Chapter 420 Acyanotic Congenital Heart Disease
The Left-to-Right Shunt Lesions
420.1 Atrial Septal Defect
Atrial septal defects (ASDs) can occur in any portion of the atrial septum (secundum, primum, or sinus venosus), depending on which embryonic septal structure has failed to develop normally (Chapter 414). Less commonly, the atrial septum may be nearly absent, with the creation of a functional single atrium. Isolated secundum ASDs account for ≈7% of congenital heart defects. The majority of cases of ASD are sporadic; autosomal dominant inheritance does occur as part of the Holt-Oram syndrome (hypoplastic or absent radii, 1st-degree heart block, ASD) or in families with secundum ASD and heart block.
An isolated valve-incompetent patent foramen ovale (PFO) is a common echocardiographic finding during infancy. It is usually of no hemodynamic significance and is not considered an ASD; a PFO may play an important role if other structural heart defects are present. If another cardiac anomaly is causing increased right atrial pressure (pulmonary stenosis or atresia, tricuspid valve abnormalities, right ventricular dysfunction), venous blood may shunt across the PFO into the left atrium with resultant cyanosis. Because of the anatomic structure of the PFO, left-to-right shunting is unusual outside the immediate newborn period. In the presence of a large volume load or a hypertensive left atrium (secondary to mitral stenosis), the foramen ovale may be sufficiently dilated to result in a significant atrial left-to-right shunt. A valve-competent but probe-patent foramen ovale may be present in 15-30% of adults. An isolated PFO does not require surgical treatment, although it may be a risk for paradoxical (right to left) systemic embolization. Device closure of these defects has been considered in young adults with a history of thromboembolic stroke.
Beda RD, Gill EAJr. Patent foramen ovale: does it play a role in the pathophysiology of migraine headache? Cardiol Clin. 2005;23:91-96.
Benson DW, Silberbach GM, Kavanaugh-McHugh A, et al. Mutations in the cardiac transcription factor NKX2.5 affect diverse cardiac developmental pathways. J Clin Invest. 1999;104:1567-1573.
Jategaonkar S, Scholtz W, Schmidt H, et al. Percutaneous closure of atrial septal defects: echocardiographic and functional results in patients older than 60 years. Circ Cardiovasc Interv. 2009;2:85-89.
Kharouf R, Luxenberg DM, Khalid O, et al. Atrial septal defect: spectrum of care. Pediatr Cardiol. 2008;29:271-280.
Masura J, Gavora P, Podnar T. Long-term outcome of transcatheter secundum-type atrial septal defect closure using Amplatzer septal occluders. J Am Coll Cardiol. 2005;45:505-507.
Radzik D, Davignon A, van Doesburg N, et al. Predictive factors for spontaneous closure of atrial septal defects diagnosed in the first 3 months of life. J Am Coll Cardiol. 1993;22:851-853.
Riggs T, Sharp SE, Batton D, et al. Spontaneous closure of atrial septal defects in premature vs full term neonates. Pediatr Cardiol. 2000;21:129-134.
Swartz EN. Is transcatheter device occlusion as good as open heart surgery for closure of atrial septal defects? Arch Dis Child. 2004;89:687-688.
Yew G, Wilson NJ. Transcatheter atrial septal defect closure with the Amplatzer septal occluder: five-year follow-up. Catheter Cardiovasc Interv. 2005;64:193-196.
420.2 Ostium Secundum Defect
An ostium secundum defect in the region of the fossa ovalis is the most common form of ASD and is associated with structurally normal atrioventricular (AV) valves. Mitral valve prolapse has been described in association with this defect but is rarely an important clinical consideration. Secundum ASDs may be single or multiple (fenestrated atrial septum), and openings ≥2 cm in diameter are common in symptomatic older children. Large defects may extend inferiorly toward the inferior vena cava and ostium of the coronary sinus, superiorly toward the superior vena cava, or posteriorly. Females outnumber males 3 : 1 in incidence. Partial anomalous pulmonary venous return, most commonly of the right upper pulmonary vein, may be an associated lesion.
Pathophysiology
The degree of left-to-right shunting is dependent on the size of the defect, the relative compliance of the right and left ventricles, and the relative vascular resistance in the pulmonary and systemic circulations. In large defects, a considerable shunt of oxygenated blood flows from the left to the right atrium (Fig. 420-1). This blood is added to the usual venous return to the right atrium and is pumped by the right ventricle to the lungs. With large defects, the ratio of pulmonary to systemic blood flow (Qp : Qs) is usually between 2 : 1 and 4 : 1. The paucity of symptoms in infants with ASDs is related to the structure of the right ventricle in early life when its muscular wall is thick and less compliant, thus limiting the left-to-right shunt. As the infant becomes older and pulmonary vascular resistance drops, the right ventricular wall becomes thinner and the left-to-right shunt across the ASD increases. The increased blood flow through the right side of the heart results in enlargement of the right atrium and ventricle and dilatation of the pulmonary artery. The left atrium may also be enlarged, but the left ventricle and aorta are normal in size. Despite the large pulmonary blood flow, pulmonary arterial pressure is usually normal because of the absence of a high-pressure communication between the pulmonary and systemic circulations. Pulmonary vascular resistance remains low throughout childhood, although it may begin to increase in adulthood and may eventually result in reversal of the shunt and clinical cyanosis.
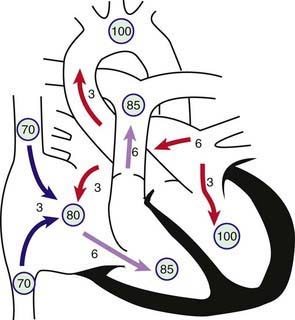
Figure 420-1 Physiology of atrial septal defect (ASD). Circled numbers represent oxygen saturation values. The numbers next to the arrows represent volumes of blood flow (in L/min/m2). This illustration shows a hypothetical patient with a pulmonary-to-systemic blood flow ratio (Qp : Qs) of 2 : 1. Desaturated blood enters the right atrium from the vena cavae at a volume of 3 L/min/m2 and mixes with an additional 3 L of fully saturated blood shunting left to right across the ASD; the result is an increase in oxygen saturation in the right atrium. Six liters of blood flows through the tricuspid valve and causes a mid-diastolic flow rumble. Oxygen saturation may be slightly higher in the right ventricle because of incomplete mixing at the atrial level. The full 6 L flows across the right ventricular outflow tract and causes a systolic ejection flow murmur. Six liters returns to the left atrium, with 3 L shunting left to right across the defect and 3 L crossing the mitral valve to be ejected by the left ventricle into the ascending aorta (normal cardiac output).
Clinical Manifestations
A child with an ostium secundum ASD is most often asymptomatic; the lesion is often discovered inadvertently during physical examination. Even an extremely large secundum ASD rarely produces clinically evident heart failure in childhood. However, on closer evaluation, in younger children, subtle failure to thrive may be present; in older children, varying degrees of exercise intolerance may be noted. Often, the degree of limitation may go unnoticed by the family until after surgical repair, when the child’s growth or activity level increases markedly.
The physical findings of an ASD are usually characteristic but fairly subtle and require careful examination of the heart, with special attention to the heart sounds. Examination of the chest may reveal a mild left precordial bulge. A right ventricular systolic lift may be palpable at the left sternal border. Sometimes a pulmonic ejection click can be heard. In most patients with an ASD, the characteristic finding is that the 2nd heart sound is widely split and fixed in its splitting during all phases of respiration. Normally, the duration of right ventricular ejection varies with respiration, with inspiration increasing right ventricular volume and delaying closure of the pulmonary valve. With an ASD, right ventricular diastolic volume is constantly increased and the ejection time is prolonged throughout all phases of respiration. A systolic ejection murmur is heard; it is medium pitched, without harsh qualities, seldom accompanied by a thrill, and best heard at the left middle and upper sternal border. It is produced by the increased flow across the right ventricular outflow tract into the pulmonary artery, not by low-pressure flow across the ASD. A short, rumbling mid-diastolic murmur produced by the increased volume of blood flow across the tricuspid valve is often audible at the lower left sternal border. This finding, which may be subtle and is heard best with the bell of the stethoscope, usually indicates a Qp : Qs ratio of at least 2 : 1.
