Endodontic Instruments
Although most instruments used in general dentistry also can be used for endodontic therapy, some hand instruments are designed specifically for endodontic procedures. In addition, many different types of instruments have been designed for procedures performed inside the pulp space. These include manually operated instruments for root canal preparation, rotary and other engine-driven and energized instruments for root canal preparation, and instruments for root canal obturation.
Standardized specifications have been established to improve instrument quality. For example, the International Standards Organization (ISO) has worked with the Fédération Dentaire Internationale (FDI) through the Technical Committee 106 Joint Working Group (TC-106 JWG-l) to define specifications. These standards are designated with an ISO number. The American Dental Association (ADA) also has been involved in this effort, as has the American National Standards Institute (ANSI); these standards are designated with an ANSI number. However, new instrument designs have resulted in a need for reconsideration of the standards.
Two ISO standards pertain to endodontic instruments. ISO No. 3630-1 deals with K-type files (as does ANSI No. 28), Hedström files (ANSI No. 58), and barbed broaches and rasps (ANSI No. 63). ISO No. 3630-3 deals with condensers, pluggers, and spreaders (ANSI No. 71).
Hand Instruments
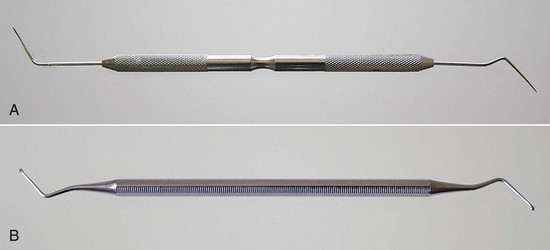
Traditional dental hand instruments have been modified for endodontic uses. A typical set of endodontic instruments might include a front-surface mouth mirror, a D-5 explorer, a D-16 endodontic explorer, cotton pliers, a spoon excavator, a series of pluggers, a plastic instrument, a hemostat, a periodontal probe, and a ruler. The endodontic explorer has two straight, very sharp ends that are angled in two different directions from the long axis of the instrument.
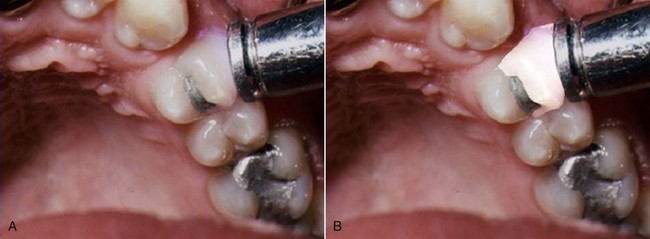
The mouth mirror should be a front-surface mirror, especially when magnifying loupes or an operating microscope are used. Several types of endodontic spoons are available. These spoons have a much longer offset from the long axis of the instrument (for better reach inside constricted pulp chambers) than regular dental spoons. The spoons are used to remove carious material and to excise pulp tissue; therefore, they should be kept well sharpened (Fig. 8-10). The cotton pliers should preferably be of the locking type that can securely hold gutta-percha cones when transferred from the dental assistant to the dentist’s hand. The periodontal probe should be a flexible one (see Fig. 8-5). The exact type and number of instruments usually depend on the techniques used and clinician’s preference.
Microinstruments
With the enhanced use of microscopes in endodontics, especially during apical surgery, many specially designed miniaturized instruments have been introduced. These include miniature mirrors, probes and pluggers (for details see Chapter 21).
Instruments for Cleaning and Shaping the Root Canal Space
The purposes of this section is to provide the principles necessary for the clinician to better understand the ideal design for current and future instruments. Most instructional materials mistakenly attempt to teach step-by-step techniques rather than explain the physics of the instruments. However, an increasing number of new products and their advocates have created confusion in the selection process. For this reason, the clinician must understand the basic mechanical, physical, and scientific principles of instrumentation (Box 8-1).
BOX 8-1 Terminology for the Physical Properties of Instruments
Successful use of an instrument depends on the ways the material, design, and technique relate to the forces exerted on the instrument. The following terms quantify the actions and reactions to these forces.
Stress: The deforming force measured across a given area.
Stress concentration point: An abrupt change in the geometric shape of a file, such as a notch, which results in a higher stress level at that point than along the surface of the file where the shape is more continuous.
Strain: The amount of deformation a file undergoes.
Elastic limit: A set value representing the maximal strain that, when applied to a file, allows the file to return to its original dimensions. After the strain is removed, the residual internal forces return to zero.
Elastic deformation: The reversible deformation that does not exceed the elastic limit.
Shape memory: A condition that exists when the elastic limit is substantially higher than is typical for conventional metals. It allows an instrument to regain its original form after being deformed.
Plastic deformation: Permanent bond displacement, which occurs when the elastic limit is exceeded. The file does not return to its original dimensions after strain is removed.
Plastic limit: The point at which a plastic-deformed file breaks.
The two primary goals of root canal instrumentation are (1) to provide a biologic environment (infection control) conducive to healing and (2) to develop a canal shape receptive to obturation. Historically, most instruments used to shape the canal were designed to be used by hand. Recently, rotary instrumentation has gained considerable popularity and may be used in conjunction with hand instruments (Box 8-2). The information in the following sections should facilitate the most efficient use of rotary instruments, minimizing the chance of instrument or procedural mishaps and allowing the clinician to achieve ideal treatment results.
BOX 8-2 Classification of Instruments Used for Cleaning and Shaping the Root Canal Space
Endodontic instruments for root canal preparation can be divided into six groups:
♦
Group I: Manually-operated instruments, such as barbed broaches and K-type and H-type instruments.
♦
Group II: Low-speed instruments with a latch-type attachment. Typical instruments in this group are Gates-Glidden (GG) burs and Peeso reamers. They are typically used in the coronal part of the canal and never used in a canal curvature.
♦
Group III: Engine-driven nickel-titanium rotary instruments. They consist of a rotating blade that can safely be operated in, and adapt itself to, curved root canals. Most engine-driven instruments available today belong to this group.
♦
Group IV: Engine-driven instruments that adapt themselves three-dimensionally to the shape of the root canal. Like other nickel-titanium instruments, they adapt to the shape of the root canal longitudinally but additionally they adapt also to the cross-section of the root canal. There is currently only one instrument in this group: the self-adjusting file (SAF; ReDent-Nova, Raanana, Israel).
♦
Group V: Engine-driven reciprocating instruments.
♦
Group VI: Ultrasonic instruments.
Group I: Manually Operated Instruments
Manually operated instruments are all instruments that are generically called files. Defining endodontic instruments by function, files are instruments that enlarge canals with reciprocal insertion and withdrawal motions. Reamers cut and enlarge canals with rotational motions. Before using either instrument, the clinician must make sure the canal is patent.
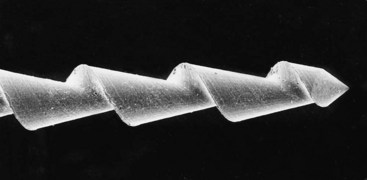
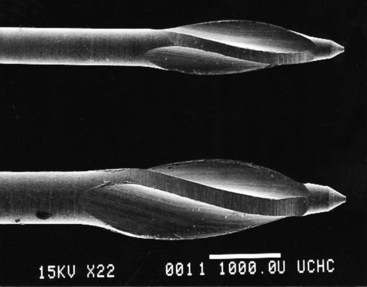
Files were first mass produced by the Kerr Manufacturing Co. of Romulus, Michigan, in the early 1900s, hence the name K-type file (or K-file) and K-type reamer (K-reamer). K-files and K-reamers originally were manufactured by the same process. Three or four equilateral, flat surfaces were ground at increasing depths on the sides of a piece of wire, producing a tapered pyramidal shape. The wire then was stabilized on one end, and the distal end was rotated to form the spiral instrument (Fig. 8-11). The number of sides and the number of spirals determine whether the instrument is best suited for filing or reaming. Generally, a three-sided configuration with fewer spirals is used for reaming; a three- or four-sided configuration with more spirals is used for filing.
Historically, root canal instruments were manufactured from carbon steel. Subsequently, the use of stainless steel greatly improved the quality of instruments. More recently, the introduction of the nickel-titanium (NiTi) alloy in the manufacture of endodontic instruments has resulted in significant improvements in canal shaping because of its increased flexibility compared to stainless steel (this metal is described later in the chapter).
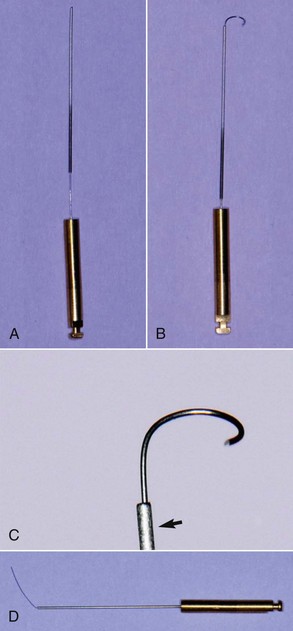
Barbed Broaches and Rasps
Dating from the early to mid-19th century, broaches and rasps were the earliest endodontic instruments used to extirpate the pulp and enlarge the canal (Fig. 8-12). Still used today, these instruments are manufactured by hacking a round, tapered wire with a blade to form sharp, projecting barbs that cut or snag tissue. Specifications have been set for both the barbed broach and the rasp (ANSI/ADA standard No. 63, ISO/FDI standard No. 3630/1).
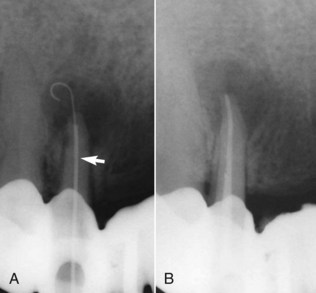
A barbed broach does not cut or machine dentin; this instrument is mostly used to engage and remove soft tissue from the canal. It is also an excellent tool for removing cotton or paper points that have accidentally become lodged in the root canal.
K-Type Instruments
The K-file and K-reamer are the oldest useful instruments for cutting and machining dentin (Fig. 8-13). They are made from a stainless steel wire that is ground to a tapered square or triangular cross-section and then twisted to create either a file or a reamer. A file has more flutes (see Components of a File) per length unit than a reamer.
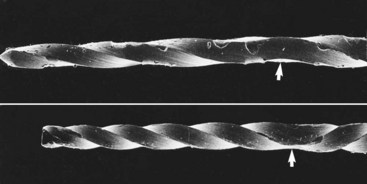
K-type instruments are useful for penetrating and enlarging root canals. The instrument works primarily by compression-and-release destruction of the dentin surrounding the canal. Generally, a reaming motion (i.e., constant file rotation) causes less transportation than a filing motion (reciprocating or “watch-winding” file rotation).406 (Transportation is the excessive loss of dentin from the outer wall of a curved canal in the apical segment. This procedural error can lead to perforation of the root canal system or an inability to negotiate the canal space apical to the canal transportation.) A stainless steel K-file can be precurved to a desired form to facilitate insertion and minimize transportation. Permanent deformation occurs when the flutes become wound more tightly or opened more widely (Fig. 8-14). When such deformation occurs, the instrument should no longer be used. Instruments fracture during clockwise motion after plastic deformation.158,345 This occurs when the instrument becomes bound while the force of rotation continues. Interestingly, although the force required for failure is the same in both directions of rotation,205,213 failure occurs in the counterclockwise direction at half the number of rotations required for failure in the clockwise direction. Therefore K-type instruments should be operated more carefully when pressure is applied in a counterclockwise direction.
H-Type Instruments
An H-type instrument has spiral edges arranged to allow cutting only during a pulling stroke (Fig. 8-15). An example is a Hedström file. An H-type instrument is better for cutting than a K-type instrument, because it has a more positive rake angle (see Components of a File) and a blade with a cutting rather than a scraping angle. Bending a Hedström file results in points of greater stress concentration than occurs with K-type instruments. These concentration points can lead to the propagation of cracks and fatigue failure.158 Clinically, fatigue happens without any external physical signs of stress, such as the flute changes seen in K-type instruments (see Fig. 8-14).
Currently all H-type instruments are ground from a tapered blank. Hedström files are formed by grinding a single continuous flute. Computer-assisted machining technology has allowed the development of H-type instruments with very complex forms. This process, called multiaxis grinding, allows adjustment of the rake angle, helix angle, multiple flutes, and tapers. H-files cut the canal wall when pulled or rotated clockwise; the file is relatively ineffective when pushed or rotated counterclockwise. Because the H-file generally has sharper edges than the K-file, it has a tendency to screw into the canal during rotation, particularly if the instrument’s blades are nearly parallel. Awareness of screwing-in forces is important for avoiding instrument failure.
Instrument Design Modifications
K-files and H-files can be modified into numerous designs. Often the instruments can be improved for more effective instrumentation by changing the geometric dimensions, using computerized multiaxis grinding machines. For example, changing the cross-sectional geometry of a K-type instrument from square to rhomboid enhances the instrument’s flexibility and rake angle. However, the possible geometries can complicate adherence to ISO and ANSI standards.
Tip Design
Studies have shown that tip design can affect file control, efficiency, and outcome in the shaping of root canal systems.266,267 The tip of the original K-file resembled a pyramid (see Fig. 8-13). Instrument tips have been described as cutting, noncutting, and partially cutting, although no clear distinction exists among the three types (Fig. 8-16).
