CHAPTER 30 Clinical Diagnosis
Proper diagnosis is essential to intelligent treatment. Periodontal diagnosis should first determine whether disease is present; then identify its type, extent, distribution, and severity; and finally provide an understanding of the underlying pathologic processes and its cause. Part 3, Sections 1 and 2 provide a detailed description of the different diseases that can afflict the periodontium. In general, they fall into the following three broad categories.
Periodontal diagnosis is determined after careful analysis of the case history and evaluation of the clinical signs and symptoms, as well as the results of various tests (e.g., probing, mobility assessment, radiographs, blood tests, and biopsies).
The interest should be in the patient who has the disease and not simply in the disease itself. Diagnosis must therefore include a general evaluation of the patient and consideration of the oral cavity.
Diagnostic procedures must be systematic and organized for specific purposes. It is not enough to assemble facts. The findings must be pieced together so that they provide a meaningful explanation of the patient’s periodontal problem. The following is a recommended sequence of procedures for the diagnosis of periodontal diseases.
First Visit
Overall Appraisal of the Patient
From the first meeting, the clinician should attempt an overall appraisal of the patient. This includes consideration of the patient’s mental and emotional status, temperament, attitude, and physiologic age.
Medical History
Most of the medical history is obtained at the first visit and can be supplemented by pertinent questioning at subsequent visits. The health history can be obtained verbally by questioning the patient and recording his or her responses on a blank piece of paper or by means of a printed questionnaire the patient completes. Figure 30-1 is the medical questionnaire form recommended by the American Dental Association.
The importance of the medical history should be clearly explained because patients often omit information that they cannot relate to their dental problems. The patient should be made aware of (1) the possible role that some systemic diseases, conditions, or behavioral factors may play in the cause of periodontal disease; (2) the presence of conditions that may require special precautions or modifications in the treatment procedure (see Chapter 37); and (3) the possibility that oral infections may have a powerful influence on the occurrence and severity of a variety of systemic diseases and conditions (see Chapter 28)
The medical history should include reference to the following:
Dental History
Current Illness
Some patients may be unaware of any problems, but many may report bleeding gums; loose teeth; spreading of the teeth with the appearance of spaces where none existed before; foul taste in the mouth; and an itchy feeling in the gums, relieved by digging with a toothpick. There may also be pain of varied types and duration, including constant, dull, gnawing pain; dull pain after eating; deep, radiating pains in the jaws; acute throbbing pain; sensitivity when chewing; sensitivity to hot and cold; burning sensation in the gums; and extreme sensitivity to inhaled air.
A preliminary oral examination is done to explore the source of the patient’s chief complaint and to determine whether immediate emergency care is required. If this is the case, the problem is addressed after consideration of the medical history (see Chapters 41 and 42).
The dental history should include reference to the following:
Intraoral Radiographic Survey
The radiographic survey should consist of a minimum of 14 intraoral films and four posterior bite-wing films (Figure 30-2).
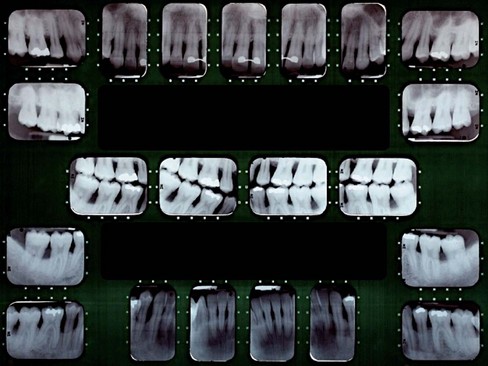
Figure 30-2 Full-mouth intraoral radiographic series (16 periapical films and four bite-wing films) used as an adjunct in periodontal diagnosis.
Panoramic radiographs are a simple and convenient method of obtaining a survey view of the dental arch and surrounding structures (Figure 30-3). They are helpful for the detection of developmental anomalies, pathologic lesions of the teeth and jaws, and fractures as well as dental screening examinations of large groups. They provide an informative overall radiographic picture of the distribution and severity of bone destruction in periodontal disease, but a complete intraoral series is required for periodontal diagnosis and treatment planning. Chapter 31 gives a detailed description of radiographic interpretation in periodontics.
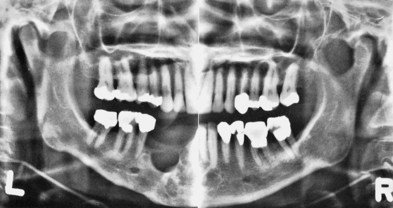
Figure 30-3 Panoramic radiograph showing temporomandibular joints and “cystic” spaces in the jaw. Areas of periodontal bone loss are not seen in detail. (Compare with Figure 30-2.)
Casts
Casts from dental impressions are useful adjuncts in the oral examination. They indicate the position of the gingival margins (recession) and the position and inclination of the teeth, proximal contact relationships, and food impaction areas. In addition, they provide a view of the lingual-cuspal relationships. Casts are important records of the dentition before it is altered by treatment. Finally, casts also serve as visual aids in discussions with the patient and are useful for pretreatment and posttreatment comparisons, as well as for reference at recall visits. They are also helpful to determine the position of implant placement if the case will require their use.
Clinical Photographs
Color photographs are useful for recording the appearance of the tissue before and after treatment. Photographs cannot always be relied on for comparing subtle color changes in the gingiva, but they do depict gingival morphologic changes. With the advent of digital clinical photography, record keeping for mucogingival problems, such as areas of gingival recession, frenum involvement, and papilla loss, has become important.
Review of the Initial Examination
If no emergency care is required, the patient is dismissed and instructed as to when to report for the second visit. Before this visit, a correlated examination is made of the radiographs, photographs, and casts to relate the radiographic changes to unfavorable conditions represented on the casts. The casts are checked for evidence of abnormal wear, plunger cusps, uneven marginal ridges, malposed or extruded teeth, crossbite relationships, or other conditions that could cause occlusal disharmony or food impaction. Such areas are marked on the casts to serve as a reference during the detailed examination of the oral cavity. The radiographs, photographs, and casts are valuable diagnostic aids; however, it is the clinical findings in the oral cavity that constitute the basis for diagnosis.
Second Visit
Oral Examination
Oral Hygiene
The cleanliness of the oral cavity is appraised in terms of the extent of accumulated food debris, plaque, and tooth surface stains (Figure 30-4). Disclosing solution may be used to detect plaque that would otherwise be unnoticed. The amount of plaque detected, however, is not necessarily related to the severity of the disease present. For example, aggressive periodontitis is a destructive type of periodontitis in which plaque is minimal. Qualitative assessments of plaque are more meaningful, and their value in diagnosis is discussed in Chapter 23.
Oral Malodor
Oral malodor, also termed fetor ex ore, fetor oris, or halitosis, is foul or offensive odor emanating from the oral cavity. Mouth odors may be of diagnostic significance, and their origin may be either oral or extraoral (remote).62 Chapter 29 discusses in detail the problems related to oral malodor.
Examination of the Oral Cavity
The entire oral cavity should be carefully examined. The examination should include the lips, floor of the mouth, tongue, palate, and oropharyngeal region, as well as the quality and quantity of saliva. Although findings may not be related to the periodontal problem, the dentist should detect all pathologic changes present in the mouth. Textbooks in oral medicine and oral diagnosis cover these topics in detail.
Examination of Lymph Nodes
Because periodontal, periapical, and other oral diseases may result in lymph node changes, the diagnostician should routinely examine and evaluate head and neck lymph nodes. Lymph nodes can become enlarged and/or indurated as a result of an infectious episode, malignant metastases, or residual fibrotic changes.
Inflammatory nodes become enlarged, palpable, tender, and fairly immobile. The overlying skin may be red and warm. Patients are often aware of the presence of “swollen glands.” Primary herpetic gingivostomatitis, necrotizing ulcerative gingivitis (NUG), and acute periodontal abscesses may produce lymph node enlargement. After successful therapy, lymph nodes return to normal in a matter of days or a few weeks.
Examination of the Teeth and Implants
The teeth are examined for caries, poor restorations, developmental defects, anomalies of tooth form, wasting, hypersensitivity, and proximal contact relationships. The stability, position, and number of implants and their relationship to the adjacent natural dentition is also examined.
