Melanoma
Melanoma is a relatively common cancer of dogs, especially those with significant levels of skin pigmentation. Melanomas in cats are relatively rare. The most common location for canine malignant melanomas (CMMs) is the haired skin, in which they grossly appear to be small brown-to-black masses but can also appear as large, flat, and/or wrinkled masses.1,2 Primary melanomas also can occur in the oral cavity, nail bed, footpad, eye, gastrointestinal tract, or mucocutaneous junction.3 Ocular melanomas of dogs and cats represent a distinct clinical syndrome and are discussed in Chapter 31. Metastatic sites can be varied, including local draining lymph nodes, the lungs, liver, meninges, adrenals, and other miscellaneous sites.
Melanoma arises from melanocytes, which are the cells that generate pigment through the melanosome by a number of melanosomal glycoproteins. In humans, cutaneous melanoma can arise due to mutations induced by repeated, intense exposure to ultraviolet (UV) light (e.g., frequent tanning or working outdoors). Melanoma is currently the most rapidly increasing incident human cancer.4 Significant recent research into the etiology of human melanoma suggests multiple causes that are independent of the aforementioned UV-associated mutagenesis.5 Since most breeds of dogs have a hair coat that likely affords them protection from sunlight, UV-associated melanoma is less likely as a primary causative agent in the dog. However, pigment cells divide every time there is injury to the skin or if there is constant trauma (e.g., areas where dogs scratch or lick). Nevertheless, risk factors for canine melanoma are not well established.
The most common oral malignancy in the dog is melanoma.2,3,6,7 Oral melanoma is most commonly diagnosed in Scottish terriers, golden retrievers, poodles, and dachshunds.2,8 Oral melanoma is primarily a disease of older dogs without gender predilection but may be seen in younger dogs.8-10 Additional oral tumor differentials include squamous cell carcinoma (SCC), fibrosarcoma, epulides/odontogenic tumors, and others.2,3,7,11-13 Melanomas in the oral cavities of dogs are found in the following locations by order of decreasing frequency: gingiva, lips, tongue, and hard palate. Feline melanoma is relatively rare but appears to be malignant in most cases.3,14-21
Melanomas in dogs have extremely diverse biologic behaviors, depending on a large variety of factors. A thorough understanding of these factors helps the clinician to delineate in advance the appropriate staging, prognosis, and treatments. The primary factors that determine the biologic behavior of an oral melanoma in a dog are site, size, stage, and histologic parameters.8-10,22,23 Unfortunately, even with a comprehensive understanding of all of these factors, there are melanomas that have an unreliable biologic behavior; thus there is a need for additional research into this relatively common, heterogeneous, and frequently extremely malignant tumor.
Pathology and Molecular Biology
Melanomas can be difficult to diagnose pathologically in some situations, especially anaplastic amelanotic melanomas, which can masquerade as soft tissue sarcomas.24,25 Numerous investigators have attempted to increase the precision of identification of melanomas predominantly through immunohistochemical means.25-29 This identification can be accomplished through the use of multiple immunohistochemical assays on suspected melanoma tissue or through the use of an immunohistochemical cocktail of antibodies. The use of PNL2 and tyrosinase, beyond the typical use of Melan A and S100, appears to hold particular promise.30,31
The molecular characterization of canine and feline melanomas remains comparatively attenuated compared to the more comprehensive evaluation of human melanomas.32 BRAF is a member of the mitogen-activated protein kinase (MAPK) signaling pathway, which is commonly mutated in human cutaneous melanoma.33 However, BRAF mutations are uncommon in canine oral malignant melanoma,34 suggesting that certain canine and/or feline malignancies can have similar molecular signatures in addition to their already well-known clinical similarities in the context of resistance to chemotherapy and irradiation and similar variable sites of metastatic propensity. A number of investigators have reported a variety of molecular abnormalities or associations in canine and feline melanoma.35-52
Biologic Behavior and Prognostic Factors
The biologic behavior of canine oral melanoma is extremely variable and best characterized based on anatomic site, size, stage, and histologic parameters. On divergent ends of the spectrum would be a low-grade 0.5-cm haired-skin melanoma, which is highly likely to be cured with simple surgical extirpation, in comparison to a 5.0-cm high-grade malignant oral melanoma with a poor-to-grave prognosis. Similar to the development of a rational staging, prognostic, and therapeutic plan for any tumor, two primary questions must be answered: what is the local invasiveness of the tumor and what is the metastatic propensity? The answers to these questions will determine the prognosis and appropriate therapies.
The anatomic site of melanoma is highly, although not completely, predictive of local invasiveness and metastatic propensity. Melanomas involving the haired-skin that are not in proximity to mucosal margins often behave in a benign manner.1,3 Surgical extirpation is often curative, but histopathologic examination is imperative for delineation of margins, as well as a description of cytologic features. The use of Ki67 immunohistochemistry (IHC) has been reported to more reliably predict potential malignant behavior compared to classic histology for cutaneous melanoma.53
Oral and/or mucosal melanoma has been considered an extremely malignant tumor with a high degree of local invasiveness and high metastatic propensity.2,8-10,22,54 This biologic behavior is similar to human oral and/or mucosal melanoma.3,55 Two recent studies have called this dogma into question and suggest benign oral melanomas can occur more frequently than previously published.56,57 Caution is necessary when assessing histopathologic descriptions, suggesting a benign course for oral melanoma. The first author has managed approximately 20 dogs over the last 7 years presenting with florid systemic metastases with an original histopathologic report, suggesting an expectation for a benign clinical course based on a high degree of differentiation. Similar to cutaneous melanoma, Ki67 appears to hold prognostic importance in canine oral melanoma as well.58
Anatomic sites of intermediate prognostic significance between the generally benign-acting haired-skin and the often malignant and metastatic oral/mucosal melanomas in dogs include the digit and footpad. Dogs with melanoma of the digits without lymph node or distant metastasis treated with digit amputation are reported to have a median survival time (MST) of approximately 12 months, with 42% to 57% alive at 1 year and 11% to 13% alive at 2 years.59,60 Unfortunately, metastasis from digit melanoma at presentation is reported to be 30% to 40%,59,61 and the aforementioned outcomes with surgery suggest that subsequent distant metastasis is common even when no overt metastasis is found at presentation or digit amputation. The prognosis for dogs with melanoma of the footpad has not been thoroughly established; the first author (PJB) has found this anatomic site to be anecdotally similar in metastatic propensity and prognosis to digit melanoma. Interestingly, human acral lentiginous melanoma (plantar surface of the foot, palms of the hand and digit) has an increased propensity for metastasis.62
A thorough review of prognostic factors in canine melanocytic neoplasms has been recently published.63 This review took a regimented, systematic approach to analyzing published reports to date in order to identify those factors that appear to be repeatable and statistically defensible, while also identifying areas in which additional work is necessary due to incomplete data. For those veterinary clinicians and/or researchers interested in canine melanoma, this publication cannot be more strongly recommended. Tables 1 and 2 from this report are particularly useful in estimating prognosis for a specific patient and subsequent identification of the most logical treatment options.
Size and Stage
For dogs with oral melanoma, primary tumor size has been found to be prognostic. The World Health Organization (WHO) staging scheme for dogs with oral melanoma is based on size and metastasis and is summarized in Box 19-1. MacEwen and colleagues reported MSTs for dogs with oral melanoma treated with surgery to be approximately 17 to 18 months, 5 to 6 months, and 3 months for stage I, II, and III disease, respectively.9 More recent reports suggest stage I oral melanoma treated with conventional therapies, including surgery, radiation, and/or chemotherapy, have MSTs of approximately 12 to 14 months, with most dogs dying of distant metastatic disease rather than local recurrence.64,65 Other investigators have found dogs with stage I oral melanoma to have median progression-free survival (PFS) times of 19 months, similar to the original MacEwen et al report.66
Staging
The staging of dogs with melanoma is relatively straightforward. A minimum database should include a thorough history and physical examination, complete blood and platelet count, biochemical profile, urinalysis, three-view thoracic radiographs, and local lymph node aspiration with cytology, whether lymphadenomegaly is present or not. Williams and Packer reported that approximately 70% of dogs with oral malignant melanoma had local lymph node metastasis when lymphadenomegaly was present, but more importantly, approximately 40% had local lymph node metastasis when no lymphadenomegaly was present.67 Additional considerations should be made for abdominal compartment testing (e.g., abdominal ultrasound) in all cases of CMM, especially in cases with moderate-to-high metastatic anatomic sites such as the oral cavity, feet, or mucosal surface of the lips, because melanoma metastasizes to the abdominal lymph nodes, liver, adrenal glands, and other sites.
The use of sentinel lymph node mapping and lymphadenectomy is of diagnostic, prognostic, and clinical benefit in human melanoma.68 Relatively few investigations have been reported to date for sentinel lymph node mapping and/or excision for dogs with malignancies,69-73 and we strongly encourage additional investigation in this area. Furthermore, the use of novel staging modalities such as gallium citrate scintigraphy is also encouraged.74
Treatment
Surgery continues to be the most effective local treatment modality for melanoma, and early detection is often necessary for a successful outcome. Tumors in the caudal aspect of the oral cavity are often large by the time they are diagnosed relative to rostral tumors; therefore it is more difficult to obtain complete surgical excision. Gross characteristics of melanomas raise suspicion for the diagnosis, and pigmented melanomas can be easily confirmed via fine needle aspiration and cytology. In dogs, small (<2 cm), mobile, well-circumscribed, slow-growing cutaneous melanomas tend to be benign and easily excisable, whereas large, poorly defined, ulcerated, and rapidly growing tumors can make surgical excision difficult. In the latter example, incisional biopsy and IHC can be an important part of the diagnostic work-up, particularly if the mass is nonpigmented and the diagnosis of melanoma is in question. In cases for which lymph node cytology results are equivocal (e.g., difficulty distinguishing between melanophages and melanoma cells), lymph nodes should be surgically excised and submitted for histopathologic evaluation (which includes IHC). Enlarged lymph nodes are often associated with metastasis, particularly with advanced tumors or tumors of high grade, and nodal effacement is common (Figure 19-1).
