CHAPTER 47 Antiinfective Therapy
It is well established that the various periodontal diseases are caused by bacterial infection. Bacteria begin reattaching to the crowns of teeth soon after the teeth have been cleaned and begin to form a biofilm. Over time, this supragingival plaque biofilm becomes more complex, leading to a succession of bacteria that are more pathogenic. Bacteria grow in an apical direction and become subgingival, and eventually, as bone is destroyed, a periodontal pocket is formed. In a periodontal pocket the bacteria form a highly structured and complex biofilm. As this process continues, the bacterial biofilm extends so far subgingivally that the patient cannot reach it during oral hygiene efforts. Additionally, this complex biofilm now may offer some protection from the host’s immunologic mechanisms in the periodontal pocket, as well as from antibiotics used for treatment. It has been suggested that an antibiotic strength 500 times greater than the usual therapeutic dose may be needed to be effective against bacteria arranged in biofilms.40
It is therefore logical to treat periodontal pockets by mechanical removal of local factors (including calculus that harbors bacteria) and also by disruption of the subgingival plaque biofilm itself. Mechanical removal includes manual instrumentation (e.g., scaling and root planing) and machine-driven instrumentation (e.g., ultrasonic scalers), and these procedures can be considered “antiinfective therapy.” Many chemotherapeutic agents are now available to clinicians treating periodontal diseases. Systemic antiinfective therapy (oral antibiotics) and local antiinfective therapy (placing antiinfective agents directly into the periodontal pocket) can reduce the bacterial challenge to the periodontium. It is also possible that systemically administered nonsteroidal antiinflammatory agents (NSAIDs) may play a role in future adjunctive therapy.68,93
Bacteria and their toxic products cause loss of attachment and loss of bone. Ultimately, however, the host’s own immunologic response to this bacterial infection can cause even more bone destruction (“indirect bone loss”) than that caused by pathogenic bacteria and their by-products. This immunologic response can be influenced by environmental (e.g., tobacco use), acquired (e.g., systemic disease), and genetic risk factors.77 Chemotherapeutic agents can modulate the host’s immune response to bacteria and reduce the host’s self-destructive immunologic response to bacterial pathogens and thus reduce bone loss.71,72,75 It is also incumbent on health care providers to counsel patients concerning the detrimental effects of systemic factors, including medications, stress, and tobacco use.40
This chapter reviews the indications and protocols for optimizing the use of antiinfective agents in the treatment of periodontal diseases.
It is important to note that significant work has been performed in a systematic evidence-based approach to evaluate the various antiinfective and host modulation therapies99 (see Chapters 78 and 79). Metaanalysis of similar research studies has given power to statistical analysis for evaluating antiinfective chemotherapeutic agents in the treatment of periodontal diseases. Unfortunately, a standardized research protocol has not yet been implemented. Therefore some studies, although relevant, have not been used in the evidence-based approach because of their study design. Further evidence-based and similar research is needed to define protocols more precisely for the use of antiinfective agents in treating periodontal diseases.
Definitions
An antiinfective agent is a chemotherapeutic agent that acts by reducing the number of bacteria present. An antibiotic is a naturally occurring, semisynthetic, or synthetic type of antiinfective agent that destroys or inhibits the growth of selective microorganisms, generally at low concentrations. An antiseptic is a chemical antimicrobial agent applied topically or subgingivally to mucous membranes, wounds, or intact dermal surfaces to destroy microorganisms and inhibit their reproduction or metabolism. In dentistry, antiseptics are widely used as the active ingredient in antiplaque and antigingivitis oral rinses and dentifrices. Disinfectants, a subcategory of antiseptics, are antimicrobial agents that are generally applied to inanimate surfaces to destroy microorganisms.21
Antiinfective agents can be administered locally or orally. When administered orally, many of these agents can be found in gingival crevicular fluid (GCF). With either approach, their purpose is to reduce the number of bacteria present in the diseased periodontal pocket. Systemic administration of antibiotics may be a necessary adjunct in controlling bacterial infection because bacteria can invade periodontal tissues, making mechanical therapy alone sometimes ineffective.4,18,20,31,76 Local administration of antiinfective agents, generally directly in the pocket, has the potential to provide greater concentrations directly to the infected area and reduce possible systemic side effects.
Additionally, a single chemotherapeutic agent can have a dual mechanism of action. For example, tetracyclines (especially doxycycline) are chemotherapeutic agents that can reduce collagen and bone destruction through their ability to inhibit the enzyme collagenase. As antibiotic agents, they also can reduce periodontal pathogens in periodontal tissues.20
Systemic Administration of Antibiotics
Background and Rationale
The treatment of periodontal diseases is based on the infectious nature of these diseases (Table 47-1). Ideally, the causative microorganism(s) should be identified and the most effective agent should be selected using antibiotic-sensitivity tests. Although this appears simple, the difficulty lies primarily in identifying specific etiologic microorganism(s) rather than microorganisms simply associated with various periodontal disorders.19,20
TABLE 47-1 Antibiotics Used to Treat Periodontal Diseases
| Category | Agent | Major Features |
|---|---|---|
| Penicillin* | Amoxicillin Augmentin† |
Extended spectrum of antimicrobial activity; excellent oral absorption; used systemically. Effective against penicillinase-producing microorganisms; used systemically. |
| Tetracyclines | Minocycline Doxycycline Tetracycline |
Effective against broad spectrum of microorganisms; used systemically and applied locally (subgingivally). Effective against broad spectrum of microorganisms; used systemically and applied locally (subgingivally). Chemotherapeutically used in sub-antimicrobial dose for host modulation (Periostat). Effective against broad spectrum of microorganisms. |
| Quinolone | Ciprofloxacin | Effective against gram-negative rods; promotes health-associated microflora. |
| Macrolide | Azithromycin | Concentrates at sites of inflammation; used systemically. |
| Lincomycin derivative | Clindamycin | Used in penicillin-allergic patients; effective against anaerobic bacteria; used systemically. |
| Nitroimidazole‡ | Metronidazole | Effective against anaerobic bacteria; used systemically and applied locally (subgingivally) as gel. |
* Indications: localized aggressive periodontitis (LAP), generalized aggressive periodontitis (GAP), medically related periodontitis (MRP), refractory periodontitis (RP).
† Amoxicillin and clavulanate potassium.
‡ Indications: LAP, GAP, MRP, RP, necrotizing ulcerative periodontitis.
An ideal antibiotic for use in prevention and treatment of periodontal diseases should be specific for periodontal pathogens, allogenic and nontoxic, substantive, not in general use for treatment of other diseases, and inexpensive.32 Currently, an ideal antibiotic for the treatment of periodontal diseases does not exist.49 Although oral bacteria are susceptible to many antibiotics, no single antibiotic at concentrations achieved in body fluids inhibits all putative periodontal pathogens.97 Indeed, a combination of antibiotics may be necessary to eliminate all putative pathogens from some periodontal pockets70 (Table 47-2).
TABLE 47-2 Common Antibiotic Regimens Used to Treat Periodontal Diseases*
| Regimen | Dosage/Duration | |
|---|---|---|
| Single Agent | ||
| Amoxicillin | 500 mg | Three times daily for 8 days |
| Azithromycin | 500 mg | Once daily for 4–7 days |
| Ciprofloxacin | 500 mg | Twice daily for 8 days |
| Clindamycin | 300 mg | Three times daily 10 days |
| Doxycycline or minocycline | 100–200 mg | Once daily for 21 days |
| Metronidazole | 500 mg | Three times daily for 8 days |
| Combination Therapy | ||
| Metronidazole + amoxicillin | 250 mg of each | Three times daily for 8 days |
| Metronidazole + ciprofloxacin | 500 mg of each | Twice daily for 8 days |
* These regimens are prescribed with a review of the patient’s medical history, periodontal diagnosis, and antimicrobial testing. Clinicians must consult pharmacology references such as Mosby’s GenRx67 or manufacturer’s guidelines for warnings, contraindications, and precautions.
