1. Abbott PV, Hetthersay GS, Hume WR. Release and diffusion through human tooth roots in vitro of corticosteroid and tetracycline trace molecules from Ledennix® paste. Endod Dent Traumatol. 1988;4:55-62.
2. Abbott PV, Hume WR, Hetthersay GS. Barriers to diffusion of Ledennix® paste in radicular dentine. Endod Dent Traumatol. 1989;5:98.
3. Abbott PV, Hume WR, Hetthersay GS. Effects of combining Ledennix® and calcium hydroxide pastes on the diffusion of corticosteroid and tetracycline through human tooth roots in vitro. Endod Dent Traumatol. 1989;5:188.
4. Accorinte ML, Loguercio AD, Reis A, Costa CA. Response of human pulps capped with different self-etch adhesive systems. Clin Oral Invest. 2008;12:119.
5. Ahmad M. An analysis of breakage of ultrasonic files during root canal instrumentation. Endod Dent Traumatol. 1989;5:78.
6. Ahmad M, Pitt-Ford TR, Crum LA. Ultrasonic debridement of root canals: acoustic streaming and its possible role. J Endod. 1987;13:490.
7. Ahmad M, Roy RA, Ghanikamarudin A, Safar M. The vibratory pattern of ultrasonic files driven piezoelectrically. Int Endod J. 1992;26:120.
8. Al-Awadhi S, Spears R, Gutmann JL, Opperman LA. Cultured primary odontoblast viability and apoptosis in the presence of root canal sealers. J Endod. 2004;30:527.
9. Alfredo E, Silva SR, Ozbrio JE, Souza-Neto MD, Brugnera-Junior A, Silva-Sousa YT. Bond strength of AH Plus and Epiphany. Int Endod J. 2008;41:733.
10. Andersen M, Lund A, Andreasen JO, Andreasen FM. In vitro solubility of human pulp tissue in calcium hydroxide and sodium hypochlorite. Endod Dent Traumatol. 1992;8:104.
11. Anjo T, Ebihara A, Takeda A, Takashima M, Sunakawa M, Suda H. Removal of two types of root canal filling material using pulsed Nd:YAG laser irradiation. Photomed Laser Surg. 2004;22:470.
12. Archer R, Reader A, Nist R, Beck M, Meyers WJ. An in vivo evaluation of the efficacy of ultrasound after step-back preparation in mandibular molars. J Endod. 1992;18:549.
13. Arisu HD, Sadik B, Bala O, Turkoz E. Computer assisted evaluation of microleakage after apical resection with laser and conventional techniques. Laser Med Sci. 2008;23:415.
14. Asboe-Jorgensen V, Attstrom R, Lang NP, Löe H. Effect of a chlorhexidine dressing on the healing after periodontal surgery. J Periodontol. 1974;45:13.
15. Autegarden JE, Pecquet C, Huet S, Bayrou O, Leynadier F. Anaphylactic shock after application of chlorhexidine to unbroken skin. Contact Dermatitis. 1999;40:215.
16. Azar NG, Heidari M, Bahrami ZS, Shokri F. In vitro cytotoxicty of a new epoxy resin root canal sealer. J Endod. 2000;26:462.
17. Azarpazhooh A, Limeback H. The application of ozone in dentistry: A systematic review of literature. J Dent. 2008;36:104.
18. Balto H. An assessment of microbial coronal leakage of temporary filling material in endodontically treated teeth. J Endod. 2002;28:762.
19. Balto H, Al-Nazha N, Al-Mansour K, Al-Otaibi M, Siddiqu Y. Microbial leakage of Cavit, IRM, and Temp Bond in post-prepared root canals using two methods of gutta-percha removal: an invitro study. J Contemp Dent Pract. 2005;6:53.
20. Barbosa CA, Goncalves RB, Siqueira JFJr., De Uzeda M. Evaluation of the antibacterial activities of calcium hydroxide, chlorhexidine, and camphorated paramonochlorophenol as intracanal medicament. A clinical and laboratory study. J Endod. 1997;23:297.
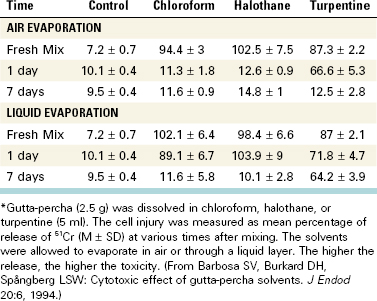
21. Barbosa SV, Burkard DH, Spångberg SW. Cytotoxic effects of gutta-percha solvents. Pediatr Dent. 1994;16:102.
22. Barkhordar RA, Kempler D. A comparison between xeroradiography and conventional radiography in the diagnosis of endodontic lesions. Oral Surg Oral Med Oral Pathol. 1988;66:489.
23. Barthel CR, Zimmer S, West G, Roulet JF. Bacterial leakage in obturated root canals following the use of different intracanal medicaments. Endod Dent Traumatol. 2000;16:282.
24. Basrani B, Ghanem A, Tjäderhane L. Physical and chemical properties of chlorhexidine and calcium hydroxide-containing medications. J Endod. 2004;30:413.
25. Basrani B, Lemonie C. Chlorhexidine gluconate. Aust Endod J. 2005;31:48.
26. Basrani B, Manek S, Sodhi R, Fillery E, Manzur A. Interaction between sodium hypochlorite and chlorhexidine gluconate. J Endod. 2007;33:966.
27. Basrani B, Santos JM, Tjäderhane L, et al. Substantive antimicrobial activity in chlorhexidine-treated human root dentin. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:240.
28. Basrani B, Tjäderhane L, Santos JM, et al. Efficacy of chlorhexidine- and calcium hydroxide-containing medicaments against Enterococcus faecalis in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:618.
29. Baumgartner JC, Johal S, Marshall JG. Comparison of the antimicrobial efficacy of 1.3% NaOCl/Biopure MTAD to 5.25% NaOCl/15% EDTA for root canal irrigation. J Endod. 2007;33:48.
30. Beach CW, Bramwell JD, Hutter JW. Use of an electronic apex locator on a cardiac pacemaker patient. J Endod. 1996;22:182.
31. Beltz RE, Torabinejad M, Pouresmail M. Quantitative analysis of the solubilizing action of MTAD, sodium hypochlorite, and EDTA on bovine pulp and dentin. J Endod. 2003;29:334.
32. Bender IB, Landau MA, Fonsecca S, Trowbridge HO. The optimum placement-site of the electrode in electric pulp testing of the 12 anterior teeth. J Am Dent Assoc. 1989;118:305-310.
33. Bergmans L, Moisiadis P, Huybrechts B, Van Meerbeek B, Quirynen M, Lambrechts P. Effect of photo-activated disinfection on endodontic pathogens ex vivo. Int Endod J. 2008;41:227.
34. Bernath M, Szabo J. Tissue reaction initiated by different sealers. Int Endod J. 2003;36:256.
35. Bodrumlu E, Alacam T. Evaluation of anitmicrobial effects of idoform-integrating gutta-percha. J Can Dent Assoc. 2006;72:733.
36. Bodrumlu E., Alacam T, Semiz M. The antimicrobial and antifungal efficacy of tetracycline-integrated gutta-percha. Indian J Dent Res. 2008;19:112.
37. Bodrumlu E, Muglali M, Sumer M, Guvenc T. The response of subcutaneous connective tissue to a new filling material. J Biomed Mater Res B Appl Biomater. 2008;84:463.
38. Bodrumlu E, Tonga U. The apical sealing ability of a new root canal filling material. Am J Dent. 2007;20:295.
39. Bodrumlu E, Uzam O, Topuz O, Semiz M. Efficacy of 3 techniques in removing root canal filling material. J Can Dent Assoc. 2008;74:721.
40. Bortnick KL, Steiman HR, Ruskin A. Comparison of nickel-titanium file distortion using electric and air-driven handpieces. J Endod. 2001;27:57.
41. Bouillaguet S, Shaw L, Barthelemy J, Krejci I, Wataha JC. Long-term sealing ability of Pulp Canal Sealer, AH-Plus, GuttaFlow and Epiphany. Int Endod J. 2008;41:219.
42. Bozza FL, Molgatini SL, Perez SB, Tejerin DP, Perez Tito RI, Kaplan AE. Antimicrobial effect in vitro of chlorhexidine and calcium hydroxide impregnated gutta-percha points. Acta Odontol Latinoam. 2005;18:51.
43. Briseňo-Marroquin B, El-Syed MAA, Willershausen-Zonnchen B. Morphology of the physiological foramen: I. Maxillary and mandibular molars. J Endod. 2004;30:321.
44. Brown BDK, Kafrawy AH, Patterson SS. Studies of Sargenti technique of endodontics: autoradiographic and scanning electron microscope studies. J Endod. 1979;5:14.
45. Brugnera A, Zanin F, Barbin EL, Spano JC, Santana R, Pecora JD. Effect of Er:YAG and Nd:YAG laser irradiation on radicular dentine permeability using different irrigating solutions. Lasers Surg Med. 2003;33:256.
46. Byström A, Claesson R, Sundqvist G. The antimicrobial effect of camphorated paramono-chlorphenol, camphorated phenol, and calcium hydroxide in the treatment of infected root canals. Endod Dent Traumatol. 1985;1:170.
47. Byström A, Sundqvist G. Bacteriologic evaluation of the effect of 0.5 percent sodium hypochlorite in endodontic therapy. Oral Surg Oral Med Oral Pathol. 1983;55:307.
48. Byström A, Sundqvist G. The antibacterial action of sodium hypochlorite and EDTA in 60 cases of endodontic therapy. Int Endod J. 1985;18:35.
49. Cabral CT, Fernandes MH. In vitro comparison of chlorhexidine and povidone-iodine on the long-term proliferation and functional activity of human alveolar bone cells. Clin Oral Invest. 2007;11:155.
50. Calan J. The use of inert plastic material in reconstructive surgery. Br J Plast Surg. 1963;16:1.
51. Camps J, Pashley D. Reliability of the dye penetration studies. J Endod. 2003;29:592.
52. Canadian Center for Occupational Health and Safety, ChemInfo, Record 728, 1994.
53. Carrilho MR, Carvalho RM, De Goes MF, et al. Chlorhexidine preserves dentine bond in vitro. J Dent Res. 2007;86:90.
54. Carrilho MR, Carvalho RM, Tay FR, Yiu C, Pashley DH. Durability of resin-dentin bonds related to water and oil storage. Am J Dent. 2005;18:315.