Diagnosis
The chest roentgenogram shows varying degrees of enlargement of the right ventricle and atrium, depending on the size of the shunt. The pulmonary artery is enlarged, and pulmonary vascularity is increased. These signs vary and may not be conspicuous in mild cases. Cardiac enlargement is often best appreciated on the lateral view because the right ventricle protrudes anteriorly as its volume increases. The electrocardiogram shows volume overload of the right ventricle; the QRS axis may be normal or exhibit right axis deviation, and a minor right ventricular conduction delay (rsR′ pattern in the right precordial leads) may be present.
The echocardiogram shows findings characteristic of right ventricular volume overload, including an increased right ventricular end-diastolic dimension and flattening and abnormal motion of the ventricular septum (Fig. 420-2). A normal septum moves posteriorly during systole and anteriorly during diastole. With right ventricular overload and normal pulmonary vascular resistance, septal motion is either flattened or reversed—that is, anterior movement in systole. The location and size of the atrial defect are readily appreciated by two-dimensional scanning, with a characteristic brightening of the echo image seen at the edge of the defect (T-artifact). The shunt is confirmed by pulsed and color flow Doppler. The normal entry of all pulmonary veins into the left atrium should be confirmed.
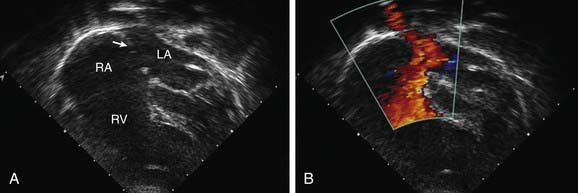
Figure 420-2 Echocardiographic findings in a secundum atrial septal defect (ASD). A, 2-D echocardiogram (apical four-chamber view) shows a moderate-sized secundum ASD (arrow). B, Color flow Doppler imaging shows left-to-right shunting (the red color represents blood moving toward the ultrasound transducer and does not indicate the level of oxygenation of the blood). LA, left atrium; RA, right atrium; RV, right ventricle.
Patients with the classic features of a hemodynamically significant ASD on physical examination and chest radiography, in whom echocardiographic identification of an isolated secundum ASD is made, need undergo diagnostic catheterization before repair, with the exception of an older patient, in whom pulmonary vascular resistance may be a concern. If pulmonary vascular disease is suspected, cardiac catheterization confirms the presence of the defect and allows measurement of the shunt ratio and pulmonary pressure and resistance.
If catheterization is performed, the oxygen content of blood from the right atrium will be much higher than that from the superior vena cava. This feature is not specifically diagnostic because it may occur with partial anomalous pulmonary venous return to the right atrium, with a ventricular septal defect (VSD) in the presence of tricuspid insufficiency, with AV septal defects associated with left ventricular to right atrial shunts, and with aorta to right atrial communications (ruptured sinus of Valsalva aneurysm). Pressure in the right side of the heart is usually normal, but small to moderate pressure gradients (<25 mm Hg) may be measured across the right ventricular outflow tract because of functional stenosis related to excessive blood flow. In children and adolescents, the pulmonary vascular resistance is almost always normal. The shunt is variable and depends on the size of the defect, but it may be of considerable volume (as high as 20 L/min/m2). Cineangiography, performed with the catheter through the defect and in the right upper pulmonary vein, demonstrates the defect and the location of the right upper pulmonary venous drainage. Alternatively, pulmonary angiography demonstrates the defect on the levophase (return of contrast to the left side of the heart after passing through the lungs).
Complications
Secundum ASDs are usually isolated, although they may be associated with partial anomalous pulmonary venous return, pulmonary valvular stenosis, VSD, pulmonary artery branch stenosis, and persistent left superior vena cava, as well as mitral valve prolapse and insufficiency. Secundum ASDs are associated with the autosomal dominant Holt-Oram syndrome. The gene responsible for this syndrome, situated in the region 12q21-q22 of chromosome 12, is TBX5, a member of the T-box transcriptional family. A familial form of secundum ASD associated with AV conduction delay has been linked to mutations in another transcription factor, Nkx2.5. Patients with familial ASD without heart block may carry a mutation in the transcription factor GATA4, located on chromosome 8p22-23.
Treatment
Surgical or transcatheter device closure is advised for all symptomatic patients and also for asymptomatic patients with a Qp : Qs ratio of at least 2 : 1 or those with right ventricular enlargement. The timing for elective closure is usually after the 1st yr and before entry into school. Closure carried out at open heart surgery is associated with a mortality rate of <1%. Repair is preferred during early childhood because surgical mortality and morbidity are significantly greater in adulthood; the long-term risk of arrhythmia is also greater after ASD repair in adults. For most patients, the procedure of choice is percutaneous catheter device closure using an atrial septal occlusion device, implanted transvenously in the cardiac catheterization laboratory (Fig. 420-3). The results are excellent and patients are discharged the following day. With the latest generation of devices, the incidence of serious complications such as device erosion is 0.1% and can be decreased by identifying high-risk patients such as those with a deficient rim of septum around the device. Echocardiography can usually determine whether a patient is a good candidate for device closure. In patients with small secundum ASDs and minimal left-to-right shunts without right ventricular enlargement, the consensus is that closure is not required. It is unclear at present whether the persistence of a small ASD into adulthood increases the risk for stroke enough to warrant prophylactic closure of all these defects.
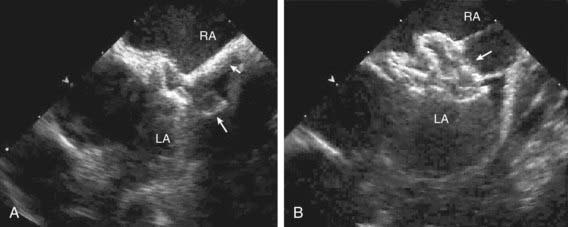
Figure 420-3 Intravascular ultrasound imaging of transcatheter occlusion of an atrial septal defect. A, A catheter (small arrow) has been advanced across the atrial defect, and the left-sided disk of the device (large arrow) has been extruded from the sheath into the left atrium (LA). B, The right atrial disk (arrow) has now been extruded into the right atrium (RA). The two halves of the device are then locked together and the catheter detached from the occluder device and removed.
Prognosis
Small- to moderate-sized ASDs detected in term infants may close spontaneously. Secundum ASDs are well tolerated during childhood, and symptoms do not usually appear until the 3rd decade or later. Pulmonary hypertension, atrial dysrhythmias, tricuspid or mitral insufficiency, and heart failure are late manifestations; these symptoms may initially appear during the increased volume load of pregnancy. Infective endocarditis is extremely rare, and antibiotic prophylaxis for isolated secundum ASDs is not recommended.
The results after surgical or device closure in children with moderate to large shunts are excellent. Symptoms disappear rapidly, and growth is frequently enhanced. Heart size decreases to normal, and the electrocardiogram shows decreased right ventricular forces. Late right heart failure and arrhythmias are less frequent in patients who have had early surgical repair, becoming more common in patients who undergo surgery after 20 yr of age. Although early and midterm results with device closure are excellent, the long-term effects are not yet known. Reports of resolution of migraine headaches in patients after device closure of ASD or PFO are intriguing, suggesting a possible thromboembolic etiology; there are also paradoxical reports of patients whose migraines began or worsened after placement of one of these devices.
420.3 Sinus Venosus Atrial Septal Defect
A sinus venosus ASD is situated in the upper part of the atrial septum in close relation to the entry of the superior vena cava. Often, one or more pulmonary veins (usually from the right lung) drain anomalously into the superior vena cava. The superior vena cava sometimes straddles the defect; in this case, some systemic venous blood enters the left atrium, but only rarely does it cause clinically evident cyanosis. The hemodynamic disturbance, clinical picture, electrocardiogram, and roentgenogram are similar to those seen in secundum ASD. The diagnosis can usually be made by two-dimensional echocardiography. If there are questions regarding pulmonary venous drainage, cardiac CT or MRI is usually diagnostic. Cardiac catheterization is rarely required, with the exception being in adult patients where assessment of pulmonary vascular resistance may be important. Anatomic correction generally requires the insertion of a patch to close the defect while incorporating the entry of anomalous veins into the left atrium. If the anomalous vein drains high in the superior vena cava, the vein can be left intact and the ASD closed to incorporate the mouth of the superior vena cava into the left atrium. The superior vena cava proximal to the venous entrance is then detached and anastomosed directly to the right atrium. This procedure avoids direct suturing of the pulmonary vein with less chance of future stenosis. Surgical results are generally excellent. Rarely, sinus venosus defects involve the inferior vena cava.
