to:
If we choose a substance that is completely removed from the blood in one pass through the kidneys, PVX is zero and RPF = UX V/PAX. If the substance x is not metabolized and is not excreted by any organ other than the kidneys, its concentration in any peripheral vessel equals PAX. Thus, RPF = UXV/PX.
PAH is filtered by the glomeruli and secreted by the peritubular capillaries into the tubules so that approximately 90% of it is removed in one pass through the kidneys. It is not metabolized or excreted by any other organ. Thus, it approximately meets the preceding assumptions and RPF = UPAHV/PPAH. Now it can be seen that the clearance of PAH is an estimate of RPF. When PAH is infused during a clearance study, it is essential that PPAH be maintained at a concentration much below the tubular transport maximum (Tmax) for PAH. If not, PVX cannot be neglected.
Some blood flows through regions of the kidneys that do not remove PAH (e.g., renal capsule, perirenal fat, and renal pelvis), and as a result, PVX is not really zero. Thus, the term effective RPF is more appropriately used when speaking of PAH clearance. Furthermore, only 90% of PAH is removed from the blood during a single pass through the kidneys. This also contributes to the fact that PVX for PAH is not really zero. A closer approximation of RPF can be determined by sampling renal arterial and venous blood and measuring their respective PAH concentrations. The extraction ratio for PAH is then determined:
A more accurate calculation of RPF is then:
The extraction ratio for PAH is 0.9 because approximately 90% of it is removed from the blood in a single pass through the kidneys. Notice that if we substitute the equation for EX into the preceding equation, we get RPF = UXV/(PAX − PVX), which is the same equation as derived before for RPF.
Another way to determine RPF is by use of the Fick principle, which states that the amount of a substance (V) removed by an organ is equal to the blood flow to the organ (Q) times the arteriovenous concentration difference of the substance in question (CA − CV):
Using the kidneys as an example and equating the amount of the substance removed to the amount excreted (UXV):
Note that this equation is identical to that derived before using the mass balance principle.
If the hematocrit is known, RBF can be calculated from the RPF by using the following equation:
In the dog and cat, normal values for RPF are 7 to 20 mL/min/kg and 8 to 22 mL/min/kg, respectively.38,39,45
If all of the plasma were filtered in one pass of blood through the glomeruli, an immovable mass of red blood cells would be all that remained behind at the efferent arteriole of the glomerular capillary. This does not occur because πGC increases along the length of the capillary and, in conjunction with PT, effectively opposes further filtration. The filtration fraction is the fraction of plasma flowing through the kidneys that is filtered into the Bowman space. It is determined by the following equation:
In the dog and cat, values for FF are 0.32 to 0.36 and 0.33 to 0.41, respectively. These values are higher than those observed in humans in whom FF is approximately 0.20.
Renal tubular function
The terms reabsorption and secretion refer to the direction of transport across an epithelium. In the kidneys, reabsorption refers to movement of water and solutes from the tubular lumen to the peritubular interstitium. Secretion refers to movement of water and solutes from the peritubular interstitium to the tubular lumen. Some substances experience reabsorption in one part of the nephron and secretion in another part (e.g., urate and potassium). The term reabsorption often is used to denote net reabsorption, which is the algebraic sum of the fluxes in both directions across the renal tubular epithelium.
The luminal membranes separate the cytoplasm of the tubular cell from the tubular fluid. The basolateral membranes separate the cytoplasm of the tubular cell from the lateral intercellular spaces and the peritubular interstitium. The transmembrane potential difference (PD) refers to the electrical PD between the outside and inside of the cell. The transepithelial or transtubular PD is the electrical PD between the tubular lumen and the peritubular interstitium and is the algebraic sum of the transmembrane PD between the tubular lumen and cell cytoplasm, and the transmembrane PD between the peritubular interstitium and cell cytoplasm. These relationships are depicted in Figure 2-8. Transmembrane PD usually is −60 to −70 mV (cell interior negative), whereas transepithelial PD is only a few millivolts. In the early proximal tubule, the tubular lumen is a few millivolts negative relative to the peritubular interstitium, whereas in the later proximal tubule, the tubular lumen is a few millivolts positive relative to the peritubular interstitium. In the thick ascending limb of Henle’s loop, the transepithelial PD is lumen positive, but in the distal tubule, the transepithelial PD is lumen negative. The transepithelial PD affects movement of charged solutes across the renal tubular epithelium and contributes to the electrochemical gradient for such solutes.The paracellular route refers to movement of solutes and water between cells (i.e., from the tubular lumen to the lateral intercellular space across tight junctions connecting epithelial cells). The transcellular route refers to movement of solutes and water through the cytoplasm of the tubular cells. The junctions between renal epithelial cells at the luminal surface are classified as leaky (proximal tubules) or tight (distal convoluted tubules, collecting ducts). Leaky epithelia do not generate large transepithelial concentration gradients, exhibit a small transepithelial PD, and have high water permeability, whereas tight epithelia can generate large transepithelial concentration gradients, exhibit a large transepithelial PD, and have low basal water permeability. The paracellular route allows movement of ions (e.g., potassium, chloride) and large, nonpolar solutes by passive diffusion and solvent drag. Electrochemical, hydrostatic, and oncotic gradients are important driving forces for reabsorption by the paracellular route. The paracellular route accounts for only 1% of the surface area available for reabsorption and 5% to 10% of water transport, whereas the transcellular route accounts for 99% of the available surface area and 90% to 95% of water transport. Both passive and active transport processes occur by the transcellular route, and all active transport processes must occur by this route.
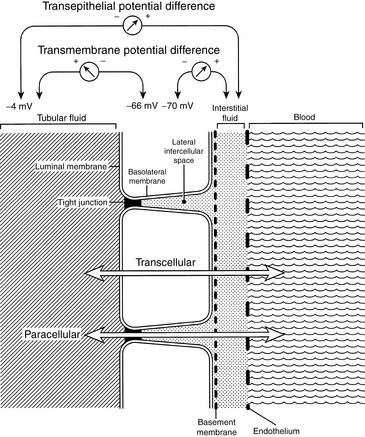
Figure 2-8 Diagram demonstrating selected terminology as applied to the renal tubular epithelium: luminal versus basolateral membranes, transmembrane versus transepithelial potential difference, and transcellular versus paracellular transport.
