CHAPTER 20 SUPPLEMENT A Masticatory System Disorders
The masticatory system consists of the temporomandibular joints (TMJs), masticatory muscles, teeth in occlusion, and neurologic and vascular supplies supporting all these structures. Research suggests that masticatory system disorders include many varied conditions with multiple possible contributing factors, rather than different manifestations of a single disease or syndrome.2,78,123 The ability to understand the anatomy and function of the masticatory system and correctly interpret relevant diagnostic information is a prerequisite to fulfilling comprehensive standards of care. Our diagnostic process must be broad based and inclusive enough to determine the most appropriate cause of masticatory dysfunction.131
Temporomandibular Joint
Harmonious function of the TMJs is a product of the coordination of the muscles of mastication by intricate mechanisms of neurologic control. Understanding the dynamics and the relationship of the TMJ to the associated muscles and nerves provides the working knowledge required for effective assessment and diagnosis.
The TMJ is one of the most complex joints in the human body. It is capable of providing both hinging (rotation) and gliding (translation) movements and is able to resist incredible forces of mastication. The TMJ is formed by the head of the condyle of the mandible as it fits into the articular fossa of the temporal bone (Supplement A Figure 20-1). The body of the mandible effectively connects both condyles so that neither condyle functions independently of the other. Interposed between the head of the condyle and the articular surface of the temporal bone is the articular disc, consisting of dense connective tissue, resulting in a compound joint with two joint cavities (Supplement A Figure 20-2). The articulating surfaces of the osseous structures are essentially convex in a healthy situation, so the biconcave configuration of the articular disc compensates for the opposing convexities. The articular surfaces of the condyles and temporal bones consist of fibrous connective tissue, rendering them resistant to breakdown and capable of repair. Deep to the superficial connective tissue layer, articular cartilage provides the cellular and structural basis for the response to the functional loading and movement of the TMJs.78,131,177 The discal ligaments and attachments to the capsule, along with the disc itself, become the means of separating the joint into superior and inferior joint spaces (see Supplement A Figures 20-1 and 20-2). Synovial lubrication of the articular surfaces is a function of synovial fluid production by endothelial cells along the borders of each joint cavity and at the anterior extent of the retrodiscal tissues.*
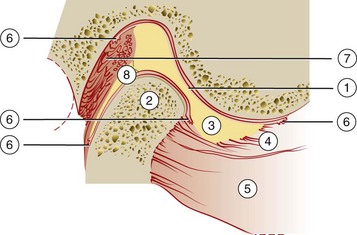
Supplement A Figure 20-1 Lateral view of cross-section through temporomandibular joint. 1, Posterior slope of articular eminence of temporal bone; 2, head of condyle; 3, articular disc (note biconcave shape); 4, superior lateral pterygoid muscle (note attachment to both head of condyle and articular disc); 5, inferior lateral pterygoid muscle; 6, synovial tissue; 7, retrodiscal tissue; 8, discal ligament attachment to the posterior surface of head of condyle.
(Modified from Dawson PE: Evaluation, diagnosis, and treatment of occlusal problems, ed 2, St Louis, 1989, Mosby.)
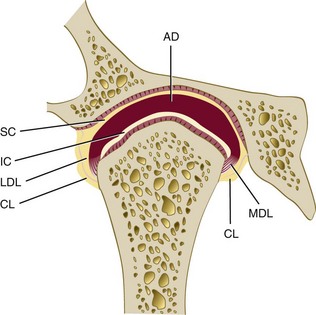
Supplement A Figure 20-2 Temporomandibular joint (anterior view), showing collateral ligaments. AD, articular disc; CL, capsular ligament; IC, inferior joint cavity; LDL, lateral discal ligament; MDL, medial discal ligament; SC, superior joint cavity.
(From Okeson JP: Management of temporomandibular joint disorders and occlusion, ed 4, St Louis, 1998, Mosby.)
Muscles and Nerves of the Masticatory System
The muscles and nerves of the masticatory system are extensively reviewed elsewhere and are only briefly discussed here for the purpose of understanding the mechanisms involved. Appropriate references are provided for further reading.
The muscles of mastication consist principally of two groups: the elevator muscles and depressor muscles. The muscles responsible for elevating the mandible are the masseter, internal pterygoid, and much of the temporal muscle. The posteriorly oriented fibers of the temporal muscle also retrude the mandible. The superficial muscle bundle of the masseter muscle may also assist in protruding the mandible while the deeper bundle serves to stabilize the condylar head against the articular eminence. Juxtaposed with the masseter muscle, the medial pterygoid forms a muscular support for the mandible at its angle. Although the primary function of elevation of the mandible, it is also active during protrusion.78,131 The lateral pterygoid muscle is now known to function as two distinct muscles, the inferior and superior lateral pterygoid muscles, with independent and almost opposite functions.6,14 The inferior lateral pterygoid muscle depresses and protrudes the mandible. The superior lateral pterygoid muscle does not contract during depression of the mandible but rather contracts along with the elevator muscles, bracing the condyle anteromedially.110,119,129,131,151
Physiologic mandibular posture and movement are products of harmonious muscular contraction among masticatory and supportive muscles. The neurologic input to produce synergy of complementary and antagonistic muscles is extremely complex. Motor and sensory innervation of the TMJs and the rest of the masticatory system are provided by structures of the trigeminal nerve. Mechanoreceptors in the skin, muscle, and ligamentous structures, especially the periodontal ligament (PDL), discern pressure differences at sensitive degrees of discrimination. Painful stimuli are perceived by nociceptors and result in both pain perception and reflex responses. The innervation of both the capsular ligaments and the discal ligaments provide essential proprioceptive input with regard to joint position. Efferent or motor neurons cause muscle contraction in response to central cortical stimulation and in response to afferent stimuli in reflex activity40,131,177
Sensory input from the PDL offers the potential to be an important component of the complex neurologic management of the masticatory system. Currently, little evidence of the existence of proprioceptive sensory organs within the neuroanatomy of the PDL is available, although it was once considered likely. Pain perception causes the nociceptive reflex to open the mouth rapidly through contraction of depressor muscles and suppression of elevator muscles, consistent with other protective reflexes within the musculoskeletal system.131 Protective reflexes may be suppressed in individuals experiencing chronic occlusal parafunction (clenching or grinding of teeth).25,49 Pressure perception is a function of the numerous mechanoreceptors within the PDL of teeth in contact. Discrimination within the dentition based on specific teeth in contact, direction of force, and intensity of force and their influence on muscle activity have been demonstrated in human study populations and animal studies.22,37,99,109,154 Both research and clinical observations suggest that elevator muscle contraction is suppressed when anterior teeth promote disclusion or separation of posterior teeth during excursive mandibular movements.173 Loss of attachment resulting from periodontitis involves the loss of some mechanoreceptors. Patients with significant bone loss, significant inflammatory disruption of the integrity of the PDL, or chronic occlusal parafunction may experience compromised regulation of muscle activity.*
Centric Relation
The mandible is suspended from the cranial base by ligaments and muscles. Understanding mandibular movement begins with an initial reference point for each condyle, usually referred to as centric relation; this clinically determined relationship of the mandible to the maxilla occurs when both condyle-disc assemblies are positioned in their most superior position in the maxillary (or glenoid) fossa and against the slope of the articular eminence of the temporal bone. Verification of centric relation is obtained by loading the TMJs bilaterally with the teeth apart, using the bimanual mandibular manipulation technique advocated by Dawson and others.40,41,42,43,165 When both condyles are in this relationship, rotation or hinging action occurs around an axis defined by the medial poles of each condyle (Supplement A Figure 20-3). The term centric relation is limited to the rotation axis through both condyles while they are seated in their respective glenoid fossae. The only occlusal consideration relative to centric relation occurs when rotation of the mandible initiates the first contact of opposing occlusal surfaces. The term initial contact in centric relation can be used to define this relationship (see Chapter 47). If the contraction of elevator muscles occurs at the point of initial occlusal contact, resulting in the distraction of one or both condyle-disc assemblies from their seated relationship, centric relation is no longer occurring.40,41,42
Supplement A Figure 20-3 In centric relation, condyles can rotate on a fixed axis. As long as the rotational axis stays fixed at the most superior position against the eminentiae, the mandible can open or close and still be in centric relation. If the condyle axis moves forward, it is no longer in centric relation.
(From Dawson PE: Evaluation, diagnosis, and treatment of occlusal problems, ed 2, St Louis, 1989, Mosby.)