420.4 Partial Anomalous Pulmonary Venous Return
One or several pulmonary veins may return anomalously to the superior or inferior vena cava, the right atrium, or the coronary sinus and produce a left-to-right shunt of oxygenated blood. Partial anomalous pulmonary venous return usually involves some or all of the veins from only one lung, more often the right one. When an associated ASD is present, it is generally of the sinus venosus type, although can be of the secundum type (Chapter 420.3). When an ASD is detected by echocardiography, one must always search for associated partial anomalous pulmonary venous return. The history, physical signs, and electrocardiographic and roentgenographic findings are indistinguishable from those of an isolated ostium secundum ASD. Occasionally, an anomalous vein draining into the inferior vena cava is visible on chest radiography as a crescentic shadow of vascular density along the right border of the cardiac silhouette (scimitar syndrome); in these cases, an ASD is not usually present, but pulmonary sequestration and anomalous arterial supply to that lobe are common findings. Total anomalous pulmonary venous return is a cyanotic lesion and is discussed in Chapter 425.7. Echocardiography generally confirms the diagnosis. MRI and CT are also useful if there is a question regarding pulmonary venous drainage or in cases of scimitar syndrome. If cardiac catheterization is performed, the presence of anomalous pulmonary veins is demonstrated by selective pulmonary arteriography and anomalous pulmonary arterial supply to the right lung is demonstrated by descending aortography.
The prognosis is excellent, similar to that for ostium secundum ASDs. When a large left-to-right shunt is present, surgical repair is performed. The associated ASD should be closed in such a way that pulmonary venous return is directed to the left atrium. A single anomalous pulmonary vein without an atrial communication may be difficult to redirect to the left atrium; if the shunt is small, it may be left unoperated.
420.5 Atrioventricular Septal Defects (Ostium Primum and Atrioventricular Canal or Endocardial Cushion Defects)
The abnormalities encompassed by AV septal defects are grouped together because they represent a spectrum of a basic embryologic abnormality, a deficiency of the AV septum. An ostium primum defect is situated in the lower portion of the atrial septum and overlies the mitral and tricuspid valves. In most instances, a cleft in the anterior leaflet of the mitral valve is also noted. The tricuspid valve is usually functionally normal, although some anatomic abnormality of the septal leaflet is generally present. The ventricular septum is intact.
An AV septal defect, also known as an AV canal defect or an endocardial cushion defect, consists of contiguous atrial and ventricular septal defects with markedly abnormal AV valves. The severity of the valve abnormalities varies considerably; in the complete form of AV septal defect, a single AV valve is common to both ventricles and consists of an anterior and a posterior bridging leaflet related to the ventricular septum, with a lateral leaflet in each ventricle. The lesion is common in children with Down syndrome.
Transitional varieties of these defects also occur and include ostium primum defects with clefts in the anterior mitral and septal tricuspid valve leaflets and small ventricular septal defects, and, less commonly, ostium primum defects with normal AV valves. In some patients, the atrial septum is intact, but an inlet VSD is similar to that found in the full AV septal defect. Sometimes AV septal defects are associated with varying degrees of hypoplasia of one of the ventricles, known as either left- or right-dominant AVSD. If the affected ventricular chamber is too small to establish a two ventricle circulation, then surgical palliation, aiming for an eventual Fontan procedure, is performed (Chapters 424.4 and 425.10).
Pathophysiology
The basic abnormality in patients with ostium primum defects is the combination of a left-to-right shunt across the atrial defect and mitral (or occasionally tricuspid) insufficiency. The shunt is usually moderate to large, the degree of mitral insufficiency is generally mild to moderate, and pulmonary arterial pressure is typically normal or only mildly increased. The physiology of this lesion is therefore similar to that of an ostium secundum ASD.
In complete AV septal defects, the left-to-right shunt occurs at both the atrial and ventricular levels (Fig. 420-4). Additional shunting may occur directly from the left ventricle to the right atrium because of absence of the AV septum. Pulmonary hypertension and an early tendency to increase pulmonary vascular resistance are common. AV valvular insufficiency increases the volume load on one or both ventricles. If the defect is large enough, some right-to-left shunting may also occur at both the atrial and ventricular levels and lead to mild arterial desaturation. With time, progressive pulmonary vascular disease increases the right-to-left shunt so that clinical cyanosis develops (Eisenmenger physiology, Chapter 427.2).
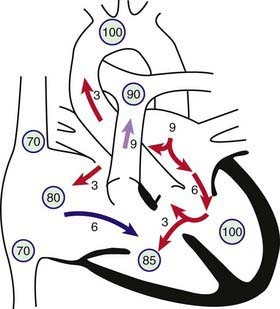
Figure 420-4 Physiology of atrioventricular septal defect (AVSD). Circled numbers represent oxygen saturation values. The numbers next to the arrows represent volumes of blood flow (in L/min/m2). This illustration shows a hypothetical patient with a pulmonary-to-systemic blood flow ratio (Qp : Qs) of 3 : 1. Desaturated blood enters the right atrium from the vena cavae at a volume of 3 L/min/m2 and mixes with 3 L of fully saturated blood shunting left to right across the atrial septal defect; the result is an increase in oxygen saturation in the right atrium. Six liters of blood flows through the right side of the common AV valve, joined by an additional 3 L of saturated blood shunting left to right at the ventricular level, further increasing oxygen saturation in the right ventricle. The full 9 L flows across the right ventricular outflow tract into the lungs. Nine liters returns to the left atrium, with 3 L shunting left to right across the defect and 6 L crossing the left side of the common AV valve and causing a mid-diastolic flow rumble. Three liters of this volume shunts left to right across the VSD, and 3 L is ejected into the ascending aorta (normal cardiac output).
Clinical Manifestations
Many children with ostium primum defects are asymptomatic, and the anomaly is discovered during a general physical examination. In patients with moderate shunts and mild mitral insufficiency, the physical signs are similar to those of the secundum ASD, but with an additional apical holosystolic murmur caused by mitral insufficiency.
A history of exercise intolerance, easy fatigability, and recurrent pneumonia may be obtained, especially in infants with large left-to-right shunts and severe mitral insufficiency. In these patients, cardiac enlargement is moderate or marked, and the precordium is hyperdynamic. Auscultatory signs produced by the left-to-right shunt include a normal or accentuated 1st heart sound; wide, fixed splitting of the 2nd sound; a pulmonary systolic ejection murmur sometimes preceded by a click; and a low-pitched, mid-diastolic rumbling murmur at the lower left sternal edge or apex, or both, as a result of increased flow through the AV valves. Mitral insufficiency may be manifested by a harsh (occasionally very high pitched) apical holosystolic murmur that radiates to the left axilla.
With complete AV septal defects, heart failure and intercurrent pulmonary infection usually appear in infancy. The liver is enlarged and the infant shows signs of failure to thrive. Cardiac enlargement is moderate to marked, and a systolic thrill is frequently palpable at the lower left sternal border. A precordial bulge and lift may be present as well. The 1st heart sound is normal or accentuated. The 2nd heart sound is widely split if the pulmonary flow is massive. A low-pitched, mid-diastolic rumbling murmur is audible at the lower left sternal border, and a pulmonary systolic ejection murmur is produced by the large pulmonary flow. The harsh apical holosystolic murmur of mitral insufficiency may also be present.
Diagnosis
Chest radiographs of children with complete AV septal defects often show moderate to severe cardiac enlargement caused by the prominence of both ventricles and atria. The pulmonary artery is large, and pulmonary vascularity is increased.