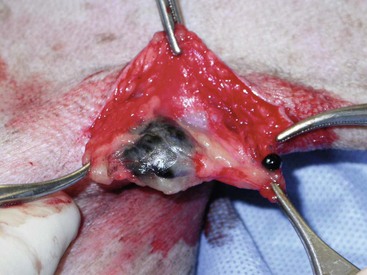
Figure 19-1 Excisional biopsy being performed on the popliteal lymph node from the dog in Figure 19-6. Note the effaced node overtaken with pigmented cells and the smaller nodule within the lymphatic vessel slightly distal to the node (toward the right in the photograph).
Benign cutaneous tumors are typically completely excised with 1-cm skin margins (and ideally one fascial plane deep). Partial mandibulectomy and maxillectomy are usually required for complete removal of oral melanomas arising from the gingiva or from other oral mucosa in close proximity to bone.75 A common error is to “shave off” a gingival mass simply because bone invasion is not observed. Due to the close proximity of the gingiva to the underlying bone, this approach typically leaves residual microscopic disease that leads to local recurrence (Figure 19-2). Advanced imaging (i.e., computed tomography [CT] scans or magnetic resonance imaging [MRI]) is critical for assessment of tumor extent and potential bone involvement. It is also helpful in the detection of enlarged regional lymph nodes (e.g., medial retropharyngeal nodes) that are difficult to palpate.
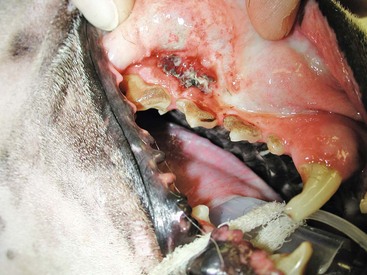
Figure 19-2 Local recurrence following incomplete resection and postoperative radiation of a gingival melanoma above the right carnassial tooth (caudal is to the left and the right canine tooth can be seen in the lower right-hand corner). When first treated, the mass was reportedly less than 1 cm diameter and wide resection (i.e., partial maxillectomy) was not performed.
Some tumors do occur in mucosal areas that are not adjacent to bone (e.g., buccal mucosa) or originate in the lip (Figure 19-3) or tongue (Figure 19-4) and are amenable to excision of the soft tissues only. The location of the tumor dictates the type of partial maxillectomy (i.e., premaxillary, unilateral rostral, central, or caudal) that is required. Complete excision of oral tumors has been shown to significantly impact prognosis.76 Dogs with tumor cells extending to the peripheral margin were 3.6 times more likely to die from tumor-related causes compared to dogs for which margins were considered complete. In that same study, dogs with tumors caudal to premolar number three (PM3) were 4.3 times more likely to die from tumor-related causes compared to dogs with tumors located rostral to PM3.
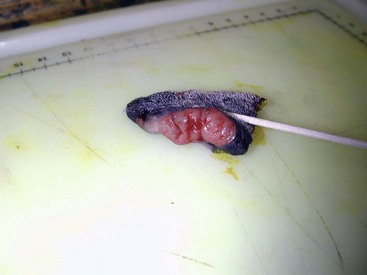
Figure 19-3 Amelanotic melanoma involving the rostral aspect of the right lip; 1- to 2-cm margins resulted in complete excision.
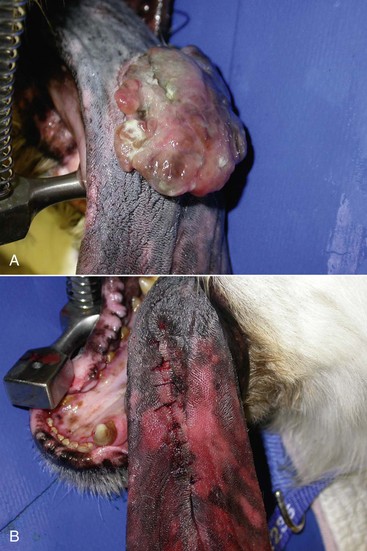
Figure 19-4 A, Lingual melanoma near the midline of the tongue of a dog. These masses are often superficial and can be easily excised with 1- to 2-cm lingual mucosal margins and a layer of muscle fibers deep to the mass. B, Closure results in little disruption of the lingual architecture and postoperative function is excellent.
For small caudal tumors lateral to the dental arcade (Figure 19-5), an oral approach provides enough exposure/access for complete excision; however, large tumors of the caudal maxilla or small tumors that extend medial to the dental arcade are best treated via a combined dorsal and intraoral approach.77 The combined approach maximizes the surgeon’s ability to obtain wide margins on tumors involving the caudal hard palate and inferior orbit. For small, superficial melanomas associated with the mandible, partial mandibulectomy is typically sufficient78; however, for large tumors that show evidence of intramedullary extension, consideration should be given to a subtotal or complete hemimandibulectomy. Local recurrence rates vary from 22% following mandibulectomy to 48% after maxillectomy75,79 and the MST for dogs treated with surgery alone varies from 150 to 318 days, with 1-year survival rates less than 35%.28,75,76,78-81
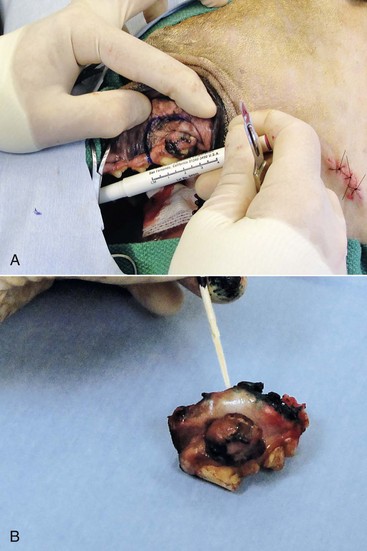
Figure 19-5 A, Intraoperative photograph of a melanoma confined to the lateral aspect of the caudal dental arcade. Surgical margin width in this case is approximately 1.5 cm around the cranial, dorsal, and caudal borders of the tumor. The incision along the hard palate was made just medial to the dental arcade. B, Surgical margins are inked with a green surgical ink and histopathologic assessment showed complete margins.
There are few objective data available to guide decision making for surgical margin width. Given the invasive nature of malignant melanomas, wide margins (2 to 3 cm) are optimal whenever possible; however, for oral lesions, wide margins are often not possible due to the limited amount of surrounding normal tissues. In the author’s experience, 1 to 2 cm margins are usually adequate for complete excision of malignant tumors with well-defined borders (Figures 19-5 and 19-6). As with any oral tumor, inspection of the gross tumor borders must be interpreted along with diagnostic imaging to determine resectability and resection lines. Wider margins are usually possible with digital melanomas because digit amputation can often be performed at a level several joints above the proximal extent of the tumor.
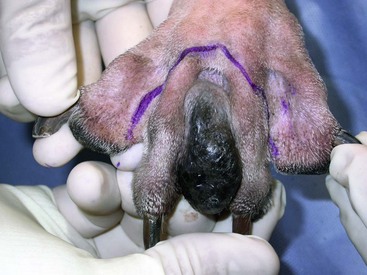
Figure 19-6 Malignant melanoma in the webbing between digits 3 and 4 (same dog as in Figure 19-1). Surgical margins were between 1 and 2 cm beyond the tumor edge. Excision was complete in this case.
With the widespread access to advanced imaging and improvements in surgical techniques (e.g., combined dorsal and intraoral approach for caudal maxillary tumors) and surgical oncologic training, complete tumor excision is more likely to be achieved. The surgical goals should be driven by the tumor stage, its location, and the surgeon’s ability to perform a wide resection in locations where surgery is difficult (e.g., large caudal tumors). Unplanned or limited attempts at excision should not be made because the first chance to operate is the best chance to achieve tumor-free margins. For patients that undergo surgical excision, quality of life is usually very good and most dogs resume eating in the first day or two following surgery. Further, owner satisfaction with functional and cosmetic results following mandibulectomy and maxillectomy is high.82 The functional outcome of single digit amputation is excellent and partial foot amputations (requiring excision of more than one digit; see Figure 19-6) are also tolerated very well and result in a good functional outcome.83
For tumors that are not amenable to wide excision or result in incomplete margins via histopathologic assessment, the combination of surgery and radiation or surgery plus adjuvant therapy should be considered. Historically, surgery has not been recommended when metastatic disease has been documented (e.g., a positive lymph node is discovered during tumor staging). However, the role of adjuvant therapy (chemotherapy or immunotherapy) in conjunction with cytoreductive surgery is being investigated for human metastatic melanoma and such approaches are now being explored in dogs (covered elsewhere in this chapter).84-86
Radiation Therapy
Radiation therapy plays an important role in the management and treatment of canine and feline oral melanomas. As with most tumor types, radiation therapy is used for the purpose of achieving local or regional control of the tumor. Radiation therapy has been described as both a primary and adjuvant therapy and both hypofractionated and definitive protocols have been used (Table 19-1).87-92
Table 19-1
Published Studies on the Treatment of Oral Melanoma with Radiation Therapy
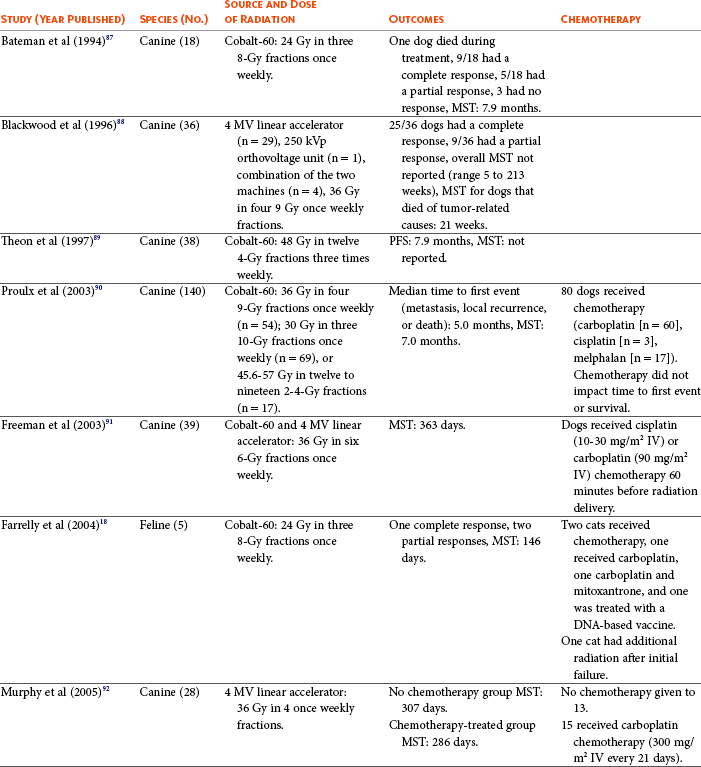
MST, Median survival time; PFS, progression-free survival; IV, intravenous.