For TMJs to maintain orthopedic stability, the condyles must remain fully seated in their respective fossae when the teeth occlude in maximal intercuspation. Orthopedic instability occurs when the occlusal relationships are such that contraction of elevator muscles is required to achieve stable occlusion in maximal intercuspal position resulting in the unseating of one or both condyles from their respective fossae (Supplement A Figure 20-4). The strain on the discal ligaments caused by a loaded joint being displaced from the fossa can lead to internal derangement of that joint, as described later. Postural and parafunctional stress can also be a source of orthopedic instability of a TMJ.
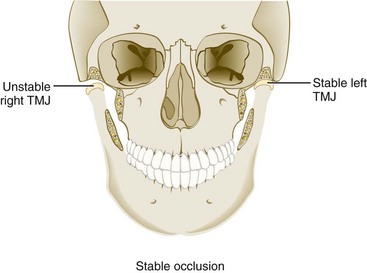
Supplement A Figure 20-4 Example of orthopedic instability. Note that with the teeth in their stable position (maximum intercuspation), the left temporomandibular joint (TMJ) is in a stable relationship with the fossa. The right TMJ, however, is not in a stable position in the fossa. When the elevator muscles contract, the right condyle moves superiorly, seeking a more stable relationship with the articular disc and fossa (the musculoskeletally stable position). This type of loading can lead to an intracapsular disorder.
(From Okeson JP: Management of temporomandibular joint disorders and occlusion, ed 4, St Louis, 1998, Mosby.)
An individual’s susceptibility to masticatory system disorders determines whether that person adapts with minimal consequence or develops dysfunction or degeneration.24,40,41,131,165
Biomechanics of the Masticatory System
Biomechanics of mandibular movement are a function of neurologic input from cortical and stomatognathic sources acting to initiate or restrict muscular contraction. Muscular action either stabilizes the condyle against the articular eminence or directs its rotational and translational movements relative to each respective temporal bone. The position and functional movement of one condyle always depend on the status or activity of the other. Because the maxillary teeth have a fixed relationship to the cranial base, just as mandibular teeth have a fixed relationship to the condyle, contact of their respective occlusal surfaces may directly influence condylar position or movement.40,41,131
The mandible can move within a range of motion (ROM) that is limited by skeletal, muscular, and ligamentous structures. Pure rotation of up to approximately 25 mm can occur before translation of the condyle is required to continue toward maximal opening of the jaw. Movement of the condyle is relative to the disc, so rotation effectively occurs within the inferior joint space (Supplement A Figure 20-5, A). Strict translation protrudes the mandible while the condyle-disc assembly moves anterior and inferior toward the articular eminence of the temporal bone.
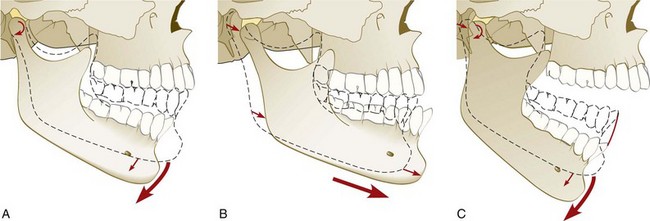
Supplement A Figure 20-5 A, Rotational movement of mandible with condyles in centric relation. This pure rotational opening can occur until the anterior teeth are about 20 to 25 mm apart. B, Translational movement of the condyle-disc assembly during protrusion of the mandible. C, Second stage of rotational movement during opening. Note the dual activity relative to the articular disc. 1, Rotation of the condylar head, relative to the disc, occurs in the inferior space. 2, Movement of the disc anteriorly and inferiorly along the articular surface of the temporal bone. The articular disc moves anteriorly and inferiorly with the head of the condyle, which continues to rotate against the disc. Translation occurs in the superior joint space, and rotation occurs in the inferior joint space.
(From Okeson JP: Management of temporomandibular joint disorders and occlusion, ed 4, St Louis, 1998, Mosby.)
The disc moves relative to the temporal bone, and movement occurs within the upper joint space (Supplement A Figure 20-5, B). In combination translation/rotation movement of the condyle, the axis of rotation for each condyle changes as the condyle translates down the articular eminence to a position inferior to its fossa (Supplement A Figure 20-5, C). Harmonious muscle function and ligament attachments keep the condyle-disc assembly properly related so that the articular disc remains loaded in its concave, avascular central portion between the condyle and the articular surface of the temporal bone. The elasticity and vascularity of the retrodiscal tissues permit anterior movement of the disc during translation of each respective condyle. Rotation and translation of the condyle can occur in the absence of any tooth-to-tooth contact because the condyle-disc assembly can be supported by the muscles of mastication against the articular eminence during rotation, translation, and combination movements. When the teeth are in contact, their ability to influence both the position and the direction of movement of condyle-disc assemblies is defined by the intensity of muscle activity and the steepness of the inclines of those teeth.*
Dysfunction and Deterioration
Ideally, function never exceeds the integrity or adaptive limits of the structural elements of the masticatory system. Clinical experience shows that the tolerance of the components of the masticatory system can be exceeded by both acute trauma and chronic trauma. Acute trauma to the head and neck region can range from a distinct event, such as an accident or a blow to the face, to a sustained overuse experience, such as a long dental appointment. Acute trauma can serve as an initiating event leading toward a chronic condition, so accurate documentation and careful monitoring may prove extremely valuable should symptoms or dysfunction persist.12,19,44
Chronic trauma is defined as any experience that repeatedly exceeds the tolerances of the affected masticatory system structure. Postural stresses and parafunctional occlusal habits, with or without occlusal discrepancies, may produce musculoskeletal disharmony and orthopedic instability of the TMJ. Occlusal relationships that disrupt the condyle during physiologic movement of rotation or translation require muscular and TMJ compensation. The extent to which the repeated loading of the teeth and the condyles during function and parafunction exceeds the tolerance of an individual will determine whether structural or muscular compromise occurs.131
Recently completed prospective research found only weak association with occlusal disharmonies, but significant correlations with both reported bruxism and tooth wear index and TMD symptoms were confirmed.23,112 When TMJ dysfunction could be correlated with specific occlusal relationships, the trend was to recognize that when inclines of posterior teeth dominated occlusal function, masticatory system harmony was disrupted.* Other researchers have found that various occlusal interferences and relationships are common among individuals with and without masticatory system disorders. They could not distinguish a particular occlusal feature as a specific etiologic or predisposing factor for the development of masticatory system disorders, although some found that several factors occurring together encouraged dysfunction.35,71,140,141,164 There seems to be less correlation between static references, such as class of malocclusion, and masticatory system disorders than when functional or extrafunctional occlusal forces exceed the tolerance of the TMJ and masticatory musculature.131,153
The general terms for occlusal parafunction used in this text include bruxism, or grinding of the teeth, and clenching, in which a person holds the teeth firmly together with significant force. Bruxism is usually confirmed by observing excessive tooth wear. Clenching type of parafunction can be distinguished from grinding the teeth and seems to be more often associated with masticatory system disorders than does bruxism.† Sleep bruxism may include both tooth grinding and clenching and seems to occur primarily in stage 1 and stage 2 (non-REM [rapid eye movement]) sleep. These episodes often occur in association with short brain and cardiac reactivations called “micro-arousals.” Rhythmic masticatory muscle activity is relatively common among non-bruxers, but the frequency and intensity of the muscle contraction is substantially greater for the sleep bruxer. The central pattern generator of the primate brainstem does not modulate or reduce muscle contraction during sleep as occurs during waking hours. Additionally, amplification of oral parafunction has been reported that is associated with a patient’s intake of selective serotonin reuptake inhibitors (SSRIs; e.g., Prozac and others).* Discrimination between occlusal function–related or parafunction-related masticatory system disorders and those with other etiology requires exacting standards of occlusal evaluation. If sufficient evidence exists to suspect that the occlusal relationships in function or parafunction may have exceeded the tolerances of that individual’s masticatory system, responsible intervention or monitoring can be initiated.41,60,134,141
Disruption of the relationship or alignment of the condyle, disc, and articular surface of the temporal bone is typically called an intracapsular disorder or internal derangement of the TMJ. The articular disc can be displaced as a result of an acute blow to the jaw, chronic trauma, or uncoordinated contraction of the lateral pterygoid muscle. When the disc cannot return to its normal relationship to the condyle on full closure of the mouth, it is considered to be displaced or dislocated. Progressive disc displacement most often begins on its lateral aspect and occurs in an anterior and medial direction because of the insertion of muscle fibers into the anteromedial aspect of the disc and the reported variability in resistance of the attachment of the lateral aspect of the disc.11,155 Stretching of the retrodiscal tissues and collateral ligaments permits the disc to be displaced and function to be limited because of pain resulting from compressive forces on retrodiscal tissues. At some stage of opening, the remaining elasticity of the retrodiscal tissues and tension of the capsular ligaments can pull the disc onto the head of the condyle, often with a discernible sound. Closing then results in the disc again becoming dislocated anteriorly, with a common joint sound often referred to as a reciprocal click* (Supplement A Figure 20-6).
