Cancer of the Gastrointestinal Tract
 Section A
Section A
Oral Tumors
Julius M. Liptak and Stephen J. Withrow
Incidence and Risk Factors
Collectively, oral cancer accounts for 6% to 7% of canine cancer and is the fourth most common cancer overall.1,2 In the cat, it accounts for 3% of all cancers.3 Oropharyngeal cancer is 2.6 times more common in dogs than cats, and male dogs have a 2.4 times greater risk of developing oropharyngeal malignancy compared to female dogs.4,5 A male sex predisposition has also been reported for dogs with malignant melanoma and tonsillar squamous cell carcinoma (SCC).6,7 Dog breeds with the highest risk of developing oropharyngeal cancer include the cocker spaniel, German shepherd dog, German shorthaired pointer, Weimaraner, golden retriever, Gordon setter, miniature poodle, Chow Chow, and Boxer.6,8,9 In one study, German shepherd dogs and Boxers had a decreased risk of developing oral melanoma.9
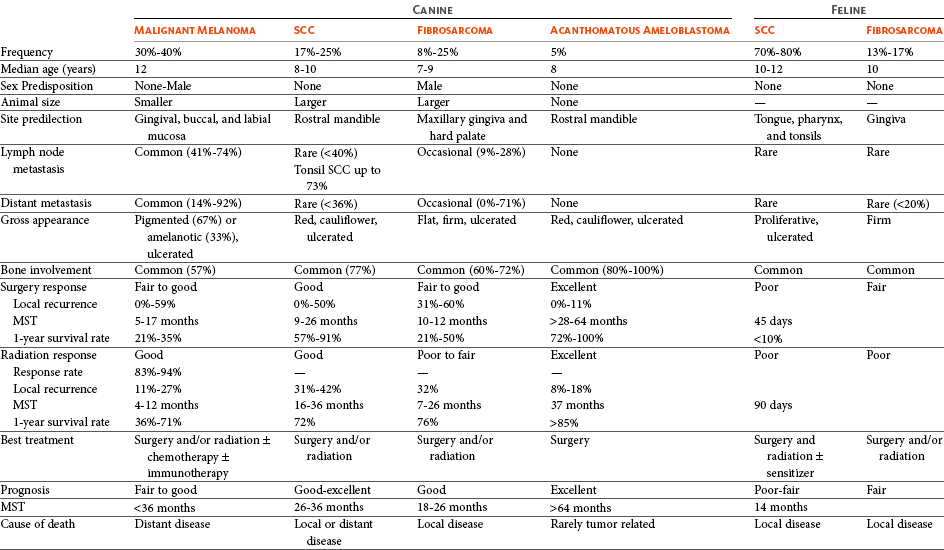
In dogs, the most common malignant oral tumors are, in descending order, malignant melanoma, SCC, and fibrosarcoma,10-21 although in other studies SCC is more common than malignant melanoma.2 SCC is the most common oropharyngeal cancer in cats, followed by fibrosarcoma, which accounts for 13% of feline oral tumors.3 Other malignant oral tumors in dogs include osteosarcoma, chondrosarcoma, anaplastic sarcoma, multilobular osteochondrosarcoma, intraosseous carcinoma, myxosarcoma, hemangiosarcoma, lymphoma, mast cell tumor, and transmissible venereal tumor.10-24 Tumors or tumorlike lesions of unusual sites, types, and biologic behavior (e.g., tonsillar SCC, tongue, malignancy of young dogs, viral papillomatosis, canine and feline eosinophilic granuloma complex, epulis, inductive fibroameloblastoma, and nasopharyngeal polyps) will be covered at the end of this chapter. A general summary of the common oral tumors is found in Table 22-1.
Pathology and Natural Behavior
The oral cavity is a very common site for a wide variety of malignant and benign cancers. Although most cancers are fairly straightforward histologically, some have confusing nomenclature or extenuating circumstances that warrant discussion.
Malignant Melanoma
In comparison to other malignant oral tumors, malignant melanoma tends to occur in smaller body weight dogs. Cocker spaniel, miniature poodle, Anatolian sheepdog, Gordon setter, Chow Chow, and golden retriever are overrepresented breeds.9 A male predisposition has been reported,7 but this is not consistent.9 The mean age at presentation is 11.4 years.9 Malignant melanoma occurs in cats but is uncommon.25
Malignant melanoma can present a confusing histopathologic picture if the tumor or the biopsy section does not contain melanin, and amelanotic melanomas represent one-third of all cases. A histopathologic diagnosis of undifferentiated or anaplastic sarcoma or even epithelial cancer should be viewed with suspicion for possible reclassification as melanoma. Melan A is an immunohistochemical stain with a moderate sensitivity and specificity for the diagnosis of melanoma in dogs and can be used to differentiate melanoma from other poorly differentiated oral tumors and may be helpful in differentiation.9
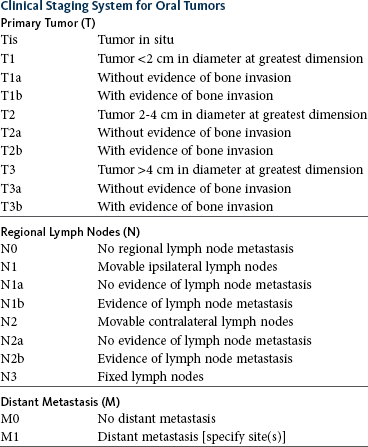
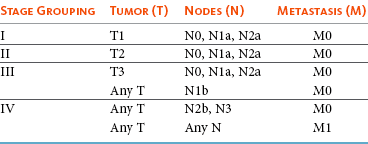
Melanoma of the oral cavity is a highly malignant tumor with frequent metastasis to the regional lymph nodes and then the lungs.7,26,27 The metastatic rate is site, size, and stage dependent and reported in up to 80% of dogs.* The World Health Organization (WHO) clinical staging system for oral tumors in dogs may have prognostic significance in dogs with oral melanoma (Table 22-2).7,26,31,36-38 Malignant melanoma is a highly immunogenic tumor, and molecular and immunomodulatory approaches to treatment are active areas of research.33,34,39-47 A review of the biology and molecular mechanisms of canine melanoma development and progression is provided in Chapter 19.48,49
Squamous Cell Carcinoma
SCC is the most common oral tumor in cats and the second most common in dogs.1,3,18-21 In cats, the risk of developing oral SCC is significantly increased by over 3.5-fold with the use of flea collars and high intake of either canned food in general or canned tuna fish specifically.50 Exposure to household smoke increases the risk of oral SCC by twofold in cats,50 and although this was not statistically significant, smoke exposure is associated with a significant increase in expression of p53 in SCC lesions compared to cats with oral SCC not exposed to environmental smoke.51 For this reason, mutations of p53 may be involved in the development and progression of smoke-related oral SCC in cats.
SCC frequently invades bone in both cats and dogs, and bone invasion is usually severe and extensive in the cat. Increased tumor expression of parathyroid hormone–related protein in cats with oral SCC may play a role in bone resorption and tumor invasion.52 Paraneoplastic hypercalcemia has also been reported in two cats with oral SCC.53 Metastasis in the cat is rare and the true incidence is unknown because so few cats have had their local disease controlled; thus an accurate estimate of the metastatic potential has not been confirmed. The metastatic rate for non–tonsillar SCC in dogs is approximately 20%,32 but the metastatic risk is site dependent—the rostral oral cavity has a low metastatic rate and the caudal tongue and tonsil have a high metastatic potential.
Fibrosarcoma
Oral fibrosarcoma is the second most common oral tumor in cats and the third most common in dogs.1,3,18-21,54 In dogs, oral fibrosarcoma tends to occur in large breed dogs, particularly golden and Labrador retrievers; at a younger age, with a median of 7.3 to 8.6 years; and with a possible male predisposition. Oral fibrosarcoma will often look surprisingly benign histologically and, even with large biopsy samples, the pathologist can find it difficult to differentiate fibroma from low-grade fibrosarcoma. This syndrome, which is common on the hard palate (Figure 22-1) and maxillary arcade between the canine and carnassial teeth of large-breed dogs, has been termed histologically low-grade but biologically high-grade fibrosarcoma.54 Even with a biopsy result suggesting fibroma or low-grade fibrosarcoma, the treatment should be aggressive, especially if the cancer is rapidly growing, recurrent, or invading bone. Fibrosarcoma is locally invasive but metastasizes to the lungs and occasionally regional lymph nodes in less than 30% of dogs.10,18-21,32
Epulides
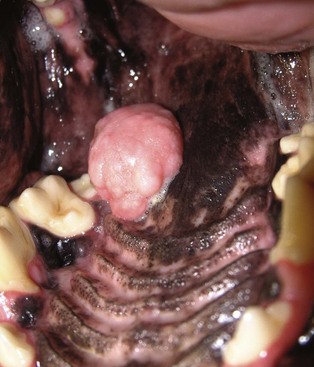
Epulides are benign gingival proliferations arising from the periodontal ligament and appear similar to gingival hyperplasia (Figure 22-2). Three types of epulides have previously been described in the dog: acanthomatous, fibromatous, and ossifying.56-58 However, the terminology for these tumors has changed; acanthomatous epulis is now termed acanthomatous ameloblastoma and peripheral odontogenic fibroma is the preferred nomenclature for fibrous and ossifying epulides.59
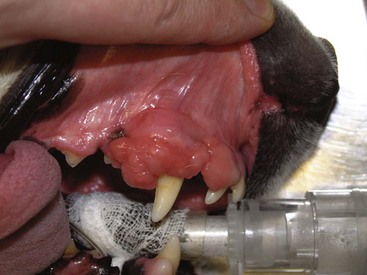
Figure 22-2 Typical appearance of a peripheral odontogenic fibroma. The mass is firmly adhered to the underlying bone but does not invade bone. Conservative resection with cautery was curative in this dog.
Peripheral Odontogenic Fibroma
Epulides are relatively common in dogs but rare in cats. Multiple epulides have been described in cats with 50% of cases occurring in cats younger than 3 years.55 The mean age at presentation for dogs with peripheral odontogenic fibromas is 8 to 9 years, and a male predisposition has been reported in one study.57,58 Peripheral odontogenic fibromas are slow-growing, firm masses that are usually covered by intact epithelium. They have a predilection for the maxilla rostral to the third premolar teeth.58,59
Acanthomatous Ameloblastoma
Acanthomatous ameloblastoma has an aggressive local behavior and frequently invades bone of the underlying mandible or maxilla. Shetland and Old English sheepdogs are predisposed.57,58 The mean age at presentation is 7 to 10 years, and a sex predisposition is unlikely with three studies reporting conflicting results.57,60,61 The rostral mandible is the most common site.60 They do not metastasize. Acanthomatous ameloblastoma is the preferred term, but some pathologists will refer to these tumors by their previous terminology of acanthomatous epulis or adamantinoma.56
History and Clinical Signs
Most cats and dogs with oral cancer present with a mass in the mouth noticed by the owner. Cancer in the caudal pharynx, however, is rarely seen by the owner, and the animal will present for signs of increased salivation, exophthalmos or facial swelling, epistaxis, weight loss, halitosis, bloody oral discharge, dysphagia or pain on opening the mouth, or occasionally cervical lymphadenopathy (especially SCC of the tonsil).18-21,62 Loose teeth, especially in an animal with generally good dentition, should alert the clinician to possible underlying neoplastic bone lysis (Figure 22-3), especially in the cat.63 Although paraneoplastic syndromes associated with oral tumors are rare, hypercalcemia has been reported in two cats with oral SCC51 and hyperglycemia in a cat with a gingival vascular hamartoma.64
Diagnostic Techniques and Work-Up
The diagnostic evaluation for oral cancers is critical due to the wide ranges of cancer behavior and therapeutic options available. If the tumor is suspected of being malignant, thoracic radiographs and lymph node cytologic assessment can be performed prior to biopsy. The most likely cancers to metastasize visibly on thoracic radiographs at the time of diagnosis are melanoma and SCC of the caudal oral, pharyngeal, and tonsillar area. Most animals will require a short general anesthesia for careful palpation, regional imaging, and a biopsy.
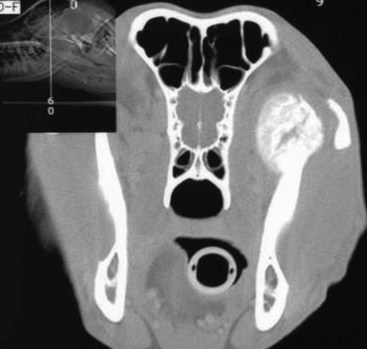
Cancers that are adherent to bone, other than peripheral odontogenic fibromas, should have regional radiographs taken under general anesthesia. Regional radiographs include open mouth, intraoral, oblique lateral, and ventrodorsal or dorsoventral projections.65 Bone lysis is not radiographically evident until 40% or more of the cortex is destroyed (Figure 22-4). However, apparently normal radiographs do not exclude bone invasion. This evaluation will assist in determining clinical staging information and the extent of resection when surgery is indicated. Computed tomography (CT) or magnetic resonance imaging (MRI) can be a very valuable staging tool, especially for evaluation of bone invasion and possible tumor extension into the nasal cavity or in the caudal pharynx and orbit, and is preferred to regional radiographs when available (Figure 22-5).66
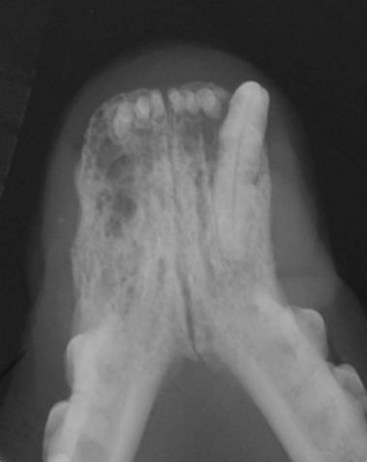
Figure 22-4 An intraoral radiograph of the rostral mandible of a cat with a SCC. Note the extensive, ill-defined bone lysis that is very common in cats with this type of tumor.
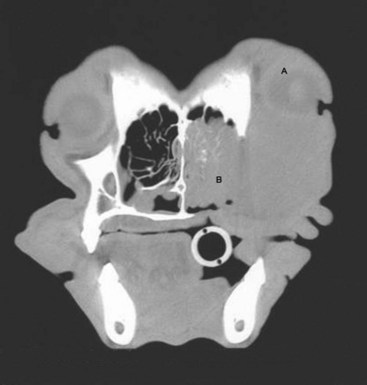
Figure 22-5 A CT image of a dog with a maxillary fibrosarcoma. Advanced imaging allows better planning of surgery and radiation therapy (RT) because the extent of bone involvement and extension into the nasal cavity is often much greater than can be appreciated grossly.
Regional lymph nodes should be carefully palpated for enlargement or asymmetry. However, caution should be exercised when making clinical judgments of whether neoplastic involvement of regional lymph nodes is present. Lymph node size is not an accurate predictor of metastasis. In one study of 100 dogs with oral melanoma, 40% of dogs with normal-sized lymph nodes had metastasis and 49% of dogs with enlarged lymph nodes did not have metastasis.27 The regional lymph nodes include the mandibular, parotid, and medial retropharyngeal lymph nodes; however, the parotid and medial retropharyngeal lymph nodes are normally not palpable.67 Furthermore, only 55% of 31 cats and dogs with metastasis to the regional lymph nodes had metastasis to the mandibular lymph node.68 Lymphoscintigraphy or contrast-enhanced ultrasonography can be used to detect the sentinel lymph nodes and guide lymph node aspirates.69 Lymph node aspiration should be performed in all animals with oral tumors, regardless of the size or degree of fixation of the lymph nodes.27,68 En bloc resection of the regional lymph nodes has been described and, although the therapeutic benefit of this approach is unknown, it may provide valuable staging information.67,68 Based on these diagnostic steps, oral tumors are then clinically staged according to the WHO staging scheme (see Table 22-2).36
The last step, under the same anesthesia, is a large incisional biopsy. Cytologic touch or aspiration preparations of oral tumors are usually not rewarding because of the necrosis and inflammation that commonly accompanies these cancers. Dogs with exophytic or ulcerated masses will generally tolerate a deep wedge or core punch biopsy without general anesthesia. Biopsy is recommended to differentiate benign from malignant disease, for owners basing their treatment options on prognosis, and when other treatment modalities such as radiation therapy (RT) are being considered. Oral cancers are commonly infected, inflamed, or necrotic, and it is important to obtain a large specimen. Electrocautery may distort the specimen and should only be used for hemostasis after blade incision or punch biopsy. Large samples of healthy tissue at the edge and center of the lesion will increase the diagnostic yield, but care must be taken not to contaminate normal tissue, which cannot be removed with surgery or included in the radiation field. Biopsies should always be performed from within the oral cavity and not through the lip to avoid seeding tumor cells in normal skin and compromising curative-intent surgical resection. For small lesions (e.g., epulides, papillomas, or small labial mucosal melanoma), curative-intent resection (excisional biopsy) may be undertaken at the time of initial evaluation. For more extensive disease, waiting for biopsy results to accurately plan treatment is strongly encouraged.
