CHAPTER 27 Influence of Systemic Conditions on the Periodontium
Many systemic diseases, disorders, and conditions have been implicated as risk indicators or risk factors in periodontal disease. Clinical and basic science research over the past several decades has led to an improved understanding and appreciation for the complexity and pathogenesis of periodontal diseases.183 Although there is clear evidence for a bacterial etiology and there are specific bacteria (periodontal pathogens) associated with destructive periodontal disease, the presence of these pathogens does not invariably cause disease. Their absence, on the other hand, appears to be consistent with periodontal health. The role of bacteria in disease etiology and pathogenesis is discussed in Chapters 23 and 25.
Perhaps the most significant advance in our understanding of the pathogenesis of periodontitis is that the host response varies between individuals and that an altered, deficient, or exaggerated host immune response to bacterial pathogens may lead to more severe forms of the disease. In other words, the individual host immune response to periodontal pathogens is very important and likely explains much of the differences in disease severity observed from one individual to another. Furthermore, systemic diseases, disorders, and conditions alter host tissues and physiology, which may impair the host’s barrier function and immune defense against periodontal pathogens creating the opportunity for destructive periodontal disease.
Recent evidence also suggests that periodontal infections can adversely affect systemic health with manifestations such as coronary heart disease, stroke, diabetes, preterm labor, low-birth-weight delivery, and respiratory disease.163 The role of periodontal infections on these systemic health conditions is discussed in Chapter 28.
The interrelationships between periodontal infections and host defense are complex. A number of environmental, physical, and psychosocial factors have the potential to alter periodontal tissues and the host immune response, resulting in more severe periodontal disease expression. It is important to recognize that the systemic diseases, disorders, or conditions themselves do not cause periodontitis, but they may predispose, accelerate, or otherwise increase its progression. This chapter discusses the influence of several notable systemic diseases, disorders, and conditions on the periodontium.
Endocrine Disorders and Hormonal Changes
Endocrine diseases, such as diabetes and hormonal fluctuations, that are associated with puberty and pregnancy are well-known examples of systemic conditions that adversely affect the condition of the periodontium. Endocrine disturbances and hormone fluctuations affect the periodontal tissues directly, modify the tissue response to local factors, and produce anatomic changes in the gingiva that may favor plaque accumulation and disease progression. This section describes the evidence supporting the relationship among endocrine disorders, hormonal changes, and periodontal disease.
Diabetes Mellitus
Diabetes mellitus is an extremely important disease from a periodontal standpoint. It is a complex metabolic disorder characterized by chronic hyperglycemia. Diminished insulin production, impaired insulin action, or a combination of both result in the inability of glucose to be transported from the bloodstream into the tissues, which in turn results in high blood glucose levels and excretion of sugar in the urine. Lipid and protein metabolism is altered in diabetes as well. Uncontrolled diabetes (chronic hyperglycemia) is associated with several long-term complications, including microvascular diseases (retinopathy, nephropathy, or neuropathy), macrovascular diseases (cardiovascular, cerebrovascular), an increased susceptibility to infections, and poor wound healing. An estimated 23.6 million individuals (children and adults), or 7.8% of the United States (US) population, have diabetes.192 Approximately 5.7 million of these individuals are unaware that they have the disease.
There are two major types of diabetes, type 1 and type 2, with several less common secondary types. Type 1 diabetes mellitus, formerly insulin-dependent diabetes mellitus (IDDM), is caused by a cell-mediated autoimmune destruction of the insulin-producing beta cells of the islets of Langerhans in the pancreas, which results in insulin deficiency. Type 1 diabetes accounts for 5% to 10% of all cases of diabetes and most often occurs in children and young adults. This type of diabetes results from a lack of insulin production and is very unstable and difficult to control. It has a marked tendency toward ketosis and coma, is not preceded by obesity, and requires injected insulin to be controlled. Patients with type 1 diabetes mellitus present with the symptoms traditionally associated with diabetes, including polyphagia, polydipsia, polyuria, and predisposition to infections.
Type 2 diabetes mellitus, formerly non–insulin-dependent diabetes mellitus (NIDDM), is caused by peripheral resistance to insulin action, impaired insulin secretion, and increased glucose production in the liver. The insulin-producing beta cells in the pancreas are not destroyed by cell-mediated autoimmune reaction. It typically begins as insulin resistance leading to reduced pancreas production of insulin as the demand increases. Type 2 diabetes is the most common form of diabetes, accounting for 90% to 95% of all cases, and usually has an adult onset. Individuals often are not aware they have the disease until severe symptoms or complications occur. Type 2 diabetes generally occurs in obese individuals and can often be controlled by diet and oral hypoglycemic agents. Ketosis and coma are uncommon. Type 2 diabetes can present with the same symptoms as type 1 diabetes but typically in a less severe form.
An additional category of diabetes is hyperglycemia secondary to other diseases or conditions. A prime example of this type of hyperglycemia is gestational diabetes associated with pregnancy. Gestational diabetes develops in 2% to 5% of all pregnancies but disappears after delivery. Women who have had gestational diabetes are at increased risk of developing type 2 diabetes later in life. Other secondary types of diabetes are those associated with diseases that involve the pancreas and destruction of the insulin-producing cells. Endocrine diseases, such as acromegaly and Cushing’s syndrome, tumors, pancreatectomy, and drugs or chemicals that cause altered insulin levels, are included in this group. Experimentally induced types of diabetes generally belong in this category rather than in type 1 or 2 diabetes mellitus.
Oral Manifestations
Numerous oral changes have been described in diabetic patients, including cheilosis, mucosal drying and cracking, burning mouth and tongue, diminished salivary flow, and alterations in the flora of the oral cavity, with greater predominance of Candida albicans, hemolytic streptococci, and staphylococci.2,22,99,158 An increased rate of dental caries has also been observed in poorly controlled diabetes.74,84 Importantly, however, these changes are not always present, are not specific, and are not pathognomonic for diabetes.161 Furthermore, these changes are less likely to be observed in well-controlled diabetic patients. Individuals with controlled diabetes have a normal tissue response, a normally developed dentition, a normal defense against infections, and no increase in the incidence of caries.233
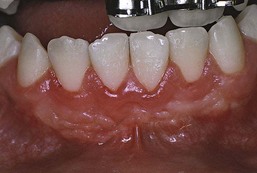
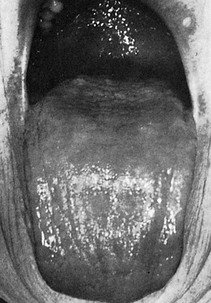
The influence of diabetes on the periodontium has been thoroughly investigated. Although it is difficult to make definitive conclusions about the specific effects of diabetes on periodontium, a variety of changes have been described, including a tendency toward enlarged gingiva, sessile or pedunculated gingival polyps, polypoid gingival proliferations, abscess formation, periodontitis, and loosened teeth112 (Figure 27-1). Perhaps the most striking changes in uncontrolled diabetes are the reduction in defense mechanisms and the increased susceptibility to infections, leading to destructive periodontal disease. In fact, periodontal disease is considered to be the sixth complication of diabetes.144 Periodontitis in type 1 diabetic patients appears to start after age 12 years.47 The prevalence of periodontitis has been reported as 9.8% in 13- to 18-year-old patients, increasing to 39% in those 19 years and older.
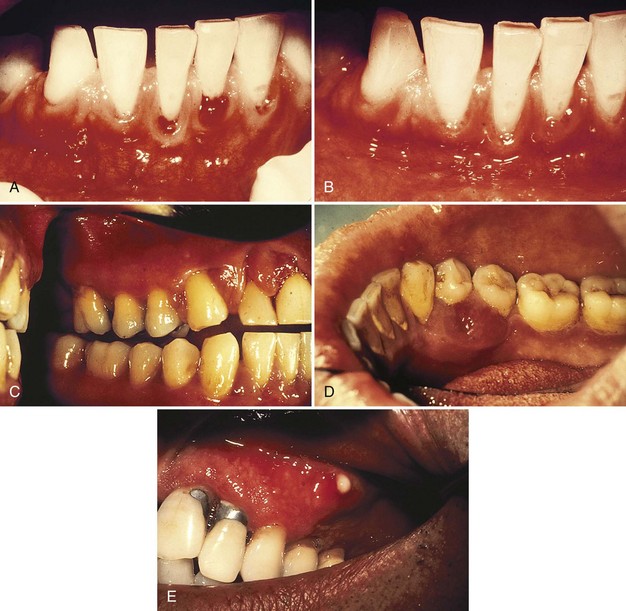
Figure 27-1 Periodontal condition in patients with diabetes. A, Adult with diabetes (blood glucose level > 400 mg/dl). Note the gingival inflammation, spontaneous bleeding, and edema. B, Same patient as in A. Improved control of diabetes following 4 days of insulin therapy (blood glucose level <100 mg/dl). The clinical periodontal condition has improved without local therapy. C, Adult patient with uncontrolled diabetes. Note the enlarged, smooth, erythematous gingival margins and papilla in the anterior area. D, Same patient as in C. Lingual view of the right mandibular area. Note the inflamed and swollen tissues in the anterior and premolars area. E, Adult patient with diabetes. Suppurating abscess on the buccal surface of the maxillary premolars in a patient with uncontrolled diabetes.
The extensive literature on this subject and the overall impression of clinicians indicate that periodontal disease in diabetic patients follows no consistent or distinct pattern. Severe gingival inflammation, deep periodontal pockets, rapid bone loss, and frequent periodontal abscesses often occur in poorly controlled diabetic patients with poor oral hygiene3 (Figures 27-2 and 27-3). Children with type 1 diabetes tend to have more destruction around the first molars and incisors than elsewhere, but this destruction becomes more generalized at older ages.47 In juvenile diabetic patients, extensive periodontal destruction often occurs as a consequence of having more severe disease at a younger age.
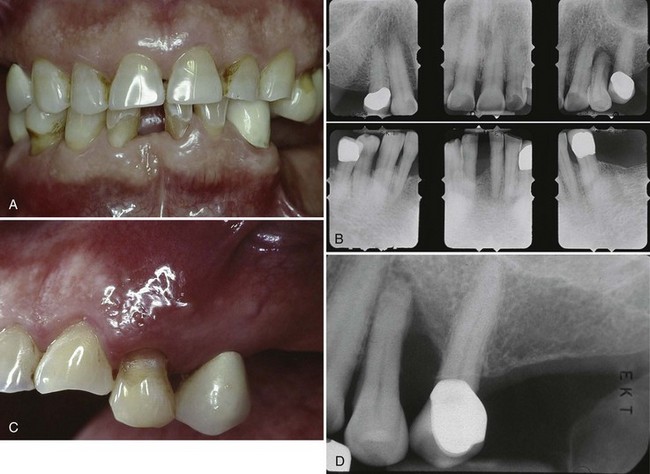
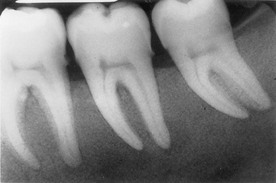
Figure 27-2 Sixty-year-old patient with long-term history of type 2 diabetes. A, Anterior retracted view of dental/periodontal condition. Note missing posterior teeth, supereruption of premolars, and mild generalized gingival inflammation. B, Periapical radiographs of remaining teeth. Note mild, generalized bone loss with localized areas of severe bone loss. Failure to replace posterior teeth adds to the occlusal burden of the remaining dentition. C, Clinical photograph of maxillary premolar area presenting with abscess. Notice diffuse erythema, inflammation surrounding abscess area. D, Periapical radiograph of maxillary premolar showing extensive bone loss associated with abscess.
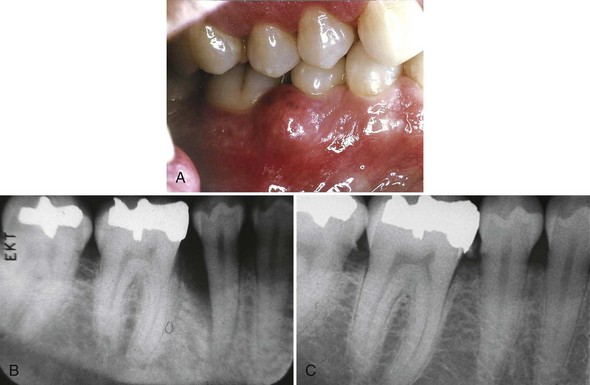
Figure 27-3 Abscess in 28-year-old patient with poorly controlled type 1 diabetes. A, Periodontal abscess in patient with poorly controlled diabetes. He presented with pain and abscess a few weeks after scaling and root planing of the area. B, Radiograph of mandibular right premolar area demonstrating severe localized destruction of bone in the area of periodontal abscess. C, Radiograph of mandibular right premolar area taken 2 months before presentation of abscess. Note presence of calculus and level of interproximal bone before abscess.
Other investigators have reported that the rate of periodontal destruction appears to be similar for those with diabetes and those without diabetes, up to 30 years of age.90,227 After age 30, diabetic patients have a greater degree of periodontal destruction, possibly related to more disease destruction over time. Patients showing overt diabetes over more than 10 years have greater loss of periodontal support than those with a diabetic history of less than 10 years.90 This destruction may also be related to the diminished tissue integrity that continues to deteriorate over time (see later section for description of altered collagen metabolism).
Although some studies have not found a correlation between the diabetic state and the periodontal condition, the majority of well-controlled studies show a higher prevalence and severity of periodontal disease in individuals with diabetes than in nondiabetic persons with similar local factors.* Findings include a greater loss of attachment, increased bleeding on probing, and increased tooth mobility. A study of risk indicators for a group of 1426 patients, ages 25 to 74, revealed that individuals with diabetes are twice as likely to exhibit attachment loss as nondiabetic individuals.106 The lack of consistency across studies is most likely related to the different degrees of diabetic involvement, variations in level of disease control and diversity of indices, and patient sampling from one study to another.
Recent studies suggest that uncontrolled or poorly controlled diabetes is associated with an increased susceptibility to and severity of infections, including periodontitis.16,207 As with other systemic conditions associated with periodontitis, diabetes mellitus does not cause gingivitis or periodontitis, but evidence indicates that it alters the response of the periodontal tissues to local factors, hastening bone loss and delaying postsurgical healing. Frequent periodontal abscesses appear to be an important feature of periodontal disease in diabetic patients.
Approximately 40% of adult Pima Indians in Arizona have type 2 diabetes. A comparison of individuals with or without diabetes in this Native American tribe has shown a clear increase in prevalence of destructive periodontitis, as well as a 15% increase in edentulousness, in diabetic patients.217 The risk of developing destructive periodontitis increases threefold in these individuals.71
Bacterial Pathogens
The glucose content of gingival fluid and blood is higher in individuals with diabetes than in those without diabetes, with similar plaque and gingival index scores.77 The increased glucose in the gingival fluid and blood of diabetic patients could change the environment of the microflora, inducing qualitative changes in bacteria that could contribute to the severity of periodontal disease observed in those with poorly controlled diabetes.
Patients with type 1 diabetes mellitus and periodontitis have been reported to have a subgingival flora composed mainly of Capnocytophaga, anaerobic vibrios, and Actinomyces species. Porphyromonas gingivalis, Prevotella intermedia, and Aggregatibacter actinomycetemcomitans, which are common in periodontal lesions of individuals without diabetes, are present in low numbers in those with the disease.108,159 Other studies, however, found scarce Capnocytophaga and abundant A. actinomycetemcomitans and black-pigmented Bacteroides, as well as P. intermedia, P. melaninogenica, and Campylobacter rectus.158,210 Black-pigmented species, especially P. gingivalis, P. intermedia, and C. rectus, are prominent in severe periodontal lesions of Pima Indians with type 2 diabetes.87,261 Although these results suggest an altered flora in the periodontal pockets of patients with diabetes, the exact role of these microorganisms has not been determined.
Polymorphonuclear Leukocyte Function
The increased susceptibility of diabetic patients to infection has been hypothesized as being caused by polymorphonuclear leukocyte (PMN) deficiencies resulting in impaired chemotaxis, defective phagocytosis, or impaired adherence.162,230 In patients with poorly controlled diabetes, the function of PMNs and monocytes/macrophages is impaired.116 As a result, the primary defense (PMNs) against periodontal pathogens is diminished, and bacterial proliferation is more likely. No alteration of immunoglobulin A (IgA), G (IgG), or M (IgM) has been found in diabetic patients.201
Altered Collagen Metabolism
Chronic hyperglycemia impairs collagen structure and function, which may directly impact the integrity of the periodontium. Decreased collagen synthesis, osteoporosis, as well as a reduction in alveolar bone height has been demonstrated in diabetic animals.91,212 Chronic hyperglycemia adversely affects the synthesis, maturation, and maintenance of collagen and extracellular matrix. In the hyperglycemic state, numerous proteins and matrix molecules undergo a nonenzymatic glycosylation, resulting in accumulated glycation end-products (AGEs). The formation of AGEs occurs at normal glucose levels as well, but in hyperglycemic environments, AGE formation is excessive. Many types of molecules are affected, including proteins, lipids, and carbohydrates. Collagen is cross-linked by AGE formation, making it less soluble and less likely to be normally repaired or replaced. Cellular migration through cross-linked collagen is impeded, and perhaps more importantly, tissue integrity is impaired as a result of damaged collagen remaining in the tissues for longer periods (i.e., collagen is not renewed at a normal rate).106 As a result, collagen in the tissues of patients with poorly controlled diabetes is older and more susceptible to pathogenic breakdown (i.e., less resistant to destruction by periodontal infections).
AGEs play a central role in the classic complications of diabetes33 and may play a significant role in the progression of periodontal disease as well. Poor glycemic control, with the associated increase in AGEs, renders the periodontal tissues more susceptible to destruction.211 The cumulative effects of altered cellular response to local factors, impaired tissue integrity, and altered collagen metabolism undoubtedly play a significant role in the susceptibility of diabetic patients to infections and destructive periodontal disease.
Female Sex Hormones
Gingival alterations during puberty, pregnancy, and menopause are associated with physiologic hormonal changes in the female patient. In puberty and pregnancy, these changes are characterized by nonspecific inflammatory reactions with a predominant vascular component, leading clinically to a marked hemorrhagic tendency. Oral changes during menopause may include thinning of the oral mucosa, gingival recession, xerostomia, altered taste, and burning mouth. The changes associated with each phase of the female life cycle from puberty to menopause are briefly described here. Chapter 38 provides a detailed description of and management considerations for periodontal manifestations of hormonal changes in the female patient.
Puberty
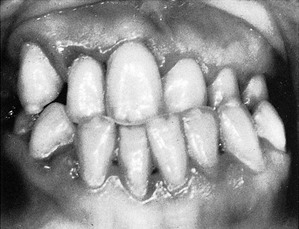
Puberty is often accompanied by an exaggerated response of the gingiva to plaque.224 Pronounced inflammation, edema, and gingival enlargement result from local factors that might ordinarily elicit a comparatively mild gingival response (Figure 27-4). As adulthood approaches, the severity of the gingival reaction diminishes, even when local factors persist. However, complete return to normal health requires removal of these factors. Although the prevalence and severity of gingival disease are increased in puberty, gingivitis is not a universal occurrence for all adolescents, with good oral hygiene, it can be prevented (see Chapter 11).