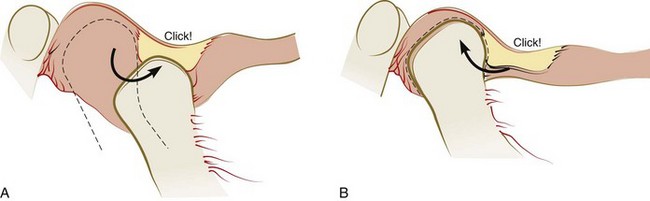
Supplement A Figure 20-6 Reciprocal click. A, Reciprocal click occurs when the condyle moves onto the articular disc from a position behind the posterior band of the disc and then, B, clicks off the disc when the condyle moves back. This occurs as the condyle translates forward and back in the opening and closing movements.
(From Dawson PE: Evaluation, diagnosis, and treatment of occlusal problems, ed 2, St Louis, 1989, Mosby.)
When the disc remains anterior to the head of the condyle during rotation and the limited range of translation possible, the condition is called closed lock or disc displacement (dislocation) without reduction (Supplement A Figure 20-7).40,131 The entire disc need not be locked anterior to the head of the condyle for this condition to limit function and cause pain. The lateral aspect of the disc would be more likely than the medial aspect to be displaced anteriorly if a partial anterior disc displacement without reduction were to occur. A history of joint sounds is usually reported, although this state of the condyle-disc assembly may not result in currently discernible sounds40,131
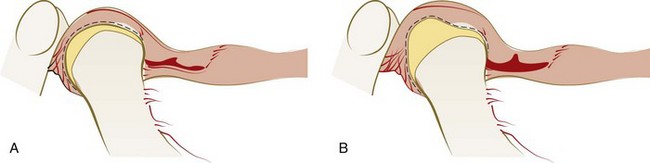
Supplement A Figure 20-7 Anterior disc displacement without reduction. A, Condyle positioned in the fossa on retrodiscal tissues with the disc remaining anterior to head of condyle. B, During translation, the disc is further misshapen, restricting full opening of the mandible.
(From Dawson PE: Evaluation, diagnosis, and treatment of occlusal problems, ed 2, St Louis, 1989, Mosby.)
The vascular portion of the retrodiscal tissues being loaded (between the condyle and the articular surface of the eminentia) accounts for most current pain or a history of pain originating within the TMJ. Adaptation of the retrodiscal tissues to completely nonvascular fibrous tissue or perforation of the disc may account for cessation of the painful symptoms.135
The presence of abnormal anatomic features of the condyle and fossa results in deviation in the shape of the affected articular surface, to which the disc then must adapt its normal anatomy, resulting in a deviation in form and function. If this type of functional limitation or irregularity is observed at a consistently occurring point in jaw opening and closing, it is often within the compensatory mechanisms of the patient and should be distinguished from the disc derangements described previously.131
When the intensity and duration of the functional and dysfunctional loading of the TMJs result in injury, molecular agents appear to be active in the degeneration of joints. Free radicals, various catabolic enzymes, neuropeptides, estrogen, cytokines, and prostaglandins are implicated in inflammatory reactions that have an impact on the articular surfaces and synovial fluid.* Loss of the ability of the synovial fluid to lubricate articular surfaces can result in adherence of the disc. Limitation of rotation occurs with adherence between the disc and the condyle, whereas fixation of the disc against the fossa permits rotation but does not allow the disc to move forward during translation.131
Hypermobility (subluxation) of the TMJ can permit the condyle to translate beyond the eminentia with both the disc and the condyle beyond its prominence. The combination of anatomic features that predisposes individuals to subluxation, often allows for self-reduction of the condyles. When condyles translate beyond the eminentia, but the discs are trapped posteriorly, the combined steepness of the disc and the eminentia prevents reduction of each condyle, and the mandible is locked open.131
Orofacial Pain
Discomfort associated with masticatory system disorders falls under the larger umbrella of orofacial pain. Pain associated with TMJ dysfunction is most frequently muscular in origin40 and may be amplified by both occlusal parafunction and stress.58 Although pain itself is a complex entity,161 working knowledge of even the uncommon sources of pain perceived in the region of the masticatory system is essential to providing comprehensive diagnosis and treatment. Sources of dental or periodontal pain should be identified by clinical, radiographic, and historic information. Nondental sources of pain include TMJ structures, muscles, cervical structures, neuropathies, vascular inflammation, all types of headache, sleep disorders, systemic disorders, and psychoimmune neurologic sources.130 A survey of 45,700 American households revealed that 22% of respondents had experienced some type of orofacial pain in the previous 6 months, establishing a meaningful probability that the periodontal patient’s list of symptoms includes pain.105 Supplement A Box 20-1 provides the current list of possible sources of orofacial pain, prepared by the American Academy of Orofacial Pain.130
SUPPLEMENT A BOX 20-1 Compiled by American Academy of Orofacial Pain.
Differential Diagnosis of Orofacial Pain
Neurogenic Pain Disorders117, 118
Headache pain is perceived primarily within the trigeminal nerve pathways, although other cranial and cervical nerves may offer painful sensory input.78,130,152 Pain originating in masticatory system structures, which are also innervated by the trigeminal nerve, requires diagnostic differentiation from headache pain.153 Headache can present in a myriad of forms and can influence perception of pain and diagnosis of origin of pain.78,130 Pain of dental and periodontal origin must be clearly defined and differentiated from heart attack, sinus pain, and myofascial pain.130,131 Pain originating in pulpal or periodontal nociceptors would be differentiated with a comprehensive clinical and radiographic evaluation. Orofacial pain originating in the TMJs or the muscles of mastication can result from neoplasm, macrotrauma, repeated microtrauma, systemic disease, and anatomic predisposition. Within the joint structures, inflammation and compression of vascularized components are the direct sources of pain. Synovitis or capsulitis, with or without osteoarthritis, and the polyarthritides are characterized by local pain, which increases with function while limiting range of motion of the affected TMJ. In addition to the potential for pain, symptoms of arthritis can include limited mandibular opening, disruption of other jaw mechanics, and joint sounds characteristic of degenerative change to and direct contact between articular surfaces.*
Orofacial pain originating in the muscles of mastication may be perceived in that region or may be referred to other structures such as individual teeth. Similarly, pain referral to the region of certain muscles requires definition of origin. Because local provocation at the origin of pain should produce symptoms at the site of pain perception, movement of the jaw would be expected to elicit pain in painful muscles of mastication. Cranial nerves experience referral on the same side, whereas skeletal nerve referral can occur to the opposite side; both sources generally refer pain centrally or superiorly.78,131,158
The muscles of mastication are subject to a variety of disorders and dysfunction, many of which can be painful. The American Academy of Orofacial Pain designates myofascial pain, myositis, muscle spasm (myospasm), local myalgia, and myofibrotic contracture as major categories of these conditions. Muscle palpation that reveals a taut band of muscle or fascia and that results in pain, which is also frequently referred, is virtually diagnostic for myofascial pain. Myositis of masticatory muscles arises from direct trauma or infection close to muscle. Associated pain increases with mandibular movement, thereby limiting range of motion. Muscle spasm is the sustained involuntary contraction resulting in pain and dramatic shortening of the affected muscle. Myospasm of masticatory muscles greatly limits mandibular movement and can change occlusion suddenly because of its rapid onset. Local myalgia, or pain specific to individual muscles, may result from ischemia or fatigue and may present as delayed-onset muscle soreness and protective co-contraction. Occlusal parafunction, extended dental appointments, metabolic imbalances, and sympathetic nervous system influences have been associated with this painful muscular reaction. An extended period of limited range of mandibular movement can result in fibrosis of the muscle and related attachments, creating a painless condition called myofibrotic contracture.130
Otolaryngologic symptoms associated with masticatory system disorders have been reported and include degrees of deafness, tinnitus, and vertigo.† Trauma and postural stress in the cervical spine can be responsible for both the perception and the origin of pain within the masticatory system.12,19,78,131
Determination of a specific origin or source of pain can become more difficult with pain referral and modulation of painful experiences by the central nervous system. Sensitization of peripheral nociceptors by higher neural centers and inflammation at the site of origin of pain can alter pain perception.81,113,156 Persistent inflammation therefore may be a contributing factor to chronic pain.81,113,156 Systemic conditions that may contribute to or predispose an individual to compromised pain regulation include sleep disorders, fibromyalgia, chronic depression, chronic fatigue syndrome, hypothyroidism, insufficient thyroid receptor activity, prolactin feedback disorder, epinephrine sensitivity related to mitral valve prolapse, premenstrual syndrome, androgen excess in women, and posttraumatic stress disorder. Although some of these situations exhibit gender bias because of hormonal factors, the influence of stress on pain experiences and the effect of variable coping skills are reported for both genders.*
Comprehensive Evaluation
Patient History and Interview
The written history and personal interview should be designed to invite open-ended responses and reflection by the patient on past experiences and the current condition. Standard dental or medical history forms may require modification to include questions regarding any history of limited or painful jaw movement, noise in either joint, and masticatory muscle symptoms (Supplement A Box 20-2). These issues should be documented with regard to timing, duration, frequency, and relationship to any history of trauma.130
SUPPLEMENT A BOX 20-2 Examples of Questions Involving Masticatory System to Include in Patient History
Are you now experiencing or have you ever experienced:
Clinical Examination
The clinical examination continues the interview process through co-discovery of the patient’s masticatory system status. The dentist leads the patient to understand the meaning of signs and symptoms of dysfunction or deterioration, seeking opportunities to expand the patient’s responses to questions. The physical examination actually begins during the interview, when asymmetries in facial form, head posture, and mandibular movement patterns can be observed. Clinical evaluation of the various structures of the masticatory system, although individual to each practitioner, should afford the patient opportunity for understanding and should include the following9,40,131:
![]() Science Transfer
Science Transfer
Stability in centric relation is an important determinant of orthopedic stability of the masticatory system because it allows for the condyles to be fully seated in their glenoid fossae. After identifying centric relation, as described in this chapter, the dentist employs procedures for establishing tooth contact by using interocclusal, hard occlusal splints and occlusal adjustment. In addition to stable centric relation, the masticatory system requires an intercuspal position that is tolerated by the joints, ligaments, and muscles and ideally, anterior disclusion to limit the contact of posterior teeth during functional and parafunctional mandibular movements. When any of these requirements is compromised, dysfunction of the masticatory system becomes more likely.
