Chapter 405 Pneumothorax
Pneumothorax is the accumulation of extrapulmonary air within the chest, most commonly from leakage of air from within the lung. Air leaks can be primary or secondary and can be spontaneous, traumatic, iatrogenic, or catamenial (Table 405-1). Pneumothorax in the neonatal period is also discussed in Chapter 95.12.
Table 405-1 CAUSES OF PNEUMOTHORAX IN CHILDREN
SPONTANEOUS
TRAUMATIC
* Associated with renal agenesis, diaphragmatic hernia, amniotic fluid leaks.
From Kuhn JP, Slovis TL, Haller JO: Caffey’s pediatric diagnostic imaging, vol 1, ed 10, Philadelphia, 2004, Mosby.
Etiology and Epidemiology
A primary spontaneous pneumothorax occurs without trauma or underlying lung disease. Spontaneous pneumothorax with or without exertion occurs occasionally in teenagers and young adults, most frequently in males who are tall, thin, and thought to have subpleural blebs. Familial cases of spontaneous pneumothorax occur and have been associated with mutations in the folliculin gene. Patients with collagen synthesis defects, such as Ehlers-Danlos disease (Chapter 651) and Marfan syndrome (Chapter 693) are unusually prone to the development of pneumothorax.
A pneumothorax arising as a complication of an underlying lung disorder but without trauma is a secondary spontaneous pneumothorax. Pneumothorax can occur in pneumonia, usually with empyema; it can also be secondary to pulmonary abscess, gangrene, infarct, rupture of a cyst or an emphysematous bleb (in asthma), or foreign bodies in the lung. In infants with staphylococcal pneumonia, the incidence of pneumothorax is relatively high. It is found in ≈5% of hospitalized asthmatic children and usually resolves without treatment. Pneumothorax is a serious complication in cystic fibrosis (CF; Chapter 395). Pneumothorax also occurs in patients with lymphoma or other malignancies, and in graft versus host disease with bronchiolitis obliterans.
External chest or abdominal blunt or penetrating trauma can tear a bronchus or abdominal viscus, with leakage of air into the pleural space. Ecstasy (methylenedioxymethamphetamine) abuse has been associated with pneumothorax.
Iatrogenic pneumothorax can complicate transthoracic needle aspiration, tracheotomy, subclavian line placement, thoracentesis, or transbronchial biopsy. It may occur during mechanical or noninvasive ventilation, acupuncture, and other diagnostic or therapeutic procedures.
Catamenial pneumothorax, an unusual condition that is related to menses, is associated with diaphragmatic defects and pleural blebs.
Pneumothorax can be associated with a serous effusion (hydropneumothorax), a purulent effusion (pyopneumothorax), or blood (hemopneumothorax). Bilateral pneumothorax is rare after the neonatal period but has been reported after lung transplantation and with Mycoplasma pneumoniae infection and tuberculosis.
Pathogenesis
The tendency of the lung to collapse, or elastic recoil, is balanced in the normal resting state by the inherent tendency of the chest wall to expand outward, creating negative pressure in the intrapleural space. When air enters the pleural space, the lung collapses. Hypoxemia occurs because of alveolar hypoventilation, ventilation-perfusion mismatch, and intrapulmonary shunt. In simple pneumothorax, intrapleural pressure is atmospheric, and the lung collapses up to 30%. In complicated, or tension, pneumothorax, continuing leak causes increasing positive pressure in the pleural space, with further compression of the lung, shift of mediastinal structures toward the contralateral side, and decreases in venous return and cardiac output.
Clinical Manifestations
The onset of pneumothorax is usually abrupt, and the severity of symptoms depends on the extent of the lung collapse and on the amount of pre-existing lung disease. Pneumothorax may cause dyspnea, pain, and cyanosis. When it occurs in infancy, symptoms and physical signs may be difficult to recognize. Moderate pneumothorax may cause little displacement of the intrathoracic organs and few or no symptoms. The severity of pain usually does not directly reflect the extent of the collapse.
Usually, there is respiratory distress, with retractions, markedly decreased breath sounds, and a tympanitic percussion note over the involved hemithorax. The larynx, trachea, and heart may be shifted toward the unaffected side. When fluid is present, there is usually a sharply limited area of tympany above a level of flatness to percussion. The presence of amphoric breathing or, when fluid is present in the pleural cavity, of gurgling sounds synchronous with respirations suggests an open fistula connecting with air-containing tissues.
