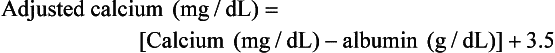Paraneoplastic Syndromes
Paraneoplastic syndromes (PNSs) are neoplasm-associated alterations in bodily structure and/or function that occur distant to the tumor. They are a diverse group of clinical aberrations that are associated with the noninvasive actions of the tumor. In many situations, the PNS parallels the underlying malignancy; therefore successful treatment of the tumor leads to disappearance of the PNS. Alternatively, recurrence of the PNS after successful treatment signals tumor recurrence and often significantly precedes clinically detectable tumor.
PNSs are often the first sign of malignancy, and the PNS may be a hallmark of a certain tumor histology. Therefore an understanding and appreciation for the types and causes of these syndromes are paramount for early cancer detection and appropriate therapy. In addition, a PNS may result in greater morbidity than that associated with the actual tumor.
The causes of PNSs are quite variable; they are usually caused by the production of small molecules (e.g., hormones, cytokines, or peptides) that are released into the circulation to cause effects at distant sites or by immune cross-reactivity between malignant and normal tissues. Some PNSs are due to functional mutations that result in overexpression of the small molecule in question, whereas many nonendocrine PNSs have no known etiology. PNSs are recognized commonly in both human and companion animal cancer patients.1 Box 5-1 summarizes the most common PNSs of dogs and cats and the tumors associated with them.
Gastrointestinal Manifestations of Cancer
An important systemic effect of cancer in animals is profound malnutrition and wasting. The weight loss and metabolic alterations observed in cancer patients despite adequate nutritional intake are termed cancer cachexia, whereas alterations observed as the result of poor nutritional intake are termed cancer anorexia. The clinical outcome of either cancer cachexia and/or anorexia is a progressive wasting (Figure 5-1). The weight loss endured by these patients is more than a simple cosmetic abnormality as human patients with cancer cachexia can have significantly reduced survival times, and many patients are unable to undergo appropriate therapy because of their poor clinical status.

Figure 5-1 Dog with lymphoma and secondary severe cachexia. Cancer cachexia can be a common paraneoplastic syndrome (PNS) in dogs and cats. The weight loss noted in cases of paraneoplastic cancer cachexia occurs despite adequate nutritional intake. The metabolic alterations associated with cancer cachexia usually occur before clinical signs of the inciting malignancy appear and unfortunately may continue after the patient is successfully treated for the tumor.
Cancer cachexia occurs frequently in human oncology, with estimated incidences from 40% to approximately 90% of hospitalized patients.2,3 Importantly, cancer cachexia accounts for approximately 20% of cancer deaths.4,5 The incidence of cancer cachexia in veterinary oncology patients is presently unknown, although this author believes that an estimated incidence of cancer cachexia in dogs is realistically 10% or less. For example, only 4% of dogs presenting to an academic oncology center were found to have cachexia, although referral bias likely results in some reporting artifact.6 The metabolic alterations associated with this PNS usually occur before weight loss is detected in both human and veterinary cancer patients.3,7-11 These metabolic alterations may last for some time after the patient is tumor free making it difficult for the clinician to reverse the weight loss.8,10 A plasmid-DNA–mediated approach utilizing growth hormone–releasing hormone (GHRH) in dogs appears to increase insulin-like growth factor-1 (IGF-1) levels (a measure of GHRH activity) and may represent a mechanism for attenuating cancer cachexia.12-15
In the clinical evaluation of a veterinary patient for the possibility of cancer cachexia/anorexia, a detailed history and physical examination are crucial. The prognostic importance of the presence of cancer cachexia in human cancer patients cannot be overstated because many studies show that this PNS is the only or one of very few independent multivariate negative prognostic factors for a variety of malignancies.2,3 A more detailed discussion of this syndrome is found in Chapter 15, Section B.
Protein-Losing Enteropathy
Protein-losing enteropathy (PLE) is a syndrome whereby excessive serum proteins are lost into the gastrointestinal (GI) tract, leading to hypoproteinemia.16 The hypoproteinemia seen in cancer patients can be due to impaired synthesis and/or increased loss into the GI tract or urine (see Renal Manifestations of Cancer later in this chapter). Once the loss of proteins becomes greater than the body’s ability to synthesize them, serum protein levels begin to decrease. The half-life of many serum proteins is long, and patients with hypoproteinemia caused by PLE or some other cancer-related protein loss may represent long-standing protein loss.2,16 PLE is thought to result from an increase in mucosal serum protein permeability because of mucosal erosion, ulceration, or lymphatic obstruction.
The diagnosis of PLE is made by noting hypoproteinemia on serum chemistry evaluation with subsequent exclusion of severe malnutrition and liver disease. Confirmation of the diagnosis is made in humans with PLE by alpha-1-antitrypsin detection,17 but this methodology has not been validated in veterinary medicine.18 In addition, nuclear scintigraphy appears to be a reliable methodology for the diagnosis of PLE.19 The incidence of PLE as a PNS is unknown in veterinary medicine but is likely to be rare. The treatment for PLE consists of treating the primary malignancy; however, those patients with a lymphangiectasia-related PLE may also be treated with medium-chain triglycerides that do not undergo transport by intestinal lymphatics.
Gastroduodenal Ulceration
The most common cause of PNS-associated gastroduodenal ulceration is mast cell tumor (MCT). The excess histamine seen in MCTs stimulates gastric H2 receptors, leading to increased gastric acid secretion. Clinical manifestations of mucosal damage and/or ulceration with gastric vessel thrombosis occur in association with gastric hyperacidity. Plasma histamine concentrations are elevated in approximately 75% of dogs with macroscopic MCT, although only 30% have GI signs.20,21 Abnormally elevated plasma histamine concentrations have also been found to be a negative prognostic factor in dogs with MCT.21 Symptomatic therapies such as proton-pump inhibitors, H2 blockers, misoprostol, sucralfate, and rehydration may be helpful in combating PNS-associated gastroduodenal ulceration. MCTs are covered in greater detail in Chapter 20.
An additional cause of PNS-associated gastroduodenal ulceration is gastrinoma (gastrin-secreting non–islet cell pancreatic tumor). Although these tumors are relatively rare, they have been reported in both dogs and cats.22-26 Gastrinomas can be associated with vomiting, lethargy, anorexia, blood loss, and abdominal pain. Many of these features are also seen in humans with gastrinoma-related Zollinger-Ellison syndrome. Gastrinomas are covered in greater detail in Chapter 25.
Endocrinologic Manifestations of Cancer
The most common cause of hypercalcemia in the dog is cancer. A variety of tumors have been associated with hypercalcemia of malignancy (HM), and neoplasia is diagnosed in approximately two-thirds of dogs27,28 and one-third of cats with hypercalcemia.29 Lymphoma is the most common cause of HM (10% to 35% occurrence). Other tumor types associated with HM in dogs and cats include anal sac apocrine gland adenocarcinoma (≥25%), thyroid carcinoma, multiple myeloma (20%), bone tumors, thymoma, squamous cell carcinoma, mammary gland carcinoma/adenocarcinoma, melanoma, primary lung tumors, chronic lymphocytic leukemia, renal angiomyxoma, and parathyroid gland tumors.30-40 The causes of HM are varied and include ectopic production of parathormone (PTH) or PTH-related peptide (PTH-rp) by the tumor, extensive and usually multifocal lytic bone metastases, primary hyperparathyroidism, tumor-associated prostaglandins (PGE1/2), interleukin-1-β (IL-1β, previously known as osteoclast-activating factor [OAF]), transforming growth factor-β (TGF-β), and receptor activator of nuclear factor kappa-B ligand (RANKL).2,34,41-46 Interestingly, TGF-β1 regulates the mRNA stability of PTH-rp.47 The HM seen in lymphoma and anal sac apocrine gland adenocarcinoma is commonly caused by tumor-associated PTH-rp.48,49 PTH-rp is a 16-kDa protein with significant sequence identity to PTH, suggesting it may act and function like PTH. In addition to HM, other hypercalcemia differential diagnoses include “lab error” (lipemia and hemolysis), acute renal failure, hypervitaminosis D, hypoadrenocorticism, and granulomatous disease.
In addition to ensuring the hypercalcemia is not due to lipemia or hemolysis, it is important to interpret the calcium value in relation to the level of serum albumin if ionized calcium determination is not available. Two commonly utilized correction formulas that controversially attempt to account for the level of serum albumin follow:
Similarly, an increase in the free ionized fraction of calcium can occur with acidosis. Acidotic HM patients may have an increase in clinical signs of hypercalcemia when compared to nonacidotic HM patients.
The primary clinical manifestations of HM are due to renal function impairment. Severe HM (calcium > 18 mg/dL) should be considered a medical emergency. An inability to concentrate urine is the initial manifestation of hypercalcemia and is due to decreased responsiveness to antidiuretic hormone (ADH) at the distal tubule; the calcium then decreases renal blood flow and glomerular filtration rate (GFR) as the result of severe vasoconstriction. Calcium salt deposition in the renal parenchyma may also contribute to renal azotemia, although this is uncommon. The urinary epithelium may then undergo degeneration, and in severe cases, necrosis results in exposure of the basement membrane of the renal tubule. The situation worsens as the patient becomes polyuric and polydipsic, resulting in progressive dehydration. In addition to effects on the renal system, severe HM may cause constipation, hypertension, twitching, weakness, shaking, depression, vomiting, bradycardia, stupor, and possibly coma and/or death.
When HM is diagnosed, appropriate steps for identification of the underlying neoplasm cause are necessary, and if azotemia accompanies HM, appropriate therapy to support renal function should be instituted quickly. The diagnostic evaluation of HM should begin with procedures used in the staging of lymphoma (as outlined in Chapter 32, Sections A and B) in addition to a rectal palpation and examination for anal sac apocrine gland adenocarcinoma. If these diagnostics do not confirm the specific cause of the HM, then the aforementioned hypercalcemia differential diagnoses should be considered and appropriately pursued. Dogs and cats with HM will typically have low PTH and high PTH-rp concentrations; however, the cause of the HM can usually be delineated with appropriate diagnostics before the return of PTH/PTH-rp assay results and such tests may not be routinely needed. Since HM is a potential medical emergency, the primary goal is elucidation of the underlying cause in order to institute the appropriate therapy for the specific tumor. Symptomatic therapy must be judiciously utilized while searching for the underlying cause of the HM. The premature administration of symptomatic therapy that includes the use of corticosteroids prior to the confirmation of the cause of the HM can have serious consequences. If lymphoma is the underlying cause of the HM, the use of corticosteroids may interfere with the ability to confirm a diagnosis, necessitating either additional diagnostics and/or waiting to determine if the lymphoma reappears after glucocorticoid withdrawal. In addition, glucocorticoids may induce resistance to other chemotherapy agents with a decrease in the ability to induce a complete remission, as well as a decrease in the length of survival.50 Therefore the use of corticosteroids in cases of undiagnosed hypercalcemia is strongly discouraged.
