Chapter 186 Haemophilus influenzae
An effective vaccine to prevent Haemophilus influenzae type b disease introduced in the USA and many other countries has resulted in a dramatic decrease in the incidence of infections caused by this organism. However, mortality and morbidity from H. influenzae type b infection remain a problem worldwide, primarily in developing countries. Occasional cases of invasive disease caused by non–type b organisms continue to occur but infrequently. Nontypable members of the species are important causes of otitis media and sinusitis.
Etiology
H. influenzae is a fastidious, gram-negative, pleomorphic coccobacillus that requires factor X (hematin) and factor V (phosphopyridine nucleotide) for growth. Some H. influenzae isolates are surrounded by a polysaccharide capsule and can be serotyped into 6 antigenically and biochemically distinct types designated by letters a-f.
Epidemiology
Before the advent of an effective type b conjugate vaccine in 1988, H. influenzae type b was a major cause of serious disease among children in all countries. There was a striking age distribution of cases, with >90% in children <5 yr of age and the majority in children <2 yr of age. The annual attack rate of invasive disease was 64-129 cases/100,000 children <5 yr of age per year. Invasive disease caused by other capsular serotypes has been much less frequent but continues to occur. The incidence of invasive disease caused by b and non–type b serotypes has been estimated at about 0.08 and 1.02 cases/100,000 children <5 yr of age per year, respectively, in the USA. Nonencapsulated (nontypable) H. influenzae organisms also occasionally cause invasive disease, especially in neonates, immunocompromised children, and children in developing countries. The estimated rate of invasive disease due to nontypable H. influenzae in the USA is 1.88/100,000 children <5 yr of age per year. Nontypable isolates are common etiologic agents in otitis media, sinusitis, and chronic bronchitis.
Humans are the only natural hosts for H. influenzae, which is part of the normal respiratory flora in 60-90% of healthy children. Most isolates are nontypable. Before the advent of conjugate vaccine immunization, H. influenzae type b could be isolated from the pharynx of 2-5% of healthy preschool and school-aged children, with lower rates among infants and adults. Asymptomatic colonization with H. influenzae type b occurs at a much lower rate in immunized populations.
The continued circulation of the type b organism despite current vaccine coverage levels suggests that elimination of type b disease may be a formidable task. The few cases of type b invasive disease in the USA now occur in both unvaccinated and fully vaccinated children. Approximately one half of cases occur in young infants too young to have received a complete primary vaccine series. Among the cases in patients who are old enough to have received a complete vaccine series, the majority are underimmunized. To highlight this point, during a recent shortage of H. influenzae type b vaccine, invasive disease developed in five children in Minnesota, all of whom were incompletely immunized. Continued efforts will be necessary to provide currently available conjugate vaccines to children in developing countries, where affordability remains an important issue.
In the pre-vaccine era, certain groups and individuals had an increased incidence of invasive type b disease, including Alaskan Eskimos, Apaches, Navajos, and African-Americans. Persons with certain chronic medical conditions were also known to be at increased risk for invasive disease, including those with sickle cell disease, asplenia, congenital and acquired immunodeficiencies, and malignancies. Unvaccinated infants with invasive H. influenzae type b infection are also at increased risk for recurrence, reflecting the fact that they typically do not develop a protective immune response to H. influenzae.
Socioeconomic risk factors for invasive H. influenzae type b disease included child care outside the home, the presence of siblings of elementary school age or younger, short duration of breast-feeding, and parental smoking. A history of otitis media was associated with an increased risk for invasive disease. Much less is known about the epidemiology of invasive disease due to non–type b strains, and it is not clear whether the epidemiologic features of type b disease apply to disease caused by non–type b isolates.
Among age-susceptible household contacts who have been exposed to a case of invasive H. influenzae type b disease, there is increased risk for secondary cases of invasive disease in the first 30 days, especially in susceptible children <24 mo of age. Whether a similar increased risk occurs for contacts of individuals with non–type b disease is unknown.
The mode of transmission is most commonly direct contact or inhalation of respiratory tract droplets containing H. influenzae. The incubation period for invasive disease is variable, and the exact period of communicability is unknown. Most children with invasive H. influenzae type b disease are colonized in the nasopharynx before initiation of antimicrobial therapy; 25-40% may remain colonized during the first 24 hr of therapy.
With the decline of disease caused by type b organisms, disease caused by other serotypes (a, c-f) and nontypable organisms has been recognized more clearly. There is no evidence that these non–type b infections have increased in frequency. However, clusters of type a and, less often, type f and type e infections have occurred.
