Chapter 404 Pleurisy, Pleural Effusions, and Empyema
Pleurisy or inflammation of the pleura is often accompanied by an effusion. The most common cause of pleural effusion in children is bacterial pneumonia (Chapter 392); heart failure (Chapter 436), rheumatologic causes, and metastatic intrathoracic malignancy are the next most common causes. A variety of other diseases account for the remaining cases, including tuberculosis (Chapter 207), lupus erythematosus (Chapter 152), aspiration pneumonitis (Chapter 389), uremia, pancreatitis, subdiaphragmatic abscess, and rheumatoid arthritis. Males and females are affected equally.
Inflammatory processes in the pleura are usually divided into 3 types: dry or plastic, serofibrinous or serosanguineous, and purulent pleurisy or empyema.
404.1 Dry or Plastic Pleurisy (Pleural Effusion)
Glenna B. Winnie and Steven V. Lossef
Etiology
Plastic pleurisy may be associated with acute bacterial or viral pulmonary infections or may develop during the course of an acute upper respiratory tract illness. The condition is also associated with tuberculosis and connective tissue diseases such as rheumatic fever.
Pathology and Pathogenesis
The process is usually limited to the visceral pleura, with small amounts of yellow serous fluid and adhesions between the pleural surfaces. In tuberculosis, the adhesions develop rapidly and the pleura are often thickened. Occasionally, fibrin deposition and adhesions are severe enough to produce a fibrothorax that markedly inhibits the excursions of the lung.
Clinical Manifestations
The primary disease often overshadows signs and symptoms. Pain, the principal symptom, is exaggerated by deep breathing, coughing, and straining. Occasionally, pleural pain is described as a dull ache, which is less likely to vary with breathing. The pain is often localized over the chest wall and is referred to the shoulder or the back. Pain with breathing is responsible for grunting and guarding of respirations, and the child often lies on the affected side in an attempt to decrease respiratory excursions. Early in the illness, a leathery, rough, inspiratory and expiratory friction rub may be audible, but it usually disappears rapidly. If the layer of exudate is thick, increased dullness to percussion and decreased breath sounds may be heard. Pleurisy may be asymptomatic. Chronic pleurisy is occasionally encountered with conditions such as atelectasis, pulmonary abscess, connective tissue diseases, and tuberculosis.
Laboratory Findings
Plastic pleurisy may be detected on radiographs as a diffuse haziness at the pleural surface or a dense, sharply demarcated shadow (Figs. 404-1 and 404-2). The latter finding may be indistinguishable from small amounts of pleural exudate. Chest radiographic findings may be normal, but ultrasonography or CT findings will be positive.
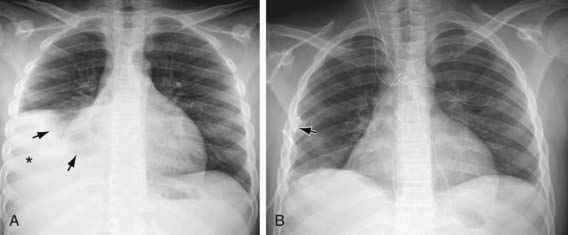
Figure 404-1 A, Right pleural effusion (asterisk) due to lupus erythematosus in a 12 yr old child. Note compressed middle and lower lobes of the right lung (arrows). B, The effusion was evacuated and the right lung was completely reexpanded after insertion of the pigtail chest tube (arrow).
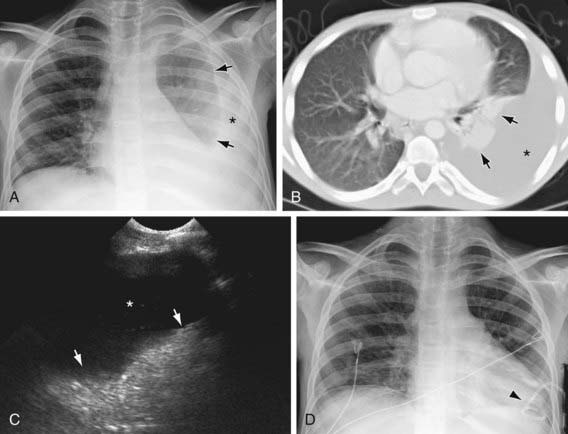
Figure 404-2 Left pleural effusion in a teenager with AIDS and mycoplasma avium intracellulare infection. The pleural effusion (asterisk) is clearly seen on the chest radiograph (A), CT scan (B), and ultrasonogram (C) of the left chest. Arrows point to the compressed and atelectatic left lung. D, A pigtail chest tube (arrowhead) was inserted, resulting in reexpansion of the left lung.
