CHAPTER 10 Acute Gingival Infections
Necrotizing Ulcerative Gingivitis
Necrotizing ulcerative gingivitis (NUG) is a microbial disease of the gingiva in the context of an impaired host response. It is characterized by the death and sloughing of gingival tissue and presents with characteristic signs and symptoms.
Clinical Features
NUG is usually identified as an acute disease. However, the term “acute” in this case is a clinical descriptor and should not be used as a diagnosis because there is no chronic form of the disease. The acronym ANUG, although frequently used, is a misnomer.59 NUG often undergoes a diminution in severity without treatment, leading to a subacute stage with milder clinical symptoms. Thus patients may have a history of repeated remissions and exacerbations, and the condition can also recur in previously treated patients. Involvement may be limited to a single tooth or group of teeth (Figure 10-1, A and B [see Figure 10-1, C]) or may be widespread throughout the mouth (see Figure 10-1, D).
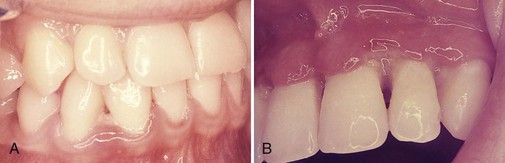
Figure 10-1 Necrotizing ulcerative gingivitis. A, Typical punched-out papilla between mandibular canine and lateral incisor, covered by grayish white pseudomembrane. B, More advanced case showing destruction of papillae resulting in irregular marginal contour.
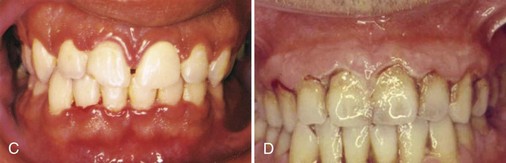
Figure 10-1 C, Typical lesions with spontaneous hemorrhage. D, Generalized involvement of papillae and marginal gingiva, with whitish necrotic lesions.
NUG can cause tissue destruction involving the periodontal attachment apparatus,41 especially in patients with long-standing disease or severe immunosuppression. When bone loss occurs, the condition is called necrotizing ulcerative periodontitis (NUP) (see Chapter 17).
History
NUG is characterized by sudden onset, sometimes after an episode of debilitating disease or acute respiratory tract infection. A change in living habits, protracted work without adequate rest, poor nutrition, tobacco use, and psychologic stress are frequent features of the patient’s history.
Oral Signs
Characteristic lesions are punched-out, craterlike depressions at the crest of the interdental papillae, subsequently extending to the marginal gingiva and rarely to the attached gingiva and oral mucosa. The surface of the gingival craters is covered by a gray, pseudomembranous slough, demarcated from the remainder of the gingival mucosa by a pronounced linear erythema (see Figure 10-1, A). In some cases the lesions are denuded of the surface pseudomembrane, exposing the gingival margin, which is red, shiny, and hemorrhagic. The characteristic lesions may progressively destroy the gingiva and underlying periodontal tissues (see Figure 10-1, B).
Spontaneous gingival hemorrhage or pronounced bleeding after the slightest stimulation are additional characteristic clinical signs (see Figure 10-1, B, and Figure 10-1, C). Other signs often found are fetid odor and increased salivation.
NUG can occur in otherwise disease-free mouths or can be superimposed on chronic gingivitis or periodontal pockets. However, NUG or NUP does not usually lead to periodontal pocket formation because the necrotic changes involve the junctional epithelium; a viable junctional epithelium is needed for pocket deepening (see Chapter 13). It is rare in edentulous mouths, but isolated spherical lesions occasionally occur in the soft palate.58
Oral Symptoms
The lesions are extremely sensitive to touch, and the patient often complains of a constant radiating, gnawing pain that is intensified by eating spicy or hot foods and chewing. There is a “metallic” foul taste, and the patient is conscious of an excessive amount of “pasty” saliva.
Extraoral and Systemic Signs and Symptoms
Patients are usually ambulatory and have a minimum of systemic symptoms. Local lymphadenopathy and a slight elevation in temperature are common features of the mild and moderate stages of the disease. In severe cases, there may be high fever, increased pulse rate, leukocytosis, loss of appetite, and general lassitude. Systemic reactions are more severe in children. Insomnia, constipation, gastrointestinal disorders, headache, and mental depression sometimes accompany the condition.
In very rare cases, severe sequelae, such as gangrenous stomatitis and noma, have been described.1,2,18,33
Clinical Course
The clinical course can vary. If untreated, NUG may lead to a progressive destruction of the periodontium and gingival recession, accompanied by an increase in the severity of systemic complications.31,51
Histopathology
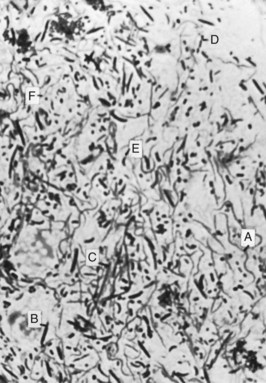
Microscopically, the NUG lesion is a nonspecific acute necrotizing inflammation of the gingival margin, involving both the stratified squamous epithelium and the underlying connective tissue. The surface epithelium is destroyed and replaced by a meshwork of fibrin, necrotic epithelial cells, polymorphonuclear leukocytes (PMNs, neutrophils), and various types of microorganisms (see Figure 10-2). This is the zone that appears clinically as the surface pseudomembrane. At the immediate border of the necrotic pseudomembrane, the epithelium is edematous, and the individual cells exhibit varying degrees of hydropic degeneration. In addition, there is an infiltration of PMNs in the intercellular spaces.
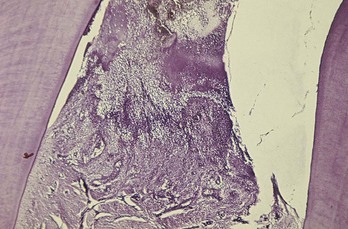
Figure 10-2 Survey section of interdental papilla in necrotizing ulcerative gingivitis. Top portion of the section shows the necrotic tissue that forms the gray marginal pseudomembrane. In lower portion, note the ulceration and accumulation of leukocytes and fibrin.
