Chapter 19 Medical Treatment of Peripheral Artery Disease
Treatment of patients with peripheral artery disease (PAD) must take into consideration the risk of adverse cardiovascular events related to systemic atherosclerosis (myocardial infarction [MI], stroke, death) and limb-related symptoms and prognosis (functional capacity, quality of life, limb viability). The risk of MI, stroke, or death related to cardiovascular disease is increased three- to sixfold in patients with PAD (see Chapter 16). Functional limitations imposed by PAD, including symptoms of limb claudication, impaired walking ability, and critical limb ischemia (CLI), adversely affect quality of life and restrict patients’ abilities to participate in many basic vocational and recreational activities. In addition, patients with PAD are at increased risk of lower-extremity ulceration and amputation, and thus foot care represents an important component of management of these patients. Medical management of the patient with PAD has three central goals: (1) prevention of cardiovascular events, (2) improvement of quality of life and functional capacity, and (3) protection and care of the limb (Fig. 19-1). In this chapter, we review the evidence to support aggressive risk factor modification and antiplatelet therapy for patients with PAD to reduce adverse cardiovascular events. We also review the physical and medical therapies used to treat patients with intermittent claudication and CLI to improve lower-extremity function and ameliorate symptoms. Foot care for PAD is briefly discussed. Catheter-based revascularization for PAD is reviewed in Chapter 20, and surgical revascularization for PAD is reviewed in Chapter 21. Multisocietal consensus guidelines for management of the patient with PAD are available and may be helpful in clinical practice.1,2
Figure 19-1 Comprehensive management of the patient with peripheral artery disease (PAD) must include three important components of care: prevention of adverse cardiovascular events, foot care, and improvement of functional capacity and quality of life (QOL).
(Reproduced with permission from Cleveland Clinic Foundation.)
Risk Factor Modification and Antiplatelet Therapy for Prevention of Cardiovascular Events
Smoking Cessation
Tobacco smoking is strongly associated with development and progression of PAD, with the risk of PAD among smokers as high as threefold that of nonsmokers (see Chapter 16). Smoking cessation is a critical component of risk factor modification for patients with PAD. Epidemiological studies have established that smoking cessation improves both cardiovascular and limb-related outcomes among patients with PAD. Given the established hazards of cigarette smoking, it would be unethical to conduct a randomized clinical trial of smoking cessation.
Smoking cessation has salutary effects on claudication symptoms, exercise physiology, and limb-related outcomes in patients with symptomatic PAD. Patients with intermittent claudication who quit smoking have longer pain-free walking times and maximal walking times compared with patients who continue to smoke.3 In a prospective study of patients with intermittent claudication followed with serial noninvasive vascular testing over a period of 10 months, patients who quit smoking had significant improvements in maximal treadmill walking distance and postexercise ankle pressure, whereas ongoing smokers had no changes in these parameters.4 Smoking cessation is also associated with improved clinical outcomes in patients with PAD. In a Swedish study, patients with intermittent claudication who were active smokers or who had quit within 6 months were followed prospectively for development of limb-related and cardiovascular outcomes.5 At 7 years of follow-up, ongoing tobacco smoking was associated with development of CLI and was also an independent predictor of the need for surgical revascularization. Indeed, only patients who continued to smoke developed rest pain during the follow-up period. Ongoing smoking was also associated with development of MI and a trend toward decreased overall survival at 10 years of follow-up.
Continued cigarette smoking is associated with adverse outcome among patients with PAD referred for vascular surgery. In a prospective study of patients referred for femoropopliteal arterial bypass grafting, ongoing tobacco use was associated with a significant reduction in the 1-year cumulative patency rate of both venous and prosthetic lower-extremity bypass grafts.6 In an Australian study of patients who underwent lumbar sympathectomy or lower-extremity bypass grafting for symptomatic PAD, patients who quit smoking following surgery had dramatically improved 5-year survival rates compared to patients who continued to smoke.7 The majority of deaths that occurred in the postoperative patients were due to a major vascular event, whereas the remaining deaths were due to other smoking-related illnesses, principally chronic obstructive pulmonary disease (COPD) and lung cancer.
Degree of ongoing tobacco use following revascularization may also be predictive of adverse events. In a registry study of patients who underwent their first arterial revascularization procedure, patients categorized as heavy smokers (>15 cigarettes/day) had significantly reduced overall survival compared to moderate smokers (<15 cigarettes/day).8 In addition, there was a 10-fold higher amputation rate among heavy smokers compared with moderate smokers at 3 years’ follow-up.
Despite the multiple benefits of smoking cessation in patients with PAD, it is an extremely difficult goal to accomplish, and initial success rates are low. The efficacy of physician advice in achieving smoking cessation is less than 5%.9 The success rate is at least 10-fold higher when smoking cessation advice and encouragement are given to patients at risk for MI, or patients who have survived an MI. Intensive counseling customized to PAD patients who continue to smoke is associated with a significant improvement in confirmed tobacco abstinence at 6 months of follow-up, compared to standard clinical smoking cessation advice.10 Smoking cessation programs may be more successful when coupled with pharmacological therapy, including both nicotine and non-nicotine agents. The antidepressant bupropion has been demonstrated to improve tobacco abstinence rates at 12 months relative to placebo when used alone or in combination with the nicotine patch.11 Recently, varenicline, a novel partial agonist of the nicotinic acetylcholine receptor (nAchR) α4β, has been shown to improve tobacco abstinence rates among subjects both with and without cardiovascular disease, including patients with PAD.12,13 Among those with cardiovascular disease, varenicline was associated with a threefold likelihood of abstinence at 1-year follow-up compared with placebo, although the absolute abstinence rate was only 19.2%.13 Side effects of varenicline include sleep abnormalities, nausea, and flatulence.13,14 Both varenicline and bupropion are associated with an increased risk of neuropsychiatric side effects. Package labeling for both agents includes a black box warning recommending observation for changes in behavior or mood or development of suicidal ideation while receiving these agents for smoking cessation treatment.14,15
Recommendations
Smoking cessation advice and encouragement of cessation efforts should be key components of each office visit. For patients motivated to quit smoking, treatment with nicotine replacement therapy, bupropion, or varenicline should be considered. These efforts may be incorporated into a formal smoking cessation program that includes longitudinal counseling on an individual basis or in a small group.
Lipid-Lowering Therapy
Dyslipidemia is a well-established risk factor for development of atherosclerotic vascular disease, including coronary artery disease (CAD) and cerebrovascular disease (CVD). In addition, epidemiological studies have established dyslipidemia—specifically high total and low-density lipoprotein (LDL) cholesterol—as a risk factor for development of atherosclerosis in the peripheral circulation16–18 (see Chapter 16). There is a less established association between low levels of high density lipoprotein (HDL) cholesterol and development of claudication.16,19,20 The association, if any, between elevated triglycerides and PAD is controversial.19,20 The association of dyslipidemia with vascular disease has led to extensive investigation of lipid-lowering therapy as a clinical strategy to prevent myocardial ischemia, stroke, and death in patients with systemic atherosclerosis. Some of these studies specifically addressed the use of lipid-lowering therapy in patients with PAD.
Given convincing epidemiological evidence that dyslipidemia is a risk factor for development of atherosclerosis and subsequent cardiovascular events, multiple randomized clinical trials investigated the use of lipid-lowering agents for prevention of death and other major cardiovascular events in high-risk patients. Development of highly effective and safe agents, particularly HMG-CoA reductase inhibitors (“statins”), has led to widespread application of lipid-lowering therapy for patients with hyperlipidemia and atherosclerotic vascular disease. In recent decades, multiple large randomized controlled trials established the role of lipid-lowering pharmacotherapy, primarily with statins, in secondary prevention of cardiovascular events among patients with CAD.21–24 Although these studies were not designed to specifically investigate the long-term benefit of lipid-lowering therapy in patients with PAD, the findings are of relevance in these patients because most patients with PAD have either symptomatic or asymptomatic coronary atherosclerosis.25–27
The Scandinavian Simvastatin Survival Study (4 S) was the first major clinical trial to demonstrate the survival benefit of aggressive lipid-lowering therapy with statins in hypercholesterolemic patients with CAD.22 In secondary analyses, the 4 S investigators examined the effect of simvastatin on development of symptoms and signs of atherosclerotic vascular disease, including claudication and the appearance of a vascular bruit, during semiannual physical examinations of the study participants.28 There was no significant difference in detection of new or worsening femoral bruits, although this endpoint was likely limited by interobserver variability and the limited sensitivity of the physical examination. There was a 38% reduction in the incidence of intermittent claudication and a 30% reduction in cerebrovascular events among patients randomized to simvastatin.22,28 Similar findings have been confirmed in subsequent studies of the use of HMG-CoA reductase inhibitors for treatment of symptomatic claudication, discussed later in this chapter.
The Heart Protection Study extended use of statins to secondary prevention of cardiovascular events in patients with atherosclerosis in any major vascular bed, and to primary prevention of cardiovascular events in high-risk patients, particularly diabetics.27,29 Patients were randomized to simvastatin or placebo and followed for a mean of 5 years. Eligible patients included those with documented CAD, CVD, or PAD, or patients without documented atherosclerotic vascular disease believed to be at high risk of a major vascular event due to diabetes or multiple cardiac risk factors. Patients enrolled in the study on the basis of PAD had intermittent claudication, had undergone lower-extremity revascularization, or had objective evidence of “leg artery stenosis.” The majority of patients randomized in the study had a history of CAD (65%); of these, 30% had PAD. There was a 13% reduction in the relative risk of all-cause mortality among patients randomized to simvastatin, due largely to a 17% reduction in the risk of vascular death. There was a 24% reduction in the first occurrence of any major vascular event among patients randomized to simvastatin. Patients enrolled on the basis of PAD alone, with no documented CAD, had a 19% reduction in incidence of first major vascular event if randomized to simvastatin. In a subsequent subset analysis of the 4588 subjects with PAD, randomization to simvastatin was associated with a 20% reduction in noncoronary revascularization procedures compared to placebo.30 There was no significant benefit of simvastatin on the incidence of lower-extremity amputation.
Non-LDL cholesterol, particularly low HDL cholesterol, has been identified as an independent risk factor for development of CAD.31,32 Pharmacological agents that raise HDL cholesterol (e.g., fibric acid derivatives, niacin) have not been as widely studied as agents that lower total and LDL cholesterol (i.e., statins) for secondary prevention of cardiovascular events in patients with atherosclerotic vascular disease, and specifically for PAD. The Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial (VA-HIT) investigators randomized men with documented CAD and low HDL cholesterol to gemfibrozil or placebo and found a 22% reduction in the primary composite endpoint of death from CAD or nonfatal MI in the gemfibrozil cohort after a median of 5.1 years of follow-up.21 The incidence of peripheral vascular surgery, the only PAD endpoint studied, was not significantly different within the treatment groups. Cardiovascular benefit has not been found in subsequent studies of other fibrates. The Bezafibrate Infarction Prevention (BIP) study was a randomized study of bezafibrate or placebo in patients with a previous MI or stable angina.33 Bezafibrate did not significantly affect the primary endpoint of fatal or nonfatal MI or sudden death. The Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study was a randomized study of fenofibrate or placebo in patients with diabetes.34 Fenofibrate did not significantly affect the primary outcome of fatal coronary heart disease (CHD) and nonfatal MI, but did decrease secondary endpoints such as nonfatal MI and coronary and peripheral revascularization. Given these limited data, the role of therapies to target non-LDL cholesterol in the management of patients with PAD is uncertain.
Recommendations
The American College of Cardiology/American Heart Association (ACC/AHA) PAD guidelines recommend treatment of patients with PAD with an HMG-CoA reductase inhibitor to an LDL goal of less than 100 mg/dL (class I). A lower LDL goal of less than 70 mg/dL is recommended as a more aggressive option for those patients at very high risk of an ischemic event (class IIa).1 Statins should be prescribed for all patients with PAD regardless of whether or not they have documented CAD. To date, no clinical trial has investigated the role of combination therapy (i.e., statin plus fibrate, statin plus ezetimibe) specifically among patients with PAD.
Treatment of Hypertension
Control of hypertension is critical for preventing stroke, MI, and congestive heart failure (CHF). Blood pressure control can be achieved with a number of pharmacological agents, either individually or in combination. Large randomized clinical trials have demonstrated the benefits of pharmacotherapy on clinical outcomes, including death.35,36 Although patients with hypertension and PAD are at increased risk of serious vascular events, few studies have specifically addressed the benefit of blood pressure lowering in this population.
Effect of blood pressure lowering on claudication
Conventional medical wisdom and small clinical trials have alleged that intensive blood pressure lowering, particularly with β-adrenergic blockers, may worsen symptoms in patients with claudication and PAD.37–41 The safety of blood pressure–lowering therapy, specifically with β-adrenergic blockers, in patients with PAD and claudication is of great importance. A substantial percentage of patients with PAD have concomitant CAD. In these patients—in particular, those who have had an MI—treatment with a β-adrenergic blocker has been demonstrated to be lifesaving therapy in large multicenter randomized clinical trials.42,43 β-Adrenergic blockers are also crucial for management of symptomatic angina pectoris in patients with CAD and patients with left ventricular (LV) dysfunction. In addition, β-adrenergic blockers are a key component of perioperative management of patients with PAD and claudication undergoing lower-extremity revascularization surgery.44,45
In a meta-analysis of small randomized controlled clinical trials of the use of β-blockers in patients with PAD and intermittent claudication, there was no significant effect on pain-free or maximal walking distance among patients treated with β-blockers.46 Of the 11 studies included in the analysis, only one study of 20 patients demonstrated an adverse effect of β-blockers on leg symptoms.40 Recently there has been interest in the potential for the β-blocker nebivolol to improve claudication symptoms.47,48 In one study of 128 patients with intermittent claudication randomized to nebivolol or metoprolol for a 48-week treatment period, there was no significant difference in pain-free or maximal walking distance between the two β-blocker groups. There were modest improvements in initial (for nebivolol) and absolute (both nebivolol and metoprolol) claudication distance compared to baseline, but in the absence of a placebo-treated control group, interpretation of these findings is limited.47
Demonstration of the safety of angiotensin-converting enzyme (ACE) inhibitors in the majority of patients with claudication is very important, particularly given the findings of recent trials that have established the importance of this class of agents in preventing cardiovascular events.49 The effect of blood pressure–lowering therapy for 6 weeks with the ACE inhibitor perindopril was studied in patients with hypertension and one of many comorbidities, including PAD.50 A subgroup of patients had significant symptomatic PAD, defined as a pain-free walking distance of 80 to 200 meters (Fontaine IIb) and an imaging study that demonstrated evidence of iliac or femoral arterial occlusive disease. Among the patients with PAD, there was no difference in the pain-free or maximal walking distance between the perindopril and placebo groups, although walking distance increased modestly in both groups above baseline. None of the PAD patients reported worsening claudication. More recently, Ahimastos et al. reported a beneficial effect of the ACE inhibitor ramipril.51 In a pilot study of 40 patients with superficial femoral artery (SFA) occlusive disease and intermittent claudication, without diabetes mellitus or hypertension, there was significant improvement in maximal walking time among patients randomized to ramipril compared with placebo.
Clinical outcomes trials
Two major clinical trials have investigated the potential benefits of blood pressure–lowering therapy in preventing cardiovascular events in patients with PAD. The Appropriate Blood Pressure Control in Diabetes (ABCD) trial studied the effect of intensive blood pressure control compared with moderate blood pressure control on the occurrence of cardiovascular events and renal insufficiency in diabetic patients.52,53 Normotensive (diastolic blood pressure 80-90 mm Hg) and hypertensive (diastolic blood pressure > 90 mm Hg) diabetic patients were enrolled. The hypertensive patients were randomized to receive either enalapril or nisoldipine as first-line therapy. The normotensive patients were randomized to either intensive blood pressure treatment (with further randomization to one of the two study drugs) to achieve a reduction in diastolic pressure of 10 mm Hg, or standard therapy, in which case they received a placebo. Patients were followed for a mean of 5 years. In a substudy of the diabetic patients in the normotensive cohort, the effect of intensive blood pressure treatment among patients with PAD was investigated.53 Among the 220 normotensive patients randomized to intensive blood pressure treatment, there was a 65% reduction in the relative risk of MI, stroke, or cardiovascular death. There was a strong inverse relationship between baseline ankle-brachial index (ABI) and risk of a cardiovascular event. Intensive blood pressure control negated this relationship and normalized the odds of a cardiovascular event toward that of patients with a normal ABI (Fig. 19-2). The benefit of intensive blood pressure control was evident even in patients with a severely decreased ABI, a subset of patients that had been excluded from prior studies.50 There were too few patients with PAD to analyze data for enalapril and nisoldipine separately. The findings of the ABCD trial emphasize the importance of intensive blood pressure control in diabetic patients with PAD.
