Chapter 21 Reconstructive Surgery for Peripheral Artery Disease
The clinical manifestations and complications of atherosclerosis are the most common therapeutic challenge encountered by vascular surgeons. The tendency for lesions to develop at specific anatomical sites and follow recognizable patterns of progression was appreciated as long ago as the late 1700 s by the extraordinary British anatomist and surgeon John Hunter. Considered one of the forefathers of vascular surgery, his dissections of atherosclerotic aortic bifurcations remain on view at the Hunterian museum in London and presage the disease process Leriche would give a name to 150 years later.1
The modern era of surgical reconstruction for complex atherosclerotic occlusive disease began in earnest in 1947 when the Portuguese surgeon J. Cid dos Santos successfully endarterectomized a heavily diseased common femoral artery (CFA).2 Four years later in San Francisco, Wylie et al. extended this new technique to the aortoiliac level.3 At the same time, and building on the pioneering work of Alexis Carrel,4 Kunlin5 would report the first long-segment vein bypass in the lower extremity. It would be another 10 years before synthetic grafts were being regularly used for aortic bypass grafting and the first efforts to extend vein grafting to the tibial level were described by McCaughan.6 Tremendous advances in our understanding of atherosclerosis biology and ability to percutaneously treat arterial occlusive disease have dramatically affected treatment algorithms for arterial insufficiency in recent years. This chapter will review the current role for surgical management of aortoiliac and infrainguinal arterial occlusive disease.
Aortoiliac Occlusive Disease
Chronic obliterative atherosclerosis of the distal aorta and iliac arteries commonly manifests as symptomatic arterial insufficiency of the lower extremities. Disease in this location is seen often in combination with occlusive disease of the femoropopliteal arteries, producing a range of symptoms from mild claudication to more severe levels of tissue loss and critical ischemia. Patients with hemodynamic impairment limited to the aortoiliac system may have intermittent claudication of the calf muscles alone or involvement of the thigh, hip, and/or buttocks. If disease distribution also targets the hypogastric vessels, patients may additionally suffer from difficulty in achieving and maintaining an erection due to inadequate perfusion of the internal pudendal arteries. The equivalent impact of impaired pelvic perfusion in women remains poorly understood but has attracted investigative attention.7 A well-characterized constellation of symptoms and signs known as Leriche’s syndrome, associated with aortoiliac occlusive disease in men, includes thigh, hip, or buttock claudication, leg muscle atrophy, impotence, and reduced femoral pulses.8
Although atherosclerotic disease limited to the aortoiliac region commonly gives rise to claudication of varying degrees, it is rarely associated with lower-extremity ischemic rest pain or ischemic tissue loss. This is largely the result of adequate collateralization around the point of obstruction via lumbar, sacral, and circumflex iliac vessels that serves to reconstitute the infrainguinal system with enough well-perfused arterial blood to ensure sufficient resting tissue perfusion (Fig. 21-1). A well-recognized exception to this general observation arises in the situation of embolic disease. Blue toe syndrome represents a situation where atherosclerotic debris breaks free from an aortic or iliac plaque and embolizes to distal vessels.9,10 Wire manipulation during coronary or peripheral angiographic procedures and cross-clamping across a calcific aortic plaque during cardiac surgery are common sources of such emboli. The terminal target of the microembolic particles, be they cholesterol crystals, calcified plaque, thrombus, or platelet aggregates, is typically the small vessels of the toes.
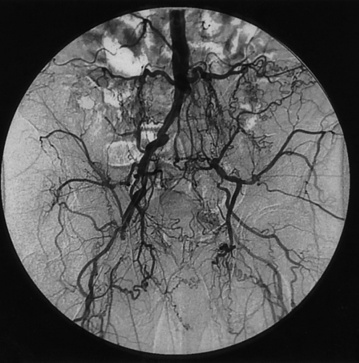
Figure 21-1 Aortoiliac occlusive disease results in a variable degree of collateralization.
Here, left hypogastric artery is reconstituted via prominent distal lumbar collaterals and right hypogastric artery. Hypogastric collaterals are in turn perfusing femoral circumflex vessels.
If aortoiliac occlusive disease is found in combination with femoropopliteal occlusive disease, ischemic rest pain or even more severe perfusion impairment leading to ischemic tissue loss or gangrene is not uncommon.11 Such progressive disease affecting multiple levels of the peripheral vasculature tree is most frequently encountered in the elderly. Approximately a third of patients operated on for symptomatic aortoiliac occlusive disease have orificial profunda femoris occlusive disease, and more than 40% have superficial femoral artery (SFA) occlusions. Aortoiliac disease typically begins at the distal aorta and common iliac artery (CIA) origins, and slowly progresses proximally and distally over time.12 This progression is quite variable but may ultimately extend to the level of the renal arteries or result in total aortic occlusion.
A particularly virulent form of atherosclerotic arterial disease is often found in young women smokers.13 Radiographic imaging in this subset of patients typically reveals atretic narrowed vasculature with diffusely calcific atherosclerotic changes. Frequently, a focal stenosis is found posteriorly near the aortic bifurcation. This particular distribution of disease and the characteristic patient profile have been referred to as small aorta syndrome14 (Fig. 21-2). Such patients invariably have an extensive smoking history, with or without other typical factors for atherosclerosis. Given the diminutive size of the aorta and iliac vessels, the durability of endovascular intervention is generally inferior in these patients, particularly in the face of continued cigarette use.
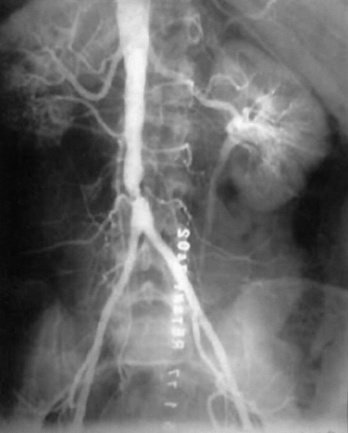
Figure 21-2 Aortoiliac occlusive disease may consist of a short-segment stenosis localized to distal aorta, a lesion particularly common in young female smokers.
The diagnosis of aortoiliac occlusive disease is generally made based on patient symptomatology, physical examination, and noninvasive tests such as segmental pressure measurements and pulse volume recordings (PVRs) (see Chapters 11 and 12). Following diagnosis of aortoiliac disease and the decision to pursue intervention, further imaging is warranted. In many centers, magnetic resonance angiography (MRA) and computed tomographic angiography (CTA) have supplanted contrast angiography as the initial imaging studies of choice. Advances have solved many of the technical limitations of earlier studies, and reliable roadmaps to guide operative planning are now reproducibly obtainable. Both MRA and CTA allow for a comprehensive view of the vascular tree with three-dimensional (3D) reconstructions (see Chapters 13 and 14). Computed tomographic angiography has the additional benefit of evaluating vessel morphology beyond the flow lumen, and allows for appreciation of degree of vessel calcification as well as anatomical localization based on surrounding structures. Angiographic findings of CTA compare favorably to standard digital subtraction angiography (DSA).15 Should a lesion amenable to percutaneous therapy be identified on MRA or CTA, formal angiography is then pursued. In cases in which a good-quality roadmap is obtained with MRA or CTA, and the clinical situation or anatomical pattern is unfavorable to a percutaneous approach, surgery can in most instances be planned directly, obviating the need for traditional subtraction angiography.16
In the minority of cases necessitating DSA for preoperative planning, a retrograde femoral approach is typically used, whereas the transbrachial approach serves as a useful alternative in patients with particularly challenging anatomy.17 Additional lateral and oblique views of the abdominal aorta are advised if concomitant mesenteric or renal occlusive disease is present, and multiple projections of the iliac and femoral bifurcations are essential in clarifying the extent of disease in these regions (see Chapter 15). Finally, full runoff views of the lower extremities are needed to assess the presence or absence of femoropopliteal or crural disease. In ambiguous cases, pullback pressure measurements, both before and after administration of a systemic vasodilator such as papaverine or nitroglycerine, or application of a tourniquet to induce reactive hyperemia can be useful in documenting the hemodynamic significance of a particular stenotic zone.18
Management
Risk factor modification remains a cornerstone of management of aortoiliac occlusive disease (see Chapter 19). Smoking cessation, blood pressure control, and aggressive efforts at cholesterol lowering should be addressed with every patient with atherosclerotic disease. Strong evidence exists supporting the benefit of a structured walking program19 in increasing walking distance of patients with claudication. The benefit of walking outside of a structured regimen with close follow-up is more debatable.20 Medical management with cilostazol has benefit in a subset of patients and is a reasonable first-line approach to improving claudication symptoms.21
A considerable change in the management approach to claudication has taken place in recent years. Patients suffering from disabling claudication, rest pain, or ischemia-related tissue loss warrant serious consideration for arteriography and either percutaneous or surgical intervention. Previously, however, such aggressive treatment would have been considered inappropriate for claudication that was not clearly disabling. As percutaneous treatment has become increasingly safer and more effective, however, and its application spread to increasingly more arterial beds, indications for endovascular revascularization have correspondingly increased (see Chapter 20). Such a sea change in the overall management approach to aortoiliac disease has had a dramatic impact on the numbers of patients now proceeding to open surgery. Just as escalating use of renal angioplasty and stenting for renal occlusive disease has led to a considerable drop in open surgical renal artery reconstructions, the rising popularity and success of aortic and iliac balloon angioplasty and stenting as first-line therapy has noticeably reduced the volume of aortoiliac reconstructive procedures performed in this country.
When medical therapy or percutaneous treatment has proven to be inadequate or is technically inadvisable, open surgical revascularization remains indicated for those patients with aortoiliac disease and disabling claudication, ischemic rest pain, and ischemic ulceration or gangrene. Patients with nighttime foot rest pain or tissue loss usually have multisegment disease, and the decision whether to perform both supra- and infrainguinal revascularization procedures or to perform only an inflow procedure is guided by severity of the ischemia.11,22–24 In general, patients presenting with significant tissue loss or gangrene are much more likely to require simultaneous or staged inflow and outflow procedures.
The numerous surgical options available to the trained vascular surgeon allow tailoring of the approach to the particular overall and anatomical situation of each patient. Historically, reconstructive options for aortoiliac occlusive disease include aortoiliac endarterectomy, aortobifemoral bypass, and so-called extra-anatomical revascularization in the form of iliofemoral, femorofemoral, or axillofemoral grafting.
Endarterectomy
Aortic endarterectomy was commonly performed in the early era of aortoiliac reconstruction.25,26 Although it is particularly suited to localized disease limited to the distal aorta or proximal iliac arteries, it has proven to be less reliable for disease involving the entire infrarenal aorta and extending into the external iliac arteries (EIAs).27,28 The obvious benefit of endarterectomy is elimination of the need for a prosthetic graft, removing the possibility of myriad late graft-related complications. Long-term patency of limited endarterectomy is excellent and on par with bypass procedures.29 However, the number of patients suitable for this reconstructive approach is small and continues to diminish in the era of endoluminal reconstruction. Experience with endarterectomy during one’s training or early surgical career is another important factor influencing the choice of therapy offered because significant technical expertise is required, and many surgeons in the current era have limited familiarity with this approach.
Aortobifemoral Bypass
Aortobifemoral bypass remains the mainstay of operative treatment for aortoiliac occlusive disease. During the last 20 years, the procedure has supplanted both aortic endarterectomy and aortoiliac bypass procedures. In the latter case, this change was largely driven by recognition of subsequent graft failure due to progression of native iliac arterial disease.27,30 Early experience with aortobifemoral grafting in the 1970s was associated with a 5% to 8% 30-day operative mortality rate.28,29,31,32 Over recent decades, mortality rates of 1% have been reported, on par with those of elective abdominal aortic aneurysm repair.33,34
Typically, half of patients proceeding to surgery for aortoiliac occlusive disease will have significant coronary artery disease, (CAD) even more will have hypertension, and almost 80% will be current or earlier cigarette smokers.33 The reduced mortality and morbidity seen in recent years are in large part due to advances in management of concomitant coronary disease. Specifically, the importance and benefit of better preoperative identification of patients in need of initial coronary revascularization, awareness of the benefit of waiting an interval period following coronary stenting before proceeding with major noncoronary vascular surgery, improved perioperative pharmacological management of patients with impaired myocardium, and more focused efforts to tailor operative and postoperative fluid administration to the individual patient’s myocardial reserve are all well recognized.35,36 General advances in postoperative intensive care unit management, including pulmonary care, infection control, and blood product utilization, have further contributed to the progress seen.
