Tumors of the Respiratory System
 Section A
Section A
Cancer of the Nasal Planum
Incidence and Risk Factors
Cancer of the nasal planum is uncommon in the dog and relatively common in the cat. The development of squamous cell carcinoma (SCC) has been correlated with ultraviolet (UV) light exposure and lack of protective pigment.1 One paper suggests a papillomavirus may be involved in feline SCC development.2 Classically, it is seen in older, lightly pigmented cats.
Pathology and Natural Behavior
By far the most common cancer is SCC. Depending on when the biopsy is performed, the tumors may be reported as carcinoma in situ, superficial SCC (<2 mm deep), or deeply infiltrative SCC. They may be very locally invasive but only rarely metastasize.
Other cancers reported in the nasal planum are lymphoma, fibrosarcoma, hemangioma, melanoma, mast cell tumor, fibroma, and eosinophilic granuloma. Immune-mediated disease may manifest as erosive or crusty lesions on the nose, but it is rarely proliferative, and other sites on the body usually are affected. Immune-mediated disease probably is not a contributing factor in tumor development.
History and Clinical Signs
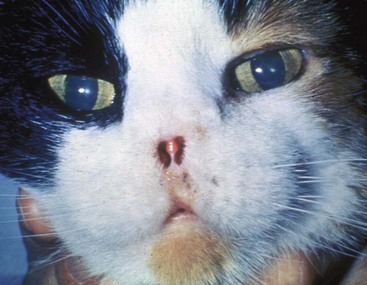
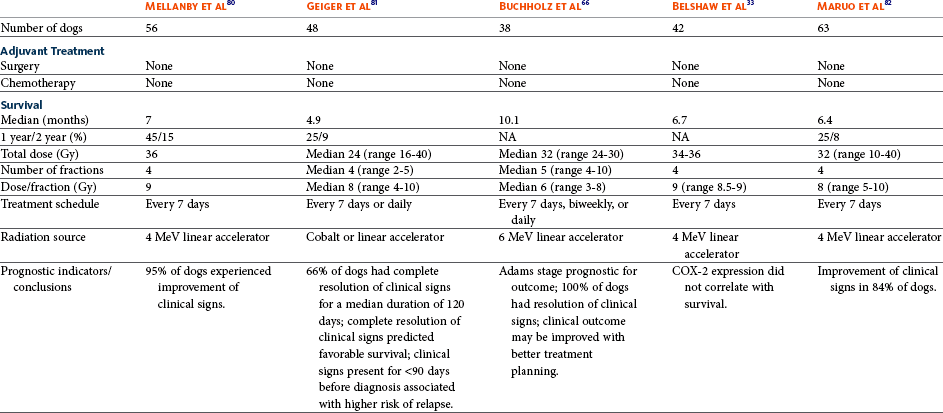
Invasive SCC usually is preceded by a protracted course of disease (months to years) that progresses through the following stages (Figure 23-1): crusting and erythema, superficial erosions and ulcers (carcinoma in situ or early SCC), and, finally, deeply invasive, erosive lesions. In cats SCC usually originates on the cornified external surface of the nasal planum, whereas in dogs it often occurs in the mucous membrane of the nostril or the external planum. Associated lesions on the eyelid, preauricular skin, and ear pinna may be seen in cats if these sites lack protective pigment. Patients often have been treated with corticosteroids or topical ointments, with minimal response.
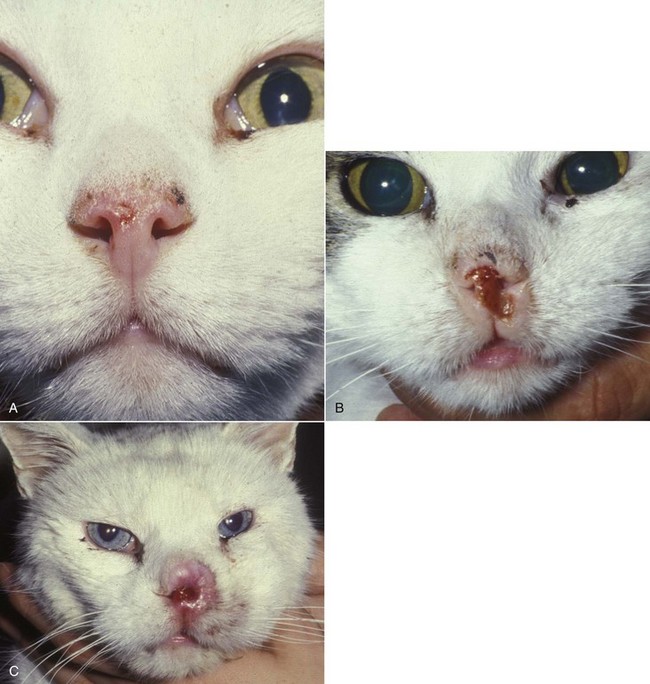
Figure 23-1 A, Crusting and erythema on the nose of a white cat. The condition had been slowly progressive for 8 months, and 6 months later, the lesion was confirmed through biopsy as carcinoma in situ. B, A cat with an invasive SCC that has caused some erosion of the nasal planum but is still confined to the nasal planum. Nosectomy was curative. C, This cat had a 2-year history of progressive nasal ulceration and deformity. The nasal planum is markedly deformed, and the surrounding skin up to the eyelids is swollen and infiltrated with SCC. Even nosectomy would not be curative. Note the concomitant eyelid lesions, which were carcinoma in situ.
Diagnostic Techniques and Work-Up
For erosive or proliferative lesions, a deep wedge biopsy may be done to determine the degree of invasion and the histologic type of disease. These biopsies require a brief general anesthetic because of the sensitivity of the nasal planum. Hemorrhage can be temporarily profuse and usually requires one or two deep sutures to appose the edges. Cytologic scrapings and superficial biopsies are of little value because they frequently reveal only inflammation, which may accompany both cancer and noncancerous conditions. Lymph nodes are rarely involved except in very advanced disease, and thoracic radiographs are almost invariably negative for metastasis. Regional radiographs generally are unrewarding. Computed tomography (CT) and magnetic resonance imaging (MRI) have proved to be valuable staging tool in dogs with SCC of the nostril. They can help define the posterior extent of the tumor and guide the posterior level of resection or the posterior extent of the radiation field.
Therapy
Limiting exposure to the sun or tattooing to add pigment protection may prevent or arrest the course of the preneoplastic disease. Topical sunscreens are easily licked off and rarely help. Maintaining the tattoo is very difficult when inflammation and ulceration are present because it is rapidly removed by macrophages. Even under the best of circumstances, tattooing must be repeated regularly and reports of success are anecdotal. Attempts to increase epithelial differentiation with synthetic derivatives of vitamin A generally have been unsuccessful for advanced disease but may be of help in reversing or limiting the growth of preneoplastic lesions.3,4
SCC and probably other neoplasms as well fall into two general categories: superficial, minimally invasive disease versus deeply infiltrating disease. Superficial cancers can be managed effectively by almost any method, including cryosurgery, lasers,5,6 photodynamic therapy,7-9 intralesional carboplatin chemotherapy,10 intralesional carboplatin combined with radiation therapy (RT),11 hyperthermia, and irradiation alone.12-19 A distinct disadvantage with these techniques is the inability to obtain a surgical margin by which to document the adequacy of treatment. RT, in particular, which would have the greatest chance of preserving the cosmetic appearance of the nose, has had poor local control rates for larger and more invasive SCC in the dog but is more effective treating SCC of the nasal planum in the cat.14,15,20 Expectations for radiation with other tumor types must be extrapolated from results achieved in more conventional sites.
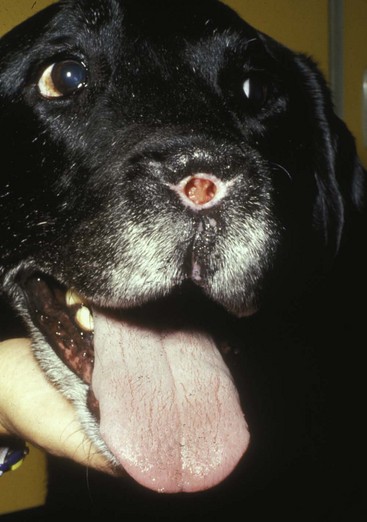
In the cat, invasive cancer of the nasal planum can be completely excised with an acceptable cosmetic result.21 The nasal planum is removed with a 360-degree skin incision that also transects the underlying turbinates (Figure 23-2). A single cutaneous purse-string suture of 3-0 nylon is placed to pull the skin into an open circle (1 cm in diameter) around the airways. The purse-string suture must not be pulled too tight or it may heal across the airways. The site subsequently crusts and scabs over. The scab is removed at suture removal (this often requires sedation or anesthesia), and healing of the skin with two patent airways (“nostrils”) is complete by 1 month. An occasional problem with “nosectomy” is stricture of the combined nasal orifice. This can be frustrating and has been variously managed with wide skin excision and removal of the nasal septum rostrally, laser ablation, rubber stents, or permanent placement of a stainless steel intraluminal expansile stent.22 Functional and cosmetic results are good in the cat (Figure 23-3), and fair to good in the dog23,24 (Figure 23-4). This procedure probably is the treatment of choice for invasive lesions that have not spread extensively to the lip or surrounding skin. The author has found that adjuvant RT has been successful if the margins of removal are incomplete. Combined removal of the premaxilla and nasal planum has been reported in the dog with extensive, invasive lesions.23,25,26
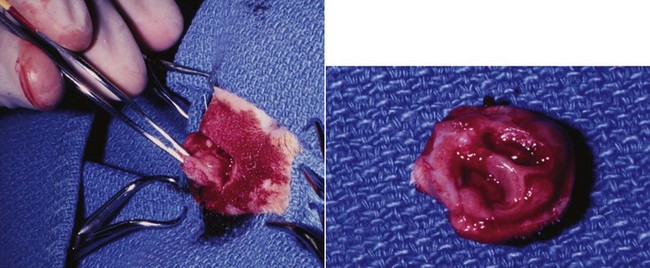
Figure 23-2 Operative view of resected nasal planum in a cat with invasive carcinoma that was confined to the nasal planum.
Prognosis
The outlook for SCC is good for early, noninvasive disease. However, the development of new sites of neoplasia on other areas of the planum after localized treatment is common because the underlying causes are not reversed.27 Later-stage disease can be cured with aggressive surgery but is poorly responsive to most other treatments.20,21,25 More than 80% of cats with invasive SCC of the nasal planum treated with wide resection (nosectomy) are free of recurrent disease at 1 year.5,21 Newer reports of proton irradiation16 and irradiation with intralesional chemotherapy11 suggest control rates in cats exceeding 60%. Strontium (Sr) 90 plesiotherapy can achieve 75% complete response (CR) rates for superficial SCC, and retreatment is possible. In a study of nosectomy (with or without removal of the pinnae) in 38 cats, the median survival time (MST) exceeded 22 months.5 Photodynamic therapy can be effective in treating cats with SCC of the nose, but the lesions must be small and minimally invasive for a good result.7-9 In dogs, the local recurrence rate after nosectomy is low.20,26 Delayed lymph node metastasis (longer than 1 year after surgery) in dogs with SCC has been successfully treated with lymphadenectomy. Because SCC rarely metastasizes from the nasal planum, even untreated animals with advanced cancer can live a long time, albeit with an ulcerated and deforming cancer.
Comparative Aspects28
As in the cat, cancer of the human nasal skin and nasal vestibule (anterior entrance to the nasal cavity) may be induced by exposure to UV light. Lack of protective pigment is also a contributing factor in humans. SCC of the vestibule is treated with irradiation (interstitial or external beam) or surgery. Surgery generally involves resection of the nasal skin and cartilage, followed by reconstruction using composite ear skin and cartilage, nasolabial flaps, or a prosthesis. Local control generally is good.
References
1. Hargis, AM. A review of solar-induced lesions in domestic animals. Comp Cont Ed Pract Vet. 1981;3:287–294.
2. Munday, JS, Dunowska, M, De Grey, S. Detection of two different papillomaviruses within a feline cutaneous squamous cell carcinoma: case report and review of the literature. N Z Vet J. 2009;57:248–251.
3. Evans, AG, Madewell, BR, Stannard, AA. A trial of 13-cis-retinoic acid for treatment of squamous cell carcinoma and preneoplastic lesions of the head in cats. Am J Vet Res. 1985;46:2553–2557.
4. Marks, SL, Song, MD, Stannard, AA, et al. Clinical evaluation of etretinate for the treatment of canine solar-induced squamous cell carcinoma and preneoplastic lesions. J Am Acad Dermatol. 1992;27:11–16.
5. Lana, SE, Ogilvie, GK, Withrow, SJ, et al. Feline cutaneous squamous cell carcinoma of the nasal planum and the pinnae: 61 cases. J Am Anim Hosp Assoc. 1997;33:329–332.
6. Olivieri, L, Nardini, G, Pengo, G, et al. Cutaneous progressive angiomatosis on the muzzle of a dog, treated by laser photocoagulation therapy. Vet Dermatol. 2010;21:517–521.
7. Peaston, AE, Leach, MW, Higgins, RJ. Photodynamic therapy for nasal and aural squamous cell carcinoma in cats. J Am Vet Med Assoc. 1993;202:1261–1265.
8. Ferreira, I, Rahal, SC, Rocha, NS, et al. Hematoporphyrin-based photodynamic therapy for cutaneous squamous cell carcinoma in cats. Vet Dermatol. 2009;20:174–178.
9. Bexfield, NH, Stell, AJ, Gear, RN, et al. Photodynamic therapy of superficial nasal planum squamous cell carcinomas in cats: 55 cases. J Vet Intern Med/Am Coll Vet Int Med. 2008;22:1385–1389.
10. Théon, AP, Madewell, BR, Shearn, VI, et al. Prognostic factors associated with radiotherapy of squamous cell carcinoma of the nasal plane in cats. J Am Vet Med Assoc. 1995;206:991–996.
11. de Vos, JP, Burm, GO, Focker, BP. Results from the treatment of advanced stage squamous cell carcinoma of the nasal planum in cats, using a combination of intralesional carboplatin and superficial radiotherapy: a pilot study. Vet Comp Oncol. 2004;2:75–81.
12. Grier, RL, Brewer, WG, Theilen, GH. Hyperthermic treatment of superficial tumors in cats and dogs. J Am Vet Med Assoc. 1980;177:227–233.
13. Shelley, BA, Bartels, KE, Ely, RW, et al. Use of the neodymium:yttrium-aluminum-garnet laser for treatment of squamous cell carcinoma of the nasal planum in a cat. J Am Vet Med Assoc. 1992;201:756–758.
14. Carlisle, CH, Gould, S. Response of squamous cell carcinoma of the nose of the cat to treatment with x-rays. Vet Radiol. 1982;23:186–192.
15. Thrall, DE, Adams, WM. Radiotherapy of squamous cell carcinomas of the canine nasal plane. Vet Radiol. 1982;23:193–195.
16. Fidel, JL, Egger, E, Blattmann, H, et al. Proton irradiation of feline nasal planum squamous cell carcinoma using an accelerated protocol. Vet Radiol Ultrasound. 2001;42:569–575.
17. Trivillin, VA, Heber, EM, Rao, M, et al. Boron neutron capture therapy (BNCT) for the treatment of spontaneous nasal planum squamous cell carcinoma in felines. Radiat Environ Biophys. 2008;47:147–155.
18. Goodfellow, M, Hayes, A, Murphy, S, et al. A retrospective study of (90)Strontium plesiotherapy for feline squamous cell carcinoma of the nasal planum. J Feline Med Surg. 2006;8:169–176.
19. Hammond, GM, Gordon, IK, Theon, AP, et al. Evaluation of strontium Sr 90 for the treatment of superficial squamous cell carcinoma of the nasal planum in cats: 49 cases (1990-2006). J Am Vet Med Assoc. 2007;231:736–741.
20. Lascelles, BD, Parry, AT, Stidworthy, MF, et al. Squamous cell carcinoma of the nasal planum in 17 dogs. Vet Rec. 2000;147:473–476.
21. Withrow, SJ, Straw, RC. Resection of the nasal planum in nine cats and five dogs. J Am Anim Hosp Assoc. 1990;26:219–222.
22. Novo, RE, Kramek, B. Surgical repair of nasopharyngeal stenosis in a cat using a stent. J Am Anim Hosp Assoc. 1999;35:251–256.
23. Gallegos, J, Schmiedt, CW, McAnulty, JF. Cosmetic rostral nasal reconstruction after nasal planum and premaxilla resection: technique and results in two dogs. Vet Surg. 2007;36:669–674.
24. Thomson, M. Squamous cell carcinoma of the nasal planum in cats and dogs. Clin Tech Small Anim Pract. 2007;22:42–45.
25. Lascelles, BD, Henderson, RA, Sequin, B, et al. Bilateral rostral maxillectomy and nasal planectomy for large rostral maxillofacial neoplasms in six dogs and one cat. J Am Anim Hosp Assoc. 2004;40:137–146.
26. Kirpensteijn, J, Withrow, SJ, Straw, RC. Combined resection of the nasal planum and premaxilla in three dogs. Vet Surg. 1994;23:341–346.
27. Théon, AP, VanVechten, MK, Madewell, BR. Intratumoral administration of carboplatin for treatment of squamous cell carcinomas of the nasal plane in cats. Am J Vet Res. 1996;57:205–210.
28. Aasi, SZ, Leffell, DJ. Cancer of the skin. In: DeVita VT, et al, eds. Cancer: principles and practices of oncology. Philadelphia: Lippincott-Raven, 2005.
 Section B
Section B
Nasosinal Tumors
Michelle M. Turek and Susan E. Lana
Canine Nasosinal Tumors
Tumors of the nasal cavity and paranasal sinuses account for approximately 1% of all neoplasms in dogs.1 The average age of dogs with this disease is approximately 10 years, although canine patients as young as 9 months have been reported.2,3 Medium-to-large breeds may be more commonly affected.2 A slight male predilection has been suggested.2,4 It has been speculated, but is unproved, that dolichocephalic breeds (long-nosed) or dogs living in urban environments, with resultant nasal filtering of pollutants, may be at higher risk for developing nasal cancer.4-6 Exposure to environmental tobacco smoke has been associated with an increased risk of nasal cancer in a group of dogs in one study,7 but the same was not true in another.6 Indoor exposure to fossil fuel combustion products, such as those produced by coal or kerosene heaters, may contribute to the suggested environmental component of this cancer.6
Pathology and Natural Behavior
Carcinomas, including adenocarcinoma, SCC, and undifferentiated carcinoma represent nearly two-thirds of canine intranasal tumors.8 Sarcomas (usually fibrosarcoma, chondrosarcoma, osteosarcoma, and undifferentiated sarcoma) comprise the bulk of the remaining cancers.9 Both carcinomas and sarcomas are characterized by progressive local invasion. The metastatic rate is generally considered low at the time of diagnosis but may be as high as 40% to 50% at the time of death, which is usually attributable to the primary disease rather than metastatic lesions.2 The most common sites of metastasis are the regional lymph nodes and the lungs.2,9,10 Less common sites include bones, kidneys, liver, skin, and brain.11-14
Rare tumors of the nasosinal region in dogs include round cell tumors, such as lymphoma (unlike in cats, in which this is a common nasal tumor); mast cell tumors; and transmissible venereal cancer. Other malignancies include hemangiosarcoma, melanoma, neuroendocrine carcinoma, nerve sheath tumor, neuroblastoma, fibrous histiocytoma, multilobular osteochondrosarcoma, hamartoma, rhabdomyosarcoma, and leiomyosarcoma.2,15-28 The biologic behavior of these less common malignancies is not well defined. Benign lesions such as polyps, fibromas, dermoid cysts, and angiomatous proliferation can also be seen.