Data from Jorgensen MG, Slots J: Compend Contin Educ Dent 21:111, 2000.
As always, the clinician in concert with the patient must make the final decision on any treatment. Thus the treatment of the individual patient must be based on the patient’s clinical status, nature of the colonizing bacteria, ability of the agent to reach the site of infection, and the risks and benefits associated with the proposed treatment plan. The clinician is responsible for choosing the correct antimicrobial agent. Some adverse reactions include allergic/anaphylactic reactions, superinfections of opportunistic bacteria, development of resistant bacteria, interactions with other medications, upset stomach, nausea, and vomiting.6 Most adverse reactions take the form of gastrointestinal upset.49 Other concerns include the cost of the medication and the patient’s willingness and ability to comply with the proposed therapy.
No consensus exists concerning the magnitude of the risk for developing bacterial resistance. Common and indiscriminate use of antibiotics worldwide has contributed to increasing numbers of resistant bacterial strains over the last 15 to 20 years, and this trend is likely to continue given the widespread use of antibiotics.96 The overuse, misuse, and widespread prophylactic application of antiinfective drugs are some of the factors that have led to the emergence of resistant microorganisms. Increasing levels of resistance of subgingival microflora to antibiotics has been correlated with the increased use of antibiotics in individual countries.91 However, researchers have noted that the subgingival microflora tends to revert to similar proportions of antibiotic-resistant isolates 3 months after therapy.29,43
Tetracyclines
Tetracyclines have been widely used in the treatment of periodontal diseases. They have been frequently used in treating refractory periodontitis, including localized aggressive periodontitis (LAP)100 (see Table 47-1). Tetracyclines have the ability to concentrate in the periodontal tissues and inhibit the growth of Aggregatibacter actinomycetemcomitans. In addition, tetracyclines exert an anticollagenase effect that can inhibit tissue destruction and may aid bone regeneration.17,59,95
Pharmacology
The tetracyclines are a group of antibiotics produced naturally from certain species of Streptomyces or derived semisynthetically. These antibiotics are bacteriostatic and are effective against rapidly multiplying bacteria. They generally are more effective against gram-positive bacteria than gram-negative bacteria. Tetracyclines are effective in treating periodontal diseases in part because their concentration in the gingival crevice is 2 to 10 times that in serum.2,7,37 This allows a high drug concentration to be delivered into periodontal pockets. In addition, several studies have demonstrated that tetracyclines at a low GCF concentration (2 to 4 µg/ml) are very effective against many periodontal pathogens.8,9
Clinical Use
Tetracyclines have been investigated as adjuncts in the treatment of LAP.82 A. actinomycetemcomitans is a frequent microorganism associated with LAP and is tissue invasive. Therefore mechanical removal of calculus and plaque from root surfaces may not eliminate this bacterium from the periodontal tissues. Systemic tetracycline can eliminate tissue bacteria and has been shown to arrest bone loss and suppress A. actinomycetemcomitans levels in conjunction with scaling and root planing.81 This combination therapy allows mechanical removal of root surface deposits and elimination of pathogenic bacteria from within the tissues. Increased posttreatment bone levels have been noted using this method.85 Because of increased resistance to tetracyclines, metronidazole or amoxicillin with metronidazole has been found more effective in treating aggressive periodontitis in children. Some investigators believe metronidazole combined with amoxicillin–clavulanic acid is the preferable antibiotic.94
Long-term use of low antibacterial doses of tetracyclines has been advocated in the past. One long-term study of patients taking low doses of tetracycline (250 mg/day for 2 to 7 years) showed persistence of deep pockets that did not bleed on probing. These sites contained high proportions of tetracycline-resistant, gram-negative rods (Fusobacterium nucleatum). After the antibiotic was discontinued, the flora was characteristic of sites with disease.49 Therefore it is not advisable to prescribe long-term regimens of tetracyclines because of the possible development of resistant bacterial strains.55 Although often used in the past as antiinfective agents, especially for LAP and other types of aggressive periodontitis, tetracyclines now tend to be replaced by more effective combination antibiotics.49
Specific Agents
Tetracycline, minocycline, and doxycycline are semisynthetic members of the tetracycline group that have been used in periodontal therapy.
Tetracycline
Tetracycline HCl requires administration of 250 mg, 4 times daily (qid). It is inexpensive, but compliance may be reduced by having to take four capsules per day. Side effects are gastrointestinal (GI) disturbances, photosensitivity, hypersensitivity, increased blood urea nitrogen (BUN), blood dyscrasias, dizziness, and headache. Also, tooth discoloration occurs when administered to children up to age 12 years.
Minocycline
Minocycline is effective against a broad spectrum of microorganisms. In patients with adult periodontitis, it suppresses spirochetes and motile rods as effectively as scaling and root planing, with suppression evident up to 3 months after therapy. Minocycline can be given twice daily (bid), thus facilitating compliance compared with tetracycline. Although associated with less phototoxicity and renal toxicity than tetracycline, minocycline may cause reversible vertigo. Minocycline administered 200 mg/day for 1 week results in a reduction in total bacterial counts, complete elimination of spirochetes for up to 2 months, and improvement in all clinical parameters.21,24
Side effects are similar to those of tetracycline; however, there is an increased incidence of vertigo. It is the only tetracycline that can discolor permanently erupted teeth and gingival tissue when administered orally.
Doxycycline
Doxycycline has the same spectrum of activity as minocycline and may be equally as effective.20 Because doxycycline can be given only once daily (qd), patients may be more compliant. Compliance is also favored because its absorption from the GI tract is only slightly altered by calcium, metal ions, or antacids, as is absorption of other tetracyclines. Side effects are similar to tetracycline HCl; however, it is the most photosensitizing agent in the tetracycline category.
The recommended dosage when used as an antiinfective agent is 100 mg bid the first day, then 100 mg qd. To reduce GI upset, 50 mg can be taken bid. When used in a sub-antimicrobial dose (to inhibit collagenase), doxycycline is recommended in a 20-mg dose twice daily.17,26 Periostat (CollaGenex Pharmaceuticals Inc, Newtown, PA) and generic forms are currently available in a dose of 20 mg of doxycycline.
Metronidazole
Pharmacology
Metronidazole is a nitroimidazole compound developed in France to treat protozoal infections. It is bactericidal to anaerobic organisms and is believed to disrupt bacterial deoxyribonucleic acid (DNA) synthesis in conditions with a low reduction potential. Metronidazole is not the drug of choice for treating A. actinomycetemcomitans infections. However, metronidazole is effective against A. actinomycetemcomitans when used in combination with other antibiotics.69,70 Metronidazole is also effective against anaerobes such as Porphyromonas gingivalis and Prevotella intermedia.39
Clinical Use
Metronidazole has been used clinically to treat gingivitis, acute necrotizing ulcerative gingivitis (NUG), chronic periodontitis, and aggressive periodontitis. It has been used as monotherapy and also in combination with both root planing and surgery or with other antibiotics. Metronidazole has been used successfully to treat NUG.61
Studies in humans have demonstrated the efficacy of metronidazole in the treatment of gingivitis and periodontitis.60 A single dose of metronidazole (250 mg orally) appears in both serum and GCF in sufficient quantities to inhibit a wide range of suspected periodontal pathogens. Administered systemically (750 to 1000 mg/day for 2 weeks), metronidazole reduces the growth of anaerobic flora, including spirochetes, and decreases the clinical and histopathologic signs of periodontitis.60 The most common regimen is 250 mg 3 times daily (tid) for 7 days.61 Currently, the critical level of spirochetes needed to diagnose an anaerobic infection, the appropriate time to give metronidazole, and the ideal dosage or duration of therapy are unknown.39 As monotherapy (no concurrent root planing), metronidazole is inferior and at best only equivalent to root planing. Therefore, if used, metronidazole should not be administered as monotherapy.