Melanoma is thought to be a relatively radioresistant tumor type often necessitating a higher dose in each fraction to achieve local control, although this point is somewhat controversial.93 Most protocols used in dogs and cats have pursued higher dose per fraction protocols accordingly, although the relative radiosensitivity of melanoma in companion animal species has not been determined.
Hypofractionated protocols have the advantage of fewer treatments with fewer anesthetic episodes, lower cost, and less time commitment for the owner. These protocols in general also result in less severe acute effects. The main disadvantage of hypofractionated protocols is a lower overall and biologic equivalent dose, which results in lower rates of local control and an increased risk for late side effects. For a discussion of side effects, see the section on radiation side effects later in this chapter and in Chapter 12.
When planning a radiation field, most clinicians will choose to treat both the oral component of the disease and the regional lymph nodes, including the mandibular and medial retropharyngeal lymph nodes due to the high rate of metastasis to these sites.
Treatment planning can be done manually or can be planned by computer to allow a more conformal and homogeneous dose distribution with better sparing of normal tissues. Most studies have reported using at least a 2-cm margin around the tumor bed or surgical incision site. The field can be shaped by using manual blocking or, if available at the treating facility, a multileaf collimator (Figure 19-7).
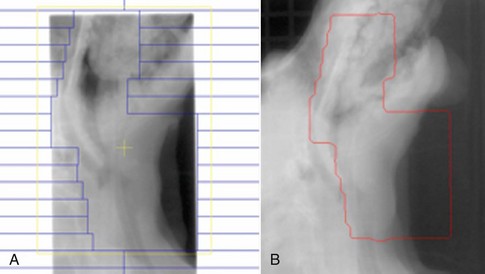
Figure 19-7 Multileaf collimator plan for a dog with an oral melanoma (A) and a portal image taken of the same dog (B). The outlined red area is the exposed field for the radiotherapy plan. A second wider exposure was taken to be able to view the surrounding anatomy and allow adjustment of the patient to ensure the correct anatomic area was treated. This plan was designed to cover the primary tumor, as well as the submandibular and medial retropharyngeal lymph nodes.
Outcomes of Dogs and Cats Treated with Radiation Therapy for Oral Melanoma
The reported range of partial and complete responses to radiation therapy are 25% to 31% and 51% to 69%, respectively, yielding an overall response rate of 82% to 94%.87,90,92 When treating gross disease, responses are generally rapid, and dramatic decreases in tumor volume can be seen within several weeks of starting therapy (Figure 19-8). PFS has been reported to range from 5 to 7.9 months.89,90 Reported recurrence rates after radiotherapy vary and are confounded by different radiation protocols and adjuvant therapies used in these studies. Proulx et al reported a local recurrence rate of 26% when treating microscopic disease after incomplete surgical resection and radiotherapy and 45% for dogs treated with radiotherapy for macroscopic disease.90
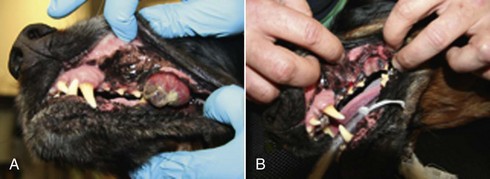
Figure 19-8 A dog with an oral melanoma treated with an 8 Gy per fraction once weekly protocol on the first day of therapy (A) and before the fourth and final fraction of radiotherapy (B). This dog went on to have a complete response.
The reported MSTs for dogs treated with radiotherapy range from 5.3 to 11.9 months (see Table 19-1).87,90-92 The majority of these studies are retrospective and use a variety of radiotherapy equipment, protocols, and adjuvant therapies, making it difficult to determine an ideal treatment regimen. Reported protocols include 2 to 4 Gy fractions daily for 12 to 19 treatments, 4 Gy per fraction 3 times weekly for 4 weeks, 8 Gy per fraction once weekly for 3 treatments, and 9 Gy per fraction once weekly for 4 fractions.
Farrelly et al reported five cats that were treated with radiotherapy for oral melanoma.18 There was one complete response and two partial responses with a MST of 146 days. One cat was treated with carboplatin, and one cat was treated with carboplatin and mitoxantrone, in addition to the radiation protocol. Another cat in this study was given a DNA-based vaccine as adjuvant to radiation.
Combining Chemotherapy with Radiation Therapy
There are conflicting data regarding the efficacy of chemotherapy used concurrently or in the adjuvant setting, and there is no clear evidence that chemotherapy decreases the risk of metastasis or local recurrence or that it extends MSTs. Published studies have reported on dogs receiving carboplatin, intralesional cisplatin, intravenous cisplatin, and melphalan.90-92,94-97
Freeman et al reported on 39 dogs with incompletely resected oral melanoma that were treated with either cisplatin (10 to 30 mg/m2 IV) or carboplatin (90 mg/m2 IV) given once weekly approximately 1 hour before receiving radiation therapy.91 The radiation protocol consisted of 6 once-weekly 6 Gy fractions. They reported a MST of 11.9 months. Fifteen percent of the dogs had local recurrence, with a reported median time to metastasis of 10.2 months. Although this is among the longest survival times reported, the majority of dogs had stage T1 (56.4%) or stage T2 (35.9%) tumors prior to surgery, which may have biased their findings for longer survival times, making this result less applicable to the general population of dogs presenting with oral melanoma.
Proulx et al retrospectively looked at dogs treated with a variety of radiation protocols in which 60 dogs received no chemotherapy and 80 dogs received a chemotherapy protocol consisting of carboplatin (n = 60), cisplatin (n = 3), or melphalan (n = 17).90 They found that chemotherapy treatment had no significant impact on either time to first event or overall survival. The mean dose of carboplatin used in this study was 225 mg/m2 IV, which is lower than the dose most often used in this species.95 This lower dose may have contributed to the lack of statistical differences in delaying time to metastasis or recurrence and impact on overall survival.
Murphy et al retrospectively evaluated a series of 28 dogs that received once weekly 8 Gy fractions to a total dose of 32 Gy.92 Fifteen dogs in this study received 300 mg/m2 IV of carboplatin every 21 days for a median of 3 doses, although chemotherapy dose reductions were allowed on subsequent doses if toxicity was found on the initial dose. Thirteen dogs received no chemotherapy with a MST of 307 days, and the 15 dogs that were treated with chemotherapy had an overall MST of 286 days. These numbers were not significantly different. The use of chemotherapy also did not reduce the proportion of dogs that died from metastasis. The dose of carboplatin in this study was chosen to decrease the risk of toxicity seen in another report that evaluated the use of carboplatin alone in dogs with melanoma in which dogs over 15 kg received 350 mg/m2 IV, and several of the dogs experienced significant toxicity requiring hospitalization, although the dose used was higher than the dose used in the Proulx study.90,96
Some concerns have been raised that using radiotherapy and chemotherapy concurrently can increase systemic side effects. Hume et al reported on 65 dogs treated with concurrent carboplatin and a definitive course of radiation therapy; six of these dogs were diagnosed with an oral melanoma.97 Carboplatin was administered at 200 to 300 mg/m2 IV. Dogs with oral melanoma in this study were prescribed 4 Gy per fraction delivered on Mondays, Wednesdays, and Fridays for a total dose of 48 Gy. They found that 21% developed grade 3 or 4 neutropenia, 20% developed grade 3 or 4 thrombocytopenia, and 10% developed grade 3 to 5 gastrointestinal toxicity according to the Veterinary Cooperative Oncology Group—Common Terminology Criteria for Adverse Events (VCOG-CTAE), version 1.0.98 Further, five of the dogs did not complete the radiation course and seven had delays in radiation therapy due to illness.
Radiation-Associated Prognostic Factors
Several possible prognostic factors have been identified that affect radiation treatment outcomes in dogs diagnosed with malignant oral melanoma. These factors must be viewed with caution because some of the data are conflicting and most are derived from retrospective case series without the use of control groups.