Differential Diagnosis
Plastic pleurisy must be distinguished from other diseases, such as epidemic pleurodynia, trauma to the rib cage (rib fracture), lesions of the dorsal root ganglia, tumors of the spinal cord, herpes zoster, gallbladder disease, and trichinosis. Even if evidence of pleural fluid is not found on physical or radiographic examination, a CT- or ultrasound-guided pleural tap in suspected cases often results in the recovery of a small amount of exudate, which when cultured may reveal the underlying bacterial cause in patients with an acute pneumonia. Patients with pleurisy and pneumonia should always be screened for tuberculosis.
Treatment
Therapy should be aimed at the underlying disease. When pneumonia is present, neither immobilization of the chest with adhesive plaster nor therapy with drugs capable of suppressing the cough reflex is indicated. If pneumonia is not present or is under good therapeutic control, strapping of the chest to restrict expansion may afford relief from pain. Analgesia with nonsteroidal anti-inflammatory agents may be helpful.
404.2 Serofibrinous or Serosanguineous Pleurisy (Pleural Effusion)
Etiology
Serofibrinous pleurisy is defined by a fibrinous exudate on the pleural surface and an exudative effusion of serous fluid into the pleural cavity. Typically it is associated with infections of the lung or with inflammatory conditions of the abdomen or mediastinum. Occasionally, it is found with connective tissue diseases such as lupus erythematosus, periarteritis, and rheumatoid, arthritis and it may be seen with primary or metastatic neoplasms of the lung, pleura, or mediastinum; tumors are commonly associated with a hemorrhagic pleurisy.
Pathogenesis
Pleural fluid originates from the capillaries of the parietal pleura and is absorbed from the pleural space via pleural stomas and the lymphatics of the parietal pleura. The rate of fluid formation is dictated by the Starling law, by which fluid movement is determined by the balance of hydrostatic and osmotic pressures in the pleural space and pulmonary capillary bed, and the permeability of the pleural membrane. Normally, only 4-12 mL of fluid is present in the pleural space, but if formation exceeds clearance, fluid accumulates. Pleural inflammation increases the permeability of the plural surface, with increased proteinaceous fluid formation; there may also be some obstruction to lymphatic absorption.
Clinical Manifestations
Because serofibrinous pleurisy is often preceded by the plastic type, early signs and symptoms may be those of plastic pleurisy. As fluid accumulates, pleuritic pain may disappear. The patient may become asymptomatic if the effusion remains small, or there may be only signs and symptoms of the underlying disease. Large fluid collections can produce cough, dyspnea, retractions, tachypnea, orthopnea, or cyanosis.
Physical findings depend on the amount of effusion. Dullness to flatness may be found on percussion. Breath sounds are decreased or absent, and there are a diminution in tactile fremitus, a shift of the mediastinum away from the affected side, and, occasionally, fullness of the intercostal spaces. If the fluid is not loculated, these signs may shift with changes in position. If extensive pneumonia is present, crackles and rhonchi may also be audible. Friction rubs are usually detected only during the early or late plastic stage. In infants, physical signs are less definite, and bronchial breathing may be heard instead of decreased breath sounds.
Laboratory Findings
Radiographic examination shows a generally homogeneous density obliterating the normal markings of the underlying lung. Small effusions may cause obliteration of only the costophrenic or cardiophrenic angles or a widening of the interlobar septa. Examinations should be performed with the patient both supine and upright, to demonstrate a shift of the effusion with a change in position; the decubitus position may be helpful. Ultrasonographic examinations are useful and may guide thoracentesis if the effusion is loculated. Examination of the fluid is essential to differentiate exudates from transudates and to determine the type of exudate (Chapter 392). Depending on the clinical scenario, pleural fluid is sent for culture for bacterial, fungal, and mycobacterial cultures; antigen testing; Gram staining; and chemical evaluation of content, including protein, lactic dehydrogenase and glucose, amylase, specific gravity, total cell count and differential, cytologic examination, and pH. Complete blood count and serum chemistry analysis should be obtained; hypoalbuminemia is often present. Exudates usually have at least one of the following features: protein level >3.0 g/dL, with pleural fluid:serum protein ratio >0.5; pleural fluid lactic dehydrogenase values >200 IU/L; or fluid:serum lactic dehydrogenase ratio >0.6. Although systemic acidosis reduces the usefulness of pleural fluid pH measurements, pH <7.20 suggests an exudate. Glucose is usually <60 mg/dL in malignancy, rheumatoid disease, and tuberculosis; the finding of many small lymphocytes and a pH <7.20 suggest tuberculosis. The fluid of serofibrinous pleurisy is clear or slightly cloudy and contains relatively few leukocytes and, occasionally, some erythrocytes. Gram staining may occasionally show bacteria; however, acid-fast staining rarely demonstrates tubercle bacilli.