Menstruation
During the menstrual period, the prevalence of gingivitis increases. Some patients may complain of bleeding gums or a bloated, tense feeling in the gums in the days preceding menstrual flow. The exudate from inflamed gingiva is increased during menstruation, suggesting that preexisting gingivitis is aggravated by menstruation, but the crevicular fluid of normal healthy gingiva is unaffected.114 Tooth mobility does not change significantly during the menstrual cycle.83 The salivary bacterial count is increased during menstruation and at ovulation up to 14 days earlier.193
Pregnancy
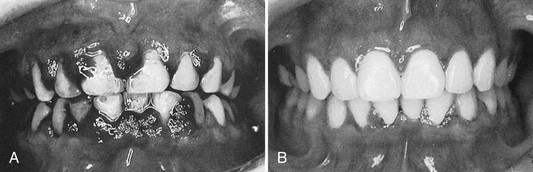
Gingival changes in pregnancy were described as early in the late 1800s, even before any knowledge about hormonal changes in pregnancy was available.25,191 As with other systemic conditions, pregnancy itself does not cause gingivitis. Gingivitis in pregnancy is caused by bacterial plaque, just as it is in nonpregnant women. The hormonal changes of pregnancy accentuate the gingival response to plaque and modify the resultant clinical picture (Figure 27-5). No notable changes occur in the gingiva during pregnancy in the absence of local factors.
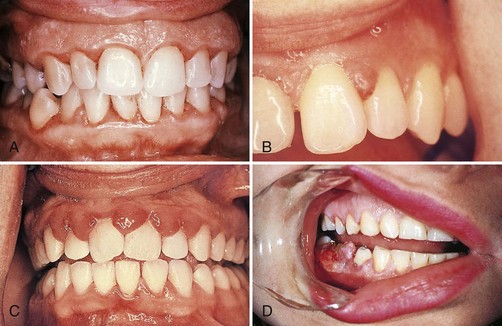
Figure 27-5 Periodontal condition in pregnancy. A, Marginal erythema and easily bleeding gingiva in a woman who is 5 months pregnant. B, Localized, incipient gingival enlargement between the maxillary central and lateral incisors in a woman who is 4 months pregnant. C, Generalized gingival enlargement of the interdental papilla and gingival margins on the facial surface of the maxillary incisors in a pregnant woman. D, Extensive gingival enlargement localized on the buccal surface of the mandibular premolars in a pregnant woman. These lesions are often referred to as “pregnancy tumors.”
The reported incidence of gingivitis in pregnancy in well-conducted studies varies from 50% to 100%.143,150 Pregnancy affects the severity of previously inflamed areas but does not alter healthy gingiva. Impressions of increased incidence may be created by the aggravation of previously inflamed but unnoticed areas. Tooth mobility, pocket depth, and gingival fluid are also increased in pregnancy.115,140,195 The severity of gingivitis is increased during pregnancy beginning in the second or third month. Patients with mild chronic gingivitis that attracted no particular attention before the pregnancy become aware of the gingiva because previously inflamed areas become enlarged, edematous, and more notably discolored (Figure 27-5, A to C).
Gingivitis becomes more severe by the eighth month and decreases during the ninth month of pregnancy.143 Plaque accumulation follows a similar pattern. Some investigators report that the greatest severity is between the second and third trimesters.51 The correlation between gingivitis and the quantity of plaque is greater after parturition than during pregnancy, which suggests that pregnancy introduces other factors that aggravate the gingival response to local factors.132
Partial reduction in the severity of gingivitis occurs by 2 months postpartum, and after 1 year the condition of the gingiva is comparable to that of patients who have not been pregnant.51 Tooth mobility, pocket depth, and gingival fluid are also reduced after pregnancy. In a longitudinal investigation of the periodontal changes during pregnancy and for 15 months postpartum, no significant loss of attachment was observed.51
Pronounced ease of bleeding is the most striking clinical feature. The gingiva is inflamed and varies in color from a bright red to bluish red.262,263 The marginal and interdental gingivae are edematous, pit on pressure, appear smooth and shiny, are soft and pliable, and sometimes present a raspberry-like appearance. The extreme redness results from marked vascularity, and there is an increased tendency to bleed. The gingival changes are usually painless unless complicated by acute infection. In some cases the inflamed gingiva forms discrete “tumorlike” masses, referred to as pregnancy tumors (Figure 27-5, D). Microscopically, gingival disease in pregnancy appears as nonspecific, vascularizing, and proliferative inflammation.150,263 Marked inflammatory cellular infiltration occurs, with edema and degeneration of the gingival epithelium and connective tissue. The epithelium is hyperplastic, with accentuated rete pegs, reduced surface keratinization, and various degrees of intracellular and extracellular edema and infiltration by leukocytes.238 Newly formed engorged capillaries are present in abundance.
The possibility that bacterial-hormonal interactions may change the composition of plaque and lead to gingival inflammation has not been extensively explored. Kornman and Loesche133 reported that the subgingival flora changes to a more anaerobic flora as pregnancy progresses. P. intermedia appears to be the only microorganism that increases significantly during pregnancy. This increase appears to be associated with elevations in systemic levels of estradiol and progesterone and to coincide with the peak in gingival bleeding. It has also been suggested that during pregnancy, a depression of the maternal T-lymphocyte response may be a factor in the altered tissue response to plaque.177
The aggravation of gingivitis in pregnancy has been attributed principally to the increased levels of progesterone, which produce dilation and tortuosity of the gingival microvasculature, circulatory stasis, and increased susceptibility to mechanical irritation, all of which favor leakage of fluid into the perivascular tissues.170,176 A marked increase in estrogen and progesterone occurs during pregnancy, with a reduction after parturition. Animal studies with radioactive estradiol have demonstrated that the gingiva is a target organ for female sex hormones.80 The severity of gingivitis varies with the hormonal levels in pregnancy.115
It has also been suggested that the accentuation of gingivitis in pregnancy occurs in two peaks: during the first trimester, when there is overproduction of gonadotropins, and during the third trimester, when estrogen and progesterone levels are highest.143 Destruction of gingival mast cells by the increased sex hormones and the resultant release of histamine and proteolytic enzymes may also contribute to the exaggerated inflammatory response to local factors.142
Hormonal Contraceptives
Hormonal contraceptives aggravate the gingival response to local factors in a manner similar to that seen in pregnancy and when taken for more than 1.5 years, increase periodontal destruction.67,131,141 Although some brands of oral contraceptives produce more dramatic changes than others, no correlation has been found to exist on the basis of differences in progesterone or estrogen content in various brands.150,189 Cumulative exposure to oral contraceptives apparently has no effect on gingival inflammation or oral debris index scores.123
Menopause
During menopause the usual rhythmic hormonal fluctuations of the female cycle are ended as estradiol ceases to be the major circulating estrogen.164 As a result, females can develop a gingivostomatitis. This condition occurs during menopause or in the postmenopausal period. Mild signs and symptoms sometimes appear, associated with the earliest menopausal changes. Menopausal gingivostomatitis is not a common condition. The term used for its designation has led to the erroneous impression that it invariably occurs associated with menopause, whereas the opposite is true. Oral disturbances are not a common feature of menopause.254
The gingiva and remaining oral mucosa are dry and shiny, vary in color from abnormal paleness to redness, and bleed easily. Fissuring occurs in the mucobuccal fold in some women, and comparable changes may occur in the vaginal mucosa.199 Microscopically, the gingiva exhibits atrophy of the germinal and prickle cell layers of the epithelium and, in some patients, areas of ulceration. The patient complains of a dry, burning sensation throughout the oral cavity, associated with extreme sensitivity to thermal changes; abnormal taste sensations described as “salty,” “peppery,” or “sour”; and difficulty with removable partial prostheses.160
The signs and symptoms of menopausal gingivostomatitis are somewhat comparable to those of chronic desquamative gingivitis (see Chapters 12 and 39). Signs and symptoms similar to those of menopausal gingivostomatitis occasionally occur after ovariectomy or sterilization by radiation in the treatment of malignant neoplasms.
Hyperparathyroidism
Parathyroid hypersecretion produces generalized demineralization of the skeleton, increased osteoclasis with proliferation of the connective tissue in the enlarged marrow spaces, and formation of bone cysts and giant cell tumors.249 The disease is called osteitis fibrosa cystica, or von Recklinghausen’s bone disease. Loss of the lamina dura and giant cell tumors in the jaws are late signs of hyperparathyroid bone disease, which in itself is uncommon. Complete loss of the lamina dura does not occur often, and clinicians may attach too much diagnostic significance to it. Loss of lamina dura may also occur in Paget’s disease, fibrous dysplasia, and osteomalacia.
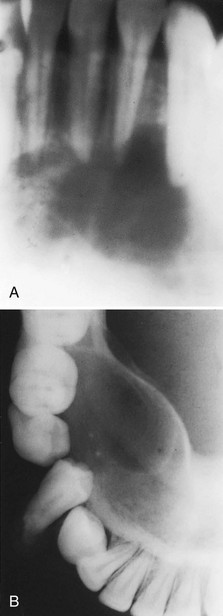
Reports have suggested that 25% to 50% of patients with hyperparathyroidism have associated oral changes.202,220,223 These changes include malocclusion and tooth mobility, radiographic evidence of alveolar osteoporosis with closely meshed trabeculae, widening of the periodontal ligament space, absence of the lamina dura (Figure 27-6), and radiolucent cystlike spaces (Figure 27-7). Bone cysts become filled with fibrous tissue with abundant hemosiderin-laden macrophages and giant cells. These cysts have been called brown tumors, but they are not tumors. More accurately, these cysts are reparative giant cell granulomas. In some cases these lesions appear in the periapical region of teeth and can lead to a misdiagnosis of a lesion of endodontic origin.145 A relationship has been suggested between periodontal disease in dogs and hyperparathyroidism secondary to calcium deficiency in the diet,110 but this has not been confirmed by other studies.225
Hematologic Disorders and Immune Deficiencies
All blood cells play an essential role in the maintenance of a healthy periodontium. White blood cells (WBCs) are involved in inflammatory reactions and are responsible for cellular defense against microorganisms as well as for proinflammatory cytokine release. Red blood cells (RBCs) are responsible for gas exchange and nutrient supply to the periodontal tissues and platelets and are necessary for normal hemostasis, as well as recruitment of cells during inflammation and wound healing. Consequently, disorders of any blood cells or blood-forming organs can have a profound effect on the periodontium.
Certain oral changes, such as hemorrhage, may suggest the existence of a blood dyscrasia. However, a specific diagnosis requires a complete physical examination and a thorough hematologic study. Comparable oral changes occur in more than one form of blood dyscrasia, and secondary inflammatory changes produce a wide range of variation in the oral signs.
Gingival and periodontal disturbances associated with blood dyscrasias must be viewed in terms of fundamental interrelationships between the oral tissues and the blood cells and blood-forming organs rather than in terms of a simple association of dramatic oral changes with hematologic disease. Hemorrhagic tendencies occur when the normal hemostatic mechanisms are disturbed. Abnormal bleeding from the gingiva or other areas of the oral mucosa that is difficult to control is an important clinical sign suggesting a hematologic disorder. Petechiae (Figure 27-8) and ecchymosis (Figure 27-9) observed most often in the soft palate area are signs of an underlying bleeding disorder. It is essential to diagnose the specific etiology to appropriately address any bleeding or immunological disorder.
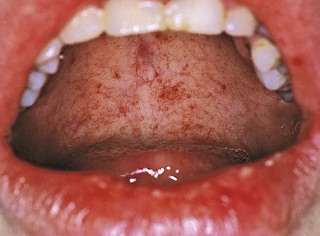
Figure 27-8 Petechiae evident on the soft palate of patient with underlying bleeding disorder (thrombocytopenia).
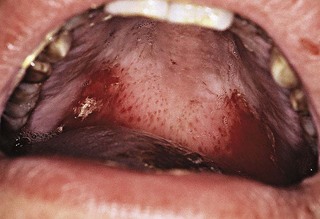
Figure 27-9 Ecchymosis evident on the lateral aspects of the soft palate and tonsillar pillars of patient with chemotherapy-induced thrombocytopenia.
Deficiencies in the host immune response may lead to severely destructive periodontal lesions. These deficiencies may be primary (inherited) or secondary (acquired), caused by immunosuppressive drug therapy, or pathologic destruction of the lymphoid system. Leukemia, Hodgkin’s disease, lymphomas, and multiple myeloma may result in secondary immunodeficiency disorders. This section discusses common hematologic and certain non–HIV/AIDS-related immunodeficiency disorders (see Chapter 19 for a detailed discussion of the HIV-infected patient).
Leukocyte (Neutrophil) Disorders
Disorders that affect production or function of leukocytes may result in severe periodontal destruction. The PMN (neutrophil) in particular plays a critical role in bacterial infections because PMNs are the first line of defense (see Chapter 25). Quantitative deficiency of leukocytes (neutropenia, agranulocytosis) are typically associated with a more generalized periodontal destruction affecting all teeth.
Neutropenia
Neutropenia is a blood disorder that results in low levels of circulating neutrophils. it is a serious condition that may be caused by diseases, medications, chemicals, infections, idiopathic conditions, or hereditary disorders. It may be chronic or cyclic, severe, or benign. It affects as many as one in three patients receiving chemotherapy for cancer. An absolute neutrophil count (ANC) of 1000 to 1500 cells/µl is diagnostic for mild neutropenia. An ANC of 500 to 1000 cells per microliter is considered moderate neutropenia and an ANC less than 500 cells/µl is a severe neutropenia. Infections are sometimes difficult to manage and may be life threatening, particularly in severe neutropenia.
Agranulocytosis
Agranulocytosis is a more severe neutropenia involving not only the neutrophil but also basophils and eosinophils. It is defined as an ANC of less than 100 cells/µl. It is characterized by a reduction in the number of circulating granulocytes and results in severe infections, including ulcerative necrotizing lesions of the oral mucosa, skin, and gastrointestinal and genitourinary tracts. Less severe forms of the disease are called neutropenia or granulocytopenia.
Drug idiosyncrasy is the most common cause of agranulocytosis, but in some cases, its cause cannot be explained. Agranulocytosis has been reported after the administration of drugs such as aminopyrine, barbiturates and their derivatives, benzene ring derivatives, sulfonamides, gold salts, or arsenical agents.134,149,166,194 It generally occurs as an acute disease. It may be chronic or periodic with recurring neutropenic cycles (e.g., cyclic neutropenia).231
The onset of disease is accompanied by fever, malaise, general weakness, and sore throat. Ulceration in the oral cavity, oropharynx, and throat is characteristic. The mucosa exhibits isolated necrotic patches that are black and gray and are sharply demarcated from the adjacent uninvolved areas.126,153 The absence of a notable inflammatory reaction caused by lack of granulocytes is a striking feature. The gingival margin may or may not be involved. Gingival hemorrhage, necrosis, increased salivation, and fetid odor are accompanying clinical features. In cyclic neutropenia, the gingival changes recur with recurrent exacerbation of the disease.49 The occurrence of generalized aggressive periodontitis has been described in patients with cyclic neutropenia215 (Figure 27-10).
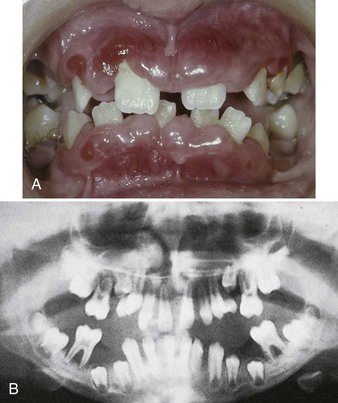
Figure 27-10 Aggressive periodontitis in 10-year-old male with cyclic neutropenia and agammaglobulinemia. A, Clinical presentation of periodontal condition. Note the severe swelling and inflammation of marginal and papillary gingiva. There is gross migration of teeth caused by loss of bone support. B, Panoramic radiograph demonstrating severe bone loss around all permanent teeth that have erupted into the oral cavity.
Because infection is a common feature of agranulocytosis, differential diagnosis involves consideration of such conditions as necrotizing ulcerative gingivitis, noma, acute necrotizing inflammation of the tonsils, and diphtheria. Definitive diagnosis depends on the hematologic findings of pronounced leukopenia and almost complete absence of neutrophils.
Leukemia is an important disease to understand and appreciate because of the seriousness of the disease and the periodontal manifestations. The leukemias are malignant neoplasias of WBC precursors characterized by (1) diffuse replacement of the bone marrow with proliferating leukemic cells, (2) abnormal numbers and forms of immature WBCs in the circulating blood, and (3) widespread infiltrates in the liver, spleen, lymph nodes, and other body sites.200
According to the cell type involved, leukemias are classified as lymphocytic or myelogenous. A subgroup of the myelogenous leukemias is monocytic leukemia. The term lymphocytic indicates that the malignant change occurs in cells that normally form lymphocytes. The term myelogenous indicates that the malignant change occurs in cells that normally form RBCs, some types of WBCs and platelets. According to their evolution, leukemias can be acute, which is rapidly fatal, subacute, or chronic. In acute leukemia, the primitive “blast” cells released into the peripheral circulation are immature and nonfunctional, whereas in chronic leukemia the abnormal cells tend to be more mature with normal morphologic characteristics and function when released into the circulation.
All leukemias tend to displace normal components of the bone marrow elements with leukemic cells, resulting in reduced production of normal RBCs, WBCs, and platelets, which leads to anemia, leukopenia (reduction in number of nonmalignant WBCs) and thrombocytopenia. Anemia results in poor tissue oxygenation, making tissues more friable and susceptible to breakdown. A reduction of normal WBCs in the circulation leads to a poor cellular defense and an increased susceptibility to infections. Thrombocytopenia leads to bleeding tendency, which can occur in any tissue but in particular affects the oral cavity, especially the gingival sulcus (Figure 27-11). Some patients may have normal blood counts while leukemic cells reside primarily in the bone marrow. This type of disease is called aleukemic leukemia.97
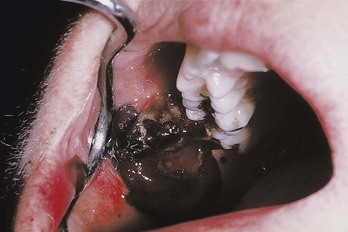
Figure 27-11 Spontaneous bleeding from the gingival sulcus in patient with thrombocytopenia. Normal coagulation is evident by the appearance of the large clot that forms in the mouth. However, platelets are inadequate to establish hemostasis at the site of hemorrhage.
The Periodontium in Leukemic Patients
Oral and periodontal manifestations of leukemia may include leukemic infiltration, bleeding, oral ulcerations, and infections. The expression of these signs is more common in acute and subacute forms of leukemia than in chronic forms.
Leukemic Infiltration
Leukemic cells can infiltrate the gingiva and less frequently the alveolar bone. Gingival infiltration often results in leukemic gingival enlargement (see Chapter 9).
A study of 1076 adult patients with leukemia showed that 3.6% of the patients with teeth had leukemic gingival proliferative lesions, with the highest incidence in patients with acute monocytic leukemia (66.7%), followed by acute myelocytic-monocytic leukemia (18.7%), and acute myelocytic leukemia (3.7%).63 It should be noted, however, that monocytic leukemia is an extremely rare form of the disease. Leukemic gingival enlargement is not found in edentulous patients or in patients with chronic leukemia, suggesting that it is the accumulation of immature leukemic blast cells in the gingiva adjacent to tooth surfaces with bacterial plaque. Leukemic gingival enlargement consists of a basic infiltration of the gingival corium by leukemic cells that increases the gingival thickness and creates gingival pockets in which bacterial plaque accumulates, initiating a secondary inflammatory lesion that contributes to the enlargement of the gingiva. It may be localized to the interdental papilla area (Figure 27-12) or expand to include the marginal gingiva and partially cover the crowns of the teeth (Figure 27-13, C and D). Clinically, the gingiva appears bluish red and cyanotic, with a rounding and tenseness of the gingival margin. The abnormal accumulation of leukemic cells in the dermal and subcutaneous connective tissue is called leukemia cutis and forms elevated and flat macules and papules63,200 (Figure 27-13, A and B).
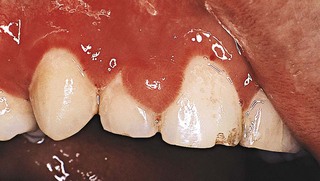
Figure 27-12 Leukemic infiltration causing localized gingival swelling of the interdental papillae between the maxillary lateral and central incisor. Note the tense induration of the area.