(Drawing by Tim Vojt.)
That renal tubular reabsorption occurs may be recognized intuitively by considering the composition of normal urine. Many low-molecular-weight solutes essential to normal physiologic function (e.g., glucose, amino acids, bicarbonate) are freely filtered at the glomerulus but do not normally appear in urine. Thus, they must have been reabsorbed along the course of the renal tubule. In the proximal tubule, water follows solute reabsorption osmotically, and solute reabsorption is said to occur isosmotically (i.e., the reabsorbed fluid has the same osmolality as extracellular fluid). Approximately two thirds of all water and solute reabsorption occurs in the proximal tubules. Almost 99% of glucose and amino acids and 90% or more of bicarbonate are reabsorbed in the early proximal tubules (Fig. 2-9). The reabsorption of bicarbonate occurs as a consequence of the tubular secretion of hydrogen ions and is crucial to renal regulation of acid-base balance (see Chapter 9).
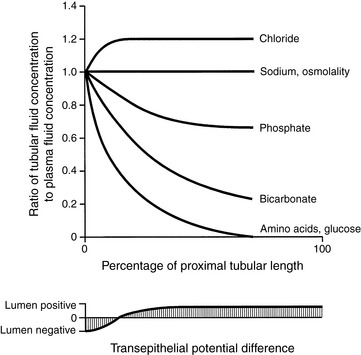
Figure 2-9 Changes in the solute composition and transepithelial potential difference along the length of the proximal nephron.
(Drawing by Tim Vojt.)
Renal transport processes
Four types of transport processes contribute to renal tubular reabsorption: passive diffusion, facilitated diffusion, primary active transport, and secondary active transport.
Passive diffusion is the movement of a substance across a membrane as a result of random molecular motion. Simple diffusion can take place directly through the lipid bilayer of the cell membrane, which occurs for substances with high lipid solubility. Simple diffusion can also occur through hydrophilic protein channels embedded in the cell membrane. Simple diffusion requires no expenditure of metabolic energy. The rate of transfer of solute is dependent on the permeability characteristics of the membrane, the electrochemical gradient (i.e., the combination of the electrical PD and chemical concentration difference across the membrane), and the hydrostatic pressure across the membrane. The rate of diffusion is linearly related to the concentration of the diffusing solute, and there is no maximal rate of transfer (Vmax). Passive diffusion is not a saturable process because a carrier is not involved.
Facilitated diffusion is the movement of a substance across a membrane down its electrochemical gradient after binding with a specific carrier protein in the membrane. The carrier protein binds the substance to be transported at one side of the cell membrane. The occupied carrier then undergoes a conformational change that causes translocation of the substance across the cell membrane. The substance is then released from the carrier on the other side of the membrane. Unlike simple diffusion, facilitated diffusion is a saturable process characterized by a maximal rate of transfer (Vmax) because a carrier is involved. The carrier has structural specificity and affinity for the substance transported, and the process is subject to competitive inhibition. Facilitated diffusion does not directly require metabolic energy, and transfer may occur in either direction across the membrane, depending on the prevailing electrochemical gradient. Examples of facilitated diffusion in the proximal tubule include the transport of glucose and amino acids at the basolateral membrane.
Primary active transport is the movement of a substance across a membrane in combination with a carrier protein but against an electrochemical gradient. Active transport requires metabolic energy, which is supplied by the hydrolysis of adenosine triphosphate (ATP). It is a saturable process characterized by a Vmax and is subject to metabolic (e.g., cellular oxidative poisons) and competitive (e.g., competition for the carrier by a structurally similar compound) inhibition. Examples of primary active transporters include Na+, K+-adenosinetriphosphatase (Na+, K+-ATPase) in basolateral membranes of tubular cells throughout the nephron, H+-ATPase in luminal membranes of tubular cells throughout the nephron, and H+, K+-ATPase in luminal membranes of α-intercalated cells in the collecting ducts.
Secondary active transport is the movement of two substances across a membrane after combination with a single carrier protein. The process is called cotransport if the transported substances are moving in the same direction across the membrane (e.g., glucose, amino acids, or phosphate with sodium at the luminal membrane of the proximal tubular cell) and countertransport if the transported substances are moving in opposite directions across the membrane (e.g., sodium and hydrogen ions at the luminal membrane of the proximal tubular cell). The “uphill” (i.e., against a concentration gradient) transport of one substance (e.g., glucose) is linked to the “downhill” (i.e., down an electrochemical gradient) transport of another substance (e.g., sodium). When the carrier is occupied by only one of the substances, it is not mobile in the cell membrane, whereas an unoccupied carrier or one that is occupied by both of the substances is mobile in the membrane. This process is saturable, demonstrates structural specificity and affinity of the carrier for the substances transported, and may be competitively inhibited. The uphill transport occurs without direct input of metabolic energy, and the substance transported uphill is said to experience secondary active transport. The metabolic energy for secondary active transport at the luminal membranes comes from the primary active transport of sodium out of the tubular cell at the basolateral membrane by Na+, K+-ATPase, a process that maintains a low intracellular sodium concentration.
Pinocytosis refers to the uptake by cells of particles too large to diffuse through the cell membrane. Filtered proteins are reabsorbed in the proximal tubule by this mechanism.
Solvent drag refers to the process, whereby water (the solvent) moving across an epithelium by osmosis can drag dissolved solutes along with it.
Morphology of the proximal tubule
Several morphologic features of proximal tubular cells suggest their primary role in the reabsorption of solutes and water. The brush border of the luminal surface of the proximal tubular cells consists of microvilli, which increase surface area, and lateral cellular interdigitations, which increase the surface area of the basolateral membranes (Fig. 2-10). Abundant mitochondria supply energy in the form of ATP required for active transport.