The electrocardiogram in patients with a complete AV septal defect is distinctive. The principal abnormalities are (1) superior orientation of the mean frontal QRS axis with left axis deviation to the left upper or right upper quadrant, (2) counterclockwise inscription of the superiorly oriented QRS vector loop (often manifest by a Q wave in leads I and aVL), (3) signs of biventricular hypertrophy or isolated right ventricular hypertrophy, (4) right ventricular conduction delay (rSR′ pattern in leads V3R and V1), (5) normal or tall P waves, and (6) occasional prolongation of the P-R interval (Fig. 420-5).
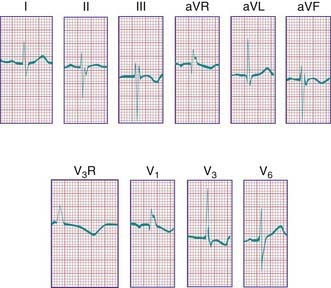
Figure 420-5 Electrocardiogram from a child with an atrioventricular septal defect. Note the QRS axis of −60 degrees and the right ventricular conduction delay with an RSR′ pattern in V1 and V3R (V3R paper speed = 50 mm/sec).
The echocardiogram (Fig. 420-6) is characteristic and shows signs of right ventricular enlargement with encroachment of the mitral valve echo on the left ventricular outflow tract; the abnormally low position of the AV valves results in a “gooseneck” deformity of the left ventricular outflow tract. In normal hearts, the tricuspid valve inserts slightly more toward the apex than the mitral valve does. In AV septal defects, both valves insert at the same level because of absence of the AV septum. In complete AV septal defects, the ventricular septum is also deficient and the common AV valve is readily appreciated. Pulsed and color flow Doppler echocardiography will demonstrate left-to-right shunting at the atrial, ventricular, or left ventricular to right atrial levels and semiquantitate the degree of AV valve insufficiency. Echocardiography is useful for determining the insertion points of the chordae of the common AV valve and for evaluating the presence of associated lesions such as patent ductus arteriosus (PDA) or coarctation of the aorta.
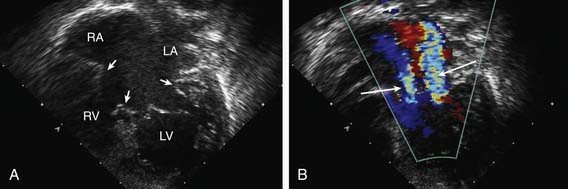
Figure 420-6 Echocardiogram of an atrioventricular septal defect. A, Subcostal four-chamber view demonstrating the common atrioventricular valve (arrows) spanning the atrial and ventricular septal defects. B, Doppler imaging shows 2 jets of regurgitation through the left side of the common atrioventricular valve (arrows). LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
Cardiac catheterization and angiocardiography is rarely required in the modern era to confirm the diagnosis unless pulmonary vascular disease is suspected, such as in a patient in whom diagnosis has been delayed beyond early infancy, especially in those with Down syndrome in whom the development of pulmonary vascular disease may be more rapid. Catheterization demonstrates the magnitude of the left-to-right shunt, the degree of elevation of pulmonary vascular resistance, and the severity of insufficiency of the common AV valve. By oximetry, the shunt is usually demonstrable at both the atrial and ventricular levels. Arterial oxygen saturation is normal or only mildly reduced unless severe pulmonary vascular disease is present. Children with ostium primum defects generally have normal or only moderately elevated pulmonary arterial pressure. Conversely, complete AV septal defects are associated with right ventricular and pulmonary hypertension and, in older patients, with increased pulmonary vascular resistance (Chapter 427.2).
Selective left ventriculography will demonstrate deformity of the mitral or common AV valve and the distortion of the left ventricular outflow tract caused by this valve (gooseneck deformity). The abnormal anterior leaflet of the mitral valve is serrated, and mitral insufficiency is noted, usually with regurgitation of blood into both the left and right atria. Direct shunting of blood from the left ventricle to the right atrium may also be demonstrated.
Treatment
Ostium primum defects are approached surgically from an incision in the right atrium. The cleft in the mitral valve is located through the atrial defect and is repaired by direct suture. The defect in the atrial septum is usually closed by insertion of a patch prosthesis. The surgical mortality rate for ostium primum defects is very low. Surgical treatment of complete AV septal defects is more difficult, especially in infants with cardiac failure and pulmonary hypertension. Because of the risk of pulmonary vascular disease developing as early as 6-12 mo of age, surgical intervention must be performed during infancy. With modern surgical techniques, full correction of these defects can be readily accomplished in infancy; palliation with pulmonary arterial banding is reserved for the subset of patients who have other associated lesions that make early corrective surgery too risky. The atrial and ventricular defects are patched and the AV valves reconstructed. Complications include surgically induced heart block requiring placement of a permanent pacemaker, excessive narrowing of the left ventricular outflow tract requiring surgical revision, and eventual worsening of mitral regurgitation requiring replacement with a prosthetic valve.
Prognosis
The prognosis for unrepaired complete AV septal defects depends on the magnitude of the left-to-right shunt, the degree of elevation of pulmonary vascular resistance, and the severity of AV valve insufficiency. Death from cardiac failure during infancy used to be frequent before the advent of early corrective surgery. In patients who survived without surgery, pulmonary vascular obstructive disease usually developed. Most patients with ostium primum defects and minimal AV valve involvement are asymptomatic or have only minor, nonprogressive symptoms until they reach the 3rd-4th decade of life, similar to the course of patients with secundum ASDs. Late postoperative complications include atrial arrhythmias and heart block, progressive narrowing of the left ventricular outflow tract requiring surgical revision, and eventual worsening of atrioventricular valve regurgitation (usually on the left side) requiring replacement with a prosthetic valve.
Becker AE, Anderson RH. Atrioventricular septal defects: what’s in a name? J Thorac Cardiovasc Surg. 1982;83:461-469.
Drinkwater DCJr, Laks H. Unbalanced atrioventricular septal defects. Semin Thorac Cardiovasc Surg. 1997;9:21-25.
Friedberg MK, Kim N, Silverman NH. Atrioventricular septal defect recently diagnosed by fetal echocardiography: echocardiographic features, associated anomalies, and outcomes. Congenit Heart Dis. 2007;2:110-114.
Malhotra SP, Lacour-Gayet F, Mitchell MB, et al. Reoperation for left atrioventricular valve regurgitation after atrioventricular septal defect repair. Ann Thorac Surg. 2008;86:147-151.
Murphy DJJr. Atrioventricular canal defects. Curr Treat Options Cardiovasc Med. 1999;1:323-334.
Sigfusson G, Ettedgui JA, Silverman NH, et al. Is a cleft in the anterior leaflet of an otherwise normal mitral valve an atrioventricular canal malformation? J Am Coll Cardiol. 1995;26:508-515.
Smedts HPM, Isaacs A, De Costa D, et al. VEGF polymorphisms are associated with endocardial cushion defects: a family-based case-control study. Pediatr Res. 2010;67:23-28.
Suzuki T, Bove EL, Devaney EJ, et al. Results of definitive repair of complete atrioventricular septal defect in neonates and infants. Ann Thorac Surg. 2008;86:596-602.
420.6 Ventricular Septal Defect
VSD is the most common cardiac malformation and accounts for 25% of congenital heart disease. Defects may occur in any portion of the ventricular septum, but most are of the membranous type. These defects are in a posteroinferior position, anterior to the septal leaflet of the tricuspid valve. VSDs between the crista supraventricularis and the papillary muscle of the conus may be associated with pulmonary stenosis and other manifestations of tetralogy of Fallot (Chapter 424.1). VSDs superior to the crista supraventricularis (supracristal) are less common; they are found just beneath the pulmonary valve and may impinge on an aortic sinus and cause aortic insufficiency. VSDs in the midportion or apical region of the ventricular septum are muscular in type and may be single or multiple (Swiss cheese septum).