Pathogenesis
The pathogenesis of disease begins with adherence to respiratory epithelium and colonization of the nasopharynx, which is mediated by pilus and non-pilus adherence factors. The mechanism of entry into the intravascular compartment is unclear but appears to be influenced by cytotoxic factors. Once in the bloodstream, H. influenzae type b and perhaps other encapsulated strains resist intravascular clearance mechanisms at least in part via the presence of a polysaccharide capsule. In the case of H. influenzae type b, the magnitude and duration of bacteremia influence the likelihood of dissemination of bacteria to sites such as the meninges and joints.
Noninvasive H. influenzae infections such as otitis media, sinusitis, and bronchitis are usually caused by nontypable strains. These organisms gain access to sites such as the middle ear and sinus cavities by direct extension from the nasopharynx. Factors facilitating spread from the pharynx include eustachian tube dysfunction and antecedent viral infections of the upper respiratory tract.
Antibiotic Resistance
Most H. influenzae isolates are susceptible to ampicillin or amoxicillin, but about a third produce a β-lactamase and are therefore resistant. β-Lactamase–negative ampicillin-resistant (BLNAR) isolates have been identified that manifest resistance by production of a β-lactam–insensitive cell wall synthesis enzyme called PBP3.
Amoxicillin-clavulanate is uniformly active against H. influenzae clinical isolates except for the rare BLNAR isolates. Among macrolides, azithromycin is active against about 99% of H. influenzae isolates; in contrast, the activity of erythromycin and clarithromycin against H. influenzae clinical isolates is poor. H. influenzae resistance to 3rd-generation cephalosporins has not been documented. Resistance to trimethoprim-sulfamethoxazole is infrequent (≈10%), and resistance to quinolones is believed to be rare.
Immunity
In the pre-vaccine era, the most important known element of host defense was antibody directed against the type b capsular polysaccharide polyribosylribitol phosphate (PRP). Anti-PRP antibody is acquired in an age-related fashion and facilitates clearance of H. influenzae type b from blood, in part related to opsonic activity. Antibodies directed against antigens such as outer membrane proteins or lipopolysaccharides may also have a role in opsonization. Both the classic and alternative complement pathways are important in defense against H. influenzae type b.
Before the introduction of vaccination, protection from H. influenzae type b infection was presumed to correlate with the concentration of circulating anti-PRP antibody at the time of exposure. A serum antibody concentration of 0.15-1.0 µg/mL was considered protective against invasive infection. Unimmunized infants >6 mo of age and young children usually lacked an anti-PRP antibody concentration of this magnitude and were susceptible to disease after encountering H. influenzae type b. This lack of antibody in infants and young children may have reflected a maturational delay in the immunologic response to thymus-independent type 2 (TI-2) antigens such as unconjugated PRP, presumably explaining the high incidence of type b infections in infants and young children in the pre-vaccine era.
The conjugate vaccines (Table 186-1) act as thymus-dependent antigens and elicit serum antibody responses in infants and young children. These vaccines are believed to prime memory antibody responses on subsequent encounters with PRP. The concentration of circulating anti-PRP antibody in a child primed by a conjugate vaccine may not correlate precisely with protection, presumably because a memory response may occur rapidly on exposure to PRP and provide protection.
Much less is known about immunity to other H. influenzae serotypes or to nontypable isolates. For nontypable isolates, evidence suggests that antibodies directed against 1 or more outer membrane proteins are bactericidal and protect against experimental challenge. A variety of antigens have been evaluated in an attempt to identify vaccine candidates for nontypable H. influenzae, including outer membrane proteins (P1, P2, P4, P5, P6, D15, and Tbp A/B), lipopolysaccharide, various adhesins, and lipoprotein D.
Diagnosis
Presumptive identification of H. influenzae is established by direct examination of the collected specimen after staining with Gram reagents. Because of its small size, pleomorphism, and occasional poor uptake of stain as well as the tendency for proteinaceous fluids to have a red background, H. influenzae is sometimes difficult to visualize. Furthermore, given that identification of microorganisms on smear by either technique requires at least 105 bacteria/mL, failure to visualize them does not preclude their presence.
Culture of H. influenzae requires prompt transport and processing of specimens because the organism is fastidious. Specimens should not be exposed to drying or temperature extremes. Primary isolation of H. influenzae can be accomplished on chocolate agar or on blood agar plates using the staphylococcus streak technique.