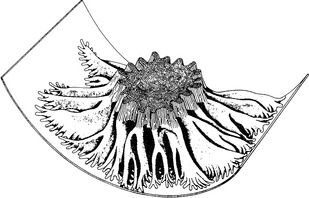
Figure 2-10 Three-dimensional model of a proximal tubular cell showing microvilli and lateral cellular interdigitations.31
The proximal tubule exhibits intrasegmental axial heterogeneity with the most proximal segments being ultrastructurally the most complex and suited for the mechanisms of solute transport described earlier.31 This morphologic complexity decreases along the length of the proximal tubule. In the first segment of the proximal tubule (S1), sodium, water, bicarbonate, amino acids, glucose, and phosphate are transported. In the second segment (S2), sodium, water, and chloride are reabsorbed, and organic acids and bases may be transported.43 Organic acids and bases may also be secreted in the third segment (S3).31 The low-specificity transport system for organic anions and cations in the proximal tubule allows elimination of many drugs and other foreign organic compounds from the body.
Sodium transport
Sodium may enter tubular cells at their luminal surface by several different mechanisms. In the proximal tubule, sodium may be cotransported across the luminal membranes of the cell with glucose, amino acids, or phosphate or may experience countertransport with hydrogen ions secreted into the tubular lumen by the Na+-H+ antiporter that facilitates bicarbonate reabsorption. In the loop of Henle, sodium enters via an Na+-K+-2Cl −carrier that is competitively inhibited by furosemide,37 and in the distal convoluted tubule, sodium enters via an Na+ Cl− cotransporter that is inhibited by thiazide diuretics. In the collecting duct, sodium enters via a luminal sodium channel that generates a lumen-negative PD favoring chloride reabsorption.
Thus, in most segments of the nephron, sodium enters the tubular cell at the luminal membrane down an electrochemical gradient that favors sodium entry into the cell (i.e., the interior of the cell has a low sodium concentration and is negative with respect to the exterior). Sodium then experiences primary active transport out of the cell and into the lateral intercellular spaces and peritubular interstitium by the Na+, K+-ATPase located in the basolateral cell membranes. This enzyme hydrolyzes ATP and translocates two potassium ions into the cell and three sodium ions out of the cell.1 It is located only in the basolateral membranes and functions to maintain a favorable electrochemical gradient for the passive entry of sodium into the tubular cells across their luminal membranes. Thus, sodium is reabsorbed in conjunction with glucose, amino acids, phosphate, and bicarbonate in the proximal tubule and with chloride in the loop of Henle and distal tubule. The different mechanisms for sodium reabsorption in the nephron and the regulation of sodium reabsorption in the kidneys are discussed in Chapter 3.
Glucose transport
Sodium attaches to a carrier in the luminal membrane of the proximal tubular cell, and this step is followed by attachment of glucose to the carrier. Translocation of the carrier occurs, and glucose is released to the interior of the cell while sodium enters down its electrochemical gradient (the interior of the cell is negative and its sodium concentration is low). As the intracellular glucose concentration increases, glucose leaves the cell by facilitated diffusion across the basolateral cell membranes. The Na+, K+-ATPase in the basolateral membranes continues to remove sodium from the cell, thus maintaining a favorable electrochemical gradient for sodium entry and expending the metabolic energy required for glucose transport. Luminal uptake of glucose is mediated by at least two transporters, a high-capacity, low-affinity transporter (SGLT2) present in the first portion of the proximal tubule (S1 and S2) and a low-capacity, high-affinity transporter (SGLT1) later in the proximal tubule (S3).53
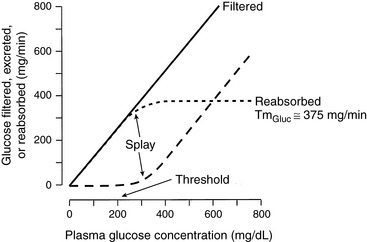
Glucose transport meets the criteria for carrier-mediated transport in that it is a saturable process. Plotting the amount filtered (Px × GFR), the amount excreted (Ux × V), and the amount handled by the tubules (Tx) for a substance against the plasma concentration of that substance (Px) yields a renal titration curve and allows determination of the renal threshold (plasma concentration at which the substance first appears in the urine) and tubular transport maximum (maximal amount of the substance that can be transported by the tubules, Tmax or TM). A renal titration curve for glucose is depicted in Figure 2-11. The Tmax for glucose is constant and relatively high, so it is usually not exceeded in health. Consequently, the kidneys do not regulate plasma glucose concentration. In humans, the Tmax for glucose is approximately 375 mg/min. In the dog, it is approximately 100 mg/min,27,47 and in the cat, 50 mg/min.32 In the renal titration curve, the Tmax for glucose is approached somewhat gradually. This characteristic is called splay and is thought to result from nephron heterogeneity. Some nephrons excrete glucose before the average Tmax is reached, whereas others continue to reabsorb glucose after the average Tmax has been reached (i.e., the Tmax for glucose differs slightly among nephrons).
Phosphate
The uptake of phosphate into the proximal tubular cell is similar to that of glucose in that it is coupled to sodium entry at the luminal membrane. The phosphate transporters NaPi-IIa and NaPi-IIc are responsible for luminal entry of phosphate in the proximal tubule.3 An important distinction from glucose transport, however, is that the Tmax for phosphate is low and readily exceeded as plasma phosphate concentration increases. Hormones also alter the Tmax for phosphate, notably parathyroid hormone (PTH). PTH decreases the Tmax for phosphate and increases renal phosphate excretion. Thus, the kidneys, acting in concert with PTH, serve as regulators of the plasma phosphate concentration.
Amino acids
The proximal tubular reabsorption of amino acids is also coupled to luminal sodium uptake. The Tmax values for the different groups of amino acids are very high, and 99% of the filtered load of amino acids is reabsorbed in the proximal tubule. Thus, the kidneys are not regulators of plasma amino acid concentrations. There are several transport systems for amino acids in the proximal tubule, including systems for neutral amino acids, cationic amino acids and cystine, anionic amino acids, imino glycine acids (proline, hydroxyproline, glycine), and ß-amino acids (e.g., taurine).6,52
Pinocytosis
Low-molecular-weight proteins (including several hormones and immunoglobulin light chains) are filtered at the glomerulus and reabsorbed by the proximal tubular cells, where they are hydrolyzed to their constituent amino acids, and these are returned to the circulation. Filtered proteins of small molecular mass may be hydrolyzed to amino acids by brush border enzymes at the luminal surface of the proximal tubular cell and their amino acids taken into the cell by cotransport with sodium. Alternatively, filtered proteins of larger molecular mass may attach to endocytic sites on the luminal cell membrane. These sites invaginate to form endosomes, which then fuse with lysosomes to form endolysosomes, in which digestion of the proteins occurs. The amino acids leave the endolysosomes and cross the basolateral membranes of the tubular cells by facilitated diffusion. This endocytic mechanism has a very high capacity, which is not normally exceeded in health.
