Chapter 175 Streptococcus pneumoniae (Pneumococcus)
Streptococcus pneumoniae (pneumococcus) is a very important pathogen that kills more than 1 million children each year worldwide. Childhood pneumococcal disease is prevalent and commonly severe, causes numerous clinical syndromes, and is a major cause of life-threatening pneumonia, bacteremia, and meningitis. Antimicrobial resistance in pneumococcus is a major public health problem, with 15-30% of isolates worldwide classified as multidrug-resistant (MDR, resistant to ≥3 classes of antibiotics). Pneumococcal polysaccharide-protein conjugate vaccines (PCVs) developed for infants have been highly successful in the control of disease caused by virulent vaccine-specific serotypes. Epidemiologic surveillance reveals a dynamic pneumococcal ecology with emergence of highly virulent, MDR serotypes. Ongoing vaccine development and distribution efforts remain our best approach to control of this threat to childhood health.
Etiology
S. pneumoniae is a gram-positive, lancet-shaped, polysaccharide encapsulated diplococcus, occurring occasionally as individual cocci or in chains. More than 90 serotypes have been identified by type-specific capsular polysaccharides. Antisera to some pneumococcal polysaccharides cross react with other pneumococcal types, defining serogroups (e.g., 6A and 6B). Encapsulated strains cause most serious disease in humans. Capsular polysaccharides impede phagocytosis. Virulence is related in part to capsular size, but pneumococcal types with capsules of the same size can vary widely in virulence.
On solid media, S. pneumoniae forms unpigmented, umbilicated colonies surrounded by a zone of incomplete (α) hemolysis. S. pneumoniae is bile soluble (i.e., 10% deoxycholate) and optochin-sensitive. S. pneumoniae is closely related to the viridans groups of Streptococcus mitis, which typically overlap phenotypically with pneumococci. The conventional laboratory definition of pneumococci continues to rely on bile and optochin sensitivity, although considerable confusion occurs in distinguishing pneumococci and other α-hemolytic streptococci. Pneumococcal capsules can be microscopically visualized and typed by exposing organisms to type-specific antisera that combine with their unique capsular polysaccharide, rendering the capsule refractile (Quellung reaction). Specific antibodies to capsular polysaccharides confer protection on the host, promoting opsonization and phagocytosis. Additionally, CD4+ T cells have a direct role in antibody-independent immunity to pneumococcal nasopharyngeal colonization. Conjugated PCVs promote T-cell immunity and protect against pneumococcal colonization, in contrast to the pneumococcal polysaccharide vaccine (PPSV23) used primarily in adults that does not affect nasopharyngeal colonization.
Epidemiology
Most healthy individuals carry various S. pneumoniae serotypes in their upper respiratory tract; >90% of children between 6 mo and 5 yr of age harbor S. pneumoniae in the nasopharynx at some time. A single serotype usually is carried by a given individual for an extended period (45 days to 6 mo). Carriage does not consistently induce local or systemic immunity sufficient to prevent later reacquisition of the same serotype. Rates of pneumococcal carriage peak during the 1st and 2nd yr of life and decline gradually thereafter. Carriage rates are highest in institutional settings and during the winter, and rates are lowest in summer. Nasopharyngeal carriage of pneumococci is common among young children attending out-of-home care, with rates of 21-59% in point prevalence studies and 65% in longitudinal studies. During the past 4 decades, serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F have constituted the majority of invasive isolates in children in the U.S. and other developed countries; strains belonging to serotypes 6B, 9V, 14, and 19F frequently have reduced susceptibility to penicillin. Since licensure of the PCVs, the prevalence of carriage and infection with vaccine serotypes has substantially declined and a shift to increased carriage or infections with nonvaccine serotypes has occurred (Fig. 175-1). Indirect protection of unvaccinated persons has occurred since PCV introduction, and this herd protection is likely due to decreases in nasopharyngeal carriage of virulent pneumococcal vaccine serotypes.