55. Carvalho CA, Valera MC, Gown-Soares S, dePaula Eduardo C. Effects of Nd:YAG and Er:YAG lasers on the sealing of root canal fillings. J Clin Laser Med Surg. 2002;20:215.
56. Caso A, Hung LK, Beirne OR. Prevention of alveolar osteitis with chlorhexidine: a meta-analytic review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:155.
57. Chen H, Teixeira FB, Ritter AL, Levin L, Trope M. The effect of intracanal anti-inflammatory medicaments on external root resorption of replanted dog teeth after extended extra-oral dry time. Dent Traumatol. 2008;24:74.
58. Cheung GS, Stock CJ. In vitro cleaning ability of root canal irrigants with and without endosonics. Int Endod J. 1993;26:334.
59. Chogle S, Mickel AK, Huffaker SK, Neibaur B. An in vitro assessment of iodoform gutta-percha. J Endod. 2005;31:814.
60. Chutich MJ, Kaminski EJ, Miller DA, Lautenschlager EP. Risk management of the toxicity of gutta-percha used in endodontic retreatment. J Endod. 1998;24:213.
61. Cobankara FK, Altinoz HC, Ergani O, Kav K, Belli S. In vitro antibacterial activities of root canal sealers using two different methods. J Endod. 2004;30:57.
62. Cohen BD, Combe ED, Lilley JD. Effects of thermal placement techniques on some physical properties of gutta-percha. Int Endod J. 1992;25:292.
63. Cohen BI, Pagnillo MK, Musikant BL, Deutsch AS. Formaldehyde evaluation from endodontic materials. Oral Health. 1998;88(12):37-39.
64. Cooley RL, Robison SF. Variables associated with electric pulp testing. Oral Surg Oral Med Oral Pathol. 1980;50:66.
65. Coronal Leakage: Clinical and Biological Implications, Endodontics: Colleagues for Excellence, American Association of Endodontics, Fall/Winter 2002.
66. Cotti E, Campisi G, Garau V, Puddu G. A new technique for the study of peripical bone lesions: Ultrasound real-time imaging. Int Endod J. 2002;35:142.
67. Cotton TP, Geisler TM, Holden DT, Schwartz SA, Schindler WG. Endodontic applications of cone 381 beam volumetric tomography. J Endod. 2007;33:1121.
68. Cunningham WT, Martin H. A scanning electron microscope evaluation of root canal debridement with the endosonic ultrasonic synergistic system. Oral Surg Oral Med Oral Pathol. 1982;53:527.
69. Dalat DM, Spångberg LSW. Comparison of apical leakage in root canals obturated with various gutta-percha techniques using a dye vacuum tracing method. J Endod. 1994;20:315.
70. Dammaschke T, Schneider U, Stratmann U, Yoo JM, Schafer E. Effect of root canal dressings on the regeneration of inflamed periapical tissue. Acta Odontol Scand. 2005;63:143.
71. Daugherty DW, Gound TG, Comer TL. Comparison of fracture rate, deformation rate, and efficiency between rotary endodontic instruments driven at 150 rpm and 350 rpm. J Endod. 2001;27:93.
72. Davies A. The mode of action of chlorhexidine. J Periodontal Res. 1973;12(Suppl):68.
73. Davis JM, Maki J, Bahcall JK. An in vitro comparison of the antimicrobial effects of various endodontic medicaments on Enterococcus faecalis. J Endod. 2007;33:567.
74. Decosta Rebeiro A, Nogueira GE, Antoniazzi JH, Moritz A, Zezell DM. Effects of diode lasers (810 nm) irradiation on root canal walls: Thermographic and morphological studies. J Endod. 2007;33:252.
75. De-Deus G, Brandao MC, Fidel RA, Fidel SR. The sealing ability of GuttaFlow in oval-shaped canals: an ex vivo study using polymicrobial leakage model. Int Endod J. 2007;40:794.
76. De-Deus G, Gurgel-Filho ED, Magalhaes KM, Coutinho-Filho T. A laboratory analysis of gutta-percha filled area obtained using Thermafil, System B and lateral condensation. Int Endod J. 2006;39:378.
77. De Gee AJ, Wu MK, Wesselink PR. Sealing properties of a Ketac-Endon glass ionomer cement an AH26 root canal sealer. Int Endod J. 1994;27:239.
78. Delany GM, Patterson SS, Miller CH, Newton CW. The effect of chlorhexidine gluconate irrigation on the root canal flora of freshly extracted necrotic teeth. Oral Surg Oral Med Oral Pathol. 1982;53:518.
79. deMoura-Netto C, deMoura AA, Davidowicz H, Aun CE, Antonio MP. Morphologic changes and removal of debris on apical dentin surfaces after Nd:YAG laser and diode laser irradiation. Photomed Laser Surg. 2008;26:263.
80. De Munck J, Van Landuyt K, Peumans M, et al. A critical review of the durability of adhesion to tooth tissue: methods and results. J Dent Res. 2005;84:118.
81. Denton G Chlorhexidine Block SS, editor. Disinfection, sterilization and preservation, 4th ed, Philadelphia: Lea & Febiger, 1991.
82. Depraet FJ, DeBruyne MA, DeMoor RJ. The sealing ability of an epoxy root canal sealer of Nd:YAG laser irradiation. Int Endod J. 2005;38:302.
83. De Rossi A, Silva LA, Leonardo MR, Rocha LB, Rossi MA. Effect of rotary or manual instrumentation, with or without a calcium hydroxide, 1% chlorhexidine intracanal dressing, on the healing of experimentally induced chronic periapical lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:628.
84. Desai P, Himel V. Comparative safety of various intracanal irrigation systems. J Endod. 2009;35:545.
85. Desai S, Chandler N. Calcium hydroxide-based root canal sealers: a review. J Endod. 2009;35:475.
86. DeSouza EB, Cai S, Simionato MR, Lage-Marques JL. High power diode laser in a disinfection in depth of the root canal dentin. Oral Surg Oral Med Oral Pathol. 2008;106:e68.
87. DeSouza EB, deAmorim CV, Marques JL. Effect of diode laser irradiation on the apical sealing of MTA retrofillings. Braz Oral Res. 2006;20:231.
88. Deveaux E, Hildebert P, Neut C, Romond C. Bacterial microleakage of Cavit, IRM, TERM, and Fermit: a 21-day in vitro study. J Endod. 1999;25:653.
89. Donley DL, Weller RN, Kulild JC, Jucak JJ. In vitro intra-canal temperatures produced by low- and high temperature thermoplasticized injectible gutta-percha. J Endod. 1991;17:307.
90. Dove SB, McDavid WD, Hamilton KE. Analysis of sensitivity and specificity of a new digital conventional radiography and computed tomography. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:682.
91. Ebihara A, Majaron B, Liaw LL, Krasieva TB, Wilder-Smith P. Er:YAG laser modification of root canal dentine: influence of pulse duration, repetitive irradiation and water spray. Laser Med Sci. 2002;17:198.
92. Ebo DG, Stevens WJ, Bridts CH, Matthieu L. Contact allergic dermatitis and life-threatening anaphylaxis to chlorhexidine. J Allergy Clin Immunol. 1998;101:128.
93. Ehrmann EH. Pulp testers and pulp testing with particular reference to the use of dry ice. Aust Dent J. 1977;22:272.
94. Eideniz AU, Ozer F, Hadimli HH, Erganis O. Bacterial efficacy of Er, Cr:YSGG laser irradiation against Enterococcus facecalis compared with NaOCl irrigation: an ex vivo pilot study. Int Endod J. 2007;40:112.
95. El Ayouti A, Weiger R, Löst C. The ability of Root ZX apex locator to reduce the frequency of overestimated radiographic working length. J Endod. 2002;28:116.
96. Ellerbruch ES, Murphy RA. Antimicrobial activity of root canal medicament vapors. J Endod. 1977;3:189.
97. Emilson CG, Ericson T, Heyden G, Magnusson BC. Uptake of chlorhexidine to hydroxyapatite. J Periodontal Res. 1973;12(Suppl):17.
98. Ercan E, Dalli M, Duulgergil CT, Yaman F. Effect of intracanal medication with calcium hydroxide and 1% chlorhexidine in endodontic retreatment cases with periapical lesions: an in vivo study. J Formos Med Assoc. 2007;106:217.
99. Ercan E, Ozekinci T, Atakul F, Gul K. Antibacterial activity of 2% chlorhexidine gluconate and 5.25% sodium hypochlorite in infected root canal: in vivo study. J Endod. 2004;30:84.
100. Estrela C, Estrela CRA, Decurcio DA, Hollanda ACB, Silva JA. Antimicrobial efficacy of ozonated water, gaseous ozone, sodium hypochlorite and chlorhexidine In infected human root canals. Int Endod J. 2007;40:85.
101. Eun HC, Lee AY, Lee YS. Sodium hypochlorite dermatitis. Contact Dermatitis. 1984;11:45.
102. Evans MD, Baumgartner JC, Khemaleelakul SU, Xia T. Efficacy of calcium hydroxide: chlorhexidine paste as an intracanal medication in bovine dentin. J Endod. 2003;29:338.
103. Fanibunda KB. The feasibility of temperature measurement as a diagnostic procedure in human teeth. J Dent. 1986;14:126.
104. Fardal O, Turnbull RS. A review of the literature on use of chlorhexidine in dentistry. J Am Dent Assoc. 1986;112:863.
105. Faria G, Celes MR, De Rossi A, Silva LA, Silva JS, Rossi MA. Evaluation of chlorhexidine toxicity injected in the paw of mice and added to cultured L929 fibroblasts. J Endod. 2007;33:715.
106. Faria MI, Souza-Gabriel Ae, Marchesan MA, Sousa-Neto MD, Silva-Sousa YT. Ultrastructural evaluation of radicular dentin after Nd:YAG irradiation combined with different chemical substances. Gen Dent. 2008;56:641.
107. Fernandes AM, Silva GA, Lopes NJr., Napimoga MH, Benatti BB, Alves JB. Direct capping of human pulps with a dentin bonding system and calcium hydroxide: an immunohistochemical analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:385.
108. Fimple JL, Fontana CR, Foschi F, et al. Photodynamic treatment of endodontic polymicrobial infection in vivo. J Endod. 2008;34:728.
109. Folk RB, Thorpe JR, McClanahan SB, Johnson JD, Strother JM. Comparison of two different direct digital radiography systems for the ability to detect artificially prepared periapical lesions. J Endod. 2005;31:304.