Therapy
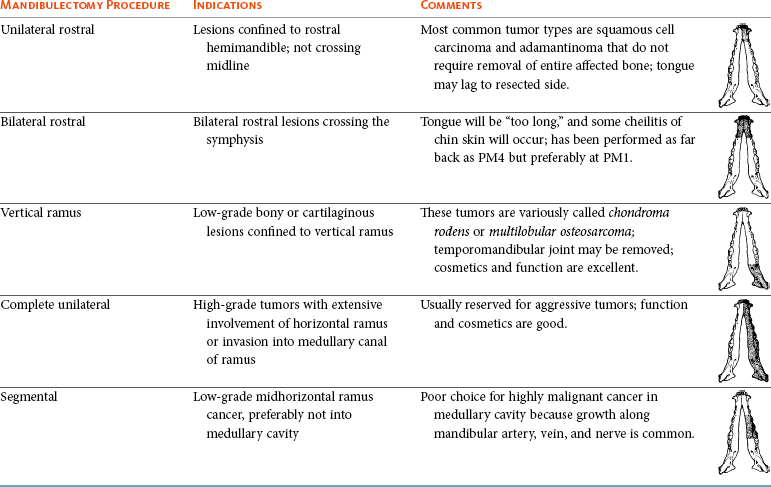
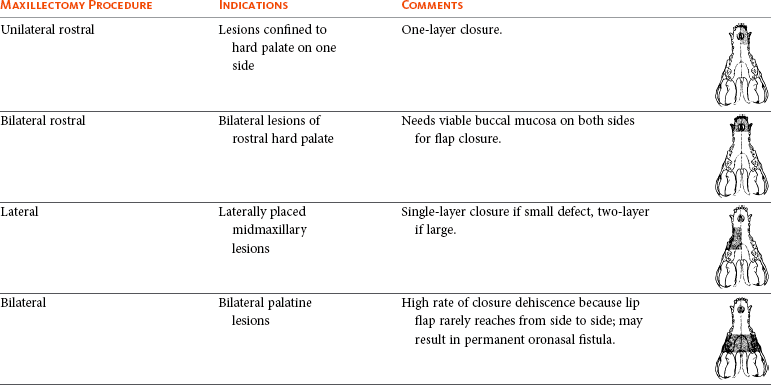
Surgery and RT are the most common treatments used for the local control of oral tumors. Surgical resection is the most economic, expeditious, and curative treatment. The type of oral surgery depends on tumor histology and location. Except for peripheral odontogenic fibromas, most oral tumors have some underlying bone involvement and surgical resection should include bony margins to increase the likelihood of local tumor control. More aggressive surgeries such as mandibulectomy, maxillectomy, and orbitectomy are generally well tolerated by cats and dogs. These procedures are indicated for all aggressive and/or invasive oral tumors, particularly lesions with extensive bone invasion, with poor sensitivity to RT, or that are too large for cryosurgery (Tables 22-3 and 22-4).11-21,70-73 Margins of at least 2 cm are necessary for malignant cancers such as SCC, malignant melanoma, and fibrosarcoma in the dog. If possible, SCC in the cat should be treated with surgical margins greater than 2 cm because of high local recurrence rates. Bone reconstruction following bony resection has been described but is rarely necessary.13,74-76 Rostral and segmental resections (e.g., mandibulectomy and maxillectomy) may be sufficient for benign lesions and rostral SCC in dogs. Rim resections with a biradial oscillating saw, in which the ventral cortex of the mandible is preserved, may be possible for small benign tumors localized to the alveolar margin of the mandible (Figure 22-6).77 Larger resections, including hemimandibulectomy, hemimaxillectomy, orbitectomy, and radical maxillectomy, are necessary for more aggressive tumors, especially fibrosarcoma, and malignant tumors with a more caudal location.11-21,70-72 Although these large resections carry some morbidity, owner satisfaction with the cosmetic and functional outcomes is in excess of 85%.11-21,71,73,78 Cosmesis is usually very good following most mandibulectomy and maxillectomy procedures (Figure 22-7) but can be challenging with aggressive bilateral rostral mandibulectomies and radical maxillectomies.11-21,70-72 Blood loss and hypotension are the most common intraoperative complications, particularly during caudal or aggressive maxillectomy procedures.19,71 Postoperative complications include incisional dehiscence, epistaxis, increased salivation, mandibular drift and malocclusion, and difficulty prehending food, particularly after bilateral rostral mandibulectomy caudal to the second premolar teeth.13-21,53,71 Elastic training, consisting of an orthodontic elastic rubber chain between an orthodontic button on the lingual aspect of the intact mandible tooth and buccal aspect of the maxillary fourth premolar tooth, has been described to maintain occlusion and prevent mandibular drift following mandibulectomy in dogs.79 Enteral feeding tubes are not usually required following oral surgery in dogs; however, they are recommended for cats treated with any type of mandibulectomy because eating can be difficult for 2 to 4 months following surgery.53,78
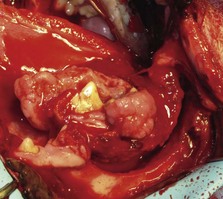
Figure 22-6 A rim resection has been performed for removal of an acanthomatous ameloblastoma with 1-cm margins. Note that the ventral cortex of the mandible has been preserved. Preservation of the ventral cortex prevents the development of mandibular drift and malocclusion.

Figure 22-7 The typical appearance of a dog 6 months postoperatively after subtotal unilateral mandibulectomy for an osteosarcoma. The tongue will often hang out and the remaining hemimandible will drift towards the resected side.
Local disease control is the goal of treatment for most animals with oral tumors. Regional lymph node resection has been described in cats and dogs; although it adds to clinical staging information, its effectiveness in controlling local and metastatic disease is unknown.67,68
Cryosurgery may be indicated for lesions less than 2 cm in diameter that are fixed or minimally invasive into bone. Larger lesions should generally be surgically resected. More extensive lesions in bone will often result in a fracture (mandible) or oronasal fistula (maxilla) if aggressively frozen. Cancer of soft tissue only should be surgically excised and not frozen.
Radiation Therapy
RT can be effective for locoregional control of oral tumors. RT can be used as a primary treatment, with either palliative or curative intent, or as an adjunct for incompletely resected tumors or tumors with an aggressive local behavior, such as oral fibrosarcoma. Malignant melanoma, canine oral SCC, and some benign tumors, such as the acanthomatous ameloblastoma, are known to be radiation responsive, and RT should be considered in the primary treatment of these tumors.31-33,80,81 For canine oral SCC, dental tumors, and fibrosarcoma, daily and alternate day protocols have been described consisting of 2.7 Gy to 4.2 Gy per fraction with a total dose ranging from 48 Gy to 57 Gy.32,82 Tumor control is better for smaller benign and malignant lesions (T1 and T2 tumors) treated with radiation alone.31,32,80 Local tumor control and survival time may be improved by combining RT with surgery and/or chemotherapy, especially for tumors considered radiation resistant, such as canine oral fibrosarcoma and feline oral SCC.53,83-88 Radiation sensitizers, such as etanidazole and gemcitabine, may improve response rates in cats with oral SCC, and platinum drugs have been used as radiation sensitizers in dogs with oral melanoma.28,37,84,87,88 However, gemcitabine is not recommended as a radiosensitizer in cats because of significant hematologic and local tissue toxicities.89
Oral melanoma is moderately responsive to coarse fractionation protocols. Four different hypofractionated radiation protocols have been described: (1) three weekly 8- to 10-Gy fractions for a total dose of 24 to 30 Gy,30,37 (2) four weekly fractions of 9 Gy for a total dose of 36 Gy,31,37 (3) six weekly 6-Gy fractions for a total dose of 36 Gy,28 and (4) eight weekly 6-Gy fractions for a total dose of 48 Gy.90 In humans, the effect of total radiation dose is controversial, but fraction size does have an impact on response rates. Doses greater than 4 Gy per fraction are recommended as response rates are significantly better with fractions greater than 8 Gy compared to less than 4 Gy.91 However, in one study of dogs with oral melanoma comparing two hypofractionated protocols of 9 to 10 Gy per fraction to a fully fractionated protocol of 2 to 4 Gy per fraction, there were no significant differences in either local recurrence rates or survival time.37 Coarse fractionation of oral melanoma has also been described in five cats with limited success, including one complete response and two partial responses.25
Acute effects are common but self-limiting. These include alopecia and moist desquamation, oral mucositis, dysphagia, and ocular changes, such as blepharitis, conjunctivitis, keratitis, and uveitis.32,80,92-94 The acute effects of coarse fractionation are less than experienced with the full-course protocols used for oral SCC and dental tumors and usually resolve rapidly.31 Late complications are rare, occurring in less than 5% of cases, but can include permanent alopecia, skin fibrosis, bone necrosis and oronasal fistula formation, development of a second malignancy within the radiation field, keratoconjunctivitis sicca, cataract formation, xerostomia, and retinal atrophy.32,61,80,95 Orthovoltage radiation may be associated with a higher incidence of second malignancies and bone necrosis than megavoltage irradiation.32,61,80
Hyperthermia offers no advantage over cryosurgery or surgery if it is used alone. In fact, bone penetration is less reproducible with heat versus cold treatment. Hyperthermia at moderate temperatures (42 to 43°C) has been used as an adjunct to irradiation.29,96,97
Chemotherapy
The major problem with most oral tumors is control of local disease. However, chemotherapy is indicated for some tumors with higher metastatic potential, especially oral melanoma in dogs and tonsillar SCC in cats and dogs. Expression of cyclooxygenase 2 (COX-2) has been noted in feline oral SCC98; however, nonsteroidal antiinflammatory drugs (NSAIDs) such as piroxicam have not been effective in the management of this disease in cats in preliminary studies.99 Piroxicam does appear to have some effect against oral SCC in dogs,100 and response rate is improved when piroxicam is combined with either cisplatin or carboplatin.101,102 Liposome-encapsulated cisplatin is not effective in cats with oral SCC,103 but mitoxantrone, in combination with RT, has shown some potential in a limited number of cats with good local responses and durable remission.85,86
The platinum drugs show the most promise, albeit modest, in treating dogs with oral melanoma, including intralesional cisplatin104 and systemic carboplatin.105 Measurable responses to melphalan have also been reported.106
Malignant melanoma is a highly immunogenic tumor. The use of immunomodulatory agents is an emerging and exciting approach for the adjunctive management of dogs with oral melanoma. A thorough discussion of the immunotherapeutic approach to management of canine melanoma is provided in Chapter 19.
Prognosis
Clinical series of over 500 dogs with various oral malignancies treated with either mandibulectomy or maxillectomy have been described.11-21,70-72 The majority of cases were treated with surgery alone. Unfortunately, the methods of reporting and outcome results vary with each paper, but an attempt to combine cases by tumor type and outcome is shown in Tables 22-5 and 22-6. Overall, the lowest rates of local tumor recurrence and best survival times are reported in dogs with acanthomatous ameloblastoma and SCC, whereas fibrosarcoma and malignant melanoma are associated with the poorest results.11-21 Most of these reports suggest that histologically complete resection, smaller diameter, and a rostral location are favorable prognostic factors. In two studies of 142 dogs treated with either mandibulectomy or maxillectomy, tumor-related deaths were 10 to 21 times more likely with malignant tumors, up to 5 times more likely with tumors located caudal to the canine teeth, and 2 to 4 times more likely following incomplete resection.20,21 Rostral locations are usually detected at an earlier stage and are more likely to be resectable with complete surgical margins. Local tumor recurrence is more frequent following incomplete resection, with 15% to 22% and 62% to 65% of tumors recurring following complete and incomplete excision, respectively.20,21 Recurrent disease negatively impacts survival time because further treatment is more difficult and the response to treatment is poorer.35 Fibrosarcoma continues to have an unacceptable local recurrence rate and needs to be addressed with wider resections or other adjuvant therapies, such as postoperative radiation.82 On the other hand, melanoma is controlled locally in 75% of cases, but metastatic disease requires more effective adjuvant therapy, such as RT, chemotherapy, or immunotherapy.
For dogs treated with megavoltage radiation, tumor type and tumor size are important factors in local tumor control for both benign and malignant oral tumors. As noted previously, acanthomatous ameloblastoma and SCC in dogs are radiation sensitive. Local recurrence is reported in 30% of oral tumors regardless of treatment, but recurrence is a function of tumor size. Compared to small tumors (T1: <2-cm diameter), recurrence is 3 times more likely in T2 tumors (2- to 4-cm diameter) and up to 8 times more likely in T3 tumors (>4-cm diameter).32,80 Tumor size is also associated with survival in dogs with malignant oral tumors, with 3-year progression-free survival (PFS) rates of 55%, 32%, and 20% for T1, T2, and T3 tumors, respectively.32
Malignant Melanoma
The prognosis for dogs with oral melanoma is guarded. Metastatic disease is the most common cause of death with metastasis to the lungs reported in 14% to 67% of dogs.* Surgery or RT can provide good local control, but strategies to manage the high metastatic potential such as chemotherapy and immunotherapy require further investigation.
Surgery is the most common treatment for management of the local tumor. The local tumor recurrence rate varies from 22% following mandibulectomy to 48% after maxillectomy.7,18,19 The median survival time (MST) for dogs with malignant melanoma treated with surgery alone varies from 150 to 318 days with 1-year survival rates less than 35%.† Regardless, tumor control and survival time are significantly better when surgery is included in the treatment plan.38 In comparison, the MST for untreated dogs with oral melanoma is 65 days.35 Variables known to have prognostic significance in dogs treated with surgery alone or in combination with other modalities include tumor size, clinical stage, and ability of the first treatment to achieve local control.‡ Dogs with tumors less than 2-cm in diameter have a MST of 511 days compared to 164 days for dogs with tumors greater than 2-cm diameter or lymph node metastasis.33 MSTs are significantly shorter for dogs with recurrent oral malignant melanomas compared to dogs with previously untreated oral melanomas.26,35
Oral melanoma is responsive to hypofractionated RT protocols. Response rates are excellent with 83% to 100% of tumors responding and a complete response observed in up to 70% of melanomas.28,30-32,90 Local recurrence is reported in 15% to 26% of dogs experiencing a complete response with a median time to local recurrence of 139 days.28,30-32 Progressive local disease was observed in all dogs that did not achieve a complete response in one study.30 The most common cause of death is metastasis and this is reported in 58% of dogs with a median time of metastasis of 311 days.28 The MST for dogs treated with RT is 211 to 363 days, with a 1-year survival rate of 36% to 48% and a 2-year survival rate of 21%.28,30-32,37 Local tumor control and survival time are significantly improved with rostral tumor location, smaller tumor volume, no radiographic evidence of bone lysis, and postoperative irradiation of microscopic disease.31,32,37 The risk factors associated with poor outcomes in dogs with melanoma include nonrostral location, bone lysis, and macroscopic disease, and in one series of 140 dogs with oral melanoma, the MST was 21 months if none of these risk factors were present compared to a MST of 11 months with one risk factor, 5 months with two risk factors, and 3 months with all three risk factors.37 Tumor size is important with median PFS for dogs with T1 oral melanomas of 19 months compared to less than 7 months for T2 and T3 tumors.32 Hypofractionated RT has also been described in five cats with oral melanoma, resulting in a 60% response rate and MST of 146 days (range: 66 to 224 days).25
Chemotherapy or immunotherapy is indicated in the adjunctive management of dogs with oral melanoma because of the high metastatic risk. A thorough discussion of malignant melanoma and its prognosis following definitive treatment with surgery, RT, chemotherapy, and/or immunomodulatory agents is provided in Chapter 19.
