Chapter 677 Septic Arthritis
Septic arthritis in infants and children has the potential to cause permanent disability. Early recognition of septic arthritis in young patients before extensive infection develops and prompt institution of appropriate medical and surgical therapy minimize further damage to the synovium, adjacent cartilage, and bone.
Etiology
Historically, Haemophilus influenzae type b (Chapter 186) accounted for more than half of all cases of bacterial arthritis in infants and young children. Since the development of the conjugate, it is now a rare cause; Staphylococcus aureus (Chapter 174.1) has emerged as the most common infection in all age groups. Methicillin-resistant S. aureus accounts for a high proportion (>25%) of community S. aureus isolates in many areas of the USA and throughout the world. Group A streptococcus (Chapter 176) and Streptococcus pneumoniae (pneumococcus) (Chapter 175) historically cause 10-20%; S. pneumoniae is most likely in the first 2 years of life. Kingella kingae is recognized as a relatively common etiology with improved culture and polymerase chain reaction (PCR) methods in children <5 yr old (Chapter 676). In sexually active adolescents, gonococcus (Chapter 185) is a common cause of septic arthritis and tenosynovitis, usually of small joints or as a monoarticular infection of a large joint (knee). Neisseria meningitidis (Chapter 184) can cause either a septic arthritis that occurs in the first few days of illness or a reactive arthritis that is typically seen several days after antibiotics have been initiated. Group B streptococcus (Chapter 177) is an important cause of septic arthritis in neonates.
Fungal infections usually occur as part of multisystem disseminated disease; Candida arthritis can complicate systemic infection in neonates with or without indwelling vascular catheters. Primary viral infections of joints are rare, but arthritis accompanies many viral (parvovirus, mumps, rubella live vaccines) syndromes, suggesting an immune-mediated pathogenesis.
A microbial etiology is confirmed in about 65% of cases of septic arthritis. Prior antibiotic therapy and the inhibitory effect of pus on microbial growth might explain the low bacterial yield. Additionally, some cases treated as bacterial arthritis are actually postinfectious (gastrointestinal or genitourinary) reactive arthritis (Chapter 151) rather than primary infection. Lyme disease produces an arthritis more like a rheumatologic disorder and not typically suppurative.
Epidemiology
Septic arthritis is more common in young children. Half of all cases occur by 2 yr of age and three fourths of all cases occur by 5 yr of age. Adolescents and neonates are at risk of gonococcal septic arthritis.
The majority of infections in otherwise healthy children are of hematogenous origin. Infection of joints can follow penetrating injuries or procedures such as trauma, arthroscopy, prosthetic joint surgery, intra-articular steroid injection, and orthopedic surgery, although this is uncommon. Immunocompromised patients and those with rheumatologic joint disease are also at increased risk of joint infection.
Pathogenesis
Septic arthritis primarily occurs as a result of hematogenous seeding of the synovial space. Less often, organisms enter the joint space by direct inoculation or extension from a contiguous focus. The synovial membrane has a rich vascular supply and lacks a basement membrane, providing an ideal environment for hematogenous seeding. The presence of bacterial products (endotoxin or other toxins) within the joint space stimulates cytokine production (tumor necrosis factor-α, interleukin-1) within the joint, triggering an inflammatory cascade. The cytokines stimulate chemotaxis of neutrophils into the joint space, where proteolytic enzymes and elastases are released by neutrophils, damaging the cartilage. Proteolytic enzymes released from the synovial cells and chondrocytes also contribute to destruction of cartilage and synovium. Bacterial hyaluronidase breaks down the hyaluronic acid in the synovial fluid, making the fluid less viscous and diminishing its ability to lubricate and protect the joint cartilage. Damage to the cartilage can occur through increased friction, especially for weight-bearing joints. The increased pressure within the joint space from accumulation of purulent material can compromise the vascular supply and induce pressure necrosis of the cartilage. Synovial and cartilage destruction results from a combination of proteolytic enzymes and mechanical factors.
Clinical Manifestations
Most septic arthritides are monoarticular. The signs and symptoms of septic arthritis depend on the age of the patient. Early signs and symptoms may be subtle, particularly in neonates. Septic arthritis in neonates and young infants is often associated with adjacent osteomyelitis caused by transphyseal spread of infection, although osteomyelitis contiguous with an infected joint can be seen at any age (Chapter 676).
Older infants and children might have fever and pain, with localizing signs such as swelling, erythema, and warmth of the affected joint. With involvement of joints of the pelvis and lower extremities, limp or refusal to walk is often seen.