Current early patency rates for aortobifemoral bypass grafting are excellent, approaching 100% in many reporting institutions. Five-year patency rates are greater than 80%,29,31–33,37 whereas 10-year rates are near 75%.29 There are multiple reasons for the improved patency. The current graft material used by most surgeons for aortoiliac reconstruction is a knitted Dacron prosthesis with enhanced hemostatic properties; it tends to have a more stable pseudointima than earlier-used woven grafts.38,39 More attention is paid to avoiding graft redundancy and ensuring a good size match between the graft and the recipient vessels. Grafts are more routinely extended beyond the iliac level to the femoral vessels, which not only improves exposure and makes for a technically easier distal anastomosis but is also associated with less graft thrombosis from unanticipated progression of atherosclerotic disease in the external iliac vessels.30 With meticulous skin preparation, close attention to draping, careful surgical technique, and judicious use of a short course of intravenous antibiotic therapy, the feared higher rate of graft infection from placing the distal dissection at the groins has not materialized.40 An exception to this general practice is recommended in certain circumstances, however. For example, patients with hostile groin creases from prior surgery or radiation therapy, or obese diabetic patients with an intertriginous rash at the inguinal crease, will all likely be better served by performing the distal anastomosis at the external iliac level if their anatomy for such is suitable.
Increased awareness of the critical role played by the deep femoral artery (DFA) in preserving long-term patency of aortobifemoral grafts29,32,41,42 has also undoubtedly contributed to the better results seen. This awareness parallels a better overall appreciation for the importance of establishing adequate outflow at the femoral level in achieving higher early and late graft patency rates and sustained symptom relief. The true impact of concomitant SFA disease is unclear from the literature. Some reports have indicated similar patency rates between those patients with and without SFA occlusion,22,23 whereas others have suggested late patency rates are reduced in this setting.40,43 What has definitely been shown is the benefit of a profundaplasty in the presence of significant superficial and profunda femoral occlusive disease.44,45 Some authors have even recommended that a profundaplasty should be carried out in every case of superficial artery occlusion, even in the absence of orificial profunda disease, arguing that a “functional” obstruction on the order of 50% stenosis is present in these patients.46 Although this position has not been universally adopted, it is now common practice to extend the hood of the distal anastomosis over the origin of the profunda femoral artery to enhance the graft outflow, especially in situations in which the SFA is occluded or severely diseased. In the presence of significant common femoral or profunda femoral origin plaque, an extensive endarterectomy and/or profundaplasty is indicated (Fig. 21-3). In these circumstances, it is preferable to close the endarterectomized recipient bed with a vein, bovine pericardial patch, or Dacron patch onto which the distal anastomosis can then be attached, rather than creating a long femoris patch with the graft limb.41
Figure 21-3 In the setting of superficial femoral artery (SFA) and orificial profunda femoral artery disease, extending common femoral arteriotomy into origin of profunda and performing a profundaplasty prior to completing distal anastomosis of aortobifemoral bypass will improve outflow and maximize graft patency.
Several technical considerations related to aortobifemoral bypass grafting are the subject of considerable and passionate debate. The first involves the manner of the proximal anastomotic creation. Advocates of an end-to-end configuration claim that it facilitates a more comprehensive thromboendarterectomy of the proximal stump and allows for a direct, more inline flow pattern with less turbulence and more favorable flow characteristics.47 Obviation of competitive flow through the excluded iliac vessels with this approach is likely more of theoretical rather than real benefit. Certainly, with concomitant aneurysmal disease or complete aortic occlusion extending up to the level of the renal arteries, end-to-end grafting is indicated. Creation of an end-to-side anastomosis can at times be technically challenging in a heavily diseased aorta partially occluded by a side-biting clamp. A lower rate of proximal suture line pseudoaneurysms and better long-term patency rates have been found in some series.48 Stapling or oversewing of the distal aorta with the end-to-end technique minimizes the immediate risk of clamp-induced emboli to the lower extremities following release of the distal clamp. Finally, those in favor of this approach claim that ability to more effectively close the retroperitoneum, particularly after resection of a short segment of the infrarenal aorta, results in lower rates of late graft infection and aortoenteric fistulae, although there is no direct evidence to support this assertion.
There are certain circumstances when an end-to-side proximal anastomotic configuration is advantageous. The most common indication involves those patients with occluded external iliac arteries, in whom interruption of forward aortic flow may result in loss of perfusion to an important hypogastric or inferior mesenteric artery (IMA) and consequent significant pelvic ischemia. Colon ischemia (1%-2%),49 or even more rarely, paraplegia secondary to cauda equina syndrome (< 1%),50 are additional complications that can be avoided by an end-to-side configuration. Although advocated by some,51 routine preservation of a patent IMA is not universally practiced.
Operative Management
The operative procedure is performed under general endotracheal anesthesia, with an epidural catheter placed for postoperative pain control. The patient is sterilely prepped and draped from the mid-chest to the mid-thighs. The femoral vessels are first exposed through bilateral longitudinal oblique incisions, thereby reducing the time in which the abdomen is open and the viscera exposed. Extent of exposure of the femoral vessels is dictated by severity of disease and level of reconstruction planned for the CFA and its bifurcation. Next, the inferior aspect of the retroperoneal tunnel through which the graft will course to reach the femoral region is begun with digital manipulation posterior to the inguinal ligament and tracking along the anterior aspect of the external iliac artery. Antibiotic-soaked sponges are then placed in the groin wounds, and attention is turned to the aortic dissection.
The proximal reconstruction is performed via a midline laparotomy. In general, aortic dissection is limited to the region between the renal arteries and the inferior mesenteric artery. This allows avoidance of extensive dissection anterior to the aortic bifurcation, where the autonomic nerve plexus regulating erection and ejaculation in men sweeps over the aorta. An intriguing recent survey indicated no significant differences in the rate of sexual dysfunction with open compared with endovascular repair of abdominal aortic aneurysms, suggesting the effects of aortic dissection in this area are perhaps less important than typically believed.52
In situations where significant aortic calcification extends up to the level of the renal arteries, it may be necessary to continue aortic dissection to the suprarenal or even supraceliac level to allow for safe proximal clamp placement. Alternatively, proximal control may be obtained by intraluminal balloon deployment. If end-to-side repair is planned, circumferential dissection of the aortic segment to be clamped is recommended; gaining control of any lumbar or accessory renal vessels encountered prior to performing the aortotomy helps avoid troublesome backbleeding. The superior aspect of the graft limb tunnels are then completed, taking care to maintain a course anterior to the common iliac vessels but posterior to the ureters. Between 5000 and 7000 units of heparin are then administered, with additional heparin given throughout the procedure to maintain the activated clotting time near the target range of 250 to 300 seconds. After allowing sufficient time for the heparin to circulate, atraumatic vascular clamps are placed above the IMA and just below the renal arteries. The distal clamp is applied first to avoid any distal embolization of plaque dislodged with placement of the proximal clamp. If an end-to-end anastomosis is planned, the aorta is transected 1 to 2 cm below the proximal clamp, and a short segment of the distal aortic cuff is excised (Fig. 21-4A). This results in better exposure of the aortic neck and a more precise proximal reconstruction, and also allows the graft to lie flat against the vertebral column rather than anteriorly oriented, facilitating later retroperitoneal coverage. If necessary, a thromboendarterectomy of the infrarenal neck is carried out at this point (Fig. 21-4B). Anastomosis is performed with a running suture of 3-0 polypropylene (Fig. 21-4C). The distal aorta is then oversewn with two layers of a running monofilament suture or stapled with a surgical stapler. If an end-to-side anastomosis is performed, an anterior longitudinal arteriotomy is carried out after placement of proximal and distal transaortic clamps. If necessary, an endarterectomy is performed and anastomosis carried out after the graft is beveled appropriately (Fig. 21-4D). If minimal plaque is present, the distal anastomosis is performed to the common femoral artery, and individual dissection of the superficial femoral and profunda femoral arteries is not necessary.
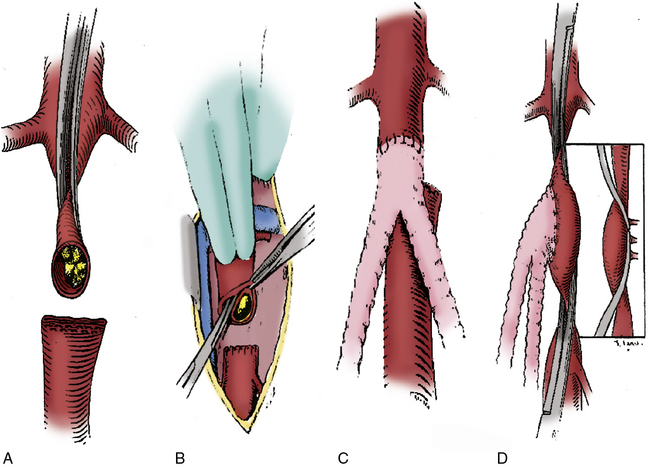
Figure 21-4 End-to-end proximal anastomosis for aortofemoral reconstruction is initiated with infrarenal aorta cross-clamp placed in anterior/posterior direction as close to origin of renal arteries as possible. Aorta is transected 1 to 2 cm below proximal clamp.
A short segment of distal aortic cuff is excised, and aorta is stapled or oversewn just proximal to origin of inferior mesenteric artery (IMA) (A). If necessary, a thromboendarterectomy of the aortic cuff is carried out (B). End-to-end configuration allows graft to lie flat against vertebral column and results in less turbulent flow (C). End-to-side configuration is required to preserve antegrade pelvic perfusion in situations where retrograde flow would be compromised due to heavily diseased or occluded external iliac arteries (EIAs) (D).
Another point of some debate concerns optimal management of patients with multilevel occlusive disease. The question frequently arises as to whether or under what circumstances a concomitant or staged outflow procedure should be performed. It is generally believed that up to 80% of patients with both inflow and outflow disease will be substantially improved following aortofemoral bypass grafting.11,22 Other reports, however, have suggested that as many as a quarter to a third of such patients will not have significant symptomatic relief with an inflow procedure alone.23 Although no single parameter exists to reliably guide the surgeon to know in which circumstances a combined procedure is optimal, severity of distal ischemia is probably the most important factor to be considered. Overall medical condition of patients and their ability to tolerate a prolonged operative procedure is also clearly important. Finally, the status of the profunda femoral artery must be taken into consideration. In the presence of SFA occlusion, a profunda that is atretic or extensively diseased may well be unable to provide sufficient collateral runoff to the foot.
If on the one hand, the bypass procedure is undertaken for claudication alone or mild rest pain, restoring adequate inflow may provide sufficient and relatively durable symptomatic relief. If on the other hand, significant tissue loss is present, a combined inflow and outflow procedure is likely warranted if limb salvage is to be achieved. If several operating teams are used, performing both procedures at the same time can be done in an acceptably timely fashion and has been found to be safe. Indeed, several recent reports found no significant differences in operative mortality or perioperative morbidity in patients undergoing concurrent inflow and outflow procedures compared with those having major inflow reconstruction alone.53,54 Although staged revascularization may be preferable in certain circumstances, both the risk of wound and graft infection resulting from redissection in the groin and the risk of progressive tissue loss during the initial recuperative period must be considered with this approach.
Results
Aortobifemoral bypass grafting is associated with patency rates that are among the highest reported for any major arterial reconstruction. As indicated earlier, 5-year primary patency rates of 70% to 88%29,31,32 and 10-year rates of 66% to 78%29 have been described. Better rates have been realized in those patients with good infrainguinal outflow operated on for claudication, compared with those with limb-threatening ischemia and associated infrainguinal occlusive disease. In general, patients with disease limited to the aortoiliac region have excellent relief of symptoms following aortobifemoral grafting, whereas those with multilevel disease have less complete levels of symptom diminution. Perioperative mortality rates average 4%, whereas 5-year survival rates between 70% and 75% have been reported.31,55,56 This latter rate is notably less than the 5-year survival rate of age-matched control population but on par with that typically seen for claudicants in general.
