CHAPTER 6 Armamentarium and Sterilization
Preparation for endodontic treatment requires a somewhat different approach compared with many other types of dental therapy. Endodontic treatment breaks through the body’s primary protective barrier, the integumentary system. Teeth are a specialized part of this system that also includes skin and mucous membranes; collectively, the integumentary system serves to protect humans from invasion by microbes, among other functions. This barrier is breached when caries, trauma, or other insults provide entry into the pulpal space of a tooth. Sterile instruments and proper technique are needed to avoid contamination by microorganisms or noxious chemicals during endodontic therapy.
The well-prepared clinician realizes that a posttreatment infection could be caused by a break in sterile technique. The astute clinician realizes that time spent before treatment in the organization and sterilization of instruments and supplies will lead to fewer posttreatment patient problems and result in a more efficient practice.
In addition to the need for impeccable sterility, endodontics differs from many other aspects of dentistry. Access and visibility are severely limited by the nature of the irregular and branching canal spaces within the roots. Individual variation means that clinicians cannot know exactly what condition is present throughout the entire root canal system (see Chapter 7 for more details). Enhanced lighting and magnification have resolved visual access problems to some extent (Figs. 6-1 to 6-4). Nevertheless, limitations remain and there continues to be a need for special instruments and techniques.
Selection of the Armamentarium
Preparation for treatment begins with selection of the armamentarium for routine endodontic patient care. Instruments used for every patient should be assembled and sterilized before use. Box 6-1 lists a typical armamentarium that can be customized for the individual clinician (Figs. 6-5 to 6-7).

FIG. 6-6 Cassette wrapped in porous autoclave paper in preparation for sterilization.
(Courtesy Hu-Friedy, Chicago, IL.)
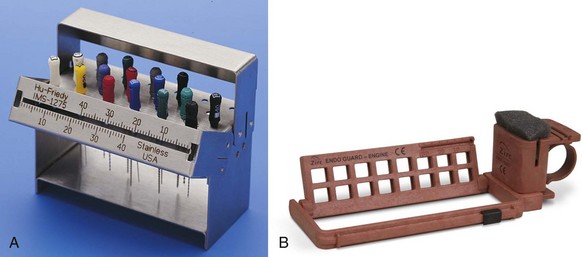
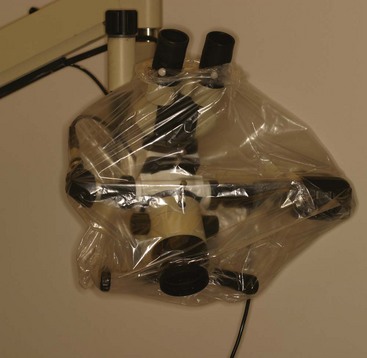
FIG. 6-7 A, File stand. B, File stand for rotary instruments.
(A, Courtesy Hu-Friedy, Chicago, IL. B, Courtesy Zirc, Buffalo, MN.)
Root canal instruments of appropriate length and type, including files, reamers, broaches, and specialized burs, may be sterilized separately because variables (e.g., tooth length, canal size, and clinical diagnosis) may affect this part of the armamentarium. Additional items that are needed occasionally (e.g., chlorhexidine and an intraosseous injection kit) should be in sterile containers and readily available to the dental assistant (Figs. 6-8 and 6-9). This approach provides a basic endodontic setup tray with optional items sterilized and ready for use in individual cases. The overarching philosophical approach is to develop a standard setup for all patients and to have readily available specialized kits for specific procedures or clinical situations.
Pretreatment Sterilization and Disinfection
The next phase after selection of the armamentarium is to develop a pretreatment sterilization plan. Sterile is defined as the absence of life forms. Disinfection means killing most life forms, especially pathogens. As commonly used, the term disinfection does not include killing spores. Manufacturers’ claims for disinfectants are based on performance against Mycobacterium, which is the genus that includes the species that causes tuberculosis (TB). For assessing virucidal efficacy, the astute clinician should be aware that agents that kill the hardy hepatitis B virus will likely be effective at destroying all viruses.