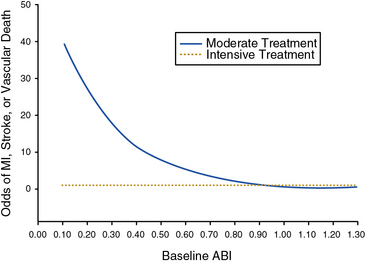
Figure 19-2 Intensive blood pressure control with enalapril or nisoldipine decreases the odds of a major vascular event among diabetic patients with peripheral artery disease (PAD).
Results from the Appropriate Blood Pressure Control in Diabetics (ABCD) Trial. The inverse correlation of ankle-brachial index (ABI) and odds of a major vascular event are not present among patients randomized to the intensive blood pressure control group. MI, myocardial infarction.
(Reproduced with permission from Mehler PS, Coll JR, Estacio R, et al: Intensive blood pressure control reduces the risk of cardiovascular events in patients with peripheral arterial disease and type 2 diabetes. Circulation 107:753–756, 2003.)53
The Heart Outcomes Prevention Evaluation (HOPE) investigators tested the efficacy of the ACE inhibitor ramipril for primary and secondary prevention of cardiovascular events in high-risk patients.49 Patients were enrolled on the basis of established atherosclerotic vascular disease (CAD, prior stroke, or PAD) or diabetes mellitus with additional cardiac risk factors. Some 44% of patients randomized had evidence of PAD, as manifested by a history of claudication with an abnormal ABI of less than 0.8, limb revascularization procedure, amputation, or angiographic evidence of arterial stenosis.54 Patients were randomized to receive ramipril or placebo. At a mean of 5 years of follow-up, there was a 22% reduction in the primary composite endpoint of MI, stroke, or cardiovascular death among patients randomized to ramipril (Fig. 19-3). In subgroup analysis, the benefit of ramipril was present regardless of baseline blood pressure. The benefit of ramipril on the composite endpoint was present among the subgroup of patients with PAD. The benefit of ramipril was likely due to cardiovascular benefits other than its blood pressure–lowering properties because the mean decrease in blood pressure among patients randomized to active therapy was very small. The findings of the HOPE trial indicate that patients with PAD should be treated with an ACE inhibitor, regardless of baseline blood pressure or the presence or absence of diabetes.
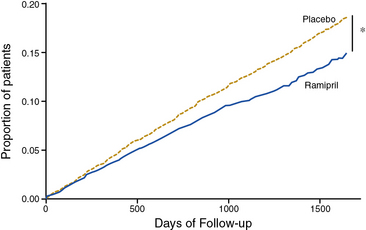
Figure 19-3 Ramipril reduces the incidence of myocardial infarction (MI), stroke, or cardiovascular death among high-risk patients with atherosclerotic vascular disease or diabetes mellitus.
(Reproduced with permission from Yusuf S, Sleight P, Pogue J, et al: Effects of an angiotensin-converting enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med 342:145–153, 2000.) 49
For patients with PAD who are intolerant of ACE inhibitors (e.g., development of cough), an angiotensin receptor blocker (ARB) is an acceptable alternative agent for cardiovascular risk reduction. In the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint (ONTARGET) Trial, 25,620 patients at high risk for cardiovascular events, a population similar to the HOPE trial, were randomized to receive ramipril, the ARB telmisartan, or a combination of the two agents.55 The ONTARGET population included 2468 patients with symptomatic PAD. After a median follow-up of 56 months, the primary cardiovascular event rates in the ramipril, telmisartan, and combination therapy groups were statistically the same (≈︀6.5%). The combination of ramipril and telmisartan, however, was associated with higher rates of renal insufficiency and hypotension than either agent alone.
Recommendations
Blood pressure control is an important component of cardiovascular risk reduction among patients with PAD, and all patients with PAD should routinely undergo blood pressure assessment. Blood pressure should be measured at least once in both upper extremities to exclude the possibility of occult subclavian stenosis leading to inaccurate blood pressure assessment in one of the arms. Any class of antihypertensive agents, including β-blockers, can be safely prescribed for blood pressure lowering, although ACE inhibitors or ARBs should be considered as first-line therapies. Caution should be exercised when managing the patient with CLI because aggressive blood pressure lowering may be detrimental in this small subset of patients. Even normotensive patients with PAD may benefit from ACE inhibitor or ARB therapy. Aggressive blood pressure control is particularly important for diabetic patients with PAD. According to the ACC/AHA PAD guidelines, target blood pressure for patients with PAD is below 140/90 mm Hg for nondiabetic and below 130/80 for diabetic patients (class I recommendation).1
Control of Diabetes Mellitus
Multiple epidemiological studies have established a strong association between diabetes mellitus and PAD16,17,56,57 (see Chapter 16). The relative risk of PAD is two to four times that of nondiabetic patients. Presence of diabetes is associated with adverse limb-related outcomes among patients with documented PAD, including the need for amputation.58,59 In the Strong Heart Study, worsening glycemic control (determined by hemoglobin A1c [HbA1c] level) was associated with increased incidence of lower-extremity amputation among diabetic Native Americans with and without PAD.60 In addition, diabetes is associated with a markedly increased risk of a major cardiovascular event, including myocardial ischemia, stroke, and death. Therapeutic options for achieving glycemic control in diabetic patients include insulin, sulfonylureas, metformin, the thiazolidinediones (“glitazones”), and novel agents that modify carbohydrate absorption and breakdown into glucose (α-glucosidase inhibitors) or increase insulin bioavailability through differing mechanisms (e.g., repaglinide, nateglinide, sitagliptin).61 Whereas clinical trials have established the vital importance of rigorous glycemic control for preventing microvascular complications in diabetic patients, the benefits of glycemic control for prevention of major cardiovascular events has not been definitively established. In addition, recent randomized controlled trials have indicated that certain high-risk patients may be more likely to experience adverse effects with very intensive glycemic control.62
Clinical outcomes trials
The Diabetes Control and Complications Trial (DCCT) Research Group investigated whether intensive glycemic control could improve clinical outcomes in diabetic patients.63 A total of 1441 young patients (aged 13-39 years) with type 1 diabetes mellitus, with and without retinopathy, were randomized to one of two glycemic control strategies: (1) intensive therapy with an insulin pump or frequent injections to maintain blood sugar as close to normal as possible, or (2) conventional therapy with once- or twice-daily insulin injections. Development or progression of retinopathy was the primary endpoint of the study, and the low cardiovascular risk profile of the study population reflected this goal. Peripheral artery disease endpoints included development of claudication, persistent loss of a pedal pulse, and need for a revascularization procedure or limb amputation. Patients were followed for a mean of 6.5 years. Among patients randomized to intensive glycemic control, there were significant reductions in development and progression of retinopathy and proteinuria and development of sensorimotor neuropathy compared to patients treated with conventional glycemic control. Owing to the young average age of the patient population, there were few deaths or major macrovascular events in either treatment group. Nonetheless, randomization to intensive glycemic control was associated with a nonsignificant 42% reduction in peripheral vascular and coronary events.64 Perhaps more importantly, however, randomization to intensive glycemic control was protective against development of elevated total and LDL cholesterol—risk factors for future development of cardiovascular events in this young patient population. Long-term follow-up data from the DCCT study were recently published.65 Additional observational follow-up data were available for 93% of patients enrolled in the original trial in the Epidemiology of Diabetes Interventions and Complications study. At a mean follow-up of 17 years, randomization to intensive glycemic control showed a persistent 42% reduction in risk for a major cardiovascular event.
The United Kingdom Prospective Diabetes Study 33 (UKPDS 33) was designed to complement the findings of the DCCT by investigating the effect of intensive glycemic control (with sulfonylureas or insulin) on the incidence of macrovascular events in type 2 diabetic patients.66 An earlier study of glycemic control strategies in type 2 diabetic persons found no cardiovascular benefit of intensive therapy with insulin and an excess of cardiovascular deaths among patients treated with the sulfonylurea tolbutamide.67 This early finding generated concern regarding the safety and potential cardiovascular toxicity of sulfonylureas with prolonged use in diabetic patients. In the UKPDS 33, patients were randomized to intensive therapy with insulin or one of three sulfonylureas, or to conventional treatment with a prescribed diabetic diet; they were followed for a median of 10 years.66 Although there were no differences in the treatment groups with regard to total mortality, cardiac mortality, or diabetes-related mortality, there was a 16% reduction in the relative risk of MI in the group randomized to intensive therapy. There was also a 39% reduction in relative risk of amputation in the intensive therapy group, which did not achieve statistical significance. There were too few deaths attributable to PAD to allow for comparison. Consistent with the findings of the DCCT, there was a highly significant 25% reduction in relative risk of microvascular complications among patients randomized to intensive glycemic control, including the need for retinal surgery.
A follow-up study evaluated a subset of participants from UKPDS 33 annually, either with clinical office visits or questionnaires, for up to 10 years after completion of the trial.68 During this period of follow-up, patients were managed at the discretion of their physicians rather than per trial protocol, and differences in HbA1c levels between the intensive and conventional treatment groups equalized. Despite this finding, the significant risk reduction in microvascular events among patients initially randomized to intensive therapy persisted (24% reduction). More importantly, there were statistically significant gains in terms of demonstration of delayed cardiovascular benefit of intensive therapy of diabetes, with reductions in all-cause mortality (27% reduction) and risk of MI (33% reduction). There was no significant reduction in risk of stroke or peripheral vascular outcomes.
Both the DCCT and UKPDS 33 trials established the importance of glycemic control in the prevention of microvascular complications in diabetic patients. The post-trial follow-up cohort studies for each of these trials have shown a sustained benefit of glycemic control on risk of microvascular complications and have also contributed data to support the possibility that intensive glycemic control (target HbA1c ≈︀ 7 g/dL) in these trials may prevent macrovascular events, particularly MI.
There has been great interest in use of the insulin-sensitizing agents, metformin and the thiazolidinediones (glitazones), for prevention of cardiovascular events in diabetic patients. In a substudy of the UKPDS (UKPDS 34), newly diagnosed, overweight, type 2 diabetic patients were randomized to intensive therapy with metformin or a prescribed diabetic diet.69 Patients were followed for a median of 10.7 years. In contrast to the sulfonylurea arm of the study, there was a significant 42% reduction in diabetes-related mortality and a 36% reduction in all-cause mortality among patients randomized to metformin therapy compared with patients randomized to diet. There was no difference in the incidence of PAD endpoints between the two groups, although the total number of clinical events related to PAD was small.
Use of thiazolidinediones for glycemic control and prevention of cardiovascular events among diabetic patients has been an area of recent study and significant controversy. In the Prospective Pioglitazone Clinical Trial in Macrovascular Events (PROactive) study, 5238 type 2 diabetic patients with established macrovascular (atherosclerotic) disease, including CAD, prior stroke, or PAD as defined by history of intermittent claudication (with abnormal ABI or toe-brachial index [TBI]) or prior major amputation were randomized to receive pioglitazone or placebo atop their established diabetes medical regimen and followed for incident cardiovascular events.70 At a mean follow-up of nearly 6 years, there was no significant difference between pioglitazone- and placebo-treated patients in the primary composite endpoint of all-cause mortality, nonfatal MI, stroke, acute coronary syndrome (ACS), coronary or leg endovascular or surgical revascularization, and amputation above the ankle. There was a 16% reduction in the secondary composite endpoint of death, nonfatal MI, or stroke in patients randomized to pioglitazone.
Recently, there have been safety concerns related to the safety of rosiglitazone, another thiazolidinedione, including risk of MI and congestive heart failure.71 The RECORD (Rosiglitazone Evaluated for Cardiovascular Outcomes in Oral Agent Combination Therapy for Type 2 Diabetes) trial randomized type 2 diabetic patients on sulfonylurea or metformin therapy with adequate glycemic control to receive added rosiglitazone therapy or added sulfonylurea and metformin in an open-label design.72 After a mean of follow-up of 5.5 years, there was no significant difference in the composite endpoint of cardiovascular death or hospitalization between the rosiglitazone and sulfonylurea/metformin combination therapy groups. There was, however, a more than twofold increase in risk of significant CHF (requiring hospitalization or leading to death) among rosiglitazone-treated patients. On the basis of previous meta-analyses and the findings of the RECORD trial, there is now a black box warning on the rosiglitazone label regarding adverse cardiovascular effects, and prescriptive access to this medication is restricted by the drug manufacturer.73
Three randomized controlled trials that have investigated the benefit of very intensive glycemic control (target HbA1c 6%-6.5% or normoglycemia) versus modern standard glycemic control (target HbA1c <7%-7.5%) among patients with type 2 diabetes.74–76 In the Action and Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trial, there was a 14% reduction in microvascular events among patients randomized to highly intensive therapy, but there was no significant reduction in macrovascular events, including risk of death, MI, or stroke.76 In the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study, not only was there no benefit of highly intensive glycemic control on a composite endpoint of cardiovascular death or nonfatal MI or stroke, but there was also an increased risk of death from any cause (22%) or cardiovascular death (35%) among individuals randomized to highly intensive therapy.74 There was a marked increase in hypoglycemic episodes requiring medical assistance (10.5% vs. 3.5%) among patients randomized to highly intensive therapy. The ACCORD trial was terminated early at the recommendation of its data safety and monitoring board based upon these findings. Finally, the Veterans Affairs Diabetes Trial (VADT) randomized type 2 diabetes patients to either standard therapy or highly intensive glycemic control (goal HbA1c <6%) using rosiglitazone plus either glimepiride or metformin, based upon body mass index (BMI; metformin for patients with BMI ≥ 27 kg/m2).75 In this trial, there was no benefit of intensive glycemic control on the risk of macrovascular, or surprisingly microvascular, complications during 5.6 years of follow-up.
Recommendations
The American Diabetes Association has published guidelines for the medical care of diabetic patients with and without PAD.77,78 The ACC/AHA PAD guidelines also address care of the diabetic patient with PAD.1 In response to the findings of ACCORD, ADVANCE, and VADT, updated multisocietal guidelines for glycemic control have been published.79 The standard target HbA1c of less than 7% stands as a recommendation for most patients. However, it is recommended that less stringent glycemic control be considered for patients with prior history of severe hypoglycemic reaction, for those with extensive micro- and macrovascular disease, and for those with limited life expectancy. A more stringent target (e.g., HbA1c <6%-6.5%) is recommended as a target for healthier patients with a long life expectancy and no major cardiovascular disease.