Although the early and late mortality rates are similar across different age groups, the 5-year primary and secondary patency rates are significantly increased with each increase in age group.33 Reed et al. reported that primary patency rates were 66%, 87%, and 96%, and secondary patency rates were 79%, 91%, and 98% (Fig. 21-5), respectively, for those younger than 50, 50 to 59, and older than 60 years of age.33 It seems prudent, based on these findings, to apply caution in the application of aortobifemoral bypass grafting for younger patients with virulent aortoiliac disease. The potential impact of graft failure and need for subsequent complex interventions should be considered, especially given the longer life expectancy of younger patients. Full utilization of all medical and endovascular options appears to be the best first-line option for younger patients with severe aortoiliac occlusive disease.
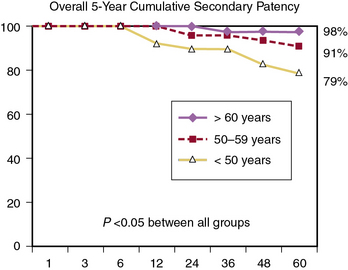
Figure 21-5 Overall 5-year cumulative secondary patency rates in a recent cohort of patients undergoing aortobifemoral bypass grafting, indicating an inverse relationship between age and graft patency.
(From Reed AB, Conte MS, Donaldson MC, et al: The impact of patient age and aortic size on the results of aortobifemoral bypass grafting, J Vasc Surg 37:1219, 2003.)
Extra-Anatomical Bypass
When comorbid disease renders a patient with aortoiliac occlusive disease particularly unsuitable for major vascular surgery and aortic cross-clamping, or when sepsis, prior surgery, or the presence of a stoma presents a hostile surgical environment for abdominal exploration, several alternatives are available to the vascular surgeon. Reconstructive options in which the thoracic aorta, axillary, iliac, or femoral arteries serve as donor vessels are generally referred to as extra-anatomical to distinguish them from the inline flow represented by an aortobifemoral procedure. The concept of extra-anatomical arterial reconstruction emerged in the 1950s during a time of many new developments in the field of vascular surgery. Freeman and Leeds provided one of the first descriptions in 1952 in their report of the use of the SFA as the conduit for a crossover femorofemoral bypass graft.57 These approaches are also called on in desperate situations represented by infection of a previously placed aortic graft.
Axillobifemoral Bypass
Axillobifemoral bypass grafting was introduced by Blaisdell58 in the early 1960s and has since enjoyed increasing popularity as an alternative to aortobifemoral bypass. This is largely due to the reliability of the axillary artery as a donor vessel and the minimal morbidity incurred, making it a particularly appealing option for patients with significant operative risk from comorbid disease. It is also appropriate in patients with significant aortoiliac occlusive disease of the distal aorta and the iliac arteries in the setting of intraabdominal sepsis, a history of multiple prior abdominal operations, intraabdominal adhesions, or prior pelvic irradiation. Of note, LoGerfo et al.59 have shown that axillobifemoral grafting has improved long-term patency compared with axillo-unifemoral grafting, presumably owing to the increased flow afforded by the second outflow limb.
Although usually performed under general anesthesia, it is possible to carry out the procedure using a combination of local anesthesia and intravenous conscious sedation. In the event that one arm has a higher blood pressure or a stronger pulse, that side should be selected as the donor site. If both sides are equal, the right axillary artery is chosen because evidence suggests there is a lower risk of arterial occlusive disease developing in the right subclavian artery compared with the left.
The axillary artery is exposed through a short infraclavicular incision parallel to the clavicle in the deltopectoral groove. The pectoralis major muscle is then bluntly separated between the clavicular and sternal heads, and the pectoralis minor muscle is identified and typically divided, enhancing exposure and allowing more space for the graft as it courses from the axilla to the subcutaneous space. The axillary artery medial to the pectoralis minor is then isolated because the proximal anastomosis is optimally placed as close to the chest as possible to minimize the risk of kinking or graft avulsion during rotational shoulder movement. Avoiding more lateral dissection further reduces the risk of injuring the medial and lateral cords of the brachial plexus as they emerge anteriorly to form the median nerve. A tunnel is created between the axillary and femoral arteries in the subcutaneous space, tracking deep to the pectoralis major muscle and inferiorly along the midaxillary line before coursing medial to the anterior superior iliac spine; this latter orientation is important to avoid kinking of the conduit in the sitting position. Long, rigid tunneling devices with a removable central obturator are specifically designed for this step and have helped lower the incidence of graft infection by obviating the need for counterincisions.
The CFAs are then dissected through standard bilateral short groin incisions, and a second subcutaneous tunnel is fashioned between them in an extrafascial suprapubic plane. A Dacron or polytetrafluoroethylene (PTFE) graft, typically 8 mm in diameter, is then drawn through the tunnel. Although there is no convincing evidence that one graft material is superior to the other, several reports support the common practice of using an externally reinforced graft.60,61 Newer grafts are available that are prefigured in an axillobifemoral configuration, thereby reducing from four to three the number of anastomoses needed. As in aortobifemoral bypass grafting, unrestricted outflow should be ensured by carrying the hood of the femoral grafts down over the profunda orifice and performing an endarterectomy or profundaplasty when necessary. If a prefigured graft is unavailable, the origin of the cross-femoral graft can be tailored to the body habitus of the patient. In most cases, the graft is taken off the distal hood of the descending axillofemoral graft. In particularly obese individuals, however, it may be preferable to move the takeoff more proximally to prevent kinking at the level of the inguinal ligament. Orienting the takeoff of the crossover graft at an acute angle to give an S-shaped final configuration has been associated with higher patency rates in some studies.62
Many of the complications following axillofemoral grafting are directly related to the graft and potentially avoidable. Disruption of the proximal anastomosis, or axillary pullout syndrome, can be minimized by proper orientation of the proximal hood and ensuring that the descending limb of the graft is free from undue tension.63 Kinking and subsequent thrombosis of the graft can be reduced by strict attention to tunnel position and use of a reinforced conduit. Given the minimal physiological insult, most patients undergoing axillofemoral grafting are ambulatory and able to tolerate a regular diet on the first postoperative day.
Reported long-term patency rates of axillofemoral grafts have varied significantly, ranging from as low as 29% to as high as 85%.60,64–66 Favorable results were reported by Passman et al.,67 who achieved 5-year patency rates of 74% and a long-term limb salvage rate of 89%, and who are vocal advocates of a wider use for this approach. In general, axillobifemoral grafting should be reserved for high-risk patients with significant tissue loss and in danger of limb loss, and not be used for treating claudication.
Femorofemoral Bypass
Femorofemoral bypass grafts are ideally suited to patients with preserved flow in both the aorta and one iliac branch, but occlusion or severe stenosis of the contralateral iliac not amenable to percutaneous treatment (Figs. 21-6 and 21-7A-B). Although possible to perform under local anesthesia in high-risk patients, it is best carried out under regional or general anesthesia. On occasion, it has been performed in an intensive care unit setting in the particular instance of a leg rendered acutely ischemic by placement of an intraaortic balloon pump. Technical details are identical to those of the crossover component of the axillobifemoral grafting discussed earlier. The suprapubic tunnel is created in a gentle C curve just superficial to the deep fascia, and can in most instances be completed by blunt finger dissection approaching from both groin incisions. Although some surgeons advocate placement of the tunnel beneath the rectus sheath, this is a minority view. Again, if warranted by the presence of significant concomitant femoral disease, an endarterectomy or profundaplasty is indicated prior to completion of the proximal or distal anastomosis.
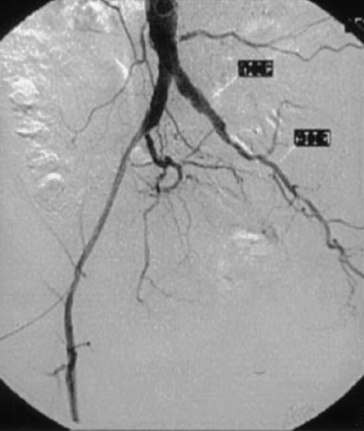
Figure 21-6 Oblique-view digital subtraction angiogram indicating a long-segment total occlusion of left external iliac artery (EIA).
Extra-anatomical left-to-right femorofemoral or iliofemoral bypass grafting would be appropriate options for this anatomical disease distribution (see also Fig. 21-7).
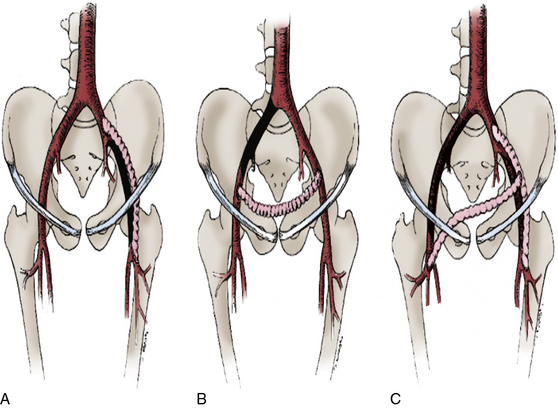
Figure 21-7 Patent common or external iliac artery (EIA) may be used as donor vessel for (A) iliofemoral, (B) ilioiliac, or (C) iliobifemoral bypass grafts depicted.
Lesions depicted in A and B would also be appropriate for femorofemoral grafts, whereas lesion in C would be appropriate for aortobifemoral or axillobifemoral grafting.
Graft failure due to progression of inflow disease following femorofemoral grafting is less problematic than one might predict. Some investigators have argued that the increased flow through the donor iliac artery following restoration of bilateral outflow, in essence shifting the aortic bifurcation to a more distal point, serves to impede further development of atherosclerotic disease. Animal studies correlating blood flow and shear stress with intimal hyperplasia lend support to this explanation.68 Maini and Mannick reported a 5-year cumulative patency rate of 80%.69 This is similar to other reports in the literature70–72 and compares favorably with the 85% rate seen with conventional aortobifemoral bypass grafting.33
With its high patency rates and low associated morbidity, cross-femoral grafting is an excellent option in patients with favorable anatomy. Given the risk of late graft failure from progression of inflow disease and the potential need to reintervene on previously dissected femoral beds should a later aortobifemoral graft be needed, however, it has traditionally been advised to proceed directly to aortobifemoral grafting in good-risk patients with any evidence of atherosclerotic disease in the aorta or patent iliac vessels. In the current era, aortic or iliac angioplasty and/or stenting in combination with cross-femoral grafting is a viable alternative in this setting, particularly for those patients at increased operative risk.
Iliofemoral Bypass
Iliofemoral grafting is another alternative to aortobifemoral grafting for a selected group of patients with hemodynamically significant disease limited to the EIA (see Fig. 21-6). Currently, most patients with this anatomical pattern of disease would typically undergo an attempt at percutaneous recanalization of a tightly stenotic or long-segment external iliac occlusion. Indeed, as the success rates with such efforts increase, the number of iliofemoral bypass grafts performed has continued to fall. However, if the percutaneous approach is unsuccessful, an iliofemoral bypass remains an excellent surgical option because it can be performed with minimal morbidity and cardiopulmonary insult and avoids the long, descending limb necessitated by an axillofemoral graft (see Fig. 21-7). Because the grafts are situated within the pelvis, they are also better protected from kinking, infection, and thrombosis than either axillofemoral or femorofemoral grafts. Less disturbance of inguinal lymph nodes and lymphatic channels typically occurs with the more limited dissection necessary.
Either the ipsilateral common iliac or proximal EIA can serve as the donor site, and if need be, a bifurcated graft can be used and taken to both femoral vessels. Alternatively, bilateral iliofemoral grafts or an ilioiliac graft can be fashioned as appropriate. Iliac exposure can be achieved through an oblique suprainguinal “transplant” incision and development of the retroperitoneal plane, which affords excellent proximal exposure even in the obese patient. Care must be taken in isolating the donor vessel and tunneling the graft to avoid injury to the ureter coursing over the iliac bifurcation. If a crossover graft is used, it can be tunneled retroperitoneally in the iliac fossa or across the properitoneum deep to the rectus sheath.
