CHAPTER 17 Necrotizing Ulcerative Periodontitis
Necrotizing ulcerative periodontitis (NUP) may be an extension of necrotizing ulcerative gingivitis (NUG) into the periodontal structures, leading to periodontal attachment and bone loss. On the other hand, NUP and NUG may be different diseases. To date, there is little evidence to support the progression of NUG to NUP or to establish a relationship between the two conditions as a single disease entity. However, numerous clinical descriptions and case reports of NUP clearly demonstrate many clinical similarities between the two conditions. A recent article reports the clinical and microscopic findings from 45 patients seen between 1965 and 2000. In this article, the authors suggest that NUG may be a precursor to NUP, citing one case of a 9-year-old malnourished male presenting with three distinct lesions that were consistent with a diagnosis of NUG, NUP, and noma.16 Until a distinction between NUG and NUP can be proved or disproved, it has been suggested that NUG and NUP be classified together under the broader category of necrotizing periodontal diseases, although with differing levels of severity.1,24
NUG has been recognized and described in the literature for centuries.27 The features of NUG are presented in Chapter 10 and briefly reviewed here. Clinically they consist of areas of ulceration and necrosis of the interdental papilla covered by a whitish yellow soft layer, or pseudomembrane, and surrounded by an erythematous halo. Lesions are typically painful and bleed easily, often without provocation. Patients may also present with oral malodor, localized lymphadenopathy, fever, and malaise.
Microscopically, NUG lesions demonstrate a nonspecific necrotizing inflammation that presents with a predominant polymorphonuclear leukocyte (PMN, neutrophil) infiltrate in the ulcerated areas and an abundant chronic infiltrate of lymphocytes and plasma cells in the peripheral and deeper areas.35
The bacterial flora associated with NUG is well known. The constant cultivable flora consists of Prevotella intermedia and Fusobacterium species, whereas the constant microscopic observations reveal the presence of Treponema and Selenomonas species. The association of these bacteria with NUG is compelling. However, the bacterial etiology has not been proven because the bacteria have not been able to transfer the disease between healthy animals (i.e., have not been able to fulfill one of Koch’s postulates). Interestingly, bacterial isolates have transmitted NUG from animal to animal in the beagle dog with a steroid-induced immunosuppression.20-22 The ability to transmit NUG with bacteria in an immunosuppressed animal (but not in immunocompetent animals) suggests that the host response or resistance is an important factor in the pathogenesis of NUG.
The lesions of NUG are confined to the gingiva without loss of periodontal attachment or alveolar bone support, a feature that distinguishes this condition from NUP. In contrast to this view, MacCarthy and Claffey19 suggested that periodontal attachment loss is one of the consequences of NUG lesions. In their evaluation of 13 patients with NUG, the mean probing attachment level for NUG-affected sites (2.2 ± 0.9 mm) was greater than control sites (0.8 ± 0.7 mm). This finding supports the concept that NUG and NUP are similar (or identical) diseases, with differences in host response or resistance rather than differences in bacterial etiology and pathogenesis.
Necrotizing Ulcerative Periodontitis
The term “necrotizing ulcerative periodontitis” was first adopted at the 1989 World Workshop in Clinical Periodontics.3 It was changed from the 1986 term of “necrotizing ulcerative gingivoperiodontitis,” which represented the condition of recurrent NUG progressing to a chronic form of periodontitis with attachment and bone loss. The 1989 adoption of NUP as a disease entity occurred when there was a heightened awareness and an increase in the number of necrotizing periodontitis cases being diagnosed and described in the literature. Specifically, more cases of NUP were being described in immunocompromised patients, especially those who were human immunodeficiency virus (HIV) positive or had acquired immunodeficiency syndrome (AIDS). In 1999 the subclassifications of NUG and NUP were included as separate diagnoses under the broader classification of “necrotizing ulcerative periodontal diseases.”1 Again, a distinction between the two conditions as separate diseases has not been clarified, but they are distinguished by the presence or absence of attachment and bone loss.
Clinical Features
Similar to NUG, clinical cases of NUP are defined by necrosis and ulceration of the coronal portion of the interdental papillae and gingival margin, with a painful, bright red marginal gingiva that bleeds easily.