A number of studies have attempted to elucidate possible molecular mechanisms associated with canine nasosinal tumorigenesis. In one study using a single polyclonal antihuman antibody, nuclear p53 accumulation was detected in nearly 60% of nasal adenocarcinomas (11 of 19), which suggests that overexpression of a mutated p53 tumor suppressor protein may play a role.29 Cyclooxygenase-2 (COX-2) expression has been detected to varying degrees in most nasosinal epithelial tumors sampled30-33 and in normal paratumoral respiratory epithelium and stromal tissue.33 In a recent study, epidermal growth factor receptor (EGFR) expression and vascular endothelial growth factor (VEGF) expression were detected in over 50% and 90% of the 24 nasal carcinomas evaluated, respectively.34 All tumors expressed either EGFR or VEGFR, but there was no association between the immunoreactivity for each protein.34 Expression of peroxisome proliferator-activated receptor γ (PPAR-γ), a nuclear receptor involved in glucose metabolism and fatty acid storage, has also been shown in canine nasal carcinomas.35 The authors of that study suggested that expression patterns may differ in tumor tissue compared to normal nasal epithelium.35 The role of these proteins in carcinogenesis is not clear, and further investigation is needed before clinical relevancy can be determined. Evaluation of the inflammatory infiltrate in 31 canine nasal carcinomas revealed an abundance of neutrophils and macrophages, which were present in greater numbers than in normal canine mucosa.36 Plasma cells were also detected in the majority of tumors but varied in number compared to normal tissue. T-lymphocytes were detected less commonly, in lower numbers than in the normal mucosa, and predominately at the periphery of the tumors. Activity of matrix metalloproteinase-2 (MMP-2) in nasal adenocarcinomas was negligible in one small study.37
History and Clinical Signs
Although many intranasal diseases will have overlapping clinical signs, a strong suspicion of cancer is appropriate for older animals with an intermittent and progressive history of unilateral (initially) epistaxis or mucopurulent discharge (or both). The average duration of clinical signs prior to diagnosis is 2 to 3 months1,38; these most commonly include epistaxis, bloody or mucopurulent nasal discharge, facial deformity due to bone erosion and subcutaneous extension of tumor, unwillingness to open the mouth, sneezing, dyspnea or stertorous breathing, exophthalmus, and ocular discharge as a result of mechanical obstruction of the nasolacrimal duct.2,8,38 Differential diagnoses for animals with these clinical signs include fungal (Aspergillus sp.) or bacterial rhinitis, idiopathic nonspecific rhinitis (usually lymphoplasmacytic), rare nasal parasites, bleeding disorders, hypertension, foreign body, trauma, and developmental anomalies (e.g., cystic Rathke’s clefts).39-41 If facial deformity is present, the diagnosis is almost always cancer42,43; however, aspergillosis, sporotrichosis, and a rare, benign condition, angiomatous proliferation of the nasal cavity, also can cause facial deformity. Dogs with epistaxis and concurrent clinical signs of systemic disease, including lethargy, inappetence, weight loss, hemorrhage at extranasal sites and concurrent neurologic disease, are more likely to have nonneoplastic systemic disorders such as bleeding disorders, hypertension, and tick-borne or bacterial infections.43,44
Clinical signs can be temporarily alleviated by a variety of symptomatic treatments, including antibiotics, steroids, and nonsteroidal antiinflammatory drugs (NSAIDs).38 An initial response to these treatments should not diminish the index of suspicion for neoplasia in older dogs with clinical signs consistent with cancer.38
On rare occasions, animals with tumors involving the caudal region of the nasal cavity may have only neurologic signs (e.g., seizures, acute blindness, behavior change, paresis, circling, and obtundation) caused by direct invasion of the cranial vault.45 However, absence of neurologic signs does not rule out tumor extension into the cranial vault because most dogs with nasal tumors that extend beyond the cribriform plate do not exhibit neurologic signs.
Diagnosis and Staging
A definitive diagnosis of nasosinal cancer requires a tissue biopsy, even though diagnostic imaging and historic information can be highly suggestive. Coagulation disorders must be ruled out before biopsy because bleeding during the procedure is to be expected. Attention should be paid to the platelet count, appropriate clotting at venipuncture sites, and the presence of hematuria, retinal hemorrhage, or petechial hemorrhages. A clotting profile (prothrombin time [PT], activated partial thromboplastin time [APTT]), bleeding time, or activated clotting time (ACT) can also be done.
The superior imaging value of CT and MRI over conventional radiographs for canine nasal disease, including neoplasia, is well documented.3,23,39,46-52 Cross-sectional imaging provides improved anatomic detail, which allows accurate determination of the extent of tumor (staging) and localization of nasal cavity abnormalities (Figure 23-5).3,23,39,46-53 It also facilitates evaluation of the integrity of the cribriform plate and identification of potential tumor extension into the cranial vault, which are important prognostic criteria. Although, in general, MRI allows for better resolution of soft tissue structures, a recent study showed no clinically relevant benefit to using MRI over CT to evaluate nasal tumors that do not extend into the cranial vault.49 CT enables better visualization of lysis of bones bordering the nasal cavity, which is important for accurate radiation treatment planning. It has been proposed that tumor signal intensity on MRI may help distinguish sarcomas from carcinomas,50 although this application requires further validation before it can be used clinically. Both modalities are useful for imaging nasal tumors, although CT is used more commonly than MRI due to its lower cost in most facilities, wider availability, and usefulness for computer planning of radiation treatments.
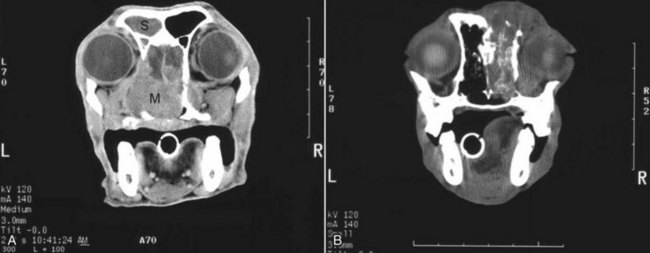
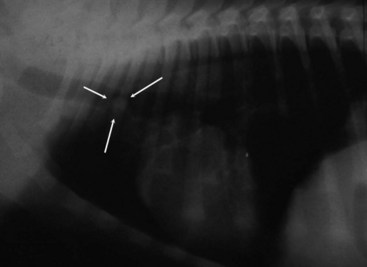
Figure 23-5 A, Contrast-enhanced CT image of a dog with nasosinal cancer taken in the transverse plane at the level of the orbit and the olfactory bulb of the brain. Note the contrast-enhancing mass (M) in the nasopharynx, which is causing erosion of the frontal and palatine bones. The tumor has invaded the left retroorbital space and the cranial vault, resulting in deviation of the falx cerebri. Non–contrast-enhancing material in the left frontal sinus (S) suggests accumulation of nasal exudates. B, Non–contrast-enhanced CT image of a dog with nasosinal cancer taken in the transverse plane at the level of the orbit. Note the soft tissue attenuating mass in the right nasal cavity. Erosion of the frontal bone has allowed the tumor to extend into the subcutaneous tissues on the dorsum of the head; erosion of the palatine bone has allowed invasion of the right retroorbital space.
Certain CT or MRI findings have been correlated with a diagnosis of cancer in dogs with nasal disease.23,39,53 None of these, alone or in combination, is definitive for neoplasia.23,39,53 These findings include bony destruction (e.g., ethmoid bones, cribriform plate, and the bones surrounding the nasal cavities), destruction of the sphenoid sinus, abnormal soft tissue in the retrobulbar space, nasopharyngeal invasion, hyperostosis of the lateral maxilla, and patchy areas of increased density within abnormal soft tissue opacity.23,53 It is important to recognize that detection of a mass in the nasal cavity of a dog is not specific for a diagnosis of neoplasia.23 Idiopathic inflammatory disease and fungal infections can present in this manner.23
Conventional radiography can still have a place in the diagnostic work-up of dogs suspected of having nasal tumors. Despite the inherent limitation of tissue superimposition, the sensitivity of radiography in detecting major nasal cavity abnormalities in dogs with nasal tumors is comparable to that of CT or MRI when tumors are sufficiently advanced to cause clinical signs.46,52,54 Certain radiographic signs have been correlated with a positive predictive value for neoplasia.55 These include the presence of a soft tissue opacity, loss of turbinate detail affecting the entire ipsilateral nasal cavity, invasion of bones surrounding the nasal cavity, and soft tissue/fluid opacities within the ipsilateral frontal sinus (Figure 23-6).55 As is true for CT and MRI changes, no radiographic sign is definitively diagnostic for neoplasia.52,55 Nasal radiography therefore represents an easily accessible and less costly screening procedure that can guide further diagnostics, including biopsy, additional imaging, or both.52,56 Standard radiographs taken under anesthesia include lateral, dorsoventral (DV), frontal sinus, and open-mouth oblique views. The most informative views are the open-mouth DV oblique view (to show the caudal nasal cavity and cribriform plate) and the isolated nasal cavity exposure with the film placed in the mouth and exposed in the DV plane.
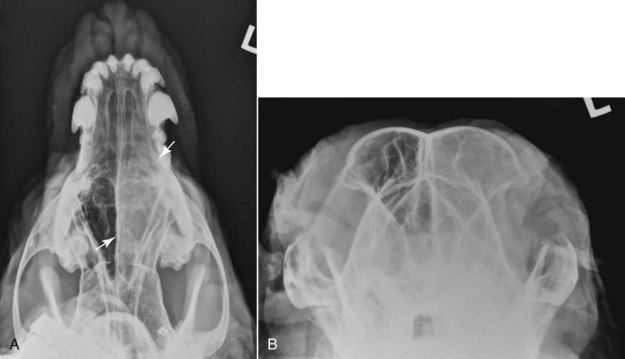
Figure 23-6 Radiographs of a dog with an intranasal anaplastic carcinoma. A, In this open mouth ventrodorsal view of the maxilla, note the asymmetry from side to side. A soft tissue opacity in the left nasal cavity (arrows) suggests the presence of a space-occupying mass. B, Rostral-caudal skyline view of the frontal sinuses. A soft tissue opacity in the left frontal sinus suggests extension of tumor or obstructive rhinitis. (Courtesy Dr. David Jimenez.)
A tissue biopsy should be obtained while the patient is under anesthesia for diagnostic imaging. Suitable samples can be acquired by a variety of techniques.57,58 These include vigorous nasal flushing to dislodge mass lesions, transnostril blind biopsy using cup forceps or a bone curette, fiberoptic-guided biopsy, fine-needle aspiration (FNA) or punch biopsy of facial deformities, rhinotomy, and transnostril aspiration and core biopsy (Figure 23-7). Transnostril aspiration and core biopsy involves passing either a punch biopsy needle or a large bore (3 to 5 mm) plastic cannula into the nasal cavity through the nostril and directing it to the tumor (Figure 23-8).57 With any transnostril technique, it is important to avoid penetrating the cribriform plate. The biopsy instrument should be marked with tape or cut off at a length that ensures that the instrument does not penetrate farther than the distance from the tip of the naris to the medial canthus of the eye (Figure 23-9). Mild-to-moderate hemorrhage is to be expected and subsides within several minutes. It is important to ensure the integrity and inflation of the endotracheal tube cuff during any of these procedures. If hemorrhage is severe, the ipsilateral carotid artery can be permanently ligated, but this is rarely indicated.59 Inadequate biopsy size or sampling outside the region of the tumor may preclude an accurate diagnosis. Further testing may be necessary when clinical and histologic findings are incongruent. In cases in which imaging results are suggestive of an aggressive process yet histopathologic changes are consistent with nonspecific inflammatory disease, a surgical biopsy obtained by rhinotomy may be required for definitive diagnosis.3,23
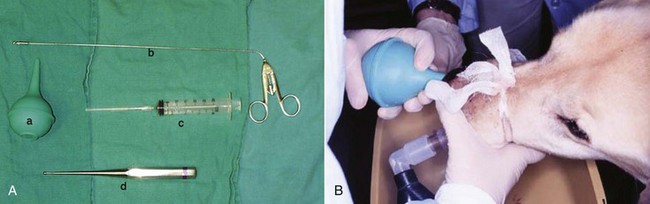
Figure 23-7 A, Several techniques can be used to procure tissue biopsy material from dogs with nasal tumors. A bulb syringe (a) can be used to flush out tumor material, or a biopsy forceps (b), plastic cannula (c), or bone curette (d) can be inserted through the nostril. B, To flush biopsy tissue in an anesthetized dog with an endotracheal tube, the contralateral passage is occluded and flushing pressure is created with a saline-filled bulb syringe; the tissue is flushed back through the nasopharynx and out the mouth into a collection bowl.
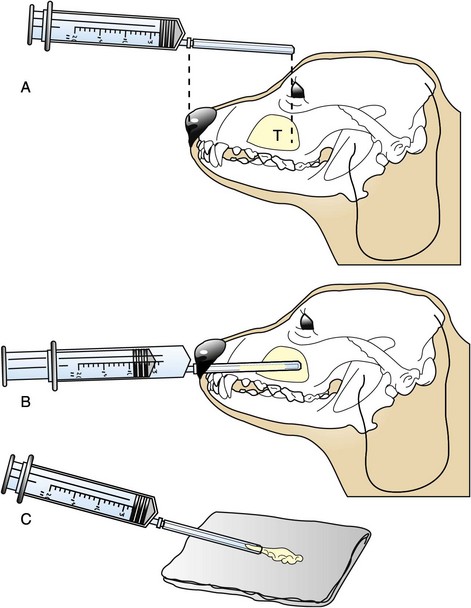
Figure 23-8 A, A caudally situated tumor (T) in a dog. To avoid injury to the brain, the plastic cannula for the core aspirate has been shortened so that it extends no deeper than the medial canthus of the eye. B, The cannula is introduced into the nasal cavity through the nares. Slight resistance usually is felt as the tumor is entered. Negative pressure is applied as the cannula is redirected at various angles. C, Tissue and blood are expelled onto a gauze sponge, where blood is separated from tissue. The tissue is submitted for histologic evaluation.
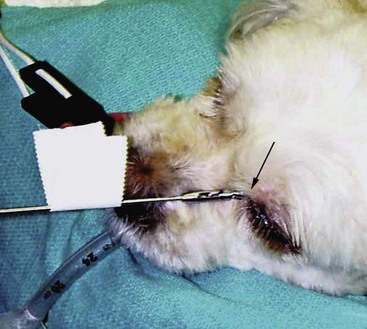
Figure 23-9 Regardless of the biopsy technique chosen, any instrument that is passed through the naris must be measured; it also should be marked with tape or cut off at a length that ensures that the instrument does not penetrate farther than the distance from the tip of the naris to the medial canthus of the eye. This ensures that the instrument does not pass through a potentially compromised cribriform plate.
Attempts at nasal washing and fluid retrieval for cytologic examination generally have been unrewarding and are not recommended as the sole means of diagnosis.1 Brush cytology also has been described, but it often is not diagnostic.60 Rhinoscopy can be used to visualize the nasal cavity, if necessary, and to guide biopsy, although samples collected in this manner often are small and superficial.61
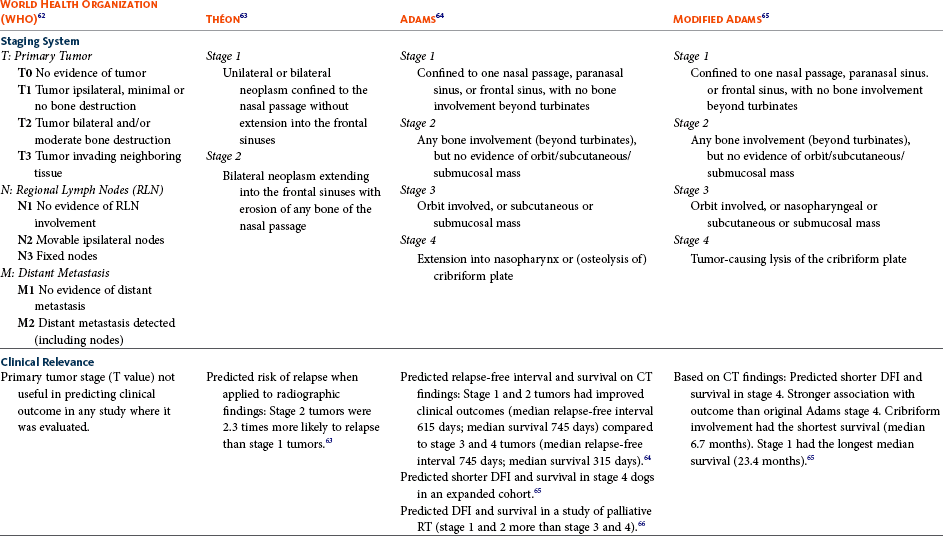
Multiple staging systems for canine nasosinal neoplasia have been proposed on the basis of local tumor extent and bony erosion (Table 23-1).4,63-65,67 The least clinically relevant is the World Health Organization (WHO) staging method because no correlation has been found between primary tumor extent and prognosis using this system.38,65,67,68 The prognostic significance of the other staging systems remains controversial. A recent review of 94 dogs with nasal tumors treated with definitive RT showed that the CT-based Adams staging system and its recently modified version in which stage 4 represents cribriform plate involvement alone correlate with disease outcome.65 Prognostic significance improved when CT findings were combined with histologic category.65
Table 23-1
Staging Systems for Canine Nasosinal Neoplasia
CT, Computed tomography; DFI, disease-free interval; RT, radiation therapy.
Regional lymph node cytology is positive for metastasis in as many as 10% to 24% of cases and is most commonly associated with carcinoma.10,38,66,68 Enlarged regional (mandibular and superficial cervical) lymph nodes should be sampled for cytology to differentiate between a reactive process and metastasis. Thoracic radiographs usually are negative for metastasis at initial presentation.1,2,10,38
Hematologic and biochemical findings generally are noncontributory in dogs with nasosinal tumors. In rare cases, paraneoplastic erythrocytosis and hypercalcemia have been documented.69-71
Treatment and Prognosis
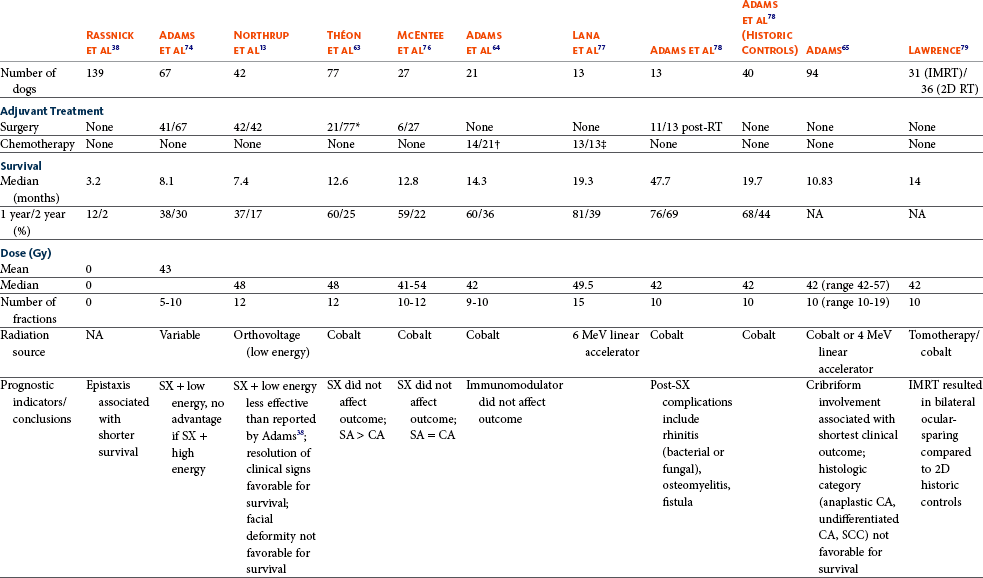
Therapy is directed primarily at control of local disease, which usually manifests in a relatively advanced stage in a critical location near the brain and eyes (Figure 23-10). Without treatment, the MST of dogs with nasal carcinoma is 95 days as reported in a retrospective case series of 139 dogs.38 Prognosis of dogs with epistaxis appears worse than in dogs without epistaxis (MST 88 days versus 224 days).38 Bone invasion occurs early, with nasosinal neoplasia, and curative surgery is virtually impossible. Although surgical removal by means of rhinotomy has been recommended, a high rate of morbidity without significant extension of life greatly limits the utility of this procedure as a sole form of treatment.1,8,10,72,73 The median survival time after surgery is approximately 3 to 6 months, similar to that reported for no treatment.1,10,72,73 In one study, surgery in conjunction with low-energy orthovoltage irradiation provided the best clinical outcome, MST, compared to other treatment modalities74; however, more recent reports have not supported this finding (Table 23-2).13,75
Table 23-2
Summary of Selected Articles on Treatment of Nasosinal Tumors in Dogs (No Treatment or Definitive Radiation)
IMRT, Intensity-modulated radiation therapy; 2D, 2-dimensional; RT, radiation therapy; NA, not applicable; SX, surgery; SA, sarcoma; CA, carcinoma; SCC, squamous cell carcinoma.
