References
1. Sasajima, K, Kawachi, T, Sano, T, et al. Esophageal and gastric cancers with metastasis induced in dogs by N-ethyl-N′-nitro-N nitrosoguanidine. J Natl Cancer Inst. 1977;58:1789–1794.
2. Scanziani, E, Giusti, AM, Gualtieri, M, et al. Gastric carcinoma in the Belgian shepherd dog. J Small Anim Pract. 1991;32:465–469.
3. Fonda, D, Gualtieri, M, Scanziani, E. Gastric carcinoma in the dog: a clinicopathological study of 11 cases. J Small Anim Pract. 1989;30:353–360.
4. Lubbes, D, Mandigers, PJ, Heuven, HC, et al. Incidence of gastric carcinoma in Dutch Tervueren shepherd dogs born between 1991 and 2002. Tijdschrift voor Diergeneeskunde. 2009;134:606–610.
5. Qvigstad, G, Kolbjornsen, O, Skancke, E, et al. Gastric neuroendocrine carcinoma with atrophic gastritis in the Norwegian lundehund. J Comp Pathol. 2008;139:194–201.
6. Sautter, JH, Hanlon, GF. Gastric neoplasms in the dog: a report of 20 cases. J Am Vet Med Assoc. 1975;166:691–696.
7. Patnaik, AK, Hurvitz, AI, Johnson, GE. Canine gastric adenocarcinoma. Vet Pathol. 1978;15:600–607.
8. Couto, CG, Rutgers, HC, Sherding, RG, et al. Gastrointestinal lymphoma in 20 dogs. J Vet Intern Med. 1989;3:73–78.
9. Patnaik, AK, Hurvitz, AI, Johnson, GF. Canine gastrointestinal neoplasms. Vet Pathol. 1977;14:547–555.
10. Kerpsack, SJ, Birchard, SJ. Removal of leiomyomas and other noninvasive masses from the cardiac region of the canine stomach. J Am Anim Hosp Assoc. 1994;30:500–504.
11. Dennis, MM, Bennett, N, Ehrhart, EJ. Gastric adenocarcinoma and chronic gastritis in two related Persian cats. Vet Pathol. 2006;43:358–362.
12. Bridgeford, EC, Marini, RP, Feng, Y, et al. Gastric Helicobacter species as a cause of feline gastric lymphoma: a viable hypothesis. Vet Immunol Immunopathol. 2008;123:106–113.
13. Swann, HM, Holt, DE. Canine gastric adenocarcinoma and leiomyosarcoma: a retrospective study of 21 cases (1986-1999) and literature review. J Am Anim Hosp Assoc. 2002;38:157–164.
14. Murray, M, Robinson, PB, McKeating, FJ, et al. Primary gastric neoplasia in the dog: a clinicopathological study. Vet Rec. 1972;91:474–479.
15. Kapatkin, AS, Mullen, HS, Matthiesen, DT, et al. Leiomyosarcoma in dogs: 44 cases (1983-1988). J Am Vet Med Assoc. 1992;201:1077–1079.
16. Ozaki, K, Yamagami, T, Nomura, K, et al. Mast cell tumors of the gastrointestinal tract in 39 dogs. Vet Pathol. 2002;39:557–564.
17. Brunnert, SR, Dee, LA, Herron, AJ, et al. Gastric extramedullary plasmacytoma in a dog. J Am Vet Med Assoc. 1992;200:1501–1502.
18. Zikes, CD, Spielman, B, Shapiro, W, et al. Gastric extramedullary plasmacytoma in a cat. J Vet Intern Med. 1998;12:381–383.
19. Frost, D, Lasota, J, Miettinen, M. Gastrointestinal stromal tumors and leiomyomas in the dog: a histopathologic, immunohistochemical, and molecular genetic study of 50 cases. Vet Pathol. 2003;40:42–54.
20. Carrasco, V, Canfran, S, Rodriguez-Franco, F, et al. Canine gastric carcinoma: immunohistochemical expression of cell cycle proteins (p53, p21, and p16) and heat shock proteins (Hsp27 and Hsp70). Vet Pathol. 2011;48:322–329.
21. Russell, KN, Mehler, SJ, Skorupski, KA, et al. Clinical and immunohistochemical differentiation of gastrointestinal stromal tumors from leiomyosarcomas in dogs: 42 cases (1990-2003). J Am Vet Med Assoc. 2007;230:1329–1333.
22. Esplin, DG, Wilson, SR. Gastrointestinal adenocarcinomas metastatic to the testes and associated structures in three dogs. J Am Anim Hosp Assoc. 1998;34:287–290.
23. Lingeman, CH, Garner, FM, Taylor, DON. Spontaneous gastric adenocarcinomas of dogs: a review. J Natl Cancer Inst. 1971;47:137–149.
24. Janke, L, Carlson, CS, St Hill, CA. The novel carbohydrate tumor antigen C2-O-sLe x is upregulated in canine gastric carcinomas. Vet Pathol. 2010;47:455–461.
25. Smith, TJ, Baltzer, WI, Ruaux, CG, et al. Gastric smooth muscle hamartoma in a cat. J Feline Med Surg. 2010;12:334–337.
26. Walter, MC, Goldschmidt, MH, Stone, EA, et al. Chronic hypertrophic pyloric gastropathy as a cause of pyloric obstruction in the dog. J Am Vet Med Assoc. 1985;186:157–161.
27. Kipnis, RM. Focal cystic hypertrophic gastropathy in a dog. J Am Vet Med Assoc. 1978;173:182–184.
28. Happe, RP, Van Der Gaag, W, Wolvekamp, THC, et al. Multiple polyps of the gastric mucosa in two dogs. J Small Anim Pract. 1977;18:179–189.
29. Culbertson, R, Branam, JE, Rosenblatt, LS. Esophageal/gastric leiomyoma in the laboratory beagle. J Am Vet Med Assoc. 1983;183:1168–1172.
30. Hayden, DW, Nielsen, SW. Canine alimentary neoplasia. Zentralbl Veterinarmed A. 1973;20:1–22.
31. Brodey, RS. Alimentary tract neoplasms in the cat: a clinicopathologic survey of 46 cases. Am J Vet Res. 1966;27:74–80.
32. Turk, MAM, Gallina, AM, Russell, TS. Nonhematopoietic gastrointestinal neoplasia in cats: a retrospective study of 44 cases. Vet Pathol. 1981;18:614–620.
33. Bagley, RS, Levy, JK, Malarkey, DE. Hypoglycemia associated with intra-abdominal leiomyoma and leiomyosarcoma in six dogs. J Am Vet Med Assoc. 1996;208:69–71.
34. Beaudry, D, Knapp, DW, Montgomery, T, et al. Hypoglycemia in four dogs with smooth muscle tumors. J Vet Intern Med. 1995;9:415–418.
35. de Brito Galvao, JF, Pressler, BM, Freeman, LJ, et al. Mucinous gastric carcinoma with abdominal carcinomatosis and hypergastrinemia in a dog. J Am Anim Hosp Assoc. 2009;45:197–202.
36. Rivers, BJ, Walter, PA, Johnston, GR, et al. Canine gastric neoplasia: utility of ultrasonography in diagnosis. J Am Anim Hosp Assoc. 1997;33:144–155.
37. Beck, C, Slocombe, RF, O’Neill, T, et al. The use of ultrasound in the investigation of gastric carcinoma in a dog. Aust Vet J. 2001;79:332–334.
38. Leib, MS, Larson, MM, Panciera, DL, et al. Diagnostic utility of abdominal ultrasonography in dogs with chronic vomiting. J Vet Intern Med. 2010;24:803–808.
39. Lecoindre, P, Chevallier, M. Findings on endoultrasonographic (EUS) and endoscopic examination of gastric tumours of dogs. Eur J Comp Gastroenterol. 1997;2:21–28.
40. Kubiak, K, Jankowski, M, Nicpon, J, et al. Gastroscopy in diagnosing gastric tumors in dogs. Medycyna Weterynaryjna. 2004;60:836–838.
41. Evans, SE, Bonczynski, JJ, Broussard, JD, et al. Comparison of endoscopic and full-thickness biopsy specimens for diagnosis of inflammatory bowel disease and alimentary tract lymphoma in cats. J Am Vet Med Assoc. 2006;229:1447–1450.
42. Beaumont, PR. Anastomotic jejunal ulcer secondary to gastrojejunostomy in a dog. J Am Anim Hosp Assoc. 1981;17:133–137.
43. Eisele, J, McClaran, JK, Runge, JJ, et al. Evaluation of risk factors for morbidity after pylorectomy and gastroduodenostomy in dogs. Vet Surg. 2010;39:261–267.
44. MacEwen, EG, Mooney, S, Brown, NO, et al. Management of feline neoplasms. In: Holzworth, J, eds. Diseases of the cat, vol. 1. Philadelphia: WB Saunders; 1987.
45. Olivieri, M, Gosselin, Y, Sauvageau, R. Gastric adenocarcinoma in a dog: six-and-one-half month survival following partial gastrectomy and gastroduodenostomy. J Am Anim Hosp Assoc. 1984;20:78–82.
46. Elliott, GS, Stoffregen, DA, Richardson, DC, et al. Surgical, medical, and nutritional management of gastric adenocarcinoma in a dog. J Am Vet Med Assoc. 1984;185:98–101.
47. Walter, MC, Matthiesen, DT, Stone, EA. Pylorectomy and gastroduodenostomy in the dog: technique and clinical results in 28 cases. J Am Vet Med Assoc. 1985;187:909–914.
48. McDonald, AE. Primary gastric carcinoma of the dog: review and case report. Vet Surg. 1978;3:70–73.
49. Sellon, RK, Bissonnette, K, Bunch, SE. Long-term survival after total gastrectomy for gastric adenocarcinoma in a dog. J Vet Intern Med. 1996;10:333–335.
50. Rolfe, DS, Twedt, DC, Seim, HB. Chronic regurgitation or vomiting caused by esophageal leiomyoma in three dogs. J Am Anim Hosp Assoc. 1994;30:425–430.
51. Beck, JA, Simpson, DS. Surgical treatment of gastric leiomyoma in a dog. Aust Vet J. 1999;77:161–162.
52. Pisters, PW, Kelsen, DP, Powel, SM, et al. Cancer of the stomach. In DeVita VT, Hellman S, Rosenberg SA, eds.: Cancer: principles and practice of oncology, ed 7, Philadelphia: Lippincott Williams & Wilkins, 2005.
 Section F
Section F
Hepatobiliary Tumors
Incidence and Risk Factors
Primary hepatic tumors are uncommon and account for less than 1.5% of all canine tumors and 1.0% to 2.9% of all feline tumors, but up to 6.9% of nonhematopoietic tumors in cats.1-4 Metastasis to the liver from nonhepatic neoplasia is more common and occurs 2.5 times more frequently than primary liver tumors in dogs, particularly from primary cancer of the spleen, pancreas, and GI tract.1,2 Primary hepatobiliary tumors are more common than metastatic disease in cats.4 The liver can also be involved in other malignant processes, such as lymphoma, malignant histiocytosis, and systemic mastocytosis.2,3 Nodular hyperplasia is a relatively common diagnosis in older dogs but is benign and probably does not represent a preneoplastic lesion.4
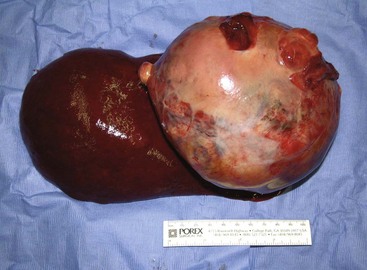
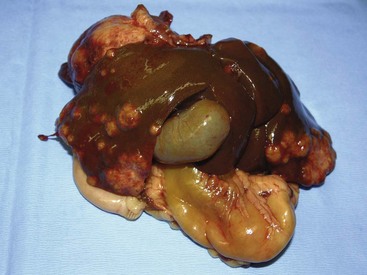
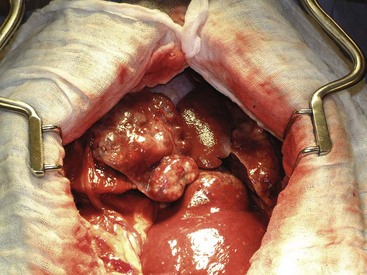
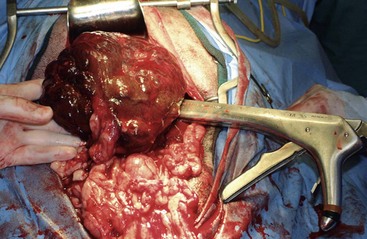
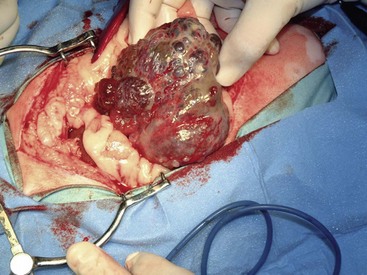
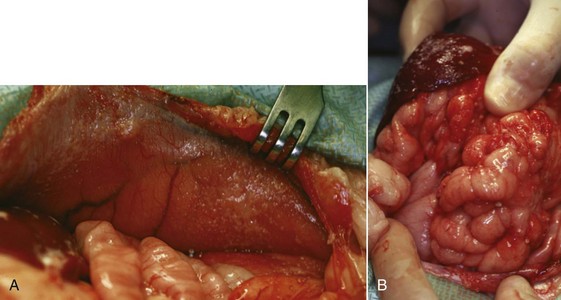
There are four basic categories of primary malignant hepatobiliary tumors in cats and dogs: hepatocellular, bile duct, neuroendocrine (or carcinoid), and mesenchymal.4 Malignant tumors are more common in dogs, whereas benign tumors occur more frequently in cats.2-8 There are three morphologic types of these primary hepatic tumors: massive, nodular, and diffuse (Table 22-7).5 Massive liver tumors are defined as a large, solitary mass confined to a single liver lobe (Figure 22-14); nodular tumors are multifocal and involve several liver lobes (Figure 22-15); and diffuse involvement may represent the final spectrum of neoplastic disease with multifocal or coalescing nodules in all liver lobes or diffuse effacement of the hepatic parenchyma (Figure 22-16).4,5
The prognosis for cats and dogs with liver tumors is determined by histology and morphology. The prognosis is good for massive hepatocellular carcinoma (HCC) and benign tumors because complete surgical resection is possible and their biologic behavior is relatively nonaggressive.7-11 In contrast, the prognosis is poor for cats with any type of malignant tumor, dogs with malignant tumors other than massive HCC, and cats and dogs with nodular and diffuse liver tumors because metastasis is more common.2-14
Pathology and Natural Behavior
Hepatocellular tumors include HCC, hepatocellular adenoma (or hepatoma), and hepatoblastoma.4 Hepatoblastoma is a rare tumor of primordial hepatic stem cells and has only been reported in one dog.15 Hepatocellular adenoma is usually an incidental finding and rarely causes clinical signs.2 Of the hepatocellular tumors, hepatocellular adenoma is more common in cats and HCC occurs more frequently in dogs.2,5,6
HCC is the most common primary liver tumor in dogs, accounting for 50% of cases, and second most common in cats.2-8 Etiologic factors implicated in the development of HCC in humans include infection with hepatitis virus B or C and cirrhosis.16 A viral etiology has also been demonstrated in woodchucks but not in cats or dogs, and cirrhosis is rare in dogs with HCC.6-9 In one study, 20% of dogs with HCC were diagnosed with additional tumors although most were benign and endocrine in origin.5
A breed and sex predisposition has not been confirmed in dogs with HCC, but miniature schnauzers and male dogs are overrepresented in some studies.5,9,11,17 Morphologically, 53% to 83% of HCCs are massive (Figure 22-17), 16% to 25% are nodular, and up to 19% are diffuse.2,5 The left liver lobes, which include the left lateral and medial lobes and papillary process of the caudate lobe, are involved in over two-thirds of dogs with massive HCC.5,9-11 Metastasis to regional lymph nodes, peritoneum, and lungs is more common in dogs with nodular and diffuse HCC.2,5,9 Other metastatic sites include the heart, kidneys, adrenal glands, pancreas, intestines, spleen, and urinary bladder.2,5,9 The metastatic rate varies from 0% to 37% for dogs with massive HCCs and 93% to 100% for dogs with nodular and diffuse HCCs.2,5-11
Bile Duct Tumors
Bile Duct Adenoma (Biliary Cystadenoma)
There are two types of bile duct tumors in cats and dogs: bile duct adenoma and carcinoma.2,5-8,12,13,18-22 Bile duct adenomas are common in cats, accounting for more than 50% of all feline hepatobiliary tumors, and are also known as biliary or hepatobiliary cystadenomas due to their cystic appearance (Figure 22-18).6-8,18-20 Male cats may be predisposed.18,20 Bile duct adenomas usually do not cause clinical signs until they reach a large size and compress adjacent organs.18-20 There is an even distribution between single and multiple lesions.6-8,18-20 Malignant transformation has been reported in humans and anaplastic changes have been observed in some feline adenomas.6,18
Bile Duct Carcinoma (Cholangiocarcinoma)
Bile duct carcinoma is the most common malignant hepatobiliary tumor in cats and the second most common in dogs.2,5-8 Bile duct carcinomas account for 22% to 41% of all malignant liver tumors in dogs.5,23 In humans, trematode infestation, cholelithiasis, and sclerosing cholangitis are known risk factors for bile duct carcinoma.24 Trematodes may also be involved in the etiology of bile duct carcinoma in cats and dogs, but they are unlikely to be a major contributor because bile duct carcinomas also occur in geographic regions outside the normal distribution of trematodes.4,8,13
A predilection for Labrador retrievers has been proposed.13 A sex predisposition has been reported for female dogs.5,12,17 In cats, however, the sex predisposition is conflicting, with both male and female cats reported to be predisposed.6-8 The distribution of morphologic types of bile duct carcinoma is similar to HCC, with 37% to 46% massive, up to 54% nodular (see Figure 22-15), and 17% to 54% diffuse.2,5,12,13 Bile duct carcinomas can be intrahepatic, extrahepatic, or within the gall bladder.2,5-8,12,13 Intrahepatic carcinomas are more common in dogs,5,12,13 whereas an equal distribution of intrahepatic and extrahepatic tumors to extrahepatic predominance has been reported in cats.6-8 Solid and cystic (or cystadenocarcinoma) bile duct carcinomas have been reported, but this distinction does not influence either treatment or prognosis.12 Bile duct carcinoma of the gall bladder is rare in both species.2,5-8,12,13
Bile duct carcinomas have an aggressive biologic behavior. Metastasis is common in dogs, with up to 88% metastasizing to the regional lymph nodes and lungs (Figure 22-19)—other sites include the heart, spleen, adrenal glands, pancreas, kidneys, and spinal cord.2,5,12,13 In cats, diffuse intraperitoneal metastasis and carcinomatosis occur in 67% to 80% of cases.6-8
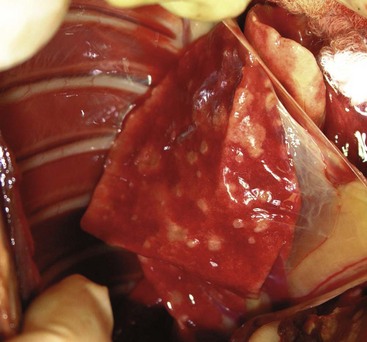
Figure 22-19 Lung metastasis in the cat with bile duct carcinoma depicted in Figure 22-15. This cat also had diffuse peritoneal metastasis.
