Chapter 60 Lower-Extremity Ulceration
Ulceration of the lower extremity is a common condition that causes significant discomfort and disability.1 An ulcer is defined as a disruption of the skin with erosion of the underlying subcutaneous tissue. This breach may extend further to the contiguous muscle and bone. The pathophysiological mechanisms underlying ulcer formation are multifactorial and include neuropathy, infection, ischemia, and abnormal foot structure and biomechanics. It is not surprising then that management of the diabetic foot is a complex clinical problem requiring an interdisciplinary approach.2,3 Minor trauma, often footwear related, is a frequent inciting event. A chronic ulcer is defined as a full-thickness skin defect with no significant reepithelialization for more than 4 weeks.
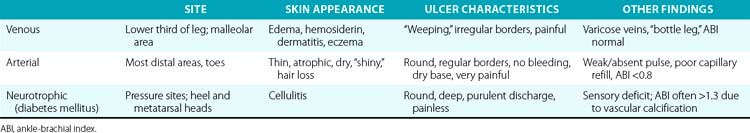
Three etiologies of leg ulcerations are responsible for almost 95% of leg ulcers: about 40% to 80% are due to underlying venous disease, 10% to 20% are due to arterial insufficiency, and 15% to 25% are secondary to diabetes mellitus; in 10% to 15% of patients, a combination of two or more causes exists. Prolonged pressure and local infection are common causes of leg ulcers with minimal vascular compromise. Rare causes are responsible for less than 5% of all leg ulcers4 (Box 60-1). The disease entities that usually underlie leg ulceration (e.g., venous insufficiency, peripheral artery disease (PAD), diabetes mellitus) are associated with significant patient morbidity and mortality. A detailed knowledge of the clinical picture, pathogenesis, relevant diagnostic tests, treatment modalities, and differential diagnosis of leg ulcerations is essential in planning the optimal treatment strategy (Table 60-1). An incorrect or delayed initial diagnosis may harm the patient and increase the risk of serious complications, including permanent disability and amputations.
The exact prevalence of lower-extremity ulcers in the United States is unknown. The prevalence of leg ulceration in the general population of Western nations is 1% to 3.5%, with the prevalence increasing to 5% in the geriatric population.5–8 Data from these studies most likely underestimate the true prevalence because they do not include patients with leg ulcers who are not known to the healthcare system.
The cost of treating leg ulceration is staggering. Epidemiological studies from Sweden estimated annual costs of treatment of lower-extremity ulcers at $25 million. In England, the estimated cost of care for patients with leg ulcers in a population of 250,000 is about $130,000 annually per patient.9 Items factored into the equation include physician visits, hospital admissions, home health care, wound care supplies, rehabilitation, time lost from work, and jobs lost. Adding to the cost is the chronic nature of these wounds, high rate of recurrence, and propensity for infection. A true accounting of the cost is difficult because of the unknown prevalence of disease.
Because the disease affects a patient’s lifestyle and attitude, the social cost of leg ulcers accrue. The ability to work may be temporarily or permanently affected by the condition,8 and the reduction in work capacity adds to the medical cost to society. An estimated 10 million workdays are lost from lower-extremity ulcers in the United States annually, and this figure may be low.10,11 A report in 1994 focused on the financial, social, and psychological implications of lower-extremity lesions in 73 patients.12 Among the study patients, 68% reported feelings of fear, social isolation, anger, depression, and negative self-image because of the ulcers. In addition, 81% of the patients felt that their mobility was adversely affected. Within the younger population that was still actively working, there was a correlation between lower-extremity ulceration and adverse effect on finances, time lost from work, and job loss. In addition, there was a strong correlation between time spent on ulcer care and feelings of anger and resentment. These factors combined to have a negative emotional impact on their lives.
Biomechanics of Walking and Ulcer Formation
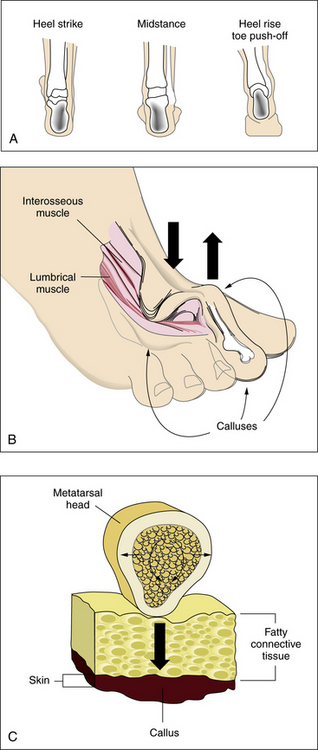
An appreciation of the biomechanics required for walking is essential to understanding the etiology of foot ulcers. The foot is a complicated biological structure containing 26 bones, numerous joints, and a network of ligaments, muscles, and blood vessels. Gait is a complex set of events that requires triplanar foot motion and control of multiple axes for complete bipedal ambulation13 (Fig. 60-1A). When the heel hits the ground, its outer edge touches first; the foot is in a supinated position, which makes it firm and rigid. The soft-tissue structures (muscles, tendons, and ligaments) then relax, allowing the foot to pronate. The foot becomes less rigid and is able to flatten, absorb the shock of touchdown, and adapt to uneven surfaces. During midstance, the heel lies below the ankle joint complex, the front and back of the foot are aligned, and the foot easily bears weight. Toward the end of midstance, the soft-tissue structures begin to tighten; the foot resupinates and regains its arch. The foot is again firm, acting as a rigid lever for propulsion. The heel lifts off the ground, swings slightly to the inside, and the toes push weight off the ground.
Sensory input from the visual, vestibular, and proprioceptive systems is necessary to modify learned motor patterns and muscular output to execute the desired action. Various external and internal forces affect foot function.14 The combination of body weight pushing down and ground reactive force pushing up creates friction and compressive forces. Shear results from the bones of the foot sliding parallel to their plane of contact during pronation and supination. Foot deformities or ill-fitting footwear enhance pressure points because they focus the forces on a smaller area. When the foot flattens too much or overpronates, the ankle and heel do not align during midstance, and some bones are forced to support more weight. The foot strains under the body’s weight, causing the muscles to pull harder on these areas, making it more difficult for tendons and ligaments to hold bones and joints in proper alignment. Over time, swelling and pain on the bottom of the foot or near the heel may occur. Bunions can form at the great toe joint, and hammertoe deformities can form at the lesser toes. Abnormal foot biomechanics resulting from limited joint mobility and foot deformities magnify shearing forces, resulting in increased plantar pressure on the foot during ambulation (see Fig. 60-1B-C). This can represent critical causes for tissue breakdown.
Pathophysiology of Ulcer Formation
Venous Disorders
Venous leg ulcers are the most frequently occurring chronic lower-extremity wounds (Fig. 60-2A) (also see Chapter 56). The prevalence of lower-extremity ulceration resulting from chronic venous disease (CVD) in European and Western populations is estimated to be 0.5% to 1%. In the United States, it is estimated that between 600,000 and 2.5 million patients have venous ulcerations; treatment costs are estimated at $2.5 to $3 billion dollars, with a corresponding loss of 2 million workdays per year.15 Ten years ago, the estimated annual cost of treatment for venous ulcer patients was almost $40,000 per patient.16 This cost has risen since then. The pathophysiology of venous ulceration is straightforward. Blood returns from the lower extremities against gravity to the inferior vena cava (IVC) through the deep and superficial venous systems. The deep veins are located within the muscles and deep fascia of the legs. The superficial system consists of the great saphenous vein and the small saphenous vein and is located within the subcutaneous fat. Valves are present within all three systems and prevent retrograde flow of blood. A portion of blood from the superficial system is directed to the deep system through the communicating perforators. While standing, about 22% of the total blood volume is localized to the lower extremities, and hydrostatic pressure in the foot veins can reach 80 mmHg. In healthy individuals with competent venous valves, the efficient calf muscle pump can reduce venous pressure by two thirds during exercise. Venous insufficiency occurs when any of these elements do not function adequately. Pressure in the venous system increases, and (most importantly) ambulatory venous pressure rises during leg exercise. The primary cause of venous hypertension is insufficiency of the valves of the deep venous system and perforating veins of the lower leg.
The exact mechanism by which ulcerations develop in patients with venous insufficiency is not clear. One theory is that ulceration is due to increased intraluminal pressure within the capillary system of the leg. The capillaries become dilated and elongated, and blood flow is sluggish, resulting in microthrombi formation and frequently leading to capillary occlusion. Fibrin, albumin, and various macromolecules leak into the dermis, where they bind to and trap growth factors, making them unavailable for the tissue repair process.17 Leakage of fibrinogen through capillary walls results in deposition of pericapillary fibrin cuffs,18 which has been suggested as a physical barrier impeding passage of oxygen.10 Iron deposition, white blood cell (WBC) accumulation, decreased fibrinolytic activity, and a myriad of inflammatory responses to vascular damage are all postulated to be the final pathways leading to venous ulcerations, but it is still not clear whether they represent causative factors.