The Centers for Disease Control and Prevention (CDC, Atlanta, GA) released new guidelines for sterilization in 2003, and these are available on the Internet (http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5217a1.htm).12 Box 6-2 lists the elements of a typical sterilization plan. These guidelines clarify the need for staff training programs to ensure that sterilization procedures are effective. Specific items include weekly monitoring of the effectiveness of the process with biologic monitors, for example, packets of spores are placed in the sterilizer for one cycle. The spores are then placed in growth medium to ensure that all have been killed. Overpacking the sterilizer is a common reason for inadequate sterilization because steam cannot penetrate as well. Thus assessment of the effectiveness of the sterilization plan is an essential feature of pretreatment planning (Fig. 6-10). The results of weekly monitoring should be kept in a log.
BOX 6-2 Elements of a Sterilization Plan
Modified from Kohn WG, Collins AS, Cleveland JL, et al: Centers for Disease Control and Prevention (CDC): Guidelines for infection control in dental health-care settings—2003. MMWR Recomm Rep 52(RR-17):1, 2003. Available at http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5217a1.htm.

FIG. 6-10 Sterilizer monitoring service. Each week an envelope containing test spores is included in a sterilizer cycle and then mailed to the test center. Sterilizer performance is confirmed when no spores grow in culture. This lab is U.S. Food and Drug Administration (FDA) approved to monitor all types of sterilizers.
(Courtesy Dr. John Ruby, University of Alabama at Birmingham.)
Monitoring the sterilizer by watching the gauges or relying on printouts of temperature, pressure, and time helps to ensure patient safety, but weekly biologic monitoring guards against sensor failure. Heat-sensitive indicators, such as special tapes that change color when exposed to sterilizer temperatures, are also ineffective to ensure proper sterilization because these heat-sensing indicators merely show that the desired temperature has been reached, not that sufficient time has passed for proper killing. Accordingly, they are best used to simply record that the package was autoclaved; they should not be interpreted to indicate sterility.
Further guidelines about sterilizers include drying of instruments before removing them from the sterilizer. Wet wraps may tear easily or allow microbes to reach the sterile contents by wicking through the wrap. In addition, cooling avoids thermal injury to personnel. Some of the new sterilizers work faster because they vacuum air from the chamber before sterilization; these models also cool faster because of a second vacuum cycle after sterilization.
Another guideline reinforces the previous policy that the pathway from the sterilizer to the point of use should not cross the path of contaminated instruments as they are brought from the treatment area, cleaned, and packaged for resterilization. Thus separate areas are required for cleaning/sorting/packaging and for sterilization and cool down.
Approved methods of sterilization include pressurized steam (autoclaving), pressurized heated chemicals, dry heat, and cold chemicals (Figs. 6-11 to 6-13). Cold chemicals are not favored because the time cycle is difficult to monitor; they are recommended only for those items that cannot be heat sterilized. Room temperature chemical sterilants are not time efficient enough to be popular in dentistry. Cold sterilants generally require extended soak times. Their proper use necessitates rinsing in sterile water to remove traces of disinfectant and handling with sterile tongs or sterile gloves to place them in a sterile storage container after treatment. Cold sterilants are poisonous and can be harmful to patients, even in small amounts.
FIG. 6-11 Rapid-cycle autoclaves. A, Automatic, programmable autoclave. B, Statim cassette autoclave.
(A, Courtesy Eschmann Equipment, Lancing, West Sussex, England. B, Courtesy SciCan, Toronto, ON, Canada.)

FIG. 6-12 Chemiclave chemical vapor sterilizer.
(From Bird DL, Robinson DS: Torres & Ehrlich Modern Dental Assisting, St. Louis, 2009, Saunders/Elsevier.)
Do not pack instrument sets tightly. Boxes with plenty of holes to admit steam through the overwrap are ideal. Closed boxes should be autoclaved with the lids ajar. Autoclaving is the typical method of sterilization for most health care facilities. The usual cycle is 30 minutes at 250° F (121° C) at 15 psi. Many offices use two autoclaves. This allows for rapid sterilization of handpieces or other expensive equipment to reduce inventory requirement, as well as serving as a backup when one machine is broken. Flash sterilization at higher temperatures of 273° F (134° C) for 10 minutes and 30 psi is also approved. The Statim brand autoclave (SciCan, Toronto, ON, Canada) uses the higher temperatures to reduce cycle time.