In early experience with iliac origin grafts reported by Couch et al., there were no operative deaths and a 77% 4-year patency rate.73 Nearly half of these patients were operated on for limb salvage in the face of critical ischemia. In patients undergoing revascularization with bilateral iliofemoral grafts, the 4-year patency rates were 92%, whereas an 85% patency rate was seen if both the superficial and deep femoral vessels were patent.73 Other reported series of iliofemoral bypass grafting have indicated similar patency rates.71,74
Thoracic Aorta–to–Femoral Artery Bypass
As early as 1961, Blaisdell et al. reported on a novel extra-anatomical bypass from the descending thoracic aorta to the femoral artery, followed by a femorofemoral bypass.75 Although carried out in the setting of sepsis after a ruptured aneurysm repair and not for occlusive disease, it provided a new alternative when the infrarenal aorta was inaccessible or inappropriate as a donor vessel. The procedure is performed through a thoracotomy incision, typically entering the chest through the eighth or ninth interspace. A muscle-sparing technique in which the latissimus dorsi muscle is not divided aids in postoperative pain management. The distal descending thoracic aorta is circumferentially dissected enough to allow for clamp control, with care taken to avoid injury to the adjacently positioned esophagus. A tunnel is fashioned by separating the diaphragm from the posterior chest wall over a distance of two finger breadths. In 1994, Criado and Keagy76 reviewed the literature and summarized 193 reconstructions taken off the descending thoracic aorta. Not unexpectedly, the majority were performed for thrombosis or infection of a previously placed aortic graft, although some primary procedures undertaken in the setting of a “hostile” abdomen were included. Cumulative 5-year primary and secondary patency rates of 73% and 83%, respectively, were obtained, and the operative mortality rate was 6%.76
Laparoscopic Revascularization
There is an increasing interest in applying laparoscopic techniques to the treatment of aortic occlusive disease, reflected in a small but growing body of literature of individual case series.77,78 Some surgeons have favored a more limited approach using hand-assisted techniques and smaller incisions,79 whereas others have championed the use of complete laparoscopic or robot-assisted revascularization.77,80,81 The purported benefits of shorter hospital stays, less perioperative pain, and fewer postoperative complications are balanced against longer operative times and lack of long-term data to support the durability of this alternative approach. It remains at present an extremely technically challenging procedure with a significant learning curve. As the technology advances and improvement is seen with anastomotic devices and instrumentation, the role of aortofemoral bypass will likely expand and become more defined. At present, however, it has failed to gain widespread acceptance and is routinely undertaken in only a limited number of centers.
Infrainguinal Arterial Occlusive Disease
Infrainguinal arterial occlusive disease is the most prevalent manifestation of chronic arterial occlusive disease encountered and treated by the vascular surgeon. Isolated disease of the SFA typically manifests as calf muscle claudication, whereas patients with multilevel disease involving the superficial femoral, popliteal, and tibial arteries generally have rest pain or ischemic tissue loss. The ischemia ulcerations usually begin as small, dry ulcers of the toes or heel area and progress to frankly gangrenous changes of the forefoot or heel, with greater degrees of arterial insufficiency. Several identifiable patterns of disease are recognized, with smokers typically having disease limited to the SFA and corresponding symptoms of claudication. Diabetes most often targets the popliteal and tibial vessels, and patients may present with frank tissue necrosis with no history of claudication.
Infrainguinal reconstruction for treatment of peripheral vascular occlusive disease has been increasingly successful for both long-term palliation of intermittent claudication and for salvage of limbs threatened by critical ischemia. There are times when primary amputation represents the safest and most advisable solution in the face of irreversible ischemia, particularly in cases where extensive infection or tissue necrosis is present. In addition, certain patient populations may have a combination of risk factors that may be predictive of a prohibitively poor outcome. This may include patients of advanced age or those in a dependent living situation on hemodialysis.82 Otherwise, an attempt at reconstruction is almost always indicated when a limb is threatened by severe ischemia. Improvements in perioperative management and surgical technique have allowed progressively more distal reconstructions to be successfully completed in an older, sicker, and more challenging patient population. In general, high rates of relief for claudication and up to an 80% to 90% limb salvage rate may be anticipated for patients with critical ischemia at institutions devoted to peripheral bypass surgery.
A large prospective randomized double-blinded multicenter trial, the Project of Ex Vivo Graft Engineering via Transfection III (PREVENT III), was recently conducted to evaluate the efficacy of edifoligide in preventing autogenous vein graft failure in lower extremity revascularization for critical limb ischemia (CLI).83 Although the trial failed to show any significant primary patency or limb salvage benefit of the studied medication, it did provide valuable contemporary information regarding infrainguinal bypass outcomes. In the study cohort of 1404 patients from 83 North American sites, the 30-day operative mortality rate was 2.7%. Assisted primary patency, limb salvage, and survival at 1 year were 77%, 88%, and 84%, respectively.83 A validated risk score subsequently created to allow stratification of patient risk factors in the setting of limb-threatening ischemia demonstrated that amputation-free survival was negatively associated with dialysis dependence, tissue loss, age older than 75, anemia, and coronary artery disease.84 The PREVENT III dataset additionally provided one of the first comprehensive evaluations of patient quality of life before and after surgical revascularization. Notably, patients undergoing successful surgical revascularization reported a significant quality-of-life improvement at 1 year compared to baseline levels.85
The two major indications for surgical intervention of infrainguinal arterial occlusive disease are claudication and limb-threatening critical ischemia. Claudication is a relative indication, given the natural history of the disease; of patients with claudication, only 1% per year will ultimately progress to limb loss.86,87 As such, it remains a subjective assessment on the parts of both patient and surgeon as to the relative degree of disability a given level of claudication pain represents.
Role of Percutaneous Transluminal Angioplasty
Of relevance in this regard is the significant shift in the indications for percutaneous intervention for infrainguinal occlusive disease witnessed in recent years. As the associated risks of balloon angioplasty and stenting have fallen and relative success rates have risen, the threshold for offering endovascular treatment to claudicants has decreased considerably. Patients once considered appropriate only for risk factor modification, exercise therapy, and medical treatment are now increasingly being offered percutaneous revascularization as a secondary or even primary treatment option (see Chapter 20). The relative merits of early intervention as opposed to traditional risk factor modification and exercise therapy for individuals with claudication remains controversial.88 Some authors have found no improvement in quality-of-life outcomes following percutaneous treatment for claudication over and above supervised exercise training programs and have also demonstrated endovascular therapy to be cost-ineffective for this indication.89 Others have noted an additive benefit when combining percutaneous intervention with supervised exercise programs and optimal medical management, as indicated by improved quality-of-life indices.90,91
In terms of patients with severe limb ischemia secondary to infrainguinal occlusive disease, the awaited long-term results of the Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial recently became available.92 The BASIL trial was a randomized controlled trial performed in 27 U.K. centers from 1999-2004. This seminal work was designed to compare a strategy of open surgical revascularization first to that of percutaneous angioplasty first in a population of patients with severe limb ischemia, and represents the only level-I evidence comparing these treatment modalities to date. The initial analysis published in 200593 found no difference in the primary endpoints of overall or amputation-free survival for open surgery vs. angioplasty, though it did find that surgery was more costly in the short term. The more recently published longer-term follow-up results also indicated the two study arms had equivalent amputation-free and overall survival by intention to treat analysis.94 However, for patients who survived beyond 2 years (representing 70% of the total cohort), open surgical bypass conferred improved overall survival and a trend toward improved amputation-free survival. The trialists concluded that for patients with available autologous vein and a life expectancy exceeding 2 years, the preferred method of revascularization is open bypass surgery. They further noted that when percutaneous angioplasty was employed as the primary intervention, it had a significantly negative impact on the outcome of future surgical revascularization attempts.
Occlusive disease of the tibial vessels, once thought to be the exclusive domain of operative bypass, is increasingly being treated percutaneously. The impact of these trends on the natural history of the disease, and to what extent the expanding reach of percutaneous therapy will affect subsequent operative management in a given patient, remains to be seen. Certainly, as enthusiasm for less invasive options has spread to include the infrapopliteal level, the relative roles of surgical and percutaneous intervention are being further redefined. Newer-generation atherectomy devices, drug-eluting balloon angioplasty, and flexible stents designed to withstand the unique torsional forces of the leg or with drug-eluting capability may significantly improve the patency and durability rates currently seen.95,96 Until the efficacy of infrainguinal percutaneous intervention is better defined, however, surgical revascularization remains the standard for any patient with critical limb ischemia. For patients with favorable anatomy and significant operative risk, and for treatment of claudication in general, percutaneous therapy has assumed a more primary role.
Duplex ultrasonography, MRA, and CTA are increasingly used as first-line modalities in the assessment of patients with infrainguinal occlusive disease (see Chapters 12 13, and 14). Although a growing body of literature supports use of duplex scanning as a stand-alone preoperative mapping modality,97 this requires a highly dedicated vascular laboratory and, to date, has not gained wide acceptance. Magnetic resonance angiography and CTA are particularly useful as noninvasive screening tests to determine patient suitability for percutaneous therapy. In some instances, operative planning may be based solely on such noninvasive radiographic information, but many surgeons are reluctant to undertake surgical reconstruction without the confirmation afforded by standard contrast angiography. This is particularly true if the distal target is at the tibial or pedal level, where CTA and MRA technology remains more limited.
Operative Management
Infrainguinal bypass can be performed under general anesthesia or, in the appropriate patient, under regional, spinal, or epidural anesthesia. The multiple sites of dissection and the harvesting of saphenous vein or an alternative vein conduit make these procedures particularly suited to a two-team approach. The time saved, particularly in cases involving potentially more tedious arm vein or lesser saphenous vein harvesting, has direct benefit in minimizing the total anesthetic load and physiological insult to the patient. Typically, the site proposed for the distal anastomosis is explored first to ascertain whether the preoperative imaging was accurate in predicting the suitability of the target vessel. On occasion, the operation is begun with an on-table angiogram to clarify the anatomy if preoperative imaging was deferred or ambiguous.
The above-knee popliteal vessel is easily exposed through a medial thigh incision, with subsequent posterolateral retraction of the sartorius muscle. The popliteal artery, with its accompanying vein and nerve, is found just posterior to the femur. The vessel is palpated to determine the presence of atherosclerotic plaque, which will guide the extent of dissection and the optimal bypass target site. The below-knee popliteal artery is also exposed through a medial incision in the proximal calf (Fig. 21-8). If the saphenous vein is to be harvested, the incision is made directly over the vein to minimize creation of devascularized skin flaps. With the exposed vein carefully protected, the incision is carried through the deep muscular fascia, and the medial head of the gastrocnemius is reflected posterolaterally to expose the below-knee popliteal fossa. The distal popliteal artery is then dissected free from the adjacent tibial nerve posteriorly and popliteal vein medially. If the distal target is the tibioperoneal trunk, the dissection is continued along the anteromedial surface of the distal popliteal artery after dividing the origin of the soleus muscle from the tibia (Fig. 21-9). In instances in which the below-knee popliteal artery has previously been exposed or where sepsis is involved, a lateral approach with excision of a segment of proximal fibula is a useful alternative approach to the below-knee popliteal artery.
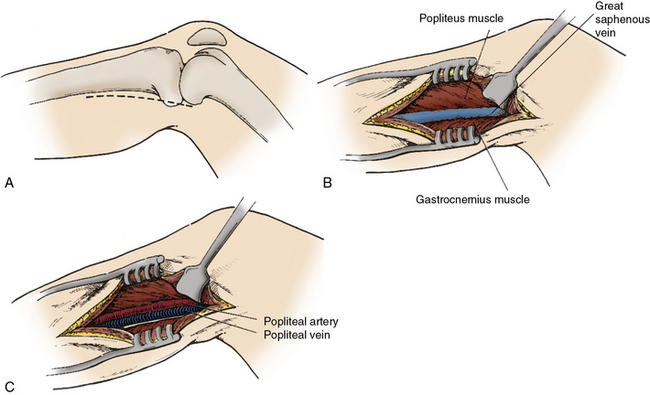
Figure 21-8 Exposure of popliteal artery below knee.