Tissue hypoxia appears to be the major underlying factor in developing venous ulceration. Unlike ulcers associated with arterial insufficiency, this hypoxic state is not caused by decreased blood flow to the legs. Patients with venous insufficiency usually have adequate blood flow to their lower extremities. Direct measurements of transcutaneous oxygen levels on the lower leg have demonstrated that exercise produces a marked rise in skin oxygen tension in normal legs, but not in those affected by venous insufficiency. Exercise reduces venous pressure in patients with competent valves, thus removing the stimulus for reflex vasoconstriction. In patients with compromised valves, venous pressure remains high during exercise, and reflex vasoconstriction persists.19
On the basis of these findings, it is clear that management of lower-extremity ulcers secondary to venous insufficiency must include measures that improve the abnormal venous blood return from the affected extremity. Leg elevation, compression therapy, local wound care, and surgical correction of selected underlying pathology are all important components of the treatment plan.
Arterial Disease
The incidence of lower-extremity ulcers caused by PAD (see Fig. 60-2B) is increasing in Western nations.8 The general aging of the population and better diagnostic techniques may provide possible explanations for this observation. Risk factors for development of atherosclerotic lesions causing leg ischemia include diabetes mellitus, smoking, hyperlipidemia, hypertension, obesity, and age.20 Lack of perfusion decreases tissue resilience, leads to rapid death of tissue, and impedes wound healing (see Chapter 17). Wound healing and tissue regeneration depend on adequate blood supply to the region. Ischemia due to vascular disease impedes healing by reducing the supply of oxygen, nutrients, and soluble mediators involved in the repair process.21
The Diabetic Foot
Persons with diabetes mellitus are particularly prone to foot ulcers. The diabetic foot is a common and serious clinical condition that has its specific characteristics. The American Diabetes Association Consensus Group found that among persons with diabetes, the risk of foot ulceration was increased among men, patients who had had diabetes for more than 10 years, and patients with poor glucose control or cardiovascular, retinal, or renal complications.22 It is estimated that 15% of U.S. patients with diabetes will develop manifestations of diabetic foot disease in their lifetime.23,24 In this population, the prevalence of lower-extremity ulcers ranges from 4% to 10%, with an annual incidence of 2% to 3%.25 Although representing only 6% of the population, patients with diabetes account for 46%25 of the 162,000 hospital admissions for foot ulcers annually. Foot ulcers occur in up to 25% of patients with diabetes and precede more than 8 in 10 nontraumatic amputations. In 2005, approximately 1.6 million people were living with limb loss; this number is expected to more than double by 2050.26 Diabetic foot ulcers and their sequelae, amputations, are the major cause of disability, morbidity, mortality, and costs for these patients.23 Ulceration and infection of lower extremities are a leading cause of hospitalization in patients with diabetes.25 Treatment of pedal soft-tissue deficits in the diabetic patient population continues to be a medical and surgical challenge, thereby extending the length of their disability and significantly increasing the cost of medical care. Nearly half of all patients who undergo amputation will develop limb-threatening ischemia in the contralateral limb, and many will ultimately require an amputation of the opposite limb within 5 years. In 2000, the Centers for Disease Control and Prevention (CDC) estimated that 12 million Americans were diagnosed with diabetes, and the estimated annual direct and indirect costs of diabetes treatment in the United States was approximately $174 billion, with 1 in 5 diabetes dollars spent on lower-extremity care. Preventing ulcerations and/or amputations is critical from both medical and economical standpoints.2
Development of diabetic foot disease can be attributed to several primary risk factors, including neuropathy, ischemia, infection, and immune impairment. Four foot-related risk factors have been identified in the genesis of pedal ulceration: altered biomechanics, limited joint mobility, bony deformity, and severe nail pathology.22
Neuropathy
Neuropathy is the most common underlying etiology of foot ulceration and frequently involves the somatic and autonomic fibers. Although there are many causes of peripheral neuropathy, diabetes mellitus is by far the most common (see Box 60-1). Neuropathy is present in 42% of diabetic patients after 20 years27 and is usually a distal symmetrical sensorimotor polyneuropathy. Peripheral neuropathy is postulated to result from abnormalities in metabolism, one of which is a deficiency in sorbitol metabolism via the polyol pathway.28,29
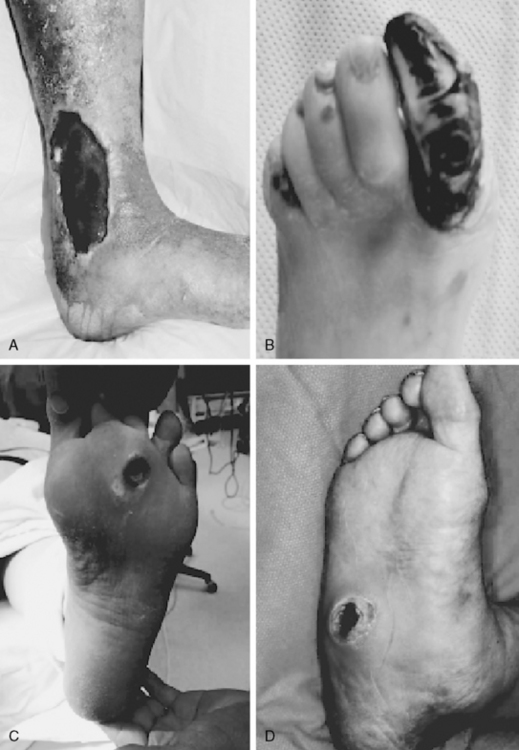
Neurotrophic ulcers typically form on the plantar aspect of the foot at areas of excessive focal pressures. These are most commonly encountered over the bony prominences of the metatarsal heads and forefoot region because of the requirements of midstance and heel-off during the gait cycle (see Fig. 60-2C). Loss of protective sensation in the foot can lead rapidly to ulceration if patient education and preventive measures are not taken. Diabetic patients are especially prone to development of a neuro-osteoarthropathy known as Charcot foot.30 This condition is thought to involve autonomic nerve dysfunction that results in abnormal perfusion to foot bones, which leads to bony fragmentation and collapse (see Fig. 60-2D). The resulting “rocker-bottom foot” is prone to tissue breakdown and ulceration.23,30
Several investigators23,30,31 have demonstrated that there is an increase in both static and dynamic foot pressures.32 To date, high pressures alone have not been shown to cause foot ulceration. Rheumatoid patients with high plantar foot pressures but no sensitivity deficit have almost no evidence of foot ulceration.33
Type A sensory fibers are responsible for light touch sensation, vibratory sensation, pressure, proprioception, and motor innervation to the intrinsic muscles of the foot. Type C sensory fibers detect painful stimuli, noxious stimuli, and temperature. When these fibers are affected, protective sensation is lost. This manifests as a distal symmetrical loss of sensation described in a “stocking” distribution and proves to be the primary factor predisposing patients to ulcers and infection.34 Patients are unable to detect increased loads, repeated trauma, or pain from shearing forces. Injuries such as fractures, ulceration, and foot deformities therefore go unrecognized. Repeat stress to high-pressure areas or bone prominences, which would be interpreted as pain in the non- neuropathic patient, also go unrecognized. Sensory dysfunction results in increased shearing forces and repeated trauma to the foot.35,36 Patients have inadequate protective sensation during all phases of gait, so high loads are undetected owing to loss of pain threshold, which results in prolonged and increased forces.31,35 These problems manifest as abnormal pressure points, increased shearing, and greater friction to the foot. Because this goes unrecognized in the insensate foot, gait patterns remain unchanged, and the stresses eventually cause tissue breakdown and ulceration.
Motor neuropathy is associated with demyelinization and motor end-plate damage, which contribute to conduction defects. The distal motor nerves are the most commonly affected, resulting in atrophy of the small intrinsic muscles of the foot. Wasting of the lumbrical and interosseous muscles of the foot results in collapse of the arch and loss of stability of the metatarsal-phalangeal joints during midstance of the gait. Overpowering by extrinsic muscles can lead to depression of the metatarsal heads, digital contractures, and cocked-up toes; equinus deformities of the ankle; or a varus hindfoot.37
Autonomic involvement causes an interruption of normal sweating at the epidermal level and arteriovenous shunting at subcutaneous and dermal levels. Hypohidrosis leads to a noncompliant epidermis that increases the risk of cracking and fissuring. Arteriovenous shunting diminishes delivery of nutrients and oxygen to tissue regions, and skin and subcutaneous tissues become more susceptible to breakdown.38
Musculoskeletal deformities
Atrophy of the small muscles within the foot results in nonfunctioning intrinsic foot muscles referred to as an intrinsic minus foot39 (see Fig. 60-1B). Muscles showing early involvement are the flexor digitorum brevis, lumbricales, and interosseous muscles. These muscle groups act to stabilize the proximal phalanx against the metatarsal head, preventing dorsiflexion at the metatarsal phalangeal joint (MTPJ) during midstance in the gait cycle. With progression of the neuropathy, these muscles atrophy and fail to function properly. This causes the MTPJs to become unstable, allowing the long flexors (flexor digitorum longus and flexor hallucis longus) and extensors (extensor digitorum longus and extensor hallucis longus) to act unchecked on the digits. Dorsal contractures develop at the MTPJs, with development of hammer digit syndrome, also known as intrinsic minus disease.