The instrument tip has two functions: to guide the file through the canal and to enlarge the canal. A clinician who is unfamiliar with the tip design of a particular instrument is apt to do either of the following: (1) transport the canal (if the tip is capable of enlarging the canal and remains too long in one position) or (2) encounter excessive torsion and break the file (if a noncutting tip is forced into a canal with a smaller diameter than the tip). Transportation of the original axis of the canal can occur by remaining too long in a curved canal with a tip that has efficient cutting ability.
The angle and radius of its leading edge and the proximity of the flute to its actual tip end determines the cutting ability of a file tip. Cutting ability and file rigidity determine the propensity to transport the canal. The clinician must keep in mind that as long as the file is engaged 360 degrees, canal transportation is unlikely to occur. Only with overuse does the file begin to cut on one side, resulting in transportation. Most instrumentation errors occur when the file tip is loose in the canal, which gives it a propensity to transport the canal.
A good beginner’s rule is this: If the canal is smaller than the file, the prudent use of a cutting tip is more efficient. If the canal is larger than the tip, using a less-effective cutting tip can help prevent transportation (see Fig. 8-16). Much has been written about the importance of various sophisticated tip modifications to prevent such ledging, but little scientific evidence exists that any one design is better than another.190,308,309,316,321
Metal Alloys
The development of nitinol, an equiatomic alloy composed of nickel and titanium,412 has proved a significant advancement in the manufacture of endodontic instruments. NiTi is called an exotic metal because it does not conform to the typical rules of metallurgy. Because it is a superelastic metal, the application of stress does not result in the usual proportional strain seen in other metals such as stainless steel. When stress is initially applied to NiTi, the result is proportional strain, but the strain remains essentially the same as the application of additional stress reaches a specific level, forming what is called a loading plateau. Eventually, of course, application of more stress results in more strain, which increases until the file breaks. This unusual property is the result of a molecular crystalline phase transformation. External stresses transform the austenitic crystalline form of NiTi into a martensitic crystalline structure that can accommodate greater stress without increasing the strain. As a result of its unique crystalline structure, a NiTi file has superelasticity, or the ability to return to its original shape after being deformed. Simply stated, NiTi alloys currently are the only readily available affordable materials with the flexibility and toughness for routine use as effective rotary endodontic files in curved canals.
Attempts to improve the NiTi alloy continue, and recent reports indicate that new NiTi alloys may be five times more flexible than currently used alloys.186 Microscopic surface defects are considered a contributing factor for crack propagation and instrument fracture. Therefore, attempts to improve surface characteristics by electropolishing, surface coatings, and surface implantation have been used for this purpose.216,310
Group II: Low-Speed Rotary Instruments
Many types of rotary instruments are used during endodontic procedures. In addition to regular burs adapted for endodontics, various types of root canal reamers are used to shape the canal space, to place or remove root canal filling materials, or to prepare a post space.
Burs
In addition to conventional burs, burs with extended shanks for low-speed contra-angle handpieces are useful for providing good visibility during deep preparation of the pulp chamber. This is particularly important when using an operating microscope when performing such procedures after access to the pulp chamber has been achieved. Straight-line access to the initial point of curvature traditionally has been accomplished using rotary instruments such as Gates-Glidden burs and Peeso instruments. These reamers are available in a 32-mm length and a 28-mm length for posterior teeth (Figs. 8-17, 8-18). Use of these instruments should be limited to the straight portion of the canal preparation. The risk of perforation with these instruments becomes a real possibility with attempts to instrument beyond the point of curvature or if the instruments are used to cut laterally. The risk of lateral cutting resulting in perforation is lower with Gates-Glidden burs than with the other instruments mentioned. This risk is especially pronounced on the furcation sides of mesial roots of molars. Gates-Glidden instruments are also available in nickel-titanium. The Peeso reamer is used mostly for post space preparation (Fig. 8-19).
Group III: Rotary Instruments for Canal Preparation
Components of a File
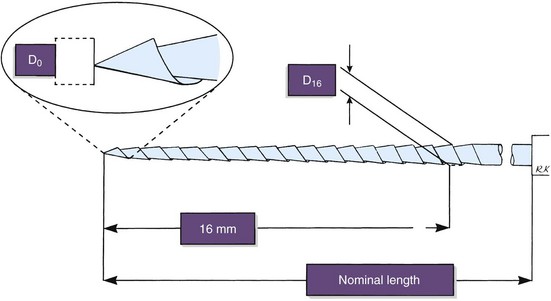
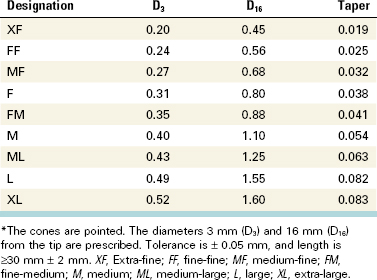
To make the best use of files, the clinician should be familiar with the parts of each file and understand how variations in design affect instrumentation (Fig. 8-20). The taper usually is expressed as the amount the file diameter increases each millimeter along its working surface from the tip toward the file handle. For example, a size #25 file with a #.02 taper would have a 0.27 mm diameter 1 mm from the tip, a 0.29 mm diameter 2 mm from the tip, and a 0.31 mm diameter 3 mm from the tip. Some manufacturers express the taper in terms of percentage (e.g., a #.02 taper is a 2% taper). Historically, as an ISO standard, a file was fluted and tapered at 2% for 16 mm, but now files incorporate a wide variation of lengths and tapers of working surfaces. The ability to determine cross-sectional diameter at a given point on a file can help the clinician determine the file size in the point of curvature and the relative stress being placed on the instrument.
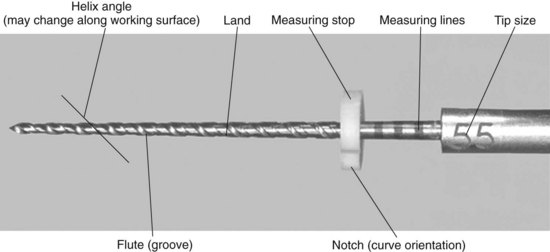
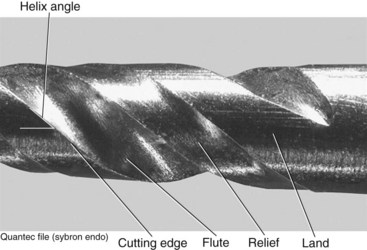
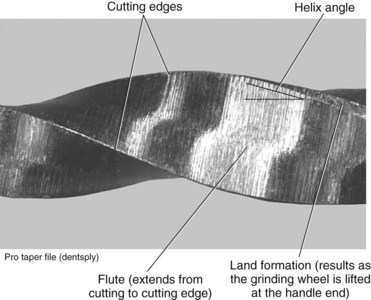
The flute of the file is the groove in the working surface used to collect soft tissue and dentin chips removed from the wall of the canal. The effectiveness of the flute depends on its depth, width, configuration, and surface finish. The surface with the greatest diameter that follows the groove (where the flute and land intersect) as it rotates forms the leading (cutting) edge, or the blade of the file. The cutting edge forms and deflects chips from the wall of the canal and severs or snags soft tissue. Its effectiveness depends on its angle of incidence and sharpness. If a surface projects axially from the central axis as far as the cutting edge between flutes, this surface is called the land (or sometimes the marginal width) (Figs. 8-21 and 8-22). The land touches the canal walls at the periphery of the file and reduces the tendency of the file to screw into the canal, reduces transportation of the canal, reduces the propagation of microcracks on its circumference, supports the cutting edge, and limits the depth of cut. Its position relative to the opposing cutting edge and its width determine its effectiveness. To reduce frictional resistance, some of the surface area of the land that rotates against the canal wall may be reduced to form the relief (Fig. 8-23). The angle the cutting edge forms with the long axis of the file, called the helix angle, augers debris collected in the flute from the canal. This angle is important for determining which file technique to use (see Fig. 8-23; Fig. 8-24).
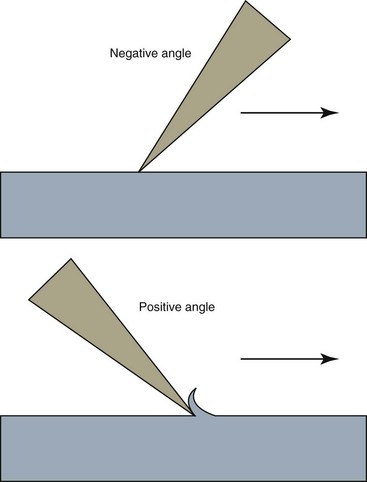
If a file is sectioned perpendicular to its long axis, the rake angle is the angle formed by the leading edge and the radius of the file. If the angle formed by the leading edge and the surface to be cut (its tangent) is obtuse, the rake angle is said to be positive or cutting. If the angle formed by the leading edge and the surface to be cut is acute, the rake angle is said to be negative or scraping (Fig. 8-25). However, the rake angle may not be the same as the cutting angle. The cutting angle, or the effective rake angle, is a better indication of a file’s cutting ability and is determined by measuring the angle formed by the cutting (leading) edge and the radius when the file is sectioned perpendicular to the cutting edge. If the flutes of the file are symmetric, the rake angle and the cutting angle are essentially the same.
The pitch of the file is the distance between a point on the leading edge and the corresponding point on the adjacent leading edge, or it may be the distance between corresponding points within which the pattern is not repeated. The smaller the pitch or the shorter the distance between corresponding points, the more spirals the file has and the greater the helix angle. Most files have a variable pitch, one that changes along the working surface. Because the diameter increases from the file tip toward the handle, the flute becomes proportionately deeper, resulting in a core taper that is different from the external taper.
The cutting angles, helix angles, and external and core tapers may vary along the working surface of the file, and the ratios of these quantities can vary between instruments of the same series. A change in any of these features can influence the file’s effectiveness or its propensity for breakage as it progresses into the canal space; this can explain why some files act uncharacteristically compared with other files in the same series. In one study, investigators using electric and air-driven handpieces with rotary NiTi instruments found no significant difference in file distortion or breakage between the two handpieces at 150 revolutions per minute (rpm).40 Other researchers have shown that the ability to select precise rpm71 and torque122 settings affects the efficiency and durability of instruments (Fig. 8-26). Determining and maintaining a file’s proper rotational speed is more difficult with an air handpiece than with an electric handpiece. For this reason, the clinician would be wise to use an electric handpiece when instrumenting with rotary files. The popularity of electric handpieces among clinicians appears to support the conclusion that regardless of the design used for rotary NiTi instruments, an electric handpiece—rather than an air-driven handpiece—should be used because it allows precise speed and torque control (see Fig. 8-26).
Instrument Designs
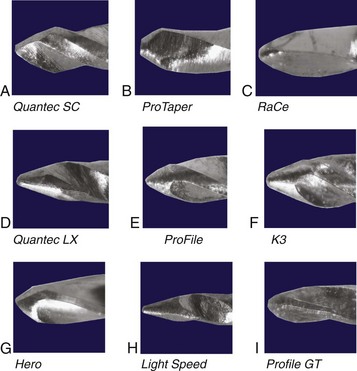
Design changes are made in endodontic instruments to help prevent procedural errors, increase efficiency, and improve the quality of canal shaping. Examples of instruments commonly used today are the ProFile and ProFile GT, the ProTaper, the Lightspeed LSX, the Quantec, the Twisted File (TF),123,212 the RaCe, the EndoSequence, and the EZ-Fill SafeSider. For more information about these instruments and others, see Chapter 9. In many patients, the apical canal is larger than the largest file used at working length, so many design changes have been directed toward enabling the clinician to increase the size of the largest file used at working length. The following design components can be used to prevent excess stress on instruments.
1.
The difference between the file’s minimum and maximum diameters can be reduced so that the torque required for rotating the larger diameter does not exceed the plastic limit of the smaller diameter.
2.
The space between the tip and the maximum diameter can be reduced so that the required torque does not exceed the ultimate strength of any part of the file.
3.
A zero taper or nearly parallel and fluted working portion of the file can be provided for curved canals so that the apical portion of the canal can be enlarged without undue file stress and compression of debris.
4.
The continuity of the blade engagement can be interrupted.
5.
The number of flute spirals can be eliminated or reduced to the smallest number necessary to prevent excessive torque, which results from the accumulation of debris.
6.
A means can be provided to complete the file function before the flutes fill with debris.
7.
Any land width can be minimized to reduce abrasion on the canal surface.
8.
The file can be given an asymmetric cross-section to help maintain the central axis of the canal.
9.
The number of flutes with similar helix angles can be reduced. When helix angles are dissimilar, screwing-in forces are reduced; when flutes have no helix angles, screwing-in forces are eliminated.
10.
Positive cutting angles can be incorporated to enhance the efficiency of canal enlargement.
11.
Blades can be made appendages or projections from the file shaft rather than ground into the shaft.
12.
Channels can be cut along the long axis of the file to facilitate its removal if it breaks.
