Chapter 100 Metabolic Disturbances
Hyperthermia in the Newborn
Elevations in temperature (38-39°C [100-103°F]) are occasionally noted on the 2nd or 3rd day of life in infants whose clinical course has been otherwise satisfactory. This disturbance is especially likely to occur in breast-fed infants whose intake of fluid has been particularly low or in infants who are overdressed or are exposed to high environmental temperatures, either in an incubator, in a bassinette near a radiator, or in the sun.
The infant may lose weight. A consistent relationship may not be seen between the fever and the extent of weight loss or inadequacy of fluid intake. Urinary output and the frequency of voiding diminish. The fontanel may be depressed. The infant takes fluids avidly, but the apparent vigor of the infant contrasts with the usual appearance of “being sick” from an infection. The rise in temperature may be associated with increases in serum levels of protein and sodium and in hematocrit. The possibility of local or systemic infection should be evaluated. Lowering the environmental temperature leads to prompt reduction of the fever and alleviation of symptoms. Oral hydration should be accomplished with additional nursing or formula and not with pure water, because of the risk of hyponatremia.
A more severe form of neonatal hyperthermia occurs in both newborn and older infants when they are warmly dressed. The diminished sweating capacity of newborn infants is a contributing factor. Warmly dressed infants left near stoves or radiators, traveling in well-heated automobiles, or left with bright sunlight shining directly on them through the windows of a closed room or automobile are likely to be victims. Body temperature may become as high as 41-44°C (106-111°F). The skin is hot and dry, and initially the infant usually appears flushed and apathetic. The extremities are warm. Tachypnea and irritability may be noted. This stage may be followed by stupor, grayish pallor, coma, and convulsions. Hypernatremia may contribute to the convulsions. Mortality and morbidity (brain damage) rates are high. Hyperthermia has been associated with sudden infant death, hemorrhagic shock, and encephalopathy syndrome (Chapter 64). The condition is prevented by dressing infants in clothing suitable for the temperature of the immediate environment. In newborn infants, exposure of the body to usual room temperature or immersion in tepid water usually suffices to bring the temperature back to normal levels. Older infants may require cooling for a longer time by repeated immersion. Attention to possible fluid and electrolyte disturbance is essential.
Hyperthermia a few days after birth can be due to infection, particularly herpes sepsis. Infants with infection appear ill with cold extremities, in contrast to those in whom hyperthermia is due to environmental causes.
Neonatal Cold Injury
Neonatal cold injury usually occurs in abandoned infants, infants in inadequately heated homes during cold spells when the outside temperature is in the freezing range, and in preterm infants (Chapter 69). The initial features are apathy, refusal of food, oliguria, and coldness to touch. The body temperature is usually between 29.5 and 35°C (85-95°F), and immobility, edema, and redness of the extremities, especially the hands and feet, and of the face are observed. Bradycardia and apnea may also occur. The facial erythema frequently gives a false impression of health and delays recognition that the infant is ill. Local hardening over areas of edema may lead to confusion with scleredema. Hypoglycemia and acidosis are common. Hemorrhagic manifestations are frequent; massive pulmonary hemorrhage is a common finding at autopsy. Hypothermia in preterm infants can be prevented with special plastic wraps that reduce evaporation and heat loss. Because of their high ratio of surface area to body mass, preterm infants are very vulnerable to evaporation heat loss. Infants at <28-30 wk should be placed inside a clear polyethylene bag without prior drying. Neonatal cold injury in preterm infants occurs in the developing world and can be prevented with skin-to-skin (kangaroo mother) care. Treatment consists of warming and paying scrupulous attention to recognition and correction of hypotension and metabolic imbalances, particularly hypoglycemia. Prevention consists of providing adequate environmental heat. The mortality rate is about 10%; about 10% of survivors have evidence of brain damage.