Symptomatic therapies that promote external loss of calcium, increase renal excretion of calcium, and inhibit bone reabsorption may be utilized in HM patients. The severity of clinical signs and associated hypercalcemia determine the preferred therapy (Box 5-2). The use of 0.9% NaCl intravenously (IV) is recommended for management of existing dehydration and to expand the extracellular fluid volume, increase GFR, increase calciuresis and natriuresis, and decrease calcium reabsorption by the kidneys. Once rehydrated, the loop diuretic furosemide with continued normosaline diuresis can be utilized to potently inhibit calcium reabsorption in the ascending loop of Henle. If the cause of the HM is determined, corticosteroids can be extremely effective as adjunct therapy for HM by their inhibition of PGE, OAF (IL-1β), vitamin D, and intestinal calcium absorption. Corticosteroids can also be cytotoxic to lymphoma cells, the most common cause of HM. The most common therapies utilized in the treatment of HM are outlined in Box 5-2. In rare cases that are unresponsive to these symptomatic therapies and treatment of the underlying cause, other treatments such as calcitonin, bisphosphonates, or gallium nitrate may be utilized.51 Bisphosphonates have become the standard of therapy for human nonhumoral HM because of their potent inhibition of bone resorption without affecting tubular calcium reabsorption.52 The use of bisphosphonates in dogs and cats appears to be a promising treatment for hypercalcemia but requires additional study.53,54 In the future, treatment options for refractory HM may include osteoprotegerins, more potent bisphosphonates, anti-PTH-rp antibodies, noncalcemic calcitriol analogs, distal tubule calcium reabsorption inhibitors, RANKL antagonists, and new bone resorption inhibitors.52,55
Hypoglycemia
The most common cause of cancer-induced hypoglycemia (<65 to 70 mg/dL serum glucose) in the dog is insulinoma (beta-islet cell tumor), and the reader is directed to Chapter 25 for a discussion of insulinomas.56 Nonislet cell tumors can also serve as sources of ectopic hormone production with resultant hypoglycemia in dogs and humans. Nonislet cell tumors with PNS hypoglycemia have been most commonly associated with hepatocellular carcinomas; however, lymphoma, hemangiosarcoma, oral melanoma, hepatoma, plasma cell tumor, multiple myeloma, smooth muscle tumors (leiomyoma and leiomyosarcoma), mammary tumors, renal tumors, and salivary gland tumors have also been reported.57-62 The hypoglycemia of extrapancreatic tumors has interestingly been associated with low insulin levels, whereas pancreatic beta-islet cell tumors (insulinomas) induce hypoglycemia by excessive circulating insulin levels. Nonislet cell tumors may induce hypoglycemia by increased tumor utilization of glucose, decreased hepatic glycogenolysis or gluconeogenesis, or the secretion of insulin or IGF-1 and IGF-2. Additional mechanisms include upregulation of insulin receptors, increased insulin binding by M proteins in myeloma, and increased production of somatomedins.2,63 The differential diagnoses of hypoglycemia include insulinoma, nonislet cell tumor hyperinsulinism, nonislet cell tumor, hypoadrenocorticism, starvation, sepsis, liver dysfunction, and laboratory error (lack of timely serum separation). Tumors associated with extrapancreatic hypoglycemia are often extremely large and therefore radiographs/ultrasound of the abdomen and/or thorax may be helpful. Exploratory laparotomy may be necessary if a space-occupying mass is not identified. Provocative testing via glucagon or glucose tolerance testing may be useful in cases when the diagnosis is uncertain. The reader is directed to Chapter 25 for an extensive discussion of the clinical consequences, diagnosis, and therapy of tumor-induced hypoglycemia.
Syndrome of Inappropriate Secretion of Antidiuretic Hormone
Syndrome of inappropriate secretion of ADH (SIADH) is a PNS widely recognized in lung, head, neck, and many other tumors in humans1,2; however, it continues to be essentially unrecognized in veterinary oncology. SIADH has been reported in dogs associated with heartworm disease, congenital hydrocephalus, and granulomatous amebic meningoencephalitis.64-66 In addition to PNS-associated SIADH, chemotherapy agents and other drugs (vincristine, cyclophosphamide, cisplatin, thiazides, morphine, and chlorpropamide), pulmonary and/or central nervous system (CNS) infections, and a variety of other conditions can cause SIADH.67,68 The initial finding in SIADH patients is hyponatremia. In addition to hyponatremia, serum hypo-osmolarity, hypernatriuresis, urine hyperosmolarity, and euvolemia with normal renal, thyroid, and adrenal function is noted.2,67 Although most human SIADH patients are asymptomatic, clinical signs can develop due to hyponatremia that result in CNS signs such as fatigue, anorexia, confusion and potentially seizures. The treatment of choice for PNS-associated SIADH is removal of the underlying cause. In addition, water restriction, demeclocycline (ADH antagonist), and hypertonic sodium chloride may be useful in SIADH cases.69
Ectopic Adrenocorticotropic Hormone Syndrome
The ectopic production of adrenocorticotropic hormone (ACTH) or ACTH-like substances is the second most common PNS reported in humans.2 This PNS is associated with small cell lung tumors, pancreatic tumors, and a wide variety of other human tumors.2,67 In the dog, this PNS is reported to occur in primary lung tumors and a single case of an abdominal neuroendocrine tumor.70,71 The predominant active molecules in this PNS are ACTH, ACTH precursors, endorphins, enkephalins, and melanocyte-stimulating hormone (MSH).2,67 All result in excessive production of steroids from the adrenal glands resulting in clinical signs similar to those seen in hyperadrenocorticism (Cushing’s disease). Invariably, tumors that cause this PNS are dexamethasone insuppressible. The diagnosis of this PNS is made by the concomitant presentation of Cushing’s-like signs with an abnormal dexamethasone suppression test and a localizable tumor. The treatment of choice is removal of the underlying cause by surgical extirpation of the tumor. When necessary, the medical therapy for this PNS centers on inhibiting cortisol production by mitotane or ketoconazole. The use of selegiline (Anipryl) in the management of this PNS in dogs has not been evaluated to date in veterinary medicine.
Hypocalcemia/Hyperglycemia
Paraneoplastic hypocalcemia and hyperglycemia are extremely rare. Tumors associated with lytic bone metastases and tumors that secrete calcitonin (medullary carcinoma of the thyroid) are the most common causes of human PNS hypocalcemia.2 A variety of nonthyroid cancers such as breast cancer, GI cancer, carcinoids, and lung cancer in humans have been reported to secrete calcitonin.72 Most cases of PNS hypocalcemia are asymptomatic, although human patients may have neuromuscular irritability and/or tetany. A gingival vascular hamartoma in a 4-month-old kitten has been reported to be associated with PNS hyperglycemia, and the hyperglycemia resolved within 24 hours on removal of the tumor.73 Treatment of choice for either PNS is eradication of the primary tumor whenever possible, calcium infusion in severe hypocalcemia cases, and diabetes-like support for severe hyperglycemia cases.
Hematologic Manifestations of Cancer
Monoclonal gammopathies can be common in animals and people with cancer and they are termed M-component disorders.1,2,74-76 The hypergammaglobulinemia seen as a PNS is due to the excessive production of proteins from a monoclonal line of immunoglobulin (Ig)-producing plasma cells or lymphocytes. When production of these Igs, partial Igs, heavy chains, and/or light chains becomes extreme, clinical signs of hyperviscosity (ataxia, depression, dementia, cardiac disease and/or failure, seizures, and coma), tissue hypoxia, bleeding (poor platelet aggregation, platelet coating with Igs, and release of platelet factor III), and/or ocular disorders (e.g., papilledema, retinal hemorrhage, detachment) may occur. These proteins may be identified by performing a protein electrophoresis on the serum and/or urine.76 Similarly, light chain production may be detected in the urine as Bence-Jones proteins. In addition to the hypergammaglobulinemia PNS seen in plasma cell tumors (multiple myeloma and extramedullary plasmacytoma), lymphomas, lymphocytic leukemias, and primary macroglobulinemia can also cause this PNS.75,76 Further discussion of plasma cell tumors, myeloma and lymphoma/leukemia can be found in Chapter 32.
Anemia
Anemia is one of the most common PNSs seen in veterinary and human oncology. Approximately 20% to 25% of human cancer patients have PNS anemia, and although the exact incidence of PNS anemia in veterinary oncology is unknown, it is thought to be a significant problem.77 There are numerous possible causes for PNS anemia in veterinary oncology patients, and the vast majority are due to either anemia of chronic disease (ACD), immune-mediated hemolytic anemia (IMHA), blood loss anemia, or microangiopathic hemolytic anemia (MAHA).
ACD is extremely common in veterinary and human oncology patients with disseminated and/or metastatic tumors. This anemia is due to disordered iron storage and metabolism, shortened red blood cell (RBC) lifespan, and occasionally decreased bone marrow response.77 The anemia seen in ACD is normocytic/normochromic, and evaluation of the bone marrow does not suggest significant problems with cellularity. Treatment of choice is removal of the tumor.
IMHA can be triggered by tumors in animals and humans. Immune mechanisms then result in the premature destruction of RBCs.78 The diagnosis of PNS IMHA is typically established by a Coombs’ slide agglutination test, and many patients will have concurrent spherocytosis and a regenerative anemia. The treatment of choice is removal of the tumor; however, if this is not immediately possible, the use of immunosuppressive dosages of prednisone (1 to 2 mg/kg daily to two times a day by mouth [BID PO]) may be indicated if a diagnosis has been established. Similar to non-PNS IMHA, the use of additional agents such as azathioprine (1 to 2 mg/kg daily for 4 to 7 days, then 0.5 to 1 mg/kg every 48 hrs PO), cyclosporine, cyclophosphamide, and others may be necessary for complicated IMHA cases.78-81
Blood loss anemia can be a sequela to many types of cancer. Due to decreased hemoglobin content, the RBCs in blood loss anemia are microcytic/hypochromic. In addition, decreased serum iron, increased total iron-binding capacity, and poikilocytosis may also be noted.82,83 The blood loss may be readily apparent in some patients (e.g., bleeding splenic tumor or bleeding superficial tumor), whereas others may not have a readily identifiable source for the loss (e.g., GI tumors). The treatment of choice is removal of the tumor, although severe anemia may necessitate blood transfusions. The use of oral and/or injectable iron may be a useful adjunct therapy.
MAHA is a secondary phenomenon to hemolysis and is typically due to fibrin deposition and/or endothelial damage.77 The most common causes of MAHA are PNS disseminated intravascular coagulation (DIC) and RBC shearing as the result of hemangiosarcoma.77,80 Schistocytosis and hemolysis are common indicators of ongoing MAHA. While any tumor can cause DIC and subsequent MAHA, hemangiosarcoma is most common.84 The treatment of choice is removal of the tumor; however, additional ancillary treatments such as aggressive supportive therapy and transfusions may be useful.
Chemotherapy-induced anemia can be quite common in people because of more aggressive chemotherapy protocols2; however, this is rarely seen in veterinary patients.77,82,83 The degree of anemia seen in veterinary cancer patients undergoing chemotherapy is generally mild, with typical packed cell volumes hovering in the 28% to 32% range for dogs and 24% to 28% range for cats. This anemia rarely necessitates therapy and resolves on discontinuation of the chemotherapy protocol.