Pathophysiology
The physical size of the VSD is a major, but not the only determinant of the size of the left-to-right shunt. The level of pulmonary vascular resistance in relation to systemic vascular resistance also determines the shunt’s magnitude. When a small communication is present (usually <5 mm), the VSD is pressure restrictive, meaning that right ventricular pressure is normal. The higher pressure in the left ventricle drives the shunt left to right and the size of the defect limits the magnitude of the shunt. In large nonrestrictive VSDs (usually >10 mm), right and left ventricular pressures are equalized. In these defects, the direction of shunting and the shunt magnitude are determined by the ratio of pulmonary to systemic vascular resistance (Fig. 420-7).
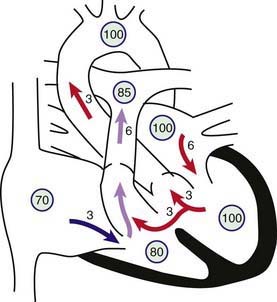
Figure 420-7 Physiology of a large ventricular septal defect (VSD). Circled numbers represent oxygen saturation values. The numbers next to the arrows represent volumes of blood flow (in L/min/m2). This illustration shows a hypothetical patient with a pulmonary-to-systemic blood flow ratio (Qp : Qs) of 2 : 1. Desaturated blood enters the right atrium from the vena cava at a volume of 3 L/min/m2 and flows across the tricuspid valve. An additional 3 L of blood shunts left to right across the VSD, the result being an increase in oxygen saturation in the right ventricle. Six liters of blood is ejected into the lungs. Pulmonary arterial saturation may be further increased because of incomplete mixing at right ventricular level. Six liters returns to the left atrium, crosses the mitral valve, and causes a mid-diastolic flow rumble. Three liters of this volume shunts left to right across the VSD, and 3 L is ejected into the ascending aorta (normal cardiac output).
After birth in patients with a large VSD, pulmonary vascular resistance may remain elevated, delaying the normal postnatal decrease, and thus the size of the left-to-right shunt may initially be limited. Because of normal involution of the media of small pulmonary arterioles, pulmonary vascular resistance begins to fall in the 1st few weeks after birth and the size of the left-to-right shunt increases. Eventually, a large left-to-right shunt develops, and clinical symptoms become apparent. In most cases during early infancy, pulmonary vascular resistance is only slightly elevated, and the major contribution to pulmonary hypertension is the large communication allowing exposure of the pulmonary circulation to systemic pressure and the large pulmonary blood flow. With continued exposure of the pulmonary vascular bed to high systolic pressure and high flow, pulmonary vascular obstructive disease eventually develops. When the ratio of pulmonary to systemic resistance approaches 1 : 1, the shunt becomes bidirectional, signs of heart failure abate, and the patient begins to show signs of cyanosis (Eisenmenger physiology, Chapter 427.2). In rare infants with a large VSD, usually those with Down syndrome, pulmonary vascular resistance never decreases, and symptoms may remain minimal until Eisenmenger physiology becomes evident.
The magnitude of intracardiac shunts is usually described by the Qp : Qs ratio. If the left-to-right shunt is small (Qp : Qs <1.5 : 1), the cardiac chambers are not appreciably enlarged and the pulmonary vascular bed is probably normal. If the shunt is large (Qp : Qs >2 : 1), left atrial and ventricular volume overload occurs, as does right ventricular and pulmonary arterial hypertension. The main pulmonary artery, left atrium, and left ventricle are enlarged.
Clinical Manifestations
The clinical findings of patients with a VSD vary according to the size of the defect and pulmonary blood flow and pressure. Small VSDs with trivial left-to-right shunts and normal pulmonary arterial pressure are the most common. These patients are asymptomatic, and the cardiac lesion is usually found during routine physical examination. Characteristically, a loud, harsh, or blowing holosystolic murmur is present and heard best over the lower left sternal border, and it is frequently accompanied by a thrill. In a few instances, the murmur ends before the 2nd sound, presumably because of closure of the defect during late systole. A short, harsh systolic murmur localized to the apex in a neonate is often a sign of a tiny VSD in the apical muscular septum. In premature infants, the murmur may be heard early because pulmonary vascular resistance decreases more rapidly.
Large VSDs with excessive pulmonary blood flow and pulmonary hypertension are responsible for dyspnea, feeding difficulties, poor growth, profuse perspiration, recurrent pulmonary infections, and cardiac failure in early infancy. Cyanosis is usually absent, but duskiness is sometimes noted during infections or crying. Prominence of the left precordium is common, as are a palpable parasternal lift, a laterally displaced apical impulse and apical thrust, and a systolic thrill. The holosystolic murmur of a large VSD is generally less harsh than that of a small VSD and more blowing in nature because of the absence of a significant pressure gradient across the defect. It is even less likely to be prominent in the newborn period. The pulmonic component of the 2nd heart sound may be increased as a result of pulmonary hypertension. The presence of a mid-diastolic, low-pitched rumble at the apex is caused by increased blood flow across the mitral valve and indicates a Qp : Qs ratio of ≥2 : 1. This murmur is best appreciated with the bell of the stethoscope.
Diagnosis
In patients with small VSDs, the chest x-ray is usually normal, although minimal cardiomegaly and a borderline increase in pulmonary vasculature may be observed. The electrocardiogram is generally normal but may suggest left ventricular hypertrophy. The presence of right ventricular hypertrophy is a warning that the defect is not small and that the patient has pulmonary hypertension or an associated lesion such as pulmonic stenosis. In large VSDs, the chest x-ray shows gross cardiomegaly with prominence of both ventricles, the left atrium, and the pulmonary artery (Fig. 420-8). Pulmonary vascular markings are increased, and frank pulmonary edema, including pleural effusions, may be present. The electrocardiogram shows biventricular hypertrophy; P waves may be notched or peaked.
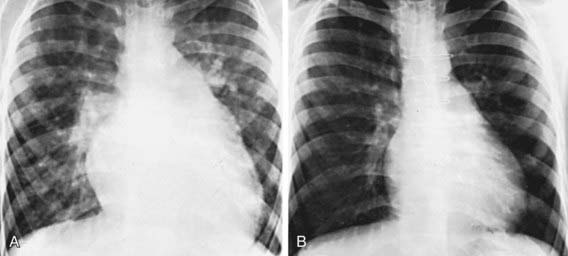
Figure 420-8 A, Preoperative roentgenogram in a patient with a ventricular septal defect with a large left-to-right shunt and pulmonary hypertension. Significant cardiomegaly, prominence of the pulmonary arterial trunk, and pulmonary overcirculation are evident. B, Three years after surgical closure of the defect, heart size is markedly decreased, and the pulmonary vasculature is normal.
The two-dimensional echocardiogram (Fig. 420-9) shows the position and size of the VSD. In small defects, especially those of the muscular septum, the defect itself may be difficult to image and is visualized only by color Doppler examination. In defects of the membranous septum, a thin membrane (called a ventricular septal aneurysm but consisting of tricuspid valve tissue) can partially cover the defect and limit the volume of the left-to-right shunt. Echocardiography is also useful for estimating shunt size by examining the degree of volume overload of the left atrium and left ventricle; in the absence of associated lesions, the extent of their increased dimensions is a good reflection of the size of the left-to-right shunt. Pulsed Doppler examination shows whether the VSD is pressure restrictive by calculating the pressure gradient across the defect. Such calculation allows an estimation of right ventricular pressure and helps determine whether the patient is at risk for the development of early pulmonary vascular disease. The echocardiogram can also be useful to determine the presence of aortic valve insufficiency or aortic leaflet prolapse in the case of supracristal VSDs.