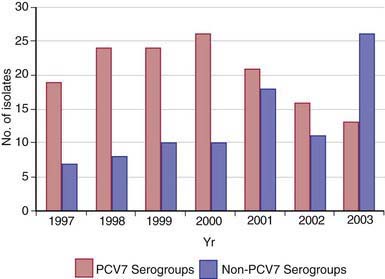
Figure 175-1 Number of isolates from Streptococcus pneumoniae serogroups included in the heptavalent pneumococcal conjugate vaccine (PCV7 [Prevnar; Wyeth Lederle Vaccines]) and from nonvaccine serogroups recovered from children treated at Primary Children’s Medical Center (Salt Lake City, UT), by year.
(From Byington CL, Samore MH, Stoddard GJ, et al: Temporal trends of invasive disease due to Streptococcus pneumoniae among children in the intermountain west: emergence of nonvaccine serogroups, Clin Infect Dis 41:21–29, 2005.)
S. pneumoniae is the most frequent cause of bacteremia, bacterial pneumonia, and otitis media and the second most common cause of meningitis in children, next to Neisseria meningitidis. The decreased ability in children <2 yr of age to produce antibody against the T-cell independent polysaccharide antigens and the high prevalence of colonization may explain an increased susceptibility to pneumococcal infection and the decreased effectiveness of polysaccharide vaccines. Males are more commonly affected than females. Native American and African-American children have rates of invasive disease that are 2- to 10-fold higher than other healthy children. Prior to the introduction of PCVs into routine childhood immunization schedules, rates of invasive pneumococcal disease in the USA peaked at 6-11 mo of age, with attack rates of >540/100,000 in healthy children. Following the universal use of PCVs, rates of infection have fallen in both high-risk and healthy children. In Tennessee, peak rates of infection have fallen from 235/100,000 to 46/100,000 in children <2 yr of age and the proportion of penicillin-resistant strains in invasive disease have fallen from 59.8% to 30.4%.
Pneumococcal disease usually occurs sporadically but can be spread from person to person by respiratory droplet transmission. The frequency and severity of pneumococcal disease are increased in patients with sickle cell disease, asplenia, deficiencies in humoral (B cell) and complement-mediated immunity, HIV infection, certain malignancies (e.g., leukemia, lymphoma), chronic heart, lung, or renal disease (particularly the nephrotic syndrome), cerebrospinal fluid (CSF) leak, and cochlear implants. Other high-risk groups are noted in Table 175-1. S. pneumoniae is an important cause of secondary bacterial pneumonia in patients with influenza. During influenza epidemics and pandemics, most deaths result from bacterial pneumonia, and pneumococcus is the predominant bacterial pathogen isolated in this setting. Pneumococcal co-pathogenicity may be important in disease caused by other respiratory viruses as well.
Table 175-1 CHILDREN AT HIGH OR MODERATE RISK OF INVASIVE PNEUMOCOCCAL INFECTION
HIGH RISK (INCIDENCE OF INVASIVE PNEUMOCOCCAL DISEASE = 150 CASES/100,000 PEOPLE PER YEAR)
Children with:
PRESUMED HIGH RISK (INSUFFICIENT DATA TO CALCULATE RATES)
Children with:
MODERATE RISK (INCIDENCE OF INVASIVE PNEUMOCOCCAL DISEASE = 20 CASES/100,000 PEOPLE PER YEAR)
From American Academy of Pediatrics: Red book: 2006 report of the Committee on Infectious Diseases, ed 27, Elk Grove Village, IL, 2006, American Academy of Pediatrics, p 527.
Pathogenesis
Invasion of the host is affected by a number of factors. Nonspecific defense mechanisms, including the presence of other bacteria in the nasopharynx, may limit multiplication of pneumococci. Aspiration of secretions containing pneumococci is hindered by the epiglottic reflex and by respiratory epithelial cilia, which move infected mucus toward the pharynx. Similarly, normal ciliary flow of fluid from the middle ear through the eustachian tube and sinuses to the nasopharynx usually prevents infection with nasopharyngeal flora, including pneumococci. Interference with these normal clearance mechanisms by allergy, viral infection, or irritants (e.g., smoke) may allow colonization and subsequent infection with these organisms in otherwise normally sterile sites.