The underlying connective tissue is markedly hyperemic, with numerous engorged capillaries and a dense infiltration of PMNs. This acutely inflamed zone appears clinically as the linear erythema beneath the surface pseudomembrane. Numerous plasma cells may appear in the periphery of the infiltrate; this is interpreted as an area of established chronic gingivitis on which the acute lesion became superimposed.30
The epithelium and connective tissue alterations decrease as the distance from the necrotic gingival margin increases, blending gradually with the uninvolved gingiva.
It is noteworthy that the microscopic appearance of NUG is nonspecific. Comparable changes result from trauma, chemical irritation, or the application of caustic medications.
Relation of Bacteria to the Necrotizing Ulcerative Gingivitis Lesion
Light and electron microscopy have been used to study the relationship of bacteria to the characteristic lesion of NUG. Light microscopy shows that the exudate on the surface of the necrotic lesion contains microorganisms that morphologically resemble cocci, fusiform bacilli, and spirochetes.76 The layer between the necrotic and living tissue contains enormous numbers of fusiform bacilli and spirochetes, in addition to leukocytes and fibrin. Spirochetes and other bacteria4,12,16,38 invade the underlying living tissue.
Spirochetes have been found as deep as 300 µm from the surface. The majority of spirochetes in the deeper zones are morphologically different from cultivated strains of Treponema microdentium. They occur in nonnecrotic tissue before other types of bacteria and may be present in high concentrations intercellularly in the epithelium adjacent to the ulcerated lesion and in the connective tissue.37
Smears from the lesions (Figure 10-3) show scattered bacteria, predominantly spirochetes and fusiform bacilli, desquamated epithelial cells, and occasional polymorphonuclear leukocytes (PMNs). Spirochetes and fusiform bacteria are usually seen with other oral spirochetes, vibrios, and filaments.
Diagnosis
Diagnosis is based on clinical findings of gingival pain, ulceration, and bleeding. A bacterial smear is not necessary or definitive because the bacterial picture is not appreciably different from that in marginal gingivitis, periodontal pockets, pericoronitis, or primary herpetic gingivostomatitis.57 Bacterial studies are useful, however, in the differential diagnosis of NUG and specific infections of the oral cavity such as diphtheria, thrush, actinomycosis, and streptococcal stomatitis.
Microscopic examination of a biopsy specimen is not sufficiently specific to be diagnostic. It can be used to differentiate NUG from specific infections, such as tuberculosis, or from neoplastic disease, but it does not differentiate between NUG and other necrotizing conditions of nonspecific origin such as those produced by trauma or caustic medications.
![]() Science Transfer
Science Transfer
The two most common acute gingival infections are necrotizing gingivitis and herpetic gingivostomatitis. These diseases occur most often in healthy patients, but patients with depressed immunologic responses such as acquired immunodeficiency syndrome (AIDS), leukemia, and cyclic neutropenia have an increased risk for these gingival entities. Necrotizing gingivitis is usually treatable with local root planing, curettage, and plaque control; however, in patients with immunologic deficiencies or patients with evidence of spread beyond the gingival tissues then systemic antibiotics are indicated. Tissue of immunologically incompetent patients can destruct rapidly and should be seen and treated on a daily basis until the necrotizing lesions are controlled.
Primary herpetic gingivostomatitis is accompanied by fever and malaise and as long as there are vesicles, the patient is infectious. Secondary infections of the virus can also cause gingival vesicles and is sometimes triggered by tissue trauma occurring with periodontal treatment such as periodontal surgery and root planing.
Differential Diagnosis
NUG should be differentiated from other conditions that resemble it in some respects, such as herpetic gingivostomatitis (see Table 10-1); chronic periodontitis; desquamative gingivitis (see Table 10-2); streptococcal gingivostomatitis; aphthous stomatitis; gonococcal gingivostomatitis; diphtheritic and syphilitic lesions (see Table 10-3); tuberculous gingival lesions; candidiasis, agranulocytosis, and dermatoses (pemphigus, erythema multiforme, and lichen planus); and stomatitis venenata. Treatment options for these diseases vary dramatically, and improper treatment may exacerbate the condition. In the case of primary herpetic gingivostomatitis, early diagnosis may result in treatment with antiviral drugs that would be ineffective for NUG, whereas treatment of a case of herpes with the debridement required for NUG could exacerbate herpes. (See Chapter 12 for a description of most of these conditions.)
TABLE 10-1 Differentiation between Necrotizing Ulcerative Gingivitis and Primary Herpetic Gingivostomatitis
| Necrotizing Ulcerative Gingivitis | Primary Herpetic Gingivostomatitis |
|---|---|
| Etiology: interaction between host and bacteria, most probably fusospirochetes. | Specific viral etiology. |
| Necrotizing condition. | Diffuse erythema and vesicular eruption. |
| Punched-out gingival margin; pseudomembrane that peels off, leaving raw areas. | Vesicles rupture and leave slightly depressed oval or spherical ulcer. |
| Marginal gingiva affected; other oral tissues rarely affected. | Diffuse involvement of gingiva; may include buccal mucosa and lips. |
| Uncommon in children. | Occurs more frequently in children. |
| No definite duration. | Duration of 7 to 10 days. |
| No demonstrated immunity. | Acute episode results in some degree of immunity. |
| Contagion not demonstrated. | Contagion. |
TABLE 10-2 Differentiation among Necrotizing Ulcerative Gingivitis, Chronic Desquamative Gingivitis, and Chronic Periodontal Disease
| Necrotizing Ulcerative Gingivitis | Desquamative Gingivitis | Chronic Destructive Periodontal Disease |
|---|---|---|
| Bacterial smears show fusospirochetal complex. | Bacterial smears reveal numerous epithelial cells, few bacterial forms. | Bacterial smears are variable. |
| Marginal gingiva affected. | Diffuse involvement of marginal and attached gingivae and other areas of oral mucosa. | Marginal gingiva affected. |
| Acute history. | Chronic history. | Chronic history. |
| Painful. | May or may not be painful. | Painless if uncomplicated. |
| Pseudomembrane. | Patchy desquamation of gingival epithelium. | Generally no desquamation, but purulent material may appear from pockets. |
| Pupillary and marginal necrotic lesions. | Papillae do not undergo necrosis. | Papillae do not undergo noticeable necrosis. |
| Affects adults of both genders, occasionally children. | Affects adults, most often women. | Generally in adults, occasionally in children. |
| Characteristic fetid odor. | None. | Some odor present but not strikingly fetid. |
TABLE 10-3 Differentiation among Necrotizing Ulcerative Gingivitis, Diphtheria, and Secondary Stage of Syphilis
| Necrotizing Ulcerative Gingivitis | Diphtheria | Secondary Stage of Syphilis (Mucous Patch) |
|---|---|---|
| Etiology: interaction between host and bacteria, most probably fusospirochetes. | Specific bacterial etiology: Corynebacterium diphtheriae. | Specific bacterial etiology: Treponema pallidum. |
| Affects marginal gingiva. | Rarely affects marginal gingiva. | Rarely affects marginal gingiva. |
| Membrane removal easy. | Membrane removal difficult. | Membrane not detachable. |
| Painful condition. | Less painful. | Minimal pain. |
| Marginal gingivae affected. | Throat, fauces, and tonsils affected. | Any part of mouth affected. |
| Serologic findings normal. | Serologic findings normal. | Serologic findings abnormal.* |
| Immunity not conferred. | Immunity conferred by an attack. | Immunity not conferred. |
| Doubtful contagiousness. | Contagion. | Only direct contact will communicate disease. |
| Antibiotic therapy relieves symptoms. | Antibiotic treatment has minimal effect. | Antibiotic therapy has excellent results. |
* Wassermann, Kahn, Venereal Disease Research Laboratories (VDRL).