The location of malignant melanoma may also have some prognostic significance. Melanomas of the lip and tongue have a lower metastatic rate and survival is more dependent on local control of the tumor. In one series of 60 dogs with oral melanomas at various sites treated with combinations of surgery, RT, chemotherapy, and immunotherapy, the MST for dogs with lip melanomas was 580 days and was not reached and greater than 551 days for dogs with tongue melanomas.7 In comparison, the MST was 319 days for maxillary melanomas and 330 days for melanomas of the hard palate.7
In another study, only 5% of 64 dogs with well-differentiated melanomas of the mucous membranes of the lips and oral cavities treated with surgery alone had died from tumor-related reasons with an overall MST of 34 months.109 This improved prognosis may reflect the location of these lesions (lip compared to oral cavity) or the degree of differentiation. Nuclear atypia and mitotic index has also been shown to be prognostic in dogs with oral malignant melanomas.110
Squamous Cell Carcinoma
Canine Oral Squamous Cell Carcinoma
The prognosis for dogs with oral SCC is good, particularly for rostral tumor locations. Local tumor control is usually the most important challenge, although metastasis to the regional lymph nodes is reported in up to 10% of dogs and to the lungs in 3% to 36% of dogs.32 In contrast, SCC of the tonsils and base of the tongue are highly metastatic, with metastasis reported in up to 73% of dogs, and locoregional recurrence is common.111-113 Surgery and RT can both be used for locoregional control of oral SCC in dogs. Photodynamic therapy has also been reported with fair-to-good results in 11 dogs with smaller oral SCC.114
Surgery is the most common treatment for non–tonsillar SCC.11 Following mandibulectomy, the local recurrence rate is 10% and the MST varies from 19 to 26 months with a 91% 1-year survival time.18 In comparison, the local recurrence rate is 29% after maxillectomy with a MST of 10 to 19 months and a 1-year survival rate of 57%.19 The higher local control and survival rates with mandibular resections probably result because the rostral mandible is the most common location for oral SCC in dogs, and complete surgical resection is more likely for rostral locations.
Full-course RT, either alone or as an adjunct following incomplete surgical resection, is also a successful treatment modality for the management of oral SCC in dogs.32,115,116 The local tumor recurrence rate is 31%. The MST for RT alone is 15 to 16 months and increases to 34 months when combined with surgery.115,116 In one series of 39 dogs with oral SCC, the overall median PFS time was 36 months with 1- and 3-year PFS rates of 72% and 55%, respectively.32 Local tumor control was more successful with smaller lesions; the median PFS time for T1 tumors (<2-cm diameter) was not reached and greater than 68 months compared to 28 months for T2 tumors (2- to 4-cm diameter) and 8 months for dogs with T3 tumors (>4-cm diameter).32 Additional favorable prognostic factors for dogs receiving orthovoltage irradiation include rostral tumor location, maxillary SCC, and young age.115 Younger age is also favorable for dogs treated with megavoltage radiation (< 9 years of age—1080 days; > 9 years of age—315 days).116
Chemotherapy is indicated for dogs with metastatic disease, dogs with bulky disease, and when owners decline surgery and RT. However, as the metastatic potential of oral SCC in dogs is relatively low, the role of chemotherapy in minimizing the risk of metastatic disease is unknown. In a series of 17 dogs treated with piroxicam alone, the response rate was 17%, with one complete response and two partial responses.100 The median progression-free interval for dogs responding to piroxicam was 180 days and significantly longer than the 102 days for dogs with stable disease.100 The outcome is better when piroxicam is combined with either cisplatin or carboplatin. In a series of nine dogs treated with piroxicam and cisplatin, the overall MST was 237 days, with the 56% of dogs responding to this chemotherapy protocol having a significantly better outcome than nonresponders with a MST of 272 days compared to 116 days.101 However, renal toxicity was reported in 41% of dogs in this study and such toxicities limit the clinical usefulness of this protocol. In another small series of seven dogs with T3 oral SCC treated with piroxicam and carboplatin, a complete response was observed in 57% of dogs and this response was sustained in all dogs at the median follow-up time of 534 days.102 Novel therapies under investigation include the combination of intralesional bleomycin and feline interleukin-12 (IL-12) DNA with translesional electroporation.107
Feline Oral Squamous Cell Carcinoma
The prognosis for cats with oral SCC is poor.14,62,117,118 There is no known effective treatment that consistently results in durable control or survival. Local control is the most challenging problem. In one series of 52 cats, the 1-year survival rate was less than 10%, with MSTs of 3 months or less for surgery alone, surgery and RT, RT and low-dose chemotherapy, or RT and hyperthermia.62 However, 42% of these cats had SCC involving the tongue, pharynx, or tonsils. In another series of 54 cats treated in general practice, the MST was 44 days with a 9.5% 1-year survival rate.119 The oncologic outcome may be better for cats with mandibular SCC. The MST for seven cats treated with a combination of mandibulectomy and RT was 14 months with a 1-year survival rate of 57%.53 Local recurrence was the cause of failure in 86% of these cats between 3 to 36 months after therapy. In another series of 22 cats treated with mandibulectomy alone, the median disease-free interval (DFI) was 340 days.78 Tumor location and extent of resection had prognostic importance with a MST of 911 days for rostral tumors, 217 days following hemimandibulectomy, and 192 days when more than 50% of the mandible was resected.78 Expansile, blastic, and discrete lesions are often more resectable than invasive, lytic, and ill-defined lesions in the experience of the authors. The use of esophagostomy or gastrostomy tubes may be necessary to provide supplemental nutrition in these cats for up to 4 months postoperatively.78
RT alone is generally considered ineffective in the management of cats with oral SCC. In nine cats treated with an accelerated radiation protocol (14 fractions of 3.5 Gy delivered twice daily for 9 days), the overall MST was 86 days and, although not significant, the MST for cats with a complete response was 298 days.81 The combination of RT with radiation sensitizers or chemotherapy improves response rates and survival times. Using the same accelerated radiation protocol with carboplatin resulted in a MST of 163 days in 14 cats.88 Intratumoral etanidazole, a hypoxic cell sensitizer, resulted in a 100% partial response rate in nine cats completing the RT course with a median decrease in tumor size of 70% and a MST of 116 days.84 Gemcitabine was used at low doses as a radiation sensitizer in eight cats with oral SCC with an overall response rate of 75%, including two cats with complete responses, for a median duration of 43 days and a MST of 112 days.87 However, gemcitabine is not recommended as a radiosensitizer in cats because of significant hematologic and local tissue toxicities.89 The combination of RT with mitoxantrone holds some promise because, in two series of 18 cats, a complete response was observed in 73% with a median duration of response of 138 to 170 days and an MST of 184 days.85,86 Palliative radiation protocols, consisting of 8 Gy fractions on days 0, 7, and 21, are not recommended because of poor disease control and radiation-induced adverse effects.120 Localized irradiation with strontium-90 may be effective for selected cats with very superficial disease.121
Chemotherapy appears ineffective in the management of cats with oral SCC. No responses were observed in 18 cats treated with liposome-encapsulated cisplatin or 13 cats treated with piroxicam.99,103 In one study, the administration of a NSAID improved survival times in cats with oral SCC.119
Fibrosarcoma
The prognosis for dogs with oral fibrosarcoma is guarded. These are locally aggressive tumors and local control is more problematic than metastasis. Metastasis is reported to the regional lymph nodes in 19% to 22% of dogs and to the lungs in up to 27% of dogs.18,19,32 Multimodality treatment of local disease appears to afford the best survival rates, with combinations of surgery and RT or RT and hyperthermia.29,78
Surgery is the most common treatment for oral fibrosarcoma. The median DFI for five cats treated with mandibulectomy was 859 days.78 Following mandibulectomy, local recurrence is reported in 59% of dogs with a MST of 11 months and a 1-year survival rate of 50%.18 The outcome is similar following maxillectomy, with local recurrence in 40% of dogs and a MST of 12 months.19 One-year survival rates rarely exceed 50% with surgery alone.11-21 The combination of surgery and RT provides the best opportunity to control local disease.
Oral fibrosarcomas are considered radiation resistant.83,122,123 The mean survival time of 17 dogs treated with RT alone was only 7 months.83 RT combined with regional hyperthermia improved local control rates to 50% at 1 year in a series of 10 cases.29 When RT is used as an adjunct to surgical resection, local tumor recurrence was reported in 32% of dogs overall and the MST increased to 18 to 26 months with a 1-year PFS rate of 76%.32,82 A smaller tumor size improves the outcome following RT, with a median PFS time of 45 months for dogs with T1 tumors compared to 31 months and 7 months for T2 and T3 tumors, respectively.32
Osteosarcoma
Osteosarcoma of axial sites is less common than appendicular osteosarcoma and represents approximately 25% of all cases.124 Of the axial osteosarcomas, the mandible and maxilla are involved in 27% and 16% to 22% of cases, respectively.124,125 The prognosis for dogs with oral osteosarcoma is better than appendicular osteosarcoma because of an apparent lower metastatic potential.119 A female sex predisposition has been reported.124
The outcome following mandibulectomy alone is variable with MSTs of 14 to 18 months and 1-year survival rates of 35% to 71%.18,124,126 In 20 dogs treated with mandibulectomy alone, the cause of death was local recurrence in 15% of dogs and metastatic disease in 35% of dogs.18 Following maxillectomy, the MST varies from 5 to 10 months with a 1-year survival rate of 17% to 27% and local tumor recurrence in 83% to 100% of dogs.19,124 The majority of dogs with maxillary osteosarcoma die as a result of local tumor recurrence with metastasis not reported in any dogs in two studies.106,125
Local tumor control is the most challenging problem and resecting oral osteosarcomas with complete surgical margins is imperative. In one study of 60 dogs with osteosarcoma of the skull, including the mandible and maxilla, the median DFI and survival times were not reached at greater than 1503 days following complete excision and significantly better than incomplete resection with a median DFI of 128 days and a MST of 199 days.127 The combination of surgery with either RT or chemotherapy did not improve the outcome in dogs with incompletely resected tumors, highlighting the necessity for an aggressive surgical approach. These results are supported by another study of 45 dogs with axial osteosarcoma in which favorable prognostic factors included complete surgical excision, mandibular location, and smaller body weight dogs.125 Chemotherapy’s role in the management of dogs with axial osteosarcoma is unknown but should be evaluated.
Epulides
Peripheral Odontogenic Fibroma
The prognosis for dogs with peripheral odontogenic fibromas is excellent following treatment with either surgery or rarely RT. These are benign tumors and metastasis has not been reported; hence local tumor control is the principal goal of therapy. For peripheral odontogenic fibromas, the local tumor recurrence rate following surgical resection without bone removal varies from 0% to 17%.57,128 RT is also effective with a 3-year PFS rate of 86%.80 However, full-course RT is usually not required because these tumors can be adequately managed with simple surgical resection.57 Local recurrence is common in cats with multiple epulides and is reported in 73% of 11 cats 3 months to 8 years after surgical resection.55
Acanthomatous Ameloblastoma
Surgery or RT is also used in the management of dogs with acanthomatous ameloblastoma. Mandibulectomy or maxillectomy is required for surgical resection of acanthomatous ameloblastomas because of frequent bone invasion by this benign tumor. Local recurrence rates following bone-removing surgery are less than 5%.* Megavoltage RT, consisting of an alternate day protocol of 4 Gy per fraction to a total of 48 Gy, results in a 3-year PFS rate of 80% in dogs with acanthomatous ameloblastomas.80 The overall local recurrence rate with RT varies from 8% to 18% in two studies of 39 dogs and recurrence was 8 times more likely with T3 tumors compared to T1 and T2 tumors.61,80 The majority of tumors recur within the radiation field, which suggests a higher radiation dose may be required to achieve higher rates of local tumor control, particularly for tumors greater than 4 cm in diameter.80 Other complications associated with RT include malignant transformation in 5% to 18% of dogs and bone necrosis in 6% of dogs.61,80 Intralesional bleomycin has been described in four dogs, and a complete response was observed in all dogs and was sustained for a minimum of 1 year with no local recurrence.129
Selected Sites or Cancer Conditions in the Oral Cavity
Tonsillar Squamous Cell Carcinoma
Tonsillar SCC is 10 times more common in animals living in urban versus rural areas, implying an etiologic association with environmental pollutants.130 Primary tonsillar cancer is often SCC. Lymphoma can affect the tonsils but is usually accompanied by generalized lymphadenopathy and is often bilateral. Other cancers, especially malignant melanoma, can metastasize to the tonsils. Cervical lymphadenopathy is a common presenting sign, even with very small primary tonsillar cancers. Fine-needle aspirates of the regional lymph nodes or excisional biopsy of the tonsil will confirm the diagnosis. Thoracic radiographs are positive for metastasis in 10% to 20% of cases at presentation. In spite of disease apparently confined to the tonsil, this disease is considered systemic at diagnosis in over 90% of cats and dogs. Simple tonsillectomy is almost never curative but probably should be done bilaterally due to the high percentage of bilateral disease.10 Cervical lymphadenectomy, especially if the regional lymph nodes are large and fixed, is rarely curative and should be considered diagnostic only. Regional RT of the pharyngeal region and cervical lymph nodes is capable of controlling local disease in over 75% of the cases; however, survival still remains poor with 1-year survival rates of only 10%.111,113 Local tumor control and survival times were significantly improved in one study of 22 dogs with tonsillar SCC when RT was combined with a variety of different chemotherapy drugs.113 Cause of death is local disease early and systemic disease (usually lung metastasis) later. To date, no known effective chemotherapeutic agents exist for canine or feline SCC, although cisplatin, carboplatin, doxorubicin, vinblastine, and bleomycin have been used with limited success.113,131 In one study of 44 dogs with tonsillar SCC treated with surgery, RT, and/or chemotherapy, the MST was 179 days and dogs presenting with either anorexia or lethargy had a significantly shorter survival time.132
Tongue
Cancer confined to the tongue is rare. In one study, 54% of tongue lesions were neoplastic and 64% of these were malignant.133 White dogs appear to be at higher risk for SCC, even though lack of pigment would not seem to be as much a problem as it is in other more exposed areas of the body (e.g., nose, eyelids, and ears).134 Other reported breed predilections include Chow Chow and Chinese Shar-Pei for malignant melanoma; poodle, Labrador retriever, and Samoyed for SCC; border collie and golden retriever for hemangiosarcoma and fibrosarcoma; and cocker spaniel for plasma cell tumors.133 The most common cancer of the canine tongue is SCC, accounting for approximately 50% of cases, followed by granular cell myoblastoma, malignant melanoma, mast cell tumor, fibrosarcoma, adenocarcinoma, neurofibrosarcoma, leiomyosarcoma, hemangiosarcoma and hemangioma, rhabdomyoma and rhabdomyosarcoma, myxoma, and lipoma.133-135 Feline tongue tumors are usually SCCs, and most are located on the ventral surface near the frenulum. Presenting signs are similar to other oral tumors. Ulceration is common with SCC.
Under general anesthesia, the tongue may be biopsied with a wedge incision and closed with horizontal mattress sutures. Biopsies are necessary to differentiate malignant tumors from nonneoplastic lesions such as eosinophilic granuloma, inflammatory disease, and calcinosis circumscripta. Ultrasonography can be useful in delineating the margins of tongue masses to determine surgical resectability.136 Regional lymph nodes should be aspirated for staging purposes and three-view thoracic radiographs evaluated for lung metastasis.