Soder et al83 showed that metronidazole was more effective than placebo in the management of sites unresponsive to root planing. Nevertheless, many patients still had sites that bled on probing despite metronidazole therapy. The existence of refractory periodontitis as a diagnostic consideration indicates that some patients do not respond to conventional therapy, including root planing, surgery, or both.
Studies have suggested that when combined with amoxicillin or amoxicillin–clavulanate potassium (Augmentin), metronidazole may be of value in the management of patients with LAP or refractory periodontitis (see later discussion).
Side Effects
Metronidazole has an Antabuse effect when alcohol is ingested. The response is generally proportional to the amount ingested and can result in severe cramps, nausea, and vomiting. Products containing alcohol should be avoided during therapy and for at least 1 day after therapy is discontinued. Metronidazole also inhibits warfarin metabolism. Patients undergoing anticoagulant therapy should avoid metronidazole because it prolongs prothrombin time.61 It also should be avoided in patients who are taking lithium. It also produces a metallic taste in the mouth, which may affect compliance.
Penicillins
Pharmacology
Penicillins are the drugs of choice for the treatment of many serious infections in humans and are the most widely used antibiotics. Penicillins are natural and semisynthetic derivatives of broth cultures of the Penicillium mold. They inhibit bacterial cell wall production and therefore are bactericidal.
Clinical Use
Penicillins other than amoxicillin and amoxicillin–clavulanate potassium (Augmentin) have not been shown to increase periodontal attachment levels, and their use in periodontal therapy does not appear to be justified.
Side Effects
Penicillins may induce allergic reactions and bacterial resistance; up to 10% of patients may be allergic to penicillin.
Amoxicillin
Amoxicillin is a semisynthetic penicillin with an extended antiinfective spectrum that includes gram-positive and gram-negative bacteria. It demonstrates excellent absorption after oral administration. Amoxicillin is susceptible to penicillinase, a β-lactamase produced by certain bacteria that breaks the penicillin ring structure and thus renders penicillins ineffective.
Amoxicillin may be useful in the management of patients with aggressive periodontitis, in both localized and generalized forms. Recommended dosage is 500 mg tid for 8 days.49,50
Amoxicillin–Clavulanate Potassium
The combination of amoxicillin with clavulanate potassium makes this antiinfective agent resistant to penicillinase enzymes produced by some bacteria. Amoxicillin with clavulanate (Augmentin) may be useful in the management of patients with LAP or refractory periodontitis.68 Bueno et al13 reported that Augmentin arrested alveolar bone loss in patients with periodontal disease that was refractory to treatment with other antibiotics, including tetracycline, metronidazole, and clindamycin.
Cephalosporins
Pharmacology
The family of β-lactams known as cephalosporins is similar in action and structure to penicillins. They are frequently used in medicine and are resistant to a number of β-lactamases normally active against penicillin.
Clinical Use
Cephalosporins are generally not used to treat dental-related infections. The penicillins are superior to cephalosporins in their range of action against periodontal pathogenic bacteria.
Side Effects
Patients allergic to penicillins must be considered allergic to all β-lactam products. Rashes, urticaria, fever, and GI upset have been associated with cephalosporins.96
Clindamycin
Pharmacology
Clindamycin is effective against anaerobic bacteria and has a strong affinity for osseous tissue.89 It is effective in situations in which the patient is allergic to penicillin.
Clinical Use
Clindamycin has shown efficacy in patients with periodontitis refractory to tetracycline therapy. Walker et al96 showed that clindamycin assisted in stabilizing refractory patients; dosage was 150 mg qid for 10 days. Jorgensen and Slots50 recommend a regimen of 300 mg bid for 8 days.
Side Effects
Clindamycin has been associated with pseudomembranous colitis, but the incidence is higher with cephalosporins and ampicillin. When needed, however, clindamycin can be used with caution, but it is not indicated in patients with a history of colitis. Diarrhea or cramping that develops during clindamycin therapy may be indicative of colitis, and clindamycin should be discontinued. If symptoms persist, the patient should be referred to an internist.
Ciprofloxacin
Pharmacology
Ciprofloxacin is a quinolone active against gram-negative rods, including all facultative and some anaerobic putative periodontal pathogens.80
Clinical Use
Because it demonstrates minimal effect on Streptococcus species, which are associated with periodontal health, ciprofloxacin therapy may facilitate the establishment of a microflora associated with periodontal health. At present, ciprofloxacin is the only antibiotic in periodontal therapy to which all strains of A. actinomycetemcomitans are susceptible. It also has been used in combination with metronidazole.70
Side Effects
Nausea, headache, metallic taste in the mouth, and abdominal discomfort have been associated with ciprofloxacin. Quinolones inhibit the metabolism of theophylline, and caffeine and concurrent administration can produce toxicity. Quinolones have also been reported to enhance the effect of warfarin and other anticoagulants.96
Macrolides
Pharmacology
Macrolide antibiotics contain a many-membered lactone ring to which one or more deoxy sugars are attached. They inhibit protein synthesis by binding to the 50S ribosomal subunits of sensitive microorganisms. Macrolides can be bacteriostatic or bactericidal, depending on the concentration of the drug and the nature of the microorganism. The macrolide antibiotics used for periodontal treatment include erythromycin, spiramycin, and azithromycin.
Clinical Use
Erythromycin does not concentrate in GCF, and it is not effective against most putative periodontal pathogens. For these reasons, erythromycin is not recommended as an adjunct to periodontal therapy.
Spiramycin is active against gram-positive organisms; it is excreted in high concentrations in saliva. It is used as an adjunct to periodontal treatment in Canada and Europe but is not available in the United States (US). Spiramycin has minimal effect on increasing attachment levels.
Azithromycin is a member of the azalide class of macrolides. It is effective against anaerobes and gram-negative bacilli. After an oral dosage of 500 mg qd for 3 days, significant levels of azithromycin can be detected in most tissues for 7 to 10 days.10,47 The concentration of azithromycin in tissue specimens from periodontal lesions is significantly higher than that of normal gingiva.62 It has been proposed that azithromycin penetrates fibroblasts and phagocytes in concentrations 100 to 200 times greater than that of the extracellular compartment. The azithromycin is actively transported to sites of inflammation by phagocytes, then released directly into the sites of inflammation as the phagocytes rupture during phagocytosis.33 Therapeutic use requires a single dose of 250 mg/day for 5 days after an initial loading dose of 500 mg.96
Recent data have suggested that azithromycin can be an effective adjunctive therapy for increasing attachment levels in patients with aggressive periodontitis,41 as well as in reducing the degree of gingival enlargement.25 At this time, these data must be carefully considered since the outcomes are derived from small subject populations. To ascertain the efficacy of azithromycin in the management of periodontal diseases, future studies will need to increase the number of subjects, improve diagnostic methods and tools, and determine appropriate dose, duration, and frequency of azithromycin therapy.
Serial and Combination Antibiotic Therapy
Rationale
Because periodontal infections may contain a wide diversity of bacteria, no single antibiotic is effective against all putative pathogens. Indeed, differences exist in the microbial flora associated with the various periodontal disease syndromes.100 These “mixed” infections can include a variety of aerobic, microaerophilic, and anaerobic bacteria, both gram-negative and gram-positive. In these cases, it may be necessary to use more than one antibiotic, either serially or in combination.69 Before combinations of antibiotics are used, however, the periodontal pathogen(s) being treated must be identified and antibiotic-susceptibility testing performed.98
Clinical Use
Antibiotics that are bacteriostatic (e.g., tetracycline) generally require rapidly dividing microorganisms to be effective. They do not function well if a bactericidal antibiotic (e.g., amoxicillin) is given concurrently. When both types of drugs are required, they are best given serially, not in combination.