Streptococcal gingivostomatitis is a rare condition characterized by a diffuse erythema of the gingiva and other areas of the oral mucosa.44 In some cases, it is confined as a marginal erythema with marginal hemorrhage. Necrosis of the gingival margin is not a feature of this disease, and there is no notable fetid odor. Bacterial smears show a preponderance of streptococcal forms, which were identified as Streptococcus viridans, but other studies report it to be group A β-hemolytic streptococcus.39
Agranulocytosis is characterized by a marked decrease in the number of circulating PMNs, lesions of the throat and other mucous membranes, and ulceration and necrosis of the gingiva, which may resemble that of NUG, and occurs most commonly after chemotherapy in cancer patients or in patients with leukemia. The oral condition in agranulocytosis is primarily necrotizing but lacks the severe inflammatory reaction seen in NUG. Blood studies serve to differentiate between NUG and the gingival necrosis in agranulocytosis.
Vincent’s angina is a fusospirochetal infection of the oropharynx and throat, as distinguished from NUG, which affects the marginal gingiva. Patients with Vincent’s angina have a painful membranous ulceration of the throat, with edema and hyperemic patches breaking down to form ulcers covered with pseudomembranous material. The process may extend to the larynx and middle ear.
NUG may occur in Leukemia patients and AIDS patients (see Chapters 19 and 20). NUG in the patient with leukemia is not produced by leukemia itself but may result from the reduced host defense mechanisms seen with leukemia. Also, NUG may be superimposed on gingival tissue alterations caused by leukemia. The differential diagnosis consists not in distinguishing between NUG and leukemic gingival changes but rather in determining whether leukemia is a predisposing factor in a mouth with NUG. For example, if a patient with necrotizing involvement of the gingival margin also has generalized diffuse discoloration and edema of the attached gingiva, the possibility of an underlying, systemically-induced gingival change should be considered. Leukemia is one of the conditions that would need to be ruled out (see Chapter 27).
NUG in the patient with human immunodeficiency virus (HIV) infection has the same clinical features, although it often follows an extremely destructive course leading to NUP, with loss of soft tissue and bone and formation of bony sequestra27 (see Chapter 19).
Etiology
Role of Bacteria
Plaut53 in 1894 and Vincent78 in 1896 postulated that NUG is caused by specific bacteria: fusiform bacillus and a spirochetal organism.
Opinions still differ regarding whether bacteria are the primary causative factors in NUG. Several observations support this concept, including that spirochetal organisms and fusiform bacilli are always found in the disease, with other organisms also involved. Rosebury et al57 described a fusospirochetal complex consisting of T. microdentium, intermediate spirochetes, vibrios, fusiform bacilli, and filamentous organisms, in addition to several Borrelia species.
Loesche et al40 described a predominant constant flora and a variable flora associated with NUG. The constant flora is composed of Prevotella intermedia, in addition to Fusobacterium, Treponema, and Selenomonas species. The variable flora consists of a heterogeneous array of bacterial types.
Treatment with metronidazole results in a significant reduction of Treponema species, Prevotella intermedia, and Fusobacterium, with resolution of the clinical symptoms.15,40 The antibacterial spectrum of this drug provides evidence for the anaerobic members of the flora as etiologic agents.
These bacteriologic findings have been supported by immunologic data8 that reported increased immunoglobulin (IgG and IgM) antibody titers for medium-sized spirochetes and P. intermedia in NUG patients compared with titers in those with chronic gingivitis and healthy controls.
Role of the Host Response
Regardless of whether specific bacteria are implicated in the etiology of NUG, the presence of these organisms appears to be insufficient to cause the disease.
In the first place, the fusiform-spirochete flora are frequently found in patients who do not have NUG. Also, exudates from NUG lesions produce fusospirochetal abscesses rather than typical NUG, when inoculated subcutaneously in experimental animals.56 Local intracutaneous injection of a hyaluronidase- and chondroitinase-containing cell-free filtrate of oral microaerophilic diphtheroid bacilli aggravated spirochetal lesions that were produced by oral treponemes.30 Only in one animal experiment has the transmission of lesions comparable with those seen in humans been reported.1
The role of an impaired host response in NUG has long been recognized. Even in the early descriptions of the disease, NUG has been associated with physical and emotional stress10,59 and decreased resistance to infection. NUG has not been produced experimentally in humans or animals by just inoculation of bacterial exudates from the lesions. In the animal model, local or systemic immunosuppression with glucocorticoids (e.g., ketoconazole) results in more characteristic lesions of NUG in infected animals. Furthermore, NUG is not found in well-nourished individuals with a fully functional immune system. All the predisposing factors for NUG are associated with immunosuppression. Cogen et al9 described a depression in host defense mechanisms, particularly in PMN chemotaxis and phagocytosis, in NUG patients. (For further details on the host-bacteria interactions in NUG, see Chapters 22, 23, and 25.)