Proulx et al reported outcomes from a total of 140 dogs treated with radiation and identified that dogs without radiologic evidence of bone destruction were found to have longer times to first event and longer overall survival times than dogs with radiographically evident bone changes.90 Destruction of underlying bone can be evaluated by using survey radiographs or by CT scan with greater sensitivity. Underlying bony involvement has been reported in up to 92% of dogs with melanoma (Figure 19-9).91
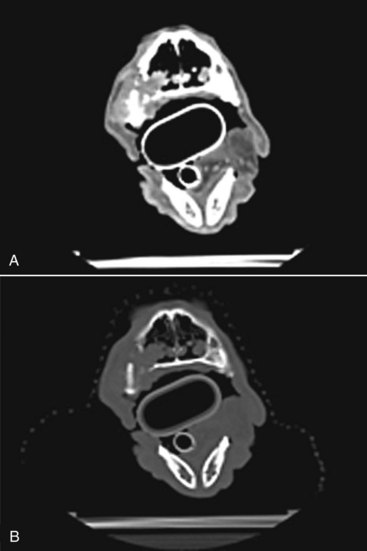
Figure 19-9 A, Postcontrast axial computed tomography (CT) image of a dog with a maxillary oral melanoma. Note the heterogeneously contrast enhancing soft tissue mass. B, Precontrast axial image at the same level as the image in A. Note the bone destruction of the maxillary bone and the invasion of the soft tissue mass into the nasal cavity.
The size of an oral tumor also affects prognosis, but more studies need to be conducted to confirm that size is a prognostic factor for dogs undergoing irradiation. Theon et al analyzed a series of 105 dogs with oral tumors treated with 4 Gy per fraction of radiation on an alternate day basis to a total dose of 48 Gy.89 Thirty-eight dogs in this report were diagnosed with oral melanoma. They found a median PFS of 7.9 months. Dogs from this group diagnosed with T1 stage lesions had a median PFS of 11.3 months, whereas dogs with stage T2 and T3 lesions had a median PFS of 6.0 and 6.7 months, respectively. They also found that distant metastasis as opposed to local failure of radiation was the major cause of first failure event. Blackwood et al also found that dogs with smaller oral melanomas treated with a 9 Gy per fraction once weekly protocol delivered over a 4-week period did better than dogs with larger tumors.88 Dogs with tumors less than 5 cm3 were more likely to achieve a complete response. Further, the volume of the tumor affected median overall survival with a MST of 86 weeks for dogs with tumors less than 5 cm3 compared to 16 weeks for dogs with tumors between 5 and 15 cm3 and 20.5 weeks for dogs with tumors greater than 15 cm3 in size. Proulx et al found that tumor size affected times to first event, pulmonary metastasis, and death.90 These studies contrast the findings of Bateman et al, who did not find an association of stage of the oral melanoma and response to radiation therapy or survival times.87
The role of vascular endothelial growth factor (VEGF) in the radiation response of melanoma has been incompletely evaluated. Several studies have found that dogs with oral melanoma have higher plasma VEGF concentrations than normal control dogs.43,99 In a preliminary study looking at plasma VEGF levels in a variety of tumor types treated with hypofractionated radiotherapy, the four dogs diagnosed with melanoma had the highest mean plasma VEGF levels of all tumor types, although no significant changes in VEGF levels were noted over the course of treatment.99 This same group also evaluated the effect that VEGF levels had on patient outcome.100,101 In a group of 39 dogs, 6 of which were diagnosed with melanoma, it was also found that VEGF levels did not significantly increase over the course of radiotherapy. However, dogs with higher plasma VEGF levels treated with hypofractionated protocols had a shorter time to treatment failure and a shorter median survival.
Side Effects of Radiation Therapy
Side effects of radiation therapy are a major concern of owners and can impact a patient’s quality of life. Prolonged side effects are particularly important to take into account when the goal of radiotherapy is palliative in nature (i.e., to improve quality of life). The severity and scope of radiation side effects and the type seen depend on several factors, including the total dose of radiation given, the dose per fraction, and the volume of tissue in the radiation field. Higher total radiation doses delivered in smaller dose per fractions tend to produce worse acute effects, whereas higher dose per fraction will increase the risk of late effects occurring. Newer treatment machines and computerized treatment planning have helped limit the volume of normal tissue that is irradiated, which in turn limits the area where these acute effects are seen. Side effects are generally broken down into acute and late effects. A standardized radiation scoring system for acute and late effects on oral mucosa and bone have been published by the Veterinary Radiation Therapy Oncology Group (VRTOG).102 Acute radiation effects on the oral cavity range from injected mucous membranes (grade 1) to patchy mucositis without evidence of pain (grade 2) to confluent fibrinous mucositis requiring the use of analgesics to ulceration, hemorrhage, and necrosis (grade 3). These can be seen on the gingival surfaces, tongue, or buccal mucosa.102 Although grade 3 toxicity is possible, most studies have not reported high-grade toxicity with either definitive or palliative protocols.87-89 The worst clinical signs associated with acute effects generally occur toward the end or just after the completion of radiotherapy and heal within 1 to 2 weeks given that the oral mucosa tends to heal rapidly.
Skin effects can also be seen with dry and moist desquamation possible. Most studies have reported these side effects to be minimal and to resolve quickly after completion of the radiation course. Other skin side effects reported include permanent alopecia in the radiation field and skin fibrosis.89
Bone necrosis is a reported late side effect of radiation therapy and probably is a greater risk in hypofractionated protocols. Blackwood et al reported three cases of bone necrosis in a series of 36 dogs treated with a hypofractionated protocol; two required surgical debridement.88 Theon et al also reported 7.6% of dogs developed bone necrosis and fistula formation in a series of oral tumors treated with a curative-intent protocol.89
Another possible late effect is a radiation-induced tumor. In order for a tumor to be classified as likely being radiation induced the following criteria are applied: (1) the tumor developed in an area that was previously irradiated, (2) an adequate period of time has passed since initial irradiation to allow for new tumor formation, (3) preirradiation evaluation revealed normal bone in cases in which the second tumor is of bone origin, and (4) histologic diagnosis of a second, different malignant tumor was confirmed at the site of initial irradiation.103
In dogs with oral melanoma treated with radiation therapy, most patients do not survive long enough for radiation-induced tumors to become a significant issue, but as more effective systemic adjuvant therapies become available, this becomes a concern. Sarcomas are thought to be the most common radiation-induced tumor, although Theon et al did report on a gingival SCC forming within a radiation field 36 months after radiation treatment.89
Salvage procedures using flaps for the surgical repair of radiation late effects have been described in dogs, although they are more likely to result in complications than the surgical flaps used in the surgical treatment of an area treated with preoperative radiotherapy or in flaps exposed to radiation in the immediate postoperative period.104
Radiation therapy is an effective modality used to achieve local-regional control for oral melanoma with minimal side effects in dogs and cats. Until more effective treatments to control and treat metastatic disease become available and the effectiveness of lower dose per fraction protocols are better studied, hypofractionated protocols will likely remain the most commonly used radiation protocols in veterinary medicine.
Treatments Other than Surgery and Radiation
Other modalities reported for local tumor control as case reports and/or case series have included intralesional cisplatin implants, intralesional bleomycin with electronic pulsing, and others, but widespread use has not been reported to date.105-108
In dogs with melanoma in the aforementioned anatomic sites predicted to have a moderate-to-high metastatic propensity or dogs with cutaneous melanoma with a high tumor score and/or increased proliferation index through increased Ki67 expression, the use of systemic therapies is warranted due to the high risk for metastasis. Rassnick and colleagues reported an overall response rate of 28% using carboplatin for dogs with malignant melanoma.96 Unfortunately, only one dog had a minimally durable complete response (approximately 150 days), and the rest were nondurable partial responses. Similarly, Boria et al reported an 18% response rate and MST of 119 days with cisplatin and piroxicam in canine oral melanoma.109 Other reports using single-agent dacarbazine, melphalan, or doxorubicin suggest poor or absent activity.110-113 More recently and importantly, two studies suggest that chemotherapy plays an insignificant role in the adjuvant treatment of canine melanoma.65,99 Although it can be argued that the studies performed to date that evaluate the activity of chemotherapy in an adjuvant setting for canine melanoma have been suboptimal due to a variety of reasons, the extensive human literature in this specific setting also suggests melanoma is an extremely chemotherapy-resistant tumor.114 It is clear that new approaches to the systemic treatment of this disease are desperately needed.
One potential therapeutic avenue that has not been reported to date in canine or feline melanoma is the use of cyclooxygenase-2 (COX-2) inhibitors. A number of authors have investigated the expression of COX-2 in canine melanoma and have found positive correlations of COX-2 expression to proliferation and survival.115-117 Studies investigating the clinical responsiveness of canine melanoma to COX-2 inhibitors (and potential correlation to COX-2 expression) are encouraged.
Immunotherapy represents one logical systemic therapeutic strategy for melanoma. A variety of immunotherapeutic strategies for the treatment of human melanoma have been reported previously, with typically poor outcomes due to a lack of breaking tolerance. Immunotherapy targets and strategies to date in canine melanoma have used autologous tumor cell vaccines (with or without transfection with immunostimulatory cytokines and/or melanosomal differentiation antigens), allogeneic tumor cell vaccines transfected with interleukin 2 (IL-2) or granulocyte-macrophage colony-stimulating factor (GM-CSF), liposomal-encapsulated nonspecific immunostimulators (e.g., L-MTP-PE), intralesional Fas ligand DNA, small interfering RNA (siRNA) against the survivin and/or Bcl-2 genes, bacterial super-antigen approaches with GM-CSF or IL-2 as immune adjuvants, PEGylated tumor-necrosis factor α (TNF-α), suicide gene therapy, adenovector CD40 ligand, and finally, canine dendritic cell vaccines loaded with melanosomal differentiation antigens.9,118-132 Although these approaches have produced some clinical antitumor responses, the methodologies for the generation of these products are expensive, time consuming, sometimes dependent on patient tumor samples being established into cell lines, and fraught with the difficulties of consistency, reproducibility, and other quality control issues. Furthermore, we are just beginning to more fully understand the native immune dysregulation potentially induced by the malignancy through increased T regulatory (Treg) cells, which suppress antitumor responses133,134 (see also Chapter 13).
