Chapter 676 Osteomyelitis
Bone infections in children are relatively common and important because of their potential to cause permanent disability. Early recognition of osteomyelitis in young patients before extensive infection develops and prompt institution of appropriate medical and surgical therapy minimize permanent damage. The risk is greatest if the physis (the growth plate of bone) is damaged.
Etiology
Bacteria are the most common pathogens in acute skeletal infections. In osteomyelitis, Staphylococcus aureus (Chapter 174.1) is the most common infecting organism in all age groups, including newborns. Community-acquired methicillin-resistant S. aureus (CA-MRSA) isolates account for >50% of S. aureus isolates recovered from children with osteomyelitis in some reports. The USA300 clone of S. aureus is the most common among CA-MRSA isolates in the USA and is more likely to cause venous thrombosis in children with acute osteomyelitis than other S. aureus clones or other bacteria for reasons that are not known.
Group B streptococcus (Chapter 177) and gram-negative enteric bacilli (Escherichia coli, Chapter 192) are also prominent pathogens in neonates; group A streptococcus (Chapter 176) constitutes <10% of all cases. After 6 yr of age, most cases of osteomyelitis are caused by S. aureus, streptococcus, or Pseudomonas aeruginosa (Chapter 197). Cases of Pseudomonas infection are related almost exclusively to puncture wounds of the foot, with direct inoculation of P. aeruginosa from the foam padding of the shoe into bone or cartilage, which develops as osteochondritis. Salmonella species (Chapter 190) and S. aureus are the two most common causes of osteomyelitis in children with sickle cell anemia. S. pneumoniae (Chapter 175) most commonly causes osteomyelitis in children <24 mo of age or children with sickle cell anemia. Bartonella henselae (Chapter 201.2) can cause osteomyelitis of any bone but especially in pelvic and vertebral bones.
Kingella kingae may be the second most common cause of osteomyelitis in children <5 yr of age in some parts of the world. K. kingae is a slow-growing, gram-negative, β-hemolytic bacterium in pairs or short chains of short bacilli. The organism, once thought to be rare, is increasingly recognized as a cause of osteomyelitis, spondylodiskitis, septic arthritis and bacteremia, and, less commonly, in endocarditis. It has been identified as the causative agent in pneumonia and meningitis. Nearly 90% of identified K. kingae infections have been in young children.
Infection with atypical mycobacteria (Chapter 209), S. aureus, or Pseudomonas can occur after penetrating injuries. Fungal infections usually occur as part of multisystem disseminated disease; Candida (Chapter 226) osteomyelitis sometimes complicates fungemia in neonates with or without indwelling vascular catheters.
A microbial etiology is confirmed in ∼60% of cases of osteomyelitis. Blood cultures are positive in ∼50% of patients. Prior antibiotic therapy and the inhibitory effect of pus on microbial growth might explain the low bacterial yield.
Epidemiology
The median age of children with musculoskeletal infections is ∼6 yr. The incidence of osteomyelitis in children is estimated to be 1 : 5,000. Bone infections are more common in boys than girls; the behavior of boys might predispose them to traumatic events. Except for the increased incidence of skeletal infection in patients with sickle cell disease, there is no predilection for osteomyelitis based on race.
The majority of osteomyelitis cases in previously healthy children are hematogenous. Minor closed trauma is a common preceding event in cases of osteomyelitis, occurring in ∼30% of patients. Infection of bones can follow penetrating injuries or open fractures. Bone infection following orthopedic surgery is uncommon. Impaired host defenses also increase the risk of skeletal infection. Other risk factors are noted in Table 676-1.