Edema
Generalized edema occurs in association with hydrops fetalis (Chapter 97.2) and in the offspring of diabetic mothers. In premature infants, edema is often a consequence of a decreased ability to excrete water or sodium, although some have considerable edema without identifiable cause. Infants with respiratory distress syndrome may become edematous without heart failure. Edema of the face and scalp may be caused by pressure from the umbilical cord around the neck, and transient localized swelling of the hands or feet may similarly be due to intrauterine pressure. Edema may be associated with heart failure. A lag in renal excretion of electrolytes and water may result in edema after a sudden large increase in intake of electrolytes, particularly with feeding of concentrated cow’s milk formulas. High-protein formulas may also cause edema as a result of the excessive renal solute load, particularly in premature infants. Rarely, idiopathic hypoproteinemia with edema lasting weeks or months is observed in term infants. The cause is unclear, and the disturbance is benign. Persistent edema of 1 or more extremities may represent congenital lymphedema (Milroy disease) or, in females, Turner syndrome. Generalized edema with hypoproteinemia may be seen in the neonatal period with congenital nephrosis and rarely with Hurler syndrome or after feeding hypoallergenic formulas to infants with cystic fibrosis of the pancreas. Sclerema is described in Chapter 639.
Hypocalcemia (Tetany) (Chapter 48)
Metabolic Bone Disease
Metabolic bone disease is a common complication in very low birthweight (VLBW) preterm infants. The smallest, sickest infants are at greatest risk. Progressive osteopenia with demineralized bones and, occasionally, pathologic fractures may develop. The major cause is inadequate intake of calcium and phosphorus to meet the requirements for growth. Poor intake of vitamin D is an additional risk factor. Contributing factors include prolonged parenteral nutrition, vitamin D and calcium malabsorption, intake of unsupplemented human milk, immobilization, and urinary calcium losses from long-term diuretic use. The serum alkaline phosphatase level is used to monitor metabolic bone disease and can be > 1,000 U/L in severe cases. Fortified human milk and formulas designed for preterm infants provide higher amounts of calcium, phosphorus, and vitamin D; promote bone mineralization; and may prevent metabolic bone disease. Many extremely LBW infants will require additional oral supplements of calcium and phosphorus. Treatment of fractures requires immobilization and administration of calcium, phosphorus, and, if needed, vitamin D (not more than 1,000 IU/day unless severe cholestasis or vitamin D resistance is present). See also Chapters 48 and 564.
Hypomagnesemia
Rarely, hypomagnesemia of unknown cause may occur in newborn infants, usually in association with hypocalcemia. It may also be associated with insufficient stores of skeletal magnesium secondary to deficient placental transfer, decreased intestinal absorption, neonatal hypoparathyroidism, hyperphosphatemia, renal loss (primary or secondary to drugs, e.g., amphotericin B), a defect in magnesium and calcium homeostasis, or iatrogenic deficiency caused by loss incurred during exchange transfusion or insufficient replacement during total intravenous alimentation. Infants of diabetic mothers may have lower than normal serum magnesium levels. The clinical manifestations of hypomagnesemia are indistinguishable from those of hypocalcemia and tetany and may, in fact, contribute to the accompanying hypocalcemia.
Hypomagnesemia occurs when serum magnesium levels fall below 1.5 mg/dL (0.62 mmol/L), although clinical signs do not usually develop until serum magnesium levels fall below 1.2 mg/dL. During exchange transfusion with citrated blood, which is low in magnesium because of binding by citrate, serum magnesium decreases about 0.5 mg/dL (0.2 mmol/L); approximately 10 days are required for return to normal. In non-iatrogenic hypomagnesemia, the serum magnesium level may be <0.5 mg/dL. Serum calcium in either instance is usually at levels noted in hypocalcemic tetany, but the serum phosphorus value is normal or high. Because the hypocalcemia accompanying hypomagnesemia is inadequately corrected by administration of calcium alone, hypomagnesemia should also be suspected in any patient with tetany not responding to calcium therapy.
Immediate treatment consists of intramuscular injection of magnesium sulfate. For newborn infants, 25-50 mg/kg/dose every 8 hr for 3-4 doses usually suffices. The accompanying hypocalcemia usually corrects itself as the hypomagnesemia resolves. The same daily dose can be given for oral maintenance therapy. Four to 5 times higher doses may be required in malabsorptive states. In most cases, the metabolic defect is transient, and treatment can be discontinued after 1-2 wk. A few patients appear to have a permanent form of the disease that requires continuous oral supplementation with magnesium to prevent recurrence of hypomagnesemia. No residual damage to the central nervous system is evident after prompt treatment.