Rams and Slots69 reviewed combination therapy using systemic metronidazole along with amoxicillin, amoxicillin-clavulanate (Augmentin), or ciprofloxacin. The metronidazole-amoxicillin and metronidazole-Augmentin combinations provided excellent elimination of many organisms in adult and LAP that had been treated unsuccessfully with tetracyclines and mechanical debridement. These drugs have an additive effect regarding suppression of A. actinomycetemcomitans. Tinoco et al87 found metronidazole and amoxicillin to be clinically effective in treating LAP, although 50% of patients harbored A. actinomycetemcomitans 1 year later. Metronidazole-ciprofloxacin combination is effective against A. actinomycetemcomitans; metronidazole targets obligate anaerobes, and ciprofloxacin targets facultative anaerobes. This is a powerful combination against mixed infections. Studies of this drug combination in the treatment of refractory periodontitis have documented marked clinical improvement. This combination may provide a therapeutic benefit by reducing or eliminating pathogenic organisms and a prophylactic benefit by giving rise to a predominantly streptococcal microflora.70
Systemic antibiotic therapy combined with mechanical therapy appears valuable in the treatment of recalcitrant periodontal infections and LAP infections involving A. actinomycetemcomitans. Antibiotic treatment should be reserved for specific subsets of periodontal patients who do not respond to conventional therapy. Selection of specific agents should be guided by the results of cultures and sensitivity tests for subgingival plaque microorganisms.
![]() Science Transfer
Science Transfer
Antiinfective agents are used either locally in delayed release products or systemically with administration by mouth. In patients with periodontitis, local antiinfective agents, such as chlorhexidine (PerioChip), doxycycline (Atridox), or minocycline (Arestin), used with scaling and root planing, have all been shown to give modest changes in average pocket depth reduction when compared to control areas in which scaling and root planing was used alone. Periimplantitis also can be treated with locally released antimicrobial agents such as doxycycline and minocycline.
Systemic antibiotics have demonstrated similar results to local agents when used in conjunction with sealing and root planing. The most effective of these is metronidazole used alone or in conjunction with amoxicillin or Augmentin on a daily basis for 1 to 2 weeks.
These antiinfective approaches are not as dramatic in their effects on advanced periodontitis as periodontal regenerative surgical techniques. For this reason, they are best used in those patients in whom periodontal surgery is contraindicated or as a precursor to surgery during initial therapy.
Pharmacologic Implications
Principles of antibiotic therapy for the proper selection of an antibiotic minimally require identification of the causative organism, determination of the antibiotic sensitivity, and an effective method of administration.45 The use of antibiotics to treat gingival diseases is contraindicated since it is a local infection that can be easily treated with scaling and appropriate home care by the patient.86 In regard to destructive periodontal diseases, there are limited data to support the use of systemic antibiotics in the treatment of these diseases. Although bacterial infections of the periodontium are considered to be important in the initiation of the disease, currently, there is no one microbe or group of microbes that have been demonstrated as the cause of these diseases. It is therefore not surprising that systemic antibiotics have had only a modest effect in the management of periodontal diseases. At this time, systemic antibiotics for the treatment of periodontal diseases have been indicated primarily for adjunctive use in the treatment of aggressive periodontal diseases.40,43
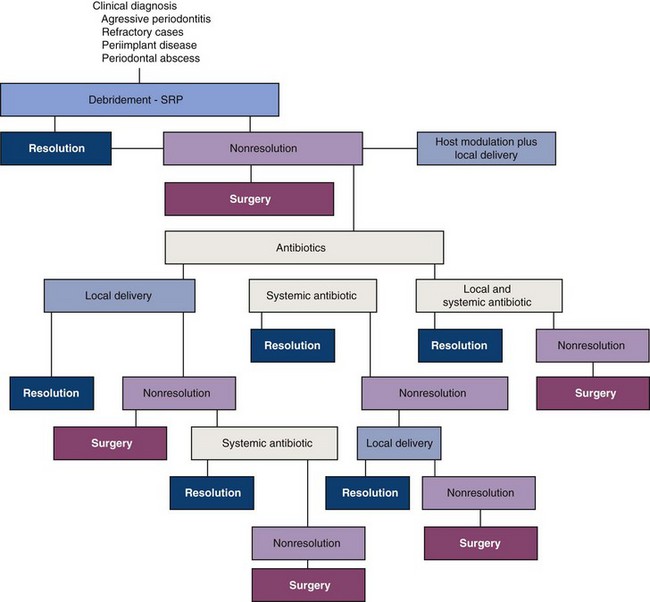
Guidelines for the use of antibiotics in periodontal therapy include the following:
The selection of an antibiotic must be made based on factors other than the empirical decisions by the clinician. Unfortunately, there is no one best choice of antibiotic at present (i.e., no “silver bullet”). Therefore the clinician must integrate history of the patient’s disease, clinical signs and symptoms, and results of radiographic examinations and possibly microbiologic sampling to determine the course of periodontal therapy. The clinician must obtain a thorough medical history, including current medications and possible adverse effects of combining these medicines, before prescribing any antibiotic therapy. The clinician must make the final decision with the patient. Risks and benefits concerning antibiotics as adjuncts to periodontal therapy should be discussed with the patient before antibiotics are used.
Local Delivery Agents
Locally delivered antimicrobial agents are available as adjuncts to scaling and root planing and as aids in the control of growth of bacteria on barrier membranes.67Although not discussed in this chapter, locally delivered NSAIDs are also being evaluated as adjunctive agents in the treatment of periodontal disease.44,92 When placed into periodontal pockets, they reduce the subgingival microflora, probing depths, and clinical signs of inflammation. A recent systematic review concluded “adjunctive local therapy generally reduced probing depth levels. Differences between treatment and SRP-only groups in the baseline to followup period typically favored treatment groups but usually only modestly (e.g., from about 0.1 mm to nearly 0.5 mm). Effects for clinical attachment level gains were smaller and statistical significance less common.”11 A report on locally delivered agents prepared by the American Academy of Periodontology stated “the clinician’s decision to use locally delivered agents (LDA) should be based upon a consideration of clinical findings, the patient’s dental and medical history, scientific evidence, patient preferences and advantages and disadvantages of alternative therapies.” The report also stated that use of LDAs may be of value when probing depths greater than 5 mm with inflammation are still present after conventional therapy. However if multiple sites are present in the same quadrant, therapy other than LDAs should be considered.5
Subgingival Chlorhexidine
A resorbable delivery system has been tested for the subgingival placement of chlorhexidine gluconate with positive clinical results (Figure 47-2). This product is available in the US and a number of other countries. PerioChip is a small chip (4.0 × 5.0 × 0.35 mm) composed of a biodegradable hydrolyzed gelatin matrix, cross-linked with glutaraldehyde and also containing glycerin and water, into which 2.5 mg of chlorhexidine gluconate has been incorporated per chip. This delivery system releases chlorhexidine and maintains drug concentrations in the GCF greater than 100 µg/ml for at least 7 days,84 concentrations well above the tolerance of most oral bacteria.12 Because the chip biodegrades in 7 to 10 days, a second appointment for removal is not needed.