A Chemiclave chemical vapor sterilizer uses a solution of 72% ethanol and 0.23% formaldehyde in place of water in its “autoclave.” This avoids the instrument corrosion typical of steam autoclaves. It uses 270° F (132° C) at 20 psi for 20 minutes, including drying time. It could be argued that a longer cycle time may be needed to meet guidelines for safety margins.18 Ventilation or filtration is required to handle formaldehyde fumes. Chemiclave chemicals can dissolve into liquids, so liquids should be steam autoclaved.
Dry heat sterilizers, either still air in an oven or forced air, also avoid corrosion of instruments. After preheating the instruments to sterilizing temperature, still air models provide sterility in 1 hour at 375° F (191° C). The Cox sterilizer (Alfa Medical, Hempstead, NY) uses forced hot air of the same temperature and sterilizes in 6 minutes. Wrapped packs increase the time needed for processing. Hot air sterilizers may require special wraps.
In-operatory sterilizers were once popular among clinicians for decontaminating instruments during treatment. Such sterilizers achieved 450° F (218° C), and typical cycles consisted of a few seconds for metal instruments.31 Glass beads or salt were used to transfer dry heat to endodontic canal instruments. These sterilizers are no longer approved for use because of the possibility that clinicians relied on them for sterilization between patients. One study found that they were ineffective at killing spores on cotton and paper products.31 Endodontic instruments used for recapitulation in a canal as it is progressively decontaminated may be disinfected chemically.16
After patient treatment, barriers should be removed and surfaces disinfected (Fig. 6-14). Aerosols generated by dental care can land anywhere, and therefore all surfaces that may be touched during treatment of the next patient should be disinfected. Special attention should be given to instrument holders and hoses. Door and drawer pulls should be included in the disinfection routine or covered with barriers. Pens, pencils, and even patient charts should be considered contaminated.
Disinfection of the treatment room includes wiping all surfaces to be used with an appropriate disinfectant. Governmental agencies catalog such disinfectants as low-level and intermediate-level disinfectants, and Table 6-1 provides examples. High-level disinfectants are cold sterilizers. Low-level disinfectants are for surfaces that are not contaminated by blood. In dentistry, blood contamination is likely, and therefore intermediate-level disinfectants seem appropriate. Many low-level disinfectants are the same chemicals found in higher-level disinfectants. Greater concentrations often allow a given chemical to be classed higher.
After disinfection of the treatment surfaces, dental assistants should wash their hands and reglove before proceeding with barrier placement and opening of sterile packs. Fresh barriers should be placed on light handles, dental unit switches, chair controls, and so on. X-ray generators and microscopes can be effectively isolated by using large plastic bags, such as dental chair covers or laundry bags. Other items for barrier consideration include electric pulp testers and other thermal pulp testers. Recycled anesthetic cartridges used for ice testing should be autoclaved before adding water and freezing.
Some people express concern that resistance to disinfectants may occur, as has happened with antibiotics. This is not likely because disinfectants (in appropriate concentration) kill rapidly, and thus bacteria are not able to adapt to them.
The current report of the CDC indicates that sterile packs will remain sterile if they remain dry in closed cabinets and if the sterile wrapping remains intact.12,21 This philosophy means that routine resterilization is not needed. Resterilization is indicated if the wrap has been exposed to fluids that may wick into the sterile side of the package or if an instrument has broken through the wrap. Packages should be dated for identification in case the biologic monitor shows incomplete spore kill.
An additional concern involves microbes in dental water lines. Chlorination or other municipal disinfection has brought a substantial improvement in public health in the last century. However, these steps do not ensure sterile water. Just as dental plaque bacteria colonize on teeth and cause problems, plastic water lines within the dental unit are a haven for bacteria to colonize. The sticky, dental plaque–like water-line biofilm grows unhindered by the slow water movement in dental water lines. Bacteria from patients can travel upstream into dental water lines. The relatively small numbers of bacteria allowable in municipal water supplies flourish within the dental unit, even in independent water bottles (Fig. 6-15). It is now known that biofilms establish themselves in these systems quickly. One approach to cleaning independent dental water lines is weekly use of strong chemicals, although staff compliance is a potential problem.27 Another approach is continuous use of low-concentration chemicals.10 Flushing water lines will not eliminate the biofilm, yet a 20- to 30-second flush between patients remains recommended to avoid microbes from one patient being transmitted to the next.12 Regular culturing of dental unit water lines remains the only method to ensure that a system meets the CDC and U.S. Environmental Protection Agency (EPA) no-more-than-minimum standard of 500 colony-forming units (CFU)/ml of water (Fig. 6-16).