Virulent pneumococci are intrinsically resistant to phagocytosis by alveolar macrophages. Pneumococcal disease frequently is facilitated by viral respiratory tract infection, which may produce mucosal injury, diminish epithelial ciliary activity, and depress the function of alveolar macrophages and neutrophils. Phagocytosis may be impeded by respiratory secretions and alveolar exudate. In the lungs and other tissues, the spread of infection is facilitated by the antiphagocytic properties of the pneumococcal capsule. Surface fluids of the respiratory tract contain only small amounts of IgG and are deficient in complement. During inflammation, there is limited influx of IgG, complement, and neutrophils. Phagocytosis of bacteria by neutrophils may occur, but normal human serum may not opsonize pneumococci and facilitate phagocytosis by alveolar macrophages. In tissues, pneumococci multiply and spread through the lymphatics or bloodstream or, less commonly, by direct extension from a local site of infection (e.g., sinuses). In bacteremia, the severity of disease is related to the number of organisms in the bloodstream and to the integrity of specific host defenses. A poor prognosis correlates with very large numbers of pneumococci and high concentrations of capsular polysaccharide in the blood and CSF.
Invasive pneumococcal disease is 30- to 100-fold more prevalent in children with sickle cell disease and other hemoglobinopathies and in children with congenital or surgical asplenia than in the general population. This risk is greatest in infants <2 yr of age since at that age antibody production to most serotypes is poor. The increased frequency of pneumococcal disease in asplenic persons is related to both deficient opsonization of pneumococci as well as absence of clearance by the spleen of circulating bacteria. Children with sickle cell disease also have deficits in the antibody-independent properdin (alternative) pathway of complement activation, in addition to functional asplenia. Both complement pathways contribute to antibody-independent and antibody-dependent opsonophagocytosis of pneumococci. With advancing age (e.g., >5 yr), children with sickle cell disease produce anticapsular antibody, augmenting antibody-dependent opsonophagocytosis and greatly reducing, but not eliminating, the risk of severe pneumococcal disease. Deficiency of many of the complement components (e.g., C2 and C3) is associated with recurrent pyogenic infection, including S. pneumonia infection. The efficacy of phagocytosis also is diminished in patients with B- and T-cell immunodeficiency syndromes (e.g., agammaglobulinemia, severe combined immune deficiency) or loss of immune globulin (e.g., nephrotic syndrome) and is largely caused by a deficiency of opsonic anticapsular antibody. These observations suggest that opsonization of pneumococci depends on the alternative complement pathway in antibody-deficient persons and that recovery from pneumococcal disease depends on the development of anticapsular antibodies that act as opsonins, enhancing phagocytosis and killing of pneumococci. Children with HIV infection also have high rates of invasive pneumococcal infection similar to or greater than that of children with sickle cell disease, although rates of invasive pneumococcal disease decreased after the introduction of highly active antiretroviral therapy (HAART).
Clinical Manifestations
The signs and symptoms of pneumococcal infection are related to the anatomic site of disease. Common clinical syndromes include otitis media (Chapter 632), sinusitis (Chapter 372), pneumonia (Fig. 175-2) (Chapter 392), and sepsis (Chapter 64). Before routine use of PCVs, pneumococci caused >80% of bacteremia episodes in infants 3-36 mo of age with fever without an identifiable source (i.e., occult bacteremia). Bacteremia may be followed by meningitis (Chapter 595), osteomyelitis (Chapter 676), suppurative arthritis (Chapter 677), endocarditis (Chapter 431), and rarely, brain abscess (Chapter 596). Primary peritonitis (Chapter 363) may occur in children with peritoneal effusions due to nephrotic syndrome and other conditions. Local complications of infection may occur, causing empyema, pericarditis, mastoiditis, epidural abscess, or meningitis. Hemolytic-uremic syndrome (Chapter 478.4) and disseminated intravascular coagulation also occur as rare complications of pneumococcal infections. Epidemic conjunctivitis caused by nonencapsulated or encapsulated pneumococci occurs as well.