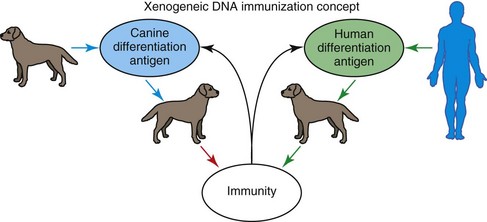
The advent of DNA vaccination circumvents many of the previously encountered hurdles in vaccine development. DNA is relatively inexpensive and simple to purify in large quantities. The gene coding for the antigen of interest is cloned into a bacterial expression plasmid with a constitutively active promoter. The plasmid is introduced into the skin or muscle with an intradermal or intramuscular injection. Once in the skin or muscle, professional antigen-presenting cells, particularly dendritic cells, are able to present the transcribed and translated antigen in the proper context of major histocompatibility complex and co-stimulatory molecules. Although DNA vaccines have induced immune responses to viral proteins, vaccinating against tissue-specific self-proteins on cancer cells is clearly a more difficult problem. One way to induce immunity against a tissue-specific differentiation antigen on cancer cells is to vaccinate with xenogeneic (different species) antigen or DNA that is homologous to the cancer antigen. As illustrated in Figure 19-10, vaccination with DNA-encoding cancer differentiation antigens is ineffective when self-DNA is used, but tumor immunity can be induced by orthologous DNA from another species.135
Tyrosinase is a melanosomal glycoprotein, essential in melanin synthesis, and routinely found to be overexpressed in a wide variety of melanomas across species.31,136-139 Immunization with xenogeneic human DNA-encoding tyrosinase family proteins induced antibodies and cytotoxic T-cells against syngeneic B16 melanoma cells in C57BL/6 mice, but immunization with mouse tyrosinase-related DNA did not induce detectable immunity.140 In particular, xenogeneic DNA vaccination induced tumor protection from syngeneic melanoma challenge and autoimmune hypopigmentation. Thus xenogeneic DNA vaccination could break tolerance against a self-tumor differentiation antigen, inducing antibody, T-cell, and antitumor responses.
Dogs with stage I to III locoregionally controlled CMM inclusive across all xenogeneic vaccine studies to date have a Kaplan-Meier (KM) MST of more than 1075 days (median not yet reached), whereas those dogs with stage I to III CMM without local tumor control have a KM MST of 553 days (p = 0.0002). The KM MST for stage II to IV dogs on the phase I trials of human tyrosinase (huTyr), murine GP75 (muGP75), and murine tyrosinase (muTyr) are 389, 153, and 224 days, respectively. The KM MST for stage II to IV dogs treated with 50 µg muTyr, 100/400/800 µg human GM-CSF, or combination muTyr/human GM-CSF are 242, 148, and more than 900 days (median not reached), respectively. For dogs that received any melanoma vaccine except for dogs on the muTyr ± GM-CSF trial (i.e., huTyr, muTyr, and muGP75), the KM MST for stages I, II, III, and IV CMM was more than 939 days (median not reached with 92.8% survival), more than 908 days (median not reached, 79% alive at 1 year, 63% alive at 2 years), more than 1646 days (median not reached; 77%, 65%, and 57% alive at 1, 2, and 3 years, respectively), and 239 days (40.5% and 18.8% alive at 1 and 2 years, respectively).141,142 The results from dogs vaccinated with huTyr were published in 2003.143
We investigated the humoral responses of dogs receiving huTyr as a potential explanation for the long-term survival seen in some of the dogs on this study. Utilizing standard enzyme-linked immunosorbent assay (ELISA) with mammalian expressed purified huTyr protein as the target of interest, we have found three of nine dogs with twofold to fivefold postvaccinal humoral responses compared to preimmune sera. We have confirmed these findings utilizing a flow-cytometric–based assay of prevaccinal and postvaccinal sera in permeabilized human SK-Mel melanoma cells expressing endogenous huTyr. Interestingly, the three dogs with postvaccinal anti-huTyr humoral responses are dogs with unexpected long-term tumor control.144 Co-investigators have also determined that normal dogs receiving the huTyr-based melanoma vaccine develop Ag-specific interferon-γ (IFN-γ) T-cells.145
The results of these trials demonstrate that xenogeneic DNA vaccination in CMM (1) is safe, (2) causes dogs to develop specific anti-Tyr immune responses, (3) is potentially therapeutic in stage II/III locoregionally controlled disease, and (4) is an attractive candidate for further evaluation in an adjuvant, minimal residual disease phase II setting for CMM. Based on these preliminary studies, a multiinstitutional safety and efficacy trial for U.S. Drug Administration (USDA) licensure in dogs with locally controlled stage II/III oral melanoma was initiated in 2006 with granting of conditional licensure in 2007. Results of this trial documented a statistically significant improvement in survival for vaccinates compared to historic controls,85 and a full licensure for the huTyr-based canine melanoma vaccine from USDA-Centers for Veterinary Biology (CVB) was received in 2009. Subsequently, we have investigated the efficacy of local tumor control and use of muTyr-based DNA vaccination in dogs with digit melanoma. These investigations have found an improvement in survival compared to historic outcomes with digit amputation only and also documented a decreased prognosis for dogs with advanced stage disease and/or increased time from digit amputation to the start of vaccination.146
A similar approach has been used in human patients with metastatic melanoma in the minimal residual disease setting. Although no clinical response data are available since these patients did not have measurable disease, several phase I trials of xenogeneic DNA vaccines have been completed. Across studies of tyrosinase and gp100 DNA immunization, approximately 40% of patients develop quantifiable CD8+ T-cell responses to the syngeneic human target antigen.147-149 Two additional exciting new approaches that appear to confer a survival benefit in human metastatic melanoma include the use of the anti-CTLA-4 antibody, ipilimumab (Yervoy, Bristol-Myers Squibb), and the selective BRAF inhibitors, vemurafenib (Zelboraf, Genentech) and dabrafenib (GSK2118436, GlaxoSmithKline), in patients who are BRAF V600 mutation positive.150-152
CMM strongly appears to be a more clinically faithful therapeutic model for human melanoma when compared to more traditional mouse systems because both human and canine diseases are chemoresistant, are radioresistant, share similar metastatic phenotypes/site selectivity, and occur spontaneously in an outbred, immunocompetent scenario. It is hoped in the future that this same vaccine also plays a role in the treatment of melanoma in other species (e.g., horses, cats, humans) due to its xenogeneic origins139 and in melanoma prevention once the genetic determinants of melanoma risk in dogs are further defined. The xenogeneic DNA vaccine platform holds promise for investigations with other antigen targets. To this end, we have recently completed a phase I study of murine CD20 DNA vaccination in dogs with B-cell non-Hodgkin’s lymphoma. We also have phase I and phase II studies initiating shortly, utilizing murine CD20 and rat HER2 DNA vaccination.
References
1. Goldschmidt, MH. Pigmented lesions of the skin. Clin Dermatol. 1994;12(4):507–514.
2. Goldschmidt, MH. Benign and malignant melanocytic neoplasms of domestic animals. Am J Dermatopathol. 1985;7(Suppl):203–212.
3. Smith, SH, Goldschmidt, MH, McManus, PM. A comparative review of melanocytic neoplasms. Vet Pathol. 2002;39(6):651–678.
4. Leiter, U, Garbe, C. Epidemiology of melanoma and nonmelanoma skin cancer: the role of sunlight. Adv Exp Med Biol. 2008;624:89–103.
5. Ragnarsson-Olding, BK. Spatial density of primary malignant melanoma in sun-shielded body sites: a potential guide to melanoma genesis. Acta Oncol. 2011;50(3):323–328.
6. Todoroff, RJ, Brodey, RS. Oral and pharyngeal neoplasia in the dog: a retrospective survey of 361 cases. J Am Vet Med Assoc. 1979;175(6):567–571.
7. Wallace, J, Matthiesen, DT, Patnaik, AK. Hemimaxillectomy for the treatment of oral tumors in 69 dogs. Vet Surg. 1992;21(5):337–341.
8. Hahn, KA, DeNicola, DB, Richardson, RC, et al. Canine oral malignant melanoma: prognostic utility of an alternative staging system. J Small Anim Pract. 1994;35(5):251–256.
9. Macewen, EG, Patnaik, AK, Harvey, HJ, et al. Canine oral melanoma: comparison of surgery versus surgery plus Corynebacterium parvum. Cancer Invest. 1986;4(5):397–402.
10. Harvey, HJ, Macewen, EG, Braun, D, et al. Prognostic criteria for dogs with oral melanoma. J Am Vet Med Assoc. 1981;178(6):580–582.
11. Bradley, RL, Macewen, EG, Loar, AS. Mandibular resection for removal of oral tumors in 30 dogs and 6 cats. J Am Vet Med Assoc. 1984;184(4):460–463.
12. Harvey, HJ. Oral tumors. Vet Clin North Am Small Anim Pract. 1985;15(3):493–500.
13. Kosovsky, JK, Matthiesen, DT, Marretta, SM, et al. Results of partial mandibulectomy for the treatment of oral tumors in 142 dogs. Vet Surg. 1991;20(6):397–401.
14. Schobert, CS, Labelle, P, Dubielzig, RR. Feline conjunctival melanoma: histopathological characteristics and clinical outcomes. Vet Ophthalmol. 2010;13(1):43–46.
15. Planellas, M, Pastor, J, Torres, MD, et al. Unusual presentation of a metastatic uveal melanoma in a cat. Vet Ophthalmol. 2010;13(6):391–394.