Group IV: Engine-Driven Three-Dimensionally Adjusting Files
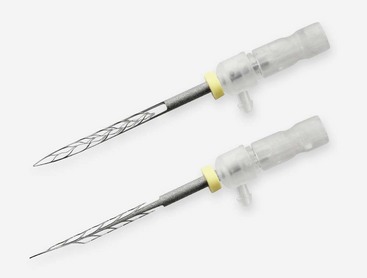
Self-Adjusting File
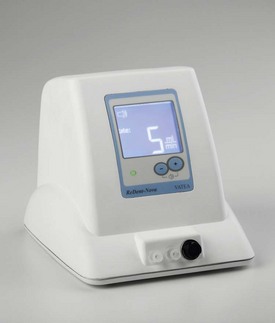
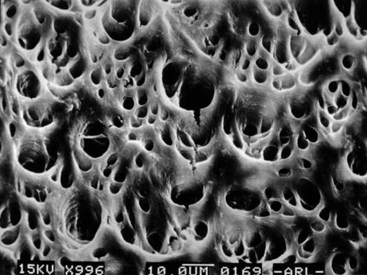
The self-adjusting file (SAF; ReDent-Nova, Raanana, Israel) represents a new approach in file design and mode of operation.260 The file is a hollow device, designed as a cylinder of thin-walled, delicate NiTi lattice with a lightly abrasive surface (Figs. 8-27 and 8-28). An initial glidepath is established with a #20 K-file to allow the insertion of the SAF file. The file is proposed to be compressed from its 1.5 mm diameter into dimensions equivalent to those of a #25 K-file (Fig. 8-29). It is operated with a modified KaVo handpiece (GENTLEpower 20LP with a 3LDSY head, KaVo Dental GmbH, Biberach/Riss, Germany) that generates in-and-out vibrations with 5000 vibrations per minute and 0.4 mm amplitude (Fig. 8-30). The overall concept is that the compressed file will adapts itself to the root canal walls, applying a uniform cutting action (see Fig. 8-28) gradually removing a uniform dentin layer from the canal walls.
As noted, the file is hollow, which allows for continuous irrigation through the file while operated in the root canal. The irrigant is delivered through a free-rotating hub to which a silicone tube is attached (see Fig. 8-30). Either a special irrigation unit (Fig. 8-31) (VATEA, ReDent-Nova, Raanana, Israel) or any appropriate dispenser-type unit may be used to deliver a constant flow of irrigant at 5 ml/min. This maintains a continuous flow of fresh, fully active irrigant that carries with its outflow tissue debris and the dentin powder generated by the file.256
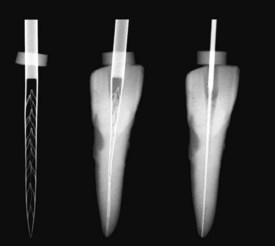
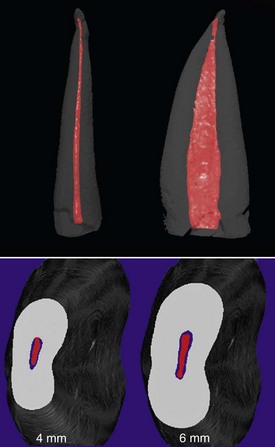
One file is used throughout the procedure. It is initially compressed into the root canal (see Fig. 8-29) and gradually enlarges while cleaning and shaping the canal. The unique feature of this file is that it adapts to the shape of the canal not only longitudinally, as every NiTi file does, but also to the cross-section of the canal. Consequently, the basic shape of the root canal is preserved: a root canal with a round cross-section is enlarged to one with a similar but larger cross-section, whereas a flat root canal will be enlarged to a flat root canal of bigger dimensions (see Fig. 8-31). This unique feature of the SAF file makes it different from any other currently available file system.
Because it has no rigid metal core, the file is also extremely adaptable longitudinally. The inherent tendency to straighten curved root canals and transport their apical part to the outer side of the curvature, which is typical of most other file systems, is greatly avoided. The absence of a metal core also makes the SAF extremely resistant to fracture. File separation—a major problem with other NiTi files—has not been reported to occur, and mechanical failure (a rare occurrence if it happens) is limited to local tears in the delicate NiTi lattice.260 It is clear that this interesting conceptual device will be subjected to extensive independent study over the next few years.
Group V: Engine-Driven Reciprocating Instruments
Endo-Eze Reciprocating Files
The Giromatic handpiece, a rotary instrument in use since 1969, delivers 3000 quarter-turn reciprocating movements per minute. Rasps and barbed broaches are most often used in Giromatic handpieces, but K-type and H-type instruments also can be used. The Endo-Eze file system (Ultradent, South Jordan, UT) is a recently introduced addition for Giromatic handpieces. The set has four instruments that are designed to clean the middle third of the canal. The sizes and tapers are 0.10 #0.025 taper, 0.13 #0.35 taper, 0.13 #0.45 taper and 0.13 #0.06 taper. The use of stainless steel hand instruments is suggested for the apical third of the canal.
Group VI: Sonic and Ultrasonic Instruments
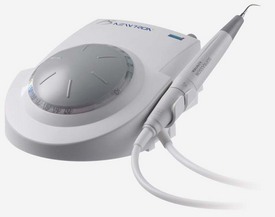
A radically different way of instrumenting root canals was introduced when clinicians became able to activate files by electromagnetic ultrasonic energy. Piezoelectric ultrasonic units are also available for this purpose (Fig. 8-32). These units activate an oscillating sinusoidal wave in the file with a frequency of about 30 kHz.
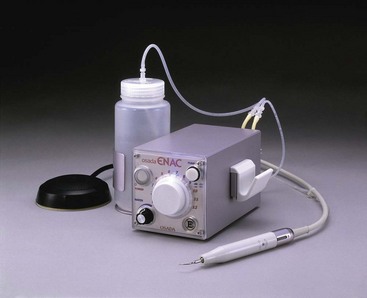
Two types of units, ultrasonic and sonic, are primarily available. Ultrasonic devices, which operate at 25 to 30 kHz, include the magnetostrictive Cavi-Endo (DENTSPLY Caulk, Milford, DE), the piezoelectric ENAC (Osada, Tokyo) (Fig. 8-33), the EMS Piezon Master 400 (Electro Medical Systems [EMS] Vallée de Joux, Switzerland), and the P5 Neutron (see Fig. 8-32) (Satelec, Merignac Cedex, France). Sonic devices, which operate at 2 to 3 kHz, include the Sonic Air MM 1500 (Micro Mega, Prodonta, Geneva, Switzerland), the Megasonic 1400 (Megasonic Corp, House Springs, MO), and the Endostar (Syntex Dental Products, Valley Forge, PA). Ultrasonic devices use regular types of instruments (e.g., K-files), whereas sonic devices use special instruments known as Rispi-Sonic, Shaper-Sonic, Trio-Sonic, or Heli-Sonic files.
Although similar in function, piezoelectric units have some advantages over the magnetostrictive systems. For example, piezoelectric devices generate little heat, so no cooling is needed for the electric handpiece. The magnetostrictive system generates considerable heat, and a special cooling system is needed in addition to the irrigation system for the root canal. The piezoelectric transducer transfers more energy to the file than does the magnetostrictive system, making it more powerful. Working with no water cooling becomes essential when using an operating microscope, because the water spray may obstruct visualization.
The file in an ultrasonic device vibrates in a sinus wave–like fashion. A standing wave has areas with maximal displacement (i.e., antinodes) and areas with no displacement (i.e., nodes). The tip of the instrument exhibits an antinode. If powered too high, especially with no contact with the canal wall, the instrument may break because of the intense vibration. Therefore files must be used only for a short time, must remain passive within the canal, and the power must be controlled carefully. The frequency of breakage in files used for longer than 10 minutes may be as high as 10%, and the breakage normally occurs at the nodes of vibrations.5
Ultrasonic devices have proved very efficient for irrigating root canal systems. During free ultrasonic vibration in a fluid, two significant physical effects are observed: cavitation and acoustic streaming. During oscillation in a fluid, a positive pressure is followed by a negative pressure. If the file’s tensile strength is exceeded during this oscillation of pressure gradients, a cavity is formed in the fluid in the negative phase. During the next positive-pressure phase, the cavity implodes with great force; this is cavitation. Under normal clinical conditions, the power of dental ultrasonic units is too low to create significant cavitation effects on the dentin walls. Acoustic streaming creates small, intense, circular fluid movement (i.e., eddy flow) around the instruments. The eddying occurs closer to the tip than in the coronal end of the file, with an apically directed flow at the tip. Acoustic streaming increases the cleaning effect of the irrigant in the pulp space through hydrodynamic shear stress. The increased amplitude that occurs with the smaller file sizes enhances the acoustic streaming.6 This has proved valuable in the cleaning of root canals because conventional irrigation solutions do not penetrate small spaces well.
Acoustic streaming has little direct antimicrobial effect. Both cavitation and acoustic streaming are dependent on the free vibration of the file. The limits of the space in a root canal significantly inhibit the practical utility of ultrasonic devices for root canal cleaning. Depending on size and power, the file tip may have amplitudes that require a canal size of at least a #30 file through a #40 file for free oscillation. Any contact with the root canal walls dampens oscillation. As the contact with the canal wall increases, the oscillation is dampened and becomes too weak to maintain acoustic streaming. Using a small file size with minimal contact to the root canal wall provides optimal cleaning conditions.7
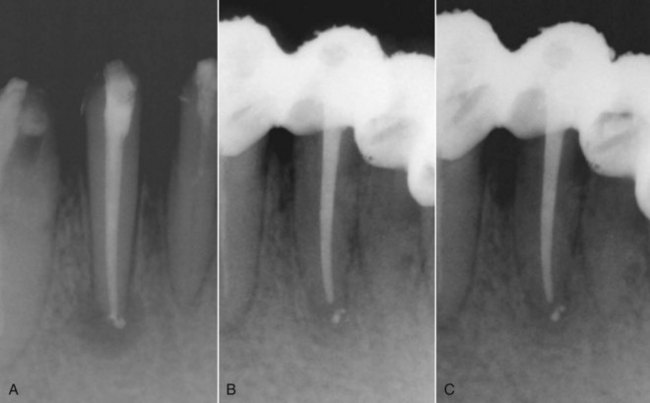
Ultrasonic devices have proved disappointing as instruments for improving the removal of dentin from the root canal walls. They do improve the ability to clean the pulp space (Fig. 8-34) and difficult-to-débride areas through acoustic streaming.12,68,402,403 However, it is unclear whether this can be achieved during regular preparation when the file is actively dampened and little acoustic streaming takes place. Cleaning is further enhanced by the excellent irrigation systems some of the devices provide. Application of a freely oscillating file with sodium hypochlorite (NaOCl) irrigation for several minutes to aid pulp space disinfection is believed to be useful after complete biomechanical instrumentation of the pulp space.361
Sonic devices are more useful for true hard-tissue removal during root canal preparation. Because the files operate like a conventional handpiece, the file vibrations are less likely to be dampened by contact with the root canal walls. Therefore the special files used in these systems are true bulk dentin removers. New tip designs for piezoelectric ultrasonic units have also been found extremely effective in dentin removal in the pulp chamber and orifice of the canals (see Fig. 8-34).
National and International Standards for Instruments
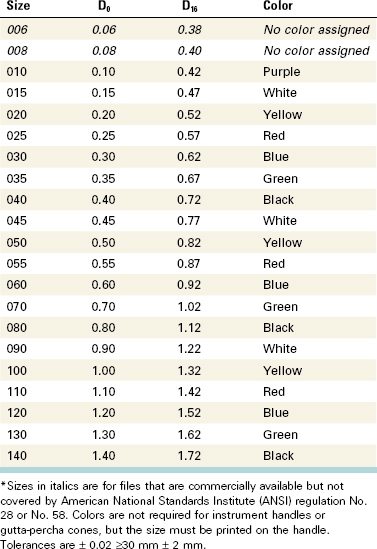
As a result of concerns that arose nearly 45 years ago, efforts were made to standardize endodontic files and root filling materials. As mentioned previously, this resulted in an international standard for endodontic files, known in the United States as ANSI standard No. 58 for Hedström files and ANSI standard No. 28 for K-files (Table 8-1). The standards have several similarities, but some important differences exist. Fig. 8-35 shows the measurements dictated by the standards. The size designation is derived from the projected diameter at the tip of the instrument. This is an imaginary measurement and is not reflected in the real size of the working part of the instrument. The taper of the instruments is designed to be a 0.02 taper, namely a diameter-increase by .02 mm for each mm of length, starting at the tip. The working diameter is the product of the taper and the length of the tip. Three standard lengths are available at 21 mm, 25 mm, and 31 mm. The working part of the instrument must be at least 16 mm. As stated previously, tapers other than .02 and working parts of instruments less than 16 mm are now available and outside the standard.
This system of numbering files with at least 15 different sizes replaced the old, somewhat imperfect system that numbered the sizes from #1 through #6. Although the new standard includes many sizes, astute clinicians may include fewer instrument sizes for their special work habits.
In recent years, suggestions to change the numbering system for files with different sizes have been implemented by several manufacturers. One system has introduced “half” sizes in the range of #15 through #60, resulting in instruments in sizes #15, #17.5, #20, #22.5, and so on. Considering the fact that most manufacturers already are unable to size their instruments within the accepted range, the introduction of half sizes seems unnecessary. However, if standards are strictly adhered to, the use of half sizes seems more reasonable for instrument systems such as the LightSpeed, in which the strength of the instrument is such that full-size increments may generate stresses beyond the tolerance of the instrument. The standards are overdue for reevaluation in light of recent technologic changes.
Effectiveness and Wear of Instruments
Although the advertising literature is rich in claims of superiority of various file designs, few of these claims can be verified by well-designed studies in the peer-reviewed endodontic literature. No standards exist for either the cutting or machining effectiveness of endodontic files, nor have clear requirements been established for resistance to wear.
In any study of the effectiveness of an instrument, two factors must be investigated: (1) effectiveness in cutting or breaking loose dentin and (2) effectiveness in machining dentin. These two parameters are radically different. Methods exist for measuring machining, but currently no good method is available for measuring cutting. Some studies have attempted to evaluate cutting, but the methodologies have involved the use of a drilling motion with K-type instruments and at a speed higher than that used for clinical procedures.