Erythema and edema of the skin and soft tissue overlying the site of infection are seen earlier in septic arthritis than in osteomyelitis, because the bulging infected synovium is usually more superficial, whereas the metaphysis is located more deeply. Septic arthritis of the hip is an exception because of the deep location of the hip joint.
Joints of the lower extremity constitute 75% of all cases of septic arthritis (Table 677-1). The elbow, wrist, and shoulder joints are involved in about 25% of cases, and small joints are uncommonly infected. Suppurative infections of the hip, shoulder, elbow, and ankle in older infants and children may be associated with an adjacent osteomyelitis of the proximal femur, proximal humerus, proximal radius, and distal tibia because the metaphysis extends intra-articularly.
Table 677-1 ANATOMIC DISTRIBUTION OF SEPTIC ARTHRITIS
| BONE | % |
|---|---|
| Knee | ∼40 |
| Hip | 22-40 |
| Ankle | 4-13 |
| Elbow | 8-12 |
| Wrist | 1-4 |
| Shoulder | ∼3 |
| Interphalangeal | <1 |
| Metatarsal | <1 |
| Sacroiliac | <1 |
| Acromioclavicular | <1 |
| Metacarpal | <1 |
| Toe | ∼1 |
Modified from Gafur OA, Copley LA, Hollmig ST, et al: The impact of the current epidemiology of pediatric musculoskeletal infection on evaluation and treatment guidelines, J Pediatr Orthop 28:777–785, 2008.
Diagnosis
Blood cultures should be performed in all cases of suspected septic arthritis. Aspiration of the joint fluid for Gram stain and culture when the history and physical findings indicate septic arthritis remains the definitive diagnostic technique and provides the optimal specimen for culture to confirm the diagnosis. Most large joint spaces are easy to aspirate, but the hip can pose technical problems; ultrasound guidance facilitates aspiration. Aspiration of joint pus provides the best specimen for bacteriologic culture of infection. If gonococcus is suspected, cervical, anal, and throat cultures should also be obtained. In addition to prompt inoculation onto solid media, inoculation of the specimen in blood culture bottles can increase recovery of K. kingae. PCR appears to be the most sensitive method for detecting K. kingae in joint fluid.
Synovial fluid analysis for cell count, differential, protein, and glucose has limited usefulness because noninfectious inflammatory diseases, such as rheumatic fever and rheumatoid arthritis, can also cause exuberant reaction with increased cells and protein and decreased glucose. Nevertheless, cell counts >50,000-100,000 cells/mm3 generally indicate an infectious process. Synovial fluid characteristics of septic arthritis can suggest infection but are not sufficiently specific to exclude infection.
The white blood cell count and differential, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) are generally elevated in children with joint infections but are nonspecific and might not be helpful in distinguishing between infection and other inflammatory processes. The leukocyte count and ESR may be normal during the first few days of infection, and normal test results do not preclude the diagnosis of septic arthritis. Monitoring elevated ESR and CRP may be of value in assessing response to therapy or identifying complications.
Radiographic Evaluation
Radiographic studies play a crucial role in evaluating septic arthritis. Conventional radiographs, ultrasonography, CT, MRI, and radionuclide studies can all contribute to establishing the diagnosis (Fig. 677-1).
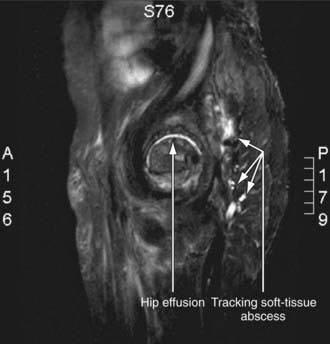
Figure 677-1 MRI of staphylococcal septic arthritis of left hip, with fluid collections between planes of gluteal muscles. Arrows indicate fluid collection.
(From Matthews CJ, Weston VC, Jones A, et al: Bacterial septic arthritis in adults, Lancet 375:846–854, 2010.)
Plain Radiographs
Plain films of septic arthritis can show widening of the joint capsule, soft-tissue edema, and obliteration of normal fat lines. Plain films of the hip can show medial displacement of the obturator muscle into the pelvis (the obturator sign), lateral displacement or obliteration of the gluteal fat lines, and elevation of the Shenton line with a widened arc.
Ultrasonography
Ultrasonography is particularly helpful in detecting joint effusion and fluid collection in the soft tissue and subperiosteal regions. Ultrasonography is highly sensitive in detecting joint effusion, particularly for the hip joint, where plain radiographs are normal in >50% of cases of septic arthritis of the hip. Ultrasonography can serve as an aid in performing hip aspiration.
Computed Tomography and Magnetic Resonance Imaging
CT and MRI may be useful in confirming the presence of joint fluid in patients with suspected osteoarthritis infections. MRI may be useful in excluding adjacent osteomyelitis.