In addition to glycemic control, periodic foot examination by healthcare providers and patient education regarding preventive foot care is critical for diabetic patients with PAD, particularly given the prevalence of peripheral neuropathy. The American Diabetic Association and the ACC/AHA recommend ABI measurement for all diabetic persons older than 50 years of age and for some younger patients with additional risk factors.1,77
Treatment of Hyperhomocysteinemia
Hyperhomocysteinemia is a disorder associated with derangements of the metabolic pathway involved in metabolism of the essential amino acid methionine.80 Hyperhomocysteinemia is due to an inherited defect of one of the enzymes involved in transsulfuration or remethylation of homocysteine, or can also occur as a result of malnutrition and deficiency of key cofactors for these enzymatic reactions (i.e., folic acid, vitamins B6 and B12). Hyperhomocysteinemia is associated with end-stage renal disease, although the mechanism by which this occurs is not well established.80 Inherited homocystinuria, the most striking form of hyperhomocysteinemia, is due to homozygous deficiency of the enzyme cystathionine β-synthase, a critical enzyme of the transsulfuration pathway of homocysteine. Homocystinuria is associated with mental retardation, ectopia lentis, and premature coronary and peripheral atherosclerosis. Among heterozygotes for cystathionine β-synthase deficiency, homocysteine levels are significantly lower (on the order of 20-40 μmol/L vs. up to 400 μmol/L for homozygotes), although there is also a predisposition to premature atherosclerosis.80 Multiple epidemiological studies have established an association between elevated plasma homocysteine levels and development and progression of CAD and CVD. Extensive epidemiological evidence has also established hyperhomocysteinemia as an independent risk factor for asymptomatic PAD and the clinical progression of symptomatic PAD.81–84 In addition to premature atherosclerosis, elevated levels of homocysteine are associated with a hypercoagulable state characterized by a tendency toward venous and arterial thrombosis.80,85
Because elevated plasma homocysteine levels are associated with low levels of the enzymatic cofactors folic acid, vitamin B6, and vitamin B12, vitamin supplementation is an obvious therapeutic consideration for treating hyperhomocysteinemia. One small trial of healthy siblings of patients with hyperhomocysteinemia and premature atherosclerotic vascular disease (PAD, CAD, or CVD before age 56) randomized siblings to combination vitamin therapy with 5 mg folic acid and 250 mg vitamin B6 or placebo.86 Siblings with and without evidence of hyperhomocysteinemia were enrolled. Subjects continued therapy for a period of 2 years and were followed for development of CAD (abnormal stress test), PAD (abnormal ABI or lower-extremity arterial duplex scan), and CVD (abnormal carotid artery duplex scan). Among patients randomized to vitamins, fasting plasma homocysteine levels fell from 14.7 to 7.4 μmol/L (a nearly 50% decline); there was an 18% decline in the placebo group. There was no significant difference in the development of PAD or asymptomatic CVD among the two groups. Among the patients randomized to aggressive vitamin therapy, there was a decrease in the incidence of an abnormal stress test.
Although the effectiveness of supplementation with folic acid and vitamin B12 for lowering plasma homocysteine levels has been established, few data show a benefit of vitamin supplementation to prevent vascular events in patients with hyperhomocysteinemia or established vascular disease. In the second Heart Outcomes and Prevention Evaluation (HOPE-2) trial, patients with atherosclerotic vascular disease were randomized to receive folic acid, vitamin B6 and B12, or placebo and followed for incident cardiovascular events.87,88 Less than 10% of patients in the study population of 5522 had known symptomatic PAD, defined as intermittent claudication or a history of bypass surgery or lower-extremity angioplasty.88 After 5 years of follow-up and despite a mean reduction in plasma homocysteine of 2.4 μmol/L in the vitamin-treated group (compared to an increase of 0.8 μmol/L in the placebo group), there was no benefit of vitamin therapy on the primary composite outcome of fatal or nonfatal MI or stroke.
In the Norwegian Vitamin Trial (NORVIT), patients with acute MI within the preceding 7 days were randomized to one of four arms in a factorial design (placebo, vitamin B6, folic acid + vitamin B12, or combination-therapy folic acid and vitamins B6 and B12).89 There was a 27% reduction in plasma homocysteine levels among individuals who received folic acid and vitamin B12. However, with a median follow-up of 40 months, there was no benefit of folic acid plus vitamin B12 therapy, with or without vitamin B6, on the composite cardiovascular endpoint. Indeed, there was a trend toward increased risk of a fatal or nonfatal cardiovascular event among individuals randomized to triple-vitamin therapy compared to placebo. The Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) trial assessed the effect of folic acid and vitamin B12 on vascular outcomes in 12,064 survivors of MI.90 Treatment with these vitamins reduced homocysteine by 3.8 μmol/L (28%) but did not affect the primary outcome of first major vascular event, defined as a major coronary event, fatal or nonfatal stroke, or noncoronary revascularization.
Recommendations
To date, there have been no large clinical outcome randomized controlled trials that have specifically investigated treatment of hyperhomocysteinemia in a PAD population. In the absence of such studies, and given the findings of the HOPE-2, NORVIT, and SEARCH trials in patients with coronary and other atherosclerotic vascular disease, use of folic acid and vitamin B6 and B12 supplementation to prevent cardiovascular events among PAD patients is not recommended.
Antiplatelet Therapy
The pivotal role of antiplatelet agents, particularly aspirin, in the secondary prevention of death and MI in patients with CAD has been established by large randomized clinical trials.91,92 The role of antiplatelet therapy in the management of patients with PAD continues to evolve, with important new data gleaned from recent large clinical trials directed primarily at the prevention of cardiovascular events in patients with and without CAD.
A recent meta-analysis of six primary prevention trials of 95,000 individuals at low to average cardiovascular risk (representing 660,000 person-years) found that aspirin reduced the risk of any vascular event by 12%.93 This was due primarily to a decrease in the risk of nonfatal MI, but not stroke or vascular mortality. This meta-analysis also included 16 secondary prevention trials of 17,000 individuals at high-average risk, representing 43,000 person-years. In the secondary prevention trials, aspirin reduced serious vascular events by 29%, including total stroke and coronary events, and was associated with a borderline nonsignificant 9% reduction in vascular mortality.
In 2002, the Antithrombotic Trialists’ Collaboration updated the 1994 meta-analysis of the evidence supporting the use of antiplatelet agents in the management of high-risk patients with atherosclerotic vascular disease.91 Studies with different antiplatelet agents (e.g., aspirin, dipyridamole, picotamide, ticlopidine) were included. The initial meta-analysis determined that antiplatelet therapy significantly reduced the odds of a major vascular event among high-risk patients with atherosclerosis by 27%.92 Also, antiplatelet therapy was effective for preventing both coronary and peripheral artery bypass graft occlusion, with a reduction in relative risk of graft occlusion of approximately one third.94 In the updated analysis, a total of 287 randomized trials with more than 200,000 patients were included.91 Confirming the original findings, there was a 22% reduction in the odds of a serious vascular event (vascular death, nonfatal MI, or nonfatal stroke) among high-risk patients treated with antiplatelet agents. The benefit appeared to be greatest among studies that enrolled patients on the basis of high-risk criteria such as prior or acute MI, PAD, or atrial fibrillation. Among the 42 studies that enrolled 9214 high-risk patients on the basis of PAD, there was a 23% reduction in the odds of a major vascular event among patients randomized to antiplatelet therapy. The benefit of antiplatelet therapy was consistent across all PAD enrollment criteria, including intermittent claudication and surgical lower-extremity revascularization. Of note, 2304 patients in this category were enrolled in a study that randomized PAD patients to treatment with a nonaspirin antiplatelet agent, picotamide, or placebo.95 Picotamide is an antiplatelet agent that both inhibits thromboxane A2 (TxA2) synthase and antagonizes the TxA2 receptor of platelets. It is also noteworthy that none of the trials in this meta-analysis investigated the benefit of aspirin alone (i.e., not in combination with dipyridamole) in standard clinical dosage (81-325 mg daily ) versus placebo, a fact that was highlighted by an updated meta-analysis of aspirin therapy for PAD by Berger et al. in 2009 (discussed later).96
The Critical Limb Ischaemia Prevention Study (CLIPS) randomized patients with ABI less than 0.85 or TBI less than 0.6 who were asymptomatic or had stable leg claudication (Fontaine stage I/II) to aspirin 100 mg daily or placebo.97 Although an initial sample size of 2000 patients was planned, the trial was terminated early because of poor enrollment, and only 366 patients were randomized, with a minority followed for more than 2 years. Aspirin was associated with a 64% reduction in the relative risk of fatal and nonfatal vascular events and a 58% reduction in fatal and nonfatal vascular events or critical limb ischemia.
In contrast to the CLIPS trial, which included patients with clinically significant PAD (claudication or asymptomatic PAD with low ABI), there have been two large randomized clinical trials that investigated the efficacy of aspirin in low-risk asymptomatic PAD patients. The Prevention of Progression of Arterial Disease and Diabetes (POPADAD) trial randomized 1276 patients older than 40 years of age who had type 1 or 2 diabetes mellitus and an “abnormal” ABI of less than 1.0, but who had no known cardiovascular disease and no symptoms of PAD.98 Subjects were randomized to aspirin 100 mg daily or placebo, as well as antioxidant vitamins, in a 2 × 2 factorial design. It is important to note that this was a population that was not only asymptomatic in terms of PAD but also had relatively mild disease, with a median ABI in the study population of 0.9, barely at the lower limit of normal. After a median follow-up of 6.7 years, there was no difference between the aspirin and placebo groups in the rate of fatal or nonfatal vascular events or amputation. In subset analysis, there was a nonsignificant trend toward benefit of aspirin among patients with ABI less than 0.9.
The largest trial of aspirin therapy for prevention of cardiovascular events among patients with PAD to date was the Aspirin for Asymptomatic Atherosclerosis (AAA) Trial.99 In this trial, nearly 29,000 Scottish patients between the ages of 50 and 75 years who had no cardiovascular disease were offered a screening ABI examination. A total of 3350 individuals with ABI below 0.95 were randomized to receive 100 mg aspirin daily or placebo and followed for an average of 8.2 years. In this study, the lower of two ankle pressures was used for calculating the ABI. There was no statistically significant difference in occurrence of the primary composite endpoint of fatal and nonfatal coronary events, stroke, or revascularization between aspirin- and placebo-treated patients (Fig. 19-4).
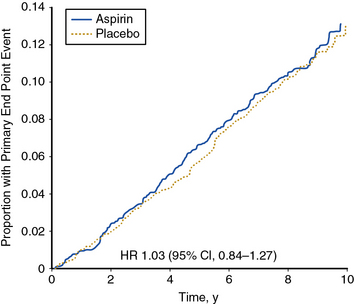
Figure 19-4 Aspirin therapy does not reduce the incidence of major vascular events among asymptomatic individuals with ankle-brachial index (ABI) <0.95.
Results from the Aspirin for Asymptomatic Atherosclerosis (AAA) trial. There was no statistically significant difference in the primary composite outcome of fatal and nonfatal myocardial infarction (MI) or stroke or revascularization between the two groups. CI, confidence interval; y, year.
(Reproduced with permission from Fowkes, FG, Price JF, Stewart, MC, et al. Aspirin for prevention of cardiovascular events in a general population screened for a low ankle-brachial index: a randomized controlled trial. JAMA 303:841–848, 2010.)99
Berger et al. performed a meta-analysis of 18 trials comprising 5269 patients with PAD that evaluated the efficacy of aspirin (± dipyridamole) for prevention of cardiovascular events including nonfatal MI, nonfatal stroke, and cardiovascular death.96 This meta-analysis included the trials incorporated into the Antithrombotic Trialists’ Collaboration 2002 meta-analysis, as well as new data from the POPADAD trial and CLIPS; the AAA study was not included. Aspirin therapy, with or without dipyridamole, did not significantly reduce the rate of cardiovascular events. In subset analyses, aspirin was associated with a significant reduction in the incidence of nonfatal stroke, but not cardiovascular mortality, MI, or major bleeding.
Large multicenter randomized clinical trials have investigated the use of the antiplatelet agent clopidogrel for secondary prevention of cardiovascular events. Clopidogrel is a thienopyridine derivative that inhibits platelet aggregation by antagonism of the adenosine diphosphate receptor.100 Clopidogrel is less likely to cause serious adverse hematological side effects, particularly neutropenia and thrombotic thrombocytopenic purpura, than its analog ticlopidine. Pooled analysis of early clinical trials suggested a trend toward a marginal benefit of ticlopidine over aspirin in the prevention of MI, stroke, or vascular death.92
The Clopidogrel versus Aspirin in Patients at Risk of Ischaemic Events (CAPRIE) study built on this finding and investigated the benefit of clopidogrel versus aspirin in the secondary prevention of cardiovascular events.101 Patients with recent MI (within 35 days), ischemic stroke (within 6 months), or symptomatic PAD were randomized to clopidogrel (75 mg daily) or aspirin (325 mg daily). Patients enrolled on the basis of PAD had intermittent claudication and an abnormal ABI (ABI ≤0.85) or had undergone leg amputation or revascularization. A total of 19,185 randomized patients were followed for development of the primary composite endpoint of first occurrence of ischemic stroke, MI, or vascular death. After a mean 1.9 years of follow-up, there was an 8.7% relative risk reduction in the annual event rate of the primary endpoint among patients randomized to clopidogrel. The benefit of clopidogrel over aspirin was greatest among the subgroup of patients enrolled on the basis of symptomatic PAD, with a relative risk reduction of the composite endpoint of 23.8% (Fig. 19-5). There was no increase in minor or major bleeding episodes associated with clopidogrel, although there was an increased incidence of gastrointestinal hemorrhage among patients randomized to aspirin.
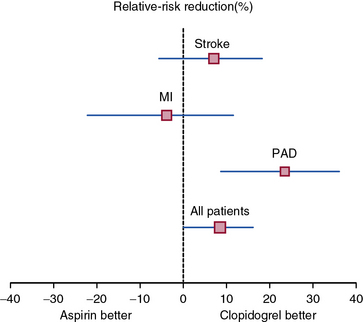
Figure 19-5 Clopidogrel decreases the risk of the composite endpoint of stroke, myocardial infarction (MI), or vascular death relative to aspirin among high-risk patients with atherosclerotic vascular disease.
Relative risk reduction for each subgroup of patients is displayed with 95% confidence intervals. The benefit of clopidogrel is particularly pronounced among the subset of patients with peripheral artery disease (PAD).
(Adapted and reproduced with permission from the CAPRIE Steering Committee: A randomised, blinded trial of clopidogrel versus aspirin in patients at risk of ischaemic events [CAPRIE]. Lancet 348:1329–1339, 1996.)101
Dual antiplatelet therapy, namely the combination of aspirin and clopidogrel, reduced the rate of cardiovascular events among patients with ACS in the Clopidogrel in Unstable Angina to Prevent Recurrent Events (CURE) trial.102 The role of dual antiplatelet therapy for secondary prevention of cardiovascular events among patients with stable atherosclerotic vascular disease was studied in the Clopidogrel and Aspirin versus Aspirin Alone for the Prevention of Atherothrombotic Events (CHARISMA) trial.103 In this trial, 15,603 patients with symptomatic atherosclerotic vascular disease or multiple high risk features (e.g., diabetes mellitus, asymptomatic abnormal ABI) were randomized to receive either aspirin (75 to 162 mg) plus clopidogrel or aspirin plus placebo and followed for incident cardiovascular events. After a median 28 months of follow-up, there was no statistically significant difference in the primary endpoint, which was the rate of first fatal or nonfatal MI, stroke, or cardiovascular death between the two groups. Dual antiplatelet therapy increased the rate of moderate bleeding (i.e., requiring blood transfusion) but did not increase the rate of fatal bleeding or intracranial hemorrhage. A number of subset analyses were performed, including a post hoc subset analysis of patients with either symptomatic or asymptomatic PAD (N = 3096).104 Among PAD patients in CHARISMA, there was no significant difference in the primary composite cardiovascular endpoint, although there was a significant 37% reduction in the rate of MI among patients randomized to dual antiplatelet therapy.
In the Clopidogrel and Acetylsalicylic Acid in Bypass Surgery for Peripheral Arterial Disease (CASPAR) trial, 551 patients undergoing lower-extremity bypass surgery for claudication or CLI were randomized to dual antiplatelet therapy with aspirin (75 to 100 mg daily) plus clopidogrel (75 mg daily) or to aspirin plus placebo and followed for limb-related events (graft occlusion, repeat revascularization, major amputation) and cardiovascular events, including death, over a period of up to 2 years.105 Compared to aspirin alone, dual antiplatelet therapy did not reduce the risk of clinical cardiovascular or limb-related events. In a post hoc analysis, dual antiplatelet therapy was associated with lower rates of graft occlusion and lower-extremity amputation among patients who had prosthetic (vs. venous) bypass grafting.
Recommendations
In light of the 20% to 25% reduction in serious vascular events attributable to antiplatelet therapy among patients with atherosclerotic vascular disease, all patients with PAD, regardless of concomitant CAD or CVD, should receive antiplatelet therapy. Antiplatelet therapy for patients with lower-extremity PAD is given a class I recommendation in the 2005 ACC/AHA PAD guidelines.1,2 The evidence base in support of antiplatelet therapy is strongest for patients with symptomatic PAD. Given the constraints of the available evidence, aspirin remains a reasonable first-line agent for PAD and should be prescribed at a dose of 81 to 325 mg daily. Aspirin therapy is particularly important among patients undergoing surgical or percutaneous revascularization procedures, and should be continued perioperatively—or initiated as soon as possible postoperatively—if it had not been prescribed previously.