Urea
Urea is passively reabsorbed in the proximal tubules, depending on tubular flow rate. Increased tubular flow, as occurs during diuresis, is the result of decreased reabsorption of water from the tubular fluid. This decreases the tubular fluid urea concentration and decreases the concentration gradient of urea across the tubular epithelium. Thus, less urea is reabsorbed at higher tubular flow rates. With decreased tubular flow, as occurs during dehydration, there is increased reabsorption of water from the tubular fluid. This increases the concentration gradient of urea across the tubular epithelium and increases passive urea reabsorption. In dehydrated patients, increased reabsorption of urea may lead to an increase in blood urea nitrogen (BUN) even before GFR is decreased. This contributes to the observation that the BUN/creatinine ratio tends to be higher in patients with prerenal azotemia than in hydrated patients with primary renal azotemia.
The renal handling of urea plays an important role in the urinary concentrating mechanism (see role of urea in The Urinary Concentrating Mechanism section). Discovery of facilitated urea transporters (UT-A and UT-B) in the kidneys has enhanced understanding of urea recycling and called into question the “passive model” of urinary concentration.15,49,54 Vasopressin (ADH)-responsive urea transporters UT-A1 and UT-A3 in the inner medullary collecting duct facilitate urea reabsorption and concentration in the interstitium, where it theoretically serves as a stimulus for passive NaCl reabsorption from the thin ascending limb of Henle’s loop. According to the “passive model” of urinary concentration,30,48 knockout mice lacking UT-A1 and UT-A3 should have impaired ability to concentrate NaCl in the inner medulla, but this does not appear to be true. Such mice have lower urea but not lower NaCl concentrations in the inner medullary interstitium, a finding inconsistent with the “passive model.15”
Urea reabsorbed from the inner medullary collecting duct via AT-A1 and AT-A3 can reenter the thin descending limb of Henle’s loop via UT-A2 and be carried back to the collecting duct. This urea is concentrated in the collecting ducts as water is reabsorbed, setting the stage for urea to be reabsorbed again back into the inner medullary interstitium via UT-A1 and UT-A3 under the influence of ADH. Reabsorbed urea enters the ascending (venous) vasa recta and then is transferred to the descending (arterial) vasa recta, which express UT-B. This recycling of urea prevents the osmotic diuresis that would occur if this urea load were excreted in the urine.
Knockout mice lacking UT-A2 do not have a reduction in medullary urea concentration or decreased urinary concentrating ability when fed a normal protein diet, whereas knockout mice lacking UT-B do have decreased medullary urea concentration, as well as decreased urinary concentrating ability and higher BUN concentrations.15 These results suggest that countercurrent exchange of urea between the ascending (venous) vasa recta and descending (arterial) vasa recta is more important for urea trapping in the inner medulla than is transfer of urea to the thin descending limbs of Henle’s loop.
The urinary concentrating mechanism
Urinary concentration is a function of the juxtamedullary nephrons with long loops of Henle that penetrate deep into the renal medulla. There are two main steps in this process. First, transport of sodium chloride without water from the ascending limb of Henle’s loop renders the medullary interstitium hyperosmotic. Second, vasopressin (ADH) increases the water permeability of the collecting duct, and tubular fluid traversing this segment of the nephron equilibrates osmotically with the hyperosmotic interstitium.
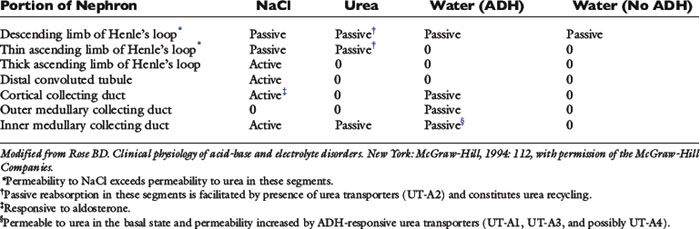
Strikingly different transport properties of various portions of the nephron form the basis for understanding the urinary concentrating mechanism (Table 2-2). The hairpin configuration of Henle’s loop is the anatomic basis for countercurrent multiplication and allows a single osmotic effect to be multiplied over the length of the loop. The vessels accompanying the loops of Henle into the medulla are called vasa recta. They prevent dissipation of the medullary osmotic gradient by a process called countercurrent exchange (see Role of the Vasa Recta section). The countercurrent multiplier concept was first applied to urine concentration by W. Kuhn, a physical chemist, in 1942.2,25 As early as 1909, however, K. Peter had noted a correlation between the length of Henle’s loop and the ability of a given species to concentrate its urine.
Role of the ascending limb of henle’s loop
The ascending limb of Henle’s loop is impermeable to water. Sodium chloride is actively transported from the thick portion of the ascending limb without accompanying water so that an osmotic gradient of approximately 200 mOsm/kg is generated. This active transport of sodium chloride is the primary energy-requiring step of the urinary concentrating mechanism.
Active sodium transport is accomplished by the Na+, K+-ATPase located in the basolateral membranes of the tubular cells. This enzyme maintains a low intracellular concentration of sodium and promotes passive entry of sodium at the luminal membrane down a concentration gradient. The luminal Na+, K+, 2Cl- carrier (NKCC2) binds one sodium ion, one potassium ion, and two chloride ions.37 Chloride delivery is the rate-limiting step in this transport process, and loop diuretics such as furosemide impair distal sodium reabsorption by competing with chloride for the luminal carrier.37
Fluid reaching the distal convoluted tubule is hypoosmotic (100 mOsm/kg) compared with the fluid entering the descending limb of Henle’s loop (300 mOsm/kg). If fluid in the loops were stationary, the active transport of sodium chloride out of the thick ascending limb without water would increase the interstitial osmolality to 400 mOsm/kg and decrease the osmolality of the fluid within the ascending limb to 200 mOsm/kg. The descending limb of Henle’s loop is highly permeable to water, and water would be extracted from this site, increasing the osmolality of the tubular fluid in this segment of the nephron to 400 mOsm/kg.