Wasting Disease of the Teeth
Wasting is defined as any gradual loss of tooth substance characterized by the formation of smooth, polished surfaces, without regard to the possible mechanism of this loss. The forms of wasting are erosion, abrasion, and attrition.46,64
Erosion, also called corrosion, is a sharply defined wedge-shaped depression in the cervical area of the facial tooth surface.53 The long axis of the eroded area is perpendicular to the vertical axis of the tooth. The surfaces are smooth, hard, and polished. Erosion generally affects a group of teeth. In the early stages, it may be confined to the enamel, but it generally extends to involve the underlying dentin, as well as the cementum.
The etiology of erosion is not known. Decalcification by acidic beverages44 or citrus fruits, combined with the effect of acid salivary secretion are suggested causes. Sognnaes70 refers to these lesions as dentoalveolar ablations and attributes them to forceful frictional actions between the oral soft tissues and the adjacent hard tissues. In patients with erosion, the salivary pH, buffering capacity, and calcium and phosphorus content have been reported as normal, with the mucin level elevated.43
Abrasion refers to the loss of tooth substance induced by mechanical wear other than that of mastication. Abrasion results in saucer-shaped or wedge-shaped indentations with a smooth, shiny surface. Abrasion starts on exposed cementum surfaces rather than on the enamel and extends to involve the dentin of the root. A sharp “ditching” around the cemento-enamel junction appears to be the result of the softer cemental surface, as compared with the much harder enamel surface.
Continued exposure to the abrasive agent, combined with decalcification of the enamel by locally formed acids, may result in loss of enamel, followed by loss of the dentin of the crown.
Toothbrushing24 with an abrasive dentifrice (Figure 30-5) and the action of clasps are frequently mentioned, but aggressive toothbrushing is the most common cause.34 Tooth position (facial) is also a major factor in the abrasive loss of the root surface. The degree of tooth wear from toothbrushing depends on the abrasive effect of the dentifrice and the angle of brushing.41,42 Horizontal brushing at right angles to the vertical axis of the teeth results in the severest loss of tooth substance. Occasionally, abrasion of the incisal edges occurs as a result of habits such as holding objects (e.g., a bobby pin or tacks) between the teeth.
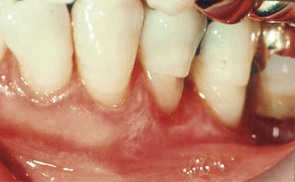
Figure 30-5 Abrasion attributed to aggressive toothbrushing. Involvement of the roots is followed by undermining of the enamel.
Attrition is occlusal wear resulting from functional contacts with opposing teeth. Such physical wear patterns may occur on incisal, occlusal, and approximal tooth surfaces. A certain amount of tooth wear is physiologic, but accelerated wear may occur when abnormal anatomic or unusual functional factors are present.
Occlusal or incisal surfaces worn by attrition are called facets. When active tooth grinding occurs, the enamel rods are fractured and become highly reflective to light.80 Thus shiny, smooth, and curviplanar facets are usually the best indicator of ongoing frictional activity. If dentin is exposed, a yellowish brown discoloration is frequently present (Figure 30-6). Facets vary in size and location depending on whether they are produced by physiologic or abnormal wear.12,78 At least one significant wear facet has been reported in 92% of adults,67 and facet prevalence approaches universality.10,79 Facets are usually not sensitive to thermal or tactile stimulation.
Facets generally represent functional or parafunctional wear, as well as iatrogenic dental treatment through coronoplasty (occlusal adjustment). Coronoplasty, however, does not appear to contribute to higher ratings of wear.68 Excessive wear may result in obliteration of the cusps and the formation of either a flat or a cuneiform (cupped-out) occlusal surface. Contrary to earlier thought, attrition in young adults from modern societies is not age related.16,68 This suggests that a significant amount of attrition, when present in young adults, is unlikely to occur from functional wear36 and is probably the result of bruxing activity.68 Attrition has been correlated with age when older adults are considered.7,66
The angle of the facet on the tooth surface is potentially significant to the periodontium. Horizontal facets tend to direct forces on the vertical axis of the tooth, to which the periodontium can adapt most effectively. Angular facets direct occlusal forces laterally and increase the risk of periodontal damage. However, gradual attrition may be compensated for by continuous tooth eruption without alveolar bone growth and is characterized by a lack of inflammatory changes on the alveolar bone surfaces.75
Another mechanism of tooth wear that has been studied recently is called abfraction and results from occlusal loading surfaces causing tooth flexure and mechanical microfractures and tooth substance loss in the cervical area.26
These four mechanisms of tooth wear (corrosion, abrasion, attrition, and abfraction) can combine with each other, resulting in increased degree of tooth wear.
Dental Stains
These are pigmented deposits on the teeth. They should be carefully examined to determine their origin (see Chapter 22).
Hypersensitivity
Root surfaces exposed by gingival recession may be hypersensitive to thermal changes or tactile stimulation. Patients often direct the clinician to the sensitive areas. These may be located by gentle exploration with a probe or cold air.
Proximal Contact Relations
Open contacts allow food impaction. The tightness of contacts should be checked by means of clinical observation and with dental floss. Abnormal contact relationships may also initiate occlusal changes such as a shift in the median line between the central incisors, with labial flaring of the maxillary canine, buccal, or lingual displacement of the posterior teeth, and an uneven relationship of the marginal ridges. Teeth opposite an edentulous site may supererupt, thus opening the proximal contacts.
Tooth Mobility
All teeth have a slight degree of physiologic mobility, which varies for different teeth and at different times of the day.50,54 It is greatest on arising in the morning and progressively decreases. The increased mobility in the morning is attributed to slight extrusion of the tooth because of limited occlusal contact during sleep. During the waking hours, mobility is reduced by chewing and swallowing forces, which intrude the teeth in the sockets. These 24-hour variations are less marked in persons with a healthy periodontium than in those with occlusal habits such as bruxism and clenching.
Single-rooted teeth have more mobility than multirooted teeth, with incisors having the most. Mobility is primarily in a horizontal direction, although some axial mobility occurs, to a lesser degree.52
Tooth mobility occurs in the following two stages:
When a force such as that applied to teeth in occlusion is discontinued, the teeth return to their original position in two stages: the first is an immediate, springlike elastic recoil; the second is a slow, asymptomatic recovery movement. The recovery movement is pulsating and is apparently associated with the normal pulsation of the periodontal vessels, which occurs in synchrony with the cardiac cycle.48
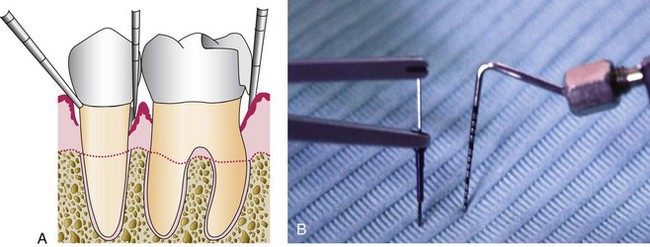
Many attempts have been made to develop mechanical or electronic devices for the precise measurement of tooth mobility.48,51,53,67 Even though standardization of grading mobility would be helpful in diagnosing periodontal disease and in evaluating the treatment outcome, these devices are not widely used. As a general rule, mobility is graded clinically by holding the tooth firmly between the handles of two metallic instruments or with one metallic instrument and one finger (Figure 30-7). An effort then is made to move it in all directions. Abnormal mobility most often occurs faciolingually. Mobility is graded according to the ease and extent of tooth movement as follows:
Mobility beyond the physiologic range is termed abnormal or pathologic. It is pathologic in that it exceeds the limits of normal mobility values; however, the periodontium is not necessarily diseased at the time of examination.
Increased mobility is caused by one or more of the following factors:
One study22 has suggested that pockets around mobile teeth harbor higher proportions of Campylobacter rectus and Peptostreptococcus micros and possibly of Porphyromonas gingivalis than nonmobile teeth. This hypothesis needs further verification.
Trauma from Occlusion
Trauma from occlusion refers to tissue injury produced by occlusal forces, not to the occlusal forces themselves (see Chapter 15). The criterion that determines that an occlusion is traumatic is whether it causes damage in the periodontal tissues; therefore the diagnosis of trauma from occlusion is made from the condition of the periodontal tissues. The periodontal findings are then used as a guide for locating the responsible occlusal relationships.
Periodontal findings that suggest the presence of trauma from occlusion include excessive tooth mobility, particularly in teeth showing radiographic evidence of a widened periodontal space; vertical or angular bone destruction; infrabony pockets; and pathologic migration, especially of the anterior teeth (see following discussion).