Table 676-1 MICROORGANISMS ISOLATED FROM PATIENTS WITH OSTEOMYELITIS AND THEIR CLINICAL ASSOCIATIONS
| MOST COMMON CLINICAL ASSOCIATION | MICROORGANISM |
|---|---|
| Frequent microorganism in any type of osteomyelitis | Staphylococcus aureus (susceptible or resistant to methicillin) |
| Foreign body–associated infection | Coagulase-negative staphylococci, other skin flora, atypical mycobacteria |
| Common in nosocomial infections | Enterobacteriaceae, Pseudomonas aeruginosa, Candida spp. |
| Decubitus ulcer | S. aureus, streptococci and/or anaerobic bacteria |
| Sickle cell disease | Salmonella spp., S. aureus, or Streptococcus pneumoniae |
| Exposure to kittens | Bartonella henselae |
| Human or animal bites | Pasteurella multocida or Eikenella corrodens |
| Immunocompromised patients | Aspergillus spp., Candida albicans, or Mycobacteria spp. |
| Populations in which tuberculosis is prevalent | Mycobacterium tuberculosis |
| Populations in which these pathogens are endemic | Brucella spp., Coxiella burnetii, fungi found in specific geographic areas (coccidioidomycosis, blastomycosis, histoplasmosis) |
Modified From Lew DP, Waldvogel FA: Osteomyelitis, Lancet 364:369–379, 2004.
Pathogenesis
The unique anatomy and circulation of the ends of long bones result in the predilection for localization of bloodborne bacteria. In the metaphysis, nutrient arteries branch into nonanastomosing capillaries under the physis, which make a sharp loop before entering venous sinusoids draining into the marrow. Blood flow in this area is thought to be “sluggish,” predisposing to bacterial invasion. Once a bacterial focus is established, phagocytes migrate to the site and produce an inflammatory exudate (metaphyseal abscess). The generation of proteolytic enzymes, toxic oxygen radicals, and cytokines results in decreased oxygen tension, decreased pH, osteolysis, and tissue destruction. As the inflammatory exudate progresses, pressure increases spread through the porous metaphyseal space via the haversian system and Volkmann canals into the subperiosteal space. Purulence beneath the periosteum may lift the periosteal membrane of the bony surface, further impairing blood supply to the cortex and metaphysis.
In newborns and young infants, transphyseal blood vessels connect the metaphysis and epiphysis, so it is common for pus from the metaphysis to enter the joint space. This extension through the physis has the potential to result in abnormal growth and bone or joint deformity. During the latter part of the 1st year of life, the physis forms, obliterating the transphyseal blood vessels. Joint involvement, once the physis forms, can occur in joints where the metaphysis is intra-articular (hip, ankle, shoulder, and elbow), and subperiosteal pus ruptures into the joint space.
In later childhood, the periosteum becomes more adherent, favoring pus to decompress through the periosteum. Once the growth plate closes in late adolescence, hematogenous osteomyelitis more often begins in the diaphysis and can spread to the entire intramedullary canal.
Clinical Manifestations
The earliest signs and symptoms of osteomyelitis, often subtle and nonspecific, are generally highly dependent on the age of the patient. Neonates might exhibit pseudoparalysis or pain with movement of the affected extremity (e.g., diaper changes). Half of neonates do not have fever and might not appear ill. Older infants and children are more likely to have fever, pain, and localizing signs such as edema, erythema, and warmth. With involvement of the lower extremities, limp or refusal to walk is seen in approximately half of patients.
Focal tenderness over a long bone can be an important finding. Local swelling and redness with osteomyelitis can mean that the infection has spread out of the metaphysis into the subperiosteal space, representing a secondary soft-tissue inflammatory response. Pelvic osteomyelitis can manifest with subtle findings such as hip, thigh, or abdominal pain. Back pain with or without tenderness to palpation overlying the vertebral processes is noted in vertebral osteomyelitis.
Long bones are principally involved in osteomyelitis (Table 676-2); the femur and tibia are equally affected and together constitute almost half of all cases. The bones of the upper extremities account for one fourth of all cases. Flat bones are less commonly affected.