110. Folwaczny M, Mehl A, Jordan C, Hickel R. Antibacterial effect of pulsed Nd:YAG laser radiation at different energy settings in the root canals. J Endod. 2002;28:24.
111. Fonseca MB, Junior PO, Pallota RC, et al. Photodynamic therapy for root canals in fected with Enterococcus faecalis. Photomed Laser Surg. 2008;26:209.
112. Foschi F, Fontana CR, Ruggeiro K, et al. Photodynamic inactivation of Enterococcus faecalis in dental root canals in vitro. Las Surg Med. 2007;39:782.
113. Fouad AF, Hopson JR, Martins JB, Krell KV, Drews TA, Jensen ME. Effects of selected electronic dental instruments on patients with cardiac pacemakers. J Endod. 1990;16:188.
114. Fouad AF, Krell KV, McKendry DJ, Koorbusch GF, Olson RA. A clinical evaluation of five electronic root canal length measuring instruments. J Endod. 1990;16:446.
115. Fouad AF, Reid LC. Effect of using electronic apex locators on selected endodontic treatment parameters. J Endod. 2000;26:364.
116. Frais S, Ng Y-L, Gulabivala K. Some factors affecting the concentration of commercially available sources of sodium hypochlorite. Int Endod J. 2001;34:206.
117. Frankenberger R, Pashley DH, Reich SM, Lohbauer U, Petschelt A, Tay FR. Characterisation of resin-dentine interfaces by compressive cyclic loading. Biomaterials. 2005;26:2043.
118. Friedman J, Marcus MI. Transillumination of the oral cavity with use of fiber optics. J Am Dent Assoc. 1970;80:801.
119. Fukumoto Y. Intracanal aspiration technique for root canal irrigation: evaluation of smear layer removal (in Japanese). Kokubyo Gakkai Zasshi. 2005;72:13.
120. Fukumoto Y, Kikuchi I, Yoshioka T, Kobayashi C, Suda H. An ex vivo evaluation of a new root canal irrigation technique with intracanal aspiration. Int Endod J. 2006;39:93.
121. Fuss Z, Trowbridge H, Bender IB, Rickoff B, Sorin S. Assessment of reliability of electrical and thermal pulp testing agents. J Endod. 1986;12:301.
122. Gabel WP, Hoen M, Steiman HR, Pink FE, Dietz R. Effect of rotational speed on nickel-titanium file distortion. J Endod. 1999;25:752.
123. Gambarini G, Pongione G, Rizzo F, Testarelli L, Cavalleri G, Gerosa R. Bending properties of nickel-titanium instruments: a comparative study. Minerva Stomatol. 2008;57:393.
124. Garcez AS, Nunez SC, Hamblin MR, Ribeiro MS. Antimicrobial effects of photodynamic therapy on patients with necrotic pulps and periapical lesions. J Endod. 2008;34:138.
125. Garcez AS, Ribeiro MS, Trope MS, et al. Antimicrobial therapy combined with conventional endodontic treatment to eliminate root canal biofilm infection. Lasers Surg Med. 2007;39:59.
126. Garofalo RR, Ede EN, Dorn SO, Kuttler S. Effect of electronic apex locators on cardiac pacemaker function. J Endod. 2002;28:831.
127. Garvey LH, Roed-Petersen J, Husum B. Anaphylactic reactions in anaesthetised patients—four cases of chlorhexidine allergy. Acta Anaesthesiol Scand. 2001;45:1290.
128. Gatewood RS, Himel VT, Dorn SO. Treatment of the endodontic emergency: a decade later. J Endod. 1990;16:284.
129. Gazelius B, Edwall B, Olgart L, Lundberg JM, Hokfelt T, Fisher JA. Vasodilatory effects and coexistence of calcitonin gene-related peptide (CGRP) and substance P in sensory nerves of cat dental pulp. Acta Physiol Scand. 1987;130:33.
130. Gazelius B, Olgart L, Edwall B, Edwall L. Non-invasive recording of blood flow in human dental pulp. Endod Dent Traumatol. 1986;2:219.
131. George R, Meyers IA, Walsh LJ. Laser activation of endodontic irrigants with improved conical laser fiber tips for removing smear layer with apical third of the root canal. J Endod. 2008;34:1524.
132. Ghori S, Gulabivala K, Premdas C, Spratt DA. Evaluation of the antimicrobial efficacy of electrochemically activated water on selected isolates from the root canal. Int Endod J. 2002;35:85.
133. Giardino L, Ambu E, Becce C, Rimondini L, Morra M. Surface tension comparison of four common root canal irrigants and two new irrigants containing antibiotic. J Endod. 2006;32:1091.
134. Giardino L, Ambu E, Savoldi E, Rimondini R, Cassanelli C, Debbia EA. Comparative evaluation of antimicrobial efficacy of sodium hypochlorite, MTAD, and tetraclean against Enterococcus faecalis biofilm. J Endod. 2007;33:852.
135. Goldberg F, De Silvio AC, Manfré S, Nastri N. In vitro measurement accuracy of an electronic apex locator in teeth with simulated apical root resorption. J Endod. 2002;28:461.
136. Goldman M, Kronman JH, Goldman LB, Clausen H, Grady J. New method of irrigation during endodontic treatment. J Endod. 1976;2:257.
137. Gomes BP, Ferraz CC, Vianna ME, Berber VB, Teixeira FB, Souza-Filho FJ. In vitro antimicrobial activity of several concentrations of sodium hypochlorite and chlorhexidine gluconate in the elimination of Enterococcus faecalis. Int Endod J. 2001;34:424.
138. Gomes BP, Sato E, Ferraz CC, Teixeira FB, Zaia AA, Souza-Filho FJ. Evaluation of time required for recontamination of coronally sealed canals medicated with calcium hydroxide and chlorhexidine. Int Endod J. 2003;36:604-609.
139. Goodman A, Schilder H, Aldrich W. The thermomechanical properties of gutta-percha II. Oral Surg Oral Med Oral Pathol. 1974;37:954.
140. Gopikrishna V, Pradeep G, Venkateshbabu N. Assessment of pulp vitality: a review. Int J Paediatr Dent. 2009;19:3.
141. Gordon MP, Chandler NP. Electronic apex locators. Int Endod J. 2004;37:425.
142. Gordon W, Atabakhsh VA, Meza F, et al. The antimicrobial efficacy of the erbium, chromium: yttrium-scandium-gallium-garnet laser with radial emitting tips on root canal dentin walls infected with Enterococcus faecalis. J Am Dent Assoc. 2007;138:992.
143. Gouw-Soares S, Staboltz A, Lage-Marques JL, Zezell DM, Groth EB, Edwardo CP. Comparative study of dentine permeability after apicoectomy and surface treatment with 9.6 micron TEA CO2 and Er:YAG laser irradiation. J Clin Laser Med Surg. 2004;22:129.
144. Goya C, Yamazaki R, Tomita Y, Kimura Y, Matsumoto K. Effects of pulsed Nd:YAG irradiation on smear layer at the apical stop and apical leakage after obturation. Int Endod J. 2000;33:266.
145. Grassi MD, Plazek DJ, Michanowicz AE, Chay IC. Changes in the physical properties of the ultrafil low temperature (70 degrees C) thermoplasticized gutta-percha system. J Endod. 1989;15:517.
146. Grawehr M, Sener B, Waltimo T, Zehnder M. Interactions of ethylenediamine tetraacetic acid with sodium hypochlorite in aqueous solutions. Int Endod J. 2003;36:411.
147. Greenstein G, Berman C, Jaffin R. Chlorhexidine. an adjunct to periodontal therapy. J Periodontol. 1986;57:370.
148. Grossman LI. Solibility of root canal cements. J Dent Res. 1978;57:927.
149. Grossman LI Clinical diagnostic methods Grossman LI, editor Endodontic practice 10th ed 1981 Lea & Febiger Philadelphia, PA 17-22
150. Grossman LI, Meiman BW. Solution of pulp tissue by chemical agent. J Am Dent Assoc. 1941;28:223.
151. Gulabivala K, personal communication, 2009.
152. Gulabivala K, Stock CJ, Lewsey JD, Ghori S, Ng YL, Spratt DA. Effectiveness of electrochemically activated water as an irrigant in an infected tooth model. Int Endod J. 2004;37:624.
153. Gundappa M, Ng SY, Whaites EJ. Comparison of ultrasound, digital and conventional radiography in the differentiation of periapical lesions. Dentomaxillofac Radiol. 2006;35:326.
154. Gurbuz T, Ozdemir Y, Kara N, Zehir C, Kurudirek M. Evaluation of root canal dentin after Nd:YAG laser irradiation and treatment with five different irrigation solutions: a preliminary study. J Endod. 2008;30:318.
155. Haapasalo HK, Siren EK, Waltimo TM, Orstavik D, Haapasalo MP. Inactivation of local root canal medicaments by dentine: An in vitro study. Int Endod J. 2000;33:126.
156. Habets JM, Geursen-Reitsma AM, Stolz E, Van Joost T. Sensitization to sodium hypochlorite causing hand dermatitis. Contact Dermatitis. 1986;15:140.
157. Haenni S, Schmidlin PR, Mueller B, Sener B, Zehnder M. Chemical and antimicrobial properties of calcium hydroxide mixed with irrigating solutions. Int Endod J. 2003;36:100.
158. Haikel Y, Gasser P, Allemann C. Dynamic fracture of hybrid endodontic hand instruments compared with traditional files. J Endod. 1991;17:217.
159. Hamblin MR, Hasan T. Photodynamic therapy: A new antimicrobial approach to infectious disease. Photochem Photobiol Sciences. 2004;3:436.
160. Hammad M, Qualtrough A, Silikas N. Effect of new obturating materials on vertical root fracture resistance of endodontically treated teeth. J Endod. 2007 Jun;33(6):732-736. (Epub 2007 Apr 16)
161. Hasselgren G, Olsson B, Cvek M. Effects of calcium hydroxide and sodium hypochlorite on the dissolution of necrotic porcine muscle tissue. J Endod. 1988;14:125.
162. Hata G, Hayami S, Weine FS, Toda T. Effectiveness of oxidative potential water as a root canal irrigant. Int Endod J. 2001;34:308.
163. Haznedaroglu F, Ersev H. Tetracycline HCl solution as a root canal irrigant. J Endod. 2001;27:738.
164. Hems RS, Gulabivala K, Ng YL, Ready D, Spratt DA. An in vitro evaluation of the ability of ozone to kill a strain of Enterococcus faecalis. Int Endod J. 2005;38:22.