Some studies of machining have evaluated the effectiveness of an instrument when used with a linear movement. These studies showed that instruments can differ significantly, not only when comparing brands and types but also within one brand and type. For K-files, effectiveness varies 2 to 12 times between files of the same brand. The variation for Hedström files is greater, ranging from 2.5 to more than 50 times.379 The greater variation among Hedström files is easy to understand because the H-file is the result of more individual grinding during manufacture than the conventional K-file, which is difficult to alter much during the manufacturing process. For example, during the grinding of a Hedström file, the rake angle can be modified to neutral or even slightly positive; this is impossible to achieve with a K-file. The Hedström file, therefore, is approximately 10 times more effective at removing dentin than the K-file.
In the machining process, the rake edge shaves off dentin that accumulates in the grooves between the rake edges. The deeper and larger this space, the longer the stroke can be before the instrument is riding on its own debris, making it ineffective. These design variations and the rake angle of the edges determine the effectiveness of a Hedström file. Of the hybrid files, the K-Flex (SybronEndo, Orange, CA) file, which is a modified K-file, shows variables similar to those of K-files. The Flex-R file (SybronEndo), which is a ground instrument, more closely resembles the H-files in its variation in effectiveness. It also is much more effective at substrate removal than the K-files but cannot measure up to the H-files’ ability to machine.
Modern endodontic stainless steel instruments are fabricated from excellent metal alloys and have considerable resistance to fracture. The clinician who practices careful application of force and a strict program of discarding instruments after use should have few instrument fractures. Stainless steel files are so inexpensive that adequate cleaning and sterilization for reuse of files in sizes up to #60 may not be cost effective. If this is the case, files in the range up to #60 may be considered disposable instruments.
Devices for Periapical Débridement Through a Root Canal Access
The Apexum device (Apexum, Or-Yehuda, Israel) is currently the single representative of this group258,259 (Fig. 8-36). It represents a totally different concept than that applied by most root canal cleaning and shaping systems. The goals of most current concepts are to maintain the original apical constriction by all means and to avoid any substantial overinstrumentation past the apical foramen. The Apexum concept requires widening the apical foramen to create an opening 330 µm in diameter by passing a #30 file 1 mm beyond the apex. This may represent some enlargement of the apical foramen in certain cases, but in other cases, the natural apical foramen diameter may be larger than this.43 Further instrumentation with larger instruments (#40 or #45) to a length 1 mm short of the apical foramen are then used to recreate an artificial apical stop and constriction.
Two miniature devices are then used to débride the periapical tissues through the root canal access. The first is the Coarse Apexum Ablator, which is made from a prebent memory-shape NiTi wire encased in a NiTi tube that serves as its sheath (see Fig. 8-36). The instrument, still encased in its sheath, is inserted to the artificial apical stop. Then the inner wire is gently pushed through the apical foramen and into the periapical lesion.
While in the periapical area, it regains its prebent shape (Figs. 8-37 and 8-38). It is then attached to a low-speed handpiece and rotated at 250 rpm for 30 seconds, thus coarsely mincing the periapical tissues. The first instrument is then removed, and the second instrument, the Fine Apexum Ablator, is inserted, passed through the apical foramen, and rotated at 7000 rpm for 30 seconds, turning the tissue into a thin suspension. The resulting suspension is then washed out coronally through the canal space using sterile saline solution delivered into the periapical area using a syringe and a thin, 30-gauge blunt needle.
The method is used as a supplementary treatment for enhancement the healing kinetics of periapical lesions for promoting faster healing (Fig. 8-39).258,259 The Apexum procedure is applied only after completion of cleaning, shaping, and disinfection of the root canal and before root canal obturation. This interesting device will clearly be subjected to additional research over the next few years.
Devices for Measuring Root Canal Length
Radiographs, tactile sensation, the presence of bleeding on paper points, and knowledge of root morphology have been used to determine the length of root canal systems. Sunada381 developed the original electronic apex locator when he suggested that the apical foramen could be localized using a direct electric current. Currently the electronic apex locator is considered an accurate tool for determining working length.114,115 One study reported that the use of electronic apex locators in a dental student clinic resulted in a higher quality of obturation length control and an overall reduction in the number of radiographs taken.115 However, these devices must not be considered flawless, because several variables are known to affect their accuracy. For example, immature roots can present problems.180 Once the roots mature (i.e., formed a narrow apical foramen) and the instruments are able to contact the canal walls, the electronic apex locator’s accuracy greatly improves. Some investigators have found no statistical difference between roots with vital and necrotic tissue.135,250 Because apical root resorption is prevalent in necrotic cases with long-standing apical lesions, these researchers also concluded that apical resorption does not have a significant effect on the accuracy of electronic apex locators.
Recently some clinicians have advocated the use of the electronically determined working length in lieu of working length estimations using the placement of a file in the canal and a radiograph. However, combined use of both of these techniques has been shown to result in greater accuracy.95 Furthermore, radiographs may also add essential anatomic information that may be missed if electronic apex locators are used exclusively.
The first two generations of electronic apex locators were sensitive to the contents of the canal and irrigants used during treatment. The development of an algorithm called the ratio measurement method distinguished the third generation of apex locators.197 To arrive at this method, the impedance of the canal was measured with two current sources of different frequencies, and a quotient was determined using the electrical potentials proportional to each impedance.197 This study found that electrolytes did not have a significant effect on the accuracy of the unit. Some third-generation apex locators are the Endex Plus, or Apit, (Osada, Los Angeles, CA),141 the Root ZX (Fig. 8-40) (J. Morita, Kyoto, Japan),114,211 and the Neosono Ultima EZ (Satelec, Mount Laurel, NJ). The Endex Plus device uses 1 and 5 kHz and provides apex location based on subtraction. The Root ZX emits currents at frequencies of 8 and 0.4 kHz and provides apex location based on the resulting quotient.
A fourth-generation apex locator was introduced with the Elements Diagnostic Unit (see Fig. 8-44), the Apex Locator (SybronEndo), and the Bingo 1020/Ray-X4 (Forum Engineering Technologies, Rishon Lezion, Israel). The Bingo uses only one of its two frequencies at a time (8 Hz or 400 Hz). According to the manufacturer, the Elements unit (which operates at frequencies of 0.5 and 4 kHz) compares the resistance and capacitance information to a database to determine the distance between the file and the apex. When the file tip reaches the area of the apical foramen, the apex locator emits a signal.
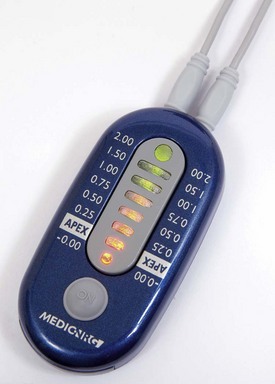
Recently, miniaturized electronic apex locators were introduced by SybronEndo (Fig. 8-41) and MedicNRG, Kibutz Afikim, Israel (the Apex NRG277,278) (Fig. 8-42).
An apex locator typically has four parts: (1) the lip clip, (2) the file clip, (3) the instrument itself, and (4) a cord connecting the other three parts. A display indicates the advancement of the file toward the apex (see Fig. 8-40).
These electrical instruments are generally safe, but manufacturers’ instructions state that they should not be used on patients with pacemakers without consulting the patient’s cardiologist.30,113 When connected directly to cardiac pacemakers in vitro, four of five electronic apex locators did not interfere with the function of the pacemaker.126
Instruments for Root Canal Obturation
After the root canal has been properly cleaned and enlarged, the space is obturated with a manufactured material. A number of obturation methods are practiced, but lateral and vertical compactions are the two most common. Many specialized instruments are available for every type of method. Spreaders and pluggers are the significant instruments for obturation. The spreader is a tapered, pointed instrument intended to displace gutta-percha laterally for insertion of additional accessory gutta-percha cones. The plugger is similar but has a blunt end. In smaller sizes, the spreader and plugger are often used interchangeably. These instruments are available with handles or as finger-held instruments. The instruments with handles are potentially dangerous because the tips of the working ends are offset from the long axes of the handles. This results in strong lateral wedging forces on the working ends if the instruments are not operated carefully. The risk of vertical damage to the root is greatly reduced with finger spreaders and pluggers. Each clinician must choose the appropriate spreader and plugger according to personal working preferences. Standardized instruments are available with the same taper as the files (e.g., #.02). Considering the greater taper of standardized accessory gutta-percha cones (Table 8-2), nonstandardized spreaders with a larger taper sometimes may be used to better accommodate the gutta-percha.
When using spreaders and accessory gutta-percha cones, the accessory cone selected must reach the depth of the penetration of the spreader. To accomplish that, the accessory cone must be thinner and/or with a smaller taper than the spreader used. If accessory cones which do not reach that depth are used, the procedure will result in voids at the apical end of each spreader penetration, leading the operator to an illusion that the root canal is properly obturated.
In recent years, spreaders and pluggers have become available in nickel-titanium. NiTi spreaders have been shown to reach deeper into canals than the stainless steel type when #.02 tapered gutta-percha is used in canals with a curvature of more than 20 degrees. When #.04 taper gutta-percha was used, the NiTi spreaders were more effective regardless of the degree of curvature.424
Heat carriers are used for vertical-compaction obturation techniques. Traditionally, heat carriers are handled similarly to pluggers. They are used to transfer heat to the gutta-percha in the root canal, allowing apical and lateral displacement of the gutta-percha. Electrical heat carriers are more common today, either as independent units such as Touch ’n Heat and System B (SybronEndo, Orange, CA) (Fig. 8-43), or incorporated into multifunctional systems such as Elements (Fig. 8-44) (SybronEndo). Recently, battery-charged, handheld heat carriers were introduced, such as the vibrating heat carrier DownPack67 (Hu-Friedy, Chicago, IL) (Fig. 8-45) or HotTip (Fig. 8-46) (Discus Dental, Culver City, CA). These devices can be heated to controlled levels with a self-contained cordless unit.
A Lentulo spiral may be used for placement of the sealer, cement, and calcium hydroxide (Ca[OH]2) dressings. The Lentulo spiral is a safe instrument if used correctly but if used carelessly, it may engage the wall of the root canal and break. It must be operated clockwise in the handpiece, inserted not rotating to working length, then retracted 1 to 2 mm to make sure it is free to rotate without engaging the canal walls. It should be started and rotated at a slow speed while being gradually withdrawn from the root canal. This instrument effectively drives the paste into the root canal. However, for optimal effect, the spiral must be as large as possible so that the paste is forced forward as the material is squeezed between the canal walls and the spiral. Endodontic files, paper points, and syringes also are commonly used to apply sealer in the root canal system, but they are all less effective than a Lentulo spiral if filling a root canal entirely with sealer is desired.
Devices for Removing Root Canal Obstructions
There are many devices available for removing natural and manufactured obstructions from within the canal. Please see Chapter 25 for details on these devices.
Materials for Disinfecting the Pulp Space
Chemomechanical Preparation
To increase the efficacy of mechanical preparation and bacteria removal, instrumentation must be supplemented with active irrigating solutions. Irrigation is defined as washing out a body cavity or wound with water or a medicated fluid. Aspiration is defined as the process of removing fluids or gases from the body with a suction device.
The objectives of irrigation are both mechanical and biologic. The mechanical objective involves flushing out debris, lubricating the canal, and dissolving organic and inorganic tissue. The biologic function of the irrigants is related to their antimicrobial effect.
Irrigation Hydrodynamics
The effectiveness of root canal irrigation in terms of debris removal and eradication of bacteria depends on several factors: penetration depth of the needle, diameter of the root canal, inner and outer diameter of the needle, irrigation pressure, viscosity of the irrigant, velocity of the irrigant at the needle tip, and type and orientation of the needle bevel (Box 8-3).181
BOX 8-3 Ideal Characteristics of an Endodontic Irrigant58,149,390
The ideal irrigant should:
1.
Be an effective germicide and fungicide.
2.
Be nonirritating to the periapical tissues.
3.
Remain stable in solution.
4.
Have a prolonged antimicrobial effect.
5.
Be active in the presence of blood, serum, and protein derivates of tissue.
6.
Have low surface tension.
7.
Not interfere with repair of periapical tissues.
8.
Not stain tooth structure.
9.
Be capable of inactivation in a culture medium.
10.
Not induce a cell-mediated immune response.
11.
Be able to completely remove the smear layer, and be able to disinfect the underlying dentin and its tubules.
12.
Be nonantigenic, nontoxic, and noncarcinogenic to tissue cells surrounding the tooth.
13.
Have no adverse effects on the physical properties of exposed dentin.
14.
Have no adverse effects on the sealing ability of filling materials.
15.
Have convenient application.
16.
Be relatively inexpensive.