16. Munday, JS, French, AF, Martin, SJ. Cutaneous malignant melanoma in an 11-month-old Russian blue cat. N Z Vet J. 2011;59(3):143–146.
17. Morges, MA, Zaks, K. Malignant melanoma in pleural effusion in a 14-year-old cat. J Feline Med Surg. 2011;13(7):532–535.
18. Farrelly, J, Denman, DL, Hohenhaus, AE, et al. Hypofractionated radiation therapy of oral melanoma in five cats. Vet Radiol Ultrasound. 2004;45(1):91–93.
19. Grahn, BH, Peiffer, RL, Cullen, CL, et al. Classification of feline intraocular neoplasms based on morphology, histochemical staining, and immunohistochemical labeling. Vet Ophthalmol. 2006;9(6):395–403.
20. Patnaik, AK, Mooney, S. Feline melanoma: a comparative study of ocular, oral, and dermal neoplasms. Vet Pathol. 1988;25(2):105–112.
21. Luna, LD, Higginbotham, ML, Henry, CJ, et al. Feline non-ocular melanoma: a retrospective study of 23 cases (1991-1999). J Feline Med Surg. 2000;2(4):173–181.
22. Bostock, DE. Prognosis after surgical excision of canine melanomas. Vet Pathol. 1979;16(1):32–40.
23. Spangler, WL, Kass, PH. The histologic and epidemiologic bases for prognostic considerations in canine melanocytic neoplasia. Vet Pathol. 2006;43(2):136–149.
24. Smedley, RC, Lamoureux, J, Sledge, DG, et al. Immunohistochemical diagnosis of canine oral amelanotic melanocytic neoplasms. Vet Pathol. 2011;48(1):32–40.
25. Choi, C, Kusewitt, DF. Comparison of tyrosinase-related protein-2, S-100, and Melan A immunoreactivity in canine amelanotic melanomas. Vet Pathol. 2003;40(6):713–718.
26. Berrington, AJ, Jimbow, K, Haines, DM. Immunohistochemical detection of melanoma-associated antigens on formalin-fixed, paraffin-embedded canine tumors. Vet Pathol. 1994;31(4):455–461.
27. Koenig, A, Wojcieszyn, J, Weeks, BR, et al. Expression of S100a, vimentin, NSE, and melan A/MART-1 in seven canine melanoma cells lines and twenty-nine retrospective cases of canine melanoma. Vet Pathol. 2001;38(4):427–435.
28. Ramos-Vara, JA, Beissenherz, ME, Miller, MA, et al. Retrospective study of 338 canine oral melanomas with clinical, histologic, and immunohistochemical review of 129 cases. Vet Pathol. 2000;37(6):597–608.
29. Sandusky, GEJ, Carlton, WW, Wightman, KA. Immunohistochemical staining for S100 protein in the diagnosis of canine amelanotic melanoma. Vet Pathol. 1985;22(6):577–581.
30. Giudice, C, Ceciliani, F, Rondena, M, et al. Immunohistochemical investigation of PNL2 reactivity of canine melanocytic neoplasms and comparison with Melan A. J Vet Diagn Invest. 2010;22(3):389–394.
31. Ramos-Vara, JA, Miller, MA. Immunohistochemical identification of canine melanocytic neoplasms with antibodies to melanocytic antigen PNL2 and tyrosinase: comparison with Melan A. Vet Pathol. 2011;48(2):443–450.
32. Palmieri, G, Capone, M, Ascierto, ML, et al. Main roads to melanoma. J Transl Med. 2009;7:86.
33. Bollag, G, Hirth, P, Tsai, J, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467(7315):596–599.
34. Shelly, S, Chien, MB, Yip, B, et al. Exon 15 BRAF mutations are uncommon in canine oral malignant melanomas. Mamm Genome. 2005;16(3):211–217.
35. Roels, S, Tilmant, K, Ducatelle, R. p53 expression and apoptosis in melanomas of dogs and cats. Res Vet Sci. 2001;70(1):19–25.
36. Roels, SL, van Daele, AJ, van Marck, EA, et al. DNA ploidy and nuclear morphometric variables for the evaluation of melanocytic tumors in dogs and cats. Am J Vet Res. 2000;61(9):1074–1079.
37. Modiano, JF, Ritt, MG, Wojcieszyn, J. The molecular basis of canine melanoma: pathogenesis and trends in diagnosis and therapy. J Vet Intern Med. 1999;13(3):163–174.
38. Ritt, MG, Or, J, Wojcieszyn, J, et al. Sustained nuclear localization of p21/WAF-1 upon growth arrest induced by contact inhibition. Cancer Lett. 2000;158(1):73–84.
39. Han, JI, Kim, DY, Na, KJ. Dysregulation of the Wnt/beta-catenin signaling pathway in canine cutaneous melanotic tumor. Vet Pathol. 2010;47(2):285–291.
40. Stell, AJ, Dobson, JM, Scase, TJ, et al. Evaluation of variants of melanoma-associated antigen genes and mRNA transcripts in melanomas of dogs. Am J Vet Res. 2009;70(12):1512–1520.
41. Thamm, DH, Huelsmeyer, MK, Mitzey, AM, et al. RT-PCR-based tyrosine kinase display profiling of canine melanoma: IGF-1 receptor as a potential therapeutic target. Melanoma Res. 2010;20(1):35–42.
42. Kent, MS, Collins, CJ, Ye, F. Activation of the AKT and mammalian target of rapamycin pathways and the inhibitory effects of rapamycin on those pathways in canine malignant melanoma cell lines. Am J Vet Res. 2009;70(2):263–269.
43. Taylor, KH, Smith, AN, Higginbotham, M, et al. Expression of vascular endothelial growth factor in canine oral malignant melanoma. Vet Comp Oncol. 2007;5(4):208–218.
44. Murua, EH, Gunther, K, Richter, A, et al. Absence of ras-gene hot-spot mutations in canine fibrosarcomas and melanomas. Anticancer Res. 2004;24(5A):3027–3028.
45. Dincer, Z, Jasani, B, Haywood, S, et al. Metallothionein expression in canine and feline mammary and melanotic tumours. J Comp Pathol. 2001;125(2-3):130–136.
46. Stiles, J, Bienzle, D, Render, JA, et al. Use of nested polymerase chain reaction (PCR) for detection of retroviruses from formalin-fixed, paraffin-embedded uveal melanomas in cats. Vet Ophthalmol. 1999;2(2):113–116.
47. Mayr, B, Eschborn, U, Schleger, W, et al. Cytogenetic studies in a canine malignant melanoma. J Comp Pathol. 1992;106(3):319–322.
48. Mayr, B, Schaffner, G, Reifinger, M, et al. N-ras mutations in canine malignant melanomas. Vet J. 2003;165(2):169–171.
49. Mayr, B, Reifinger, M, Grohe, D, et al. Cytogenetic alterations in feline melanoma. Vet J. 2000;159(1):97–100.
50. Mayr, B, Wilhelm, B, Reifinger, M, et al. Absence of p21 WAF1 and p27 kip1 gene mutations in various feline tumours. Vet Res Commun. 2000;24(2):115–124.
51. Sulaimon, SS, Kitchell, BE. The basic biology of malignant melanoma: molecular mechanisms of disease progression and comparative aspects. J Vet Intern Med. 2003;17(6):760–772.
52. Murakami, A, Mori, T, Sakai, H, et al. Analysis of KIT expression and KIT exon 11 mutations in canine oral malignant melanomas. Vet Comp Oncol. 2011;9(3):219–224.
53. Laprie, C, Abadie, J, Amardeilh, MF, et al. MIB-1 immunoreactivity correlates with biologic behaviour in canine cutaneous melanoma. Vet Dermatol. 2001;12(3):139–147.
54. Millanta, F, Fratini, F, Corazza, M, et al. Proliferation activity in oral and cutaneous canine melanocytic tumours: correlation with histological parameters, location, and clinical behaviour. Res Vet Sci. 2002;73(1):45–51.
55. Vail, DM, Macewen, EG. Spontaneously occurring tumors of companion animals as models for human cancer. Cancer Invest. 2000;18(8):781–792.
56. Esplin, DG. Survival of dogs following surgical excision of histologically well-differentiated melanocytic neoplasms of the mucous membranes of the lips and oral cavity. Vet Pathol. 2008;45(6):889–896.
57. Spangler, WL, Kass, PH. The histologic and epidemiologic bases for prognostic considerations in canine melanocytic neoplasia. Vet Pathol. 2006;43(2):136–149.
58. Bergin, IL, Smedley, RC, Esplin, DG, et al. Prognostic evaluation of Ki67 threshold value in canine oral melanoma. Vet Pathol. 2011;48(1):41–53.
59. Henry, CJ, Brewer, WG, Jr., Whitley, EM, et al. Canine digital tumors: a veterinary cooperative oncology group retrospective study of 64 dogs. J Vet Intern Med. 2005;19(5):720–724.
60. Wobeser, BK, Kidney, BA, Powers, BE, et al. Diagnoses and clinical outcomes associated with surgically amputated canine digits submitted to multiple veterinary diagnostic laboratories. Vet Pathol. 2007;44(3):355–361.
61. Marino, DJ, Matthiesen, DT, Stefanacci, D, et al. Evaluation of dogs with digit masses: 117 cases (1981-1991). J Am Vet Med Assoc. 1995;207:726–728.
62. Piliang, MP. Acral lentiginous melanoma. Clin Lab Med. 2011;31(2):281–288.
63. Smedley, RC, Spangler, WL, Esplin, DG, et al. Prognostic markers for canine melanocytic neoplasms: a comparative review of the literature and goals for future investigation. Vet Pathol. 2011;48(1):54–72.