Diagnosis and Differential Diagnosis
The diagnosis of pneumothorax is usually established by radiographic examination (Figs. 405-1 to 405-6). The amount of air outside the lung varies with time. A radiograph that is taken early shows less lung collapse than one taken later if the leak continues. Expiratory views accentuate the contrast between lung markings and the clear area of the pneumothorax (see Fig. 405-1). When the possibility of diaphragmatic hernia is being considered, a small amount of barium may be necessary to demonstrate that it is not free air but is a portion of the gastrointestinal tract that is in the thoracic cavity. Ultrasound can also be used to establish the diagnosis.
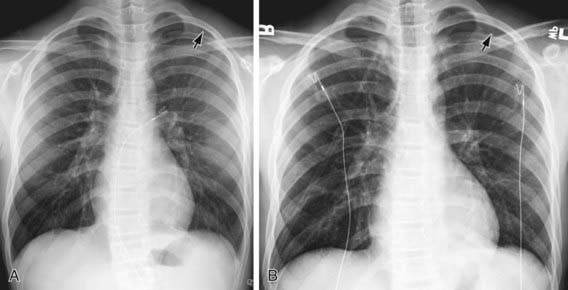
Figure 405-1 Utility of an expiratory film in detection of a small pneumothorax. A, Teenage boy with stab wound and subtle radiolucency in the left apical region (arrow) on inspiratory chest radiograph. The margin of the visceral pleura is very faintly visible. B, On an expiratory film, the pneumothorax (arrow) is more obvious as the right lung has deflated and become more opaque, providing better contrast with the air in the pleural space.
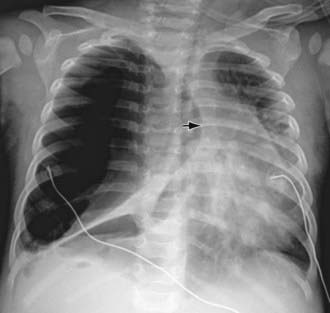
Figure 405-2 Right pneumothorax, with lung collapse of a compliant lung. Shift of the mediastinum to the left (arrow) indicates that this is a tension pneumothorax.
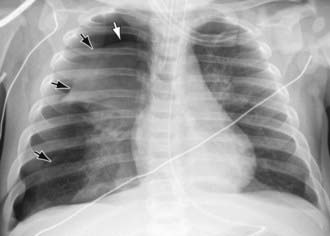
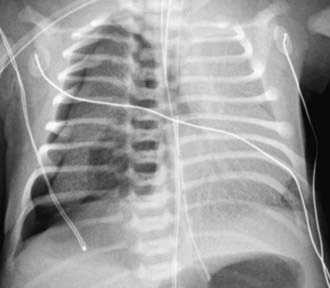
Figure 405-4 Pneumothorax, with collapse of right lung (arrows) caused by barotrauma in a 7 month old child who was intubated for respiratory failure.
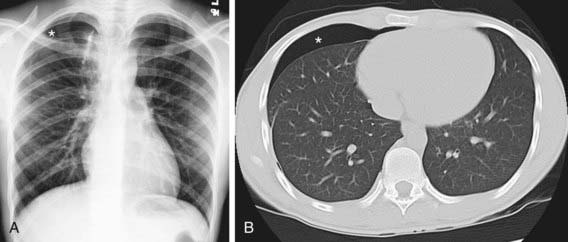
Figure 405-5 Teenager in whom a spontaneous right pneumothorax developed because of a bleb. He had a persistent air leak despite recent surgical resection of the causative apical bleb. Chest radiograph (A) and CT scan (B) clearly show the persistent pneumothorax (asterisk).
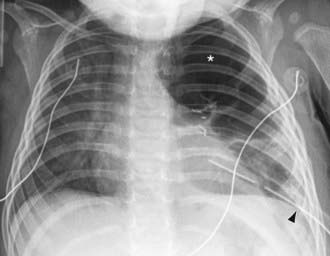
Figure 405-6 Bronchopleural fistula following surgical resection of the left upper lobe due to congenital lobar emphysema. Chest radiograph shows localized pneumothorax (asterisk) that persisted despite prior insertion of a large-bore chest tube (arrowhead).
It may be difficult to determine whether a pneumothorax is under tension. Evidence of tension includes shift of mediastinal structures away from the side of the air leak. A shift may be absent in situations in which the other hemithorax resists the shift, such as in the case of bilateral pneumothorax. When the lungs are both stiff, such as in CF or respiratory distress syndrome, the unaffected lung may not collapse easily and shift may not occur (see Fig. 405-3). On occasion, the diagnosis of tension pneumothorax is made only on the basis of evidence of circulatory compromise or on hearing a “hiss” of rapid exit of air under tension with the insertion of the thoracostomy tube.
Pneumothorax must be differentiated from localized or generalized emphysema, an extensive emphysematous bleb, large pulmonary cavities or other cystic formations, diaphragmatic hernia, compensatory overexpansion with contralateral atelectasis, and gaseous distention of the stomach. In most cases, chest radiography or CT differentiates among these possibilities.