Radionuclide Imaging
Radionuclide imaging compared to radiographs is more sensitive in providing supportive evidence of the diagnosis of septic arthritis; a scan may be positive within 2 days of the onset of symptoms. Three-phase imaging with technetium-99 methylene diphosphonate shows symmetric uptake on both sides of the joint, limited to the bony structures adjacent to the joint. Radionuclide imaging is also useful for evaluating the sacroiliac joint.
Differential Diagnosis
The differential diagnosis of septic arthritis depends on the joint or joints involved and the age of the patient. For the hip, toxic synovitis, Legg-Calvé-Perthes disease, slipped capital femoral epiphysis, psoas abscess, and proximal femoral, pelvic, or vertebral osteomyelitis as well as diskitis should be considered. For the knee, distal femoral or proximal tibial osteomyelitis, pauciarticular rheumatoid arthritis, and referred pain from the hip should be considered. Other conditions such as trauma, cellulitis, pyomyositis, sickle cell disease, hemophilia, and Henoch-Schönlein purpura can mimic purulent arthritis. When several joints are involved, serum sickness, collagen vascular disease, rheumatic fever, and Henoch-Schönlein purpura should be considered. Arthritis is one of the extraintestinal manifestations of inflammatory bowel disease. Reactive arthritis following a variety of bacterial (gastrointestinal or genital) and parasitic infections, streptococcal pharyngitis, or viral hepatitis can resemble acute septic arthritis (Chapter 151).
Treatment
Optimal treatment of septic arthritis requires cooperation of pediatricians, orthopedic surgeons, and radiologists to benefit the patient.
Antibiotic Therapy
The initial empirical antibiotic therapy is based on knowledge of likely bacterial pathogens at various ages, the results of the Gram stain of aspirated material, and additional considerations. In neonates, an antistaphylococcal penicillin, such as nafcillin or oxacillin (150-200 mg/kg/24 hr divided q6h IV), and a broad-spectrum cephalosporin, such as cefotaxime (150-225 mg/kg/24 hr divided q8h IV), provide coverage for the S. aureus, group B streptococcus, and gram-negative bacilli. If MRSA is a concern, vancomycin is selected in favor of nafcillin or oxacillin. If the neonate is a small premature infant or has a central vascular catheter, the possibility of nosocomial bacteria (Pseudomonas aeruginosa or coagulase-negative staphylococci) or fungi (Candida) should be considered.
In older infants and children with septic arthritis, empirical therapy to cover for S. aureus, streptococci, and K. kingae includes cefazolin (100-150 mg/kg/24 hr divided q8h) or nafcillin (150-200 mg/kg/24 hr divided q6h).
In areas where methicillin resistance is noted in ≥10% of community S. aureus strains (CA-MRSA), including an antibiotic that is effective against CA-MRSA isolates is suggested. Clindamycin (30-40 mg/kg divided q8h) and vancomycin (15 mg/kg q6-8h IV) are alternatives when treating CA-methicillin-resistant S. aureus infections. For immunocompromised patients, combination therapy is usually initiated, such as with vancomycin and ceftazidime or with extended-spectrum penicillins and β-lactamase inhibitors with an aminoglycoside. Adjunct therapy with dexamethasone for 4 days with antibiotic therapy appeared to benefit children with septic arthritis in one study but has not been studied in children with CA-MRSA septic arthritis.
When the pathogen is identified, appropriate changes in antibiotics are made, if necessary. If a pathogen is not identified and a patient’s condition is improving, therapy is continued with the antibiotic selected initially. If a pathogen is not identified and a patient’s condition is not improving, re-aspiration or the possibility of a noninfectious condition should be considered.
Duration of antibiotic therapy is individualized depending on the organism isolated and the clinical course. Ten to 14 days is usually adequate for streptococci, S. pneumoniae, and K. kingae; longer therapy may be needed for S. aureus and gram-negative infections. Normalization of ESR and CRP in addition to a normal examination supports discontinuing antibiotic therapy. In selected patients, obtaining a plain radiograph of the joint before completing therapy can provide evidence (typically periosteal new bone) of a previously unappreciated contiguous site of osteomyelitis that would likely prolong antibiotic treatment. Oral antibiotics can be used to complete therapy once the patient is afebrile for 48-72 hr and is clearly improving.
Surgical Therapy
Infection of the hip is generally considered a surgical emergency because of the vulnerability of the blood supply to the head of the femur. For joints other than the hip, daily aspirations of synovial fluid may be required. Generally, one or two subsequent aspirations suffice. If fluid continues to accumulate after 4-5 days, arthrotomy or video assisted arthroscopy is needed. At the time of surgery, the joint is flushed with sterile saline solution. Antibiotics are not instilled because they are irritating to synovial tissue, and adequate amounts of antibiotic are achieved in joint fluid with systemic administration.