Surgical resection is recommended, whereas RT is reserved for melanomas, inoperable cancer, or tumors metastatic to the regional lymph nodes. Partial glossectomy of up to 60% of the tongue has been recommended for unilateral tumors not crossing the midline or tumors confined to the rostral mobile portion of the tongue. However, 54% of canine tongue tumors are located in the midline or are bilaterally symmetrical, which limits the ability to achieve complete surgical resection.112 Recently, 50% to 100% resection or avulsion of the tongue was reported in five dogs with minimal postoperative problems, which suggests more aggressive resections may be possible without compromising quality of life.137 Feeding tubes are recommended for enteral nutrition during postoperative recovery but, in the long term, eating and drinking are usually only mildly impaired and good hydration and nutrition can be maintained postoperatively.134,137 Hypersalivation is the most common complaint following aggressive resections.137 Thermoregulation can be a problem in hot and humid environments. Grooming in cats will be compromised and may result in poor hair-coat hygiene.
The prognosis for tongue tumors depends on the site, type, and grade of cancer.134 Tongue SCCs in dogs are graded from I (least malignant) to III (most malignant) based on histologic features such as degree of differentiation and keratinization, mitotic rate, tissue and vascular invasion, nuclear pleomorphism, and scirrhous reaction.134 The MST for dogs with grade I tongue SCC is 16 months following surgical resection, which is significantly better than the MSTs of 4 and 3 months reported for grade II and III SCC, respectively.134 The 1-year survival rate is 50% following complete surgical resection and approaches 80% with complete histologic resection of low-grade SCC.134 Cancer in the rostral (mobile) tongue has a better prognosis possibly because rostral lesions are detected at an earlier stage, the caudal tongue may have richer lymphatic and vascular channels to allow metastasis, and rostral tumors are easier to resect with wide margins. Long-term control of feline tongue tumors is rarely reported with 1-year survival rates for tongue SCC less than 25%.
Granular cell myoblastoma is a curable cancer.138 These cancers may look large and invasive but are almost always removable by conservative and close margins (Figure 22-8). Permanent local control rates exceed 80%. They may recur late, but serial surgeries are usually possible. Metastasis is rare with this cancer. Local control in four of five tongue melanomas was obtained by surgery, and the metastatic rate was less than 50% in this small series.117 In another series of dogs with tongue melanoma, the MST was not reached and was greater than 551 days.7 The biologic behavior of other tongue cancers is generally unknown due to the rarity of these conditions.117
Undifferentiated Malignancy of Young Dogs
Undifferentiated malignancy is seen in dogs under 2 years of age (range: 6 to 22 months).139 Most dogs are large breeds and there is no sex predilection. The disease is manifest by a rapidly growing mass in the area of the hard palate, upper molar teeth, maxilla, and/or orbit. Biopsies reveal an undifferentiated malignancy of undetermined histiogenesis. The majority of dogs present with metastasis to the regional lymph nodes and distant sites beyond the head and neck. An effective treatment has not been identified, although chemotherapy would be necessary considering the high metastatic rate. Most dogs are euthanatized within 30 days of diagnosis due to progressive and uncontrolled tumor growth.
Papillary SCC has been reported to occur in the oral cavity of very young dogs (2 to 5 months of age). Treatment recommendations include complete surgical resection or surgical cytoreduction and curettage followed by radiation (40 Gy in 20 fractions). Using this latter combination therapy, no dog had metastasis and long-term control was achieved in all dogs for periods up to 4 years.140
Multilobular Osteochondrosarcoma
Multilobular osteochondrosarcoma is an infrequently diagnosed bony and cartilaginous tumor that usually arises from the canine skull, including the mandible, maxilla, hard palate, orbit, and calvarium.23,24 Histologically, these tumors are characterized by multiple lobules with a central cartilaginous or bone matrix surrounded by a thin layer of spindle cells.23,24 On imaging, multilobular osteochondrosarcoma is characterized by a typical “popcorn” appearance (Figure 22-9). Surgery is recommended for management of the local tumor, although there are anecdotal reports that multilobular osteochondrosarcoma may also be responsive to RT. The overall rate of local recurrence following surgical resection is 47% to 58% and depends on completeness of surgical resection and histologic grade.23,24 The median DFI for completely resected multilobular osteochondrosarcoma is 1332 days and significantly better than the 330 days reported for incompletely excised tumors.24 In terms of tumor grade, the local recurrence rate for grade III tumors is 78% and significantly worse than the recurrence rates of 30% and 47% for grade I and II multilobular osteochondrosarcoma, respectively.24 This tumor has a moderate metastatic potential (usually to the lung), which is grade dependent, but usually occurs late in the course of disease. Metastasis is reported in up to 58% of dogs with the median time to metastasis of 426 to 542 days.23,24 Metastasis is significantly more likely following incomplete surgical resection, with a 25% metastatic rate in completely excised tumors and 75% following incomplete resection.24 Tumor grade also has a significant impact on metastatic rate with metastasis reported in 78% of grade III multilobular osteochondrosarcoma compared to 30% of grade I and 60% of grade II tumors.24 There is no known effective chemotherapy treatment for metastatic disease, but survival times greater than 12 months have been reported with pulmonary metastasectomy because of the slow-growing nature of this tumor.24 The overall MST is 21 months and is grade dependent, with reported MSTs of 50 months, 22 months, and 11 months for grade I, II, and III tumors, respectively.23,24 Tumor location also has prognostic significance because the outcome for dogs with mandibular multilobular osteochondrosarcoma is significantly better with a MST of 1487 days compared to 587 days for these tumors at other sites.24
Viral Papillomatosis
Viral papillomatosis is horizontally transmitted by a DNA viral agent (papovavirus) from dog to dog.141 Affected animals are generally young. The lesions appear wartlike and are generally multiple in the oral cavity, pharynx, tongue, or lips. A biopsy can be performed if necessary, but visual examination is usually diagnostic. Most patients never suffer any significant side effects of this disease, although an occasional dog will have such marked involvement as to require surgical or cryosurgical cytoreduction in order to permit swallowing. The majority of patients will undergo a spontaneous regression of disease within 4 to 8 weeks. For resistant cases, an immunodeficiency etiology is suggested and a wide variety of treatments have been attempted, including crushing lesions in situ to “release” viral antigens and using several immunomodulators, including autogenous vaccines, interleukins, levamisole, thiabendazole, imiquimod, and L-MTP-PE.142 However, these methods are seldom required or effective. Furthermore, autogenous vaccines are not recommended because malignant skin tumors have been reported at the site of inoculation.56 The prognosis is usually excellent.
Odontogenic Tumors
Odontogenic tumors originate from epithelial cells of the dental lamina and account for up to 2.4% of all feline oral tumors.3 They are broadly classified into two groups depending on whether the tumors are able to induce a stromal reaction.143 Inductive odontogenic tumors include ameloblastic fibroma, dentinoma, and ameloblastic, complex, and compound odontomas. Ameloblastomas and calcifying epithelial odontogenic tumors are examples of noninductive odontogenic tumors.143
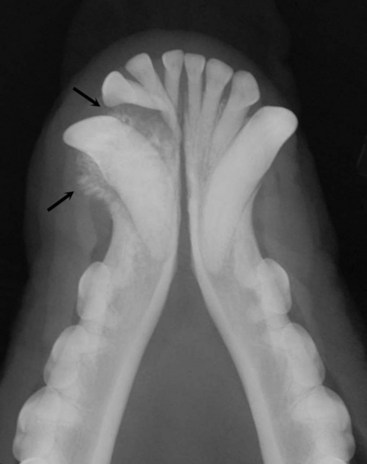
Inductive fibroameloblastoma is the most common odontogenic tumor in cats, usually occurs in cats less than 18 months of age, and has a predilection for the region of the upper canine teeth and maxilla.3,56,143-145 Radiographically the tumor site shows variable degrees of bone destruction, production, and expansion of the mandibular or maxillary bones (Figure 22-10). Teeth deformity is common. Smaller lesions are treated with surgical debulking and cryosurgery or premaxillectomy. Larger lesions will respond to radiation. Local treatment needs to be aggressive, but control rates are good and metastasis has not been reported.3,56
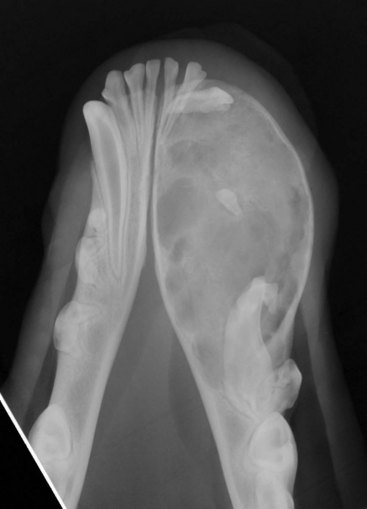
Figure 22-10 An intraoral radiograph of the rostral mandible in a dog with an ameloblastoma. Note the smooth expansile mandibular mass. The tumor was curetted and filled with cancellous bone graft and the dog was tumor-free 1 year after surgery.
Odontomas are benign tumors arising from the dental follicle during the early stages of tooth development.146 Odontomas induce both enamel and dentin within the tumor. Odontomas have a similar biologic behavior to ameloblastomas.
Dentigerous cysts are nonneoplastic, circumscribed cystic lesions originating from islands of odontogenic epithelium.143 They contain one or more teeth embedded in the cyst wall. Radiographs show a characteristic radiolucent halo surrounding the nonerupted tooth originating at the cementoenamel junction and enveloping the crown of the tooth.65 Odontogenic cysts may represent an early stage of malignant epithelial tumors.143 Surgical treatment is recommended, consisting of surgical removal of nonerupted teeth and the cyst lining with possible cancellous bone grafting, to prevent local tumor recurrence.65
Eosinophilic Granulomas
Eosinophilic Granulomas in Dogs
Canine oral eosinophilic granulomas affect young dogs (1 to 7 years of age) and may be heritable in the Siberian husky and Cavalier King Charles spaniel.147-149 It is histologically similar to the feline disease, with eosinophils and granulomatous inflammation predominating. The granulomas typically occur on the lateral and ventral aspects of the tongue. They are raised, frequently ulcerated, and may mimic more malignant cancers in gross appearance. Treatment with corticosteroids or surgical excision is generally curative, although spontaneous regression may occur. Local recurrences are uncommon.
Eosinophilic Granuloma in the Cat
Eosinophilic granuloma, a condition also known as rodent ulcer or indolent ulcer, occurs more commonly in female cats with a mean age of 5 years.150-153 The etiology is unknown. Any oral site is at risk, but it is most common on the upper lip near the midline (Figure 22-11). The history is usually that of a slowly progressive (months to years) erosion of the lip. Biopsies are often necessary to differentiate the condition from true cancers.
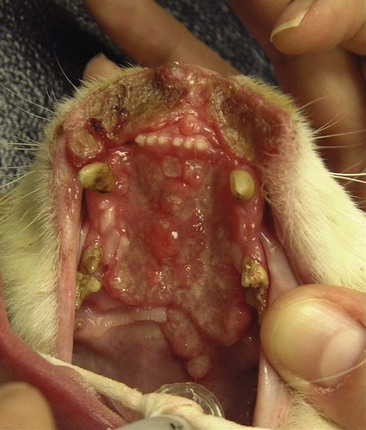
Figure 22-11 An extensive eosinophilic granuloma in a cat involving the lips and hard palate. These lesions can appear aggressive and similar to malignant oral tumors such as squamous cell carcinomas (SCCs). An incisional biopsy is often required to differentiate this benign nonsurgical disease from malignant diseases.
Various treatments are proposed, including (in order of author preference): oral prednisone at 1 to 2 mg/kg twice a day (BID) for 30 days or subcutaneous methylprednisolone acetate* at 20 mg/cat every 2 weeks, megestrol acetate,† hypoallergenic diets, RT, surgery, immunomodulation, or cryosurgery. The prognosis for complete and permanent recovery is fair, although rare cases may undergo spontaneous regression.
Nasopharyngeal Polyps in Cats
Nasopharyngeal polyps are nonneoplastic, inflammatory masses originating from either the middle ear or eustachian tube and can extend into the external ear canal or nasopharynx.154,155 Young cats are usually affected, with a mean age of 13.6 months in one series of 31 cats.154,156 The cause is unknown, but a viral etiology and congenital abnormality of the branchial arches have been suggested.155 Clinical signs include sneezing, change in voice, swallowing problems, rhinitis, and difficulty in breathing. Firm, fleshy masses can be seen or palpated in the caudal pharynx or above the soft palate. Occasionally, masses can be visualized in the external ear canal.157,158 Radiographs or advanced imaging of the skull may reveal fluid or tissue in the tympanic bullae.159 Traction on the stalk of the inflammatory polyp, either through the oral cavity or external ear canal depending on accessibility, is recommended for initial treatment of inflammatory polyps. However, recurrences have been reported in four of eight cats treated with this method alone.154 A ventral bulla osteotomy is recommended for cats with either recurrent inflammatory polyps or evidence of tympanic bulla involvement on skull imaging. Less than 4% of cats have recurrence of inflammatory polyps following bulla osteotomy.154,158,160
Comparative Aspects161
SCC accounts for the vast majority of oral cancer in humans. Oral tumors are associated with alcohol and tobacco use and usually occur in patients over 40 years old. Patients with oral cancer have an increased risk of developing esophageal and lung cancer. Tumors are staged similar to animals and clinical stage influences both treatment options and prognosis.
Surgery and RT are the only options that provide the opportunity for a cure. Surgery and radiation are occasionally combined, especially since neither modality is likely to achieve a cure rate greater than 70% when used as sole therapy. Chemotherapy has a limited role for control of local disease but has shown promise, often in combination with radiation, for advanced-stage cancer.
Prognosis is strongly correlated to histologic grade, stage, and site. Metastasis, particularly to the regional lymph nodes, is more frequent with tonsillar and pharyngeal SCC and larger sized tumors. Tumors of the pharynx and caudal tongue are associated with a worse prognosis than cancers of the rostral tongue and oral cavity because of the higher incidence of nodal metastasis and difficulty in controlling disease once it has spread beyond the primary site.
References
1. Hoyt, RF, Withrow, SJ. Oral malignancy in the dog. J Am Anim Hosp Assoc. 1984;20:83.
2. Bronden, LB, Eriksen, T, Kristensen, AT. Oral malignant melanomas and other head and neck neoplasms in Danish dogs: data from the Danish Veterinary Cancer Registry. Acta Vet Scand. 2009;51:54.
3. Stebbins, KE, Morse, CC, Goldschmidt, MH. Feline oral neoplasia: a ten year survey. Vet Pathol. 1989;26:121.
4. Dorn, CR, Taylor, DON, Frye, FL, et al. Survey of animal neoplasms in Alameda and Contra Costa Counties, California. I. Methodology and description of cases. J Natl Cancer Inst. 1968;40:295.
5. Dorn, CR, Taylor, DON, Schneider, R, et al. Survey of animal neoplasms in Alameda and Contra Costa Counties, California. II. Cancer morbidity in dogs and cats from Alameda County. J Natl Cancer Inst. 1968;40:307.
6. Cohen, D, Brodey, RS, Chen, SM. Epidemiologic aspects of oral and pharyngeal neoplasms in the dog. Am J Vet Res. 1964;25:1776.
7. Kudnig, ST, Ehrhart, N, Withrow, SJ, et al. Survival analysis of oral melanoma in dogs. Vet Cancer Soc Proc. 2003;23:39.
8. Dorn, CR, Priester, WA. Epidemiologic analysis of oral and pharyngeal cancer in dogs, cats, horses and cattle. J Am Vet Med Assoc. 1976;169:1202.
9. Ramos-Vara, JA, Beissenherz, ME, Miller, MA, et al. Retrospective study of 338 canine oral melanomas with clinical, histologic, and immunohistochemical review of 129 cases. Vet Pathol. 2000;37:597.