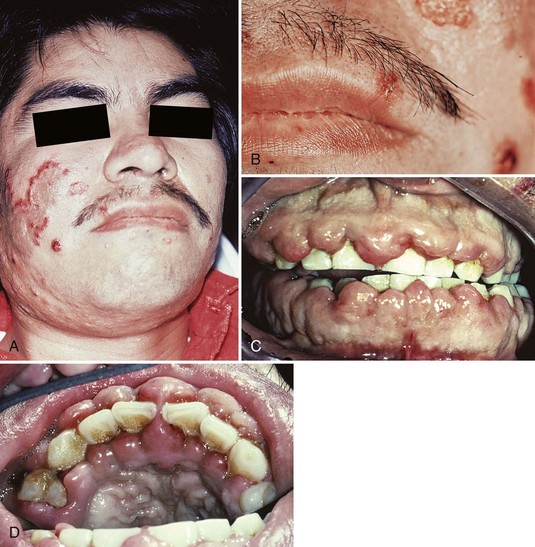
Figure 27-13 Adult male with acute myelocytic leukemia. A, View of patient’s face. Note the elevated, flat macules and papules (leukemia cutis) on the right cheek. B, Close-up view of skin lesions. C, Intraoral view showing pronounced gingival enlargements of the entire gingival margin and interdental papilla areas of both arches. D, Occlusal view of maxillary anterior teeth. Note the marked enlargement in both the facial and the palatal aspects.
(Courtesy Dr. Spencer Woolfe, Dublin, Ireland.)
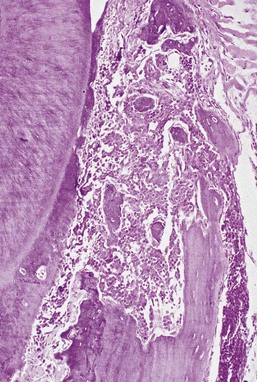
Microscopically, the gingiva exhibits a dense, diffuse infiltration of predominantly immature leukocytes in the attached and marginal gingiva. Occasionally, mitotic figures indicative of ectopic hematopoiesis may be seen. The normal connective tissue components of the gingiva are displaced by the leukemic cells (Figure 27-14). The nature of the cells depends on the type of leukemia. The cellular accumulation is denser in the entire reticular connective tissue layer. In almost all cases the papillary layer contains comparatively few leukocytes. The blood vessels are distended and contain predominantly leukemic cells, and the RBCs are reduced in number. The epithelium presents a variety of changes and may be thinned or hyperplastic. Common findings include degeneration associated with intercellular and intracellular edema and leukocytic infiltration with diminished surface keratinization.
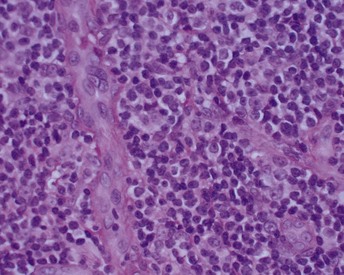
Figure 27-14 Human histologic appearance of leukemic infiltrate with dense, diffuse infiltration of predominantly immature leukocytes. The normal connective tissue components of the gingiva are displaced by the leukemic cells. The cellular accumulation is denser in the entire reticular connective tissue layer.
(Courtesy of Dr. Russell Christensen, University of California, Los Angeles.)
The microscopic picture of the marginal gingiva differs from that of other gingival locations in that it usually exhibits a notable inflammatory component, in addition to the leukemic cells. Scattered foci of plasma cells and lymphocytes with edema and degeneration are common findings. The inner aspect of the marginal gingiva is usually ulcerated, and marginal necrosis with pseudomembrane formation may also be seen.
The periodontal ligament and alveolar bone may also be involved in acute and subacute leukemia. The periodontal ligament may be infiltrated with mature and immature leukocytes. The marrow of the alveolar bone exhibits a variety of changes, such as localized areas of necrosis, thrombosis of the blood vessels, infiltration with mature and immature leukocytes, occasional RBCs, and replacement of the fatty marrow by fibrous tissue.
In leukemic mice the presence of infiltrate in marrow spaces and the periodontal ligament results in osteoporosis of the alveolar bone with destruction of the supporting bone and disappearance of the periodontal fibers31,39 (Figure 27-15).
Bleeding
Gingival hemorrhage is a common finding in leukemic patients (see Figure 27-11), even in the absence of clinically detectable gingivitis. Bleeding gingiva can be an early sign of leukemia. It is caused by the thrombocytopenia resulting from replacement of the bone marrow cells by leukemic cells and from the inhibition of normal stem cell function by leukemic cells or their products.200 This bleeding tendency can also manifest in the skin and throughout the oral mucosa, where petechiae are often found, with or without leukemic infiltrates. A more diffuse submucosal bleeding manifests as ecchymosis (see Figure 27-9). Oral bleeding has been reported as a presenting sign in 17.7% of patients with acute leukemia and in 4.4% of patients with chronic leukemia.148 Bleeding may also be a side effect of the chemotherapeutic agents used to treat leukemia.
Oral Ulceration and Infection
In leukemia, the response to bacterial plaque or other local irritation is altered. The cellular component of the inflammatory exudate differs both quantitatively and qualitatively from that in nonleukemic individuals in that there is a pronounced infiltration of immature leukemic cells in addition to the usual inflammatory cells. As a result, the normal inflammatory response may be diminished.
Granulocytopenia (diminished WBC count) results from the displacement of normal bone marrow cells by leukemic cells, which increases the host susceptibility to opportunistic microorganisms and leads to ulcerations and infections. Discrete, punched-out ulcers penetrating deeply into the submucosa and covered by a firmly attached white slough can be found on the oral mucosa.15 These lesions occur in sites of trauma such as the buccal mucosa in relation to the line of occlusion or on the palate. Patients with past history of herpesvirus infection may develop recurrent herpetic oral ulcers, often in multiple sites, and large atypical forms, especially after chemotherapy is instituted102 (Figure 27-16).
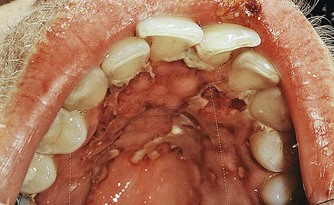
Figure 27-16 Large ulcerations on the palate of patient with granulocytopenia secondary to leukemia. These atypical ulcerations are caused by herpesvirus opportunistic infection. Notice the smaller, discrete, round ulcerations that have coalesced into the larger lesion.
A gingival (bacterial) infection in leukemic patients can be the result of an exogenous bacterial infection or an existing bacterial infection (e.g., gingival or periodontal disease). Acute gingivitis and lesions resembling necrotizing ulcerative gingivitis are more frequent and severe in terminal cases of acute leukemia21 (Figures 27-17 and 27-18). The inflamed gingiva in patients with leukemia differs clinically from that in nonleukemic individuals. Gingiva is a peculiar bluish red, is spongelike and friable, and bleeds persistently on the slightest provocation or even spontaneously in leukemic patients. This greatly altered and degenerated tissue is extremely susceptible to bacterial infection, which can be so severe as to cause acute gingival necrosis with pseudomembrane formation (Figure 27-19) or bone exposure (Figure 27-20). These are secondary oral changes superimposed on the oral tissues altered by the blood dyscrasia. They produce associated disturbances that may be a source of considerable difficulty to the patient, such as systemic toxic effects, loss of appetite, nausea, blood loss from persistent gingival bleeding, and constant gnawing pain. Eliminating or reducing local factors (e.g., bacterial plaque) can minimize severe oral changes in leukemia. In some patients with severe acute leukemia, symptoms may only be relieved by treatment that leads to remission of the disease.
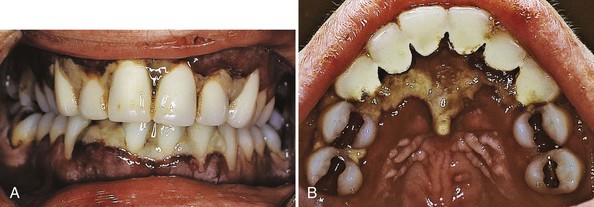
Figure 27-17 Adult female with acute myelocytic leukemia. A, Anterior view of patient with acute myelocytic leukemia. Interdental papillae are necrotic with a highly inflamed and swollen gingival tissue at the base of the lesions. B, Palatal view demonstrating extensive necrosis of interdental and palatal tissues behind the maxillary incisors.
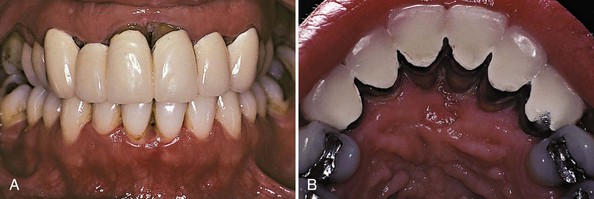
Figure 27-18 Same patient as in Figure 27-17 after chemotherapy that resulted in remission of leukemia. A, Anterior view reveals dramatic improvement in gingival health following remission of leukemia. Note the loss of interdental papillae as well as gingival recession in the anterior areas. B, Palatal view shows extensive loss of gingival tissue around maxillary incisors.
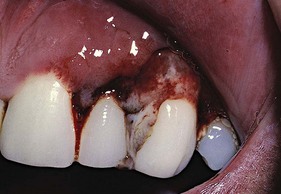
Figure 27-19 Opportunistic bacterial infection of gingiva in patient with hospitalized with leukemia. Gingival tissue is highly inflamed, bleeding, and necrotic with pseudomembrane formation.
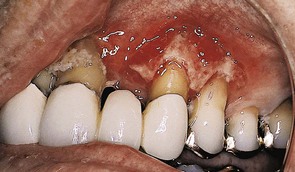
Figure 27-20 Opportunistic bacterial infection in immunosuppressed patient caused complete destruction of gingiva, exposing underlying alveolar bone.
In chronic leukemia, oral changes suggesting a hematologic disturbance are rare. The microscopic changes in chronic leukemia may consist of replacing the normal fatty marrow of the jaws with islands of mature lymphocytes or lymphocytic infiltration of the marginal gingiva without dramatic clinical manifestations.
The existence of leukemia is sometimes revealed by a gingival biopsy performed to clarify the nature of a troublesome gingival condition. In such cases the gingival findings must be corroborated by medical examination and hematologic study. In patients with diagnosed leukemia, gingival biopsy may indicate the extent to which leukemic infiltration is responsible for the altered clinical appearance of the gingiva. Although such findings are of interest, their benefit to the patient is insufficient to warrant routine gingival biopsy studies in patients with leukemia. Furthermore, it is important to note that the absence of leukemic involvement in a gingival biopsy specimen does not rule out the possibility of leukemia. A gingival biopsy in a patient with chronic leukemia may reveal typical gingival inflammation without any suggestion of a hematologic disturbance.
Anemia
Anemia is a deficiency in the quantity or quality of the blood, as manifested by a reduction in the number of erythrocytes and in the amount of hemoglobin. Anemia may be the result of blood loss, defective blood formation, or increased RBC destruction. Anemias are classified according to cellular morphology and hemoglobin content as (1) macrocytic hyperchromic anemia (pernicious anemia), (2) microcytic hypochromic anemia (iron deficiency anemia), (3) sickle cell anemia, and (4) normocytic-normochromic anemia (hemolytic or aplastic anemia).
Pernicious anemia results in tongue changes in 75% of patients. The tongue appears red, smooth, and shiny because of atrophy of the papillae (Figure 27-21). There is also marked pallor of the gingiva (Figure 27-22). Iron deficiency anemia induces similar tongue and gingival changes. A syndrome consisting of glossitis and ulceration of the oral mucosa and oropharynx, inducing dysphagia (Plummer-Vinson syndrome) has been described in patients with iron deficiency anemia. Sickle cell anemia is a hereditary form of chronic hemolytic anemia that occurs almost exclusively in blacks. It is characterized by pallor, jaundice, weakness, rheumatoid manifestations, and leg ulcers. Oral changes include generalized osteoporosis of the jaws, with a peculiar stepladder alignment of the trabeculae of the interdental septa, along with pallor and yellowish discoloration of the oral mucosa. Periodontal infections may precipitate sickle cell crisis.194 Aplastic anemia results from a failure of the bone marrow to produce erythrocytes. The etiology is usually the effect of toxic drugs on the marrow or displacement of RBCs by leukemic cells. Oral changes include pale discoloration of the oral mucosa and increased susceptibility to infection because of the concomitant neutropenia.
Thrombocytopenia
Thrombocytopenia is a term used to describe the condition of reduced platelet count resulting from either lack of platelet production or increased loss of platelets. Purpura refers to the purplish appearance of the skin or mucous membranes where bleeding has occurred as a result of decreased platelets. Thrombocytopenic purpura may be idiopathic (i.e., of unknown etiology, as in Werlhof’s disease) or may occur secondary to some known etiologic factor responsible for a reduced amount of functioning marrow and a resultant reduction in the number of circulating platelets. Such etiologic factors include aplasia of the marrow; displacement of the megakaryocytes in the marrow, as in leukemia; replacement of the marrow by tumor; and destruction of the marrow by irradiation or radium or by drugs such as benzene, aminopyrine, and arsenical agents.
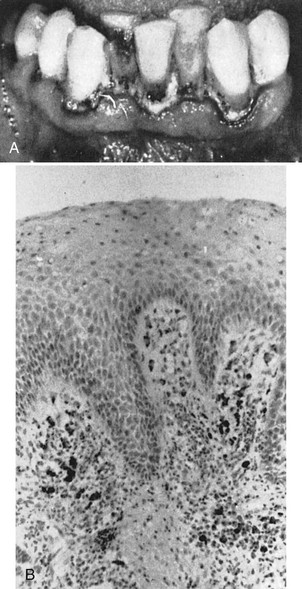
Thrombocytopenic purpura is characterized by a low platelet count, a prolonged clot retraction and bleeding time, and a normal or slightly prolonged clotting time. There is spontaneous bleeding into the skin or from mucous membranes. Petechiae and hemorrhagic vesicles occur in the oral cavity, particularly in the palate, tonsillar pillars, and the buccal mucosa. The gingivae are swollen, soft, and friable. Bleeding occurs spontaneously or on the slightest provocation and is difficult to control. Gingival changes represent an abnormal response to local irritation. The severity of the gingival condition is dramatically alleviated by removal of the local factors (Figure 27-23).
Antibody Deficiency Disorders
Agammaglobulinemia
Agammaglobulinemia, or hypogammaglobulinemia, is an immune deficiency resulting from inadequate antibody production caused by a deficiency in B cells. It can be congenital (X-linked or Bruton’s agammaglobulinemia) or acquired (common variable immunodeficiency).
Congenital agammaglobulinemia is caused by an X-linked, recessive gene (Bruton’s tyrosine kinase). It affects approximately 1 : 100,000 population. Because the defect is recessive and linked to the X chromosome, only males have the disease. The gene is responsible for B-cell development. In the absence of mature B cells, patients lack lymphoid tissue and fail to develop plasma cells. Thus production of antibodies is deficient. Germinal centers where B cells proliferate and differentiate are poorly developed in all lymphoid tissues. Tonsils, adenoids, and peripheral lymph nodes are small or absent.
Acquired or late-onset agammaglobulinemia is most often known as common variable immunodeficiency disease (CVID). The disorder is characterized by the onset of recurrent bacterial infections in the second and third decades of life, resulting from drastic decrease in immunoglobulin and antibody levels. The basic immunologic defect in CVID is failure of B-lymphocyte differentiation into plasma cells. In contrast to patients with the X-linked form of the disease, patients with CVID typically have an enlarged spleen and swollen glands or lymph nodes. Along with other autoimmune problems, some patients develop autoantibodies against their blood cells. Causes of the disease are unknown. Unlike the X-linked early-onset form of the disease, CVID is not genetic, and both males and females are susceptible.
T-cell function remains normal in agammaglobulinemia. The disease (congenital or acquired) is characterized by recurrent bacterial infections, especially ear, sinus, and lung infections. Patients are also susceptible to periodontal infections. Aggressive periodontitis is a common finding in children diagnosed with agammaglobulinemia (see Figure 27-10).
Genetic Disorders
Many systemic conditions associated with or predisposing to periodontal destruction include genetic disorders that result in an inadequate number or function of circulating neutrophils. This underscores the importance of the neutrophil in the protection of the periodontium against infection. Severe periodontitis has been observed in individuals with primary neutrophil disorders such as neutropenia, agranulocytosis, Chédiak-Higashi syndrome, and lazy leukocyte syndrome. In addition, severe periodontitis has also been observed in individuals who exhibit secondary neutrophil impairment, as seen in Down syndrome, Papillon-Lefèvre syndrome, and inflammatory bowel disease. This section discusses conditions with primary and secondary leukocyte disorders.
Chédiak-Higashi Syndrome
Chédiak-Higashi syndrome is a rare disease that affects the production of organelles found in almost every cell. It affects mostly the melanocytes, platelets, and phagocytes. It causes partial albinism, mild bleeding disorders, and recurrent bacterial infections. Neutrophils contain abnormal, giant lysosomes that can fuse with the phagosome, but their ability to release their contents is impaired. As a result, killing of ingested microorganisms is delayed. Patients with Chédiak-Higashi syndrome are susceptible to repeated infections that can be serious and life-threatening. Aggressive periodontitis has been described in these patients. Chédiak-Higashi syndrome has been described as a genetically transmitted disease in ranch-raised mink11,185 (see Chapter 24).
Lazy Leukocyte Syndrome
Lazy leukocyte syndrome is characterized by susceptibility to severe microbial infections, neutropenia, defective chemotactic response by neutrophils, and an abnormal inflammatory response.188 Individuals diagnosed with lazy leukocyte syndrome are susceptible to aggressive periodontitis with destruction of bone and early tooth loss.
Leukocyte Adhesion Deficiency
Leukocyte adhesion deficiency (LAD) is a very rare genetic disorder. Only a few hundred cases have been diagnosed. Because LAD is an inherited disease, it is categorized as a primary immunodeficiency most often diagnosed at birth. Many children do not survive.
LAD results from the inability to produce or failure to normally express an important cell surface integrin (CD18), which is necessary for leukocytes to adhere to the vessel wall at the site of infection. When leukocytes cannot effectively adhere to the vessel wall near the site of infection, they cannot migrate to the infection. As a result, bacterial infections are able to continue to destroy host tissues unimpeded by the normal host immune response. Infections act similarly to those observed in neutropenic patients because phagocytes are unable to reach the site of infection.
Cases of periodontal disease attributed to LAD are rare. They begin during or immediately after eruption of the primary teeth. Extremely acute inflammation and proliferation of the gingival tissues with rapid destruction of bone are found. Profound defects in peripheral blood neutrophils and monocytes and an absence of neutrophils in the gingival tissues have been noted in patients with LAD.184,185 These patients also have frequent respiratory tract infections and sometimes otitis media. Both primary and permanent teeth are affected, often resulting in early tooth loss.247
Papillon-Lefèvre Syndrome
Papillon-Lefèvre syndrome, first described by French physicians Papillon and Lefèvre,186 is a very rare inherited condition that appears to follow an autosomal recessive pattern. Parents are not affected, and both must carry the autosomal genes for the syndrome to appear in the offspring. It may occur in siblings and has no gender predilection. The estimated frequency is 1 to 4 cases per 1 million individuals. Rare cases of adult onset of this syndrome, although with mild periodontal lesions, have also been described.35
The syndrome is characterized by hyperkeratotic skin lesions, severe destruction of the periodontium, and in some cases, calcification of the dura.40,75 The cutaneous and periodontal changes usually appear together between the ages of 2 and 4 years. The skin lesions consist of hyperkeratosis and ichthyosis of localized areas on palms, soles, knees, and elbows (Figure 27-24).