The size and length of the irrigation needle relative to the canal space dimension is of utmost importance for the effectiveness of proper irrigation. If the external diameter of the needle is too large or rigid, it may inhibit introduction of the irrigant into the more apical extent of the root canal or into areas of curved canals. The internal diameter of the needle correlates to the necessary pressure for moving the syringe plunger and the velocity at which the irrigant is expressed. Narrow needles require more pressure of the plunger and will extrude the irrigant with higher velocity than large needle diameters. Although a needle with a larger internal diameter will extrude the irrigant with a larger volume over time, the irrigant cannot be introduced as deeply. Moser and Heuer276 measured the pressure necessary for activation of the plunger in different sizes and types of irrigation needles. They determined lower pressure was necessary for irrigation with larger needle diameter sizes (23 to 24 gauge) compared with smaller diameter sizes (24 to 30 gauge). However, larger needles placed at a greater distance from the apex were less efficient during irrigation than smaller diameter needles placed closer to the apex.174 Common injection needles have an external diameter of 0.40 mm (27 gauge), but special irrigation tips with external diameters of 0.30 mm (30 gauge) are available as well (Table 8-3). To improve safety of irrigation and prevent apical extrusion of the irrigant, some of these needles release the solution through lateral openings and have a closed, safe-ended tip (Fig. 8-47). The Stropko Flexi-Tip (30 gauge) needle is fabricated out of NiTi to improve its flexibility and penetration into curved canals.
TABLE 8-3 Sizes and Manufacturers of Needles Used for Root Canal Irrigation
| Manufacturer |
Product |
Gauge |
| DENTSPLY |
Max-I-Probe |
21–30 |
| Ultradent, South Jordan, UT, USA |
NaviTip |
29, 30 |
| NaviTip FX Tip (brush-covered needle) |
30 |
| |
Capillary Tip |
25, 28 |
| |
Endo-Eze Tip/Deliver Eze |
18, 19, 20, 22, 30, 31 |
| |
Endo-Eze Irrigator Tip/Deliver Eze Spülkanüle |
27 |
| KerrHawe, Bioggio, Switzerland |
NaviTip |
21–30 |
| Hager & Werken, Duisburg, Germany |
Miraject Endotec |
21–25 |
| Vista Dental Products |
Stropko Flexi-Tip (NiTi) |
30 |
| KerrHawe |
KerrHawe irrigation probe |
|
| Transcoject, Neumünster, Germany |
Spülkanülen Endo |
23, 25, 27, 30 |
Adapted from Hülsmann M, Rödig T, Nordmeyer S: Complications during root canal irrigation. Endod Topics 16:27, 2007.
Sodium Hypochlorite
NaOCl is the most commonly used irrigating solution. It is an excellent antibacterial agent, capable of dissolving necrotic tissue, vital pulp tissue, and the organic components of dentin and biofilms.343
NaOCl solution, commonly known as bleach, is frequently used as a disinfectant or a bleaching agent. It is the irrigant of choice in endodontics, owing to its efficacy against pathogenic organisms and pulp digestion, and satisfies most of the preferred characteristics stated earlier.
History
Hypochlorite was first produced in 1789 in Javelle, France, by passing chlorine gas through a solution of sodium carbonate. The resulting liquid, known as “Eau de Javelle” or “Javelle water” was a weak solution of NaOCl. However, this process was not very efficient, and alternate production methods were sought. One such method involved the extraction of chlorinated lime (known as bleaching powder) with sodium carbonate to yield low levels of available chlorine. This method was commonly used to produce hypochlorite solutions for use as a hospital antiseptic that was sold under the trade names “Eusol” and “Dakin’s solution.” NaOCl as a buffered 0.5% solution was recommended for the irrigation of wounds during World War I by Dakin.74 Coolidge69 later introduced NaOCl to endodontics.
Mode of Action
Please see Chapter 9 for further information on this subject.
Allergic Reactions to Sodium Hypochlorite
Although few reports have been published101,156 on allergic-like reactions to NaOCl, real allergies to NaOCl are unlikely to occur, since both sodium and chlorine are essential elements in the physiology of the human body. Nevertheless, hypersensitivity and contact dermatitis may occur in rare cases. In cases of hypersensitivity to NaOCl, chlorhexidine should not be used either (because of its chlorine content). Use of an alternative irrigant with high antimicrobial efficacy, such as iodine potassium iodide, should be considered, assuming there is no known allergy to the irrigant. Irrigants such as alcohol or tap water are less effective against microorganisms and do not dissolve vital or necrotic tissue. Ca(OH)2 could be used as a temporary medicament because it dissolves both vital and necrotic tissue.10,161
Increasing the Efficacy of Hypochlorite Preparations
One alternative approach to improving the effectiveness of hypochlorite irrigants in the root canal system439 could be to increase the temperature of low-concentration NaOCl solutions, which improves their immediate tissue-dissolution capacity. Furthermore, heated hypochlorite solutions remove organic debris from dentin shavings more efficiently. The antimicrobial properties of heated NaOCl solutions have also been evaluated. The bactericidal rates for NaOCl solutions are more than doubled for each 5° C rise in temperature in the range of 5° C to 60° C. This was corroborated in a recent study359 using steady-state planktonic Enterococcus faecalis cells. A temperature rise of 25° C increased NaOCl efficacy by a factor of 100. The capacity to dissolve human dental pulp using 1% NaOCl at 45° C was found to be equal to that of a 5.25% solution at 20° C. Additionally, the systemic toxicity of preheated low concentrations of NaOCl irrigants should be less than that of a more concentrated unheated solution. However, there are no clinical studies available at this point to support the use of heated NaOCl.
In this context, it should also be noted that time is a factor that has gained little attention in endodontic studies. Even fast-acting biocides such as NaOCl require an adequate working time to reach their potential. Chlorine, which is responsible for the dissolving and antibacterial capacity of NaOCl, is unstable and consumed rapidly during the first phase of tissue dissolution, probably within 2 minutes.268 Continuous replenishment of the irrigant is essential, especially since rotary root canal preparation techniques have expedited the shaping process. The optimal time a hypochlorite irrigant needs to remain in the canal system is an issue yet to be resolved.
Chlorhexidine
History
Chlorhexidine (CHX) was developed more than 50 years ago at Imperial Chemical Industries in England and first marketed in the United Kingdom in 1953 as an antiseptic cream.104 Since 1957 it has been used for general disinfection purposes and the treatment of skin, eye, and throat infections in both humans and animals.104,231
Molecular Structure
CHX belongs to the polybiguanide antibacterial family, consisting of two symmetric four-chlorophenyl rings and two bisguanide groups connected by a central hexamethylene chain. CHX is a strongly basic molecule and is stable as a salt. CHX digluconate salt is easily soluble in water.147
Mode of Action
CHX is a wide-spectrum antimicrobial agent, active against gram-positive and gram-negative bacteria as well as yeasts.81 Owing to its cationic nature, CHX is capable of electrostatically binding to the negatively charged surfaces of bacteria,72 damaging the outer layers of the cell wall and rendering it permeable.165,178,179
Depending on its concentration, CHX can have both bacteriostatic and bactericidal effects. At high concentrations CHX acts as a detergent; by damaging the cell membrane, it causes precipitation of the cytoplasm and thereby exerts a bactericidal effect. At low sublethal concentrations, CHX is bacteriostatic, causing low molecular-weight substances (i.e., potassium and phosphorous) to leak out without the cell being irreversibly damaged. It also can affect bacterial metabolism in several other ways, such as abolishing sugar phosphotransferase system (PTS) transport activity and inhibiting acid production in some bacteria.25
Substantivity
Due to the cationic nature of the CHX molecule, it can be absorbed by anionic substrates such as the oral mucosa.237,240 CHX has the ability to bind to proteins such as albumin, present in serum or saliva, pellicle found on the tooth surface, salivary glycoproteins, and mucous membranes.317,398 This reaction is reversible.169 CHX can also be adsorbed onto hydroxyapatite and teeth. Studies have shown that the uptake of CHX onto teeth is also reversible. This reversible reaction of uptake and release of CHX leads to substantive antimicrobial activity and is referred to as substantivity. This effect depends on the concentration of CHX. At low concentrations of 0.005% to 0.01%, a stable monolayer of CHX is adsorbed and formed on the tooth surface, which might change the physical and chemical properties of the surface and may prevent or reduce bacterial colonization. At higher concentrations (>0.02%), a multilayer of CHX is formed on the surface, providing a reservoir of CHX which can rapidly release the excess into the environment as the concentration of CHX in the surrounding environment decreases.97
The antibacterial substantivity of three concentrations of CHX solution (4%, 2%, and 0.2%) after 5 minutes of application has shown a direct relationship between the concentration of CHX and its substantivity.271 On the contrary, Lin et al.226 attributed the substantivity of CHX to its ability to adsorb onto the dentin during the first hour. They stated that it is only after the saturation point is reached after the first hour that the antimicrobial capability of CHX increases with time. Furthermore, Komorowski et al.199 revealed that a 5-minute application of CHX did not induce substantivity, so dentin should be treated with CHX for 7 days. Taken together, it seems that residual antimicrobial activity of CHX in the root canal system remains for up to 12 weeks.
Cytotoxicity
In the medical field, CHX is normally used at concentrations between 0.12% and 2.0%. According to Löe,231 at these concentrations, CHX has a low level of tissue toxicity, both locally and systemically. In another report, when 2% CHX was used as a subgingival irrigant, no apparent toxicity was noted on gingival tissues.232,369 Moreover, CHX rinses have been reported to promote the healing of periodontal wounds.14 Based on these reports, Jeansonne et al.184 assumed that the periapical tissues would be as tolerant to CHX as gingival tissues. In two studies when CHX and NaOCl were injected into subcutaneous tissues of guinea pigs and rats, an inflammatory reaction developed; however, the toxic reaction from CHX was less than that of NaOCl.289,432 Furthermore, when CHX was applied as a rinse in the extraction sites of the third molars on the day of surgery and several days after, it was reported to reduce the incidence of alveolar osteitis.56 There are only a few reports in the literature of allergic and anaphylactic reactions to CHX.127,286
Conversely, some studies have reported unfavorable effects of CHX on the tissues. Hidalgo and Dominguez168 demonstrated that CHX is cytotoxic to some lines of cultured human skin fibroblasts. Recently the behavior of osteoblastic human alveolar bone cells in the presence of CHX and povidone iodine (PI) has been investigated. It has been reported that CHX has a higher cytotoxicity profile than povidone iodine.49 Faria et al.105 also demonstrated that CHX injected in the hind paw of mice could induce severe toxic reactions. They reported that CHX induced apoptosis at lower concentrations and necrosis at higher concentrations when added to cultured L929 fibroblast cells.
Another interesting observation has recently been reported whereby the byproduct of combining CHX with NaOCl is the formation of toxic breakdown products such as parachloroaniline (PCA) that may have a negative impact on tissues.26 The toxicity level of CHX on periapical tissues when applied in the root canals, especially with other irrigants, merits further investigation.
Chlorhexidine Application in Endodontics
CHX has been extensively studied as an endodontic irrigant and intracanal medication, both in vivo20,228,239,293 and in vitro.* In vitro, CHX has at least as good or even better antimicrobial efficacy than Ca(OH)2.354 Notably, 2% CHX was very effective in eliminating a biofilm of E. faecalis.223 In vivo, it inhibits experimentally induced inflammatory external root resorption when applied for 4 weeks.228 In infected root canals, it reduces bacteria as effectively as Ca(OH)2 when applied for 1 week.20 Unlike Ca(OH)2, CHX has substantive antimicrobial activity that, if imparted onto the root dentin, has the potential to prevent bacterial colonization of root canal walls for prolonged periods of time.184,199 This effect depends on the concentration of CHX, but not on its mode of application, which may be as a liquid, gel, or controlled-release device.27
Chlorhexidine as an Endodontic Irrigant
CHX in liquid and gel form has been recommended as an irrigant solution, and its different properties have been tested in several studies, both in vitro137 and in vivo.* Many investigations have conducted studies on the antibacterial effectiveness of CHX in different concentrations. It has been demonstrated that 2% CHX as an irrigant has a better antibacterial efficacy than 0.12% CHX in vitro, so the antibacterial efficacy of CHX evidently depends on its concentration level.28 Since NaOCl is still the most commonly used irrigant, the antibacterial efficacy of CHX has been compared to that of NaOCl. The results from these studies are inconclusive, but in general, no significant difference between the two solutions has been reported. Unlike NaOCl, CHX lacks a tissue-dissolving property. Therefore, NaOCl is still considered the primary irrigating solution in endodontics.
*References 99, 208, 220, 356, 357, 386, 405, and 437.
The cleanliness of root canals by CHX in gel and liquid forms was evaluated using scanning electron microscopy in two separate experiments. In an in vitro study, the canals treated with 2% CHX gel were cleaner than those treated with 2% CHX liquid or 5.25% NaOCl, and it was suggested that the mechanical action of the gel might have facilitated the cleansing of the canals. Another in vitro study showed that the 2% CHX liquid was inferior to 2.5% NaOCl in cleaning the canals.431
The antibacterial effectiveness of CHX in infected root canals has been investigated in several studies. Investigators315 reported that 2.5% NaOCl was significantly more effective than 0.2% CHX when the infected root canals were irrigated for 30 minutes with either of the solutions.
In a controlled and randomized clinical trial, the efficacy of 2% CHX liquid was tested against saline using culture technique. All the teeth were initially instrumented and irrigated using 1% NaOCl. Then either 2% CHX liquid or saline was applied as a final rinse. The authors reported a further reduction in the proportion of positive cultures in the CHX group. Their results showed a better disinfection of the root canals using CHX compared to saline as a final rinse.437
In a recent study, the antibacterial efficacy of 2% CHX gel was tested against 2.5% NaOCl in teeth with apical periodontitis, with the bacterial load assessed using real-time quantitative polymerase chain reaction (RTQ-PCR) and colony forming units (CFU). The bacterial reduction in the NaOCl group was significantly greater than the CHX group when measured by RTQ-PCR. Based on culture technique, bacterial growth was detected in 50% of the CHX-group cases compared to 25% in the NaOCl group.405 On the other hand, another study based on this culture technique revealed no significant difference between the antibacterial efficacy of 2.5% NaOCl and 0.12% CHX liquid when used as irrigants during the treatment of infected canals.356,357
Chlorhexidine as an Intracanal Medication
CHX in liquid, gel, or in a controlled-release device has been suggested as an alternative intracanal medication to replace Ca(OH)2. This has been the focus of many in vitro78,199,217,354 and in vivo studies.* The results of in vitro experiments were mostly in favor of CHX regardless of its mode of application. The results have also demonstrated the potential antibacterial substantivity of CHX in the root canals.