10. Todoroff, RJ, Brodey, RS. Oral and pharyngeal neoplasia in the dog: a retrospective survey of 361 cases. J Am Vet Med Assoc. 1979;175:567.
11. Withrow, SJ, Holmberg, DL. Mandibulectomy in the treatment of oral cancer. J Am Anim Hosp Assoc. 1983;19:273.
12. Withrow, SJ, Nelson, AW, Manley, PA, et al. Premaxillectomy in the dog. J Am Anim Hosp Assoc. 1985;21:49.
13. White, RAS, Gorman, NT, Watkins, SB, et al. The surgical management of bone-involved oral tumours in the dog. J Small Anim Pract. 1985;26:693.
14. Bradley, RL, MacEwen, EG, Loar, AS. Mandibular resection for removal of oral tumors in 30 dogs and 6 cats. J Am Vet Med Assoc. 1984;184:460.
15. Salisbury, SK, Richardson, DC, Lantz, GC. Partial maxillectomy and premaxillectomy in the treatment of oral neoplasia in the dog and cat. Vet Surg. 1986;15:16.
16. Salisbury, SK, Lantz, GC. Long-term results of partial mandibulectomy for treatment of oral tumors in 30 dogs. J Am Anim Hosp Assoc. 1988;24:285.
17. White, RAS. Mandibulectomy and maxillectomy in the dog: long-term survival in 100 cases. J Small Anim Pract. 1991;32:69.
18. Kosovsky, JK, Matthiesen, DT, Marretta, SM, et al. Results of partial mandibulectomy for the treatment of oral tumors in 142 dogs. Vet Surg. 1991;20:397.
19. Wallace, J, Matthiesen, DT, Patnaik, AK. Hemimaxillectomy for the treatment of oral tumors in 69 dogs. Vet Surg. 1992;21:337.
20. Schwarz, PD, Withrow, SJ, Curtis, CR, et al. Mandibular resection as a treatment for oral cancer in 81 dogs. J Am Anim Hosp Assoc. 1991;27:601.
21. Schwarz, PD, Withrow, SJ, Curtis, CR, et al. Partial maxillary resection as a treatment for oral cancer in 61 dogs. J Am Anim Hosp Assoc. 1991;27:617.
22. Richardson, RC. Canine transmissible venereal tumor. Compend Contin Educ Pract Vet. 1981;3:951.
23. Straw, RC, LeCouteur, RA, Powers, BE, et al. Multilobular osteochondrosarcoma of the canine skull: 16 cases (1978-1988). J Am Vet Med Assoc. 1989;195:1764.
24. Dernell, WS, Straw, RC, Cooper, MF, et al. Multilobular osteochondrosarcoma in 39 dogs: 1979-1993. J Am Anim Hosp Assoc. 1998;34:11.
25. Farrelly, J, Denman, DL, Hohenhaus, AE, et al. Hypofractionated radiation therapy of oral melanoma in five cats. Vet Radiol Ultrasound. 2004;45:91.
26. Overley, B, Goldschmidt, M, Shofer, F, et al. Canine oral melanoma: a retrospective study. Vet Cancer Soc Proc. 2001;21:43.
27. William, LE, Packer, RA. Association between lymph node size and metastasis in dogs with oral malignant melanoma: 100 cases (1987-2001). J Am Vet Med Assoc. 2003;222:1234.
28. Freeman, KP, Hahn, KA, Harris, FD, et al. Treatment of dogs with oral melanoma by hypofractionated radiation therapy and platinum-based chemotherapy (1987-1997). J Vet Intern Med. 2003;17:96.
29. Brewer, WG, Turrel, JM. Radiotherapy and hyperthermia in the treatment of fibrosarcomas in the dog. J Am Vet Med Assoc. 1982;181:146.
30. Bateman, KE, Catton, PA, Pennock, PW, et al. Radiation therapy for the treatment of canine oral melanoma. J Vet Intern Med. 1994;8:267.
31. Blackwood, L, Dobson, JM. Radiotherapy of oral malignant melanomas in dogs. J Am Vet Med Assoc. 1996;209:98.
32. Théon, AP, Rodriguez, C, Madewell, BR. Analysis of prognostic factors and patterns of failure in dogs with malignant oral tumors treated with megavoltage irradiation. J Am Vet Med Assoc. 1997;210:778.
33. MacEwen, EG, Patnaik, AK, Harvey, HJ, et al. Canine oral melanoma: comparison of surgery versus surgery plus Corynebacterium parvum. Cancer Invest. 1986;4:397.
34. MacEwen, EG, Kurzman, ID, Vail, DM, et al. Adjuvant therapy for melanoma in dogs: results of randomized clinical trials using surgery, liposome-encapsulated muramyl tripeptide and granulocyte-macrophage colony-stimulating factor. Clin Cancer Res. 1999;5:4249.
35. Harvey, HJ, MacEwen, GE, Braun, D, et al. Prognostic criteria for dogs with oral melanoma. J Am Vet Med Assoc. 1981;178:580.
36. Owen, LN. TNM classification of tumors in domestic animals. Geneva: WHO; 1980.
37. Proulx, DR, Ruslander, DM, Dodge, RK, et al. A retrospective analysis of 140 dogs with oral melanoma treated with external beam radiation. Vet Radiol Ultrasound. 2003;44:352.
38. Hahn, KA, DeNicola, DB, Richardson, RC, et al. Canine oral malignant melanoma: prognostic utility of an alternative staging system. J Small Anim Pract. 1994;35:251.
39. Moore, AS, Theilen, GH, Newell, AD, et al. Preclinical study of sequential tumor necrosis factor and interleukin 2 in the treatment of spontaneous canine neoplasms. Cancer Res. 1991;51:233.
40. Elmslie, RE, Dow, SW, Potter, TA. Genetic immunotherapy of canine oral melanoma. Vet Cancer Soc Proc. 1994;14:111.
41. Elmslie, RE, Potter, TA, Dow, SW. Direct DNA injection for the treatment of malignant melanoma. Vet Cancer Soc Proc. 1995;15:52.
42. Dow, SW, Elmslie, RE, Willson, AP, et al. In vivo tumor transfection with superantigen plus cytokine genes induces tumor regression and prolongs survival in dogs with malignant melanoma. J Clin Invest. 1998;101:2406.
43. Hogge, G, Burkholder, J, Culp, J, et al. Development of human granulocyte-macrophage colony-stimulating factor-transfected tumor cell vaccines for the treatment of spontaneous cancer. Human Gene Ther. 1998;9:1851.
44. Quintin-Colonna, F, Devauchelle, P, Fradelizi, D, et al. Gene therapy of spontaneous canine melanoma and feline fibrosarcoma by intratumoral administration of histoincompatible cells expressing human interleukin-2. Gene Ther. 1996;3:1104.
45. Bergman, PJ, Camps-Palau, MA, McKnight, JA, et al. Development of a xenogeneic DNA vaccine program for canine malignant melanoma with prolongation of survival in dogs with locoregionally controlled stage II-III disease. Vet Cancer Soc Proc. 2003;23:40.
46. Bergman, PJ, McKnight, J, Novosad, A, et al. Long-term survival of dogs with advanced malignant melanoma after DNA vaccination with xenogeneic human tyrosinase: a phase I trial. Clin Cancer Res. 2003;9:1284.
47. Bergman, PJ, Camps-Palau, MA, McKnight, JA, et al. Phase I & IB trials of murine tyrosinase ± human GM-CSF DNA vaccination in dogs with advanced malignant melanoma. Vet Cancer Soc Proc. 2004;24:55.
48. Modiano, JF, Ritt, MG, Wojcieszyn, J. The molecular basis of canine melanoma: pathogenesis and trends in diagnosis and therapy. J Vet Intern Med. 1999;13:163.
49. Sulaimon, SS, Kitchell, BE. The basic biology of malignant melanoma: molecular mechanisms of disease progression and comparative aspects. J Vet Intern Med. 2003;17:760.
50. Bertone, ER, Snyder, LA, Moore, AS, et al. Environmental and lifestyle risk factors for oral squamous cell carcinoma in domestic cats. J Vet Intern Med. 2003;17:557.
51. Snyder, LA, Bertone, ER, Jakowski, RM, et al. p53 expression and environmental tobacco smoke exposure in feline oral squamous cell carcinoma. Vet Pathol. 2004;41:209.
52. Martin, CK, Tannehill-Gregg, SH, Wolfe, TD, et al. Bone-invasive oral squamous cell carcinoma in cats: pathology and expression of parathyroid hormone-related protein. Vet Pathol. 2011;48:302.
53. Hutson, CA, Willauer, CC, Walder, EJ, et al. Treatment of mandibular squamous cell carcinoma in cats by use of mandibulectomy and radiotherapy: seven cases (1987-1989). J Am Vet Med Assoc. 1992;201:777.
54. Ciekot, PA, Powers, BE, Withrow, SJ, et al. Histologically low grade yet biologically high grade fibrosarcomas of the mandible and maxilla of 25 dogs (1982-1991). J Am Vet Med Assoc. 1994;204:610.
55. Colgin, LMA, Schulman, FY, Dubielzig, RR. Multiple epulides in 13 cats. Vet Pathol. 2001;38:227.
56. Dubielzig, RR. Proliferative dental and gingival disease of dogs and cats. J Am Anim Hosp Assoc. 1982;18:577.
57. Bjorling, DE, Chambers, JN, Mahaffey, EA. Surgical treatment of epulides in dogs: 25 cases (1974-1984). J Am Vet Med Assoc. 1987;190:1315.
58. Yoshida, K, Yanai, T, Iwasaki, T, et al. Clinicopathological study of canine oral epulides. J Vet Med Sci. 1999;61:897.
59. Fiani, N, Vertstraete, FJ, Kass, PH, et al. Clinicopathologic characterization of odontogenic tumors and focal fibrous hyperplasia in dogs: 152 cases (1995-2005). J Am Vet Med Assoc. 2011;238:495.
60. White, RAS, Gorman, NT. Wide local excision of acanthomatous epulides in the dog. Vet Surg. 1989;18:12.
61. Thrall, DE. Orthovoltage radiotherapy of acanthomatous epulides in 39 dogs. J Am Vet Med Assoc. 1984;184:826.
62. Reeves, NCP, Turrel, JM, Withrow, SJ. Oral squamous cell carcinoma in the cat. J Am Anim Hosp Assoc. 1993;29:438.
63. Madewell, BR, Ackerman, N, Sesline, DH. Invasive carcinoma radiographically mimicking primary bone cancer in the mandibles of two cats. J Am Vet Radiol Soc. 1976;27:213.
64. Padgett, SL, Tillson, DM, Henry, CJ, et al. Gingival vascular hamartoma with associated paraneoplastic hyperglycemia in a kitten. J Am Vet Med Assoc. 1997;210:914.
65. Dhaliwal, RS, Kitchell, BE, Marretta, SM. Oral tumors in dogs and cats. Part I. Diagnosis and clinical signs. Compend Contin Educ Pract Vet. 1998;20:1011.
66. Gendler, A, Lewis, JR, Reetz, JA, et al. Computed tomographic features of oral squamous cell carcinoma in cats: 18 cases (2002-2008). J Am Vet Med Assoc. 2010;236:319.
67. Smith, MM. Surgical approach for lymph node staging of oral and maxillofacial neoplasms in dogs. J Am Anim Hosp Assoc. 1995;31:514.
68. Herring, ES, Smith, MM, Robertson, JL. Lymph node staging of oral and maxillofacial neoplasms in 31 dogs and cats. J Vet Dent. 2002;19:122.
69. Lurie, DM, Seguin, B, Verstraete, FJ, et al. Contrast-assisted ultrasound for sentinel node detection in canine head and neck neoplasia. Invest Radiol. 2006;41:415.
70. Kirpensteijn, J, Withrow, SJ, Straw, RC. Combined resection of the nasal planum and premaxilla in three dogs. Vet Surg. 1994;23:341.
71. Lascelles, BD, Thomson, MJ, Dernell, WS, et al. Combined dorsolateral and intraoral approach for the resection of tumors of the maxilla in the dog. J Am Anim Hosp Assoc. 2003;39:294.
72. Lascelles, BDX, Henderson, RA, Seguin, B, et al. Bilateral rostral maxillectomy and nasal planectomy for large rostral maxillofacial neoplasms in six dogs and one cat. J Am Anim Hosp Assoc. 2004;40:137.
73. Fox, LE, Geoghegan, SL, Davis, LH, et al. Owner satisfaction with partial mandibulectomy or maxillectomy for treatment of oral tumors in 27 dogs. J Am Anim Hosp Assoc. 1997;33:25.
74. Boudrieau, RJ, Tidwell, AS, Ullman, SL, et al. Correction of mandibular nonunion and malocclusion by plate fixation and autogenous cortical bone grafts in two dogs. J Am Vet Med Assoc. 1994;204:774.
75. Boudrieau, RJ, Mitchell, SL, Seeherman, H. Mandibular reconstruction of a partial hemimandibulectomy in a dog with severe malocclusion. Vet Surg. 2004;33:119.
76. Bracker, KE, Trout, NJ. Use of a free cortical ulnar autograft following en bloc resection of a mandibular tumor. J Am Anim Hosp Assoc. 2000;36:76.
77. Arzi, B, Verstraete, FJ. Mandibular rim excision in seven dogs. Vet Surg. 2010;39:226.
78. Northrup, NC, Selting, KA, Rassnick, KM, et al. Outcomes of cats with oral tumors treated with mandibulectomy. J Am Anim Hosp Assoc. 2006;42:350.
79. Bar-Am, Y, Verstraete, FJM. Elastic training for the prevention of mandibular drift following mandibulectomy in dogs: 18 cases (2005-2008). Vet Surg. 2010;39:574.
80. Théon, AP, Rodriguez, C, Griffey, S, et al. Analysis of prognostic factors and patterns of failure in dogs with periodontal tumors treated with megavoltage irradiation. J Am Vet Med Assoc. 1997;210:785.
81. Fidel, JL, Sellon, RK, Houston, RK, et al. A nine-day accelerated radiation protocol for feline squamous cell carcinoma. Vet Radiol Ultrasound. 2007;48:482.
82. Forrest, LJ, Chun, R, Adams, WM, et al. Postoperative radiation therapy for canine soft tissue sarcoma. J Vet Intern Med. 2000;14:578.
83. Thrall, DE. Orthovoltage radiotherapy of oral fibrosarcomas in dogs. J Am Vet Med Assoc. 1981;172:159.
84. Evans, SM, LaCreta, F, Helfand, S, et al. Technique, pharmacokinetics, toxicity, and efficacy of intratumoral etanidazole and radiotherapy for treatment of spontaneous feline oral squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 1991;20:703.
85. Personal Communication LaRue, SM, Vail, DM, Ogilvie, GK, et al. Shrinking-field radiation therapy in combination with mitoxantrone chemotherapy for the treatment of oral squamous cell carcinoma in the cat. Vet Cancer Soc Proc. 1991;11:99.
86. Ogilvie, GK, Moore, AS, Obradovich, JE, et al. Toxicoses and efficacy associated with administration of mitoxantrone to cats with malignant tumor. J Am Vet Med Assoc. 1993;202:1839.
87. Jones, PD, de Lorimier, LP, Kitchell, BE, et al. Gemcitabine as a radiosensitizer for nonresectable feline oral squamous cell carcinoma. J Am Anim Hosp Assoc. 2003;39:463.
88. Fidel, J, Lyons, J, Tripp, C, et al. Treatment of oral squamous cell carcinoma with accelerated radiation therapy and concomitant carboplatin in cats. J Vet Intern Med. 2011;25:504.
89. LeBlanc, AL, LaDue, TA, Turrel, JM, et al. Unexpected toxicity following use of gemcitabine as a radiosensitizer in head and neck carcinomas: a Veterinary Radiation Therapy Oncology Group pilot study. Vet Radiol Ultrasound. 2004;45:466.