Serotyping of H. influenzae is accomplished by slide agglutination with type-specific antisera. Accurate serotyping is essential to monitor progress toward elimination of type b invasive disease. Timely reporting of cases to public health authorities should be ensured.
Clinical Manifestations and Treatment
The initial antibiotic therapy of invasive infections possibly due to H. influenzae should be a parenterally administered antimicrobial agent effective in sterilizing all foci of infection and effective against ampicillin-resistant strains, usually an extended-spectrum cephalosporin such as cefotaxime or ceftriaxone. These antibiotics have achieved popularity because of their relative lack of serious adverse effects and ease of administration. After the antimicrobial susceptibility of the isolate has been determined, an appropriate agent can be selected to complete the therapy. Ampicillin remains the drug of choice for the therapy of infections due to susceptible isolates. If the isolate is resistant to ampicillin, ceftriaxone can be administered once daily in selected circumstances for outpatient therapy.
Oral antimicrobial agents are sometimes used to complete a course of therapy initiated by the parenteral route and are typically initial therapy for noninvasive infections such as otitis media and sinusitis. If the organism is susceptible, amoxicillin is the drug of choice. An oral 3rd-generation cephalosporin (e.g., cefixime, cefdinir) or amoxicillin-clavulanate may be used when the isolate is resistant to ampicillin.
Meningitis
In the pre-vaccine era, meningitis accounted for more than half of invasive H. influenzae disease. Clinically, meningitis caused by H. influenzae type b cannot be differentiated from meningitis caused by Neisseria meningitidis or Streptococcus pneumoniae (Chapter 595.1). It may be complicated by other foci of infection such as the lungs, joints, bones, and pericardium.
Antimicrobial therapy should be administered intravenously for 7-14 days for uncomplicated cases. Cefotaxime, ceftriaxone, and ampicillin cross the blood-brain barrier during acute inflammation in concentrations adequate to treat H. influenzae meningitis. Intramuscular therapy with ceftriaxone is an alternative in patients with normal organ perfusion.
The prognosis of H. influenzae type b meningitis depends on the age at presentation, duration of illness before appropriate antimicrobial therapy, cerebrospinal fluid (CSF) capsular polysaccharide concentration, and rapidity with which organisms are cleared from CSF, blood, and urine. Clinically manifested inappropriate secretion of antidiuretic hormone and evidence of focal neurologic deficits at presentation are poor prognostic features. About 6% of patients with H. influenzae type b meningitis are left with some hearing impairment, probably because of inflammation of the cochlea and the labyrinth. Dexamethasone (0.6 mg/kg/day divided every 6 hours for 2 days), particularly when given shortly before or concurrent with the initiation of antimicrobial therapy, decreases the incidence of hearing loss. Major neurologic sequelae of H. influenzae type b meningitis include behavior problems, language disorders, delayed development of language, impaired vision, mental retardation, motor abnormalities, ataxia, seizures, and hydrocephalus.
Cellulitis
Children with H. influenzae cellulitis often have an antecedent upper respiratory tract infection. They usually have no prior history of trauma, and the infection is thought to represent seeding of the organism to the involved soft tissues during bacteremia. The head and neck, particularly the cheek and preseptal region of the eye, are the most common sites of involvement. The involved region generally has indistinct margins and is tender and indurated. Buccal cellulitis is classically erythematous with a violaceous hue, although this sign may be absent. H. influenzae may often be recovered directly from an aspirate of the leading edge, although this procedure is seldom performed. The blood culture may also reveal the causative organism. Other foci of infection may be present concomitantly, particularly in children <18 mo of age. A diagnostic lumbar puncture should be considered at the time of diagnosis in these children.
Parenteral antimicrobial therapy is indicated until patients become afebrile, after which an appropriate orally administered antimicrobial agent may be substituted. A 7- to 10-day course is customary.
Preseptal Cellulitis
Infection involving the superficial tissue layers anterior to the orbital septum is termed preseptal cellulitis, which may be caused by H. influenzae. Uncomplicated preseptal cellulitis does not imply a risk for visual impairment or direct central nervous system extension. However, concurrent bacteremia may be associated with the development of meningitis. H. influenzae preseptal cellulitis is characterized by fever, edema, tenderness, warmth of the lid, and, occasionally, purple discoloration. Evidence of interruption of the integument is usually absent. Conjunctival drainage may be associated. S. pneumoniae, Staphylococcus aureus, and group A streptococcus cause clinically indistinguishable preseptal cellulitis. The latter two pathogens are more likely when fever is absent and the integument is interrupted (e.g., an insect bite or trauma).