Table 676-2 SITES OF OSTEOMYELITIS IN CHILDREN
| BONE | % |
|---|---|
| Femur | 23-28 |
| Tibia | 20-24 |
| Humerus | 5-13 |
| Radius | 5-6 |
| Phalanx | 3-5 |
| Pelvis | 4-8 |
| Calcaneus | 4-8 |
| Ulna | 4-8 |
| Metatarsal | ∼2 |
| Vertebrae | ∼2 |
| Sacrum | ∼2 |
| Clavicle | ∼2 |
| Skull | ∼1 |
| Carpal bone | <1 |
| Rib | <1 |
| Metacarpal | <1 |
| Cuboid | <1 |
| Cuneiform | <1 |
| Pyriform aperture | <1 |
| Olecranon | <1 |
| Maxilla | <1 |
| Mandible | <1 |
| Scapula | <1 |
| Sternum | <1 |
| Foot | 1 |
Modified from Gafur OA, Copley LA, Hollmig ST, et al: The impact of the current epidemiology of pediatric musculoskeletal infection on evaluation and treatment guidelines, J Pediatr Orthop 28(7):777–785, 2008.
There is usually only a single site of bone or joint involvement. Several bones are infected in <10% of cases; the exception is osteomyelitis in neonates, in whom two or more bones are involved in almost half of the cases. Children with subacute symptoms and focal finding in the metaphyseal area (usually of tibia) might have a Brodie abscess, with radiographic lucency and surrounding reactive bone.
Diagnosis
The diagnosis of osteomyelitis is clinical; blood cultures should be performed in all suspected cases. Depending on the results of imaging studies (see later) aspiration or biopsy of bone or subperiosteal abscess for Gram stain, culture, and possibly bone histology provides the optimal specimen for culture to confirm the diagnosis. These specimens are often obtained by the interventional radiologist or at the time of surgical drainage by the orthopedic surgeon. Direct inoculation of clinical specimens into aerobic blood culture bottles can improve the recovery of K. kingae, particularly if held for 1 wk. Polymerase chain reaction appears to be the most sensitive technique to detect K. kingae, with detection up to 6 days after antibiotics are initiated.
There are no specific laboratory tests for osteomyelitis. The white blood cell count and differential, erythrocyte sedimentation rate (ESR), or C-reactive protein (CRP) are generally elevated in children with bone infections but are nonspecific and not helpful in distinguishing between skeletal infection and other inflammatory processes. The leukocyte count and ESR may be normal during the first few days of infection, and normal test results do not preclude the diagnosis of skeletal infection. However, most children with acute hematogenous osteomyelitis have elevations in the ESR and/or CRP. Monitoring elevated ESR and CRP may be of value in assessing response to therapy or identifying complications.
Radiographic Evaluation
Radiographic studies play a crucial role in the evaluation of osteomyelitis. Conventional radiographs, ultrasonography, CT, MRI, and radionuclide studies can all contribute to establishing the diagnosis. Plain radiographs are often used for initial evaluation to exclude other causes such as trauma and foreign bodies. MRI has emerged as the most sensitive and specific test and is widely used for diagnosis. The sequence of radionuclide studies or MRI is often determined by age, site, and clinical presentation.
Plain Radiographs
Within 72 hr of onset of symptoms of osteomyelitis, plain radiographs of the involved site using soft-tissue technique and compared to the opposite extremity, if necessary, can show displacement of the deep muscle planes from the adjacent metaphysis caused by deep-tissue edema. Lytic bone changes are not visible on radiographs until 30-50% of the bony matrix is destroyed. Tubular long bones do not show lytic changes for 7-14 days after onset of infection. Infection in flat and irregular bones can take longer to appear.