*Measurable disease remained in all dogs after the surgical procedure.
†Immunotherapy given using liposome-encapsulated MTP-PE.
‡Slow-release formulation of cisplatin given as chemotherapy.
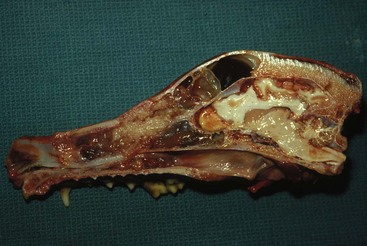
Figure 23-10 Cross-section of a dog’s skull with a typical intranasal carcinoma. Note the middle to caudal position of the tumor in the nasal cavity, the erosion of the dorsal nasal bones, the dark mucus in the frontal sinus secondary to obstruction, and the proximity of the tumor to the cribriform plate. Complete surgical resection is impossible.
RT using high-energy megavoltage (MeV) equipment (cobalt source or linear accelerator) as the sole treatment modality has become the therapy of choice for canine nasosinal tumors (Table 23-3; see also Table 23-2). It has the advantage of treating the entire nasal cavity, including bone, and its use has been associated with the greatest improvement in survival. Although surgical removal of the tumor before MeV irradiation has not been shown to improve clinical outcome,10,74,83 controlled studies have not been done for this combination. MST after MeV irradiation with curative intent (i.e., full-course or definitive radiation) alone ranges from 8 to 19.7 months.* The 1- and 2-year survival rates range from 43% to 68% (1 year) and 11% to 44% (2 years).63,64,76-78,86 Doses of 42 to 54 Gy are usually delivered in 10 to 18 treatments of 3- to 4.2-Gy fractions over 2 to 4 weeks to the nasal cavity and frontal sinuses as dictated by imaging.63-65,74,77-79 True statistical comparisons between reports are not possible because of inconsistencies in methodology (even within individual reports) with respect to total dose, fraction number, dose per fraction, treatment schedules, use of CT staging, use of computerized treatment planning, response monitoring, and statistical assessment. Furthermore, differences in tumor type and tumor stage also affect patient outcome.65 CT-based computerized treatment planning can greatly enhance normal tissue sparing while ensuring optimized dose distribution within the tumor. The MST ranges from 11 to 19.7 months in dogs treated with CT-staged, computer-planned MeV irradiation to a minimum of 41 Gy.* As computer-based radiation planning becomes more advanced in veterinary medicine, it follows that clinical outcomes may continue to improve.66
Table 23-3
Summary of Selected Articles Describing Palliative Radiation Therapy for Nasosinal Tumors in Dogs
RT can induce normal tissue complications in the radiation treatment field (see Chapter 12). Acute and late toxicities affect rapidly and slowly renewing tissues, respectively, and depend on daily dose to the tissue, total dose, overall treatment time, and volume of tissue treated.87 The severity of side effects can therefore vary among protocols and also between individuals on the same treatment schedule, depending on the tissues included in the radiation field. Acute toxicities associated with definitive, full-course irradiation of nasosinal tumors typically involve the oral cavity (oral mucositis), eye (keratoconjunctivitis and blepharitis), nasal cavity (rhinitis), and skin (desquamation) (Figure 23-11).63,64,76,79,88 Acute effects develop during the course of RT and resolve within 2 to 8 weeks after treatment.63,64,76,88 Oral antibiotics, pain medication, and/or ocular medication including artificial tears may be needed to support the patient during this period. Rarely, temporary esophagostomy or gastrostomy tube feedings may be indicated if oral mucositis is severe in order to maintain adequate nutritional intake.
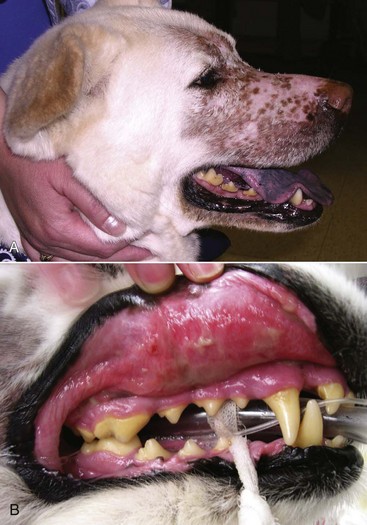
Figure 23-11 Potential acute effects of radiation therapy (RT) in dogs. A, Resolving desquamation one month after definitive RT for a nasal tumor. Note the hair loss in the radiation field. B, Oral mucositis on the last day of a definitive radiation protocol. Note the yellowish material on inner aspect of the upper lip. This represents dead epithelium that is being shed from the mucosa.
Late radiation effects, although less common than acute effects, are more detrimental and long lasting. Their development should be prevented, if possible, with thoughtful radiation treatment planning. Tissues that may be affected include the ocular lens (cataracts), cornea (keratitis, atrophy, keratoconjunctivitis sicca), anterior uvea (uveitis), retina (hemorrhage and degeneration), neuronal tissue (brain necrosis, causing neurologic changes and/or seizures, and optic nerve degeneration), bone (osteonecrosis), and skin (fibrosis).* Late complications develop months to years after therapy and are generally irreversible. The most commonly observed clinically relevant late effects in dogs treated with definitive RT for nasosinal tumors are those affecting ocular structures.68,76 Late ocular changes in the dog typically occur 6 to 9 months following RT and most often include keratoconjunctivitis sicca, cataract formation, and blindness in the irradiated eye if radiation doses are not limited.79 Other late effects are rare.
Overall, although most dogs with nasosinal neoplasia experience a favorable tumor response to RT, the long-term prognosis is poor (see Table 23-2). Even when treated with a definitive (curative-intent) radiation protocol, most dogs die or are euthanized as a result of local disease progression. An investigation of treatment failure patterns following full-course MeV irradiation showed that the median duration of local control in 24 dogs was 312 days.92 Marked tumor regression (90% reduction in size) was observed using CT in 46% of cases and was associated with a longer duration of local control than that seen in dogs in which tumor response to radiation was less favorable (389 versus 161 days).92 Most of the dogs in that series experienced local progressive disease, which affirms the need for more effective treatment strategies.
The following approaches have been investigated in an attempt to improve local control .
1. Full-course preoperative RT followed by surgical exenteration of residual or recurrent disease showed promise in a small series of dogs (n = 13), with a MST of 47 months compared to 19 months for dogs treated with radiation alone (see Table 23-2).78 The combination treatment was associated with an increased incidence of late effects, including rhinitis (bacterial and fungal), osteomyelitis, and fistula formation.78 A larger group of dogs must be treated in this manner to confirm these findings.
2. A logical and intuitive approach to improve local control is to increase radiation dose. This has been investigated using a boost technique in one study in which the total radiation dose was escalated to 57 Gy without an increase in overall treatment time. The treatment proved too toxic with respect to acute effects and resulted in radiation-related deaths in one-third of evaluated dogs.88 It appears that normal tissue tolerance may not allow dose escalation within standard overall treatment times using conventional radiation planning and delivery techniques.
3. The use of radiosensitizers in conjunction with ionizing radiation has been reported. A Veterinary Radiation Therapy Oncology Group (VRTOG) pilot study described the use of gemcitabine as a radiosensitizer for nasosinal carcinoma.93 Gemcitabine was given intravenously at a dosage of 50 mg/m2 twice weekly before daily RT. The authors reported significant hematologic toxicity (neutropenia) and local acute tissue complications associated with this dose and schedule. In another report, low-dose cisplatin (7.5 mg/m2 given intravenously every other day) administered in conjunction with full-course RT was well tolerated and did not appear to cause an increase in acute or late radiation effects.84 The efficacy of this approach with respect to improvement of clinical outcome is not known. A combination of radiation and cisplatin, administered intramuscularly throughout therapy using a slow-release polymer system (open cell polylactic acid polymer impregnated with platinum [OPLA-Pt]), has been shown to be well tolerated; however, survival times were similar to those in other studies that used RT alone (MST: 474 days).77
4. The goal of RT is to deliver the maximum radiation dose to the tumor while limiting the dose to surrounding normal tissues to their tolerance level.94 Currently, in veterinary medicine, maximally optimized radiation delivery is most widely achieved through the use of conventional computerized treatment planning that allows the use of multiple treatment fields directed at the patient from different angles, with differential weighting of beams, and beam shape modulators to tailor the distribution of radiation dose to each patient as much as possible. Even with the development of more advanced planning systems (i.e., 3-dimensional [3D] versus 2-dimensional [2D]), these conventional methods have limitations that prevent escalation of the radiation dose without also increasing complications.88
A potential solution to this challenge is the rapidly emerging technology called intensity-modulated RT (IMRT). IMRT achieves conformal distribution of radiation dose to the tumor while sparing sensitive normal tissues.79,95-98 Multiple radiation fields of nonuniform beam intensity are delivered with the goal of distributing the radiation dose among larger volumes of normal tissue. This results in modest (tolerable) doses to normal tissues and integration of the multiple beams into a higher total dose throughout the volume of the tumor. IMRT treatment plans are generated by “automated optimization” in which the computer itself determines the optimal nonuniform radiation exposure that must be delivered to give the desired conformal dose pattern.
Through specialized software, the delivery of IMRT is achieved by using a large number of beams of varying radiation intensity that are modulated spatially and temporally.95 Modulation of beam intensity is realized using a multileaf collimator, which is a collimator that is made of multiple leaves that move rapidly and independently under computer control, customizing beam shape and intensity to the patient. As a result, IMRT has the potential to achieve a much higher degree of target conformity and normal tissue sparing than conventional (2D or 3D conformal) treatment-planning techniques.79,95-97 The complex shape of nasosinal tumors and the surrounding critical structures, including eyes and brain, provide a strong rationale for application of IMRT with this tumor type. RT-related morbidity can be significantly reduced in dogs with nasosinal tumors when IMRT is used.79,96 A recent clinical study compared radiation-induced ocular toxicity in dogs with nasal tumors treated with IMRT to that of historical controls treated with conventional 2D RT.79 IMRT reduced the radiation dose delivered to the eyes and resulted in bilateral ocular sparing. This was in contrast to profound ocular morbidity observed in the historical control group. MSTs for both groups were similar. Paramount to the success of highly conformal RT is precise daily patient positioning.95 Millimeter variation in patient setup can result in underdosing of the tumor or overdosing of normal tissues. A recent study suggested that daily setup variation associated with conventional nonrigid immobilization techniques used in veterinary RT may be insufficient to ensure high-quality IMRT delivery over a multiple-week course of treatment for nasal tumors.98 In that study, daily image guidance was necessary to ensure daily setup precision. Rigid immobilization techniques (head and cranial body fixation devices) have been developed for veterinary patients99-102 and, in conjunction with daily image guidance, permit successful implementation of highly conformal therapy.98,99 Image-guided RT (IGRT) refers to the use of “on-board” imaging, most commonly CT, integrated into IMRT delivery units. Advanced IGRT systems, such as Varian’s Trilogy, Tomotherapy, and CyberKnife, are in use at select veterinary RT facilities,98,99,103 and availability is growing. IMRT/IGRT provides the theoretical opportunity for tumor dose escalation without increasing the number of radiation treatments and overall treatment time. This in principle could translate into improved tumor control.97 A newly emerging application of IMRT/IGRT in veterinary medicine is stereotactic RT (SRT). SRT refers to the delivery of high-dose (ablative) radiation in a single dose or a few fractions.104 The treatment is delivered with extreme accuracy, and the dose is pinpointed to the tumor, minimizing the effect on nearby organs. This technique is optimally suited for small, well-defined tumor targets, although the advancement of radiation delivery technology has allowed for successful application in larger tumors.104 The high degree of precision is achieved by delivering many (up to hundreds) irregular subfields using specialized software and sophisticated multileaf collimation to create a complex intensity pattern.104 SRT fractionation schedules usually involve daily treatment for 1 to 3 days, which has obvious practical advantages compared to conventionally fractionated schedules that take weeks to complete. SRT requires highly reproducible patient immobilization, high-fidelity image guidance and an intensity modulated radiation beam to achieve extreme precision.104 Nasal tumors provide an excellent rationale for use of SRT; however, efficacy with respect to tumor control and survival rates associated with this hypofractionated treatment has not yet been proved. Initial experiences in dogs with nasal tumors include treatment with doses up to 30 Gy delivered in three fractions or in single fraction treatments of up to 15 Gy.105,106 Toxicity and tumor control data are preliminary but appear favorable.105,106 Despite the multiple apparent advantages of IMRT and SRT, the high financial costs and logistical challenges associated with optimal use of this technology must be balanced. Controlled clinical trials that rigorously examine normal tissue effects and tumor responses are needed to validate superiority of IMRT/IGRT to conventional RT in veterinary medicine.
In contrast to curative-intent radiation protocols, treatment schedules that are less intensive can be used to palliate tumor-related clinical signs. The goal of palliative radiation is to improve quality of life without aiming to maximize tumor sterilization. Most palliative protocols involve coarse fractionated treatments (6 to 9 Gy) delivered weekly or biweekly.33,66,80-82 The result of this approach is temporary improvement of clinical signs in 66% to 100% of dogs and limited morbidity associated with acute side effects.66,80-82 The reported median duration of control of clinical signs ranges from 120 to 300 days.66,81 Acute toxicities affecting the oral mucosa and skin are generally mild and short lived.66,80-82 A multiinstitutional retrospective study reported development of chronic ocular toxicities in 13% of 39 dogs that had at least one eye irradiated.81 Reported MSTs range from 146 days to 300 days.33,66,80-82 Tumor stage (Adams stage I) and duration of clinical signs (>90 days) have been correlated with longer survival in cases receiving this type of radiation.66,81 While true statistical comparisons between studies are not possible because of inconsistencies in methodology, the best clinical outcome in terms of duration of resolution of clinical signs and survival (approximately 300 days for both) is reported in a study of 38 dogs in which radiation treatment planning was performed using CT-staging and 3D-conformal computerized treatment planning software.66 In contrast to other studies, radiation delivery was planned using standardized dose and volume specifications ensuring uniform tumor dose distributions. The authors suggested that sophisticated radiation treatment planning in canine nasal tumor patients treated palliatively may result in more favorable clinical outcomes than previously reported.66
A recent report suggested that some dogs with recurrent nasal tumors can experience a second clinical remission following reirradiation of the tumor.107 The study evaluated a selected cohort of nine dogs in which the median time to tumor progression after a first full course of RT was 513 days. The first course of radiation delivered a median of 50 Gy in a median of 18 fractions. The median total dose used in the second protocol was 36 Gy, delivered in approximately 2-Gy fractions. The overall MST for this small, selection-biased cohort of dogs was 927 days (95% confidence interval [CI] 423 days to 1767 days). All dogs developed one or more late effects involving the skin, eyes, and nasal cavity. Late effects in seven of the nine dogs were considered mild and did not affect quality of life. Severe late effects were reported in two dogs, and both had sudden blindness that led to euthanasia. The authors concluded that there is a population of canine nasal tumor patients (i.e., those with slowly progressive tumors) that will benefit from reirradiation. Further work is needed to refine time-dose prescriptions to minimize radiation-induced late effects. Conformal techniques such as IMRT could play a role in this setting to limit morbidity.
Chemotherapy is used rarely as a sole therapy for canine nasosinal tumors. Treatment with cisplatin alone has been shown to benefit some dogs.108 A clinical response rate of 27%, including one radiographically confirmed complete remission, was reported in a small series of 11 dogs.108 All of the dogs experienced alleviation of clinical signs, and the MST was 5 months. Another report evaluated a combination chemotherapy protocol of doxorubicin, carboplatin, and oral piroxicam in eight dogs with advanced nasal tumors.109 A clinical response rate of 75% was observed, including 4 CRs confirmed by CT imaging. All dogs experienced resolution of clinical signs, and the protocol was well tolerated. MST in these eight dogs was 210 days (range 150 days to 960 days). These preliminary results are favorable, but the case number is small and more dogs need to be treated in this manner to confirm these findings.
Other therapeutic approaches to nasal tumors that have been investigated include proton-beam therapy, brachytherapy, immunotherapy, cryotherapy, and photodynamic therapy (PDT). Charged particles like protons have a well-defined tissue range and sharp dose fall off, which could potentially be exploited for conformal tumor targeting and normal tissue sparing.110 A small clinical trial involving nine dogs with nasal tumors treated with proton-beam therapy resulted in tumor responses and survival times similar to those reported for MeV irradiation.110 Acute effects of the skin and eyes were pronounced in some dogs, and 50% of dogs developed radiation-induced cataracts. Due to limited availability of proton irradiators, this technology is unlikely to be optimized for use in dogs. Intracavitary radiation using radioactive isotopes (brachytherapy) has been evaluated after surgical removal of nasosinal tumors in dogs.111,112 Potential problems associated with this type of radiation include dose distribution and radiation exposure to personnel. The question of whether brachytherapy improves survival over traditional external-beam radiation has not been answered. Immunotherapy and cryosurgery have not improved survival times.1,113 A recent case report described the use of image-guided cryotherapy in the treatment of a rapidly recurrent nasal carcinoma that resulted in long-term tumor control and survival.114 The authors suggested that further investigation of this technique may be warranted for the management of focal residual or recurrent nasal tumors. Reports evaluating PDT have been published; however, results are too preliminary to draw any conclusions.115,116
When all treatments fail to control epistaxis, unilateral or bilateral carotid artery ligation can palliate the symptoms in dogs for up to 3 months or longer without damage to the brain.58
The importance of prognostic factors in the treatment of canine nasosinal tumors remains controversial. Negative predictors of survival from various studies include age (>10 years),68 epistaxis,38 duration of clinical signs,81 advanced local tumor stage,63-66,68 metastatic disease,10,68 histologic subtype (carcinoma, particularly squamous cell or undifferentiated),63,65,74 and failure to achieve resolution of clinical signs.81 An analysis of 94 dogs with varying subtypes of nasal carcinoma or sarcoma treated with curative RT at three veterinary facilities was recently performed.65 A correlation was demonstrated between clinical outcome and the original Adams tumor staging scheme,64 as well as the modified Adams tumor system.65 Based on these findings, dogs with cribriform plate involvement as determined by CT imaging have the shortest disease-free survival (DFS) and overall survival (OS). This subset of dogs had a median DFS of 3.8 months and a median OS of almost 7 months, compared to dogs with unilateral tumors without bone involvement in which DFS and OS were 6.5 and 23 months, respectively. The study also showed that prognosis varied based on histologic category. When grouped together, dogs with anaplastic carcinoma, undifferentiated carcinoma, and SCC had a shorter DFS. There was no effect on clinical outcome when all carcinomas were compared with all sarcomas. The statistically strongest association with clinical outcome was made when clinical stage was combined with histologic findings.
A major pitfall of many veterinary studies with respect to assessment of treatment efficacy in canine nasosinal cancer is the evaluable endpoint. Tumor response and time to tumor progression are the most representative measures of treatment efficacy. Regular diagnostic imaging, ideally CT or MRI, would be required to allow accurate determination of these endpoints. Due to high costs associated with imaging and the need for anesthesia, follow-up is rarely done in this manner. Analyzing the return of clinical signs as an indication of tumor recurrence is problematic since similar signs can result from rhinitis secondary to therapy (radiation and/or surgery) or residual tumor.117 Assessment of the survival time, as is commonly done in most veterinary nasosinal cancer studies, may be biased by the use of additional treatments on suspicion of progressive disease and by the decision for euthanasia, which can vary greatly from one pet owner to another. Furthermore, inconsistencies in methodology between studies and even within individual reports, as well as lack of controlled studies, are other limitations that have prevented the informed development of the optimal treatment approach to nasosinal tumors in dogs.
Feline Nasosinal Tumors
Nasal and sinus cavity tumors in the cat are malignant in more than 90% of the histologically diagnosed cases. They occur in an older population of cats with a mean age reported between 9 and 10 years.118-120 In general, these tumors are locally invasive and associated with a low metastatic rate at the time of diagnosis.119,121
Clinical signs related to nasosinal tumors in cats overlap with those of other causes of chronic nasal disease.118,120 These include nasal discharge, upper respiratory tract dyspnea, sneezing, epistaxis, facial swelling, ocular discharge, and weight loss.118-120,122-124 Although each of these signs can occur with both neoplasia and rhinitis, in some reports, certain signs are more commonly associated with neoplasia such as unilateral discharge or epistaxis,118 whereas in others, the character of the clinical signs does not distinguish the underlying cause.120 The median duration of clinical signs prior to diagnosis is several months, and many cats will experience temporary alleviation of clinical signs with use of antibiotics and or glucocorticosteroids.118-120 Differential diagnoses for chronic nasal signs include chronic rhinitis, infectious rhinitis, foreign body, nasal polyp, nasopharyngeal stenosis, and trauma.