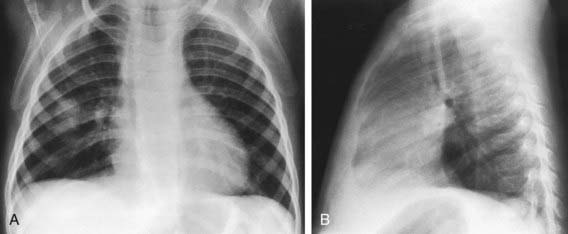
Figure 175-2 Bacterial pneumonia: “round” pneumonia (Streptococcus pneumoniae) in an 11 mo old girl with a 2 day history of cough and spiking fever. There was leukocytosis with a shift to the left. A, The anteroposterior view shows a round, nodular area of consolidation in the right midlung. B, On the lateral projection, the nodule lying in the right middle lobe appears somewhat triangular. Such round pneumonias are usually caused by one of the common bacterial pathogens and are most commonly located in the superior segment of a lower lobe. Confusion with a metastatic lesion is possible, but the clinical findings are those of pneumonia. An important radiographic clue is that the consolidation typically appears round on the frontal projection, a shape that does not persist on the lateral view (here the infiltrate appears triangular).
(From Hilton SVW, Edwards DK, editors: Practical pediatric radiology, ed 3, Philadelphia, 2006, Elsevier, p 329.)
Diagnosis
The diagnosis of pneumococcal infection is established by recovery of S. pneumoniae from the site of infection or the blood. Although pneumococci may be found in the nose or throat of patients with otitis media, pneumonia, septicemia, or meningitis, cultures of these locations are generally not helpful for diagnosis as they are not indicative of causation. Blood cultures should be obtained in children with pneumonia, meningitis, arthritis, osteomyelitis, peritonitis, pericarditis, or gangrenous skin lesions. Due to the implementation of universal vaccination with PCVs, there has been a substantial decrease in the incidence of occult bacteremia, but blood cultures should still be considered in febrile patients with clinical toxicity or significant leukocytosis. Leukocytosis often is pronounced, with total white blood cell counts frequently >15,000/mm3. In severe cases of pneumococcal disease, white blood cell count may be low.
Pneumococci can be identified in body fluids as gram-positive, lancet-shaped diplococci. Early in the course of pneumococcal meningitis, many bacteria may be seen in relatively acellular cerebrospinal fluid. With current methods of continuously monitored blood culture systems, the average time to isolation of pneumococcal organisms is 14-15 hr. Pneumococcal latex agglutination tests for urine or other body fluids suffer from poor sensitivity and add little to Gram-stained fluids and standard cultures.
Treatment
The incidence of high-level β-lactam resistance and MDR strains have expanded dramatically during the past several decades. Spread of pneumococcal β-lactam and macrolide resistance is largely due to global spread of worldwide clones primarily of serotypes 6A, 6B, 9V, 14, 19F, and 23F, and introduction of PCVs appears to have decreased the overall incidence of pneumococcal resistance. In contrast, fluoroquinolone resistance is more commonly due to spontaneous mutation than clonal spread. Widespread use of antibiotics contributes to the spread of resistant strains. Some serotypes can undergo capsule switching (i.e., change from one serotype to another) in association with the development of antibiotic resistance.
Resistance in pneumococcal organisms to penicillin and the extended spectrum cephalosporins cefotaxime and ceftriaxone is defined by the minimum inhibitory concentration (MIC) as well as clinical syndrome. Pneumococci are considered susceptible, intermediate, or resistant to various antibacterial agents based on specific MIC breakpoints. For patients with pneumococcal meningitis, penicillin-susceptible strains have an MIC ≤0.06 µg/mL and penicillin resistant strains have an MIC ≥0.12 µg/mL. For patients with pneumococcal pneumonia, breakpoints are higher; in particular, penicillin susceptible strains have an MIC ≤2 µg/mL, and penicillin resistant strains have an MIC ≥8 µg/mL. For patients with meningitis, cefotaxime and ceftriaxone susceptible strains have an MIC ≤0.5 µg/mL and resistant strains have an MIC ≥2.0 µg/mL. For patients with nonmeningeal pneumococcal disease, breakpoints are higher, and cefotaxime- and ceftriaxone-susceptible strains have an MIC ≤1.0 µg/mL and resistant strains have an MIC ≥2 µg/mL. In cases where the pneumococcus is resistant to erythromycin but sensitive to clindamycin, a D-test should be performed to determine whether clindamycin resistance can be induced; if the D-test is positive, clindamycin should not be used to complete treatment of the patient. More than 30% of pneumococcal isolates are resistant to trimethoprim-sulfamethoxazole; levofloxacin resistance has also been reported. All isolates from children with severe infections should be tested for antibiotic susceptibility given widespread pneumococcal MDR strains. Resistance to vancomycin has not been seen to date, but vancomycin-tolerant pneumococci that are killed at a slower rate have been reported, and these tolerant pneumococci may be associated with a worse clinical outcome. Linezolid is an oxazolidinone antibacterial with activity against MDR gram-positive organisms including pneumococcus and has been used in the treatment of MDR pneumococcal pneumonia, meningitis, and severe otitis. Despite early favorable studies, use of this drug is limited by myelosuppression and high cost, and linezolid resistance in pneumococcus has been reported.