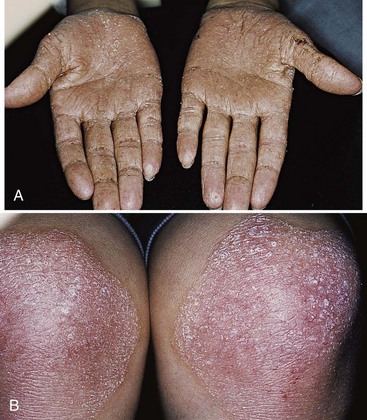
Figure 27-24 Papillon-Lefèvre syndrome in a 17-year-old male. A, Clinical view of palms demonstrating hyperkeratosis of skin. B, Clinical view of knees with hyperkeratotic, scaly lesions.
Periodontal involvement consists of early inflammatory changes that lead to bone loss and exfoliation of teeth. Primary teeth are lost by 5 or 6 years of age. The permanent dentition then erupts normally, but within a few years, the permanent teeth are also lost because of destructive periodontal disease (Figure 27-25). At a very early age, usually 15 to 20 years, patients are often edentulous except for the third molars. These may be lost as well a few years after eruption. Tooth extraction sites heal uneventfully.76
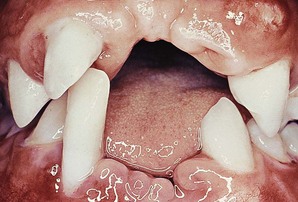
Figure 27-25 Same case as in Figure 27-24. Intraoral clinical view of 17-year-old boy with Papillon-Lefèvre syndrome showing early tooth loss and severe bone loss around remaining teeth as evidenced by gingival recession. The missing teeth were exfoliated.
There are few case reports of successful tooth retention in patients with Papillon-Lefèvre syndrome.235,252 Successful retention of permanent teeth may be associated with timing treatment (antibiotics and extraction of erupted teeth) with severity of syndrome symptoms.252 Extraction of all primary teeth followed by a period of edentulousness may partially explain the lack of recurrent infection. In their review of the literature, Wiebe et al reported that the symptoms of Papillon-Lefèvre syndrome diminish with age and teeth that erupt later may not be lost.252
The microscopic changes reported include marked chronic inflammation of the lateral wall of the pocket with a predominantly plasma cell infiltrate, considerable osteoclastic activity and apparent lack of osteoblastic activity, and an extremely thin cementum.154 Bacterial flora studies of plaque in a patient with Papillon-Lefèvre syndrome revealed a similarity to bacterial flora in chronic periodontitis.146 Spirochete-rich zones in the apical portion of the pockets, as well as spirochete adherence to the cementum and microcolony formation of Mycoplasma species, have been reported in Papillon-Lefèvre syndrome.122 Gram-negative cocci and rods appear at the apical border of plaque.243 Although Schroeder et al214 failed to show defects in peripheral blood neutrophils in a 10-year-old male patient with Papillon-Lefèvre syndrome, others have implicated neutrophil defects as a contributing factor. Firatli et al78 reported depressed chemotaxis of peripheral neutrophils and suggested that it explained the pathogenesis of the Papillon-Lefèvre syndrome. Ghaffar et al found significantly depressed neutrophil function in probands with Papillon-Lefèvre syndrome with respect to phagocytic and lytic activity.89
Down Syndrome
Down syndrome (mongolism, trisomy 21) is a congenital disease caused by a chromosomal abnormality and characterized by mental deficiency and growth retardation. The prevalence of periodontal disease in Down syndrome is high (occurring in almost 100% of patients younger than 30 years).52,59 Although plaque, calculus, and local irritants (e.g., diastemata, crowding of teeth, high frenum attachments, and malocclusion) are present and oral hygiene is poor, the severity of periodontal destruction exceeds that explainable by local factors alone.48,50,52,198,226
Periodontal disease in Down syndrome is characterized by formation of deep periodontal pockets associated with substantial plaque accumulation and moderate gingivitis (Figure 27-26). These findings are usually generalized, although they tend to be more severe in the lower anterior region. Moderate recession is sometimes seen in this region as well. The disease progresses rapidly. The high prevalence and increased severity of periodontal destruction associated with Down syndrome is most likely explained by poor PMN chemotaxis, phagocytosis, and intracellular killing.*
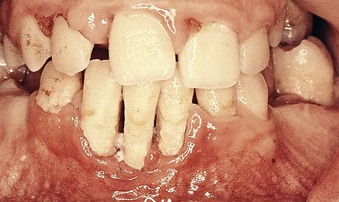
Figure 27-26 Severe periodontal destruction in 14-year-old patient with Down syndrome. Note the extensive loss of periodontal support around the mandibular incisors as evidenced by severe gingival recession. Moderate-heavy bacterial plaque is associated with moderate gingival inflammation in the area.
Stress and Psychosomatic Disorders
Psychologic conditions, particularly psychosocial stress, have been implicated as risk indicators for periodontal disease.85 The most notable example is the documented relationship between stress (e.g., experienced by soldiers at war or by students during examinations) and acute necrotizing ulcerative gingivitis (NUG) (see Chapters 10 and 17). The presence of NUG in soldiers stressed by wartime conditions in the trenches led to one of the early diagnostic terms used to describe this condition, namely “trench mouth.” Despite this well-known association between stress and NUG, confirming the connection between psychologic conditions and other forms of periodontal disease (e.g., chronic periodontitis) has been elusive. These relationships are difficult to elucidate because, as with many common diseases, the etiology and pathogenesis of periodontal disease is multifactorial and the role of individual risk factors is difficult to define.
Some studies have failed to recognize a relationship between psychological conditions and periodontal disease despite specific efforts to identify them. In a study of 80 patients (40 with aggressive periodontitis and 40 with chronic periodontitis), Monteiro da Silva et al failed to find a relationship between psychologic factors and periodontal disease.172 They were able to identify depression and smoking as marginally significant in the aggressive periodontitis group. Their inability to find a relationship may be attributed to a lack of significant differences in psychologic characteristics between the two groups in the study. In an earlier study, the same group identified depression and loneliness as significant factors associated with aggressive periodontal disease in 50 patients compared with 50 periodontally healthy individuals and 50 individuals with chronic periodontitis.171 Another challenge in defining a relationship between psychosocial status and periodontitis is the myriad of confounding factors and the difficulty in controlling for them.65
Psychosocial Stress, Depression, and Coping
Recently, several clinical studies and a systematic review of the subject have documented a positive relationship between psychosocial stress and chronic forms of periodontal disease.190 In case-controlled studies, individuals with stable lifestyles (based on family structure and employment status) and minimal negative life events had less periodontal disease destruction than individuals with less stable lifestyles (e.g., unmarried, unemployed) and more negative life events.56 Interestingly, it is now becoming apparent that the effect is not simply a matter of the presence of stress versus a lack of stress but rather the type of stress as well as the ability of the individual to cope with stress that correlate with destructive periodontal disease.
All individuals experience stress, but these events do not invariably result in destructive periodontitis. The types of stress that lead to periodontal destruction appear to be more chronic or long term and less likely to be controlled by the individual. Life events, such as the loss of a loved one (e.g., spouse or family member), a failed relationship, loss of employment, and financial difficulties, are examples of stressful life events that are typically not controllable by the individual or not perceived by the individual as being under his or her control, rendering the person with a feeling of “helplessness.” The duration of the stressful life event will also have an influence on the total impact of the stress-induced disease destruction.
Financial stress is an example of a long-term, constant pressure that may exacerbate periodontal destruction in susceptible individuals. Genco et al86 found that individuals with high levels of financial stress and poor coping skills had twice as much periodontal disease as those with minimal stress and good coping skills. Psychologic tests were used to identify and weigh the causes of stress, such as children, spouse, finances, single life, and work, and to measure individual coping skills. Individuals with problem-focused (practical) coping skills fared better than individuals with emotion-focused (avoidance) coping skills with respect to periodontal disease. As part of their analysis, the researchers also found that chronic stress and inadequate coping could lead to changes in daily habits, such as poor oral hygiene, clenching, and grinding, as well as physiologic changes such as decreased saliva flow and suppressed immunity.
Comparing 89 patients with periodontal disease to 63 periodontally healthy individuals, Wimmer et al253 found that patients with defensive (emotional) coping skills were more likely to refuse responsibility and downplay their condition. All patients completed a comprehensive stress assessment questionnaire (German language) to evaluate their coping behavior. Patients with periodontal disease were less likely to use “active” coping skills (i.e., situation control) and more likely to cope with stress by averting blame (emotional) than were periodontally healthy individuals.
These studies support the concept that one of the most important aspects related to the influence of stress on periodontal disease destruction is the manner in which the individual copes with the stress. Emotional coping methods appear to render the host more susceptible to the destructive effects of periodontal disease than do practical coping methods. Furthermore, emotional coping is more common in situations that must be accepted and by individuals who feel helpless in the situation.
Stress-Induced Immunosuppression
Stress and psychosomatic disorders most likely impact the periodontal health through changes in the individual’s behavior and through complex interactions among the nervous, endocrine, and immune systems. Individuals under stress may have poorer oral hygiene, may start or increase clenching and grinding of their teeth, and may smoke more frequently. All these behavioral changes increase their susceptibility to periodontal disease destruction. Likewise, individuals under stress may be less likely to seek professional care.
In addition to the many behavioral changes that may influence periodontal disease destruction, psychosocial stress may also impact the disease through alterations in the immune system. The influence of stress on the immune system and systemic health conditions, such as cardiovascular disease, is well known. Stress-related immune system changes clearly have the potential to affect the pathogenesis of periodontal disease as well. One possible mechanism involves the production of cortisol. Stress increases cortisol production from the adrenal cortex by stimulating an increase in the release of adrenocorticotropic hormone (ACTH) from the pituitary gland. Increased cortisol suppresses the immune response directly through suppression of neutrophil activity, IgG production, and salivary IgA secretion. All these immune responses are critical for the normal immunoinflammatory response to periodontal pathogens (see Chapter 21). The resulting “stress-induced” immunosuppression increases the potential for destruction by periodontal pathogens. Stress may also affect the cellular immune response directly through an increased release of neurotransmitters, including epinephrine, norepinephrine, neurokinin, and substance P, which interact directly with lymphocytes, neutrophils, and monocytes/macrophages via receptors causing an increase in their tissue-destructive function. Thus, in a manner similar to cortisol production, the “stress-induced” release of these neurotransmitters results in an upregulated immune response that increases the potential for destruction by the cellular response to periodontal pathogens.
It is important to remember that although stress may predispose an individual to more destruction from periodontitis, the presence of periodontal pathogens remains as the essential etiologic factor (i.e., stress alone does not cause or lead to periodontitis in the absence of periodontal pathogens).
Influence of Stress on Periodontal Therapy Outcomes
Psychologic conditions, such as stress and depression, may also influence the outcome of periodontal therapy. In a large-scale retrospective study of 1299 dental records from a health maintenance organization (HMO) database, 85 individuals with depression had posttherapy outcomes that were less favorable (below median) compared to those without depression.70 More than half (697) were complete enough for a comprehensive evaluation, including both periodontal diagnosis and psychologic profiles. The authors concluded that depression might have a negative effect on periodontal treatment outcomes.
A recent study investigating the relationship between psychologic stress and wound repair in patients after routine surgery (inguinal hernia open incision repair) revealed that stress impairs the inflammatory response and matrix degradation.30 Forty-seven adults were given a standardized questionnaire before surgery to assess their psychologic stress before surgery. Wound fluids were collected over the first 20 hours after surgery to measure inflammatory markers (interleukin-1 [IL-1], IL-6, and matrix metalloproteinase-9 [MMP-9]). Greater psychologic stress was significantly associated with lower levels of IL-1 and MMP-9, as well as significantly more painful, poorer, and slower recovery.
Another study compared the psychiatric characteristics of individuals with different outcomes to periodontal therapy.10 Two groups were compared to evaluate the psychologic characteristics of 11 individuals who were responsive to periodontal treatment compared with 11 individuals who were not responsive to periodontal treatment. The responsive group had a more rigid personality, whereas the nonresponsive group had a more passive, dependent personality. Furthermore, the nonresponsive group reported more stressful life events in their past.
These studies suggest that both stressful life events and the individual’s personality and coping skills are factors to consider in assessing the risk of periodontal disease destruction and the potential for successful periodontal therapy. If patients are identified with emotional or defensive coping skills, care should be taken to ensure that they receive information in a manner that does not elicit a “defensive” reaction.
Psychiatric Influence of Self-Inflicted Injury
Psychosomatic disorders may result in harmful effects to the health of tissues in the oral cavity through the development of habits that are injurious to the periodontium. Neurotic habits, such as grinding or clenching the teeth, nibbling on foreign objects (e.g., pencils, pipes), nail biting, and excessive use of tobacco, are all potentially injurious to the teeth and the periodontium. Self-inflicted gingival injuries, such as gingival recession, have been described in both children and adults (Figure 27-27). However, these types of self-inflicted, factitious injuries do not appear to be common in psychiatric patients.209
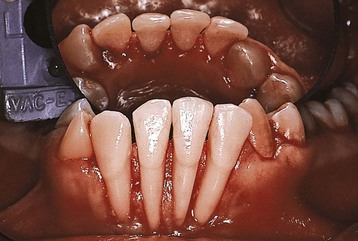
Figure 27-27 Severe gingival recession localized to the labial surface of all mandibular incisors. This finding was discovered under general anesthesia in an uncooperative, institutionalized adult with mental disorders. The patient was known to pace around the home with all four fingers inside his lower lip.
Nutritional Influences
Some clinicians enthusiastically adhere to the theory in periodontal disease that assigns a key role to nutritional deficiencies and imbalances. Previous research did not support this view, but numerous problems in experimental design and data interpretation could be responsible for making these research findings inadequate.5,208 The majority of opinions and research findings regarding the effects of nutrition on oral and periodontal tissues point to the following:
The role of nutrition in periodontal disease may be related to the effect of nutrition on inflammation. A recent review of the literature evaluating the effect of nutritional factors on inflammation demonstrated that subtle shifts in nutritional status are associated with the prevalence of periodontitis.43 More specifically, they reported that the results of contemporary animal and human studies have demonstrated the role of specific micronutrients in the modulation of the host’s inflammatory response by reducing inflammatory biomarkers, which may in turn be responsible for periodontal destruction. The evidence for the effect of nutrition on inflammation is significant. Data suggest that diets containing foods rich in antioxidants are beneficial, whereas foods containing high levels of refined carbohydrates are detrimental to the inflammatory process.43
Fat-Soluble Vitamin Deficiency
Vitamins A, D, and E are fat-soluble vitamins required in the human diet.
Vitamin A Deficiency
A major function of vitamin A is to maintain the health of epithelial cells of the skin and mucous membranes. Deficiency of vitamin A results in dermatologic, mucosal, and ocular manifestations. In the absence of vitamin A, degenerative changes occur in epithelial tissues, resulting in a keratinizing metaplasia. Because epithelial tissues provide a primary barrier function to protect against invading microorganisms, vitamin A may play an important role in protecting against microbial invasion by maintaining epithelial integrity.
Little information is available regarding the effects of vitamin A deficiency on the oral structures in humans. Several epidemiologic studies have failed to demonstrate any relationship between this vitamin and periodontal disease in humans.246
In experimental animals, vitamin A deficiency results in hyperkeratosis and hyperplasia of the gingiva with a tendency for increased periodontal pocket formation. The following periodontal changes have been reported in vitamin A–deficient rats: hyperplasia and hyperkeratinization of the gingival epithelium, with proliferation of the junctional epithelium, and retardation of gingival wound healing.61,82 In the presence of local factors, vitamin A–deficient rats develop periodontal pockets that are deeper than those in animals not deficient in vitamin A and that exhibit associated epithelial hyperkeratosis.29,94
Vitamin D Deficiency
Vitamin D, or calciferol, is essential for the absorption of calcium from the gastrointestinal tract and the maintenance of the calcium-phosphorus balance. Deficiency in vitamin D and imbalance in calcium-phosphorus intake result in rickets in young children and osteomalacia in adults. No studies have demonstrated a relationship between vitamin D deficiency and periodontal disease.
The effect of vitamin D deficiency or imbalance on the periodontal tissues of young dogs results in osteoporosis of alveolar bone. Osteoid forms at a normal rate but remains uncalcified and fails to resorb, which leads to excessive accumulation. A reduction in the width of the periodontal ligament space results from a normal rate of cementum formation coupled with defective calcification and distortion of the growth pattern of alveolar bone.18,250
In osteomalacic animals, there is rapid, generalized, severe osteoclastic resorption of alveolar bone and proliferation of fibroblasts that replace bone and marrow as well as new bone formation around the remnants of unresorbed bony trabeculae.62 Radiographically, there is generalized partial to complete disappearance of the lamina dura and reduced density of the supporting bone, loss of trabeculae, increased radiolucency of the trabecular interstices, and increased prominence of the remaining trabeculae. Microscopic and radiographic changes in the periodontium are almost identical with those seen in experimentally induced hyperparathyroidism.
Vitamin E Deficiency
Vitamin E serves as an antioxidant to limit free-radical reactions and to protect cells from lipid peroxidation. Cell membranes, which are high in polyunsaturated lipids, are the major site of damage in vitamin E deficiency. No relationship has been demonstrated between deficiencies in vitamin E and oral disease, but systemic vitamin E appears to accelerate gingival wound healing in the rat.128,187
Water-Soluble Vitamin Deficiency
Vitamins B and C are water-soluble vitamins required in the human diet.
B-Complex Deficiency
The vitamin B complex includes thiamin, riboflavin, niacin, pyridoxine (B6), biotin, folic acid, and cobalamin (B12). Oral disease is rarely caused by a deficiency in just one component of the B-complex group; the deficiency is generally multiple.
Oral changes common to B-complex deficiencies are gingivitis, glossitis, glossodynia, angular cheilitis, and inflammation of the entire oral mucosa. The gingivitis in vitamin B deficiencies is nonspecific because it is caused by bacterial plaque rather than by the deficiency, but gingivitis is subject to the deficiency’s modifying effect.
The human manifestations of thiamin deficiency, called beriberi, are characterized by paralysis; cardiovascular symptoms, including edema; and loss of appetite. Frank beriberi is rare in the United States. Oral disturbances that have been attributed to thiamin deficiency include hypersensitivity of the oral mucosa; minute vesicles (simulating herpes) on the buccal mucosa, under the tongue, or on the palate; and erosion of the oral mucosa.100,151
The symptoms of riboflavin deficiency (ariboflavinosis) include glossitis, angular cheilitis, seborrheic dermatitis, and a superficial vascularizing keratitis. The glossitis is characterized by a magenta discoloration and atrophy of the papillae. In mild-to-moderate cases the dorsum exhibits a patchy atrophy of the lingual papillae and engorged fungiform papillae, which project as pebblelike elevations.258 In severe deficiency the entire dorsum is flat, with a dry and often fissured surface. Changes observed in riboflavin-deficient monkeys include severe lesions of the gingiva, periodontal tissues, and oral mucosa, including noma.42,237
Angular cheilitis begins as an inflammation of the commissure of the lips, followed by erosion, ulceration, and fissuring. Riboflavin deficiency is not the only cause of angular cheilitis. Loss of vertical dimension, together with drooling of saliva into the angles of the lips, may produce a condition similar to angular cheilitis. Candidiasis may develop in the commissures of debilitated persons; this lesion has been termed perlèche.98 Supplemental riboflavin is ineffective to resolve cases of glossitis and angular cheilitis that are not caused by vitamin deficiency.258
Niacin deficiency results in pellagra, which is characterized by dermatitis, gastrointestinal disturbances, neurologic and mental disturbances (dermatitis, diarrhea, or dementia), glossitis, gingivitis, and generalized stomatitis. This condition is rare but may occasionally result from malabsorption or alcoholism. Glossitis and stomatitis may be the earliest clinical signs of niacin deficiency.152 The gingiva may be involved in aniacinosis with or without tongue changes.129 The most common finding is NUG, usually in areas of local irritation.