Clopidogrel is a therapeutic alternative among patients intolerant of aspirin (e.g., gastrointestinal distress, allergy, bronchospasm), and can also be considered as the initial choice for patients with PAD. With the exception of patients with recent ACS or those undergoing percutaneous coronary or peripheral interventions, there is currently insufficient evidence to support dual antiplatelet therapy with aspirin and clopidogrel for the secondary prevention of cardiovascular events.
Anticoagulant Therapy
Several clinical trials have addressed the role of anticoagulant therapy, typically with the vitamin K antagonist warfarin, in the management of patients with PAD. These include several small studies that explored the potential effect of oral anticoagulation on limb-related outcomes. A review of three small trials found that oral anticoagulation therapy had no benefit on walking capacity or limb-related outcomes, nor on cardiovascular outcomes among patients with intermittent claudication.106 Another trial, the Dutch Bypass Oral Anticoagulants or Aspirin investigators compared the efficacy of high-intensity warfarin (target international normalized ratio [INR] 3.0-4.5) with aspirin (80 mg daily) therapy on graft occlusion in patients following infrainguinal bypass grafting.107 After a mean 21 months of follow-up, there was no significant difference in the rate of graft occlusion between the warfarin and aspirin groups. Subset analyses, however, demonstrated a significant reduction (31%) in the rate of graft occlusion of venous conduit bypass grafts, but not prosthetic grafts, among patients randomized to warfarin.
The Warfarin Antiplatelet Vascular Evaluation (WAVE) trial studied the potential benefit of oral anticoagulation therapy in addition to aspirin for patients with noncoronary atherosclerotic vascular disease.108 The study included 2161 patients with atherosclerotic vascular disease (symptomatic lower-extremity PAD, symptomatic or asymptomatic carotid artery disease (CAD), or subclavian artery stenosis) who were randomized to receive aspirin (dose 81-325 mg) or aspirin plus warfarin (INR goal of 2-3). More than 80% of patients enrolled had lower-extremity PAD. The co-primary endpoints of the trial were: (1) cardiovascular death, nonfatal MI, and nonfatal stroke, and (2) the above plus need for urgent coronary or peripheral revascularization. Over a follow-up period of 35 months, there was no significant benefit of oral anticoagulation therapy in either of the two co-primary endpoints. There was 3.4-fold relative risk of life-threatening bleeding, and a 15.2-fold relative risk for hemorrhagic stroke among patients randomized to oral anticoagulation therapy.
Recommendations
Based on the lack of compelling efficacy data for warfarin and the associated risk of bleeding, both the American College of Chest Physicians Conference on Antithrombotic and Thrombolytic Therapy and the ACC/AHA PAD guidelines have issued recommendations opposing routine use of oral anticoagulant therapy in patients with PAD.1,2,109 Anticoagulation therapy may be considered following an episode of acute limb ischemia that had been treated with thrombolytic therapy, or may be warranted when there is a comorbid condition associated with increased risk for thromboembolism (e.g., atrial fibrillation, mechanical heart valve, prior venous thromboembolism [VTE]). In such cases involving patients with PAD, oral anticoagulation should be combined with low-dose aspirin.
Care and Protection of the Feet
Careful attention to foot care is indicated to reduce the likelihood of skin breakdown and infection, and is particularly important in diabetic persons with vascular disease and in patients with critical limb ischemia. Treatment of lower-extremity ulcers is discussed in Chapter 60. The feet of patients with PAD should be kept clean, and moisturizing lotion applied to prevent drying and fissuring. Stockings should be made of absorbent natural fibers. Well-fitted shoes are recommended to reduce the risk of pressure-induced necrosis. In some patients, customized footwear or orthotic devices (e.g., for the patient with excessive callous formation or bony deformities) are indicated. The patient is advised to inspect the skin of the feet frequently so minor abrasions can be addressed promptly. High-grade graduated elastic stockings should generally be avoided because they can restrict cutaneous blood flow.
In patients with ischemia at rest, conservative measures include placing the affected limb in a dependent position (i.e., below heart level) to increase perfusion pressure and oxygen tension in ischemic tissues. If there is edema, which may impair healing, the limb is kept horizontal instead. Sheepskin should be placed beneath the heels of the feet to prevent skin breakdown at these sites. A footboard should be used to cradle the blankets over the feet in a fashion that minimizes frictional trauma. Alternatively, protective boots may be used. Wisps of cotton or lambswool inserted between the toes help protect the digits from intertriginous friction and moisture. Gentle warmth is recommended to minimize vasoconstriction, but excessive heat should be avoided. Tinea pedis should be treated with appropriate antimicrobial preparations to reduce the risk of cutaneous breakdown leading to bacterial superinfection. Caution is advised in the use of topical medications because of the possibility of local inflammatory reactions. Open sores should be kept clean, and deep cultures should be obtained (also see Chapter 60). Antibiotic medications are not always effective, in part because of impaired delivery to ischemic tissue. Plain roentgenographic examinations or magnetic resonance imaging (MRI) of underlying bone may be helpful in assessing the possibility of osteomyelitis, for which antibiotic therapy generally is given.
Improvement of Function and Quality of Life
Treatment of Intermittent Claudication and Critical Limb Ischemia
Physical and pharmacological therapies should be considered in the treatment plan of patients with symptomatic PAD. These include supervised exercise rehabilitation and pharmacotherapy. Drugs available for the treatment of intermittent claudication include cilostazol and pentoxifylline. Investigative pharmacotherapies include prostaglandins (PGs), metabolic agents, angiogenic growth factors, stem cell therapy, and statins. The evidence supporting and refuting the efficacy of drug therapy for intermittent claudication and CLI is reviewed subsequently. Endovascular and surgical reconstructions for the treatment of disabling claudication are reviewed in Chapters 20 and 21.
Exercise Training
Supervised exercise training programs improve walking duration, speed, and walking distance in patients with intermittent claudication. Although supervised exercise therapy has been shown to be highly cost-effective compared to catheter-based revascularization for treatment of lower-extremity claudication, it is not widely available owing to a lack of reimbursement by most third-party payers in the United States.110,111 Two separate meta-analyses have supported the efficacy of supervised exercise training. One meta-analysis of 21 randomized and nonrandomized trials found that pain-free walking distance and maximal walking distance increased by 180% and 120%, respectively.112 Another meta-analysis that included 10 randomized trials found that supervised exercise training improved maximal walking distance by 150%.113 Strength or resistance training is not as effective as treadmill training in improving walking distances in patients with claudication.114, In the recently published Claudication: Exercise Versus Endoluminal Revascularization (CLEVER) study, randomization to supervised exercise training was associated with improved maximal walking time at 6 months follow-up compared to endovascular therapy or optimal medical therapy among patients with aortoiliac occlusive disease115. Supervised exercise training has also been shown to improve quality of life among patients with PAD and either claudication or atypical leg symptoms.114 Unsupervised exercise training programs are not as effective as supervised programs.116,117 Most comparative studies have found that home-based unsupervised exercise is not as effective as supervised exercise training. A recent study, however, did observe comparable improvement in walking time between home-based exercise coupled with activity monitoring and supervised exercise training.116
Several mechanisms have been proposed to explain the improvement in walking distance that results from exercise training. These include collateral blood vessel development, enhancement in endothelium-dependent nitric oxide (NO)-mediated vasodilation of the microcirculation, improved hemorrheology, increased oxidative capacity of calf skeletal muscle, and better walking biomechanics.119 Exercise training has been found to improve collateral blood flow in animal models of hindlimb ischemia, as well as capillary density in humans.120–122 Exercise-induced angiogenesis has been attributed to up-regulation of angiogenic growth factors such as vascular endothelial growth factor (VEGF).123,124 Increased expression and activity of endothelial nitric oxide synthase (eNOS) and production of NO may contribute to angiogenesis.121,123,125,126 In humans, several studies have found that exercise training improves maximal calf blood flow following exercise.127,128 Similarly, exercise training improves calf blood flow during reactive hyperemia following an ischemic stimulus. There was no effect of exercise training on resting calf blood flow in these studies. Exercise training enhances endothelium-dependent vasodilation in peripheral conduit arteries, and thereby may contribute to improved blood flow and walking time in claudicants.114,129,130 Improvement in skeletal muscle metabolic function, and specifically oxidative capacity, occurs with exercise training and may be relevant to the improvement in walking capacity experienced by patients with intermittent claudication.131,132 Also, exercise training may improve walking distance by altering biomechanics and adapting patterns of walking that are more efficient, engaging skeletal muscle less affected by ischemia, and improving gait stability.133
A number of novel approaches to exercise training for improvement of functional capacity for patients with PAD are currently under investigation, including supervised interval training, home-based monitored exercise programs, and arm ergometry.118,134–137
Recommendations
In the ACC/AHA PAD guidelines, a program of supervised exercise training is recommended as an initial treatment modality for patients with intermittent claudication (class I).1 Exercise programs should use a treadmill or a track and take place in sessions of 45 to 60 minutes at least 3 times a week for a minimum of 12 weeks. Prior to beginning exercise rehabilitation, patients should undergo a comprehensive cardiovascular risk assessment that includes a history, physical examination, and ascertainment of all relevant atherosclerotic risk factors. An electrocardiogram (ECG)-monitored exercise tolerance test tailored to the patient’s symptoms and ability should be performed to assess development of any exercise-induced cardiac symptoms, heart rate and blood pressure responses, ST-segment depression, and arrhythmias. It also may serve as a baseline evaluation of the time to onset of claudication, and maximal walking time tolerated on the treadmill. During the training session, patients should be encouraged to walk until symptoms of moderate severity develop. Following a rest period and resolution of symptoms, walking should resume until symptoms recur. This cycle should be repeated as many times as needed during the 45- to 60-minute period. For patients unable to participate in a supervised exercise training program, a home-based walking program is recommended.
Vasodilator Drugs
Vasodilator drugs have undergone extensive investigation for treatment of patients with claudication and critical limb ischemia. It is tempting to assume that treatment with a medication that reduces arteriolar resistance would be as effective for patients with PAD as nitrates and calcium channel blockers are for patients with angina. In general, however, vasodilator drug therapy has been disappointing for relief of intermittent claudication. Differences in the pathophysiology of limb and myocardial ischemia may promote insight into the disparate efficiency of vasodilator drugs. Vasodilator therapy may decrease myocardial oxygen demand, but is not likely to affect skeletal muscle oxygen demand. Therefore, to be effective, vasodilators would have to improve the blood supply to exercising muscle.
When an obstructive arterial lesion produces critical stenosis, distal perfusion pressure is reduced (see Chapter 17). Intramuscular (IM) arterioles normally dilate in response to the metabolic demands of limb exercise, but flow augmentation is blunted in patients with proximal stenotic disease. Also, distal pressure falls during exercise, leading to accumulation of the ischemic metabolites believed to mediate the symptom of claudication. These substances potentiate local vasodilation and perfusion pressure falls, no longer balancing the extravascular compressive force exerted by exercising muscle within the tissue compartment. The distal vasculature virtually collapses under these circumstances, and this mechanism may not be mitigated by vasodilator therapy. Indeed, vasodilator drugs, including β-adrenergic agonists (isoproterenol, nylidrin), α-adrenergic antagonists (reserpine, guanethidine, tolazoline), calcium channel blockers, nitrates, and others (isoxsuprine, cyclandelate) have been evaluated in clinical trials. None increases blood flow in exercising skeletal muscle subtended by significant arterial obstructive lesions, nor do any of these improve symptoms of intermittent claudication or objective measures of exercise capacity. Nonetheless, one drug with vasodilator properties, cilostazol, has been reported to improve walking distance in patients with claudication.
Cilostazol
Cilostazol was approved by the U.S. Food and Drug Administration (FDA) in 1999 for use in patients with intermittent claudication. As a phosphodiesterase (PDE) III inhibitor, cilostazol increases cyclic adenosine monophosphate (cAMP), causes vasodilation, and inhibits platelet aggregation.138–141 The precise mechanism of action whereby cilostazol may improve symptoms of claudication, however, is not known. In a pooled analysis of 2491 patients in nine trials, cilostazol (100 mg twice daily) increased maximal walking distance by approximately 50% compared to 24% for placebo, corresponding to an absolute improvement of 42 meters more than the improvement with placebo142 (Fig. 19-6). The efficacy of cilostazol compared to placebo among patients with CLI has not been evaluated, although observational studies have reported improvements in lower-extremity microcirculation and amputation-free survival among cilostazol-treated patients.143,144
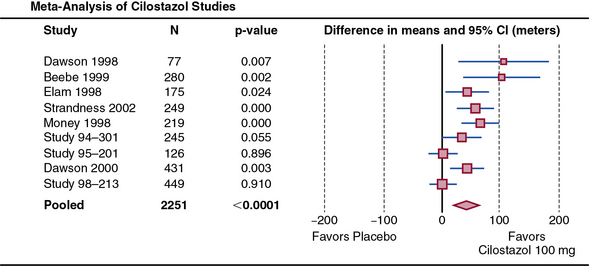
Figure 19-6 Cilostazol improves maximal walking distance in patients with claudication.
Meta-analysis of nine randomized controlled trials including 1258 subjects. Shown are random effects–weighted differences in maximal walking distance, with 95% confidence intervals (CI) for the nine trials and the pooled treatment effect. There was a pooled improvement of 42.1 meters in maximal walking distance compared to placebo over a mean follow-up of 20.4 weeks.
(Reproduced with permission from Pande RL, Hiatt WR, Zhang P, et al: A pooled analysis of the durability and predictors of treatment response of cilostazol in patients with intermittent claudication. Vasc Med 15:181–188, 2010.)142
Cilostazol is primarily metabolized in the liver by the CYP3A4 and CYP2C19 isoenzymes. Prescription of low-dose (50 mg twice daily) cilostazol is recommended for patients who concurrently take medications that are known to inhibit CYP3A4 or CYP2C19, including diltiazem, fluoxetine, fluconazole, erythromycin, and other macrolide antibiotics (CYP3A4), as well as omeprazole (CYP2C19).145 Side effects of cilostazol are relatively common and include headache in approximately 25%, palpitations in approximately 15%, and diarrhea or abnormal stool in 15% to 20%.146 The FDA has advised that cilostazol not be administered to patients with CHF of any severity.145 The reason for this advisory is that when studied in patients with congestive heart failure, other PDE III inhibitors, such as milrinone or vesnarinone, were associated with increased mortality.147,148 Cilostazol has not been associated with increased mortality rates, but it has not been studied in a heart failure population.
In the Cilostazol: A Study in Long-Term Effects (CASTLE) trial, 1435 patients with claudication were randomized to receive cilostazol (100 mg twice daily) or placebo and followed for adverse events.149 At approximately 3 years of follow-up, there was no excess of death or serious bleeding events among those randomized to cilostazol compared to placebo. Importantly, the discontinuation rate of study medication was very high in this trial (>60%), perhaps reflective of the side effect profile of cilostazol.
Recommendations
Cilostazol is an effective therapy to improve walking distance in patients with intermittent claudication, and a therapeutic trial of cilostazol is recommended in the ACC/AHA PAD guidelines (class I).1 Physicians should be aware of the side effect profile of this drug and are advised not to administer this medication to patients with congestive heart failure.