Treatment
Therapy varies with the extent of the collapse and the nature and severity of the underlying disease. A small (<5%) or even moderate-sized pneumothorax in an otherwise normal child may resolve without specific treatment, usually within about 1 wk. A small pneumothorax complicating asthma may also resolve spontaneously. Administering 100% oxygen may hasten resolution, but patients with chronic hypoxemia should be monitored closely during administration of supplemental oxygen. Pleural pain deserves analgesic treatment. Needle aspiration may be required on an emergency basis for tension pneumothorax and is as effective as tube thoracostomy in the emergency room management of primary spontaneous pneumothorax. If the pneumothorax is recurrent, secondary, or under tension, or there is >5% collapse, chest tube drainage is necessary. Pneumothorax complicating CF frequently recurs, and definitive treatment may be justified with the 1st episode. Similarly, if pneumothorax complicating malignancy does not improve rapidly with observation, chemical pleurodesis or surgical thoracotomy is often necessary.
Closed thoracotomy (simple insertion of a chest tube) and drainage of the trapped air through a catheter, the external opening of which is kept in a dependent position under water, is adequate to reexpand the lung in most patients; pigtail catheters are frequently used. When there have been previous pneumothoraces, it may be indicated to induce the formation of strong adhesions between the lung and chest wall by a sclerosing procedure to prevent recurrence. This can be carried out by the introduction of talc, doxycycline, or iodopovidone into the pleural space (chemical pleurodesis). Open thoracotomy through a limited incision, with plication of blebs, closure of fistula, stripping of the pleura (usually in the apical lung, where the surgeon has direct vision), and basilar pleural abrasion is also an effective treatment for recurring pneumothorax. Stripping and abrading the pleura leaves raw, inflamed surfaces that heal with sealing adhesions. Postoperative pain is comparable to that with chemical pleurodesis, but the chest tube can usually be removed in 24-48 hr, compared with the usual 72-hr minimum for closed thoracotomy and pleurodesis. VATS is a preferred therapy for blebectomy, pleural stripping, pleural brushing, and instillation of sclerosing agents, with somewhat less morbidity than occurs with traditional open thoracotomy.
Pleural adhesions help prevent recurrent pneumothorax, but they also make subsequent thoracic surgery difficult. When lung transplantation may be a future consideration (e.g., in CF), a stepwise approach to treatment of pneumothorax has been proposed. This approach begins with observation and progresses through chest tube drainage and thoracoscopic and then open surgery, and finally to chemical or mechanical pleurodesis. At any step during this approach, the patient and family are given the option of the definitive procedure if they understand that its performance may make lung transplantation difficult or impossible. It should also be kept in mind that the longer a chest tube is in place, the greater the chance of pulmonary deterioration, particularly in a patient with CF, in whom strong coughing, deep breathing, and postural drainage are important. These are all difficult to accomplish with a chest tube in place.
Treatment of the underlying pulmonary disease should begin on admission and should be continued throughout the course of treatment directed at the air leak.
Agarwal R, Aggarwal AN, Gupta D, et al. Efficacy and safety of iodopovidone in chemical pleurodesis: a meta-analysis of observational studies. Respir Med. 2006;100:2043-2047.
Amin R, Noone PG, Ratien F: Chemical pleurodesis versus surgical intervention for persistent and recurrent pneumothoraces in cystic fibrosis, Cochrane Database Syst Rev (2):CD007481, 2009.
Bialas RC, Weiner TM, Phillips JD. Video-assisted thoracic surgery for primary spontaneous pneumothorax in children: is there an optimal technique? J Pediatr Surg. 2008;43:2151-2155.
Chen JS, Hsu HH, Tsai KT, et al. Salvage for unsuccessful aspiration of primary pneumothorax: thoracoscopic surgery or chest tube drainage? Ann Thorac Surg. 2008;85:1908-1913.
Miller MP, Sagy M. Pressure characteristics of mechanical ventilation and incidence of pneumothorax before and after the implementation of protective lung strategies in the management of pediatric patients with severe ARDS. Chest. 2008;134:969-973.
Posner K, Needleman J. Pneumothorax. Pediatr Rev. 2008;29:69-70.
Ren HZ, Zhu CC, Yang C, et al. Mutation analysis of the FLCN gene in Chinese patients with sporadic and familial isolated primary spontaneous pneumothorax. Clin Genet. 2008;74:178-183.
Treasure T. Minimal access surgery for pneumothorax. Lancet. 2007;370:294-295.
Zehtabchi S, Rios CL. Management of emergency department patients with primary spontaneous pneumothorax: needle aspiration or tube thoracostomy? Ann Emerg Med. 2008;51:91-100.