It is essential for the clinician to determine the predisposing factors leading to immunodeficiency in NUG in order to address the continued susceptibility of the patient and to determine whether an underlying systemic disease is present. Immunodeficiency may be related to varying levels of nutritional deficiency, fatigue caused by chronic sleep deprivation, other health habits (e.g., alcohol or drug abuse), psychosocial factors, or systemic disease. Importantly, NUG may be the presenting symptom for patients with immunosuppression related to human immunodeficiency virus (HIV) infection.
Local Predisposing Factors
Preexisting gingivitis, injury to the gingiva, and smoking are important predisposing factors. Although NUG may appear in an otherwise disease-free mouth, it most often occurs superimposed on preexisting chronic gingival disease and periodontal pockets. Deep periodontal pockets and pericoronal flaps are particularly vulnerable areas because they offer a favorable environment for the proliferation of anaerobic fusiform bacilli and spirochetes. Areas of the gingiva traumatized by opposing teeth in malocclusion, such as the palatal surface behind the maxillary incisors and the labial gingival surface of the mandibular incisors, may predispose to NUG.
The relationship between NUG and smoking has often been mentioned in the literature. Pindborg51 reported that 98% of his patients with NUG were smokers and that the frequency of this disease increases with an increasing exposure to tobacco smoke. The effect of smoking on periodontal disease in general has been the subject of numerous studies in the past two decades, and smoking has been established as a high risk factor for disease (see Chapter 26).
Systemic Predisposing Factors
NUG is not found in a well-nourished individual with a fully functional immune system. Therefore it is important for the clinician to determine the predisposing factors leading to immunodeficiency. Again, immunodeficiency may be related to varying levels of nutritional deficiency; fatigue caused by chronic sleep deficiency; other health habits (e.g., alcohol or drug abuse), and systemic disease (e.g., diabetes, debilitating infection).
Nutritional Deficiency
Necrotizing gingivitis has been produced by giving animals nutritionally deficient diets.6,34,47,74,77 Several researchers found an increase in the fusospirochetal flora in the mouths of the experimental animals, but the bacteria were regarded as opportunists, proliferating only when the tissues were altered by the deficiency. A poor diet has been cited as a predisposing factor in NUG and its sequelae in developing African countries, although the effects appear primarily to diminish the effectiveness of the immune response.19,20,35
In a controlled study of malnourished Nigerian children with and without NUG, those with NUG had a 38% increase in cortisol levels and higher levels of proinflammatory cytokines (interleukin [IL]-6, IL-8, IL-10, IL-18, and IL1 β) as compared to controls whose values were close to normal.20
Nutritional deficiencies (e.g., vitamin C, vitamin B2) accentuate the severity of the pathologic changes induced when the fusospirochetal bacterial complex is injected into animals.72
Debilitating Disease
Debilitating systemic disease may predispose patients to the development of NUG. Such systemic disturbances include chronic diseases (e.g., syphilis, cancer), severe gastrointestinal disorders (e.g., ulcerative colitis), blood dyscrasias (e.g., leukemia, anemia), and acquired immunodeficiency syndrome (AIDS). Nutritional deficiency resulting from debilitating disease may be an additional predisposing factor. Experimentally-induced leukopenia in animals may produce ulcerative gangrenous stomatitis.47,75,76 Ulceronecrotic lesions appear in the gingival margins of hamsters exposed to total-body irradiation42; these lesions can be prevented with systemic antibiotics.43
Psychosomatic Factors
Psychologic factors appear to be important in the etiology of NUG. The disease often occurs in association with stressful situations (e.g., induction into the armed forces, school examinations).25 Psychologic disturbances,26 as well as increased adrenocortical secretion,64 are common in patients with the disease.
Significant correlation between disease incidence and two personality traits, dominance and abasement, suggests the presence of an NUG-prone personality.23
The mechanisms whereby psychologic factors create or predispose to gingival damage have not been established, but alterations in digital and gingival capillary responses suggesting increased autonomic nervous activity have been demonstrated in patients with NUG.24
Cohen-Cole et al10 suggested that a psychiatric disturbance (e.g., trait anxiety, depression, or psychopathic deviance) and the impact of negative life events (stress) may lead to activation of the hypothalamic-pituitary-adrenal axis. This results in elevation of serum and urine cortisol levels, which is associated with a depression of lymphocyte and PMN function that may predispose to NUG.
It can be concluded that opportunistic bacteria are the primary etiologic agents of NUG in patients who demonstrate immunosuppression. Stress, smoking, and preexisting gingivitis are common predisposing factors.
Epidemiology and Prevalence
The prevalence of NUG appears to have been rather low in the United States and Europe before 1914. During World Wars I and II, numerous “epidemics” broke out among the Allied troops, but German soldiers did not seem to have been similarly affected. Epidemic-like outbreaks have also occurred among civilian populations. A study at a dental clinic in Prague, Czech Republic, reported the incidence of NUG as 0.08% in patients age 15 to 19 years, 0.05% in those age 20 to 24, and 0.02% in those age 25 to 29.67
NUG occurs at all ages, with the highest incidence reported between ages 20 and 30 years14,36,72 and ages 15 and 20 years.67 It is not common in children in the United States, Canada, and Europe, but it has been reported in children from low socioeconomic groups in underdeveloped countries.33 In India, 54%46 and 58%52 of the patients in two studies were under age 10 years.
In a random school population in Nigeria, NUG occurred in 11.3% of children between ages 2 and 6 years65; in a Nigerian hospital population, it was present in 23% of children under age 10 years.18 It has been reported in several members of the same family in low socioeconomic groups. NUG is more common in children with Down syndrome than in other children with mental deficiencies.3
Opinions differ as to whether NUG is more common during the winter,36,49 summer, or fall65 and whether there is a peak seasonal incidence.13
Communicability
NUG often occurs in groups in an epidemic pattern. At one time, NUG was considered contagious and had to be reported to the community health department, but it was later concluded not to be communicable.55,61 A distinction must be made between “communicability” and “transmissibility” when referring to the characteristics of disease. The term transmissible denotes a capacity for the maintenance of an infectious agent in successive passages through a susceptible animal host.55 The term communicable signifies a capacity for the maintenance of infection by natural modes of spread such as direct contact through drinking water, food, and eating utensils; via the airborne route; or by means of arthropod vectors. A disease that is communicable is described as contagious. It has been demonstrated that disease associated with the fusospirochetal bacterial complex is transmissible; however, it has not been shown to be communicable or contagious.