Two multicenter, randomized, double-blind, parallel-group, controlled clinical trials of this chip were conducted in the US with a total of 447 patients in 10 centers.48 In these studies, patients received a supragingival prophylaxis for up to 1 hour, followed by scaling and root planing for 1 hour. Chips were placed in target sites with probing depth of 5 to 8 mm at baseline that bled on probing and again at 3 and 6 months if probing depth remained at 5 mm or greater. Sites in control subjects received either a placebo chip (inactive) with scaling and root planing or scaling and root planing alone. Sites in test subjects received either a chlorhexidine chip (active) with scaling and root planing or scaling and root planing alone. Examinations were performed at baseline and again at 3, 6, and 9 months. At 9 months, significant decreases in probing depth from baseline favoring the active chip compared with controls were observed: chlorhexidine chip with scaling and root planing, −0.95 ± 0.05 mm; placebo chip with scaling and root planing, −0.69 ± 0.05 mm (p = 0.001); scaling and root planing alone, −0.65 ± 0.05 mm (p = 0.00001). Although statistically significant, the net clinical changes were limited. The proportion of pocket sites with a probing depth reduction of 2 mm or more was increased in the chlorhexidine chip group (30%) compared with scaling and root planing alone (16%), a statistically significant difference on a per-patient basis (p < 0.0001).
No signs of staining were noted in any of the previous three studies as a result of the chlorhexidine chip treatment, as measured by a stain index. Adverse effects were minimal, with a few patients who complained of slight pain and swelling in the first 24 hours after chip placement.
Tetracycline-Containing Fibers
The first local delivery product available in the US, an ethylene/vinyl acetate copolymer fiber (diameter, 0.5 mm) containing tetracycline, 12.7 mg per 9 inches, was extensively studied. When packed into a periodontal pocket, it was well tolerated by oral tissues, and for 10 days it sustained tetracycline concentrations exceeding 1300 µg/ml, well beyond the 32 to 64 µg/ml required to inhibit the growth of pathogens isolated from periodontal pockets.* In contrast, GCF concentrations of only 4 to 8 µg/ml were reported after systemic tetracycline administration, 250 mg qid for 10 days (total oral dose, 10 g).36,37
Studies demonstrated that tetracycline fibers applied with or without scaling and root planing reduced probing depth, bleeding on probing, and periodontal pathogens and provided gains in clinical attachment level. Such effects were significantly better than those attained with scaling and root planing alone or with placebo fibers. No change in antibiotic resistance to tetracycline was found after tetracycline fiber therapy among the tested putative periodontal pathogens. However, these fibers are no longer commercially available.
Subgingival Doxycycline
A gel system using a syringe with 10% doxycycline (Atridox) (Figure 47-3) is available. It was the only local delivery system accepted by the American Dental Association (ADA) when the ADA had an acceptance program for professional products, although that program was terminated in 2008. This product is available in the US and a number of other countries.
In a 9-month multicenter study of 180 patients, treatment with 10% doxycycline gel alone was more effective than the other treatments at all time periods, with the exception of the 3-month clinical attachment level value. For the 10% doxycycline gel group, the reduction in clinical attachment level at 9 months showed a gain of 0.4 mm compared with vehicle control; the reduction in probing depth was 0.6 mm greater than vehicle control; and the reduction of bleeding on probing was 0.2 units greater than vehicle control. The differences were clinically small but statistically significant. Although resistance was not evaluated in this study, the local application of doxycycline has previously been reported to show transient increases in resistance in oral microbes and no overgrowth of foreign pathogens.58
Two multicenter clinical trials each studied 411 patients with moderate-to-severe periodontitis.30 At baseline, patients were randomized to one of four treatment groups: 10% doxycycline gel, vehicle control, oral hygiene only, and scaling and root planing. Sites with probing depth of 5 mm or greater that bled on probing were treated at baseline and then again with the same treatment at 4 months. Clinical assessments were made for 9 months, measuring clinical attachment level, probing depth, and bleeding on probing. All treatment groups in both studies showed clinical improvements from baseline over the 9-month period. The results for all parameters measured were significantly better in the 10% doxycycline gel group compared with vehicle control and oral hygiene only. Compared with scaling and root planing, the effects of 10% doxycycline gel as monotherapy on clinical attachment level gain and probing depth reduction were equivalent (see Chapter 48). Most recently, a 6-month, randomized, multicenter, placebo-controlled examiner-masked study66 in 171 subjects evaluated the combined use of systemically delivered doxycycline hyclate (20 mg, bid) plus locally delivered doxycycline hyclate gel (10%) in combination with scaling and root planing versus scaling and root planing plus placebo. The results of the study showed that the combination therapy provided statistically significantly greater clinical benefits than control therapy for all clinical measures at both 3 and 6 months. The clinical parameters measured were probing depth, clinical attachment level, bleeding on probing, and gingival health.66
Subgingival Minocycline
A locally delivered, sustained-release form of minocycline microspheres (Arestin) is available for subgingival placement as an adjunct to scaling and root planing (Figure 47-4). The 2% minocycline is encapsulated into bioresorbable microspheres in a gel carrier. This product is available in the US and a number of other countries. When compared to controls (scaling and root planing with nonactive vehicle as subgingival irrigant), there was a statistically significant increase in clinical attachment levels in patients who presented with pockets of 6 mm or greater probing depth.22
In a four-center, double-blind, randomized trial, patients with periodontal pockets at least 5 mm deep were selected, and either 2% minocycline gel or vehicle were applied once every 2 weeks for 4 applications after initial scaling and root planing.90A total of 343 teeth (976 sites) were included in the minocycline group, with 299 teeth (810 sites) in the control group. Reductions in P. gingivalis and P. intermedia at weeks 2, 4, 6, and 12 and at weeks 6 and 12 for A. actinomycetemcomitans were statistically significant. These results demonstrated the advantages of supplementing standard subgingival debridement with minocycline gel application. The three primary clinical efficacy variables in this study were probing depth, clinical attachment level, and bleeding index. There was a trend toward clinical improvement in both treatment groups for all three measures, and the reduction in probing depth was significantly greater with minocycline gel. When sites with probing depth of at least 7 mm and significant bleeding at baseline were considered, the improvements were greater than with 5-mm pockets. The improvements with minocycline were statistically significantly better than the control group.
Applications of 2% minocycline were also evaluated in a 3-month study in 30 patients.38 Active or placebo gel was placed subgingivally at planed sites in each subject according to a double-blind protocol, immediately after scaling and root planing and 2 and 4 weeks later. Differences between groups in mean probing depth did not reach statistical significance at any visit, but mean clinical attachment levels favored the minocycline group (p < 0.05) at both reassessments. The number of sites that bled after deep probing at 12 weeks also favored the minocycline group (p < 0.05).
Subgingival Metronidazole
A topical medication containing an oil-based metronidazole 25% dental gel (glyceryl mono-oleate and sesame oil) has been tested in a number of studies.1 This product is not available in the US. It is applied in viscous consistency to the pocket, where it is liquidized by the body heat and then hardens again, forming crystals in contact with water. As a precursor, the preparation contains metronidazole-benzoate, which is converted into the active substance by esterases in GCF. Two 25% gel applications at a 1-week interval have been used.54
Studies have shown that metronidazole gel is equivalent to scaling and root planing but have not shown adjunctive benefits with scaling and root planing. In a 6-month study of 30 patients, treatment consisted of 2 applications of the dental gel in 2 randomly selected quadrants at 1-week intervals, as well as simultaneous subgingival scaling of the remaining quadrants.85 Oral hygiene instructions were given on day 21. Statistical analyses showed that both treatments were effective in reducing probing depth and bleeding on probing over the 6-month period. At the end of the follow-up period, the mean reduction in probing depth was 1.3 mm after gel treatment and 1.5 mm after subgingival scaling. Bleeding on probing was reduced by 35% and 42%, respectively. No significant differences between the two treatments were detected. Dark-field microscopy showed a shift toward a seemingly healthier microflora for both treatment modalities; this effect persisted throughout the 6-month period.