64. Freeman, KP, Hahn, KA, Harris, FD, et al. Treatment of dogs with oral melanoma by hypofractionated radiation therapy and platinum-based chemotherapy (1987-1997). J Vet Intern Med. 2003;17(1):96–101.
65. Proulx, DR, Ruslander, DM, Dodge, RK, et al. A retrospective analysis of 140 dogs with oral melanoma treated with external beam radiation. Vet Radiol Ultrasound. 2003;44(3):352–359.
66. Theon, AP, Rodriguez, C, Madewell, BR. Analysis of prognostic factors and patterns of failure in dogs with malignant oral tumors treated with megavoltage irradiation. J Am Vet Med Assoc. 1997;210(6):778–784.
67. Williams, LE, Packer, RA. Association between lymph node size and metastasis in dogs with oral malignant melanoma: 100 cases (1987-2001). J Am Vet Med Assoc. 2003;222(9):1234–1236.
68. Leong, SP, Accortt, NA, Essner, R, et al. Impact of sentinel node status and other risk factors on the clinical outcome of head and neck melanoma patients. Arch Otolaryngol Head Neck Surg. 2006;132(4):370–373.
69. Herring, ES, Smith, MM, Robertson, J. Lymph node staging of oral and maxillofacial neoplasms in 31 dogs and cats. J Vet Dent. 2002;19(3):122–126.
70. Nwogu, CE, Kanter, PM, Anderson, TM. Pulmonary lymphatic mapping in dogs: use of technetium sulfur colloid and isosulfan blue for pulmonary sentinel lymph node mapping in dogs. Cancer Invest. 2002;20(7-8):944–947.
71. Yudd, AP, Kempf, JS, Goydos, JS, et al. Use of sentinel node lymphoscintigraphy in malignant melanoma. Radiographics. 1999;19(2):343–353.
72. Wells, S, Bennett, A, Walsh, P, et al. Clinical usefulness of intradermal fluorescein and patent blue violet dyes for sentinel lymph node identification in dogs. Vet Comp Oncol. 2006;4(2):114–122.
73. Suga, K, Karino, Y, Fujita, T, et al. Cutaneous drainage lymphatic map with interstitial multidetector-row computed tomographic lymphography using iopamidol: preliminary results. Lymphology. 2007;40(2):63–73.
74. Liuti, T, de Vos, J, Bosman, T, et al. 67Gallium citrate scintigraphy to assess metastatic spread in a dog with an oral melanoma. J Small Anim Pract. 2009;50(1):31–34.
75. Wallace, J, Matthiesen, DT, Patnaik, AK. Hemimaxillectomy for the treatment of oral tumors in 69 dogs. Vet Surg. 1992;21(5):337–341.
76. Schwarz, PD, Withrow, SJ, Curtis, CR, et al. Partial maxillary resection as a treatment for oral cancer in 61 Dogs. J Am Anim Hosp Assoc. 1991;27:617–624.
77. Lascelles, BD, Thomson, MJ, Dernell, WS, et al. Combined dorsolateral and intraoral approach for the resection of tumors of the maxilla in the dog. J Am Anim Hosp Assoc. 2003;39(3):294–305.
78. Schwarz, PD, Withrow, SJ, Curtis, CR, et al. Mandibular resection as a treatment for oral cancer in 81 dogs. J Am Anim Hosp Assoc. 1991;27:601–610.
79. Kosovsky, JK, Matthiesen, DT, Marretta, SM, et al. Results of partial mandibulectomy for the treatment of oral tumors in 142 dogs. Vet Surg. 1991;20(6):397–401.
80. Harvey, HJ, MacEwen, EG, Braun, D, et al. Prognostic criteria for dogs with oral melanoma. J Am Vet Med Assoc. 1981;178(6):580–582.
81. Kurzman, ID, MacEwen, EG, Rosenthal, RC, et al. Adjuvant therapy for osteosarcoma in dogs: results of randomized clinical trials using combined liposome-encapsulated muramyl tripeptide and cisplatin. Clin Cancer Res. 1995;1:1595–1601.
82. Fox, LE, Geoghegan, SL, Davis, LH, et al. Owner satisfaction with partial mandibulectomy or maxillectomy for treatment of oral tumors in 27 dogs. J Am Anim Hosp Assoc. 1997;33:25–31.
83. Liptak, JM, Dernell, WS, Rizzo, SA, et al. Partial foot amputation in 11 dogs. J Am Anim Hosp Assoc. 2005;41(1):47–55.
84. Bergman, PJ, Wolchok, JD. Of mice and men (and dogs): Development of a xenogeneic DNA vaccine for canine oral malignant melanoma. Cancer Therapy. 2008;6:817–826.
85. Grosenbaugh, DA, Leard, AT, Bergman, PJ, et al. Safety and efficacy of a xenogeneic DNA vaccine encoding for human tyrosinase as adjunctive treatment for oral malignant melanoma in dogs following surgical excision of the primary tumor. Am J Vet Res. 2011;72(12):1631–1638.
86. Morton, DL. Cytoreductive surgery and adjuvant immunotherapy in the management of metastatic melanoma. Tumori. 2001;87(4):S57–S59.
87. Bateman, KE, Catton, PA, Pennock, PW, et al. 0–7-21 radiation therapy for the treatment of canine oral melanoma. J Vet Intern Med. 1994;8(4):267–272.
88. Blackwood, L, Dobson, JM. Radiotherapy of oral malignant melanomas in dogs. J Am Vet Med Assoc. 1996;209(1):98–102.
89. Theon, AP, Rodriguez, C, Madewell, BR. Analysis of prognostic factors and patterns of failure in dogs with malignant oral tumors treated with megavoltage irradiation. J Am Vet Med Assoc. 1997;210(6):778–784.
90. Proulx, DR, Ruslander, DM, Dodge, RK, et al. A retrospective analysis of 140 dogs with oral melanoma treated with external beam radiation. Vet Radiol Ultrasound. 2003;44(3):352–359.
91. Freeman, KP, Hahn, KA, Harris, FD, et al. Treatment of dogs with oral melanoma by hypofractionated radiation therapy and platinum-based chemotherapy (1987-1997). J Vet Intern Med. 2003;17(1):96–101.
92. Murphy, S, Hayes, AM, Blackwood, L, et al. Oral malignant melanoma: the effect of coarse fractionation radiotherapy alone or with adjuvant carboplatin therapy. Vet Comp Oncol. 2005;3(4):222–229.
93. Khan, N, Khan, MK, Almasan, A, et al. The evolving role of radiation therapy in the management of malignant melanoma. Int J Radiat Oncol Biol Phys. 2011;80(3):645–654.
94. Theon, AP, Madewell, BR, Moore, AS, et al. Localized thermo-cisplatin therapy: a pilot study in spontaneous canine and feline tumours. Int J Hyperthermia. 1991;7(6):881–892.
95. Bergman, PJ, MacEwen, EG, Kurzman, ID, et al. Amputation and carboplatin for treatment of dogs with osteosarcoma: 48 cases (1991 to 1993). J Vet Intern Med. 1996;10(2):76–81.
96. Rassnick, KM, Ruslander, DM, Cotter, SM, et al. Use of carboplatin for treatment of dogs with malignant melanoma: 27 cases (1989-2000). J Am Vet Med Assoc. 2001;218(9):1444–1448.
97. Hume, KR, Johnson, JL, Williams, LE. Adverse effects of concurrent carboplatin chemotherapy and radiation therapy in dogs. J Vet Intern Med. 2009;23(1):24–30.
98. Veterinary Co-operative Oncology Group—Common Terminology Criteria for Adverse Events (VCOG-CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.0. Vet Comp Oncol. 2004;2(4):195–213.
99. Wergin, MC, Ballmer-Hofer, K, Roos, M, et al. Preliminary study of plasma vascular endothelial growth factor (VEGF) during low- and high-dose radiation therapy of dogs with spontaneous tumors. Vet Radiol Ultrasound. 2004;45(3):247–254.
100. Wergin, MC, Kaser-Hotz, B. Plasma vascular endothelial growth factor (VEGF) measured in seventy dogs with spontaneously occurring tumours. In Vivo. 2004;18(1):15–19.
101. Wergin, MC, Roos, M, Inteeworn, N, et al. The influence of fractionated radiation therapy on plasma vascular endothelial growth factor (VEGF) concentration in dogs with spontaneous tumors and its impact on outcome. Radiother Oncol. 2006;79(2):239–244.
102. Ladue, T, Klein, MK. Toxicity criteria of the veterinary radiation therapy oncology group. Vet Radiol Ultrasound. 2001;42(5):475–476.
103. Cahan, WG, Woodard, HQ. Sarcoma arising in irradiated bone; report of 11 cases. Cancer. 1948;1(1):3–29.
104. Seguin, B, McDonald, DE, Kent, MS, et al. Tolerance of cutaneous or mucosal flaps placed into a radiation therapy field in dogs. Vet Surg. 2005;34(3):214–222.
105. Kitchell, BE, Brown, DM, Luck, EE, et al. Intralesional implant for treatment of primary oral malignant melanoma in dogs. J Am Vet Med Assoc. 1994;204(2):229–236.
106. Theon, AP, Madewell, BR, Moore, AS, et al. Localized thermo-cisplatin therapy: a pilot study in spontaneous canine and feline tumours. Int J Hyperthermia. 1991;7(6):881–892.
107. Spugnini, EP, Dragonetti, E, Vincenzi, B, et al. Pulse-mediated chemotherapy enhances local control and survival in a spontaneous canine model of primary mucosal melanoma. Melanoma Res. 2006;16(1):23–27.