Computed Tomography and Magnetic Resonance Imaging
CT can demonstrate osseous and soft-tissue abnormalities and is ideal for detecting gas in soft tissues. In selected children who cannot remain still or tolerate sedation, CT is a valuable imaging modality. MRI is more sensitive than CT or radionuclide imaging in acute osteomyelitis and is the best radiographic imaging technique for identifying abscesses and for differentiating between bone and soft-tissue infection. MRI provides precise anatomic detail of subperiosteal pus and accumulation of purulent debris in the bone marrow and metaphyses for possible surgical intervention. In acute osteomyelitis, purulent debris and edema appear dark, with decreased signal intensity on T1-weighted images, with fat appearing bright (Fig. 676-1). The opposite is seen in T2-weighted images. The signal from fat can be diminished with fat-suppression techniques to enhance visualization. Gadolinium administration can also enhance MRI. Cellulitis and sinus tracts appear as areas of high signal intensity on T2-weighted images. MRI can also demonstrate a contiguous septic arthritis, pyomyositis, or venous thrombosis.
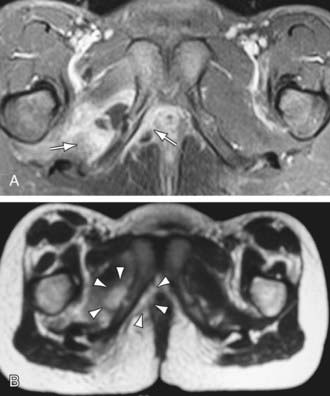
Figure 676-1 MRI of an 8 yr old girl with acute pelvic hematogenous osteomyelitis. A, Axial T1-weighted contrast-enhanced MRI with fat saturation reveals a nonenhancing fluid collection adjacent to the inflamed pubic synchondrosis. B, The fluid collection appears hyperintense on the corresponding T2-weighted image (arrowheads). In addition, a contrast enhancement within the adjacent internal obturator muscle is seen (arrow), indicating pelvic acute pelvic hematogenous osteomyelitis with complicating adjacent abscess formation and soft tissue inflammation.
(From Weber-Chrysochoou C, Corti N, Goetschel P, et al: Pelvic osteomyelitis: a diagnostic challenge in children, J Pediatr Surg 42:553–557, 2007.)
Radionuclide Studies
Radionuclide imaging can be valuable in suspected bone infections, especially early in the course of infection and/or if multiple foci are suspected or an unusual site is suspected, as in the pelvis. Technetium-99 methylene diphosphonate (99mTc), which accumulates in areas of increased bone turnover, is the preferred agent for radionuclide bone imaging (three-phase bone scan). Osteomyelitis causes increased vascularity, inflammation, and increased osteoblastic activity, resulting in an increased concentration of 99mTc. Any areas of increased blood flow or inflammation can cause increased uptake of 99mTc in the first and second phases, but osteomyelitis causes increased uptake of 99mTc in the third phase (4-6 hr). Three-phase imaging with 99mTc has excellent sensitivity (84-100%) and specificity (70-96%) in hematogenous osteomyelitis and can detect osteomyelitis within 24-48 hr after onset of symptoms. The sensitivity in neonates is much lower, due to poor bone mineralization. Advantages include infrequent need for sedation, lower cost, and the ability to image the entire skeleton for detection of multiple foci.
Differential Diagnosis
Distinguishing osteomyelitis from cellulitis or trauma (accidental or abuse) is the most common clinical circumstance. Myositis or pyomyositis can also appear similar to osteomyelitis with fever, warm and swollen extremities, and limping; tenderness to palpation of the affected soft tissue area is generally more diffuse than noted in acute osteomyelitis. Nevertheless, distinguishing myositis and pyomyositis from osteomyelitis clinically may be difficult. Myositis and pyomyositis often are found adjacent to an osteomyelitis on MRI. Pyomyositis is often caused by S. aureus or group A streptococcus. The pelvic muscles are a common site of infection and can mimic a pelvic osteomyelitis. MRI is the definitive study to identify and localize pelvic pyomyositis. An iliopsoas abscess can manifest with thigh pain, limp, and fever and must be considered in the differential diagnosis of osteomyelitis. The iliopsoas abscess may be primary (hematogenous: S. aureus) or secondary to infection in adjacent bone (S. aureus), kidney (E. coli) or intestine (E. coli, Bacteroides spp.). M. tuberculosis has been reported in patients with HIV infection.