The masticatory system consists of the temporomandibular joints (TMJs), masticatory muscles, oral structures, and all their corresponding vascular and neurologic components. As such, many conditions exist where various aspects of these structures change because of wear or inflammation. As changes occur, various components are affected, and in many cases, pain results. Unfortunately, however, the pain is often diffuse and not well localized. This makes definitive diagnosis extremely difficult, and although improved, imaging of masticatory structures is still problematic. Thus masticatory system disorders remain a difficult area for diagnosis and treatment.
Evaluation of the TMJ begins with ROM analysis. Observation of departure from a straight path of opening and closing the mandible suggests an intracapsular disorder or masticatory muscle incoordination.40,41,131 An average maximal opening of 50 mm is common; findings of less than 40 mm of opening suggest limited opening caused by a masticatory system disorder. The range of right and left lateral excursions is usually about 9 mm, and protrusion of mandible is typically 7 mm. Limitation in ROM may be normal for some patients, but for most, these observations are of diagnostic value.9,47,68,131,160 Auscultation of the joint by listening with a stethoscope or Doppler instrument, which amplifies joint sound for both the patient and the dentist, can reveal noises diagnostic for numerous conditions. The intensity and nature of any sounds, clicks, pops, or crepitus (grinding, grating, or rubbing sounds) should be recorded accurately. Any sound detected as part of the initial evaluation should be tracked consistently to detect any change. Diagnostic interpretation and management based on specific sounds correlated with status of the TMJ can be found in other references.7,9,40,41,131,170
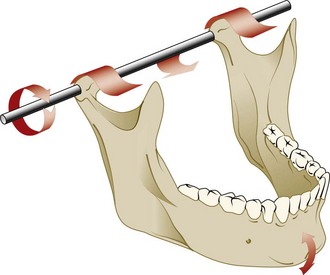
Firm palpation of the TMJ with the mouth closed can be uncomfortable for the patient with inflammation in joint structures or superficial muscles. Palpation while opening may become more uncomfortable if retrodiscal tissues are also inflamed. Load testing of the TMJ is essentially a means to palpate the head of the condyle, surface of the glenoid fossa, and tissue interposed between them, except in the case of bone-to-bone contact. With bimanual mandibular manipulation, the dentist loads the joints equally and may detect resistance or tension on either side. The patient is in a supine position in the dental chair to minimize postural influence on muscle activity. The patient’s head is braced by the support of the chair and by being cradled against the dentist’s arm or abdomen. The dentist’s middle fingers locate the notch in the mandible just anterior to the angle, and the thumbs are placed near the midline in the mental region of the mandible (Supplement A Figure 20-8). Initially, the dentist provides very gentle guidance to the hinging action of the mandible, with a slight lifting force applied by the fingers and slight depressing force applied with the thumbs. If the patient remains comfortable, increasing force can be applied at both points, ultimately with enough pressure to load-test the joints. With the avascular fibrous discs interposed, the condyles are in centric relation, and the loading of both joints is comfortable. Discomfort may occur with muscle incoordination or bracing or with an anterior displaced disc and attempted loading of vascular retrodiscal tissues of either TMJ. When performed properly, the patient can bite firmly as part of guided loading of the TMJs and report the nature or absence of pain or tension.9,40,62,131
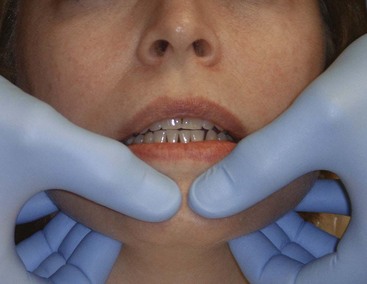
Supplement A Figure 20-8 Bimanual manipulation load testing in centric relation with the teeth apart.
Muscle palpation is also a learned technique that requires both experience and expertise to derive the most reliable information. Too little pressure is not diagnostic of modest muscle pain or spasm, whereas too much pressure can hurt even when normal musculature is palpated.56 Externally, the muscles palpated include the anterior, middle, and posterior temporalis; superficial masseter; anterior and posterior digastric; sternocleidomastoid; trapezius; posterior cervical muscles; and insertion of the medial pterygoid muscle. Intraorally, the deep masseter is tested with moderate squeezing pressure, and the medial pterygoid muscle is palpated directly in the general region of the insertion point for local anesthesia with mandibular block. The lateral pterygoid is difficult to palpate because of the dominance of the medial pterygoid in the same region. Its palpation superior and distal to the palpation point for the medial pterygoid can be attempted distal to the maxillary tuberosity. Offering manual resistance to the patient’s efforts to protrude the mandible is also a test of the lateral pterygoid muscle. Neither method of evaluation of the possible soreness of the lateral pterygoid muscle is completely reliable, although both provide some insight into muscle status.*
During muscle palpation, the dentist may be able to detect a particularly taut and uncomfortable band of muscle fibers. This condition represents regional myofascial pain or trigger point myalgia, which can be responsible for referral of pain to the teeth and other orofacial regions. Diagnostic injection of local anesthesia can be effective in identifying trigger point pain and pain referral patterns.131,158
The occlusal analysis is a logical extension of the evaluation of the teeth and periodontium. Tooth mobility is assessed in both static and dynamic modes. Pressure applied to a tooth with a firm object allows detection of movement through both visual and tactile evaluation (see Chapters 30 and 49). Asking the patient to move in excursions of the mandible while maintaining firm contact of opposing teeth also permits visual and tactile assessment. Sources of tooth mobility include inadequate periodontal support, inflammation of the periodontium, and excessive occlusal loading of the teeth with adequate periodontium, resulting in adaptive mobility. The physical evaluation of the teeth and any restorations can reflect history of trauma or wear. Visual observation, registration with marking paper or wax, and electronic assessment of tooth contacts as the patient moves in all excursions may reveal disharmonies sufficient to cause orthopedic instability of either TMJ. If the teeth are found to be relatively firm, the relationship between the maxillary and mandibular teeth may influence the direction of condylar movement as soon as tooth-to-tooth contact is made.