Pathologic Migration of the Teeth
Alterations in tooth position should be carefully noted, particularly with a view toward identifying abnormal forces, a tongue-thrusting habit, or other habits that may be contributing factors (see Chapter 15). Premature tooth contacts in the posterior region that deflect the mandible anteriorly contribute to destruction of the periodontium of the maxillary anterior teeth and to pathologic migration (Figure 30-8; see also Figure 15-10, A and B). The loss of posterior teeth can lead to the facial “flaring” of the maxillary anterior dentition. This is due to the increased trauma that the mandibular anterior dentition places against the palatal surface of the maxillary anterior dentition. Pathologic migration of anterior teeth in young persons may be a sign of localized aggressive (juvenile) periodontitis.
Sensitivity to Percussion
Sensitivity to percussion is a feature of acute inflammation of the periodontal ligament. Gentle percussion of a tooth at different angles to the long axis often aids in localizing the site of inflammatory involvement.
Dentition with the Jaws Closed
Examination of the dentition with the jaws closed can detect conditions, such as irregularly aligned teeth, extruded teeth, improper proximal contacts, and areas of food impaction, all of which may favor plaque accumulation.
Excessive overbite, seen most often in the anterior region, may cause impingement of the teeth on the gingiva and food impaction, followed by gingival inflammation, gingival enlargement, and pocket formation. The real significance of the detrimental effect of excessive anterior overbite on gingival health is still controversial.2
In open-bite relationships, abnormal vertical spaces exist between the maxillary and mandibular teeth. The condition occurs most often in the anterior region, although posterior open bite is occasionally seen. Reduced mechanical cleansing by the passage of food may lead to accumulation of plaque, debris, calculus formation, and extrusion of teeth.
In crossbite, the normal relationship of the mandibular teeth to the maxillary teeth is reversed, with the maxillary teeth being lingual to the mandibular teeth. Crossbite may be bilateral or unilateral, or it may affect only a pair of antagonists. Trauma from occlusion, food impaction, spreading of the mandibular teeth, and associated gingival and periodontal disturbances may be caused by crossbite.
Functional Occlusal Relationships
Examination of functional occlusal relationships is an important part of the diagnostic procedure. Dentitions that appear normal when the jaws are closed may present marked functional abnormalities. Systematic procedures for the detection and correction of functional abnormalities are presented in Chapter 49.
Examination of the Periodontium
The periodontal examination should be systematic, starting in the molar region in either the maxilla or the mandible and proceeding around the arch. This prevents overemphasis of unusual findings at the expense of other conditions that although less striking, may be equally important. It is important to detect the earliest signs of gingival and periodontal disease.
Charts to record the periodontal and associated findings provide a guide for a thorough examination and record of the patient’s condition (Figure 30-9, A and B). They are also used to evaluate the response to treatment and for comparison at recall visits. However, excessively complicated mouth charting may lead to a frustrating maze of minutiae rather than clarification of the patient’s problem.
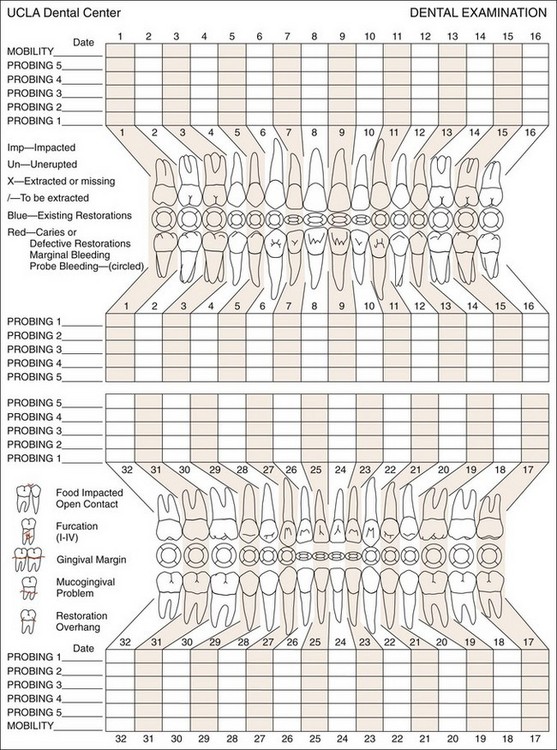
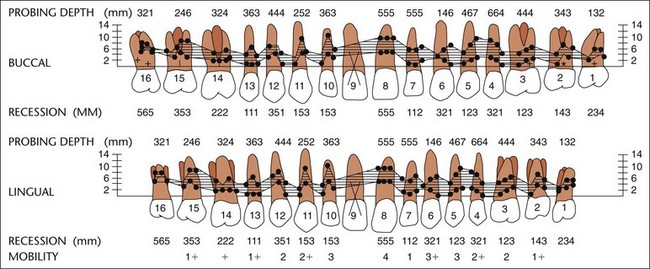
Figure 30-9 A, UCLA periodontal chart. B, Computerized diagram showing various periodontal parameters.
(Courtesy University of California, Los Angeles School of Dentistry.)
In the last decade, electronic clinical records have been developed and are increasingly being used by general dentists and periodontists. Most systems provide a rapid and easy access to information and allow the incorporation of digital clinical and radiographic images.63 Computerized dental examination systems using high-resolution graphics and voice-activated technology permit easy retrieval and comparison of data.11
Plaque and Calculus
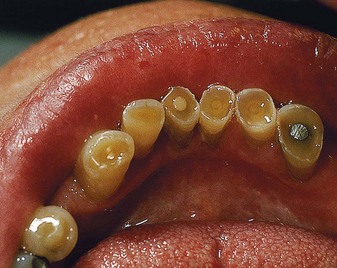
There are many methods available for assessing plaque and calculus accumulation.18 The presence of supragingival plaque and calculus can be directly observed and the amount measured with a calibrated probe. For the detection of subgingival calculus, each tooth surface is carefully checked to the level of the gingival attachment with a no. 17 or no. 3A explorer (Figure 30-10). Warm air may be used to deflect the gingiva and aid in visualization of the calculus.
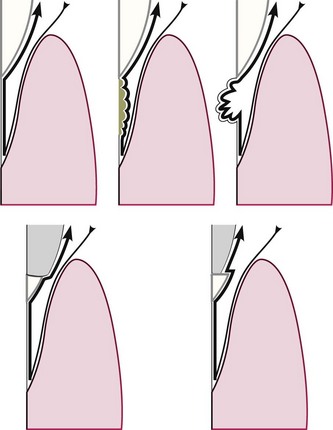
Figure 30-10 Top left, Detection of smoothness of various irregularities on the root surface with outward motion of a probe or explorer. Top center, Calculus. Top right, Caries. Bottom left and right, Irregular margins of restorations.
Although the radiograph may sometimes reveal heavy calculus deposits interproximally and even on the facial and lingual surfaces, it cannot be relied on for the thorough detection of calculus.
Gingiva
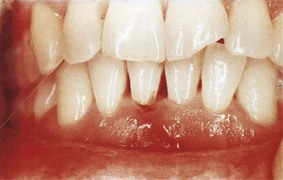
The gingiva must be dried before accurate observations can be made (Figure 30-11). Light reflection from moist gingiva obscures detail. In addition to visual examination and exploration with instruments, firm but gentle palpation should be used for detecting pathologic alterations in normal resilience, as well as for locating areas of exudate.
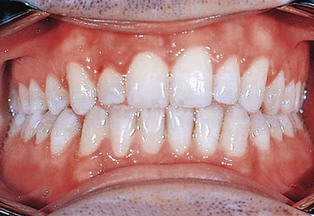
Figure 30-11 Normal gingiva with incipient gingivitis in tooth #7. Normal surface features are better revealed by drying gingiva.
Features of the gingiva to consider are: color, size, contour, consistency, surface texture, position, ease of bleeding, and pain (see Chapter 8). Any deviation from the norm should be evaluated and not overlooked. The distribution of gingival disease and its acute or chronic nature should also be noted.
Clinically gingival inflammation can produce two basic types of tissue response: edematous and fibrotic. Edematous tissue response is characterized by a smooth, glossy, soft, red gingiva. In the fibrotic tissue response, some of the characteristics of normalcy persist; the gingiva is more firm, stippled, and opaque, it is usually thicker, and the margin appears rounded.