A relatively uncommon cause of PNS-associated anemia is myelophthisis (bone marrow invasion/crowding out), and it is most commonly caused by leukemias.2 Another uncommon cause of PNS-associated anemia is bone marrow hypoplasia resulting from hyperestrogenism. Sertoli cell tumors in the male dog and granulosa cell tumors of the female dog are commonly associated with hyperestrogenism, and anemia is a common presenting feature.85-87
Erythrocytosis
Erythrocytosis is a relatively uncommon PNS. Tumors that have been associated with PNS erythrocytosis include renal tumors (primary and secondary), lymphoma (including renal origin), lung or liver tumors, cecal leiomyosarcoma, nasal fibrosarcoma, and transmissible venereal tumor (TVT).62,88-93 The erythrocytosis seen in cancer patients can be due directly to overproduction of erythropoietin, indirect excess erythropoietin from renal hypoxia, or increased production of hypoxia-inducible factors such as HIF-1.94 Interestingly, some tumor suppressor genes (TSG) are important proteosomal regulators of HIF-1, which may explain some of the vascular diseases seen in human patients with certain TSG mutations.95 Other differential diagnoses for polycythemia include arteriovenous shunts, severe dehydration, hyperadrenocorticism, polycythemia vera (primary polycythemia), and a variety of cardiac and/or pulmonary diseases.
It is important to differentiate primary and secondary causes of erythrocytosis. Polycythemia vera is a myeloproliferative disorder resulting in clonal proliferation of RBCs with splenomegaly and possibly pancytosis most commonly associated with acquired recurrent mutation in JAK2.1,2,96 Secondary polycythemia results from decreased arterial oxygen saturation. PNS erythrocytosis is best treated by removal of the erythropoietin-producing tumor whenever possible; phlebotomy can be a useful temporary adjunct therapy. Unfortunately, the volumes typically needed for a therapeutic phlebotomy in PNS erythrocytosis cases necessitates administration of fluids and potentially readministration of plasma. The use of hydroxyurea (40 to 50 mg/kg divided BID PO)91 as a chemotherapeutic agent for polycythemia vera has been previously recommended; however, this author has noted limited benefit with use of this agent.
Neutrophilic Leukocytosis
Increases in the number of circulating neutrophils have occasionally been associated with a variety of tumors in humans and dogs. Unfortunately, a leukemoid reaction of this nature can be difficult to distinguish from a true leukemia without extensive diagnostics. Neutrophilic leukocytosis has been reported in dogs with lymphoma, renal carcinoma, primary lung tumor, rectal polyp, and metastatic fibrosarcoma.97-100 The exact mechanism of PNS leukemoid reactions is unknown; however, the production of a colony-stimulating factor such as granulocyte colony-stimulating factor (G-CSF) or granulocyte-macrophage colony-stimulating factor (GM-CSF) is considered likely. Reports have documented tumor-produced G-CSF and GM-CSF in dogs with primary lung tumor and renal transitional cell carcinoma, whereas tumor-produced G-CSF has been documented in a cat with dermal adenocarcinoma and suspected in a cat with pulmonary squamous cell carcinoma.101-103 This PNS is generally of minimal clinical significance, and normalization of the PNS is possible following removal of the inciting tumor.
Thrombocytopenia
Thrombocytopenia in human and veterinary cancer patients is typically secondary to chemotherapy administration; however, the incidence of thrombocytopenia in tumor-bearing dogs prior to chemotherapy administration has been reported to be as high as 36%.80 Dogs with lymphoproliferative tumors have been reported to have thrombocytopenia in 58% of cases.104 Dogs with Sertoli cell tumors (or occasionally seminomas) that excessively produce estrogen are also prone to thrombocytopenia.85,87 Twenty percent of the cases of thrombocytopenia in cats have been reported to be due to cancer, most commonly associated with lymphoma.105
Numerous mechanisms for PNS thrombocytopenia are possible and include platelet destruction, sequestration, or consumption and/or decreased platelet production. The most common tumors associated with PNS thrombocytopenia are vascular tumors of the spleen and tumors infiltrating the marrow, such as lymphoma or leukemias.106 Immune-mediated thrombocytopenia (ITP) is an additional significant cause of thrombocytopenia.107 The treatment of PNS-associated thrombocytopenia is removal of the inciting tumor; however, adjunctive therapies such as intravenous fluids, plasma, and heparin may be beneficial. For those cases secondary to ITP, the use of immunosuppressive drugs such as corticosteroids (≥2 mg/kg PO daily) and azathioprine (2 mg/kg PO daily then 0.5 to 1 mg/kg PO every other day) may be necessary.
Coagulopathies and Disseminated Intravascular Coagulation
Alterations in hemostasis can be common in human and veterinary cancer patients. PNS coagulopathies are most commonly associated with tumors that cause thrombocytopenia, thrombocytosis, DIC, platelet dysfunction, changes in platelet aggregation, or hyperheparinemia (due to MCT).108,109 Trousseau’s syndrome is a carcinoma-associated human coagulopathy that appears to be related to excess production of hypoxia factors leading to angiogenesis and a procoagulant state.110 PNS-associated DIC has been reported to be the cause of consumptive thrombocytopenia in almost 40% of DIC cases,80 suggesting this PNS may be an important clinical syndrome based on the potentially devastating morbidity and mortality associated with DIC. The diagnosis of DIC is made when the patient has thrombocytopenia, prolongation of activated partial thromboplastin time (aPTT), elevated fibrin degradation products (FDP), and hypofibrinogenemia.80,111,112 A reduction in serum antithrombin III levels appears to be one of the most reliable and prognostic measurements for the presence of DIC.104,113,114
The incidence of DIC in dogs with malignant tumors is approximately 10%.115 A variety of tumors have been associated with DIC; hemangiosarcoma is the most common in dogs. Other associated tumors include inflammatory mammary gland tumors, thyroid carcinomas, primary lung tumors, and intraabdominal carcinomas.113,115-117 DIC has not been evaluated as a prognostic factor in veterinary cancer patients to date; however, the presence and severity of DIC in human cancer patients is a significant negative prognostic factor. Although not specifically related to DIC, the hyperheparinemia associated with MCTs can be associated with prolonged bleeding times and poor hemostasis during and after biopsy or surgery.20
Miscellaneous
Thrombocytosis is a common PNS in humans with lymphomas and leukemias, although it is rarely noted in veterinary oncology. Myeloproliferative disorders appear to be the most common cause of PNS thrombocytosis in dogs and cats.118-120 Important differential diagnoses include causes of primary thrombocytosis such as inflammatory processes, some hemolytic anemias, posthemorrhage, iron deficiency, and postsplenectomy.
PNS eosinophilia is rarely reported in veterinary medicine. Dogs with mammary tumors, leiomyosarcoma, T-cell lymphosarcoma (LSA), or fibrosarcoma and cats with a variety of tumors (lymphoma, sarcomas, MCT, and bladder tumors) have been reported to have tumor-associated eosinophilia.121-125 In addition, eosinophilic effusions have been documented in dogs and cats with cancer and nonneoplastic conditions.126 The cause of eosinophilia in these cases is poorly understood; however, the production of eosinophilic substances such as GM-CSF, various interleukins (e.g., IL-5, -13, and -17), and eotaxins are likely. PNS eosinophilia should be distinguished from eosinophilic leukemia and hypereosinophilia syndrome. The treatment for PNS eosinophilia is removal of the inciting tumor; PNS-associated eosinophilia is typically of little clinical significance.
Although lymphocytosis can be noted with various lymphocytic neoplasms and reactive nonneoplastic conditions, it is rarely noted as a PNS. T-cell lymphocytosis has been recently reported as a PNS in association with thymoma in a dog.127
Platelets may play a role in cancer progression and metastasis attributable to platelet aggregation–mediated augmentation of tumor cell extravasation, survival, and angiogenesis. Platelet aggregation was investigated in a study of 59 dogs with cancer.109 When compared to control dogs, the platelets of dogs with cancer exhibited significantly higher maximum aggregation, higher adenosine triphosphate (ATP) secretion, and shorter delays in the aggregation response. In addition to aiding the metastatic process, platelet hyperaggregation may also lead to thromboembolism.128
Cutaneous Manifestations of Cancer
A wide variety of cutaneous syndromes are associated with malignancies in humans,129 whereas relatively few are noted in veterinary medicine.130 For example, Sweet syndrome is a human PNS associated with hematologic and solid tumors that causes acute febrile neutrophilic dermatosis but has not been reported to date in veterinary patients.131 As with any PNS, cutaneous-associated PNS lesions may precede, coexist with, or follow the diagnosis of the underlying tumor.
Alopecia
Pancreatic carcinoma has been reported in cats as the cause of a progressive, nonscarring PNS alopecia.132-135 The alopecia is acute, bilaterally symmetric (ventrum and limbs), and ventrally glistening. The hair easily epilates from nonalopecic areas and histologically exhibits severe follicular and adnexal atrophy with absence of stratum corneum in many areas, including the foot pads. Clinical signs include anorexia, weight loss, lethargy, and difficulty walking and/or standing, which is most likely due to the aforementioned foot pad histologic changes. In two reports, a similar presentation was noted in cats with bile duct carcinoma.134,136 The cause of this PNS is presently unknown.
Cutaneous Flushing
When the skin episodically turns various shades of red because of changes in cutaneous blood vessel vasodilation, it is termed cutaneous flushing. Intermittent or paroxysmal cutaneous flushing can be associated with pheochromocytoma (see Chapter 25).2,137,138 This PNS has also been reported in a dog with primary lung tumor and concomitant intrathoracic MCT.139 Dogs that undergo MCT degranulation may also have cutaneous flushing. Important nonneoplastic diagnoses to rule out for this PNS include drug reactions, demodicosis, and systemic lupus erythematosus. In addition to pheochromocytoma, humans may experience cutaneous flushing caused by Zollinger-Ellison syndrome, carcinoids, leukemias, renal cell carcinoma, and many other conditions.2,140
Nodular Dermatofibrosis
Nodular dermatofibrosis (ND) is a well-recognized PNS of multiple, slowly growing cutaneous nodules in dogs with bilateral renal cysts or cystadenocarcinomas (Figure 5-2).141-144 The nodules are composed of dense but well-differentiated collagen tissue (collagenous nevi) and are found predominately on the limbs, although the head and trunk may be affected in advanced cases. ND appears to be inherited in an autosomal dominant fashion and is most commonly seen in middle-aged German shepherd dogs.141-143,145 The ND-associated mutation was mapped to exon 7 of the Birt-Hogg-Dube (BHD) locus on canine chromosome 5, which is the same locus for a phenotypically similar human disease.146 Intact females with ND are also at increased risk for the development of uterine leiomyomas, and the BHD mutation appears to be homozygous lethal.143,146,147 The pathogenesis of ND is unknown; however, the BHD mutation in humans leads to a novel protein called folliculin, which may be related to the mTOR pathway.148,149 Currently, there is no effective therapy for the underlying tumor; however, palliative therapy via surgical removal of nodules may be utilized in cases in which the nodules are ulcerated, cosmetically displeasing, or interfering with function.