Diagnosis and Differential Diagnosis
Thoracentesis should be performed when pleural fluid is present or is suggested, unless the effusion is small and the patient has a classic-appearing lobar pneumococcal pneumonia. Thoracentesis can differentiate serofibrinous pleurisy, empyema, hydrothorax, hemothorax, and chylothorax. Exudates are usually associated with an infectious process. In hydrothorax, the fluid has a specific gravity <1.015, and evaluation reveals only a few mesothelial cells rather than leukocytes. Chylothorax and hemothorax usually have fluid with a distinctive appearance, but differentiating serofibrinous from purulent pleurisy is impossible without microscopic examination of the fluid. Cytologic examination may reveal malignant cells. Serofibrinous fluid may rapidly become purulent.
Complications
Unless the fluid becomes purulent, it usually disappears relatively rapidly, particularly with appropriate treatment of bacterial pneumonia. It persists somewhat longer if due to tuberculosis or a connective tissue disease and may recur or remain for a long time if due to a neoplasm. As the effusion is absorbed, adhesions often develop between the 2 layers of the pleura, but usually little or no functional impairment results. Pleural thickening may develop and is occasionally mistaken for small quantities of fluid or for persistent pulmonary infiltrates. Pleural thickening may persist for months, but the process usually disappears, leaving no residua.
Treatment
Therapy should address the underlying disease. With a large effusion, draining the fluid makes the patient more comfortable. When a diagnostic thoracentesis is performed, as much fluid as possible should be removed for therapeutic purposes. Rapid removal of ≥1 L of pleural fluid may be associated with the development of reexpansion pulmonary edema (Chapter 388). If the underlying disease is adequately treated, further drainage is usually unnecessary, but if sufficient fluid reaccumulates to cause respiratory embarrassment, chest tube drainage should be performed. In older children with suspected parapneumonic effusion, tube thoracostomy is considered necessary if the pleural fluid pH is <7.20 or the pleural fluid glucose level is <50 mg/dL. If the fluid is clearly purulent, tube drainage with thrombolytic therapy or video-assisted thoracoscopic surgery (VATS) is indicated. Patients with pleural effusions may need analgesia, particularly after thoracentesis or insertion of a chest tube. Those with acute pneumonia may need supplemental oxygen in addition to specific antibiotic treatment.
404.3 Purulent Pleurisy or Empyema
Etiology
Empyema is an accumulation of pus in the pleural space. It is most often associated with pneumonia (Chapter 392) due to Streptococcus pneumoniae, although Staphylococcus aureus is most common in developing nations and Asia as well as in post-traumatic empyema. The relative incidence of Haemophilus influenzae empyema has decreased since the introduction of the Hib vaccination. Group A streptococcus, gram-negative organisms, tuberculosis, fungi, and malignancy are less common causes. The disease can also be produced by rupture of a lung abscess into the pleural space, by contamination introduced from trauma or thoracic surgery, or, rarely, by mediastinitis or the extension of intra-abdominal abscesses.
Epidemiology
Empyema is most frequently encountered in infants and preschool children. It is increasing in frequency. It occurs in 5-10% of children with bacterial pneumonia and in up to 86% of children with necrotizing pneumonia.
Pathology
Empyema has 3 stages: exudative, fibrinopurulent, and organizational. During the exudative stage, fibrinous exudate forms on the pleural surfaces. In the fibrinopurulent stage, fibrinous septa form, causing loculation of the fluid and thickening of the parietal pleura. If the pus is not drained, it may dissect through the pleura into lung parenchyma, producing bronchopleural fistulas and pyopneumothorax, or into the abdominal cavity. Rarely, the pus dissects through the chest wall (i.e., empyema necessitatis). During the organizational stage, there is fibroblast proliferation; pockets of loculated pus may develop into thick-walled abscess cavities or the lung may collapse and become surrounded by a thick, inelastic envelope (peel).