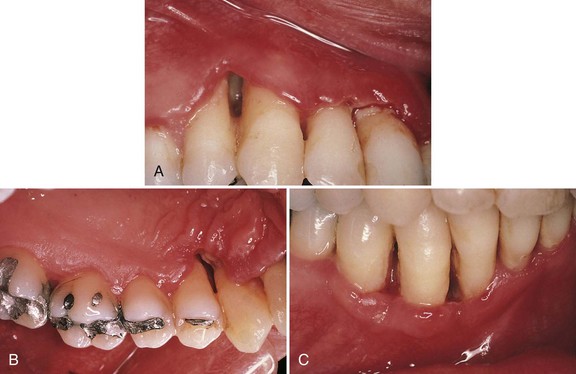
The distinguishing feature of NUP is the destructive progression of the disease that includes periodontal attachment and bone loss. Deep interdental osseous craters typify periodontal lesions of NUP (Figure 17-1). However, “conventional” periodontal pockets with deep probing depth are not found because the ulcerative and necrotizing nature of the gingival lesion destroys the marginal epithelium and connective tissue, resulting in gingival recession. Periodontal pockets are formed because the junctional epithelial cells remain viable and can therefore migrate apically to cover areas of connective tissue loss. The necrosis of the junctional epithelium in NUG and NUP creates an ulcer that prevents this epithelial migration, and a pocket cannot form. Advanced lesions of NUP lead to severe bone loss, tooth mobility, and ultimately tooth loss. In addition to these manifestations, as previously mentioned, NUP patients may present with oral malodor, fever, malaise, or lymphadenopathy.
Microscopic Findings
In a study using transmission (TEM) and scanning electron microscopy (SEM) of the microbial plaque overlying the necrotic gingival papillae, Cobb et al4 demonstrated striking histologic similarities between NUP in HIV-positive patients and previous descriptions of NUG lesions in non-HIV patients. Biopsies of involved posterior papillae from 10 male and 6 female HIV-positive patients with NUP were evaluated. Microscopic examination revealed a surface biofilm composed of a mixed microbial flora with different morphotypes and a subsurface flora with dense aggregations of spirochetes (bacterial zone). Below the bacterial layers were dense aggregations of PMNs (neutrophil-rich zone) and necrotic cells (necrotic zone). The biopsy technique used in this study did not allow observation of the deepest layer and thus was not able to identify the spirochetal infiltration zone, which is classically described in NUG lesions. In addition to the NUG-like microscopic features of NUP described in this study, high levels of yeasts and herpes-like viruses were observed. This latter finding is most likely indicative of the conditions afforded to opportunistic microbes in the immunocompromised host (HIV-positive patients).
HIV/AIDS Patients
Gingival and periodontal lesions with distinctive features are frequently found in patients with HIV infection and AIDS. Many of these lesions are atypical manifestations of inflammatory periodontal diseases that arise in the course of HIV infection and the patient’s concomitant immunocompromised state. Linear gingival erythema (LGE), NUG, and NUP are the most common HIV-associated periodontal conditions reported in the literature.25 Chapter 19 provides detailed descriptions of these and other atypical periodontal diseases that occur in the HIV-infected patient.
NUP lesions found in HIV-positive/AIDS patients can present with similar features to those seen in HIV-negative patients. On the other hand, NUP lesions in HIV-positive/AIDS patients can be much more destructive and frequently result in complications that are extremely rare in non-HIV/AIDS patients. For example, periodontal attachment and bone loss associated with HIV-positive NUP may be extremely rapid. Winkler et al37 reported cases of NUP in HIV-positive patients (formerly referred to as “HIV-P”) with teeth that lost more than 90% of periodontal attachment and 10 mm of bone over a 3- to 6-month period. Ultimately, many of these lesions resulted in tooth loss. Other complications reported in this population include a progression of the lesions to involve large areas of soft tissue necrosis, with exposure of bone and sequestration of bone fragments. This type of severe, progressive lesion with extension into the vestibular area and the palate is referred to as necrotizing ulcerative stomatitis (see Figure 19-31).
The reported prevalence of NUP in HIV-infected patients varies.6,13,25,27 Riley et al28 reported only two cases of NUP in 200 HIV-positive patients (1%), whereas Glick et al13 found a prevalence of 6.3% for NUP cases in a prospective study of 700 HIV-positive patients. Variations in reported findings may be related to differences in the populations (e.g., intravenous drug users versus homosexuals versus patients with hemophilia) and differences in the immune status of the study subjects.