Appendicitis, urinary tract infection, and gynecologic disease are among the conditions in the differential diagnosis of pelvic osteomyelitis. Children with leukemia commonly have bone pain or joint pain as an early symptom. Neuroblastoma with bone involvement may be mistaken for osteomyelitis. Primary bone tumors need to be considered, but fever and other signs of illness are generally absent except in Ewing sarcoma. In patients with sickle cell disease, distinguishing bone infection from infarction may be challenging. Chronic recurrent multifocal osteomyelitis (CRMO) and synovitis, acne, pustulosis, hyperostosis, and osteitis syndrome are rare noninfectious conditions in children characterized by recurrent osteoarticular inflammation and different skin conditions, palmoplantar pustulosis, psoriasis, severe acne, neutrophilic dermatosis (Sweet syndrome, Chapter 163), and pyoderma gangrenosum.
Treatment
Optimal treatment of skeletal infections requires collaborative efforts of pediatricians, orthopedic surgeons, and radiologists. Obtaining material for culture (blood, periosteal abscess, bone) before antibiotics are given is essential. Because most patients with osteomyelitis have an indolent, non–life-threatening condition, cultures should be obtained even if there is a delay of a few hours in initiating antibiotics.
Antibiotic Therapy
The initial empirical antibiotic therapy is based on knowledge of likely bacterial pathogens at various ages, the results of the Gram stain of aspirated material, and additional considerations. In neonates, an antistaphylococcal penicillin, such as nafcillin or oxacillin (150-200 mg/kg/24 hr divided q6h IV), and a broad-spectrum cephalosporin, such as cefotaxime (150-225 mg/kg/24 hr divided q8h IV), provide coverage for the S. aureus, group B streptococcus, and gram-negative bacilli. If methicillin-resistant Staphylococcus is suspected, vancomycin is substituted for nafcillin. If the neonate is a small premature infant or has a central vascular catheter, the possibility of nosocomial bacteria (Pseudomonas or coagulase-negative staphylococci) or fungi (Candida) should be considered. In older infants and children, the principal pathogens are S. aureus and streptococcus.
A major factor influencing the selection of empirical therapy is the rate of methicillin resistance among community S. aureus isolates. If MRSA accounts for ≥10% of community S. aureus isolates, including an antibiotic effective against CA-MRSA in the initial empirical antibiotic regimen is suggested. Vancomycin (45 mg/kg/24 hr divided q8h or 60 mg/kg/24 hr divided q6h IV) is the gold standard agent for treating invasive MRSA infections, especially when the child is critically ill. Clindamycin (30-40 mg/kg/24 hr q8hr) is also recommended when the rate of clindamycin resistance is ≤10% among community S. aureus isolates and the child is not severely ill. Cefazolin (100 mg/kg/24 hr divided q8h IV) or nafcillin (150-200 mg/kg/24 hr divided q6h) is the agent of choice for parenteral treatment of osteomyelitis caused by methicillin-susceptible S. aureus. Penicillin is first-line therapy for treating osteomyelitis due to susceptible strains of S. pneumoniae as well as all group A streptococcus. Cefotaxime or ceftriaxone is recommended for pneumococcal isolates with resistance to penicillin or for most Salmonella spp.
Special situations dictate deviations from the usual empirical antibiotic selection. In patients with sickle cell disease with osteomyelitis, gram-negative enteric bacteria (Salmonella) are common pathogens as well as S. aureus, so a broad-spectrum cephalosporin such as cefotaxime (150-225 mg/kg/24 hr divided q8h) is used in addition to vancomycin or clindamycin. Clindamycin (40 mg/kg/24 hr divided q6h IV) is a useful alternative drug for patients allergic to β-lactam drugs. In addition to good antistaphylococcal activity, clindamycin has broad activity against anaerobes and is useful for treating infections secondary to penetrating injuries or compound fractures. For immunocompromised patients, combination therapy is usually initiated, such as with vancomycin and ceftazidime, or with piperacillin-tazobactam and an aminoglycoside. K. kingae usually responds to β-lactam antibiotics, including cefotaxime. Although the efficacy of treating osteomyelitis caused by B. henselae is uncertain, azithromycin plus rifampin may be considered.