Attempts have been made to spread NUG from human to human, without success.62 King35 traumatized an area in his gingiva and introduced debris from a patient with a severe case of NUG. There was no response until he happened to fall ill shortly thereafter; subsequent to his illness, he observed the characteristic lesion in the experimental area. It may be inferred with reservation from this experiment that systemic debility is a prerequisite for the contagion of NUG.
It is a common impression that because NUG often occurs in groups using the same kitchen facilities, the disease is spread by bacteria on eating utensils. Growth of fusospirochetal organisms requires carefully controlled conditions and an anaerobic environment; they do not ordinarily survive on eating utensils.11,29
The occurrence of NUG in epidemic-like outbreaks does not necessarily mean that it is contagious. The affected groups may be afflicted by the disease because of common predisposing factors rather than because of its spread from person to person. In all likelihood, both a predisposed immunocompromised host and the presence of appropriate bacteria are necessary for the production of this disease.
Primary Herpetic Gingivostomatitis
Primary herpetic gingivostomatitis is an infection of the oral cavity caused by the herpes simplex virus type 1 (HSV-1).14,44,45,60 It occurs most often in infants and children younger than 6 years of age,5,60,63 but it is also seen in adolescents and adults. It occurs with equal frequency in male and female patients. In most persons, however, the primary infection is asymptomatic.
As part of the primary infection, the virus ascends through sensory and autonomic nerves, where it persists as latent HSV in neuronal ganglia that innervate the site. In approximately one-third of the world’s population, secondary manifestations result from various stimuli such as sunlight, trauma, fever, and stress. These secondary manifestations include herpes labialis (Figure 10-4), herpetic stomatitis, herpes genitalis, ocular herpes, and herpetic encephalitis. Secondary herpetic stomatitis can occur on the palate, gingiva (see Figure 10-5), or on the mucosa as a result of dental treatment that traumatizes or stimulates the latent virus in the ganglia innervating the area and may present as pain away from the site of treatment 2 to 4 days later. Careful inspection for characteristic vesicles may be diagnostic (see Figure 10-4).
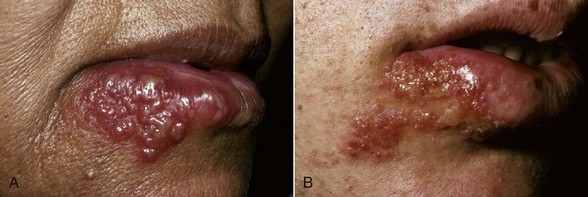
Figure 10-4 Recurrent herpetic vesicles in the lip. A, Early stage. B, Late stage showing brownish crusted lesions.
(From Sapp JP, Eversole, LR, Wysocki GP: Contemporary oral and maxillofacial pathology, ed 2, St Louis, 2002, Mosby.)
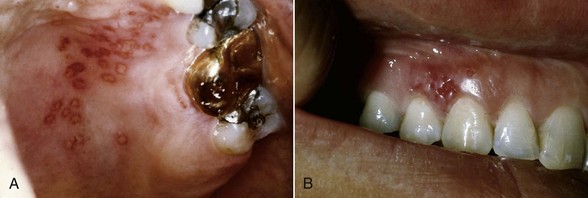
Figure 10-5 Recurrent intraoral herpetic vesicles in the palate (A) and in the gingiva (B). The latter location is rare.
(From Sapp JP, Eversole, LR, Wysocki GP: Contemporary oral and maxillofacial pathology, ed 2, St Louis, 2002, Mosby.)
Clinical Features
Oral Signs
Primary herpetic gingivostomatitis appears as a diffuse, erythematous, shiny involvement of the gingiva and the adjacent oral mucosa, with varying degrees of edema and gingival bleeding. In its initial stage, it is characterized by the presence of discrete, spherical gray vesicles, which may occur on the gingiva, labial and buccal mucosae, soft palate, pharynx, sublingual mucosa, and tongue (see Figure 10-6). After approximately 24 hours, the vesicles rupture and form painful, small ulcers with a red, elevated, halolike margin and a depressed, yellowish or grayish white central portion. These occur either in widely separated areas or in clusters where confluence occurs (see Figure 10-7).
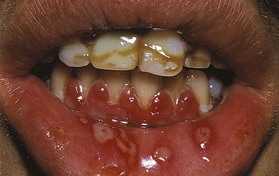
Figure 10-6 Primary herpetic gingivostomatitis in 12-year-old male, with diffuse erythematous involvement of the gingiva and spherical gray vesicle in the lip.
(Courtesy Dr. Heddie Sedano, University of California, Los Angeles, and University of Minnesota.)
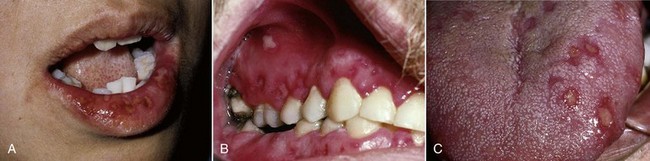
Figure 10-7 Involvement of the lip, gingiva, and tongue in primary herpetic gingivostomatitis.
(From Sapp JP, Eversole, LR, Wysocki GP: Contemporary oral and maxillofacial pathology, ed 2, St Louis, 2002, Mosby.)
Occasionally, primary herpetic gingivitis may occur without overt vesiculation. The clinical picture consists of diffuse, erythematous, shiny discoloration and edematous enlargement of the gingivae with a tendency toward bleeding.
The course of the disease is limited to 7 to 10 days. The diffuse gingival erythema and edema that appear early in the disease persist for several days after the ulcerative lesions have healed. Scarring does not occur in the areas of healed ulcerations.
Oral Symptoms
The disease is accompanied by generalized “soreness” of the oral cavity, which interferes with eating, drinking, and oral hygiene. The ruptured vesicles are the focal sites of pain and are particularly sensitive to touch, thermal changes, foods such as condiments and fruit juices, and the action of coarse foods. In infants, the disease is marked by irritability and refusal to take food.
Extraoral and Systemic Signs and Symptoms
Cervical adenitis, fever as high as 101° F to 105° F (38° C to 40.6° C), and generalized malaise are common.
