Chapter 422 Acyanotic Congenital Heart Disease
Regurgitant Lesions
422.1 Pulmonary Valvular Insufficiency and Congenital Absence of the Pulmonary Valve
Pulmonary valvular insufficiency most often accompanies other cardiovascular diseases or may be secondary to severe pulmonary hypertension. Incompetence of the valve is an expected result after surgery for right ventricular outflow tract obstruction, for example, pulmonary valvotomy in patients with valvular pulmonic stenosis or valvotomy with infundibular resection in patients with tetralogy of Fallot. Isolated congenital insufficiency of the pulmonary valve is rare. These patients are usually asymptomatic because the insufficiency is generally mild.
The prominent physical sign is a decrescendo diastolic murmur at the upper and midleft sternal border, which has a lower pitch than the murmur of aortic insufficiency because of the lower pressure involved. Roentgenograms of the chest show prominence of the main pulmonary artery and, if the insufficiency is severe, right ventricular enlargement. The electrocardiogram is normal or shows minimal right ventricular hypertrophy. Pulsed and color Doppler studies demonstrate retrograde flow from the pulmonary artery to the right ventricle during diastole. Cardiac magnetic resonance angiography (MRA) is useful for quantifying both right ventricular volume and the regurgitant fraction. Isolated pulmonary valvular insufficiency is generally well tolerated and does not require surgical treatment. When pulmonary insufficiency is severe, especially if significant tricuspid insufficiency has begun to develop, replacement with a homograft valve may become necessary to preserve right ventricular function.
Congenital absence of the pulmonary valve is usually associated with a ventricular septal defect, often in the context of tetralogy of Fallot (Chapter 424.1). In many of these neonates, the pulmonary arteries become widely dilated and compress the bronchi, with subsequent recurrent episodes of wheezing, pulmonary collapse, and pneumonitis. The presence and degree of cyanosis are variable. Florid pulmonary valvular incompetence may not be well tolerated, and death may occur from a combination of bronchial compression, hypoxemia, and heart failure. Correction involves plication of the massively dilated pulmonary arteries, closure of the ventricular septal defect, and placement of a homograft across the right ventricular outflow tract.
McDonnell BE, Raff GW, Gaynor JW, et al. Outcome after repair of tetralogy of Fallot with absent pulmonary valve. Ann Thorac Surg. 1999;67:1391-1395.
Pinsky WW. Absent pulmonary valve syndrome. In: Garson A, Bricker JT, Fisher DJ, et al, editors. The science and practice of pediatric cardiology. Baltimore: Williams & Wilkins; 1998:1413-1419.
422.2 Congenital Mitral Insufficiency
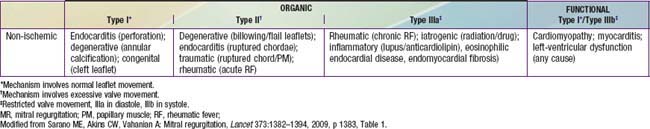
Congenital mitral insufficiency is rare as an isolated lesion and is more often associated with other anomalies. It is most commonly encountered in combination with an atrioventricular septal defect, either an ostium primum defect, or a complete AV septal defect (Chapter 420.5). Mitral insufficiency is also seen in patients with dilated cardiomyopathy (Chapter 433.1) as their left ventricular function deteriorates, secondary to dilatation of the valve ring. Mitral insufficiency may also be encountered in conjunction with coarctation of the aorta, ventricular septal defect, corrected transposition of the great vessels, anomalous origin of the left coronary artery from the pulmonary artery, or Marfan syndrome. In the absence of other congenital heart disease, endocarditis or rheumatic fever should be suspected in a patient with isolated severe mitral insufficiency (Table 422-1).
In isolated mitral insufficiency, the mitral valve annulus is usually dilated, the chordae tendineae are short and may insert anomalously, and the valve leaflets are deformed. When mitral insufficiency is severe enough to cause clinical symptoms, the left atrium enlarges as a result of the regurgitant flow, and the left ventricle becomes hypertrophied and dilated. Pulmonary venous pressure is increased, and the increased pressure ultimately results in pulmonary hypertension and right ventricular hypertrophy and dilatation. Mild lesions produce no symptoms; the only abnormal sign is the apical holosystolic murmur of mitral regurgitation. Severe regurgitation results in symptoms that can appear at any age, including poor physical development, frequent respiratory infections, fatigue on exertion, and episodes of pulmonary edema or congestive heart failure. Often, a diagnosis of reactive airway disease will have been made because of the similarity in pulmonary symptoms, including wheezing, which may be a dominant finding in infants and young children.
The typical murmur of mitral insufficiency is a high-pitched, apical holosystolic murmur. If the insufficiency is moderate to severe, it is usually associated with a low-pitched, apical mid-diastolic rumbling murmur indicative of increased diastolic flow across the mitral valve. The pulmonary component of the 2nd heart sound will be accentuated in the presence of pulmonary hypertension. The electrocardiogram usually shows bifid P waves consistent with left atrial enlargement, signs of left ventricular hypertrophy, and sometimes signs of right ventricular hypertrophy. Roentgenographic examination shows enlargement of the left atrium, which at times is massive. The left ventricle is prominent, and pulmonary vascularity is normal or prominent. The echocardiogram demonstrates the enlarged left atrium and ventricle. Color Doppler demonstrates the extent of the insufficiency, and pulsed Doppler of the pulmonary veins detects retrograde flow when mitral insufficiency is severe. Cardiac catheterization shows elevated left atrial pressure. Pulmonary artery hypertension of varying severity may be present. Selective left ventriculography reveals the severity of mitral regurgitation.