Lymphoma is the most commonly diagnosed tumor type in the feline nasal cavity and sinuses followed by epithelial neoplasms (carcinoma, adenocarcinoma, SCC). Less frequently reported tumor types include sarcomas (fibrosarcoma, osteosarcoma, chondrosarcoma), mast cell tumor, melanoma, plasmacytoma, olfactory neuroblastoma, and benign lesions such as nasal hamartoma, chondroma, and neurofibroma.118-120,125,126
Diagnostic principles are similar to those in the dog. A tissue sample is required to make a definitive diagnosis of cancer in most cases. DeLorenzi et al evaluated cytology from squash preparations obtained from endoscopic biopsies of nasopharyngeal masses in cats and found that cytologic results were in good agreement with histopathology with an overall accuracy of 90%.127 However, distinguishing lymphoma from lymphoid inflammatory disease was not as accurate and histopathologic confirmation was recommended.127 Another study evaluated the histopathologic and cytologic features of nasal lymphoma in 50 cases. These authors found that 91% of cases were classified as immunoblastic lymphoma according to the National Institutes of Health (NIH) working formulation. The majority of cases were B-cell (68%) and 20% were T-cell, with 12% having a mixed population of B- and T-cells.125
Radiographs of the nasal cavity have been reported as a diagnostic tool in cats with both chronic rhinitis and nasal neoplasia. Although no radiographic sign is entirely specific for neoplasia, findings with the highest predictive value for cancer include displacement of midline structures, unilateral changes such as soft tissue opacity and loss of turbinate detail, and evidence of bone invasion.128 Similarly, as CT scan becomes a common imaging tool, reports of the characteristic findings of scans from cats with sinonasal disease of all etiologies have been published.129-131 One retrospective assessment of CT imaging in 62 cats with nasosinal disease showed that, although certain findings such as osteolysis of paranasal bones, extension of disease into the orbit of facial soft tissues, the presence of a space-occupying mass, and turbinate destruction may suggest a CT diagnosis of neoplasia over rhinitis, nasal biopsy is necessary for confirmation.129 Another study evaluated the clinical characteristics and CT findings in 43 cats and found that those with neoplasia were significantly more likely to have unilateral lysis of the ethmoturbinates as well as dorsal and lateral maxilla, lysis of the vomer bone and ventral maxilla, and unilateral soft tissue or fluid in the sphenoid recess, frontal sinus, or retrobulbar space. Interestingly, in that study population, cribriform plate lysis was not significantly associated with neoplasia.130 In another report describing CT findings in cats with confirmed fungal rhinitis, they found that some of the features overlap with those seen in neoplasia patients, including older age at the time of diagnosis, soft tissue mass, and osteolysis. Although these studies confirm the utility of CT scan determining extent of disease, no one group of features replaces histopathology for definitive diagnosis.
Even though the metastatic rate of feline nasosinal cancer at the time of diagnosis is reportedly low, any enlarged regional lymph nodes should be evaluated cytologically to differentiate a reactive process from metastasis. In a recent report of 123 cases of feline nasosinal cancer, 21 cats had regional lymphadenopathy. None showed cytologic evidence of metastasis.119
As in the dog, RT continues to be the predominant local therapy of choice for this disease. Reports of treatment for feline nonlymphoma nasosinal tumors are few, and case numbers are small.121,132 In the largest published study, 16 cats with nonlymphoproliferative neoplasms were treated using a definitive course of radiation to a total dose of 48 Gy. The therapy was well tolerated, and MST was 12 months with 44% and 16% of cats alive at 1 and 2 years, respectively.121 In another report of radiation treatment for nonlymphoproliferative tumors, this time treated using a coarse fractionation regime, results showed that the protocol was well tolerated, with a median survival of almost 13 months and a 63% 1-year survival rate reported.132
Recent reports of feline nasal and nasal pharyngeal lymphoma treated with radiation and/or chemotherapy indicate the potential for long-term survival (Table 23-4).122-125,133 Although treatment protocols vary between reports, in general, overall response rate for feline nasal lymphoma is high, between 70% and 90%. The inclusion of RT appears to enhance overall survival with median survival for combination therapy ranging from 174 to 955 days.123,124 Interestingly, systemic failure of disease at nonnasal sites is also reported in 13% to 16% of cases, indicating that a combination of RT and chemotherapy may be warranted in some patients. However, the timing of such treatment remains to be determined (concurrent versus sequential), as does which patients are at highest risk for local failure. Furthermore, improved radiation treatment planning along with optimized radiation treatment schedules and doses may prove to ameliorate local control rates and reduce the risk of systemic failure without the addition of chemotherapy.
Comparative Aspects134
In humans, cancer of the nasal cavity and paranasal sinuses is classified with other cancers of the upper aerodigestive tract under the all-encompassing title, tumors of the head and neck. Tumors of four anatomically defined regions are included: nasal cavity and paranasal sinuses, nasopharynx, oral cavity, and oropharynx. The majority of tumors affecting these sites are SCCs (head and neck SCC [HNSCC]). However, a large variety of histologically distinct cancers can develop in the nasal cavity and paranasal sinuses, including adenocarcinoma, esthesioneuroblastoma, lymphoma, melanoma, angiosarcoma, and tumors of connective tissues.
Tumors specifically of the nasal cavity and paranasal sinuses are rare in humans and represent the smallest proportion of head and neck cancers, with a yearly risk of 1 in every 100,000 people. They generally affect persons between the ages of 45 and 85 years old and are twice as common in males as females. The etiologic factors for the disease are predominantly environmental and workplace in nature and include exposure to wood, textile, and leather dusts, as well as flour, nickel, chromium, and radium. Other factors associated with increased risk for all head and neck cancers include smoking and alcohol use, which may have an additive effect.
Tumors at this site are locally invasive. They are typically advanced at the time of diagnosis because lesions in this area can go undetected for extended periods of time and clinical signs can mimic those of infectious or inflammatory disease. The likelihood of lymph node metastasis at the time of diagnosis is low—reportedly between 10% and 15%, depending on the location of the primary tumor.
Surgery and RT are the mainstay of curative treatment when considered possible, based on stage and location of disease. Chemotherapy can be useful in an adjuvant setting, but it is not curative when used alone. Both radiation and surgery have advantages and disadvantages as primary therapies. Overall, survival at 5 years is approximately 60% and 30% for nasal cavity and parasinal tumors, respectively. More favorable prognoses are associated with smaller lesions in which complete surgical resection is achievable. The main form of treatment failure is local recurrence, which is caused by incomplete local control of advanced tumors in many cases. When adequate margins cannot be obtained, surgery and radiation are often combined.
The role of chemotherapy in cancer of the nasal cavity and paranasal sinuses is not clear because these cases are usually reported in conjunction with other head and neck tumors. Active chemotherapy agents include the taxanes, 5-fluorouracil (5-FU), and the platinum drugs. Several studies have reported improved local control and, in some cases, improved overall survival in treatment protocols that combine traditional fractionated radiation and chemotherapy; however, the toxicity may also be increased. The optimal case selection, chemotherapy agents, and radiation schedule has yet to be defined.
Clinical trials of targeted therapies for head and neck cancer have been done and most involve EGFR antagonists. Cetuximab (Erbitux), a chimeric (mouse/human) monoclonal antibody against EGFR, has been shown to improve locoregional control and survival when used as a radiation sensitizer in people with HNSCC undergoing RT with curative intent. It has been approved by the Food and Drug Administration (FDA) for this indication, as well as for use as a single agent in patients whose cancer has progressed on platinum-containing therapy. Positive results associated with cetuximab have had a significant impact on the standard of care for advanced HNSCC. The subset of patients who might benefit from these types of treatments will need to be identified in order to maximize their use and improve outcome for this disease.
References
1. MacEwen, EG, Withrow, SJ, Patnaik, AK. Nasal tumors in the dog: retrospective evaluation of diagnosis, prognosis, and treatment. J Am Vet Med Assoc. 1977;170:45–48.
2. Patnaik, AK. Canine sinonasal neoplasms: Clinicopathological study of 285 cases. J Am Anim Hosp Assoc. 1989;25:103–114.
3. Lefebvre, J, Kuehn, NJ, Wortinger, A. Computed tomography as an aid in the diagnosis of chronic nasal disease in dogs. J Small Anim Pract. 2005;46:280–285.
4. Strunzi, H, Hauser, B. Tumors of the nasal cavity. Bull World Health Organ. 1976;53:257–263.
5. Reif, JS, Cohen, D. The environmental distribution of canine respiratory tract neoplasms. Arch Environ Health. 1971;22:136–140.
6. Bukowski, JA, Wartenberg, D. Environmental causes for sinonasal cancers in pet dogs, and their usefulness as sentinels of indoor cancer risk. J Toxicol Environ Health. 1998;54:579–591.
7. Reif, JS, Bruns, C, Lower, KS. Cancer of the nasal cavity and paranasal sinuses and exposure to environmental tobacco smoke in pet dogs. Am J Epidemiol. 1998;147:488–492.
8. Madewell, BR, Priester, WA, Gillette, EL, et al. Neoplasms of the nasal passages and paranasal sinuses in domesticated animals as reported by 13 veterinary colleges. Am J Vet Res. 1976:851–856.
9. Patnaik, AK, Lieberman, PH, Erlandson, RA, et al. Canine sinonasal skeletal neoplasms: Chondrosarcomas and osteosarcomas. Vet Pathol. 1984;21:475–482.
10. Henry, CJ, Brewer, WG, Tyler, JW, et al. Survival in dogs with nasal adenocarcinoma: 64 cases (1981-1995). J Vet Intern Med. 1998;12:436–439.
11. Hahn, KA, Matlock, CL. Nasal adenocarcinoma metastatic to bone in two dogs. J Am Vet Med Assoc. 1990;197(4):491–494.
12. Hahn, KA, McGavin, MD, Adams, WH. Bilateral renal metastases of nasal chondrosarcoma in a dog. Vet Pathol. 1997;34:352–355.
13. Northrup, NC, Etue, SM, Ruslander, DM, et al. Retrospective study of orthovoltage radiation therapy for nasal tumors in 42 dogs. J Vet Intern Med. 2001;15:183–189.
14. Snyder, MK, Lipitz, L, Skorupski, KA, et al. Secondary intracranial neoplasia in the dog: 177 cases (1986-2003). J Vet Intern Med. 2008;22:172–177.
15. Kaldrymidou, E, Papaioannou, N, Poutahidis, T, et al. Malignant lymphoma in nasal cavity and paranasal sinuses of a dog. J Vet Med A Physiol Pathol Clin Med. 2000;47(8):457–462.
16. Naganobu, K, Ogawa, H, Uchida, K, et al. Mast cell tumor in the nasal cavity of a dog. J Vet Med Sci. 2000;62(9):1009–1011.
17. Weir, EC, Pond, MJ, Duncan, JR, et al. Extragenital occurrence of transmissible venereal tumor in the dog: literature review and case reports. J Am Anim Hosp Assoc. 1978;14:532–536.
18. Perez, J, Bautista, MJ, Carrasco, L, et al. Primary extragenital occurrence of transmissible venereal tumors: three case reports. Can Pract. 1994;19(1):7–10.
19. Ginel, PJ, Molleda, JM, Novales, M, et al. Primary transmissible venereal tumour in the nasal cavity of a dog. Vet Rec. 1995;136:222–223.
20. Papzoglou, LG, Koutinas, AF, Plevraki, AG, et al. Primary intranasal transmissible venereal tumour in the dog: a retrospective study of six spontaneous cases. J Vet Med A Physiol Pathol Clin Med. 2001;48(7):391–400.
21. Patnaik, AK. Canine sinonasal neoplasms: soft tissue tumors. J Am Anim Hosp Assoc. 1989;25:491–497.
22. Patnaik, AK, Ludwig, LL, Erlandson, RA. Neuroendocrine carcinoma of the nasopharynx in a dog. Vet Pathol. 2002;39:496–500.
23. Miles, MS, Dhaliwal, RS, Moore, MP, et al. Association of magnetic resonance imaging findings and histologic diagnosis in dogs with nasal disease: 78 cases (2001-2004). J Am Vet Med Assoc. 2008;232:1844–1849.
24. Hicks, DG, Fidel, JL. Intranasal malignant melanoma in a dog. J Am Anim Hosp Assoc. 2006;42:472–476.
25. Ueno, H, Kobayashi, Y, Yamada, K. Olfactory esthesioneuroblastoma treated with orthovoltage radiotherapy in a dog. Aust Vet J. 2007;85:271–275.
26. Kitagawa, M, Okada, M, Yamamura, H, et al. Diagnosis of olfactory neuroblastoma in a dog by magnetic resonance imaging. Vet Rec. 2006;159:288–289.
27. LeRoith, T, Binder, EM, Graham, AH, et al. Respiratory epithelial adenomatoid hamartoma in a dog. J Vet Diagn Invest. 2009;21:918–920.
28. Fujita, M, Takaishi, Y, Yasuda, D, et al. Intranasal hemangiosarcoma in a dog. J Vet Med Sci. 2008;70:525–528.
29. Gamblin, FM, Sagartz, JE, Guillermo, C. Overexpression of p53 tumor suppressor protein in spontaneously arising neoplasms of dogs. Am J Vet Res. 1997;58(8):857–863.
30. Kleiter, MK, Malarkey, DE, Ruslander, DE, et al. Expression of cyclooxygenase-2 in canine epithelial nasal tumors. Vet Radiol Ultrasound. 2004;45(3):255–260.
31. Borzacchiello, G, Paciello, O, Papparella, S. Expression of cyclooxygenase-1 and -2 in canine nasal carcinomas. J Comp Pathol. 2004;131(1):70–76.
32. Impellizeri, JA, Esplin, DG. Expression of cyclooxygenase-2 in canine nasal carcinomas. Vet J. 2008;176:408–410.
33. Belshaw, Z, Constantio-Casas, F, Brearley, MJ, et al. COX-2 expression and outcome in canine nasal carcinomas treated with hypofractionated radiotherapy. Vet Comp Oncol. 2010;9:141–148.
34. Shiomitsu, K, Johnson, CL, Malarkey, DE, et al. Expression of epidermal growth factor receptor and vascular endothelial growth factor in malignant canine epithelial nasal tumours. Vet Comp Oncol. 2009;7:106–114.
35. Paciello, O, Borzacchiello, G, Varricchio, E, et al. Expression of peroxisome proliferator-activated receptor gamma (PPAR-γ) in canine nasal carcinomas. J Vet Med A Physiol Pathol Clin Med. 2007;54:406–410.
36. Vanherberghen, M, Day, MJ, Delvaux, F, et al. An Immunohistochemical study of the inflammatory infiltrate associated with nasal carcinoma in dogs and cats. J Comp Pathol. 2009;141:17–26.
37. Nakaichi, M, Yunuki, T, Okuda, M, et al. Activity of matrix metalloproteinase-2 (MMP-2) in canine oronasal tumors. Res Vet Sci. 2007;82:271–279.
38. Rassnick, KM, Goldkamp, CE, Erb, HN, et al. Evaluation of factors associated with survival in dogs with untreated nasal carcinomas: 139 cases (1993-2003). J Am Vet Med Assoc. 2006;229:401–406.
39. Saunders, JH, Van Bree, H, Gielen, I, et al. Diagnostic value of computed tomography in dogs with chronic nasal disease. Vet Radiol Ultrasound. 2003;44(4):409–413.
40. Burrow, RD. A nasal dermoid sinus in an English bull terrier. J Small Anim Pract. 2004;45(11):572–574.
41. Beck, JA, Hunt, GB, Goldsmid, SE, et al. Nasopharyngeal obstruction due to cystic Rathke’s clefts in two dogs. Aust Vet J. 1999;77:94–96.
42. Lobetti, RG. A retrospective study of chronic nasal disease in 75 dogs. J S Afr Vet Assoc. 2009;80:224–228.
43. Strasser, JL, Hawkins, EC. Clinical features of epistaxis in dogs: a retrospective study of 35 cases (1999-2002). J Am Anim Hosp Assoc. 2005;41:179–184.
44. Bissett, SA, Drobatz, KJ, McKnight, A, et al. Prevalence, clinical features, and causes of epistaxis in dogs: 176 cases (1996-2001). J Am Vet Med Assoc. 2007;231:1843–1850.
45. Smith, MO, Turrel, JM, Bailey, CS, et al. Neurologic abnormalities as the predominant signs of neoplasia of the nasal cavity in dogs and cats: Seven cases (1973-1986). J Am Vet Med Assoc. 1989;195:242–245.
46. Thrall, DE, Robertson, ID, McLeod, DA, et al. A comparison of radiographic and computed tomographic findings in 31 dogs with malignant nasal cavity tumors. Vet Radiol. 1989;30:59–66.
47. Park, RD, Beck, ER, LeCouteur, RA. Comparison of computed tomography and radiography for detecting changes induced by malignant nasal neoplasia in dogs. J Am Vet Med Assoc. 1992;201:1720–1724.
48. Codner, EC, Lurus, AG, Miller, JB, et al. Comparison of computed tomography with radiography as a noninvasive diagnostic technique for chronic nasal disease in dogs. J Am Vet Med Assoc. 1993;202:1106–1110.
49. Drees, R, Forrest, LJ, Chappell, R. Comparison of computed tomography and magnetic resonance imaging for the evaluation of canine intranasal neoplasia. J Small Anim Pract. 2009;50:334–340.
50. Avner, A, Dobson, JM, Sales, JI, et al. Retrospective review of 50 canine nasal tumours evaluated by low-field magnetic resonance imaging. J Small Anim Pract. 2008;49:233–239.
51. Agthe, P, Caine, AR, Gear, RNA, et al. Prognostic significance of specific magnetic resonance imaging features in canine nasal tumours treated by radiotherapy. J Small Anim Pract. 2009;50:641–648.
52. Petite, AFB, Dennis, R. Comparison of radiography and magnetic resonance imaging for evaluating the extent of nasal neoplasia in dogs. J Small Anim Pract. 2006;47:529–536.
53. Burk, RL. Computed tomographic imaging of nasal disease in 100 dogs. Vet Radiol Ultrasound. 1992;33(3):177–180.
54. Gibbs, C, Lane, JG, Denny, HR. Radiological features of intra-nasal lesions in the dog: A review of 100 cases. J Small Anim Pract. 1979;20:515–535.
55. Russo, M, Lamb, CR, Jakovljevic, S. Distinguishing rhinitis and nasal neoplasia by radiography. Vet Radiol Ultrasound. 2000;41(2):118–124.
56. Forrest, LJ, Thrall, DE. Oncologic applications of diagnostic imaging techniques. Vet Clin North Am Small Anim Pract. 1995;25(1):185–205.
57. Withrow, SJ, Susaneck, SJ, Macy, DW, et al. Aspiration and punch biopsy techniques for nasal tumors. J Am Anim Hosp Assoc. 1985;21:551–554.
58. Rudd, RG, Richardson, DC. A diagnostic and therapeutic approach to nasal disease in dogs. Compend Contin Educ Pract Vet. 1985;7:103–112.
59. Clendenin, MA, Conrad, MC. Collateral vessel development after chronic bilateral common carotid artery occlusion in the dog. Am J Vet Res. 1979;40:1244.
60. Clercx, C, Wallon, J, Gilbert, S, et al. Imprint and brush cytology in the diagnosis of canine intranasal tumours. J Small Anim Pract. 1996;37:423–427.
61. Lent, SEF, Hawkins, EC. Evaluation of rhinoscopy and rhinoscopy-assisted mucosal biopsy in diagnosis of nasal disease in dogs: 199 cases (1985-1989). J Am Vet Med Assoc. 1992;201:1425–1429.
62. Owen, LN. TNM Classification of tumors in domestic animals. Geneva: World Health Organization; 1980.
63. Theon, AP, Madewell, BR, Harb, MF, et al. Megavoltage irradiation of neoplasms of the nasal and paranasal cavities in 77 dogs. J Am Vet Med Assoc. 1993;202:1469–1475.