Neuroendocrine Tumors
Neuroendocrine tumors, also known as carcinoids, are rare in cats and dogs.2,5-8,25 These tumors arise from neuroectodermal cells and are histologically differentiated from carcinomas with the use of silver stains.3,14 Neuroendocrine hepatobiliary tumors are usually intrahepatic, although extrahepatic tumors have been reported in the gall bladder.14,21,22,25 Carcinoids tend to occur at a younger age than other primary hepatobiliary tumors.5,14 Morphologically, carcinoids are nodular in 33% and diffuse in the remaining 67% of cases.5,14 Primary hepatic neuroendocrine tumors have an aggressive biologic behavior with frequent involvement of more than one liver lobe and metastasis to the regional lymph nodes, peritoneum, and lungs in cats and dogs.5,14,25 Other metastatic sites include the heart, spleen, kidneys, adrenal glands, and pancreas.14
Sarcomas
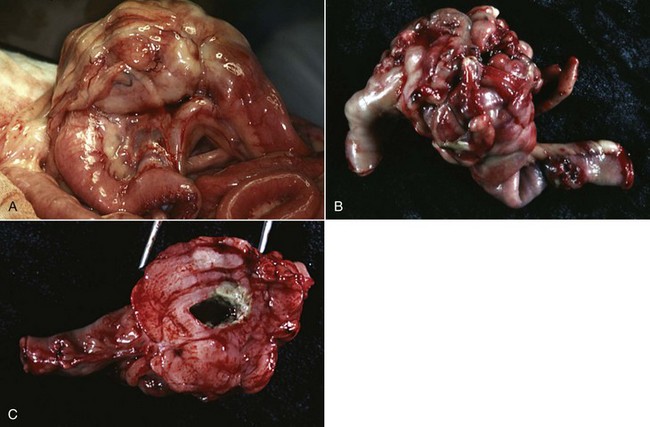
Primary and nonhematopoietic hepatic sarcomas are rare in cats and dogs.2,5-8,24 The most common primary hepatic sarcomas are hemangiosarcoma, leiomyosarcoma (see Figure 22-14), and fibrosarcoma, with hemangiosarcoma the most frequently diagnosed primary hepatic sarcoma in cats and leiomyosarcoma the most common in dogs.* The liver is a common site for metastatic hemangiosarcoma in dogs, whereas only 4% to 6% of hemangiosarcomas occur primarily in the liver.28,29 Other primary hepatic sarcomas include rhabdomyosarcoma, liposarcoma, osteosarcoma, and malignant mesenchymoma.2-8 The liver, with lungs, lymph nodes, spleen, and bone marrow, is commonly involved in dogs with disseminated histiocytic sarcoma.30,31 Benign mesenchymal tumors such as hemangiomas are rare.2-8 There are no known breed predispositions, although a male predilection has been reported.5 Diffuse morphology has not been reported with massive and nodular types accounting for 36% and 64% of sarcomas, respectively.5,24 Hepatic sarcomas have an aggressive biologic behavior, with metastasis to the spleen and lungs reported in 86% to 100% of dogs.5,24
Other Primary Hepatic Tumors
Myelolipoma is a benign hepatobiliary tumor in cats.3,4 Histologically, myelolipomas are composed of well-differentiated adipose tissue intermixed with normal hematopoietic elements.4 Chronic hypoxia has been proposed as an etiologic factor because myelolipomas have been reported in liver lobes entrapped in diaphragmatic herniae.4 Myelolipomas can be either single or multifocal.4
History and Clinical Signs
Hepatobiliary tumors are symptomatic in approximately 50% of cats and 75% of dogs, especially in animals with malignant tumors.1-15 The most common presenting signs are nonspecific, such as inappetence, weight loss, lethargy, vomiting, polydipsia-polyuria, and ascites.1-15 Weakness, ataxia, and seizures are uncommon and may be caused by hepatic encephalopathy, paraneoplastic hypoglycemia, or central nervous system metastasis.5,9,32 Icterus is more common in dogs with extrahepatic bile duct carcinomas and diffuse neuroendocrine tumors.2,5,12 Hemoperitoneum secondary to rupture of massive HCC has been reported in two dogs.33 However, these symptoms rarely assist in differentiating primary and metastatic liver tumors from nonneoplastic hepatic diseases.3 Physical examination findings can be equally unrewarding. A cranial abdominal mass is palpable in up to three-quarters of cats and dogs with liver tumors, although palpation can be misleading because hepatic enlargement may be either absent in nodular and diffuse forms of liver tumors or missed due to the location of the liver in the cranial abdominal cavity deep to the costal arch.1-15
Diagnostic Techniques and Work-Up
Hematologic and serum biochemical abnormalities are usually nonspecific. Leukocytosis, anemia, and thrombocytosis are common in dogs with liver tumors.1-14 Leukocytosis is probably caused by inflammation and necrosis associated with large liver masses.9,10 Anemia is usually mild and nonregenerative.5,11 The cause of anemia is unknown, although anemia of chronic disease, inflammation, red blood cell sequestration, microangiopathic destruction, and iron deficiency may be involved.34 Thrombocytosis, defined as a platelet count greater than 500 × 103/µL, is seen in approximately 50% of dogs with massive HCC.11 Proposed causes of thrombocytosis include anemia, iron deficiency, inflammatory cytokines, and paraneoplastic production of thrombopoietin.35-37 Anemia and thrombocytopenia are relatively common in dogs with primary and metastatic hepatic hemangiosarcomas.3 Prolonged coagulation times (e.g., increased prothrombin time, thrombin time, and activated partial thromboplastin time) and specific clotting factor abnormalities (e.g., decreased factor VIII:C and increased factor VIII:RA and fibrinogen degradation products) have been identified in dogs with hepatobiliary tumors, although these are rarely clinically relevant.38
Liver enzymes are commonly elevated in dogs with hepatobiliary tumors (Table 22-8). Increased activity of liver enzymes probably reflects hepatocellular damage or biliary stasis and is not specific for hepatic neoplasia.4 There is also no correlation between the degree of hepatic involvement and magnitude of liver enzyme alterations.4,11 The type of liver enzyme abnormalities may provide an indication of the type of tumor and differentiate primary and metastatic liver tumors.39 Alkaline phosphatase (ALP) and alanine transferase (ALT) are commonly increased in dogs with primary hepatic tumors, whereas aspartate aminotransferase (AST) and bilirubin are more consistently elevated in dogs with metastatic liver tumors.1,39 Furthermore, an AST-to-ALT ratio less than 1 is consistent with HCC or bile duct carcinoma, whereas a neuroendocrine tumor or sarcoma is more likely when the ratio is greater than 1.5 In general, however, liver enzyme elevations are not specific for the diagnosis of hepatobiliary diseases.41 Other changes in the serum biochemical profile in dogs with hepatic tumors may include hypoglycemia, hypoalbuminemia, hyperglobulinemia, and increased preprandial and postprandial bile acids.1,2,5,9-14 Hypoglycemia is a paraneoplastic syndrome reported secondary to hepatic adenoma and management is described in more detail in Chapter 5. In contrast to dogs, azotemia is often present in cats with hepatobiliary tumors and may be the only biochemical abnormality, although liver enzyme abnormalities, especially ALT, AST, and total bilirubin, are also common and are significantly higher in cats with malignant tumors.6-8
Table 22-8
Common Clinicopathologic Abnormalities in Cats and Dogs with Hepatobiliary Tumors
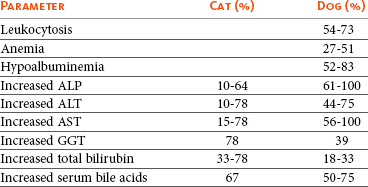
ALP, Alkaline phosphatase; ALT, alanine transferase; AST, aspartate aminotransferase; GGT, γ-glutamyltransferase.
α-Fetoprotein, an oncofetal glycoprotein, is used in the diagnosis, monitoring response to treatment, and prognostication of HCC in humans.16 In dogs, serum levels of α-fetoprotein are increased in 75% of HCC and 55% of bile duct carcinomas.42,43 However, α-fetoprotein has limited value in the diagnosis and treatment monitoring of canine HCC as serum levels of α-fetoprotein are also increased in other types of liver tumors, such as bile duct carcinoma and lymphoma, and nonneoplastic hepatic disease.43,44 Hyperferritinemia is common in dogs with histiocytic sarcoma and immune-mediated hemolytic anemia (IMHA); thus, once IMHA has been excluded, serum ferritin levels may be useful in differentiating histiocytic sarcoma from other causes of liver disease.45
Imaging
Radiographs, ultrasonography, and advanced imaging can be used for the diagnosis, staging, and surgical planning of cats and dogs with hepatobiliary tumors. A cranial abdominal mass, with caudal and lateral displacement of the stomach, is frequently noted on abdominal radiographs of cats and dogs with massive liver tumors.10,11,17 Mineralization of the biliary tree is a rare finding in dogs with bile duct carcinoma.4 Sonographic examination is recommended because these radiographic findings are not specific for the diagnosis of a hepatic mass and do not provide information on the relationship of the hepatic mass with regional anatomic structures.
Abdominal ultrasonography is the preferred method for identifying and characterizing hepatobiliary tumors in cats and dogs.20,46-50 Sonographic examination is useful in determining the presence of a hepatic mass and defining the tumor as massive, nodular, or diffuse46-50 and, in the case of cats, whether the tumor is cystic or not.20 If focal, the size and location of the mass and its relationship with adjacent anatomic structures, such as the gall bladder or caudal vena cava, can be assessed.20,46-50 Tumor vascularization can be determined using Doppler imaging techniques.4 The ultrasonographic appearance of hepatobiliary tumors varies and does not correlate with histologic tumor type.20,46-50 However, contrast-enhanced ultrasonography is useful in differentiating malignant tumors from benign lesions.51-53
Ultrasound-guided FNA or needle core biopsy of hepatic masses is a useful, minimally invasive technique to obtain cellular or tissue samples for diagnostic purposes.47-50 A coagulation profile is recommended prior to hepatic biopsy because mild-to-moderate hemorrhage is the most frequent complication, occurring in approximately 5% of cases.47-50 A correct diagnosis is obtained in up to 60% of hepatic aspirates and 90% of needle core biopsies.47-50,54 More invasive techniques, such as laparoscopy and open keyhole approaches, can also be used for the biopsy and staging of cats and dogs with suspected liver tumors. In humans, laparoscopy is recommended for local staging as up to 20% of cases do not proceed with open surgery because of either nodular or diffuse tumors or unresectable disease.55 However, for solitary and massive hepatic masses, surgical resection can be performed without a preoperative biopsy because both diagnosis and treatment can be achieved in a single procedure.
Advanced imaging techniques, such as CT and MRI, are preferred in humans for the diagnosis and staging of liver tumors.16 Unlike ultrasonography, imaging appearance may provide an indication of tumor type.16 Furthermore, CT and MRI are more sensitive for the detection of small hepatic lesions and determining the relationship of liver masses with adjacent vascular and soft tissue structures.16 The use of advanced imaging in cats and dogs with hepatobiliary tumors has not been evaluated.
Imaging is also important for the staging of cats and dogs with liver tumors. Local extension and regional metastasis can be assessed with abdominal ultrasonography, CT, MRI, or laparoscopy. The sonographic and sometimes gross appearance of nodular hyperplasia and metastatic disease is similar. In two studies, 25% to 36% of dogs with ultrasonographically detectable focal hepatic lesions were diagnosed with nodular hyperplasia.47,56 Biopsy of such lesions is recommended prior to definitively diagnosing metastatic disease and excluding animals from curative-intent surgery.57 Although rare at the time of diagnosis, three-view thoracic radiographs or advanced imaging techniques should be assessed for evidence of lung metastasis prior to treatment.
Therapy and Prognosis
Liver lobectomy is recommended for cats and dogs with any hepatic tumor that has a massive morphologic appearance, particularly HCC. Surgical techniques for liver lobectomy include finger fracture, mass ligation, mattress sutures, bipolar vessel sealant devices, and surgical stapling.58 Mass ligation is not recommended for large dogs, tumors involving either the central or right liver divisions, or tumors with a wide base.58 The finger-fracture technique, involving blunt dissection through hepatic parenchyma and individual ligation of bile ducts and vessels, is acceptable for smaller lesions. Surgical staplers or bipolar vessel sealant devices are preferred for liver lobectomy because operative time is shorter with fewer complications (Figure 22-20).11,58 A hilar dissection technique may be required for larger tumors extending to the hilus of the liver lobe because adequate margins may not be achievable with a surgical stapler.59 Advanced imaging and intraoperative ultrasonography may provide information on the relationship of right-sided and central liver tumors with the caudal vena cava prior to liver lobectomy. Right-sided liver tumors can be excised even if intimately associated with the caudal vena cava, with or without an ultrasonic aspirator, but the surgeon should be very familiar with the course of the caudal vena cava through the hepatic parenchyma. En bloc resection of the caudal vena cava with a right-sided HCC has been reported.60 In one report of 42 dogs with massive HCC treated with liver lobectomy, the intraoperative mortality rate was 4.8% and the complication rate was 28.6%.11 Complications include hemorrhage, vascular compromise to adjacent liver lobes, and transient hypoglycemia and reduced hepatic function.4,11,58
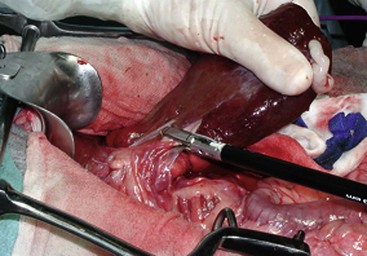
Figure 22-20 Liver lobectomy using a bipolar vessel sealant device. (Courtesy Univ. Prof. Dr. Gilles Dupré, University of Vienna, College of Veterinary Medicine.)
Prognostic factors in dogs with massive HCC include surgical treatment, side of liver involvement, ALT and AST activity, and ratios of ALP-to-AST and ALT-to-AST.11 The MST for 42 dogs with massive HCC following liver lobectomy was not reached after more than 1460 days of followup because the majority of dogs were either still alive or died of diseases unrelated to their liver tumor (Figure 22-21).11 In comparison, the MST of 270 days was significantly decreased for six dogs managed conservatively and these dogs were 15.4 times more likely to die of tumor-related causes than dogs treated surgically.11 Right-sided liver tumors, involving either the right lateral lobe or caudate process of the caudate lobe, had a poorer prognosis because intraoperative death was more likely due to caudal vena cava trauma during surgical dissection.11 There was no difference in survival time if dogs with right-sided massive HCC survived surgery.11 Increased ALT and AST were associated with a poor prognosis, which may reflect more severe hepatocellular injury secondary to either large tumor size or more aggressive biologic behavior.11

Figure 22-21 Kaplan-Meier survival curve for dogs with massive hepatocellular carcinoma. The median survival time (MST) for dogs with surgically resected tumors is significantly better than dogs not treated with curative-intent liver lobectomy. Rights were not granted to include this figure in electronic media. Please refer to the printed book. (Reprinted with permission from Liptak JM, Dernell WS, Monnet E, et al: Massive hepatocellular carcinoma in dogs: 48 cases (1992-2002), J Am Vet Med Assoc 225:1225, 2004.) J Am Vet Med Assoc
The prognosis for dogs with massive HCC is good. Local tumor recurrence is reported in 0% to 13% of dogs with massive HCC following liver lobectomy.10,11 Metastasis to other regions of the liver and lungs has been documented in 0% to 37% of dogs, but metastasis is rare in recent clinical reports and most deaths are unrelated to HCC.5,10,11
In contrast, the prognosis for dogs with nodular and diffuse HCC is poor. Surgical resection is usually not possible due to involvement of multiple liver lobes. Treatment options for nodular and diffuse HCC in humans include liver transplantation or minimally invasive procedures for regional control, such as ablation or embolization.16 Bland embolization and chemoembolization have been reported with moderate success in the palliation of four dogs with HCC.61,62 The role of radiation and chemotherapy in the management of HCC is largely unknown. RT is unlikely to be effective as the canine liver cannot tolerate cumulative doses greater than 30 Gy.4,16 Hepatocellular carcinoma is considered chemoresistant in humans because response rates are usually less than 20%.4,16 The poor response to systemic chemotherapy is probably a result of rapid development of drug resistance due to either the role of hepatocytes in detoxification or expression of P-glycoprotein, a cell membrane efflux pump associated with multidrug resistance.4 However, single-agent gemcitabine has been investigated in dogs with unresectable HCC with encouraging results.63 Novel treatment options currently being investigated in human medicine include immunotherapy, hormonal therapy with tamoxifen, and antiangiogenic agents.16
Bile Duct Tumors
Bile duct adenomas can present as either single (e.g., massive) or multifocal lesions. Liver lobectomy is recommended for cats with single bile duct adenoma (cystadenoma) or multifocal lesions confined to one to two lobes.6-8,18-20 The prognosis is very good following surgical resection with resolution of clinical signs and no reports of local recurrence or malignant transformation.8,18,19
Liver lobectomy is also recommended for cats and dogs with massive bile duct carcinoma. However, survival time has been poor in cats and dogs treated with liver lobectomy because the majority have died within 6 months due to local recurrence and metastatic disease.8,64 There is no known effective treatment for cats and dogs with nodular or diffuse bile duct carcinomas because these lesions are not amenable to surgical resection and other treatments are often not successful.
Neuroendocrine Tumors
Carcinoids have an aggressive biologic behavior and are usually not amenable to surgical resection because solitary lesions and massive morphology are rare.5,14 The efficacy of RT and chemotherapy is unknown. Prognosis is poor because metastasis to the regional lymph nodes, peritoneum, and lungs occurs in 93% of dogs and usually early in the course of disease.5,14
Sarcomas
Liver lobectomy can be attempted for solitary and massive sarcomas. However, prognosis is poor because metastatic disease is often present at the time of surgery.5,24 Chemotherapy has not been investigated in the treatment of primary hepatic sarcomas, although, similar to other solid sarcomas, response rates are likely to be poor. Doxorubicin-based protocols and ifosfamide have shown some promise with sarcomas in other locations and warrant consideration for cats and dogs with primary hepatic sarcomas.65,66
Other Primary Hepatic Tumors
Surgical resection with liver lobectomy is recommended for cats with primary hepatic myelolipoma, and the prognosis is excellent with prolonged survival time and no reports of local recurrence.4
Comparative Aspects
Hepatocellular carcinoma is one of the most common malignancies in humans as a result of viral infections with hepatitis viruses B and C and cirrhosis induced by alcohol consumption and other disease.16 A number of paraneoplastic syndromes have been described including hypoglycemia, erythrocytosis, and hypercalcemia.16 Ultrasonography is considered a good screening imaging modality, but advanced imaging with contrast-enhanced CT or MRI is preferred to determine the location, size, and extent of hepatic lesions.16 Other tests include serum α-fetoprotein, serologic tests for hepatitis B and C viruses, and histologic confirmation with core liver biopsies.16 Unlike HCC in dogs, the morphology of HCC in humans is often nodular or diffuse, which makes definitive treatment more problematic. Treatment options depend on the stage of disease and include surgery (e.g., liver lobectomy and liver transplantation), local ablative therapies (e.g., cryosurgery, ethanol or acetic acid injection, and microwave or radiofrequency ablation), regional therapies (e.g., transarterial chemotherapy, embolization, chemoembolization, or RT), and systemic treatment with chemotherapy or immunotherapy.16 Response rates to single- and multiple-agent chemotherapy protocols are less than 25%, and chemotherapy is no longer recommended for human patients with HCC.16
Bile duct carcinomas are rare and, similar to cats and dogs, often associated with a poor prognosis.26 Risk factors include primary sclerosing cholangitis, the liver flukes Opisthorchis viverrini and Clonorchis sinensis in endemic areas of Southeast Asia and China, and cholelithiasis.26 Surgical resection is preferred but, because of the high rate of local or regional recurrence, adjuvant treatment with RT or chemotherapy is recommended.26 However, because of the rarity of this tumor, studies supporting the efficacy of these adjuvant treatments are lacking. Papillary histology, extrahepatic location, and complete resection are favorable prognostic factors in humans with bile duct carcinomas.67
References
1. Strombeck, DR. Clinicopathologic features of primary and metastatic neoplastic disease of the liver in dogs. J Am Vet Med Assoc. 1978;173:267.
2. Cullen, JM, Popp, JA. Tumors of the liver and gall bladder. In Meuten DJ, ed.: Tumors in domestic animals, ed 4, Ames, Iowa: Iowa State Press, 2002.
3. Hammer, AS, Sikkema, DA. Hepatic neoplasia in the dog and cat. Vet Clin North Am Small Anim Pract. 1995;25:419.
4. Thamm, DH. Hepatobiliary tumors. In Withrow SJ, MacEwen EG, eds.: Small animal clinical oncology, ed 3, Philadelphia: WB Saunders, 2001.
5. Patnaik, AK, Hurvitz, AI, Lieberman, PH. Canine hepatic neoplasms: a clinicopathological study. Vet Pathol. 1980;17:553.
6. Patnaik, AK. A morphologic and immunohistochemical study of hepatic neoplasms in cats. Vet Pathol. 1992;29:405.
7. Post, G, Patnaik, AK. Nonhematopoietic hepatic neoplasms in cats: 21 cases (1983-1988). J Am Vet Med Assoc. 1992;201:1080.
8. Lawrence, HJ, Erb, HN, Harvey, HJ. Nonlymphomatous hepatobiliary masses in cats: 41 cases (1972 to 1991). Vet Surg. 1994;23:365.
9. Patnaik, AK, Hurvitz, AI, Lieberman, PH, et al. Canine hepatocellular carcinoma. Vet Pathol. 1981;18:427.
10. Kosovsky, JE, Manfra-Marretta, S, Matthiesen, DT, et al. Results of partial hepatectomy in 18 dogs with hepatocellular carcinoma. J Am Anim Hosp Assoc. 1989;25:203.
11. Liptak, JM, Dernell, WS, Monnet, E, et al. Massive hepatocellular carcinoma in dogs: 48 cases (1992-2002). J Am Vet Med Assoc. 2004;225:1225.
12. Patnaik, AK, Hurvitz, AI, Lieberman, PH, et al. Canine bile duct carcinoma. Vet Pathol. 1981;18:439.
13. Hayes, HM, Morin, MM, Rubenstein, DA. Canine biliary carcinoma: epidemiological comparisons with man. J Comp Pathol. 1983;93:99.