Oral manifestations of vitamin B–complex and niacin deficiency in experimental animals include black tongue and gingival inflammation, with destruction of the gingiva, periodontal ligament, and alveolar bone.19,60 Necrosis of the gingiva and other oral tissues and leukopenia are terminal features of niacin deficiency in experimental animals.
Folic acid deficiency results in macrocytic anemia with megaloblastic erythropoiesis, accompanied by oral changes, gastrointestinal lesions, diarrhea, and intestinal malabsorption.61 Folic acid–deficient animals demonstrate necrosis of the gingiva, periodontal ligament, and alveolar bone without inflammation.216 The absence of inflammation is the result of deficiency-induced granulocytopenia. In humans with sprue and other folic acid deficiency states, generalized stomatitis occurs, which may be accompanied by ulcerated glossitis and cheilitis. Ulcerative stomatitis is an early indication of the toxic effect of folic acid antagonists (e.g., methotrexate) used in the treatment of leukemia.
In a series of human studies, Vogel et al241,242 reported a significant reduction of gingival inflammation after systemic or local use of folic acid compared with placebo. This reduction occurred with no change in plaque accumulation. The authors also postulated that the gingival changes associated with pregnancy and oral contraceptives may be partly related to suboptimal levels of folic acid in the gingiva.240 In a clinical study of pregnant women, a reduction in gingival inflammation occurred with the use of topical folate mouth rinses; no change was found with systemic folic acid.182 A relationship has also been suggested between phenytoin-induced gingival overgrowth and folic acid, based on the interference of folic acid absorption and utilization of phenytoin.239 However, a more recent double-blind, randomized, placebo-controlled study of 20 institutionalized epileptic adults taking phenytoin found no difference in gingival conditions (gingival overgrowth, health or plaque index) between the group taking 3 mg folic acid daily versus the placebo group at 4-week intervals over a 16-week period.32
Vitamin C (Ascorbic Acid) Deficiency
Severe vitamin C deficiency in humans results in scurvy, a disease characterized by hemorrhagic diathesis and delayed wound healing. Vitamin C is required in the human diet, as well as in other primates, guinea pigs, and some rare flying mammals.54 Because vitamin C is abundant in fruits and vegetables, scurvy is uncommon in developed countries. It may occur in infants in their first year of life if formulas are not fortified with vitamins and in older persons, especially those living alone and with restricted diets.54 Malnutrition associated with alcoholism may predispose an individual to scurvy.
Scurvy results in defective formation and maintenance of collagen, impairment or cessation of osteoid formation, and impaired osteoblastic function.79,255 Vitamin C deficiency is also characterized by increased capillary permeability, susceptibility to traumatic hemorrhages, hyporeactivity of the contractile elements of the peripheral blood vessels, and sluggishness of blood flow.136 Clinical manifestations of scurvy include hemorrhagic lesions into the muscles of the extremities, the joints, and sometimes the nail beds; petechial hemorrhages, often around hair follicles; increased susceptibility to infections; and impaired wound healing.54 Bleeding, swollen gingiva, and loosened teeth are common features of scurvy.
Possible Etiologic Factors
Ascorbic acid may play a role in periodontal disease through one or more of the following suggested mechanisms256:
Epidemiologic Studies
Several studies in large populations have analyzed the relationship between gingival or periodontal status and ascorbic acid levels.246 These studies used different methods for the biochemical analysis of ascorbic acid and various indices for the assessment of periodontal changes; they were made in persons of different socioeconomic status, different races, and various ages. All the epidemiologic surveys failed to establish a causal relationship between the levels of vitamin C and the prevalence or severity of periodontal disease.36,72,205 Megadoses of ascorbic acid have also been found to be unrelated to better periodontal health.118,257
Gingivitis
The legendary association of severe gingival disease with scurvy led to the presumption that vitamin C deficiency is an etiologic factor in gingivitis, which is common at all ages. Gingivitis with enlarged, hemorrhagic, bluish red gingiva is described as one of the classic signs of vitamin C deficiency, but gingivitis is not caused by vitamin C deficiency. Vitamin C–deficient patients do not necessarily have gingivitis. Acute vitamin C deficiency does not cause or increase the incidence of gingival inflammation, but it does increase its severity.44,92,93 Gingivitis in vitamin C–deficient patients is caused by bacterial plaque. Vitamin C deficiency may aggravate the gingival response to plaque and worsen the edema, enlargement, and bleeding. In addition, although correcting the deficiency may reduce the severity of the disorder, gingivitis will remain as long as bacterial factors are present.
Periodontitis
Changes in the supporting periodontal tissues and gingiva in vitamin C deficiency have been documented extensively in experimental animals.92,245 Acute vitamin C deficiency results in edema and hemorrhage in the periodontal ligament, osteoporosis of the alveolar bone, and tooth mobility; hemorrhage, edema, and degeneration of collagen fibers occur in the gingiva. Vitamin C deficiency also impairs gingival healing. The periodontal fibers that are least affected by vitamin C deficiency are those just below the junctional epithelium and above the alveolar crest, which explains the infrequent apical downgrowth of the epithelium.245
Vitamin C deficiency alone does not cause periodontal destruction. Local bacterial factors are required for increased probing depth and attachment loss to occur. However, acute vitamin C deficiency accentuates the destructive effect of gingival inflammation on the underlying periodontal ligament and alveolar bone.93
Experimental studies conducted in humans failed to show the dramatic clinical changes that have traditionally been described in scurvy.55,113,197 A case report by Charbeneau and Hurt44 showed worsening of a preexisting moderate periodontitis with the development of scurvy. In a retrospective analysis of 12,419 adults studied in the third National Health and Nutrition Examination Survey (NHANES III), Nishida et al174 found that there was a weak but statistically significant dose-response relationship between the levels of dietary vitamin C intake and periodontal disease in current and former smokers as measured by clinical attachment. This suggests that vitamin C deficiency has its greatest impact on periodontal disease when preexisting disease and other co-destructive factors are present.
Summary
Analysis of the literature indicates that the microscopic signs of vitamin C deficiency are quite different from those that occur in plaque-induced periodontal disease in humans. Patients with acute or chronic vitamin C–deficient states and no plaque accumulation show minimal, if any, changes in their gingival health status.
Protein Deficiency
Protein depletion results in hypoproteinemia with many pathologic changes, including muscular atrophy, weakness, weight loss, anemia, leukopenia, edema, impaired lactation, decreased resistance to infection, slow wound healing, lymphoid depletion, and reduced ability to form certain hormones and enzyme systems. Protein deprivation has been shown to cause changes in the periodontium of experimental animals.45 The following observations have been made in protein-deprived animals: degeneration of the connective tissue of the gingiva and periodontal ligament, osteoporosis of alveolar bone, impaired deposition of cementum, delayed wound healing, and atrophy of the tongue epithelium.39,221,222 Similar changes occur in the periosteum and bone in other nonoral areas. Osteoporosis results from reduced deposition of osteoid, reduction in the number of osteoblasts, and impairment in the morphodifferentiation of connective tissue cells to form osteoblasts, rather than from increased osteoclastic activity.
These observations are of interest in that they reveal a loss of alveolar bone resulting from the inhibition of normal bone-forming activity rather than from the introduction of destructive factors. Protein deficiency also accentuates the destructive effects of bacterial plaque and occlusal trauma on the periodontal tissues, but the initiation of gingival inflammation and its severity depend on the bacterial plaque.222 In other words, protein deprivation results in periodontal tissues that lack integrity and as a result, are more vulnerable to breakdown when challenged by bacteria.
Medications
Some medications prescribed to cure, manage, or prevent diseases may have an adverse effect on periodontal tissues, wound healing, or the host immune response. Bisphosphonates are a class of widely prescribed medications that have recently been associated with osteonecrosis of the jaw (ONJ). Corticosteroids have long been prescribed to suppress the immune system in the control and management of autoimmune disease, in cancer treatment, and as an antirejection medication in transplant patients. This section discusses the effect of bisphosphonates and corticosteroids on the periodontium.
Bisphosphonates
Bisphosphonate medications are primarily used to treat cancer (intravenous [IV] administration) and osteoporosis (oral administration). They act by inhibiting osteoclastic activity, which leads to less bone resorption, less bone remodeling, and less bone turnover.206 The use of bisphosphonates in cancer treatment is aimed at preventing the often lethal imbalance of osteoclastic activity. In the treatment of osteoporosis, the goal is simply to harness osteoclastic activity to minimize or prevent bone loss and in many cases, to increase bone mass by creating an advantage for osteoblastic activity. The major difference in the use of bisphosphonates for cancer versus osteoporosis is the potency and route of administration of the specific bisphosphonate medication used. Potency is influenced by the chemical properties, as well as the binding and release pharmacokinetic properties, of these agents with bone. Specifically, the strength of binding and the ease of release of bisphosphonates with hydroxyapatite make them more or less potent.
Bisphosphonates were first synthesized in the 1950s as a substitute for pyrophosphate, a compound used in detergent. The ability of bisphosphonates to increase bone mass was discovered after animal studies in 1966, but the potential advantage of using bisphosphonates in humans with low bone mass was not appreciated until 1984.244 The Food and Drug Administration (FDA) approved the use of alendronate for osteoporosis in 1995.
The chemical structure of bisphosphonate consists of two phosphate groups covalently bonded to a central carbon (Figure 27-28). In addition to the two phosphate groups, the central carbon also has two side chains, R1 and R2. Both the short R1 side chain and the long R2 side chain influence the chemical properties and pharmacokinetics. The long R2 side chain also influences the mode of action and determines the strength or potency of the medication. Bisphosphonates inhibit osteoclasts by two mechanisms that depend on whether the R2 side chain contains nitrogen. Non-aminobisphosphonates are metabolized by osteoclasts to form an adenosine triphosphate (ATP) analog that interferes with energy production and causes osteoclast apoptosis. Aminobisphosphonates (risedronate, zoledronate, ibandronate, and alendronate) are more potent and have multiple effects on osteoclasts, including (1) inactivation of ATP, (2) osteoclast cytoskeletal disruption, (3) impairment of osteoclast recruitment, and (4) induction of osteoblasts to produce osteoclast-inhibiting factor.181 Table 27-1 lists some of the common bisphosphonate medications used for osteoporosis and cancer treatment that are currently available in the US.
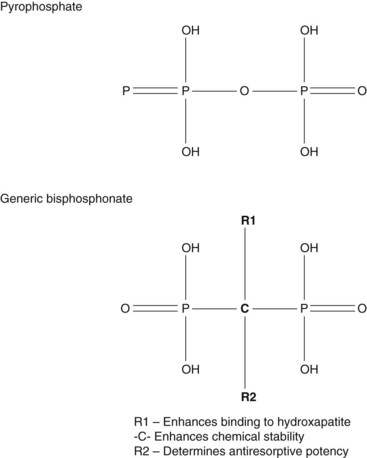
Figure 27-28 Chemical structure of bisphosphonate molecule. Two phosphate groups are covalently bonded with a central carbon. The carbon also has two side chains, R1 and R2.
TABLE 27-1 Current Nonaminobisphosphonate and Aminobisphosphonate Medications and Common Therapeutic Uses
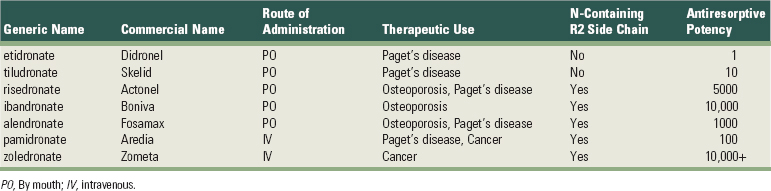
Bisphosphonates have a high affinity for hydroxyapatite and are rapidly absorbed in bone, especially in areas of high activity, which may explain why bisphosphonate-induced osteonecrosis is only found in the jaws.244 The bisphosphonate molecule gets incorporated into bone without being metabolized or modified. During osteoclastic resorption of bone, the trapped bisphosphonate is released and able to affect osteoclasts again. As a result, the half-life of bisphosphonates in the bone is estimated to be 10 years or more.
Osteonecrosis of the jaw associated with bisphosphonates was first reported in 2003 by Marx in a report of 36 cases of patients with avascular necrosis of the jaws who were treated with IV bisphosphonate for malignant tumors.155 Several case series reporting an association between bisphosphonates and osteonecrosis of the jaws were published in the months and years after this initial report.157,204 Various terms have been used to describe this type of osteonecrosis of the jaw, including avascular necrosis, bisphosphonate-related or -associated ONJ (BRONJ), and bisphosphonate-induced ONJ (BIONJ).244 Today, with the widespread recognition of BIONJ, it is important to remember that necrotic bone exposure of the jaw (ONJ) is a condition with multiple possible etiopathogenic factors, including systemic medications, radiation, infection, trauma, direct chemical toxicity, or other idiopathic mechanisms, and clinicians should carefully consider all factors before concluding a diagnosis of BIONJ.8
The condition of BIONJ has been defined as exposure/necrosis of portions of the jaw bone in patients exposed to bisphosphonates that has persisted more than 8 weeks with no past history of radiation therapy to the jaws.203 The stage of osteonecrosis is used to categorize patients and make treatment decisions.1,156,157 Stage 0 is defined as patients at risk who have been treated with IV or oral bisphosphonates but have no apparent exposed/necrotic bone. Stage 1 is exposed/necrotic bone in patients who are asymptomatic with no infection. Stage 2 is exposed/necrotic bone in patients with pain and clinical evidence of infection. Stage 3 is exposed/necrotic bone in patients with pain, infection, and one or more of the following: pathologic fracture, extraoral fistula, or osteolysis extending to the inferior border.
Clinically, BIONJ presents as exposed alveolar bone occurring spontaneously or after a dental procedure (Figures 27-29 and 27-30). The sites may be painful with surrounding soft tissue induration and inflammation. Infection with drainage may be present. Radiographically, lesions appear radiolucent with sclerosis of lamina dura, loss of lamina dura, or widening of periodontal ligament in areas where teeth are present. Histologically, bone appears necrotic with empty lacunae demonstrating a lack of living osteocytes. In advanced cases, pathologic fracture may be present through the area of exposed/necrotic bone.
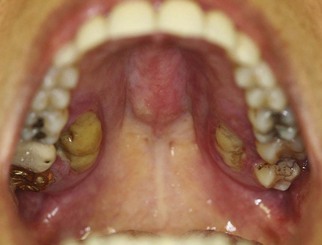
Figure 27-29 Clinical photograph of exposed bone on palatal surface of maxilla adjacent to molar root in 60-year-old female with bisphosphonate-induced osteonecrosis of bone (maxilla). The bone exposures were noted about 1 year during treatment with bisphosphonate (Aredia and Zometa).
(Courtesy of Drs. Eric S. Sung and Evelyn M. Chung, University of California, Los Angeles.)
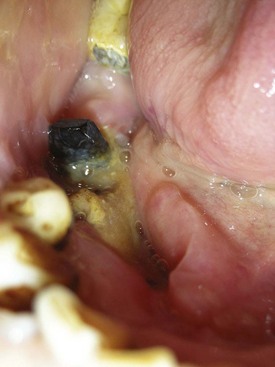
Figure 27-30 Clinical photograph of exposed bone on lingual surface of posterior mandible in 70-year-old male with bisphosphonate-induced osteonecrosis of bone (mandible). The bone exposure was noted after 3 years of bisphosphonate (Aredia and Zometa) for multiple myeloma.
(Courtesy of Drs. Eric S. Sung and Evelyn M. Chung, University of California, Los Angeles.)
The high potency of nitrogen-containing bisphosphonates, especially those administered via IV for cancer treatment (e.g., zoledronate), may explain the high incidence of BIONJ in these patients as compared to osteoporotic patients taking oral bisphosphonates. The incidence in patients treated for cancer has been reported to range from 2.5% to 5.4%.248 Estimating the incidence in patients taking oral bisphosphonates for osteoporosis is more difficult due to the large number of patients taking this medication (prescriptions) and lack of good reporting or documentation for these patients. Estimates from different sources project the incidence to range from 0.007% to 0.04%.244 Clearly, the incidence is low.
In addition to bisphosphonate therapy, other factors make individuals susceptible to BIONJ. Potential risk factors that may contribute to BIONJ include systemic corticosteroid therapy, smoking, alcohol, poor oral hygiene, chemotherapy, radiotherapy, diabetes, and hematologic disease.28 The precipitating factors or events leading to BIONJ are reported to include extractions, root canal treatment, periodontal infections, periodontal surgery, and dental implant surgery, yet some cases appear to be idiopathic with spontaneous exposures.157 In a retrospective evaluation of patients treated with IV bisphosphonates for metastatic bone cancer from 1996 to 2006, Estilo et al73 found that type of cancer, duration of bisphosphonate therapy, sequential IV bisphosphonate treatment with pamidronate followed by zoledronate, comorbid osteoarthritis, rheumatoid arthritis, and benign hematologic conditions were significantly associated with an increased likelihood of ONJ. In their study, systemic administration of corticosteroids was not found to be associated with an increased risk BRONJ.73
Patients being treated for cancer with IV bisphosphonates are at greater risk than patients being treated for osteoporosis with oral bisphosphonates. Dental health care providers should evaluate patients carefully, consider the risks, communicate with medical health care providers, inform patients, and consider treatment options and risks carefully.
Not surprisingly, the bone-preserving action of bisphosphonates has been studied and advocated for use in the prevention of bone loss from periodontal disease.232 Several animal studies have shown that bisphosphonates, applied topically or administered systemically, have the potential to prevent alveolar bone loss caused by periodontitis.* The use of bisphosphonates in bone regeneration has been proposed as well.232 Although some studies have demonstrated bone preservation with low doses of bisphosphonate, higher doses and longer administration may have a neutral or detrimental effect on bone loss caused by periodontitis.34,41 In a 2 to 3 year follow-up report of 4 female patients with periodontitis treated with etidronate (200 mg daily for periods of 2 weeks with 10-week “off drug” periods), the potential of this agent to prevent periodontal bone loss was reported.229 In a 2-year randomized, placebo-controlled clinical trial of 335 patients treated with alendronate (70 mg once weekly), no significant difference in alveolar bone loss or alveolar bone density was found.120 Interestingly, alendronate was found to significantly reduce bone loss relative to controls in a subset of this group (i.e., patients with low mandibular bone mineral density at baseline), suggesting that the effect may be more perceptible in cases with less bone mass or less bone density. In another clinical trial of 24 patients (12 experimental and 12 control), alendronate was shown to have a significant positive effect (bone preserving) on bone density in jaws.68 Bone mineral density of the maxilla and mandible was measured for all patients using dual energy x-ray absorptiometry (DEXA) at baseline and following 6 months of treatment (10 mg daily for 6 months).
Corticosteroids
In humans, systemic administration of cortisone and ACTH appears to have no effect on the incidence or severity of gingival and periodontal disease. However, renal transplant patients receiving immunosuppressive therapy (prednisone or methylprednisone, azathioprine, or cyclophosphamide) have significantly less gingival inflammation than control subjects with similar amounts of plaque.20,125,180,236135
Exogenous cortisone may have an adverse effect on bone quality and physiology. The systemic administration of cortisone in experimental animals results in osteoporosis of alveolar bone.95 There was capillary dilation and engorgement with hemorrhage into the periodontal ligament and gingival connective tissue, as well as a degeneration and reduction in the number of collagen fibers in the periodontal ligament and increased destruction of the periodontal tissues associated with inflammation.95
Stress increases circulating endogenous cortisol levels through stimulation of the adrenal glands (hypothalamic-pituitary-adrenal axis). This increased exposure to endogenous cortisol may have adverse effects on the periodontium by diminishing the immune response to periodontal bacteria (see section on psychosocial stress).