90. Turrel, JM. Principles of radiation therapy. In: Thielen GH, Madewell BR, eds. Veterinary cancer medicine. Philadelphia: Lea & Febiger, 1987.
91. Overgaard, J, Overgaard, M, Hansen, PV, et al. Some factors of importance in the radiation treatment of malignant melanoma. Radiother Oncol. 1986;5:183.
92. Roberts, SM, Lavach, SD, Severin, GA, et al. Ophthalmic complications following megavoltage irradiation of the nasal and paranasal cavities in dogs. J Am Vet Med Assoc. 1987;190:43.
93. Jamieson, VE, Davidson, MG, Naisse, MP, et al. Ocular complications following cobalt 60 radiotherapy of neoplasms in the canine head region. J Am Anim Hosp Assoc. 1991;27:51.
94. LaRue, SM, Gillette, EL. Radiation therapy. In: Withrow SJ, MacEwen EG, eds. Small animal clinical oncology. Philadelphia: Saunders, 2001.
95. Thrall, DE, Goldschmidt, MH, Biery, DN. Malignant tumor formation at the site of previously irradiated acanthomatous epulides in four dogs. J Am Vet Med Assoc. 1981;178:127.
96. Gillette, EL, McChesney, SL, Dewhirst, MW, et al. Response of canine oral carcinomas to heat and radiation. Int J Radiat Oncol Biol Phys. 1987;13:1861.
97. Dewhirst, MW, Sim, DA, Forsyth, K, et al. Local control and distant metastases in primary canine malignant melanomas treated with hyperthermia and/or radiotherapy. Int J Hyperthermia. 1985;1:219.
98. Hayes, A, Scase, T, Miller, J, et al. COX-1 and COX-2 expression in feline oral squamous cell carcinoma. J Comp Pathol. 2006;135:93.
99. DiBernardi, L, Dore, M, Davis, JA, et al. Study of feline oral squamous cell carcinoma: potential target for cyclooxygenase inhibitor treatment. Prostaglandins Leukot Essent Fatty Acids. 2007;76:245.
100. Schmidt, BR, Glickman, NW, DeNicola, DB, et al. Evaluation of piroxicam for the treatment of oral squamous cell carcinoma in dogs. J Am Vet Med Assoc. 2001;218:1783.
101. Boria, PA, Murry, DJ, Bennett, PF, et al. Evaluation of cisplatin combined with piroxicam for the treatment of oral malignant melanoma and oral squamous cell carcinoma in dogs. J Am Vet Med Assoc. 2004;224:388.
102. de Vos, JP, Burm, AGD, Focker, BP, et al. Piroxicam and carboplatin as a combination treatment of canine oral non-tonsillar squamous cell carcinoma: a pilot study and a literature review of a canine model of human head and neck squamous cell carcinoma. Vet Comp Oncol. 2005;3:16.
103. Fox, LE, Rosenthal, RC, King, RR, et al. Use of cis-bis-neodecanoato-trans-R,R-1,2-diaminocyclohexane platinum (II), a liposomal cisplatin analogue, in cats with oral squamous cell carcinoma. Am J Vet Res. 2000;61:791.
104. Kitchell, BE, Brown, DM, Luck, EE, et al. Intralesional implant for treatment of primary oral malignant melanoma in dogs. J Am Vet Med Assoc. 1994;204:229.
105. Rassnick, KM, Ruslander, DM, Cotter, SM, et al. Use of carboplatin for treatment of dogs with malignant melanoma: 27 cases (1989-2000). J Am Vet Med Assoc. 2001;218:1444.
106. Page, RL, Thrall, DE, Dewhirst, MW, et al. Phase I study of melphalan alone and melphalan plus whole body hyperthermia in dogs with malignant melanoma. Int J Hyperthermia. 1991;7:559.
107. Reed, SD, Fulmer, A, Buckholz, J, et al. Bleomycin/interleukin-12 electrochemogenetherapy for treating naturally occurring spontaneous neoplasms in dogs. Cancer Gene Ther. 2010;17:571.
108. Spugnini, EP, Dragonetti, E, Vincenzi, B, et al. Pulse-mediated chemotherapy enhances local control and survival in a spontaneous canine model of primary mucosal melanoma. Melanoma Res. 2006;16:23.
109. Esplin, DG. Survival of dogs following surgical excision of histologically well-differentiated melanocytic neoplasms of the mucous membranes of the lips and oral cavity. Vet Pathol. 2008;45:889.
110. Spangler, WL, Kass, PH. The histologic and epidemiologic bases for prognostic considerations in canine melanocytic neoplasia. Vet Pathol. 2006;43:136.
111. MacMillan, R, Withrow, SJ, Gillette, EL. Surgery and regional irradiation for treatment of canine tonsillar squamous cell carcinoma: retrospective review of eight cases. J Am Anim Hosp Assoc. 1982;18:311.
112. Beck, ER, Withrow, SJ, McChesney, AE, et al. Canine tongue tumors: a retrospective review of 57 cases. J Am Anim Hosp Assoc. 1986;22:525.
113. Brooks, MB, Matus, RE, Leifer, CE, et al. Chemotherapy versus chemotherapy plus radiotherapy in the treatment of tonsillar squamous cell carcinoma in the dog. J Vet Intern Med. 1988;2:206.
114. McCaw, DL, Pope, ER, Payne, JT, et al. Treatment of canine oral squamous cell carcinomas with photodynamic therapy. Br J Cancer. 2000;82:1297.
115. Evans, SM, Shofer, F. Canine oral nontonsillar squamous cell carcinoma. Vet Radiol. 1988;29:133.
116. LaDue-Miller, T, Price, S, Page, RL, et al. Radiotherapy for canine non-tonsillar squamous cell carcinoma. Vet Radiol Ultrasound. 1996;37:74.
117. Bostock, DE. The prognosis in cats bearing squamous cell carcinoma. J Small Anim Pract. 1972;13:119.
118. Cotter, SM. Oral pharyngeal neoplasms in the cat. J Am Anim Hosp Assoc. 1981;17:917.
119. Hayes, AM, Adams, VJ, Scase, TJ, et al. Survival of 54 cats with oral squamous cell carcinoma in United Kingdom general practice. J Small Anim Pract. 2007;48:394.
120. Bregazzi, VS, LaRue, SM, Powers, BE, et al. Response of feline oral squamous cell carcinoma to palliative radiation therapy. Vet Radiol Ultrasound. 2001;42:77.
121. Nagata, K, Selting, KA, Cook, CR, et al. 90Sr therapy for oral squamous cell carcinoma in two cats. Vet Radiol Ultrasound. 2011;52:114.
122. Hilmas, DE, Gillette, EL. Radiotherapy of spontaneous fibrous connective-tissue sarcomas in animals. J Natl Cancer Inst. 1976;56:365.
123. Creasey, WA, Phil, D, Thrall, DE. Pharmacokinetic and anti-tumor studies with the radiosensitizer misonidazole in dogs with spontaneous fibrosarcomas. Am J Vet Res. 1982;43:1015.
124. Heyman, SJ, Diefenderfer, DL, Goldschmidt, MH, et al. Canine axial skeletal osteosarcoma: a retrospective study of 116 cases (1986 to 1989). Vet Surg. 1992;21:304.
125. Hammer, AS, Weeren, FR, Weisbrode, SE, et al. Prognostic factors in dogs with osteosarcomas of the flat and irregular bones. J Am Anim Hosp Assoc. 1995;31:321.
126. Straw, RC, Powers, BE, Klausner, J, et al. Canine mandibular osteosarcoma: 51 cases (1980-1992). J Am Anim Hosp Assoc. 1996;32:257.
127. Kazmierski, KJ, Dernell, WS, Lafferty, MH, et al. Osteosarcoma of the canine head: a retrospective study of 60 cases. Vet Cancer Soc Proc. 2002;22:30.
128. Bostock, DE, White, RAS. Classification and behaviour after surgery of canine epulides. J Comp Pathol. 1987;97:197.
129. Yoshida, K, Watarai, Y, Sakai, Y, et al. The effect of intralesional bleomycin on canine acanthomatous epulis. J Am Anim Hosp Assoc. 1998;34:457.
130. Reif, JS, Cohen, D. The environmental distribution of canine respiratory tract neoplasms. Arch Environ Health. 1971;22:136.
131. Buhles, WC, Theilan, GH. Preliminary evaluation of bleomycin in feline and canine squamous cell carcinomas. Am J Vet Res. 1973;34:289.
132. Mas, A, Blackwood, L, Cripps, P, et al. Canine tonsillar squamous cell carcinoma: a multicentre retrospective review of 44 clinical cases. J Small Anim Pract. 2011;52:359.
133. Dennis, MM, Ehrhart, N, Duncan, CG, et al. Frequency of and risk factors associated with lingual lesions in dogs: 1,196 cases (1995-2004). J Am Vet Med Assoc. 2006;228:1533.
134. Carpenter, LG, Withrow, SJ, Power, BE, et al. Squamous cell carcinoma of the tongue in 10 dogs. J Am Anim Hosp Assoc. 1993;29:17.
135. Brodey, RS. A clinical and pathologic study of 130 neoplasms of the mouth and pharynx in the dog. Am J Vet Res. 1960;21:787.
136. Solano, M, Penninck, DG. Ultrasonography of the canine, feline and equine tongue: normal findings and case history reports. Vet Radiol Ultrasound. 1996;37:206.
137. Dvorak, LD, Beaver, DP, Ellison, GW, et al. Major glossectomy in dogs: a case series and proposed classification system. J Am Anim Hosp Assoc. 2004;40:331.
138. Turk, MAM, Johnson, GC, Gallina, AM. Canine granular cell tumour (myoblastoma): a report of four cases and review of the literature. J Small Anim Pract. 1983;24:637.
139. Patnaik, AK, Lieberman, PH, Erlandson, RA, et al. A clinicopathologic and ultrastructural study of undifferentiated malignant tumors of the oral cavity in dogs. Vet Pathol. 1986;23:170.
140. Ogilvie, GK, Sundberg, JP, O’Banion, MK, et al. Papillary squamous cell carcinoma in three young dogs. J Am Vet Med Assoc. 1988;192:933.
141. Norris, AM, Withrow, SJ, Dubielzig, RR. Oropharyngeal neoplasms. In: Harvey CE, ed. Oral disease in the dog and cat: veterinary dentistry. Philadelphia: Saunders, 1985.
142. Calvert, CA. Canine viral and transmissible neoplasias. In: Greene CE, ed. Clinical microbiology and infectious diseases of the dog and cat. Philadelphia: Saunders, 1984.
143. Poulet, FM, Valentine, BA, Summers, BA. A survey of epithelial odontogenic tumors and cysts in dogs and cats. Vet Pathol. 1992;29:369.
144. Dubielzig, RR, Adams, WM, Brodey, RS. Inductive fibroameloblastoma, an unusual dental tumor of young cats. J Am Vet Med Assoc. 1979;174:720.
145. Dernell, WS, Hullinger, GH. Surgical management of ameloblastic fibroma in the cat. J Small Anim Pract. 1994;35:35.
146. Figueiredo, C, Barros, HM, Alvares, LC, et al. Composed complex odontoma in a dog. Vet Med Small Anim Clin. 1974;69:268.
147. Potter, KA, Tucker, RD, Carpenter, JL. Oral eosinophilic granuloma of Siberian huskies. J Am Anim Hosp Assoc. 1980;16:595.
148. Madewell, BR, Stannard, AA, Pulley, LT, et al. Oral eosinophilic granuloma in Siberian husky dogs. J Am Vet Med Assoc. 1980;177:701.
149. Bredal, WP, Gunnes, G, Vollset, I, et al. Oral eosinophilic granuloma in three Cavalier King Charles spaniels. J Small Anim Pract. 1996;37:499.
150. Scott, DW. Chapter 11: Disorders of unknown or multiple origin. J Am Anim Hosp Assoc. 1980;16:406.
151. McClelland, RB. X-ray therapy in labial and cutaneous granulomas in cats. J Am Vet Med Assoc. 1954;125:469.
152. MacEwen, EG, Hess, PW. Evaluation of effect of immunomodulation on the feline eosinophilic granuloma complex. J Am Anim Hosp Assoc. 1987;23:519.
153. Song, MD. Diagnosing and treating feline eosinophilic granuloma complex. Vet Med. 1994;December:1141.
154. Kapatkin, AS, Matthiesen, DT, Noone, KE, et al. Results of surgery and long-term follow-up in 31 cats with nasopharyngeal polyps. J Am Anim Hosp Assoc. 1990;26:387.
155. Kudnig, ST. Nasopharyngeal polyps in cats. Clin Tech Small Anim Pract. 2002;17:174.
156. Bradley, RL, Noone, KE, Saunders, GK, et al. Nasopharyngeal and middle ear polypoid masses in five cats. Vet Surg. 1985;14:141.
157. Harvey, CE, Goldschmidt, MH. Inflammatory polypoid growths in the ear canal of cats. J Small Anim Pract. 1978;19:669.
158. Faulkner, JE, Budsberg, SC. Results of ventral bulla osteotomy for treatment of middle ear polyps in cats. J Am Anim Hosp Assoc. 1990;26:496.
159. Parker, NR, Binnington, AG. Nasopharyngeal polyps in cats: three case reports and a review of the literature. J Am Anim Hosp Assoc. 1985;21:473.
160. Trevor, PB, Martin, RA. Tympanic bulla osteotomy for treatment of middle ear disease in cats: 19 cases (1984-1991). J Am Vet Med Assoc. 1993;202:123.
161. Mendenhall, WM, Riggs, CE, Cassissi, NJ. Treatment of head and neck cancers. In: DeVita RT, Hellman S, Rosenberg SA, eds. Cancer: Principles and practice of oncology. Philadelphia: Lippincott Williams & Wilkins, 2005.
 Section B
Section B
Salivary Gland Cancer
Incidence and Risk Factors
Primary salivary gland cancer is rare in the dog and cat. Most cases are reported in older patients (10 to 12 years of age), and no specific breed or sex predilection has been determined in dogs.1-3 The Siamese cat may be at higher risk than other breeds, with male cats being affected twice as often as female cats.4
Pathology
The vast majority of salivary cancers are adenocarcinomas, but a wide range of specific histologic types including osteosarcoma, mast cell tumor, sebaceous carcinoma, oncocytoma, and malignant fibrous histiocytoma have been reported.1,5-9 They can arise from major (parotid, mandibular, sublingual, zygomatic) or minor accessory glands throughout the oral cavity. Of 245 submissions of salivary tissue for histologic diagnosis in both species, 30% were neoplastic.3 Benign salivary gland tumors, including pleomorphic adenoma, are rare in animals10,11 compared to humans. The mandibular gland is most commonly affected.3,4 Malignancies are variably locally invasive, and metastasis to regional lymph nodes is common (39% for cats and 17% for dogs).4,12 Distant metastasis has been reported (16% for cats and 8% for dogs at presentation) but may be slow to develop.4,13 Benign lipomatous infiltration of the canine salivary gland has also been reported, and surgical excision was curative in all treated cases.14,15
History and Clinical Signs
Symptoms are nonspecific and generally include halitosis, dysphagia, exophthalmus, or a unilateral, firm, painless swelling of the upper neck (mandibular and sublingual), base of the ear (parotid), upper lip or maxilla (zygomatic), or mucous membrane of the lip or tongue (accessory salivary tissue). Major differential diagnoses are mucoceles, abscesses, salivary gland infarction, sialadenitis, lymphoma, or reactive or metastatic lymphadenopathy.3,16
Diagnostic Techniques and Work-Up
Fine-needle aspiration (FNA) cytology of masses in these locations should help differentiate noncancer diseases from cancer. Regional radiographs will usually be normal but may reveal periosteal reaction on adjacent bones or displacement of surrounding structures. CT imaging may be helpful in determining extent of disease and infiltration into surrounding structures. Needle core or wedge biopsies will usually be necessary to make a definitive diagnosis.