While maintaining centric relation, the dentist continues the physical examination by positioning the condyles in a fully seated relationship without tooth-to-tooth contact, using bimanual manipulation or a leaf gauge technique. The mandible is manually guided to close until the first tooth-to-tooth contact is made. If that position is also maximum intercuspation, optimum seating of the condyles is maintained. If the initial contact in centric relation is not maximum intercuspation, the condyles will be directed from a fully seated position to an inferior position relative to their respective fossae, resulting in an orthopedic instability. The direction and extent of the accommodation of the mandible should be carefully measured and recorded at the initial evaluation and consistently evaluated at subsequent appointments to discover any trend.*
To increase the reliability of this evaluation, a muscle-deprogramming effort may be employed. The simplest approach is to use cotton rolls placed between the anterior teeth for 5 to 15 minutes to allow possible muscle relaxation through avoidance of proprioceptive or pressure neurologic input. A prefabricated or directly fabricated acrylic or composite bite stop for anterior teeth offers the same advantage.9,10,131 A more complex means of confirming the seating of the condyles in centric relation is achieved with a maxillary or mandibular muscle relaxation occlusal appliance. These appliances provide full coverage for the respective arch and occlusal contact for at least one cusp or incisal edge of the opposing teeth so that teeth are protected from spontaneous shifting. The occlusal design provides immediate disclusion of all the posterior teeth in every protrusive or lateral excursion. This allows for the progressive deprogramming of muscles through the advantage of reduced muscle contractions and limited noxious neurologic input.42,95,172
Models of the dentition must be accurately mounted to be diagnostic. The facebow transfer relates the maxillary cast to the axis of rotation of the articulator as the maxillary teeth relate to the cranial case. With careful bimanual manipulation or the use of an anterior bite stop, a transfer wafer is generated with the condyles fully seated in their glenoid fossa. The maxillary and mandibular teeth of each diagnostic model then relate to one another in centric relation, as determined by condylar position. They may reveal an occlusal discrepancy in centric relation, which would require compensation by the patient.9,40
Imaging
When the clinical evaluation, panoramic radiography, and patient history indicate the possibility of structural masticatory system disorders or the possible presence of pathology, especially neoplasm, appropriate imaging of the TMJ is warranted.102 The state-of-the-art technique for imaging of soft tissue, especially the articular disc, is magnetic resonance imaging (MRI). The current highest standard for imaging of hard tissue, such as the condyle or the temporal bone, is computed tomography (CT). Cone-beam CT (CBCT) has become much more readily available to dentistry in the last few years, with software systems able to display the data gathered as both anteroposterior and cross-sectional depiction of the condyle and cranial structures with the same or better image quality as spiral CT. Less radiation exposure and lower cost to the patient are both reasons to favor CBCT over conventional CT. The interpretation of MRI and CT images usually requires specialized training for the clinician or access to a radiologist. Arthrography is still being used for certain diagnostic situations, such as suspected perforation of the articular disc, and nuclear medicine has developed protocols to image the TMJ to determine if active deterioration is occurring.†
Although plain-film tomography is occasionally a feature of some of the newer radiographic equipment, the technique most readily available to a majority of practitioners is panoramic radiography. The image produced depicts only general relationships and gross anatomy, so the information provided should be used only for screening purposes. When pathology or marked deformation is suggested by a panoramic radiograph, further diagnostic imaging and procedures may be warranted.18,78
Diagnostic Decision Making
Complete evaluation of every patient’s periodontal status must include the diagnostic components required to reveal any form of masticatory system disorder. The existence of factors responsible for historical, current, or potential impairment of masticatory system function can be integrated into a comprehensive treatment plan. Patients who require substantial periodontal therapy or have advanced periodontal disease may be at increased risk for masticatory system disorders, so diagnostic processes must remain consistently thorough and inclusive for all patients.24,149 For the patient presenting with a symptomatic masticatory system disorder, the diagnostic strategy would logically begin with the inclusion of all potential sources of pain or dysfunction, followed by the systematic exclusion of possible causative or contributing factors, beginning with the least likely. When no symptoms are reported, the history and clinical examination still need to be thorough because some patients tend to tolerate modest dysfunction or mild transient discomfort. The diagnostic strategy for the patient presenting minimal or no signs and symptoms of masticatory system disorders is to attempt to confirm a stable condition while identifying risk factors. Careful documentation of past or current trauma and disharmony provide the basis for trend analysis and anticipation of possible future problems.*
Consistent professional maintenance care has been clearly demonstrated to be a key ingredient in successful management of a patient’s periodontal condition.67,118 Complementing any treatment sequence, these appointments afford dentists the opportunity at every stage of comprehensive care to provide continuing evaluation of the status of the entire masticatory system and to provide timely and appropriate intervention when needed (see Chapter 49).
Acknowledgments
I would like to acknowledge the encouragement and the recommendation of references provided by Dr. Henry Gremillion during the revision of this chapter.
1 Al-Hadi LA. Prevalence of temporomandibular disorders in relation to some occlusal parameters. J Prosthet Dent. 1993;70:345.
2 Allen EP, Bayne SC, Becker IM, et al. Annual review of selected dental literature: Report of the Committee of Scientific Investigation of the American Academy of Restorative Dentistry. J Prosthet Dent. 1999;82:39.
3 Atwood MJ, Dixon DC, Talcott GW, et al. Comparison of two scales in the assessment of muscle and joint palpation tenderness in chronic temporomandibular disorders. J Orofac Pain. 1993;7:403.
4 Austin DG. Special considerations in orofacial pain and headache. Dent Clin North Am. 1997;41:325.
5 Auvenshine RC. Psychoneuroimmunology and its relationship to the differential diagnosis of temporomandibular disorders. Dent Clin North Am. 1997;41:279.
6 Aziz MA, Cowie RJ, Skinner CE, et al. Are the two heads of the human lateral pterygoid separate muscles? A perspective based on their nerve supply. J Orofac Pain. 1998;12:226.
7 Bade DM, Lovasko JH, Dimitroff M, et al. Clinical comparison of temporomandibular joint sound auscultation and emission imaging studies. J Orofac Pain. 1994;8:55.
8 Bates RE, Gremillion HA, Stewart CM. Degenerative joint disease. Part II. Symptoms and examination findings. J Craniomandib Pract. 1994;12:88.
9 Becker IM, Tarantola GJ. Parameters of care: temporomandibular disorders. Key Biscayne, FL: Pankey Institute of Advanced Dental Education; 1994.
10 Becker I, Tarantola G, Zambrano J, et al. Effect of a prefabricated anterior bite stop on electromyographic activity of masticatory muscles. J Prosthet Dent. 1999;82:22.
11 Ben Amor F, Carpentier P, Foucart JM, et al. Anatomic and mechanical properties of the lateral disc attachment of the temporomandibular joint. J Oral Maxillofac Surg. 1998;56:1164.
12 Benoliel R, Eliav E, Elishoov H, et al. Diagnosis and treatment of persistent pain after trauma to the head and neck. J Oral Maxillofac Surg. 1994;52:1138.
13 Bergdahl J, Anneroth G, Anneroth I. Clinical study of patients with burning mouth. Scand J Dent Res. 1994;102:229.
14 Bertilsson O, Ström D. A literature survey of a hundred years of anatomic and functional lateral pterygoid muscle research. J Orofac Pain. 1995;9:17.
15 Bjorne A, Agerberg G. Craniomandibular disorders in patients with Ménière’s disease: a controlled study. J Orofac Pain. 1996;10:28.
16 Bouquot J, Roberts A. NICO (neuralgia-inducing cavitational osteonecrosis): radiographic appearance of the “invisible” osteomyelitis. Oral Surg. 1992;74:600.
17 Bouquot JE, Roberts AM, Person P, et al. NICO (neuralgia-inducing cavitational osteonecrosis): osteomyelitis in 224 jawbone samples from patients with facial neuralgias. Oral Surg. 1992;73:307.
18 Brooks SL, Brand JW, Gibbs SJ, et al. Imaging of the temporomandibular joint: a position paper of the American Academy of Oral and Maxillofacial Radiology. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:609.
19 Burgess JA, Kolbinson DA, Lee PT, et al. Motor vehicle accidents and TMDs: assessing the relationship. J Am Dent Assoc. 1996;127:1767.
20 Bush FM, Harkins SW. Pain-related limitation in activities of daily living in patients with chronic orofacial pain: psychometric properties of a disability index. J Orofac Pain. 1995;9:57.
21 Bush FM, Harkins SW, Harrington WG. Otalgia and aversive symptoms in temporomandibular disorders. Ann Otol Rhino Laryngol. 1999;108:884.
22 Byers MR, Dong WK. Comparison of trigeminal receptor location and structure in the periodontal ligament of different types of teeth from the rat, cat, and monkey. J Comp Neurol. 1989;279:117.
23 Carlsson GA, Egermark I, Magnusson T. Predictors of signs and symptoms of temporomandibular disorders: a 20-year follow-up study form childhood to adulthood. Acta Odontol Scand. 2002;60:180.
24 Carranza FA, Newman MG. Clinical periodontology, ed 8. Philadelphia: Saunders; 1996.
25 Cathelineau G, Yardin M. The relationship between tooth vibratory sensation and periodontal disease. J Periodontol. 1982;53:704.
26 Chan SWY, Reade PC. Tinnitus and temporomandibular pain-dysfunction disorder. Clin Otolaryngol. 1994;19:370.
27 Chole RA, Parker WS. Tinnitus and vertigo in patients with temporomandibular disorder. Arch Otolaryngol Head Neck Surg. 1992;118:817.