Children with preseptal cellulitis in whom H. influenzae and S. pneumoniae are etiologic considerations (young age, high fever, intact integument) should undergo blood culture, and a diagnostic lumbar puncture should be considered.
Parenteral antibiotics are indicated for preseptal cellulitis. Because methicillin-susceptible and methicillin-resistant S. aureus, S. pneumoniae, and group A β-hemolytic streptococci are other causes, empirical therapy should include agents active against these pathogens. Patients with preseptal cellulitis without concurrent meningitis should receive parenteral therapy for about 5 days, until fever and erythema have abated. In uncomplicated cases, antimicrobial therapy should be given for 10 days.
Orbital Cellulitis
Infections of the orbit are infrequent and usually develop as complications of acute ethmoid or sphenoid sinusitis. Orbital cellulitis may manifest as lid edema but is distinguished by the presence of proptosis, chemosis, impaired vision, limitation of the extraocular movements, decreased mobility of the globe, or pain on movement of the globe. The distinction between preseptal and orbital cellulitis may be difficult and is best delineated by CT.
Orbital infections are treated with parenteral therapy for at least 14 days. Underlying sinusitis or orbital abscess may require surgical drainage and more prolonged antimicrobial therapy.
Supraglottitis or Acute Epiglottitis
Supraglottitis is a cellulitis of the tissues comprising the laryngeal inlet (Chapter 377). It has become exceedingly rare since the introduction of conjugate type b vaccines. Direct bacterial invasion of the involved tissues is probably the initiating pathophysiologic event. This dramatic, potentially lethal condition can occur at any age. Because of the risk of sudden, unpredictable airway obstruction, supraglottitis is a medical emergency. Other foci of infection, such as meningitis, are rare. Antimicrobial therapy directed against H. influenzae and other etiologic agents should be administered parenterally but only after the airway is secured, and therapy should be continued until patients are able to take fluids by mouth. The duration of antimicrobial therapy is typically 7 days.
Pneumonia
The true incidence of H. influenzae pneumonia in children is unknown because invasive procedures required to obtain culture specimens are seldom performed (Chapter 392). In the pre-vaccine era, type b bacteria were believed to be the usual cause. The signs and symptoms of pneumonia due to H. influenzae cannot be differentiated from those of pneumonia due to many other microorganisms. Other foci of infection may be present concomitantly.
Children < 12 mo of age in whom H. influenzae pneumonia is suspected should receive parenteral antimicrobial therapy initially because of their increased risk for bacteremia and its complications. Older children who do not appear severely ill may be managed with an orally administered antimicrobial. Therapy is continued for 7-10 days. Uncomplicated pleural effusion associated with H. influenzae pneumonia requires no special intervention. However, if empyema develops, surgical drainage is indicated.
Suppurative Arthritis
Large joints, such as the knee, hip, ankle, and elbow, are affected most commonly (Chapter 677). Other foci of infection may be present concomitantly. Although single joint involvement is the rule, multiple joint involvement occurs in about 6% of cases. The signs and symptoms of septic arthritis caused by H. influenzae are indistinguishable from those of arthritis caused by other bacteria.
Uncomplicated septic arthritis should be treated with an appropriate antimicrobial administered parenterally for at least 5-7 days. If the clinical response is satisfactory, the remainder of the course of antimicrobial treatment may be given orally. Therapy is typically given for 3 wk for uncomplicated septic arthritis, but it may be continued beyond 3 wk, until the C-reactive protein concentration is normal.
Pericarditis
H. influenzae is a rare cause of pericarditis (Chapter 434). Affected children often have had an antecedent upper respiratory tract infection. Fever, respiratory distress, and tachycardia are consistent findings. Other foci of infection may be present concomitantly.
The diagnosis may be established by recovery of the organism from blood or pericardial fluid. Gram stain or detection of PRP in pericardial fluid, blood, or urine (when type b organisms are the cause) may aid the diagnosis. Antimicrobials should be provided parenterally in a regimen similar to that used for meningitis (Chapter 595.1). Pericardiectomy is useful for draining the purulent material effectively and preventing tamponade and constrictive pericarditis.