Children 1 mo of age or older with suspected pneumococcal meningitis should be treated with combination therapy using vancomycin (60 mg/kg/24 hr divided q 6 hr IV), and high-dose cefotaxime (300 mg/kg/24 hr divided q 8 hr IV) or ceftriaxone (100 mg/kg/24 hr divided q 12 hr IV). Proven pneumococcal meningitis can be treated with penicillin alone, or cefotaxime or ceftriaxone alone, if the isolate is penicillin-susceptible. If the organism is nonsusceptible (i.e., intermediate or full resistance) to penicillin but susceptible to cefotaxime and ceftriaxone, pneumococcal meningitis can be treated with cefotaxime or ceftriaxone alone. However, if the organism is nonsusceptible to penicillin and to cefotaxime or ceftriaxone, pneumococcal meningitis should be treated with combination vancomycin plus cefotaxime or ceftriaxone, not with vancomycin alone, and consideration should be given to the addition of rifampin.
For invasive infections outside the central nervous system (e.g., lobar pneumonia with or without bacteremia), high-dose cefotaxime and ceftriaxone are effective, even for those infections caused by cephalosporin-intermediate or -resistant strains. For individuals who are allergic to penicillin, clindamycin, erythromycin (or related macrolides, e.g., azithromycin or clarithromycin), cephalosporins (standard dosing), and trimethoprim-sulfamethoxazole may provide effective alternative therapy for susceptible strains, depending on the site of infection (e.g., clindamycin may be effective for pneumococcal infections other than meningitis). Higher doses of amoxicillin (80-100 mg/kg/24 hr) have been successful in the treatment of otitis media caused by penicillin-nonsusceptible strains. Empirical treatment of pneumococcal disease should be based on knowledge of susceptibility patterns in specific communities.
Prognosis
Prognosis depends on the integrity of host defenses, virulence and numbers of the infecting organism, the age of the host, the site and extent of the infection, and the adequacy of treatment. The mortality rate for pneumococcal meningitis is approximately 10% in most studies. Pneumococcal meningitis results in sensorineural hearing loss in 20-30% of patients and can cause other serious neurologic sequelae including paralysis, epilepsy, blindness, and intellectual deficits.
Prevention
Immunologic responsiveness and efficacy following administration of pneumococcal polysaccharide vaccines is unpredictable in children <2 yr of age. PPSV23 contains purified polysaccharide of 23 pneumococcal serotypes responsible for >95% of cases of invasive disease. The clinical efficacy of these vaccines is controversial and studies have yielded conflicting results. In contrast, PCVs (see Table 175-2) provoke “protective” antibody responses in 90% of infants given these vaccines at 2, 4, and 6 mo of age, and greatly enhanced responses (e.g., immunologic memory) are apparent after “booster” doses given at 12-15 mo of age. In addition, PCVs reduce nasopharyngeal carriage of vaccine serotypes by up to 60-70%. In efficacy trials in the USA, infant immunization with PCV7 decreased invasive infections from pneumococcal vaccine serotypes by >93% and lobar pneumonias by >73%. Its administration was associated with a 6-7% decrease in otitis media, but greater reduction in complications of otitis media such as tympanostomy tube placement. From 2000 (PCV7 introduction) to 2005, invasive pneumococcal disease in U.S. children <5 yr of age decreased by 94%. PCV7 has significantly decreased rates of invasive pneumococcal disease in children with sickle cell disease, and preliminary studies suggest substantial protection for HIV-infected children and splenectomized adults. Adverse events after the administration of PCV7 have included local swelling and redness and slightly increased rates of fever, when used in conjunction with other childhood vaccines. There have been multiple reports of increases of empyema due to serotypes 1, 3, and 19A; necrotizing pneumonia due to serotype 3 and serogroup 19; bacteremia due to serotypes 3 and 8; and mastoiditis and recalcitrant acute otitis media due to MDR serotype 19A. These observations inform the serotype composition of new PCVs (Table 175-2).