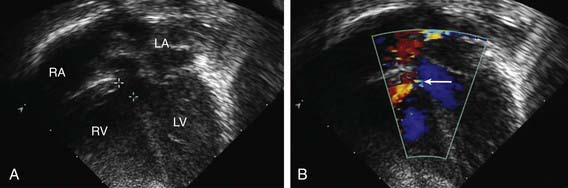
Figure 420-9 Echocardiogram in a patient with a perimembranous ventricular septal defect (VSD). A, Apical four-chamber view showing the location of the defect (outlined between two crosshatches) beneath the aortic valve. B, Color Doppler imaging shows the left-to-right shunt (arrow) through the defect (the red color represents blood moving toward the ultrasound transducer and does not indicate the level of oxygenation of the blood). LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
The hemodynamics of a VSD can also be demonstrated by cardiac catheterization, although catheterization is today performed only when laboratory data do not fit well with the clinical findings or when pulmonary vascular disease is suspected. Oximetry demonstrates increased oxygen content in the right ventricle; because some defects eject blood almost directly into the pulmonary artery (streaming), the full magnitude of the oxygen saturation increase is occasionally apparent only when pulmonary arterial blood is sampled. Small, restrictive VSDs are associated with normal right heart pressures and pulmonary vascular resistance. Large, nonrestrictive VSDs are associated with equal or nearly equal pulmonary and systemic systolic pressure and variable elevations in pulmonary vascular resistance. Pulmonary blood flow may be 2 to 4 times systemic blood flow. In patients with such “hyperdynamic pulmonary hypertension,” pulmonary vascular resistance is only minimally elevated because resistance is equal to pressure divided by flow. However, if left untreated until Eisenmenger syndrome is present, pulmonary artery systolic and diastolic pressure will be elevated but the degree of left-to-right shunting minimal. In these cases, desaturation of blood in the left ventricle is usually encountered. The size, location, and number of ventricular defects can be demonstrated by left ventriculography. Contrast medium passes across the defect or defects to opacify the right ventricle and pulmonary artery. Administration of 100% oxygen with and without nitric oxide can be used to determine whether the pulmonary vascular resistance, if elevated, is still reactive and therefore more likely to drop after surgical repair.
Treatment
The natural course of a VSD depends to a large degree on the size of the defect. A significant number (30-50%) of small defects close spontaneously, most frequently during the 1st 2 yr of life. Small muscular VSDs are more likely to close (up to 80%) than membranous VSDs (up to 35%). The vast majority of defects that close do so before the age of 4 yr, although spontaneous closure has been reported in adults. VSDs that close often have ventricular septal aneurysm (accessory tricuspid valve) tissue that limits the magnitude of the shunt. Most children with small defects remain asymptomatic, without evidence of an increase in heart size, pulmonary arterial pressure, or resistance. A long-term risk is infective endocarditis. Some long-term studies of adults with unoperated small VSDs show an increased incidence of arrhythmia, subaortic stenosis, and exercise intolerance. The Council on Cardiovascular Disease in the Young of the American Heart Association states that an isolated, small, hemodynamically insignificant VSD is not an indication for surgery. The declining risk of open heart surgery has led others to suggest that all VSDs be closed electively by mid-childhood.
It is less common for moderate or large VSDs to close spontaneously, although even defects large enough to result in heart failure may become smaller and up to 8% may close completely. More commonly, infants with large defects have repeated episodes of respiratory infection and heart failure despite optimal medical management. Heart failure may be manifested in many of these infants primarily as failure to thrive. Pulmonary hypertension occurs as a result of high pulmonary blood flow. These patients are at risk for pulmonary vascular disease if the defect is not repaired during early infancy.
Patients with VSD are also at risk for the development of aortic valve regurgitation, the greatest risk occurring in patients with a supracristal VSD (Chapter 420.7). A small number of patients with VSD develop acquired infundibular pulmonary stenosis, which then protects the pulmonary circulation from the short-term effects of pulmonary overcirculation and the long-term effects of pulmonary vascular disease. In these patients, the clinical picture changes from that of a VSD with a large left-to-right shunt to a VSD with pulmonary stenosis. The shunt may diminish in size, become balanced, or even become a net right-to-left shunt. These patients must be carefully distinguished from those in whom an Eisenmenger physiology develops (Chapter 427.2).
In patients with small VSDs, parents should be reassured of the relatively benign nature of the lesion, and the child should be encouraged to live a normal life, with no restrictions on physical activity. Surgical repair is currently not recommended. As protection against infective endocarditis, the integrity of primary and permanent teeth should be carefully maintained; with the latest revision of the American Heart Association guidelines, antibiotic prophylaxis is no longer recommended for dental visits or surgical procedures (Chapter 431). These patients can be monitored by a combination of clinical examination and noninvasive laboratory tests until the VSD has closed spontaneously. Echocardiography is used to estimate pulmonary artery pressure, screen for the development of left ventricular outflow tract pathology (subaortic membrane or aortic regurgitation), and to confirm spontaneous closure.
In infants with a large VSD, management has 2 aims: to get the symptoms of heart failure under control (Chapter 436) and prevent the development of pulmonary vascular disease. If early treatment is successful, sometimes the shunt may diminish in size with spontaneous improvement, especially during the 1st yr of life. The clinician must be alert not to confuse clinical improvement caused by a decrease in defect size with clinical changes caused by the development of Eisenmenger physiology. Because surgical closure can be carried out at low risk in most infants, medical management should not be pursued in symptomatic infants after an initial unsuccessful trial. Since pulmonary vascular disease can usually be prevented when surgery is performed within the 1st yr of life, even infants with well controlled heart failure should not have surgery delayed inordinately unless there is evidence that the defect is becoming pressure restrictive.
Indications for surgical closure of a VSD include patients at any age with large defects in whom clinical symptoms and failure to thrive cannot be controlled medically; infants between 6 and 12 mo of age with large defects associated with pulmonary hypertension, even if the symptoms are controlled by medication; and patients older than 24 mo with a Qp : Qs ratio greater than 2 : 1. Patients with a supracristal VSD of any size are usually referred for surgery because of the high risk for aortic valve regurgitation (Chapter 420.7). Severe pulmonary vascular disease nonresponsive to pulmonary vasodilators is a contraindication to closure of a VSD.
Prognosis
The results of primary surgical repair are excellent, and complications leading to long-term problems (residual ventricular shunts requiring reoperation or heart block requiring a pacemaker) are rare. Pulmonary arterial palliative banding with repair in later childhood, once the standard of care, is now reserved for extremely complicated cases or very premature infants. Surgical risks are somewhat higher for defects in the muscular septum, particularly apical defects and multiple (Swiss cheese–type) VSDs. These patients may require pulmonary arterial banding if symptomatic, with subsequent debanding and repair of multiple VSDs at an older age. Catheter occlusion devices are in clinical trials as a means of closing apical muscular VSDs and other devices are being tested for closing the more common perimembranous defects. Sometimes these devices are placed during surgery in what is known as a hybrid approach to repair.
After surgical obliteration of the left-to-right shunt, the hyperdynamic heart becomes quiet, cardiac size decreases toward normal (see Fig. 420-8), thrills and murmurs are abolished, and pulmonary artery hypertension regresses. The patient’s clinical status improves markedly. Most infants begin to thrive, and cardiac medications are no longer required. Catch-up growth occurs in most patients within the next 1-2 yr. In some instances after successful surgery, systolic ejection murmurs of low intensity persist for months. The long-term prognosis after surgery is excellent. Patients with a small VSD and those who have undergone surgical closure without residua are considered to be at standard risk for health and life insurance.
Beekman RHIII. Closing the ventricular septal defect because you can: evidence-averse care? J Pediatr. 2007;150:569-570.
Glen S, Burns J, Bloomfield P. Prevalence and development of additional cardiac abnormalities in 1448 patients with congenital ventricular septal defects. Heart. 2004;90:1321-1325.
Holzer R, Balzer D, Cao QL, et al. Device closure of muscular ventricular septal defects using the Amplatzer muscular ventricular septal defect occluder: immediate and mid-term results of a U.S. registry. J Am Coll Cardiol. 2004;43:1257-1263.
Hornberger LK, Sahn DJ, Krabill KA, et al. Elucidation of the natural history of ventricular septal defects by serial Doppler color flow mapping studies. J Am Coll Cardiol. 1989;13:1111-1118.
Kenny D, Morgan G, Bajwa A, et al. Evolution of transcatheter closure of perimembranous ventricular septal defects in a single centre. Catheter Cardiovasc Interv. 2009;73:568-575.
Lim DS, Forbes TJ, Rothman A, et al. Transcatheter closure of high-risk muscular ventricular septal defects with the CardioSEAL occluder: initial report from the CardioSEAL VSD registry. Catheter Cardiovasc Interv. 2007;70:740-744.
Ramaciotti C, Keren A, Silverman NH. Importance of pseudoaneurysms of the ventricular septum in the natural history of isolated perimembranous ventricular septal defects. Am J Cardiol. 1986;57:268-272.