Mitral valvuloplasty can result in striking improvement in symptoms and heart size, but in some patients, installation of a prosthetic mechanical mitral valve may be necessary. Before surgery, associated anomalies must be identified.
422.3 Mitral Valve Prolapse
Mitral valve prolapse results from an abnormal mitral valve mechanism that causes billowing of one or both mitral leaflets, especially the posterior cusp, into the left atrium toward the end of systole. The abnormality is predominantly congenital but may not be recognized until adolescence or adulthood. Mitral valve prolapse is usually sporadic, is more common in girls, and may be inherited as an autosomal dominant trait with variable expression. It is common in patients with Marfan syndrome, straight back syndrome, pectus excavatum, scoliosis, Ehlers-Danlos syndrome, osteogenesis imperfecta, and pseudoxanthoma elasticum. The dominant abnormal signs are auscultatory, although occasional patients may have chest pain or palpitations. The apical murmur is late systolic and may be preceded by a click, but these signs may vary in the same patient and, at times, only the click is audible. In the standing or sitting position, the click may occur earlier in systole, and the murmur may be more prominent in late systole. Arrhythmias may occur and are primarily unifocal or multifocal premature ventricular contractions.
The electrocardiogram is usually normal but may show biphasic T waves, especially in leads II, III, aVF, and V6; the T-wave abnormalities may vary at different times in the same patient. The chest roentgenogram is normal. The echocardiogram shows a characteristic posterior movement of the posterior mitral leaflet during mid- or late systole or demonstrates pansystolic prolapse of both the anterior and posterior mitral leaflets. These echocardiographic findings must be interpreted cautiously because the appearance of minimal mitral prolapse may be a normal variant. Prolapse is more precisely defined by single or bileaflet prolapse of >2 mm beyond the long axis annular plane with or without leaflet thickening. Prolapse with valve thickening >5 mm is “classic,” a lesser degree is “non-classic.” Two-dimensional real-time echocardiography shows that both the free edge and the body of the mitral leaflets move posteriorly in systole toward the left atrium. Doppler can assess the presence and severity of mitral regurgitation.
This lesion is not progressive in childhood, and specific therapy is not indicated. Antibiotic prophylaxis is no longer recommended during surgery and dental procedures (Chapter 431).
Adults (men more often than women) with mitral valve prolapse are at increased risk for cardiovascular complications (sudden death, arrhythmia, cerebrovascular accidents, progressive valve dilatation, heart failure, and endocarditis) in the presence of thickened (>5 mm) and redundant mitral valve leaflets. Risk factors for morbidity also include poor left ventricular function, moderate to severe mitral regurgitation, and left atrial enlargement.
Often, confusion exists concerning the diagnosis of mitral valve prolapse. The high frequency of mild prolapse on the echocardiogram in the absence of clinical findings suggests that, in these cases, true mitral valve prolapse syndrome is not present. These patients and their parents should be reassured of this fact, and no special recommendations should be made regarding management or frequent laboratory studies.
American Academy of Pediatrics Committee on Sports Medicine and Fitness. Mitral valve prolapse and athletic competition of children and adolescents. Pediatrics. 1995;95:789.
Bouknight DP, O’Rourke RA. Current management of mitral valve prolapse. Am Fam Physician. 2000;61:3343-3350. 3353–3354
Briffa N. Surgery for degenerative mitral valve disease. BMJ. 2010;341:c5339.
Hepner ADS, Morrell H, Greaves S, et al. Prevalence of mitral valve prolapse in young athletes. Cardiol Young. 2008;18:402-404.
Jacobs W, Chamoun A, Stouffer GA. Mitral valve prolapse: a review of the literature. Am J Med Sci. 2001;321:401-410.
Maron BJ, Ackerman MJ, Nishimura RA, et al. Task Force 4: HCM and other cardiomyopathies, mitral valve prolapse, myocarditis, and Marfan syndrome. J Am Coll Cardiol. 2005;45:1340-1345.
Sarano ME, Akins CW, Vahanian A. Mitral regurgitation. Lancet. 2009;373:1382-1394.
422.4 Tricuspid Regurgitation
Isolated tricuspid regurgitation is generally associated with Ebstein anomaly of the tricuspid valve. Ebstein anomaly may occur either without cyanosis or with varying degrees of cyanosis, depending on the severity of the tricuspid regurgitation and the presence of an atrial-level communication (patent foramen ovale or atrial septal defect). Older children tend to have the acyanotic form, whereas if detected in the newborn period, Ebstein anomaly is usually associated with severe cyanosis (Chapter 424.7).
Tricuspid regurgitation often accompanies right ventricular dysfunction. When the right ventricle becomes dilated because of volume overload or intrinsic myocardial disease, or both, the tricuspid annulus also enlarges, with resultant valve insufficiency. This form of regurgitation may improve if the cause of the right ventricular dilatation is corrected, or it may require surgical plication of the valve annulus. Tricuspid regurgitation is also encountered in newborns with perinatal asphyxia. The cause may be related to an increased susceptibility of the papillary muscles to ischemic damage and subsequent transient papillary muscle dysfunction. Finally, tricuspid regurgitation is seen in up to 30% of children after heart transplantation, which can be a risk factor for graft dysfunction but is also seen as a consequence of valve injury due to endomyocardial biopsy.
Ben Sivarajan V, Chrisant MR, Ittenbach RF, et al. Prevalence and risk factors for tricuspid valve regurgitation after pediatric heart transplantation. J Heart Lung Transplant. 2008;27:494-500.
Sachdeva R, Fiser RT, Morrow WR, et al. Ruptured tricuspid valve papillary muscle: a treatable cause of neonatal cyanosis. Ann Thorac Surg. 2007;83:680-682.