14. Patnaik, AK, Lieberman, PH, Hurvitz, AI, et al. Canine hepatic carcinoids. Vet Pathol. 1981;18:439.
15. Shiga, A, Shirota, K, Shida, T, et al. Hepatoblastoma in a dog. J Vet Med Sci. 1997;59:1167.
16. Bartlett, DL, Carr, BI, Marsh, JW. Cancer of the liver. In: DeVita VT, Hellman S, Rosenberg SA, eds. Cancer: principles and practice of oncology. Philadelphia: Lippincott Williams & Wilkins, 2005.
17. Evans, SM. The radiographic appearance of primary liver neoplasia in dogs. Vet Radiol. 1987;28:192.
18. Adler, R, Wilson, DW. Biliary cystadenomas of cats. Vet Pathol. 1995;32:415.
19. Trout, NJ, Berg, J, McMillan, MC, et al. Surgical treatment of hepatobiliary cystadenomas in cats: five cases (1988-1993). J Am Vet Med Assoc. 1995;206:505.
20. Nyland, TG, Koblik, PD, Tellyer, SE. Ultrasonographic evaluation of biliary cystadenomas in cats. Vet Radiol Ultrasound. 1999;40:300.
21. Willard, MD, Dunstan, RW, Faulkner, J. Neuroendocrine carcinoma of the gall bladder in a dog. J Am Vet Med Assoc. 1988;192:926.
22. Morrell, CN, Volk, MV, Mankowski, JL. A carcinoid tumor in the gallbladder of a dog. Vet Pathol. 2002;39:756.
23. Trigo, FJ, Thompson, H, Breeze, RG, et al. The pathology of liver tumors in the dog. J Comp Pathol. 1982;92:21.
24. Kapatkin, AS, Mullen, HS, Matthiesen, DT, et al. Leiomyosarcoma in dogs: 44 cases (1983-1988). J Am Vet Med Assoc. 1992;201:1077.
25. Patnaik, AK, Lieberman, PH, Erlandson, RA, et al. Hepatobiliary neuroendocrine carcinoma in cats: a clinicopathologic, immunohistochemical, and ultrastructural study of 17 cases. Vet Pathol. 2005;42:331.
26. Bartlett, DL, Ramanathan, RK, Deutsch, M. Cancer of the biliary tree. In DeVita VT, Hellman S, Rosenberg SA, eds.: Cancer: principles and practice of oncology, ed 7, Philadelphia: Lippincott Williams & Wilkins, 2005.
27. Scavelli, TD, Patnaik, AK, Mehlhaff, CJ, et al. Hemangiosarcoma in the cat: retrospective evaluation of 31 surgical cases. J Am Vet Med Assoc. 1985;187:817–819.
28. Brown, NO, Patnaik, AK, MacEwen, EG. Canine hemangiosarcoma: retrospective analysis of 104 cases. J Am Vet Med Assoc. 1985;186:56–58.
29. Srebernik, N, Appleby, EC. Breed prevalence and sites of haemangioma and haemangiosarcoma in dogs. Vet Rec. 1991;129:408–409.
30. Affolter, VK, Moore, PF. Canine cutaneous and systemic histiocytosis: reactive histiocytosis of dermal dendritic cells. Am J Dermatopathol. 2000;22:40.
31. Affolter, VK, Moore, PF. Localized and disseminated histiocytic sarcoma of dendritic cell origin in dogs. Vet Pathol. 2002;39:74.
32. Leifer, CE, Peterson, ME, Matus, RE, et al. Hypoglycemia associated with nonislet cell tumor in 13 dogs. J Am Vet Med Assoc. 1985;186:53.
33. Arohnson, MG, Dubiel, B, Roberts, B, et al. Prognosis for acute nontraumatic hemoperitoneum in the dog: a retrospective analysis of 60 cases (2003-2006). J Am Anim Hosp Assoc. 2009;45:72.
34. Rogers, KS. Anemia. In Ettinger SJ, Feldman EC, eds.: Textbook of veterinary internal medicine, ed 5, Philadelphia: WB Saunders, 2000.
35. Helfand, SC. Platelets and neoplasia. Vet Clin North Am Small Anim Pract. 1988;18:131.
36. Baatout, S. Interleukin-6 and megakaryocytopoiesis: an update. Ann Hematol. 1996;73:157.
37. Jelkmann, W. The role of the liver in the production of thrombopoietin compared with erythropoietin. Eur J Gastroenterol Hepatol. 2001;13:791.
38. Badylak, SF, Dodds, WJ, van Vleet, JF. Plasma coagulation factor abnormalities in dogs with naturally occurring hepatic disease. Am J Vet Res. 1983;44:2336.
39. McConnell, MF, Lumsden, JH. Biochemical evaluation of metastatic liver disease in the dog. J Am Anim Hosp Assoc. 1983;19:173.
40. Reference deleted in pages.
41. Center, SA, Slater, MR, Manwarren, T, et al. Diagnostic efficacy of serum alkaline phosphatase and γ-glutamyltransferase in dogs with histologically confirmed hepatobiliary disease: 270 cases (1980-1990). J Am Vet Med Assoc. 1992;201:1258.
42. Lowseth, LA, Gillett, NA, Chang, IY, et al. Detection of serum α-fetoprotein in dogs with hepatic tumors. J Am Vet Med Assoc. 1991;199:735.
43. Yamada, T, Fujita, M, Kitao, S, et al. Serum alpha-fetoprotein values in dogs with various hepatic diseases. J Vet Med Sci. 1999;61:657.
44. Hahn, KA, Richardson, RC. Detection of serum alpha-fetoprotein in dogs with naturally occurring malignant neoplasia. Vet Clin Pathol. 1995;24:18.
45. Friedrichs, KR, Thomas, C, Plier, M, et al. Evaluation of serum ferritin as a tumor marker for canine histiocytic sarcoma. J Vet Intern Med. 2010;24:904.
46. Feeney, DA, Johnston, GR, Hardy, RM. Two-dimensional, gray-scale ultrasonography for assessment of hepatic and splenic neoplasia in the dog and cat. J Am Vet Med Assoc. 1984;184:68.
47. Vörös, K, Vrabély, T, Papp, L, et al. Correlation of ultrasonographic and pathomorphological findings in canine hepatic diseases. J Small Anim Pract. 1991;32:627.
48. Newell, SM, Selcer, BA, Girard, E, et al. Correlations between ultrasonographic findings and specific hepatic disease in cats: 72 cases (1985-1997). J Am Vet Med Assoc. 1998;213:94.
49. Leveille, R, Partington, BP, Biller, DS, et al. Complications after ultrasound-guided biopsy of abdominal structures in dogs and cats: 246 cases (1984-1991). J Am Vet Med Assoc. 1993;203:413.
50. Barr, F. Percutaneous biopsy of abdominal organs under ultrasound guidance. J Small Anim Pract. 1995;36:105.
51. O’Brien, RT, Iani, M, Matheson, J, et al. Contrast harmonic ultrasound of spontaneous liver nodules in 32 dogs. Vet Radiol Ultrasound. 2004;45:547.
52. Kutara, K, Asano, K, Kito, A, et al. Contrast harmonic imaging of canine hepatic tumors. J Vet Med Sci. 2006;68:433.
53. Nakamura, K, Takagi, S, Sasaki, N, et al. Contrast-enhanced ultrasonography for characterization of canine focal liver lesions. Vet Radiol Ultrasound. 2010;51:79.
54. Roth, L. Comparison of liver cytology and biopsy diagnoses in dogs and cats: 56 cases. Vet Clin Pathol. 2001;30:35.
55. D’Angelica, M, Fong, Y, Weber, S, et al. The role of staging laparoscopy in hepatobiliary malignancy: prospective analysis of 401 cases. Ann Surg Oncol. 2003;10:183.
56. Cuccovillo, A, Lamb, CR. Cellular features of sonographic target lesions of the liver and spleen in 21 dogs and a cat. Vet Radiol Ultrasound. 2002;43:275.
57. Stowater, JL, Lamb, CR, Schelling, SH. Ultrasonographic features of canine hepatic nodular hyperplasia. Vet Radiol. 1990;31:268.
58. Martin, RA, Lanz, OI, Tobias, KM. Liver and biliary system. In Slatter DH, ed.: Textbook of small animal surgery, ed 3, Philadelphia: WB Saunders, 2003.
59. Covey, JL, Degner, DA, Jackson, AH, et al. Hilar liver resection in dogs. Vet Surg. 2009;38:104.
60. Seki, M, Asano, K, Ishigaki, K, et al. En block resection of a large hepatocellular carcinoma involving the caudal vena cava in a dog. J Vet Med Sci. 2011;73(5):693–696.
61. Weisse, C, Clifford, CA, Holt, D, et al. Percutaneous arterial embolization and chemoembolization for treatment of benign and malignant tumors in three dogs and a goat. J Am Vet Med Assoc. 2002;221:1430.
62. Cave, TA, Johnson, V, Beths, T, et al. Treatment of unresectable hepatocellular adenoma in dogs with transarterial iodized oil and chemotherapy with and without an embolic agent: a report of two cases. Vet Comp Oncol. 2003;1:191.
63. Elpiner, A, Brodsky, E, Hazzah, T, et al. Single agent gemcitabine chemotherapy in dogs with hepatocellular carcinoma. Vet Comp Oncol. 2011;9(4):260–268.
64. Fry, PD, Rest, JR. Partial hepatectomy in two dogs. J Small Anim Pract. 1993;34:192.
65. Ogilvie, GK, Powers, BE, Mallinckrodt, CH, et al. Surgery and doxorubicin in dogs with hemangiosarcoma. J Vet Intern Med. 1996;10:379.
66. Rassnick, KM, Frimberger, AE, Wood, CA, et al. Evaluation of ifosfamide for treatment of various canine neoplasms. J Vet Intern Med. 2000;14:271.
67. Chung, C, Bautista, N, O’Connell, TX. Prognosis and treatment of bile duct carcinoma. Am Surg. 1998;64:921.
 Section G
Section G
Intestinal Tumors
Incidence and Risk Factors
Reports vary, but overall intestinal tumors are rare in dogs and cats.1-3 In a survey of insured dogs in the United Kingdom, a standardized incidence rate of 210/100,000 dogs was reported for alimentary tumors. This accounted for 8% of all tumor submissions.4 Incidence of feline digestive neoplasia in a South African survey comprised 13.5% of all tumors, which likely included oral tumors.5 In the United States, a query of over 300,000 cat submissions to the Veterinary Medical Database (VMDB) found 8% to relate to cancer and less than 1% (13% of the cancer cases) to be intestinal neoplasia.6 Less than 1% of over 10,000 dogs submitted for necropsy at one institution were diagnosed with intestinal adenocarcinoma, which agrees with other reports.2,7,8 Regarding specific tumor types, lymphoma comprises nearly 30% of all feline tumors and 6% of all canine tumors and is the most common intestinal tumor in most reports.2,9-11 Adenocarcinoma is the second most frequent tumor in both species, with mast cell tumors in cats and leiomyosarcomas or GISTs in dogs next.
As with many cancers, incidence of intestinal neoplasia increases in older dogs and cats. Mean ages of affected cats for small and large intestinal neoplasia generally range between 10 and 12 years, and increasing risk after age 7 has been reported.2,6,12-20 Dogs are also usually middle aged or older, with mean ages most often between 6 and 9 years, possibly older (12 years) for dogs with leiomyosarcoma.11,21-24 Some earlier studies of feline lymphoma report younger median ages, most likely a result of a larger percentage of FeLV-positive cats in the study population.25,26
There is a slight sex predilection for males to develop intestinal tumors in some studies for both dogs and cats. Many studies report a near equal incidence among male and female dogs,24,27-29 although one study did find 76% of dogs with intestinal adenocarcinoma to be male.30 Males also appear overrepresented in smooth muscle tumors, comprising 82% of GI leiomyomas31 and 76% of dogs with leiomyosarcoma.23 Additionally, 90% of dogs with GI lymphoma were male.22 Furthermore, there is a slight male predominance in nonlymphomatous small intestinal tumors in dogs.11,32,33
In cats, there also is a predominance of males in some studies,17,34 with males equaling or only slightly exceeding females in others,* although three of four cats with large granular lymphoma were female.14
Siamese cats are 1.8 times more likely to develop intestinal neoplasia.6 Siamese cats are overrepresented in studies of intestinal adenocarcinoma, up to eight times greater than other breeds, suggesting a predisposition for this disease.2,6,15,32,38 Although small numbers of Siamese cats are included in many series of feline intestinal lymphoma, one study did show a significant overrepresentation.13 Otherwise, there is no breed predilection for intestinal lymphoma in cats.
In dogs, few studies of intestinal neoplasia report an overrepresentation of specific breeds. Large breed dogs in general constituted most cases in a series of smooth muscle tumors.28 Collies and German shepherd dogs are overrepresented in some reports for intestinal tumors, especially adenocarcinoma and rectal carcinoma and polyps.21,39 It is interesting to note, however, that in 104 benign and malignant tumors diagnosed in a cohort of military working dogs (German shepherd dogs and Belgian Malinois), only one (a leiomyosarcoma) was intestinal.40 Mast cell tumors have been reported primarily in Maltese, among other miniature breeds. Although these reports came from Japan where small breeds are popular, over 50% of reported cases in two series were in Maltese dogs, with a male predominance.41,42
With the exception of retroviral influence on the development of feline lymphoma, there are no known etiologic organisms or chemical agents that reliably contribute to the development of spontaneously occurring intestinal neoplasia in dogs and cats. There is a known association of FeLV and feline immunodeficiency virus (FIV) with feline lymphoma. Older cats with intestinal lymphoma are usually negative for FeLV on serology, although evaluation of feline intestinal lymphoma by polymerase chain reaction (PCR) for the long terminal repeat region and immunohistochemistry (IHC) for gp70 antigen has shown some tumors to be positive for viral DNA, even when seronegative for FeLV p27 antigen. For intestinal lymphoma, PCR was more often positive than IHC.43,44 These results suggest FeLV exposure in the development of lymphoma in some cats serologically negative for FeLV, and they support PCR as the most useful of these tests for identifying possible occult, latent, replication-deficient, or partial genome virus infection in tissue. With younger cats more often IHC positive and IHC correlating well with seropositivity, PCR may identify cats with lymphoma that have been exposed to FeLV but are seronegative.43 There is no association between retroviral infection and nonlymphomatous intestinal neoplasia in cats, with most cats testing negative for FeLV and/or FIV serologically.15,32
In other species, type-A retrovirus particles have been found in a metastatic intestinal adenocarcinoma in a boa, and cytomegalovirus has been associated with GI epithelial masses in macaque monkeys infected with simian immunodeficiency virus.45,46
Helicobacter pylori infection is associated with increased risk of gastric cancer in humans, although no such association has been confirmed in domestic animals. Concurrent lymphoma and Helicobacter infection has been reported in a cat, but causal association was not proved.47 Multiple gastroduodenal adenocarcinomas and a rectal adenoma were found in a cougar with concurrent Helicobacter-like organisms and spirochetes.48 Some cats shed Helicobacter species in the feces and thus may represent normal flora rather than pathogens.49
Finally, lymphoma (although not specifically intestinal) has been reported in a dog 4 weeks after initiation of cyclosporine and ketoconazole therapy for anal furunculosis.50 There is an association between cyclosporine use in human transplant patients and the development of lymphoma.
Pathology and Natural Behavior
Epithelial, mesenchymal, neuroendocrine, and discrete/round cell neoplasias can all be found in the intestinal tract. In both cats and dogs, lymphoma is the most common type of small intestinal neoplasia, followed by adenocarcinoma. Subtypes of feline intestinal lymphoma include lymphocytic, lymphoblastic, epitheliotropic, and large granular lymphocyte (LGL) types. Because of advances in novel targeted receptor tyrosine kinase inhibitors (TKIs) in human medicine, characteristics of GISTs have also been reported in dogs. Other tumors include leiomyosarcoma in both dogs and cats, carcinoids in dogs, and mast cell tumors in cats. There are scattered case reports of uncommon tumors, such as extramedullary plasmacytoma, extraskeletal osteosarcoma, and hemangiosarcoma. Although most small intestinal neoplasia is malignant in dogs, most rectal tumors are benign polyps, adenomas, or carcinoma in situ29,51 (Figure 22-22).
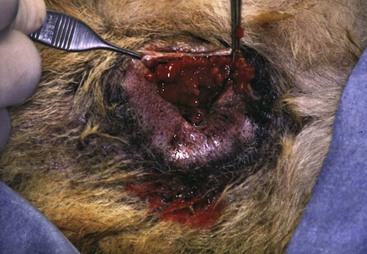
Figure 22-22 Cobblestone appearance to a rectal adenocarcinoma. Dogs with this tumor type live an average of 12 months following surgical excision.52 (Courtesy Dr. Eric Pope, Ross University, College of Veterinary Medicine.)
Most alimentary adenocarcinoma in cats is found in the small intestine.1,30,37 However, the colon and rectum are a more common site in dogs.7,8 Of colorectal adenocarcinomas, the rectum is a more common site than the colon.52 The cecum is more likely to develop leiomyosarcoma or GIST than adenocarcinoma.8,23 Histologic descriptors for carcinoma of the intestine include adeno- (forming glands), mucinous (>50% mucin), signet ring (>50% of cells have intracellular mucin), and undifferentiated or solid (no evidence of gland formation).7 Grossly, colorectal adenocarcinomas may demonstrate a pedunculated (especially in the distal rectum), cobblestone (middle rectum), or annular (middle rectum) appearance, which may relate to behavior and prognosis8,52,53 (Figure 22-23).
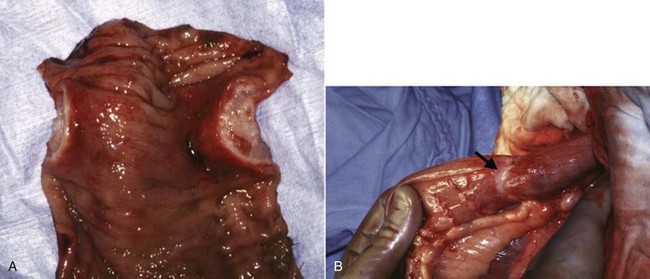
Figure 22-23 An annular form of clonic adenocarcinoma causing a stricture. The thick band of tissue (B) creating the stricture is seen on cross-section (A). In one study, dogs with this type of tumor survived an average of only 1.6 months.52 (Courtesy Dr. Eric Pope, Ross University, College of Veterinary Medicine.)
Adenomatous polyps are found in the rectum of dogs, and carcinoma in situ is found in both the colon and rectum. Most lesions are solitary, although multiple and diffuse lesions can be seen and are associated with increased recurrence rates.29 In cats, polyps are more common in the duodenum.
The term carcinoid refers to tumors that arise from the diffuse endocrine system rather than the intestinal epithelium, despite histologic similarity to carcinomas. Carcinoid cells arise from enterochromaffin cells of the intestinal mucosa and contain secretory granules that may contain substances such as 5-hydroxytryptamine (serotonin), secretin, somatostatin, and gastrin, among others.7 IHC for cytokeratin and for secretory substances such as serotonin may be positive, and serum concentration of serotonin has been documented at 10 times the normal range in one dog with a carcinoid.54 Described in many species, carcinoids may occur in both the large and the small intestines and frequently metastasize to the liver.2,8,54 Carcinoids may follow an aggressive and debilitating clinical course.54
GISTs are well documented in humans and have been reported in dogs.55 These nonlymphoid tumors of mesenchymal origin were originally diagnosed as leiomyosarcomas and some but not all were leiomyomas. Histologically, GISTs are highly cellular mesenchymal tumors that do not show ultrastructural characteristics consistent with smooth muscle differentiation. GISTs are thought to arise from multipotential stem cells phenotypically similar to interstitial cells of Cajal, driven by activating mutations of Kit. These cells regulate intestinal motility via an autonomic pacemaker effect. Although these cells can differentiate into smooth muscle cells if deprived of Kit, GISTs are a discrete clinical entity from leiomyosarcoma.56 GISTs are distinguished by high vimentin immunoreactivity, low alpha smooth muscle actin reactivity, CD117 (Kit) reactivity, and a site predilection for the large intestine (compared to the stomach for leiomyoma).31,57 Activating mutations were identified in Kit exon 11 encoding the juxtamembrane domain in two of four cases examined.31 CD117 reactivity is considered a major diagnostic criteria and in many studies is used to distinguish GISTs from leiomyosarcomas.58,59 When stratified as such, 28 of 42 leiomyosarcomas in dogs were reclassified as GISTs and only 2 of the 28 cases of GIST metastasized (7%). These investigators also found that GISTs were significantly more likely to occur in the large intestine, specifically the cecum, and leiomyosarcomas in the stomach and small intestine.58 Considering these findings, the incidence of true leiomyosarcoma is likely low because many previously reported cases may have actually been GISTs. Leiomyomas occur more commonly in the stomach but have also been reported in the esophagus, small intestine, and colorectum.31
Intestinal lymphoma in dogs occurs in the stomach and small intestine equally and more often in both of these sites than the large intestine. Lesions are typically diffuse, and neoplastic cells infiltrate the submucosa and lamina propria. Additional visceral and systemic involvement may be seen.