FIG. 6-15 Independent water source. Water is pressurized by compressed air.
(Courtesy DentalEZ, Bay Minette, AL.)

FIG. 6-16 Waterline monitoring service. This laboratory uses U.S. Environmental Protection Agency (EPA) methods to determine heterotrophic bacterial counts for drinking water.
(Courtesy Dr. John Ruby, University of Alabama at Birmingham.)
The American Dental Association goal is less than 200 CFU/ml, and the European Union strives for less than 100 CFU/ml.
One can readily see that organization and quality control monitoring are essential to avoid contamination of the endodontic treatment field. Organization should be a key item in assembling the armamentarium, sterilization of instruments before treatment, and preparation of the treatment area with disinfectants and barriers. Attention to every detail is needed to avoid microbial contamination.
Treatment
Collection of a proper medical history is mandatory for proper treatment planning. Many examples illustrate the need for thorough consideration of medical aspects unique to each patient. For example, mutations of the tubercle bacillus, Mycobacterium tuberculosis, now endow it with resistance to multiple antibiotics. Infectious microscopic droplets hang in the air for hours after dental treatment, and this is a particular concern when treating patients with TB. These droplets pose a serious risk for staff and other patients. For public health reasons, patients with active TB must be treated in a special negative–air pressure room. Treatment room air must be vented outside through high-efficiency filters that are then properly treated. The use of pretreatment oral irrigants containing chlorhexidine gluconate has been recommended for reduction of microflora in aerosols.12
Patients with heart defects may also need special treatment.9 Oral bacteria that escape from the oral cavity or from an endodontic infection can cause serious complications or even initiate disease in healthy hearts.
Clothing worn by office personnel is a concern for staff safety. The purpose of protective fabric is to avoid contamination of skin and street clothes. Regulations mandate coverage of forearms. The garb should be changed if visibly soiled or wet by potentially infectious matter. The protective clothing should not be worn outside the office. Laundering at home is a potential source of contamination of family members and is not allowed.
Eye protection and masks should be donned before washing and gloving. Glasses and masks protect thin mucous membranes from possible contamination. Documentation of virus transmission via the conjunctiva of the eye has resulted in the recommendation for protection of these membranes.26 Risk of human immunodeficiency virus (HIV) transfer to a health care worker exposed by mucous membrane is estimated to be about 0.1%.15 Eyeglasses should have side shields.
Microscope controls should be covered with a barrier, such as a large plastic bag used for laundry or a dental chair cover. The plastic can be torn and stretched over the objective lens. The eyepieces should be adjusted for use with glasses (Fig. 6-17). Those who wear magnifying loupes should not adjust them during treatment unless followed by regloving. An assistant not involved with treatment may do so, or a barrier that can be immediately discarded may be used to adjust glasses or replace wet masks.
Masks commonly available for use in dentistry protect the wearer only partially. Small droplets containing bacteria can pass through most clinical masks. Wet masks are even less efficient and should be changed immediately. Masks that do not seal around the face fail to give maximal protection. The ideal mask has not yet been developed.
Dental treatment produces large amounts of potentially contaminated droplets during treatment. Rubber dam use reduces the aerosol, but infected pulp tissue can serve as a source for aerosol-borne biocontaminants. Most droplets from dental treatment quickly fall out of the air because of their relatively large size, but small particles can remain suspended in the air for long periods. Microbes are carried via these small droplets deep into lungs, where they cause infection. Airborne transmission definitely accounts for the spread of Legionnaires’ disease, TB, severe acute respiratory syndrome (SARS), and influenza. Other diseases may spread by droplets. Use of a preprocedural mouth rinse reduces the aerosol along with proper high-velocity evacuation common in large-bore dental evacuators.14,19
Hand washing should precede gloving. Long fingernails and jewelry that could puncture glove material or shelter bacteria from the washing process should be avoided. Recently, a new latex glove material has been tested. Apparently, guayule (pronounced why-YOU-lee; Yulex, Maricopa, AZ) rubber proteins have low allergenic potential. It is derived from a plant in the desert of southwestern United States.1
Sterile gloves should be worn for surgical procedures. Examination-type gloves may harbor microbes from the point of manufacture or from contamination at the point of use. Hand lotions may degrade the rubber of gloves and should not be used until treatment has concluded for the day. Sharp jewelry and artificial nails can penetrate the gloves, even if such penetrations are too small to be seen; thus they should not be worn with gloves. The glove material weakens during use, especially when using hand files. Gloves should be changed between patients and more often if harshly used. Interruption of treatment to answer the telephone or to develop a radiograph presents an opportune time for fresh gloves.