Researchers155 developed an experimental model using dentin powder particles to investigate the possible inactivation of some antibacterial medicaments when they come in contact with dentin. The medicaments tested were Ca(OH)2, 1% NaOCl, 0.5% and 0.05% CHX acetate, and different concentrations of iodine potassium iodide (IKI). They showed that dentin powder had inhibitory effects on all medicaments tested. The effect was dependent on the concentration of the medicament and the duration of contact. The effect of Ca(OH)2 was totally abolished by the presence of dentin powder. The effect of 0.05% CHX and 1% NaOCl was reduced but not totally eliminated by the presence of dentin. No inhibition could be measured when full-strength solutions of CHX and IKI were used.
Contradicting results have been reported when evaluating the effect of different intracanal medications on sealing of the root canals. In an in vitro study using extracted human teeth, all root canals, after 10 days of intracanal medication, were obturated and then tested for microbial leakage. The root canals medicated with CHX gel demonstrated less resistance against bacterial leakage compared to the root canals medicated with Ca(OH)2.23 In contrast, Wuerch et al.430 found no significant difference in leakage between the canals medicated with CHX and Ca(OH)2 and reported that 2% CHX gel and Ca(OH)2 paste did not adversely affect the apical seal of the root canal system.
In a human ex vivo model, the antibacterial efficacy of 0.2% CHX liquid applied for 24 hours was tested against a saline solution. After extraction, the infected teeth were endodontically treated using CHX or saline for irrigation and then further medicated with either of the solutions. Samples were obtained and evaluated using culture technique at each treatment step. In both groups, the numbers of bacteria were decreased after instrumentation and irrigation of the canals. However, after 24 hours of medication, it was reported that the numbers of bacteria were further decreased in the CHX group, in contrast to the saline group, where an increase was noted.78 CHX may also have an effect on reduction of inflammatory external root resorption caused by infection. Lindskog et al.228 assessed the therapeutic effect of a 4-week intracanal application of CHX gel on inflammatory root resorption in replanted infected teeth of monkeys. They reported that the extent of inflammatory resorption was significantly reduced compared to nonmedicated teeth, suggesting that CHX may be a useful adjunct in the management of inflammatory root resorption.
These in vitro and animal studies suggest that CHX has the potential to replace Ca(OH)2 as an intracanal medicament, but the limitations of the in vitro experiments make the conclusions obtained difficult to extrapolate to actual clinical situations. In vivo human experiments are therefore required to test the efficacy of CHX as an intracanal medication.
Compared to the number of in vitro studies, very few in vivo studies have been conducted to assess the effectiveness of CHX as an intracanal medication. An in vivo investigation20 assessed the antibacterial efficacy of three different intracanal medications: camphorated paramonochlorophenol, Ca(OH)2, and 0.12% CHX liquid by applying them for 1 week in single-rooted teeth of patients. Using a culture method, it was reported that the proportions of positive cultures were not significantly different among the tested medications, but they were slightly lower in teeth medicated with CHX (0.12%) liquid than those medicated with camphorated paramonochlorophenol or Ca(OH)2.20
Another in vivo study evaluated the antibacterial effectiveness of 2% CHX liquid as an intracanal medication in teeth with apical periodontitis.293 The results showed a moderate increase in bacterial counts during a medication period of 7 to 14 days that was similar to outcomes seen and reported for Ca(OH)2 by Peters et al.301 It was speculated that the CHX liquid may have partially escaped from the apical foramen, and that the higher-viscosity gel form might have been better suited as an intracanal medication.293
However, a different study239 demonstrated that intracanal medication with Ca(OH)2, 2% CHX gel, or a mixture of Ca(OH)2/CHX applied for 7 days did not reduce the bacterial concentration beyond what was achieved after chemomechanical preparation using 1% NaOCl. The results were not significantly different among the medication groups. Similar results were found by other investigators,238 where after a randomized controlled trial of 30 patients, they concluded that a final rinse with MTAD (mixture of tetracycline, acid, and detergent) and intracanal application of 2% CHX gel did not reduce bacterial counts beyond levels achieved by chemomechanical preparation using NaOCl.
Chlorhexidine and Calcium Hydroxide
During the last few years, researchers have studied the combination of Ca(OH)2 and CHX, with the concept that their antimicrobial properties interact in a synergistic fashion that enhances their efficacy. The high pH of Ca(OH)2 was unaffected when combined with CHX.26 However, the results have not been conclusive. Some in vitro studies have reported an improved antibacterial action when both agents were combined,28,102,438 while other studies reported conflicting results.157
Recent animal studies have evaluated the tissue reactions to the mix of Ca(OH)2/CHX, showing that the combination exerts good antimicrobial properties364 and improves healing of the periapical tissues. In vivo studies have shown that the mix is at least as good as both agents applied separately in necrotic teeth with apical periodontitis,239 as well as in previously treated cases with persistent apical periodontitis. A more recent study utilizing a CHX-based protocol of 0.12% CHX as an irrigant followed by a 7-day intracanal medication of Ca(OH)2/0.12% CHX has shown promising results. The authors concluded that chemomechanical canal shaping using 0.12% CHX solution as an irrigant significantly reduced the number of intracanal bacteria, but it failed to render the canals bacteria free. Further intracanal medication with a Ca(OH)2/CHX paste significantly improved the results by reducing the number of bacteria.353 Taken together, it seems that the usefulness of mixing Ca(OH)2 with CHX remains unclear and controversial.
Chlorhexidine and Coronal Penetration of Bacteria
Because of its antimicrobial substantivity, it seems that CHX preparations delay entry of bacteria through the coronal portion of the tooth into the root canal system. A laboratory study138 investigated the time required for recontamination of the root canal system of teeth with coronal restorations medicated with Ca(OH)2, 2% CHX gel, or a combination of both. The canals without a coronal restoration but medicated with CHX showed recontamination after an average time of 3.7 days, the group with Ca(OH)2 after 1.8 days, and the group with CHX + Ca(OH)2 after 2.6 days. The canals medicated with CHX and restored with intermediate restorative material (IRM) showed recontamination within 13.5 days, the group with Ca(OH)2 + IRM after 17.2 days, and the group with CHX + Ca(OH)2 + IRM after 11.9 days. The group with no medication but restored with IRM showed recontamination after an average time of 8.7 days. There were statistically significant differences between the groups (P < 0.05). All groups without a coronal restoration were recontaminated significantly more quickly than those restored with IRM, except those teeth that had a restoration but no medicament. The groups with intracanal medication and a coronal restoration were not significantly different from each other.
An ex vivo study408 assessed coronal dye penetration of extracted human teeth after root canal treatment using 1% NaOCl, 1% NaOCl + 17% ethylenediamine tetra-acetic acid (EDTA), 2% CHX gel, 2% CHX gel + 1% NaOCl and distilled water. Results revealed that the least dye penetration occurred with 1% NaOCl + 17% EDTA and 2% CHX gel. NaOCl, distilled water, and 2% CHX gel + 1% NaOCl had more dye penetration with a significant difference compared with NaOCl + 17% EDTA and 2% CHX gel and compared with one another.
Lambrianidis and colleagues210 have shown that viscous irrigants, including those containing CHX gluconate, were less soluble substances that can leave residue on root canal surfaces and potentially affect root canal obturation. The efficiency of removing Ca(OH)2/CHX gel, Ca(OH)2/CHX solution and Ca(OH)2/saline pastes with the use of instrumentation and irrigation with NaOCl and EDTA solutions has been assessed. None of the techniques used in this study removed the interappointment root canal medicaments effectively. Overall, Ca(OH)2/CHX (gel) paste was associated with significantly larger amount of residue, whereas the Ca(OH)2/CHX mixture was associated with less residue than the other two medicaments. Overall, because of its substantivity, CHX as an intracanal medicament/irrigant may delay the coronal recontamination of the root canal system.
Interaction Between CHX, NaOCl, and EDTA
A suggested clinical protocol439 for treating dentin prior to root canal obturation consists of irrigation with NaOCl to dissolve the organic components, irrigation with EDTA to eliminate the smear layer, and irrigation with CHX to increase the antimicrobial spectrum of activity and impart substantivity. Although such a combination of irrigants may enhance the overall antimicrobial effectiveness,208 possible chemical interactions between the irrigants must be considered. Some studies have reported the occurrence of color change and precipitation when NaOCl and CHX are combined26,439 (Fig. 8-48). Furthermore, concerns have been raised that the color change may have some clinical relevance because of staining, and the resulting precipitate might interfere with the seal of the root obturation. The formation of a precipitate could be explained by the acid-base reaction that occurs when NaOCl and CHX are mixed together. CHX, a dicationic acid, has the ability to donate protons. NaOCl is alkaline and can accept protons from the dicationic acid. This proton exchange results in the formation of a neutral and insoluble substance referred to as the precipitate.26 Basrani et al.26 evaluated the chemical nature of this precipitate and reported that there was an immediate reaction when 2% CHX was combined with NaOCl, even at the low concentration (0.023%). They found that the amount of PCA directly increased with the increasing concentration of NaOCl. PCA has been shown to be toxic in humans with short-term exposure, resulting in cyanosis, which is a manifestation of methemoglobin formation. In another study, the effect on root dentin and dentinal tubules was evaluated subsequent to irrigating root canals with a combination of NaOCl and CHX. They concluded that the NaOCl/CHX precipitate tends to occlude the dentinal tubules and suggested that until this precipitate is studied further, caution should be exercised when irrigating with both NaOCl and CHX. In summary, the combination of NaOCl and CHX causes color changes and formation of a neutral and possibly toxic insoluble precipitate that may interfere with the seal of the root obturation. Alternatively, the canal can be dried using paper points before the final CHX rinse.439
The combination of CHX and EDTA produces a white precipitate, so a group of investigators311 did a study to determine whether the precipitate involves the chemical degradation of CHX. The precipitate was produced and redissolved in a known amount of dilute trifluoroacetic acid. Based on the results, CHX was found to form a salt with EDTA rather than undergoing a chemical reaction.
Dentin Bonding of Chlorhexidine
During the last 2 decades, chemical and technical advances have contributed to increases in resin-dentin bond strength. However, the premature loss of bond strength is one of the problems that still affects adhesive restorations270 and markedly reduces their durability.54,80,117 Researchers53 evaluated the effect of CHX on resin-dentin bond stability ex vivo. They concluded that autodegradation of collagen matrices can occur in resin-infiltrated dentin, but this may be prevented by the application of a synthetic protease inhibitor such as CHX.53 Because of its broad-spectrum matrix metalloproteinase (MMP)-inhibitory effect, CHX may significantly improve resin-dentin bond stability.
Allergic Reactions to Chlorhexidine
CHX, although reported to be a relatively safe solution, may induce allergic reactions. The sensitization rate has been reported in several studies to be approximately 2%.202 One case of an anaphylactic shock was reported after the application of 0.6% CHX to intact skin that had signs of a rash following a minor accident.15 Further allergic reactions such as anaphylaxis, contact dermatitis, and urticaria have been reported following direct contact to mucosal tissue or open wounds.92,303,340,363 There are no publications reporting allergic reactions following root canal irrigation with CHX.181
MTAD and Tetraclean
Recently, MTAD (Fig. 8-49) and Tetraclean, two new irrigants based on a mixture of antibiotics, citric acid, and a detergent, have been developed. MTAD is the first irrigating solution created which is capable of removing the both the smear layer and disinfecting the root canal system.392 It is a mixture of 3% doxycycline hyclate, 4.25% citric acid, and 0.5% polysorbate-80 (Tween 80) detergent.392 Commercially available as BioPure MTAD (DENTSPLY Tulsa Dental, Tulsa, OK), it is mixed as a liquid and powder prior to use. MTAD has been recommended in clinical practice as a final rinse after completion of conventional chemomechanical preparation.*
Tetraclean (Ogna Laboratori Farmaceutici, Muggio, Italy) is another combination product similar to MTAD. The two irrigants differ in the concentration of antibiotics (doxycycline 150 mg/5 ml for MTAD and 50 mg/5 ml for Tetraclean) and the kind of detergent (Tween 80 for MTAD, polypropylene glycol for Tetraclean).
Mode of Action
There is no detailed information on the exact mechanism of action of MTAD in the removal of the smear layer and the killing of bacteria. In most studies, its effect on the smear layer is attributed to the existence of doxycycline and citric acid. These two components in solution have been separately reported as being effective in the removal of the smear layer.163 Its antibacterial effect is mostly attributed to the doxycycline, an isomer of tetracycline. Tetracyclines—including tetracycline HCl, minocycline, and doxycycline—are broad-spectrum antibiotics that are effective against a wide range of microorganisms. Tetracycline is a bacteriostatic antibiotic which exerts its effect through the inhibition of protein synthesis. According to Torabinejad et al.,393 this property may be advantageous because in the absence of bacterial cell lysis, antigenic byproducts (i.e., endotoxin) are not released. In high concentrations, tetracycline may also have a bactericidal effect. The role of citric acid in killing bacteria is not well known. Tween 80, the other component of MTAD, seems to have limited antibacterial activity, yet it may increase the antibacterial effect of some substances by directly affecting the bacterial cell membrane. It may also facilitate the penetration of MTAD into dentin. On the contrary, Tween 80 may also be a nutrient for some bacteria, and it may inactivate the antibacterial properties of some disinfecting agents such as CHX and povidone iodine. Doxycycline, citric acid, and Tween 80 together may have a synergistic effect on the disruption of the bacterial cell wall and on the cytoplasmic membrane.