Necrolytic Migratory Erythema/Superficial Necrolytic Dermatitis
Superficial necrolytic dermatitis (SND) is a rare PNS in humans and dogs characterized by circinate and gyrate areas of erosive blistering and erythema as the result of glucagonomas (glucagon-secreting pancreatic alpha-cell tumors).2,150-153 Marked fissuring, ulceration, and crusting of foot pads have also been noted in dogs. Nonneoplastic differential diagnoses for SND include hepatic disease and diabetes mellitus. Necrolytic migratory erythema, metabolic epidermal necrosis, diabetic dermatopathy, and hepatocutaneous syndrome are terms that have been previously used to describe SND. This PNS resolves in people after surgical extirpation of the glucagonoma, although the prognosis may be poor in dogs due to multiple postoperative complications.154 Octreotide (2 to 3 µg/kg BID) has been reported to successfully treat paraneoplastic SND.155 Although not specifically related to hepatocutaneous syndrome, a dog with sarcomatoid renal cell carcinoma and paraneoplastic hepatopathy with similarities to Stauffer’s syndrome in humans has been reported.152,156
Miscellaneous Syndromes
The number of recognized cutaneous PNSs in humans easily reaches into the dozens; however, the specificity of which we are able to delineate similar PNS in veterinary oncology is limited.130 Ischemic necrosis of the digits and/or feet is a common human PNS associated with lymphoma, adenocarcinoma, and occasionally other malignancies.157 Symmetric cutaneous necrosis of the hind feet has been reported in a cat with multicentric follicular lymphoma.158 Interestingly, the necrosis was not associated with a neoplastic infiltrate and thrombotic/vasculitic causes were not seen histologically, suggesting it was of paraneoplastic origin.
Malassezia-associated dermatitis was first reported in a cat with paraneoplastic alopecia from a metastatic exocrine pancreatic carcinoma.159 A study of over 500 feline skin biopsies found fifteen (2.7%) of the submissions contained Malassezia organisms. Ten of the 15 cats also had neoplasia, suggesting that Malassezia yeast in feline skin biopsies should prompt a clinical workup for neoplasia.160
Pemphigus vulgaris (PV) is a dermatopathy characterized by intraepidermal bullae and erosions of the skin and oral mucosa.161,162 In humans, paraneoplastic PV (PPV) can be associated with lymphoma, Kaposi’s sarcoma, and various carcinomas.2 PPV has been reported in association with mediastinal lymphoma and splenic sarcoma in dogs.163,164 Canine paraneoplastic pemphigus may be an excellent comparative model to human pemphigus PNS.165 Recent reports suggest that paraneoplastic pemphigus is due to circulating IgG autoantibodies against desmoglein 3 across species.161,166,167
Erythema multiforme has been associated with thymoma in a dog.168 The erythema multiforme resolved after thymectomy, suggesting it was paraneoplastic in origin and may be similar to feline thymoma-associated exfoliative dermatitis.169,170 The variety noted in miscellaneous reports should serve as a reminder that there are likely many other uncharacterized cutaneous PNSs in veterinary oncology.
Renal Manifestations of Cancer
Human and veterinary cancer patients alike can develop important renal complications. Most are iatrogenic in nature and include chemotherapy-related toxicity (e.g., cisplatin), antibiotic toxicity (e.g., aminoglycoside), and contrast-associated nephropathy. In addition, infiltrative diseases such as lymphoma can have renal consequences. Biochemical alterations that can lead to nephrotoxicity include tubular precipitations from hypercalcemia, protein casts, glomerulopathies due to amyloidosis and/or membranous glomerulopathy, and fluid and electrolyte disorders due to hypercalcemia, hyponatremia, and acute tumor lysis syndrome. Nephrogenic diabetes insipidus may be a renal PNS in dogs with intestinal leiomyosarcoma.171
Approximately 6% to 10% of human cancer patients have significant glomerulonephritis and protein loss in the urine.172,173 Similarly, 11% of humans with nephrotic syndrome have a concurrent diagnosis of cancer.174 The most common malignancies associated with PNS glomerulonephritis in humans are carcinomas of the lung and GI tract.175 Immune complexes are thought to play a central role.176,177 The prevalence of glomerulonephritis in veterinary cancer patients is unknown; however, immune complex glomerulonephritis has been reported in a dog with polycythemia vera and a dog with lymphocytic leukemia.178,179
Neurologic Manifestations of Cancer
Greater than 50% of human cancer patients have a mild degree of neuromuscular dysfunction (myopathy and/or peripheral neuropathy); however, the frequency of a specific neurologic PNS is low.1,2,180-182 Human neurologic PNSs are separated into anatomic categories (brain, spinal cord, peripheral nerve, muscle, neuromuscular junction). The prevalence of neurologic PNSs in veterinary medicine is unknown, and presently there are examples of neurologic PNSs reported in the dog for the brain, peripheral nerves, and neuromuscular junction.183-189 Many non-PNS causes of neurologic complications from cancer such as metabolic encephalopathy, brain metastasis, cerebrovascular incidents, neurotoxicity from radiation and/or chemotherapy, and neurologic infections due to altered immunity are possible.
Myasthenia Gravis
Myasthenia gravis (MG) is an acquired or congenital disorder of the neuromuscular junction that results from a failure of synaptic transmission. Antibodies to nicotinic acetylcholine receptors (nACHRs) can be documented in dogs with MG and are useful in the diagnosis and follow-up.183,190 In a similar fashion, Lambert-Eaton syndrome in humans occurs as the result of calcium-channel autoantibody formation from predominately lung tumors, which then result in an MG-like syndrome caused by poor calcium influx from the presynaptic neuromuscular junction.191,192
The most common cause of acquired MG in the dog is thymoma, although it has also been reported in association with osteosarcoma, lymphoma, and bile duct carcinoma.184,193-199 The clinical signs revolve around intermittent mild-to-severe muscular weakness, exercise intolerance, dysphagia, and megaesophagus (and possible secondary aspiration pneumonia). Rapid clinical improvement and decreases in nACHR antibodies have been noted after surgical extirpation of thymoma.194,200 The use of immunosuppressive doses of prednisone (>2 mg/kg PO daily) may be a useful adjunct in the treatment of paraneoplastic MG.195
Peripheral Neuropathy
Peripheral nerve lesions that are the result of cancer are a relatively common event in humans and animals. When nerve fibers were analyzed from dogs with a wide variety of malignancies, a significant percentage of abnormal findings such as demyelination, myelin globulation, and axonal degeneration were noted in some specific malignancies.185 Tumors associated with large numbers of peripheral nerve changes included primary lung tumors, insulinoma, MCT, thyroid adenocarcinoma, melanoma, and mammary tumors.185 In contrast, clinically apparent PNS of the peripheral nerves in veterinary medicine is rare. Tumors associated with PNS peripheral neuropathy in dogs and cats include primary lung tumor, leiomyosarcoma, undifferentiated sarcoma, hemangiosarcoma, mammary tumor, multiple myeloma, lymphoma, and insulinoma.185-187,201-203 A multisystemic PNS in humans caused by plasma cell dyscrasia or tumor is termed POEMS syndrome for polyneuropathy, organomegaly, endocrinopathy, M protein, and skin changes. The treatment for paraneoplastic peripheral neuropathy is removal of the inciting tumor.
Diencephalic Syndrome and Miscellaneous Neuromuscular Syndromes
Diencephalic syndrome is a PNS seen in infants with rostral hypothalamic tumors that undergo extreme emaciation despite normal to increased caloric intake.2 All cases occur in association with a tumor in the diencephalic region that secretes excess growth hormone. Only one case has been reported in the veterinary literature to date (affecting a 3-year-old Doberman pinscher).188 Similar to human cases of diencephalic syndrome, this dog had extreme emaciation despite increased caloric intake with a growth-hormone producing astrocytoma in the diencephalic region. The cause for the lack of acromegalic and accelerated growth signs in this case is unknown.
Miscellaneous Manifestations of Cancer
Hypertrophic osteopathy (HO) is a syndrome characterized by periosteal proliferation of new bone along the shafts of long bones in response to malignant and nonmalignant diseases (Figure 5-3). HO has been present in the medical literature for over 25 centuries—Hippocrates described “Hippocratic fingers” (digital clubbing seen in humans with HO). As a PNS, HO is reported most commonly with primary lung tumors2,204; however, in clinical veterinary practice, HO secondary to pulmonary metastatic disease (especially osteosarcoma) is common. It has also been reported with urinary bladder rhabdomyosarcoma, esophageal tumors, malignant Sertoli cell tumor, renal TCC, and nephroblastoma and in cats with renal adenoma, papillary carcinoma, and adrenocortical carcinoma.102,205-213 Nonmalignant conditions such as heartworms, heart disease, focal lung atelectasis, pregnancy, abscesses, granulomas, foreign bodies, and pneumonia have also been associated with HO.
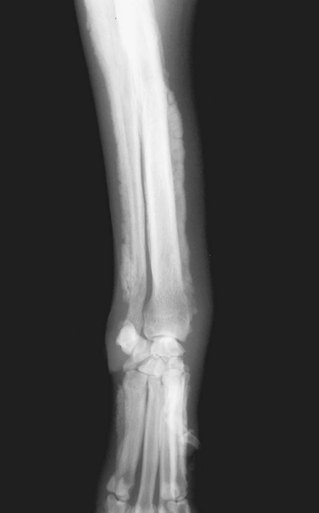
Figure 5-3 Hypertrophic osteopathy (HO) in the front limb of a dog with a primary lung tumor. This paraneoplastic syndrome (PNS) is a bony disease that results in periosteal proliferation and subsequent lameness. This syndrome is seen in a wide variety of malignant and nonmalignant diseases.
The clinical signs of dogs or cats afflicted with HO typically include a history of shifting-leg lameness and/or reluctance to move with all four limbs affected. The limbs are typically warm to the touch and swollen with occasional cases having involvement of the ribs and/or pelvis. The diagnosis of HO is made by radiography of the affected bones (starts distally and moves proximally) and finding the unique 90-degree periosteal reaction seen in Figure 5-3. The search for the inciting tumor begins with radiographs of the thorax, and if negative, radiographs and/or ultrasound of the abdomen with blood work and urinalysis should be pursued.
The etiology of HO is still unknown; however, based on measurable increase in blood flow to extremities and resultant connective tissue and periosteal proliferation, it has been well accepted that HO likely develops in part as a result of afferent neurologic stimulation.214 This is further supported by the resolution of HO after vagotomy; however, this therapy is not routinely used in veterinary patients.215 Recent work suggests that excess production of GHRH and/or vascular endothelial growth factor (VEGF) by the tumor may also contribute to HO.12,216 The treatment for HO is removal of the inciting tumor when possible, and reports have been published documenting resolution of HO in dogs treated for their primary tumor.217,218 Prednisone (1 to 2 mg/kg PO daily) or nonsteroidal antiinflammatory drugs (NSAIDs) may be a useful adjunct therapy for HO when the inciting tumor cannot be removed (i.e., diffuse metastasis). Other treatments for HO such as intercostal nerve resection, unilateral vagotomy, bilateral cervical vagotomy, analgesics, and subperiosteal rib resection have been suggested; however, these therapies have not been evaluated extensively in veterinary patients.204,219 The use of bisphosphonates has become more common for human patients with HO220 and likely represents an exciting new therapeutic modality in veterinary medicine for a variety of indications.53,54 Furthermore, the use of agents such as toceranib phosphate (Palladia),221 in light of its antiangiogenic mechanisms of action, may warrant investigation for patients with resistant HO.