Medial incision is made (A) directly overlying course of great saphenous vein (B). After posterior reflection of the gastrocnemius muscle the tibial nerve, popliteal vein, and popliteal artery are encountered in the deep posterior compartment.
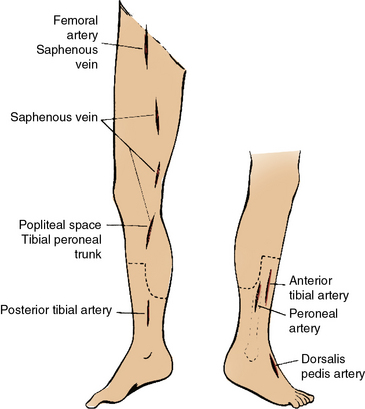
Figure 21-9 Placement of incisions for femoropopliteal and femorotibial bypass and for greater saphenous vein harvest.
These should avoid the incision lines for a below-knee amputation.
Although exposure of the proximal posterior and peroneal vessels can be gained by extending the tibioperoneal trunk dissection distally, more distal exposure of these vessels is best gained through targeted medial incisions. The posterior tibial artery is found more medially on the reflected soleus muscle, whereas the peroneal artery is deeper and more lateral. The posterior tibial artery at the level of the ankle is a relatively easier target given the proximity of the vessel to the skin surface. The initial incision is made just posterior to the medial malleolus, and the artery is exposed by division of the overlying retinaculum. Further distal dissection allows access to the bifurcation and medial and lateral plantar branches.98 The anterior tibial artery is typically approached from the anterolateral aspect of the calf (see Fig. 21-9) and is found deep within the anterior compartment with the adjacent deep peroneal nerve and anterior tibial veins. The dorsalis pedis artery is easily exposed through an axial incision on the dorsum of the foot just lateral to the extensor hallucis longus tendon (see Fig. 21-9).
Following exposure of the distal anastomotic target vessel, the site of the proximal anastomosis is dissected. For patients with SFA disease, this will most commonly be at the level of the common femoral artery. The artery is mobilized as already described, from the level of the inguinal ligament to its terminal bifurcation. The distal extent of this dissection is dictated by the presence of concomitant femoral plaque. Lymphatic tissue overlying the femoral vessels is best ligated and divided to prevent postoperative development of lymph fistulas or lymphoceles. If an extensive endarterectomy or profundaplasty is required, the proximal profunda femoral artery is dissected along its proximal length accordingly.
If all or part of the SFA is spared of significant atherosclerotic involvement, the proximal anastomosis can be moved distally as dictated by the particular anatomical pattern of disease, and a so-called distal origin graft can be fashioned99 (Fig. 21-10). This situation is particularly applicable to the diabetic population, where infrapopliteal disease is the rule, and sparing of the superficial femoral and popliteal arteries is not uncommon. It is also used in situations where conduit is sparse and a moderately diseased proximal vessel is accepted as an inflow source for a more distal origin bypass graft in the interests of performing a fully autologous vein graft rather than using prosthetic material. An increasingly popular approach when only limited conduit is available is to combine, either concurrently in the operating room or as a staged preoperative procedure, catheter-based treatment of the superficial femoral or popliteal artery inflow with more distal bypass.99
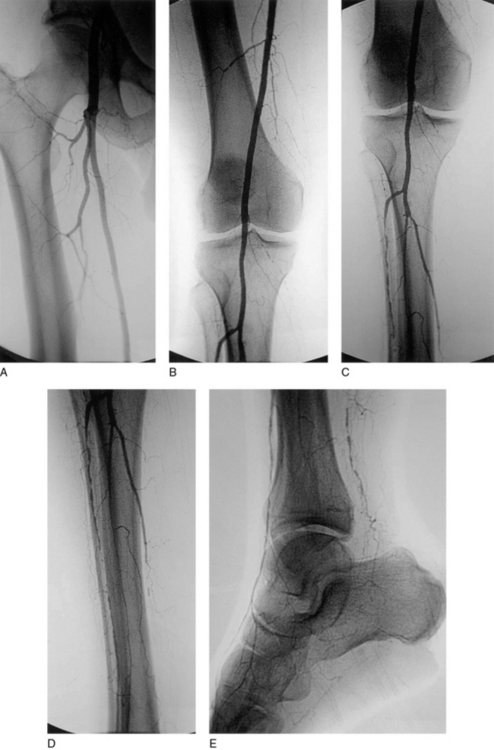
Arteriogram indicating preservation of superficial femoral artery (SFA) and popliteal arteries with mid-calf occlusions of all three infrageniculate vessels. This anatomical pattern of disease is amenable to “distal origin” vein grafting from below-knee popliteal or proximal posterior tibial artery to dorsalis pedis artery.
Autogenous Vein Bypass
In general, infrainguinal bypass surgery is best performed with autogenous vein conduit, preferably the ipsilateral greater saphenous vein if available.100 This is particularly true for grafts extending below the knee, where prosthetic conduits of Dacron or PTFE have significantly poorer patency rates. The first report of a femoropopliteal bypass graft using autogenous greater saphenous vein in a reversed orientation was by Kunlin in 1951.5 Given the orientation of the vein valves, the vein is reversed such that the distal end of the vein is sewn to the proximal inflow artery, and the larger proximal end of the vein is sewn to the distal outflow artery. The vein is harvested through a long incision overlying the course of the vein or by more tedious but less invasive sequential skip incisions with intervening cutaneous skin bridges (see Fig. 21-9). All side branches are ligated, and after harvest, the vein is cannulated and gently dilated with a solution containing heparin and papaverine to assess its suitability. Veins with chronic fibrosis or that fail to dilate to a diameter of 3 mm or greater will likely have poor long-term function.
For prosthetic grafts, a tunnel is usually fashioned through the subsartorial plane between the groin incision and the above-knee popliteal space in the interests of protecting the graft from subsequent infection. For vein conduits, it remains the surgeon’s preference as to whether the graft is tunneled deeply or in a superficial location in the subcutaneous space. The more superficial configuration greatly facilitates ongoing clinical examination and ultrasonographic surveillance as well as later surgical revision, but it carries a risk of graft exposure should there be wound-healing problems. Occlusion from trauma to grafts placed superficially has been of theoretical but not practical concern.
The order of anastomoses is surgeon dependent, with strong feelings expressed in each camp. Before occluding the target vessel, the patient is systemically anticoagulated with 5000 to 10,000 units of heparin. The artery is then clamped proximally and distally and incised, the vein spatulated, and a beveled anastomosis is carried out. Typically, a 5-0 monofilament suture of Prolene is used for the femoral anastomosis, a 6-0 suture is used at the popliteal level, and a very fine 7-0 suture is used at the tibial or pedal level. If the target tibial vessel is deep within the calf and visibility is challenging, a technique of “parachuting” the heel of the distal anastomosis is often employed. After completing the first anastomosis, the graft is carefully marked to ensure against mechanical twisting or kinking of the graft during the tunneling process. One of the benefits of performing the proximal anastomosis first is that following release of the clamps, adequacy of flow through the graft can be assessed.
Occasionally, such extensive calcification of the target vessel is encountered that the risk of a significant injury from clamping, even with the minimally traumatic clamps in use today, is prohibitively high. In such cases, proximal inflow and distal artery backbleeding can be controlled by occlusion balloons placed intraluminally. For distal anastomoses at the knee or more distal level, another alternative technique is use of a proximally placed sterile pneumatic tourniquet. This is particularly advantageous when sewing to diminutive distal tibial or pedal targets, where the impact of a crush injury or plaque dislodgment on graft function could be considerable. Removing the need for clamps by using the tourniquet has two more advantages. First, it improves operative visibility. Second, and more importantly, given that less longitudinal and circumferential dissection are needed, the degrees of vessel spasm and venous bleeding that frequently accompany vessel exposure at this level are kept to a minimum.
Flow through the graft and outflow arteries is assessed with continuous-wave Doppler ultrasound following completion of the bypass. Ideally, a contrast angiogram is also performed after directly cannulating the proximal graft (Fig. 21-11). This allows for immediate repair of any technical defects—for example, intraluminal thrombus, twisting or kinking of the graft, or retained valve cusps, that are identified101 (Fig. 21-12). Intraoperative completion duplex ultrasonography is a sensitive screen for hemodynamically significant abnormalities within the graft.102,103
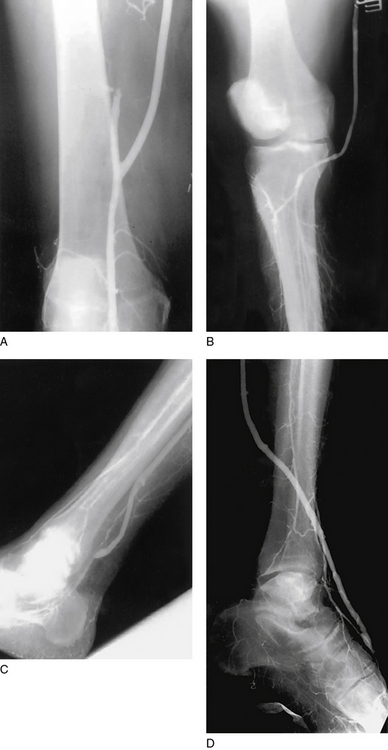
Figure 21-11 Intraoperative completion arteriograms of distal anastomoses to above-knee popliteal (A), below-knee popliteal (B), distal posterior tibial (C), and dorsalis pedis (D) arteries.
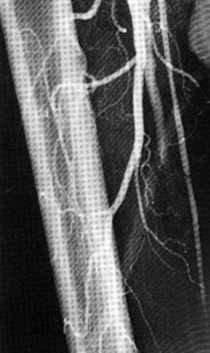
Figure 21-12 Intraoperative completion arteriogram of in situ femoropopliteal vein graft indicating retained valve, visualized as a filling defect in graft, and persistent arteriovenous fistula (AVF).
Current reports of the 5-year results of reversed saphenous vein graft using modern techniques have been excellent, with primary and secondary patency rates of 75% and 80%, respectively, and limb salvage rates of 90%.104,105
In Situ Grafting
There has been ongoing enthusiasm in some circles for in situ vein bypass grafting, whereby except for its proximal and distal extent, the greater saphenous vein is left undisturbed in its native bed. This technique was first described in 1962106 but was later popularized by Leather and Karmody in the late 1970s.107 Recent reports of in situ saphenous vein grafting have indicated 5-year graft patency rates approaching 80% and limb salvage rates of 84% to 90%.105,108–110
The approach minimizes trauma to the vein during excision and handling, and in theory enhances preservation of the vasa vasorum and endothelium. It further lowers the considerable risk of wound healing complications seen with traditional vein harvesting and facilitates creation of more technically precise anastomoses because the proximal and distal vein diameters are more closely matched to those of the inflow and outflow target vessels (Fig. 21-13). Extent of proximal vein mobilization is dictated by location of the saphenofemoral junction relative to theproposed site of the proximal anastomosis. It may at times be necessary to perform an endarterectomy of the SFA if the length of proximal vein is insufficient. Lysis of the valve cusps is obligatory given the nonreversed configuration, and is facilitated by newer less traumatic valvulotomes that function safely through the blinded segments of undissected graft. Critics of this technique argue that the advantages listed have not translated into improved graft function or patency. They further argue that the time required and dissection involved in finding and ligating substantial side branches—which can develop into physiologically important arteriovenous fistulae (AVF) that “steal” distal flow—obviates the stated benefits of this approach. Newer techniques using angioscopy and endoluminal coiling111 of larger side branches may help minimize these concerns.