Surface Tension
According to Grossman and Meiman,150 low surface tension is one of the ideal characteristics of an irrigant. Lower surface tension may help by allowing the irrigant to penetrate better into the dentinal tubules and inaccessible areas of the root canal system.388
To be more effective in debris removal and to penetrate more readily into the root canal system, irrigants must be in contact with the dentin walls. The closeness of this contact is directly related to its surface tension.133 Irrigants with a low surface tension are more suitable as endodontic irrigants. To decrease the surface tension, Tween 80 has been added to the MTAD solution.
Smear Layer Removal
In two studies, the efficacy of MTAD or EDTA in the removal of the smear layer was confirmed, but no significant difference between these two solutions was reported.389,390
Antibacterial Efficacy
Reported results regarding the antibacterial properties of MTAD are conflicting. The studies measuring zones of inhibition on agar plates have shown consistently that MTAD was an effective antibacterial agent against E. faecalis.73,201,393 Tay et al.389,390 also found larger zones of bacterial inhibition using dentin cores irrigated with MTAD compared to NaOCl-irrigated dentin cores. When they applied MTAD to dentin that was already irrigated with 1.3% NaOCl, however, they noticed a contradictory result: the diameters of the zones of inhibition were significantly smaller than those of MTAD alone but comparable to those irrigated with 1.3% NaOCl alone. They concluded that the antimicrobial effect of MTAD was lost due to oxidation of the MTAD by NaOCl.
A study using extracted human teeth contaminated with saliva showed that MTAD was more effective than 5.25% NaOCl in disinfection of the teeth.347 In contrast, Krause et al.,201 using bovine tooth sections, showed that 5.25% NaOCl was more effective than MTAD in disinfection of dentin discs inoculated with E. faecalis.
In another study performed on extracted human teeth inoculated with E. faecalis, a protocol of 1.3% NaOCl followed by 5 minutes of MTAD was more effective in the disinfection of canals than a protocol of 5.25% NaOCl followed by 1 minute of 17% EDTA and then 5 minutes of 5.25% NaOCl as a final rinse.347 In a series of studies, MTAD has failed to show superior antibacterial efficacy against bacterial biofilms. Bacteria collected from the teeth of patients diagnosed with apical periodontitis were grown as a biofilm on hemisections of root apices. MTAD was an effective antibacterial agent in this model, but it was unable to completely disrupt the bacterial biofilm, compared to 6% NaOCl. NaOCl (5.25%) was the most effective irrigant against a biofilm of E. faecalis generated on cellulose nitrate membrane filters, whereas the bacterial load reduction using MTAD was not significant.134 The results of testing antibacterial efficacy of medicaments obtained from in vitro studies should be analyzed with caution. They may be influenced by factors such as the test environment, bacterial susceptibility, and the different methodologies used to evaluate the results.354
In vivo Clinical Trial
With the exception of one study394 that evaluated the effect of MTAD on postoperative discomfort, there have been no other in vivo studies to address the other characteristics of MTAD. Malkhassain et al.238 in a clinical controlled trial of 30 patients reported that the final rinse with MTAD did not reduce the bacterial counts in infected canals beyond levels achieved by chemomechanical preparation using NaOCl alone.
Protocol for Use
The clinical protocol for the use of MTAD was developed on the basis of a pilot project.346 The results of this project showed consistent disinfection of the infected root canals could occur after chemomechanical preparation using 1.3% NaOCl as a root canal irrigant followed by a 5-minute rinse of MTAD.
Ethylenediamine Tetra-Acetic Acid
EDTA is often suggested as an irrigation solution because it can chelate and remove the mineralized portion of the smear layer (Fig. 8-50). It is a polyaminocarboxylic acid with the formula [CH2N(CH2CO2H)2]2. This colorless, water-soluble solid is produced on a large scale for many applications. Its prominence as a chelating agent arises from its ability to sequester di- and tricationic metal ions such as Ca2+ and Fe3+. After being bound by EDTA, metal ions remain in solution but exhibit diminished reactivity.
History
The compound was first described in 1935 by Ferdinand Munz, who prepared the compound from ethylenediamine and chloroacetic acid. Today, EDTA is mainly synthesized from ethylenediamine (1, 2-diaminoethane), formaldehyde (methanal), and sodium cyanide.
Mode of Action
On direct exposure for extended time, EDTA extracts bacterial surface proteins by combining with metal ions from the cell envelope, which can eventually lead to bacterial death.
Applications in Endodontics
EDTA alone normally cannot remove the smear layer effectively; a proteolytic component (e.g., NaOCl) must be added to remove the organic components of the smear layer.136 Commercial products with such combinations are available. EndoDilator N-Ø (Union Broach, York, PA) is a combination of EDTA and a quaternary ammonium compound. Such an irrigation fluid has a slight detergent effect in addition to the chelating effect. Two newer irrigating solutions, MTAD (discussed earlier) and Smear Clear (SybronEndo), have recently been studied. Smear Clear is a clear, odorless, water-soluble solution containing water, 17% EDTA salts, a cationic surfactant (cetrimide), and anionic surfactants.
EDTA is normally used in a concentration of 17% and can remove the smear layers when in direct contact with the root canal wall for less than 1 minute. The decalcifying process is self-limiting. For root canal preparation, EDTA has limited value alone as an irrigation fluid. Since it is capable of decalcifying up to 50 µm, it can open up an occluded, very fine canal. This depth, added from two opposite canal walls, will result in a 100-µm opening, equivalent to the tip of a #010 file.
Although citric acid appears to be slightly more potent at similar concentration than EDTA, both agents show high efficiency in removing the smear layer. In addition to their cleaning ability, chelators may detach biofilms adhering to root canal walls.151 This may explain why an EDTA irrigant proved to be highly superior to saline in reducing intracanal microbiota despite the fact that its antiseptic capacity is relatively limited.151 Although never shown in a randomized clinical trial, an alternating irrigating regimen of NaOCl and EDTA may be more efficient in reducing bacterial loads in root canal systems than NaOCl alone. Antiseptics such as quaternary ammonium compounds (EDTAC) or tetracycline antibiotics (MTAD) have been added to EDTA and citric acid irrigants, respectively, to increase their antimicrobial capacity. The clinical value of this, however, is questionable. EDTAC shows similar smear-removing efficacy as EDTA, but it is more caustic.
Interaction of EDTA and NaOCl
Investigators146 studied the interactions of EDTA with NaOCl. They concluded that EDTA retained its calcium-complexing ability when mixed with NaOCl, but EDTA caused NaOCl to lose its tissue-dissolving capacity, with virtually no free chlorine detected in the combinations. Clinically, this suggests that EDTA and NaOCl should be used separately. In an alternating irrigating regimen, copious amounts of NaOCl should be administered to wash out remnants of the EDTA.
Intracanal Medication
When treatment cannot be completed in one appointment [see Chapters 4 and 15], the surviving intracanal bacteria often proliferate between appointments.47,415 To curtail bacterial regrowth and possibly even improve bacterial suppression, an intracanal medication can be advantageous. Interappointment antimicrobial medication acts to inhibit proliferation and further eliminate surviving bacteria, as well as minimize ingress through a leaking restoration.
Phenolic Preparations
Phenol (C6H5OH), or carbolic acid, is one of the oldest antimicrobial agents used in medicine. Despite the severe toxicity of phenolic preparations, derivatives of phenol, such as paramonochlorophenol (C6H4OHCl), thymol (C6H3OHCH3C3H7), and cresol (C6H4OHCH3), remain available. One survey noted a decrease in the use of classic phenolic intracanal medicaments with a corresponding increase in the use of Ca(OH)2 or no medication.128
Phenol is a nonspecific protoplasm poison that has an optimal antibacterial effect at 1% to 2%. Many dental preparations use much too high a concentration of phenol (e.g., in the range of 30%). At such a concentration, the antimicrobial effect in vivo is lower than optimal and of very short duration.254
Derivatives of phenol are stronger antiseptics and toxins than phenol. Phenolic compounds are often available as camphorated solutions. Camphoration results in a less toxic phenolic compound because it slows the release of toxins to the surrounding tissues.
Studies in vitro have shown that phenol and phenol derivatives are highly toxic to mammalian cells, and their antimicrobial effectiveness does not sufficiently balance their toxicity.370,371 Phenols are ineffective antiseptics under clinical conditions.46,96
Formaldehyde
Formaldehyde, used as formocresol, has been used extensively in endodontic therapy despite its high toxicity and mutagenic and carcinogenic potential.221 The formaldehyde component of formocresol may vary substantially between 19% and 37%. Tricresol formalin, another formaldehyde preparation, contains 10% tricresol and 90% formaldehyde. All of these preparations have a formaldehyde content well above the 10% normally used for fixation of pathologic specimens. Formaldehyde is volatile and releases antimicrobial vapors when applied to a cotton pellet for pulp chamber disinfection. All formaldehyde preparations are potent toxins with an antimicrobial effectiveness much lower than their toxicity.371,372
There is no clinical reason to use formocresol as an antimicrobial agent for endodontic treatment, based on what is known at this time. The alternatives are better antiseptics with significantly lower toxicity.
Halogens
Chlorinated solutions have been used for many years to irrigate root canals. They are also used as an intracanal dressing in the form of chloramine-T, an N-chloro tosylamide sodium salt. Iodine, in the form of IKI, is a very effective antiseptic solution with low tissue toxicity. IKI is an effective disinfectant for infected dentin and can kill bacteria in infected dentin in 5 minutes in vitro.324 IKI releases vapors with a strong antimicrobial effect. The solution can be prepared by mixing 2 g of iodine in 4 g of potassium iodide; this mixture then is dissolved in 94 ml of distilled water. Tincture of iodine (5%) has proved to be one of the few reliable agents for disinfection of rubber dam and tooth surfaces during the preparation of an aseptic endodontic workfield.272
Calcium Hydroxide
Hermann introduced166,167 the use of Ca(OH)2 in endodontics in 1920. Although its use was well documented for its time, the clinical applications over the next 25 years were not well known. Its use in root canal treatment as an intracanal medication has been associated with periradicular healing and few adverse reactions. Its use in root canal treatment was promoted by a series of papers46,48 documenting the antibacterial efficacy of Ca(OH)2 in human root canals. Subsequent studies substantiated these reports,290,360 and the routine use of Ca(OH)2 as an interappointment intracanal medicament became widespread.327
Ca(OH)2 cannot be categorized as a conventional antiseptic, but it kills bacteria in the root canal space. The value of Ca(OH)2 in endodontic treatment of necrotic, infected teeth is now well documented. Ca(OH)2 normally is used as slurry of Ca(OH)2 in a water base. At body temperature, less than 0.2% of the Ca(OH)2 is dissolved into Ca++ and OH− ions. Because Ca(OH)2 needs water to dissolve, water should be used as the vehicle for the Ca(OH)2 paste. In contact with air, Ca(OH)2 forms calcium carbonate (CaCO3). However, this is an extremely slow process and of little clinical significance. Ca(OH)2 paste, with a significant amount of calcium carbonate, feels granular because the carbonate has a very low solubility.
Ca(OH)2 is a slow-acting antiseptic. Direct-contact experiments in vitro show that a 24-hour contact period is required for complete killing of enterococci.324,360 Another study of 42 patients found that NaOCl canal irrigation reduced the bacteria level by only 61.9%, but use of Ca(OH)2 in the canals for 1 week resulted in a 92.5% reduction.350 These researchers concluded that Ca(OH)2 should be used in infected cases to more predictably obtain disinfection.
In addition to killing bacteria, Ca(OH)2 has the extraordinary ability to hydrolyze the lipid moiety of bacterial lipopolysaccharides (LPS), thereby inactivating the biologic activity of the lipopolysaccharide and reducing its effect.322,323 This is a very desirable effect because dead cell wall material remains after the bacteria have been killed and can continue to stimulate inflammatory responses in the periradicular tissue.
Ca(OH)2 may be mixed with sterile water or saline; this formula is also available commercially from a number of manufacturers in sterile, single-dose packages (e.g., Calasept [J.S. Dental, Ridgefield, CT]; Calcijet [Centrix, Shelton, CT]; and DT Temporary Dressing, [Global Dental Products, North Bellmore, NY]) (Fig. 8-51). The mixture should be thick to carry as many Ca(OH)2 particles as possible. This slurry is best applied with a Lentulo spiral. For maximum effectiveness, the root canal must be filled homogeneously to the working length. Saturated Ca(OH)2 solution mixed with a detergent is an effective antimicrobial agent suitable for irrigation.