Hypermagnesemia
Hypermagnesemia may occur in newborn infants of mothers treated with magnesium sulfate during labor. At high serum levels, the central nervous system is depressed and infants have respiratory depression that may require mechanical ventilation. Lower levels may result in hypoventilation, lethargy, flaccidity, hyporeflexia, and poor sucking. Hypermagnesemia may be associated with failure to pass meconium. The upper limit of normal magnesium is 2.8 mg/dL (1.15 mmol/L), but serious symptoms rarely occur at levels < 5 mg/dL (2.1 mmol/L). In most cases, no specific therapy (beyond supportive care and maintenance of respiratory support) is required. Intravenous calcium and diuresis will reduce the magnesium levels. In rare cases, exchange transfusion has been used for rapid removal of magnesium ion from the blood.
Substance Abuse and Neonatal Abstinence (Withdrawal)
Substance abuse during pregnancy is a serious problem for both the mother and her newborn. The mother may suffer adverse consequences of her addiction, including episodes of drug withdrawal during pregnancy and illnesses related to high-risk behavior. Effects on the fetus and newborn include chronic or intermittent drug exposure, poor maternal nutrition, acute withdrawal shortly after birth, and long-term effects on physical growth and neurodevelopment. Because infants with in utero drug exposure often have social and environmental risk factors and may have been exposed to multiple substances, it may be difficult to evaluate the effects of specific in utero drug exposure on long-term neurodevelopmental outcome.
Pregnancies in women who use illegal drugs or alcohol are high risk. Prenatal care is usually inadequate, and these women have a higher incidence of sexually transmitted infections, including syphilis, HIV, and hepatitis. In addition, the risk of preterm labor, intrauterine growth restriction, premature rupture of membranes, and perinatal morbidity and mortality is higher. Physiologic addiction to narcotics occurs in most infants born to actively addicted mothers because opiates cross the placenta. Withdrawal may manifest even before birth as increased activity of the fetus when the mother feels a need for the drug or withdrawal symptoms develop. Heroin and methadone are the drugs most frequently associated with withdrawal syndromes, but such syndromes may also occur with alcohol, nicotine, phenobarbital, pentazocine, codeine, propoxyphene, hydroxyzine, amphetamines, neuroleptics, antidepressants, and benzodiazepines.
Heroin addiction results in a 50% incidence of LBW infants, half of whom are small for gestational age. Chronic infections, maternal undernutrition, and a direct fetal growth–inhibiting effect are possible causes. The rate of stillbirths is increased, but not the incidence of congenital anomalies. Clinical manifestations of withdrawal occur in 50-75% of infants, usually beginning within the 1st 48 hr, depending on the daily maternal dose (<6 mg/24 hr is associated with no or mild symptoms), the duration of addiction (duration > 1 yr has a > 70% incidence of withdrawal), and the time of the last maternal dose (the incidence is higher if the last dose was taken within 24 hr of birth). Rarely, symptoms may appear as late as 4-6 wk of age. The incidence of respiratory distress syndrome and hyperbilirubinemia may be decreased in preterm infants of heroin users; accelerated production of pulmonary surfactant may explain the former, and enzyme induction of hepatic glucuronyl transferase the latter.
Tremors and hyperirritability are the most prominent symptoms. The tremors may be fine or jittery and indistinguishable from those of hypoglycemia, but they are more often coarse, “flapping,” and bilateral; the limbs are frequently rigid, hyperreflexic, and resistant to flexion and extension. Irritability and hyperactivity are generally marked and may lead to skin abrasions. Other signs include wakefulness, hyperacusis, hypertonicity, tachypnea, diarrhea, vomiting, high-pitched cry, fist sucking, poor feeding with weight loss (disorganized sucking), and fever. Sneezing, yawning, hiccups, myoclonic jerks, convulsions, abnormal sleep cycles, nasal stuffiness, apnea, flushing alternating rapidly with pallor, and lacrimation are less common. The Neonatal Intensive Care Unit Network Neurobehavioral Scale (NNNS) is a useful way to evaluate neonates exposed to opiates or other drugs (Table 100-1). The risk of sudden infant death syndrome is higher in such neonates. The diagnosis is generally established from the history and clinical findings. Examining the urine for opiates may reveal only low levels during withdrawal, but quinine, which is often mixed with heroin, may be present in higher concentrations. Meconium testing is more accurate than neonatal urine drug testing. Hypoglycemia and hypocalcemia should be excluded.