Table 175-2 COMPARISON OF PNEUMOCOCCAL VACCINES LICENSED IN USA OR IN ADVANCED DEVELOPMENT (PCV7 SEROTYPES IN BOLD)
| CARRIER PROTEIN | PNEUMOCOCCAL CAPSULAR POLYSACCHARIDES | MANUFACTURER |
|---|---|---|
| Diphtheria CRM197 protein | 4,6B, 9V, 14, 18C, 19F, 23F | Wyeth Lederle (PCV7, Prevnar) |
| Diphtheria CRM197 protein | 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 23F | Wyeth Lederle (PCV13, Prevnar 13) |
| Haemophilus influenzae protein D Tetanus and diphtheria toxoids |
1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, 23F | GlaxoSmithKline (PCV10, Synflorix) |
| None | 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F, 33F | Sanofi Pasteur MSD (PPSV23, Pneumovax II) |
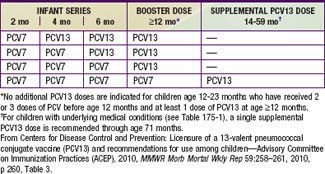
Immunization with PVC13 is recommended for all infants on a schedule for primary immunization, in previously unvaccinated infants, and for transition for those partially vaccinated with PCV7 (Tables 175-3 and 175-4). High-risk children ≥2 yr of age, such as those with asplenia, sickle cell disease, some types of immune deficiency (e.g., antibody deficiencies), HIV infection, cochlear implant, CSF leak, diabetes mellitus, and chronic lung, heart, or kidney disease (including nephrotic syndrome), may benefit also from PPSV23 administered after 2 yr of age following priming with the scheduled doses of PCV13. Thus, it is recommended that children ≥2 yr of age with these underlying conditions receive supplemental vaccination with PPSV23. A second dose of PPSV23 is recommended 5 yr after the first dose of PPSV23 for persons aged ≥2 yr who are immunocompromised, have sickle cell disease, or functional or anatomic asplenia.
Table 175-3 RECOMMENDED ROUTINE VACCINATION SCHEDULE FOR 13-VALENT PNEUMOCOCCAL CONJUGATE VACCINE (PCV13) AMONG INFANTS AND CHILDREN WHO HAVE NOT RECEIVED PREVIOUS DOSES OF 7-VALENT VACCINE (PCV7) OR PCV13, BY AGE AT FIRST DOSE—ADVISORY COMMITTEE ON IMMUNIZATION PRACTICES (ACIP), USA, 2010
| AGE AT FIRST DOSE (MO) | PRIMARY PCV13 SERIES* | PCV13 BOOSTER DOSE† |
|---|---|---|
| 2-6 | 3 doses | 1 dose at age 12-15 mo |
| 7-11 | 2 doses | 1 dose at age 12-15 mo |
| 12-23 | 2 doses | — |
| 24-59 (healthy children) | 1 dose | — |
| 24-71 (children with certain chronic diseases or immunocompromising conditions) | 2 doses | — |
* Minimum interval between doses is 8 weeks except for children vaccinated at age <12 months for whom minimum interval between doses is 4 weeks. Minimum age for administration of first dose is 6 weeks.
† Given at least 8 weeks after the previous dose.