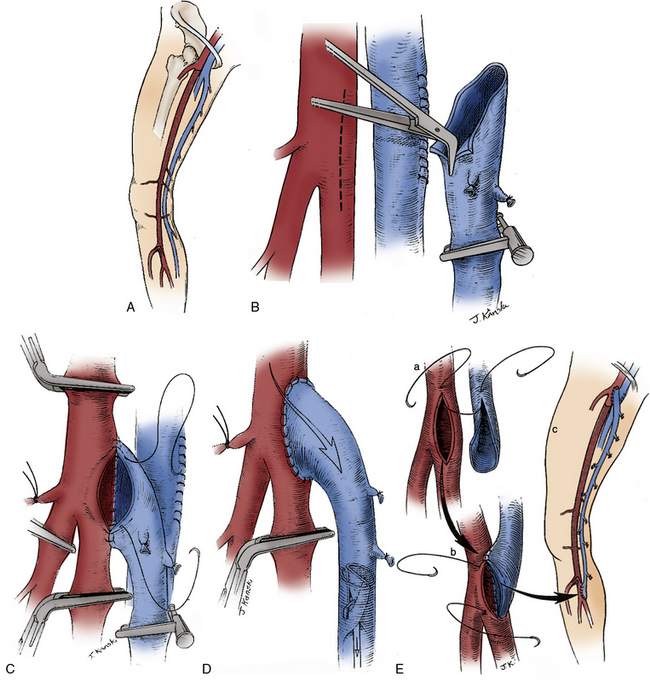
Figure 21-13 In situ method of infrainguinal reconstruction.
Saphenous vein is left undisturbed in its native bed, except at proximal and distal anastomotic sites—in this case, common femoral artery (CFA) and tibioperoneal trunk, respectively (A). Saphenofemoral junction is transected in groin, venotomy in femoral vein is oversewn, and proximal end of saphenous vein is spatulated in preparation for anastomosis (B). After first venous valve is excised under direct vision, graft is anastomosed end-to-side to femoral artery (C). Flow is then restored through vein graft, and valvulotome passed from distal end to lyse residual valves (D) before distal anastomosis is performed (E).
Angioscopic-assisted valve lysis has been employed for more than a decade but has not gained widespread favor. Although there is a significant learning curve with this technology, and operative times—at least initially—are significantly prolonged, advocates cite fewer wound complications, shorter hospital stays, and decreased recuperative periods as potential benefits. Proponents of routine angioscopy for direct visualization of valve lysis stress its particular utility in demonstrating such unsuspected endoluminal venous pathology as phlebitic strictures, webs, and fibrotic valve cusps.112 This adjunct may be particularly useful in cases in which arm vein is used, when endoluminal pathology is more frequently encountered and is presumably partly responsible for suboptimal results.113
Nonreversed Saphenous Vein Grafts
Recognizing the many practical advantages inherent to the in situ technique, Belkin et al. and others have modified the approach to infrainguinal bypass grafting with venous conduit to incorporate several of the same principles.114 In particular, if the harvested vein is tapered to any significant extent, it is used in a nonreversed fashion. By optimizing the size matching between the artery and vein at both the proximal and distal anastomosis sites as discussed earlier, one can often use smaller veins than would be suitable for reversed vein grafting. The nonreversed configuration also allows preservation of the saphenous vein hood, which extends the available conduit length and is especially beneficial when the femoral artery is thick walled and diseased.
The vein is harvested and dilated in a similar fashion to reversed vein grafts, and the cusps of the proximal valve of the greater saphenous vein are excised under direct vision with fine Potts scissors. There are currently two main types of valvulotomes available. The modified Mills valvulotome is a short, metal, hockey stick–shaped cutter that can be introduced through the distal end of the vein or through the side branches. After the proximal anastomosis is performed, and with the perfused conduit on gentle stretch, the valves are carefully lysed in a sequential fashion by pulling the valvulotome inferiorly. An alternative recently designed self-centering valvulotome allows lysis of all valves in a single pass and is believed by some to be less traumatic. Once acceptable pulsatile flow is ensured, the distal anastomosis is performed in the standard fashion.
It is important to note that similar patency rates have consistently been demonstrated regardless of which technique is applied,109,110 so surgeon preference and comfort level are acceptable reasons for choosing one method over another.
Alternative Vein Sources
The ipsilateral greater saphenous vein (GSV) remains the conduit of choice for infrainguinal arterial reconstructions. However, the ipsilateral GSV may be unusable or absent in as many as 20% to 40% of patients requiring surgical revascularization.115,116 In patients without adequate ipsilateral GSV, alternative vein sources include the contralateral GSV, the small saphenous vein, and the cephalic and basilic arm veins. Some groups advocate preserving the contralateral GSV and preferentially utilize upper-extremity veins as the most appealing ectopic autologous conduit,117 but the majority of vascular surgeons, the present authors included, favor the use of contralateral GSV in this setting, citing quite favorable patency and morbidity profiles.115 Regardless of the strategy employed, the quality of the vein conduit chosen is of paramount importance. Preoperative duplex ultrasound surveillance can be used to reliably assess the presence of available venous conduit, as well as the relative quality with regard to wall thickness, compressibility, and diameter. The ultimate viability of the vein, however, is determined intraoperatively following cannulation and gentle dilation with heparinized saline.118
In situations in which an adequate single length of vein necessary to achieve inline pulsatile flow to the ischemic limb is unavailable, composite grafts whereby shorter usable vein lengths are spliced together in an end-to-end fashion can be used. Graft patency and limb salvage rates of such composite grafts are reduced compared to results with single-segment saphenous vein but have historically been better than those of prosthetic grafts (see Reoperative Bypass Surgery).118 Cryopreserved cadaver vein allografts (CVG) remain a conduit of last resort, reserved for highly selective cases given their extremely poor patency rates in comparison to other conduit choices.119
Prosthetic Bypass
As stated, it is recommended that infrainguinal bypass surgery be performed with saphenous vein or an autologous substitute whenever feasible, given the clearly demonstrated enhanced patency rates.100,120 Despite the ample published data supporting this strategy, some institutions and surgeons more frequently rely on prosthetic grafts. When the distal target is the above-knee popliteal artery and the tibial outflow is relatively well preserved, this is an acceptable approach; patency rates in this situation approach those of vein grafts.121 A variety of surgical adjunctive procedures, from patching the distal anastomotic target vessel, to creation of a distal AVF, to use of various autogenous vein cuffs interposed between the distal prosthetic and the target artery have all been attempted as a means of improving patency rates of grafts extending below the knee.122 More recently, flared grafts designed to minimize turbulence and shear stress between the prosthetic and native vessel have gained some popularity. Polyester (Dacron) and PTFE grafts are the two main types of prosthetics available, and as in other anatomical positions, available data show generally equal results with either choice. The entire procedure is carried out through two small proximal and distal incisions between which the graft is tunneled anatomically. The selection of a 6- or 8-mm graft is dictated by the size of the native vessels.
Reoperative Bypass Surgery
As the patient population treated by vascular surgeons has increased in age, and more and more challenging cases are accepted for primary treatment, there has been a corresponding increase in the incidence of reoperative bypass surgery performed for infrainguinal arterial occlusive disease. Such reoperative procedures are particularly challenging, both because of the scarring present at the inflow and outflow target sites and because there is typically a lack of ipsilateral greater saphenous vein. Whenever possible, the first problem is addressed by choosing anastomotic sites just above or below the previous touchdown points, thereby avoiding dissection through often densely scarred tissue planes. When ipsilateral greater saphenous vein is absent due to prior infrainguinal or coronary artery bypass surgery or prior saphenous vein stripping, there are a number of alternative conduit sites available, as already mentioned. Chew et al. studied the consequence of using the contralateral greater saphenous vein in these situations and found it to be the optimal conduit. Despite the presumably high incidence of contralateral lower extremity as well as coronary occlusive disease in this population, short- and long-term impacts were found to be minimal.115
Use of arm veins, in general, can be extremely technically challenging and for that reason has not been universally adopted. Often the arm veins distal to the antecubital crease are scarred and of small caliber, but their more proximal counterparts are often of excellent size and quality. Dissection of the basilic vein can be particularly tedious because it has multiple side branches and lies adjacent to several important nerves. Because arm veins are often relatively short, a venovenostomy is often required to create composite grafts long enough to complete the arterial reconstruction (Fig. 21-14). This is performed with generous spatulation of each vein hood to create a widely patent vein-to-vein anastomosis. Given their thin-walled nature, arm vein grafts are also quite prone to twisting and kinking, and special care must be taken during the tunneling process to avoid these problems. The more proximal arm veins can be relatively large, and it is often advantageous to use one or more of the segments in a nonreversed fashion to better match the graft to the inflow vessel size.
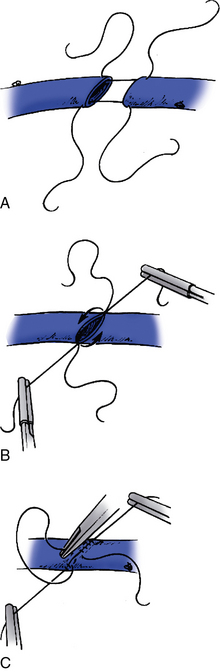
Figure 21-14 Creation of a composite graft by venovenostomy.
A widely spatulated venovenostomy is optimal (A). The posterior wall (B) and anterior wall (C) are aligned with separate strands of suture to avoid a “purse string” effect on the suture line.
Not surprisingly, the results of reoperative infrainguinal bypass surgery do not match those of primary reconstruction. With autogenous vein, 5-year patency rates of 60% and limb salvage rates of 70% to 80% have been reported.115,123 Coumadin is often used postoperatively in patients with compromised outflow or in whom the conduit was of marginal quality and has been associated with improved long-term patency.124
Post-Reconstruction Management
Many patients undergoing surgical reconstruction for arterial insufficiency will require one or more adjunctive operative procedures of their foot. Small uninfected ulcerations of the toe or foot often can be safely managed conservatively. However, larger gangrenous lesions of the toe, forefoot, or heel usually require débridement of all necrotic tissue at completion of the revascularization procedure. If the ischemia is particularly severe or infection is present, toe or transmetatarsal amputation may be necessary to achieve a margin of healthy tissue. This is particularly important in patients with diabetes or end-stage renal disease, in whom persistent infection or necrosis can result in limb loss despite the presence of a well-revascularized extremity. Wounds are usually left open and treated with saline wet-to-dry dressings or newer vacuum sponge dressings. Serial débridements on the ward or in the operating room are often necessary for larger wounds, which can then be surgically closed after an interval healing period or allowed to slowly close via secondary intention.
Unless otherwise contraindicated, all patients are maintained indefinitely on an antiplatelet regimen with either aspirin or clopidogrel following surgical bypass. As stated earlier, in cases in which a graft is at increased risk of failure, such as in the redo setting or when compromised outflow or a marginal conduit was accepted, the antiplatelet agent may be supplemented with Coumadin.124 Aggressive risk factor modification in the form of smoking cessation, lipid reduction, exercise, blood pressure management, and diabetic blood sugar control is of further paramount importance in minimizing the risk of disease progression or recurrence.125 More immediately, aggressive rehabilitation maximizes the chances of and shortens the time to a return to full function after extensive reconstructive surgery.
Graft Failure and Surveillance
Postoperative graft failures are typically classified according to the time interval from surgery as early, intermediate, or late. Graft thrombosis occurring within 30 days, so-called early graft failures, are generally believed to be due to technical or judgment errors by the surgeon. Included in this list would be such technical errors as twists, kinks, incompletely lysed valves, or anastomotic defects, as well as judgment errors in using a poor-quality vein or targeting an outflow vessel with inadequate runoff to support the graft. Intermediate graft failures include those between 30 days and 2 years and are generally attributed to the proliferation of intimal hyperplasia at the anastomoses or prior valve sites within the graft (Fig. 21-15). Randomized trials are currently underway to determine the impact of genetic modulation of vein grafts on the development of intimal hyperplasia, and hold some promise in reducing or minimizing this significant cause of vein graft failure.126 Late graft failures occurring beyond 2 years are typically due to progression of atherosclerotic occlusive disease within the inflow or outflow arteries.