However, the fluid within Henle’s loops is not stationary. New tubular fluid with an osmolality of 300 mOsm/kg is constantly entering the descending limb of Henle’s loop from the proximal tubule. As fluid continues to move through the loops and an osmotic gradient of 200 mOsm/kg is generated, this single osmotic effect is multiplied over the length of Henle’s loop (Fig. 2-12). The magnitude of the gradient from the beginning of the loop to its hairpin turn is a function of the length of the loop itself. Thus, the vertical osmotic gradient greatly exceeds the horizontal gradient at any given level. This is the countercurrent multiplier concept of urinary concentration.
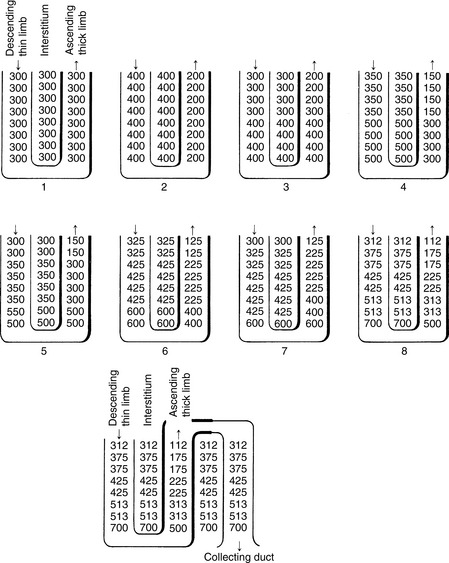
Figure 2-12 Stepwise operation of the countercurrent multiplier mechanism of urinary concentration. Numbers refer to osmolalities (mOsm per kg H2O) of tubular fluid and interstitium.
(Reprinted with permission from Valtin H. Renal function: mechanisms preserving fluid and solute balance in health, 2nd ed. Boston: Little, Brown, 1983: 166.)
Roles of the collecting ducts and antidiuretic hormone
The collecting duct is divided into three segments: the cortical collecting duct, outer medullary collecting duct, and inner medullary collecting duct. These segments differ in their permeability to sodium and urea (see Table 2-2). The main role of the cortical collecting duct is delivery of fluid with a very high urea concentration to the outer medullary collecting duct. This occurs because sodium chloride and water are removed from this segment of the nephron, but urea is not. The main functions of the inner medullary collecting duct are to add urea to the inner medullary interstitium and to produce maximally concentrated urine by osmotic equilibration of tubular fluid with the hyperosmotic interstitium under the influence of ADH.9,29 This segment of the nephron is permeable to urea, and its urea permeability is increased by ADH (see previous section on urea).
As just described, fluid entering the distal tubule is hypoosmotic to plasma (approximately 100 mOsm/kg). Without the collecting duct, the so-called countercurrent multiplier would dilute tubular fluid. In the presence of ADH, this hypoosmotic fluid equilibrates osmotically with the cortical interstitium (osmolality, 300 mOsm/kg) as the tubular fluid flows through the cortical collecting duct. By this process, approximately two thirds of the tubular water is removed before delivery to the medullary collecting duct. For example, 100 mOsm of solute in 1 L of tubular fluid is reduced to 100 mOsm of solute in 0.33 L of tubular fluid (300 mOsm/kg) with 0.67 L of water reabsorbed. Even more water can be reabsorbed, depending on how much active sodium reabsorption occurs in the cortical collecting duct in response to aldosterone stimulation. These effects markedly reduce fluid delivery to the medullary collecting duct. Tubular fluid entering the medullary collecting duct is thus isosmotic with plasma but much reduced in volume. It is in the medullary collecting duct that the final concentration of urine occurs.
The water permeability of the epithelium of the collecting duct is dependent on the action of ADH. In the presence of ADH, water is removed from the collecting duct as the fluid osmotically equilibrates with a progressively hyperosmotic medullary interstitium, and the final osmolality of the urine may approximate that of the papillary interstitium. In humans, this maximal urine osmolality is 900 to 1400 mOsm/kg.44 In dogs and cats, however, urine osmolality can approach 2800 and 3000 mOsm/kg, respectively.24,45 Water reabsorption in the distal convoluted tubule and connecting tubule is minimal because of their relative impermeability to water, regardless of the presence or absence of ADH. Thus, water reabsorption in the cortical collecting duct under the influence of ADH is important in reducing the fluid load delivered to the medullary collecting duct.
In the absence of ADH, the collecting duct is impermeable to water. The fluid entering this portion of the nephron has an osmolality of approximately 100 mOsm/kg. Under these conditions, additional sodium chloride without water is removed from the tubular fluid during its course through the cortical collecting duct and inner medullary collecting duct so that the final urine osmolality can be as low as 50 mOsm/kg. However, the outer medullary collecting duct is impermeable to sodium.
Even in the absence of ADH, urine osmolality may be greater than 50 mOsm/kg if the animal is dehydrated. The GFR is decreased by dehydration, and there is an increase in the proximal tubular reabsorption of sodium chloride and water. Less tubular fluid reaches the distal nephron, and urine osmolality can approach 400 mOsm/kg.50
Role of the vasa recta
If the water removed from the medullary collecting duct in the presence of ADH were allowed to remain in the medullary interstitium, the hyperosmotic gradient would dissipate rapidly. However, this does not occur because of the countercurrent exchange function of the vasa recta. Plasma in the vasa recta entering the medulla from the cortex encounters an increasingly hyperosmotic medullary interstitium. As a result, water is removed from the vessels and solutes (e.g., sodium chloride and urea) enter the vessels. After passing the hairpin turn of the loop, the vasa recta climb back toward the renal cortex. Now they encounter a medullary interstitium of progressively decreasing osmolality so that water enters the vessels and solutes are removed. In this way, water is removed from and solutes are recycled back into the medullary interstitium, thus preventing dissipation of the osmotic gradient. This process is known as countercurrent exchange. That the vasa recta can effectively remove water and recycle solute may be appreciated by considering the different flow rates in the vasa recta and medullary collecting duct. Although only 5% of RPF goes to the renal medulla, this flow is much greater than the approximately 3% of GFR that enters the medullary collecting ducts. Consider, for example, a 10-kg dog with a GFR of 4 mL/min/kg and an RPF of 12 mL/min/kg. RPF in the medulla would be 6 mL/min (5% of 120), and tubular fluid flow in the renal medulla would be 1.2 mL/min (3% of 40), a fivefold difference. These factors contribute to the effective removal of water from the medullary interstitium and prevent dissipation of the osmotic gradient in this region of the kidneys.