Fever
Although the most common causes of fever are infection, inflammation, autoimmune disease, or drug/blood product reactions, cancer can cause fever as a PNS and should remain an important differential diagnosis. Paraneoplastic fever can accompany a wide variety of tumors in human and veterinary patients. The incidence of fever as a PNS in veterinary medicine is unknown; however, in human patients presenting with fever of unknown origin, cancer is the cause in over one-third of the cases, with the most commonly diagnosed cancers being lymphoma, hepatoma, and renal cell carcinoma.2 Approximately 10% of human cancer patients develop noninfectious/inflammation-related fever at some point during the course of their disease.222 The pathogenesis of PNS fever is predominately due to excess production of cytokines (IL-1, IL-6, TNF-α, and interferons) and febrile-promoting prostaglandins.1,222,223
The most important point in managing fever in veterinary patients with cancer is the evaluation for the presence of concurrent infection. Cancer patients with neutropenia and fever represent a medical emergency. Cancer patients with fever in the absence of neutropenia are not generally medical emergencies but should still be worked up to determine the presence or absence of an infectious and/or inflammatory nidus. If one is not found, then PNS fever is more likely the cause. If the fever is severe and threatens quality of life, the use of an NSAID such as indomethacin or naproxen is commonly utilized in humans with PNS fever. The best therapy for PNS fever is removal of the inciting tumor; however, if this cannot be accomplished, NSAIDs may be useful.
References
1. Pelosof, LC, Gerber, DE. Paraneoplastic syndromes: an approach to diagnosis and treatment. Mayo Clin Proc. 2010;85(9):838–854.
2. John, WJ, Patchell, RA, Foon, KA. Paraneoplastic syndromes. In: DeVita VT, Hellman S, Rosenberg SA, eds. Cancer: Principles & Practice of Oncology. ed 5. Philadelphia: Lippincott-Raven Publishers; 1997:2397–2422.
3. Coss, CC, Bohl, CE, Dalton, JT. Cancer cachexia therapy: a key weapon in the fight against cancer. Curr Opin Clin Nutr Metab Care. 2011;14(3):268–273.
4. Muscaritoli, M, Bossola, M, Bellantone, R, et al. Therapy of muscle wasting in cancer: what is the future? Curr Opin Clin Nutr Metab Care. 2004;7(4):459–466.
5. Crowe, SE, Oliver, J. Cancer cachexia, Compend. Contin Educ Pract Vet. 1981;3:681–690.
6. Michel, KE, Sorenmo, K, Shofer, FS. Evaluation of body condition and weight loss in dogs presented to a veterinary oncology service. J Vet Intern Med. 2004;18(5):692–695.
7. Ogilvie, GK, Walters, LM, Salman, MD, et al. Resting energy expenditure in dogs with nonhematopoietic malignancies before and after excision of tumors. Am J Vet Res. 1996;57:1463–1467.
8. Chlebowski, RT, Herber, D. Metabolic abnormalities in cancer patients: carbohydrate metabolism. Surg Clin North Am. 1986;66:957–968.
9. Herber, D, Byerly, LO, Chi, J, et al. Pathophysiology of malnutrition in the adult cancer patient. Cancer. 1986;58:1867–1873.
10. McAndrew, PF. Fat metabolism and cancer. Surg Clin North Am. 1986;66:1003–1012.
11. Ogilvie, GK, Vail, DM, Wheeler, SL, et al. Effects of Chemotherapy and Remission on Carbohydrate Metabolism in Dogs with Lymphoma. Cancer. 1992;69(1):233–238.
12. Mito, K, Maruyama, R, Uenishi, Y, et al. Hypertrophic pulmonary osteoarthropathy associated with non-small cell lung cancer demonstrated growth hormone-releasing hormone by immunohistochemical analysis. Intern Med. 2001;40(6):532–535.
13. Draghia-Akli, R, Hahn, KA, King, GK, et al. Effects of plasmid-mediated growth hormone-releasing hormone in severely debilitated dogs with cancer. Mol Ther. 2002;6(6):830–836.
14. Tone, CM, Cardoza, DM, Carpenter, RH, et al. Long-term effects of plasmid-mediated growth hormone releasing hormone in dogs. Cancer Gene Ther. 2004;11(5):389–396.
15. Bodles-Brakhop, AM, Brown, PA, Pope, MA, et al. Double-blinded, Placebo-controlled plasmid GHRH trial for cancer-associated anemia in dogs. Mol Ther. 2008;16(5):862–870.
16. Fossum, TW. Protein-losing enteropathy. Semin Vet Med Surg (Small Anim). 1989;4(3):219–225.
17. Strygler, B, Nicor, MJ, Santangelo, WC, et al. Alpha1-anti-trypsin excretion in stool in normal subjects and in patients with gastrointestinal disorders. Gastroenterology. 1990;99:1380–1387.
18. Ruaux, CG, Steiner, JM, Williams, DA. Protein-losing enteropathy in dogs is associated with decreased fecal proteolytic activity. Vet Clin Pathol. 2004;33(1):20–22.
19. Berry, CR, Guilford, WG, Koblik, PD, et al. Scintigraphic evaluation of four dogs with protein-losing enteropathy using 111indium-labeled transferrin. Vet Radiol Ultrasound. 1997;38(3):221–225.
20. Fox, LE, Rosenthal, RC, Twedt, DC, et al. Plasma histamine and gastrin concentrations in 17 dogs with mast cell tumors. J Vet Intern Med. 1990;4(5):242–246.
21. Ishiguro, T, Kadosawa, T, Takagi, S, et al. Relationship of disease progression and plasma histamine concentrations in 11 dogs with mast cell tumors. J Vet Intern Med. 2003;17(2):194–198.
22. English, RV, Breitschwerdt, EB, Grindem, CB, et al. Zollinger-Ellison syndrome and myelofibrosis in a dog. J Am Vet Med Assoc. 1988;192:1430–1434.
23. Drazner, FH. Canine gastrinoma: a condition analogous to the Zollinger-Ellison syndrome in man. California Vet. 1981;11:6–11.
24. Middleton, DJ. Duodenal ulceration associated with gastrin-secreting pancreatic tumor in a cat. J Am Vet Med Assoc. 1983;183:461–462.
25. Straus, E, Johnson, GF, Yalow, RS. Canine Zollinger-Ellison syndrome. Gastroenterology. 1977;72:380–381.
26. Hayden, DW, Henson, MS. Gastrin-secreting pancreatic endocrine tumor in a dog (putative Zollinger-Ellison syndrome). J Vet Diagn Invest. 1997;9:100–103.
27. Uehlinger, P, Glaus, T, Hauser, B, et al. [Differential diagnosis of hypercalcemia–a retrospective study of 46 dogs]. Schweiz Arch Tierheilkd. 1998;140(5):188–197.
28. Elliott, J. Hypercalcemia in the dog: A study of 40 cases. J Small Anim Pract. 1991;32:564–567.
29. Savary, KC, Price, GS, Vaden, SL. Hypercalcemia in cats: a retrospective study of 71 cases (1991-1997). J Vet Intern Med. 2000;14(2):184–189.
30. Sheafor, SE, Gamblin, RM, Couto, CG. Hypercalcemia in two cats with multiple myeloma. J Am Anim Hosp Assoc. 1996;32:503–508.
31. Pressler, BM, Rotstein, DS, Law, JM, et al. Hypercalcemia and high parathyroid hormone-related protein concentration associated with malignant melanoma in a dog. J Am Vet Med Assoc. 2002;221(2):263–265. [240].
32. Kleiter, M, Hirt, R, Kirtz, G, Day, MJ. Hypercalcaemia associated with chronic lymphocytic leukaemia in a Giant Schnauzer. Aust Vet J. 2001;79(5):335–338.
33. Anderson, TE, Legendre, AM, McEntee, MM. Probable hypercalcemia of malignancy in a cat with bronchogenic adenocarcinoma. J Am Anim Hosp Assoc. 2000;36(1):52–55.
34. Weller, RE, Hoffman, WE. Renal function in dogs with lymphosarcoma and associated hypercalcemia. J Small Anim Pract. 1992;33:61–66.
35. Klausner, JS, Bell, FW, Hayden, DW, et al. Hypercalcemia in two cats with squamous cell carcinoma. J Am Vet Med Assoc. 1990;196:103–105.
36. Elliott, J, Dobson, JM, Dunn, JK, et al. Hypercalcemia in the dog: a study of 40 cases. J Small Anim Pract. 1991;32:564–571.
37. Messinger, JS, Windham, WR, Ward, CR. Ionized hypercalcemia in dogs: a retrospective study of 109 cases (1998-2003). J Vet Intern Med. 2009;23(3):514–519.
38. Gajanayake, I, Priestnall, SL, Benigni, L, et al. Paraneoplastic hypercalcemia in a dog with benign renal angiomyxoma. J Vet Diagn Invest. 2010;22(5):775–780.
39. Ross, JT, Scavelli, TD, Matthieson, DT, et al. Adenocarcinoma of the apocrine glands of the anal sac in dogs: a review of 32 cases. J Am Anim Hosp Assoc. 1991;27:349–355.
40. Meuten, DJ, Cooper, BJ, Capen, CC, et al. Hypercalcemia associated with an adenocarcinoma derived from the apocrine glands of the anal sac. Vet Pathol. 1981;18:454–471.
41. Hofbauer, LC, Neubauer, A, Heufelder, AE. Receptor activator of nuclear factor-kappaB ligand and osteoprotegerin: potential implications for the pathogenesis and treatment of malignant bone diseases. Cancer. 2001;92(3):460–470.
42. Rosol, TJ, Nagode, LA, Couto, CG, et al. Parathyroid hormone (PTH)-related protein, PTH, and 1,25-dihydroxyvitamin D in dogs with cancer-associated hypercalcemia. Endocrinology. 1992;131:1157–1164.
43. Forrester, SD, Fallin, EA. Diagnosing and managing the hypercalcemia of malignancy. Vet Med. 1992;1(26):39.
44. Cryer, PE, Kissane, JM. Clinicopathologic conference: Malignant hypercalcemia. Am J Med. 1979;65:486–494.
45. Weir, EC, Norrdin, RW, Matus, RE, et al. Humoral hypercalcemia of malignancy in canine lymphosarcoma. Endocrinology. 1988;122:602–608.
46. Barger, AM, Fan, TM, de Lorimier, LP, et al. Expression of receptor activator of nuclear factor kappa-B ligand (RANKL) in neoplasms of dogs and cats. J Vet Intern Med. 2007;21(1):133–140.
47. Sellers, RS, Capen, CC, Rosol, TJ. Messenger RNA stability of parathyroid hormone-related protein regulated by transforming growth factor-beta1. Mol Cell Endocrinol. 2002;188(1-2):37–46.
48. Bolliger, AP, Graham, PA, Richard, V, et al. Detection of parathyroid hormone-related protein in cats with humoral hypercalcemia of malignancy. Vet Clin Pathol. 2002;31(1):3–8.
49. Weir, EC, Burtis, WJ, Morris, CA, et al. Isolation of 16000-dalton parathyroid hormone-like proteins from two animal tumors causing humoral hypercalcemia of malignancy. Endocrinology. 1988;123(6):2744–2751.
50. Price, GS, Page, RL, Fischer, B, et al. Efficacy and toxicity of doxorubicin/cyclophosphamide maintenance therapy in dogs with multicentric lymphosarcoma. J Vet Intern Med. 1991;5:259–262.