Prostaglandins
Vasodilator PGs have undergone somewhat extensive investigation for the treatment of patients with intermittent claudication or critical limb ischemia. This class of drugs includes prostaglandin E1 (PGE1), prostacyclin (PGI2), and its analogs beraprost and iloprost. The efficacy of vasodilator PGs administered intraarterially (IA) or intravenously (IV) has been assessed in patients with intermittent claudication and in patients with critical limb ischemia. A systematic review of clinical trials of prostanoids for treatment of claudication found that short-term IA or IV administration of PGE1 to patients with intermittent claudication appeared to increase walking distance, whereas IV PGI2 or its analog taprostene was found not to improve walking distance.150 Two placebo-controlled trials of oral beraprost administered for 6 months had conflicting results, one showing improvement and one showing no change in pain-free or maximal walking distance.151,152 In one multicenter placebo-controlled trial, iloprost administered for 6 months was no more effective than placebo in improving pain-free or maximal walking distance among patients with intermittent claudication.153
Short-term (i.e., 3-4 days) IA or IV administration of PGE1, iloprost, or ciprostene is not effective in ameliorating critical limb ischemia,154 but when administered parenterally for longer periods of time (7-28 days) may reduce pain, ulcer size, or risk of amputation.154 The Ischemia Cronica degli Arti Inferiori study assessed the efficacy of IV PGE1 or placebo in 1560 patients with critical limb ischemia. The relative risk of death, major amputation or persistence of critical limb ischemia, acute MI, or stroke was significantly reduced by 13% at the time of hospital discharge but not at 6 months. After 6 months of treatment, there was no difference between groups in death or amputation, but there was a greater chance of resolution of CLI in those survivors who did not have amputation.155 Two randomized placebo-controlled studies assessed the effect of oral iloprost on critical limb ischemia.156 There was no apparent benefit of iloprost in terms of reducing the risk of the primary endpoint of amputation or death, but there was a modest benefit in terms of resolution of ulcers and rest pain in those who survived without amputation. In a recent trial of patients with critical limb ischemia, twice-daily infusion of taprostene compared with placebo did not improve pain control, wound healing, or amputation rates.157 Side effects of PGs include flushing, headache, and gastrointestinal distress.
Recommendations
The use of oral or IV vasodilator PGs, such as beraprost and iloprost, is not recommended to improve walking distance in patients with intermittent claudication.1 In addition, these drugs are not effective therapy to reduce the risk of amputation or death in patients with critical limb ischemia.
Calcium channel blockers
The efficacy of calcium channel blockers in patients with intermittent claudication has been evaluated in several small clinical trials. Nifedipine failed to improve exercise tolerance as assessed by pedal ergometry in a randomized placebo-controlled trial.158 In another placebo-controlled trial comparing nifedipine with atenolol, alone or in combination, nifedipine did not improve pain-free or absolute walking distance.41 In one placebo-controlled dose-ranging crossover trial, verapamil improved pain-free walking distance by 29% and maximal walking distance by 49%.159 There have been no subsequent trials with verapamil reported to confirm these findings.
Hemorrheological Agents
Pentoxifylline
Pentoxifylline is a methylxanthine derivative that was approved by the FDA in 1984 for treatment of patients with intermittent claudication. It is purported to decrease blood and plasma viscosity, improve red and white blood cell deformability, inhibit neutrophil adhesion and activation, and decrease plasma fibrinogen, although not all studies have confirmed these findings.160–162 One meta-analysis of 11 trials comprising 612 patients found that pentoxifylline (600-1200 mg daily) improved pain-free walking distance by 29 meters and maximal claudication distance by 48 meters.163 Another meta-analysis of six randomized double-blinded placebo-controlled trials found that pentoxifylline improved pain-free walking distance by 21 meters and maximal claudication distance by 44 meters.164 The meta-analyses did not include a large randomized placebo-controlled trial of 698 patients with intermittent claudication, of whom 232 received pentoxifylline, 227 received cilostazol, and 239 received placebo.146 In this trial, there was no difference in pain-free walking distance or maximal walking distance between the pentoxifylline- and placebo-treated groups after 24 weeks of treatment. Pain-free and maximal walking distance increased in the cilostazol group compared with both placebo and pentoxifylline. In a trial of iloprost versus pentoxifylline versus placebo among patients with intermittent claudication, there was a 14% improvement in maximal walking distance among patients randomized to pentoxifylline compared to placebo.153
The efficacy of pentoxifylline to improve CLI has been studied in two placebo-controlled trials. 165,166 In one study, patients with CLI were randomized to treatment with IV pentoxifylline (600 mg twice daily) or placebo for up to 21 days. Severity of rest pain was significantly reduced in patients treated with pentoxifylline compared with those who received a placebo.165 Yet, in another placebo-controlled trial in which pentoxifylline (600 mg intravenously) was administered to patients with critical limb ischemia, there was no significant difference in pain relief between the placebo and pentoxifylline treatment groups.166 It is not known whether pentoxifylline facilitates ulcer healing or reduces risk of amputation in patients with critical limb ischemia. Side effects of pentoxifylline include dyspepsia, nausea, vomiting, eructation, flatus, bloating, and dizziness. Side effects requiring discontinuation of the drug occur in up to 9.6% of patients.
Recommendations
The clinical efficacy of pentoxifylline for intermittent claudication is not well established. Improvement in claudication distance is very modest at best, and falls within a range that may not be clinically recognized by the patient. Nonetheless, pentoxifylline may be considered as a second-line alternative to cilostazol in the ACC/AHA PAD guidelines for treating intermittent claudication (class IIb) and is the only other FDA-approved agent for this indication.1 Pentoxifylline is not useful in the treatment of critical limb ischemia.
Metabolic Agents
Symptoms of intermittent claudication may be related in part to abnormalities in skeletal muscle metabolism (see Chapter 17). Therefore, drugs that favorably affect oxidative metabolism may confer benefit by enhancing skeletal muscle function even without improving blood supply. For example, L-carnitine and its derivative propionyl-L-carnitine enhance glucose oxidation and oxidative metabolism via the Krebs cycle by providing a source of carnitine.167 Three placebo-controlled trials have assessed the efficacy of propionyl-L-carnitine in patients with intermittent claudication.168–170 In these studies, propionyl-L-carnitine administered as a 1-g oral dose twice daily improved maximal walking distance by 54% to 73%, whereas those randomized to placebo increased maximal walking distance 25% to 46%. Propionyl-L-carnitine improved physical function, walking speed, and distance as assessed by quality-of-life questionnaires.168,171 Propionyl-L-carnitine was not associated with any significant adverse side effects.
A recent trial assessed the potential benefit of propionyl-L-carnitine therapy (vs. placebo) as adjunctive treatment to home-based monitored exercise training for patients with intermittent claudication.172 In this trial, maximal walking time increased in both the propionyl-L-carnitine and placebo groups after 6 months of exercise training, with a nonsignificant trend toward improved walking in the propionyl-L-carnitine group. Propionyl-L-carnitine has potential merit for treating intermittent claudication, but should be considered investigational at this time.
Ranolazine is a piperazine derivative that inhibits fatty acid oxidation, activates pyruvate dehydrogenase, and shifts metabolism toward carbohydrate oxidation, thereby increasing efficiency of oxygen utilization.173 Ranolazine improves exercise capacity and decreases angina frequency in patients with CAD and was associated with improvement in pain-free walking time (vs. placebo) among patients with intermittent claudication in a single-center pilot study.174–176
Angiogenic Growth Factors
Angiogenic growth factors have undergone investigation for treatment of PAD. This class of drugs includes VEGF, fibroblast growth factor (FGF), hepatocyte growth factor (HGF), and hypoxia-inducible factor-1α (HIF-1α). They may be administered as recombinant proteins or by gene transfer using plasmid deoxyribonucleic acid (DNA) or an adenoviral vector that encodes the angiogenic growth factor.177 Vascular endothelial growth factor, FGF, and HIF-1α increase collateral blood vessels and improve blood flow in animal models of hindlimb ischemia.178–181 Therefore, angiogenic growth factors have the potential to promote collateral blood vessel formation and thereby increase blood flow to the ischemic limbs of patients with PAD.
Vascular endothelial growth factor
Several nonrandomized open-label studies found that IA gene transfer therapy of pHVEGF165 increased collateral blood vessels, as assessed by MRI or digital subtraction angiography (DSA) in patients with PAD.182,183 Also, pHVEGF165 administration improved blood flow and increased ABI in some patients who participated in these trials.182 A randomized placebo-controlled trial of IM VEGF121 was conducted (DSA) in patients with unilateral intermittent claudication. There was no significant improvement in pain-free or maximal walking distance after 12 or 26 weeks.184
Fibroblast growth factor
Recombinant FGF2 administered directly into the femoral artery was evaluated in a placebo-controlled study.185 Patients were randomized to receive FGF2 on one occasion only, on two occasions 30 days apart, or placebo. One-time administration of FGF2 increased peak walking time at 90 days by 34% compared with 14% for placebo. Yet, there was no significant improvement in peak walking time compared with placebo when FGF2 was administered on two occasions. A nonrandomized study of patients with CLI observed that IM injection of plasmid DNA encoding FGF1 reduced pain and ulcer size and increased the ABI.186 In a phase 2 placebo-controlled study of patients with critical limb ischemia, IM administration of FGF1 using a plasmid vector did not improve ulcer healing, the primary endpoint, but did decrease secondary endpoints including all amputations and the composite of major amputation and death.187 However, in a follow-up phase 3 study of patients with critical limb IM, FGF1 did not decrease amputation-free survival.188
Other angiogenic growth factors
Hepatocyte growth factor using a plasmid vector and given as an IM injection increased TcPO2 but did not improve pain, heal ulcers, or decrease amputations in a placebo-controlled trial of patients with critical limb ischemia.189
Hypoxia-inducible factor-1α is an inducible transcriptional regulatory factor. In conditions of low oxygen tension, HIF-1α binds to hypoxia-responsive elements in the promoter/enhancer region of target genes, inducing those encoding VEGF-A, platelet-derived growth factor (PDGF), angiotensin-1, and inducible nitric oxide synthase (iNOS).190–192 In a phase 1 study, 34 patients with CLI with no revascularization options received an IM injection of an adenovirus encoding HIF-1α. The injections were well tolerated and associated with complete wound healing in 5 of 18 patients in 1 year.193 A subsequent trial randomized patients with intermittent claudication to placebo or one of three doses of the adenovirus encoding HIF-1α and found no benefit of HIF-1α on peak walking time, claudication onset time, or ABI up to 1 year after randomization.194
Stem Cell Therapy
Infusion of endothelial progenitor cells into mice in an experiment model of hindlimb ischemia has been shown to improve blood flow and capillary density in the ischemic hindlimb and reduce the rate of limb loss.195 Bone marrow mononuclear cells include endothelial progenitor cells. Intramuscular injection of autologous bone marrow–derived mononuclear cells improved collateral blood vessel formation in animal models of myocardial and hindlimb ischemia.196,197 Consequently, the effect of autologous implantation of bone marrow–derived mononuclear cells was studied in patients with PAD manifested as limb ischemia.198 Injection of bone marrow mononuclear cells, compared with peripheral blood mononuclear cells, reduced rest pain and improved pain-free walking time. The improvement was sustained for 24 weeks. Angiographic evidence of collateral blood vessel formation was present in many of the patients who received bone marrow–derived mononuclear cells. Additional data supporting potential angiogenic benefit of IM injection or IA infusion of bone marrow–derived mononuclear cells or bone marrow–derived mesenchymal cells for patients with CLI and limited revascularization options have been reported in multiple small single-center early phase trials.199–204 Many of these studies reported noninvasive testing (e.g., ABI, TBI, plethysmography) rather than angiography to document evidence of angiogenesis.
Several trials are planned or are in progress. The Use of Vascular Repair Cells in Patients with Peripheral Arterial Disease to Treat Critical Limb Ischemia (RESTORE-CLI) trial has randomized 86 patients to stem and progenitor cell therapy with lower-extremity (IM injection) versus a sham control procedure (injection of an electrolyte solution) at 18 U.S. centers.205 In a published interim analysis, stem cell therapy was associated with improved amputation-free survival, and the results of the completed study are awaited. The Rejuvenating Endothelial Progenitor Cells via Transcutaneous Intraarterial Supplementation (JUVENTAS) trial is another multicenter randomized controlled trial of stem cell therapy in CLI that is currently underway.206 In JUVENTAS, bone marrow–derived mononuclear cells (vs. placebo) will be administered to the lower extremities through IA infusion, and patients will be followed for the primary endpoint of lower-extremity amputation at 6 months.
Statins
As discussed earlier in this chapter, lipid-lowering therapy with statins reduces the risk of adverse cardiovascular events in patients with atherosclerosis, including those with PAD. Post hoc analysis of the 4S found that simvastatin reduced the risk of new or worsening claudication.28 Several prospective studies found that statin therapy improved symptoms of claudication in patients with intermittent claudication. One placebo-controlled trial found that atorvastatin (80 mg/day) for 12 months improved pain-free walking time by 63%, compared with 38% in the placebo group207 (Fig. 19-7). In other studies, 6 and 12 months of treatment with simvastatin (40 mg/day) improved pain-free and maximal walking distance.208,209 The efficacy of lipid lowering therapy on walking time was not demonstrated, however, in another trial of patients with claudication who were randomized to lovastatin (40 mg) plus niacin 2000 mg daily, lovastatin plus niacin 1000 mg daily, or diet intervention.210 After 28 weeks of follow-up, there was no significant difference in treadmill walking times among the three treatment groups.
Figure 19-7 Atorvastatin improves pain-free walking time (PFWT) in patients with intermittent claudication.
*, P = 0.025 for 80-mg dose at 12 months.
(Reproduced with permission from Mohler ER 3 rd, Hiatt WR, Creager MA: Cholesterol reduction with atorvastatin improves walking distance in patients with peripheral arterial disease. Circulation 108:1481–1486, 2003.)207
There are several potential mechanisms whereby statin therapy may improve symptoms of claudication. These include reduction in plaque size, improvement in vasomotor regulation of blood flow (particularly in the microcirculation), promotion of angiogenesis, and increased skeletal muscle metabolic function. It is not likely that reduction of plaque size accounts for the improvement in symptoms. This is because angiographic studies have shown that treatment with statins induces only very mild changes in vascular lumen size, and these are unlikely to affect blood flow through a stenotic artery.211 In the studies that have examined the effect of statins on patients with claudication, there have been no, or only very minor, changes in the ABI.207,209 Endothelial function, particularly in the peripheral resistance vessels, is impaired in patients with atherosclerosis, including those with PAD.212 Statin therapy has been shown to improve endothelial function in patients with CAD.213,214 Therefore, statin therapy may improve blood flow to the microcirculation and thereby ameliorate symptoms of claudication. Animal studies have found that hypercholesterolemia inhibits angiogenesis.215 This inhibition may be reduced when cholesterol concentration is reduced by statin therapy, enabling collateral formation to occur. In addition, statins have been shown to increase circulating endothelial progenitor cells independent of cholesterol reduction, and thereby may have a proangiogenic effect.216 Favorable effects on metabolic function are not likely to account for the observed effects on walking time. Therapy with simvastatin, alone or in combination with ezetimibe, did not improve phosphocreatine recovery time, as assessed by phosphorus-31 magnetic resonance spectroscopy (MRS).217
Miscellaneous Pharmacological Agents
A number of additional pharmacological agents have been studied as potential therapies for intermittent claudication in recent clinical trials, but unfortunately none has yielded a consistently positive efficacy signal. Multiple serotonin receptor antagonists have been studied with mixed, and largely negative, clinical results to date.218,219 The antichlamydial antibiotic rifalazil had no significant effect on treadmill walking times or quality-of-life parameters compared to placebo.220
Nutraceuticals and Alternative Therapies
Dietary supplements with nutrients, herbs, and vitamins such as L-arginine, ginkgo biloba, and vitamin E, as well as ethylenediaminetetraacetic acid (EDTA), have been studied as complementary therapeutic strategies to improve functional capacity in patients with intermittent claudication. The available evidence supporting and refuting these holistic therapies is reviewed here because vascular specialists are frequently queried by their patients about these remedies.
L-arginine
Endothelium-derived NO is synthesized from its precursor, L-arginine, by eNOS.221 Endothelium-dependent vasodilation, mediated by NO, contributes to the physiological regulation of blood flow at rest and during exercise.222–224 Nitric oxide activates guanylyl cyclase on subjacent vascular smooth muscle and increases cyclic guanosine monophosphate (cGMP) and thereby causes vasodilation. Endothelium-dependent vasodilation is abnormal in conduit and resistance vessels in patients with PAD.212
In one study, L-arginine administered IV at a dose of 8 g twice daily for 3 weeks improved pain-free walking distance by 230% and maximal walking distance by 155%.225 Another study examined the effect of a food bar containing L-arginine (3.3 g) and B-complex and antioxidant vitamins on walking distance in patients with intermittent claudication.226 Pain-free and maximal walking distance improved by 66% and 23%, respectively, following 2 weeks of therapy in patients eating two bars per day. In a larger placebo-controlled trial, 80 patients with intermittent claudication were randomized to one of four daily L-arginine doses (0, 3, 6, or 9 g) administered in thrice-daily divided doses over a 12-week period.227 There was less of a beneficial trend of L-arginine therapy on maximal walking distance among the patients randomized to the 6-g and 9-g treatment arms. The subsequent Nitric Oxide in Peripheral Arterial Insufficiency (NO-PAIN) randomized 133 claudicants to placebo versus 3 grams daily of oral L-arginine therapy that was continued for 6 months.228 The findings of this trial were dramatic: not only was this a negative study, but randomization L-arginine treatment was associated with less brachial artery flow-mediated vasodilation and less improvement in treadmill walking distance than in the placebo group. In light of these data, long-term L-arginine supplementation is not recommended for patients with intermittent claudication.