Other Systemic Conditions
Osteoporosis
Osteoporosis is a disease characterized by low bone mass and structural deterioration leading to an increased risk of bone fracture that affects approximately 10 million people in the US with a higher predilection for females (80%) as compared to males (20%). An additional 34 million individuals in the US are estimated to have osteopenia or low bone density. Loss of bone mass and the incidence of osteoporosis increases with age for both men and women, with women affected earlier than men. The rate of bone loss is greatest for women during the perimenopausal years when estrogen levels decrease.
A bone mineral density (BMD) test is used to measure an individual’s bone mass. BMD is measured using a DEXA scan. The DEXA value, or T-score, is a comparison of the patient’s BMD to that of a healthy 30-year-old adult with peak bone mass. The World Health Organization (WHO) defines osteoporosis and osteopenia by measures of “standard deviations” as compared to that of a normal healthy young adult. Thus a T-score between +1 and −1 is considered normal, whereas a T-score between −1 and −2.5 indicates low bone density or osteopenia and a T-score less than −2.5 (i.e., more than 2.5 standard deviations below normal) is diagnostic for osteoporosis (Table 27-2).
TABLE 27-2 Definitions of Osteoporosis and Osteopenia Indicated by T-Scores
| T-Score | Bone Mineral Density (BMD) Category |
|---|---|
| ≥ −1 | Normal |
| −1 to −2.5 | Low (osteopenia) |
| ≤ −2.5 | Osteoporosis |
Ranges of T-scores are based on standard deviations outside of normal, which is based on the BMD of a healthy 30-year-old adult.
Adapted from World Health Organization definitions.
One of the most significant consequences of osteoporosis is the increased risk of bone fracture. All bones affected by osteoporosis are susceptible to fracture, but fractures of the pelvis and vertebrae carry the most serious risk of morbidity and mortality. Studies have reported that approximately 20% of patients with hip fractures die within a year and 50% experience significant disability (e.g., inability to walk unassisted).137
Although it is logical to surmise a relationship between osteoporosis and periodontitis, it has been a challenging hypothesis to prove. One of the difficulties is the fact that both osteoporosis and periodontitis are chronic, multifactorial diseases that result in bone loss, and bone loss in each condition is exacerbated by local and systemic factors.179 Gender, genetic predisposition, inactivity, deficient diets (e.g., deficient in calcium or vitamin D), alcohol, smoking, hormones, and medications put individuals at risk for osteoporosis, with some of these factors putting them at risk for the progression of periodontitis as well. Variations in populations studied, measurements used to assess disease, and study design have also hindered the ability to clearly define a relationship between osteoporosis and periodontitis.
Attempts to use tooth loss as an indirect measure of periodontitis in individuals with osteoporosis have offered mixed conclusions. Several studies have reported greater tooth loss, more alveolar bone loss and edentulism in individuals with osteoporosis.58,64,107,260 However, some other studies have suggested that tooth loss is not correlated to osteoporosis or BMD.27,66,168 One large study (1365 Caucasian postmenopausal females) investigating systemic BMD (lumbar spine and proximal femur) and tooth loss, found no significant correlation.66 Part of the challenge and the reason for conflicting results in these studies may be attributed to differences in measurement methods. Another problem in translating any tooth loss study conclusions to the question of whether osteoporosis contributes to periodontitis is that the cause of tooth loss in these studies is often unknown (i.e., tooth loss may or may not be related to periodontal disease).
Studies that attempt to correlate osteoporosis with periodontitis are fraught with similar, if not greater, challenges. Again, some studies have concluded or suggested that osteoporosis does contribute to the progression of periodontitis,117,130,169,234 whereas others refute this conclusion.69,147 One study by Elders et al failed to show a correlation between BMD (lumbar BMD) and clinical parameters of periodontitis in a dentate group of 46- to 55-year-old females.69 They used intraoral periodontal examinations (probing depth, bleeding on probing, and missing teeth) and bitewing radiographs to assess clinical parameters of periodontitis and interproximal alveolar bone loss. This study included edentulous subjects (286) as well, but no significant difference in BMD was found between edentulous (60) and dentate (226) subjects, suggesting that neither periodontitis nor tooth loss was related to osteoporosis. The mean age of subjects in this study was relatively young, which may have contributed to the lack of correlation.88
Most studies citing a positive correlation between osteoporosis or BMD and periodontitis are cross-sectional studies of postmenopausal females. Klemetti et al evaluated 227 healthy postmenopausal women aged 48 to 56 years and found that women with higher skeletal BMD were more likely to retain teeth with periodontitis (deep periodontal pockets) than those with osteoporosis.130 Tezal et al concluded that skeletal BMD (measured by DEXA at multiple sites) is related to alveolar bone loss and to a lesser extent to clinical attachment loss, implicating postmenopausal osteopenia as a risk indicator for periodontal disease in 70 postmenopausal white women aged 51 to 78 years.234 Periodontal parameters included probing depth, supragingival plaque, calculus, bleeding on probing, clinical attachment loss, and interproximal alveolar bone loss. Analysis was adjusted for age, age at menopause, estrogen supplementation, cigarette smoking, body mass, and supragingival plaque. Inagaki et al evaluated the association between periodontal conditions, tooth loss, and metacarpal BMD (m-BMD) in a cross-sectional study of 356 Japanese women (171 premenopausal, mean age 37.9 years and 185 postmenopausal, mean age 63.3 years) and concluded that periodontal status and tooth loss after menopause could be a useful indicator of m-BMD loss in Japanese women.117 These cross-sectional studies are suggestive but do not consider the periodontal condition or existence of periodontitis before the onset of osteoporosis or loss of systemic bone mineral density.
The effect of estrogen deficiency and osteopenia/osteoporosis on periodontitis is not known but may be an important factor to consider. Reinhardt et al196 conducted a 2-year, prospective, longitudinal study of postmenopausal women (59 with moderate/advanced periodontitis and 16 with no periodontitis) to evaluate the effect of estrogen (serum estradiol) levels on periodontitis in these patients. Clinical measurements, including supragingival plaque, bleeding on probing, and relative clinical attachment, were taken at baseline and every 6 months for 2 years. They concluded that estrogen deficiency and osteopenia/osteoporosis together are risk factors for alveolar bone loss in postmenopausal women with a history of periodontitis.196 Lerner138 also proposed that estrogen deficiency might play a significant role in the progression of periodontitis in osteopenic/osteoporotic women citing that the cytokines believed to be involved in inflammation-induced remodeling are very similar to those suggested to play crucial roles in postmenopausal osteoporosis. In patients with periodontal disease and concomitant postmenopausal osteoporosis, the possibility exists that the lack of estrogen influences the activities of bone cells and immune cells in such a way that the progression of alveolar bone loss will be enhanced.138 In fact, there are numerous studies that report less risk for tooth loss and improved oral health in postmenopausal women on hormone replacement therapy.* More prospective, longitudinal studies evaluating the effect of estrogen and osteopenia/osteoporosis on periodontitis are needed to improve our understanding.
Congenital Heart Disease
Congenital heart disease occurs in about 1% of live births. Approximately 40% of individuals born with heart defects would die without treatment. However, the prognosis has been dramatically improved with advances in cardiac surgery. Cardiac defects can involve the heart, the adjacent vessels, or a combination of both. The most striking feature of congenital heart disease is cyanosis caused by shunting of deoxygenated blood from the right to left, resulting in a return of poorly oxygenated blood to systemic circulation. In severe cases, cyanosis is obvious at birth, particularly in tetralogy of Fallot. Chronic hypoxia causes impaired development, compensatory polycythemia (increase in RBCs/hemoglobin) and clubbing edema of toes and fingers (Figure 27-31). Polycythemia is significant because it can result in hemorrhagic or thrombotic tendencies. Patients with congenital heart defects are often at risk for infective endocarditis because of turbulent blood flow in the heart and associated cardiovascular defects. The need for prophylactic antibiotics should be evaluated before dental therapy.
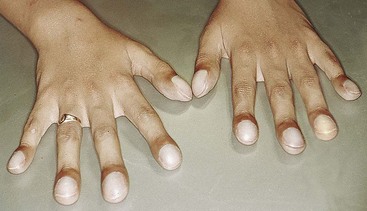
Figure 27-31 Characteristic clubbing of the fingers in adolescent patient with tetralogy of Fallot, consistent with untreated congenital cyanotic heart disease.
In addition to the obvious cyanosis of lips and oral mucosa, oral abnormalities associated with cyanotic congenital heart disease include delayed eruption of both primary and permanent dentitions, increased positional abnormalities, and enamel hypoplasia. The teeth often have a bluish white appearance with an increased pulp vascular volume. Gingival disease and other oral symptoms have been reported in children with congenital heart disease.26,124 Reports seem to indicate more severe caries and periodontal disease in patients with cyanotic congenital heart defects. However, the apparent increase in dental disease may be attributed to poor oral hygiene and a general lack of dental care rather than a disease-related etiology.
Tetralogy of Fallot
As its name implies, tetralogy of Fallot is characterized by four cardiac defects: (1) ventricular septal defect, (2) pulmonary stenosis, (3) malposition of the aorta to the right, and (4) compensatory right ventricular enlargement. Clinical features include severe cyanosis, audible heart murmurs, and breathlessness. Cyanosis and breathlessness cause cerebral anoxia and syncope. Oral changes include a purplish red discoloration of the lips and gingiva. Severe marginal gingivitis and periodontal destruction have been reported (Figure 27-32). The discoloration of the lips and gingiva corresponds to the general degree of cyanosis and returns to normal after corrective heart surgery. The tongue appears coated, fissured, and edematous, and there is extreme reddening of the fungiform and filiform papillae. The number of subepithelial capillaries is increased but also returns to normal after heart surgery.81
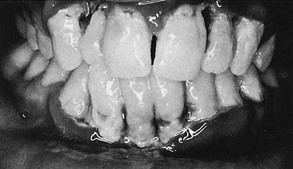
Figure 27-32 Extensive marginal inflammation with ulceronecrotic lesions and periodontal destruction in patient with tetralogy of Fallot shown in Figure 27-31.
Eisenmenger’s Syndrome
Among patients with ventricular septal defects, about half with large defects (>1.5 cm in diameter) develop Eisenmenger’s syndrome. This syndrome is distinguished by a greater blood flow from the stronger left ventricle to the right ventricle (backward flow) through the septal defect causing increased pulmonary blood flow, which in turn leads to progressive pulmonary fibrosis, small-vessel occlusion, and high pulmonary vascular resistance. With increasing pulmonary resistance, the right ventricle hypertrophies, the shunt becomes bidirectional, and ultimately, blood flow is reversed (right-to-left). The increased vascular resistance builds pressure in the right ventricle, causing right ventricular hypertrophy, and a reverse in the direction of the blood flow, resulting in a right-to-left shunt.
The natural history of a patient with untreated Eisenmenger’s syndrome is a gradual increase in cyanosis over many years, eventually leading to cardiac failure. Cyanosis of the lips, cheeks, and buccal mucous membranes is observed in these patients but is much less severe than in those with tetralogy of Fallot. Severe, generalized periodontitis has been reported in patients with Eisenmenger’s syndrome.46 However, similar to patients with other types of congenital heart disease, the incidence of periodontal disease reported in those with Eisenmenger’s syndrome may be related more to poor oral hygiene and a general lack of dental care than to any specific, syndrome-related etiology.
Hypophosphatasia
Hypophosphatasia is a rare familial skeletal disease characterized by rickets, poor cranial bone formation, craniostenosis, and premature loss of primary teeth, particularly the incisors. Patients have a low level of serum alkaline phosphatase, and phosphoethanolamine is present in serum and urine.
Teeth are lost with no clinical evidence of gingival inflammation and show reduced cementum formation.23 In patients with minimal bone abnormalities, premature loss of deciduous teeth may be the only symptom of hypophosphatasia. In adolescents, this disease resembles localized “juvenile” (aggressive) periodontitis.259
Metal Intoxication
The ingestion of metals, such as mercury, lead, and bismuth, in medicinal compounds and through industrial contact may result in oral manifestations caused by either intoxication or absorption without evidence of toxicity.
Bismuth Intoxication
Chronic bismuth intoxication is characterized by gastrointestinal disturbances, nausea, vomiting, and jaundice, as well as by an ulcerative gingivostomatitis, generally with pigmentation and accompanied by a metallic taste and burning sensation of the oral mucosa. The tongue may be sore and inflamed. Urticaria, different types of exanthematous eruptions, bullous and purpuric lesions, and herpes zoster–like eruptions and pigmentation of the skin and mucous membranes are among the dermatologic lesions attributed to bismuth intoxication. Acute bismuth intoxication, which is seen less frequently, is accompanied by methemoglobin formation, cyanosis, and dyspnea.111
Bismuth pigmentation in the oral cavity usually appears as a narrow, bluish black discoloration of the gingival margin in areas of preexisting gingival inflammation (Figure 27-33) (see Chapter 8). Such pigmentation results from the precipitation of particles of bismuth sulfide associated with vascular changes in inflammation; it is not evidence of intoxication but simply indicates the presence of bismuth in the bloodstream. Bismuth pigmentation in the oral cavity also occurs in cases of intoxication; it assumes a linear form if the marginal gingiva is inflamed.
Lead Intoxication
Lead is slowly absorbed, and toxic symptoms are not particularly definitive when they do occur.121 There is pallor of the face and lips and gastrointestinal symptoms consisting of nausea, vomiting, loss of appetite, and abdominal colic. Peripheral neuritis, psychologic disorders, and encephalitis have been reported. Oral signs include salivation, coated tongue, a peculiar sweetish taste, gingival pigmentation, and ulceration. Gingival pigmentation is linear (burtonian line), steel gray, and associated with local inflammation. Oral signs may occur without toxic symptoms.
Mercury Intoxication
Mercury intoxication is characterized by headache, insomnia, cardiovascular symptoms, pronounced salivation (ptyalism), and a metallic taste.4 Gingival pigmentation in linear form results from the deposition of mercuric sulfide. The chemical also acts as an irritant, which accentuates the preexisting inflammation and often leads to notable ulceration of the gingiva and adjacent mucosa and destruction of the underlying bone. Mercurial pigmentation of the gingiva also occurs in areas of local irritation in patients without symptoms of intoxication.
Other Chemicals
Other chemicals, such as phosphorus, arsenic, and chromium, may cause necrosis of the alveolar bone with loosening and exfoliation of the teeth.139,213 Inflammation and ulceration of the gingiva are usually associated with destruction of the underlying tissues. Benzene intoxication is accompanied by gingival bleeding and ulceration with destruction of the underlying bone.213
![]() Science Transfer
Science Transfer
Dentists need to appreciate the wide range of systemic conditions that have periodontal implications to modify the treatment of affected patients, and in some cases, the dentist may be the first doctor to diagnose systemic disease based on its oral presentation. Recurrent periodontal abscesses and accentuated gingival inflammation and periodontal bone loss occur more frequently in diabetic patients. Some cases of leukemia have been first diagnosed in the dental office because of the abnormal patterns of gingival hyperplasia, necrosis, and bleeding caused by the associated thrombocytopenia, which can result in the unusual sign of internal bleeding and ecchymosis of the gingival tissues.
Other diseases associated with deficient polymorphonuclear leukocyte function, such as Papillon Lefèvre syndrome, Down syndrome, and Chédiak-Higashi syndrome, present with aggressive periodontitis.
Hormonal changes of the estrogen progesterone complex can accentuate plaque-induced gingivitis. This is most profound when it occurs in pregnancy, but cyclic menstrual changes, puberty, and the use of hormonal-based contraceptives can make gingivitis more severe.
Nutritional deficiencies need to be severe and prolonged before any dramatic periodontal manifestations are observed, and this includes vitamin C dietary deficiency, which only causes significant changes to gingival health in the late stages of scurvy.
Osteoporosis does not have dramatic periodontal implications, but edentulous alveolar processes may be more affected than alveolar bone supporting the teeth. Recent widespread utilization of bisphosphonate therapy has focused attention on bisphosphonate-induced osteonecrosis of the jaw (BIONJ), which can occur spontaneously or follow surgical procedures such as tooth extraction, periodontal surgery, or implant surgery. The risk for this is particularly high in patients subjected to intravenous administration, and these patients need to be managed by dental specialists. Oral bisphosphonates carry a lower risk of osteonecrotic sequelae, but even these patients may be better treated by nonsurgical therapy.
1 American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg. 2007;65:369-376.
2 Adler P, Wegner H, Bohatka L. Influence of age and duration of diabetes on dental development in diabetic children. J Dent Res. 1973;52:535-537.
3 Ainamo J, Lahtinen A, Uitto VJ. Rapid periodontal destruction in adult humans with poorly controlled diabetes. A report of 2 cases. J Clin Periodontol. 1990;17:22-28.
4 Akers L. Ulcerative stomatitis following therapeutic use of mercury and bismuth. J Am Dent Assoc. 1936:23.
5 Alfano MC. Controversies, perspectives, and clinical implications of nutrition in periodontal disease. Dent Clin North Am. 1976;20:519-548.
6 Alfano MC, Miller SA, Drummond JF. Effect of ascorbic acid deficiency on the permeability and collagen biosynthesis of oral mucosal epithelium. Ann N Y Acad Sci. 1975;258:253-263.
7 Allen IE, Monroe M, Connelly J, et al. Effect of postmenopausal hormone replacement therapy on dental outcomes: systematic review of the literature and pharmacoeconomic analysis. Manag Care Interface. 2000;13:93-99.
8 Almazrooa SA, Woo SB. Bisphosphonate and nonbisphosphonate-associated osteonecrosis of the jaw: a review. J Am Dent Assoc. 2009;140:864-875.
9 Alvares O, Siegel I. Permeability of gingival sulcular epithelium in the development of scorbutic gingivitis. J Oral Pathol. 1981;10:40-48.
10 Axtelius B, Soderfeldt B, Nilsson A, et al. Therapy-resistant periodontitis. Psychosocial characteristics. J Clin Periodontol. 1998;25:482-491.
11 Babior B, Disorders of neutrophil function, Wyngaarden JB, Smith LHJ, editors, Cecil textbook of medicine, 198818
12 Barkin RM, Weston WL, Humbert JR, Maire F. Phagocytic function in Down syndrome–I. Chemotaxis. J Ment Defic Res. 1980;24(Pt 4):243-249.
13 Barkin RM, Weston WL, Humbert JR, Sunada K. Phagocytic function in Down syndrome–II. Bactericidal activity and phagocytosis. J Ment Defic Res. 1980;24(Pt 4):251-256.
14 Barnett ML, Baker RL, Yancey JM, et al. Absence of periodontitis in a population of insulin-dependent diabetes mellitus (IDDM) patients. J Periodontol. 1984;55:402-405.
15 Barrett AP. Gingival lesions in leukemia. A classification. J Periodontol. 1984;55:585-588.
16 Bartolucci EG, Parkes RB. Accelerated periodontal breakdown in uncontrolled diabetes. Pathogenesis and treatment. Oral Surg Oral Med Oral Pathol. 1981;52:387-390.
17 Becker AR, Handick KE, Roberts WE, Garetto LP. Osteoporosis risk factors in female dental patients. A preliminary report. J Indiana Dent Assoc. 1997;76:15-19. quiz 20
18 Becks H, Collins DA, Freytog RM. Changes in oral structures of the dog persisting after chronic overdoses of vitamin D. AM J Orthod. 1946;32:463.
19 Becks H, Wainwright WW, Morgan AF. Comparative study of oral changes in dogs due to deficiencies of pantothenic acid, nicotine acid and unknowns of vitamin B complex,. AM J Orthod. 1943;29:183.
20 Been V, Engel D. The effects of immunosuppressive drugs on periodontal inflammation in human renal allograft patients. J Periodontol. 1982;53:245-248.