64. Adams, WM, Miller, PE, Vail, DM, et al. An accelerated technique for irradiation of malignant canine nasal and paranasal sinus tumors. Vet Radiol Ultrasound. 1998;39:475–481.
65. Adams, WM, Kleiter, MM, Thrall, DE, et al. Prognostic significance of tumor histology and computed tomographic staging for radiation treatment response of canine nasal tumors. Vet Radiol Ultrasound. 2009;50:330–335.
66. Buchholz, J, Hagen, R, Leo, C, et al. 3D conformal radiation therapy for palliative treatment of canine nasal tumors. Vet Radiol Ultrasound. 2009;50:679–683.
67. Kondo, Y, Matsunaga, S, Mochizuki, M, et al. Prognosis of canine patients with nasal tumors according to modified clinical stages based on computed tomography: a retrospective study. J Vet Med Sci. 2008;70:207–212.
68. LaDue, TA, Dodge, R, Page, RL, et al. Factors influencing survival after radiotherapy of nasal tumors in 130 dogs. Vet Radiol Ultrasound. 1999;40:312–317.
69. Couto, CF, Boudrieau, RJ, Zanjani, ED. Tumor-associated erythrocytosis in a dog with nasal fibrosarcoma. J Vet Intern Med. 1989;3:183–185.
70. Wilson, RB, Bronstad, DC. Hypercalcemia associated with nasal adenocarcinoma in a dog. J Am Vet Med Assoc. 1983;182:1246–1247.
71. Anderson, GM, Lane, I, Fischer, J, et al. Hypercalcemia and parathyroid hormone-related protein in a dog with undifferentiated nasal carcinoma. Can Vet J. 1999;40:341–342.
72. Laing, EJ, Binnington, AG. Surgical therapy of canine nasal tumors: a retrospective study (1982-1986). Can Vet J. 1988;29:809–813.
73. Holmberg, DL, Fries, C, Cockshutt, J, et al. Ventral rhinotomy in the dog and cat. Vet Surg. 1989;18:446–449.
74. Adams, WM, Withrow, SJ, Walshaw, R, et al. Radiotherapy of malignant nasal tumors in 67 dogs. J Am Vet Med Assoc. 1987;191:311–315.
75. Evans, SM, Goldschmidt, M, McKee, LJ, et al. Prognostic factors and survival after radiotherapy for intranasal neoplasms in dogs: 70 cases (1974-1985). J Am Vet Med Assoc. 1989;194:1460–1463.
76. McEntee, MC, Page, RL, Heidner, GL, et al. A retrospective study of 27 dogs with intranasal neoplasms treated with cobalt radiation. Vet Radiol Ultrasound. 1991;32:135–139.
77. Lana, SE, Dernell, WS, Lafferty, MS, et al. Use of radiation and a slow-release cisplatin formulation for treatment of canine nasal tumors. Vet Radiol Ultrasound. 2004;45:1–5.
78. Adams, WM, Bjorling, DE, McAnulty, JF, et al. Outcome of accelerated radiotherapy alone or accelerated radiotherapy followed by exenteration of the nasal cavity in dogs with intranasal neoplasia: 53 cases (1993-2002). J Am Vet Med Assoc. 2005;227:936–941.
79. Lawrence, JA, Forrest, LJ, Turek, MM, et al. Proof of principle of ocular sparing in dogs with sinonasal tumors treated with intensity-modulated radiation therapy. Vet Radiol Ultrasound. 2010;51:561–570.
80. Mellanby, RJ, Stevenson, RK, Herrtage, ME, et al. Long-term outcome of 56 dogs with nasal tumours treated with four doses of radiation at intervals of seven days. Vet Rec. 2002;151:253–257.
81. Geiger, T, Rassnick, K, Siegel, S, et al. Palliation of clinical signs in 48 dogs with nasal carcinomas treated with coarse-fraction radiation therapy. J Am Anim Hosp Assoc. 2008;44:116–123.
82. Maruo, T, Shida, T, Fukuyama, Y, et al. Retrospective study of canine nasal tumor treated with hypofractionated radiotherapy. J Vet Med Sci. 2011;73:193–197.
83. Morris, JS, Dunn, KJ, Dobson, JM, et al. Effects of radiotherapy alone and surgery and radiotherapy on survival of dogs with nasal tumours. J Small Anim Pract. 1994;35:567–573.
84. Nadeau, M, Kitchell, BE, Rooks, RL, et al. Cobalt radiation with or without low-dose cisplatin for treatment of canine naso-sinus carcinomas. Vet Radiol Ultrasound. 2004;45(4):362–367.
85. Yoon, JH, Feeney, DA, Jessen, CR, et al. External-beam Co-60 radiotherapy for canine nasal tumors: a comparison of survival by treatment protocol. Res Vet Sci. 2008;84:140–149.
86. Hunley, DW, Mauldin, GN, Shiomitsu, K, et al. Clinical outcome in dogs with nasal tumors treated with intensity-modulated radiation therapy. Can Vet J. 2010;51:293–300.
87. Hall, EJ, Giaccia, AJ. Time, dose, and fractionation in radiotherapy. In Hall EJ, et al, eds.: Radiobiology for the radiologist, ed 6, Philadelphia: Lippincott Williams & Wilkins, 2006.
88. Thrall, DE, McEntee, MC, Novotney, C. A boost technique for irradiation of malignant canine nasal tumors. Vet Radiol Ultrasound. 1993;34(4):295–300.
89. Jamieson, VE, Davidson, MG, Nasisse, MP, et al. Ocular complications following cobalt 60 radiotherapy of neoplasms in the canine head region. J Am Anim Hosp Assoc. 1991;27:51–55.
90. Ching, SV, Gillette, SM, Powers, BE, et al. Radiation-induced ocular injury in the dog: a histological study. Int J Radiation Oncology Biol Phys. 1990;19:321–328.
91. Roberts, SM, Lavach, JD, Severin, GA, et al. Ophthalmic complications following megavoltage irradiation of the nasal and paranasal cavities in dogs. J Am Vet Med Assoc. 1987;100:43–47.
92. Thrall, DE, Heidner, GL, Novotny, CA, et al. Failure patterns following cobalt irradiation in dogs with nasal carcinoma. Vet Radiol Ultrasound. 1993;34(2):126–133.
93. LeBlanc, AK, LaDue, TA, Turrel, JM, et al. Unexpected toxicity following use of gemcitabine as a radiosensitizer in head and neck carcinomas: a veterinary radiation therapy oncology group pilot study. Vet Radiol Ultrasound. 2004;45(5):466–470.
94. McEntee, MC. Veterinary radiation therapy: Review and current state of the art. J Am Anim Hosp Assoc. 2006;42:94–109.
95. Hong, TS, Ritter, MA, Tome, WA, et al. Intensity-modulated radiation therapy: emerging cancer treatment technology. Br J Cancer. 2005;92:1819–1824.
96. Vaudaux, C, Schneider, U, Kaser-Hotz, B. Potential for intensity-modulated radiation therapy to permit dose escalation for canine nasal cancer. Vet Radiol Ultrasound. 2007;48:475–481.
97. Guttierrez, AN, Deveau, M, Forrest, LJ, et al. Radiobiological and treatment planning study of a simultaneously integrated boost for canine nasal tumors using helical tomotherapy. Vet Radiol Ultrasound. 2007;48:594–602.
98. Deveau, MA, Gutierrez, AN, Mackie, TR, et al. Dosimetric impact of daily setup variations during treatment of canine nasal tumors using intensity-modulated radiation therapy. Vet Radiol Ultrasound. 2010;51:90–96.
99. Harmon, J, Van Ufflen, D, LaRue, S. Assessment of a radiotherapy patient cranial immobilization device using daily on-board kilovoltage imaging. Vet Radiol Ultrasound. 2009;50:230–234.
100. Rohrer Bley, C, Blattmann, H, Roos, M, et al. Assessment of a radiotherapy patient immobilization device using single plane port radiographs and a remote computed tomography scanner. Vet Radiol Ultrasound. 2003;44:470–475.
101. Kippenes, H, Gavin, PR, Sande, RD, et al. Comparison of the accuracy of positioning devices for radiation therapy of canine and feline head tumors. Vet Radiol Ultrasound. 2000;41:371–376.
102. Kent, MS, Gordon, IK, Benavides, I, et al. Assessment of the accuracy and precision of a patient immobilization device for radiation therapy in canine head and neck tumors. Vet Radiol Ultrasound. 2009;50:550–554.
103. Forrest, LJ, Mackie, TR, Ruchala, K, et al. The utility of megavoltage computed tomography images from a helical tomotherapy system for setup verification purposes. Int J Radiat Oncol Biol Phys. 2004;60:1639–1644.
104. Martin, A, Gaya, A. Stereotactic body radiotherapy: A review. Clin Oncol. 2010;22:157–172.
105. Communication, Personal, Custis, J, Harmon, J, Ryan, S, et al. Stereotactic radiation therapy for the treatment of canine nasal tumors. Vet Cancer Soc Proc. 2010;30:46.
106. Personal CommunicationCharney, S, Witten, M, Ettinger, S, et al. Cyber knife radiosurgery for irradiation of tumors in dogs and cats. Vet Cancer Soc Proc. 2010;30:97.
107. Bommarito, DA, Kent, MS, Selting, KA, et al. Reirradiation of recurrent canine nasal tumors. Vet Radiol Ultrasound. 2011;52:207–212.
108. Hahn, KA, Knapp, DW, Richardson, RC, et al. Clinical response of nasal adenocarcinoma to cisplatin chemotherapy in 11 dogs. J Am Vet Med Assoc. 1992;200:355–357.
109. Langova, V, Mutsaers, AJ, Phillips, B, et al. Treatment of eight dogs with nasal tumours with alternating doses of doxorubicin and carboplatin in conjunction with oral piroxicam. Aust Vet J. 2004;82:676–680.
110. Mayer-Stankeova, S, Fidel, J, Wergin, MC, et al. Proton spot scanning radiotherapy of spontaneous canine tumors. Vet Radiol Ultrasound. 2009;50:314–318.
111. White, R, Walker, M, Legendre, AM, et al. Development of brachytherapy technique for nasal tumors in dogs. Am J Vet Res. 1990;51:1250–1256.
112. Thompson, JP, Ackerman, N, Bellah, JR, et al. 192Iridium brachytherapy, using an intracavitary afterload device, for treatment of intranasal neoplasms in dogs. Am J Vet Res. 1992;53:617–622.
113. Withrow, SJ. Cryosurgical therapy for nasal tumors in the dog. J Am Anim Hosp Assoc. 1982;18:585–589.
114. Murphy, SM, Lawrence, JA, Schmiedt, CW, et al. Image-guide transnasal cryoablation of a recurrent nasal adenocarcinoma in a dog. J Small Anim Pract. 2011;52:329–333.
115. Lucroy, MD, Long, KR, Blaik, MA, et al. Photodynamic therapy for the treatment of intranasal tumors in 3 dogs and 1 cat. J Vet Intern Med. 2003;17:727–729.
116. Osaki, T, Takagi, S, Hoshino, Y, et al. Efficacy of antivascular photodynamic therapy using benzoporphyrin derivative monoacid ring A (BPD-MA) in 14 dogs with oral and nasal tumors. J Vet Med Sci. 2009;71:125–132.
117. Thrall, DE, Harvey, CE. Radiotherapy of malignant nasal tumors in 21 dogs. J Am Vet Med Assoc. 1983;183:663–666.
118. Henderson, SM, Bradley, K, Day, MJ, et al. Investigation of nasal disease in the cat—a retrospective study of 77 cases. J Feline Med Surg. 2004;6:245–257.
119. Mukaratirwa S, van der Linde-Sipman JS, Gruys E: Feline nasal and paranasal sinus tumors: clinicopathological study, histomorphological description and diagnostic immunohistochemistry of 123 cases. J Feline Med Surg. 2001;3:235–245.
120. Demko, JL, Cohn, LA. Chronic nasal discharge in cats: 75 cases (1993-2004). J Am Vet Med Assoc. 2007;230:1032–1037.
121. Theon, AP, Peaston, AE, Madewell, BR, et al. Irradiation of nonlymphoproliferative neoplasms of the nasal cavity and paranasal sinuses in 16 cats. J Am Vet Med Assoc. 1994;204:78–83.
122. Taylor, SS, Goodfellow, MR, Browne, WJ, et al. Feline extranodal lymphoma: response to chemotherapy and survival in 110 cats. J Small Anim Pract. 2009;50:584–592.
123. Sfiligoi, G, Theon, AP, Kent, MS. Response of nineteen cats with nasal lymphoma to radiation therapy and chemotherapy. Vet Radiol Ultrasound. 2007;48:388–393.
124. Haney, SM, Beaver, L, Turrel, J, et al. Survival analysis of 97 cats with nasal lymphoma: a multi institutional retrospective study (1986-2006). J Vet Intern Med. 2009;23:287–294.
125. Little, L, Patel, R, Goldschmidt, M. Nasal and nasopharyngeal lymphoma in cats: 50 cases (1989-2005). Vet Pathol. 2007;44:885–892.
126. Greci, V, Mortellaro, CM, Olivero, D, et al. Inflammatory polyps of the nasal turbinates of cats: an argument for designation of feline mesenchymal nasal hamartoma. J Feline Med Surg. 2011;13:213–219.
127. DeLorenzi, D, Bertoncello, D, Bottero, E. Squash preparation cytology from nasopharyngeal masses in the cat: cytological results and histological correlations in 30 cases. J Feline Med Surg. 2008;10:55–60.
128. Lamb, CR, Richbell, S, Mantis, P. Radiographic signs in cats with nasal disease. J Feline Med Surg. 2003;5(4):227–235.
129. Schoenborn, WC, Wisner, ER, Kass, PP, et al. Retrospective assessment of computed tomographic imaging of feline sinonasal disease in 62 cats. Vet Radiol Ultrasound. 2003;44(2):185–195.
130. Tromblee, TC, Jones, JC, Etue, AE, et al. Association between clinical characteristics, computed tomography characteristics, and histologic diagnosis for cats with sinonasal disease. Vet Radiol Ultrasound. 2006;47:241–248.
131. Karnik, K, Riechle, JK, Fischetti, AJ, et al. Computed tomographic findings of fungal rhinitis and sinusitis in cats. Vet Radiol Ultrasound. 2009;50:65–68.
132. Mellanby, RJ, Herrtage, ME, Dobson, JM. Long-term outcome of eight cats with non-lymphoproliferative nasal tumours treated by megavoltage radiotherapy. J Feline Med Surg. 2002;4:77–81.
133. Elmslie, RE, Ogilvie, GK, Gillette, EL, et al. Radiotherapy with and without chemotherapy for localized lymphoma in 10 cats. Vet Radiol. 1991;32:277–280.
134. Mendenhall, WM, Werning, JW, Pfister, DG. Cancer of the head and neck. In: DeVita VT, Lawrence TS, Rosenberg SA, eds. Cancer: Principles and practice of oncology. Philadelphia: Lippincott Williams & Wilkins, 2008.
 Section C
Section C
Cancer of the Larynx and Trachea
Incidence and Risk Factors
Cancer in either the larynx or the trachea is rare in dogs and cats.1,2 Young patients with active osteochondral ossification sites are at higher risk for benign tracheal osteocartilaginous tumors, which grow in synchrony with the rest of the musculoskeletal system.1,3 Laryngeal oncocytomas also appear to occur in younger mature dogs.4,5 No breed or gender predilection is known for either site.
Pathology and Natural Behavior
Reported canine laryngeal tumors include rhabdomyoma (oncocytoma), osteosarcoma, extramedullary plasmacytoma,4,6 chondrosarcoma, osteosarcoma, undifferentiated carcinoma, fibrosarcoma, granular cell tumor, mast cell tumor, adenocarcinoma, and SCC.7-10 Rhabdomyomas in the dog may be large, are minimally invasive, and do not appear to metastasize.4,5,11,12 A 6-month-old dog with a laryngeal cyst has been reported.13 Most other laryngeal tumors are very locally invasive and have a significant metastatic potential. Feline laryngeal neoplasms most commonly are lymphomas, although SCC and adenocarcinoma have been reported.2,8,10,14,15 Benign tracheal tumors and cysts in cats are rare.2,14,16
Reported tracheal cancer includes lymphoma, chondrosarcoma, histiocytic sarcoma, adenocarcinoma, and SCC.17,18 Tracheal leiomyomas and polyps have also been reported.19-21 Several reports of benign tracheal osteochondral tumors exist in the literature.3,22 These lesions grow from the cartilaginous rings and are composed of cancellous bone capped by cartilage. They may reflect a malfunction of osteogenesis rather than true cancer and are benign.22 The larynx and trachea may be secondarily invaded by neoplasms such as lymphoma and thyroid adenocarcinoma.
History and Clinical Signs
Patients with laryngeal tumors usually have a progressive change in voice or bark, exercise intolerance, or dysphagia. Patients with tracheal tumors usually have coughing and exercise intolerance. Because osteochondral lesions of young dogs grow at the same rate as the rest of the skeleton, symptoms become most noticeable during the skeletal growth spurt.
Diagnostic Techniques and Work-Up
Laryngeal tumors usually can be biopsied under direct visualization. Small samples or cytology alone may yield false negative results.2 Regional radiographs may reveal the general location of the lesion, but CT images will yield more precise localization.23
Tracheal tumors offer more of a diagnostic challenge but can be biopsied with the use of fiberoptic instruments or a rigid bronchoscope (Figure 23-12, A). Alternatively, open surgical biopsy, often coupled with excision, can be performed. Plain radiographs or a tracheogram may reveal a mass narrowing the lumen (Figure 23-12, B). CT or MRI may aid localization of the lesion.23
Therapy
Benign laryngeal cancers such as rhabdomyomas and cysts can be removed successfully with preservation of function.4,11-13,16 Temporary tracheostomy will provide short-term relief of upper airway obstruction and is well tolerated in dogs and some cats.24 Complete laryngectomy with a permanent tracheostomy is another option used in humans, but it has had limited use in veterinary medicine.11,25-29 Depending on their suspected radioresponsiveness, invasive cancers can be treated with irradiation to better preserve laryngeal function. Radiation should control lymphoma or plasmacytoma in the dog or cat larynx or trachea. Chemotherapy, with or without surgery, may also be effective.6,30
Tracheal tumors should be treated with resection, especially benign osteochondral tumors (Figure 23-13).3,31 Full thickness removal with end-to-end anastomosis can easily be performed on up to three or four rings. Experimentally, up to 50% of tracheal length has been removed with successful closure.32 Intraluminal stents may provide temporary palliation.
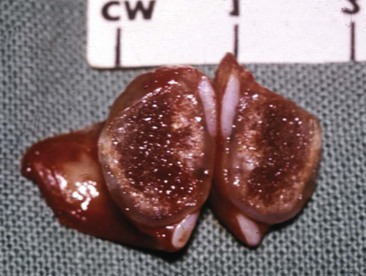
Figure 23-13 Resected tumor and associated tracheal cartilage rings of the dog in Figure 23-12. The tumor is bisected and has the typical appearance of cancellous bone with a cartilaginous cap. The patient recovered uneventfully from surgery and survived for longer than 5 years, when it was lost to follow-up.
Phototherapy via bronchoscopy has been used successfully in humans for small lesions (carcinoma in situ or early carcinoma), but these lesions are only rarely recognized in the dog or cat.
Prognosis
Benign lesions of the trachea and larynx have a good prognosis if they can be resected.16 Most dogs with resectable rhabdomyomas live longer than 1 year and may be presumed cured.4,11,12 Long-term outcomes may be expected with treatment of extramedullary plasmacytomas or granular cell tumors.6,9 Very limited information is available for the malignancies because very few have been treated and reported.10,17 In one study in cats, the MST for 27 cats with a variety of laryngeal and tracheal lesions was 5 days and only 7% were alive at 1 year.2
Comparative Aspects33
Laryngeal cancer is common in humans (2% of all cancers) and is related to smoking and alcohol consumption. The lesion is nearly always SCC. Earlier detection prompted by voice changes makes treatment more feasible. In humans, the disease appears to progress through stages of development from dysphagia, to carcinoma in situ, to minimally invasive carcinoma, to invasive carcinoma. Of patients with carcinomas, 60% have local disease only, 30% have regional nodal metastasis, and 10% have distant metastasis. The disease is treated with surgery (partial or complete laryngectomy) or irradiation. Local control and cure rates are good to excellent.