165. Hennessey TS. Some antibacterial properties of chlorhexidine. J Periodontal Res. 1973;12(Suppl):61.
166. Hermann BW. Calciumhydroxyd als mittel zum behandel und füllen von zahnwurzelkanälen, Dissertation. Germany: University of Würzburg; 1920. Med Diss V
167. Hermann BW. Dentin obliteration der wurzelkanäle nach behandlung mit calcium. Zahnärtzl Rundschau. 1930;39:888.
168. Hidalgo E, Dominguez C. Mechanisms underlying chlorhexidine-induced cytotoxicity. Toxicol In Vitro. 2001;15:271.
169. Hjeljord LG, Rolla G, Bonesvoll P. Chlorhexidine-protein interactions. J Periodontal Res. 1973;12(Suppl):11.
170. Holland G, Walton R. Diagnosis and treatment planning. In Torabinejad M, Walton R, editors: Endodontics: principles and practice, ed 4, St. Louis: Saunders, 2009.
171. Holloway GA. Laser doppler measurement of cutaneous blood flow. In: Rolfe P, editor. Non-invasive physiological measurements. London: Academic Press Inc.; 1983:11.
172. Horiba N, Hiratsuka K, Onoe T, et al. Bactericidal effect of electrolysed neutral water on bacteria isolated from infected root canals. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:83.
173. Howell RM, Duell RC, Mullaney TP. The determination of pulp vitality by thermographic means using cholesteric liquid crystals. Oral Surg Oral Med Oral Pathol. 1970;29:763.
174. Hsieh YD, Gau CH, Kung Wu SF, Shen EC, Hsu PW, Fu E. Dynamic recording of irrigating fluid distribution in root canals using thermal image analysis. Int Endod J. 2007;40:11.
175. Huang FM, Chang YC. Prevention of the epoxy resin-based root canal sealer-infused cyclooxygenase-2 expression and cytotoxicity of human oseteoblast cells by various antioxidants. Biomaterials. 2005;26:1849.
176. Huang FM, Tai KW, Chou MY, Chang YC. Cytotixicty of resin-, zinc oxide-eugenol-, and calcium hydroxide-based root canal sealers on human periodontal ligament cells and permanent V79 cells. Int Endod J. 2002;35:153.
177. Hugh CL, Walton RE, Facer SR. Evaluation of intracanal sealer distribution with 5 different obturation techniques. Quintessence Int. 2005;36:712.
178. Hugo WB, Longworth AR. The effect of chlorhexidine on the electrophoretic mobility, cytoplasmic constituents, dehydrogenase activity and cell walls of Excherichia coli and Staphylococcus aureus. J Pharm Pharmacol. 1966;18:569.
179. Hugo WB, Longworth ARL. Some aspects of the mode of action of chlorhexidine. J Pharm Pharmacol. 1964;16:655.
180. Hülsmann M, Pieper K. Use of an electronic apex locator in the treatment of teeth with incomplete root formation. Endod Dent Traumatol. 1989;5:238.
181. Hülsmann M, Rödig T, Nordmeyer S. Complications during root canal irrigation. Endod Topics. 2007;16:27.
182. Iatha H, Sandeep M, Kulkarni S, Yakub SS. Evaluation of fracture resistance in simulated immature teeth using Resilon and Ribbond as root reinforcements—an in vitro study. Dent Traumatol. 2009;25:433-438.
183. Ichikawa K, Nakamura HK, Ogawa N, Sakimura T, Kuroda M. R&D of long-term life-support system by using electrochemically activated biofilm reactor of aquatic animals for space examinations. Biological Science in Space. 1999;13:348.
184. Jeansonne MJ, White RR. A comparison of 2.0% chlorhexidine gluconate and 5.25% sodium hypochlorite as antimicrobial endodontic irrigants. J Endod. 1994;20:276.
185. Jha D, Guerrero A, Ngo T, Helfer A, Hasselgren G. Inability of laser and rotary instrumentation to eliminate root canal infection. J Am Dent Assoc. 2006;137:67.
186. Johnson E, Lloyd A, Kuttler S, Namerow K. Comparison between a novel nickel-titanium alloy and 508 Nitinol on the cyclic fatigue life of ProFile 25/.04 rotary instruments. J Endod. 2008;34:1406.
187. Kaplan AE, Ormaechea MF, PIcca M, Canzobre MC, Ubios AM. Rheological properties and biocompatibility of endodontic sealers. Int Endod J. 2003;36:527.
188. Karlovic Z, Pezelj-Ribaric S, Miletic I, Jukic S, Grgurevic J, Anic I. Erbium:YAG laser versus ultrasonic in preparation of root-end cavities. J Endod. 2005;31:821.
189. Katz A, Tagger M, Tamse A. Rejuvenation of brittle gutta-percha cones – a universal technique? J Endod. 1987;13:65.
190. Keate KC, Wong M. A comparison of endodontic file tip quality. J Endod. 1990;16:486.
191. Kim S, Dorscher-Kim J, Liu MT, Trowbridge H. Biphasic pulp blood flow response to substance P in the dog as measured with radiolabeled, microsphere injection method. Arch Oral Biol. 1988;33:305.
192. Kim S, Schuessler G, Chien S. Measurement of blood flow in the dental pulp of dogs with Xe-133 washout method. Arch Oral Biol. 1983;28:501.
193. Kimura Y, Onaga K, Yokoyama K, Kinoshita J, Ogata Y, Matsumoto K. Root surface temperature increase during Er:YAG laser irradiation on root canals. J Endod. 2002;28:76.
194. Kimura Y, Wilder-Smaith P, Matsumoto K. Lasers in endodontics: a review. Int Endod J. 2000;33:173.
195. Klotz MD, Gerstein HS, Bahn HN. Bacteraemia after topical use of prednisolone in infected pulps. J Am Dent Assoc. 1965;71:871.
196. Koba K, Kimura Y, Matsumoto K, et al. Post-operative symptoms and healing after endodontic treatment of infected teeth using pulsed Nd:YAG laser. Endod Dent Traumatol. 1999;15:68.
197. Kobayashi C, Suda H. New electronic canal measuring device based on the ratio method. J Endod. 1994;20:111.
198. Kolokuris I, Avanitoyannis I, Robinson C, Blanshard JM. Effect of moisture and aging on gutta-percha. J Endod. 1992;18:583.
199. Komorowski R, Grad H, Yu-Wu X, Friedman S. Antimicrobial substantivity of chlorhexidine-treated bovine root dentin. J Endod. 2000;26:315.
200. Kontakiotis EG, Tzanetakis GN, Loizides AL. A 12 month longitudinal in vitro leakage study on a new silicon-based root canal filling material (Gutta-Flow). Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:854.
201. Krause TA, Liewehr FR, Hahn CL. The antimicrobial effect of MTAD, sodium hypochlorite, doxycycline, and citric acid on Enterococcus faecalis. J Endod. 2007;33:28.
202. Krautheim AB, Jermann TH, Bircher AJ. Chlorhexidine anaphylaxis: case report and review of the literature. Contact Dermatitis. 2004;50:113.
203. Krell KV. The effects of CO2 ice on PFM restorations. J Endod. 1985;11:51.
204. Krell KV, Johnson RJ, Madison S. Irrigation patterns during ultrasonic canal instrumentation. Part I: K-type files. J Endod. 1988;14:65.
205. Krupp JD, Brantley WA, Gerstein H. An investigation of the torsional and bending properties of seven brands of endodontic files. J Endod. 1984;10:372.
206. Kulid J. Diagnostic testing. In: Ingle JI, Bakland L, Baumgartner C, editors. Endodontics. ed 6. Hamilton: BC Decker Inc.; 2008:542-543.
207. Kullendorff B, Petersson K, Rohlin M. Direct digital radiography for the detection of periapical bone lesions: a clinical study. Endod Dent Traumatol. 1997;13:183.
208. Kuruvilla JR, Kamath MP. Antimicrobial activity of 2.5% sodium hypochlorite and 0.2% chlorhexidine gluconate separately and combined, as endodontic irrigants. J Endod. 1998;24:472.
209. Lai YY, Pai L, Chen cp. Marginal leakage of different temporary restorations in standardized complex endodontic access preparations. J Endod. 2007;33:875.
210. Lambrianidis T, Kosti E, Boutsioukis C, Mazinis M. Removal efficacy of various calcium hydroxide/chlorhexidine medicaments from the root canal. Int Endod J. 2006;39:55-61.
211. Lambrianidis T, Margelos J, Beltes P. Removal efficiency of calcium hydroxide dressing from the root canal. J Endod. 1999;25:85.
212. Larsen CM, Watanabe I, Glickman GN, He J. Cyclic fatigue analysis of a new generation of nickel titanium rotary instruments. J Endod. 2009;35:401.
213. Lautenschlager EP, Jacobs JJ, Marshall GW, Heuer MA. Brittle and ductile torsional failures of endodontic instruments. J Endod. 1977;3:175.
214. Lee DH, Kim NR, Lim BS, Lee YK, Hwang KK, Yang HC. Effects of root canal sealers on lipopolysaccharide expression of cyclooxygenase-2 in murine macrophage cells. J Endod. 2007;33:1329.
215. Lee DH, Lim BS, Lee YK, Kim NR, Yang HC. Inhibitory effects of root canal sealers on the expression of inducible nitric oxide synthase in lipopolysaccharide-stimulated murine macrophage cells. J Biomed Mater Res. 2007;83:91. B
216. Lee DH, Park B, Saxena A, Serene TP. Enhanced surface hardness by boron implantation in nitinol alloy. J Endod. 1996;22:543.
217. Lenet BJ, Komorowski R, Wu XY, et al. Antimicrobial substantivity of bovine root dentin exposed to different chlorhexidine delivery vehicles. J Endod. 2000;26:652.
218. Leonardo MR, Bezerra da Silva LA, Filho MT, Santana da Silva R. Release of formaldehyde by 4 endodontic sealers. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:221-225.
219. Leonardo MR, Guillen-Carlas MG, Pecora JD, Ito IY, Silva LA. Er:YAG laser antimicrobial effects in the root canals of dogs’ teeth with pulp necrosis and chronic periapical lesions. Photomed Laser Surg. 2005;38:295.
220. Leonardo MR, Tanomaru Filho M, Silva LA, Nelson Filho P, Bonifacio KC, Ito IY. In vivo antimicrobial activity of 2% chlorhexidine used as a root canal irrigating solution. J Endod. 1999;25:167.
221. Lewis BB, Chester SB. Formaldehyde in dentistry: a review of mutagenic and carcinogenic potential. J Am Dent Assoc. 1981;103:429.