The deformity acts to plantarflex the metatarsals, making the heads more prominent and increasing the plantar pressure created beneath them (see Fig. 60-1B). It also acts to decrease the amount of toe weight-bearing during the gait cycle, which also increases pressure on the metatarsal heads. In normal foot anatomy, a metatarsal fat pad located plantar to the MTPJs helps dissipate pressures on the metatarsal heads from the ground. When hammer digit deformity occurs, this fat pad migrates distally and becomes nonfunctional, resulting in elevated plantar pressures that increase the risk of skin breakdown and ulceration due to shearing forces.1
Overpowering by the extrinsic foot muscles also leads to an equinus deformity at the ankle and a varus hindfoot. A cavovarus foot type can develop, leading to decreased range of motion of the pedal joints, inability to adapt to terrain, and low tolerance to shock. In essence, a mobile adapter is converted to a rigid lever. Pressure is equal to body weight divided by surface area, thus decreasing surface area below a metatarsal head with concomitant rigid deformities and leading to increased forces or pressure to the sole of the foot. When neuropathic foot disease is associated with congenital foot deformities such as long or short metatarsals, a plantarflexed metatarsal, abnormalities in the metatarsal parabola, or a Charcot foot30 (see Fig. 60-2D), there is a higher propensity toward breakdown as a result of increased and abnormal plantar foot pressures.
Increased body weight and decreased surface area of contact of the foot components with the ground increase pressure. A low pressure but constant insult over an extended period can have the same ulcerogenic effect as high pressure over a shorter period. This is typical of the effect of tight-fitting shoes. If the magnitude of these forces in a given area is large enough, either skin loss or hypertrophy of the stratum corneum (callus) occurs (see Fig. 60-1C). Presence of callus in patients with neuropathy should raise a red flag because the risk of ulceration in a callused area is increased by two orders of magnitude.
Arterial disease
One of the major factors affecting diabetic foot disease is development of lower-extremity arterial disease.26 Peripheral artery disease is estimated to be two to four times more common in persons with diabetes than in others23,40 (see Chapter 14). Atherosclerosis occurs at a younger age in persons with diabetes than in others, and its hallmark is involvement of the tibioperoneal vessels, with sparing of the pedal vessels. In addition to being more prevalent in diabetes, atherosclerosis is more accelerated and results in a higher rate of amputations.41–43 Lesions in persons with diabetes tend to localize to the infracrural region. Relative sparing of the pedal vessels often assists pedal bypass. Occlusive lesions affecting the foot and precluding revascularization are not common in diabetic patients.23
Purely ischemic diabetic foot ulcers are uncommon, representing only 10% to 15% of ulcers in patients with diabetes. More commonly, ulcers have a mixed ischemic and neuropathic origin, representing 33% of diabetic foot ulcers.23 Initiation of an ischemic ulcer usually requires a precipitating factor such as mechanical stress. Ulcers often develop on the dorsum of the foot over the first and fifth metatarsal heads. A heel ulcer can develop from constant pressure applied while the heel is in a dependent position or during prolonged immobilization and bed rest. Once formed, the blood supply necessary to allow healing of an ulcer is greater than that needed to maintain intact skin. This leads to chronic ulcer development unless the blood supply is improved.
Infection
Patients with diabetes appear to be more prone to various infections than their nondiabetic counterparts.44 Several factors increase the risk of developing diabetic foot infections, including diabetic neuropathy, peripheral artery disease, and immunological impairment. Several defects in immunological response relate to increased infection risk in diabetics. Diabetic patients demonstrate a decrease in function of polymorphonuclear leukocytes that can manifest as a decrease in migration, phagocytosis, and decreased intracellular activity. Evidence suggests impaired cellular immune response as well as abnormalities in complement function.45,46 Some of the defects appear to improve with control of hyperglycemia.47
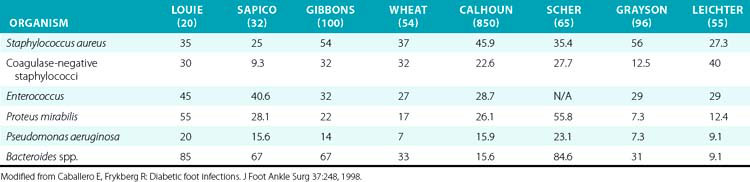
Undiagnosed clean neuropathic foot ulcers often convert to acute infections with abscess and/or cellulitis.48 Diabetic foot infections can be classified into those that are nonthreatening and those that are life or limb threatening. Non–limb-threatening diabetic foot infections are often mild infections associated with a superficial ulcer. They often have less than 2 cm of surrounding cellulitis and demonstrate no signs of systemic toxicity. These infections have on average 2.1 organisms48 (Table 60-2). Aerobic gram-positive cocci are the sole pathogens in 42% of these cases, with the most notable organisms being Staphylococcus aureus, coagulase-negative S. aureus, and streptococci. These less severe infections can often be managed with local wound care, rest, elevation, and oral antibiotics on an outpatient basis. A foot infection in a diabetic patient can present with a more severe, life- or limb-threatening picture. In these patients, there is usually a deeper ulceration or an undrained abscess, gangrene, or necrotizing fasciitis. Methicillin-resistant S. aureus (MRSA) is an increasingly common isolate.44 They tend to have greater than 2 cm of surrounding cellulitis, as well as lymphangitis and edema of the affected limb. These more severe cases generally present with fever, leukocytosis, and hyperglycemia.
In contrast to nondiabetic individuals, complex foot infections in diabetic patients usually involve multiple organisms with complex biofilm environments.49 Studies report an average of five to eight different species per specimen.50–53 These include a combination of gram-positive and gram-negative aerobic and anaerobic organisms. The most prevalent organisms identified were S. aureus, coagulase-negative Staphylococcus, group B Streptococcus, Proteus, Escherichia coli, Pseudomonas, and Bacteroides. Recently, MRSA infection has become more common in diabetic foot ulcers and is associated with previous antibiotic treatment and prolonged time to healing.44,54,55 Anaerobic infections with Clostridium are also common. These patients require immediate hospitalization, broad-spectrum intravenous (IV) antibiotics, and aggressive surgical débridement. Superficial wound cultures are often unreliable because they may demonstrate organisms responsible for colonization that do not affect the associated infection. Deep wound or bone cultures are the best way to accurately assess the microbiology in a diabetic foot infection and assess for osteomyelitis.
Assessment of the Patient with a Lower-Extremity Ulcer
Accurate diagnosis of the underlying cause of lower-extremity ulceration is essential for successful treatment. The etiology of most leg ulcers can be ascertained quite accurately by careful problem-focused history taking and physical examination.32 Diagnostic and laboratory studies are occasionally necessary to establish the diagnosis, but are more often performed to guide treatment strategy.56
History
Patients with ulcers due to venous insufficiency usually complain of aching and swelling of the legs (see Chapter 55). They may recount a history of recurrent cellulitis, previous deep vein thrombosis (DVT), or previous superficial venous surgery. Symptoms are often worse at the end of the day, exacerbated when the leg is dependent, and relieved by leg elevation.
Arterial insufficiency is suggested by a history of underlying cardiac or cerebrovascular disease, complaints of leg claudication or impotence, or pain in the distal foot when supine (rest pain; see Chapter 18). Symptoms of arterial insufficiency are due to inadequate perfusion to the lower extremity relative to its metabolism. Tissue hypoxia and the subsequent increase in concentration of lactic acid produce pain. Patients may complain of pain in the buttocks or calves brought on with activity and relieved with rest (intermittent claudication), or pain in the forefoot aggravated by elevation and relieved by dependency (rest pain). Presence of an extremity ulcer is an easily recognized but late sign of peripheral vascular insufficiency. Patients with lower-extremity ulcers resulting from atherosclerotic disease usually have a risk factor profile that includes older age, male sex, smoking, diabetes mellitus, hypertension, hypercholesterolemia, and obesity.20,23 Patients with leg ulcers and multiple atherosclerotic risk factors often have atherosclerosis in other arterial beds.57
Up to one third of patients with diabetes mellitus can have significant atherosclerotic disease without specific symptoms. The common complaints are those of neuropathic disease, which include history of numbness, paresthesias, and burning pain in the lower extremities. Patients often report previous episodes of foot ulcers and chronic skin infections.