51. Nelson, KA, Walsh, D, Abdullah, O, et al. Common complications of advanced cancer. Semin Oncol. 2000;27(1):34–44.
52. Hurtado, J, Esbrit, P. Treatment of malignant hypercalcaemia. Expert Opin Pharmacother. 2002;3(5):521–527.
53. Milner, RJ, Farese, J, Henry, CJ, et al. Bisphosphonates and cancer. J Vet Intern Med. 2004;18(5):597–604.
54. Hostutler, RA, Chew, DJ, Jaeger, JQ, et al. Uses and effectiveness of pamidronate disodium for treatment of dogs and cats with hypercalcemia. J Vet Intern Med. 2005 Ja;19(1):29–33.
55. Sato, K, Onuma, E, Yocum, RC, et al. Treatment of malignancy-associated hypercalcemia and cachexia with humanized anti-parathyroid hormone-related protein antibody. Semin Oncol. 2003;30(5 Suppl 16):167–173.
56. Caywood, DD, Klausner, JS, O’Leary, TP, et al. Pancreatic insulin-secreting neoplasms:clinical, diagnostic, and prognostic features in 73 dogs. J Am Anim Hosp Assoc. 1988;24:577–584.
57. Boari A, Venturoli M, Minuto F: Non-islet cell tumor hypoglycemia in a dog associated with high levels of insulin-like growth factor II. XVII World Small Animal Veterinary Association Proceedings 678–679, 1992.
58. Beaudry, D, Knapp, DW, Montgomery, T, et al. Smooth muscle tumors associated with hypoglycemia in four dogs. Clinical presentation, treatment, and tumor immunohistochemical staining. J Vet Intern Med. 1995;9:415–418m.
59. Rossi, G, Errico, G, Perez, P, Rossi, G, et al. Paraneoplastic hypoglycemia in a diabetic dog with an insulin growth factor-2-producing mammary carcinoma. Vet Clin Pathol. 2010;39(4):480–484.
60. Zini, E, Glaus, TM, Minuto, F, et al. Paraneoplastic hypoglycemia due to an insulin-like growth factor type-II secreting hepatocellular carcinoma in a dog. J Vet Intern Med. 2007;21(1):193–195.
61. Battaglia, L, Petterino, C, Zappulli, V, et al. Hypoglycaemia as a paraneoplastic syndrome associated with renal adenocarcinoma in a dog. Vet Res Commun. 2005;29(8):671–675.
62. Snead, EC. A case of bilateral renal lymphosarcoma with secondary polycythaemia and paraneoplastic syndromes of hypoglycaemia and uveitis in an English Springer Spaniel. Vet Comp Oncol. 2005;3(3):139–144.
63. Zapf, J. Role of insulin-like growth factor (IGF) II and IGF binding proteins in extrapancreatic tumour hypoglycaemia. J Intern Med. 1993;234(6):543–552.
64. Breitschwerdt, EB, Root, CR. Inappropriate secretion of antidiuretic hormone in a dog. J Am Vet Med Assoc. 1979;175(2):181–186.
65. Brofman, PJ, Knostman, KA, DiBartola, SP. Granulomatous amebic meningoencephalitis causing the syndrome of inappropriate secretion of antidiuretic hormone in a dog. J Vet Intern Med. 2003;17(2):230–234.
66. Shiel, RE, Pinilla, M, Mooney, CT. Syndrome of inappropriate antidiuretic hormone secretion associated with congenital hydrocephalus in a dog. J Am Anim Hosp Assoc. 2009;45(5):249–252.
67. Pierce, ST. Paraendocrine syndromes. Curr Opin Oncol. 1993;5(4):639–645.
68. Sorensen, JB, Andersen, MK, Hansen, HH. Syndrome of inappropriate secretion of antidiuretic hormone (SIADH) in malignant disease. J Intern Med. 1995;238(2):97–110.
69. Kinzie, BJ. Management of the syndrome of inappropriate secretion of antidiuretic hormone. Clin Pharm. 1987;6(8):625–633.
70. Ogilvie, GK, Weigel, RM, Haschek, WM, et al. Prognostic factors for tumor remission and survival in dogs after surgery for primary lung tumor: 76 cases (1975-1985). JAVMA. 1989;195:109–112.
71. Galac, S, Kooistra, HS, Voorhout, G, et al. Hyperadrenocorticism in a dog due to ectopic secretion of adrenocorticotropic hormone. Domest Anim Endocrinol. 2005;28(3):338–348.
72. Hillyard, V, Coombes, RC, Greenberg, PB, et al. Calcitonin in breast and lung cancer. Clin Endocrinol. 1976;5:1–8.
73. Padgett, SL, Tillson, DM, Henry, CJ, et al. Gingival vascular hamartoma with associated paraneoplastic hyperglycemia in a kitten. J Am Vet Med Assoc. 1997;210:914–915.
74. Forrester, SD, Greco, DS, Relford, RL. Serum hyperviscosity syndrome associated with multiple myeloma in two cats. JAVMA. 1992;200(1):79–82.
75. Forrester, SD, Reeford, RL. Serum hyperviscosity syndrome: Its diagnosis and treatment. Vet Med. 1992;1:48–54.
76. MacEwen, EG, Hurvitz, AI. Diagnosis and management of monoclonal gammopathies. Vet Clin North Am. 1977;7:119–132.
77. Madewell, BR, Feldman, BF. Characterization of anemias associated with neoplasia in small animals. J Am Vet Med Assoc. 1980;176:419–425.
78. Dodds, WJ. Autoimmune hemolytic disease and other causes of immune-mediated anemia: an overview. J Am Anim Hosp Assoc. 1977;13:437–441.
79. Ogilvie, GK, Felsberg, PJ, Harris, SW. Short term effect of cyclophosphamide and azathioprine on the selected aspects of the canine immune system. J Vet Immunol Immunopathol. 1988;18:119–127.
80. Madewall, BR, Feldman, BF, O’Neil, S. Coagulation abnormalities in dogs with neoplastic disease. Thromb Haemost. 1980;44:35–38.
81. Mason, N, Duval, D, Shofer, FS, et al. Cyclophosphamide exerts no beneficial effect over prednisone alone in the initial treatment of acute immune-mediated hemolytic anemia in dogs: a randomized controlled clinical trial. J Vet Intern Med. 2003;17(2):206–212.
82. Feldman, BF. Management of the anemic dog. In: Kirk RW, ed. Current Veterinary Therapy. VIII ed. Philadelphia: Saunders; 1983:395–400.
83. Comer, KM. Anemia as a feature of prmary gastrointestinal neoplasia. Compend Contin Educ Pract Vet. 1990;12:13–19.
84. Hammer, AS, Couto, CG, Swardson, C, et al. Hemostatic abnormalities in dogs with hemangiosarcoma. J Vet Intern Med. 1991;5:11–14.
85. Sherding, RG, Wilson, GP. Bone marrow hypoplasia in eight dogs with Sertoli cell tumor. J Am Vet Med Assoc. 1982;178:497–501.
86. Sanpera, N, Masot, N, Janer, M, et al. Oestrogen-induced bone marrow aplasia in a dog with a Sertoli cell tumour. J Small Anim Pract. 2002;43(8):365–369.
87. Morgan, RW. Blood dyscrasias associated with testicular tumors in the dog. J Am Anim Hosp Assoc. 1982;18:971–975.
88. Peterson, ME. Inappropriate erythropoietin production from a renal carcinoma in a dog with polycythemia. J Am Vet Med Assoc. 1981;179:995–996.
89. Scott, RC, Patnaik, AK. Renal carcinoma with secondary polycythemia in the dog. J Am Anim Hosp Assoc. 1972;8:275–283.
90. Nelson, RW, Hager, D. Renal lymphosarcoma with inappropriate erythropoietin production in a dog. J Am Vet Med Assoc. 1983;182:1396–1397.
91. Couto, CG, Boudrieau, RJ, Zanjani, ED. Tumor-associated erthrocytosis in a dog with a nasal fibrosarcoma. J Vet Intern Med. 1989;3:183–185.
92. Gorse, MJ. Polycythemia associated with renal fibrosarcoma in a dog. J Am Vet Med Assoc. 1988;192:793–794.
93. Durno, AS, Webb, JA, Gauthier, MJ, et al. Polycythemia and inappropriate erythropoietin concentrations in two dogs with renal T-cell lymphoma. J Am Anim Hosp Assoc. 2011;47(2):122–128.
94. Yeo, EJ, Chun, YS, Park, JW. New anticancer strategies targeting HIF-1. Biochem Pharmacol. 2004;68(6):1061–1069.
95. Czyzyk-Krzeska, MF, Meller, J. von Hippel-Lindau tumor suppressor: not only HIF’s executioner. Trends Mol Med. 2004;10(4):146–149.
96. Beurlet, S, Krief, P, Sansonetti, A, et al. Identification of JAK2 mutations in canine primary polycythemia. Exp Hematol. 2011;39(5):542–545.
97. Lappin, MR, Lattimer, KS. Hematuria and extreme neutrophilic leukocytosis in a dog with renal tubular carcinoma. J Am Vet Med Assoc. 1988;192:1289–1292.
98. Thompson, JP, Christopher, MM, Ellison, GW, et al. Paraneoplastic leukocytosis associated with a rectal adenomatous polyp in a dog. J Am Vet Med Assoc. 1992;201:737–738.
99. Chinn, DR, Myers, RK, Matthews, JA. Neutrophilic leukocytosis associated with metastatic fibrosarcoma in a dog. J Am Vet Med Assoc. 1985;186:806–809.
100. Madewell, BR, Wilson, DW, Hornoff, WJ, et al. Leukemoid blood response and bone infarcts in a dog with renal tubular adenocarcinoma. J Am Vet Med Assoc. 1990;197:1623–1625.
101. Sharkey, LC, Rosol, TJ, Grone, A, et al. Production of granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor by carcinomas in a dog and a cat with paraneoplastic leukocytosis. J Vet Intern Med. 1996;10:405–408.
102. Peeters, D, Clercx, C, Thiry, A, et al. Resolution of paraneoplastic leukocytosis and hypertrophic osteopathy after resection of a renal transitional cell carcinoma producing granulocyte-macrophage colony-stimulating factor in a young Bull Terrier. J Vet Intern Med. 2001;15(4):407–411.
103. Dole, RS, MacPhail, CM, Lappin, MR. Paraneoplastic leukocytosis with mature neutrophilia in a cat with pulmonary squamous cell carcinoma. J Feline Med Surg. 2004;6(6):391–395.
104. Ruslander, D, Page, RL. Perioperative management of paraneoplastic syndromes. Vet Surg. 1995;25:47–62.
105. Jordan, HL, Grindem, CB, Breitschwerdt, EB. Thrombocytopenia in cats: a retrospective study of 41 cases. J Vet Intern Med. 1993;7:261–265.
106. Hatgis, AM, Feldman, BF. Evaluation of hemostatic defects secondary to vascular tumors in dogs: 11 cases (1983-1988). J Am Vet Med Assoc. 1991;198:891–894.
107. Helfand, SC, Couto, CG, Madewell, BR. Immune-mediated thrombocytopenia associated with solid tumors in dogs. J Am Anim Hosp Assoc. 1985;21:787–794.
108. Rogers, KS. Coagulation disorders associated with neoplasia in the dog. Vet Med. 1992;1:55–61.
109. McNiel, EA, Ogilvie, GK, Fettman, MJ, et al. Platelet hyperfunction in dogs with malignancies. J Vet Intern Med. 1997;11:178–182.