Vitamin E
Vitamin E (α-tocopherol) is a lipid-soluble antioxidant that has undergone evaluation in patients with intermittent claudication. It inhibits oxidation of polyunsaturated fatty acid. Vitamin E may improve erythrocyte deformability and improve blood flow through the microcirculation because polyunsaturated fatty acids are incorporated into the erythrocyte membrane. A Cochrane systematic review evaluated five placebo-controlled trials of vitamin E in patients with intermittent claudication.229 The trials, conducted between 1953 and 1975, were small and measured different outcomes, precluding any conclusions regarding the efficacy of vitamin E for intermittent claudication. Both the Heart Protection Study (HPS) and the HOPE study failed to demonstrate any efficacy of vitamin E on adverse cardiovascular events in patients with atherosclerosis, including those with PAD.27,49 Therefore, vitamin E is not recommended as therapy for patients with PAD, including those with intermittent claudication.
Ginkgo biloba
Ginkgo biloba is an herb whose major constituents include flavonoids and terpene lactones, such as ginkgolides and bilobalide. Ginkgo may have antioxidant, antiplatelet, and hemorrheological actions.230 It is one of the top-selling herbal medicinal products in the United States.
A meta-analysis of eight randomized placebo-controlled trials found that in recipients of ginkgo, the pain-free claudication distance was 34 meters more than in patients receiving placebo.231 In the largest trial, 24 weeks of ginkgo treatment improved pain-free and maximal walking distance by 45 and 61 meters, respectively, whereas placebo improved these by 21 and 25 meters, respectively.232 Doses employed in clinical trials range from 120 to 320 mg/day. The most common dosage is 40 mg 3 times daily. Potential adverse effects of ginkgo include gastrointestinal symptoms, headache, nausea, vomiting, bleeding, or allergic skin reactions.230 Ginkgo biloba is also associated with adverse interactions with many common prescription medications. It has been associated with cerebral hemorrhage in case reports.233 Ginkgo biloba may be considered as an alternative therapy for treatment of claudication, but its efficacy is probably of marginal clinical importance.
Disodium ethylenediaminetetraacetic acid (EDTA)
EDTA combines with polyvalent cations, including calcium ions, to form a soluble nonionic complex that can be excreted. It requires IV administration and is usually administered two or more times a week. The rationale for using EDTA in patients with atherosclerosis, including those with PAD, is to leech calcium out of atherosclerotic plaque, induce plaque regression, and reduce the severity of stenosis. Also, EDTA may decrease metal ion-dependent formation of reactive oxygen species (ROS) and metal ion-dependent lipid peroxidation.234 There is limited biological evidence to support its efficacy in atherosclerosis.235
The Program to Assess Alternative Treatment Strategies to Achieve Health (PATCH) assessed the effect of EDTA on endothelium- dependent vasodilation in patients with CAD. Up to 33 treatments of IV EDTA over a 6-month period caused no changes in peripheral endothelial function.236 One clinical trial found no effect of EDTA on the severity of atherosclerosis in patients with PAD.237 Two systematic reviews evaluated four placebo-controlled trials that assessed the efficacy of EDTA in patients with intermittent claudication.238,239 These reviews found no evidence that EDTA improves pain-free or maximal walking distance in patients with intermittent claudication.
Potential serious adverse effects of EDTA include hypocalcemia, renal insufficiency, and proteinuria. Additional side effects include gastrointestinal and musculoskeletal symptoms, hypertension, tachycardia, and fever. Based on lack of efficacy as well as safety concerns, EDTA should not be used to treat patients with intermittent claudication.
Conclusions
Patients with PAD are at increased risk for adverse cardiovascular events such as MI, stroke, and death and have impaired functional capacity and qualify of life. In severe circumstances, limb viability is threatened. Comprehensive care of the PAD patient must address all aspects of care: prevention of cardiovascular events, foot care, and therapies to improve leg symptoms. Risk factor modification and antiplatelet therapy are critical components of the management of all patients with PAD. Supervised exercise rehabilitation and the PDE inhibitor cilostazol improve walking distance in patients with claudication. Unfortunately, the pharmacological armamentarium for treatment of claudication is limited. Available medical therapies are not effective in preserving limb viability for patients with critical limb ischemia, so these patients should be considered for revascularization, as reviewed in the next two chapters. Promising new therapies for claudication and critical limb ischemia, particularly stem cell therapy, are undergoing extensive clinical investigation.
1 Hirsch A.T., Haskal Z.J., Hertzer N.R., et al. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113:e463–e654.
2 Rooke T.W., Hirsch A.T., Misra S, et al. 2011 ACCF/AHA focused update of the guideline for the management of patients with peripheral artery disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;124:2020–2045.
3 Gardner A.W. The effect of cigarette smoking on exercise capacity in patients with intermittent claudication. Vasc Med. 1996;1:181–186.
4 Quick C.R., Cotton L.T. The measured effect of stopping smoking on intermittent claudication. Br J Surg. 1982;69(Suppl):S24–S26.
5 Jonason T, Bergstrom R. Cessation of smoking in patients with intermittent claudication. Effects on the risk of peripheral vascular complications, myocardial infarction and mortality. Acta Med Scand. 1987;221:253–260.
6 Powell J.T., Greenhalgh R.M. Changing the smoking habit and its influence on the management of vascular disease. Acta Chir Scand Suppl. 1990;555:99–103.
7 Faulkner K.W., House A.K., Castleden W.M. The effect of cessation of smoking on the accumulative survival rates of patients with symptomatic peripheral vascular disease. Med J Aust. 1983;1:217–219.
8 Lassila R, Lepantalo M. Cigarette smoking and the outcome after lower limb arterial surgery. Acta Chir Scand. 1988;154:635–640.
9 Law M, Tang J.L. An analysis of the effectiveness of interventions intended to help people stop smoking. Arch Intern Med. 1995;155:1933–1941.
10 Hennrikus D, Joseph A.M., Lando H.A., et al. Effectiveness of a smoking cessation program for peripheral artery disease patients: a randomized controlled trial. J Am Coll Cardiol. 2010;56:2105–2112.
11 Jorenby D.E., Leischow S.J., Nides M.A., et al. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. N Engl J Med. 1999;340:685–691.
12 Jorenby D.E., Hays J.T., Rigotti N.A., et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs. placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296:56–63.
13 Rigotti N.A., Pipe A.L., Benowitz N.L., et al. Efficacy and safety of varenicline for smoking cessation in patients with cardiovascular disease: a randomized trial. Circulation. 2011;121:221–229.
14 Varenicline package insert. Pfizer, Inc; 2011.
15 Bupropion (Zyban) package insert. GlaxoSmithKline; 2011.
16 Bowlin S.J., Medalie J.H., Flocke S.A., et al. Epidemiology of intermittent claudication in middle-aged men. Am J Epidemiol. 1994;140:418–430.
17 Newman A.B., Siscovick D.S., Manolio T.A., et al. Ankle-arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Cardiovascular Heart Study (CHS) Collaborative Research Group. Circulation. 1993;88:837–845.
18 Murabito J.M., D’Agostino R.B., Silbershatz H, et al. Intermittent claudication. A risk profile from the Framingham Heart Study. Circulation. 1997;96:44–49.
19 Fowkes F.G., Housley E, Riemersma R.A., et al. Smoking, lipids, glucose intolerance, and blood pressure as risk factors for peripheral atherosclerosis compared with ischemic heart disease in the Edinburgh Artery Study. Am J Epidemiol. 1992;135:331–340.
20 Mowat B.F., Skinner E.R., Wilson H.M., et al. Alterations in plasma lipids, lipoproteins and high density lipoprotein subfractions in peripheral arterial disease. Atherosclerosis. 1997;131:161–166.
21 Rubins H.B., Robins S.J., Collins D, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med. 1999;341:410–418.
22 Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344:1383–1389.
23 Sacks F.M., Pfeffer M.A., Moye L.A., et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335:1001–1009.
24 Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. N Engl J Med. 1998;339:1349–1357.
25 Hirsch A.T., Criqui M.H., Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–1324.
26 Zheng Z.J., Sharrett A.R., Chambless L.E., et al. Associations of ankle-brachial index with clinical coronary heart disease, stroke and preclinical carotid and popliteal atherosclerosis: the Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis. 1997;131:115–125.
27 Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22.
28 Pedersen T.R., Kjekshus J, Pyorala K, et al. Effect of simvastatin on ischemic signs and symptoms in the Scandinavian simvastatin survival study (4S). Am J Cardiol. 1998;81:333–335.
29 MRC/BHF Heart Protection Study of cholesterol-lowering therapy and of antioxidant vitamin supplementation in a wide range of patients at increased risk of coronary heart disease death: early safety and efficacy experience. Eur Heart J. 1999;20:725–741.
30 Heart Protection Study Collaborative Group. Randomized trial of the effects of cholesterol-lowering with simvastatin on peripheral vascular and other major vascular outcomes in 20,536 people with peripheral arterial disease and other high-risk conditions. J Vasc Surg. 2007;45:645–654. discussion 653–644
31 Goldbourt U, Yaari S, Medalie J.H. Isolated low HDL cholesterol as a risk factor for coronary heart disease mortality. A 21-year follow-up of 8000 men. Arterioscler Thromb Vasc Biol. 1997;17:107–113.
32 Gordon D.J., Rifkind B.M. High-density lipoprotein–the clinical implications of recent studies. N Engl J Med. 1989;321:1311–1316.
33 Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease: the Bezafibrate Infarction Prevention (BIP) study. Circulation. 2000;102:21–27.
34 Keech A, Simes R.J., Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366:1849–1861.
35 Psaty B.M., Lumley T, Furberg C.D., et al. Health outcomes associated with various antihypertensive therapies used as first-line agents: a network meta-analysis. JAMA. 2003;289:2534–2544.
36 Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs. diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288:2981–2997.
37 Lip G.Y., Makin A.J. Treatment of hypertension in peripheral arterial disease. Cochrane Database Syst Rev. 2003. CD003075
38 Frishman W.H. Beta-adrenergic receptor blockers. Adverse effects and drug interactions. Hypertension. 1988;11:II21–II29.
39 Rodger J.C., Sheldon C.D., Lerski R.A., et al. Intermittent claudication complicating beta-blockade. BMJ. 1976;1:1125.
40 Roberts D.H., Tsao Y, McLoughlin G.A., et al. Placebo-controlled comparison of captopril, atenolol, labetalol, and pindolol in hypertension complicated by intermittent claudication. Lancet. 1987;2:650–653.
41 Solomon S.A., Ramsay L.E., Yeo W.W., et al. Beta blockade and intermittent claudication: placebo controlled trial of atenolol and nifedipine and their combination. BMJ. 1991;303:1100–1104.
42 Timolol-induced reduction in mortality and reinfarction in patients surviving acute myocardial infarction. N Engl J Med. 1981;304:801–807.
43 A randomized trial of propranolol in patients with acute myocardial infarction. I. Mortality results. JAMA. 1982;247:1707–1714.
44 Mangano D.T., Layug E.L., Wallace A, et al. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. Multicenter Study of Perioperative Ischemia Research Group. N Engl J Med. 1996;335:1713–1720.
45 Poldermans D, Boersma E, Bax J.J., et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high-risk patients undergoing vascular surgery. Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. N Engl J Med. 1999;341:1789–1794.
46 Radack K, Deck C. Beta-adrenergic blocker therapy does not worsen intermittent claudication in subjects with peripheral arterial disease. A meta-analysis of randomized controlled trials. Arch Intern Med. 1991;151:1769–1776.
47 Espinola-Klein C, Weisser G, Jagodzinski A, et al. Beta-blockers in patients with intermittent claudication and arterial hypertension: results from the nebivolol or metoprolol in arterial occlusive disease trial. Hypertension. 2011;58:148–154.
48 Diehm C, Pittrow D, Lawall H. Effect of nebivolol vs. hydrochlorothiazide on the walking capacity in hypertensive patients with intermittent claudication. J Hypertens. 2011;29:1448–1456.
49 Yusuf S, Sleight P, Pogue J, et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–153.
50 Overlack A, Adamczak M, Bachmann W, et al. ACE-inhibition with perindopril in essential hypertensive patients with concomitant diseases. The Perindopril Therapeutic Safety Collaborative Research Group. Am J Med. 1994;97:126–134.
51 Ahimastos A.A., Lawler A, Reid C.M., et al. Brief communication: ramipril markedly improves walking ability in patients with peripheral arterial disease: a randomized trial. Ann Intern Med. 2006;144:660–664.
52 Estacio R.O., Jeffers B.W., Hiatt W.R., et al. The effect of nisoldipine as compared with enalapril on cardiovascular outcomes in patients with non-insulin-dependent diabetes and hypertension. N Engl J Med. 1998;338:645–652.
53 Mehler P.S., Coll J.R., Estacio R, et al. Intensive blood pressure control reduces the risk of cardiovascular events in patients with peripheral arterial disease and type 2 diabetes. Circulation. 2003;107:753–756.
54 The HOPE (Heart Outcomes Prevention Evaluation) Study: the design of a large, simple randomized trial of an angiotensin-converting enzyme inhibitor (ramipril) and vitamin E in patients at high risk of cardiovascular events. The HOPE Study Investigators. Can J Cardiol. 1996;12:127–137.
55 Yusuf S, Teo K.K., Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559.
56 Adler A.I., Stevens R.J., Neil A, et al. UKPDS 59: hyperglycemia and other potentially modifiable risk factors for peripheral vascular disease in type 2 diabetes. Diabetes Care. 2002;25:894–899.
57 Meijer W.T., Hoes A.W., Rutgers D, et al. Peripheral arterial disease in the elderly: the Rotterdam Study. Arterioscler Thromb Vasc Biol. 1998;18:185–192.
58 Jude E.B., Oyibo S.O., Chalmers N, et al. Peripheral arterial disease in diabetic and nondiabetic patients: a comparison of severity and outcome. Diabetes Care. 2001;24:1433–1437.
59 Bowers B.L., Valentine R.J., Myers S.I., et al. The natural history of patients with claudication with toe pressures of 40 mmHg or less. J Vasc Surg. 1993;18:506–511.
60 Resnick H.E., Carter E.A., Sosenko J.M., et al. Incidence of lower-extremity amputation in American Indians: the Strong Heart Study. Diabetes Care. 2004;27:1885–1891.
61 Tahrani A.A., Bailey C.J., Del Prato S, et al. Management of type 2 diabetes: new and future developments in treatment. Lancet. 2011;378:182–197.
62 Dluhy R.G., McMahon G.T. Intensive glycemic control in the ACCORD and ADVANCE trials. N Engl J Med. 2008;358:2630–2633.
63 The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–986.
64 Effect of intensive diabetes management on macrovascular events and risk factors in the Diabetes Control and Complications Trial. Am J Cardiol. 1995;75:894–903.
65 Nathan D.M., Cleary P.A., Backlund J.Y., et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–2653.
66 Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–853.
67 Knatterud G.L., Klimt C.R., Levin M.E., et al. Effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. VII. Mortality and selected nonfatal events with insulin treatment. JAMA. 1978;240:37–42.
68 Holman R.R., Paul S.K., Bethel M.A., et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589.
69 Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:854–865.
70 Dormandy J.A., Charbonnel B, Eckland D.J., et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–1289.
71 Nissen S.E., Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471.
72 Home P.D., Pocock S.J., Beck-Nielsen H, et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet. 2009;373:2125–2135.
73 . Rosiglitazone maleate package insert. 2011.