Physical Examination
A complete examination can only be performed with the patient supine in an examination gown. The patient’s vital signs are recorded and abnormalities noted; temperature, respiratory rate, heart rate, and blood pressure in both upper extremities should be obtained. Fever may indicate the presence of an infected ulcer, and tachycardia and tachypnea may support the diagnosis of a septic foot.
A classic look, listen, and feel examination includes inspection of the skin of the extremities, palpation of all peripheral pulses, measurement of ankle-brachial indices (ABIs), assessment of extremity temperature, auscultation for bruits, and a thorough neurological examination.32
Visual inspection coupled with an accurate history can determine the presence of a chronic vascular condition (Fig. 60-3A). Color of the skin is conferred by the blood in the subpapillary layer and varies with position of the extremity, temperature of the skin, and degree of blood oxygenation (reduced hemoglobin (Hb) → blue). Also in chronic arterial insufficiency, the arterioles are maximally dilated as a compensatory response to the chronic ischemia, intensifying color changes. In acute arterial occlusion, the venules empty, leading to a chalky white appearance regardless of extremity position. Partial but inadequate perfusion from either an incomplete acute or chronic occlusion allows for pooling of blood in the venules, which may be red in the cold or blue at higher temperatures.
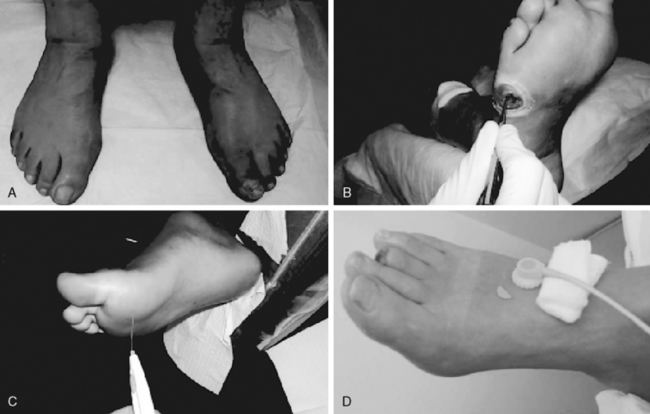
Figure 60-3 Examination of the foot.
A, Visual. B, Probing the wound. C, Using Semmes-Weinstein monofilament. D, Transcutaneous oximetry.
When the extremity is at the level of the heart, the pooled blood masks the color imparted by arterial flow. Elevation of the extremity above the level of the central venous pressure (rarely >25 cm) allows the pooled venous blood to drain, enabling an accurate assessment of the degree of arterial flow. A normal extremity remains pink, whereas one with arterial insufficiency becomes pallid. Conversely, allowing the extremity to become dependent causes an intense rubor or cyanosis. Time of return of blood to the dependent extremity is a useful marker of the severity of the deficit (normally <20 seconds). With diminished nutritional supply to the skin, there is thinning and functional loss of dermal appendages, evident as dry, shiny, and hairless skin. Nails may become brittle and ridged. Comparison of color and trophic changes between extremities gives a good indication of the severity of the process unless a bilateral deficit is present, in which case the experience of the examiner is required to make an accurate diagnosis.
Skin temperature is a reliable indicator of blood flow rate in the dermal vessels, although flow is governed primarily by constriction or dilation of the arterioles to maintain a constant core temperature. Nevertheless, the temperature of the skin as a marker of perfusion is useful and can be assessed by lightly palpating the skin with the back of the hand and comparing similar sites from one extremity to the other. An ischemic limb is cool, and demarcation of temperature gives a rough indication of the level of the occlusion. Again, assessment of temperature differences is confounded when both extremities are affected.
In limbs of patients with venous insufficiency, there is evidence of chronic edema. Venous hypertension causes transudation of serous fluid and red blood cells (RBCs) into the subcutaneous tissue. Hemoglobin from the RBCs breaks down to produce the pigment hemosiderin, leading to hyperpigmentation, especially in the medial paramalleolar areas. Patients with venous insufficiency commonly develop stasis dermatitis. This eczematic process may spread from the area of the medial malleolus and involve the leg circumferentially. Recurrent cellulitis can cause contraction of subcutaneous tissue in the lower third of the leg below the knee, and together with the chronic edema can produce a “bottle leg” appearance.
Ulcer evaluation
A thorough evaluation of ulcers of the lower extremity is critical in ascertaining etiology and instituting an appropriate treatment strategy. Specific characteristics of the ulcer, such as location, size, depth, and appearance, should be recorded during the initial evaluation and with each subsequent follow-up visit to record progress and evaluate the treatment regimen.58 Ulcers of the foot should be gently examined with a cotton-tipped probe to establish the presence of a sinus tract. The margins of the ulcer should be undermined to evaluate the extent of tissue destruction. Ulcer extension to tendon, bone, or joint should be sought. A positive probe- to-bone finding (see Fig. 60-3B) has a high predictive value for osteomyelitis and is an extremely sensitive and cost-effective screen.59
Extremity ulcerations have a characteristic appearance depending on their origin. Ulcerations due to ischemia are typically located on the tips of the toes (see Fig. 60-2B) and between the digits. These lesions often appear punched out and are painful but exhibit little bleeding. Ischemic ulcers are characterized by absence of bleeding, pain, precipitating trauma, or underlying foot deformity. They also often develop on the dorsum of the foot and over the first and fifth metatarsal heads. Ischemic ulcers are uncommon on the plantar surface because pressure is usually less sustained and perfusion better. A heel ulcer can develop from constant pressure applied while the heel is in a dependent position or during prolonged immobilization and bed rest. It should not be a surprise that a patient with relatively mild symptoms of arterial insufficiency develops limb-threatening extremity ulcers. This is because once an ulcer is present, the blood supply necessary to heal the wound is greater than that needed to maintain intact skin. A chronic ulcer will develop unless blood supply is improved.
Elevated venous pressure due to perforator or deep vein incompetency or venous thrombosis reduces the pressure gradient for perfusion. Inadequate tissue perfusion results because elevated venous pressure and venous stasis hinder clearance of breakdown products. However, venous ulcers rarely present in the foot; they are more commonly located in the “gaiter” distribution of the leg around the medial malleolus, where venous pressures are highest. These are associated with a swollen leg with a distinctive skin appearance (see Fig. 60-2A). Venous ulcerations occur most commonly on the medial aspect of the ankle and are surrounded by areas with induration and brown pigmentation of the surrounding area (brawny induration) and scaling skin. These ulcers are often exquisitely tender and weep copious serous fluid.
The appearance of the extremity in venous insufficiency is distinctive and rarely poses a problem distinguishing between it and arterial insufficiency. It is important to differentiate the rubor associated with vascular insufficiency and cellulitis accompanying an infective process. Cellulitic color changes will persist despite extremity elevation. With isolated venous insufficiency, the extremity is warm and variably swollen, with the characteristic skin changes described earlier. Acute or chronic arterial vascular insufficiency may be superimposed on the changes of chronic venous insufficiency (CVI), impairing healing of the venous ulcer. In these situations, lower-extremity revascularization may be required to assist in healing a venous ulceration that is not responding appropriately to compression therapy. Furthermore, the presence of significant lower-extremity swelling or skin changes can complicate arterial reconstructions by altering the surgical approach to distal arterial target sites.
Neuropathic ulcerations typically occur at the heel or over the metatarsal heads on the plantar surface at pressure points (mal perforans ulcer; see Fig. 60-2C) but may also occur in less characteristic locations secondary to trauma. They usually are painless. Sensory neuropathy in the diabetic patient may allow the destructive process to go unchecked, with extension into the deep plantar space and minimal appreciation by the patient.
In addition to ulcers, patients may present with varying degrees of tissue loss or frankly gangrenous digits, forefoot, or hindfoot. Presence of dry gangrene is a relatively stable process allowing for a complete vascular evaluation; however, any progression to an infected wet gangrene requires immediate surgical débridement.
Vascular examination
A careful physical examination should be performed in patients with leg ulcers to elucidate their underlying cause (see Chapter 11). Handheld Doppler ultrasound should be used in case of inability to easily palpate a given vessel. These can be supplemented with noninvasive vascular tests (see Chapter 12) and other diagnostic tests as necessary for each clinical situation. An ABI is an important tool for assessing perfusion to the foot. Patients with an ABI less than 0.6 often experience claudication, and those with an ABI less than 0.3 may complain of rest pain; in patients with tissue loss, the ABI is often less than 0.5.60 In patients with diabetes and renal failure due to calcification of the vessel, ABI may be falsely elevated and is unreliable for evaluating level of ischemia.