Intestinal lymphoma in cats was originally thought to be predominantly of B-cell origin, resulting from its origin in Peyer’s patches and germinal centers; however, some reports suggest that the incidence of T-cell lymphoma may equal or exceed that of B-cell lymphoma.20,34,44 IHC and PCR for antigen receptor rearrangement (PARR) can be useful in identifying predominant immunophenotype and clonality, as well as distinguishing lymphoma from severe inflammation.60,61 There is no clear association between the presence of FeLV antigens in tissue and clonality (B-cell versus T-cell).44 Incidence of feline intestinal lymphoma appears to have increased over the past 2 decades to an extent that may exceed that attributed to an aging cat population.62 Within a diagnosis of intestinal lymphoma, subtype also impacts behavior. In one series, cats with lymphocytic/small cell lymphoma experienced a 69% complete remission rate with prednisone and chlorambucil for a MST of nearly 2 years, whereas cats with lymphoblastic lymphoma had only an 18% complete remission rate with combination chemotherapy for a MST of less than 3 months. Cats with lymphoblastic lymphoma were more likely to have a palpable abdominal mass and require surgery for intestinal obstruction than cats with lymphocytic lymphoma.35
Other unique subsets of feline intestinal lymphoma include epitheliotropic and LGL lymphoma. Most of these cats are serologically negative for FeLV. Immunohistochemical evaluation of feline epitheliotropic lymphoma shows these tumors to be strictly T-cell in origin and 80% small/lymphocytic.34,63 In one study, although great overlap of values occurred, there was a significantly greater percentage of intraepithelial lymphocytes in neoplastic compared to normal cats and inflammatory bowel disease cases. The determination of epitheliotropism depends on the pathologist’s interpretation. As with epitheliotropic cutaneous lymphoma, microabscesses are often seen. Intraepithelial lymphocytes are richer in villous than crypt epithelium, suggesting that this diagnosis may be reliably made with endoscopic biopsies. This disease may represent a continuum from inflammatory bowel disease.34
By contrast, LGL lymphoma of the intestine (also called globule leukocyte and granulated round cell tumor) often has a rapidly progressive and fatal course.64,65 These tumors are distinguished by heterogeneous cytoplasmic granules (azurophilic on cytology and eosinophilic on histopathology with routine hematoxylin and eosin [H&E] staining) and are commonly seen in the intestinal tract (especially jejunum), occasionally with leukemic cells.66,67 Perforin-like immunoreactivity has been demonstrated and may help distinguish these from other lymphomas.14
Extramedullary plasmacytoma (EMP) refers to solitary tumors with no evidence of systemic multiple myeloma. Case reports of GI EMP in dogs and cats exist, though uncommon. In one series, one-fourth of EMPs were found in the digestive system, most in the mouth.68 One case report in a dog with EMP of the colon and rectum was associated with monoclonal gammopathy.69 Another uncommon tumor type is extraskeletal osteosarcoma, which has been reported in the duodenum of a cat. This cat had no evidence of metastasis at diagnosis but did well for only 4 months after surgery when clinical signs recurred and the cat died.70 Three of 55 extraskeletal and 145 total cases of feline osteosarcoma were of intestinal origin.71 A series of four cats was reported with intestinal hemangiosarcoma arising from four different locations within the intestines, with none surviving greater than 1 week.72 Finally, one dog was diagnosed with ganglioneuroma of the rectum and experienced long-term survival following surgical resection.73
Intestinal mast cell tumors are cited as the third most common tumor following lymphoma and adenocarcinoma in cats, but incidence and behavior are poorly reported. They have been confused with carcinoids but are distinct.12 They may present as an eosinophilic enteritis, and conversely, eosinophilic enteritis may mimic intestinal tumors.74,75 Intestinal sclerosing mast cell tumor in the cat is a potentially aggressive variant characterized by moderate-to-abundant dense stromal tissue, marked eosinophilic infiltrates, and some cases with tryptase and c-kit immunoreactivity. Ultrasonographic changes were transmural, and tumors were most commonly located in the small intestine. Outcome was reported for 25 of 50 cats, and survival was less than 2 months for 23/25; however, outcome was unknown for the remaining 25 cats.76 In dogs, intestinal mast cell tumors occur primarily in the stomach and small intestine, are typically poorly granulated, and are often immunohistochemically positive for toluidine blue, c-kit, and tryptase. Mucosal mast cells may be structurally distinct from cutaneous mast cells.41
When tumors of the GI system metastasize, sites of predilection in decreasing frequency include mesenteric lymph nodes (especially adenocarcinoma), liver (especially leiomyosarcoma), mesentery, omentum, spleen, kidney, bone, peritoneum/carcinomatosis, and lung.11,32,37,53 Interestingly, metastasis from intestinal adenocarcinoma was discovered in three dogs initially presented for testicular masses.77 One dog was presented for multiple cutaneous masses that suggested round cell or epithelial malignancy on cytology but for which IHC confirmed epithelial origin. A primary small intestinal adenocarcinoma with additional visceral metastasis was identified at necropsy.78 Lymphoma is often a systemic disease, and one-fourth of dogs and four-fifths of cats with GI lymphoma will have concurrent involvement of other organs.13,22
Molecular Aspects
With an increasing armamentarium of molecular diagnostics, insights as to the pathogenesis, progression, and prognosis of tumors are constantly emerging. Cellular adhesion and invasion (e.g., Tenascin-C,51,79 versica, hyaluronan,80 β-catenin, and E-cadherin81-83), stromal remodeling, and alterations in tumor suppressor genes (e.g., p5381,83-85) may play a role in the development and progression of intestinal neoplasia. The importance of the relationship between a tumor cell and its stroma should not be overlooked. Although molecular markers/targets likely play an important role in intestinal tumors, the utility of these in diagnostics, prognostication, and therapy in companion animal species, with the exception of GIST and CD117 expression, is limited until further interrogations characterize their importance and provide avenues for their utility.55
Measures of cellular proliferation include markers such as argyrophilic nucleolar organizer regions (AgNORs). In feline intestinal lymphoma, AgNORs did not correlate with remission rate or duration or with survival time.19
COX enzymes are responsible for prostaglandin synthesis, and COX-2 is overexpressed in many head/neck and genitourinary tumors, creating a possible therapeutic target. COX-2 has been identified in both benign and malignant small intestinal and colorectal epithelial tumors in dogs, although the number of positive cells varies and in some studies was very low.86,87 Additionally, one study found no COX-2 staining in 13 intestinal tumors in cats.88 COX inhibitors are thus of questionable value in treating intestinal tumors.
History and Clinical Signs
The duration of clinical signs prior to presentation typically averages 6 to 8 weeks but can range from less than 1 day to several months.11,22,23 Clinical signs include (in varying order of frequency): weight loss, diarrhea, vomiting, and anorexia and less frequently melena, anemia, and hypoglycemia (with smooth muscle tumors).* Clinical signs often relate to location of the tumor within the GI tract. Proximal lesions more commonly result in vomiting, small intestinal lesions in weight loss, and large bowel lesions in hematochezia and tenesmus.30,32 Although carcinoids may secrete endocrine substances, clinical signs do not always reflect hypersecretion.7 Dogs and cats may present with clinical signs relating to intestinal obstruction, such as anorexia, weight loss, and vomiting. Although uncommon, perforation and septic peritonitis can occur.34 Smooth muscle tumors are located within the muscular layer of the intestines and not within the lumen and evidence of GI bleeding is often absent, but anemia and melena have been reported.23,24
Paraneoplastic Syndromes
One dog was presented for alopecia and Cheyletiella infection within 2 months of euthanasia for abdominal carcinomatosis from intestinal carcinoma. The neoplasia was not identified with abdominal ultrasound at the original work-up, but immunosuppression resulting from an underlying neoplasia was thought to lead to opportunistic Cheyletiella infection. While pruritus resolved with ivermectin therapy, alopecia persisted, suggesting paraneoplastic origin.89 Neutrophilic leukocytosis (in one dog associated with monocytosis and eosinophilia) has been reported in dogs with rectal tumors. Resolution or improvement of hematologic abnormalities occurred following treatment for adenomatous rectal polyps.90,91 Hypereosinophilia and eosinophilic tumor infiltrates have been reported in a cat and several dogs with intestinal T-cell lymphoma; the suggested cause was IL-5 secretion by the neoplastic lymphocytes.92-94 Extramedullary plasmacytoma may lead to a hyperviscosity syndrome resulting from overproduction of immunoglobulin.95
Erythrocytosis managed with periodic phlebotomy was related to a cecal leiomyosarcoma in a 14-year-old dog. The diagnosis was made at postmortem 2 years later, and erythropoietin mRNA and protein were isolated from tumor cells, suggesting ectopic erythropoietin production as the cause of the erythrocytosis.96 Hypoglycemia is also reported with intestinal smooth muscle tumors as a paraneoplastic syndrome.97 Nephrogenic diabetes insipidus has also been documented in one dog with intestinal leiomyosarcoma.98
Diagnostic Techniques and Work-Up
An abdominal mass may be palpated on initial examination in approximately 20% to 40% of dogs with lymphoma22,27 and 20% to 50% of dogs with nonlymphomatous solid intestinal tumors.11,30,32 Pain and fever were reported in 20% of dogs with lymphoma in one report.22 Digital rectal examination may identify masses or annular strictures due to rectal tumors or polyps in as high as 63% of dogs.30,52
Abdominal masses are also often readily palpated in cats with both lymphoma and adenocarcinoma. Approximately 50% of cats with nonlymphomatous tumors will be presented with a palpable mass.15,32 Abnormal abdominal palpation is common in cats with lymphoma with up to 86% having a palpable mass.16,34 Dehydration is also common, occurring in 30% to 60% of cats with nonlymphomatous tumors.15,32
Clinical Pathology
Complete Blood Count
Anemia is common in dogs and cats with intestinal tumors and is often not characterized but may occur in conjunction with melena and elevated blood urea nitrogen (BUN). Anemia affects nearly 40% of dogs in most studies and as low as 15% but up to 70% of cats.* Leukogram changes are also common including leukocytosis in 25% to 70% of dogs and 40% of cats.11,15,24,32 Left shift may be seen as well as monocytosis in some patients.32,34
Chemistry Profile
Biochemical abnormalities are similar between dogs and cats with intestinal tumors. As a result of malabsorption, hypoproteinemia may be present in one-fourth to one-third of patients.† Other common abnormalities include elevated liver enzymes, specifically alkaline phosphatase in 15% to 33% of dogs and up to 85% of cats with nonlymphomatous neoplasia.11,24,30,32,34 In one series a high cholesterol was seen in 41% of cats with nonlymphomatous tumors.32 An elevated blood urea nitrogen has been reported in 13% of dogs and 30% of cats with intestinal adenocarcinoma.11,15 This may be a result of concurrent renal insufficiency or intestinal bleeding due to the tumor or of dehydration. While some cats may have hyperglycemia,32 smooth muscle tumors can cause up to 55% of patients to be hypoglycemic as a result of insulin-like growth factor secretion.23 Dogs may also have increased amylase and electrolyte disturbances,30 and patients with lymphoma may be hypercalcemic.16
Serum alpha 1-acid glycoprotein (AGP), an acute phase reactant protein, may be increased in cats with cancer but lacks specificity and prognostic relevance.99,100
Cytology and Histopathology
As with other anatomic sites, cytology of the intestinal tract can help differentiate among major tumor types. Additionally, lymphocyte accumulations can be tested using PARR for clonality. If amplification of variable regions in the genome of lymphocytes using PARR reveals monotony consistent with a clonal expansion, then a diagnosis of lymphoma can be confirmed. This test can be performed using either stained or unstained slides from ultrasound-guided aspirates.60,101 PARR may detect clonal expansions not yet evident (as lymphoma) on histopathology of the same sample.102 Because of reported eosinophilia with intestinal lymphoma and reports of mast cell tumor with concurrent small T-cell lymphoma in cats, it may be challenging to distinguish between the two.103 Despite concerns of complications following surgery in cats with intestinal lymphoma for the purpose of obtaining diagnostic samples for histopathology or for resection of a mass, the risk of perioperative dehiscence appears to be low.104
Imaging
Plain and Contrast Abdominal Radiographs
In dogs and cats with intestinal lymphoma, concurrent enlargement of liver, spleen, and/or mesenteric lymph nodes may be seen.22 Plain abdominal radiographs may reveal an abdominal mass in approximately 40% of both dogs and cats, although some reports are higher for solid tumor types and lower for lymphoma.* Intestinal lymphoma may be more difficult to identify on plain radiography because of other organ involvement, peritoneal effusion, or diffuse intestinal lesions. An obstructive pattern may also be seen on plain radiographs, with incidence ranging from 10% to 75%.11,24,30,32 Other abnormalities may include poor serosal detail and thickened stomach wall.16
Contrast radiography, although used less following advances in ultrasound, has often been used to evaluate patients with signs of primary GI disease. Contrast radiography can help rule in or out an obstruction, localize a tumor, and view areas of the GI tract that are difficult to image with ultrasonography because of gas accumulation (Figure 22-24). Contrast radiographs may reveal filling defects in approximately half the cats and dogs with GI neoplasia.32 In dogs with GI lymphoma, all 12 dogs examined had abnormal contrast series.22 In one series, 87% of cats with intestinal adenocarcinoma showed evidence of partial or complete obstruction.15
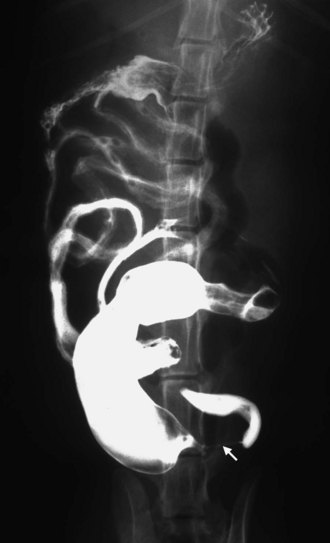
Figure 22-24 The arrow indicates an obstructing tumor on contrast radiography. The thin trail of barium is all that will pass through the lumen of the tumor and the obstruction is evidenced by the dilated segment of small bowel adjacent and oral to the tumor. (Courtesy Dr. Jimmy Lattimer, University of Missouri, College of Veterinary Medicine.)
Thoracic Radiographs
Thoracic radiographs are critical to the complete evaluation of the cancer patient. For dogs with nonlymphomatous intestinal tumors, yield is low with very few patients presenting with pulmonary metastasis.11 This may be due to a bias in reporting because many reports detail outcome of treatment and patients with metastatic disease may not receive treatment. In fact, many case series report no evidence of metastasis on initial evaluation for solid tumors of the intestine in dogs.11,23,24,30,32 In cats, 2 of 14 cats in one series and no cats in another had pulmonary nodules at initial evaluation.15,32 For cats and dogs with lymphoma, enlarged sternal or perihilar lymph nodes, pleural effusion, or diffuse interstitial changes may be seen.16,22
Abdominal Ultrasound
Ultrasound allows noninvasive localization of the tumor and identification of other sites of metastasis/involvement. It also can guide needle aspiration or needle biopsy or assist in treatment planning. Ultrasound is a more sensitive diagnostic test than radiographs for identifying a mass.11,23,28,105 Ultrasound is also less time consuming than contrast radiography, and the increased use, availability, and operator skill for the former has diminished the need for the latter.
Ultrasound findings in dogs and cats with intestinal neoplasia most consistently include bowel wall thickening and loss of normal wall layers.30,105,106 Degree of thickening, distribution of lesion(s), and symmetry are also used to help differentiate neoplasia from nonneoplastic disease.107 Intestinal lymphoma in dogs more often results in long segments of involved bowel and either a solitary mass or diffusely thickened bowel loops with thickening of the muscularis propria in cats.35,106,108 Adenocarcinoma in cats has been described as having mixed echogenicity and was asymmetric in three of five cats.105 In one study, two-thirds of dogs with intestinal adenocarcinoma had hypoechoic tumors, and most had decreased motility. Masses averaged 4-cm long with a median wall thickness of 1.2 cm.8,30 Mast cell tumors have an eccentric appearance with alteration but not loss of wall layering, commonly involving the muscularis propria.103 Smooth muscle tumors are characteristically large (median diameter 4.8 cm) and anechoic/hypoechoic, and a muscular layer origin may be identified. Leiomyomas may have a smooth contour.28
Ultrasound has also proven useful in differentiating neoplastic from nonneoplastic intestinal disease. Dogs with tumors have significantly thicker intestinal walls, and 99% have a loss of wall layering compared to a maintenance of wall layering in 88% of dogs with nonneoplastic disease (Figure 22-25). In fact, dogs with a loss of wall layering are more than 50 times more likely to have a tumor than enteritis. Additionally, dogs with walls thicker than 1 cm are nearly 4 times as likely to have a tumor, and those with focal lesions are nearly 20 times as likely.106 Nevertheless, possible differential diagnoses include fungal (Pythiosis and histoplasmosis) masses that can mimic neoplasia.107 Lymphadenopathy can also be seen with both neoplasia (lymphoma and solid tumors), as well as with infectious or inflammatory bowel disease. In general, neoplasia exhibits more dramatic thickening with loss of wall layering and greater lymph node enlargement, as well as more frequent focal lesions than nonneoplastic intestinal disease.107
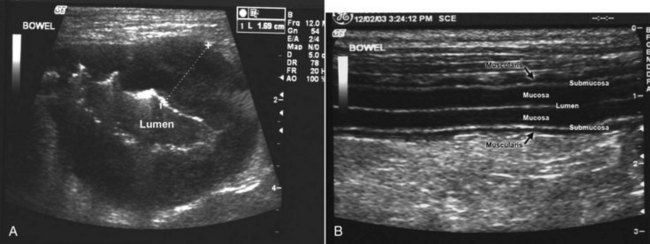
Figure 22-25 A cross-sectional ultrasound image of a segment of small intestine with lymphoma (A) is compared to a longitudinal view of a segment of normal small intestine (B). Note that the clearly defined intestinal layers in the normal tissue are completely effaced in the tumor tissue. A loss of layering is strongly supportive of neoplasia. The diseased bowel is also markedly thickened, suggesting neoplasia. (Courtesy Dr. Stephanie Essman, University of Missouri, College of Veterinary Medicine.)
In a series of 14 cats with carcinomatosis, 3 of which were a result of small intestinal tumors (2 carcinomas and 1 lymphoma), the hallmark ultrasonographic finding (100% of cats) was the presence of masses in the double sheet portion of peritoneum that connects the visceral and parietal portions. All cats also had free peritoneal fluid.36
Endoscopy and Laparoscopy
Minimally invasive methods of collecting tissues to aid in diagnosis are increasingly used. Endoscopic findings in dogs with intestinal lymphoma include an irregular cobblestone or patchy erythematous appearance to the duodenal mucosa and poor distensibility and elasticity of the duodenal wall.27
Significant interobserver variation may occur in the interpretation of biopsy samples. In one study, blinded pathologists assigned a degree of mucosal cellular infiltrate as severe as neoplasia in five clinically normal research dogs.109 Interobserver variation is likely to be more pronounced with small tissue samples and this is a limitation of these less invasive approaches.