History
Primary herpetic gingivostomatitis is the result of an acute infection by HSV and has an acute onset.
Histopathology
The virus targets the epithelial cells, which show “ballooning degeneration” that consists of acantholysis, nuclear clearing, and nuclear enlargement. These cells are called Tzanck cells. Infected cells fuse, forming multinucleated cells, and intercellular edema leads to formation of an intraepithelial vesicles that rupture and develop a secondary inflammatory response with a fibropurulent exudate48 (see Figure 10-8). Discrete ulcerations resulting from rupture of the vesicles have a central portion of acute inflammation, with varying degrees of purulent exudate, surrounded by engorged blood vessels.
Diagnosis
It is critical to arrive at a diagnosis as early as possible in primary herpetic infections. Treatment with antiviral medications can dramatically alter the course of the disease, reducing symptoms and potentially reducing recurrences. The diagnosis is usually established from the patient’s history and the clinical findings. Material may be obtained from the lesions and submitted to the laboratory for confirmatory tests, including virus culture and immunologic tests using monoclonal antibodies or deoxyribonucleic acid (DNA) hybridization techniques.5,54 This should not delay treatment if strong clinical evidence exists for primary gingivostomatitis.
Differential Diagnosis
Primary herpetic gingivostomatitis should be differentiated from several conditions. NUG can be differentiated in different ways (see Table 10-1).
Erythema multiforme can be differentiated because its vesicles are generally more extensive than those in primary herpetic gingivostomatitis and on rupture demonstrate a tendency toward pseudomembrane formation. In addition, the tongue is usually very involved in erythema multiforme, with infection of the ruptured vesicles resulting in varying degrees of ulceration. Oral involvement in erythema multiforme may be accompanied by skin lesions. The duration of erythema multiforme may be comparable with that of primary herpetic gingivostomatitis, but prolonged involvement may occur for weeks.
Stevens-Johnson syndrome is a comparatively rare form of erythema multiforme, characterized by vesicular hemorrhagic lesions in the oral cavity, hemorrhagic ocular lesions, and bullous skin lesions.
Bullous lichen planus is a very rare and painful condition. It is characterized by large blisters on the tongue and cheek that rupture and undergo ulceration; it runs a prolonged, indefinite course. Patches of linear, gray, lacelike lesions of lichen planus are often interspersed among the bullous eruptions. Lichen planus involvement of the skin may coexist with the oral lesions and facilitate differential diagnosis.
Desquamative gingivitis is characterized by diffuse involvement of the gingiva, with varying degrees of “peeling” of the epithelial surface and exposure of the underlying tissue. It is a chronic condition (see Chapter 12).
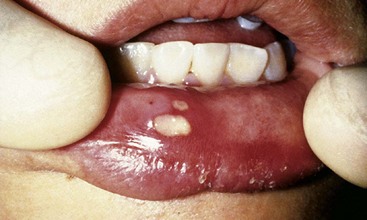
Lesions of recurrent aphthous stomatitis (RAS)21 range from occasional small (0.5 to 1 cm in diameter), well-defined, round or ovoid, shallow ulcers with a yellowish gray central area surrounded by an erythematous halo, which heal in 7 to 10 days without scarring, to larger (1 to 3 cm in diameter) oval or irregular ulcers, which persist for weeks and heal with scarring (Figure 10-9). The cause is unknown, although immunopathologic mechanisms appear to play a role. RAS is a different clinical entity from primary herpetic gingivostomatitis. The ulcerations may look the same in the two conditions, but diffuse erythematous involvement of the gingiva and acute toxic systemic symptoms do not occur in RAS. A history of previous episodes of painful mucosal ulcerations suggests RAS rather than primary HSV.
Communicability
Primary herpetic gingivostomatitis is contagious.7,39 Most adults have developed immunity to HSV as the result of infection during childhood, which in most cases is subclinical. For this reason, acute herpetic gingivostomatitis usually occurs in infants and children. Recurrent herpetic gingivostomatitis has been reported,28 although it is not often clinically significant unless immunity is destroyed by debilitating systemic disease. Studies demonstrating HSV in periodontal pockets suggest more recurrence of viral replication than previously recognized.68 Secondary herpetic infection of the skin, such as herpes labialis, does recur.66
Pericoronitis
The term pericoronitis refers to inflammation of the gingiva in relation to the crown of an incompletely erupted tooth (Figure 10-10). It occurs most often in the mandibular third molar area. Pericoronitis may be acute, subacute, or chronic.
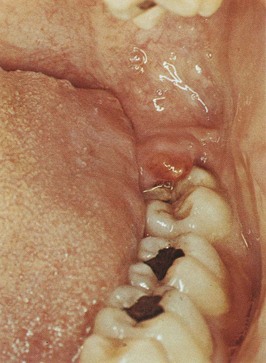
Figure 10-10 Pericoronitis. Inflamed coronal flap covering disto-occlusal surface of impacted mandibular third molar. Note swelling and redness.
(From Glickman I, Smulow J: Periodontal disease: clinical, radiographic and histopathologic features, Philadelphia, 1974, Saunders.)
Clinical Features
The partially erupted or impacted mandibular third molar is the most common site of pericoronitis. The space between the crown of the tooth and the overlying gingival flap (operculum) is an ideal area for the accumulation of food debris and bacterial growth. Even in patients with no clinical signs or symptoms, the gingival flap is often chronically inflamed and infected and has varying degrees of ulceration along its inner surface. Acute inflammatory involvement is a constant possibility and may be exacerbated by trauma, occlusion, or a foreign body trapped underneath the tissue flap (e.g., popcorn husk, nut fragment).
Acute pericoronitis is identified by varying degrees of inflammatory involvement of the pericoronal flap and adjacent structures, as well as by systemic complications. The inflammatory fluid and cellular exudate increase the bulk of the flap, which then may interfere with complete closure of the jaws and can be traumatized by contact with the opposing jaw, aggravating the inflammatory involvement.
The resultant clinical picture is a red, swollen, suppurating lesion that is exquisitely tender, with radiating pains to the ear, throat, and floor of the mouth. The patient is extremely uncomfortable because of a foul taste and an inability to close the jaws, in addition to the pain. Swelling of the cheek in the region of the angle of the jaw and lymphadenitis are common findings. Trismus may also be a presenting complaint. The patient may also have systemic complications such as fever, leukocytosis, and malaise.