Role of urea
Although there is evidence for active transport of sodium chloride from the thick ascending limb of Henle’s loop, active transport has not been demonstrated in the thin descending and ascending limbs. A two-solute model of the urinary concentrating mechanism was developed simultaneously in 1972 by Stephenson and by Kokko and Rector.26,30,48 This model requires an important contribution by urea as the second solute.
The thin descending limb of Henle’s loop has a low passive permeability for sodium chloride and limited permeability to urea (except in segments that express UT-A2), but it is highly permeable to water. The permeability of the inner medullary collecting duct to urea is enhanced by ADH via UT-A1, UT-A3, and possibly UT-A4. The distal convoluted tubule, cortical collecting duct, and outer medullary collecting duct are relatively impermeable to urea, even in the presence of ADH. Thus, the urea concentration of tubular fluid increases markedly in this portion of the nephron.
During a state of water conservation (i.e., antidiuresis), the plasma ADH concentration is high. More urea is removed from the inner medullary collecting duct and enters the medullary interstitium. In dogs, urea constitutes more than 40% of the total medullary solute concentration during antidiuresis (after 24 hours of water deprivation) but less than 10% during water diuresis.8,33
Urea increases medullary interstitial osmolality without a change in the sodium concentration in this region. Thus, water is removed osmotically from the thin descending limb of Henle’s loop by the high concentration of urea in the medullary interstitium. The sodium concentration of the tubular fluid in the descending limb of Henle’s loop eventually exceeds the medullary interstitial sodium concentration because the thin descending limb of Henle’s loop has a low permeability for sodium. The sodium permeability of the thin ascending limb of Henle’s loop is high, and as the tubular fluid rounds the hairpin turn and enters this portion of the nephron, sodium can be removed passively into the medullary interstitium down a concentration gradient (Fig. 2-13). Recent findings in knockout mice lacking UT-A1 and UT-A3 have raised questions about the “passive model” of sodium chloride concentration in the inner medulla (see previous discussion).
Endocrine functions of the kidneys
The kidneys are responsible for endocrine functions that play essential roles in the regulation of red cell production by the bone marrow, defense of the extracellular fluid volume (ECFV), and maintenance of calcium homeostasis. Gradual loss of these endocrine functions occurs during the progression of chronic renal disease and contributes to specific manifestations of the uremic syndrome, such as nonregenerative anemia, systemic hypertension, and renal secondary hyperparathyroidism.
Erythropoietin production
Erythropoietin (EPO) is a glycoprotein hormone with a molecular mass of 35,000 Da that stimulates red blood cell production by the bone marrow. In the fetus, EPO is produced in the liver, but shortly after birth production switches to the kidneys, which become the major source of EPO in the adult animal. Decreased oxygen delivery to the kidneys is the major stimulus for EPO production. An oxygen sensor (thought to be a heme protein) detects decreased oxygen tension and activates transcriptional factors that increase transcription of the EPO gene. Peritubular interstitial fibroblasts in the renal cortex and outer medulla are the primary site of EPO synthesis in the kidneys.35 EPO binds to receptors on erythroid progenitor cells in the bone marrow preventing apoptosis, and allows them to proliferate and differentiate into reticulocytes.13,23
Absolute or relative deficiency of EPO is the primary cause of the anemia of chronic renal failure.28 Recombinant human EPO has been used successfully to correct the anemia of chronic renal failure in human patients.14 Although initially effective in correcting the anemia of renal failure in dogs and cats, use of recombinant human EPO is associated with antibody formation in up to 50% of treated dogs and cats after 1 to 3 months of treatment.11 The resulting anemia can be more severe than that present before treatment because the induced antibodies can cross-react with the animal’s native EPO. The canine EPO gene has been isolated,34 and recombinant canine EPO has been used to stimulate erythropoiesis in normal dogs42 and in those with naturally occurring chronic renal failure.41 It is not as effective when used in dogs that have developed red cell aplasia from previous treatment with recombinant human EPO. Recombinant feline EPO also has been synthesized and used effectively to treat cats with anemia of chronic renal failure.40 Unexpectedly, some cats that initially responded to recombinant feline EPO later developed anemia that was refractory to additional treatment with recombinant feline EPO.
Renin-angiotensin system
The main role of the renin-angiotensin system (RAS) is defense of the ECFV via sodium homeostasis. The role of the kidneys in maintenance of sodium balance is discussed further in Chapter 3.
Renin is an enzyme synthesized and stored in the granular cells of the JGA (specialized smooth muscle cells in the afferent arterioles). The kidneys are the most important source of renin, but renin is also found in many other tissues (e.g., vascular endothelium, adrenal gland, and brain). Local production of angiotensin II in some tissues may be important in the regulation of local processes without having a systemic effect. The RAS of the brain may be involved in control of systemic blood pressure, secretion of ADH, catecholamine release, and thirst.
There are three major stimuli for renin release. Decreased renal perfusion pressure caused by systemic hypotension (pressure below 80 to 90 mm Hg) or ECFV depletion is sensed in the afferent arterioles by the granular cells, which increase their secretion of renin. Stimulation of cardiac and arterial baroreceptors by systemic hypotension leads to increased sympathetic neural activity and increased concentrations of circulating catecholamines, which in turn stimulate renin release via β1-adrenergic receptors on granular cells. Lastly, changes in distal tubular flow and delivery of chloride affect renin release. Decreased ECFV or chronic NaCl depletion decreases distal tubular flow and delivery of chloride to the macula densa (partly as a consequence of enhanced proximal reabsorption of water and NaCl), which in turn stimulates renin release. Expansion of the ECFV or NaCl loading increases distal tubular flow and delivery of chloride to the macula densa, which inhibits renin release. The release of renin is inhibited by a direct effect of angiotensin II on the granular cells, which constitutes a negative feedback loop.