Table 100-1 NEUROBEHAVIORAL SCALE
| DOMAIN | ITEMS |
|---|---|
| Physiological | Labored breathing |
| Nasal flaring | |
| Autonomic | Sweating |
| Spit-up | |
| Hiccoughing | |
| Sneezing | |
| Nasal stuffiness | |
| Yawning | |
| CNS | Abnormal sucking |
| Choreiform movements | |
| Athetoid postures and movements | |
| Tremors | |
| Cogwheel movements | |
| Startles | |
| Hypertonia | |
| Back arching | |
| Fisting | |
| Cortical thumb | |
| Myoclonic jerks | |
| Generalized seizures | |
| Abnormal posture | |
| Skin | Pallor |
| Mottling | |
| Lividity | |
| Overall cyanosis | |
| Circumoral cyanosis | |
| Periocular cyanosis | |
| Visual | Gaze aversion during orientation |
| Pull-down during orientation | |
| Fuss/cry during orientation | |
| Obligatory following during orientation | |
| End point nystagmus during orientation | |
| Sustained spontaneous nystagmus | |
| Visual locking | |
| Hyperalertness | |
| Setting sun sign | |
| Roving eye movements | |
| Strabismus | |
| Tight blinking | |
| Other abnormal eye signs | |
| Gastrointestinal | Gagging/choking |
| Loose stools, watery stools | |
| Excessive gas, bowel sounds | |
| State | High-pitched cry |
| Monotone-pitch cry | |
| Weak cry | |
| No cry | |
| Extreme irritability | |
| Abrupt state changes | |
| Inability to achieve quiet awake state (state 4) |
CNS, central nervous system.
From Lester BM, Tronick EZ, Brazelton TB: The Neonatal Intensive Care Unit Network Neurobehavioral Scales procedures, Pediatrics 113:641–667, 2004.
Methadone addiction is associated with severe withdrawal symptoms, the incidence varying from 20 to 90%. Mothers taking methadone usually have better prenatal care than those taking heroin; these mothers have a high incidence of polysubstance abuse, including alcohol, barbiturates, and tranquilizers, and they are often heavy smokers. The incidence of congenital anomalies is not increased. The average birthweight of infants of mothers taking methadone is higher than that of infants of heroin-addicted mothers; the clinical manifestations are similar, except that the former group has a higher incidence of seizures (10-20%) and later onset (2-6 wk of age) of withdrawal. Women who continue to abuse heroin, even if they enter a methadone program, are more likely to have preterm and/or low birthweight infants than those born to women who stop using heroin. They are also more likely to suffer withdrawal and have a higher risk of neonatal mortality
Alcohol withdrawal is uncommon. Infants of women who have been drinking immediately before delivery may have alcohol on their breath for several hours because it rapidly crosses the placenta. Blood levels in the infant are similar to those in the mother. Hypoglycemia and metabolic acidosis may be present. Infants in whom withdrawal symptoms develop often become agitated and hyperactive, with marked tremors lasting 72 hr, followed by about 48 hr of lethargy before return to normal activity. Seizures may develop.
Phenobarbital withdrawal usually occurs in infants of mothers addicted to the drug. Symptoms begin at a median age of 7 days (range, 2-14 days). Infants may have a brief acute stage consisting of irritability, constant crying, sleeplessness, hiccups, and mouthing movements, followed by a subacute stage consisting of voracious appetite, frequent regurgitation and gagging, episodic irritability, hyperacusis, sweating, and a disturbed sleep pattern, all of which may last 2-4 mo.
Cocaine abuse in pregnant women is common, but withdrawal in their infants is unusual; the pregnancy may be complicated by premature labor, abruptio placentae, and fetal asphyxia. Infants may have intrauterine growth restriction and neurobehavioral deficits characterized by impaired state regulation, impaired auditory information processing, developmental delay, and learning disabilities. At 24 mo of age, they score lower on the mental portion of the Bayley Scales of Infant Development and are twice as likely to have developmental delay. Family disorganization, polysubstance abuse, sexually transmitted infections, and child abuse and neglect may also be present. At 4 yr of age, children exposed prenatally to cocaine demonstrate specific cognitive impairments (visual-spatial and math skills; general knowledge) and are less likely to have an IQ above the normative mean. With a more enriching home environment, IQ scores of cocaine-exposed children are similar to those of nonexposed children.