Complications
Involvement may be localized in the form of a pericoronal abscess. It may spread posteriorly into the oropharyngeal area and medially to the base of the tongue, making it difficult for the patient to swallow. Depending on the severity and extent of the infection, there is involvement of the submaxillary, posterior cervical, deep cervical, and retropharyngeal lymph nodes.32,50 Peritonsillar abscess formation, cellulitis, and Ludwig’s angina are infrequent but potential sequelae of acute pericoronitis.
1 Berke JD. Experimental study of acute ulcerative stomatitis. J Am Dent Assoc. 1961;63:86.
2 Box HK. Necrotic gingivitis. Toronto: University of Toronto Press; 1930.
3 Brown RH. Necrotizing ulcerative gingivitis in mongoloid and nonmongoloid retarded individuals. J Periodontal Res. 1973;8:290.
4 Cahn LR. The penetration of the tissue by Vincent’s organisms: a report of a case. J Dent Res. 1929;9:695.
5 Cawson RA. Infections of the oral mucous membrane. In: Cohen B, Kramer IRH, editors. Scientific foundations of dentistry. Chicago: Year Book Medical Publishers, 1976.
6 Chapman OD, Harris AE. Oral lesions associated with dietary deficiencies in monkeys. J Infect Dis. 1941;69:7.
7 Chilton NW. Herpetic stomatitis. Am J Orthod Oral Surg. 1944;30:335.
8 Chung CP, Nisengard RJ, Slots J, et al. Bacterial IgG and IgM antibody titers in acute necrotizing ulcerative gingivitis. J Periodontol. 1983;54:557.
9 Cogen RB, Stevens AWJr, Cohen-Cole SA, et al. Leukocyte function in the etiology of acute necrotizing ulcerative gingivitis. J Periodontol. 1983;54:402.
10 Cohen-Cole SA, et al. Psychiatric, psychosocial and endocrine correlates of acute necrotizing ulcerative gingivitis (trench mouth): a preliminary report. Psychiatr Med. 1983;1:215.
11 Coutley RL. Vincent’s infection. Br Dent J. 1943;74:34.
12 Curtois GJIII, Cobb CM, Killoy WJ. Acute necrotizing ulcerative gingivitis: a transmission electron microscope study. J Periodontol. 1983;54:671.
13 Daley FH. Studies of Vincent’s infection at the clinic of Tufts College Dental School from October 1926 to February 1928. J Dent Res. 1928;8:408.
14 Dodd K, Johnston LM, Budding GJ. Herpetic stomatitis. J Pediatr. 1938;12:95.
15 Duckworth R, Waterhouse JP, Britton DG, et al. Acute ulcerative gingivitis: a double blind controlled clinical trial with metronidazole. Br Dent J. 1966;120:599.
16 Ellerman V. Vincent’s organisms in tissue. Z Hyg Infekt Pr. 1907;56:453.
17 Emslie RD. Cancrum oris. Dent Pract. 1963;13:481.
18 Enwonwu CO. Epidemiological and biochemical studies of necrotizing ulcerative gingivitis and noma (cancrum oris) in Nigerian children. Arch Oral Biol. 1972;17:1357.
19 Enwonwu CO. Cellular and molecular effects of malnutrition and their relevance to periodontal diseases. J Clin Periodontol. 1994;21:643.
20 Enwonwu CO, Phillips RS, Savage KO. Inflammatory cytokine profile and circulating cortisol levels in malnourished children with necrotizing ulcerative gingivitis. Eur Cytokine Netw. 2005;16:240.
21 Eversole LR. Diseases of the oral mucous membranes. Review of the literature. In: Millard HD, Mason DK, editors. World workshop on oral medicine. Chicago: YearBook Medical Publishers, 1989.
22 Falkler WAJr, Martin SA, Vincent JW, et al. A clinical, demographic and microbiologic study of NUG patients in an urban dental school. J Clin Periodontol. 1987;14:307.
23 Formicola AJ, Witte ET, Curran PM. A study of personality traits and acute necrotizing ulcerative gingivitis. J Periodontol. 1970;41:36.
24 Giddon DB. Psychophysiology of the oral cavity. J Dent Res. 1966;45(Suppl 6):1627.
25 Giddon DB, Zackin SJ, Goldhaber P. Acute necrotizing gingivitis in college students. J Am Dent Assoc. 1964;68:381.
26 Goldhaber P, Giddon DB. Present concepts concerning the etiology and treatment of acute necrotizing ulcerative gingivitis. Int Dent J. 1964;14:468.
27 Greenspan D, Pindborg JJ, Greenspan JS, et al. AIDS and the dental team. Copenhagen: Munksgaard; 1986.
28 Griffin JW. Recurrent intraoral herpes simplex virus infection. Oral Surg. 1965;19:209.
29 Hampp EG, Mergenhagen SE. Experimental infection with oral spirochetes. J Infect Dis. 1961;109:43.
30 Hooper PA, Seymour GJ. The histopathogenesis of acute ulcerative gingivitis. J Periodontol. 1979;50:419.
31 Horning GM, Cohen ME. Necrotizing ulcerative gingivitis, periodontitis and stomatitis: clinical staging and predisposing factors. J Periodontol. 1995;66:990.
32 Jacobs MH. Pericoronal and Vincent’s infections: bacteriology and treatment. J Am Dent Assoc. 1953;30:392.
33 Jimenez M, Baer PN. Necrotizing ulcerative gingivitis in children: a 9-year clinical study. J Periodontol. 1975;46:715.
34 Johnson BD, Engel D. Acute necrotizing gingivitis. A review of diagnosis, etiology and treatment. J Periodontol. 1986;7:141.
35 King JD. Nutritional and other factors in trench mouth with special reference to the nicotinic acid component of vitamin B complex. Br Dent J. 1943;74:113.
36 Lado RA, Carranza FAJr. Estudio sobre la incidencia de la gingivitis ulceronecrotizante. Rev Asoc Odont Argentina. 1961;49:87.
37 Listgarten MA. Electron microscopic observations on the bacterial flora of acute necrotizing ulcerative gingivitis. J Periodontol. 1965;36:328.