Renin converts the α2-globulin angiotensinogen (which is synthesized and released by the liver) to angiotensin I, and this is the rate-limiting step of the RAS cascade. Angiotensin-converting enzyme is found in vascular endothelium and cleaves the carboxyl-terminal (C-terminal) two amino acids from the inactive decapeptide angiotensin I to yield the active octapeptide angiotensin II. This step in the RAS cascade is not rate limiting, and most of the angiotensin I is rapidly converted to angiotensin II.
The effects of angiotensin II restore ECFV. Angiotensin II causes arteriolar vasoconstriction in many organs (renal, splanchnic, and cutaneous vascular beds are most sensitive), which increases systemic blood pressure. It enhances the sensitivity of vascular smooth muscle to and facilitates the release of norepinephrine from the adrenal medulla and sympathetic nerve terminals, thus secondarily affecting systemic blood pressure. Angiotensin II causes increased proximal tubular reabsorption of sodium by stimulating the Na+-H+ antiporter in luminal membranes of proximal tubular cells. It causes increased secretion of aldosterone from the zona glomerulosa of the adrenal cortex, and aldosterone in turn causes increased reabsorption of sodium chloride in the cortical collecting duct. Lastly, angiotensin II causes alterations in glomerular and postglomerular hemodynamics that enhance sodium and water reabsorption. Angiotensin II causes constriction of the efferent and afferent arterioles, an effect thought to be mediated by thromboxane A2. The efferent arteriole constricts more than the afferent so that the FF increases (i.e., RPF decreases more than GFR). Renal hemodynamic changes favoring salt and water reabsorption occur in the postglomerular capillary beds secondary to these glomerular hemodynamic changes. These changes include decreased peritubular capillary hydrostatic pressure and increased peritubular capillary oncotic pressure. Angiotensin II can cause glomerular mesangial cells to contract, potentially reducing the surface area for filtration and decreasing the ultrafiltration coefficient, Kf. Angiotensin II stimulates release of vasodilator prostaglandins (e.g., PGE2 and PGI2) from glomeruli. By this mechanism, the potentially harmful vasoconstrictive effects of angiotensin II on the kidneys are minimized.
Activation of vitamin D
Vitamin D3 (cholecalciferol) is obtained in the diet or by ultraviolet irradiation of the compound 7-dehydrocholesterol in the skin. The liver hydroxylates cholecalciferol to 25-hydroxycholecalciferol, which is the predominant form of vitamin D3 in plasma. In the kidneys, 25-hydroxycholecalciferol is converted to the active form of vitamin D3, 1,25-dihydroxycholecalciferol (calcitriol), by the enzyme 25-hydroxycholecalciferol-1a-hydroxylase, which is found in the mitochondria of the proximal tubular cells. Calcitriol interacts with its high-affinity receptor (the vitamin D receptor [VDR]) in target tissues forming a ligand-activated transcription factor that travels to the nucleus of the cell and interacts with specific DNA sequences in vitamin D-responsive genes.
The activity of the 1α-hydroxylase system is closely regulated by PTH, calcium, phosphate, and calcitriol itself, which exerts negative feedback inhibition on 1α-hydroxylase. Hypocalcemia and PTH stimulate calcitriol synthesis. There is an inverse relationship between calcium concentrations and the activity of the 1α-hydroxylase enzyme system that may arise directly or may be secondary to changes in the secretion of PTH in response to alterations in serum calcium concentration. Some evidence exists to support a direct suppressive effect of calcium on 1α-hydroxylase activity in proximal tubular cells.4 The 1α-hydroxylase enzyme system is stimulated by hypophosphatemia and inhibited by hyperphosphatemia.
The major effects of 1,25-dihydroxycholecalciferol (calcitriol) are increased intestinal absorption of calcium and phosphate, a permissive effect on PTH-mediated bone resorption of calcium and phosphate, and negative feedback control on PTH synthesis and secretion by the parathyroid glands. The actions of vitamin D are discussed in more detail in Chapter 6.
1 Avison M.J., Gullans S.R., Ogino T., et al. Measurement of Na-K coupling ratio of Na-K ATPase in rabbit proximal tubules. Am J Physiol. 1987;253:C126.
2 Berliner R.W. Mechanisms of urine concentration. Kidney Int. 1982;22:202.
3 Biber J., Hernando N., Forster I., et al. Regulation of phosphate transport in proximal tubules. Pflugers Arch. 2009;458:39-52.
4 Bland R., Walker E.A., Hughes S.V., et al. Constitutive expression of 25-hydroxyvitamin D3-1 alpha-hydroxylase in a transformed human proximal tubule cell line: evidence for direct regulation of vitamin D metabolism by calcium. Endocrinology. 1999;140:2027-2034.
5 Bovee K.C., Joyce T. Clinical evaluation of glomerular function: 24-hour creatinine clearance in dogs. J Am Vet Med Assoc. 1979;174:488.
6 Bröer S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol Rev. 2008;88:249-286.
7 Brown S.A. Determinants of glomerular ultrafiltration in cats. Am J Vet Res. 1993;54:970.
8 Bulger R.E. Composition of renal medullary tissue. Kidney Int. 1987;31:557.
9 Chandhoke P.S., Saidel C.M., Knepper M.A. Role of inner medullary collecting duct NaCl transport in urinary concentration. Am J Physiol. 1985;249:F688.
10 Clive D.M., Stoff J.S. Renal syndromes associated with nonsteroidal antiinflammatory drugs. N Engl J Med. 1984;310:563.
11 Cowgill L.D., James K.M., Levy J.K., et al. Use of recombinant human erythropoietin for the management of anemia in dogs and cats with renal failure. J Am Vet Med Assoc. 1998;212:521-528.
12 Dunn M.J. Nonsteroidal antiinflammatory drugs and renal function. Annu Rev Med. 1984;35:411.
13 Ebert B.L., Bunn H.F. Regulation of the erythropoietin gene. Blood. 1999;94:1864-1877.
14 Eschbach J.W., Egrie J., Downing M., et al. Correction of anemia of end-stage renal disease with recombinant human erythropoietin. N Engl J Med. 1987;316:73-78.
15 Fenton R.A. Essential role of vasopressin-regulated urea transport processes in the mammalian kidney. Pflugers Arch. 2009;458:169-177.
16 Fettman M.J., Allen T.A., Wilke W.L., et al. Single-injection method for evaluation of renal function with 14C-inulin and 3H-tetraethylammonium bromide in dogs and cats. Am J Vet Res. 1985;46:482.