Treatment
The decision to use drug therapy for neonatal drug withdrawal should be based on the presence of signs of withdrawal. Infants with confirmed drug exposure who do not have signs of withdrawal do not require pharmacologic treatment. Drug withdrawal is a self-limiting process. However, withdrawal from sedative-hypnotic drugs or narcotics can be life-threatening. Indications for drug treatment include seizures, poor feeding, diarrhea, excessive vomiting, inability to sleep, and fever. Several methods to assess severity of the withdrawal are available.
Infants who are undergoing opiate withdrawal require care in a quiet environment with reduction of external stimuli and swaddling. Treatment of heroin and methadone withdrawal using methadone has been successful. Methadone withdrawal may require larger amounts of medication for longer periods to control clinical manifestations than are needed for heroin withdrawal. Paregoric at a beginning dose of 0.05-0.1 mL/kg is given every 3-4 hr and increased by 0.05 mL every 4 hr if necessary, depending on the size and response of the infant. Paregoric abolishes most withdrawal symptoms, especially diarrhea. Tincture of opium (10 mg/mL) diluted 25-fold results in the same morphine equivalency as paregoric. The recommended dose of diluted tincture of opium is 0.1 mL/kg (≈2 drops/kg) with feedings every 4 hr. The dose may be increased by 2 drops every 4 hr if needed. The dose and duration of therapy may be adjusted according to the clinical response. A combination of an opiate plus phenobarbital may be the most effective approach to an opiate withdrawal. Parenteral administration of fluids may be necessary to prevent aspiration or dehydration until the symptoms are brought under control. Buprenorphine, rather than methadone, treatment during pregnancy reduces the severity and duration of withdrawal.
Mortality from withdrawal is <5% and may be negligible with early recognition and treatment. The prognosis for normal development is affected by the adverse circumstances of high-risk pregnancy and delivery and by the environment to which the infant is returned after recovery, as well as by the effects of the particular drug on fetal and subsequent neonatal development.
100.1 Maternal Selective Serotonin Reuptake Inhibitors and Neonatal Behavioral Syndromes
Women of childbearing age have a combined incidence of depression and anxiety of approximately 19%. Selective serotonin reuptake inhibitors (SSRIs; fluoxetine, paroxetine, sertraline, citalopram, fluvoxamine) and, less often, serotonin norepinephrine reuptake inhibitors (SNRIs; venlafaxine, duloxetine) have been used to treat pregnant women with depression or anxiety disorders. Exposure to these agents during pregnancy may inconsistently produce congenital malformations (Chapter 90). In addition, poor neonatal adaptation has been noted with the use of many of these agents but most often with paroxetine and fluoxetine.
It is unclear whether poor neonatal adaptation is due to serotonin overstimulation (serotonin syndrome) or to withdrawal (serotonin discontinuation syndrome). Indeed, both conditions may occur with different agents. Paroxetine has a short half-life and few if any active metabolites and is also a potent muscarinic blocking agent. Serum paroxetine levels after birth decline rapidly. Neonatal adaptive symptoms after late pregnancy exposure to paroxetine may be withdrawal with cholinergic overdrive. Symptoms may also be delayed. In contrast, fluoxetine and its active metabolite (nor-fluoxetine) have long half-lives and may produce a serotonin syndrome of acute toxicity. Onset may be at birth or in the 1st 24 hr of life. The cord blood level of fluoxetine is equal to blood level in the mother. All agents cross the placental and blood-brain barriers.
A neonatal behavioral syndrome that has features of both direct serotonin toxicity and withdrawal (cholinergic overdrive) is noted in Figure 100-1 and is characterized by central nervous system (irritability, excess or restless sleep), motor (agitation, tremor, hyperreflexia, rigidity, hypotonia or hypertonia), respiratory (nasal congestion, respiratory distress, tachypnea), gastrointestinal (diarrhea, emesis, poor feeding) and systemic (hypothermia or hyperthermia, hypoglycemia) manifestations. Most infants have only mild symptoms that resolve within 2 wk; a severe syndrome characterized by seizures, dehydration, weight loss, hyperpyrexia, and respiratory failure is present in 1%. No deaths have been reported.