110. Denko, NC, Giaccia, AJ. Tumor hypoxia, the physiological link between Trousseau’s syndrome (carcinoma-induced coagulopathy) and metastasis. Cancer Res. 2001;61(3):795–798.
111. Ratnoff, OD. Hemostatic emergencies in malignancy. Semin Oncol. 1989;16(6):561–571.
112. Sarris, AH, Kempin, S, Berman, E, et al. High incidence of disseminated intravascular coagulation during remission induction of adult patients with acute lymphoblastic leukemia. Blood. 1992;79:1305–1310.
113. Feldman, BF, Madewell, BR, O’Neil, S. Disseminated intravascular coagulation: anti-thrombin, plasminogen, and coagulation abnormalities in 41 dogs. J Am Vet Med Assoc. 1981;179:151–154.
114. Green, RA. Clinical Implications of antithrombin III deficiency in animal diseases. Comp Cont Ed. 1984;6(6):537–546.
115. Maruyama, H, Miura, T, Sakai, M, et al. The incidence of disseminated intravascular coagulation in dogs with malignant tumor. J Vet Med Sci. 2004;66(5):573–575.
116. Stockhaus, C, Kohn, B, Rudolph, R, et al. Correlation of haemostatic abnormalities with tumour stage and characteristics in dogs with mammary carcinoma. J Small Anim Pract. 1999;40(7):326–331.
117. Susaneck, SJ, Allen, TA, Hoopes, J, et al. Inflammatory mammary carcinoma in the dog. J Am Anim Hosp Assoc. 1983;19:971–976.
118. Hammer, AS, Couto, CG, Bailey, MQ. Essential thrombocythemia in a cat. Proc Vet Cancer Soc. 1988;8:18.
119. Hogan, DF, Dhaliwal, RS, Sisson, DD, et al. Paraneoplastic thrombocytosis-induced systemic thromboembolism in a cat. J Am Anim Hosp Assoc. 1999;35(6):483–486.
120. Degen, MA, Feldman, BF, Turrel, JM, et al. Thrombocytosis associated with myeloproliferative disorder in a dog. J Am Vet Med Assoc. 1989;194:1457–1459.
121. Sellon, RK, Rottman, JB, Jordan, HL, et al. Hypereosinophilia associated with transitional cell carcinoma in a cat. J Am Vet Med Assoc. 1992;201:591–593.
122. Barrs, VR, Beatty, JA, McCandlish, IA, et al. Hypereosinophilic paraneoplastic syndrome in a cat with intestinal T cell lymphosarcoma. J Small Anim Pract. 2002;43(9):401–405.
123. Couto, CG. Tumor associated eosinophilia in a dog. J Am Vet Med Assoc. 1984;184:837–838.
124. Fews, D, Scase, TJ, Battersby, IA. Leiomyosarcoma of the pericardium, with epicardial metastases and peripheral eosinophilia in a dog. J Comp Pathol. 2008;138(4):224–228.
125. Marchetti, V, Benetti, C, Citi, S, et al. Paraneoplastic hypereosinophilia in a dog with intestinal T-cell lymphoma. Vet Clin Pathol. 2005;34(3):259–263.
126. Fossum, TW, Wellman, M, Relford, RL, et al. Eosinophilic pleural or peritoneal effusions in dogs and cats: 14 cases (1986-1992). J Am Vet Med Assoc. 1993;202:1873–1876.
127. Batlivala, TP, Bacon, NJ, Avery, AC, et al. Paraneoplastic T cell lymphocytosis associated with a thymoma in a dog. J Small Anim Pract. 2010;51(9):491–494.
128. Honn, KV, Tang, DG, Crissman, JD. Platelets and cancer metastasis: a causal relationship? Cancer Metastasis Rev. 1992;11(3-4):325–351.
129. Boyce, S, Harper, J. Paraneoplastic dermatoses. Dermatol Clin. 2002;20(3):523–532.
130. Turek, MM. Cutaneous paraneoplastic syndromes in dogs and cats: a review of the literature. Vet Dermatol. 2003;14(6):279–296.
131. Cohen, PR, Holder, WR, Tucker, SB, et al. Sweet syndrome in patients with solid tumors. Cancer. 1993;72:2723–2731.
132. Brooks, DG, Campbell, KL, Dennis, JS, et al. Pancreatic paraneoplastic alopecia in three cats. J Am Anim Hosp Assoc. 1994;30:557–563.
133. Tasker, S, Griffon, DJ, Nuttall, TJ, et al. Resolution of paraneoplastic alopecia following surgical removal of a pancreatic carcinoma in a cat. J Small Anim Pract. 1999;40(1):16–19.
134. Pascal-Tenorio, A, Olivry, T, Gross, TL, et al. Paraneoplastic alopecia associated with internal malignancies in the cat. Vet Dermatol. 1997;8:47–51.
135. Godfrey, DR. A case of feline paraneoplastic alopecia with secondary Malassezia-associated dermatitis. J Small Anim Pract. 1998;39:394–396.
136. Barrs, VR, Martin, P, France, M, et al. What is your diagnosis? Feline paraneoplastic alopecia associated with pancreatic and bile duct carcinomas. J Small Anim Pract. 1999;40(12):559. [595, 596].
137. Barthez, PY, Marks, SL, Woo, J, et al. Pheochromocytoma in dogs: 61 cases (1984-1995). J Vet Intern Med. 1997;11:272–278.
138. Herrera, MF, Stone, E, Deitel, M, Asa, SL. Pheochromocytoma producing multiple vasoactive peptides. Arch Surg. 1992;127:105–108.
139. Miller, WH. Cutaneous flushing associated with intrathoracic neoplasia in a dog. J Am Anim Hosp Assoc. 1992;28:217–219.
140. Shepherd, JJ, Challis, DR, Davies, PF, et al. Multiple endocrine neoplasm, type 1: Gastrinomas, pancreatic neoplasms, microcarcinoids, the Zollinger-Ellison syndrome, lymph nodes, and hepatic metastases. Arch Surg. 1993;128:1133–1142.
141. Gilbert, PA, Griffin, CE, Walder, EJ. Nodular dermatofibrosis and renal cystadenoma in a German Shepherd dog. J Am Anim Hosp Assoc. 1990;26:253–256.
142. Lium, G, Moe, E. Hereditary multifocal renal cystadenocarcinomas and nodular dermatofibrosis in the German Shepherd dog: macroscopic and histopathologic changes. Vet Pathol. 1985;22:447–455.
143. Atlee, BA, DeBoer, DJ, Ihrke, PJ, et al. Nodular dermatofibrosis in German Shepherd dogs as a marker for renal cystadenocarcinoma. J Am Anim Hosp Assoc. 1991;27:481–487.
144. Suter, M, Lott-Stoltz, G, Wild, P. Generalized nodular dermatofibrosis in six Alsatians. Vet Pathol. 1983;20:632–634.
145. Jonasdottir, TJ, Mellersh, CS, Moe, L, et al. Genetic mapping of a naturally occurring hereditary renal cancer syndrome in dogs. Proc Natl Acad Sci U S A. 2000;97(8):4132–4137.
146. Lingaas, F, Comstock, KE, Kirkness, EF, et al. A mutation in the canine BHD gene is associated with hereditary multifocal renal cystadenocarcinoma and nodular dermatofibrosis in the German Shepherd dog. Hum Mol Genet. 2003;12(23):3043–3053.
147. Moe, L, Lium, B. Hereditary multifocal renal cystadenocarcinomas and nodular dermatofibrosis in 51 German shepherd dogs. J Small Anim Pract. 1997;38(11):498–505.
148. Warren, MB, Torres-Cabala, CA, Turner, ML, et al. Expression of Birt-Hogg-Dube gene mRNA in normal and neoplastic human tissues. Mod Pathol. 2004;17(8):998–1011.
149. Menko, FH, van Steensel, MA, Giraud, S, et al. Birt-Hogg-Dube syndrome: diagnosis and management. Lancet Oncol. 2009;10(12):1199–1206.
150. Allenspach, K, Arnold, P, Glaus, T, et al. Glucagon-producing neuroendocrine tumour associated with hypoaminoacidaemia and skin lesions. J Small Anim Pract. 2000;41(9):402–406.
151. Byrne, KP. Metabolic epidermal necrosis-hepatocutaneous syndrome. Vet Clin North Am Small Anim Pract. 1999;29(6):1337–1355.
152. Gross, TL, Song, MD, Havel, PJ, et al. Superficial necrolytic dermatitis (necrolytic migratory erythema) in dogs. Vet Pathol. 1993;30:75–81.
153. Cave, TA, Evans, H, Hargreaves, J, et al. Metabolic epidermal necrosis in a dog associated with pancreatic adenocarcinoma, hyperglucagonaemia, hyperinsulinaemia and hypoaminoacidaemia. J Small Anim Pract. 2007;48(9):522–526.
154. Torres, SMF, Caywood, DD, O’Brien, TD, et al. Resolution of superficial necrolytic dermatitis following excision of a glucagon-secreting pancreatic neoplasm in a dog. J Am Anim Hosp Assoc. 1997;33:313–319.
155. Oberkirchner, U, Linder, KE, Zadrozny, L, et al. Successful treatment of canine necrolytic migratory erythema (superficial necrolytic dermatitis) due to metastatic glucagonoma with octreotide. Vet Dermatol. 2010;21(5):510–516.
156. Zini, E, Bovero, A, Nigrisoli, E, et al. Sarcomatoid renal cell carcinoma with osteogenic differentiation and paraneoplastic hepatopathy in a dog, possibly related to human Stauffer’s syndrome. J Comp Pathol. 2003;129(4):303–307.
157. Poszepczynska-Guigne, E, Viguier, M, et al. Paraneoplastic acral vascular syndrome: epidemiologic features, clinical manifestations, and disease sequelae. J Am Acad Dermatol. 2002;47(1):47–52.
158. Ashley, PA, Bowman, LA. Symmetric cutaneous necrosis of the hind feet and multicentric follicular lymphoma in a cat. J Am Vet Med Assoc. 1999;214:211–214.
159. Godfrey, DR. A case of feline paraneoplastic alopecia with secondary Malassezia-associated dermatitis. J Small Anim Pract. 1998;39(8):394–396.
160. Mauldin, EA, Morris, DO, Goldschmidt, MH. Retrospective study: The presence of Malassezia in feline skin biopsies. A clinicopathological study. Vet Dermatol. 2002;13(1):7–13.
161. Schmidt, E, Zillikens, D. Modern diagnosis of autoimmune blistering skin diseases. Autoimmun Rev. 2010;10(2):84–89.
162. Olivry, T, Linder, KE. Dermatoses affecting desmosomes in animals: a mechanistic review of acantholytic blistering skin diseases. Vet Dermatol. 2009;20(5-6):313–326.
163. Lemmens, P, De Bruin, A, De Meulemeester, J, et al. Paraneoplastic pemphigus in a dog. Vet Dermatol. 1998;9:127–134.
164. Elmore, SA, Basseches, J, Anhalt, GJ, et al. Paraneoplastic pemphigus in a dog with splenic sarcoma. Vet Pathol. 2005;42(1):88–91.
165. De Bruin, A, Muller, E, Wyder, M, et al. Periplakin and envoplakin are target antigens in canine and human paraneoplastic pemphigus. J Am Acad Dermatol. 1999;40(5 Pt 1):682–685.