Tracheal cancer independent of lung cancer is very rare in humans.
References
1. Brown, MR, Rogers, KS. Primary tracheal tumors in dogs and cats. Comp Pract Vet. 2003;25:854–860.
2. Jakubiak, MJ, Siedlecki, CT, Zenger, E, et al. Laryngeal, laryngotracheal, and tracheal masses in cats: 27 cases (1998-2003). J Am Anim Hosp Assoc. 2005;41:310–316.
3. Withrow, SJ, Holmberg, DL, Doige, CE, et al. Treatment of a tracheal osteochondroma with an overlapping end-to-end tracheal anastomosis. J Am Anim Hosp Assoc. 1978;14:469–473.
4. Meuten, DJ, Calderwood-Mays, MB, Dillman, RC, et al. Canine laryngeal rhabdomyoma. Vet Pathol. 1985;22:533–539.
5. Pass, DA, Huxtable, CR, Cooper, BJ, et al. Canine laryngeal oncocytomas. Vet Pathol. 1980;17:672–677.
6. Hayes, AM, Gregory, SP, Murphy, S, et al. Solitary extramedullary plasmacytoma of the canine larynx. J Small Anim Pract. 2007;48:288–291.
7. Wheeldon, EB, Suter, PF, Jenkins, T. Neoplasia of the larynx in the dog. J Am Vet Med Assoc. 1982;180:642–647.
8. Saik, JE, Toll, SL, Diters, RW, et al. Canine and feline laryngeal neoplasia: a 10-year survey. J Am Anim Hosp Assoc. 1986;22:359–365.
9. Rossi, G, Tarantino, C, Taccini, E, et al. Granular cell tumour affecting the left vocal cord in a dog. J Comp Pathol. 2007;136:74–78.
10. Carlisle, CH, Biery, DN, Thrall, DE. Tracheal and laryngeal tumors in the dog and cat: literature review and 13 additional patients. Vet Radiol. 1991;32:229–235.
11. Henderson, RA, Powers, RD, Perry, L. Development of hypoparathyroidism after excision of laryngeal rhabdomyosarcoma in a dog. J Am Vet Med Assoc. 1991;198:639–643.
12. Calderwood-Mays, MB. Laryngeal oncocytoma in two dogs. J Am Vet Med Assoc. 1984;185:677–679.
13. Cuddy, LC, Bacon, NJ, Coomer, AR, et al. Excision of a congenital laryngeal cyst in a five-month-old dog via a lateral extraluminal approach. J Am Vet Med Assoc. 2010;236:1328–1333.
14. Vasseur, PB. Laryngeal adenocarcinoma in a cat. J Am Anim Hosp Assoc. 1981;17:639–641.
15. Sheaffer, KA, Dillon, AR. Obstructive tracheal mass due to an inflammatory polyp in a cat. J Am Anim Hosp Assoc. 1996;32:431–434.
16. Rudorf, H, Lane, JG, Brown, PJ, et al. Ultrasonographic diagnosis of a laryngeal cyst in a cat. J Small Anim Pract. 1999;40:275–277.
17. Evers, P, Sukhiani, HR, Sumner-Smith, G, et al. Tracheal adenocarcinoma in two domestic shorthaired cats. J Small Anim Pract. 1994;35:217–220.
18. Bell, R, Philbey, AW, Martineau, H, et al. Dynamic tracheal collapse associated with disseminated histiocytic sarcoma in a cat. J Small Anim Pract. 2006;47:461–464.
19. Byran, RD, Frame, RW, Kier, AB. Tracheal leiomyoma in a dog. J Am Vet Med Assoc. 1981;178:1069–1070.
20. Black, AP, Liu, S, Randolph, JF. Primary tracheal leiomyoma in a dog. J Am Vet Med Assoc. 1981;179:905–907.
21. Hendricks, JC, O’Brien, JA. Tracheal collapse in two cats. J Am Vet Med Assoc. 1985;187:418–419.
22. Carb, A, Halliwell, WH. Osteochondral dysplasias of the canine trachea. J Am Anim Hosp Assoc. 1981;17:193–199.
23. Stadler, K, Hartman, S, Matheson, J, et al. Computed tomographic imaging of dogs with primary laryngeal or tracheal airway obstruction. Vet Radiol Ultrasound. 2011;52(4):377–384.
24. Guenther-Yenke, CL, Rozanski, EA. Tracheostomy in cats: 23 cases (1998-2006). J Feline Med Surg. 2007;9:451–457.
25. Nelson, AW. Upper respiratory system. In: Slatter DH, ed. Textbook of small animal surgery. Philadelphia: WB Saunders, 1993.
26. Harvey, CE. Speaking out. J Am Anim Hosp Assoc. 1986;22:568.
27. Crowe, DT, Goodwin, MA, Greene, CE. Total laryngectomy for laryngeal mast cell tumor in a dog. J Am Anim Hosp Assoc. 1986;22:809–816.
28. Block, G, Clarke, K, Salisbury, SK, et al. Total laryngectomy and permanent tracheostomy for treatment of laryngeal rhabdomyosarcoma in a dog. J Am Anim Hosp Assoc. 1995;31:510–513.
29. Salisbury, SK. Aggressive cancer surgery and aftercare. In: Morrison WB, ed. Cancer in dogs and cats: medical and surgical management. Baltimore: Williams & Wilkins, 1998.
30. Schneider, PR, Smith, CW, Feller, DL. Histiocytic lymphosarcoma of the trachea in the cat. J Am Anim Hosp Assoc. 1979;15:485–487.
31. Pearson, GR, Lane, JG, Holt, PE, et al. Chondromatous hamartomas of the respiratory tract in the dog. J Small Anim Pract. 1987;28:705–712.
32. Nelson, AW. Lower respiratory system. In: Slatter DH, ed. Textbook of small animal surgery. Philadelphia: WB Saunders, 1993.
33. Mendenhall, WM, Riggs, CE, Cassisi, NJ. Treatment of head and neck cancers. In: DeVita VT, et al, eds. Cancer: principles and practices of oncology. Philadelphia: Lippincott-Raven, 2005.
 Section D
Section D
Pulmonary Neoplasia
Robert B. Rebhun and William T.N. Culp
Incidence and Risk Factors
Lung cancer is the leading cause of cancer-related human deaths worldwide, but primary lung cancer remains relatively uncommon in pet dogs and cats. The incidence of primary lung cancer in pet dogs and cats presenting for necropsy is less than 1%.1-4 Incidence rates in pet dogs range from 4.2 per 10,000 dogs per year in the United States to 15 per 100,000 dogs per year in the United Kingdom.5,6 In contrast, the incidence of pulmonary neoplasia was 8.8% in a closed colony of “normal” beagles and was dominated by a high incidence of pulmonary tumors in dogs dying after the median lifespan of 13.6 years.7
The average age of pet dogs diagnosed with primary lung tumors is approximately 11 years,1,8 with the exception of anaplastic carcinomas that occur at an average age of 7.5 years.9 The Boxer, Doberman, Australian shepherd, Irish setter, and Bernese Mountain dog breeds are possibly overrepresented.1,8,10 The average age of cats with pulmonary tumors is 12 to 13 years.3,9,11 No breed or gender predisposition has been reported in cats with the possible exception of Persian cats.4
In people, the risk of developing primary lung cancer is strongly associated with smoking, but no such definitive risk factors have been identified in the pet population. Urban living and second-hand smoke exposure have both been implicated as potential causes of lung cancer in dogs but are yet to be clearly demonstrated.12,13 An increased risk of lung cancer was found in dogs with increased amounts of anthracosis, suggesting an association between inhalation of polluted air and lung cancer.14 Cytologic analysis of bronchoalveolar lavage fluid also revealed increased anthracosis in dogs that had been exposed to passive tobacco smoke when compared to dogs without a history of exposure.15 In the experimental setting, laboratory dogs trained to smoke cigarettes through a tracheostoma (in the presence or absence of asbestos exposure) did develop lung cancer at a higher rate than control dogs.16,17 Experimentally induced exposure to radiation such as that found in plutonium also significantly increases the occurrence of lung cancer when inhaled as an aerosol in research dogs.18,19
Pathology and Natural Behavior
Pulmonary tumors can arise from any tissue in the lung but most commonly originate from epithelium of the airways or alveolar parenchyma. Tumors derived from epithelium of large airways are typically located near the hilus, whereas parenchymally derived tumors tend to be peripherally located. However, the most recent WHO guidelines on classification of pulmonary neoplasms largely classify lung tumors of domestic animals by histologic pattern and not by site of origin.10
Approximately 85% of canine lung tumors are bronchoalveolar in origin, whereas adenocarcinoma, adenosquamous carcinoma, and SCC collectively comprise the remaining 13% to 15% of primary lung tumors.7,8 Adenocarcinoma represents 60% to 70% of feline lung tumors, whereas bronchoalveolar carcinoma, SCC, and adenosquamous carcinoma are less common.4,10,11 Small cell carcinoma represents approximately 25% of human pulmonary neoplasms but rarely occurs in the dog or cat.
Lung tumors can spread by local invasion or hematogenous and lymphatic routes, resulting in locoregional spread to other areas of the lung or lymph nodes or distant metastasis. Intrapulmonary metastases are believed to occur through vascular and lymphatic invasion or intra-airway seeding. Seventy-one percent of canine pulmonary malignant tumors had evidence of local vascular or lymphatic invasion, and 23% had distant metastasis beyond hilar lymph nodes.8 SCC and anaplastic carcinomas have metastatic rates that exceed 50% and 90% of cases, respectively, and thus are believed to be more likely to metastasize than adenocarcinoma or bronchoalveolar carcinoma.3
Metastasis is common in the cat, with a metastatic rate of 76% in feline patients with pulmonary tumors.4,11 The incidence of lymph node metastasis and the incidence of intrathoracic metastasis are equivalent at 30% of patients, whereas extrathoracic metastases occurred in only 16% of cats. Metastasis to bone or the nervous system is not uncommon in dogs or cats. Metastasis to the digits is a common and well-described clinical phenomenon in cats.20
Distinguishing poorly differentiated primary lung tumors from metastatic carcinoma can occasionally provide a diagnostic challenge. Immunohistochemistry using antibodies directed against thyroid transcription factor-1, cytokeratin, or vimentin may be useful in differentiating primary lung tumors from metastatic disease.21-23 Antibodies against CD18 may also be useful for differentiating pulmonary tumors of histiocytic origin.24
Clinical Signs and Physical Examination Findings
Veterinary patients will often be diagnosed with a primary pulmonary tumor incidentally during a routine geriatric screen.1,7,25 Up to 30% of cases of primary pulmonary tumors will be diagnosed without the presence of clinical signs.25 The most common clinical sign reported in dogs with pulmonary neoplasia is coughing, which is noted in 52% to 93% of dogs.1,25-28 Other clinical signs that have been recorded include dyspnea (6% to 24%), lethargy (12% to 18%), anorexia (13%), weight loss (7% to 12%), hemoptysis (3% to 9%) and lameness likely secondary to hypertrophic osteopathy (4%).25-27
The clinical signs in cats are similar to dogs; however, the occurrence of these signs is variable and gastrointestinal signs may be noted as well.29,30 As in dogs, signs referable to the respiratory tract are common, with dyspnea (24% to 65%), cough (29% to 53%), tachypnea (14%), and hemoptysis (10%) being noted regularly.29,30 Nonspecific signs such as lethargy and anorexia can be seen in 24% to 43% and 19% to 71% of cats, respectively.30,31 Vomiting/regurgitation and diarrhea are seen in approximately 19% of cats with primary pulmonary neoplasia.29,30
Abnormal physical examination findings consistent with pulmonary neoplasia are often not seen.25 Increased bronchovesicular sounds may be auscultated in dogs with extensive pulmonary involvement.26 As pulmonary effusion can occur in many cases, dull lung and heart sounds may also be noted.25,29,30 Metastasis to the nervous system has been reported in several cases of primary pulmonary tumors, and neurologic abnormalities can be diagnosed on physical examination.31-34
Lameness can occur in dogs and cats associated with primary pulmonary neoplasia for a variety of reasons. Hypertrophic osteopathy is a paraneoplastic syndrome that has been most commonly associated with primary and metastatic lung tumors, although other malignant and nonmalignant diseases have resulted in hypertrophic osteopathy.27,35-39 The disease is characterized by periosteal new bone formation at a site distant to the primary tumor. Lameness may improve with removal of the lung tumor.35,38
Several reports of cats with concurrent pulmonary neoplasia and digital metastasis can be found in the veterinary literature; this phenomenon has been noted with both pulmonary adenocarcinoma and SCC.4,20,40-43 Of 36 cats with metastatic bronchogenic carcinoma to the digit, all were presented for lameness and none were presented for respiratory signs.43 The authors of that study concluded that thoracotomy with lung lobectomy and digital amputation should not be recommended because nonrespiratory disease often progressed and metastatic lesions in other digits resulted in continued lameness.43 The MST for cats undergoing digital amputation alone was only 67 days.43
Diagnostic Techniques and Work-Up
A complete blood count (CBC) and serum chemistry panel are unlikely to signal a clinician to the presence of a pulmonary mass. Nonspecific changes in these diagnostics have been reported in veterinary cases, and routine blood tests are often within normal limits.11,43 These diagnostics, however, are essential to the preanesthetic and overall evaluation of a patient undergoing treatment for a pulmonary neoplasm.
The presence of pleural effusion at diagnosis is less common in dogs than cats. When pleural effusion is noted, a sample should be obtained via thoracocentesis. The pleural fluid tends to be a clear or blood-tinged modified transudate.25,29 Thirty percent (26/86) of cats in one study were noted to have pleural effusion, and thoracocentesis was performed in 13; fluid analysis was diagnostic for a primary lung tumor in 12/13 cats.11 However, in a separate feline study, fluid analysis was diagnostic for a malignant effusion secondary to a malignant neoplasm in only 1/8 cases.29 Of three dogs with pleural effusion in one study, one was diagnosed with carcinoma based on evaluation of fluid obtained by thoracocentesis.25
Bronchoalveolar lavage (BAL) and transtracheal washes have been advocated as a method of diagnosing pulmonary neoplasia.25,44-46 In a series of cases that underwent BAL to aid in the diagnosis of respiratory tract diseases, 14 carcinomas were identified. Of those 14 cases, the BAL was definitive, supportive, or not helpful in eight, four, and two cases, respectively.45 Transtracheal washes have been less successful; in one study, in six dogs with confirmed primary pulmonary neoplasia, none of the washes yielded neoplastic cells.25
The majority of pulmonary tumors are diagnosed on thoracic radiographs (see Chapter 6). When evaluating 277 canine cases from two large case series, 83% of pulmonary tumors were viewed on thoracic radiographs.25,27 In cats with primary pulmonary tumors, 67% to 91% will have radiographic evidence of solitary or multiple pulmonary masses on thoracic radiographs.11,30
Several studies have evaluated tumor location and number within the lungs.1,26,27 In a series of 210 dogs with primary pulmonary tumors, tumor location was determined in 191 cases.27 Of the total dogs in that study, 53.8% were found in a single lung lobe and 37.1% were found in multiple lung lobes.27 Only 2 dogs were found to have a tumor in multiple lobes in a smaller study of 15 total dogs; of the 17 affected lobes in that study, 13 were on the right and 4 were on the left.26 Of 29 dogs with primary pulmonary tumors in a separate study, the side of the tumor was recorded in 26 with 13 left-sided and 13 right-sided tumors.1 One study has reported that clinical signs were not noted until the pulmonary tumor grew to at least 3 cm in size.7
In a study of 86 cats with primary pulmonary neoplasia, the location was determined in all cases.11 Tumors were left-sided in 26 cats, right-sided in 27 cats, and bilateral in 33 cats.11 In 45 cats, the tumors were found in a single lung lobe and the right and left caudal lobes were more commonly diagnosed with pulmonary tumors (34) versus the right and left cranial (9) and right middle lung lobes (2), which was likely the result of increased tissue at risk in caudal lobes.11 Nineteen cats in one study were found to have a single lung lobe affected, whereas two cats had multiple lesions.30 Of 17 cats in a separate study, all single lesions were left-sided; however, 10/17 had multiple lesions (both right- and left-sided).29
The radiographic pattern of primary pulmonary neoplasia has been variably reported in dogs and cats. Four radiographic patterns were described in a study evaluating 41 cats with primary pulmonary tumors: focal, localized, diffuse, and normal.47 In the focal group, 65% demonstrated solitary masses and 35% demonstrated multiple masses.47 Of the cases with focal (solitary and multiple) and localized masses, 17 were on the right side and 20 were on the left side.47
Thoracic ultrasound may be employed to assess pulmonary neoplasia or to obtain a sample of tissue via FNA or pretreatment biopsy (Figure 23-14). The ultrasonographic appearance of pulmonary neoplasia has been described in several studies.48-50 Pulmonary masses may be hypoechoic or may exhibit variable echogenicity, and tumors are generally considered to have both a lack of discernable bronchi and normal branching vessels.48,49
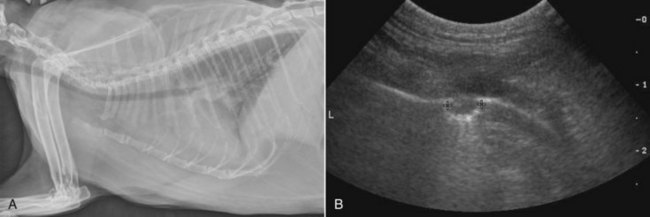
Figure 23-14 A, Right lateral radiograph. B, Ultrasound image of a pulmonary carcinoma in the left caudal lung lobe of a cat.
Thoracic CT scans are gaining popularity for the preoperative assessment of pulmonary neoplasia and many clinicians feel this diagnostic is a necessity (Figure 23-15). Recently, the CT findings of primary lung tumors were described.51 Several characteristics of solitary lung tumors were noted with CT in a majority of cases; 17/18 were well circumscribed, 16/19 were located in a cranial or caudal lobe, and 14/18 were located in the center to periphery of the lobe.51 Internal mineralization was an uncommon finding being diagnosed in only 3/19 cases.51
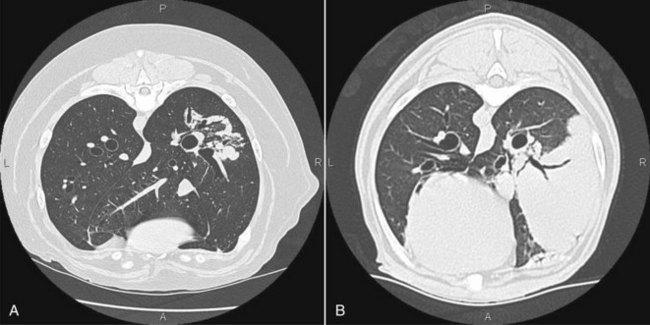
Figure 23-15 Transverse CT scan images of a right caudal lung lobe histiocytic sarcoma (A) in a 9-year-old Bernese Mountain dog and a right caudal pulmonary carcinoma (B) in a 10-year-old Fox terrier.