Evaluation of sterile technique often discloses contamination early in the treatment process. Procedures such as diagnosis or application of the rubber dam contaminate gloves. Gloves should be considered only a barrier for staff protection. Patient protection comes from careful attention to sterile technique, including not allowing nonsterile gloves to touch anything that will go into the root canal.
Because of the lack of total protection with gloves, it is recommended that a disinfectant hand wash be used. Chlorhexidine has a property called substantivity, meaning that it bonds to skin and maintains antibacterial action longer than other surgical scrubs. Alcohol-based hand rubs have recently become popular for hospital workers. Presently available waterless techniques appear less effective than a surgical soap-and-water method.7
Reducing bacteria within the oral cavity before treatment should be of concern to every clinician for every procedure. Degerming the oral cavity with an appropriate mouthwash before treatment has been recommended by Bender and Montgomery2 as a means of reducing bacteremia.11 Sterile technique should be used. Application of the rubber dam will typically contaminate gloves, and one approach is to change gloves after dam application. Another possibility is to have the assistant place the rubber dam and then reglove.20
Sharps should be handled with caution. Needles should be recapped with one hand to avoid possible injury to the hand holding the needle cover. Needles are available that recap automatically. Scalpel blades should be placed and removed from the handle by means of a hemostat or needle holder, with consideration to where hands are positioned, to avoid an injury in case something slips.
Disinfection of the canal continues to be a heavily researched topic. At present the only agent that dissolves pulp tissue is sodium hypochlorite. This explains its continued popularity among clinicians. Studies have demonstrated that 7 minutes of tissue contact time with sodium hypochlorite dissolves 75% of a tissue plug in vitro.6,13,17 Dilute concentrations of sodium hypochlorite are less effective for dissolving remaining tissue, and therefore the full-strength concentration is popular. However, it should be remembered that even dilute solutions can cause untoward reactions if injected periapically or exposed to oral tissues. Another clinical use for sodium hypochlorite is for disinfection of gutta-percha cones. A 1-minute exposure to 1% or a 5-minute exposure to 0.5% has been shown to be an effective gutta-percha disinfectant.3
Small instruments such as canal files may be dropped into a chairside container of disinfectant when no longer needed. This approach will provide some protection to staff members if they are accidentally stuck by an instrument in the cleaning and repackaging process. Such an approach will not positively ensure protection from infectious agents but will certainly reduce risk. Some clinicians have adopted the approach of using files as disposable instruments. Files require sterilization before treatment, except those few sterilized by the manufacturer.25 After single use, files can be discarded in the chairside sharps container. This philosophy of single use has the added advantage of reducing the possibility of fracture of instruments caused by repeated use (see also Chapters 8 and 9).
An often overlooked clinical problem concerns decontamination of radiographs. The use of digital radiography has improved patient protection with single-use sleeves covering the digital sensor. Proper technique dictates placing the barrier over the digital sensor when other barriers are placed before treatment commences. The barrier should completely cover the holder, or a sterilized holder or disposable holder should be used for each patient.
Conventional radiographic films or gloves worn from the treatment area into the developing area can contaminate this area. Staff should be required to disinfect conventional films and deglove before leaving the treatment room. Hand washing and regloving should occur before treatment continues.
Reusable instruments should be taken to a dedicated area for removal of gross debris by scrubbing or preferably by ultrasonic bath. Heavy gloves along with eye protection and a mask should be worn during instrument processing for sterilization. The ultrasonic bath should be a germicide to minimize infection spread to office workers. The ultrasonic bath cover should be used to reduce aerosols. Automatic instrument washers are also available for added cleaning efficiency and worker protection. Dedicated washers use copious amounts of high-temperature water to rapidly prepare instrument packs for autoclaving. The difficulty of thoroughly cleaning endodontic instruments was reported by researchers, who found that 76% of endodontic files from dental offices were contaminated with proteinaceous material.28
If time is not available to clean instruments immediately, they should be placed in a disinfectant bath to prevent drying of foreign material on the instruments because dried material is more difficult to remove.