74 Gerstein H.C., Miller M.E., Byington R.P., et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559.
75 Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139.
76 Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572.
77 Peripheral arterial disease in people with diabetes. Diabetes Care. 2003;26:3333–3341.
78 . Standards of medical care for patients with diabetes mellitus. Diabetes Care. Suppl 1. 2003:S33–S50.
79 Skyler J.S., Bergenstal R, Bonow R.O., et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA Diabetes Trials: a position statement of the American Diabetes Association and a Scientific Statement of the American College of Cardiology Foundation and the American Heart Association. J Am Coll Cardiol. 2009;53:298–304.
80 Welch G.N., Loscalzo J. Homocysteine and atherothrombosis. N Engl J Med. 1998;338:1042–1050.
81 van den Bosch M.A., Bloemenkamp D.G., Mali W.P., et al. Hyperhomocysteinemia and risk for peripheral arterial occlusive disease in young women. J Vasc Surg. 2003;38:772–778.
82 Malinow M.R., Kang S.S., Taylor L.M., et al. Prevalence of hyperhomocysteinemia in patients with peripheral arterial occlusive disease. Circulation. 1989;79:1180–1188.
83 Darius H, Pittrow D, Haberl R, et al. Are elevated homocysteine plasma levels related to peripheral arterial disease? Results from a cross-sectional study of 6880 primary care patients. Eur J Clin Invest. 2003;33:751–757.
84 Taylor L.M.Jr, DeFrang R.D., Harris E.J.Jr, et al. The association of elevated plasma homocysteine with progression of symptomatic peripheral arterial disease. J Vasc Surg. 1991;13:128–136.
85 den Heijer M, Koster T, Blom H.J., et al. Hyperhomocysteinemia as a risk factor for deep-vein thrombosis. N Engl J Med. 1996;334:759–762.
86 Vermeulen E.G., Stehouwer C.D., Twisk J.W., et al. Effect of homocysteine-lowering treatment with folic acid plus vitamin B6 on progression of subclinical atherosclerosis: a randomised, placebo-controlled trial. Lancet. 2000;355:517–522.
87 Lonn E, Yusuf S, Arnold M.J., et al. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354:1567–1577.
88 Lonn E, Held C, Arnold J.M., et al. Rationale, design and baseline characteristics of a large, simple, randomized trial of combined folic acid and vitamins B6 and B12 in high-risk patients: the Heart Outcomes Prevention Evaluation (HOPE)-2 trial. Can J Cardiol. 2006;22:47–53.
89 Bonaa K.H., Njolstad I, Ueland P.M., et al. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006;354:1578–1588.
90 Armitage J.M., Bowman L, Clarke R.J., et al. Effects of homocysteine-lowering with folic acid plus vitamin B12 vs. placebo on mortality and major morbidity in myocardial infarction survivors: a randomized trial. JAMA. 2010;303:2486–2494.
91 Antithrombotic Trialists Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71.
92 Collaborative overview of randomised trials of antiplatelet therapy–I: prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Antiplatelet Trialists’ Collaboration. BMJ. 1994;308:81–106.
93 Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–1860.
94 Collaborative overview of randomised trials of antiplatelet therapy–II: maintenance of vascular graft or arterial patency by antiplatelet therapy. Antiplatelet Trialists’ Collaboration. BMJ. 1994;308:159–168.
95 Balsano F, Violi F. Effect of picotamide on the clinical progression of peripheral vascular disease. A double-blind placebo-controlled study. The ADEP Group. Circulation. 1993;87:1563–1569.
96 Berger J.S., Krantz M.J., Kittelson J.M., et al. Aspirin for the prevention of cardiovascular events in patients with peripheral artery disease: a meta-analysis of randomized trials. JAMA. 2009;301:1909–1919.
97 Catalano M, Born G, Peto R. Prevention of serious vascular events by aspirin amongst patients with peripheral arterial disease: randomized, double-blind trial. J Intern Med. 2007;261:276–284.
98 Belch J, MacCuish A, Campbell I, et al. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ. 2008;337:a1840.
99 Fowkes F.G., Price J.F., Stewart M.C., et al. Aspirin for prevention of cardiovascular events in a general population screened for a low ankle brachial index: a randomized controlled trial. JAMA. 2010;303:841–848.
100 Drugs for the heart. Philadelphia: WB Saunders; 2001.
101 A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet. 1996;348:1329–1339.
102 Yusuf S, Zhao F, Mehta S.R., et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494–502.
103 Bhatt D.L., Fox K.A., Hacke W, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706–1717.
104 Cacoub P.P., Bhatt D.L., Steg P.G., et al. Patients with peripheral arterial disease in the CHARISMA trial. Eur Heart J. 2009;30:192–201.
105 Belch J.J., Dormandy J, Biasi G.M., et al. Results of the randomized, placebo-controlled clopidogrel and acetylsalicylic acid in bypass surgery for peripheral arterial disease (CASPAR) trial. J Vasc Surg. 2010;52:825–833. 833 e821–833 e822
106 Cosmi B, Conti E, Coccheri S. Anticoagulants (heparin, low molecular weight heparin and oral anticoagulants) for intermittent claudication. Cochrane Database Syst Rev. 2001. CD001999
107 Efficacy of oral anticoagulants compared with aspirin after infrainguinal bypass surgery (the Dutch Bypass Oral Anticoagulants or Aspirin Study): a randomised trial. Lancet. 2000;355:346–351.
108 Anand S, Yusuf S, Xie C, et al. Oral anticoagulant and antiplatelet therapy and peripheral arterial disease. N Engl J Med. 2007;357:217–227.
109 Sobel M, Verhaeghe R. Antithrombotic therapy for peripheral artery occlusive disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition). Chest. 2008;133:815S–843S.
110 O’Brien-Irr M.S., Harris L.M., Dosluoglu H.H., et al. Endovascular intervention for treatment of claudication: is it cost-effective? Ann Vasc Surg. 2010;24:833–840.
111 Treesak C, Kasemsup V, Treat-Jacobson D, et al. Cost-effectiveness of exercise training to improve claudication symptoms in patients with peripheral arterial disease. Vasc Med. 2004;9:279–285.
112 Gardner A.W., Poehlman E.T. Exercise rehabilitation programs for the treatment of claudication pain. A meta-analysis. JAMA. 1995;274:975–980.
113 Leng G.C., Fowler B, Ernst E. Exercise for intermittent claudication. Cochrane Database Syst Rev. 2000. CD000990
114 McDermott M.M., Ades P, Guralnik J.M., et al. Treadmill exercise and resistance training in patients with peripheral arterial disease with and without intermittent claudication: a randomized controlled trial. JAMA. 2009;301:165–174.
115 Murphy T.P., Cutlip D.E., Regensteiner J.G., et al. CLEVER Study Investigators: Supervised exercise versus primary stenting for claudication resulting from aortoiliac peripheral artery disease: six-month outcomes from the claudication: exercise versus endoluminal revascularization (CLEVER) study. Circulation. 2012;125:130–139.
116 Regensteiner J.G., Meyer T.J., Krupski W.C., et al. Hospital vs. home-based exercise rehabilitation for patients with peripheral arterial occlusive disease. Angiology. 1997;48:291–300.
117 Degischer S, Labs K.H., Hochstrasser J, et al. Physical training for intermittent claudication: a comparison of structured rehabilitation versus home-based training. Vasc Med. 2002;7:109–115.
118 Gardner A.W., Parker D.E., Montgomery P.S., et al. Efficacy of quantified home-based exercise and supervised exercise in patients with intermittent claudication: a randomized controlled trial. Circulation. 2011;123:491–498.
119 Stewart K.J., Hiatt W.R., Regensteiner J.G., et al. Exercise training for claudication. N Engl J Med. 2002;347:1941–1951.
120 Duscha B.D., Robbins J.L., Jones W.S., et al. Angiogenesis in skeletal muscle precede improvements in peak oxygen uptake in peripheral artery disease patients. Arterioscler Thromb Vasc Biol. 2011;31:2742–2748.
121 Lloyd P.G., Yang H.T., Terjung R.L. Arteriogenesis and angiogenesis in rat ischemic hindlimb: role of nitric oxide. Am J Physiol Heart Circ Physiol. 2001;281:H2528–H2538.
122 Mathien G.M., Terjung R.L. Muscle blood flow in trained rats with peripheral arterial insufficiency. Am J Physiol. 1990;258:H759–H765.
123 Laufs U, Werner N, Link A, et al. Physical training increases endothelial progenitor cells, inhibits neointima formation, and enhances angiogenesis. Circulation. 2004;109:220–226.
124 Prior B.M., Yang H.T., Terjung R.L. What makes vessels grow with exercise training? J Appl Physiol. 2004;97:1119–1128.
125 Niebauer J, Maxwell A.J., Lin P.S., et al. Impaired aerobic capacity in hypercholesterolemic mice: partial reversal by exercise training. Am J Physiol. 1999;276:H1346–H1354.
126 Buckwalter J.B., Curtis V.C., Valic Z, et al. Endogenous vascular remodeling in ischemic skeletal muscle: a role for nitric oxide. J Appl Physiol. 2003;94:935–940.
127 Gardner A.W., Katzel L.I., Sorkin J.D., et al. Exercise rehabilitation improves functional outcomes and peripheral circulation in patients with intermittent claudication: a randomized controlled trial. J Am Geriatr Soc. 2001;49:755–762.
128 Hiatt W.R., Regensteiner J.G., Hargarten M.E., et al. Benefit of exercise conditioning for patients with peripheral arterial disease. Circulation. 1990;81:602–609.
129 Gokce N, Vita J.A., Bader D.S., et al. Effect of exercise on upper and lower extremity endothelial function in patients with coronary artery disease. Am J Cardiol. 2002;90:124–127.
130 Brendle D.C., Joseph L.J., Corretti M.C., et al. Effects of exercise rehabilitation on endothelial reactivity in older patients with peripheral arterial disease. Am J Cardiol. 2001;87:324–329.
131 Brass E.P. Skeletal muscle metabolism as a target for drug therapy in peripheral arterial disease. Vasc Med. 1996;1:55–59.
132 Hiatt W.R., Regensteiner J.G., Wolfel E.E., et al. Effect of exercise training on skeletal muscle histology and metabolism in peripheral arterial disease. J Appl Physiol. 1996;81:780–788.
133 Gardner A.W., Forrester L, Smith G.V. Altered gait profile in subjects with peripheral arterial disease. Vasc Med. 2001;6:31–34.
134 Collins T.C., Lunos S, Carlson T, et al. Effects of a home-based walking intervention on mobility and quality of life in people with diabetes and peripheral arterial disease: a randomized, controlled trial. Diabetes Care. 2011;34:2174–2179.
135 Villemur B, Marquer A, Gailledrat E, et al. New rehabilitation program for intermittent claudication: interval training with active recovery. Pilot study. Ann Phys Rehabil Med. 2011;54:275–281.
136 Bronas U.G., Treat-Jacobson D, Leon A.S. Comparison of the effect of upper body-ergometry aerobic training vs. treadmill training on central cardiorespiratory improvement and walking distance in patients with claudication. J Vasc Surg. 2011;53:1557–1564.
137 Saxton J.M., Zwierska I, Blagojevic M, et al. Upper- versus lower-limb aerobic exercise training on health-related quality of life in patients with symptomatic peripheral arterial disease. J Vasc Surg. 2011;53:1265–1273.
138 Oida K, Ebata K, Kanehara H, et al. Effect of cilostazol on impaired vasodilatory response of the brachial artery to ischemia in smokers. J Atheroscler Thromb. 2003;10:93–98.
139 Tanaka T, Ishikawa T, Hagiwara M, et al. Effects of cilostazol, a selective cAMP phosphodiesterase inhibitor on the contraction of vascular smooth muscle. Pharmacology. 1988;36:313–320.
140 Woo S.K., Kang W.K., Kwon K.I. Pharmacokinetic and pharmacodynamic modeling of the antiplatelet and cardiovascular effects of cilostazol in healthy humans. Clin Pharmacol Ther. 2002;71:246–252.
141 Igawa T, Tani T, Chijiwa T, et al. Potentiation of anti-platelet aggregating activity of cilostazol with vascular endothelial cells. Thromb Res. 1990;57:617–623.
142 Pande R.L., Hiatt W.R., Zhang P, et al. A pooled analysis of the durability and predictors of treatment response of cilostazol in patients with intermittent claudication. Vasc Med. 2010;15:181–188.
143 Soga Y, Iida O, Hirano K, et al. Impact of cilostazol after endovascular treatment for infrainguinal disease in patients with critical limb ischemia. J Vasc Surg. 2011;54:1659–1667.
144 Miyashita Y, Saito S, Miyamoto A, et al. Cilostazol increases skin perfusion pressure in severely ischemic limbs. Angiology. 2011;62:15–17.
145 . Cilostazol package insert. 2011.
146 Dawson D.L., Cutler B.S., Hiatt W.R., et al. A comparison of cilostazol and pentoxifylline for treating intermittent claudication. Am J Med. 2000;109:523–530.
147 Cohn J.N., Goldstein S.O., Greenberg B.H., et al. A dose-dependent increase in mortality with vesnarinone among patients with severe heart failure. Vesnarinone Trial Investigators. N Engl J Med. 1998;339:1810–1816.
148 Packer M, Carver J.R., Rodeheffer R.J., et al. Effect of oral milrinone on mortality in severe chronic heart failure. The PROMISE Study Research Group. N Engl J Med. 1991;325:1468–1475.
149 Hiatt W.R., Money S.R., Brass E.P. Long-term safety of cilostazol in patients with peripheral artery disease: the CASTLE study (Cilostazol: A Study in Long-term Effects). J Vasc Surg. 2008;47:330–336.
150 Reiter M, Bucek R.A., Stumpflen A, et al. Prostanoids for intermittent claudication. Cochrane Database Syst Rev. 2004. CD000986
151 Mohler E.R.3rd. Hiatt WR, Olin JW, et al: Treatment of intermittent claudication with beraprost sodium, an orally active prostaglandin I2 analogue: a double-blinded, randomized, controlled trial. J Am Coll Cardiol. 2003;41:1679–1686.
152 Lievre M, Morand S, Besse B, et al. Oral beraprost sodium, a prostaglandin I(2) analogue, for intermittent claudication: a double-blind, randomized, multicenter controlled trial. Beraprost et Claudication Intermittente (BERCI) Research Group. Circulation. 2000;102:426–431.
153 Creager M.A., Pande R.L., Hiatt W.R. A randomized trial of iloprost in patients with intermittent claudication. Vasc Med. 2008;13:5–13.
154 Second European Consensus Document on chronic critical leg ischemia. Circulation. 1991;84:IV1–IV26.
155 Prostanoids for chronic critical leg ischemia. A randomized, controlled, open-label trial with prostaglandin E1. The ICAI Study Group. Ischemia Cronica degli Arti Inferiori. Ann Intern Med. 1999;130:412–421.
156 Two randomised and placebo-controlled studies of an oral prostacyclin analogue (iloprost) in severe leg ischaemia. The Oral Iloprost in severe Leg Ischaemia Study Group. Eur J Vasc Endovasc Surg. 2000;20:358–362.
157 Belch J.J., Ray S, Rajput-Ray M, et al. The Scottish-Finnish-Swedish PARTNER study of taprostene versus placebo treatment in patients with critical limb ischemia. Int Angiol. 2011;30:150–155.
158 Lewis P, Psaila J.V., Davies W.T., et al. Nifedipine in patients with peripheral vascular disease. Eur J Vasc Surg. 1989;3:159–164.
159 Bagger J.P., Helligsoe P, Randsbaek F, et al. Effect of verapamil in intermittent claudication A randomized, double-blind, placebo-controlled, cross-over study after individual dose-response assessment. Circulation. 1997;95:411–414.
160 Dawson D.L., Zheng Q, Worthy S.A., et al. Failure of pentoxifylline or cilostazol to improve blood and plasma viscosity, fibrinogen, and erythrocyte deformability in claudication. Angiology. 2002;53:509–520.
161 Schratzberger P, Dunzendorfer S, Reinisch N, et al. Mediator-dependent effects of pentoxifylline on endothelium for transmigration of neutrophils. Immunopharmacology. 1999;41:65–75.