Thoracic CT has been shown to be more accurate than thoracic radiographs in the assessment of tracheobronchial lymph node metastasis.28 In 14 dogs diagnosed with primary pulmonary tumors, the accuracy of CT to determine tracheobronchial lymph node metastasis was 93% compared with 57% for thoracic radiography.28 Additionally, five dogs deemed to be free of pulmonary metastasis with thoracic radiographs were found to have pulmonary metastasis with CT.28
In a study evaluating the assessment of pulmonary metastatic disease in 18 dogs (two of which had bronchoalveolar carcinoma) by thoracic radiography and CT, only 9% of CT-detected pulmonary nodules were noted on thoracic radiographs.52 CT was able to detect pulmonary nodules down to approximately 1 mm in size, whereas thoracic radiography required a size of 7 to 9 mm for detection.51 Overall, CT was significantly more sensitive than thoracic radiography in the identification of pulmonary nodules.52,53
Fine-Needle Aspiration
FNA of a pulmonary mass may be performed prior to lung lobectomy to attempt to obtain a cytologic diagnosis. FNA is often performed with ultrasound or CT guidance; however, blind aspirates have been reported.11,50,54,55 Sedation is generally required to prevent iatrogenic trauma during the aspiration process. In dogs, preoperative FNA with cytology has resulted in a diagnosis in 38% to 90% of cases in which it is performed.25-27 Diagnosis of primary pulmonary neoplasia in cats is reportedly higher, with 80% to 100% of cases being diagnosed by FNA and cytology.11,30,54
A pretreatment biopsy (biopsy performed prior to definitive therapy) can be performed prior to lung lobectomy, although the clinical relevance is questionable.56 If there is suspicion that a pulmonary neoplasm is not a primary lesion (i.e., either metastatic or systemic neoplasia such as lymphoma), a pretreatment biopsy may be considered; however, if the eventual goal is to treat the pulmonary tumor by lung lobectomy, a pretreatment biopsy is not necessary. Multiple techniques for performing a pretreatment biopsy have been described, including utilizing a biopsy needle, bronchoscopic biopsy, keyhole incision with staple application, and thoracoscopy.*
To perform a pretreatment biopsy with a biopsy needle, sedation or anesthesia is required. Additionally, the use of ultrasound, fluoroscopic, or CT guidance is recommended to improve the targeting of the lesion and decrease the chance of iatrogenic trauma to normal structures.50,55-56 In a study of dogs and cats undergoing CT-guided tissue core biopsy of intrathoracic lesions (including pulmonary tumors), the accuracy of diagnosis for the biopsy technique was 92% and the sensitivity for neoplasia was 80%.55 A procedural complication rate of 43% was reported in that cohort; pulmonary hemorrhage and pneumothorax were seen in 30% and 27% of cases, respectively.55
Bronchoscopic biopsy has been performed in dogs and cats with pulmonary neoplasia with mixed success.11,27 Findings that have been reported during bronchoscopic examination in veterinary patients with primary pulmonary neoplasia include narrowing of bronchi, mucosal erosions, and mucosal swelling and hyperemia.58 In one series of cats, 5/7 cases were successfully diagnosed by means of endoscopic bronchiolar brushing.11
A keyhole lung biopsy technique has been described for nonneoplastic lung disease.46 For this technique, a small thoracotomy (3 to 7 cm) was performed and a surgical stapler was applied across the lung lobe to seal vessels and small airways.46 Thoracoscopy is a minimally invasive surgical option that may be utilized to obtain a pretreatment biopsy.59 In a study describing the use of thoracoscopy to determine the cause of pleural effusion, biopsies of several suspicious intrathoracic lesions were performed.60 In 8/18 cases, neoplasia was the cause of the pleural effusion, although the number of primary pulmonary tumors resulting in pleural effusion was unknown.60 Performing a biopsy with thoracoscopy allowed for sufficient sample acquisition and the opportunity to perform an exploration of the thoracic cavity.60
Treatment
Surgery is the treatment of choice for primary pulmonary tumors. The surgical approach to a pulmonary tumor is clinician dependent, but certain overarching criteria exist. For unilateral tumors, a lateral thoracotomy or median sternotomy may be performed. Thoracoscopic lung lobectomy can be considered if the mass is deemed to be peripherally located and is in a suitable location. If nodules in multiple lobes are found bilaterally and the goal is to remove all gross disease, a median sternotomy should be performed.
Both partial and complete lung lobectomies have been described, and the elected technique is based on the location of the tumor. In general, a complete lung lobectomy should be performed; however, partial lung lobectomy may be an option for small tumors located in a peripheral position on the lung lobe. A cuff of normal tissue should be removed with the tumor to increase the chance of obtaining a wide margin.
Partial and complete lung lobectomies are generally performed with either a suturing method or the use of a surgical stapler. When performing a partial lung lobectomy with the suture method, the area to be removed is delineated by placing crushing forceps proximal to the lesion.61 A continuous overlapping suture can then be placed proximal to the clamps.61 For complete lung lobectomy utilizing suture ligation, the pulmonary artery and vein are individually ligated, and the bronchi are oversewn to prevent air leakage. Pneumonectomy can be performed when indicated.62
Several studies have evaluated the use of stapling equipment for lung lobectomy.26,63,64 In a study of 37 dogs and cats undergoing resection of pulmonary lesions (67% were neoplastic) by surgical staples, the complications were minimal and the surgery was determined to be safe, fast, and efficient.63 Use of surgical staplers is considered by many to be the technique of choice for partial and complete lung lobectomy (Figure 23-16).
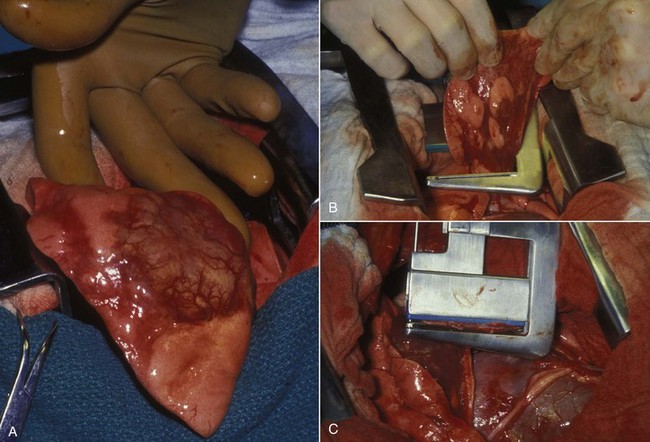
Figure 23-16 A, Operative view of lung cancer. Note the typical raised lesion with superficial neovascularization. B, The lung lobe is lifted up, and surgical staples are placed at the level of the proximal mainstem bronchus. C, Firing the stapler releases a double row of B-shaped, stainless steel staples. The lung mass then is removed, and the instrument is released. The artery, vein, and bronchus are inspected for any small leaks, which are sutured.
Thoracoscopy is being employed more commonly for both diagnostic and treatment purposes. Thoracoscopy can be utilized to localize pulmonary lesions and to obtain biopsies.59,60 Additionally, thoracoscopic lung lobectomy and thoracoscopic-assisted lung lobectomy have been recently described in several reports.65-67 In one study of nine dogs with pulmonary neoplasia (seven metastatic and two primary), successful thoracoscopic lung lobectomy was performed in five cases.65 Cases that were deemed to be good candidates for thoracoscopic removal in that study included those with small masses located away from the hilus. Caudal lung lobectomy was determined to be easier than cranial lung lobectomy; however, both were performed successfully.65 Conversion from a thoracoscopic technique to an open procedure was most commonly performed because of poor operative vision.65 One-lung ventilation should be considered in cases of thoracoscopic lung lobectomy to improve visualization.65,66,68
Biopsy of hilar lymph nodes is recommended as a staging tool because metastatic disease to the lymph nodes significantly affects prognosis.28 Lymph node biopsy is relatively easy to perform via a lateral thoracotomy, but the position of the lymph nodes during a median sternotomy increases the difficulty. In the largest thoracoscopic lung lobectomy study in veterinary patients to date, none of the hilar lymph nodes were biopsied; however, thoracoscopic lymph node biopsy is performed regularly in human patients.65,69
Cisplatin-based chemotherapy protocols are considered the standard of care for human patients that receive chemotherapy in the adjuvant or palliative setting.70 Relatively little is known about the efficacy of chemotherapy treatment for pulmonary tumors in domestic animals; however, chemotherapy treatment has been largely unrewarding in the gross disease setting. Early clinical trials evaluating the safety and efficacy of doxorubicin in cancer-bearing pet dogs included one evaluable dog with papillary pulmonary adenocarcinoma that experienced progressive disease.71 No responses were seen in three dogs with lung adenocarcinoma that were treated with mitoxantrone.72 Minimal responses were reported in two dogs treated with vindesine, whereas two dogs treated with the combination of vindesine and cisplatin both experienced greater than 50% reduction in measurable disease.26 Treatment with vinorelbine resulted in partial responses in two out of seven dogs with measurable bronchoalveolar carcinoma.73 Three additional dogs with microscopic disease were treated with adjuvant vinorelbine in the microscopic disease setting and those patients achieved survival times of 113, 169, and greater than 730 days.73 Pharmacokinetic studies in humans have concluded that vinorelbine treatment results in 300-fold higher concentration in the lung when compared to plasma, which is 3.4- to13.8-fold higher than lung concentrations of vindesine and vincristine, respectively.74 Based on observed partial responses in dogs and pharmacokinetic data in humans, treatment with vinorelbine or cisplatin appears to hold the most promise.
Delivery of aerosolized chemotherapy or cytokines has been described and appears well tolerated in dogs with primary or metastatic pulmonary neoplasia. Complete and partial responses have been described in dogs treated with inhalational therapy for metastatic tumors, whereas stable or progressive disease was reported in dogs with primary lung tumors.75,76
Treatment with monoclonal antibodies or small-molecule tyrosine kinase inhibitors (TKIs) directed against cell signaling pathways has been shown to be beneficial in distinct subpopulations of human patients with non–small-cell lung cancer (NSCLC). Such targeted therapies are yet to be thoroughly examined in dogs with this disease. With the recent availability of receptor TKIs toceranib (Palladia) and masitinib (Kinavet) for use in veterinary patients, future interrogation of such therapeutics may be warranted. In a phase I study, monotherapy with toceranib resulted in stable disease for more than 10 weeks in the only dog with primary lung carcinoma.77
Malignant pleural effusions can be responsive to systemic chemotherapy, intrapleural chemotherapy, or a combination of both routes. Cisplatin, carboplatin, or mitoxantrone have been used successfully for this purpose, resulting in temporary palliation of clinical signs.78-81 Sclerosing agents such as talc or tetracyclines have been also been used in a palliative setting.82
RT in physician-based medicine is most often reserved for tumors that are unresectable.70 Elective nodal irradiation for locally advanced NSCLC of human patients has traditionally been recommended but is a current source of controversy. Technologies such as IMRT, gamma-knife, or tomotherapy can now be used to provide more precise delivery of radiation while sparing unaffected tissues, especially when used together with respiratory gating or breath-hold techniques. Use of such modalities in veterinary patients is now becoming more accessible but remains to be investigated in dogs and cats with lung tumors.
Interventional oncology is a specialty that utilizes minimally invasive, image-guided techniques to perform diagnostic and therapeutic procedures in the treatment of cancer. Interventional oncologic techniques for the treatment of primary pulmonary neoplasia have not been described in the veterinary literature; however, several treatment options exist in human medicine for pulmonary neoplasia.83-86 These options are generally reserved for cases of pulmonary metastatic disease, cases of nonresectable pulmonary tumors, or patients in which surgery is contraindicated due to existing comorbidities.84-87 Significant research and proper case selection would need to be performed before these techniques could be routinely performed in clinical veterinary patients.
Radiofrequency ablation (RFA) and regional chemotherapy administration are two interventional oncology techniques that have been reported in the treatment of pulmonary neoplasia. RFA is performed by placing an electrode within a tumor and generating heat; the heat results in coagulative necrosis in a defined region.83,85,86 The goal is to produce a 360-degree region of necrosis around the tumor, with a 1-cm thick margin of normal tissue included.83,85
RFA has been performed in dogs with experimentally induced transmissible venereal tumor of the lung.88 In this group of five dogs, RFA was applied percutaneously with CT guidance to 14 tumors. On harvest of the affected lung lobes, gross and histopathologic evaluations demonstrated complete thermal coagulation necrosis of all the lesions that had been treated; no viable tumor was identified in any dogs.88
Regional chemotherapy has been developed with the goal of increasing the efficacy of chemotherapy agents and decreasing systemic side effects.89 Regional delivery allows for increased concentrations of chemotherapy to be delivered to the tumor by selective catheterization of the tumoral arterial supply.89 Regional techniques involving the administration of chemotherapy into the bronchial arteries and pulmonary arteries have been described in several human studies.87,89,90 Recently, a study demonstrated that significantly higher concentrations of chemotherapy agents (gemcitabine and carboplatin) were noted in pulmonary tissue that had been treated with regional techniques (selective pulmonary artery perfusion) as compared to the concentration after intravenous administration in an experimental model.90 As stated previously, the indications for these treatments remain to be determined in veterinary patients.
Prognosis
Several studies have evaluated prognostic variables in both dogs and cats with primary pulmonary neoplasia (Table 23-5).* Dogs with clinical signs at the time of diagnosis have been found to have significantly shorter disease-free intervals (DFIs) and survival times versus those in which a pulmonary tumor was found incidentally.25 Metastatic disease within the lymph nodes is also associated with a significantly decreased DFI and survival time in dogs.25,27,28 In one study, dogs with well-differentiated tumors lived longer and had longer DFIs than dogs with moderately or poorly differentiated tumors.25 Similarly, histologic grade is predictive of outcome in cats. The MST for cats undergoing surgical resection of pulmonary tumors was 2.5 months and 23 months for poorly differentiated and well differentiated tumors, respectively.30
One study evaluated the effect of several variables on remission and survival in dogs with primary lung tumors.91 Dogs for which surgery was successful in rendering them free of macroscopic disease lived significantly longer than dogs that had gross disease postoperatively.91 Factors significantly associated with remission included limited degree of primary tumor involvement, normal-sized lymph nodes, and lack of metastatic disease.91 In a separate study of 15 dogs, trends for longer survival times were noted in dogs with adenocarcinoma versus SCC, in dogs with peripheral lesions versus lesions that involved an entire lobe, and in dogs with tumor volume smaller than 100 cm3 when compared to dogs with tumor volume larger than 100 cm3.26
Dogs without clinical signs at the time of diagnosis have a MST of 545 days versus 240 days in dogs with clinical signs.25 Tumor stage (T) is also prognostic for MST, with T1 (solitary), T2 (multiple), and T3 (invasive into adjacent tissues) tumors having MSTs of 26 months, 7 months, and 3 months, respectively.25 Dogs with lymph node metastasis had a MST of 1 month compared with dogs without lymph node involvement that survived for a median of 15 months.25 In another study, dogs with lymph node enlargement diagnosed prior to surgery survived for a median time of 60 days, whereas dogs without lymph node enlargement had a MST of 285 days.91 A similar finding was noted in a more recent study in which dogs with lymphadenopathy had MSTs of 126 days versus a MST that had not been reached in the dogs without lymphadenopathy.28 In dogs for which a surgical remission could be achieved, the MST is 330 days versus 28 days in dogs that could not be rendered free of visible disease.91 Although not statistically significant, the MST of dogs with SCC was 8 months and the MST of dogs with adenocarcinoma was 19 months in one study.26 Finally, dogs with well-differentiated, moderately differentiated, and poorly differentiated lung tumors have MSTs of 790 days, 251 days, and 5 days, respectively.25
A recent study evaluated 42 dogs with primary lung tumors and based the prognostic evaluation on the WHO classification scheme.89 In these cases, 34 tumors were determined to be carcinomas (26 papillary adenocarcinomas) and eight were determined to be sarcomas. Fourteen of the carcinomas and two of the sarcomas were T1N0M0. Dogs with papillary adenocarcinomas and a clinical stage of T1N0M0 had the best overall prognosis with an MST of 555 days; this survival time was significantly longer than dogs with any other tumor type or dogs with a worse clinical stage.92 Dogs with other tumor types had a MST of 72 days.92
Comparative Aspects70
Lung cancer is the leading cause of cancer deaths in the United States and worldwide.93 Approximately 85% of human lung cancers are NSCLCs, with the remainder being small-cell lung cancer. NSCLCs are comprised of three distinct histologic subtypes: SCC, adenocarcinoma, and large-cell lung cancer. Large airway origin tumors predominated in humans through the 1960s and were commonly associated with smoking cigarettes. Adenocarcinoma arising from the smaller airways now predominates in human patients, which is likely a result of changes to tobacco blends and the use of cigarette filters.94
If curative-intent resection can be performed, prognosis for human patients with NSCLC is largely dependent on stage, with 5-year survival rates greater than 60% to 70% for patients with stage I disease. Five-year survival rates are roughly 30% to 40%, 10% to 30%, and less than 5% for patients with stage II, stage III, and stage IV disease, respectively.70
Inherited cancer syndromes caused by germline p53 mutations, retinoblastoma, EGFR, and other genes have been reported to increase the risk of lung cancer. Furthermore, an association between single-nucleotide polymorphism variations has been linked to lung carcinogenesis and may cooperate with nicotine exposure. DNA synthesis and repair genes may also play a role in the development and prognosis of lung cancer. Commonly acquired molecular abnormalities in human lung cancer include but are not limited to microsatellite instabilities, EGFR mutations, p53 inactivation, RB inactivation, P16INK4a inactivation, allelic loss, and high telomerase activity. K-ras mutations have been found in up to 30% of human NSCLCs.95 Close homology exists between canine and human K-ras. Interestingly, K-ras mutations were detected in 5 of 21 canine NSCLC specimens by direct sequencing. Further studies concluded that the frequency and type of mutations in canine NSCLC tissues more closely matched those for tumors from human nonsmokers with K-ras mutations than those for smokers.8
The FDA has approved the use of two EGFR-targeted molecules, gefitinib (Iressa, Astra Zeneca) and erlotinib (Tarceva, Genentech/Roche), in the second- and third-line treatment of human lung cancer. Gefitinib monotherapy confers substantial clinical benefit in terms of progression-free survival and overall survival in NSCLC patients with EGFR mutations. These mutations were commonly found in patients fitting the responsive profile observed in initial and subsequent clinical studies: specifically female, nonsmokers, Asian descent, and adenocarcinoma, which suggest a distinctive biology in this subgroup of individuals.96
Miscellaneous Lung Conditions
Canine Pulmonary Lymphomatoid Granulomatosis
Canine pulmonary lymphomatoid granulomatosis is a poorly understood disease occurring most commonly in young to middle-aged dogs with no breed or gender predilection.97 The most common laboratory abnormalities occurring in seven cases included basophilia and leukocytosis. Lung lobe consolidation or large pulmonary granulomas and tracheobronchial lymph node enlargement are typically seen on thoracic radiographs. Transthoracic FNA is not often diagnostically rewarding. Differential diagnoses include heartworm granulomas, metastatic neoplasia, and primary lung tumors. Traditionally, a definitive diagnosis requires biopsy and histopathology. Characteristic histopathology often demonstrates angiocentric and angiodestructive infiltration of the pulmonary parenchyma by large lymphoreticular and plasmacytoid cells in addition to normal-appearing lymphocytes, eosinophils, and plasma cells. This infiltrate is typically centered around small-to-medium arteries and veins.
The etiology of this disease is not known but is suspected to be neoplastic or preneoplastic. A recent case report indicates evidence of clonality based on polymerase chain reaction testing for antigen-receptor rearrangements (PARR), but this finding needs to be investigated in a larger series of cases.98 It is not known whether flow cytometry or PARR testing may improve the diagnostic power of FNA.
In a very limited number of cases, the response to chemotherapy is quite variable.99,100 Of five dogs that were treated with cyclophosphamide, vincristine, and prednisone, three achieved a CR. The remaining dogs showed either worsening in clinical signs or progressed to lymphoid leukemia within 2 months. Dogs achieving a complete response were alive at 7, 12, and 32 months.
Histiocytic Sarcoma
Histiocytic sarcoma (HS) was originally reported in a series of 11 Bernese Mountain dogs and is believed to be inherited with a polygenic mode of inheritance in this breed101,102 (see Chapter 33, Section F). Histiocytic sarcoma is currently the preferred term for identifying malignant tumors of antigen-presenting dendritic cell origin, whereas the original term malignant histiocytosis refers to the systemic (disseminated) form of the disease. Other breeds at risk include the Rottweiler, golden retriever, and flat-coated retriever. Dogs often present with respiratory signs but nonspecific presenting complaints of weight loss, lethargy, fever, and anorexia are also common. HS commonly metastasizes to lymph nodes, the kidneys, the liver, and the central nervous system. HS can occur in a hemophagocytic form, resulting in anemia, hypoalbuminemia, thrombocytopenia, and leukopenia. Paraneoplastic hypercalcemia can also occur with this disease. Dogs presenting with the hemophagocytic form of HS have a particularly poor prognosis, with hypoalbuminemia and thrombocytopenia being associated with a dismal prognosis.103,104 In contrast to dogs with disseminated HS, dogs with localized HS that underwent surgical excision and adjuvant therapy with CCNU had a reported MST of 568 days.105 Interestingly, 5 out of 16 dogs within that study had localized pulmonary lesions that were resected prior to therapy with CCNU.
References
1. Brodey, RS, Craig, PH. Primary pulmonary neoplasms in the dog: a review of 29 cases. J Am Vet Med Assoc. 1965;147:1628–1643.