Bacteremia without an Associated Focus
Bacteremia due to H. influenzae may be associated with fever without any apparent focus of infection (Chapter 170). In this situation, risk factors for “occult” bacteremia include the magnitude of fever (≥39°C) and the presence of leukocytosis (≥15,000 cells/µL). In the pre-vaccine era, meningitis developed in about 25% of children with occult H. influenzae type b bacteremia if left untreated. In the vaccine era, this H. influenzae infection has become exceedingly rare. When it does occur, the child should be re-evaluated for a focus of infection and a second blood culture performed. In general, the child should be hospitalized and given parenteral antimicrobial therapy after a diagnostic lumbar puncture and chest radiograph are obtained.
Miscellaneous Infections
Urinary tract infection, epididymo-orchitis, cervical adenitis, acute glossitis, infected thyroglossal duct cysts, uvulitis, endocarditis, endophthalmitis, primary peritonitis, osteomyelitis, and periappendiceal abscess are rarely caused by H. influenzae.
Invasive Disease in Neonates
Neonates rarely have invasive H. influenzae infection. In the infant with illness within the first 24 hr of life, especially in association with maternal chorioamnionitis or prolonged rupture of membranes, transmission of the organism to the infant is likely to have occurred through the maternal genital tract, which may be (<1%) colonized with nontypable H. influenzae. Manifestations of neonatal invasive infection include bacteremia with sepsis, pneumonia, respiratory distress syndrome with shock, conjunctivitis, scalp abscess or cellulitis, and meningitis. Less commonly, mastoiditis, septic arthritis, and congenital vesicular eruption may occur.
Otitis Media
Acute otitis media is one of the most common infectious diseases of childhood (Chapter 632). It results from the spread of bacteria from the nasopharynx through the eustachian tube into the middle ear cavity. Usually because of a preceding viral upper respiratory tract infection, the mucosa in the area becomes hyperemic and swollen, resulting in obstruction and an opportunity for bacterial multiplication in the middle ear.
The most common bacterial pathogens are S. pneumoniae, H. influenzae, and Moraxella catarrhalis. Most H. influenzae isolates causing otitis media are nontypable. Ipsilateral conjunctivitis may also be present. Amoxicillin (80-90 mg/kg/day) is a suitable first-line oral antimicrobial agent, because the probability that the causative isolate is resistant to amoxicillin and the risk for invasive potential are sufficiently low to justify this approach. Alternatively, in certain cases, a single dose of ceftriaxone constitutes adequate therapy.
In the case of treatment failure or if a β-lactamase–producing isolate is obtained by tympanocentesis or from drainage fluid, amoxicillin-clavulanate (Augmentin) and erythromycin-sulfisoxazole (Pediazole) are among the available alternatives. Erythromycin-sulfisoxazole is useful for patients allergic to β-lactam antibiotics.
Conjunctivitis
Acute infection of the conjunctivae is common in childhood (Chapter 618). In neonates, H. influenzae is an infrequent cause. However, it is an important pathogen in older children, as are S. pneumoniae and S. aureus. Most H. influenzae isolates associated with conjunctivitis are nontypable, although type b isolates and other serotypes are occasionally found. Empirical treatment of conjunctivitis beyond the neonatal period usually consists of topical antimicrobial therapy with sulfacetamide. Topical fluoroquinolone therapy is to be avoided because of its broad spectrum, high cost, and high rate of emerging resistance among many bacterial species. Ipsilateral otitis media caused by the same organism may be present and requires oral antibiotic therapy.
Sinusitis
H. influenzae is an important cause of acute sinusitis in children, second in frequency only to S. pneumoniae (Chapter 372). Chronic sinusitis lasting >1 yr or severe sinusitis requiring hospitalization is often caused by S. aureus or anaerobes such as Peptococcus, Peptostreptococcus, and Bacteroides. Nontypable H. influenzae and viridans group streptococci are also frequently recovered.
For uncomplicated sinusitis, amoxicillin is acceptable initial therapy. However, if clinical improvement does not occur, a broader-spectrum agent, such as amoxicillin-clavulanate, may be appropriate. A 10-day course is sufficient for uncomplicated sinusitis. Hospitalization for parenteral therapy is rarely required; the usual reason is suspicion of progression to orbital cellulitis.
Prevention
Universal immunization with H. influenzae type b conjugate vaccine is recommended for all infants. Prophylaxis is indicated if close contacts of an index patient with type b disease are unvaccinated. The contagiousness of non–type b H. influenzae infections is not known, and prophylaxis is not recommended.