A large, multicenter study of 206 subjects investigated 2 applications of metronidazole gel in 2 randomly selected quadrants versus 2 quadrants of scaling.1 Probing depths were reduced by 1.2 mm in the gel and 1.5 mm in the scaling group. At 6 months, the differences between treatments were statistically but not clinically significant. Also, bleeding on probing was reduced by 88% for both treatment groups.
Comparative Studies
Few researchers have attempted to compare these devices side-by-side, but in one study, doxycycline polymer, metronidazole gel, and PerioChip were compared in 47 periodontal patients. The study found that all controlled release polymer devices increased gingival attachment levels but that there was a slightly greater improvement in patients treated with the doxycycline polymer.78
Local Delivery of Antimicrobial Agents and Periimplant Mucositis/Implantitis
Since there are a number of similarities between the pathogens involved in periodontitis periimplant mucositis and periimplantitis, it is logical that similar clinical approaches should be implemented for periimplant mucositis and periimplantitis. An early study by Mombelli et al,63 utilizing tetracycline-containing fibers that are no longer available, demonstrated in 25 patients with periimplant mucositis that there was a significant reduction of four periopathogenic species, but other species, such as P. gingivalis and A. actinomycetemcomitans, had very low baseline and were not significantly affected. There was a slight but not significant improvement in the probing depth at 12 months. Mombelli’s study showed that the local delivery of tetracycline from polymeric fibers may be used to improve the clinical and microbiologic parameters around infected implants when compared with scaling and root planing alone.
In another study,14 investigators applied the controlled release of doxycycline (Atridox) into periimplant pockets and noted differences from scaling and root planing alone. This study also showed the efficacy of such devices in treating periimplant mucositis. Patients who received the doxycycline treatment showed significantly greater probing attachment levels and lesser pocket probing depth and bleeding index (p < 0.05) than those who received scaling/root planing alone.14
A study by Persson et al79 evaluated 31 implants with periimplantitis in 25 patients and monitored clinical and radiographic changes over 12 months after adjunctive placement of minocycline microspheres (Arestin). Minocycline microspheres (Arestin) were locally delivered to each implant site with bone loss and a probing pocket depth (PPD) equal to or greater than 5 mm. Rescue therapy with Arestin was allowed at days 180 and 270 at any site exhibiting an increase in PPD equal or greater than 2 mm from the previous visit. Six implants in six subjects were either rescued or exited because of persisting active periimplantitis. Successful implants showed a statistically significant reduction in both PPD and percentage of sites with bleeding on probing between baseline and day 360 (p < 0.05). At mesial implant sites, the mean PPD reduction amounted to 1.6 mm (95% CI: 0.9 to 2.2 mm, p < 0.001) and was accompanied by a statistically significant reduction of the bleeding on probing value (p < 0.001).
Although studies evaluating the use of the PerioChip (chlorhexidine) for periimplant mucositis were not identified, one 12-month study compared the clinical and microbiologic results after application of minocycline microspheres as an adjunct to mechanical treatment of incipient periimplant infections compared with an adjunctive treatment using application of 1 ml of a 1% chlorhexidine gel.73 Sixteen patients in the minocycline group and 14 in the chlorhexidine group completed the study. The findings of the study were that the adjunctive use of minocycline microspheres resulted in improvements of probing depths and bleeding scores, whereas the adjunctive use of chlorhexidine only resulted in limited reduction of bleeding scores. For the deepest sites of the treated implants in the minocycline group, the mean probing depth was reduced from 5.0 to 4.4 mm at 12 months. This study could not show any significant difference in the levels of bacterial species or groups at any time point between the two antimicrobial agents tested.
Conclusion
Scaling and root planing alone are effective in reducing pocket depths, gaining increases in periodontal attachment levels, and decreasing inflammation levels (bleeding on probing). When scaling and root planing are combined with the subgingival placement of sustained-release vehicles, however, additional clinical benefits are possible, including further reduction in pocket depths, additional gain in clinical attachment level, (e.g., 0.39 mm for minocycline gel), and further decrease in inflammation. Improvement in clinical attachment levels also occurs with the chlorhexidine chip (0.16 mm) and doxycycline gel (0.34 mm).42 When systemic antibiotics are used as adjuncts to scaling and root planing, the evidence indicates that some systemic antibiotics (e.g., metronidazole and tetracycline) provide additional improvement in attachment levels (0.35 mm for metronidazole; 0.40 mm for tetracycline) when used as adjuncts to scaling and root planing.43 Use of antiinfective chemotherapeutic treatment adjuncts does not result in significant patient-centered adverse effects.
The decision as to when to use local or systemic antimicrobials should be based on the clinicians consideration of the clinical findings, the patient’s medical and dental history,27,28 patient preferences, and potential benefits of adjunctive therapy with these agents.
There are extensive reviews of the local delivery agents available for periodontitis.42,46,52,53,74
1 Ainamo J, Lie T, Ellingsen BH, et al. Clinical responses to subgingival application of a metronidazole 25% gel compared to the effect of subgingival scaling in adult periodontitis. J Clin Periodontol. 1992;19(pt II):723.
2 Alger FA, Solt CW, Vuddhankanok S, et al. The histologic evaluation of new attachment in periodontally diseased human roots treated with tetracycline hydrochloride and fibronectin. J Periodontol. 1990;61:447.
3 American Academy of Periodontology. Parameters of care. J Periodontol. 2000;71(suppl):847.
4 American Academy of Periodontology, Research, Science and Therapy Committee. Systemic antibiotics in periodontics. J Periodontol. 2004;75:1553.
5 American Academy of Periodontology Statement on Local Delivery of Sustained or Controlled Release Antimicrobials as Adjunctive therapy in the treatment of Periodontitis. Journal of Periodontology. 2006;77:1458.
6 American Dental Association, Council on Scientific Affairs. Combating antibiotic resistance. J Am Dent Assoc. 2004;135:484.
7 Bader HI, Goldhaber P. The passage of intravenously administered tetracycline into the gingival sulcus of dogs. J Oral Ther Pharmacol. 1968;2:324.
8 Baker PJ, Evans RT, Slots J, et al. Antibiotic susceptibility of anaerobic bacteria from the oral cavity. J Dent Res. 1985;65:1233.
9 Baker PJ, Evans RT, Slots J, et al. Susceptibility of human oral anaerobic bacteria to antibiotics suitable for topical use. J Clin Periodontol. 1985;12:201.
10 Blandizzi C, Tecla M, Lupetti A, et al. Periodontal tissue disposition of azithromycin in patients affected by chronic inflammatory periodontal diseases. J Periodontol. 1999;70:960.
11 Bonito A, Lux L, Lohr KN. Impact of local adjuncts to scaling and root planing in periodontal disease therapy: A systematic review. J. Periodontal. 2005;76:1227-1236.
12 Briner WW, Kayrouz GA, Chanak MX. Comparative antimicrobial effectiveness of a substantive (0.12% chlorhexidine) and a nonsubstantive (phenolic) mouthrinse in vivo and in vitro. Compend Contin Educ Dent. 1994;15:1158.
13 Bueno L, Walker C, van Ness W, et al. Effect of Augmentin on microbiota associated with refractory periodontitis. J Dent Res. 1988;67:246. (abstract 1064)
14 Buchter A, Meyer U, Kruse-Losler B, et al. Sustained release of doxycycline for the treatment of periimplantitis: Randomised controlled trial. Br J Oral Maxillofac Surg. 2004;42:439-444.
15 Carranza FAJr, Saglie R, Newman MG, et al. Scanning and transmission electron microscopic study of tissue-invading microorganisms in localized juvenile periodontitis. J Periodontol. 1983;54:598.
16 Caton J, Bleiden T, Adams D, et al. Subantimicrobial doxycycline therapy for periodontitis. J Dent Res. 1997;76:1307. (abstract)
17 Caton JG, Ciancio SG, Blieden TM, et al. Treatment with subantimicrobial dose doxycycline improves the efficacy of scaling and root planing in patients with adult periodontitis. J Periodontol. 2000;71:521.