Use of Clinical Indices in Dental Practice
There has been a tendency to extend the use of indices originally designed for epidemiologic studies into dental practice (see Chapter 5). Of all the indices proposed, the gingival index and the sulcus bleeding index appear to be the most useful and most easily transferred to clinical practice.
The gingival index (Löe and Silness) provides an assessment of the gingival inflammatory status that and can be used in practice to compare gingival health before and after phase I therapy or before and after surgical therapy. It can also be used to compare the gingival status at recall visits. Attaining good intraexaminer and interexaminer calibration is imperative in the dental office.
The sulcus bleeding index (Mühlemann and Son) provides an objective, easily reproducible assessment of the gingival status. It is extremely useful for detecting early inflammatory changes and the presence of inflammatory lesions located at the base of the periodontal pocket, an area inaccessible to visual examination. Patients can easily understand this index; therefore it can be used to enhance the patient’s motivation for plaque control.
Periodontal Pockets
Examination for periodontal pockets must include their presence and distribution on each tooth surface, pocket depth, level of attachment on the root, and type of pocket (suprabony or infrabony).
Signs And Symptoms
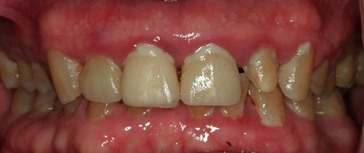
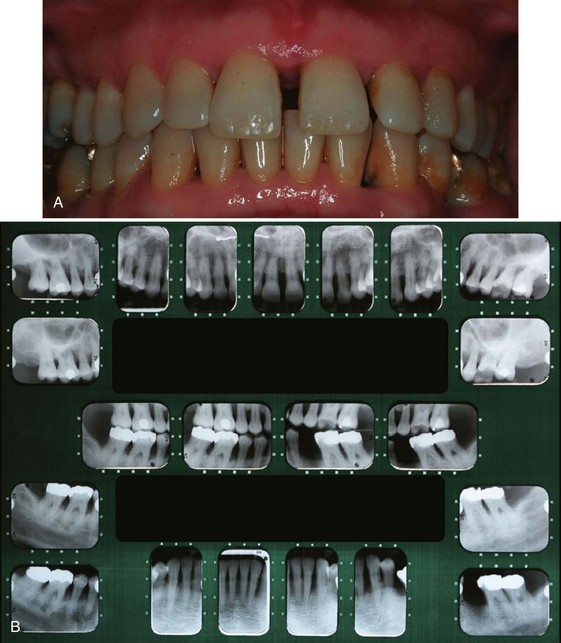
Although probing is the only reliable method of detecting pockets, clinical signs, such as color changes (bluish red marginal gingiva or bluish red vertical zone extending from the gingival margin to the attached gingiva); a “rolled” edge separating the gingival margin from the tooth surface; or an enlarged, edematous gingiva, may suggest their presence. The presence of bleeding, suppuration, and loose, extruded teeth may also denote the presence of a pocket (Figures 30-12 to 30-15).
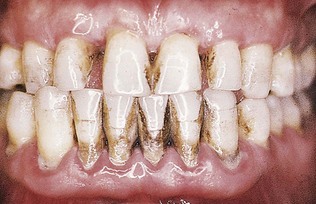
Figure 30-12 Periodontal pockets around mandibular anterior teeth, showing rolled margins, edematous inflammatory changes, and abundant calculus and plaque.
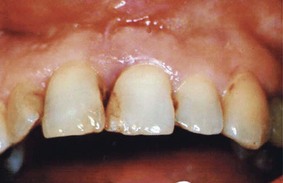
Figure 30-14 Periodontal pocket between maxillary central incisors produced bluish discoloration extending apically. Probing reveals presence of deep pocket.
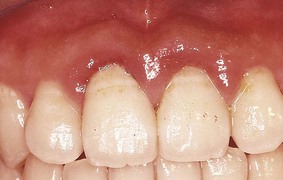
Figure 30-15 Severe generalized gingival inflammation. Note the dark hue in the marginal areas of the central incisors, which is caused in part by dark subgingival calculus and a deep pocket.
Periodontal pockets are generally painless but may give rise to symptoms such as localized or sometimes radiating pain or sensation of pressure after eating, which gradually diminishes. A foul taste in localized areas, sensitivity to hot and cold, and toothache in the absence of caries is also sometimes present.
Detection Of Pockets
The only accurate method of detecting and measuring periodontal pockets is careful exploration with a periodontal probe. Pockets are not detected by radiographic examination. The periodontal pocket is a soft tissue change. Radiographs indicate areas of bone loss in which pockets may be suspected, but they do not show pocket presence or depth and consequently they show no difference before and after pocket elimination unless bone has been modified.
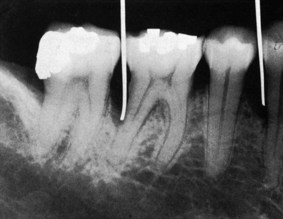
Gutta percha points or calibrated silver points29 can be used with the radiograph to assist in determining the level of attachment of periodontal pockets (Figure 30-16). They may be used effectively for individual pockets or in clinical research, but their routine use throughout the mouth would be difficult to manage. Clinical examination and probing are more direct and efficient. See Chapter 31 for detailed information regarding radiographic examination.
Pocket Probing
There are two different pocket depths: (1) the biologic or histologic depth and (2) the clinical or probing depth28 (Figure 30-17).
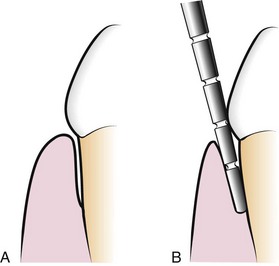
Figure 30-17 A, Biologic or histologic pocket depth is the actual distance between the gingival margin and the attached tissues (bottom of pocket). B, Probing or clinical pocket depth is the depth of penetration of the probe.
The biologic depth is the distance between the gingival margin and the base of the pocket (the coronal end of the junctional epithelium). This can be measured only in carefully prepared and adequately oriented histologic sections. The probing depth is the distance to which a probe penetrates into the pocket. Probes presently used are described in Chapter 45.
Probe penetration can vary, depending on the force of introduction, the shape and size of the probe tip, the direction of penetration, resistance of the tissues, convexity of the crown, and the degree of tissue inflammation.4 Several studies have been made to determine the depth of penetration of a probe in a sulcus or pocket. Armitage and colleagues6 used beagle dogs to evaluate the penetration of a probe using a standardized force of 25 g. They reported that in healthy gingiva the probe penetrated the epithelium to about two-thirds of its length; in gingivitis, it stopped 0.1-mm short of its apical end; and in periodontitis, the probe tip consistently went past the most apical cells of the junctional epithelium (Figure 30-18).
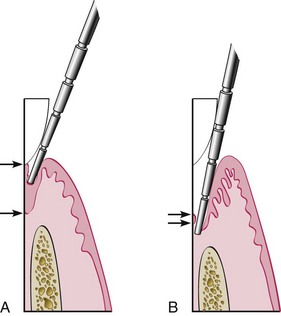
Figure 30-18 A, In a normal sulcus with a long junctional epithelium (between arrows), the probe penetrates about one third to one half the length of the junctional epithelium. B, In a periodontal pocket with a short junctional epithelium (between arrows), the probe penetrates beyond the apical end of the junctional epithelium.
In human periodontal pockets, the probe tip penetrates to the most coronal intact fibers of the connective tissue attachment.38,71 The depth of penetration of the probe in the connective tissue apical to the junctional epithelium in a periodontal pocket is about 0.3 mm.38,65,71 This is important in evaluating differences in probing depth before and after treatment, as the reduction in probe penetration may be a result of reduced inflammatory response rather than gain in attachment.37,39
The probing forces have been explored by several investigators19,76,77; forces of 0.75 N have been found to be well tolerated and accurate.73 Interexaminer error (depth discrepancies between examiners) was reported to be as much as 2.1 mm, with an average of 1.5 mm, in the same areas.28
Probing Technique
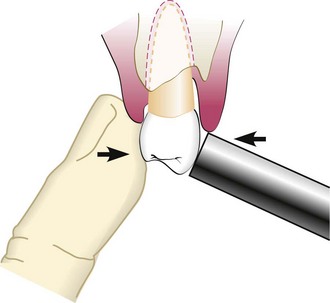
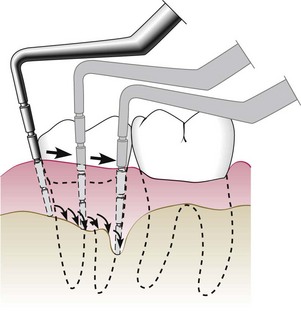
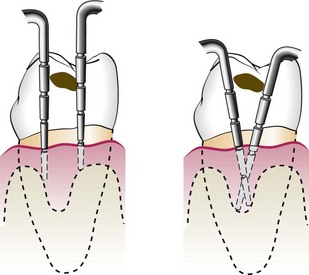
The probe should be inserted parallel to the vertical axis of the tooth and “walked” circumferentially around each surface of each tooth to detect the areas of deepest penetration (Figure 30-19).