162 Rao K.M., Simel D.L., Cohen H.J., et al. Effects of pentoxifylline administration on blood viscosity and leukocyte cytoskeletal function in patients with intermittent claudication. J Lab Clin Med. 1990;115:738–744.
163 Hood S.C., Moher D, Barber G.G. Management of intermittent claudication with pentoxifylline: meta-analysis of randomized controlled trials. CMAJ. 1996;155:1053–1059.
164 Girolami B, Bernardi E, Prins M.H., et al. Treatment of intermittent claudication with physical training, smoking cessation, pentoxifylline, or nafronyl: a meta-analysis. Arch Intern Med. 1999;159:337–345.
165 Intravenous pentoxifylline for the treatment of chronic critical limb ischaemia. The European Study Group. Eur J Vasc Endovasc Surg. 1995;9:426–436.
166 Efficacy and clinical tolerance of parenteral pentoxifylline in the treatment of critical lower limb ischemia. A placebo controlled multicenter study. Norwegian Pentoxifylline Multicenter Trial Group. Int Angiol. 1996;15:75–80.
167 Broderick T.L., Quinney H.A., Lopaschuk G.D. Carnitine stimulation of glucose oxidation in the fatty acid perfused isolated working rat heart. J Biol Chem. 1992;267:3758–3763.
168 Hiatt W.R., Regensteiner J.G., Creager M.A., et al. Propionyl-L-carnitine improves exercise performance and functional status in patients with claudication. Am J Med. 2001;110:616–622.
169 Brevetti G, Diehm C, Lambert D. European multicenter study on propionyl-L-carnitine in intermittent claudication. J Am Coll Cardiol. 1999;34:1618–1624.
170 Brevetti G, Perna S, Sabba C, et al. Propionyl-L-carnitine in intermittent claudication: double-blind, placebo-controlled, dose titration, multicenter study. J Am Coll Cardiol. 1995;26:1411–1416.
171 Brevetti G, Perna S, Sabba C, et al. Effect of propionyl-L-carnitine on quality of life in intermittent claudication. Am J Cardiol. 1997;79:777–780.
172 Hiatt W.R., Creager M.A., Amato A, et al. Effect of propionyl-L-carnitine on a background of monitored exercise in patients with claudication secondary to peripheral artery disease. J Cardiopulm Rehabil Prev. 2011;31:125–132.
173 Stanley W.C. Myocardial energy metabolism during ischemia and the mechanisms of metabolic therapies. J Cardiovasc Pharmacol Ther. 2004;9(Suppl 1):S31–S45.
174 Ma A, Garland W.T., Smith W.B., et al. A pilot study of ranolazine in patients with intermittent claudication. Int Angiol. 2006;25:361–369.
175 Chaitman B.R., Skettino S.L., Parker J.O. v: Anti-ischemic effects and long-term survival during ranolazine monotherapy in patients with chronic severe angina. J Am Coll Cardiol. 2004;43:1375–1382.
176 Chaitman B.R., Pepine C.J., Parker J.O., et al. Effects of ranolazine with atenolol, amlodipine, or diltiazem on exercise tolerance and angina frequency in patients with severe chronic angina: a randomized controlled trial. JAMA. 2004;291:309–316.
177 Isner J.M., Asahara T. Angiogenesis and vasculogenesis as therapeutic strategies for postnatal neovascularization. J Clin Invest. 1999;103:1231–1236.
178 Vincent K.A., Shyu K.G., Luo Y, et al. Angiogenesis is induced in a rabbit model of hindlimb ischemia by naked DNA encoding an HIF-1alpha/VP16 hybrid transcription factor. Circulation. 2000;102:2255–2261.
179 Takeshita S, Zheng L.P., Brogi E, et al. Therapeutic angiogenesis. A single intraarterial bolus of vascular endothelial growth factor augments revascularization in a rabbit ischemic hind limb model. J Clin Invest. 1994;93:662–670.
180 Yang H.T., Deschenes M.R., Ogilvie R.W., et al. Basic fibroblast growth factor increases collateral blood flow in rats with femoral arterial ligation. Circ Res. 1996;79:62–69.
181 Tsurumi Y, Takeshita S, Chen D, et al. Direct intramuscular gene transfer of naked DNA encoding vascular endothelial growth factor augments collateral development and tissue perfusion. Circulation. 1996;94:3281–3290.
182 Isner J.M., Baumgartner I, Rauh G, et al. Treatment of thromboangiitis obliterans (Buerger’s disease) by intramuscular gene transfer of vascular endothelial growth factor: preliminary clinical results. J Vasc Surg. 1998;28:964–973. discussion 973–965
183 Baumgartner I, Pieczek A, Manor O, et al. Constitutive expression of phVEGF165 after intramuscular gene transfer promotes collateral vessel development in patients with critical limb ischemia. Circulation. 1998;97:1114–1123.
184 Rajagopalan S, Mohler E.R.3rd. Lederman RJ, et al: Regional angiogenesis with vascular endothelial growth factor in peripheral arterial disease: a phase II randomized, double-blind, controlled study of adenoviral delivery of vascular endothelial growth factor 121 in patients with disabling intermittent claudication. Circulation. 2003;108:1933–1938.
185 Lederman R.J., Mendelsohn F.O., Anderson R.D., et al. Therapeutic angiogenesis with recombinant fibroblast growth factor-2 for intermittent claudication (the TRAFFIC study): a randomised trial. Lancet. 2002;359:2053–2058.
186 Comerota A.J., Throm R.C., Miller K.A., et al. Naked plasmid DNA encoding fibroblast growth factor type 1 for the treatment of end-stage unreconstructible lower extremity ischemia: preliminary results of a phase I trial. J Vasc Surg. 2002;35:930–936.
187 Nikol S, Baumgartner I, Van Belle E, et al. Therapeutic angiogenesis with intramuscular NV1FGF improves amputation-free survival in patients with critical limb ischemia. Mol Ther. 2008;16:972–978.
188 Belch J, Hiatt W.R., Baumgartner I, et al. Effect of fibroblast growth factor NV1FGF on amputation and death: a randomised placebo-controlled trial of gene therapy in critical limb ischaemia. Lancet. 2011;377:1929–1937.
189 Powell R.J., Simons M, Mendelsohn F.O., et al. Results of a double-blind, placebo-controlled study to assess the safety of intramuscular injection of hepatocyte growth factor plasmid to improve limb perfusion in patients with critical limb ischemia. Circulation. 2008;118:58–65.
190 Wang G.L., Jiang B.H., Rue E.A., et al. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92:5510–5514.
191 Guillemin K, Krasnow M.A. The hypoxic response: huffing and HIFing. Cell. 1997;89:9–12.
192 Wenger R.H., Gassmann M. Oxygen(es) and the hypoxia-inducible factor-1. Biol Chem. 1997;378:609–616.
193 Rajagopalan S, Olin J, Deitcher S, et al. Use of a constitutively active hypoxia-inducible factor-1alpha transgene as a therapeutic strategy in no-option critical limb ischemia patients: phase I dose-escalation experience. Circulation. 2007;115:1234–1243.
194 Creager M.A., Olin J.W., Belch J.J., et al. Effect of hypoxia-inducible factor-1{alpha} gene therapy on walking performance in patients with intermittent claudication. Circulation. 2011;124:1765–1773.
195 Kalka C, Masuda H, Takahashi T, et al. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci U S A. 2000;97:3422–3427.
196 Kamihata H, Matsubara H, Nishiue T, et al. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation. 2001;104:1046–1052.
197 Shintani S, Murohara T, Ikeda H, et al. Augmentation of postnatal neovascularization with autologous bone marrow transplantation. Circulation. 2001;103:897–903.
198 Tateishi-Yuyama E, Matsubara H, Murohara T, et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002;360:427–435.
199 Forbes T.L. Rationale and design of the JUVENTAS trial for repeated intra-arterial infusion of autologous bone marrow-derived mononuclear cells in patients with critical limb ischemia. Commentary. J Vasc Surg. 2010;51:1568.
200 Walter D.H., Krankenberg H, Balzer J.O., et al. Intraarterial administration of bone marrow mononuclear cells in patients with critical limb ischemia: a randomized-start, placebo-controlled pilot trial (PROVASA). Circ Cardiovasc Interv. 2011;4:26–37.
201 Wester T, Jorgensen J.J., Stranden E, et al. Treatment with autologous bone marrow mononuclear cells in patients with critical lower limb ischaemia. A pilot study. Scand J Surg. 2008;97:56–62.
202 Lara-Hernandez R, Lozano-Vilardell P, Blanes P, et al. Safety and efficacy of therapeutic angiogenesis as a novel treatment in patients with critical limb ischemia. Ann Vasc Surg. 2010;24:287–294.
203 Lu D, Chen B, Liang Z, et al. Comparison of bone marrow mesenchymal stem cells with bone marrow-derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: a double-blind, randomized, controlled trial. Diabetes Res Clin Pract. 2011;92:26–36.
204 Perin E.C., Silva G, Gahremanpour A, et al. A randomized, controlled study of autologous therapy with bone marrow-derived aldehyde dehydrogenase bright cells in patients with critical limb ischemia. Catheter Cardiovasc Interv. 2011;78:1060–1067.
205 Powell R.J., Comerota A.J., Berceli S.A., et al. Interim analysis results from the RESTORE-CLI, a randomized, double-blind multicenter phase II trial comparing expanded autologous bone marrow-derived tissue repair cells and placebo in patients with critical limb ischemia. J Vasc Surg. 2011;54:1032–1041.
206 Sprengers R.W., Moll F.L., Teraa M, et al. Rationale and design of the JUVENTAS trial for repeated intra-arterial infusion of autologous bone marrow-derived mononuclear cells in patients with critical limb ischemia. J Vasc Surg. 2010;51:1564–1568.
207 Mohler E.R.3rd, Hiatt W.R., Creager M.A. Cholesterol reduction with atorvastatin improves walking distance in patients with peripheral arterial disease. Circulation. 2003;108:1481–1486.
208 Aronow W.S., Nayak D, Woodworth S, et al. Effect of simvastatin versus placebo on treadmill exercise time until the onset of intermittent claudication in older patients with peripheral arterial disease at six months and at one year after treatment. Am J Cardiol. 2003;92:711–712.
209 Mondillo S, Ballo P, Barbati R, et al. Effects of simvastatin on walking performance and symptoms of intermittent claudication in hypercholesterolemic patients with peripheral vascular disease. Am J Med. 2003;114:359–364.
210 Hiatt W.R., Hirsch A.T., Creager M.A., et al. Effect of niacin ER/lovastatin on claudication symptoms in patients with peripheral artery disease. Vasc Med. 2010;15:171–179.
211 Brown B.G., Zhao X.Q., Sacco D.E., et al. Lipid lowering and plaque regression. New insights into prevention of plaque disruption and clinical events in coronary disease. Circulation. 1993;87:1781–1791.
212 Liao J.K., Bettmann M.A., Sandor T, et al. Differential impairment of vasodilator responsiveness of peripheral resistance and conduit vessels in humans with atherosclerosis. Circ Res. 1991;68:1027–1034.
213 Anderson T.J., Meredith I.T., Yeung A.C., et al. The effect of cholesterol-lowering and antioxidant therapy on endothelium-dependent coronary vasomotion. N Engl J Med. 1995;332:488–493.
214 Treasure C.B., Klein J.L., Weintraub W.S., et al. Beneficial effects of cholesterol-lowering therapy on the coronary endothelium in patients with coronary artery disease. N Engl J Med. 1995;332:481–487.
215 Van Belle E, Rivard A, Chen D, et al. Hypercholesterolemia attenuates angiogenesis but does not preclude augmentation by angiogenic cytokines. Circulation. 1997;96:2667–2674.
216 Vasa M, Fichtlscherer S, Adler K, et al. Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation. 2001;103:2885–2890.
217 West A.M., Anderson J.D., Epstein F.H., et al. Low-density lipoprotein lowering does not improve calf muscle perfusion, energetics, or exercise performance in peripheral arterial disease. J Am Coll Cardiol. 2011;58:1068–1076.
218 Norgren L, Jawien A, Matyas L, et al. Sarpogrelate, a 5-hT2A receptor antagonist in intermittent claudication. A phase II European study. Vasc Med. 2006;11:75–83.
219 Hiatt W.R., Hirsch A.T., Cooke J.P., et al. Randomized trial of AT-1015 for treatment of intermittent claudication. A novel 5-hydroxytryptamine antagonist with no evidence of efficacy. Vasc Med. 2004;9:18–25.
220 Jaff M.R., Dale R.A., Creager M.A., et al. Anti-chlamydial antibiotic therapy for symptom improvement in peripheral artery disease: prospective evaluation of rifalazil effect on vascular symptoms of intermittent claudication and other endpoints in Chlamydia pneumoniae seropositive patients (PROVIDENCE-1). Circulation. 2009;119:452–458.
221 Gornik H.L., Creager M.A. Arginine and endothelial and vascular health. J Nutr. 2004;134:2880S–2887S. discussion 2895 S
222 Maxwell A.J., Schauble E, Bernstein D, et al. Limb blood flow during exercise is dependent on nitric oxide. Circulation. 1998;98:369–374.
223 Duffy S.J., New G, Tran B.T., et al. Relative contribution of vasodilator prostanoids and NO to metabolic vasodilation in the human forearm. Am J Physiol. 1999;276:H663–H670.
224 Gordon M.B., Jain R, Beckman J.A., et al. The contribution of nitric oxide to exercise hyperemia in the human forearm. Vasc Med. 2002;7:163–168.
225 Boger R.H., Bode-Boger S.M., Thiele W, et al. Restoring vascular nitric oxide formation by L-arginine improves the symptoms of intermittent claudication in patients with peripheral arterial occlusive disease. J Am Coll Cardiol. 1998;32:1336–1344.
226 Maxwell A.J., Anderson B.E., Cooke J.P. Nutritional therapy for peripheral arterial disease: a double-blind, placebo-controlled, randomized trial of HeartBar. Vasc Med. 2000;5:11–19.
227 Oka R.K., Szuba A, Giacomini J.C., et al. A pilot study of L-arginine supplementation on functional capacity in peripheral arterial disease. Vasc Med. 2005;10:265–274.
228 Wilson A.M., Harada R, Nair N, et al. L-arginine supplementation in peripheral arterial disease: no benefit and possible harm. Circulation. 2007;116:188–195.
229 Kleijnen J, Mackerras D. Vitamin E for intermittent claudication. Cochrane Database Syst Rev. 2000. CD000987
230 Ernst E. The risk-benefit profile of commonly used herbal therapies: Ginkgo, St. John’s wort, ginseng, echinacea, saw palmetto, and kava. Ann Intern Med. 2002;136:42–53.
231 Pittler M.H., Ernst E. Ginkgo biloba extract for the treatment of intermittent claudication: a meta-analysis of randomized trials. Am J Med. 2000;108:276–281.
232 Peters H, Kieser M, Holscher U. Demonstration of the efficacy of ginkgo biloba special extract EGb 761 on intermittent claudication–a placebo-controlled, double-blind multicenter trial. Vasa. 1998;27:106–110.
233 De Smet P.A. Herbal remedies. N Engl J Med. 2002;347:2046–2056.
234 Lamas G.A., Ackermann A. Clinical evaluation of chelation therapy: is there any wheat amidst the chaff? Am Heart J. 2000;140:4–5.
235 Evans D.A., Tariq M, Sujata B, et al. The effects of magnesium sulphate and EDTA in the hypercholesterolaemic rabbit. Diabetes Obes Metab. 2001;3:417–422.
236 Anderson T.J., Hubacek J, Wyse D.G., et al. Effect of chelation therapy on endothelial function in patients with coronary artery disease: PATCH substudy. J Am Coll Cardiol. 2003;41:420–425.
237 Sloth-Nielsen J, Guldager B, Mouritzen C, et al. Arteriographic findings in EDTA chelation therapy on peripheral arteriosclerosis. Am J Surg. 1991;162:122–125.
238 Villarruz M.V., Dans A, Tan F. Chelation therapy for atherosclerotic cardiovascular disease. Cochrane Database Syst Rev. 2002. CD002785
239 Ernst E. Chelation therapy for peripheral arterial occlusive disease: a systematic review. Circulation. 1997;96:1031–1033.