If scrubbing is the only feasible means of cleaning, heavy puncture-resistant gloves should be worn and brushes should be long handled to minimize the risk of percutaneous injury. After the ultrasonic bath the instruments should be rinsed in tap water and wrapped for sterilization. Many offices favor cassette systems to lessen the risk of a stick injury. Staff should be cautioned about the risk of a sharp instrument protruding through an opening in a cassette, even one that is wrapped.
As instruments move along a path from dirty to clean and then sterile, the path should not cross itself in order to avoid cross-contamination.
General Considerations for the Office
Government guidelines spell out requirements for staff education.12 Staff meetings are strongly recommended because of the many advantages of well-educated personnel in the dental office. Proof of staff meetings should be kept in a log. Box 6-3 includes items for consideration.
BOX 6-3 Written Records for Consideration by Clinicians
Modified from Table 5 of Kohn WG, Collins AS, Cleveland JL, et al: Centers for Disease Control and Prevention (CDC): Guidelines for infection control in dental health-care settings—2003. MMWR Recomm Rep 52(RR-17):1, 2003.
Vaccinations for all employees working in the treatment room must include the hepatitis B series of three injections. Other vaccinations are recommended as a means of preventing disease. Such expense is minimal considering lost time and possible sequelae to valuable workers. Box 6-4 includes the 2005 vaccination recommendations of the CDC.12
It is now evident that antibody levels to hepatitis B wane over time. CDC findings suggest that 60% of those vaccinated will have no detectable antibodies after 12 years.4 Hepatitis B is a serious threat to unprotected individuals. The risk of clinical hepatitis B to an unvaccinated health care worker stuck with a needle from a patient positive for both hepatitis B surface antigen and hepatitis B e antigen is 22% to 31%.5
Janitors and those who dispose of dental office wastes must be protected from harm. They should be protected from sharps such as canal instruments that are misplaced in an area they will clean. Janitors need education in use of personal protective equipment, such as heavy gloves and appropriate disinfectants.
Dental offices do not generate large volumes of medical wastes.23 Dental offices are estimated to generate only 1% to 2% of their waste as medical waste. The majority of waste is not subject to regulation. Used gloves, masks, and protective garb and barriers are not regulated waste.
Sharps are part of regulated medical waste. Sharps include needles, scalpel blades, endodontic instruments, and other items capable of breaking the skin. Used anesthetic cartridges are classified as sharps because they could break and cause an injury if placed in regular waste. Sharps should be discarded at the point of use in a rigid container with a biohazard label. After sealing this puncture-resistant container with a tamper-proof seal, the container can be discarded in normal fashion.
Regulated medical waste includes items soaked in blood or saliva, such as 2 × 2 inch gauze or cotton rolls. This type of medical waste must be sealed in a leak-resistant biohazard bag that does not contain sharps. This type of waste could be placed in a sharps container. State regulations should be consulted before discarding biohazard bags in regular waste.
Liquid medical wastes, such as contents of a vacuum system separator, can be disposed of in a sanitary sewer, although care should be taken to avoid splatter when pouring into a toilet or sink. Normal protective gear including office garb, eyeglasses, and mask is needed for pouring liquid medical waste into a drain.
Some municipalities mandate mercury separators (endodontic offices may be exempt). These separators remove the heavy metal before it enters the city waste system and help the local government comply with regulations regarding mercury in solid waste and effluent from sewage treatment.
Nitrous oxide scavengers remove some of the gas exhaled by patients. These devices reduce but do not eliminate the gas from the office. Scavengers are connected to the central dental vacuum system and require a high flow rate. The vacuum system exhaust should be piped outside rather than into the mechanical room, where it may reenter the office air, because nitrous oxide interferes with deoxyribonucleic acid (DNA) synthesis. Further, nitrous oxide increases the rate of congenital abnormalities in children of both males and females exposed to the gas in the dental office environment.8
Future Considerations
Patient concerns will likely surface as more knowledge about existing pathogens becomes apparent and new pathogens arise. Clinicians should be ready to modify their practices and procedures to allay fears. Increased use of disposable products is likely. Many clinicians are already using new canal files for each patient.