Exploratory Laparotomy
When noninvasive or minimally invasive diagnostics fail to confirm a diagnosis, an exploratory laparotomy may be indicated for a dog or cat with persistent signs of GI disease. Benefits include direct visualization of all abdominal viscera and the ability to collect full thickness biopsies of all segments of intestines and other viscera. Patients with resectable solid tumors may be both diagnosed and treated in one procedure with resection and anastomosis. In a series of dogs with GI lymphoma, endoscopic biopsies were sometimes difficult to interpret because of lymphoplasmacytic infiltrate, but biopsies obtained by laparotomy confirmed the diagnosis in all cases undergoing surgery.22 It should be noted that carcinomatosis should not always be seen as an indication for euthanasia (Figure 22-26). Following removal of the primary intestinal adenocarcinoma, two cats with malignant effusion lived 4.5 and 28 months after surgery.15
Therapy and Prognosis
With the exception of lymphoma, surgical resection is the primary treatment for intestinal tumors. As long as severe extraserosal invasion and/or adhesions do not complicate the surgical approach, complete excision is often possible. For dogs and cats without evidence of local or distant metastasis, long-term survival is possible, although some tumors may later metastasize. Overall, the 1-year survival rate is approximately 40% for dogs with solid small intestinal tumors.11 For cats with adenocarcinoma, approximately 50% will metastasize to the local lymph nodes, 30% to the peritoneal cavity (carcinomatosis), and 20% or less to the lungs.2,32,37 Dogs have similar rates of metastasis to lymph nodes for both adenocarcinoma and leiomyosarcoma, although liver is usually the second most frequent site.8,11,32 Perioperative mortality can approach 30% to 50% as a result of sepsis, peritonitis, or owner decision for euthanasia when nonresectable tumors are present.11,23
The benefit of surgery is questionable for dogs with intestinal mast cell tumors. In two case series, most dogs died within the first month. Only 2 of 49 dogs (combined total for two series, almost all GI) lived past 180 days and prednisone was not helpful in most cases.41,42
Various surgical techniques have been used to treat small and large intestinal tumors. For dogs with colorectal adenocarcinoma, local excision via anal approach and rectal prolapse (when amenable) or other methods yielded a median survival of 2 to almost 4 years compared to 15 months for stool softeners alone52,110-112; colorectal plasmacytomas and polyps also fare well with MSTs of 15 months and 2 years or more, respectively, following surgical excision.39,113 This is in contrast to small intestinal adenocarcinoma in which 12 days mean survival was reported without treatment, and a mean survival of only 114 days for dogs with surgical resection, though others report 7 and 10 months.11,30,32 Regarding surgical techniques for resection and anastomosis of small intestine, stapling techniques have been shown to be equivalent to hand suturing.114 Dogs with leiomyosarcoma who survive the perioperative period fare somewhat better with MSTs of 1.1 and almost 2 years.23,24 One case series found the median postoperative survival for 28 dogs with GIST to be approximately 38 months (1 year if postoperative deaths were included) versus 8 months for 10 dogs with leiomyosarcoma, although the difference was not statistically significant.58 Additionally, another study found no difference between GIST and leiomyosarcoma with 1-year survival of approximately 80%.59 Colostomy use has been reported to aid in management of dogs with nonresectable rectal tumors. In one report, skin excoriation was the most common complication, but colostomy bags were managed for up to 7 months.115
Transrectal endoscopic removal of benign canine rectal tumors that would have otherwise required rectal pull-through surgery or pubic osteotomy afforded five of six dogs significant improvement in quality of life, with three dogs cured.116 Standard treatment with surgery yields a 41% recurrence of clinical signs, and 18% of dogs experienced transition to malignancy with tumor recurrence.29 Surgical removal of duodenal polyps in cats typically also affords a cure.17
In cats with small intestinal adenocarcinoma, there is significant perioperative risk, but cats that live beyond 2 weeks may experience long-term control with surgery alone (Figure 22-27). In two series, all cats that did not have their tumors resected were euthanized or died within 2 weeks of surgery.15,32 One-half of cats in one report and all cats in another that had their tumors resected died within 2 weeks of surgery, and 4 of 11 died within 2 months in another of complications or nontumor causes.15,32,37 For the remaining cats that survived 2 weeks beyond surgery, mean survival was 15 months, although only 20 weeks in another report (median 5 weeks).15,38
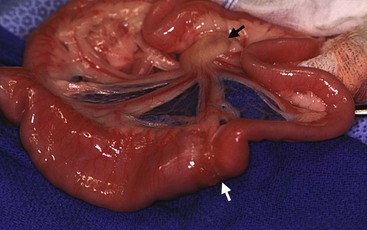
Figure 22-27 Intestinal obstruction as a result of adenocarcinoma (white arrow). Note the distension of the jejunum oral to the mass as compared to the normal diameter aboral to the mass. There is also an enlarged lymph node (black arrow). (Courtesy Dr. Eric Pope, Ross University, College of Veterinary Medicine.)
In cats with large intestinal neoplasia, survival following surgery alone was approximately 3.5 months for lymphoma, 4.5 months for adenocarcinoma, and 6.5 months for mast cell tumor. Adjuvant chemotherapy improved survival for cats with adenocarcinoma but not for cats with lymphoma.18
Lymphoma is treated primarily with chemotherapy, except when intestinal perforation or the need for a biopsy necessitates surgery (Figure 22-28). Surgery and chemotherapy did not improve survival compared to chemotherapy alone for cats with alimentary lymphoma.20 In cats with LGL lymphoma of the intestines, few attempts at therapy have been reported in the literature. Chemotherapy induced partial or complete remission in some cats, but MST was only 57 days.64 One cat did well for over a year following surgical resection until the tumor recurred.
Chemotherapy
No randomized studies exist to confirm or deny any benefit of adjuvant chemotherapy following resection of epithelial intestinal tumors in dogs and cats. The benefit of adjuvant chemotherapy in humans is questionable, although current fluorouracil-based regimens are often considered to be the standard of care. One retrospective study in cats with colonic adenocarcinoma did show a significant survival advantage for cats receiving adjuvant doxorubicin, with a median survival of 280 days with and 56 days without chemotherapy.18 For carcinomatosis, intracavitary therapy may be helpful with carboplatin for cats or cisplatin or 5-fluorouracil (5-FU) for dogs.117 When attempted, adjuvant chemotherapy typically includes doxorubicin in veterinary medicine. Two dogs with leiomyosarcoma received adjuvant chemotherapy following surgical resection. One had metastasis at surgery and died 4 months later, and the other was lost at over 2 years, but there were several other dogs in that series that were long lived that did not receive chemotherapy.23 Two dogs with adenocarcinoma also received chemotherapy following surgery and both survived over 17 months.30
In dogs with GI lymphoma, of the eight dogs treated in one report, all were euthanized by 14 weeks.22 In cats with intestinal lymphoma, MST is typically 6 to 9 months, although many studies comment that a subset of cats did very well, living over 2 years.16,25,35 This may relate to histologic subtype because cats with lymphocytic lymphoma (75% of cases, 70% to 90% response rate, approximately 2-year MST with chlorambucil and prednisone) fared vastly better than those with lymphoblastic lymphoma (25% of cases, <20% response rate, 2.7-month MST with multiagent chemotherapy).35,63 General treatment of feline lymphoma is discussed elsewhere (see Chapter 33, Section B), but treatment regimens reported typically include vincristine, doxorubicin, prednisone, and cyclophosphamide for lymphoblastic lymphoma and chlorambucil and prednisone for cats with lymphocytic lymphoma.35
A reduction in the size and clinical signs of rectal polyps in eight dogs was noted following piroxicam therapy, either orally or in suppository form. Clinical response did not relate to whether there was inflammation associated with the tumor.91
Radiation Therapy
Because of concern for toxicity to surrounding abdominal viscera, the ability to often completely excise intestinal tumors for adequate local control, and the inability to reliably irradiate the same tissue each day because intestines are mobile, RT is seldom used in the treatment of intestinal tumors.
Prognostic Factors
Intestinal perforation does not appear to be a negative prognostic factor for leiomyosarcoma because dogs surviving the perioperative period enjoyed prolonged survival in one series.23 For colorectal tumors, treatment is prognostic with local excision significantly better than palliative care. Gross appearance, although not statistically examined, may determine outcome because dogs with annular, obstructing masses survived a mean of 1.6 months; nodular or cobblestone masses 12 months; and single, pedunculated masses 32 months.52
For nonlymphomatous small intestinal tumors in dogs, metastasis at the time of surgery resulted in significantly shorter survival times (3 months versus 15 months). One-year survival for dogs with lymph node metastasis was 20% as compared to 67% without.11 In another study, however, dogs with and without visceral metastasis from leiomyosarcoma survived equally long following surgical resection (21 months).23 In one study, males fared significantly better than females for small intestinal adenocarcinoma, although the number of females was small.30
The strongest prognostic factor for cats with intestinal lymphoma is response to treatment. Cats achieving a complete resolution of clinical signs typically fare significantly better than those that do not.20,118 For cats with epitheliotropic lymphoma, those that did not achieve remission (euthanized within 3.5 months) had a worse survival than those that achieved remission (median 11 months).34 For 103 cats with lymphoma, 28 of which were intestinal, negative prognostic factors were FeLV positivity (median survival 3 months if positive, 17 months if negative for early stage disease, not prognostic for advanced stages) and advanced stage of disease.26 FeLV status did not affect outcome in other studies.20,25 For large intestinal neoplasia, cats with surgery for lymphoma fared equally poorly with and without adjuvant chemotherapy (median survival of just over 3 months in both groups). Cats with adenocarcinoma, however, survived significantly longer if they were treated with subtotal colectomy (138 days versus 68 days with mass excision), received postoperative doxorubicin (280 days with versus 56 days without), and had negative lymph nodes at surgery (259 days negative versus 49 days positive).18
Comparative Aspects
Although cancer of the large intestine and rectum is well characterized in humans, small intestinal neoplasia is rare. Theories for this discrepancy include more rapid small intestinal transit time as compared to the large intestine (creating less contact time for carcinogens), dilution of carcinogens with fluid as compared to solid stool, differences in pH, relative lack of bacteria to allow transformation of procarcinogens, presence of detoxifying enzymes, and increased presence of immunoglobulin A promoting local immunosurveillance of damaged cells as a result of increased lymphocytes in the small intestine. Intake of red meat, salt-cured foods, and fat are associated with an increased risk of small intestinal neoplasia, although tobacco and alcohol use were not.119 This is in contrast to veterinary medicine where in cats and sometimes dogs, malignant neoplasia is more common in the small than the large intestine. This may reflect differences in physiology, diet, or genetics among species. The difference in species may also be a matter of proportion in that humans develop a large number of colorectal cancers as a result of diet and genetic influences. As in animals, tumors of the small intestine of humans are usually malignant. Diagnostic evaluation is similar to that described in animals, although advanced imaging such as CT is more often used. Most diagnoses are made at surgery and 5-year survival rates average just over 20%.119
In contrast to the rarity of small bowel neoplasia, large bowel/colorectal cancer is one of the most frequently diagnosed cancers in both men and women. Risk factors include genetic predisposition/familial history, tobacco and alcohol intake, advanced age, and predisposing medical conditions, among others. Colorectal cancer development may further be influenced by intake of red meat (especially fried), low-fiber and/or high-fat diet, obesity, fecal pH, and fecal mutagens. Among genetic risk factors, polymorphism in colonic enzymes and mutations leading to familial adenomatous syndromes are uncommon but are important as models of carcinogenesis. In most familial polyposis syndromes, the adenomatous polyposis coli (APC) gene is mutated. The multistage progression from benign polyp to carcinoma is well understood and underscores the importance of early detection.120 In contrast, hereditary nonpolyposis colon cancer (HNPCC) develops without known premalignant polyps. It is inherited via autosomal dominance with high penetration and is characterized by microsatellite instability.121
Severe celiac disease in humans is associated with an increased risk of lymphoma. Progression to lymphoma from inflammatory bowel disease (IBD), especially in cats, has been postulated but not confirmed. Two of 97 cats with lymphoma had a history of IBD in a population of cats examined for comparison to a group of cats with IBD at one institution during the same time period.122
The most clinically important aspects of comparative oncology when considering intestinal neoplasia in humans are the use of COX inhibitors in treatment, the prevention of colorectal neoplasia, and the use of TKIs. In people, KIT mutations in GI stromal tumors have led to the use of imatinib mesylate, a TKI that inhibits KIT.123 This illustrates the notion of therapy directed at the molecular defect rather than the histologic diagnosis. KIT is mutated in some canine GISTs, and thus TKIs may benefit this population as well.
COX inhibition by NSAIDs will decrease the incidence of colorectal cancer and decrease mortality by 40% to 50%.124 Among the proposed mechanisms of action, prostaglandin production is thought to be related to tumor progression and therefore inhibition leads to cancer prevention. Additionally, non-COX pathways include inhibition of transcription factors and induction of nuclear hormone receptors that lead to cellular differentiation.124 Interestingly, a recent retrospective study found a significantly reduced incidence of cancer in dogs with a history of NSAID use (71% reduced risk).125
Therapy in humans is similar to that in companion animals. Surgical resection is the primary mode of therapy, with adjuvant targeted or traditional chemotherapy in many cases, especially if patients present with lymph node metastasis or unresectable disease. Transanal endoscopic resection is used when possible for rectal tumors, and this technique has been performed with some success in dogs. TKIs may improve prognosis for unresectable and metastatic GISTs.123 Adjuvant chemotherapy is used in colon cancer, with 5-FU–based combinations providing the best control often in combination with a platinum agent, with some studies showing decreased local recurrence but not increased overall survival.126 RT is used primarily for areas of the GI tract that are not very mobile, such as the stomach and rectum.
References
1. Cotchin, E. Some tumours of dogs and cats of comparative veterinary and human interest. Vet Rec. 1959;71(45):1040–1050.
2. Patnaik, AK, Liu, SK, Johnson, GF. Feline intestinal adenocarcinoma. Vet Pathol. 1976;13:1–10.
3. Engle, GG, Brodey, RS. A retrospective study of 395 feline neoplasms. J Am Anim Hosp Assoc. 1969;5:21–31.
4. Dobson, JM, Samuel, S, Milstein, H, et al. Canine neoplasia in the UK: estimates of incidence rates from a population of insured dogs. J Small Anim Pract. 2002;43(6):240–246.
5. Bastianello, SS. A survey of neoplasia in domestic species over a 40-year period from 1935 to 1974 in the republic of South Africa. V. Tumours occurring in the cat. Onderstepoort J Vet Res. 1983;50:105–110.
6. Rissetto, K, Villamil, JA, Selting, KA, et al. Recent trends in feline intestinal neoplasia: an epidemiologic study of 1,129 cases in the veterinary medical database from 1964 to 2004. J Am Anim Hosp Assoc. 2011;47:28–36.
7. Head, KW, Else, RW, Dubielzig, RR. Tumors of the intestines. In Meuten DJ, ed.: Tumors in domestic animals, ed 4, Ames, Iowa: Iowa State Press, 2002.
8. Patnaik, AK, Hurvitz, AI, Johnson, GF. Canine intestinal adenocarcinoma and carcinoid. Vet Pathol. 1980;17:149–163.
9. Dorn, CR, Taylor, DON, Schneider, R, et al. Survey of animal neoplasms in Alameda and Contra Costa Counties, California. II. Cancer morbidity in dogs and cats from Alameda County. J Natl Cancer Inst. 1968;40:307–318.
10. Veterinary Cancer Registry. www.vetcancerregistry.com. [Searched March 2005, by permission from Dr. Steve Steinberg].
11. Crawshaw, J, Berg, J, Sardinas, JC, et al. Prognosis for dogs with nonlymphomatous, small intestinal tumors treated by surgical excision. J Am Anim Hosp Assoc. 1998;34:451–456.
12. Alroy, J, Leav, I, DeLellis, RA, et al. Distinctive intestinal mast cell neoplasms of domestic cats. Lab Invest. 1975;33(2):159–167.
13. Gabor, LJ, Malik, R, Canfield, PJ. Clinical and anatomical features of lymphosarcoma in 118 cats. Aust Vet J. 1998;76(11):725–732.
14. Kariya, K, Konno, A, Ishida, T. Perforin-like immunoreactivity in four cases of lymphoma of large granular lymphocytes in the cat. Vet Pathol. 1997;34:156–159.
15. Kosovsky, JE, Matthiesen, DT, Patnaik, AK. Small intestinal adenocarcinoma in cats: 32 cases (1978-1985). J Am Vet Med Assoc. 1988;192(2):233–235.
16. Mahony, OM, Moore, AS, Cotter, SM, et al. Alimentary lymphoma in cats: 28 cases (1988-1993). J Am Vet Med Assoc. 1995;207(12):1593–1598.
17. MacDonald, JM, Mullen, HS, Moroff, SD. Adenomatous polyps of the duodenum in cats: 18 cases (1985-1990). J Am Vet Med Assoc. 1993;202(4):647–651.
18. Slaweinski, MJ, Mauldin, GE, Mauldin, GN, et al. Malignant colonic neoplasia in cats: 46 cases (1990-1996). J Am Vet Med Assoc. 1997;211:878–881.
19. Rassnick, KM, Mauldin, GN, Moroff, SD, et al. Prognostic value of argyrophilic nucleolar organizer region (AgNOR) staining in feline intestinal lymphoma. J Vet Intern Med. 1999;13:187–190.
20. Zwahlen, CH, Lucroy, MD, Kraegel, SA, et al. Results of chemotherapy for cats with alimentary malignant lymphoma: 21 cases (1993-1997). J Am Vet Med Assoc. 1998;213:1144–1149.
21. Patnaik, AK, Hurvitz, AI, Johnson, GF. Canine gastrointestinal neoplasms. Vet Pathol. 1977;14:547–555.
22. Couto, CG, Rutgers, HC, Sherding, RG, et al. Gastrointestinal lymphoma in 20 dogs: a retrospective study. J Vet Intern Med. 1989;3:73–78.
23. Cohen, M, Post, GS, Wright, JC. Gastrointestinal leiomyosarcoma in 14 dogs. J Vet Intern Med. 2003;17:107–110.
24. Kapatkin, AS, Mullen, HS, Matthiesen, DT, et al. Leiomyosarcoma in dogs: 44 cases (1983-1988). J Am Vet Med Assoc. 1992;201(7):1077–1079.
25. Jeglum, KA, Whereat, A, Young, K. Chemotherapy of lymphoma in 75 cats. J Am Vet Med Assoc. 1987;190(2):174–178.
26. Mooney, SC, Hayes, AA, MacEwen, EG, et al. Treatment and prognostic factors in lymphoma in cats: 103 cases (1977-1981). J Am Vet Med Assoc. 1989;194(5):696–699.
27. Miura, T, Maruyama, H, Sakai, M, et al. Endoscopic findings on alimentary lymphoma in 7 dogs. J Vet Med Sci. 2004;66(5):577–580.
28. Myers, NC, Penninck, DG. Ultrasonographic diagnosis of gastrointestinal smooth muscle tumors in the dog. Vet Radiol Ultrasound. 1994;35(5):391–397.
29. Valerius, KD, Powers, BE, McPherron, MA, et al. Adenomatous polyps and carcinoma in situ of the canine colon and rectum: 34 cases (1982-1994). J Am Anim Hosp Assoc. 1997;33:156–160.
30. Paoloni, MC, Penninck, DG, Moore, AS. Ultrasonographic and clinicopathologic findings in 21 dogs with intestinal adenocarcinoma. Vet Radiol Ultrasound. 2002;43(6):562–567.
31. Frost, D, Lasota, J, Miettinen, M. Gastrointestinal stromal tumors and leiomyomas in the dog: a histopathologic, immunohistochemical, and molecular genetic study of 50 cases. Vet Pathol. 2003;40:42–54.
32. Birchard, SJ, Couto, CG, Johnson, S. Nonlymphoid intestinal neoplasia in 32 dogs and 14 cats. J Am Anim Hosp Assoc. 1986;22:533–537.
33. Wolf, JC, Ginn, PE, Homer, B, et al. Immunohistochemical detection of p53 tumor suppressor gene protein in canine epithelial colorectal tumors. Vet Pathol. 1997;34:394–404.
34. Carreras, JK, Goldschmidt, M, Lamb, M, et al. Feline epitheliotropic intestinal malignant lymphoma: 10 cases (1997-2000). J Vet Intern Med. 2003;17:326–331.
35. Fondacaro, JV, Richter, KP, Carpenter, JL, et al. Feline gastrointestinal lymphoma: 67 cases (1988-1996). Eur J Comp Gastroenterol. 1999;4(2):5–11.
36. Monteiro, CB, O’Brien, RT. A retrospective study on the sonographic findings of abdominal carcinomatosis in 14 cats. Vet Radiol Ultrasound. 2004;45(6):559–564.
37. Cribb, AE. Feline gastrointestinal adenocarcinoma: a review and retrospective study. Can Vet J. 1988;29:709–712.
38. Turk, MAM, Gallina, AM, Russell, TS. Nonhematopoietic gastrointestinal neoplasia in cats: a retrospective study of 44 cases. Vet Pathol. 1981;18:614–620.
39. Seiler, RJ. Colorectal polyps of the dog: a clinicopathologic study of 17 cases. J Am Vet Med Assoc. 1979;174:72–75.
40. Peterson, MR, Frommelt, RA, Dunn, DG. A study of the lifetime occurrence of neoplasia and breed differences in a cohort of German Shepherd Dogs and Belgian Malinois military working dogs that died in 1992. J Vet Intern Med. 2000;14:140–145.
41. Ozaki, K, Yamagami, T, Nomura, K, et al. Mast cell tumors of the gastrointestinal tract in 39 dogs. Vet Pathol. 2002;39:557–564.
42. Takahashi, T, Kadosawa, T, Nagase, M, et al. Visceral mast cell tumors in dogs: 10 cases (1982-1997). J Am Vet Med Assoc. 2000;216:222–226.
43. Jackson, ML, Haines, DM, Meric, SM, et al. Feline leukemia virus detection by immunohistochemistry and polymerase chain reaction in formalin-fixed, paraffin-embedded tumor tissue from cats with lymphosarcoma. Can J Vet Res. 1993;57:269–276.
44. Jackson, ML, Wood, SL, Misra, V, et al. Immunohistochemical identification of B and T lymphocytes in formalin-fixed, paraffin-embedded feline lymphosarcomas: relation to feline leukemia virus status, tumor site, and patient age. Can J Vet Res. 1996;60:199–204.
45. Oros, J, Lorenzo, H, Andrada, M, et al. Type A-like retroviral particles in a metastatic intestinal adenocarcinoma in an emerald tree boa (Corallus caninus). Vet Pathol. 2004;41:515–518.
46. Hendricks-Hutto, E, Anderson, DC, Mansfield, KG. Cytomegalovirus-associated discrete gastrointestinal masses in macaques infected with the simian immunodeficiency virus. Vet Pathol. 2004;41:691–695.