21 Bergmann OJ, Ellegaard B, Dahl M, Ellegaard J. Gingival status during chemical plaque control with or without prior mechanical plaque removal in patients with acute myeloid leukaemia. J Clin Periodontol. 1992;19:169-173.
22 Bernick SM, Cohen DW, Baker L, Laster L. Dental disease in children with diabetes mellitus. J Periodontol. 1975;46:241-245.
23 Beumer J 3rd, Trowbridge HO, Silverman SJr, Eisenberg E. Childhood hypophosphatasia and the premature loss of teeth. A clinical and laboratory study of seven cases. Oral Surg Oral Med Oral Pathol. 1973;35:631-640.
24 Birkenfeld L, Yemini M, Kase NG, Birkenfeld A. Menopause-related oral alveolar bone resorption: a review of relatively unexplored consequences of estrogen deficiency. Menopause. 1999;6:129-133.
25 Biro S. Studies regarding the influence of pregnancy upon caries. Vierteljahrschr Zahnheilk. 1898;14:371.
26 Blitzer B, Sznajder N, Carranza FA. [Periodontal disease risks in children with congenital cardiopathies]. Rev Asoc Odontol Argent. 1975;63:169-172.
27 Bollen AM, Taguchi A, Hujoel PP, Hollender LG. Number of teeth and residual alveolar ridge height in subjects with a history of self-reported osteoporotic fractures. Osteoporos Int. 2004;15:970-974.
28 Borgioli A, Viviani C, Duvina M, et al. Biphosphonates-related osteonecrosis of the jaw: clinical and physiopathological considerations. Ther Clin Risk Manag. 2009;5:217-227.
29 Boyle P. Effect of Vitamin A deficiency on the periodontal tissues. AM J Orthod. 1947;33:744.
30 Broadbent E, Petrie KJ, Alley PG, Booth RJ. Psychological stress impairs early wound repair following surgery. Psychosom Med. 2003;65:865-869.
31 Brown LR, Roth GD, Hoover D, et al. Alveolar bone loss in leukemic and nonleukemic mice. J Periodontol. 1969;40:725-730.
32 Brown RS, Di Stanislao PT, Beaver WT, Bottomley WK. The administration of folic acid to institutionalized epileptic adults with phenytoin-induced gingival hyperplasia. A double-blind, randomized, placebo-controlled, parallel study. Oral Surg Oral Med Oral Pathol. 1991;71:565-568.
33 Brownlee M. Glycation and diabetic complications. Diabetes. 1994;43:836.
34 Brunsvold MA, Chaves ES, Kornman KS, et al. Effects of a bisphosphonate on experimental periodontitis in monkeys. J Periodontol. 1992;63:825-830.
35 Bullon P, Pascual A, Fernandez-Novoa MC, et al. Late onset Papillon-Lefevre syndrome? A chromosomic, neutrophil function and microbiological study. J Clin Periodontol. 1993;20:662-667.
36 Buzina R, Brodarec A, Jusic M, et al. Epidemiology of angular stomatitis and bleeding gums. Int J Vitam Nutr Res. 1973;43:401-415.
37 Cabrini RL, Carranza FAJr. Adenosine triphosphatase in normal and scorbutic wounds. Nature. 1963;200:1113.
38 Campbell MJ. Epidemiology of periodontal disease in the diabetic and the non-diabetic. Aust Dent J. 1972;17:274-278.
39 Carranza FAJr, Gravina O, Cabrini RL. Periodontal and pulpal pathosis in leukemic Mice. Oral Surg Oral Med Oral Pathol. 1965;20:374-379.
40 Carvel RI. Palmo-plantar hyperkeratosis and premature periodontal destruction. (Papillon-Lefevre Syndrome: a 30 year study of two affected sisters). J Oral Med. 1969;24:73-82.
41 Cetinkaya BO, Keles GC, Ayas B, Gurgor P. Effects of risedronate on alveolar bone loss and angiogenesis: a stereologic study in rats. J Periodontol. 2008;79:1950-1961.
42 Chapman O, Harris AE. Oral lesions associated with dietary deficiencies in monkeys. J Infect Dis. 1941;69:7.
43 Chapple IL. Potential mechanisms underpinning the nutritional modulation of periodontal inflammation. J Am Dent Assoc. 2009;140:178-184.
44 Charbeneau TD, Hurt WC. Gingival findings in spontaneous scurvy. A case report. J Periodontol. 1983;54:694-697.
45 Chawla TN, Glickman I. Protein deprivation and the periodontal structures of the albino rat. Oral Surg Oral Med Oral Pathol. 1951;4:578-602.
46 Chung EM, Sung EC, Sakurai KL. Dental management of the Down and Eisenmenger syndrome patient. J Contemp Dent Pract. 2004;5:70-80.
47 Cianciola LJ, Park BH, Bruck E, et al. Prevalence of periodontal disease in insulin-dependent diabetes mellitus (juvenile diabetes). J Am Dent Assoc. 1982;104:653-660.
48 Cichon P, Crawford L, Grimm WD. Early-onset periodontitis associated with Down’s syndrome–clinical interventional study. Ann Periodontol. 1998;3:370-380.
49 Cohen DW, Friedman LA, Shapiro J, et al. Diabetes mellitus and periodontal disease: two-year longitudinal observations. I. J Periodontol. 1970;41:709-712.
50 Cohen DW, Friedman LA, Shapiro J, et al. Periodontal manifestations of cyclic neutropenia. J Periodontal. 1961;32:159.
51 Cohen DW, Shapiro J, Friedman L, et al. A longitudinal investigation of the periodontal changes during pregnancy and fifteen months post-partum. II. J Periodontol. 1971;42:653-657.
52 Cohen MM, Winer RA, Schwartz S, Shklar G. Oral aspects of mongolism. I. Periodontal disease in mongolism. Oral Surg Oral Med Oral Pathol. 1961;14:92-107.
53 Costello C, Webber A. White cell function in Down’s syndrome. Clin Genet. 1976;9:603-605.
54 Cotran R, Kumar V, Robbins SR: Robbins’ pathologic basis of disease, 1989.
55 Crandon JH, Lund CC, Dill DB. Experimental human scurvy. N Engl J Med. 1940;223:353.
56 Croucher R, Marcenes WS, Torres MC, et al. The relationship between life-events and periodontitis. A case-control study. J Clin Periodontol. 1997;24:39-43.
57 Cutler CW, Wasfy MO, Ghaffar K, et al. Impaired bactericidal activity of PMN from two brothers with necrotizing ulcerative gingivo-periodontitis. J Periodontol. 1994;65:357-363.
58 Daniell HW. Postmenopausal tooth loss. Contributions to edentulism by osteoporosis and cigarette smoking. Arch Intern Med. 1983;143:1678-1682.
59 Deas DE, Mackey SA, McDonnell HT. Systemic disease and periodontitis: manifestations of neutrophil dysfunction. Periodontol 2000. 2003;32:82-104.
60 Denton J. A study of tissue changes in experimental black tongue of dogs compared with similar changes in pellagra. AM J Patho. 1928;4:341.
61 Dreizen S. Oral manifestations of human nutritional anemias. Arch Environ Health. 1962;5:66-75.
62 Dreizen S, Levy BM, Bernick S, et al. Studies of the biology of the periodontium of marmosets. III. Periodontal bone changes in marmosets with osteomalacia and hyperparathyroidism. Isr J Med Sci. 1967;3:371.
63 Dreizen S, McCredie KB, Keating MJ, Luna MA. Malignant gingival and skin “infiltrates” in adult leukemia. Oral Surg Oral Med Oral Pathol. 1983;55:572-579.
64 Drozdzowska B, Pluskiewicz W, Michno M. Tooth count in elderly women in relation to their skeletal status. Maturitas. 2006;55:126-131.
65 Dumitrescu AL. Psychological perspectives on the pathogenesis of periodontal disease. Rom J Intern Med. 2006;44:241-260.
66 Earnshaw SA, Keating N, Hosking DJ, et al. Tooth counts do not predict bone mineral density in early postmenopausal Caucasian women. EPIC study group. Int J Epidemiol. 1998;27:479-483.
67 El-Ashiry GM, El-Kafrawy AH, Nasr MF, Younis N. Comparative study of the influence of pregnancy and oral contraceptives on the gingivae. Oral Surg Oral Med Oral Pathol. 1970;30:472-475.
68 El-Shinnawi UM, El-Tantawy SI. The effect of alendronate sodium on alveolar bone loss in periodontitis (clinical trial). J Int Acad Periodontol. 2003;5:5-10.
69 Elders PJ, Habets LL, Netelenbos JC, et al. The relation between periodontitis and systemic bone mass in women between 46 and 55 years of age. J Clin Periodontol. 1992;19:492-496.
70 Elter JR, White BA, Gaynes BN, Bader JD. Relationship of clinical depression to periodontal treatment outcome. J Periodontol. 2002;73:441-449.
71 Emrich LJ, Shlossman M, Genco RJ. Periodontal disease in non-insulin-dependent diabetes mellitus. J Periodontol. 1991;62:123-131.
72 Enwonwu CO, Edozien JC. Epidemiology of periodontal disease in Western Nigerians in relation to socio-economic status. Arch Oral Biol. 1970;15:1231-1244.
73 Estilo CL, Van Poznak CH, Wiliams T, et al. Osteonecrosis of the maxilla and mandible in patients with advanced cancer treated with bisphosphonate therapy. Oncologist. 2008;13:911-920.
74 Falk H, Hugoson A, Thorstensson H. Number of teeth, prevalence of caries and periapical lesions in insulin-dependent diabetics. Scand J Dent Res. 1989;97:198-206.
75 Fardal O, Drangsholt E, Olsen I. Palmar plantar keratosis and unusual periodontal findings. Observations from a family of 4 members. J Clin Periodontol. 1998;25:181-184.
76 Farzim I, Edalat M. Periodontosis with hyperkeratosis palmaris et plantaris (The Papillon Lefèvre Syndrome): a case report. J Periodontol. 1974;45:316-318.
77 Ficara AJ, Levin MP, Grower MF, Kramer GD. A comparison of the glucose and protein content of gingival fluid from diabetics and nondiabetics. J Periodontal Res. 1975;10:171-175.
78 Firatli E, Gurel N, Efeoglu A, Badur S. Clinical and immunological findings in 2 siblings with Papillon-Lefèvre syndrome. J Periodontol. 1996;67:1210-1215.
79 Folis R. The pathology of nutritional disease. Springfield: Charles C. Thomas; 1948.
80 Formicola AJ, Weatherford T3rd, Grupe HJr. The uptake of H3-estradiol by the oral tissues of rats. J Periodontal Res. 1970;5:269-275.
81 Forsslund G. The occurrence of subepithelial gingival blood vessels in patients with morbus caeruleus (tetralogy of Fallot). Acta Odontol Scand. 1962;20:301-306.
82 Frandsen AM. Periodontal tissue changes in vitamin A deficient young rats. Acta Odontol Scand. 1963;21:19-34.
83 Friedman LA. Horizontal tooth mobility and the menstrual cycle. J Periodontal Res. 1972;7:125-130.
84 Galea H, Aganovic I, Aganovic M. The dental caries and periodontal disease experience of patients with early onset insulin dependent diabetes. Int Dent J. 1986;36:219-224.
85 Genco RJ. Current view of risk factors for periodontal diseases. J Periodontol. 1996;67:1041-1049.
86 Genco RJ, Ho AW, Grossi SG, et al. Relationship of stress, distress and inadequate coping behaviors to periodontal disease. J Periodontol. 1999;70:711-723.
87 Genco RJ, Shlossman M, Zambon JJ. Immunologic studies of periodontitis parents with type II diabetes. J Dent Res. 1987;66:257.
88 Geurs NC, Lewis CE, Jeffcoat MK. Osteoporosis and periodontal disease progression. Periodontol 2000. 2003;32:105-110.
89 Ghaffer KA, Zahran FM, Fahmy HM, Brown RS. Papillon-Lefèvre syndrome: neutrophil function in 15 cases from 4 families in Egypt. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:320-325.
90 Glavind L, Lund B, Loe H. The relationship between periodontal state and diabetes duration, insulin dosage and retinal changes. J Periodontol. 1968;39:341-347.
91 Glickman I. The periodontal structures in experimental diabetes. NY J Dent. 1946;16:226.
92 Glickman I. Acute vitamin C deficiency and periodontal disease; the periodontal tissues of the guinea pig in acute vitamin C deficiency. J Dent Res. 1948;27:9-23.
93 Glickman I. Acute vitamin C deficiency and the periodontal tissues; the effect of acute vitamin C deficiency upon the response of the periodontal tissues of the guinea pig to artificially induced inflammation. J Dent Res. 1948;27:201-210.
94 Glickman I, Stoller M. The periodontal tissues of the albino rat in vitamin A deficiency. J Dent Res. 1948;27:758.
95 Glickman I, Stone IC, Chawla TN. The effect of cortisol acetate upon the periodontium of white mice. J Periodontal. 1953;24:161.
96 Goetzl EJ, Wasserman SI, Gigli I, Austen KF. Enhancement of random migration and chemotactic response of human leukocytes by ascorbic acid. J Clin Invest. 1974;53:813-818.
97 Goldman HM. Acute aleukemic leukemia. AM J Orthod. 1940;26:89.
98 Goodman H. Perleche: a consideration of its etiology and pathology. Bull Johns Hopkins Hosp. 1943;51:263.
99 Gottsegen R. Dental and oral considerations in diabetes mellitus. N Y State J Med. 1962;62:389-395.
100 Govier WM, Greig ME. Prevention of oral lesions in B1 avitaminotic dogs. Science. 1943;98:216-217.
101 Goya JA, Paez HA, Mandalunis PM. Effect of topical administration of monosodium olpadronate on experimental periodontitis in rats. J Periodontol. 2006;77:1-6.
102 Greenberg MS, Cohen SG, Boosz B, Friedman H. Oral herpes simplex infections in patients with leukemia. J Am Dent Assoc. 1987;114:483-486.
103 Gregory L, Williams R, Thompson E. Leucocyte function in Down’s syndrome and acute leukaemia. Lancet. 1972;1:1359-1361.
104 Grodstein F, Colditz GA, Stampfer MJ. Post-menopausal hormone use and tooth loss: a prospective study. J Am Dent Assoc. 1996;127:370-377. quiz 392
105 Grodstein F, Colditz GA, Stampfer MJ. Tooth loss and hormone use in postmenopausal women. Compend Contin Educ Dent Suppl. 1998:S9-S16.
106 Grossi SG, Zambon JJ, Ho AW, et al. Assessment of risk for periodontal disease. I. Risk indicators for attachment loss. J Periodontol. 1994;65:260-267.
107 Gur A, Nas K, Kayhan O, et al. The relation between tooth loss and bone mass in postmenopausal osteoporotic women in Turkey: a multicenter study. J Bone Miner Metab. 2003;21:43-47.
108 Gusberti FA, Syed SA, Bacon G, et al. Puberty gingivitis in insulin-dependent diabetic children. I. Cross-sectional observations. J Periodontol. 1983;54:714-720.
109 Hayden P, Buckley LA. Diabetes mellitus and periodontal disease in an Irish population. J Periodontal Res. 1989;24:298-302.
110 Henrikson PA. Periodontal disease and calcium deficiency. An experimental study in the dog. Acta Odontol Scand. 1968;26(Suppl 50):51-132.
111 Higgins W. Systematic poisoning with bismuth. JAMA. 1916;66:648.
112 Hirschfeld I. Periodontal symptoms associated with diabetes. J Periodontal. 1934;5:37.
113 Hodges RE, Baker EM, Hood J, et al. Experimental scurvy in man. Am J Clin Nutr. 1969;22:535-548.
114 Holm-Pederson P, Loe H. Flow of gingival exudate as related to menstruation and pregnancy. J Periodontal. 1967;2:13.
115 Hugoson A. Gingival inflammation and female sex hormones. A clinical investigation of pregnant women and experimental studies in dogs. J Periodontal Res Suppl. 1970;5:1-18.
116 Iacopino AM. Periodontitis and diabetes interrelationships: role of inflammation. Ann Periodontol. 2001;6:125-137.
117 Inagaki K, Kurosu Y, Yoshinari N, et al. Efficacy of periodontal disease and tooth loss to screen for low bone mineral density in Japanese women. Calcif Tissue Int. 2005;77:9-14.
118 Ismail AI, Burt BA, Eklund SA. Relation between ascorbic acid intake and periodontal disease in the United States. J Am Dent Assoc. 1983;107:927-931.
119 Izumi Y, Sugiyama S, Shinozuka O, et al. Defective neutrophil chemotaxis in Down’s syndrome patients and its relationship to periodontal destruction. J Periodontol. 1989;60:238-242.
120 Jeffcoat MK, Cizza G, Shih WJ, et al. Efficacy of bisphosphonates for the control of alveolar bone loss in periodontitis. J Int Acad Periodontol. 2007;9:70-76.
121 Jones R. Symptoms in early stages of industrial plumbism. JAMA. 1935;104:105.
122 Jung J, Carranza FAJr, Newman MG. Scanning electron-microscopy of plague in Papillon-Lefèvre syndrome. J Periodontol. 1981;52:442-446.
123 Kalkwarf KL. Effect of oral contraceptive therapy on gingival inflammation in humans. J Periodontol. 1978;49:560-563.
124 Kaner A, Losch P, Green M. Oral manifestations of congenital heart disease. J Pediatr. 1946;29:269.
125 Kardachi BJ, Newcomb GM. A clinical study of gingival inflammation in renal transplant recipients taking immunosuppressive drugs. J Periodontol. 1978;49:307-309.
126 Kastlin G. Agranulocytic angina. Am J Med Sci. 1927;173:799.
127 Khan AJ, Evans HE, Glass L, et al. Defective neutrophil chemotaxis in patients with Down syndrome. J Pediatr. 1975;87:87-89.
128 Kim JE, Shklar G. The effect of vitamin E on the healing of gingival wounds in rats. J Periodontol. 1983;54:305-308.
129 King J. Vincent’s diesease treated with nicotinic acid. Lancet. 1940;2:32.
130 Klemetti E, Collin HL, Forss H, et al. Mineral status of skeleton and advanced periodontal disease. J Clin Periodontol. 1994;21:184-188.
131 Knight GM, Wade AB. The effects of hormonal contraceptives on the human periodontium. J Periodontal Res. 1974;9:18-22.
132 Kolodzinski E, Munoa N, Malatesta E. Clinical study of gingival tissue in pregnant women. J Dent Res. 1974;53:693.
133 Kornman KS, Loesche WJ. The subgingival microbial flora during pregnancy. J Periodontal Res. 1980;15:111-122.
134 Kracke R: Granulopenia as associated with amidopyrine administration presented at annual session of American Medical Association, 1934.
135 Krohn S. The effect of the administration of steroid hormones on the gingival tissues. J Periodontal. 1958;29:300.
136 Lee R, Lee NZ. The peripheral vascular system and its reactions in scurvy: an experimental study. Am J Physiol. 1947;149:465.
137 Leibson CL, Tosteson AN, Gabriel SE, et al. Mortality, disability, and nursing home use for persons with and without hip fracture: a population-based study. J Am Geriatr Soc. 2002;50:1644-1650.
138 Lerner UH. Inflammation-induced bone remodeling in periodontal disease and the influence of post-menopausal osteoporosis. J Dent Res. 2006;85:596-607.
139 Liberman H. Chromone ulcerations of the nose and throat. N Engl J Med. 1941;225:132.
140 Lindhe J, Attsfrom R. Gingival exudation during the menstrual cycle. J Periodontal Res. 1967;2:194-198.
141 Lindhe J, Bjorn AL. Influence of hormonal contraceptives on the gingiva of women. J Periodontal Res. 1967;2:1-6.
142 Lindhe J, Branemark PI. Changes in microcirculation after local application of sex hormones. J Periodontal Res. 1967;2:185-193.