2. Nielsen, SW, Horava, A. Primary pulmonary tumors of the dog. A report of sixteen cases. Am J Vet Res. 1960;21:813–830.
3. Moulton, JE, von Tscharner, C, Schneider, R. Classification of lung carcinomas in the dog and cat. Vet Pathol. 1981;18:513–528.
4. D’Costa, S, Yoon, B-I, Kim, DY, et al. Morphologic and molecular analysis of 39 spontaneous feline pulmonary carcinomas. Vet Pathol. 2011. [DOI 10.117710300985811419529].
5. Dorn, CR, Taylor, DO, Frye, FL, et al. Survey of animal neoplasms in Alameda and Contra Costa Counties, California. I. Methodology and description of cases. J Natl Cancer Inst. 1968;40:295–305.
6. Dobson, JM, Samuel, S, Milstein, H, et al. Canine neoplasia in the UK: estimates of incidence rates from a population of insured dogs. J Small Anim Pract. 2002;43:240–246.
7. Hahn, FF, Muggenburg, BA, Griffith, WC. Primary lung neoplasia in a beagle colony. Vet Pathol. 1996;33:633–638.
8. Griffey, SM, Kraegel, SA, Madewell, BR. Rapid detection of K-ras gene mutations in canine lung cancer using single-strand conformational polymorphism analysis. Carcinogenesis. 1998;19:959–963.
9. Stunzi, H, Head, KW, Nielsen, SW. Tumours of the lung. Bull World Health Organ. 1974;50:9–19.
10. Meuten, DJ. Tumors in domestic animals, ed 4. Ames, Iowa: Iowa State University Press; 2002.
11. Hahn, KA, McEntee, MF. Primary lung tumors in cats: 86 cases (1979-1994). J Am Vet Med Assoc. 1997;211:1257–1260.
12. Reif, JS, Cohen, D. The environmental distribution of canine respiratory tract neoplasms. Arch Environ Health. 1971;22:136–140.
13. Reif, JS, Dunn, K, Ogilvie, GK, et al. Passive smoking and canine lung cancer risk. Am J Epidemiol. 1992;135:234–239.
14. Bettini, G, Morini, M, Marconato, L, et al. Association between environmental dust exposure and lung cancer in dogs. Vet J. 2009;186(3):364–369.
15. Roza, MR, Viegas, CA. The dog as a passive smoker: effects of exposure to environmental cigarette smoke on domestic dogs. Nicotine Tob Res. 2007;9:1171–1176.
16. Auerbach, O, Hammond, EC, Kirman, D, et al. Effects of cigarette smoking on dogs. II. Pulmonary neoplasms. Arch Environ Health. 1970;21:754–768.
17. Humphrey, EW, Ewing, SL, Wrigley, JV, et al. The production of malignant tumors of the lung and pleura in dogs from intratracheal asbestos instillation and cigarette smoking. Cancer. 1981;47:1994–1999.
18. Gillett, NA, Stegelmeier, BL, Kelly, G, et al. Expression of epidermal growth factor receptor in plutonium-239-induced lung neoplasms in dogs. Vet Pathol. 1992;29:46–52.
19. Wilson, DA, Mohr, LC, Frey, GD, et al. Lung, liver and bone cancer mortality after plutonium exposure in beagle dogs and nuclear workers. Health Phys. 2010;98:42–52.
20. van der Linde-Sipman, JS, van den Ingh, TS. Primary and metastatic carcinomas in the digits of cats. Vet Q. 2000;22:141–145.
21. Ramos-Vara, JA, Miller, MA, Johnson, GC. Usefulness of thyroid transcription factor-1 immunohistochemical staining in the differential diagnosis of primary pulmonary tumors of dogs. Vet Pathol. 2005;42:315–320.
22. Bettini, G, Marconato, L, Morini, M, et al. Thyroid transcription factor-1 immunohistochemistry: diagnostic tool and malignancy marker in canine malignant lung tumours. Vet Comp Oncol. 2009;7:28–37.
23. Burgess, HJ, Kerr, ME. Cytokeratin and vimentin co-expression in 21 canine primary pulmonary epithelial neoplasms. J Vet Diagn Invest. 2009;21:815–820.
24. Affolter, VK, Moore, PF. Localized and disseminated histiocytic sarcoma of dendritic cell origin in dogs. Vet Pathol. 2002;39:74–83.
25. McNiel, EA, Ogilvie, GK, Powers, BE, et al. Evaluation of prognostic factors for dogs with primary lung tumors: 67 cases (1985-1992). J Am Vet Med Assoc. 1997;211:1422–1427.
26. Mehlhaff, CJ, Leifer, CE, Patnaik, AK, et al. Surgical treatment of primary pulmonary neoplasia in 15 dogs. J Am Anim Hosp Assoc. 1984;20:5.
27. Ogilvie, GK, Haschek, WM, Withrow, SJ, et al. Classification of primary lung tumors in dogs: 210 cases (1975-1985). J Am Vet Med Assoc. 1989;195:106–108.
28. Paoloni, MC, Adams, WM, Dubielzig, RR, et al. Comparison of results of computed tomography and radiography with histopathologic findings in tracheobronchial lymph nodes in dogs with primary lung tumors: 14 cases (1999-2002). J Am Vet Med Assoc. 2006;228:1718–1722.
29. Barr, F, Gruffydd-Jones, TJ, Brown, PJ, et al. Primary lung tumours in the cat. J Small Anim Pract. 1987;1987:11.
30. Hahn, KA, McEntee, MF. Prognosis factors for survival in cats after removal of a primary lung tumor: 21 cases (1979-1994). Vet Surg. 1998;27:307–311.
31. MacCoy, DM, Trotter, EJ, deLahunta, A, et al. Pelvic limb paralysis in a young miniature pinscher due to metastatic bronchogenic adenocarcinoma. J Am Anim Hosp Assoc. 1976;1976:4.
32. Sorjonen, DC, Braund, KG, Hoff, EJ. Paraplegia and subclinical neuromyopathy associated with a primary lung tumor in a dog. J Am Vet Med Assoc. 1982;180:1209–1211.
33. Moore, JA, Taylor, HW. Primary pulmonary adenocarcinoma with brain stem metastasis in a dog. J Am Vet Med Assoc. 1988;192:219–221.
34. Mori, T, Yamagami, T, Umeda, M, et al. Small cell anaplastic carcinoma of the lung with cerebral metastasis in a dog. J Vet Med Sci. 1991;53:1129–1131.
35. Brodey, RS. Hypertrophic osteoarthropathy in the dog: a clinicopathologic survey of 60 cases. J Am Vet Med Assoc. 1971;159:1242–1256.
36. Halliwell, WH, Ackerman, N. Botryoid rhabdomyosarcoma of the urinary bladder and hypertrophic osteoarthropathy in a young dog. J Am Vet Med Assoc. 1974;165:911–913.
37. Caywood, DD, Kramek, BA, Feeney, DA, et al. Hypertrophic osteopathy associated with a bronchial foreign body and lobar pneumonia in a dog. J Am Vet Med Assoc. 1985;186:698–700.
38. Liptak, JM, Monnet, E, Dernell, WS, et al. Pulmonary metastatectomy in the management of four dogs with hypertrophic osteopathy. Vet Comp Oncol. 2004;2:1–12.
39. Grillo, TP, Brandao, CV, Mamprim, MJ, et al. Hypertrophic osteopathy associated with renal pelvis transitional cell carcinoma in a dog. Can Vet J. 2007;48:745–747.
40. Moore, AS, Middleton, DJ. Pulmonary adenocarcinoma in 3 cats with non-respiratory signs only. J Small Anim Pract. 1982;23(9):501–509.
41. Pollack, M, Martin, RA, Diters, RW. Metastatic squamous cell carcinoma in multiple digits of a cat. J Am Anim Hosp Assoc. 1984;20:835–839.
42. Scott-Moncrieff, JCGSE, Radovsky, A, Blevins, WE. Pulmonary squamous cell carcinoma with multiple digital metastases in a cat. J Small Anim Pract. 1989;30:696–699.
43. Gottfried, SD, Popovitch, CA, Goldschmidt, MH, et al. Metastatic digital carcinoma in the cat: a retrospective study of 36 cats (1992-1998). J Am Anim Hosp Assoc. 2000;36:501–509.
44. Hawkins, EC, DeNicola, DB, Kuehn, NF. Bronchoalveolar lavage in the evaluation of pulmonary disease in the dog and cat. State of the art. J Vet Intern Med. 1990;4:267–274.
45. Hawkins, EC, DeNicola, DB, Plier, ML. Cytological analysis of bronchoalveolar lavage fluid in the diagnosis of spontaneous respiratory tract disease in dogs: a retrospective study. J Vet Intern Med. 1995;9:386–392.
46. Norris, CR, Griffey, SM, Walsh, P. Use of keyhole lung biopsy for diagnosis of interstitial lung diseases in dogs and cats: 13 cases (1998-2001). J Am Vet Med Assoc. 2002;221:1453–1459.
47. PD, K. Radiographic appearance of primary lung tumors in cats. Vet Radiol. 1986;27:8.
48. Schwarz, LA, Tidwell, AS. Alternative imaging of the lung. Clin Tech Small Anim Pract. 1999;14:187–206.
49. Reichle, JK, Wisner, ER. Non-cardiac thoracic ultrasound in 75 feline and canine patients. Vet Radiol Ultrasound. 2000;41:154–162.
50. Larson, MM. Ultrasound of the thorax (noncardiac). Vet Clin North Am Small Anim Pract. 2009;39:733–745.
51. Marolf, AJ, Gibbons, DS, Podell, BK, et al. Computed tomographic appearance of primary lung tumors in dogs. Vet Radiol Ultrasound. 2011;52:168–172.
52. Nemanic, S, London, CA, Wisner, ER. Comparison of thoracic radiographs and single breath-hold helical CT for detection of pulmonary nodules in dogs with metastatic neoplasia. J Vet Intern Med. 2006;20:508–515.
53. Eberle, N, Fork, M, von Babo, V, et al. Comparison of examination of thoracic radiographs and thoracic computed tomography in dogs with appendicular osteosarcoma. Vet Pathol. 2011. [DOI 10.1111/j.1476-5829.2010.00241.x].
54. Wood, EF, O’Brien, RT, Young, KM. Ultrasound-guided fine-needle aspiration of focal parenchymal lesions of the lung in dogs and cats. J Vet Intern Med. 1998;12:338–342.
55. Zekas, LJ, Crawford, JT, O’Brien, RT. Computed tomography-guided fine-needle aspirate and tissue-core biopsy of intrathoracic lesions in thirty dogs and cats. Vet Radiol Ultrasound. 2005;46:200–204.
56. Bauer, TG. Lung biopsy. Vet Clin North Am Small Anim Pract. 2003;30:1207–1225.
57. Norris, CR, Griffey, SM, Samii, VF, et al. Comparison of results of thoracic radiography, cytologic evaluation of bronchoalveolar lavage fluid, and histologic evaluation of lung specimens in dogs with respiratory tract disease: 16 cases (1996-2000). J Am Vet Med Assoc. 2001;218:1456–1461.
58. Venker-van Haagen, AJVM, Heijn, A, et al. Bronchoscopy in small animal clinics: an analysis of the results of 228 bronchoscopies. J Am Anim Hosp Assoc. 1985;24:6.
59. Faunt, KK, Jones, BD, Turk, JR, et al. Evaluation of biopsy specimens obtained during thoracoscopy from lungs of clinically normal dogs. Am J Vet Res. 1998;59:1499–1502.
60. Kovak, JR, Ludwig, LL, Bergman, PJ, et al. Use of thoracoscopy to determine the etiology of pleural effusion in dogs and cats: 18 cases (1998-2001). J Am Vet Med Assoc. 2002;221:990–994.
61. Slatter, DH. Textbook of small animal surgery, ed 3. Philadelphia: Saunders; 2003.
62. Liptak, JM, Monnet, E, Dernell, WS, et al. Pneumonectomy, four case studies and a comparative review. J Small Anim Prac. 2004;45:441–447.
63. LaRue, SM, Withrow, SJ, Wykes, PM. Lung resection using surgical staples in dogs and cats. Vet Surg. 1987;16:238–240.
64. Walshaw, R. Stapling techniques in pulmonary surgery. Vet Clin North Am Small Anim Pract. 1994;24:335–366.
65. Lansdowne, JL, Monnet, E, Twedt, DC, et al. Thoracoscopic lung lobectomy for treatment of lung tumors in dogs. Vet Surg. 2005;34:530–535.
66. Levionnois, OL, Bergadano, A, Schatzmann, U. Accidental entrapment of an endo-bronchial blocker tip by a surgical stapler during selective ventilation for lung lobectomy in a dog. Vet Surg. 2006;35:82–85.
67. Dhumeaux, MP, Haudiquet, PR. Primary pulmonary osteosarcoma treated by thoracoscopy-assisted lung resection in a dog. Can Vet J. 2009;50:755–758.
68. Mosing, M, Iff, I, Moens, Y. Endoscopic removal of a bronchial carcinoma in a dog using one-lung ventilation. Vet Surg. 2008;37:222–225.
69. Denlinger, CE, Fernandez, F, Meyers, BF, et al. Lymph node evaluation in video-assisted thoracoscopic lobectomy versus lobectomy by thoracotomy. Ann Thorac Surg. 2010;89:1730–1735. [discussion 1736].
70. DeVita, VT, Lawrence, TS, Rosenberg, SA. DeVita, Hellman, and Rosenberg’s cancer: principles & practice of oncology, ed 8. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins; 2008.
71. Ogilvie, GK, Reynolds, HA, Richardson, RC, et al. Phase II evaluation of doxorubicin for treatment of various canine neoplasms. J Am Vet Med Assoc. 1989;195:1580–1583.
72. Ogilvie, GK, Obradovich, JE, Elmslie, RE, et al. Efficacy of mitoxantrone against various neoplasms in dogs. J Am Vet Med Assoc. 1991;198:1618–1621.
73. Poirier, VJ, Burgess, KE, Adams, WM, et al. Toxicity, dosage, and efficacy of vinorelbine (Navelbine) in dogs with spontaneous neoplasia. J Vet Intern Med. 2004;18:536–539.
74. Chabner, B, Longo, DL. Cancer chemotherapy and biotherapy: principles and practice, ed 4. Philadelphia: Lippincott Williams & Wilkins; 2006.
75. Hershey, AE, Kurzman, ID, Forrest, LJ, et al. Inhalation chemotherapy for macroscopic primary or metastatic lung tumors: proof of principle using dogs with spontaneously occurring tumors as a model. Clin Cancer Res. 1999;5:2653–2659.
76. Khanna, C, Vail, DM. Targeting the lung: preclinical and comparative evaluation of anticancer aerosols in dogs with naturally occurring cancers. Curr Cancer Drug Targets. 2003;3:265–273.
77. London, CA, Hannah, AL, Zadovoskaya, R, et al. Phase I dose-escalating study of SU11654, a small molecule receptor tyrosine kinase inhibitor, in dogs with spontaneous malignancies. Clin Cancer Res. 2003;9:2755–2768.
78. Moore, AS, Kirk, C, Cardona, A. Intracavitary cisplatin chemotherapy experience with six dogs. J Vet Intern Med. 1991;5:227–231.
79. Spugnini, EP, Crispi, S, Scarabello, A, et al. Piroxicam and intracavitary platinum-based chemotherapy for the treatment of advanced mesothelioma in pets: preliminary observations. J Exp Clin Cancer Res. 2008;27:6.
80. Kelly, J, Holmes, EC, Rosen, G. Mitoxantrone for malignant pleural effusion due to metastatic sarcoma. Surg Oncol. 1993;2:299–301.
81. Sparkes, A, Murphy, S, McConnell, F, et al. Palliative intracavitary carboplatin therapy in a cat with suspected pleural mesothelioma. J Feline Med Surg. 2005;7:313–316.
82. Laing, EJNA. Pleurodesis as a treatment for pleural effusion in the dog. J Am Anim Hosp Assoc. 1986;2:4.
83. Rose, SC, Thistlethwaite, PA, Sewell, PE, et al. Lung cancer and radiofrequency ablation. J Vasc Interv Radiol. 2006;17:927–951. [quiz 951].
84. Okuma, T, Matsuoka, T, Yamamoto, A, et al. Frequency and risk factors of various complications after computed tomography-guided radiofrequency ablation of lung tumors. Cardiovasc Intervent Radiol. 2008;31:122–130.
85. Crocetti, L, Lencioni, R. Radiofrequency ablation of pulmonary tumors. Eur J Radiol. 2010;75:23–27.
86. Duncan, M, Wijesekera, N, Padley, S. Interventional radiology of the thorax. Respirology. 2010;15:401–412.
87. Grootenboers, MJ, Schramel, FM, van Boven, WJ, et al. Selective pulmonary artery perfusion followed by blood flow occlusion: new challenge for the treatment of pulmonary malignancies. Lung Cancer. 2009;63:400–404.
88. Ahrar, K, Price, RE, Wallace, MJ, et al. Percutaneous radiofrequency ablation of lung tumors in a large animal model. J Vasc Interv Radiol. 2003;14:1037–1043.
89. Muller, H, Guadagni, S. Regional chemotherapy for carcinoma of the lung. Surg Oncol Clin N Am. 2008;17:895–917.
90. van Putte, BP, Grootenboers, M, van Boven, WJ, et al. Selective pulmonary artery perfusion for the treatment of primary lung cancer: Improved drug exposure of the lung. Lung Cancer. 2009;65:208–213.
91. Ogilvie, GK, Weigel, RM, Haschek, WM, et al. Prognostic factors for tumor remission and survival in dogs after surgery for primary lung tumor: 76 cases (1975-1985). J Am Vet Med Assoc. 1989;195:109–112.
92. Polton, GA, Brearley, MJ, Powell, SM, et al. Impact of primary tumour stage on survival in dogs with solitary lung tumours. J Small Anim Pract. 2008;49:66–71.
93. Jemal, A, Center, MM, DeSantis, C, et al. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19:1893–1907.
94. Valaitis, J, Warren, S, Gamble, D. Increasing incidence of adenocarcinoma of the lung. Cancer. 1981;47:1042–1046.
95. Westra, WH, Slebos, RJ, Offerhaus, GJ, et al. K-ras oncogene activation in lung adenocarcinomas from former smokers. Evidence that K-ras mutations are an early and irreversible event in the development of adenocarcinoma of the lung. Cancer. 1993;72:432–438.
96. Pugh, TJ, Bebb, G, Barclay, L, et al. Correlations of EGFR mutations and increases in EGFR and HER2 copy number to gefitinib response in a retrospective analysis of lung cancer patients. BMC Cancer. 2007;7:128.
97. Postorino, NC, Wheeler, SL, Park, RD, et al. A syndrome resembling lymphomatoid granulomatosis in the dog. J Vet Intern Med. 1989;3:15–19.
98. Shimazaki, T, Nagata, M, Goto-Koshino, Y, et al. A case of canine lymphomatoid granulomatosis with cutaneous lesions. J Vet Med Sci. 2010;72(8):1067–1069.
99. Berry, CR, Moore, PF, Thomas, WP, et al. Pulmonary lymphomatoid granulomatosis in seven dogs (1976-1987). J Vet Intern Med. 1990;4:157–166.
100. Hatoya, S, Kumagai, D, Takeda, S, et al. Successful management with CHOP for pulmonary lymphomatoid granulomatosis in a dog. J Vet Med Sci. 2011;73(4):527–530.
101. Rosin, A, Moore, P, Dubielzig, R. Malignant histiocytosis in Bernese Mountain dogs. J Am Vet Med Assoc. 1986;188:1041–1045.
102. Padgett, GA, Madewell, BR, Keller, ET, et al. Inheritance of histiocytosis in Bernese mountain dogs. J Small Anim Pract. 1995;36:93–98.
103. Moore, PF, Affolter, VK, Vernau, W. Canine hemophagocytic histiocytic sarcoma: a proliferative disorder of CD11d+ macrophages. Vet Pathol. 2006;43:632–645.
104. Skorupski, KA, Clifford, CA, Paoloni, MC, et al. CCNU for the treatment of dogs with histiocytic sarcoma. J Vet Intern Med. 2007;21:121–126.
105. Skorupski, KA, Rodriguez, CO, Krick, EL, et al. Long-term survival in dogs with localized histiocytic sarcoma treated with CCNU as an adjuvant to local therapy. Vet Comp Oncol. 2009;7:139–144.