When the pathogen is identified and antibiotic susceptibilities are determined, appropriate adjustments in antibiotics are made as necessary. If a pathogen is not identified and a patient’s condition is improving, therapy is continued with the initially selected antibiotic. This selection is more complicated currently owing to the presence of MRSA isolates in the community. If a pathogen is not identified and a patient’s condition is not improving, reaspiration or biopsy and the possibility of a noninfectious condition should be considered.
Duration of antibiotic therapy is individualized depending on the organism isolated and clinical course. For most infections including those caused by S. aureus, the minimal duration of antibiotics is 21-28 days, provided that the patient shows prompt resolution of signs and symptoms (within 5-7 days) and the CRP and ESR have normalized; a total of 4-6 wk of therapy may be required. For group A streptococcus, S. pneumoniae, or H. influenzae type b, treatment duration maybe shorter. A total of 7-10 postoperative days of treatment is adequate for Pseudomonas osteochondritis when thorough curettage of infected tissue has been performed. Immunocompromised patients generally require prolonged courses of therapy, as do patients with mycobacterial or fungal infection.
Changing antibiotics from the intravenous route to oral administration when a patient’s condition clearly has improved and the child is afebrile for ≥48-72 hr, may be considered. For the oral antibiotic regimen with β-lactam drugs for susceptible staphylococcal or streptococcal infection, cephalexin (80-100 mg/kg/24 hr q8h) or oral clindamycin (30-40 mg/kg/24 hr q8h) can be used to complete therapy for children with clindamycin-susceptible CA-MRSA or for patients who are seriously allergic or cannot tolerate β-lactam antibiotics. The oral regimen decreases the risk of complications related to prolonged intravenous therapy, is more comfortable for patients, and permits treatment outside the hospital if adherence to treatment can be ensured. Outpatient intravenous antibiotic therapy via a central venous catheter can be used for completing therapy at home, as an alternative; however, catheter-related complications, including infection or mechanical problems, can lead to readmission or emergency department visits.
In children with venous thrombosis complicating osteomyelitis, anticoagulants generally are administered under the supervision of a hematologist until the thrombus has resolved.
Surgical Therapy
When frank pus is obtained from subperiosteal or metaphyseal aspiration or is suspected based on MRI findings, a surgical drainage procedure is usually indicated. Surgical intervention is also often indicated after a penetrating injury and when a retained foreign body is possible. In selected cases, catheter drainage performed by an interventional radiologist is adequate.
Treatment of chronic osteomyelitis consists of surgical removal of sinus tracts and sequestrum, if present. Antibiotic therapy is continued for several months or longer until clinical and radiographic findings suggest that healing has occurred. Monitoring the CRP or ESR is not helpful in most cases of chronic osteomyelitis.
Physical Therapy
The major role of physical medicine is a preventive one. If a child is allowed to lie in bed with an extremity in flexion, limitation of extension can develop within a few days. The affected extremity should be kept in extension with sandbags, splints, or, if necessary, a temporary cast. Casts are also indicated when there is a potential for pathologic fracture. After 2-3 days, when pain is easing, passive range of motion exercises are started and continued until the child resumes normal activity. In neglected cases with flexion contractures, prolonged physical therapy is required.