38 Listgarten MA, Lewis DW. The distribution of spirochetes in the lesion of acute necrotizing ulcerative gingivitis: an electron microscopic and statistical survey. J Periodontol. 1967;38:379.
39 Littner MM, Dayan D, Kaffe I, et al. Acute streptococcal gingivostomatitis: report of five cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1982;53:144.
40 Loesche WJ, Syed SA, Langhorn BE, Stoll J. The bacteriology of acute necrotizing ulcerative gingivitis. J Periodontol. 1982;53:223.
41 Lopez R, Baelum V. Necrotizing ulcerative gingival lesions and clinical attachment loss. Eur J Oral Sci. 2005;112:105.
42 Mayo J, Carranza FAJr, Cabrini RL. Comparative study of the effect of antibiotics, bone marrow and cysteamine on oral lesions produced in hamsters by total body irradiation. Experientia. 1964;20:403.
43 Mayo J, Carranza FAJr, Epper CE, et al. The effect of total-body irradiation on the oral tissues of the Syrian hamster. Oral Surg. 1962;15:739.
44 McCarthy PL, Shklar G. Diseases of the oral mucosa, ed 2. Philadelphia: Lea & Febiger; 1980.
45 McNair ST. Herpetic stomatitis. J Dent Res. 1950;29:647.
46 Miglani DC, Sharma OP. Incidence of acute necrotizing gingivitis and periodontosis among cases seen at the government hospital, Madras. J All India Dent Assoc. 1965;37:183.
47 Miller DK, Rhoads CP. The experimental production in dogs of acute stomatitis associated with leukopenia and a maturation defect of the myeloid elements of the bone marrow. J Exp Med. 1935;61:173.
48 Neville BW, Damm DD, Allen CM, et al. Oral and maxillofacial pathology. Philadelphia: Saunders; 1995.
49 Pedler JA, Radden BG. Seasonal influence of acute ulcerative gingivitis. Dent Pract. 1957;8:23.
50 Perkins AE. Acute infections around erupting mandibular third molar. Br Dent J. 1944;76:199.
51 Pindborg JJ. Gingivitis in military personnel with special reference to ulceromembranous gingivitis. Odontol Tidskr. 1951;59:407.
52 Pindborg JJ, Bhat M, Devanath KR, et al. Occurrence of acute necrotizing gingivitis in South Indian children. J Periodontol. 1966;37:14.
53 Plaut HC. Studienzur bakterielle diagnostik der diphtheriae un der aginen. Dtsch Med Wochenschr. 1894;20:920.
54 Regezi JA, Sciubba JJ. Oral pathology: clinico-pathologic correlations. Philadelphia: Saunders; 1989.
55 Rosebury T. Is Vincent’s infection a communicable disease? J Am Dent Assoc. 1942;29:823.
56 Rosebury T, Foley G. Experimental Vincent’s infection. J Am Dent Assoc. 1939;26:1978.
57 Rosebury T, MacDonald JB, Clark A. A bacteriologic survey of gingival scrapings from periodontal infections by direct examination, guinea pig inoculation and anaerobic cultivation. J Dent Res. 1950;29:718.
58 Rosenthal SL, Gootzeit EH. The incidence of B. Fusiformis and Spirochetes in the edentulous mouth. J Dent Res. 1942;21:373.
59 Rowland R. Necrotizing ulcerative gingivitis. Ann Periodontol. 1999;4:65.
60 Sapp JP, Eversole LR, Wysocki GP. Contemporary oral and maxillofacial pathology, ed 2. St. Louis: Mosby (Elsevier); 2004.
61 Schluger S. Necrotizing ulcerative gingivitis in the army: incidence, communicability, and treatment. J Am Dent Assoc. 1949;38:174.
62 Schwartzman J, Grossman L. Vincent’s ulceromembranous gingivostomatitis. Arch Pediatr. 1941;58:515.
63 Scott TFM, Steigman AS, Convey JH. Acute infectious gingivostomatitis: etiology, epidemiology, and clinical picture of common disorders caused by virus of herpes simplex. JAMA. 1941;117:999.
64 Shannon IL, Kilgore WG, Leary TJ. Stress as a predisposing factor in necrotizing ulcerative gingivitis. J Periodontol. 1969;40:240.
65 Sheiham A. An epidemiological study of oral disease in Nigerians. J Dent Res. 1965;44:1184.
66 Ship II, Brightman VJ, Laster LL. The patient with recurrent aphthous ulcers and the patient with recurrent herpes labialis: a study of two population samples. J Am Dent Assoc. 1967;75:645.
67 Skach M, Zabrodsky S, Mrklas L. A study of the effect of age and season on the incidence of ulcerative gingivitis. J Periodontal Res. 1970;5:187.
68 Slots J, Contreras A. Herpesviruses: a unifying causative factor in periodontitis? Oral Microbiol Immunol. 2000;5:277.
69 Smith DT. Spirochetes and related organisms in fusospirochetal disease. Baltimore: Williams & Wilkins; 1932.
70 Stammers AF. Vincent’s infection. Br Dent J. 1944;76:171.
71 Stevens AWJ, Cogen RB, Cohen-Cole SA, et al. Demographic and clinical data associated with acute necrotizing ulcerative gingivitis in a dental school population. J Clin Periodontol. 1984;11:487.
72 Swenson HM. Induced Vincent’s infection in dogs. J Dent Res. 1944;23:190.
73 Swenson HM, Muhler JC. Induced fusospirochetal infection in dogs. J Dent Res. 1947;26:161.
74 Topping NH, Fraser HF. Mouth lesions associated with dietary deficiencies in monkeys. US Public Health Rep. 1939;54:431.
75 Tunnicliff R, Fink EB, Hammond C. Significance of fusiform bacilli and spirilla in gingival tissue. J Am Dent Assoc. 1936;23:1959.
76 Tunnicliff R, Hammond C. Abscess production by fusiform bacilli in rabbits and mice by the use of scillaren-B or mucin. J Dent Res. 1937;16:479.
77 Underhill FP, Mendel LB. Further experiments on the pellagra-like syndrome in dogs. Am J Physiol. 1928;83:589.
78 Vincent H. Sur l’etiologie et sur les lesions anatomopathologiques de la pourritute d’hopital. Ann l’Inst Pasteur. 1896;10:448.