Necrotizing forms of periodontitis appear to be more prevalent in patients with more severe immunosuppression.25,26 Case reports have depicted NUP as a progressive extension of HIV periodontitis (i.e., chronic to necrotic progression).29 Glick et al13,14 found a high correlation between the diagnosis of NUP and immunosuppression in HIV-positive patients. Those patients presenting with NUP were 20.8 times more likely to have CD4+ counts below 200 cells/mm3 compared with HIV-positive patients without NUP. The authors consider a diagnosis of NUP to be a marker for immune deterioration and a predictor for the diagnosis of AIDS.13 Others have suggested that NUP may be used as an indicator of HIV infection in undiagnosed patients. Shangase et al32 reported that a diagnosis of NUG or NUP in systemically healthy, asymptomatic South Africans was strongly correlated with HIV infection. Of patients presenting with NUG or NUP, 39 of 56 (69.6%) were subsequently found to be HIV positive (see Chapter 19).
Etiology of Necrotizing Ulcerative Periodontitis
The etiology of NUP has not been determined, although a mixed fusiform-spirochete bacterial flora appears to play a key role. Because bacterial pathogens are not solely responsible for causing the disease, some predisposing “host” factor(s) may be necessary. Numerous predisposing factors have been attributed to NUG, including poor oral hygiene, preexisting periodontal disease, smoking, viral infections, immunocompromised status, psychosocial stress, and malnutrition.
![]() Science Transfer
Science Transfer
Necrotizing ulcerative gingivitis and necrotizing ulcerative periodontitis are more prevalent and more severe in patients who are HIV positive. These patients require urgent treatment as untreated lesions can progress rapidly and in a few days, severe bone loss around affected teeth can be seen.
Smoking, malnutrition, and high plaque levels all increase the risk of necrotizing ulcerative gingivitis and need to be changed so that treatment success is obtained.
Many necrotizing ulcerative gingivitis lesions respond well to initial therapy and the gingival tissues can heal and return to health. Patients need to be thoroughly reevaluated 4 to 6 weeks after managing the acute stages of necrotizing ulcerative gingivitis to ascertain if additional periodontal surgical treatment is needed to treat residual soft tissue and bony defects.
NUP is frequently associated with a diagnosis of AIDS or a positive HIV status. Therefore clinicians should check all patients presenting with NUP to ascertain their HIV status. NUP can progress rapidly and lead to tooth exfoliation, so treatment should include local debridement, local antiplaque agents, and systemic antibiotics. Early diagnosis and treatment of NUP are crucial because the osseous defects that occur in the late stages of the disease are extremely difficult to resolve, even with extensive regenerative surgical procedures. If a child presents with NUP, severe systemic abnormalities, such as advanced malnutrition, are often present.
Microbial Flora
Assessment of the microbial flora of NUP lesions is almost exclusively limited to studies involving HIV-positive and AIDS patients, with some conflicting evidence. Murray et al24 reported that cases of NUP in HIV-positive patients demonstrated significantly greater numbers of the opportunistic fungus Candida albicans and a higher prevalence of Actinobacillus (Aggregatibacter) actinomycetemcomitans, Prevotella intermedia, Porphyromonas gingivalis, Fusobacterium nucleatum, and Campylobacter species compared with HIV-negative controls. Further, they reported a low or variable level of spirochetes, which is inconsistent with the flora associated with NUG. Citing differences in microbial flora, they refuted the notion that the destructive lesions seen in HIV-positive patients were related to NUG lesions; they suggested that the flora of NUP lesions in HIV-positive patients is comparable to that of chronic periodontitis lesions, thus supporting their concept that necrotizing periodontitis in the HIV-positive patient is an aggressive manifestation of chronic periodontitis in the immunocompromised host.