Prognosis
When pus is drained and appropriate antibiotic therapy is given, the improvement in signs and symptoms is rapid. Failure to improve or worsening by 72 hr requires review of the appropriateness of the antibiotic therapy, the need for surgical intervention, and the correctness of the diagnosis. Acute-phase reactants may be useful as monitors. Failure of either of these acute-phase reactants to follow the usual course should raise concerns about the adequacy of therapy. Recurrence of disease and development of chronic infection after treatment occur in <10% of patients.
Because children are in a dynamic state of growth, sequelae of skeletal infections might not become apparent for months or years; therefore, long-term follow-up is necessary, with close attention to range of motion of joints and bone length. Although firm data about the impact of delayed treatment on outcome are not available, it appears that initiation of medical and surgical therapy within 1 wk of onset of symptoms provides a better prognosis than delayed treatment.
Arnold SR, Elias D, Buckingham SC, et al. Changing patterns of acute hematogenous osteomyelitis and septic arthritis. Emergence of community-associated methicillin-resistant Staphylococcus aureus. J Pediatr Orthop. 2006;26:703-708.
Bonheffer J, Haeberle B, Schaad UB, et al. Diagnosis of hematogenous osteomyelitis and septic arthritis: 20 years experience at the University Children’s Hospital Basel. Swiss Med Wkly. 2001;131:575-581.
Carrillo-Marquez M, Hulten KG, Hammerman WA, et al. USA300 is the predominant genotype causing Staphylococcus aureus septic arthritis in children. Pediatr Infect Dis J. 2009;28:1076-1080.
Centers for Disease Control and Prevention. Osteomyelitis/septic arthritis caused by Kingella kingae among day care attendees—Minnesota. MMWR Morb Mortal Wkly Rep. 2003;53:241-243.
Cheer K, Pearce S. Osteoarticular infection of the symphysis public and sacroiliac joints in active young sportsmen. BMJ. 2010;340:362-364.
Dubnov-Raz G, Scheuerman O, Chodick G, et al. Invasive Kingella kingae infections in children: clinical and laboratory characteristics. Pediatrics. 2008;122:1305-1309.
Harel L, Prais D, Bar-On E, et al. Dexamethasone therapy for septic arthritis in children. J Pediatr Orthop. 2011;31(2):211-215.
Howard A, Wilson M. Septic arthritis in children. BMJ. 2010;341:776-777.
Kang SN, Sanghera T, Mangwani J, et al. The management of septic arthritis in children. J Bone Joint Surg Br. 2009;91:1127-1133.
Matthews CJ, Weston VC, Jones A, et al. Bacterial septic arthritis in adults. Lancet. 2010;375:846-854.
Nelson JD. Bugs, drugs and bones: a pediatric infectious disease specialist reflects on management of musculoskeletal infections. J Pediatr Orthop. 1999;19:141-142.
Odio CM, Ramirez T, Arias G, et al. Double blind, randomized, placebo-controlled study of dexamethasone therapy for hematogenous septic arthritis in children. Pediatr Infect Dis J. 2003;22:883-888.
Peltola H, Paakkonen M, Kallio P, et al. Prospective, randomized trial of 10 days versus 30 day of antimicrobial treatment, including a short-term course of parenteral therapy, for childhood septic arthritis. Clin Infect Dis. 2009;48:1201-1210.
Ross JJ, Hu LT. Septic arthritis of the pubic symphysis. Medicine. 2003;82:340-345.
Saavedra-Lozano J, Mejías A, Ahmed N, et al. Changing trends in acute osteomyelitis in children: impact of methicillin-resistant Staphylococcus aureus infections. J Pediatr Orthop. 2008;28:569-575.
Schirtliff ME, Mader JT. Acute septic arthritis. Clin Microbiol Rev. 2002;15:527-554.
Sultan J, Hughes PJ. Septic arthritis or transient synovitis of the hip in children. J Bone Joint Surg Br. 2010;92:1289-1293.
Taekema HC, Landham PR, Maconochie I. Towards evidence based medicine for paediatricians. distinguishing between transient synovitis and septic arthritis in the limping child: how useful are clinical prediction tools? Arch Dis Child. 2009;94:167-168.
Wang CL, Wang SM, Yang YJ, et al. Septic arthritis in children: relationship of causative pathogens, complications, and outcome. J Microbiol Immunol Infect. 2003;36:41-46.