28 Christensen GJ. Abnormal occlusal conditions: a forgotten part of dentistry. J Am Dent Assoc. 1995;126:1667.
29 Christensen GJ. Treating bruxism and clenching. J Am Dent Assoc. 2000;131:233.
30 Christensen LV, Rassouli NM. Experimental occlusal interferences. Part I. A review. J Oral Rehabil. 1995;22:515.
31 Christensen LV, Rassouli NM. Experimental occlusal interferences. Part V. Mandibular rotations versus hemimandibular translations. J Oral Rehabil. 1995;22:865.
32 Christensen LV, McKay DC. TMD diagnostic decision-making and probability theory. Part I. J Craniomandib Pract. 1996;14:240.
33 Christensen LV, McKay DC. TMD diagnostic decision-making and probability theory. Part II. J Craniomandib Pract. 1996;14:312.
34 Clark GT, Ram S. Four oral motor disorders: bruxism, dystonia, dyskinesia and drug-induced dystonic extrapyramidal reactions. Dent Clin N Am. 2007;51:225.
35 Clark GT, Tsukiyama Y, Baba K, et al. Sixty-eight years of experimental occlusal interference studies: what have we learned? J Prosthet Dent. 1999;82:704.
36 Ciancaglini R, Loreti P, Radaelli G. Ear, nose, and throat symptoms in patients with TMD: the association of symptoms according to severity of arthropathy. J Orofac Pain. 1994;8:293.
37 Coffey JP, Williams WN, Turner GE, et al. Human bite force discrimination using specific maxillary and mandibular teeth. J Oral Rehabil. 1989;16:529.
38 Cooper BC, Cooper DL. Recognizing otolaryngologic symptoms in patients with temporomandibular disorders. J Craniomandib Pract. 1993;11:260.
39 Dao TTT, Reynolds WJ, Tenenbaum HC. Comorbidity between myofascial pain of the masticatory muscle and fibromyalgia. J Orofac Pain. 1997;11:232.
40 Dawson PE. Evaluation, diagnosis, and treatment of occlusal problems, ed 2. St Louis: Mosby; 1989.
41 Dawson PE. Functional occlusion from TMJ to smile design. St. Louis: Mosby; 2007.
42 Dawson PE. New definition for relating occlusion to varying conditions of the temporomandibular joint. J Prosthet Dent. 1995;74:619.
43 Dawson PE. A classification system that relates maximal intercuspation to the position and condition of the temporomandibular joint. J Prosthet Dent. 1996;75:60.
44 De Boever JA, Keersmaeker K. Trauma in patients with temporomandibular disorders: frequency and treatment outcomes. J Oral Rehabil. 1996;23:91.
45 De Bont LGM, Stengenga B. Pathology of temporomandibular joint internal derangement and osteoarthrosis. Int J Oral Maxillofac Surg. 1993;22:71.
46 De Wijer A, Lobbezoo-Scholte AM, Steenks MH, et al. Reliability of clinical findings in temporomandibular disorders. J Orofac Pain. 1995;9:181.
47 Dimitroulis G, Dolwick MF, Gremillion HA. Temporomandibular disorders. 1. Clinical evaluation. Aust Dent J. 1995;40:301.
48 Donaldson KW. Rheumatoid diseases and the temporomandibular joint: a review. J Craniomandib Pract. 1995;13:264.
49 Dong WK, Shiwaku T, Kawakami Y, et al. Static and dynamic responses of periodontal ligament mechanoreceptors and intradental mechanoreceptors. J Neurophysiol. 1993;69:1567.
50 Ehrlich R, Garlick D, Ninio M. The effect of jaw clenching on the electromyographic activities of 2 neck and 2 trunk muscles. J Orofac Pain. 1999;13:115.
51 Ferrario VF, Sforza C, Sigurta D, et al. Temporomandibular joint dysfunction and flat lateral guidances: a clinical association. J Prosthet Dent. 1996;75:534.
52 Ferrario VF, Sforza C, Colombo A, et al. An electromyographic investigation of masticatory muscle symmetry in normo-occlusion subjects. J Oral Rehabil. 2000;27:33.
53 Fu K, Ma X, Zhang Z, et al. Interleukin-6 in synovial fluid and HLA-DR expression in synovium from patients with temporomandibular disorders. J Orofac Pain. 1995;9:131.
54 Gelb H, Gelb ML, Wagner ML. The relationship of tinnitus to craniocervical mandibular disorders. J Craniomandib Pract. 1997;15:136.
55 Glaros AG, Baharloo L, Glass EG. Effect of parafunctional clenching and estrogen on temporomandibular disorder pain. J Craniomandib Pract. 1998;16:78.
56 Glaros AG, Glass EG, Williams KB. Clinical examination findings of temporomandibular disorder patients: a factor analytic study. J Orofac Pain. 1998;12:193.
57 Glaros AG, Tabacchi KN, Glass EG. Effect of parafunctional clenching on TMD pain. J Orofac Pain. 1998;12:145.
58 Glaros AG, Williams K, Lausten L. The role of parafunctions, emotions and stress in predicting facial pain. J Am Dent Assoc. 2005;136:451.
59 Goupille P, FouQuet B, Goga D, et al. The temporomandibular joint in rheumatoid arthritis: correlations between clinical and tomographic features. J Dent. 1993;21:141.
60 Gremillion HA. TMD and maladaptive occlusion: does a link exist? J Craniomandib Pract. 1995;13:205.
61 Gynther GW, Holmlund AB, Reinholt FP, et al. Temporomandibular joint involvement in generalized osteoarthritis and rheumatoid arthritis: a clinical, arthroscopic, histologic, and immunohistochemical study. Int J Oral Maxillofac Surg. 1997;26:10.
62 Harper RP, Schneiderman E. Condylar movement and centric relation in patients with internal derangement of the temporomandibular joint. J Prosthet Dent. 1996;75:67.
63 Haskin CL, Milam SB, Cameron IL. Pathogenesis of degenerative joint disease in the human temporomandibular joint. Crit Rev Oral Biol Med. 1995;6:248.
64 Heir GM. Differentiation of orofacial pain related to Lyme disease from other dental and facial pain disorders. Dent Clin North Am. 1997;41:243.
65 Hickman DM, Cramer R. The effect of different condylar positions on masticatory muscle electromyography activity in humans. Oral Surg Oral Med Oral Pathol Radiol Endod. 1998;85:18.
66 Hintze H, Weise M, Wenzel A. Con beam CT and conventional tomography for the detection of morphological temporomandibular joint changes. Dentomaxillofacial Radiogr. 2007;36:192.
67 Hirschfield L, Wasserman B. A long-term survey of tooth loss in 600 treated periodontal patients. J Periodontol. 1978;49:225.
68 Hochstedler JL, Allen JD, Follmar MA. Temporomandibular joint range of motion: a ratio of interincisal opening to excursive movement in a healthy population. J Craniomandib Pract. 1996;14:296.
69 Honey OB, Scarfe WC, Hilgers MJ, Klueber K, Silveira AM, Haskel BS, Farman AG. Accuracy of cone-beam computed tomography imaging of the temporomandibular joint: comparisons with panoramic radiology and linear tomography. Am J Orthrod Dentofacial Orthop. 2007;132:429.
70 Huntley TA, Wiesenfeld D. Delayed diagnosis of the cause of facial pain in patients with neoplastic disease: a report of eight cases. J Oral Maxillofac Surg. 1994;52:81.
71 Ingervall B, Hähner R, Kessi S. Pattern of tooth contacts in eccentric mandibular positions in young adults. J Prosthet Dent. 1991;66:169.
72 Isberg A, Westesson PL. Steepness of articular eminence and movement of the condyle and disk in asymptomatic temporomandibular joints. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;86:152.
73 Israel HA, Diamond B, Saed-Nejad F, et al. Osteoarthritis and synovitis as major pathoses of the temporomandibular joint: comparison of clinical diagnosis with arthroscopic morphology. J Oral Maxillofac Surg. 1998;56:1023.
74 Ito T, Gibbs CH, Marguelles-Bonnet R, et al. Loading on the temporomandibular joints with five occlusal conditions. J Prosthet Dent. 1986;56:478.
75 Jacobs R, van Steenberghe D. Role of periodontal ligament receptors in the tactile function of teeth: a review. J Periodont Res. 1994;29:153.
76 Kahn J, Tallents RH, Katzberg RW, et al. Association between dental occlusal variables and intraarticular temporomandibular joint disorders: horizontal and vertical overlap. J Prosthet Dent. 1998;79:658.