Roos-Hesselink JW, Meijboom FJ, Spitaels SE, et al. Outcome of patients after surgical closure of ventricular septal defect at young age: longitudinal follow-up of 22–34 years. Eur Heart J. 2004;25:1057-1062.
420.7 Supracristal Ventricular Septal Defect with Aortic Insufficiency
A supracristal VSD is complicated by prolapse of the aortic valve into the defect and aortic insufficiency, which may eventually occur in 50-90% of patients. Although supracristal VSD accounts for ≈5% of all patients with VSD, the incidence is higher in Asian children. The VSD, which may be small or moderate in size, is located anterior to and directly below the pulmonary valve in the outlet septum, superior to the muscular ridge known as the crista supraventriculari, which separates the trabecular body of the right ventricle from the smooth outflow portion. The right or, less often, the noncoronary aortic cusp prolapses into the defect and may partially or even completely occlude it. Such occlusion may limit the amount of left-to-right shunting and give the false impression that the defect is not large. Aortic insufficiency is most often not recognized until late in the 1st decade of life or beyond. Of note, aortic insufficiency is occasionally associated with VSDs located in the membranous septum.
Early heart failure secondary to a large left-to-right shunt rarely occurs, but without surgery, severe aortic insufficiency and left ventricular failure may ensue. The murmur of a supracristal VSD is usually heard at the mid to upper left sternal border, as opposed to the lower left sternal border, and it is sometimes confused with that of pulmonic stenosis. A decrescendo diastolic murmur will be appreciated at the upper right or mid left sternal borders if there is aortic insufficiency. More advanced degrees of aortic insufficiency will be associated with a wide pulse pressure. These clinical findings must be distinguished from PDA or other defects associated with aortic runoff.
The clinical manifestations vary widely from trivial aortic regurgitation and small left-to-right shunts in asymptomatic children to florid aortic insufficiency and massive cardiomegaly in symptomatic adolescents. Closure of all supracristal ventricular VSDs at the time of diagnosis is commonly recommended to prevent the development of aortic regurgitation, even in an asymptomatic child. Patients who already have significant aortic insufficiency require surgical intervention to prevent irreversible left ventricular dysfunction. Surgical options depend on the degree of damage to the valve. If the insufficiency is mild, they may include simple closure of the defect to bolster the valve apparatus without touching the valve itself, valvuloplasty for more significant degrees of involvement, and replacement with a prosthesis or homograft or aortopulmonary translocation for severe involvement.
420.8 Patent Ductus Arteriosus
During fetal life, most of the pulmonary arterial blood is shunted right-to-left through the ductus arteriosus into the aorta (Chapter 415). Functional closure of the ductus normally occurs soon after birth, but if the ductus remains patent when pulmonary vascular resistance falls, aortic blood then is shunted left-to-right into the pulmonary artery. The aortic end of the ductus is just distal to the origin of the left subclavian artery, and the ductus enters the pulmonary artery at its bifurcation. Female patients with patent ductus arteriosus (PDA) outnumber males 2 : 1. PDA is also associated with maternal rubella infection during early pregnancy, a now uncommon occurrence. However, PDA is a common problem in premature infants, as the smooth muscle in the wall of the preterm ductus is less responsive to high PO2 and therefore less likely to constrict after birth. In these infants, it can cause severe hemodynamic derangements and several major sequelae (Chapter 95.3).
When a term infant is found to have a PDA, the wall of the ductus is deficient in both the mucoid endothelial layer and the muscular media, whereas in the premature infant, the PDA usually has a normal structure. Thus, a PDA persisting beyond the 1st few weeks of life in a term infant rarely closes spontaneously or with pharmacologic intervention, whereas if early pharmacologic or surgical intervention is not required in a premature infant, spontaneous closure occurs in most instances. A PDA is seen in 10% of patients with other congenital heart lesions and often plays a critical role in providing a source of pulmonary blood flow when the right ventricular outflow tract is stenotic or atretic (Chapter 421.6) or in providing systemic blood flow in the presence of aortic coarctation or interruption (Chapter 421.8).
Pathophysiology
As a result of the higher aortic pressure postnatally, blood shunts left to right through the ductus, from the aorta to the pulmonary artery. The extent of the shunt depends on the size of the ductus and on the ratio of pulmonary to systemic vascular resistance. If the PDA is small, pressures within the pulmonary artery, the right ventricle, and the right atrium are normal. If the PDA is large, pulmonary artery pressure may be elevated to systemic levels during both systole and diastole. Thus, patients with a large PDA are at high risk for the development of pulmonary vascular disease if left unoperated.
Clinical Manifestations
A small PDA is usually asymptomatic. A large PDA will result in heart failure similar to that encountered in infants with a large VSD. Retardation of physical growth may be a major manifestation in infants with large shunts. A small PDA is associated with normal peripheral pulses, and a large PDA results in bounding peripheral arterial pulses and a wide pulse pressure, due to runoff of blood into the pulmonary artery during diastole. The heart is normal in size when the ductus is small, but moderately or grossly enlarged in cases with a large communication. In these cases, the apical impulse is prominent and, with cardiac enlargement, is heaving. A thrill, maximal in the 2nd left interspace, is often present and may radiate toward the left clavicle, down the left sternal border, or toward the apex. It is usually systolic but may also be palpated throughout the cardiac cycle. The classic continuous murmur is described as being like machinery in quality. It begins soon after onset of the 1st sound, reaches maximal intensity at the end of systole, and wanes in late diastole. It may be localized to the 2nd left intercostal space or radiate down the left sternal border or to the left clavicle. When pulmonary vascular resistance is increased, the diastolic component of the murmur may be less prominent or absent. In patients with a large left-to-right shunt, a low-pitched mitral mid-diastolic murmur may be audible at the apex as a result of the increased volume of blood flow across the mitral valve.
Diagnosis
If the left-to-right shunt is small, the electrocardiogram is normal; if the ductus is large, left ventricular or biventricular hypertrophy is present. The diagnosis of an isolated, uncomplicated PDA is untenable when right ventricular hypertrophy is present.
Radiographic studies in patients with a large PDA show a prominent pulmonary artery with increased pulmonary vascular markings. Cardiac size depends on the degree of left-to-right shunting; it may be normal or moderately to markedly enlarged. The chambers involved are the left atrium and left ventricle. The aortic knob may be normal or prominent.
The echocardiographic view of the cardiac chambers is normal if the ductus is small. With large shunts, left atrial and left ventricular dimensions are increased. The ductus can easily be visualized directly and its size estimated. Color and pulsed Doppler examinations demonstrate systolic or diastolic (or both) retrograde turbulent flow in the pulmonary artery, and aortic retrograde flow in diastole (Fig. 420-10) in the presence of a large shunt.
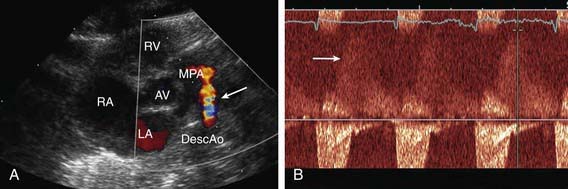
Figure 420-10 Echocardiogram in a newborn with a small- to moderate-sized patent ductus arteriosus (PDA). A, Color Doppler performed in a parasternal short axis view shows flow (arrow) from the aorta into the main pulmonary artery. B, Doppler evaluation demonstrates retrograde diastolic flow into the pulmonary artery. AV, aortic valve; DescAo, descending aorta; LA, left atrium; MPA, main pulmonary artery; RA, right atrium; RV, right ventricle.
The clinical pattern is sufficiently distinctive to allow an accurate diagnosis by noninvasive methods in most patients. In patients with atypical findings, cardiac catheterization may be indicated. Cardiac catheterization will demonstrate either normal or increased pressure in the right ventricle and pulmonary artery, depending on the size of the ductus. The presence of oxygenated blood shunting into the pulmonary artery confirms the left-to-right shunt. The catheter may pass from the pulmonary artery through the ductus into the descending aorta. Injection of contrast medium into the ascending aorta shows opacification of the pulmonary artery from the aorta and identifies the ductus.