A potential threat on the horizon are prions (PREE-onz). Prions are glycoproteins found on cell surfaces. Modified versions of normal prions cause various slow-moving, often fatal diseases of the nervous system. Prions are almost impossible to sterilize in a dental office and they have an affinity for stainless steel. They can be killed by certain caustic chemical treatments, such as sodium hydroxide. Current World Health Organization (WHO, Geneva, Switzerland) recommendations for reprocessing heat-resistant instruments call for keeping instruments moist until decontamination begins, because drying strongly stabilizes the bond of prions to the instruments. Prions are also resistant to formalin or glutaraldehyde fixatives, with their bond strengthened as well. The WHO describes several methods to rid dirty instruments of prions before repackaging and routine sterilization.33 The following methods, performed prior to routine sterilization, yield the best protection for office worker as well as patients.
Note that 1 N sodium hydroxide is made by adding 40 g of the crystal to 1 liter of distilled water.
Note that freshly opened 5.25% bleach (sodium hypochlorite) contains 25,000 ppm available chlorine. The WHO recommendations call for 20,000 ppm available chlorine. Today many commercial concentrations are 6%.
Research based on new technology will surely define more expeditious means of destroying prions. Investigations have explored the use of an industrial fabrication method, that is, low-pressure oxygen–argon plasma made by radiofrequency energy, to decontaminate and sterilize in one quick process. One study32 found organic matter, about 1 µm thick, on endodontic files that had been decontaminated and sterilized by a local dental practice. One third of the files had contaminant thicknesses exceeding 50 µm. After plasma exposure for unspecified times, no nitrogen was present, suggesting the destruction of proteinaceous matter. Calcium remaining was interpreted as lingering dentin.32 Although prions have high resistance to proteases, endopeptidases, such as keratinase, have been suggested as potential aids.22 Disposal of contaminated files is best accomplished by incineration.33
Because of fears of the public about disease transmission in the dental office, many endodontists consider all canal instruments as single-use items. This overcomes the difficulty of staff injury during cleaning and repackaging. Apparently, complete cleaning of endodontic files is quite difficult with current procedures. Another study reports that ultrasonic baths are better than hand brushing, and that disinfectant washers are also a step in the right direction, albeit not completely effective.30 Investigators found that even after cleaning by hand and ultrasonication, plus aggressive 24-hour immersion in disinfectants concentrated enough to corrode nickel–titanium files, residual debris remained.29
At present no vaccination exists against hepatitis C. Fortunately, the rate of disease transmission to health care workers is low. The estimates of disease transmission from a needle stick range up to 7%.24
Other hazards may also arise. Clinicians must keep abreast of research findings and changes in guidelines from regulatory agencies. New inventions and techniques will help clinicians deal with the current threats, as immunizations and innovations become available to make the workplace safer for staff and patients.
Air in the treatment room may contain spores. Although this has not been found to be problematic at present, it presents a possible concern for the future.
1. http://www.ada.org/prof/resources/pubs/adanews/adanewsarticle.asp?articleid=2991 Accessed August 30, 2009
2. Bender IB, Montgomery A. Nonsurgical endodontic procedures for the patient at risk for infective endocarditis and other systemic disorders. J Endod. 1986;12:400.
3. Cardoso CL, Kotaka CR, Redmerski R, Guilhermetti M, Queiroz AF. Rapid decontamination of gutta-percha cones with sodium hypochlorite. J Endod. 1999;25:498.
4. Centers for Disease Control and Prevention (CDC). Immunization of health-care workers: recommendations of the Advisory Committee on Immunization Practices and the Hospital Infection Control Practices Advisory Committee. MMWR Recomm Rep. 1997;46(RR-18):1.
5. Centers for Disease Control and Prevention (CDC). Updated U.S. Public Health Service guidelines for the management of occupational exposures to HBV, HCV, and HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep. 2001;50(RR-11):1.
6. Chu GT, Fleury AAP, Murray P, Flax MD. The ability of different concentrations of NaOCl to dissolve and reduce the weight of bovine pulp tissue in vitro [abstract PR6]. J Endod. 2004;30:266.
7. Clinton KD, Eleazer P. A comparison of bacteria on hands after alcohol based hand rub alone versus chlorhexidine hand scrub with water [abstract PR7]. J Endod. 2004;30:275.
8. Cohen EN, Brown BW, Bruce DL, et al. A survey of anesthetic health hazards among dentists. J Am Dent Assoc. 1975;90:1291.