222. Leyhausen G, Heil J, Reifferscheid G, Waldmann P, Geurtsen W. Genotoxicity of the epoxy resin-based root canal sealer AH Plus. J Endod. 1999;25:109.
223. Lima KC, Fava LR, Siqueira JFJr. Susceptibilities of Enterococcus faecalis biofilms to some antimicrobial medications. J Endod. 2001;27:616.
224. Limkangwalmongkol S, Burtscher P, Abbot PV, Sandler AB, Bishop BM. A comparative study of the apical leakage of four root canal sealers and laterally condensed gutta-percha. J Endod. 1991;17:495.
225. Lin J, Chandler NP, Purton D, Monteith B. Appropriate electrode placement site for electric pulp testing first molar teeth. J Endod. 2007;33:1296.
226. Lin S, Zuckerman O, Weiss EI, Fuss Z. Antibacterial efficacy of a new chlorhexidine slow-releasing device to disinfect dentinal tubules. J Endod. 2003;29:416.
227. Lindberg LG, Tamura T, Öberg PÅ. Photoplethysmography. Part 1: comparison with laser doppler flowmetry. Med Biol Eng Comput. 1991;29:40.
228. Lindskog S, Pierce AM, Blomlof L. Chlorhexidine as a root canal medicament for treating inflammatory lesions in the periodontal space. Endod Dent Traumatol. 1998;14:186.
229. Lipski M. In vitro infrared thermographic assessment of root surface temperatures generated by high-temperature thermo-plasticized injectible gutta-percha obturation technique. J Endod. 2006;32:438.
230. Lipski M. Root surface temperature rises in vitro during root canal obturation with thermoplasticized gutta-percha on a carrier or by injection. J Endod. 2004;30:441.
231. Löe H. Does chlorhexidine have a place in the prophylaxis of dental diseases? J Periodontal Res. 1973;12(Suppl):93.
232. Löe H, Schiott CR. The effect of mouthrinses and topical application of chlorexidine on the development of dental plaque and gingivitis in man. J Periodontal Res. 1970;5(Suppl):79.
233. Lohbaur U, Dahl U, Dasch W, Petschelt A. Calcium release and pH of gutta-percha points containing calcium hydroxide. J Dent Res. 2001;80:272.
234. Loshon CA, Melly E, Setlow B, Setlow P. Analysis of the killing of spores of Bacillus subtilis by a new disinfectant, Sterilox®. J Appl Microbiol. 2001;91:1051.
235. Lumley PJ, Walmsley AD. Effect of precurving on the performing of endosonic K files. J Endod. 1992;18:232.
236. Maden M, Gorgul G, Tinaz AC. Evaluation of apical leakage of root canals obturated with Nd:YAG laser-softened gutta-percha, System B, and lateral condensation techniques. J Contemp Dent Pract. 2002;3:16.
237. Magnusson B, Heyden G. Autoradiographic studies of 14C-chlorhexidine given orally in mice. J Periodontal Res. 1973;Suppl 12(Suppl):49.
238. Malkhassian G, Manzur AJ, Legner M, et al. Antibacterial efficacy of MTAD final rinse and two percent chlorhexidine gel medication in teeth with apical periodontitis: a randomized double-blinded clinical trial. J Endod. 2009;35:1483-1490.
239. Manzur A, Gonzalez AM, Pozos A, Silva-Herzog D, Friedman S. Bacterial quantification in teeth with apical periodontitis related to instrumentation and different intracanal medications: a randomized clinical trial. J Endod. 2007;33:114.
240. Marais JT, Brozel VS. Electro-chemically activated water in dental unit water lines. Br Dent J. 1999;187:154.
241. Marais JT, Williams WP. Antimicrobial effectiveness of electro-chemically activated water as an endodontic irrigation solution. Int Endod J. 2001;34:237.
242. Marciano J, Michailesco PM. Dental gutta-percha: chemical composition, x-ray identification, enthalpic studies, and clinical implications. J Endod. 1989;15:149.
243. Margelos J, Eliades G, Verdelis C, Palaghias G. Interaction of calcium hydroxide with zinc oxide-eugenol type sealers: a potential clinical problem. J Endod. 1997;23:43.
244. Margelos J, Verdelis K, Eliades G. Chloroform uptake by gutta-percha and assessment of its concentration in air during the chloroform-dip technique. J Endod. 1996;22:547.
245. Margolis HC, Moreno EC, Murphy BJ. Importance of high pKa acids in cariogenic potential of plaque. J Dent Res. 1985;64:786-792.
246. Marmary Y, Koter T, Heling I. The effect of periapical rarefying osteitis on cortical and cancellous bone. A study comparing conventional radiographs with computed tomography. Dentomaxillofac Radiol. 1999;28:267.
247. Martin H, Ferris C, Mazzella W. An evaluation of media used in electric pulp testing. Oral Surg Oral Med Oral Pathol. 1969;27:374.
248. Matsumoto K. Lasers in endodontics. Dent Clin North Am. 2000;44:889.
249. Matsuya Y, Matsuya S. Effect of abietic acid and poly-methyl methacrylate on the dissolution process of zinc oxide-eugenol cement. Biomaterials. 1994;15:307.
250. Mayeda DL, Simon JH, Aimar DF, Finley K. In vivo measurement accuracy in vital and necrotic canals with the Endex apex locator. J Endod. 1993;19:545.
251. Melker KB, Vertucci FJ, Rojas MF, Progulske-Fox A, Belanger M. Antimicrobial efficacy of medicated root canal filling materials. J Endod. 2006;32:148.
252. Magnifying the surgical field with an OM. In: Merino EM, editor. Endodontic Microsurgery. London: Quintessence Pub Co; 2009:5-9.
253. Mesaros SV, Trope M. Revascularization of traumatized teeth assessed by laser doppler flowmetry: case report. Endod Dent Traumatol. 1997;13:24.
254. Messer HH, Feigal RJ. A Comparison of the antibacterial and cytotoxic effects of parachlorophenol. J Dent Res. 1985;64:818.
255. Metzger Z, Better H, Abramovitz I. Immediate root canal disinfection with ultraviolet light: an ex vivo feasibility study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:425.
256. Metzger Z, Teperovich E, Cohen R, Zary R, Paué F, Hülsmann M. Removal of apical smear layer by the Self Adjusting File (SAF): a scanning electron microscope study. J Endod. 2009. Submitted for publication
257. Metzger Z, Dotan M, Better H, Abramovitz I. Sensitivity of oral bacteria to 254 nm ultraviolet light. Int Endod J. 2007;40:120.
258. Metzger Z, Huber R, Slavescu D, Dragomirescu D, Tobis I, Better H. Healing kinetics of periapical lesions enhanced by the Apexum procedure: a clinical trial. J Endod. 2009;35:153.
259. Metzger Z, Huber R, Tobis I, Better H. Enhancement of healing kinetics of periapical lesions in dogs by the Apexum procedure. J Endod. 2009;35:40.
260. Metzger Z, Teperovich E, Zary R, Cohen R, Hof R. Respecting the root canal: a new concept of a Self Adjusting File (SAF). J Endod. 2009. Submitted for publication
261. Michaelson RE, Seidberg BH, Guttuso J. An in vivo evaluation of interface media used with the electric pulp tester. J Am Dent Assoc. 1975;91:118.
262. Mickel AK, Lindquist KAD, Chogle S, Jones JJ, Curd F. Electric pulp tester conductance through various interface media. J Endod. 2006;32:1178.
263. Mickel AK, Nguyen TH, Chogle S. Antimicrobial activity of endodontic sealers on Enterococcus faecalis. J Endod. 2003;29:257.
264. Mickel AK, Wright ER. Growth inhibition of Stretococcus anginosus (Milleri) by three calcium hydroxide sealer and one zinc oxide-eugenol sealer. J Endod. 1999;25:34.
265. Mikrogeorgis G, Lyroudia K, Moyvdas I, Nikolaidis N, Pitas I. Digital radiograph registration and subtraction: a useful tool for the evaluation of the progress of chronic apical periodontitis. J Endod. 2004;30:513.
266. Miserendino LJ, Moser JB, Heuer MA, Osetek EM. Cutting efficiency of endodontic instruments. I. A quantitative comparison of the tip and fluted region. J Endod. 1985;11:435.
267. Miserendino LJ, Moser JB, Heuer MA, Osetek EM. Cutting efficiency of endodontic instruments. II. An analysis of the design of the tip. J Endod. 1986;12:8.
268. Miwa Z, Ikawa M, Iijima H, Saito M, Takagi Y. Pulpal blood flow in vital and nonvital young permanent teeth measured by transmitted-light photoplethysmography: a pilot study. Pediatr Dent. 2002;24:594.
269. Miyagak DC, de Carvalho EM, Robazza CR, Chavasco JK, Levorato GL. In vitro evaluation of the antimicrobial activity of endodontic sealers. Braz Oral Res. 2006;20:303.
270. Mjör IA, Moorhead JE, Dahl JE. Reasons for replacement of restorations in permanent teeth in general dental practice. Int Dent J. 2000;50:361.
271. Mohammadi Z, Khademi AA, Davari AR. Evaluation of the antibacterial substantivity of three concentrations of chlorhexidine in bovine root dentine. Iranian Endod J. 2008;2:113.
272. Möller ÅJR. Microbiological examination of root canals and periapical tissues of human teeth. Odontol Tidskr. 1966;74:1. (special issue)
273. Monticelli F, Sadek FT, Schuster CG, et al. Efficacy of two contemporary single-cone filling techniques in preventing bacterial leakage. J Endod. 2007;33:310.
274. Monticelli F, Sword J, Martin RL, et al. Sealing properties of two contemporary single-cone obturation systems. Int Endod J. 2007;40:374.
275. Mora MA, Mol A, Tyndall DA, Rivera EM. In vitro assessment of local computed tomography for the detection of longitudinal tooth fractures. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:825.
276. Moser JB, Heuer MA. Forces and efficacy in endodontic irrigation systems. Oral Surg Oral Med Oral Pathol. 1982;53:425.
277. Moshonov J, Slutzky-Goldberg I. Apex locators: an update and prospects for the future. Int J Comput Dent. 2004;7:359.
278. Moshonov J, Slutzky-Goldberg I. Apex locators: update and prospects for the future. Int J Comp Dent. 2004;7(4):359.
279. Muller P, Guggenheim B, Schmidlin PR. Efficacy of gasiform ozone and photodynamic therapy on a multispecies oral biofilm in vitro. Eur J Oral Sci. 2007;115:77.