Clinical Manifestations
The initial signs and symptoms are primarily those of bacterial pneumonia. Children treated with antibiotic agents may have an interval of a few days between the clinical pneumonia phase and the evidence of empyema. Most patients are febrile, develop increased work of breathing or respiratory distress, and often appear more ill. Physical findings are identical to those described for serofibrinous pleurisy, and the 2 conditions are differentiated only by thoracentesis, which should always be performed when empyema is suspected.
Laboratory Findings
Radiographically, all pleural effusions appear similar, but the absence of a shift of the fluid with a change of position indicates a loculated empyema (Figs. 404-3 to 404-5). Septa may be confirmed by ultrasonography or CT. The maximal amount of fluid obtainable should be withdrawn by thoracentesis and studied as described in Chapter 404.2. The effusion is empyema if bacteria are present on Gram staining, the pH is <7.20, and there are >100,000 neutrophils/µL. The appearance of pus produced by different organisms is not distinctive; cultures of the fluid must always be performed. In pneumococcal empyema, the culture is positive in 58% of cases. In patients with negative culture results for pneumococcus, the pneumococcal polymerase chain reaction (PCR) analysis is most helpful to making a diagnosis. Blood cultures have a high yield, possibly higher than cultures of the pleural fluid. Leukocytosis and an elevated sedimentation rate may be found.
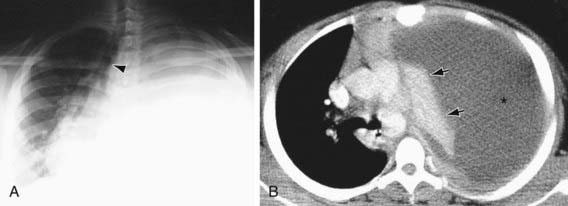
Figure 404-3 Empyema and pneumonia in a teenager. A, Chest radiograph shows opacification of the left thorax. Note shift of mediastinum and trachea (arrowhead) to right. B, Thoracic CT scan shows massive left pleural effusion (asterisk). Note the compression and atelectasis of the left lung (arrows) and shift of the mediastinum to the right.
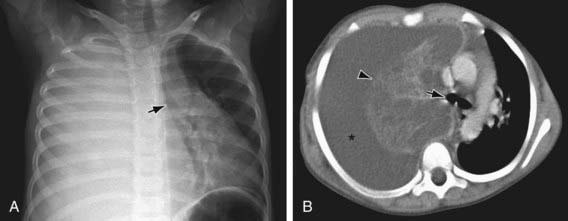
Figure 404-4 Pneumonia and parapneumonic effusion in a 4 yr old child. A, Chest radiograph shows complete opacification of the right thorax due to a large pleural effusion. Note the shift of the mediastinum and trachea (arrow) to the left. B, Thoracic CT scan shows a large right pleural effusion (asterisk) surrounding and compressing the consolidated right lung (arrowhead). Note the shift of the mediastinum and tracheal carina (arrow) to the left.
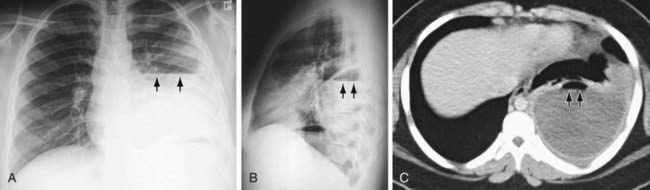
Figure 404-5 Loculated hydropneumothorax. Frontal (A) and lateral (B) chest radiographs show loculated hydropneumothorax that complicated pneumonia in a 14 yr old child. Arrows point to the horizontal air-fluid level at the interface between the intrapleural effusion and air. C, Thoracic CT scan helps to localize the loculated hydropneumothorax, with its air-fluid level (arrows).
Complications
With staphylococcal infections, bronchopleural fistulas and pyopneumothorax commonly develop. Other local complications include purulent pericarditis, pulmonary abscesses, peritonitis from extension through the diaphragm, and osteomyelitis of the ribs. Septic complications such as meningitis, arthritis, and osteomyelitis may also occur. Septicemia is often encountered in H. influenzae and pneumococcal infections. The effusion may organize into a thick “peel,” which may restrict lung expansion and may be associated with persistent fever and temporary scoliosis.