Therapy
When possible, aggressive surgical removal should be performed. Unfortunately, many lesions are extracapsular and widely extensive throughout the regional area, which contains numerous vital structures. However, complete extirpation of the ipsilateral neck can be performed with good functional outcome. The primary clinical impairment after a “radical” neck resection is the inability to blink the eye, which can be managed with a tarsorrhaphy or eye drops. If this surgery can be performed to the level of microscopic residual disease and RT is planned postoperatively, this complication is acceptable.
Postoperative RT resulted in good local control and prolonged survival in three reported cases.6 Chemotherapy for salivary gland adenocarcinoma has been largely unreported.
Prognosis
The prognosis for salivary gland cancer is generally unknown. Clinical experience on a limited number of cases would indicate that aggressive local resection (usually histologically incomplete) followed by adjuvant radiation can attain permanent local control and long-term survival.4,17 Incomplete removal will invariably result in local recurrence.2 Histologic grade was not prognostic for outcome, whereas advanced stage was a negative prognosticator.4 Median survival for 24 dogs and 30 cats treated with surgical resection with or without adjuvant radiation was 550 and 516 days, respectively.4 The prognosis for long-term survival for cats is worse than for dogs.4,18 Another report of six dogs with salivary carcinoma treated with surgery alone had a median survival of only 74 days, and all six dogs developed pulmonary metastasis.19
Comparative Aspects20
Salivary gland tumors are more common in older humans than in animals and account for 4% of head and neck neoplasms. The parotid gland is most commonly affected. A wide variety of benign and malignant neoplasms are recognized. Treatment is with surgical excision, and radiation is utilized for inoperable disease or after incomplete removal. Five-year survival usually exceeds 75% but depends on stage and histologic type.
References
1. Koestner, A, Buerger, L. Primary neoplasms of the salivary glands in animals compared to similar tumors in man. Pathol Vet. 1965;2:201–226.
2. Carberry, CA, Glanders, JA, Harvey, HJ, et al. Salivary gland tumors in dogs and cats: a literature and case review. J Am Anim Hosp Assoc. 1988;24:561–567.
3. Spangler, WL, Culbertson, MR. Salivary gland disease in dogs and cats: 245 cases (1985-1988). J Am Vet Med Assoc. 1991;198:465–469.
4. Hammer, A, Getzy, D, Ogilvie, G, et al. Salivary gland neoplasia in the dog and cat: survival times and prognostic factors. J Am Vet Med Assoc. 2001;37:478–482.
5. Thomsen, BV, Myers, RK. Extraskeletal osteosarcoma of the mandibular salivary gland in a dog. Vet Pathol. 1999;36:71–73.
6. Carberry, CA, Flanders, JA, Anderson, WI, et al. Mast cell tumor in the mandibular salivary gland in a dog. Cornell Vet. 1987;77:362–366.
7. Sozmen, M, Brown, PJ, Eveson, JW. Sebaceous carcinoma of the salivary gland in a cat. J Vet Med A Physiol Pathol Clin Med. 2002;49:425–427.
8. Perez-Martinez, C, Garcia Fernandez, RA, Reyes Avila, LE, et al. Malignant fibrous histiocytoma (giant cell type) associated with a malignant mixed tumor in the salivary gland of a dog. Vet Pathol. 2000;37:350–353.
9. Brocks, BA, Peeters, ME, Kimpfler, S. Oncocytoma in the mandibular salivary gland of the cat. J Feline Med Surg. 2008;10:188–191.
10. Boydell, P, Pike, R, Crossley, D, et al. Sialadenosis in dogs. J Am Vet Med Assoc. 2000;216:872–874.
11. Canapp, SO, Jr., Cohn, LA, Maggs, DJ, et al. Xerostomia, xerophthalmia, and plasmacytic infiltrates of the salivary glands (Sjögren’s-like syndrome) in a cat. J Am Vet Med Assoc. 2001;218:59–65.
12. Mazzullo, G, Sfacteria, A, Ianelli, N, et al. Carcinoma of the submandibular salivary glands with multiple metastases in a cat. Vet Clin Pathol. 2005;34:61–64.
13. Sozmen, M, Brown, PJ, Eveson, JW. Salivary duct carcinoma in five cats. Comp Path. 1999;121:311–319.
14. Kitshoff, AM, Millward, IR, Williams, JH, et al. Infiltrative angiolipoma of the parotid salivary gland in a dog. J S Afr Vet Assoc. 2010;81:258–261.
15. Brown, PJ, Lucke, VM, Sozmen, M, et al. Lipomatous infiltration of the canine salivary gland. J Small Anim Pract. 1997;28:234–236.
16. McGill, S, Lester, N, McLachlan, A, et al. Concurrent sialocele and necrotizing sialadenitis in a dog. J Small Anim Pract. 2009;50:151–156.
17. Evans, SM, Thrall, DE. Postoperative orthovoltage radiation therapy of parotid salivary gland adenocarcinoma in three dogs. J Am Vet Med Assoc. 1983;182:993–994.
18. Volmer, C, Benal, Y, Caplier, F, et al. Atypical vimentin expression in a feline salivary gland adenocarcinoma with widespread metastases. J Vet Med Sci. 2009;71:1681–1684.
19. Hahn, KA, Nolan, ML. Surgical prognosis for canine salivary gland neoplasms. Proc Annu Conf Am Coll Vet Radiol Vet Cancer Soc. 1997.
20. Mendehall, WM, Riggs, CE, Cassisi, NJ. Cancer of the head and neck, section 2. In DeVita VT, ed.: Cancer: principles and practice of oncology, ed 7, Philadelphia: Lippincott Williams & Wilkins, 2005.
 Section C
Section C
Esophageal Cancer
Incidence and Risk Factors
Cancer of the esophagus is very rare and accounts for less than 0.5% of all cancer in the dog and cat.1 Sarcomas, secondary to Spirocerca lupi infestation, have been reported in indigenous areas (Africa, Israel, and the southeastern United States).2-5 No cause is known for the more common carcinomas. Most animals are older, and no gender or breed predilection is evident.
Pathology and Natural Behavior
The more commonly reported histologic types include SCC, leiomyosarcoma, fibrosarcoma, and osteosarcoma. Rarely, benign neoplasms such as leiomyoma, adenomatous polyp,6 and plasmacytoma may be encountered, especially in the area of the terminal esophagus and cardia.7-10 Paraesophageal tumors such as thymic, heart base, or thyroid may also invade the esophagus.1 In cats, SCCs are usually seen in females and are located in the middle third of the esophagus just caudal to the thoracic inlet.10-13 Most esophageal cancers are locally invasive, and metastasis is to draining lymph nodes, direct extension, or hematogenously to distant sites.
History and Clinical Signs
Signs other than those of general debilitation and weight loss include pain on swallowing, dysphagia, and regurgitation of undigested food. Pneumonia, secondary to aspiration, may also be noted. Hypertrophic osteopathy has been reported, especially with Spirocerca lupi–induced sarcomas.2,4 Spirocerca usually affects the caudal thoracic esophagus and can be associated with thoracic spondylitis and a microcytic hypochromic anemia and a neutrophilia.3,4
Diagnostic Techniques and Work-Up
It is generally evident from the history that the patient is suffering from a partial or complete upper gastrointestinal (GI) obstruction. Plain radiographs may reveal retention of gas within the esophageal lumen, a mass, or esophageal dilatation proximal to the cancer. A positive-contrast esophagogram with or without fluoroscopy will generally reveal a stricture or mass lesion in the lumen. Esophagoscopy allows visualization of the lesion, which is frequently ulcerated. Several biopsies should be taken because necrosis and inflammation are often prominent. The risk of esophageal perforation during the biopsy is generally minimal. Leiomyomas appear as circumscribed submucosal masses, with a normal and freely movable mucosa making endoscopic biopsy unrewarding. Advanced imaging such as CT or MRI may be helpful to determine extent of the lesion, especially when minimal mucosal involvement is evident on endoscopic evaluation.
Open surgical biopsy via thoracotomy or cervical exploration is another option to obtain tissue for a diagnosis. Spirocerca lupi ova may be detected in the feces.3
Therapy
Therapy for malignant cancer of the esophagus is difficult at best because of the advanced stage of disease in most cases.14 Intrathoracic resections are further complicated by poor exposure, lengthy resections, tension on the anastomosis, and unique healing problems of the thoracic esophagus. Low-grade leiomyosarcomas can be marginally excised with successful outcomes. They generally do not invade the mucosa and the muscular defect in the esophageal wall can be plicated with good postoperative function.15 For lesions in the caudal esophagus or cardia, gastric advancement through the diaphragm can be considered but carries a high complication rate, including reflux esophagitis.
Various procedures to partially replace the resected esophagus have been described, including microvascular transfer of colon or small bowel, but clinical reports on their use in the cancer patient are lacking.16-18 Chemotherapy has rarely been attempted. RT for the cervical esophagus can be attempted but is of limited value for the intrathoracic esophagus because of the poor tolerance of surrounding normal tissues such as the lung and heart. Short-term palliation can be achieved via esophagotomy or gastrostomy tubes. Benign leiomyomas of the esophagus or cardia can be approached via thoracotomy or celiotomy.9
Prognosis
Except for benign lesions,7,8 lymphoma, or low-grade leiomyosarcoma,15 the prognosis for esophageal tumors (primarily carcinomas) is very poor for cure or palliation due to poor resection options and high metastatic rate. One report describes a series of six dogs treated with partial esophagectomy for esophageal sarcomas associated with Spirocerca. Five of the six also received doxorubicin, and the median survival was 267 days.19
Comparative Aspects20
Esophageal cancer (principally SCC) is rare in humans but still accounts for 7000 deaths per year in the United States. Marked geographic variance in worldwide incidence implies numerous environmental influences on development, including tobacco, alcohol, hot food, and nitrosamines.
Most esophageal cancers have extensive local tumor growth and lymph node involvement precluding curative treatment. Combinations or single use of surgery, radiation, and chemotherapy have resulted in 5-year survivals of less than 20% of the more common advanced-stage disease. A variety of palliative bypass procedures for inoperable disease are also performed (esophagogastrostomy, intraluminal intubation, dilatation, and feeding gastrostomies).
References
1. Ridgeway, RL, Suter, PF. Clinical and radiographic signs in primary and metastatic esophageal neoplasms of the dog. J Am Vet Med Assoc. 1979;174:700–704.
2. Bailey, WS. Spirocerca lupi: a continuing inquiry. J Parasitol. 1972;58:3–22.
3. Ranen, E, Lavy, E, Aizenbert, I, et al. Spirocercosis-associated esophageal sarcomas in dogs: a retrospective study of 17 cases (1997-2003). Vet Parasitol. 2004;119:209–221.
4. Dvir, E, Kirberger, RM, Mukorera, V, et al. Clinical differentiation between dogs with benign and malignant spirocercosis. Vet Parasitol. 2008;155:80–88.
5. Lindsay, N, Kirberger, R, Williams, M. Imaging diagnosis—spinal cord chondrosarcoma associated with spirocercosis in a dog. Vet Radiol Ultrasound. 2010;51:614–616.
6. Gibson, CJ, Parry, NM, Jakowski, RM, et al. Adenomatous polyp with intestinal metaplasia of the esophagus (Barrett esophagus) in a dog. Vet Pathol. 2010;47:116–119.
7. Culbertson, R, Branam, JE, Rosenblatt, LS. Esophageal/gastric leiomyoma in the laboratory beagle. J Am Vet Med Assoc. 1983;183:1168–1171.
8. Hamilton, TA, Carpenter, JL. Esophageal plasmacytoma in a dog. J Am Vet Med Assoc. 1994;204:1210–1211.
9. Rolfe, DS, Twedt, DC, Seim, HB. Chronic regurgitation or vomiting caused by esophageal leiomyoma in three dogs. J Am Anim Hosp Assoc. 1994;30:425–430.
10. Carpenter, JL, Andrews, LN, Holzworth, J. Tumor and tumor-like lesion. In: Holzworth J, ed. Diseases of the cat. Philadelphia: WB Saunders, 1987.
11. Cotchin, E. Neoplasms in cats. Proc R Soc Med. 1952;45:671.
12. Gualtieri, M, Monzeglio, MG, Di Giancamillo, M. Oesophageal squamous cell carcinoma in two cats. J Small Anim Pract. 1999;40:79–83.
13. Shinozuka, J, Nakayama, H, Suzuki, M, et al. Esophageal adenosquamous carcinoma in a cat. J Vet Med Sci. 2001;63:91–93.
14. McCaw, D, Pratt, M, Walshaw, R. Squamous cell carcinoma of the esophagus in a dog. J Am Anim Hosp Assoc. 1980;16:561–563.
15. Farese, JP, Bacon, NJ, Ehrhart, NP, et al. Oesophageal leiomyosarcoma in dogs: surgical management and clinical outcome of four cases. Vet Comp Oncol. 2008;6:31–38.
16. Gregory, CR, Gourley, IM, Bruyette, DS, et al. Free jejunal segment for treatment of cervical esophageal stricture in a dog. J Am Vet Med Assoc. 1988;193:230–232.
17. Kuzma, AB, Holmberg, DL, Miller, CW, et al. Esophageal replacement in the dog by microvascular colon transfer. Vet Surg. 1989;18:439–445.
18. Straw, RC, Tomlinson, JL, Constantinescu, G, et al. Use of a vascular skeletal muscle graft for canine esophageal reconstruction. Vet Surg. 1987;16:155–156.
19. Ranen, E, Shamir, MH, Shahar, R, et al. Partial esophagectomy with single layer closure for treatment of esophageal sarcomas in 6 dogs. Vet Surg. 2004;33:428–434.
20. Posner, MC, Forastiere, AA, Minsky, BD. Cancer of the esophagus. In DeVita VT, Hellman S, Rosenberg SA, eds.: Cancer: principles and practice of oncology, ed 7, Philadelphia: Lippincott Williams & Wilkins, 2005.
 Section D
Section D
Exocrine Pancreatic Cancer
Incidence and Risk Factors
Cancer of the exocrine pancreas is very rare (<0.5% of all cancers) in the dog and the cat.1,2 Older female dogs and spaniels have been described as being at higher risk.3-5 Experimentally, N-ethyl-N′-nitro-N-nitrosoguanidine has been shown to induce pancreatic duct adenocarcinoma when administered intraductally in dogs.6
Pathology and Natural Behavior
Almost all cancers of the pancreas are epithelial and most are adenocarcinoma of ductular or acinar origin. Nodular hyperplasia is a common asymptomatic finding in older dogs or cats. “Benign” pancreatic pseudocysts and adenomas have been diagnosed by ultrasonography or surgery in dogs and cats.2,7 In the vast majority of cases, malignant cancer has metastasized to regional or distant sites before a diagnosis can be made.1,4,8
History and Clinical Signs
The history and clinical signs of exocrine pancreatic cancer are vague and nonspecific and may mimic or be accompanied by pancreatitis. Weight loss and anorexia (marked in cats), paraneoplastic alopecia in cats,9,10 vomiting, rare associated diabetes mellitus,11 abdominal distension due to mass effect or abdominal effusions secondary to tumor implantation on the peritoneum (i.e., carcinomatosis; common in cats), icterus (with common bile duct obstruction), and depression are common symptoms. Alternatively, patients may present for symptoms of metastatic disease.
Diagnostic Techniques and Work-Up
Most hematologic and biochemical evaluations are nonspecific but may include mild anemia, hyperglycemia, neutrophilia, and bilirubinemia (if occluding the common bile duct.).1,2 Elevations of serum amylase and lipase are inconsistent.12 In extreme cases, signs of pancreatic insufficiency may be exhibited.13 Positive-contrast upper GI radiographs may reveal slowed gastric emptying and occasionally compression or invasion of the duodenum.
Ascites may be a clinical sign and, when present, may reveal malignant cells on cytologic examination (carcinomatosis). Flow cytometry can be considered to help distinguish malignant from nonmalignant abdominal effusions. In the dog, most tumors are not palpable through the abdominal wall. In the cat, late stage, large palpable masses may be present.