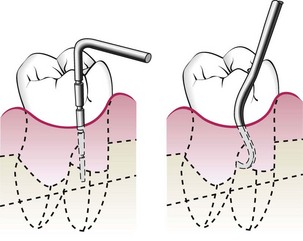
In addition, special attention should be directed to detecting the presence of interdental craters and furcation involvements. To detect an interdental crater, the probe should be placed obliquely from both the facial and lingual surfaces so as to explore the deepest point of the pocket located beneath the contact point (Figure 30-20). In multirooted teeth, the possibility of furcation involvement should be carefully explored. The use of specially designed probes (e.g., Nabers probe) allows an easier and more accurate exploration of the horizontal component of furcation lesions (Figure 30-21).
Level of Attachment Versus Pocket Depth
Pocket depth is the distance between the base of the pocket and the gingival margin. It may change from time to time even in untreated periodontal disease because of changes in the position of the gingival margin, and therefore it may be unrelated to the existing attachment of the tooth.
The level of attachment, on the other hand, is the distance between the base of the pocket and a fixed point on the crown such as the cementoenamel junction (CEJ). Changes in the level of attachment can be the result of gain or loss of attachment and afford a better indication of the degree of periodontal destruction (or gain). Shallow pockets attached at the level of the apical third of the root connote more severe destruction than deep pockets attached at the coronal third of the roots (see Chapter 13 and Figure 13-17 Figure 13-18 Figure 13-19 ).
Determining The Level of Attachment
When the gingival margin is located on the anatomic crown, the level of attachment is determined by subtracting from the depth of the pocket the distance from the gingival margin to the CEJ. If both are the same, the loss of attachment is zero.
When the gingival margin coincides with the CEJ, the loss of attachment equals the pocket depth.
When the gingival margin is located apical to the CEJ, the loss of attachment is greater than the pocket depth. Therefore the distance between the CEJ and the gingival margin should be added to the pocket depth. Drawing the gingival margin on the chart where pocket depths are entered helps clarify this important point.69
Bleeding on Probing
The insertion of a probe to the bottom of the pocket elicits bleeding if the gingiva is inflamed and the pocket epithelium is atrophic or ulcerated. Noninflamed sites rarely bleed. In most cases, bleeding on probing is an earlier sign of inflammation than gingival color changes45 (see Chapter 8). However, color changes may present without bleeding on probing.24 Depending on the severity of inflammation, bleeding can vary from a tenuous red line along the gingival sulcus to profuse bleeding.1 If periodontal treatment is successful, bleeding on probing will cease.3
To test for bleeding after probing, the probe is carefully introduced to the bottom of the pocket and gently moved laterally along the pocket wall. Sometimes bleeding appears immediately after removal of the probe; other times it may be delayed for a few seconds. Therefore the clinician should recheck for bleeding 30 to 60 seconds after probing.
As a single test, bleeding on probing is not a good predictor of progressive attachment loss; however, its absence is an excellent predictor of periodontal stability.4 When bleeding is present in multiple sites of advanced disease, bleeding on probing is a good indicator of progressive attachment loss.4,20 Armitage analyzed the literature on this subject up to 1996, performing a metaanalysis of the various papers and concluded that the presence of bleeding on probing in a “treated and maintained patient population” is an important risk predictor for increased loss of attachment.4
Insertion of a soft wooden interdental stimulator in the interdental space produces a similar bleeding response3 and can be used by the patient to self-examine the gingiva for the presence of inflammation.13
When to Probe
Probing of pockets is done at various times for diagnosis and for monitoring the course of treatment and maintenance. The initial probing of moderate or advanced cases is usually hampered by the presence of heavy inflammation and abundant calculus and cannot be done very accurately. Probing at this stage is also difficult as the result of the discomfort and pain that occurs when the gingival tissues are inflamed.
The purpose of this initial probing, together with the clinical and radiographic examination, is done to determine whether the tooth can be saved or should be extracted. After the patient has performed an adequate plaque control for some time and calculus has been removed, the major inflammatory changes disappear and an accurate probing of the pockets can be performed. The purpose of this second probing is to accurately establish the level of attachment and degree of involvement of roots and furcations. Data obtained from this probing provides valuable information for treatment decisions.
Later in periodontal treatment, probings are done to determine changes in pocket depth and to ascertain healing progress after different procedures.
Probing Around Implants
Since periimplantitis can create pockets around implants, probing around them becomes part of examination and diagnosis. To prevent scratching of the implant surface, plastic periodontal probes should be used instead of the steel probes used for the natural dentition.
Automatic and Electronic Periodontal Probing
The use of the periodontal probe is the classic method to detect pocket depth and loss of attachment. However, it presents some problems in terms of reproducibility of the measurements. Accuracy and reproducibility depend not only on root morphology and tissue changes but also, very importantly, on the probing technique, the probing force, the size of the probe, the angle of insertion of the probe, and the precision of probe calibration (Figure 30-22).
Probing Force
One of the main problems in reproducibility has been the variation of probing force. The development of pressure-sensitive probes has helped overcome this. It has been shown that with forces of up to 30 g, the tip of the probe remains within the junctional epithelium,5 whereas forces of up to 50 g are necessary to reach the bone level.31
Probe Angulation
Standardization of the probe tip (<1 mm) and the addition of registration stents to maintain reproducible probe angulation have been used to overcome this error.8 New, commercially available computer-assisted technology has been used to improve probing accuracy and reproducibility. In addition to more accurate measurement, the data derived from automatic probing can become part of the electronic record. Once incorporated, clinical changes and patterns of disease activity can be easily determined. These records also provide feedback to the patient. The Florida Probe System2 (Figure 30-23) consists of a probe handpiece, digital readout, foot switch, computer interface, and computer. The end of the probe is 0.4 mm, and it reciprocates through a sleeve that provides a reference by which measurements are made.
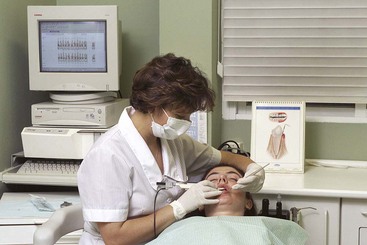
Figure 30-23 Automated periodontal probes: Florida Probe System. Integration of direct electronic measurements with constant probing force with computer storage and online data readout.
These measurements are made electronically and transferred automatically when the foot switch is pressed or a voice command is given. Constant probing force is provided by coil springs inside the probe handpiece and digital readout (Figure 30-24). This method combines the advantages of a constant probing force with precise electronic measurement and computer storage of data, thus eliminating the potential errors associated with visual reading and the need for an assistant to record the measurements.
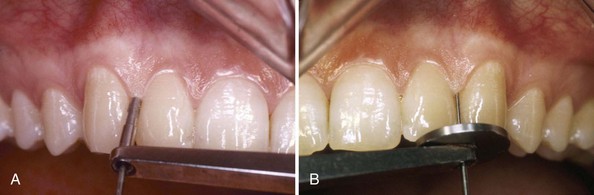
Figure 30-24 Florida Probe System. A, Handpiece for assessing probing pocket depths. B, Handpiece for assessing relative clinical attachment levels.
Several studies have been made comparing the Florida Probe with conventional probing. The automatic probe appears to underestimate deep probing depths55 but show less variability than conventional probing.15,61,81 The automatic probe also has the problem of providing little tactile sensitivity thus making it more difficult to “walk” the probe.
Other electronic systems, such as the Interprobe and the Periprobe, provide constant probing force and computer storage of data, but their reproducibility is only slightly better than conventional methods.61,81
The lack of standardization between probings by different individuals and at different times by the same individual depend not only on root morphology and tissue changes but also on the probing technique, the probing force, the size of the probe, the angle of insertion of the probe, and precision of the probe calibration. For measurements of clinical attachment loss, it is necessary to identify the location of the CEJ, and this offers another hurdle for the standardization of measurements.