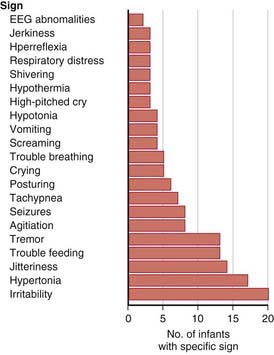
Figure 100-1 Neonatal signs after late in utero exposure to serotonin reuptake inhibitors. Frequencies of specific signs reported to the U.S. Food and Drug Administration (FDA) Adverse Events Reporting System. Ordered by frequency of occurrence (n = 57 infants). EEG, electroencephalographic.
(From Moses-Kolko EL, Bogen D, Perel J, et al: Neonatal signs after late in utero exposure to serotonin reuptake inhibitors, JAMA 293:2372–2383, 2005.)
Treatment is directed at the individual manifestations and accompanied by supportive therapies. A method of prevention of neonatal SSRI withdrawal has been proposed that consists of weaning the mother from the SSRI in the 3rd trimester of pregnancy. The advantages of this approach for the fetus must be weighed against the risk for the mother of recurrence of psychiatric symptoms during the last trimester and postpartum period.
Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295:499-507.
Ferriera E, Carceller AM, Agogue C, et al. Effects of selective serotonin reuptake inhibitors and venlafaxine during pregnancy in term and preterm neonates. Pediatrics. 2007;119:52-59.
Kallen B. Neonate characteristics after maternal use of antidepressants in late pregnancy. Arch Pediatr Adolesc Med. 2004;158:312-316.
Koren G. Discontinuation syndrome following late pregnancy exposure to antidepressants. Arch Pediatr Adolesc Med. 2004;158:307-308.
Laine K, Heikkinen T, Ekblad U, et al. Effects of exposure to selective serotonin reuptake inhibitors during pregnancy on serotonergic symptoms in newborns and cord blood monoamine and prolactin concentrations. Arch Gen Psychiatry. 2003;60:720-726.
Moses-Kolko EL, Bogen D, Percel J, et al. Neonatal signs after late in utero exposure to serotonin reuptake inhibitors. JAMA. 2005;293:2372-2383.
Sanz EJ, De-las-Cuevas C, Kiuru A, et al. Selective serotonin reuptake inhibitors in pregnant women and neonatal withdrawal syndrome: a database analysis. Lancet. 2005;365:482-487.
100.2 Fetal Alcohol Syndrome
High levels of alcohol ingestion during pregnancy can be damaging to embryonic and fetal development. A specific pattern of malformation identified as fetal alcohol syndrome has been documented, and major and minor components of the syndrome are expressed in 1-2 infants/1,000 live births (see Table 100-2). Both moderate and high levels of alcohol intake during early pregnancy may result in alterations in growth and morphogenesis of the fetus; the greater the intake, the more severe the signs. The risk of abnormality for infants born to heavy drinkers is twice that for infants born to moderate drinkers; in one study, 32% of infants born to heavy drinkers had congenital anomalies, compared with 9% of those born to abstinent mothers and 14% of those born to moderate drinkers. Additional maternal risk factors associated with fetal alcohol syndrome are advanced maternal age, low socioeconomic status, poor psychologic indicators, and binge drinking.
Characteristics of fetal alcohol syndrome include (1) prenatal onset and persistence of growth deficiency for length, weight, and head circumference; (2) facial abnormalities, including short palpebral fissures, epicanthal folds, maxillary hypoplasia, micrognathia, smooth philtrum, and a thin, smooth upper lip (Fig. 100-2); (3) cardiac defects, primarily septal defects; (4) minor joint and limb abnormalities, including some restriction of movement and altered palmar crease patterns; and (5) delay of development and mental deficiency varying from borderline to severe (Table 100-2). Fetal alcohol syndrome is a common identifiable cause of mental retardation. The severity of dysmorphogenesis may range from severely affected infants with full manifestations of fetal alcohol syndrome to those mildly affected with only a few manifestations.