In contrast to these findings, Cobb et al4 reported that the microbial composition of NUP lesions in HIV-positive patients was very similar to that of NUG lesions, as discussed earlier. Using electron microscopy, they described a mixed microbial flora with various morphotypes in 81.3% of specimens. The subsurface microbial flora featured dense aggregations of spirochetes in 87.5% of specimens. They also reported opportunistic yeasts and herpes-like viruses in 65.6% and 56.5% of NUP lesions, respectively. The differences between these reports may be explained by the limitations in obtaining viable cultures of spirochetes24 compared with the more definitive electron microscopic observation of spirochetes.4
In a recent review article, Feller and Lemmer suggested that spirochetes, herpesviruses, candida, and HIV have a potential pathogenic role in NUP lesions in the HIV-seropositive individual.12 Spirochetes have the ability to modulate host innate and adaptive immune responses and to stimulate host inflammatory reactions,8 which may reduce the local immune competence and facilitate the development of necrotizing disease.12 Activated herpesviruses have the capacity to deregulate the host immune system, which may lead to an increase in the colonization and activity of other pathogenic microorganisms. Candida albicans has been reported to produce eicosanoids leading to the release of proinflammatory mediators, which may facilitate spirochete colonization and invasion, promoting the development of necrotizing periodontal diseases.11,12
Immunocompromised Status
Clearly, both NUG and NUP lesions are more prevalent in patients with compromised or suppressed immune systems. Numerous studies, particularly those evaluating HIV-positive and AIDS patients, support the concept that a diminished host response is present in those individuals diagnosed with necrotizing ulcerative periodontal diseases.37 Whereas a compromised immune system (“immune compromise”) in the HIV-infected patient is driven by impaired T-cell function and altered T-cell ratios, evidence indicates that other forms of compromised immunity predispose individuals to NUG and NUP as well.
Cutler et al6 described impaired bactericidal activity of PMNs in two children with NUP. In a comparative assay of PMNs against periodontal pathogens, two brothers (ages 9 and 14 years) showed significant depression of PMN phagocytosis and killing function compared with gender- and age-matched controls. Further, Batista et al2 reported periodontal findings and NUP in an adolescent with a rare genetic disease (multifactorial congenital immunodeficiency [CVID]) that causes impaired secretion of immunoglobulin; the oral lesions resolved with administration of intravenous immunoglobulin (IVIG).
Psychologic Stress
Most clinical and animal studies evaluating the role of stress on necrotizing periodontal disease have evaluated subjects with NUG7,15,33,34 and thus have not specifically addressed the role of stress on NUP.
NUG patients have been found to have had significantly more anxiety, higher depression scores, a greater magnitude of recent stressful events, more overall distress and adjustments related to these events, and more negative life events.5,14 Although the role of stress in the development of NUP has not been reported specifically, the many similarities between NUG and NUP would suggest that similar relationships to stress may exist.
The mechanisms that predispose an individual with stress to necrotizing ulcerative periodontal diseases have not been established. However, it is well known that stress increases systemic cortisol levels, and sustained increases in cortisone have a suppressive effect on the immune response. In an investigation of 474 military personnel, Shannon et al33 found that urinary levels of 17-hydroxycorticosteroid were higher in subjects with NUG than in all other subjects diagnosed with periodontal health, gingivitis, or periodontitis. Experimentally, noma-like lesions have been produced in rats by administering cortisone and causing mechanical injury to the gingiva30 and in hamsters by total body irradiation.20 Thus, stress-induced immunosuppression may be one mechanism that impairs the host response and leads to necrotizing periodontal disease. The scientific evidence supporting an etiologic role of stress in chronic periodontitis is not as clear (see Chapter 27).
Malnutrition
Direct evidence of the relationship between malnutrition and necrotizing periodontal disease is limited to descriptions of necrotizing infections in severely malnourished children. Lesions resembling NUG but with progression to become gangrenous stomatitis, or noma, have been described in children with severe malnutrition in underdeveloped countries. Jimenez and Baer17 reported cases of NUG in children and adolescents ages 2 to 14 years with malnutrition in Colombia. In the advanced stages, NUG lesions extended from the gingiva to other areas of the oral cavity, becoming gangrenous stomatitis (noma) and causing exposure, necrosis, and sequestration of the alveolar bone. Later, Jimenez et al reported that 44 of the 45 cases of necrotizing disease (NUG = 29, NUP = 7, noma = 9) documented from 1965 to 2000 were from a low socioeconomic group and that malnutrition was associated with nearly all of the necrotizing conditions (29/29 NUG, 6/7 NUP and 9/9 noma cases).16 In a study of socioeconomically deprived Nigerian children with NUG (153 cases), Enwonwu et al confirmed malnutrition by measuring circulating micronutrients.10 Compared with neighborhood counterparts, the children with NUG and micronutrient deficiencies demonstrated dysregulated cytokine production with a complex interplay of elevated proinflammatory and antiinflammatory mediators.