9. Dajani AS, Taubert KA, Wilson W, et al. Prevention of bacterial endocarditis: recommendations of the American Heart Association. JAMA. 1997;277:1794.
10. Eleazer PD, Schuster GS, Weathers DW. Chemical regimen to reduce bacterial contamination in dental waterlines. J Am Dent Assoc. 1997;128:617.
11. Fine DH, Korik A, Furgang D, et al. Assessing pre-procedural subgingival irrigation and rinsing with an antiseptic mouthrinse to reduce bacteremia. J Am Dent Assoc. 1996;127:641.
12. Kohn WG, Collins AS, Cleveland JL, et al. Centers for Disease Control and Prevention (CDC): Guidelines for infection control in dental health-care settings—2003. MMWR Recomm Rep. 2003;52(RR-17):1.
13. Hand RE, Smith ML, Harrison JW. Analysis of the effect of dilution on the necrotic tissue dissolution property of sodium hypochlorite. J Endod. 1978;4:60.
14. Harrell SK, Molinari J. Aerosols and splatter in dentistry. J Am Dent Assoc. 2004;135:429.
15. Ippolito G, Puro V, De Carli G. The risk of occupational human immunodeficiency virus in health care workers. Ann Intern Med. 1993;153:1451.
16. Izu KH, Thomas SJ, Zhang P, Izu AE, Michalek S. Effectiveness of sodium hypochlorite in preventing inoculation of periapical tissues with contaminated patency files. J Endod. 2004;30:92.
17. Johnson BR, Munaretto R, Chruszczyk A, Daniel J, BeGole EA. Tissue solvent activity of high concentration NaOCl on bovine tendon collagen [abstract OR48]. J Endod. 2004;30:274.
18. Kolstad RA. How well does the Chemiclave sterilize handpieces? J Am Dent Assoc. 1998;129:985.
19. Logothethis DD, Martinez-Welles JM. Reducing bacterial aerosol contamination. J Am Dent Assoc. 1995;126:1634.
20. Luckey JB, Barfield RD, Eleazer PD. Bacterial count comparisons on examination gloves from freshly opened boxes versus nearly empty boxes and from examination gloves before treatment versus after dental dam isolation. J Endod. 2006;32:646-648.
21. Mayworm D. Sterile shelf life and expiration dating. J Hosp Supply Process Distrib. 1984;2:32.
22. McDonnell GE. Antisepsis, Disinfection, and Sterilization. Washington, DC: ASM Press; 2007. p 147
23. Palenik CJ. Managing medical waste in dental environments. J Contemp Dent Pract. 2003;4:76.
24. Puro V, Petrosillo N, Ippolito G. Risk of hepatitis C seroconversion after occupational exposures in health care workers: Italian Study Group on Occupation Risk of HIV and Other Bloodborne Infections. Am J Infect Control. 1995;23:273.
25. Roth TP, Whitney SI, Walker SG, Friedman S. Microbial contamination of endodontic files received from manufacturers. J Endod. 2006;32:649.
26. Sartori M, La Terra G, Aglietta M, Manzin A, Navino C, Verzetti G. Transmission of hepatitis C via blood splash into conjunctiva. Scand J Infect Dis. 1993;25:270.
27. Shepherd PA, Shojaei MA, Eleazer PD, Stewart AV, Staat RH. Clearance of biofilms from dental unit waterlines through the use of hydroperoxide-ion phase transfer catalysts. Quintessence Int. 2001;32:755.
28. Smith A, Dickson M, Aitken J, Bagg J. Contaminated dental instruments. J Hosp Infect. 2002;51(3):233-235.
29. Sonntag D, Peters OA. Effect of prion decontamination protocols in nickel–titanium rotary surfaces. J Endod. 2007;33(4):442-446.
30. Walker JT, Dickinson J, Sutton JM, Raven ND, Marsh PD. Cleanability of dental instruments: implications of residual protein and risks from Creutzfeldt-Jacob disease. Br Dent J. 2007;203(7):395-401.
31. Windeler AS, Walter RG. The sporicidal activity of glass bead sterilizers. J Endod. 1975;1:273.
32. Whittaker AG, Graham EM, Baxter RL, et al. Plasma cleaning of dental instruments. J Hosp Infect. 2004;56:37-41.
33. World Health Organization http://whqlibdoc.who.int/hq/2000/WHO_CDS_CSR_APH_2003.3.pdf Accessed 6 July, 2008