Ultrasonography should be a useful diagnostic tool for localization of the primary tumor, documentation, and aspiration of fluid, as well as metastasis to liver and regional lymph nodes.14 A large (>2 cm), apparently single mass is suggestive of pancreatic cancer versus nodular hyperplasia in cats.15 The utility of advanced imaging such as CT and MRI has not been documented for pancreatic tumors in veterinary patients. At present, most diagnoses are made at exploratory laparotomy.
Therapy
Most non–islet cell carcinomas of the pancreas are metastatic (regional lymph nodes and liver) or locally and extensively invasive at the time of diagnosis. If the liver, peritoneal cavity, or draining lymph nodes are positive for tumor, aggressive surgery should generally not be performed. Complete pancreatectomy or pancreaticoduodenectomy (Whipple’s procedure) has been described in humans and dogs,16 but it carries a high operative morbidity and mortality without significant cure rates. Palliative GI bypass (gastrojejunostomy) is a short-term option if bowel obstruction is imminent. Radiation and chemotherapy have shown limited value in humans and animals. Occasionally, uncomfortable effusions from carcinomatosis can be diminished with systemic or intracavitary chemotherapy (see Chapter 11); however, the palliative response tends to be short lived.
Prognosis
The outlook for this disease in animals is very poor due to its critical location and advanced stage at diagnosis. One-year survival after diagnosis, regardless of treatment, has not been reported.
Comparative Aspects17
Pancreatic exocrine carcinoma accounts for more than 27,000 deaths per year in the United States. Most patients have disease progression beyond the pancreas at the time of initial diagnosis. Seventy-five percent are located in the head of the pancreas and the remainder in the body and tail. Direct extension to duodenum, bile duct, and stomach, as well as common metastasis to lymph node and liver, make treatment difficult.
When possible, pancreaticoduodenectomy (Whipple’s procedure) or complete pancreatectomy are the treatments of choice. However, operative mortality ranges from 5% to 30%. Palliative bypass of the biliary tree and duodenum is commonly performed for inoperable lesions.
Traditional external beam RT is generally palliative rather than curative. Intraoperative and interstitial radiation are being explored as means for high-dose delivery to the tumor while sparing normal radiosensitive structures.18,19 Chemotherapy alone (especially the taxols and gemcitabine) or in combination with radiation or surgery has demonstrated some improvement in quality of life, yet only very modest improvements in survival. Overall, 5-year survival for all patients remains less than 5%.
References
1. Davenport D: Pancreatic carcinoma in twenty dogs and five cats, Personal communication.
2. Seaman, RL. Exocrine pancreatic neoplasia in the cat: a case series. J Am Anim Hosp Assoc. 2004;40:238–245.
3. Kircher, CH, Nielsen, SW. Tumours of the pancreas. Bull WHO. 1976;53:195–202.
4. Anderson, NV, Johnson, KH. Pancreatic carcinoma in the dog. J Am Vet Med Assoc. 1967;150:286–295.
5. Brown, PJ, Mason, KV, Merrett, DJ, et al. Multifocal necrotizing steatitis associated with pancreatic carcinoma in three dogs. J Small Anim Pract. 1994;35:129–132.
6. Kamano, T, Azuma, N, Katami, A, et al. Preliminary observation on pancreatic duct adenocarcinoma induced by intraductal administration of N1-ethyl-N′-nitro-N-nitrosoguanidine in dogs. Jpn J Cancer Res. 1988;79:1–4.
7. VanEnkevort, BA, O’Brien, RT, Young, KM. Pancreatic pseudocysts in 4 dogs and 2 cats: ultrasonographic and clinicopathologic findings. J Vet Intern Med. 1999;13:309–313.
8. Chang, SC, Liao, JW, Lin, YC, et al. Pancreatic acinar cell carcinoma with intracranial metastasis in a dog. J Vet Med Sci. 2007;69:91–93.
9. Tasker, S, Griffon, DJ, Nuttall, TJ, et al. Resolution of paraneoplastic alopecia following surgical removal of a pancreatic carcinoma in a cat. J Small Anim Pract. 1999;40:16–19.
10. Brooks, DG, Campbell, KL, Dennis, JS, et al. Pancreatic paraneoplastic alopecia in three cats. J Am Anim Hosp Assoc. 1994;30:557–563.
11. Kipperman, BS, Nelson, RW, Griffey, SM, et al. Diabetes mellitus and exocrine pancreatic neoplasia in two cats with hyperadrenocorticism. J Am Anim Hosp Assoc. 1992;28:415–418.
12. Quigley, KA, Jackson, MI, Haines, DM. Hyperlipasemia in 6 dogs with pancreatic or hepatic neoplasia: evidence for tumor lipase production. Vet Clin Pathol. 2001;30:114–120.
13. Bright, JM. Pancreatic adenocarcinoma in a dog with a maldigestion syndrome. J Am Vet Med Assoc. 1985;187:420–421.
14. Bennett, PF, Hahn, KA, Toal, RL, et al. Ultrasonographic and cytopathological diagnosis of exocrine pancreatic carcinoma in the dog and cat. J Am Anim Hosp Assoc. 2001;37:466–473.
15. Hecht, S, Penninck, DG, Keating, JH. Imaging findings in pancreatic neoplasia and nodular hyperplasia in 19 cats. Vet Radiol Ultrasound. 2007;48:45–50.
16. Cobb, LF, Merrell, RC. Total pancreatectomy in dogs. J Surg Res. 1984;37:235–240.
17. Yeo, CJ, Yeo, TP, Hruban, RH, et al. Cancer of the pancreas. In: DeVita VT, Hellman S, Rosenberg SA, eds. Cancer: principles and practice of oncology. Philadelphia: Lippincott Williams & Wilkins, 2005.
18. Ahmadu-Suka, F, Gillette, EL, Withrow, SJ, et al. Pathologic response of the pancreas and duodenum to experimental intraoperative irradiation. Int J Radiat Oncol Biol Phys. 1988;14:1197–1204.
19. Dobelbower, RR, Konski, AA, Merrick, HW, et al. Intraoperative electron beam radiation therapy (IOEBRT) for carcinoma of the exocrine pancreas. Int J Radiat Oncol Biol Phys. 1991;20:113–119.
 Section E
Section E
Gastric Cancer
Incidence and Risk Factors
Gastric cancer is more common than esophageal cancer but still accounts for less than 1% of all malignancies. No definitive etiology is known, although long-term administration of nitrosamines may induce carcinomas in dogs.1 Several articles have described a high incidence of gastric carcinoma in related Belgian shepherds, Norwegian lundehunds, and Dutch Tervueren shepherd dogs, implying a genetic mechanism in development of this disease.2-5 The average age of affected carcinoma patients is 8 years, with a 2.5:1 male-to-female ratio.6,7 Males are also more commonly affected with gastric lymphoma than females.8 Leiomyomas tend to occur in very old dogs (average age 15 years).9,10 Chronic gastritis, Helicobacter, and common descent have been implicated in feline gastric cancer.11,12
Pathology and Natural Behavior
Adenocarcinoma accounts for 70% to 80% of cancer of the canine stomach.13 It is often scirrhous (firm and white on the serosal surface) and has been termed linitis plastica (leather bottle) because of its firm and nondistensible texture. Lesions can be diffusely infiltrative, expansile (often with a central crater and ulceration), or may look more polypoid.14 Other reported malignancies include leiomyosarcoma,15 lymphoma,8 mast cell tumor,16 extramedullary plasmacytoma,17,18 and fibrosarcoma. GI stromal tumors (GISTs) have been described in the GI tract of the dog, with 20% occurring in the stomach, and were likely previously classified as leiomyomas.19-21 In humans and in approximately 50% of cases in dogs, GISTs express CD117 (c-kit), a tyrosine kinase receptor, and mutations in c-kit likely play a role in tumorigenesis of this cancer.19 In one series, one-third of GISTs in dogs had metastasized at the time of diagnosis.19 Adenocarcinoma will frequently spread to regional lymph nodes (70% to 80% at necropsy) followed by liver and lung.2,7,12,16 An unusual occurrence of metastasis of gastric adenocarcinomas to the testes has been reported in three dogs.22 Adenocarcinomas have been described as diffuse or interstitial, but little clinical significance can be associated with these variants.7,23 The tumor antigen C2-O-sLe(X) is unregulated in canine gastric carcinoma and may play a role in the metastatic potential.24 Benign lesions are generally leiomyomas, hypertrophic gastropathy, hamartomas,25 or adenomas.10,26-31 Feline gastric adenocarcinoma is rare, and the stomach is the least commonly affected GI site in the cat.31,32 Lymphoma is the most common gastric tumor in the cat and may be solitary or one component of systemic involvement. Most cats with gastric lymphoma are negative for feline leukemia virus (FeLV).
History and Clinical Signs
The most common history is one of progressive vomiting (often blood tinged or “coffee grounds” in nature), anorexia, and weight loss. The weight loss may be a result of poor digestion, loss of protein and blood from an ulcer, or generalized tumor cachexia. Duration of symptoms is from weeks to many months.
Diagnostic Techniques and Work-Up
Routine laboratory tests and noncontrast radiographs are generally not diagnostic. Hypoglycemia may be a paraneoplastic syndrome associated with leiomyomas or leiomyosarcomas.33,34 A microcytic hypochromic anemia is common. Occult blood in the feces may be detected. Liver “enzymes” may be elevated because of hepatic metastasis or obstruction of the common bile duct. Elevated gastrin levels are occasionally detected.5,35 Thoracic radiographs are only rarely positive for metastasis at the time of initial presentation.
Positive- or double-contrast gastric radiographs may reveal a mass lesion extending into the lumen (Figure 22-12). Ulceration is also a common sign. Delayed gastric emptying, poor motility, or delayed adherence of contrast material to an ulcerated tumor may also be detected. Fluoroscopy may aid in determining motility alterations. Ultrasonography may also be a useful imaging modality in concert with fine-needle or needle core biopsy.36,37 Ultrasound was of diagnostic utility in 27% of dogs with gastric adenocarcinoma of gastric lymphoma.38 Malignancies tend to be sessile, and adenocarcinoma tends to occur most commonly on the lesser curvature and gastric antrum (Figure 22-13). Benign lesions may be pedunculated or well circumscribed. Leiomyomas often occur at the cardia and will grow into the lumen as a smooth circumscribed mass.10
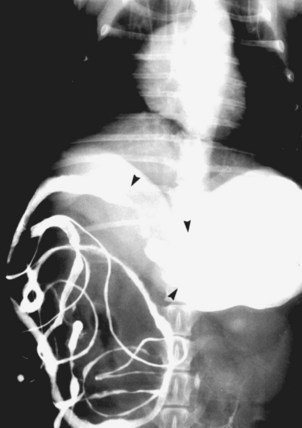
Figure 22-12 Ventrodorsal view of a dog with gastric cancer. Note the filling defect and partial outflow obstruction of gastric antrum and pylorus.
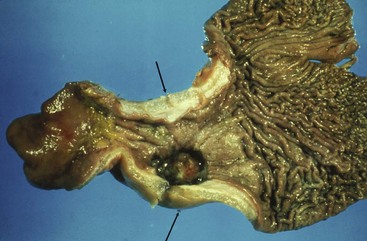
Figure 22-13 Gross specimen of the stomach from a dog with gastric adenocarcinoma. Note the large ulcer and fibrous thickening of stomach wall in area of gastric antrum (arrows). This patient had metastasis to regional lymph nodes and liver.
Gastroscopy with a flexible endoscope will generally reveal larger lesions that can be biopsied.39,40 Several “large” samples should be taken because most gastric tumors have superficial necrosis, inflammation, and ulceration. Endoscopic biopsies were more likely to detect gastric lymphoma than intestinal lymphoma in cats.41 In some patients, the gastric lesions are submucosal only, making endoscopic biopsy difficult. False-negative biopsies through the gastroscope are common. Open surgical biopsy is the most definitive method of diagnosis. For suspected GIST tumors, a CD117 immunohistochemical stain should be applied for confirmation.
Therapy
Except for lymphoma, surgery is the most common form of treatment for gastric cancer. As with esophageal cancer, curative resection is complicated by advanced stage disease in a difficult operative area (lesser curvature, antrum, and pylorus) with a frequently debilitated patient. At the time of surgery, a careful evaluation of liver and regional lymph nodes should be made to adequately stage the cancer. Lymph node metastasis can be quite varied, and all abdominal lymph nodes should be examined. If the cancer is felt to be localized to the stomach at laparotomy, a curative resection may be attempted. If possible, wide partial gastrectomy or antrectomy followed by a gastroduodenostomy (Billroth I) should be performed because of the increased morbidity associated with more extensive surgery such as gastrectomy and gastrojejunostomy (Billroth II).42,43 Lesions requiring biliary bypass and very extensive surgery (complete gastrectomy) are generally too advanced to make these procedures worthwhile in terms of survival. For obstructive lesions felt to be inoperable for cure or for those that are metastatic, it is possible to perform a palliative gastrojejunostomy to allow passage of food into the intestine, although this procedure may be associated with significant postoperative mobidity.42
Leiomyomas are usually discrete, solitary lesions in the area of the cardia. They are not premalignant but can cause symptoms because of a mass effect. They can be easily “shelled out” via a midline laparotomy, gastrotomy, and submucosal removal or via intercostal thoracotomy if the side of the lesion can be clearly delineated.10 In humans, GIST tumors may be responsive to inhibitors of c-Kit (e.g., toceranib, imatinib). The recent development of these agents in veterinary oncology could theoretically have efficacy for this tumor.
RT is rarely utilized due to the poor radiation tolerance of surrounding normal tissue (liver and intestine). Nonresectable lymphoma may be dramatically reduced with lower doses of irradiation than required for other tumors. No effective chemotherapy is known for adenocarcinoma.
Lymphoma may be excised if localized but does not generally respond well to conventional chemotherapy, although several exceptions exist in cats with gastric lymphoma (see Chapter 32, Section B).8,44 The need for postoperative chemotherapy after “complete” resection of lymphoma is unknown.
Prognosis
The prognosis for most malignant gastric cancer is poor. Even if surgery can be performed, most patients are dead within 6 months as a result of recurrent or metastatic cancer.3,11,45-49 In a series of 24 dogs undergoing pylorectomy and gastroduodenostomy, the perioperative mortality was 24% with a MST of 33 days. Preoperative weight loss was a negative indicator of outcome.43 Few adenocarcinoma patients are operable for cure, and the short-term morbidity with radical resection can be high. Of 17 dogs treated with surgery for gastric adenocarcinoma, the median survival was 2 months.11 Rare cases have survived as long as 3 years.3 Palliation via bypass can be achieved for 1 to 6 months. The median survival for seven dogs with gastric leiomyosarcoma that lived at least 2 weeks postoperatively was 1 year.15 Another paper suggested median survivals for GI leiomyosarcomas, or GISTs, of 8 to 12 months.21 The hypoglycemia seen with some smooth muscle tumors is reversible after tumor resection. Patients with benign lesions can be cured with complete surgical excision.10,50,51 Gastric extramedullary plasmacytomas appear to carry an excellent prognosis with surgery and chemotherapy. GI mast cell tumors were usually metastatic to regional nodes, and fewer than 10% of patients survived 6 months.16-18
Comparative Aspects52
Gastric cancer is the sixth most common cause of cancer death in humans. Adenocarcinoma constitutes over 90% of all malignant gastric cancer. Multiple socioeconomic, geographic, and environmental factors are associated with risk of tumor development. Helicobacter has been associated with the development of human gastric carcinoma and lymphoma but has not been correlated with carcinogenesis in the dog or cat, even though the organism is prevalent in the species.
Most lesions will be firm, ulcerative, and located in the antrum or lower third of the stomach, as in the dog. Most lesions are detected late in the course of disease and have direct tumor extension to surrounding organs, lymph node metastasis, or systemic metastasis, as with the dog.
Treatment is with surgical resection when possible or with less effective radiation and chemotherapy. Five-year survival for all patients is less than 10%, with a 30% survival for patients deemed operatively to have “localized” disease.