77 Kampe T, Tagdae T, Bader G, et al. Reported symptoms and clinical findings in a group of subjects with longstanding bruxing behavior. J Oral Rehabil. 1997;24:581.
78 Kaplan AS, Assaed LA. Temporomandibular disorders: diagnosis and treatment. Philadelphia: Saunders; 1991.
79 Kato T, Rompre P, Montplaisir JY, et al. Sleep bruxism: an oromotor activity secondary to micro-arousal. J Dent Res. 2001;80:1940.
80 Kato T, Thie NMR, Huynh N, et al. Topical review: sleep bruxism and the role of peripheral sensory influences. J Orofac Pain. 2003;17:191.
81 Katz MA. Approach to the management of nonmalignant pain. Am J Med. 1996;101(suppl 1A):54S.
82 Katzberg RW, Westesson P. Diagnosis of the temporomandibular joint. Philadelphia: Saunders; 1993.
83 Keersmaeker K, De Boever JA, van Den Berghe L. Otalgia in patients with temporomandibular joint disorders. J Prosthet Dent. 1996;75:72.
84 Kenworthy CR, Morrish RB, Mohn C, et al. Bilateral condylar movement patterns in adult subjects. J Orofac Pain. 1997;11:328.
85 Kerstein RB. Disclusion time measurement studies: a comparison of disclusion time between chronic myofascial pain dysfunction patients and nonpatients: a population analysis. J Prosthet Dent. 1994;72:473.
86 Kerstein R. Disclusion time measurement studies: stability of disclusion time—a 1-year follow-up. J Prosthet Dent. 1994;72:164.
87 Kleinegger CL, Lilly GE. Cranial arteritis: a medical emergency with orofacial manifestations. J Am Dent Assoc. 1999;130:1203.
88 Kelmetti E, Vainio P, Kroger H. Craniomandibular disorders and skeletal mineral status. J Craniomandib Pract. 1995;13:89.
89 Koh ET, Yap AU, Koh CK, et al. Temporomandibular disorders in rheumatoid arthritis. J Rheumatol. 1999;26:1918.
90 Könönen M, Wenneberg B, Kallenberg A. Craniomandibular disorders in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: a clinical study. Acta Odontol Scand. 1992;50:281.
91 Kopp S. The influence of neuropeptides, serotonin, and interleukin 1B on temporomandibular joint pain and inflammation. J Oral Maxillofac Surg. 1998;56:189.
92 Kuboki T, Azuma Y, Orsini MG, et al. Effects of sustained unilateral molar clenching on the temporomandibular joint space. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82:616.
93 Kubota E, Kubota T, Matsumoto J, et al. Synovial fluid cytokines and proteinases as markers of temporomandibular joint disease. J Oral Maxillofac Surg. 1998;56:192.
94 Kumar KL, Cooney TG. Headaches. Med Clin North Am. 1995;79:261.
95 Kurita H, Ikeda K, Kurashina K. Evaluation of the effect of a stabilization splint on occlusal force in patient occlusions with masticatory muscle disorders. J Oral Rehabil. 2000;27:79.
96 Larheim TA. Current trends in temporomandibular joint imaging. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;80:555.
97 Lavigne GJ, Kato T, Koltra A, Sessle BJ. Neurobiological mechanisms involved in sleep bruxism. Crit Rev Oral Biol Med. 2003;14:30.
98 Lavigne GJ, Khoury S, Abe S, Yamaguchi T, Raphael K. Bruxism physiology and pathology: an overview for clinicians. J Oral Rehabil. 2008;35:476.
99 Lavigne G, Kim JS, Valiquette C, et al. Evidence that periodontal pressoreceptors provide positive feedback to jaw closing muscles during mastication. J Neurophysiol. 1987;58:342.
100 Levitt SR, McKinney MW. Appropriate use of predictive values in clinical decision making and evaluating diagnostic tests for TMD. J Orofac Pain. 1994;8:298.
101 Levitt SR, McKinney MW. Validating the TMJ scale in the national sample of 10,000 patients: demographic and epidemiologic characteristics. J Orofac Pain. 1994;8:25.
102 Lewis EL, Dolwick MF, Abramowicz S, Reeder SL. Contemporary imaging of the temporomandibular joint. Dent Clin N Am. 2008;52:875.
103 Lindauer SJ, Sabol G, Isaccson RJ, et al. Condylar movement and mandibular rotation during jaw opening. Am J Orthod Dentofac Orthop. 1995;107:573.
104 Lipton JA, Ship JA, Larach-Robinson D. Estimated prevalence and distribution of reported orofacial pain in the United States. J Am Dent Assoc. 1993;124:115.
105 Liu ZJ, Yamagata K, Kashara Y, et al. Electromyographic examination of jaw muscles in relation to symptoms and occlusion of patients with temporomandibular joint disorders. J Oral Rehabil. 1999;26:33.
106 Lobbezoo F, Lavigne GJ. Do bruxism and temporomandibular disorders have a cause-and-effect relationship? J Orofac Pain. 1997;11:15.
107 Lobbezoo F, Naeije M. Bruxism is regulated centrally not peripherally. J Oral Rehabil. 2001;28:1085.
108 Locher MC, Felder M, Sailer HF. Involvement of the temporomandibular joints in ankylosing spondylitis (Bechterew’s disease). J Craniomaxillofac Surg. 1996;24:205.
109 Louca C, Cadden SW, Linden RWA. The roles of periodontal ligament mechanoreceptors in the reflex control of human jaw-closing muscles. Brain Res. 1996;731:63.
110 Loughner BA, Gremillion HA, Larkin LH, et al. Muscle attachment to the lateral aspect of the articular disc of the human temporomandibular joint. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82:139.
111 Lyssy KJ, Escalante A. Perioperative management of rheumatoid arthritis: areas of concern for primary care physicians. Postgrad Med Rheum Arthritis. 1996;99:191.
112 Magnusson T, Egermark I, Carlsson GE. A prospective investigation over two decades on signs and symptoms of temporomandibular disorders and associated variables. A final summary. Acta Odontol Scand. 2005;63:99.
113 Maixner W, Fillingham R, Kincaid S, et al. Relationship between pain sensitivity and resting arterial blood pressure in patients with painful temporomandibular disorders. Psychosom Med. 1997;59:503.
114 Major P, Ramos-Remus C, Suarez-Almazor ME, et al. Magnetic resonance imaging and clinical assessment of temporomandibular joint pathology in ankylosing spondylitis. J Rheumatol. 1999;26:616.
115 Marbach JL. Is phantom tooth pain a deafferentation (neuropathic) syndrome? Oral Surg Oral Med Oral Pathol. 1993;75:225.
116 Marbach JL. Orofacial phantom pain: theory and phenomenology. J Am Dent Assoc. 1996;127:221.
117 Marbach JJ. Medically unexplained chronic orofacial pain: temporomandibular pain and dysfunction syndrome, orofacial phantom pain, burning mouth syndrome, and trigeminal neuralgia. Med Clin North Am. 1999;83:691.
118 McGuire MK, Nunn ME. Prognosis versus actual outcome: a long-term survey of 100 treated periodontal patients under maintenance care. J Periodontol. 1991;62:51.
119 Merida-Velasco JR, Rodriguez-Vazquez JF, Jimenez-Collado J. The relationships between the temporomandibular joint disc and related masticatory muscles in humans. J Oral maxillofac Surg. 1993;51:390.
120 Milam SB, Schmitz JP. Molecular biology of temporomandibular joint disorders: proposed mechanisms of disease. J Oral Maxillofac Surg. 1995;53:1448.
121 Milam SB, Zardeneta G, Schmitz JP. Oxidative stress and degenerative temporomandibular joint disease: a proposed hypothesis. J Oral Maxillofac Surg. 1998;56:214.
122 Minagi S, Ohtsuki H, Sato T, et al. Effect of balancing-side occlusion on the ipsilateral TMJ dynamics under clenching. J Oral Rehabil. 1997;24:57.
123 Mohl ND. Reliability and validity of diagnostic modalities for temporomandibular disorders. Adv Dent Res. 1993;7:113.
124 Mohl ND, Ohrbach R. Clinical decision making for temporomandibular disorders. J Dent Educ. 1992;56:823.
125 Morgan DH, Goode RL, Christiansen RL, et al. The TMJ-ear connection. J Craniomandib Pract. 1995;13:42.
126 Moses AJ. Scientific methodology in temporomandibular disorders. Part I. Epidemiology. J Craniomandib Pract. 1994;12:114.
127 Murakami K, Kubota E, Maeda H, et al. Intraarticular levels of prostaglandin E2, hyaluronic acid, and chrondroitin-4 and -6 sulfates in the temporomandibular joint synovial fluid of patients with internal derangements. J Oral Maxillofac Surg. 1998;56:199.