From Centers for Disease Control and Prevention: Licensure of a 13-valent pneumococcal conjugate vaccine (PCV13) and recommendations for use among children—Advisory Committee on Immunization Practices (ACEP), 2010, MMWR Morb Mortal Wkly Rep 59:258–261, 2010, p 260, Table 3.
Table 175-4 RECOMMENDED TRANSITION SCHEDULE FROM 7-VALENT PNEUMOCOCCAL CONJUGATE VACCINE (PCV7) TO 13-VALENT VACCINE (PCV13) VACCINATION AMONG INFANTS AND CHILDREN, ACCORDING TO NUMBER OF PREVIOUS PCV7 DOSES RECEIVED—ADVISORY COMMITTEE ON IMMUNIZATION PRACTICES (ACIP), USA, 2010
Immunization with pneumococcal vaccines also may prevent pneumococcal disease caused by nonvaccine serotypes that are serotypically related to a vaccine strain (e.g., 6A and 6B). However, because current vaccines do not eliminate all pneumococcal invasive infections, penicillin prophylaxis is recommended for children at high risk of invasive pneumococcal disease, including children with asplenia or sickle cell disease. Oral penicillin V potassium (125 mg bid for children <3 yr; 250 mg bid for children ≥3 yr) decreases the incidence of pneumococcal sepsis in children with sickle cell disease. Once monthly intramuscular benzathine penicillin G (600,000 U q 3-4 wk for children <60 lb; 1,200,000 U q 3-4 wk for children ≥60 lb) may also provide prophylaxis. Erythromycin may be used in children with penicillin allergy, but its efficacy is unproved. Prophylaxis in sickle cell disease has been safely discontinued after the 5th birthday in children who have received all recommended pneumococcal vaccine doses and who had not experienced invasive pneumococcal disease. Prophylaxis is often administered for at least 2 yr after splenectomy or up to 5 yr of age. Efficacy in children >5 yr of age and adolescents is unproved. If oral antibiotic prophylaxis is used, strict compliance must be encouraged. Given the rapid emergence of penicillin-resistant pneumococci, especially in children receiving long-term, low-dose therapy, prophylaxis cannot be relied on to prevent disease. High-risk children with fever should be promptly evaluated and treated regardless of vaccination or penicillin prophylaxis history.
American Academy of Pediatrics. Recommendations for the prevention of Streptococcus pneumoniae infections in infants and children: use of 13-valent pneumococcal conjugate vaccine (PCV13) and pneumococcal polysaccharide vaccine (PPSV23). Pediatrics. 2010;126:186-190.
American Academy of Pediatrics. Red book: 2009 report of the Committee on Infectious Diseases, ed 28. Elk Grove Village, IL: American Academy of Pediatrics; 2009. 524–535
Centers for Disease Control and Prevention. Licensure of 1 13-valent pneumococcal conjugate vaccine (PCV13) and recommendations for use among children—ACIP, 2010. MMWR Morb Mortal Wkly Rep. 2010;59:258-261.
Centers for Disease Control and Prevention. Effects of new penicillin susceptibility breakpoints for Streptococcus pneumoniae—United States, 2006–2007. MMWR Morb Mortal Wkly Rep. 2008;57:1353-1357.
Centers for Disease Control and Prevention. Direct and indirect effects of routine vaccination of children with 7-valent pneumococcal conjugate vaccine on incidence of invasive pneumococcal disease—United States, 1998–2003. MMWR Morb Mortal Wkly Rep. 2005;54:893-897.
Centers for Disease Control and Prevention. Emergence of antimicrobial-resistant serotype 19A Streptococcus pneumonia—Massachusetts, 2001–2006. MMWR Morb Mortal Wkly Rep. 2007;56:1077-1080.
Halasa NB, Shankar SM, Talbot TR, et al. Incidence of invasive pneumococcal disease among individuals with sickle cell disease before and after the introduction of the pneumococcal conjugate vaccine. Clin Infect Dis. 2007;44:1428-1433.
Hendrickson DJ, Blumberg DA, Joad JP, et al. Five-fold increase in pediatric parapneumonic empyema since introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2008;27:1030-1032.