If the physical examination suggests venous insufficiency, a Trendelenburg test should be performed to assess valve function of the deep venous system and perforators. The patient is placed in a supine position and the legs elevated. After decompression of the superficial veins occurs, a tourniquet is placed around the patient’s thigh and he or she is asked to stand. If the varicose veins do not fill within 60 seconds below the tourniquet, the valves in the deep system and perforators are not compromised, and proximal saphenous vein incompetence is likely.
Neurological examination
The lower-extremity neurological examination is essential and should include testing for motor strength, deep-tendon reflexes, and vibratory, proprioceptive, and protective sensation.61 Chronic ischemia can cause varying patterns of sensory loss that is usually within the affected arterial distribution. Neuropathy occurs in 42% of patients with diabetes within 20 years after diagnosis of the disease27 and alters motor, sensory, and autonomic function, which directly affect the dynamic function of the foot during gait. The gait of the patient should be observed to detect any gross asymmetry or unsteadiness.
Motor neuropathy is associated with demyelinization and motor end-plate damage, which contribute to conduction defects. Atrophy of the small intrinsic muscles of the foot occurs secondary to distal motor nerve damage. Wasting of the lumbrical and interosseous muscles of the foot results in collapse of the arch and loss of stability of metatarsal-phalangeal joints during midstance of the gait.1 Overpowering by extrinsic muscles can lead to depression of the metatarsal heads, digital contractures, and cocked-up toes. These changes result in abnormal pressure points, increased shearing, and ulcer formation.
Diabetic sensory neuropathy is typically a stocking-glove distribution and is associated with a decrement in vibration and two-point discrimination. Loss of protective sensation due to peripheral neuropathy is the most common cause of ulceration in the diabetic population. The use of monofilament gauges (Semmes-Weinstein) is a good objective way of assessing diabetic neuropathy61 (see Fig. 60-3C). Patients with normal foot sensation usually can feel a 4.17 monofilament (equivalent to 1 g of linear pressure). Patients who cannot detect a 5.07 monofilament when it buckles (equivalent to 10 g of linear pressure) are considered to have lost protective sensation.62,63 Several cross-sectional studies have indicated that foot ulceration is strongly associated with elevated cutaneous pressure perception thresholds.61 Magnitudes of association, however, were provided in a case-control study64 where an unadjusted sevenfold risk of ulceration was observed in those patients (97% male) with insensitivity to the 5.07 monofilament. Screening is vital in identifying diabetic neuropathy early, thus enabling earlier intervention and management to reduce the risk of ulceration and lower-extremity amputation. Although a nerve conduction velocity test (also called nerve conduction study [NCS]) is the gold standard, its expense and limited availability prevent its widespread application as a screening tool for diabetic neuropathy. Semmes-Weinstein monofilament is a convenient, inexpensive, painless alternative to NCS that should be used in the initial evaluation of all patients with diabetes mellitus as a screen for peripheral neuropathy. A positive Semmes-Weinstein monofilament result is a significant predictor of future ulceration and likely lower-extremity amputation as well in patients with diabetes mellitus.65 If diabetic patients have positive monofilament results, their chances of ulceration increase by 10% to 20%, corresponding to a 2.5 to 5 times higher risk than patients with normal sensation as determined by monofilament. Additionally, the risks of leg amputation increase 5% to 15%, which corresponds with a 1.5 to 15 times higher risk for patients with diabetes mellitus with positive monofilament results compared to those with negative monofilament results. The Semmes-Weinstein monofilament is an important evidence-based tool for determining which patients are at increased risk of complications during follow-up, leading to improved patient selection for early intervention and management. Ultimately, screening with Semmes-Weinstein monofilament may lead to improved clinical outcomes for patients with diabetes.65
The presence of neuropathy mandates attention to the biomechanics of the foot. The role of the podiatrist or foot surgeon in evaluating these patients cannot be underscored enough.2 Use of a computerized gait analysis system to assess abnormally high-pressure areas has led to greater use of orthotic devices to prevent skin breakdown. For example, an F-scan system uses an ultrathin Tekscan sensor (Tekscan Inc., South Boston, Mass.) consisting of 960 sensor cells (5 mm2 each). The sensor is used in a floor mat system designed to measure barefoot or stocking-foot dynamic plantar pressures, indicating those individuals with pressures of 6 kg/cm2 or greater. Abnormal mechanical forces that can result in ulcerations should be addressed with the use of offloading devices or other modalities to assist wound healing.
Particular attention should be paid to documenting a complete neurological examination on patients who have suffered from a previous stroke; much of the rationale for extremity salvage hinges on potential for rehabilitation. The remainder of the physical examination should be undertaken with attention to the presence of comorbidities that may influence the decision-making process.
Tests and Imaging Techniques
Use of nondiagnostic imaging techniques by duplex ultrasound has been covered in depth in Chapter 12. Other noninvasive imaging methods useful in the assessment of patients with leg ulcers include plain radiography, magnetic resonance imaging (MRI), and magnetic resonance angiography (MRA; see Chapter 13).66 Imaging techniques can be used to diagnose osteomyelitis and confirm the presence of bony deformities. Plain film radiography is used primarily to exclude bony lesions as a cause of a patient’s pain complaints, assess the presence of osteomyelitis beneath a ulcerated foot lesion, and assess the degree of vascular wall calcification (usually in concert with standard IV contrast angiography). Plain films of the foot are relatively inexpensive and can show soft-tissue swelling, disruption of bone cortex, and periosteal elevation. Magnetic resonance imaging can provide details of pathological anatomical features and has high sensitivity for assessment of deep space infection and presence of osteomyelitis in the diabetic foot.
Assessment of a patient with foot ulcers stemming from PAD encompasses a thorough history and physical examination with adjunctive use of the noninvasive vascular laboratory to confirm, localize, and grade lesions.60 Although multiple noninvasive and invasive methods are available to assess the peripheral vasculature, it should be obvious that not every patient requires an exhaustive battery of tests to evaluate his or her vascular status. In general, only those tests likely to provide information that alters the course of action should be performed. Differing clinical syndromes mandate the extent of peripheral vascular testing. It is imperative that flow-limiting arterial lesions be evaluated and reconstructed or bypassed if ischemic foot ulcers are to heal.
Management of Ulcers
General
Aggressive mechanical débridement, systemic antibiotic therapy, and strict non–weight-bearing are the cornerstones for effective wound care.67 Sharp débridement in the operating room or at the bedside, when applicable, allows for thorough removal of all necrotic material and optimizes the wound environment.68 All necrotic bone plus a small portion of the uninvolved bone, soft tissue, and devascularized structures should be excised, and the degree of penetration of the infection should be established.68 Curettage of any exposed or remaining cartilage is important to prevent this avascular structure from becoming a nidus of infection. Foot soaks, whirlpool therapy, or enzymatic débridement provide modest benefit but are rarely effective and may lead to further skin maceration or wound breakdown. No prospective randomized studies have demonstrated the superiority of dressing products compared with standard saline wet-to-dry sterile gauze in establishing a granulation bed. Use of moist dressings in clean granulating wounds is recommended to enhance the wound environment.21,69 An ideal dressing not only provides protection against further bacterial contamination but also maintains moisture balance, optimizes wound pH, absorbs fibrinous fluids, and reduces local pain. Various dressings are currently available to target specific characteristics of the wound70; however, moist normal saline dressings are probably sufficient for most wounds. These inexpensive dressings are highly absorptive of exudative drainage and maintain the moist environment.
In the presence of infection and cellulitis, oral antimicrobial therapy should be instituted on the basis of the suspected pathogen and clinical findings. Severe infections should be treated with broad-spectrum IV antibiotics,71 with particular emphasis on the role of biofilms.49 After bacterial contamination has been controlled, small ulcers can usually be excised and closed immediately. Large open wounds, however, are treated with a staged approach with frequent débridement and establishment of a granulation base. The clean wounds can then be closed with healthy tissue, with the use of local or free-flap coverage and soft-tissue repair. Meticulous surgical reconstruction of these wounds can help avert the production of inelastic scar tissue over weight-bearing surfaces. Any remaining extrinsic or intrinsic pressures can be reduced with the postoperative use of orthoses.