Other conditions can produce systolic and diastolic murmurs in the pulmonic area in an acyanotic patient and are described in Chapter 416. An aorticopulmonary window defect may rarely be clinically indistinguishable from a patent ductus, although, in most cases, the murmur is only systolic and is loudest at the right rather than the left upper sternal border. A sinus of Valsalva aneurysm that has ruptured into the right side of the heart or pulmonary artery, coronary arteriovenous fistulas, and an aberrant left coronary artery with massive collaterals from the right coronary display dynamics similar to that of a PDA with a continuous murmur and a wide pulse pressure. Truncus arteriosus with torrential pulmonary flow also has an “aortic runoff” physiology. A peripheral arteriovenous fistula also results in a wide pulse pressure, but the distinctive precordial murmur of a PDA is not present. VSD with aortic insufficiency, repaired tetralogy of Fallot, and combined aortic and mitral insufficiency (usually due to rheumatic fever) may be confused with a PDA, but the murmurs should be differentiated by their to-and-fro rather than continuous nature. The combination of a large VSD and a PDA results in findings more like those of an isolated VSD. Echocardiography should be able to eliminate these other diagnostic possibilities.
Prognosis and Complications
Spontaneous closure of the ductus after infancy is extremely rare. Patients with a small PDA may live a normal span with few or no cardiac symptoms, but late manifestations may occur. In patients with a large PDA, cardiac failure most often occurs in early infancy but may occur later in life, even with a moderate-sized communication.
Infective endarteritis may be seen at any age. Pulmonary or systemic emboli may occur. Rare complications include aneurysmal dilatation of the pulmonary artery or the ductus, calcification of the ductus, noninfective thrombosis of the ductus with embolization, and paradoxical emboli. Pulmonary hypertension (Eisenmenger syndrome) usually develops in patients with a large PDA who do not undergo ductal closure (Chapter 427.2).
Treatment
Irrespective of age, patients with PDA require surgical or catheter closure. In patients with a small PDA, the rationale for closure is prevention of bacterial endarteritis or other late complications. In patients with a moderate to large PDA, closure is accomplished to treat heart failure or prevent the development of pulmonary vascular disease, or both. Once the diagnosis of a moderate to large PDA is made, treatment should not be unduly postponed after adequate medical therapy for cardiac failure has been instituted.
Transcatheter PDA closure is routinely performed in the cardiac catheterization laboratory (Fig. 420-11). Small PDAs are generally closed with intravascular coils. Moderate to large PDAs may be closed with an umbrella-like device or with a catheter-introduced sac into which several coils are released. Surgical closure of PDA can be accomplished by a standard left thoracotomy or using thoracoscopic minimally invasive techniques. Because the case fatality rate with interventional or surgical treatment is considerably less than 1% and the risk without it is greater, closure of the ductus is indicated in asymptomatic patients, preferably before 1 yr of age. Pulmonary hypertension is not a contraindication to surgery at any age if it can be demonstrated at cardiac catheterization that the shunt flow is still predominantly left to right and that severe pulmonary vascular disease is not present. After closure, symptoms of cardiac failure rapidly disappear. Infants who had failed to thrive usually have immediate improvement in physical development. The pulse and blood pressure return to normal, and the machinery-like murmur disappears. A functional systolic murmur over the pulmonary area may persist; it may represent turbulence in a persistently dilated pulmonary artery. The radiographic signs of cardiac enlargement and pulmonary overcirculation disappear over a period of several months, and the electrocardiogram becomes normal.
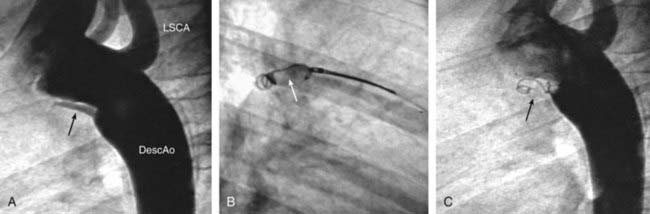
Figure 420-11 Transcatheter closure of a small patent ductus arteriosus (PDA) using a coil. A, Angiogram of transverse and descending aorta shows small PDA (arrow). B, Coil (arrow) has been extruded from sheath and is being positioned in ductal lumen. C, Angiogram demonstrating total occlusion of PDA by coil (arrow). DescAo, descending aorta; LSCA, left subclavian artery.
Alagarsamy S, Chhabra M, Gudavalli M, et al. Comparison of clinical criteria with echocardiographic findings in diagnosing PDA in preterm infants. J Perinat Med. 2005;33:161-164.
Bergwerff M, DeRuiter MC, Gittenberger-de Groot AC. Comparative anatomy and ontogeny of the ductus arteriosus, a vascular outsider. Anat Embryol (Berl). 1999;200:559-571.
Gittenberger-de Groot AC, Van Ertbruggen I, Moulaert A, et al. The ductus arteriosus in the preterm infant: histologic and clinical observations. J Pediatr. 1980;96:88-93.
Rothman A, Lucas VW, Sklansky MS, et al. Percutaneous coil occlusion of patent ductus arteriosus. J Pediatr. 1997;130:447-454.
420.9 Aorticopulmonary Window Defect
An aorticopulmonary (AP) window defect consists of a communication between the ascending aorta and the main pulmonary artery. The presence of pulmonary and aortic valves and an intact ventricular septum distinguishes this anomaly from truncus arteriosus (Chapter 425.8). Symptoms of heart failure appear during early infancy; occasionally, minimal cyanosis is present. The defect is usually large, and the cardiac murmur is usually systolic with an apical mid-diastolic rumble as a result of the increased blood flow across the mitral valve. In the rare instance when the communication is smaller and pulmonary hypertension is absent, the findings on examination can mimic those of a PDA (wide pulse pressure and a continuous murmur at the upper sternal borders). The electrocardiogram shows either left ventricular or biventricular hypertrophy. Radiographic studies demonstrate cardiac enlargement and prominence of the pulmonary artery and intrapulmonary vasculature. The echocardiogram shows enlarged left-sided heart chambers; the window defect can best be delineated with color flow Doppler. CT or MRI angiography can also be utilized to visualize the defect (see Fig. 417-20).
Cardiac catheterization, usually performed in older children to evaluate pulmonary vascular resistance, reveals a left-to-right shunt at the level of the pulmonary artery, as well as hyperkinetic pulmonary hypertension, because the defect is almost always large. Selective aortography with injection of contrast medium into the ascending aorta demonstrates the lesion, and manipulation of the catheter from the main pulmonary artery directly to the ascending aorta is also diagnostic.
An aorticopulmonary window defect is surgically corrected during infancy. If surgery is not carried out in infancy, survivors carry the risk of progressive pulmonary vascular obstructive disease, similar to that of other patients who have large intracardiac or great vessel communications.
420.10 Coronary-Cameral Fistula
A congenital fistula may exist between a coronary artery and an atrium, ventricle (especially the right), or pulmonary artery. Sometimes, multiple fistulas exist. Regardless of the recipient chamber, the clinical signs are similar to those of PDA, although the machinery-like murmur may be more diffuse. If the flow is substantial, the involved coronary artery may be dilated or aneurysmal. The anatomic abnormality is usually demonstrable by color flow Doppler echocardiography and, during catheterization, by injection of contrast medium into the ascending aorta. Small fistulas may be hemodynamically insignificant and may even close spontaneously. If the shunt is large, treatment consists of either transcatheter coil embolization or, for lesions not amenable to catheter intervention, surgical closure of the fistula.
420.11 Ruptured Sinus of Valsalva Aneurysm
When one of the sinuses of Valsalva of the aorta is weakened by congenital or acquired disease, an aneurysm may form and eventually rupture, usually into the right atrium or ventricle. This condition is extremely rare in childhood. The onset is usually sudden. The diagnosis should be suspected in a patient in whom symptoms of acute heart failure develop in association with a new loud to-and-fro murmur. Color Doppler echocardiography and cardiac catheterization demonstrate the left-to-right shunt at the atrial or ventricular level. Urgent surgical repair is generally required. This condition is often associated with infective endocarditis of the aortic valve.