A plausible explanation is that malnutrition, particularly when extreme, contributes to a diminished host resistance to infection and necrotizing disease. It is well documented that many of the host defenses, including phagocytosis; cell-mediated immunity; and complement, antibody, and cytokine production and function, are impaired in malnourished individuals.9 Depletion of nutrients to cells and tissues results in immunosuppression and increases disease susceptibility. Thus it is reasonable to conclude that malnutrition can predispose an individual to opportunistic infections or intensify the severity of existing oral infections.
Summary
NUP and NUG share many clinical and microbiologic features, but NUP is distinguished by a more severe condition with periodontal attachment and bone loss. Indeed, some patients with NUP, particularly those with compromised immunity, can have severe and rapidly progressive disease. It appears that an impaired immune response and lowered host resistance to infection are significant factors in the onset and progression of NUP. The best example of an immunocompromised host with a predisposition for NUP is the HIV-positive/AIDS patient. As with the other infection-related complications of HIV/AIDS, the immunocompromised status of these patients renders them vulnerable to opportunistic periodontal infections, including NUP. Several other factors have been identified, specifically in cases of NUG, that may also play a role in NUP, including smoking, viral infections, psychosocial stress, and malnutrition. Although none of these factors alone is sufficient to cause necrotizing disease, in combination with other immunocompromising conditions, they undoubtedly have the potential to adversely influence the host response or resistance to infection.
1 Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. Dec 1999;4(1):1-6.
2 Batista ELJr, Novaes ABJr, Calvano LM, et al. Necrotizing ulcerative periodontitis associated with severe congenital immunodeficiency in a prepubescent subject: clinical findings and response to intravenous immunoglobulin treatment. J Clin Periodontol. Aug 1999;26(8):499-504.
3 Caton J: Consensus report on periodontal diagnosis and diagnostic aids. Paper presented at: Proceedings of the World Workshop in Clinical Periodontics, 1989, Chicago.
4 Cobb CM, Ferguson BL, Keselyak NT, et al. A TEM/SEM study of the microbial plaque overlying the necrotic gingival papillae of HIV-seropositive, necrotizing ulcerative periodontitis. J Periodontal Res. Apr 2003;38(2):147-155.
5 Cohen-Cole SA, Cogen RB, Stevens AWJr, et al. Psychiatric, psychosocial, and endocrine correlates of acute necrotizing ulcerative gingivitis (trench mouth): a preliminary report. Psychiatr Med. Jun 1983;1(2):215-225.
6 Cutler CW, Wasfy MO, Ghaffar K, et al. Impaired bactericidal activity of PMN from two brothers with necrotizing ulcerative gingivo-periodontitis. J Periodontol. Apr 1994;65(4):357-363.
7 Da Silva AM, Newman HN, Oakley DA. Psychosocial factors in inflammatory periodontal diseases: a review. J Clin Periodontol. 1995:22.
8 Ellen RP, Galimanas VB. Spirochetes at the forefront of periodontal infections. Periodontol 2000. 2005;38:13-32.
9 Enwonwu CO, Phillips RS, Falkler WAJr. Nutrition and oral infectious diseases: state of the science. Compend Contin Educ Dent. May 2002;23(5):431-434. 436, 438 passim; quiz 448
10 Enwonwu CO, Phillips RS, Savage KO. Inflammatory cytokine profile and circulating cortisol levels in malnourished children with necrotizing ulcerative gingivitis. Eur Cytokine Netw.. Sep 2005;16(3):240-248.
11 Feller L, Buskin A, Blignaut E. A review of candida and periodontal disease in immunocompetent and HIV-infected subjects. Sadj. May 2005;60(4):152-154.
12 Feller L, Lemmer J. Necrotizing periodontal diseases in HIV-seropositive subjects: pathogenic mechanisms. J Int Acad Periodontol. Jan 2008;10(1):10-15.
13 Glick M, Muzyka BC, Lurie D, et al. Oral manifestations associated with HIV-related disease as markers for immune suppression and AIDS. Oral Surg Oral Med Oral Pathol. Apr 1994;77(4):344-349.