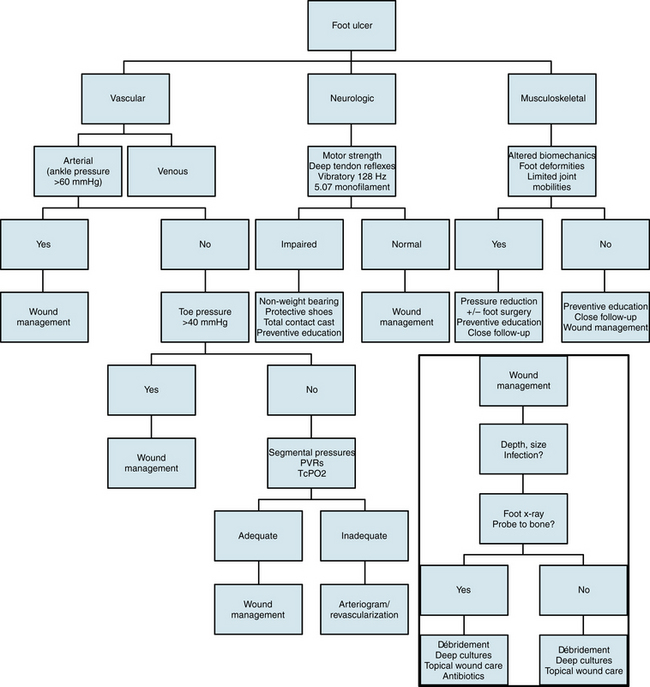
Surgical correction of biomechanical defects, plastic and soft-tissue reconstruction, and appropriate measures to minimize foot pressure are all essential to enable the patient to walk effectively again. In cases of gross wound infections and rampant cellulitis, use of a silver-containing medication such as Silvadene may be necessary in the initial setting to reduce bacterial load. Oral antimicrobial therapy should be instituted on the basis of the suspected pathogen and clinical findings. Intravenous antimicrobials should be administered for severe infections. Use of bioactive drugs (e.g., recombinant platelet-derived growth factor [PDGF], Regranex) or skin substitutes (e.g., Apligraf, Dermagraft) show promising results and have proven useful under specific circumstances. Likewise, the use of negative pressure wound therapy has been a big advance in the care of advanced wounds.72–74 A clinical practice algorithm for foot ulcers75 is seen in Figure 60-4.
Off-loading strategies such as total contact casting or removable walkers has resulted in significant decreases in healing times.76,77 Stresses placed on the foot can be intrinsic, as was previously described with respect to digital contractures, or extrinsic in nature. These external forces can result from inappropriate footwear, traumatic injury, or foreign bodies. Shoes that are too tight or too shallow are a frequent yet preventable component to development of neuropathic ulcers. Various shoe modifications such as the rocker-sole design and different types of insoles have made it possible to reduce plantar foot pressures, thus decreasing the risks of ulceration.78–80
Venous Ulcers
Elevation of the leg is a simple maneuver that can effectively (but temporarily) eliminate venous hypertension. All patients should be encouraged to elevate the affected leg above the level of the heart for 2 to 3 hours during the day and when lying in bed at night. Compression therapy is also effective in controlling edema and accelerates healing of ulcerations. However, before compression is applied to the limb, significant occlusive arterial disease should be excluded. Compression therapy is generally contraindicated in patients with an ABI less than 0.7 or with other signs and symptoms of compromised blood supply to the leg. Many different types of compression devices are available, including elastic and nonelastic bandages, graduated compression stockings (GCS), and compression pumps. The most effective way of delivering compression must be decided on an individual basis. Compression should be applied just before arising from bed and removed at bedtime.
Treatment of stasis dermatitis minimizes further trauma to the skin from scratching. Pruritus can be controlled by topically applied corticosteroids, orally administered antihistamines, or both. The goal of local wound care in patients with venous ulcers is to minimize stasis, decrease bacterial contamination of the ulcer, and provide a healthy moist wound environment that promotes healing. Heavily contaminated venous ulcers with surrounding cellulitis may require systemic antibiotic therapy in addition to local wound control. The predominant organisms cultured from chronic ulcers are gram-positive pathogens like S. aureus and Streptococcus pyogenes. The most common gram-negative bacteria are Pseudomonas aeruginosa, especially in the diabetic population. Various moisture retentive dressings can be used in conjunction with compression therapy to relieve pain, débride necrotic tissue, and promote granulation tissue formation.
The goal of surgical treatment in venous insufficiency is to correct underlying pathology. Surgical intervention can result in healing up to 90% of ulcers and modest long-term results if diagnostic studies can adequately characterize and localize the incompetent superficial or perforating system valves.81 Ulcer recurrence is significantly less after superficial venous surgery and use of compression stockings when compared with compression therapy alone.82,83 If reflux exists in the deep venous system, ligation and stripping of the superficial veins has a poor result and high ulcer recurrence rate. For patients who are young and understand the importance of long-term compression therapy and adjunctive antiplatelet or anticoagulant therapy, reconstruction of vein valves can be recommended.84,85
Ischemic Ulcers
Management of ischemic ulcers follows some basic guiding principles. It is imperative that flow-limiting arterial lesions be evaluated and reconstructed or bypassed.86 In general, the optimal strategy is to perform revascularization, if indicated, as soon as possible. Closure of the ulcer by primary healing or secondary reconstructive surgery will then be expedited. If revascularization of an ischemic ulcer is not possible for medical or technical reasons, amputation of the foot or limb will most likely result. Contraindications to revascularization include nonambulatory patients and a foot phlegmon with sepsis or excessive foot gangrene, precluding a functional foot despite adjunctive plastic surgical procedures such as skin grafts and free flaps.
Nonoperative management of patients with lower-extremity ischemia consists of general wound care measures. As a rule, however, severe ischemia of the lower limb generally requires an interventional approach. The method of revascularization of the affected limb depends on several factors, among the most important being the indications for surgery, the patient’s operative risk, arteriographic findings, and available graft material. Chapter 21 reviews these important issues.
Diabetic Ulcers
The role of a multidisciplinary group of consultants in the management of diabetic ulcers cannot be overemphasized.87 Successful management of foot ulcers involves recognition and correction of the underlying etiology, as well as appropriate wound care and prevention of recurrence. Assessment of the ulcer consists of determining the size and depth of the wound and inspection of the surrounding area for local signs of infection or gangrene. Several classification systems have been devised for descriptive purposes and act as prognostic indicators.88
The absence of systemic manifestations such as fever, chills, or leukocytosis is an unreliable indicator of underlying infection, especially in the diabetic immunocompromised population. The use of plain films to rule out osteomyelitis or deep culture of the wound is frequently necessary.
Neuropathic and Musculoskeletal Management
To avoid major amputations in these chronic neuropathic wounds, reconstructive foot surgery may often become the conservative treatment. The endpoint for chronic diabetic foot wounds should include reduction in the number of major amputations, prevention of infection, decreased probability of ulceration, maintenance of skin integrity, and improvement of function. Successful outcomes for diabetic foot reconstruction should result in less intrinsic pressures via minor amputations, arthroplasties, osteotomies, condylectomies, exostosectomies, tendon procedures, and joint arthrodesis.87 Open wounds can be treated in one stage and are primarily closed with premorbid tissue using local flap reconstruction and soft-tissue repair.89 Plastic surgical repair of these wounds can help avoid production of inelastic scar tissue over weight-bearing surfaces.90 Extrinsic and intrinsic pressures can be further neutralized with postoperative accommodative shoe gear.76,77 Prophylactic diabetic foot surgery is an increasingly used option to prevent recurrent ulceration and reduce the risk of major amputations.91,92 Surgical biomechanics, plastic and soft-tissue reconstruction, and appropriate offloading are all essential to creating a stable platform from which to keep these difficult patients free from tissue breakdown and as functional as possible.
Treatment of pedal soft-tissue deficits in the diabetic patient population continues to be a medical and surgical challenge that extends the length of the patient’s disability and significantly increases the cost of medical care. Simple closure of these wounds is often difficult because of preexisting bone deformity, tissue inelasticity, location of the defect, and superimposed osteomyelitis. Clinical pathways related to diabetic foot ulcers frequently involve persistent sharp débridement, expensive wound care products, long-term IV antibiotics, total contact casting, total contact casting with Achilles tendon lengthening, use of skin equivalents, electrical stimulation, multiple offloading orthopedic devices, and even amputation.
Wounds are often allowed to granulate, contract, and heal by secondary intention. Use of negative pressure wound therapy has increased the armamentarium of wound specialists and has significantly improved outcomes.74,87 When these wounds occur on the plantar aspect of the foot, they frequently recur, since the resulting scar has decreased extensibility and mobility. Attempted primary wound closure of diabetic pedal defects is frequently unsuccessful and may be a sequela of inadequate wound assessment, lack of proper evaluation of comorbidities, and an inadequate treatment plan.93 Reconstructive surgery has traditionally been performed on selected patients with severe deformities that cannot be accommodated by custom footwear. Some authors have stressed the importance of addressing the underlying bony pathology in treating diabetic foot problems and have dispelled the unfounded fear of performing surgery on diabetic feet.89,91,92
Reconstructive surgery can range from simple metatarsal head resections to subtotal calcanectomies. Local flaps that are often difficult to elevate and inset are more easily mobilized and incised when concomitant bone resection is achieved at the time of flap creation. In addition, a local flap results in greater exposure and direct visualization of the underlying osseous structures compared with a single linear or semielliptical incision. Implementation of local random flaps can eliminate the need for additional incisions often deemed necessary to gain access to a forefoot, midfoot, or rearfoot bony defect. Use of negative pressure wound therapy has greatly enabled salvage of these complex limb wounds.74,87
Summary
Chronic leg ulcers are frequently encountered in clinical practice. The cost of chronic nonhealing wounds is enormous. Considerable morbidity and mortality are associated with ulcerations of the lower limbs in both diabetic and nondiabetic patients. The role of the primary care physician in evaluation, diagnosis, and management of lower-extremity wounds is critical. Careful assessment of vascular disease, evaluation and management of biomechanical and metabolic abnormalities, and aggressive treatment of any infections are required. A multidisciplinary approach provides a comprehensive treatment protocol and significantly increases the chances of successfully healing the ulcer and preventing recurrence.