280. Mumford JM. Evaluation of gutta percha and ethyl chloride in pulp-testing. Br Dent J. 1964;116:338.
281. Narhi MVO, Virtanen A, Kuhta J, Huopaniemi T. Electrical stimulation of teeth with a pulp tester in the cat. Scand J Dent Res. 1979;87:32.
282. Nagayoshi M, Kitamura C, Fukuizumi T, Nishihara T, Terashita M. Antimicrobial effect of ozonated water on bacteria invading dentinal tubules. J Endod. 2004;30:778.
283. Nielsen BA, Craig BJ. Comparison of the Endovac System to needle irrigation of root canals. J Endod. 2007;33:611.
284. Nissan R, Trope M, Zheng C-D, Chance B. Dual wavelength spectrophotometry as a diagnostic test of the pulp chamber contents. Oral Surg Oral Med Oral Pathol. 1992;74:508.
285. Noiri Y, Katsumoto T, Azakami H, Ebisu S. Effects of Er:YAG laser radiation on biofilm-forming bacteria associated with endodontic pathogens in vitro. J Endod. 2008;34:826.
286. Okano M, Nomura M, Hata S, et al. Anaphylactic symptoms due to chlorhexidine gluconate. Arch Dermatol. 1989;125:50.
287. Oliveira RG, Gouw-Soares S, Baldochi SL, Edwardo CP. Scanning electron microscopy: effect of Er:YAG and Nd:YAG lasers on apical seals after apicoectomy and retrofill. Photomed Laser Surg. 2004;23:533.
288. Onay EO, Unger M, Ozdemir BH. In vivo evaluation of the biocompatibility of a new resin-based obturation material. Oral Surg Oral Med Oral Pathol. 2007;104:e60.
289. Oncag O, Hosgor M, Hilmioglu S, Zekioglu O, Eronat C, Burhanoglu D. Comparison of antibacterial and toxic effects of various root canal irrigants. Int Endod J. 2003;36:423.
290. Ørstavik D, Haapasalo M. Disinfection by endodontic irrigants and dressings of experimentally infected dentinal tubules. Endod Dent Traumatol. 1990;6:142.
291. Oztan MD, Kiyan M, Gerceker D. Antimicrobial effect, in vitro, of gutta-percha points containing root canal medications against yeasts and Enterococcus faecalis. Oral surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:410.
292. Pantera EA, Anderson RW, Pantera CT. Reliability of electric pulp testing after pulpal testing with dichlorodi-fluoromethane. J Endod. 1993;19:312.
293. Paquette L, Legner M, Fillery ED, Friedman S. Antibacterial efficacy of chlorhexidine gluconate intracanal medication in vivo. J Endod. 2007;33:788.
294. Park DS, Lee HJ, Yoo HM, Oh TS. Effect of Nd:YAG laser irradiation of the apical leakage of obturated root canals: an electrochemical study. Int Endod J. 2001;34:318.
295. Pascon E, Spångberg LS. In vitro cytotoxicity of root canal filling materials. Part I. Gutta-percha. J Endod. 1990;16:429.
296. Paurazas SB, Geist JR, Pink FE, Hoen MM, Steiman HR. Comparison of diagnostic accuracy of digital imaging by using CCD and CMOS-APS sensors with E-speed film in the detection of periapical bony lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:356.
297. Pawinska M, Skrzydlewska E. Release of hyroxcyl ions from calcium hydroxide preparations used in endodontic treatment. Rocz Akad Med Bialymst. 2003;48:145.
298. Perez SB, Tejerina DP, Perez Tito RI, Bozza FL, Kaplan AE, Molgatini SL. Endodontic microorganisms susceptabilty by direct contact test. Acta Odontol Latinoam. 2008;21:169.
299. Perin FM, Franca SC, Silva-Sousa YT, et al. Evaluation of the microbial effect of Er:YAG laser irradiation versus 1% sodium hypochlorite irrigation for root canal disinfection. Aust Endod J. 2004;30:20.
300. Peters DD. Evaluation of the effects of carbon dioxide used as a pulp test. Part III: in vivo effect on human enamel. J Endod. 1986;12:13.
301. Peters LB, Van Winkelhoff AJ, Buijs JF, Wesselink PR. Effects of instrumentation, irrigation and dressing with calcium hydroxide on infection in pulpless teeth with periapical bone lesions. Int Endod J. 2002;35:13.
302. Peters OA. Current challenges and concepts in the preparation of root canal systems: a review. J Endod. 2004;30:559.
303. Pham NH, Weiner JM, Reisner GS, Baldo BA. Anaphylaxis to chlorhexidine. Case report. Implication of immunoglobulin E antibodies and identification of an allergenic determinant. Clin Exp Allergy. 2000;30:1001.
304. Pizzo G, Giammanco GM, Cumbo E, Nicolosi G, Gallina G. In vitro antibacterial activity of endodontic sealers. J Dent. 2006;34:35.
305. Podbielski A, Spahr A, Haller B. Additive anitmiccrobial activity of calcium hydroxide and chlorhexidine on common endodontic bacterial pathogens. J Endod. 2003;29:340.
306. Popescu IG, Popescu M, Man D. Drug allergy: incidence in terms of age and some drug allergens. Med Intern. 1984;22:195.
307. Portenier I, Waltimo T, Orstavik D, Haapasalo M. Killing of Enterococcus faecalis by MTAD and chlorhexidine digluconate with or without cetrimide in the presence or absence of dentine powder or BSA. J Endod. 2006;32:138.
308. Powell SE, Simon JHS, Maze B. A comparison of the effect of modified and nonmodified instrument tips on apical canal configuration, Part 1. J Endod. 1986;12:293.
309. Powell SE, Wong PD, Simon JHS. A comparison of the effect of modified and nonmodified instrument tips on apical canal configuration. Part 2. J Endod. 1988;14:224.
310. Rapisarda E, Bonaccorso A, Tripi TR, Condorelli GG, Torrisi L. Wear of nickel-titanium endodontic instruments evaluated by scanning electron microscopy: effect of ion implantation. J Endod. 2001;27:588.
311. Rasimick BJ, Nekich M, Hladek MM, Musikant BL, Deutsch AS. Interaction between chlorhexidine digluconate and EDTA. J Endod. 2008;34:1521.
312. Richman MJ. The use of ultrasonics in root canal therapy and root resection. J Med. 1957;12:12.
313. Rickard GD, Richardson R, Johnson T, Mccoll D, Hooper L: Ozone therapy for the treatment of dental caries. Cochrane Database Syst Rev, CD004153, 2004.
314. Rickoff B, Trowbridge H, Baker J, Fuss Z, Bender IB. Effects of thermal vitality tests on human dental pulp. J Endod. 1988;14:482.
315. Ringel AM, Patterson SS, Newton CW, Miller CH, Mulhern JM. In vivo evaluation of chlorhexidine gluconate solution and sodium hypochlorite solution as root canal irrigants. J Endod. 1982;8:200.
316. Roane JB, Sabala CL, Duncanson MG. The “balanced force” concept for instrumentation of curved canals. J Endod. 1985;11:203.
317. Rölla G, Löe H, Schiott CR. The affinity of chlorhexidine for hydroxyapatite and salivary mucins. J Periodont Res. 1970;5(Suppl):90.
318. Roy RA, Ahmad M, Crum LA. Physical mechanisms governing the hydrodynamic response of an oscillating ultrasonic file. Int Endod J. 1994;27:197.
319. Rowe AHR, Pitt Ford TR. The assessment of pulpal vitality. Int Endod J. 1990;23:77.
320. Ruddle CJ. The ProTaper endodontic system: geometries, features and guidelines for use. Dent Today. 2001;20:60.
321. Sabala CL, Roane JB, Southard LZ. Instrumentation of curved canals using a modified tipped instrument: a comparison study. J Endod. 1988;14:59.
322. Safavi KE, Nichols FC. Alteration of biological properties of bacterial lipopolysaccharide by calcium hydroxide treatment. J Endod. 1994;20:127.
323. Safavi KE, Nichols FC. Effect of calcium hydroxide on bacterial lipopolysaccharide. J Endod. 1993;19:76.
324. Safavi KE, Spångberg L, Langeland K. Root canal dentinal tubule disinfection. J Endod. 1990;16:207.
325. Saleh IM, Ruyter IE, Haapasalo M, Ørstavik D. The effects of dentin pretreatment on the adhesion of root canal sealers. Int Endod J. 2002;35:859.
326. Santos C, Sousa-Neto MD, Alfredo E, Guerisoli DM, Percora JD, Comelli Lea RF. Morphologic evaluation of the radicular dentin irradiated with Nd:YAG laser under different parameters and angles of incidence. Photomed Laser Surg. 2005;23:590.
327. Sathorn C, Parashos P, Messer HH. Effectiveness of single- versus multiple-visit endodontic treatment of teeth with apical periodontitis: a systematic review and meta-analysis. Int Endod J. 2005;38:347.
328. Sato I, Ando-Kurihara N, Kota K, Iwaku M, Hoshino E. Sterilization of infected root-canal dentine by topical application of a mixture of ciprofloxacin, metronidazole and minocycline in situ. Int Endod J. 1996;29:118.
329. Saunders EM. In vivo findings associated with heat generation during thermomechanical compaction of gutta-percha. 1. Temperature levels at the external surface of the root. Int Endod J. 1990;23:263.
330. Schäfer E. Irrigation of the root canal. ENDO. 2007;1:11-28.
331. Schäfer E, Zandbiglari T. Solubility of root canal sealers in water and artificial saliva. Int Endod J. 2003;36:660.
332. Schilder H, Goodman A, Aldrich W. The thermomechanical properties of gutta-percha I. The compressibility of gutta-percha. Oral Surg Oral Med Oral Pathol. 1974;37:946.
333. Schilder H, Goodman A, Aldrich W. The thermomechanical properties of gutta-percha, III. Determination of phase transition temperatures for gutta-percha. Oral Surg Oral Med Oral Pathol. 1974;38:109.
334. Schilder H, Goodman A, Aldrich W. The thermomechanical properties of gutta-percha, V. Volume changes in bulk gutta-percha as a function of temperature and its relationship to molecular phase transformation. Oral Surg Oral Med Oral Pathol. 1985;59:285.
335. Schnettler JM, Wallace JA. Pulse oximetry as a diagnostic tool of pulpal vitality. J Endod. 1991;17:488.
336. Schoop U, Goharkhay K, Klimscha J, et al. The use of erbium, chromium:yttrium-scandium-gallium-garnet laser in endodontic treatment: the results of an in vitro study. J Am Dent Assoc. 2007;138:949.