128 Murray H, Locker D, Mock D, et al. Pain and the quality of life in patients referred to a craniofacial pain unit. J Orofac Pain. 1996;10:316.
129 Neff PA. TMJ occlusion and function. Washington, DC: Georgetown University School of Dentistry; 1975.
130 Okeson JP. Orofacial pain: guidelines for assessment, diagnosis, and management. Carol Stream, Ill: Quintessence; 1996.
131 Okeson JP. Management of temporomandibular joint disorders and occlusion, ed 4. St Louis: Mosby; 1998.
132 Parker MW. The significance of occlusion in restorative dentistry. Dent Clin North Am. 1993;37:341.
133 Parker WS, Chole RA. Tinnitus, vertigo, and temporomandibular disorders. Am J Orthod Dentofac Orthop. 1995;107:153.
134 Pavone BW. Bruxism and its effect on the natural teeth. J Prosthet Dent. 1985;53:692.
135 Pereira FL, Lundh H, Eriksson L, et al. Microscopic changes in the retrodiscal tissues of painful temporomandibular joints. J Oral Maxillofac Surg. 1996;54:461.
136 Pertes RA. Differential diagnosis of orofacial pain. Mt Sinai J Med. 1998;65:348.
137 Pharoah MJ. The prescription of diagnostic images for temporomandibular joint disorders. J Orofac Pain. 1999;13:251.
138 Pleash O, Wolfe F, Lane N. The relationship between fibromyalgia and temporomandibular disorders: prevalence and symptom severity. J Rheumatol. 1996;23:1948.
139 Pullinger AG, Seligman DA. The degree to which attrition characterizes differentiated patient groups of temporomandibular disorders. J Orofac Pain. 1993;7:196.
140 Pullinger AG, Seligman DA. Quantification and validation of predictive values of occlusal variables in temporomandibular disorders using multifactorial analysis. J Prosthet Dent. 2000;83:66.
141 Pullinger AG, Seligman DA, Gornbein JA. A multiple logistic regression analysis of the risk and relative odds of temporomandibular disorders as a function of common occlusal features. J Dent Res. 1993;72:968.
142 Rammelsberg P, Pospiech PR, Jäger L, et al. Variability of disc position in asymptomatic volunteers and patients with internal derangements of the TMJ. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:393.
143 Raphael KG, Marbach JJ, Klausner J. Myofascial face pain: clinical characteristics of those with regional versus widespread pain. J Am Dent Assoc. 2000;131:161.
144 Rassouli NM, Christensen LV. Experimental occlusal interferences. Part III. Mandibular rotations induced by a rigid interference. J Oral Rehabil. 1995;22:781.
145 Ratcliffe A, Israel H, Saed-Nejad F, et al. Proteoglycans in the synovial fluid of the temporomandibular joint as an indicator of changes in cartilage metabolism during primary and secondary osteoarthritis. J Oral Maxillofac Surg. 1998;56:204.
146 Raustia AM, Pirttiniemi PM, Pyhtinen J. Correlation of occlusal factors and condyle position asymmetry with signs and symptoms of temporomandibular disorders in young adults. J Craniomandib Pract. 1995;13:152.
147 Ren Y, Isberg A. Tinnitus in patients with temporomandibular joint internal derangement. J Craniomandib Pract. 1995;13:75.
148 Rodriguez-Vazquez JF, Merida-Velasco JR, Merida-Velasco JA, et al. Anatomical considerations on the discomalleolar ligament. J Anat. 1998;192:617.
149 Rosenbaum RS. The possible effects of periodontal disease on occlusal function. Curr Opin Periodontol. 1993:163-169. (review)
150 Ruf S, Cecere F, Kupfer J, et al. Stress-induced changes in the functional electromyographic activity of the masticatory muscles. Acta Odontol Scand. 1997;55:44.
151 Sarnat BG, Laskin DM. The temporomandibular joint: biologic basis for clinical practice, ed 4. Philadelphia: Saunders; 1992.
152 Schiffman E, Haley D, Baker C, et al. Diagnostic criteria for screening headache patients for temporomandibular disorders. Headache. 1995;35:121.
153 Schiffman EL, Fricton JR, Haley D. The relationship of occlusion, parafunctional habits and recent life events to mandibular dysfunction in a nonpatient population. J Oral Rehabil. 1992;19:201.
154 Schindler JH, Stengel E, Spiess WE. Feedback control during mastication of solid food textures: a clinical-experimental study. J Prosthet Dent. 1998;80:330.
155 Schmolke C. The relationship between the temporomandibular joint capsule, articular disc, and jaw muscles. J Anat. 1994;184:335.
156 Sessle BJ. The neural basis of the temporomandibular joint and masticatory muscle pain. J Orofac Pain. 1999;13:238.
157 Shibata T, Murakami K, Kubota E, et al. Glycosaminoglycan components in temporomandibular joint synovial fluid as markers of joint pathology. J Oral Maxillofac Surg. 1998;56:209.
158 Simons DG, Travell JG, Simons LS. Myofascial pain and dysfunction: the trigger point manual. Baltimore: Williams & Wilkins; 1999.
159 Stegenga B, de Bont LGM, de Leeuw R, et al. Assessment of mandibular function impairment associated with temporomandibular joint osteoarthrosis and internal derangement. J Orofac Pain. 1993;7:183.
160 Stratmann U, Mokyrs K, Meyer U, et al. Clinical anatomy and palpability of the inferior lateral pterygoid muscle. J Prosthet Dent. 2000;83:548.
161 Suvinen TI, Reade PC. Temporomandibular disorders: A critical review of the nature of pain and its assessment. J Orofacial Pain. 1995;9:317.
162 Svensson P, Jadidi F, Arima T, Baad-Hansen L, Sessle BJ. Relationships between craniofacial pain and bruxism. J Oral Rehabil. 2008;35:524.
163 Taddey JJ. Musicians and temporomandibular disorders: prevalence and occupational etiologic considerations. J Craniomandib Pract. 1992;10:241.
164 Tanne K, Tanaka E, Sakuda M. Association between malocclusion and temporomandibular disorders in orthodontic patients before treatment. J Orofacial Pain. 1993;7:156.
165 Tarantola GJ, Becker IM, Gremillion H. The reproducibility of centric relation: a clinical approach. J Am Dent Assoc. 1997;128:1245.
166 Tasaki MM, Westesson P, Isberg AM, et al. Classification and prevalence of temporomandibular joint disc displacement in patients and symptom-free volunteers. Am J Orthod Dentofac Orthop. 1996;109:249.
167 Tenenbaum HC, Freeman BV, Psutka DJ, et al. Temporomandibular disorders: disc displacements. J Orofac Pain. 1999;13:285.
168 Tsolka P, Walter JD, Wilson RF, et al. Occlusal variables, bruxism and temporomandibular disorders: a clinical and kinesiographic assessment. J Oral Rehabil. 1995;22:849.
169 Turp JG, Gobetti JP. Trigeminal neuralgia: an update. Compendium. 2000;21:279.
170 Wabeke KB, Spruijt RJ, van der Zaag J. The reliability of clinical methods for recording temporomandibular joint sounds. J Dent Res. 1994;73:1157.
171 Watanabe EK, Yatani H, Kuboki T, et al. The relationship between signs and symptoms of temporomandibular disorders and bilateral occlusal contact patterns in lateral excursions. J Oral Rehabil. 1998;25:409.
172 Wilkinson TM, Crowley CM. A histologic study of retrodiscal tissues of the human temporomandibular joint in the open and closed position. J Orofac Pain. 1994;8:7.
173 Williamson EH, Ludquist DO. Anterior guidance: its effect on electromyographic activity of the temporal and masseter muscles. J Prosthet Dent. 1983;49:816.
174 Wish-Baratz S, Ring GD, Hiss J, et al. The microscopic structure and function of the vascular retrodiscal pad of the human temporomandibular joint. Arch Oral Biol. 1993;38:265.
175 Wright EF, Bifano SL. Tinnitus improvement through TMD therapy. J Am Dent Assoc. 1997;128:1424.
176 Wright EF, Gullickson DC. Identifying acute pulpalgia as a factor in TMD pain. J Am Dent Assoc. 1996;127:773.
177 Zarb GA, Carlsson GE, Sessle BJ, et al. Temporomandibular joint and masticatory muscle disorders. St Louis: Mosby; 1994.
178 Zarb GA, Carlsson GE. Temporomandibular disorders: osteoarthritis. J Orofac Pain. 1999;13:295.
179 Zimers PL, Gobetti JP. Head and neck lesions commonly found in musicians. J Am Dent Assoc. 1994;125:1487.