Vaccine
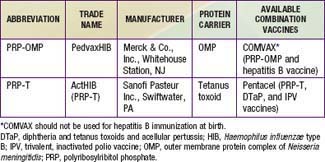
Two H. influenzae type b conjugate vaccines are currently marketed in the USA, PRP–outer membrane protein (PRP-OMP) and PRP–tetanus toxoid (PRP-T), which differ in the carrier protein used and the method of conjugating the polysaccharide to the protein (see Table 186-1 and Chapter 165). They are often sold in combination with other vaccines. One combination vaccine, which consists of PRP-OMP combined with hepatitis B vaccine (COMVAX, Merck & Co., Inc., Whitehouse Station, NJ), can be used for doses recommended at 2, 4, and 12-15 mo of age. Another consists of DTaP vaccine (diphtheria and tetanus toxoids and acellular pertussis), IPV vaccine (trivalent, inactivated polio vaccine) and PRP-T, (Pentacel, Sanofi Pasteur Inc., Swiftwater, PA) that can be used for doses recommended at 2, 4, 6, and 12-15 mo of age.
Prophylaxis
Unvaccinated children <48 mo of age who are in close contact with an index case of invasive H. influenzae type b infection are at increased risk for invasive infection. The risk for secondary disease for children >3 mo of age is inversely related to age. About half of the secondary cases among susceptible household contacts occur in the first week after hospitalization of the patient with the index case. Because many children are now protected against H. influenzae type b by prior immunization, the need for prophylaxis has greatly decreased. When prophylaxis is used, rifampin is indicated for all members of the household or close contact group, including the index patient, if the group includes ≥1 child <48 mo of age who is not fully immunized.
Parents of children hospitalized for invasive H. influenzae type b disease should be informed of the increased risk for secondary infection in other young children in the same household if they are not fully immunized. Parents of children exposed to a single case of invasive H. influenzae type b disease in a child-care center or nursery school should be similarly informed, although there is disagreement about the need for rifampin prophylaxis for these children.
For prophylaxis, children should be given rifampin orally (0-1 mo of age, 10 mg/kg/dose; >1 mo of age, 20 mg/kg/dose, not to exceed 600 mg/dose) once a day for 4 consecutive days. The adult dose is 600mg once daily. Rifampin prophylaxis is not recommended for pregnant women.
Adderson EE, Byington CL, Spencer L, et al. Invasive serotype a Haemophilus influenzae infections with a virulence genotype resembling Haemophilus influenzae type b: emerging pathogen in the vaccine era? Pediatrics. 2001;108:18-24.
Adegbola RA, Secka O, Lahai G, et al. Elimination of Haemophilus influenzae type b (BHb) disease from the Gambia after the introduction of routine immunization with a Hib conjugate vaccine: a prospective study. Lancet. 2005;366:144-150.
Centers for Disease Control and Prevention. Invasive Haemophilus influenzae type b disease in five young children—Minnesota, 2008. MMWR Morbid Mortal Wkly Rep. 2009;58:58-60.
Gessner BD, Sutanto A, Linehan M, et al. Incidences of vaccine-preventable Haemophilus influenzae type b pneumonia and meningitis in Indonesian children: hamlet-randomised vaccine-probe trial. Lancet. 2005;365:43-52.
McIntyre PB, Berkey CS, King SM, et al. Dexamethasone as adjunctive therapy in bacterial meningitis: a meta-analysis of randomized clinical trials since 1988. JAMA. 1997;278:925-931.
Prymula P, Peeters P, Chrobok V, et al. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typeable Haemophilus influenzae: a randomized double-blind efficacy study. Lancet. 2006;367:740-748.
Saha SK, Baqui AH, Darmstadt GL, et al. Invasive Haemophilus influenzae type b diseases in Bangladesh, with increased resistance to antibiotics. J Pediatr. 2005;146:227-233.
Triden L, Glennen A, Juni B, et al. Invasive Haemophilus influenzae disease and antibiotic susceptibility of invasive isolates in Minnesota, 2002–2005. Infect Dis Clin Pract. 2007;15:373-376.
Watt JP, Wolfson LJ, O’Brien KL, et al. Burden of disease caused by Haemophilus influenzae type b in children younger than 5 years: global estimates. Lancet. 2009;374:903-910.
Yaro S, Lourd M, Naccro B, et al. The epidemiology of Haemophilus influenzae type b meningitis in Burkina Faso. Pediatr Infect Dis J. 2006;25:415-419.