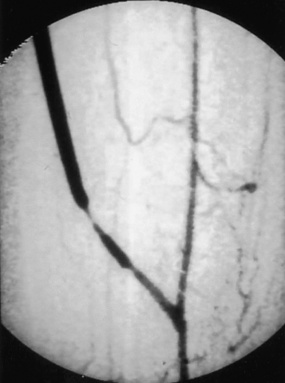
Figure 21-15 Arteriogram demonstrating severe stenosis of distal graft from intimal hyperplasia, likely at prior valve site.
Given the known incidence of graft failure and the potentially dire consequence in terms of limb salvage or preservation of limb function in a patient with limited options for secondary or tertiary bypass, the ability to maintain graft patency through early identification and prompt correction of graft stenoses is of paramount importance.127 Serial postoperative surveillance scanning with a duplex ultrasound has proved an excellent means of accurately identifying hemodynamically significant stenoses within the vein graft that threaten the graft patency.128 Subsequent confirmation by angiography and prophylactic treatment by percutaneous cutting balloon angioplasty, surgical patch angioplasty, or interposition grafting of significant lesions minimizes the risk of graft thrombosis and ensures optimal long-term graft patency.
1 Gray EA. Portrait of a surgeon: a biography of John Hunter. London: Robert Hale; 1952.
2 dos Santos JC. Sur la desobstion des thromboses arterielles anciennes. Mem Acad Chir (Paris). 1947;73:409.
3 Wylie EJ, Kerr E, Davies O. Experimental and clinical experiences with the use of fascia lata applied as a graft about major arteries after thromboendarterectomy and aneurysmorrhaphy. Surg Gynecol Obstet. 1951;93:257.
4 Carrel A. The surgery of blood vessels, etc. John Hopkins Hosp Bull. 1907;190:18.
5 Kunlin J. Le traitement de l’ischemie arteritique par la greffe veineuse longue. Rev Chir. 1951;70:206.
6 McCaughan JJJr. Surgical exposure of the distal popliteal artery. Surgery. 1958;44:536.
7 Berman JR, Berman LA, Goldstein I. Female sexual dysfunction: incidence, pathophysiology, evaluation and treatment options. Urology. 1999;54:385.
8 Leriche R, Morel A. The syndrome of thrombotic obliteration of the aortic bifurcation. Ann Surg. 1948;127:193.
9 Wingo JP, Nix ML, Greenfield LJ, et al. The blue toe syndrome: hemodynamic and therapeutic correlates of outcome. J Vasc Surg. 1986;3:475.
10 Karmody AM, Powers SR, Monaco VJ, et al. “Blue toe” syndrome. Arch Surg. 1976;111:1263.
11 Brewster DC, Perler BA, Robison JG, et al. Aortofemoral graft for multilevel occlusive disease: predictors of success and need for distal bypass. Arch Surg. 1982;117:1593.
12 Imparata AM, Kim G, Davidson T, et al. Intermittent claudication: its natural course. Surgery. 1975;78:795.
13 Cronenwett JL, Davis JT, Gooch JB, et al. Aortoiliac occlusive disease in women. Surgery. 1980;88:775.
14 Caes F, Cham B, Van den Brande P, et al. Small artery syndrome in women. Surg Gynecol Obstet. 1985;161:165.
15 Shareghi S, Gopal A, Gul K, et al. Diagnostic accuracy of 64 multidetector computed tomography angiography in peripheral vascular disease. Catheter Cardiovasc Intervent. 2010;75:23–31.
16 Carpenter JP, Owen RS, Holland GA, et al. Magnetic resonance angiography of the aorta, iliac and femoral arteries. Surgery. 1994;116:17.
17 Seldinger SE. Catheter replacement of needle in percutaneous arteriography: new technique. Acta Radiol. 1953;39:368.
18 Udoff EJ, Barth KH, Harrington DP, et al. Hemodynamic significance of iliac artery stenosis: pressure measurements during angiography. Radiology. 1979;132:289.
19 Nehler MR, Hiatt WR. Exercise therapy for claudication. Ann Vasc Surg. 1999;13:109.
20 Regensteiner JG, Meyer TJ, Krupski WC. Hospital versus home-based exercise rehabilitation for patients with peripheral arterial occlusive disease. Angiology. 1997;48:291.
21 Dawson DL, Cutler BS, Hiatt WR, et al. A comparison of cilostazol and pentoxifylline for treating intermittent claudication. Am J Med. 2000;109:523.
22 Martinez BD, Hertzer NR, Beven EG. Influence of distal arterial occlusive disease on prognosis following aortobifemoral bypass. Surgery. 1980;88:795.
23 Hill DA, McGrath MA, Lord RSA, et al. The effect of superficial femoral artery occlusion on the outcome of aortofemoral bypass for intermittent claudication. Surgery. 1980;87:133.
24 Harris PL, Cave-Bigley DJ, McSweeney L. Aortofemoral bypass and the role of concomitant femorodistal reconstruction. Br J Surg. 1985;72:317.
25 Darling RC, Linton RR. Aortoiliofemoral endarterectomy for atherosclerotic occlusive disease. Surgery. 1975;110:1458.
26 Barker WF, Cannon JA. An evaluation of endarterectomy. Arch Surg. 1953;66:488.
27 Crawford ES, Manning LG, Kelly TF. “Redo” surgery after operations for aneurysm and occlusion of the abdominal aorta. Surgery. 1977;81:41.
28 Perdue GD, Long WD, Smith RBIII, et al. Perspective concerning aortofemoral arterial reconstruction. Ann Surg. 1971;173:940.
29 Brewster DC, Darling RC. Optimal methods of aortoiliac reconstruction. Surgery. 1978;84:739.
30 Baird RJ, Feldman P, Miles JT, et al. Subsequent downstream repair after aorta-iliac and aorta-femoral bypass operations. Surgery. 1977;82:785.
31 Crawford ES, Bomberger RA, Glaeser DH, et al. Aortoiliac occlusive disease: factors influencing survival and function following reconstructive operation over a twenty-five year period. Surgery. 1981;90:1055.
32 Malone JM, Moore WS, Goldstone J. The natural history of bilateral aortofemoral bypass grafts for ischemia of the lower extremities. Arch Surg. 1975;110:1300.
33 Reed AB, Conte MS, Donaldson MC, et al. The impact of patient age and aortic size on the results of aortobifemoral bypass grafting. J Vasc Surg. 2003;37:1219.
34 Menard MT, Chew DK, Chan RK, et al. Outcome in patients at high risk after open surgical repair of abdominal aortic aneurysm. J Vasc Surg. 2003;37:285.
35 Whittemore AD, Clowes AW, Hechtman HB, et al. Aortic aneurysm repair: reduced operative mortality associated with maintenance of optimal cardiac performance. Ann Surg. 1971;173:940.
36 Kaluza GL, Joseph J, Lee JR, et al. Catastrophic outcomes of noncardiac surgery soon after coronary stenting. J Am Coll Cardiol. 2000;35:1288.
37 Mozersky DJ, Summer DS, Strandness DE. Long-term results of reconstructive aortoiliac surgery. Am J Surg. 1972;123:503.
38 Cooley DA, Wukasch DC, Bennett JG, et al. Double velour knitted Dacron grafts for aortoiliac vascular replacements, Paper presented at Vascular Graft Symposium,. Bethesda, Md: National Institutes of Health. November 5, 1976.
39 Yates SG, Barros D’Sa AA, Berger K, et al. The preclotting of porous arterial prosthesis. Ann Surg. 1978;188:611.
40 Nevelsteen A, Wouters L, Suy R. Aorto-femoral Dacron reconstruction for aortoiliac occlusive disease: a 25-year survey. Eur J Vasc Surg. 1991;5:179.
41 Malone JM, Goldstone J, Moore WS. Autogenous profundaplasty: the key to long-term patency in secondary repair of aortofemoral graft occlusion. Ann Surg. 1978;188:817.
42 Morris GCJr., Edwards W, Cooley DA, et al. Surgical importance of profunda femoris artery. Arch Surg. 1961;82:32.
43 Rutherford RB, Jones DN, Martin MS, et al. Serial hemodynamic assessment of aortobifemoral bypass. J Vasc Surg. 1986;4:428.
44 Bernhard VM, Ray LI, Militello JP. The role of angioplasty in the profunda femoris artery in revascularization of the ischemic limb. Surg Gynecol Obstet. 1976;142:840.
45 Malone JM, Goldstone J, Moore WS. Autogenous profundaplasty: the key to long-term success and need for distal bypass. Arch Surg. 1982;117:1593.
46 Berguer R, Higgins RF, Cotton LT. Geometry, blood flow, and reconstruction of the deep femoral artery. Am J Surg. 1975;130:68.
47 Juleff RS, Brown OW, McKain MM, et al. The influence of competitive flow on graft patency. J Cardiovasc Surg. 1992;33:415.
48 Pierce GE, Turrentine M, Stringfield S, et al. Evaluation of end-to-side vs. end-to-end proximal anastomosis in aortobifemoral bypass. Arch Surg. 1982;117:1580.
49 Brewster DC, Franklin DP, Cambria RP, et al. Intestinal ischemia complicating abdominal aortic surgery. Surgery. 1991;109:447.
50 Gloviczki P, Cross SA, Stanson AW, et al. Ischemic injury to the spinal cord or lumbosacral plexus after aorto-iliac reconstruction. Am J Surg. 1991;162:131.
51 Seegar JM, Coe DA, Kaelin LD, et al. Routine reimplantation of patent inferior mesenteric arteries limits colon infarction after aortic reconstructions. J Vasc Surg. 1992;15:635.
52 Prinssen M, Buskens E, Blankensteijn JD. Sexual dysfunction after conventional or endovascular AAA repair: results of a randomized trial, February 12, 2004. Paper presented at 17th International Congress of Endovascular Interventions, Phoenix
53 Nypaver TJ, Ellenby MI, Mendoza O, et al. A comparison of operative approaches and parameters of success in multilevel arterial occlusive disease. J Am Coll Surg. 1994;179:449.
54 Dalman RL, Taylor LMJr., Moneta GL, et al. Simultaneous operative repair of multilevel lower extremity occlusive disease. J Vasc Surg. 1991;13:211.
55 Malone JM, Moore WS, Goldstone J. Life expectancy following aortofemoral arterial grafting. Surgery. 1977;81:551.
56 Szilagyi DE, Hageman JH, Smith RF, et al. A thirty-year survey of the reconstructive surgical treatment of aortoiliac occlusive disease. J Vasc Surg. 1986;3:421.
57 Freeman NE, Leeds FH. Operations on large arteries. Calif Med. 1952;77:229.
58 Blaisdell FW, Hall AD. Axillary-femoral artery bypass for lower extremity ischemia. Surgery. 1963;54:563.
59 LoGerfo FW, Johnson WC, Carson JD, et al. A comparison of the late patency rates of axillo-bilateral femoral and axillo-unilateral femoral grafts. Surgery. 1977;81:33.
60 Harris EJ, Taylor LM, McConnell DB, et al. Clinical results of axillobifemoral bypass using externally supported polytetrafluoroethylene. J Vasc Surg. 1990;12:416.
61 El-Massry S, Saad E, Sauvage LR, et al. Axillofemoral bypass using externally-supported, knitted Dacron grafts: a follow-up through twelve years. J Vasc Surg. 1993;17:107.
62 Broome A, Christenson JT, Ekloff B, et al. Axillofemoral bypass reconstructions in sixty-one patients with leg ischemia. Surgery. 1980;88:673.
63 Taylor LM, Park TC, Edwards JM, et al. Acute disruption of polytetrafluoroethylene grafts adjacent to axillary anastomoses: a complication of axillofemoral grafting. J Vasc Surg. 1994;20:520.
64 Donaldson MC, Louras JC, Buckman CA. Axillofemoral bypass: a tool with a limited role. J Vasc Surg. 1986;3:757.
65 Ascer E, Veith FJ, Gupta SK, et al. Comparison of axillounifemoral and axillobifemoral bypass operations. Surgery. 1985;97:169.