143 Loe H. Periodontal changes in pregnancy. J Periodontal. 1965;36:209.
144 Loe H. Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care. 1993;16:329-334.
145 Loushine RJ, Weller RN, Kimbrough WF, Liewehr FR. Secondary hyperparathyroidism: a case report. J Endod. 2003;29:272-274.
146 Lundgren T, Renvert S, Papapanou PN, Dahlen G. Subgingival microbial profile of Papillon-Lefèvre patients assessed by DNA-probes. J Clin Periodontol. 1998;25:624-629.
147 Lundstrom A, Jendle J, Stenstrom B, Toss G, Ravald N. Periodontal conditions in 70-year-old women with osteoporosis. Swed Dent J. 2001;25:89-96.
148 Lynch MA, ShipII. Initial oral manifestations of leukemia. J Am Dent Assoc. 1967;75:932-940.
149 Madison F, Squier TL. Primary granulocytopenia after administration of benzene chain derivatives. Jama. 1934;102:755.
150 Maier AW, Orban B. Gingivitis in pregnancy. Oral Surg Oral Med Oral Pathol. 1949;2:334-373.
151 Mann A, Spies TD, Springer M. Oral manifestations of vitamin B complex deficiencies. J Dent Res. 1941;20:269.
152 Manson-Bahr P, Ransford ON. Stomatitis of vitamin B2 deficiency treated with nicotinic acid. Lancet. 1938;2:426.
153 Mark H. Agranulocytic angina: its oral manifestations. J Am Dent Assoc. 1934;21:119.
154 Martinez Lalis R, Lopez Otero R, Carranza FAJr. A case of Papillon-Lefèvre syndrome. Periodontics. 1965;3:292.
155 Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003;61:1115-1117.
156 Marx RE. Oral & intravenous bisphosphonate-induced osteonecrosis of the jaws: history, etiology, prevention, and treatment, pp viii. Chicago: Quintessence Pub. Co.; 2007. p 150
157 Marx RE, Sawatari Y, Fortin M, Broumand V. Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg. 2005;63:1567-1575.
158 Mascola RFJr. The oral manifestations of diabetes mellitus. A review. N Y State Dent J. 1970;36:139-142.
159 Mashimo PA, Yamamoto Y, Slots J, et al. The periodontal microflora of juvenile diabetics. Culture, immunofluorescence, and serum antibody studies. J Periodontol. 1983;54:420-430.
160 Massler M. Oral manifestations during the female climacteric (the postmenopausal syndrome). Oral Surg Oral Med Oral Pathol. 1951;4:1234-1243.
161 McCarthy P, Shklar G. Disease of the oral mucosa, ed 2. Philadelphia: Lea & Febiger; 1980.
162 McMullen JA, Van Dyke TE, Horoszewicz HU, Genco RJ. Neutrophil chemotaxis in individuals with advanced periodontal disease and a genetic predisposition to diabetes mellitus. J Periodontol. 1981;52:167-173.
163 Mealey BL. Influence of periodontal infections on systemic health. Periodontol 2000. 1999;21:197-209.
164 Mealey BL, Moritz AJ. Hormonal influences: effects of diabetes mellitus and endogenous female sex steroid hormones on the periodontium. Periodontol 2000. 2003;32:59-81.
165 Menezes AM, Rocha FA, Chaves HV, et al. Effect of sodium alendronate on alveolar bone resorption in experimental periodontitis in rats. J Periodontol. 2005;76:1901-1909.
166 Meyer AH. Agranulocytosis: Report of case caused by sulfadiazine. Cal West Med. 1944;60:277.
167 Mitsuta T, Horiuchi H, Shinoda H. Effects of topical administration of clodronate on alveolar bone resorption in rats with experimental periodontitis. J Periodontol. 2002;73:479-486.
168 Mohammad AR, Bauer RL, Yeh CK. Spinal bone density and tooth loss in a cohort of postmenopausal women. Int J Prosthodont. 1997;10:381-385.
169 Mohammad AR, Hooper DA, Vermilyea SG, et al. An investigation of the relationship between systemic bone density and clinical periodontal status in post-menopausal Asian-American women. Int Dent J. 2003;53:121-125.
170 Mohammed A, Waterhouse JP, Friederici HH. The microvasculature of the rat gingiva as affected by progesterone. an ultrastructural study J Periodontal. 1974;45:50.
171 Montiero da Silva A, Newman HN, Oakley DA, et al. Psychosocial factors and adult onset rapidly progressive periodontitis. J Clin Periodontol. 1996;23:789.
172 Montiero da Silva A, Newman HN, Oakley DA, et al. Psychosocial factors, dental plaque levels, and smoking in periodontitis patients. J Clin Periodontol. 1998;25:217.
173 Nichols C, Laster LL, Bodak-Gyovai LZ. Diabetes mellitus and periodontal disease. J Periodontol. 1978;49:85-88.
174 Nishida M, Grossi SG, Dunford RG, et al. Dietary vitamin C and the risk for periodontal disease. J Periodontol. 2000;71:1215-1223.
175 Novaes ABJr, Pereira AL, de Moraes N, Novaes AB. Manifestations of insulin-dependent diabetes mellitus in the periodontium of young Brazilian patients. J Periodontol. 1991;62:116-122.
176 O’Leary T, Shannon I, Prigmore JR. Clinical and systematic findings in periodontal disease. J Periodontal. 1962;32:243.
177 O’Neil TC. Maternal T-lymphocyte response and gingivitis in pregnancy. J Periodontol. 1979;50:178-184.
178 O’Uchi N, Nishikawa H, Yoshino T, et al. Inhibitory effects of YM175, a bisphosphonate, on the progression of experimental periodontitis in beagle dogs. J Periodontal Res. 1998;33:196-204.
179 Oh T-J, Bashutski J, Giannobile WV. The interrelationship between osteoporosis and oral bone loss. Grand Rounds in Oral Systemic Medicine. 2007:2.
180 Oshrain HI, Mender S, Mandel ID. Periodontal status of patients with reduced immunocapacity. J Periodontol. 1979;50:185-188.
181 Otomo-Corgel J. Implants and oral bisphosphonates: risky business? J Periodontol. 2007;78:373-376.
182 Pack AR, Thomson ME. Effects of topical and systemic folic acid supplementation on gingivitis in pregnancy. J Clin Periodontol. 1980;7:402-414.
183 Page RC. The pathobiology of periodontal diseases may affect systemic diseases: inversion of a paradigm. Ann Periodontol. 1998;3:108-120.
184 Page RC, Bowen T, Altman L, et al. Prepubertal periodontitis. I. Definition of a clinical disease entity. J Periodontol. 1983;54:257-271.
185 page RC, Schroeder HE. Periodontitis in man and other animal a comparative review. Basel Karger. 1982.
186 Papillion MM, Lefèvre LP. Deux cas de keratodermie palmaire et plantaire symmetrique famiale (maladie de Meleda) chez le frère et la soeur: coéxistance dans les deux cas d’altérations dentaires graves. Bull Soc Derma Symp. 1924;31:82-87.
187 Parrish JHJr, DeMarco TJ, Bissada NF. Vitamin E and periodontitis in the rat. Oral Surg Oral Med Oral Pathol. 1977;44:210-218.
188 Patrone F, Dallegri F, Rebora A, Sacchetti C. Lazy leukocyte syndrome. Blut. 1979;39:265-269.
189 Perry DA. Oral contraceptives and periodontal health. J West Soc Periodontol Periodontal Abstr. 1981;29:72-80.
190 Peruzzo DC, Benatti BB, Ambrosano GM, et al. A systematic review of stress and psychological factors as possible risk factors for periodontal disease. J Periodontol. 2007;78:1491-1504.
191 Pinard A. Gingivitis in pregnancy. Dent Register. 1877;31:258.
192 Prevention CDC, National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2007, Atlanta, GA, 2008, U.S. Department of Health and Human Services Centers for Disease Control and Prevention
193 Prout RE, Hopps RM. A relationship between human oral bacteria and the menstrual cycle. J Periodontol. 1970;41:98-101.
194 Randall C. Granulocytopenia following barbiturates and amidopyrine. JAMA. 1934;102:1137.
195 Rateitschak KH. Tooth mobility changes in pregnancy. J Periodontal Res. 1967;2:199-206.
196 Reinhardt RA, Payne JB, Maze CA, et al. Influence of estrogen and osteopenia/osteoporosis on clinical periodontitis in postmenopausal women. J Periodontol. 1999;70:823-828.
197 Restarski J, Pijoan M. Gingivitis and vitamin C. J Am Dent Assoc. 1944;31:1323.
198 Reuland-Bosma W, van Dijk J. Periodontal disease in Down’s syndrome: a review. J Clin Periodontol. 1986;13:64-73.
199 RIchman J, Abarbanel AR. Effects of estradiol, testosterone, diethylstilbestrol and several of their derivatives upon the human mucous membrane. J Am Dent Assoc. 1943;30:913.
200 Robbins SL, Cotran RS, Kumar V. Pathologic basis of disease, ed 4. Philadelphia: Saunders; 1989.
201 Robertson HD, Polk HCJr. The mechanism of infection in patients with diabetes mellitus: a review of leukocyte malfunction. Surgery. 1974;75:123-128.
202 Rosenberg E, Guralnick WC. Hyperparathyroidism. Oral Surg. 1962;15(suppl 2):84.
203 Ruggiero SL, Dodson TB, Assael LA, et al. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws–2009 update. J Oral Maxillofac Surg. 2009;67:2-12.
204 Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg. 2004;62:527-534.
205 Russell AL. International nutrition surveys: a summary of preliminary dental findings. J Dent Res. 1963;42(1)Pt 2:233-244.
206 Russell RG. Bisphosphonates: mode of action and pharmacology. Pediatrics. 2007;119(Suppl 2):S150-S162.
207 Safkan-Seppala B, Ainamo J. Periodontal conditions in insulin-dependent diabetes mellitus. J Clin Periodontol. 1992;19:24-29.
208 Salvi GE, Lawrence HP, Offenbacher S, Beck JD. Influence of risk factors on the pathogenesis of periodontitis. Periodontol 2000. 1997;14:173-201.
209 Sandhu HS, Sharma V, Sidhu GS. Role of psychiatric disorders in self-inflicted periodontal injury: a case report. J Periodontol. 1997;68:1136-1139.
210 Sastrowijoto SH, Hillemans P, van Steenbergen TJ, et al. Periodontal condition and microbiology of healthy and diseased periodontal pockets in type 1 diabetes mellitus patients. J Clin Periodontol. 1989;16:316-322.
211 Schmidt AM, Weidman E, Lalla E, et al. Advanced glycation endproducts (AGEs) induce oxidant stress in the gingiva: a potential mechanism underlying accelerated periodontal disease associated with diabetes. J Periodontal Res. 1996;31:508-515.
212 Schneir M, Imberman M, Ramamurthy N, Golub L. Streptozotocin-induced diabetes and the rat periodontium: decreased relative collagen production. Coll Relat Res. 1988;8:221-232.
213 Schour I, Sarnat BG. Oral manifestations of occupational origin. JAMA. 1942;120:1197.
214 Schroeder HE, Seger RA, Keller HU, Rateitschak-Pluss EM. Behavior of neutrophilic granulocytes in a case of Papillon-Lefèvre syndrome. J Clin Periodontol. 1983;10:618-635.
215 Scully C, MacFadyen E, Campbell A. Oral manifestations in cyclic neutropenia. Br J Oral Surg. 1982;20:96-101.
216 Shaw J. The relation of nutrition to periodontal disease. J Dent Res. 1962;41(suppl 1):264.
217 Sheridan P. Diabetes and oral health. J Am Dent Assoc. 1987;115:741-742.
218 Shilotri PG, Bhat KS. Effect of mega doses of vitamin C on bactericidal ativity of leukocytes. Am J Clin Nutr. 1977;30:1077-1081.
219 Shoji K, Horiuchi H, Shinoda H. Inhibitory effects of a bisphosphonate (risedronate) on experimental periodontitis in rats. J Periodontal Res. 1995;30:277-284.
220 Silverman SJr, Gordan G, Grant T, et al. The dental structures in primary hyperparathyroidism. Studies in forty-two consecutive patients. Oral Surg Oral Med Oral Pathol. 1962;15:426-436.
221 Stahl SS. The effect of a protein-free diet on the healing of gingival wounds in rats. Arch Oral Biol. 1962;7:551-556.
222 Stahl SS, Sandler HC, Cahn L. The effects of protein deprivation upon the oral tissues of the rat and particularly upon periodontal structures under irritation. Oral Surg Oral Med Oral Pathol. 1955;8:760-768.
223 Strock M. The mouth in hyperparathyroidsim. N Engl J Med. 1945;224:1019.
224 Sutcliffe P. A longitudinal study of gingivitis and puberty. J Periodontal Res. 1972;7:52-58.
225 Svanberg G, Lindhe J, Hugoson A, Grondahl HG. Effect of nutritional hyperparathyroidism on experimental periodontitis in the dog. Scand J Dent Res. 1973;81:155-162.
226 Sznajder N, Carraro JJ, Otero E, Carranza FAJr. Clinical periodontal findings in trisomy 21 (mongolism). J Periodontal Res. 1968;3:1-5.
227 Sznajder N, Carraro JJ, Rugna S, Sereday M. Periodontal findings in diabetic and nondiabetic patients. J Periodontol. 1978;49:445-448.
228 Taguchi A, Sanada M, Suei Y, et al. Effect of estrogen use on tooth retention, oral bone height, and oral bone porosity in Japanese postmenopausal women. Menopause. 2004;11:556-562.
229 Takaishi Y, Miki T, Nishizawa Y, Morii H. Clinical effect of etidronate on alveolar pyorrhoea associated with chronic marginal periodontitis: report of four cases. J Int Med Res. 2001;29:355-365.
230 Tan JS, Anderson JL, Watanakunakorn C, Phair JP. Neutrophil dysfunction in diabetes mellitus. J Lab Clin Med. 1975;85:26-33.
231 Telsey B, Beube FE, Zegarelli EV, Kutscher AH. Oral manifestations of cyclical neutropenia associated with hypergammaglobulinemia; report of a case. Oral Surg Oral Med Oral Pathol. 1962;15:540-543.
232 Tenenbaum HC, Shelemay A, Girard B, et al. Bisphosphonates and periodontics: potential applications for regulation of bone mass in the periodontium and other therapeutic/diagnostic uses. J Periodontol. 2002;73:813-822.
233 Tervonen T, Knuuttila M, Pohjamo L, Nurkkala H. Immediate response to nonsurgical periodontal treatment in subjects with diabetes mellitus. J Clin Periodontol. 1991;18:65-68.
234 Tezal M, Wactawski-Wende J, Grossi SG, et al. The relationship between bone mineral density and periodontitis in postmenopausal women. J Periodontol. 2000;71:1492-1498.
235 Tinanoff N, Tempro P, Maderazo EG. Dental treatment of Papillon-Lefèvre syndrome: 15-year follow-up. J Clin Periodontol. 1995;22:609-612.
236 Tollefsen T, Saltvedt E, Koppang HS. The effect of immunosuppressive agents on periodontal disease in man. J Periodontal Res. 1978;13:240-250.
237 Topping N, Fraser HF. Mouth lesions associated with dietary deficiencies in monkeys. Public Health Rep. 1939;54:416.
238 Turesky S, Fisher B, Glickman I. A histochemical study of the attached gingiva in pregnancy. J Dent Res. 1958;37:1115-1122.
239 Vogel RI. Relationship of folic acid to phenytoin-induced gingival overgrowth. In: Hassel TM, Johnson M, Dudley K, editors. Phenytoin-induced teratology and gingival pathology. New York: Raven Press, 1980.
240 Vogel RI, Deasy M, Alfano M, et al. The effect of folic acid on gingival health of women taking oral contraceptives. J Prev Dental. 1980;6:221.
241 Vogel RI, Fink RA, Frank O, Baker H. The effect of topical application of folic acid on gingival health. J Oral Med. 1978;33:20-22.
242 Vogel RI, Fink RA, Schneider LC, Frank O, Baker H. The effect of folic acid on gingival health. J Periodontol. 1976;47:667-668.
243 Vrahopoulos TP, Barber P, Liakoni H, Newman HN. Ultrastructure of the periodontal lesion in a case of Papillon-Lefèvre syndrome (PLS). J Clin Periodontol. 1988;15:17-26.
244 Wade ML, Suzuki JB. Issues related to diagnosis and treatment of bisphosphonate-induced osteonecrosis of the jaws. Grand Rounds in Oral Systemic Medicine. 2007;2:46-53.
245 Waerhaug J. Effect of C-avitaminosis on the supporting structures of the teeth. J Periodontal. 1958;47:667.
246 Waerhaug J. Epidemiology of periodontal disease. American Academy of Periodontology University Michigan Press; 1966.
247 Waldrop TC, Anderson DC, Hallmon WW, et al. Periodontal manifestations of the heritable Mac-1, LFA-1, deficiency syndrome. Clinical, histopathologic and molecular characteristics. J Periodontol. 1987;58:400-416.
248 Wang EP, Kaban LB, Strewler GJ, et al. Incidence of osteonecrosis of the jaw in patients with multiple myeloma and breast or prostate cancer on intravenous bisphosphonate therapy. J Oral Maxillofac Surg. 2007;65:1328-1331.
249 Weinmann J, Schour I. The effect of parathyroid hormone on the alveolar bone and teeth of the normal and rachitic rat. AM J Patho. 1945;21:857.
250 Weinmann J, Schour I. Experimental studies in calcification. AM J Patho. 1945;21:821.
251 Weinreb M, Quartuccio H, Seedor JG, et al. Histomorphometrical analysis of the effects of the bisphosphonate alendronate on bone loss caused by experimental periodontitis in monkeys. J Periodontal Res. 1994;29:35-40.
252 Wiebe CB, Hakkinen L, Putnins EE, et al. Successful periodontal maintenance of a case with Papillon-Lefèvre syndrome: 12-year follow-up and review of the literature. J Periodontol. 2001;72:824-830.
253 Wimmer G, Janda M, Wieselmann-Penkner K, et al. Coping with stress: its influence on periodontal disease. J Periodontol. 2002;73:1343-1351.
254 Wingrove FA, Rubright WC, Kerber PE. Influence of ovarian hormone situation on atrophy, hypertrophy, and/or desquamation of human gingiva in premenopausal and postmenopausal women. J Periodontol. 1979;50:445-449.
255 Wolbach S, Bessey OA. Tissue changes in vitamin deficiencies. Physiol Rev. 1942;22:233.
256 Woolfe SN, Hume WR, Kenney EB. Ascorbic acid and periodontal disease: a review of the literature. J West Soc Periodontol Periodontal Abstr. 1980;28:44-56.
257 Woolfe SN, Kenney EB, Hume WR, Carranza FAJr. Relationship of ascorbic acid levels of blood and gingival tissue with response to periodontal therapy. J Clin Periodontol. 1984;11:159-165.
258 Wray D, Lowe GDO, Dagg JH, et al. Systemic disease: oral manifestations and effects on oral health. Edinburgh: Churchill Livingstone; 1999.
259 Yendt ER. The parathyroids and calcium metabolism. Philadelphia: Harper and Row; 1986.
260 Yoshihara A, Seida Y, Hanada N, et al. The relationship between bone mineral density and the number of remaining teeth in community-dwelling older adults. J Oral Rehabil. 2005;32:735-740.
261 Zambon JJ, Reynolds H, Fisher JG, et al. Microbiological and immunological studies of adult periodontitis in patients with noninsulin-dependent diabetes mellitus. J Periodontol. 1988;59:23-31.
262 Ziskin D, Blackberg SN. A study of the gingivae during pregnancy. J Dent Res. 1933;13:253.
263 Ziskin D, Blackberg SN, Stout A. The gingivae during pregnancy: an experimental study and a histopathological interpretation. Surg Gyneocol Obstet. 1933;57:1933.