108. Rassnick, KM, Ruslander, DM, Cotter, SM, et al. Use of carboplatin for treatment of dogs with malignant melanoma: 27 cases (1989-2000). J Am Vet Med Assoc. 2001;218(9):1444–1448.
109. Boria, PA, Murry, DJ, Bennett, PF, et al. Evaluation of cisplatin combined with piroxicam for the treatment of oral malignant melanoma and oral squamous cell carcinoma in dogs. J Am Vet Med Assoc. 2004;224(3):388–394.
110. Page, RL, Thrall, DE, Dewhirst, MW, et al. Phase I study of melphalan alone and melphalan plus whole body hyperthermia in dogs with malignant melanoma. Int J Hyperthermia. 1991;7(4):559–566.
111. Ogilvie, GK, Reynolds, HA, Richardson, RC, et al. Phase II evaluation of doxorubicin for treatment of various canine neoplasms. J Am Vet Med Assoc. 1989;195(11):1580–1583.
112. Aigner, K, Hild, P, Breithaupt, H, et al. Isolated extremity perfusion with DTIC: an experimental and clinical study. Anticancer Res. 1983;3(2):87–93.
113. Murphy, S, Hayes, AM, Blackwood, L, et al. Oral malignant melanoma: the effect of coarse fractionation radiotherapy alone or with adjuvant carboplatin therapy. Vet Comp Oncol. 2005;3(4):222–229.
114. O’Day, S, Boasberg, P. Management of metastatic melanoma 2005. Surg Oncol Clin N Am. 2006;15(2):419–437.
115. Martinez, CM, Penafiel-Verdu, C, Vilafranca, M, et al. Cyclooxygenase-2 expression is related with localization, proliferation, and overall survival in canine melanocytic neoplasms. Vet Pathol. 2011;48(6):1204–1211.
116. Pires, I, Garcia, A, Prada, J, et al. COX-1 and COX-2 expression in canine cutaneous, oral and ocular melanocytic tumours. J Comp Pathol. 2010;143(2-3):142–149.
117. Paglia, D, Dubielzig, RR, Kado-Fong, HK, et al. Expression of cyclooxygenase-2 in canine uveal melanocytic neoplasms. Am J Vet Res. 2009;70(10):1284–1290.
118. Alexander, AN, Huelsmeyer, MK, Mitzey, A, et al. Development of an allogeneic whole-cell tumor vaccine expressing xenogeneic gp100 and its implementation in a phase II clinical trial in canine patients with malignant melanoma. Cancer Immunol Immunother. 2006;55(4):433–442.
119. Hogge, GS, Burkholder, JK, Culp, J, et al. Preclinical development of human granulocyte-macrophage colony-stimulating factor-transfected melanoma cell vaccine using established canine cell lines and normal dogs. Cancer Gene Ther. 1999;6(1):26–36.
120. Macewen, EG, Kurzman, ID, Vail, DM, et al. Adjuvant therapy for melanoma in dogs: results of randomized clinical trials using surgery, liposome-encapsulated muramyl tripeptide, and granulocyte macrophage colony-stimulating factor. Clin Cancer Res. 1999;5(12):4249–4258.
121. Bianco, SR, Sun, J, Fosmire, SP, et al. Enhancing antimelanoma immune responses through apoptosis. Cancer Gene Ther. 2003;10(9):726–736.
122. Dow, SW, Elmslie, RE, Willson, AP, et al. In vivo tumor transfection with superantigen plus cytokine genes induces tumor regression and prolongs survival in dogs with malignant melanoma. J Clin Invest. 1998;101(11):2406–2414.
123. Gyorffy, S, Rodriguez-Lecompte, JC, Woods, JP, et al. Bone marrow-derived dendritic cell vaccination of dogs with naturally occurring melanoma by using human gp100 antigen. J Vet Intern Med. 2005;19(1):56–63.
124. Helfand, SC, Soergel, SA, Modiano, JF, et al. Induction of lymphokine-activated killer (LAK) activity in canine lymphocytes with low dose human recombinant interleukin-2 in vitro. Cancer Biother. 1994;9(3):237–244.
125. Mayayo, SL, Prestigio, S, Maniscalco, L, et al. Chondroitin sulfate proteoglycan-4: a biomarker and a potential immunotherapeutic target for canine malignant melanoma. Vet J. 2011;190(2):e26–e30.
126. Moriyama, M, Kano, R, Maruyama, H, et al. Small interfering RNA (siRNA) against the survivin gene increases apoptosis in a canine melanoma cell line. J Vet Med Sci. 2010;72(12):1643–1646.
127. Watanabe, Y, Kano, R, Maruyama, H, et al. Small interfering RNA (siRNA) against the Bcl-2 gene increases apoptosis in a canine melanoma cell line. J Vet Med Sci. 2010;72(3):383–386.
128. Thamm, DH, Kurzman, ID, Clark, MA, et al. Preclinical investigation of PEGylated tumor necrosis factor alpha in dogs with spontaneous tumors: phase I evaluation. Clin Cancer Res. 2010;16(5):1498–1508.
129. Finocchiaro, LM, Fiszman, GL, Karara, AL, et al. Suicide gene and cytokines combined nonviral gene therapy for spontaneous canine melanoma. Cancer Gene Ther. 2008;15(3):165–172.
130. Finocchiaro, LM, Glikin, GC. Cytokine-enhanced vaccine and suicide gene therapy as surgery adjuvant treatments for spontaneous canine melanoma. Gene Ther. 2008;15(4):267–276.
131. Gil-Cardeza, ML, Villaverde, MS, Fiszman, GL, et al. Suicide gene therapy on spontaneous canine melanoma: correlations between in vivo tumors and their derived multicell spheroids in vitro. Gene Ther. 2010;17(1):26–36.
132. von Euler, H, Sadeghi, A, Carlsson, B, et al. Efficient adenovector CD40 ligand immunotherapy of canine malignant melanoma. J Immunother. 2008;31(4):377–384.
133. Horiuchi, Y, Tominaga, M, Ichikawa, M, et al. Relationship between regulatory and type 1 T cells in dogs with oral malignant melanoma. Microbiol Immunol. 2010;54(3):152–159.
134. Tominaga, M, Horiuchi, Y, Ichikawa, M, et al. Flow cytometric analysis of peripheral blood and tumor-infiltrating regulatory T cells in dogs with oral malignant melanoma. J Vet Diagn Invest. 2010;22(3):438–441.
135. Guevara-Patino, JA, Turk, MJ, Wolchok, JD, et al. Immunity to cancer through immune recognition of altered self: studies with melanoma. Adv Cancer Res. 2003;90:157–177.
136. Bouchard, B, Vijayasaradhi, S, Houghton, AN. Production and characterization of antibodies against human tyrosinase. J Invest Dermatol. 1994;102(3):291–295.
137. Bouchard, B, Del, MV, Jackson, IJ, et al. Molecular characterization of a human tyrosinase-related-protein-2 cDNA. Patterns of expression in melanocytic cells. Eur J Biochem. 1994;219(1-2):127–134.
138. Cangul, IT, van Garderen, E, van der Poel, HJ, et al. Tyrosinase gene expression in clear cell sarcoma indicates a melanocytic origin: insight from the first reported canine case. APMIS. 1999;107(11):982–988.
139. Phillips, JC, Lembcke, LM, Noltenius, CE, et al. Evaluation of tyrosinase expression in canine and equine melanocytic tumors. Am J Vet Res. 2012;73(2):272–278.
140. Weber, LW, Bowne, WB, Wolchok, JD, et al. Tumor immunity and autoimmunity induced by immunization with homologous DNA. J Clin Invest. 1998;102(6):1258–1264.
141. Bergman, PJ, Camps-Palau, MA, McKnight, JA, et al. Development of a xenogeneic DNA vaccine program for canine malignant melanoma at the Animal Medical Center. Vaccine. 2006;24:4582–4585.
142. Bergman, PJ. Cancer immunotherapy. Vet Clin North Am Small Anim Pract. 2010;40(3):507–518.
143. Bergman, PJ, McKnight, J, Novosad, A, et al. Long-term survival of dogs with advanced malignant melanoma after DNA vaccination with xenogeneic human tyrosinase: a phase I trial. Clin Cancer Res. 2003;9:1284–1290.
144. Liao, JC, Gregor, P, Wolchok, JD, et al. Vaccination with human tyrosinase DNA induces antibody responses in dogs with advanced melanoma. Cancer Immun. 2006;6:8.
145. Goubier, A, Fuhrmann, L, Forest, L, et al. Superiority of needle-free transdermal plasmid delivery for the induction of antigen-specific IFNgamma T cell responses in the dog. Vaccine. 2008;26:2186–2190.
146. Manley, CA, Leibman, NF, Wolchok, JD, et al. Xenogeneic murine tyrosinase DNA vaccine for malignant melanoma of the digit of dogs. J Vet Intern Med. 2011;25(1):94–99.
147. Wolchok, JD, Yuan, J, Houghton, AN, et al. Safety and immunogenicity of tyrosinase DNA vaccines in patients with melanoma. Mol Ther. 2007;15:2044–2050.
148. Yuan, J, Ku, GY, Gallardo, HF, et al. Safety and immunogenicity of a human and mouse gp100 DNA vaccine in a phase I trial of patients with melanoma. Cancer Immun. 2009;9:5.
149. Ginsberg, BA, Gallardo, HF, Rasalan, TS, et al. Immunologic response to xenogeneic gp100 DNA in melanoma patients: comparison of particle-mediated epidermal delivery with intramuscular injection. Clin Cancer Res. 2010;16(15):4057–4065.
150. Postow, MA, Callahan, MK, Wolchok, JD. The antitumor immunity of ipilimumab: (T cell) memories to last a lifetime? Clin Cancer Res. 2012;18(7):1821–1823.