Determination of Disease Activity
Currently, there are no accurate methods to determine activity or inactivity of a lesion. Inactive lesions may show little or no bleeding on probing and minimal amounts of gingival fluid. Active lesions bleed more readily on probing and have large amounts of fluid and exudates although active and nonactive sites may show no differences in bleeding on probing, even in patients with aggressive periodontitis.31 The bacterial flora in a healthy gingival sulcus, as revealed by dark-field microscopy, consists mostly of coccoid cells. The bacterial flora of a periodontal pocket shows a greater number of spirochetes and motile bacteria.25
For the determination of pocket depth or attachment levels to provide information on whether the lesion is in an active or inactive state, measurements taken at different times have to be compared. The precise assessment and comparison of the clinical attachment level (CAL) at different intervals of time can determine whether the attachment is being lost, which indicates that the lesion is active. The exact measurement of this important clinical parameter is done from the CEJ to the bottom of the pocket and therefore requires to determine exactly and reproducibly the location of this landmark.
The Florida Probe provides a means of recording relative CAL changes over time. A later model of the probe has a modified sleeve with a prominent 0.125-mm edge to facilitate a “catch” of the CEJ. The width of this edge is considered small enough not to interfere with probing-depth measurements, thus providing concurrent measurements of CAL and pocket depth.32,66 Of course, this measurement does not predict the future but only shows what happened in the period of time between the two measurements. See Chapters 23 and 25 for other methods of determination of disease activity still in a developmental stage. The precise determination of disease activity will have a direct influence on diagnosis, prognosis, and therapy. The goals of therapy may change, depending on the state of the periodontal lesion.
Amount of Attached Gingiva
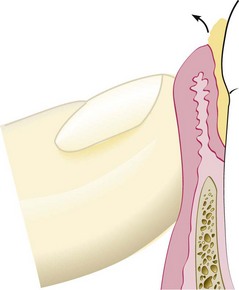
It is important to establish the relation between the bottom of the pocket and the mucogingival line. The width of the attached gingiva is the distance between the mucogingival junction and the projection on the external surface of the bottom of the gingival sulcus or the periodontal pocket. It should not be confused with the width of the keratinized gingiva, because the latter also includes the marginal gingiva (Figure 30-25).
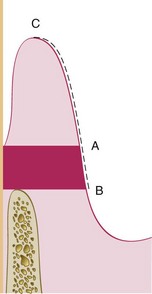
Figure 30-25 Shaded area shows the attached gingiva, which extends between the projection on the external surface of the bottom of the pocket (A) and the mucogingival junction (B). The keratinized gingiva may extend from the mucogingival junction (B) to the gingival margin (C).
The width of the attached gingiva is determined by subtracting the sulcus or pocket depth from the total width of the gingiva (gingival margin to mucogingival line). This is done by stretching the lip or cheek to demarcate the mucogingival line while the pocket is being probed (Figure 30-26). The amount of attached gingiva is generally considered to be insufficient when stretching of the lip or cheek induces movement of the free gingival margin.
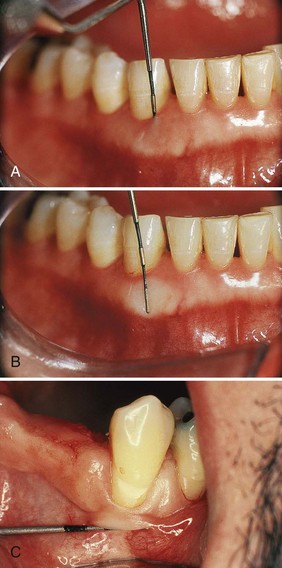
Figure 30-26 To determine the width of the attached gingiva, the pocket is probed (A), and then the probe is placed on the outer surface (B) while the lip (or cheek) is extended to demarcate the mucogingival line. C, Another method to demarcate the mucogingival line is pushing the lip (cheek) coronally.
Other methods used to determine the amount of attached gingiva include pushing the adjacent mucosa coronally with a dull instrument or painting the mucosa with Schiller’s potassium iodide solution, which stains keratin.
Degree of Gingival Recession
During periodontal examination, it is necessary to record the data regarding the amount of gingival recession. This measurement is taken with a periodontal probe from the CEJ to the gingival crest, and it is drawn on the patient’s chart (see Chapter 8).
Alveolar Bone Loss
Alveolar bone levels are evaluated by clinical and radiographic examination. Probing is helpful for determining (1) the height and contour of the facial and lingual bones obscured on the radiograph by the roots and (2) the architecture of the interdental bone. Transgingival probing, performed after the area is anesthetized, is a more accurate method of evaluation and provides additional information on bone architecture23,34,73 (see Chapter 60).
Palpation
Palpating the oral mucosa in the lateral and apical areas of the tooth may help locate the origin of radiating pain that the patient cannot localize. Infection deep in the periodontal tissues and the early stages of a periodontal abscess may also be detected by palpation.
Suppuration
The presence of an abundant number of neutrophils in the gingival fluid transforms it into a purulent exudate.4 Several studies9,10,13,30 have evaluated the association between suppuration and the progression of periodontitis and reported that this sign is present in a very low percentage of diseased sites (3% to 5%).4 Therefore it is not by itself a good indicator.
Clinically, the presence of exudate in a periodontal pocket is determined by placing the ball of the index finger along the lateral aspect of the marginal gingiva and applying pressure in a rolling motion toward the crown (Figure 30-27). Visual examination without digital pressure is not enough. The purulent exudate is formed in the inner pocket wall, and therefore the external appearance may give no indication of its presence. Exudate formation does not occur in all periodontal pockets, but digital pressure often reveals it in pockets where its presence is not suspected.
Periodontal Abscess
A periodontal abscess is a localized accumulation of exudate within the gingival wall of a periodontal pocket (see Chapter 13). Periodontal abscesses may be acute or chronic.
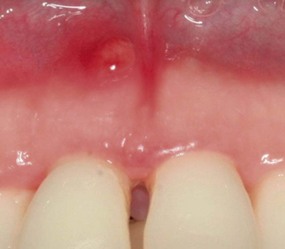
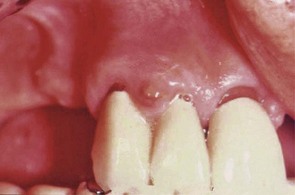
The acute periodontal abscess appears as an ovoid elevation of the gingiva along the lateral aspect of the root (Figures 30-28 to 30-30). The gingiva is edematous and red, with a smooth, shiny surface. The shape and consistency of the elevated area vary; the area may be domelike and relatively firm, or pointed and soft. In most cases, exudate may be expressed from the gingival margin with gentle digital pressure.
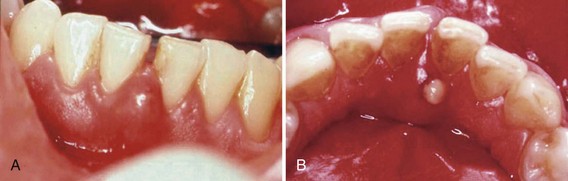
Figure 30-28 A, Facial view of acute periodontal abscess between the lower central incisors. B, Lingual view of the same patient with a suppurating draining sinus.
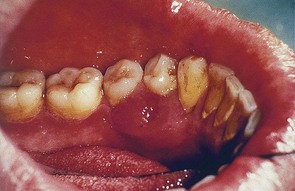
Figure 30-29 Acute periodontal abscess in the wall of a deep pocket in the lingual surface of lower premolars.
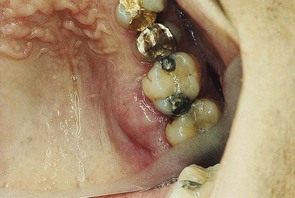
Figure 30-30 Acute periodontal abscess associated with a deep periodontal pocket in palatal area of first and second upper molars. Note how the fibrotic character of the palatal tissue masks the typical changes of the abscess.
The acute periodontal abscess is accompanied by symptoms such as throbbing, radiating pain and tenderness of the gingiva to palpation. Other symptoms may include sensitivity of the tooth to palpation; tooth mobility and lymphadenitis; and less frequently, systemic effects such as fever, leukocytosis, and malaise. Occasionally, the patient may have symptoms of an acute periodontal abscess without any notable clinical lesion or radiographic changes.