337. Schoop U, Moritz A, Kluger W, et al. The Er:YAG laser in endodontics: results of an in vitro study. Lasers Surg Med. 2002;30:360.
338. Schuur AH, Moorer WR, Wesselink PR. Solvents for the removal of gutta-percha from root canals. 2. Side effects of Chloroform, halothane and xylene. Ned Tijdschr Tandheelkd. 2004;111:303.
339. Schroeder A. Ledermix 1962–Ledermix today. Evaluation after 13 years of experience. Zahnarztl Prax. 1975;26(9):195-196. May 2
340. Scully C, Ng YL, Gulabivala K. Systemic complications due to endodontic manipulations. Endod Topics. 2003;4:60.
341. Seltzer S, Bender IB, Ziontz M. The dynamics of pulpal inflammation: correlation between diagnostic data and actual histological findings in the pulp. Oral Surg Oral Med Oral Pathol. 1963;16:973.
342. Senia ES, Marraro RV, Mitchell JL, Lewis AG, Thomas L. Rapid sterilization of gutta-percha cones with 5.25% sodium hypochlorite. J Endod. 1975;1:136.
343. Senia ES, Marshal FJ, Rosen S. The solvent action of sodium hypochlorite on pulp tissue of extracted teeth. Oral SurgOral Med Oral Pathol. 1971;31:96.
344. Serper A, Calt S, Dogan AL, Gue D, Ozcelik B, Kuraner T. Comparison of the cytotoxic effects and smear layer removing capacity of oxidative potential water, NaOCl, EDTA. J Oral Sci. 2001;43:233.
345. Seto BG, Nicholls JI, Harrington GW. Torsional properties of twisted and machined endodontic files. J Endod. 1990;16:355.
346. Shabahang S, Pouresmail M, Torabinejad M. In vitro antimicrobial efficacy of MTAD and sodium hypochlorite. J Endod. 2003;29:450.
347. Shabahang S, Torabinejad M. Effect of MTAD on Enterococcus faecalis-contaminated root canals of extracted human teeth. J Endod. 2003;29:576.
348. Shipper G, Ørstavik D, Teixeira F, Trope M. An evaluation of microbial leakage in roots filled with a thermoplastic synthetic polymer-based root canal filling material (Resilon). J Endod. 2004;30:342.
349. Short RD, Dorn SO, Kutter S. The crystallization of sodium hypochlorite on gutta-percha cones after the rapid-sterilization technique: an SEM study. J Endod. 2003;29:670.
350. Shuping GB, Ørstavik D, Sigurdsson A, Trope M. Reduction of intracanal bacteria using nickel-titanium rotary instrumentation and various medications. J Endod. 2000;26:751.
351. Shur Al, Sedgley CM, Fenno JC. The antimicrobial efficacy of ‘MPG’ gutta-percha in vitro. Int Endod J. 2003;36:616.
352. Sigurdsson A. Pulpal diagnosis. Endod Topics. 2003;5:12.
353. Sipert CR, Hussne RP, Nishiyama CK, Torres SA. In vitro antimicrobial activity of Fill Canal, Sealarex, Mineral Trioxide Aggregate, Portland Cement and EndoRez. Int Endod J. 2005;38:539.
354. Siqueira JFJr, De Uzeda M. Intracanal medicaments: evaluation of the antibacterial effects of chlorhexidine, metronidazole, and calcium hydroxide associated with three vehicles. J Endod. 1997;23:167.
355. Siqueira JFJr, Favieri A, Gahyva SM, Moraes SR, Lima KC, Lopes HP. Antimicrobial activity and flow rate or newer and established root canal sealers. J Endod. 2000;26:274.
356. Siqueira JFJr, Paiva SS, Rocas IN. Reduction in the cultivable bacterial populations in infected root canals by a chlorhexidine-based antimicrobial protocol. J Endod. 2007;33:541.
357. Siqueira JFJr, Rocas IN, Paiva SS, Guimaraes-Pinto T, Magalhaes KM, Lima KC. Bacteriologic investigation of the effects of sodium hypochlorite and chlorhexidine during the endodontic treatment of teeth with apical periodontitis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:122.
358. Siren EK, Lavonious E, Kontakiotis E. Effects of Ca(OH)2 gutta-percha points on bacteria in root canals. J Dent Res. 2000;79:543.
359. Sirtes G, Waltimo T, Schaetzle M, Zehnder M. The effects of temperature on sodium hypochlorite short-term stability, pulp dissolution capacity, and antimicrobial efficacy. J Endod. 2005;31:669-671.
360. Sjögren U, Figdor D, Spångberg LSW, Sundqvist G. The antimicrobial effect of calcium hydroxide as a short-term intracanal dressing. Int Endod J. 1991;24:119.
361. Sjögren U, Sundqvist G. Bacteriologic evaluation of ultrasonic root canal instrumentation. Oral Surg Oral Med Oral Pathol. 1987;63:366.
362. Sjögren U, Sundquist G, Nair PNR. Tissue reaction to gutta-percha particles of various sizes when implanted subcutaneously in guinea pigs. Eur J Oral Sci. 1995;103:313.
363. Snellman E, Rantanen T. Severe anaphylaxis after a chlorhexidine bath. J Am Acad Dermatol. 1999;40:771.
364. Soares JA, Leonardo MR, Da Silva LA, Tanomaru Filho M, Ito IY. Effect of rotary instrumentation and of the association of calcium hydroxide and chlorhexidine on the antisepsis of the root canal system in dogs. Braz Oral Res. 2006;20:120.
365. Solovyeva AM, Dummer PMH. Cleaning effectiveness of root canal irrigation with electrochemically activated anolyte and catholyte solutions: a pilot study. Int Endod J. 2000;33:494.
366. Soukos NS, Chen PS, Morris JT, et al. Photodynamic therapy for endodontic disinfection. J Endod. 2006;32:979.
367. Sousa-Neto MD, Marchesan MA, Pecora JD, Junior AB, Silva-Souse YT, Saquy PC. Effect of Er:YAG laser on adhesion of root canal sealers. J Endod. 2002;28:185.
368. Sousa-Neto MD, Silva Coelho FI, Marchesan MA, Alfredo E, Silva-Sousa YT. Ex vivo study of the adhesion of an epoxy based sealer to human dentin submitted to irradiation with Er:YAG and Nd:YAG lasers. Int Endod J. 2005;38:866.
369. Southard SR, Drisko CL, Killoy WJ, Cobb CM, Tira DE. The effect of 2% chlorhexidine digluconate irrigation on clinical parameters and the level of Bacteroides gingivalis in periodontal pockets. J Periodontol. 1989;60:302.
370. Spångberg L, Engström B, Langeland K. Biologic effects of dental materials. 3. Toxicity and antimicrobial effect of endodontic antiseptics in vitro. Oral Surg Oral Med Oral Pathol. 1973;36:856.
371. Spångberg L, Rutberg M, Rydinge E. Biological effects of endodontic antimicrobial agents. J Endod. 1979;5:166.
372. Spångberg LSW, Barbosa SV, Lavigne GD. AH26 releases formaldehyde. J Endod. 1993;19:596.
373. Spratt DA, Pratten J, Wilson M, Gulabivala K. An in vitro evaluation of the antimicrobial efficacy of irrigants on biofilms of root canal isolates. Int Endod J. 2001;34:300.
374. Stabholtz A, Sahar-Helft S, Moshonov J. Laser in endodontics. Dent Clin North Am. 2004;48:809.
375. Stabholz A, Moshonov J, Sahar-Helft S, Rocca JP. Lasers in endodontics. In Ingle JI, Bakland LK, Baumgartener JC, editors: Ingle’s endodontics, ed 6, Hamilton, ON, Canada: BC Decker Inc, 2008.
376. Stabholz A, Khayat A, Ravanshad SH, McCarthy DWD, Neev J, Torabinejad M. Effects of Nd:YAG laser on apical seal of teeth after apicoectomy and retrofill. J Endod. 1992;18:371.
377. Stabholz A, Khayat A, Weeks DA, Neev J, Torabinejad M. A scanning electron microscopic study of the apical dentine surfaces lased with Nd:YAG laser following apicectomy and retrofill. Int Endod J. 1992;25:288.
378. Staehle HJ, Spiess V, Heinecke A, Muller HP. Effect of root canal filling materials containing calcium hydroxide and the alkalinity of root dentin. Endod Dent Traumatol. 1995;11:163.
379. Stenman E, Spångberg LSW. Machining efficiency of Flex-R, K-Flex, Trio-Cut, and S-Files. J Endod. 1990;16:575.
380. Stock CJR. Current status of the use of ultrasound in endodontics. Int Dent J. 1991;41:175.
381. Sunada I. New method for measuring the length of the root canal. J Dent Res. 1962;41:375.
382. Sweatman TL, Baumgartner JC, Sakaguchi RL. Radicular temperatures associated with thermoplasticized gutta-percha. J Endod. 2001;27:512.
383. Szep S, Grumann L, Ronge K, Schriever A, Schultz M, Heidemann D. In vitro cytoxicity of medicated and nonmedicated gutta-percha points in cultures of gingival fibroblasts. J Endod. 2003;29:36.
384. Takeda FH, Harashima T, Kimura Y, Matsumoto K. A comparative study of the removal of smear layer by three endodontic irrigants and two types of lasers. Int Endod J. 1999;32:32.
385. Tani-Ishii N, Teranara T. Clinical and radiographic evaluation of root canal obturation with Obtura II. J Endod. 2003;29:739.
386. Tanomaru Filho M, Leonardo MR, Da Silva LA. Effect of irrigating solution and calcium hydroxide root canal dressing on the repair of apical and periapical tissues of teeth with periapical lesion. J Endod. 2002;28:295.
387. Tanomaru-Filho M, Tanomaru JM, Barros DB, Wantanabe E, Ito IY. In vitro antimicrobial activity of endodontic sealers, MTA-based cements and Portland cement. J Oral Sci. 2007;49:41.
388. Tasman F, Cehreli ZC, Ogan C, Etikan I. Surface tension of root canal irrigants. J Endod. 2000;26:586.
389. Tay FR, Hosoya Y, Loushine RJ, Pashley DH, Weller RN, Low DC. Ultrastructure of intraradicular dentin after irrigation with biopure MTAD. II. The consequence of obturation with an epoxy resin-based sealer. J Endod. 2006;32:473.