Limitations of Calcium Hydroxide
There are some concerns regarding the use of Ca(OH)2. The handling and proper placement of Ca(OH)2 present a challenge to the average clinician.233,358 Also, the removal of Ca(OH)2 is frequently incomplete, resulting in a residue covering 20% to 45% of the canal wall surfaces, even after copious irrigation with saline, NaOCl, or EDTA.211 Residual Ca(OH)2 can shorten the setting time of zinc oxide eugenol–based endodontic sealers.243 Most notably, it may interfere with the seal of the root filling and compromise the quality of treatment. An additional concern is that Ca(OH)2 is not totally effective against several endodontic pathogens, including E. faecalis and Candida albicans.416 Recently the ability of Ca(OH)2 to completely eradicate bacteria from the root canal has been questioned. For example, in vitro studies have shown that dentin can inactivate the antibacterial activity of Ca(OH)2,155,307 and one clinical study301 has shown that the number of bacteria-positive canals actually increased after Ca(OH)2 medication. Other studies have also indicated that Ca(OH)2 could not predictably eliminate bacteria or that cultures changed from negative to positive after Ca(OH)2 placement.301,415
When different studies report inconsistent results, a systematic review and meta-analysis technique can clarify conflicting research data and the current state of knowledge regarding specific issues. Therefore, based on the current best available evidence, Ca(OH)2 has limited effectiveness in eliminating bacteria from human root canals when assessed by culture techniques. The quest for better antibacterial protocols and sampling techniques must continue to ensure that bacteria can be been reliably eradicated prior to obturation.
Chlorhexidine
CHX is also used as an intracanal medication and has already been discussed extensively under its own heading.
Ledermix
Ledermix (Lederle Pharmaceuticals, Wolfratshausen, Germany) is a corticosteroid-antibiotic paste. Medicaments such as Ledermix paste have been recommended as routine intracanal medicaments. Ledermix paste has been advocated as an initial dressing, particularly if the patient presents with endodontic symptoms.
Schroeder developed the material that is now commercially marketed as Ledermix paste.339 Ledermix paste contains triamcinolone acetonide as an antiinflammatory agent, at a concentration of 1%. When the Ledermix materials and other similar corticosteroid-containing formulations were commercially released, there was a considerable amount of opposition to their use.195
Ledermix paste is a nonsetting, water-soluble paste material for use as root canal medicament or as a direct or indirect pulp capping agent. The release and dentin diffusion characteristics of triamcinolone from Ledermix paste when used as a root canal medicament have been investigated under different conditions.1-3 These studies show that triamcinolone is released from Ledermix paste in the root canal and can reach the systemic circulation via diffusion through dentinal tubules, lateral canals, and the apical foramen. After the first 24 hours, 30% of the triamcinolone was released. By the end of 14 weeks, the remaining 70% had been released.
Immediate intracanal placement of Ledermix has been studied for replanted teeth, because it has been shown to inhibit root resorption after extended dry time. In a recent study done in dogs, the groups treated with Ledermix, triamcinolone and demeclocycline had significantly more favorable healing and more remaining root structure than the group filled with gutta-percha and sealer (positive control).57
The Triple-Antibiotics Paste
The triple-antibiotics regimen, composed of metronidazole, ciprofloxacin, and minocycline, was first tested328 for its effectiveness against Escherichia coli–infected dentin in vitro. The same research group also tested its bactericidal efficacy against microbes from carious dentin and infected pulp. They found that the mixture of antibiotics is sufficiently potent to eradicate the bacteria.391 The clinical effectiveness of the triple-antibiotic paste in the disinfection of immature teeth with apical periodontitis has been reported.425 One potential concern of using an intracanal antibiotic paste is that it may cause bacterial resistance. Additionally, intracanal use of minocycline can cause tooth discoloration, creating potential cosmetic complications.
Bioactive Glass
Research is underway in the use of bioactive glass as an intracanal medicament. In one study,440 the glass used was composed of 53% SiO2 (w/w), 23% Na2O, 20% CaO, and 4% P2O5 and was prepared from reagent-grade Na2CO3, CaHPO4, 2H2O, CaCO3, and Belgian sand. When used in root canals, bioactive glass was found to kill bacteria, but the mechanism of action was not pH related, and dentin did not seem to alter its effect. Some new obturating materials (e.g., Resilon [Pentron Clinical Technologies, Wallingford, CT]) contain bioactive glass.
New Irrigation and Disinfection Techniques and Devices
Other than conventional irrigation, additional techniques for endodontic disinfection have been proposed and tested, including laser systems and gaseous ozone. Recently several new devices for endodontic irrigation and/or disinfection have been introduced, among which are the EndoActivator System (DENTSPLY Tulsa Dental Specialties, Tulsa, OK), passive ultrasonic irrigation, EndoVac (Discus, Culver City, CA), the Safety-Irrigator (Vista Dental Products, Racine, WI), the self-adjusting file (ReDent, Raanana, Israel), photoactivation disinfection, and ozone. These new devices use pressure, vacuum, oscillation, and/or a combination with suction. For most of these devices and techniques, no data exists on the clinical efficacy, risk, frequency, and intensity of apical irrigant extrusion or the problems encountered with the use of negative pressure or oscillation.
EndoActivator
The EndoActivator System uses safe, noncutting polymer tips in an easy-to-use subsonic handpiece to quickly and vigorously agitate irrigant solutions during endodontic therapy (Fig. 8-52).
In a recent study,84 the safety of various intracanal irrigation systems was analyzed by measuring the apical extrusion of irrigant. They concluded that EndoActivator had a minimal, although statistically insignificant, amount of irrigant extruded out of the apex when delivering the subsonic activation of the irrigant into the pulp chamber and canal. Manual, ultrasonic, and Rinsendo (Dürr Dental, Bietigheim-Bissingen, Germany) groups had significantly greater amount of extrusion compared with EndoVac and EndoActivator.
Passive Ultrasonic Activation
Ultrasonic devices were first introduced in endodontics by Richman.312 Ultrasonically activated files have the potential to prepare and débride root canals mechanically. The files are driven to oscillate at ultrasonic frequencies of 25 to 30 kHz. They operate in a transverse vibration, setting up a characteristic pattern of nodes and antinodes along their length.413,414 Unfortunately, it proved difficult to control the cutting of dentin during ultrasonic preparation, which resulted in apical perforations and irregular canal shapes.235,380
However, it has been shown that ultrasonically driven files are effective for the irrigation of root canals. Two types of ultrasonic irrigation have been described in the literature6,420: one where irrigation is combined with simultaneous ultrasonic instrumentation (UI) and another without simultaneous instrumentation, called passive ultrasonic irrigation (PUI). During UI, the file is intentionally brought into contact with the root canal wall. UI has been shown to be less effective in removing simulated pulp tissue from the root canal system or smear layer from the root canal wall than PUI. This can be explained by a reduction of acoustic streaming and cavitation.6 Because the root canal anatomy is complex,302 an instrument will never contact the entire root canal wall.429 Thus, UI could result in uncontrolled cutting of the root canal wall without effective cleaning.
Passive ultrasonic irrigation was first described by Weller et al.420 The term passive does not adequately describe the process, since it is in fact active. When it was first introduced, the term passive related to the noncutting action of the ultrasonically activated file. PUI relies on the transmission of acoustic energy from an oscillating file or smooth wire to an irrigant in the root canal. The energy is transmitted by means of ultrasonic waves and can induce acoustic streaming and cavitation of the irrigant.6 After the root canal has been shaped to the master apical file (irrespective of the preparation technique used), a small file or smooth wire (for example, size #15) is introduced in the center of the root canal, as far as the apical region. The root canal is then filled with an irrigant solution, and the ultrasonically oscillating file activates the irrigant. Since the root canal has already been shaped, the file or wire can move freely, and the irrigant can penetrate more easily into the apical part of the root canal system,204 with the cleaning effect being more significant.235 Using this noncutting methodology, the potential to create aberrant shapes within the root canal are reduced to a minimum. A file larger than a #15 or #20 will only oscillate freely in a wide root canal. A #25 file may in fact produce less acoustic streaming than a #15 and #20 file.6 Consequently, using a file larger than a #20 may be considered fundamentally different from the basic principle of PUI. The cleaning efficacy of PUI implies the effective removal of dentin debris, microorganisms (planktonic or in biofilm), and organic tissue from the root canal. Because of the active streaming of the irrigant, its potential to contact a greater surface area of the canal wall will be enhanced.
If ultrasonic activation of the hypochlorite irrigant is to be used, it appears important to apply the ultrasonic instrument after canal preparation has been completed. A freely oscillating instrument will cause more ultrasound effects in the irrigating solution than a counterpart that binds to canal walls.318 In addition, ultrasonic files can cause uncontrolled cutting of the canal walls, especially if used during preparation.380 Therefore, it appears best to insert a slim, noncutting instrument in a controlled fashion after canal preparation.402,403 As of recently, smooth wires fitting to an ultrasonic device have been commercially available. However, objective guidelines regarding their risks and benefits have not been ascertained.439
EndoVac
EndoVac is a combined irrigation/evacuation system. The irrigant is extruded from the system with pressure at the root canal orifice. The evacuator, a microcannula, extends to the apical region of the root canal; the dimensions of the needle are size #55 with a 2% taper. High-volume suction from the dental unit results in negative apical pressure and thus passively sucks the irrigant from the orifice to the apical part of the root canal, which is passively evacuated from the canal space. Apical extrusion of the irrigant will probably be reduced, since the canal is irrigated with negative (as opposed to positive) pressure.283 There is little research to demonstrate this device is safe and effective when there is an open (immature) apex or in cases where there is a pathologic perforating root resorption.
A similar device has been presented by Fukumoto et al.119,120 The irrigant is delivered by a needle (outer diameter 0.41 mm, inner diameter 0.19 mm) and a tubing pump (Masterflex, Cole Palmer Instruments, Vernon Hills, IL) to the coronal or middle part of the root canal and aspirated by a second needle (outer diameter 0.55 mm, inner diameter 0.30 mm), which is introduced into the apical part of the root canal. The aspiration pressure is −20 kPa. The irrigant is flushed from the coronal to the apical part of the root canal and there finally suctioned. This technique in an in vitro experiment on teeth with resected apices resulted in significantly less overextrusion of irrigant than a conventional irrigation technique with the needle tip placed 2 mm short of the apex. When the needle was placed 3 mm from the apex, the results did not significantly differ (Figs. 8-53 to 8-56).
Safety-Irrigator
The Safety-Irrigator is an irrigation/evacuation system that apically delivers the irrigant under positive pressure through a thin needle containing a lateral opening, and evacuates the solution through a large needle at the root canal orifice. As of this printing, no information on its safety or efficacy is available (Fig. 8-57). Absent clear evidence of safety and efficacy through peer-reviewed literature, the reader is advised to use caution in choosing an irrigating device.
Self-Adjusting File
The SAF was recently introduced primarily as an endodontic file (discussed at length earlier under Instruments for Cleaning and Shaping the Root Canal Space). It should also be considered as an irrigation device since the file is hollow, which allows for continuous irrigation. The irrigant is delivered through a free-rotating hub to which a silicone tube is attached (Fig. 8-58). Either a special irrigation unit (see Fig. 8-30) (VATEA, ReDent, Raanana, Israel) or any physio-dispenser-type unit may be used to deliver a constant flow of irrigant at 5 ml/min. This maintains a continuous flow of fresh, fully active irrigant, facilitating an outflow of tissue debris and dentin powder that is generated by the file use. No positive pressure is thought to be created in the canal during this continuous irrigation procedure. The open metal lattice allows the irrigant to escape freely, minimizing the risk of irrigant transportation beyond the apical foramen.260 The replacement of irrigation fluid in the apical part of curved canals is demonstrated in Fig. 8-59. Further research on this device is underway.
HealOzone
Ozone (also known as triatomic oxygen or trioxygen) is a naturally occurring compound consisting of three oxygen atoms. It is found in nature in the form of a gas in the stratosphere at a concentration of 1 to 10 ppm, being continually created from and destroyed into molecular O2. The reliable microbiologic and metabolic properties of ozone, in either the gaseous or aqueous phases, make it a useful disinfectant with a wide range of activity. Ozone, in the gaseous or aqueous phase, has been shown to be a powerful and reliable antimicrobial agent against bacteria, fungi, protozoa, and viruses. It is generally accepted that the oxidant potential of ozone induces the destruction of cell walls and cytoplasmic membranes of bacteria and fungi. During this process, ozone attacks glycoproteins, glycolipids, and other amino acids and inhibits the enzymatic control system of the cell. This results in increased membrane permeability, the key element of cell viability, leading to immediate functional cessation. The ozone molecules then readily enter the cell and cause the microorganism to die. Ozone can also attack many biomolecules, such as the cysteine, methionine, and histidine residues of proteins. By oxidizing the biomolecules featured in dental diseases, ozone has a severely disruptive effect on cariogenic bacteria, resulting in the elimination of acidogenic bacteria. The strongest naturally occurring acid—produced by acidogenic bacteria during cariogenesis—is pyruvic acid. Ozone can decarboxylate this acid to acetic acid. It has been shown that remineralization of incipient carious lesions can be encouraged when the production of acetic acid, or other high pKa acids found in resting plaque, buffers plaque fluid.245
E.A. Fisch (1899–1966) was the first dentist to use ozonated water in his practice and introduced it to the German surgeon Erwin Payr (1871–1946) who used it from that time forward in surgery. He reported his results at the 59th Congress of the German Surgical Society in Berlin (1935). In dental surgery, ozonated water was used to promote hemostasis, enhance local oxygen supply, and inhibit bacterial proliferation. Theoretically, ozone can reduce the bacterial count in active carious lesions and therefore may temporarily arrest the progression of caries, resulting in preventing or delaying the need for tooth restorations.313 For review on ozone use in dentistry see Azarpazhooh and Limeback.17