18 Christersson LA, Slots J, Rosling BG, et al. Microbiological and clinical effects of surgical treatment of localized juvenile periodontitis. J Clin Periodontol. 1985;12:465.
19 Ciancio SG. Use of antibiotics in periodontal therapy. In: Newman MG, Goodman A, editors. Antibiotics in dentistry. Chicago: Quintessence, 1983.
20 Ciancio SG. Antibiotics in periodontal therapy. In: Newman MG, Kornman K, editors. Antibiotic/antimicrobial use in dental practice. Chicago: Quintessence, 1990.
21 Ciancio SG. Antiseptics and antibiotics as chemotherapeutic agents for periodontitis management. Compend Contin Educ Dent. 2000;21:59.
22 Ciancio SG, editor: FDA approves Arestin marketing. In: Biological therapies in dentistry, 17(suppl 1):s1, Hamilton, Ontario, Canada, 2001, BC Decker (newsletter).
23 Ciancio SG, Adams D, Blieden T, et al: Subantimicrobial dose doxycycline: a new adjunctive therapy for adult periodontitis. Presented at annual meeting of American Academy of Periodontology, Boston, 1998.
24 Ciancio SG, Slots J, Reynolds HS, et al. The effect of short-term administration of minocycline HCl administration on gingival inflammation and subgingival microflora. J Periodontol. 1982;53:57.
25 Clementini M, Vittorini G, Crea A, et al. Efficacy of AZM therapy in patients with gingival overgrowth induced by cyclosporine A: a systematic review. BMC Oral Health.. 2008;8:34.
26 Crout R, Adams D, Blieden T, et al: Safety of doxycycline hyclate 20 mg bid in patients with adult periodontitis. Presented at annual meeting of American Academy of Periodontology, Boston, 1998.
27 Edelson H, Kaplan H, Korchak H. Differing effects of nonsteroidal anti-inflammatory agents on neutrophil functions. Clin Res. 1982;30:469A.
28 Feldman R, Szeto B, Chauncey H, et al. Nonsteroidal anti-inflammatory drugs in the reduction of human alveolar bone loss. J Clin Periodontol. 1983;10:131.
29 Feres M, Haffajee AD, Allard K, et al. Antibiotic resistance of subgingival species during and after periodontal therapy. J Clin Periodontol. 2002;28:12.
30 Garrett S, Adams D, Bandt C, et al. Two multicenter clinical trials of subgingival doxycycline in the treatment of periodontitis. J Dent Res. 1997;76:153. (abstract 1113)
31 Genco RJ, Cianciola JJ, Rosling H. Treatment of localized juvenile periodontitis. J Dent Res. 1981;60:527. (abstract 872)
32 Gibson W. Antibiotics and periodontal disease: a selective review of the literature. J Am Dent Assoc. 1982;104:213.
33 Gladue RP, Snyder ME. Intracellular accumulation of azithromycin by cultured human fibroblasts. Antimicrob Agents Chemother. 1990;34:1056.
34 Goodson JM, Tanner A. Antibiotic resistance of the subgingival microbiota following local tetracycline therapy. Oral Microbiol Immunol. 1992;7:113.
35 Goodson JM, Cugini M, Kent RL, et al. Multicenter evaluation of tetracycline fiber therapy. II. Clinical response. J Periodontal Res. 1991;26:371.
36 Gordon JM, Walker CB, Murphy JC. Concentration of tetracycline in human gingival fluid after single doses. J Clin Periodontol. 1981;8:117.
37 Gordon JM, Walker CB, Murphy CJ, et al. Tetracycline: levels achievable in gingival crevice fluid and in vitro effect on subgingival organisms. Part I. Concentrations in crevicular fluid after repeated doses. J Periodontol. 1981;52:609.
38 Grace MA, Watts TL, Wilson RF, et al. A randomized controlled trial of a 2% minocycline gel as an adjunct to nonsurgical periodontal treatment, using a design with multiple matching criteria. J Clin Periodontol. 1997;24:249.
39 Greenstein G. The role of metronidazole in the treatment of periodontal diseases. J Periodontol. 1993;64:1.
40 Greenstein G. Changing periodontal concepts: treatment considerations. Compend Contin Educ Dent. 2005;26:81.
41 Haas AN, de Castro GD, Moreno T, et al. Azithromycin as an adjunctive treatment of aggressive periodontitis: 12-months randomized clinical trial. J Clin Periodontol. 2008;35:696.
42 Hanes PJ, Purvis JP. Local anti-infective therapy: pharmacological agents. A systematic review. Ann Periodontol. 2003;8:79.
43 Haffajee AD, Socransky SS, Gunsolley JC. Systemic anti-infective periodontal therapy: a systematic review. Ann Periodontol. 2003;8:115.
44 Heasam PA, Benn DK, Kelly PJ, et al. The use of topical flurbiprofen as an adjunct to non-surgical management of periodontal disease. J Clin Periodontol. 1993;20:457.
45 Hessen MT, Kaye D. Principles of use of antibacterial agents. Infect Dis Clin N Amer. 2004;18:435.
46 Hill M, Moore R. Locally acting oral chemotherapeutic agents. In: Rose LF, Mealey BL, Genco RJ, Cohen DW, editors. Periodontics: medicine, surgery, and implants. St. Louis: Elsevier; 2004:277-286. Chapter 16
47 Hoepelman IM, Schneider MM. Azithromycin: the first of the tissue-selective azalides. Int J Antimicrob Agents. 1995;5:145.
48 Jeffcoat M, Bray KS, Ciancio SG, et al. Adjunctive use of a subgingival controlled-release chlorhexidine chip reduces probing depth and improves attachment level compared with scaling and root planing alone. J Periodontol. 1998;69:989.
49 Jorgensen MG, Slots J. Practical antimicrobial periodontal therapy. Compend Contin Educ Dent. 2000;21:111.
50 Jorgensen MG, Slots J. Responsible use of antimicrobials in periodontics. J Cal Dent Assoc. 2000;28:185.
51 Kaplan H, Edelson H, Korchak H, et al. Effects of nonsteroidal anti-inflammatory agents on neutrophil functions in vitro and in vivo. Biochem Pharmacol. 1984;33:371.
52 Killoy WJ. Chemical treatment of periodontitis: local delivery of antimicrobials. Int Dent J. 1998;48(Suppl 1):305-315.
53 Killoy WJ, Polson AM. Controlled local delivery of antimicrobials in the treatment of periodontitis. Dent Clin North Am. 1998;42:263-283.
54 Klinge B, Attström R, Karring T, et al. Three regimens of topical metronidazole compared with subgingival scaling on periodontal pathology in adults. J Clin Periodontol. 1992;19(pt II):708.
55 Kornman KS, Karl EH. The effect of long-term low-dose tetracycline therapy on the subgingival microflora in refractory adult periodontitis. J Periodontol. 1982;53:604.
56 Lang NP, Adler R, Joss A, et al. The absence of bleeding on probing: an indicator of periodontal stability. J Clin Periodontol. 1990;17:7144.
57 Lang NP, Joss A, Orsanic T, et al. Bleeding on probing: a predictor for the progression of periodontal disease? J Clin Periodontol. 1986;13:590.
58 Larsen T. Occurrence of doxycycline-resistant bacteria in the oral cavity after local administration of doxycycline in patients with periodontal disease. Scand J Infect Dis. 1991;23:89.
59 Lee HM, Ciancio SG, Tuter G, et al. Subantimicrobial dose doxycycline efficacy as a matrix metalloproteinase inhibitor in chronic periodontitis is enhanced when combined with a non-steroidal anti-inflammatory drug. J Periodontol. 2004;75:453.