Acknowledgment
Bauer E. Sumpio is supported by grants from the National Institutes of Health (R01-HL47345) and the Veterans Administration (Merit Review). This work was supported in part by an unrestricted grant from the North American Foundation for Limb Preservation.
1 Sumpio B.E. Foot ulcers. N Engl J Med. 2000;343:787–793.
2 Sumpio B.E., Armstrong D.G., Lavery L.A., et al. The role of interdisciplinary team approach in the management of the diabetic foot: a joint statement from the Society for Vascular Surgery and the American Podiatric Medical Association. J Vasc Surg. 2010;51:1504–1506.
3 Sumpio B.E., Aruny J., Blume P.A. The multidisciplinary approach to limb salvage. Acta Chir Belg. 2004;104:647–653.
4 Mekkes J.R., Loots M.A., Van Der Wal A.C., et al. Causes, investigation and treatment of leg ulceration. Br J Dermatol. 2003;148:388–401.
5 Beauregard S, G.B. A survey of skin problems and skin care regiments in the elderly. Arch Dermatol. 1987;123:1638–1643.
6 Clement D.L. Venous ulcer reappraisal: insights from an international task force. Veines International Task Force. J Vasc Res. 1999;36(Suppl 1):42–47.
7 De Wolfe V. The prevention and management of chronic venous insufficiency. Pract Cardiol. 1980;6:187–202.
8 Phillips T.J. Chronic cutaneous ulcers: etiology and epidemiology. J Invest Dermatol. 1994;102:38S–41S.
9 Ellison D.A., Hayes L., Lane C., et al. Evaluating the cost and efficacy of leg ulcer care provided in two large UK health authorities. J Wound Care. 2002;11:47–51.
10 Browse N.L. The etiology of venous ulceration. World J Surg. 1986;10:938–943.
11 Goldman M., Fronek A. The Alexander House Group: consensus paper on venous leg ulcers. J Dermatol Surg Oncol. 1992;18:592.
12 Phillips T., Stanton B., Provan A. A study of the impact of leg ulcers on quality of life: financial, social, and psychological implications. J Am Acad Dermatol. 1994;31:49–53.
13 Hutton W., Stokes I. The mechanics of the foot. In: Klenerman L., ed. The foot and its disorders. Oxford: Blackwell Scientific Publications; 1991:11.
14 Murray H., Boulton A. The pathophysiology of diabetic foot ulceration. Clin Podiatr Med Surg. 1995;12:1.
15 Phillips T.J. Leg ulcer management. Dermatol Nurs. 1996;8:333–340. quiz 41–42
16 O’Donnell T.F.Jr, Browse N.L., Burnand K.G., et al. The socioeconomic effects of an iliofemoral venous thrombosis. J Surg Res. 1977;22:483–488.
17 Falanga V., Eaglstein W.H. The “trap” hypothesis of venous ulceration. Lancet. 1993;341:1006–1008.
18 Burnand K.G., Clemenson G., Whimpster I., et al. Proceedings: extravascular fibrin deposition in response to venous hypertension-the cause of venous ulcers. Br J Surg. 1976;63:660–661.
19 Dodd H.J., Gaylarde P.M., Sarkany I. Skin oxygen tension in venous insufficiency of the lower leg. J R Soc Med. 1985;78:373–376.
20 Sumpio B., Pradhan S. Atherosclerosis: biological and surgical considerations. In: Ascher E., Hollier L., Strandness D.E.Jr. Haimovici’s vascular surgery. Malden, Mass: Blackwell Science; 2004:137.
21 Singer A.J., Clark R.A. Cutaneous wound healing. N Engl J Med. 1999;341:738–746.
22 American Diabetes Association. Preventive foot care in people with diabetes [position statement]. Diabetes Care. 2003;26(Suppl 1):78.
23 Knox R.C., Dutch W., Blume P., et al. Diabetic foot disease. Int J Angiol. 2000;9:1–6.
24 Reiber G.E., Lipsky B.A., Gibbons G.W. The burden of diabetic foot ulcers. Am J Surg. 1998;176(2A Suppl):5S–10S.
25 Boulton A.J. The diabetic foot: a global view. Diabetes Metab Res Rev. 2000;16(Suppl 1):S2–S5.
26 Weiss J.S., Sumpio B.E. Review of prevalence and outcome of vascular disease in patients with diabetes mellitus. Eur J Vasc Endovasc Surg. 2006;31:143–150.
27 O’Brien I.A., Corrall R.J. Epidemiology of diabetes and its complications. N Engl J Med. 1988;318:1619–1620.
28 Kamal K., Powell R.J., Sumpio B.E. The pathobiology of diabetes mellitus: implications for surgeons. J Am Coll Surg. 1996;183:271–289.
29 Laing P. The development and complications of diabetic foot ulcers. Am J Surg. 1998;176(2A Suppl):11S. 9S
30 Lee L., Blume P.A., Sumpio B. Charcot joint disease in diabetes mellitus. Ann Vasc Surg. 2003;17(5):571–580.
31 Veves A., Fernando D., Walewski P., et al. A study of plantar pressures in a diabetic clinic population. Foot. 1991;2:89.
32 Boulton A.J., Armstrong D.G., Albert S.F., et al. Comprehensive foot examination and risk assessment: a report of the task force of the foot care interest group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care. 2008;31:1679–1685.
33 Masson E., Hay E., Stockley I., et al. Abnormal foot pressures alone may not cause ulceration. Diabet Med. 1989;6:426–428.
34 Levin M.E. Diabetes and peripheral neuropathy. Diabetes Care. 1998;21:1.
35 Boulton A.J., Hardisty C.A., Betts R.P., et al. Dynamic foot pressure and other studies as diagnostic and management aids in diabetic neuropathy. Diabetes Care. 1983;6:26–33.
36 Fernando D.J., Masson E.A., Veves A., et al. Relationship of limited joint mobility to abnormal foot pressures and diabetic foot ulceration. Diabetes Care. 1991;14:8–11.
37 Morag E., Pammer S., Boulton A., et al. Structural and functional aspects of the diabetic foot. Clin Biomech (Bristol, Avon). 1997;12:S9–S10.
38 Saltzman C., Pedowitz W. Diabetic foot infection. AAOS Instructional Course Lectures. 1999;48:317–323.
39 Habershaw G., Chzran J. Management of diabetic foot problems. Biomechanical considerations of the diabetic foot. Philadelphia: WB Saunders. 1995:53–65.
40 Bullock G., Stavosky J. Surgical wound management of the diabetic foot. Surg Technol Int. 1997;6:301–310.
41 Bild D.E., Selby J.V., Sinnock P., et al. Lower-extremity amputation in people with diabetes. Epidemiology and prevention. Diabetes Care. 1989;12:24–31.
42 Kannel W.B., McGee D.L. Diabetes and cardiovascular disease. The Framingham study. JAMA. 1979;241:2035–2038.
43 Melton L.J.3rd, Macken K.M., Palumbo P.J., et al. Incidence and prevalence of clinical peripheral vascular disease in a population-based cohort of diabetic patients. Diabetes Care. 1980;3:650–654.
44 Dang C.N., Prasad Y.D., Boulton A.J., et al. Methicillin-resistant Staphylococcus aureus in the diabetic foot clinic: a worsening problem. Diabet Med. 2003;20:159–161.
45 Hostetter M.K. Handicaps to host defense. Effects of hyperglycemia on C3 and Candida albicans. Diabetes. 1990;39(3):271–275.
46 Hostetter M.K., Krueger R.A., Schmeling D.J. The biochemistry of opsonization: central role of the reactive thiol ester of the third component of complement. J Infect Dis. 1984;150:653–661.
47 MacRury S.M., Gemmell C.G., Paterson K.R., et al. Changes in phagocytic function with glycaemic control in diabetic patients. J Clin Pathol. 1989;42:1143–1147.