47. Fry, DR, Slocombe, RF, Beck, C. Gastric lymphoma associated with the presence of helicobacter in a cat. Aust Vet Pract. 2003;33(3):126–131.
48. Yamazaki, Y, Aono, I, Ohya, T, et al. Gastroduodenal adenocarcinomas and rectal adenoma in a cougar (Felis concolor) infected with Helicobacter-like organisms and spirochetes. J Vet Med Sci. 2002;64(2):149–153.
49. Fox, JG, Shen, Z, Xu, S, et al. Helicobacter marmotae sp. nov. isolated from livers of woodchucks and intestines of cats. J Clin Microbiol. 2002;40(7):2513–2519.
50. Blackwood, L, German, AJ, Stell, AJ, et al. Multicentric lymphoma in a dog after cyclosporine therapy. J Small Anim Pract. 2004;45:259–262.
51. Mukaratirwa, S, de Witte, E, van Ederen, AM, et al. Tenascin expression in relation to stromal tumour cells in canine gastrointestinal epithelial tumours. J Comp Path. 2003;129:137–146.
52. Church, EM, Mehlhaff, CJ, Patnaik, AK. Colorectal adenocarcinoma in dogs: 78 cases (1973-1984). J Am Vet Med Assoc. 1987;191(6):727–730.
53. Prater, MR, Flatland, B, Newman, SJ, et al. Diffuse annular fusiform adenocarcinoma in a dog. J Am Anim Hosp Assoc. 2000;36:169–173.
54. Sako, T, Uchida, E, Okamoto, M, et al. Immunohistochemical evaluation of a malignant intestinal carcinoid in a dog. Vet Pathol. 2003;40:212–215.
55. Gillespie, V, Baer, K, Farrelly, J, et al. Canine gastrointestinal stromal tumors: immunohistochemical expression of CD34 and examination of prognostic indicators including proliferation markers Ki67 and AgNOR. Vet Pathol. 2011;48:283.
56. Miettinen, M, Majidi, M, Lasota, J. Pathology and diagnostic criteria of gastrointestinal stromal tumors (GISTs): a review. Eur J Cancer. 2002;38(Suppl 5):S39–S51.
57. LaRock, RG, Ginn, PE. Immunohistochemical staining characteristics of canine gastrointestinal stromal tumors. Vet Pathol. 1997;34:303–311.
58. Russell, KN, Mehler, SJ, Skorupski, KA, et al. Clinical and immunohistochemical differentiation of gastrointestinal stromal tumors from leiomyosarcomas in dogs: 42 cases (1990-2003). J Am Vet Med Assoc. 2007;230(9):1329–1333.
59. Maas, CPHJ, Haar, G, van der Gaag, I, et al. Reclassification of small intestinal and cecal smooth muscle tumors in 72 dogs: clinical, histologic, and immunohistochemical evaluation. Vet Surg. 2007;36(4):302–313.
60. Waly, NE, Gruffydd-Jones, TJ, Stokes, CR, et al. Immunohistochemical diagnosis of alimentary lymphomas and severe intestinal inflammation in cats. J Comp Pathol. 2005;133(4):253–260.
61. Kiupel, M, Smedley, RC, Pfent, C, et al. Diagnostic algorithm to differentiate lymphoma from inflammation in feline small intestinal biopsy samples. Vet Pathol. 2011;48(1):212–222.
62. Louwerens, M, London, CA, Pedersen, NC, et al. Feline lymphoma in the post-feline leukemia virus era. J Vet Intern Med. 2005;19(3):329–335.
63. Stein, TJ, Pellin, M, Steinberg, H, et al. Treatment of feline gastrointestinal small-cell lymphoma with chlorambucil and glucocorticoids. J Am Anim Hosp Assoc. 2010;46(6):413–417.
64. Snead, ECR. Large granular intestinal lymphosarcoma and leukemia in a dog. Can Vet J. 2007;48(8):848–851.
65. Krick, EL, Little, L, Patel, R, et al. Description of clinical and pathological findings, treatment and outcome of feline large granular lymphocyte lymphoma (1996-2004). Vet Comp Oncol. 2008;6(2):102–110.
66. Wellman, ML, Hammer, AS, DiBartola, SP, et al. Lymphoma involving large granular lymphocytes in cats: 11 cases (1982-1991). J Am Vet Med Assoc. 1992;201(8):1265–1269.
67. McEntee, MF, Horton, S, Blue, J, et al. Granulated round cell tumor of cats. Vet Pathol. 1993;30:195–203.
68. Platz, SJ, Breuer, W, Pfleghaar, S, et al. Prognostic value of histopathological grading in canine extramedullary plasmacytomas. Vet Pathol. 1999;36:23–27.
69. Trevor, PB, Saunders, GK, Waldron, DR, et al. Metastatic extramedullary plasmacytoma of the colon and rectum in a dog. J Am Vet Med Assoc. 1993;203(3):406–409.
70. Stimson, EL, Cook, WT, Smith, MM, et al. Extraskeletal osteosarcoma in the duodenum of a cat. J Am Anim Hosp Assoc. 2000;36:332–336.
71. Heldmann, E, Anderson, MA, Wagner-Mann, C. Feline osteosarcoma: 145 cases (1990-1995). J Am Anim Hosp Assoc. 2000;36:518–521.
72. Sharpe, A, Cannon, MJ, Lucke, VM, et al. Intestinal haemangiosarcoma in the cat: clinical and pathological features of four cases. J Small Anim Pract. 2000;41(9):411–415.
73. Reimer, ME, Reimer, MS, Saunders, GK, et al. Rectal ganglioneuroma in a dog. J Am Anim Hosp Assoc. 1999;35:107–110.
74. Howl, JH, Peterson, MG. Intestinal mast cell tumor in a cat: presentation as eosinophilic enteritis. J Am Anim Hosp Assoc. 1995;31:457–461.
75. Regnier, A, Delverdier, M, Dossin, O. Segmental eosinophilic enteritis mimicking intestinal tumors in a dog. Canine Practice. 1996;21(6):25–29.
76. Halsey, CHC, Powers, BE, Kamstock, DA. Feline intestinal sclerosing mast cell tumour: 50 cases (1997-2008). Vet Comp Oncol. 2010;8(1):72–79.
77. Esplin, DG, Wilson, SR. Gastrointestinal adenocarcinomas metastatic to the testes and associated structures in three dogs. J Am Anim Hosp Assoc. 1998;34:287–290.
78. Juopperi, TA, Cesta, M, Tomlinson, L, et al. Extensive cutaneous metastases in a dog with duodenal adenocarcinoma. Vet Clin Pathol. 2003;32:88–91.
79. Mukaratirwa, S, Gruys, E, Nederbragt, H. Relationship between cell proliferation and tenascin-C expression in canine gastrointestinal tumours and normal mucosa. Res Vet Sci. 2004;76:133–138.
80. Mukaratirwa, S, van Ederen, AM, Gruys, E, et al. Versican and hyaluronan expression in canine colonic adenomas and carcinomas: relation to malignancy and depth of tumour invasion. J Comp Path. 2004;131:259–270.
81. McEntee, MF, Brenneman, KA. Dysregulation of beta-catenin is common in canine sporadic colorectal tumors. Vet Pathol. 1999;36:228–236.
82. Restucci, B, Martano, M, De Vico, G, et al. Expression of E-cadherin, beta-catenin and APC protein in canine colorectal tumours. Anticancer Res. 2009;29(8):2919–2925.
83. Aresu, L, Pregel, P, Zanetti, R, et al. E-cadherin and β-catenin expression in canine colorectal adenocarcinoma. Res Vet Sci. 2010;89(3):409–414.
84. Gamblin, RM, Sagartz, JE, Couto, CG. Overexpression of p53 tumor suppressor protein in spontaneously arising neoplasms of dogs. Am J Vet Res. 1997;58(8):857–863.
85. Mayr, B, Reifinger, M. Canine tumour suppressor gene p53 mutation in a case of anaplastic carcinoma of the intestine. Acta Vet Hung. 2002;50(1):31–35.
86. Knottenbelt, C, Mellor, D, Nixon, C, et al. Cohort study of COX-1 and COX-2 expression in canine rectal and bladder tumours. J Small Anim Pract. 2006;47(4):196–200.
87. McEntee, MF, Cates, JM, Neilsen, N. Cyclooxygenase-2 expression in spontaneous intestinal neoplasia of domestic dogs. Vet Pathol. 2002;39:428–436.
88. Beam, SL, Rassnick, KM, Moore, AS, et al. An immunohistochemical study of cyclooxygenase-2 expression in various feline neoplasms. Vet Pathol. 2003;40:496–500.
89. Muller, A, Guaguere, E, Degorce-Rubiales, F. Cheyletiellosis associated with a bowel carcinoma in an old dog. Prat Medic Chirurg. 2002;37(5):405–406.
90. Thompson, JP, Christopher, MM, Ellison, GW, et al. Paraneoplastic leukocytosis associated with a rectal adenomatous polyp in a dog. J Am Vet Med Assoc. 1992;201(5):737–738.
91. Knottenbelt, CM, Simpson, JW, Tasker, S, et al. Preliminary clinical observations on the use of piroxicam in the management of rectal tubulopapillary polyps. J Small Anim Pract. 2000;41(9):393–397.
92. Barrs, VR, Beatty, JA, McCandlish, IA, et al. Hypereosinophilic paraneoplastic syndrome in a cat with intestinal T cell lymphosarcoma. J Small Anim Practice. 2002;43:401–405.
93. Ozaki, K, Yamagami, T, Nomura, K, et al. T-cell lymphoma with eosinophilic infiltration involving the intestinal tract in 11 dogs. Vet Pathol. 2006;43(3):339–344.
94. Marchetti, V, Benetti, C, Citi, S, et al. Paraneoplastic hypereosinophilia in a dog with intestinal T-cell lymphoma. Vet Clin Pathol. 2005;34(3):259–263.
95. Jackson, MW, Helfand, SC, Smedes, SL, et al. Primary IgG secreting plasma cell tumor in the gastrointestinal tract of a dog. J Am Vet Med Assoc. 1994;204(3):404–406.
96. Sato, K, Hikasa, Y, Morita, T, et al. Secondary erythrocytosis associated with high plasma erythropoietin concentrations in a dog with cecal leiomyosarcoma. J Am Vet Med Assoc. 2002;220(4):486–490.
97. Bagley, RS, Levy, JK, Malarkey, DE. Hypoglycemia associated with intra-abdominal leiomyoma and leiomyosarcoma in six dogs. J Am Vet Med Assoc. 1996;208(1):69–71.
98. Cohen, M, Post, GS. Nephrogenic diabetes insipidus in a dog with intestinal leiomyosarcoma. J Am Vet Med Assoc. 1999;215(12):1818–1820.
99. Selting, KA, Ogilvie, GK, Lana, SE, et al. Serum Alpha 1-acid glycoprotein concentrations in healthy and tumor-bearing cats. J Vet Intern Med. 2000;14(5):503–506.
100. Correa, SS, Mauldin, GN, Mauldin, GE, et al. Serum Alpha 1-acid glycoprotein concentration in cats with lymphoma. J Am Anim Hosp Assoc. 2001;37:153–158.
101. Avery, PR, Avery, AC. Molecular methods to distinguish reactive and neoplastic lymphocyte expansions and their importance in transitional neoplastic states. Vet Clin Pathol. 2004;33(4):196–207.
102. Kaneko, N, Yamamoto, Y, Wada, Y, et al. Application of polymerase chain reaction to analysis of antigen receptor rearrangements to support endoscopic diagnosis of canine alimentary lymphoma. J Vet Med Sci. 2009;71(5):555–559.
103. Laurenson, MP, Skorupski, KA, Moore, PF, et al. Ultrasonography of intestinal mast cell tumors in the cat. Vet Radiol Ultrasound. 2011;52(3):330–334.
104. Smith, AL, Wilson, AP, Hardie, RJ, et al. Perioperative complications after full-thickness gastrointestinal surgery in cats with alimentary lymphoma. Vet Surg. 2011;40:849–852.
105. Rivers, BJ, Walter, PA, Feeney, DA, et al. Ultrasonographic features of intestinal adenocarcinoma in five cats. Vet Radiol. 1997;38(4):300–306.
106. Penninck, DG, Smyers, B, Webster, CRL, et al. Diagnostic value of ultrasonography in differentiating enteritis from intestinal neoplasia in dogs. Vet Radiol Ultrasound. 2003;44(5):570–575.
107. Gaschen, L. Ultrasonography of small intestinal inflammatory and neoplastic diseases in dogs and cats. Vet Clin North Am Small Anim Pract. 2011;41(2):329–344.
108. Zwingenberger, AL, Marks, SL, Baker, TW, et al. Ultrasonographic evaluation of the muscularis propria in cats with diffuse small intestinal lymphoma or inflammatory bowel disease. J Vet Intern Med. 2010;24(2):289–292.
109. Willard, MD, Jergens, AE, Duncan, RB, et al. Interobserver variation among histopathologic evaluations of intestinal tissues from dogs and cats. J Am Vet Med Assoc. 2002;220:1177–1182.
110. Danova, NA, Robles-Emanuelli, JC, Bjorling, DE. Surgical excision of primary canine rectal tumors by an anal approach in twenty-three dogs. Vet Surg. 2006;35(4):337–340.
111. Morello, E, Martano, M, Squassino, C, et al. Transanal pull-through rectal amputation for treatment of colorectal carcinoma in 11 dogs. Vet Surg. 2008;37(5):420–426.
112. Swiderski, J, Withrow, S. A novel surgical stapling technique for rectal mass removal: a retrospective analysis. J Am Anim Hosp Assoc. 2009;45(2):67–71.
113. Kupanoff, PA, Popovitch, CA, Goldschmidt, MH. Colorectal plasmacytomas: a retrospective study of nine dogs. J Am Anim Hosp Assoc. 2006;42(1):37–43.
114. Coolman, BR, Ehrhart, N, Pijanowski, G, et al. Comparison of skin staples with sutures for anastomosis of the small intestine in dogs. Vet Surg. 2000;29:293–302.
115. Hardie, EM, Gilson, SD. Use of colostomy to manage rectal disease in dogs. Vet Surg. 1997;26(4):270–274.
116. Holt, PE, Durdey, P. Transanal endoscopic treatment of benign canine rectal tumours: preliminary results in six cases (1992 to 1996). J Small Anim Prac. 1999;40:423–427.
117. Moore, AS, Kirk, C, Cardona, A. Intracavitary cisplatin chemotherapy experience with six dogs. J Vet Intern Med. 1991;5(4):227–231.
118. Malik, R, Gabor, LJ, Foster, SF, et al. Therapy for Australian cats with lymphosarcoma. Aust Vet J. 2001;79:808–817.
119. Coit, DG. Cancer of the small intestine. In DeVita VT, Hellman S, Rosenberg SA, eds.: Cancer: principles and practice of oncology, ed 6, Philadelphia: Lippincott Williams & Wilkins, 2001.
120. Skibber, JM, Minsky, BD, Hoff, PM. Cancer of the colon. In DeVita VT, Hellman S, Rosenberg SA, eds.: Cancer: principles and practice of oncology, ed 6, Philadelphia: Lippincott Williams & Wilkins, 2001.
121. Squire, JA, Whitmore, GF, Phillips, RA. Genetic basis of cancer. In Tannock IF, Hill RP, eds.: The basic science of oncology, ed 3, New York: McGraw-Hill, 1998.
122. Hart, JR, Shaker, E, Patnaik, AK, et al. Lymphocytic-plasmacytic enterocolitis in cats: 60 cases (1988-1990). J Am Anim Hosp Assoc. 1994;30:505–514.
123. Sawaki, A, Yamao, K. Imatinib mesylate acts in metastatic or unresectable gastrointestinal stromal tumor by targeting KIT receptors- a review. Cancer Chemother Pharmacol. 2004;54(suppl 1):S44–S49.
124. Peek, RM. Prevention of colorectal cancer through the use of COX-2 selective inhibitors. Cancer Chemother Pharmacol. 2004;54(suppl 1):S50–S56.
125. Personal communication: Oberthaler K, Shofer F, Bowden A, et al: Chemoprevention using NSAIDs in dogs: A preliminary epidemiological survey, Proceedings 24th annual conference of the Veterinary Cancer Society, p 3.
126. Anonymous: Adjuvant chemotherapy for localised colon cancer. Fluorouracil + folinic acid for node-positive, non-metastatic disease. Prescrire Int. 2011;20(113):46–49.
 Section H
Section H
Perianal Tumors
Michelle M. Turek and Stephen J. Withrow
The perianal area of dogs contains several glands and structures from which tumors may develop. Perianal, or circumanal, glands are located in the dermis in a circular fashion around the anus and are also scattered in areas on the prepuce, tail, pelvic limbs, and trunk.1 These are commonly referred to as hepatoid glands as a result of their cellular morphologic resemblance to hepatocytes and are considered nonsecretory, sebaceous glands in the adult dog.1-3 The anal sacs represent blind cutaneous diverticula that are located on each side of the anus at the four o’clock and eight o’clock positions. Located in the connective tissue surrounding these diverticula are distinct apocrine sweat glands that empty their secretions into the lumen of the anal sacs. The most frequently observed tumors of this region in dogs include perianal sebaceous adenomas, perianal sebaceous adenocarcinomas, and apocrine gland adenocarcinomas of the anal sac (Table 22-9).
Table 22-9
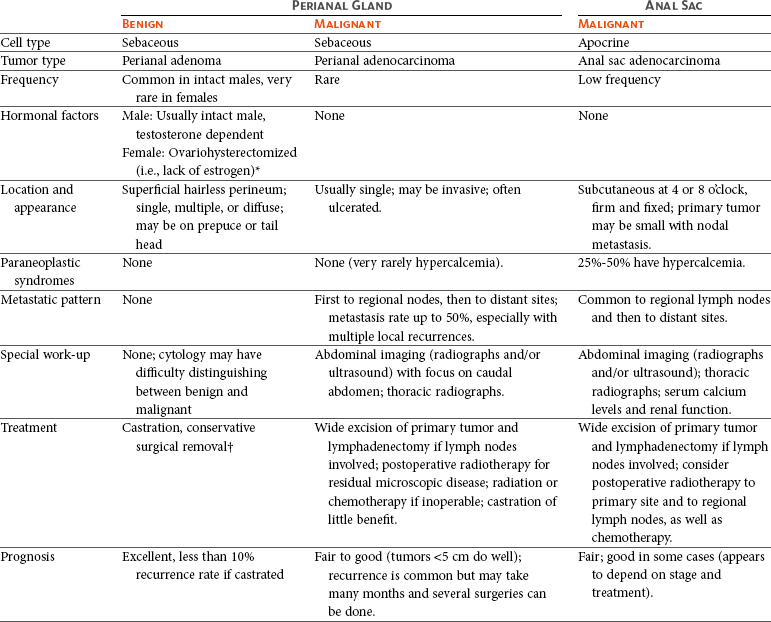
*If multiple, recurrent, or large (male-like), consider testosterone secretion from adrenal glands; Cushing’s signs may or may not be present.
†Estrogens will cause regression but carry risk of bone marrow suppression. Adenomas will respond to radiation therapy, but surgery is cheaper, faster, and safer. Electrochemotherapy and cryosurgery have been reported.
Because cats do not have glands analogous to the perianal sebaceous glands in the dog, perianal adenoma and perianal adenocarcinoma are uncommonly recognized in this species. Apocrine gland adenocarcinoma of the anal sac occurs rarely in the cat.4-10
Incidence and Risk Factors
Perianal adenomas (circumanal, hepatoid tumors) represent the majority, 58% to 96%, of canine perianal tumors.1,11 Development and progression of these benign tumors appear to be sex hormone–dependent, with growth stimulated by androgenic hormones and depressed by estrogenic hormones.1,12,13 The older, intact male is at high risk.1,11,12 The mean reported age is 10 years.11 A high incidence of associated testicular interstitial cell tumors has been reported for males with adenomas, supporting testosterone production as a cause.12 However, a true cause-and-effect relationship has not been clarified since interstitial cell tumors are a common incidental finding in non–adenoma-bearing, older intact males. Perianal adenomas in the female occur almost exclusively in ovariohysterectomized animals in which low levels of estrogen do not suppress tumor growth. Rarely, testosterone secretion from the adrenal glands, occasionally accompanied by signs of hyperadrenocorticism, may stimulate perianal adenoma formation in the female.14,15 Cocker spaniels, beagles, bulldogs, and Samoyeds may be predisposed.1,12
Perianal adenocarcinoma, a malignant tumor of the perianal glands, occurs much less frequently than its benign counterpart, representing only 3% to 21% of all tumors in this region.1,11 Average age of affected dogs is 11 years.11,16 Tumors occur in castrated or intact males, as well as in females, implying no hormonal dependency; however, this does not preclude earlier hormonal initiation.12,16 Large-breed males appear to be overrepresented.16
Apocrine gland adenocarcinoma of the anal sacs occurs at relatively low frequency in the dog, representing 17% of perianal malignancies and 2% of all skin and subcutaneous tumors.11,17 A breed survey of a large cohort of British dogs with anal sac adenocarcinoma suggests that spaniels, in particular English cocker spaniels, are at increased risk.18 Earlier reports suggested a female predilection11,19-21; however, approximately equal sex distribution has been shown in multiple larger series.18,22-24 In male dogs, it has been proposed that neutering may be associated with increased incidence of anal sac apocrine gland adenocarcinoma.18 The average age of dogs diagnosed with this disease is 9 to 11 years.11,19,20,22-26 Dogs as young as 5 years have been reported,19,20,23,25,27 suggesting that evaluation of the perineum and palpation of the anal sacs should be a routine part of the physical examination in every adult dog.23 Benign tumors of the anal sac are very rare.