Prognosis
When pus is drained and appropriate antibiotic therapy is given, the improvement in signs and symptoms is rapid. Failure to improve or worsening by 72 hr requires review of the appropriateness of the antibiotic therapy, the need for surgical intervention, or the correctness of the diagnosis. Acute-phase reactants may be useful as monitors. The serum CRP typically normalizes within 7 days after start of treatment, whereas the ESR typically rises for 5-7 days, and then falls slowly, dropping sharply after 10-14 days. Failure of either of these acute-phase reactants to follow the usual course should raise concerns about the adequacy of therapy. Recurrence of disease and development of chronic infection after treatment occur in <10% of patients.
Because children are in a dynamic state of growth, sequelae of skeletal infections might not become apparent for months or years; therefore, long-term follow-up is necessary with close attention to range of motion of joints and bone length. Although firm data about the impact of delayed treatment on outcome are not available, it appears that initiation of medical and surgical therapy within 1 wk of onset of symptoms provides a better prognosis than delayed treatment.
Arnold SR, Elias D, Buckingham SC, et al. Changing patterns of acute hematogenous osteomyelitis and septic arthritis. Emergence of community-associated methicillin-resistant Staphylococcus aureus. J Pediatr Orthop. 2006;26:703-708.
Bocchini C, Hulten KG, Mason EOJr, et al. Panton-Valentine leukodicin genes are associated with enhanced inflammatory response and local disease in acute hematogenous Staphylococcus aureus osteomyelitis in children. Pediatrics. 2006;117:433-440.
Browne LP, Mason EO, Kaplan SL, et al. Optimal imaging strategy for community-acquired Staphylococcus aureus musculoskeletal infections in children. Pediatr Radiol. 2008;38:841-847.
Chen CJ, Chiu CH, Lin TY, et al. Experience with linezolid therapy in children with osteoarticular infections. Pediatr Infect Dis J. 2007;26:985-988.
Chometon S, Benito Y, Chaker M, et al. Specific real-time polymerase chain reaction places Kingella kingae as the most common cause of osteoarticular infections in young children. Pediatr Infect Dis J. 2007;26:377-381.
Connolly LP, Connolly SA, Druback LA, et al. Acute hematogenous osteomyelitis of children: assessment of skeletal scintigraphy-based diagnosis in the era of MRI. J Nucl Med. 2002;43:1310-1316.
Dubnov-Raz G, Ephros M, Garty BZ, et al. Invasive pediatric Kingella kingae infections. Pediatr Infect Dis J. 2010;29(7):639-642.
Dubnov-Raz G, Scheuerman O, Chodick G, et al. Invasive Kingella kingae infections in children: clinical and laboratory characteristics. Pediatrics. 2008;122:1305-1309.
Fernandez M, Carrol CL, Baker CJ. Discitis and vertebral osteomyelitis in children: an 18-year review. Pediatrics. 2000;105:1299-1304.
Floyed RL, Steele RW. Culture-negative osteomyelitis. Pediatr Infect Dis J. 2003;22:731-735.
Gafur OA, Copley LA, Hollmig ST, et al. The impact of the current epidemiology of pediatric musculoskeletal infection on evaluation and treatment guidelines. J Pediatr Orthop. 2008;28:777-785.
Gomez M, Maraqa N, Alvarez A, et al. Complications of outpatient parenteral antibiotic therapy in childhood. Pediatr Infect Dis J. 2001;20:541-543.
Gonzales BE, Teruya J, Mahoney DHJr, et al. Venous thrombosis associated with staphylococcal osteomyelitis in children. Pediatrics. 2006;117:1675-1679.
Gray PEA, McMullan B, Ziegler JB. Getting to the bones of the matter. Arch Dis Child Educ Pract Ed. 2010;95:178-182.
Hajjaji N, Hocqueloux L, Kerdraon R, et al. Bone infection in cat-scratch disease: a review of the literature. J Infect. 2007;54:417-421.
Ibia EO, Imoisili M, Pikis A. Group A β-hemolytic streptococcal osteomyelitis in children. Pediatrics. 2003;112:e22-e26.