60 Lekovic V, Kenney EB, Carranza FAJr, et al. Effect of metronidazole on human periodontal disease: a clinical and microbiologic study. J Periodontol. 1983;54:476.
61 Lozdan J, Sheiham A, Pearlman BA, et al. The use of nitroimidazine in the treatment of acute ulcerative gingivitis: a double-blind controlled trial. Br Dent J. 1971;130:294.
62 Malizia T, Tejada MR, Ghelardi E, et al. Periodontal tissue disposition of azithromycin. J Periodontol. 1997;68:1206.
63 Mombelli A, Feloutzis A, Bragger U, Lang NP. Treatment of Peri-implantitis by local delivery of tetracycline. Clinical, microbiological and radiological results. Clin Oral Implants Res. 2001;4:287.
64 Morrison SL, Cobb CM, Kazakos GM, et al. Root surface characteristics associated with subgingival placement of monolithic tetracycline-impregnated fibers. J Periodontol. 1992;63:137.
65 Niederman R, Holborow D, Tonetti M, et al. Reinfection of periodontal sites following tetracycline fiber therapy. J Dent Res. 1990;69:277. (abstract 1345)
66 Novak MJ, Dawson DR3rd, Magnusson I, et al. Combining host modulation and topical antimicrobial therapy in the Management of moderate to severe periodontititis: a randomized multicenter trial. J Periodontal. 2008;79(1):33-41.
67 Nowzari H, McDonald ES, Flynn J, et al. The dynamics of microbial colonization of barrier membranes for guided tissue regeneration. J Periodontol. 1996;67:694.
68 Offenbacher S, Braswell L, Loos A, et al. Effects of flurbiprophen on the progression of periodontitis in Macaca mulatta. J Periodontal Res. 1987;22:473.
69 Rams TE, Slots J. Antibiotics in periodontal therapy: an update. Compend Contin Educ Dent. 1992;13:1130.
70 Rams TE, Feik D, Slots J. Ciprofloxacin/metronidazole treatment of recurrent adult periodontitis. J Dent Res. 1992;71:319. (abstract)
71 Reddy MS, Geurs NC, Gunsolley JC. Periodontal host modulation with antiproteinase, anti-inflammatory, and bone sparing agents: a systematic review. Ann Periodontol. 2003;8:12.
72 Research, Science and Therapy Committee. Pharmacologic blocking of host responses as an adjunct in the management of periodontal diseases: a research update. Chicago: American Academy of Periodontology; 1992.
73 Renvert S, Lessem J, Dahle’n G, Lindahl C, Svensson M. Topical minocycline microspheres versus topical chlorhexidine gel as an adjunct to mechanical debridement of incipient peri-implant infections: a randomized clinical trial. J Clin Periodontol. 2006;33:362-369.
74 Rose LF, Mealey BL, Genco RJ, Cohen DW, editors, Periodontics: medicine, surgery, and implants, 2004, Elsevier, St. Louis277-286, Chapter 16
75 Rosling BG, Slots J, Christersson LA, et al. Topical antimicrobial therapy and diagnosis of subgingival bacteria in the management of inflammatory periodontal disease. J Clin Periodontol. 1986;13:975.
76 Saglie FR, Carranza FAJr, Newman MG, et al. Identification of tissue invading bacteria in human periodontal disease. J Periodontal Res. 1982;17:452.
77 Salvi GE, Lawrence HP, Offenbacher S, Beck JD. Influence of risk factors on the pathogenesis of periodontal disease. Periodontol 2000. 1997;14:173.
78 Salvi GE, Mombelli A, Mayfield L, et al. Local antimicrobial therapy after initial periodontal treatment. J Clin Periodontol. 2002;29:540-550.
79 Persson GR, Salvi GE, Heitz-Mayfield LJ, Lang MP. Antimicrobial therapy using a local drug delivery system (Arestin) in the treatment of peri-implantitis. I. Microbiological outcomes. Clin Oral Implants Res. 2006 Aug;17(4):386-393.
80 , Mosby’s GenRx, ed 10, 2001, Mosby, St Louis
81 Slots J, Rams TE. Antibiotics and periodontal therapy: advantages and disadvantages. J Clin Periodontol. 1990;17:479.
82 Slots J, Rosling BG. Suppression of periodontopathic microflora in localized juvenile periodontitis by systemic tetracycline. J Clin Periodontol. 1983;10:465.
83 Soder P, Frithiof L, Wikner S, et al. The effects of systemic metronidazole after non-surgical treatment in moderate and advanced periodontitis in young adults. J Periodontol. 1990;61:281.
84 Soskolne WA, Heasman PA, Stabholz A, et al. Sustained local delivery of chlorhexidine in the treatment of periodontitis: a multi-center study. J Periodontol. 1997;68:32.
85 Stelzel M, Flores-De-Jacoby L. Topical metronidazole application compared with subgingival scaling: a clinical and microbiological study on recall patients. J Clin Periodontol. 1996;23:24.
86 Tatakis DN, Trombelli L. Modulation of clinical expression of plaque-induced gingivitis. L. Background review and rationale. J Clin Periodontol. 2004;31:220.
87 Tinoco EM, Beldi M, Campedelli F, et al. Clinical and microbiologic effects of adjunctive antibiotics in treatment of localized aggressive periodontitis: a controlled clinical study. J Periodontol. 1998;69:1355.
88 Tonetti M, Cugini AM, Goodson JM. Zero order delivery with periodontal placement of tetracycline loaded ethylene vinyl acetate fibers. J Periodontal Res. 1990;25:243.
89 Tyler K, Walker CB, Gordon J, et al. Evaluation of clindamycin in adult refractory periodontitis: antimicrobial susceptibilities. J Dent Res. 1985;64:360. (special issue; abstract 1667)
90 Van Steenberghe D, Bercy P, Kohl J. Subgingival minocycline hydrochloride ointment in moderate to severe chronic adult periodontitis: a randomized, double-blind, vehicle-controlled, multicenter study. J Periodontol. 1993;64:637.
91 Van Winkelhoff AJ, Gonzales DH, Winkel EG, et al. Antimicrobial resistance in the subgingival microflora in patients with adult periodontitis: a comparison between the Netherlands and Spain. J Clin Periodontol. 2000;27:79.
92 Vogel R, Schneider L, Goteinter D. The effects of a topical nonsteroidal anti-inflammatory drug on ligature induced periodontal disease in the squirrel monkey. J Clin Periodontol. 1986;12:139.
93 Waite I, Saxon C, Young A, et al. The periodontal status of subjects receiving nonsteroidal anti-inflammatory drugs. J Periodontal Res. 1981;16:100.
94 Walker C, Karpinia K. Rationale for use of antibiotics in periodontics. J Periodontol. 2002;73:1188.
95 Walker C, Thomas J: The effect of subantimicrobial doses of doxycycline on the microbial flora and antibiotic resistance in patients with adult periodontitis. Presented at Annual meeting of American Academy of Periodontology, Boston, 1998.
96 Walker CB. Selected antimicrobial agents: mechanisms of action, side effects and drug interactions. Periodontol 2000. 1996;10:12.
97 Walker CB. The acquisition of resistance of antibiotic resistance in the periodontal microflora. Periodontol 2000. 1996;10:79.
98 Walker CB, Gordon JM, Socransky SS. Antibiotic susceptibility testing of subgingival plaque samples. J Clin Periodontol. 1983;10:422.
99 Walker CB, Gordon JM, Mcquilkin SJ, et al. Tetracycline: levels achievable in gingival crevice fluid and in vitro effect on subgingival organisms. Part II. Susceptibilities of periodontal bacteria. J Periodontol. 1981;52:613.
100 Walker CB, Gordon JM, Magnusson I, et al. A role for antibiotics in the treatment of refractory periodontitis. J Periodontol. 1993;64:772.