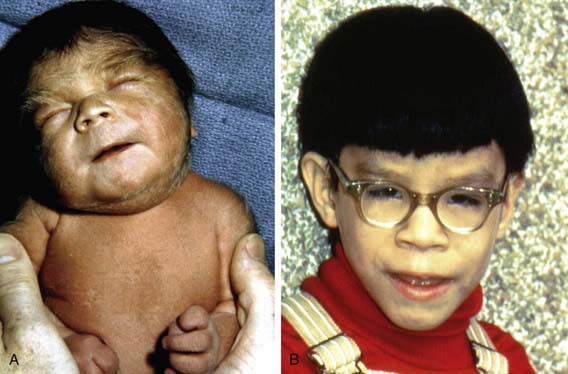
Figure 100-2 At birth (A) and at 4 yr of age (B). Note the short palpebral fissures; long, smooth philtrum with vermillion border; and hirsutism in the newborn.
(From Jones KL, Smith DW: Recognition of the fetal alcohol syndrome in early infancy, Lancet 2:999–1001, 1973.)
The detrimental effects may be due to the alcohol itself or to one of its breakdown products. Some evidence suggests that alcohol may impair placental transfer of essential amino acids and zinc, both of which are necessary for protein synthesis, an effect that may account for the intrauterine growth restriction.
Treatment of infants with fetal alcohol syndrome is difficult because no specific therapy exists. These infants may remain hypotonic and tremulous despite sedation, and the prognosis is poor. Counseling with regard to recurrence is important. Prevention is achieved by eliminating alcohol intake after conception.
Centers for Disease Control. Alcohol use among pregnant and nonpregnant women of childbearing age—United States, 1991–2005. MMWR Morb Mortal Wkly Rep. 2009;58:529-532.
Guerrini I, Jackson S, Keaney F. Pregnancy and alcohol misuse. BMJ. 2009;338:b845.
Iveli MF, Morales S, Rebolledo A, et al. Effects of light ethanol consumption during pregnancy: increased frequency of minor anomalies in the newborn and altered contractility of umbilical cord artery. Pediatr Res. 2007;61:456-461.
Jacobson SW, Carr LG, Croxford J, et al. Protective effects of the alcohol dehydrogenase-ASH1B allele in children exposed to alcohol during pregnancy. J Pediatr. 2006;148:30-37.
Kable JA, Coles CD. The impact of prenatal alcohol exposure on neurophysiological encoding of environmental events at six months. Alcohol Clin Exp Res. 2004;28:489-496.
Malisza KL, Allman AA, Shiloff D, et al. Evaluation of spatial working memory function in children and adults with fetal alcohol spectrum disorders: a functional magnetic resonance imaging study. Pediatr Res. 2005;58:1150-1157.
Moore ES, Ward RE, Jamison PL, et al. The subtle facial signs of prenatal exposure to alcohol: an anthropometric approach. J Pediatr. 2001;139:215-219.
Mukherje R, Eastman N, Turk J, Hollins S. Fetal alcohol syndrome: law and ethics. Lancet. 2007;369:1149-1150.
O’Donnell M, Nassar N, Leonard H, et al. Increasing prevalence of neonatal withdrawal syndrome: population study of maternal factors and child protection involvement. Pediatrics. 2009;123:e614-e621.
Rivkin MJ, Davis PE, Lemaster JL, et al. Volumetric MRI study of brain in children with intrauterine exposure of cocaine, alcohol, tobacco, and marijuana. Pediatrics. 2008;121:741-750.
Sood B, Delaney-Black V, Covington C, et al. Prenatal alcohol exposure and childhood behavior at age 6 to 7 years I: dose-response effect. Pediatrics. 2001;108:e34.
Backstrom MC, Kuusela AL, Maki R. Metabolic bone disease of prematurity. Ann Med. 1996;28:275-282.
Bandstra ES, Morrow CE, Anthony JC, et al. Intrauterine growth of full-term infants: impact of prenatal cocaine exposure. Pediatrics. 2001;108:1309-1319.
Coyle MG, Ferguson A, Lagasse L, et al. Diluted tincture of opium (DTO) and phenobarbital versus DTO alone for neonatal opiate withdrawal in term infants. J Pediatr. 2002;140:561-564.
Coyle MG, Ferguson A, Lagasse L, et al. Neurobehavioral effects of treatment for opiate withdrawal. Arch Dis Child Fetal Neonatal Ed. 2005;90:F73-F74.
Fewtrell MS, Cole TJ, Bishop NJ, et al. Neonatal factors predicting childhood height in preterm infants: evidence for a persisting effect of early metabolic bone disease? J Pediatr. 2000;137:668-673.