390. Tay FR, Pashley DH, Loushine RJ, et al. Ultrastructure of smear layer-covered intraradicular dentin after irrigation with biopure MTAD. J Endod. 2006;32:218.
391. Thibodeau B, Teixeira F, Yamauchi M, Caplan DJ, Trope M. Pulp revascularization of immature dog teeth with apical periodontitis. J Endod. 2007;33:680.
392. Torabinejad M, Johnson WB (Inventors, Assignee): Irrigation solution and methods for use, US Patent and Trademark Office, December 25, 2003.
393. Torabinejad M, Shabahang S, Aprecio RM, Kettering JD. The antimicrobial effect of MTAD: an in vitro investigation. J Endod. 2003;29:400.
394. Torabinejad M, Shabahang S, Bahjri K. Effect of MTAD on postoperative discomfort: a randomized clinical trial. J Endod. 2005;31:171.
395. Treudler R, Richter G, Geier J, Schnuch A, Orfanos CE, Tebbe B. Increse in sensitization to oil of turpentime: recent data from a Multicenter Study on 45,005 patients from the German-Austrian Information Network of Departments of Dermatology (IVDK). Contact Dermatitis. 2001;42:68.
396. Trope M, Jaggi J, Barnett F, Tronstad L. Vitality testing of teeth with a radiation probe using 133 xenon radioisotope. Endod Dent Traumatol. 1986;2:215.
397. Trope M, Sigurdsson A. Clinical manifestations and diagnosis. In: Ørstavik D, Pitt Ford TR, editors. Essential endodontology: prevention and treatment of apical periodontitis. Oxford: Blackwell Science, 1998.
398. Turesky S, Warner V, Lin PS, Soloway B. Prolongation of antibacterial activity of chlorhexidine adsorbed to teeth. Effect of sulfates. J Periodontol. 1977;48:646.
399. Tyndall DA, Kapa SF, Bagnell CP. Digital subtraction radiography for detecting cortical and cancellous bone changes in the periapical region. J Endod. 1990;16:173.
400. Usacheva MN, Teichert MC, Biel MA. Comparison of the methylene blue and toluidine blue photobactericidal efficacy against gram-positive and gram-negative microorganisms. Lasers Surg Med. 2001;29:165.
401. Vahdaty TR, Ford Pitt, Wilson RF. Efficacy of chlorhexidine in disinfecting dentinal tubules in vitro. Endod Dent Traumatol. 1993;9:243-248.
402. van der Sluis LWM, Wu MK, Wesselink PR. A comparison between a smooth wire and a K-file in theremoval of artificially placed dentine debris in plastic blocks. Int Endod J. 2005;38:593.
403. van der Sluis LWM, Wu MK, Wesselink PR. The efficacy of ultrasonic irrigation to remove artificially placed dentine debris from human root canals prepared using instruments of varying taper. Int Endod J. 2005;38:764.
404. Vezzani MS, Pietro R, Silva-Sousa YT, Brugnera-Junior A, Sousa-Neto MD. Disinfection of root canals using Er:YAG laser at different frequencies. Photomed Laser Surg. 2006;24:499.
405. Vianna ME, Horz HP, Gomes BP, Conrads G. In vivo evaluation of microbial reduction after chemo-mechanical preparation of human root canals containing necrotic pulp tissue. Int Endod J. 2006;39:484.
406. Villalobos RL, Moser JB, Heuer MA. A method to determine the cutting efficiency of root canal instruments in rotary motion. J Endod. 1980;6:667.
407. Virducic D, Jukietc S, Karlovic Z, Miletic L, Anic I. Removal of gutta-percha from root canals using Nd:YAG laser. Int Endod J. 2003;36:670.
408. Vivacqua-Gomes N, Ferraz CC, Gomes BP, Zaia AA, Teixeira FB, Souza-Filho FJ. Influence of irrigants on the coronal microleakage of laterally condensed gutta-percha root fillings. Int Endod J. 2002;35:791-795.
409. Wainwright M. Photodynamic antimicrobial chemotherapy (PACT). J Antimicrob Chemother. 1998;42:13.
410. Wainwright M, Crossley KB. Photosensitising agents—circumventing resistance and breaking down biofilms: a review. Int Biodeterioration Biodegradation. 2004;53:119.
411. Wainwright M, Phoenix DA, Laycock SL, Wareing DR, Wright PA. Photobactericidal activity of phenothiazinium dyes against methicillin-resistant strains of Staphylococcus aureus. FEMS Microbiol Lett. 1998;160:177.
412. Walia H, Brantley WA, Gerstein H. An initial investigation of the bending and torsional properties of nitinol root canal files. J Endod. 1988;14:246.
413. Walmsley AD. Ultrasound and root canal treatment: the need for scientific evaluation. Int Endod J. 1987;20:105.
414. Walmsley AD, Williams AR. Effects of constraint on the oscillatory pattern of endosonic files. J Endod. 1989;15:189.
415. Waltimo T, Trope M, Haapasalo M, Ørstavik D. Clinical efficacy of treatment procedures in endodontic infection control and one year follow-up of periapical healing. J Endod. 2005;31:863.
416. Waltimo TM, Ørstavik D, Siren EK, Haapasalo MP. In vitro susceptibility of Candida albicans to four disinfectants and their combinations. Int Endod J. 1999;32:421.
417. Wang QQ, Zhang CF, Yim XZ. Evaluation of the bacteriocidal effect of Er,Cr:YSGG and Nd:YAG lasers in experimentally infected root canals. J Endod. 2007;33:630.
418. Wang X, Sun Y, Kimura Y, Kinoshita J, Ishizaki NT, Matsumo T. Effects of diode laser irradiation on smear layer removal from root canal walls and apical leakage after obturation. Photomed Laser Surg. 2005;23:575.
419. Webber RT, del Rio CE, Brady JM, Segall RO. Sealing quality of a temporary filling material. Oral Surg Oral Med Oral Pathol. 1978;46:123.
420. Weller RN, Brady JM, Bernier WE. Efficacy of ultrasonic cleaning. J Endod. 1980;6:740.
421. Wilder-Smith PE. A new method for the non invasive recording of blood flow in human dental pulp. Int Endod J. 1988;21:307.
422. Willershausen B, Hagedorm B, Tekyatan H, Briseno Marroquin B. Effect of calcium hydroxide and chlorhexidine based gutta-percha points on gingival fibroblasts and epithelial tumor cells. Eur J Med Res. 2004;9:345.
423. Wilkinson K, Beeson T, Kirkpatrick T. Fracture resistance of simulated immature teeth filled with Resilon, gutta-percha, or composite. J Endod. 2007;33:480-483.
424. Wilson BL, Baumgartner JC. Comparison of spreader penetration during lateral compaction of .04 and .02 tapered gutta-percha. J Endod. 2003;29:828.
425. Windley W3rd, Teixeira F, Levin L, Sigurdsson A, Trope M. Disinfection of immature teeth with a triple antibiotic paste. J Endod. 2005;31:439.
426. Winik R, Araki AT, Negrao JA, Bello-Silva MS, Lage-Marques JL. Sealer penetration and marginal permability after apicoectomy varying retrocavity preparation and retrofilling material. Braz Dent J. 2006;17:323.
427. Winrow MJ. Metabolic studies with radiolabelled chlorhexidine in animals and man. J Periodontal Res. 1973;12(Suppl):45.
428. Wolcott JF, Himel VT, Hicks ML. Thermafil retreatment using a new “System B” technique or a solvent. J Endod. 1999;25:761.
429. Wu MK, Van Der Sluis LWM, Wesselink PR. The capability of two hand instrumentation techniques to remove the inner layer of dentine in oval canals. Int Endod J. 2003;36:218.
430. Wuerch RM, Apicella MJ, Mines P, Yancich PJ, Pashley DH. Effect of 2% chlorhexidine gel as an intracanal medication on the apical seal of the root-canal system. J Endod. 2004;30:788.
431. Yamashita JC, Tanomaru Filho M, Leonardo MR, Rossi MA, Silva LA. Scanning electron microscopic study of the cleaning ability of chlorhexidine as a root-canal irrigant. Int Endod J. 2003;36:391.
432. Yesilsoy C, Whitaker E, Cleveland D, Phillips E, Trope M. Antimicrobial and toxic effects of established and potential root canal irrigants. J Endod. 1995;21:513.
433. Yoshioka T, Kobayashi C, Suda H, Sasaki T. An observation of the healing process of periapical lesions by digital subtraction radiography. J Endod. 2002;28:589.
434. Yu DG, Kimura Y, Tomita Y, Nakamura Y, Watanabe H, Matsumoto K. Study on removal effects of filling materials and broken files from root canals using pulsed Nd:YAG laser. J Clin Laser Med Surg. 2000;18:23.
435. Zaia AA, Nakagawa R, Quadros I, et al. An in vitro evaluation of four materials as barriers to coronal microleakage in root-filled teeth. Int Endod J. 2002;35:729.
436. Zakariasen KL, Brayton SM, Collison DM. Efficient root canal placement without chloroform. J Can Dent Assoc. 1990;56:509.
437. Zamany A, Safavi K, Spångberg LSW. The effect of chlorhexidine as an endodontic disinfectant. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:578.
438. Zehnder M, Grawehr M, Hasselgren G, Waltimo T. Tissue-dissolution capacity and dentin-disinfecting potential of calcium hydroxide mixed with irrigating solutions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:608.
439. Zehnder M. Root canal irrigants. J Endod. 2006;32:389.
440. Zehnder M, Söderling E, Salonen J, Waltimo T. Preliminary evaluation of bioactive glass S53p4 as an endodontic medication in vitro. J Endod. 2004;30:220.
441. Zerella JA, Fouad AF, Spångberg LS. Effectiveness of a calcium hydroxide and chlorhexidine digluconate mixture as disinfectant during retreatment of failed endodontic cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:756.
442. Zmener O, Banegas G, Pameijer CH. Coronal micro-leakage of three temporary restorative materials: an in vitro study. J Endod. 2004;30:582.
443. Zmener O, Dominquez FV. Tissue response to a glass ionomer used as an endondontic cement. Oral Surg Oral Med Oral Pathol. 1983;56:198.
444. Zmener O, Pameijer CH. Clinical and radiographic evaluation of a resin-based root canal sealer. Am J Dent. 2004;17:19.
445. Zmener O, Pameijer CH, Serrano SA, Vidueira M, Macchi RL. Significance of moist root canal dentin with the use of methacrylate-based endodonitc sealers: an in vitro coronal dye leakage study. J Endod. 2008;34:76.