Hsu HE, Shutt KA, Moore MR, et al. Effect of pneumococcal conjugate vaccine on pneumococcal meningitis. N Engl J Med. 2009;360:244-256.
Huss A, Scott P, Stuck AE, et al. Efficacy of pneumococcal vaccination in adults: a meta-analysis. CMAJ. 2009;180:48-58.
Kaplan SL, Barson WJ, Lin PL, et al. Serotype 19A is the most common serotype causing invasive pneumococcal infections in children. Pediatrics. 2010;125:429-436.
Kaplan SL, Mason EO, Barson WJ, et al. Outcome of invasive infections outside the central nervous system caused by Streptococcus pneumoniae isolates nonsusceptible to ceftriaxone in children treated with beta-lactam antibiotics. Pediatr Infect Dis J. 2001;20:392-396.
Kyaw MH, Lynfield R, Schaffner W, et al. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumonia. N Engl J Med. 2006;354:1455-1463.
Lucero MG, Nohynek H, Williams G, et al. Efficacy of an 11-valent pneumococcal conjugate vaccine against radiologically confirmed pneumonia among children less than 2 years of age in the Philippines. Pediatr Infect Dis J. 2009;28:455-462.
Lynch JP, Zhanel GG. Streptococcus pneumonia: does antimicrobial resistance matter? Semin Respir Crit Care Med. 2009;30:210-238.
Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis. 2008;198:962-970.
Musher DM, Rueda-Jaimes AM, Graviss EA, et al. Effect of pneumococcal vaccination: a comparison of vaccination rates in patients with bacteremic and nonbacteremic pneumococcal pneumonia. Clin Infect Dis. 2006;43:1004-1008.
Neuman MI, Harper MB. Time to positivity of blood cultures for children with Streptococcus pneumoniae bacteremia. Clin Infect Dis. 2001;33:1324-1328.
O’Brien KL, Wolfson LJ, Watt JP, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374:893-902.
Ongkasuwan J, Valdez TA, Hulten KG, et al. Pneumococcal mastoiditis in children and the emergence of multidrug-resistant serotype 19A isolates. Pediatrics. 2008;122:34-39.
Park SY, Van Beneden CA, Pilishvili T, et al. invasive pneumococcal infections among vaccinated children in the United States. J Pediatr. 2010;156:478-483.
Pelton SI, Huot H, Finkelstein JA, et al. Emergence of 19A as virulent and multidrug resistant pneumococcus in Massachusetts following universal immunization of infants with pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2007;26:468-472.
Pichichero ME, Casey JR. Emergence of a multiresistant serotype 19A pneumococcal strain not included in the 7-valent conjugate vaccine as an otopathogen in children. JAMA. 2007;298:1772-1778.
Poehling KA, Lafleur BJ, Szilagyi PS, et al. Population-based impact of pneumococcal conjugate vaccine in young children. Pediatrics. 2004;114:755-761.
Reefhuis J, Honein MA, Whitney CG, et al. Risk of bacterial meningitis in children with cochlear implants. N Eng J Med. 2003;349:335-345.
Reinert P, Benkerrou M, Montalembert M, et al. Immunogenicity and safety of a pneumococcal conjugate 7-valent vaccine in infants with sickle cell disease. Pediatr Infect Dis J. 2007;26:1105-1109.
Rello J, Lisbon T, Lujan M, et al. Severity of pneumococcal pneumonia associated with genomic bacterial load. Chest. 2009;136:832-840.
Talbot TR, Poehling KA, Hartert T, et al. Reduction in high rates of antibiotic-nonsusceptible invasive pneumococcal disease in Tennessee after introduction of the pneumococcal conjugate vaccine. Clin Infect Dis. 2004;39:641-651.
Von Gottberg A, Klugman KP, Cohen C, et al. Emergence of levofloxacin-non-susceptible Streptococcus pneumoniae and treatment for multidrug-resistant tuberculosis in children in South Africa: a cohort observational surveillance study. Lancet. 2008;371:1108-1113.
Waters AM, Kerecuk L, Luk D, et al. Hemolytic uremic syndrome associated with invasive pneumococcal disease: the United Kingdom experience. J Pediatr. 2007;151:140-144.