14 Glick M, Muzyka BC, Salkin LM, et al. Necrotizing ulcerative periodontitis: a marker for immune deterioration and a predictor for the diagnosis of AIDS. J Periodontol. May 1994;65(5):393-397.
15 Hildebrand HC, Epstein J, Larjava H. The influence of psychological stress on periodontal disease. J West Soc Periodontol Periodontal Abstr. 2000;48(3):69-77.
16 Jimenez LM, Duque FL, Baer PN, et al. Necrotizing ulcerative periodontal diseases in children and young adults in Medellin, Colombia, 1965–2000. J Int Acad Periodontol. Apr 2005;7(2):55-63.
17 Jimenez M, Baer PN. Necrotizing ulcerative gingivitis in children: a 9 year clinical study. J Periodontol. Dec 1975;46(12):715-720.
18 Listgarten MA. Electron microscopic observations on the bacterial flora of acute necrotizing ulcerative gingivitis. J Periodontal. 36, 1965.
19 MacCarthy D, Claffey N. Acute necrotizing ulcerative gingivitis is associated with attachment loss. J Clin Periodontol. 1991;18:776-779.
20 Mayo J, Carranza FAJr, Epper CE, et al. The effect of total-body irradiation on the oral tissues of the Syrian hamster. Oral Surg Oral Med Oral Pathol. Jun 1962;15:739-745.
21 Mikx F, van Campen GJ. Preliminary evaluation of the microflora in spontaneous and induced necrotizing ulcerative gingivitis in the beagle dog. J Periodontal Res. Sep 1982;17(5):460-461.
22 Mikx FH, Hug HU, Maltha JC. Necrotizing ulcerative gingivitis in beagle dogs. I. Attempts at unilateral induction and intraoral transmission of NUG, a microbiological and clinical study. J Periodontal Res. Jan 1984;19(1):76-88.
23 Mikx FH, van Campen GJ. Microscopical evaluation of the microflora in relation to necrotizing ulcerative gingivitis in the beagle dog. J Periodontal Res. Nov 1982;17(6):576-584.
24 Murray PA, Winkler JR, Sadkowski L, et al. Microbiology of HIV-associated gingivitis and periodontitis. Littleton, MA: PSG Publishing; 1988.
25 Novak MJ. Necrotizing ulcerative periodontitis. Ann Periodontol. Dec 1999;4(1):74-78.
26 Pistorius A, Willershausen B. Cases of HIV-associated characteristic periodontal diseases. Eur J Med Res. Mar 26 1999;4(3):121-125.
27 Plaut HC. [Bacterial diagnostic studies of diphtheria and oral diseases]. Dtsch Med Wochnschr. 20, 1894.
28 Riley C, London JP, Burmeister JA. Periodontal health in 200 HIV-positive patients. J Oral Pathol Med. Mar 1992;21(3):124-127.
29 Rowland RW. Necrotizing ulcerative gingivitis. Ann Periodontol. Dec 1999;4(1):65-73. discussion 78
30 SanGiacomo TR, Tan PM, Loggi DG, et al. Progressive osseous destruction as a complication of HIV-periodontitis. Oral Surg Oral Med Oral Pathol. Oct 1990;70(4):476-479.
31 Selye H. Effect of cortisone and somatotrophic hormone upon the development of a noma-like condition in the rat. Oral Surg Oral Med Oral Pathol. Apr 1953;6(4):557-561.
32 Shangase L, Feller L, Blignaut E. Necrotising ulcerative gingivitis/periodontitis as indicators of HIV-infection. Sadj. Apr 2004;59(3):105-108.
33 Shannon IL, Kilgore WG, O’Leary TJ. Stress as a predisposing factor in necrotizing ulcerative gingivitis. J Periodontol. Apr 1969;40(4):240-242.
34 Shields WD. Acute necrotizing ulcerative gingivitis. A study of some of the contributing factors and their validity in an Army population. J Periodontol. Jun 1977;48(6):346-349.
35 Vincent H. [The etiology and the histopathology of hospital rot]. Ann de l’Insti Pastuer. 1896;10:488.
36 Wade DN, Kerns DG. Acute necrotizing ulcerative gingivitis-periodontitis: a literature review. Mil Med. May 1998;163(5):337-342.
37 Winkler JR, Grassi M, Murray PA. Clinical description and etiology of HIV-associated periodontal diseases. Littleton, MA: PSG Publishing; 1988.