Anal gland apocrine gland adenocarcinoma occurs rarely in the cat, representing 0.5% of all feline skin and subcutaneous neoplasms.7 Median age of affected cats is 12 years, although animals as young as 6 years have been reported.7,10 Siamese cats may be at higher risk.7
Pathology and Natural Behavior
Almost any tumor can occasionally affect the perianal region, including lymphoma, soft tissue sarcoma, SCC, melanoma, leiomyoma, transmissible venereal tumor, and mast cell tumor. However, the most common tumors in the dog are those that arise from the sebaceous glands of the perineum. The histologic distinction between perianal adenoma and adenocarcinoma may not always be clear, and clinically there may be an intermediate condition called invasive perianal adenoma, which may look benign microscopically, yet be moderately invasive in the patient.
Perianal adenomas follow a benign clinical course. The tumors are slow-growing, and although local disease may be extensive, metastasis does not occur.1,11,12
Perianal adenocarcinoma is generally associated with a low rate of metastasis (15%) at the time of original diagnosis.16 It has been suggested that metastasis is most likely to develop later in the course of disease as the primary tumor becomes larger and more invasive.16 The most frequent site of metastasis is the regional sublumbar and pelvic lymph nodes, including the iliac, hypogastric, and sacral nodes.1,16 Distant metastases may rarely affect lungs, liver, kidney, and bone.12,16 These tumors tend to be more rapidly growing, fixed, and firmer than the more common benign perianal adenomas.
Anal sac apocrine gland adenocarcinoma in the dog is distinct from perianal sebaceous gland adenocarcinoma clinically and histologically. Usually, only one anal sac is affected; however, bilateral tumors can occur.19,20,25,28 The metastatic potential of this tumor is well established with most reports identifying about 50% metastasis (range 46% to 96%) at the time of initial presentation.11,20-26,28 Regional sublumbar lymph nodes and pelvic nodes are by far the most common sites of metastasis early in the course of disease.22-26,28 The primary anal sac tumor may be as small as 0.5 cm, with greatly enlarged metastatic nodes. Distant metastatic sites, including lungs, liver, spleen, bone, and, less commonly, heart, adrenal glands, pancreas, kidneys, and/or the mediastinum, may rarely develop later.11,19,20,22-25 The association between anal sac apocrine gland adenocarcinoma and paraneoplastic hypercalcemia of malignancy, mediated by tumor secretion of parathyroid hormone–related peptide,29-31 has been well documented.* Incidence of hypercalcemia is approximately 27% based on an analysis of 113 dogs with anal sac adenocarcinoma.23 Along with lymphoma, plasma-ionized calcium concentrations tend to be higher with this tumor than with other types of neoplasia.34
The pathogenesis of canine perianal tumors is not known. In a large study evaluating tumor growth characteristics of 240 perianal gland tumors, cell proliferation and apoptosis were quantified by proliferating cell nuclear antigen (PCNA) staining and microscopic detection of apoptotic corpuscles.35 An increase in both parameters was observed in malignant lesions compared to benign ones.35 Various other immunohistochemical studies have attempted to elucidate possible molecular mechanisms involved in canine perianal gland tumorigenesis. Using a single polyclonal antihuman antibody, nuclear p53 accumulation was detected in 50% (8 of 16) of perianal sebaceous gland adenocarcinomas in one study, suggesting that expression of a mutated p53 tumor suppressor protein may play a role.36 Discordant results were reported in another study in which p53 reactivity was found in none of 11 perianal gland adenocarcinomas and in only a small percentage of adenomas.37 In the same tumor samples, Mdm2 expression was observed in both adenomas and adenocarcinomas.37 A study that evaluated androgen receptor expression found no difference between perianal adenomas and their malignant counterparts; the authors concluded that the mechanism by which androgens may influence carcinogenesis is still unknown.38 Finally, another proposed mediator of tumor growth or evolution is growth hormone. Growth hormone was detected immunohistochemically in 96% (23 of 24) of perianal adenomas and 100% (5 of 5) of perianal sebaceous gland adenocarcinomas.39 With respect to anal sac adenocarcinoma, expression of E-cadherin, a protein known to mediate adhesion and communication between cells and the extracellular matrix, was evaluated in tumor biopsies from dogs using a polyclonal antihuman antibody that has been validated for use in canines.26 E-cadherin expression correlated with the presence of metastasis and shorter survival time, suggesting that it may play a role in tumor progression.26 p53 expression has been detected immunohistochemically in a low-to-moderate proportion of anal gland adenocarcinoma tissue biopsies; however, no clinical implications have come from these findings.36 A recent genetic analysis study in English cocker spaniels showed an association between tumor development and major histocompatibility complex haplotype (DLA-DQB1 allele), suggesting that a genetic factor may play a role in tumor development in this at-risk breed.40
The biologic behavior of anal sac apocrine gland adenocarcinoma in the cat has not been clearly defined. Most reports suggest that it is a locally invasive disease associated with a moderate-to-high risk of tumor recurrence following surgery.5-8,10 The rate of metastasis is variable between studies ranging from low to high.6,7,10 Described sites of metastasis include sublumbar lymph nodes, liver, diaphragm, and lungs.6,7,10 Paraneoplastic hypercalcemia is not a common complication of this disease in cats.7,10 Bilateral tumors have not been reported.
History and Clinical Signs
The history for benign perianal adenoma is that of a slow-growing (over months to years) mass or masses that are nonpainful and usually asymptomatic. These may be single, multiple, or diffuse (similar to generalized hyperplasia or hypertrophy of the perianal tissue; Figure 22-29).1 Most occur on the hairless skin area around the anus, although they may extend to haired regions and can develop on the prepuce, scrotum, or tail head (stud tail or “caudal tail gland”).1 Benign lesions may ulcerate and become infected but are rarely adherent or fixed to deeper structures.1 They are usually fairly well circumscribed, on average 0.5 to 3 cm in diameter, and elevated from the perineum.1
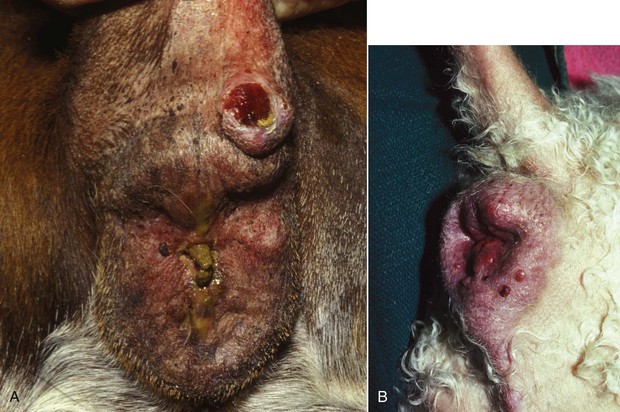
Figure 22-29 A, Typical small and ulcerated perianal adenoma can be seen at 1 o’clock. Treatment with castration and cryosurgery was curative. B, Diffuse 360-degree involvement of the perianal region with perianal adenoma. Aggressive resection or cryosurgery should not be performed; instead, castration, a waiting period of several months for partial regression, and the local treatment for residual disease were applied.
The male perianal adenocarcinoma may look similar to adenomas but tends to grow more rapidly, be more firm, become ulcerated, adhere to underlying tissues, recur following conservative surgery, and generally be larger than its benign counterpart.16 Obstipation, dyschezia, or perianal pain/irritation can be seen with larger masses.16 Rarely, signs are related to obstruction of the pelvic canal by lymph node metastasis. Tumors can be multiple.16 Castrated males with new or recurrent perianal tumors should raise the suspicion for malignant rather than benign disease because adenocarcinomas are not hormonally dependent.
Clinical signs of dogs with anal sac adenocarcinoma are often referable to either the presence of the primary mass (perianal discomfort, swelling [Figure 22-30], bleeding; scooting), to obstruction of the pelvic canal by regional nodal metastasis (tenesmus, constipation [Figure 22-31]), or to hypercalcemia (polyuria, polydipsia; anorexia; lethargy; vomiting).22,23 In as many as 39% of dogs, the primary tumor can be an incidental finding on physical examination.23 Rarely, regional bone metastasis or direct extension of tumor from sublumbar lymph nodes into the lumbar vertebrae with associated pain or fracture may be seen.
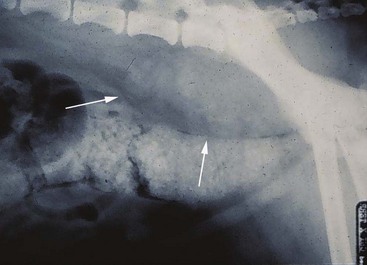
Figure 22-31 Lateral radiograph of the caudal abdomen in a male dog with anal sac adenocarcinoma. Note the metastatic involvement of sublumbar/iliac lymph nodes (arrows) and downward displacement of large bowel.
In cats, the most common history and clinical signs associated with anal sac adenocarcinoma are related to the presence of local disease. Like dogs, cats may be presented for tenesmus, constipation, scooting, presence of a mass, hemorrhagic discharge, or excessive grooming of the perineal area.6-8,10 Lethargy and/or inappetence may be secondary to severe constipation.5 Not all cats present with clinical signs, and tumors can be detected incidentally during a routine physical examination.7 Clinicians should recognize anal sac adenocarcinoma as a distinct clinical entity in the cat and include it as a differential diagnosis for animals with anal sac disease.7 It is not uncommon for anal sac adenocarcinoma to be misdiagnosed as an anal sac abscess based on the presence of a mass, ulceration, or a fistulous tract in the perineal region.7
Diagnostic Techniques and Work-Up
In the intact male with suspected perianal adenoma, a routine geriatric work-up prior to anesthesia is desirable. Thoracic radiographs to evaluate for lung metastasis may not be cost effective unless indicated for other cardiopulmonary evaluation given the benign biologic behavior of these tumors. Evaluation of regional lymph node size is indicated if one suspects perianal adenocarcinoma based on signalment (neutered male), recurrent disease, or physical examination characteristics of malignancy. Ultrasonographic evaluation of regional lymph nodes is a superior staging tool in comparison to plain radiography which can both underestimate and overestimate lymph node disease.41 Although distant metastasis is uncommon, thoracic radiographs to evaluate for lung metastasis are recommended in dogs with perianal adenocarcinoma. FNA and cytology to differentiate benign from malignant tumors in the male can be unrewarding, although they are helpful in ruling out other forms of cancer or mass development. Tissue biopsy is necessary to make the distinction in most cases, and the most important histologic criteria supporting a diagnosis of perianal sebaceous gland adenocarcinoma is invasiveness of tumor cells into surrounding tissue.42 Disorderly arrangement of cells, increased nuclear pleomorphism, and increased numbers of mitotic figures also favor a diagnosis of malignancy.42 The use of monoclonal antibodies, MAbs4A9 and 1A10, developed against a canine mammary cell line and screened for reactivity with perianal adenocarcinoma, has been proposed as a diagnostic tool to help distinguish benign perianal tumors from adenocarcinoma when routine light microscopy is not conclusive.43 Clinical validation is needed to confirm the utility of this approach.
Since dogs with anal sac adenocarcinoma may present with signs unrelated to perianal disease (i.e., polyuria, polydipsia due to hypercalcemia), assessment of animals with suspicious clinical signs requires a careful rectal examination, including palpation of the anal sacs and evaluation for possible regional lymphadenopathy. Definitive diagnosis must be made cytologically or histologically, but a strong presumption of anal sac adenocarcinoma can be made for animals in which rectal examination identifies the presence of a firm and discrete mass in the area of the anal sac. This tumor has a characteristic cytologic appearance and is made up of polyhedral cells that have uniform round nuclei and light blue-gray, slightly granular cytoplasm. Features of malignancy may be subtle. In addition to suggesting the presence of cancer in the anal sacs, FNA is valuable for ruling out infection or inflammatory disease of the anal sac. The clinician should be aware that anal sac tumors can become secondarily infected or inflamed. Staging of disease includes thoracic radiographs for rare detection of pulmonary or mediastinal involvement. Abdominal radiographs may reveal regional lymphadenopathy in advanced cases (see Figure 22-31). Ultrasonographic evaluation of regional lymph nodes is a superior staging evaluation because plain radiography can both underestimate and overestimate lymph node disease.41 A recent study evaluating sonographic features of abdominal lymph nodes found that alterations in shape, contour, cavitation, echogenicity and parenchymal uniformity do not reliably distinguish neoplastic from benign nodes. The only significant difference identified between neoplastic and benign lymph nodes was size.44 Ultrasound also allows for evaluation of other possible sites of abdominal metastasis, particularly liver and spleen. Advanced imaging, including CT and MRI, may yield a more complete assessment of abdominal involvement.45 In rare instances, pulmonary metastasis can be present without obvious regional lymph node disease. Lameness or bone pain should be evaluated with radiography and/or nuclear scintigraphy to rule out bone metastasis. Work-up also includes complete blood count, serum chemistry panel, and urinalysis. Hypercalcemia of malignancy can result in renal damage, which may modify prognosis and anesthetic risk. Medical management of hypercalcemia or impaired renal function may be necessary prior to surgery (see Chapter 5).
Careful rectal examination should be performed on all dogs with perianal tumors to detect the clinical degree of fixation prior to surgery, the probability of resection, and the risk of postoperative complications, particularly incontinence, although this is very rare with even aggressive unilateral resection. It is also important to palpate for the presence of enlarged sacral lymph nodes located along the ventral surface of the vertebrae because these are not easily detectable by ultrasound due to their position in the caudal pelvic canal.
Similarly to dogs, feline anal sac adenocarcinoma can be diagnosed cytologically and histologically. Complete staging of this tumor in cats includes thoracic radiographs to evaluate for pulmonary metastasis and abdominal imaging.4-6,8 Abdominal ultrasound is more sensitive than radiography for detection of metastasis involving abdominal organs or lymph nodes. Positive immunoreactivity with anticytokeratin monoclonal antibody, CAM 5.2, was recently reported in a series of 12 cats with anal sac adenocarcinoma.7
Therapy and Prognosis
Due to the hormonal dependence of perianal adenomas, the vast majority of these tumors will regress (at least partially) following castration, and recurrence is uncommon.12 Surgery is recommended in males with ulcerated tumors or recurrence, as well as in females with typical small and focal masses.12 For diffuse or large benign lesions situated on or in the anal sphincter, castration followed by an observation period of several months to allow reduction in tumor volume may permit safer and easier mass removal. This will only be effective for benign lesions that are hormone dependent. Over 90% of male dogs will be cured with castration and mass removal.1,12 Adenomas can be excised with minimal margins. In addition to standard surgical techniques, mass removal can be achieved using cryosurgery or carbon dioxide laser, which should only be used for focal lesions less than 1 to 2 cm in diameter.46,47 Use of either of these techniques precludes evaluation of the surgical margins for completeness of excision, which may be acceptable for benign masses; however, as the most consistent criterion for malignancy is invasiveness, lack of margin assessment is not without risk. Hyperthermia and RT have also been used successfully.48,49 The cost, added morbidity, and limited availability of these techniques make them a poor alternative to standard blade excision. Electrochemotherapy has been recently described50,51 and consists of intratumoral injections of chemotherapy (cisplatin and/or bleomycin in recent reports) followed by local delivery of electric pulses to potentiate drug uptake. Treatments are delivered in one or two weekly sessions. Based on limited studies, the reported overall response rate is greater than 90%, with 65% complete responses.50,51 Smaller tumors (<5 cm) generally respond better than larger tumors.50,51 Larger tumors are more likely to develop local complications, including focal necrosis, erythema, and inflammation.50,51 Systemic effects are not reported.50,51 Castration and surgical excision remain the treatments of choice for adenomas. Adenomas may also regress following estrogen therapy; however, its use is contraindicated by the risk of myelosuppression. Cyclosporin is reported to have had a palliative effect in a single dog with multiple ulcerated perianal adenomas and a measurable reduction in tumor size was observed.52
Perianal sebaceous gland adenocarcinoma is more locally invasive than its benign counterpart and generally does not respond to castration.12 Aggressive surgical removal with adequate margins is indicated. Removal of one-half or more of the anal sphincter is possible with only rare transient loss of continence. Recurrent disease becomes more difficult to resect. Unfortunately, most perianal adenocarcinomas are not suspected or known until after a conservative resection, which contaminates further tissue planes and can make the second resection problematic. Due to common local recurrence, this tumor is difficult to cure and may require numerous palliative resections over several years. Preoperative diagnosis of malignancy (i.e., incisional biopsy) and more aggressive initial resection should improve local control and should be strongly encouraged. The utility of postsurgical RT should also improve local control; however, data for this approach are lacking. The use of electrochemotherapy has been reported in a small series of dogs.51 Although the study suggested a favorable outcome in the adjuvant setting, additional clinical studies are needed for validation of the efficacy of the approach.51
In a series of 41 dogs with perianal adenocarcinoma, stage of tumor had a significant influence on DFI and overall survival (Figure 22-32).16 Tumors less than 5 cm in diameter (T2) were associated with 2-year tumor control rates in excess of 60%, suggesting that surgical removal of these masses at an early stage is relatively successful with respect to disease control. Fifteen percent of dogs had evidence of metastasis at the time of diagnosis, which related negatively to survival (see Figure 22-32).16 MST for dogs with lymph node or distant metastasis was 7 months; however, aggressive treatment was not attempted in five of six cases.16 If present, regional lymph node metastasis may be excised. Large nodal volume is not a contraindication to caudal abdominal exploratory and lymphadenectomy because some nodes “shell out” readily, whereas others are invasive. Enlarged lymph nodes should be considered for removal, especially if they are causing obstruction of the pelvic canal. The use of radiation and/or chemotherapy, including actinomycin D, has been reported anecdotally, but their role in local or distant control is largely undefined.16,53,54 Controlled clinical trials have not been performed. It has been suggested that nuclear size measured by computer-assisted image analysis in cytologic tumor samples may correlate with biologic behavior in this tumor type.55
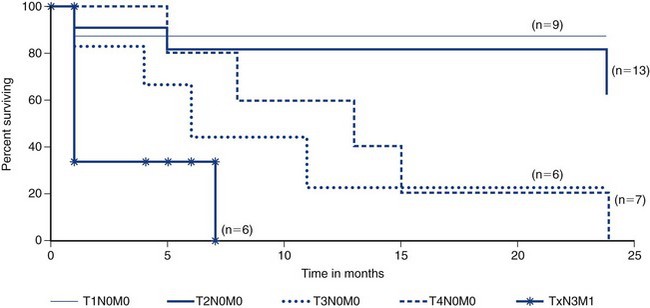
Figure 22-32 Survival duration in 41 male dogs with perianal adenocarcinoma based on stage. Note that dogs with small tumors (T1 or T2) without lymph node involvement will do well after aggressive removal of the primary tumor. T1 = tumor <2 cm, T2 = tumor 2-5 cm, T3 = tumor >5 cm, T4 = tumor invading other structures, such as fascia, muscle, or bone; N0 = no evidence of regional lymph node involvement, N3 = fixed lymph nodes; M0 = no evidence of distant metastasis, M1 = distant metastasis detected.
Apocrine gland adenocarcinoma of the anal sac is generally locally invasive, and aggressive surgical excision is recommended. Recurrence rate is high after marginal surgery alone.20,22 Complete resection of large perianal malignancies is difficult due to proximity to the rectum and poor definition of perineal tissue to define an adequate margin. Metastasis is present at the time of diagnosis in approximately 50% to 80% of cases,19,20,22,23,25 with regional lymph nodes being the most common site.
Enlarged lymph nodes should be considered for removal, especially if they are causing obstruction of the pelvic canal or contributing to hypercalcemia. Radiation and/or chemotherapy may be used adjuvantly or as the sole treatment.22-25,28
Large-scale studies controlled for tumor stage and treatment are lacking in dogs with anal sac adenocarcinoma. There is discordance in the outcome and prognosis reported in the literature. Earlier small studies suggest a MST of 6 to 12 months.20,22,27 This is in contrast to recent reports in which the MST ranges from 16 to 18 months.23,24 These later studies involve larger groups of dogs (113 dogs and 80 dogs, respectively).23,24 However, there is variability in stage of disease and treatment in the study populations that leads to difficulty accurately predicting the prognosis of individual dogs with this tumor type. Negative prognostic indicators gleaned from these and other studies include increasing primary tumor size,23,24 presence of lymph node metastasis,24 presence of distant metastasis,23,24,26 advanced clinical stage,24 nonpursuit of surgery,23 treatment with chemotherapy alone,23 and lack of any therapy at all.24 In one study, extirpation of involved sublumbar lymph nodes in dogs without distant metastasis correlated with improved survival compared to animals in which nodal metastases were not removed.24 E-cadherin expression detected immunohistochemically in tumor biopsies has also been suggested to directly correlate with survival.26 These prognostic variables should be confirmed in controlled clinical trials.