Treatment
Treatment includes systemic antibiotics and thoracentesis and possibly chest tube drainage with or without a fibrinolytic agent, video-assisted thorascopic surgery (VATS), or open decortication (Chapter 392); controlled studies are needed. If empyema is diagnosed early, antibiotic treatment plus thoracentesis achieves a complete cure. The selection of antibiotic should be based on the in vitro sensitivities of the responsible organism. See Chapters 174, 175, and 186 for treatment of infections by Staphylococcus, S. pneumoniae, and H. influenzae, respectively. Clinical response in empyema is slow; even with optimal treatment, there may be little improvement for as long as 2 wk. With staphylococcal infections, resolution is very slow, and systemic antibiotic therapy is required for 3-4 wk. Instillation of antibiotics into the pleural cavity does not improve results.
When pus is obtained by thoracentesis, it is unclear whether optimal management is closed-chest tube drainage or VATS followed by chest tube drainage. Multiple aspirations of the pleural cavity should not be attempted. If pleural fluid septa are detected on ultrasound, immediate VATS can be associated with a shortened hospital course. Closed-chest tube drainage is controlled by an underwater seal or continuous suction; sometimes more than 1 tube is required to drain loculated areas. Closed drainage is usually continued for about 1 wk. Chest tubes that are no longer draining are removed.
Instillation of fibrinolytic agents into the pleural cavity via the chest tube may promote drainage, decrease fever, lessen need for surgical intervention, and shorten hospitalization; it does not shorten the course of disease when used after VATS, however. The optimal drug and dosage have not been determined. Streptokinase 15,000 U/kg in 50 mL of 0.9% saline daily for 3-5 days and urokinase 40,000 U in 40 mL saline every 12 hr for 6 doses have been evaluated in randomized trials in children. There is a risk of anaphylaxis with streptokinase, and both drugs can be associated with hemorrhage and other complications.
Extensive fibrinous changes may take place over the surface of the lungs owing to empyema, but they eventually resolve. In the child who remains febrile and dyspneic >72 hr after initiation of therapy with intravenous antibiotics and thoracostomy tube drainage, surgical decortication via VATS or, less often, open thoracotomy may speed recovery. If pneumatoceles form, no attempt should be made to treat them surgically or by aspiration, unless they reach sufficient size to cause respiratory embarrassment or become secondarily infected. Pneumatoceles usually resolve spontaneously with time. The long-term clinical prognosis for adequately treated empyema is excellent, and follow-up pulmonary function studies suggest that residual restrictive disease is uncommon, with or without surgical intervention.
Akhan O, Ozkan O, Akinci D, et al. Image-guided catheter drainage of infected pleural effusions. Diagn Interv Radiol. 2007;13:204-209.
Aydogan M, Aydogan A, Ozcan A, et al. Intrapleural streptokinase treatment in children with empyema. Eur J Pediatr. 2008;167:739-744.
Aziz A, Healey JM, Qureshi F, et al. Comparative analysis of chest tube thoracostomy and video-assisted thoracoscopic surgery in empyema and parapneumonic effusion associated with pneumonia in children. Surg Infect (Larchmt). 2008;9:317-323.
Cameron R, Davies HR: Intra-pleural fibrinolytic therapy versus conservative management in the treatment of adult parapneumonic effusions and empyema, Cochrane Database Syst Rev (2):CD002312, 2008.
Chiu CY, Wong KS, Huang YC, et al. Echo-guided management of complicated parapneumonic effusion in children. Pediatr Pulmonol. 2006;41:1226-1232.
Kurt BA, Winterhalter KM, Connors RH, et al. Therapy of parapneumonic effusions in children: video-assisted thoracoscopic surgery versus conventional thoracostomy drainage. Pediatrics. 2006;118:e547-e553.
Nyambat B, Kilgore PE, Yong DE, et al. Survey of childhood empyema in Asia. BMC Infect Dis. 2008;8:90.
Sawicki GS, Lu FL, Valim C, et al. Necrotising pneumonia is an increasingly detected complication of pneumonia in children. Eur Respir J. 2008;31:1285-1291.