The chronic periodontal abscess usually presents a sinus that opens onto the gingival mucosa along the length of the root. There may be a history of intermittent exudation. The orifice of the sinus may appear as a difficult-to-detect pinpoint opening, which, when probed, reveals a sinus tract leading deep into the periodontium. The sinus may be covered by a small, pink, beadlike mass of granulation tissue. The chronic periodontal abscess is usually asymptomatic. However, the patient may report episodes of dull, gnawing pain; slight elevation of the tooth; and a desire to bite down and grind the tooth. The chronic periodontal abscess often undergoes acute exacerbations, with all the associated symptoms.
Diagnosis of the periodontal abscess requires correlation of the history and clinical and radiographic findings. The suspected area should be probed carefully along the gingival margin in relation to each tooth surface to detect a channel from the marginal area to the deeper periodontal tissues. Continuity of the lesion with the gingival margin is clinical evidence that the abscess is periodontal.
The abscess is not necessarily located on the same surface of the root as the pocket from which it is formed. A pocket at the facial surface may give rise to a periodontal abscess interproximally. It is common for a periodontal abscess to be located at a root surface other than that along which the pocket originated because drainage is more likely to be impaired when a pocket follows a tortuous course.
In children a sinus orifice along the lateral aspect of a root is usually the result of periapical infection of a deciduous tooth. In the permanent dentition, such an orifice may be caused by a periodontal abscess, as well as by apical involvement. The orifice may be patent and draining, or it may be closed and appear as a red, nodular mass (Figure 30-31). Exploration of such masses with a probe usually reveals a pinpoint orifice that communicates with an underlying sinus.
Periodontal Abscess and Gingival Abscess
The principal differences between the periodontal abscess and the gingival abscess are location and history (see Chapter 13). The gingival abscess is confined to the marginal gingiva, and it often occurs in previously disease-free areas (Figure 30-32). It is usually an acute inflammatory response to forcing of foreign material into the gingiva. The periodontal abscess involves the supporting periodontal structures and generally occurs in the course of chronic destructive periodontitis.
Periodontal Abscess and Periapical Abscess
Several characteristics can be used as guidelines in differentiating a periodontal abscess from a periapical abscess. If the tooth is nonvital, the lesion is most likely periapical. However, a previously nonvital tooth can have a deep periodontal pocket that can abscess. Moreover, a deep periodontal pocket can extend to the apex and cause pulpal involvement and necrosis.
An apical abscess may spread along the lateral aspect of the root to the gingival margin. However, when the apex and lateral surface of a root are involved by a single lesion that can be probed directly from the gingival margin, the lesion is more likely to have originated as a periodontal abscess.
Radiographic findings are helpful in differentiating between a periodontal and a periapical lesion (see Chapter 31). Early acute periodontal and periapical abscesses present no radiographic changes. Ordinarily, a radiolucent area along the lateral surface of the root suggests the presence of a periodontal abscess, whereas apical rarefaction suggests a periapical abscess. However, acute periodontal abscesses that show no radiographic changes often cause symptoms in teeth with long-standing, radiographically detectable periapical lesions that are not contributing to the patient’s complaint. Clinical findings, such as the presence of extensive caries, pocket formation, lack of tooth vitality, and the existence of continuity between the gingival margin and the abscess area, often prove to be of greater diagnostic value than radiographic appearance.
A draining sinus on the lateral aspect of the root suggests periodontal rather than apical involvement; a sinus from a periapical lesion is more likely to be located further apically. However, sinus location is not conclusive. In many instances, particularly in children, the sinus from a periapical lesion drains on the side of the root rather than at the apex (see Chapter 51).
Laboratory Aids to Clinical Diagnosis
When unusual gingival or periodontal problems are detected that cannot be explained by local causes, the possibility of contributing systemic factors must be explored. The signs and symptoms of oral manifestations of systemic disease have to be clearly understood and analyzed and their presence discussed with the patient’s physician. Parts 3 and 5 present discussions of many of these problems, and the reader is also directed to standard texts on medical diagnosis for more detailed descriptions of the necessary tests and their interpretation.
Numerous laboratory tests aid in the diagnosis of systemic diseases that may contribute to periodontal and oral diseases and will also be needed for the treatment decisions when dealing with medically compromised patients. Chapter 37 describes the use of these tests for the evaluation and management of medically compromised patients. Analyses of blood smears, blood cell counts, white blood cell differential counts, and erythrocyte sedimentation rates are used to evaluate the presence of blood dyscrasias and generalized infections. Determination of coagulation time, bleeding time, clot retraction time, prothrombin time, capillary fragility test, and bone marrow studies may be required at times. The reader is referred to books on medical diagnoses for a consideration of this subject.
![]() Science Transfer
Science Transfer
All patients should have a comprehensive periodontal evaluation that is recorded in the chart. This, at a minimum, includes probing data on six surfaces on each tooth, as well as recording gingival recession and bleeding on probing. Other parameters that need to be recorded include mobility, furcation involvement, mucogingival deficiencies, plaque scores, open contacts, and examination of functional occlusal relationships. A comprehensive medical and dental history must accompany the clinical protocol. A full-mouth series of radiographs taken within the last 2 years with any needed updates is necessary to achieve a correct diagnosis and prepare the appropriate treatment plan.
Periodontal screening and recording systems have little value in managing patients in a private practice because they do not give sufficient specific data for individual patient treatment planning.
References
1 Abrams K, Caton J, Polson AM. Histologic comparisons of interproximal gingival tissues related to the presence or absence of bleeding. J Periodontol. 1984;55:629.
2 Alexander AG, Tipnis AK. The effect of irregularity of teeth and the degree of overbite and overjet on the gingival health. Br Dent J. 1970;128:539.
3 Amato R, Caton J, Polson A, et al. Interproximal gingival inflammation related to the conversion of a bleeding to a non-bleeding state. J Periodontol. 1986;57:63.
4 Armitage GC. Periodontal diseases: Diagnosis. Ann Periodontol. 1996;1:37.
5 Armitage GC, Jeffcoat MK, Chadwick DE, et al. Longitudinal evaluation of elastase as a marker for the progression of periodontitis. J Clin Periodontol. 1994;65:120.
6 Armitage GC, Svanberg GK, Löe H. Microscopic evaluation of clinical measurements of connective tissue attachment levels. J Clin Periodontol. 1977;4:173.
7 Arstad T. The capsular ligaments of the temporomandibular joint and retrusion facets of the dentition in relationship to mandibular movements. Oslo, Norway: Akademisk Forlag; 1954.
8 Badersten A, Nilveus R, Egelberg J. Reproducibility of probing attachment levels measurements. J Clin Periodontol. 1984;11:475.
9 Badersten A, Nilvéus R, Egelberg J. Effect of nonsurgical therapy. VII. Bleeding, suppuration and probing depth in sites with probing attachment loss. J Clin Periodontol. 1985;12:432.
10 Badersten A, Nilvéus R, Egelberg J. Scores of plaque, bleeding, suppuration and probing depth to predict probing attachment loss. Five years of observation following nonsurgical periodontal therapy. J Clin Periodontol. 1990;7:102.
11 Baumgartner HS. A voice-input computerized dental examination system using high resolution graphics. Compend Contin Educ Dent. 1988;9:446.
12 Beyron HI. Occlusal changes in the adult dentition. J Am Dent Assoc. 1954;48:674.
13 Caton J, Polson A. The interdental bleeding index: a simplified procedure to monitor gingival health. Compend Contin Educ Dent. 1985;6:89.
15 Clark WB, Yang MC, Magnusson I. Measuring clinical attachment level reproducibility and relative measurements with an electronic probe. J Periodontol. 1992;63:831.
16 Egermark-Erikson I, Carlsson GE, Ingervall B. Prevalence of mandibular dysfunction and orofacial parafunction in 7-, 11-, and 15-year old Swedish children. Eur J Orthop. 1981;l3:163.
18 Fischman SL, Picozzi A. Review of the literature: the methodology of clinical calculus evaluation. J Periodontol. 1969;40:607.
19 Gabathuler H, Hassel TM. A pressure sensitive periodontal probe. Helv Odontol Acta. 1971;15:114.
20 Gibbs RA. DNA amplification by the polymerase chain reaction. Anal Chem. 1990;62:1202.
21 Gibbs CH, Hirschfeld JW, Lee JG, et al. Description and clinical evaluation of a new computerized periodontal probe—the Florida probe. J Clin Periodontl. 1988;15:137.
22 Grant DA, Grant DA, Flynn MJ, et al. Periodontal microbiota of mobile and non-mobile teeth. J Periodontol. 1995;66:386.