166. Nishifuji, K, Olivry, T, Ishii, K, et al. IgG autoantibodies directed against desmoglein 3 cause dissociation of keratinocytes in canine pemphigus vulgaris and paraneoplastic pemphigus. Vet Immunol Immunopathol. 2007;117(3-4):209–221.
167. Nishifuji, K, Tamura, K, Konno, H, et al. Development of an enzyme-linked immunosorbent assay for detection of circulating IgG autoantibodies against canine desmoglein 3 in dogs with pemphigus. Vet Dermatol. 2009;20(5-6):331–337.
168. Tepper, LC, Spiegel, IB, Davis, GJ. Diagnosis of erythema multiforme associated with thymoma in a dog and treated with thymectomy. J Am Anim Hosp Assoc. 2011;47(2):e19–e25.
169. Singh, A, Boston, SE, Poma, R. Thymoma-associated exfoliative dermatitis with post-thymectomy myasthenia gravis in a cat. Can Vet J. 2010;51(7):757–760.
170. Rottenberg, S, Von, TC, Roosje, PJ. Thymoma-associated exfoliative dermatitis in cats. Vet Pathol. 2004;41(4):429–433.
171. Cohen, M, Post, GS. Nephrogenic diabetes insipidus in a dog with intestinal leiomyosarcoma. J Am Vet Med Assoc. 1999;215(12):1818–1820. [1806].
172. Zimmerman, SW, Vishnu-Moorthy, A, Burkholder, PM. Glomerulopathies associated with neoplastic disease. In: Rieselbach RE, Garnick NB, eds. Cancer in the kidney. Philadelphia: Lea & Febiger, 1982.
173. Lien, YH, Lai, LW. Pathogenesis, diagnosis and management of paraneoplastic glomerulonephritis. Nat Rev Nephrol. 2011;7(2):85–95.
174. Lee, JC, Yamauchi, H, Hopper, J. The association of cancer and nephrotic syndrome. Ann Intern Med. 1966;64:41–47.
175. Alpers, CE, Cotran, RS. Neoplasia and glomerular injury. Kidney Int. 1986;30(4):465–473.
176. Dinh, BL, Brassard, A. Renal lesion associated with the Walker 256 adenocarcinoma in the rat. Br J Exp Pathol. 1968;49:145–148.
177. Norris, SH. Paraneoplastic glomerulopathies. Semin Nephrol. 1993;13(3):258–272.
178. Page, RL, Stiff, ME, McEntee, MC, et al. Transient glomerulonephropathy associated with primary erythrocytosis in a dog. J Am Vet Med Assoc. 1990;196:620–622.
179. Willard, MD, Krehbiel, JD, Schmidt, GM, et al. Serum and urine protein abnormalities associated with lymphocytic leukemia and glomerulonephritis in a dog. J Am Anim Hosp Assoc. 1981;17:381–386.
180. Waterhouse, DM, Natale, RB, Cody, RL. Breast cancer and paraneoplastic cerebellar degeneration. Cancer. 1991;68:1835–1841.
181. Nishi, Y, Yufu, Y, Shinomiya, S, et al. Polyneuropathy in acute megakaryoblastic leukemia. Cancer. 1991;68:2033–2036.
182. Braik, T, Evans, AT, Telfer, M, et al. Paraneoplastic neurological syndromes: unusual presentations of cancer. A practical review. Am J Med Sci. 2010;340(4):301–308.
183. Shelton, GD, Willard, MD, Cardinet, GH, et al. Acquired myasthenia gravis: selective involvement of esophageal, pharyngeal, and facial muscles. J Vet Intern Med. 1990;4:281–284.
184. Ridyard, AE, Rhind, SM, French, AT, et al. Myasthenia gravis associated with cutaneous lymphoma in a dog. J Small Anim Pract. 2000;41(8):348–351.
185. Braund, KG. Remote effects of cancer on the nervous system. Semin Vet Med Surg (Sm Anim). 1990;5:262–270.
186. Bergman, PJ, Bruyette, DS, Coyne, BE, et al. Canine clinical peripheral neuropathy associated with pancreatic islet cell carcinoma. Prog Vet Neurol. 1994;5:57–62.
187. Braund, KG, Steiss, JE, Amling, KA, et al. Insulinoma and subclinical peripheral neuropathy in two dogs. J Vet Intern Med. 1987;1:86–90.
188. Nelson, RW, Morrison, WB, Lurus, AG, et al. Diencephalic syndrome secondary to intracranial astrocytoma in a dog. J Am Vet Med Assoc. 1981;179:1004–1010.
189. Inzana, KD. Paraneoplastic neuromuscular disorders. Vet Clin North Am Small Anim Pract. 2004;34(6):1453–1467.
190. Shelton, GD. Routine and specialized laboratory testing for the diagnosis of neuromuscular diseases in dogs and cats. Vet Clin Pathol. 2010;39(3):278–295.
191. Leys, K, Lang, B, Johnston, I, et al. Calcium channel autoantibodies in the Lambert-Eaton myasthenic syndrome. Ann Neurol. 1991;29:307–310.
192. Mareska, M, Gutmann, L. Lambert-Eaton myasthenic syndrome. Semin Neurol. 2004;24(2):149–153.
193. Krotje, LJ, Fix, AS, Potthoff, AD. Acquired myasthenia gravis and cholangiocellular carcinoma in a dog. J Am Vet Med Assoc. 1990;197(4):488–490.
194. Lainesse, MFC, Taylor, SM, Myers, SL, et al. Focal myasthenia gravis as a paraneoplastic syndrome of canine thymoma: Improvement following thymectomy. J Am Anim Hosp Assoc. 1996;32:111–117.
195. Moore, AS, Madewell, BR, Cardinet III, GH, et al. Osteogenic sarcoma and myasthenia gravis in a dog. J Am Vet Med Assoc. 1990;197(2):226–227.
196. Joseph, RJ, Carrillo, JM, Lennon, VA. Myasthenia gravis in the cat. J Vet Intern Med. 1988;2:75–79.
197. Klebanow, ER. Thymoma and acquired myasthenia gravis in the dog: a case report and review of 13 additional cases. J Am Anim Hosp Assoc. 1992;28:63–69.
198. Atwater, SW, Powers, BE, Park, RD, et al. Thymoma in dogs: 23 cases (1980-1991). J Am Vet Med Assoc. 1994;205(7):1007–1013.
199. Moffet, AC. Metastatic thymoma and acquired generalized myasthenia gravis in a beagle. Can Vet J. 2007;48(1):91–93.
200. Zitz, JC, Birchard, SJ, Couto, GC, et al. Results of excision of thymoma in cats and dogs: 20 cases (1984-2005). J Am Vet Med Assoc. 2008;232(8):1186–1192.
201. Villiers, E, Dobson, J. Multiple myeloma with associated polyneuropathy in a German shepherd dog. J Small Anim Pract. 1998;39(5):249–251.
202. Mariani, CL, Shelton, SB, Alsup, JC. Paraneoplastic polyneuropathy and subsequent recovery following tumor removal in a dog. J Am Anim Hosp Assoc. 1999;35(4):302–305.
203. Cavana, P, Sammartano, F, Capucchio, MT, et al. Peripheral neuropathy in a cat with renal lymphoma. J Feline Med Surg. 2009;11(10):869–872.
204. Ogilvie, GK. Paraneoplastic syndromes. In: Withrow SJ, MacEwen EG, eds. Small animal clinical oncology. ed 2. Philadelphia: Saunders; 1996:32–42.
205. Gram, WD, Wheaton, LG, Snyder, PW, et al. Feline hypertrophic osteopathy associated with pulmonary carcinoma. J Am Anim Hosp Assoc. 1990;26:425–428.
206. Seaman, RL, Patton, CS. Treatment of renal nephroblastoma in an adult dog. J Am Anim Hosp Assoc. 2003;39(1):76–79.
207. Barrand, KR, Scudamore, CL. Canine hypertrophic osteoarthropathy associated with a malignant Sertoli cell tumour. J Small Anim Pract. 2001;42(3):143–145.
208. Becker, TJ, Perry, RL, Watson, GL. Regression of hypertrophic osteopathy in a cat after surgical excision of an adrenocortical carcinoma. J Am Anim Hosp Assoc. 1999;35(6):499–505.
209. Randolph, JF, Center, SA, Flanders, JA, et al. Hypertrophic osteopathy associated with adenocarcinoma of the esophageal glands in a dog. J Am Vet Med Assoc. 1984;184:98–99.
210. Halliwell, WH, Ackerman, N. Botryoid rhabdomyosarcoma of the urinary bladder and hypertrophic osteoarthropathy in a young dog. J Am Vet Med Assoc. 1974;165:911–913.
211. Caywood, DD, Osborne, CA, Stevens, JB, et al. Hypertrophic osteoarthropathy associated with an atypical nephroblastoma in a dog. J Am Anim Hosp Assoc. 1980;16:855–865.
212. Rendano, VT, Slauson, DO. Hypertrophic osteopathy in a dog with prostatic adenocarcinoma and without thoracic metastasis. J Am Anim Hosp Assoc. 1982;18:905–909.
213. Johnson, RL, Lenz, SD. Hypertrophic osteopathy associated with a renal adenoma in a cat. J Vet Diagn Invest. 2011;23(1):171–175.
214. Uchiyama, G, Ishizuka, M, Sugiura, N. Hypertrophic pulmonary osteoarthropathy inactivated by antitumor chemotherapy. Radiat Med. 1985;3(1):25–28.
215. Hara, Y, Tagawa, M, Ejima, H, et al. Regression of hypertrophic osteopathy following removal of intrathoracic neoplasia derived from vagus nerve in a dog. J Vet Med Sci. 1995;57(1):133–135.
216. Abe, Y, Kurita, S, Ohkubo, Y, et al. A case of pulmonary adenocarcinoma associated with hypertrophic osteoarthropathy due to vascular endothelial growth factor. Anticancer Res. 2002;22(6B):3485–3488.
217. Madewell, BR, Nyland, TG, Weigel, JE. Regression of hypertrophic osteopathy following pneumonectomy in a dog. J Am Vet Med Assoc. 1978;172:818–821.
218. Hahn, KA, Richardson, RC. Use of cisplatin for control of metastatic malignant mesenchymoma and hypertrophic osteopathy in a dog. J Am Vet Med Assoc. 1989;195:351–353.
219. Brodey, RS. Hypertrophic osteoarthropathy. Spontaneous animal models of human disease. San Diego: Academic Press; 1980.
220. Amital, H, Applbaum, YH, Vasiliev, L, et al. Hypertrophic pulmonary osteoarthropathy: control of pain and symptoms with pamidronate. Clin Rheumatol. 2004;23(4):330–332.
221. London, CA, Malpas, PB, Wood-Follis, SL, et al. Multi-center, placebo-controlled, double-blind, randomized study of oral toceranib phosphate (SU11654), a receptor tyrosine kinase inhibitor, for the treatment of dogs with recurrent (either local or distant) mast cell tumor following surgical excision. Clin Cancer Res. 2009;15(11):3856–3865.
222. Greenberg, SB, Taber, L. Fever of unknown origin. In: Mackowiak PA, ed. Fever: Mechanisms and management. New York: Raven Press; 1991:183.
223. Saper, CB, Breder, CD. The neurologic basis of fever. N Engl J Med. 1994;330:1880–1886.