Although chemotherapy and RT are generally considered standard adjuvant treatment options for anal sac adenocarcinoma, the role of these modalities alone or in combination is not well defined. Chemotherapy is theoretically indicated given the high rate of metastasis. Chemotherapeutics with demonstrated antitumor activity in the gross disease setting include carboplatin, cisplatin, and actinomycin D.22,24,52 Other drugs reported in the adjuvant setting include mitoxantrone and melphalan.25,28 Gross tumor responses have not been described for these agents when used alone, and their true role in combination therapies is not known. Toceranib phosphate, a TKI, has been associated with modest tumor responses with median response durations of 19 to 25 weeks in a series of 33 dogs with multiple prior failed therapies.56 This antitumor effect may be mediated through inhibition of the platelet-derived growth factor receptor β (PDGFR-β) based on immunoreactivity of tumor biopsies to anti-PDGFR antibody.57 Controlled clinical trials are needed to determine the role of toceranib in the treatment of anal sac adenocarcinoma. Adjuvant electrochemotherapy using cisplatin is reported in a single dog following an incomplete primary tumor excision.58
The optimal combination of therapies for anal sac adenocarcinoma is not known. In three clinical studies with controlled treatment regimes, MSTs ranged from 17 to 31 months.24,25,28 A study of 14 dogs that underwent surgical excision and melphalan chemotherapy reported an overall MST of 20 months (95% CI, 11.6 to 28.4 months).28 Seven dogs had known nodal metastasis at the time of diagnosis, and nodal metastasis was not prognostic for survival in this small study. A significant relationship between a modified clinical staging scheme and survival was found in a study of 50 dogs treated prospectively according to a predetermined management algorithm.24 Treatment included various combinations of surgery, carboplatin chemotherapy, and hypofractionated (9 Gy × 4 weekly fractions) RT. Fifteen dogs had sublumbar lymphadenopathy and/or distant metastasis at the time of treatment. MST was 17 months (95% CI, 7 to 27 months). Toxicity associated with the treatment was not reported. The third study utilized a standardized RT protocol with concurrent chemotherapy in a cohort of 15 dogs.25 Dogs were treated with combination surgery (primary tumor ± metastatic lymph nodes), curative-intent radiation (3.2 Gy × 15 daily fractions for a total dose of 48 Gy) to the primary tumor site and regional lymph node beds, and mitoxantrone chemotherapy. Seven dogs had lymph node metastasis at diagnosis. Median event-free survival was 9 months (range: 2.5 to 35 months), and overall survival was 31 months (range: 5 to 47 months). Measurable responses were observed in bulky metastatic lymph nodes. The relative importance of radiation versus mitoxantrone chemotherapy is not clear from this study. Without more rigorously controlled clinical trials, strong statements about the “best” treatment and prognosis are difficult to support. In a case series of five dogs selected for resectability of metastatic lymph nodes, a MST of 20 months was achieved with surgery alone.59 One dog underwent repeated lymph node debulking surgeries and survived for 54 months. Another dog survived 18 months following omentalization of cystic sublumbar lymph node metastasis for palliation of tenesmus and dysuria.60 Taken together, these findings of prolonged survival, despite varying tumor stages and varying treatments, strongly suggest the need for prospective, controlled clinical trials to elucidate any benefits of treatment modalities, such as radiation, chemotherapy, molecular-targeted drugs, and their combinations. Identification of reliable prognostic criteria predictive of outcome or treatment response is also needed. It has been suggested in a small study using computer-assisted analysis that nuclear size of tumor cells may correlate with biologic behavior; nuclear size parameters were larger in metastatic anal gland adenocarcinomas than in nonmetastatic tumors.61 Further validation of this approach is needed before it can be considered clinically useful. Finally, when short-term palliation is the therapeutic goal, treatment options include surgery, palliative RT, chemotherapy and/or toceranib, even though efficacy of these approaches has not been definitively established.
Curative-intent RT of the perineal and pelvic regions is associated with self-limiting, acute radiation effects, including mild-to-severe moist desquamation, colitis, and perineal discomfort lasting 2 to 4 weeks posttherapy. Radiation prescriptions should be designed to minimize the risk of late effects, including rectal stricture or perforation. Two studies have demonstrated that radiation doses of less than 3 Gy per fraction should be used to minimize this risk.62,63 In the only report of standardized curative-intent RT for anal sac adenocarcinoma,25 none of the side effects were reported as life-threatening, and all dogs maintained reasonable quality of life as assessed by owners. Chronic complications included tenesmus and narrow stool, diarrhea, and rectal stricture. The daily radiation dose in this study was 3.2 Gy. Future studies should be designed with daily radiation doses of less than 3 Gy to reduce the risk of chronic complications.
There is discordance in the data reported regarding the prognostic significance of hypercalcemia of malignancy accompanying anal sac adenocarcinoma. Hypercalcemia has generally been associated with shorter survival20,23; however, other studies have not supported this finding.22,25,26,28 Complete or near-complete removal of the tumor results in reversal of hypercalcemia. Return of hypercalcemia after therapy usually signals recurrence or metastasis.
Prognosis associated with feline anal sac adenocarcinoma has not been clearly established and some reports are conflicting. Local recurrence after surgical resection appears relatively common. The reported rate of metastasis is variable. In a study involving 39 cats that underwent incisional biopsy or tumor resection, survival time ranged from 0 to 23 months.7 Eighty-five percent of cats succumbed to local disease or presumptive metastasis. MST in this group was 3 months; 1- and 2-year survival rates were 19% and 0%, respectively.7 This is in contrast to a recent retrospective study of 23 cats that reported a median DFI and survival time of over 300 days.10 The role of chemotherapy and RT is not known. A short-lived partial response to carboplatin was reported in a cat with recurrent anal sac adenocarcinoma.8 In another report, outcome following concurrent adjuvant curative-intent RT (48 Gy) and chemotherapy (carboplatin) resulted in local recurrence and/or metastatic disease within 6 months of treatment in two cats.6 In both cases, RT was well tolerated with minimal acute effects.6 Although these survival data are not encouraging, more studies are needed to determine the true role of radiation and chemotherapy in this disease. Based on individual cases of long-term survival, it has been suggested that early detection and completeness of excision may positively impact outcome.6,7
Comparative Aspects64-66
No similar hormonally dependent perianal disease state exists in humans. The most common cancer of the anal margin is squamous cell (epidermoid) carcinoma. These tumors arise from the junction of haired skin and mucous membrane of the anal canal. Risk of developing cancer in this location is positively correlated with sexual activity, and most tumors are associated with human papillomavirus infection. Precancerous changes (dysplasia) in the epithelium of the anal canal may precede tumor development. Regional lymph nodes are the most common site of metastasis. Previously considered a surgical disease requiring a permanent colostomy, improved outcomes have been achieved with definitive chemoradiation. The standard approach to therapy is concomitant radiation and chemotherapy using 5-FU and mitomycin C. Surgery is reserved for locally recurrent or persistent disease. Mean 5-year disease-free survival and overall survival are 60% and 75%, respectively. Size and degree of invasion of the primary tumor, regional lymph node involvement, and presence of distant metastases are important prognostic factors. Identification of biomarkers that may serve as predictors of outcome or targets for therapy is being explored.
References
1. Nielsen, SW, Aftosmis, J. Canine perianal gland tumors. J Am Vet Med Assoc. 1964;144:127–135.
2. Esplin, DG, Wilson, SR, Hullinger, GA. Squamous cell carcinoma of the anal sac in five dogs. Vet Pathol. 2003;40:332–334.
3. Maita, K, Ishida, K. Structure and development of the perianal gland of the dog. Jpn J Vet Sci. 1975;37:349–356.
4. Chun, R, Jakovljevic, S, Morrison, WB, et al. Apocrine gland adenocarcinoma and pheochromocytoma in a cat. J Am Anim Hosp Assoc. 1997;33:33–36.
5. Mellanby, RJ, Foale, R, Friend, E, et al. Anal sac adenocarcinoma in a Siamese cat. J Feline Med Surg. 2002;4:205–207.
6. Elliott, JW, Blackwood, L. Treatment and outcome of four cats with apocrine gland carcinoma of the anal sac and review of the literature. J Feline Med Surg. 2011;13:712–717.
7. Shoieb, AM, Hanshaw, DM. Anal sac gland carcinoma in 64 cats in the United Kingdom (1995-2007). Vet Pathol. 2009;46:677–683.
8. Wright, ZM, Fryer, JS, Calise, DV. Carboplatin chemotherapy in a cat with a recurrent anal sac apocrine gland adenocarcinoma. J Am Anim Hosp Assoc. 2010;46:66–69.
9. Parry, NMA. Anal sac gland carcinoma in a cat. Vet Pathol. 2006;43:1008–1009.
10. Personal communication Cavanaugh, R, Bacon, N, Schallberger, S, et al. Biologic behavior and clinical outcome of cats with anal sac adenocarcinoma: a Veterinary Society of Surgical Oncology retrospective study of 23 cats. Vet Cancer Soc Proc. 2008;28:63.
11. Berrocal, A, Vos, JH, van den Ingh, TS, et al. Canine perineal tumours. J Vet Med Ser A. 1989;36:739–749.
12. Wilson, GP, Hayes, HM. Castration for treatment of perianal gland neoplasms in the dog. J Am Vet Med Assoc. 1979;174(12):1301–1303.
13. Chaisiri, N, Pierrpoint, CG. Steroid-receptor interaction in a canine anal adenoma. J Small Anim Pract. 1979;20:405–416.
14. Dow, SW, Olson, PN, Rosychuk, RA, et al. Perianal adenomas and hypertestosteronemia in a spayed bitch with pituitary-dependent hyperadrenocorticism. J Am Vet Med Assoc. 1988;192:1439–1441.
15. Hill, KE, Scott-Montrieff, CR, Koshko, MA, et al. Secretion of sex hormones in dogs with adrenal dysfunction. J Am Vet Med Assoc. 2005;226:556–561.
16. Vail, DM, Withrow, SJ, Schwarz, PD, et al. Perianal adenocarcinoma in the canine male: a retrospective study of 41 cases. J Am Anim Hosp Assoc. 1990;26:329–334.
17. Goldschmidt, MH, Shofer, FS. Skin tumors of the dog and cat. Oxford, UK: Pergamon Press; 1992.
18. Polton, GA, Mowat, V, Lee, HC, et al. Breed, gender and neutering status of British dogs with anal sac gland carcinoma. Vet Comp Oncol. 2006;4(3):125–131.
19. Goldschmidt, MH, Zoltowski, C. Anal sac gland adenocarcinoma in the dog: 14 cases. J Small Anim Pract. 1981;22:119–128.
20. Ross, JT, Scavelli, TD, Matthiesen, DT, et al. Adenocarcinoma of the apocrine glands of the anal sac in dogs: a review of 32 cases. J Am Anim Hosp Assoc. 1991;27:349–355.
21. Meuten, DJ, Cooper, BJ, Capen, CC, et al. Hypercalcemia associated with an adenocarcinoma derived from the apocrine glands of the anal sac. Vet Pathol. 1981;18:454–471.
22. Bennett, PF, DeNicola, DB, Bonney, P, et al. Canine anal sac adenocarcinomas: clinical presentation and response to therapy. J Vet Intern Med. 2002;16:100–104.
23. Williams, LE, Gliatto, JM, Dodge, RK, et al. Carcinoma of the apocrine glands of the anal sac in dogs: 113 cases (1985-1995). J Am Vet Med Assoc. 2003;223:825–831.
24. Polton, GA, Brearley, MJ. Clinical stage, therapy, and prognosis in canine anal sac gland carcinoma. J Vet Intern Med. 2007;21:274–280.
25. Turek, MM, Forrest, LJ, Adams, WM, et al. Postoperative radiotherapy and mitoxantrone for anal sac adenocarcinoma in the dog: 15 cases (1991-2001). Vet Comp Oncol. 2003;1(2):94–104.
26. Polton, GA, Brearley, MJ, Green, LM, et al. Expression of E-cadherin in canine anal sac gland carcinoma and its association with survival. Vet Comp Oncol. 2007;5(4):232–238.
27. White, RAS, Gorman, NT. The clinical diagnosis and management of rectal and pararectal tumours in the dog. J Small Anim Pract. 1987;28:87–107.
28. Emms, SG. Anal sac tumours of the dog and their response to cytoreductive surgery and chemotherapy. Aust Vet J. 2005;83(6):340–343.
29. Rosol, TJ, Capen, CC, Danks, JA, et al. Identification of parathyroid hormone-related protein in canine apocrine adenocarcinoma of the anal sac. Vet Pathol. 1990;27:89–95.
30. Gröne, A, Werkmeister, JR, Steinmeyer, CL, et al. Parathyroid hormone-related protein in normal and neoplastic canine tissues: immunohistochemical localization and biochemical extraction. Vet Pathol. 1994;31:308–315.
31. Mellanby, RJ, Craig, R, Evans, H, et al. Plasma concentrations of parathyroid hormone-related protein in dogs with potential disorders of calcium metabolism. Vet Rec. 2006;159:833–838.
32. Hause, WR, Stevenson, S, Meuten, DJ, et al. Pseudohyperparathyroidism associated with adenocarcinomas of anal sac origin in four dogs. J Am Anim Hosp Assoc. 1981;17:373–379.
33. Meuten, DJ, Segre, GV, Capen, CC, et al. Hypercalcemia in dogs with adenocarcinoma derived from apocrine glands of the anal sac. Lab Invest. 1983;48(4):428–434.
34. Messinger, JS, Windham, WR, Ward, CR. Ionized hypercalcemia in dogs: a retrospective study of 109 cases (1998-2003). J Vet Intern Med. 2009;23:514–519.
35. Martins, AMCRPF, Vasques-Peyser, A, Torres, LN, et al. Retrospective-systematic study and quantitative analysis of cellular proliferation and apoptosis in normal, hyperplastic and neoplastic perianal glands in dogs. Vet Comp Oncol. 2008;6(2):71–79.
36. Gamblin, RM, Sagartz, JE, Couto, CG. Overexpression of p53 tumor suppressor protein in spontaneously arising neoplasms of dogs. Am J Vet Res. 1997;58(8):857–863.
37. Nakano, M, Taura, Y, Inoue, M. Protein expression of Mdm2 and p53 in hyperplastic and neoplastic lesions of the canine circumanal gland. J Comp Path. 2005;132:27–32.
38. Pisani, G, Millanta, F, Lorenzi, D, et al. Androgen receptor expression in normal, hyperplastic and neoplastic hepatoid glands in the dog. Res Vet Sci. 2006;81:231–236.
39. Petterino, C, Martini, M, Castagnaro, M. Immunohistochemical detection of growth hormone (GH) in canine hepatoid gland tumors. J Vet Med Sci. 2004;66(5):569–572.
40. Aguirre-Hernandez, J, Polton, G, Kennedy, LJ, et al. Association between anal sac gland carcinoma and dog leukocyte antigen-DQB1 in the English Cocker Spaniel. Tissue Antigens. 2010;76:476–481.
41. Llabrés-Díaz, FJ. Ultrasonography of the medial iliac lymph nodes in the dog. Vet Radiol Ultrasound. 2004;45(2):156–165.
42. Stannard, AA, Pulley, LT. Tumors of the skin and soft tissues. In: Moulton JE, ed. Tumors in domestic animals. Berkeley: University of California Press, 1978.
43. Ganguly, A, Wolfe, LG. Canine perianal gland carcinoma-associated antigens defined by monoclonal antibodies. Hybridoma. 2006;25(1):10–14.
44. De Swarte, M, Alexander, K, Rannou, B, et al. Comparison of sonographic features of benign and neoplastic deep lymph nodes in dogs. Vet Radiol Ultrasound. 2011;52:451–456.
45. Personal communication Anderson, C, Fidel, J, Sellon, R. Comparison of abdominal ultrasound and magnetic resonance imaging for detection of abdominal lymphadenopathy in dogs with metastatic anal sac gland adenocarcinoma. Vet Cancer Soc Proc. 2010;30:124.
46. Liska, WD, Withrow, SJ. Cryosurgical treatment of perianal gland adenomas in the dog. J Am Anim Hosp Assoc. 1978;14:457–463.
47. Shelley, BA. Use of the carbon dioxide laser for perianal and rectal surgery. Vet Clin North Am Small Anim Pract. 2002;32:621–637.
48. Grier, RL, Brewer, WG, Theilen, GH. Hyperthermic treatment of superficial tumors in cats and dogs. J Am Vet Med Assoc. 1980;177(3):227–233.
49. Gillette, EL. Veterinary radiotherapy. J Am Vet Med Assoc. 1970;157(11):1707–1712.
50. Tozon, N, Kodre, V, Sersa, G, et al. Effective treatment of perianal tumors in dogs with electrochemotherapy. Anticancer Res. 2005;25:839–846.
51. Spugnini, EP, Dotsinsky, I, Mudrov, N, et al. Biphasic pulses enhance bleomycin efficacy in a spontaneous canine perianal tumor model. J Exp Clin Cancer Res. 2007;26(4):483–487.
52. Park, C, Yoo, JH, Kim, HJ, et al. Cyclosporine treatment of perianal gland adenoma concurrent with benign prostatic hyperplasia in a dog. Can Vet J. 2010;51:1279–1282.
53. Hammer, AS, Couto, CG, Ayl, RD, et al. Treatment of tumor-bearing dogs with actinomycin D. J Vet Intern Med. 1994;8(3):236–239.
54. Bley, CR, Stankeova, S, Sumova, A, et al. Metastases of perianal gland carcinoma in a dog: palliative tumor therapy. Schweiz Arch Tierheilkd. 2003;145(2):89–94.
55. Simeonov, R, Simeonova, G. Computer-assisted nuclear morphometry in the cytological evaluation of canine perianal adenocarcinomas. J Comp Path. 2008;139:226–230.
56. London, C, Mathie, T, Stingle, N, et al. Preliminary evidence for biologic activity of toceranib phosphate (Palladia) in solid tumours. Vet Comp Oncol. 2011. [Epub ahead of print].
57. Brown, RJ, Newman, SJ, Durtschi, DC, et al. Expression of PDGFR-β and kit in canine anal sac apocrine gland adenocarcinoma using tissue immunohistochemistry. Vet Comp Oncol. 2012;10(1):74–79.
58. Spugnini, EP, Dotsinsky, I, Mudrov, N, et al. Adjuvant electrochemotherapy for incompletely excised anal sac carcinoma in a dog. In Vivo. 2008;22:47–50.
59. Hobson, HP, Brown, MR, Rogers, KS. Surgery of metastatic anal sac adenocarcinoma in five dogs. Vet Surg. 2006;35:267–270.
60. Hoelzler, MG, Bellah, JR, Donofro, MC. Omentalization of cystic sublumbar lymph node metastases for long-term palliation of tenesmus and dysuria in a dog with anal sac adenocarcinoma. J Am Vet Med Assoc. 2001;219(12):1729–1731.
61. Simeonov, R, Simeonova, G. Quantitative analysis in spontaneous canine anal sac gland adenomas and carcinomas. Res Vet Sci. 2008;85:559–562.
62. Anderson, CR, McNiel, EA, Gillette, EL, et al. Late complications of pelvic irradiation in 16 dogs. Vet Radiol Ultrasound. 2002;43(2):187–192.
63. Arthur, JJ, Kleiter, MM, Thrall, DE. Characterization of normal tissue complications in 51 dogs undergoing definitive pelvic region irradiation. Vet Radiol Ultrasound. 2008;49(1):85–89.
64. Lampejo, T, Davanagh, D, Clark, J. Prognostic biomarkers in squamous cell carcinoma of the anus: a systematic review. Br J Cancer. 2010;103:1858–1869.
65. Chan, E, Kachnic, LA, Thomas, CR, Jr. Anal cancer: Progress on combined-modality and organ preservation. Curr Prob Cancer. 2009;33:302–326.
66. Shia, J. An update on tumors of the anal canal. Arch Pathol Lab Med. 2010;134:1601–1611.
*References 7, 10, 18, 19, 26-35.
*References 7, 10, 18, 19, 26-35.
†References 7, 9, 18-21, 26, 34, 35, 58.
‡References 7, 26, 28, 29, 31-35.
*References 11, 13, 18, 19, 57, and 60.
*Depo-Medrol, Upjohn.
†Ovaban, Schering.
*References 2, 5-8, 24, 26-29.
*References 12, 13, 15, 16, 18-20, 25, 26, 35-37.
*References 2, 11, 15, 25, 27, 34, 35, 38.
*References 11, 16, 23, 24, 30, 32, 34.
†References 11, 15, 16, 24, 27, 30.