66 Rutherford RB, Patt A, Pearce WH. Extra-anatomic bypass: a closer look. J Vasc Surg. 1987;6:437.
67 Passman MA, Taylor LM, Moneta GL, et al. Comparison of axillofemoral and aortofemoral bypass for aortoiliac occlusive disease. J Vasc Surg. 1996;23:263.
68 Berguer R, Higgins RJ, Reddy DJ. Intimal hyperplasia: an experimental study. Arch Surg. 1980;115:332.
69 Maini BS, Mannick JA. Effect of arterial reconstruction on limb salvage. Arch Surg. 1978;113:1297.
70 Plecha FR, Plecha FM. Femorofemoral bypass grafts: ten years experience. J Vasc Surg. 1984;1:555.
71 Harrington ME, Harrington EB, Haimov M, et al. Iliofemoral versus femorofemoral bypass: the case for an individualized approach. J Vasc Surg. 1992;16:841.
72 Schneider JR, Besso SR, Walsh DB, et al. Femorofemoral versus aortobifemoral bypass: outcome and hemodynamic results. J Vasc Surg. 1994;19:43.
73 Couch NP, Clowes AW, Whittemore AD, et al. The iliac-origin graft arterial graft: a useful alternative for iliac occlusive disease. Surgery. 1985;97:183.
74 Kalman PG, Hosang M, Johnston KW, et al. Unilateral iliac disease: the role of iliofemoral bypass. J Vasc Surg. 1987;6:139.
75 Blaisdell FW, DeMattei GA, Gauder PG. Extraperitoneal thoracic aorta to femoral bypass grafts as replacement for an infected aortic bifurcation prosthesis. Am J Surg. 1961;102:583.
76 Criado E, Keagy BA. Use of the descending thoracic aorta as an inflow source in aortoiliac reconstruction: indications and long-term results. Ann Vasc Surg. 1994;8:38.
77 Dion YM, Gracia CR. A new technique for laparoscopic aortobifemoral grafting in occlusive aortoiliac disease. J Vasc Surg. 1997;26:685.
78 Ahn SS, Hiyama DT, Rudkin GH, et al. Laparoscopic aortobifemoral bypass. J Vasc Surg. 1997;26:128.
79 Kelly JJ, Kercher KW, Gallagher KA, et al. Hand-assisted laparoscopic aortobifemoral bypass versus open bypass for occlusive disease. J Laparoendosc Adv Surg Tech A. 2002;12:339.
80 Wisselink W, Cuesta MA, Gracia C, et al. Robot-assisted laparoscopic aortobifemoral bypass for aortoiliac occlusive disease: a report of two cases. J Vasc Surg. 2002;36:1079.
81 Stadler P, Dvoracek L, Vitasek P, et al. Is robotic surgery appropriate for vascular procedures? Report of 100 aortoiliac cases. Eur J Vasc Endovasc Surg. 2008;36:405–406.
82 Crawford RS, Cambria RP, Abularrage CJ, et al. Preoperative functional status predicts perioperative outcomes after infrainguinal bypass surgery. J Vasc Surg. 2010;51:351–358.
83 Conte MS, Bandyk DF, Clowes AW, et al. Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J Vasc Surg. 2006;43:742–751.
84 Schanzer A, Mega J, Meadows J, et al. Risk stratification in critical limb ischemia: derivation and validation of a model to predict amputation-free survival using multicenter surgical outcomes data. J Vasc Surg. 2008;48:1464–1471.
85 Nguyen LL, Moneta GL, Conte MS, et al. Prospective multicenter study of quality of life before and after lower extremity vein bypass in 1404 patients with critical limb ischemia. J Vasc Surg. 2006;44:977–984.
86 McAllister FF. The fate of patients with intermittent claudication managed non-operatively. Am J Surg. 1976;132:593.
87 Walsh DB, Gilbertson JJ, Zwolak RM, et al. The natural history of superficial femoral artery stenoses. J Vasc Surg. 1991;14:299.
88 Murphy TP, Hirsch AT, Ricotta JJ, et al. The Claudication: Exercise Vs. Endoluminal Revascularization (CLEVER) study: rationale and methods. J Vasc Surg. 2008;47:1356–1363.
89 Spronk S, Bosch JL, den Hoed PT, et al. Cost-effectiveness of endovascular revascularization compared to supervised hospital-based exercise training in patients with intermittent claudication: a randomized controlled trial. J Vasc Surg. 2008;48:1472–1480.
90 Greenhalgh RM, Belch JJ, Brown LC, et al. The adjuvant benefit of angioplasty in patients with mild to moderate intermittent claudication (MIMIC) managed by supervised exercise, smoking cessation advice and best medical therapy: results from two randomized trials for stenotic femoropopliteal and aortoiliac arterial disease. Eur J Vasc Endovasc Surg. 2008;36:680–688.
91 Nylaende M, Abdelnoor M, Stranden E, et al. The Oslo balloon angioplasty versus conservative treatment study (OBACT)—the 2 years results of a single centre, prospective, randomised study in patients with intermittent claudication. Eur J Vasc Endovasc Surg. 2007;33:3–12.
92 Bradbury AW, Adam DJ, Bell J, et al. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: analysis of amputation free and overall survival by treatment received. J Vasc Surg. 2010;51:18S–31S.
93 Adam DJ, Beard JD, Cleveland T, et al. Bypass versus Angioplasty in Severe Limb Ischemia of the Leg (BASIL) trial: multicentre, randomised controlled trial. Lancet. 2005;366:1925–1934.
94 Bradbury AW, Adam DJ, Bell J, et al. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: an intention-to-treat analysis of amputation-free and overall survival in patients randomized to a bypass surgery-first or a balloon angioplasty-first revascularization strategy. J Vasc Surg. 2010;51:5S–17S.
95 Faries PF, Morrisey NJ, Teodorescu V, et al. Recent advances in peripheral angioplasty and stenting. Angiology. 2002;53:617.
96 Duda SH, Poerner TC, Wiesenger B, et al. Drug-eluting stents: potential applications for peripheral arterial occlusive disease. J Vasc Interv Radiol. 2003;14:291.
97 Grassbaugh JA, Nelson PR, Rzucidlo EM, et al. Blinded comparison of preoperative duplex ultrasound scanning and contrast arteriography for planning revascularization at the level of the tibia. J Vasc Surg. 2003;37:1186.
98 Ascher E, Veith FJ, Gupta SK. Bypasses to plantar arteries and other tibial branches: an extended approach to limb salvage. J Vasc Surg. 1988;8:434.
99 Reed AB, Conte MS, Belkin M, et al. Usefulness of autogenous bypass grafts originating distal to the groin. J Vasc Surg. 2002;35:48.
100 Veith FJ, Gupta SK, Ascer E, et al. Six-year prospective multicenter randomized comparison of autologous saphenous vein and expanded polytetrafluoroethylene graft in infrainguinal arterial reconstruction. J Vasc Surg. 1986;3:104.
101 Baxter BT, Rizzo RJ, Flinn WR, et al. A comparative study of intraoperative angioscopy and completion arteriography following femorodistal bypass. Arch Surg. 1990;125:997.
102 Gilbertson JJ, Walsh DB, Zwolak RM, et al. A blinded comparison of angiography, angioscopy, and duplex scanning in the intraoperative evaluation of in situ saphenous vein bypass grafts. J Vasc Surg. 1992;15:121.
103 Bandyk D, Johnson B, Gupta A, et al. Nature and management of duplex abnormalities encountered during infrainguinal vein bypass grafting. J Vasc Surg. 1996;24:430.
104 Taylor LM, Edwards JM, Porter JM. Present status of reversed vein bypass grafting: five-year results of a modern series. J Vasc Surg. 1990;11:193.
105 Fogle MA, Whittemore AD, Couch NP, et al. A comparison of in situ and reversed saphenous vein grafts for infrainguinal reconstruction. J Vasc Surg. 1987;5:46.
106 Hall KV. The great saphenous vein used “in situ” as an arterial shunt after extirpation of vein valves. Surgery. 1962;51:492.
107 Leather RP, Powers SR, Karmody AM. A reappraisal of the in situ saphenous vein arterial bypass: its use in limb salvage. Surgery. 1979;86:453.
108 Donaldson MC, Mannick JA, Whittemore AD. Femoral-distal bypass with in situ greater saphenous vein: long-term results using Mills valvulotome. Ann Surg. 1991;213:457.
109 Moody AP, Edwards PR, Harris PL. In situ versus reversed femoropoplitial vein grafts: long-term follow-up of a prospective, randomized trial. Br J Surg. 1992;79:750.
110 Wengerter KR, Veith FJ, Gupta SK, et al. Prospective randomized multicenter comparison of in situ and reversed vein infrapopliteal bypasses. J Vasc Surg. 1991;13:189.
111 Rosenthal D, Dickson C, Rodriquez F, et al. Infrainguinal endovascular in situ saphenous vein bypass: ongoing results. J Vasc Surg. 1994;20:389.
112 Tf Panetta, Marin ML, Veith FJ, et al. Unsuspected pre-existing saphenous vein pathology: an unrecognized cause of vein bypass failure. J Vasc Surg. 1992;15:102.
113 Marcaccio EJ, Miller A, Tannenbaum GA, et al. Angioscopically directed interventions improve arm vein bypass grafts. J Vasc Surg. 1993;17:994.
114 Belkin M, Knox J, Donaldson MC, et al. Infrainguinal arterial reconstruction with nonreversed greater saphenous vein. J Vasc Surg. 1996;24:957.
115 Chew DK, Owens CD, Belkin M, et al. Bypass in the absence of ipsilateral greater saphenous vein: safety and superiority of the contralateral greater saphenous vein. J Vasc Surg. 2002;35:1085–1092.
116 Taylor LM, Edwards JM, Brant B. Autogenous reversed vein bypass for lower extremity ischemia in patients with absent or inadequate greater saphenous vein. Am J Surg. 1987;153:505–510.
117 Holzenbein TJ, Pomposelli FBJr., Miller A. Results of a policy with arm veins used as the first alternative to an unavailable ipsilateral greater saphenous vein for infrainguinal bypass. J Vasc Surg. 1996;23:130–140.
118 Chew DK, Contes MS, Donaldson MC, et al. Autogenous composite vein bypass graft for infrainguinal arterial reconstruction. J Vasc Surg. 2001;33:259–265.
119 Bannazadeh M, Sarac TP, Bena J. Reoperative lower extremity revascularization with cadaver vein for limb salvage. Ann Vasc Surg. 2009;23:24–31.
120 Whittemore AD, Kent KC, Donaldson MC, et al. What is the proper role of polytetrafluoroethylene grafts in infrainguinal reconstruction? J Vasc Surg. 1989;10:299.
121 Quinones-Baldrich WJ, Prego AA, Ucelay-Gomez R, et al. Long-term results of infrainguinal revascularization with polytetrafluoroethylene: a ten-year experience. J Vasc Surg. 1992;16:209.
122 Miller JH, Foreman RK, Ferguson L, et al. Interposition vein cuff for anastomosis of prosthesis to small artery. Aust N Z J Surg. 1984;54:283.
123 Belkin M, Conte MS, Donaldson MC, et al. Preferred strategies for secondary infrainguinal bypass: lessons learned from 300 consecutive reoperations. J Vasc Surg. 1995;21:282.
124 Sarac TP, Huber TS, Back MR, et al. Warfarin improves the outcome of infrainguinal vein bypass grafting at high risk for failure. J Vasc Surg. 1998;28:446.
125 Creager MA. Medical management of peripheral arterial disease. Cardiol Rev. 2001;9:238.
126 Mann MJ, Whittemore AD, Donaldson MC, et al. Ex-vivo gene therapy of human vascular bypass grafts with E2F: the PREVENT single-centre, randomized, controlled trial. Lancet. 1999;354:1493.
127 Veith FJ, Weiser RK, Gupta SK, et al. Diagnosis and management of failing lower extremity arterial reconstructions prior to graft occlusion. J Cardiovasc Surg. 1984;25:381.
128 Bandyk DF, Schmitt DD, Seabrook GR, et al. Monitoring functional patency of in situ saphenous vein bypasses: the impact of a surveillance protocol and elective revision. J Vasc Surg. 1989;9:286.