Jacobs RF, McCarthy RE, Elser JM. Pseudomonas osteochondritis complicating puncture wounds of the foot in children: a 10-year evaluation. J Infect Dis. 1989;160:657-661.
Kaplan SL. Osteomyelitis in children. Infect Dis Clin North Am. 2005;19:787-797.
Lew DP, Waldvogel FA. Osteomyelitis. Lancet. 2004;364:369-379.
López VN, Ramos JM, Meseguer V, et al. Microbiology and outcomes of iliopsoas abscess in 124 patients. Medicine. 2009;88:120-130.
Maraqa NF, Gomez MM, Rathore MH. Outpatient parenteral antimicrobial therapy in osteoarticular infections in children. J Pediatr Orthop. 2002;22:506-510.
Marschall J, Bhavan KP, Olsen MA, et al. The impact of prebiopsy antibiotics on pathogen recovery in hematogenous vertebral osteomyelitis. Clin Infect Dis. 2010;52:867-872.
Martinez-Aguilar G, Hammerman WA, Mason EOJr, et al. Clindamycin treatment of invasive infections caused by community-acquired, methicillin-resistant and methicillin-susceptible S. aureus in children. Pediatr Infect Dis J. 2003;22:593-598.
Nelson JD. Bugs, drugs and bones: a pediatric infectious disease specialist reflects on management of musculoskeletal infections. J Pediatr Orthop. 1999;19:141-142.
Nguyen S, Pasquet A, Legout L, et al. Efficacy and tolerance of rifampicin-linezolid compared with rifampicin-cotrimoxazole combinations in prolonged oral therapy for bone and joint infections. Clin Microbiol Infect. 2009;15:1163-1169.
Pääkkönen M, Kalio MJT, Kallio PE, et al. Sensitivity of erythrocyte sedimentation rate and C-reactive protein in childhood bone and joint infections. Clin Orthop Relat Res. 2009;468:861-866.
Pannaraj PS, Hulten KG, Gonzalez BE, et al. Infective pyomyositis and myositis in children in the era of community-acquired, methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis. 2006;43:953-960.
Peltola H, Paakkonen M, Kallio P, et al. Short- versus long-term antimicrobial treatment for acute hematogenous osteomyelitis of childhood. Pediatr Infect Dis J. 2010;29(12):1123-1128.
Saavedra-Lozano J, Mejías A, Ahmed N, et al. Changing trends in acute osteomyelitis in children: impact of methicillin-resistant Staphylococcus aureus infections. J Pediatr Orthop. 2008;28:569-575.
Saigal G, Azouz EM, Abdenour G. Imaging of osteomyelitis with special reference to children. Semin Musculoskelet Radiol. 2004;8:255-265.
Shih HN, Shih LY, Wong YC. Diagnosis and treatment of subacute osteomyelitis. J Trauma. 2005;58:83-87.
Verdier I, Gayet-Ageron A, Ploton C, et al. Contribution of a broad range polymerase chain reaction to the diagnosis of osteoarticular infections caused by Kingella kingae. Pediatr Infect Dis J. 2005;24:692-696.
Weber-Chrysochoou C, Corti N, Goetschel P, et al. Pelvic osteomyelitis: a diagnostic challenge in children. J Pediatr Surg. 2007;42:553-557.
Yagupsky P. K. kingae infections of the skeletal system in children: diagnosis and therapy. Expert Rev Anti Infect Ther. 2004;2:787-794.
Yagupsky P. Kingella kingae: from medical rarity to an emerging paediatric pathogen. Lancet Infect Dis. 2004;4:358-367.
Yagupsky P, Porsch E, St Geme JW3rd. Kingella kingae: an emerging pathogen in young children. Pediatrics. 2011;127(3):557-565.
Zaoutis T, Localio AR, Leckerman K, et al. Prolonged intravenous therapy versus early transition to oral antimicrobial therapy for acute osteomyelitis in children. Pediatrics. 2009;123:636-642.