Godding V, Bonnier C, Fiasse L, et al. Does in utero exposure to heavy maternal smoking induce nicotine withdrawal symptoms in neonates? Pediatr Res. 2004;55:645-651.
Goldschmidt L, Richardson GA, Willford J, et al. Prenatal marijuana exposure and intelligence test performance at age 6. J Am Acad Child Adolesc Psychiatry. 2008;47:254-263.
Hurt H, Giannetta JM, Korczykowski M, et al. Functional magnetic resonance imaging and working memory in adolescents with gestational cocaine exposure. J Pediatr. 2008;152:371-377.
Jackson L, Ting A, Mckay S, et al. A randomized controlled trial of morphine versus phenobarbitone for neonatal abstinence syndrome. Arch Dis Child Fetal Neonatal Ed. 2004;89:F300-F304.
Jansson LM, Choo R, Velez ML, et al. Methadone maintenance and breastfeeding in the neonatal period. Pediatrics. 2008;121:106-114.
Jentink J, Dolk H, Loane MA, et al. Intrauterine exposure to carbamazepine and specific congenital malformations: systematic review and case-control study. BMJ. 2010;341:c6581.
Jentink J, Loane MA, Dolk H, et al. Valproic acid monotherapy in pregnancy and major congenital malformations. N Engl J Med. 2010;362:2185-2193.
Johnson K, Gerada C, Greenough A. Treatment of neonatal abstinence syndrome. Arch Dis Child Fetal Neonatal Ed. 2003;88:F2-F5.
Jones HE, Kaltenbach K, Heil SH, et al. Neonatal abstinence syndrome after methasone or buprenopphine exposure. N Engl J Med. 2010;363(24):2320-2330.
Kraft WK, Gibson E, Dysart K, et al. Sublingual buprenorphine for treatment of neonatal abstinence syndrome: a randomized trial. Pediatrics. 2008;122:e601-e607.
Lester BM, Tronick EZ, Brazelton TB. The Neonatal Intensive Care Unit Network Neurobehavioral Scale procedures. Pediatrics. 2004;113:641-667.
Levine TP, Liu J, Dad A, et al. Effects of prenatal cocaine exposure on special education in school-aged children. Pediatrics. 2008;122:e83-e91.
McCall EM, Alderdice FA, Halliday HL, et al: Interventions to prevent hypothermia at birth in preterm and /or low birthweight infants, Cochrane Database Syst Rev (23):CD004210, 2008.
Osborn DA, Jeffery HE, Cole MJ: Opiate treatment for opiate withdrawal in newborn infants, Cochrane Database Syst Rev (3):CD002059, 2005.
Osborn DA, Jeffery HE, Cole MJ: Sedatives for opiate withdrawal in newborn infants, Cochrane Database Syst Rev (3):CD002053, 2005.
Ostrea EMJr, Knapp DK, Tannenbaum L, et al. Estimates of illicit drug use during pregnancy by maternal interview, hair analysis, and meconium analysis. J Pediatr. 2001;138:344-348.
Pedersen LH, Henriksen TB, Olsen J. Fetal exposure to antidepressants and normal milestone development at 6 and 19 months of age. Pediatrics. 2010;125:e600-e608.
Ryan S. Nutritional aspects of metabolic bone disease in the newborn. Arch Dis Child. 1996;74:F145-F148.
Shankaran S, Das A, Bauer CR, et al. Association between patterns of maternal substance use and infant birth weight, length, and head circumference. Pediatrics. 2004;114:e226-e234.
Singer LT, Nelson S, Short E, et al. Prenatal cocaine exposure: drug and environmental effects at 9 years. J Pediatr. 2008;153:105-111.
Smith LM, La Gasse LL, Derauf C, et al. The infant development, environment, and lifestyle study: effects of prenatal methamphetamine exposure, polydrug exposure, and poverty or intrauterine growth. Pediatrics. 2006;118:1149-1156.
Tunell R. Prevention of neonatal cold injury in preterm infants. Acta Paediatr. 2004;93:308-310.
Velez ML, Jansson LM, Schroeder J, et al. Prenatal methadone exposure and neonatal neurobehavioral functioning. Pediatr Res. 2009;66:704-709.
Wang LH, Lin HC, Lin CC, et al. Increased risk of adverse pregnancy outcomes in women receiving zolpidem during pregnancy. Clin Pharm Therapeutics. 2010;88(3):369-374.