17 Finco D.R. Simultaneous determination of phenolsulfonphthalein excretion and endogenous creatinine clearance in the normal dog. J Am Vet Med Assoc. 1971;159:336.
18 Finco D.R., Barsanti J.A. Mechanism of urinary excretion of creatinine by the cat. Am J Vet Res. 1982;43:2207.
19 Finco D.R., Brown S.A., Crowell W.A., et al. Exogenous creatinine clearance as a measure of glomerular filtration rate in dogs with reduced renal mass. Am J Vet Res. 1991;52:1029.
20 Finco D.R., Coulter D.B., Barsanti J.A. Simple, accurate method for clinical estimation of glomerular filtration rate in the dog. Am J Vet Res. 1981;42:1874.
21 Finco D.R., Duncan J.R. Evaluation of blood urea nitrogen and serum creatinine concentrations as indicators of renal dysfunction: a study of 111 cases and a review of related literature. J Am Vet Med Assoc. 1976;168:593.
22 Finco D.R., Tabaru H., Brown S.A., et al. Endogenous creatinine clearance measurement of glomerular filtration rate in dogs. Am J Vet Res. 1993;54:1575.
23 Fried W. Erythropoietin and erythropoiesis. Exp Hematol. 2009;37:1007-1015.
24 Hardy R.M., Osborne C.A. Water deprivation test in the dog: Maximal normal values. J Am Vet Med Assoc. 1979;174:479.
25 Jamison R.J. The renal concentrating mechanism. Kidney Int. 1987;32(Suppl. 21):S43.
26 Jamison R.L., Maffly R.H. The urinary concentrating mechanism. N Engl J Med. 1976;295:1059.
27 Keyes J.L., Swanson R.E. Dependence of glucose Tm on GFR and tubular volume. Am J Physiol. 1971;221:1.
28 King L.G., Giger U., Diserens D., et al. Anemia of chronic renal failure in dogs. J Vet Intern Med. 1992;6:264-270.
29 Kokko J.P. The role of the collecting duct in urinary concentration. Kidney Int. 1987;31:606.
30 Kokko J.P., Rector F.C.Jr. Countercurrent multiplication system without active transport in inner medulla. Kidney Int. 1972;2:214.
31 Koushanpour E., Kriz W. Renal physiology: principles, structure, and function. New York: Springer-Verlag; 1986.
32 Kruth S.A., Cowgill L.D. Renal glucose transport in the cat (abstract). Proc Am Coll Vet Intern Med. 78, 1982.
33 Levitin H., Goodman A., Pigeon G., et al. Composition of the renal medulla during water diuresis. J Clin Invest. 1962;41:1145.
34 MacLeod J.N., Tetreault J.W., Lorschy K.A.S., et al. Expression and bioactivity of recombinant canine erythropoietin. Am J Vet Res. 1998;59:1144-1148.
35 Maxwell P.H., Ferguson D.J.P., Nicholls L.G., et al. Sites of erythropoietin production. Kidney Int. 1997;51:393.
36 Navar L.G., Bell P.D., Crowell W.A., et al. Evaluation of the single nephron glomerular filtration coefficient in the dog. Kidney Int. 1977;12:137.
37 O’Grady S.M., Palfrey H.C., Field M. Characteristics and function of Na-K-2Cl cotransport in epithelial tissues. Am J Physiol. 1987;253:C177.
38 Osbaldiston G.W., Fuhrman W. The clearance of creatinine, inulin, para-aminohippurate and phenolsulfonphthalein in the cat. Can J Comp Med. 1970;34:138.
39 Powers T.E., Powers J.D., Garg R.C. Study of the double isotope single-injection method for estimating renal function in purebred beagle dogs. Am J Vet Res. 1977;38:1933.
40 Randolph J.F., Scarlett J.M., Stokol T., et al. Expression, bioactivity, and clinical assessment of recombinant feline erythropoietin. Am J Vet Res. 2004;65:1355-1366.
41 Randolph J.F., Scarlett J., Stokol T., et al. Clinical efficacy and safety of recombinant canine erythropoietin in dogs with anemia of chronic renal failure and dogs with recombinant human erythropoietin-induced red cell aplasia. J Vet Intern Med. 2004;18:81-91.
42 Randolph J.F., Stokol T., Scarlett J.M., et al. Comparison of biological activity and safety of recombinant canine erythropoietin with that of recombinant human erythropoietin in clinically normal dogs. Am J Vet Res. 1999;60:636-642.
43 Rose B.D. Clinical physiology of acid-base and electrolyte disorders. New York: McGraw-Hill, 1994;94.
44 Rose B.D. Clinical physiology of acid-base and electrolyte disorders. New York: McGraw-Hill, 1994;115.
45 Ross L.A., Finco D.R. Relationship of selected clinical renal function tests to glomerular filtration rate and renal blood flow in cats. Am J Vet Res. 1981;42:1704.
46 Schnermann J., Briggs J.P. Tubuloglomerular feedback: mechanistic insights from gene-manipulated mice. Kidney Int. 2008;74:418-426.
47 Shannon J., Farber S., Troast L. The measurement of glucose Tm in the normal dog. Am J Physiol. 1941;133:752.
48 Stephenson J.L. Concentration of urine in a central core model of the renal counterflow system. Kidney Int. 1972;2:85.
49 Tsukaguchi H., Shayakul C., Berger U.V., et al. Urea transporters in kidney: molecular analysis and contribution to the urinary concentrating process. Am J Physiol. 1998;275:F319-F324.
50 Valtin H., Edwards B.R. GFR and the concentration of urine in the absence of vasopressin, Berliner-Davidson re-explored. Kidney Int. 1987;31:634.
51 Valtin H., Schafer J.A. Renal function. Boston: Little, Brown and Company, 1995;98.
52 Verry F., Singer D., Ramadan T., et al. Kidney amino acid transport. Pflugers Arch. 2009;458:53-60.
53 Wright E.M. Renal Na+-glucose cotransporters. Am J Physiol Renal Physiol. 2001;280:F10-F18.
54 Yang B., Bankir L. Urea and urine concentrating ability: new insights from studies in mice. Am J Physiol Renal Physiol. 2005;288:F881-F896.