23 Greenberg J, Laster L, Listgarten MA. Transgingival probing as a potential estimator of alveolar bone level. J Periodontol. 1976;47:514.
24 Greenstein G. The role of bleeding upon probing in the diagnosis of periodontal disease. A literature review. J Periodontol. 1984;55:684.
25 Greenstein G, Caton J, Polson AM. Histologic characteristics associated with bleeding after probing and visual signs of inflammation. J Periodontol. 1981;52:420.
26 Grippo JO, Simring M, Schreiner S. Attrition, abrasion, corrosion and abfraction revisited. JADA. 2004;135:1109.
28 Hassel TM, German MA, Saxer UP. Periodontal probing: interinvestigator discrepancies and correlations between probing force and recorded depth. Helv Odontol Acta. 1973;17:38. 1973
29 Hirschfeld L. A calibrated silver point for periodontal diagnosis and recording. J Periodontol. 1953;24:94.
30 Kaldahl WB, Kalkwarf KL, Patil KD, et al. Relationship of gingival bleeding, gingival suppuration and supragingival plaque to attachment loss. J Periodontol. 1990;61:347.
31 Kalkwarf KI, Kahldal WD, Patil KD. Comparison of manual and pressure controlled periodontal probing. J Periodontol. 1986;57:467.
32 Karpinia K, Magnusson I, Gibbs C, et al. Accuracy of probing attachment levels using CEJ probe versus traditional probes. J Clin Periodontol. 2004;31:173.
33 Kitchen PC. The prevalence of tooth root exposure and the relation of the extent of such exposure to the degree of abrasion in differing age classes. J Dent Res. 1941;20:565.
34 Kim HY, Yi SW, Choi SH, et al. Bone probing measurement as a reliable evaluation of the bone level in periodontal defects. J Periodontol. 2000;71:729.
35 Kurashima K. Viscoelastic properties of periodontal tissue. Bull Tokyo Med Dent Univ. 1965;12:240.
36 Lambrechts P, Braem M, Vuylsteke-Wanters M, Vanherle G. Quantitative in vivo wear of human enamel. J Dent Re4s. 1989;68:1752.
37 Listgarten MA. Periodontal probing: what does it mean? J Clin Periodontol. 1980;7:165.
38 Listgarten MA, Mao R, Robinson PJ. Periodontal probing: the relationship of the probe tip to periodontal tissues. J Periodontol. 1976;47:511.
39 Magnusson I, Listgarten MA. Histological evaluation of probing depth following periodontal treatment. J Clin Periodontol. 1980;7:26.
40 Mandell RL, Ebersole JL, Socransky SS. Clinical, immunologic and microbiologic features of active disease sites in juvenile periodontitis. J Clin Periodontol. 1987;14:534.
41 Manly RS. Abrasion of cementum and dentin by modern dentifrices. J Dent Res. 1941;20:583.
42 Manly RS. Factors influencing tests on the abrasion of dentin by brushing with dentifrices. J Dent Res. 1944;23:59.
43 Mannerberg F. Saliva factors in cases of erosion. Odont Rev. 1963;14:156.
44 McCay CM, Wills L. Erosion of molar teeth by acid beverages. J Nutr. 1949;39:313.
45 Meitner SW, Zander HA, Iker HP, et al. Identification of inflamed gingival surfaces. J Clin Periodontol. 1979;6:93.
46 Miller WD. Experiments and observations on the wasting of tooth tissue variously designated as erosion, abrasion, chemical abrasion, denudation, etc. D Cosmos. 1907;49:1.
47 Mühlemann HR. Ten years of tooth mobility measurements. J Periodontol. 1960;31:110.
48 Mühlemann HR. Tooth mobility: a review of clinical aspects and research findings. J Periodontol. 1967;38:686.
49 Mühlemann HR, Savdir S, Rateitschak KH. Tooth mobility—its causes and significance. J Periodontol. 1965;36:148.
50 O’Leary TJ. Tooth mobility. Dent Clin North Am. 1969;3:567.
51 O’Leary TJ, Rudd KD. An instrument for measuring horizontal mobility. Periodontics. 1963;1:249.
52 Parfitt GJ. Measurement of the physiologic mobility of individual teeth in an axial direction. J Dent Res. 1960;39:608.
53 Parfitt GJ. The dynamics of a tooth in function. J Periodontol. 1961;32:102.
54 Perlitsch MJ. A systematic approach to the interpretation of tooth mobility and clinical implications. Dent Clin North Am. 1980;24:177.
55 Perry DA, Taggart EJ, Leung A, et al. Comparison of a conventional probe with electronic and manual pressure-regulated probes. J Periodontol.. 1994;65:908.
56 Persson R. Assessment of tooth mobility using small loads. II. Effect of oral hygiene procedures. J Clin Periodontol. 1980;7:506.
57 Persson R. Assessment of tooth mobility using small loads. III. Effect of periodontal treatment including a gingivectomy procedure. J Clin Periodontol. 1981;8:4.
58 Persson R. Assessment of tooth mobility using small loads. IV. The effect of periodontal treatment including gingivectomy and flap procedures. J Clin Periodontol. 1981;8:88.
59 Persson R, Svensson A. Assessment of tooth mobility using small loads. I. Technical devices and calculations of tooth mobility in periodontal health and disease. J Clin Periodontol. 1980;7:259.
60 Preshaw PM, Kupp L, Hefti AF, et al. Measurement of clinical attachment levels using a constant-force periodontal probe modified to detect the cemento-enamel junction. J Clin Periodontol. 1999;26:434.
61 Quyrinen M, Callens A, van Steenberghe D, et al. Clinical evaluation of a constant for5ce electronic probe. J Periodfontol. 1993;64:35.
62 Ratcliff PA, Johnson PW. The relationship between malodor, gingivitis and periodontitis. A review. J Periodontol. 1999;70:485.
63 Rhodes PR. The electronic patient record. In: Rose LF, Mealey BL, Genco RJ, Cohen DW, editors. Periodontics; medicine, surgery and implants. S Louis, Mo: Elsevier Mosby, 2004.
64 Robinson HBG. Abrasion, attrition and erosion of teeth. Health Center J Ohio State Univ. 1949;3:21.
65 Saglie R, Johanson JR, Flotra L. The zone of completely and partially destructed periodontal fibers in pathological pockets. J Clin Periodontol. 1975;2:198.
66 Salonen L, Hellden L. Prevalence of signs and symptoms of dysfunction in the masticatory system: an epidemiologic study in an adult Swedish population. J Craniomandib Dis Fac oral pain. 1990;4:241.
67 Schulte W, D’Hoedt B, Scholz F, et al. Periotest-neues Messverfahren der Funktion des Parodontiums. Zahnarztl Mitt. 1983;73:1229.
68 Seligman DA, Pullinger AG, Solberg AK. The prevalence of dental attrition and its association with factors of age, gender and TMJ symptomatology. J Dent Res. 1988;67:1323.
69 Sivertson JF, Burgett FG. Probing of pockets related to the attachment level. J Periodontol. 1976;47:281.
70 Sognnaes RF. Periodontal significance of intraoral frictional ablation. J West Soc Periodontol. 1977;25:112.
71 Spray JR, Garnick JJ, Doles LR, et al. Microscopic demonstration of the position of periodontal probes. J Periodontol. 1978;49:148.
73 Tibbetts LS. Use of diagnostic probes for detection of periodontal disease. J Am Dent Assoc. 1969;78:549.
75 Van der Bilt A, Olthoff LW, Bosman F, Osterhaven SP. The effect of missing postcanine teeth on chewing performance in man. Arch Oral Biol. 1993;38:423.
76 Van der Velden U. Probing force and the relationship of the probe tip to the periodontal tissues. J Clin Periodontol. 1979;6:106.
77 Van der Velden U, De Vries JH. Introduction of a new periodontal probe. The pressure probe. J Clin Periodontol. 1978;5:188.
78 Weinberg LA. Diagnosis of facets in occlusal equilibration. J AM Dent Assoc. 1956;52:26.
79 Woda A, Gourdon AM, Faraj M. Occlusal contact and tooth wear. J Prosthet Dent. 1987;57:85.
80 Xhonga FA. Bruxism and its effect on teeth. J Oral Rehabil. 1977;4:65.
81 Yang MCK, Bochacki V, Genco RJ. Immunological assays for putative periodontal pathogens. Oral Microbiol Immunol. 1986;1:39.