48 Caballero E., Frykberg R.G. Diabetic foot infections. J Foot Ankle Surg. 1998;37:248–255.
49 Davis S.C., Martinez L., Kirsner R. The diabetic foot: the importance of biofilms and wound bed preparation. Curr Diab Rep. 2006;6:439–445.
50 Louie T.J., Bartlett J.G., Tally F.P., et al. Aerobic and anaerobic bacteria in diabetic foot ulcers. Ann Intern Med. 1976;85:461–463.
51 Sapico F.L., Canawati H.N., Witte J.L., et al. Quantitative aerobic and anaerobic bacteriology of infected diabetic feet. J Clin Microbiol. 1980;12:413–420.
52 Sapico F.L., Witte J.L., Canawati H.N., et al. The infected foot of the diabetic patient: quantitative microbiology and analysis of clinical features. Rev Infect Dis. 1984;6(Suppl 1):S171–S176.
53 Wheat L.J., Allen S.D., Henry M., et al. Diabetic foot infections. Bacteriologic analysis. Arch Intern Med. 1986;146:1935–1940.
54 Day M.R., Armstrong D.G. Factors associated with methicillin resistance in diabetic foot infections. J Foot Ankle Surg. 1997;36:322–325. discussion 31
55 Tentolouris N., Jude E.B., Smirnof I., et al. Methicillin-resistant Staphylococcus aureus: an increasing problem in a diabetic foot clinic. Diabet Med. 1999;16:767–771.
56 Adam D.J., Naik J., Hartshorne T., et al. The diagnosis and management of 689 chronic leg ulcers in a single-visit assessment clinic. Eur J Vasc Endovasc Surg. 2003;25:462–468.
57 Weitz J.I., Byrne J., Clagett G.P., et al. Diagnosis and treatment of chronic arterial insufficiency of the lower extremities: a critical review. Circulation. 1996;94:3026–3049.
58 Pressley Z., Foster J., Kolm P., et al. Digital image analysis: a reliable tool in the quantitative evaluation of cutaneous lesions and beyond. Arch Dermatol. 2007;143:1331–1333.
59 Grayson M.L., Gibbons G.W., Balogh K., et al. Probing to bone in infected pedal ulcers. A clinical sign of underlying osteomyelitis in diabetic patients. JAMA. 1995;273:721–723.
60 Collins K.A., Sumpio B.E. Vascular assessment. Clin Podiatr Med Surg. 2000;17:171–191.
61 Feng Y., Schlosser F.J., Sumpio B.E. The Semmes-Weinstein monofilament examination as a screening tool for diabetic peripheral neuropathy. J Vasc Surg. 2009;50:675–682. 682 e1
62 Armstrong D.G., Lavery L.A. Diabetic foot ulcers: prevention, diagnosis and classification. Am Fam Physician. 1998;57(1325–1332):37–38.
63 Birke J., Sims D. Plantar sensory threshold in the ulcerative foot. Leprosy Rev. 1986;57:261.
64 McNeely M., Boyko E., Ahroni J., et al. The independent contributions of diabetic neuropathy and vasculopathy in foot ulceration: how great are the risks? Diabetes Care. 1995;18:216–219.
65 Feng Y., Schlosser F.J., Sumpio B.E. The Semmes Weinstein monofilament examination is a significant predictor of the risk of foot ulceration and amputation in patients with diabetes mellitus. J Vasc Surg. 2010;53:220–226. e1–5
66 Sumpio B.E., Lee T., Blume P.A. Vascular evaluation and arterial reconstruction of the diabetic foot. Clin Podiatr Med Surg. 2003;20:689–708.
67 Steed D.L., Donohoe D., Webster M.W., et al. Effect of extensive debridement and treatment on the healing of diabetic foot ulcers. Diabetic Ulcer Study Group. J Am Coll Surg. 1996;183:61–64.
68 Granick M., Boykin J., Gamelli R., et al. Toward a common language: surgical wound bed preparation and debridement. Wound Repair Regen. 2006;14(Suppl 1):S1–S10.
69 Bergstrom N., Bennett M., Carlson C. Treatment of pressure ulcers. Clinical practice guidelines, no. 15. (AHCPR publication no. 95-0652.). 1994. Rockville, Md., 1994, Agency for Health Care Policy and Research, pp 1-102.
70 Bello Y.M., Phillips T.J. Recent advances in wound healing. JAMA. 2000;283:716–718.
71 Joshi N., Caputo G.M., Weitekamp M.R., et al. Infections in patients with diabetes mellitus. N Engl J Med. 1999;341:1906–1912.
72 Orgill D.P., Manders E.K., Sumpio B.E., et al. The mechanisms of action of vacuum assisted closure: more to learn. Surgery. 2009;146:40–51.
73 Sumpio B.E., Allie D.E., Horvath K.A., et al. Role of negative pressure wound therapy in treating peripheral vascular graft infections. Vascular. 2008;16:194–200.
74 Blume P.A., Walters J., Payne W., et al. Comparison of negative pressure wound therapy using vacuum-assisted closure with advanced moist wound therapy in the treatment of diabetic foot ulcers: a multicenter randomized controlled trial. Diabetes Care. 2008;31:631–636.
75 Frykberg R.G., Armstrong D.G., Giurini J., et al. Diabetic foot disorders. A clinical practice guideline. For the American College of Foot and Ankle Surgeons and the American College of Foot and Ankle Orthopedics and Medicine. J Foot Ankle Surg. 2000;39:S1–S60.
76 Bus S.A., Valk G.D., van Deursen R.W., et al. The effectiveness of footwear and offloading interventions to prevent and heal foot ulcers and reduce plantar pressure in diabetes: a systematic review. Diabetes Metab Res Rev. 2008;24:S162–S180.
77 Bus S.A., Valk G.D., van Deursen R.W., et al. Specific guidelines on footwear and offloading. Diabetes Metab Res Rev. 2008;24:S192–S193.
78 Barrow J., Hughes J., Clark P. A study of the effect of wear on the pressure-relieving properties of foot orthosis. Foot. 1992;1:195–199.
79 Nawoczenski D., Birke J., Coleman W. Effect of rocker sole design on plantar forefoot pressures. J Am Podiatr Med Assoc. 1988;78:455–460.
80 Tang P.C., Ravji K., Key J.J., et al. Let them walk! Current prosthesis options for leg and foot amputees. J Am Coll Surg. 2008;206:548–560.
81 Bello M., Scriven M., Hartshorne T., et al. Role of superficial venous surgery in the treatment of venous ulceration. Br J Surg. 1999;86:755–759.
82 Barwell J.R., Taylor M., Deacon J., et al. Surgical correction of isolated superficial venous reflux reduces long-term recurrence rate in chronic venous leg ulcers. Eur J Vasc Endovasc Surg. 2000;20:363–368.
83 Ghauri A.S., Nyamekye I., Grabs A.J., et al. Influence of a specialised leg ulcer service and venous surgery on the outcome of venous leg ulcers. Eur J Vasc Endovasc Surg. 1998;16:238–244.
84 Eriksson I. Reconstruction of deep venous valves of the lower extremity. Surg Annu. 1992;24(Pt 2):211–229.
85 Plagnol P., Ciostek P., Grimaud J.P., et al. Autogenous valve reconstruction technique for post-thrombotic reflux. Ann Vasc Surg. 1999;13:339–342.
86 Sarage A., Yui W., Blume P., et al. Aggressive revascularization options using cryoplasty in patients with lower extremity vascular disease. In: Geroulakos G., ed. Re-do vascular surgery. London: Springer Verlag; 2009:79–84.
87 Sumpio B., Driver V., Gibbons G., et al. A Multidisciplinary approach to limb preservation- the role of VAC Therapy. Wounds Oct Suppl. 2009:1–19.
88 Frangos S., Kilaru S., Blume P., et al. Classification of diabetic foot ulcers: improving communication. Int J Angiol. 2002;11:158–164.
89 Blume P., Partagas L., Attinger C., et al. Single stage surgical treatment of noninfected diabetic foot ulcers. J Plastic Reconstr Surg. 2002;109:601–609.
90 Blume P., Salonga C., Garbalosa J., et al. Predictors for the healing of transmetatarsal amputations: retrospective study of 91 amputations. Vascular. 2007;15:126–133.
91 Armstrong D., Lavery L., Stern S., et al. Is prophylactic diabetic foot surgery dangerous? J Foot Ankle Surg. 1996;35:585–589.
92 Catanzariti A., Blitch E., Karlock L. Elective foot and ankle surgery in the diabetic patient. J Foot Ankle Surg. 1995;34:23–41.
93 Blume P.A., Key J.J., Thakor P., et al. Retrospective evaluation of clinical outcomes in subjects with split-thickness skin graft: comparing V.A.C. therapy and conventional therapy in foot and ankle reconstructive surgeries. Int Wound J. 2010;7:480–487.